CHAPTER 80 Lymphoma in the Cat and Dog
Lymphoma (i.e., malignant lymphoma, lymphosarcoma) is a lymphoid malignancy that originates from solid organs (e.g., lymph nodes, liver, spleen); this distinguishes lymphomas from lymphoid leukemias, which originate in the bone marrow (see Chapter 81).
Etiology and Epidemiology
Early reports stated that approximately 70% of cats with lymphoma have feline leukemia virus (FeLV) infection (Table 80-1). Although the prevalence of viremia in cats with lymphoma varies with the anatomic form of presentation (see later discussion), young cats with lymphoma generally are FeLV-positive, whereas older cats are FeLV-negative. Over the past few years, the prevalence of FeLV infection in cats with lymphoma seen at our clinic has been decreasing. Feline immunodeficiency virus (FIV) infection increases the risk of developing lymphoma in cats; cats infected with FIV are almost six times more likely to develop lymphoma than noninfected cats, whereas cats coinfected with FeLV and FIV are more than 75 times more likely to develop lymphoma than noninfected cats (Shelton et al., 1990). Recently, Louwerens et al. (2005) reported an increase in the prevalence of feline lymphoma, despite the decrease in the prevalence of FeLV infection; this increase was associated with a high prevalence of the gastrointestinal form, extranodal or atypical forms, and FeLV-negative mediastinal forms in young to middle-aged Siamese and oriental breeds.
 TABLE 80-1 Prevalence of Feline Leukemia Virus Infection in Cats with Lymphoma
TABLE 80-1 Prevalence of Feline Leukemia Virus Infection in Cats with Lymphoma
| ANATOMIC FORM | FeLV POSITIVE (%) |
|---|---|
| Alimentary | 30 |
| Mediastinal | 90 |
| Multicentric | 80 |
| Cutaneous | 0 |
In dogs the etiology of lymphomas is considered multifactorial because no single etiologic agent has been identified. However, a genetic component is evident, in that the neoplasm is highly prevalent in certain breeds and bloodlines (Modiano et al., 2005). There is also a distinct breed-related predisposition to lymphoma in dogs, with some breeds, such as the Boxer, Basset Hound, Rottweiler, Cocker Spaniel, Saint Bernard, Scottish Terrier, Airedale Terrier, English Bulldog, and Golden Retriever, being at high risk. At our clinic the breeds most commonly affected are Golden Retrievers, Cocker Spaniels, and Rottweilers.
The age of cats with lymphoma at the time of presentation is bimodal, with the first peak occurring in cats that are approximately 2 years of age and the second one occurring in cats that are approximately 10 to 12 years of age. The cats that make up the first peak are mainly FeLV-positive, whereas those that make up the second peak are predominantly FeLV-negative. As mentioned before, the prevalence of FeLV-positive cats with lymphoma continues to decrease at our clinic. The mean age of FeLV-positive cats with lymphoma when first seen is 3 years, whereas the mean age of FeLV-negative cats with lymphoma is 7 to 8 years. Most dogs with lymphoma are middle-age or older (6 to 12 years of age).
Clinical Features
There are four anatomic forms of presentation in cats and dogs with lymphoma:
The distribution of the different anatomic forms differs between cats and dogs. The multicentric form is the most common in dogs, accounting for more than 80% of all the lymphomas in this species. In cats the alimentary form is the most common. At our clinic, alimentary lymphoma is found in more than 70% of the cats with this neoplasm.
The clinical findings in cats and dogs with lymphoma are related to the anatomic form of the presentation. Animals with the generalized or multicentric form are evaluated because of vague, nonspecific clinical signs; frequently, the owners detect one or more subcutaneous masses (i.e., enlarged lymph nodes) during grooming in an otherwise healthy pet, and this prompts them to seek veterinary care. Occasionally, dogs and cats with lymphoma are evaluated because of nonspecific clinical signs, such as weight loss, anorexia, and lethargy. If the enlarged lymph nodes mechanically obstruct lymphatic drainage, edema occurs; if they compress the airway, coughing is the main presenting complaint.
Physical examination of cats and dogs with multicentric lymphoma usually reveals massive generalized lymphadenopathy, with or without hepatomegaly, splenomegaly, or extranodal lesions (e.g., ocular, cutaneous, renal, neural). The affected lymph nodes are markedly enlarged (5 to 15 times their normal size), painless, and freely movable. A syndrome of reactive (hyperplastic) lymphadenopathy that occurs in cats can mimic the clinicopathologic features of multicentric lymphoma.
Cats and dogs with mediastinal lymphoma are usually evaluated because of dyspnea, coughing, or regurgitation (the latter is more common in cats) of recent onset. Polyuria and polydipsia are common presenting complaints in dogs with mediastinal lymphoma and hypercalcemia; tumor-associated hypercalcemia is extremely rare in cats with lymphoma. The respiratory and upper digestive tract signs are caused by compression from enlarged anterior mediastinal lymph nodes, although malignant pleural effusion can contribute to the severity of the respiratory tract signs. On physical examination the abnormalities are usually confined to the thoracic cavity and consist of decreased bronchovesicular sounds, normal pulmonary sounds displaced to the dorsocaudal thoracic cavity, a dull sound heard on percussion of the ventral thoracic cavity, and a noncompressible anterior mediastinum (in cats). Unilateral or bilateral Horner’s syndrome may occur in cats (and occasionally dogs) with mediastinal lymphoma. Some dogs with mediastinal lymphoma have marked head and neck edema caused by compression from enlarged lymph nodes (anterior vena cava syndrome).
Cats and dogs with an alimentary lymphoma usually display gastrointestinal tract signs, such as vomiting, anorexia, diarrhea, and weight loss. Occasionally, signs compatible with an intestinal obstruction or peritonitis (caused by rupture of a lymphomatous mass) occur. Physical examination typically reveals an intraabdominal mass or masses (e.g., enlarged mesenteric or ileocecocolic lymph nodes or intestinal masses) and thickened bowel loops (in patients with diffuse small intestinal lymphoma). Rarely, polypoid lymphomatoid masses can protrude through the anus in dogs with colorectal lymphoma.
The clinical signs and physical examination findings in cats and dogs with extranodal lymphomas are extremely variable and depend on the location of the mass or masses. In general, the clinical signs stem from the compression or displacement of normal parenchymal cells in the affected organ (e.g., azotemia in renal lymphoma, variable neurologic signs in central nervous system [CNS] lymphoma). The typical clinical signs and physical examination findings in cats and dogs with extranodal lymphomas are summarized in Table 80-2. Common extranodal forms in dogs include cutaneous and ocular lymphomas; in cats they include nasopharyngeal, ocular, renal, and neural lymphomas.
 TABLE 80-2 Clinical Signs and Physical Examination Findings in Dogs and Cats with Extranodal Lymphomas
TABLE 80-2 Clinical Signs and Physical Examination Findings in Dogs and Cats with Extranodal Lymphomas
| ORGAN INVOLVED | CLINICAL PRESENTATION | PHYSICAL FINDING(S) |
|---|---|---|
| CNS | Solitary or multifocal CNS signs | Any neurologic finding |
| Eye | Blindness, infiltrates, photophobia | Infiltrates, uveitis, RD, glaucoma |
| Kidney | PU/PD, azotemia, erythrocytosis* | Renomegaly, renal masses |
| Lung | Coughing, dyspnea | None, radiographic changes |
| Skin | Any primary or secondary lesion | Any primary or secondary lesion |
CNS, Central nervous system; RD, retinal detachment; PU/PD, polyuria/polydipsia.
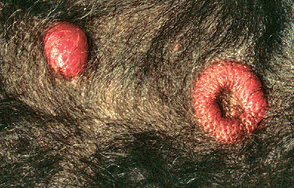
Cutaneous lymphoma is one of the most common extranodal forms of lymphoma in dogs; it is the most common extranodal lymphoma in dogs at our clinic, but it is rare in cats. The clinical signs and characteristics of the lesions are extremely variable, and they can mimic any primary or secondary skin lesion. Dogs with mycosis fungoides (an epidermotropic T-cell lymphoma) are usually first evaluated because of chronic alopecia, desquamation, pruritus, and erythema, eventually leading to plaque and tumor formation (Fig. 80-1). Mucocutaneous and mucosal lesions are relatively common, but generalized lymph node involvement may not be seen initially. A characteristic lesion in dogs with this form of lymphoma is a circular, raised, erythematous, donut-shaped, dermoepidermal mass that contains normal skin in the center (Fig. 80-2). Most of the cats with cutaneous lymphoma reported in the literature have been negative for FeLV viremia.
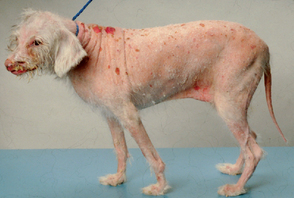
FIG 80-1 Diffuse desquamative dermatopathy in a 13-year-old female spayed dog with mycosis fungoides (a specific type of epidermotropic cutaneous T-cell lymphoma). Clinical signs and lesions were present for almost 2 years.
Ocular lymphoma occurs in both dogs and cats. Ocular involvement in dogs is commonly associated with the multicentric form, whereas both primary ocular involvement and ocular involvement associated with the multicentric form are common in cats. A variety of signs and lesions may be present in these animals, including photophobia, blepharospasm, epiphora, hyphema, hypopyon, ocular masses, third eyelid infiltration, anterior uveitis, chorioretinal involvement, and retinal detachment.
Nasopharyngeal lymphoma is relatively common in cats but is extremely rare in dogs. Clinical signs are similar to those seen in cats with any upper respiratory tract disorder and include sneezing, unilateral or bilateral nasal discharge (ranging from mucopurulent to frankly hemorrhagic), stertorous breathing, exophthalmos, and facial deformity; this is one of the most common forms of presentation of extranodal lymphoma seen in cats at our clinic.
Renal lymphoma is relatively common in cats but rare in dogs. Cats with this anatomic form are first evaluated because of vague clinical signs, usually secondary to chronic renal failure. On physical examination the cat is emaciated and usually anemic and has large, irregular, and firm kidneys; both kidneys are commonly affected. There is a purported association between renal and CNS lymphoma in cats, so some oncologists recommend using antineoplastic drugs that achieve high CNS concentrations (i.e., cytosine arabinoside, lomustine) in the treatment of cats with renal involvement in an attempt to prevent secondary CNS dissemination. This association has not been recognized at our clinic.
Cats and dogs with neural lymphoma are evaluated because of a variety of neurologic signs that reflect the location and extent of the neoplasms. Although CNS signs are most common, peripheral nerve involvement may occur occasionally in cats. Three forms of presentation are clinically recognized: solitary epidural lymphoma, neuropil (intracranial or intraspinal) lymphoma (also called true CNS lymphoma), and peripheral nerve lymphoma. The solitary epidural lymphoma is common in young FeLV-positive cats. Neural lymphomas can be primary (e.g., epidural lymphoma), or they may be secondary to the multicentric form; as discussed earlier, secondary CNS lymphoma may ocur in cats with the renal form. A relatively common presentation is that of a CNS relapse in dogs that have been receiving chemotherapy for multicentric lymphoma for months to years; these patients develop acute onset of neurologic signs, typically while the multicentric neoplasm is still in remission. This late CNS relapse is likely related to the fact that most drugs used to treat lymphoma do not cross the blood-brain barrier when used at standard doses; thus the CNS becomes a sanctuary for tumor cells.
A variety of differential diagnoses should be considered in a cat or dog with suspected lymphoma. The clinician should always bear in mind that lymphomas are great imitators; they can mimic numerous different neoplastic and nonneoplastic disorders. The differential diagnoses in cats and dogs with lymphoma are similar to those in patients with leukemia (see Chapter 81).
Occasionally, dogs with lymphoma are evaluated because of clinical signs secondary to a paraneoplastic syndrome (i.e., molecularly mediated distant effects of the neoplasm). Paraneoplastic syndromes that have been encountered in dogs with lymphoma include hypercalcemia, monoclonal and polyclonal gammopathies, immune cytopenias, polyneuropathy, and hypoglycemia. Only hypercalcemia and gammopathies have been documented in cats with this neoplasm, although they are considerably less frequent than in dogs. Of all these syndromes, only humoral hypercalcemia of malignancy in dogs is of clinical relevance.
Hematologic and serum biochemical features
A variety of nonspecific hematologic and serum biochemical abnormalities can be detected in cats and dogs with lymphoma. The hematologic abnormalities result from the infiltration of bone marrow with neoplastic cells, splenic hypofunction or hyperfunction (caused by neoplastic infiltrates), chronic disease, or paraneoplastic immune-mediated abnormalities (i.e., immune hemolytic anemia or thrombocytopenia, both of which are extremely rare). Certain hematologic abnormalities (i.e., monocytosis, leukemoid reactions) may result from the local or systemic production of bioactive substances by the tumor cells (e.g., hematopoietic growth factors, interleukins). The serum biochemical abnormalities result either from the production of bioactive substances by the tumor cells (i.e., paraneoplasia) or from organ failure secondary to neoplastic infiltration. In general, the complete blood count (CBC) and biochemical profile are not diagnostic in cats and dogs with lymphoma.
Common hematologic abnormalities include nonregenerative anemia, leukocytosis, neutrophilia (with or without a left shift), monocytosis, abnormal lymphoid cells in peripheral blood (i.e., lymphosarcoma cell leukemia), thrombocytopenia, isolated or combined cytopenias, and leukoerythroblastic reactions, among others. Lymphocytosis is rare in dogs and cats with lymphoma; when present, it is usually of low magnitude (i.e., <10,000 to 12,000/μl).
Serum biochemical abnormalities are more common in dogs than in cats with lymphoma and consist mainly of hypercalcemia and gammopathies. Hypercalcemia is one of the most common paraneoplastic abnormalities in dogs with lymphoma, occurring in approximately 20% to 40% of the patients; it is extremely rare in cats, and it is more prevalent in dogs with mediastinal lymphoma than in those with the multicentric, alimentary, or extranodal forms. In most dogs with lymphoma and hypercalcemia, the tumor is of T-cell origin.
There are numerous molecular mechanism underlying hypercalcemia in dogs with lymphoma, but in most cases hypercalcemia is thought to occur as a result of the production of a parathormone-like protein, called PTHrp (PTH-related protein), by the neoplastic cells. Markedly increased serum concentrations of 1,25-vitamin D have been documented in human patients with lymphoma and hypercalcemia. We have recently recognized a similar condition in dogs with lymphoma and hypercalcemia (most of the dogs were Boxers with mediastinal T-cell lymphoma).
Hyperproteinemia is another paraneoplastic abnormality that rarely occurs in cats and dogs with lymphoma. It may be secondary to the production of a monoclonal protein by the lymphoma cells and can result in the development of hyperviscosity syndromes. Polyclonal gammopathies may also be present in cats and dogs with lymphoma.
Imaging
Radiographic abnormalities in cats and dogs with lymphoma vary with the different anatomic forms but in general are secondary to lymphadenopathy or organomegaly (i.e., hepatomegaly, splenomegaly, renomegaly); occasionally, the infiltration of other organs (e.g., lungs) may lead to the appearance of additional radiographic abnormalities.
Radiographic changes in cats and dogs with multicentric lymphoma include sternal or tracheobronchial lymphadenopathy or both; interstitial, bronchoalveolar, or mixed pulmonary infiltrates; pleural effusion (rare); intraabdominal lymphadenopathy (e.g., mesenteric or iliac); hepatomegaly; splenomegaly; renomegaly; or intraabdominal masses. Rarely, lytic or proliferative bone lesions are identified on plain abdominal or thoracic radiographs.
In cats and dogs with mediastinal lymphoma, radiographic changes are usually limited to the finding of an anterior (or, more rarely, posterior) mediastinal mass, with or without pleural effusion. In cats and dogs with alimentary lymphoma, abnormalities are rarely detected on plain abdominal radiographs (<50%). When present, they vary in nature but include mainly hepatomegaly, splenomegaly, and midabdominal masses. Positive contrast–enhanced radiography of the upper gastrointestinal tract usually reveals abnormalities in most animals. In a series of dogs with alimentary lymphoma evaluated at our clinic, abnormalities were found in all dogs that underwent positive contrast–enhanced radiography of the upper gastrointestinal tract and included mucosal irregularities, luminal filling defects, and irregular thickening of the wall, suggestive of infiltrative mural disease.
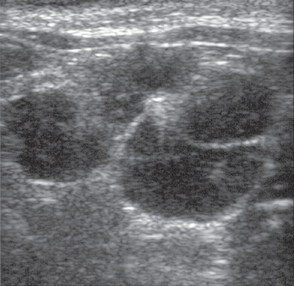
Ultrasonography constitutes an invaluable tool for evaluating cats or dogs with suspected or confirmed intraabdominal lymphoma. The technique is also helpful in the evaluation of mediastinal masses in both species (see Chapter 79). Changes in the echogenicity of parenchymal organs (i.e., liver, spleen, kidneys) detected by this technique usually reflect changes in organ texture secondary to neoplastic infiltration. In addition, enlarged lymphoid structures or organs can easily be identified using this technique. Several abnormalities are commonly detected ultrasonographically in cats and dogs with intraabdominal lymphoma; these include hepatomegaly, splenomegaly, changes in the echogenicity of liver or spleen (mixed echogenicity or multiple hypoechoic areas), intestinal thickening, lymphadenopathy (Fig. 80-3), splenic masses, and effusion. In a study of 11 cats with alimentary lymphoma evaluated ultrasonographically at our clinic, we found hypoechoic masses of the gastric or intestinal wall, focal or diffuse gastric wall thickening, a symmetrical thickening of the intestinal wall, loss of the normal layered appearance of the gastrointestinal wall, and abdominal lymphadenopathy (Grooters et al., 1994). Fine-needle aspiration (FNA) and needle biopsy can also be easily performed using this technique to guide the placement of the needle.
Diagnosis
The clinical signs and physical examination findings described in preceding paragraphs are usually suggestive of lymphoma. However, before instituting therapy, the clinician must confirm the diagnosis cytologically, histopathologically, or molecularly. In addition, a minimum database consisting of a CBC, serum biochemistry profile, and urinalysis should be obtained if the owners are contemplating treatment.
In most cats and dogs with multicentric, superficial extranodal, mediastinal, or alimentary lymphoma, a diagnosis can easily be obtained by FNA cytologic studies of the affected organs or lymph nodes. The techniques for FNA and the cytologic features of lymphoma are described in detail in Chapter 75.
In our practice lymphomas can be diagnosed cytologically in approximately 90% of dogs and 70% to 75% of cats evaluated (i.e., usually in only 10% of the dogs and 25% to 30% of the cats is it necessary to perform a histopathologic, flow cytoemetric, or molecular evaluation of a lymph node or mass to establish a diagnosis). Until there is conclusive evidence that the histopathologic classification of canine and feline lymphomas offers prognostic information, the surgical removal of a lymph node or extranodal mass for histopathologic evaluation in an animal with a cytologic diagnosis of lymphoma is not necessarily indicated. A diagnosis based on cytologic findings rather than histopathologic findings yielded by an excisional lymph node biopsy also offers two major benefits: (1) It is associated with minimal or no morbidity, and (2) it is financially acceptable to most owners (i.e., approximate cost of a lymph node aspirate is $70 to $100; the cost for biopsy and histopathologic evaluation is $300 to $400).
New diagnostic methodologies commonly used in patients with lymphoma in our clinic include immunophenotyping by flow cytometry and clonal analysis by polymerase chain reaction (PCR). In the former, a sample of the affected organ/tissue is obtained by FNA and placed in an appropriate transport media. In the laboratory these cells are incubated with specific antibodies that recognize epitopes specific for T- or B-cells. Flow cytometric evaluation of the sample allows to immunophenotype the cell population. Clonal analysis by PCR also requires an FNA or a small biopsy specimen. Specific laboratories will evaluate the population of cells in question by PCR to determine if they are B- or T-cell in origin and if they are monoclonal or poyclonal. This technique has high sensitivity and specificity for distinguishing reactive lymphadenopathy from lymphoma (Lana et al., 2006).
After a diagnosis of lymphoma is confirmed, it is customary to stage the disease to obtain a prognosis. A staging system devised by the World Health Organization has been used for the past two decades for the staging of cats and dogs with lymphoma (Table 80-3). In this system, derived from the TNM (tumor, node, metastasis) staging system for neoplasms in humans, clinical and clinicopathologic information from the patient is used in an attempt to determine the extent of disease and correlate it with the prognosis. Unfortunately, it cannot be used prognostically (i.e., animals with stage I disease have survival times similar to those of animals with stage IV disease). The only prognostic information of clinical relevance in this system is the fact that asymptomatic (i.e., substage a) dogs with lymphoma have better prognosis than “sick” (i.e., substage b) dogs. A staging system that takes into account tumor bulk and FeLV status in cats with lymphoma provides some prognostic information when cats are treated with a specific chemotherapy protocol (Mooney et al., 1989). Until a new system is devised, it is advisable to determine the prognosis on the basis of the patient’s overall clinical condition, the FeLV status (in cats), and any constitutional signs or severe hematologic and biochemical abnormalities the patient may have. Another important issue is that even though a specific staging protocol may be of some prognostic value in patients treated with a given chemotherapy protocol, it may not be so when a different drug combination is used. Moreover, at this time the effectiveness of more aggressive protocols in dogs and cats with advanced-stage lymphoma is unknown.
 TABLE 80-3 TNM Staging System for Dogs and Cats with Lymphoma
TABLE 80-3 TNM Staging System for Dogs and Cats with Lymphoma
| STAGE | CLINICAL FEATURES |
|---|---|
| I | Solitary lymph node involvement |
| II | More than one lymph node enlarged but on one side of the diaphragm (i.e., cranial or caudal) |
| III | Generalized lymph node involvement |
| IV | Stage III findings, plus hepatomegaly and/or splenomegaly |
| V | Any of the above, plus bone marrow or extranodal involvement substage a: asymptomatic substage b: sick |
TNM, Tumor, node, metastasis.
At least a CBC, a serum biochemistry profile, and a urinalysis should be performed in all cats and dogs with lymphoma whose owners are contemplating therapy. In addition, FeLV and FIV tests should be performed in cats. The resulting minimum database can provide a wealth of information that can help the owner (and the clinician) decide whether to treat the patient. In addition, once a decision to treat the pet has been made, the nature of any clinicopathologic abnormalities usually dictates the treatment or treatments used. For example, in a dog with pronounced cytopenias caused by lymphomatous infiltration of the bone marrow, a highly myelosuppressive chemotherapy combination almost certainly will result in severe neutropenia and sepsis; it should therefore be avoided.
In cats and dogs with suspected CNS lymphoma, it is advisable to perform cerebrospinal fluid (CSF) analysis and advanced imaging (i.e., computed tomography [CT] scan or magnetic resonance imaging [MRI]). The finding of high numbers of neoplastic lymphoid cells and an increased protein concentration in a CSF sample is diagnostic for lymphoma. Because of their poor accessibility, the diagnosis of extradural masses usually requires the collection of a surgical specimen for cytologic or histopathologic evaluation.
As previously discussed, immunophenotyping of canine and feline lymphoma has become routine for most oncologists. This can be done by immunocytochemistry, immunohistochemistry, flow cytometry, or PCR for clonality. Published reports suggest that dogs with T-cell lymphoma treated with standard combination chemotherapy have a worse prognosis for remission and survival than dogs with B-cell tumors; however, in our experience, this is not the case. In a recent study we demonstrated that T-cell phenotype was not a negative prognostic factor in dogs with lymphoma treated with COP- or CHOP-based protocols (Hosoya et al., 2007). This is likely because most dogs with T-cell lymphoma received lomustine (CCNU), a drug that in our experience is effective in patients with T-cell phenotype.
Treatment
Once a diagnosis of lymphoma is established, the prognosis and potential therapeutic options should be discussed with the pet’s owner. Remission rates in cats and dogs with lymphoma treated with various chemotherapy protocols are approximately 65% to 75% and 80% to 90%, respectively. Most cats with lymphoma treated with multiple-agent chemotherapy protocols are expected to live 6 to 9 months; approximately 20% of the cats live more than 1 year. Most dogs with lymphoma treated in a similar fashion are expected to live 12 to 16 months; approximately 20% to 30% of the dogs are alive 2 years after diagnosis. The approximate survival time in untreated cats and dogs with lymphoma is 4 to 8 weeks. Probably the most important reason for the shorter survival times in cats than in dogs with lymphoma is that remissions appear to be difficult to reinduce once the tumor has relapsed. In addition, the retrovirus-associated nonlymphomatous disorders that affect cats with lymphoma lead to shortened survival times (i.e., FeLV infection is a negative prognostic factor in cats with lymphoma).
In my experience, even if an animal has stage I nodal or extranodal lymphoma at the time of presentation, systemic dissemination of the disease usually occurs within weeks to months of diagnosis. However, occasionally solitary oral or cutaneous lymphomas may behave as true stage I diseases (i.e., there is no systemic dissemination). Therefore the mainstay of treatment for animals with lymphoma is chemotherapy, given the fact that lymphomas are (or will become) systemic neoplasms. Surgery, radiotherapy, or both can be used to treat localized lymphomas before or during chemotherapy. Half-body irradiation or chemotherapy and bone marrow transplantation have also been recently used to treat dogs with lymphoma (see Suggested Readings). General guidelines for the management of patients with lymphoma are presented here. The protocols recommended in this chapter have been used at our clinic with a success rate comparable to those of other treatments published in the literature.
There are two main chemotherapeutic approaches in dogs and cats with lymphoma: induction chemotherapy, followed by maintenance (and reinduction) or more aggressive chemotherapy for a finite period of time, at the end of which no maintenance chemotherapy is used. The former is usually done with a less aggressive COP (cyclophosphamide, vincristine, and prednisone)-based protocol, whereas the latter is usually based on CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone)-type protocols. An example of the latter is one of several University of Wisconsin (UW) protocols. CHOP-based protocols are similar to those used in people with high-grade lymphomas.
COP-Based Protocols
When using COP-based protocols, the treatment of cats and dogs with lymphoma is divided into several phases, or strategies: induction of remission, intensification, maintenance, and reinduction of remission or “rescue” (Box 80-1). Immediately after diagnosis, a relatively nonaggressive multiple-agent COP-based chemotherapy protocol is used to induce remission; in our clinic we frequently use the COAP protocol, with the addition of cytosine arabinoside to the COP protocol. During this phase, which lasts 6 to 8 weeks, patients are evaluated weekly by a veterinarian, at which time they receive an intravenous (IV) injection of an antimitotic agent (vincristine) in addition to undergoing a routine physical examination (with or without a CBC). If at the end of this phase the patient is considered to be in complete remission (CR; i.e., all neoplastic masses have completely disappeared), the maintenance phase is initiated. During this phase, a multiple-agent chemotherapy protocol consisting of three drugs (chlorambucil [Leukeran], methotrexate, prednisone [LMP]) administered orally is used, so that the patient requires less intensive monitoring (once every 6 to 8 weeks). In my experience, maintenance chemotherapy is necessary when using COP-based protocols. Over the past few years, we have instructed the owners of pets with multicentric lymphoma to closely monitor the size of the lymph nodes in their pets; when the nodes start enlarging (i.e., relapse), we add a fourth drug to the LMP protocol (usually vincristine, at a dosage of 0.5-0.75 mg/m2, IV, q1-2 weeks). This usually suffices to reinduce remission and maintain it for several weeks or months.
 BOX 80-1 Chemotherapy Protocols Used to Treat Dogs and Cats* with Lymphoma at the Ohio State University Veterinary Teaching Hospital
BOX 80-1 Chemotherapy Protocols Used to Treat Dogs and Cats* with Lymphoma at the Ohio State University Veterinary Teaching Hospital
PO, By mouth; IV, intravenous; SC, subcutaneous; BSA, body surface area; IM, intramuscular.
Cyclophosphamide: 50 mg/m2 PO q48h in dogs or 200-300 mg/m2 PO q3 weeks in cats
Vincristine: 0.5 mg/m2 IV weekly
Cytosine arabinoside: 100 mg/m2 daily as an IV drip or SC for only 2 days in cats and 4 days in dogs
Prednisone: 50 mg/m2 PO q24h for 1 week, then 20 mg/m2 PO q48h
Cyclophosphamide (Cytoxan®): 50 mg/m2 BSA, PO, q48h; or 300 mg/m2 BSA, PO, every 3 weeks (dogs or cats)
Vincristine (Oncovin®): 0.5 mg/m2 BSA, IV, once a week
Prednisone: 40-50 mg/m2 BSA, PO, q24h for a week; then 20-25 mg/m2 BSA, PO, every other day.
| Week 1: | Vincristine 0.5-0.75 mg/m2, IV |
| Asparaginase 400 IU/KG IM or SC | |
| Prednisone 2 mg/kg PO q24h | |
| Week 2: | Cyclophosphamide 200-250 mg/m2, IV |
| Prednisone 1.5 mg/kg PO q24h | |
| Week 3: | Vincristine 0.5-0.75 mg/m2, IV |
| Prednisone 1 mg/kg PO q24h | |
| Week 4: | Doxorubicin 30 mg/m2 (or 1 mg/kg if <10 kg) IV |
| Prednisone 0.5 mg/kg PO q24h | |
| Week 5: | No treatment |
| Week 6: | Vincristine 0.5-0.75 mg/m2, IV |
| Week 7: | Cyclophosphamide 200-250 mg/m2, IV |
| Week 8: | Vincristine 0.5-0.75 mg/m2, IV |
| Week 9: | Doxorubicin 30 mg/m2 (or 1 mg/kg if <10 kg) IV |
| Week 10: | No treatment |
| Week 11: | Vincristine 0.5-0.75 mg/m2, IV |
| Week 12: | Cyclophosphamide 200-250 mg/m2, IV |
| Week 13: | Vincristine 0.5-0.75 mg/m2, IV |
| Week 14: | Doxorubicin 30 mg/m2 (or 1 mg/kg if <10 kg) IV |
| Week 15: | No treatment |
| Week 16: | Vincristine 0.5-0.75 mg/m2, IV |
| Week 17: | Cyclophosphamide 200-250 mg/m2, IV |
| Week 18: | Vincristine 0.5-0.75 mg/m2, IV |
| Week 19: | Doxorubicin 30 mg/m2 (or 1 mg/kg if <10 kg) IV |
l-Asparaginase (Elspar): 10,000-20,000 IU/m2 IM (one or two doses)
Vincristine (Oncovin): 0.5-0.75 mg/m2 IV q1-2 weeks
Dexamethasone: 0.5 mg/lb (0.23 mg/kg) PO or SC on days 1 and 8
Actinomycin D: 0.75 mg/m2 as IV push on day 1
Cytosine arabinoside: 200-300 mg/m2 as IV drip over 4 hours or SC on day 1
Melphalan: 20 mg/m2 PO on day 8§
Doxorubicin (Adriamycin): 30 mg/m2 (or 1 mg/kg for dogs under 10 kg) IV on day 1
Cyclophosphamide (Cytoxan): 100-150 mg/m2 PO on days 15 and 16
Cyclophosphamide (Cytoxan): 200-300 mg/m2 PO on day 10
Doxorubicin (Adriamycin): 30 mg/m2 (or 1 mg/kg for dogs under 10 kg) IV on day 1
Doxorubicin (Adriamycin): 1 mg/kg IV on day 1
Cyclophosphamide (Cytoxan): 200-300 mg/m2 PO on day 10 or 11
Dexamethasone (4 mg/cat q1-2 weeks can be added to this protocol)
Mitoxantrone (Novantrone): 4-6 mg/m2 as IV drip over 4-6 hours on day 1
Cyclophosphamide (Cytoxan): 200-300 mg/m2 PO on day 10 or 11
Dexamethasone (4 mg/cat q1-2 weeks can be added to this protocol)
Mitoxantrone (Novantrone): 4-6 mg/m2 in IV drip over 4-6 hours on day 1
Cyclophosphamide (Cytoxan): 200-300 mg/m2 PO on day 10 or 11
Cytosine arabinoside (Cytosar-U): 200 mg/m2 in IV drip over 4-6 hours (mixed in the same bag with mitoxantrone) on day 1
Dexamethasone (4 mg/cat q1-2 wks can be added to this protocol)
The maintenance or modified maintenance phase continues until the tumor relapses (i.e., is out of remission), at which time the reinduction phase begins. This phase is similar to the induction phase in that intensive treatments are used. Once remission is obtained, the patient is started again on a modified maintenance protocol. If at the end of the induction phase the patient is not in CR, we recommend that intensification with l-Asparaginase be done before the maintenance phase is initiated. In addition to the chemotherapeutic approach discussed in this section, a variety of protocols have been used successfully in the treatment of cats and dogs with lymphoma. (See Suggested Readings for additional information.)
Induction of remission
As previously discussed, my protocol of choice for the induction of remission is COAP. The agents in this protocol consist of cyclophosphamide, vincristine, cytosine arabinoside, and prednisone; these four drugs are currently available as generic products. The dosages are specified in Box 80-1. These drugs belong to four different categories, have different mechanisms of action, and do not have superimposed toxicities (with the exception of cyclophosphamide and cytosine arabinoside, both of which are myelosuppressive; however, the latter is used only for a short period); thus they fulfill the basic criteria of multiple-agent chemotherapy described in Chapter 77. The cytosine arabinoside is usually administered by the subcutaneous (SC) route because, given its short half-life and S-phase–specific mechanism of action, an IV bolus injection results in minimal cell kill; SC administration of this drug is painful in cats (and in some dogs). IV infusion of the agent is also associated with myelosuppression. The induction phase lasts 6 to 8 weeks, and weekly visits to the veterinarian are necessary during this time.
During the induction phase toxicity is minimal (<15%) and client compliance is high because most of the toxic signs are hematologic (i.e., cytopenias) and usually do not result in clinical signs that can be detected by the owners. The dose-limiting toxicity of this induction protocol is hematologic (i.e., myelosuppression leading to neutropenia); the neutrophil nadir usually occurs around day 7 or 8 because two myelosuppressive agents (i.e., cyclophosphamide and cytosine arabinoside) are given during the initial 2 to 4 days of treatment. In most cases the neutropenia is mild (2000 to 3500 cells/μl). The neutropenia is severe if the animals have neoplastic bone marrow infiltration before the initiation of treatment, have FeLV- or FIV-associated myelodysplasia or other retrovirus-associated bone marrow disorders, or receive the cytosine arabinoside by constant-rate IV infusion rather than by the SC route. Also, anecdotally, neutropenia appears to be common in Cocker Spaniels receiving this protocol. Dosage adjustments in cats and dogs that develop neutropenia are described in Chapter 78. Gastrointestinal toxicity is minimal to nonexistent; however, cats receiving cyclophosphamide occasionally become anorectic. Consequently, this drug should be administered once every 3 weeks in cats (as opposed to every other day in dogs; see Box 80-1). If anorexia develops, treatment with cyproheptadine (Periactin; Merck Sharp & Dohme, West Point, Pa.), an antiserotonin compound, at a dosage of 1 to 2 mg per cat PO q8-12 hours is indicated. Hair loss is also minimal, and it occurs primarily in woolly-haired dogs (e.g., Poodle, Bichon Frise); cats (and some dogs) may shed their tactile hairs during treatment.
During this phase, owners are instructed to monitor their pet’s appetite and activity level, measure their lymph nodes (if superficial lymphadenopathy was present initially), and take their pet’s rectal temperature daily (pyrexia is usually secondary to neutropenia and bacteremia or sepsis). If pyrexia develops, owners are instructed to contact their veterinarian immediately so that their pet can undergo a complete physical examination and CBC (for additional information, see Chapter 78). Treatment with COAP results in CR within 1 to 14 days of the start of therapy in most animals (>85% in dogs, >70% in cats). This remission is usually maintained throughout the induction phase.
In dogs with diffuse alimentary lymphoma we use a more aggressive doxorubicin-containing protocol (CHOP; see Box 80-1) because, in my experience, the response rate to COAP is low. This protocol is more expensive and more likely to cause adverse effects than the COAP protocol. We typically use lomustine (CCNU) in dogs with epidermotropic T-cell lymphoma (see Box 80-1).
In dogs and cats with multicentric (or any other anatomic form of) lymphoma coexisting with neurologic signs, we usually use the COAP protocol but administer the cytosine arabinoside as a continuous IV infusion (200-400 mg/m2 as an IV infusion over 24 hours for 1 to 4 days) in order to attain high concentrations of this drug in the CNS. This protocol tends to cause marked myelosuppression in cats, so we typically administer cytosine arabinoside as a 12- to 24-hour infusion (200 mg/m2) in this species. More information on the treatment of dogs and cats with suspected or confirmed CNS lymphoma is given later in this chapter.
Maintenance
The protocol recommended for the maintenance phase of treatment is LMP (“lump”), which consists of chlorambucil, methotrexate, and prednisone (see Box 80-1). These three drugs also act by three different mechanisms of action and have different toxicities. The advantages of this protocol include its reduced cost compared with the cost of the induction phase; its ease of administration (all the drugs are administered orally by the owners); its minimal toxicity; and the fact that intensive monitoring by a veterinarian is not necessary.
The toxicities associated with LMP maintenance chemotherapy are minimal. Of the three drugs in this protocol, methotrexate is the only one that is associated with moderate to severe toxicity. In approximately 25% of dogs and cats receiving methotrexate, gastrointestinal tract signs consisting of anorexia, vomiting, or diarrhea develop. Anorexia and vomiting are more common than diarrhea and usually occur after the patient has been receiving the drug for more than 2 weeks. In these cases treatment with an antiemetic, such as metoclopramide, on the days the animal receives the methotrexate, at a dosage of 0.1 to 0.3 mg/kg PO every 8 hours, alleviates or eliminates the upper gastrointestinal tract signs. We have recently used maropitant (Cerenia, Pfizer Animal Health, Kalamazoo, Mich.) at a dosage of 2 mg/kg PO every 24 hours to prevent chemotherapy-associated nausea and vomiting. Gastroprotectants, such as famotidine (0.5 mg/kg PO q24h) may also be effective in preventing or minimizing this adverse effect. In cases of methotrexate-associated diarrhea, treatment with a bismuth subsalicylate–containing product (Pepto-Bismol) may also alleviate or eliminate the signs; however, it may be necessary to discontinue the drug. Hematologic toxicity associated with LMP therapy is minimal to nonexistent. In a very small proportion of cats (i.e., <5%) receiving chlorambucil for weeks to months, serum biochemical abnormalities consistent with cholestasis that resolve on discontinuation of the drug may develop. Recently, tonic or tonic-clonic convulsions have been decribed in cats receiving chlorambucil.
During this phase the patient is examined every 6 to 8 weeks, at which time a complete physical examination and a CBC are performed. As with the induction protocols, owners are instructed to monitor their pet’s activity, appetite, behavior, rectal temperature, and lymph node size. As previously discussed, over the past few years we have been instructing the owners of pets with multicentric lymphoma to closely monitor the size of the lymph nodes in their pets; when the nodes start enlarging (i.e., relapse), a fourth drug is added to the LMP protocol (usually vincristine, at a dosage of 0.5-0.75 mg/m2, IV, q1-2 weeks). This usually suffices to reinduce remission and maintain it for several weeks or months.
Most animals treated with this protocol remain in remission for approximately 3 to 6 months. If a relapse occurs, reinduction of remission (as discussed next) is instituted. After remission is reinduced, animals can be treated with a modified maintenance protocol, as described in previous paragraphs.
Reinduction of remission or rescue
Virtually every dog and cat with lymphoma treated with induction followed by maintenance chemotherapy eventually relapses; this generally occurs 3 to 6 months after the start of induction therapy (median: approximately 4 months), but it can occur within weeks of starting the maintenance phase or years after the original diagnosis was made. At this time, reinduction of remission is indicated. In my experience, remission can be reinduced one to four additional times in most dogs with relapsing lymphoma. Reinduction of remission is usually not as successful in cats as in dogs (i.e., remission cannot be reinduced in most cats with relapsing lymphoma). Therefore the following discussion on “rescue” pertains mostly to dogs with lymphoma.
There are numerous “rescue” protocols described in the literature, and as a general rule, the practitioner may have difficulty deciding what protocol to choose. We currently use the D-MAC protocol (see Box 80-1), which consists of dexamethasone, melphalan (Alkeran; Burroughs Wellcome, Research Triangle Park, N.C.), cytosine arabinoside (Cytosar-U), and actinomycin D (Cosmegen; Merck Sharp & Dohme, West Point, Pa.) as our trump card for rescue (Alvarez et al., 2006). This protocol results in an over 70% remission rate in dogs with relapsing lymphoma; it has a relatively low toxicity compared with that of doxorubicin-containing protocols, and it is necessary for the owner to go the veterinarian only once every 2 weeks (instead of every week). The median duration of remission using the D-MAC protocol was 61 days (range 2 to 467+ days). Previous use of doxorubicin and failure to induce remission with the induction protocol were negative prognostic factors for response to this protocol. Thrombocytopenia occurred in 56% of the dogs, neutropenia in 17%, and gastrointestinal toxicity in 22%; three of the 56 dogs required hospitalization because of toxicity. Because the long-term use of melphalan is associated with severe chronic thrombocytopenia, chlorambucil (Leukeran), 20 mg/m2, is substituted for melphalan after four cycles. If complete or partial remissions are achieved after the administration of four to six cycles of D-MAC, the patient can be started on a maintenance protocol again.
If the response to D-MAC is poor (i.e., the disease progresses), the CHOP protocol is recommended (see Box 80-1). Our protocol calls for two or three cycles of CHOP once the tumor has relapsed; if CR is obtained, the patient is started on maintenance chemotherapy at the end of the second or third CHOP cycle. The maintenance protocol in these animals also includes LMP, with the possible addition of vincristine (0.5 to 0.75 mg/m2 IV once weekly to every other week, alternating weeks with the chlorambucil) or cytosine arabinoside (200 to 400 mg/m2 subcutaneously every other week, alternating weeks with the chlorambucil).
After a second relapse occurs, D-MAC or CHOP is administered for two additional cycles, as described in the preceding paragraph. In our experience, after the second and third relapses, the percentage of animals in which remission can be easily reinduced decreases with each subsequent cycle. This likely stems from the development of multiple-drug resistance by the tumor cells. Other protocols that have been successful in reinducing remission in dogs with lymphoma are listed in Box 80-1. Although the probability of reinducing remission is considerably lower in cats than in dogs, one of the protocols listed in Box 80-1 can be used for this purpose.
In cats doxorubicin- or mitoxantrone-containing protocols have been used with some degree of success (see Box 80-1); asparaginase-containing protocols may also be used but generally are not as effective as in dogs.
Intensification
If a dog is undergoing induction therapy but only partial remission (PR) is obtained, intensification with one or two doses of l-Asparaginase (Elspar; 10,000 to 20,000 IU/m2 IM, repeated once at a 2- to 3-week interval) may be indicated. This drug can rapidly induce CR in most dogs with lymphoma that have shown only PR while receiving COP-based protocols. Asparaginase should not be used in dogs with a history of pancreatitis or in those that are at high risk for acute pancreatitis (i.e., obese, middle-age female dogs). In my experience, l-Asparaginase appears to be less effective in cats than in dogs; doxorubicin (1 mg/kg IV q3 weeks) or mitoxantrone (4 to 6 mg/m2 IV q3 weeks; Novantrone; Lederle, Wayne, N.J.) can be used as intensifying agents in cats. In a recent study only two of thirteen (15%) cats with lymphoma treated with l-Asparaginase underwent CR, and two of thirteen (15%) underwent PR; these response rates are quite a bit lower than those reported in dogs (i.e., >70%) (LeBlanc et al., 2007).
CHOP-Based Protocols
Although I do not personally use CHOP-based protocols, such as the UW-19 or UW-25, to treat dogs with multicentric lymphoma, I occasionally use them in dogs with diffuse small intestinal lymphoma. However, numerous articles on CHOP-based protocols in dogs with lymphoma have appeared in the literature in the last two decades. The most attractive aspect of using CHOP-based protocols is that the patient is under treatment for a finite period of time (i.e., 19 weeks for the UW-19 and 25 weeks for the UW-25); when the protocol ends, the patient is closely monitored but does not receive additional chemotherapy (i.e., no maintenance). This feature is extremely important in humans undergoing chemotherapy, in whom the prevalence of adverse effects is extremely high and the patient is looking forward to a life without chemotherapy. However, people considering chemotherapy for their pets may not share this sentiment. As a general rule, the probability and severity of toxicity with CHOP-based protocols are higher than with COP-based protocols. Box 80-1 lists the UW-19 protocol, commonly used by numerous oncologists.
Should You Use COP-Based or CHOP-Based Protocols?
Clinicians have been debating the relative merits of COP- and CHOP-based protocols for several years. However, because most institutions or clinicians prefer one protocol over the other, because most of the reports on COP-based protocols are 10 to 20 years old, and because in most reports of COP- or CHOP-based chemotherapy studies the endpoint has been remission times, rather than survival times, a definitive answer is not readily available.
However, in our clinic we have a similar number of patients treated with COP- and CHOP-based (UW-19) protocols; these patients are cared for by the same group of clinicians and technicians. We recently published the results of a retrospective study of 101 dogs with multicentric lymphoma treated with either COP-based protocols with maintenance chemotherapy (n = 71) or CHOP-based protocol (UW-19, n = 30) in our clinic (Hosoya et al., 2007). The probability of achieving CR or PR was similar for both protocols (92% for dogs treated with COP versus 100% for dogs treated with CHOP). Although the median duration of remission was significantly longer in dogs treated with CHOP than in those treated with COP (174 versus 94 days), the median survival times (MST) were not statistically different between groups (Figs. 80-4 and 80-5). The MST in dogs receiving COP was 309 days, compared with 275 days in dogs receiving the UW-19 protocol. The MST was similar for dogs with B- or T-cell lymphoma treated with the COP-based protocols (321 versus 378 days, respectively); there were not enough dogs with T-cell phenotype treated with the UW-19 to perform statistical analysis.
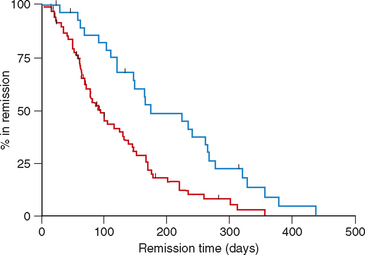
FIG 80-4 Kaplan Meier curves for duration of first remission in dogs with multicentric lymphoma treated with COAP (red line) or CHOP (blue line). The median duration of remission was significantly longer in dogs treated with CHOP chemotherapy (p < 0.01).
(From Hosoya et al., 2008.)
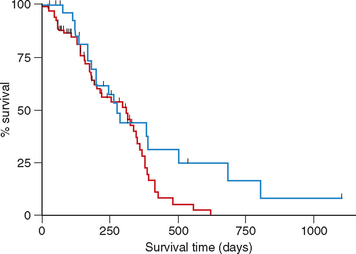
FIG 80-5 Kaplan Meier survival curves in dogs with multicentric lymphoma treated with COAP (red line) or CHOP (blue line). The median duration of remission was not significantly different between groups (p = 0.09).
(From Hosoya et al., 2008.)
The prevalence of severe myelosuppression and adverse gastrointestinal effects was significantly higher in dogs receiving CHOP chemotherapy. The cost of treatment using both protocols was similar. Therefore there is no advantage of one protocol over the other one, and the clinician must make a decision based on a variety of factors (e.g., the owner’s perception, the patient’s clinical signs and other concurrent illnesses, cost).
Management of solitary and extranodal lymphomas
The clinician faces a dilemma when confronted with a dog or cat with a solitary lymphoma, regardless of whether it is nodal (i.e., stage Ia disease) or extranodal (i.e., a solitary cutaneous or oral mass). Should the mass (or lymph node) be treated in the same manner as other solitary malignancies (i.e., by wide surgical excision)? Should the patient be treated primarily with chemotherapy? Should the patient be treated with a combination of surgery, irradiation, and chemotherapy? Unfortunately, there are no correct answers to these questions.
In my experience, seemingly solitary lymphomas become (or already are) systemic in most animals. Exceptions include some oral and some cutaneous solitary T-cell lymphomas. Although cures have been achieved through the surgical excision or irradiation of solitary lymphomas, they are extremely rare. Therefore it is important not to underestimate the malignant behavior of this neoplasm by treating the patient only with a local treatment modality, such as surgery or radiotherapy. The following guidelines can be used in this subset of patients:
Radiotherapy constitutes an excellent treatment modality for dogs and cats with solitary lymphomas because lymphoma cells are extremely radiosensitive. Marked responses (CR or PR) are seen within hours or days of the start of such treatment. Different sources and protocols have been used in cats and dogs with lymphoma, but in general 3 to 5 Gy (300 to 500 rad) per fraction is delivered daily or thrice weekly for a total of six to ten fractions (total dose, 30 to 50 Gy [3000 to 5000 rad]). We have successfully used coarse fractionation radiotherapy (7 Gy once a week for 4 treatments) followed by maintenance chemotherapy (discussed later) in dogs with solitary oral T-cell lymphomas. Special settings in which radiotherapy is beneficial include CNS lymphomas (see following paragraphs) and upper airway lymphomas that cause respiratory compromise.
Another decision the clinician must make if chemotherapy is to be used is which protocol to use and for how long. There are also no specific guidelines for this. We use a standard induction chemotherapy protocol (COAP) in most cats and dogs with solitary lymphoma after they have undergone surgical excision or irradiation. After completion of the induction phase, the animals are treated with a maintenance protocol (LMP) and remission is reinduced as necessary (as in other forms of lymphoma). In our experience, early relapses occur in most animals treated with only maintenance chemotherapy protocols after the surgical excision of solitary lymphomas.
Central nervous system lymphoma
The treatment of choice for cats and dogs with primary or secondary epidural lymphoma is radiotherapy plus multiple-agent chemotherapy. If radiotherapy facilities are not available, multiple-agent chemotherapy is an effective alternative approach. It is my clinical impression that the surgical excision of such masses does not provide a significant advantage over chemotherapy alone or radiotherapy plus chemotherapy, given the fact that the latter two forms of treatment consistently induce rapid remissions (i.e., within 12 to 36 hours of the initiation of therapy). However, because surgery may be necessary to confirm the diagnosis, surgical excision of the mass is usually attempted at that time. If radiotherapy is available, three to five doses weekly of 3 to 4 Gy, to a total of 25 to 30 Gy, are indicated. The COAP protocol alone has been effective in inducing remission in cats with epidural lymphoma.
In cats and dogs with lymphoma of the neuropil (i.e., true CNS lymphoma), chemotherapy with or without radiotherapy is the preferred protocol. In animals in which it is possible to localize the lesion (i.e., by neurologic examination, CT, or MRI), radiotherapy should be used in conjunction with chemotherapy. If this is not possible, diffuse craniospinal irradiation can be performed.
Intrathecal chemotherapy can be used in cats and dogs with confirmed or highly likely neuropil lymphoma. The drug of choice is cytosine arabinoside (Cytosar-U) because it is almost nontoxic, it is inexpensive, and it is easy to administer. However, IV administration of this drug as a constant rate infusion (CRI) at dosages of 200 to 600 mg/m2 over 24 to 72 hours achieves similar results and is our preferred approach. Responses to intrathecal or IV CRI cytosine arabinoside are usually quite spectacular. Dogs and cats that are tetraparetic, demented, or comatose usually regain normal neurologic status within 6 to 48 hours of receiving the first dose of this agent. In addition, disappearance of the neoplastic cells from the CSF can be documented within hours of the injection.
We frequently induce clinical and cytologic remission (i.e., normal neurologic status and disappearance of neoplastic cells from CSF) in cats and dogs with primary or secondary CNS lymphoma treated with COAP (using cytosine arabinoside as an IV infusion). As previously discussed, an alternative drug that crosses the blood-brain barrier and is effective in eliminating lymphoma cells is lomustine (CCNU; see Box 80-1) administered at a dosage of 60 mg/m2 PO every 3 weeks in dogs and at a dosage of 10 mg/cat every 3 weeks in cats; we have seen marked improvement or disappearance of neurologic signs in dogs and cats with lymphoma treated with this drug.
Despite the fact that remissions are easily attained in dogs and cats with CNS lymphoma, they are relatively short in duration compared with the duration of remissions in dogs and cats with disease in other anatomic locations. Most dogs and cats with CNS lymphoma relapse within 2 to 4 months of diagnosis; however, prolonged remissions (i.e., 6 to 12 months) are possible.
Ocular lymphoma
Ocular lymphoma can be treated using a variety of modalities. However, the eye behaves similarly to the blood-brain barrier in that adequate intraocular concentrations of chemotherapeutic agents are usually difficult to attain. If the clinician and owner want to try to preserve the animal’s eye, there are several alternatives to enucleation. As in animals with CNS lymphoma, the administration of cytosine arabinoside as a slow IV drip usually results in remission of the tumor. Lomustine is also effective in dogs and cats with intraocular lymphoma.
Cutaneous lymphoma
Cutaneous lymphoma is the most common extranodal form of lymphoma in dogs seen at the Veterinary Teaching Hospital of The Ohio State University. In dogs with cutaneous involvement secondary to multicentric lymphoma, we use a standard chemotherapy protocol (i.e., COAP). In dogs with epitheliotropic T-cell lymphomas we use either doxorubicin-containing (i.e., CHOP; see Box 80-1) or lomustine (CCNU)-containing protocols. In a recent study of 46 dogs with epidermotropic cutaneous T-cell lymphoma, 15 (33%) underwent CR and 23 (50%) underwent PR, for a response rate of 83% (Risbon et al., 2006). The median number of treatments to achieve a response was 1 (range, 1-6). The overall median duration of response was 94 days (range, 22-282). Sixteen dose reductions were required because of neutropenia (10/46), thrombocytopenia (1/46), anemia (1/46), increased liver enzyme activity (3/46), or unspecified reasons (1/46).
Alimentary lymphoma
We use standard chemotherapy protocols (i.e., COAP) in dogs and cats with solitary mural or nodal (e.g., mesenteric or ileocecocolic lymph node) involvement. Even though surgery is not necessarily indicated for these dogs and cats, a fair number are referred after exploratory surgery and an incisional or excisional biopsy has been performed. In general, the response in these animals is good. Dogs and cats with diffuse intestinal lymphoma usually respond poorly to chemotherapy. Responses to doxorubicin-containing protocols (i.e., CHOP) appear to be better than those to COAP, although survival times are short (4 to 6 months). Dogs with colorectal lymphoma and cats with gastric lymphoma tend to respond extremely well to COAP chemotherapy; we have documented remission times in excess of 3 years in these subsets of patients. In cats this may be related to the fact that Helicobacter spp. may play a role in the development of gastric lymphoma, as H. pilori does in people; we treat all cats with gastric lymphoma with combination chemotherapy and antibiotics proven effective in cats with Helicobacter infection.
In cats with epitheliotropic intestinal lymphoma, a common, small lymphocytic form of the disease in older individuals, we have used a very conservative approach with excellent results. We administer a combination of chlorambucil (20 mg/m2, PO q2 weeks) plus prednisone (1-2 mg/kg, PO q24-48h) or dexamethasone (4 mg/cat, PO q1-2 weeks); if clinical signs do not improve within 3 or 4 weeks, we add vincristine (0.5 mg/m2, IV, q1-2 weeks). Most cats treated with this protocol have marked improvement of the clinical signs and typically gain weight. Interestingly, some of the cats exhibit no appreciable decrease in mesenteric lymph node size, despite the remarkable clinical improvement. For these cats I use the approach of “treating the patient, not the disease” (i.e., as long as the patient feels well and is free of clinical signs, the current treatment is continued).
“Low-Budget” Lymphoma Protocols
Quite frequently, the clinician is evaluating a dog or cat with lymphoma that should benefit from chemotherapy, but because of finances or other issues (e.g., time commitment) the owners are not interested in the standard multiagent chemotherapy approach. Because most of these patients are asymptomatic, they would benefit from some form of therapy. In our clinic we have used one of the following quite successfully: prednisone alone, prednisone and chlorambucil, chlorambucil alone, lomustine alone, or prednisone and lomustine. Although the duration of remission is shorter than when using COP-based protocols, most of these patients (and their owners) enjoy prolonged (i.e., months), good-quality survival times. These protocols are listed in Box 80-1.
Alvarez FJ, et al. Dexamethasone, melphalan, actinomycin D, cytosine arabinoside (DMAC) protocol for dogs with relapsed lymphoma. J Vet Intern Med. 2006;20:1178.
Axiak SM, et al. Hematologic changes associated with half-body irradiation in dogs with lymphoma. J Vet Intern Med. 2006;20:1398.
Baskin CR, et al. Factors influencing first remission and survival in 145 dogs with lymphoma: a retrospective study. J Am Anim Hosp Assoc. 2000;36:404.
Carter RF, et al. Chemotherapy of canine lymphoma with histopathologic correlation: doxorubicin alone compared to COP as first treatment regimen. J Am Anim Hosp Assoc. 1987;23:587.
Chun R, et al. Evaluation of a high-dose chemotherapy protocol with no maintenance therapy for dogs with lymphoma. J Vet Intern Med. 2000;14:120.
Cotter SM. Treatment of lymphoma and leukemia with cyclophosphamide, vincristine, and prednisone. I. Treatment of dogs. J Am Anim Hosp Assoc. 1983;19:159.
Cotter SM. Treatment of lymphoma and leukemia with cyclophosphamide, vincristine, and prednisone. II. Treatment of cats. J Am Anim Hosp Assoc. 1983;19:166.
Couto CG, et al. Gastrointestinal lymphoma in 20 dogs. J Vet Intern Med. 1989;3:73.
Couto CG. Extranodal lymphomas. In: Kirk RW, editor. Current veterinary therapy IX: small animal practice. Philadelphia: WB Saunders, 1986.
Dervisis NG, et al. Efficacy of temozolomide or dacarbazine in combination with an anthracycline for rescue chemotherapy in dogs with lymphoma. J Am Vet Med Assoc. 2007;231:563.
Frimberger AE, et al. A combination chemotherapy protocol with dose intensification and autologous bone marrow transplant (VELCAP-HDC) for canine lymphoma. J Vet Intern Med. 2006;20:355.
Greenberg CB, et al. Phase II clinical trial of combination chemotherapy with dexamethasone for lymphoma in dogs. J Am Anim Hosp Assoc. 2007;43:27.
Grooters AM, et al. Ultrasonographic appearance of feline alimentary lymphoma. Vet Radiol Ultrasound. 1994;35:468.
Hosoya K, et al. COAP or UW-19 Treatment of dogs with multicentric lymphoma. J Vet Intern Med. 2007;21:1355.
Jeglum KA, et al. Chemotherapy for lymphoma in cats. J Am Vet Med Assoc. 1987;190:174.
Keller E, et al. Evaluation of prognostic factors and sequential combination chemotherapy for canine lymphoma. J Vet Intern Med. 1993;7:289.
Lana SE, et al. Utility of polymerase chain reaction for analysis of antigen receptor rearrangement in staging and predicting prognosis in dogs with lymphoma. J Vet Intern Med. 2006;20:329.
LeBlanc AK, et al. Effects of l-Asparaginase on plasma amino acid profiles and tumor burden in cats with lymphoma. J Vet Intern Med. 2007;21:760.
Loar AS. The management of feline lymphosarcoma. Vet Clin North Am. 1984;14:1299.
Louwerens M, et al. Feline lymphoma in the post-feline leukemia virus era. J Vet Intern Med. 2005;19:329.
MacEwen EG, et al. Some prognostic factors for advanced multicentric canine lymphosarcoma. J Am Vet Med Assoc. 1987;190:564.
Madewell BR. Diagnosis, assessment of prognosis, and treatment of dogs with lymphoma: sentinel changes (1973–1999). J Vet Intern Med. 1999;13:393.
Modiano JF, et al. Distinct B-cell and T-cell lymphoproliferative disease prevalence among dog breeds indicates heritable risk. Cancer Res. 2005;65:5654.
Mooney SC, et al. Renal lymphoma in cats: 28 cases (1997–1984). J Am Vet Med Assoc. 1987;191:1473.
Mooney SC, et al. Treatment and prognostic factors in lymphoma in cats: 103 cases (1977–1981). J Am Vet Med Assoc. 1989;194:696.
Moore AS, et al. Lomustine (CCNU) for the treatment of resistant lymphoma in dogs. J Vet Intern Med. 1999;13:395.
Postorino N, et al. Single-agent therapy with adriamycin for canine lymphoma. J Am Anim Hosp Assoc. 1989;25:221.
Risbon RE, et al. Response of canine cutaneous epitheliotropic lymphoma to lomustine (CCNU): a retrospective study of 46 cases (1999-2004). J Vet Intern Med. 2006;20:1389.
Saba CF, Thamm DH, Vail DM. Combination chemotherapy with l-Asparaginase, lomustine, and prednisone for relapsed or refractory canine lymphoma. J Vet Intern Med. 2007;21:127.
Shelton GH, et al. Feline immunodeficiency virus and feline leukemia virus infection and their relationships to lymphoid malignancies in cats: a retrospective study. J AIDS. 1990;3:623.
Teske E, et al. Prognostic factors for treatment of malignant lymphoma in dogs. J Am Vet Med Assoc. 1994;205:1722.
Vail DM. Recent advances in chemotherapy for lymphoma in dogs and cats. Compend Cont Educ Pract Vet. 1993;15:1031.
Wellman ML, et al. Lymphoma involving large granular lymphocytes in cats: 11 cases (1982–1991). J Am Vet Med Assoc. 1992;201:1265.