chapter 15 Gait awareness
In the management of a stroke survivor, gait analysis and gait training traditionally have been the responsibility of physical therapists. Because of the interdisciplinary approach used to rehabilitate the stroke survivor, much sharing of information occurs between team members regarding the patient’s functional and mobility status. Occupational and physical therapists often “cotreat” to enhance problem-solving regarding specific barriers to independence in activities of daily living.
Just as physical therapists have much to gain by familiarizing themselves with terminology and treatments used by occupational therapists (e.g., in the area of perceptual motor deficits), occupational therapists should benefit from having a basic understanding of normal gait components, common gait deviations after a stroke, and gait retraining. An integrated approach to treatment of the stroke survivor necessitates a working knowledge of the terminology, evaluation techniques, and rationale for treatment of other disciplines.
The physical therapist should perform a thorough examination before gait analysis and retraining. This examination includes factors such as range of motion, posture and bony alignment, strength, motor control, coordination, sensation, and balance. The therapist notes any deficits in these areas and is then ready to observe and analyze gait and to speculate on which of the deficits may be contributing to a specific gait deviation. The therapist can address specific deficits with appropriate treatment interventions and modalities.
Gait analysis is the objective documentation of gait71 and ranges in complexity from observational assessment to quantitative analysis using instrumented gait analysis systems. These systems can include tools such as videotaping, three-dimensional motion analysis, dynamic electromyograms, and force plates. A variety of such quantitative systems is available and differs widely in sophistication and price.11,60
Kinematic analysis evaluates movement patterns, including the movement of the body, and specific angles between body segments (joint angles) as the body moves through the gait cycle. Observational gait analysis is a qualitative method of kinematic analysis. When kinematics is measured by instrumented analysis, it is considered a quantitative gait analysis.60 Observational gait analysis is the visual inspection of walking.88 Although not as reliable as quantitative gait analysis, observational gait analysis is the method most often used by practitioners. Most physical therapists do not have access to highly technical evaluation equipment, although videotaping is now more commonly available. Perry developed a systematic method for observational gait analysis that helps standardize this evaluation.77
Observational gait analysis is an acquired skill that requires much practice and repetition. The physical therapist must learn how to look at nine different points on the body (head, shoulders, arms, trunk, pelvis, hips, knees, ankles, and feet) while simultaneously comparing the observed gait with normal gait features, in three body planes. When one is first learning gait analysis, observation of as many normal gaits as possible is necessary. When one is first performing observational gait analysis in the clinic, the recommendation is that the physical therapist choose patients who can tolerate walking for several minutes. This allows the therapist to apply Perry’s approach to viewing trunk and limb excursions during the gait cycle.
Observational gait analysis should take place in the sagittal and frontal planes. The frontal or coronal view must include anterior and posterior vantage points. Certain motions such as leg rotation and foot abduction and adduction take place in the transverse or horizontal plane, although the therapist usually is not in a position to observe motion specifically in this plane. In normal gait, most movement occurs in the sagittal plane, whereas in abnormal gait, many of the deviations are observed as compensations in the frontal (coronal) and transverse (horizontal) planes71 (Fig. 15-1).
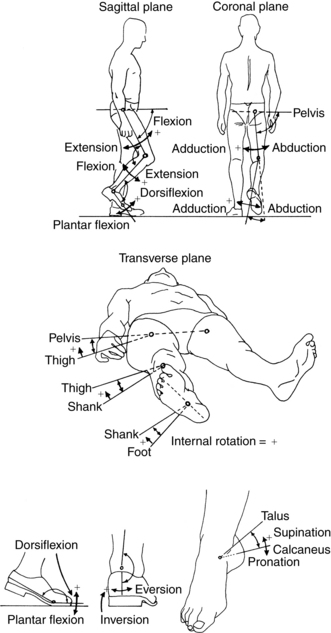
Figure 15-1 System of naming angular motion.
(From Inman VT, Ralston HJ: Human walking, Philadelphia, 1981, Williams & Wilkins.)
Terminology
Physical therapists must first familiarize themselves with the components of the normal gait cycle and with the terminology used to describe these components before they can analyze the gait of a person who has had a stroke. A cycle begins when the heel of one foot touches the ground and ends after the leg and body have advanced through space and time and the heel of that same foot hits the ground again.
The cycle includes a period when the leg is in contact with the ground, which is followed by a period when it is advancing through space. Thus the gait cycle of one leg can be divided into two phases: the stance phase (in which the leg is in contact with the ground) and the swing phase (in which the leg is off the ground). The stance phase makes upto 60% of the gait cycle, and the swing phase makes upto 40% (Fig. 15-2). In a normal gait, the opposite leg also is going through a gait cycle simultaneously (i.e., has a stance phase and a swing phase). Each leg has two periods at the beginning and end of stance when the opposite leg is also in contact with the ground. These are called the periods of double support. Together they account for 10% of the initial stance phase and 10% of the end of stance for both legs.
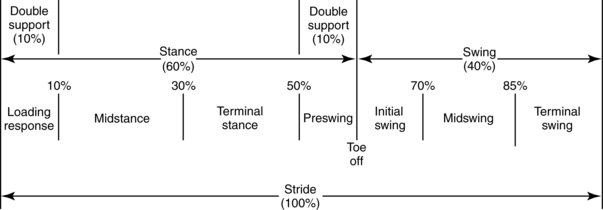
Figure 15-2 Phases of gait cycle and their proportions as percentages of gait cycle.
(From Ounpuu S: Evaluation and management of gait disorders, New York, 1995, Marcel Dekker.)
The phases of swing and stance are further divided into substages. The language used to describe these subdivisions uses the traditional terms or the terms developed at Rancho Los Amigos Medical Center (Table 15-1). Because the terms are similar, physical therapists often use a mixture of old and new terms unless the facility in which they work advocates strict adherence to one terminology. Most physical therapists are familiar with the Rancho Los Amigos terminology because of the abundance of research, literature, and gait assessment forms that have been produced by the pathokinesiology service and physical therapy department at that facility.73
| TRADITIONAL | RANCHO LOS AMIGOS | |
|---|---|---|
| Stance phase | Heel strike: The beginning of the stance phase when the heel contacts the ground; the same as initial contact | Initial contact: The beginning of the stance phase when the heel or another part of the foot contacts the ground |
| Foot flat: Occurs immediately following heel strike when the sole of the foot contacts the floor; occurs during loading response | Loading response: The portion of the first double support period of the stance phase from initial contact until the contralateral extremity leaves the ground | |
| Midstance: The point at which the body passes directly over the reference extremity | Midstance: The portion of the single limb support stance phase that begins when the contralateral extremity leaves the ground and ends when the body is directly over the supporting limb | |
| Heel off: The point following midstance when the heel of the reference extremity leaves the ground; occurs prior to terminal stance | Terminal stance: The last portion of the single limb support stance phase that begins with heel rise and continues until the contralateral extremity contacts the ground | |
| Toe off: The point following heel off when only the toe of the reference extremity is in contact with the ground | Preswing: The portion of stance that begins the second double support period from the initial contact of the contralateral extremity to lift off of the reference extremity | |
| Swing phase | Acceleration: The portion of beginning swing from the moment the toe of the reference extremity leaves the ground to the point when the reference extremity is directly under the body | Initial swing: The portion of swing from the point when the reference extremity leaves the ground to maximum knee flexion of the same extremity |
| Midswing: The portion of the swing phase when the reference extremity passes directly below the body: extends from the end of acceleration to the beginning of deceleration | Midswing: The portion of the swing phase from maximum knee flexion of the reference extremity to a vertical tibial position | |
| Deceleration: The swing portion of the swing phase when the reference extremity is decelerating in preparation for the heel strike | Terminal swing: The portion of the swing phase from a vertical position of the tibia of the reference extremity to just before initial contact |
From O’Sullivan SB, Schmitz TJ, editors: Physical rehabilitation assessment and treatment, Philadelphia, 1994, FA Davis.
The Rancho Los Amigos definition of swing phase is divided into the substages of initial swing, midswing, and terminal swing. The stance phase is divided into initial contact, loading response, midstance, terminal stance, and preswing (Fig. 15-3). Within these substages, the physical therapist observes the joint displacements and movements occurring at the trunk, pelvis, hip, knee, ankle, and toes. Fig. 15-4 illustrates the phases of the gait cycle and the corresponding normal joint displacements that occur as the body moves through the sagittal plane.
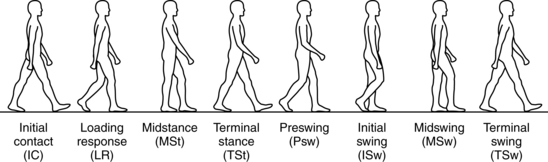
Figure 15-3 Phases of gait cycle shown with corresponding body position for sagittal plane motion.
(From Ounpuu S: Evaluation and management of gait disorders, New York, 1995, Marcel Dekker.)
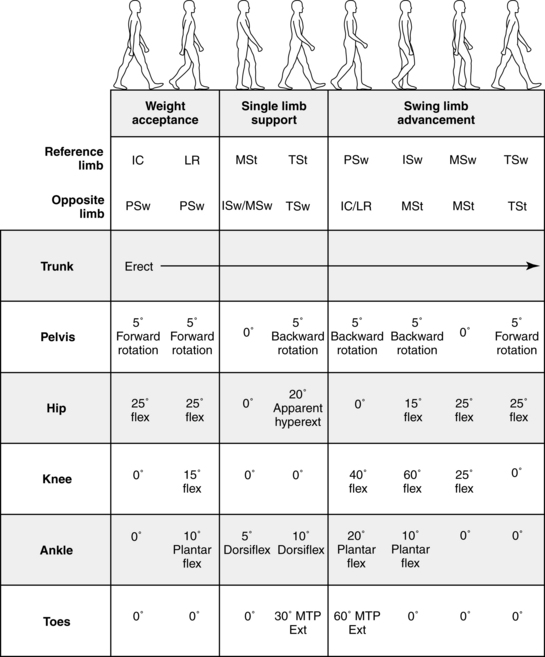
Figure 15-4 Range of motion summary.
(Courtesy Rancho Los Amigos Medical Center Physical Therapy Department and Pathokinesiology Laboratory, Downey, CA)
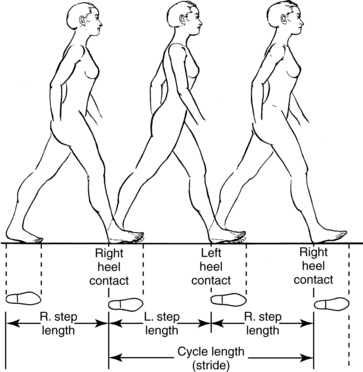
Other terms used in describing gait cycles are stride, step, cadence, and velocity. A stride is equal to a gait cycle (i.e., from heel strike of one leg to the next heel strike of the same leg). Stride can refer to distance (stride length) or time (stride time) in the gait cycle of one leg. A step is described as the distance (step length) or time (step time) from the heel strike of one leg to the heel strike of the opposite leg (Fig. 15-5).
Reliable gait parameters
Cadence is the number of steps or strides per unit of time. Walking velocity equals speed: the distance walked divided by time. Because time-distance variables are the components of gait that can be measured most reliably, therapists can use them in assessing improvement in stroke patients.39,83,84 For example, persons who have had a stroke with a resulting hemiparesis typically walk with a slower than normal gait.56,67 Routine recording of the cadence and velocity of these patients is an objective way of documenting change over time. Velocity measures are traditionally taken during a standard 10 minute walk test and show improvement for physical therapists that do not have access to the instrumented gait analysis systems mentioned previously.
It is important for therapists to have reference values from the healthy able-bodied population for cadence (100 to 120 steps/min) and velocity (1.2 to 1.5 m/sec) at their disposal, so a quick comparison with their patients’ values can be made if one has the goal of recovery.23 In addition, comparing an individual’s cadence and velocity measure at two points in time may help to document improvement objectively.
Improvements in cadence and velocity also can be an indication of functional improvement and limb recovery. A study of hemiplegic patients by Harro and Giuliani43 showed positive correlations between high scores (greater than 90) on the motor portion of the Fugl-Meyer motor assessment scale and the ability to increase walking speeds. Richards and colleagues83 studied 18 hemiplegic subjects divided into three subgroups: slow, intermediate, and fast walkers. They found that the fast walkers had movements and muscle activations more like those of able-bodied subjects than the slow or intermediate speed walkers.
In recent years, the six-minute walk test has been used in the stroke population. While it was first used in the cardiopulmonary population to assess functional capacity,6 its utility in the stroke population has been demonstrated. Dean and other separate investigators have consistently reported that this population’s ambulation endurance is very limited.26,58 The initial ambulation speed is not maintained throughout the six-minute walk test, and an individual’s final walk distance is both lower than predicted from their 10-minute walk velocity and below the value used to identify heart transplant patients.26 Thus, emphasizing the need to measure and train for both ambulation speed and endurance in this population.
Shaughnessy and colleagues have shown that monitoring step activity throughout the day using a portable microprocessor is another tool for demonstrating improvement in ambulation tolerance.87 They demonstrated an 80% improvement in step activity across a three-month outpatient rehabilitation period. Clinicians could monitor step activity during the course of a day by placing pedometers on their patients.
Perry has shown that ambulation speed differentiates level of ambulatory functioning and that individuals ambulatory in the community (0.58 m/sec) ambulate at speeds higher than independent household ambulators (0.4 m/sec) do. She has further classified levels within household (most and least limited) and community (most and least limited) ambulation based on speed and independence of ambulation while performing activities in the home and outside.78 Common threads to community ambulation were increased ambulation distance/endurance, the ability to change level and terrain irregularity, obstacle avoidance, and the manual handling of loads. All these threads are essential for successful full access community ambulation. Thus, when they work on gait recovery in the stroke population, therapists must routinely take quantified measures of gait speed and endurance during a variety of ambulation tasks to assess household and community ambulation feasibility and reentry.
Hemiplegic gaits
The type of gait of a person who has had a stroke depends on where in the brain the insult has occurred and which systems are affected, such as motor, sensory, balance, coordination, perceptual, and visual systems. If a motor area in the cortex or a motor track is involved, hemiplegia or hemiparesis is manifested in the contralateral limbs. The location of the infarction within these areas determines whether the arm or the leg is more impaired. Not all stroke patients are hemiplegic or hemiparetic, nor do all hemiparetic patients have the same degree of motor deficits. Unfortunately, the term hemiplegic gait frequently is applied to all individuals with hemiparesis, although many varieties and degrees of deficits exist.39 Individuals who have suffered ischemia in areas of the brain supplied by the anterior cerebral artery usually have greater deficits in the leg. Those with ischemic lesions in areas supplied by the middle cerebral artery have greater arm involvement, although leg weakness is usually also present in varying degrees. Middle cerebral artery infarctions are the most common type of stroke.16 The gait deviations seen with these lesions are those most often described by the generic term hemiplegic gait. Following are descriptions of some of the more common alterations.
During the stance phase of the hemiparetic leg, a patient may exhibit “foot flat” or even a “forefoot first” at the initial contact instead of a heel strike with adequate ankle dorsiflexion. The patient also may exhibit plantar flexion (forefoot first) and supination (in the frontal plane) at initial contact and then begin to bear weight precariously on the lateral border of the foot.17,39,60,70
During the loading response, while the patient is still in double limb support, weight is being “loaded,” or accepted, onto the leg. Normally, 10 to 15 degrees of knee flexion is needed to absorb the forces of momentum and body weight. This flexion may be absent, in which case the knee remains extended or even hyperextends (genu recurvatum) during midstance, as the body moves forward. In this instance, no tibial advancement occurs over the foot because no dorsiflexion is occurring at the ankle (Fig. 15-6).

Figure 15-6 Genu recurvatum in midstance caused by a rigid plantar flexion contracture (greater than 15 degrees). Tibia is prevented from advancing forward, driving the knee posteriorly into recurvatum, impeding progression, and reducing momentum.
(From Adams J, Perry J: Human walking, Philadelphia, 1994, Williams & Wilkins.)
Midstance begins the period of single limb support. In addition to knee hyperextension, the therapist also may observe trunk and hip flexion as the body attempts to move its center of mass forward over a stiff knee. The problem may be compounded by pelvic retraction. Other patients may display the opposite scenario during midstance on the paretic leg; knee flexion may be excessive in the sagittal plane, with concurrent excessive dorsiflexion and hip flexion.3,17,60,70
In the frontal plane, lateral trunk lean may be excessive over the ipsilateral leg during midstance or a positive Trendelenburg sign may be evident, both of which indicate weak hip abductors of the stance leg. A positive Trendelenburg sign is present when excessive lateral displacement of the pelvis occurs over the stance leg, with an excessive lowering of the pelvis on the contralateral swing leg.63,71
During the terminal stance phase, which is still a period of single limb support, normal hip extension may be absent along with the ability to transfer weight onto the forefoot in preparation for push off. Dorsiflexion at the ankle joint may continue to be excessive or diminished. Lack of heel rise can occur in the sagittal plane, combined with excessive dorsiflexion, and the contralateral leg makes initial contact early.3,39,60,71
The preswing phase is the final stance stage and the second double support period. A lack of knee flexion (normally between 30 and 40 degrees) often occurs in the paretic leg, accompanied by a lack of ankle joint plantar flexion at the end of preswing.3,17,70,73
Many of the deviations observed in the hemiparetic limb during stance can contribute to a decreased step length by the opposite leg. The body is not able to complete its normal excursion forward because of lack of movement, or ineffective movement, of the pelvis, hip, knee, or ankle of the hemiparetic limb. The opposite limb may “step to” instead of stepping past the paretic limb. Step length also can be reduced in the hemiparetic leg.
The therapist sometimes can see the swing phase of the paretic limb as a mass flexion movement instead of a series of sequential flexion movements.39,56 More often the swing phase is characterized by a stiff-legged swing, with a decrease in hip flexion and in the velocity and amount of reciprocal knee flexion and extension. The velocity of the entire paretic limb is often decreased.39,66 The decrease in hip flexion, together with the lack of knee flexion and dorsiflexion, often results in circumduction to advance the stiff limb.3,39,56,63,70,73 Circumduction occurs when the patient swings the leg through in a semicircle and is most noticeable when looking at the patient in the frontal plane (Fig. 15-7). The patient combines external rotation and abduction at the hip to lift the leg out to the side and then adducts and often internally rotates the leg to bring it back in.63 In a normal gait pattern, no abduction, adduction, or external or internal rotation occurs in the frontal plane during the swing phase.73

Figure 15-7 Supination of foot during swing phase resulting from uninhibited activity in the tibialis anterior. Circumduction of hip is also present during this swing phase.
(From Davies P: Steps to follow: the comprehensive treatment of patients with hemiplegia, New York, 2000, Springer-Verlag.)
The limited knee flexion in the preswing phase persists into the initial swing phase and often throughout the entire swing phase. The toe drag first seen in the initial swing phase may continue because of the decreased knee swing but also may be a consequence of decreased hip flexion and decreased ankle dorsiflexion. The patient can initiate compensatory hip hiking at this stage to assist with clearing the toes as the leg advances.3,39,60,66,70 Other compensations used to counteract toe drag are increased hip and knee flexion or vaulting by the opposite (stance) leg. Vaulting occurs when the person rises up on the toes of the stance foot for better clearance of the swing leg.3
In the midswing phase, the pelvis may remain retracted instead of rotating forward to neutral. Hip hiking and leg circumduction may continue, especially if knee flexion and dorsiflexion remain limited. Dorsiflexion may be decreased or absent, with the ankle assuming a plantar-flexed (foot-drop) position. The foot may supinate during midswing because of an imbalance in ankle dorsiflexor muscle function17,24,60,73 (see Fig. 15-7). Normally, the anterior tibialis and long toe extensors dorsiflex the foot symmetrically. Some stroke patients have overactive anterior tibialis muscles and weak long toe extensors, causing the medially placed anterior tibialis tendon to pull the foot into supination.24
As the limb progresses toward the terminal swing phase, many patients are unable to extend the knee while simultaneously flexing the hip and ankle. Instead, knee extension is decreased, and the foot initially contacts the ground with the knee flexed.60,66 The pelvis still may be retracted or may not have rotated forward past neutral. This, in addition to the decrease in knee extension, results in a decreased step length by the paretic leg. Other subjects may exhibit knee extension with plantar flexion during the terminal swing phase, instead of the normal dorsiflexion seen in preparation for upcoming heel strike.39,73 In other persons, adduction of the hip with knee extension can be so pronounced as to cause the swing leg to cross in front of the stance foot. Patients literally end up tripping over themselves.
Causes of gait deviations
One cannot overemphasize that the causes of the aforementioned observed gait deviations may vary from patient to patient. For example, a common deviation at initial contact is foot flat or forefoot first instead of heel strike. This abnormality could result from weak dorsiflexor muscles,29,30,56,59,73 excessive activity of the plantar flexors,3,55,56,73 a decreased ability to perform fast reciprocal movements,39,53,56 disruption in the central generation of preprogrammed muscle activation,45 noncontractile soft tissue tightness in the plantar flexors,3,21,29,73 or a pathological condition of the ankle joint. Even when soft tissue tightness and joint contractures are ruled out, hypotheses vary and often conflict about the precipitating factor. This is especially true when the issue of voluntary versus reflex skeletal muscle activation is addressed. A number of recent papers and publications provide an abbreviated review of the literature on this topic.21,29,34,39,43,44,52,53,56
Osteoporosis
Stroke survivors have a fourfold increased risk of falling compared to the healthy community dwelling population.64 Falls in this population have an increased risk, from 1.2% to 6%, of resulting in fractures to the distal radius, humeral head, and hip.15,81,100 Fractures occur predominantly on the paretic side and hip fractures in particular and accelerate the downward spiral toward increased morbidity and mortality.80,81 Risk factors for fracture include reduced mobility, strength of the paretic leg, and reduced bone mineral density (BMD).82 In Chapter 14, falls in the stroke population are explored; however, an analysis of the timeline for bone density demineralization is warranted so interventions to minimize this loss and possibly lessen the fracture risk can be developed.
In the spinal cord injury (SCI) population, bone demineralization occurs within the first three months postinjury and proceeds up to 16 months after injury. The demineralization has been attributed to prolonged bedrest, immobility, and lack of muscle contraction and gravitational loading below the level of SCI.37,101 In the stroke population, investigations of BMD loss have been compared within a limb and between limbs (paretic vs. nonparetic) in longitudinal fashion for up to 12 months after stroke.46,47,82 The rate of bone demineralization over the first year and the factors that might alter the loss are explored next.
As early as one month after stroke, significant BMD loss for the paretic upper limb (UL) compared to the nonparetic UL has been shown for the humerus (4%) and total arm (4%).82 The paretic limb’s distal radius loss reaches significance when compared to the other side at four months (3%). However, all three sites of the paretic UL continue to decline over the year (total arm 3%, humerus 14%, distal radius 3%), which puts the paretic UL at risk for fracture if used to break a fall. The nonparetic UL’s distal radius demonstrates a 2% increase in BMD for the first year, which may be attributed to increased loading activity associated with the nonparetic UL during ambulation, although this hypothesis has not been tested.
Ranmnemark and colleagues82 demonstrated that, at four months after stroke, a significant BMD loss for the proximal femur had already occurred in the paretic limb (6%), and loss continued throughout the remainder of the first year (12%). The nonparetic limb appeared to lose BMD as well, although at a slower rate compared to the other side (4% at 12 months). Studies reported that when BMD loss rate was analyzed across the first year, the most loss occurred within the first seven months in both the paretic (10%) and nonparetic legs (2%) of stroke survivors.46,47,82
Jorgensen46,47 has demonstrated that ambulatory status and weight-bearing load on the paretic limb after stroke affect the rate of BMD loss. Using the 6 level ordinal scale for the Functional Ambulation Category (FAC) to qualify ambulation status, a linear relationship of ambulation assistance to BMD loss was demonstrated. Thus, if subjects ambulated independently or with assistance (FAC 2 to 6) within the first two weeks after stroke, subjects lost less BMD at one year (2%) compared to those who achieved ambulation by two months (7%) and to those still nonambulatory at two months (10%).47 In addition, the amount of weight on the paretic limb during 30 seconds of static standing was linearly related to walking onset after stroke. Subjects who walked within two weeks of stroke had a higher percentage of body weight (51%) loaded through the paretic limb versus those who walked by seven months (43%) and those immobile at seven months (35%).46
BMD loss has been demonstrated for the paretic upper and lower limb throughout the first year after stroke. The upper extremity loss occurs sooner than the lower extremity loss, but both limbs show significant loss that could contribute to fracture risk during a fall. Early ambulation after stroke has been shown to modulate bone demineralization of the paretic limb during the first year. Therapists should use interventions that promote independent ambulation as early as possible after stroke with the knowledge that the sooner independent ambulation is achieved, the less bone loss occurs.
Treatment interventions
The physical therapist first addresses deficits identified during the physical assessment that are contributing to the abnormal gait, such as decreased range of motion and strength. Interventions can include basic modalities and therapeutic exercise and a variety of approaches to address the lack of movement and voluntary control. Many interventions are based on theories that advocate facilitation of normal movement and sensory stimulation of the patient by the therapist. In this context, the patient is a passive recipient of the therapist’s efforts. However, during the past 20 years, therapists gradually have shifted away from using these more traditional therapeutic approaches to using the motor control perspective. The motor control approach also is based on a theoretical model, but it does not advocate specific treatment techniques that are done by the therapist to the patient. In the motor control model the main task of the therapist is not to facilitate normal movement but to structure the environment in such a way that the patient actively will relearn to use the affected limbs functionally. The motor control relearning theory is based on research from a variety of fields: neurophysiology, muscle physiology, biomechanics, and psychology.20,42 Patients are believed to learn by actively trying to solve problems (see Chapter 6). Therefore, therapists should structure tasks to promote acquisition of the movements needed to solve specific motor control problems in a variety of situations (see Chapters 4 and 5). This pertains not only to patients with a hemiplegic gait but also to patients with motor control deficits described in the following sections.
In the last ten years, the research directed at improving gait function in patients after stroke have supported a basic tenet of motor skill acquisition. In order to improve gait functioning, the individual must practice the task of gait. The part practice intervention of weight shifting activities in standing with two feet in contact with the ground was not superior to the conventional neurodevelopmental treatment (NDT) based physical therapy intervention at improving gait.102 Thus, suggesting that improvements in gait may not be amenable to part practice in standing positions where 2 feet are always in contact with the ground.
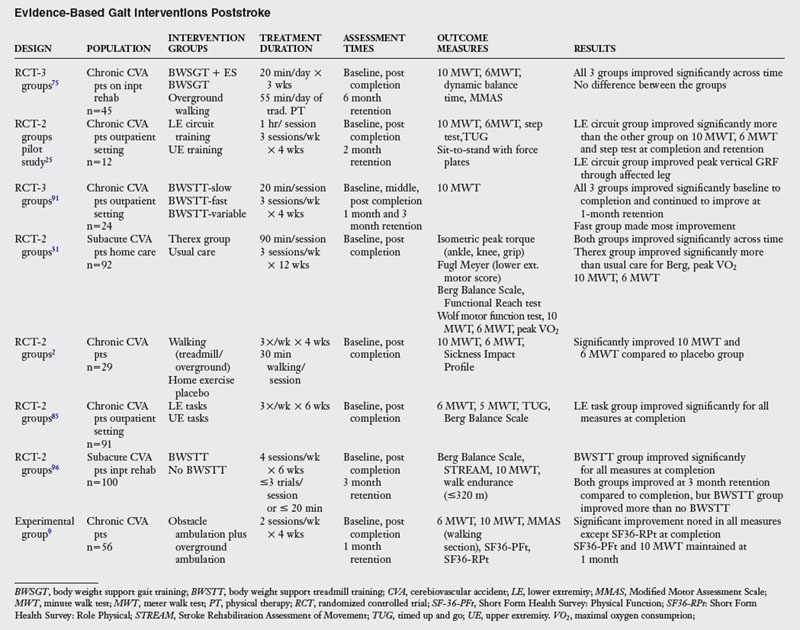
The question becomes if ambulation practice is required to improve ambulation, then how much practice does a patient require to improve gait? The recent literature shows that at least twenty minutes of ambulation practice is the minimum amount of time per session needed to note improved ambulation. Table 15-2 shows that the amount of practice for any of the ambulatory intervention groups is a considerable increase from what is presently observed in the rehabilitation clinic. This increase in time on task results in significant gains in overground walking speed and endurance.
Recent ambulatory intervention advancements have been the use of body weight support treadmill training (BWSTT), task-related circuit training, BWS + electric stimulation, overground walking practice, obstacle training, and home-based exercise programs (see Table 15-2). Visitin and Barbeau96 first demonstrated that BWSTT was better than non-BWS walking for recent stroke survivors. At the end of a six week inpatient rehabilitation unit stay, those individuals who received BWSTT ambulated at a faster overground speed (0.34 m/sec) than individuals who received the non-BWS (0.25 m/sec) (control group). At the three-month retention, while both groups continued to improve, the BWSTT group was clearly superior (0.52 m/sec) to the control group (0.30 m/sec) (Fig. 15-8).
(From Mobility Research, LiteGait, PO Box, 3141, Tempe, AZ 85280; 1-800-332-WALK; www.litegait.com.)
Sullivan’s group91 demonstrated that the speed which therapists train ambulation may be a critical factor in enhancing ambulation recovery. While BWSTT was performed for all groups, the training speeds for each group was different (fast vs. slow vs. variable). While all groups improved, the fast group (2.0 m/hr) made the most gains in overground walking speed. This speaks to the specificity of speed training and suggests that training should occur at the speed to with the therapist wants the patient ultimately ambulate.
An additional innovation to the BWS device is the incorporation of an electric stimulation component during gait training. However, when compared to overground ambulation training, both groups of chronic stroke survivors made similar gains in walking velocity and endurance.75 Ada’s group2 in their study of treadmill vs. overground walking programs found similar results in a community dwelling population of stroke survivors, and Nilsson’s group62 corroborated these results in acute stroke survivors, which suggests that the practice of ambulation is the common critical element for patients already somewhat ambulatory.
Dean25 in her pilot study and Salbach85 in a larger study found that training individuals in upright dynamic activities through a circuit system was more beneficial than no treatment or conventional treatment. The circuit included stations for walking overground at comfortable and fast speeds, walking over obstacles, transitions of sit-to-stand from varying height chairs, dynamic upright balance activities, and lower extremity strengthening activities performed in standing. Bassile9 demonstrated that an obstacle ambulation training program was feasible and improved the gait and quality of life in chronic stroke survivors. Lastly, Duncan’s specific home-based therapy program for acute stroke survivors that incorporated dynamic balance and LE strengthening performed in upright along with ambulation and aerobic training was found to be better than standard of care.31
In conclusion to this section, some common intervention themes are noted. First the task of ambulation must be practiced for much longer periods in the clinical setting if one wishes to improve this function. Results of this longer practice yield improved ambulation endurance (distance) and speed (velocity). Ambulation practice should occur at faster speeds to meet community ambulation activities. Both LE strength and balance (see Chapter 8) play a role in ambulation enhancement, and the literature supports the notion that performing task specific practice in upright dynamic postures along with ambulation practice, not in place of it, contributes to enhanced locomotion.
Other abnormal gait patterns
The list of abnormal gait patterns that can appear after stroke is too extensive to be covered completely in a single chapter. Therefore, what follows are examples of abnormal gaits that are particularly challenging to the physical therapist. Each deficit results from damage in the particular part of the brain described.
Cerebellar strokes
A person who has an infarct in the cerebellum caused by occlusion or hemorrhage of a vertebral or a cerebellar artery may exhibit completely different gait deviations than a hemiparetic patient. The cerebellum is composed of three parts or lobes: the flocculonodular lobe, the anterior lobe, and the posterior lobe. The flocculonodular lobe also is called the vestibulocerebellum because most of its input is from the vestibular nuclei in the pons. The anterior lobe also is known as the spinocerebellum because most of its input is from the spinocerebellar tracts via the inferior cerebellar peduncle and the superior cerebellar peduncle. The posterior lobe also is known as the neocerebellum and contains most of the cerebellar hemispheres. The hemispheres receive their major input from the cortex via the middle cerebellar peduncle.
In addition, the cerebellum can be divided longitudinally into functional zones perpendicular to the horizontal fissures dividing the lobes. The medial structure is the vermis. Adjacent to the vermis, on either side, is the pars intermedia (intermediate section) of the cerebellar hemisphere. Lateral to this is the bulk of the cerebellar hemisphere.
Gait is influenced most by the flocculonodular and anterior lobes. Consequently, infarcts in these areas lead to difficulty maintaining a proper stance and walking.65 Damage to the flocculonodular lobe (vestibulocerebellum) causes head and neck ataxia. Truncal tremor is often severe. The patient often uses a wide-based stance with the feet apart to increase stability. Any attempt to bring the feet together or walk with one foot directly in front of the other causes loss of balance. Ataxia or dysmetria of the limbs is not common.
Damage to the anterior lobe, especially the medial aspect, causes a disruption in the sensory input (via the spinocerebellar tracts) that is related to agonist-antagonist muscle activity. Lower limb ataxia or dysmetria is also present, but upper limb ataxia is usually absent. Lesions in a cerebellar hemisphere result in ipsilateral limb dysmetria or hypotonia, in addition to other deficits. Although the damage does not affect postural stability, the gait appears ataxic and staggering because of the limb dysmetria.60
The cerebellum is supplied by three main arteries: the posterior inferior cerebellar artery, the anterior inferior cerebellar artery, and the superior cerebellar artery. These arteries are part of the posterior circulation—the vertebrobasilar system. The posterior inferior cerebellar artery is a branch of the vertebral artery, whereas the anterior inferior cerebellar artery and superior cerebellar artery are branches of the basilar artery. Chapter 1 describes in detail the territories supplied by these arteries and their associated areas.4,5 In general, these arteries supply the areas of the cerebellum that their names imply, in addition to parts of the brainstem. Some areas of vascularization in the cerebellum overlap because of the many free cortical anastomoses5 (Fig. 15-9). Although one artery may supply one particular lobe predominantly, this overlapping may result in additional blood coming from the distal branches of another artery. However, as a rule, the superior cerebellar artery supplies the superior cerebellar peduncle, the anterior inferior cerebellar artery supplies the middle cerebellar peduncle, and the posterior inferior cerebellar artery supplies the inferior cerebellar peduncle.5
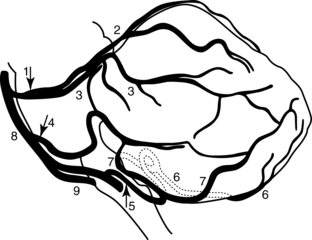
Figure 15-9 Lateral view of cerebellar arteries. 1, Superior cerebellar artery; 2, medial branch of superior cerebellar artery; 3, lateral branch of superior cerebellar artery; 4, anterior inferior cerebellar artery; 5, posterior inferior cerebellar artery; 6, medial branch of posterior inferior cerebellar artery; 7, lateral branch of posterior inferior cerebellar artery; 8, basilar artery; 9, vertebral artery.
(From Bogousslavsky J, Caplan L, editors: Stroke syndromes, Cambridge, UK, 1995, Cambridge University Press.)
A cerebellar stroke resulting from occlusion of the posterior inferior cerebellar artery usually is referred to in the literature as a lateral medullary syndrome (Wallenberg syndrome)12,38,95 because it was believed that the posterior inferior cerebellar artery supplied the lateral medulla and parts of the cerebellum. Recently this term has been disputed, based on evidence that the lateral medulla is supplied less frequently by the posterior inferior cerebellar artery than previously thought.4 If the lateral medulla is spared, an infarct of the posterior inferior cerebellar artery territory is apparent as a headache on the ipsilateral side, vertigo, nausea and vomiting, nystagmus, and limb and gait ataxia. If the lateral medulla is involved, the foregoing signs and symptoms are present. In addition, interruption of the sympathetic nerve fibers can cause Horner syndrome. Cranial nerves V, IX, and X also are affected.4,94
Involvement of cranial nerves V, IX, and X results in ipsilateral loss of pain and temperature in the face (V), dysphagia (IX), and dysphonia (X). Pain and temperature may be decreased on the opposite side of the body because of the interruption of the ascending spinothalamic tracts. This combination of cerebellar and medullary signs constitutes Wallenberg lateral medullary syndrome. In either type of posterior inferior cerebellar artery infarct, the inferior cerebellar peduncle and the inferior aspect of the cerebellum are affected. The result is ipsilateral limb ataxia and gait ataxia.4,94 In addition, the patient tends to fall to the side of the lesion (ipsilateral axial lateropulsion) and has difficulty shifting weight toward the contralateral leg.4
Earlier texts reported that posterior inferior cerebellar artery infarcts are the most common,12 but recent findings have shown that superior cerebellar artery infarcts occur as frequently.4,5 Superior cerebellar artery infarcts have several different clinical manifestations. Dysarthria is one of the most frequent. Limb dysmetria, gait ataxia, and ipsilateral axial lateropulsion are also common symptoms.4 Anterior inferior cerebellar artery infarcts are the least common. In addition to vertigo and ataxia, tinnitus and deafness are present. Auditory involvement and peripheral facial palsy are classic signs of anterior inferior cerebellar artery infarcts, which differentiate them from superior cerebellar artery or posterior inferior cerebellar artery infarcts.4,94
Gait retraining after a cerebellar stroke is focused on relearning the way to correct balance losses. Patients first must learn the point in space where their center of gravity is positioned optimally over their base of support for stability. Then they must relearn the way to realign their center of gravity constantly with their base of support. This task is most difficult during ambulation when the center of gravity is shifted anterior to the base of support as the body moves forward.93
Balance retraining should encourage active problem-solving by the patient (see Chapter 8). Being held upright by the therapist while walking does not promote functional independence. Likewise, assistive devices that require upper extremity weight-bearing (e.g., walkers) may prevent loss of balance but do not promote functional improvement because they do not challenge the patient to relearn balance control.7,13 The patient is merely stabilized externally and is not required to use or integrate postural reflexes.
Activities that require active weight shifting and goal-oriented reaching are encouraged and practiced while the patient is standing (see Chapter 14). The therapist can introduce progressively more challenging exercises and activities as the patient becomes more adept.7 Initially, some patients benefit from walking with their nonaffected side next to a high mat. The hand of the nonaffected side is placed on the surface of the mat for support. The patient can advance the dysmetric limb more easily if the opposite (sound side) hip maintains contact with the high mat during stance. Later, the patient uses a cane only to prevent loss of balance or as a cue to shift weight to the less affected side, not as a maximal assistive device.
Contraversive pushing/pusher syndrome
An unusual motor behavior that hemiplegic patients sometimes display in the clinic is ipsilateral pushing. The patients tend to push away from the unaffected side in any position. Davies24 described the syndrome in 1985 and called it the pusher syndrome. The original description of the pusher syndrome was based solely on a practitioner’s observation and was most often thought to be associated with left hemiplegia and perceptual deficits (especially left neglect), left visual field neglect with or without homonymous hemianopsia, impaired body scheme and body image, and visuospatial deficits.24 Recent research activity has attempted to identify the neural correlates and mechanisms for this clinical disorder. Unilateral lesions of the posterior lateral thalamus have been implicated in recent imaging studies.49,51 Also, diminished perfusion for the intact areas of inferior frontal, middle temporal, and inferior parietal lobes have resulted in pusher syndrome.92
The original description of the pusher syndrome was based solely on a practitioner’s observation. The behavior was seen in as many as 10% of the 327 stroke patients in the study by Pedersen, Wandell, and Jorgensen.74 The syndrome appears in both right and left hemisphere damage.50,74 Neglect and aphasia are also highly associated with pushing behavior.50
Karnath and colleagues have suggested through their research that the task of the brain areas damaged or receiving low perfusion in patients with pusher syndrome appears to be control of upright body posture.49,50,92 They demonstrated that patients with pusher syndrome show normal perception of visual vertical but a severe tilt of perceived body posture in relation to gravity. While seated in a tilting chair, patients with pusher syndrome oriented their bodies upright when they were actually 18 degrees tilted towards the side of the brain lesion. However, they were able to orient the visual world vertically appropriate. In addition, they were able to align their bodies to earth’s vertical when they used visual cues from the laboratory surroundings. In the dark, they were also able to orient to visual vertical, suggesting that both visual and vestibular inputs were unaffected. 24
Karnath and Broetz48 have identified three characteristic behaviors associated with pusher syndrome (see Chapter 7 for the Clinical Assessment Scale for Contraversive Pushing). First, the patient’s longitudinal body axis is tilted toward the paretic side when sitting or standing. Second, the patient actively pushes (abduction and extension of arm or leg) with the nonaffected extremities, which results in a lean toward the hemiplegic side and loss of balance. Third, the patient resists any attempt by the examiner to correct the tilted body axis.
The rehabilitation literature is scant on outcomes and intervention.72,74,76 Karnath51 found patients with pusher syndrome have a good prognosis. The behavior was rarely observed after six months of stroke. However, rehabilitation did take 3.6 weeks longer for the patients with contraversive pushing as compared to other stroke patients to achieve similar functional outcomes.
Gait training for patients with contraversive pushing is a definite challenge, as is transfer training. During sit-to-stand activities, some patients project themselves quickly out of a chair toward their hemiparetic side. If left unguarded, they fall. Transferring toward the stronger side is difficult because they always push away from that side. Although easier, transfers toward the hemiparetic side are dangerous because of the lack of motor control on that side. Standing requires assistance to prevent falling to the weak side.
Walking with an assistive device, such as a cane in the stronger hand, is initially unproductive, because these patients tend to use the cane to push themselves toward the hemiparetic leg. They appear unable actively to shift weight onto the strong leg. The more these patients are supported (to prevent falling to the paretic side), the more they push into the helper.
Gait retraining is based on the same principles discussed in the ataxic gaits section. Patients must relearn the way to adjust their center of gravity over their base of support while standing. The patients must regain proper positioning of their trunk in relation to gravitational forces so their center of mass stays within the limits of their strength and base of support (cone of stability). This implies a need for conscious awareness of their loss of balance. Trial and error is encouraged to promote active problem-solving. Two interventions are suggested for specific use with patients who demonstrate contraversive pushing. Karnath48 proposed that, since patients’ perception of visual vertical are intact but their perception of body vertical is inaccurate, the patients must use the visual vertical to align their bodies. They must be taught that the visual alignment information is correct and the body’s perception (feeling) of alignment is incorrect. This can be done through visual feedback of their bodies aligned to an external vertical axis. For example, patients can align their trunks to the vertical axis in a mirror with tape along the vertical line bisecting their body halves. Patients can also use door and window frames to align their trunks. However, they may require external feedback from the therapist simultaneously with a “conscious awareness” that balance is achieved in this position. Using the visual vertical axis for postural alignment takes care of a behavior seen with pusher syndrome.
During dynamic activities such as transferring sit to stand and ambulation, the unaffected upper and lower extremities are called into play to assist with the activity. Active pushing by the nonaffected extremities in a lateral direction toward the hemiplegic side occurs, and often the patient falls to this side when transferring, standing, or walking if not prevented from doing so by the therapist.
The second intervention has been used by clinicians but has not been evaluated systematically in the clinic. Therapists should remove all firm pushing surfaces from patient contact during activities. Thus, when performing sitting activities, the feet may be unsupported initially. In sitting and standing, the patient is not allowed to hold a firm external support with the nonaffected hand, so assistive devices and parallel bars are counterproductive. For example, the patient may be asked to hold a cup of water while transferring from sit-to-stand. When standing or transferring, the patient might be asked to simultaneously perform reach, grasp, and place activities with the nonaffected upper extremity. The items are retrieved or placed on movable surfaces (e.g., hospital tray table or rolling stools). This intervention eliminates the success of the pushing arm in destabilizing the patient, and the therapist can assist the patient to realign the vertical axis of body more easily. If the patient can perform these activities while preferentially shifting their center of mass toward the nonaffected side while receiving external visual and verbal feedback about vertical alignment then he or she can consciously be aware of what body positions create stability (e.g., objects are placed or retrieved from midline and in the direction of the nonaffected side).
Relearning to maintain balance while walking is a formidable task for patients with ipsilateral pushing. The degree of difficulty in relearning to maintain balance while walking is compounded by changes in somatic sensation, strength, motor control, and feedback circuits following the infarction. Patients must regain some control of trunk in dynamic standing activities before ambulation can proceed safely. Visual and tactile goals can be helpful. Having patients walk around a high mat or table while observing themselves in a mirror vertically bisected with a tape may cue patients where to shift their weight to avoid falling. The use of parallel bars is discouraged; patients must learn to weight shift with the trunk to correct balance losses and not merely to pull on a bar to remain upright. If safe for both patient and therapist, using the mirror and ambulating in free space may be possible with the therapist guarding and stabilizing the affected lower limb and trunk side. Patients can advance to using a cane once they have mastered trunk control. Hands-on techniques used by the therapist to facilitate movement are discouraged. The patients simply will push into the hands of the therapist.
At times, leg weakness interferes with a pusher syndrome patient’s ability to relearn postural control and weight shifting. Davies24 advocated splinting the hemiparetic knee in extension while having the patient work on active weight shifting during functional standing activities. Splinting the knee this way might increase loading in the affected patient’s affected leg while standing. One can assume that the added stability somehow reassures patients and gives them time to assess accurately whether they are balanced. Perhaps the degrees of freedom have been limited, allowing patients to concentrate on one task, weight shifting, to achieve a functional goal without having to concern themselves with an unstable knee. At this time, only speculations can be made about what reduces the pushing tendency and why. Although treatment techniques were suggested for gait training patients with contraversive pushing, no controlled studies have been done to verify their efficacy, and they are based solely on this and other practitioners’ clinical experiences.
Proprioceptive deficits
Loss of sensation after a stroke can compound motor deficits. In particular, loss of proprioception can greatly impede motor recovery after stroke.32 Proprioception is conveyed to the cerebellum and to the cerebral cortex. Information about joint position and muscle activity is sent to both, but the information projected to the cerebellum is not recorded as conscious perception. The information is used to ensure coordinated limb movements. In contrast, the information sent to the cortex can be perceived consciously and provides awareness of limb position and movement.38
Proprioceptive input from muscle spindles, joint receptors, and cutaneous touch receptors reaches the cerebellum through the inferior cerebellar peduncle via the ipsilateral dorsal spinocerebellar tracts. The same information reaches the somatosensory area of the cerebral cortex via the ipsilateral posterior columns of the spinal cord, which cross in the medulla and ascend in the medial lemniscus to the thalamus and then to the cortex.
Middle cerebral artery strokes can impair awareness of proprioception at the cortical level. Although all sensations can be affected, proprioception and two-point discrimination are usually more impaired than pain and temperature perception.12 The deficits are manifested in the contralateral arm and leg. Cerebellar artery strokes cause loss of the unconscious, rapid proprioceptive input required for the smooth, automatic movements of gait. Loss of sensory input regarding agonist-antagonist muscle activity disrupts the continuous modulation of these muscles that is required for coordinated gait movements.
A study by Kusoffsky, Wadell, and Nilsson54 found that patients with proprioceptive loss after cortical stroke were able to regain a greater amount of function in the leg than in the arm. One explanation they gave for this was that gait greatly depends on centrally generated activation patterns, and these patterns in turn do not depend on peripheral sensory mechanisms. These central pattern generators originate in the spinal cord and are controlled by locomotor centers in the brainstem. These centers are influenced by the cerebellum, the basal ganglia, and the cerebral cortex.40 The physical therapist can take advantage of this phenomenon by emphasizing functional gait as much as possible, as with BWSTT.
Along with vestibular and visual input, proprioceptive information contributes to a patient’s ability to maintain a stable upright position. Input from muscle spindles and joint receptors provides valuable information not only about the position of a limb in space but also about the environment.45,93 The ability to react to uneven surfaces or changes in ground texture depends on this input, and its impairment puts a patient at higher risk of falling. Coordinated limb movements may be decreased, and the person may be unable to judge the step length or limb joint excursions needed for maneuvering in the environment.
Vision can help to compensate for the proprioceptive loss.38,45,69,93 As with other deficits, the physical therapist should encourage a problem-solving approach. The patient must learn consciously to use visual input, which was not necessary before. Occasionally mirrors are useful, although the therapist should evaluate these aids individually for each patient. Mirrors can hinder as often as they help patients, especially those with visuospatial deficits.
The therapist’s role is to provide a variety of settings in which the person can practice using visual cues. In addition, biofeedback can be used to provide auditory cues. One type of biofeedback unit is a limb load monitor that can signal a person when the foot contacts the ground. Standard biofeedback units provide information about the force of muscle contraction during strengthening exercises (see Chapter 10).
Visual deficits
Visual impairments from strokes also can affect gait. The most common visual deficit in hemiplegic patients is homonymous hemianopsia,95 which occurs when an infarction involves the optic tract, the lateral geniculate body, or the optic radiation to one occipital cortex. A branch of the internal carotid artery, the anterior choroidal artery, supplies most of the optic tract and the optic radiation, with some coverage by branches of the middle cerebral artery and the posterior cerebral artery.95 The visual cortex is supplied mainly by the posterior cerebral artery but also is supplied by some middle cerebral artery collaterals.18 Homonymous hemianopsia also can result from an isolated occlusion of the calcarine branch of the posterior cerebral artery, but in this case, no concurrent hemiplegia or hemisensory loss occurs.36
When homonymous hemianopsia is present, visual information about one half of a person’s environment is missing. The temporal half of the visual field of one eye and the nasal half of the visual field of the other eye are absent. Loss of the left half of the visual field accompanies left hemiplegia, and loss of right visual field accompanies right hemiplegia. As mentioned previously, balance is maintained by an intricate communication network between the visual, vestibular, and proprioceptive systems. If vision is impaired, one aspect of this network is functioning abnormally. The ability to maintain balance is at risk if the patient does not learn to use other systems for feedback about the environment.22
Self-awareness of the visual deficit is crucial for patients. They must test this new awareness in a variety of situations and environments to ensure safety on discharge from the hospital and maximize functional independence (see Chapter 16).
Perceptual deficits
Perceptual deficits such as left neglect or visual neglect are neurobehavioral deficits that can affect gait. These phenomena and their manifestations, causes, and clinical implications are discussed elsewhere (see Chapters 18 and 19).10,36 Ipsilateral pushing also may be classified as a neurobehavioral deficit.
Hemineglect and hemianopsia are separate entities that can often coexist.8 Likewise, neglect and sensory loss can develop together or independently. Communication between the occupational and physical therapists concerning a patient’s perceptual status is a necessity and helps determine the best treatment approach to maximize function and ensure consistency of treatment interventions. Information obtained from formal testing by the occupational therapist can provide valuable insights for the physical therapist formulating the gait retraining program.
Orthotic interventions
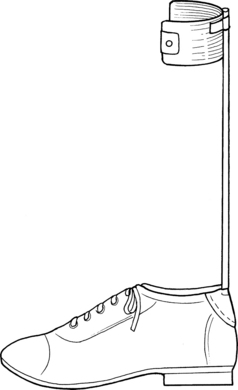
An orthosis (from the Greek adjective orthos, meaning “straight”) is an external device that improves a person’s function when applied to a body part.57 The more commonly used term for an orthosis is a brace. Orthoses now are named according to the joints they encompass. Short leg braces are known as ankle-foot orthoses (AFOs). A long leg brace is known as a knee-ankle-foot orthosis (KAFO) or a hip-knee-ankle-foot orthosis if it contains a hip joint and a knee joint. The newer terminology is more descriptive and specific and avoids confusion.
Orthotic devices are prescribed by a physician and fabricated by an orthotist. The physical therapist provides input to the physician and orthotist about which temporary devices have been assessed in the clinic before a permanent orthosis is prescribed. The physical therapist is also responsible for gait training the individual with the orthotic device. Training includes donning and doffing instructions, skin inspections, and patient education as well as the actual gait training.
Orthotic devices are classified in four categories: stabilizing (supportive), functional (assistive), corrective, and protective. All orthoses are used to increase function.
Stabilizing and functional orthoses are the two types most often used with stroke survivors. Stabilizing orthoses are used to prevent unwanted motion such as plantar flexion at the ankle or knee buckling. Functional orthoses have an element that compensates for lost muscle strength by assisting with movement. Stabilizing orthoses are not intended as a way to correct a fixed deformity in an adult; they only can stabilize and accommodate a deformity. Corrective orthoses are used to correct or realign parts of a limb. They are used for infants and young children to help correct flexible skeletal deformities. These orthoses should not be used to correct a fixed deformity in an adult. A stabilization orthosis can be used, but only to support the fixed deformity. Protective orthoses protect a portion of a limb from weight-bearing forces (e.g., a limb with a fracture).35
The orthotist adheres to basic physical principles when fabricating an orthosis to control a weak joint. An orthosis that provides three points of pressure is the most common type.90 One of the three forces is directed toward the joint itself, and the other two end forces are directed opposite to the main force (Fig. 15-10). This principle is important for the occupational therapist to learn because of its relevance to adaptive shoe equipment. Fig. 15-10, B illustrates the three points of pressure used with an AFO that is providing a dorsiflexion assist. The main point of pressure is on the dorsum of the foot. The two counter pressures are at the posterior calf and the distal plantar surface of the foot. Elastic laces, often used to facilitate donning a shoe with stroke survivors, eliminate the main point of pressure and result in loss of orthotic effectiveness. Therefore, elastic laces should not be used with dorsiflexion-assist braces. Elastic laces should be used cautiously with solid ankle AFOs that prevent dorsiflexion (see Fig. 15-10, A) because the foot needs to be held snugly in the AFO and shoe. This is especially true if plantar flexion spasticity is present.

Figure 15-10 A, Three points of pressure of an ankle-foot orthosis with dorsiflexion stop. B, Three points of pressure of a dorsiflexion assist ankle-foot orthosis. C, Three points of pressure of a locked knee-ankle-foot orthosis.
(These illustrations are diagrammatic only.)
Another orthotic principle states that the longer the lever arms, the less force needs to be applied at the three points of pressure. Therapists need to consider bony landmarks and superficial nerves when implementing these principles.90 The orthotic joint axis of motion should be aligned with the skeletal joint; otherwise, abnormal pressures can be applied in the wrong areas, such as under calf bands, with movement or positioning.35,90
Orthotic devices can be made of a variety of materials, the most common of which are metal and plastic. Plastic orthoses are in total contact with a limb and are worn inside the shoe. Metal orthoses are attached to a shoe and held in place on the limb with straps or bands.
An AFO is the most commonly used orthosis for patients with a hemiplegic gait and is the most appropriate.60,77,98 An AFO can affect knee motion and ankle motion. Knee buckling can be reduced, in stance, by adjusting the amount of dorsiflexion at the ankle joint. Similarly, knee hyperextension (genu recurvatum) can be avoided by controlling the amount of plantar flexion. Therefore, the therapist can avoid using a heavier KAFO to control the knee.
Plastic orthoses usually are made from high-temperature thermoplastic materials such as polypropylene. They require high temperatures for molding and therefore are shaped over a model, such as a plaster cast impression of the patient’s leg. They are more resistant to continued stress than the low-temperature thermoplastics used for UL orthoses.
The simplest and most commonly used plastic AFO is the posterior leaf splint or spring35 (Fig. 15-11, A ). The leaf spring is used when the main gait deviation is “foot drop” during the swing phase. The orthosis functions as a dorsiflexion assist device because of its flexibility. The plastic of the calf portion is displaced in stance and then springs back to a 90-degree angle during swing. The ankle joint is held at this 90-degree angle during swing. Foot drop and toe drag are avoided. This orthosis, however, does not afford any mediolateral stability at the ankle joint. If this is of concern, then the therapist can try a more substantial orthosis.
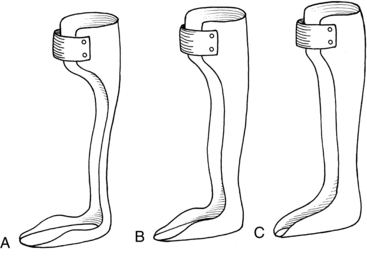
Figure 15-11 A, Posterior leaf splint or posterior leaf orthosis. B, Modified ankle-foot orthosis. C, Solid ankle-foot orthosis.
A modified AFO has a wide calf upright with lateral trimline borders that are just posterior to the malleoli (Fig. 15-11, B). Usually the foot plate encompasses more of the lateral and medial borders of the foot. This results in more control of calcaneal and forefoot inversion and eversion. The increased width of the calf portion offers somewhat more resistance to plantar flexion in swing and stance.
The most supportive AFO is the solid ankle AFO (Fig. 15-11, C). The lateral trim lines extend even farther forward, anterior to the malleoli. Because of its construction, the solid ankle AFO is designed to prevent ankle motion and foot motion in any plane. The device controls dorsiflexion, plantar flexion, inversion, and eversion.
A variety of hinged plastic AFOs are now available to allow certain motions and to block others. The ankle joint components are too numerous to mention, and newer components are being designed continuously. The orthotist can use different combinations of joints and stops to allow, limit, or prevent movement. For example, the therapist may wish to allow dorsiflexion past neutral (90 degrees) in stance to allow normal tibial advancement over the foot but block plantar flexion at neutral to prevent foot drop in swing and knee hyperextension in stance.
Another group of plastic AFOs is referred to as tone-inhibiting AFOs. Most of these AFOs initially were designed for use with children with cerebral palsy.28 Several types have been designed more specifically for use with adult hemiplegics.61 The common denominator is the flexibility allowed by these orthoses, in the foot and in the ankle. In theory, this flexibility allows more normal weight-bearing contacts on the plantar surface of the foot throughout stance, which promotes normal mobility in the foot during stance rather than having the foot held in one position. Mueller and colleagues61 documented the foot-loading patterns obtained when using two different tone-inhibiting AFOs. They assessed biomechanical alignment and foot stability, and one orthosis—the dynamic ankle-foot orthosis—was found to have had significant effects at the lateral forefoot with respect to force. The authors concluded that this effect might support the medial longitudinal arch of the foot and increase the stability of the forefoot as it is loaded. They theorized that this in turn might allow the forefoot to be loaded at a faster velocity. They did not investigate the effects of correct biomechanical alignment on muscle electromyographic activity.
The use of this type of AFO is based on the same principles that underlie the use of serial casting.19,28 Both were believed, by some practitioners, to reduce abnormal muscle activity. However, the scientific literature so far does not confirm that the prolonged stretch afforded by serial casting has a central inhibitory effect.1,14,19,27,97 Changes in sarcomere number and connective tissue caused by immobilization, positioning, and stretch can influence muscle contraction force.1,14,21,41 In addition, muscle length also can influence the manifestation of hyperreflexia.1,20,21 Perhaps these mechanical properties of muscle are influenced by tone-inhibiting orthoses. By promoting better biomechanical alignment and normal muscle length, these AFOs may exert an effect on peripheral rather than central factors that, over time, could otherwise augment stretch reflexes. Further research is needed—especially long-term, controlled studies—to investigate the many variables that influence motor control and muscle function. The term tone inhibiting may have to be reconsidered until a more complete and universally accepted definition of tone exists along with what contributes to normal and abnormal tone.
Metal orthoses were the main type of orthotic devices used before the 1970s.35 Metal AFOs still are used for certain stroke survivors who cannot tolerate the total contact of a plastic AFO for whatever reason. The components usually consist of two metal uprights attached to an ankle joint. The metal is usually aluminum, but sometimes heavier steel is needed for control. The ankle joint is attached to a stirrup that is fastened beneath the heel of the shoe. The proximal ends of the upright are attached to a calf band.
The metal ankle joint is usually a single- or double-channel (chamber) type (Fig. 15-12). Other types of ankle joints are described in detail elsewhere.21,35,56 A single-channel ankle joint can assist dorsiflexion with a spring placed in the channel. Plantar flexion also can be limited to prevent genu recurvatum by placing a pin in the channel. A double-channel ankle joint can prevent dorsiflexion and plantar flexion by using pins in both channels. Small screws hold the pins in the chambers. The degree of dorsiflexion or plantar flexion (i.e., the ankle joint angle) can be determined by the degree to which the pins are driven into the channels by tightening the screws. Springs and pins can be used in combination to stop one movement and assist another.

Figure 15-12 A, Single-channel (chamber) metal ankle joint. B, Double-channel (chamber) metal ankle joint.
The metal uprights attached to the ankle joint and stirrup offer a certain amount of foot and ankle mediolateral control. However, if additional support is needed (e.g., to prevent severe foot inversion), a strap can be added that applies pressure to the lateral malleolus in a medial direction and is secured around the medial upright. Because it prevents varus positioning of the ankle, the strap is called a varus correction strap. Force can be applied in the opposite direction with a strap to prevent foot eversion and a valgus foot position. This strap then is called a valgus correction strap. A varus correction strap is more common.
The simplest type of metal AFO is the Veterans Administration Prosthetic Center shoe clasp orthosis, which consists of a single narrow metal upright that attaches to the heel counter of a shoe with a metal clasp and a calf strap (Fig. 15-13). The orthosis offers dorsiflexion assist only, with no mediolateral or plantar flexion control.
Occasionally a KAFO with knee locks may be prescribed for a patient who requires additional knee control. However, the additional weight, the prevention of normal knee joint excursions during swing, and increased energy cost caused by these factors greatly limit the potential for functional ambulation.60,77,98 In addition, donning and doffing a KAFO are difficult for hemiplegic patients (see Fig. 15-10, C).98
A KAFO combines the features of an AFO with a knee joint and (in the case of a metal orthosis) metal uprights that extend proximally up the thigh. Thigh bands secure the KAFO on the upper leg. The simplest knee joint is a hinge, and the most common locks to maintain knee extension are drop ring locks.35 The thigh component of plastic KAFOs usually is made of the same thermoplastic material as the AFO component. Metal and plastic combinations also can be used.33,35,56
As previously mentioned, KAFOs are seldom used for hemiparetic patients. Occasionally, a preexisting knee joint deformity or ligamentous laxity is exacerbated by walking because of the now weak muscular support. In such instances, no alternative may be available to using a KAFO to allow minimal household ambulation. A KAFO or a knee extension splint sometimes is used as an initial training device to enhance stability. These are used only as temporary measures and not as long-term orthotic devices.20,60,98
The physical therapist has the responsibility of reevaluating the orthotic device on an ongoing basis, especially in the outpatient or home therapy setting. In this era of decreased length of hospital stays, patients sometimes are prescribed an orthotic device while still in the early stages of recovery. As motor control improves, the orthotic device may need to be modified or discontinued to allow more active movement by the patient.
Assistive devices
The assistive devices most commonly used with stroke patients are canes, walkers, and occasionally two crutches. Hemiparetic patients whose balance is impaired minimally and who have functional strength in the opposite upper extremity may use a cane. Two crutches or a walker require at least some functional use of both upper extremities. Both devices provide more external stability, with the walker providing more stability than the crutches. The main function of a cane is to increase the base of support and thereby improve balance.86 The base of support is increased by providing another contact with the floor. Canes also decrease the need for abductor muscle tension to stabilize the pelvis in stance on the paretic side.68,86 This in turn helps to prevent dropping of the contralateral pelvis (a positive Trendelenburg sign) in stance when the cane is used in the hand opposite the hemiparetic leg. Using the opposite hand also helps simulate the reciprocal arm and leg movements of a normal gait.
A variety of canes are on the market, ranging from a simple wooden straight cane to a tripod “walk cane” (also called a hemiwalker). At a level in between these two canes are the narrow- and wide-based quadruped canes (quad canes) (Figs. 15-14 to 15-17). Widening the base of support provides more stability. Physical therapists may begin training with a wide-based cane because of hemiparesis and impaired balance. They should advance patients as quickly as possible to the least amount of assistance required to ensure a safe, stable gait. Patients often are kept inadvertently on a maximally wide base of support cane when it is no longer needed. This prevents the patient from maximizing functional ambulation for two reasons: (1) normal weight shifting to the hemiparetic leg is limited, and (2) cadence is slower than it is with a smaller device86 or no device. The key word is safety. Maximum use of the involved leg should be encouraged along with normal trunk and pelvic movement, if patient safety is not compromised.
Two crutches occasionally are used: axillary or (more often) forearm (Lofstrand) crutches (Fig. 15-18). Certain cerebellar stroke patients or others who have impaired balance but functional use of both arms and hands may be trained with these devices. These patients require the extra postural support afforded by the second crutch but have enough motor control to be able to advance the crutches reciprocally.
Therapists may use walkers for training stroke patients who have functional use of both arms and hands but need greater outside support than that afforded by two crutches. Occasionally a walker may allow functional use of a hemiparetic arm even though balance is sufficient with a cane. In this case, the patient also should practice gait training with a cane to promote optimum postural control. If patients have sufficient control of the paretic arms, they also may use walkers when it is necessary for them to transport objects around the house (e.g., in the kitchen).
Standard walkers are the most stable assistive devices because they provide four points of contact with the ground. The base of support is greatly increased. A variety of walkers are available as well. In addition to standard walkers with four legs, rolling walkers with front wheels only, with four wheels, and platform attachments are also available. Rolling walkers allow a more normal reciprocal gait, but the therapist must take care to prevent the walker from “running away” with the patient. A stroke survivor with insufficient arm and hand strength to lift a walker may have the ability to maintain a grip on the rolling walker and push it forward. Some walkers have pressure-sensitive brakes that prevent forward movement when the patient pushes down on the walker.
As mentioned in the cerebellar stroke section, postural control sometimes is sacrificed for stability when a walker is used. The patient has no need to relearn balance and control if the walker provides needed support. As mentioned, safety is the ultimate concern. If safe, functional ambulation is not possible without a walker, then safe, independent ambulation with a walker is the preferred choice.
The type of gait pattern taught to the stroke survivor depends on a number of factors, including balance, strength, and coordination.68,86 The therapist also should consider cognitive and perceptual deficits, including apraxias.
Smidt and Mommens89 suggested terminology for describing walking patterns. Point refers to the number of contacts made with the floor, including with feet and assistive devices, during the forward progression of the gait cycle (Figs. 15-19 and 15-20). For example, a four-point contralateral gait indicates that two feet and two assistive devices (such as canes being advanced one at a time) are being used (see Fig. 15-20, A). The more contacts on the floor at any given moment, the more stable the person is while walking. In addition, the pattern can be called a delayed pattern if the assistive device is advanced before the limbs. Delayed patterns provide more stability than moving a limb concurrently with an assistive device. Following are the most common gait patterns taught to stroke patients.
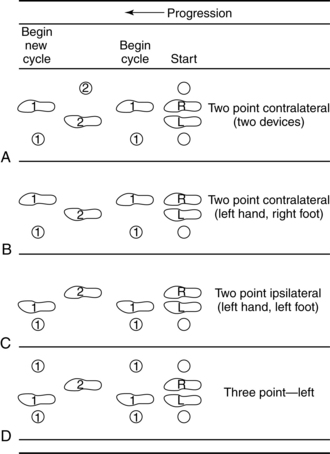
Figure 15-19 A to D, Diagrammatic view of assisted gaits.
(From Smidt G, Mommens MA: Gait patterns. Phys Ther 60(5):553, 1980.)
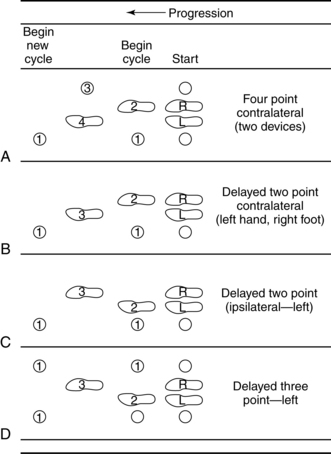
Figure 15-20 A to D, Diagrammatic view of assisted gaits.
(From Smidt G, Mommens MA: Gait patterns. Phys Ther 60(5):553, 1980.)
Gait patterns
Two-point contralateral gait pattern using one device
Hemiparetic patients with a nonfunctional arm often are taught a two-point contralateral gait pattern using one assistive device. A device, such as a cane, is held in the unaffected hand. The cane and the paretic leg are advanced together (one point), and then the unaffected leg is advanced alone (second point) (see Fig. 15-19, B). The cane may be advanced first and then the paretic limb followed by the unaffected limb for a more stable pattern. This pattern is a delayed contralateral two-point gait pattern (see Fig. 15-20, B). In Figs. 15-19, B and 15-20, B the right leg is the hemiparetic leg.
Four-point contralateral gait pattern using two devices
The devices used in a four-point contralateral gait pattern could be canes or crutches. The therapist might choose this type of gait for stroke patients who have functional use of all four limbs but have impaired balance. They require bilateral support but are able to advance each device (two points) and each leg (two points) individually and reciprocally. Although this is a stable gait pattern, it is not used often with hemiparetic patients. Sometimes a patient recovering from a cerebellar stroke will be taught this pattern to encourage coordinated reciprocal arm and leg movements and postural control.
Two-point contralateral gait pattern using two devices
If the previously mentioned patients regain sufficient postural control, they might be advanced to using a two-point contralateral pattern (see Fig. 15-19, A). They still would be using two crutches or canes but would be moving one device and the opposite leg simultaneously (one point) followed by the other device and opposite leg (second point).
Five-point gait pattern using one device
Therapists may train patients with a walker if they have functional control of all four extremities but require greater trunk control than that afforded by a cane. For example, certain patients who have had cerebellar strokes may never recover adequate postural stability to be able to use two canes. A walker allows the patient to use five points of contact: the four legs of the walker and one of the patient’s legs. The patient advances the walker simultaneously with one leg and then places all four of the walker legs firmly on the floor at the same time as the patient’s foot. This pattern is called a five-point gait pattern (Fig. 15-21, B). If the patient moves the walker first, followed by the patient’s leg, the pattern is called a five-point delayed gait pattern (Fig. 15-21, A). Other authors refer to this gait pattern as a “3-1-point” or “modified 3-point” pattern.79 The basic sequence is the same, however.
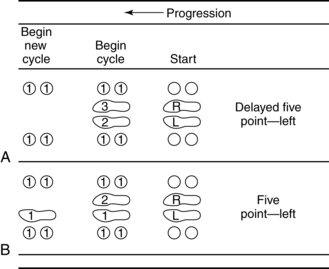
Figure 15-21 A and B, Diagrammatic view of assisted gaits.
(From Smidt G, Mommens MA: Gait patterns. Phys Ther 60(5):554, 1980.)
Therapists may train previously mentioned patients with a rolling walker. They may choose this device for two reasons: (1) the walker is in constant contact with the floor while being advanced, therefore affording maximal postural control; and (2) the walker is in constant motion, and the patient is able to take equal step lengths and to increase speed. With the standard walker, the patient is forced to use a “step-to” type of gait pattern (the walker is advanced, then the foot, then the other foot) that prevents a normal stride and limits velocity.89 In making the decision to use a rolling walker, the physical therapist also must consider the patient’s ability to control the continuous forward motion of the walker, as mentioned previously.
Three-point gait pattern using two devices
Three-point gait patterns seldom are used with stroke patients and are used more often with patients who have orthopedic conditions requiring weight relief on one leg (see Figs. 15-19, D, and 15-20, D).
Guarding techniques
The goal of gait training after stroke is to have the patient walk as efficiently, safely, and independently as possible. To promote optimum functional ambulation, it is important for the patient to experience postural instability and to relearn the way to correct these imbalances.
With this in mind, the therapist must be as close to patients as necessary to prevent them from falling or injuring themselves and yet not inhibit them from learning the way to right themselves. Therapists must allow patients to take some risks without jeopardizing the patients’ safety or their own safety. This is not an easy task, especially for new therapists. The ultimate horror for any therapist is having a patient fall. Obviously, until therapists are comfortable with patients and know how much, if any, outside support they need, guarding too much is better than guarding too little. Regardless, the goal always should be safe, optimal function, and the therapist needs to reevaluate on an ongoing basis how much guarding is needed and in what type of setting and on what type of surface activities should be performed.
Hemiparetic patients walking with a cane most often are guarded on the weaker side. The therapist stands slightly posterior and lateral to the affected side.79,86 The therapist is then in the best position to assist the patient. Should patients lose their balance or stumble, they may have difficulty preventing a fall to the weaker side because of decreased sensation and decreased strength and control of the paretic leg. The therapist can control patients with the hand closest to them at the hip or pelvis and can control patients’ shoulder and trunk with the other hand if necessary.
The use of gait belts or guarding belts varies from therapist to therapist and from institution to institution, but most facilities advocate their use in the initial stages of gait training and on stairs. The patient’s safety is of the utmost concern. At times, a patient is uncontrollable without a gait belt. Other times, the belt can be a hindrance to patients relearning postural control if the therapist is inadvertently tugging on the belt with every step. Therapists must evaluate each patient individually. The size of the patient in comparison to the therapist also may need to be considered. The therapist should decide which anticipatory actions need to be taken to protect the patient from harm based on clinical assessment and sound judgment.
When guarding a patient who is ascending stairs, the therapist is positioned posterior and to the weaker side. The patient should be trained using a railing at the stronger side. Initially, the therapist may teach the patient to ascend one step at a time, leading with the stronger leg. When the patient is descending, the therapist stands in front of and lateral to the affected side so as to provide assistance if the patient’s knee buckles. Using the railing, the patient steps down one step at a time, leading with the paretic leg. A patient who regains functional strength of the paretic leg may advance to the step-over-step method of stair climbing, with close guarding by the therapist. Ascending and descending stairs with only a cane or two canes is difficult and requires excellent balance. Some home environments may necessitate such training, but it should be undertaken with sufficient guarding, and the therapist carefully should weigh the safety risks.
Guarding techniques need to be taught to family members as soon as possible during inpatient rehabilitation. Family participation in gait training provides the opportunity for practice and repetition of newly learned techniques.
CASE STUDY Gait Training after Stroke
This case study in no way reflects the patient’s whole treatment program because emphasis also is placed on increasing strength and function in the trunk and left arm and in the leg. In addition, frequent sessions of cotreating by the occupational and physical therapists occurred to enhance communication about specific treatment concerns (e.g., the subluxed shoulder) and functional goals.
H.C. is a 54-year-old male who was admitted to the emergency room of a university medical center with sudden onset of left-sided weakness. Two weeks earlier, he had undergone a mitral valve repair and a single coronary artery bypass with a left saphenous vein graft. He had had an uneventful postoperative recovery course and was discharged to his home and prescribed a β-blocker.
On admission, the neurological workup and results included: (1) a computerized tomography scan of the head showing early lucency in the right subcortical area; (2) noninvasive flow studies (on the second day) showing accelerated flow velocities in the right middle cerebral artery suggestive of stenosis and normal flows in the anterior cerebral arteries, posterior cerebral arteries, and basal artery; and (3) a transesophageal echocardiogram revealing trace mitral regurgitation, normal left ventricular function, and no intracardiac or aortic mass or thrombus.
The attending neurologist concluded that H.C. had suffered an infarct in the right corona radiata and putamen in the territory supplied by the lenticulostriate branches of the right middle cerebral artery. The cause of the infarct was probably an embolus of cardiac origin that developed after the mitral valve repair. H.C. was prescribed anticoagulant medication and was stabilized medically. Twelve days later, he was transferred to the rehabilitation unit of the same medical center.
On admission to the rehabilitation unit, H.C. had symptoms of a pure motor syndrome with left upper extremity weakness that was greater than the left leg weakness, minimal left lower facial droop, and no sensory loss. He was alert and oriented and most cooperative although somewhat deconditioned because of the previous cardiac surgery.
Physical assessment revealed normal passive range of motion of the left arm and leg, although both legs manifested tight hamstrings and could perform only a straight leg raise to barely 60 degrees. A finger-width subluxation was present in the left shoulder. Strength testing revealed that the left arm was grossly 2 to 3 out of 5 throughout. He was able to extend the left knee completely while sitting (3 out of 5), but the hip flexors were weaker (2 out of 5). He exhibited no isolated voluntary ankle movement, although dorsiflexion was 2 out of 5 with simultaneous flexion of the hip and knee, and plantar flexion was 2 out of 5 during simultaneous extension of these proximal joints. He did not at that time (or ever) exhibit any spasticity in the limbs during passive testing by the therapist, with the exception of mild, unsustained ankle clonus. He exhibited no ankle edema despite the leg weakness and previous vein graft for the coronary artery bypass graft surgery.
His gait was evaluated initially while he was walking around a high mat with his right side next to the mat, using his right arm and the mat to “unload” the left leg. During static standing, he required contact guarding and verbal cues to extend the left hip and knee actively. He had a tendency to bear most of his weight on the stronger right leg. When cued to stand with equal weight on both legs, he was unable to maintain an upright posture and would fall to the left because the knee would buckle. He required minimal assistance to maintain the hip and knee in extension when bearing weight symmetrically.
Initially he was able to take 10 steps around the mat with minimal assistance. His gait analysis was as follows: Uneven step lengths were observed; the left was greater although less controlled than the right. The shorter step with the right leg resulted in a “step-to” type of gait pattern—the right leg stepping to meet, instead of pass, the left leg. He exhibited a decrease in single-limb stance time on the left leg. His cadence was slow—approximately 40 steps per minute.
During the left leg stance, the heel did not strike at initial contact; the foot was flat. The loading response resulted in excessive knee flexion that was greater than the normal 10 to 15 degrees. To prevent buckling in midstance, the knee snapped back into hyperextension (genu recurvatum). Instead of bringing his body forward by allowing the tibia to advance over the foot (dorsiflexion), he kept the ankle angle fixed and flexed the hip and trunk over the foot. He did not push off at the end of stance. Instead, he quickly took a short step with the right leg to unload the left one as soon as possible.
Because the resulting right leg position was next to the left leg instead of beyond it, the left leg was unable to assume the normal preswing position of hip extension and 40-degree knee flexion (see Fig. 15-4). Instead, the left hip and knee were in full extension, and he was forced to initiate swing on the left from this position.
During the swing phase of the left leg, H.C. exhibited decreased hip and knee flexion and a foot drop because of the weak dorsiflexors. This resulted in his toes scraping the floor. He displayed mild lateral trunk flexion to the right in an attempt first to initiate swing from the previously mentioned abnormal preswing position and then to clear the toes throughout the swing phase.
H.C. was put on a program of active assistive range of motion and strengthening exercises for the left arm and leg. Treatment of the leg emphasized functional strengthening in weight-bearing positions (e.g., sit-to-stand exercises for hip and knee strengthening).
During the initial stage of gait training, a posterior leaf splint orthosis was used to assist with dorsiflexion during the swing phase on the left side. This splint was chosen to encourage a more efficient swing phase and to discourage the patient from leaning to the right to clear the left leg during swing. Because of its flexibility, the posterior leaf splint did not restrict activity at the left ankle or knee during stance. Although the knee was unstable, H.C. was still in the early stages of recovery. Sacrificing mobility for stability (i.e., blocking any ankle dorsiflexion and knee flexion in stance) was not beneficial. Doing so would have forced him to move compensatorily because it is normal to dorsiflex up to 10 degrees at the ankle during midstance and terminal stance. The therapist offered close supervision to contact guarding at the knee because of possible knee buckling resulting from excessive dorsiflexion. H.C. was taught to be aware of the difference between excessive knee flexion and recurvatum. He was soon able to identify correctly when he was in either of these abnormal positions even if he could not always prevent them.
H.C. quickly advanced from ambulation around the high mat to ambulation with a narrow-based quad cane and then a straight cane. He had the advantage of recovering much of his hip extension and abduction strength, which meant he did not require a large degree of outside support from an assistive device for these muscles.
Functional training included standing balance retraining in single-limb and double-limb weight-bearing positions. Modified versions of activities that the patient previously had enjoyed (soccer) and a few new ones (golf putting and baseball) were introduced. H.C. practiced ambulation in a variety of environments and on both even and uneven terrains in preparation for discharge. H.C. even practiced getting through busy revolving doors.
After 6 weeks of inpatient rehabilitation, H.C. was evaluated for a permanent AFO. His left leg strength had improved enough to allow him to isolate dorsiflexion and plantar flexion in any position grossly in the 2 out of 5 range, ankle inversion and eversion in the 2 out of 5 range, and toe flexion and extension in the 1+ out of 5 range. His hip flexors improved minimally to 2+ out of 5, and knee extension also improved minimally to 3+ out of 5.
During ambulation, H.C. continued to manifest knee recurvatum in stance and did not push off at the end of stance because of weak plantar flexors. During swing, he continued to exhibit a foot drop and toe drag. The physiatrist, physical therapist, and orthotist performed a joint observational gait analysis. Because of the continued plantar flexor and dorsiflexor weakness during the swing and stance phases and less than normal knee extension strength, they decided that H.C. required minimal knee control and ankle control from an AFO. In addition, the weak ankle invertors and evertors necessitated mediolateral control by an orthosis. Therefore a posterior leaf splint was deemed insufficient. However, because H.C. was continuing to progress and did not require maximum support at the knee, a solid ankle AFO was also inappropriate. The general consensus was that H.C. should be allowed to have as much movement at the ankle as possible without jeopardizing his safety to promote development of a normal gait pattern.
For this reason the team decided to order a hinged polypropylene AFO with free dorsiflexion at the ankle and a plantar flexion stop at 90 degrees. The hinged ankle with free dorsiflexion allowed him to move the tibia normally over the foot (dorsiflexion) in midstance and terminal stance. The plantar flexion stop at 90 degrees prevented foot drop in swing and recurvatum in stance. The orthosis improved his gait by allowing the normal joint excursions at the knee and ankle in stance while preventing abnormal movements in stance and swing. The promotion of normal joint excursions at the ankle in stance allowed him to take equal step lengths with both legs.
On discharge to his home, H.C. was able to ambulate independently indoors with a straight cane and the hinged AFO, but he required supervision outdoors. He was able to ascend and descend stairs step-over-step using a railing and ascend and descend curbs and ramps with the straight cane, all with distant supervision. He could perform simple home exercises independently for left leg and arm strengthening. He returned to work as a full-time university professor and continued with outpatient physical therapy three times a week for six months after discharge. He regained full functional use of the left upper extremity including finger function (albeit with decreased coordination), fine motor control, and strength. He also continued to receive occupational therapy for several months as an outpatient.
Summary
The aim of this chapter is to familiarize the occupational therapist with processes used by physical therapists during gait evaluation and training of patients who have had a stroke. The most common type of gait disorders are those resulting from a middle cerebral artery infarction.
The application of orthotic devices is not an exact science. To assume that a particular abnormal gait always requires one specific type of orthotic device is not accurate. Therapists must evaluate devices on an individual trial basis. Use of a specific device or pattern requires individualized attention.
Those well-versed and experienced in motor control research20,39,42,44,99 believe that the trend in physical therapy is moving away from earlier theoretical models of treatment techniques and toward a motor control model. The emphasis is no longer on specific treatment techniques to “facilitate” movement but on active problem-solving by the patient to promote skilled movement and motor relearning. The practice of specific tasks is to be emphasized during intervention. Treatment programs need to be based on specific motor control deficits, which may require modification or emphasis in the practice of a task which is meaningful to the patient and which takes place in numerous environments. All of the gait intervention research presented in this chapter support this model.
One can no longer assume that certain treatment techniques are effective. Effectiveness needs to be validated by research. As presented earlier, Weinstein and colleagues99 examined the effect that balance-training and weight shifting activities during standing had on the hemiplegic gait. Although patients who received training improved their standing symmetry significantly, training did not translate into improved weight shifting during ambulation. This study clearly demonstrates the hazards of assuming that transfer of training occurs from a part of one functional task to another. For example, it would be convenient to assume that the techniques used to improve the standing balance of a patient with contraversive pushing will improve the ability to walk. However, no evidence as yet supports this theory. Further research such as that of Weinstein and colleagues99 is imperative for therapists to validate the rationales for their treatment procedures for stroke patients. To do otherwise denies the patient the most beneficial treatment approach.
The case study was unusual because the patient exhibited no spasticity and had voluntary, isolated control of all muscles but had decreased strength. However, several authors questioned the role of spasticity in preventing normal movement1,20,21,44 and pointed to weakness as the more limiting factor. Spasticity is well-known to increase the incidence of muscle contracture and thereby alter the biomechanical efficiency of a muscle.1,20,21,29,41,44 In this respect, only the ankle joint was at risk and minimally so. The patient was a model patient for other reasons. He was not cognitively impaired, and he was motivated to return to work. He was aware (although grudgingly at times) of the need for faithful adherence to a regular exercise program of repeated practice of newly learned motor skills.
Therapists always should be aware of the need for careful physical assessment, individualized treatment programs that are based on research findings, and ongoing reevaluation of the effectiveness of the treatment program in promoting optimum function.
Review questions
1. What constitutes a gait cycle?
2. What are the phases and subphases of the gait cycle?
3. What are step, stride, and cadence?
4. What tests would you perform to assess gait speed and gait endurance?
5. What type of cerebral infarct is associated with the typical “hemiplegic gait”?
6. What are some of the variables that can cause a deviation from the normal joint excursions during a gait cycle?
7. In what way does the motor control model differ from the more traditional theoretical models underlying the different therapeutic techniques?
8. How might ambulation recovery be related to hip osteoporosis?
9. What are some of the manifestations of a posterior inferior cerebellar stroke?
10. What makes treatment of patients demonstrating contraversive pushing so challenging?
11. What helps compensate for proprioceptive loss after stroke?
12. What are the main differences between metal and plastic orthotic devices?
13. What orthotic device is used most commonly with stroke patients?
14. What assistive devices are used most commonly with stroke patients?
15. What determines the type of gait pattern that will be taught to a stroke patient?
1. Ada L, Canning C. Anticipating and avoiding muscle shortening. In: Ada L, Canning C, editors. Key issues in neurological physiotherapy. Boston: Butterworth-Heinemann, 1990.
2. Ada L, Dean CM, Hall JM, et al. A treadmill and overground walking program improves walking in persons residing in the community after stroke. A placebo-controlled, randomized trial. Arch Phys Med Rehabil. 2003:84;10:1486-1491.
3. Adams JM, Perry J. Gait analysis. clinical application. Rose J, Gamble JG, editors. Human walking. Baltimore: Williams & Wilkins, 1994.
4. Amarenco P. Cerebellar stroke syndromes. In: Bogousslavsky J, Caplan L, editors. Stroke syndromes. Cambridge, UK: Cambridge University Press, 2001.
5. Amarenco P. The spectrum of cerebellar infarcts. Neurology. 1991;41(7):973.
6. American Thoracic Society Board of Directors. ATS statement. Guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002:166;1:111-117.
7. Balliet R, Harbst KB, Kim D, et al. Retraining of functional gait through the reduction of upper extremity weight bearing in chronic cerebellar ataxia. Int Rehabil Med. 1987;8(4):148-153.
8. Barton JJS, Caplan LR. Cerebral visual dysfunction. In: Bogousslavsky J, Caplan L, editors. Stroke syndromes. Cambridge, UK: Cambridge University Press, 2001.
9. Bassile CC, Dean C, Boden-Albala B, et al. Obstacle training programme for individuals post stroke. feasibility study. Clin Rehabil. 2003:17;2:130-136.
10. Bingman VP, Zucchi M. Spatial orientation. In: Cohen H, editor. Neuroscience for rehabilitation. Philadelphia: JB Lippincott, 1993.
11. Bontrager E. Instrumented gait analysis. In: DeLisa JA, editor. Gait analysis in the science of rehabilitation monograph 002. Baltimore: Department of Veterans Affairs, Veterans Health Administration, Rehabilitation Research and Development Service, Scientific and Technical Section, 1998.
12. Branch EF. The neuropathology of stroke. In: Duncan P, Badke MB, editors. Stroke rehabilitation. Chicago: Mosby, 1987.
13. Brandt T, Krafczyk S, Malsbenden I. Postural imbalance with head extension. improvement by training as a model for ataxia therapy. Ann N Y Acad Sci. 1981;374:636.
14. Brower B, Davidson LK, Olney SJ. Serial casting in idiopathic toe walkers. J Pediatr Orthop. 2000;20(2):221-225.
15. Brown DL, Morgenstern LB, Majersik JJ, et al. Risk of Fractures after stroke. Cerebrovasc Dis. 2008;25(1-2):95-99.
16. Brust JB. Circulation of the brain. In Kandel ER, Schwartz JH, editors: Principles of neural science, ed 4, New York: McGraw-Hill, 2000.
17. Burdett RG, Borello-France D, Blatchly C, et al. Gait comparison of subjects with hemiplegia walking unbraced, with ankle-foot orthosis, and Air-Stirrup brace. Phys Ther. 1998;68(8):1197.
18. Caplan LR. Visual perceptual abnormalities. In: Bogousslavsky J, Caplan L, editors. Stroke syndromes. Cambridge, UK: Cambridge University Press, 2001.
19. Carlson. A neurophysiological analysis of inhibitive casting. Phys Occup Ther Pediatr. 1984;4(4):31-42.
20. Carr JH, Shepherd RB. A motor learning model for rehabilitation. In: Carr JH, Shepherd RB, editors. Movement science foundations for physical therapy in rehabilitation. Gaithersburg, MD: Aspen, 2000.
21. Carr JH, Shepherd RB, Ada L. Spasticity. research findings and implications for intervention. Physiotherapy. 1995:81;8:421.
22. Cohen H. Special senses 2. the vestibular system. Cohen H, editor. Neuroscience for rehabilitation. Philadelphia: JB Lippincott, 1999.
23. Craik RL, Dutterer L. Spatial and temporal characteristics of foot fall patterns. In: Craik RL, Oatis CA, editors. Gait analysis, theory and application. St. Louis: Mosby, 1995.
24. Davies PM. Steps to follow. Berlin: Springer-Verlag; 1985.
25. Dean CM, Richards CL, Malouin F. Task-related circuit training improves performance of locomotor tasks in chronic stroke. A randomized, controlled pilot trial. Arch Phys Med Rehabil. 2000:81;4:409-417.
26. Dean CM, Richards CL, Malouin F. Walking speed over 10 metres overestimates locomotor capacity after stroke. Clin Rehabil. 2001;15(4):415-421.
27. De Deyne PG. Application of passive stretch and its implications for muscle fibers. Phys Ther. 2001;81(2):819-827.
28. Diamond M, Ottenbacher K. Effect of tone-inhibiting DAFO on stride characteristics of an adult with hemiparesis. Phys Ther. 1981;70(7):423.
29. Dietz V, Quintern J, Berger W. Electrophysiological studies of gait in spasticity and rigidity. evidence that altered mechanical properties of muscle contribute to hypertonia. Brain. 1981:104;3:431-449.
30. Dimitrijevic MR, Faganel J, Sherwood AM, et al. Activation of paralysed leg flexors and extensors during gait in patients after stroke. Scand J Rehabil Med. 1981;13(4):109.
31. Duncan P, Studenski S, Richards L, et al. Randomized clinical trial of therapeutic exercise in subacute stroke. Stroke. 2003;34(9):2173-2180.
32. Duncan PW, Badke MB. Determinants of abnormal motor control. In: Duncan PW, Badke MB, editors. Stroke rehabilitation. Chicago: Mosby, 1987.
33. Edelstein J. Orthotic management and assessment. In O’Sullivan S, Schmitz TJ, editors: Physical rehabilitation: assessment and treatment, ed 4, Philadelphia: FA Davis, 2001.
34. Engardt M, Knutsson E, Jonsson M, et al. Dynamic muscle strength training in stroke patients. effect on knee extension torque, EMG activity, and motor function. Arch Phys Med Rehabil. 1995:76;5:419-425.
35. Faculty of Prosthetics and Orthotics. New York University School of Medicine and Post Graduate Medical School. Lower limb orthotics. New York: New York University School of Medicine and Post Graduate Medical School. 1986.
36. Ferro JM. Neurobehavioral aspects of deep hemispheric stroke. In: Bogousslavsky J, Caplan L, editors. Stroke syndromes. Cambridge, UK: Cambridge University Press, 2001.
37. Garland DE, Stewart CA, Adkins RH, et al. Osteoporosis after spinal cord injury. J Orthop Res. 1992;10(3):371-378.
38. Gilman S, Newman SW. Clinical neuroanatomy, ed 8. Philadelphia: FA Davis; 1992.
39. Giuliani CA. Adult hemiplegic gait. In: Smidt GL, editor. Gait in rehabilitation. New York: Churchill Livingstone, 1990.
40. Glatt SL, Koller WS. Gait apraxia. In: Spivack BS, editor. Evaluation and management of gait disorders. New York: Marcel Dekker, 1995.
41. Goldspink G, Williams P. Muscle fiber and connective tissue changes associated with use and disuse. In: Ada L, Canning C, editors. Key issues in neurological physiotherapy. Boston: Butterworth-Heinemann, 1990.
42. Gordon J. Assumptions underlying physical therapy intervention. In: Carr JH, Shepherd RB, editors. Movement science foundations for physical therapy in rehabilitation. Gaithersburg, MD: Aspen, 2000.
43. Harro CC, Giuliani CA. Kinematic and EMG analysis of hemiplegic gait patterns during free and fast walking speeds. Neurol Rep. 1987;11:57.
44. Held JM. Recovery of function after brain damage. theoretical implications for therapeutic intervention. Carr JH, Shepherd RB, editors. Movement science foundations for physical therapy in rehabilitation. Gaithersburg, MD: Aspen, 2000.
45. Jones LA. Somatic senses 3. proprioception. Cohen H, editor. Neuroscience for rehabilitation. Philadelphia: JB Lippincott, 1999.
46. Jorgensen L, Crabtree NJ, Reeve J, et al. Ambulatory level and asymmetrical weight bearing after stroke affects bone loss in the upper and lower part of the femoral neck differently. Bone Adaptation after decreased mechanical loading. Bone. 2000:27;5:701-707.
47. Jorgensen L, Jacobsen BK, Wilsgarrd T, et al. Walking after stroke. Does it matter? Changes in bone mineral density within the first 12 months after stroke. A longitudinal study. Osteoporos Int. 2000:11;5:381-387.
48. Karnath HO, Broetz D. Understanding and treating “Pusher Syndrome. Phys Ther. 2003;83(12):1119-1125.
49. Karnath HO, Ferber S, Dichgans J. The origin of contraversive pushing. evidence for a second graviceptive system in humans. Neurology. 2000:55;9:1298-1304.
50. Karnath HO, Ferber S, Dichgans J. The neural representation of postural control in humans. Proc Natl Acad Sci U S A. 2000;97(25):13931-13936.
51. Karnath HO, Johannsen L, Broetz D, et al. Prognosis of contraversive pushing. J Neurol. 2002;249(9):1250-1253.
52. Knutsson E. Gait control in hemiparesis. Scand J Rehabil Med. 1981;13(2–3):101-108.
53. Knutsson E, Martensson A. Dynamic motor capacity in spastic paresis and its relation to prime mover dysfunction, spastic reflexes, and antagonist co-activation. Scand J Rehabil Med. 1980;12(3):93.
54. Kusoffsky A, Wadell I, Nilsson BY. The relationship between sensory impairment and motor recovery in patients with hemiplegia. Scand J Rehabil Med. 1982;14(1):27-32.
55. Lehmann JF. Lower limb orthotics. In Redford JB, editor: Orthotics etc, ed 3, Baltimore: Williams & Wilkins, 1986.
56. Lehmann JF, Condon SM, Price R, et al. Gait abnormalities in hemiplegia. Arch Phys Med. 1987;68(11):763-771.
57. Licht S. Preface to the first edition. In Redford JB, editor: Orthotics etc, ed 3, Baltimore: Williams & Wilkins, 1986.
58. Macko RF, Ivey FM, Forrester LW, et al. Treadmill exercise rehabilitation improves ambulatory function and cardiovascular fitness in patients with chronic stroke. A randomized controlled trial. Stroke. 2005:36;10:2206-2211.
59. McComas AJ, Sica RE, Upton AR, et al. Functional changes in motor neurons of hemiparetic patients. J Neurol Neurosurg Psychiatry. 1973;36(2):183-193.
60. Montgomery J. Assessment and treatment of locomotor deficits in stroke. In: Duncan PW, Badke MB, editors. Stroke rehabilitation: recovery of motor control. Chicago: Mosby, 1987.
61. Mueller K, Cornwall MW, McPoil TG, et al. Effect of two contemporary tone-inhibiting AFOs on foot-loading patterns in adult hemiplegics. a small group study. Top Stroke Rehabil. 1995:1;4:1-16.
62. Nilsson L, Carlsson J, Danielsson A, et al. Walking training of patients with hemiparesis at an early stage after stroke. A comparison of walking raining on a treadmill with body weight support and walking training on the ground. Clin Rehabil. 2001;15:515-527.
63. Norkin C. Gait analysis. In O’Sullivan S, Schmitz TJ, editors: Physical rehabilitation: assessment and treatment, ed 4, Philadelphia: FA Davis, 2001.
64. Nyberg L, Gustafson Y. Patient falls in stroke rehabilitation. A challenge to rehabilitation strategies. Stroke. 1995;26(5):838-842.
65. Oestreich L, Troost BT. Cerebellar dysfunction and disorders of posture and gait. In: Spivack BS, editor. Evaluation and management of gait disorders. New York: Marcel Dekker, 1995.
66. Olney SJ, Griffin MP, Monga TN, et al. Work and power in gait of stroke patients. Arch Phys Med Rehabil. 1991;72(5):309-314.
67. Olney SJ, Richards CL. Hemiplegic gait following stroke. Gait Posture. 1996;4(2):36.
68. Olsson EC, Smidt GL. Assistive devices. In: Smidt GL, editor. Gait in rehabilitation. New York: Churchill Livingstone, 1990.
69. O’Sullivan SB. Motor control assessment. In O’Sullivan SB, Schmitz TJ, editors: Physical rehabilitation: assessment and treatment, ed 4, Philadelphia: FA Davis, 2001.
70. O’Sullivan SB. Stroke. In O’Sullivan SB, Schmitz TJ, editors: Physical rehabilitation: assessment and treatment, ed 4, Philadelphia: FA Davis, 2001.
71. Ounpuu S. Clinical gait analysis. In: Spivack BS, editor. Evaluation and management of gait disorders. New York: Marcel Dekker, 1995.
72. Paci M, Nannetti L. Physiotherapy for pusher behavior in a patient with post-stroke hemiplegia. J Rehabil Med. 2004;36(4):183-185.
73. Pathokinesiology Service and Physical Therapy Department. Observational gait analysis handbook. Downey, CA: Professional Staff Association of Rancho Los Amigos Medical Center; 1991.
74. Pedersen PM, Wandell A, Jorgensen HS. Ipsilateral pushing in stroke. incidence, relation to neuropsychological symptoms, and impact on rehabilitation—the Copenhagen stroke study. Arch Phys Med. 1996:77;1:25-28.
75. Peraula SH, Tarkka IM, Pitkanen K, et al. The effectiveness of body weight-supported gait training and floor walking in patients with chronic stroke. Arch Phys Med Rehabil. 2005;86(8):1557-1564.
76. Perennou DA, Amblard B, Laassel EM, et al. Understanding the pusher behavior of some stroke patients with spatial deficits. a pilot study. Arch Phys Med Rehabil. 2002:83;4:570-575.
77. Perry J. The mechanics of walking. In: Perry J, Hislop H, editors. Principles of lower extremity bracing. Washington, DC: American Physical Therapy Association, 1977.
78. Perry J, Garrett M, Gronley JK, et al. Classification of walking handicap in the stroke population. Stroke. 1995;26(6):982-989.
79. Pierson FM. Ambulation aids, patterns and activities. In Pierson FM, editor: Principles and techniques of patients care, ed 2, Philadelphia: Saunders, 1999.
80. Ramnemark A, Nilsson M, Borssen B, et al. Stroke, a major and increasing risk factor for femoral neck fracture. Stroke. 2000;31(7):1572-1577.
81. Ramnemark A, Nyberg L, Borssen B, et al. Fractures after stroke. Osteoporos Int. 1998;8(1):92-95.
82. Ramnemark A, Nyberg L, Lorentzon R, et al. Progressive hemiosteoporosis on the paretic side and increased bone mineral density in the nonparetic arm the first year after severe stroke. Osteoporos Int. 1999;9(3):269-275.
83. Richards CL, Malouin F, Dumas F. Gait velocity as an outcome measure of locomotor recovery after stroke. In: Craik RL, Oatis C, et al, editors. Gait analysis: theory and application. St Louis: Mosby, 1995.
84. Richards CL, Olney SJ. Hemiparetic gait following stroke. II. Recovery and physical therapy. Gait Posture. 1996;4(2):49.
85. Salbach NM, Mayo NE, Wood-Dauphinee S. A task orientated intervention enhances walking distance and speed in the first year post stroke. a randomized controlled trial. Clin Rehabil. 2004:18;5:509-519.
86. Schmitz TJ. Preambulation and gait training. In O’Sullivan S, Schmitz TJ, editors: Physical rehabilitation: assessment and treatment, ed 4, Philadelphia: FA Davis, 2001.
87. Shaughnessy M, Michael KM, Sorkin JD, et al. Steps after stroke. Capturing ambulatory recovery. Stroke. 2005:36;6:1305-1307.
88. Sisto SA. An overview of the value of information resulting from instrumented gait analysis for the physical therapist. In: DeLisa JA, editor. Gait analysis in the science of rehabilitation monograph 002. Baltimore: Department of Veterans Affairs, Veterans Health Administration, Rehabilitation Research and Development Service, Scientific and Technical Section, 1998.
89. Smidt GL, Mommens MA. System of reporting and comparing influence of ambulatory aids on gait. Phys Ther. 1980;60(5):551-558.
90. Smith E, Juvinall RC. Mechanics of orthotics. In Redford JB, editor: Orthotics etc, ed 3, Baltimore: Williams & Wilkins, 1986.
91. Sullivan KJ, Knowlton BJ, Dobkin BH. Step training with body weight support. Effect of treadmill speed and practice paradigms on poststroke locomotor recovery. Arch Phys Med Rehabil. 2002:83;5:683-691.
92. Ticini L, Klose U, Nagele T, Karnath HO. Perfusion imaging in Pusher Syndrome to investigate the neural substrates involved in controlling upright body position. PLoS One. 2009;4(5):e5737.
93. Tiderksaar R. Falls in older persons. In: Spivack BS, editor. Evaluation and management of gait disorders. New York: Marcel Dekker, 1995.
94. Timmann D, Diener HC. Cerebellar ataxia. In: Bogousslavsky J, Caplan L, editors. Stroke syndromes. Cambridge, UK: Cambridge University Press, 2001.
95. Toole JF. Cerebrovascular disorders, ed 5. Philadelphia: Lippincott Williams & Wilkins; 1999.
96. Visitin M, Barbeau H, Korner-Bitensky N, et al. A new approach to retrain gait in stroke patients through body weight support and treadmill stimulation. Stroke. 1998;29(6):1122-1128.
97. Walsh EG, Wright GW, Brown K, et al. Biodynamics of the ankle in spastic children. effect of chronic stretching on the calf musculature. Exp Physiol. 1990:75;3:423-425.
98. Walters RL, Garland DE, Montgomery J. Orthotic prescription for stroke and head injury. American Academy of Orthopedic Surgeons. Atlas of orthotics, ed 2. St Louis: Mosby. 1985.
99. Weinstein CJ, Gardner ER, McNeal DR, et al. Standing balance training. effects on balance and locomotion in hemiparetic adults. Arch Phys Med. 1989:70;10:755-762.
100. Whitson HE, Pieper CF, Sanders L, et al. Adding injury to insult. Fracture risk after stroke in veterans. J Am Geriatr Soc. 2006:54;7:1082-1088.
101. Wilmet E, Ismail AA, Heilporn A, et al. Longitudinal study of the one mineral content and of soft tissue composition after spinal cord section. Paraplegia. 1995;33(11):674-677.
102. Winstein CJ, Gardner ER, McNeal DR, et al. Standing balance training. Effect on balance and locomotion in hemiparetic adults. Arch Phys Med Rehabil. 1989:70;10:755-762.