chapter 9 Vestibular rehabilitation and stroke
The vestibular system is one of the special senses; it has receptors on the head and signals the brain via a cranial nerve. The end organs for the vestibular system, the vestibular labyrinths, detect head acceleration, or a change in the rate at which the head is moving. This information is converted to a velocity signal (velocity is speed plus direction), so the signal received by the brain really represents the speed and direction at which the head moves. The labyrinths are located within cavities inside the temporal bones of the skull on either side of the head, so the end organs are inaccessible from the outside world. One cannot see it or otherwise examine it without drilling into the temporal bone to expose it. Because the end organ is not obvious and because the roles of the vestibular system—contributions to postural control, oculomotor control, and spatial orientation, and modulation of some autonomic function—are subtle, the vestibular system was the last of the special senses to be discovered, and many people still do not understand it.
Overview of the vestibular system
Peripheral vestibular labyrinth
A detailed discussion of the anatomy and physiology of the vestibular system is beyond the scope of this chapter. The vestibular system has been reviewed many times in journal articles and textbooks. For excellent reviews, the reader is encouraged to examine other texts. 3,5,7,9,11,24 To put the topic of this chapter in context requires a brief reviews of some main points about the vestibular system.
The vestibular labyrinth has two sets of motion detectors: three semicircular canals (lateral, posterior, and superior) that act as rotatory accelerometers to detect turning motions of the head, and two saclike otoliths (utricle and saccule) that act as linear accelerometers to detect linear acceleration of the head (Fig. 9-1). Since gravity is a fixed linear acceleration, the otoliths also detect static tilt with reference to gravity. This gravitational signal is very important for spatial orientation, because it acts as an earth-fixed reference.
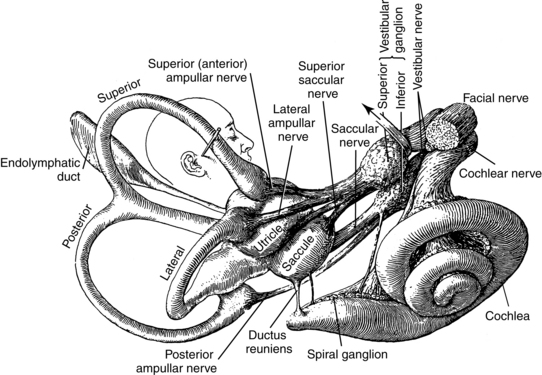
Figure 9-1 Gross anatomy of the vestibular labyrinth.
(From Brödel M: Three unpublished drawings of the anatomy of the human ear, Philadelphia, 1946, Saunders.)
This information, and the complex anatomical structures associated with it, is needed to keep the head erect and see where one are going as one moves through space, plots a course toward a particular location or target, and generates appropriate autonomic responses when one encounters a perturbation; that is, when one is inadvertently thrown off balance for some reason. These motor skills help some lower animals capture and eat their prey and help other animals avoid becoming prey. These skills serve similar purposes in humans as they move through space, while avoiding or encountering obstacles, performing purposeful activities that involve manipulating objects while they move their heads in all planes. People with impaired vestibular systems complain of vertigo and poor spatial navigation skills, blurred vision, impaired postural control, and nausea and other signs of autonomic involvement.
Inertial mechanism
The mechanism of the vestibular labyrinth is based on the principle of inertia, that is, that an object remains at rest until an asymmetrical force acts on it, and then it continues to move until another asymmetrical force acts on it to stop it. The semicircular canals are narrow (imagine a curved tube approximately the width of a hair on your head), so they provide a large amount of resistance to any fluid that fills it. The canals are then filled with a thick fluid known as endolymph. Endolymph has a high specific gravity, so it has high inertia. The inertial properties of endolymph and the high resistance of the canals, combined, mean that the vestibular system is not sensitive to extremely slow head movements. It is somewhat responsive to slow head movements, but it responds most accurately to moderate to rapid head movements in the range of 0.1 to 7.0 Hz. Not surprisingly, this frequency bandwidth is the range of most normal head movements. When an individual rotates his or her head, while shaking his or her head “no,” tiny cilia attach to specialized hair cells, located on a miniscule hillock that blocks one end of the canal, and bend backward in response to movement of the endolymph over the gelatinous cup or cupula into which they protrude. This motion of the cilia starts a chain of events within the hair cells in which ions are exchanged; the cell membrane either hyperpolarizes or depolarizes. If the cell membrane depolarizes, then neurotransmitter is released, and the adjacent vestibular nerve fires which signals to the related neurons in the vestibular nuclei, located in the medulla, that the person turned his or her head.
In the otoliths, the hair cells are located in patches either in the base of the utricle or on the side of the saccule. Their cilia protrude into the otoconial membrane, which is a protein matrix containing many microscopic crystals of calcium carbonate known as otoconia. The otoconia act as an inertial mass. The otoconial membrane slides back and forth over the cilia in response to linear acceleration. For example, when a person accelerates his or her car going forward, the otoconial membrane virtually slides backward over the underlying cilia, bending them backward and commencing the transduction process described in the preceding paragraph.
Innervation and blood supply
All of this hardware in the temporal bone is supplied by nerves and arteries. The vestibular labyrinth is innervated by the vestibular nerve, which is half of cranial nerve VIII. The vestibular nerve has two branches. The superior branch innervates the superior and horizontal semicircular canals and the utricle, and the inferior branch innervates the posterior canal and the saccule.
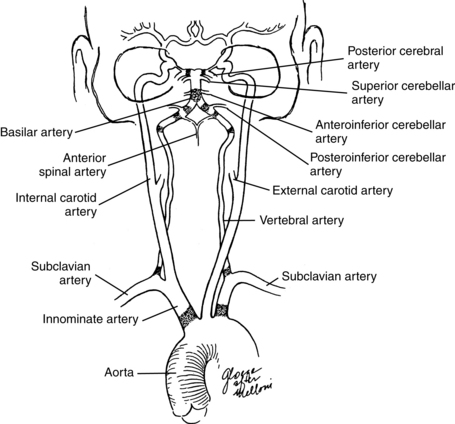
The arterial supply to the vestibular labyrinth is similar to the innervation. The entire labyrinth receives its blood supply from one artery, the anterior inferior cerebellar artery (AICA), which is a branch off the basilar artery. A major branch from the AICA, the labyrinthine artery, supplies the entire inner ear. Inside the inner ear, it bifurcates to form the common cochlear artery and anterior vestibular artery (AVA). The AVA supplies the area primarily innervated by the superior vestibular nerve, i.e., the superior and horizontal semicircular canals and the utricle. These areas drain into the anterior vestibular vein. The common cochlear artery bifurcates and forms the cochlear artery and the posterior vestibular artery (PVA). The PVA innervates the posterior semicircular canal and the saccule. These areas drain into the posterior vestibular vein. Both veins join with the vein from the round window, elsewhere in the inner ear, and form the vestibulocochlear vein, eventually draining into the cochlear aqueduct and then the inferior petrosal sinus. Other small veins from the semicircular canals join to form the vein of the vestibular aqueduct, eventually draining into the lateral venous sinus (Fig. 9-2).
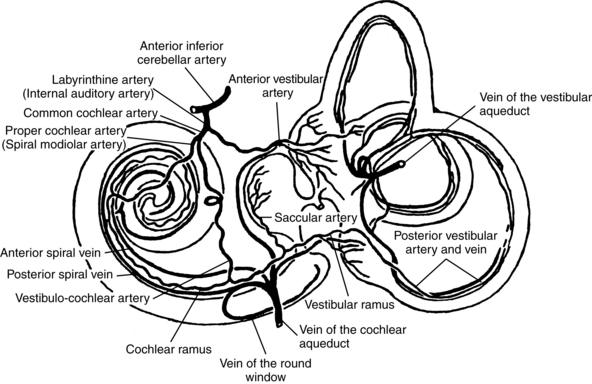
Figure 9-2 Arterial supply to the vestibular labyrinth.
(Modified from Nabeya D: Study in comparative anatomy of blood-vascular system of internal ear in mammalian and in homo, Acta Schol Med Imp Kioto 6:1, 1923.)
Interruption to the blood supply to the vestibular labyrinth can cause the usual manifestations of vestibular weakness, including vertigo, disequilibrium, blurred vision, and nausea. The blood supply can be interrupted by ischemia or infarction. When the AVA is involved, the patient does not have hearing loss, since the loss of blood supply is distal to the bifurcation of the labyrinthine artery. When the labyrinthine artery is involved, hearing loss is more likely. During the acute phase of sudden, dramatic, and incapacitating symptoms, which may last hours to days, patients are treated with palliative care. After the acute phase is over, patients who have not compensated spontaneously may be referred for vestibular rehabilitation. These patients are often rehabilitated successfully.
Central projections
The vestibular nerve projects to the vestibular nuclei in the rostral medulla (Fig. 9-3). The projection has some spatial specificity in that different nerves project to different areas of the vestibular nuclei. From there projections project to the dentate and fastigial nuclei of the cerebellum. Eventually those signals make their way to the flocculus, nodulus, and ventral uvula in the cerebellar vermis, the so-called vestibulocerebellum. Projections out of the cerebellum return to the vestibular nuclei. From there, some signals descend the vestibulospinal tracts to cervical and lumbosacral levels of the spinal cord. Those pathways are involved in postural control and are especially important in the absence of vision. Patients with vestibular weakness caused by peripheral or central lesions often have impaired balance.
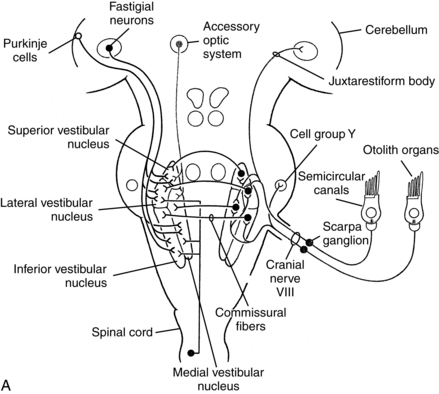
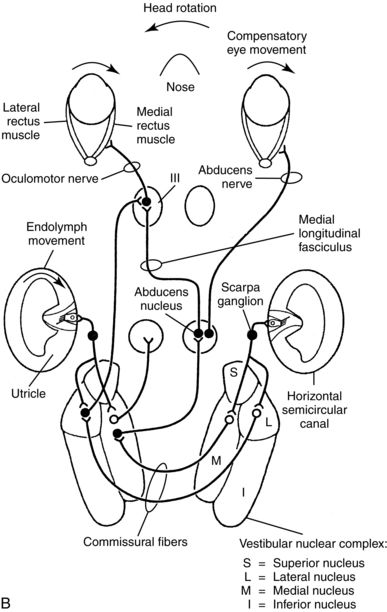
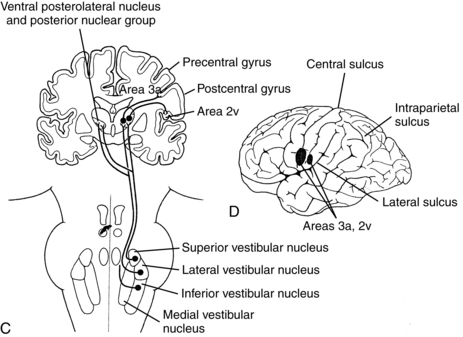
Figure 9-3 Central vestibular projections. Closed cell bodies are excitatory, and open cell bodies are inhibitory. A, Afferent projections of the vestibular nerve. B, Projections mediating the horizontal vestibulo-ocular reflex. C, Vestibulo-cortical projections. D, Likely vestibular projection areas in the cerebral cortex.
(From Dickman JD: The vestibular system. In Haines DE, editor: Fundamental neuroscience, New York, 1997, Churchill Livingstone.)
After receiving input from oculomotor-related neurons in other nuclei, other tracts ascend the medial longitudinal fasciculus in a complex set of crossed and uncrossed pathways to synapse on the nuclei from cranial nerves III, IV, and IV. Those cranial nerves control the extraocular muscles of the eyes, so those vestibuloocular pathways control the vestibuloocular reflex (VOR). The VOR is an eye movement made in response to head movement, which stabilizes the position of the eye in space. The head is relatively large and sits atop a flexible neck, so as an individual moves his or her body through space, the head moves. To see clearly while the person moves the head, he or she generates the VOR in the direction opposite the head movement. Patients with unilateral vestibular weakness caused by peripheral or central lesions often complain of blurred vision during head movement, due to decreased amplitude of the VOR. Also, some patients with central vestibular lesions have other unusual or abnormal eye movement patterns. Neurologists sometimes use these patterns of eye movements to help localize cerebellar and brainstem lesions.
A few pathways, which are still poorly mapped, ascend via the thalamus to some poorly defined, probably small areas in the cerebral cortex, mostly around some auditory projection areas in the temporal lobe, near the junction of the temporal and parietal lobes and into the insula8,22 (see Fig. 9-3). The functions of these projections are not clear, but they may mediate the conscious perception of motion or the vestibular contributions to spatial orientation. For example, reports in humans have shown that stimulation to those brain regions in patients undergoing neurosurgery elicits a sense of motion.26,40 Lesions to the posterolateral thalamus impair upright body orientation30 and cause perceived tilt of the visual vertical and deviations of the eyes.44 Lesions to the putative vestibular cortex impair spatial perception by affecting perception of the subjective visual vertical.43 Research on the ascending projections from the vestibular nuclei has progressed considerably with improvements in brain mapping techniques, so the vestibular thalamic projections and vestibular cortical projections will probably be mapped and studied more thoroughly in the future.
A fourth set of projections, also still poorly mapped, are involved in mediating some aspects of autonomic function. Therefore, some patients with vestibular weakness complain of autonomic signs such as nausea, sweating, increased heart rate, or anxiety.1,2 Recent research has shown differential uses of rotational and linear vestibular inputs in modulating muscle sympathetic nerve activity vs. skin sympathetic nerve activity.12 For a good review of vestibular autonomic mechanisms and clinical implications, see the paper by Yates and Bronstein.42
Central arterial supply
The vestibular nuclei receive their blood supply from the anterior and posterior cerebellar arteries (AICA and PICA, respectively). The AICA arises from the basilar artery and supplies the cerebellopontine angle, part of the anterior cerebellum, part of the vermis and the vestibulocerebellum, part of the rostral pons, the middle cerebellar peduncle, and cranial nerve VII (facial nerve) and cranial nerve VIII. The PICA is a branch off the rostral section of the vertebral artery. It supplies the lateral medulla and part of the cerebellum, including part of the vermis, where the nodulus and ventral uvula are located (Fig. 9-4). The vestibular cortical projection is probably supplied by the middle cerebral artery off the branches that supply the temporal lobe. Since the vestibular cortex is still being investigated, the exact blood supply may be a matter for some debate.
Stroke syndromes
In approximately 20% of patients who complain of vertigo, in general, the cause is vascular in nature (stroke, vertebrobasilar migraine headache, or transient ischemic attack).39 Vestibular lesions in stroke patients, as indicated by complaints of vertigo, are rare, however. In one study of 474 confirmed strokes in which patients were hospitalized, only 2% complained of vertigo.36 More than half of all brainstem strokes are in the pons,23 and strokes in that area can cause lesions of the vestibular nuclei. Of the overall population of patients seen in the emergency department and subsequently admitted for stroke, however, the percentage of patients presenting with vertigo is quite small.32
Lateral medullary syndrome
The most common stroke of the vestibular system, first reported in the late 19th century,34 is lateral medullary syndrome, also known as Wallenberg syndrome.3 This syndrome is caused by a stroke of either the PICA or AICA. Therefore, it is a lateral brainstem stroke. Because both arteries that supply the vestibular nuclei also supply other areas, lateral medullary syndrome is manifested by mixed sensory and motor loss, including vertigo, lateropulsion, disequilibrium, ataxia, contralateral loss of pain and temperature sensation in the trunk and limbs, and the following ipsilateral signs: facial numbness, Horner syndrome (drooping of the upper eyelid, constriction of the pupil, and decreased sweating), and dysphagia. Involvement of the PICA also includes hoarseness and skew deviation of the eyes. Involvement of the AICA also includes ipsilateral tinnitus, hearing loss, facial weakness, and reduced peripheral vestibular responses on objective diagnostic tests. These patients have vertigo, difficulty standing and walking, sensory loss on the ipsilateral side of the face and on the contralateral side of the body, difficulty speaking and swallowing, abnormal eye movements, and hearing impairments. In addition to thrombosis and ischemia, dissection of the vertebral artery caused by sports injuries or by chiropractic manipulation of the neck can cause this syndrome.37
Lateral medullary syndrome is relatively common. These patients may be referred for rehabilitation, although many of them recover spontaneously. No studies have evaluated the effectiveness of rehabilitation in this population, but these patients usually respond well to therapy. Therapy should involve functional skills, balance therapy, and habituation exercises to reduce vertigo, which are the kinds of exercises used to reduce vertigo in patients with peripheral vestibular disorders.25,31
Cerebellar infarcts
Cerebellar lesions without involvement in the brainstem can be caused by occlusion of the PICA, AICA, or vertebral artery. These patients are rarely seen for vestibular rehabilitation. According to noted authorities Baloh and Harker, the acute episodes of vertigo, disequilibrium, and nausea accompanied by typical cerebellar signs, of ataxia, disdiadochokinesia, and gaze nystagmus are often followed by edema of the cerebellum.4 Cerebellar edema can be fatal because, when the cerebellum becomes compressed, the nearby brainstem structures can be damaged unless the area is surgically decompressed.
Lesions of vestibular areas in cerebral cortex
Strokes affecting just the insular cortex are rare. One paper reported that, of 4,800 new strokes in a database, four (less than 0.001%) were restricted to the insula. The three patients with anterior insula lesions all had transiently poor balance, and some had transient aphasia and dizziness.13 These people all recovered spontaneously. More frequently, vertigo and balance problems can be part of the syndrome seen in large middle cerebral artery strokes. In that case, general principles of vestibular rehabilitation should be incorporated in the rehabilitation treatment plan as needed.
Vestibular rehabilitation
Although isolated central vestibular impairments are unusual, patients with strokes sometimes present with symptoms of vestibular disorder along with their other symptoms. Any patient who complains of vertigo should be evaluated to determine if the problem is central or peripheral. A detailed discussion of vestibular rehabilitation is beyond the scope of this chapter, but many reviews of this topic have been published. The American Occupational Therapy Association has defined the necessary entry-level skills for this subspecialty.14 A brief overview of this topic follows.
Intervention such as habituation exercises and activities for vertigo,15,18,19,27 balance therapy, repositioning maneuvers for benign paroxysmal positional vertigo (BPPV),28 functional skills training (see Chapters 7 and 8), adaptive safety equipment, and home modifications (see Chapters 27 and 28) can be incorporated into the treatment plan for stroke rehabilitation as needed. The goals of vestibular rehabilitation are usually to reduce or eliminate vertigo when present, to reduce oscillopsia (illusory movement of the visual world) when present, to improve safety and to decrease falls (see Chapter 14), and, as in all rehabilitation, to increase independence. Habituation exercises and activities involve repetitive rotations of the head to elicit vertigo in an attempt to desensitize the system to the sensation. Current practice incorporates a visual target, i.e., the patient should be looking at something while moving the head. Therefore, tasks involving repetitive head movements (sorting tasks in which the containers are on different sides) are therapeutic. A recent metaanalysis indicates that habituation treatments are effective, although they have not been tested in stroke patients.27 This area of practice is dynamic, and new research studies continue to expand our understanding of treatment in this specialty,16 so the interested therapist should search the literature periodically to learn what is new.
BPPV is a very common peripheral vestibular disorder35,41 and might occur when small vessels are compromised. This disorder occurs when otoconia, small particles of calcium carbonate located in the otoliths of the vestibular labyrinth, become displaced from the utricle in one of the semicircular canals. Theoretically, disease of the small vessels that supply the vestibular labyrinth could damage the membrane that holds these particles in place and allow them to be released into the semicircular canals. Also, in the emergency department, the physician may not be able to differentiate the acute symptoms of stroke from the acute symptoms of vestibular disorder. Therefore, some patients are admitted to the stroke unit but are later found to have BPPV. For this reason, some therapists in a stroke rehabilitation unit may need to treat patients with BPPV. The “repositioning maneuvers” used to treat BPPV are quite effective and are easily learned,17,21,28,38 so the astute stroke therapist should learn these techniques. Central positional vertigo has been described in the literature.6,29 If a stroke patient appears to have BPPV but does not respond to repositioning treatments, the therapist should consult the neurologist to determine if the patient’s symptoms might be central in origin.
Graduated balance training exercises and activities are used when patients complain of disequilibrium. These programs usually increase in difficulty from standing still to standing on an unstable surface to moving through space while moving the head about and manipulating objects. Movement in anteroposterior, mediolateral, and off-axis planes should be incorporated.
Patients with vertigo and balance problems are at risk for falling, so therapy should involve some discussion of home modifications, such as bathtub seats, bathroom grab bars, night lights, and tacking down throw rugs (see Chapter 27). These issues can be incorporated into discharge planning for most stroke patients seen as inpatients or during routine discussions during outpatient care.
Review questions
1. What is the most common stroke to disrupt the function of the vestibular system? List the symptoms associated with this stroke.
2. What are the signs and symptoms of a cerebellar stroke?
3. What are the two major goals of a vestibular rehabilitation program after a stroke?
4. What are the specific interventions used to improve function during a vestibular rehabilitation program?
1. Balaban CD. Vestibular autonomic regulation (including motion sickness and the mechanism of vomiting). Curr Opin Neurol. 1999;12(1):29-33.
2. Balaban CD, Thayer JF. Neurological bases for balance-anxiety links. J Anxiety Disord. 2001;15(1-2):53-79.
3. Baloh RW, Halmagyi GM, editors. Disorders of the vestibular system. New York: Oxford University Press, 1996.
4. Baloh RW, Harker LA. Central vestibular disorders. In Cummings CW, Fredrickson JM, Krause CJ, et al, editors: Otolaryngology: head & neck surgery, ed 3, St. Louis: Mosby, 1998.
5. Baloh RW, Honrubia V. Clinical neurophysiology of the vestibular system, ed 2. Philadelphia: FA Davis; 1990.
6. Brandt T. Positional and positioning vertigo and nystagmus. J Neurol Sci. 1990;95(1):3-28.
7. Brandt T. Vertigo. its multisensory syndromes, ed 2. London: Springer. 1999.
8. Brandt T, Dieterich M, Danek A. Vestibular cortex lesions affect the perception of verticality. Ann Neurol. 1994;35(4):403-412.
9. Brandt T, Dietrich M, Strupp M. Vertigo and dizziness. common complaints. London: Springer. 2005.
10. Brödel M. Three unpublished drawings of the anatomy of the human ear. Philadelphia: Saunders; 1946.
11. Bronstein AM, Lempert T. Dizziness. a practical approach to diagnosis and management. New York: Cambridge. 2007.
12. Carter JR, Ray CA. Sympathetic responses to vestibular activation in humans. Am J Physiol Regul Integr Comp Physiol. 2008;294(3):R681-R688.
13. Cereda C, Ghika J, Maeder P, Bogousslavsky J. Strokes restricted to the insular cortex. Neurology. 1996;59(12):1950-1955.
14. Cohen HS, Burkhardt A, Cronin GW, et al. Specialized knowledge and skills in adult vestibular rehabilitation for occupational therapy practice. Am J Occ Ther. 2006;60(6):669-678.
15. Cohen H, Kane-Wineland M, Miller LV, Hatfield CL. Occupation and visual/ vestibular interaction in vestibular rehabilitation. Otolaryngol Head Neck Surg. 1995;112(4):526-532.
16. Cohen HS. Disability and rehabilitation in the dizzy patient. Curr Opin Neurol. 2006;19(1):49-54.
17. Cohen HS, Kimball KT. Effectiveness of treatments for benign paroxysmal positional vertigo of the posterior canal. Otol Neurotol. 2005;26(5):1034-1040.
18. Cohen HS, Kimball KT. Increased independence and decreased vertigo after vestibular rehabilitation. Otolaryngol Head Neck Surg. 2003;128(1):56-66.
19. Cohen HS, Kimball KT. Changes in gait ataxia and balance after vestibular rehabilitation. Otolaryngol Head Neck Surg. 2004;130:418-425.
20. Dickman JD. The vestibular system. In: Haines DE, editor. Fundamental neuroscience. New York: Churchill Livingstone, 1997.
21. Epley JM. The canalith repositioning procedure. for treatment of benign paroxysmal positional vertigo. Otolaryngol Head Neck Surg. 1992:107;3:399-404.
22. Fasold O, von Brevern M, Kuhberg M, et al. Human vestibular cortex as identified with caloric stimulation in functional magnetic resonance imaging. Neuroimage. 2002;17(3):393-1384.
23. Fritschi JA, Reulen HJ, Spetzler RF, Zabramski JM. Cavernous malformations of the brain stem. A review of 139 cases. Acta Neurochirurgica. 1994;130(1-4):35-46.
24. Furman JM, Cass SP. Vestibular disorders. a case-study approach. New York: Oxford. 2003.
25. Furman JM, Whitney SL. Central causes of dizziness. Phys Ther. 2000;80(2):179-187.
26. Hawrylyshyn PA, Rubin AM, Tasker RR, et al. Vestibulo-thalamic projections in man—a sixth primary sensory pathway. J Neurophys. 1978;41(2):394-401.
27. Hillier SL, Hollohan V. Vestibular rehabilitation for unilateral peripheral vestibular dysfunction. Cochrane Database Syst Rev. 4, 2007. CD005397
28. Hilton M, Pinder D. The Epley (canalith repositioning) manoeuvre for benign paroxysmal positional vertigo. Cochrane Database Syst Rev. (2):2004. CD003162
29. Johkura K. Central paroxysmal positional vertigo. isolated dizziness caused by small cerebellar hemorrhage. Stroke. 2007:38;6:e26-e27.
30. Karnath HO, Ferber S, Dichgans J. The neural representation of postural control in humans. Proc Natl Acad Sci USA. 2000;97(25):13931-13936.
31. Kelly PJ, Stein J, Shafqat S, et al. Functional recovery after rehabilitation for cerebellar stroke. Stroke. 2001;32(2):530-534.
32. Kerber KA, Brown DL, Lisabeth LD, et al. Stroke among patients with dizziness, vertigo, and imbalance in the emergency department. a population-based study. Stroke. 2006:37;1:2484-2487.
34. Lysakowski A, McCrea RA, Tomlinson RD. Anatomy of vestibular end organs and neural pathways. In: Cummings CW, Fredrickson JM, Krause CJ, et al, editors. Otolaryngology: head & neck surgery. St. Louis: Mosby, 1998.
35. Neuhauser HK, von Brevern M, Radtke A, et al. Epidemiology of vestibular vertigo. a neurotologic survey of the general population. Neurology. 2005:65;6:898-904.
35. Rathore SS, Hinn AR, Cooper LS, et al. Characterization of incident stroke signs and symptoms. findings from the Atherosclerosis Risk in Communities Study. Stroke. 2002:33;11:2718-2721.
36. Saeed AB, Shuaib A, Al-Sulaiti G, Emery D. Vertebral dissection. warning symptoms, clinical features and prognosis in 26 patients. Can J Neurol Sci. 2000:27;4:292-296.
37. Semont A, Freyss G, Vitte E. Curing the BPPV with a liberatory maneuver. Adv Otorhinolaryngol. 1988;42:290-293.
38. Solomon D. Distinguishing and treating causes of central vertigo. Otolaryngol Clin North Am. 2000;33(3):579-601.
39. Tasker RR, Organ LW. Stimulation mapping of the upper human auditory pathway. J Neurosurg. 1973;38(3):320-325.
40. von Brevern M, Radtke A, Lezius F, et al. Epidemiology of benign paroxysmal positional vertigo. A population based study. J Neurol Neurosurg Psychiatry. 2007;78(7):710-715.
41. Yates BJ, Bronstein AM. The effects of vestibular system lesions on autonomic regulation. observations, mechanisms, and clinical implications. J Vestib Res. 2005:15;3:119-129.
42. Yelnik AP, Lebreton FO, Bonan IV, et al. Perception of verticality after recent cerebral hemispheric stroke. Stroke. 2002;33(9):2247-2253.
43. Zwergal A, Buttner-Ennever J, Brandt T, Strupp M. An ipsilateral vestibulothalamic tract adjacent to the medial lemniscus in humans. Brain. 2008;131(Pt 11):2928-2935.