Chapter 13 Plant cell and tissue culture; biochemical conversions; clonal propagation
One of the rapidly expanding areas of pharmacognosy has involved the application of the artificial culture of plant cells, tissues and organs to the study of medicinal plants. Principal topics include the development of commercial production of expensive biomedicaments, the discovery of new metabolites, the selection of superior strains of medicinal plants, the elucidation of biosynthetic pathways of secondary metabolites with isolation of corresponding enzymes, and the improvement of medicinal plant species by genetic engineering.
INDUSTRIAL SIGNIFICANCE
A number of factors militate against dependence by the pharmaceutical industry on the use of botanical sources of drugs, and these have been, to some extent, responsible for the reluctance of industry to invest in the exploitation of the plant kingdom. These factors include the following.
Following from the above, it is not surprising that industry world-wide takes a close interest in the commercial possibilities of cultivating particular species of plant cells, under conditions analogous to the production of antibiotics, that will yield biomedicinals. By this means, production could at all times be geared to demand, and a product of standard quality assured. Furthermore, a highly sophisticated and specific method of production can be patented.
Shikonin, a dye and antibacterial, is commercially produced by the cultivation of Lithospermum plant cells and the production of the ginsenosides and antitumour alkaloids of Catharanthus roseus are currently being developed; Japanese patents exist concerning the manufacture of many secondary metabolites including the purple pigment from Melissa officinalis, the production of catharanthine and ajmalicine by cell cultures derived from C. roseus anthers, the manufacture of an analogue of taxol (q.v.) by callus cultures of Taxus spp., and tropane alkaloids from Duboisia tissue cultures. A vast amount of work has been reported during the last decade and the majority of common medicinal plants, and many less common ones, have been subjected to cell culture investigation. Nevertheless, in the majority of cases, yields of metabolites have been commercially disappointing. Staba, a pioneer in the investigation of medicinal plant cell culture, stated (J. Nat. Prod., 1985, 48, 203) ‘there are arguably as many gravestones as milestones along the way in developing plant tissue culture systems for the production of secondary metabolites’. Ultimately, of course, as Fowler pointed out over 25 years ago (Chem. Ind., 1981, 229), ‘no matter how elegant the science, the fundamental criterion has to be price comparability coupled with profitability’, a fact clearly obviated by subsequent events.
CULTIVATION OF PLANT CELLS
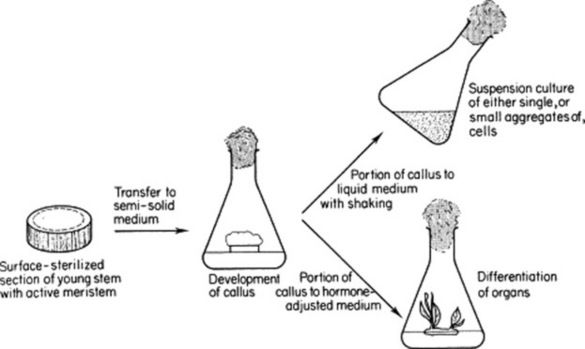
Although the feasibility of artificially cultivating plant cells had long been recognized, and White had propagated isolated tomato roots for periods of over 30 years, it was only some few decades ago that modern developments in the cultivation of cells of higher plants as a callus, or as a suspension liquid culture, really began. In this connection the publication of P. R. White’s Cultivation of Plant and Animal Cells in 1954 and H. E. Street’s developmental work at Leicester University deserve mention.
Cultures of single cells growing under controlled conditions in a liquid medium, or callus cultures consisting of undifferentiated masses of cells developing on a semi-solid medium, can be initiated from parenchymatous tissues of shoots, roots and other plant structures (see Fig. 13.1). The maintenance of such cultures depends on an adequate supply of nutrients, including growth factors, and a controlled sterile environment. The cells, although undifferentiated, contain all the genetic information present in the normal plant. By suitable manipulation of the hormone content of the medium, it is possible to initiate the development of roots, shoots, and complete plants from the callus cell culture and to encourage the division of cells in a suspension culture.
Several forms of suspension culture are commonly utilized, as follows.
The problems associated with plant-cell culture are not completely identical with those encountered in the fermentation of microorganisms and fungi. With the former there is much slower cell proliferation and it takes about 3–6 weeks to progress from the shake-flask level (300 ml) to production capacity (20 000 litres). Also in plant cell cultures, large aggregates of cells may form, which then exist under different environmental conditions from the suspended cells. Dispersal of aggregates by the use of the usual fermenter paddles was originally considered too vigorous for fragile plant cells, resulting in their rupture. To overcome this, various designs of low-shear fermenters including the air-lift or drum types were designed. However, such refinements are not always necessary and the shikonin production mentioned above is, in fact, carried out in conventional stirred-tank vessels. As R. Verpoorte et al. have pointed out (J. Nat. Prod., 1993, 56, 186), for the industrial application of plant cell cultures, the recognition that shear- tolerant plant cell cultures exist is important because the fermentation industry at present uses stirred tanks almost exclusively. Investment in new ingenious bioreactors, as reported for experimental cell cultures in recent years, would place a major constraint on the commercialization of plant cell biotechnology.
To maximize on the cell mass produced, the cell suspension culture eventually becomes very dense and this presents problems of even aeration.
Most pilot studies have utilized fermenters of 5–15 litres capacity and the reported scaling up to 20 000 and more recently 75 000 litres in West Germany and Japan presents chemical engineering problems of considerable complexity.
PRODUCTION OF SECONDARY METABOLITES
The genetic information required for the manufacture of secondary metabolites is also present in the undifferentiated cells of the species concerned, and when activated should lead to the production of these materials. Much interest has been aroused by this aspect of cell culture with the aim of growing particular plant cells on a commercial scale for the production of valuable metabolites.
A pioneer in the cell culture of medicinal plants was E. J. Staba of Minnesota University, and his group was the first to demonstrate that many medicinal plants did produce in cell culture their characteristic secondary metabolites, albeit often in low yield. Notable advances were made by Zenk and colleagues who in 1975 demonstrated a 10% (dry weight) production of anthraquinone derivatives in a Morinda citrifolia culture—at that date the highest yield of secondary metabolite achieved by cell culture. By 1991 (M. H. Zenk, Phytochemistry, 1991, 30, 3861) almost 1000 species of callus were deposited in the collection at Braunschweig. Commercially orientated research has concentrated on those species that produce high-value speciality phytochemicals. Obvious examples are Catharanthus roseus (dimeric antitumour alkaloids), ginseng (ginsenosides) and Taxus species (taxol).
Apart from the general problem of low yield of product other factors which need to be addressed with cell cultures as a source of phytopharmaceuticals are: instability of cell lines, compartmentalization and isolation of the products, and the nature of the metabolites produced. Some points concerning these problems are given below.
Low production of desired metabolites
Knowledge of the enzymology of secondary metabolite formation, although rapidly expanding, is still incomplete. Secondary metabolic processes compete with primary metabolism for precursors and potential bottlenecks for the former may involve those enzymes linking the primary and secondary pathways, for example, tryptophan decarboxylase converting tryptophan to tryptamine in the formation of indole alkaloids and cyclase enzymes involved in the synthesis of cyclohexanoid monoterpenes from geranylpyrophosphate. With cell cultures, as distinct from whole plants, particular genes may be repressed and need to be activated by suitable elicitors, a technique which is currently an important area of research and is discussed below.
The compositions of the media in which culture cells are grown have been extensively investigated with a view to increasing both the biomass and secondary metabolites. Often, as reported with Dioscorea deltoidea for instance, rapidly dividing cells produce little or no metabolites of interest and a change from a growth medium (high biomass) to a production medium is required to effect the necessary biosynthesis. In this connection Zenk’s ‘alkaloid production medium’ for ajmalicine in C. roseus may be noted, together with the effects of long-term starvation of phosphate on levels of purine nucleotides and related compounds (F. Shimano and H. Ashihara, Phytochemistry, 2006, 67, 132).
Variations in the relative hormonal contents of the growth medium can also affect metabolism. It has been reported that reduced concentrations of 2,4-D increased alkaloid formation in C. roseus cultures and that abscisic acid and antigibberellin compounds have similar effects. With Thalictrum minus, ethylene has been shown to activate the production of berberine in cell cultures from the key intermediate (S)-reticuline and the ethylene-producing reagent 2-chloroethylphosphoric acid stimulates anthraquinone production in callus cultures of Rheum palmatum. Conversely, cardenolide accumulation in Digitalis lanata tissue cultures is decreased by ethylene. Cytokinins have been found to enhance secondary metabolite accumulation in a number of tissue culture studies—indole alkaloids (C. roseus), condensed tannin (Onobrychis sp.), coumarins (Nicotiana sp.), rhodozanthin (Ricinus sp.), berberine (Thalictrum minus). Rhodes et al. found a five-fold increase in alkaloid content of a culture of Cinchona ledgeriana occurs when cells are transferred from a 2,4-D, benzyladenine medium to one containing IAA and zeatin riboside. For information on plant hormones, see Chapter 12.
Although alkaloids from a wide range of medicinal plants have been produced satisfactorily by cell culture in the laboratory, a singular lack of success has been experienced in obtaining quinine and quinidine from Cinchona cultures and morphine and codeine from those of Papaver somniferum although, in both cases, other alkaloids are formed. To some extent the problems with the former are being overcome by the use of transformed roots (see below) but the growth rate is very slow. With morphine biosynthesis it appears that lack of developed laticiferous tissue in the unorganized cell culture may be responsible because cytodifferentiation leading to latificer-type cells leads to morphinan alkaloid production. One problem with Catharanthus cell cultures has been their inability to dimerize the requisite indole monomers to form the medicinally important anticancer alkaloids vinblastine and vincristine. In a similar way the accumulation of monoterpenes in cell cultures of some volatile oil-producing plants is severely limited, probably because of the absence of such storage structures as glands, ducts and trichomes. Thus, in Rosa damascena callus and suspension cultures, negligible amounts of monoterpenes are accumulated, although enzymes with high activity for the conversion of mevalonate and IPP into geraniol and nerol (see Chapter 18) are extractable from the apparently inactive callus. In this case, non-compartmentalization of the metabolites probably leads to their further metabolism. This is supported by the finding that, when added to cultures of Lavandula angustifolia, the monoterpenoid aldehydes geranial, neral and citronellal are reduced to their corresponding alcohols, geraniol, nerol and cirtronellol which, once formed, disappear from the cultures over about 15 h.
In studies on the phenolic antioxidant compounds produced by in vitro cultures of rosemary, A. Kuhlmann and C. Rohl (Pharm. Biol., 2006, 44, 401) find the content of carnosic acid, carnosol and rosmaric acid to be dependent on the differentiation grade of the cell culture type. Higher concentrations of rosmaric acid were measured in suspension cultures than in shoot and callus cultures, whereas the former on average produced three-fold less carnosic acid than the two latter cultures. Carnesol could not be detected in suspension cultures.
With Ginkgo biloba although a satisfactory biomass of undifferentiated cells could be produced on a manufacturing scale, the poor level of ginkgolides produced renders it of scant importance.
It has been observed that the origin (stem, root, etc.) of the callus can play an important part in determining the biochemistry of the subsequent culture.
Improved metabolite production may sometimes be achieved by the addition of precursors to the culture medium. Thus, addition of coniferin (a phenylpropane) to cell suspension cultures of Podophyllum hexandrum improved podophyllotoxin production 12.8-fold and an increase in quinoline alkaloids was obtained with Cinchona ledgeriana cultures fed with L-tryptophan. With transformed root cultures of Catharanthus roseus, the addition of the precursor loganin to the culture medium has been shown to increase the production of both ajmalicine and serpentine at the early stationary phase of growth, although it produced no increases during the early and late exponential growth phases. Catharanthine production was unaffected but was increased, together with the other alkaloids, by multiple feedings of loganin (see E. N. Gaviraj and C. Veeresham, Pharm. Biol., 2006, 44, 371 and references cited therein).
Light intensity
and selective wavelengths of light have been shown to have a stimulating effect on the production of some secondary metabolites in various tissue cultures. Thus, in one report (1990) blue light enhanced, whereas red light decreased, diosgenin production in Dioscorea deltoidea callus cultures. A recent example of the stimulant effect of UV-B radiation on secondary metabolism in callus cultures is the research of F. Antognoni et al. (Fitoterapia, 2007, 78, 345) on Passiflora quadrangularis. Daily doses of UV-B radiation (12.6, 25.3, 37.9 KJ m−2) produced increases in the flavonoid production of orientin, isoorientin, vitexin and isovitexin. Isoorientin accumulation in the callus after 7 days reached levels comparable to those found in the fresh leaves of greenhouse-raised plants. However, such beneficial treatments are difficult to accommodate with conventional stirred-tank fermentors.
The selection of high-yielding cell lines has been a major factor in countering low productivity. Such selection, perhaps involving a few plants from several thousand, has been greatly facilitated by the use of modern immunoassays (q.v.). In the case of Catharanthus roseus cultures, for example, recent research has concentrated on the production of the dimeric alkaloids vinblastine and vincristine (q.v.), the important anticancer drugs. The alkaloids are produced at the end of a complex biogenetic pathway in which the monomers are first produced. The latter, as corynanthe-, strychnos- and aspidosperma-type alkaloids can all be produced (0.1–1.5%) in culture using Zenk’s alkaloid production medium. Different cell cultures derived from any one species of plant may vary enormously in their synthetic capacities, so that, in the above case, distinct high ajmalicine-producing and high serpentine-producing strains are possible.
Examples of other plants for which somaclonal variation has been exploited include Nicotiana rustica (nicotine) (of no commercial interest), Coptis japonica (berberine), Anchusa officinalis (rosmarinic acid), Lithospermum erythrorhizon (shikonin) and Hyoscyamus muticus (hyoscine). For Thalictrum minus (berberine) a strain giving a 350-fold increase in alkaloid production has been reported.
Instability of cell lines
It is well known that changes in the genetic characteristics of cells occur within a culture so that callus selected for specific biochemical properties may need reselection after a period of time. In a few cases, for example anthraquinone formation, selection may be achieved on a colour basis but, more usually, assays such as radio-immunoassay are necessary. Gross changes in chromosome number may occur in cultured cells; thus, Tabata and colleagues noted in 1974 that with a particular suspension culture of Datura innoxia cells there was a 32% level of diploid cells, with the remainder mostly at the tetraploid level, ranging in constitution from 4n − 5 to 4n + 3 (see Chapter 14 for extra chromosomal types). Another strain contained no diploid cells but cells with 46 or 44 chromosomes occurring in the proportions of 79% and 21%, respectively. Nevertheless, for alkaloid production, Kibler and Neumann in 1979 found that haploid (1n) and diploid (2n) cell suspension cultures of D. innoxia showed no difference in tropane alkaloid production (c.f. leaves of 1n and 2n plants; Table 14.1), but for protoplast-derived cell-culture clones of Hyoscyamus muticus, Oksman-Caldentey et al. have found that cultures from 1n plants are richer in hyoscine than those from 2n plants.
Isolation of product
For continuous cultivation and production of active metabolites it is preferable, for isolation purposes, that the metabolites be excreted into the medium rather than be retained within the cells. The biomass can then be separated from the nutrient liquid from which the active constituents are extracted. Two-phase culture systems have been described. With these an immiscible nontoxic liquid phase, e.g. a silicone product, is added to the fermentation tank to extract the metabolites and in this way the development of the culture is not disturbed. The removal of entrapped metabolites from immobilized cells (q.v.) without killing the cells is another innovation.
Nature of metabolites produced
Sometimes compounds not detected in the original plant appear in the cultures; thus, a new coumarin, rutacultin, has been isolated from suspension cell cultures of Ruta graveolens, two new chalcones have been characterized from static (callus) cultures of Glycyrrhiza echinata, sesquiterpene lactones from Andrographis paniculata cultures, new minor alkaloids and anthraquinones from Cinchona ledgeriana and C. pubescens, and tropane alkaloids, not previously obtained from the species, from belladonna root-cell suspension cultures. Recently the novel compound (2-glyceryl)-O-coniferaldehyde has been obtained from cultures of Artemisia annua and Tanacetum parthenium (L. K. Sy and G. D. Brown, Phytochemistry, 1999, 50, 781) and the quinone- methide triterpenes, tingenone and 22-hydroxytingenone, from callus cultures of Catha edulis (E. Abdel Sattar et al., Saudi Pharm. J., 1998, 6, 242). Other plants yield cultures which produce a different spectrum of secondary metabolites from those found in the intact plant. These aspects of cell culture, although generally unhelpful for the promotion of this technique for industrial purposes, have important implications for other areas of phytochemistry. However, in the case of cell cultures of Papaver somniferum and P. bracteatum which do not produce morphinan alkaloids, sanguinarine, a benzophenanthridine alkaloid of commercial importance, is obtained in yields sufficiently high to allow industrial exploitation.
INDUCED SECONDARY METABOLISM IN CELL CULTURES
Although the undifferentiated cells of a plant suspension culture are generally totipotent, i.e. they possess the complete genetic make-up of the whole plant, many genes, including those involved in secondary metabolism, are repressed with the consequence that the yields of desired compounds in such cultures are disappointingly low. However, it is becoming increasingly apparent that a large number of secondary metabolites belong to a class of substances termed phytoalexins. These are stress-related compounds produced in the normal plant as a result of damaging stimuli from physical, chemical or microbiological factors. When cell cultures are subjected to such elicitors, some genes are derepressed, resulting, among other things, in the formation of the secondary metabolites which are found in the entire plant. The technique is being increasingly employed in cell-culture studies and examples giving a range of both abiotic acid and biotic inducers are given in Table 13.1.
Table 13.1 Induced production of metabolites in cell cultures by various elicitors.
| Elicitor | Plant-cell suspension culture | Effect |
|---|---|---|
| Arachidonic acid | Taxus spp. | Production of taxol |
| Chitosan | Polygonum tinctorium | Production of indirubin |
| Colchicine | Valeriana wallichii | Sixty-fold increase in valepotriates with six new compounds (not due to higher ploidy level) |
| Copper sulphate | Lithospermum erythrorhizon | Greatly increased shikonin production |
| Various Solanaceae | Induced formation of sesquiterpene phytoalexins of lubimin type | |
| Calcium | Alkanna tinctora | Stimulation of sanguinarine and chelerythrine biosynthesis |
| Acetylsalicylic acid | Catharanthus roseus | Increased production of tumour cell suspensions (505%), total phenolics (1587%), furanocoumarins (612%), anthocyanins (1476%) |
| Methyl jasmonate | Sanguinaria canadensis | Dihydrobenzophenanthridine oxidase activated in last step of sanguinarine biogenesis |
| Cinchona robusta | Production of novel anthraquinones (robustaquinones) with a rare oxygenation pattern in ring A | |
| Nicotiana tabacum | Production of anatalline | |
| Thiosemicarbazide | Panax ginseng | Promotes biosynthesis of saponins and inhibits phytosterol production |
| Sterilized fungal mycelia (Pythium, Phytophthora, Verticillium), etc. or extracts | Pimpinella anisum Petroselinium crispum Ammi majus | Stimulation of coumarin synthesis |
| Catharanthus roseus | Production of catharanthine and other major indole alkaloids stimulated | |
| Cephalotaxus harringtonia | Dramatic increase in alkaloid content | |
| Cinchona ledgeriana | Increase in anthraquinone production | |
| Gossypium arboreum | One hundredfold increase in gossypol after 120 h incubation | |
| Yeast, yeast extracts and carbohydrate preparations | Eschscholtzia californica | Large and rapid increase of benzophenanthridine alkaloid production |
| Thalictrum rugosum | Up to fourfold enhancement of berberine | |
| Ruta graveolens | Increased production of acridone expoxides but not rutacridone | |
| Orthosiphon aristatus | Stimulation of rosmarinic acid production |
BIOCHEMICAL CONVERSIONS BY PLANT CELL CULTURES
In much the same way that modification of a particular substrate can be effected by microbial fermentation so, too, can plant suspension cultures be employed for the same purpose.
Of possible commercial significance is the ability of some cell cultures of Digitalis lanata to effect glucosylations, hydroxylations, and acetylations. Reinhard and colleagues demonstrated that their cell culture, strain 291, cultivated in air-lift bioreactors, was particularly efficient in the conversion of β-methyldigitoxin into β-methyldigoxin (a 12β-hydroxylation). Commercial exploitation of this process would enable utilization of the large stocks of digitoxin which accumulate as a byproduct in the manufacture of digoxin from D. lanata. More recently, Stricker (Planta Med., 1986, 418) reported on a highly efficient 12β-hydroxylation of digitoxin itself using D. lanata cell suspensions with a two-stage process involving first, the proliferation of cells in a growth medium and then a transfer to a suitable production medium. With cell cultures of both D. lanata and Thevetia neriifolia a number of new cardenolides have been biosynthesized from added precursors. Cell suspension cultures of Strophanthus gratus will effect various biochemical conversions of digitoxigenin, as will cultured ginseng cells.
Monoterpene bioconversions have been demonstrated with Mentha cell lines capable of transforming pulegone to isomethone, and (−)-menthone to (+)-neomenthol. Cultured cells of Eucalyptus perriniana have been shown to biotransform thymol, carvacrol and eugenol into glycosides (glucosides and gentiobiosides), which accumulated within the cells (K. Shimoda et al., Phytochemistry, 2006, 67, 2256).
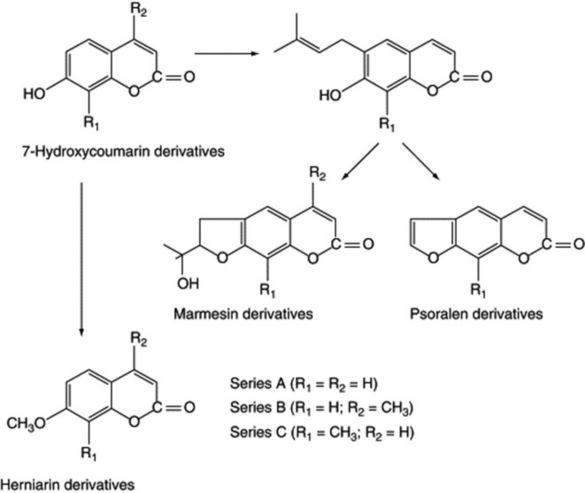
The rue plant (Ruta graveolens) and its normal tissue cultures contain a number of constituents, including furanocoumarins derived from 7-hydroxycoumarin (Fig. 13.2, series A). In 1974, Steck and Constabel showed that two chemical mimics of the 7-hydroxycoumarin precursor, the 4-methyl and 8-methyl derivatives, when fed to the Ruta cell culture, gave rise to a number of the corresponding unnatural analogues (Fig. 13.2, series B and C).
Possibilities for the production of anticancer drugs are illustrated by the biotransformation of synthetic dibenzylbutanolides to lignans suitable for conversion to etopside (Chapter 27) by a semi-continuous process involving cultures of Podophyllum peltatum (J. P. Kutney et al. Heterocycles, 1993, 36, 13). Interesting and potentially useful hydroxylation and oxidation reactions have also been demonstrated for the biotransformation of podophyllum lignans in cell suspension cultures of Forsythia intermedia (A. J. Broomhead and P. M. Dewick, Phytochemistry, 1991, 30, 1511).
The principal alkaloid produced by the cultivation of Rauwolfia serpentina cells is the glucoalkaloid raucaffricine. However, feeding with high levels of ajmaline leads to the production of a new group of alkaloids, the raumaclines.
Some biotransformations are stereo-specific and have potential for the isolation of optically active compounds from the racemate; thus, Nicotiana tabacum cell cultures can selectively hydrolyse the R-configurational forms of monoterpenes such as bornyl acetate and isobornyl acetate.
Many other biochemical transformations by cell cultures have been demonstrated, and include epoxidations, ester formation and saponification, glycosylation, hydroxylation, isomerization, methylation, and demethylation and oxidation.
For this technique to be commercially viable, the product must be sufficiently important, the substrate must be available in reliable amounts, and the reaction should not be one that is more easily performed by microorganisms or by chemical means.
IMMOBILIZED PLANT CELLS
Immobilized plant cells can be used in the same way as immobilized enzymes to effect chemically difficult reactions. Reinhard and coworkers have reported on the immobilization of Digitalis lanata cells by suspending them in a sodium alginate solution, precipitating the alginate plus entrapped cells with calcium chloride solution, pelleting, and allowing the product to harden. The granules catalysed the conversion of digitoxin to purpurea glycoside A (formula, see Table 23.7) and hydroxylated β-methyldigitoxin to give β-methyldigoxin. Although the hydroxylating activity of the entrapped cells was about half that of suspended cells, the pellets had the advantage that the biocatalyst was re-usable for periods extending to over 60 days. Other workers report the biotransformation of codeinone to codeine by immobilized cells of Papaver somniferum and the release of papaverine, codeine and morphine to the medium (3240–4050 mg 1−1); decarboxylation of L-tyrosine and L-DOPA has also been recorded. Japanese workers have studied seaweed immobilized cell cultures for the production of flavour constituents. Glyoxal cross-linked polyacrylamide-hydrazide has been used as the immobilizing agent for Mentha cells which effect the monoterpene reductions mentioned above and the technique has been further developed by arresting unwanted cell division of the entrapped cells by gamma irradiation. Other immobilization supports which have been used include polyurethane foam, acrylamide and xanthan-acrylamide.
A technique which has been found useful for the study of alkaloid formation in Coffea arabica is to use a membrane of polypropylene sheeting of specified pore size, porosity and thickness on which to immobilize the cells in a 3-mm thick layer; the nutrient medium circulates below the membrane. Catharanthus roseus cells have also been considerably studied and progress has been made in developing methods for the release of alkaloids sequestrated in the cell vacuoles without killing the culture.
It has been suggested that the immobilization of mycelia-forming microorganisms (e.g. Claviceps paspali) by alginic acid might be effective in the biotechnological production of complex metabolites such as the ergot alkaloids.
ORGAN CULTURE
In the same way that it is possible to culture undifferentiated plant cells, so aseptic suspension cultures of leaves and roots can be maintained. The organs can be obtained in the culture either by differentiation from callus tissue cultures by suitable hormonal manipulation, or by the use of sterilized roots or growing points from whole plants or seedlings. Often, these cultured organs will synthesize secondary metabolites which may be either non-existent or in poor yield in the normal cell culture. Thus, cardenolide production in Digitalis lanata and D. purpurea cultures increases as tissue differentiation proceeds. Enhanced production of alkaloids occurs when roots develop from the callus cultures of the tropane alkaloid-producing Solanaceae. In contrast to the latter, in which leaf differentiation alone produces little alkaloid, leaf organ cultures of Catharanthus roseus and Rauwolfia serpentina synthesize a variety of alkaloids. Dimeric alkaloids have been detected in organ cultures of C. roseus, suggesting the possibility of an efficient production system for these valuable alkaloids. The dimers occurred only in those cultures which also contained vindoline and catharanthine. Whereas cell suspension cultures of Papaver bracteatum were found to synthesize orientalidine and sanguinarine, the root and shoot cultures produced thebaine. For ginsenoside production, ginseng root cultures have been grown in 20 000-litre bioreactors.
As with cell culture, elicitation can give increased yields of secondary metabolites. Thus both normal and hairy (q.v. below) root cultures of Hyoscyamus muticus when treated with jasmonic acid and its methyl ester at concentrations of 0.001 to 10 μM produced large increases in the levels of methyl putrescine and conjugated polyamines. However the increase of tropane alkaloid production was not remarkable (S.-Bionde et al., Plant Cell Rep., 2000, 19, 691).
Transformed root culture—‘hairy root’ culture
Certain soil bacteria of the genus Agrobacterium cause a transformation of plant cells by introducing into their genome t-DNA from a bacterial plasmid. Such transformed roots, produced by inoculating the host plant, when grown in a hormone-free medium give rise to copious roots referred to as ‘transformed roots’ or ‘hairy roots’ (see Chapter 14). On removal of the Agrobacterium the roots continue to develop profusely and for some plants which normally produce secondary metabolites the hairy roots accumulate these metabolites in quantities comparable to those found in the normal intact plant.
Agrobacterium rhizogenes and A. tumefaciens are the bacterial species most commonly used to effect transformation.
Compared with the few examples given in the 13th edition of this book there is now a considerable bibliography on transformed root cultures of medicinal plants. Some examples are given in Table 13.2.
Table 13.2 Metabolites of some transformed (hairy) roots.
| Alkaloid-containing plants | |
| Atropa belladonna | Increase in growth rate compared with untreated roots |
| Catharanthus roseus | Optimization of selected lines may give source of catharanthine and vindoline |
| Cinchona ledgeriana | Quinoline alkaloid production comparable with that of intact plants |
| Datura stramonium | Alkaloid content comparable with normal roots of intact plants; increased by use of various elicitors, e.g. methyl jasmonate |
| Hyoscyamus albus | New piperidone alkaloid obtained together with tropane alkaloids |
| H. muticus | Culture treated with chitosan accumulated hyoscyamine 2.5 to 3-fold compared with untreated hairy roots |
| Narcissus confusus | Improved production of galanthamine in shake cultures by use of methyl jasmonate as elicitor |
| Solanum laciniatum | Solasodine yield increased about four-fold |
| Flavonoids | |
| Glycyrrhiza glabra | Known and new flavonoids produced; also a new prenylated biaurone |
| Iridoids | |
| Valeriana officinalis | Yield of valepotriates four times that found in normal 9-month-old roots |
| Lignans | |
| Linum flavum | 5-Methoxypodophyllotoxin production 2–5 times that of untransformed roots; 5–12 times higher than cell suspension culture; comparable with natural roots |
| Polyacetylenes | |
| Lobelia inflata | Polyacetylenes produced, some having a gentiobiose moiety. These compounds have anticancer activity |
| Quinones | |
| Sesamum indicum | Yield of antimicrobial naphthoquinone increased by more than 50 times that found in intact plant |
| Steroids | |
| Panax ginseng | Japanese patent for production of ginsenosides; diol and triol type ginsenosides produced. Production more effective than with ordinary root cultures |
| Trigonella foenum-graecum | By optimization of the cultural conditions, up to three times the production of diosgenin compared with non-elicited roots |
As with ordinary cell cultures and root cultures it is possible to utilize transformed roots to carry out biological conversions not normally associated with the intact plant. The rapid growth rate of hairy roots offers the possibility of rapid conversions. Ginseng hairy root cultures have been shown to convert digitoxigenin (the aglycone of a number of Digitalis cardiac glycosides, q.v.) into new compounds by esterification at C-3 with stearate, palmitate and myristate, and by the formation of gentiobiosides and sophorosides. Parr et al. (Phytochemistry, 1991, 30, 2607) in studies on the biosynthesis of tropane alkaloids fed the S-analogue of tropinone (8-thiabicyclo[3.2.1]octan-3-one) to transformed root cultures of Datura stramonium and obtained the S-analogue of tropine, together with the 3-O-acetyl ester.
Cell cultures derived from A. rhizogenes-transformed Papaver somniferum tissue have been studied for alkaloid production (R. D. Williams and B. E. Ellis, Phytochemistry, 1993, 32, 719). As with normal cultures, no morphinan alkaloids were produced but large amounts of sanguinarine were.
CLONAL PROPAGATION
As noted earlier (Fig. 13.1), by adjustment of the plant growth regulators in the cell culture medium it is possible to promote differentiation of organs from callus tissues and to carry these forward to produce entire plants. As all cells of the callus are derived from a single meristem, all regenerated plants should be genetically identical. This fact has obvious commercial implications for the production, in a short period of time, of uniform crops derived from a small number of desirable plants (or even a single individual).
In 1980, Levy reported from Ecuador on the first large-scale commercial application of in vitro culture for the mass propagation of clones of pyrethrum plants. The scale of such projects is evident from the original objective, which was to produce, from 57 superior clones isolated over a 10-year research period up to 1976, 12 million plants per year to reach a plantation target of the number required for 1000 ha of land over 4 years. Further details of the process are given in the 15th edition of this book. Similar techniques are now practised for other crops of pharmaceutical interest, benefits being the ability to introduce selected high-yielding strains on a commercial scale in a short time, the creation of improved growth characteristics and a greater ease of plantation planning.
Clonal propagation is a potentially valuable method for producing high-yielding crops of species which tend to be variable when grown from seed. Fennel (Foeniculum vulgare), for instance, is genetically heterozygous and produces wide variations in oil yield and composition. In 1987, Miura et al. (Planta Med., 1987, 53, 92) recorded a 2-year trial involving the production of uniform clonal plants derived from somatic embryoids of suitable parent plants. In the normal sexually propagated plants the anethol content of fruits varied from 0 to 12.9% (mean 2.82%), whereas in the clonal plants it showed a narrower distribution of 0.7–3.0% (mean 1.92%).
Genetic homogeneity
In practice, genetic changes can occur in cells during their artificial culture, and careful regulation of the medium is necessary. However, it has been shown, at least for Datura innoxia, that on differentiation it is the normal 2n plants, as distinct from the abnormal chromosomal types, that are favoured. Clonally propagated species for which the homogeneity of the secondary metabolites has been shown include Aconitum carmichaelii, Angelica acutiloba, Bupleurum falcatum, Gentiana scabra, Rehmannia glutinosa and Stevia rehaubiana.
Verpoorte R, Heijden R van der, Schripsema J. Plant cell biotechnology for the production of alkaloids: present status and prospects (review with 130 refs). Journal of Natural Products. 1993;56:186.
Zafar R, Aeri V, Datta A. Application of plant tissue and cell culture for production of secondary metabolites (review with 80 refs). Fitoterapia. 1992;63:33.