Chapter 40 Pesticides of natural origin
Pesticides may be classified according to the type of organism against which they are effective, namely, fungicides, herbicides, insecticides, molluscicides, nematocides, rodenticides. The origin of the use of natural products in these respects is lost in antiquity (see Further Reading) and a large number of such materials, of local use, still remain to be chemically investigated and evaluated. Although the majority of pesticides used in modern agriculture are synthetic, plant products still contribute to the insecticides and rodenticides. Phytochemicals can also serve as lead compounds from which others, exhibiting, for example, a greater toxicity towards the pest, a wide spectrum of activity such as the inclusion of mites, a lowered mammalian toxicity and a decrease in photodecomposition, can be developed.
ACARICIDES
Mites and ticks are small arachnids of the order Acarina (Acari). Specific mites infest crude drugs and food (Chapter 13) and the house-dust mite, Dermatophagoides pteronyssinus, is well known for its possible association with asthma. Ticks are the largest members of the order and economically the most important. They are all blood-sucking parasites responsible for microbial infections, e.g the spirochaete infection causing Lyme disease, and protozoal diseases in animals.
The control of mites by plant products has centred largely on essential oils. In a report (Pharm. J., 1998, 261, 406) on the laboratory testing of three oils by I. Burgess and colleagues, tea tree oil was the most effective, giving 100% immobilization of house-dust mites at 30 min, and 100% mortality at 2 h; for the same exposure times lavender oil gave figures of 87% and 87% and lemon oil 63% and 80% respectively. Australian workers have demonstrated that for laundering purposes several essential oils are effective acaricides when emulsified in low concentrations of the laboratory detergent Tween and that a simple washing procedure with eucalyptus oil, without the use of very hot water, controlled house-dust mites and their allergens in clothing and bedding (E. R. Tovey and L. G. McDonald, J. Allergy Clin. Immunol., 1997, 100, 464).
For Third World countries where synthetic acaricides are relatively expensive the exploitation of suitable local plants is important. The essential oils of some members of the Capparidaceae have been shown to be effective antitick agents and the situation is outlined by W. Lwande et al. (Phytochemistry, 1999, 50, 40) in their studies on the tick-repellent properties of the essential oil of Gynandropsis gynandron. This East African annual species has been proposed as an anti-tick pasture plant as it disrupts the free-living stages of Rhipicephalus appendiculatus, the vector of the pathogen causing East Coast fever in animals. Twenty-eight compounds were identified in the essential oil, carvacrol, phytol and linalool being the major constituents, although greatest repellency towards the tick was shown by a number of minor constituents. Methyl isothiocyanate was also identified in the oil (2.1%) and could contribute towards the activity. It may be noted here that G. gynandron is also employed in traditional medicine for a number of conditions and its essential oil is used as a repellant for head-lice.
INSECTICIDES
PYRETHRUM FLOWER
Pyrethrum flowers (Insect flowers, Dalmatian insect flowers) are the dried flower-heads of Chrysanthemum cinerariifolium (Trev.) Vis. [Tanacetum cinerariifolium (Trev.) Sch. Bip., Pyrethrum cinerariifolium Trev.] (Compositae). The plant is perennial, about 1 m high, and indigenous to the Balkans. Principal cultivated sources are Kenya, Tasmania, Tanzania and Rwanda. Smaller amounts are grown in Japan, Eastern Europe, Brazil and India.
History
The insecticidal properties of Persian or Caucasian insect flowers (Chrysanthemum coccineum Willd. and C. marshallii Aschers) have long been known in their country of origin, but the use of the Yugoslavian species dates from the middle of the last century. Persian insect flowers are now rarely seen in British commerce, and Kenya, the largest exporter, produces flowers of the Dalmatian type, the original Dalmatian seed being introduced by Gilbert Walker in 1928.
Collection
Conditions for pyrethrum cultivation are particularly favourable in Kenya; the producing areas have an altitude of 1900–2700 m and an annual rainfall of 76–180 cm. The altitude is important, giving a low night temperature (5–15°C), which stimulates maximum bud production. Collection takes place for about 9 months of the year. As about 90% of the insecticidal activity of the plant is present in the flowers, only these are collected. Before drying they are not toxic to insects. In Kenya all the flowers are delivered to the Pyrethrum Marketing Board at Nakuru. Here all samples are analysed and the growers paid on the pyrethrin content of their deliveries. The thousands of African smallholders are organized on cooperative lines and all the profits of the Board are returned to the growers. At the factory of the Board some of the flowers are baled for export but the majority are made either into powder or into standardized liquid extract. Current developments may be followed in the biannual journal Pyrethrum Post.
Characters
The closed flower-heads are about 6–9 mm in diameter and the open ones about 9–12 mm in diameter. They bear a short peduncle which is striated longitudinally. The involucre consists of two or three rows of yellowish or greenish-yellow, lanceolate, hairy bracts. The receptable is nearly flat and devoid of palae. It bears numerous, yellow tubular florets and a single row of cream or straw-coloured ligulate florets. The ligulate corollas are 10–20 mm in length and have about 17 veins and three rounded teeth, the central one very small (distinction from ox-eye daisies, C. leucanthemum, in which the ligulate corollas have seven veins and three teeth, the centre one being the largest). The achenes are five-ribbed (achenes of Persian flowers usually 10-ribbed). The flowers have a slightly aromatic odour and a bitter, acrid taste.
Characters of powders
The species used as insecticides are C. cinerariifolium, C. coccineum and C. marshallii, the powders from which show the following elements: parenchyma often containing aggregate crystals, T-shaped hairs, numerous spherical pollen grains, sclerenchymatous cells (particularly from Persian flowers), tracheids and epidermal cells having a striated, papillose cuticle. Kenya flowers are guaranteed to contain not less than 1.3% of pyrethrin; Japanese usually contain 0.9–1.0% and Dalmatian about 0.7–0.8%.
Constituents
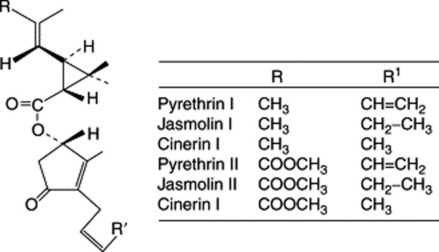
Pyrethrum owes its insecticidal properties to esters which are reportedly produced by a number of different cell types (oil glands, resin ducts and mesophyll cells). Pyrethrin I, jasmolin I and cinerin I are esters of chrysanthemic acid (chrysanthemum monocarboxylic acid), while pyrethrin II, jasmolin II and cinerin II are esters of pyrethric acid (monomethyl ester of chrysanthemum dicarboxylic acid). The alcohol component of the pyrethrins is the keto-alcohol pyrethrolone and of the cinerins the keto-alcohol cinerolone. Pyrethrum flowers also contain sesquiterpene lactones and the triterpenoid pyrethrol. The biosynthesis of pyrethrin I in seedlings of C. cinerariifolium has been studied using [1-13C]-D-glucose as a precursor; the acid portion of the molecule is derived from D-glucose and the alcohol moiety possibly from linoleic acid (K. Matsuda et al., Phytochemistry, 2005, 66, 1529).
Pyrethrum Extract of the BP (Vet.) contains 24.5–25.5% of pyrethrins; it may be prepared extemporaneously from the flower-heads and is used for the preparation of the BP (Vet.) dusting powder and spray. The dusting powder (pyrethrum extract, diatomite, talc) has a pyrethrin content of 0.36–0.44%, of which not less than half consists of pyrethrin I. It is assayed by titrimetry for both pyrethrin I and II. Extracts containing 50% more active material compared with commercial extracts can be obtained by extraction of the plant material with liquified carbon dioxide (100 bar). The extract is usually diluted on farms with kerosene to a pyrethrin strength of about 0.2%. For work on Pyrethrum hybrids, see Chapter 14.
The popularity of pyrethrum derived from its rapid knock-down action (largely due to pyrethrin II), lethality to insects (pyrethrin I) and low mammalian toxicity. However, synthetic analogues of natural pyrethrins with higher insecticidal activity (over 1000 times that of pyrethrin I), more photostability and a similar low toxicity have virtually displaced pyrethrin from the market, particularly in the area of domestic insecticidal sprays. There continues, however, to be a market for natural pyrethrins in special areas such as food processing plants and insecticidal spraying of edible fruits and vegetables shortly before harvest.
Derris and lonchocarpus
The roots of many species of Derris and Lonchocarpus (Leguminosae) have insecticidal properties which are usually, but not invariably, due to the presence of rotenone. The former British Veterinary Codex included monographs on ‘Derris’, the dried rhizome and roots of Derris elliptica, D. malaccensis and possibly other species, and on ‘Lonchocarpus’, the dried roots of Lonchocarpus utilis, L. urucu and possibly other species. Other genera of the same family with rotenoid-producing species are Millettia, Neorautanenia and Tephrosia.
Derris is indigenous to Malaya and is cultivated there and in Burma, Thailand and tropical Africa. Lonchocarpus is indigenous to Peru and Brazil and it is the usual source of material on the UK and USA markets, frequently being sold as a black resinous extract containing about 30% of isolatable rotenone and about 20% of the structurally related deguelin.
Characters
Derris roots are up to 2 m long and 1 cm or more diameter. They are sometimes attached to short pieces of rhizome. The outer surface is greyish-brown to reddish-brown and bears fine longitudinal furrows and, in the larger pieces, elongated lenticles. The drug is flexible and breaks with a fibrous fracture. It has a slight aromatic odour; and when chewed, gradually produces a feeling of numbness in the tongue and throat. Prolonged grinding of the drug is necessary on account of its fibrous nature, and special precautions are necessary, owing to the objectionable properties of the dust. A transverse section shows a thin brown bark and a cream to pale-brown wood which in the larger pieces show three or four concentric rings.
Lonchocarpus usually occurs in pieces 4–30 cm long and 1.5–2.5 cm in diameter. The outer surface is brownish-grey, with wrinkles and scars and, in the larger pieces, transverse lenticles.
Constituents
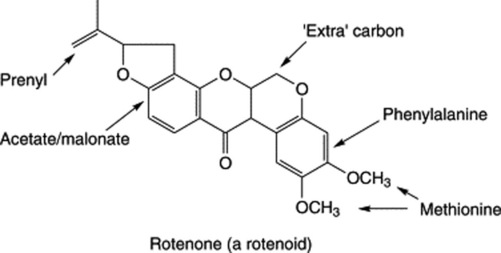
Derris and lonchocarpus contain about 3–10% of rotenone, a colourless crystalline substance which is insoluble in water but soluble in many organic solvents. However, rotenone is not the only constituent with insecticidal properties, and the evaluation of the drug depends both on rotenone content and on the amount of chloroform extractive it contains.
Rotenone is an isoflavone derivative and is biosynthesized from acetate, mevalonate and phenylalanine with an extra carbon arising from a C-1 pool. Its toxicity to mammals limits its usefulness.
The roots also contain deguelin which is similar to rotenone but possesses a gem-dimethylpyran moiety. Flavonoids and stilbenes with the same moiety are minor components.
Rotenoid derivatives, having a larvicidal property, have been isolated from Derris trifoliata; activity is due mainly to rotenone (A. Yenesew et al., Phytochemistry, 2006, 67, 988).
Nicotinoids
As early as 1763 nicotine, in the form of a tea prepared from tobacco, was recommended for the destruction of aphids.
The genus Nicotiana (Solanaceae) comprises about 100 species. Tobacco for smoking, chewing and snuffing is prepared by a curing process, largely of the cultivated Virginian tobacco, N. tabacum, and the Turkish tobacco, N. rustica. Tobacco is believed to be a native of tropical America, and was cultivated and used by the native inhabitants before the discovery of the American continent by Europeans. N. tabacum is of hybrid origin, and various ‘synthetic tobaccos’, somewhat resembling it, have been raised by crossing and breeding wild, possibly ancestral, species. Nicotine (structure and biogenetic origin, Fig. 26.2) is the characteristic alkaloid of the genus and is prepared commercially from waste material of the tobacco industry; it has long been used as an effective insecticide but is gradually being replaced by safer products. Other species (e.g. N. glutinosa) produce nornicotine by demethylation of nicotine in the leaves, whereas some (e.g. N. glauca) contain, in addition to the nicotine alkaloids, the homologous anabasine (structure and biogenetic origin, Fig. 26.11). Nornicotine and anabasine are also insecticidal. An interesting report (J. E. Huesing and D. Jones, Phytochemistry, 1987, 26, 1381) indicated that extracts of species of Nicotiana, section Repandae, caused high levels of mortality in Manduca sexta, the tobacco hornworm, a tobacco-associated insect which is not susceptible to the toxic effects of nicotine. The insecticidal component is an N-acylnornicotine which is found only in this section of the genus and is absent from the other 65 spp.
The nicotinoids are also found in some other members of the Solanaceae (spp. of Duboisia, Anthocercis, Cyphanthera and Crenidium), a few Erythroxylum spp., Asclepias syriaca and Anabasis aphylla.
Cevadilla seed
Cevadilla or sabadilla consists of the seeds of Schoenocaulon officinale (Liliaceae), a plant found from Mexico to Venezuela. The seeds are dark brown to black, sharply pointed and about 6 mm long. They contain about 2–4% of mixed alkaloids known as ‘veratrine’. The chief alkaloids, cevadine and veratridine, are closely related to the ester alkaloids of veratrum (q.v.). The powdered seeds and preparations of ‘veratrine’ are used as a dust or spray to control thrips and various true bugs which attack vegetables.
Ryania
The roots and stems of Ryania speciosa (Flacourtiaceae), a plant native to South America, contain 0.16%–0.2% of alkaloids having insecticidal properties. Ryanodine, the principal alkaloid, is a complex ester involving 1-pyrrole-carboxylic acid. The plant is used in the control of various lepidopterous larvae which attack fruits, and particularly the European corn borer.
Miscellaneous
A number of other plants containing insecticidal compounds with scope for synthetic improvement include Mammea spp., Guttiferae (coumarins); Ebenaceous spp., containing the naphthoquinone plumbagin (q.v.) and Phryma leptostachya, Verbenaceae (a highly active lignan, haedoxan A). Melia azedarach (Meliaceae), native to N.W. India, has been long recognized for its insecticidal properties and is still the subject of considerable research. Three diacylated meliacarpin derivatives with strong insecticidal activity against the larvae of Spodoptera littoralis have been isolated from the leaves (F. I. Bohnenstengel et al., Phytochemistry, 1999, 50, 977); two insecticidal tetranortriterpenoids have potential for further development (B. S. Siddiqui et al., Phytochemistry, 2000, 53, 371) and positive antifeedant properties have been demonstrated with extracts of unripe fruits and green or senescent leaves against mature adults of the elm leaf beetle, Xanthogaleruca luteola (M. Defagó et al., Fitoterapia, 2006, 77, 500).
RODENTICIDES
Red squill
Red squill and white squill (see ‘Cardioactive Drugs’, Chapter 23) are both varieties of Urginea maritima (Liliaceae). The red squill may be distinguished in either the whole or powdered state by the reddish-brown outer scales and the white to deep purple inner ones. In addition to other cardioactive glycosides, the bulb of the red squill also contains the glucosides scilliroside and scillirubroside. Strains selected for high scilliroside content have been developed from plants introduced to southern California in 1946. Unlike other mammals, rodents do not regurgitate the squill bulb, and death follows convulsions and respiratory failure.
MOLLUSCICIDES
Pharmaceutical interest in molluscicides is concerned primarily with the control of schistosomiasis (bilharzia), a parasitic disease of humans in which certain freshwater snails act as intermediate hosts for the blood flukes, Schistosoma haemotobium, S. mansoni and S. japonicum. The disease, which causes intestinal and bladder damage, is prevalent in S. America, Africa and the Far East and is increasing as a result of the construction of dams and irrigation systems which provide enlarged breeding areas for snails. Eggs are eliminated in the faeces or urine of infected humans and, in water, hatch as miracidia which enter the host snails (Biomphalaria pfeiffer (S. America), B. glabrata, Bulinus globosus, etc.) where numerous cercaria are produced. The cercaria emerge into the water and infect humans by passing through the skin into the bloodstream. Synthetic drugs are available to combat the infection but for the general control of the disease the eradication of the intermediate stages of the life-cycle of the fluke is necessary together with improved sanitary arrangements. In 1998 it was estimated that there were over 20 million severely diseased individuals in the tropics and some ten times that number infected to some degree.
During the last two decades it has been shown that a wide range of phytochemicals exhibit molluscicidal activity. Prominent families in this connection are the Leguminosae, Araliaceae, Compositae and Liliaceae. However, before a plant, shown to possess molluscicidal activity in laboratory tests, can be utilized on a large scale a number of other, fairly obvious, criteria need to be satisfied. Thus, the plant material must be available in sufficient quantity and, if necessary, capable of easy propagation in the region where required; the active constituents should be water-soluble and easily extractable from the plant source; the molluscicidal activity should be high and the toxicity towards other organisms, including humans, low. Few plants as yet examined appear to have satisfied all of these requirements.
The berries of the Ethiopian plant Phytolacca dodecandra (Phytolaccaceae) have proved effective in clearing stretches of waterways of snails, but cultivation in areas outside of the natural habitat has produced disappointingly low yields of fruits. The most active components of this plant are triterpenoid saponins composed of oleanolic acid (Fig. 23.10) with a branched sugar side-chain at C-3; they are liberated by the enzymatic cleavage of the ester-bound saccharide chains of non-molluscicidal bidesmodic saponins (S. T. Thiilborg et al., Phytochemistry, 1993, 32, 1167). A plant the pods of which contain similar saponins is Swartzia madagascariensis (Leguminosae), a tree widespread throughout Africa; it has local medicinal, insecticidal and piscicidal uses. The leaves of the S. America species S. simplex have a similar activity to those of the African plant and glycosides of oleanolic acid, gypsogenin (Fig. 23.10) and gypsogenic acid have been isolated as active constituents. Saponins are also present in Tetrapleura tetraptera (Leguminosae), a promising Nigerian molluscicide. A number of these plants containing effective saponins are also well established piscicides.
Spirostanol saponins, as found in Balanites aegyptica (Zygophyllaceae), are potent molluscicides. This plant contains balanitin-1, -2 and -3; balanitin-1, for example, possesses a yamogenin aglycone (q.v.) with a branched glucose and rhamnose side-chain. In the same family, saponins from the pericarps of Guaiacum officinale have molluscicidal activity. In an evaluation of plant molluscicides against the freshwater snail Lymnaea luteola, the vector of animal schistosomiasis in India, Sapindus trifoliatus (Sapindaceae) was the most effective of the species tested (D. Sukumaran et al., Pharm. Biol., 2002, 40, 450). The aqueous extracts of three other Indian-grown plants (Thevetia peruviana, Alstonia scholaris and Euphorbia pulcherrina) have also been shown to possess considerable molluscicidal activity (A. Singh and S. K. Singh, Fitoterapia, 2005, 76, 747).
Tannins constitute the active principles of some Leguminosae e.g. Acacia spp., and napthoquinones of the juglone and plumbagin type (p. 251) constitute those of the Malawi Ebenaceous species Diospyros usambarensis. The disadvantage of the latter source, investigated by Hostettman et al., is that the naphthoquinones are at their highest concentration in the root-bark.
Other phytochemical groups of compounds having recognized molluscicidal activity are isobutylamides of the Asteraceae, Rutaceae and Piperaceae, steroidal glycoalkaloids (Solanum mammosum), anthraquinones (Morinda lucida, Rubiaceae) and flavonoids of various families. Two N.E. Brazilian species of Solanum (S. jabrense and S. stipulaceum) have shown promising activity (T. M. S. Silva et al., Fitoterapia, 2006, 77, 449).
Some of the most active substances known are the unsaturated anacardic acids of cashew nut shells (Anacardium occidentale), but unfortunately field trials carried out in Mozambique showed the treated water to give rise to dermatitis.
Continued progress in this area is to be expected; other plants tested and shown to possess molluscicidal activity include Ambrosia maritima, Ammi majus, Azolla pinnata, Calendula micrantha officinalis, Croton campestris, Cucumis prophetarum, Euphorbia splendens, Millettia thonningii and Rhynchosia minimum.
Casida JE, Quistad GB, editors. Pyrethrum flowers, production, chemistry, toxicology and uses. New York: Oxford University Press, 1995.
Dales MJ. A review of plant materials used for controlling insect pests of stored products. Chatham, UK: Bulletin 65, Natural Resources Institute, 1996.
Regnault-Roger C, Philogène BJR. Past and current prospects for the use of botanicals and plant allelochemicals in integrated pest management. Pharmaceutical Biology. 2008;46(1–2):41-52.