Diabetes mellitus
Control of blood glucose
Glucose occupies a central position in metabolism as the predominant substrate for energy production. Cells receive their supply of glucose from blood, and control mechanisms ensure that the blood glucose concentration remains within narrow limits. Glucose enters the blood by absorption from the gut and from breakdown of stored glycogen or gluconeogenesis in the liver. At physiological concentrations, glucose is transferred from the blood into cells almost entirely by active transport. In most tissues this transfer is dependent on the action of the polypeptide hormone insulin.
Insulin is a protein that is secreted rapidly from the β-cells of the islets of Langerhans in the pancreas in response to a small rise in blood glucose, and its secretion is inhibited by a fall in blood glucose (Table 40.1). Insulin consists of two peptide chains, A and B, connected by two disulphide bridges. In the β-cell, insulin aggregates into hexamers with zinc, and after release from the cell it dissociates into dimers and eventually into the active monomeric form.
Table 40.1
Control of insulin release from pancreatic islets of Langerhans β-cells
| Stimulants of insulin release | Inhibitors of insulin release |
| Parasympathetic stimulation (muscarinic receptors) | Sympathetic stimulation of α2-adrenoceptors |
| Increased glucose | Decreased glucose |
| Amino acids | Somatostatin |
| Fatty acids | |
| Cortisol | |
| Gastrin | |
| Secretin | |
| Glucagon | |
| Incretins (GLP-1, GIP) |
GIP, glucose-dependent insulinotropic peptide; GLP-1, glucagon-like peptide.
The presence of nutrients in the small intestine stimulates the release from gut endocrine cells of peptide hormones called incretins, which promote insulin secretion. The principal incretins are glucose-dependent insulinotropic peptide (GIP), secreted by the upper gut, and glucagon-like peptide-1 (GLP-1), which is released from the distal gut. Release is triggered by neural signals from the upper gut and direct interaction of nutrients with the secretory cells. GLP-1 has several actions that regulate glucose homeostasis:
 enhanced glucose-dependent insulin secretion; incretins are probably responsible for about 60% of the insulin that is secreted in response to a meal,
enhanced glucose-dependent insulin secretion; incretins are probably responsible for about 60% of the insulin that is secreted in response to a meal,
 inhibition of glucagon release,
inhibition of glucagon release,
 promotion of satiety by an action on the hypothalamus (Ch. 37).
promotion of satiety by an action on the hypothalamus (Ch. 37).
The actions of GLP-1 are brief as it has a very short plasma half-life of 1–2 min due to rapid degradation by dipeptidyl peptidase-4 (DPP-4).
Insulin is secreted into the blood under fasting conditions, with pulses every 10–14 min, and a slower cycle of release every 105–120 min. In response to a rise in plasma glucose (both the actual concentration and the rate of change) there is a superimposed biphasic pattern of insulin release.
 The first phase of release occurs within seconds, peaks at 3–5 min and lasts for about 10 min. This is achieved by the release of a small pool of insulin in secretory vesicles.
The first phase of release occurs within seconds, peaks at 3–5 min and lasts for about 10 min. This is achieved by the release of a small pool of insulin in secretory vesicles.
 The second phase of release is more gradual, rising to a lower peak than in phase 1, and is due to synthesis of new insulin.
The second phase of release is more gradual, rising to a lower peak than in phase 1, and is due to synthesis of new insulin.
Insulin secretion from pancreatic β-cells is modulated by K+ channels in the cell membrane that are sensitive to ATP (KATP channels). The KATP channel has subunits known as sulfonylurea receptors (SURs), various isoforms of which act as regulatory proteins in the response to ATP in different tissues. First-phase insulin release is triggered when glucose enters the β-cell via the GLUT2 glucose transporter, and undergoes glycolysis with generation of intracellular ATP. Activation of the SUR1 receptor isoform by ATP closes the KATP channel, which reduces membrane K+ efflux and depolarises the β-cell. Depolarisation opens voltage-gated L-type Ca2+ channels in the cell membrane, and an influx of Ca2+ ions into the cell triggers second messengers that lead to exocytosis of insulin granules. (See Fig. 5.4 for description of the KATP channel in vascular smooth muscle.) In addition to glucose, many other factors influence insulin secretion (Table 40.1).
Peripheral tissues express specific cell surface insulin receptors that are linked to a tyrosine kinase (insulin receptor kinase) (Ch. 1). Stimulation of these receptors leads to translocation of the GLUT4 glucose transporter to the cell surface, allowing glucose uptake, and activates pathways involved in glycogen synthesis, glycolysis and fatty acid synthesis. Metabolic effects of insulin include the following.
 Glucose metabolism: promotion of active transport of glucose into cells, particularly in skeletal muscle and adipose tissue, accompanied by K+. Insulin enhances storage of glucose as glycogen in liver and muscle and inhibits the breakdown of glycogen (glycogenolysis). Insulin also inhibits gluconeogenesis from amino acids in the liver. The overall effect is to increase glycogen stores.
Glucose metabolism: promotion of active transport of glucose into cells, particularly in skeletal muscle and adipose tissue, accompanied by K+. Insulin enhances storage of glucose as glycogen in liver and muscle and inhibits the breakdown of glycogen (glycogenolysis). Insulin also inhibits gluconeogenesis from amino acids in the liver. The overall effect is to increase glycogen stores.
 Lipid metabolism: reduced plasma free fatty acids and increased adipocyte triglyceride storage. Insulin increases hydrolysis of circulating triglycerides from lipoproteins by enhancing the activity of lipoprotein lipase, and promotes fatty acid uptake by adipose cells. Glucose entry into adipocytes provides glycerol phosphate for esterification of fatty acids to triglycerides. In adipose tissue, insulin inhibits lipases and prevents triglyceride breakdown.
Lipid metabolism: reduced plasma free fatty acids and increased adipocyte triglyceride storage. Insulin increases hydrolysis of circulating triglycerides from lipoproteins by enhancing the activity of lipoprotein lipase, and promotes fatty acid uptake by adipose cells. Glucose entry into adipocytes provides glycerol phosphate for esterification of fatty acids to triglycerides. In adipose tissue, insulin inhibits lipases and prevents triglyceride breakdown.
 Protein metabolism: inhibition of the catabolism of amino acids in the liver and increased amino acid transport into muscle with enhanced protein synthesis.
Protein metabolism: inhibition of the catabolism of amino acids in the liver and increased amino acid transport into muscle with enhanced protein synthesis.
The effects of insulin on different tissues are summarised in Table 40.2.
Table 40.2
| Site | Effect |
| Liver | Increased glucose storage as glycogen |
| Decreased protein catabolism | |
| Increased protein synthesis | |
| Decreased gluconeogenesis | |
| Muscle | Increased protein synthesis |
| Increased glycogen synthesis | |
| Increased glucose uptake | |
| Increased amino acid uptake | |
| Adipose tissue | Increased triglyceride storage |
| Increased triglyceride synthesis | |
| Decreased lipolysis |
Several hormones inhibit the anabolic actions of insulin, particularly on carbohydrate metabolism, although effects on protein metabolism vary. These include glucagon, growth hormone, cortisol and catecholamines. Most of these hormones are released in stressful situations that require the breakdown of glycogen reserves to provide energy.
Diabetes mellitus
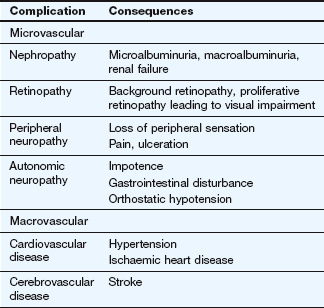
Failure to secrete sufficient insulin to control the normal level of blood glucose results in diabetes mellitus. The condition is diagnosed when the fasting plasma glucose concentration exceeds 7 mmol⋅L−1. The long-term consequences include increased risk of the development of vascular and neuropathic disease (Table 40.3). Two patterns of diabetes mellitus are recognised: type 1 and type 2. There is still dispute over whether they represent distinct entities, or different manifestations of the same disease process. There is a strong genetic predisposition for both conditions.
Type 1 diabetes mellitus
Type 1 diabetes represents a severe deficiency of insulin production caused by autoimmune destruction of pancreatic β-cells, and usually presents in younger people. Type 1 diabetes typically presents with a short history of feeling tired and unwell, together with weight loss, polyuria and polydipsia. There is a high risk of ketoacidosis because of breakdown of fatty acids and amino acids in the liver to provide an energy source, which generates ketone bodies.
Type 2 diabetes mellitus
Type 2 diabetes, which usually presents later in life, is the consequence of a relative deficiency of insulin. It accounts for 90% of cases of diabetes in the Western world. In established type 2 diabetes the first phase of insulin secretion is absent or attenuated, and the second phase is slowed.
People with type 2 diabetes are often overweight (the average body mass index at diagnosis is 30 kg⋅m−2). This increases cellular resistance to insulin in the liver, muscles and adipose tissue so that less glucose is transported into cells. In addition to reduced tissue uptake of glucose, glycogen in the liver is broken down and glucose is released into the circulation. In adipose tissue, triglycerides are broken down to free fatty acids which are released into the circulation.
In type 2 diabetes there is reduced or absent GLP-1 secretion in response to oral glucose, and a reduced sensitivity to the peptide at pancreatic β-cells. In addition, high circulating free fatty acids and the production of reactive oxygen species in response to a sustained high plasma glucose concentration reduce insulin secretion.
Insulin resistance characteristically precedes overt diabetes by several years, but for some time insulin secretion by the pancreas is sufficient to overcome cellular resistance. Eventually, β-cell dysfunction with loss of the first-phase insulin response to a glucose load results in loss of compensation for insulin resistance. In people with type 2 diabetes who are not obese the major defect is inadequate insulin secretion.
In type 2 diabetes, postprandial hyperglycaemia is the major defect in blood glucose control, with excess glucose outside the cells rather than a shortage inside. People with type 2 diabetes do not usually develop ketoacidosis, because sufficient glucose enters cells to permit adequate energy production for most situations. The ideal approach to treatment would be an intervention that restores the early phase of insulin secretion in response to a glucose load.
Insulins and insulin analogues
Normal insulin secretion from the pancreas is into the portal circulation and is strictly regulated to meet metabolic needs. Sixty per cent of the insulin that is released from the pancreas is extracted by the liver before it reaches the systemic circulation. In contrast, therapeutic delivery of insulin is to the systemic circulation, and the relationship to metabolic needs can only be approximated by the dosages used and their timing in relation to meals.
Natural insulin formulations
Insulins for therapeutic administration were originally extracted from either bovine or porcine pancreas. Bovine insulin differs chemically from human insulin in three amino acid residues, and porcine in one, but their actions are very similar to human insulin. These insulins are now rarely used.
Human-sequence insulin is produced either by enzymatic modification of porcine insulin, or by recombinant DNA technology using bacteria or yeast. All current insulin preparations have a low impurity content, which has caused problems in the past, and have low immunogenic potential.
All insulins (and insulin analogues; see below) are formulated at a standard strength of 100 units⋅mL−1 to reduce confusion over doses. The choice of injection device, usually a form of prefilled syringe, is important to facilitate use.
Pharmacokinetics
Currently available insulins must be given parenterally, because insulin is a protein and would otherwise be digested in the gut. At present, subcutaneous injection is used for routine treatment, with intravenous infusion for emergency situations. Recommended subcutaneous injection sites include upper arms, thigh, buttocks and abdomen. Absorption is faster from the abdomen than from the limbs, although strenuous exercise can increase absorption from the limbs.
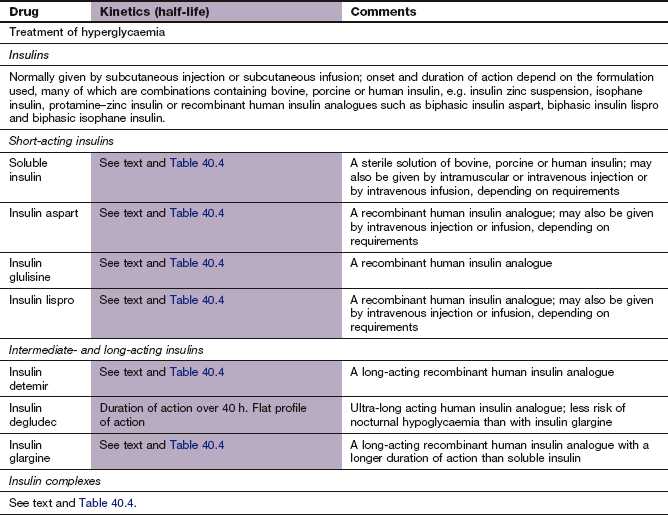
The half-life of insulin in plasma is very short (about 8–16 min), and to avoid the need for frequent injections during maintenance treatment the absorption of insulin from injection sites must be prolonged. Insulin is formulated either in a soluble preparation or complexed with a substance to delay absorption from the injection site (Table 40.4).
Table 40.4
Characteristics of insulins following subcutaneous administration
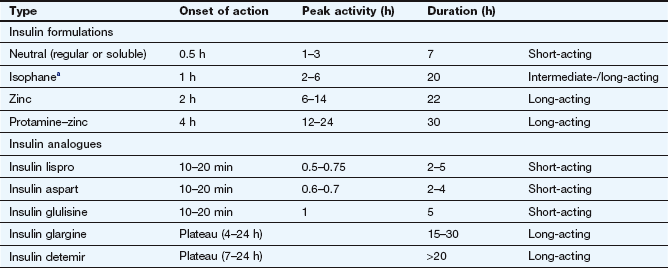
aSometimes called NPH (neutral protamine Hagedorn) insulin.
Soluble insulins aggregate to form hexamers, which delays their absorption from the injection site. After subcutaneous injection, the maximum plasma concentration of soluble insulin (also called neutral insulin) is achieved about 2 h later, compared with minutes after intravenous injection. To limit the increase in plasma glucose concentration generated by a meal, subcutaneous soluble insulin must be given 15–30 min before eating. The action of intravenous soluble insulin lasts less than an hour and is mainly terminated by degradation in the kidney. Continued absorption from a subcutaneous injection site prolongs the duration of action after injection to about 5 h.
To generate intermediate- or long-acting formulations, insulin is complexed with the following.
 Protamine: to create an intermediate-acting complex isophane insulin. Isophane insulin can also be formulated together with a non-complexed solution of soluble insulin (biphasic isophane insulin). The ratio of soluble to isophane insulin in biphasic insulin varies from 10 : 90 through 20 : 80; 30 : 70 and 40 : 60 to 50 : 50.
Protamine: to create an intermediate-acting complex isophane insulin. Isophane insulin can also be formulated together with a non-complexed solution of soluble insulin (biphasic isophane insulin). The ratio of soluble to isophane insulin in biphasic insulin varies from 10 : 90 through 20 : 80; 30 : 70 and 40 : 60 to 50 : 50.
 Zinc: to create the intermediate-acting insulin zinc suspension or the long-acting crystalline insulin zinc suspension. Insulin molecules form hexamers which are stabilized by zinc, and the size of these molecular aggregates determines the rate of diffusion from the site of injection. Such complexes act as modified-release formulations for subcutaneous administration (Table 40.4).
Zinc: to create the intermediate-acting insulin zinc suspension or the long-acting crystalline insulin zinc suspension. Insulin molecules form hexamers which are stabilized by zinc, and the size of these molecular aggregates determines the rate of diffusion from the site of injection. Such complexes act as modified-release formulations for subcutaneous administration (Table 40.4).
 Protamine and zinc: to create the long-acting protamine–zinc insulin. This is only available as a bovine insulin and is now used rarely because it binds soluble insulin if given in the same syringe.
Protamine and zinc: to create the long-acting protamine–zinc insulin. This is only available as a bovine insulin and is now used rarely because it binds soluble insulin if given in the same syringe.
Unwanted effects
 The main problem is an excessive action producing hypoglycaemia. Neuroglycopenia with confusion and coma can occur. Treatment is with sugary foods or drinks, oral glucose or glucose gel (Hypostop®; 10–20 g) if the person is conscious. Intravenous injection of 20% glucose is used if the person is unconscious. Glucagon (see below) can be given intramuscularly if venous access is not available, followed by a sugary drink on waking. All people with diabetes who take insulin should carry a card with details of their treatment (‘insulin passport’). Although most people experience warning symptoms of hypoglycaemia, some do not and are prone to sudden hypoglycaemia with loss of consciousness. Frequent hypoglycaemic attacks can reduce the awareness of the onset of symptoms.
The main problem is an excessive action producing hypoglycaemia. Neuroglycopenia with confusion and coma can occur. Treatment is with sugary foods or drinks, oral glucose or glucose gel (Hypostop®; 10–20 g) if the person is conscious. Intravenous injection of 20% glucose is used if the person is unconscious. Glucagon (see below) can be given intramuscularly if venous access is not available, followed by a sugary drink on waking. All people with diabetes who take insulin should carry a card with details of their treatment (‘insulin passport’). Although most people experience warning symptoms of hypoglycaemia, some do not and are prone to sudden hypoglycaemia with loss of consciousness. Frequent hypoglycaemic attacks can reduce the awareness of the onset of symptoms.
 Rebound hyperglycaemia can occur after an episode of hypoglycaemia, especially at night (Somogyi effect). This results from the compensatory release of hormones such as adrenaline. It can produce ketonuria, leading to a mistaken belief that too little insulin has been given.
Rebound hyperglycaemia can occur after an episode of hypoglycaemia, especially at night (Somogyi effect). This results from the compensatory release of hormones such as adrenaline. It can produce ketonuria, leading to a mistaken belief that too little insulin has been given.
 Animal insulins produce circulating antibodies, although this is less common with current, highly purified preparations. These could diminish the activity of the insulin (insulin resistance) or produce local reactions (lipoatrophy) at injection sites.
Animal insulins produce circulating antibodies, although this is less common with current, highly purified preparations. These could diminish the activity of the insulin (insulin resistance) or produce local reactions (lipoatrophy) at injection sites.
 Insulins can cause local fat hypertrophy at the injection site, which can be minimized by rotating the site of injection.
Insulins can cause local fat hypertrophy at the injection site, which can be minimized by rotating the site of injection.
Insulin analogues
Mechanism of action and effects
The insulin analogues are recombinant chemical modifications of naturally occurring insulin. These changes have no effect on the binding of the molecule to cellular insulin receptors.
Short-acting insulin analogues: Unlike soluble insulins, these do not readily form dimers and hexamers, and therefore they are rapidly absorbed from an injection site with a faster onset and a shorter duration of action.
 Insulin aspart has one amino acid substitution of aspartic acid for proline at position B28.
Insulin aspart has one amino acid substitution of aspartic acid for proline at position B28.
 Insulin glulisine has two amino acid changes involving substitution with glutamic acid for lysine at B29 and lysine for asparagine at B3.
Insulin glulisine has two amino acid changes involving substitution with glutamic acid for lysine at B29 and lysine for asparagine at B3.
 Insulin lispro has the amino acids lysine and proline reversed at positions B28 and B29.
Insulin lispro has the amino acids lysine and proline reversed at positions B28 and B29.
Insulin aspart and lispro are available complexed with protamine to give an intermediate duration of action, and in this form are combined with the short-acting formulations as ready mixed biphasic insulins.
Long-acting insulin analogues:
 Insulin detemir has threonine at B30 omitted and a fatty acid chain added to the amino acid B29. This increases the formation of insulin complexes and enhances binding to albumin, which slows absorption from the injection site.
Insulin detemir has threonine at B30 omitted and a fatty acid chain added to the amino acid B29. This increases the formation of insulin complexes and enhances binding to albumin, which slows absorption from the injection site.
 Insulin glargine has two amino acid changes involving substitution of glycine for asparagine at A21 and addition of two arginines to the C-terminus of the B chain. This makes the molecule more soluble at acid pH, and less soluble at physiological pH. Insulin glargine precipitates after subcutaneous injection, slowly redissolves and is then absorbed.
Insulin glargine has two amino acid changes involving substitution of glycine for asparagine at A21 and addition of two arginines to the C-terminus of the B chain. This makes the molecule more soluble at acid pH, and less soluble at physiological pH. Insulin glargine precipitates after subcutaneous injection, slowly redissolves and is then absorbed.
Pharmacokinetics
Compared with standard soluble insulin, absorption of short-acting insulin analogues from a subcutaneous injection site occurs faster and leads to an early peak plasma concentration (Table 40.4). The duration of action is also shorter, at almost 3 h. They are usually given just before a meal, but can be used immediately after eating. They can be mixed with long-acting standard insulins, and are also available as biphasic formulations (insulin aspart with insulin aspart protamine in a 30 : 70 ratio; insulin lispro with insulin lispro protamine in a 25 : 75 or 50 : 50 ratio). Insulin analogues can be given by subcutaneous injection or infusion or by intravenous injection or infusion.
Long-acting insulin analogues are slowly and uniformly absorbed after subcutaneous injection, which avoids plasma insulin peaks.
Unwanted effects
 Unwanted effects are similar to those of other insulins. Despite the structural modifications, there is no reported excess of immunogenic reactions compared with standard insulin.
Unwanted effects are similar to those of other insulins. Despite the structural modifications, there is no reported excess of immunogenic reactions compared with standard insulin.
 There is a slightly reduced frequency of hypoglycaemia with insulin aspart or lispro compared with soluble insulin, because of the shorter duration of action.
There is a slightly reduced frequency of hypoglycaemia with insulin aspart or lispro compared with soluble insulin, because of the shorter duration of action.
Therapeutic regimens for insulin
The choice of regimen for insulin administration depends on the age, lifestyle, circumstances and preference of the individual. The general principle is to maintain a background (basal) level of insulin and then to give insulin boluses prior to meals to deal with the glucose load (basal-bolus regimens). Options include the following.
 Single daily injections before breakfast or at bedtime: used mainly for elderly people with type 2 diabetes who require insulin, and in whom the long-term complications of diabetes are less relevant. An intermediate- or long-acting insulin is used which can be combined with a short-acting insulin to improve control.
Single daily injections before breakfast or at bedtime: used mainly for elderly people with type 2 diabetes who require insulin, and in whom the long-term complications of diabetes are less relevant. An intermediate- or long-acting insulin is used which can be combined with a short-acting insulin to improve control.
 Twice-daily injections before breakfast and evening meal: suitable for people who have a reasonably stable pattern of activity and eating habits. Short- and intermediate-acting insulins are combined, either in fixed ratios provided by the manufacturer (see above), or in varying ratios according to individual requirements.
Twice-daily injections before breakfast and evening meal: suitable for people who have a reasonably stable pattern of activity and eating habits. Short- and intermediate-acting insulins are combined, either in fixed ratios provided by the manufacturer (see above), or in varying ratios according to individual requirements.
 Multiple injections before breakfast and evening meal and at bedtime are increasingly used in younger, active people who require more flexibility in their lifestyle. A number of tailored regimens can be employed using short- and intermediate-acting insulins. Examples include long-acting insulin at bedtime or intermediate-acting insulin at breakfast and at bedtime to ensure a ‘background’ level, and then short-acting insulin before breakfast and midday and evening meals.
Multiple injections before breakfast and evening meal and at bedtime are increasingly used in younger, active people who require more flexibility in their lifestyle. A number of tailored regimens can be employed using short- and intermediate-acting insulins. Examples include long-acting insulin at bedtime or intermediate-acting insulin at breakfast and at bedtime to ensure a ‘background’ level, and then short-acting insulin before breakfast and midday and evening meals.
There are also situations in which a basal-bolus regimen is not appropriate.
 Continuous subcutaneous infusion of short-acting insulin via a portable syringe pump and catheter is used if there is a problem with recurrent hypoglycaemia, unpredictable daily lives or hyperglycaemia before breakfast despite optimisation of a multiple-injection insulin regimen. The rate of infusion can be programmed, and boluses given before meals.
Continuous subcutaneous infusion of short-acting insulin via a portable syringe pump and catheter is used if there is a problem with recurrent hypoglycaemia, unpredictable daily lives or hyperglycaemia before breakfast despite optimisation of a multiple-injection insulin regimen. The rate of infusion can be programmed, and boluses given before meals.
 Intravenous infusion is used for treatment of ketoacidotic crises, in labour, during and after surgery, or at other times when the person's usual routine cannot be adhered to. Short-acting insulin is infused in 5% glucose solution with added potassium chloride (unless there is hyperkalaemia).
Intravenous infusion is used for treatment of ketoacidotic crises, in labour, during and after surgery, or at other times when the person's usual routine cannot be adhered to. Short-acting insulin is infused in 5% glucose solution with added potassium chloride (unless there is hyperkalaemia).
 Intraperitoneal infusion: people with diabetes who are being treated for chronic renal failure by continuous ambulatory peritoneal dialysis can add their insulin to the dialysis fluid. Some implantable insulin pumps also use this route. This is the only therapeutic regimen in which insulin has direct access to the portal circulation.
Intraperitoneal infusion: people with diabetes who are being treated for chronic renal failure by continuous ambulatory peritoneal dialysis can add their insulin to the dialysis fluid. Some implantable insulin pumps also use this route. This is the only therapeutic regimen in which insulin has direct access to the portal circulation.
Other parenteral hypoglycaemic drugs
Mechanism of action
Exenatide and liraglutide are peptides that share part of their amino acid sequences with the naturally occurring incretin, glucagon-like peptide-1 (GLP-1). They bind to and activate the GLP-1 receptor, leading to an increase in glucose-dependent synthesis of insulin and its secretion from β-cells. They restore the first-phase insulin response to an oral glucose load and, unlike insulin, promote weight loss. Unlike GLP-1 they are resistant to the enzymatic action of DPP-4.
Oral hypoglycaemic drugs
The main sites of action of oral hypoglycaemic drugs are shown in Figure 40.1.
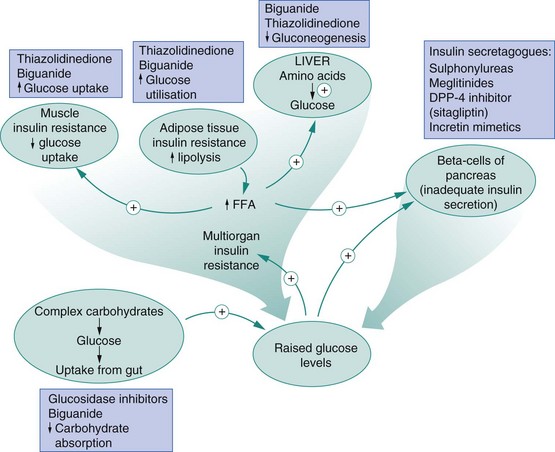
Fig. 40.1 Metabolic dysfunctions in type 2 diabetes and sites of drug action.
The metabolic dysfunctions seen in type 2 diabetes result from inadequate insulin secretion and tissue resistance to the effects of insulin. Drug classes used to overcome the metabolic dysfunctions are ‘insulin sensitisers’ or increase insulin secretion and are shown with their main actions. DPP-4, dipeptidyl peptidase-4; FFA, free fatty acids.
Sulfonylureas
Mechanism of action
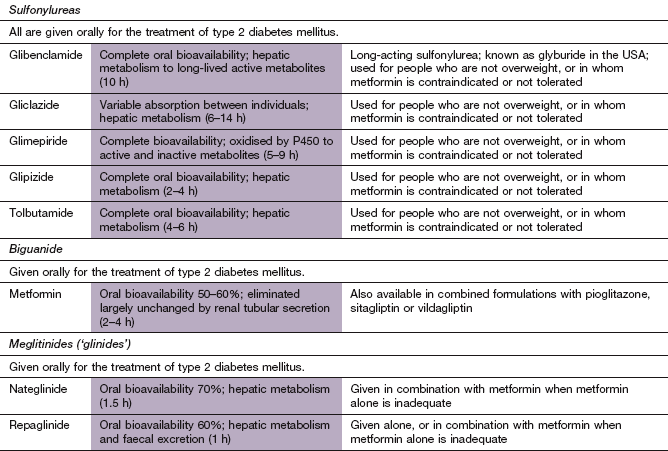
Sulfonylureas act mainly by increasing the release of insulin from the pancreatic β-cells in response to stimulation by glucose (Fig. 40.1). They bind to the sulfonylurea (SUR1) receptor and close the KATP channel in the β-cell membrane. The resultant membrane depolarisation increases both first- and second-phase insulin secretion in response to glucose. Compounds with a short duration of action are usually preferred, to minimise the risk of hypoglycaemia. The long duration of action of glibenclamide carries a greater risk of hypoglycaemia and it is not recommended for treatment of the elderly.
Pharmacokinetics
Sulfonylureas are structurally related to sulphonamides. They are absorbed rapidly (although the rate of absorption is reduced when taken with food), are highly protein-bound and are metabolised by the liver. Tolbutamide, glipizide, glimepiride and gliclazide have half-lives of less than 10 h and short durations of action. Glibenclamide has a longer duration of action because of slow dissociation from the SUR1 receptor.
Unwanted effects
 Gastrointestinal disturbance, with nausea, vomiting, diarrhoea, constipation.
Gastrointestinal disturbance, with nausea, vomiting, diarrhoea, constipation.
 Hypoglycaemia (particularly nocturnal) is most frequent with the longer-acting drugs or with excessive dosage since the drugs continue to work at low plasma glucose concentrations.
Hypoglycaemia (particularly nocturnal) is most frequent with the longer-acting drugs or with excessive dosage since the drugs continue to work at low plasma glucose concentrations.
 Weight gain is almost inevitable unless dietary restrictions are observed.
Weight gain is almost inevitable unless dietary restrictions are observed.
 Hypersensitivity reactions (usually in the first 6–8 weeks of therapy) include skin rashes and, rarely, blood disorders.
Hypersensitivity reactions (usually in the first 6–8 weeks of therapy) include skin rashes and, rarely, blood disorders.
 Glipizide and glimepiride can increase renal sensitivity to antidiuretic hormone and produce water retention with dilutional hyponatraemia.
Glipizide and glimepiride can increase renal sensitivity to antidiuretic hormone and produce water retention with dilutional hyponatraemia.
 Sulfonylureas (except glipizide) should be avoided in people with acute porphyria.
Sulfonylureas (except glipizide) should be avoided in people with acute porphyria.
 Concerns have been raised that sulfonylureas might increase cardiovascular mortality in type 2 diabetes, possibly as a result of binding to SUR2 receptors in the heart and inhibiting cardiac KATP channels. These channels have a cardioprotective role in ischaemic tissue, preventing cell depolarisation to conserve intracellular energy stores. Inhibition of the channels could lead to arrhythmias in people who have diabetes and ischaemic heart disease (see Ch. 5). However, recent clinical studies have failed to confirm the original concerns about cardiovascular mortality. Glimepiride and gliclazide bind less avidly than other sulfonylureas to cardiac SUR2 receptors.
Concerns have been raised that sulfonylureas might increase cardiovascular mortality in type 2 diabetes, possibly as a result of binding to SUR2 receptors in the heart and inhibiting cardiac KATP channels. These channels have a cardioprotective role in ischaemic tissue, preventing cell depolarisation to conserve intracellular energy stores. Inhibition of the channels could lead to arrhythmias in people who have diabetes and ischaemic heart disease (see Ch. 5). However, recent clinical studies have failed to confirm the original concerns about cardiovascular mortality. Glimepiride and gliclazide bind less avidly than other sulfonylureas to cardiac SUR2 receptors.
 There is some evidence that sulfonylureas may accelerate the rate of pancreatic β-cell loss.
There is some evidence that sulfonylureas may accelerate the rate of pancreatic β-cell loss.
Meglitinides
Mechanism of action
The sulfonylurea moiety of glibenclamide is called meglitinide, and the currently available meglitinides are derived from this. Nateglinide binds to the SUR1 receptor on the β-cell, while repaglinide binds to a different nearby site. They stimulate insulin release in the same way as sulfonylureas. Nateglinide, unlike repaglinide, has a greater effect on insulin secretion when plasma glucose levels are rising and therefore produces little stimulation of insulin secretion in the fasting state. They have a rapid onset of action and a short duration of activity, and thus are taken shortly before main meals.
Pharmacokinetics
Both nateglinide and repaglinide are rapidly and well absorbed from the gut. They are metabolised in the liver and have short half-lives (1–2 h).
Unwanted effects
Biguanide
Mechanism of action and effects
Metformin does not affect insulin secretion. Its molecular target is the protein kinase LKB1, which activates the hepatic enzyme 5′-AMP-activated protein kinase (AMPK). AMPK is a key regulator of the metabolism of fat and glucose. The major actions of metformin are as follows.
 Suppression of hepatic gluconeogenesis is the most important effect. Since some gluconeogenic activity remains, the risk of hypoglycaemia is minimal.
Suppression of hepatic gluconeogenesis is the most important effect. Since some gluconeogenic activity remains, the risk of hypoglycaemia is minimal.
 Facilitation of glucose uptake in skeletal muscle and adipocytes. The full effect requires the presence of insulin. Metformin increases cell surface expression and activity of the membrane glucose transporter GLUT4.
Facilitation of glucose uptake in skeletal muscle and adipocytes. The full effect requires the presence of insulin. Metformin increases cell surface expression and activity of the membrane glucose transporter GLUT4.
 Decreased glucose absorption from the gut.
Decreased glucose absorption from the gut.
 Improvement in the adverse plasma lipid profile found in diabetes. Metformin increases fatty acid oxidation and reduces plasma triglycerides. It also raises plasma high-density lipoprotein (HDL) cholesterol (Ch. 48).
Improvement in the adverse plasma lipid profile found in diabetes. Metformin increases fatty acid oxidation and reduces plasma triglycerides. It also raises plasma high-density lipoprotein (HDL) cholesterol (Ch. 48).
Metformin can suppress appetite and causes less weight gain than the sulfonylureas, which is useful in overweight people with diabetes.
Pharmacokinetics
Metformin is slowly and incompletely absorbed from the gut and excreted unchanged by the kidney.
Unwanted effects
 Gastrointestinal upset, including anorexia, nausea, abdominal discomfort and diarrhoea (usually transient).
Gastrointestinal upset, including anorexia, nausea, abdominal discomfort and diarrhoea (usually transient).
 Decreased vitamin B12 absorption.
Decreased vitamin B12 absorption.
 Inhibition of pyruvate metabolism encourages lactate accumulation. Lactic acidosis can result in situations that lead to an increase in anaerobic metabolism (e.g. shock with hypoxaemia), and metformin should be avoided in these situations. Lactic acidosis is more common in the presence of renal impairment, although the degree of renal impairment at which this becomes a significant risk is unclear.
Inhibition of pyruvate metabolism encourages lactate accumulation. Lactic acidosis can result in situations that lead to an increase in anaerobic metabolism (e.g. shock with hypoxaemia), and metformin should be avoided in these situations. Lactic acidosis is more common in the presence of renal impairment, although the degree of renal impairment at which this becomes a significant risk is unclear.
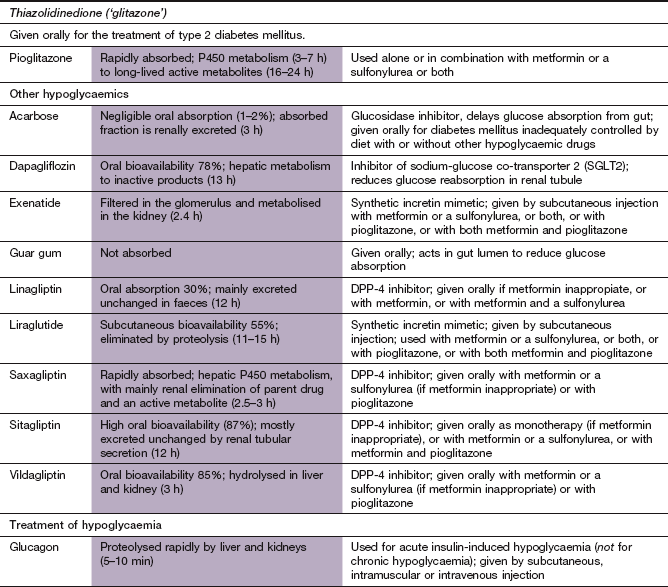
Thiazolidinedione
Mechanisms of action and effects
Pioglitazone has no effect on insulin secretion but is an insulin sensitiser. The effects are mediated through binding to peroxisome proliferator-activated receptor γ (PPAR-γ) in the cell nucleus. PPAR-γ associates as a heterodimer with the retinoid X receptor (RXR) (Ch. 1) in the cell nucleus and binds to PPAR-γ response elements in the promoter domains of target genes. In the absence of a ligand this heterodimer is further associated with a multiprotein co-repressor complex that has histone deacetylase activity and inhibits gene transcription. When a PPAR ligand binds to the PPAR–RXR heterodimer the co-repressor complex dissociates and a co-activator complex with histone acetylase activity is recruited. In the case of PPAR-γ this results in the expression of genes that control adipocyte differentiation, and may increase the number of small adipocytes which are more insulin-sensitive.
The actions of pioglitazone include:
 enhanced insulin sensitivity and glucose utilisation in peripheral tissues, especially in adipocytes but also skeletal muscle and hepatocytes. Adipose tissue more readily takes up triglycerides from the blood. A secondary effect of the reduced availability of non-esterified fatty acids is improvement of insulin sensitivity in muscle cells. Other effects on adipocyte cell signalling may also influence tissue insulin sensitivity. These include reduced synthesis of pro-inflammatory cytokines that interfere with the insulin signalling cascade, such as tumour necrosis factor α and interleukin-6, and an increase in the insulin-sensitising and anti-inflammatory cytokine adiponectin,
enhanced insulin sensitivity and glucose utilisation in peripheral tissues, especially in adipocytes but also skeletal muscle and hepatocytes. Adipose tissue more readily takes up triglycerides from the blood. A secondary effect of the reduced availability of non-esterified fatty acids is improvement of insulin sensitivity in muscle cells. Other effects on adipocyte cell signalling may also influence tissue insulin sensitivity. These include reduced synthesis of pro-inflammatory cytokines that interfere with the insulin signalling cascade, such as tumour necrosis factor α and interleukin-6, and an increase in the insulin-sensitising and anti-inflammatory cytokine adiponectin,
 suppression of gluconeogenesis in the liver by inhibition of fructose-1,6-bisphosphatase,
suppression of gluconeogenesis in the liver by inhibition of fructose-1,6-bisphosphatase,
 in addition to reducing the plasma glucose concentration, the effect on triglycerides also improves diabetic dyslipidaemia. Plasma HDL cholesterol concentration is increased, due to increased lipolysis of triglycerides in very-low-density lipoprotein (VLDL). The plasma low-density lipoprotein (LDL) fraction may also become larger and less dense, which may further reduce atherogenesis (Ch. 48). Overall, fat is redistributed from visceral to subcutaneous stores,
in addition to reducing the plasma glucose concentration, the effect on triglycerides also improves diabetic dyslipidaemia. Plasma HDL cholesterol concentration is increased, due to increased lipolysis of triglycerides in very-low-density lipoprotein (VLDL). The plasma low-density lipoprotein (LDL) fraction may also become larger and less dense, which may further reduce atherogenesis (Ch. 48). Overall, fat is redistributed from visceral to subcutaneous stores,
 a small reduction in blood pressure, possibly by improving endothelial function and reducing sympathetic nervous system activity. There is also a reduction in diabetic microalbuminuria.
a small reduction in blood pressure, possibly by improving endothelial function and reducing sympathetic nervous system activity. There is also a reduction in diabetic microalbuminuria.
Pharmacokinetics
Pioglitazone is well absorbed from the gut and is metabolised in the liver. The half-life (3–7 h) is not related to the duration of action. Since the mechanism of action involves gene transcription, the onset of the hypoglycaemic effect is gradual over 6–8 weeks.
Unwanted effects
 Gastrointestinal disturbances.
Gastrointestinal disturbances.
 Headache, dizziness, visual disturbances.
Headache, dizziness, visual disturbances.
 Fluid retention leading to oedema, which can cause decompensation in heart failure.
Fluid retention leading to oedema, which can cause decompensation in heart failure.
 Weight gain because of fat-cell differentiation.
Weight gain because of fat-cell differentiation.
 Increased risk of fractures, especially in women.
Increased risk of fractures, especially in women.
 Liver dysfunction has been reported rarely, and liver function tests should be monitored during treatment.
Liver dysfunction has been reported rarely, and liver function tests should be monitored during treatment.
Dipeptidyl peptidase-4 inhibitors
Mechanism of action
The ‘gliptins’ are competitive inhibitors of DPP-4 and reduce the ability of the enzyme to inactivate the incretin hormones GLP-1 and GIP. As a consequence, insulin synthesis and secretion are increased and therefore blood glucose levels are decreased and β-cell function is improved.
Pharmacokinetics
DPP-4 inhibitors are rapidly absorbed from the gut. Sitagliptin is excreted by the kidney and linagliptin is excreted largely unchanged in faeces; they have half-lives of about 12 h. Saxagliptin is cleared mainly by P450 metabolism in the liver, whereas vildagliptin undergoes P450-independent hydrolysis in the liver and kidney; both have a half-life of about 3 h. The long duration of action of DPP-4 inhibitors is due to extended binding to the target enzyme.
Glucosidase inhibitor
Mechanism of action and effects
Carbohydrate digestion in the intestine involves several enzymes that sequentially degrade complex polysaccharides such as starch into monosaccharides like glucose. Initial digestion of carbohydrates in the gut lumen is carried out by amylases from the saliva and pancreas. The final digestion of oligosaccharides is carried out by β-galactosidases (including lactase) and various α-glucosidase enzymes (such as maltase, isomaltase, glucoamylase and sucrase, which hydrolyse oligosaccharides) in the small-intestinal brush border. Acarbose competes with dietary oligosaccharides for α-glucosidase enzymes, and has a higher affinity for these enzymes. Binding to the enzymes is reversible, so that digestion and absorption of glucose after a meal is slower than usual but not prevented. As a result, the postprandial peak of blood glucose is reduced and blood glucose concentrations are more stable through the day. Acarbose has no effect on insulin secretion or its tissue action and is less effective for achieving glycaemic control than other oral hypoglycaemic agents. Its use is limited by the high incidence of unwanted effects.
Inhibitor of renal glucose transport
Mechanism of action
Dapagliflozin is a competitive reversible inhibitor of the sodium-glucose co-transporter 2 (SGLT2) in the proximal convoluted tubule of the kidney. It reduces glucose absorption from the tubular filtrate and increases urinary glucose excretion. It does not predispose to hypoglycaemia, and its use is associated with modest weight loss. The place of dapagliflozin in therapy of type 2 diabetes is currently uncertain.
Drugs to increase plasma glucose levels
Mechanism of action and use
Glucagon is a polypeptide synthesised by the α-cells of the pancreatic islets of Langerhans. It binds to specific hepatocyte receptors and activates membrane-bound adenylyl cyclase. The consequent increase in intracellular cAMP leads to inhibition of glycogen synthase. This blocks the effect of insulin on hepatocytes and mobilises stored liver glycogen. Glucagon is used to raise blood glucose in severe acute insulin-induced hypoglycaemia.
Management of type 1 diabetes
The aim of treatment is to maintain a plasma glucose concentration as close to normal as possible. Maintenance treatment of type 1 diabetes should include an appropriate diet with a regulated carbohydrate intake distributed throughout the day. Excess dietary saturated fat should be avoided. The complications of type 1 diabetes can be reduced by close control of the blood glucose concentration using insulin, usually in a basal-bolus regimen (see above). The preferred regimens are those with multiple short-acting insulin doses before meals. Short-acting insulin analogues are used in place of soluble insulin for those who wish to inject shortly before or immediately after meals or when there are episodes of hypoglycaemia at night or between meals. Long-acting insulin analogues are recommended in place of isophane insulin if there is recurrent nocturnal hypoglycaemia or morning hyperglycaemia with difficult daytime control.
The success of the chosen approach can be monitored by measurement of the blood glucose concentrations, often carried out on a finger-prick blood specimen using a blood glucose reagent strip. If peak or trough blood glucose estimations are outside an acceptable range, the insulin regimen should be adjusted, although this should not be done more than once or twice a week. Long-term control of diabetes is usually assessed by the plasma concentration of glycosylated haemoglobin (HbA1c). An HbA1c level greater than 53 mmol⋅mol−1 (upper limit of normal is 42 mmol⋅mol−1) is associated with a higher risk of developing microvascular and neuropathic complications. Hyperglycaemia leads to the glycosylation of proteins which inhibits their function and may promote vascular and neurological damage. Several other mechanisms of vascular and neurological damage may also contribute to the complications of hyperglycaemia. In older people with type 1 diabetes, management of cardiovasular risk factors (see type 2 diabetes below) becomes increasingly important.
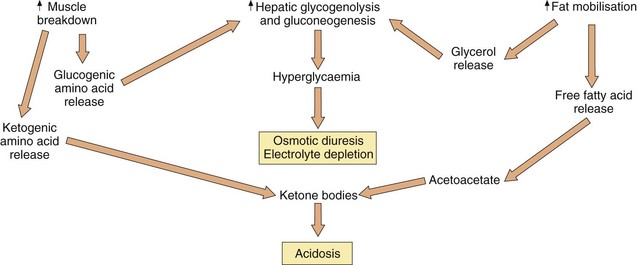
The most dramatic complication of untreated or poorly controlled type 1 diabetes is diabetic ketoacidosis (Fig. 40.2), which can lead to coma if it is severe. Systemic infection, dietary indiscretion or inappropriate insulin dose reduction or omission can precipitate ketoacidosis in a person with treated type 1 diabetes. Apart from the treatment of any precipitating cause, the management of ketoacidosis includes:
 restoration of extracellular volume: hyperglycaemia leads to an osmotic diuresis with excessive urinary salt and water loss. Replacement by isotonic (0.9%) saline is essential,
restoration of extracellular volume: hyperglycaemia leads to an osmotic diuresis with excessive urinary salt and water loss. Replacement by isotonic (0.9%) saline is essential,
 potassium replacement: the osmotic diuresis results in excessive urinary potassium loss. Potassium is also shifted from within cells into extracellular fluid in exchange for hydrogen ions in the ketoacidotic state. Correction of the extracellular acidosis reverses this shift and can produce profound hypokalaemia. Once a good urine flow has been established, intravenous potassium supplements are usually required,
potassium replacement: the osmotic diuresis results in excessive urinary potassium loss. Potassium is also shifted from within cells into extracellular fluid in exchange for hydrogen ions in the ketoacidotic state. Correction of the extracellular acidosis reverses this shift and can produce profound hypokalaemia. Once a good urine flow has been established, intravenous potassium supplements are usually required,
 intravenous insulin until the ketosis is abolished and the plasma glucose is below 15 mmol⋅L−1. An intermediate-acting insulin should be continued subcutaneously during the infusion to maintain a basal blood insulin concentration. The metabolic acidosis will usually correct with treatment of the hyperglycaemia and fluid replacement. Intravenous sodium bicarbonate is occasionally required if the arterial pH is less than 7.0, but should be used with caution.
intravenous insulin until the ketosis is abolished and the plasma glucose is below 15 mmol⋅L−1. An intermediate-acting insulin should be continued subcutaneously during the infusion to maintain a basal blood insulin concentration. The metabolic acidosis will usually correct with treatment of the hyperglycaemia and fluid replacement. Intravenous sodium bicarbonate is occasionally required if the arterial pH is less than 7.0, but should be used with caution.
Management of type 1 diabetes in special situations
 Close attention to diabetic control is important before conception and during pregnancy because poor control will affect the fetus, leading to increased intra-uterine and perinatal mortality.
Close attention to diabetic control is important before conception and during pregnancy because poor control will affect the fetus, leading to increased intra-uterine and perinatal mortality.
 At times of intercurrent illness, the dose of insulin will need to be increased, guided by blood glucose monitoring, to counteract the hyperglycaemic action of hormones released during stress reactions.
At times of intercurrent illness, the dose of insulin will need to be increased, guided by blood glucose monitoring, to counteract the hyperglycaemic action of hormones released during stress reactions.
 During and after surgery, soluble insulin should be given in 10% glucose solution by intravenous infusion, dosage being guided by the blood glucose concentrations. Subcutaneous insulin can be restarted as soon as the person is able to eat and drink.
During and after surgery, soluble insulin should be given in 10% glucose solution by intravenous infusion, dosage being guided by the blood glucose concentrations. Subcutaneous insulin can be restarted as soon as the person is able to eat and drink.
Management of type 2 diabetes mellitus
The mainstays of treatment are lifestyle and dietary modifications. As for type 1 diabetes, close control of the blood glucose concentration in type 2 diabetes reduces the risk of microvascular complications, although the effect on macrovascular complications such as myocardial infarction is less convincing. The target HbA1c concentration is 48 mmol⋅mol−1.
More than 75% of people with newly diagnosed type 2 diabetes are obese. Weight reduction not only improves blood glucose levels but also reduces other cardiovascular disease risk factors. Dietary advice should include:
 reducing energy intake if obese (an average weight loss of 18 kg is required to control blood glucose),
reducing energy intake if obese (an average weight loss of 18 kg is required to control blood glucose),
 ensuring that more than half the total energy intake is from carbohydrates, total fat contributing less than 35% of total energy intake,
ensuring that more than half the total energy intake is from carbohydrates, total fat contributing less than 35% of total energy intake,
 encouraging high-fibre foods and limiting sucrose and alcohol intake.
encouraging high-fibre foods and limiting sucrose and alcohol intake.
This should be combined with advice to exercise regularly and to stop smoking (because of the contribution to vascular disease) as appropriate. Lifestyle and dietary advice should initially be encouraged for obese people, with use of metformin if this fails. There is some evidence that metformin may reduce the risk of cardiovascular disease in obese people with type 2 diabetes. Combination therapy with a sulfonylurea and metformin can be useful if a single drug is insufficient to reduce the blood glucose concentration. By contrast, underweight people with type 2 diabetes often require early treatment with an oral hypoglycaemic agent, usually a sulfonylurea.
Pioglitazone should be considered if there is intolerance to combination therapy with metformin plus a sulfonylurea (replacing the drug to which there is intolerance). Failure of such combinations usually indicates declining insulin release and the need for exogenous insulin, although for some overweight people the combination of a sulfonylurea, metformin and pioglitazone may be helpful. The meglitinides are used alone for non-obese people with diabetes or when metformin is contraindicated. In other individuals meglitinides can be given in combination with metformin. A DPP-4 inhibitor such as sitagliptin can be considered when metformin alone is inadequate, but preferably in people without marked obesity. They can also be added to metformin with a sulfonylurea if insulin is not acceptable.
Within 3 years of diagnosis, 50% of individuals with type 2 diabetes will need combination therapy to achieve glycaemic control. Failure of oral treatment usually implies β-cell ‘exhaustion’ and up to 30% of those with type 2 diabetes require insulin with or without an oral hypoglycaemic drug. The most effective combination is a basal dose of intermediate-acting insulin at bedtime combined with metformin during the day. If the HbA1c is greater than 75 mmol⋅mol−1 then biphasic insulin should be considered. A sulfonylurea can be used with insulin if metformin is contraindicated, but with a greater risk of hypoglycaemia and eventual loss of efficacy as β-cell exhaustion progresses. Combination therapy with insulin and a glitazone or a meglitinide has not been well studied. There is some evidence that insulin therapy is more likely to be successful if used early in type 2 diabetes to preserve β-cell function.
A GLP-1 mimetic, such as exenatide, is most appropriate for obese people for whom insulin is being considered. It can be added to metformin with a sulfonylurea, or used in combination with insulin. A GLP-1 mimetic is less likely to be effective if the diabetes has been present for many years, because β-cell exhaustion is often present.
Acarbose is of limited value when used alone or in combination with metformin. It may be most effective in early diabetes, when there is still sufficient insulin secretion for it to influence glycaemic control.
Intensive management of risk factors for cardiovascular disease is of crucial importance because the major complications of type 2 diabetes are vascular. In particular, control of raised blood pressure reduces both microvascular and macrovascular complications. There is little to choose between antihypertensive drugs for use in diabetes, except that thiazides and β-adrenoceptor antagonists may aggravate diabetes and should probably be avoided in the few people who are managed with dietary control alone. Use of an angiotensin-converting enzyme (ACE) inhibitor or an angiotensin II receptor antagonist reduces the risk of renal failure when there is evidence of diabetic nephropathy (either overt or with microalbuminuria) (Ch. 6). Treatment of the abnormal atherogenic plasma lipid profile that is common in type 2 diabetes is recommended for people over the age of 40 years (Ch. 48). Once a person with diabetes has developed coronary artery disease then management of all risk factors (Ch. 48) will reduce the risk of subsequent myocardial infarction or death to the same extent as for someone without diabetes.
True/false questions
1. Oral hypoglycaemic drugs are only used in type 2 diabetes.
2. Glibenclamide is the drug of choice when there is no residual insulin secretion.
3. Sulfonylureas should be administered in conjunction with a dietary regimen in obese people.
4. Glibenclamide can cause hypoglycaemia, particularly in the elderly.
5. Metformin and the sulfonylurea gliclazide cannot be taken together.
6. The meglitinides are structurally related to glibenclamide.
7. Oral hypoglycaemics given to a pregnant mother can cause hypoglycaemia in the fetus.
8. Sulfonylureas should not be given together with the antibacterial trimethoprim.
9. Insulin lispro has a longer duration of action than isophane insulin.
10. Dipeptidyl peptidase-4 (DPP-4) synthesises incretin hormones.
11. Acarbose is completely absorbed from the gut after oral administration.
One-best-answer (OBA) question
Ms JJ is a 55-year-old housewife with a body mass index (BMI) of 35 kg⋅m−2. She was diagnosed with type 2 diabetes mellitus. Choose the correct statement below.
Case-based questions
A 25-year-old teacher, Mr JAH, was admitted to hospital as an emergency. He had developed a sore throat a week previously. His GP prescribed penicillin, but the soreness persisted and Mr JAH noticed profuse white spots on the back of his throat. He drank fluids copiously and passed more urine than usual. Two days before admission, he began to vomit, and on the day before admission he became drowsy and confused. He had lost approximately 12 kg in weight, despite eating more than usual. His great uncle had diabetes mellitus. Mr JAH was clinically dehydrated and ketones could be smelt on his breath. Results of blood tests indicated that he had diabetic ketoacidosis.
A Which type of diabetes does Mr JAH have?
B What was the significance of his sore throat?
C Was it significant that his great uncle suffered from diabetes mellitus?
D Explain his polydipsia and polyuria.
E What treatments should have been instituted rapidly?
F After he had recovered from the acute illness, what general advice should have been given about diet?
G Mr JAH was a ‘three-meals-a-day’ man whose only exercise was walking a mile to work and back each day. Although insulin regimens vary widely, suggest a possible regimen and the types of insulin that could be given.
H How long before meals should subcutaneous injection of soluble insulin have been given?
I In addition to blood glucose levels, what other indicator could have been measured to signify good control in diabetes?
Mr JAH became more active, joined a health club and met a partner who liked to party. His eating became more irregular with hurried meals. His glycaemic control deteriorated.
J What alterations to his insulin regimen could have been helpful?
1. True. Oral hypoglycaemic drugs (sulfonylureas, biguanides, meglitinides, thiazolidinediones, dipeptidyl peptidase-4 inhibitors) are only used in type 2 diabetes and act by different mechanisms to control glucose levels.
2. False. Glipizide is a sulfonylurea which stimulates insulin secretion from the islet β-cells and would be ineffective in the absence of any insulin-secreting ability.
3. True. Sulfonylureas cause weight gain partly by stimulating appetite; metformin might be a better choice.
4. True. Glibenclamide has a long duration of action and active metabolites can accumulate when renal function declines; hypoglycaemia is a greater problem in the elderly.
5. False. These drugs act in part by different mechanisms and can be combined. Unlike the sulfonylureas, metformin has a neutral or suppressive effect on appetite.
6. True. Meglitinides (glinides) chemically resemble the sulfonylurea moiety of glibenclamide.
7. True. Neonates born to mothers with diabetes who are taking oral hypoglycaemics in pregnancy have problems with hypoglycaemia; insulin is normally substituted in pregnancy.
8. True. Sulfonylureas have some structural similarities to the sulphonamides and trimethoprim and can produce severe hypoglycaemia when given together.
9. False. Isophane insulin is complexed with protamine and has a duration of action of 20 h, whereas synthetic insulin lispro is modified structurally and has a faster onset of action and shorter duration.
10. False. DPP-4 breaks down the incretin glucagon-like peptide-1 (GLP-1), so DPP-4 inhibitors such as sitagliptin enhance incretin activity on the islet β-cells.
11. False. Acarbose is very poorly absorbed and acts within the gut to reduce the digestion of glicose by α-glucosidases.
12. True. Glucagon reverses the effect of insulin on liver glycogen storage and is used to increase blood glucose in severe acute hypoglycaemia induced by insulin.
OBA answer
A Correct. Diet and exercise should be tried for 3 months before suggesting other treatments.
B Incorrect. Treatment, support and advice should take place over many months.
C Incorrect. Sulfonylureas can stimulate appetite by increasing insulin secretion and cause further weight gain.
D Incorrect. The use of pioglitazone as second-line therapy added to either metformin or a sulfonylurea is not recommended, except for people who are unable to tolerate metformin and sulfonylurea combination therapy, or people in whom either drug is contraindicated; in such cases, the thiazolidinedione should replace the poorly tolerated or contraindicated drug.
E Incorrect. Metformin has a cardioprotective effect which is not wholly explicable by its effects on glucose and may be due to improvements in the lipid profile.
Case-based answers
A The ketoacidosis indicates that Mr JAH has type 1 diabetes mellitus.
B An upper respiratory tract infection can be all that is necessary to precipitate ketoacidosis. Aggravating factors include the candidiasis in his throat, and overbreathing, causing dryness.
C There is a strong familial tendency, but neither type 1 nor type 2 diabetes mellitus is a single-gene disorder, so there is no classic pattern of inheritance.
D Once the tubular transport maximum for glucose reabsorption in the kidneys is exceeded the glucose in the distal tubules causes an osmotic diuresis, leading to polyuria and then to thirst.
E Insulin, fluids and salts should be given to correct dehydration, glucose levels, ketoacidosis and electrolyte imbalances. Ketoacidosis can lead to coma.
F A dietary regimen should be agreed to create a stable pattern of eating habits commensurate with his lifestyle. Diets low in animal fat and high in fibre are recommended, ideally with carbohydrate intake distributed throughout the day.
G Initiate a stable pattern of eating habit and activity, and twice-daily subcutaneous insulin injections before breakfast and evening meal. The insulin regimen would contain a mixture of short- and long-acting insulins, the ratios of which vary depending upon his glucose levels. Insulins frequently used are soluble insulin and isophane insulin.
H The time to onset of activity of neutral soluble insulin is 30 min, with peak activity at 1–3 h.
I The amount of glycosylated haemoglobin (HbA1c) can be measured. High concentrations indicate an increased risk of microvascular and neuropathic complications.
J Rapid-acting monomeric insulin lispro may be helpful. This has a time to onset of only 15 min and a time to peak plasma levels of 0.5–0.75 h, so should be given immediately before a meal. Insulin lispro during the day with insulin isophane in the evening is a possible regimen, but education about eating and lifestyle would probably provide greater benefit than a change of insulin regimen.
Bailey, CJ. The challenge of managing coexistent type 2 diabetes and obesity. BMJ. 2011;342:d1996.
Beckman, JA, Creager, MA, Libby, P. Diabetes and atherosclerosis. Epidemiology, pathophysiology, and management. JAMA. 2002;287:2570–2581.
Beigi, FI. Glycemic management of type 2 diabetes mellitus. N Engl J Med. 2012;366:1319–1327.
Bennett, WL, Maruthur, SS, Singh, S, et al. Comparative effectiveness and safety of medications for type 2 diabetes: update including new drugs and 2-drug combinations. Ann Intern Med. 2011;154:602–613.
Blood Pressure Lowering Treatment Trialists’ Collaboration. Effects of different blood pressure-lowering regimens on major cardiovascular events in individuals with and without diabetes mellitus. Arch Intern Med. 2005;165:1410–1419.
Daneman, D. Type 1 diabetes. Lancet. 2006;367:847–858.
De Witt, DE, Hirsch, IB. Outpatient insulin therapy in type 1 and type 2 diabetes mellitus. JAMA. 2003;289:2254–2264.
Friedland, SN, Leong, A, Filion, KB, et al. The cardiovascular effects of peroxisome proliferator-activated receptor antagonists. Am J Med. 2012;125:126–133.
Gale, EAM. Newer insulins in type 2 diabetes. BMJ. 2012;345:e4611.
Heine, RJ, Diamant, M, Mbanya, J-C, et al. Management of hyperglycaemia in type 2 diabetes. BMJ. 2006;333:1200–1204.
Kelly, TN, Bazzano, LA, Fonseca, VA, et al. Systematic review: glucose control and cardiovascular disease in type 2 diabetes. Ann Intern Med. 2009;151:394–403.
Metchick, LN, Petit, WA, Jr., Inzucchi, SE. Inpatient management of diabetes mellitus. Am J Med. 2002;113:317–323.
Pickup, JC. Insulin-pump therapy for type 1 diabetes mellitus. N Engl J Med. 2012;366:1616–1624.
Plank, J, Siebenhofer, A, Berghold, A, et al. Systematic review and meta-analysis of short-acting insulin analogues in patients with diabetes mellitus. Arch Intern Med. 2005;165:1337–1344.
Selvin, E, Bolen, S, Yeh, H-C, et al. Cardiovascular outcomes in trials of oral diabetes medications: a systematic review. JAMA. 2008;168:2070–2080.
Snow, V, Weiss, KB, Mottur-Pilson, C, et al. Evidence for tight blood pressure control in type 2 diabetes mellitus. Ann Intern Med. 2003;138:587–592.
Stumvoll, M, Goldstein, BJ, van Haeften, TW. Type 2 diabetes: principles of pathogenesis and therapy. Lancet. 2005;365:1333–1346.
Tahrani, AA, Bailey, CJ, Del Prato, S, et al. Management of type 2 diabetes: new and future developments in treatment. Lancet. 2011;378:182–197.
Verspohl, EJ. Novel therapeutics for type 2 diabetes: incretin hormone mimetics (glucagon-like peptide-1 receptor agonists) and dipeptidyl peptidase-4 inhibitors. Pharmacol Ther. 2009;124:113–138.
Wilding, JPH, Hardy, K. Glucagon-like peptide-1 analogues for type 2 diabetes. BMJ. 2011;342:d410.