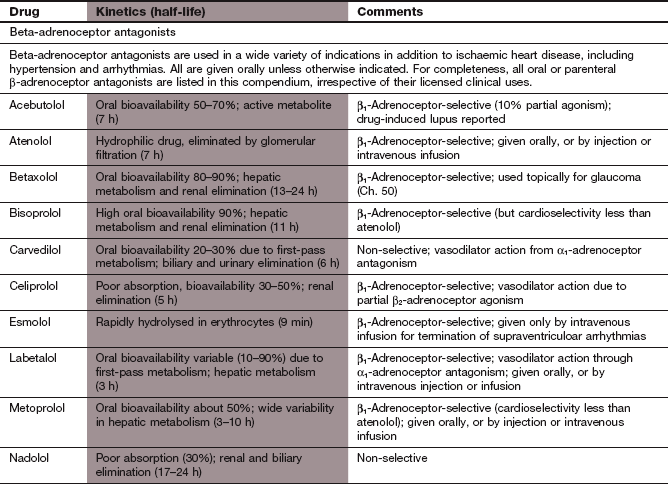
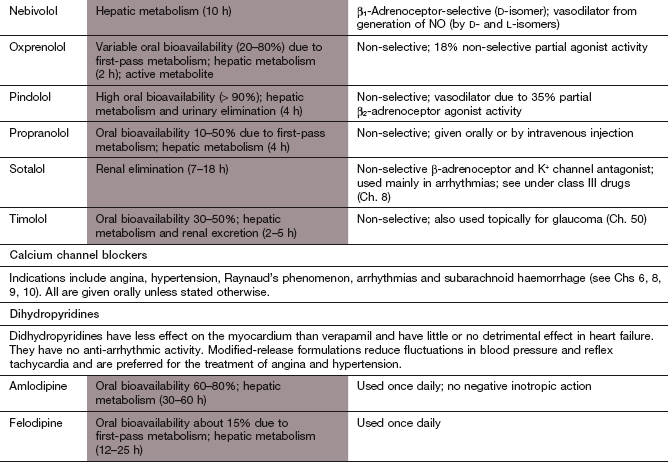
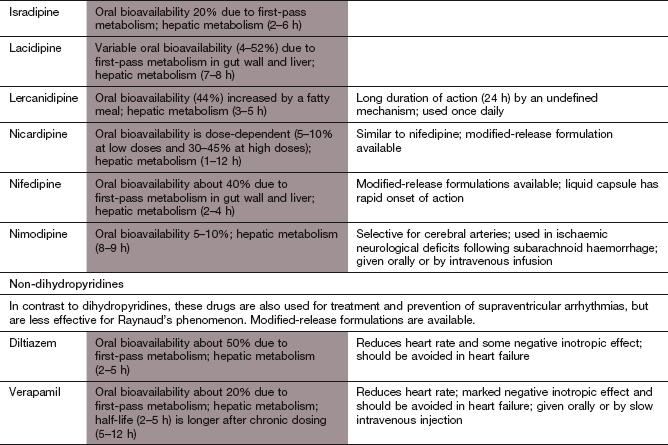
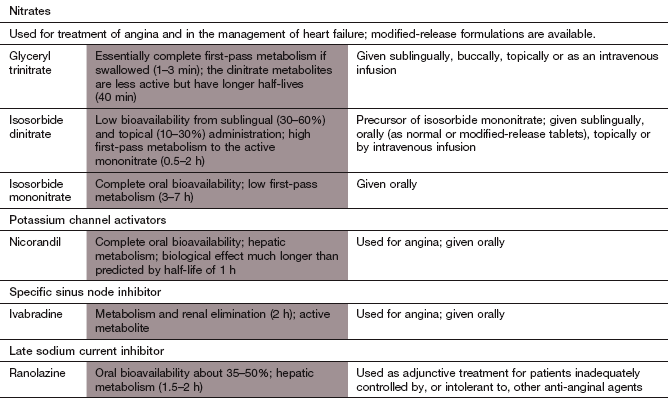
Ischaemic heart disease
The heart receives about 5% of the cardiac output at rest via the coronary arteries, and extracts about 75% of the oxygen from the perfusing blood. When the metabolic demand from the myocardium becomes greater (for example with exercise) there is little increase in the percentage of oxygen extracted from the blood passing through the myocardium and coronary artery blood flow increases by up to three- to fourfold to supply the necessary oxygen. Myocardial perfusion occurs largely during diastole, when the muscle of the heart is relaxed and not compressing the intramyocardial vessels. Therefore, unlike for other organs, myocardial perfusion is reliant on the diastolic blood pressure.
Ischaemic heart disease most frequently arises as a result of restriction of blood flow to cardiac muscle by development of atheromatous plaques in the large epicardial coronary arteries (Fig. 5.1). Myocardial ischaemia can sometimes occur in the presence of structurally normal epicardial coronary arteries. In this situation it arises either from abnormal regulation of the microvascular circulation within the myocardium, or from intense vasoconstriction of an epicardial artery (coronary vasospasm).
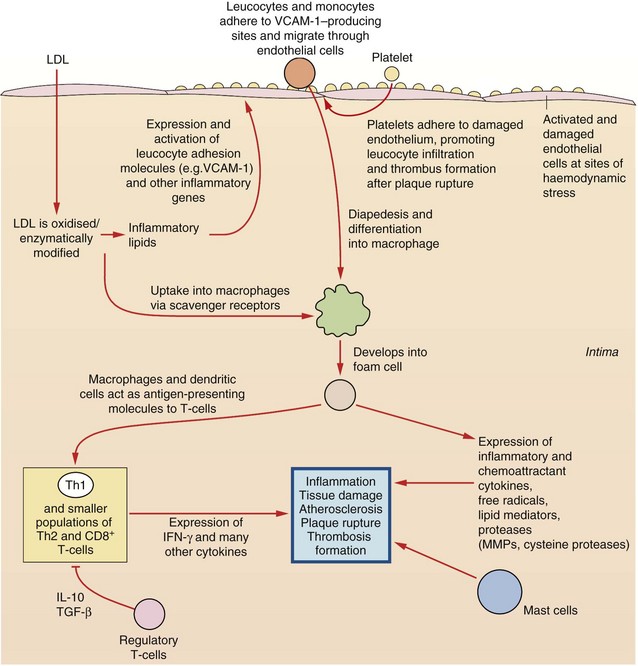
Fig. 5.1 Aspects of inflammatory processes that contribute to coronary heart disease.
Multifactorial processes contribute to coronary heart disease; endothelium is damaged and activated; platelets adhere and promote leucocyte infiltration and thrombus formation; low-density lipoprotein (LDL) is oxidised and taken up via scavenger receptors into monocyte-macrophages, subsequently forming foam cells. Dysfunctional expression of a host of cytokines, lipid mediators, free radicals and proteases exacerbates inflammation, endothelial damage, atheroma formation, plaque rupture and thrombus formation. These processes are influenced by risk factors such as smoking, heredity, hypercholesterolaemia, hypertension, obesity, diabetes, age and gender. IFN-γ, interferon-γ; IL-10, interleukin-10; MMPs, matrix metalloproteases; TGF-β, tumour growth factor β; Th, T-helper cell; VCAM-1, vascular cell adhesion molecule 1.
Atheromatous plaques tend to form in areas of flow disturbance, such as bends in the vessels or near branching vessels. The major risk factors for atheromatous coronary artery disease (in common with atheroma in other parts of the vascular tree) are male sex, smoking, hypertension, hypercholesterolaemia and diabetes mellitus. The effects of these risk factors are additive, and when several are present coronary atheroma occurs more extensively and at a younger age.
Early atheromatous plaques enlarge by stretching the medial smooth muscle (remodelling) and do not narrow the lumen of the vessel until 40–50% of the cross-sectional area of the vessel is diseased. Even when luminal narrowing occurs, symptoms only arise when 75% of the cross-sectional area of the vessel lumen is occluded.
Although atheroma can diffusely involve a long segment of the vessel, plaques are often confined to a small segment of the coronary artery. Localised plaques frequently involve only part of the circumference of the arterial wall, leaving the rest free of significant disease and still able to respond to vasoconstrictor and vasodilator influences. At the site of an atheromatous plaque there is turbulent blood flow. The consequent changes in shear stress at the endothelial surface impair endothelial function and reduce local generation of vasodilator substances such as nitric oxide (see organic nitrates below). Therefore, diseased segments of an artery are particularly prone to vasospasm, which produces dynamic flow limitation superimposed on the fixed atheromatous narrowing. If the coronary artery disease is long-standing, then collateral vessels can develop around the atheromatous narrowing and improve perfusion distal to the diseased segment of the artery.
There are two morphological types of atheromatous plaque. Some have a lipid-rich core, with a substantial infiltration of inflammatory cells and a thin fibrous cap. Such plaques are relatively unstable (‘vulnerable’ plaques) and are more prone to plaque disruption by ulceration or rupture of the cap, leading to thrombus formation (see below). Other plaques have a fibrotic core, with a thick fibrous cap, and are more stable. The reasons why both stable and unstable plaques can coexist in the coronary circulation is not well understood.
Reversible myocardial ischaemia is the consequence of an imbalance between oxygen supply and oxygen demand in a part of the myocardium (Fig. 5.2) due to an inability to increase coronary blood flow sufficiently to meet the metabolic demands of the heart. Rupture of an atheromatous plaque is responsible for most acute ischaemic cardiac events (presenting as an acute coronary syndrome).
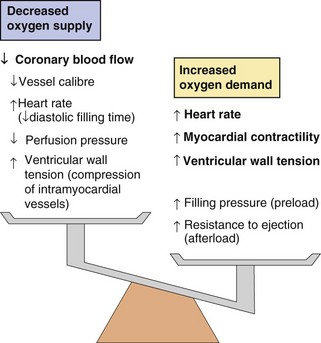
Fig. 5.2 Factors increasing myocardial oxygen demand and decreasing myocardial oxygen supply in angina.
Anti-anginal drugs act at many different sites to reduce oxygen demand and increase oxygen supply.
Clinical manifestations of myocardial ischaemia
Angina pectoris is pain arising from heart muscle after it switches to anaerobic metabolism, and is a symptom of reversible myocardial ischaemia. Ischaemia occurs once the coronary artery lumen is narrowed sufficiently to restrict maximal blood flow to a level that cannot deliver adequate oxygen to meet the metabolic needs of the myocardium. Stable angina is relatively predictable ischaemic chest pain that is most frequently experienced as chest pain on exertion or with emotional stress and is rapidly relieved by rest. Reversible myocardial ischaemia can also present with shortness of breath (due to diastolic stiffening of the left ventricle when a reduced cellular energy supply impairs the uptake of Ca2+ by the sarcoplasmic reticulum; see also heart failure with preserved ejection fraction, Ch. 7), or it can occur without symptoms (silent ischaemia). Vasospasm at the site of an atheromatous plaque accentuates the reduction in flow produced by a fixed atheromatous obstruction, and when it is present angina occurs at a lower workload.
People with stable angina have an increased risk of subsequent myocardial infarction or sudden cardiac death, due to rupture of an unstable atheromatous plaque (see below). On average the annual rate of such events is about 2%.
Acute coronary syndromes (unstable angina, myocardial infarction and sudden death)
Acute coronary syndromes have a common pathophysiological origin, arising from disruption of an unstable atheromatous plaque (vulnerable plaque) in a coronary artery. Plaque disruption can be precipitated by sudden stresses on the cap produced by pulsatile blood flow across the plaque, by elastic recoil of the vessel in diastole or by vasospasm. As a consequence of these stresses the thin cap over the plaque fissures or ulcerates, leading to plaque rupture and exposure of the core of the plaque to circulating blood. Plaque rupture initiates platelet adhesion and then aggregation (Ch. 11), followed by thrombus formation and local vasospasm. These processes lead to a sudden reduction in blood flow. Platelet–thrombin microemboli can break off from the thrombus and become lodged in small distal vessels downstream from the thrombus, contributing to ischaemia.
Unstable angina
Unstable angina occurs if there is incomplete occlusion of the coronary artery following plaque rupture, but with critical reduction in blood flow so that oxygen supply is inadequate at rest or on minimal stress. Angina may then occur at rest or with very little exertion. Unstable angina is distinguished pathologically from other acute coronary syndromes because perfusion of the ischaemic tissue remains sufficient to prevent necrosis of myocytes. Unlike myocardial infarction, symptoms of unstable angina are usually relieved by glyceryl trinitrate (see below), or resolve spontaneously within 30 min.
Following an episode of unstable angina the thrombus may become incorporated into the plaque so that after healing the plaque is substantially larger, leading to greater long-term luminal narrowing.
Myocardial infarction and sudden cardiac death
Myocardial infarction most commonly arises from complete coronary artery occlusion following disruption of an unstable atheromatous plaque. Occlusion often occurs at the site of an atheromatous lesion that previously was only producing minor or moderate stenosis of the artery and may not have caused symptoms prior to disruption. Muscle necrosis begins when the occlusion lasts for longer than 20–30 min.
Myocardial infarction is usually associated with intense, prolonged chest pain and sympathetic nervous stimulation which increases cardiac work. However, about 15% of infarctions do not present with pain, and may go unrecognised (silent infarction). The diagnosis of acute myocardial infarction requires a rise in the plasma concentrations of sensitive biochemical markers, such as cardiac-specific myoglobin or troponin, which are released from necrotic myocytes. Cell death begins in the subendocardial muscle which is furthest from the epicardial blood supply (the endocardium receives its oxygen from the ventricular cavity), and, unless perfusion is restored, it progressively extends across the full thickness of the myocardium (transmurally) over the next few hours. Activation of endogenous fibrinolysis (Ch. 11) and the presence of a good collateral circulation are factors that favour reperfusion of the ischaemic area and naturally limit the size of the infarct. If very early reperfusion occurs the damage is usually confined to the subendocardial myocardium.
A full-thickness (or transmural) myocardial infarction often produces characteristic changes on the electrocardiograph (ECG), with early ST-segment elevation and eventually pathological Q waves. The resulting infarction is referred to as an ST-elevation myocardial infarction (STEMI). A subendocardial infarction often presents without diagnostic ECG changes. In these cases the ECG may show ST-segment depression or T-wave inversion (consistent with myocardial ischaemia), or even be normal. The resulting infarction is classified as a non-ST-elevation myocardial infarction (NSTEMI), because of the absence of the characteristic ST-segment changes usually found with more extensive myocardial damage.
Myocardial infarction principally affects left ventricular muscle, and the amount of muscle lost correlates well with both early and late survival. Infarction of the anterior muscle of the left ventricle (usually resulting from an occlusion in the left coronary artery system) causes greater myocardial loss than does inferior infarction of the ventricle (usually from right coronary artery occlusion). The amount of muscle loss also determines the extent of left ventricular remodelling (a geometrical change in the left ventricle that begins with healing of the infarct), which determines the risk of subsequent heart failure.
Sudden cardiac death results when fatal ventricular arrhythmias arise from ischaemic tissue, or from ventricular rupture.
Drug treatment of angina
Drug treatment for angina is directed either:
Drugs can be taken to relieve the ischaemia rapidly during an acute attack or as regular prophylaxis to reduce the risk of subsequent episodes. Several classes of drug are used to treat angina.
Organic nitrates
Mechanism of action and effects
The organic nitrates are vasodilators that relax vascular smooth muscle by mimicking the effects of endogenous nitric oxide (NO). Enzymatic degradation of the nitrate releases NO, which combines with thiol groups in vascular endothelium to form nitrosothiols. Nitrosothiols activate guanylyl cyclase, which generates the second messenger cyclic guanosine monophosphate (cGMP; Fig. 5.3). cGMP activates protein kinase G, which reduces the availability of intracellular Ca2+ to the contractile mechanism of vascular smooth muscle, causing relaxation and vasodilation. Vasodilation is produced in three main vascular beds.
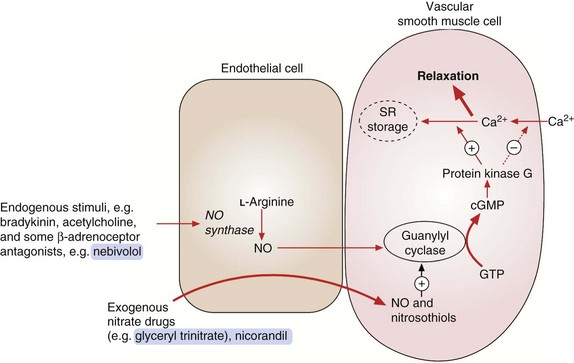
Fig. 5.3 Actions of endogenous nitric oxide (NO) and exogenous nitrates.
Endogenous NO from endothelial cells relaxes vascular smooth muscle by activating guanylyl cyclase with subsequent formation of cGMP. This activates protein kinase G, which decreases Ca2+ influx into the cell, increases Ca2+ storage in the sarcoplasmic reticulum (SR) and increases myosin light-chain dephosphorylation. Exogenous agents such as organic nitrates and nicorandil react with tissue thiols, generating NO or nitrosothiols, which then activate guanylyl cyclase and increase cGMP.
 Venous capacitance vessels, leading to peripheral pooling of blood and reduced venous return to the heart. This lowers left ventricular filling pressure (preload), decreases ventricular wall tension and therefore reduces myocardial oxygen demand. Venous dilation is produced at moderate plasma nitrate concentrations, and tolerance to this action occurs rapidly during continued treatment.
Venous capacitance vessels, leading to peripheral pooling of blood and reduced venous return to the heart. This lowers left ventricular filling pressure (preload), decreases ventricular wall tension and therefore reduces myocardial oxygen demand. Venous dilation is produced at moderate plasma nitrate concentrations, and tolerance to this action occurs rapidly during continued treatment.
 Arterial resistance vessels, leading to reduced resistance to left ventricular emptying (afterload). This lowers blood pressure, decreases cardiac work and contributes to a reduced myocardial oxygen demand. Arterial dilation occurs at higher plasma nitrate concentrations than venodilation, but tolerance arises less readily during long-term treatment.
Arterial resistance vessels, leading to reduced resistance to left ventricular emptying (afterload). This lowers blood pressure, decreases cardiac work and contributes to a reduced myocardial oxygen demand. Arterial dilation occurs at higher plasma nitrate concentrations than venodilation, but tolerance arises less readily during long-term treatment.
 Coronary arteries. Nitrates have little effect on total coronary blood flow in angina; indeed, flow may be reduced because of a decrease in perfusion pressure. However, blood flow through collateral vessels may be improved, and nitrates also relieve coronary artery vasospasm. The net effect is increased blood supply to ischaemic areas of the myocardium. Coronary artery dilation occurs at low plasma nitrate concentrations, and tolerance is slow to develop.
Coronary arteries. Nitrates have little effect on total coronary blood flow in angina; indeed, flow may be reduced because of a decrease in perfusion pressure. However, blood flow through collateral vessels may be improved, and nitrates also relieve coronary artery vasospasm. The net effect is increased blood supply to ischaemic areas of the myocardium. Coronary artery dilation occurs at low plasma nitrate concentrations, and tolerance is slow to develop.
Pharmacokinetics
Glyceryl trinitrate (GTN) is the most widely used organic nitrate. It is well absorbed from the gut but undergoes extensive first-pass metabolism in the liver to inactive metabolites. To increase its bioavailability, GTN is given by one of four routes that avoid first-pass metabolism.
 Sublingual: the tablet is placed under the tongue and is absorbed rapidly across the buccal mucosa. The very short half-life of GTN (less than 5 min) limits the duration of action to approximately 30 min. Tablets lose their potency with prolonged storage, and a metered-dose aerosol spray is a more stable delivery method.
Sublingual: the tablet is placed under the tongue and is absorbed rapidly across the buccal mucosa. The very short half-life of GTN (less than 5 min) limits the duration of action to approximately 30 min. Tablets lose their potency with prolonged storage, and a metered-dose aerosol spray is a more stable delivery method.
 Buccal: a tablet containing GTN in an inert polymer matrix is held between the upper lip and gum, which permits slow release of drug to prolong the duration of action.
Buccal: a tablet containing GTN in an inert polymer matrix is held between the upper lip and gum, which permits slow release of drug to prolong the duration of action.
 Transdermal: GTN is absorbed well through the skin and can be delivered from an adhesive patch via a rate-limiting membrane or matrix. Steady release of the drug maintains a stable blood concentration for at least 24 h after application of the patch.
Transdermal: GTN is absorbed well through the skin and can be delivered from an adhesive patch via a rate-limiting membrane or matrix. Steady release of the drug maintains a stable blood concentration for at least 24 h after application of the patch.
 Intravenous: the short duration of action of GTN is an advantage for intravenous dose titration.
Intravenous: the short duration of action of GTN is an advantage for intravenous dose titration.
Isosorbide 5-mononitrate is well absorbed from the gut and does not undergo first-pass metabolism. It has a half-life of 3–7 h so modified-release formulations are often used to prolong the duration of action.
Unwanted effects
 Venodilation can produce postural hypotension, dizziness, syncope and reflex tachycardia. Tachycardia can be reduced by concurrent use of a β-adrenoceptor antagonist.
Venodilation can produce postural hypotension, dizziness, syncope and reflex tachycardia. Tachycardia can be reduced by concurrent use of a β-adrenoceptor antagonist.
 Arterial dilation causes throbbing headaches and flushing, but tolerance to these effects is common during treatment with long-acting nitrates.
Arterial dilation causes throbbing headaches and flushing, but tolerance to these effects is common during treatment with long-acting nitrates.
 Tolerance to the therapeutic effects of nitrates develops rapidly if there is a sustained high plasma nitrate concentration. Tolerance is therefore a particular problem with delivery of GTN via transdermal patches or with long-acting nitrates. The cause is incompletely understood, but an important mechanism may be increased degradation of NO by oxygen free radicals (e.g. superoxides). There is limited evidence that co-administration of an angiotensin-converting enzyme (ACE) inhibitor, angiotensin receptor antagonist or hydralazine (Ch. 6) may reduce nitrate tolerance by impairing superoxide formation. Reflex activation of the sympathetic nervous system and the renin–angiotensin system in response to hypotension may also counteract the vasodilator actions of the nitrates. Tolerance can be avoided by a ‘nitrate-low’ period of several hours in each 24 h. This is preferable to a ‘nitrate-free’ period, which carries a risk of rebound angina. A nitrate-low period is achieved by asymmetric dosing with conventional formulations of isosorbide mononitrate (e.g. twice daily, at 8 a.m. and 1 p.m.) or by using a once-daily formulation that allows plasma nitrate concentrations to fall overnight. Transdermal GTN patches must be removed for part of each 24 h (e.g. overnight) to prevent tolerance.
Tolerance to the therapeutic effects of nitrates develops rapidly if there is a sustained high plasma nitrate concentration. Tolerance is therefore a particular problem with delivery of GTN via transdermal patches or with long-acting nitrates. The cause is incompletely understood, but an important mechanism may be increased degradation of NO by oxygen free radicals (e.g. superoxides). There is limited evidence that co-administration of an angiotensin-converting enzyme (ACE) inhibitor, angiotensin receptor antagonist or hydralazine (Ch. 6) may reduce nitrate tolerance by impairing superoxide formation. Reflex activation of the sympathetic nervous system and the renin–angiotensin system in response to hypotension may also counteract the vasodilator actions of the nitrates. Tolerance can be avoided by a ‘nitrate-low’ period of several hours in each 24 h. This is preferable to a ‘nitrate-free’ period, which carries a risk of rebound angina. A nitrate-low period is achieved by asymmetric dosing with conventional formulations of isosorbide mononitrate (e.g. twice daily, at 8 a.m. and 1 p.m.) or by using a once-daily formulation that allows plasma nitrate concentrations to fall overnight. Transdermal GTN patches must be removed for part of each 24 h (e.g. overnight) to prevent tolerance.
 Drug interactions are most troublesome with phosphodiesterase inhibitors, such as sildenafil, used in the treatment of erectile dysfunction. These inhibit cGMP metabolism (Ch. 16) and co-administration can result in marked hypotension.
Drug interactions are most troublesome with phosphodiesterase inhibitors, such as sildenafil, used in the treatment of erectile dysfunction. These inhibit cGMP metabolism (Ch. 16) and co-administration can result in marked hypotension.
Beta-adrenoceptor antagonists (β-blockers)
Mechanism of action and effects in angina
All β-adrenoceptor antagonists (often simply referred to as β-blockers) act as competitive antagonists of catecholamines at β-adrenoceptors. They achieve their therapeutic effect in angina by blockade of the cardiac β1-adrenoceptor with reduced generation of intracellular cAMP. As a result they:
 decrease heart rate (by inhibition of the cardiac If pacemaker current in the sinoatrial node; see Ch. 8); this is most marked during exercise, when the rate of rise in heart rate is blunted,
decrease heart rate (by inhibition of the cardiac If pacemaker current in the sinoatrial node; see Ch. 8); this is most marked during exercise, when the rate of rise in heart rate is blunted,
 reduce the force of cardiac contraction (see Ch. 7),
reduce the force of cardiac contraction (see Ch. 7),
 lower blood pressure by reducing cardiac output (a consequence of both the decreased heart rate and force of myocardial contraction).
lower blood pressure by reducing cardiac output (a consequence of both the decreased heart rate and force of myocardial contraction).
The overall effect is to reduce myocardial oxygen demand. The slower heart rate also lengthens diastole and gives more time for coronary perfusion, which effectively improves myocardial oxygen supply.
Certain β-adrenoceptor antagonists have additional properties, which might reduce the incidence of unwanted effects or enhance their blood pressure-lowering actions (see below and also Chs 6 and 8), as follows.
 Cardioselectivity. Some β-adrenoceptor antagonists, for example atenolol, bisoprolol and metoprolol, are selective antagonists at the β1-adrenoceptor. They are usually called cardioselective drugs since the most important site of action on β1-adrenoceptors is the heart. Other β-adrenoceptor antagonists, for example propranolol, have equal or greater antagonist activity at β2-adrenoceptors; these drugs are referred to as ‘non-selective’ β-adrenoceptor antagonists. The cardioselectivity of all β-adrenoceptor antagonists is dose-related, with progressively more β2-adrenoceptor blockade at higher doses.
Cardioselectivity. Some β-adrenoceptor antagonists, for example atenolol, bisoprolol and metoprolol, are selective antagonists at the β1-adrenoceptor. They are usually called cardioselective drugs since the most important site of action on β1-adrenoceptors is the heart. Other β-adrenoceptor antagonists, for example propranolol, have equal or greater antagonist activity at β2-adrenoceptors; these drugs are referred to as ‘non-selective’ β-adrenoceptor antagonists. The cardioselectivity of all β-adrenoceptor antagonists is dose-related, with progressively more β2-adrenoceptor blockade at higher doses.
 Partial agonist activity (PAA) or intrinsic sympathomimetic activity (ISA). Certain β1-adrenoceptor antagonists also act as partial agonists at either β1- or β2-adrenoceptors. For example, pindolol is a β1-adrenoceptor antagonist that also has weak agonist activity at β2-adrenoceptors, and as such it will produce vasodilation in some vascular beds (see Fig. 6.6). Drugs with PAA at the β1-adrenoceptor have less inhibitory effect on heart rate and force of contraction and may be less effective than full antagonists in the treatment of severe angina, but their PAA means they are less likely to cause a resting bradycardia. Beta-adrenoceptor antagonists with PAA are not widely used.
Partial agonist activity (PAA) or intrinsic sympathomimetic activity (ISA). Certain β1-adrenoceptor antagonists also act as partial agonists at either β1- or β2-adrenoceptors. For example, pindolol is a β1-adrenoceptor antagonist that also has weak agonist activity at β2-adrenoceptors, and as such it will produce vasodilation in some vascular beds (see Fig. 6.6). Drugs with PAA at the β1-adrenoceptor have less inhibitory effect on heart rate and force of contraction and may be less effective than full antagonists in the treatment of severe angina, but their PAA means they are less likely to cause a resting bradycardia. Beta-adrenoceptor antagonists with PAA are not widely used.
 Vasodilator activity. Pure β1-adrenoceptor antagonists do not cause vasodilation. Indeed, the reflex response to β1-adrenoceptor blockade is vasoconstriction, mediated in part by the reflex sympathetic nervous system stimulation of α1-adrenoceptors in response to the fall in cardiac output. However, some β-adrenoceptor antagonists have additional properties that produce arterial vasodilation. Mechanisms of vasodilation include β2-adrenoceptor partial agonist activity (e.g. pindolol; see above), α1-adrenoceptor blockade (e.g. carvedilol, labetalol) or an increase in endothelial NO synthesis (e.g. nebivolol) (see Fig. 6.6). Vasodilation does not have any proven advantage for the treatment of angina, but may be useful when β-adrenoceptor antagonists are given for the treatment of hypertension (Ch. 6).
Vasodilator activity. Pure β1-adrenoceptor antagonists do not cause vasodilation. Indeed, the reflex response to β1-adrenoceptor blockade is vasoconstriction, mediated in part by the reflex sympathetic nervous system stimulation of α1-adrenoceptors in response to the fall in cardiac output. However, some β-adrenoceptor antagonists have additional properties that produce arterial vasodilation. Mechanisms of vasodilation include β2-adrenoceptor partial agonist activity (e.g. pindolol; see above), α1-adrenoceptor blockade (e.g. carvedilol, labetalol) or an increase in endothelial NO synthesis (e.g. nebivolol) (see Fig. 6.6). Vasodilation does not have any proven advantage for the treatment of angina, but may be useful when β-adrenoceptor antagonists are given for the treatment of hypertension (Ch. 6).
Pharmacokinetics
Highly lipophilic β-adrenoceptor antagonists, such as propranolol and metoprolol, are well absorbed from the gut but undergo extensive first-pass metabolism in the liver, with considerable variability among individuals. Reduction in heart rate during exercise is closely related to the plasma concentration of the drug, so dose titration of lipophilic β-adrenoceptor antagonists is usually necessary to achieve an optimal clinical response. Most lipophilic β-adrenoceptor antagonists have short half-lives (see Compendium of drugs used to treat ischaemic heart disease at the end of this chapter), and are often available in modified-release formulations to prolong their duration of action.
Hydrophilic β-adrenoceptor antagonists, such as atenolol, are incompletely absorbed from the gut, and are eliminated unchanged in the urine. The dose range to maintain effective plasma concentrations is narrower than for those drugs that undergo metabolism. The half-lives of hydrophilic β-adrenoceptor antagonists are usually longer than those of lipophilic drugs (see Compendium at the end of this chapter).
Unwanted effects
 Blockade of β1-adrenoceptors. A large dose of a β-adrenoceptor antagonist can precipitate acute pulmonary oedema if there is pre-existing poor left ventricular function, when high sympathetic nervous activity is necessary to maintain cardiac output. However, there is a paradox that when used at low doses with gradual-dose titration a β-adrenoceptor antagonist is part of the core therapy of heart failure (Ch. 7). A reduction in cardiac output can also impair blood supply to peripheral tissues, which can be detrimental in critical leg ischaemia (Ch. 10) or can provoke Raynaud's phenomenon (Ch. 10). Excessive bradycardia occasionally occurs, and β-adrenoceptor antagonists should be used with caution or avoided in the presence of advanced atrioventricular conduction defect (heart block). Drugs with partial agonist activity produce less bradycardia or reduction of cardiac output.
Blockade of β1-adrenoceptors. A large dose of a β-adrenoceptor antagonist can precipitate acute pulmonary oedema if there is pre-existing poor left ventricular function, when high sympathetic nervous activity is necessary to maintain cardiac output. However, there is a paradox that when used at low doses with gradual-dose titration a β-adrenoceptor antagonist is part of the core therapy of heart failure (Ch. 7). A reduction in cardiac output can also impair blood supply to peripheral tissues, which can be detrimental in critical leg ischaemia (Ch. 10) or can provoke Raynaud's phenomenon (Ch. 10). Excessive bradycardia occasionally occurs, and β-adrenoceptor antagonists should be used with caution or avoided in the presence of advanced atrioventricular conduction defect (heart block). Drugs with partial agonist activity produce less bradycardia or reduction of cardiac output.
 Bronchospasm can be precipitated in people with asthma (including those with chronic obstructive pulmonary disease and some bronchodilator reversibility). People who are susceptible to this problem can experience bronchospasm even with cardioselective drugs.
Bronchospasm can be precipitated in people with asthma (including those with chronic obstructive pulmonary disease and some bronchodilator reversibility). People who are susceptible to this problem can experience bronchospasm even with cardioselective drugs.
 Hypoglycaemia may be prolonged by non-selective β-adrenoceptor antagonists, which may be a problem in people with diabetes mellitus who are treated with insulin (Ch. 40). Gluconeogenesis, a component of the metabolic response to hypoglycaemia, is dependent upon β2-adrenoceptor stimulation in the liver. Beta-adrenoceptor antagonists also blunt the autonomic response that alerts the person to the onset of hypoglycaemia.
Hypoglycaemia may be prolonged by non-selective β-adrenoceptor antagonists, which may be a problem in people with diabetes mellitus who are treated with insulin (Ch. 40). Gluconeogenesis, a component of the metabolic response to hypoglycaemia, is dependent upon β2-adrenoceptor stimulation in the liver. Beta-adrenoceptor antagonists also blunt the autonomic response that alerts the person to the onset of hypoglycaemia.
 Central nervous system effects. These include sleep disturbance, vivid dreams and hallucinations, and are more common with lipophilic drugs, which readily cross the blood–brain barrier. Other consequences of CNS action include fatigue and subtle psychomotor effects, for example lack of concentration and sexual dysfunction.
Central nervous system effects. These include sleep disturbance, vivid dreams and hallucinations, and are more common with lipophilic drugs, which readily cross the blood–brain barrier. Other consequences of CNS action include fatigue and subtle psychomotor effects, for example lack of concentration and sexual dysfunction.
 Effects on blood lipid levels. Most β-adrenoceptor antagonists raise the plasma concentration of triglycerides and lower the concentration of high-density lipoprotein (HDL) cholesterol (Ch. 48). These changes are modest but potentially atherogenic. They are most marked with non-selective β-adrenoceptor antagonists, and least if the drug has partial agonist activity.
Effects on blood lipid levels. Most β-adrenoceptor antagonists raise the plasma concentration of triglycerides and lower the concentration of high-density lipoprotein (HDL) cholesterol (Ch. 48). These changes are modest but potentially atherogenic. They are most marked with non-selective β-adrenoceptor antagonists, and least if the drug has partial agonist activity.
 Sudden withdrawal syndrome. Upregulation of β-adrenoceptors (Ch. 1) during long-term treatment makes the heart more sensitive to catecholamines. Palpitation due to a greater awareness of the heart is common on withdrawal. Beta-adrenoceptor antagonists should be stopped gradually in people with ischaemic heart disease, to avoid precipitating unstable angina or myocardial infarction.
Sudden withdrawal syndrome. Upregulation of β-adrenoceptors (Ch. 1) during long-term treatment makes the heart more sensitive to catecholamines. Palpitation due to a greater awareness of the heart is common on withdrawal. Beta-adrenoceptor antagonists should be stopped gradually in people with ischaemic heart disease, to avoid precipitating unstable angina or myocardial infarction.
 Drug interactions. The calcium channel blocker verapamil and, to a lesser extent, diltiazem (see below) have potentially hazardous additive effects with β-adrenoceptor antagonists, since both reduce the force of cardiac contraction and slow heart rate.
Drug interactions. The calcium channel blocker verapamil and, to a lesser extent, diltiazem (see below) have potentially hazardous additive effects with β-adrenoceptor antagonists, since both reduce the force of cardiac contraction and slow heart rate.
Calcium channel blockers
Mechanism of action and effects
Calcium is essential for excitation/contraction coupling in muscle cells. The following mechanisms of regulating intracellular free Ca2+ concentration are important pharmacologically (Figs 5.4 and 5.5).
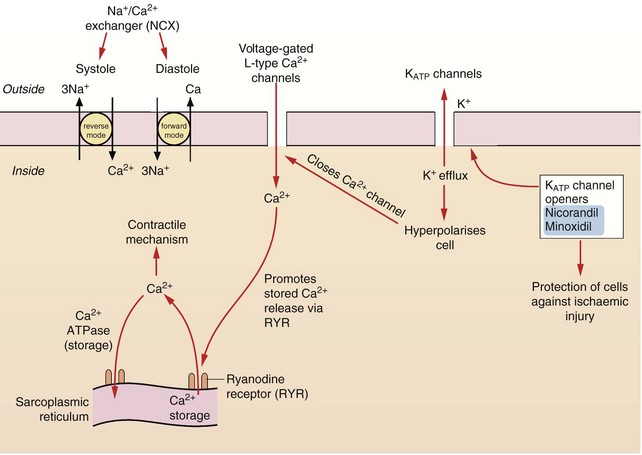
Fig. 5.4 The control of calcium regulation and actions of potassium channel openers in cardiac myocytes and blood vessels.
Calcium concentrations in cardiac cells and in vascular smooth muscle are under the control of a number of different mechanisms. Calcium entry through voltage-gated L-type Ca2+ channels stimulates ryanodine receptors (RYR) in the sarcoplasmic reticulum, releasing stored Ca2+ (known as Ca2+-induced calcium release, CICR). Intracellular Ca2+ is also regulated by exchange with Na+ via the Na+/Ca2+ exchangers (NCX) in the cell membrane. Vascular smooth muscle cells have ATP-sensitive inward rectifier K+ channels (KIR) which combine with sulfonylurea receptors to form ATP-sensitive K+ channels (KATP). Hyperpolarisation of the cell by drugs which open KATP channels, such as nicorandil, closes voltage-gated L-type Ca2+ channels and causes relaxation.
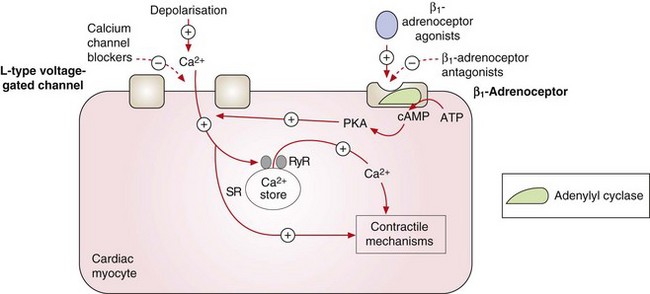
Fig. 5.5 Contraction of the cardiac myocyte by voltage-gated and receptor-operated channels.
Depolarisation during the action potential activates the voltage-gated L-type Ca2+ channels and the influx of Ca2+ into the cell results in myosin phosphorylation and muscle contraction. It also promotes further Ca2+ release from the sarcoplasmic reticulum (SR) by stimulation of ryanodine receptors (RyR). Stimulation of the β1-adrenoceptors by catecholamines activates adenylyl cyclase and the generated cAMP binds to subunits of protein kinase A (PKA), which phosphorylates the L-type Ca2+ channels, increasing their opening time and facilitating Ca2+ entry. The L-type Ca2+ channels can also be activated by other pathways, such as phospholipase C-dependent signalling triggered by agonism of α1-adrenoceptors (not shown). The activity of the voltage-gated L-type Ca2+ channels can therefore be reduced directly by calcium channel blockers or indirectly by antagonists of β1-adrenoceptors or other receptors. +, Stimulates activity; –, inhibits activity.
 Ca2+ can enter cells through transmembrane voltage-gated and ligand-gated channels in smooth muscle and cardiac muscle cells (Figs 5.4 and 5.5).
Ca2+ can enter cells through transmembrane voltage-gated and ligand-gated channels in smooth muscle and cardiac muscle cells (Figs 5.4 and 5.5).
 A rise in intracellular free Ca2+ promotes release of Ca2+ from the sarcoplasmic reticulum in striated and cardiac muscle cells through actions at ryanodine receptors (Figs 5.4 and 5.5).
A rise in intracellular free Ca2+ promotes release of Ca2+ from the sarcoplasmic reticulum in striated and cardiac muscle cells through actions at ryanodine receptors (Figs 5.4 and 5.5).
 Ligand-gated channels linked to G-protein-coupled receptors release Ca2+ from intracellular stores in the sarcoplasmic reticulum.
Ligand-gated channels linked to G-protein-coupled receptors release Ca2+ from intracellular stores in the sarcoplasmic reticulum.
 Ca2+ can exit cells in exchange for Na+ via the Na+/Ca2+ exchanger (Fig. 5.4).
Ca2+ can exit cells in exchange for Na+ via the Na+/Ca2+ exchanger (Fig. 5.4).
Therefore, in striated muscle, free Ca2+ in the cytosol comes from the sarcoplasmic reticulum, while in smooth muscle it enters the cell through transmembrane Ca2+ channels. Cardiac muscle uses both mechanisms. There are at least five different types of transmembrane Ca2+ channel, two of which are found in cardiovascular tissues. These are listed here.
 Voltage-gated L-type Ca2+ channels (long-acting, high-threshold-activated, slowly inactivated): these are important therapeutically and are found in the cell membranes of a large number of excitable cells, including cardiac and vascular smooth muscle. Ca2+ enters the cell through these channels when the cell membrane is depolarised. The cardiac and vascular L-type Ca2+ channels have different subunit structures.
Voltage-gated L-type Ca2+ channels (long-acting, high-threshold-activated, slowly inactivated): these are important therapeutically and are found in the cell membranes of a large number of excitable cells, including cardiac and vascular smooth muscle. Ca2+ enters the cell through these channels when the cell membrane is depolarised. The cardiac and vascular L-type Ca2+ channels have different subunit structures.
 Voltage-gated T-type Ca2+ channels (transient, low-threshold-activated, fast inactivated): these are found in pacemaker cells of the sinoatrial and atrioventricular nodes, and are also present in vascular smooth muscle.
Voltage-gated T-type Ca2+ channels (transient, low-threshold-activated, fast inactivated): these are found in pacemaker cells of the sinoatrial and atrioventricular nodes, and are also present in vascular smooth muscle.
Calcium channel blockers (sometimes referred to inaccurately as calcium antagonists) have widely different chemical structures, but their common action is to reduce Ca2+ influx through voltage-gated L-type Ca2+ channels in smooth and cardiac muscle. None of the currently available calcium channel blockers affect T-type channels to any important extent, or influence ligand-gated Ca2+ channels (which are involved in neurotransmitter release and respond to endogenous agonists such as noradrenaline; Fig. 5.5).
There are clinically important differences among the calcium channel blockers, which bind to discrete receptors on the L-type Ca2+ channel. The receptor for verapamil is intracellular, while diltiazem and the dihydropyridines (e.g. nifedipine, amlodipine) have extracellular binding sites; however, the receptor domains for verapamil and diltiazem overlap. Verapamil and diltiazem exhibit frequency-dependent receptor binding and gain access to the Ca2+ channel when it is in the open state; in contrast, the dihydropyridines preferentially bind to the channel in its inactivated state. As more Ca2+ channels are in the inactive state in relaxed vascular smooth muscle than in cardiac muscle, dihydropyridines selectively bind to Ca2+ channels in vascular smooth muscle. These receptor binding characteristics account for the relative vascular selectivity of the dihydropyridines and for the anti-arrhythmic properties of verapamil and diltiazem (Ch. 8).
Calcium channel blockers produce a number of effects that are important in the treatment of angina.
 Arteriolar dilation. Although all calcium channel blockers are vasodilators, dihydropyridine derivatives such as nifedipine and amlodipine are the most potent and show the greatest vascular selectivity. Arterial dilation reduces peripheral resistance and lowers the blood pressure. This reduces the work of the left ventricle, and therefore reduces myocardial oxygen demand. Most dihydropyridines have a rapid onset of action and produce rapid vasodilation and reduction in blood pressure. This leads to reflex sympathetic nervous system activation and tachycardia (Fig. 5.6). Amlodipine or modified-release formulations of short-acting dihydropyridines are more slowly absorbed and gradually reduce blood pressure with little reflex tachycardia.
Arteriolar dilation. Although all calcium channel blockers are vasodilators, dihydropyridine derivatives such as nifedipine and amlodipine are the most potent and show the greatest vascular selectivity. Arterial dilation reduces peripheral resistance and lowers the blood pressure. This reduces the work of the left ventricle, and therefore reduces myocardial oxygen demand. Most dihydropyridines have a rapid onset of action and produce rapid vasodilation and reduction in blood pressure. This leads to reflex sympathetic nervous system activation and tachycardia (Fig. 5.6). Amlodipine or modified-release formulations of short-acting dihydropyridines are more slowly absorbed and gradually reduce blood pressure with little reflex tachycardia.
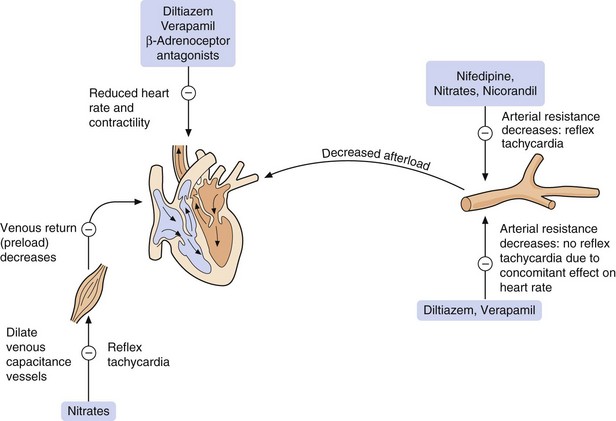
Fig. 5.6 The major sites of action of anti-anginal drugs.
Reflex tachycardia results from the actions of dihydropyridine calcium channel blocker nifedipine and the potassium channel opener nicorandil. Nifedipine causes a rapid fall in blood pressure, triggering the reflex. This is not a problem with the non-dihydropyridines diltiazem and verapamil, which concomitantly slow the heart rate. Reflex tachycardia to nifedipine can be minimised with a modified-release formulation, or a more slowly acting dihydropyridine compound such as amlodipine can be used.
 Coronary artery dilation. Prevention or relief of coronary vasospasm improves myocardial blood flow.
Coronary artery dilation. Prevention or relief of coronary vasospasm improves myocardial blood flow.
 Negative chronotropic effect. Verapamil and diltiazem (but not the dihydropyridines) slow the rate of firing of the sinoatrial node and slow conduction of the electrical impulse through the atrioventricular node (see also Ch. 8). Thus, reflex tachycardia is not seen with these drugs and they also slow the rate of rise in heart rate during exercise.
Negative chronotropic effect. Verapamil and diltiazem (but not the dihydropyridines) slow the rate of firing of the sinoatrial node and slow conduction of the electrical impulse through the atrioventricular node (see also Ch. 8). Thus, reflex tachycardia is not seen with these drugs and they also slow the rate of rise in heart rate during exercise.
 Reduced cardiac contractility. Most calcium channel blockers (but particularly verapamil) have some negative inotropic effect. Amlodipine does not impair myocardial contractility.
Reduced cardiac contractility. Most calcium channel blockers (but particularly verapamil) have some negative inotropic effect. Amlodipine does not impair myocardial contractility.
Pharmacokinetics
Most calcium channel blockers are lipophilic compounds with similar pharmacokinetic properties. They are almost completely absorbed from the gut lumen, and variable first-pass metabolism can limit bioavailability. Their half-lives are mostly in the range of 2 to 12 h, and modified-release formulations are widely used to prolong their duration of action. However, amlodipine is slowly absorbed and does not undergo first-pass metabolism. It has a high volume of distribution, due to extensive membrane partitioning in cells, and slower metabolism by the liver, which together result in a very long half-life of about 1–2 days. Verapamil can be given intravenously, a route that is usually reserved for the treatment of supraventricular arrhythmias (Ch. 8).
Unwanted effects
 Arterial dilation can produce headache, flushing and dizziness, although tolerance often occurs with continued use. Ankle oedema, which is frequently resistant to diuretics, probably arises from increased transcapillary hydrostatic pressure. Tolerance to oedema does not occur. All these unwanted effects are most common with the dihydropyridines.
Arterial dilation can produce headache, flushing and dizziness, although tolerance often occurs with continued use. Ankle oedema, which is frequently resistant to diuretics, probably arises from increased transcapillary hydrostatic pressure. Tolerance to oedema does not occur. All these unwanted effects are most common with the dihydropyridines.
 Reduced cardiac contractility can precipitate heart failure in people with pre-existing poor left ventricular function, particularly with verapamil. Amlodipine does not depress cardiac contractility.
Reduced cardiac contractility can precipitate heart failure in people with pre-existing poor left ventricular function, particularly with verapamil. Amlodipine does not depress cardiac contractility.
 Tachycardia and palpitations can arise with dihydropyridines, especially with rapid-release formulations.
Tachycardia and palpitations can arise with dihydropyridines, especially with rapid-release formulations.
 Bradycardia and heart block with verapamil and diltiazem.
Bradycardia and heart block with verapamil and diltiazem.
 Altered gut motility: constipation is most common with verapamil, less so with diltiazem. Amlodipine and other dihydropyridines can cause nausea and heartburn.
Altered gut motility: constipation is most common with verapamil, less so with diltiazem. Amlodipine and other dihydropyridines can cause nausea and heartburn.
 Drug interactions: verapamil and diltiazem can slow the heart rate excessively if they are used in combination with other drugs that have similar effects on atrioventricular nodal conduction; for example, digoxin (Ch. 8) or β-adrenoceptor antagonists. Metabolism of many calcium channel blockers can be inhibited or accelerated by drugs that affect the liver P450 cytochrome enzymes.
Drug interactions: verapamil and diltiazem can slow the heart rate excessively if they are used in combination with other drugs that have similar effects on atrioventricular nodal conduction; for example, digoxin (Ch. 8) or β-adrenoceptor antagonists. Metabolism of many calcium channel blockers can be inhibited or accelerated by drugs that affect the liver P450 cytochrome enzymes.
Potassium channel openers
Mechanism of action
There are many different K+ channels in cell membranes (Ch. 8, Table 8.1). Of these, the ATP-inhibited KATP channels are the target for nicorandil (Fig. 5.4). KATP channels are found in many tissues, but have a variety of tissue-specific subunit configurations making targeted drug action on the channels possible. Nicorandil opens vascular smooth muscle KATP channels, so that K+ leaves the cell and the efflux of positive ions hyperpolarises the cell. Hyperpolarisation means that the cell will be more difficult to depolarise and the membrane voltage-gated L-type Ca2+ channels are less likely to open (see calcium channel blockers, above). The consequence is that less Ca2+ is available to the muscle contractile mechanism, leading to vasodilation in systemic and coronary arteries (Fig. 5.4). Prevention of coronary vasospasm improves myocardial perfusion and blood pressure will fall, which reduces myocardial oxygen demand. In addition, enhanced KATP channel activity may protect myocardial cells against ischaemic injury.
Nicorandil also carries a nitrate moiety, and part of its vasodilator action is via generation of NO in vascular smooth muscle (see organic nitrates, above). This may account for the venodilation produced by the drug, which reduces venous return and further reduces myocardial oxygen demand.
Pharmacokinetics
Nicorandil is rapidly and almost completely absorbed from the gut. It is eliminated by hepatic metabolism and has a short half-life of 1 h. However, the tissue effects correlate poorly with the plasma concentration and the biological effect lasts up to 12 h.
Specific sinus node inhibitors
Mechanism of action
In cardiac pacemaker cells (especially the sinoatrial node) the pacemaker If current is responsible for spontaneous depolarisation (Ch. 8). This is an inward current of positive ions through f-channels that carry both Na+ and K+, that are activated by the negative intracellular potential in diastole or by cyclic nucleotides. Ivabradine is a specific inhibitor of the If current, and its major effect is to slow sinus heart rate. The degree of channel inhibition is use-dependent, since ivabradine binds to the open channel from the internal side of the cell membrane. As a result, the efficacy of ivabradine increases with the frequency of channel opening and is greatest at higher heart rates. Unlike β-adrenoceptor antagonists, ivabradine has no effect on myocardial contractility.
Pharmacokinetics
Ivabradine is well absorbed from the gut and undergoes extensive first-pass metabolism in the gut wall and liver to an active metabolite. It has a half-life of 2 h.
Unwanted effects
Late sodium current inhibitors
Mechanism of action
Transmembrane Na+ channels are activated during the initial electrical excitation of myocardial cells, and are mainly inactivated during the plateau phase of the action potential. However, a small proportion of the Na+ channels remain open, giving rise to the late Na+ current. In hypoxic tissues this current is increased and the consequent rise in intracellular Na+ concentration activates the reverse mode of the Na+/Ca2+ exchanger in the cell membrane, leading to removal of Na+ from the cell, intracellular Ca2+ accumulation and increased diastolic myocardial tension (Fig. 5.4). Ranolazine attenuates the late transcellular Na+ current in ischaemic myocardial cells, and reduces Ca2+ accumulation. There are two potentially beneficial consequences of this effect: the lower wall tension in the ventricles should reduce myocardial oxygen demand, and it will also reduce compression of small intramyocardial coronary vessels, thus improving myocardial perfusion.
Pharmacokinetics
Ranolazine is partially absorbed from the gut and extensively metabolised in the liver. It has a short elimination half-life of about 2 h and a modified-release formulation is used.
Unwanted effects
 Nausea, dyspepsia, constipation.
Nausea, dyspepsia, constipation.
 Headache, dizziness, lethargy.
Headache, dizziness, lethargy.
 Prolongation of the QT interval on the ECG (Ch. 8), with the potential to provoke cardiac arrhythmias if used with other drugs that have the same effect.
Prolongation of the QT interval on the ECG (Ch. 8), with the potential to provoke cardiac arrhythmias if used with other drugs that have the same effect.
Management of stable angina
The principal aims of treatment for stable angina are to relieve symptoms and to improve prognosis. Angina has a pronounced circadian rhythm and occurs most frequently in the hours after waking, so a drug given for prevention of symptoms should ideally be effective at this time. There is no evidence that control of symptoms will affect either survival or the risk of a subsequent myocardial infarction. Improvement in prognosis is achieved mainly by using drugs that do not directly affect symptoms.
There are several important principles of management.
 Lifestyle changes: stopping smoking reduces the progression of coronary atheroma. It also reduces coronary vasospasm, and may improve symptoms, but importantly reduces the risk of developing an acute coronary syndrome by up to 50%. Symptoms may be improved by weight loss in people who are obese by reducing cardiac work. Regular exercise will improve fitness and attenuate the rise in heart rate on exercise, which will increase exercise duration before the onset of angina.
Lifestyle changes: stopping smoking reduces the progression of coronary atheroma. It also reduces coronary vasospasm, and may improve symptoms, but importantly reduces the risk of developing an acute coronary syndrome by up to 50%. Symptoms may be improved by weight loss in people who are obese by reducing cardiac work. Regular exercise will improve fitness and attenuate the rise in heart rate on exercise, which will increase exercise duration before the onset of angina.
 Reduction of high blood pressure and control of diabetes will reduce progression of atheroma.
Reduction of high blood pressure and control of diabetes will reduce progression of atheroma.
 Treatment of provoking or exacerbating factors for angina, such as anaemia, arrhythmias or thyrotoxicosis.
Treatment of provoking or exacerbating factors for angina, such as anaemia, arrhythmias or thyrotoxicosis.
 Sublingual GTN remains the treatment of choice for an acute anginal attack. It relieves symptoms within minutes, but gives only short-lived protection (20–30 min). GTN can also be taken before an activity that is likely to produce angina.
Sublingual GTN remains the treatment of choice for an acute anginal attack. It relieves symptoms within minutes, but gives only short-lived protection (20–30 min). GTN can also be taken before an activity that is likely to produce angina.
 If anginal attacks are frequent, a prophylactic anti-anginal drug should be used. A rise in heart rate is one of the main precipitating factors for angina, and a drug that lowers heart rate, such as a β-adrenoceptor antagonist or a rate-limiting calcium channel blocker like verapamil or diltiazem, is first-line treatment. Ivabradine can be used if other heart-rate limiting drugs are not tolerated. Nitrates are less suitable as first-line prophylactic agents because of the risk of tolerance. If symptoms are not controlled by optimal doses of a single drug then a combination of a β-adrenoceptor antagonist with a calcium channel blocker (not verapamil) can be used. Alternatively, a β-adrenoceptor antagonist or calcium channel blocker can be combined with a long-acting nitrate. If two drugs do not control symptoms, then coronary angiography should be considered with a view to revascularisation. ‘Triple therapy’ (e.g. β-adrenoceptor antagonist, calcium channel blocker and a long-acting nitrate) has not been shown convincingly to be better than two agents, but such combinations may give further symptomatic benefit if coronary revascularisation is not being considered or while awaiting coronary angiography. Nicorandil is generally used in combination therapy. Ranolazine may be helpful when symptomatic hypotension precludes the use of other drugs.
If anginal attacks are frequent, a prophylactic anti-anginal drug should be used. A rise in heart rate is one of the main precipitating factors for angina, and a drug that lowers heart rate, such as a β-adrenoceptor antagonist or a rate-limiting calcium channel blocker like verapamil or diltiazem, is first-line treatment. Ivabradine can be used if other heart-rate limiting drugs are not tolerated. Nitrates are less suitable as first-line prophylactic agents because of the risk of tolerance. If symptoms are not controlled by optimal doses of a single drug then a combination of a β-adrenoceptor antagonist with a calcium channel blocker (not verapamil) can be used. Alternatively, a β-adrenoceptor antagonist or calcium channel blocker can be combined with a long-acting nitrate. If two drugs do not control symptoms, then coronary angiography should be considered with a view to revascularisation. ‘Triple therapy’ (e.g. β-adrenoceptor antagonist, calcium channel blocker and a long-acting nitrate) has not been shown convincingly to be better than two agents, but such combinations may give further symptomatic benefit if coronary revascularisation is not being considered or while awaiting coronary angiography. Nicorandil is generally used in combination therapy. Ranolazine may be helpful when symptomatic hypotension precludes the use of other drugs.
 Low-dose aspirin reduces the risk of subsequent myocardial infarction by about 35%. Clopidogrel is an alternative if aspirin is not tolerated, but the combination has not been shown to have any additive benefit in stable coronary artery disease (see Ch. 11).
Low-dose aspirin reduces the risk of subsequent myocardial infarction by about 35%. Clopidogrel is an alternative if aspirin is not tolerated, but the combination has not been shown to have any additive benefit in stable coronary artery disease (see Ch. 11).
 Lowering the total plasma cholesterol to <4.0 mmol⋅L−1 by diet and a statin (Ch. 48) reduces the risk of subsequent non-fatal myocardial infarction, cardiac death and the need for a coronary artery revascularisation procedure by more than 30%.
Lowering the total plasma cholesterol to <4.0 mmol⋅L−1 by diet and a statin (Ch. 48) reduces the risk of subsequent non-fatal myocardial infarction, cardiac death and the need for a coronary artery revascularisation procedure by more than 30%.
 ACE inhibitors (Ch. 6) have no anti-anginal action, but reduce the risk of subsequent myocardial infarction and death by about 15%, especially in people at high risk of an event.
ACE inhibitors (Ch. 6) have no anti-anginal action, but reduce the risk of subsequent myocardial infarction and death by about 15%, especially in people at high risk of an event.
 Percutaneous coronary intervention (PCI) consists of angioplasty and usually insertion of a stent to maintain vessel patency. PCI improves symptoms, but only reduces cardiovascular death or myocardial infarction if there is a large area of ischaemic myocardium. Insertion of a coronary artery stent is followed by combination antiplatelet therapy with aspirin and clopidogrel (Ch. 11) for up to a year to minimise stent thrombosis which carries a high risk of myocardial infarction or death. Short-term use of a glycoprotein IIb/IIIa antagonist such as abciximab (Ch. 11) at the time of angioplasty further improves outcome for high-risk procedures. Re-stenosis after angioplasty is due to intimal hyperplasia and smooth muscle proliferation encroaching on the lumen of the vessel, and usually occurs within 6 months of the procedure. Angioplasty alone is followed by a re-stenosis rate of about 40% at 6 months, which can be reduced to about 20% by the use of a bare-metal stent. The most recent development is drug-eluting stents, which are coated with a polymer matrix containing an antiproliferative drug such as sirolimus or tacrolimus (Ch. 38). Drug-eluting stents reduce the risk of re-stenosis at 6 months to about 6%.
Percutaneous coronary intervention (PCI) consists of angioplasty and usually insertion of a stent to maintain vessel patency. PCI improves symptoms, but only reduces cardiovascular death or myocardial infarction if there is a large area of ischaemic myocardium. Insertion of a coronary artery stent is followed by combination antiplatelet therapy with aspirin and clopidogrel (Ch. 11) for up to a year to minimise stent thrombosis which carries a high risk of myocardial infarction or death. Short-term use of a glycoprotein IIb/IIIa antagonist such as abciximab (Ch. 11) at the time of angioplasty further improves outcome for high-risk procedures. Re-stenosis after angioplasty is due to intimal hyperplasia and smooth muscle proliferation encroaching on the lumen of the vessel, and usually occurs within 6 months of the procedure. Angioplasty alone is followed by a re-stenosis rate of about 40% at 6 months, which can be reduced to about 20% by the use of a bare-metal stent. The most recent development is drug-eluting stents, which are coated with a polymer matrix containing an antiproliferative drug such as sirolimus or tacrolimus (Ch. 38). Drug-eluting stents reduce the risk of re-stenosis at 6 months to about 6%.
 Coronary artery bypass grafting (CABG) improves long-term prognosis compared with medical treatment in people with a left mainstem coronary artery stenosis, and in those with ‘triple vessel disease’ (significant stenoses of the left anterior descending, left circumflex and right coronary arteries) who have impaired left ventricular function. In less severe disease it is used for symptom relief.
Coronary artery bypass grafting (CABG) improves long-term prognosis compared with medical treatment in people with a left mainstem coronary artery stenosis, and in those with ‘triple vessel disease’ (significant stenoses of the left anterior descending, left circumflex and right coronary arteries) who have impaired left ventricular function. In less severe disease it is used for symptom relief.
Symptoms, and their response to treatment, are a poor guide to the severity of coronary artery disease, and quantifying the amount of ischaemic myocardium with non-invasive myocardial perfusion imaging is a more accurate predictor. A large area of ischaemic myocardium, or failure to respond to two prophylactic drugs in adequate dosages, should lead to consideration of coronary angiography, with a view to CABG or PCI.
Management of acute coronary syndromes
Management of acute coronary syndromes without ST-segment elevation
Acute coronary syndromes require urgent treatment even if there is no ECG evidence of myocardial infarction at presentation. Unstable angina, if left untreated, progresses in about 10% of cases to myocardial infarction or death. The management of an acute coronary syndrome is initially determined by the ECG. In the absence of ST-segment elevation on the ECG, management is based on the assumption that a myocardial infarction has occurred until a sensitive marker of myocardial damage such as plasma troponin I or T is obtained about 10–12 h after the onset of pain. A rise in one of these markers will differentiate NSTEMI from unstable angina. If there is no evidence of myocardial damage and the ECG does not show ischaemic changes, then the risk of subsequent myocardial infarction or sudden death is low and treatment can then be less intensive.
 Initial treatment is with sublingual or intravenous GTN which may reduce pain by relief of coronary artery vasospasm at the site of the arterial occlusion and increase coronary blood flow. Analgesia with an intravenous opioid such as morphine (Ch. 19), together with an antiemetic, is used for pain that does not settle with a nitrate. Intramuscular injection of morphine should be avoided, since a low cardiac output and poor tissue perfusion often delay absorption. Supplementary oxygen may be required if the arterial oxygen saturation is below 94% with the aim of maintaining it between 94 and 98%, unless there is a risk of type 2 respiratory failure. Oxygen should be avoided if there is no hypoxaemia since it may increase myocardial damage.
Initial treatment is with sublingual or intravenous GTN which may reduce pain by relief of coronary artery vasospasm at the site of the arterial occlusion and increase coronary blood flow. Analgesia with an intravenous opioid such as morphine (Ch. 19), together with an antiemetic, is used for pain that does not settle with a nitrate. Intramuscular injection of morphine should be avoided, since a low cardiac output and poor tissue perfusion often delay absorption. Supplementary oxygen may be required if the arterial oxygen saturation is below 94% with the aim of maintaining it between 94 and 98%, unless there is a risk of type 2 respiratory failure. Oxygen should be avoided if there is no hypoxaemia since it may increase myocardial damage.
 A loading dose of aspirin and clopidogrel (Ch. 11) should be given. If myocardial infarction is confirmed, then dual platelet inhibition with clopidogrel and low-dose aspirin is continued for up to 1 year. This reduces the risk of subsequent myocardial infarction by a further 20% compared to aspirin alone. After a year, the clopidogrel is stopped, but aspirin is continued indefinitely. Low-dose aspirin is used alone after an episode of unstable angina, when dual therapy has no advantage.
A loading dose of aspirin and clopidogrel (Ch. 11) should be given. If myocardial infarction is confirmed, then dual platelet inhibition with clopidogrel and low-dose aspirin is continued for up to 1 year. This reduces the risk of subsequent myocardial infarction by a further 20% compared to aspirin alone. After a year, the clopidogrel is stopped, but aspirin is continued indefinitely. Low-dose aspirin is used alone after an episode of unstable angina, when dual therapy has no advantage.
 Full anticoagulation with fondaparinux (Ch. 11) is initially used together with dual antiplatelet therapy. Fondaparinux has largely replaced low-molecular-weight heparin (which carries a higher risk of bleeding) unless coronary angiography is planned within 24 h of admission. The risk of further myocardial infarction or death within 14 days is reduced by about 60% using combined treatment with antiplatelet therapy and an anticoagulant. If PCI is carried out the direct thrombin inhibitor bivalirudin (Ch. 11) is used in combination with clopidogrel and aspirin to reduce the risk of ischaemic events during and after the procedure. Heparin combined with a glycoprotein IIb/IIIa antagonist such as tirofiban (Ch. 11) can be used instead of bivalirudin, but there is a higher risk of bleeding.
Full anticoagulation with fondaparinux (Ch. 11) is initially used together with dual antiplatelet therapy. Fondaparinux has largely replaced low-molecular-weight heparin (which carries a higher risk of bleeding) unless coronary angiography is planned within 24 h of admission. The risk of further myocardial infarction or death within 14 days is reduced by about 60% using combined treatment with antiplatelet therapy and an anticoagulant. If PCI is carried out the direct thrombin inhibitor bivalirudin (Ch. 11) is used in combination with clopidogrel and aspirin to reduce the risk of ischaemic events during and after the procedure. Heparin combined with a glycoprotein IIb/IIIa antagonist such as tirofiban (Ch. 11) can be used instead of bivalirudin, but there is a higher risk of bleeding.
 A β-adrenoceptor antagonist is the first-choice anti-anginal treatment, and can reduce subsequent ischaemic events after presentation with unstable angina. A heart rate-limiting calcium channel blocker, such as verapamil or diltiazem, can be used if a β-adrenoceptor antagonist is contraindicated or not tolerated. If further treatment is needed, then a dihydropyridine calcium channel blocker such as nifedipine, or nicorandil or a nitrate via a buccal tablet or by intravenous infusion can be used with a β-adrenoceptor antagonist. Apart from β-adrenoceptor antagonists, anti-anginal drugs do not improve prognosis in unstable angina.
A β-adrenoceptor antagonist is the first-choice anti-anginal treatment, and can reduce subsequent ischaemic events after presentation with unstable angina. A heart rate-limiting calcium channel blocker, such as verapamil or diltiazem, can be used if a β-adrenoceptor antagonist is contraindicated or not tolerated. If further treatment is needed, then a dihydropyridine calcium channel blocker such as nifedipine, or nicorandil or a nitrate via a buccal tablet or by intravenous infusion can be used with a β-adrenoceptor antagonist. Apart from β-adrenoceptor antagonists, anti-anginal drugs do not improve prognosis in unstable angina.
 In the acute phase of an acute coronary syndrome, angiography (followed when appropriate by CABG or PCI) is carried out for the 10% of people whose pain is refractory to full medical treatment. If the symptoms settle, those who had evidence of myocardial damage during the acute episode (an increase in the plasma concentration of troponin I or troponin T), and therefore had an NSTEMI, or those who have ongoing evidence of reversible myocardial ischaemia, should also be investigated by angiography.
In the acute phase of an acute coronary syndrome, angiography (followed when appropriate by CABG or PCI) is carried out for the 10% of people whose pain is refractory to full medical treatment. If the symptoms settle, those who had evidence of myocardial damage during the acute episode (an increase in the plasma concentration of troponin I or troponin T), and therefore had an NSTEMI, or those who have ongoing evidence of reversible myocardial ischaemia, should also be investigated by angiography.
Management of ST-segment elevation myocardial infarction
The presence of ST-segment elevation on the ECG usually heralds a more extensive myocardial infarction (STEMI) and, in contrast to NSTEMI, assisted early opening of the occluded artery to reperfuse the myocardium limits the extent of myocardial damage and improves long-term outcomes. Reperfusion should be considered if there is characteristic ST-segment elevation in two or more contiguous leads or left bundle branch block on the ECG and a good history of acute myocardial infarction. In the latter situation, an acute myocardial infarction cannot be easily diagnosed from the ECG but mortality is high. The greatest reduction in mortality is achieved in people at highest risk of death (i.e. anterior infarcts rather than inferior), older people (>65 years of age) and those with a presenting systolic blood pressure below 100 mmHg. Reperfusion therapy significantly reduces mortality if given within 12 h of the onset of pain, but the survival advantage is greater the earlier treatment is given.
 Analgesia and oxygen may be given as described above for unstable angina/NSTEMI. An intravenous β-adrenoceptor antagonist can be given to reduce cardiac work, especially if there is hypertension, but should be avoided if there are signs of heart failure.
Analgesia and oxygen may be given as described above for unstable angina/NSTEMI. An intravenous β-adrenoceptor antagonist can be given to reduce cardiac work, especially if there is hypertension, but should be avoided if there are signs of heart failure.
 ‘Primary’ PCI (coronary angioplasty, usually with insertion of a stent) is the treatment of choice for reperfusion in STEMI if it can be started within 120 min of presentation. There is a greater reduction in mortality than using thrombolytic therapy. ‘Rescue’ PCI can be considered if thrombolysis has failed to reperfuse the infarct-related vessel. Anticoagulation with bivalirudin or a combination of heparin and a glycoprotein IIb/IIIa antiplatelet drug (Ch. 11) is essential at the time of primary PCI, in addition to dual oral antiplatelet therapy. A combination of aspirin with an oral ADP receptor antagonist (either prasugrel, ticagrelor or clopidogrel; see Ch. 11) is continued for at least 12 months after primary PCI, especially if a drug-eluting stent has been inserted.
‘Primary’ PCI (coronary angioplasty, usually with insertion of a stent) is the treatment of choice for reperfusion in STEMI if it can be started within 120 min of presentation. There is a greater reduction in mortality than using thrombolytic therapy. ‘Rescue’ PCI can be considered if thrombolysis has failed to reperfuse the infarct-related vessel. Anticoagulation with bivalirudin or a combination of heparin and a glycoprotein IIb/IIIa antiplatelet drug (Ch. 11) is essential at the time of primary PCI, in addition to dual oral antiplatelet therapy. A combination of aspirin with an oral ADP receptor antagonist (either prasugrel, ticagrelor or clopidogrel; see Ch. 11) is continued for at least 12 months after primary PCI, especially if a drug-eluting stent has been inserted.
 Natural fibrinolysis can be enhanced by intravenous fibrinolytic therapy (Ch. 11) to rapidly reperfuse the occluded artery and limit the size of the infarct. Treatment with thrombolytic therapy is now limited mainly to people who cannot be rapidly transferred to a centre that carries out primary PCI. The preferred agents are alteplase (recombinant tissue plasminogen activator, rt-PA) or a synthetic rt-PA analogue such as tenecteplase. Alteplase and related compounds are relatively short-acting, and subsequent anticoagulation reduces reocclusion of the artery. Fondaparinux (Ch. 11) for 8 days after thrombolysis reduces mortality and reinfarction by up to 25% more than heparin. Streptokinase is rarely used now, since it has a lower success rate for opening occluded arteries and produces symptomatic hypotension during about 10% of administrations.
Natural fibrinolysis can be enhanced by intravenous fibrinolytic therapy (Ch. 11) to rapidly reperfuse the occluded artery and limit the size of the infarct. Treatment with thrombolytic therapy is now limited mainly to people who cannot be rapidly transferred to a centre that carries out primary PCI. The preferred agents are alteplase (recombinant tissue plasminogen activator, rt-PA) or a synthetic rt-PA analogue such as tenecteplase. Alteplase and related compounds are relatively short-acting, and subsequent anticoagulation reduces reocclusion of the artery. Fondaparinux (Ch. 11) for 8 days after thrombolysis reduces mortality and reinfarction by up to 25% more than heparin. Streptokinase is rarely used now, since it has a lower success rate for opening occluded arteries and produces symptomatic hypotension during about 10% of administrations.
In addition to the management discussed above, complications of myocardial infarction may need specific treatment (Box 5.1).
Secondary prophylaxis after myocardial infarction
Secondary prophylaxis to reduce late mortality after myocardial infarction requires a broad-based approach. These interventions are additive and not mutually exclusive.
 Stopping smoking is of major benefit, and reduces mortality after a myocardial infarction by up to 50%.
Stopping smoking is of major benefit, and reduces mortality after a myocardial infarction by up to 50%.
 Rehabilitation programmes which include exercise reduce mortality by up to 25% and improve psychological recovery.
Rehabilitation programmes which include exercise reduce mortality by up to 25% and improve psychological recovery.
 Low-dose aspirin combined with clopidogrel (Ch. 11) inhibit platelet aggregation and reduce mortality in the first few weeks when started within 24 h of the onset of pain. Overall mortality is reduced by at least 25%. The combination is effective following both STEMI and NSTEMI.
Low-dose aspirin combined with clopidogrel (Ch. 11) inhibit platelet aggregation and reduce mortality in the first few weeks when started within 24 h of the onset of pain. Overall mortality is reduced by at least 25%. The combination is effective following both STEMI and NSTEMI.
 Beta-adrenoceptor antagonists, started orally soon after the infarct, reduce both death and reinfarction by about 25%. The mechanism is unknown. Greatest benefit is seen in those at highest risk, for example following anterior infarction and in those who have had serious post-infarct arrhythmias, post-infarct angina or heart failure. Heart failure should be controlled before a β-adrenoceptor antagonist is given (Ch. 7).
Beta-adrenoceptor antagonists, started orally soon after the infarct, reduce both death and reinfarction by about 25%. The mechanism is unknown. Greatest benefit is seen in those at highest risk, for example following anterior infarction and in those who have had serious post-infarct arrhythmias, post-infarct angina or heart failure. Heart failure should be controlled before a β-adrenoceptor antagonist is given (Ch. 7).
 An ACE inhibitor (Ch. 6) is of greatest benefit if there is clinical or radiological evidence of heart failure after myocardial infarction, with a reduction in mortality of about 25% over the subsequent year. There is a smaller survival advantage if there is significant left ventricular dysfunction after the infarction (an ejection fraction of 40% or less) without clinical evidence of heart failure, with a 20% reduction in mortality over 3–5 years. This is accompanied by a significant reduction in non-fatal reinfarction, the mechanism of which is unknown. ACE inhibitors also reduce both non-fatal reinfarction and death when there is well-preserved left ventricular function, although the absolute benefits are smaller. The effects of an ACE inhibitor are greatest with high doses. An angiotensin receptor antagonist (Ch. 6) has similar efficacy following myocardial infarction, and should be considered if an ACE inhibitor is poorly tolerated.
An ACE inhibitor (Ch. 6) is of greatest benefit if there is clinical or radiological evidence of heart failure after myocardial infarction, with a reduction in mortality of about 25% over the subsequent year. There is a smaller survival advantage if there is significant left ventricular dysfunction after the infarction (an ejection fraction of 40% or less) without clinical evidence of heart failure, with a 20% reduction in mortality over 3–5 years. This is accompanied by a significant reduction in non-fatal reinfarction, the mechanism of which is unknown. ACE inhibitors also reduce both non-fatal reinfarction and death when there is well-preserved left ventricular function, although the absolute benefits are smaller. The effects of an ACE inhibitor are greatest with high doses. An angiotensin receptor antagonist (Ch. 6) has similar efficacy following myocardial infarction, and should be considered if an ACE inhibitor is poorly tolerated.
 Verapamil and diltiazem produce a small reduction in reinfarction, but do not reduce mortality. They may be detrimental if there have been symptoms or signs of heart failure. These drugs should be considered as an option only for those at high risk who cannot tolerate a β-adrenoceptor antagonist and who do not have significant left ventricular dysfunction. Dihydropyridine calcium channel blockers have no effect on prognosis after myocardial infarction.
Verapamil and diltiazem produce a small reduction in reinfarction, but do not reduce mortality. They may be detrimental if there have been symptoms or signs of heart failure. These drugs should be considered as an option only for those at high risk who cannot tolerate a β-adrenoceptor antagonist and who do not have significant left ventricular dysfunction. Dihydropyridine calcium channel blockers have no effect on prognosis after myocardial infarction.
 Long-term anticoagulation with warfarin (Ch. 11) reduces mortality and reinfarction to a similar extent to low-dose aspirin. In combination with aspirin, warfarin produces an additional reduction in both fatal and non-fatal events but with an increased risk of bleeding.
Long-term anticoagulation with warfarin (Ch. 11) reduces mortality and reinfarction to a similar extent to low-dose aspirin. In combination with aspirin, warfarin produces an additional reduction in both fatal and non-fatal events but with an increased risk of bleeding.
 Plasma cholesterol should be reduced to <4.0 mmol⋅L−1 by diet and a statin (Ch. 48). Statins reduce reinfarction and cardiac death by 25–30%. Fibrates are less effective, but may be useful if the total cholesterol is not greatly raised but the high-density lipoprotein cholesterol is low.
Plasma cholesterol should be reduced to <4.0 mmol⋅L−1 by diet and a statin (Ch. 48). Statins reduce reinfarction and cardiac death by 25–30%. Fibrates are less effective, but may be useful if the total cholesterol is not greatly raised but the high-density lipoprotein cholesterol is low.
 A Mediterranean diet reduces mortality after myocardial infarction. A further reduction in mortality can be achieved with supplementary omega-3 fatty acids. An anti-arrhythmic effect may be responsible for these benefits (Ch. 48).
A Mediterranean diet reduces mortality after myocardial infarction. A further reduction in mortality can be achieved with supplementary omega-3 fatty acids. An anti-arrhythmic effect may be responsible for these benefits (Ch. 48).
True/false questions
1. The increased oxygen demand produced by a rise in cardiac workload is met by an increase in coronary blood flow.
2. Nitric oxide (NO) causes vasodilation by increasing cAMP in vascular smooth muscle cells.
3. In angina, glyceryl trinitrate (GTN) increases total coronary blood flow.
4. Topical absorption of GTN from a transdermal patch avoids first-pass metabolism.
5. GTN can be taken safely with a β-adrenoceptor antagonist.
6. All β-adrenoceptor antagonists have vasodilator activity.
7. Resting bradycardia is less likely with a β-adrenoceptor antagonist with intrinsic sympathomimetic activity.
8. The anti-anginal action of amlodipine arises from its negative chronotropic and inotropic effects on the heart.
9. Enhancing the efflux of K+ ions hyperpolarises vascular smooth muscle cells.
10. Ivabradine slows the heart rate and reduces myocardial contractility.
11. Percutaneous coronary intervention (PCI) reduces mortality after a ST-elevation myocardial infarction (STEMI) more than thrombolytic therapy.
12. Drug-eluting stents reduce the risk of re-stenosis after angioplasty.
One-best-answer (OBA) questions
1. Which statement about angina and myocardial infarction is incorrect?
A Isosorbide mononitrate does not undergo first-pass metabolism.
B Verapamil can reduce arterial blood pressure without causing reflex tachycardia.
C Antiplatelet drugs such as tirofiban can reduce the risk of myocardial infarction in high-risk individuals with unstable angina.
D Cholesterol reduction is of little benefit in reducing the risk of recurrence of myocardial infarction.
E Nifedipine does not improve prognosis after myocardial infarction.
2. Which change to the treatment regimen would is most likely to reduce the risk of tolerance developing to isosorbide mononitrate?
A Switch to its longer-acting precursor, isosorbide dinitrate.
B Give GTN in addition by the buccal route when necessary.
C Switch to a continuously applied transdermal GTN patch.
D Schedule doses so that there is a period of low plasma concentration of isosorbide mononitrate each day.
E Administer isosorbide mononitrate together with a β-adrenoceptor antagonist
3. Which drug combination is most likely to have an adverse effect on cardiac function in a person suffering from angina?
Case-based questions
Mr TK, a 65-year-old man who works as a landscape gardener, has been having episodes of chest pain that he likened to indigestion. They were brought on by moderately strenuous exercise and relieved by rest but not by antacids. The symptoms had been present for approximately 1 year, but recently the frequency and intensity of the pains had become worse and were now occurring several times a week. He is hypertensive and his serum cholesterol is 6.6 mmol⋅L−1. He smokes 40 cigarettes per day and is overweight. He drinks about 10 units of alcohol a week. He had a good exercise tolerance during a diagnostic exercise test but his ECG showed anterolateral ST-segment depression at peak exercise. There is no evidence of heart failure. A diagnosis of angina is made, and medical treatment started.
Six months later, despite continuing medication, Mr TK awoke with severe chest pains and dyspnoea that was not relieved by GTN. An ECG showed an acute STEMI.
A How could his acute attacks of angina be treated?
B The frequency of his attacks requires prophylactic treatment. What options are available to reduce the frequency of anginal attacks?
C What other drugs could be useful to improve his prognosis?
D Would lifestyle changes help Mr TK?
E In unstable angina, which drug treatments would be likely to reduce the progression of the episodes to myocardial infarction or sudden death?
F What is the likely cause of the myocardial infarction?
G Why is it important to give fibrinolytic therapy as quickly as possible?
H Mr TK is given the fibrinolytic agent altepase (recombinant tissue plasminogen activator, rt-PA) because of fears that he would get an allergic response to streptokinase. Is this justified?
I He is given 150 mg aspirin orally, after an initial loading dose. Would this have any added benefit if fibrinolytic therapy is also given?
J The normal therapeutic dose of aspirin for headache is about 650 mg. Why is the dose given to Mr TK so small?
K Consideration is given to giving fondaparinux to Mr TK, but this is considered unnecessary because he had been given rt-PA. Is this decision correct?
L Following his myocardial infarction, long-term prophylactic treatment of his condition was considered. Which of the following drugs would have been likely to be of benefit: an ACE inhibitor, low-dose aspirin, a β-adrenoceptor antagonist, diltiazem, verapamil or warfarin?
1. True. Increased coronary blood flow meets the increased myocardial oxygen demand, as the proportion of oxygen removed from the coronary artery blood is high (75%) even at rest.
2. False. Nitric oxide vasodilates by increasing synthesis of cGMP, which reduces intracellular Ca2+. Organic nitrates act by the same pathway.
3. False. Although GTN can reduce coronary vasospasm or dilate collateral blood vessels, it does not increase total coronary blood flow. Its main actions are peripheral venodilation, which reduces venous return (preload), and reduced peripheral resistance (afterload), both of which reduce cardiac work and oxygen demand.
4. True. GTN taken orally undergoes extensive first-pass metabolism due to delivery to the liver in the hepatic portal circulation; this is avoided by transdermal or sublingual administration, from which the drug gains direct access to the systemic circulation.
5. True. The main anti-anginal action of β-adrenoceptor antagonists is to reduce myocardial oxygen demand by reducing heart rate and contractility. They thus reduce the reflex tachycardia that may occur with GTN and other nitrates.
6. False. βlockade of β1-adrenoceptors produces reflex vasoconstriction, but some β-adrenoceptor antagonists produce peripheral vasodilation by partial agonism at β2-adrenoceptors (e.g. pindolol), by α1-adrenoceptor blockade (e.g. carvedilol) or by activation of NO synthesis (nebivolol).
7. True. Drugs with intrinsic sympathomimetic (partial agonist) activity at β1-adrenoceptors produce less bradycardia at rest, but may be less effective than full antagonists in reducing heart rate on exertion.
8. False. Dihydropyridine calcium channel blockers mainly reduce myocardial oxygen demand by reducing peripheral arterial resistance and venous return; they do not reduce heart rate and amlodipine does not reduce myocardial contractility.
9. True. Potassium channel openers such as nicorandil hyperpolarise vascular myocytes by enhancing K+ efflux; this closes voltage-gated L-type Ca2+ channels, leading to smooth muscle relaxation.
10. False. Ivabradine slows the heart by a direct action at the sinoatrial node without effect on myocardial contractility, unlike β-adrenoceptor antagonists.
11. True. PCI with stent insertion within 120 min of a STEMI is preferred to thrombolytic therapy and can also be used when thrombolysis has failed.
12. True. Stents coated with polymers that elute an antiproliferative drug (e.g. tacrolimus) can reduce re-stenosis compared to bare-metal stents.
OBA answers
A Correct. Isosorbide mononitrate can therefore give a more predictable response of greater duration than the dinitrate.
B Correct. Unlike the dihydropyridines, verapamil also acts on the heart and reflex tachycardia does not occur.
C Correct. Platelet inhibition with tirofiban, an intravenous glycoprotein IIb/IIIa antagonist, reduces the risk of MI or death in those at high risk (see also Ch. 11). These agents are most effective after a non-ST-elevation myocardial infarction.
D Incorrect. Cholesterol reduction to <4.0 mmol⋅L−1 should be attempted and statins reduce reinfarction and cardiac death by 25–30% (Ch. 48).
E Correct. Dihydropyridine calcium channel antagonists do not improve prognosis after myocardial infarction. Verapamil and diltiazem produce a small reduction in reinfarction, but do not reduce mortality and may be detrimental if there are signs of heart failure.
The only way to reduce tolerance is to allow daily periods with low plasma concentrations of organic nitrate. Tolerance will develop to all the organic nitrates independent of the route given if plasma concentrations remain high continuously. A β-adrenoceptor antagonist will reduce reflex tachycardia but not the development of tolerance.
A Unlikely. Atenolol prevents reflex tachycardia caused by the nitrate.
B Most likely. Verapamil and atenolol both have a negative inotropic effect and this could be a problem, particularly if there are signs of heart failure. They also have a negative chronotropic effect and the combination can cause severe bradycardia and heart block.
C Unlikely, although amlodipine and atenolol might cause excessive hypotension.
D Unlikely. Nicorandil does not have a direct effect on the heart.
E Unlikely. Aspirin will reduce the likelihood of development of myocardial infarction.
Case-based answers
A Sublingual GTN is the first-choice drug for rapid relief of Mr TK's acute anginal attacks, although protection is only short-lived.
B For prophylaxis, a β-adrenoceptor antagonist is often the treatment of first choice, or a calcium channel blocker if a β-adrenoceptor antagonist is contraindicated. If symptoms are not well controlled with either agent alone, a combination of both drugs, or the addition of a long-acting nitrate could be used, but the benefit of triple therapy is not convincing. Dual therapy with atenolol and diltiazem could significantly decrease the number of anginal attacks compared with either drug alone, and will also lower blood pressure and heart rate, which are precipitating factors for angina. The combination should be carefully monitored, however, because of the risk of compounding bradycardia or heart failure.
C Additional therapy to improve prognosis includes low-dose aspirin or a statin drug to lower plasma cholesterol, both of which have been shown to reduce the risk of subsequent myocardial infarction.
D Smoking, lack of exercise and obesity are all risk factors for coronary heart disease. TK is exposed to these increased risks and should address them by lifestyle changes.
E The most consistent evidence is for combined use of aspirin with a β-adrenoceptor antagonist.
F Coronary artery occlusion at the site of a ruptured atheromatous plaque, causing myocardial necrosis.
G The benefit of fibrinolytic therapy is strongly dependent upon the delay between symptoms and administration. The benefit is particularly great if fibrinolytic therapy can be administered within 6 h from the onset of pain, but there is good evidence for benefit until at least 12 h.
H Allergic reactions to streptokinase are extremely rare. Mr TK had not had a previous myocardial infarction and had not previously been given streptokinase, so would be unlikely to have high titres of streptokinase-neutralising antibodies. It would, therefore, be safe to give him streptokinase unless he had severe symptomatic hypotension. However, alteplase or an rt-PA derivative is usually preferred.
I Aspirin and fibrinolytic therapy have additive benefit for treating acute myocardial infarction, reducing subsequent reinfarction or death.
J Low doses of aspirin selectively reduce production of the platelet-aggregating agent thromboxane A2 by platelets, while having less effect on the production of the platelet-disaggregating agent prostacyclin (prostaglandin I2, PGI2) from endothelial cells. Large doses of aspirin do not produce additional benefit, and the risk of gastric irritation or ulceration is increased.
K The decision is incorrect. Giving fondaparinux or heparin is necessary to improve the long-term patency of the artery as rt-PA is short-acting; it would not be necessary with streptokinase, which has a longer duration of action than rt-PA.
L ACE inhibitors, low-dose aspirin and β-adrenoceptor antagonists all reduce mortality and the risk of reinfarction. The β-adrenoceptor antagonist will need to be given under close observation, since it carries a risk of worsening heart failure. Verapamil and diltiazem may also be detrimental in people who have signs of heart failure. Warfarin reduces mortality and reinfarction to a similar extent as low-dose aspirin but with a greater risk of bleeding, so is not required unless aspirin is poorly tolerated.
Hansson, GK. Inflammation, atherosclerosis and coronary heart disease. N Engl J Med. 2005;352:1685–1695.
Tamargo, J, Caballero, R, Gomez, R, et al. Pharmacology of cardiac potassium channels. Cardiovasc Res. 2004;62:9–33.
Abrams, J. Chronic stable angina. N Engl J Med. 2005;352:2524–2533.
Deedwania, PC, Carbajal, EV. Medical therapy versus myocardial revascularization in chronic coronary syndrome and stable angina. Am J Med. 2011;124:681–688.
Ben-Dor, I, Battler, A. Treatment of stable angina. Heart. 2007;93:868–874.
Fayers, KE, Cummings, MH, Shaw, KM, et al. Nitrate tolerance and the links with endothelial dysfunction and oxidative stress. Br J Clin Pharmacol. 2003;56:620–628.
Feher, MD. Lipid lowering to delay the progression of coronary artery disease. Heart. 2003;89:451–458.
Heidenreich, PA, McDonald, KM, Haslie, T, et al. Meta-analysis of trials comparing β-blockers, calcium antagonists and nitrates for stable angina. JAMA. 1999;281:1927–1936.
Knight, CJ. Antiplatelet treatment in stable coronary artery disease. Heart. 2003;89:1273–1278.
Ko, DT, Hebert, PR, Coffey, CS, et al. β-blocker therapy and symptoms of depression, fatigue, and sexual dysfunction. JAMA. 2002;288:351–357.
Nash, DT, Nash, SD. Ranolazine for chronic stable angina. Lancet. 2008;372:1335–1341.
Ong, HT. β blockers in hypertension and cardiovascular disease. BMJ. 2007;334:946–949.
Opie, LH, Commerford, PJ, Gersh, BJ. Controversies in stable coronary heart disease. Lancet. 2007;367:69–78.
Pfisterer, ME, Zellweger, MJ, Gersh, BJ. Management of stable coronary artery disease. Lancet. 2010;375:763–772.
Toda, N. Vasodilating β-adrenoceptor blockers as cardiovascular therapeutics. Pharmacol Ther. 2003;100:215–234.
Wei, J, Wu, T, Yang, Q, et al. Nitrates for stable angina: A systematic review and meta-analysis of randomized clinical trials. Int J Cardiol. 2011;146:4–12.
Aronow, WS. Office management of myocardial infarction. Am J Med. 2010;1213:593–595.
Chan, MY, Becker, RC, Harrington, RA, et al. Noninvasive, medical management for non-ST-elevation acute coronary syndromes. Am Heart J. 2008;155:397–407.
Peters, RJG, Mehta, S, Yusuf, S. Acute coronary syndromes without ST segment elevation. BMJ. 2007;334:1256–1259.
Rothberg, MB, Celestin, C, Fiore, LD, et al. Warfarin plus aspirin after myocardial infarction or acute coronary syndrome: meta-analysis with estimates of risk and benefit. Ann Intern Med. 2005;143:241–250.
Brouwer, MA, Clappers, N, Verheugt, FWA. Adjunctive treatment in patients treated with thrombolytic therapy. Heart. 2004;90:581–588.
Dalal, H, Evans, PH, Campbell, JL. Recent developments in secondary prevention and cardiac rehabilitation after acute myocardial infarction. BMJ. 2004;328:693–697.
Keeley, EC, Hillis, LD. Primary PCI for myocardial infarction with ST-segment elevation. N Engl J Med. 2007;356:47–54.
Lee, VC, Rhew, DC, Dylan, M, et al. Meta-analysis: angiotensin-receptor blockers in chronic heart failure and high-risk acute myocardial infarction. Ann Intern Med. 2004;41:693–704.
White, HD, Chew, DP. Acute myocardial infarction. Lancet. 2008;372:570–584.