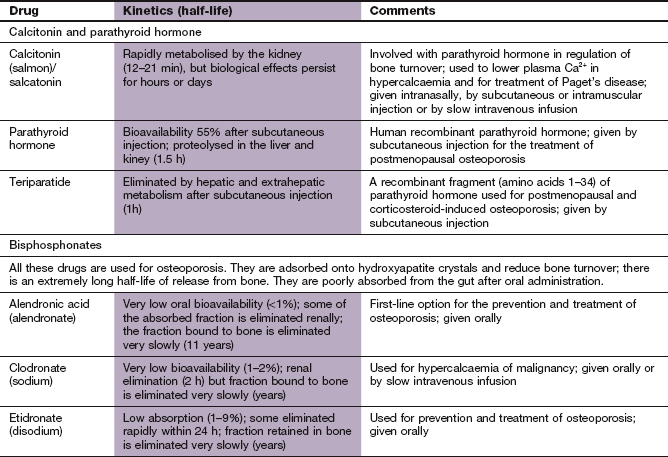
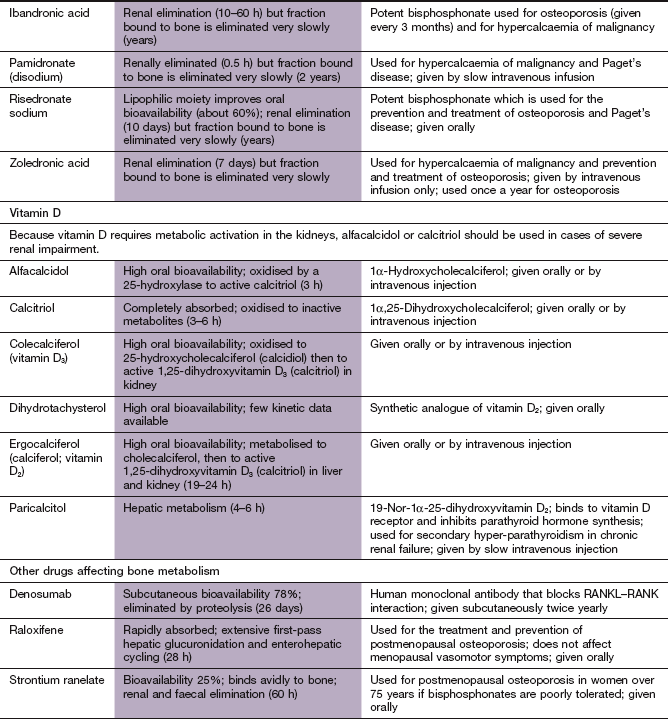
Calcium metabolism and metabolic bone disease
Regulation of calcium metabolism
Calcium ions play a part in a large number of cellular activities, including stimulus–response coupling in striated and smooth muscle, and in endocrine and exocrine glands. Calcium modulates the actions of intracellular cAMP and is a cofactor for numerous intracellular enzymes and for blood clotting. However, more than 98% of Ca2+ in the body is in the form of hydroxyapatite crystals deposited on the protein matrix of bone, which provides its mechanical strength.
Calcium circulates in plasma partly bound to protein (approximately 50%) and the rest in the free ionised (and therefore ‘active’) form. The free fraction in plasma is maintained precisely within narrow limits principally by the actions of parathyroid hormone (PTH) and 1,25-dihydroxyvitamin D3 (calcitriol). Calcitonin secretion (see below) also reacts to changing plasma Ca2+ concentrations but it is less important in overall control of Ca2+ homeostasis. Calcium in plasma is in dynamic exchange with Ca2+ in the gut, renal tubules and bone. This is illustrated with the main controlling factors in Figure 42.1.
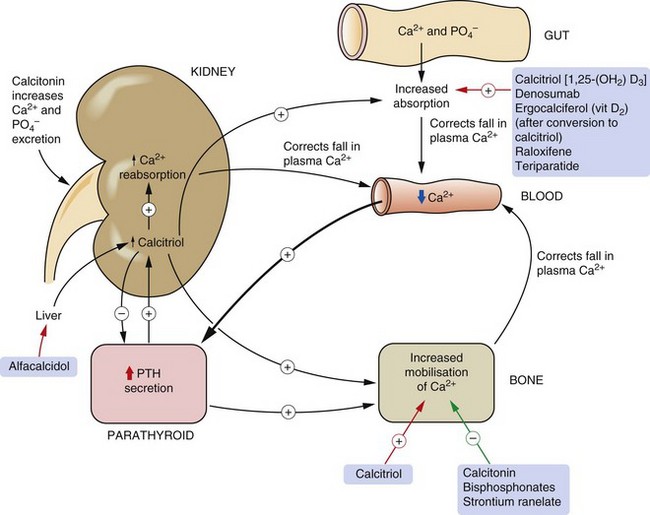
Fig. 42.1 Regulation of calcium metabolism.
A fall in plasma Ca2+ leads to increased release of parathyroid hormone (PTH) from the parathyroid gland, which increases calcitriol [1,25-(OH2)-D3] formation in the kidney. This in turn increases gut absorption of Ca2+. PTH further increases bone mobilisation of Ca2+ to return plasma Ca2+ to normal. An increase in plasma Ca2+, conversely, decreases PTH secretion. Calcitonin, secreted by the thyroid, decreases Ca2+ reabsorption from the kidney and decreases bone turnover. Drugs used for hypercalcaemia are indicated by green arrows and drugs for hypocalcaemia by red arrows.
PTH is a polypeptide hormone which is the main physiological regulator of Ca2+ in blood. Its secretion from parathyroid chief cells is stimulated by a reduction of ionised Ca2+ in plasma. PTH secretion is inhibited when the plasma Ca2+ concentration rises.
The main actions of PTH relating to calcium homeostasis are:
 stimulation of the synthesis of the biologically active form of vitamin D (calcitriol) in the kidney by upregulation of the enzyme responsible for 1α-hydroxylation,
stimulation of the synthesis of the biologically active form of vitamin D (calcitriol) in the kidney by upregulation of the enzyme responsible for 1α-hydroxylation,
 enhanced reabsorption of Ca2+ from the kidney distal tubules and enhancement of urinary phosphate excretion. The rise in plasma Ca2+/phosphate ratio also increases plasma free Ca2+,
enhanced reabsorption of Ca2+ from the kidney distal tubules and enhancement of urinary phosphate excretion. The rise in plasma Ca2+/phosphate ratio also increases plasma free Ca2+,
 mobilisation of Ca2+ and phosphate from bone through stimulation of osteoclasts, which increases bone resorption. Osteoclasts do not have a receptor for PTH. PTH binds to osteoblasts and increases their expression of the surface molecule human receptor activator of nuclear factor-κB ligand (RANKL) and inhibits their expression of the surface receptor osteoprotegerin. Osteoprotegerin is the natural inhibitor of osteoclast activity by acting as a decoy receptor for binding RANKL. RANKL that is not bound to osteoprotegerin interacts with and activates RANK on osteoclasts and stimulates differentiation of osteoclast precursors to mature osteoclasts.
mobilisation of Ca2+ and phosphate from bone through stimulation of osteoclasts, which increases bone resorption. Osteoclasts do not have a receptor for PTH. PTH binds to osteoblasts and increases their expression of the surface molecule human receptor activator of nuclear factor-κB ligand (RANKL) and inhibits their expression of the surface receptor osteoprotegerin. Osteoprotegerin is the natural inhibitor of osteoclast activity by acting as a decoy receptor for binding RANKL. RANKL that is not bound to osteoprotegerin interacts with and activates RANK on osteoclasts and stimulates differentiation of osteoclast precursors to mature osteoclasts.
The effect of PTH on the kidney occurs within minutes of PTH release, whereas that on bone begins after 1–2 h.
Vitamin D (calciferol) is a group of compounds that have secosteroid nuclei (a steroid nucleus with one bond in the steroid ring broken). There are two precursors of active vitamin D. Ergocalciferol (vitamin D2) is derived from food and absorbed from the gut. However, given adequate ultraviolet B sunlight, the major source of vitamin D is conversion of 7-dehydrocholesterol in the skin to cholecalciferol (vitamin D3). Therefore, vitamin D is really a skin-derived hormone rather than a vitamin but this source was discovered after the dietary origins. Vitamins D2 and D3 are further metabolised in the liver to 25-hydroxyvitamin D3 (calcidiol), and then in the kidney to 1,25-dihydroxyvitamin D3 (calcitriol). 1α-Hydroxylation is an essential step for activation of vitamin D, and PTH stimulates 1α-hydroxylase activity in the kidney, increasing the formation of calcitriol. Calcitriol binds to specific vitamin D receptors in the target cell nucleus (Ch. 1) and the vitamin D–receptor complex acts as a transcription factor that increases the synthesis of Ca2+ transport proteins in the gut. The main effect of active forms of vitamin D is to increase the plasma concentration of Ca2+ by:
 facilitating absorption of Ca2+ from the small intestine,
facilitating absorption of Ca2+ from the small intestine,
 enhancing Ca2+ mobilisation from bone by increasing osteoclastic numbers and activity
enhancing Ca2+ mobilisation from bone by increasing osteoclastic numbers and activity
In the kidney, vitamin D promotes phosphate retention, in contrast to the action of PTH. Therefore, vitamin D maintains the plasma Ca2+ and phosphate concentrations to allow normal osteoblast function. These actions of vitamin D affect Ca2+ turnover in bone over periods of days to weeks.
Calcitonin is a peptide secreted by the parafollicular cells of the thyroid when its calcium-sensing receptors detect a rise in plasma Ca2+. The main target cell for calcitonin is the osteoclast, which it inhibits by stimulation of adenylyl cyclase, thus reducing bone turnover. Calcitonin also decreases Ca2+ and phosphate reabsorption by the kidney, thereby increasing their renal excretion.
Physiology of bone turnover
Bone is constantly undergoing remodelling (bone turnover), which involves resorption and replacement of small areas of bone. Up to 10% of bone undergoes remodelling at any point in time, and trabecular bone (in the ends of long bones and in the vertebrae) undergoes greater turnover than cortical bone. Resorption leaves trenches on the bone surface and osteoclasts recruit osteoblasts to refill the trenches. This process is essential for maintenance of Ca2+ homeostasis, replacement of apoptotic osteoclasts and repair of microfractures. There are many factors that regulate bone turnover, but the final pathway is via the balance between RANKL expressed by osteoblasts and activated T- and B-lymphocytes, and osteoprotegerin expressed by osteoblasts (see above). The balance between osteoblast and osteoclast activity and therefore the extent of bone remodelling is modulated by an interaction between various cells in the immune system (lymphocytes and dendritic cells), cytokines and circulating hormones.
Hypercalcaemia
The main causes of hypercalcaemia are:
 increased resorption of Ca2+ from bone; for example, primary hyperparathyroidism, secretion of parathyroid-related hormone by cancer cells and bony metastases,
increased resorption of Ca2+ from bone; for example, primary hyperparathyroidism, secretion of parathyroid-related hormone by cancer cells and bony metastases,
 increased absorption of Ca2+ from the gut through excessive use of vitamin D or in sarcoidosis,
increased absorption of Ca2+ from the gut through excessive use of vitamin D or in sarcoidosis,
 reduced renal excretion of Ca2+; for example, as caused by thiazide diuretics (Ch. 14).
reduced renal excretion of Ca2+; for example, as caused by thiazide diuretics (Ch. 14).
Hypercalcaemia occurs when the mobilisation of Ca2+ into the extracellular space exceeds the capacity to remove it. Chronic moderate hypercalcaemia leads to a progressive decline in renal function, formation of renal stones and ectopic calcification (e.g. cornea, blood vessels). Severe hypercalcaemia causes anorexia, nausea, vomiting, constipation, drowsiness and confusion, eventually leading to coma. Hypercalcaemia impairs the ability of the kidney to reabsorb salt and water which in conjunction with vomiting can lead to depletion of plasma volume and pre-renal failure. Urgent treatment is indicated when the plasma Ca2+ concentration rises above 3.5 mmol⋅L−1 (normal <2.6 mmol⋅L−1), since sudden death from cardiac arrest can occur.
Antiresorptive drugs for hypercalcaemia
Mechanisms of action and effects: Bisphosphonates are pyrophosphate analogues that bind to hydroxyapatite crystals in bone matrix. They are preferentially deposited under osteoclasts, and are taken up by these cells and inhibit their resorptive action on bone. There are two different cellular actions of the drugs on osteoclasts, depending on the structure of the bisphosphonate.
 Amino-bisphosphonates (nitrogen-containing drugs: alendronic acid, disodium pamidronate, risedronate sodium, zoledronic acid) act by inhibition of the ATP-dependent enzyme farnesyl pyrophosphate synthase in the synthetic pathway from mevalonic acid to cholesterol; this reduces the production of lipids that are essential for signalling processes required for normal osteoclast function, and leads to impaired differentiation of osteoclast precursors, reduced ability of mature osteoclasts to reabsorb bone by altering the permeability of osteoclast membranes to small ions and, eventually, osteoclast apoptosis.
Amino-bisphosphonates (nitrogen-containing drugs: alendronic acid, disodium pamidronate, risedronate sodium, zoledronic acid) act by inhibition of the ATP-dependent enzyme farnesyl pyrophosphate synthase in the synthetic pathway from mevalonic acid to cholesterol; this reduces the production of lipids that are essential for signalling processes required for normal osteoclast function, and leads to impaired differentiation of osteoclast precursors, reduced ability of mature osteoclasts to reabsorb bone by altering the permeability of osteoclast membranes to small ions and, eventually, osteoclast apoptosis.
 Non-nitrogen-containing drugs (sodium clodronate, disodium etidronate) affect metabolism within the cell by forming a toxic analogue of ATP that induces osteoclast apoptosis. These drugs have a relatively weak antiresorptive action.
Non-nitrogen-containing drugs (sodium clodronate, disodium etidronate) affect metabolism within the cell by forming a toxic analogue of ATP that induces osteoclast apoptosis. These drugs have a relatively weak antiresorptive action.
The actions of most bisphosphonates are relatively short-lived unless taken regularly, but zoledronic acid can suppress bone resorption for up to a year after a single dose.
Pharmacokinetics: Bisphosphonates are poorly absorbed from the gut, and oral formulations are best taken once weekly with the stomach empty to avoid binding by Ca2+ in food. Alendronic acid and risedronate sodium are only available in oral formulations while disodium pamidronate and zoledronic acid are only formulated for intravenous use. Removal of most bisphosphonates from blood via the kidney is rapid, but their effect is prolonged since a fraction remains tightly bound to Ca2+ in bone.
 Gastrointestinal disturbance, particularly nausea, abdominal pain, diarrhoea or constipation with the oral treatments.
Gastrointestinal disturbance, particularly nausea, abdominal pain, diarrhoea or constipation with the oral treatments.
 Headache, dizziness, musculoskeletal pain.
Headache, dizziness, musculoskeletal pain.
 Alendronic acid and risedronate sodium can cause severe oesophagitis and oesophageal strictures. To reduce the risk, the tablets should be swallowed intact with a full glass of water at least 30 min before food and followed by standing or sitting (but not lying down) for at least 30 min after ingestion. Once-weekly dosing also reduces the risk of oesophageal damage.
Alendronic acid and risedronate sodium can cause severe oesophagitis and oesophageal strictures. To reduce the risk, the tablets should be swallowed intact with a full glass of water at least 30 min before food and followed by standing or sitting (but not lying down) for at least 30 min after ingestion. Once-weekly dosing also reduces the risk of oesophageal damage.
 Transient pyrexia and influenza-like symptoms after intravenous infusion.
Transient pyrexia and influenza-like symptoms after intravenous infusion.
 Osteonecrosis of the jaw, especially after intravenous use, and atypical femoral fractures.
Osteonecrosis of the jaw, especially after intravenous use, and atypical femoral fractures.
Calcitonin
Mechanism of action and effects: The actions of calcitonin on bone and the kidney to reduce plasma Ca2+ concentrations have been discussed above. Calcitonin begins to act within a few hours of administration, with a maximum effect within 12–24 h. However, the hypocalcaemic effect produced by repeated administrations only lasts between 2 and 3 days. The loss of clinical response results from downregulation of calcitonin receptors on osteoclasts, leading to a rebound increase in bone resorption.
Treatment of hypercalcaemia
When possible, the primary cause should be corrected, for example removal of a parathyroid adenoma or treatment of myeloma. Oral Ca2+ supplements, vitamin D and thiazide diuretics should be discontinued. Additional measures may include correction of dehydration, enhancing renal excretion of Ca2+ and inhibiting bone resorption.
Most people with severe hypercalcaemia are fluid-depleted at presentation. Rehydration with intravenous isotonic saline is essential; this also promotes a sodium-linked Ca2+ diuresis in the proximal and distal renal tubules. Loop diuretics such as furosemide (Ch. 14) increase renal Ca2+ elimination but should only be given with high volumes of intravenous isotonic saline and intensive monitoring of fluid balance to avoid dehydration.
A bisphosphonate such as disodium pamidronate or zoledronic acid by intravenous infusion is the drug treatment of first choice for severe hypercalcaemia. Initial intravenous rehydration is essential to avoid precipitation of calcium bisphosphonate in the kidney. Oral bisphosphonate treatment may be sufficient for less severe hypercalcaemia. Following a single intravenous infusion of bisphosphonate the plasma Ca2+ concentration falls gradually after 2–4 days, with a maximum effect after 4–7 days and a response that persists for 1–4 weeks after treatment. Because of the delay in onset of action of the bisphosphonates, calcitonin can be given concurrently for an early effect. Corticosteroids such as prednisolone (Ch. 44) are effective for lowering plasma Ca2+ when vitamin D excess is an important factor, for example in sarcoidosis and for acute treatment of vitamin D overdose, or for hypercalcaemia associated with haematological malignancy such as myeloma or lymphoma. Corticosteroids probably act by reducing the effect of vitamin D on intestinal Ca2+ transport, but can take several days to work.
Hypocalcaemia
There are two major underlying causes of hypocalcaemia:
 deficiency of PTH; for example, idiopathic hypoparathyroidism, after surgical parathyroid removal,
deficiency of PTH; for example, idiopathic hypoparathyroidism, after surgical parathyroid removal,
 deficiency of vitamin D; for example, dietary deficiency, limited exposure to sunlight, renal failure (failure of 1α-hydroxylation).
deficiency of vitamin D; for example, dietary deficiency, limited exposure to sunlight, renal failure (failure of 1α-hydroxylation).
Hypocalcaemia produces neuromuscular irritability with paraesthesiae of the extremities or around the mouth, muscle cramps and tetany. When severe, it can produce seizures. Chronic hypocalcaemia, especially in congenital hypoparathyroidism, is associated with mental deficiency, seizures, intracranial calcification (e.g. choroid plexus) and ocular cataracts.
Drugs for hypocalcaemia
Mechanism of action: This is discussed above. A dose-related increase in Ca2+ and phosphate absorption from the gut occurs at lower concentrations of vitamin D than those which stimulate bone resorption. Ergocalciferol is inactive and can only be used if 1α-hydroxylation by the kidney is intact. In renal impairment, the hydroxylated active forms alfacalcidol or calcitriol should be used. Paricalcitol is a synthetic vitamin D analogue used in chronic renal failure; it binds to the vitamin D receptor and inhibits PTH synthesis and secretion, but has less effect than natural vitamin D on the plasma Ca2+ concentration.
Pharmacokinetics: The fat-soluble D vitamins are well absorbed orally in the presence of bile. They can also be given intravenously. Both alfacalcidol and calcitriol are active forms of vitamin D; they have short half-lives (about 3 h) and are metabolised and excreted mainly in the bile. Paricalcitol requires intravenous injection.
Treatment of hypocalcaemia
Mild hypocalcaemia can be treated with oral Ca2+ supplements, taken between meals to avoid binding to dietary phosphate and oxalate which forms salts that are poorly absorbed. In the absence of reversible pathology such as malabsorption due to coeliac disease, the mainstay of treatment for more severe hypocalcaemia is vitamin D supplements. The few individuals who have vitamin D deficiency from inadequate diet or lack of exposure to sunlight (such as may be found in Asian women in the UK) will respond to small doses of vitamin D. Most causes of hypocalcaemia, however, require much larger doses (usually given as ergocalciferol) to maintain normocalcaemia. Oral Ca2+ supplements (as carbonate or citrate salts) are often used with vitamin D for the treatment of chronic hypocalcaemia.
For treatment of hypoparathyroidism, alfacalcidol is given; PTH is not used for replacement therapy (although PTH and a synthetic PTH fragment, teriparatide, are now licensed for treatment of osteoporosis; see below). Large doses of ergocalciferol could be used, but carry a risk of hypercalcaemia. The action of vitamin D begins after 2–4 weeks of treatment, because there is deficient renal hydroxylation of vitamin D in hypoparathyroidism; the action of calcitriol is much more rapid, beginning after 1–2 days, but it is rarely required unless a very rapid onset of action is necessary.
Acute severe hypocalcaemia (sometimes occurring after parathyroidectomy) must be treated with intravenous Ca2+ (as gluconate, gluceptate or chloride salt).
Metabolic bone disease
Osteomalacia is the bone disease resulting from failure of adequate bone mineralisation due to lack of vitamin D. Bone pain is prominent and low plasma concentrations of Ca2+ and phosphate produce proximal muscle weakness. In developing children the bones become distorted (rickets). Treatment is with vitamin D (ergocalciferol) supplements, but it will take at least a year to achieve a normal bone structure.
Screening for vitamin D deficiency
Vitamin D deficiency is most common in people with pigmented skin. In adults there is an additional risk in the elderly who have less exposure to sunlight, obese people, those with malabsorption or renal disease, or those who take anticonvulsants, rifampicin or highly active antiretroviral drugs. The rising incidence of vitamin D deficiency in infants has led to a recommendation that vitamin D supplements should be routinely given to children under 5 years old. In adults who are at risk, deficiency of vitamin D is usually suspected from bone pain affecting the ribs, hips and pelvis together with proximal muscle weakness.
Renal bone disease
Chronic renal disease is associated with deficient activation of vitamin D and hypocalcaemia. At the same time, reduced renal phosphate excretion leads to hyperphosphataemia. The low serum Ca2+ stimulates PTH secretion (secondary hyperparathyroidism) in an attempt to maintain the plasma Ca2+ concentration. The result is demineralisation of bone (renal bone disease) and soft tissue calcification from the increased plasma calcium–phosphorus product. Vascular calcification is associated with increased cardiovascular disease and mortality.
Treatment of renal bone disease requires a 1α-hydroxylated vitamin D derivative (such as alfacalcidol or calcitriol), which will increase plasma Ca2+ but does not affect the plasma phosphate. An oral non-Ca2+-containing phosphate binder such as aluminium hydroxide is necessary to reduce the plasma phosphate concentration if this is raised to avoid tissue calcification. People who are undergoing haemodialysis or ambulatory peritoneal dialysis cannot readily excrete absorbed aluminium, and an alternative phosphate binder such as sevelamer or lanthanum is used. Despite the symptomatic benefit of vitamin D in renal disease there is little evidence for any improvement in long-term mortality.
Osteoporosis
Osteoporosis is the loss of bone mass due to reduced organic bone matrix and, consequently, mineral content, which decreases the mechanical strength of bone. It results from an imbalance between bone resorption and formation, and affects trabecular bone more than cortical bone. It is a natural and inevitable part of the ageing process (beginning from age 30–35 years), and in females a marked increase in bone loss occurs after the menopause. Other predisposing factors include smoking, heavy alcohol intake, malnutrition, malabsorption and lack of exercise. Osteoporosis in younger people is associated with trabecular bone loss and predisposes to spontaneous vertebral fractures. In older people, cortical bone is also lost, increasing the risk of low-impact traumatic fracture, particularly of the neck of the femur. Sometimes osteoporosis is secondary to other conditions such as myeloma or thyrotoxicosis or occurs as a result of prolonged corticosteroid therapy (Ch. 44).
The diagnosis of osteoporosis is usually made by estimating bone mineral density from dual-energy X-ray absorptiometry (DEXA) scanning. Bone mineral density is then compared to mean bone density in a young adult reference population, and a T-score calculated (standard deviations of bone mineral density from the mean of the reference population):
Once established, osteoporosis is difficult to reverse, and emphasis should be placed on prevention where possible. Management of osteoporosis includes:
 non-pharmacological approaches, removing factors that increase the risk of demineralisation,
non-pharmacological approaches, removing factors that increase the risk of demineralisation,
 the use of either antiresorptive therapies (with an associated decrease in markers of bone formation and bone resorption: bisphosphonates, raloxifene, oestrogen, denosumab) or,
the use of either antiresorptive therapies (with an associated decrease in markers of bone formation and bone resorption: bisphosphonates, raloxifene, oestrogen, denosumab) or,
 anabolic therapies that lay down new bone (with increases in markers of bone formation and bone resorption: calcitonin, teriparatide),
anabolic therapies that lay down new bone (with increases in markers of bone formation and bone resorption: calcitonin, teriparatide),
 supplementary vitamin D which increases the laying down of hydroxyapatite on bone collagen organic matrix,
supplementary vitamin D which increases the laying down of hydroxyapatite on bone collagen organic matrix,
 strontium ranelate, which does not conform to any of the above patterns of effect on bone metabolism (see below).
strontium ranelate, which does not conform to any of the above patterns of effect on bone metabolism (see below).
Prevention of osteoporosis
Preventive strategies are important in those identified as being at high risk of osteoporosis. Use of a prediction tool for calculating the risk of future fractures can help to target treatment. Early use of preventive treatments is important if prolonged corticosteroid treatment is planned (Ch. 44).
 Oral calcium supplements increase bone mineral density in the spine in postmenopausal women, but with an uncertain effect on the risk of vertebral fractures. The addition of vitamin D (ergocalciferol) confers greater benefit, with a reduction in the risk of non-vertebral fractures. Recently, concern has been raised that high-dose Ca2+ supplements may increase the risk of myocardial infarction, but any increase in risk is modest.
Oral calcium supplements increase bone mineral density in the spine in postmenopausal women, but with an uncertain effect on the risk of vertebral fractures. The addition of vitamin D (ergocalciferol) confers greater benefit, with a reduction in the risk of non-vertebral fractures. Recently, concern has been raised that high-dose Ca2+ supplements may increase the risk of myocardial infarction, but any increase in risk is modest.
 Oral bisphosphonates (see above) are the treatment of choice for prevention of postmenopausal osteoporosis and corticosteroid-induced osteoporosis.
Oral bisphosphonates (see above) are the treatment of choice for prevention of postmenopausal osteoporosis and corticosteroid-induced osteoporosis.
 Hormone-replacement therapy (HRT) with oestrogen (Ch. 45) in peri- and postmenopausal women was once the mainstay of preventative treatment for osteoporosis. However, 5–10 years of oestrogen therapy may be required and long-term use of HRT increases the risk of breast cancer and thromboembolic events. As a consequence, the use of HRT for this indication has declined.
Hormone-replacement therapy (HRT) with oestrogen (Ch. 45) in peri- and postmenopausal women was once the mainstay of preventative treatment for osteoporosis. However, 5–10 years of oestrogen therapy may be required and long-term use of HRT increases the risk of breast cancer and thromboembolic events. As a consequence, the use of HRT for this indication has declined.
Treatments for established osteoporosis
The choice of treatment depends on the clinical circumstances. Pain relief is important if there are fractures, and salmon calcitonin given subcutaneously can aid pain relief when used for up to 3 months after a vertebral fracture. Drug treatment to prevent bone loss can reduce the risk of further fractures by up to 50%. Options include the following.
 Oral bisphosphonates increase bone density, with the best evidence in postmenopausal women. Alendronic acid and risedronate sodium have been shown to reduce hip, vertebral and wrist fractures. Bisphosphonates are also first-line treatment for the management of corticosteroid-induced osteoporosis. If there has been a good response in bone mineral density after 5 years then treatment is usually stopped for 3–5 years while monitoring markers of bone turnover. Such a strategy does not increase the risk of subsequent fractures. Intravenous bisphosphonates such as zoledronic acid once a year or ibandronic acid every 3 months are used when oral treatment is poorly tolerated.
Oral bisphosphonates increase bone density, with the best evidence in postmenopausal women. Alendronic acid and risedronate sodium have been shown to reduce hip, vertebral and wrist fractures. Bisphosphonates are also first-line treatment for the management of corticosteroid-induced osteoporosis. If there has been a good response in bone mineral density after 5 years then treatment is usually stopped for 3–5 years while monitoring markers of bone turnover. Such a strategy does not increase the risk of subsequent fractures. Intravenous bisphosphonates such as zoledronic acid once a year or ibandronic acid every 3 months are used when oral treatment is poorly tolerated.
 Raloxifene is a selective oestrogen receptor (ER) modulator. It binds to both types of oestrogen receptor (ERα and ERβ; Ch. 45) and is a partial agonist of ERα (which acts as a gene activator) but also an antagonist of ERβ by recruiting co-repressor molecules (which suppresses genes). The distribution of the two receptors may explain the tissue specificity of raloxifene, which has oestrogen receptor agonist effects on bone and lipids (reducing low-density lipoprotein [LDL] cholesterol) but acts as an anti-oestrogen on the breast and endometrium (Ch. 45). Raloxifene reduces the risk of oestrogen receptor-positive breast cancer in postmenopausal women by 75%, but its effects on pre-existing breast cancer are unknown. It does not affect menopausal vasomotor symptoms. Raloxifene reduces the risk of vertebral fractures by 40%, but has no effect on non-vertebral fractures, which may reflect the tissue distribution of oestrogen receptor subtypes. It is recommended for women who have had a vertebral fragility fracture who cannot take a bisphosphonate, or who have a fragility fracture after at least 1 year of treatment with a bisphosphonate. Unwanted effects include hot flushes and leg cramps. In addition, raloxifene doubles the risk of venous thromboembolism, particularly during the first 4 months of treatment.
Raloxifene is a selective oestrogen receptor (ER) modulator. It binds to both types of oestrogen receptor (ERα and ERβ; Ch. 45) and is a partial agonist of ERα (which acts as a gene activator) but also an antagonist of ERβ by recruiting co-repressor molecules (which suppresses genes). The distribution of the two receptors may explain the tissue specificity of raloxifene, which has oestrogen receptor agonist effects on bone and lipids (reducing low-density lipoprotein [LDL] cholesterol) but acts as an anti-oestrogen on the breast and endometrium (Ch. 45). Raloxifene reduces the risk of oestrogen receptor-positive breast cancer in postmenopausal women by 75%, but its effects on pre-existing breast cancer are unknown. It does not affect menopausal vasomotor symptoms. Raloxifene reduces the risk of vertebral fractures by 40%, but has no effect on non-vertebral fractures, which may reflect the tissue distribution of oestrogen receptor subtypes. It is recommended for women who have had a vertebral fragility fracture who cannot take a bisphosphonate, or who have a fragility fracture after at least 1 year of treatment with a bisphosphonate. Unwanted effects include hot flushes and leg cramps. In addition, raloxifene doubles the risk of venous thromboembolism, particularly during the first 4 months of treatment.
 Teriparatide is a synthetic recombinant fraction of PTH (amino acids 1–34) that is used for the treatment of postmenopausal osteoporosis. It is given daily by subcutaneous injection. The most common unwanted effects are nausea, oesophageal reflux, postural hypotension, dyspnoea, depression and dizziness. It is recommended for postmenopausal women who cannot tolerate a bisphosphonate. There is also evidence that it is effective for osteoporosis treatment in men, and corticosteroid-induced osteoporosis. Human recombinant PTH can also be given by subcutaneous injection.
Teriparatide is a synthetic recombinant fraction of PTH (amino acids 1–34) that is used for the treatment of postmenopausal osteoporosis. It is given daily by subcutaneous injection. The most common unwanted effects are nausea, oesophageal reflux, postural hypotension, dyspnoea, depression and dizziness. It is recommended for postmenopausal women who cannot tolerate a bisphosphonate. There is also evidence that it is effective for osteoporosis treatment in men, and corticosteroid-induced osteoporosis. Human recombinant PTH can also be given by subcutaneous injection.
 Strontium ranelate is preferentially taken up by trabecular bone and incorporated into bone in the same way as Ca2+. It stimulates osteoblast activity and inhibits osteoclast differentiation and resorptive activity. Strontium ranelate reduces the risk of both hip and vertebral fractures. The pattern of bone remodelling, with increases in markers of bone formation and a decrease in bone resorption, differs from that seen with both antiresorptive and anabolic therapies. Unwanted effects include nausea, diarrhoea, headache and rashes. Strontium ranelate is recommended for use in postmenopausal women who cannot tolerate a bisphosphonate, and may be the first-choice treatment for women older than 80 years.
Strontium ranelate is preferentially taken up by trabecular bone and incorporated into bone in the same way as Ca2+. It stimulates osteoblast activity and inhibits osteoclast differentiation and resorptive activity. Strontium ranelate reduces the risk of both hip and vertebral fractures. The pattern of bone remodelling, with increases in markers of bone formation and a decrease in bone resorption, differs from that seen with both antiresorptive and anabolic therapies. Unwanted effects include nausea, diarrhoea, headache and rashes. Strontium ranelate is recommended for use in postmenopausal women who cannot tolerate a bisphosphonate, and may be the first-choice treatment for women older than 80 years.
 Denosumab is a human monoclonal antibody which binds specifically to RANKL and prevents activation of the RANK receptor found on osteoclasts and their precursors. As a result, formation of osteoclasts is reduced, their function is inhibited and their survival reduced. Resorption of both cortical and trabecular bone is decreased, and denosumab reduces both vertebral and non-vertebral fractures with a greater efficacy than oral bisphosphonates in postmenopausal women. Denosumab is also effective for treatment of osteoporosis in men taking androgen-depletion therapy for prostate cancer. Denosumab has a very long half-life of about 26 days and is given subcutaneously twice a year. Unwanted effects include diarrhoea, constipation, dyspnoea, increased frequency of urinary tract and upper respiratory tract infections, limb pains, sciatica, hypocalcaemia, hypophosphataemia, rash, sweating and cataracts.
Denosumab is a human monoclonal antibody which binds specifically to RANKL and prevents activation of the RANK receptor found on osteoclasts and their precursors. As a result, formation of osteoclasts is reduced, their function is inhibited and their survival reduced. Resorption of both cortical and trabecular bone is decreased, and denosumab reduces both vertebral and non-vertebral fractures with a greater efficacy than oral bisphosphonates in postmenopausal women. Denosumab is also effective for treatment of osteoporosis in men taking androgen-depletion therapy for prostate cancer. Denosumab has a very long half-life of about 26 days and is given subcutaneously twice a year. Unwanted effects include diarrhoea, constipation, dyspnoea, increased frequency of urinary tract and upper respiratory tract infections, limb pains, sciatica, hypocalcaemia, hypophosphataemia, rash, sweating and cataracts.
 Calcitriol is used when bisphosphonates are unsuitable, and is given for postmenopausal and corticosteroid-induced osteoporosis.
Calcitriol is used when bisphosphonates are unsuitable, and is given for postmenopausal and corticosteroid-induced osteoporosis.
 Testosterone (Ch. 46) is sometimes used for prophylaxis and treatment of corticosteroid-induced osteoporosis in men.
Testosterone (Ch. 46) is sometimes used for prophylaxis and treatment of corticosteroid-induced osteoporosis in men.
Paget's disease of bone
Paget's disease of bone is a disturbance of bone remodelling characterised by both excessive bone reabsorption by osteoclasts and an increase in formation of poor-quality bone. The new bone matrix is non-lamellar woven bone (haphazard organization of the collagen matrix due to rapid bone formation) with areas of osteosclerosis, leaving bone that is structurally weakened. Paget's disease mainly affects the skull and long bones. The aetiology is unknown but there is a genetic predisposition and a slow virus infection may initiate the disease.
About a third of pagetic bone lesions are asymptomatic, but the remainder can produce bone pain and deformity, nerve entrapment and pathological fractures. Active treatment should be given if symptoms are present or a risk of complications is identified. Apart from symptomatic measures such as analgesics, two main treatments are used, as follows.
 Bisphosphonates are effective by inhibiting bone resorption. They can relieve pain to a greater extent than analgesics but may not reduce bone deformity or decrease the risk of fractures. Oral treatment is usually sufficient, with intravenous treatment reserved for severe disease.
Bisphosphonates are effective by inhibiting bone resorption. They can relieve pain to a greater extent than analgesics but may not reduce bone deformity or decrease the risk of fractures. Oral treatment is usually sufficient, with intravenous treatment reserved for severe disease.
 Calcitonin, by reducing osteoclastic bone resorption, can reduce pain and then improve the structural abnormalities in pagetic bone. Pain relief usually begins within 2 weeks, but treatment may be necessary for several months to improve bone remodelling. Approximately 50% of people will relapse on stopping treatment. Calcitonin is often used for initial treatment while awaiting a response to a bisphosphonate.
Calcitonin, by reducing osteoclastic bone resorption, can reduce pain and then improve the structural abnormalities in pagetic bone. Pain relief usually begins within 2 weeks, but treatment may be necessary for several months to improve bone remodelling. Approximately 50% of people will relapse on stopping treatment. Calcitonin is often used for initial treatment while awaiting a response to a bisphosphonate.
True/false questions
1. Hypocalcaemia develops when there is a deficiency in parathyroid hormone (PTH) or vitamin D activity.
2. Vitamin D deficiency can lead to hypoparathyroidism.
3. Calcitonin decreases Ca2+ resorption in the kidney.
4. Bisphosphonates lower blood Ca2+ levels rapidly.
5. Oestrogens maintain bone density by directly enhancing Ca2+ absorption from the intestine.
6. Raloxifene stimulates oestrogen receptors on bone, breast and uterine tissue.
7. Denosumab binds to RANK ligand and reduces osteoclast activity.
8. Teriparatide is used in the treatment of postmenopausal osteoporosis.
One-best-answer (OBA) questions
1. Identify the inaccurate statement below concerning osteoporosis.
A High doses of oral prednisolone increase the risk of osteoporosis.
B Raloxifene has oestrogenic activity at all oestrogen receptors.
C Oral bisphosphonates reduce Ca2+ mobilisation in bone.
2. Identify the inaccurate statement below concerning osteomalacia and rickets.
1. True. Deficiencies in the production of PTH or vitamin D or in the responsiveness of their target tissues cause hypocalcaemia.
2. False. Vitamin D deficiency leads to hyperparathyroidism, which may assist in reducing the worst excesses of vitamin D deficiency. PTH increases calcitriol formation and calcitriol has a negative feedback effect on PTH.
3. True. Calcitonin reduces Ca2+ resorption and inhibits bone turnover.
4. False. Bisphosphonates inhibit bone dissolution and their effects occur slowly; plasma Ca2+ concentrations fall slowly with a maximum effect after about a week.
5. False. Oestrogens inhibit the cytokines that recruit the bone-resorbing osteoclasts. Oestrogens also inhibit the actions of PTH.
6. False. Raloxifene has been licensed to increase bone density in postmenopausal women. It is an oestrogen receptor agonist selective for its actions on oestrogen receptors in bone and without stimulant effects on oestrogen receptors in breast and uterus.
7. True. Denosumab prevents activation of RANK receptors on osteoclasts by RANK ligand (RANKL), and reduces osteoclast proliferation, function and survival.
8. True. Teriparatide is a recombinant version of amino acids 1–34 of PTH and is given daily by subcutaneous injection for postmenopausal osteoporosis.
OBA answers
1. Answer B is the inaccurate statement.
A Correct. Corticosteroids can reduce the number of bone-forming cellular units (osteoclasts/osteoblasts), decrease Ca2+ absorption, increase renal Ca2+ excretion and increase bone resorption.
B Incorrect. Raloxifene is oestrogenic on bone but anti-oestrogenic on receptors in the breast and uterus.
C Correct. Bisphosphonates reduce bone Ca2+ mobilisation and are particularly useful in corticosteroid-induced osteoporosis.
D Correct. The anti-oestrogenic effects of raloxifene can result in hot flushes and thromboembolism in some women.
2. Answer E is the inaccurate statement.
A Correct. Sunlight is involved in the formation of cholecalciferol in the skin, which is then converted to active vitamin D compounds in the liver and kidneys.
B Correct. Ergocalciferol is hydroxylated in the kidney to calcitriol before it can exert its biological activity. In renal failure, if 1α-hydroxylase activity is defective, alfacalcidol or calcitriol may have to be substituted.
C Correct. Because of the lack of active vitamin D formed in the kidney, less Ca2+ and phosphate will be absorbed from the gut.
D Correct. Vitamin D promotes bone mineralisation by promoting the laying down of hydroxyapatite on the collagen organic matrix.
E Incorrect. Low levels of Ca2+ and lack of vitamin D may result in higher levels of PTH (secondary hyperparathyroidism).
Andress, DL, Coyne, DW, Kalantar-Zadeh, K, et al. Management of secondary hyperparathyroidism in stages 3 and 4 chronic kidney disease. Endocr Pract. 2008;14:18–27.
Canalis, E, Giustina, A, Bilezikian, JP. Mechanisms of anabolic therapies for osteoporosis. N Engl J Med. 2007;357:905–916.
Cooper, MS, Gittoes, NJL. Diagnosis and management of hypocalcaemia. BMJ. 2008;336:1298–1302.
Favus, MJ. Bisphosphonates for osteoporosis. N Engl J Med. 2010;363:2027–2035.
Holick, MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281.
Laroche, M. Treatment of osteoporosis: all the questions we still cannot answer. Am J Med. 2008;121:744–747.
MacLean, C, Newberry, S, Maglione, M, et al. Systematic review: comparative effectiveness of treatments to prevent fractures in men and women with low bone density or osteoporosis. Ann Intern Med. 2008;148:197–213.
Mallick, S, Kanthety, R, Rahman, M. Vitamin D: bone and beyond, rationale and recommendations for supplementation. Am J Med. 2009;122:793–802.
Marcocci, C, Cetani, F. Primary hyperparathyroidism. N Engl J Med. 2011;365:2389–2397.
Mazziotti, G, Canalis, E, Giustina, A. Drug-induced osteoporosis: mechanisms and clinical implications. Am J Med. 2010;123:877–884.
Pallan, S, Omair Rahman, M, Khan, AA. Diagnosis and management of primary hyperparathyroidism. BMJ. 2012;344:e1013.
Palmer, SC, McGregor, DO, Macaskill, P, et al. Meta-analysis: vitamin D compounds in chronic kidney disease. Ann Intern Med. 2007;147:840–853.
Poole, KES, Compston, JE. Osteoporosis and its management. BMJ. 2006;333:1251–1256.
Rachner, TD, Khosla, S, Hofbauer, LC. Osteoporosis: now and the future. Lancet. 2011;377:1276–1287.
Ralston, SH, Langston, AL, Reid, IR. Pathogenesis and management of Paget's disease of bone. Lancet. 2008;372:155–163.
Rosen, CJ. Postmenopausal osteoporosis. N Engl J Med. 2005;353:595–603.
Rosen, CJ. Vitamin D insufficiency. N Engl J Med. 2011;364:248–254.
Shoback, D. Hypoparathyroidism. N Engl J Med. 2008;359:391–403.
Sitges-Serra, A, Bergenfelz, A. Clinical update: sporadic primary hyperparathyroidism. Lancet. 2007;370:468–470.
Stewart, AF. Hypercalcemia associated with cancer. N Engl J Med. 2005;352:373–379.
Whyte, MP. Paget's disease of bone. N Engl J Med. 2006;355:593–600.