Muscle
Introduction
Although all cells are capable of some sort of movement, the dominant function of several cell types is to generate force through contraction. In these specialised contractile cells, movement is generated by interaction of the proteins actin and myosin (contractile proteins). Certain forms of contractile cell function as single-cell contractile units:
• Myoepithelial cells are an important component of certain secretory glands (see Ch. 5), where they function to expel secretions from glandular acini.
• Pericytes are smooth muscle-like cells that surround blood vessels (see Ch. 8).
• Myofibroblasts are cells that have a contractile role in addition to being able to secrete collagen. This type of cell is generally inconspicuous in normal tissues but becomes important following tissue damage during the process of healing and repair, leading to formation of a scar.
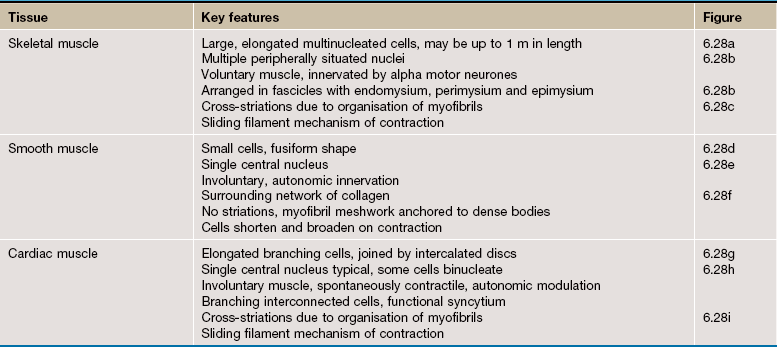
Other forms of contractile cells function by forming multicellular contractile units termed muscles. Such muscle cells can be divided into three types:
• Skeletal muscle is responsible for the movement of the skeleton as well as organs such as the globe of the eye and the tongue. Skeletal muscle is often referred to as voluntary muscle since it is capable of voluntary (conscious) control. The arrangement of the contractile proteins gives rise to the appearance of prominent cross-striations in some histological preparations and so the name striated muscle is often applied to skeletal muscle. The highly developed functions of the cytoplasmic organelles of muscle cells has led to the use of a special terminology for some muscle cell components: plasma membrane or plasmalemma = sarcolemma; cytoplasm = sarcoplasm; endoplasmic reticulum = sarcoplasmic reticulum.
• Smooth muscle is so named because, unlike other forms of muscle, the arrangement of contractile proteins does not give the histological appearance of cross-striations. This type of muscle forms the muscular component of visceral structures such as blood vessels, the gastrointestinal tract, the uterus and the urinary bladder, giving rise to the alternative name of visceral muscle. Since smooth muscle is under inherent autonomic and hormonal control, it is also described as involuntary muscle.
• Cardiac muscle has many structural and functional characteristics intermediate between those of skeletal and smooth muscle and provides for the continuous rhythmic contractility of the heart. Although striated in appearance, cardiac muscle is readily distinguishable from skeletal muscle and should not be referred to by the term ‘striated muscle’.
Muscle cells of all three types are surrounded by an external lamina (see Ch. 4). In all muscle cell types, contractile forces developed from the internal contractile proteins are transmitted to the external lamina via link proteins which span the muscle cell membrane. The external lamina binds individual muscle cells into a single functional mass.
Skeletal Muscle
Skeletal muscles have a wide variety of morphological forms and modes of action; nevertheless all have the same basic structure. Skeletal muscle is composed of extremely elongated, multinucleate contractile cells, often described as muscle fibres, bound together by collagenous supporting tissue. Individual muscle fibres vary considerably in diameter from 10 to 100 µm and may extend throughout the whole length of a muscle and, in some sites, may be many centimeters in length.
Skeletal muscle contraction is controlled by large motor nerves, individual nerve fibres branching within the muscle to supply a group of muscle fibres, collectively described as a motor unit. Excitation of any one motor nerve results in simultaneous contraction of all the muscle fibres of the corresponding motor unit. The structure of neuromuscular junctions is described in Fig. 7.12. The vitality of skeletal muscle fibres is dependent on maintenance of their nerve supply which, if damaged, results in atrophy of the fibres (see below). Skeletal muscle contains highly specialised stretch receptors known as neuromuscular spindles which are shown in Fig. 7.23.
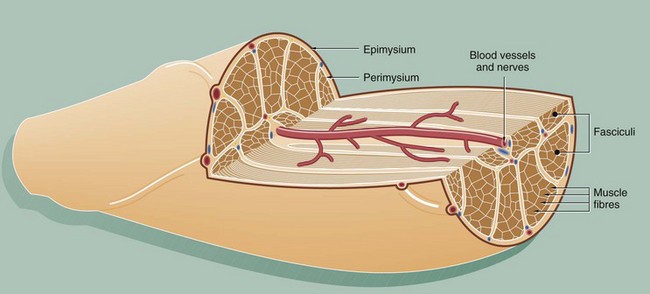
FIG. 6.1 Skeletal muscle
This diagram illustrates the arrangement of the basic components which make up a typical skeletal muscle. The individual muscle cells (muscle fibres) are grouped together into elongated bundles called fasciculi or fascicles with delicate supporting tissue called endomysium occupying the spaces between individual muscle fibres.
Each fascicle is surrounded by loose collagenous tissue called perimysium. Most muscles are made up of many fasciculi, and the whole muscle mass is invested in a dense collagenous sheath called the epimysium. Large blood vessels and nerves enter the epimysium and divide to ramify throughout the muscle in the perimysium and endomysium.
The size of the fasciculi reflects the function of the muscle concerned. Muscles responsible for fine, highly controlled movements (e.g. external muscles of the eye) have small fasciculi and a relatively greater proportion of perimysial supporting tissue. In contrast, muscles responsible for gross movements only (e.g. muscles of the buttocks) have large fasciculi and relatively little perimysial tissue. Muscle fibres are anchored to the supporting tissue so that contractile force can be transmitted. The connective tissue framework contains both collagen and elastic fibres. This connective tissue becomes continuous with that of the tendons and muscle attachments (see Ch. 10), which distribute and direct the motive forces of the muscle to bone, skin, etc., as appropriate.
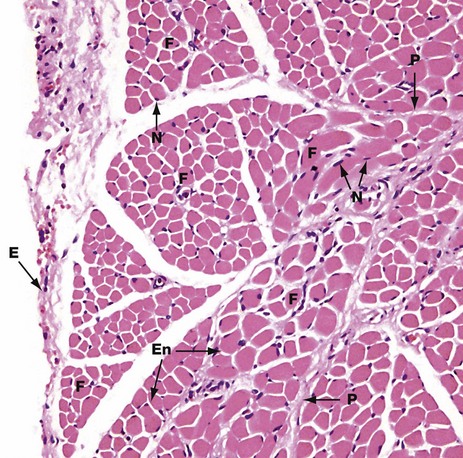
FIG. 6.2 Skeletal muscle
H&E (MP)
This micrograph shows the general arrangement of muscle fibres in skeletal muscle. Here, there are several distinct fasciculi.
The individual pink-stained muscle cells (fibres) are cut in transverse section and appear polygonal in shape, with nuclei N lying at the peripheries of the cells. The spaces between the cells are occupied by small amounts of barely visible endomysial supporting tissue. The endomysium, which consists mainly of reticulin fibres and a small amount of collagen, conveys numerous small blood vessels, lymphatics and nerves throughout the muscle.
Surrounding the individual fasciculi F is the perimysium P, composed of collagen and through which larger vessels and nerves run. The epimysium E is a collagenous sheath that binds the fascicles into a single muscle. The endomysium En is barely visible as the delicate support tissue surrounding each muscle fibre.
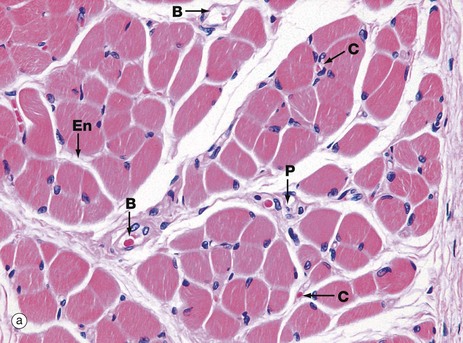
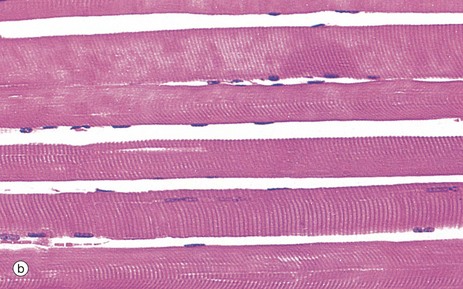
FIG. 6.3 Skeletal muscle
(a) H&E, TS (HP) (b) H&E, LS (HP)
These micrographs show skeletal muscle from human limb muscles. Micrograph (a) in transverse section shows the muscle to be made up of numerous small fasciculi. The spaces between the fasciculi are filled with loose collagenous tissue, the perimysium P, which is continuous with the delicate endomysium En, separating individual muscle fibres in each fasciculus. The supporting tissue of skeletal muscle also contains elastin fibres (not distinguishable in this preparation) which are most numerous in muscles attached to soft tissues as in the tongue and face. Note the rich network of capillaries C in the endomysium. Small blood vessels B and nerves run in the perimysium.
Micrograph (b) demonstrates the characteristic histological features of skeletal muscle fibres in longitudinal section. Skeletal muscle fibres are extremely elongated, unbranched cylindrical cells with numerous flattened nuclei located at fairly regular intervals just beneath the sarcolemma (plasma membrane).
Each muscle fibre has multiple nuclei arranged at the cell periphery. In transverse section, as in micrograph (a), most muscle fibre profiles appear to contain only a single nucleus, while some do not include any because the plane of section has cut between the zones containing a nucleus.
In routine histological preparations stained with H&E, it is often possible to see the striations in skeletal muscle when cut in longitudinal section. Special stains are required for better resolution of these structures (see Fig. 6.6a).
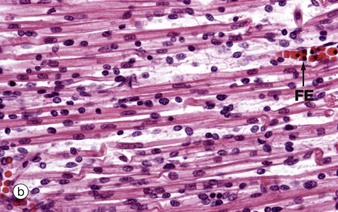
FIG. 6.4 Skeletal muscle embryogenesis
(a) H&E (MP) (b) H&E (HP)
During embryological development, mesenchymal cells in each myotome differentiate into long, mononuclear skeletal muscle precursors called myoblasts, which then proliferate by mitosis. Subsequently, the myoblasts fuse end to end forming elongated multinucleate cells called myotubes which may eventually contain up to 100 nuclei. These myotubes then synthesise contractile proteins to form myofilaments and so cross-striations gradually become visible. Proliferating myoblasts and early developing myotubes are illustrated in micrograph (a). Micrograph (b) illustrates a slightly later stage in development, and here there is a suggestion of very early cross-striation in some of the myotubes. Note the nucleated fetal erythrocytes FE within a small vessel on one edge of the image.
Mature muscle cells can regenerate if damaged, by proliferation of stem cells which remain in adult muscles. These muscle stem cells resemble myoblasts and are called satellite cells. They enter mitosis after muscle damage, and several fuse to form differentiated muscle fibres. Muscle fibres which have formed as a result of regeneration after damage have nuclei in the centre of the fibre rather than at the periphery.
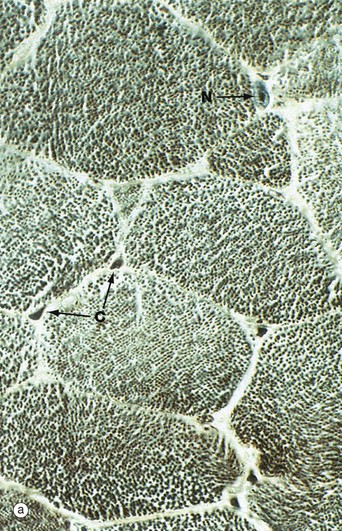
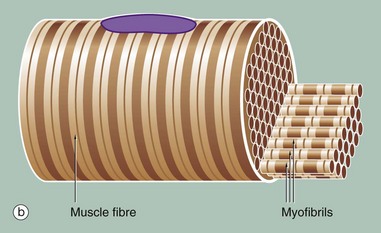
FIG. 6.5 Skeletal muscle
(a) Iron haematoxylin, TS (HP) (b) Schematic diagram
Micrograph (a) shows a transverse section through several skeletal muscle fibres at very high magnification. The plane of section includes only one skeletal muscle cell nucleus N. Note the presence of erythrocytes in endomysial capillaries C.
In preparations such as this, the transversely sectioned muscle fibres appear packed with numerous dark dots. These represent the cut ends of myofibrils, elongated cylindrical structures which lie parallel to one another in the sarcoplasm.
Figure (b) shows part of a single muscle fibre. The diagram illustrates that each myofibril exhibits a repeating pattern of cross-striations which is a product of the highly ordered arrangement of the contractile proteins within it; detail of this arrangement can only be seen using electron microscopy (see Fig. 6.6). Furthermore, the parallel myofibrils are each arranged with their cross-striations in register, giving rise to the regular striations which may be seen with light microscopy in longitudinal sections of skeletal muscle as in Fig. 6.3.
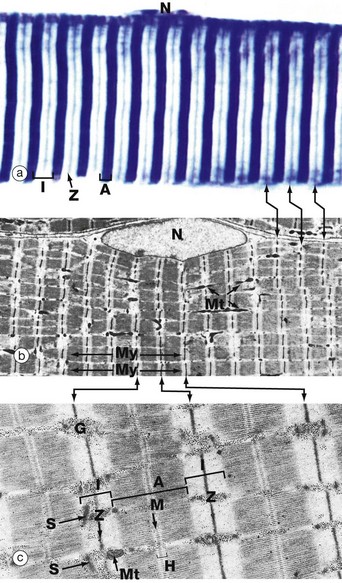
FIG. 6.6 Skeletal muscle
(a) Heidenhain's haematoxylin (HP) (b) EM ×2860 (c) EM ×18 700
This series of micrographs shows the arrangement of the contractile proteins within skeletal muscle and explains the striations seen with light microscopy.
Micrograph (a) shows the striations of a skeletal muscle fibre at a magnification close to the limit of resolution. They are composed of alternating broad light I bands (isotropic in polarised light) and dark (anisotropic) A bands. Fine dark lines called Z lines (Zwischenscheiben) Z can be seen bisecting the I bands. Note the nucleus N at the periphery of the cell.
Micrograph (b) shows the electron microscopic appearance of muscle with a nucleus N situated in a similar position. The sarcoplasm is filled with myofibrils My oriented parallel to the long axis of the cell. These are separated by a small amount of sarcoplasm containing rows of mitochondria Mt in a similar orientation. Each myofibril has prominent regular cross-striations arranged in register with those of the other myofibrils and corresponding to the I, A and Z bands seen in light microscopy. The Z bands are the most electron-dense and divide each myofibril into numerous contractile units called sarcomeres, arranged end to end.
With further magnification in micrograph (c), the arrangement of the contractile proteins (myofilaments) may be seen in each sarcomere. The dark A band is bisected by the lighter H (Heller) band, which is further bisected by a more dense M (Mittelscheibe) line. Irrespective of the degree of contraction of the muscle fibre, the A band remains constant in width. In contrast, the I and H bands narrow during contraction, and the Z lines are drawn closer together. These findings are explained by the sliding filament theory (Fig. 6.7). Mitochondria Mt and numerous glycogen granules G provide a rich energy source in the scanty cytoplasm between the myofibrils. The mature muscle cell contains little rough endoplasmic reticulum; it contains, however, a smooth membranous system S which is involved in activation of the contractile mechanism (see Figs 6.8 to 6.10).
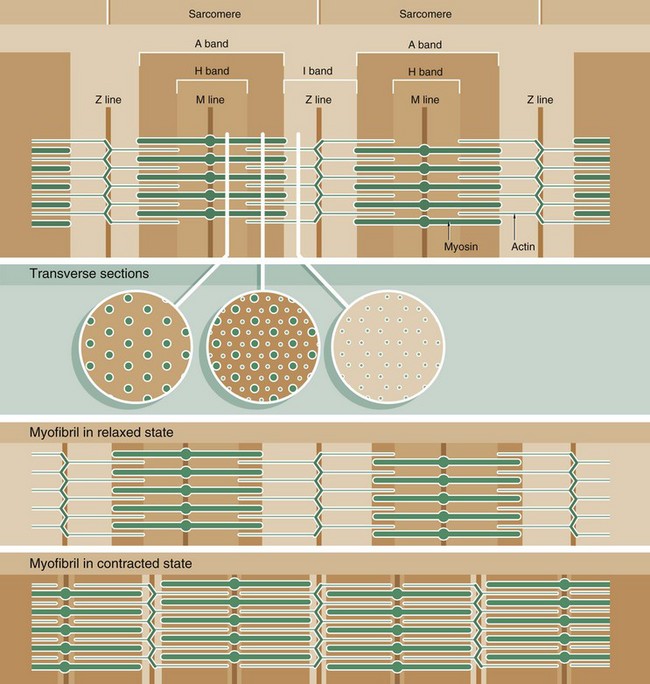
FIG. 6.7 The arrangement of myofilaments in the sarcomere
Muscle function is intimately linked to its microscopic structure. The sarcomere consists of two types of myofilaments, thick filaments and thin filaments. Each type remains constant in length irrespective of the state of contraction of the muscle. The thick filaments, which are composed mainly of the protein myosin, are maintained in register by their attachment to a disc-like zone represented by the M line. Similarly, the thin filaments, which are composed mainly of the protein actin, are attached to a disc-like zone represented by the Z line. The I and H bands, both areas of low electron density, represent areas where the thick and thin filaments do not overlap one another.
The sliding filament mechanism of muscle contraction proposes that contraction occurs through a sliding movement of the thick and thin filaments over one another as illustrated above. Myosin molecules possess ATP-activated side projections or head groups which can bind to actin to form cross-bridges, temporary physical linkages between the thick and thin filaments. Once bound to the adjacent actin molecules, these myosin head groups generate movement by a change in protein configuration, triggered by energy from the hydrolysis of ATP, pulling the myosin thick filaments over the thin actin filaments and so shortening the length of the sarcomere. When another ATP molecule binds to the myosin head group, it detaches from actin, breaking the cross-bridge, and the head group then moves back to its original configuration, ready for the next cross-bridge cycle. This process can be likened to multiple small strokes from the oars of a boat steadily producing movement of the craft.
A large number of accessory proteins are also present in the sarcomere where they play roles in filament alignment and in regulation of contraction.
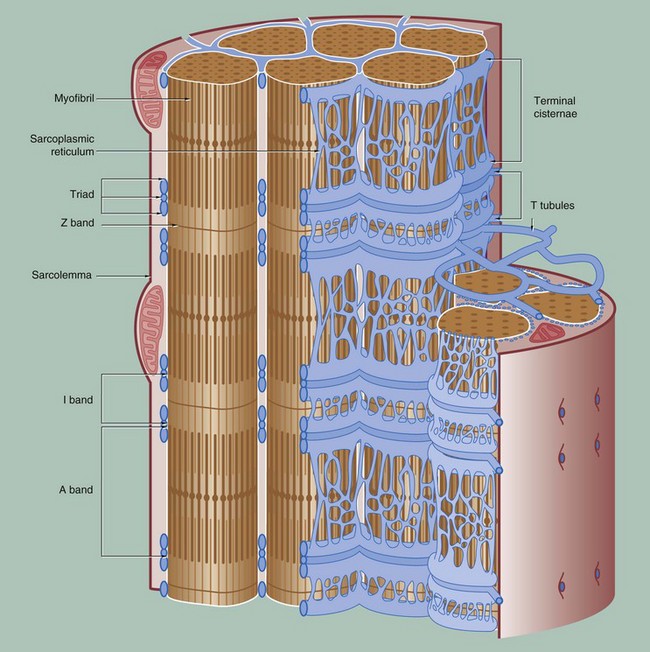
FIG. 6.8 The conducting system for contractile stimuli in skeletal muscle
To permit the synchronous contraction of all sarcomeres in the muscle fibre, a system of tubular extensions of the muscle cell plasma membrane (sarcolemma) extends transversely into the muscle cell to surround each myofibril at the region of the junction of the A and I bands. Known as the T tubule system, its lumen is continuous with the extracellular space. (In amphibian skeletal muscle, which was the first to be studied, the T tubules are disposed at the Z bands and the same applies in cardiac muscle.)
Between the T tubules, a second membrane system derived from smooth endoplasmic reticulum, the sarcoplasmic reticulum, forms a membranous network which embraces each myofibril. On either side of each T tubule, the sarcoplasmic reticulum exhibits a flattened cisternal arrangement, each pair of terminal cisternae and a T tubule forming a triad near the junction of the I and A bands of each sarcomere.
Calcium ions are concentrated within the lumen of the sarcoplasmic reticulum. Depolarisation of the sarcolemma of the muscle fibre is rapidly disseminated throughout the sarcoplasm by the T tubule system. This promotes the release of Ca2+ ions from the sarcoplasmic reticulum into the sarcoplasm surrounding the myofilaments; Ca2+ ions then activate the sliding filament mechanism as described above, resulting in muscle contraction.

FIG. 6.9 Skeletal muscle (LS)
EM × 33 000
This electron micrograph of mammalian skeletal muscle cut in longitudinal section demonstrates the main elements of the conducting system. In the vicinity of the junction of the A and I bands (and depending on the state of contraction) are tubular triads Td each comprising a central flattened tubule of the T tubule system T and a pair of terminal cisternae TC of the sarcoplasmic reticulum. Within the A bands there are tubular elements of the sarcoplasmic reticulum SR connecting the terminal cisternae. Likewise within the I bands, similar though less regular longitudinal tubular profiles of sarcoplasmic reticulum are seen. The conducting system of ‘slow-twitch’ (red) fibres as shown here (also see Fig. 6.14) is more regular than that of ‘fast-twitch’ (white) fibres where this pattern is more difficult to discern. Note the distribution of mitochondria M, regularly arranged between the sarcomeres within the I bands in immediate association with those parts of the actin and myosin filaments which interact during the process of contraction. The reason for this appearance is evident in the following micrograph.
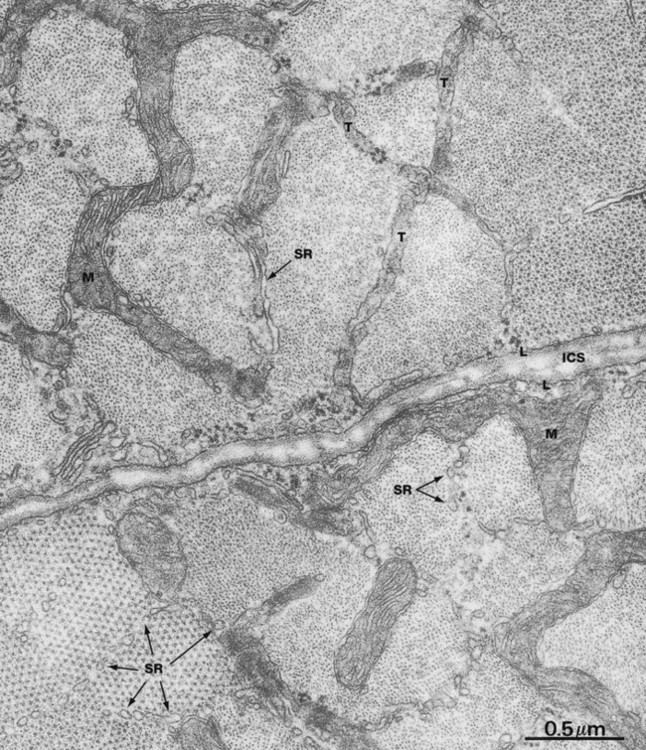
FIG. 6.10 Skeletal muscle
EM × 44 000
This micrograph shows parts of two skeletal muscle cells cut in transverse section in the vicinity of the junction of A and I bands; the intercellular space ICS bisects the field. Note the external lamina L adjacent to the sarcolemma. Sarcomeres at the upper right and lower left of this field have been sectioned through the end part of the A band and thus show both actin and myosin filaments. The remaining sarcomeres are cut through the I band and contain only actin filaments. This results from the fact that the bands of all sarcomeres within any one muscle cell are not exactly in register with one another.
Each sarcomere is ensheathed by a network of tubules of the sarcoplasmic reticulum SR. The plane of section has also included a part of a broader-diameter T tubule system T which branches to encompass several different sarcomeres. Direct communication of T tubules with the intercellular space is difficult to see here, as the tubules appear to ramify into a complex tubular system just beneath the plasmalemma, but continuity of the T tubule lumen and the intercellular space has been convincingly demonstrated by experimental techniques. Note the extraordinary serpentine branched mitochondria M which lie between the sarcomeres within the I bands, giving rise to the mitochondrial appearance seen in longitudinal section in the previous micrograph.
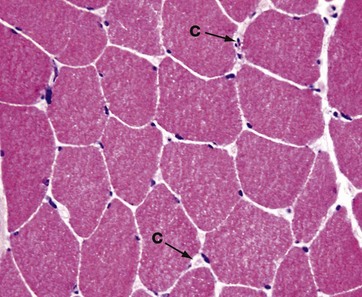
FIG. 6.11 Skeletal muscle
Frozen section, H&E (HP)
In research and clinical diagnosis of muscle disease, formalin-fixed paraffin-embedded tissues are not always used, as they cause artefact to develop and can limit investigation of muscle structure. Fresh frozen section is often the preferred method for preparation of muscle for investigation by light microscopy.
This micrograph shows a frozen section of skeletal muscle from human vastus lateralis muscle. Tissue shrinkage is minimised. In this transverse section, the extreme peripheral location of the nuclei of the skeletal muscle fibres is well seen. In cross-section, muscle fibres appear polyhedral with flattening of adjacent cells. In normal muscle, the cross-sectional areas of individual fibres are approximately the same. In the endomysial space, numerous minute capillaries C are just recognisable. Compare the huge diameter of the muscle fibres, which may be as great as 0.1 mm in diameter, with that of the capillaries, the latter being approximately 7 µm across.

FIG. 6.12 Skeletal muscle
Immunohistochemical stain for dystrophin (HP)
The proteins of the myofilaments actin and desmin are critical for normal muscle function. Another group of proteins which have a key role in muscle function are those associated with the cell membrane of skeletal muscle. A complex of large proteins act to link the contractile proteins within the cell through the cell membrane with structural proteins in the external lamina. Thus, contractile forces within each muscle fibre are transmitted to the collagenous support tissues to bring about movement.
One of the most important and well understood of these linkage proteins is called dystrophin. Immunohistochemical staining for this protein, seen here as a brown stain, shows it to be closely associated with the muscle cell membrane (see below).
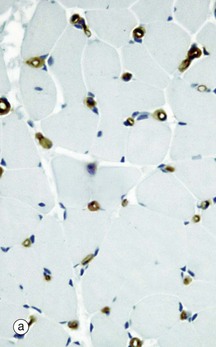
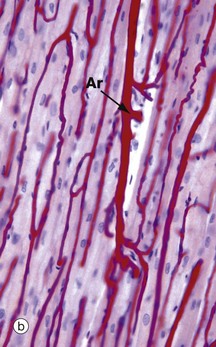
FIG. 6.13 Skeletal muscle blood supply
(a) Immunohistochemical stain for CD 34, TS (HP) (b) Perfusion preparation/haematoxylin, LS (HP)
Micrograph (a) shows skeletal muscle cut in transverse section and stained to show endothelial cells using an immunohistochemical technique. This highlights capillaries. Each muscle fibre is in close contact with 1 to 3 capillaries, seen as small circular brown profiles.
The capillaries run along the muscle fibres, a feature best illustrated in micrograph (b) which is a perfusion preparation of muscle in which the vessels have been injected with red gel. Muscle nuclei can be seen stained blue with haematoxylin. A small artery Ar running in the perimysium can be seen, giving off capillaries which branch out and run along the length of the muscle fibres. The high energy requirement of skeletal muscle demands an extensive capillary network.

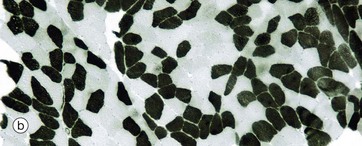

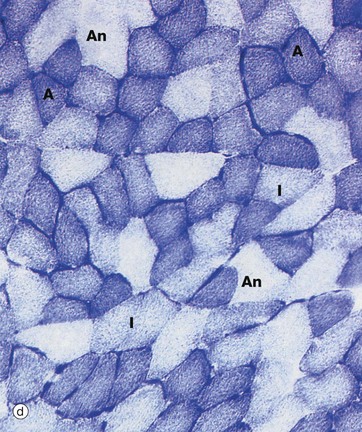
FIG. 6.14 Skeletal muscle
Histochemical techniques, TS (a) PAS (HP) (b) ATPase pH 4.2 (MP) (c) ATPase pH 9.4 (HP) (d) Succinate dehydrogenase (HP)
The mode of activity of skeletal muscle varies from one part of the body to another. Some muscles, such as those involved in the maintenance of posture, are required to contract almost continuously while others, such as the extra-ocular muscles, make rapid short-lived movements. In humans, distinction between these types cannot be made on gross examination of the muscle. In domestic poultry, however, the extremes are easily identified by a difference in colours; for example, leg muscles are red, and flight (breast) muscles are white.
Correspondingly, ‘slow-twitch’ and ‘fast-twitch’ muscle fibre types can be demonstrated by nerve stimulation studies. The metabolic requirements of each fibre type differ markedly, the slow red fibres mainly relying on aerobic metabolism and the fast white fibres using predominantly anaerobic pathways. Most muscles contain a mixture of these extreme fibre types as well as an intermediate type. There are significant interspecies differences.
Aerobic (type I) muscle fibres A contain abundant mitochondria. They also have a large content of myoglobin, an oxygen-storage molecule analogous to haemoglobin, which accounts for the red colour of such fibres.
In contrast, anaerobic (type II) muscle fibres An contain few mitochondria and relatively little myoglobin. These muscle fibres are, however, rich in glycogen and glycolytic enzymes. These characteristics account for the ‘white’ colour of such fibres. In micrograph (a), the PAS stain demonstrates this difference in glycogen content. The type II fibres stain dark pink due to the presence of abundant glycogen granules, whilst the aerobic type I fibres contain little glycogen and appear paler in colour. Anaerobic fibres predominate in muscles responsible for intense but sporadic contraction such as the biceps and triceps of the arms.
Type I and II fibres can also be identified by the nature of their myosin ATPase, which differs in its protein structure between different fibre types. In the preparation in micrograph (b), type I fibres are dark and type II fibres are light. This low magnification shows the checkerboard pattern of the fibre types within a muscle fascicle. Micrograph (c) has been stained at a pH that reveals type I fibres as pale and type II fibres as dark (the opposite to micrograph b). Such histochemical staining is used routinely in diagnosis of muscle disease.
The activity of the specific mitochondrial enzyme succinate dehydrogenase, which catalyses one of the stages of the Krebs cycle, demonstrates the relative proportions of mitochondria within the muscle fibres. In micrograph (d), note the presence of intensely stained small-diameter aerobic fibres A, poorly stained large-diameter anaerobic fibres An and intermediate fibres I.
The type of metabolism of each fibre is determined by the frequency of impulses in its motor nerve supply. Any one motor nerve supplies fibres of one type only, and all the fibres of a particular motor unit are of the same metabolic type. Indeed, if the motor nerve supply to one type of fibre is experimentally transplanted to supply another fibre type, this fibre type will become converted to the metabolic pattern of the former.
Smooth Muscle
Skeletal muscle is specialised for relatively forceful contractions of short duration under voluntary control. In contrast, smooth muscle is specialised for continuous contractions of relatively low force, producing diffuse movements through contraction of the whole muscle mass, rather than contraction of individual motor units. Contractility is an inherent property of smooth muscle, occurring independently of innervation, often in a rhythmic or wave-like fashion. Superimposed on this inherent contractility are the influences of the autonomic nervous system, hormones and local metabolites which modulate contractility to accommodate changing functional demands. For example, the smooth muscle of the intestinal wall undergoes continuous rhythmic contraction (peristalsis), propelling the luminal contents distally. This activity is enhanced by parasympathetic stimulation and influenced by a variety of hormones which are released in response to changes in the nature and volume of the gut contents. The structure of autonomic neuromuscular junctions is described in Ch. 7.
The cells of smooth muscle are relatively small, with only a single nucleus. The fibres are bound together in irregular branching fasciculi, the arrangement varying considerably from one organ to another according to functional requirements.
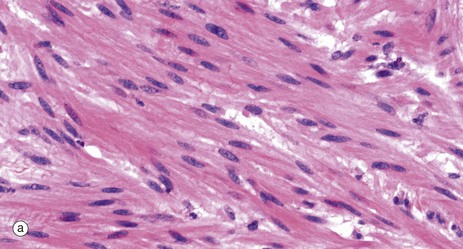
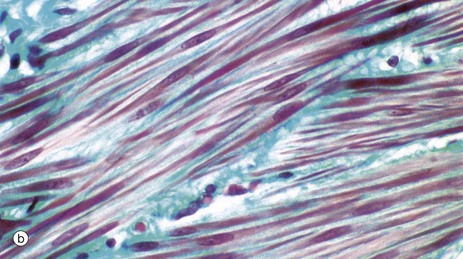
FIG. 6.15 Smooth muscle
(a) H&E, LS (HP) (b) Masson trichrome, LS (HP)
As seen in these micrographs, smooth muscle fibres are elongated, spindle-shaped cells with tapered ends which may occasionally be bifurcated. Smooth muscle fibres are generally much shorter than skeletal muscle fibres and contain only one nucleus which is elongated and centrally located in the cytoplasm at the widest part of the cell; however, depending on the contractile state of the fibres at fixation, the nuclei may sometimes appear to be spiral-shaped.
Smooth muscle fibres are bound together in irregular branching fasciculi and these fasciculi, rather than individual fibres, are the functional contractile units. Within the fasciculi, individual muscle fibres are arranged roughly parallel to one another with the thickest part of one cell lying against the thin parts of adjacent cells.
The contractile proteins of smooth muscle are not arranged in myofibrils, as in skeletal and cardiac muscle, and thus visceral muscle cells are not striated.
Between the individual muscle fibres and between the fasciculi, there is a network of supporting collagenous tissue. This is well demonstrated in micrograph (b) in which the collagen is stained blue-green.
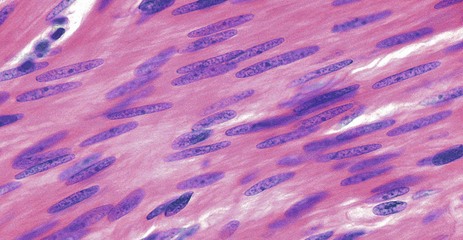
FIG. 6.16 Smooth muscle
H&E, LS (HP)
This micrograph illustrates smooth muscle from the bowel wall, cut in longitudinal section. In this case, the fibres are arranged in a highly regular manner and packed so closely that it is difficult to identify individual cell outlines, although cell shape can be deduced from that of the nuclei.
Smooth muscle cell nuclei are elongated and are typically described as being ‘cigar-shaped’ with rather blunt ends.
The cytoplasm is eosinophilic but lacks the cross-striations seen in skeletal and cardiac muscle.
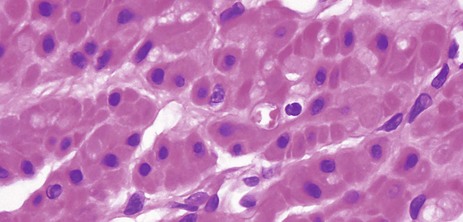
FIG. 6.17 Smooth muscle
H&E, TS (HP)
This micrograph shows smooth muscle in transverse section at very high magnification. The spindle-shaped cells are sectioned at various different points along their length, which gives the erroneous impression that they are of differing diameters. Nuclei are only included in the plane of section where fibres have been cut through their widest diameter. Note the plump nuclear shape and the central location of the nuclei within the cytoplasm.
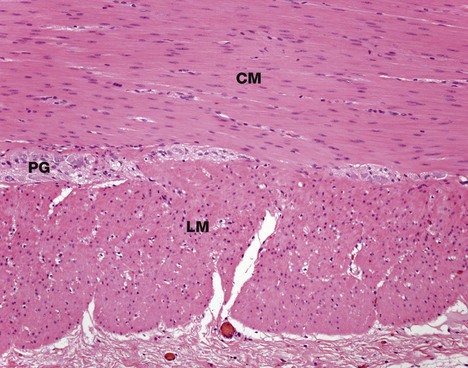
FIG. 6.18 Smooth muscle
H&E, TS (MP)
This micrograph illustrates smooth muscle from the bowel wall, cut in transverse section. In many tubular visceral structures, such as the ileum as shown in this micrograph, smooth muscle is disposed in layers, with the cells of one layer arranged at right angles to those of the adjacent layer. This arrangement permits a wave of contraction to pass down the tube, propelling the contents forward; this action is called peristalsis.
Typically, the longitudinal outer smooth muscle layer L (cut in transverse section here) is closely applied to the inner circular layer CM, with only a minimal amount of supporting tissue between. In this specimen, the collagen is stained blue.
The supporting tissue here contains clumps of large cells with pale nuclei which represent parasympathetic ganglia PG (see Fig. 7.22). The parasympathetic nervous system of the gastrointestinal tract modulates the intensity of peristalsis.
Smooth Muscle Contraction
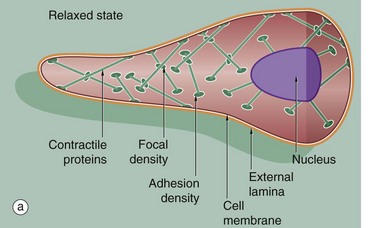
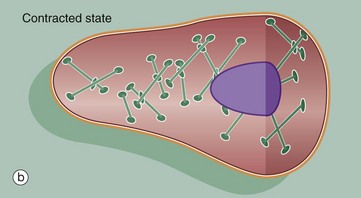
Smooth muscle does not show the longitudinally organised system of contractile proteins that is seen in striated muscle, but has an arrangement where bundles of contractile proteins criss-cross the cell, being inserted into anchoring points (focal densities) within the cytoplasm, as well as anchoring to the cell membrane as focal adhesion densities.
Tension generated by contraction is transmitted through anchoring densities in the cell membrane to the surrounding external lamina, thus allowing a mass of smooth muscle cells to function as one unit. The intermediate filaments of smooth muscle, desmin, are also inserted into the focal densities (Fig. 6.20).
The contraction mechanism of smooth muscle differs from that for striated muscle. Because the contractile proteins are arranged in a criss-cross lattice inserted around the cell membrane, contraction results in shortening of the cell, which assumes a globular shape in contrast to its elongated shape in the relaxed state (see Fig. 6.20).
The mechanism of smooth muscle contraction is as follows:
• Thin filaments of actin are associated with tropomyosin.
• Thick filaments composed of myosin only bind to actin if one chain is phosphorylated.
• Calcium ions in the cytosol of smooth muscle cells cause contraction, as in striated muscle, but the control of Ca2+ ion movements is different. In relaxed smooth muscle, free Ca2+ ions are normally sequestered in sarcoplasmic reticulum throughout the cell. On membrane excitation, free Ca2+ ions are released into the cytoplasm and bind to a protein called calmodulin (a calcium-binding protein). The calcium-calmodulin complex then activates an enzyme called myosin light-chain kinase, which phosphorylates myosin and permits it to bind to actin. Actin and myosin subsequently interact by filament sliding to produce contraction in a similar way to that for skeletal muscle.
• Contraction of smooth muscle can be modulated by surface receptors activating internal second messenger systems. Expression of different receptors allows smooth muscle in different sites to respond to several different hormones.
• Compared with skeletal muscle, smooth muscle is able to maintain a high force of contraction for very little ATP usage.
Most smooth muscle is present in the walls of hollow viscera (e.g. gut, ureter, Fallopian tube) where it is arranged in sheets with cells aligned circumferentially or longitudinally, with contraction resulting in reduction of the lumen diameter.
In these so-called unitary smooth muscles, cells tend to generate their own low level of rhythmic contraction, which may also be stimulated by stretch and is transmitted from cell to cell via the gap junctions. Such smooth muscle is richly innervated by the autonomic nervous system (see Ch. 7), which increases or decreases levels of spontaneous contraction rather than actually initiating it. Physiologically, this is termed tonic smooth muscle and is characterised by slow contraction, no action potentials and a low content of fast myosin.
A second arrangement of smooth muscle is typified by that of the iris of the eye. Here, rather than simply modulating spontaneous activity, autonomic innervation precisely controls contraction, resulting in opening and closing of the pupil. Similar neurally controlled or multi-unit smooth muscle is found in the vas deferens and in some large arteries. Physiologically, this is termed phasic smooth muscle, and it is characterised by rapid contraction associated with an action potential.
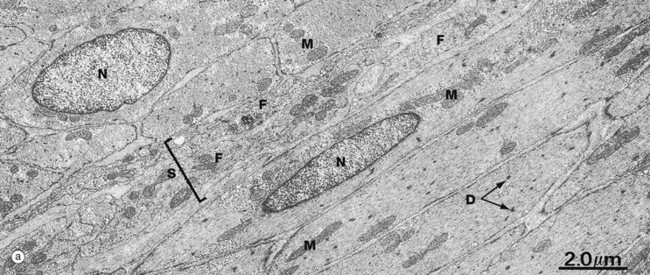
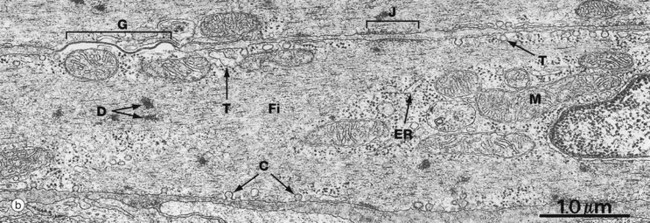
FIG. 6.19 Smooth muscle
(a) EM ×8000 (b) EM ×21 000
At low magnification, micrograph (a) demonstrates the spindle-shaped and elongated central nuclei N of smooth muscle cells. The cells at the lower right are cut longitudinally, and those at the upper left transversely. Between them is a band of supporting tissue S containing the cytoplasmic processes of fibroblasts F. Note the relative sparsity of mitochondria M and other intracellular organelles.
At high magnification in micrograph (b), details of the plasma membrane and endomembrane system can be seen. The plasma membrane contains numerous flask-shaped invaginations. In some areas, these are irregular in shape and size and may be involved in pinocytosis. In other areas, the invaginations are regular in shape and distribution and are called caveolae C. The endomembrane system contains some elements which represent a poorly developed Golgi and endoplasmic reticulum ER. Other vesicular and tubular structures T are seen near the plasma membrane, often in association with caveolae; these probably constitute a system analogous to the sarcoplasmic reticulum of skeletal muscle, with caveolae being analogous to the T tubule system.
Thick and thin filaments Fi of myosin and actin criss-cross the cytoplasm of each cell and are anchored to the cell membrane at attachment junctions (focal adhesion densities) J. Filaments are also attached within the cytoplasm to focal densities D which are believed to hold filaments in register.
The narrow intercellular spaces are of almost uniform width, but at numerous sites the plasma membranes of adjacent cells form specialised cell junctions. Nexus (gap) junctions G mediate spread of excitation throughout visceral muscle (see Fig. 5.12).
Cardiac Muscle
Cardiac muscle or myocardium exhibits many structural and functional characteristics intermediate between those of skeletal and visceral muscle. Like the former, its contractions are strong and utilise a great deal of energy; like the latter, the contractions are continuous and initiated by inherent mechanisms, although they are modulated by external autonomic and hormonal stimuli.
Cardiac muscle fibres are essentially long, cylindrical cells with one or at most two nuclei which are centrally located within the cell. The ends of the fibres are split longitudinally into a small number of branches, the ends of which abut onto similar branches of adjacent cells, giving the impression of a continuous three-dimensional cytoplasmic network; this was formerly described as a syncytium before the discrete intercellular boundaries were recognised.
Between the muscle fibres, delicate collagenous tissue analogous to the endomysium of skeletal muscle supports the extremely rich capillary network necessary to meet the high metabolic demands of strong, continuous activity.
Cardiac muscle fibres have an arrangement of contractile proteins similar to that of skeletal muscle and are consequently striated in a similar manner. However, this is often difficult to see with light microscopy due to the irregular branching shape of the cells and their myofibrils. Cardiac muscle fibres also have a system of T tubules and sarcoplasmic reticulum analogous to that of skeletal muscle. In the case of cardiac muscle, however, there is a slow leak of Ca2+ ions into the cytoplasm from the sarcoplasmic reticulum after recovery from the preceding contraction; this causes a succession of automatic contractions independent of external stimuli. The rate of this inherent rhythm is then modulated by external autonomic and hormonal stimuli.
Between the ends of adjacent cardiac muscle cells are specialised intercellular junctions called intercalated discs which not only provide points of anchorage for the myofibrils but also permit extremely rapid spread of contractile stimuli from one cell to another. Thus, adjacent fibres are triggered to contract almost simultaneously, thereby acting as a functional syncytium. In addition, a system of highly modified cardiac muscle cells constitutes the pacemaker regions of the heart and ramifies throughout the organ as the Purkinje system, thus coordinating contraction of the myocardium as a whole in each cardiac cycle. This is illustrated and described in more detail in Ch. 8.
Cardiac muscle cells in certain locations in the heart are responsible for secreting hormones into the bloodstream.
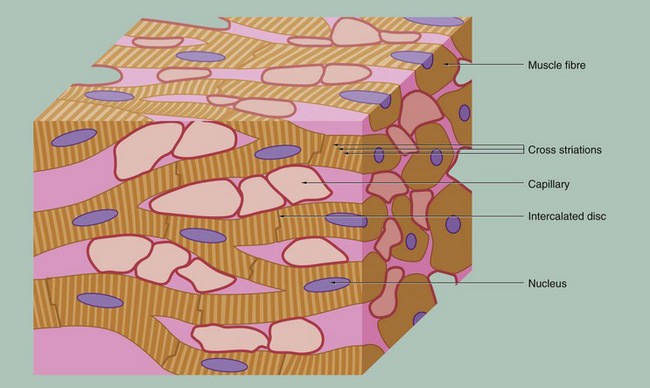
FIG. 6.21 Cardiac muscle
This schematic representation highlights the key features of cardiac muscle. The cardiac myocytes are long, branching cells with central nuclei. The cells are joined together end to end via specialised intercellular junctions termed intercalated discs. These produce both structural and electrical coupling between the myocytes, allowing them to act as a functional syncytium. There are large numbers of capillaries between the cells, reflecting the high metabolic requirements of cardiac muscle.
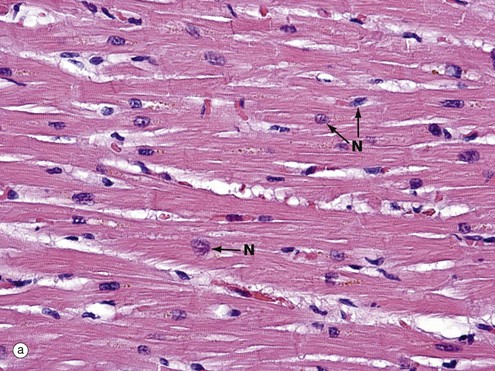
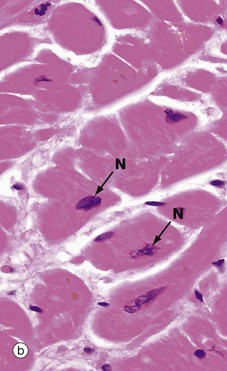
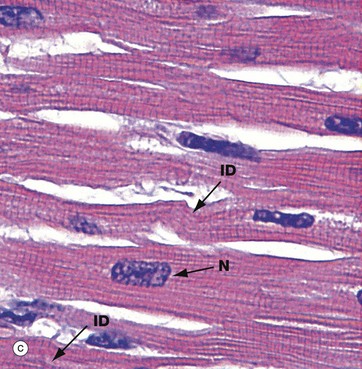
FIG. 6.22 Cardiac muscle
(a) H&E, LS (MP) (b) H&E, TS (HP) (c) H&E, polarised light, LS (HP)
In longitudinal section in micrograph (a), cardiac muscle cells are seen to contain one or two nuclei N and an extensive eosinophilic cytoplasm which branches to give the appearance of a continuous three-dimensional network. The elongated nuclei are mainly centrally located, a characteristic well demonstrated in transverse section as shown in micrograph (b).
Fine wisps of collagenous tissue run between fibres, together with an extensive capillary supply which is not seen at this resolution.
Micrograph (c) has been taken from an H&E-stained section but viewed using polarised light. This creates improved optical contrast so as to reveal the cross-striations. In routine light microscopy, striations in cardiac muscle are generally not as easy to demonstrate as in skeletal muscle. The branching cytoplasmic network is readily seen. Intercalated discs ID mark the intercellular boundaries and are just visible in this micrograph. Note the delicate supporting tissue filling the intercellular spaces.
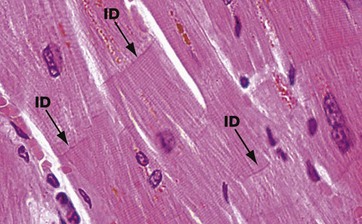
FIG. 6.23 Cardiac muscle
H&E, LS (HP)
The branching pattern of cardiac muscle cells is well demonstrated in this image. Individual cells are attached to each other end to end by specialised cell junctions termed intercalated discs (see also Figs 6.26 and 6.27). These can just be seen as transverse bands ID within the muscle cells. A red-brown pigment seen in these cardiac cells is termed lipofuscin and is derived from turnover of cell material within lysosomes, so-called wear-and-tear pigment. This pigment gradually accumulates in the human heart with age and can be responsible for the heart muscle appearing brown in colour (see also Ch. 1).
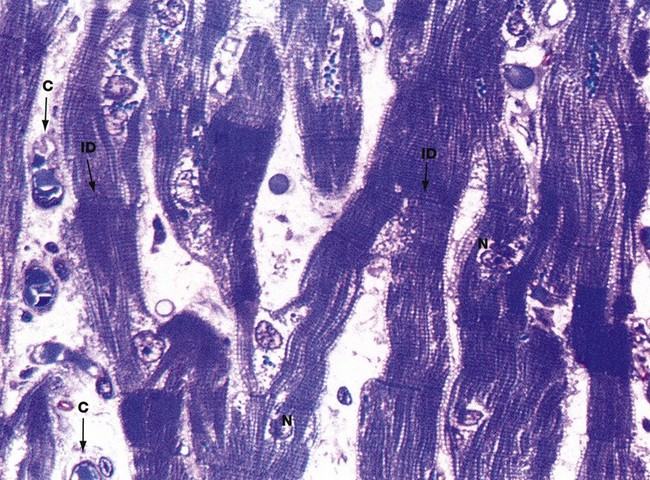
FIG. 6.24 Cardiac muscle
Thin section, toluidine blue, LS (HP)
This micrograph illustrates an extremely thin, resin-embedded section at very high magnification. The branching cytoplasmic network of cardiac muscle cells is readily seen, with prominent intercalated discs ID marking the intercellular boundaries.
With this method of preparation, it is easy to see the typical cross-striations and central nuclei N. Also note the delicate supporting tissue filling the intercellular spaces, containing an extensive network of blood capillaries C.
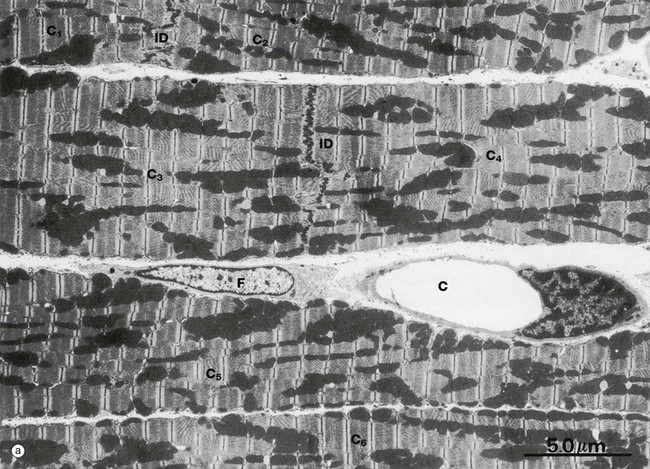
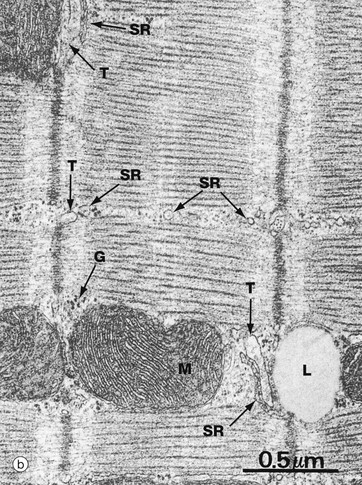
FIG. 6.25 Cardiac muscle
(a) EM ×5000 (b) EM ×38 000
Micrograph (a) illustrates portions of six cardiac muscle cells labelled C1 to C6. None of their nuclei is included in the plane of section. Cells C1 and C2 abut one another end to end and are demarcated by an intercalated disc ID. Cells C3 and C4 are demarcated similarly. The intercellular space contains a capillary C and a fibroblast F.
The sarcomeres of cardiac muscle have an identical banding pattern to that of skeletal muscle. The sarcomeres are not, however, arranged into single columns making up cylindrical myofibrils as in skeletal muscle, but form a branching myofibrillar network, continuous in three dimensions throughout the cytoplasm. The branching columns of sarcomeres are separated by sarcoplasm containing rows of mitochondria and sarcoplasmic reticulum. The great abundance of mitochondria in cardiac muscle reflects the enormous metabolic demands of continuous cardiac muscle activity.
Conduction of excitatory stimuli to the sarcomeres of cardiac muscle is mediated by a system of T tubules and sarcoplasmic reticulum, essentially similar in arrangement to that of skeletal muscle. The T tubules, however, ramify throughout the cardiac muscle cytoplasm at the Z lines, and their origins are seen as indentations in the sarcolemma, which thus has a somewhat scalloped outline.
The conducting system of the cardiac myocyte can be seen at high magnification in micrograph (b). T tubules T and sarcoplasmic reticulum SR form poorly defined triads compared with those of skeletal muscle. Note the typical closely packed cristae of the mitochondria M, a lipid droplet L and glycogen granules G.
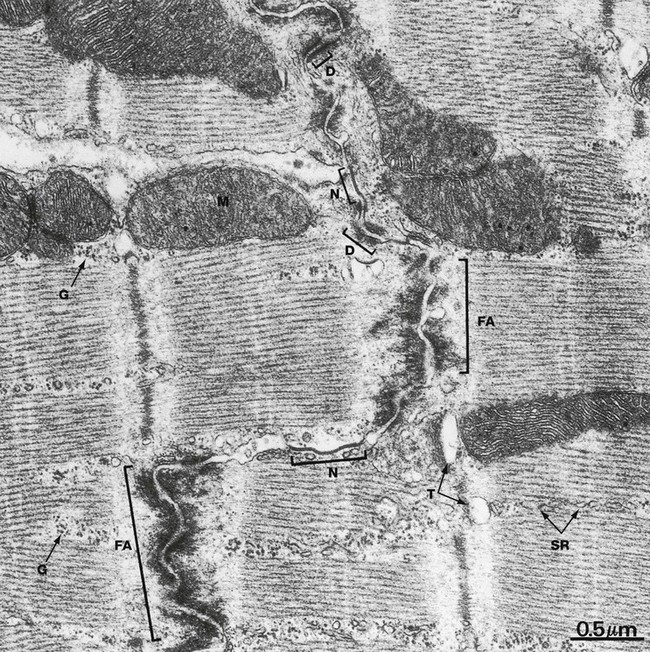
FIG. 6.26 Cardiac muscle, intercalated disc, LS
EM ×31 000
Intercalated discs are specialised transverse junctions between cardiac muscle cells at sites where they meet end to end; they always coincide with the Z lines. Intercalated discs bind the cells, transmit forces of contraction and provide areas of low electrical resistance for the rapid spread of excitation throughout the myocardium.
The intercalated disc is an interdigitating junction and consists of three types of membrane-to-membrane contact. The predominant type of contact, the fascia adherens FA, resembles the zonula adherens of epithelial junctional complexes (see Fig. 5.11) but is more extensive and less regular. The actin filaments at the ends of terminal sarcomeres insert into the fasciae adherentes and thereby transmit contractile forces from cell to cell. Desmosomes D occur less frequently and provide anchorage for intermediate filaments of the cytoskeleton. Gap (nexus) junctions N (see Fig. 5.12) are present mainly in the longitudinal portions of the interdigitations and are sites of low electrical resistance through which excitation passes from cell to cell.
Note the similarity of the sarcomeres of cardiac and skeletal muscle (see Fig. 6.9). The mitochondria M are elongated or spheroidal and have abundant closely packed cristae rich in oxidative enzyme systems. The sarcoplasm within and between the sarcomeres is rich in glycogen granules G. Lace-like profiles of sarcoplasmic reticulum SR and parts of T tubules T can be identified.
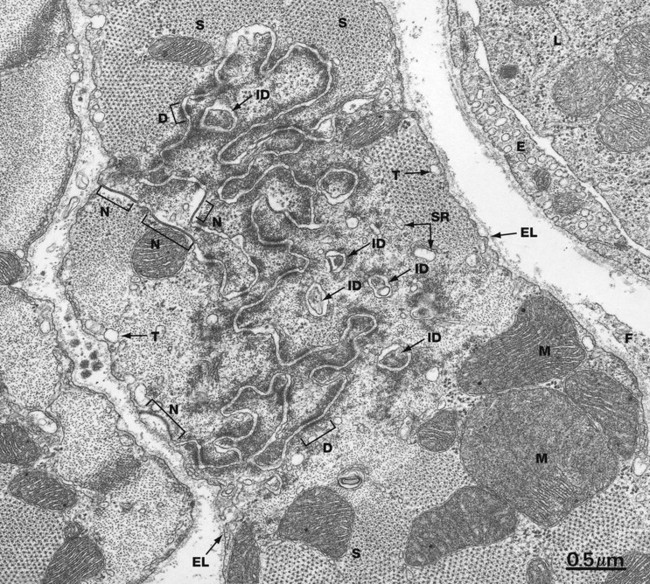
FIG. 6.27 Cardiac muscle, intercalated disc, TS
EM ×27 000
The end-to-end junction between cardiac muscle cells is not simply a flat surface. In reality, each junction is a highly convoluted set of finger-like interdigitations, maximising the surface area in contact between each cell.
This rich structure can be appreciated in this micrograph which shows a transverse section through cardiac muscle in the vicinity of a Z line incorporating an intercalated disc. The tortuous course of the intercellular junction as well as islands of apparently isolated paired cell membranes ID reflect the manner in which the ends of the cardiac muscle cells interdigitate with one another. Most of the intercellular junction comprises fasciae adherentes with interspersed desmosomes D and some nexus junctions N.
The regular dot-like lattice represents the contractile proteins of the sarcomeres S, with the thick filaments surrounded by thin filaments cut in transverse section. Mitochondria M are prominent.
Beneath the cell membrane are dilated tubules T representing part of the T tubule system. Elements of the sarcoplasmic reticulum SR can be seen surrounding the sarcomeres. Beyond the plasma membrane lies the external lamina EL. A fibroblast cytoplasmic extension F is present in the extracellular space at the right of the field. At the upper right is an endomysial capillary, its endothelium E containing numerous pinocytotic vesicles. The lumen contains a leucocyte.
Review
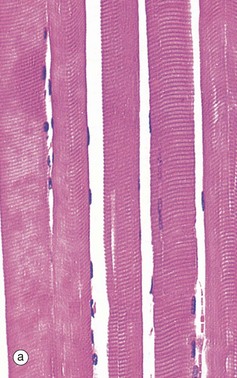
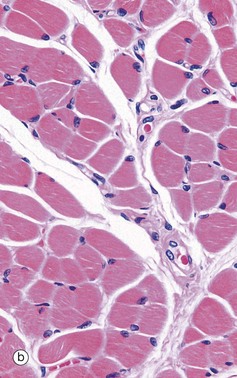

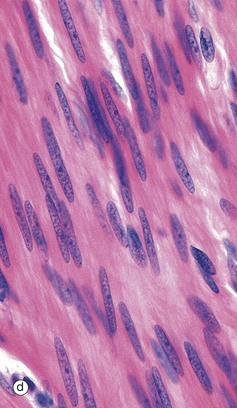
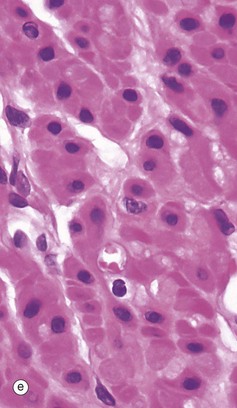
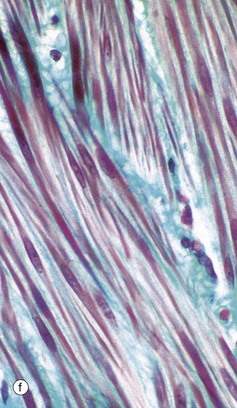
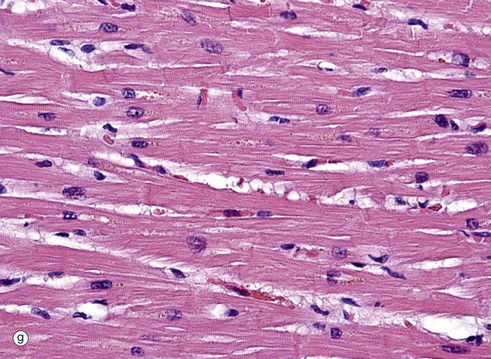
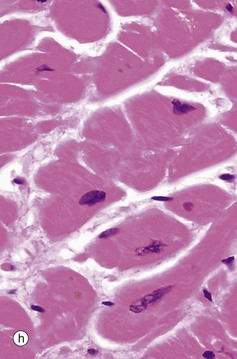
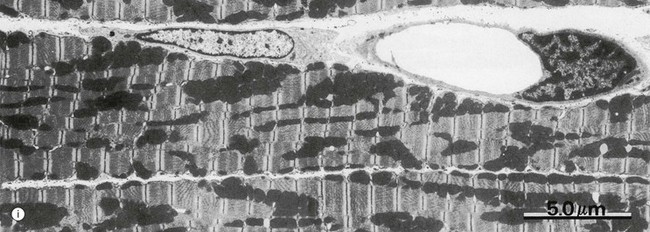
FIG. 6.28 Examples of skeletal, smooth, and cardiac muscle (illustrations (d-i) opposite)
(a) Skeletal muscle, LS, H&E (HP) (b) Skeletal muscle, TS, H&E (HP) (c) Skeletal muscle, LS, Heidenhain’s haematoxylin (HP) (d) Smooth muscle, LS, H&E (HP) (e) Smooth muscle, TS, H&E (HP) (f) Smooth muscle, Masson trichrome (HP) (g) Cardiac muscle, LS, H&E (MP) (h) Cardiac muscle, TS, H&E (HP) (i) Cardiac muscle, EM ×5000