Liver and pancreas
Liver and Biliary System
The liver, like the pancreas, develops embryologically as a glandular outgrowth of the primitive foregut. The major functions of the liver may be summarised as follows:
• Converting carbohydrates and proteins into fatty acids and triglyceride
• Regulation of blood glucose concentration by glycogenesis, glycogenolysis and gluconeogenesis
The main functional cell in the liver is a type of epithelial cell called the hepatocyte. These cells are arranged as thin plates separated by fine vascular sinusoids through which blood flows. The close association of liver cells and the circulation allows absorption of nutrients from digestion, as well as secretion of products into the blood.
Blood flow into the liver sinusoids comes from terminal branches of both the hepatic portal vein and hepatic artery. The liver is therefore unusual in having both arterial and venous blood supplies, as well as separate venous drainage.
With the exception of most lipids, absorbed food products pass directly from the gut to the liver via the hepatic portal vein. This brings blood that is rich in amino acids, simple sugars and other products of digestion but relatively poor in oxygen. The oxygen required to support liver metabolism is supplied via the hepatic artery. After passing through the sinusoids, venous drainage of blood from the liver occurs via the hepatic vein into the vena cava.
The main blood vessels and ducts run through the liver within a branched collagenous framework termed the portal tracts. These tracts also contain the bile ducts that transport bile away from the liver to be secreted into the small bowel.
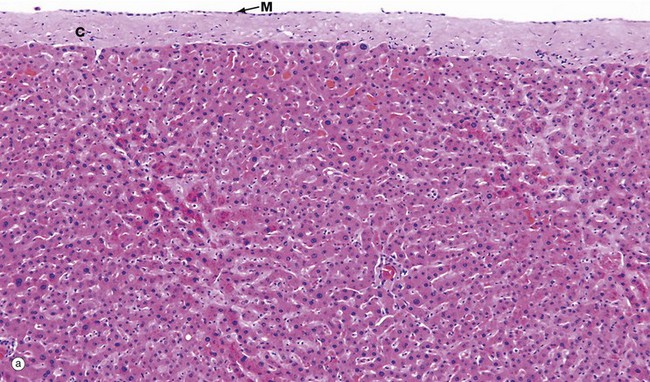
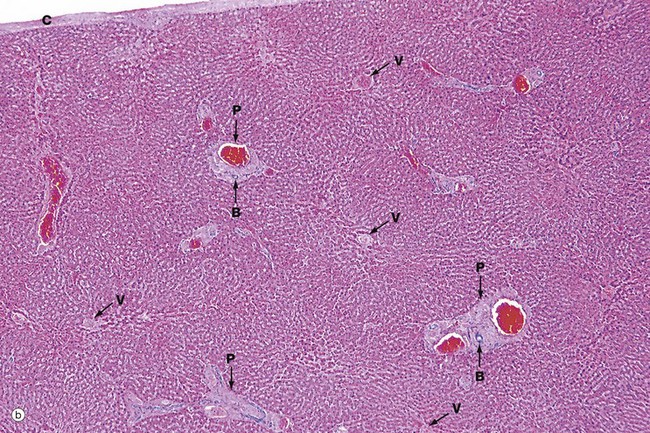
FIG. 15.1 Liver (caption and illustration (b) opposite)
(a) Capsule and parenchyma, H&E (MP) (b) Architecture, H&E (LP)
Micrograph (a) shows the structure of the liver, which is a solid organ composed of tightly packed pink-staining plates of hepatocytes. The outer surface of the liver is covered by a capsule composed of collagenous tissue C called Glisson's capsule, covered by a layer of mesothelial cells M from the peritoneum.
The sinusoids can just be seen as pale-stained spaces between the plates of liver cells. The hepatic sinusoids form a very low-resistance system of vascular channels that allows blood to come into contact with the hepatocytes over a huge surface area.
Micrograph (b) shows the overall architecture of the liver at a slightly lower magnification. The liver does not contain much in the way of connective tissue. Most of the collagenous connective tissue in the liver is found in the portal tracts P which contain the main blood vessels running into the liver. Larger vessels can be seen containing bright red blood, even at this low magnification. The other structures that run in the portal tracts are branches of the bile ducts B.
Less conspicuous than the portal tracts are the centrilobular venules (hepatic venules) V that drain the liver. These are tributaries of the hepatic vein and take blood away from the liver.
The very close association of the sinusoidal vasculature of the liver with the hepatocytes is essential for normal function. Certain diseases of the liver cause obliteration of the normal sinusoidal arrangement and this then causes impairment of liver function.
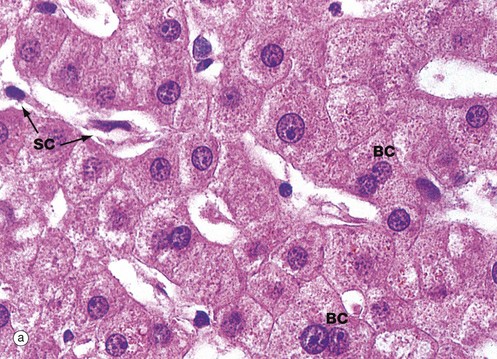
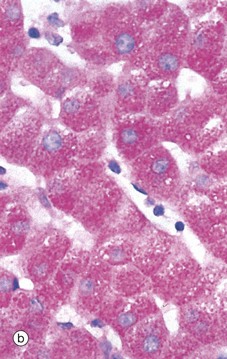
FIG. 15.2 Hepatocytes
(a) H&E (HP) (b) PAS/haematoxylin (HP)
Hepatocytes are large polyhedral cells with round nuclei, peripherally dispersed chromatin and prominent nucleoli. The nuclei vary greatly in size, reflecting an unusual cellular feature; more than half of the hepatocytes contain twice the normal (diploid) complement of chromosomes within a single nucleus (i.e. they are tetraploid) and some contain four or even eight times this amount (polyploid). Binucleate cells BC are also common in normal liver.
The extensive cytoplasm has a variable appearance, depending on the nutritional status of the individual. When well-nourished, hepatocytes store significant quantities of glycogen and process large quantities of lipid. Both of these metabolites are partially removed during routine histological preparation, leaving irregular unstained areas within the cytoplasm. The cytoplasm is otherwise strongly eosinophilic due to numerous mitochondria, with a fine basophilic granularity due to extensive free ribosomes and rough endoplasmic reticulum. Fine brown granules of the ‘wear-and-tear’ pigment lipofuscin (see Fig. 1.25) are present in variable amounts, increasing with age. All of these features are seen in micrograph (a).
The sinusoids are lined by flat endothelial lining cells SC which are readily distinguishable from hepatocytes by their flattened condensed nuclei and attenuated poorly stained cytoplasm.
Micrograph (b) shows glycogen in hepatocytes which, being polysaccharide, is PAS-positive (i.e. stains magenta). In this preparation, the nuclei are counterstained blue with haematoxylin.
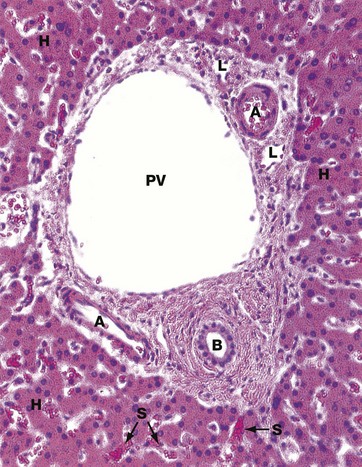
FIG. 15.3 Portal tract
H&E (MP)
This micrograph shows a typical portal tract containing three main structures. The largest is a terminal branch of the hepatic portal vein PV (terminal portal venule) which has a thin wall lined by endothelial cells. Smaller-diameter thick-walled vessels are terminal branches of the hepatic artery A with the structure of arterioles.
A network of bile canaliculi is located within each plate of hepatocytes, but these are far too small to be seen at this magnification. These drain into bile collecting ducts lined by simple cuboidal or columnar epithelium, known as the canals of Hering, which in turn drain into the bile ductules B. The bile ductules are usually located at the periphery of the tract. The bile ductules merge to form larger, more centrally located trabecular ducts which drain via intrahepatic ducts into the right and left hepatic ducts, the common hepatic duct and then to the duodenum via the common bile duct.
Because these three structures are always found in the portal tracts, the tracts are often referred to as portal triads. Lymphatics L are also present in the portal tracts but, since their walls are delicate and often collapsed, they are less easily identified.
Surrounding the portal tract are anastomosing plates of hepatocytes H, between which are the hepatic sinusoids S. These receive blood from both the hepatic portal and hepatic arterial systems. The layer of hepatocytes immediately bordering the portal tract is known as the limiting plate.
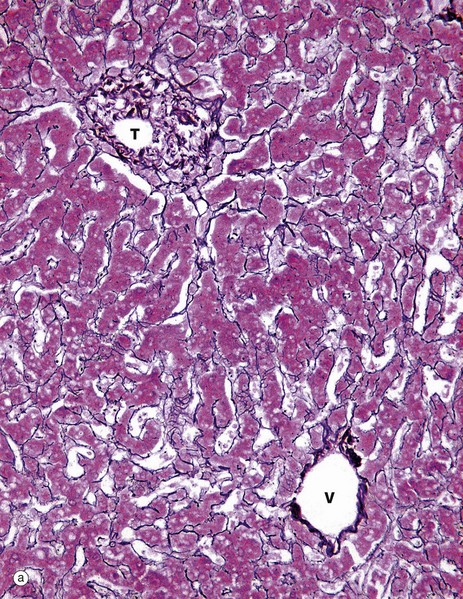
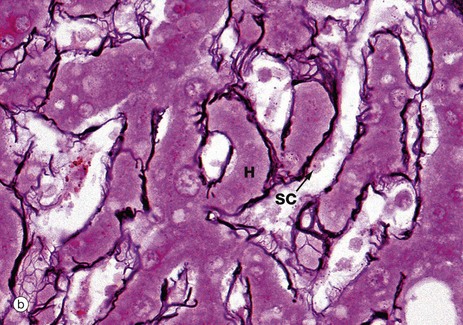
FIG. 15.4 Liver
(a) Reticulin (MP) (b) Reticulin (HP)
The structural integrity of the liver is maintained by a delicate meshwork of extracellular matrix in the form of a fine meshwork of reticulin fibres (collagen type III). The reticulin meshwork supports both the hepatocytes and the sinusoidal lining cells (endothelial cells). These micrographs have both been stained by a silver method that shows reticulin as a black-stained material.
Micrograph (a) shows how reticulin is present on both sides of liver cell plates. The sinusoids are also bounded by the same reticulin framework. The reticulin merges with the sparse collagenous supporting tissue of the portal tract T and terminal hepatic venule V. At the periphery of the liver, the reticulin becomes continuous with Glisson's capsule, which invests the external surface of the liver.
Micrograph (b) shows more detail of the reticulin scaffolding. Single layers of hepatocytes in the liver cell plates H lie immediately upon the reticulin framework. On the other side of the reticulin layer are the hepatic sinusoidal spaces. Some sinusoidal lining cells SC can just be seen.
The sinusoids are lined by a discontinuous fenestrated endothelium which has no basement membrane and is separated from the hepatocytes by a narrow space (the space of Disse) which drains into the lymphatics of the portal tracts.
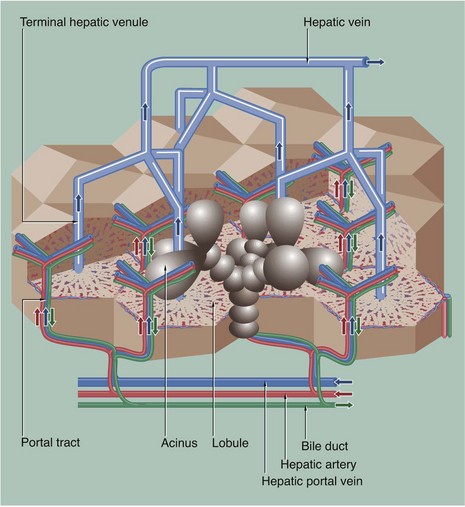
FIG. 15.5 Hepatic vasculature and biliary system
This diagram shows the hepatic vascular system and the bile collecting system. The hepatic portal vein and hepatic artery branch repeatedly within the liver. Their terminal branches run within the portal tracts and empty into the sinusoids. Blood from both systems percolates between plates of hepatocytes in the sinusoids, which converge to drain into a terminal hepatic (centrilobular) venule. These drain to intercalated veins and then to the hepatic vein, which drains into the inferior vena cava.
Bile is secreted into a network of minute bile canaliculi situated between the plasma membranes of adjacent hepatocytes. These canaliculi are too small to be represented in this diagram. The canalicular network drains into a system of bile ducts located in the portal tracts. Bile then flows through the extrahepatic biliary tree and is finally discharged into the second part of the duodenum. The hepatic lobule and acinus are explained in Fig. 15.7.
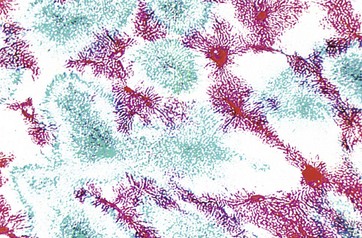
FIG. 15.6 Perfusion method
(LP)
This preparation shows one of the techniques used by early histologists in mapping hepatic blood flow. The hepatic portal vein (supplying the liver) has been perfused with a red dye, and the hepatic vein (draining the liver) has been back-perfused with a blue dye. Thus it can be seen how liver units can be defined by a number of portal tracts peripherally (stained red), with blood draining to a single terminal hepatic venule (stained blue) at the centre.
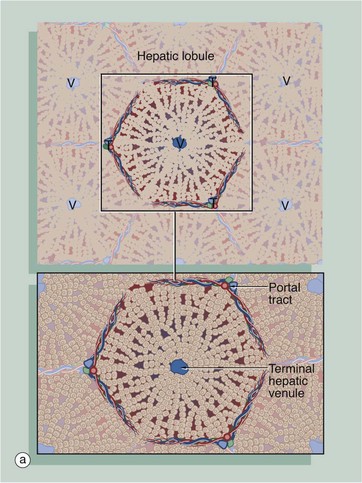
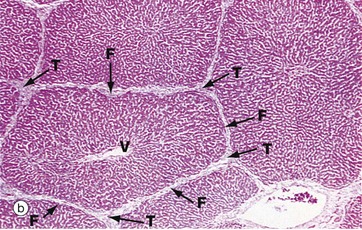
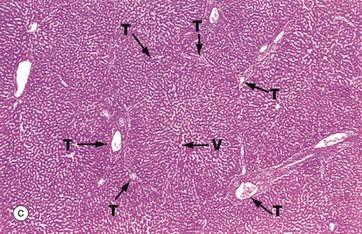
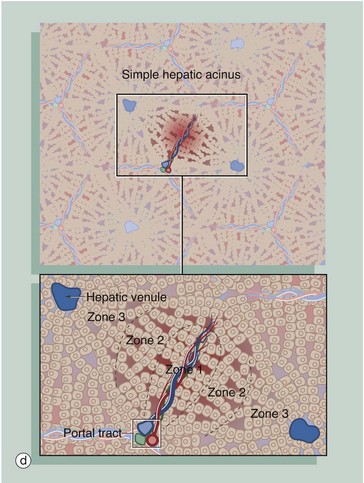
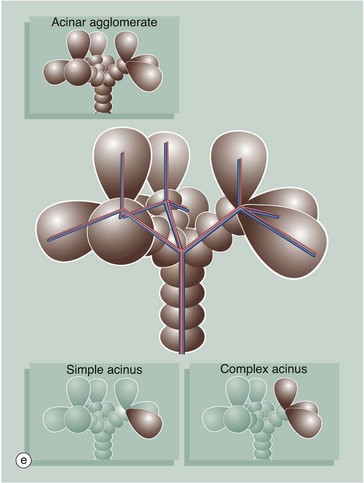
FIG. 15.7 Liver architecture (illustrations opposite)
(a) Diagram of the liver lobule (b) Pig, H&E (LP) (c) Human, H&E (LP) (d) Diagram of the simple acinus (e) Diagram of acinar agglomerate
The structural unit of the liver can be considered as a conceptually simple hepatic lobule. However, the physiology of the liver is more accurately represented by a unit structure known as the hepatic acinus.
The hepatic lobule (a) is roughly hexagonal in shape and is centred on a terminal hepatic venule (centrilobular venule) V. The portal tracts T are positioned at the angles of the hexagon. The blood from the portal vein and hepatic artery branches flows away from the portal tract to the adjacent central veins. In some species, such as the pig (b), the lobule is outlined by bands of fibrous tissue F, giving a well-defined structural unit. In humans (c) and most other species, no such clear structural definition exists, although lobules can be roughly outlined as an hexagonal array of portal tracts T arranged around a terminal hepatic venule V.
The hepatic acinus (d) is a more physiologically useful model of liver anatomy, although more difficult to define histologically. The acinus is a roughly berry-shaped unit of liver parenchyma centered on a portal tract. The acinus lies between two or more terminal hepatic venules and blood flows from the portal tracts through the sinusoids to the venules. The acinus is divided into zones 1, 2 and 3 and the hepatocytes in these zones have different metabolic functions.
Zone 1 is closest to the portal tract and receives the most oxygenated blood, while zone 3 is furthest away and receives the least oxygen. Liver cells in zone 3 contain high levels of esterases and low levels of oxidative enzymes. Large branches of the portal vein and hepatic artery supply an agglomerate of acini, each of which is in turn composed of several complex acini which, at the lowest level, are made of simple acini, each supplied by terminal vascular branches. Although the structure looks on paper like a bunch of grapes, it must be remembered that this is a functional grouping and in reality the hepatic parenchyma is uniform and continuous.
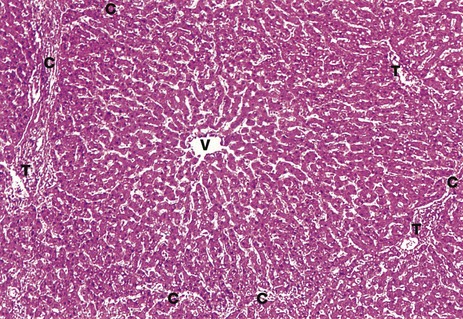
FIG. 15.8 Liver lobule
H&E (MP)
This micrograph illustrates a single human liver lobule and includes parts of a number of hepatic acini, each centred on a portal tract. The irregular hexagonal boundary of the lobule is defined by portal tracts T and sparse collagenous tissue C. Sinusoids originate at the lobule margin and course between plates of hepatocytes to converge upon the terminal hepatic (centrilobular) venule V. The plates of hepatocytes are usually only one cell thick and so each hepatocyte is exposed to blood on at least two sides. The plates of hepatocytes branch and anastomose to form a three-dimensional structure like a sponge.
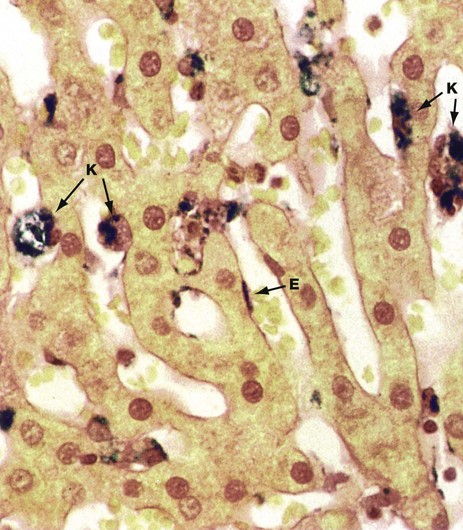
FIG. 15.9 Sinusoid lining cells
Perls Prussian blue (HP)
The sinusoid lining cells include at least three cell types. The majority of cells lining the hepatic sinusoids are endothelial cells E with flat darkly stained nuclei and thin fenestrated cytoplasm.
Scattered among the endothelial cells are large plump phagocytic cells with ovoid nuclei. Known as Küpffer cells K, these form part of the monocyte-macrophage defence system (see Chs. 3 and 4) and, with the spleen, participate in the removal of spent erythrocytes and other particulate debris from the circulation. The phagocytic capability of the Küpffer cells can be demonstrated when they are ‘fed’, either artificially or under pathological conditions, with appropriate particulate matter. The animal used for this preparation was injected intravenously with a particulate iron-sugar compound which, with this staining method, is demonstrated as a dark deposit within the sinusoid lining cells.
The third cell type, known as stellate cells, Ito cells or hepatic lipocytes, cannot be easily distinguished by light microscopy. This cell type has lipid droplets containing vitamin A in their cytoplasm. These cells have the dual functions of vitamin A storage and production of extracellular matrix and collagen. During liver injury, these cells are thought to produce greatly increased amounts of collagen, causing the fibrosis which is a characteristic of hepatic cirrhosis.
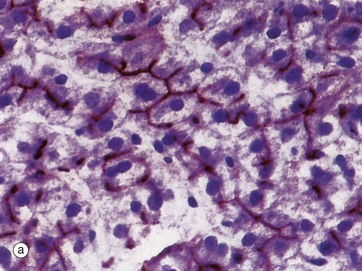
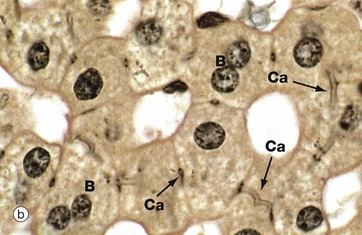
FIG. 15.10 Bile canaliculi
(a) Enzyme histochemical staining for ATPase (HP) (b) Iron haematoxylin (HP)
Bile is synthesised by all hepatocytes and is secreted into a system of minute canaliculi which form an anastomosing network within the plates of hepatocytes. The canaliculi have no discrete structure of their own but consist merely of fine channels formed by the plasma membranes of adjacent hepatocytes. The ultrastructural features are shown in Fig. 15.12. Bile canaliculi of adjacent hepatocyte plates merge to form canals of Hering before draining into the bile ductules of the portal tracts.
The hepatocyte plasma membranes forming the walls of the canaliculi contain the enzyme ATPase, which suggests that bile secretion is an energy-dependent process. A histochemical method for ATPase has been used in micrograph (a) to demonstrate bile canaliculi (stained brown), which are difficult to demonstrate with routine light microscopy methods. Within each hepatocyte plate, the canaliculi form a regular hexagonal network reminiscent of chicken wire, each hexagon enclosing a single hepatocyte.
In micrograph (b), a black stain has been deposited in the walls of the bile canaliculi Ca. Note two binucleate hepatocytes B. The biliary canalicular membrane also contains alkaline phosphatase. In diseases which cause obstruction of bile flow, this enzyme is released from the hepatocyte canalicular membrane into the blood, where it can be detected. Measurement of the serum alkaline phosphatase level forms part of a typical set of liver function tests, a common biochemical assay used in routine practice. Elevated blood levels of hepatic alkaline phosphatase are therefore a feature of obstructive jaundice (see textbox).
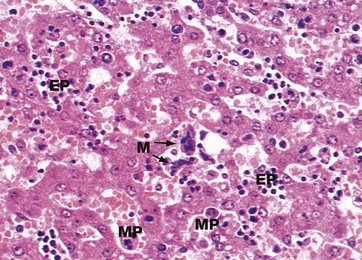
FIG. 15.11 Fetal liver
H&E (MP)
In fetal life, the liver and spleen are important sites of haematopoiesis (see Ch. 3). When haematopoiesis in bone marrow begins, some time after the fourth month of gestation, the importance of the liver and spleen for this function gradually declines. In this micrograph of fetal liver, the hepatocyte plates are two cells thick, a normal finding up to the age of about 7 years. The sinusoids are packed with blood precursors including megakaryocytes M and erythroid EP and myeloid precursors MP.
In adult life, the liver does not normally have haematopoietic tissue. If the capacity of the bone marrow is inadequate for demand, then the fetal function of hepatic haematopoiesis can be re-established. Important causes include fibrosis of bone marrow, replacement of bone marrow by malignancy or some genetic disorders of haemoglobin formation. Haematopoiesis occurs in liver and spleen, both of which may become abnormally enlarged.
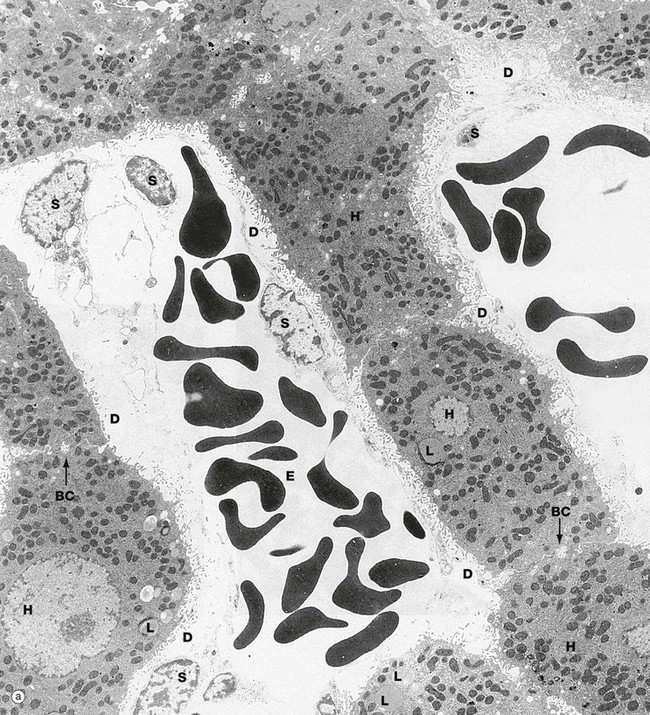
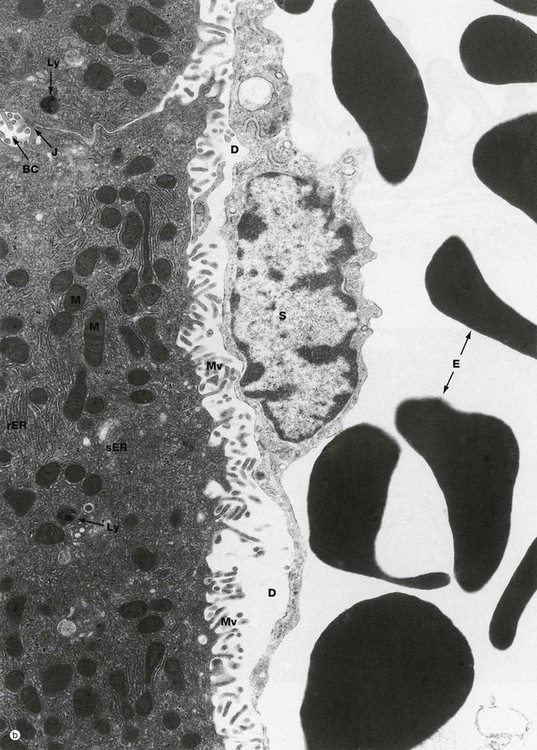
FIG. 15.12 Liver (illustration (b) opposite)
(a) EM ×4400 (b) EM ×15 200
These micrographs demonstrate the main ultrastructural features of the liver. Hepatocytes H are exposed on each side to the sinusoids which are lined by a discontinuous layer of sinusoid lining cells S. These are supported by the fine reticulin framework of the liver (see Fig. 15.4), with the space of Disse D between the lining cells and the hepatocyte surface. Via the gaps in the sinusoid lining, the space of Disse is continuous with the sinusoid lumen, thus bathing the hepatocyte surface with plasma. Numerous irregular microvilli Mv extend from the hepatocyte surface into the space of Disse, greatly increasing the surface area for metabolic exchange. Between the bases of the microvilli, there are coated pits involved in endocytosis. Erythrocytes E can be seen within the sinusoids.
Reflecting their extraordinary range of biosynthetic and degradative activities, the hepatocyte cytoplasm (b) is crowded with organelles, particularly rough endoplasmic reticulum rER, smooth endoplasmic reticulum sER, Golgi stacks, free ribosomes, mitochondria M, lysosomes Ly and peroxisomes. Lipid droplets L and glycogen rosettes are present in variable numbers depending on nutritional status.
Bile canaliculi BC are seen to be formed from the plasma membranes of adjacent hepatocytes, the plasma membranes being tightly bound by junctional complexes J. Small microvilli project into the canaliculi. The subjacent cytoplasm contains a network of actin filaments, contraction of which reduces canalicular diameter, thus reducing flow rate.
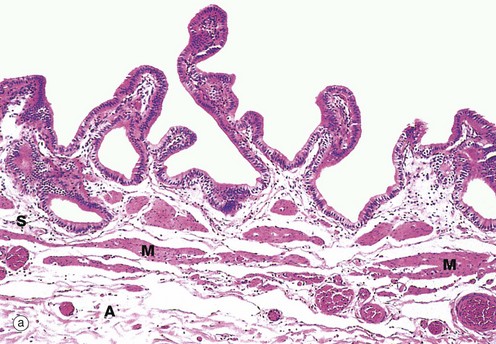
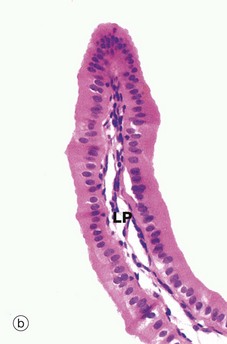
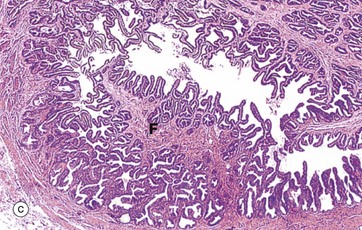
FIG. 15.13 Gallbladder
(a) H&E (LP) (b) H&E (MP) (c) H&E (LP)
The intrahepatic bile collecting system merges to form right and left hepatic ducts which join, creating a single large duct, the common hepatic duct. On leaving the liver, this is joined by the cystic duct which drains the gallbladder. The common bile duct so formed joins the pancreatic duct to form the short ampulla of Vater before entering the duodenum. Bile draining down the common hepatic duct is shunted into the gallbladder where it is stored and concentrated. The major bile ducts outside the liver are collectively called the extrahepatic biliary tree.
The gallbladder is a muscular sac lined by a simple columnar epithelium. It has a capacity of about 100 mL in humans. The presence of lipid in the duodenum promotes the secretion of the hormone cholecystokinin-pancreozymin (CCK) by neuroendocrine cells of the duodenal mucosa, stimulating contraction of the gallbladder and forcing bile into the duodenum. Bile is an emulsifying agent, facilitating the hydrolysis of dietary lipids by pancreatic lipases.
Micrograph (a) shows the wall of a gallbladder in the non-distended state in which the mucosa is thrown up into many folds. The relatively loose submucosa S is rich in elastic fibres, blood vessels and lymphatics which drain water reabsorbed from bile during the concentration process. The fibres of the muscular layer M are arranged in longitudinal, transverse and oblique orientations but do not form distinct layers. Externally, there is a thick collagenous adventitial (serosal) coat A, conveying the larger blood and lymphatic vessels. In the neck of the gallbladder and in the extrahepatic biliary tree, mucous glands are found in the submucosa. Mucus may provide a protective surface film for the biliary tract.
At high magnification in micrograph (b), the simple epithelial lining of the gallbladder is seen to consist of very tall columnar cells with basally located nuclei. Numerous short, irregular microvilli account for the unevenness of the luminal surface. The lining cells concentrate bile 5- to 10-fold by an active process, the resulting water passing into lymphatics in the lamina propria LP.
Micrograph (c) illustrates the wall of the cystic duct, which is formed into a twisted mucosa-covered fold F known as the spiral valve of Heister.
The flow of bile and pancreatic juice into the duodenum is controlled by the complex arrangement of smooth muscle known as the sphincter of Oddi. The components of this structure include the choledochal sphincter at the distal end of the common bile duct, the pancreatic sphincter at the end of the pancreatic duct, and a meshwork of muscle fibres around the ampulla. This arrangement controls the flow of bile and pancreatic juice into the duodenum and, at the same time, prevents reflux of bile and pancreatic juice into the wrong parts of the duct system. When the choledochal sphincter is closed, bile is directed into the gallbladder where it is concentrated.
Pancreas
The pancreas is a large gland which, like the liver, develops embryologically as an outgrowth of the primitive foregut. The pancreas has both exocrine and endocrine components. The endocrine pancreas is described in detail in Ch. 17. The exocrine pancreas, which forms the bulk of the gland, secretes an enzyme-rich alkaline fluid into the duodenum via the pancreatic duct. The high pH of pancreatic secretions is due to a high content of bicarbonate ions and serves to neutralise the acidic chyme as it enters the small intestine from the stomach. The pancreatic enzymes degrade proteins, carbohydrates, lipids and nucleic acids by the process of luminal digestion (see Fig. 14.18). Like pepsin in the stomach, the pancreatic proteolytic enzymes trypsin and chymotrypsin are secreted in an inactive form. Enterokinase, an enzyme secreted by the duodenal mucosa, activates protrypsin to form trypsin. Trypsin then activates prochymotrypsin to form chymotrypsin. This mechanism prevents autodigestion of the pancreas. The other pancreatic enzymes are secreted in the active form.
Pancreatic secretion occurs continuously, the rate being modulated by hormonal and nervous influences. Secretin, a hormone released by neuroendocrine cells scattered in the duodenum, promotes the secretion of copious watery fluid rich in bicarbonate. Cholecystokinin-pancreozymin (CCK), also derived from duodenal neuroendocrine cells, stimulates the secretion of enzyme-rich pancreatic fluid. Gastrin, secreted by neuroendocrine cells of the gastric pylorus, has a similar action on the pancreas to that of CCK. The pancreas is richly innervated by the autonomic nervous system, which also modulates secretory activity.
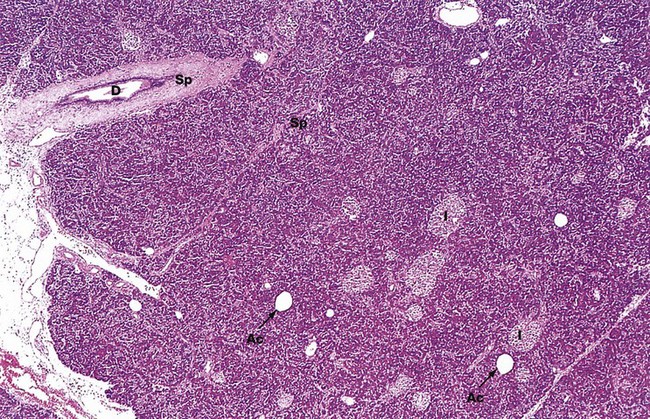
FIG. 15.14 Pancreas
H&E (LP)
The pancreas is a lobulated gland covered by a thin collagenous capsule which extends as delicate septa Sp between the lobules. The exocrine component of the pancreas consists of closely packed secretory acini which drain into a highly branched duct system. Most of the secretion drains into the main pancreatic duct, which joins the common bile duct to drain into the duodenum via the ampulla of Vater. In most people, a small accessory pancreatic duct drains into the duodenum more proximally. Interlobular ducts D can be seen in this micrograph. Their surrounding supporting tissue reinforces the septal framework.
The endocrine tissue of the pancreas forms islets of Langerhans I of various sizes scattered throughout the exocrine tissue. Occasional adipocytes Ac are scattered throughout the parenchyma. These are scanty in young adults but are seen in increasing numbers in older people, reflecting the natural atrophy of the gland with age.
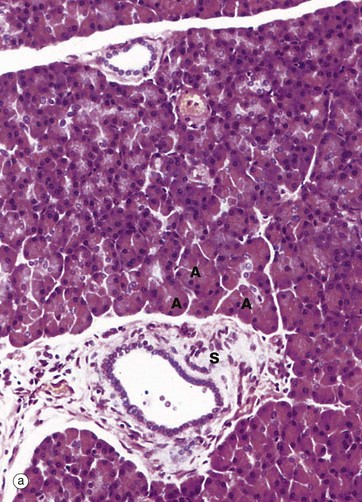
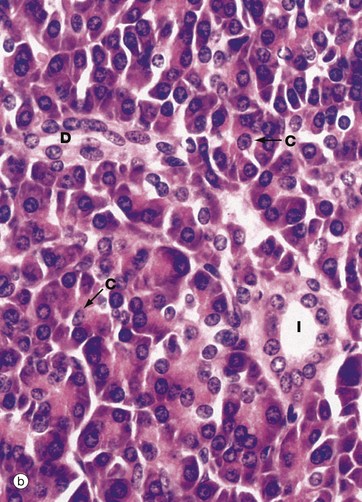
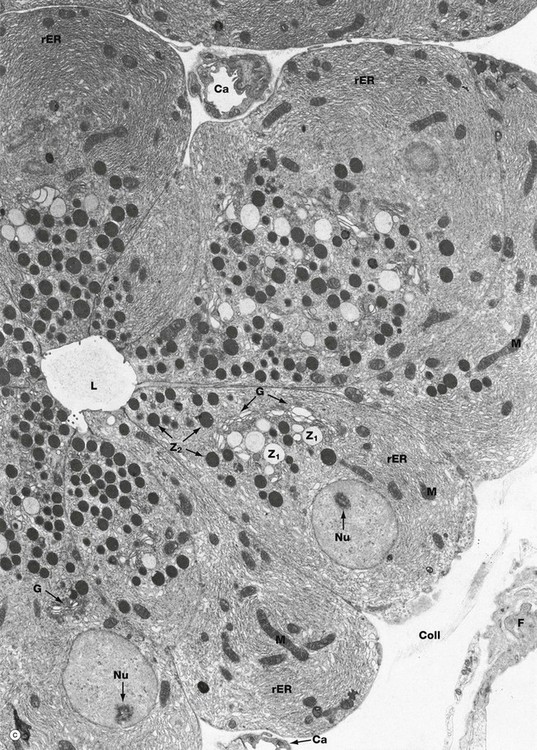
FIG. 15.15 Exocrine pancreas (illustration (c) opposite)
(a) H&E (MP) (b) H&E (HP) (c) EM ×8500
Details of the pancreatic acini and duct system can be seen in these micrographs. Each acinus is made up of an irregular cluster of pyramid-shaped secretory cells, the apices of which surround a minute central lumen which represents the end of the duct system. The smallest of the tributaries are known as intercalated ducts. Adjacent acini are separated by inconspicuous supporting tissue containing numerous capillaries. In histological sections, the interacinar spaces tend to appear wider than they do in vivo, due to a fixation artefact.
The intercalated ducts drain into small intralobular ducts, which in turn drain into the interlobular ducts in the septa of the gland. The intercalated ducts are lined by simple low cuboidal epithelium, which becomes stratified cuboidal in the larger ducts. With increasing size, the ducts are invested by a progressively thicker layer of dense collagenous supporting tissue. The wall of the main pancreatic duct contains smooth muscle.
Micrograph (a) shows the general arrangement of the glandular acini A. An intralobular duct is seen in upper midfield and a larger interlobular duct in lower midfield, the latter having a much broader sheath of supporting tissue S.
At higher magnification in micrograph (b), the cells of each pancreatic acinus have a roughly triangular shape in section, their apices projecting towards a central lumen of a minute duct. The acinar cells are typical protein-secreting cells. The nuclei are basally located and surrounded by basophilic cytoplasm which is crammed with rough endoplasmic reticulum. The apices of the cells are packed with eosinophilic secretory granules containing proenzymes. The centres of the acini frequently contain one or more nuclei of centroacinar cells C with pale nuclei and sparse pale-stained cytoplasm. These represent the terminal lining cells of intercalated ducts. Cells of similar appearance can be seen between the acini and those of intercalated ducts D passing to join the larger intralobular ducts I. The cells lining the intercalated ducts secrete water and bicarbonate ions into the pancreatic juice.
Electron micrograph (c) illustrates part of a pancreatic acinus with its central lumen L. The pyramid-shaped secretory cells have round, basally located nuclei with dispersed chromatin and prominent nucleoli Nu, both characteristic features of highly active cells. The basal cytoplasm is packed with lamellar profiles of rough endoplasmic reticulum rER, among which elongated mitochondria M are scattered. A large Golgi apparatus G is located in a supranuclear position and is responsible for packaging enzymes synthesized on the rough endoplasmic reticulum to form zymogen granules. Newly packed secretory or zymogen granules Z1 are large and much less electron-dense than the smaller mature granules Z2 which aggregate in the apical cytoplasm. Zymogen granules are released into the acinar lumen by exocytosis. Small irregular microvilli associated with this process are seen projecting into the lumen. Note small capillaries Ca, a fibroblast F and collagen Coll in the fine supporting tissue which surrounds the acinus.
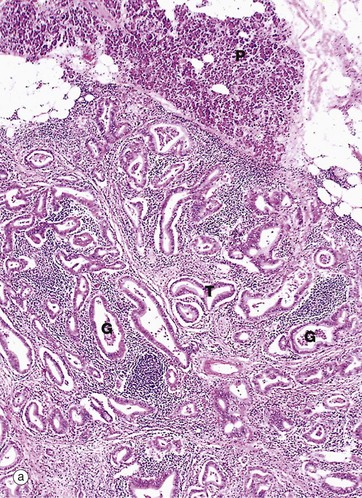
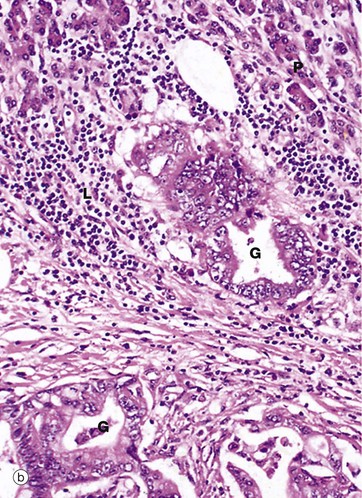
FIG. 15.16 Adenocarcinoma of pancreas
(a) H&E (LP) (b) H&E (HP)
Pancreatic adenocarcinomas usually arise in the head of the gland and present with obstructive jaundice. Micrograph (a) shows tumour T, with normal pancreas P in the upper field. Note the characteristic tortuous irregular glands G within a dense collagenous stroma.
At higher magnification in micrograph (b), the tumour is seen to have a ductal pattern, with marked variation in the size and shape of neoplastic glands G. The cells of these malignant glands have large nuclei with prominent nucleoli. It may be helpful to compare the nuclear size against the normal pancreatic acinar cells P and the surrounding small lymphocytes L.