Gastrointestinal tract
Introduction
The function of the gastrointestinal system is to break down food for absorption into the body. This process occurs in five main phases: ingestion, fragmentation, digestion, absorption and elimination of waste products. Digestion is the process by which food is enzymatically broken down into molecules that are small enough to be absorbed into the circulation. As an example, ingested proteins are first reduced to polypeptides and then further degraded to small peptides and amino acids that can be absorbed.
The gastrointestinal system is essentially a muscular tube lined by a mucous membrane that exhibits regional variations, reflecting the changing functions of the system from mouth to anus. The mucous membrane is protective, secretory, absorptive or a combination of these in different parts of the tract (see Fig. 14.3). The muscle gives strength to the wall of the tract as well as moving the food along. Muscle is arranged somewhat differently in different areas of the tract. Because of its continuity with the external environment, the gastrointestinal system is a potential portal of entry for pathogenic organisms. As a result, the system incorporates a number of defence mechanisms which include prominent aggregations of lymphoid tissue, known as the gut-associated lymphoid system (GALT), distributed throughout the tract (see Ch. 11).
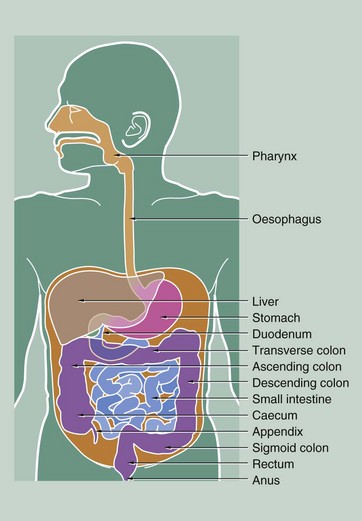
FIG. 14.1 Parts of the gastrointestinal tract
Ingestion and initial fragmentation of food occur in the oral cavity, resulting in the formation of a bolus of food. This is then conveyed to the oesophagus by the action of the tongue and pharyngeal muscles during swallowing. Secretion of saliva from major and minor salivary glands (see Ch. 13) aids fragmentation and lubricates the food for swallowing.
The oesophagus conducts food from the oral cavity to the stomach where fragmentation is completed and digestion begins. Initial digestion, accompanied by the intense muscular action of the stomach wall, converts the stomach contents to a semi-digested liquid called chyme. Chyme is squirted through a muscular sphincter, the pylorus, into the duodenum, the short first part of the small intestine. Digestive enzymes from a large exocrine gland, the pancreas, enter the duodenum together with bile from the liver via the common bile duct (see Ch. 15). Bile contains excretory products of liver metabolism, some of which act as emulsifying agents necessary for fat digestion. Duodenal contents pass along the rest of the small intestine where the process of digestion is completed and the main absorptive phase occurs. The middle segment of the small intestine is called the jejunum and the distal segment the ileum. There is no distinct anatomical boundary between these parts of the small bowel.
The liquid residue from the small intestine passes through the ileocaecal valve into the large intestine. Here, water is absorbed from the liquid residue, which becomes progressively more solid as it passes towards the anus. The capacious first part of the large intestine is called the caecum, from which projects a blind-ended sac, the appendix. The next part of the large intestine, the colon, is divided anatomically into ascending, transverse, descending and sigmoid segments, although histologically the segments are indistinguishable from one another. The terminal portion of the large intestine, the rectum, is a holding chamber for faeces prior to defaecation via the anal canal.
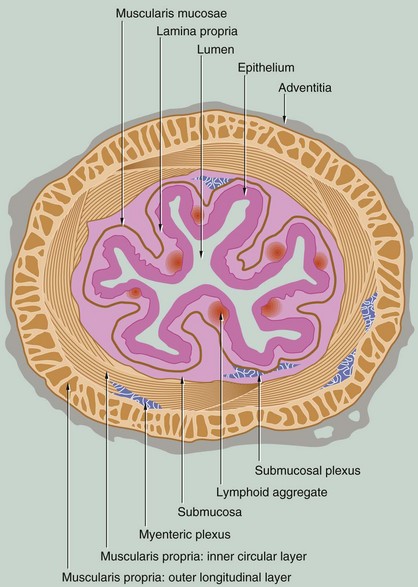
FIG. 14.2 Structure of the gastrointestinal tract
The structure of the gastrointestinal tract conforms to a general plan that is clearly evident from the oesophagus to the anus. The tract is essentially a muscular tube lined by a mucous membrane. There are minor variations in the arrangement of the muscular component in different parts of the gut, but much more striking are the marked changes in the structure and therefore function of the mucosa in the different regions of the tract.
The gastrointestinal tract has four distinct functional layers: mucosa, submucosa, muscularis propria and adventitia.
• Mucosa. The mucosa is made up of three components: the epithelium, a supporting lamina propria and a thin smooth muscle layer, the muscularis mucosae, which produces local movement and folding of the mucosa. At four points along the tract, the mucosa undergoes abrupt transition from one form to another: the gastro-oesophageal junction, the gastroduodenal junction, the ileocaecal junction and the rectoanal junction.
• Submucosa. This layer of loose collagenous connective tissue supports the mucosa and contains the larger blood vessels, lymphatics and nerves.
• Muscularis propria. The muscular wall proper consists of smooth muscle that is usually arranged as an inner circular layer and an outer longitudinal layer. In the stomach only, there is an inner oblique layer of muscle. The action of the two layers, at right angles to one another, is the basis of peristaltic contraction (see textbox).
• Adventitia. This outer layer of loose supporting tissue conducts the major vessels, nerves and contains variable adipose tissue. Where the gut lies within the abdominal cavity (peritoneal cavity), the adventitia is referred to as the serosa (visceral peritoneum) and is lined by a simple squamous epithelium (mesothelium). Elsewhere, the adventitial layer merges with retroperitoneal tissues.
Food is propelled along the gastrointestinal tract by two main mechanisms: voluntary muscular action in the oral cavity, pharynx and upper third of the oesophagus is succeeded by involuntary waves of smooth muscle contraction called peristalsis. Peristalsis and the secretory activity of the entire gastrointestinal system are modulated by the autonomic nervous system and a variety of hormones, some of which are secreted by neuroendocrine cells located within the gastrointestinal tract itself. These cells constitute a diffuse neuroendocrine system, with cells producing a variety of locally acting hormones found scattered along the whole length of the tract (see also Ch. 17).
Autonomic regulation of certain glandular secretions and the smooth muscle of the gut and its blood vessels is mediated by the enteric nervous system, comprising postganglionic sympathetic fibres and ganglia and postganglionic fibres of the parasympathetic nervous system, supplied by the vagus nerve. Contraction of the smooth muscle of the bowel is initiated by pacemaker cells known as interstitial cells of Cajal, modulated by the autonomic nervous system, particularly the parasympathetic nervous system. As in other organs of the body, parasympathetic efferent fibres synapse with effector neurones in small ganglia located in or close to the organ involved. In the gastrointestinal tract, parasympathetic ganglia are concentrated in plexuses in the wall of the tract. In the submucosa, isolated or small clusters of parasympathetic ganglion cells give rise to postganglionic fibres which supply the mucosal glands and the smooth muscle of the muscularis mucosae. This submucosal plexus, Meissner plexus, also contains postganglionic sympathetic fibres arising from the superior mesenteric plexus. Larger clusters of parasympathetic ganglion cells are found between the two layers of the muscularis propria, the postganglionic fibres mainly supplying the surrounding smooth muscle. This plexus is known as the myenteric plexus or Auerbach plexus.
Glands are found throughout the tract at various levels in its wall. In some parts of the tract (i.e. stomach, small and large intestine), the mucosa is arranged into glands that secrete mucus for lubrication among other things. In the lower oesophagus and duodenum, glands penetrate the muscularis mucosae to lie in the submucosa. The pancreas and liver are large glands draining into the gastrointestinal lumen but lying entirely outside its wall (see Ch. 15).
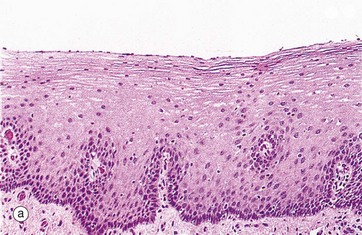
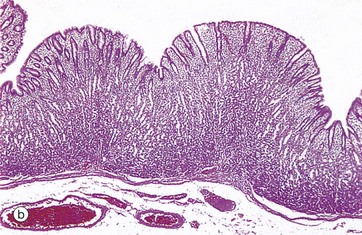
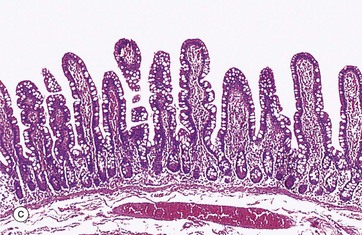
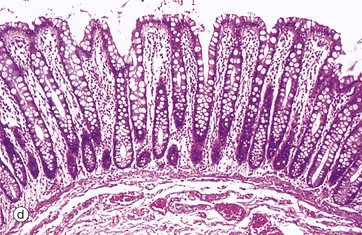
FIG. 14.3 Basic mucosal types in the gastrointestinal tract
(a) Squamous mucosa, H&E (MP) (b) Gastric type secretory mucosa, H&E (LP) (c) Intestinal type absorptive mucosa, H&E (MP) (d) Colorectal type absorptive/protective mucosa, H&E (MP)
Four basic mucosal types are found lining the gastrointestinal tract and these can be classified according to their main function:
• Protective. This type is found in the oral cavity, pharynx, oesophagus and anal canal and is illustrated in micrograph (a). The surface epithelium is of stratified squamous type and, although not keratinised in humans, it may be keratinised in some animals that have a coarse diet (e.g. rodents, herbivores). A stratified mucosal lining of this type is well suited to sites of potential frictional trauma, such as that associated with the passage of food during mastication and swallowing, or during the passage of faeces through the anal canal.
• Secretory. This type of mucosa occurs only in the stomach and is illustrated in micrograph (b). It consists of long, closely packed tubular glands that are simple or branched, depending on the region of the stomach. These glands act to produce various combinations of acid and digestive enzymes in order to facilitate digestion of food whilst also secreting mucus to protect the mucosa itself from injury.
• Absorptive. This mucosal form is typical of the entire small intestine and is illustrated in image (c). The mucosa is arranged into finger-like projections called villi which serve to dramatically increase surface area of the mucosa, with intervening short glands called crypts. In the duodenum, some crypts extend through the muscularis mucosae to form submucosal glands called Brunner's glands. This is the major histological feature that differentiates the duodenum from the jejunum and ileum.
• Absorptive/protective. This form lines the entire large intestine and is shown in micrograph (d). The mucosa is arranged into closely packed, straight tubular glands consisting of cells specialised for water absorption, as well as mucus-secreting goblet cells to lubricate the passage of faeces.
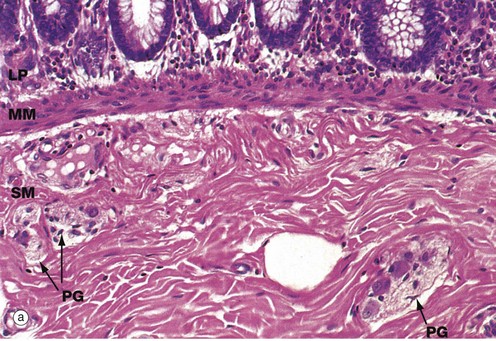
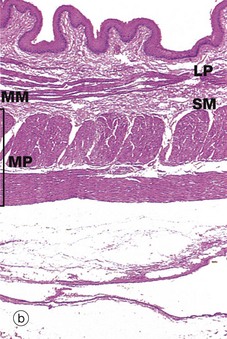
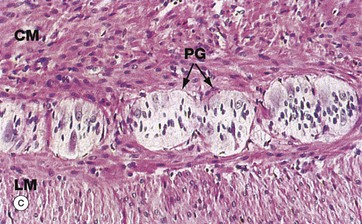
FIG. 14.4 Components of the wall of the gastrointestinal tract
(a) Colon, H&E (HP) (b) Oesophagus, H&E (LP) (c) Colon, H&E (HP)
This series of micrographs illustrates the deeper layers of the wall of the gastrointestinal tract.
Micrograph (a) illustrates the muscularis mucosae MM, clearly demarcating the delicate lamina propria LP from the more robust underlying submucosa SM. This arrangement is typical of the whole of the gastrointestinal tract.
In most of the gut, the lamina propria consists of loose supporting tissue with a diffuse population of lymphocytes and plasma cells. The exception is the stomach which normally has few, if any, resident lymphoid cells. At intervals throughout the oesophagus, small and large bowels and appendix, prominent aggregates of lymphocytes with lymphoid follicles are found. There are also smaller numbers of eosinophils and histiocytes (see Fig. 4.21) to deal with any microorganisms breaching the intestinal epithelium until a specific immune response can be mounted. In the oesophagus, where the function of the mucosa is to protect against friction, the lamina propria is more collagenous than elsewhere and the muscularis mucosae is more prominent. The lamina propria is also typically rich in blood and lymphatic capillaries necessary to support the secretory and absorptive functions of the mucosa.
The muscularis mucosae consists of several layers of smooth muscle fibres, those in the deeper layers orientated parallel to the luminal surface. The more superficial fibres are oriented at right angles to the surface; in the small intestine, the fibres extend up into the villi (see Fig. 14.22). The activity of the muscularis mucosae keeps the mucosal surface and glands in a constant state of gentle agitation which expels secretions from the deep glandular crypts, prevents clogging and enhances contact between epithelium and luminal contents for absorption.
The submucosa consists of collagenous and adipose connective tissue that binds the mucosa to the main bulk of the muscular wall. The submucosa contains the larger blood vessels and lymphatics, as well as the nerves supplying the mucosa. Tiny parasympathetic ganglia PG are scattered throughout the submucosa, forming the submucosal (Meissner) plexus from which postganglionic fibres supply the muscularis mucosae.
The typical arrangement of the two layers of the muscular wall proper is seen in micrograph (b), which shows a longitudinal section of the oesophagus. The muscularis propria MP is made up of an outer longitudinal layer and a somewhat broader inner circular layer. There has been some artefactual separation of the layers in this micrograph, making them easier to visualise. The submucosa SM is separated from the lamina propria LP by the muscularis mucosae MM.
Micrograph (c) illustrates, at high magnification, the junction of outer longitudinal LM and inner circular CM layers of the muscularis propria in the large intestine. Between the layers, there are clumps of pale-stained parasympathetic ganglion cells of the myenteric (Auerbach) plexus. The two layers of the muscularis propria undergo synchronised rhythmic contractions that pass in peristaltic waves down the tract, propelling the contents distally. Peristalsis is initiated by the pacemaker cells, the interstitial cells of Cajal, but the level of activity is modulated by the autonomic nervous system, by locally produced gastrointestinal tract hormones and by other environmental factors. Parasympathetic activity enhances peristalsis while sympathetic activity slows gut motility.
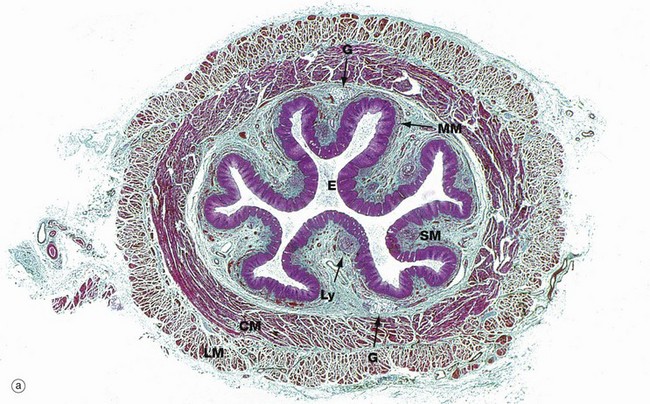
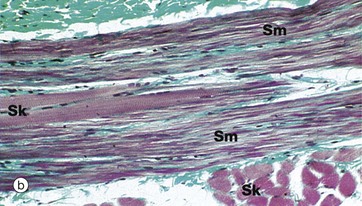
FIG. 14.5 Oesophagus
(a) Masson trichrome stain (LP) (b) Masson trichrome stain (HP)
The oesophagus is a strong muscular tube that conveys food from the oropharynx to the stomach. The initiation of swallowing is a voluntary act involving the skeletal muscles of the oropharynx. This is then succeeded by a strong peristaltic reflex that conveys the bolus of food or fluid to the stomach. Food and fluid do not normally remain in the oesophagus for more than a few seconds and reflux is usually prevented by a physiological sphincter at the gastro-oesophageal junction (see textbox).
Below the diaphragm, the oesophagus passes a centimetre or so into the abdominal cavity before joining the stomach at an acute angle. Sphincter control appears to involve four complementary factors: diaphragmatic contraction, greater intra-abdominal pressure than intragastric pressure being exerted upon the abdominal part of the oesophagus, unidirectional peristalsis and maintenance of correct anatomical arrangements of the structures.
Micrograph (a) shows the lower third of the oesophagus. In the relaxed state, the oesophageal mucosa is deeply folded, an arrangement that allows marked distension during the passage of a food bolus. The lumen of the oesophagus is lined by a thick protective stratified squamous epithelium E (see Fig. 14.3). The underlying lamina propria is quite narrow and contains scattered lymphoid aggregates Ly. The muscularis mucosae MM is barely visible at this magnification.
The submucosa SM is quite loose with many elastin fibres, allowing for considerable distension during passage of a food bolus. The submucosa also contains small seromucous glands G, similar to salivary glands, which aid lubrication and are most prominent in the upper and lower thirds of the oesophagus.
The muscularis propria is thick, and inner circular CM and outer longitudinal LM layers of smooth muscle are clearly distinguishable. Since the first part of swallowing is under voluntary control, bundles of skeletal muscle predominate in the muscularis propria of the upper third of the oesophagus. In the middle third of the oesophagus, there is gradual transition from striated to smooth muscle and, in the lower oesophagus, the muscularis propria consists entirely of smooth muscle.
Micrograph (b) shows part of the muscularis propria of the upper oesophagus at high magnification in the area of transition from skeletal to smooth muscle fibres. A bundle of smooth muscle fibres Sm is seen, with two skeletal muscle fibres Sk in their midst. Other skeletal muscle fibres are seen in transverse section in the lower right of the micrograph. The cross-striations of the skeletal muscle are just visible at this magnification. The collagen of the endomysial supporting tissue stains green with this method.
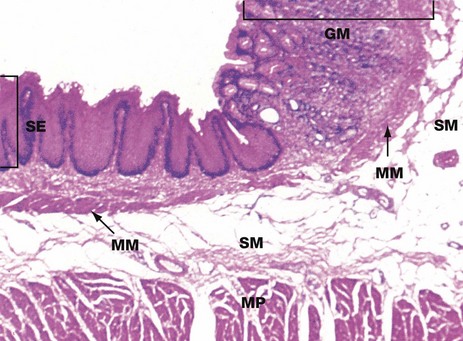
FIG. 14.6 Oesophago-gastric junction
H&E (LP)
At the junction of the oesophagus with the stomach, the mucosa of the tract undergoes an abrupt transition from a protective stratified squamous epithelium SE to a tightly packed glandular secretory mucosa GM.
The muscularis mucosae MM is continuous across the junction, although it is less easily seen in the stomach where it lies immediately beneath the base of the gastric glands. The underlying submucosa SM and muscularis propria MP continue uninterrupted beneath the mucosal junction. The muscularis propria does not form a defined anatomical sphincter, but rather a physiological sphincter mechanism as described in Fig. 14.5.
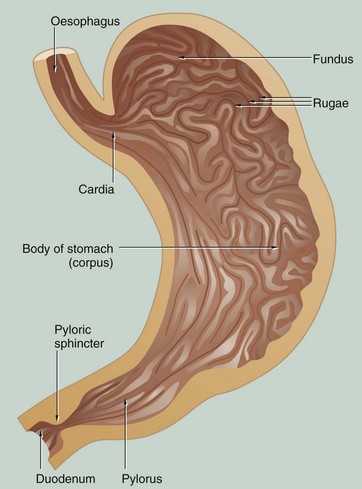
FIG. 14.7 Stomach
Food passes from the oesophagus into the stomach, a distensible organ, where it may be retained for 2 hours or more. In the stomach, the food undergoes mechanical and chemical breakdown to form chyme. Solid foods are broken up by a strong muscular churning action while chemical breakdown is produced by gastric juices secreted by the glands of the stomach mucosa.
There is little absorption from the stomach except for water, alcohol and some drugs. Once chyme formation is completed, the pyloric sphincter relaxes and allows the liquid chyme to be squirted into the duodenum.
In the non-distended state, the stomach mucosa is thrown into prominent longitudinal folds called rugae that allow distension after eating. Anatomically, the stomach is divided into four regions: the cardia, fundus, body (corpus) and pylorus (pyloric antrum). The pylorus terminates in a strong muscular sphincter at the gastroduodenal junction.
The mucosa of the entire stomach has a tubular glandular form, but there are three distinctly different histological zones:
• The cardia is a small area of mucus-secreting glands surrounding the entrance of the oesophagus. In some individuals the cardia measures only a few millimetres or may be incomplete or absent altogether.
• The mucosa of the fundus and body forms the major histological region and consists of glands that secrete acid-pepsin gastric juices as well as some protective mucus.
• The glands of the pylorus secrete mucus of two different types and there are associated endocrine cells which secrete the hormone gastrin.
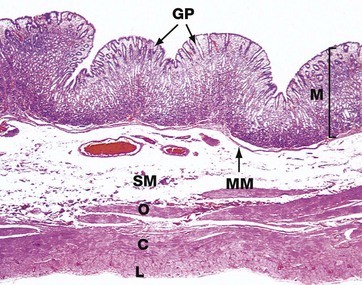
FIG. 14.8 Body of the stomach
H&E (LP)
This micrograph illustrates the body of the stomach in the non-distended state. The mucosa M is thrown into prominent folds or rugae and consists of gastric glands that extend from the level of the muscularis mucosae MM to open into the stomach lumen via gastric pits or foveolae GP.
The muscularis propria comprises the usual inner circular C and outer longitudinal L layers, but the inner circular layer is reinforced by a further inner oblique layer O.
The submucosa SM is relatively loose and distensible and contains the larger blood vessels. The serosal layer, which covers the peritoneal surface, is thin and barely visible at this magnification.
The adipose tissue of the lesser and greater omentum is attached along the lesser and greater curvature of the stomach (not illustrated in this micrograph). Lymph nodes and large blood vessels lie within this omental fatty tissue.
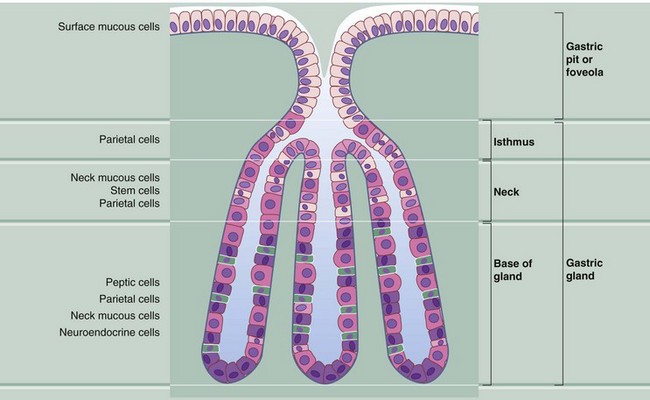
FIG. 14.9 Body of the stomach: structure of the gastric glands
The mucosa of the fundus and body of the stomach consists of straight tubular glands that synthesise and secrete gastric juice. The gastric pits occupy about one-quarter of the thickness of the gastric mucosa and each has between one and seven gastric glands opening into it. Gastric juice is a watery secretion containing hydrochloric acid (pH 0.9–1.5) and the digestive enzyme pepsin, which hydrolyses proteins into polypeptide fragments. The stomach mucosa is protected from self-digestion by a thick surface covering of mucus, which is maintained at a higher pH than the gastric juice by the secretion of bicarbonate ions by the gastric surface mucous cells. The gastric glands contain a mixed population of cells:
• Surface mucous cells cover the luminal surface of the stomach and partly line the gastric pits. The cytoplasmic mucigen granules that pack these cells are stained poorly by the standard H&E stain. These cells have short surface microvilli and secrete protective bicarbonate ions directly into the deeper layers of the surface mucous coat.
• Neck mucous cells are squeezed between the parietal cells in the neck and base of the gastric glands. These cells have larger secretory granules and more polyribosomes than surface mucous cells.
• Parietal or oxyntic cells are distributed along the length of the glands but tend to be most numerous in the isthmus of the glands. These large rounded cells have an extensive eosinophilic (oxyntic) cytoplasm and a centrally located nucleus. Parietal cells secrete gastric acid as well as intrinsic factor, a glycoprotein necessary for the absorption of vitamin B12 in the terminal ileum.
• Chief, peptic or zymogenic cells are located towards the bases of the gastric glands. Peptic cells are recognised by their condensed, basally located nuclei and strongly basophilic granular cytoplasm. This reflects their large content of ribosomes. These are the pepsin-secreting cells.
• Neuroendocrine cells, part of the diffuse neuroendocrine system, are also found in the base of the gastric glands. They secrete 5-HT (serotonin) and other hormones (see also Fig. 14.12).
• Stem cells are found mainly in the neck of the gastric glands. These undifferentiated cells divide continuously to replace all other types of cell in the glands. The maturing cells then migrate up or down as appropriate. These cells are not easily identified in sections of normal gastric mucosa but become very prominent with plentiful mitotic figures after damage to the mucosa has occurred, such as after an episode of gastritis (see textbox).
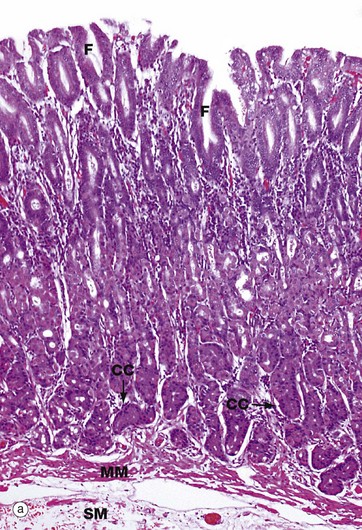
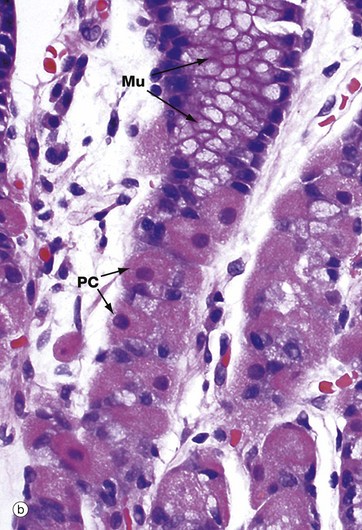
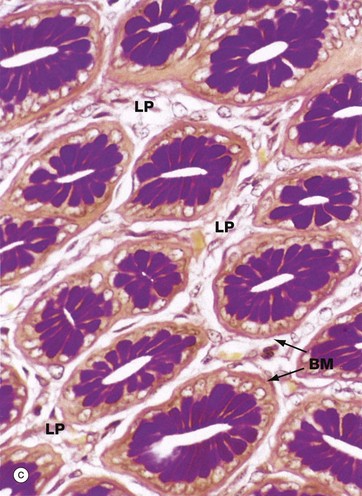
FIG. 14.10 Gastric body mucosa
(a) H&E (LP) (b) H&E (HP) (c) PAS/haematoxylin/orange G (HP)
Micrograph (a) shows the full thickness of the gastric body mucosa and includes a small amount of submucosa SM. The gastric pits or foveolae F, lined by pale-stained surface mucous cells, are easily identifiable. The isthmus and neck of the glands also appear pale due to the predominance of neck mucous cells and parietal cells PC. The base of the glands, where chief (zygomatic) cells CC predominate, are stained darker in this H&E preparation. The glands extend down to the muscularis mucosae MM. Normal gastric mucosa is virtually devoid of lymphoid cells.
Micrograph (b) is a high-power view of the neck and isthmus of a gastric body gland. The neck mucous cells Mu and parietal cells PC are easily visualised at this magnification. The tall columnar mucus-secreting cells of the stomach are not of the goblet cell type which are found in small and large intestines. The mucus produced by these mucous cells protects the epithelium from autodigestion by acid gastric juice. The parietal cells are recognised by their copious eosinophilic cytoplasm and central nucleus, which is often described as a ‘fried egg’ appearance.
In transverse section, as in micrograph (c), the tubular nature of the gastric pits is clearly evident. Between one and seven gastric glands may open into each gastric pit. Note the loose vascular but scanty lamina propria LP which supports the gastric pits and glands. The lightly PAS-positive basement membrane BM can be distinguished between the epithelium and lamina propria. The mucus of the neck mucous cells stains a strong magenta colour with this staining method.
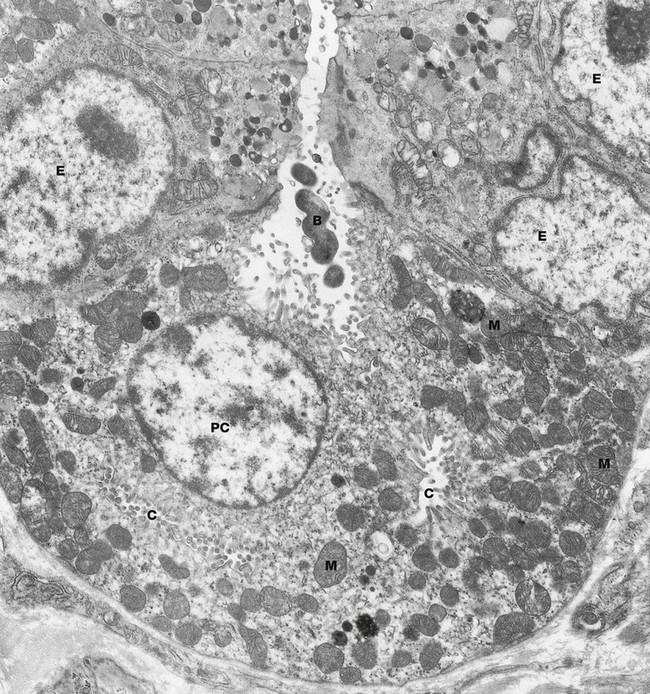
FIG. 14.11 Parietal cell, rat
EM ×9600
This micrograph shows a parietal cell PC within a gastric gland. The luminal plasma membrane of the parietal cell forms deep, branching canaliculi C that extend throughout the cytoplasm and between adjacent cells. Numerous short microvilli project into the lumina of the intracellular canaliculi, greatly increasing the surface area.
The canaliculi are closely related to a tubulovesicular membrane complex (not well seen in this micrograph). This system consists of numerous small, membrane-bound vesicles which include a transmembrane ‘proton pump’ in the form of a H+-K+ ATPase. When there is a physiological increase in the demand for acid secretion, mediated via histamine, acetylcholine and gastrin (see textbox), the vesicles of this tubulovesicular complex fuse with the canalicular membranes of the parietal cell to facilitate active secretion of hydrogen ions into the gastric lumen. In actively secreting cells, as in this case, the canalicular system becomes more prominent due to the large increase in its surface area from fusion with these pre-formed vesicles, whereas in resting parietal cells the canalicular system is inconspicuous and the complex is more prominent. This morphological change reflects altered functional demand and it is believed that when demand for acid secretion falls again, membrane-bound vesicles bearing proton pumps are endocytosed to re-form the tubulovesicular complex.
Secretion of hydrochloric acid begins with the production of carbonic acid in the cytoplasm of the parietal cell, catalyzed by the enzyme carbonic anhydrase. Carbonic acid then dissociates into hydrogen and bicarbonate ions. The hydrogen ions are actively transported into the canalicular lumen and chloride ions follow passively. The end result is a hydrogen ion concentration in gastric juice about 1 million times that in plasma. This process is fuelled by the many mitochondria M of the parietal cells.
Parietal cells also secrete a glycoprotein called intrinsic factor which is essential for the absorption of vitamin B12 in the terminal ileum. Also seen in this micrograph are several neuroendocrine cells E, recognised by their small electron-dense secretory granules. Note the coiled bacterium B in the lumen of the gland; this is most likely Helicobacter pylori.
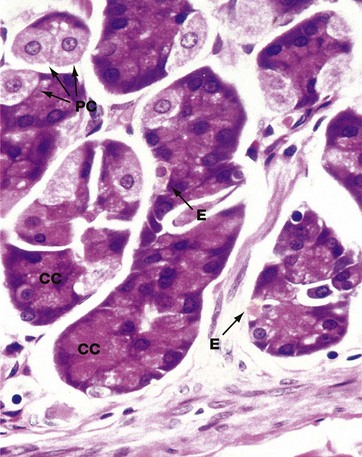
FIG. 14.12 Base of gastric gland
H&E (HP)
Chief (zymogen or peptic) cells CC, which synthesise and secrete the proteolytic enzyme pepsin, are the principal cell type in the basal third of the gastric glands, although some parietal cells PC are also found at this level.
Chief cells have basally located nuclei and extensive granular cytoplasm packed with rough endoplasmic reticulum, the ribosomes accounting for the cytoplasmic basophilia. The inactive pepsin precursor, pepsinogen, is synthesised by the ribosomes and stored in numerous secretory granules located towards the luminal surface. Pepsinogen remains inactive until it reaches the lumen of the stomach where it is activated by the low pH of the gastric juices. Secretion of an inactive precursor molecule prevents autodigestion of the gastric glands.
The much larger parietal cells are round with large, centrally located nuclei and eosinophilic (pink-stained) cytoplasm due to the numerous mitochondria that are a feature of highly metabolically active cells.
The secretory activity of both parietal and peptic cells is controlled by the autonomic nervous system and via the hormone gastrin, which is secreted by neuroendocrine cells of the pyloric region (see textbox).
A variety of other neuroendocrine cells of the gastrointestinal endocrine system are also scattered in the gastric body mucosa and elsewhere in the gastrointestinal tract. Occasionally, the neuroendocrine cells E can be identified in sections fixed with chromium-containing fixatives, as in this example.
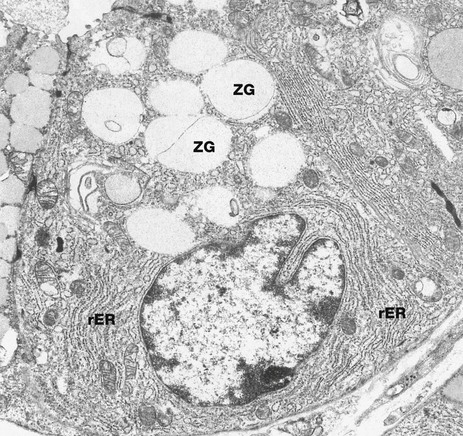
FIG. 14.13 Chief cell
EM ×7200
This electron micrograph illustrates a chief (zymogen) cell at the base of a gastric gland. The typical ultrastructural features of chief cells are those of protein-secreting cells in general. These features include an extensive rough endoplasmic reticulum rER and membrane-bound secretory vesicles (zymogen granules) ZG containing pepsinogen. These are crowded in the apical cytoplasm, thus restricting the nucleus to the base of the cell. The extensive rough endoplasmic reticulum accounts for the basophilia of chief cells in H&E sections.
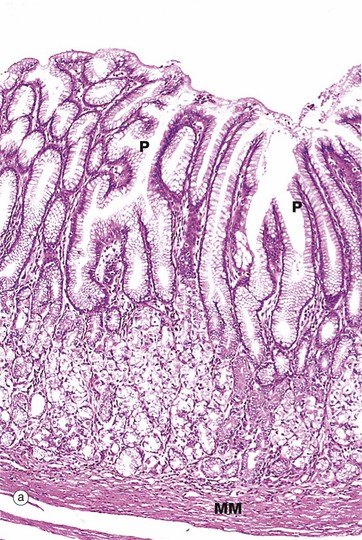
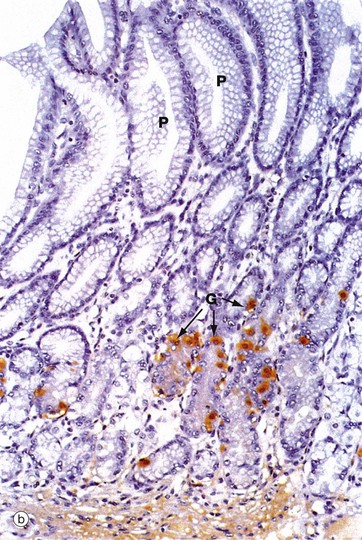
FIG. 14.14 Pyloric stomach
(a) H&E (LP) (b) Immunohistochemical staining for gastrin (MP)
In contrast to the simple tubular glands of the fundus and body, the pyloric glands are branched and coiled and the gastric pits P occupy about half the thickness of the pyloric mucosa (a). The glands are lined almost exclusively by mucus-secreting cells which are similar to the neck mucous cells of the gastric body and fundus. A small number of acid-secreting parietal cells are also scattered among the pyloric glands. Note the prominent muscularis mucosae MM separating the glands from the underlying submucosa. As in the body of the stomach, stem cells are found in the neck of the glands but cannot be easily identified by light microscopy.
Scattered among the pyloric mucous cells are neuroendocrine cells that secrete the peptide hormone gastrin and are thus called G cells. In micrograph (b), an antibody to gastrin has been used to highlight the G cells, which contain gastrin in secretory granules in their cytoplasm. The G cells are stained brown G and are found mainly in the neck of the glands. The presence of food in the stomach stimulates the secretion of gastrin into the bloodstream. Gastrin then promotes secretion of pepsin and acid by the gastric glands of the fundus and body, as well as enhancing gastric motility.
Other neuroendocrine cells in the pylorus secrete various other hormonal products, including somatostatin, which is involved in the regulation of insulin, glucagon, gastrin and growth hormone secretion.
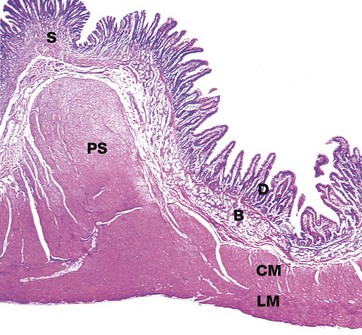
FIG. 14.15 Pyloric stomach
H&E (LP)
The pyloric sphincter PS marks a sharp transition from the glandular secretory type mucosa of the stomach S to the villous absorptive type mucosa of the duodenum D and the remainder of the small intestine. In addition, the duodenum is distinguished from the jejunum and ileum by the presence of numerous mucus-secreting glands B. These glands, known as Brunner's glands, are predominantly found in the submucosa but may extend into the mucosa. These glands secrete a thin, alkaline mucus. The pyloric sphincter consists of a marked thickening of the circular layer of the muscularis at the gastroduodenal junction. Note the continuity of both the circular CM and longitudinal LM layers of the muscularis between the pylorus and duodenum.
The inner oblique layer of the muscularis propria is found only in the body of the stomach.
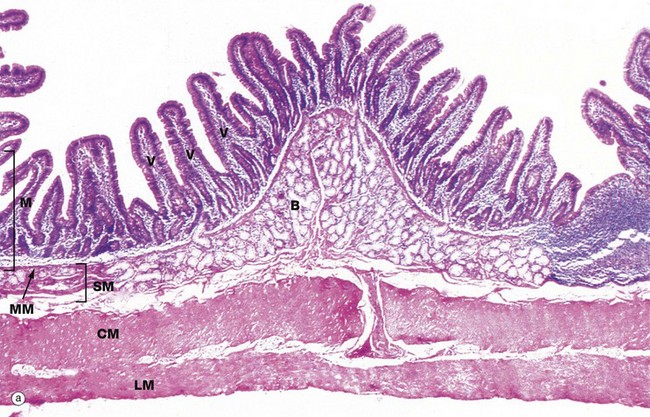
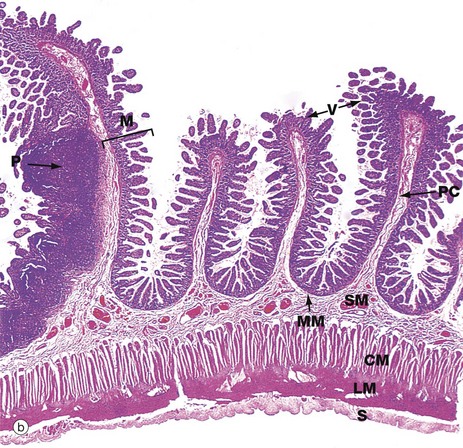
FIG. 14.16 Small intestine, monkey (caption continues opposite)
(a) Duodenum, H&E (LP) (b) Ileum, H&E (MP)
The duodenum, seen in micrograph (a), represents the first part of the small intestine and receives partly digested food in the form of acidic chyme from the stomach via the pyloric canal. The main function of the duodenum is to neutralise gastric acid and pepsin and to initiate further digestive processes.
Micrograph (a) illustrates monkey duodenum, the wall of the human duodenum being too thick to be photographed in its entirety. The mucosa M has the characteristic villous form of the whole of the small intestine, interspersed with short glands, known as crypts of Lieberkühn, extending down to the muscularis mucosae MM.
The feature unique to the duodenum is the extensive mass of coiled branched tubular Brunner's glands B, found mainly in the submucosa SM. The ducts of the Brunner's glands pass through the muscularis mucosae to open into the crypts between the mucosal villi V. The muscularis propria of the duodenum consists of an inner circular layer CM and an outer longitudinal layer LM, as in the rest of the small intestine.
The tall columnar cells of Brunner's glands have extensive poorly stained mucigen-filled cytoplasm and basally located nuclei. The presence of chyme in the duodenum stimulates Brunner's glands to secrete a thin, alkaline mucus that helps to neutralise the acidic chyme and protect the duodenal mucosa from autodigestion. Other products of Brunner's glands include lysozyme and epidermal growth factor.
Chyme also stimulates the release of two peptide hormones, secretin and cholecystokinin-pancreozymin (CCK) from neuroendocrine cells scattered throughout the duodenal mucosa. Secretin and CCK promote pancreatic exocrine secretion into the duodenal lumen via the pancreatic duct. CCK also stimulates contraction of the gallbladder, thus propelling bile into the common bile duct. The pancreatic and common bile ducts merge to empty their contents into the duodenum via a single short duct that opens into the second part of the duodenum via the ampulla of Vater.
Pancreatic juice is alkaline due to a high content of bicarbonate ions and thus helps to neutralise the acidic gastric contents entering the duodenum. The pancreas also secretes a variety of digestive enzymes, including the proteolytic enzymes trypsin and chymotrypsin. Like pepsin in the stomach, these are secreted in an inactive pro-enzyme form. On entering the duodenal lumen, trypsin is activated by the enzyme enterokinase, secreted by the duodenal mucosa. Activated trypsin in turn activates chymotrypsin. The pancreatic enzymes, which also include amylase and lipases, initiate the processes of luminal digestion (see textbox overleaf). The biliary secretions contain bile acids which act as emulsifying agents and are particularly important in the absorption of lipids.
Micrograph (b) shows a section of ileum at very low magnification. The mucosa M is thrown into transverse folds, the plicae circulares PC (also called valvulae conniventes or folds of Kerckring), covered with villi V. The muscularis mucosae MM lies immediately beneath the crypts and is difficult to see at this magnification. The vascular submucosa SM extends into the plicae circulares. The inner circular CM and outer longitudinal LM layers of the muscularis propria lie deep to this and there is an outer layer of serosa S. Peyer's patches P (see Ch. 11) dominate the mucosa at the left of the field.
The small intestine has the same basic structure throughout, except for the following features:
• Brunner's glands are only found in the duodenum.
• The villi tend to be longest in the duodenum and become shorter towards the ileum.
• Lymphoid tissue becomes more prominent in the ileum and is fairly inconspicuous in the duodenum.
• The proportion of goblet cells in the epithelium increases distally.
• Plicae circulares are most prominent and numerous in the jejunum and proximal ileum and are generally absent in the proximal duodenum and distal ileum.
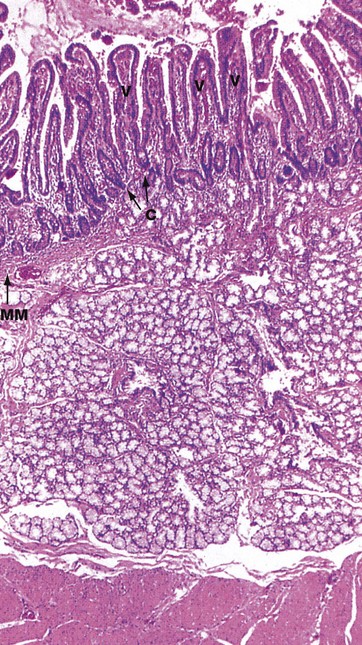
FIG. 14.17 Duodenum
H&E (LP)
This micrograph of the human duodenum is stained by the standard H&E method. The duodenal mucosa has the typical form found elsewhere in the small intestine, with numerous elongated villi V, between the bases of which are shorter crypts C. In the distal duodenum, the height of the villi is about four times the length of the crypts, while in the more distal small bowel the villus/crypt ratio is 2–3 : 1.
The pale-stained Brunner's glands occupy the entire submucosa SM deep to the muscularis mucosae MM. A small component of the Brunner's gland is sometimes found in the lamina propria where the duct of the gland empties into the base of a mucosal crypt. The Brunner's glands secrete alkaline mucins into the lumen of the small intestine.
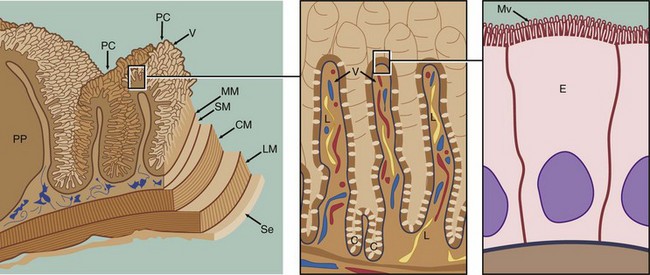
FIG. 14.18 Small intestine
The small intestine, comprising the duodenum, jejunum and ileum, is the principal site for absorption of digestion products from the gastrointestinal tract. Digestion begins in the stomach and is completed in the small intestine in association with the absorptive process. Four factors combine to provide an enormous surface area:
• The small intestine is extremely long (4 to 6 m in humans).
• The mucosa and submucosa are thrown up into circularly arranged folds called plicae circulares PC or valves of Kerckring which are particularly numerous in the jejunum.
• The mucosal surface is made up of numerous finger-like projections called villi V.
• Thousands of microvilli Mv are present at the luminal surface of the enterocytes E, the columnar cells covering the villi. These cells are responsible for the process of absorption and some digestion.
The muscularis mucosae MM lies immediately beneath the mucosal crypts and separates the mucosa from the submucosa SM. The vascular submucosa extends into, and forms the core of, the plicae circulares. Inner circular CM and outer longitudinal LM layers of the muscularis are responsible for continuous peristaltic activity of the small intestine. The peritoneal aspect of the muscularis is invested by the loose collagenous serosa Se, which is lined on its peritoneal surface by mesothelium identical in appearance to the mesothelial lining of the pleura (see Fig. 12.22).
Lymphoid aggregations known as Peyer's patches PP are a prominent feature within the lamina propria of the small intestine (see Fig. 11.16).
The products of protein and carbohydrate digestion (see textbox), namely amino acids and monosaccharides, respectively, enter the intestinal capillaries and pass via the portal vein to the liver. In contrast, reconstituted triglycerides pass into intestinal lymphatics known as lacteals L, and thence via the thoracic duct to the general circulation, bypassing the liver. For lymphatic transport, the triglycerides become coated with phospholipids and proteins to form fine globules known as chylomicrons. A minority of lipid digestion products, such as short-chain fatty acids and glycerol, pass in the portal system to the liver, along with almost all the bile acids which are reabsorbed and recirculated.
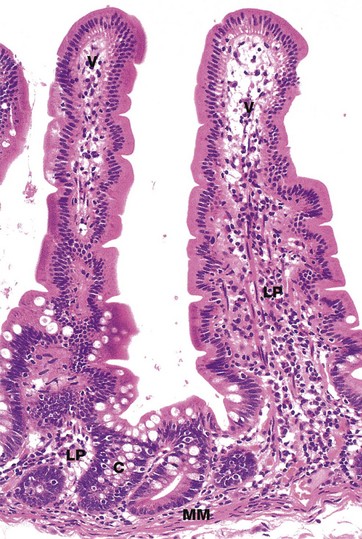
FIG. 14.19 Intestinal villi and crypts
H&E (MP)
The intestinal villi V are lined by a simple columnar epithelium which is continuous with that of the crypts C. As in other parts of the gastrointestinal tract, the epithelium includes a variety of cell types, each with its own specific function. Cell types in the small intestine epithelium include:
• Enterocytes, the most numerous cell type, are tall columnar cells with surface microvilli that are seen as a brush border in light micrographs. These cells are the main absorptive cells.
• Goblet cells are scattered among the enterocytes and produce mucin for lubrication of the intestinal contents and protection of the epithelium.
• Paneth cells are found at the base of the crypts and are distinguished by their prominent eosinophilic apical granules. These cells have a defensive function.
• Neuroendocrine cells produce locally acting hormones that regulate gastrointestinal motility and secretion.
• Stem cells, found at the base of the crypts, divide continuously to replenish all of the above four cell types.
• Intraepithelial lymphocytes, which are mostly T cells, provide defence against invasive organisms.
The lamina propria LP extends between the crypts and into the core of each villus and contains a rich vascular and lymphatic network into which digestive products are absorbed. The muscularis mucosae MM lies immediately beneath the base of the crypts.
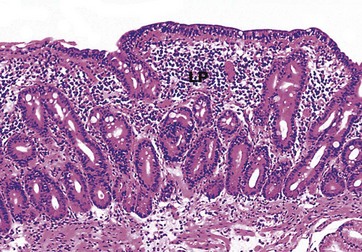
FIG. 14.20 Coeliac disease, atrophic small intestine
H&E (MP)
Biopsies of the small bowel in coeliac disease typically reveal flattening or loss of the normal intestinal villi (compare Figs 14.17 and 14.19) as well as a marked increase in the number of lymphocytes and plasma cells in the lamina propria LP. In addition, there is usually a marked increase in the number of intraepithelial T lymphocytes in the surface epithelium, suggesting that the condition is at least partly due to a cell-mediated immune response against the small bowel enterocytes. In order to maintain the integrity of the mucosal surface in the face of this greatly increased enterocyte turnover, there must be increased proliferation of stem cells in the bases of the crypts and, as a result, the crypts appear greatly elongated.
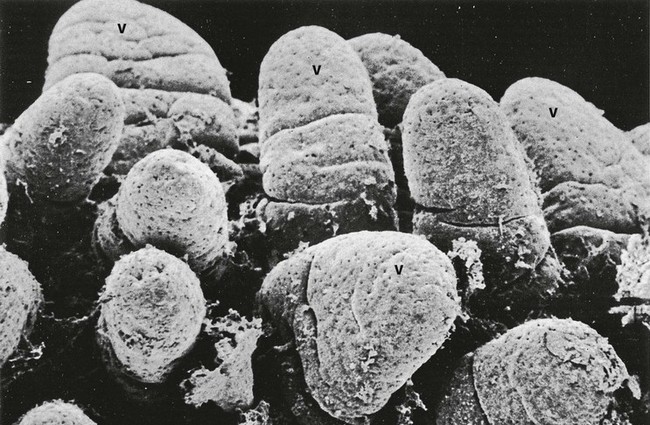
FIG. 14.21 Intestinal villi
SEM ×100
This low-power scanning electron micrograph shows villi V along the crest of a plica circularis in the small intestine. Note the variability of the shape of the villi; some are finger-shaped while others have a broader leaf-like profile. Surface openings of scattered goblet cells stud the villous surface. Fragments of mucus can be seen trapped between the villi.
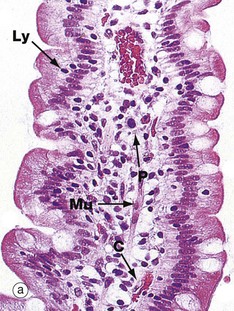
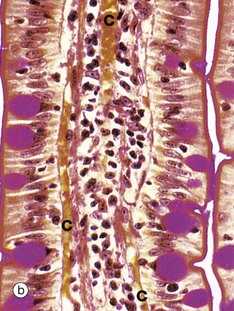
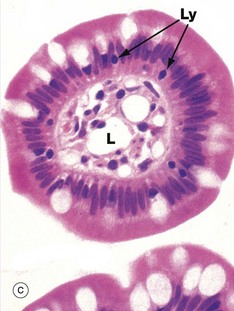
FIG. 14.22 Intestinal villi
(a) H&E, LS (MP) (b) PAS/iron-haematoxylin/orange G, LS (HP) (c) H&E, TS (HP)
These micrographs illustrate the tall columnar enterocytes that cover the intestinal villi, as well as the goblet cells scattered among them. The luminal surface of the enterocytes seen in micrograph (b) is strongly PAS-positive due to a particularly thick glycocalyx and a surface layer of goblet cell–derived mucus. Both features protect against autodigestion. The glycocalyx is also the site for adsorption of pancreatic digestive enzymes.
T lymphocytes Ly are scattered among the enterocytes. Plasma cells P in the villous core secrete IgA into the intestinal lumen by transcytosis across epithelial cells.
The cores of the villi are extensions of the lamina propria and consist of loose supporting tissue. Capillaries C lie immediately beneath the basement membrane and transport most digestive products to the hepatic portal vein. Tiny lymphatic vessels drain into a single larger vessel called a lacteal L at the centre of the villus. The lacteals transport absorbed lipid into the circulatory system via the thoracic duct. Smooth muscle fibres Mu are seen in the long axis of the villous core in micrograph (a) and represent extensions of the muscularis mucosae.
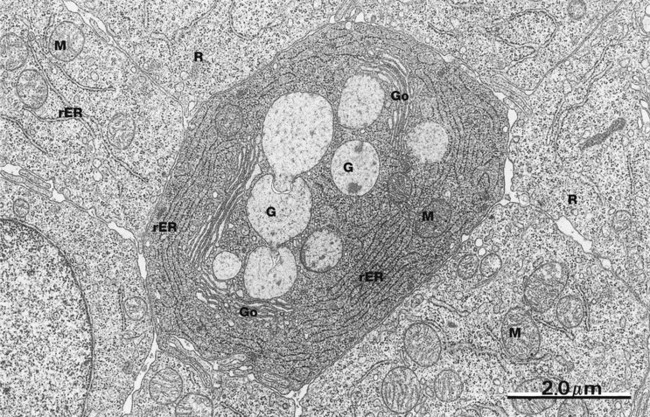
FIG. 14.23 Duodenal epithelium
EM ×14 500
This low-power electron micrograph of a horizontal section through the duodenal epithelium demonstrates several important features. In the central area, there is a goblet cell containing several mucin-containing granules G. The goblet cell appearance by conventional light microscopy is actually an artefact of preparation whereby water is taken up by the granules, causing them to expand and compress the surrounding cytoplasm. Adjacent to the mucin granules there are three Golgi apparatuses Go with plentiful rough endoplasmic reticulum rER, features typical of secretory cells. Occasional mitochondria M are also seen.
Surrounding the goblet cell are a number of enterocytes. These have much less prominent rER but contain large numbers of free ribosomes R and mitochondria M (see Fig. 14.25). This micrograph is of duodenal epithelium, but jejunal and ileal epithelium are identical.
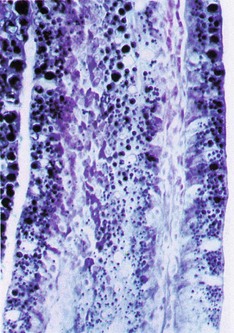
FIG. 14.24 Intestinal villus
Sudan black (HP)
This frozen section from the intestine of a rat fed with milk is stained to demonstrate the presence of absorbed lipids.
Ingested triglycerides are emulsified by bile and hydrolysed by the pancreatic enzyme lipase. The degradation products, mainly free fatty acids and monoglycerides, are absorbed by enterocytes where they are resynthesised into triglycerides in the smooth endoplasmic reticulum. Here, the triglycerides are reconstituted into small globules and form a lipoprotein complex, incorporating protein, cholesterol and phospholipids. Membrane-bound vesicles containing multiple droplets bud from the smooth endoplasmic reticulum and pass towards the base of the cell where they are released by exocytosis into the intercellular clefts. From here, the small lipoprotein droplets known as chylomicrons pass into the lacteals and then into larger lymphatics, eventually entering the general circulation.
Note the high concentration of black-stained lipid in the enterocyte cytoplasm and in the chylomicrons within the central lacteal.
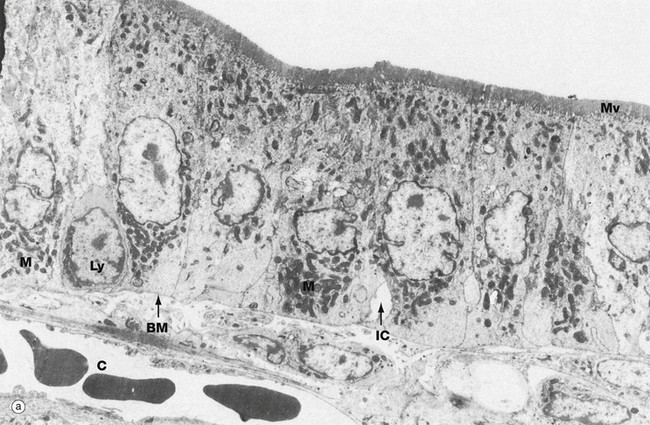
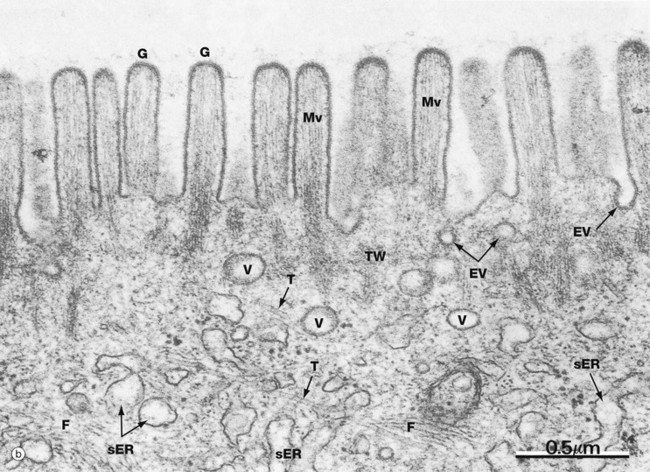
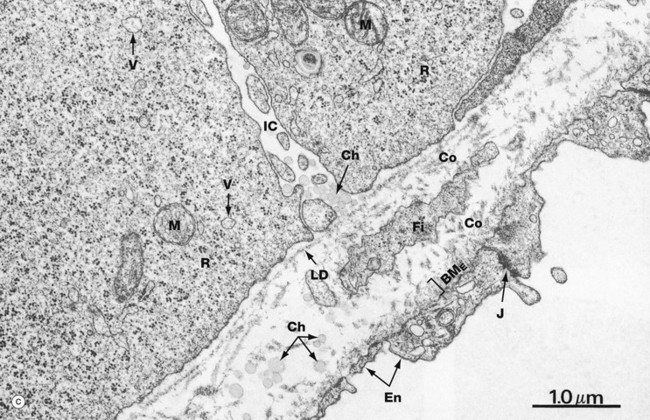
FIG. 14.25 Enterocytes (illustrations (a) and (b) opposite)
(a) EM ×4540 (b) EM ×5600 (c) EM ×22 000
These micrographs illustrate the main ultrastructural features of enterocytes, the absorptive cells of the small intestine. Micrograph (a) shows the enormous number of microvilli Mv (up to 3000 per cell), which increase the surface area of the plasma membrane exposed to the lumen by some 30 times. The microvilli are of uniform length (approximately 1 µm) and constitute the brush border of light microscopy (see Figs 14.22 and 5.14). Most absorption in the small intestine occurs by direct passage of low molecular weight digestion products across the luminal plasma membrane. Mitochondria M are particularly abundant within enterocytes, reflecting the high energy demands of such processes. Chylomicrons, assembled in the enterocytes, pass first into the intercellular clefts IC, then across the basement membrane BM into the core of the villus and finally into the lacteal. Lymphocytes Ly are commonly found in the intercellular clefts between enterocytes, where they play an important part in the immunological defence of the gastrointestinal tract. Note the close proximity of a blood capillary C to the enterocyte basement membrane.
As seen in micrograph (b), the glycocalyx G of the enterocyte microvilli is unusually prominent. It provides protection against autodigestion and acts as the site for adsorption of pancreatic digestive enzymes. This micrograph also shows the microfilament cytoskeleton of the microvilli Mv extending into the superficial cytoplasm. Here, in the terminal web TW, it becomes integrated into the cytoskeleton of the body of the cell. Deeper in the cell, microfilaments F and microtubules T are readily identified. Enterocytes are tightly bound near their luminal surface by junctional complexes (see Fig. 5.9) which prevent direct access of luminal contents into the intercellular spaces, as well as holding the epithelium together.
Endocytotic vesicles EV are often seen between the bases of microvilli and transport vesicles V are common in the superficial cytoplasm. Endocytosis with transfer to the extracellular fluid at the base of the cell (transcytosis) is an important mechanism of uptake of macromolecules from the gut lumen into the blood. An example of transcytosis is the uptake of maternal antibodies from the milk in breast fed infants. Smooth endoplasmic reticulum sER is seen deeper in the cytoplasm.
Micrograph (c) illustrates the basal aspect of two enterocytes, separated by an intercellular cleft IC. Their basement membrane is thin and the lamina densa LD appears to be discontinuous. Close beneath the base of the enterocytes is a tiny lymphatic tributary of the central lacteal, its endothelial lining En being thin and fenestrated. Note the junctional complex J binding adjacent endothelial cells and the thin discontinuous endothelial basement membrane BME. The delicate supporting tissue between the basement membrane and lymphatic contains fibroblasts Fi and fine collagen fibrils Co.
The main feature of the basal enterocyte cytoplasm is numerous free ribosomes R, scattered mitochondria M and membranous vesicles V containing lipoprotein droplets en route for exocytosis into the intercellular cleft. The cleft contains numerous small chylomicrons Ch that cluster near the lamina densa, as if temporarily held up in their passage towards the lymphatic. In the lamina propria, the chylomicrons are larger, probably due to fusion of smaller ones coursing through from the intercellular cleft. Note that the chylomicrons in the extracellular environment are not membrane-bound but have a fine electron-dense limiting layer of protein.
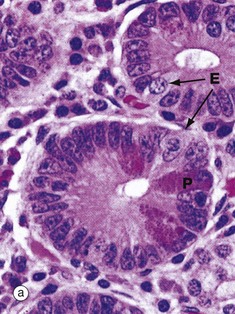
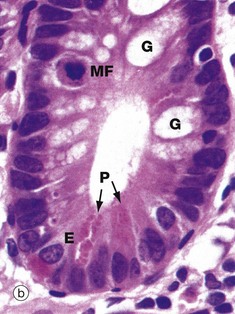
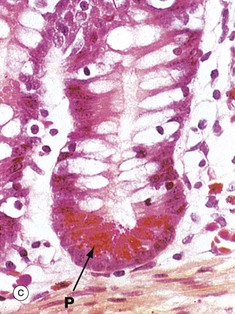
FIG. 14.26 Crypts of Lieberkühn
(a) H&E, TS (HP) (b) H&E, LS (HP) (c) Phloxine-tartrazine, LS (HP)
The majority of cells in the crypt bases are stem cells that divide regularly to replenish the epithelial cells of the villi. Immature goblet cells G are readily seen in micrograph (b). A single mitotic figure MF is identifiable.
With H&E staining in micrograph (a), Paneth cells P, which form part of the innate immune system, exhibit intensely eosinophilic apical cytoplasmic granules. These are stained bright scarlet by the phloxine-tartrazine method in micrograph (c). The granules of Paneth cells contain antimicrobial peptides (defensins) and protective enzymes such as lysozyme and phospholipase A. These products, secreted into the small bowel, provide the first line of defence against any pathogens that survive passage through the stomach. The lumen of the small bowel is virtually sterile. Paneth cells are long-lived (weeks) in comparison to the short lifespan (3-5 days) of enterocytes and goblet cells.
Endocrine cells E also contain eosinophilic cytoplasmic granules which are found in a subnuclear position, in contrast to the apical granules of Paneth cells. Secretory products of gut endocrine cells include hormones such as secretin, somatostatin and 5-HT (serotonin). In general, each endocrine cell produces only one hormone.
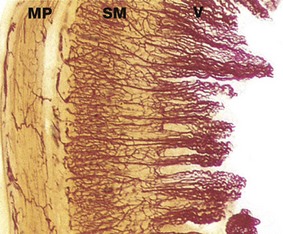
FIG. 14.27 Intestinal villi
Carmine perfused (LP)
This specimen of small intestine has been perfused before fixation with a red dye to demonstrate the blood supply of the mucosa. Long loops of branching capillaries originating from a dense capillary network in the submucosa SM extend up to the tips of the villi V. Note also the capillary network supplying the muscularis propria MP. Most of the absorbed food products, with the exception of triglycerides, enter the capillaries and pass via the portal vein to the liver.
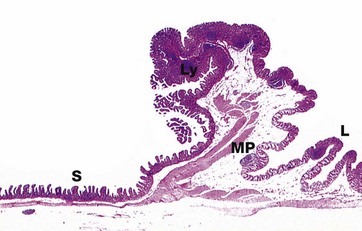
FIG. 14.28 Ileocaecal junction
H&E (LP)
Indigestible food residues from the ileum are propelled by peristalsis into the distended first part of the large intestine, the caecum, through the cone-shaped ileocaecal valve. There is an abrupt transition in the lining of the valve from the small intestinal villiform pattern S to the glandular form in the large intestine L. The ileocaecal valve consists of a thickened extension of the muscularis propria MP that provides robust support for the mucosa. Lymphoid tissue Ly in the form of large Peyer's patches is found in the mucosa.
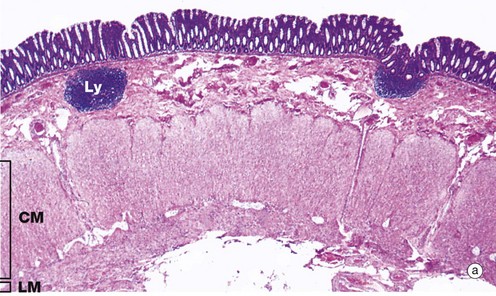
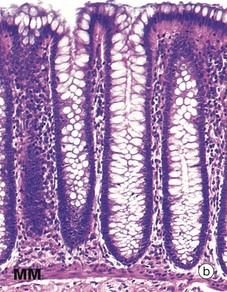
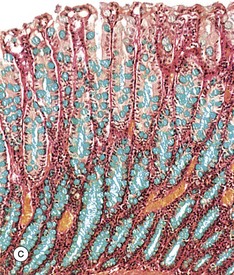
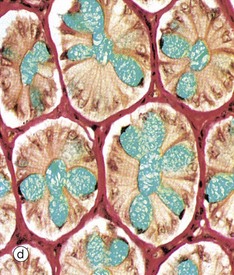
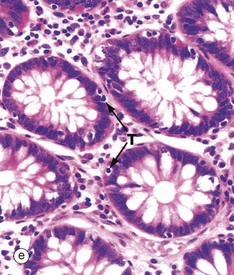
FIG. 14.29 Colon
(a) H&E (LP) (b) H&E (MP) (c) Alcian blue/van Gieson (MP) (d) Alcian blue/van Gieson (HP) (e) H&E (HP)
The principal functions of the large intestine are the recovery of water and salt from faeces and the propulsion of increasingly solid faeces to the rectum prior to defaecation.
As shown in micrograph (a), the muscular wall is consequently thick and capable of powerful peristaltic activity. As in the rest of the gastrointestinal tract, the muscularis propria of the large intestine consists of inner circular CM and outer longitudinal layers LM but, except in the rectum, the longitudinal layer forms three separate longitudinal bands called taeniae coli.
The mucosa is the same from caecum to rectum. It is folded in the non-distended state but does not exhibit distinct plicae circulares like those of the small intestine. Immediately above the anal valves, the mucosa forms longitudinal folds called the columns of Morgagni. The muscularis mucosae is a prominent feature of the large intestinal mucosa. Rhythmic contractions prevent clogging of the glands and enhance expulsion of mucus.
Consistent with its functions of water absorption and faecal lubrication, the mucosa consists of cells of two types: absorptive cells and mucus-secreting goblet cells. As seen in micrograph (b), these are arranged in closely packed straight tubular glands or crypts, which extend down to sit on to the muscularis mucosae MM. As faeces pass along the large intestine and become progressively dehydrated, the mucus becomes increasingly important in protecting the mucosa from trauma. The Alcian blue method shown in micrograph (c) stains goblet cell mucus a greenish-blue colour, while the absorptive cells remain poorly stained. Goblet cells predominate in the base of the glands, whereas the luminal surface is almost entirely lined by columnar absorptive cells.
Micrographs (d) and (e) show transverse sections through the upper part of large intestinal glands, highlighting the closely packed arrangement of the glands in the mucosa. The tall columnar absorptive cells have oval basal nuclei. In contrast, goblet cell nuclei are small and condensed. Stem cells at the base of the glands continually replace the epithelium. Intraepithelial T lymphocytes T are easily seen in image (e).
Lamina propria fills the space between the glands and contains numerous blood vessels into which water is absorbed. In the lamina propria, lymphatics are very scantly, if present at all. The lamina propria also contains collagen, which is stained red in micrographs (c) and (d), as well as lymphocytes and plasma cells. These form part of the defence mechanisms against invading pathogens, along with intraepithelial lymphocytes and the lymphoid aggregates Ly, which are smaller than Peyer's patches. These are found in the lamina propria and submucosa, as seen at low power in image (a).
The large intestine is inhabited by a variety of commensal bacteria that further degrade food residues. Bacterial degradation is an important mechanism for the digestion of cellulose in ruminants but, in humans, most cellulose is excreted. Small quantities of fat-soluble vitamins derived from bacterial activity are absorbed in the large intestine.
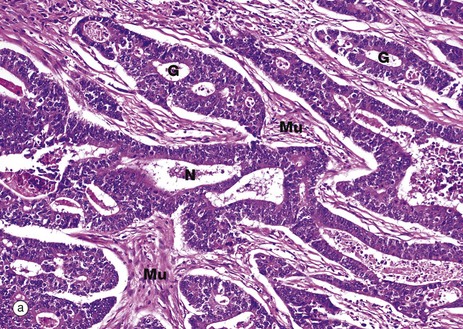
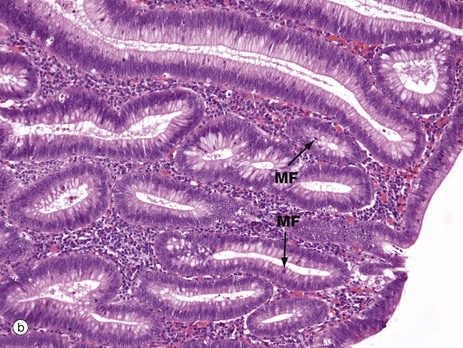
FIG. 14.30 The adenoma-carcinoma sequence
(a) Colonic adenocarcinoma, H&E (MP) (b) Colonic adenoma, H&E (MP)
Micrograph (a) shows a typical adenocarcinoma of the colon. Compare this with the normal colonic mucosa seen in Fig. 14.29. In adenocarcinoma, the malignant epithelial cells form disorganised abnormal glands that invade into the adjacent tissues. The depth of invasion and indeed the spread to the draining mesenteric lymph nodes is the basis for staging the tumour, i.e. giving a prediction of the likely behaviour of the tumour based on the extent of the tumour. Obviously, the further the tumour has spread, the worse the outcome is likely to be. A small superficial adenocarcinoma confined to the colon has a good chance of cure by surgery, whereas a tumour that has spread far and wide (metastasised) will have a much worse outlook. In image (a) the malignant glands G of the tumour have invaded into the muscularis propria. Small bundles of smooth muscle Mu can be identified and there are also areas of necrosis N (dead tissue), another feature commonly seen in cancers.
In contrast, micrograph (b) shows an adenoma, a benign but pre-cancerous tumour of the colon. These tumours exhibit epithelial dysplasia, showing abnormal disordered growth. When compared against the normal mucosa shown in Fig. 14.29, the epithelial cells are seen to have larger nuclei and there are obvious mitotic figures MF, indicating increased cell proliferation. There are fewer mucus-containing goblet cells, reflecting a lack of normal cellular differentiation. If such pre-malignant changes remain untreated, a significant number of adenomas will progress over time to acquire further genetic abnormalities and invasive adenocarcinoma may develop.
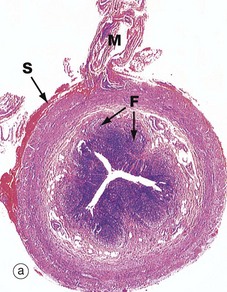
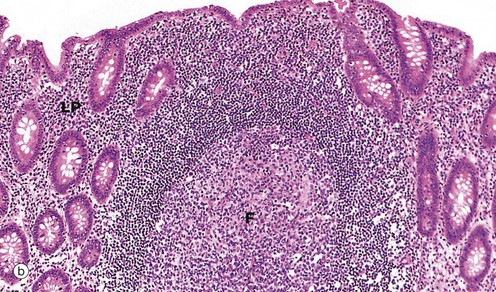
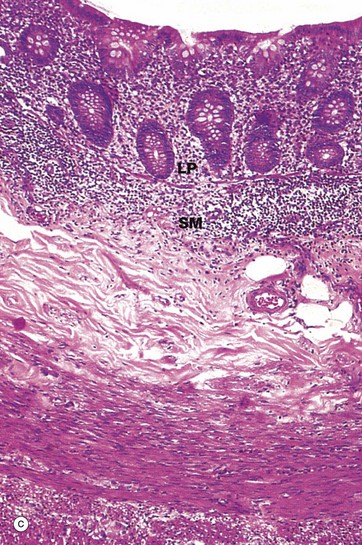
FIG. 14.31 Appendix
(a) H&E (LP) (b) H&E (MP) (c) H&E (MP)
The appendix is a small, blind-ended, tubular sac extending from the caecum just distal to the ileocaecal junction. The general structure of the appendix conforms to that of the rest of the large intestine. In some mammals, the appendix is capacious and is involved in prolonged digestion of cellulose, but in humans its function is unknown.
Micrograph (a) illustrates the suspensory mesentery or mesoappendix M, in continuity with the outer serosal layer S. The serosa contains extravasated blood due to haemorrhage during surgical removal. The mesenteries of the gastrointestinal tract conduct blood vessels, lymphatics and nerves to and from the gastrointestinal tract.
The most characteristic feature of the appendix, particularly in the young, is the presence of masses of lymphoid tissue in the mucosa and submucosa. As seen in micrographs (b) and (c), the lamina propria LP and upper submucosa SM are diffusely infiltrated with lymphocytes. Note that the mucosal glands are much less closely packed than in the large intestine. As seen in micrographs (a) and (b), the lymphoid tissue also forms follicles F, often containing germinal centres (see Ch. 11). These follicles bulge into the lumen of the appendix and, like the follicles of Peyer's patches in the small intestine, are invested by a simple epithelium of M cells (see Fig. 11.16), which presumably facilitates sampling of antigen in the lumen.
The most common disorder affecting the appendix is acute appendicitis (inflammation of the appendix). This typically presents with severe abdominal pain, initially centred in the middle of the abdomen and then later localising to the right iliac fossa. Appendicitis is a fairly common acute surgical emergency. If it is left untreated, the appendix may rupture and discharge infected pus into the peritoneal cavity, resulting in acute peritonitis.
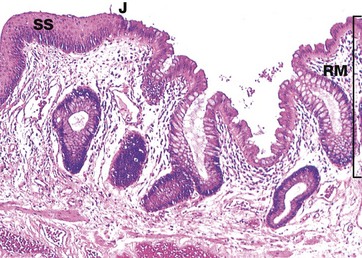
FIG. 14.32 Anorectal junction
H&E (MP)
The rectum is the short, dilated, terminal portion of the large intestine. The rectal mucosa RM is the same as the rest of the large bowel except that it has even more numerous goblet cells. At the anorectal junction J, it undergoes an abrupt transition to become stratified squamous epithelium SS in the anal canal. Branched tubular circumanal glands open at the recto-anal junction into small pits at the distal ends of the columns of Morgagni.
The anal canal forms the last 2 or 3 cm of the gastrointestinal tract and is surrounded by voluntary muscle that forms the anal sphincter. Here, the stratified squamous epithelium undergoes a gradual transition to skin containing sebaceous glands and large apocrine sweat glands (see Ch. 9).
Review
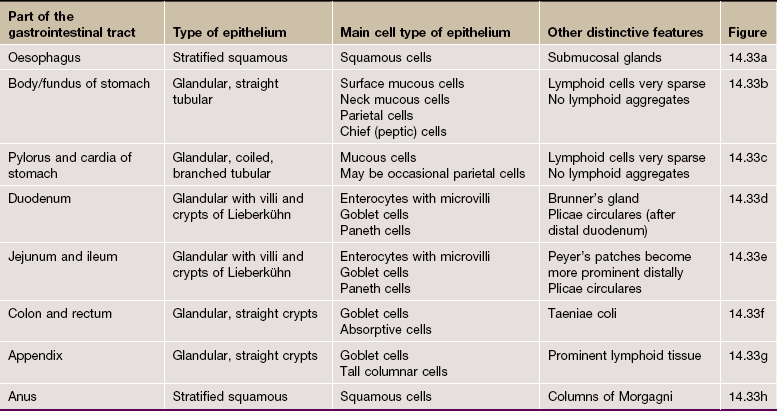
Table 14.1 outlines the main structural features of the different components of the gastrointestinal tract for easy reference and revision. Please note that the epithelium of all segments includes stem cells and neuroendocrine cells, which have not been included in the table for simplicity. Each line of the table refers to the correspondingly labelled micrograph opposite.
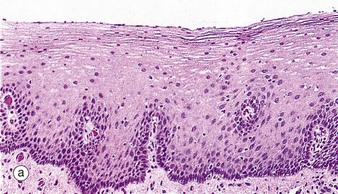
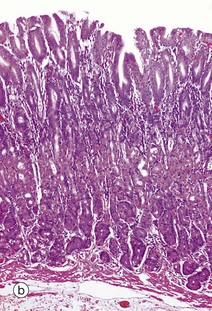
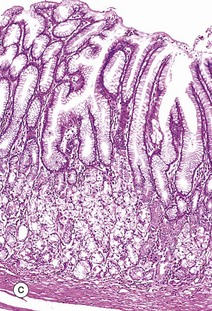
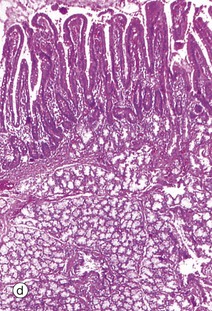
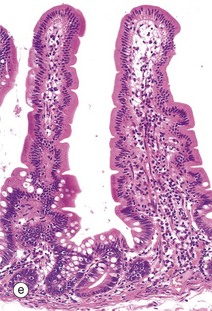
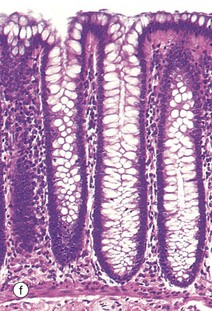
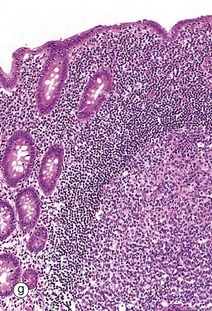
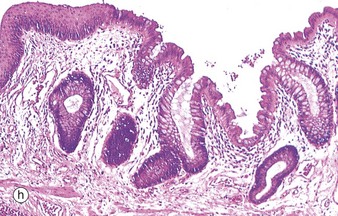
FIG. 14.33 Comparison of histological features throughout the gastrointestinal tract (see Table 14.1 opposite)