Pain Assessment and Management in Children
On completion of this chapter the reader will be able to:
 Identify measures to assess pain in children.
Identify measures to assess pain in children.
 List various types of pain assessment tools for use with children.
List various types of pain assessment tools for use with children.
 Outline essential pain management strategies to reduce pain in children.
Outline essential pain management strategies to reduce pain in children.
 Review common types of pain experienced by children.
Review common types of pain experienced by children.
 Discuss evidence to support specific pain management strategies.
Discuss evidence to support specific pain management strategies.
PAIN ASSESSMENT
Although the ability to measure pain in children has improved dramatically in recent years, assessment of pain in children continues to be complex and challenging. Children’s ability to describe pain changes as they grow older and as they cognitively and linguistically mature (Box 7-1). Three types of measures—behavioral, physiologic, and self-report—have been developed to measure children’s pain, and their applicability depends on the child’s cognitive and linguistic ability.
BEHAVIORAL MEASURES
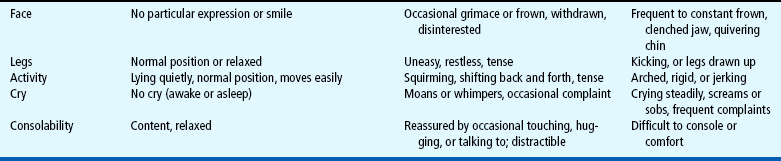
Distress behaviors, such as vocalization, facial expression, and body movement, have been associated with pain (Figs. 7-1 and 7-2). These behaviors are helpful in evaluating pain in infants and children with limited communication skills. However, discriminating between pain behaviors and reactions from other sources of distress, such as hunger, anxiety, or other types of discomfort, is not always easy. These factors decrease the specificity and sensitivity of behavioral measures (Table 7-1).
TABLE 7-1
Selected Behavioral Pain Assessment Scales for Young Children
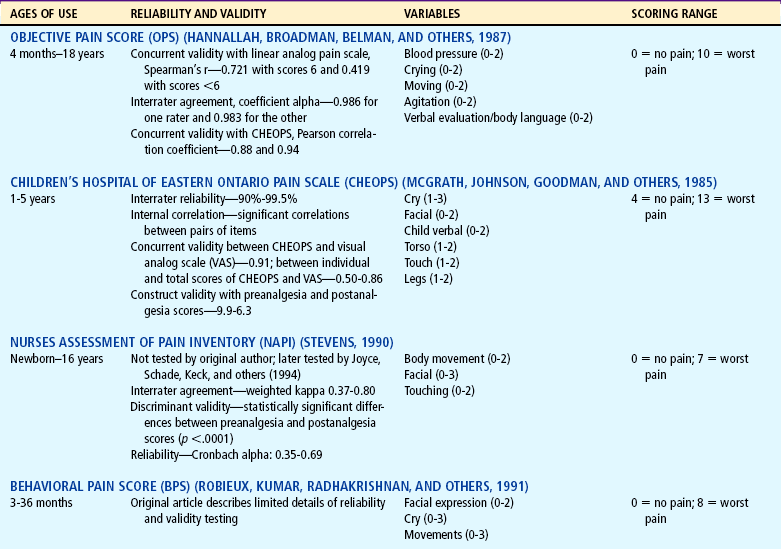
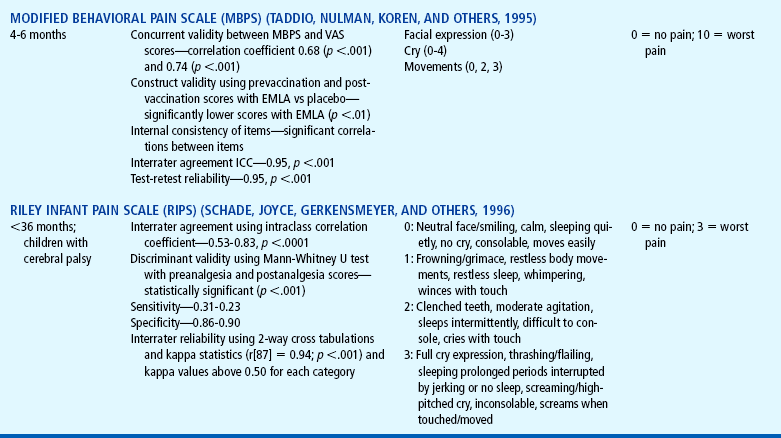
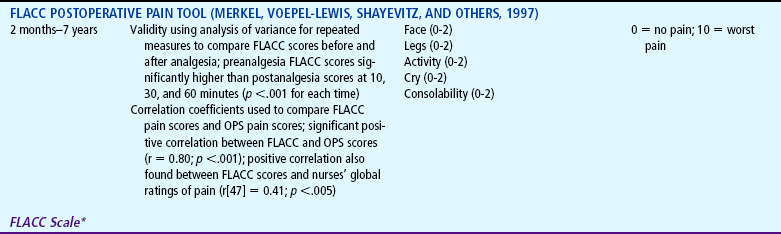
*From Merkel SI, Voepel-Lewis T, Shayevitz JR, and others: The FLACC: a behavioral scale for scoring postoperative pain in young children, Pediatr Nurs 23(3):293-297, 1997. Used with permission of Jannetti Publications, Inc., and the University of Michigan Health System. Can be reproduced for clinical and research use.
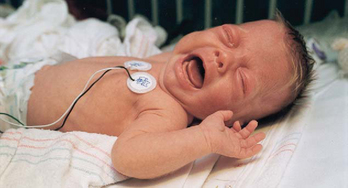
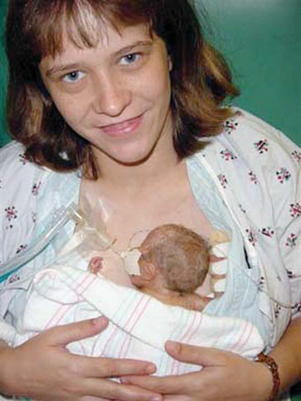
FIG. 7-1 Full, robust crying of preterm infant after heel stick. (Courtesy Halbouty Premature Nursery, Texas Children’s Hospital, Houston; photo by Paul Vincent Kuntz.)
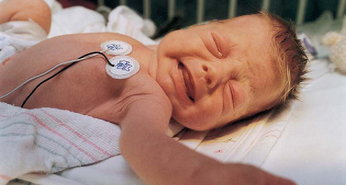
FIG. 7-2 The face of pain after heel stick. Note eye squeeze, brow bulge, nasolabial furrow, and widespread mouth. (Courtesy Halbouty Premature Nursery, Texas Children’s Hospital, Houston; photo by Paul Vincent Kuntz.)
Behavioral assessment is useful for measuring pain in infants and preverbal children who do not have the language skills to communicate that they are in pain, or in children with mental clouding and confusion that limit their ability to communicate meaningfully (McGrath, 1998). Behavior provides important information that cannot be obtained from self-report. Behavioral assessment may provide a more complete picture of the total pain experience when administered in conjunction with a subjective self-report measure. However, behavioral pain scales may be more time consuming than self-reports. These measures depend on a trained observer to watch and record children’s behaviors such as vocalization, facial expression, and body movements that suggest discomfort.
Behavioral measures are most reliable when measuring short, sharp procedural pain, such as during injections or lumbar punctures. They are less reliable when measuring longer-lasting pain. In older children pain scores on behavioral measures do not always correlate with the children’s own reports of pain intensity. The four most commonly used behavioral pain measures are the FLACC, CHEOPS, TPPPS, and PPPRS (see Table 7-1).
The FLACC Pain Assessment Tool is an interval scale that includes five categories of behavior: Facial expression, Leg movement, Activity, Cry, and Consolability (Manworren and Hynan, 2003). It measures pain by quantifying pain behaviors with scores ranging from 0 (no pain behaviors) to 10 (most possible pain behaviors). The FLACC observational pain tool has been revised and validated to include behaviors specific to those with cognitive impairment (Malviya, Voepel-Lewis, Burke, and others, 2006). The revised FLACC includes an open-ended descriptor under each category to allow parents or caregivers to record individual pain behaviors. In 52 children (4 to 19 years) with cognitive impairment, interrater reliability was supported with intraclass correlation coefficients 0.76 to 0.90. Construct validity was demonstrated by the decrease in FLACC scores following analgesic administration (Malviya, Voepel-Lewis, Burke, and others, 2006).
The Children’s Hospital of Eastern Ontario Pain Scale (CHEOPS) was developed in collaboration with experienced recovery room nurses who were queried as to what behaviors they most frequently observed to determine whether a child is in pain (McGrath, Johnson, Goodman, and others, 1985). Six categories of behaviors are identified: cry, facial, verbal, torso, touch, and legs. Scoring was devised as: 0 = behavior that is the antithesis of pain, 1 = behavior that is not indicative of pain and is not the antithesis of pain, 2 = behavior indicating mild or moderate pain, and 3 = behavior indicating severe pain. The range of the total score is 4 to 13.
The Toddler-Preschooler Postoperative Pain Scale (TPPPS) is an observational scale developed for measuring postoperative pain in children ages 1 to 5 years (Tarbell, Cohen, and Marsh, 1992). It consists of seven items divided among three pain behavior categories: (1) vocal pain expression (verbal pain complaints—cry, scream, groan, moan, grunt), (2) facial pain expression (open mouth, lips pulled back at corners, squint, closed eyes, furrowed forehead, brow bulge), and (3) bodily pain expression (restless behavior).
The Parent’s Postoperative Pain Rating Scale (PPPRS) is a scale that parents may use to rate their children’s pain by noting changes in the frequency of a number of behaviors (Chambers, Reid, McGrath, and others, 1996).
PHYSIOLOGIC MEASURES
Physiologic measures are not able to distinguish between physical responses to pain and other forms of stress to the body (Sweet and McGrath, 1998). Profound physiologic changes often accompany the experience of pain. Physiologic parameters such as heart rate, respiratory rate, blood pressure, palmar sweating, cortisone levels, transcutaneous oxygen, vagal tone, and endorphin concentrations reflect a generalized and complex response to stress. They are not localized responses to pain, but they provide useful information about the general distress levels of children who are experiencing pain. Like behavioral scales, physiologic measures may be useful for infants and children who are not able to communicate verbally. The physiologic parameters provide indirect estimates of pain, and the presence and strength of pain can only be inferred from the changes in these parameters. Most of the studies on the physiologic parameters involved predominantly infants.
SELF-REPORT MEASURES
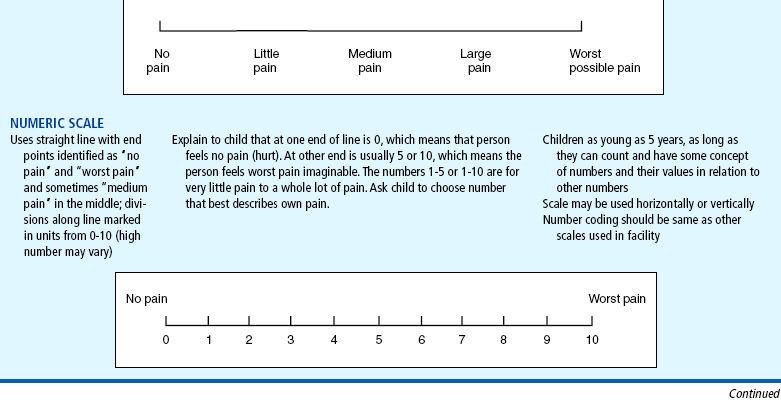
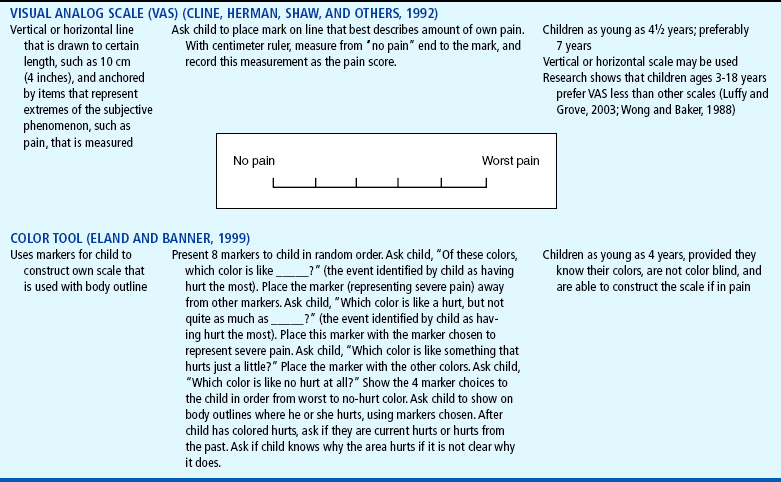
Although children who are 4 and 5 years old are able to use self-report measures (Table 7-2), their ability to use them may be influenced by the cognitive characteristics of the preoperational stage (Stanford, Chambers, and Craig, 2006). The child’s thinking tends to be egocentric, concrete, and perceptually dominated. Simple, concrete anchor words, such as “no hurt” to “biggest hurt,” are more appropriate than “least pain sensation to worst intense pain imaginable.”
TABLE 7-2
Pain Rating Scales for Children
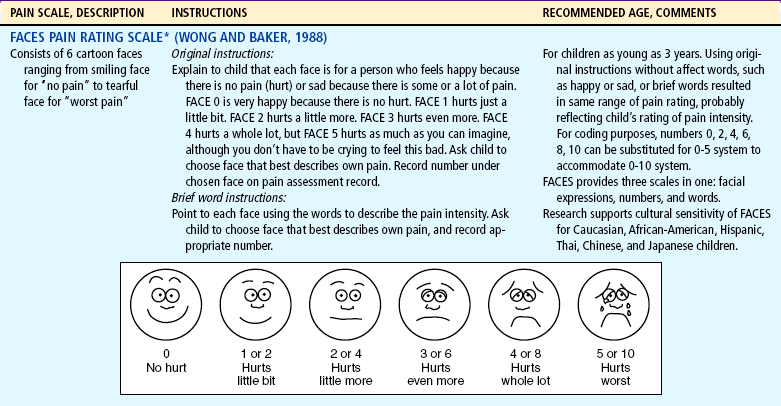


*Wong-Baker FACES Pain Rating Scale reference manual describing development and research of the scale is available from City of Hope Pain and Palliative Care Resource Center, 1500 East Duarte Road, Duarte, CA 91010, (626) 359-8111, ext. 3829; fax (626) 301-8941.
†Instructions for Word-Graphic Rating Scale from Acute Pain Management Guideline Panel: Acute pain management in infants, children, and adolescents: operative and medical procedures; quick reference guide for clinicians, ACHPR Pub No 92-0020, Rockville, Md, 1992, Agency for Healthcare Research and Quality, US Department of Health and Human Services. Word-Graphic Rating Scale is part of the Adolescent Pediatric Pain Tool and is available from Pediatric Pain Study, University of California, School of Nursing, Department of Family Health Care Nursing, San Francisco, CA 94143-0606.
The ability to discriminate degrees of pain in facial expressions appears to be reasonably established by 3 years of age (Stanford, Chambers, and Craig, 2006). Faces pain scales that were developed for young children may be a measure of pain intensity, pain affect, or both, particularly when the faces are anchored by a smiling face on one end and a face with tears on the other end (Chambers, Giesbrecht, Craig, and others, 1999; Chambers, Hardial, Craig, and others, 2005). Although clinicians may think that the smiling face anchor confounds the emotion of “feeling happy” with being “pain free,” there is no evidence to support this notion. Researchers looked at the effects of the smiling face (e.g., the Wong-Baker [WB] FACES Pain Scale) vs the neutral anchor faces (e.g., Bieri Faces Pain Scale—Revised) on measurement of pain. Chambers, Hardial, Craig, and others (2005) demonstrated a high correlation between the two forms of faces scales, with r = 0.91 between the Bieri Faces (neutral anchor) and WB-FACES Pain Scale (smiling anchor). These data suggest that children are able to use either scale for communicating the amount of pain they experience.
MULTIDIMENSIONAL MEASURES
Several cognitive skills, such as measurement, classification, and seriation (the ability to accurately place in ascending or descending order), become explicit between approximately 7 and 10 years of age. Older children are able to use the 0 to 10 numeric rating scale that is currently used by adolescents and adults. However, the use of the 0 to 10 numeric rating scale is only an assessment of pain intensity, which may not change in some pain states (Jacob, Miaskowski, Savedra, and others, 2003a). Other dimensions such as pain quality, pain location, and spatial distribution of pain may change without a change in pain intensity.
Two multidimensional assessment tools have been well validated in children 8 years and older that assess not only pain intensity, but also pain location and pain quality. Modeled after the McGill Pain Questionnaire (Melzack, 1975), the Adolescent Pediatric Pain Tool (APPT) is a multidimensional pain instrument for children and adolescents that is used to assess three dimensions of pain: location, intensity, and quality (Fig. 7-3). The APPT is a one-page, two-sided instrument with a front and back body outline on one side (Savedra, Holzemer, Tesler, and others, 1993, 1989). On the back side is a 100 mm word-graphic rating scale (Tesler, Savedra, Holzemer, and others, 1991) and a pain descriptor list (Wilkie, Holzemer, Tesler, and others, 1990). Each of the three components of the APPT is scored separately. The body outline is scored by placing a clear plastic template overlay with 43 body areas on the body outline diagram. An estimate of the pervasiveness of the pain is made by counting the number of body areas marked. A ruler or micrometer preprinted on the APPT is used to score the word-graphic rating scale. The number of millimeters from the left side of the scale to the point marked by the child is measured; and the numeric value provides an overall evaluation of the amount of pain the child is experiencing. The total number of words on the descriptor list is counted, and scores range from 0 to 56. The clinician then counts the number of words selected in each of three categories—evaluative, sensory, and affective—and calculates a percentage score for each one (Savedra, Holzemer, Tesler, and others, 1993).
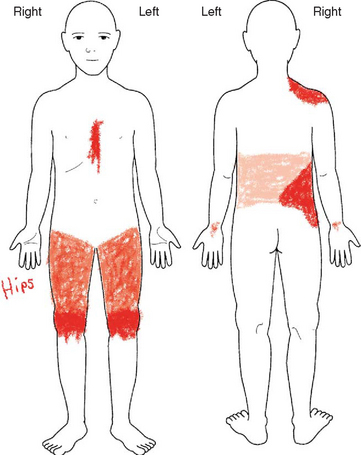
FIG. 7-3 Adolescent Pediatric Pain Tool: body outlines for pain assessment. Instructions: “Color in the areas on these drawings to show where you have pain. Make the marks as big or as small as the place where the pain is.” Tool has been completed by a child with sickle cell disease. (From Savedra MC, Tesler MD, Holzemer WL, Ward JA, School of Nursing, University of California-San Francisco; copyright 1989, 1992.)
The Pediatric Pain Questionnaire (PPQ) is a multidimensional pain instrument to assess patient and parental perceptions of the pain experience in a manner appropriate for the cognitive-developmental level of children and adolescents. The PPQ represents an attempt to assess the complexities of pediatric chronic, recurrent pain and targeted chronic musculoskeletal pain in children with juvenile rheumatoid arthritis. It consists of eight questions: (1) the pain history, (2) pain language, (3) the colors children associate with pain, (4) the emotions they experience, (5) their worst pain experiences, (6) the ways they cope with pain, (7) the positive aspects of pain, and (8) the location of their current pain. The PPQ includes three components: (1) visual analog scales; (2) color-coded rating scales; and (3) verbal descriptors to provide information about the sensory, affective, and evaluative dimensions of chronic pain (Varni, Thompson, and Hanson, 1987). It also has information about the child’s and family’s pain history, symptoms, pain relief interventions, and socioenvironmental situations that may influence pain. The child, parent, and physician complete the form separately.
PAIN ASSESSMENT IN SPECIFIC POPULATIONS
Assessment of pain in the preverbal child is difficult, especially in the neonate, since the most reliable indicator of pain, self-report, is not possible. Evaluation must be based on physiologic changes and behavioral observations (Box 7-2). Although behaviors such as vocalizations, facial expressions, and body movements are common to all infants, they vary with different situations. Crying associated with pain is more intense and sustained (see Fig. 7-1). Facial expression is the most consistent and specific characteristic; scales are available to systematically evaluate facial features, such as eye squeeze, brow bulge, open mouth, and taut tongue (Hadjistavropoulos, Craig, Grunau, and others, 1997). Most infants respond with increased body movements, but the infant may be experiencing pain even when lying quietly with eyes closed. The preterm infant’s response to pain may be behaviorally blunted or absent; however, there is ample evidence that such infants are neurologically capable of feeling pain. In addition, infants in awake or alert states demonstrate a more robust reaction to painful stimuli than infants in sleep states. Also, an infant receiving a muscle-paralyzing agent such as vecuronium will be incapable of a behavioral or visible pain response.
Although regular use of pain assessment tools can assist caregivers in determining whether the infant is in pain, caregivers must consider the infant’s maturity, behavioral state, energy resources available to respond, and risk factors for pain. In infants with diminished ability to respond robustly to pain, it is imperative to presume that pain exists in all situations that are usually considered painful for adults and children, even in the absence of behavioral or physiologic signs (Sweet and McGrath, 1998).
Several pain assessment tools have been developed for the assessment of pain in the neonate (Table 7-3). One pain assessment tool used by nurses who work with preterm and full-term infants in the neonatal intensive care setting is called CRIES, which is an acronym for the tool’s physiologic and behavioral indicators of pain: Crying, Requiring increased oxygen, Increased vital signs, Expression, and Sleeplessness. Each indicator is scored from 0 to 2’similar to the Apgar score for neonates. The total possible pain score, representing the worst pain, is 10. A pain score greater than 4 should be considered significant. This tool has been tested for reliability and validity for postoperative pain in infants between the ages of 32 weeks of gestation up to 20 weeks postterm (60 weeks) (Sweet and McGrath, 1998).
TABLE 7-3
Pain Assessment Scales for Infants
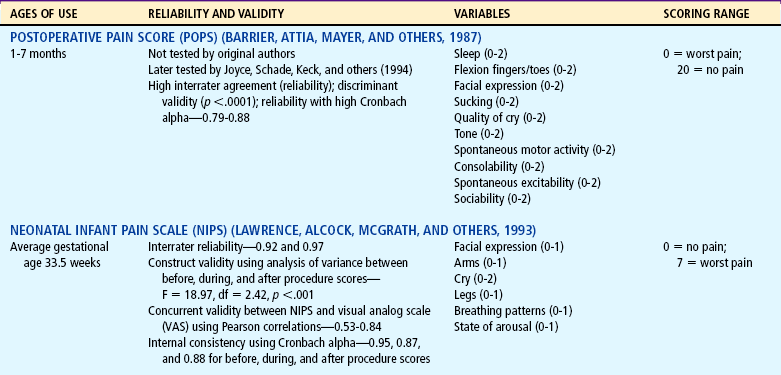

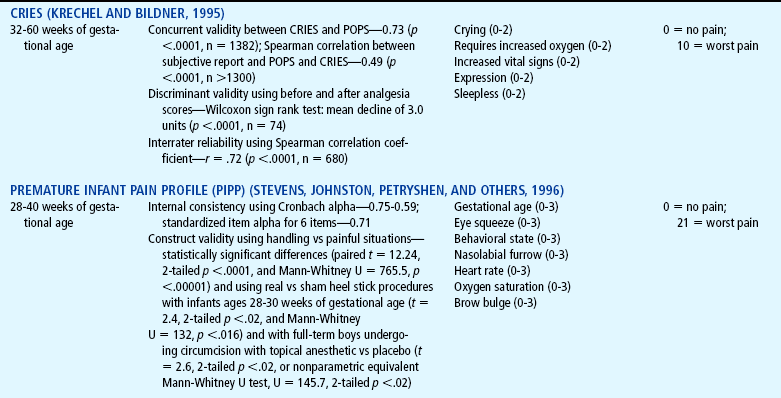
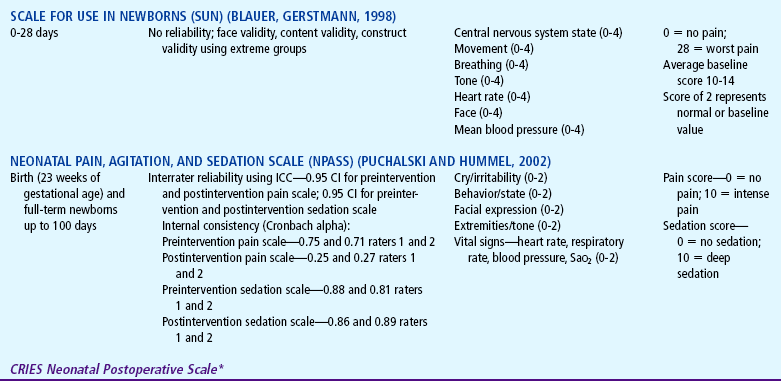

*Neonatal pain assessment tool developed at the University of Missouri–Columbia (Krechel SW, Bildner J: CRIES: a new neonatal postoperative pain measurement score: initial testing of validity and reliability, Pediatr Anaesth 5:53-61, 1995). Copyright S Krechel, MD, and J Bildner, RNC, CNS, 1995. Used with permission.
The Premature Infant Pain Profile (PIPP) is unique because it has been developed specifically for preterm infants (Sweet and McGrath, 1998). The category “gestational age at time of observation” gives a higher pain score to infants with lower gestational age. Infants who are asleep 15 seconds before the painful procedure also receive additional points for their blunted behavioral responses to painful stimuli.
The Neonatal Pain, Agitation, and Sedation Scale (NPASS) was originally developed to measure pain or sedation in preterm infants after surgery. It measures five criteria (see Table 7-3) in two dimensions (pain and sedation) and may be used in neonates as young as 23 weeks of gestation up to infants 100 days old. Extra points are added in the pain scale dimension for preterm infants based on gestational age (Sweet and McGrath, 1998).
CHILDREN WITH COMMUNICATION AND COGNITIVE IMPAIRMENT
The assessment of pain in children with communication and cognitive impairment can be challenging. Children who have significant difficulties in communicating with others about their pain include those with significant neurologic impairments (e.g., cerebral palsy), mental retardation, metabolic disorders, autism, severe brain injury, and communication barriers (e.g., critically ill children who are on ventilators or heavily sedated or have neuromuscular disorders, loss of hearing, or loss of vision). These children are at greater risk than other children for undertreatment of pain because they have medical problems that may cause pain during numerous painful procedures. Their behaviors include moaning, inconsistent patterns of play and sleep, changes in facial expression, and other physical problems that may mask expression of pain and be difficult to interpret (Hadden and von Baeyer, 2002). These children often experience spasticity, contractures, and orthopedic surgical treatment that may be painful.
The mother or primary caregiver is an important source of information during assessment (Breau, MacLaren, McGrath, and others, 2003). Up to 60% of parents of children with severe cognitive impairment reported that their child experienced pain or severe discomfort that was not being effectively managed (Lenton, Stallard, Lewis, and others; 2001; Stallard, Williams, Velleman, and others, 2002a). The most frequently reported pain behaviors are crying; being less active; seeking comfort; moaning; not cooperating; being irritable; being stiff, spastic, tense, or rigid; sleeping less; being difficult to satisfy or pacify; flinching or moving body part away; and being agitated or fidgety (Hadden and von Baeyer, 2002). Parents also reported pain during some daily living activities such as assisted stretching and walking, independent standing, toileting, putting on splints, occupational therapy, range of motion, and physical therapy.
Stallard, Williams, Lenton, and others (2001) asked the parents of cognitively impaired and noncommunicative children to assess the presence, severity, and duration of their pain during a 2-week observation period. Parents reported that 84% experienced pain on 5 or more separate days, with 32% experiencing pain on 12 or more days. Of the 74 episodes that lasted longer than 30 minutes, 33.8% occurred at night. Most pain episodes were judged to last longer than 10 minutes, with 48% of the children having episodes lasting longer than 10 minutes on 5 or more days. Although the experience of pain was common among this group of children, none was receiving treatment for relief or management of pain.
The Non-communicating Children’s Pain Checklist is a pain measurement tool specifically designed for children with cognitive impairments (Breau, McGrath, Camfield, and others, 2002). The scale discriminates between periods of pain and calm and can predict behavior during subsequent episodes of pain (Fig. 7-4). The scale consists of six subscales (vocal, social, facial, activity, body and limbs, physiologic), which are scored based on the number of times the items are observed over a 10-minute period (0 = not at all; 1 = just a little; 2 = fairly often; 3 = very often).
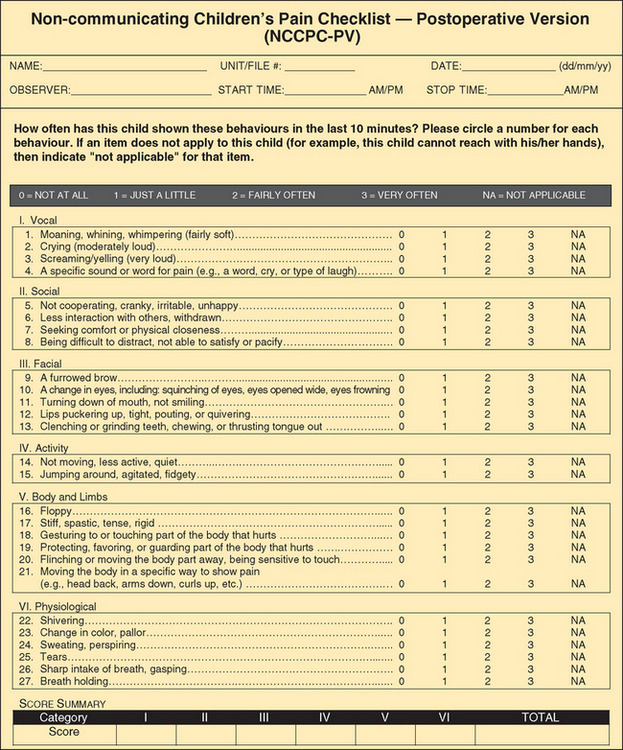

FIG. 7-4 Non-communicating Children’s Pain Checklist. (Reprinted with permission from Breau L, McGrath P, Finley A, and others: Psychometric properties of the Non-communicating Children’s Pain Checklist revised, Pain 99[1-2]:349-357, 2002.)
Another tool, the Pain Indicator for Communicatively Impaired Children (PICIC), distinguishes between pain and nonpain in communicatively impaired children with life-threatening illness (Stallard, Williams, Velleman, and others, 2002a, 2002b). The PICIC has six core pain cues: (1) crying with or without tears; (2) screaming, yelling, groaning, or moaning; (3) screwed up or distressed looking face; (4) body appearing stiff or tense; (5) difficulty in comforting or consoling; and (6) flinching or moving away if touched. The items are rated using a 4-point Likert scale (1 = not at all, 2 = a little, 3 = often, 4 = all the time).
CULTURAL ISSUES IN PAIN ASSESSMENT
A major challenge in the assessment and management of pain in children is the cultural appropriateness of pain assessment tools that have been validated only in Caucasian and English-speaking children. Observational scales and interview questionnaires for pain may not be as reliable for pain assessment as self-report scales in Hispanic children. In Chinese children who learned to read Chinese characters vertically downward and from right to left, the use of vertically oriented visual analog scales resulted in less error than horizontally oriented scales. Cultural background may therefore influence the reliability of pain assessment tools developed in a single cultural context (Bernstein and Pachter, 2003).
The Oucher Pain Scale (see Table 7-2), originally developed and validated as a self-report of pain intensity for Caucasian children 3 to 12 years old, now features culturally specific photographs with pictures of children who more closely match the physical characteristics of African-American and Hispanic children (Beyer and Knott, 1998). The Oucher Faces Pain Scale consists of six photographs on the right side and a 0 to 100 scale marked off in tens on the left side. The photographs show the face of one child with the pictures arranged to show increasing levels of discomfort. Each version has been tested primarily with children in the ethnic group (Caucasian, African American, Hispanic) depicted in the photographs. It has been used by children ages 3 to 12 years old. Children use the Oucher by selecting a photograph or number that most closely represents the level of pain intensity they are experiencing (Beyer and Knott, 1998). These tools address cultural issues in accuracy of pain assessment and were designed to promote cultural sensitivity and self-esteem for minority, non-Caucasian children.
CHILDREN WITH CHRONIC ILLNESS AND COMPLEX PAIN
Questionnaires and pain assessment scales do not always provide the most meaningful means of assessing pain in children, particularly for those with complex pain. Some children cannot relate to a face or a number that describes their pain and may not be able to isolate pain from other symptoms they are experiencing. In children with cancer, experiencing multiple symptoms makes it difficult to isolate the pain symptom from other symptoms. Rating the pain does not always accurately convey to others how they really feel (Woodgate and Yanofsky, 2004).
In children with chronic illness, particularly those with complex pain, the most important aspect during assessment is to develop a trusting relationship with the child and the family, so that a deeper understanding of the pain experience may be obtained. The pain experience may be complicated by pain processes that occur in the central nervous system (such as hyperalgesia, central sensitization, windup), by other symptoms (such as fatigue, nausea, vomiting, diarrhea, constipation) that accompany medical treatments, and by complications (such as infections, unexpected development of fistulas, typhlitis) from disease or treatments (Turner, 2005). The pain experience may interfere with the child’s ability to eat, sleep, and perform daily activities and routines (Miaskowski and Lee, 1999; Morin, Gibson, and Wade, 1998).
Other important components of assessment include the onset of pain; pain duration or pattern; the effectiveness of the current treatment; factors that aggravate or relieve the pain; other symptoms and complications concurrently felt; and interference with the child’s mood, function, and interactions with family (Turner, 2005). In addition to asking the child or parent when the pain started and how long the pain lasts, the nurse can assess variations and rhythms by asking if the pain is better or worse at certain times during the day or night. If the child has had pain for a while, the child or parent may know which medications and doses are helpful. They may also have found some nonpharmacologic methods that have helped. The nurse may ask the child or parent if there are activities, positions, and other events that may increase the pain. Pain may be accompanied by other symptoms such as nausea and poor appetite.
Other factors warranting careful assessment that may pose barriers to effective management include family issues and relationships, fears and concerns about addiction (see Community Focus box, p. 189), the clinician’s and family’s lack of knowledge about pain, inappropriate use of pain medications, ineffective management of adverse effects from medications, and the use of different pain interventions (Turner, 2005).
PAIN MANAGEMENT
Unrelieved pain may lead to potential long-term physiologic, psychosocial, and behavioral consequences (Goldschneider and Anand, 2003; Weisman, Bernstein, and Schechter, 1998). Management of pain should be a priority for all clinicians.
NONPHARMACOLOGIC MANAGEMENT
Pain is often associated with fear, anxiety, and stress (Kain, Mayes, Caldwell-Andrews, and others, 2006). A number of nonpharmacologic techniques (see Nursing Care Guidelines box), such as distraction, relaxation, guided imagery, and cutaneous stimulation, provide coping strategies that may help reduce pain perception, make pain more tolerable, decrease anxiety, and enhance the effectiveness of analgesics or reduce the dosage required (Rusy and Weisman, 2000). In addition, these techniques decrease the perceived threat of pain, provide a sense of control, enhance comfort, and promote rest and sleep (Greco and Berde, 2005). Although there is a paucity of research on the effectiveness of many of these interventions, the strategies are safe, noninvasive, and inexpensive, and most are independent nursing functions. Environmental and psychologic factors may exert a powerful influence on children’s pain perceptions and may be modified by using psychosocial strategies, education, parental support, and cognitive-behavioral interventions. For children undergoing repeated painful procedures, cognitive-behavioral interventions are effective for decreasing anxiety and distress (McGrath and Hillier, 2003).
If the child cannot identify a familiar coping technique, the nurse can describe several strategies and let the child select the most appealing one. Experimentation with several strategies that are suitable to the child’s age, pain intensity, and abilities is often necessary to determine the most effective approach. Parents should be involved in the selection process; they may be familiar with the child’s usual coping skills and can help identify potentially successful strategies. Involving parents also encourages their participation in learning the skill with the child and acting as coach. If the parent cannot assist the child, other appropriate persons may include a grandparent, older sibling, nurse, or child life specialist (McGrath and Hillier, 2003).
Children should learn to use a specific strategy before pain occurs or before it becomes severe. Children are responsive to pain-controlling strategies that involve their imaginations and senses of play (Gerik, 2005). To reduce the child’s effort, instructions for a strategy, such as distraction or relaxation, can be audiotaped and played during a period of comfort. However, even after they have learned an intervention, children often need help using it during a painful procedure. The intervention can also be used after the procedure. This gives the child a chance to recover, feel mastery, and cope more effectively (McGrath and Hillier, 2003).
Virtual reality has been identified as a potentially effective tool for pain distraction (Gold, Kim, Kant, and others, 2006). The participant’s attention is drawn away from the “real world” and into the “virtual world” with the incorporation of visual, auditory, and tactile stimuli.
Several studies have documented the effectiveness of nonpharmacologic analgesia, such as containment, positioning, nonnutritive sucking (Fig. 7-5), and kangaroo holding during painful procedures in neonates. Containment is achieved through positioning and blanket rolls (Cole and Jorgensen, 1997). It provides a “nest” that enhances the infant’s feelings of security and decreases stress. Comforting measures and swaddling have been demonstrated to reduce crying and heart rate after procedures such as heel punctures and injections. In infants between 27 and 34 weeks of gestational age, those infants who were swaddled after a routine heel stick procedure were able to calm crying immediately, decrease heart rate, and return to a sleep state; in comparison, infants who were not swaddled took a minimum of 10 minutes to return to baseline physiologic and behavioral levels (Fearon, Kisilevsky, Hains, and others, 1997). Proper positioning with the infant held in a midline orientation, hand to mouth activity, and proper flexion can promote self-soothing behaviors. Facilitated tucking, which is holding the infant’s extremities flexed and contained close to the trunk, during heel lance procedures has been demonstrated to decrease heart rate, decrease crying time, and promote stability in the sleep-wake cycles after the lance.
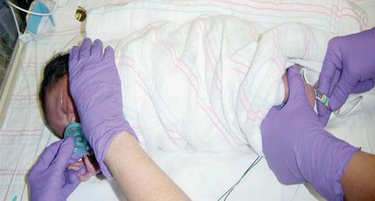
FIG. 7-5 Sucking following oral sucrose can enhance analgesia before a heel stick in a preterm infant.
Nonnutritive sucking (pacifier) attenuates behavioral, physiologic, and hormonal responses to pain from procedures, such as heel punctures, venipuncture, and immunization injections. The administration of concentrated sucrose with and without nonnutritive sucking has been demonstrated to have calming and pain-relieving effects for invasive procedures in neonates (see Evidence-Based Practice box). The amount of time crying was decreased with the oral administration of 2 ml of a 12% to 24% sucrose solution, 2 minutes before a heel lance or venipuncture (Stevens, Yamada, and Ohlsson, 2005).
Kangaroo care is skin-to-skin holding of infants dressed only in diapers against their mother’s or father’s chest (Gray, Watt, and Blass, 2000; Johnston, Stevens, Pinelli, and others, 2003). Infants who spent 1 to 3 hours in kangaroo care showed increased frequency in quiet sleep, longer duration of quiet sleep, and decreased crying in the neonatal intensive care unit. They also cried less at the age of 6 months when compared with neonates who did not receive skin-to-skin contact. Significant differences were found in pain responses during heel lancing between infants who were kangaroo held and those who were not. In the study by Gray, Watt, and Blass (2000), heart rate increased by 8 to 10 beats/min in the kangaroo care group vs an increase by 36 to 38 beats/min in the control group of neonates who were swaddled in bassinets. Grimacing was 64% less, and crying was 82% less frequent.
In another study, infant responses to pain during heel lance procedures were compared using kangaroo holding (Fig. 7-6), with the neonate held upright at a 60-degree angle between the mother’s breasts for maximal skin-to-skin contact (Johnston, Stevens, Pinelli, and others, 2003). A blanket was placed over the neonate’s back, and the mother’s clothes were wrapped around the neonate for 30 minutes before the lancing procedure, during, and at least 30 minutes after the heel stick. Another group remained in the isolette in a prone position, swaddled with a blanket and the heel accessible, for 30 minutes before the heel lancing procedure. Pain scores were significantly lower in kangaroo-held infants.
COMPLEMENTARY PAIN MEDICINE
Many terms are used to describe approaches to health care that are outside the realm of conventional medicine as practiced in the United States. Complementary and alternative medicine (CAM), as defined by the National Center for Complementary and Alternative Medicine, is a group of diverse medical and health care systems, practices, and products that are not currently considered part of conventional medicine (Myers, Stuber, Bonamer-Rheingans, and others, 2005). Although some scientific evidence exists regarding the efficacy of some CAM therapies, questions are yet to be answered through well-designed scientific studies, such as whether these therapies are safe and whether they work for the diseases or medical conditions for which they are used.
CAM therapies may be grouped into five classes:
1. Biologically based—foods, special diets, herbal or plant preparations, vitamins, other supplements
2. Manipulative treatments—chiropractic, osteopathy, massage
3. Energy based—Reiki, bioelectric or magnetic treatments, pulsed fields, alternating and direct currents
4. Mind-body techniques—mental healing, expressive treatments, spiritual healing, hypnosis, relaxation
5. Alternative medical systems—homeopathy; naturopathy; ayurvedic; and traditional Chinese medicine, including acupuncture and moxibustion
Current estimates of pediatric CAM use range from 10% to 15%, derived from children sampled at health care facilities, with chronic conditions, and/or from countries other than the United States. For the U.S. population, pediatric CAM use was estimated to be 31% to 84% (Myers, Stuber, Bonamer-Rheingans, and others, 2005; Rusy and Weisman, 2000). Those who used CAM were found in each age-group, and the mean age was 10.3 years. The majority used unconventional therapy for chronic, as opposed to life-threatening, medical conditions. The therapies that are increasingly used include herbal medicine, massage, megavitamins, self-help groups, folk remedies, energy healing, and homeopathy (Myers, Stuber, Bonamer-Rheingans, and others, 2005; Rusy and Weisman, 2000).
PHARMACOLOGIC MANAGEMENT
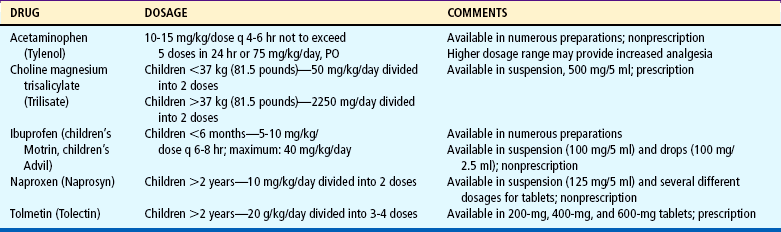
Nonopioids, including acetaminophen (Tylenol, Paracetamol) and nonsteroidal antiinflammatory drugs (NSAIDs), are suitable for mild to moderate pain (Table 7-4); opioids are needed for moderate to severe pain (Table 7-5). A combination of the two analgesics acts on the pain system on two levels: nonopioids primarily act at the peripheral nervous system and opioids primarily act at the central nervous system. The combination of NSAIDs and opioids provides increased analgesia without increased side effects. Several combinations, such as acetaminophen with codeine, may have increasing doses of the opioid but a constant dose of the nonopioid (Table 7-6). Before increasing the opioid, it may be preferable to increase the nonopioid component, for example, adding one regular-strength acetaminophen tablet (325 mg) to acetaminophen 300 mg with codeine 15 mg (Tylenol No. 2) before advancing to acetaminophen 300 mg with codeine 30 mg (Tylenol No. 3) or codeine 60 mg (Tylenol No. 4). However, if this approach is not successful, pain management will require a stronger opioid (see Table 7-5).
TABLE 7-4
Nonsteroidal Antiinflammatory Drugs (NSAIDs) Approved for Children*
note: Newer formulations of NSAIDs selectively inhibit one of the enzymes of cyclooxygenase (COX-2, which is responsible for pain transmission) but do not inhibit the other (COX-1). Inhibition of COX-1 decreases prostaglandin production, which is necessary for normal organ function. For example, prostaglandins help maintain gastric mucosal blood flow and barrier protection, regulate blood flow to the liver and kidneys, and facilitate platelet aggregation and clot formation. Theoretically, the COX-2 NSAIDs provide similar analgesic and antiinflammatory benefits with fewer gastric and platelet side effects than the nonselective agents. COX-2 NSAIDs are approved for use in patients older than 18 years of age.
Acetylsalicylic acid (aspirin) is also an NSAID but is not recommended for children because of its possible association with Reye syndrome. The NSAIDs in this table have no known association with Reye syndrome. However, caution should be exercised in prescribing any salicylate-containing drug (e.g., choline magnesium trisalicylate) for children with known or suspected viral infection. Side effects of ibuprofen, naproxen, and tolmetin include nausea, vomiting, diarrhea, constipation, gastric ulceration, bleeding nephritis, and fluid retention.
Acetaminophen and choline magnesium trisalicylate are well tolerated in the gastrointestinal tract and do not interfere with platelet function. NSAIDs (except acetaminophen) should not be given to patients with allergic reactions to salicylates. All the NSAIDs should be used cautiously in patients with renal impairment.
*All NSAIDs in this table (except acetaminophen) have significant antiinflammatory, antipyretic, and analgesic actions. Acetaminophen has a weak antiinflammatory action, and its classification as an NSAID is controversial. Patients respond differently to various NSAIDs; therefore changing from one drug to another may be necessary for maximum benefit.
Data from: Drug facts and comparisons, Philadelphia, 2008, Lippincott Williams & Wilkins.
TABLE 7-5
Dosage of Selected Opioids for Children
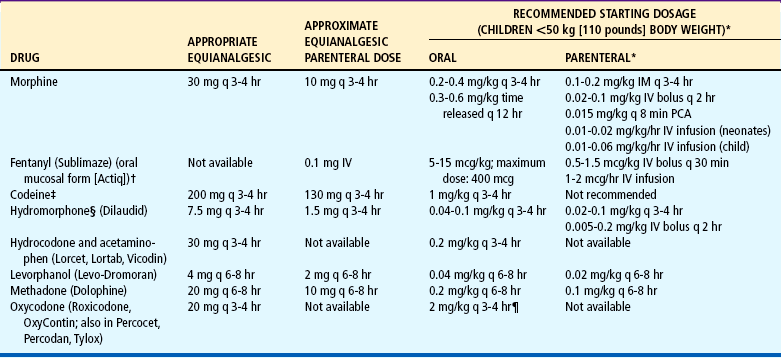
IM, Intramuscular; IV, intravenous; PCA, patient-controlled analgesia; q, every.
note: Published tables vary in suggested doses that are equianalgesic to morphine. Clinical response is criterion that must be applied for each patient; titration to clinical response is necessary. Because there is not complete cross-tolerance among these drugs, it is usually necessary to use a lower than equianalgesic dose when changing drugs and to retitrate to response. caution: Recommended doses do not apply to patients with renal or hepatic insufficiency or other conditions affecting drug metabolism and kinetics.
*caution: Doses listed for patients with body weight <50 kg (110 pounds) cannot be used as initial starting doses in infants <6 months of age. For nonventilated infants <6 months, the initial opioid dose should be about ¼ to ⅓ of the dose recommended for older infants and children. For example, morphine could be used at a dose of 0.03 mg/kg instead of the traditional 0.1 mg/kg.
†Actiq is indicated only for management of breakthrough cancer pain in patients with malignancies who are already receiving and are tolerant to opioid therapy, but it can be used for preop-erative or preprocedural sedation and analgesia.
‡CAUTION: Codeine doses above 65 mg often are not appropriate because of diminishing incremental analgesia with increasing doses but continually increasing constipation and other side effects.
§For morphine, hydromorphone, and oxymorphone, rectal administration is an alternate route for patients unable to take oral medications, but equianalgesic doses may differ from oral and parenteral doses because of pharmacokinetic differences.
¶caution: Doses of aspirin and acetaminophen in combination with opioid or nonsteroidal antiinflammatory drug preparations must also be adjusted to patient’s body weight. Daily dose of acetaminophen should not exceed 75 mg/kg, or 4000 mg.
Data from Acute Pain Management Guideline Panel: Acute pain management: operative or medical procedures and trauma: clinical practice guideline, AHCPR Pub No 92-0032, Rockville, Md, 1992, Agency for Health Care Policy and Research, Public Health Service, US Department of Health and Human Services; and Berde C, Albin A, Glazer J, and others: American Academy of Pediatrics report of the Subcommittee on Disease-Related Pain in Childhood Cancer, Pediatrics 86(5 pt 2):818-825, 1990. Codeine dosages from McCaffery M, Pasero C: Pain: a clinical manual, ed 2, St Louis, 1999, Mosby.
TABLE 7-6
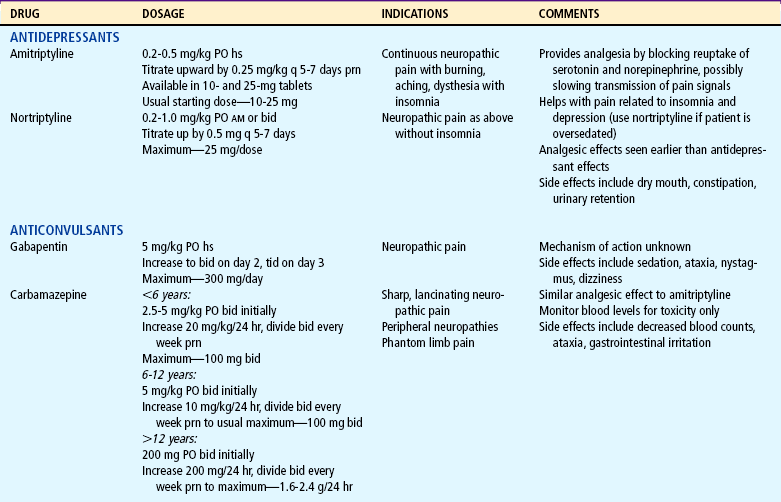

bid, Twice a day; hs, at bedtime; IV, intravenous; NSAIDs, nonsteroidal antiinflammatory drugs; PO, by mouth; prn, as needed; q, every; tid, three times a day.
Oxycodone is available without a nonopioid in an immediate release and controlled release preparation (OxyContin). The oxycodone dose can be safely increased without the risk of toxicity from excessive acetaminophen use. Actions of various opioids differ. Morphine is considered the gold standard for the management of severe pain. When morphine is not a suitable opioid, drugs such as hydromorphone (Dilaudid) and fentanyl (Sublimaze) are effective substitutes. Although fentanyl is used as an anesthetic in the operating room, it is classified as an analgesic. It can be safely administered by nurses by the intravenous (IV), intramuscular (IM), transmucosal, and transdermal routes (Algren, Gursoy, Johnson, and others, 1998; Golianu, Krane, Galloway, and others, 2000).
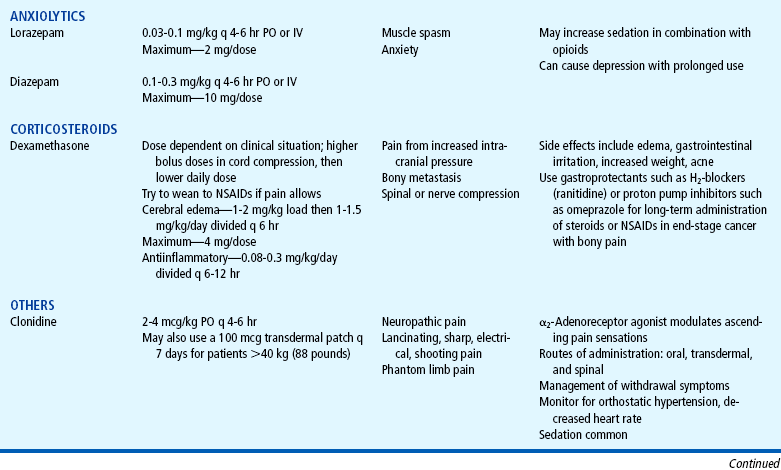
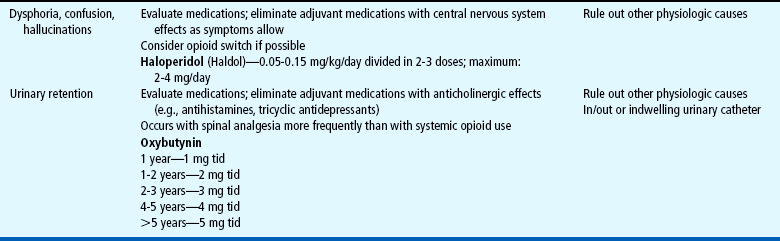
Several drugs, known as coanalgesics or adjuvant analgesics, may be used alone or with opioids to control pain symptoms and opioid side effects. Drugs frequently used to relieve anxiety, cause sedation, and provide amnesia are diazepam (Valium) and midazolam (Versed); however, these drugs are not analgesics and should be used to enhance the effects of analgesics, not as a substitute for analgesics. Other adjuvants include tricyclic antidepressants (e.g., amitriptyline, imipramine) and antiepileptics (e.g., gabapentin, carbamazepine, clonazepam) for neuropathic pain (see Table 7-6), stool softeners and laxatives for constipation, antiemetics for nausea and vomiting, diphenhydramine for itching, steroids for inflammation and bone pain, and dextroamphetamine and caffeine for possible increased analgesia and decreased sedation (Table 7-7) (Greco and Berde, 2005).
TABLE 7-7
Management of Opioid Side Effects

bid, Twice a day; hs, at bedtime; IV, intravenous; PO, by mouth; PR, by rectum; prn, as needed; q, every; tid, three times a day.
The use of placebos to determine whether the patient is having pain is unjustified and unethical; a positive response to a placebo, such as a saline injection, is common in patients who have a documented organic basis for pain. Therefore the deceptive use of placebos does not provide useful information about the presence or severity of pain. The use of placebos can cause side effects similar to those of opioids, can destroy the patient’s trust in the health care staff, and raises serious ethical and legal questions. The American Society for Pain Management Nursing has issued a position statement against the use of placebos to treat pain (McCaffery and Pasero, 1999).
Children (except infants younger than about 3 to 6 months) metabolize drugs more rapidly than adults; younger children may require higher doses of opioids to achieve the same analgesic effect. Therefore the therapeutic effect and duration of analgesia vary. Children’s dosages are usually calculated according to body weight, except in children with a weight greater than 50 kg (110 pounds), where the weight formula may exceed the average adult dosage. In this case the adult dosage is used.
A reasonable starting dose of opioid for infants under 6 months who are not mechanically ventilated is one fourth to one third of the recommended starting dose for older children. The infant is monitored closely for signs of pain relief and respiratory depression. The dose is titrated to effect. Because tolerance can develop rapidly, large doses may be needed for continued severe pain (Greco and Berde, 2005). If pain relief is inadequate, the initial dose is increased (usually by 25% to 50% if pain is moderate, or by 50% to 100% if pain is severe) to provide greater analgesic effectiveness. Decreasing the interval between doses may also provide more continuous pain relief. A major difference between opioids and nonopioids is that nonopioids have a ceiling effect, which means that doses higher than the recommended dose will not produce greater pain relief. Opioids do not have a ceiling effect other than that imposed by side effects; therefore larger dosages can be safely given for increasing severity of pain.
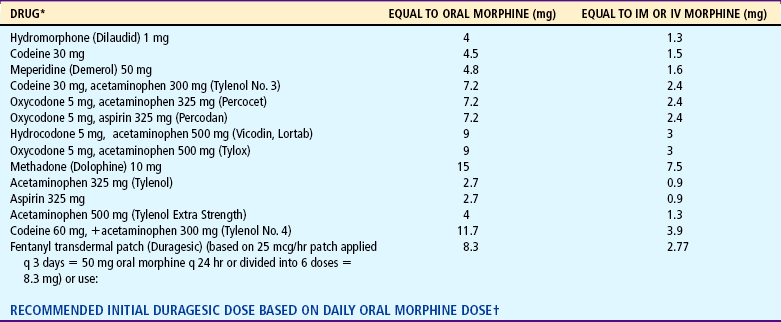
Parenteral and oral dosages of opioids are not the same. Because of the first-pass effect, an oral opioid is rapidly absorbed from the gastrointestinal tract and is partially metabolized in the liver before reaching the central circulation. Therefore oral dosages must be larger to compensate for the partial loss of analgesic potency to achieve equianalgesia (equal analgesic effect). Conversion factors (Table 7-8) for selected opioids must be used when a change is made from IV (preferred) or IM to oral administration. Immediate conversion from IM or IV to the suggested equianalgesic oral dose may result in a substantial error. For example, the dose may be significantly more or less than what the child requires. Small changes ensure small errors. Several routes of analgesic administration can be used (Box 7-3); the most effective and least traumatic should be selected.
TABLE 7-8
Equianalgesia of Selected Analgesics
IM, Intramuscular; IV, intravenous; q, every.
Note: When converting to oral oxycodone from oral morphine, an appropriate conservative estimate is 15-20 mg oxycodone per 30 mg morphine; however, when converting to oral morphine from oral oxycodone, an appropriate conservative estimate is 30 mg morphine per 30 mg oxycodone (McCaffery m, Pasero C: Pain: a clinical manual, ed 2, St Louis, 1999, Mosby).
*Oral medication with exception of fentanyl.
†Data from Duragesic package insert, Janssen Pharmaceutical Products, Titusville, NJ, 2001.
Courtesy Betty R. Ferrell, PhD, FAAN, 1999. Used with permission.
Patient-Controlled Analgesia
A significant advance in the administration of IV, epidural, or subcutaneous analgesics is the use of patient-controlled analgesia (PCA). As the name implies, the patient controls the amount and frequency of the analgesic, which is typically delivered through a special infusion device. Children who are physically able to “push a button” (i.e., 5 to 6 years of age) and who can understand the concept of pushing a button to obtain pain relief can use PCA (Maxwell and Yaster, 2000). Although it is controversial, parents and nurses have used the IV PCA system for the child. Nurses can efficiently use the infusion device on a child of any age to administer analgesics to avoid signing for and preparing opioid injections every time one is needed (Fig. 7-7). When PCA is used as “nurse- or parent-controlled” analgesia, the concept of patient control is negated, and the inherent safety of PCA needs to be monitored. Research has reported safe and effective analgesia in children when the PCA was controlled by patient, parent, or nurse (Algren, Gursoy, Johnson, and others, 1998; Maxwell and Yaster, 2000).
PCA infusion devices typically allow for three methods or modes of drug administration to be used alone or in combination:
 Patient-administered boluses that can only be infused according to the preset amount and lockout interval (time between doses). More frequent attempts at self-administration usually mean the patient may need the dose and time adjusted for better pain control.
Patient-administered boluses that can only be infused according to the preset amount and lockout interval (time between doses). More frequent attempts at self-administration usually mean the patient may need the dose and time adjusted for better pain control.
 Nurse-administered boluses that are typically used to give an initial loading dose to increase blood levels rapidly and to relieve breakthrough pain (pain not relieved with the usual programmed dose).
Nurse-administered boluses that are typically used to give an initial loading dose to increase blood levels rapidly and to relieve breakthrough pain (pain not relieved with the usual programmed dose).
 Continuous basal rate infusion that delivers a constant amount of analgesic and prevents pain from returning during those times, such as sleep, when the patient cannot control the infusion.
Continuous basal rate infusion that delivers a constant amount of analgesic and prevents pain from returning during those times, such as sleep, when the patient cannot control the infusion.
As with any type of analgesic management plan, continued assessment of the child’s pain relief is essential for the greatest benefit from PCA. Typical uses of PCA are for controlling pain from surgery, sickle cell crisis, trauma, and cancer. Morphine is the drug of choice for PCA and is usually prepared in a concentration of 1 mg/ml (Table 7-9). Other options are hydromorphone (0.2 mg/ml) and fentanyl (0.01 mg/ml). Hydromorphone is often used when patients are not able to tolerate side effects such as pruritus and nausea from the morphine PCA (Algren, Gursoy, Johnson, and others, 1998; Maxwell and Yaster, 2000).
TABLE 7-9
Suggested Intravenous Patient-Controlled Analgesia Opioid Infusion Orders

From Yaster M, Krance EJ, Kaplan R F, and others: Pediatric pain management and sedation handbook, St Louis, 1997, Mosby.
Some physicians may still prescribe meperidine. However, meperidine is the least potent and shortest-acting of the synthetic opioids and the least effective in providing analgesia for severe pain. More important, it may increase the risk of seizures when administered chronically because of the excitatory effects on the nervous system of its metabolite, normeperidine. Some authors (Nadvi, Sarnaik, and Ravindranath, 1999) have argued that the incidence of meperidine-associated seizures is extremely small (0.4% of patients; 0.06% of admissions) and the risk of seizures should not dissuade clinicians from using this drug. However, the American Pain Society recommends that meperidine be reserved for brief treatment courses for patients who have reported and demonstrated its effectiveness, or who have allergies or uncorrectable intolerances to other opioids. Meperidine should not be used for longer than 48 hours or in doses greater than 600 mg/24 hr (Max, Byas-Smith, Gracely, and others, 1995).
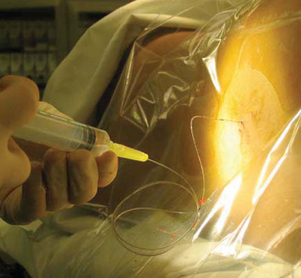
Epidural Analgesia
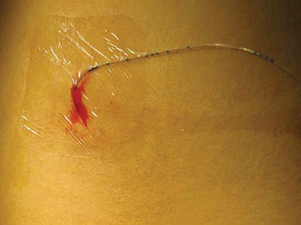
Epidural analgesia may also be used to manage pain in selected cases. Although an epidural catheter may be inserted at any vertebral level, it is usually placed into the epidural space of the spinal column at the lumbar or caudal level (Fig. 7-8). The thoracic level is usually reserved for older children or adolescents who have had an upper abdominal or thoracic procedure, such as a lung transplant. An opioid (usually fentanyl, hydromorphone, or preservative-free morphine, which is often combined with a long-acting local anesthetic such as bupivacaine or ropivacaine) is instilled via single or intermittent bolus, continuous infusion, or patient-controlled epidural analgesia. Analgesia results from the drug’s effect on opiate receptors in the dorsal horn of the spinal cord, rather than the brain. As a result, respiratory depression is rare, but if it occurs, it develops slowly, typically 6 to 8 hours after administration (Golianu, Krane, Galloway, and others, 2000). Properly securing the epidural catheter with an occlusive dressing decreases the possibility of soiling or inadvertently displacing the catheter (Fig. 7-9). Careful monitoring of sedation level and respiratory status is critical to prevent opioid-induced respiratory depression. Assessment of pain and the skin condition around the catheter site are important aspects of nursing care (Golianu, Krane, Galloway, and others, 2000).
Transmucosal and Transdermal Analgesia
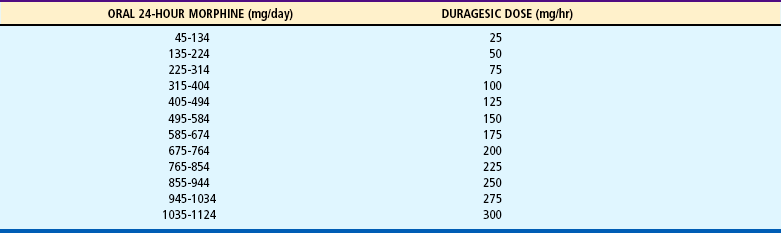
Fentanyl is also available as a transdermal patch (Duragesic). Although contraindicated for acute pain management, it may be used for older children and adolescents who have cancer pain or sickle cell pain or for patients who are opioid tolerant.
One of the most significant improvements in the ability to provide atraumatic care to children is the anesthetic cream LMX (a 4% liposomal lidocaine cream) or EMLA (an eutectic mixture of local anesthetics) (Abdelkefi, Abdennebi, Mellouli, and others, 2004; Choi, Irwin, Hui, and others, 2003; Egekvist and Bjerring, 2000; Gad, Olsen, Lysgaard, and others, 2005; Rogers and Ostrow, 2004; Santiago, Abad, Fernandez, and others, 2000; Uziel, Berkovitch, Gazarian, and others, 2003). The eutectic mixture (lidocaine 2.5% and prilocaine 2.5%), whose melting point is lower than that of the two anesthetics alone, permits effective concentrations of the drug to penetrate intact skin (see Evidence-Based Practice box and Fig. 7-10).
In some situations there is not ample time for topical preparations like LMX or EMLA to take effect, and refrigerant sprays such as ethyl chloride and fluorimethane can be used (Reis and Holubkov, 1997). When sprayed on the skin, these sprays vaporize, rapidly cool the area, and provide superficial anesthesia. Hospital formularies may have other products with lidocaine, prilocaine, or amethocaine topical preparations that require less time for application.
A randomized controlled trial compared the efficacy and safety of amethocaine gel (which is applied for 30 minutes) and EMLA cream (which is applied for 60 minutes) before a port-a-cath puncture. Children rated the pain following the puncture using the FACES rating scale (coded 0 to 5). Both groups had low pain scores, less than or equal to 2, and it did not make a significant difference whether the amethocaine or the EMLA cream was used. The researchers concluded that the amethocaine was clinically equivalent to EMLA, but the amethocaine gel required less time for anesthesia (Bishai, Taddio, Bar-Oz, and others, 1999). Another transdermal option is Numby Stuff, which uses iontophoresis (mild electrical current) to actively push the drug into the skin. This preparation of Iontocaine (lidocaine hydrochloride 2% with epinephrine 1:100,000 topical solution) provides dermal anesthesia to a depth of 10 mm in approximately 10 minutes (Squire, Kirchhoff, and Hissong, 2000). It can be used for IV placement, insertion of percutaneous central catheters lines, lumbar punctures, implantable port needle insertion, and pulsed dye laser therapy. It is important to provide explanations and let the child become familiar with the equipment. Some children may find the tingling sensation uncomfortable or frightening while the drug is administered (Squire, Kirchhoff, and Hissong, 2000).
The LidoSite Topical System is another method to help reduce needlestick pain associated with procedures such as IV cannulation, venipuncture, or laser ablation of superficial skin lesions for patients age 5 years and older. The LidoSite system delivers numbing medication to the procedure site quickly and effectively after a 10-minute application (Fig. 7-11 and Evidence-Based Practice box). The system consists of a single-use, prefilled LidoSite patch, filled with lidocaine hydrochloride 10% and epinephrine 0.1%, and the LidoSite controller, an easy-to-use preprogrammed device that activates the patch. It provides pain reduction equivalent to a lidocaine (Xylocaine) injection without the needlestick. Through iontophoresis, a mild current from the controller activates the patch to accelerate delivery of lidocaine—the anesthetic medication—to the injection site. Epinephrine contained in the LidoSite patch helps focus the anesthetic effect directly under the patch and extends the duration of the effect for an hour.
The intradermal route is sometimes used to inject a local anesthetic, typically lidocaine, into the skin to reduce the pain from a lumbar puncture, bone marrow aspiration, or venous or arterial access. One problem with the use of lidocaine is the stinging and burning that initially occur. However, the used of buffered lidocaine with sodium bicarbonate (see Evidence-Based Practice box) reduces the stinging sensation (Wong and Pasero, 1997a, 1997b). Warming the lidocaine to 37° C (98.6° F) may accomplish the same effect.
Timing of Analgesia
The right timing for administering analgesics depends on the type of pain. For continuous pain control, such as for postoperative or cancer pain, a preventive schedule of medicationaround the clock (ATC) is effective. The ATC schedule avoids the low concentrations of medications in plasma that permit breakthrough pain. If analgesics are administered only when pain returns (a typical use of the prn, or “as needed,”, order), pain relief may take several hours. This may require higher doses, leading to a cycle of undermedication of pain alternating with periods of overmedication and drug toxicity. This cycle of erratic pain control also promotes “clock watching,” which may be erroneously equated with addiction. Nurses can effectively use prn orders by giving the drug at regular intervals, since “as needed” should be interpreted as “as needed to prevent pain,” not “as little as possible.”
Preventive pain control is best provided through continuous IV infusion rather than intermittent boluses. If intermittent boluses are given, the intervals between doses should not exceed the drug’s expected duration of effectiveness. For extended pain control with fewer administration times, drugs that provide longer duration of action(e.g., some NSAIDs, time-released morphine or oxycodone, methadone, levorphanol) can be used.
Continuous analgesia is not always appropriate, since not all pain is continuous. Frequently, temporary pain control or conscious sedation is needed to provide analgesia before a scheduled procedure. When pain can be predicted, the drug’s peak effect should be timed to coincide with the painful event. For example, with opioids the peak effect is approximately a half hour for the IV route; with nonopioids the peak effect occurs about 2 hours after oral administration. Forrapid onset and peak of action, opioids that quickly penetrate the blood-brain barrier (e.g., IV fentanyl) provide excellent pain control.
Monitoring Side Effects
Both NSAIDs and opioids have side effects, although the major concern is with those from opioids (Box 7-4). Respiratory depression is the most serious complication and is most likely to occur in sedated patients. The respiratory rate may decrease gradually, or respirations may cease abruptly. Lower limits of normal are not established for children, but any significant change from a previous rate calls for increased vigilance. A slower respiratory rate does not necessarily reflect decreased arterial oxygenation; an increased depth of ventilation may compensate for the altered rate. If respiratory depression or arrest occurs, the nurse must be prepared to intervene quickly (see Nursing Care Guidelines box).
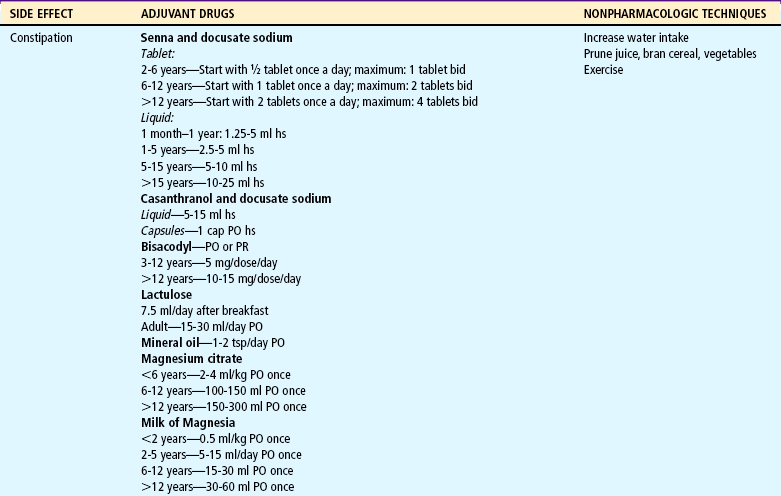
Although respiratory depression is the most feared side effect, constipation is a common, and sometimes serious, side effect of opioids, which decrease peristalsis and increase anal sphincter tone. Prevention with stool softeners and laxatives is more effective than treatment once constipation occurs. Dietary treatment, such as increased fiber, is usually not sufficient to promote regular bowel evacuation. However, dietary measures, such as increased fluid and fruit intake, and physical activity are encouraged. Pruritus from epidural or IV infusion can be treated with low doses of IV naloxone, nalbuphine, or diphenhydramine. Nausea, vomiting, and sedation usually subside after two days of opioid administration; however, oral or rectal antiemetics may be necessary.
Both tolerance and physical dependence can occur with prolonged use of opioids (see Community Focus box). Physical dependence is a normal, natural, physiologic state of neuroadaptation. When opioids are abruptly discontinued without weaning, withdrawal symptoms occur. Symptoms of withdrawal occur at 24 hours after abrupt discontinuation and reach a peak within 72 hours. Symptoms of withdrawal include signs of neurologic excitability (irritability, tremors, seizures, increased motor tone, insomnia), gastrointestinal dysfunction (nausea, vomiting, diarrhea, abdominal cramps), and autonomic dysfunction (sweating, fever, chills, tachypnea, nasal congestion, rhinitis). Withdrawal symptoms can be anticipated and prevented by weaning patients from opioids that were administered for more than 5 to 10 days. Adherence to a weaning protocol to prevent or minimize withdrawal symptoms from opioids will be required. A weaning flow sheet (Fig. 7-12) may be used to assess the efficacy of opioid weaning in neonates (Franck and Vilardi, 1995; Franck, Vilardi, Durand, and others, 1998).
Tolerance occurs when the dose of an opioid needs to be increased to achieve the same analgesic effects that was previously achieved at a lower dose (see Community Focus box).Tolerance may develop after 10 to 21 days of morphine administration. Treatment of tolerance involves increasing the dose or decreasing the duration between doses. Treatment of physical dependence involves gradually reducing the dose over several days to prevent withdrawal symptoms. The following are guidelines for treating physical dependence from morphine (Max, Byas-Smith, Gracely, and others, 1995):
 Gradually reduce dose (similar to tapering of steroids).
Gradually reduce dose (similar to tapering of steroids).
 Give one half of previous daily dose every 6 hours for first 2 days.
Give one half of previous daily dose every 6 hours for first 2 days.
 Then reduce dose by 25% every 2 days. Continue this schedule until total daily dose of 0.6 mg/kg/day of morphine (or equivalent) is reached. After 2 days on this dose, discontinue opioid.
Then reduce dose by 25% every 2 days. Continue this schedule until total daily dose of 0.6 mg/kg/day of morphine (or equivalent) is reached. After 2 days on this dose, discontinue opioid.
 A switch to oral methadone may also be done, using one fourth of equianalgesic dose as initial weaning dose and proceeding as described above.
A switch to oral methadone may also be done, using one fourth of equianalgesic dose as initial weaning dose and proceeding as described above.
Parents and older children may fear addiction when opioids are prescribed. The nurse should address these concerns with assurance that any such risk is extremely low. It may be helpful to ask the question, “If you did not have this pain, would you want to take this medicine?” The answer is invariably no, which reinforces the solely therapeutic nature of the drug. It is also important to avoid making statements to the family such as “We don’t want you to get used to this medicine,” or “By now you shouldn’t need this medicine,” which may reinforce the fear of becoming addicted. Whereas both physical dependence and tolerance are physiologic states, addiction or psychologic dependence is a psychologic state and implies a “cause-effect” mode of thinking, such as “I need the drug because it makes me feel better.” Infants and children do not have the cognitive ability to make the cause-effect association and therefore cannot become addicted. The use of opioid analgesics early in life has not been demonstrated to increase the risk for addiction later in life. Nurses need to explain to parents the differences among physical dependence, tolerance, and addiction and allow parents to express concerns about the use and duration of use of opioids. Infants and children, when treated appropriately with opioids, may be at risk for physical tolerance and physical dependence, but not psychologic dependence or addiction (Turner, 2005; Greco and Berde, 2005).
Evaluation of Effectiveness of Pain Regimen
The effectiveness of analgesics can be enhanced by a supportive attitude toward the child. By reinforcing the cause and effect of the medication and analgesia, the nurse can condition the child to expect pain relief, provided the regimen is likely to be effective. A pain relief scale or periodic ratings of pain intensity should be used for evaluation of effectiveness of pain regimens.
The response to therapy should be evaluated 15 to 30 minutes after each dose, and titration should continue to the highest achievable amount of relief (Max, Byas-Smith, Gracely, and others, 1995). In a retrospective study that examined the pain experience of children with sickle cell disease, evidence of pain relief from medications was documented for less than half (44.8%) of the patients in the emergency department (ED) (Jacob and Mueller, 2006). Even though The Joint Commission required documentation of pain assessments with vital signs, evidence of pain relief was not documented in 41.4% of the episodes. Titration methods in the ED or during the course of hospitalization, if used, were not reflected in the amount of medications received by the children (Jacob, Miaskowski, Savedra, and others, 2003a; 2003b).
Several harmful effects occur with unrelieved pain, particularly when pain is prolonged. A number of physiologic stress responses in the body are triggered during pain, and they lead to negative consequences that involve multiple systems. Unrelieved pain may prolong the stress response and adversely affect an infant’s or child’s recovery, whether it is from trauma, surgery, or disease. In a landmark study by Anand and Hickey (1992), 30 neonates received deep intraoperative anesthesia with high doses of the opioid sufentanil, followed postoperatively by an infusion of opioids for 24 hours, and 15 neonates received lighter anesthesia with halothane and morphine followed postoperatively by intermittent morphine and diazepam. The 15 neonates who received the lighter anesthesia and intermittent postoperative opioids had more severe hyperglycemia and lactic acidemia, and four postoperative deaths occurred in the group. The 30 neonates who received deep anesthesia had a lower incidence of complications (sepsis, metabolic acidosis, disseminated intravascular coagulation) and no deaths.
Poorly controlled acute pain can predispose patients to chronic pain syndromes. A guiding principle in pain management is that prevention of pain is always better than treatment (Benjamin, Swinson, and Nagel, 2000). Pain that is established and severe is often more difficult to control. When pain is unrelieved, sensory input from injured tissues reaches spinal cord neurons and may enhance subsequent responses. Long-lasting changes in cells within spinal cord pain pathways may occur after a brief painful stimulus and may lead to the development of chronic pain conditions. Basbaum (1999a, 1999b) reported a series of studies that emphasize a distinct neurochemistry of acute and persistent pain and concluded that persistent pain is not merely a prolonged acute pain symptom of some other disease. Underlying physiologic mechanisms lead to the persistence of pain (Marx, 2004; Woolf and Salter, 2000).
In a study of nursing practice related to pain assessment and management in different pediatric specialty units (Jacob and Puntillo, 2000), complaints of pain were noted, but specific pain scores or notations about responses to analgesics after administration were seldom documented. Pain scores were not available before and after analgesics, and it was therefore not possible to conclude whether analgesics were effective. Nurses therefore need to evaluate and monitor pain in a timely fashion after administration of analgesics; titrate dosage to effect; or make recommendations for an alternate analgesic, for addition of another analgesic, or for a combination of analgesics, adjuvants, and nonpharmacologic strategies.
PAINFUL AND INVASIVE PROCEDURES
Several painful and invasive procedures require the administration of anesthetics and analgesics. For circumcision pain, caudal or penile blocks are employed before the procedure. Parents are then instructed how to apply lidocaine gels for the first 24 to 36 hours after the circumcision. For open wounds, bupivacaine may be instilled with or without epinephrine onto the dressing applied to skin to minimize pain for up to 48 hours after the procedure. For graft donor sites, analgesia is maintained by using a foam dressing soaked with bupivacaine (0.25%, 2 mg/kg; 0.8 ml/kg) applied to the donor surface.A continuous infusion of 0.25% bupivacaine at 1 to 3 ml/hr via a standard 18-gauge epidural catheter is then curled on the outer or inner surface of the foam (Cousins and Power, 2003). Wound perfusion of bupivacaine is useful for iliac crest bone graft donor sites (used for alveolar bone grafting in some techniques of cleft palate repair). A standard 18-gauge epidural catheter is also used with a very low infusion rate (1 to 3 ml/hr) of bupivacaine. For minor and some intermediate procedures, the local anesthetic infiltration with bupivacaine is commonly used. Some examples of these procedures include surface wounds and tunneling procedures in the anesthetized child requiring inguinal surgery; insertion of ventriculoperitoneal shunts, central venous lines, or central venous catheter-reservoir systems; and similar procedures.
Nitrous oxide, which is taken up rapidly and eliminated by the lungs, is highly insoluble in the blood and is delivered quickly to the brain to produce an analgesic effect equivalent to that of IV morphine. After approximately 2 minutes of inhalation, maximum pain relief can be achieved. The child breathes via face mask, nasal mask, or mouthpiece. It is not suitable for children younger than 3 years old and works best for children older than 5 years of age. Nitrous oxide inhalations are used frequently for a wide variety of procedures that require potent analgesia for a short time, such as suture insertion or removal, dressing removal or changes (including burns), drain or catheter removal, venipuncture or cannulation, lumbar puncture, physical therapy, and biopsies (skin, muscle, renal, or bone marrow).However, in children with pneumothorax, bowel obstruction, abnormal airway, recent head injury (especially with an intracranial air pocket), chronic respiratory disease with air trapping or bullous changes in the lung, and some types of uncorrected congenital heart disease (such as pulmonary hypertension), the use of nitrous oxide is contraindicated. Nitrous oxide may lead to expansion of air pockets in confined spaces (chest, cranial cavities, bowel lumens) and create an increased pressure and tension effect. Tension pneumothorax, ischemia or shift of intracranial contents, and bowel distention with risk of perforation could occur. In addition, because nitrous oxide produces a degree of sedation and potentiates the sedative effects of other central nervous system depressants, caution is required when concurrently giving opioids, benzodiazepines, antihistamines, and similar drugs. Inspired concentrations of up to 50% nitrous oxide used for less than 30 minutes do not affect airway reflexes. Only trained personnel may administer nitrous oxide and monitor the child(Cousins and Power, 2003).
POSTOPERATIVE PAIN
Surgery and traumatic injuries (e.g., fractures, dislocations, strains, sprains, lacerations, burns) generate acatabolic state as a result of increased secretion of catabolic hormones. Consequently, alterations in blood flow, coagulation, fibrinolysis, substrate metabolism, and water and electrolyte balance can occur and increase the demands on the cardiovascular and respiratory systems (Cousins and Power, 2003). The major endocrine and metabolic changes are elicited during the first48 hours after surgery or trauma. Local anesthetics and opioid neural blockade may effectively modify the responses to surgical injury and mitigate the physiologic response.
Pain associated with surgery to the chest (e.g., repair of congenital heart defects, chest trauma) or abdominal regions (e.g., appendectomy, cholecystectomy, splenectomy) may result in pulmonary complications.Pain leads to decreased muscle movement in the thorax and abdominal area and leads to decreased tidal volume, vital capacity, functional residual capacity, and alveolar ventilation. The patient is unable to cough and clear secretions, and the risk for complications such as pneumonia and atelectasis is high. Severe postoperative pain also results in sympathetic overactivity, which leads to increases in heart rate, peripheral resistance, blood pressure, and cardiac output. The patient eventually experiences an increase in cardiac demand and myocardial oxygen consumption and a decrease in oxygen delivery to the tissues.
The basis for good postoperative pain control in children is preemptive analgesia.Preemptive analgesia involves administration of medications (e.g., local and regional anesthetics, analgesics) before the child experience the pain or before surgery is performed so that the sensory activation and changes in the pain pathways of the peripheral and central nervous system can be controlled. Preemptive analgesia has been demonstrated to lower postoperative pain, lower analgesic requirement, lower hospital stay, lower complications after surgery, and minimize the risks for peripheral and central nervous system sensitization that can lead to persistent pain (Cousins and Power, 2003).
A combination of medications(multimodal or balanced analgesia) is used for postoperative pain and may include NSAIDs, local anesthetics, nonopioids, and opioid analgesics to achieve optimum relief and minimize side effects. Opioids (see Table 7-5) administered ATC during the first 48 hours or administered via PCA (see Table 7-9) are commonly prescribed postoperatively. The duration of use is frequently limited to days, since the cause of pain usually resolves. The combination of the IV NSAID ketorolac and morphine using a PCA device is frequently prescribed after thoracic surgery. Morphine delivered by PCA leads to a lower total dosage of opioid analgesia when compared with the administration of intermittent doses of analgesic as required. After bowel surgery, a mixture of local anesthetics (bupivacaine) and a low-dose opioid(fentanyl) delivered by epidural route improvesthe rate of recovery and minimizes the gastrointestinal effects (e.g., bowel stasis, nausea, vomiting). Once bowel function has been restored, oral opioids such as immediate release and controlled release preparations are preferred in older children. Controlled release opioids facilitate ATC dosing and improve sleep. They are also associated with lower incidence of nausea, sedation, and breakthrough pain.
RECURRENT HEADACHES IN CHILDREN
Recurrent headaches in children can be caused by several factors, including tension, dental braces, and imbalance or weakness of eye muscles causing deviation in alignment and refractive errors. Headaches may be the sequelae to accidents, sinusitis and other cranial infection or inflammation, increased intracranial pressure, epileptic attacks, drugs, obstructive sleep apnea, and rarely hypertension. Other causes may include arteriovenous malformations, disturbances in cerebrospinal fluid flow or absorption, intracranial hemorrhages, ocular and dental diseases, bacterial infections, and brain tumors.Severe pain is the most disturbing symptom in migraine. Tension-type headache is usually mild or moderate, often producing a pressing feeling in the temples, like a “tight band around the head.” Continuous, daily, or near-daily headache with no specific cause occurs in a small subgroup of children. In epilepsy, headaches commonly occur before, during, or after a seizure attack.
Treatment of recurrent headaches requires an understanding of the antecedents and consequences of headache pain. A headache diary can allow the child to record the time of onset, activities before the onset, any worries or concerns as far back as 24 hours before the onset, severity and duration of pain, pain medications taken, and activity pattern during headache episodes. The headache diary allows ongoing monitoring of headache activity, indicates the effects of interventions, and guides treatment planning.
There are two main approaches to prevention of headache using behavioral approaches:
 Teaching patients self-control skills to prevent headache (e.g., biofeedback techniques and relaxation training)
Teaching patients self-control skills to prevent headache (e.g., biofeedback techniques and relaxation training)
 Modifying behavior patterns that increase the risk of headache occurrence or reinforce headache activity (e.g., cognitive-behavioral stress management techniques)
Modifying behavior patterns that increase the risk of headache occurrence or reinforce headache activity (e.g., cognitive-behavioral stress management techniques)
Biofeedback is atechnology-based form of relaxation therapy and can be useful in assessing and reinforcing learning of relaxation skills such as progressive muscle relaxation, deep breathing, and imagery. Children as young as 7 years of age have been taught these skills and with 2 to 3 weeks of practice are able to decrease the time needed to achieve relaxation.
To modify behavior patterns that increase the risk of headache occurrence or reinforce headache activity, the nurse instructs parents to avoid giving excessive attention to their child’s headache and to respond matter-of-factly to pain behavior and requests for special attention (Holden, Deichmann, and Levy, 1999). Parents are taught to assess whether school or social performance demands are being avoided because of headache. Parents are taught to focus attention on adaptive coping such as the use of relaxation techniques and maintenance of normal activity patterns. When using cognitive-behavioral stress management techniques, the parents identify negative thoughts and situations that may be associated with increased risk for headache. The child is then taught to activate positive thoughts and engage in adaptive behavior appropriate to the situation.
RECURRENT ABDOMINAL PAIN IN CHILDREN
Recurrent abdominal pain (RAP) or functional abdominal pain is defined as pain that occurs at least once per month for 3 consecutive months, accompanied by pain-free periods, and is severe enough that it interferes with a child’s normal activities. Management of RAP is highly individualized to reflect the causes of the pain and the psychosocial needs of the child and family. A clear understanding of the child’s characteristics (anxiety, physical health, temperament, coping skills, experience, learned response, depression), child’s disability (school attendance, activities with family, social interactions, pain behaviors), environmental factors (family attitudes and behavioral patterns, school environment, community, friendships), and the pain stimulus (disease, injury, stress) is important in planning management strategies (Collins and Weisman, 2003).
Before any workup of the pain, the nurse informs the family that RAP is common in children and only 10% of children with RAP have an identifiable organic cause for their pain symptom. Medical workup is dictated by the child’s symptoms and signs in combination with knowledge about common organic causes of RAP. If an organic cause is found, it will be treated appropriately. Even if no organic cause is found, the nurse needs to communicate to the child and family a belief that the pain is real. Usually the abdominal pain goes away, but even if problems are identified, they may not be the actual cause and pain may persist, may be replaced by another symptom, or may go away on its own. The management plan includes regular follow-up at a 3- to 4-month intervals, a list of symptoms that call for earlier contact, and biobehavioral pain management techniques. The goal is to minimize the impact the pain has on the child’s activities and the family’s life (Collins and Weisman, 2003).
Case reports have demonstrated the effectiveness of implementing a time-out procedure, token systems, and positive reinforcement based on operant theory treatment modalities. Stress management and cognitive-behavioral strategies have also been reported to be successful. Parent training in how to avoid positive reinforcement of sick behaviors and focus on rewarding healthy behaviors is important. Over the course of several sessions, parents are educated about RAP, how to distinguish between sick and well behaviors, a reward system for well behaviors, and the importance of reinforcing relaxation and stressing coping skills taught to children for pain management. Treatment may consist of a varying number of sessions over 1 to 6 months and may include various components such as monitoring of symptoms, limited parent attention, relaxation training, increased dietary fiber, and requirements of school attendance. Response rates are 25% without abdominal pain and 56% to 75% improvements in symptoms (Collins and Weisman, 2003).
The use of cognitive-behavioral therapy has been documented to reduce or eliminate pain in children with RAP and highlights the involvement of parents in supporting their child’s self-management behavior. No negative side effects of symptom substitution occurred with the interventions. The combination of self-regulation and cognitive-behavioral interventions along with fiber intervention is more effective fortreating RAP than using fiber alone (Weydert, Ball, and Davis, 2003).
Famotidine is an H2-receptor antagonist that may be given twice daily (0.5 mg/kg/dose). It was demonstrated to improve pain in 68% of children and decreased the peptic index score. The peptic index score is a composite score for nausea, vomiting, appetite, epigastric pain, weight loss, and nocturnal awakening. Pizotifen is a serotonin antagonist that was tested in 14 children given at 5 ml (0.25 mg) twice daily for 1 month. Patients who received pizotifen had fewer days of abdominal pain, a lower index of severity, and a lower index of misery when compared with those receiving a placebo. The only side effects noted were slight drowsiness and slight weight gain. Pizotifen is available worldwide but not approved for use in the United States (Symon and Russell, 1995).
CANCER PAIN IN CHILDREN
Pain in children with cancer was reported to be the most prevalent symptom (84.4%) and was rated to be moderate to severe (86.6%) and highly distressing (52.8%) (Collins, Byrnes, Dunkel, and others, 2000). Pain may be related to an operation; mucositis; a phantom limb; infection; chemotherapy; and procedures such as bone marrow aspiration, needle puncture, or lumbar puncture. Tumor-related pain frequently occurs when the child relapses or when tumors become resistant to treatment. Intractable pain may occur in patients with solid tumors that metastasize to the central or peripheral nervous system. In young adult survivors of childhood cancer, chronic pain conditions may develop, including complex regional pain syndrome of the lower extremity, phantom limb pain, avascular necrosis, mechanical pain related to bone that failed to unite after tumor resection, and postherpetic neuralgia.
Other treatment-related pain includes (1) abdominal pain after allogeneic bone marrow transplantation, which may be associated with acute graft-versus-host disease; (2) abdominal pain associated with typhlitis (infection of the cecum), which occurs when the patient is immunocompromised; (3) phantom sensations and phantom limb pain after an amputation; (4) peripheral neuropathy after administration of vincristine; and (5) medullary bone pain, which may be associated with administration of granulocyte colony—stimulating factor (Collins and Weisman, 2003).
Almost 40% of all pain episodes in children with cancer may be attributed to procedures (Ljungman, Gordh, Sorensen, and others, 2001, 2000, 1999; Ljungman, Kreuger, Andreasson, and others, 2000). Survivors of childhood cancer describe vivid memories of their experience with repeated painful procedures during treatment. These procedures include needle puncture for IM chemotherapy (l-asparaginase), IV lines, port access and blood draws, lumbar puncture, bone marrow aspiration and biopsy, removal of central venous catheters, and other invasive diagnostic procedures. Fear and anxiety related to these procedures may be minimized with parent and child preparation. The preparation starts with obtaining information from the parent about the child’s coping styles, explaining the procedure, and enlisting their support. Topical analgesics (e.g., cold sprays, EMLA, amethocaine gels), as discussed previously, have been effective in providing analgesia before needle procedures.
Lumbar puncture for administration of chemotherapy (e.g., cytarabine, methotrexate) and collection of cerebrospinal fluid may lead to a leak at the puncture site and low intracranial pressure (Collins and Weisman, 2003). Some children may experience postdural puncture headache, which may be treated by administering nonopioid analgesics and placing the patient in the supine position for 1 hour after the procedure. The pain related to bone marrow aspiration is due to the insertion of a large needle into the posterior iliac space and the unpleasant sensation experienced at the time of marrow aspiration. Cognitive-behavioral therapy (e.g., guided imagery, relaxation, music therapy, hypnosis), conscious sedation, and general anesthesia have been proven effective in decreasing pain and distress during the procedure.
If the patient is neutropenic (absolute neutrophil count, 500/mm3), the antipyretic action of acetaminophen may mask a fever. In patients with thrombocytopenia (platelet count, 50,000/mm3), who may be at risk for bleeding, NSAIDs are contraindicated. Morphine is the most widely used opioid for moderate to severe pain and may be administered via the oral (including sustained release formulations such as MS Contin and Kadian), IV, subcutaneous, epidural, and intrathecal routes. When dose-limiting side effects of morphine develop, hydromorphone has been reported to be effective in several studies of children with cancer (Drake, Longworth, and Collins, 2004). In children and adolescents with mucositis after bone marrow transplantation, hydromorphone was well tolerated and had a potency ratio of approximately 6:1 relative to morphine (Drake, Longworth, and Collins, 2004).
The most common clinical syndrome of neuropathic pain is painful peripheral neuropathy caused by chemotherapeutic agents, particularly vincristine and cisplatin, and rarely cytarabine (Collins and Weisman, 2003). Withdrawal of the chemotherapy may resolve the neuropathy over weeks to months, or it may persist even after withdrawal. Neuropathic pain is associated with at least one of the following: (1) pain that is described as electric or shocklike, stabbing, or burning; (2) signs of neurologic involvement (e.g., paralysis, neuralgia, pain hypersensitivity) other than those associated with the progression of the tumor; and (3) the location of the solid organ cancer consistent with neurologic damage that could give rise to neuropathic pain. Dying children with cancer who experience neuropathic pain have higher baseline requirements of morphine and require rapid increases of morphine than dying children without neuropathic pain (Dougherty and DeBaun, 2003). Children with neuropathic pain often require massive opioid infusion (>3 mg/kg/hr of IV morphine dose equivalent, or approximately 100-fold greater than standard starting infusion rates). An epidural or subarachnoid infusion may be initiated if the patient experiences dose-limiting side effects of opioids or if pain was resistant to opioids.
Tricyclic antidepressants (amitriptyline, desipramine) and anticonvulsants (gabapentin, carbamazepine) have demonstrated effectiveness in neuropathic cancer pain. Randomized controlled trials showed that 60% to 70% of patients with neuropathic pain achieve relief with tricyclic antidepressants (Sindrup, Otto, Finnerup, and others, 2005). The tricyclic antidepressants have many actions that could be involved in their pain-relieving effect and have been considered the mainstay of therapy for neuropathic pain (Sindrup, Otto, Finnerup, and others, 2005).
Klepstad, Borchgrevink, Hval, and others (2001) reported the pain experience of a 12-year-old girl with severe neuropathicpain caused by a cervical spinal tumor. Two weeks after resection of the tumor, the child experienced increased pain in her neck, which was superficial and distributed in the dermatomes below the cervical medullary lesion. Pain was provoked by touch and did not decrease in intensity despite a subcutaneous infusion of morphine at 160 mg/24 hr. The child screamed from increased pain when her parents or siblings tried to comfort her with bodily contact. Pain was relieved after administration of 7.5 to 10 mg IV ketamine. Ketamine is an N-methyl-d-aspartate (NMDA) antagonist, which has undesirable side effects (e.g., sedation, nausea, dissociative reactions, muteness, dizziness, and visual distortions) and short duration of action (Sang, 2000). After administration of ketamine, the child was able to tolerate touch without pain paroxysms. A continuous IV infusion was eventually initiated for convenience, and benzodiazepines were added to avoid the psychomimetic effects associated with ketamine.
Although ketamine is frequently used to ensure analgesia and sedation during painful procedures in children, the long-term use of ketamine for the treatment of neuropathic pain in children has not been systematically studied and is not of clinical benefit for all patients (Klepstad, Borchgrevink, Hval, and others, 2001). In randomized studies of patients with chronic neuropathic pain, only some patients had a beneficial response to ketamine (Haines and Gaines, 1999; Max, Byas-Smith, Gracely, and others, 1995; Mitchell, 2001).
PAIN AND SEDATION IN END-OF-LIFE CARE
Many patients require doses of opioids that make them sedated but arousable as their disease progresses (e.g., cancer, human immunodeficiency virus, cystic fibrosis, neurodegenerative disease) at the end of life. Comfort can be achieved with a combination of opioids and adjuvant analgesics in most situations. Parents need reassurance that the opioids are treating pain but not causing the child’s death and the child’s advancing disease is the cause of death.
A small group of patients have intolerable side effects or inadequate analgesia despite extremely aggressive use of medications to relieve pain and side effects. Continuous sedation may be a means of relieving suffering when there is no feasible or acceptable means of providing analgesia that preserves alertness. A continuing high-dose infusion of opioids along with sedation is prescribed to reduce the possibility that a child might experience unrelieved pain but be too sedated to report it. Sedation in these situations is widely regarded as providing comfort, not euthanasia. Clinicians and ethicists have a range of views regarding assisted suicide and euthanasia, but they all agree that no child or parent should choose death because of inadequate efforts to relieve pain and suffering (Berde and Collins, 2003).
REFERENCES
Abdelkefi, A, Abdennebi, YB, Mellouli, F, et al. Effectiveness of fixed 50% nitrous oxide oxygen mixture and EMLA cream for insertion of central venous catheters in children. Pediatr Blood Cancer. 2004;43(7):777–779.
Algren, JT, Gursoy, F, Johnson, TD, et al. The effect of nitrous oxide diffusion on laryngeal mask airway cuff inflation in children. Paediatr Anaesth. 1998;8(1):31–36.
Anand, KJ, Hickey, PR. Halothane-morphine compared with high-dose sufentanil for anesthesia and postoperative analgesia in neonatal cardiac surgery. N Engl J Med. 1992;326(1):1–9.
American Pain Society. Principles of analgesic use in the treatment of acute pain and chronic cancer pain, ed 4. Glenview, Ill: The Society, 1999.
Barrier, G, Attia, J, Mayer, MN, et al. Measurement of postoperative pain and narcotic administration in infants using a new clinical scoring system. Anesthesiology. 1987;67(3A):A532.
Basbaum, AI. Distinct neurochemical features of acute and persistent pain. Proc Natl Acad Sci USA. 1999;96(14):7739–7743.
Basbaum, AI. Spinal mechanisms of acute and persistent pain. Reg Anesth Pain Med. 1999;24(1):59–67.
Benjamin, L, Swinson, G, Nagel, R. Sickle cell anemia day hospital: an approach for the management of uncomplicated painful crises. Blood. 2000;95:1130–1137.
Berde, C, Collins, J. Cancer pain and palliative care in children. In: Melzack R, Wall P, eds. Handbook of pain management. St Louis: Churchill Livingstone, 2003.
Bernstein, B, Pachter, L. Cultural considerations in children’s pain. In: Schechter N, Berde C, Yaster M, eds. Pain in infants, children, and adolescents. Philadelphia: Lippincott Williams & Wilkins, 2003.
Beyer, JE, Denyes, MJ, Villarruel, AM, The creation, validation and continuing development of the Oucher: a measure of pain intensity in children. J Pediatr Nurs 1992;7(5):335–346. http://www.oucher.org [retrieved May 3, 2007, from].
Beyer, JE, Knott, CB. Construct validity estimation for the African-American and Hispanic versions of the Oucher scale. J Pediatr Nurs. 1998;13(1):20–31.
Bishai, R, Taddio, A, Bar-Oz, B, et al. Relative efficacy of amethocaine gel and lidocaine-prilocaine cream for port-a-cath puncture in children. Pediatrics. 1999;104(3):e31.
Blauer, T, Gerstmann, D. A simultaneous comparison of three neonatal pain scales during common NICU procedures. Clin J Pain. 1998;14(1):39–47.
Breau, LM, MacLaren, J, McGrath, PJ, et al. Caregivers’ beliefs regarding pain in children with cognitive impairment: relation between pain sensation and reaction increases with severity of impairment. Clin J Pain. 2003;19(6):335–344.
Breau, LM, McGrath, PJ, Camfield, CS, et al. Psychometric properties of the Non-communicating Children’s Pain Checklist–Revised. Pain. 2002;99:349–357.
Chambers, CT, Giesbrecht, K, Craig, KD, et al. A comparison of faces scales for the measurement of pediatric pain: children’s and parents’ ratings. Pain. 1999;83:25–35.
Chambers, CT, Hardial, J, Craig, KD, et al. Faces scales for the measurement of postoperative pain intensity in children following minor surgery. Clin J Pain. 2005;21(3):277–285.
Chambers, CT, Reid, GJ, McGrath, PJ, et al. Development and preliminary validation of a postoperative pain measure for parents. Pain. 1996;68:307–313.
Choi, WY, Irwin, MG, Hui, TW, et al. EMLA cream versus dorsal penile nerve block for postcircumcision analgesia in children. Anesth Analg. 2003;96(2):396–399.
Cline, ME, Herman, J, Shaw, ER, et al. Standardization of the visual analogue scale. Nurs Res. 1992;41(6):378–380.
Cole, J, Jorgensen, K. Medical, developmental, and pharmacologic intervention: the essence of collaboration. Neonatal Netw. 1997;16:56–58.
Collins, JJ, Byrnes, ME, Dunkel, IJ, et al. The measurement of symptoms in children with cancer. J Pain Symptom Manage. 2000;19(5):363–377.
Collins, J, Weisman, S. Management of pain in childhood cancer. In: Schechter N, Berde C, Yaster M, eds. Pain in infants, children, and adolescents. Philadelphia: Lippincott Williams & Wilkins, 2003.
Cousins, M, Power, I. Acute and postoperative pain. In: Melzack R, Wall P, eds. Handbook of pain management. St Louis: Churchill Livingstone, 2003.
Dougherty, M, DeBaun, MR. Rapid increase of morphine and benzodiazepine usage in the last 3 days of life in children with cancer is related to neuropathic pain. J Pediatr. 2003;142(4):373–376.
Drake, R, Longworth, J, Collins, JJ. Opioid rotation in children with cancer. J Palliat Med. 2004;7(3):419–422.
Egekvist, H, Bjerring, P. Effect of EMLA cream on skin thickness and subcutaneous venous diameter: a randomized, placebo-controlled study in children. Acta Dermatol Venereol. 2000;80(5):340–343.
Eland, JA, Banner, W. Analgesia, sedation, and neuromuscular blockage in pediatric critical care. In: Hazinski ME, ed. Manual of pediatric critical care. St Louis: Mosby, 1999.
Fearon, I, Kisilevsky, BS, Hains, SM, et al. Swaddling after heel lance: age-specific effects on behavioral recovery in preterm infants. Develop Behav Pediatr. 1997;18:222–232.
Franck, L, Vilardi, J. Assessment and management of opioid withdrawal in ill neonates. Neonatal Netw. 1995;14(2):39–48.
Franck, LS, Vilardi, J, Durand, D, et al. Opioid withdrawal in neonates after continuous infusions of morphine or fentanyl during extracorporeal membrane oxygenation. Am J Crit Care. 1998;7(5):364–369.
Gad, LN, Olsen, KS, Lysgaard, AB, et al. Optimized use of EMLA cream in children—secondary publication: a randomized, prospective, controlled comparison of two application regimes. Ugeskr Laeger. 2005;167(4):404–407.
Gerik, SM. Pain management in children: developmental considerations and mind-body therapies. South Med J. 2005;98(3):295–302.
Gold, JI, Kim, SH, Kant, AJ, et al. Effectiveness of virtual reality for pediatric pain distraction during i.v. placement. Cyberpsychol Behav. 2006;9(2):207–212.
Goldschneider, K, Anand, K. Long-term consequences of pain in neonates. In: Schechter N, Berde C, Yaster M, eds. Pain in infants, children, and adolescents. Philadelphia: Lippincott Williams & Wilkins, 2003.
Gray, L, Watt, L, Blass, E. Skin-to-skin contact is analgesic in healthy newborns. Pediatrics. 2000;105(1):110–111.
Greco, C, Berde, C. Pain management for the hospitalized pediatric patient. Pediatr Clin North Am. 2005;52(4):995–1027.
Hadden, KL, von Baeyer, CL. Pain in children with cerebral palsy: common triggers and expressive behaviors. Pain. 2002;99(1-2):281–288.
Hadjistavropoulos, HD, Craig, KD, Grunau, RE, et al. Judging pain in infants: behavioural, contextual, and developmental determinants. Pain. 1997;73(3):319–324.
Haines, DR, Gaines, SP. N of 1 randomised controlled trials of oral ketamine in patients with chronic pain. Pain. 1999;83(2):283–287.
Hannallah, RS, Broadman, LM, Belman, AB, et al. Comparison of caudal and ilioinguinal/iliohypogastric nerve blocks for control of post-orchiopexy pain in pediatric ambulatory surgery. Anesthesiology. 1987;66:832–834.
Hester, NO, Foster, RL, Jordan-Marsh, M, et al, Putting pain measurement into clinical practice. Finley, GA, McGrath, PJ, eds. Measurement of pain in infants and children, vol 10. Seattle: IASP Press, 1998.
Hodgkinson, K, Bear, M, Thorn, J, et al. Measuring pain in neonates: evaluating an instrument and developing a common language. Aust J Adv Nurs. 1994;12(1):17–22.
Holden, E, Deichmann, M, Levy, J. Empirically supported treatments in pediatric psychology: recurrent pediatric headache. J Pediatr Psychol. 1999;24:91–100.
Jacob, E, Miaskowski, C, Savedra, M, et al. Changes in intensity, location, and quality of vaso-occlusive pain in children with sickle cell disease. Pain. 2003;102(1-2):187–193.
Jacob, E, Miaskowski, C, Savedra, M, et al. Management of vaso-occlusive pain in hospitalized children with sickle cell disease. J Pediatr Hematol Oncol. 2003;25(4):307–311.
Jacob, E, Mueller, BU, Pain experience of children with sickle cell disease who had prolonged hospitalizations for acute painful episodes. Pain Med 2006 10.1111/j.1526-4637.2006.00252.x [(Online Early Articles).].
Jacob, E, Puntillo, KA. Variability of analgesic practices for hospitalized children on different pediatric specialty units. J Pain Symptom Manage. 2000;20(1):59–67.
Johnston, CC, Stevens, B, Pinelli, J, et al. Kangaroo care is effective in diminishing pain response in preterm neonates. Arch Pediatr Adolesc Med. 2003;157(11):1084–1088.
Jordan-Marsh, M, Yoder, L, Hall, D, et al. Alternate Oucher form testing: gender ethnicity, and age variations. Res Nurs Health. 1994;17:111–118.
Joyce, BA, Schade, JG, Keck, JF, et al. Reliability and validity of preverbal pain assessment tools. Issues Comp Pediatr Nurs. 1994;17:121–135.
Kain, ZN, Mayes, LC, Caldwell-Andrews, AA, et al. Preoperative anxiety, postoperative pain, and behavioral recovery in young children undergoing surgery. Pediatrics. 2006;118(2):651–658.
Klepstad, P, Borchgrevink, P, Hval, B, et al. Long-term treatment with ketamine in a 12-year-old girl with severe neuropathic pain caused by a cervical spinal tumor. J Pediatr Hematol Oncol. 2001;23(9):616–619.
Krechel, SW, Bildner, J. CRIES: a new neonatal postoperative pain measurement score: initial testing of validity and reliability. Pediatr Anaesth. 1995;5:53–61.
Lawrence, J, Alcock, D, McGrath, P, et al. The development of a tool to assess neonatal pain. Neonatal Netw. 1993;12(6):59–66.
Lenton, S, Stallard, P, Lewis, M, et al. Prevalence and morbidity associated with non-malignant, life-threatening conditions in childhood. Child Care Health Devel. 2001;27(5):389–398.
Ljungman, G, Gordh, T, Sorensen, S, et al. Lumbar puncture in pediatric oncology: conscious sedation vs. general anesthesia. Med Pediatr Oncol. 2001;36(3):372–379.
Ljungman, G, Gordh, T, Sorensen, S, et al. Pain variations during cancer treatment in children: a descriptive survey. Pediatr Hematol Oncol. 2000;17(3):211–221.
Ljungman, G, Gordh, T, Sorensen, S, et al. Pain in paediatric oncology: interviews with children, adolescents and their parents. Acta Paediatr. 1999;88(6):623–630.
Ljungman, G, Kreuger, A, Andreasson, S, et al. Midazolam nasal spray reduces procedural anxiety in children. Pediatrics. 2000;105(1 Pt 1):73–78.
Luffy, R, Grove, SK. Examining the validity, reliability, and preference of three pediatric pain measurement tools in African-American children. Pediatr Nurs. 2003;29(1):54–60.
Malviya, S, Voepel-Lewis, T, Burke, C, et al. The revised FLACC observational pain tool: improved reliability and validity for pain assessment in children with cognitive impairment. Paediatr Anaesth. 2006;16(3):258–265.
Manworren, R, Hynan, L. Clinical validation of FLACC: Preverbal Patient Pain Scale. Pediatr Nurs. 2003;29(2):140–146.
Marx, J. Pain research: prolonging the agony. Science. 2004;305(5682):326–329.
Max, MB, Byas-Smith, MG, Gracely, RH, et al. Intravenous infusion of the NMDA antagonist, ketamine, in chronic posttraumatic pain with allodynia: a double-blind comparison to alfentanil and placebo. Clin Neuropharmacol. 1995;18(4):360–368.
Maxwell, L, Yaster, M. Perioperative management issues in pediatric patients. Anesthesiol Clin North Am. 2000;18(3):601–632.
McCaffery, M, Pasero, C. Pain clinical manual. St Louis: Mosby, 1999.
McGrath, P. Behavioral measures of pain. In: Finley G, McGrath P, eds. Measurement of pain in infants and children. Seattle: IASP Press, 1998.
McGrath P, Hillier L, eds. Modifying the psychologic factors that intensify children’s pain and prolong disability. Philadelphia: Lippincott Williams & Wilkins, 2003.
McGrath, PJ, Johnson, G, Goodman, JT, et al. The CHEOPS: a behavioral scale to measure postoperative pain in children. In: Fields H, Dubner R, Cervero F, eds. Advances in pain research and therapy. New York: Raven Press, 1985.
Melzack, R. The McGill pain questionnaire: major properties and scoring methods. Pain. 1975;1:277–299.
Merkel, SI, Voepel-Lewis, T, Shayevitz, JR, et al. The FLACC: a behavioral scale for scoring postoperative pain in young children. Pediatr Nurs. 1997;23(3):293–297.
Miaskowski, C, Lee, K. Pain, fatigue, and sleep disturbances in oncology outpatients receiving radiation therapy for bone metastasis: a pilot study. J Pain Symptom Manage. 1999;17(5):320–332.
Mitchell, AC. An unusual case of chronic neuropathic pain responds to an optimum frequency of intravenous ketamine infusions. J Pain Symptom Manage. 2001;21(5):443–446.
Morin, C, Gibson, D, Wade, J. Self-reported sleep and mood disturbance in chronic pain patients. Clin J Pain. 1998;14(4):311–314.
Myers, C, Stuber, ML, Bonamer-Rheingans, JI, et al. Complementary therapies and childhood cancer. Cancer Control. 2005;12(3):172–180.
Nadvi, SZ, Sarnaik, S, Ravindranath, Y. Low frequency of meperidine-associated seizures in sickle cell disease. Clin Pediatr (Phila). 1999;38(8):459–462.
Puchalski, M, Hummel, P. The reality of neonatal pain. Adv Neonatal Care. 2002;2(5):233–244.
Reis, E, Holubkov, R. Vapocoolant spray is equally effective as EMLA cream in reducing immunization pain in school-aged children. Pediatrics. 1997;100(6):E5.
Robieux, I, Kumar, R, Radhakrishnan, S, et al. Assessing pain and analgesia with a lidocaine-prilocaine emulsion in infants and toddlers during venipuncture. J Pediatr. 1991;118(6):971–973.
Rogers, TL, Ostrow, CL. The use of EMLA cream to decrease venipuncture pain in children. J Pediatr Nurs. 2004;19(1):33–39.
Rusy, L, Weisman, S. Complementary therapies for acute pediatric pain management. Pediatr Clin North Am. 2000;47(3):589–599.
Sang, CN. NMDA-receptor antagonists in neuropathic pain: experimental methods to clinical trials. J Pain Symptom Manage. 2000;19(1 Suppl):S21–S25.
Santiago, A, Abad, P, Fernandez, C, et al. Premedication with EMLA cream for ambulatory surgery in children. Ambul Surg. 2000;8(3):157.
Savedra, MC, Holzemer, WL, Tesler, MD, et al. Assessment of postoperation pain in children and adolescents using the adolescent pediatric pain tool. Nurs Res. 1993;42(1):5–9.
Savedra, MC, Tesler, MD, Holzemer, WL, et al. Pain location: validity and reliability of body outline markings by hospitalized children and adolescents. Res Nurs Health. 1989;12:307–314.
Schade, JG, Joyce, BA, Gerkensmeyer, J, et al. Comparison of three preverbal scales for postoperative pain assessment in a diverse pediatric sample. J Pain Symptom Manage. 1996;12(6):348–359.
Sindrup, SH, Otto, M, Finnerup, NB, et al. Antidepressants in the treatment of neuropathic pain. Basic Clin Pharmacol Toxicol. 2005;96(6):399–409.
Squire, SJ, Kirchhoff, KT, Hissong, K. Comparing two methods of topical anesthesia used before intravenous cannulation in pediatric patients. J Pediatr Health Care. 2000;14(2):68–72.
Stallard, P, Williams, L, Lenton, S, et al. Pain in cognitively impaired, non-communicating children. Arch Dis Child. 2001;85(6):460–462.
Stallard, P, Williams, L, Velleman, R, et al. The development and evaluation of the pain indicator for communicatively impaired children (PICIC). Pain. 2002;98(1-2):145–149.
Stallard, P, Williams, L, Velleman, R, et al. Intervening factors in caregivers’ assessments of pain in non-communicating children. Devel Med Child Neurol. 2002;44(3):213–214.
Stanford, EA, Chambers, CT, Craig, KD. The role of developmental factors in predicting young children’s use of a self-report scale for pain. Pain. 2006;120(1-2):16–23.
Stevens, B. Development and testing of a pediatric pain management sheet. Pediatr Nurs. 1990;16(6):543–548.
Stevens, B, Johnston, C, Petryshen, P, et al. Premature Infant Pain Profile: development and initial validation. Clin J Pain. 1996;12:13–22.
Stevens, B, Yamada, J, Ohlsson, A. Sucrose for analgesia in newborn infants undergoing painful procedures. http://www.thecochranelibrary.com. [(review), 2005. In Cochrane Neonatal Collaboration, retrieved May 4, 2007, from].
Sweet, S, McGrath, P. Physiological measures of pain. In: Finley G, McGrath P, eds. Measurement of pain in infants and children. Seattle: IASP Press, 1998.
Symon, DN, Russell, G. Double blind placebo controlled trial of pizotifen syrup in the treatment of abdominal migraine. Arch Dis Child. 1995;72(1):48–50.
Taddio, A, Nulman, I, Koren, BS, et al. A revised measure of acute pain in infants. J Pain Symptom Manage. 1995;10(6):456–463.
Tarbell, SE, Cohen, IT, Marsh, JL. The Toddler-Preschooler Postoperative Pain Scale: an observational scale for measuring postoperative pain in children aged 1-5: preliminary report. Pain. 1992;50(3):273–280.
Tesler, MD, Savedra, MC, Holzemer, WL, et al. The word-graphic rating scale as a measure of children’s and adolescents’ pain intensity. Res Nurs Health. 1991;14:361–371.
Turner, HN. Complex pain consultations in the pediatric intensive care unit. AACN Clin Issues. 2005;16(3):388–395.
Uziel, Y, Berkovitch, M, Gazarian, M, et al. Evaluation of eutectic lidocaine/prilocaine cream (EMLA) for steroid joint injection in children with juvenile rheumatoid arthritis: a double blind, randomized, placebo controlled trial. J Rheumatol. 2003;30(3):594–596.
Varni, JW, Thompson, KL, Hanson, V. The Varni/Thompson Pediatric Pain Questionnaire, part I, Chronic musculoskeletal pain in juvenile rheumatoid arthritis. Pain. 1987;28:27–38.
Villarruel, AM, Denyes, MJ. Pain assessment in children: theoretical and empirical validity. Adv Nurs Sci. 1991;14(2):32–41.
Weisman, S, Bernstein, B, Schechter, N. Consequences of inadequate analgesia during painful procedures in children. Arch Pediatr Adolesc Med. 1998;152:147–149.
Weydert, J, Ball, T, Davis, M. Systematic review of treatments for recurrent abdominal pain. Pediatrics. 2003;111(1):1–3.
Wilkie, DJ, Holzemer, WL, Tesler, MD, et al. Measuring pain quality: validity and reliability of children’s and adolescents’ pain language. Pain. 1990;41(2):151–159.
Wong, DL, Baker, CM. Pain in children: comparison of assessment scales. Pediatr Nurs. 1988;14(1):9–17.
Wong, D, Pasero, CL. Reducing the pain of lidocaine. Am J Nurs. 1997;97(1):17–18.
Wong, D, Pasero, CL. Using local anesthetics to control procedural pain. Am J Nurs. 1997;97(1):17.
Woodgate, R, Yanofsky, R. A different perspective to approaching cancer symptoms in children. J Pain Symptom Manage. 2004;26(3):800–817.
Woolf, CJ, Salter, MW. Neuronal plasticity: increasing the gain in pain. Science. 2000;288(5472):1765–1769.
Yaster, M, Krance, EJ, Kaplan, RF, et al. Pediatric pain management and sedation handbook. St Louis: Mosby, 1997.
 IN TEXT
IN TEXT COMMUNITY FOCUS
COMMUNITY FOCUS