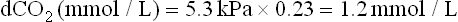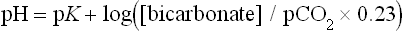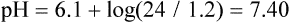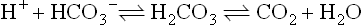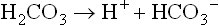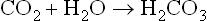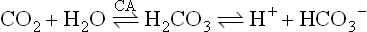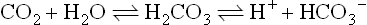Regulation of Hydrogen Ion Concentration (Acid–Base Balance)
Introduction
Acids are produced in the course of metabolism
Metabolism generates carbon dioxide within cells. Carbon dioxide dissolves in water, forming carbonic acid, which in turn dissociates releasing hydrogen ion. The acids derived from sources other than CO2 are known as nonvolatile; by definition, they cannot be removed through the lungs, and must be excreted via the kidney. The net production of nonvolatile acids is in the order of 50 mmol/24 h.
Lactic acid is produced during anaerobic glycolysis and its concentration in plasma is the hallmark of hypoxia. Ketoacids (acetoacetic and β-hydroxybutyric acid) are important in diabetes (Chapter 21). The metabolism of sulfur-containing amino acids and phosphorus-containing compounds also generates inorganic acids.
In spite of the amount of hydrogen ion produced, its blood concentration (or its negative logarithm, the pH), is remarkably constant: it remains between 35 and 45 nmol/L (pH 7.35–7.45). Maintenance of stable pH is essential because it affects the ionization of proteins (Chapter 2) and, consequently, the activity of many enzymes and other biologically active molecules such as ion channels. Changes in pH together with the partial pressure of carbon dioxide (pCO2) affect the shape of the hemoglobin saturation curve, and thus tissue oxygenation (Chapter 5). Also, a decrease in pH increases sympathetic tone and may lead to cardiac dysrhythmias.
Maintaining the acid–base balance involves lungs, erythrocytes and kidneys
The acid–base balance involves the lungs, the erythrocytes, and the kidneys (Fig. 25.1). The lungs control the exchange of carbon dioxide and oxygen between the blood and the atmosphere; the erythrocytes transport gases between lungs and tissues; and the kidneys control plasma bicarbonate synthesis and the excretion of the hydrogen ion.
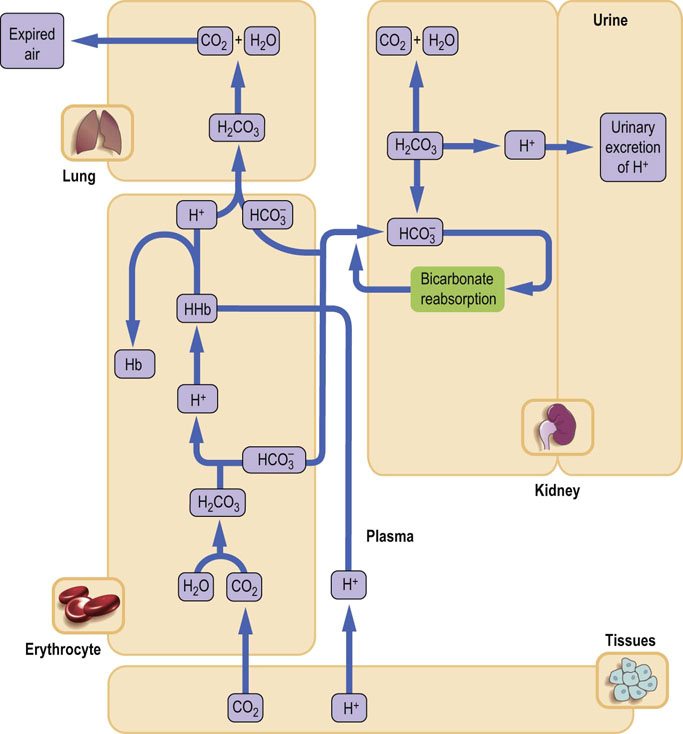
Fig. 25.1 Acid–base balance.
Lungs, kidneys, and erythrocytes contribute to the maintenance of the acid–base balance. The lungs control the gas exchange with the atmospheric air. Carbon dioxide generated in tissues is transported in plasma as bicarbonate; the erythrocyte hemoglobin contributes to CO2 transport. Hemoglobin also buffers the hydrogen ion derived from carbonic acid. The kidneys reabsorb filtered bicarbonate in the proximal tubules and generate new bicarbonate in the distal tubules, where there is a net secretion of hydrogen ion. Hb, hemoglobin.
Body Buffer systems: respiratory and metabolic components of the acid–base balance
Blood and tissues contain buffer systems that minimize changes in hydrogen ion concentration
The main buffer that neutralizes hydrogen ions released from cells is the bicarbonate buffer. Another important buffer is hemoglobin, which contributes to buffering of hydrogen ion generated from the carbonic anhydrase reaction. Within cells, the hydrogen ion is neutralized by intracellular buffers, mainly proteins and phosphates (Table 25.1 and Chapter 2).
Table 25.1
The main buffers in the human body
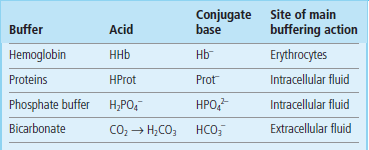
See Chapter 2 for the principles of buffering action. The Brønsted–Lowry definition of an acid is ‘a molecular species that has a tendency to lose a hydrogen ion, forming a conjugate base’.
Bicarbonate buffer is an open system which remains at equilibrium with atmospheric air
Buffering capacity of the bicarbonate buffer exceeds all the ‘closed’ buffer systems. The metabolically produced CO2 diffuses through cell membranes and dissolves in plasma. The plasma solubility coefficient of CO2 is 0.23 if pCO2 is measured in kPa (0.03 if pCO2 is measured in mmHg; 1 kPa = 7.5 mmHg or 1 mmHg = 0.133 kPa)). Thus, at the normal pCO2 of 5.3 kPa (40 mmHg), the concentration of dissolved CO2 (dCO2) is:
CO2 equilibrates with H2CO3 in plasma in the course of a slow, nonenzymatic reaction. Normally plasma H2CO3 concentration is very low, about 0.0017 mmol/L. However, because of the equilibrium between H2CO3 and dissolved CO2 (theoretically all dissolved CO2 could eventually convert into H2CO3), this component of the bicarbonate buffer can be taken as equal to the sum of the H2CO3 and the dissolved CO2. The key equation describing the behavior of the bicarbonate buffer is the Henderson–Hasselbalch equation (Chapter 2). It expresses the relationship between pH and the components of the buffer:
The equation demonstrates that blood pH is determined by the ratio between the concentration of plasma bicarbonate (the ‘base’ component of the buffer) and the concentration of dissolved CO2 (the ‘acid’ component). Normally, at pCO2 of 5.3 kPa and dCO2 concentration 1.2 mmol/L (see above) the plasma bicarbonate concentration is about 24 mmol/L. The pK of the bicarbonate buffer is 6.1. Let's insert the actual concentrations of buffer components into the preceding equation:
Thus the normal concentration of bicarbonate and normal partial pressure of CO2 correspond to pH 7.40 (hydrogen ion concentration 40 nmol/L). The bicarbonate buffer minimizes changes in hydrogen ion concentration when acid is added to blood.
When the H+ concentration in the system increases, the bicarbonate component of the buffer accepts (H+), forming carbonic acid, which is subsequently converted into CO2 and H2O in the reaction catalyzed by carbonic anhydrase:
The CO2 is eliminated through the lungs. The excess hydrogen ion has been neutralized and the [bicarbonate]/pCO2 ratio brought back towards normal.
On the other hand, when the H+ concentration decreases, the carbonic acid component of the buffer will dissociate to supply H+ for a reaction that will yield water and bicarbonate ion (at this stage the increase in plasma bicarbonate is minimal):
Subsequently, the ventilation rate will decrease, retaining CO2 and attempting to normalize the [bicarbonate]/pCO2 ratio:
Thus the denominator in the Henderson–Hasselbalch equation (pCO2) is controlled by the lungs. For this reason it is called ‘the respiratory component of the acid–base balance’. On the other hand, plasma bicarbonate concentration is controlled by the kidneys and erythrocytes and, consequently, it is called ‘the metabolic component of the acid–base balance’ (Fig. 25.2).
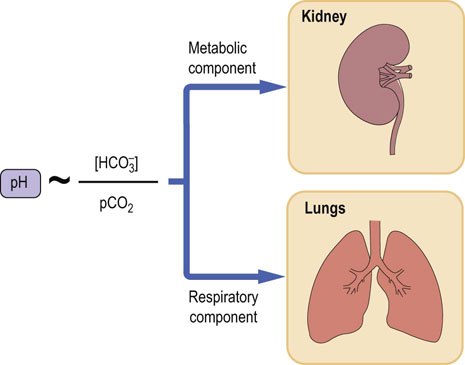
Fig. 25.2 The bicarbonate buffer.
Blood pH is proportional to the ratio of plasma bicarbonate to the partial pressure of carbon dioxide (pCO2). The components of the bicarbonate buffer are thus the carbon dioxide and the bicarbonate. The pCO2 is the respiratory component of acid–base balance, and bicarbonate is the metabolic component.
Carbonic anhydrase converts the dissolved CO2 into carbonic acid
Erythrocytes and renal tubular cells contain a zinc-containing enzyme, carbonic anhydrase (CA), which converts dissolved CO2 into carbonic acid. Carbonic acid dissociates, yielding hydrogen and bicarbonate ions:
This is how renal tubular cells and erythrocytes produce bicarbonate. The kidneys regulate bicarbonate reabsorption and synthesis, and the erythrocytes adjust its concentration in response to changes in pCO2.
Respiratory and metabolic components of the acid–base balance are interlinked
The respiratory and metabolic components of the acid–base balance are closely inter-dependent: one tends to compensate for the untoward changes in the other. When the primary disorder is respiratory (such as, for instance, a severe chronic obstructive airway disease, COAD) and causes accumulation of CO2, a compensatory increase in bicarbonate reabsorption by the kidney takes place. Conversely, the decrease in pCO2 (as happens during hyperventilation in an asthmatic attack) would cause a kidney response, leading to decreased bicarbonate reabsorption.
Conversely, when the primary problem is metabolic (for instance, diabetic ketoacidosis), a decrease in bicarbonate concentration and the resulting decrease in pH stimulate the respiratory center to increase the ventilation rate. The CO2 is blown off and plasma pCO2 decreases. This is why patients with metabolic acidosis hyperventilate. On the other hand, an increase in plasma bicarbonate (causing an increase in pH) leads to a decrease in the ventilation rate, and to CO2 retention. Thus, the compensatory change always tends to normalize the [bicarbonate]/pCO2 ratio, helping to bring the pH towards normal (Fig. 25.3).
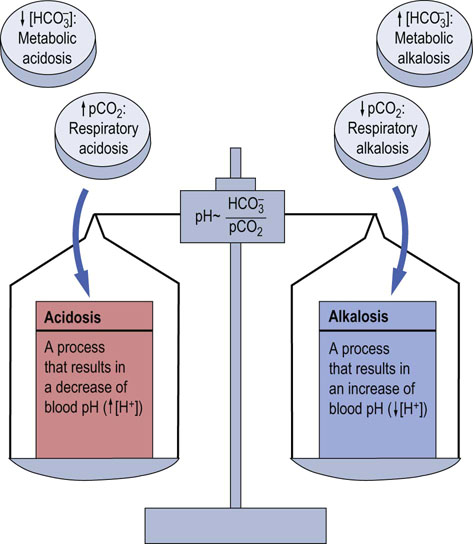
Fig. 25.3 Disorders of the acid–base balance.
A primary increase in pCO2, or a decrease in plasma bicarbonate concentration, lead to acidosis. A decrease in pCO2, or an increase in plasma bicarbonate, lead to alkalosis. If the primary change is in pCO2, the disorder is called respiratory, and if the primary change is in plasma bicarbonate, it is called metabolic.
Intracellular buffering
Intracellular buffers are proteins and phosphates
The two main intracellular buffers are proteins and phosphates, and the buffering is governed by the HPO42–/H2PO4– and [protein]/protein-H } ratios. Hemoglobin is an important extracellular protein buffer.
Note that when the hydrogen ion is present in plasma in excess, it enters cells in exchange for potassium and this may result in an increase in plasma potassium concentration. Conversely, a decrease in plasma hydrogen ion, or bicarbonate excess, would be buffered by cell-derived hydrogen ion. Hydrogen ion would enter plasma in exchange for potassium, decreasing plasma potassium concentration. Thus acidemia, (low blood pH) may be associated with hyperkalemia, and alkalemia (high blood pH) is associated with hypokalemia (Fig. 25.4).
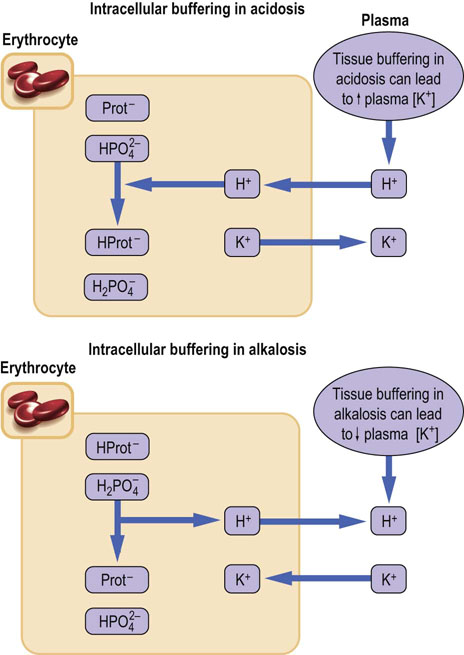
Fig. 25.4 Intracellular buffers: proteins, phosphates, and the potassium–hydrogen ion exchange.
Intracellular buffers are primarily proteins and phosphates. However, the hydrogen ion enters cells in exchange for potassium. Therefore, an accumulation of the hydrogen ion in the plasma (acidemia) and the consequent entry of excess of hydrogen ion into cells increase plasma potassium concentration. Conversely, a deficit of hydrogen ion in plasma (alkalemia) may lead to a low plasma potassium concentration. Prot, protein.
The measurement of blood gases
The ‘blood gas measurement’ is an important first-line laboratory investigation. In respiratory failure, it is also essential to guide oxygen therapy and assisted ventilation.
The measurements are performed on a sample of arterial blood, taken usually from the radial artery in the forearm. The jargon term ‘blood gases’ means the measurements of pO2, pCO2, and pH (or hydrogen ion concentration) from which the concentration of bicarbonate is calculated using the Henderson–Hasselbalch equation. Several other indices are also computed: they include the total amount of buffers in the blood (the buffer base) and the difference between the desired (normal) amount of buffers in the blood and the actual amount (base excess). The reference values for pH, pCO2, and O2 are given in Table 25.2.
Table 25.2
Reference ranges for blood gas results
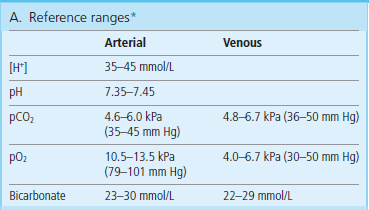
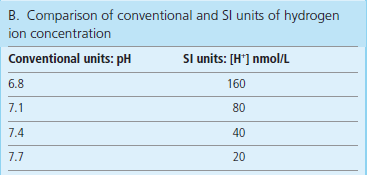
*The measured values in ‘blood gases’ are pH, pCO2 and pO2; the bicarbonate concentration is calculated from pH and pCO2 values; pH below 7.0 or above 7.7 is life threatening. (Adapted with permission from Hutchinson AS. In Dominiczak MH, editor. Seminars in clinical biochemistry, Glasgow, 1997, Glasgow University Press.)
Lungs: the gas exchange
The lungs supply oxygen necessary for tissue metabolism and remove the generated CO2
Approximately 10,000 L of air pass through the lungs of an average person each day. Lungs lie in the thoracic cavity surrounded with the pleural sac, a thin ‘bag’ of tissue that lines the thoracic cage at one end, and attaches to the external surface of the lungs at the other. When the thoracic cage expands during inspiration, negative pressure created in the expanding pleural sac inflates the lung.
The airways are ‘tubes’ of progressively decreasing diameter. They consist of the trachea, large and small bronchi, and even smaller bronchioles (Fig. 25.5). At the end of the bronchioles, there are pulmonary alveoli – structures lined with endothelium and covered with a film of surfactant – the main component of which is dipalmitoylphosphatidylcholine (Chapter 26). Surfactant decreases the surface tension of the alveoli. The gas exchange takes place in the alveoli.
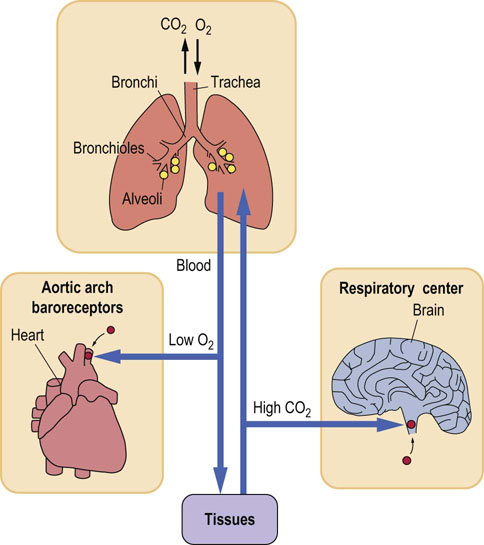
Fig. 25.5 Control of the respiratory rate by pCO2 and pO2.
Lung ventilation and perfusion are main factors controlling gas exchange. The pCO2 regulates the ventilation rate through central chemoreceptors in the brainstem. When pO2 decreases, this control switches to pO2-sensitive peripheral receptors in the carotid bodies and in the aortic arch.
Respiration rate is controlled by the respiratory center located in the brainstem
Both the partial pressures of oxygen (pO2) and carbon dioxide (pCO2) affect the ventilation rate: the respiratory center has chemoreceptors sensitive to pCO2 and to pH. Under normal circumstances it is not the pO2 that stimulates ventilation, but the increase in pCO2 or the decrease in pH. However, when the pO2 falls and hypoxia develops, it begins to control ventilation through a set of receptors located in the carotid bodies in the aortic arch. When arterial pO2 decreases to less than 8 kPa (60 mmHg), this ‘hypoxic drive’ becomes the main controller of the ventilation rate. Persons who suffer from hypoxia due to chronic lung disease depend on hypoxic drive to maintain their ventilation rate (see Clinical Box on this page).
Ventilation and lung perfusion together determine gas exchange
Blood supply to the pulmonary alveoli is provided by the pulmonary arteries that carry deoxygenated blood from the periphery and through the right ventricle. After its oxygenation in the lungs, the blood flows through pulmonary veins to the left atrium. In the alveolar capillaries of the lungs, it accepts oxygen, which diffuses through the alveolar wall from the inspired air; at the same time the CO2 diffuses from the blood into the alveoli (Fig. 25.5) and is removed with the expired air.
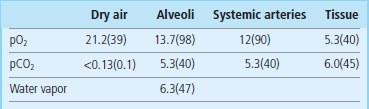
The rate of diffusion of gases in and out of the blood is determined by the difference in partial pressures between alveolar air and blood. Table 25.3 shows the pO2 and pCO2 in the lungs. Compared with the atmospheric air, pCO2 in the alveolar air is slightly higher and pO2 slightly lower (this is due to the water vapor pressure). Carbon dioxide is much more soluble in water than oxygen, and equilibrates with blood more rapidly. Therefore, when problems develop, one first notices a decrease in blood pO2 (hypoxia). An increase in pCO2 (hypercapnia) occurs later and usually indicates a more severe disease. The other major factor determining gas exchange is the rate at which the blood flows through the lungs (the perfusion rate). Normally the alveolar ventilation rate is approximately 4 L/min and the perfusion 5 L/min (the ratio of ventilation to perfusion (Va/Q) is 0.8).
Different combinations of disturbed ventilation and perfusion may occur
In pathologic conditions, some parts of the lung may be well-perfused, but poorly ventilated. This occurs when some alveoli collapse and are unable to exchange gases. As a result, the blood pO2 decreases, because there is no diffusion of oxygen from the alveolar air. The presence of oxygen-poor blood in the arterial circulation is known as the ‘shunt’ condition. On the other hand, when ventilation is adequate but perfusion poor, gas exchange cannot take place: in such cases, part of the lung behaves as if it had no alveoli at all, forming the ‘physiologic dead space’. Examples of conditions related to poor ventilation, poor perfusion, or the combination of both, are given below:
 Rib-cage deformities impair ventilation by limiting lung movement.
Rib-cage deformities impair ventilation by limiting lung movement.
 Chest trauma may decrease ventilation as a result of lung collapse; impairing ventilation.
Chest trauma may decrease ventilation as a result of lung collapse; impairing ventilation.
 Alveoli may be actually destroyed in pulmonary emphysema.
Alveoli may be actually destroyed in pulmonary emphysema.
 Inadequate synthesis of surfactant leads to the collapse of alveoli, impairment of ventilation and to the respiratory distress syndrome.
Inadequate synthesis of surfactant leads to the collapse of alveoli, impairment of ventilation and to the respiratory distress syndrome.
 Obstruction or narrowing of the bronchial tree (a mechanical obstruction by inhaled objects, or narrowing by a growing tumor): this impairs ventilation.
Obstruction or narrowing of the bronchial tree (a mechanical obstruction by inhaled objects, or narrowing by a growing tumor): this impairs ventilation.
 Constriction of the bronchi occurs in asthma impairing ventilation.
Constriction of the bronchi occurs in asthma impairing ventilation.
 The efficiency of ventilation may be reduced by impaired elasticity of the lung or dysfunction of relevant muscles (the diaphragm and intercostal muscles of the chest wall).
The efficiency of ventilation may be reduced by impaired elasticity of the lung or dysfunction of relevant muscles (the diaphragm and intercostal muscles of the chest wall).
 Fluid present in the alveoli (pulmonary edema) impairs ventilation by affecting diffusion of gases.
Fluid present in the alveoli (pulmonary edema) impairs ventilation by affecting diffusion of gases.
 Defects in the neural control impair ventilation through affecting lung movement.
Defects in the neural control impair ventilation through affecting lung movement.
 Lung perfusion is compromised in circulatory problems such as shock and heart failure.
Lung perfusion is compromised in circulatory problems such as shock and heart failure.
Table 25.4 lists pathologic conditions related to gas exchange.
Table 25.4
Blood partial pressures of oxygen and carbon dioxide depend on lung perfusion and ventilation

*Depending on a degree of shunt.
Handling of carbon dioxide by erythrocytes
Erythrocytes transport CO2 to the lungs in a ‘fixed’ form – as bicarbonate
Human metabolism produces CO2 at a rate of 200–800 mL/min. The CO2 dissolves in water and generates carbonic acid, which in turn dissociates into hydrogen and bicarbonate ions. Thus, CO2 generates large numbers of hydrogen ions:
In plasma, the above reaction is nonenzymatic and proceeds slowly, generating only minute amounts of carbonic acid, which remains in equilibrium with a large amount of dissolved CO2. However, the same reaction in the erythrocytes is catalyzed by carbonic anhydrase, which ‘fixes’ CO2 as bicarbonate. The generated hydrogen ion is buffered by hemoglobin.
The bicarbonate ion produced by erythrocyte carbonic anhydrase reaction moves to plasma in exchange for chloride ion (the ‘chloride shift’) (Fig. 25.6). As much as 70% of all CO2 produced in tissues becomes bicarbonate; approximately 20% is carried ‘fixed’ to hemoglobin as carbamino groups, and only 10% remains dissolved in plasma.
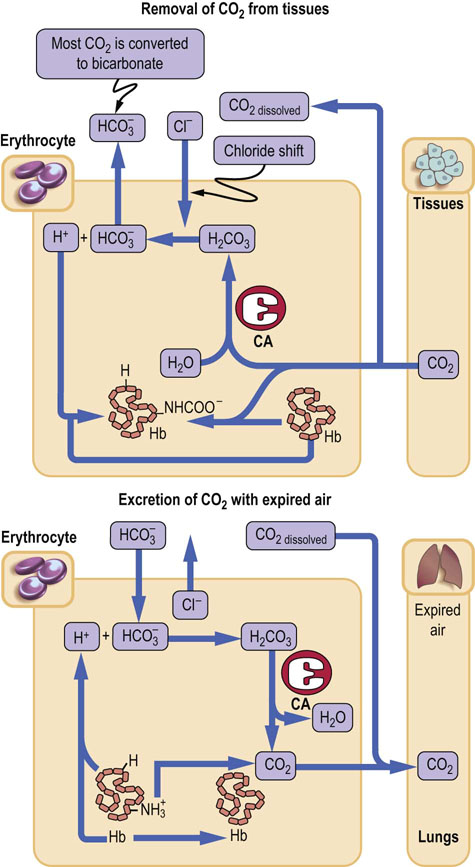
Fig. 25.6 CO2 transport by the erythrocytes.
Erythrocyte carbonic anhydrase converts approximately 70% of the CO2 produced in tissues into bicarbonate for transport to the lungs: approximately 20% of the total amount is transported bound to hemoglobin, as carbamates (-NHCOO−) and the rest as dissolved gas in plasma. CA, carbonic anhydrase.
In the lungs, higher pO2 facilitates dissociation of CO2 from hemoglobin. This is known as the Haldane effect. The hemoglobin releases its hydrogen ion, which reacts with bicarbonate, and forms carbonic acid, which, in turn, releases CO2.
Handling of bicarbonate by the kidneys
The kidneys control plasma bicarbonate concentration and the removal of the hydrogen ion. In common with erythrocytes, renal tubular cells (proximal and distal) contain carbonic anhydrase.
Proximal tubules reabsorb bicarbonate in the process aided by carbonic anhydrase
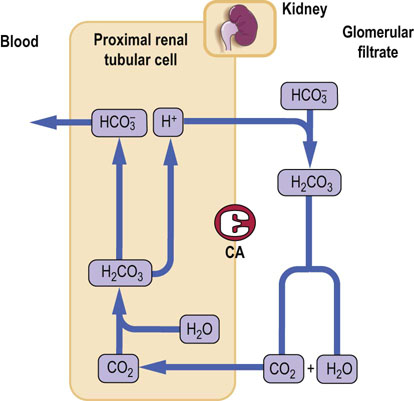
Normally, bicarbonate is reabsorbed in the proximal tubule and the urine is almost bicarbonate-free. The surfaces of the renal tubular cells facing the lumen are impermeable to bicarbonate. The filtered bicarbonate combines with hydrogen ion secreted by the cells, and forms carbonic acid, which is converted into CO2 by carbonic anhydrase located on the luminal membrane. The CO2 diffuses into cells, where intracellular carbonic anhydrase converts it back into carbonic acid, dissociating into hydrogen and bicarbonate ions. Bicarbonate is returned to the plasma, and the hydrogen ion is secreted into the lumen of the tubule to trap more filtered bicarbonate. Note that, in this process, hydrogen ion is used exclusively to aid bicarbonate reabsorption, and there is no net excretion (Fig. 25.7).
Distal tubules generate new bicarbonate and excrete hydrogen
The situation is different in the distal tubule where bicarbonate is generated. The mechanism is identical to that of bicarbonate reabsorption, but this time there is both a net loss of hydrogen ions from the body and a net gain of bicarbonate. The CO2 diffuses into cells. The distal tubule carbonic anhydrase converts it into carbonic acid, which dissociates into hydrogen ion and bicarbonate. Bicarbonate is transported to the plasma, and hydrogen ion is secreted into the tubule lumen. However, no bicarbonate is present in the lumen of the distal tubule (all has been reabsorbed earlier) and the hydrogen ion is buffered (trapped) by phosphate ions present in the filtrate, and by ammonia synthesized by the proximal tubules. It is subsequently excreted in the urine (Fig. 25.8).
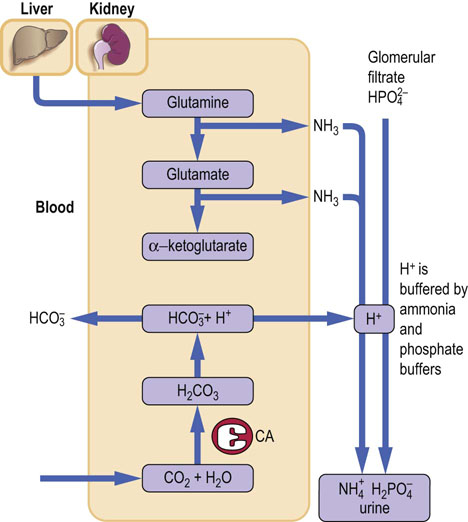
Fig. 25.8 Hydrogen ion excretion by the kidney.
Excretion of the hydrogen ion takes place in the distal tubules. Hydrogen ion reacts with ammonia, forming the ammonium ion. Hydrogen ion is also buffered in the tubule lumen by phosphate. Approximately 50 mmol of hydrogen ion is excreted daily. CA, carbonic anhydrase.
Ammonia generated by glutaminase reaction participates in the excretion of hydrogen ion
Ammonia is generated during the transformation of glutamine into glutamic acid catalyzed by glutaminase. Ammonia diffuses across the luminal membrane, allowing hydrogen ion to be trapped inside the tubule as the ammonium ion (NH4+), to which the membrane is impermeable.
Disorders of the acid–base balance
Classification of the acid–base disorders
The concept of respiratory and metabolic components of the acid–base balance forms a basis for the classification of acid–base balance disorders (Fig. 25.3). They are divided into acidosis or alkalosis. Acidosis is a process that leads to the accumulation of hydrogen ion. Alkalosis causes a decrease in hydrogen ion concentration (acidemia and alkalemia are terms that simply describe blood pH). Thus, acidosis and alkalosis result in academia and alkalemia, respectively (see the Clinical Box on this page).
There are four main disorders of acid–base balance
The key to further classification is the ‘location’ of the primary cause within the respiratory and metabolic components of the system. If the primary cause is the change in pCO2, the acidosis or alkalosis is called respiratory, and if it is bicarbonate, the acidosis or alkalosis is called metabolic. Thus there are four main disorders of acid–base balance: respiratory acidosis, metabolic acidosis, respiratory alkalosis, and metabolic alkalosis (Fig. 25.3). However, mixed disorders can also develop; we consider them later.
Lung and the kidney work in a concerted way to minimize changes in plasma pH: this is known as the compensation of acid–base disorders
Acidosis is accompanied by a decreased ratio of plasma bicarbonate to pCO2, and alkalosis by an increased ratio. Whenever a problem occurs, the compensating mechanisms are triggered to bring the hydrogen ion concentration back towards normal. This translates into normalizing the [bicarbonate]/pCO2 ratio in the Henderson–Hasselbalch equation.
Consequently, when the respiratory acidosis causes an increase in pCO2, the kidney will increase the generation of bicarbonate, increasing its plasma concentration and normalizing the ratio. Conversely, when diabetic ketoacidosis causes depletion of plasma bicarbonate, ventilation rate increases, pCO2 decreases and the ratio of bicarbonate/pCO2 changes towards normal. While respiratory compensation can occur within minutes, metabolic compensation takes hours to days to develop fully (Table 25.5).
Table 25.5
Respiratory and metabolic compensation in the acid–base disorders
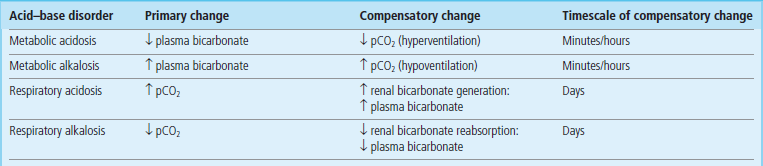
Respiratory and metabolic compensation in the acid–base disorders minimizes changes in the blood pH. A change in the respiratory component leads to metabolic compensation, and a change in the metabolic component stimulates respiratory compensation.
Acidosis
Respiratory acidosis occurs most often in lung disease and results from decreased ventilation
The most common cause is the chronic obstructive airways disease (COAD). Severe asthmatic attack can result in respiratory acidosis because of bronchial constriction. Respiratory acidosis often accompanies hypoxia (respiratory failure); in such a case, an increase in pCO2 often parallels the decrease in pO2 (Table 25.6, see Clinical Box on page 336).
Table 25.6
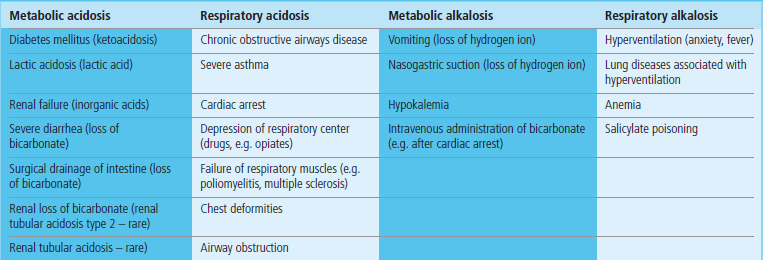
Respiratory acidosis is common and is caused primarily by diseases of the lung that affect gas exchange. Respiratory alkalosis is rarer and is caused by hyperventilation, which decreases pCO2. Metabolic acidosis is common and results from either overproduction or retention of nonvolatile acids in the circulation. Metabolic alkalosis is rarer: its most common causes are vomiting and gastric suction, both causing loss of hydrogen ion from the stomach.
Metabolic acidosis results from excessive production, or inefficient metabolism or excretion, of nonvolatile acids
A classic example of metabolic acidosis is the diabetic ketoacidosis, when ketoacids, acetoacetic acid, and β-hydroxybutyric acid accumulate in the plasma (Chapter 21). Acidosis may also occur during extreme physical exertion, when there is accumulation of lactic acid generated from muscle metabolism; in normal circumstances, lactate would be quickly metabolized on cessation of exercise. However, when large amounts of lactate are generated as a consequence of hypoxia, lactic acidosis may become life-threatening, as happens, for instance, in shock (Table 25.6).
Excretion of nonvolatile acids is also impaired in renal failure, and this also leads to metabolic acidosis. Renal failure develops when the perfusion of the kidneys is inadequate (e.g. in trauma, shock, or dehydration) or if there is an intrinsic kidney disease such as glomerulonephritis (inflammatory reaction in the renal tubular tissue).
Excessive loss of bicarbonate can also be a cause of metabolic acidosis. This is common when bicarbonate present in the intestinal fluid is lost as a result of severe diarrhea or surgical drainage after bowel surgery.
Impaired bicarbonate reabsorption and hydrogen ion secretion causes rare renal tubular acidoses
Defects in renal handling of bicarbonate and hydrogen ion lead to a group of relatively rare disorders known as renal tubular acidoses (RTA). The proximal RTA is caused by impaired reabsorption of bicarbonate, and the distal RTA by impaired hydrogen ion excretion. Proximal RTA is usually accompanied by other defects in proximal transport mechanisms (this is known as the Fanconi syndrome).
Generally, acidosis is a much more common condition than alkalosis.
Alkalosis
Alkalosis is rarer than acidosis
A mild respiratory alkalosis may be a consequence of hyperventilation during exercise, anxiety attack, or fever. It also occurs in pregnancy. Metabolic alkalosis is often associated with abnormally low serum potassium concentration, as a result of cellular buffering. Cellular entry or exit of potassium ion is associated with the movement of hydrogen ion in an opposite direction. Thus, alkalosis can cause hypokalemia, and hypokalemia (Chapter 22) may lead to alkalosis. Severe metabolic alkalosis may also occur as a result of the massive loss of hydrogen ion from the stomach during vomiting (see Clinical Box on this page), or as a result of nasogastric suction after surgery. Lastly, it may occur when too much bicarbonate is given intravenously: for instance, during resuscitation from cardiac arrest (Table 25.6).
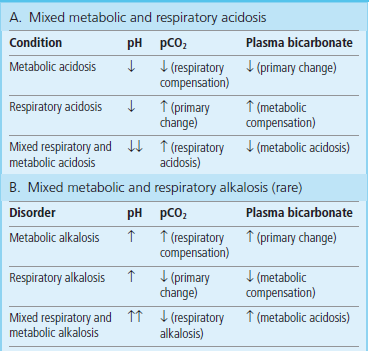
Mixed acid–base disorders
More than one acid–base disorder can exist in one patient. The result of this is a mixed acid–base disorder, sometimes quite difficult to diagnose (see Clinical Box below and Table 25.7).
Summary
 Maintenance of the hydrogen ion concentration within a narrow range is vital for survival.
Maintenance of the hydrogen ion concentration within a narrow range is vital for survival.
 Acid–base balance is regulated by the concerted action of lungs and kidneys. The erythrocytes play a key role in the transport of carbon dioxide in blood.
Acid–base balance is regulated by the concerted action of lungs and kidneys. The erythrocytes play a key role in the transport of carbon dioxide in blood.
 Main buffers in blood are hemoglobin and bicarbonate, whereas in the cells they include proteins and phosphate. The bicarbonate buffer system communicates with atmospheric air.
Main buffers in blood are hemoglobin and bicarbonate, whereas in the cells they include proteins and phosphate. The bicarbonate buffer system communicates with atmospheric air.
 Acid–base disorders are acidosis and alkalosis and each of them can be either metabolic or respiratory.
Acid–base disorders are acidosis and alkalosis and each of them can be either metabolic or respiratory.
 Measurement determination of pH, pCO2 and bicarbonate, and pO2, is a first-line investigation and is frequently required in emergencies.
Measurement determination of pH, pCO2 and bicarbonate, and pO2, is a first-line investigation and is frequently required in emergencies.
Appel, SJ, Downs, CA, Understanding acid–base balance. Nursing: Fall 2008; 38:9–1. http://journals.lww.com/nursing/Fulltext/2008/09002/Understanding_acid_base_balance.3.aspx
McNamara, J, Worthley, LIG, Acid–Base Balance: Part I. Physiology. Critical Care and Resuscitation 2001; 3:181–18. http://cicm.org.au/journal/2001/september/ABa1.pdf
Neligan, PJ, Deutschman, CS. Acid–base balance in critical care medicine. www.ccmtutorials.com/renal/Acid%20Base%20Balance%20in%20Critical%20Care%20Medicine-NELIGAN.pdf.
Respiratory Acid/Base Balance, Gas Transport and Erythrocytes. www.sci.utah.edu/~macleod/bioen/be6000/notes/L14-acid–base.pdf
 ska-Konkel
ska-Konkel