Bone Metabolism and Calcium Homeostasis
Introduction
Many cell functions depend on tight control of extracellular calcium concentration.
These include neural transmission, cellular secretion, contraction of muscle cells, cell proliferation, the permeability of cell membranes, blood clotting, and the mineralization of bone. Bone serves as a reservoir of calcium when deficiency exists, and as its store when the body is calcium replete. The skeleton contains 99% of the calcium present in the body in the form of hydroxyapatite; the remainder is distributed in the soft tissues, teeth, and the extracellular fluid (ECF).
Bone structure and bone remodeling
Bone is a specialized connective tissue that, along with cartilage, forms the skeletal system
In addition to serving a supportive and protective role, bone is the site of substantial metabolic activity. There are two types of bone: the thick, densely calcified external bone (cortical or compact bone) and a thinner, honeycomb network of calcified tissue (trabecular bone).
Collagen and hydroxyapatite are the main components of the bone matrix
Within the bone matrix, the major protein (90%) is type 1 collagen (see Box on p. 12). The calcium-rich crystals of hydroxyapatite (Ca10[PO4]6[OH]2) are found on, within, and between the collagen fibers. The attachment of hydroxyapatite to collagen and the calcification of bone are, in part, controlled by the presence of glycoproteins and proteoglycans with a high ion-binding capacity. Collagen fibers orientate so that they have the greatest density per unit volume and are packed in layers, giving bone the lamellar structure observed on microscopy. Post-translational modifications of collagen result in the formation of intra- and intermolecular pyridinoline and pyrrole crosslinks. This microarchitecture allows bone to function as the major reservoir of calcium for the body.
The noncalcified organic matrix within bone, known as osteoid, becomes mineralized through two mechanisms. Within the extracellular space of the bone, plasma membrane-derived matrix vesicles act as a focus for deposition of calcium phosphate. Crystallization eventually obliterates the vesicle membrane, leaving a collection of clustered hydroxyapatite crystals. Within this environment, the bone-forming cells (osteoblasts) secrete packets of matrix proteins that rapidly mineralize, and these combine with matrix vesicle-derived crystals. Pyrophosphate present in the matrix inhibits this process. The alkaline phosphatase secreted by the osteoblasts destroys pyrophosphate, allowing mineralization to proceed. Mineralization is highly dependent on an adequate supply of calcium and phosphate. When mineral deprivation exists, there is an increase in the percentage of the nonmineralized organic matrix (osteoid) within bone, resulting in the clinical condition of osteomalacia.
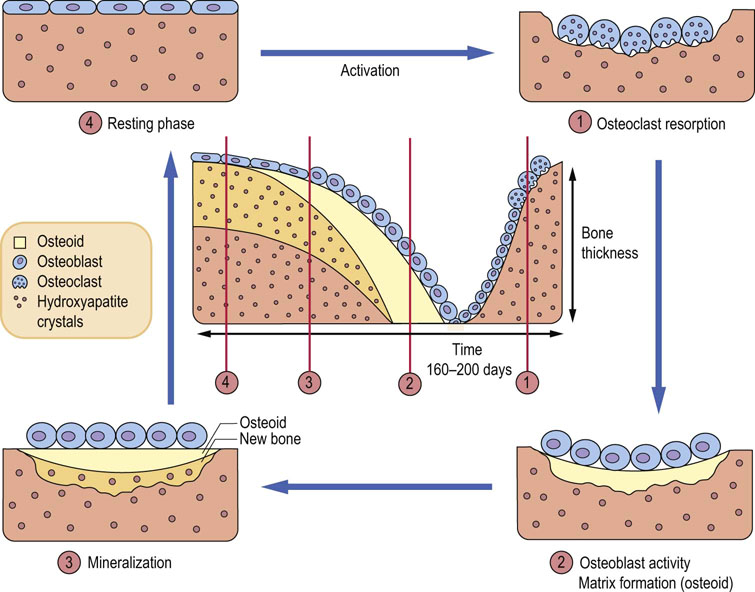
Bone constantly changes its structure through remodeling
Small amounts of calcium are exchanged daily between bone and the ECF as a result of constant bone remodeling, i.e. coupled processes of resorption by the bone-resorbing cells (osteoclasts) and formation by the osteoblasts (Fig. 26.1). This exchange maintains a relative calcium balance between newly formed and older bone. Bone undergoes constant mechanical adaptation. Increased mechanical load stimulates bone formation and excess osteoclastic activity underpins several diseases, in particular osteoporosis, rheumatoid arthritis, and metastatic cancers.
Osteoclasts are bone-resorbing cells
Osteoclasts are multinucleated, giant tissue-specific macrophages. They are derived from pluripotent hematopoietic mononuclear cells in the bone marrow and remain in contact with a calcified surface.
RANK receptor and its ligand RANKL are essential for differentiation, maturation, and regulation of osteoclasts
The maturation of osteoclasts from their progenitor cells is directed by growth factors, particularly monocyte-colony stimulating factor (M-CSF). Another essential factor is the membrane receptor protein structurally related to the tumor necrosis factor receptor, called receptor activator of nuclear factor NFκB (RANK). It binds the TNF-related cytokine called RANK ligand (RANKL). The binding of RANKL to RANK can be decreased when it binds instead to osteoprotegerin (OPG), a protein which also belongs to the TNF receptor superfamily. RANKL and OPG together control differentiation and activation of osteoclasts. RANKL stimulates and OPG inhibits bone resorption. Importantly, estrogens induce OPG synthesis.
RANK controls osteoclasts through intracellular signaling cascades and transcription factors
Essentially, RANK prepares the osteoclast to resorb bone. It stimulates intracellular signaling cascades (Chapter 40), which in turn activate transcription factors that control genes. It also cooperates with other immunoglobulin-like membrane receptors. Intracellular signaling involves, among others, adaptor molecules known as TNF receptor-associated cytoplasmic factors (TRAFs). TRAFs assemble further signaling proteins and activate pathways involving NFκB and activator protein-1 (AP-1). Other pathways involve c-Jun terminal kinase, p38 stress-activated protein kinase, extracellular signal-regulated kinase (ERK) and the src pathway, which involves phosphatidylinositol-3-kinase (PI3K) and Akt kinase (Chapter 40). The effect of this is activation of a transcription factor known as the nuclear factor of activated T cells-2 (NFAT2). The end result is the induction of genes coding for tartrate-resistant acid phosphatase, cathepsin K, calcitonin and the β2 integrin, which directly control bone resorption. Osteoclast resorption of bone releases a range of molecules: collagen peptides, pyridinoline crosslink fragments, and calcium from the bone matrix (through the action of lysosomal enzymes, collagenases and cathepsins). Collagen breakdown products (hydroxyproline) in serum and urine, and collagen fragments (amino-terminal and carboxy-terminal telopeptides NTX and CTX, respectively) can be measured in clinical laboratories.
Parathyroid hormone contributes to osteoclast activation
Parathyroid hormone (PTH) activates osteoclasts indirectly via osteoblasts and calcitonin. Local factors such as the cytokines interleukin-1 (IL-1), tumor necrosis factor (TNF), transforming growth factor-β (TGF-β) and interferon-α (INF-α) are also important regulators of osteoclasts and act through RANKL and OPG.
Osteoblasts are bone-forming cells
The osteoblast is derived from mesenchyme. Mature osteoblasts synthesize type 1 collagen, osteocalcin, cell attachment proteins (thrombospondin, fibronectin, bone sialoprotein, osteopontin), proteoglycans, and growth-related proteins. They control bone mineralization.
The function of the osteoblasts is regulated by several hormones and growth factors
PTH binds to a specific receptor and stimulates production of cyclic adenosine monophosphate (cAMP), ion and amino acid transport, and collagen synthesis. Calcitriol (1,25-dihydroxycholecalciferol; 1,25(OH)2D3) stimulates synthesis of alkaline phosphatase, matrix, and bone-specific proteins, and can decrease osteocalcin secretion. Growth factors such as osteoblast stimulating factor 1 (OSF-1), TGF-β, insulin-like growth factors (IGF-1 and IGF-2), and platelet-derived growth factor (PDGF) serve as autocrine regulators of osteoblast function. There are also the bone morphogenetic proteins (BMP) which belong to the TGF-β superfamily. Serum biochemical markers reflecting osteoblast function are bone-specific alkaline phosphatase, osteocalcin, and markers of collagen formation: carboxy-terminal procollagen extension peptide (ICTP) and amino or carboxy-terminal procollagen extension peptides (PINP, P1CP).
A protein that is a member of the LDL-receptor family plays an important role in osteoblast differentiation
Two signaling pathways are important in skeletal development: the Wnt/β-catenin pathway and the TGF-β/BMP pathway (Wnt is a widely present signalling glycoprotein involved particularly in embryo development). The LDL-receptor-related protein 5 (LRP5, Chapter 18), together with another receptor, activates the Wnt pathway. Mutation of the gene encoding for LRP5 was shown to increase bone mass and the formation of dense bone. On the other hand, the loss-of-function mutation caused osteoporosis.
Serum calcium
The total plasma calcium concentration is maintained between 2.2 and 2.60 mmol/L (8.8–10.4 mg/dL). Calcium exists in the circulation in three forms. The ionized Ca2+ is the most important, physiologically active form (50% of total calcium). The majority of the remaining calcium, is protein bound, mainly to negatively charged albumin (40%), and the rest is complexed to substances such as citrate and phosphate (10%).
If plasma protein concentration increases (as, for instance, in dehydration), protein-bound calcium and total serum calcium increase. In conditions of reduced plasma proteins (e.g. liver disease, nephrotic syndrome or malnutrition), the protein-bound calcium concentration is reduced, decreasing the total calcium, although ionized calcium is maintained within the reference range. In many acute and chronic illnesses the albumin concentration decreases. While this decreases the total calcium concentration, it does not change the concentration of the ionized fraction. Therefore, in clinical laboratories, the ‘adjusted calcium’ concept is used: the measured value is extrapolated to albumin concentration of 40 g/L (4 g/dL).
Calcium homeostasis
Parathyroid hormone (PTH) responds to changes in ionized calcium and phosphate
PTH is an 84-amino acid, single-chain peptide hormone secreted by the chief cells of the parathyroid glands. A decrease in extracellular ionized calcium or an increase in serum phosphate concentration stimulates its secretion. Chronic severe magnesium deficiency can inhibit its release from secretory vesicles, and low concentrations of calcitriol interfere with its synthesis. PTH(1–84) is mainly metabolized into a biologically active PTH(1–34) amino-terminal fragment and an inactive carboxy-terminal fragment, PTH(35–84) (Fig. 26.2). Most of the cellular actions of PTH are mediated by G-protein and cAMP signaling.
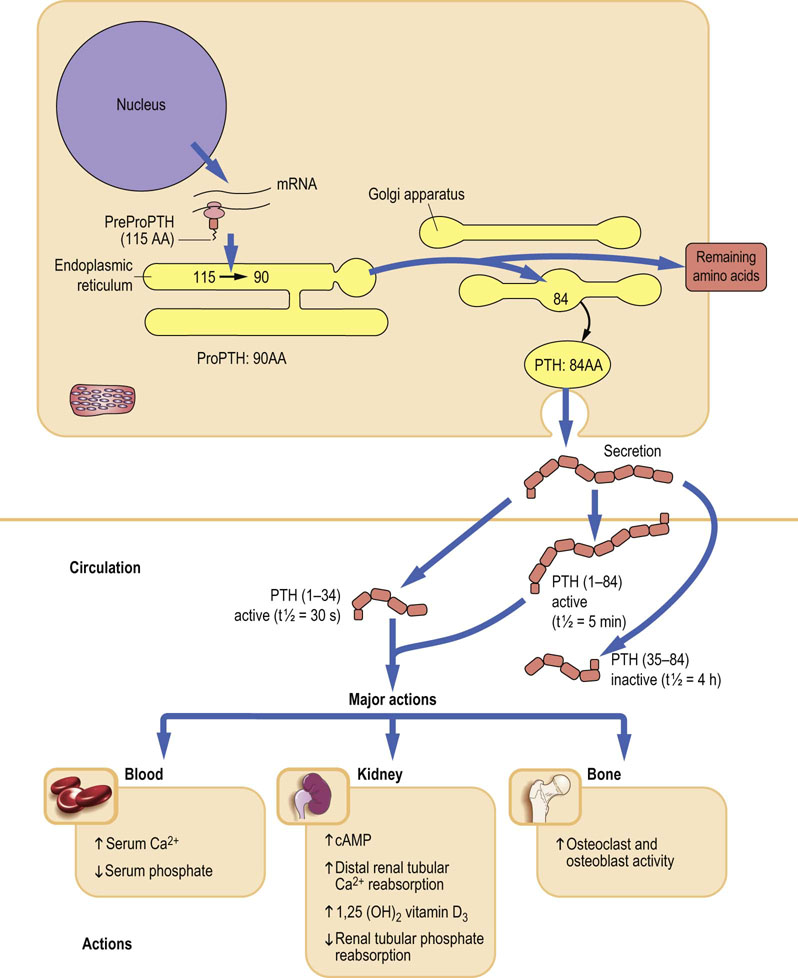
Fig. 26.2 Synthesis and major actions of the parathyroid hormone (PTH).
PTH mobilizes calcium from all available sources and decreases its renal excretion. AA, amino acids.
When plasma calcium decreases, PTH is released from the parathyroid glands, stimulating osteoclast-mediated bone resorption, renal reabsorption of calcium, and its absorption in the small intestine (mediated by calcitriol). PTH secretion is feedback-regulated by calcium: an increase in calcium decreases PTH secretion (Fig. 26.3).
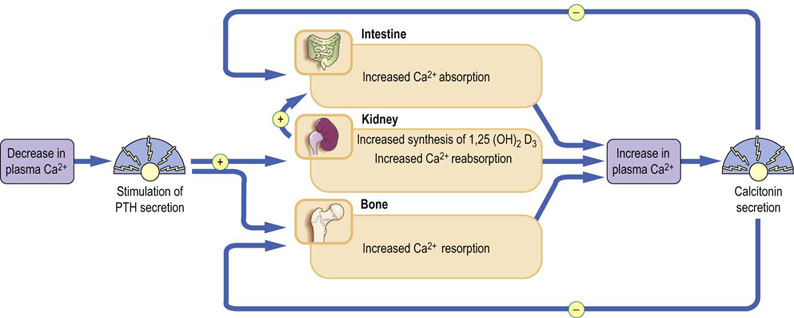
Fig. 26.3 Major hormones influencing calcium homeostasis.
A decrease in plasma ionized calcium stimulates release of PTH; this promotes Ca2+ reabsorption from the kidney, resorption from bone, and absorption by the gut via increased production of 1,25(OH)2D3. As a result, plasma calcium increases. Conversely, an increase in plasma ionized calcium stimulates release of calcitonin, which inhibits reabsorption of calcium by the kidney and osteoclast-mediated bone resorption.
Calcium-sensing receptor is a cell surface G-protein coupled receptor
Ionized calcium is maintained within a narrow range through an extracellular calcium-sensing receptor (CaSR), which is a cell-surface G-protein-coupled receptor present in the chief cells of the parathyroid gland, the thyroidal C cells, and along the kidney tubules. Minute changes in ionized calcium modulate cellular function to maintain normocalcemia.
Vitamin D is synthesized in the skin by UV radiation
Vitamin D2 (ergocalciferol) is synthesized in the skin by UV radiation of ergosterol, and vitamin D3 (cholecalciferol) by UV irradiation of 7-dehydrocholesterol. Vitamin D3 and its hydroxylated metabolites are transported in the plasma bound to a specific globulin, vitamin D-binding protein (DBP). Cholecalciferol is also found in the diet, where its absorption is associated with other fats, and it is transported to the liver in chylomicrons. It is released from chylomicrons in the liver by DBP and hydroxylated at the 25-position, forming calcidiol (25-hydroxycholecalciferol; 25(OH)D3). The metabolism of vitamin D is illustrated in Figure 26.4.
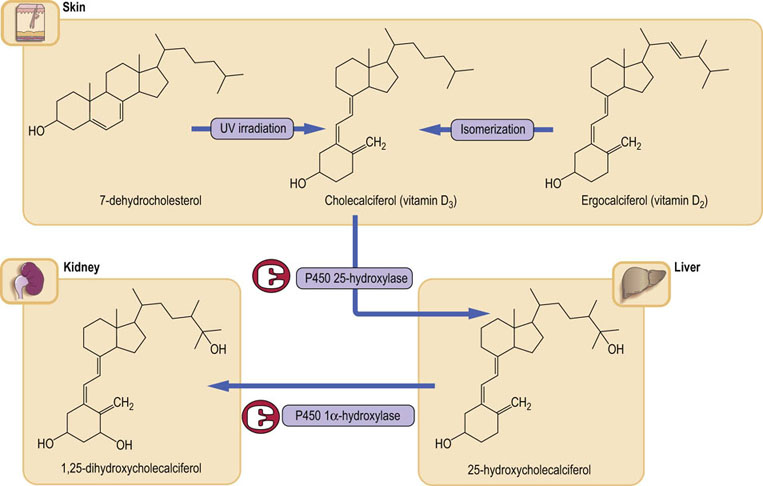
Fig. 26.4 Vitamin D metabolism.
Vitamin D is mainly synthesized in response to the action of sunlight on the skin; a smaller component comes from the diet. Normal liver and kidney function are essential to the formation of the active form 1,25(OH)2D3. Plasma calcium concentration controls the level of 1,25(OH)2D3 through the parathyroid hormone. Note that both hydroxylase enzymes belong to the cytochrome P450 superfamily. 1,25(OH)2D3, 1,25-dihydroxycholecalciferol, calcitriol,1,25(OH)2D3; 25-hydroxycholecalciferol, calcidiol, 25(OH)D3
Calcidiol is the main liver storage form of vitamin D
The 25-hydroxylation step is carried out by a hepatic microsomal enzyme. The hepatic content of 25(OH)D3 regulates the rate of 25-hydroxylation. 25(OH)D3 is the major form of the vitamin found in both the liver and the circulation, in each case bound to DBP, Its levels in the circulation reflect hepatic stores of the vitamin. A significant proportion of 25(OH)D3 is subject to enterohepatic circulation, being excreted in the bile and reabsorbed in the small bowel. Disturbance in the enterohepatic circulation can lead to deficiency of this vitamin.
Calcitriol (1α,25-dihydroxycholecalciferol; 1,25(OH)2D3)is the active metabolite of vitamin D
The main sites for further hydroxylation of the 25(OH)D3 at the 1-position are the renal tubules, although bone and the placenta can also carry out this reaction. The 25(OH)D3 1α-hydroxylase is a mitochondrial enzyme. Its product, calcitriol (1,25(OH)2D3) is the most potent of the vitamin D metabolites and the only naturally occurring form of vitamin D that is active at physiologic concentrations. The 1α-hydroxylase activity is stimulated by PTH, low serum concentrations of phosphate or calcium, vitamin D deficiency, calcitonin, growth hormone, prolactin, and estrogen. Conversely, its activity is feedback inhibited by 1,25(OH)2D3, hypercalcemia, high phosphate concentration and hypoparathyroidism.
1,25(OH)2D3 is transported in plasma also bound to DBP. Vitamin D may be described as a hormone. In the intestinal epithelial cells it binds to a cytoplasmic receptor like other steroid hormones (Chapters 17 and 35) and this ligand–protein complex is transported to the nucleus where it induces gene expression.
The renal tubules, cartilage, intestine, and placenta also contain a 24-hydroxylase, producing the inactive 24,25-dihydroxycholecalciferol (24,25[OH]2D3). The concentration of the 24,25[OH]2D3 in the circulation is reciprocally related to the level of the 1,25(OH)2D3.
1,25(OH)2D3 increases the absorption of calcium and phosphate from the gut via active transport by calcium-binding proteins
Together with PTH, 1,25(OH)2D3 stimulates bone resorption by osteoclasts. These effects increase plasma calcium and phosphate concentrations. Low 1,25(OH)2D3 causes abnormal mineralization of newly formed osteoid as a result of low calcium and phosphate availability and reduced osteoblast function. It leads to the development of rickets in children or osteomalacia in adults.
Calcitonin inhibits bone resorption
Calcitonin is a 32-amino acid peptide synthesized and secreted primarily by the parafollicular cells of the thyroid gland (C cells). Its secretion is regulated by serum calcium through the calcium-sensing receptor (CaSR): an increase in serum calcium results in a proportional increase in calcitonin, and a decrease elicits a corresponding reduction in calcitonin. Chronic stimulation results in exhaustion of the secretory reserve of the C cells. The precise biological role of calcitonin is not known, but the main effect is inhibition of osteoclastic bone resorption (Fig. 26.3).
Calcium is absorbed in the small intestine and is excreted in urine and feces
Calcium is absorbed predominantly in the proximal small intestine. This is regulated through the quantity of calcium ingested in the diet, and two cellular calcium transport processes: the active, saturable transcellular absorption stimulated by 1,25(OH)2D3, and the nonsaturable paracellular absorption controlled by the concentration of calcium in the intestinal lumen relative to the serum concentration.
In a normal adult taking a Western diet, the amount of calcium intake and its deposition in bone are matched by the excretion in urine and feces. During growth, a child is in positive calcium balance, whereas an elderly person may be in negative calcium balance. Changes in calcium absorption reflect alterations in dietary calcium intake, intestinal calcium solubility, and vitamin D metabolism.
Generally, as serum calcium increases, its excretion increases. When hypercalcemia is caused by hyperparathyroidism, PTH will act on the renal tubule, promoting reabsorption of filtered calcium and thus diminishing the effects of the increased filtration and inhibition of renal tubular reabsorption that are caused by increased serum calcium. Decreasing serum calcium is associated with a reduction in urinary excretion, mainly as a result of decreased amounts of filtered calcium. In hypoparathyroid patients, who lack PTH, renal tubular reabsorption of calcium is reduced.
Several hormones directly or indirectly affect calcium homeostasis
Thyroid hormone stimulates osteoclast-mediated resorption of bone. Adrenal and gonadal steroids, particularly estrogens in women and testosterone in men, stimulate osteoblast and inhibit osteoclast function. They also decrease renal calcium and phosphate excretion and increase intestinal calcium absorption.
Growth hormone has anabolic effects on bone, promoting the growth of the skeleton. Its effects are mediated by insulin-like growth factors (IGF-1 and IGF-2) acting on cells of the osteoblast lineage. Growth hormone increases the urinary excretion of calcium and hydroxyproline, whilst decreasing the urinary excretion of phosphate.
Recent data suggest that the central nervous system may also be involved in bone homeostasis. Leptin, an adipokine, which regulates adipose tissue mass and controls appetite (Chapter 22), has been shown to have an inhibitory effect on bone formation. Leptin-deficient animals display high bone mass. However, mutations in the signaling pathway stimulated by leptin have no effect on the bone mass, which suggests that it is a central effect, probably mediated by the sympathetic nervous system.
Disorders of calcium metabolism
Hypercalcemia is most commonly caused either by primary hyperparathyroidism or by malignancy
In practice, 90% of cases of hypercalcemia are due to either primary hyperparathyroidism or malignancy; a greater diagnostic challenge is presented when it becomes necessary to differentiate occult malignancy from the less common causes of hypercalcemia. There is a wide individual variation in the development of symptoms and signs of hypercalcemia (Fig. 26.5).
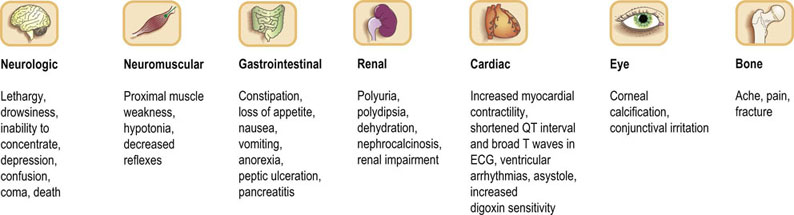
Fig. 26.5 Symptoms and signs of hypercalcemia.
Symptoms are more likely as the serum concentration of calcium increases.
The measurement of PTH has enabled the clinician to discriminate primary hyperparathyroidism from nonparathyroid causes of hypercalcemia (particularly malignancy): an increased or inappropriately detectable intact PTH in the presence of hypercalcemia is observed in primary hyperparathyroidism, whereas a PTH below the limit of detection of the assay is usually observed in nonparathyroid causes of hypercalcemia.
Vitamin D excess or overdose may be obvious from the history, but sometimes only becomes apparent after measurement of the concentrations of ergocalciferol, cholecalciferol and 1,25(OH)2D3.
Primary hyperparathyroidism is relatively common
Primary hyperparathyroidism is a relatively common endocrine disease, characterized by hypercalcemia associated with an increased or inappropriate concentration of PTH. It has an incidence ranging from 1 in 500 to 1 in 1000 of the population. In 80–85% of patients, a solitary parathyroid gland adenoma is present, and the condition is curable by successful removal of the adenoma. The signs and symptoms of parathyroidism are describe d in the box on p. 347.
Hypercalcemia tends to occur late in the course of malignant disease, and is usually a poor prognostic sign
Current evidence indicates that the most common cause of the hypercalcemia of malignancy (HCM) is the production, by tumors or their metastases, of the parathyroid hormone-related protein (PTHrP) that can circulate in blood and exert its effects on the skeleton and kidneys. Production of PTHrP is common in breast, lung, kidney or other solid tumors, but is more rare in hematologic, gastrointestinal, and head and neck malignancies. The amino-terminal portion of PTHrP possesses PTH-like activity that results in hypercalcemia, hypophosphatemia, phosphaturia, increased renal calcium reabsorption, and osteoclast activation.
The second type of hypercalcemia is the result of increased bone resorption by osteoclasts stimulated by factors produced by the primary tumor or, more usually, by metastases, that stimulate osteoclasts by alteration of the RANKL/OPG balance. Mediators such as PTHrP, cytokines, and growth factors (e.g. IL-1, TNF-α, lymphotoxin, and TGF-β) have all been shown to possess osteoclast-stimulating activity that results in significant bone resorption. Production of prostaglandins, especially PGE2, has been demonstrated in several classes of tumors, particularly the breast cancer. Prostaglandins stimulate osteoclastic bone resorption.
Excess of vitamin D is toxic
Increasing therapeutic use of potent vitamin D analogues, hydroxylated at position 1 or at positions 1 and 25, make vitamin D toxicity the third most common cause of hypercalcemia.
Hypocalcemia is common in clinical practice
Changes in ionized calcium can result from pH changes in plasma. Alkalemia (Chapter 25) increases protein binding of calcium, decreasing the ionized calcium concentration. The clinical signs of hypocalcemia are, in the main, due to neuromuscular irritability and are more obvious and severe when the onset of hypocalcemia is acute. In some cases, this irritability may be demonstrated by eliciting specific clinical signs. The Chvostek's sign is the presence of twitching of the muscles around the mouth (circumoral muscles) in response to tapping the facial nerve anterior to the ear, and the Trousseau's sign is the typical contraction of the hand in response to reduced blood flow in the arm induced by inflation of a blood pressure cuff. Numbness, tingling, cramps, tetany and even seizures may occur. Causes of hypocalcemia can be divided into those associated with low PTH(1–84), those in which the decreased serum calcium causes secondary hyperthyroidism, and rare cases in which there is PTH resistance. The most common cause of hypoparathyroidism is a complication of neck surgery.
Pseudohypoparathyroidism syndromes are characterized by hypocalcemia, hyperphosphatemia, and increased concentrations of PTH(1–84).The classic type of pseudohypoparathyroidism is due to end-organ resistance to PTH, caused by a genetic defect resulting in an abnormal regulatory subunit of the G-protein. Confirmation of the diagnosis is made by demonstrating a lack of increase in plasma or urinary cAMP in response to the infusion of PTH. The main causes of hypocalcemia are listed in Table 26.1.
Hypocalcemia may be a result of abnormalities in vitamin D metabolism
Deficiency, acquired or inherited disorders of its metabolism, and vitamin D resistance may occur. The most common causes of vitamin D deficiency are:
Reduced exposure to sunlight, which is common in institutionalized elderly persons and immigrants to Western Europe from the Middle East or Indian subcontinent who wear traditional dress.
Poor dietary intake, i.e. diets such as strict vegetarian, which have inadequate vitamin D content and, in the long term, may result in deficiency.
Malabsorption of vitamin D caused by celiac disease, Crohn's disease, pancreatic insufficiency, inadequate bile salt secretion, and nontropical sprue.
Liver disease (deficient 25-hydroxylation), and renal failure (1α-hydroxylase deficiency).
Metabolic bone disease
Osteoporosis is a common age-related disease of bone
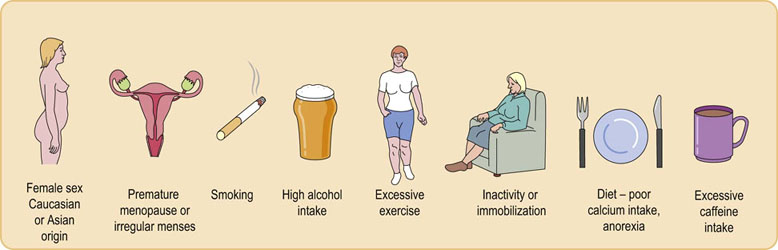
Osteoporosis is defined as a significant reduction of bone mineral density compared with age- and sex-matched reference ranges, with an increased susceptibility to fractures. Seventy million people worldwide are at risk of osteoporosis. Osteoporosis is associated with aging. Bone density decreases from a peak achieved by the age of 30 years in men and women, and the rate of bone loss is accelerated in women after loss of estrogen secretion at the menopause. The progressive loss of bone is a result of uncoupling of bone turnover over a prolonged period of time, with a relative increase of bone resorption or decrease in bone formation. A number of factors have been recognized as contributing to an increased risk of osteoporosis (Fig. 26.6).
Paget's disease of bone is characterized by areas of accelerated bone turnover
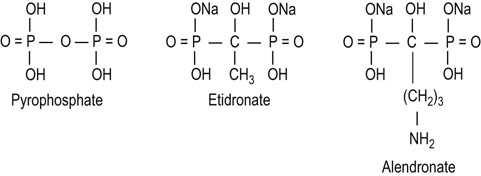
Osteoclasts in Paget's disease are large, numerous, and multinucleate; their activity is coupled to increased osteoblast number and activity. A common biochemical abnormality in this disease is increased serum alkaline phosphatase, indicating increased osteoblast activity. Increased collagen breakdown by osteoclasts results in a high serum and urine concentration of hydroxyproline, pyridinolines, and collagen telopeptides. The bisphosphonates (see Fig. 26.7) have significant antiosteoclastic activity, and are the drugs of first choice for treating Paget's disease.
Summary
 Bone is a metabolically active tissue that undergoes constant remodeling.
Bone is a metabolically active tissue that undergoes constant remodeling.
 The major cells involved in the remodeling process are osteoclasts and osteoblasts.
The major cells involved in the remodeling process are osteoclasts and osteoblasts.
 Bone metabolism is closely interrelated with the metabolism of calcium, which also involves the intestine and kidney.
Bone metabolism is closely interrelated with the metabolism of calcium, which also involves the intestine and kidney.
 Calcium balance is hormonally regulated by parathyroid hormone, vitamin D metabolites and calcitonin.
Calcium balance is hormonally regulated by parathyroid hormone, vitamin D metabolites and calcitonin.
 The measurement of calcium in serum is an important test in clinical laboratories, because both hypercalcemia and hypocalcemia lead to clinical symptoms.
The measurement of calcium in serum is an important test in clinical laboratories, because both hypercalcemia and hypocalcemia lead to clinical symptoms.
 Hypocalcemia is common in clinical practice.
Hypocalcemia is common in clinical practice.
 The main causes of hypercalcemia are primary hyperparathyroidism, malignancy and vitamin D excess.
The main causes of hypercalcemia are primary hyperparathyroidism, malignancy and vitamin D excess.
 Osteoporosis, a decrease in bone density leading to bone fractures, is a major health problem.
Osteoporosis, a decrease in bone density leading to bone fractures, is a major health problem.
Boyle, WJ, Simonet, WS, Lacey, DL. Osteoclast differentiation and activation. Nature. 2003; 423:337–341.
Buckley, KA, Fraser, WD. Receptor activator for nuclear factor kappaB ligand and osteoprotegrin: regulators of bone physiology and immune responses/potential therapeutic agents and biochemical markers. Ann Clin Biochem. 2002; 39:551–556.
Datta, HK, Ng, WF, Walker, JA, et al. The cell biology of bone metabolism. J Clin Pathol. 2008; 61:577–587.
Fraser, WD. Paget's disease of bone. CPD Rheumatology. 2001; 2:8–13.
Fraser, WD. Hyperparathyroidism. Lancet. 2009; 374:145–158.
Harada, S, Rodan, GA. Control of osteoblast function and regulation of bone mass. Nature. 2003; 423:349–355.
Rachner, TD, Khosla, S, Hofbauer, L. Osteoporosis: now and the future. Lancet. 2011; 377:1276–1287.
Richards, JB, Rivadeneira, F, Pastinen, TM, et al. Bone mineral density, osteoporosis, and osteoporotic fractures: a genome-wide association study. Lancet. 2008; 371:1505–1512.
Wharton, B, Bishop, N. Rickets. Lancet. 2003; 362:1389–1400.
Peacock, M, Calcium metabolism in health and disease. CJASN. 2010;5(Suppl 1):523–53. http://cjasn.asnjournals.org/content/5/Supplement_1/S23.long