Complex Lipids
Introduction
Complex lipids encompass the glycerophospholipids, introduced in Chapter 3, and the sphingolipids. These molecules are found mostly in two locations, either embedded in biological membranes or in circulating lipoproteins. The sphingolipids are almost exclusively in cell membranes, primarily the plasma membrane. They carry a wide range of carbohydrate structures which face into the exterior environment and, like glycoproteins, have a range of recognition functions. A major difference between these two classes of lipids is that glycerophospholipids are saponifiable (except plasmalogens), while sphingolipids contain no alkali-labile ester bonds. Thus, it was convenient to isolate sphingolipids from tissues by saponification, and then extract the remaining lipids into organic solvent. Once isolated, the characterization of the glycan structure of the sphingolipids was technically challenging. Therefore, the structures were, for a long time, unknown and mysterious, leading to their name: sphinx-like or sphingolipids.
This chapter discusses the structure, biosynthesis, and function of the two major classes of polar lipids: glycerophospholipids and sphingolipids. In preparation for this chapter, it might help to review the structure of phospholipids in Chapter 3.
Synthesis and turnover of glycerophospholipids
Synthesis of glycerophospholipids
There are many species of glycerophospholipids with a distinct composition of polar head groups and hydrophobic acyl groups (see Chapter 3). With regard to the acyl groups, saturated fatty acids are usually esterified at the sn-1 position, whereas unsaturated fatty acids are esterified at the sn-2 position. Biosynthesis of glycerophospholipids first proceeds by the de novo pathway, and subsequently the originally attached fatty acids in the de novo pathway are replaced with new ones in the remodeling pathway. Through this remodeling pathway, diversity and asymmetry of the acyl groups are generated.
De novo pathway
Phospholipids are in a constant state of synthesis, turnover, and remodeling
The de novo pathway begins with sequential reactions, in which glycerol-3-P is acylated by transfer of two fatty acids from acyl-CoA to produce phosphatidic acid (PA) via the intermediate, lysophosphatidic acid (see Fig. 28.1). Then PA is dephosphorylated to diacylglycerol (DAG) by a specific cytosolic phosphatase. Alternatively, PA reacts with cytidine triphosphate (CTP) to yield the activated phosphatidic acid, cytidine diphosphate (CDP)-DAG. Phosphatidic acid and DAG are common intermediates in the synthesis of both triglycerides (triacylglycerols) and phospholipids. All animal cells, except for erythrocytes, are able to synthesize phospholipids de novo, whereas triglyceride synthesis occurs mainly in liver, adipose tissue, and intestinal cells. The starting material, glycerol-3-phosphate, is formed in most tissues by reduction of the glycolytic intermediate dihydroxyacetone phosphate (DHAP). In liver, kidney and intestine, glycerol-3-P can also be formed directly via phosphorylation of glycerol by a specific kinase. DHAP may also be acylated by addition of a fatty acid to the 1-hydroxyl group; this intermediate is then reduced and acylated to PA.
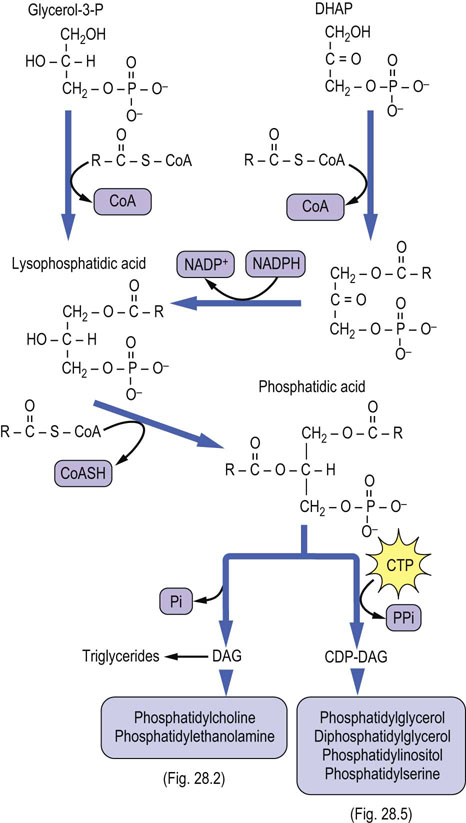
Fig. 28.1 De novo pathway to synthesis of glycerophospholipids.
CDP, cytidine diphosphate; CDP-DAG, CDP-diacylglycerol; CTP, cytidine triphosphate; CoAS, coenzyme A; DHAP, dihydroxyacetone phosphate; Pi, inorganic phosphate; PPi, inorganic pyrophosphate.
The biosynthesis of the major phospholipid phosphatidylcholine (PC; also known as lecithin) from DAG requires activation of choline to CDP-choline. In this series of reactions, shown in Figure 28.2, the choline ‘head group’ is converted to phosphocholine and then activated to CDP-choline by a pyrophosphorylase reaction. The pyrophosphate bond is cleaved and phosphocholine (choline phosphate) is transferred to DAG to form PC. This reaction is analogous to the transfer of GlcNAc-6-P to dolichol or the high-mannose core of lysosomal enzymes – both the sugar and a phosphate are transferred from the nucleotide derivative. Phosphatidylethanolamine (PE) is formed by a similar pathway using CTP and phosphoethanolamine, to form CDP-ethanolamine. Both PC and PE can react with free serine by an exchange reaction to form phosphatidylserine (PS) and the free base, choline or ethanolamine (Fig. 28.3).
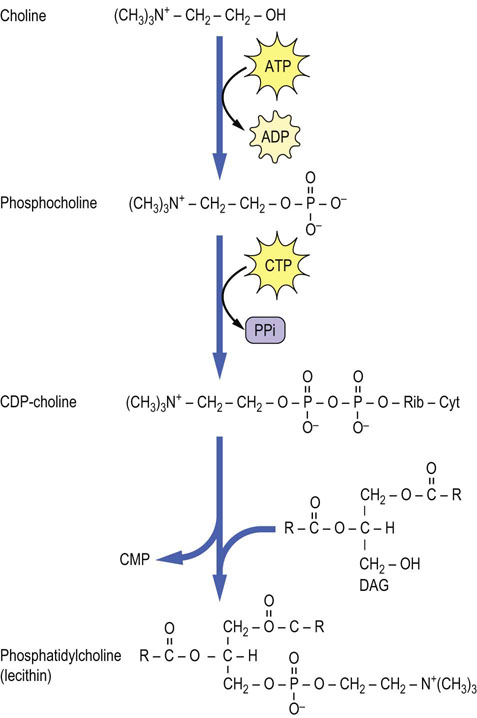
Fig. 28.2 Formation of phosphatidylcholine by the CDP-choline pathway.
This pathway is an extension of the bottom left side of Figure 28.1. Cyt, cytosine; CDP, cytidine diphosphate; CMP, cytidine monophosphate; DAG, diacylglycerol; Rib, ribose; CTP, cytidine triphosphate.
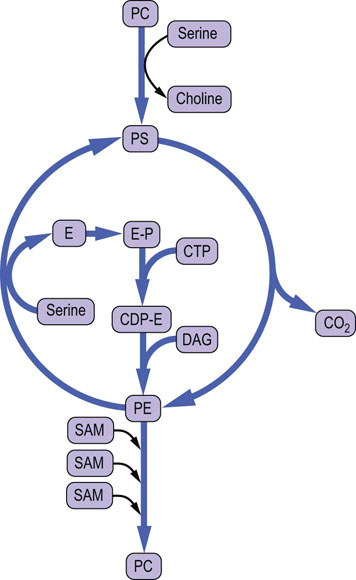
Fig. 28.3 Pathways of interconversion of phospholipids by exchange of head groups, by methylation or by decarboxylation.
CDP, cytidine diphosphate; CTP, cytidine triphosphate; DAG, diacylglycerol; E, ethanolamine; PC, phosphatidylcholine; PE, phosphatidylethanolamine; PC, phosphatidylserine; SAM, S-adenosylmethionine.
Large amounts of PC are needed in liver for biosynthesis of lipoproteins and bile in liver. In a secondary pathway, which is a necessary supplement to the above CDP-choline pathway in liver in times of starvation, PC can also be formed by methylation of PE with the methyl donor S-adenosylmethionine (SAM) (Figs 28.3 and 28.4). This methylation pathway involves the sequential transfer of three activated methyl groups from three molecules of SAM. PE used in this pathway is supplied from PS by a specific mitochondrial decarboxylase (Fig. 28.3).
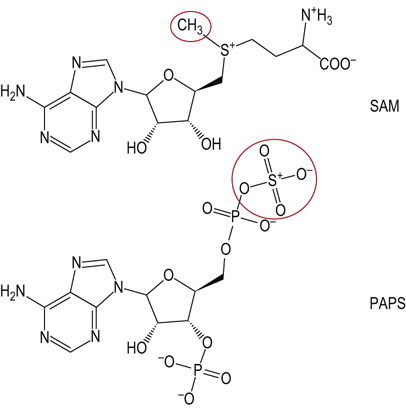
Fig. 28.4 Structures of the methyl and sulfate donors involved in the synthesis of membrane lipids.
SAM, S-adenosylmethionine; PAPS, 3'-phosphoadenosine-5'-phosphosulfate (active sulfate).
PS and other phospholipids with an alcohol head group, e.g. phosphatidylglycerol (PG) and phosphatidylinositol (PI), are synthesized by an alternative pathway. In this case, PA is activated by CTP, yielding CDP-DAG (see Fig. 28.5); the PA group is then transferred to free serine, glycerol or inositol, to form PS, PG or PI, respectively. A second PA may also be added to phosphatidylglycerol to form 1,3-diphosphatidylglycerol (DPG). This lipid, known commonly as cardiolipin, is found almost exclusively in the inner mitochondrial membrane; it represents about 20% of phospholipids in heart mitochondria and is required for efficient activity of electron transport complexes III and IV and the ATP:ADP translocase.
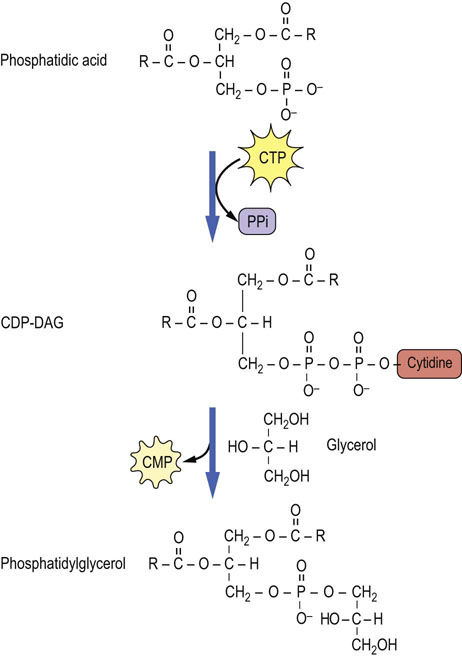
Fig. 28.5 Formation of phosphatidylglycerol by activation of phosphatidic acid to form CDP-DAG, and transfer of DAG to glycerol.
This pathway is an extension of the lower right side of Figure 28.1. CMP, cytidine monophosphate; CTP, cytidine triphosphate.
Plasmalogens are a second major class of mitochondrial lipids, and are enriched in nerve and muscle tissue; in the heart they account for nearly 50% of total phospholipids. The biosynthesis of plasmalogens proceeds from DHAP: it is first acylated at C-1; then the acyl group exchanges with a lipid alcohol to form an ether lipid. The ether lipid is desaturated, leading eventually to a 1-alkenylether-2-acyl-phospholipid. The function of plasmalogens versus diacylphospholipids is not clear, but there is some evidence that they are more resistant to oxidative damage, which may provide protection against oxidative stress in tissues with active aerobic metabolism (see Chapter 37).
Remodeling pathway
The acyl groups of glycerophospholipids are highly diverse and distributed in an asymmetric manner between the sn-1 and sn-2 position of glycerol; polyunsaturated fatty acids, such as arachidonate, are found predominately at the sn-2 position. The composition of fatty acyl groups in phospholipids also varies among tissues and membranes, and with the nature of the head group: choline, ethanolamine, serine, inositol or glycerol. The diversity and asymmetry of phospholipids are not explained by the de novo pathway since phosphatidic acid and DAG are common precursors of both triglycerides and phospholipids. Instead, the redistribution of fatty acids in phospholipids is accomplished by remodeling pathways through the concerted action of phospholipase A2 (PLA2) and lysophospholipid acyltransferases (LPLAT), which remove, replace and, in the process, redistribute fatty acids in phospholipids. Not until the last decade have the LPLAT enzymes been identified; they play an essential role in (re)incorporation of polyunsaturated fatty acids into phospholipids.
Turnover of phospholipids
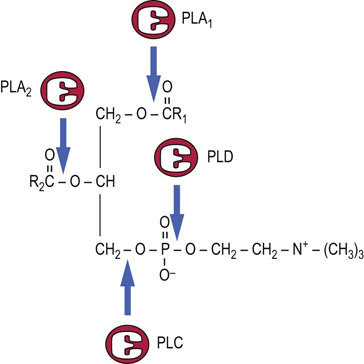
Phospholipids are in a continuous state of turnover in most membranes. This occurs as a result of oxidative damage, during inflammation, and through activation of phospholipases, particularly in response to hormonal stimuli. As shown in Figure 28.6, there are a number of phospholipases that act on specific bonds in the phospholipid structure. Phospholipase A2 (PLA2) and phospholipase C (PLC) are particularly active during the inflammatory response and in signal transduction. Phospholipase B (not shown) is a lysophospholipase that removes the second acyl group after action of PLA1 or PLA2. The lysophospholipids may be degraded or recycled (reacylated).
Sphingolipids
Structure and biosynthesis of sphingosine
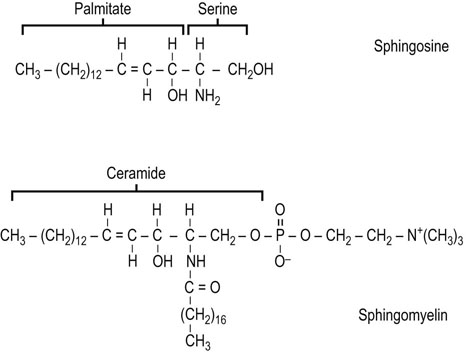
Sphingolipids are a complex group of amphipathic, polar lipids. They are built on a core structure of the long-chain amino alcohol sphingosine, which is formed by oxidative decarboxylation and condensation of palmitate with serine. In all sphingolipids, the long-chain fatty acid is attached to the amino group of the sphingosine in an amide linkage (Fig. 28.8). Because of the alkaline stability of amides, compared to esters, sphingolipids are nonsaponifiable, which facilitates their separation from alkali-labile glycerolipids.
The synthesis of the sphingosine base of sphingolipids involves condensation of palmitoyl-CoA with serine, in which the carbon-1 of serine is lost as carbon dioxide. The product of this reaction is converted in several steps to sphingosine, which is then N-acylated to form ceramide (N-acylsphingosine). Ceramide (see Fig. 28.9) is the precursor and backbone structure of both sphingomyelin and glycosphingolipids.
Sphingomyelin
Sphingomyelin is the only sphingolipid that contains phosphate and is the major phospholipid in the myelin sheath of nerves
Sphingomyelin (see Fig. 28.8) is found in plasma membranes, subcellular organelles, endoplasmic reticulum, and mitochondria. It comprises 5–20% of the total phospholipids in most cell types, and is mostly localized in the plasma membrane. The phosphocholine group in sphingomyelin is transferred to the terminal hydroxyl group of sphingosine by a transesterification reaction with phosphatidylcholine. The fatty acid composition varies, but long-chain fatty acids are common, including lignoceric (24 : 0), cerebronic (2-hydroxylignoceric) and nervonic (24 : 1) acids.
Glycolipids
Sphingolipids containing covalently bound sugars are known as glycosphingolipids or glycolipids. As with glycoconjugates, in general, the structure of the oligosaccharide chains is highly variable. In addition, the glycosyltransferase distribution and glycosphingolipid content of cells varies during development and in response to regulatory processes.
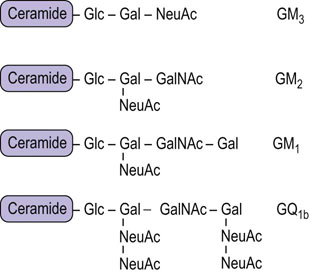
Glycolipids can be classified into three main groups: neutral glycolipids, sulfatides and gangliosides. In all of these compounds, the polar head-group, comprising the sugars, is attached to ceramide by a glycosidic bond at the terminal hydroxyl group of sphingosine. Figure 28.9 illustrates the structure and biosynthesis of some of the simpler glycolipids. Neutral glycolipids contain only neutral and amino sugars. Glucosylceramide (GlcCer) and galactosylceramide (GalCer) are the smallest members of this class of compounds and serve as the nucleus for elaboration of more complex structures. Sulfatides are formed by addition of sulfate from the sulfate donor, 3'-phosphoadenosine-5'-phosphosulfate (PAPS) (Fig. 28.4), yielding, for example, GalCer 3-sulfate. Finally, glycolipids containing sialic acids (N-acetylneuraminic acid, NeuAc) are termed gangliosides.
Structure and nomenclature of gangliosides
Gangliosides are glycosphingolipids containing sialic (N-acetylneuraminic) acid
The term ‘ganglioside’ refers to glycolipids that were originally identified in high concentrations in ganglionic cells of the central nervous system. In general, more than 50% of the sialic acid in these cells is present in gangliosides. Gangliosides are also found in the surface membranes of cells of most extraneural tissues, but in these tissues they account for less than 10% of the total sialic acid.
The nomenclature used to identify the various gangliosides is based on the number of sialic acid residues contained in the molecule, and on the sequence of the carbohydrates (Fig. 28.10). GM refers to a ganglioside with a single (mono) sialic acid, whereas GD, GT and GQ would indicate two, three and four sialic acid residues in the molecule, respectively. The number after the GM, e.g. GM1, refers to the structure of the oligosaccharide. These numbers were derived from the relative mobility of the glycolipids on thin layer chromatograms; the larger, GM1, gangliosides migrate the most slowly.
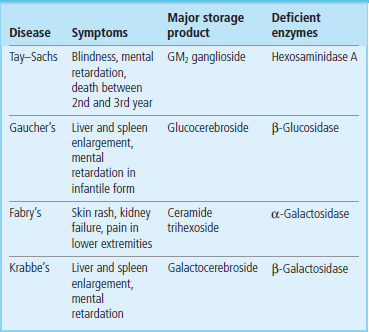
Lysosomal storage diseases resulting from defects in glycolipid degradation
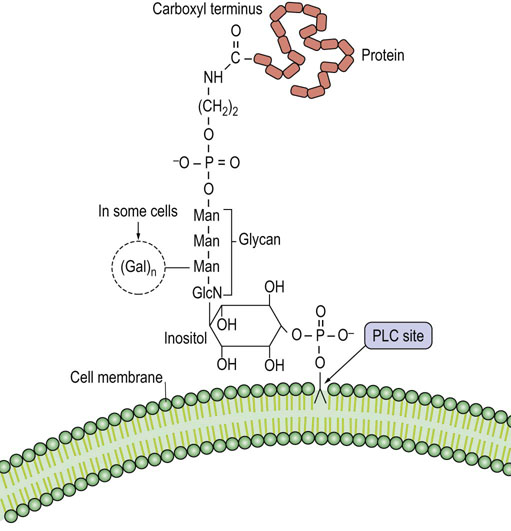
The complex oligosaccharides on glycolipids are built up, one sugar residue at a time, in the Golgi apparatus and are degraded in a similar stepwise fashion but in the opposite direction by a series of exoglycosidases in lysosomes (Fig. 28.11). Defects in sequential degradation of glycolipids lead to a number of lysosomal storage diseases, known as sphingolipidoses and gangliosidoses (Table 28.1). These diseases are autosomal recessive in inheritance. Heterozygotes are asymptomatic, indicating that a single copy of the gene for a functional enzyme is sufficient for apparently normal turnover of glycolipids. Like I-cell disease (Chapter 27), the sphingolipidoses are characterized by accumulation of undigested lipids in inclusion bodies in the cells.
ABO blood group antigens
Blood transfusion replenishes the oxygen-carrying capacity of blood in persons who suffer from blood loss or anemia (see Chapter 5). The term ‘blood transfusion’ is something of a misnomer, because it involves only the infusion of washed and preserved red cells. The membranes of red blood cells contain a number of blood group antigens, of which the ABO blood group system is the best understood and most widely studied.
The ABO blood group antigens are complex carbohydrates present as components of glycoproteins or glycosphingolipids of red cell membranes (Fig. 28.12). The H locus codes for a fucosyltransferase, which adds fucose to a galactose residue in a glycan chain. Individuals with type A blood have, in addition to the H substance, an A gene that codes for a specific GalNAc transferase that adds GalNAc α1,3 to the galactose residue of H substance, to form the A antigen. Individuals with type B blood have a B gene that codes for a galactosyl transferase that adds galactose α1,3 to the galactose residue of H substance, to form the B antigen. Individuals with type AB blood have both the GalNAc and the Gal transferases, and their red blood cells contain a mixture of A and B substances. Those with type O blood have only H substance on their red cell membranes; they do not make either enzyme. Enzymes such as coffee bean α-galactosidase can remove the galactose from type B red cells, an approach that is being tested for increasing the supply of type O (universal donor) red cells.
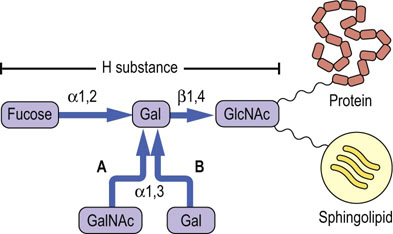
Fig. 28.12 Relationship between the H, A, and B blood group substances.
The terminal oligosaccharide is linked through other sugars to proteins and lipids of the red cell membrane. GlcNAc, N-acetylglucosamine; GalNAc, N-acetylgalactosamine; Gal, galactose.
An individual may have type A, type B, type AB or type O blood. Individuals with type A cells develop natural antibodies in their plasma that are directed against and will agglutinate type B and type AB red blood cells; those with type B red cells develop antibodies against A substance and will agglutinate type A and type AB blood. Persons with type AB blood have neither A nor B antibodies and are called ‘universal recipients’, as they can be transfused with cells of either blood type. Individuals with type O blood have only H substance, not A or B substance, on their red blood cells and are ‘universal donors’, as their red blood cells are not agglutinated by either A or B antibodies; however, they may accept blood only from a type O donor.
The ABO antigens are present on most cells in the body but they are referred to as blood group antigens because of their association with transfusion reactions. The transfusion reaction is the result of reaction of host antibodies with transfused red cells, resulting in complement-mediated hemolysis (see Chapter 38). While the transfusion reaction demonstrates the role of carbohydrates in recognition of the foreign red cells, the physiologic function of blood group substances is unclear. Persons with an O genotype are generally as healthy as those with A or B genotype. However, there is some evidence that specific phenotypes may confer differential resistance to disease; for example, people with type A and type O blood appear to be more susceptible to smallpox and cholera, respectively.
Other blood group substances
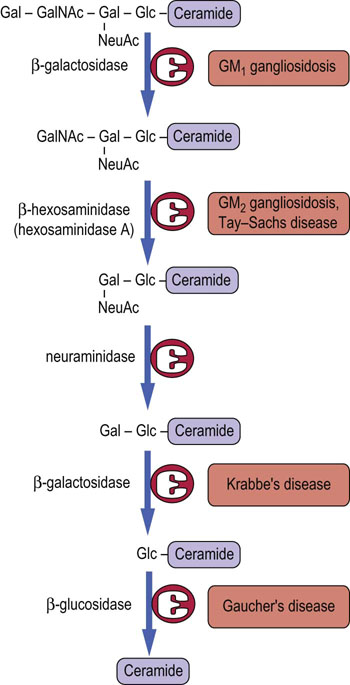
The Lewis blood group antigens correspond to a set of fucosylated glycan structures. The Lewis-A antigen (Lewisa) is synthesized by a fucosyltransferase that transfers a fucose residue to a GlcNAc residue in a glycan chain (Fig. 28.13), while the Lewisb antigen is synthesized by the action of a second fucosyltransferase which transfers fucose to the terminal galactose residue in the same glycan chain. Note the similarity of these structures to the sialyl Lewis-X antigen in Figure 27.9 and the ABO antigens in Figure 28.12. There are 13 fucosyltransferase genes in the human genome. Changes in fucosylation of glycans are associated with differentiation, development, carcinogenesis and metastasis.
The P blood group antigens expressed on the globo-series glycosphingolipids are distributed in red cells and other tissues. Again, the glycans in this blood group are synthesized by the sequential action of distinct glycosyltransferases. The physiologic function of these blood groups is unknown, but P antigens are associated with urinary tract infections and parvovirus infections. Uropathogenic strains of E. coli express lectins that bind to the Galα1,4Gal moiety of the Pk and P1 antigens. More work is needed to understand the genetics and biochemistry of these and other blood group antigens as well as their roles in physiology and disease.
Summary
 Complex polar lipids are essential components of all cell membranes.
Complex polar lipids are essential components of all cell membranes.
 Phospholipids are the major structural lipids of all membranes, but they also have important functional properties as surfactants, as cofactors for membrane enzymes, and as components of signal transduction systems.
Phospholipids are the major structural lipids of all membranes, but they also have important functional properties as surfactants, as cofactors for membrane enzymes, and as components of signal transduction systems.
 The primary route for de novo biosynthesis of phospholipids involves the activation of one of the components (either DAG or the head group) with CTP to form a high-energy intermediate, such as CDP-diacylglycerol or CDP-choline.
The primary route for de novo biosynthesis of phospholipids involves the activation of one of the components (either DAG or the head group) with CTP to form a high-energy intermediate, such as CDP-diacylglycerol or CDP-choline.
 Phospholipids undergo maturation in the remodeling pathway, in which acyl groups at the sn-2 position are replaced with new ones, yielding diversity and asymmetry of the hydrophobic moiety of phospholipids.
Phospholipids undergo maturation in the remodeling pathway, in which acyl groups at the sn-2 position are replaced with new ones, yielding diversity and asymmetry of the hydrophobic moiety of phospholipids.
 Glycosphigolipids function as receptors for cell–cell recognition and interactions, and as binding sites for symbiotic and pathogenic bacteria and for viruses. Carbohydrate structures on glycosphingolipids of red cell membranes are also antigenic determinants responsible for the ABO and other blood types.
Glycosphigolipids function as receptors for cell–cell recognition and interactions, and as binding sites for symbiotic and pathogenic bacteria and for viruses. Carbohydrate structures on glycosphingolipids of red cell membranes are also antigenic determinants responsible for the ABO and other blood types.
 Glycosphingolipids are degraded in lysosomes by a sequence of reactions that involve a stepwise removal of sugars from the nonreducing end of the molecule, with each step involving a specific lysosomal exoglycosidase. A number of inherited lysosomal storage diseases result from defects in degradation of sphingolipids.
Glycosphingolipids are degraded in lysosomes by a sequence of reactions that involve a stepwise removal of sugars from the nonreducing end of the molecule, with each step involving a specific lysosomal exoglycosidase. A number of inherited lysosomal storage diseases result from defects in degradation of sphingolipids.
Beck, M. Therapy for lysosomal storage diseases. IUBMB Life. 2010; 62:33–40.
Brown, JR, Crawford, BE, Esko, JD. Glycan antagonists and inhibitors: a fount for drug discovery. Crit Rev Biochem Mol Biol. 2007; 42:481–515.
Cipolla, L, Araujo, AC, Bini, D, et al. Discovery and design of carbohydrate-based therapeutics. Expert Opin Drug Discov. 2010; 5:721–737.
Desnick, RJ, Schuchman, EH. Enzyme replacement therapy for lysosomal diseases: lessons from 20 years of experience and remaining challenges. Annu Rev Hum Genet. 2012; 13:307–335.
Kulkarni, AA, Weiss, AA, Iyer, SS. Glycan-based high affinity ligands for toxins and pathogen receptors. Med Res Review. 2010; 30:327–393.
Mignani, R, Feriozzi, S, Schaefer, RM, et al. Dialysis and transplantation in Fabry disease: indications for enzyme replacement therapy. Clin J Am Soc Nephrol. 2010; 5:379–385.
Sindou, H, Shimizu, T. Acyl-CoA:lysophospholipid acyltransferases. J Biol Chem. 2009; 284:1–5.
Van Meer, G, Voelker, DR, Feigenson, GW. Membrane lipids: where they are and how they behave. Nat Rev Mol Cell Biol. 2008; 9:112–124.
Yamamoto, F, Cid, E, Yamamoto, M, Blancher, A. ABO research in the modern era of genomics. Transfus Med Rev. 2012; 26:103–118.
Xu, YH, Barnes, S, Sun, Y, Grabowski, GA. Multi-system disorders of glycosphingolipid and ganglioside metabolism. J Lipid Res. 2010; 51:1643–1675.
Blood groups. www.bloodbook.com/type-sys.html.
Gaucher's disease. www.ninds.nih.gov/disorders/gauchers/gauchers.htm.
Paroxysmal nocturnal hemoglobinuria. http://emedicine.medscape.com/article/207468-overview.
Tay–Sachs disease. www.ninds.nih.gov/disorders/taysachs/taysachs.htm.