Neurochemistry
Introduction
The brain is, in many ways, a chemist's delight. This is so because it illustrates various general principles of biology applied to a highly specialized tissue that ultimately regulates all the other tissues of the body. This chapter highlights the differences between the central nervous system – that is, the brain and spinal cord – and the peripheral nervous system, which is outside the dura (the thick fibrous covering that contains the cerebrospinal fluid (CSF)).
Brain and peripheral nerve
The distinction between brain and peripheral nerve essentially reflects the division between the central nervous system (CNS) and the peripheral nervous system (PNS): a convenient dividing line being the confines of the dura, within which watertight compartment is the CSF, partially produced (about one-third of the total volume) through the action of the blood–brain barrier. Myelin insulates the axons of nerves; the chemical composition of CNS myelin is quite distinct from that of PNS myelin, not least because the two forms are produced by two different types of cells: the oligodendrocytes within the CNS and the Schwann cells within the PNS. The distinction between the functions of the CNS and those of the PNS is fundamental to differential diagnosis in neurology. A typical example is the difference between the demyelination of the CNS that occurs in multiple sclerosis, and the demyelination of the PNS that occurs in Guillain–Barré syndrome.
The blood–brain barrier
The term blood–brain ‘barrier’ is a slight misnomer, in that the barrier is not absolute but relative: its permeability depends on the size of the molecule in question
Initially, experiments based upon use of a dye (Evans blue) bound to albumin showed that, over a period of hours, an animal progressively turned blue in all tissues, with the notable exception of the brain, which remained white. It subsequently became clear that 1 molecule in 200 of serum albumin passed normally into the CSF, which is analogous to lymph. It also became obvious that, for any given protein, the ratio of its concentrations in CSF and serum was a linear function of the molecular radius of the molecules in solution.
There are a total of six sources of the CSF
Under normal and pathologic conditions, proteins pass from these cellular or tissue sources into the CSF, and their degree of filtration and/or rate of local synthesis vary. The total quantity of the CSF therefore constitutes the algebraic summation of these six sources (Fig. 41.2.1).
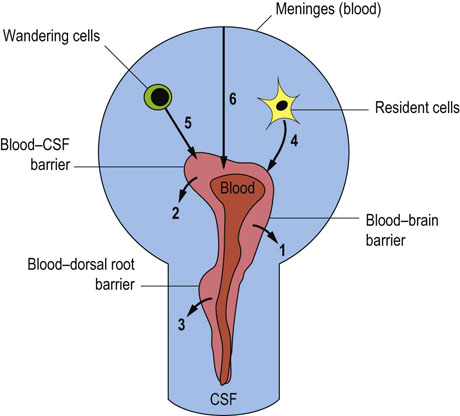
Fig. 41.2.1 The six main sources of cerebrospinal fluid (CSF).
The involved processes comprise passage across barriers (from the blood, i.e. 1, 2, 3) and direct sources of local production (CNS cells, i.e. 4, 5, 6).
 The blood–brain barrier (the parenchymal capillaries) gives rise to about one-third of the volume of CSF, and is known as the interstitial fluid source.
The blood–brain barrier (the parenchymal capillaries) gives rise to about one-third of the volume of CSF, and is known as the interstitial fluid source.
 The blood–CSF barrier provides the bulk of CSF (almost all of the remaining two-thirds), termed choroidal fluid because it is principally provided by the choroid plexi (capillary tufts) situated in the lateral ventricles and, to a lesser degree, the plexi situated in the third and fourth ventricles.
The blood–CSF barrier provides the bulk of CSF (almost all of the remaining two-thirds), termed choroidal fluid because it is principally provided by the choroid plexi (capillary tufts) situated in the lateral ventricles and, to a lesser degree, the plexi situated in the third and fourth ventricles.
 The dorsal root ganglia contain capillaries that have a much greater degree of permeability.
The dorsal root ganglia contain capillaries that have a much greater degree of permeability.
 The brain parenchyma of the CNS produces a number of brain-specific proteins. These include prostaglandin synthase (formerly called β-trace protein), and transthyretin (a protein formerly called prealbumin).
The brain parenchyma of the CNS produces a number of brain-specific proteins. These include prostaglandin synthase (formerly called β-trace protein), and transthyretin (a protein formerly called prealbumin).
 The CSF circulating cells, mainly lymphocytes within the CNS, synthesize local antibodies. However, in the CNS there is strong presence of immune suppressor cells. Because of this, in brain infections such as meningitis, steroids are given in addition to antibiotics, to suppress the potentially devastating effects, within this confined space, of inflammation associated with the intrathecal immune response.
The CSF circulating cells, mainly lymphocytes within the CNS, synthesize local antibodies. However, in the CNS there is strong presence of immune suppressor cells. Because of this, in brain infections such as meningitis, steroids are given in addition to antibiotics, to suppress the potentially devastating effects, within this confined space, of inflammation associated with the intrathecal immune response.
 The meninges represent a sixth source of CSF under pathologic conditions; they can give rise to dramatic increase in the concentrations of CSF proteins.
The meninges represent a sixth source of CSF under pathologic conditions; they can give rise to dramatic increase in the concentrations of CSF proteins.
Cells of the nervous system
Fewer than 10% of the cells of the nervous system are large neurons. The three major cell types in the nervous system (which each constitute about 30%) are astrocytes, which also make up part of the blood–brain barrier; oligodendrocytes, which are principally composed of fat and serve to insulate the axons; and microglia, which are essentially resident macrophages (scavengers).
These different cell types are associated with predominant protein molecules that are important in various brain pathologies (Table 41.2.1). Other minor constituents of the nervous system include the ependymal cells, which are ciliated cells secreting brain-specific proteins such as prostaglandin synthase. The brain endothelial cells, unlike other tissue capillaries, have tight junctions that bind them together; this feature is believed also to contribute to the blood–brain barrier, although it is the basement membrane which is the major source of molecular sieving of the different-sized proteins.
Table 41.2.1
The different cells of the CNS, and their protein markers indicating brain pathologies
| Cell | Protein | Pathology |
| Neuron | Neuron-specific enolase | Brain death |
| Astrocyte | GFAP | Plaque (or scar) |
| Oligodendrocyte | Myelin basic protein | De/remyelination |
| Microglia | Ferritin | Stroke |
| Choroid plexi | Asialotransferrin | CSF leak (rhinorrhea) |
Neurons
The significant features of neurons are their length, their many interconnections, and the fact that they do not divide postpartum
There is an archetypal notion of the electrical activity of the nervous system – in particular, of the electrical activity of neurons. However, three other biological features of neurons are particularly worthy of note: their length, their prolific interconnections, and the fact that they do not divide postpartum.
Because of their great length, neurons depend upon an efficient system of axonal transport
Neurons can typically be 1 m long; thus the nucleus, the source of information for the synthesis of neurotransmitters, is typically quite remote from the synaptic terminal, the site of release of those transmitters. Because of this extensive length, a crucial requirement is the neuron's ability to transport material both from the nucleus towards the synapse (anterograde transport) and from the synapse to the nucleus (retrograde transport). Neurons have evolved special characteristics to deal with this separation of their two functional sites, and to maintain electrical activity at the nodes of Ranvier (Fig. 41.2.2).
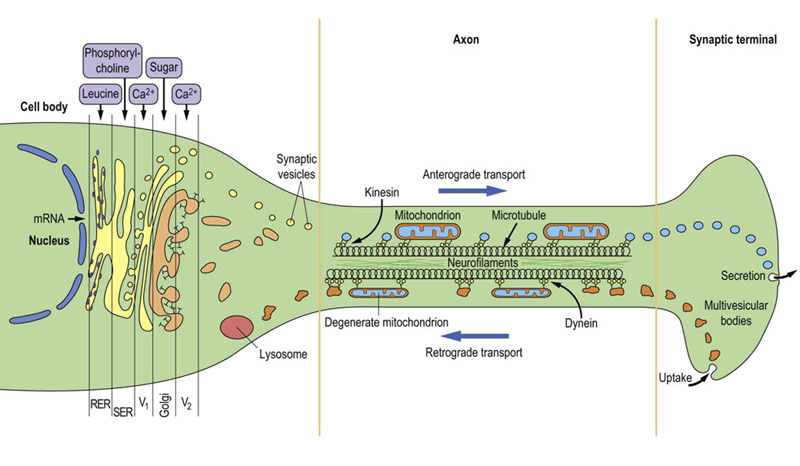
Fig. 41.2.2 The functional structure of a neuron.
Within the cell body, there is specialized movement through the Golgi stack by the components required to form synaptic vesicles (V1, V2). In the axon, there is fast axonal transport along microtubules via the motile proteins, kinesin (in anterograde transport) or dynein (in retrograde transport). RER, rough endoplasmic reticulum; SER, smooth endoplasmic reticulum.
The normal ‘resting’ movement within the axon is mediated by separate molecular ‘motors’ (motile proteins): kinesin in the case of anterograde transport and dynein in retrograde transport. The materials being transported in each direction are also rather different, and the different components of axonal structure shown in Figure 41.2.2 possess the capacity for different speeds of transportation. During growth, a separate form of transport (toward the synapse) occurs that takes place at the rate of about 1 mm/day; this flow constitutes bulk movement of the building blocks such as the filamentous proteins.
Neurotransmission is an energy-demanding process
Brain constitutes 2% of body mass, yet under resting conditions it responsible for 20% of the overall glucose consumption. Glucose is almost exclusive energy substrate for the brain. The brain does not utilize fatty acids for energy production. Under certain conditions it may utilize β-hydroxybutyrate or lactate derived from circulation. Large amount of energy is required to maintain plasma membrane resting potentials of the neurons, that are subject to continuous (10–60 Hz frequency) depolarization cycles (action potentials). To meet these demands, over 70% of brain energy is produced and utilized by neurons. Astrocytes utilizing glucose release significant amounts lactate to extracellular brain compartment, and it serves as complementary energy source for the neurons. However, neither exogenous nor endogenous lactate, can fully replace glucose as the principal energy source.
Neuroglial structures
Astrocytes and oligodendrocytes comprise the neuroglial structures
In the cortex, or gray matter, one typically finds a protoplasmic astrocyte with one set of processes surrounding the endothelial cells, thereby helping to ‘filter’ materials from the blood, and a separate set of processes surrounding the neurons, which are thereby being ‘fed’ selected substances that have been extracted from the blood for passage to the neurons.
In the white matter, the astrocytes have a rather more fibrous appearance and have more of a structural role. When there is injury to the CNS, astrocytes can play a major part in the reaction, synthesizing large amounts of the glial fibrillary acidic protein (GFAP). This is the cellular equivalent of scar tissue and is found in diseases such as multiple sclerosis, in which it is the major constituent of the characteristic plaques. Astrocytes are not present in the PNS.
The oligodendrocytes present in the CNS can wrap round as many as 20 axons, forming the myelin sheath that insulates these neuronal processes from one another and stops cross-talk between neurons. There is also intense oligodendrocyte mitochondrial activity at the nodes of Ranvier, which are parallel to the sites of depolarization within the underlying axon. In the PNS, the Schwann cells form the myelin and, typically, wrap round only a single axon.
Synaptic transmission
One of the unique chemical characteristics of the brain is the massively high density of synapses between different neurons
Thus a locally acting neurohormone is released by one axon onto many other cell bodies. On the receiving end, a given cell body will typically receive myriad cellular products via its profusely branched dendritic tree: each branch can be smothered in synapses. The ‘neurotransmitter’ (neurohormone) is released by the axon's nerve terminals of the first neuron onto the dendrite of the second neuron or a nonneuronal cell. This is mediated by a neurotransmitter receptor on the respondent cell. There is usually a second messenger, such as a cyclic nucleotide, which may stimulate protein phosphorylation. Typically, G-proteins are found just under the neurotransmitter receptor protein spanning the cell membrane, where they act to ‘couple’ the first messenger (e.g. norepinephrine) to a second messenger (e.g. cyclic AMP, cAMP) (Chapter 40). Some neurotransmitter receptors are coupled with ion channels.
Neurotransmitters are normally inactivated after their actions on the target cell. Hydrolysis is a major mechanism by which this is achieved. The best-studied example is that of the enzyme acetylcholinesterase. There can also be regulation at the level of the second messenger, such as cAMP, which is broken down by the enzyme phosphodiesterase. Phosphodiesterase is inhibited by caffeine and other methylxanthines, and they thereby mimic many of the effects of adrenergic neurotransmission.
Synaptic transmission involves the recycling of membrane components
In addition to release of a specific neurohormone, there is also an extensive system for recycling of membrane constituents associated with this process. The synaptic vesicles contain a very high concentration of the relevant neurotransmitter, which is bounded by a membrane (Chapter 42.1). During synaptic release of the transmitter, there is fusion of the synaptic vesicle membrane (containing the neurotransmitter) with the presynaptic membrane. This increase in total membrane mass is redressed by invagination of the lateral aspects of the nerve terminals, where an inward puckering movement of the membrane is effected by contractile movements of the protein clathrin. There then follows a form of pinocytosis of the excess membrane, which is transported in retrograde fashion toward the nucleus, to be digested in lysosomes.
Types of synapse
Because of the multitude of different synaptic inputs to a given neuron, the final algebraic summation results in a ‘decision’ at the level of the axon hillock (the site of origin of the axon from the cell body) as to whether or not to transmit an action potential down the axon as an all-or-nothing phenomenon. However, even before this decision is made, the input of a particular neurotransmitter can essentially be classified as excitatory or inhibitory.
In addition to the relatively short-term decisions concerning action potentials (Chapter 42.1), there is a longer-term modulation of the resting membrane potential, moving it either closer to (excitation) or further from (inhibition) the critical membrane potential, which is the level at which the resting membrane potential will finally trigger an action potential at the axon hillock. Many drugs have a longer-term effect on modulation, in addition to the short-term effect, which partially explains their addictive effect; this can be seen with alcohol or the opioid drugs. There are also long-term effects during treatment with various drugs: for example, those used to treat endogenous depression, such that it may be weeks before any beneficial effects are seen.
Cholinergic transmission
The best-studied neurotransmitter is acetylcholine
Acetylcholine (ACh) is synthesized in the cytoplasmic compartment of cholinergic nerve terminals from acetyl-CoA and choline by choline acetyltransferase. The enzyme is expressed exclusively in the cholinergic neurons. Acetyl-CoA is synthesized from pyruvate derived from glycolysis, whereas choline is taken up from extracellular compartment by plasma membrane-potential-driven high-affinity choline uptake system.
As a model system, this transmitter can have two rather different effects, depending upon its site of origin within the nervous system (i.e. central or peripheral). The effects originally demonstrated by experiments with nicotine are characteristic of the nicotinic receptors, whereas those demonstrated with muscarine characterize the muscarinic receptors. The nicotinic type of transmission is exerted by motor neurons located in the brainstem and anterior horns of the medulla oblongata. Another group of central cholinergic neurons located in the brain septum plays a key role in the basic and higher cognitive functions through activation of postsynaptic muscarinic receptors. There is a complex picture of the agonists and antagonists associated with the regional actions of ACh (Fig. 41.2.3). The classic antagonist of the muscarinic effect is atropine and the best-studied blocker for the nicotinic receptor is the poisonous snake venom α-bungarotoxin.
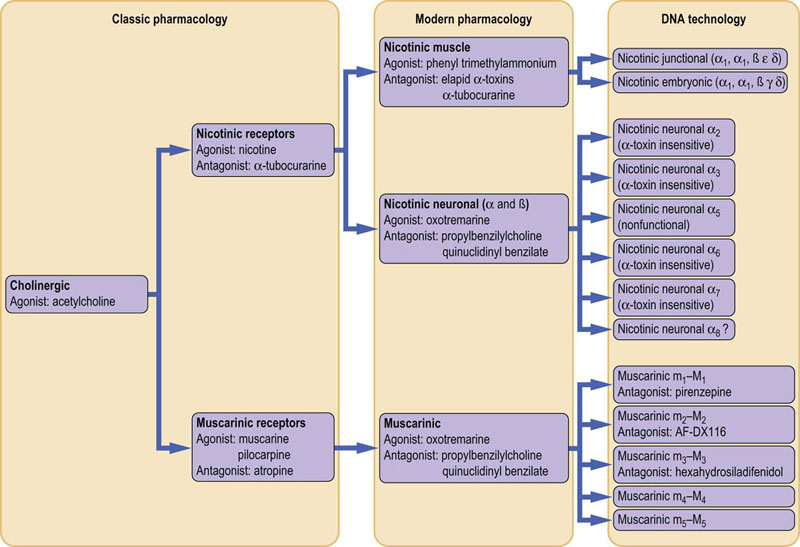
Fig. 41.2.3 The history of naming: acetylcholine agonists and antagonists.
The changes in nomenclature, for the agonists and antagonists of the different central (neuronal) versus peripheral (muscle) regional actions of acetylcholine (ACh).
In Alzheimer's disease, amyloid-β(1–42), in combination with other neurotoxic factors, causes preferential impairment of cholinergic neurons in the brain septum, yielding progressive loss of cognitive function, which leads to dementia. In the early stages of this disease, the inhibitors of acetylcholinesterase with M2 receptor agonist properties improved cognitive functions but had no effect on disease progress. The antagonists of glutamatergic NMDA receptors are employed to reduce excitotoxic effects of excessive activation of glutamatergic neurons.
In myasthenia gravis, autoantibodies are formed against the nicotinic receptor. However, by blocking the hydrolysis of ACh, for example by means of the drug edrophonium (which inhibits acetylcholinesterase), the concentration of ACh can be effectively increased (Chapter 42.1).
Peripheral cholinergic neurons are located in parasympathetic ganglia and innervate all visceral tissues. They dilate blood vessels of the gatrointestinal tract and enhance salivation and peristalsis. They also constrict airways, control heart function, constrict the pupils and regulate lens accommodation, and stimulate sexual arousal and genital erection.
Catecholamine transmission
Catecholamines, epinephrine and norepinephrine are synthesized from L-tyrosine in the sequence of reactions catalyzed by tyrosine hydroxylase/aromatic amino acid decarboxylase, then dopamine β-hydroxylase and phentolamine-N-methyltransferase, yielding dopamine, norepinephrine, and epinephrine, respectively. Dopamine is a precursor of norepinephrine and epinephrine.
Dopamine is a transmitter in the brain dopaminergic neurons located in its several areas, including substantia nigra. They are involved in reward-driven learning, regulation of mood, attention, learning, and prolactin release through different classes of dopamine receptors (D1–5). Disturbances of dopamine metabolism are associated with several central nervous system pathologies including Parkinson's disease, schizophrenia, and restless legs syndrome. To overcome dopamine deficits in some of these diseases L-DOPA, its precursor, is administered, as it easily crosses blood–brain barrier. Dopamine is given to patients in shock and heart failure to elevate cardiac output and increase blood pressure and renal filtration. Several drugs, including amphetamines, cocaine, and nicotine, exert their behavioral and addictive effects through excessive stimulation of the release and the increase of dopamine level in the synaptic cleft. They also stimulate serotoninergic and norepinephrinergic transmission in the brain.
Norepinephrine and epinephrine are synthesized in the brain and peripheral sympathetic ganglia by respective groups of neurons acting as neurotransmitters. On the other hand, catecholamines released from chromaffin cells into circulation exert endocrine effects. In the brain, they exert regulatory functions in decision-making processes. Peripherally, they increase blood pressure (they cause vasoconstriction and increase the rate and force of cardiac muscle contraction), cause bronchial and pupil dilatation, inhibit peristalsis, increase sweating and renin secretion, and promote ejaculation. Their actions are mediated through two separate receptors: α-adrenergic receptor, blocked by phentolamine, and β-adrenergic receptor, blocked by propranolol. The latter drug was commonly used by cardiologists (other β-blockers are the mainstay of treatment in coronary heart disease), but neurologists also use it as part of the treatment of Parkinson's disease. Many adrenergic effects are mediated by cAMP (Chapter 42.1).
The action of catecholamines is terminated by their reuptake and degradation to aldehydes by mitochondrial monoamine oxidases and subsequent methylation by catechol-O-methyltransferase to homovanillic or vanillylmandelic acids, which are excreted with urine. Excess of these compounds in urine may indicate the presence of adrenal medullar tumor, pheochromocytoma.
Glutamate: glutamatergic transmission
Depending on the brain region, 50–80% of the neuronal population is glutamatergic
The mean L-glutamate level in the brain is in the range of 5–10 mmol/L. It is synthesized from α-ketoglutarate by glutamate dehydrogenase and aminotransferases or from glutamine by phosphate-activated glutaminase. The L-glutamate/glutamate–zinc complex is taken up by synaptic vesicles of glutamatergic presynaptic nerve terminals, where it reaches concentrations exceeding 100 mmol/L. Glutamate is released upon depolarization, transiently reaching high concentrations in the synaptic clefts. Its binding to different classes of receptors, including NMDA (the principal one), causes depolarization/activation of postsynaptic recipient neurons. Glutamatergic receptor stimulation is subject to multiple regulations, which play an important role in synaptic plasticity, termed long-term potentiation. This phenomenon takes place in the hippocampus and in the different regions of brain cortex, and is involved in learning, memory formation, and other cognitive functions.
Glutamate is quickly taken up from the synaptic space by specific transporters expressed mainly on the adjacent astroglial cells. There, glutamine synthetase converts glutamate to glutamine, which is subsequently transported back to glutamatergic neurons.
Excessive glutamate release or its impaired uptake, which takes place, among others, in ischemia, hypoglycemia and exposure to neurotoxic xenobiotics, may cause its excessive accumulation in the extracellular space. This in turn causes prolonged depolarization of the recipient cells and, consequently, excitotoxic injury. Epilepsy is the pathologic condition caused by excessive glutamate release by pathologically stimulated glutamatergic neurons and/or the deficiency of inhibitory GABAergic transmission (see below).
γ-Aminobutyric acid (GABA): GABAergic transmission
GABA is the chief inhibitory neurotransmitter in the brain
The inhibitory effect of GABA on postsynaptic neurons results from binding to specific GABAA receptors. GABA concentration remains in the range of 4–6 mmol/L. It is the ligand for gated chloride channels. Their opening upon GABA binding causes flow of Cl– ions into the neuron, causing its hyperpolarization and inhibition of transmitter function. GABA is synthesized by L-glutamate decarboxylase present in the cytoplasm of GABAergic neurons. GABA action is terminated mainly by its uptake by presynaptic terminals through high-affinity GABA transporter. GABA may then be either loaded again into vesicles or metabolized to succinate – a TCA cycle intermediate. Several GABAA receptor agonists and GABA uptake or GABA-transaminase inhibitors are used as sedatives, tranquilizers or anxiolytic drugs. The most common groups include barbiturates, benzodiazepines, chloral hydrate and valproate. Ethanol also acts as the GABAA receptor agonist.
Ion channels
Even at rest, the neuron is working to pump ions along ionic gradients
The ‘resting’ neuron is, nevertheless, continually pumping sodium out of the cell and potassium in, through ion channels. During an action potential, there is a momentary reversal of these ionic movements: sodium enters the cell and potassium then leaves, effectively repolarizing the resting membrane (Chapter 41.1). Mutations of sodium channels can occur at different sites and give rise to hyperkalemic periodic paralysis. The negative ion chloride moves through separate channels, which are implicated in specific pathologic states such as myotonia.
Calcium ions have an important role in the synchronization of neuronal activity
The movement of calcium ions within cells often provides a ‘trigger’ for the cells to synchronize an activity such as synaptic release of neurotransmitter; this synchronization also has a prominent role in the sarcoplasmic reticulum of muscle (Chapter 20). Within the central nervous system, the Lambert–Eaton syndrome is a disease that affects predominantly the P/Q subtype of calcium channels, in an example of molecular mimicry. The patient may have a primary oat cell carcinoma of the lung; the immune system responds by making antibodies against these malignant cells. However, the malignant cells and the calcium channels possess a common epitope, the effect of which is that the immune response causes the release of neurotransmitter to be blocked at the presynaptic site. This is analogous to, but nevertheless can be clearly distinguished from, the condition in myasthenia gravis, in which the block is postsynaptic.
It is also worth noting that blockade of the presynaptic release of neurotransmitter may be usefully exploited by therapeutic application of botulinum toxin (a protein derived from anerobic bacteria), which contains enzymes to hydrolyze the presynaptic proteins involved in release of neurotransmitters. This toxin is used in special cases of spasticity such as torticollis, in which the patient can be relieved of the excessive contractures of the neck muscles, which turn the head chronically to one side and thus cause pain and distraction if untreated.
Mechanism of vision
The mechanism by which the human eye can detect a single photon of light provides a fascinating example of the chemical processes underlying neuronal function
The mechanism of vision involves both trapping of photons and the transducer effect, whereby the energy of light is converted into a chemical form, which is then ultimately transmuted into an action potential by a retinal ganglion neuron. A number of the intermediates are as yet not precisely known, but the underlying hypothesis is that the receptor protein, rhodopsin, is coupled to the G-protein. There are several sequence homologies of rhodopsin with the adrenergic β-receptor and with the muscarinic ACh receptor. The main steps (Fig. 41.2.4) take place in the following order):
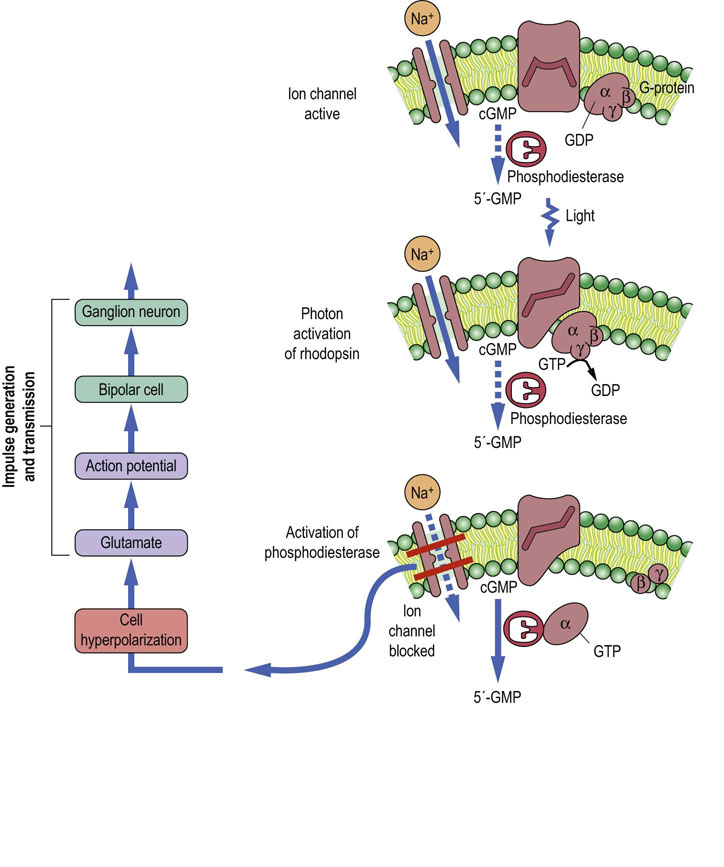
Fig. 41.2.4 Neurochemistry of synaptic transmission in the mechanism of vision.
The figure shows the consequences of photon activation of rhodopsin via G-protein coupling in a rod cell. Phosphodiesterase is activated and hydrolyzes the second messenger, cGMP, thereby blocking the entry of sodium and causing hyperpolarization of the cell. Currently, the steps through which neurotransmission subsequently proceeds to produce the final action potential in the ganglion neuron are not known in detail. Compare the activities of the G-protein coupled receptors. Dotted lines indicate an inactive process.
Summary
 The nervous system contains a number of distinct cells, each of which synthesizes its own individual proteins.
The nervous system contains a number of distinct cells, each of which synthesizes its own individual proteins.
 The specialized functions of the nervous system mean that these proteins are effectively compartmentalized in different loci.
The specialized functions of the nervous system mean that these proteins are effectively compartmentalized in different loci.
 In order to facilitate communication within the brain, there are two specialized methods of moving cells, organelles and proteins: the cerebrospinal fluid and axonal transport.
In order to facilitate communication within the brain, there are two specialized methods of moving cells, organelles and proteins: the cerebrospinal fluid and axonal transport.
 The blood–brain barrier is diverse in anatomic origin and is not absolute but relative (specifically based on molecular size of the transferred molecules).
The blood–brain barrier is diverse in anatomic origin and is not absolute but relative (specifically based on molecular size of the transferred molecules).
 Synthesis of antibodies within the CSF, with no parallel presence in plasma is unequivocal evidence for the brain as the source of antigenic stimulation.
Synthesis of antibodies within the CSF, with no parallel presence in plasma is unequivocal evidence for the brain as the source of antigenic stimulation.
 Neurotransmitters in the brain are synthesized and released into synaptic clefts from axonal terminals of specific groups of neurons (glutamatergic, GABA-ergic, catecholaminergic, cholinergic etc).
Neurotransmitters in the brain are synthesized and released into synaptic clefts from axonal terminals of specific groups of neurons (glutamatergic, GABA-ergic, catecholaminergic, cholinergic etc).
 The activity of neurons depends on the function of ion channels both in the central nervous system and the peripheral nerves.
The activity of neurons depends on the function of ion channels both in the central nervous system and the peripheral nerves.
Barry, DM, Millecamps, S, Julien, JP, et al. New movements in neurofilament transport, turnover and disease. Exp Cell Res. 2007; 313:2110–2120.
Bettens, K, Sleegers, K, Van Broeckhoven, C. Genetic insights in Alzheimer's disease. Lancet Neurol. 2013; 12:92–104.
Bos, JL, Rehmann, H, Wittinghofer, A. GEFs and GAPs: critical elements in the control of small G proteins. Cell. 2007; 129:865–877.
Cannon, SC. Physiologic principles underlying ion channelopathies. Neurotherapeutics. 2007; 4:174–183.
de Leon, MJ, Mosconi, L, Blennow, K, et al. Imaging and CSF studies in the preclinical diagnosis of Alzheimer's disease. Ann N Y Acad Sci. 2007; 1097:114–145.
George, DR, Whitehouse, PJ, D'Alton, S, et al. Through the amyloid gateway. Lancet. 2012; 380:1986–1987.
Honig, LS. Translational research in neurology. Dementia. Arch Neurol. 2012; 69:969–977.
Stein-Streilein, J, Taylor, AW. An eye's view of T regulatory cells. J Leukoc Biol. 2007; 81:593–598.