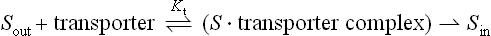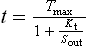Membranes and Transport
Introduction
Biomembranes are not rigid or impermeable, but highly mobile and dynamic structures
The plasma membrane is the gatekeeper of the cell. It controls not only the access of inorganic ions, vitamins and nutrients but also the entry of drugs and the exit of waste products. Integral transmembrane proteins have important roles in transporting these molecules through the membrane and often maintain concentration gradients across the membranes. K+, Na+ and Ca2+ concentrations in the cytoplasm are maintained at ∼140, 10, and 10−4 mmol/L, respectively, by transport proteins, whereas those outside (in the blood) are ∼5, 145, and 1–2 mmol/L, respectively. The driving force for transport of ions and maintenance of ion gradients is directly or indirectly provided by ATP. The transport properties of membranes will be illustrated by several important examples.
Types of transport processes
Simple diffusion through the phospholipid bilayer
Some small, neutral molecules can traverse biomembranes by simple diffusion
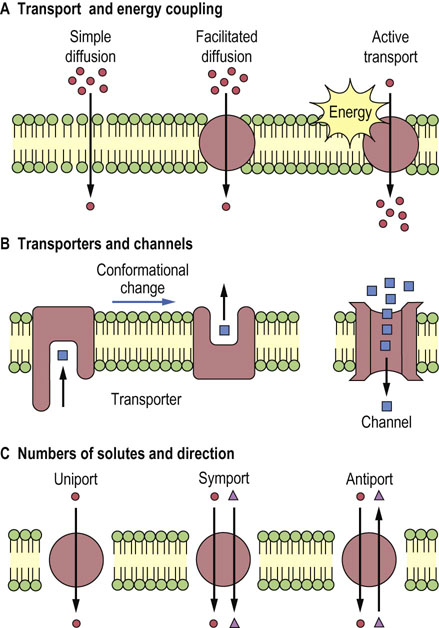
Small, nonpolar molecules (such as O2, CO2, N2) and uncharged polar molecules (such as urea, ethanol, and small organic acids) move through membranes by simple diffusion without the aid of membrane proteins (Table 8.1 and Fig. 8.1A). The direction of net movement of these species is always ‘downhill’, along the concentration gradient, from high to low concentration to establish equilibrium.
Table 8.1
Transport systems of biomembranes
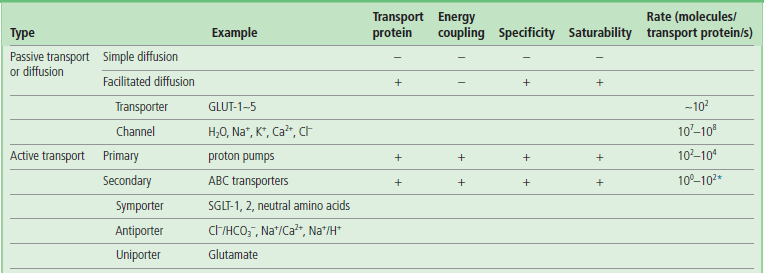
Transport systems are classified according to the role of transport proteins and energy coupling. Typical substrates for various types of transporters and channels are shown in the parentheses.
*The Cl−/HCO3− antiporter seems to be an exception to secondary active transport systems, as its transport rate is high, at 105 molecules/transport protein/s.
The hydrophobicity of the molecules is an important requirement for simple diffusion across the membrane, as the interior of the phospholipid bilayer is hydrophobic. The rate of transport of these molecules is, in fact, closely related to their partition coefficient between oil and water.
Although water molecules can be transported by simple diffusion, channel proteins (see below) are believed to control the movement of water across most membranes, especially in the kidney for concentration of the urine. Mutation in a water channel protein gene (aquaporin-2) causes diuresis in patients with nephrogenic diabetes insipidus, a disease characterized by excessive urination but without the hyper-glycemia characteristic of diabetes mellitus (see Chapter 23).
Transport mediated by membrane proteins
Membrane proteins are required for transport of larger molecules across biomembranes
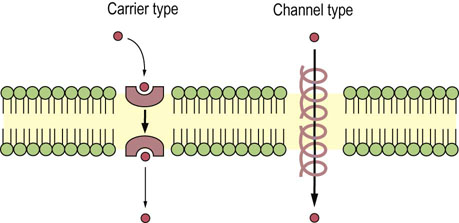
Transport of larger, polar molecules, such as amino acids or sugars, into a cell requires the involvement of membrane proteins known as transporters, also called porters, permeases, translocases or carrier proteins. The term ‘carrier’ is also applied to ionophores, which move passively across the membrane together with the bound ion (Fig. 8.2). Transporters are as specific as enzymes for their substrates, and work by one of two mechanisms: facilitated diffusion or active transport. Facilitated diffusion catalyzes the movement of a substrate through a membrane down a concentration gradient and does not require energy. In contrast, active transport is a process in which substrates are transported uphill, against their concentration gradient. Active transport must be coupled to an energy-producing reaction (see Fig. 8.1A).
Saturability and specificity are important characteristics of membrane transport systems
The rate of facilitated diffusion is generally much greater than that of simple diffusion: transport proteins catalyze the transport process. In contrast to simple diffusion, in which the rate of transport is directly proportional to the substrate concentration, facilitated diffusion is a saturable process, having a maximum transport rate, Tmax (Fig. 8.3). When the concentration of extracellular molecules (transport substrates) becomes very high, the Tmax is achieved by saturation of the transport proteins with substrate. The kinetics of facilitated diffusion for substrates can be described by the same equations that are used for enzyme catalysis (e.g. Michaelis–Menten and Lineweaver–Burk type equations) (see Chapter 6):

Fig. 8.3 Comparison of the transport kinetics of facilitated diffusion and simple diffusion.
The rate of transport of substrate is plotted against the concentration of substrate in the extracellular medium. In common with enzyme catalysis, transporter-catalyzed uptake has a maximum transport rate, Tmax (saturable). Kt is the concentration at which the rate of substrate uptake is half-maximal. For simple diffusion, the transport rate is slower and directly proportional to substrate concentration.
where Kt is the dissociation constant of the substrate–transporter complex, and Sout is the concentration of transport substrate. Then the transport rate, t, can be calculated as:
where the Kt is the concentration that gives the half-maximal transport rate. The Kt for a transporter is conceptually the same as the Km for an enzyme (Chapter 6).
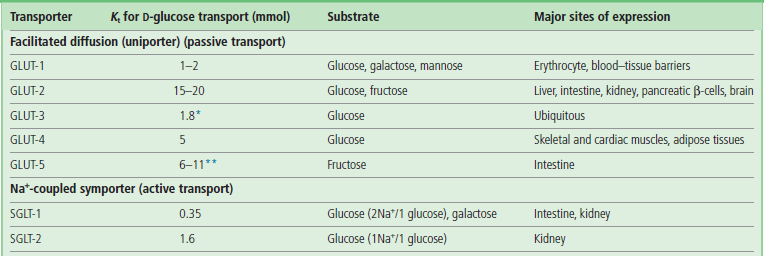
The transport process is usually highly specific: each transporter transports only a single species of molecules or structurally related compounds. The red blood cell GLUT-1 transporter has a high affinity for D-glucose, but 10–20 times lower affinity for the related sugars, D-mannose and D-galactose. The enantiomer L-glucose is not transported; its Kt is more than 1000 times higher than that of the D-form.
Characteristics of glucose transporters (uniporters)
Glucose transporters catalyze downhill transport of glucose into and out of cells
Glucose transporters are essential for facilitated diffusion of glucose into cells. The GLUT family of glucose transporters includes GLUT-1 to GLUT-5 (Table 8.2) and others. They are transmembrane proteins similar in size, all having about 500 amino acid residues and 12 transmembrane helices. GLUT-1, in red blood cells, has a Km of ∼2 mmol/L. The GLUT-1 transporter operates at about 40% of Tmax under fasting conditions (blood glucose concentration of 5 mmol/L; 90 mg/dL); this level of activity is sufficient to meet the needs of the red cell (Chapter 12). In contrast, pancreatic islet β-cells express GLUT-2, with a Km of more than 10 mmol/L (180 mg/dL). In response to the intake of food and the resulting increase in blood glucose concentration, GLUT-2 molecules respond by increasing the uptake of glucose into β-cells, stimulating insulin secretion (Chapter 21). Cells in insulin-sensitive tissues such as muscle and adipose have GLUT-4. Insulin stimulates translocation of GLUT-4 from intracellular vesicles to the plasma membrane, facilitating glucose uptake during meals.
Transport by channels and pores
Membrane channels or pores are open, less selective conduits for transport of ions, metabolites and even proteins across biomembranes
Channels are often pictured as tunnels across the membrane, in which binding sites for substrates (ions) are accessible from either side of the membrane at the same time (see Fig. 8.1B). Conformational changes are not required for the translocation of substrates entering from one side of the membrane to exit on the other side. However, voltage changes and ligand binding induce conformational changes in channel structure that have the effect of opening or closing the channels – processes known as voltage or ligand ‘gating’. Movement of molecules through channels is fast in comparison with the rates achieved by transporters (see Table 8.1).
The terms ‘channel’ and ‘pore’ are sometimes used interchangeably. However, ‘pore’ is used most frequently to describe more open, somewhat nonselective structures that discriminate between substrates, e.g. peptides or proteins, on the basis of size. The term ‘channel’ is usually applied to more specific ion channels.
Three examples of pores important for cellular physiology
The gap junction between endothelial, muscle, and neuronal cells is a cluster of small pores, in which two cylinders of six connexin subunits in the plasma membranes join each other to form a pore about 1.2–2.0 nm (12–20 Å) in diameter. Molecules smaller than about 1 kDa can pass between cells through these gap junctions. Such cell–cell interchange is important for physiologic communication or coupling, for example in the concerted contraction of uterine muscle during labor and delivery. Mutations of the genes encoding connexin 26 and connexin 32 cause deafness and Charcot–Marie–Tooth disease, respectively.
Nuclear pores have a radius of about 9.0 nm (90 Å) through which larger proteins and nucleic acids enter and leave the nucleus.
A third class of pores is important for protein sorting. Mitochondrial proteins encoded by nuclear genes are transported to this organelle through pores in the outer mitochondrial membrane. Nascent polypeptide chains of secretory proteins and plasma membrane proteins also pass through pores in the endoplasmic reticulum membrane during biosynthesis of the peptide chain.
Active transport
Primary active transport systems use ATP directly to drive transport; secondary active transport uses an electrochemical gradient of Na+ or H+ ions, or a membrane potential produced by primary active transport processes
ATP is a high-energy product of metabolism and is often described as the ‘energy currency’ of the cell (Chapter 9). The phosphoanhydride bond of ATP releases free energy when it is hydrolyzed to produce adenosine diphosphate (ADP) and inorganic phosphate. Such energy is used for biosynthesis, cell movement, and uphill transport of molecules against concentration gradients. Primary active transport systems use ATP directly to drive transport; secondary active transport uses an electrochemical gradient of Na+ or H+ ions, or a membrane potential produced by primary active transport processes. Sugars and amino acids are generally transported into cells by secondary active transport systems.
Primary active transport systems use ATP to drive ion pumps (ion transporting ATPases or pump ATPases)
The pump ATPases are classified into four groups (Table 8.3). Coupling factor ATPases (F-ATPases) in mitochondrial, chloroplast, and bacterial membranes hydrolyze ATP and transport hydrogen ions (H+). As discussed in detail in the next chapter, the mitochondrial F-ATPase works in the backward direction, synthesizing ATP from ADP and phosphate as protons move down an electrochemical (concentration and charge) gradient generated across the inner mitochondrial membrane during oxidative metabolism. The product, ATP, is released into the mitochondrial matrix, but is needed for biosynthetic reactions in the cytoplasm. ATP is transported to the cytoplasm through an ATP-ADP translocase in the mitochondrial inner membrane. This translocase is an example of an antiport system (see Fig. 8.1C); it allows one molecule of ADP to enter only if one molecule of ATP exits simultaneously.
Table 8.3
Primary active transporters in eukaryotic cells
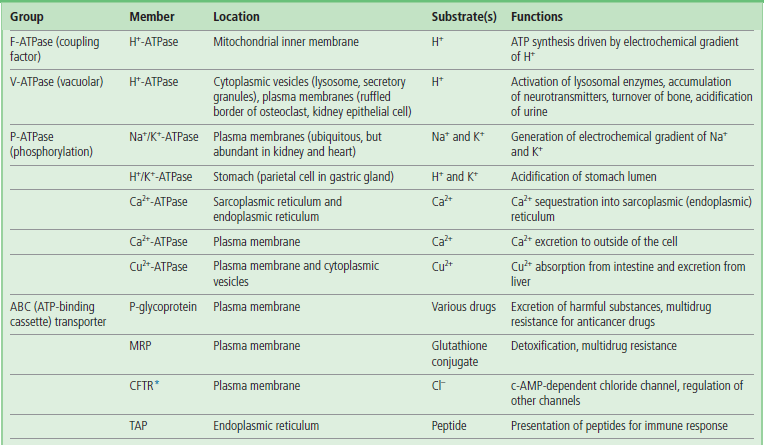
Various examples of primary active transporters (ATP-powered pump ATPases) are listed, together with their location.
*Some ABC transporters function as channels or channel regulators. MRP, multidrug resistance-associated protein; CFTR, cystic fibrosis transmembrane conductance regulator; TAP, transporter associated with antigen processing.
Cytoplasmic vesicles, such as lysosomes, endosomes, and secretory granules, are acidified by a V-type (vacuolar) H+-ATPase in their membranes. Acidification by this V-ATPase is important for the activity of lysosomal enzymes that have acidic pH optima, and for the accumulation of drugs and neurotransmitters in secretory granules. The V-ATPase also acidifies the extracellular environments of osteoclasts and renal epithelial cells. Defects in the osteoclast plasma membrane V-ATPase result in osteopetrosis (increased bone density), while mutation of the ATPase in collecting ducts of the kidney causes renal tubular acidosis. F- and V-type ATPases are structurally similar, and seem to be derived from a common ancestor. The ATP-binding catalytic subunit and the subunit forming the H+ pathway are conserved between these ATPases.
P-ATPases form phosphorylated intermediates that drive ion translocation: the ‘P’ refers to the phosphorylation. These transporters have an active-site aspartate residue that is reversibly phosphorylated by ATP during the transport process. The P-type Na+/K+-ATPase in various tissues and the Ca2+-ATPase in the sarcoplasmic reticulum have important roles in maintaining cellular ion gradients. Na+/K+-ATPases also create an electrochemical gradient of Na+ that produces the driving force for uptake of nutrients from the intestine (below). The discharge of this electrochemical gradient is also fundamental to the process of nerve transmission. Mutations of P-ATPase genes cause Brody cardiomyopathy (Ca2+-ATPase), familial hemiplegic migraine type 2 (Na+/K+-ATPase), and Menkes and Wilson's diseases (Cu2+-ATPases).
The ATP-binding cassette (ABC) transporters comprise the fourth active transporter family. ‘ABC’ is the abbreviation for ‘ATP-binding cassette’, referring to an ATP-binding region in the transporter (see Table 8.3). P-glycoprotein (‘P’ = permeability) and MRP (multidrug resistance-associated protein), which have a physiologic role in excretion of toxic metabolites and xenobiotics, contribute to resistance of cancer cells to chemotherapy. TAP transporters, a class of ABC transporters associated with Antigen Presentation, are required for initiating the immune response against foreign proteins; they mediate antigen peptide transport from the cytosol into endoplasmic reticulum. Some ABC transporters are present in peroxisomal membrane where they appear to be involved in the transport of peroxisomal enzymes necessary for oxidation of very long-chain fatty acids. Defects of ABC transporters are associated with a number of diseases (see box on next page).
Uniport, symport, and antiport are examples of secondary active transport
Transport processes may be classified into three general types: uniport (monoport), symport (cotransport) and antiport (countertransport) (see Fig. 8.1). Transport substrates move in the same direction during symport, and in opposite directions during antiport. Uniport of charged substrates may be electrophoretically driven by the membrane potential of the cell. The movement of one substrate uphill, against its concentration gradient, can be driven by antiport of another substrate (usually a cation such as Na+ or H+) down a gradient. The proteins participating in these transport systems are termed uniporters, symporters, and antiporters, respectively (see Table 8.1). Some examples are presented below.
In the case of Na+ ions, the concentration difference between outside (145 mmol/L) and inside (12 mmol/L) the cell is about a factor of 10, being maintained by the Na+/K+-ATPase. The Na+/K+-ATPase is electrogenic, pumping out three Na+ and pumping in two K+ ions, generating an inside-negative membrane potential. K+ leaks out through K+ channels, down its concentration gradient (140 mmol/L to 5 mmol/L), further increasing the electric potential. The concentration gradient of Na+ ions and the electric potential (inside negative) power the import and export of other molecules with Na+ against their concentration gradient by symporters and antiporters, respectively.
Examples of transport systems and their coupling
Ca2+ transport and mobilization in muscle
Membrane depolarization opens up voltage dependent ion channels at the neuromuscular junction
Striated muscle (skeletal and cardiac) is composed of bundles of muscle cells (Chapter 20). Each cell is packed with bundles of actin and myosin filaments (myofibrils) that produce contraction. During muscle contraction, nerves at the neuromuscular junction stimulate local depolarization of the membrane by opening voltage-dependent Na+ channels. The depolarization spreads rapidly into invaginations of the plasma membrane called the transverse (T) tubules, which extend around the myofibrils (see Fig. 20.5).
Voltage-dependent Ca2+ channels (VDCCs) located in the T tubules of skeletal muscle change their conformation in response to membrane depolarization, and directly activate a Ca2+-release channel in the sarcoplasmic reticulum membrane, a network of flattened tubules that surrounds each myofibril in the muscle cell cytoplasm. The escape of Ca2+ from the lumen (interior compartment) of the sarcoplasmic reticulum increases the cytoplasmic concentration of Ca2+ (depolarization-induced Ca2+ release) about 100-fold, from 10−4 mmol/L (0.0007 mg/L) to about 10−2 mmol/L (0.07 mg/dL), triggering ATP hydrolysis by myosin, which initiates muscle contraction. A Ca2+-ATPase in the sarcoplasmic reticulum then hydrolyzes ATP to transport Ca2+ back out of the cytoplasm into the lumen of the sarcoplasmic reticulum, decreasing the cytoplasmic Ca2+ and allowing the muscle to relax (Fig. 8.4, left).
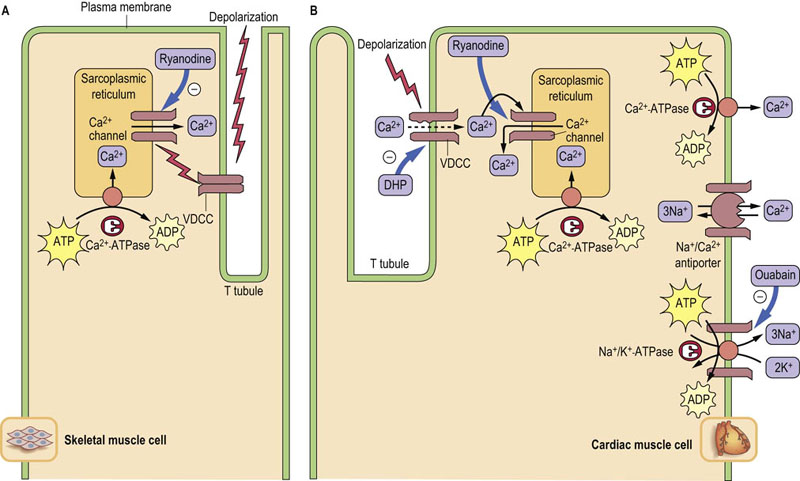
Fig. 8.4 Ca2+ movement in muscle contraction cycle.
Roles of transporters in Ca2+ movements in skeletal (A) and cardiac (B) muscle cells during contraction. Thick arrows indicate the binding sites for inhibitors. In skeletal muscle, VDCCs directly activate release of Ca2+ from the sarcoplasmic reticulum. The increased cytoplasmic Ca2+ concentration triggers muscle contraction. A Ca2+-ATPase in the sarcoplasmic reticulum pumps Ca2+ back into the lumen, decreasing the cytoplasmic Ca2+ concentration, and the muscle relaxes. In heart muscle, VDCCs allow entry of a small amount of Ca2+, which induces release of Ca2+ from the lumen of sarcoplasmic reticulum. Two types of Ca2+-ATPases and an Na+/Ca2+-antiporter are responsible for pumping cytoplasmic Ca2+ out of the muscle cell. The Na+/Ca2+-antiporter uses the sodium (Na+) gradient produced by Na+/K+-ATPase to antiport Ca2+. DHP, dihydropyridine, nifedipine, a calcium channel blocker used for treatment of hypertension. Ryanodine is a potent inhibitor of the Ca++ channel in the sarcoplasmic reticulum.
In cardiac muscle, VDCCs permit the entry of a small amount of Ca2+, which then stimulates Ca2+ release through the Ca2+ channel from the lumen of the sarcoplasmic reticulum (Ca2+-induced Ca2+ release). Not only the sarcoplasmic reticulum Ca2+-ATPase but also an Na+/Ca2+-antiporter and a plasma membrane Ca2+-ATPase are responsible for pumping out cytoplasmic Ca2+ from heart muscle (Fig. 8.4, right). The rapid restoration of ion gradients allows for rhythmic contraction of the heart.
Active transport of glucose in the intestines
A Na+/K+-ATPase drives uptake of glucose into intestinal and renal epithelial cells
The transport of blood glucose into cells is generally by facilitated diffusion, as the intracellular concentration of glucose is typically less than that of blood (see Table 8.2). In contrast, the transport of glucose from the intestine into blood involves both facilitated diffusion and active transport processes (Fig. 8.5). Active transport is especially important for maximal recovery of sugars from the intestine when the intestinal concentration of glucose falls below that in the blood.
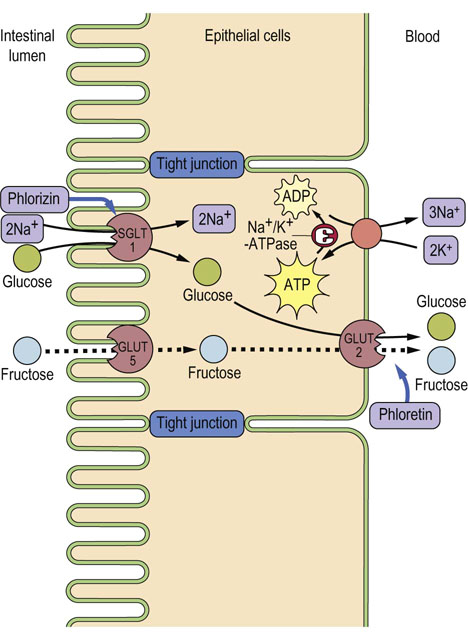
Fig. 8.5 Glucose transport from intestinal lumen into the blood.
Glucose is pumped into the cell through the Na+-coupled glucose symporter (SGLT1), and passes out of the cell by facilitated diffusion mediated by the GLUT-2 uniporter. The Na+ gradient for glucose symport is maintained by the Na+/K+-ATPase, which keeps the intracellular concentration of Na+ low. SGLT1 is inhibited by phlorizin and GLUT-2 by phloretin. Phloretin-insensitive GLUT-5 catalyzes the uptake of fructose by facilitated diffusion. The fructose is then exported through GLUT-2. A defect of SGLT1 causes glucose/galactose malabsorption. Adjacent cells are connected by impermeable tight junctions, which prevent solutes from crossing the epithelium.
An Na+-coupled glucose symporter SGLT1, driven by a Na+ gradient formed by Na+/K+-ATPase, transports glucose into the intestinal epithelial cell, while GLUT-2 facilitates the downhill movement of glucose into the portal circulation (see Fig. 8.5). A similar pathway operates in the kidney.
The renal glomerulus is an ultrafiltration system that filters small molecules from blood. However, glucose, amino acids, many ions, and other nutrients in the ultrafiltrate are almost completely reabsorbed in the proximal tubules, by symport processes. Glucose is reabsorbed primarily by sodium glucose transporter 2 (SGLT2; one-to-one Na+:Glc stoichiometry) into renal proximal tubular epithelial cells. Much smaller amounts of glucose are recovered by SGLT1 in a later segment of the tubule, which couples transport of one molecule of glucose to two sodium ions. The concentration of Na+ in the filtrate is 140 mmol/L (322 mg/dL), while that inside the epithelial cells is 30 mmol/L (69 mg/dL), so that Na+ flows ‘downhill’ along its gradient, dragging glucose ‘uphill’ against its concentration gradient. As in intestinal epithelial cells, the low intracellular concentration of Na+ is maintained by an Na+/K+-ATPase on the opposite side of the tubular epithelial cell, which antiports three cytoplasmic sodium ions for two extracellular potassium ions, coupled with hydrolysis of a molecule of ATP.
Acidification of gastric juice by a proton pump in the stomach
A P-ATPase in gastric parietal cells maintains the low pH of the stomach
The lumen of the stomach is highly acidic (pH ≈1) because of the presence of a proton pump (H+/K+-ATPase; P-ATPase in Table 8.3) that is specifically expressed in gastric parietal cells. The gastric proton pump is localized in intracellular vesicles in the resting state. Stimuli such as histamine and gastrin induce fusion of the vesicles with the plasma membrane (Fig. 8.6A). The pump antiports two cytoplasmic protons and two extracellular potassium ions, coupled with hydrolysis of a molecule of ATP; thus it is called an H+/K+-ATPase. The counter-ion C1– is secreted through a Cl– channel, producing hydrochloric acid (HCl) (gastric acid) in the lumen (Fig. 8.6B).
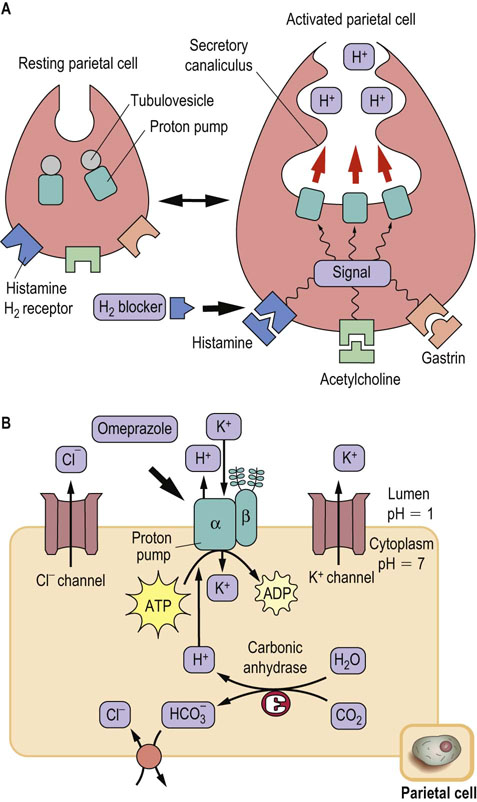
Fig. 8.6 Acid secretion from gastric parietal cells.
(A) Acid secretion is stimulated by extracellular signals and accompanied by morphologic changes in parietal cells, from resting (left) to activated (right). The proton pump (H+/K+-ATPase) moves to the secretory canaliculus (plasma membrane) from cytoplasmic tubulovesicles. H2-blockers compete with histamine at the histamine H2-receptor. (B) Ion balance in the parietal cell. The H+ transported by the proton pump are supplied by carbonic anhydrase. Bicarbonate, the other product of this enzyme, is antiported with Cl–, which is secreted through a Cl– channel. The potassium ions imported by the proton pump are again excreted by a K+ channel. The proton pump has catalytic α- and glycosylated β-subunits. The drug omeprazole covalently modifies cysteine residues located in the extracytoplasmic domain of the α-subunit and inhibits the proton pump. Thick arrows indicate the binding sites for inhibitors.
Renal Na+ transport and renin–angiotensin–aldosterone system
The kidney is a major site for regulation of Na+ homeostasis; several transport systems participate in Na+ handling in the kidney tubules
The kidney plays important parts in preservation of water and electrolyte homeostasis (Chapter 23). The primary urine is formed by glomerular filtration. Salts passing through the glomerular filter are reabsorbed in the renal tubule. Most of the Na+ reabsorption in the tubules occurs in proximal segments. Although <10% of Na+ reabsorption occurs in the distal nephron, this site is recognized to be essential for fine-tuning of plasma volume since Na+ reabsorption in the distal nephron is sensitive to the steroid hormone aldosterone.
As indicated in Figure 8.7, several Na+ transport proteins participate in Na+ reabsorption in the kidney tubules. Among them, Na+/K+-ATPase expressed in the basolateral membranes of epithelial cells transports Na+ across the membrane into the blood and generates a low intracellular Na+ concentration. The Na+ ions in the urine are transported into the epithelial cells through apical membranes by segment-specific Na+ transport proteins. Na+/H+-antiporter (NHE3) mediates Na+ entry in the proximal tubule. Furosemide-sensitive Na+-K+-2Cl–-symporter (NKCC2) and thiazide-sensitive Na+-Cl–-cotransporter (NCCT) are localized in thick ascending loop of Henle and distal convoluted tubules, respectively. Amiloride-sensitive epithelial Na+ channel (ENaC) is expressed in the aldosterone-sensitive distal nephron. The ionic balance of epithelial cells is also regulated by the renal outer medullary K+ channel (ROMK) and the Cl– channel (ClC).
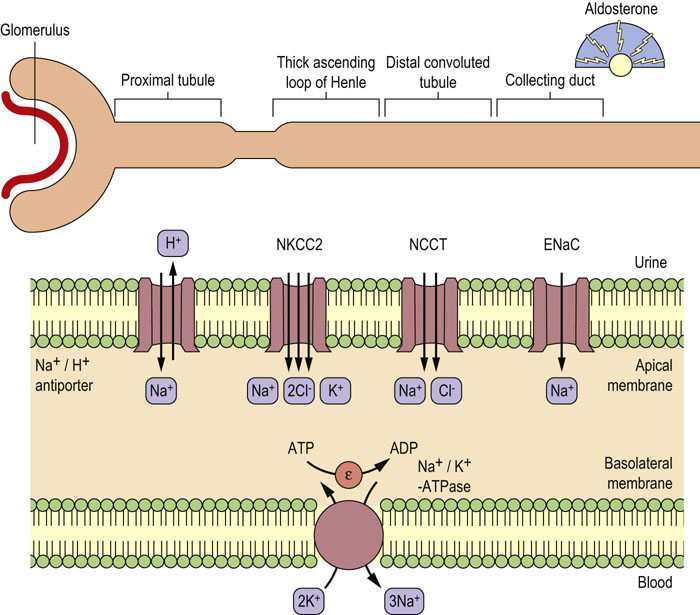
Fig. 8.7 Segment-specific expression of renal Na+ transport proteins.
Various Na+ transport proteins expressed in the apical membranes of renal epithelial cells transport Na+ from the urine into the cells. Na+ in the cells is excreted into the blood by an Na+/K+-ATPase expressed in the basolateral membranes. K+ ions are recycled through basolateral ROMK in the thick ascending loop of Henle and aldosterone sensitive distal nephron. Chloride ions leaves the cells in the thick ascending loop of Henle and distal convoluted tubule through ClCs.
The renin-angiotensin-aldosterone system plays a central role in the regulation of circulating volume, extracellular fluid osmolality and blood pressure
As discussed in more detail in Chapters 24 and 25, renin is produced in the juxtaglomerular apparatus of the kidney upon decrease of circulating volume, and it converts angiotensinogen produced in the liver to angiotensin I. Angiotensin I is converted to angiotensin II by angiotensin-converting enzyme (ACE). Angiotensin II produced in the blood promotes the release of aldosterone from the adrenal glands. Aldosterone promotes renal Na+ and water retention and elevated blood pressure by increasing Na+ reabsorption in the cortical collecting duct. Angiotensin II also promotes elevations in blood pressure by direct vasoconstriction and by increasing Na+ reabsorption in the proximal tubule.
Summary
 Most of the permeability properties of the membrane are determined by transport proteins, which are integral membrane proteins.
Most of the permeability properties of the membrane are determined by transport proteins, which are integral membrane proteins.
 Protein-mediated transport is a saturable process with high substrate specificity.
Protein-mediated transport is a saturable process with high substrate specificity.
 Facilitated diffusion is catalyzed by transporters that permit the movement of ions and molecules down concentration gradients, whereas uphill or active transport requires energy.
Facilitated diffusion is catalyzed by transporters that permit the movement of ions and molecules down concentration gradients, whereas uphill or active transport requires energy.
 Primary active transport is catalyzed by pump ATPases that use energy produced by ATP hydrolysis.
Primary active transport is catalyzed by pump ATPases that use energy produced by ATP hydrolysis.
 Secondary active transport uses electrochemical gradients of Na+ and H+, or membrane potential produced by primary active transport processes. Uniport, symport, and antiport are examples of secondary active transport.
Secondary active transport uses electrochemical gradients of Na+ and H+, or membrane potential produced by primary active transport processes. Uniport, symport, and antiport are examples of secondary active transport.
 Numerous substrates such as ions, nutrients, small organic molecules including drugs and peptides, and proteins are transported by various transporters.
Numerous substrates such as ions, nutrients, small organic molecules including drugs and peptides, and proteins are transported by various transporters.
 All these transporters are indispensable for homeostasis. The expression of unique sets of transporters is important for specific cell functions such as muscle contraction, nutrient and ion absorption by intestinal epithelial cells, resorption of nutrients by kidney cells, and secretion of acid from gastric parietal cells.
All these transporters are indispensable for homeostasis. The expression of unique sets of transporters is important for specific cell functions such as muscle contraction, nutrient and ion absorption by intestinal epithelial cells, resorption of nutrients by kidney cells, and secretion of acid from gastric parietal cells.
Camargo, SM, Brockenhauer, D, Kleta, R. Aminoacidurias: clinical and molecular aspects. Kidney Int. 2008; 73:918–925.
Hinz, A, Tampe, R. ABC transporters and immunity: mechanism of self-defense. Biochemistry. 2012; 51:4981–4989.
Linton, KJ. Structure and function of ABC transporters. Physiology. 2007; 22:122–130.
Prassas, I, Diamandis, EP. Novel therapeutic applications of cardiac glycosides. Nat Rev Drug Discov. 2008; 7:926–935.
Seyberth, HW, Schlingmann, KP. Bartter- and Gitelman-like syndromes: salt-losing tubulopathies with loop or DCT defects. Pediatr Nephrol. 2011; 26:1789–1802.
Thorens, B, Mueckler, M. Glucose transporters in the 21st century. Am J Physiol Endocrinol Metab. 2010; 298:E141–E145.
Transport across cell membrane. http://users.rcn.com/jkimball.ma.ultranet/BiologyPages/D/Diffusion
Membrane transpor. www.rpi.edu/dept/bcbp/molbiochem/MBWeb/mb1/part2/carriers.htm
Human ABC transporter. http://nutrigene.4t.com/humanabc.htm
P-ATPas. www.traplabs.dk/patbase