Neurotransmitters
Introduction
Neurotransmitters are molecules that act as chemical signals between nerve cells
Nerve cells communicate with each other and with target tissues by secreting chemical messengers, called neurotransmitters. This chapter describes the various classes of neurotransmitters and how they interact with their target cells. It will discuss their effects on the body, how alterations in their signaling may cause disease, and how pharmacologic manipulation of their concentrations may be used therapeutically.
Definition of a neurotransmitter
Traditionally, for a molecule to be labeled as a neurotransmitter, a number of criteria have to be met:
 Synthesis of the molecule occurs within the neuron, i.e. all biosynthetic enzymes, substrates, cofactors, etc., must be present for de novo synthesis.
Synthesis of the molecule occurs within the neuron, i.e. all biosynthetic enzymes, substrates, cofactors, etc., must be present for de novo synthesis.
 Storage of the molecule occurs within the nerve ending prior to release, e.g. in synaptic vesicles.
Storage of the molecule occurs within the nerve ending prior to release, e.g. in synaptic vesicles.
 Release of the molecule from the presynaptic ending occurs in response to an appropriate stimulus such as an action potential.
Release of the molecule from the presynaptic ending occurs in response to an appropriate stimulus such as an action potential.
 There is binding and recognition of the putative neurotransmitter molecule on the postsynaptic target cell.
There is binding and recognition of the putative neurotransmitter molecule on the postsynaptic target cell.
 Mechanisms exist for the inactivation and termination of the biological activity of the neurotransmitter.
Mechanisms exist for the inactivation and termination of the biological activity of the neurotransmitter.
Rigorous adherence to the above criteria means that some molecules that are involved in the cross-talk between neurons are not in the strict sense classified as neurotransmitters. Thus, nitric oxide (NO), adenosine, neurosteroids, polyamines, etc., are often termed neuromodulators rather than neurotransmitters.
Classification of neurotransmitters
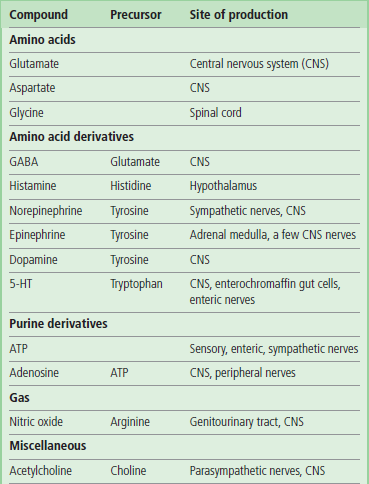
A classification of neurotransmitters based on chemical composition is shown in Table 41.1.1. Many are derived from simple compounds, such as amino acids (Table 41.1.2), but peptides are also now known to be extremely important. The principal transmitters in the peripheral nervous system are norepinephrine and acetylcholine (ACh) (Fig. 41.1.1).
Table 41.1.1
Classification of neurotransmitters
| Group | Examples |
| Amines | Acetylcholine (ACh), norepinephrine, epinephrine, dopamine, 5-HT |
| Amino acids | Glutamate, GABA |
| Purines | ATP, adenosine |
| Gases | Nitric oxide |
| Peptides | Endorphins, tachykinins, many others |
5-HT, 5-hydroxytryptamine; GABA, γ-amino butyric acid.
Neurotransmitters can be classified in several ways. The scheme shown relies on chemical similarities. All except the peptides are synthesized at the nerve ending and packaged into vesicles there; peptides are synthesized in the cell body and transported down the axon.
Table 41.1.2
Neurotransmitters of low molecular weight
Many neurotransmitters are simple compounds, often derived from common amino acids.
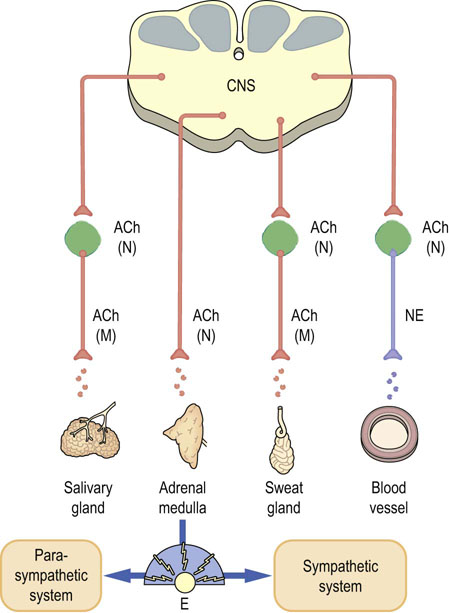
Fig. 41.1.1 Transmitters in the autonomic nervous system.
Catecholamines and acetylcholine (ACh) are transmitters in the sympathetic and parasympathetic nervous systems. Preganglionic nerves all release ACh, which binds to nicotinic (N) receptors. Most postganglionic sympathetic nerves release norepinephrine (NE), whereas postganglionic parasympathetic nerves release ACh, which acts at muscarinic (M) receptors. Adrenal glands release epinephrine. Motor neurons release ACh, which acts at distinct nicotinic receptors. E, epinephrine (see also Fig. 41.1.3).
Several transmitters may be found in one nerve
An early dogma of nerve function held that one nerve contained one transmitter. However, this is now known to be an oversimplification, and combinations of transmitters are the rule. The pattern of cellular transmitters may characterize a particular functional role, but details of this also remain unclear. A major low-molecular-weight transmitter such as an amine is often present, along with several peptides, an amino acid, and a purine. Sometimes, there may even be more than one possible transmitter in a particular vesicle, as is believed to be the case for adenosine triphosphate (ATP) and norepinephrine in sympathetic nerves. In some cases, the intensity of stimulation may control which transmitter is released, peptides often requiring greater levels of stimulus. Furthermore, different transmitters may have a different timescale of action. Sympathetic nerves are good examples of nerves for which this is the case: it is believed that ATP causes their rapid excitation, whereas norepinephrine and the neuromodulator neuropeptide Y (NPY) cause a slower phase of action. In some tissues, NPY on its own may be able to produce a very slow excitation.
Neurotransmission
Action potentials are caused by changes in ion flows across cell membranes
The signal carried by a nerve cell reflects an abrupt change in the voltage potential difference across the cell membrane. The normal resting potential difference is a few millivolts, with the inside of the cell being negative, and is caused by an imbalance of ions across the plasma membrane: the concentration of K+ ion is much greater inside cells than outside, whereas the opposite is true for Na+ ion. This difference is maintained by the action of the Na+/K+-ATPase (Chapter 24). Only those ions to which the membrane is permeable can affect the potential, as they can come to an electrochemical steady state under the combined influence of concentration and voltage differences. Because the membrane in all resting cells is comparatively permeable to K+ as a result of the presence of voltage-independent (leakage) K+ channels, this ion largely controls the resting potential.
A change in voltage which tends to drive the resting potential towards zero from the normal negative voltage is known as a depolarization, whereas a process that increases the negative potential is called hyperpolarization
So far, this picture is common to all cells. However, nerve cells contain voltage-dependent sodium channels that open very rapidly when a depolarizing change in voltage is applied. When they open, they allow the inward passage of huge numbers of Na+ ions from the extracellular fluid (Fig. 41.1.2), which swamps the resting voltage and drives the membrane potential to positive values. This reversal of voltage is the action potential. Almost immediately afterwards, the sodium channels close and so-called delayed potassium channels open. These restore the normal resting balance of ions across the membrane and, after a short refractory period, the cell can conduct another action potential. Meanwhile, the action potential has spread by electrical conductance to the next segment of nerve membrane, and the entire cycle starts again.
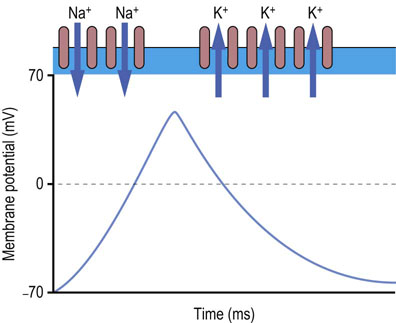
Fig. 41.1.2 Generation of action potential.
Action potential is formed as follows. At the start of an action potential, the membrane is at its resting potential of about −70 mV. This is maintained by voltage-independent K+ channels. When an impulse is initiated by a signal from a neurotransmitter, voltage-dependent Na+ channels open. These allow inflow of Na+ ions, which alter the membrane potential to positive values. The Na+ channels then close and K+ channels, called delayed rectifier channels, open to restore the initial balance of ions and the negative membrane potential.
Neurotransmitters alter the activity of various ion channels to cause changes in the membrane potential
Excitatory neurotransmitters cause a depolarizing change in voltage, in which case an action potential is more likely to occur. In contrast, inhibitory transmitters hyperpolarize the membrane and an action potential is then less likely to occur.
Neurotransmitters act at synapses
Neurotransmitters are released into the space between cells at a specialized area known as a synapse (Fig. 41.1.3). In the simplest case, they diffuse from the presynaptic membrane across the synaptic space or cleft, and bind to receptors at the postsynaptic membrane. However, many neurons, particularly those containing amines, have several varicosities along the axon, containing transmitter. These varicosities may not be close to any neighboring cell, so transmitter released from them has the possibility of affecting many neurons. Nerves innervating smooth muscle are commonly of this kind.
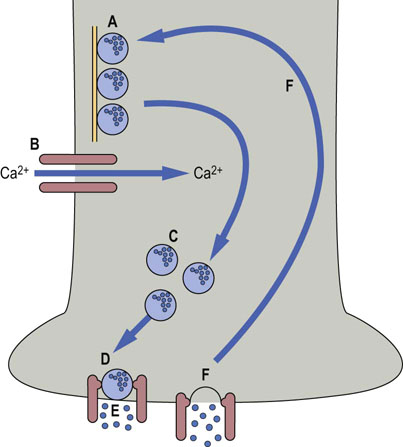
Fig. 41.1.3 Release of neurotransmitters.
Neurotransmitters are released from vesicles at the synaptic membrane. (A) In the resting state, vesicles are attached to microtubules. (B) When an action potential is received, calcium channels open. (C) Vesicles move to the plasma membrane, and (D) bind to a complex of docking proteins. (E) Neurotransmitter is released, and (F) vesicles are recycled.
When the action potential arrives at the end of the axon, the change in voltage opens calcium channels. Calcium entry is essential for mobilization of vesicles containing transmitter, and for their eventual fusion with the synaptic membrane and release through it.
Because transmitters are released from vesicles, impulses arrive at the postsynaptic cell in individual packets, or quanta. At the neuromuscular junction between nerves and skeletal muscle cells, a large number of vesicles are discharged at a time, and a single impulse may therefore be enough to stimulate contraction of the muscle cell. The number of vesicles released at synapses between neurons, however, is much smaller; consequently, the recipient cell will be stimulated only if the total algebraic sum of the various positive and negative stimuli exceeds its threshold. As each cell in the brain receives input from a huge number of neurons, this implies that there is a far greater capability for the fine control of responses in the central nervous system (CNS) than there is at the neuromuscular junction.
Receptors
Neurotransmitters act by binding to specific receptors, and opening or closing ion channels
There are several mechanisms by which receptors for excitatory neurotransmitters can cause the propagation of an action potential in a postsynaptic neuron. Directly or indirectly, they cause changes in ion flow across the membrane, until the potential reaches the critical point, or threshold, for initiation of an action potential. Receptors that directly control the opening of an ion channel are called ionotropic, whereas metabotropic receptors cause changes in second messenger systems, which in turn alter the function of channels that are separate from the receptor.
Ionotropic receptors (ion channels)
Ionotropic receptors contain an ion channel within their structure (Fig. 41.1.4; see also Chapter 8). Examples include the nicotinic ACh receptor and some glutamate and γ-amino butyric acid (GABA) receptors. These are transmembrane proteins, with several subunits, usually five, surrounding a pore through the membrane. Each subunit has four transmembrane regions. When the ligand binds, there is a change in the three-dimensional structure of the complex, which allows the flow of ions through it. The effect on membrane potential depends on the particular ions that are allowed to pass: the nicotinic ACh receptor is comparatively nonspecific towards sodium and potassium and causes depolarization, whereas the GABAA receptor is a chloride channel and causes hyperpolarization.
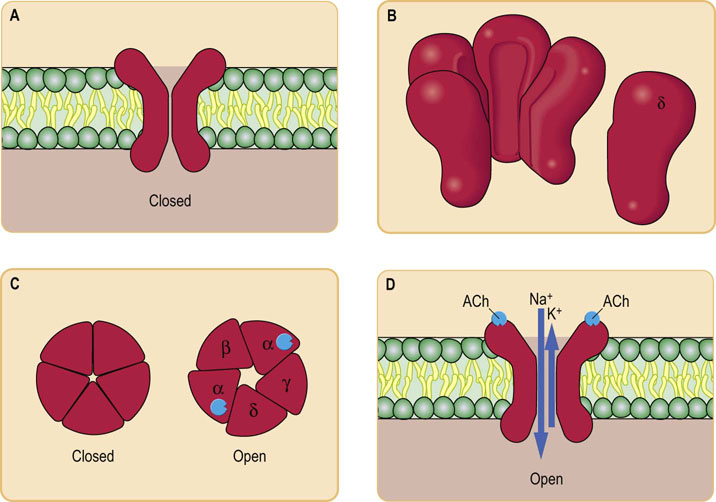
Fig. 41.1.4 Mechanism of action of ionotropic receptors.
Ionotropic receptors directly open ion channels (in fact, they are themselves ion channels). The best studied example is the nicotinic ACh receptor. This is a transmembrane protein (A) consisting of five nonidentical subunits (B), each one passing right through the membrane. The subunits surround a pore (C) that selectively allows certain ions through when it is opened by a ligand (D).
Metabotropic receptors
All known metabotropic receptors are coupled to G-proteins
Metabotropic receptors are coupled to second messenger pathways and act more slowly than ionotropic receptors. All known metabotropic receptors are coupled to G-proteins (Chapter 40) and, like hormone receptors, have seven transmembrane regions. Typically, they then couple either to adenylate cyclase, altering the production of cyclic adenosine monophosphate (cAMP), or to the phosphatidyl inositol pathway, which alters calcium fluxes. Ion channels that are separate from the receptor are then usually modified by phosphorylation. For instance, the β-adrenergic receptor, which responds to norepinephrine and epinephrine (Fig. 13.5), causes an increase in cAMP, which stimulates a kinase to phosphorylate and activate a calcium channel. Some of the muscarinic class of ACh receptors have similar effects on K+ channels.
Regulation of neurotransmitters
The action of transmitters must be halted by their removal from the synaptic cleft
When transmitters have served their function, they must be removed from the synaptic space. Simple diffusion is probably the major mechanism of removal of neuropeptides. Enzymes such as acetylcholinesterase, which cleaves ACh, may destroy any remaining transmitter. Surplus transmitters may also be taken back up into the presynaptic neuron for reuse, and this is a major route of removal for catecholamines and amino acids. Interference with uptake causes an increase in the concentration of transmitter in the synaptic space; this often has useful therapeutic consequences.
Concentrations of neurotransmitters may be manipulated
The effects of neurotransmitters can be altered by changing their effective concentrations or the number of receptors. Concentrations can be altered by:
Changes in the number of receptors may be involved in long-term adaptations to the administration of drugs.
Classes of neurotransmitters
Amino acids
It has been particularly difficult to prove that amino acids are true neurotransmitters; they are present in high concentrations because of their other metabolic roles, and therefore simple measurement of their concentrations did not provide conclusive evidence. Pharmacologic studies of responses to different analogues and the cloning of specific receptors finally provided the proof.
Glutamate
Glutamate is the most important excitatory transmitter in the CNS
Glutamate acts on both ionotropic and metabotropic receptors. Clinically, the receptor characterized in vitro by N-methyl-D-aspartate (NMDA) binding is particularly important (Fig. 41.1.5).
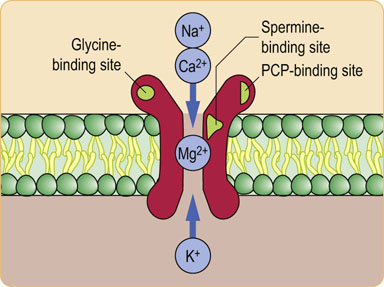
Fig. 41.1.5 The NMDA glutamate receptor.
The glutamate receptor that binds N-methyl-D-aspartate (NMDA) is complex. This receptor is clinically important because it may cause damage to neurons after stroke (excitotoxicity). It contains several modulatory binding sites, so it may be possible to develop drugs that could alter its function. Glycine is an obligatory cofactor, as are polyamines such as spermine. Magnesium physiologically blocks the channel at the resting potential, so the channel can open only when the cell has been partially depolarized by a separate stimulus. It therefore causes a prolongation of the excitation. This receptor also binds phencyclidine (PCP). Because this drug of abuse can cause psychotic symptoms, it is possible that dysfunction of pathways involving NMDA receptors causes some of the symptoms of schizophrenia.
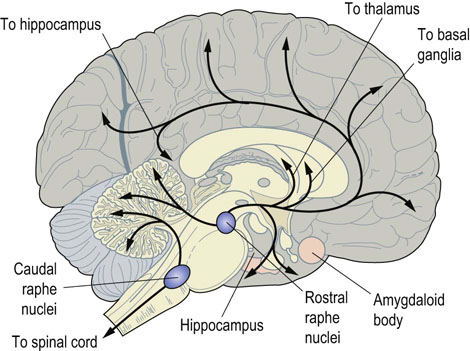
The hippocampus (Fig. 41.1.6) is an area of the limbic system of the brain that is involved in emotion and memory. Certain synaptic pathways there become more active when chronically stimulated, a phenomenon known as long-term potentiation. This represents a possible model of how memory is laid down, and it requires activation of the NMDA receptor and the consequent influx of calcium.
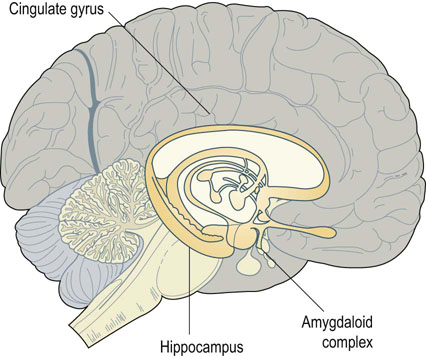
Fig. 41.1.6 Limbic system.
The limbic system of the brain is involved in emotions and memory. It consists of various areas surrounding the upper brainstem, including the hippocampus, the amygdaloid body, and the cingulate gyrus. Removal of the hippocampus prevents the laying down of short-term memory, while intact amygdaloid function is required for the emotion of fear.
Glutamate is recycled by high-affinity transporters into both neurons and glial cells. The glial cells convert it into glutamine, which then diffuses back into the neuron. Mitochondrial glutaminase in the neuron regenerates glutamate for reuse.
Glutamate and excitotoxicity
Extracellular glutamate concentration is increased after trauma and stroke, during severe convulsions, and in some organic brain diseases such as Huntington's chorea, AIDS-related dementia, and Parkinson's disease. This is because of release of glutamate from damaged cells and damage to the glutamate uptake pathways.
Excess glutamate is toxic to nerve cells
The activation of NMDA receptor allows calcium entry into cells. This activates various proteases, which in turn initiate the pathway of programmed cell death or apoptosis (see Chapter 42). There may, in addition, be changes in other ionotropic glutamate receptors that also cause aberrant calcium uptake. Uptake of sodium ions is also implicated and causes swelling of cells. Activation of NMDA receptors also increases the production of nitric oxide, which may in itself be toxic. Cell death in some models of excitotoxicity can be prevented by inhibitors of nitric oxide production, but the mechanism of toxicity is not clear.
Attempts are being made to develop drugs to inhibit NMDA activation and suppress excitotoxicity. The hope is that damage caused by stroke can be limited or even reversed. Unfortunately, many of the drugs have side effects because they bind to the phencyclidine-binding site and have unpleasant psychologic effects such as paranoia and delusions.
γ-Amino butyric acid (GABA)
GABA is synthesized from glutamate by the enzyme glutamate decarboxylase
GABA (Fig. 41.1.7) is the major inhibitory transmitter in the brain. There are two known GABA receptors: the GABAA receptor is ionotropic and the GABAB receptor is metabotropic. The GABAA receptor consists of five subunits that arise from several gene families, giving an enormous number of potential receptors with different binding affinities. This receptor is the target for several useful therapeutic drugs. Benzodiazepines bind to it and cause a potentiation of the response to endogenous GABA; these drugs reduce anxiety and also cause muscle relaxation. Barbiturates also bind to the GABA receptor and stimulate it directly in the absence of GABA; because of this lack of dependence on endogenous ligand, they are more likely to cause toxic side effects in overdose.
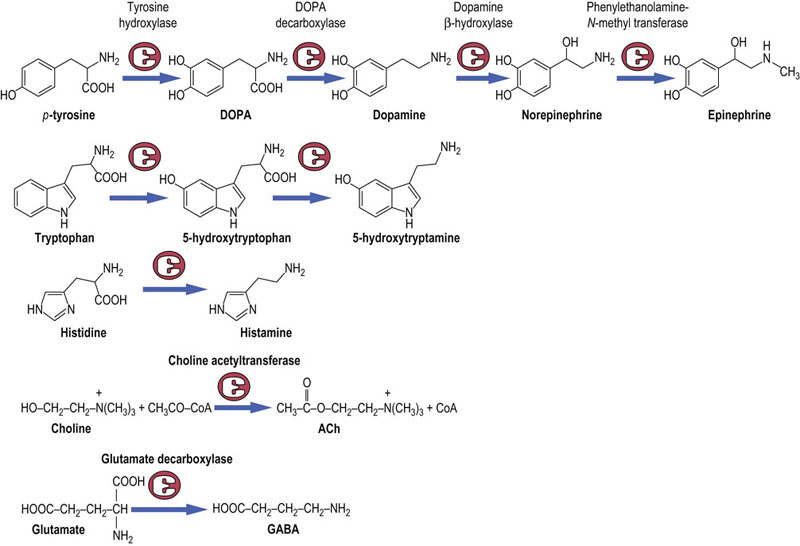
Fig. 41.1.7 Synthesis of neurotransmitters and their precursors.
The amino acid tyrosine is the precursor of dopamine,norepinephrine and epinephrine.Tryptophan is the precursor of serotonin (5-hydroxytryptamine), and histamine derives from the amino acid histidine. Choline, an amino alcohol is the precursor of acetylcholine, and the common amino acid, glutamic acid, is the precursor of the GABA.
Glycine
Glycine is primarily found in inhibitory interneurons in the spinal cord, where it blocks impulses traveling down the cord in motor neurons to stimulate skeletal muscle. The glycine receptor on motor neurons is ionotropic and is blocked by strychnine; motor impulses can then be passed without negative control, which accounts for the rigidity and convulsions caused by this toxin.
Catecholamines
Norepinephrine, epinephrine, and dopamine, known as catecholamines, are all derived from the amino acid tyrosine (Fig. 41.1.7). In common with other compounds containing amino groups, such as serotonin, they are also known as biogenic amines. Nerves that release catecholamines have varicosities along the axon, instead of a single area of release at the end. Transmitter is released from the varicosities and diffuses through the extracellular space until it meets a receptor. This allows it to affect a wide area of tissue, and these compounds are believed to have a general modulatory effect on overall brain functions such as mood and arousal.
Norepinephrine and epinephrine
Norepinephrine (also known as noradrenaline) is a major transmitter in the sympathetic nervous system
Sympathetic nerves arise in the spinal cord and run to ganglia situated close to the cord, from which postganglionic nerves run to the target tissues. Norepinephrine (Fig. 41.1.7) is the transmitter for these postganglionic nerves, whereas the transmitter at the intermediate ganglia is ACh. Stimulation of these nerves is responsible for various features of the ‘fight or flight’ response, such as stimulation of the heart rate, sweating, vasoconstriction in the skin, and bronchodilation.
There are also norepinephrine-containing neurons in the CNS, largely in the brainstem (Fig. 41.1.8). Their axons extend in a wide network throughout the cortex and alter the overall state of alertness or attention. The stimulatory effects of amphetamines are caused by their close chemical similarity to catecholamines.
Epinephrine (also known as adrenaline) is produced by the adrenal medulla under the influence of ACh-containing nerves, analogous to the sympathetic preganglionic nerves
Epinephrine is more active than norepinephrine on the heart and lungs, causes redirection of blood from the skin to skeletal muscle, and has important stimulatory effects on glycogen metabolism in the liver. In response to epinephrine, a sudden extra supply of glucose is delivered to muscle, the heart and lungs work harder to pump oxygen round the circulation, and the body is then prepared to run or to defend itself (Chapter 21). Epinephrine is not essential for life, however, as it is possible to remove the adrenal medulla without serious consequences.
The receptors for norepinephrine and epinephrine are called adrenoceptors (see Fig. 13.5). They are divided into α- and β-receptor classes and subclasses on the basis of their pharmacology. Epinephrine acts on all classes of the receptors but norepinephrine is more specific for α-receptors. β-Blockers, such as atenolol, are used to treat hypertension and chest pain (angina) in ischemic heart disease because they antagonize the stimulatory effects of catecholamines on the heart. Nonspecific α-blockers have limited use, although the more specific β1-blockers, such as prazosin, and α2-blockers, such as clonidine, can be used to treat hypertension. Certain subclasses of β-receptors are found in particular tissues; for instance, the β2-receptor is present in lung and β2-receptor agonists such as salbutamol are therefore used to produce bronchial dilatation in asthma without stimulating the β1-receptor in the heart.
Norepinephrine is taken up into cells by a high-affinity transporter and catabolized by the enzyme monoamine oxidase (MAO). Further oxidation and methylation by catecholamine-O-methyltransferase (COMT) convert the products to metanephrines and vanillylmandelic acid (4-hydroxy-3-methoxymandelic acid) (Fig. 41.1.9), which can be measured in the urine as indices of the function of the adrenal medulla. They are particularly increased in patients who have the tumor of the adrenal medulla known as pheochromocytoma. This tumor causes hypertension because of the vasoconstrictor action of the catecholamines it produces.
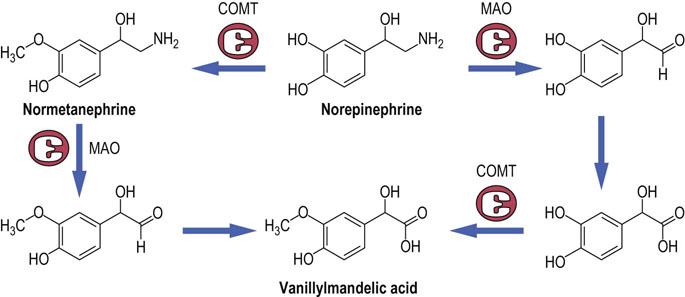
Fig. 41.1.9 Catabolism of catecholamines.
Catecholamines are degraded by oxidation of the amino group by the enzyme monoamine oxidase (MAO), and by methylation by catecholamine-O-methyltransferase (COMT). The pathway shown is for norepinephrine but the pathways for epinephrine, dopamine, and 5-HT are analogous.
Dopamine
Dopamine is both an intermediate in the synthesis of norepinephrine and a neurotransmitter
It is a major transmitter in nerves that interconnect the nuclei of the basal ganglia in the brain and control voluntary movement (Fig. 41.1.10). Damage to these nerves causes Parkinson's disease, which is characterized by tremor and difficulties in initiating and controlling movement. Dopamine is also found in pathways affecting the limbic systems of the brain, which are involved in emotional responses and memory. Defects in dopaminergic systems are implicated in schizophrenia, because many antipsychotic drugs used to treat this disease have been found to bind to dopamine receptors.
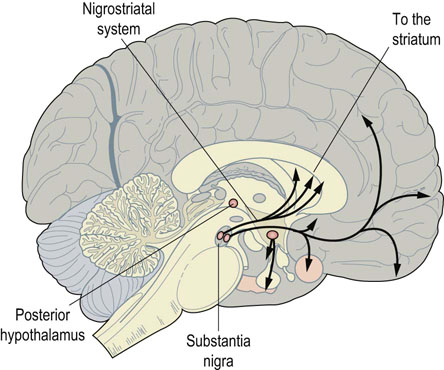
Fig. 41.1.10 Dopamine in the nigrostriatal tract.
Nerves containing dopamine run in well-defined tracts. One of the most important tracts, the nigrostriatal, connects the substantia nigra in the midbrain with the basal ganglia below the cortex. Damage to this causes Parkinson's disease, with loss of fine control of movement.
In the periphery, dopamine causes vasodilatation and it is therefore used clinically to stimulate renal blood flow, and is important in the treatment of renal failure (Chapter 23). The catabolism of dopamine is comparable to norepinephrine. However, the major metabolite formed is homovanillic acid (HVA).
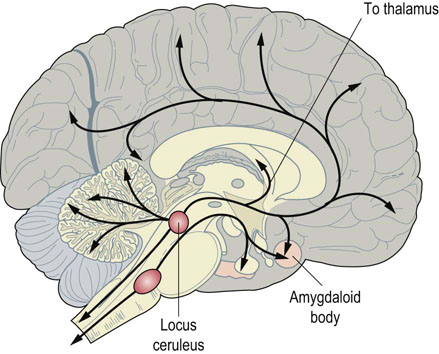
Serotonin (5-hydroxytryptamine)
Serotonin, also called 5-hydroxytryptamine (5-HT), is derived from tryptophan (Fig. 41.1.7)
In addition, serotonin biosynthesis has a number of biochemical similarities to dopamine synthesis. Thus, tryptophan hydroxylase, like tyrosine hydroxylase, displays a cofactor requirement for tetrahydrobiopterin (BH4) (see below). Furthermore, 5-hydroxytryptophan is converted to serotonin by dopa decarboxylase (also known as aromatic amino acid decarboxylase).
Serotoninergic neurons are concentrated in the raphe nuclei in the upper brainstem (Fig. 41.1.11), but project up to the cerebral cortex and down to the spinal cord. They are more active when subjects are awake than when they are asleep, and serotonin may control the degree of responsiveness of motor neurons in the spinal cord. In addition, it is implicated in so-called vegetative behaviors such as feeding, sexual behavior, and temperature control.
Acetylcholine
Acetylcholine (ACh) is the transmitter of the parasympathetic autonomic nervous system and of the sympathetic ganglia (Fig. 41.1.3)
Stimulation of the parasympathetic system produces effects that are broadly opposite to those of the sympathetic system, such as slowing of the heart rate, bronchoconstriction, and stimulation of intestinal smooth muscle. ACh also acts at neuromuscular junctions, where motor nerves contact skeletal muscle cells and cause them to contract. Apart from these roles, ACh may be involved in learning and memory, as neurons containing this transmitter also exist in the brain.
ACh is synthesized from choline by the enzyme choline acetyl transferase. After it is secreted into the synaptic cleft, it is largely broken down by acetylcholinesterase. The remainder is taken back up into the nerve cell by transporters similar to those for amines.
There are two main classes of ACh receptors: nicotinic and muscarinic (see Chapter 41.2, Fig. 41.2.3).
Both respond to ACh but can be distinguished by their associated agonists and antagonists; they are quite different structurally and differ in their mechanisms of action.
 Nicotinic receptors are ionotropic. They bind nicotine and are found on ganglia and at the neuromuscular junction. When ACh or nicotine binds, a pore opens, which allows both Na+ and K+ to pass through. Because the action of the ligand on the channel is direct, action is rapid.
Nicotinic receptors are ionotropic. They bind nicotine and are found on ganglia and at the neuromuscular junction. When ACh or nicotine binds, a pore opens, which allows both Na+ and K+ to pass through. Because the action of the ligand on the channel is direct, action is rapid.
 Muscarinic receptors, responding to the fungal toxin muscarine, are metabotropic. They are much more widespread in the brain than are nicotinic receptors, and are also the major receptors found on smooth muscle and glands innervated by parasympathetic nerves. Atropine specifically inhibits these receptors. There are several separate muscarinic receptors, differing in their tissue distribution and signaling pathways. As yet, no clear pattern has emerged as to their specific functions.
Muscarinic receptors, responding to the fungal toxin muscarine, are metabotropic. They are much more widespread in the brain than are nicotinic receptors, and are also the major receptors found on smooth muscle and glands innervated by parasympathetic nerves. Atropine specifically inhibits these receptors. There are several separate muscarinic receptors, differing in their tissue distribution and signaling pathways. As yet, no clear pattern has emerged as to their specific functions.
Clinically, ACh agonists, in common with acetylcholinesterase inhibitors, are used to treat glaucoma, an eye disease characterized by high intraocular pressure, by increasing the tone of the muscles of accommodation of the eye. They are also used to stimulate intestinal function after surgery. On the other hand, when acetylcholinesterase is inhibited by organophosphate insecticides or nerve gases, a toxic syndrome is caused by the resulting excess of ACh. There may be diarrhea, increased secretory activity of several glands, and bronchoconstriction. This syndrome can be antagonized by atropine, although longer-term treatment involves the use of drugs that can remove the insecticide from the enzyme, such as pralidoxime.
Nitric oxide gas
In autonomic and enteric nerves, nitric oxide (NO) is produced from arginine by the tetrahydrobiopterin-dependent nitric oxide synthases
NO has a number of attributed physiologic functions including relaxation of both vascular and intestinal smooth muscle, and the possible regulation of mitochondrial energy production. Furthermore, within the brain, NO may have a role in memory formation. However, excessive NO formation has been implicated in the neurodegenerative process associated with Parkinson's and Alzheimer's disease. Whilst the exact mechanism whereby excessive NO causes neuronal death is not known, a growing body of evidence suggests that irreversible damage to the mitochondrial electron transport chain may be an important factor.
NO is not stored in vesicles, but released directly into the extracellular space
Consequently, NO does not, in the strictest sense, meet all the current criteria to be labeled as a neurotransmitter. NO itself diffuses comparatively easily between cells, and binds directly to heme groups in the enzyme guanylate cyclase, stimulating the production of cyclic guanosine monophosphate.
Other small molecules
ATP and other purine-containing molecules derived from it are now known to have transmitter functions
ATP is present in synaptic vesicles of sympathetic nerves, along with norepinephrine, and is responsible for rapid excitatory potentials in smooth muscle. Adenosine receptors are widespread in the brain and in vascular tissue. Adenosine is largely inhibitory in the CNS, and inhibition of adenosine receptors is believed to underlie the stimulatory effects of caffeine.
Study of histamine in nerves is complicated by the large amounts that are present in mast cells
Histamine is found in a small number of neurons, mainly in the hypothalamus, although their projections are widespread throughout the brain. It has been shown to control the release of pituitary hormones, arousal, and food intake. Antihistamines designed to control allergies caused by release from mast cells act on the H1 receptor and tend to be sedative, suggesting that other central functions also probably exist. The histamine receptor in the stomach is of the H2 class; therefore, the H2 inhibitors, such as cimetidine and ranitidine, that are used to treat peptic ulcers have no effect on allergy.
Peptides
Many peptides act as neurotransmitters
It is an open question whether all the peptides that have been described are really true neurotransmitters. Nevertheless, more than 50 small peptides have now been shown to influence neural function. All known peptide receptors are metabotropic and coupled to G-proteins (Chapter 40), and so act comparatively slowly. There are no specific uptake pathways or degradative enzymes, and the main route of disposal is simple diffusion followed by cleavage by a number of peptidases in the extracellular fluid. This allows a peptide to affect a number of neurons before it is finally degraded.
Vasoactive intestinal peptide (VIP) is one of many peptides that affect the function of the intestine through the enteric nervous system. It was originally described as a gut hormone that affected blood flow and fluid secretion, but it is now known to be an important enteric neuropeptide, inhibiting smooth muscle contraction. It also causes vasodilatation in several secretory glands, and potentiates stimulation by ACh.
Many neuropeptides belong to a multigene family
The opioid peptides and opioid receptors provide a good example of a multigene family. They are the endogenous ligands for opiate analgesics such as morphine and codeine. The control of pain is complex, and opioid peptides and receptors are found both in the spinal cord and in the brain itself. There are at least three genes that code for these peptides, and each contains the sequences for several active molecules:
 proopiomelanocortin contains β-endorphin, which binds to opiate µ-receptors, and also adrenocorticotropic hormone (ACTH) and the melanocyte-stimulating hormones (MSH), which are pituitary hormones (Chapter 39);
proopiomelanocortin contains β-endorphin, which binds to opiate µ-receptors, and also adrenocorticotropic hormone (ACTH) and the melanocyte-stimulating hormones (MSH), which are pituitary hormones (Chapter 39);
 proenkephalin A contains the sequences for Met- and Leu-enkephalins, which bind to δ-receptors and are involved in pain regulation at local levels in the brain and spinal cord;
proenkephalin A contains the sequences for Met- and Leu-enkephalins, which bind to δ-receptors and are involved in pain regulation at local levels in the brain and spinal cord;
 prodynorphin contains sequences for dynorphin and several other peptides, which bind to the κ-class of receptors.
prodynorphin contains sequences for dynorphin and several other peptides, which bind to the κ-class of receptors.
Opiates affect pleasure pathways in the brain, which explains their euphoriant effects, and they also have side effects, such as respiratory depression, that limit their use. In excess, they cause contraction of the muscles of the eye, resulting in ‘pinpoint’ pupils. It has been shown that endorphins are released after strenuous exercise, giving the so-called ‘jogger’s high’. It is hoped that increased knowledge of the specific opioid receptors and neural opioid pathways will allow the development of analgesics with fewer side effects and less likelihood of abuse.
Substance P is another example of a member of a multigene family, known as the tachykinin family. It is present in afferent fibers of sensory nerves and transmits signals in response to pain. It is also involved in so-called neurogenic inflammation stimulated by nerve impulses, and is an important neurotransmitter in the intestine.
Neuropeptides can act as neuromodulators
Some peptides do act as true neurotransmitters, but they also have many other actions. They often alter the action of other transmitters, acting as neuromodulators, but have no action of their own. For instance, VIP enhances the effect of ACh on salivary gland secretion in cat submandibular glands (glands located under the jawbone) by causing vasodilatation and potentiating the cholinergic component. NPY causes inhibition of the release of norepinephrine at autonomic nerve terminals, acting at presynaptic autoreceptors, and potentiates the action of norepinephrine in certain arteries while having only weak actions itself. Opioid peptides also are capable of modulating neurotransmitter release.
Summary
 Neurons communicate at synapses by means of neurotransmitters.
Neurons communicate at synapses by means of neurotransmitters.
 A large number of compounds, whether of low molecular weight, such as the biogenic amines, or larger peptides, can act as neurotransmitters.
A large number of compounds, whether of low molecular weight, such as the biogenic amines, or larger peptides, can act as neurotransmitters.
 They act on specific receptors and there is normally more than one receptor for each neurotransmitter.
They act on specific receptors and there is normally more than one receptor for each neurotransmitter.
 The presence of several transmitters in the same nerves and the identification of multiple receptors suggest that there is a high degree of flexibility and complexity in the signals that can be produced in the nervous system.
The presence of several transmitters in the same nerves and the identification of multiple receptors suggest that there is a high degree of flexibility and complexity in the signals that can be produced in the nervous system.
Aitkenhead, H, Heales, SJ. Establishment of paediatric age-related reference intervals for serum prolactin to aid in the diagnosis of neurometabolic conditions affecting dopamine metabolism. Ann Clin Biochem. 2013; 50:156–158.
Clayton, PT. B6-responsive disorders: a model of vitamin dependency. J Inherit Metab Dis. 2006; 29:317–326.
Hyland, K. Inherited disorders affecting dopamine and serotonin: critical neurotransmitters derived from aromatic amino acids. J Nutr. 2007; 137(6 suppl 1):1568S–1572S.
Kurian, MA, Zhen, J, Meyer, E, et al. Clinical and molecular characterisation of hereditary dopamine transporter deficiency syndrome: an observational cohort and experimental study. Lancet Neurol. 2011; 10:54–62.
Lam, AAJ, Hyland, K, Heales, SJR. Tetrahydrobiopterin availability, nitric oxide metabolism and glutathione status in the hph-1 mouse: implications for the pathogenesis and treatment of tetrahydrobiopterin deficiency states. J Inherit Metab Dis. 2007; 30:256–262.
The AADC Research Trust. www.aadcresearch.org.
Databases of PKU and PND variations this website hosts databases of pediatric neurotransmitter disorders (PND). www.BioPKU.org.
The PND Association is a disease organization representing children and families who are affected by a pediatric neurotransmitter disease. www.pndassoc.org.