Central Nervous System
Brain
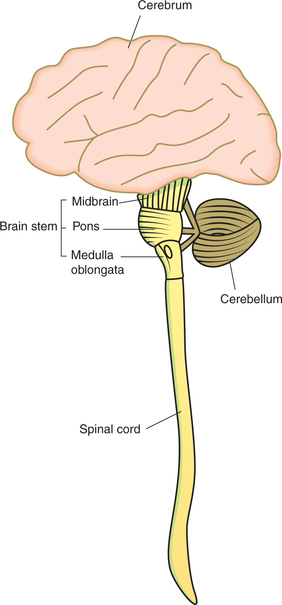
The central nervous system (CNS) consists of the brain and the spinal cord (Fig. 10.1-1). The brain is the largest and most complex mass of nervous tissue in the body. It is contained in cranial cavity and weighs about 1380 g in the adult male and about 1250 g in the adult female.
Parts of brain
Spinal cord traced upwards becomes medulla oblongata. Above the medulla, a broad bridge—the pons—connects the two hemispheres of the cerebellum, which lie behind the pons and medulla. The midbrain connects the forebrain with hindbrain. Medulla oblongata, pons and midbrain together constitute the brain stem.
Cerebrum
External features
Cerebrum consists of two cerebral hemispheres which are separated from each other in the upper part by a median longitudinal fissure in which the falx cerebri (a fold of dura mater) invaginates. In the lower part the two cerebral hemispheres are connected by the largest white commissure called corpus callosum.
Sulci and gyri
The surface of cerebral hemisphere is covered by a thin layer (2–4-mm thick) of grey matter called the cerebral cortex. The entire surface of cerebral hemisphere is folded with intervening grooves of fissures. The folds or convolutions are called gyri and the intervening fissures are called sulci. The cerebral cortex follows the contour of the sulci and gyri of the hemisphere. As a result of the folding of the cerebral surface, the cerebral cortex acquires a much larger surface area (about 2200 cm2).
Lobes and functional areas
Each cerebral hemisphere is divided into four lobes (Fig. 10.1-2A):
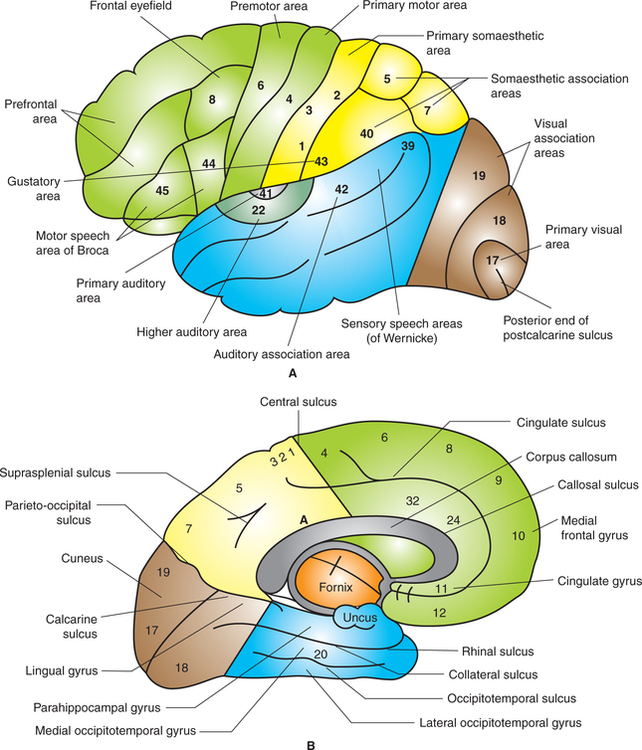
Fig. 10.1-2 Left cerebral hemisphere showing lobes, sulci and gyri: A, superolateral surface and B, medial surface.
Frontal lobe
The frontal lobe lies in front of the central sulcus and above the posterior ramus of the lateral sulcus (Fig. 10.1-2). It forms about one-third of cortical surface. On the basis of functions, the frontal lobe is subdivided into two main areas:
I Precentral cortex
Precentral cortex refers to posterior part of the frontal lobe that includes lip of central sulcus, precentral gyrus and posterior part of superior, middle and inferior frontal gyri. The precentral cortex is also called excitomotor area of cortex.
Nowadays, the motor cortex and sensory cortex are together known as sensorimotor cortex.
The precentral cortex includes following important areas:
1. Primary motor area (area 4). It lies in the precentral gyrus extending into the paracentral lobule on the medial surface (Fig. 10.1-2). Different parts of the contralateral half of the body are represented separately in more or less inverted order. Those parts of the body which carry out the most skilled movements, e.g. the fingers and thumb, have the largest areas of cortical representation. The body is represented upside down (however face is not represented in inverted manner) (Fig. 10.1-3A).
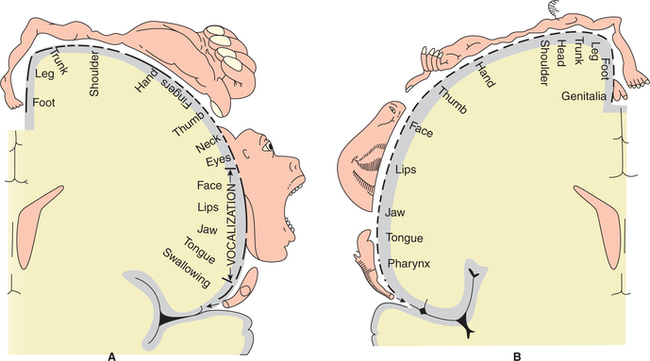
Fig. 10.1-3 Topographical representation (homunculus) of motor (A) and sensory (B) areas in cerebral cortex.
Functions. It is concerned with initiation of voluntary movements of the contralateral half of the body and initiation of speech.
2. Premotor area. Premotor area lies anterior to the primary motor area and includes Brodmann's area 6, 8, 44 and 45.
• Area 6. It abuts on the primary motor cortex both above and behind. Cells from this area contribute fibres to pyramidal tracts. Topographical organization of this area is roughly the same as that of primary motor cortex.
• Area 8. It is also called frontal eyefield. It lies anterior to area 6. It is concerned with control of eye movements.
• Area 44 and 45 or Broca's motor speech area. It is special region of premotor cortex situated in inferior frontal gyrus.
Functions. This area, specially in dominated hemisphere (left hemisphere in right-handed person), is concerned with movements of those structures which are responsible for the production of voice and articulation of speech.
II Prefrontal cortex
Location. Prefrontal cortex, also called prefrontal lobe or orbitofrontal cortex, is the anterior part of frontal lobe lying anterior to area 8 and 44 (Fig. 10.1-2).
Major areas. Prefrontal cortex has different Brodmann's areas such as 9–14, 23, 24, 29 and 32, 44–47.
Functions of prefrontal cortex
1. Centre for planned actions. Prefrontal-association areas in close association with motor cortex plan complex patterns and sequence of motor movements.
2. Centre for higher functions. This forms the centre for higher functions like emotions, learning, memory and social behaviour.
3. Seat of intelligence. Short-term memories are registered in prefrontal cortex. It can keep track of many bits of information and also has ability to recall this information bit by bit for subsequent thoughts. It is therefore called seat of intelligence or an organ of mind.
4. Control of intellectual activities. The prefrontal cortex has the following intellectual abilities:
• It allows the person to concentrate on central theme of thought. It helps in depth and abstractness of thought, and thereby in elaboration of thought.
• It allows to delay action in response to incoming sensory signals so that sensory information can be weighed until the best response is obtained.
• It allows to consider the consequence of motor actions before their performance.
• It plays role in solution of complicated mathematical, legal and philosophical problems.
• It allows to correct avenues of information in diagnosis of rare disease.
• It allows to control one's activity according to the moral laws.
Parietal lobe
Parietal lobe (Fig. 10.1-2) lies between the central sulcus and parieto-occipital sulcus, and at the upper part of first imaginary line.
Areas of parietal lobe
Functionally parietal lobe can be divided into three parts:
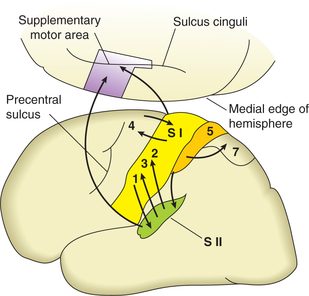
Primary sensory area (first somatic sensory area)
Location. The first somatic sensory area (SI) occupies the posterior wall of the central sulcus, the post-central gyrus and the post-central part of the paracentral lobule (Fig. 10.1-4).
Major areas. It includes Brodmann's area 3, 1 and 2.
Topographical organization. The primary sensory cortex receives sensory inputs from the opposite half of the body. The representation of the body within this area is similar to that already noted in primary motor cortex (Fig. 10.1-3B).
Location. Secondary sensory area, also called second somatic sensory area (SII), is situated in post-central gyrus below the area of face of first somatic sensory area. Most of it is buried in the superior wall of the sylvian fissure (lateral cerebral sulcus).
Topographical representation. The secondary sen sory (SII) area receives sensory impulses from primary sensory area (SI) as well as from thalamus directly (Fig. 10.1-4). Like SI, the SII area also manifests a dermatomal (point-topoint) sequence of representation (although there is more overlap). Thus the body is represented twice in the somatic sensory cortex, i.e. in area SI as well as in area SII.
Temporal lobe
Temporal lobe (Fig. 10.1-2) lies below the posterior ramus of lateral sulcus and its continuation to the second imaginary line.
Areas of temporal lobe
The major areas in the temporal lobe are (Fig. 10.1-2) given below.
Primary auditory area, also called audiosensory area, includes Brodmann's area 41 and 42, and forms the centre for hearing.
Functions. This area perceives the nerve impulses as sound, i.e. auditory information such as loudness, pitch, source and direction of sound.
Auditory-association area corresponds to Brodmann's areas 22, 21 and 20.
Area 22, also called Wernicke's area, is a sensory speech centre situated in the posterior part of superior temporal gyrus behind the area 41 and 42 in the categorical hemisphere, i.e. dominant hemisphere.
Location. Areas 21 and 20 are located in the middle and inferior temporal gyrus, respectively.
Functions. These areas receive impulses from primary area, and are concerned with interpretation and integration of auditory impulses.
Lesions of these areas impair auditory, short-term memory without impairing visual memory.
Occipital lobe
Occipital lobe lies behind the parieto-occipital sulcus and its continuation down an imaginary line (Fig. 10.1-2).
Areas of occipital lobe
Occipital lobe is mostly formed of sensory and association areas. It contains visual cortex having three areas (Fig. 10.1-2):
Primary visual cortex is also called striate area (area 17). It lies on the medial surface of the occipital lobe in and near the calcarine sulcus occupying parts of lingual gyrus and cuneus.
Peristriate area, also called visual-association area (area 18), lies in the walls of lunate sulcus.
Parastriate area (area 19) is also a visual-association area. It lies in the cortex in front of the lunate sulcus.
Functions
• Primary visual area (area 17) is concerned with perception of visual impulses.
• Visual-association areas (area 18 and area 19) are concerned with interpretation of visual impulses. These are involved in the recognition and identification of objects in the light of past experience.
• Occipital eyefield area (area 19) is concerned with the movements of eyeball.
Summary of cortical functional areas
Classically, cortical functional areas are subdivided into following areas (Fig. 10.1-2).
• Primary somaesthetic areas (area 3, 1 and 2),
• Secondary (supplementary) somaesthetic area and
• Somaesthetic-association areas (area 5, 7 and higher association area 40).
• Primary auditory area (area 41) or auditory area I,
• Auditory-association area (area 42) or auditory area II and
• Primary visual area (area 17) or visuostriate area of visual area I,
Histological structure of cerebral cortex
The cerebral cortex, also known as pallium, is divided phylogenetically into three types: allocortex, mesocortex and neocortex.
1. Allocortex or old cortex forms about 10% of the entire cortex.
Since most of the allocortex is located around the peripheral margin of the diencephalon in the form of a ring, it is also called limbic cortex.
2. Mesocortex, which is the transitional zone between allocortex and neocortex, comprises the cingulate gyrus, part of parahippocampal gyrus and subiculum.
3. Neocortex, also called an isocortex, comprises rest of 90% of the cerebral cortex in human brain. The extent of neocortex has increased with the evolution of mammals.
Histologically, the cerebral cortex is composed of nerve cells and fibres. Three types of cells may be identified in the cerebral cortex:
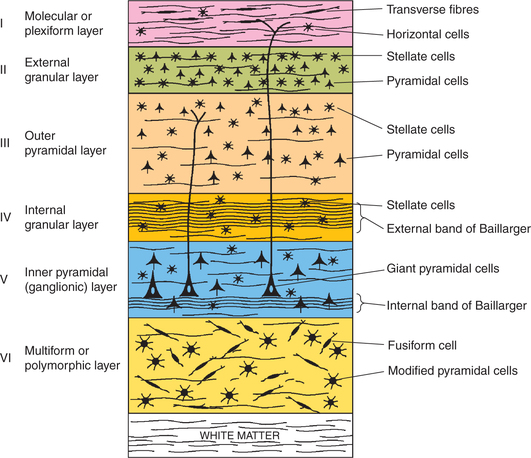
The six laminae of neocortex numbered I–VI are (Fig. 10.1-5) as follows.
1. Molecular or plexiform layer. It mainly consists of transverse nerve fibres dispersed with occasional horizontal cells.
2. External granular layer. It contains numerous stellate or granule cells. It is traversed by afferent and efferent projection fibres.
3. Outer pyramidal layer. It consists mainly of pyram idal neurons, and some stellate and basket cells.
4. Internal granular layer. This layer consists of densely packed stellate cells. The inner zone of this layer is traversed by transversely running fibres called external band of Baillarger.
5. Inner pyramidal (ganglionic) layer. This layer consists of large pyramidal cells. It is specially developed in the motor cortex, where these cells are called giant cells or Betz cells. This layer is traversed by aggregation of transversely running fibres called internal band of Baillarger.
6. Polymorphous or multiform layer. This layer contains many spindle-shaped cells called fusiform cells. This layer merges with the white matter of cerebral cortex.
Interior of cerebrum
The interior of each cerebrum, below the thick layer of grey matter (cerebral cortex), consists of:
Basal ganglia
Components of basal ganglia
Basal ganglia are subcortical nuclear masses. They are so named, as they develop in the basal part of cerebral hemisphere. From the physiological viewpoint, the term basal ganglia includes:
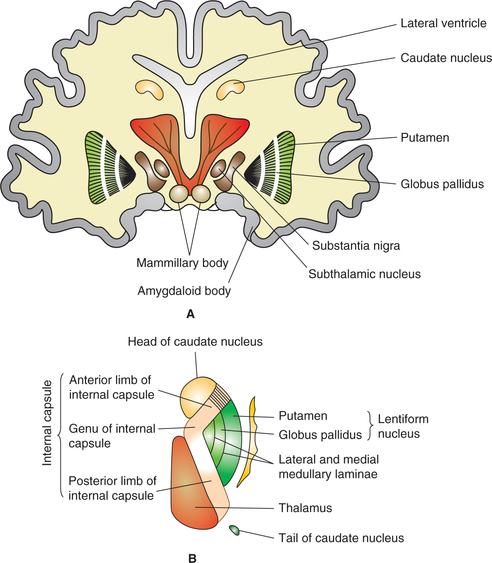
Corpus striatum (Fig. 10.1-6) comprises subcortical masses of grey matter, which is divided almost completely by the fibres of internal capsule into two parts.
(i) Caudate nucleus (medial part) and
(ii) Lenticular nucleus (lateral part), which is further subdivided into two parts:
Phylogenetically and functionally the corpus striatum can be divided into two parts:
1. Neostriatum or striatum. Phylogenetically, the caudate nucleus and putamen are of more recent origin and hence called neostriatum or striatum.
2. Paleostriatum refers to globus pallidus, which is older and primitive part. It is also called pallidum, as it is pale (pallid).
Subthalamic nucleus (body of Luys) is a biconvex mass of grey matter, which is situated lateral to red nucleus and dorsal to substantia nigra in the mesencephalon.
Substantia nigra is a sheet made up of pigmented nerve cells. It appears dark as the neurons contain the pigment neuromelanin.
Functions of basal ganglia
Control of voluntary motor activity
Basal ganglia control the voluntary movements which are initiated by the motor cortex.
The role of basal ganglia in control of voluntary motor activity includes the following.
(i) Cognitive control of motor activity. The basal ganglia, like the cerebellum, are involved in the planning and programming of the movement.
Most of the motor actions occur as a consequence of thoughts generated in mind. This process is known as cognitive control of motor activity.
(ii) Timing and scaling of the intensity of movements. Two important capabilities of brain in controlling the movements are:
• Timing of the movements, i.e. how rapidly the movements should be performed and
• Scaling of the intensity of movements, i.e. how large the movement should be.
(iii) Subconscious execution of some movements. Basal ganglia subconsciously execute some movements during the performance of trained motor activities, i.e. skilled activities. Examples of movements executed subconsciously at the level of basal ganglia are:
Control of clutch and brake while driving (constant attention is required during initial stages, however, they are carried out subconsciously by basal ganglia as they become routine).
Importance. By subconscious control of activities the basal ganglia relieve cortex from routine acts so that cortex can be free to plan its actions.
Control of reflex muscular activity
The basal ganglia exert inhibitory effect on spinal reflexes and regulate activity of muscles which maintain posture.
Muscle spindles and the γ motor neurons of spinal cord (which are responsible for maintaining the tone of the muscles) are controlled by basal ganglia, especially substantia nigra. In lesion of basal ganglia muscle tone increases. Rigidity (lead-pipe type) is a characteristic feature of Parkinson's disease.
Disorders of basal ganglia
Parkinson's disease, also called paralysis agitans or shaking palsy was first described by James Parkinson in 1817. Parkinson's disease occurs in elderly people due to a steady loss of dopamine and dopamine receptors with age in the basal ganglia in normal individuals.
Pathogenesis. A current view of the pathogenesis of Parkinson's disease is that there is an imbalance between excitation and inhibition in the basal ganglia created by the loss of the dopaminergic inhibition of the putamen.
Clinical features. Parkinson's disease has both hypokinetic and hyperkinetic features. Its cardinal features are a triad of akinesia, rigidity and tremor of which akinesia is a hypokinetic feature while rigidity and tremors are hyperkinetic features.
1. Akinesia or hypokinesia. The patient is unable to initiate the voluntary movements (akinesia) or the voluntary movements are decreased (hypokinesia).
Manifestations of akinesia or hypokinesia include:
• Slow performance of voluntary movements (bradykinesia).
• Mask-like facial expression due to decrease in movements of facial muscles.
• Absence of normal associated movements, e.g. swinging of arms during walking.
• Shuffling or festinant-type gait, in which patient is bent forward and walks quickly with short steps as if trying to catch up centre of gravity or preventing himself from falling.
• Retropulsion, i.e. when a walking patient is suddenly pulled backwards, he begins to walk backwards and is unable to stop.
2. Rigidity. It refers to an increase in tone of the muscles.
Characteristic features of rigidity occurring in Parkinson's disease are:
• Due to rigidity, posture becomes that of flexion attitude in which back is flexed, arms are abducted and flexed, and the knees are bent.
• In advanced cases, the rigidity may increase to such an extent that a statue-like appearance is produced with complete absence of movements.
• Rigidity differs from spasticity seen in lesions of pyramidal tracts (see Table 10.1-1).
Table. 10.1-1
Differences between spasticity and rigidity
| Feature | Spasticity | Rigidity |
| 1. Lesion | Occurs in pyramidal tract lesions, commonest site being internal capsule. |
Occurs in basal ganglia lesion, therefore, called the extrapyramidal rigidity. |
| 2. Muscles involved | One group of muscles either agonist or antagonist (usually antigravity muscles) are involved. |
Both agonist and antagonist muscles are involved producing a uniform hypertonia often resulting in general attitude of flexion of the limbs and trunk. |
| 3. Characteristics of Hypertonia |
Clasp-knife type of hypertonia is seen in muscles involved, i.e. on passive flexion initially there is marked resistance but then there is sudden completion of movement without much resistance (similar to closure of a pocket knife). |
Usually there occurs a uniform resistance to flexion giving a feeling as if lead pipe is being bent (lead-pipe rigidity). Sometimes, there is a series of catches during passive motion of the limb (cogwheel rigidity). |
| 4. Relation of hypertonia to stretch |
Spasticity is stretch sensitive, i.e. degree of hypertonia developed during any passive stretch is proportional to the speed of stretch applied. |
Rigidity is not stretch sensitive. |
Cause of rigidity. An increased discharge of γ-efferents supplying the muscle spindle causes rigidity.
In patients with Parkinson's disease, lack of dopaminergic activity due to degeneration of neurons in the substantia nigra shifts the balance towards excitatory cholinergic fibres. As a result, hyperkinetic features of Parkinson's disease appear.
3. Tremors. Tremors (i.e. involuntary rhythmic oscillatory movements of the distal parts of limbs and head) seen in Parkinson's disease have following characteristics:
• The tremors are present at rest, but disappear during activity. It is hallmark of Parkinson's disease and so popularly known as resting (static) tremors.
• Frequency of tremors ranges from 4–6 times/s.
• It is frequently seen as frill-rolling movements of the hand, i.e. rhythmic contraction of thumb over first two fingers.
• Tremors are suppressed during sleep and exaggerated by stress, anxiety and excitement.
The tremors are observed as rhythmic movements of pronation and supination in fingers, hands, lips or tongue.
L-dopa is used in the treatment of Parkinson's disease. It can cross the blood–brain barrier and reaches the brain tissue, where it is concentrated into dopamine and thus compensates its deficiency.
White matter of cerebrum
Passing through, between and around the subcortical masses of grey matter of cerebrum are tracts of white fibres. The white fibres of cerebrum are of three types (Fig. 10.1-7).
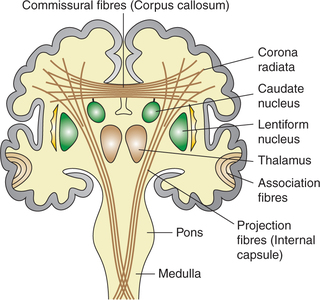
Fig. 10.1-7 Frontal view of coronal section of brain showing the position of association, commissural and projection fibres.
1. Association fibres. Association fibres connect the different gyri of the same hemisphere (Fig. 10.1-7).
2. Commissural fibres. Commissural fibres connect the corresponding parts of two cerebral hemispheres with each other. There are five bundles of commissural fibres (Fig. 10.1-7):
3. Projection fibres. Projection fibres connect the cerebral hemispheres with other parts of CNS, e.g. thalamus, brain stem and spinal cord. Projection fibres include the afferent and efferent tracts contained in corona radiata and internal capsule.
Corona radiata. Corona radiata (fountain of fibres) refers to that part of projection fibres that radiates from the upper end of internal capsule to cerebral cortex (Fig. 10.1-7). It contains both the ascending and descending fibres.
Internal capsule. Internal capsule is a thick curved band of projection fibres (ascending and descending) that occupy the space between the thalamus and caudate nucleus medially and the lentiform nucleus laterally. Superiorly, it fans out as corona radiata and inferiorly the fibres descend into the crus cerebri.
Parts. The internal capsule can be divided into following parts (Fig. 10.1-8):
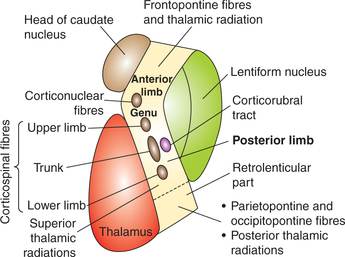
Fig. 10.1-8 Parts of internal capsule; disposition of motor fibres and thalamic radiations passing through it.
Applied aspects. In internal capsule fibres are densely crowded in a narrow area. Pyramidal fibres being compressed in this little space are particularly vulnerable to effects of even a pinpoint vascular lesion.
Damage to internal capsule from infarction and haemorrhage is a common form of stroke, resulting in loss or decrease in sensations and movements of the opposite half of the body (hemianaesthesia and hemiplegia).
Thalamus
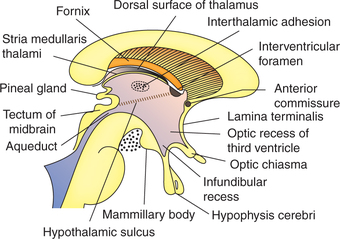
The thalamus is a large ovoid structure placed immediately lateral to the third ventricle (Figs 10.1-9 and 10.1-10).
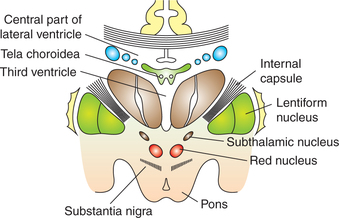
Fig. 10.1-10 Coronal section through the brain passing through the basilar part of pons showing the relations of ventral surface of thalamus and subthalamic structures.
• Posterior end (or pole) is expanded and is called pulvinar.
• Dorsal or superior surface of the thalamus is convex and forms the part of floor of the central part of lateral ventricle (Fig. 10.1-10).
• Medial surface forms the greater part of the lateral wall of third ventricle, and is lined by ependyma. The medial surfaces of the two thalami are connected by a short bar of grey matter called the interthalamic adhesion. Inferiorly, the medial surface is separated from the hypothalamus by hypothalamic sulcus (Fig. 10.1-9).
• Lateral surface of thalamus is related to the posterior limbs of internal capsule.
Internal structure
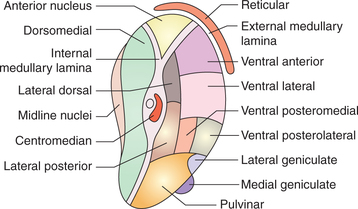
Like other parts of brain, thalamus consists of grey matter (mainly) and white matter (Fig. 10.1-11).
White matter. White matter is scanty in thalamus and includes:
• Stratum zonale, a thin layer of white matter covering the superior surface of thalamus.
• External medullary lamina is a thin layer of white matter covering the lateral surface of thalamus. It consists of thalamocortical and corticothalamic fibres.
• Internal medullary lamina is a Y-shaped sheet of white matter placed vertically in the grey matter of thalamus. It consists mainly of internuclear thalamic connections.
Grey matter. Grey matter of thalamus is divided into three masses of nuclei by the Y-shaped internal medullary lamina:
Functionally, the thalamic nuclei can be grouped under two divisions:
Functions and connections
Various functions of thalamus along with the connections through which these functions are mediated are summarized below.
1. Sensory relay centre. Almost all the sensory impulses (except olfactory) reach the thalamic nuclei, which relay them to the cerebral cortex by thalamic radiations (ascending thalamocortical system). Because of this thalamus is usually considered the head ganglion of all the sensory system.
2. Centre for integration of sensory impulses. The thalamus also forms a major centre for integration and modification of peripheral sensory impulses before the impulses are projected to specific areas of cerebral cortex. Because of this thalamus is usually considered as a functional gateway of cerebral cortex.
3. Crude centre for perception of sensations. Thal amus also acts as a crude centre for sense perception. Pain sensation is perceived in the thalamus itself. Whether a sensation is pleasant or unpleasant and agreeable or disagreeable is the function of thalamus.
4. Centre for integration of motor function. Thalamus receives the output from the basal ganglia and the cerebellum before projecting it to the motor cortex, thereby helping in integration of motor functions by unconscious regulation of muscle tone.
5. Role in arousal and alertness reaction. Majority of non-specific ascending impulses from reticular activating system are relayed to thalamus before proceeding to cortex. Through these fibres the thalamus is involved in controlling the level of consciousness and maintaining state of alertness and wakefulness.
6. Role in emotional aspect of behaviour. Because of intimate connections between thalamus, frontal cortex and hypothalamus, the thalamus is involved in subjective feeling of various emotions.
7. Role in language. Thalamus is also concerned with language (speech) function. Integration between different cortical parts by subcortical connections in the thalamus helps to achieve speech.
8. Role in synchronization of electroencephalogram. Thalamus also plays an important role in the genesis of synchronization of electroencephalogram (EEG).
9. Centre for integration of visceral and somatic function. Thalamus receives somatic as well as autonomic sensations, and is also connected with hypothalamus. Because of this it also acts as a centre for integration of visceral and somatic functions.
10. Centre for sexual sensations. Thalamus also acts as a centre for perception of sexual sensations.
11. Centre for reflex activity. All the sensory fibres relay in thalamus, so it forms the centre for many reflex activities.
Applied aspects
Thalamic syndrome. The thalamic syndrome is a disturbance of emotional responses to sensory experience characterized by symptoms and signs which occur on the opposite side of the body. These include the following.
1. Astereognosis, which occurs due to loss of tactile localization, tactile discrimination and stereognosis.
2. Thalamic over-reaction, i.e. the threshold for pain, touch and temperature is decreased and the sensations become exaggerated and disagreeable.
3. Ataxia, decreased muscle tone and profound muscular weakness.
Hypothalamus
The hypothalamus is a bilateral diencephalic diffuse nuclear mass situated below the thalamus. It is the most important organ of integration in the homeostatic control of internal environment.
Subdivisions and nuclei of hypothalamus
For convenience of description the hypothalamus can be divided as follows.
From medial to lateral into two zones:
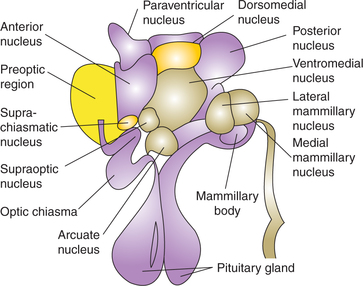
From anterior to posterior, the hypothalamic nuclear mass is arranged in four regions (Fig. 10.1-12):
Connections of hypothalamus (Fig. 10.1-13)
The hypothalamus serves as the main integrator of the autonomic nervous system, is concerned with visceral functions, and is, therefore, connected to other areas having a similar function. These include the various parts of the limbic system, the reticular formation, and autonomic centres in the brain stem and spinal cord.
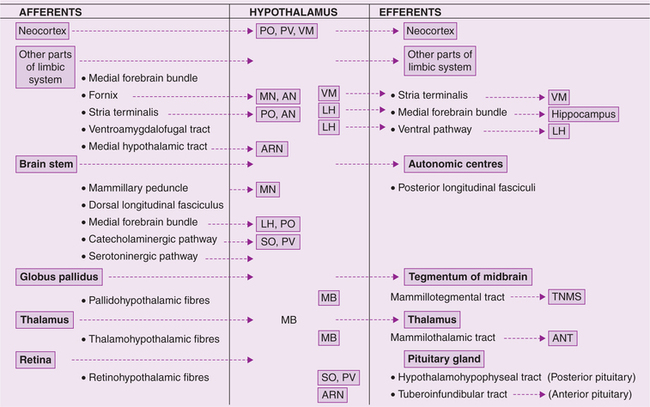
Fig. 10.1-13 Simplified scheme of main afferent and efferent connections of hypothalamus. (PO = Preoptic; PV = paraventricular; VM = ventromedial; MN = medial nucleus; AN = anterior nucleus; LH = lateral hypothalamus; SO = supraoptic; ARN = arcuate nucleus; MB = mammillary body.)
Apart from its neural connections, the hypothalamus also acts by releasing secretions into the blood stream and into the CSF.
Functions of hypothalamus
1. Autonomic functions subserved by the hypothalamus are:
(i) Cardiovascular regulation through cardiovascular control centres in the reticular regions of medulla and pons.
(ii) Regulation of peristaltic and secretomotor functions of alimentary tract.
2. Endocrinal functions include the following.
(i) Control of anterior pituitary. Hypothalamus does the following functions through the releasing hormones:
• Controls the metabolism by controlling thyroid gland.
• Through its influence over adrenal cortex, it controls the metabolism of different foodstuffs and maintains electrolyte balance.
• Keeps the gonads inhibited till the physical growth is complete. After physical growth is complete this inhibition is removed so that gonads start functioning and gametes are produced (propagation of species).
• Controls the formation of milk by the breasts by controlling prolactin secretion (see page 461).
(ii) Regulation of posterior pituitary functions. Neural control of posterior pituitary with the secretion of antidiuretic hormone (ADH) in regulation of water balance by controlling water excretion by kidneys (see page 280).
(iii) Regulation of uterine contractility and regulation of milk ejection from the breast. Oxytocin secretion increases the contractility of uterus especially at end of the pregnancy and thus helps during parturition. It also contracts the myoepithelial cells that surround the alveoli of breast and cause milk ejection. When the baby suckles the breast, signals from nipple to hypothalamus cause reflex oxytocin release, which causes expulsion of milk through nipples.
3. Control of circadian rhythm (biological clock). Circadian rhythm refers to rhythmic fluctuations in certain physiological parameters of the body. These are called circadian rhythms because they often show 24-h cycles (circadian around a day). Common rhythmic variations in homeostatic regulatory mechanism are:
• Rhythmic secretion of ACTH (see page 401),
• Rhythmic secretion of growth hormone (see page 372),
• Rhythmic secretion of melatonin (see page 420),
• Sleep–wake cycles (see page 551),
• Rhythmic gonadotropin secretion (see page 449).
4. Regulation of food intake. The regulation of food intake is an essential vegetative function of the hypothalamus, which maintains the body weight of an individual relatively constant over a long period. To regulate the food intake, hypothalamus has two centres, namely, the feeding centre and satiety centre, located in the tuberal region.
Feeding centre. The lateral hypothalamic nucleus subserves as the feeding centre or hunger centre. When this is stimulated, in animals, it creates a sensation of hunger and leads to increased food intake (hyperphagic).
Normally, the feeding centre is always active and its activity is inhibited by the satiety centre after food intake. The destruction of feeding centre leads to loss of appetite (anorexia).
Satiety centre. Satiety is opposite to hunger, i.e. it is a feeling of fulfilment after food intake. The ventromedial nucleus of hypothalamus acts as a satiety centre. Stimulation of this in animals causes sensation of food intake (fulfilment).
The cells of satiety centre act as glucoreceptors (also called glucostats), therefore the activity of satiety centre is governed by glucose utilisation of these cells.
Role of neurotransmitters in food intake. Food intake is increased by the stimulation of α2 adrenergic receptors in medial hypothalamus and centrally acting opioids.
Food intake is decreased by the stimulation of β adrenergic and dopaminergic in lateral hypothalamus and by stimulation of serotonergic pathways.
5. Regulation of sexual behaviour and reproduction. In animals, hypothalamus plays an important role in maintaining the sexual functions, especially in females. A decorticate female animal will have regular oestrous cycle provided the hypothalamus is intact.
6. Emotional and instinctual behaviour. The emotional and instinctual behaviour is mainly regulated by limbic cortex. The two centres in hypothalamus involved in such a behaviour and emotional changes are called reward centre and punishment centre (see page 548).
Reward centre is located along the course of medial forebrain bundle, especially in lateral and ventromedial nucleus of hypothalamus.
Punishment centre is located in the medial hypothalamus (periventricular zone).
Role of reward and punishment centres. Almost anything that we do is related in some way to reward and punishment. If we do something that is rewarding, we continue to do it. Electrical stimulation of this area encourages the animal to seek more of such stimulation. If we do something that is punishing, we cease to do it.
The electrical stimulation of this area leads to pain, fear, defence, escape reactions and the other elements of punishment. The experimental animal avoids further stimulation of this area.
Therefore, reward and punishment centres constitute one of the most important of all the controllers of our bodily activities, our drives, our aversions and our motivation.
7. Regulation of body temperature. The hypothalamus acts as principal integrating centre for heat regulation. By adjusting a balance between heat production and heat loss, it helps to maintain body temperature at 37°C. Hypothalamus accomplishes this function by two centres: heat loss centre and heat gain centre (see page 195).
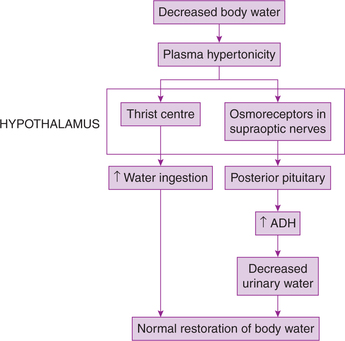
8. Role in regulation of water balance. Hypothalamus regulates water balance of the body by two mechanisms (Fig. 10.1-14):
• Through thirst centre by controlling water intake and
• Through osmoreceptors in supraoptic nucleus by controlling water loss.
(i) Through thirst centre. Thirst centre located in the lateral nucleus of hypothalamus is stimulated by plasma hypertonicity (which occurs when the water content of the body is reduced). This causes intense desire for water, and animal drinks large quantities of water.
(ii) Through osmoreceptors in supraoptic nucleus. The increased plasma osmolality also stimulates osmoreceptors in supraoptic nucleus. The stimulated neurons of supraoptic nucleus in turn send impulses to posterior pituitary gland to secrete hormone ADH. This hormone reaches the kidney tubules through blood and causes increased absorption of water from the collecting ducts of the kidneys.
Thus water loss is decreased. When body has excess water exactly opposite events occur.
Applied aspects
Disturbances in hypothalamic lesions include:
• Disturbances of body temperature regulation
• Sleep disturbances due to lesions in mammillary body and anterior hypothalamus
• Endocrine abnormalities, e.g. hypogonadism and hypothyroidism
• Disturbance in sexual functions due to involvement of mid hypothalamus
• Disturbance of body water balance due to damage to supraoptic nuclei or infundibular stalk, characterized by excessive thirst and polyuria
• Emotional disturbances leading to sham rage due to lesions in ventromedial and posterolateral parts.
Brain stem
The brain stem consists (from below upwards) of the medulla oblongata, pons and midbrain.
Medulla oblongata
The medulla oblongata is conical in shape and connects to the pons above and the spinal cord below.
Functions
1. Pathway for ascending and descending tracts. The medulla oblongata forms the main pathway for the ascending and descending tracts of spinal cord.
2. House of vital centres. The medulla oblongata houses many important centres which control the vital functions of the body:
• Respiratory centres (inspiratory and expiratory) control the normal rhythmic respiration (page 241)
• Vasomotor and cardiac centres control the blood pressure and functions of heart and vascular system (page 178)
• Deglutition centre controls the pharyngeal and oesophageal phase of deglutition (page 314)
• Vomiting centre is responsible for inducing vomiting in disorders of gastrointestinal tract
• Superior and inferior salivary nuclei, located in the medulla, control the salivary secretion (page 313).
3. Cranial nerve nuclei located in the medulla control following functions:
• Twelfth cranial (hypoglossal) nerve controls the movements of tongue.
• Eleventh cranial (accessory) nerve controls the movements of shoulder.
• Tenth cranial (vagus) nerve controls the functions of important viscera, viz. heart, lungs and GIT.
• Eighth cranial nerve controls the auditory function (cochlear division of the nerve has the relay in medulla oblongata) and vestibular function (medial and inferior vestibular nuclei extend through much of the medulla).
Pons
The pons is situated on the anterior surface of the cerebellum below the midbrain and above the medulla oblongata. Pons is divisible into two parts.
Functions
Pons subserves following functions:
1. Connecting pathway between cerebral cortex and cerebellum.
2. Pathway for ascending and descending tracts of spinal cord and medulla oblongata.
3. Joining station for medial lemniscus with fibres of 5th, 7th, 9th and 10th cranial nerves.
4. Contains pneumotaxic and apneustic centres for regulation of respiration (page 242).
Midbrain
The midbrain is a narrow part of the brain that connects forebrain to hindbrain.
Midbrain can be divided into two parts (Fig. 10.1-15).
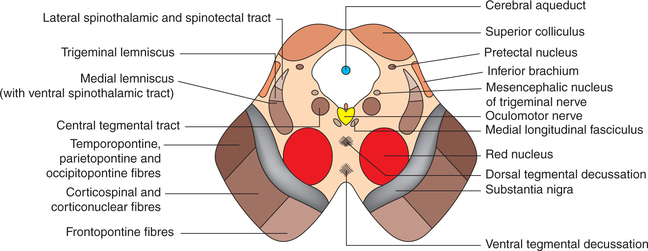
Fig. 10.1-15 Main features of internal structure of midbrain exhibited by transverse section at the level of superior colliculi.
1. Tectum. It consists of superior and inferior colliculi.
2. Cerebral peduncles. Each cerebral peduncle, in turn, consists of three parts which from anterior to posterior side.
(i) Crus cerebri (or basis pedunculi). It consists of large mass of vertically running descending fibres from cerebral cortex which include frontopontine fibres, corticospinal and corticonuclear fibres, and temporopontine, parietopontine and occipitopontine fibres (occupying the lateral one-sixth of crus).
(ii) Substantia nigra is a mass of pigmented grey matter (therefore appears dark in colour).
(iii) Tegmentum of the two sides is continuous across the midline. It contains following important masses of grey matter and nerve fibres:
Hindbrain
Cerebellum
Cerebellum, the largest part of hindbrain, consists of two lateral parts called the cerebellar hemispheres connected in the midline by a narrow central region called the vermis. The cerebellum are thrown into numerous transverse folds called folia. The surface of the cerebellum presents three main fissures (Fig. 10.1-16):
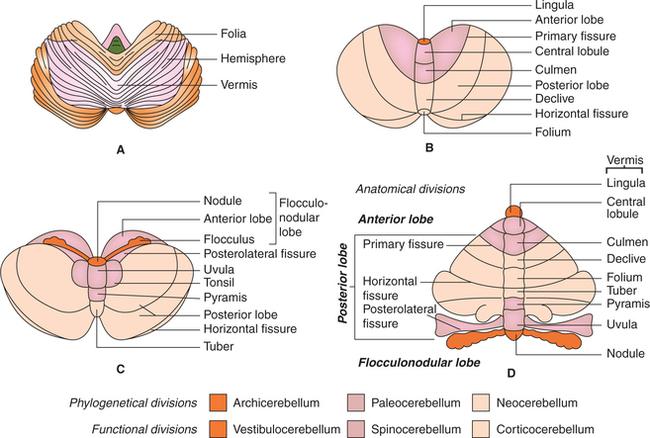
Fig. 10.1-16 Gross anatomy of cerebellum: A, superior surface showing folia; B and C, superior and inferior surface showing fissures, lobes and parts of vermis and D, schematic diagram to show the parts of vermis and hemisphere in a opened unrolled cerebellum.
• Primary fissure. It is V-shaped, with opening being forwards. It forms the posterior limit of the anterior lobe.
• Horizontal fissure separates the superior surface of the cerebellum from its inferior surface.
• Posterolateral fissure is situated anteriorly on the inferior surface of the cerebellar hemisphere, and separates the posterior lobe from the flocculonodular lobe.
Vermis is so named because it resembles a worm which is bent on itself to form a complete circle. It consists of following parts: lingula, central lobule, culmen, declive, folium, tuber, pyramis, uvula and nodule (Table 10.1-2).
Table. 10.1-2
Lobes of cerebellum and parts of vermis and hemisphere forming them
| Lobes of cerebellum | Part of vermis | Part of hemisphere |
| Anterior lobe | Lingula Central lobule Culmen |
No lateral projection Alae Anterior quadrangular lobule |
| Primary fissure Posterior lobe |
Declive Folium |
Posterior quadrangular lobule Superior semilunar lobule |
| Horizontal fissure | Tuber Pyramis Uvula |
Inferior semilunar lobule Biventral lobule Tonsil |
| Posterolateral fissure Flocculo-nodular lobe |
Nodule |
Flocculus |
Anatomical divisions
Anatomically the cerebellum has been divided into three lobes.
1. Anterior lobe is that part of cerebellum which lies in front of the primary fissure on the superior surface (Table 10.1-2).
2. Posterior lobe is that part of cerebellum which lies between the primary fissure and posterolateral fissure.
3. Flocculonodular lobe is that part of the cerebellum which lies anterior to the posterolateral fissure on the inferior surface. It consists of (Fig. 10.1-16 and Table 10.1-2):
Phylogenetical divisions
Phylogenetically, i.e. according to evolutionary stages, the cerebellum consists of three subdivisions.
1. Archicerebellum. It is the oldest part to develop. It consists of (Fig. 10.1-16):
2. Paleocerebellum. Phylogenetically, it is the next part to appear. It consists of (Fig. 10.1-16):
3. Neocerebellum. It is the latest part to develop. It consists of whole of the posterior lobe except pyramis and uvula (Fig. 10.1-16).
Functional divisions
Functionally, cerebellum is divided into three divisions:
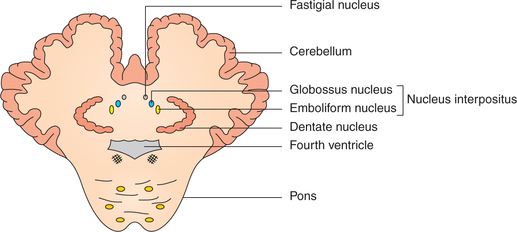
1. Vestibulocerebellum. It includes the flocculonodular lobe, which is its principal component and has vestibular connections only.
• Nucleus fastigial is its effector nucleus.
• It is concerned with control of body posture and equilibrium.
2. Spinocerebellum. It includes the parts forming paleocerebellum, i.e. entire anterior lobe except lingula and some parts of posterior lobe (pyramis, uvula and paraflocculus).
• Nucleus interpositus, i.e. nucleus globossus and nucleus emboliformis are its effector nuclei.
• It receives proprioceptive inputs from the spinal cord and is concerned with control of axial (trunk) and limb muscles postural reflexes.
3. Corticocerebellum. Corticocerebellum, also called as central cerebellum, includes whole of the posterior lobe except pyramis and uvula.
Histological structure
Histologically, cerebellum consists of (Fig. 10.1-17):
• Cerebellar cortex (outer grey matter layer),
• White matter (formed by afferent and efferent nerve fibres of cerebellum) forming medullary core and
• Deep cerebellar nuclei (masses of grey matter embedded in the medullary core).
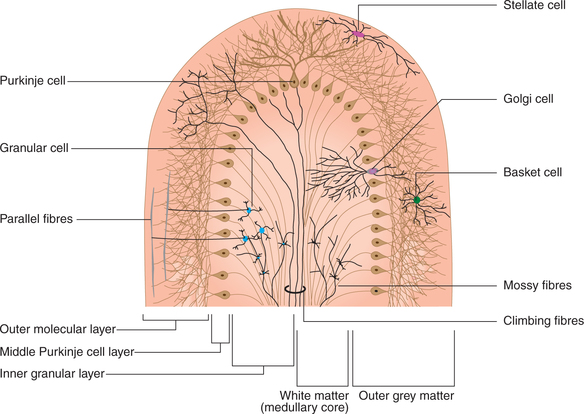
Microscopically the grey matter of cerebellar cortex consists of five main types of neurons (stellate cells, basket cells, Purkinje cells, granule cells and Golgi cells) which are arranged in three layers:
• Molecular layer (most superficial) is composed of two types of neurons (stellate and basket cells) and unmyelinated nerve fibres.
• Purkinje cell layer. It is composed of a single layer of large, flask-shaped Purkinje cells and dendrites of Purkinje cells.
• Granule cell layer consists of granule and Golgi cells, with their processes and sensory mossy fibres with their synaptic glomeruli.
The cerebellar cortex, i.e. outer grey matter surrounds inner medullary core of white matter (an arrangement opposite to what is seen in spinal cord). White matter is formed by both afferent and efferent fibres. These fibres can be classified in three groups.
1. Projection fibres of the cerebellum leave or enter the cerebellum. These are arranged in three bundles:
• Inferior cerebellar peduncle consists of fibres connecting cerebellum with medulla,
• Middle cerebellar peduncle contains the fibres connecting cerebellum with pons and
• Superior cerebellar peduncle connecting the cerebellum with midbrain.
2. Association fibres connect different regions of the same cerebellar hemisphere.
3. Commissural fibres connect the areas of two halves of cerebellar cortex with each other.
Within the white matter of medullary core of cerebellum are embedded four pairs of masses of grey matter called deep cerebellar nuclei (Fig. 10.1-18):
Neural circuits and neuronal activity in cerebellum
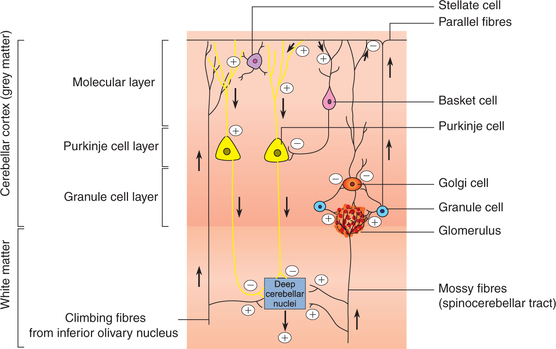
Cerebellum executes its functions through excitatory output of the deep cerebellar nuclei to the brain stem and thalamus. Neural connections within the cerebellar cortex, i.e. intrinsic cerebellar circuit (Fig. 10.1-19), is basically concerned with modulating or timing the excitatory output of deep cerebellar nuclei via the fibres of Purkinje cells. This is done in accordance with the signals received by the cerebellar cortex from different parts of the brain and body. The entire process can be discussed, for the purpose of understanding only, under following headings:
Afferents to cerebellar cortex
Afferents to cerebellar cortex reach via two types of fibres:
1. Climbing fibres. These fibres represent terminations of axons reaching the cerebellum from the inferior olivary nucleus (Fig. 10.1-19). The climbing fibres excite Purkinje cells and the deep cerebellar nuclei.
2. Mossy fibres. All the afferent fibres of cerebellum, other than the olivocerebellar, are called mossy fibres. The mossy fibres excite the Purkinje cells, granule cells and the deep cerebellar nuclei.
Neuronal activity of intrinsic cerebellar circuitry
As a result of excitatory input from the climbing fibres or mossy fibres (Fig. 10.1-19) following activity is set up in the intrinsic cerebellar circuitry:
Neuronal activity of deep cerebellar nuclei
The deep cerebellar nuclei receive excitatory inputs via collaterals from the mossy fibres, climbing fibres and also other excitatory inputs.
The neuronal activity in the cerebellar cortex plays an important role in modulating the excitatory signals of following pathways:
• From deep cerebellar nuclei to thalamus, then to cerebral cortex and motor pathway to spinal cord and
Because of this modulating or timing effect the cerebellar cortex is able to well organize and co-ordinate the different movements of the body.
Functions of cerebellum
1. Control of body posture and equilibrium. Vestibulocerebellum which includes flocculonodular lobe as its principal component and nucleus fastigii (as its effector nucleus) and vermal region of the cerebellum are concerned with control of body posture and equilibrium.
2. Control of muscle tone and stretch reflexes. Spinocerebellum regulates the postural reflexes by modifying muscle tone. It facilitates the γ motor neurons in the spinal cord. The γ motor neurons reflexly modify the activity of α motor neurons, and thus regulate the muscle tone. Thus cerebellum forms an important site of linkage of α–γ systems responsible for muscle tone.
3. Control of voluntary movements. Cerebellum is not able to initiate any motor activity, but co-ordinates movements initiated by the motor cortex.
Control of movements by cerebellum includes regulation of time, rate, range (extent), force and direction of muscular activity.
4. Other functions of cerebellum. Recent studies have shown that the importance of the cerebellum is as follows:
(i) Influence on autonomic system. It has been pos tulated that the cerebellum may influence autonomic functions and thus respiratory, cardio vascular, pupillary and urinary bladder responses.
(ii) Influence on conduction in ascending sensory pathway may be exerted by the cerebellum through the reticular formation and thalamus.
(iii) Control of eyeball movements. The oculomotor, trochlear and abducent nuclei, which supply extraocular muscles of eye movements, are brought under the cerebellar control through vestibular nuclei. Medial longitudinal fasciculus is involved in these connections.
Cerebellar lesions
Common signs observed in patients with cerebellar dysfunction due to lesions of cerebellum are the following.
1. Disturbances in tone and posture include:
(i) Atonia or hypotonia. Hypotonia refers to reduction and atonia to loss of tone in muscles. It occurs due to reduction of the facilitatory neocerebellar output to the descending inhibitory reticular formation.
(ii) Attitude changes. Trunk is bent with concavity towards the affected side; this is because the weight of the body is thrown on the unaffected leg.
(iii) Deviation movement. The arm held straight out in front of the body deviates laterally when the eyes are closed. In bilateral lesions both arms deviate.
(iv) Effect on deep reflexes. The deep or tendon reflexes become weak and pendular.
2. Disturbances in equilibrium. The patient suffering from disturbance of equilibrium walks on a wide base, sways from side-to-side (drunken-like gait), and is unable to maintain the upright posture due to involvement of vestibular system.
3. Disturbances in movements include:
(i) Ataxia, i.e. lack of co-ordination of movements, is the hallmark of cerebellar disorder.
(ii) Intention tremors become evident during purposeful movements and diminish or disappear with rest. These tremors become more marked as the hand approaches the object (i.e. are observed at the end of movement) and are coarse, oscillating, to-and-fro and rhythmic.
(iii) Nystagmus refers to regular and rhythmic to-and-fro involuntary oscillatory movements of the eyes, occurring due to inco-ordination of extrao cular muscles.
(iv) Dysarthria or scanning speech occurs due to incoordination of various muscles and structures involved in speech.
Spinal cord
Anatomy of spinal cord
Gross anatomy
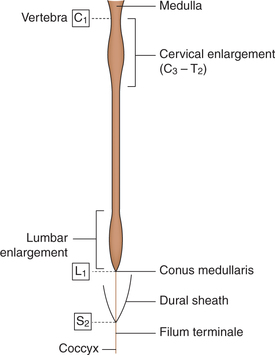
• The spinal cord (Fig. 10.1-20) extends from the upper border of the first cervical vertebra to the lower border of the first lumbar vertebra.
• Its upper end becomes continuous with medulla oblongata and its lower end called conus medullaris becomes continuous with a fibrous cord called filum terminale.
• The spinal cord, like the brain, is surrounded by three meninges: the dura mater, the arachnoid mater and the pia mater.
Internal structure
As seen on cross-section (Fig. 10.1-21), the spinal cord presents inner grey matter and outer white matter. Grey matter is constituted by the nerve cell bodies, dendrites and parts of axons, while white matter is formed by the myelinated and unmyelinated nerve fibres.
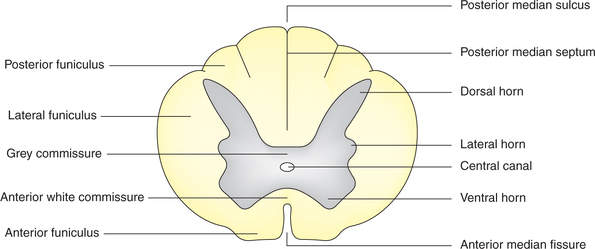
In transverse section the grey matter of spinal cord forms an H-shaped mass in the centre of which is present a canal called the spinal canal. The spinal grey matter exhibits following parts:
Dorsal horn or posterior grey column refers to the posterior horn-like projection of the H-shaped grey matter in each lateral half of the cord.
The dorsal horn neurons of spinal grey matter are involved in sensory functions.
Ventral horn or anterior grey column refers to the anterior projection of the grey matter in each lateral half of the cord.
The ventral horn neurons of spinal grey matter are involved in motor functions and send motor nerve fibres to the muscles and other effector organs.
Lateral horn or intermediate horn or lateral column refers to small lateral projection between the ventral and dorsal grey columns present in the thoracic segments and first two lumbar segments only.
The lateral horn cells of spinal grey matter extend from T1 to L2 segments and from S2 to S4 segments of the spinal cord.
Grey commissure is the part of the grey matter which connects the two (right and left) symmetrical halves of spinal grey matter across the midline. It is traversed by the central canal.
White matter is formed by the nerve fibres which are arranged as ascending and descending tracts (described later). In general, the white matter of spinal cord is divided into right and left halves, in front by a deep anterior median fissure and behind by the posterior median septum (Fig. 10.1-21). In each half the spinal white matter exhibits following parts:
Tracts of spinal cord
Ascending tracts convey impulses arising in various parts of body to different parts of the brain. The ascending tracts present in the spinal cord (Fig. 10.1-22) can be grouped as:
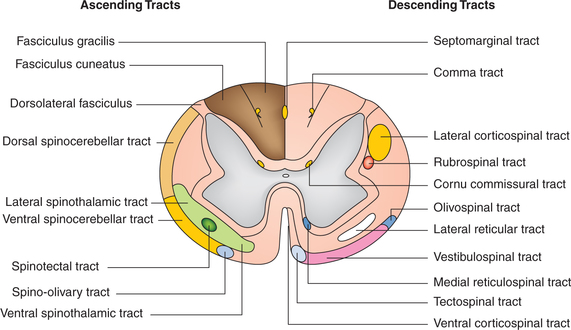
Fig. 10.1-22 Schematic diagram to show the position of the main ascending and descending tracts of the spinal cord and brain stem.
1. Posterior (dorsal) column–medial lemniscus pathway. Fasciculus gracilis and fasciculus cuneatus are often referred to as the posterior column tracts. Fasciculus gracilis is situated medial to fasciculus cuneatus (see page 511).
2. Spinothalamic pathways. Anterior and lateral spinothalamic tracts. The spinothalamic tracts are formed by the axons of the second-order sensory neurons of the pathway of crude touch and pressure (anterior spinothalamic tract) and pain and temperature (lateral spinothalamic tract). For details see page 512.
3. Spinoreticular tract. This tract is located in anterolateral white funiculus (Fig. 10.1-22). The fibres of the spinoreticular tract are the components of ascending reticular activating system (RAS) and are concerned with arousing consciousness or alertness.
4. Spinotectal tract. This tract is located in the lateral side of lateral white funiculus anterior to the lateral spinothalamic tract (Fig. 10.1-22). These fibres form alternate route for conduction of slow pain, and are also concerned with spinovisual reflexes.
5. Spino-olivary tract. This tract is located in anterolateral part of white funic ulus and occupies mostly the anterior white funiculus (Fig. 10.1-22). This tract is concerned with proprioception.
6. Spinocerebellar tracts. The spinocerebellar tracts carry proprioceptive impulses arising in the lower part of the body to the cerebellum. Some exteroceptive sensations (e.g. touch) may reach the cerebellum through these pathways. The spinocerebellar pathway is organized into the ventral and dorsal tracts.
Ventral spinocerebellar tract. It is located in lateral white funiculus of the spinal cord (Fig. 10.1-22) and is constituted by the second-order neurons of proprio ceptive pathway.
Dorsal (posterior) spinocerebellar tract. This tract is located in the lateral funiculus. It is situated posterior to ventral spinocerebellar tract (Fig. 10.1-22).
The peripheral processes of first-order neurons receive impulses from the muscle spindles, Golgi tendon organs and other proprioceptive receptors.
Cuneocerebellar tract. The central processes of some firstorder neurons (related to cervical segments) reach the accessory cuneate nucleus in the medulla. The central processes of the second-order neurons located in the accessory cuneate nucleus form the cuneocerebellar tract. This tract brings the conscious proprioception impulses from the upper limb. Thus, it may be regarded as the forelimb equivalent of the dorsal spinocerebellar tract.
The descending tracts are concerned with the various motor activities of the body and formed by the motor nerve fibres arising from the brain and descending into the spinal cord and brain stem (Fig. 10.1-22).
Descending tracts ending in spinal cord. Traditionally, the descending tracts ending in the spinal cord have been divided into two groups:
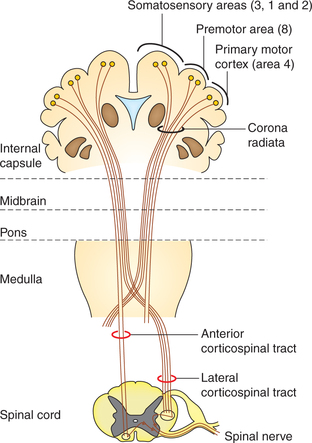
1. Pyramidal tracts. The pyramidal tracts refer to the corticospinal tracts, which are constituted by the axons that transmit motor signals directly from the cortex to spinal cord (Fig. 10.1-23). Corticospinal tract fibres originate from the following nerve cells in the cerebral cortex as:
• Primary motor cortex (area 4) – 30%,
• Premotor area (area 8) and supplementary motor area – 30% and
All the above fibres form the fibres of upper motor neurons of the motor pathway. In the lower part of medulla about 90% fibres decussate in the mid line to reach opposite side. From here downwards the fibres of corticospinal tracts are divided into two separate tracts:
• Lateral corticospinal tract is constituted by 80% of fibres which have crossed to opposite side.
• Anterior corticospinal tract is formed by 20% uncrossed pyramidal fibres. These fibres descend down through the anterior white funiculus of the same side.
The neurons giving origin to the fibres of pyramidal tract along with their axons constitute the upper motor neurons. The ventral motor neurons in the spinal cord along with their axons constitute the lower motor neurons.
Functions. The cerebral cortex controls voluntary fine skilled movements of the body through the corticospinal tracts. Interruption of the tract anywhere in its course leads to paralysis of the muscles concerned.
2. Extrapyramidal tracts. The descending tracts of spinal cord other than the pyramidal tracts are collectively called extrapyramidal tracts. These include (Figs 10.1-22 and 10.1-24):
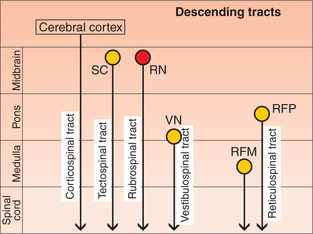
Fig. 10.1-24 Schematic drawing to show the various descending tracts ending in the spinal cord and brain stem. (SC = Superior colliculus; RN = red nucleus; VN = vestibular nucleus, RFP = reticular formation of pons; RFM = reticular formation of medulla.)
Descending tracts ending in the brain stem
Corticonuclear tracts. These arise from the cerebral cortex along with the corticospinal tracts. The nuclei of cranial nerves that supply skeletal muscles are functionally equivalent to ventral horn cells of the spinal cord. These are controlled by corticonuclear fibres.
Cortico–ponto–cerebellar pathway. This pathway consists of the fibres arising in the cerebral cortex of the frontal, temporal, parietal and occipital lobes. This pathway forms the anatomical basis for control of cerebellar activity of cerebral cortex.
Other fibres ending in the brain stem
Other fibres arising from the cerebral cortex end in the following masses of grey matter of brain stem:
• Red nucleus (corticorubral fibres),
• Tectum (corticotectal fibres),
The above fibres ultimately form part of extrapyramidal system.
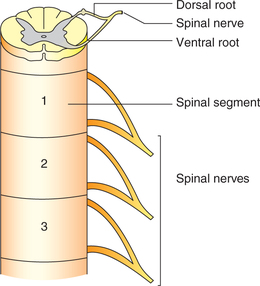
Spinal segments and spinal nerves
Spinal cord, though a continuous structure, can be considered to consist of 31 spinal segments, each giving attachment to rootlets of the ventral and dorsal root of each spinal nerve (Fig. 10.1-25). The 31 segments of spinal cord correspond symmetrically to 31 spinal nerves and are named as:
Each spinal nerve is a mixed nerve formed by union of two roots: a dorsal (sensory) root and a ventral (motor) root (Fig. 10.1-25).
1. Dorsal nerve root. The dorsal nerve root is formed by several rootlets. All sensory fibres reach the spinal cord through dorsal nerve roots. Each dorsal nerve root is marked by a swelling called dorsal nerve root ganglion or spinal ganglion. Dorsal root ganglion is composed of T-shaped unipolar neurons with peripheral and central processes.
Peripheral process of dorsal root ganglion cells extend up to sensory receptors in the skin. The area of the skin supplied by a spinal nerve is called dermatome.
Central processes of dorsal root ganglion cells constitute the dorsal nerve root, which is attached to spinal cord through various rootlets. Each rootlet just before entering the spinal cord divides into medial and lateral divisions.
2. Ventral nerve root. The ventral nerve root is composed of axons of motor neurons present in the ventral grey horn. The ventral root also contains the autonomic fibres originating from the lateral (intermediate) horn of the spinal grey matter.
Functions of spinal cord
1. Sensory functions. All the somatic afferent impulses enter the spinal cord through the dorsal nerve root.
After entering the spinal cord, all the somatic sensations are conveyed to the brain (post-central gyrus) by ascending tracts (see page 492).
2. Motor functions. Spinal cord performs motor functions through the pyramidal and extrapyramidal tracts. Motor functions served by spinal cord are:
• Control of tone and power of muscles,
• Control of movement of muscles and joints,
3. Autonomic functions. Visceral afferent impulses in spinal cord travel through dorsal nerve roots to lateral horns of T1–L2 and S2–S4 spinal segments.
Autonomic efferents travelling through spinal cord supply the visceral organs and control the activity of smooth muscles, heart, glands of GIT, sweat glands and adrenals. The spinal cord also regulates the body temperature. In other words, spinal cord helps in maintaining the optimal internal environment of the body through its autonomic function.
Lesions of spinal cord
Transection of spinal cord
Complete transection of spinal cord
The effects (symptoms and signs) produced by complete transection of the spinal cord occur in following three stages.
A. Stage of spinal shock. Spinal shock refers to cessation of all the functions and activity below the level of the section immediately after injury. Characteristic effects during shock are summarized:
• Paralysis of the muscles below the level of section.
• Loss of tone in the paralysed muscles. So, the muscles become atonic or flaccid. This is called state of flaccid paralysis.
• Areflexia, i.e. all the superficial and deep reflexes are markedly decreased or lost.
2. Sensory effects. All the sensations are lost below the level of transections.
3. Vasomotor effects. The sympathetic vasoconstrictor fibres leave the spinal cord between T1 and L2. Therefore, depending upon the site of lesion the vasomotor effects produced are:
B. Stage of reflex activity. After about 3-weeks period, depending largely upon the general health of the patient, the reflex activity begins to return to the isolated segments of spinal cord below the level of lesion. Various developments which take place are:
• Smooth muscles regain functional activity first of all.
• Sympathetic tone of the blood vessels is regained and blood pressure is restored to normal.
• Skeletal muscle tone then recovers slowly after 3–4 weeks.
• Reflex activity begins to return after few weeks of recovery of muscle tone.
• Flexer reflexes return first. The reflex which elicited first is Babinski's reflex (Babinski sign is positive).
• Extensor reflexes return after variable period of 1–5 weeks.
• Mass reflex can be elicited in some cases by scratching the skin of the lower limb or anterior abdominal wall depending on the level of lesion.
• Note. The mass reflex can be utilized to provide paraplegic patients a degree of bladder and bowel control. Patients can be trained to initiate urination and defaecation by intentionally producing mass reflex with the help of a stroke or a pinch on their thighs.
C. Stage of reflex failure. The failure of reflex activity may occur when general condition of the patient starts deteriorating.
Hemisection of the spinal cord (brown-sequard syndrome)
Hemisection of the spinal cord refers to a lesion involving one lateral half of the spinal cord.
The effects of hemisection of the spinal cord can be described in two stages:
Immediate effects
Immediate effects following hemisection of the spinal cord are those of ‘spinal shock’.
Late effects
If the patient survives, typical motor and sensory changes develop after recovery from the spinal shock. These changes constitute the Brown-Sequard syndrome and can be described as:
Changes at the level of hemisection
• All the sensations are lost (complete anaesthesia) at the level of hemisection on the same side because of complete damage to posterior nerve root, posterior horn cells and spinothalamic fibres (which cross to the opposite side).
• Lower motor neuron (LMN) type paralysis (flaccid paralysis) is seen due to damage to the anterior horn cells.
• Permanent vasomotor paralysis occurs due to damage of the lateral horn cells.
 Cerebrum
Cerebrum Lobes and functional areas
Lobes and functional areas