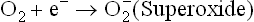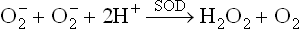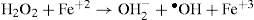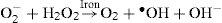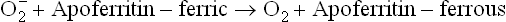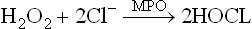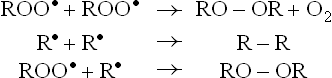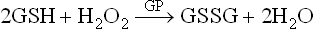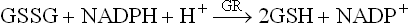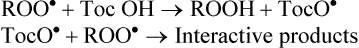Free Radicals In Health And Disease
Free radical is a molecule or molecular fragment that contains one or more unpaired electrons in its outer orbit and has an independent existence. A free radical is designated by a superscript dot(R•). In body, the oxidative reactions normally ensure that molecular oxygen is completely reduced to water. Incomplete reduction of oxygen which generates oxygen-free radicals is an infrequent occurrence but plays vital role in health and disease, as discussed in this chapter. Some oxygen free-radicals of biological importance include superoxide radical(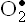 ), hydroperoxyl radical(
), hydroperoxyl radical( 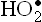 ), and hydroxy radical(OH•)
), and hydroxy radical(OH•)
After going through this chapter, the student should be able to understand:
• Incomplete reduction of oxygen and other sources of free radicals; generation of free radicals; the chain reaction; the damaging and the beneficial effects of reactive oxygen metabolites.
• Scavenging of oxygen free radicals, the antioxidant defences and their role in aetiology of diseases.
I Incomplete Reduction of Oxygen
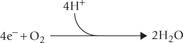
Oxygen is the final acceptor of electrons in the electron transport chain (ETC). Normally, four electrons are transferred to molecular oxygen to form a water molecule; this is the final event of the electron transport chain.
Thus, a complete reduction of the molecular oxygen requires four electrons. Sometimes oxygen settles for a fewer electrons at a time, thereby undergoing incomplete reduction. The products of incomplete reduction are referred to as reactive oxygen intermediates. They are highly reactive, being capable of reacting with a number of biomolecules. Because of their strong oxidant and cytotoxic properties, they are implicated in a number of pathological processes.
A Reactive Oxygen Intermediates and Free Radicals
The reactive oxygen intermediates are referred to as oxygen free radicals (OFR), reactive oxygen metabolites (ROM), or simply active oxygen (AO). These include, among others, superoxide, hydrogen peroxide, and hydroxy radicals.
1. Superoxide radical is produced when a single electron is transferred to oxygen. It is, therefore, referred to as the single electron reduction product of oxygen. It is both an anion and a free radical.
2. Hydrogen peroxide: The two-electron reduction product of oxygen is hydrogen peroxide (H2O2).
Since this reaction generates a non-radical product (H2O2) from the free radical reactants, it is called dismutation.
The dismutation reaction may occur spontaneously or it is catalyzed by the enzyme superoxide dismutase (SOD). When spontaneous, its rate is very slow.
3. Hydroxy radical: The three-electron reduction product of oxygen is hydroxy radical (‘OH) which is themost powerful free radical. It is produced when an electronis transferred from ferrous ion to hydrogen peroxide.This results in lysis of O-O bond; one of the fragmentsappears as the hydroxy radical, as shown below:
The above reaction is referred to as the Fenton reaction.
A summary of all these reduction steps of oxygen is presented in Figure 27.1 .
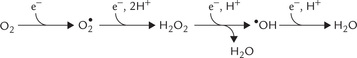
Fig. 27.1 Partial reduction of molecular oxygen by fewer than four electrons generates highly reactive products 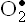 is one electron reduction product, H2O2 is 2 electron reduction product •OH is three electron reduction product.
is one electron reduction product, H2O2 is 2 electron reduction product •OH is three electron reduction product. 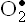 = superoxide radical, ‘OH = hydroxy radical, H2O2 = hydrogen peroxide.
= superoxide radical, ‘OH = hydroxy radical, H2O2 = hydrogen peroxide.
Another iron-catalyzed reaction for generation of hydroxy radicals is Haber-Weiss reaction.
B Transition Metals
Like ferrous iron, copper in the cuprous state can also react with hydrogen peroxide to yield the hydroxy radical. The ferrous iron and cuprous copper are more reactive than their oxidized counterparts, ferric and cupric, respectively. They are referred to as the transition metals. Evidently they are important in oxygen-free-radical biochemistry. In their absence, the superoxide and hydrogen peroxide are harmless and rapidly removed.
Because ferrous iron plays an important role in generating the deleterious free radicals, it is never found in free or loosely chelated form in healthy state. When transported in plasma, it is bound with apotransferrin (to form transferrin) and, while being stored, it is bound with apoferritin (to form ferritin). However, superoxide radical is capable of releasing the ferritin bound iron by causing ferric to ferrous conversion.
Since ferrous has low affinity for apoferritin, it is liberated and, in turn, seriously exacerbates the tissue damage.
II Generation of Oxygen Free Radicals
A Electron Leakage
Major source of free radicals in cells is “electron leakage” from the electron transport chain. Normally, efficiency of the electron transfer reactions is so high that most of the oxygen is completely reduced to form water and less than 2% forms oxygen free radicals (OFR).
Simply stated, the electron leakage is minimum under normal circumstances. Transition metals which can amplify the free radical response, are kept sequestered. However, these measures are not completely effective and some “leakage” is inevitable due to improper operation of electron transport chain. This results in generation of OFRs, even under normal circumstances.
B Normal Oxidation-reduction Reactions
Generation of free radicals is common during normal metabolism. Examples include:
1. Autoxidation of certain compounds including adrenaline, thiols, ascorbic acid, etc. Such reactions are greatly amplified through participation of transition metals.
2. Flavin coenzymes, present in the peroxisomes, are especially active in generating H2O2 (Box 27.1).
3. Enzymes, such as xanthine oxidase, aldehyde oxidase, and dihydro-orotate dehydrogenase can also generate OFRs.
C Exogenous Agents
Toxic compounds, such as carbon tetrachloride, are capable of generating oxidative stress (Chapter 12). Exposure to ionizing radiations damages tissues by producing free radicals. Lights of certain wavelengths can cause photolysis of covalent bonds to produce free radicals. Cigarette smoke also contains dangerously high concentration of free radicals.
D Respiratory Burst
In all the examples discussed so far, free radicals are accidently generated and are potentially hazardous for the body. However, free radical production is sometimes required in biological systems. For example, phagocytes generate free radicals with a specific purpose of destroying the engulfed bacteria. Certain enzymes (such as, ribonucleotide reductase) generate free radicals at their active site, which helps the catalytic process (Chapter 20). Certainly, free radicals have beneficial effects as well.
Phagocytosis by white blood cells:
Phagocytosis is an important defence mechanism, especially against the invading bacteria. Neutrophils and monocytes are important components of this mechanism. Both can engulf the microorganisms, foreign particles and cellular debris, and subsequently kill the invading microorganisms through generation (and participation) of the free radicals.
1. The enzyme NADPH oxidase catalyzes formation of superoxide radical from oxygen and NADPH (Fig. 27.2 ).
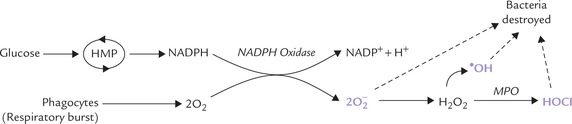
Fig. 27.2 Generation of superoxide radicals in neutrophils by NADPH oxidase (MPO = myeloperoxidase).
2. By dismutation, H2O2 is generated, as discussed earlier.
3. The hydrogen peroxide is involved in generation of another lethal oxygen species: hypochlorous acid (HOCl) and hydroxy radicals. Hypochlorous acid is formed by combination of chloride ions with the hydrogen peroxide. The enzyme catalyzing this conversion, myeloperoxidase (MPO) is present in neutrophil granules.
Both hypochlorous acid and hydroxy radicals have potent microbicidal action. Thus, they strengthen body defences by destroying the invading microbes. If the above pathway is impaired due to some reason, the neutrophils are unable to generate free radicals. Such patients are highly susceptible to bacterial infections because although their phagocytes ingest the bacteria normally, they are not able to destroy the ingested pathogen. The condition is fatal in early life (Case 27.1).
Hydrogen peroxide is not a free radical:
All reactive oxygen metobolites are erroneously believed to be free radicals, though technically speaking, the latter term is reserved for only those substances which contain a single, unpaired electron. Thus, superoxide is a free radical but hydrogen peroxide is not.
The respiratory burst:
It has been observed that cellular consumption of oxygen is greatly increased following ingestion of bacteria by the phagocytic cells (or following interaction of these cells with some external stimuli such as membrane binding of immunoglobulins). In neutrophils, the oxygen consumption may rise up to 50-fold, i.e. the respiratory burst.
III Damage Produced by Free Radicals
All types of biomolecules can be damaged by free radicals because of their extremely high reactivity; for example:
Lipids
Lipids are most susceptible to damaging effects of free radicals. Cell membranes, being rich in polyunsaturated fatty acids (PUFAs), are especially prone to the damage. This is because the oxidative damage to PUFA proceeds in a self-amplifying manner, which is also referred to as the chain reaction. Initial reaction of the free radical with PUFA generates more free radicals. Each free radical, in turn, generates more of free radicals.
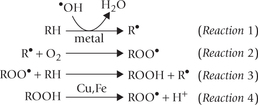
RH = target PUFA; R• = initiating radical (or carbon centred radical; ROO• = fatty acid peroxyl radical, ROOH = Lipid hydroperoxide.
The chain reaction is initiated with interaction of PUFA with a free radical 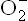 ,
, 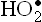 , or •OH). Removal of hydrogen atom follows to generate R•—the carbon-centred radical (Reaction 1). It promptly adds oxygen to form the fatty acid peroxy radical (ROO•, Reaction 2). The latter is termed carrier of the chain-reaction since it can attack another polyunsaturated lipid molecule (Reaction 3). This reaction generates a lipid hydroperoxide (ROOH). Thus, net result of Reactions 2 and 3 is the conversion of R’ to ROOH. The latter can break to form more of oxidizing species, e.g. peroxyl radical (Reaction 4) and aldehydes. Damage by these compounds is not confined to their site of production since they can diffuse to other parts of the cell.
, or •OH). Removal of hydrogen atom follows to generate R•—the carbon-centred radical (Reaction 1). It promptly adds oxygen to form the fatty acid peroxy radical (ROO•, Reaction 2). The latter is termed carrier of the chain-reaction since it can attack another polyunsaturated lipid molecule (Reaction 3). This reaction generates a lipid hydroperoxide (ROOH). Thus, net result of Reactions 2 and 3 is the conversion of R’ to ROOH. The latter can break to form more of oxidizing species, e.g. peroxyl radical (Reaction 4) and aldehydes. Damage by these compounds is not confined to their site of production since they can diffuse to other parts of the cell.
Reaction 1 falls into the initiation phase; and Reaction 2–4 make up the propagation phase of this chain reaction.
Termination of this reaction chain occurs when a peroxyl radical reacts with another such radical to form inactive products.
Proteins
Proteins are far less susceptible than PUFA to free radical damage. Mechanism of protein damage is still not very clear. It is possible that protein conformation may be altered because of oxidation of sulfhydryl group, or modification of certain amino acid residues such as proline or methionine.
IV Free Radical Scavenger Systems
Several protective mechanisms have evolved in cells that serve to minimize the toxic effects of free radicals. These free radical scavenger systems or antioxidant defence mechanisms can be divided into two categories:
• Preventive mechanisms which prevent the generation of free radicals.
• Interceptive mechanisms which destroy the free radicals that are accidentally generated.
A Preventive Mechanisms
The most important preventive mechanisms against free radical generation are (a) the efficiency of electron transport, and (b) sequestration of transition metals, as discussed earlier. In addition, the peroxide decomposing enzymes, such as glutathione peroxidase and catalase, play important roles:
1. Glutathione peroxidase (GP), a cytosolic enzyme, reductively eliminates hydrogen peroxide and organic hydroperoxides, thus preventing unchecked buildup of peroxides in cells. Peroxides are eliminated by reaction with reduced glutathione (GSH), catalyzed by GP.
The oxidized glutathione (GSSG) is reconverted to reduced glutathione by the enzyme glutathione reductase (GR), using the reducing power of NADPH.
Detoxification of H2O2 in this way prevents generation of free radicals from it (remembering that, by definition, H2O2 is not a free radical). Some types of glutathione peroxidase require selenium (Se) this is the reason why Se appears to have antioxidant activity.
2. Catalase is another enzyme causing decomposition of peroxide to yield water and oxygen. It is a haemcontaining enzyme, present in blood and tissues (which is why hydrogen peroxide bubbles when applied to wounds). Highest concentration of catalase is present in peroxisomes (up to 40% of the total proteins). This enzyme is used when large quantities of H2O2 are generated.
B Interceptive Mechanisms
These exist in both aqueous and membrane compartments of the cell and comprise an enzyme (superoxide dismutase) and several non-enzyme substances.
1. Superoxide dismutase (SOD) is the only known enzyme that takes a free radical (superoxide) as its substrate and hence acts as a scavenger. Different isoenzymes of SOD are described. The isoenzyme of SOD present in mitochondria is Mn2+-dependent, and the other, present in cytosol requires copper. An extracellular copperzinc-dependent isoenzyme has also been reported.
2. Non-enzyme substances include vitamin E, retinoids, ascorbate, ceruloplasmin, transferrin, ferritin, ubiquinone, uric acid and bilirubin.
(a) Vitamin E and retinoids being lipophilic, act in biological membranes.
(b) The others are aqueous phase antioxidants.
(c) Some of these agents are also called chain-breaking antioxidants since they terminate the lipid peroxidation chain reactions. For instance, α-tocopherol (Toc OH, a vitamin E compound) does so by intercepting lipid peroxyl radical (ROO•).
Tocopherol is the most potent chain-breaking antioxidant in tissues. The other example is β-carotene. It is, however, less effective.
The antioxidant action of vitamin C and β-carotene have been explained in Chapter 18. Consumption of foods rich in the above vitamin nutrients has been recommended for reducing risk of certain chronic health problems. For example, the populations that consume foods rich in vitamin C are known to have reduced risk of stomach cancer; foods containing β -carotene may offer protection against many cancers, including those of lungs, stomach, oesophagus and oral cavity.
C Others
Caffeine and ceruloplasmin are good antioxidants. Vitamin A and cysteine are mild, non-specific antioxidants.
Action of antioxidants is supplemented by processes that repair the damage caused by free radicals, e.g. DNA repair enzymes (Chapter 21).
V Free Radicals in Aetiology of Disease
Free radicals have been implicated in degeneration of aging and a number of human diseases such as cancer, inflammatory disease, and reperfusion injury, and in the aging process (Table 27.1 ).
Table 27.1
Disorders caused by free radicals
| 1. Age-related diseases | Parkinsonism, Alzheimer’s disease |
| 2. Diseases of the eye* | Retrolenral fibroplasia, retinopathy of prematurity, cataractogenesis |
| 3. Respiratory diseases | Adult respiratory distress syndrome, bronchopulmonary dysplasia in premature infants |
| 4. Chronic inflammatory disease | Rheumatoid arthritis |
| 5. Cancers | |
| 6. Atherosclerosis and myocardial infarction | |
| 7. Diabetes mellitus | |
| 8. Reperfusion injury and shock-related injury |
*Eye tissue has highest concentration of free radical scavenging enzymes.
The list is growing and there is tendency to implicate free radicals in pathogenesis of several diseases where no other pathogenic mechanism can be elucidated. To establish or rule out involvement of free radicals in a disease is difficult because of their extremely short lifetime.
Specific role of oxygen derivatives in pathogenesis of several diseases is, however, established beyond doubt:
Parkinsonism
Excessive production of hydrogen peroxide by monoamino oxidase (MAO) has been implicated as a major factor for the neuronal degeneration in patients with Parkinson’s disease. The dopaminergic nigrostriatal neurons that are destroyed in this disease have shown high MAO activity. Thus, treatment with MAO inhibitors has been found to be an effective mode of therapy in this disorder.
Atherosclerosis
Oxidized low density lipoproteins (LDL), formed by action of free radicals, are readily taken up by the macro-phages, producing foam cells. These cells accumulate beneath the endothelial layer of the arterial wall, and this triggers onset of atherogenesis (Chapter 12).
Diabetes Mellitus
Free radicals induce destruction of the pancreatic (3-cells, and this has been implicated in etiopathogenesis of the type-1 diabetes.
Cancer
It is firmly established that when mutations occur within DNA, cancer may arise. Free radicals cause mutations by inflicting chromosomal damage and inhibiting the DNA repair processes.
Alzheimer’s Disease
It is a chronic and progressive neuro-degenerative disorder, characterized by loss of neurons in motor, sensory and cognitive systems in elderly people (onset: 7th to 9th decade of life). The neuropathological hallmarks (senile plaque and neurofibrillary tangles) were first described by Alois Alzheimer a German psychiatrist, in 1907. The disease has genetic predisposition, and free radicals play important role in causing the neuronal damage.
Male Infertility
Males are at a greater risk of incurring free radical damage because they tend to accumulate large body stores of iron (iron plays important role in free radical generation, as discussed earlier). These free radicals are known to reduce sperm viability and motility, hence contributing to male sterility. Women are protected till menopause.
Rheumatoid Arthritis
Excessive generation of free radicals by macrophages (respiratory burst) is one of the major causes in the pathogenesis of several inflammatory diseases, including rheumatoid arthritis.
Thus, medical importance of free radicals is widely acknowledged, and yet not entirely clear. Some consider oxygen as the most important and least avoidable environmental mutagen. Laboratory animals are known to die in an atmosphere of oxygen, and premature infants have developed retrolental fibroplasia. Unfortunately, oxygen therapy of this condition results in blindness.
 The incomplete reduction of oxygen during aerobic metabolism generates reactive oxygen intermediates such as the superoxide and hydroxy radicals.
The incomplete reduction of oxygen during aerobic metabolism generates reactive oxygen intermediates such as the superoxide and hydroxy radicals.