14 INFECTION
INTRODUCTION
Humans appear to have had some understanding of the factors involved in the transmission of infectious diseases from a very early time. Evidence of purposeful sanitation has been found in ancient civilisations. During the fourteenth century, people infected with the Black Death (a bubonic plague involving an infection of the lymph glands) were isolated because, although the transmission method was unknown, it was realised that infected individuals were contagious. But it was only when Robert Hooke first saw fungi under a rudimentary microscope in 1665 that our understanding of microorganisms and their infectious consequences started to become clearer. The pioneering nurse, Florence Nightingale, obviously understood infection control and the ability to limit the impact of microorganism contamination. During the Crimean War (1853–1856) she instituted fundamental infection-control procedures that dramatically reduced the infection rate of wounded soldiers and therefore the mortality rate.1 Joseph Lister, a surgeon in the 1800s, implemented the use of antiseptics for surgery in operating theatres, greatly reducing surgical infections, which were catastrophic in patients and caused massive mortality rates.2
However, it was not until Alexander Fleming’s discovery of penicillin in 1923 and the subsequent extraction of commercial quantities by the Australian pharmacologist, Howard Florey, in the 1940s that our ability to eradicate microorganisms using antibiotics occurred. This discovery paved the way for modern healthcare methods to treat and prevent infectious diseases in individuals who may otherwise die. Coupled with this discovery, sanitary living conditions, clean water and adequate nutrition in developed countries like Australia and New Zealand have greatly reduced the mortality rate from infectious diseases.
This chapter examines what microorganisms are and which ones are harmful to humans. We also explore treatment methods, including antimicrobials and vaccines, as well as the more common infections and those acquired in healthcare settings, particularly hospitals. The final section looks at a growing problem in Australia and New Zealand: antimicrobial resistance to drug therapies.
INFECTION RATES
Currently, the mortality rate attributable to infectious diseases in Australia is very low compared to 80 years ago (see Figure 14-1). There were just over 1700 deaths due to infectious diseases in 2005 — septicaemia accounted for most of these deaths (1053), followed by viral hepatitis (162).3 The majority of deaths in Australia and New Zealand reflect those of other developed countries, where people die predominantly from cardiovascular disease and cancers. Nonetheless, when other contributing causes of death are included, pneumonia and influenza were evident in about 13% of all deaths.3
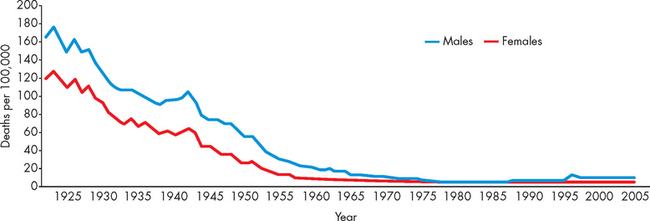
FIGURE 14-1 Mortality rate from infectious diseases in Australia, 1922–2005.
Source: Australian Institute of Health and Welfare. Australia’s health 2008. Cat. no. AUS 99. Canberra: AIHW; 2008.
In contrast, infectious diseases remain the number one cause of death worldwide, accounting for 26% of all deaths in 2005.4 In Africa alone, 62% of deaths were attributable to infection. While the reasons for this are multifactoral, developing countries do not have the infrastructure and living standards of countries such as Australia and New Zealand, and with high concentrations of people living in close quarters, infectious contamination occurs more readily. In addition, vaccination coverage is not as extensive in developing countries and unfortunately children acquire diseases that are eradicated in Western countries, which significantly impacts on the mortality rate.
Developing countries with dense populations and poor sanitation suffer from plague, cholera, malaria, tuberculosis, leprosy and schistosomiasis. Only smallpox has been eradicated worldwide by vaccination. Although vaccines and antimicrobials have altered the prevalence of some infectious diseases, mutant strains of bacteria and viruses have emerged showing resistance to the protection provided by drug therapy. The emergence of new diseases, such as West Nile virus, severe acute respiratory syndrome (SARS), Lyme disease, Hantavirus and drug-resistant tuberculosis, indicates the current intense challenges being faced in the struggle to prevent and control infectious diseases.
DEFINITIONS
To gain a fuller understanding of the infectious disease process, you first need to understand several terms, as the interaction between microorganisms and humans is complex. These terms are used throughout the chapter and so understanding them is vital.
 Antimicrobial: a chemical agent that acts to inhibit, grow or destroy microorganisms. The most common antimicrobial is antibiotics and these are effective against pathogenic bacteria.
Antimicrobial: a chemical agent that acts to inhibit, grow or destroy microorganisms. The most common antimicrobial is antibiotics and these are effective against pathogenic bacteria. Colonisation: refers to the presence, growth and multiplication of an organism without observable clinical symptoms or immune reaction in a patient.
Colonisation: refers to the presence, growth and multiplication of an organism without observable clinical symptoms or immune reaction in a patient. Epidemic: a sudden increase in the incidence of a disease, which many people acquire within a short time frame, and usually within a particular location — for example, an outbreak of gastroenteritis.
Epidemic: a sudden increase in the incidence of a disease, which many people acquire within a short time frame, and usually within a particular location — for example, an outbreak of gastroenteritis. Flora: microorganisms that inhabit a body region without causing infection — for example, bacteria within the gastrointestinal system.
Flora: microorganisms that inhabit a body region without causing infection — for example, bacteria within the gastrointestinal system. Healthcare-acquired infection: an infection acquired by an individual in a healthcare facility. Such infections are often antibiotic-resistant bacteria in hospitals and were previously known as nosocomial infections.
Healthcare-acquired infection: an infection acquired by an individual in a healthcare facility. Such infections are often antibiotic-resistant bacteria in hospitals and were previously known as nosocomial infections. Microorganisms: these include bacteria, viruses, fungi and parasites. Microbe is another commonly used term that is synonymous with microorganism. Many microorganisms do not harm humans: in fact, they live on our skin, in our bodies and in the environment. However, others cause infections that range from being irritating to the individual to life-threatening.
Microorganisms: these include bacteria, viruses, fungi and parasites. Microbe is another commonly used term that is synonymous with microorganism. Many microorganisms do not harm humans: in fact, they live on our skin, in our bodies and in the environment. However, others cause infections that range from being irritating to the individual to life-threatening. Opportunistic infection: a microorganism causing infection in an individual that would not normally harm a healthy individual. For example, an immune-compromised person receiving chemotherapy acquires infections more readily.
Opportunistic infection: a microorganism causing infection in an individual that would not normally harm a healthy individual. For example, an immune-compromised person receiving chemotherapy acquires infections more readily. Pandemic: a disease that spreads rapidly and widely throughout an entire country or continent, or even the world. This is greater in magnitude that an epidemic. For example, the spread of swine flu, influenza A H1N1, across several continents in 2009 from a base in Mexico was a pandemic (an explanation of H1N1 terminology is provided later in this chapter).
Pandemic: a disease that spreads rapidly and widely throughout an entire country or continent, or even the world. This is greater in magnitude that an epidemic. For example, the spread of swine flu, influenza A H1N1, across several continents in 2009 from a base in Mexico was a pandemic (an explanation of H1N1 terminology is provided later in this chapter). Pathogen: a microorganism that causes disease. The common cold is one of the most common viral infections in humans.5
Pathogen: a microorganism that causes disease. The common cold is one of the most common viral infections in humans.5MICROORGANISMS
Normal flora
For many microorganisms, the human body is a very hospitable site to grow and flourish. The microorganisms are provided with nutrients and appropriate conditions of temperature and humidity. In many cases a mutual relationship exists in which humans and microorganisms benefit. For instance, the human gut is colonised by a large variety of microorganisms. At birth, the gastrointestinal tract becomes progressively colonised with microorganisms that provide vital functions throughout the individual’s life span. The large intestine contains numerous bacteria, yeasts and parasites.6,7 In fact, it has been estimated that in excess of 1011 bacteria (that is, 100,000,000,000 or one hundred billion) are contained in each gram of human faeces, so there are more bacteria in one gram of faeces than there are people in the world or cells in your entire body!
Table 14-1 summarises the microorganisms that are the normal flora of different body regions. These bacteria are provided with nutrients from ingested food and in exchange they produce (1) enzymes that facilitate the digestion and utilisation of many molecules in the human diet, (2) antibacterial factors that prevent colonisation by pathogenic microorganisms and (3) usable metabolites (e.g. vitamin K is needed in clotting and is produced by bacteria in the large intestine). This relationship normally is maintained through the physical integrity of the skin and mucosal epithelium (lining of the gastrointestinal tract) and other mechanisms that guarantee that the immune system, including the inflammatory process, does not attack these microorganisms comprising the normal body flora. If any location in the body that normally contains microorganisms is compromised, many of these microorganisms may leave the site and cause infection. Individuals with deficiencies in their immune system become easily infected with opportunistic microorganisms — those that normally would not cause disease but seize the opportunity provided by the person’s decreased immune or inflammatory responses.
Table 14-1 NORMAL INDIGENOUS FLORA OF THE HUMAN BODY
| LOCATION | MICROORGANISMS |
|---|---|
| Skin | |
| Nose | |
| Mouth | |
| Pharynx | |
| Distal intestine | |
| Colon | |
| Urethra | |
| Vagina |
Source: Grimes DE. Infectious diseases. Mosby’s Clinical Nursing Series. St Louis: Mosby; 1991.
Pathogens
True pathogens have devised means to circumvent the normal controls provided by the host’s main defensive barriers, the inflammatory system and the immune system.8 Infection by a pathogen is influenced by several factors:
 Mechanism of action: direct damage of cells, interference with cellular metabolism and rendering a cell dysfunctional because of the accumulation of pathogenic substances and toxin production.
Mechanism of action: direct damage of cells, interference with cellular metabolism and rendering a cell dysfunctional because of the accumulation of pathogenic substances and toxin production. Infectivity: the ability of the pathogen to invade and multiply in the individual — for example, coagulase (an enzyme) that causes coagulation and allows some microorganisms, such as Staphylococcus, to clot and form a sticky layer around themselves, protecting themselves against host defences.
Infectivity: the ability of the pathogen to invade and multiply in the individual — for example, coagulase (an enzyme) that causes coagulation and allows some microorganisms, such as Staphylococcus, to clot and form a sticky layer around themselves, protecting themselves against host defences. Pathogenicity: the ability of an agent to produce disease — success depends on its speed of reproduction, extent of tissue damage and production of toxins.
Pathogenicity: the ability of an agent to produce disease — success depends on its speed of reproduction, extent of tissue damage and production of toxins. Virulence: the potency of a pathogen measured in terms of the number of microorganisms or micrograms of toxin required to kill a host — for example, measles is of low virulence; the rabies virus is highly virulent.
Virulence: the potency of a pathogen measured in terms of the number of microorganisms or micrograms of toxin required to kill a host — for example, measles is of low virulence; the rabies virus is highly virulent. Toxigenicity: a factor important in determining a pathogen’s virulence, such as production of soluble toxins or endotoxin.
Toxigenicity: a factor important in determining a pathogen’s virulence, such as production of soluble toxins or endotoxin.The portal of entry is the way the pathogen enters the body. Humans have several defence mechanisms that preclude entry of microorganisms into the body and if these are overcome sophisticated defences can be activated immediately. The pathogen can enter the body via direct contact, inhalation, ingestion or penetration of the skin. Direct contact typically occurs when human-to-human contact permits transmission of the pathogen to the host — sexually transmitted infections are an example when close contact occurs between humans. Inhalation is a common form of pathogen transmission despite several anatomical and physiological barriers in the upper respiratory tract. Infections that occur from inhalational entry include pneumonia, the common cold and tuberculosis. Ingestion of pathogenic microorganisms provides a direct entry into the body. However, the acidity of the stomach and the normal flora of the gastrointestinal tract must be overcome by the pathogen. Lastly, direct penetration of the skin may occur due to puncturing of the dermal layer — for example, a bite — or due to a breakdown in the integrity of skin. Either way, pathogens can infect wounds or cause skin lesions that can be infective.
Spread of infection is facilitated further by the ability of pathogens to attach to cell surfaces, release enzymes that dissolve protective barriers, escape the action of phagocytes or resist the effect of low pH, such as in the stomach. After penetrating protective barriers, pathogens spread through the lymph and blood for invasion of tissues and organs, where they multiply and cause disease. In humans the route of entrance of many pathogenic microorganisms also becomes the site of shedding of new infectious agents to other individuals, completing a cycle of infection (see Figure 14-2).
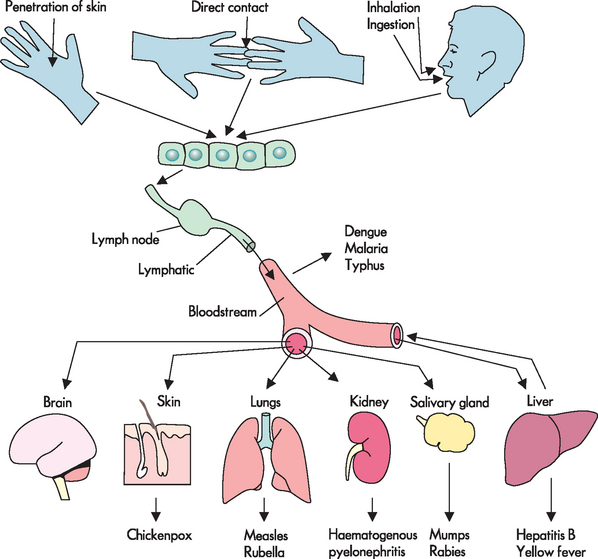
FIGURE 14-2 The route of entry and spread of microorganisms that cause infection in the body.
Source: Based on Kumar V et al. Robbins & Cotran pathologic basis of disease. 7th edn. Philadelphia: Saunders; 2005. Adapted from Mims CA. The pathogenesis of infectious disease. 4th edn. San Diego: Academic Press; 1996
Classes of microorganisms
Infectious disease can be caused by microorganisms that range in size from 20 nanometres (1 nanometre is 0.000001 of a millimetre) — for example, the poliovirus, which causes poliomyelitis — to 10 metres — for example, a tapeworm. There are six main classes of microorganisms: bacteria, virus, fungi, parasites, protozoa and algae. In reality, algal, parasitic and protozoan infections are not common (compared to bacterial and viral infections) in Australia and New Zealand and so they are not discussed further in this chapter. However, Giardia intestinalis is a common intestinal microorganism found in many parts of the world in contaminated drinking water. It causes diarrhoea and abdominal pain.
Table 14-2 provides an overview of the most common classes of pathogenic microorganisms and their characteristics.
Table 14-2 CLASSES OF HUMAN INFECTIOUS MICROORGANISMS
| CLASS | SIZE | EXAMPLES OF DISEASE |
|---|---|---|
| Viruses | 20–30 nm | |
| Bacteria | 0.8–15 μm | |
| Chlamydia | 200–1000 nm | Sexually transmitted infection (Chlamydia) |
| Rickettsiae | 300–1200 nm | Australian tick typhus |
| Mycoplasma | 125–350 nm | Mycoplasma pneumonia |
| Mycobacterium | 1–10 μm | Tuberculosis |
| Fungi | 2–200 μm | |
| Protozoa | 1–360 μm | |
| Parasitic worms (Helminths) | 3 mm to 10 m | |
| Note: Chlamydia, Rickettsiae, Mycoplasma and Mycobacterium are classified as bacteria. | ||
Bacteria
There are many different bacteria and the majority are harmless to humans. However, of those that are pathogenic, some can be particularly destructive to humans and can cause death in a short period of time. In hospitals, bacteria remain the number one cause of infections, acquired both outside and within the hospital.
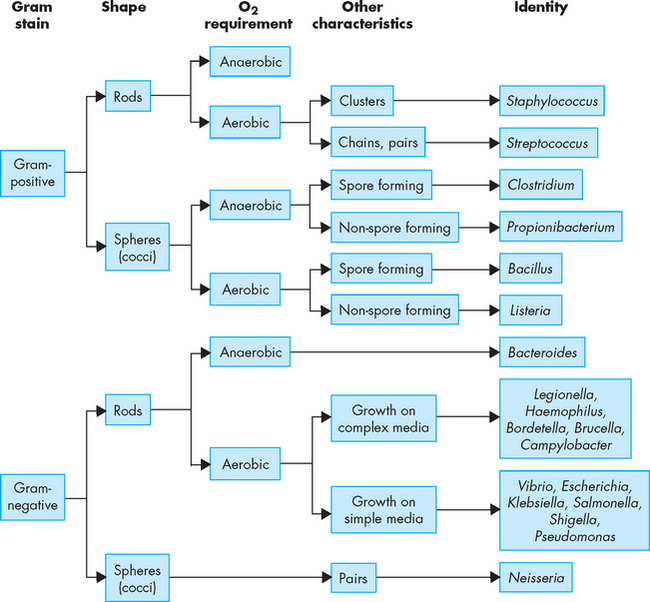
Bacteria are prokaryocytes (lacking a discrete nucleus) and are relatively small. They can be aerobic (meaning they need oxygen) or anaerobic (meaning they can survive without oxygen). In addition, they can be motile (meaning they can move) or non-motile (meaning they cannot move). There are three main bacterial shapes: (1) spherical, called cocci; (2) rod-shaped, called bacilli; and (3) spiral-shaped, termed spirochetes. These can be viewed under a microscope and provide a simple identification method (see Figure 14-3). The other primary classification method for bacteria is the difference in cell wall constituents.
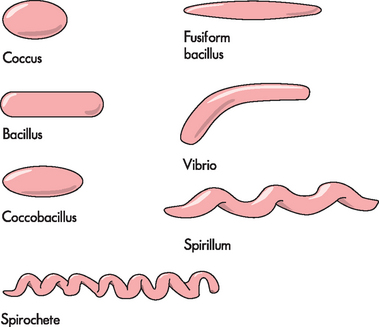
FIGURE 14-3 Morphology of bacteria.
The different shapes of bacteria, which provide a means of identification.
Source: Based on Murray PR et al. Medical microbiology. 6th edn. Philadelphia: Mosby; 2009.
A test called the gram stain was devised to differentiate between the two broad categories of bacteria, namely gram-positive and gram-negative. Briefly, the gram stain involves attaching bacteria to a glass slide and applying crystal violet (purple dye), which is then washed off. Iodine is then applied, which colours all bacteria a deep purple. Next, alcohol is applied and this washes off the purple colour from some bacteria, while others retain the purple colour. Finally, a red counter stain is applied which colours gram-negative bacteria (that did not retain the purple dye) red. Under a microscope, the difference in colours is used to identify gram-positive and gram-negative bacteria. Bacteria that are gram-positive have a thick layer of peptidoglycan, which is found in the cell walls of bacteria. The iodine of the gram stain becomes trapped in the peptidoglycan layer, hence the name gram-positive. In contrast, gram-negative bacteria have only a thin peptidoglycan layer. The thin layer absorbs the red counter stain, which provides the red appearance, hence the name gram-negative. The cellular structure of gram-positive and gram-negative bacteria and the gram stain are illustrated in Figure 14-4, and different gram-positive and gram-negative bacteria are shown in Figure 14-5. The gram stain provides useful information, as broad categories of antibiotics can be chosen, based on whether the bacteria are gram-positive or gram-negative.
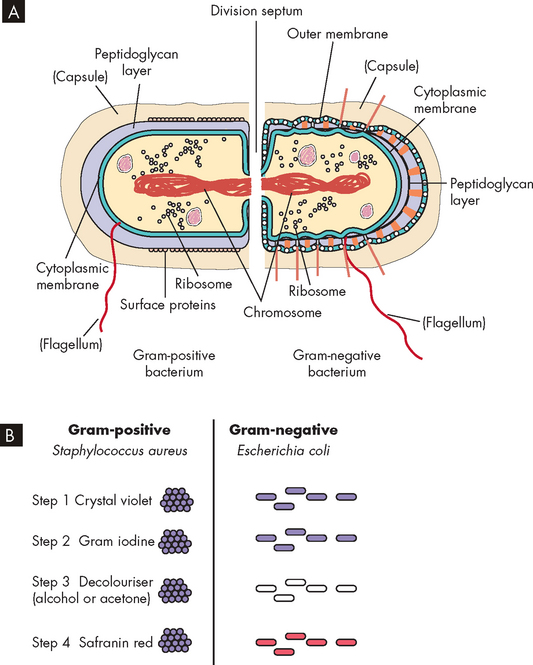
FIGURE 14-4 Gram-positive and gram-negative bacteria and the gram stain.
A Gram-positive bacteria have a thick layer of peptidoglycan (filling the purple space), whereas gram-negative bacteria have a thin peptidoglycan layer (single black line) and an outer membrane. B The iodine of the gram stain is trapped in the thick peptidoglycan layer in gram-positive bacteria. Gram-negative bacteria take up the counterstain, which provides the red appearance.
Source: Murray PR et al. Medical microbiology. 6th edn. Philadelphia: Mosby; 2009.
Bacterial survival and growth depends on the effectiveness of the body’s defence mechanisms and on the ability of the bacteria to resist these defences. Many pathogens have devised ways of preventing destruction by the inflammatory and immune systems. For example, some bacteria produce thick capsules of carbohydrate or protein that are antiphagocytic, preventing efficient opsonisation and phagocytosis (see Chapter 13 for more details on phagocytosis). Such coatings include the thick polysaccharide covering of the pneumococcus and the waxy capsule surrounding the tubercle bacillus.
Other bacteria survive and proliferate in the body by producing toxins — poisonous substances that are produced during metabolism and growth of the microorganism. The toxins have the ability to cause direct damage to the host cells or change normal cellular function. Two broad groups of toxins are produced by bacteria:
 Exotoxins are proteins released during bacterial growth. They are usually enzymes and have highly specific effects on host cells. They include cytotoxins, neurotoxins, pneumotoxins, enterotoxins and haemolysins. Exotoxins can damage cell membranes, activate second messengers and inhibit protein synthesis. Exotoxins are immunogenic and elicit the production of antibodies known as antitoxins. Fortunately, vaccines are available for many of the exotoxins (i.e. tetanus, diphtheria and pertussis). Some strains of toxin-producing group A streptococci cause destructive skin infections (e.g. flesh-eating bacteria syndrome or necrotising fasciitis) and pneumonia, which may kill an individual within 2 days.
Exotoxins are proteins released during bacterial growth. They are usually enzymes and have highly specific effects on host cells. They include cytotoxins, neurotoxins, pneumotoxins, enterotoxins and haemolysins. Exotoxins can damage cell membranes, activate second messengers and inhibit protein synthesis. Exotoxins are immunogenic and elicit the production of antibodies known as antitoxins. Fortunately, vaccines are available for many of the exotoxins (i.e. tetanus, diphtheria and pertussis). Some strains of toxin-producing group A streptococci cause destructive skin infections (e.g. flesh-eating bacteria syndrome or necrotising fasciitis) and pneumonia, which may kill an individual within 2 days. Endotoxins (lipopolysaccharides) are contained in the cell walls of gram-negative bacteria and are released during cell lysis or destruction of the bacteria. Bacteria that produce endotoxins are called pyrogenic bacteria because they activate inflammatory, immunity and clotting processes and produce fever (see Figure 14-6).
Endotoxins (lipopolysaccharides) are contained in the cell walls of gram-negative bacteria and are released during cell lysis or destruction of the bacteria. Bacteria that produce endotoxins are called pyrogenic bacteria because they activate inflammatory, immunity and clotting processes and produce fever (see Figure 14-6).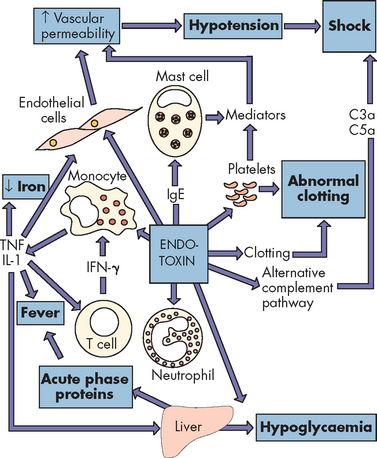
FIGURE 14-6 Endotoxin release causes activation of every immune mechanism and the clotting cascade.
This bacterial endotoxin activates almost every immune mechanism, as well as the clotting pathway. IFN-γ = interferon-γ; IgE = immunoglobulin E; IL-1 = interleukin-1; TNF = tumour necrosis factor.
Source: Based on Murray PR et al. Medical microbiology. 6th edn. Philadelphia: Mosby; 2009. Redrawn from Mims C et al. Medical microbiology. London: Mosby-Wolfe; 1993.
Inflammation is the body’s initial response to the presence of bacteria. Vascular permeability is increased, allowing blood-borne substances (i.e. the complement system) involved in bacterial destruction to access the site of infection. Endotoxins increase capillary permeability further by activating the complement cascade. Capillary permeability may increase sufficiently to permit the escape of large volumes of plasma, contributing to hypotension and, in severe cases, shock (see Chapter 23). Endotoxins can also activate the coagulation cascade (see Figure 14-6).
Septicaemia refers to the presence of microorganisms in the blood, while bacteraemia is the presence of bacteria in the blood and is caused by a failure of the body’s inflammation and immune defence mechanisms. The usual cause is proliferation of gram-negative bacteria, although a few gram-positive bacteria and fungi can cause it. Symptoms of gram-negative septic shock are produced by the release of endotoxins. Once in the blood, endotoxins cause the release of vasoactive peptides and cytokines that affect blood vessels, producing vasodilation, which reduces blood pressure, causes decreased oxygen delivery and produces septic shock (see Chapter 23).
Viruses
The word virus comes from the Latin meaning poison or toxin. Viruses are much smaller than bacteria and are the smallest pathogenic microorganisms (see Figure 14-7). Viruses are very simple microorganisms consisting of nucleic acid (genetic material) protected from the environment by a layer of protein, called a capsid (see Figure 14-8). The genetic information is in either RNA (ribonucleic acid) or DNA (deoxyribonucleic acid). Viral diseases are the most common causes of illness in humans and include a variety of diseases ranging from the common cold to several types of cancers and human immunodeficiency virus (HIV), which leads to acquired immunodeficiency syndrome (AIDS). Viruses are sensitive to many environmental factors and cannot survive for long outside of a host cell.
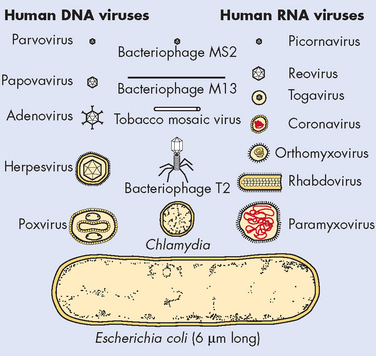
FIGURE 14-7 Comparison of viral and bacterial shapes and sizes.
Source: Murray PR et al. Medical microbiology. 6th edn. Philadelphia: Mosby; 2009. Courtesy the Upjohn Company, Kalamazoo, Mich.
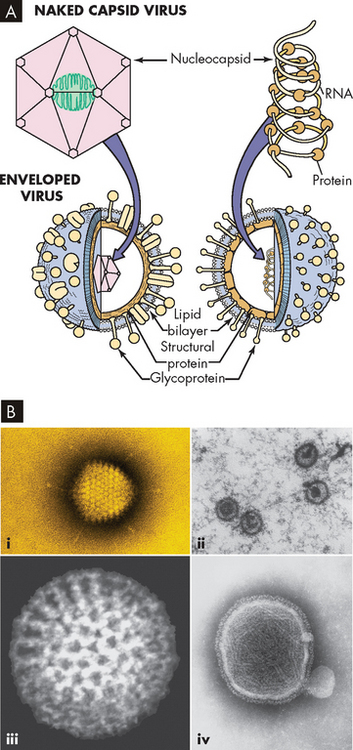
FIGURE 14-8 The structure of a virus.
A Note the enveloped virus on the left, which refers to a lipid membrane surrounding the nucleic acid. Naked viruses (right) do not have this layer. B Electron microscope images of viruses: (i) adenovirus, (ii) Epstein Barr virus, (iii) rotavirus and (iv) paramyxovirus.
Source: A Murray PR et al. Medical microbiology. 6th edn. Philadelphia: Mosby; 2009. B Courtesy of Science Source. © Photo Researchers, Inc. New York.
Virions (viral particles) do not possess any of the metabolic organelles found in bacteria or human cells. Therefore, viruses have no intrinsic metabolism — that is, they do not perform activities that are consistent with life when outside of a host cell. Some scientists consider that viruses are not living cells as they cannot survive without a host. The host provides the capability for reproduction and metabolic processes. Reproduction can occur, but it depends totally on the ability of a virus to infect a susceptible host cell — a cell that cannot resist viral invasion and replication.
Infection with a virus begins with a virion binding to a specific receptor on the plasma membrane of a host cell. The virus then changes the activity of the host cell such that viral replication occurs. The clinical symptoms will reflect the alteration of the function of the infected cells (see Figure 14-9) — for example, the influenza virus binds to a receptor on respiratory epithelial cells, causing symptoms of an upper respiratory tract infection.
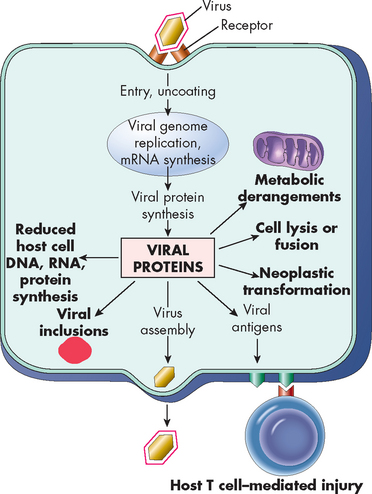
FIGURE 14-9 Mechanisms of virus injury to host cells.
Viral injury can cause multiple events to be altered within the cell.
Source: Kumar V. Robbins & Cotran pathologic basis of disease. 8th edn. Philadelphia: Saunders; 2010.
The viral capsid (protein coat) must be removed in the cytoplasm of the infected host cell (uncoating). The viral genetic material may be processed by one of several paths, depending on the particular virus. Generally, all RNA viruses, except influenza and retroviruses, replicate their genetic material in the cytoplasm of the infected cell, and all DNA viruses, except poxviruses, require the DNA to enter the nucleus and use the cell’s DNA enzyme to replicate. Poxviruses provide their own DNA polymerase and replicate their DNA in the cytoplasm of the infected cell. Retroviruses generally convert their RNA genetic information to DNA using an enzyme contained in the virion — reverse transcriptase.
After infection, viruses usually make multiple copies of their genetic material and produce the necessary viral proteins for replication. New virions are assembled in the host cell’s cytoplasm and are released from the cell for transmission of the viral infection to other host cells. This cycle is referred to as the productive or lytic cycle because a large number of offspring are produced and the result is often destruction of the host cell.
Some viruses are not productive initially, but instead initiate a latency phase during which the host cell is transformed. During this phase, the viral DNA may be integrated into the DNA of the host cell and become a permanent passenger in that cell and its offspring. In response to stimuli — such as stress, hormonal changes or disease — the virus may exit latency and enter a productive cycle and cause signs and symptoms of infection in the individual. An example of this is the varicella zoster virus, which causes chickenpox. Although the initial infection subsides rapidly, the virus can lie dormant in the individual and then reappear later to produce the painful illness herpes zoster, more commonly known as shingles.
Besides taking over the host cell’s metabolic machinery, viral infection can injure cells. In some viral infections, cellular destruction results from large quantities of virus being released from the cell’s plasma membrane. Alteration of the plasma membrane by the expression of new antigens as a result of viral infection can incite an immune response against the individual’s infected cells (e.g. hepatitis B virus). Once inside the human host cell, virions have many harmful effects, including the following:
 disruption of lysosomal membranes, resulting in release of ‘digestive’ lysosomal enzymes that can kill the cell (e.g. herpes virus)
disruption of lysosomal membranes, resulting in release of ‘digestive’ lysosomal enzymes that can kill the cell (e.g. herpes virus) alteration of the antigenic properties or ‘identity’ of the infected cell, causing the individual’s immune system to attack the cell as if it were foreign (e.g. hepatitis B virus)
alteration of the antigenic properties or ‘identity’ of the infected cell, causing the individual’s immune system to attack the cell as if it were foreign (e.g. hepatitis B virus) transformation of host cells into cancerous cells, resulting in uninhibited and unregulated growth (e.g. human papilloma virus)
transformation of host cells into cancerous cells, resulting in uninhibited and unregulated growth (e.g. human papilloma virus)Examples of human diseases caused by specific viruses are listed in Table 14-3.
Table 14-3 DISEASE-CAUSING VIRUSES
| DNA VIRUSES | |
|---|---|
| FAMILY | VIRAL MEMBERS |
| Adenoviridae | Human adenoviruses |
| Hepadnaviridae | Hepatitis B virus |
| Herpesviridae | Herpes simplex 1 and 2, varicella zoster virus, cytomegalovirus, Epstein-Barr virus, human herpes viruses 6, 7 and 8 |
| Papillomaviridae | Human papilloma viruses |
| Parvoviridae | Parvovirus B-19 |
| Polyomaviridae | BK and JC-polyomaviruses |
| Poxviridae | Variola, vaccinia, orf, molluscum contagiosum, monkeypox |
| RNA VIRUSES | |
|---|---|
| FAMILY | VIRAL MEMBERS |
| Arenaviridae | Lymphocytic choriomeningitis virus, Lassa fever virus |
| Astroviridae | Gastroenteritis-causing astroviruses |
| Bunyaviridae | Arboviruses including California encephalitis and Lacrosse viruses; non-arboviruses including sin nombre and related hantaviruses |
| Caliciviridae | Noroviruses and hepatitis E virus |
| Coronaviridae | Coronaviruses, including SARS coronavirus |
| Filoviridae | Ebola and Marburg haemorrhagic fever viruses |
| Flaviviridae | Arboviruses including yellow fever, dengue, West Nile, Japanese encephalitis and St Louis encephalitis viruses; non-arboviruses including hepatitis C virus |
| Orthomyxoviridae | Influenza A, B and C viruses |
| Paramyxoviridae | Parainfluenza viruses, mumps virus, measles virus, respiratory syncytial virus, metapneumovirus, Nipah virus |
| Picornaviridae | Polio viruses, coxsackie A viruses, coxsackie B viruses, echoviruses, enteroviruses 68–71, enterovirus 72 (hepatitis A virus), rhinoviruses |
| Reoviridae | Rotavirus, Colorado tick fever virus |
| Retroviridae | Human immunodeficiency viruses (HIV-1 and HIV-2), human T-lymphotropic viruses (HTLV-1 and HTLV-2) |
| Rhabdoviridae | Rabies virus |
| Togaviridae | Eastern, Western and Venezuela equine encephalitis viruses, rubella virus |
Source: Forbes BA et al. Bailey & Scott’s diagnostic microbiology. 12th edn. St Louis: Mosby; 2007.
Fungi
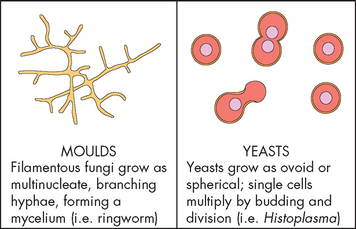
Fungi are relatively large microorganisms with thick walls that grow as either single-celled yeasts (spheres) or multicelled moulds (filaments or hyphae) (see Figure 14-10). Some fungi can exist in either form and are called dimorphic fungi. The cell walls of fungi are rigid and multilayered. The wall is composed of polysaccharides different from the bacterial peptidoglycans. Importantly for clinical practice, the lack of peptidoglycans allows fungi to resist the action of bacterial cell wall inhibitors such as penicillins and cephalosporins. In contrast to bacteria, the cytoplasm of fungi contains organelles: mitochondria, ribosomes, Golgi apparatus, microtubules, microvesicles, endoplasmic reticulum and nuclei. Moulds are aerobic and live in a variety of environments, such as bread, vegetables, soil and water. Yeasts are facultative anaerobes, which produce ATP using aerobic respiration if present; however, they have the capacity to adapt to anaerobic conditions and can survive if needed. They usually reproduce by simple division orbudding.
Pathological fungi cause disease by adapting to the host environment. Fungi that colonise the skin can digest keratin (the protein in the skin that provides structure). Other fungi can grow with wide temperature variations in lower oxygen environments. Still other fungi have the capacity to suppress host immune defences. Phagocytes and T lymphocytes are important in controlling fungi, while low white blood cell counts promote fungal infection.
Diseases caused by fungi are called mycoses. Mycoses can be superficial, deep or opportunistic (remember, these microorganisms would not normally harm a healthy individual). Superficial mycoses occur on or near skin or mucous membranes and usually produce mild and superficial disease. Fungi that invade the skin, hair or nails are known as dermatophytes. The diseases they produce are called tineas (ringworm) — for example, tinea capitis (scalp), tinea pedis (feet) and tinea cruris (groin). Superficial dermatophytes grow in a ringlike, erythematous patch with a raised border. Itching is often intense and cracking of tissue can occur and lead to secondary bacterial infection. Infections of the scalp are accompanied by scaling and hair loss. (Chapter 19 discusses the various skin disorders caused by fungi.)
Deep infections involving internal organs can be life-threatening and are most common in association with other diseases or as an opportunistic infection in immunosuppressed individuals. Fungi causing deep infection enter the body through inhalation or through open wounds. Filamentous forms can multiply extracellularly, but the spherical yeasts multiply within cells, including leucocytes (white blood cells).
Some fungi are a part of the normal body flora and become pathological only when immunity is compromised, allowing exaggerated growth and translocation. For example, Candida albicans is usually found in the mouth, gastrointestinal tract and vagina of normal individuals. Changes in pH and the use of antibiotics that kill bacteria which normally inhibit Candida growth permit rapid proliferation and overgrowth, which can lead to superficial or deep infection.
Methods of infection
Microorganisms use a diversity of methods to invade the host and promote growth, often resulting in infection (see Table 14-4). Because primary immune responses may take a week to develop adequately, some pathogens proliferate at rates that surpass the development of a protective response.
Table 14-4 MECHANISMS OF TISSUE DAMAGE AND ASSOCIATED MICROORGANISMS
| PATHOGENS THAT CAUSE TISSUE DAMAGE | |
|---|---|
| Infectious agent | Disease |
| Produce exotoxin | |
| Streptococcus pyogenes | Tonsillitis, scarlet fever |
| Staphylococcus aureus | Skin abscess (boils), toxic shock syndrome, food poisoning |
| Corynebacterium diphtheriae | Diphtheria |
| Clostridium tetani | Tetanus |
| Vibrio cholerae | Cholera |
| Produce endotoxin | |
| Escherichia coli | Gram-negative sepsis |
| Haemophilus influenzae | Meningitis, pneumonia |
| Salmonella typhi | Typhoid |
| Shigella | Bacillary dysentery |
| Pseudomonas aeruginosa | Wound infections |
| Yersinia pestis | Plague |
| Cause direct damage with invasion | |
| Variola | Smallpox |
| Varicella zoster | Chickenpox, shingles |
| Hepatitis B virus | Hepatitis |
| Poliovirus | Poliomyelitis |
| Measles virus | Measles, subacute sclerosing panencephalitis |
| Influenza virus | Influenza |
| Herpes simplex virus | Herpes labialis (cold sores) |
| PATHOGENS ASSOCIATED WITH TISSUE DAMAGE | |
|---|---|
| Infectious agent | Disease |
| Produce immune complexes | |
| Hepatitis B virus | Kidney disease |
| Malaria | Vascular deposits |
| Streptococcus pyogenes | Glomerulonephritis |
| Treponema pallidum | Kidney damage in secondary syphilis |
| Most acute infections | Transient renal deposits |
| Produce antibody against cells or tissues (autoantibody) | |
| Streptococcus pyogenes | Rheumatic fever |
| Mycoplasma pneumonia | Haemolytic anaemia |
| Cause cell-mediated immunity | |
| Mycobacterium tuberculosis | Tuberculosis |
| Mycobacterium leprae | Tuberculoid leprosy |
| Lymphocytic choriomeningitis virus | Aseptic meningitis |
| Borrelia burgdorferi | Lyme arthritis |
| Schistosoma mansoni | Schistosomiasis |
| Herpes simplex virus | Herpes stromal keratitis |
Source: Janeway CA et al. Immunobiology: the system in health and disease. 5th edn. New York: Garland; 2001.
Viral pathogens bypass many defence mechanisms by developing intracellularly, thus hiding within cells and away from normal inflammatory or immune responses. In many cases, however, because viral agents must spread from cell to cell, the developing immune response eventually cures the infection so the disease is usually self-limiting, in that it resolves without the need for medications. However, many viruses (e.g. measles, herpes) are inaccessible to antibodies after initial infection because they are not in the bloodstream but instead remain inside infected cells, spreading by direct cell-to-cell contact. Some viruses will persist and a state of unapparent infection may result. In persistent infections, cellular injury may be minimal and the virus persists until it is activated to replicate (e.g. the cold sores of herpes virus infection). Immunity may limit recurrent outbreaks and protect the individual from an acute exacerbation only, or may be sufficiently strong to prevent disease.
Some viruses elude the immune response by undergoing antigenic variation — changing their appearance by altering surface antigens. For example, the influenza virus undergoes yearly antigenic drift resulting from mutations in key surface antigens, haemagglutinin (H antigen) and neuraminidase (N antigen), allowing the emergence of new strains of influenza virus. Thus, immunity against the previous year’s viruses is no longer completely protective, creating the need for new vaccines every year. Antigenic shifts are major changes in antigenicity that occur from recombination of genes for H and N among different strains of viruses, and can result in major worldwide pandemics. For example, the spread of swine flu in 2009 was due to H1N1, while the avian (bird) flu of 2004–05 was due to H5N1. Other pathogens, such as some parasitic microorganisms, use a similar approach and change surface antigens by gene switching.
Table 14-5 contains examples of microorganisms that fight off the immune system or cause it to attack the host.
Table 14-5 PATHOGEN RESISTANCE TO IMMUNE FUNCTION
| MECHANISMS | EFFECT ON IMMUNITY | EXAMPLE |
|---|---|---|
| Destroys or blocks component of immune system | ||
| Produce toxins | Kills phagocyte or interferes with chemotaxis Prevents phagocytosis by inhibiting fusion between phagosome and lysosomal granules | Staphylococcus, Streptococcus, Mycobacterium tuberculosis |
| Produce antioxidants (e.g. catalase, superoxide dismutase) | Prevents killing by oxygen-dependent mechanisms | Mycobacterium spp., Salmonella typhi |
| Produce protease to digest IgA | Promotes bacterial attachment | Neisseria gonorrhoeae (urinary tract infection), Haemophilus influenzae, Streptococcus pneumoniae (pneumonia) |
| Produce surface molecules that mimic Fc receptors and bind antibody | Prevents activation of complement system Prevents antibody functioning as opsonin | Staphylococcus, Herpes simplex virus |
| Mimic self-antigens | ||
| Produce surface antigens(e.g. M protein, red blood cell antigens) that are similar to self-antigens | Pathogen resembles the individual’s own tissue; in some individuals, antibodies can be formed against the self-antigen, leading to hypersensitivity disease (e.g. antibody to M protein also reacts with cardiac tissue, causing rheumatic heart disease; antibody to red blood cell antigens can cause anaemia) | Group A streptococcus (M protein), Mycoplasma pneumonia (red cell antigens) |
| Change antigenic profile | ||
| Undergo mutation of antigens or activate genes that change surface molecules | Immune response delayed because of failure to recognise new antigen | Influenza, HIV, some parasites |
Clinical manifestations of infection
This section provides an overview of the general clinical manifestations of infections. The progression from infection to infectious disease follows predictable stages (infection, incubation, symptoms, shedding of the microorganism), as demonstrated by the pathogenesis of measles illustrated in Figure 14-11. Clinical manifestations of infectious diseases vary, depending on the pathogen and the organ system affected. Manifestations can arise directly from the infecting microorganism or its products; however, the majority of clinical symptoms result from the host’s inflammatory and immune responses. Infectious diseases typically begin with nonspecific or general symptoms of fatigue, malaise (general unwell feeling), weakness and loss of concentration. Generalised aching and loss of appetite (anorexia) are common complaints. The hallmark of most infectious diseases is fever and this is covered in detail in Chapter 13.
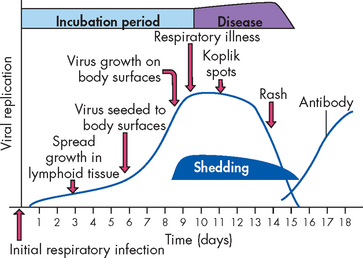
FIGURE 14-11 The progression of measles.
The pathogenesis of measles is representative of most viral infections in unimmunised individuals. The virus enters through the oropharynx, from where it infects the regional lymph nodes. After 5 to 7 days, virus enters the blood (viraemia) and spreads to the body surfaces (respiratory, gastrointestinal and urinary tracts, and the skin). The measles virus replicates in these tissues, leading to upper respiratory tract symptoms, with the appearance of red spots with bluish-white specks (Koplik spots) in the oral mucosa and later to an extensive rash involving most parts of the skin. At or near the onset of overt symptoms, the infected individual is shedding virus and is highly infectious to others. Antibodies against the measles virus are primarily responsible for resolving the infection. They are produced within 10 to 11 days but are immediately absorbed by viral particles in the blood so that free antibody is not measurable until about 2 weeks after the initial infection.
Detection and treatment of microorganisms
The detection and treatment of pathogenic microorganisms are challenging and often difficult tasks. Our immune and inflammatory systems are far superior to any manufactured drug at both detecting and eradicating pathogens from our bodies. However, our systems are sometimes unable to cope with the proliferation of pathogens. In addition, for both the very young, whose immune systems are immature, and the ageing population, who have more co-morbidities, treatment with antimicrobial agents is often necessary and in some cases life-saving.
There are two requirements to diagnosing pathogenic microorganisms in humans:
 a detailed clinical history and physical examination, which often provides clues to the origin of the infection and the likely pathogen
a detailed clinical history and physical examination, which often provides clues to the origin of the infection and the likely pathogenBacteria are detected from body fluids and specimens using cultures. Growth medium, such as agar, promotes the growth of bacteria such that it can be identified. The gram stain, described previously, is the mainstay of bacterial determination. The bacteria are viewed under a microscope and their shape and gram stain provide evidence of the particular type of bacteria (see Figures 14-3 and 14-4). Sensitivity analysis of which antimicrobial agents are both resistant to and sensitive to the bacteria also guides the clinician in treatment. Viruses are harder to detect due to their size. They are not observable under a light microscope and most diagnoses are based on clinical manifestations only. However, various techniques can be used to identify viruses, including immunofluorescence, serology (antigens and antibody detection e.g. hepatitis) and cell cultures. Fungi are diagnosed by microscopic observation of specimens to visualise either spheres or filaments.
The treatment of pathogenic microorganisms has been one of the greatest advances in healthcare over the last 50 years. The advent of penicillin and other antimicrobial agents has significantly reduced mortality due to infectious diseases. Antimicrobial agents destroy or neutralise the pathogen once the disease process has started. In addition, prophylactic procedures (namely, vaccines) have been developed to prevent pathogens from initiating disease. The majority of vaccine development has focused on preventing the most severe and common infections. With the initial success of antibiotic therapy, there was no perceived need for vaccination against many common and non-life-threatening infections. However, the increasing problem of antibiotic-resistant pathogens has forced a reappraisal of that strategy and a greater emphasis now is being placed on the development of new vaccines.
ANTIMICROBIALS
Since initiation of the widespread use of penicillin during World War II, antibiotics have had the greatest impact on successful resistance to infection. Antibiotics are natural products of fungi, bacteria and related microorganisms that kill or inhibit the growth of other microorganisms. Numerous chemicals or antimicrobials have been identified that either prevent the growth of microorganisms or directly destroy them (see Figure 14-12). Antibiotics generally act by preventing the function of enzymes or cell structures that are unique to the infecting agent. Because viruses use the enzymes of the host’s cells, there has been far less success in developing antiviral antibiotics.
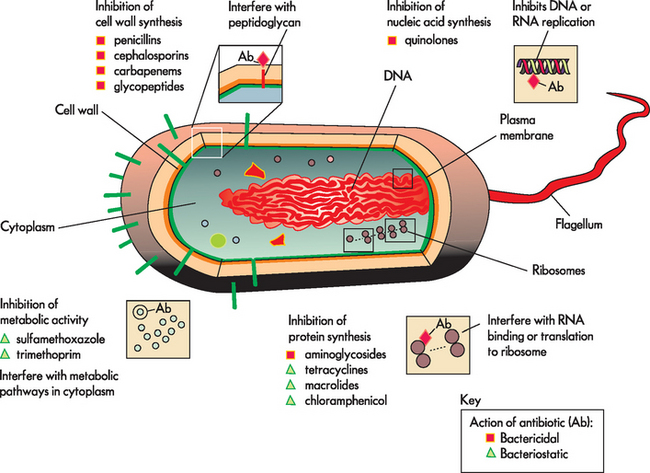
FIGURE 14-12 Mechanism of action and type of antibiotics.
Note that the four main classes of antibiotics are both bactericidal (directly kill bacteria) and bacteriostatic (neutralise further bacterial growth). The antibiotic groups break down the cell wall, interfere with nucleic acid activity, stop protein synthesis or interfere with cellular metabolic activities.
Antiviral agents are a more recent drug compared to antibiotics. As viruses reside in the host’s cells, using a drug to interfere or stop viral replication will also interfere with the host’s cellular reproduction. Since the advent of human immunodeficiency virus (HIV) in the early 1980s, an enormous amount of research has been conducted into antiviral medication. Antiviral drugs have now been developed to treat influenza, HIV, hepatitis and herpesvirus, although not all drugs provide a cure. The majority of these mimic a section of viral DNA, rendering viral replication inactive. Figure 14-13 provides an example of the antiviral drug aciclovir, which is used to treat genital herpes and shingles.
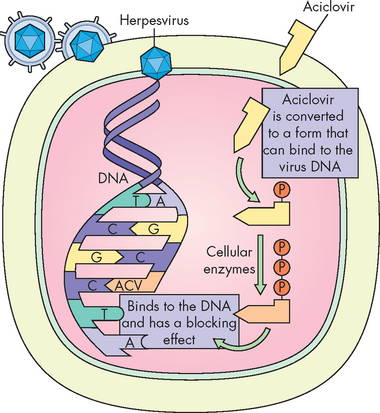
FIGURE 14-13 The mechanism of action for the antiviral drug aciclovir.
Aciclovir binds with an enzyme that combines with the viral DNA to block the effects of the virus.
Source: Based on Cohen J, Powderly WG. Infectious diseases. 2nd edn. St Louis: Mosby; 2004.
At the present time, no vaccines are available to prevent fungal disease effectively. Many of the antifungal drugs (e.g. amphotericin B, ketoconazole, fluconazole) used to treat deep or systemic infections are toxic to the host because the fungal cell composition is similar to the host cell. Therefore, it is imperative that use of these drugs be restricted.
VACCINES
The purpose of vaccination is to induce long-lasting protective immune responses under conditions that will not result in disease in a healthy recipient of the vaccine. The primary immune response from vaccination is generally short lived; therefore, booster injections are used to push the immune response through multiple secondary responses, resulting in large numbers of memory cells and sustained protective levels of antibody or T cells, or both.
Development of a successful vaccine is costly and depends on several factors. These include identification of the protective immune response and the appropriate antigen to induce that response. For instance, individuals with ongoing HIV infection produce a great deal of antibody against several HIV antigens. But, for development of a successful vaccine, we must first understand which antibody will protect against an initial infection.
When an antigen is identified that can be used in a vaccine, it must be developed into an effective, cost-efficient, stable and safe vaccine. For instance, most vaccines against viral infection (measles, mumps, rubella and varicella (chickenpox)) contain live viruses that are attenuated (weakened so as to not cause infection when administered to the recipient) so they continue to express appropriate antigens but establish only a limited and easily controlled infection. For most common vaccines against viral infections, limited replication of the virus appears to afford better long-term protection than using a viral antigen. One current exception is the hepatitis B vaccine, which uses a recombinant viral protein. The hepatitis A vaccine is an inactivated (killed) virus and normally should not cause an infection.
Even attenuated viruses can establish life-threatening infections in individuals whose immune system is congenitally deficient or suppressed.9 The risk of infection by the vaccine strain of virus is extremely small, but it may affect the choice of recommended vaccines. For instance, two different vaccines were developed against poliovirus, which causes poliomyelitis:
 The Sabin vaccine was an attenuated virus that was administered orally. It provided systemic protection and induced a secretory immune response to prevent growth of the poliovirus in the intestinal tract. Being a live virus, the vaccine could cause poliomyelitis in some children who had unsuspected immune deficiencies (about 1 case in 2.4 million doses).
The Sabin vaccine was an attenuated virus that was administered orally. It provided systemic protection and induced a secretory immune response to prevent growth of the poliovirus in the intestinal tract. Being a live virus, the vaccine could cause poliomyelitis in some children who had unsuspected immune deficiencies (about 1 case in 2.4 million doses). The Salk vaccine was a completely inactivated virus administered by injection. It induced protective systemic immunity but did not provide adequate secretory immunity. Therefore, even if the individual was protected from systemic infection by poliovirus, the virus could transiently infect their intestinal mucosa, be shed and spread to others.
The Salk vaccine was a completely inactivated virus administered by injection. It induced protective systemic immunity but did not provide adequate secretory immunity. Therefore, even if the individual was protected from systemic infection by poliovirus, the virus could transiently infect their intestinal mucosa, be shed and spread to others.When poliomyelitis was epidemic, the oral vaccine was preferred.
Vaccination programs have been extremely effective against poliovirus. Poliovirus has been eliminated from Western countries — the Western Pacific region, including Australia and New Zealand, was declared to be completely free in October 2000.10 As of 2010, only four countries (Afghanistan, India, Nigeria and Pakistan) were classified as endemic, with 1249 new cases of polio in 2009. In contrast, in 1988, 125 countries on five continents were considered endemic and more than 1000 children per day were paralysed due to poliovirus.11 This huge reduction is due to the World Health Organization (WHO) charter to eradicate poliovirus globally.
Some common bacterial vaccines are killed microorganisms or extracts of bacterial antigens. The vaccine against pneumococcal pneumonia consists of a mixture of capsular polysaccharides from 10 strains of Streptococcus pneumoniae. Of the more than 90 known strains of this microorganism, only these 10 cause the most severe illnesses. However, the capsular vaccine is not very immunogenic in young children. A conjugated vaccine is available that contains capsular polysaccharides from 7 strains that are conjugated (linked chemically) to carrier proteins in order to increase immunogenicity. A similar vaccine is available for Haemophilus influenzae type b (Hib).
Some bacterial pathogens are not invasive, but do colonise mucosal membranes or wounds and release potent toxins that act locally or systemically. These include the bacteria that cause diphtheria, cholera and tetanus. Vaccination against systemic toxins (e.g. diphtheria, tetanus) has been achieved using toxoids — purified toxins that have been chemically detoxified without loss of immunogenicity. Pertussis (whooping cough) vaccine has been changed from a killed whole-cell vaccine to a cellular extract (acellular) vaccine that contains the pertussis toxin and additional bacterial antigens. This change has dramatically reduced the adverse side effects of the previous vaccine (fever and local inflammatory reactions).
Additional difficulties associated with vaccination include allergic reactions to the vaccine antigen itself or other components of the preparation. For instance, some viral vaccines are grown in chicken eggs and many elicit a reaction in individuals who are allergic to eggs. Thimerosal is a mercury-containing compound that was used as a preservative in vaccines. Although no cases of mercury toxicity have been reported secondary to vaccination, thimerosal was removed from all childhood vaccines in 2000.12
A more common problem is compliance of the susceptible population. Depending on the microorganism, a certain percentage of the population should be immunised to protect the total population. If this level of immunisation is not achieved, outbreaks of infection can occur. For instance, in the United States, an effective measles vaccine was made available in 1963 and resulted in a dramatic decrease in the number of measles cases. Many parents became complacent and did not obtain measles vaccination for their preschool children. As a result, a large increase occurred in the number of cases and deaths in 1989 and 1990, which initiated a re-emphasis on complete immunisation before children could start school. Even with successful development of a vaccine, however, a certain percentage of the population will be genetically unresponsive to vaccination and therefore will not produce a protective immune response. With most vaccines, the percentage of unresponsive individuals is low and they will benefit from successful immunisation of the rest of the population.
Australia and New Zealand have extensive childhood immunisation programs.13,14 These health programs have high immunisation rates; however, there have been some delays in the immunisation of children younger than two years of age, especially in Indigenous populations.15 The vaccination programs of Australia and New Zealand are detailed in Tables 14-6 and 14-7.
Table 14-6 RECOMMENDED IMMUNISATION SCHEDULE OF AUSTRALIA
| AGE | VACCINE |
|---|---|
| Birth | |
| 2 months | |
| 4 months | |
| 6 months | |
| 12 months | |
| 12–24 months | |
| 18 months | |
| 18–24 months | |
| 4 years | |
| 10–13 years | |
| 12–13 years (girls only) | |
| 15–17 years | |
| 15–49 years | |
| 50 years and over | |
| 65 years and over |
Source: Department of Health and Ageing. National Immunisation Program (NIP) schedule. Canberra: Commonwealth of Australia; 2007.
Table 14-7 RECOMMENDED IMMUNISATION SCHEDULE OF NEW ZEALAND
| AGE | DISEASES COVERED AND VACCINES |
|---|---|
| 6 weeks | Diphtheria/tetanus/whooping cough/polio/hepatitis B/Haemophilus influenzae type b 1 injection |
| Pneumococcal 1 injection | |
| 3 months | Diphtheria/tetanus/whooping cough/polio/Hepatitis B/Haemophilus influenzae type b 1 injection |
| Pneumococcal 1 injection | |
| 5 months | Diphtheria/tetanus/whooping cough/polio/Hepatitis B/Haemophilus influenzae type b 1 injection |
| Pneumococcal 1 injection | |
| 15 months | Haemophilus influenzae type b 1 injection |
| Measles/mumps/rubella 1 injection | |
| Pneumococcal 1 injection | |
| 4 years | Diphtheria/tetanus/whooping cough/polio 1 injection |
| Measles/mumps/rubella 1 injection | |
| 11 years | Diphtheria/tetanus/whooping cough 1 injection |
| 12 years (girls only) | Human papillomavirus 3 doses over 6 months |
Source: Ministry of Health. National Immunisation Schedule: health provider booklet. Wellington: Ministry of Health; 2008.
INFECTIONS
Common infections
In this section we outline some of the more common infections in children and adults in Australia and New Zealand. The majority of these common infections do not cause sustainable harm — the severity of most infections ranges from mild irritation to serious bed-bound conditions that take days or weeks to overcome. However, a tremendous amount of research is being conducted into the links between infection and other conditions. For instance, it has been suggested that the common acute infections of childhood (cough, cold, fever and sore throat) are linked to endothelial dysfunction and that this may lead to the pathogenesis of early atherosclerosis lesions.16,17 This is an area of our knowledge that is growing and clinicians need to keep abreast of advancements.
More serious infections can be quite problematic, especially in the young and the elderly, as their capacity to combat infection is limited. As we have discussed, there are a variety of antimicrobial agents and their prescription and administration should be limited to known causes of infection and when infection is incapacitating. The overuse of prescriptions and unwarranted administration of antibiotics has led to a major problem in healthcare settings — the emergence of drug-resistant microorganisms. Indeed, infections within a hospital setting are a major battle for patients and staff, and the ability to prevent unwarranted infections and treat and contain existing infections is one of the core domains of nursing practice.
The following sections look at some common microorganisms and associated infections and injury. We have limited the scope of infections in the examples listed here, as others are covered in specific chapters elsewhere in the text.
The common cold
The common cold is one of the most frequent infections in humans.18 It is usually a viral infection, such as rhinovirus, coronavirus, parainfluenza virus, respiratory syncytial virus (RSV) or adenovirus. RSV infection is the single most common cause of upper respiratory tract infections in children up to the age of two years.5 Such viruses are spread easily and so large numbers of community members are infected, such as in schools and workplaces. Diagnosis is made using the clinical signs and symptoms, rather than by identification of the pathogen, as specific viral detection is unwarranted in most cases. It has been suggested that antibiotics are incorrectly prescribed to common cold infections caused by a virus more than 50% of the time.19 Most treatments involve mitigating symptoms, if required, or vaccination for high-risk groups.20
Helicobacter pylori infection
Helicobacter pylori are gram-negative rod bacteria that are found in individuals with chronic inflammation of the stomach lining (gastritis; see Figure 14-14). They are the predominant causative agent in the development of peptic ulcers of the stomach and duodenum and gastric adenocarcinoma.6 Infection with Helicobacter pylori is extremely common. In developing countries, 70–90% of the population are colonised before the age of 10 years.6 In developed countries, the prevalence of Helicobacter pylori infection increases with age, with 40% or more of the population infected at age 60 years.21 The transmission appears to be person to person,22 via the faecal–oral route, demonstrating the importance of basic hygiene.
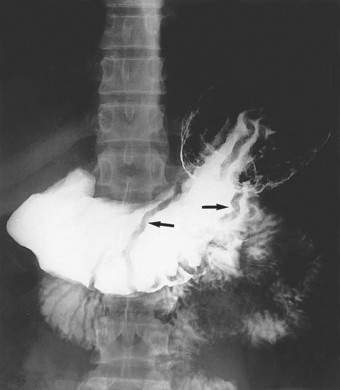
In the body of the stomach, thickened mucosal folds (arrows), the result of inflammation, can be seen.
Source: Mettler FA. Essentials of Radiology. 2nd edn. Philadelphia: Saunders; 2005.
The causal link between Helicobacter pylori colonisation and gastritis was found in the 1980s by the Australian researchers Professor Barry Marshall and Dr Robin Warren (who were awarded a Nobel prize for this research in 2005). The exact pathogenesis of chronic gastritis due to Helicobacter pylori is not fully understood, but the bacteria blocks acid production in the stomach by producing an enzyme (urease) that breaks down urea and neutralises the gastric acids. The bacteria also cause apoptosis (programmed cell death) of gastric epithelial cells, accelerating the inflammatory process.6 Diagnosis is made by endoscopic biopsy or a non-invasive test that measures urease in an individual’s breath.
Pharmacological treatment is very successful with the use of a proton pump inhibitor and antibiotic. In most cases this will cure the individual of the bacteria. The production of a vaccine against Helicobacter pylori would greatly reduce colonisation and major research is currently underway to develop a safe and effective vaccine.
Urinary tract infection
Urinary tract infection is another of the most common infections affecting humans.23 A urinary tract infection occurs when there are significant numbers of bacteria in the urinary tract, which can cause lower and upper tract infections. Urinary tract infections afflict the young and the old and, after infancy, females are more commonly infected than males. Such infections are common in young females who are sexually active, as the pressure around the genital region during intercourse can force local bacteria into the urethra, which is very close anatomically to the opening of the vagina. After 65 years of age, about 40% of men and women become infected.23 Bacteriuria (bacteria in the urine) is usually asymptomatic in older individuals.
The gram-negative anaerobe, Escherichia coli, a normal intestinal flora, accounts for up to 90% of uncomplicated community-based urinary tract infections in both adults and children.23,24 Contamination occurs due to the close proximity of the anus to the urethral opening. Signs and symptoms include dysuria (painful urination), urinary urgency and frequency (wanting to urinate frequently and needing to pass urine quickly), and bacteriuria accompanied by smelly, cloudy urine.23,25,26 The treatment depends on the severity of the infection, the number of infections and other complications (e.g. possible renal infection). Antibiotics that are prescribed include penicillin and cephalosporins.
Healthcare-acquired infections
Infections are a scourge of modern healthcare facilities. Approximately 6% of all patients admitted to Australian hospitals will develop a hospital-acquired infection,27 which equates to about 150,000 infections in Australian hospitals each year.28 In addition, there has been an increase in the number of hospitalisations for pneumonia, urinary tract infections and gastrointestinal infections.29
Healthcare-acquired infections are acquired by individuals either during a stay in a healthcare setting or afterwards. Such infections are relatively common in Australian hospitals, particularly those on the eastern seaboard. Furthermore, they may be caused by microorganisms from an endogenous source (a body site of the individual, such as the skin, nose, mouth, gastrointestinal tract) or an exogenous source (external to the patient, such as healthcare personnel, visitors, patient care equipment, medical devices or the healthcare environment).30
The most common healthcare-acquired infections include:
Urinary tract infections are the most common healthcare-acquired infections, usually related to in-dwelling urinary catheters in hospitalised patients. The length of time the in-dwelling urinary catheter remains in a patient is the best predictor of whether the patient will acquire bacteriuria (bacteria in the urine).27 Surgical wound infections are the second most common healthcare-acquired infections in Australian hospitals and significantly contribute to an increased duration of hospital stay and mortality.31
Pathogens on stethoscopes and other healthcare equipment
Potential sources of pathogenic contamination from healthcare staff to patients have been examined. In recent times the search for pathogens that cause healthcare-acquired infections has been more intense. An examination of 155 doctors and medical students found that of the 250 stethoscopes used, both personal and ward-based, almost all (92.7%) isolated Staphylcoccus, which was potentially pathogenic. It was also found that cleaning regularity had no effect on bacterial count. Other prior studies confirm these findings and suggest that objects such as blood pressure cuffs, pens and medical record notes are potential sources of infection.
Source: Denholm JT et al. A microbiological survey of stethoscopes in Australian teaching hospitals: potential for nosocomial infection? Australian Infection Control 2007; 10(3):79–86; Datz C et al. What’s on doctors’ ball point pens? Lancet 1997; 350(9094):1824; Fishman M et al. Medical records contaminated with dried blood: a quality issue. American Journal of Infection Control 1997; 27(5):438–443.
One of the main healthcare-acquired infections and multi-resistant bacteria in Australian and New Zealand hospitals is methicillin-resistant Staphylococcus aureus (MRSA).32,33 The increased prevalence of MRSA infections is alarming. MRSA now accounts for 63% of all staphylococcus infections, and the fact that MRSA accounted for only 2% of Staphylococcus infections in 1974 indicates that infection control procedures have not been successful.34 In addition, most MRSA infections (85%) occur in healthcare settings.35
The prevention of MRSA contamination in hospitals should be fundamental. Simple infection-control procedures, such as hand-washing between examining patients and isolating MRSA-infected patients, should be enough to prevent the spread. However, this is often not the case and the prevalence of MRSA infections has been increasing in Australia and New Zealand. Fortunately, MRSA remains treatable with antibiotics (vancomycin); however, with increasing levels of antimicrobial resistance, this may not always be the case (see the box ‘Health alert: pathogens on stethoscopes and other healthcare equipment’).
ANTIMICROBIAL RESISTANCE
Microbial pathogens have emerged that have developed mechanisms for circumventing the most modern techniques for destroying or controlling infection.36,37 These include microorganisms that attack the immune system (e.g. HIV) and those that are resistant to multiple antibiotics (e.g. MRSA). HIV is one of the few microorganisms that directly attacks the central processes involved in the development of an immune response and is discussed in Chapter 15.
Many pathogens have mutated and developed resistance to particular antibiotics. Resistance occurs primarily through inactivation of the drug, alteration of the bacterial membrane that prevents the antibiotic from being taken up, alteration of the target molecule, or reduced uptake or active efflux of the antibiotic. These changes result from genetic mutations and can be transmitted directly to neighbouring microorganisms. Penicillin resistance, for example, results from the production of an enzyme (beta (β)-lactamase) that breaks down the antibiotic.
A rapid emergence of multiple antibiotic–resistant bacteria has been observed. These microorganisms are resistant to almost all currently available antibiotics. For example, Streptococcus pneumoniae, which causes pneumonia, meningitis and acute otitis media (ear infections), was once routinely susceptible to penicillin. Since the 1980s, however, the incidence of penicillin-resistant microorganisms in Australia has risen dramatically.38,39 In addition, many of these microorganisms are resistant to multiple antibiotics. In some areas, more than 20% of tuberculosis cases are caused by multiple antibiotic–resistant Mycobacterium tuberculosis. Also, the incidence of drug-resistant gonorrhoea, malaria, pneumococcal disease, salmonellosis, shigellosis and staphylococcal infections has increased dramatically.
Why have multiple antibiotic–resistant microorganisms appeared? Overuse of antibiotics can lead to the destruction of the normal flora, allowing the selective overgrowth of antibiotic-resistant strains or pathogens that had previously been kept under control. Let us use a common example to illustrate how antibiotic-resistant microorganisms may occur. An individual develops a cough and fever, necessitating a visit to their local general practitioner. The general practitioner examines the individual and diagnoses an upper respiratory tract infection, with the offending pathogen considered to be bacterial in origin. Accordingly, the general practitioner prescribes an antibiotic to treat the infection. However, no pathogenic bacterium was isolated from sputum (mucus coughed from the airways) cultures, nor was there any direct evidence that the individual had a bacterial infection. In reality, the individual was infected with a virus, such as a rhinovirus, a frequent cause of influenza. Therefore, this individual was unnecessarily exposed to an antibiotic, which provides an opportunity for the bacteria to develop resistance.
The antibiotics amoxycillin and cephalexin are the third and eleventh most prescribed medications in Australia, respectively. In fact, almost 5 million prescriptions are made annually for amoxycillin alone. In addition, 4 of the 10 most frequently prescribed medications by general practitioners are from the antibiotic group — amoxycillin, cephalexin, timentin and roxithromycin. Alone, these four antibiotics account for more than 10% of all prescriptions by general practitioners each year.3
Unfortunately, antibiotics have a limited capacity and bacterial resistance to treatment is likely to develop over time. For instance, after eight years of widespread use, penicillin was found to be ineffective against the majority of Staphylococcus aureus cases in hospitals;40 and Escherichia coli has recently developed an increasing resistance to many antibiotics, including cephalosporins, macrolides and aminoglycosides.41 Therefore, the need to be vigilant in the prescription of antimicrobial drugs is crucial, and measures to guide the practitioner in many cases are now in place.
Microorganisms
 A microorganism is a term used to describe bacteria, viruses, fungi and parasites. Many microorganisms do not harm humans, but others cause infections that range from being irritating to the individual to life-threatening.
A microorganism is a term used to describe bacteria, viruses, fungi and parasites. Many microorganisms do not harm humans, but others cause infections that range from being irritating to the individual to life-threatening. Pathogens enter the body through a variety of means, including direct contact, inhalation, ingestion and direct penetration of the skin.
Pathogens enter the body through a variety of means, including direct contact, inhalation, ingestion and direct penetration of the skin. Bacteria lack a discrete nucleus and are relatively small. They can be aerobic or anaerobic, motile or non-motile.
Bacteria lack a discrete nucleus and are relatively small. They can be aerobic or anaerobic, motile or non-motile. Bacteria are identified according to their shape: cocci, bacilli and sphirochete. In addition, bacteria are classified according to a gram stain as gram-positive or gram-negative.
Bacteria are identified according to their shape: cocci, bacilli and sphirochete. In addition, bacteria are classified according to a gram stain as gram-positive or gram-negative. Viruses are simple structures with a protein layer, called a capsid, surrounding one type of nucleic acid.
Viruses are simple structures with a protein layer, called a capsid, surrounding one type of nucleic acid. Viruses can survive for extended periods of time but infect people when they enter the host cells. They can bypass many defence mechanisms by developing intracellularly, thus hiding within cells and away from normal inflammatory or immune responses.
Viruses can survive for extended periods of time but infect people when they enter the host cells. They can bypass many defence mechanisms by developing intracellularly, thus hiding within cells and away from normal inflammatory or immune responses. Fungi are relatively large microorganisms with thick walls that grow as either single-celled yeasts (spheres) or multicelled moulds (filaments or hyphae).
Fungi are relatively large microorganisms with thick walls that grow as either single-celled yeasts (spheres) or multicelled moulds (filaments or hyphae). Deep fungal infections involving internal organs can be life-threatening and are most common in association with other diseases or as an opportunistic infection in immunosuppressed individuals.
Deep fungal infections involving internal organs can be life-threatening and are most common in association with other diseases or as an opportunistic infection in immunosuppressed individuals.Antimicrobials
Infections
 The common cold is one of the most frequent infections in humans. It is usually a viral infection, such as rhinovirus, coronavirus, parainfluenza virus, respiratory syncytial virus and adenovirus.
The common cold is one of the most frequent infections in humans. It is usually a viral infection, such as rhinovirus, coronavirus, parainfluenza virus, respiratory syncytial virus and adenovirus. Urinary tract infection is another common infection affecting humans. The gram-negative anaerobe, Escherichia coli, a normal bowel flora, accounts for up to 90% of uncomplicated community-based urinary tract infections in both adults and children.
Urinary tract infection is another common infection affecting humans. The gram-negative anaerobe, Escherichia coli, a normal bowel flora, accounts for up to 90% of uncomplicated community-based urinary tract infections in both adults and children.Leila is a 76-year-old woman who is experiencing a urinary tract infection. She has had a fever for 2 days, painful urination (dysuria), foul-smelling urine that is cloudy and, on occasions, leaking urine (urinary incontinence). Leila has 5 children and 10 years ago was diagnosed with diabetes mellitus type II. This is her third urinary tract infection for the year. Her previous infections resulted from Escherichia coli, which was cultured from the urine.