19 ALTERATIONS OF THE INTEGUMENTARY SYSTEM ACROSS THE LIFE SPAN
INTRODUCTION
The integumentary system is the most visible organ system and so alterations to the skin are usually noticeable. Disorders and diseases of the integumentary system are common across the life span, such as nappy rash and acne in the younger population and skin tears in the elderly. In addition, skin alterations are particularly relevant to clinicians in Australia and New Zealand, as we have the highest rates of skin cancer in the world.
The skin is the largest organ in the body and is exposed to many potentially damaging agents including ultraviolet radiation, chemicals and physical trauma. Alterations of the integument include inflammatory conditions due to infections, allergies or unknown causes; benign and malignant neoplasms; traumatic conditions; and vascular disorders. Although the number of disorders affecting the integumentary system is vast, in the following sections we discuss only the commonly encountered conditions.
SKIN LESIONS
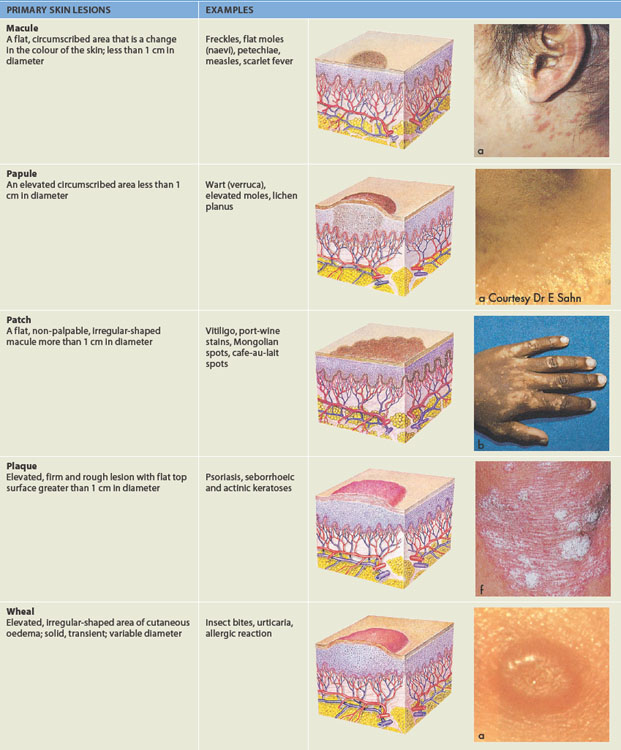
Physical description of the skin when diagnosing a skin disorder includes detail about the skin lesion. Skin lesions are either primary (original appearance) or secondary (the appearance has been changed by normal progress over time). Identification of the morphological structure and physical appearance of skin lesions is important to understand the underlying pathophysiology. Descriptions and examples of primary and secondary lesions are listed in Table 19-1. This list can be used as a reference tool when referring to the pathophysiological conditions throughout the rest of the chapter.
SKIN CANCER
Skin cancer is the most common form of cancer in both Australia and New Zealand and skin cancer rates in these two countries are the highest in the world. Interestingly, clinicians are not required to report the incidence of some types of benign skin cancers to health authorities, so skin cancers are actually much more common than suggested by publications that list the incidence of diseases. This high rate is attributed primarily to the high ambient solar ultraviolet (UV) radiation. Fair-skinned people are the most susceptible to developing skin cancer — skin cancer is rare in Aboriginal and Torres Strait Islander peoples and the Maori population. The most frequently occurring skin cancers are non-melanoma skin cancers (basal cell carcinoma and squamous cell carcinoma) and melanoma.
Basal cell carcinoma
Basal cell carcinoma is a malignant tumour of the integument that arises from pluripotential cells (cells that can develop into many different types of cells) in the basal layer of the epidermis. It is the most common form of skin cancer in Australia and New Zealand and accounts for at least two-thirds of non-melanoma skin cancers. Since neither Australian nor New Zealand registries record non-melanoma skin cancers, incidence rates have been determined from national population surveys. The Australian 2002 survey estimated the incidence rate of basal cell carcinoma to be 1041 per 100,000 in males and 745 per 100,000 in females.1 Data collected from general practices throughout Australia has shown that basal cell carcinoma accounted for 0.6% of all general practitioner–patient encounters between April 2005 and March 2007.2
The predominant cause of basal cell carcinoma is repeated exposure to solar UV radiation, especially in the form of ultraviolet B (UVB) with wavelengths 280–320 nanometres (nm).3 The sun emits several types of electromagnetic radiation: UV, visible and infrared. These are emitted as waves and have different wavelengths. For instance, UV light has a wavelength that is shorter than visible light. The sun emits three types of ultraviolet light: UVA, UVB and UVC. UVA has the longest wavelength, followed by UVB and UVC. The highest energy rays are UVC, but fortunately UVC is filtered out by the earth’s atmosphere and does not reach the surface. However, UVB is particularly harmful to the skin and with the destruction of the ozone layer UVB radiation can penetrate the earth’s atmosphere, thereby increasing the risk for UV-induced photocarcinogenesis (cancer arising from light). Exposure to UV radiation during childhood and adolescence significantly increases the risk of developing basal cell carcinoma. A very small number of basal cell carcinomas can be attributed to other causes, such as ionising radiation therapy and when individuals are immunosuppressed.1
Basal cell carcinoma arises from stem cells located in the basal layer of hair follicles or the epidermis. The precise pathogenesis of basal cell carcinoma is not known. It probably begins with a gene mutation (concepts of cancer are discussed in Chapter 36). Both UVA and UVB can damage DNA. UVB is absorbed directly by DNA, causing changes in a number of genes. Also, UVA causes DNA damage by generating oxygen radicals (see Chapter 4), which causes breaks in the DNA molecule.4 Studies suggest that alterations in particular tumour suppressor genes may be related to the formation of basal cell carcinoma.5,6 Changes in genes may decrease repair of UV-induced DNA damage and induce cell cycle proliferation, leading to the formation of basal cell carcinoma.
Basal cell carcinomas are slow-growing tumours that rarely spread to other parts of the body. They occur most frequently on body areas that are exposed to the sun, such as the face, head, neck, shoulders and back. There are three common growth patterns (superficial, nodular and morphoeic), each with different clinical features:
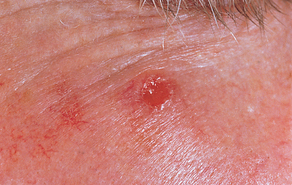
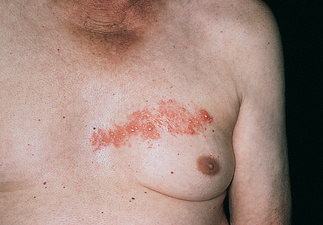
FIGURE 19-1 Basal cell carcinoma.
Ulcerated lesion with raised pearly border.
Source: Courtesy of Department of Dermatology, School of Medicine, University of Utah.
The most common treatment of basal cell carcinomas is surgical excision. Complete excision with a 4–5 mm margin cures the majority of patients. For highly aggressive basal cell carcinomas Mohs surgery can be used. In this procedure all margins of the excised tumour are examined to ensure a high cure rate.7 Cryotherapy, the destruction of tissues by the application of a cryogenic agent such as liquid nitrogen, can be used to treat well-defined lesions of non-aggressive basal cell carcinomas on sites other than the head and neck. Imiquimod 5% cream can be used to treat superficial basal cell carcinoma. The cream is applied five times a week for six weeks to the tumour and a 5 mm margin of normal skin surrounding it. Imiquimod is a cytokine and interferon-inducer that stimulates an immune response.
For superficial basal cell carcinomas on the trunk and limbs, curettage and diathermy/electrodesiccation can be used. Curettage is the medical term for surgical scraping — the tumour tissue is scraped away, and this is followed by the application of diathermy (production of local heat, which can stop bleeding or destroy tissue) to the base. Photodynamic therapy is an effective treatment for superficial and thin nodular basal cell carcinomas. Light is used to activate a photo-sensitiser such as methyl aminolevulinate (Metvix®), which is localised in tumour tissue to form cytotoxic reactive oxygen species, to cause destruction of the carcinoma. Radiation therapy is generally used only to treat basal cell carcinomas not suited to surgery or as an adjuvant to surgery for persistent or advanced basal cell carcinomas.
Although basal cell carcinomas rarely metastasise (spread to other areas), they may destroy local healthy tissue. Basal cell carcinomas have a long recurrence period of 10 to more than 20 years. Therefore, follow-up monitoring is important. Since the main cause of basal cell carcinomas is exposure to sun, prevention is aimed at improving sun-protective behaviours. Both the Cancer Council of Australia and the Cancer Society of New Zealand have devised strategies to improve sun protection and reduce the incidence of skin cancers. Such strategies include avoiding exposure in the middle of the day, staying in the shade, wearing clothing and hats that protect exposed skin, using sunscreen and avoiding the use of solariums.
Squamous cell carcinoma
Squamous cell carcinoma (see Figure 19-2) is a cancer arising from keratinocytes in the outer layers of the epidermis. It may arise from skin or other sites lined by squamous epithelium, such as the oesophagus, mouth and vagina. Squamous cell carcinoma has precursor lesions, is invasive and may metastasise (spread to other body regions; refer to Chapter 36).
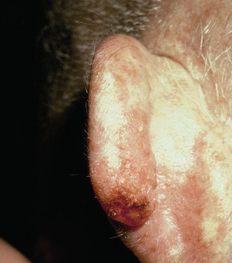
FIGURE 19-2 Squamous cell carcinoma on the ear.
The lesion has irregular borders.
Source: Talley NJ, O’Connor S. Clinical examination: a systematic guide to physical diagnosis. 6th edn. Sydney: Elsevier; 2010.
The incidence of squamous cell carcinoma, the second most common of the non-melanocyte skin cancers, continues to rise both in Australia and worldwide. In Australia in 2002 it was estimated that the incidence rate was 387 per 100,000 people.1
The predominant cause of squamous cell carcinomas and related squamous keratinocyte tumours is cumulative sun exposure. Other risk factors include infection with human papillomavirus, immune suppression, smoking, chronic ulcers and genetic syndromes such as xeroderma pigmentosum in which the mechanism for DNA repair is defective. In addition, a number of precancerous conditions including solar keratosis and squamous cell carcinoma in situ (Bowen’s disease) can develop into squamous cell carcinoma.
Solar keratosis
The majority of squamous cell carcinomas arise from solar keratoses. Solar keratoses are lesions that have atypical nuclei in the epidermal basal layer and hyperkeratosis (see Figure 19-3). They appear as irregular plaques with a rough, hard, hyperkeratotic surface. Solar keratoses are associated mainly with middle age and are found most commonly on the face, ears and back of hands. The lesions can remit spontaneously, persist or progress into squamous cell carcinoma. Solar keratoses can be treated with cryotherapy, topical medications including 5% 5-fluorouracil cream (an antimetabolite that stops the cell cycle), imiquimod 5% cream (which stimulates the immune response), 3% diclofenac gel (which induces apoptosis, inhibits cell proliferation and suppresses angiogenesis (blood vessel growth)) or photodynamic therapy.
Squamous cell carcinoma in situ (Bowen’s disease)
Squamous cell carcinoma in situ (meaning remaining where it originated) consists of abnormal keratinocytes confined to the epidermis. The lesions involve the full thickness of the epidermis and are well-demarcated, erythematous scaly patches up to several centimetres in diameter. They are found most commonly on the ears, face, hands and lower legs. Squamous cell carcinoma in situ is more likely to progress to invasive disease than solar keratosis, with about 5% progressing into invasive squamous cell carcinoma.8 Development of a lump or bleeding may indicate that the lesion has progressed to invasive squamous cell carcinoma. Treatment includes cryotherapy, removal of the lesion and cauterisation to the base, 5-flourouracil cream, imiquimod cream and photodynamic therapy.
The events involved in the pathogenesis of squamous cell carcinoma (both in situ and invasive) are largely unknown. Damage to DNA results in monoclonal differentiation within a keratinocyte, meaning the cell line that produces keratinocytes is damaged. Excessive UV exposure overwhelms the mechanisms that repair DNA and mutations can occur in several tumour-suppressor genes including p53.9 Mutations lead to uncontrolled cell proliferation and loss of apoptosis (see Chapter 4). Inflammation is a major component of the neoplastic progression. Tumour cells co-opt signalling molecules of the innate immune system that maintain chronic levels of lymphocytes and mast cells promoting tumour growth, invasion and metastasis.10,11
Squamous cell carcinoma in situ begins as an erythematous papule (small, raised skin region) or nodule. Invasive squamous cell carcinomas are usually slow-growing nodules that may develop ulcers that do not heal. The borders are irregular and bleed easily. About 5% of squamous cell carcinomas metastasise. Since squamous cell carcinomas are aggressive, have potential for recurrence and may spread to lymph nodes and distant sites, treatment is usually surgical excision.
Melanoma
Melanoma is a malignant tumour that arises from melanocytes. It progresses rapidly and has a high rate of metastasis. Australia and New Zealand have the highest incidence rates of melanoma in the world, being three times higher than the rate in the United States and four times higher than the rate in the United Kingdom. Melanoma is the fourth most common malignant cancer in Australia and New Zealand, and the incidence in Australia has been increasing over the last decade, with a 16% increase in males and a 24% increase in females.12
The cause of melanoma involves both environmental and genetic factors. The primary environmental factor is intermittent exposure to UV radiation in sunlight. This link is very evident, as individuals who have had sunburn are twice as likely to develop melanoma as those who have never had sunburn. The highest risk occurs if the sunburn occurs in childhood. People with fair skin and blonde or red hair have a greater risk of melanoma that may be associated with genetic variation in the melanocortin receptor (a receptor involved in the production of melanin).13 Other risk factors include a previous history of skin cancer, the presence of melanocytic naevi (a benign accumulation of melanocytes) and a family history with the most common genetic abnormality being mutation of the p16 gene.
PATHOPHYSIOLOGY
Although the exact pathogenesis of the transformation of a melanocyte into melanoma is not fully known, it is believed to be a multistep process of progressive genetic mutations.13–15 4 6 Abnormalities of the genetic pathways within the melanocyte promote melanocyte proliferation and apoptosis leading to the development of melanoma (see Figure 19-4). The different genetic components include changes to the melanocortin 1 receptor (MC1R), mutations of the CDKN2A, CDK4 and N-ras genes, with a loss of the phosphatase and tensin (PTEN) homologue on chromosome 10 (homologue refers to chromosomes with the same genes). Enzymes such as cathepsin then mediate degradation of matrix proteins allowing invasion and metastasis.16
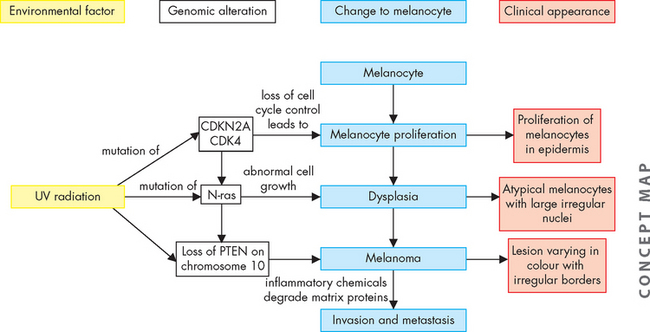
FIGURE 19-4 Proposed pathogenesis of melanoma.
The development of melanoma is believed to be a multistep process resulting from complex interactions between UV light and genes. Genetic alterations include melanocortin 1 receptor (MC1R) variants, mutations of CDKN2A and CDK4 genes (involved in controlling cell division), mutations of N-ras genes that can promote resistance to apoptosis and loss of the phosphatase and tensin (PTEN) homologue gene on chromosome 10. Enzymes such as cathepsin then mediate degradation of the matrix proteins, allowing invasion and metastasis.
Source: Based on Demierre M, Sondak VK. Cutaneous melanoma: pathogenesis and rationale for chemoprevention. Clinical Reviews in Oncology/Haematology 2005; 53(3): 225–239.
CLINICAL MANIFESTATIONS
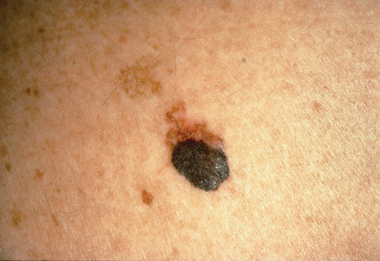
Melanomas differ in size, shape and colour. They range in size from a few millimetres in diameter to a few centimetres. They have irregular borders and may be dark brown, black, blue or red in colour. During the horizontal growth phase they are flat and during the vertical growth phase they become raised. Some melanomas may be itchy or bleed.
There are four main types of melanomas:
EVALUATION AND TREATMENT
Diagnosis of melanoma is based on biopsy results. Melanomas are classified and staged using the classification and staging system for melanoma published in 2002 by the American Joint Committee on Cancer and the International Union Against Cancer. The TNM (tumour, lymph node, metastasis) staging includes melanoma thickness and ulceration, the number of metastatic lymph nodes and the site of distant metastases (a general description of the TNM system is in Chapter 36).
Early diagnosis and treatment are essential, as invasion of melanoma into deeper skin tissue and ulceration results in a poor prognosis. Therefore, the standard treatment for primary melanoma is wide local surgical excision of the skin and subcutaneous tissue.12 If the melanoma invades deeper tissue, there is a risk of spread to the regional lymph nodes. The risk of metastasis is linked to the Breslow thickness of the primary melanoma (a standardised method to determine the thickness of melanomas). The risk of metastasis is related to the thickness of the melanoma. Patients with a melanoma greater than 1 mm in thickness are given the opportunity to discuss sentinal lymph node (the node receiving lymphatic drainage from the tumour) biopsy. If the biopsy is positive, treatment may include lymph node dissection and adjuvant therapy (other cancer therapies, such as chemotherapy).
Benign skin lesions: keratocanthoma
Although keratocanthomas are benign lesions that resolve spontaneously, because of their similarity in appearance to squamous cell carcinomas, they are excised to ensure correct diagnosis. Keratocanthomas (see Figure 19-7) initially appear as a small papule that grows rapidly forming an erythematous nodule with a central keratin plug. The keratin plug is expelled and the edges eventually resolve, leaving a scar.
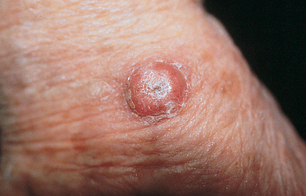
A fully developed keratocanthoma presents as a raised, round lesion with a central keratin plug.
Source: Courtesy of Department of Dermatology, School of Medicine, University of Utah.
INFLAMMATORY DISORDERS OF THE SKIN
Dermatitis
Dermatitis, also called eczema, is the most common inflammatory condition of the skin and is caused by both exogenous and endogenous agents. Exogenous dermatitis includes irritant contact dermatitis and allergic contact dermatitis. Endogenous dermatitis includes atopic dermatitis and seborrhoeic dermatitis. Acute dermatitis is associated with pruritis, erythema, vesicles and scales. The manifestations of chronic dermatitis include pruritis, dryness of the skin, skin thickening, hyperpigmentation and sometimes fissures.
Irritant contact dermatitis
Irritant contact dermatitis is a non-allergic inflammatory response to chemical or physical agents. The inflammatory response may occur after the first exposure or it may result from chronic cumulative exposures.
Common substances causing irritant contact dermatitis include water, soap, detergents, oils, acids, alkalis and rubber. As well as exposure to the irritant, environmental factors such as heat, low humidity and UV radiation and mechanical factors such as friction, occlusion and pressure may also contribute to the development of contact dermatitis. Heat, for example, causes sweating, which facilitates the penetration of irritants. Occlusive gloves enhance irritation from heat and sweating. Low humidity decreases the level of ceramide, a major lipid in the stratum corneum, essential for maintaining the water permeability barrier of the skin.
Irritant contact dermatitis is the most common occupational skin disorder, usually affecting the hands. People in occupations that involve frequent hand cleansing, wearing occlusive gloves for more than two hours per day or exposing skin to liquid for more than two hours per day have a high risk of developing irritant contact dermatitis. Occupational irritant contact dermatitis caused by wet work is particularly common in nurses.
The pathogenesis of irritant contact dermatitis consists of exposure to the irritant, which triggers a cascade of skin barrier disruptions, cellular damage and release of chemical mediators resulting in an inflammatory response (refer to Chapter 13).17 Irritants damage the skin by a number of mechanisms, including direct cytotoxicity, lipid-barrier removal, cell membrane damage and the breakdown of proteins. Once the irritant penetrates the damaged stratum corneum, keratinocytes release cytokines and stimulate MHC class II antigens.18 Although a number of cytokines are released, it has been suggested that tumour necrosis factor-alpha (TNF-α) is the major mediator of inflammation (see Figure 19-8).
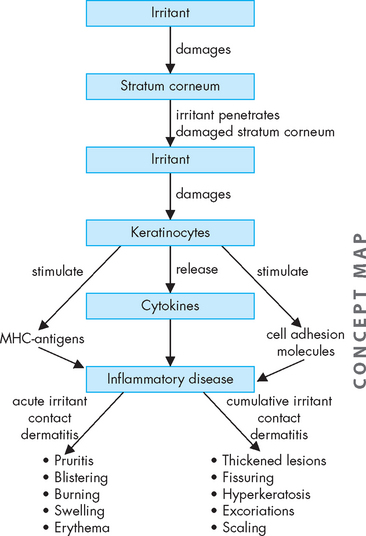
FIGURE 19-8 The pathogenesis of irritant contact dermatitis.
Irritant contact dermatitis is a pathophysiological cascade of skin barrier disruptions, cellular damage and release of chemical mediators following exposure to an irritant.
The clinical manifestations of irritant contact dermatitis are a result of the inflammatory response. Acute irritant contact dermatitis develops rapidly after exposure to highly irritating substances and manifests with pruritis, burning, pain, erythema, blistering and swelling. The rash is contained to the area of exposure. Cumulative irritant dermatitis following repeated exposure to mild irritants results in thickened lesions with fissuring, hyperkeratosis, excoriations and scaling.
Treatment includes avoiding contact with the irritant, topical corticosteroids that have anti-inflammatory and immunosuppressive effects and emollients that, by providing a surface film of lipids, restore some of the barrier function.
Allergic contact dermatitis
Allergic contact dermatitis is a type IV delayed T-cell mediated hypersensitivity reaction. The inflammatory reaction occurs in sensitised individuals when they are later exposed to the antigen.
The development of allergic contact dermatitis occurs in two phases. During the initial phase sensitisation is acquired. This is followed by a subcutaneous inflammatory reaction when subsequently exposed to the same antigen.
Allergens including metals, chemicals, microorganisms, drugs and latex can form sensitising antigens. For the immune response to be induced, the allergen must penetrate the epidermis. A number of risk factors that damage the skin’s barrier function enhancing penetration of allergens have been associated with the development of allergic contact dermatitis. These include friction, heat exposure, humidity and frequent hand washing. Once the allergen has penetrated the epidermis it binds to a protein. Langerhans’ cells transport the antigen to lymph nodes and present it to T lymphocytes, which become sensitised to the antigen. When the chemical allergen is encountered again a rapid, more aggressive immune response occurs.19,20
The sensitised T lymphocytes secrete cytokines that elicit the inflammatory response causing a rash with pruritis, erythema, oedema and vesicular lesions. The lesions may weep, increasing the risk of secondary infections.
Patch testing is used to diagnose allergic contact dermatitis. Management includes removal of the irritant and topical corticosteroids. If the reaction is severe, oral corticosteroids may be required.
Nappy rash
Nappy rash is the most common dermatitis of infancy and early childhood. Nappy dermatitis is confined to areas covered by the nappy — that is, the lower aspect of the abdomen, genitalia, buttocks and upper portion of the thighs. It is a contact dermatitis caused by a combination of factors including maceration by wet nappies; irritants such as ammonia (produced from urine), faeces, chemical agents present in nappy wipes and nappy soaking solutions; Candida albicans; airtight plastic pants; and friction between the nappy and skin. The lesions caused by nappy rash vary from mild erythema to blistering and ulcers (see Figure 19-9).
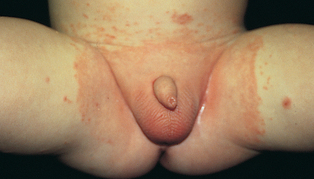
FIGURE 19-9 Nappy rash with Candida albicans as a secondary infection.
This is an erythematous rash over the area covered by the nappy. It has spread to the groin folds, which usually occurs with a secondary infection.
Source: Courtesy of Department of Dermatology, School of Medicine, University of Utah.
Treatment includes frequent changing of the nappy to keep the skin clean and dry, using disposable nappies that absorb the urine, applying a barrier cream at each change and exposing the area to air. If there is Candida infection, topical antifungal medication such as imidazole or nystatin should be applied. Low-potency topical steroids can be used in the short term when the condition has failed to respond to other approaches.
Latex allergy
As use of latex gloves has increased over recent decades, so latex sensitivity has increased. Latex consists of a protein-based structure to which individuals may become sensitised. It can be found in a number of products including some catheters and condoms. As a result of high exposure to powdered latex gloves, healthcare workers have a high risk of becoming sensitised. The latex allergen leaches out of the gloves and into the powder, which carries the latex into the environment. Exposure to latex may result in two types of responses:
 a type I hypersensitivity reaction that causes anaphylaxis; this response is mediated by immunoglobulin E.
a type I hypersensitivity reaction that causes anaphylaxis; this response is mediated by immunoglobulin E.Repeated exposure to latex causes rupture of mast cells with release of histamine. The histamine increases capillary permeability resulting in tissue swelling and a fall in blood pressure. Histamine release from the mast cells in the airways causes oedema in the airways, increased resistance to airflow and wheezing.21 For more details on hypersensitivity reactions, see Chapter 15.
Sensitised individuals should not be exposed to latex. In the workplace, for example, non-latex gloves should be worn. People with latex allergy are advised to wear a MedicAlert bracelet and, if necessary, carry an adrenaline auto-injector (Epipen®). They should also be aware that cross-reactivity exists between latex and a number of plant-derived foods such as nuts, avocados, potatoes, tomatoes and bananas.22
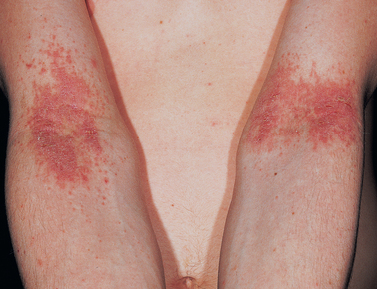
Atopic dermatitis
Atopic dermatitis is an inherited chronic inflammatory skin disorder. Together with asthma and allergic rhinitis it is a condition of the atopic state. It occurs mainly in children, with at least 60% of cases arising within the first year of life. Although atopic dermatitis can persist into adulthood, symptoms usually diminish by puberty.
The cause of atopic dermatitis involves a complex interplay between environmental triggers and genetic factors including altered innate and adaptive immune responses.23 Although genetically determined, factors such as stress may exacerbate the disease.
PATHOPHYSIOLOGY
Although the exact pathogenesis of atopic dermatitis remains unclear, it is believed that epidermal barrier dysfunction has a primary role. Genetic aberrations in constituents of the epidermal barrier including mutations in the filaggrin gene have been recently identified.24 The filaggrin gene encodes for filaggrin, a protein necessary for the formation and hydration of the skin barrier. Defects in the epidermal barrier may also result from changed lipid composition such as a decrease in ceremides, a major water-retaining molecule. The resulting damage to the epidermal barrier causes increased transdermal water loss and allows the entrance of antigens, pathogens and nonspecific irritants that cause activation of the inflammatory response.25 The resulting dermatitis involves complex actions of Langerhans’ cells, natural killer cells and eosinophils and IgE production by B cells.
CLINICAL MANIFESTATIONS
Clinical manifestations result from both epidermal barrier dysfunction and the inflammatory response. The most commonly affected sites in infants with atopic dermatitis are the face, scalp and extensor surfaces. In older children and adults flexural surfaces such as the antecubital and popliteal fossae are more commonly affected. Skin dryness is a consequence of transepidermal water loss. Mild cases are associated with erythema in localised areas of the skin (see Figure 19-10). Acute lesions are erythematous, itchy and scaling. Weeping and crusted lesions may develop. Chronic lesions are thickened plaques, papules or nodules. Dermal fibrosis is a feature of chronic atopic dermatitis. Due to disturbance of the antimicrobial barrier, recurrent bacterial, viral and fungal infections occur. More than 90% of people with atopic dermatitis chronically carry Staphylococcus aureus on their skin.
EVALUATION AND TREATMENT
The standard treatment for acute exacerbations of atopic dermatitis is topical corticosteroids. Other management strategies include the use of mild, non-alkali soaps and emollients, as well as barrier repair therapy.
Seborrhoeic dermatitis
Seborrhoeic dermatitis is a chronic inflammatory skin disorder affecting areas of the body where sebaceous glands are most prominent, such as the scalp, eyebrows, nasolabial folds, behind the ears, axillae and chest. Although the cause is unknown, the trigger appears to be from the Malassezia yeasts. When affecting the scalp in infants it manifests as cradle cap and in adults as dandruff. The lesions are scaly, white or yellow plaques. It is treated with shampoos containing sulfur, salicylic acid, zinc pyrithione or tar.
Acne vulgaris
Acne vulgaris is an inflammatory disorder of the pilosebaceous follicle that mainly affects adolescents: 85% of teenagers and young adults develop acne vulgaris. It affects both males and females, but the most intense and severe clinical cases occur in males. Spontaneous regression usually occurs by the age of 20, but in some people it may persist during adult life. Acne vulgaris causes polymorph cutaneous lesions including comedones, papules, cysts, pustules and abscesses that may leave scars (see Figure 19-11). The areas of the body most affected are the face, anterior trunk and upper back, where there are greater concentrations of sebaceous glands.
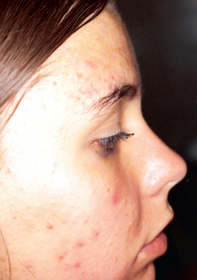
FIGURE 19-11 Acne vulgaris with comedones and inflammatory pustules.
Source: Christensen BL, Kockrow EO. Adult health nursing. 6th edn. St Louis: Mosby; 2011.
Factors causing acne include overactivity of the pilosebaceous ducts, blockage of the ducts, excessive sebum production and proliferation of Propionibacterium acnes. The initial trigger is thought to be the increased hormonal influences of androgens at puberty.26
Several factors are involved in the pathogenesis of acne vulgaris. Androgens stimulate the sebaceous glands. Although the androgens are in normal quantity, the pilosebaceous unit overreacts to them. The increase of sebaceous secretion is the initial alteration. Retention of sebum due to hyperkeratosis leads to the formation of comedones. With overgrowth of bacteria and release of free fatty acids, a papula-pustule is formed.27 When the follicular wall of a closed comedone ruptures, inflammation is initiated. Pustules form when the inflammation is close to the surface; papules and cystic nodules when the inflammation is deeper. After regression of the lesions scars may be left.
Topical treatments including benzoyl peroxide, salicylic acid and tretinoin are used as the first line of therapy. In severe and topical-resistant cases oral medications may be used, including antibiotics, isotretinoin and hormones. Severe or persistent acne can be controlled and scarring avoided with oral isotretinoin. However, the most serious problem with this drug is its teratogenic effect with a high incidence of miscarriage.
Acne rosacea
Acne rosacea is a chronic inflammatory skin disease that affects adults and is characterised by central facial erythema, telangiectasia (small dilated blood vessels on the skin), lesions, oedema and flushing (see Figure 19-12). There are periods of exacerbation and remission.
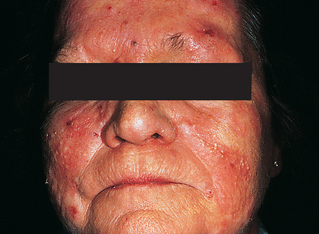
Erythema and pustules on the forehead, nose and cheeks.
Source: Courtesy of Department of Dermatology, School of Medicine, University of Utah.
Both genetic and environmental factors seem to be involved in the pathogenesis of acne rosacea. Environmental factors including sunlight, heat, cold, alcohol and emotional stress trigger exacerbation of acne rosacea in people with a genetic predisposition to the condition. Chronic photodamage caused by light exposure causes degradation of the dermal collagen and elastic tissue. This is due to production of reactive oxygen species and an increase in enzymes that degrade the dermal layer.28 In addition, there is damage to the superficial cutaneous vasculature. Peptides are abnormal in the skin of people with rosacea and have a role in the skin’s inflammatory responses.29
The clinical manifestations of acne rosacea result from the damage to the dermis and the inflammatory response. Increased loss of water from the epidermis causes skin dryness and scaling. Erythema occurs on the nose and cheeks and telangectasia develops. After a prolonged period acne rosacea may develop into bullous hyperplasia of the nose, known as rhynophyma.
Management includes avoiding UV exposure, avoiding known triggers and appropriate skin care to help repair and maintain skin barrier integrity. In patients with inflammatory rosacea, topical agents such as metronidazole and azelaic acid are used.
Cutaneous lupus erythematosus
Cutaneous lupus erythematosus is an inflammatory autoimmune disease. Lupus may be limited to the skin (cutaneous lupus erythematosus) or have multi-organ involvement (systemic lupus erythematosus). In a small percentage of cases, patients with cutaneous lupus erythematosus can develop systemic lupus erythematosus. Mucocutaneous manifestations such as malar (cheek) rash, alopecia and oral ulcers are common in patients with systemic lupus erythematosus.
Cutaneous lupus erythematosus is subdivided into chronic cutaneous lupus erythematosus, subacute cutaneous lupus erythematosus and acute cutaneous lupus erythematosus.30 The cause of lupus erythematosus is related to both genetic and environmental factors (see Chapter 15 for more details). The pathogenesis is still not well understood. It is believed that predisposition to the different subsets of cutaneous lupus erythematosus is related to different genes.31 A major environmental factor triggering cutaneous lupus erythematosus is UV light. Other triggering factors include medications, hormones, stress, viruses and skin trauma.31 The initiation of the autoimmune reaction cascade has been attributed to UV-induced keratinocyte apoptosis (programmed cell death).32 Complex mechanisms involving apoptosis, autoantibodies, T cells, B cells and vascular changes induce the lesions of cutaneous lupus erythematosus.31
Rashes vary with the type of cutaneous lupus erythematosus. In acute cutaneous lupus erythematosus there is an erythematous rash on both cheeks and across the nose in a butterfly pattern. Subacute cutaneous lupus erythematosus has a maculopapular rash that may occur on any part of the body. The lesions of chronic cutaneous lupus erythematosus are usually located on areas of the skin exposed to light. They are erythematous, raised lesions with scaling. As the lesion progresses the centre becomes hypopigmented and the border hyperpigmented. The lesions spread and may merge. As the lesions resolve, scarring may occur.
The management of cutaneous lupus erythematosus includes protection from sunlight and avoidance of photosensitising drugs. Lesions may be treated with topical corticosteroids. For patients with widespread lesions anti-malarial drugs are used. Treatment of severe cutaneous lupus erythematosus includes immunosuppressive drugs, retinoids and thalidomide.
Papulosquamous disorders
Papulosquamous disorders are a group of skin disorders that present with scaly papules and plaques. Psoriasis is a common papulosquamous disorder; it is a chronic immune-mediated inflammatory disorder of the skin characterised by hyperproliferation of keratinocytes, infiltration by T lymphocytes and vascular changes in the dermal layer. There is hyperplasia (increase in cell numbers) of the epidermal spinosum layer and incomplete differentiation of the granular and cornified layers. It affects both males and females and commonly presents by the age of 20 years.
The aetiology consists of both genetic and environmental changes. At least 10 genes have been found to be associated with psoriasis, three of which are involved in the interleukin pathway.33 Environmental factors that can trigger the condition include stress, alcohol, trauma, infections and some medications such as ACE inhibitors and β-adrenergic receptor blocking drugs.
T lymphocytes and the cytokines that they release are involved in the pathogenesis of psoriasis. Antigens are presented to the T lymphocyte receptors to begin activation of the T lymphocytes. The activated T lymphocytes migrate into the skin and when they encounter the initiating antigen they release cytokines. TNF-α, interleukin-23 and interferon are considered key cytokines in causing keratinocyte activation, hyperproliferation and abnormal growth of dermal blood vessels.34
Psoriasis is characterised by increased epidermal cell turnover, with the time for epidermal shedding reduced to 3 days. Increased cell proliferation causes the epidermis to thicken and plaque to form. As a result of the loosely cohesive keratin the lesions have a silvery appearance. The blood vessels in the dermal papillae become tortuous and dilated with increased permeability, causing erythema. The scales are loosely adherent and when removed minute bleeding points are revealed.
There are several forms of psoriasis. Plaque-type psoriasis (psoriasis vulgaris) is the most common type. In this form, lesions are red plaque with silver scale. The lesions have well-defined edges and vary in size and shape. The lesions most commonly occur on the knees, elbows and scalp (see Figure 19-13).
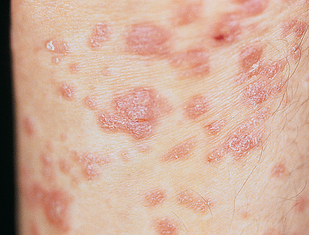
Lesions have well-defined edges and silver scale, and vary in size and shape.
Source: Courtesy of Department of Dermatology, School of Medicine, University of Utah.
Guttate psoriasis is characterised by small pink papules occurring on the trunk and limbs. This form of psoriasis usually occurs a few weeks after a streptococcal respiratory infection and may resolve spontaneously in weeks or months, but may return with recurrent streptococcal infections.
In milder forms of psoriasis topical treatments alone can be used. Creams or ointments that contain vitamin D and corticosteroids have been found to be the most effective in treating psoriasis.35 Corticosteroids inhibit epidermal proliferation, enhance normal differentiation and inhibit inflammation. Vitamin D inhibits keratinocyte proliferation and enhances differentiation. Sun exposure may also be beneficial to supplement topical treatment. Moderate-to-severe psoriasis can be treated with topical therapy in combination with oral systemic agents or UV light. Systemic agents include immunosuppressants such as methotrexate and cyclosporin. Biological therapies (bioactive substances that act at the cellular level) are also used to treat moderate-to-severe psoriasis or psoriatic arthritis. These drugs target specific immune responses associated with psoriasis. Biological therapies currently used for the treatment of psoriasis are alefacept (blocks T cell activation), etanercept (binds to TNF-α thus lowering the amount available), adalimumab (binds to and blocks the action of TNF-α) and infliximab (blocks the effects of TNF-α).36
INFECTIONS OF THE INTEGUMENTARY SYSTEM
Infections of the integument may present alone or as a complication of underlying conditions such as psoriasis or atopic dermatitis. Integumentary infections may be caused by bacteria, viruses, fungi or parasites. Although most infections occur superficially, systemic signs and symptoms may develop. Continuity of epidermal keratinocytes, normal skin flora, sebum and immune responses often provides protection against pathogens that cause skin infections.
There is a high prevalence of skin infections in the Indigenous population in Australia, particularly those living in remote communities. The most common skin infections affecting Indigenous population are scabies and streptococcal pyoderma. In some remote communities up to 50% of children may be infected with scabies.37 Although skin infections do not usually directly cause death, they are a cause of serious complications such as acute post-streptococcal glomerulonephritis and acute rheumatic fever. The rates of hospitalisation for infectious skin disease are twice as high in the Indigenous population as in the non-Indigenous population.38 The high incidence of infectious skin diseases in the Indigenous population has been attributed to overcrowding and poor housing, inadequate water supply, heat and humidity, poor education and poor hygiene.37,39
Bacterial infections
When pathogenic bacteria invade the skin, superficial or systemic infections may develop.
Folliculitis
Folliculitis is an inflammation of the hair follicle, occurring most prominently on the scalp and extremities. It usually occurs in children and adults with a predisposing factor that increases the number of bacteria on the skin surface. The most common bacterium causing folliculitis is Staphylococcus aureus. Bacteria invade the follicle and release chemotactic factors and enzymes that cause inflammation. The superficial lesions consist of small pustules with a surrounding area of erythema. The pustules develop in clusters and form crusts. Topical antibiotics are used to treat the condition. Although the pustules usually heal in a few days, they may develop into furuncles.
Furuncles and carbuncles
Furuncles (or boils) result from the spread of bacterial infection through the follicular wall into the surrounding dermis. The most common infecting organism is Staphylococcus aureus. Furuncles most commonly affect young adult males and are usually located on the face, back of the neck, chest, axillae, buttocks and thighs. The lesions are small, painful red nodules that become pustular and develop central necrosis (see Figure 19-14). Scarring may occur following discharge of necrotic tissue and pus.
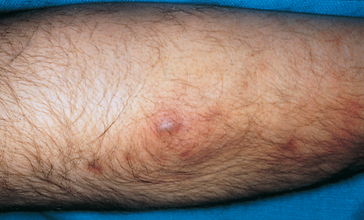
The lesion is a red pustular nodule.
Source: Christensen BL, Kockrow EO. Adult health nursing. 6th edn. St Louis: Mosby; 2011.
Carbuncles are collections of infected hair follicles that most commonly occur on the back of the neck, shoulders or thighs. A carbuncle extends into the lower dermis and subcutaneous tissue and forms an erythematous, painful swollen nodule that drains pus through multiple openings of the skin. Fever and malaise often occur during lesion development. Abscesses may form which require incision and drainage. Recurrent infections are treated with antibiotics such as beta lactams.
Cellulitis
Cellulitis is a spreading infection of the dermis and subcutaneous tissues. It is usually caused by streptococci and Staphylococcus aureus. It often occurs on the lower limbs and is most commonly seen in the elderly. People who have leg ulcers or wounds and those with venous disease are at risk of developing cellulitis. Cellulitis usually affects one leg only. The infected area is red, hot, swollen and painful. There may be demarcation between the affected skin and normal skin. A lesion that has allowed entry of the bacteria is often present. Systemic manifestations such as fever, malaise and vomiting may also be present. Systemic antibiotics, such as flucloxacillin, are used to treat the disorder. In milder conditions antibiotics are administered orally and in more severe cases they are given intravenously. Further treatment options use a combination of high stretch bandaging to aid lymphatic drainage and oedema control, and an intravenous antibiotic regimen that can be managed as an outpatient.40
Impetigo
Impetigo is a contagious superficial skin infection that occurs mainly in children but may occur at any age. It frequently occurs in childcare centres and schools and hence is referred to as ‘school sores’. The main causative organisms are Staphylococcus aureus, Streptococcus pyogenes and group A beta-haemolytic streptococci. The organisms enter through damaged skin and are transmitted through direct contact. The incubation period is 1–3 days for Streptococcus pyogenes and 4–10 days for Staphylococcus aureus.
Clinical manifestations usually appear 4–10 days after infection. The infection begins with small blisters that become pustular and burst quickly leaving a yellow crust. The skin underneath is red and inflamed (see Figure 19-15). If left untreated the lesions can develop into skin ulcers that penetrate the dermis, called ecthyma. These lesions are painful and can leave scars.
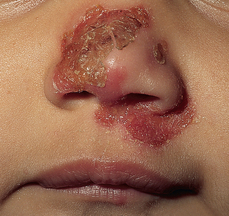
Lesions consist of pustules that burst, leaving a yellow crust.
Source: Hockenbury MJ. Wong’s essentials of pediatric nursing. 8th edn. St Louis: Mosby; 2009.
Management of impetigo involves removal of crusts using soap and water, aluminium acetate or potassium permanganate solution. Antibiotic treatment consists of penicillin where Streptococcus pyogenes is the infecting pathogen and mupirocin ointment where Staphylococcus aureus is the infecting pathogen. For severe or longstanding infections, flucloxacillin, cephalexin or roxithromycin can be used. Most cases of impetigo are no longer infectious after 24 hours of appropriate antibiotic therapy. Sores on exposed surfaces must be covered with a watertight dressing and children with impetigo are excluded from school or childcare until antibiotic treatment has commenced.41
Staphylococcal scalded skin syndrome
Staphylococcal scalded skin syndrome is a toxin-mediated condition caused by Staphylococcus aureus that causes blistering and desquamation of the skin. It occurs mainly in children under five who have not yet developed antibodies against the staphylococcal toxins or in adults who are immunosuppressed. Neonates have the highest risk because of their lack of immunity, not having prior exposure to the toxin.
The strains of Staphylococcus aureus that cause staphylococcal syndrome produce toxins that cause epidermolysis (destruction of the epidermis) by interfering with the cell-to-cell adhesion of keratinocytes.42 As a result there is separation of the skin just below the granular layer of the epidermis.
Clinical manifestations begin with fever and malaise often associated with a respiratory tract infection. Discrete erythematous areas develop that spread from the face and trunk to cover the entire body except the palms, soles and mucous membranes. Large fragile bullae form in the erythematous area and the pain is severe. The bullae rupture causing large areas of epidermis to slough off. Loss of skin leads to hypothermia, fluid loss causing dehydration and secondary infection.
Before treatment is commenced, culture and histology are undertaken to differentiate staphylococcal scalded skin syndrome from toxic epidermal necrolysis. Once confirmed, the infection is treated with oral or intravenous antibiotics. The aseptic technique should be used for dressings. Regular analgesia is needed for pain relief. Healing usually occurs in 10–14 days without scarring.
Paronychia
Paronychia is an infection of the nail fold. Acute paronychia is usually caused by the bacteria staphylococci or streptococci. Damage to the nail fold allows bacteria to enter, causing a painful inflammation. Pus may accumulate in the nail fold and under the nail, forming an abscess that may need to be incised and drained.
Chronic paronychia develops slowly and is most commonly caused by Candida. It often follows damage to the cuticle by water and detergents. The space between the nail fold and nail plate opens, allowing a warm, moist environment for growth of the microorganism. The skin around the nail becomes red, swollen and painful. Pus may be expressed from the nail fold. Although the nail plate is usually not infected, it may become discoloured. Treatment involves keeping the hands dry.
Viral infections
Herpes simplex virus
There are two types of herpes simplex virus (HSV): type 1 and type 2. HSV-1 generally infects the oral and respiratory mucosa and HSV-2 the genitalia, although infections can occur anywhere on the skin. HSV-1 is transmitted by respiratory droplets or direct contact with infected saliva, whereas HSV-2 is spread by sexual contact. After entering the mucous membranes or abraded skin, the virus enters the epithelial cells and replicates within them. The virus moves along sensory nerve pathways to the dorsal root ganglia where latent infection is established. The primary infection may be relatively severe with systemic manifestations such as fever and malaise. Antibodies are produced so that recurrent infections are less severe.
A burning, tingling or stinging sensation at the site of the lesion often precedes recurrent infection with HSV-1. This is followed by the appearance of small, inflamed painful vesicles that ulcerate and crust (see Figure 19-16). The lesions most commonly occur on the lips, in the mouth and around the nose. UV exposure, stress, fatigue or skin irritation may trigger reactivation of the virus. Treatment is symptomatic and the lesions usually resolve within 2 weeks. Over-the-counter topical aciclovir (an antiviral agent) creams can be used, which may reduce the duration of the infection.
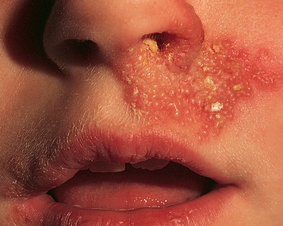
Lesions consist of vesicles that ulcerate and crust.
Source: Christensen BL, Kockrow EO. Adult health nursing. 6th edn. St Louis: Mosby; 2011.
Prodromal manifestations (symptoms that occur early before specific symptoms appear) such as pain, tingling or itching may also precede reactivation of HSV-2. In females vesicles occur on the vulva, perianal skin, vagina and cervix. In the male the vesicles occur on the penile shaft, prepuce and glans and the perianal area. Between 24 and 72 hours after appearing the vesicles rupture forming painful, weeping ulcers. Regional lymph nodes may enlarge. Antiviral medications including aciclovir and famciclovir may reduce the severity and duration of symptoms.
Herpes zoster and varicella
Herpes zoster and varicella are both caused by the varicella-zoster virus. Varicella (chickenpox) occurs as the primary infection. Following the infection the virus remains dormant in trigeminal and dorsal root ganglia and may be reactivated many years later to cause herpes zoster (commonly referred to as shingles).
Varicella occurs most commonly in children, with the peak incidence being in 5–9-year-olds. In adults the infection tends to be more severe. Chickenpox is highly contagious and is transmitted by direct contact with fluid from the vesicles or airborne respiratory droplets. The person is infectious from up to 4 days prior to the appearance of the rash until 5–6 days after the appearance of the rash. The rash can be preceded by systemic manifestations such as fever, headache, sore throat and malaise. The rash begins as maculopapular lesions (containing both macules and papules) that become vesicles. The vesicles progress to pustules that crust and are very itchy. Lesions are most numerous on the trunk and less so on the face, scalp and limbs. It is recommended in Australia that children be immunised against chickenpox when they are 18 months old or when they are between 10 and 14 years old if they have not had chickenpox or been immunised against it (see Chapter 14 for immunisation schedules).
Herpes zoster results from reactivation of the varicella virus that entered the cutaneous nerves from an episode of chickenpox. Although it can affect anyone who has had chickenpox it is more prevalent in older people whose immune responses have declined with age. Trigger factors include stress and illness. Individuals with shingles are potentially contagious to people who have not had chickenpox and it is possible to develop chickenpox from contact with shingles. The rash usually occurs on the skin of a single dermatome (an area of skin innervated by a single nerve), but one or two adjacent dermatomes may also be involved. Thoracic dermatomes are most commonly affected, but cervical and lumbosacral dermatomes may also be affected. If the ophthalmic branch of the trigeminal nerve is infected, severe and permanent eye damage can occur.42
The first sign of shingles is tingling, itching or pain in the area of the affected nerve. A rash of vesicles with an erythematous base occurs, following the line of the affected nerve (see Figure 19-17). New lesions continue to form over 3–5 days, with pustulation over 4–6 days and scabbing over 7–10 days.43 In 2–4 weeks healing occurs and the crusts fall off. However, pain termed postherpetic neuralgia can persist for 3–6 months.
A rash of vesicles on the skin of a thoracic dermatome of the affected nerve.
Source: Christensen BL, Kockrow EO. Adult health nursing. 6th edn. St Louis: Mosby; 2011.
Treatment includes antiviral medications such as famciclovir or aciclovir. If medications are started within 72 hours of the appearance of the rash, pain can be reduced and healing accelerated. Topical creams and lotions such as aluminium acetate lotion can be used to help relieve the discomfort. Analgesics such as paracetamol should be given for pain relief. In cases of severe pain or postherpetic pain, more potent analgesics may be required. There is a vaccine, Zostavax®, available for people over 50 years old. However, although this vaccine will decrease the incidence of herpes zoster, its efficacy is only 50% and its duration of protection beyond 4 years is currently not known.44
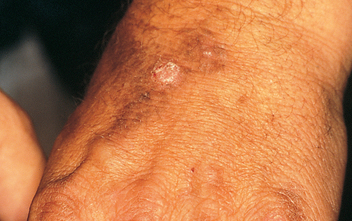
Warts
Warts (verrucae) are benign epidermal lesions caused by the human papillomavirus. Warts most commonly occur in children and are transmitted by direct contact. Different types of warts are caused by different types of the human papillomavirus. Warts vary in size, shape and location depending on the type of wart. Common warts (verruca vulgaris) occur most commonly on the hands and are raised lesions with rough surfaces. Flat warts (verruca plana) occur most commonly on the face, hands and lower legs, and are flat smooth lesions. Plantar warts occur on the soles of the feet. Most wart infections resolve without medical intervention. They may be treated with topical agents such as salicylic acid or by cryotherapy.
Genital warts (condylomata acuminata) are a sign of genital human papillomavirus infection and are one of the most commonly sexually transmitted infections. More than 40 types of papillomaviruses can infect the anogenital tract of men and women causing genital warts. The warts are soft, raised lesions that may appear in clusters with a cauliflower-like appearance. They occur on the external genitalia including the penis, scrotum, vulva, perineum and perianal area. They may also appear in the vagina, on the cervix or in the anus. Treatment involves removal of the warts by cryotherapy or topical preparations such as imiquimod cream or podophyllotoxin solution. Human papillomavirus vaccine (Gardasil®) prevents cervical cancer, precancerous lesions and genital warts due to the human papillomavirus.
Molluscum contagiosum
Molluscum contagiosum is a contagious infection of the skin caused by the poxvirus. It occurs most commonly in children and is transmitted by direct skin-to-skin contact or indirect contact with contaminated towels, bedclothes or clothing. After attaching to the surface of the epithelial cells the viral DNA is engulfed by the host cell and becomes incorporated into the host cell DNA. The virus uses the host cell DNA to replicate itself. When the host cell dies it ruptures, releasing the virus particles, which infect new epidermal cells. The infected cells form pearly, umbilicated dome-shaped papules 1–5 mm in diameter. The central plug, consisting of dead epithelial cells and virus particles, is highly contagious. The lesions are mainly found on the trunk, face and extremities. The individual lesions form a crust after 6–12 weeks and gradually disappear leaving a small pitted scar.45 All lesions usually disappear in 6–9 months. No specific treatment has been developed. Imiqimod cream can be used to treat the lesions.
Fungal infections
Fungal infections of the skin and nails are caused by dermatophytes, a group of fungi that invade keratin. The three most common species infecting the skin are the Epidermophyton, Microsporum and Trchophyton genera. When caused by dermatophytes the superficial fungal or mycotic infections of the skin are termed tinea or dermaphytosis.
Tinea infections
Tinea is a superficial skin infection caused by dermatophytes. The infection may be transmitted from person to person, animal to person or soil to person. The fungi infect the dead, keratinised cells of the epidermis. They emit enzymes that digest the keratin causing scaling and hair breakage. They may also produce an immune response. Tinea infections are classified according to the body region infected:
 Tinea corporis (ringworm of the body) in Australia is caused mainly by the Microsporum species. The lesions are flat, red and circular. They may be either dry and scaly or moist and crusted (see Figure 19-18).
Tinea corporis (ringworm of the body) in Australia is caused mainly by the Microsporum species. The lesions are flat, red and circular. They may be either dry and scaly or moist and crusted (see Figure 19-18). Tinea capitis (scalp) occurs most frequently in children. The lesions are small papules that spread leaving scaly patches of temporary baldness. The infected hairs become brittle and break easily.
Tinea capitis (scalp) occurs most frequently in children. The lesions are small papules that spread leaving scaly patches of temporary baldness. The infected hairs become brittle and break easily. Tinea pedis (‘athlete’s foot’) occurs in both adults and children. It is often spread by using communal showers. The lesions are found between the toes and plantar surfaces where the skin is scaly, itchy and painful with fissures or blisters filled with a watery fluid.
Tinea pedis (‘athlete’s foot’) occurs in both adults and children. It is often spread by using communal showers. The lesions are found between the toes and plantar surfaces where the skin is scaly, itchy and painful with fissures or blisters filled with a watery fluid. Tinea manus (hand) occurs on the palms and finger webs. The lesions are dry, scaly, erythematous lesions or moist vesicles.
Tinea manus (hand) occurs on the palms and finger webs. The lesions are dry, scaly, erythematous lesions or moist vesicles. Tinea cruris (groin) is an infection of the groin and pubic area. The lesions are small, erythematous scaling vesicular patches with well-defined borders.
Tinea cruris (groin) is an infection of the groin and pubic area. The lesions are small, erythematous scaling vesicular patches with well-defined borders. Tinea unguium (onchomycosis) is a fungal infection of the nails. It affects both the toenails and fingernails. It is characterised by nail plate separation from the nail bed and yellow-brown accumulations of brittle keratin over the nail (see Figure 19-19).
Tinea unguium (onchomycosis) is a fungal infection of the nails. It affects both the toenails and fingernails. It is characterised by nail plate separation from the nail bed and yellow-brown accumulations of brittle keratin over the nail (see Figure 19-19).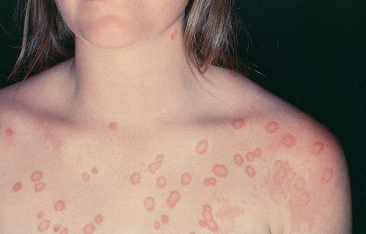
The lesions are flat, red and circular.
Source: Callen PP et al. Color atlas of dermatology. Philadelphia: Saunders; 1993.
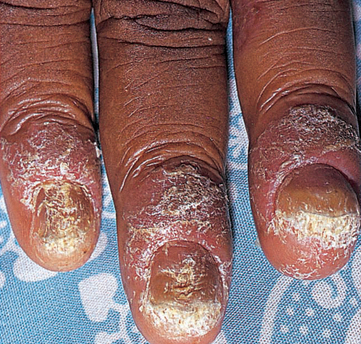
A fungal infection of the nails. The nail plate has separated from the nail bed and there are accumulations of yellow-brown keratin over the nail.
Source: Talley NJ, O’Connor S. Clinical examination: a systematic guide to physical diagnosis. 6th edn. Sydney: Elsevier; 2010.
Diagnosis consists of microscopy and culture of skin scrapings, or observation of the skin with UV light — the spores and filaments (hyphae) of fungi fluoresce blue-green when exposed to ultraviolet light. Treatment depends on the type of fungi causing the infection. Most fungal skin infections are treated with topical antifungal drugs such as clotrimazole (Canesten®), ketoconazole (Nizoral®) and miconazole (Daktarin®). Topical antifungal therapy may be used for chronic fungal infections or infections of the nails. For resistant tinea capitis infection griseofulvin is the treatment of choice and for tinea unguium oral terbinafine is given.
Candidiasis
Candidiasis infections are caused by the yeast-like fungus, Candida albicans. This fungus inhabits the skin and mucous membranes as part of the normal flora. However, an opportunistic infection caused by Candida albicans can occur when an individual has suppressed immune function or depleted numbers of bacteria that normally reside on the skin and keep the candidal organisms in check. Factors that predispose to infection are occluded moist skin surfaces that rub together, systemic administration of antibiotics, pregnancy, diabetes mellitus, Cushing’s syndrome, debilitated states, immunosuppression and infants younger than 6 months of age because of decreased immune activity. Candidiasis occurs in skin folds, the mouth, vagina and penis:
 Skin candidiasis is most common in infants, debilitated adults and obese patients. It occurs when occluded moist areas of skin rub together and become macerated.46 Common sites include under the breasts, the axillae, groin and webs of fingers and toes. The rash appears red, inflamed and pustular and sometimes has a white exudate. The area is painful.
Skin candidiasis is most common in infants, debilitated adults and obese patients. It occurs when occluded moist areas of skin rub together and become macerated.46 Common sites include under the breasts, the axillae, groin and webs of fingers and toes. The rash appears red, inflamed and pustular and sometimes has a white exudate. The area is painful. Oral candidiasis appears as white patches on the tongue and oral mucosa. Under the white plaque the tongue is red, swollen and painful.
Oral candidiasis appears as white patches on the tongue and oral mucosa. Under the white plaque the tongue is red, swollen and painful. Vaginal candidiasis appears as red, swollen vaginal and labial membranes with a non-offensive whitish vaginal discharge. It may be associated with itching and pain. In more severe cases the rash can extend to the perianal and groin areas.
Vaginal candidiasis appears as red, swollen vaginal and labial membranes with a non-offensive whitish vaginal discharge. It may be associated with itching and pain. In more severe cases the rash can extend to the perianal and groin areas.Treatment of candidiasis depends on the site and severity of the infection. Candidiasis of the skin is treated with antifungal creams. Oral candidiasis is treated with oral gels, suspensions or lozenges such as nystatin, clotrimazole or fluconazole. To treat vaginal candidiasis antifungal pessaries are available.
Parasitic infestations
Scabies
Scabies is a contagious parasitic infestation caused by the microscopic mite Sarcoptes scabiei. It is transmitted either by direct skin-to-skin contact or indirectly from contaminated items such as bedclothes and towels. Scabies often spreads in conditions where there is close contact between people such as childcare centres, schools and aged-care facilities. Other high-risk groups include Indigenous communities in Australia and New Zealand.
The female mite burrows into the stratum corneum and lays 2–3 eggs per day. The eggs hatch into larvae and travel back up to the surface of the skin. The signs and symptoms of scabies are the result of an adaptive immune response.47 The predominant manifestation is intense itching. It is more severe at night and occurs over most of the body. Pruritis is the result of a hypersensitive reaction to components of saliva, eggs and faecal material of the mites. The diagnostic sign of scabies is the burrow, the track made by the female. The burrows occur mainly between the fingers, wrists, elbows, axillae, lower abdomen, penis, breasts and shoulder blades and appear as short, grey, wavy lines. The scabies rash consists of papules, vesicles and erythema (see Figure 19-20). Scratching may lead to secondary infections.
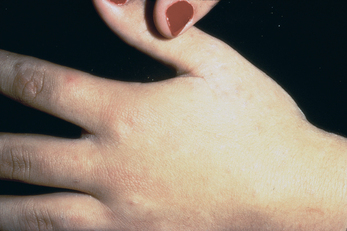
The burrows of the scabies mite between the fingers.
Source: Talley NJ, O’Connor S. Clinical examination: a systematic guide to physical diagnosis. 6th edn. Sydney: Elsevier; 2010.
Crusted scabies (‘Norwegian scabies’) is a very contagious variant of scabies in which there is hyperinfestation with thousands of mites present in exfoliating scales. This form of scabies often occurs in immunocompromised people, institutionalised elderly people and Aboriginal Australians. It appears to occur when the immune system is unable to adequately attack the mites via the T helper-2 cell response resulting in very large numbers of mites.48 It presents as a scaly, erythematous rash with crusts and hyperkeratinisation, making it difficult to differentiate from psoriasis or eczema.
Treatment includes topical permethrin preparations (e.g. Lyclear® cream or Quellada® lotion) or benzyl benzoate 25% preparations (e.g. Ascabiol® or Benzemul® 25%). For moderate to severe infections the treatment may be repeated 14 days after the first treatment. Prior to treatment bed linen, towels and clothing should be machine washed in hot water as the mite can survive on these items.
Pediculosis
Pediculosis is an infestation of blood-sucking lice. The lice have three pairs of legs with powerful claws. The female louse lays up to 300 eggs, called nits, during her lifetime. The nits hatch in 7–10 days giving rise to nymphs that become adults in 10 days. Three species of lice infest humans: Pediculus humanus capitis (head lice), Pediculus humanus corporis (body lice) and Phthirus pubis (pubic lice).
Head lice are the most common type of louse. They occur most commonly in schoolchildren. Transmission is through direct head-to-head contact with a person with head lice. Head lice grip the shaft of the hair with their claws. The female lays eggs, usually at night, at the base of the hair shaft. The eggs adhere to the hair shaft and hatch about 8 days later (see Figure 19-21). Itching is the primary manifestation and is caused by a reaction to louse saliva. Scratching can cause crusted lesions to form. Lice, which are about 3 mm in length, can be identified on the hair. Unhatched eggs are dark-coloured and are found close to the scalp. Hatched eggs are white and are found further away from the scalp and are therefore easier to see. Head infestation is diagnosed by the presence of lice or viable eggs. Treatment consists of physical methods using a fine-toothed comb to remove nits and lice and application of topical antiparasitics. A number of antiparasitic agents such as permethrin (e.g. Quellada® head lice treatment) and benzyl benzoate may be applied topically. Two applications are required.

FIGURE 19-21 Eggs of the pediculosis adhering to the hair shaft.
Source: Christensen BL, Kockrow EO. Adult health nursing. 6th edn. St Louis: Mosby; 2011.
Pubic lice are oval and white to grey in colour. Transmission is by direct contact. Heavy infestation can also involve axillary hair, chest hair, facial hair, eyebrows and eyelashes. The most common manifestation is pruritis. Lice and nits are visible. Treatment involves application of a topical antiparasitic agent and washing all bed linen, towels and clothing in hot water. Sexual partners should also be treated.
Body lice range in size from 2 mm to 4 mm and live in clothing, coming onto the skin to feed. Body lice tend to be associated with poverty and poor hygiene. Treatment consists of topical antiparasitic agents and washing clothes in hot water.
Ticks
Ticks are bloodsucking external parasites that cause dermatological disease both directly by their bite and indirectly as vectors of bacterial, rickettsial, protozoal and viral diseases. There are two main families of ticks: soft ticks with a wrinkled, soft appearance; and hard ticks with a hard dorsal plate. In Australia, there are approximately 75 species of tick, with the most medically important one being the paralysis tick, Ixodes holocylus. This tick has four stages of development in its life cycle: egg, larva, nymph and adult. Before dying, the female deposits a large number of eggs in moist leaf litter. The larvae hatch after 40–60 days. The larvae, nymphs and adults all need to feed on blood to survive, with the hosts being either animal or human. Ticks use their mouthparts to penetrate the skin and attach to the host. They break through the blood vessels of the dermis to obtain blood, increasing their body weight by more than one hundred times. From their saliva they secrete a number of substances including anticoagulants and prostaglandins that modulate the flow of blood and suppress the immune response of the host.49
The manifestations of tick bite are caused by the physical trauma to the skin, salivary secretions, toxins and excretions.49 The lesions are itchy, painful, erythematous macules, papules or nodules. Granuloma, lichenification and secondary infections may occur. Allergic reactions are the most serious condition associated with ticks.50 They are caused by allergens secreted by the tick. The allergic reaction can vary from localised itching and swelling to severe widespread reactions including anaphylaxis. Tick paralysis in humans is rare but is most likely to occur in children. It is caused by neurotoxins secreted by the tick. Clinical manifestations include unsteady gait, weakness of the limbs, rashes, flu-like symptoms and ascending flaccid paralysis. Even after removing the tick, the condition may continue to deteriorate for a time and recovery is slow.51 Ticks can transmit infectious diseases through salivary secretions. The main tick-borne infectious disease in Australia is spotted fever caused by bacteria belonging to the Rickettsia family.
Although treatment involves removing the tick as soon as possible, evidence is lacking in relation to tick removal.49 The whole tick should be removed using fine-tipped forceps, firmly gripping the mouth part and lifting gently to detach the tick. The body should not be squeezed. In people with a history of allergic reactions ticks should be removed by a doctor and where resuscitation facilities are available.52
TRAUMATIC CONDITIONS OF THE INTEGUMENTARY SYSTEM
Pressure ulcers
A pressure ulcer is an area of localised injury to the skin and/or underlying tissue, usually over a bony prominence, as a result of pressure or pressure in combination with shear and/or friction.56 Most pressure ulcers occur over bony prominences such as the sacrum, heels and ischial tuberosities. A number of factors including immobility, poor nutritional status, incontinence and debilitation increase the risk of developing a pressure ulcer.
PATHOPHYSIOLOGY
A growing body of evidence suggests that most pressure ulcers are the result of deep tissue injury.53 Muscle is less resistant than skin to pressure changes and therefore may necrose prior to skin breakdown. The most significant extrinsic factors causing pressure ulcers are the duration and amount of pressure applied to skin and soft tissues over bony prominences. Pressure is defined as the force (patient’s body weight) exerted on a unit of area (skin contact area). When applied over a bony prominence the pressure will compress all the tissues lying between the skin and underlying skeleton. As a result blood vessels will be occluded. If pressure is relieved within a few hours there is a brief period of reactive hyperaemia (redness) and blood recirculates to the area, delivering oxygen and nutrients and thus preventing tissue damage. However, sustained pressure leads to decreased capillary blood flow, occlusion of blood vessels and tissue ischaemia. Capillary pressure over 32 mmHg (normal range 12–32 mmHg) compromises oxygenation and microcirculation,54 leading to tissue necrosis and ulceration.
Another factor implicated in the pathogenesis of pressure ulcers is shear. Shear refers to the mechanical force acting in parallel to a plane. This can occur when a patient slides down a chair or bed. As the patient slides, shear force is created by the motion of the deep fascia and skeletal muscle relative to the skin, which is restrained from moving as a result of frictional forces. The resulting distortion of capillaries and soft tissue causes ischaemia. Friction opposes the movement of one surface against another such as the skin against a bed sheet. Frictional forces may lead to superficial skin erosions that initiate pressure ulceration.
A moist environment such as that caused by incontinence can increase the effects of pressure, shear and friction.
CLINICAL MANIFESTATIONS
The clinical manifestations of pressure ulcers result from tissue ischaemia and the inflammatory response. However, there is still a lack of knowledge about the complex biochemical, cellular and genetic changes that occur.55 Ischaemia leads to necrosis and ulcer formation. Pressure ulcers range from discoloured areas of skin to large necrotic areas of tissue involving muscle, tendon and bone. Pressure ulcers are generally classified by four stages, as defined by the European Pressure Ulcer Advisory Panel (EPUAP) and National Pressure Ulcer Advisory Panel (NPUAP; see Table 19-2).56
Table 19-2 EPUAP/NPUAP PRESSURE ULCER CLASSIFICATION SYSTEM
| Category I | Non-blanchable erythema | Intact skin with non-blanchable redness of a localised area, usually over a bony prominence. Darkly pigmented skin may not have visible blanching; its colour may differ from the surrounding area. The area may be painful, firm, soft, warmer or cooler as compared to adjacent tissue. Category 1 may be difficult to detect in individuals with dark skin tones. May indicate at-risk persons. |
| Category II | Partial thickness | Partial thickness loss of dermis presenting as a shallow open ulcer with a red pink wound bed, without slough. May also present as an intact or open/ruptured serum-filled or sero-sanguinous filled blister. Presents as a shiny or dry shallow ulcer without slough or bruising. |
| Category III | Full-thickness skin loss | Full-thickness tissue loss. Subcutaneous fat may be visible but bone, tendon or muscle is not exposed. Slough may be present but does not obscure the depth of tissue loss. May include undermining and tunnelling. The depth of a category III pressure ulcer varies by anatomical location. The bridge of the nose, ear, occiput and malleolus do not have (adipose) subcutaneous tissue and category III ulcers can be shallow. In contrast, areas of significant adiposity can develop extremely deep category III pressure ulcers. |
| Category IV | Full-thickness tissue loss | Full-thickness tissue loss with exposed bone, tendon or muscle. Slough or eschar may be present. Often includes undermining and tunnelling. The depth of a category IV pressure ulcer varies by anatomical location. The bridge of the nose, ear, occiput and malleolus do not have (adipose) subcutaneous tissue and these ulcers may be shallow. Category IV ulcers can extend into muscle and/or supporting structures (e.g. fascia, tendon or joint capsule) making osteomyelitis or osteitis likely to occur. Exposed bone/muscle is visible or directly palpable. |
Source: European Pressure Ulcer Advisory Panel and National Pressure Ulcer Advisory Panel. Prevention and treatment of pressure ulcers: quick reference guide. Washington, DC: National Pressure Ulcer Advisory Panel; 2009.
The inflammatory response causes hyperaemia, pain, fever and leucocytosis (see Chapter 13). If the ulceration is large, toxicity and pain lead to loss of appetite, debility and renal insufficiency. Proteolytic enzymes from bacteria that colonise the dead tissue and from the macrophages dissolve the necrotic tissue, causing a foul-smelling discharge. Infection and inflammation of surrounding tissue may develop, particularly if the individual is immunosuppressed or has diabetes mellitus.
EVALUATION AND TREATMENT
An estimated 95,695 pressure ulcers develop annually in Australia,57 which adds significantly to the cost of healthcare. The first step in preventing pressure ulcers is to identify patients at risk. A number of risk assessment scales such as the Norton scale, Braden scale and Waterloo scale have been developed to determine a patient’s potential for pressure ulcer development. The Braden scale was found to have the best reliability and is the most widely used.58 Recently the Glamorgan scale has been developed to identify paediatric patients at risk of developing pressure ulcers.59 One of the most effective methods of preventing pressure ulcers is repositioning to reduce the duration of pressure on an area of the body. Support surfaces that redistribute pressure or relieve pressure such as alternating pressure mattresses are also used to reduce the amount or duration of pressure between the individual and the support surface.
When treating a pressure ulcer a moist environment should be maintained on the wound bed. Dressings that create an optimal healing environment should be used — such as hydrocolloids, hydrogels, hydrofibres, foams, films, alginates and soft silicones.60 Surgical management consists of debridement and is generally reserved to treat category III/IV pressure ulcers. After debridement the wound may either be left open to heal or repaired using skin flaps or grafting.
Skin tears
Skin tears are wounds caused as a result of shearing, friction or blunt trauma to the skin. Payne and Martin have defined a skin tear as ‘a traumatic wound occurring principally on the extremities of older adults, as a result of friction alone or shearing and friction forces, which separate the epidermis from the dermis (partial thickness wound) or which separate both the epidermis and dermis from underlying structures (full thickness wound)’.61
Skin tears occur most frequently in the elderly. The ageing process causes epidermal thinning, loss of dermal and subcutaneous tissue, flattening of the dermal–epidermal ridge, decreased collagen and elastin, decreased number of sweat glands and reduced sebum production. These changes cause the skin to be fragile and dry, making it more prone to tear when subjected to trauma, shearing or frictional forces. Risk factors associated with skin tears include impaired mobility, dependence for activities of daily living, a history of previous skin tears, cognitive deficit, poor nutritional status, fluid volume deficit, decreased sensation, ecchymosis or senile purpura, and corticosteroid therapy.
The reduced cohesion between the epidermis and dermis resulting from the flattening of the rete ridges and the dermis and subcutaneous tissue is a major contributing factor in the pathogenesis of skin tears. Loss of cohesion enables skin layers to slide across each other, breaking blood vessels when subjected to tractional forces. This results in a traumatic wound that usually bleeds and is painful.
Skin tears can present from a simple linear laceration to extensive tissue loss and necrosis. The most widely used classification of skin grades the wound on a scale of I to III (see Table 19-3). Although other classification systems have been devised,62,63 they generally have not been widely accepted.
Table 19-3 THE PAYNE-MARTIN CLASSIFICATION FOR SKIN TEARS
| Linear type (full-thickness) | Epidermis and dermis are pulled in one layer from supporting structures. The wound is incision-like in appearance. | |
| Flap type (partial thickness) | Epidermis and dermis are separated. Flap can be completely approximated or approximated to expose no more than 1 mm of the dermis. | |
| Scant tissue loss type | 25% or less of the epidermis flap is lost. | |
| Moderate-to-large tissue loss type | More than 25% of the epidermis flap is lost. | |
| Complete tissue loss | The epidermal flap is absent. |
A number of strategies are used to prevent the occurrence of skin tears. These include careful handling of at-risk patients, padding leg supports on wheelchairs and bed rails, providing a safe environment to prevent trauma, protecting fragile skin by wearing long sleeves and pants, keeping the skin well-hydrated, avoiding the use of soaps and using emollients, keeping the skin clean and free of urine and faeces, and not placing adhesives directly onto the skin.64
Although there is no universal consensus for the management of skin tears, generally accepted principles include cleaning the wound with 0.9% normal saline or nontoxic wound cleanser, approximating the skin tear flap as closely as possible and applying a moist dressing.62,65
Burns
In the 2004 National Health Survey 182,500 Australians were reported to have received a burn or scald injury,66 and in 2004–2005 there were 156 deaths in Australia as a result of a burn injury.67 A burn injury occurs when heat damages the body’s tissues including the skin, muscle, bone and subcutaneous structures.
Burns can be caused by thermal, electrical, chemical or radiation sources:
 Thermal burns include scald injuries, flame burns and contact burns.
Thermal burns include scald injuries, flame burns and contact burns.
 Electrical burns can cause injuries from direct contact with the electrical source or from the source’s arc. As the electricity passes through the body, the current is converted to heat, which damages the tissue between the points of entry and exit. Low-voltage injuries tend to cause burns at the sites of entry and exit, whereas high-voltage injuries cause extensive tissue damage with soft tissue and bone necrosis.
Electrical burns can cause injuries from direct contact with the electrical source or from the source’s arc. As the electricity passes through the body, the current is converted to heat, which damages the tissue between the points of entry and exit. Low-voltage injuries tend to cause burns at the sites of entry and exit, whereas high-voltage injuries cause extensive tissue damage with soft tissue and bone necrosis. Chemical burns occur when the skin is exposed to acids, alkalis or other corrosive chemicals. When in contact with the skin, chemical energy is converted into thermal energy, causing tissue damage. Chemical burns cause necrosis and continue to cause damage until completely removed.
Chemical burns occur when the skin is exposed to acids, alkalis or other corrosive chemicals. When in contact with the skin, chemical energy is converted into thermal energy, causing tissue damage. Chemical burns cause necrosis and continue to cause damage until completely removed. Radiation burns commonly occur due to exposure to UV radiation from sunlight, as discussed earlier. Also, radiotherapy treatments for cancer may cause radiation burns to the skin.
Radiation burns commonly occur due to exposure to UV radiation from sunlight, as discussed earlier. Also, radiotherapy treatments for cancer may cause radiation burns to the skin.PATHOPHYSIOLOGY
The pathogenesis of burns involves both local and systemic responses. A burn has three zones of injury:
In addition to the local effects of the burn there are systemic effects. The extent of the systemic effects depends on the total body surface area of the burn and the depth of the burn. Burns exceeding 20% of total body surface area are considered to be major burns and are associated with massive fluid shifts, oedema and release of inflammatory mediators.
Immediately after the burn injury, the systemic microcirculation loses its vessel wall integrity and plasma leaks out of the damaged capillaries into the surrounding tissues. There is also a systemic inflammatory response resulting from cytokine and inflammatory mediator release from the damaged cells. This further increases vascular permeability, causing additional fluid loss from the bloodstream. With the loss of plasma from the bloodstream, the blood becomes more viscous, impairing the microcirculation, and the haematocrit rises. Intravascular hypovolaemia (low blood volume) and burn shock result from the massive fluid losses from the circulating fluid volume. With the loss of plasma proteins from the vascular space, hypoproteinaemia (low protein levels) may result. Damage to the cells causes a shift of electrolytes, with sodium moving into the injured cells and potassium moving out, leading to hyperkalaemia. There is also a decrease in transmembrane potential involving non-thermally damaged cells, which contributes to the fluid and electrolyte shifts.
As a result of the systemic inflammatory response and hypovolaemia, most organ systems of the body are affected. Reduced cardiac output results from decreased plasma volume, increased afterload and decreased contractility (refer to Chapter 22 for a detailed description). Cardiac contractility is decreased in the first 24 hours after injury and is thought to be caused by circulating mediators such as TNF-α as well as impaired calcium.68 With reduced cardiac contractility, the blood flow to the liver, gastrointestinal organs and kidneys is reduced, resulting in slowed peristalsis and gastric emptying and reduced urine output. A burn injury is followed by a hypermetabolic state that is mediated by elevated levels of catecholamines (adrenaline or noradrenaline) and cortisol. The hypermetabolic state with increased oxygen consumption is related to an increase in and resetting of the thermal regulatory set point, resulting in a higher body temperature. Immune function is suppressed with impaired phagocytosis, decreased opsonins and impaired cellular and humoral immunity, thereby increasing susceptibility to systemic wound sepsis.
As a result of the loss of skin integrity, the skin’s functions of protection from invasion of harmful substances, loss of fluids and electrolytes and regulation of body temperature are altered. Following a burn the normal protective defence mechanisms, including defensins derived from the keratinocytes and acidic secretions from the sweat and sebaceous glands, are lost, resulting in the wound becoming colonised and invaded by microorganisms.69
Two key factors when assessing the burn wound are (1) the total body surface area that has been burnt and (2) the depth of the burn. A number of tools are used to determine the surface area burned. One of these is the rule of nines, in which the adult body is divided into 11 sections with each section representing 9% of the total body surface area (see Figure 19-22). A more accurate assessment that considers the varying rate of growth in the head, thighs and lower legs of different age groups is the Lund and Browder chart.
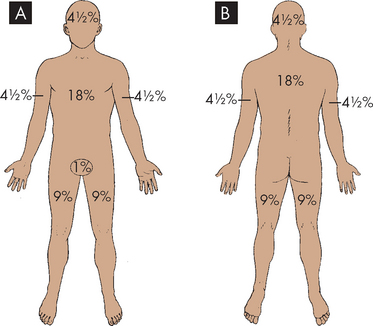
FIGURE 19-22 Estimation of burn injury: rule of nines.
The total surface area burned is often assessed using the rule of nines, whereby the body is divided into sections with each section representing 9% of the total surface area. A Adults (anterior view). B Adults (posterior view).
Burns are also classified according to depth of injury (see Figure 19-23). Burn depth may be described in terms of degree (first, second, third or fourth degree) or in term of thickness (superficial, partial thickness or full-thickness). In Australia and New Zealand burn depth is most frequently described as:
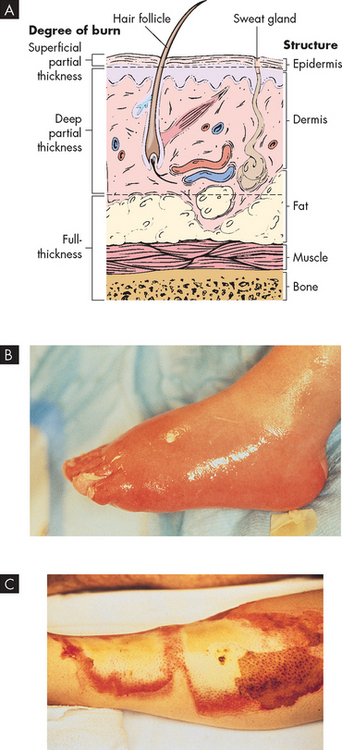
A A cross-section of skin indicating the degree of burn and structures involved. B A superficial partial-thickness injury following a scald. C A full-thickness burn destroys all layers of the skin. The area is waxy white and insensate.
Source: A Brown D, Edwards H. Lewis’s medical-surgical nursing. 2nd edn. Sydney: Elsevier; 2008. B & C Courtesy of Intermountains Burn Center, University of Utah.
Table 19-4 DEPTH ASSESSMENT OF BURNS
| CLASSIFICATION | DESCRIPTION |
|---|---|
| Epidermal | Dry and red, blanches with pressure, no blisters. May be painful. Heals with no scarring. |
| Superficial dermal | Pale pink with fine blistering, blanches with pressure. Usually extremely painful. Can have colour-match defect. Low risk of hypertrophic scarring. |
| Mid-dermal | Dark pink with large blisters. Capillary refill sluggish. May be painful. Moderate risk of hypertrophic scarring. |
| Deep dermal | Blotchy red, may blister, no capillary refill. In child may be dark lobster red with mottling. High risk of hypertrophic scarring. |
| Full-thickness | White, waxy or charred. No blisters. No capillary refill. Will scar. |
Source: Adapted from Australian and New Zealand Burn Association Limited. Emergency management of severe burns. 8th edn. Wellington: Ministry of Health; 2004.
CLINICAL MANIFESTATIONS
Due to the complexity of a burn injury, the clinical manifestations and complications are widespread. As a result of the shock, the patient’s heart rate will increase to circulate the remaining blood in an effort to maintain cardiac output. Loss of skin integrity leads to a loss of body temperature, which leads to increased metabolism. With loss of skin integrity, sensations perceived by the skin are affected. Irritation of nerves causes pain. Although in a full-thickness burn nerves are destroyed and the burned tissue itself is often insensate, the patient will still suffer pain. The modalities of touch, two-point discrimination, warming and vibration are significantly reduced because of damage to the sensory receptors.72 Another sensation patients often suffer is itch. Loss of the protective barrier function, together with immunosuppression, places the patient at risk of developing localised and systemic infection.
EVALUATION AND TREATMENT
The immediate management of a burn injury involves airway maintenance and fluid resuscitation. The goal of fluid therapy is to maintain tissue perfusion without causing fluid overload. Calculation of fluid replacement is commonly based on the Parkland formula (4 mL of intravenous solution (Ringer’s lactate solution) × percentage of total body surface area burned) and modified according to clinical variables such as urine output. Large quantities of protein are lost as a result of a burn injury, increasing nutritional requirements. Pain management is also an important aspect of treatment, and a number of different medications and pain management modalities are used (refer to Chapter 7).
Another problem that may arise is compartment syndrome of the extremities or trunk associated with circumferential burns. As a result of constricting eschar forming around a limb or trunk, blood flow can be restricted and, in the case of the chest, ventilation can be compromised. Escharotomy (surgical division of the necrotic tissue) may be required to allow for muscle movement and return of blood flow (see Figure 19-24). Necrotic tissue provides an environment for the growth of microorganisms, placing the patient at the risk of infection. Infections are mostly caused by staphylococci, streptococci and pseudomonas, thus meticulous wound management is required. For the patient with deep partial thickness and full-thickness burns, surgery to remove the burn eschar and provide wound coverage is undertaken as soon as the patient’s condition has stabilised. Wounds are covered with an autograft, split skin grafts or skin substitute material such as Transcyte or Integra.73 Cultured cellular epithelial autografts can also be used to cover the wounds. More recently, a cellular epithelial autograft suspension has been developed that takes only 5 days to culture and can be sprayed onto the wound.74 This technique has been found to improve scar quality.
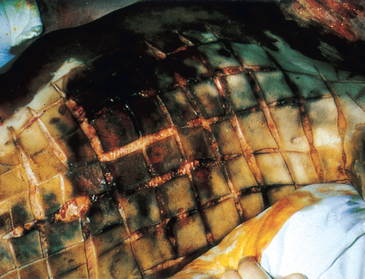
Necrotic tissue has been surgically divided to allow for muscle movement.
Source: Christensen BL, Kockrow EO. Adult health nursing. 6th edn. St Louis: Mosby; 2011.
VASCULAR DISORDERS
Vascular disorders may be associated with skin diseases, may be congenital or may involve responses to local or systemic vasoactive substances.
Cutaneous vasculitis
Cutaneous vasculitis is an inflammation of the blood vessels in the skin. It can affect the capillaries, venules, arterioles and lymphatics. The inflammation is thought to arise through:
 indirect injury by activation of antibodies, which then generate inflammation within the vessel wall
indirect injury by activation of antibodies, which then generate inflammation within the vessel wallCauses of the inflammation include bacterial or viral infections or a toxic response to drugs.
Vasculitis can have a varied appearance and it usually presents on the lower limbs. Lesions may include petechiae, ecchymoses, purpura and haemorrhagic bullae. In severe cases the vasculitis may impair the blood supply causing necrosis and ulceration.
Generally, the rash spontaneously resolves but it may recur at variable intervals after the initial episode. Treatment includes resting and elevating the affected limb, and finding and removing the cause. If the symptoms are severe, prednisone may be used.
Scleroderma
Scleroderma is an autoimmune disease of the connective tissue. It is characterised by fibrosis in the skin and organs of the body. Large amounts of collagen are deposited in the organs, and this is accompanied by inflammatory reactions and vascular changes. Scleroderma is more prominent in women and although it is found in every age group, the age of onset is most frequently between 25 and 55 years. Although the cause of the disease is not known, it is believed that both genetic and environmental factors are involved in activation of the immune system causing injury and formation of scar tissue.
There are two main types of scleroderma: (1) localised scleroderma, which affects only the skin, related tissues and the muscles below the tissues; and (2) systemic scleroderma, which affects the skin and organs. The clinical manifestations depend on the type of scleroderma and the extent of involvement. Redness and swelling lead to the skin becoming hard, shiny, taut and tightly connected to the underlying tissue. Skin changes most often occur in the fingers, feet, face and neck. Skin tightness can lead to a decreased range of movement of the fingers, face and toes. Raynaud’s phenomenon with episodes of vasoconstriction can cause ulcers on the fingers. Calcium deposits develop in the subcutaneous tissue and erupt through the skin, appearing as hard nodules.
There is no cure for scleroderma and treatment depends on the type of scleroderma and the symptoms. Drug treatment aims to inhibit tissue fibrosis, vascular changes and immune system changes. Drugs used include glucocorticoids, immunosuppressive agents, vasodilator drugs and antifibrotic agents.
Haemangioma
Haemangiomas are benign lesions of capillary endothelium. They are not usually present at birth but emerge in the first few months of life. The incidence of haemangiomas is three times higher in female infants than in male infants and is higher in premature infants. Haemangiomas are classified as superficial or deep, or a combination of the two. Superficial haemangiomas, historically called strawberry haemangiomas, are raised and bright red. These are the most common. Deep haemangiomas are slightly raised and bluish in colour. Most haemangiomas occur on the face and neck, with fewer occurring on the trunk or extremities.
Haemangiomas proliferate rapidly, plateau and most spontaneously involute. Proliferation generally occurs in the first 6–8 months of life but deep lesions can grow from 12 to 24 months. By 5 years of age, 50% of haemangiomas will maximally regress and by 9 years of age 90% will have regressed.
Doppler ultrasound, CT scanning and MRI can be used to diagnose haemangiomas.
Treatment of haemangiomas is controversial. Often they are not treated, because when they are allowed to spontaneously involute there is little scarring. However, indications for treatment include life-threatening or function-threatening lesions, lesions in locations likely to permanently scar, large facial haemangiomas and peduncled haemangiomas. Treatments include corticosteroids, intralesional interferon alpha, imiquimod, vincristine, and laser and debulking surgery.76
Port-wine stain
A port-wine stain (naevus flammeus) is a congenital capillary malformation composed of dilated vessels in the papillary dermis. It presents as pink, red or purplish macules. Port-wine stains can occur on any surface of the body but are most common on the face and neck. In the adult the lesion often darkens and sometimes becomes nodular.
The standard treatment for port-wine stains is pulsed dye laser. The pulsed dye laser emits yellow light at a wavelength that corresponds to the absorption peak of oxyhaemoglobin. The energy emitted is converted to heat that causes thermal coagulation of the vessel wall.
Describe the cause, pathogenesis and clinical manifestations of cutaneous vasculitis, scleroderma and port-wine stain.
Skin cancer
 Basal cell carcinoma arises from stem cells in the basal layer of the epidermis. The predominant cause of basal cell carcinoma is repeated exposure to UV radiation, which damages the DNA. Basal cell carcinomas are slow-growing tumours that rarely spread. Treatment includes surgical excision, cryotherapy and imiquimod 5% cream.
Basal cell carcinoma arises from stem cells in the basal layer of the epidermis. The predominant cause of basal cell carcinoma is repeated exposure to UV radiation, which damages the DNA. Basal cell carcinomas are slow-growing tumours that rarely spread. Treatment includes surgical excision, cryotherapy and imiquimod 5% cream. Squamous cell carcinoma arises from keratinocytes in the outer layers of the epidermis. The predominant cause of squamous cell carcinoma is repeated UV exposure, which damages DNA, and mutations in the genes, which cause uncontrolled cell proliferation and loss of apoptosis.
Squamous cell carcinoma arises from keratinocytes in the outer layers of the epidermis. The predominant cause of squamous cell carcinoma is repeated UV exposure, which damages DNA, and mutations in the genes, which cause uncontrolled cell proliferation and loss of apoptosis.Inflammatory disorders of the skin
 Irritant contact dermatitis develops from exposure to chemical or physical agents that cause an inflammatory response. Manifestations include a rash over the exposed area, pruritis, burning, pain, erythema, blistering and swelling. Treatment of irritant contact dermatitis is avoidance of contact with the irritant, topical corticosteroids and emollients.
Irritant contact dermatitis develops from exposure to chemical or physical agents that cause an inflammatory response. Manifestations include a rash over the exposed area, pruritis, burning, pain, erythema, blistering and swelling. Treatment of irritant contact dermatitis is avoidance of contact with the irritant, topical corticosteroids and emollients. Allergic contact dermatitis is a type IV delayed T-cell mediated hypersensitivity reaction occurring in two stages: (1) initial sensitisation to an allergen; and (2) inflammatory reaction when subsequently exposed to the same antigen.
Allergic contact dermatitis is a type IV delayed T-cell mediated hypersensitivity reaction occurring in two stages: (1) initial sensitisation to an allergen; and (2) inflammatory reaction when subsequently exposed to the same antigen. Latex allergy may cause two types of responses: (1) a type IV delayed T-cell mediated hypersensitivity; or (2) a type I hypersensitivity reaction.
Latex allergy may cause two types of responses: (1) a type IV delayed T-cell mediated hypersensitivity; or (2) a type I hypersensitivity reaction. Atopic dermatitis is associated with a family history of atopic conditions such as asthma and allergic rhinitis. The cause involves both genetic and environmental factors. Damage to the epidermal layer of the skin results in water loss and entry of antigens that activate the inflammatory response.
Atopic dermatitis is associated with a family history of atopic conditions such as asthma and allergic rhinitis. The cause involves both genetic and environmental factors. Damage to the epidermal layer of the skin results in water loss and entry of antigens that activate the inflammatory response. Acne vulgaris is an inflammation of the pilosebaceous follicle. The initial trigger causing overactivity of the pilosebaceous ducts, blockage of the ducts and excessive sebum production is thought to be hormonal.
Acne vulgaris is an inflammation of the pilosebaceous follicle. The initial trigger causing overactivity of the pilosebaceous ducts, blockage of the ducts and excessive sebum production is thought to be hormonal. Acne rosacea, an inflammatory condition affecting the face, is caused by both genetic and environmental factors. The clinical manifestations of skin dryness, erythema on the nose and cheeks, and telangiectasia result from damage to the dermis and an inflammatory response.
Acne rosacea, an inflammatory condition affecting the face, is caused by both genetic and environmental factors. The clinical manifestations of skin dryness, erythema on the nose and cheeks, and telangiectasia result from damage to the dermis and an inflammatory response. Lupus erythematosus can affect only the skin (cutaneous lupus erythematosus) or have multi-organ involvement (systemic lupus erythematosus). The cause involves both genetic and environmental factors with triggers including sunlight, medications, hormones, stress, viruses and skin trauma. The lesions vary depending on the type of lupus erythematosus.
Lupus erythematosus can affect only the skin (cutaneous lupus erythematosus) or have multi-organ involvement (systemic lupus erythematosus). The cause involves both genetic and environmental factors with triggers including sunlight, medications, hormones, stress, viruses and skin trauma. The lesions vary depending on the type of lupus erythematosus.Infections of the integumentary system
 Cellulitis is a spreading infection of the dermis and subcutaneous tissues. Local manifestations include redness, heat, swelling and pain in the infected area. Systemic manifestations such as fever, malaise and vomiting may also be present.
Cellulitis is a spreading infection of the dermis and subcutaneous tissues. Local manifestations include redness, heat, swelling and pain in the infected area. Systemic manifestations such as fever, malaise and vomiting may also be present. Impetigo is a contagious superficial skin infection caused by Staphylococcus and Streptococcus. Lesions begin as small blisters that become pustular, rupture and form crusts.
Impetigo is a contagious superficial skin infection caused by Staphylococcus and Streptococcus. Lesions begin as small blisters that become pustular, rupture and form crusts. Staphylococcal scalded skin syndrome is a toxin-mediated condition caused by Staphylococcus aureus. Toxins produced by the bacteria cause separation of the skin just below the granular layer of the epidermis. It occurs mainly in young children who have not developed antibodies against the toxins or adults who are immunosuppressed. It is treated with antibiotics.
Staphylococcal scalded skin syndrome is a toxin-mediated condition caused by Staphylococcus aureus. Toxins produced by the bacteria cause separation of the skin just below the granular layer of the epidermis. It occurs mainly in young children who have not developed antibodies against the toxins or adults who are immunosuppressed. It is treated with antibiotics. Herpes simplex type I is a viral infection that affects the oral and respiratory mucosa, and Herpes simplex type 2 affects the genitalia. Lesions are small, inflamed painful vesicles that ulcerate and crust. Latent infection is established in dorsal root ganglia.
Herpes simplex type I is a viral infection that affects the oral and respiratory mucosa, and Herpes simplex type 2 affects the genitalia. Lesions are small, inflamed painful vesicles that ulcerate and crust. Latent infection is established in dorsal root ganglia. Herpes zoster (shingles) and varicella (chickenpox) are both caused by the same herpes virus. Varicella occurs most commonly in children and causes a vesicular rash on the trunk, limbs, face and scalp. Herpes zoster occurs mainly in adults and results from reactivation of the varicella virus. It causes a painful vesicular rash, usually on the skin of a single dermatome.
Herpes zoster (shingles) and varicella (chickenpox) are both caused by the same herpes virus. Varicella occurs most commonly in children and causes a vesicular rash on the trunk, limbs, face and scalp. Herpes zoster occurs mainly in adults and results from reactivation of the varicella virus. It causes a painful vesicular rash, usually on the skin of a single dermatome. Warts are benign epidermal lesions caused by the human papillomavirus. Condylomata acuminata are genital warts transmitted by sexual contact.
Warts are benign epidermal lesions caused by the human papillomavirus. Condylomata acuminata are genital warts transmitted by sexual contact. Molluscum contagiosum is a contagious condition of the skin caused by the poxvirus. Lesions are pearly papules with a central plug consisting of dead epithelial cells and virus particles found on the trunk, face and extremities.
Molluscum contagiosum is a contagious condition of the skin caused by the poxvirus. Lesions are pearly papules with a central plug consisting of dead epithelial cells and virus particles found on the trunk, face and extremities. Tinea infections are superficial skin infections caused by dermatophytes. They are classified by location: tinea corporis (body), tinea capitis (scalp), tinea pedis (feet), tinea manus (hands), tinea cruris (groin) and tinea unguium (nails). They are treated with antifungal drugs.
Tinea infections are superficial skin infections caused by dermatophytes. They are classified by location: tinea corporis (body), tinea capitis (scalp), tinea pedis (feet), tinea manus (hands), tinea cruris (groin) and tinea unguium (nails). They are treated with antifungal drugs. Candidiasis is an infection caused by the yeast-like fungus, Candida albicans. It occurs on the skin and mucous membranes.
Candidiasis is an infection caused by the yeast-like fungus, Candida albicans. It occurs on the skin and mucous membranes. Scabies is a contagious parasitic infection caused by Sarcoptes scabiei. The characteristic feature of scabies is the burrow, a track made by the female where she lays her eggs.
Scabies is a contagious parasitic infection caused by Sarcoptes scabiei. The characteristic feature of scabies is the burrow, a track made by the female where she lays her eggs. Ticks are blood-sucking parasites that secrete a number of substances including anticoagulants and prostaglandins into the host. The manifestations of the tick bite include itchy, painful, erythematous lesions. Allergic reactions are caused by both the physical trauma to the skin and the salivary secretions, toxins and excretions of the tick.
Ticks are blood-sucking parasites that secrete a number of substances including anticoagulants and prostaglandins into the host. The manifestations of the tick bite include itchy, painful, erythematous lesions. Allergic reactions are caused by both the physical trauma to the skin and the salivary secretions, toxins and excretions of the tick.Traumatic conditions of the integumentary system
 Pressure ulcers are areas of localised injury to the skin resulting from pressure or pressure in combination with shear and/or friction. As a result of pressure, underlying blood vessels are occluded, leading to ischaemia, tissue necrosis and ulceration.
Pressure ulcers are areas of localised injury to the skin resulting from pressure or pressure in combination with shear and/or friction. As a result of pressure, underlying blood vessels are occluded, leading to ischaemia, tissue necrosis and ulceration. Skin tears caused by shearing, friction or blunt trauma result in separation of the epidermis from the dermis or the epidermis and dermis from the underlying structures. They may present from a simple linear tear to extensive tissue loss and necrosis.
Skin tears caused by shearing, friction or blunt trauma result in separation of the epidermis from the dermis or the epidermis and dermis from the underlying structures. They may present from a simple linear tear to extensive tissue loss and necrosis. Burns can be caused by thermal, electrical, chemical or radiation sources. There are both local and systemic responses to a burn injury. Burns exceeding 20% of the total body surface area are associated with fluid shifts, oedema and release of inflammatory mediators. As a result of the loss of skin integrity, the normal protective functions of the skin are lost. The two key factors when assessing a burn are the total body surface area burnt and the depth of the burn.
Burns can be caused by thermal, electrical, chemical or radiation sources. There are both local and systemic responses to a burn injury. Burns exceeding 20% of the total body surface area are associated with fluid shifts, oedema and release of inflammatory mediators. As a result of the loss of skin integrity, the normal protective functions of the skin are lost. The two key factors when assessing a burn are the total body surface area burnt and the depth of the burn.Vascular disorders
 Cutaneous vasculitis is an inflammation of the blood vessels of the skin caused by bacterial or viral infections or a toxic response to drugs.
Cutaneous vasculitis is an inflammation of the blood vessels of the skin caused by bacterial or viral infections or a toxic response to drugs. Scleroderma is an autoimmune disease of the connective tissue characterised by fibrosis in the skin and organs of the body.
Scleroderma is an autoimmune disease of the connective tissue characterised by fibrosis in the skin and organs of the body.Mark is 39 years old and was referred to a dermatologist for a suspicious-looking mole on the back of his lower left leg. Mark is a sales manager who lives with his wife and two children. He has lived near the beach all his life and when younger spent most weekends surfing. He is fit and still enjoys surfing and jogging along the beach. Although he now wears sunscreen when outdoors, he admits that when he was younger he just smeared zinc across his nose.
The dermatologist noted that Mark has light skin and blond hair. Mark explained that the mole at the back of his leg had become itchy, and that it had become slightly raised and changed colour over the past 4–6 weeks. Although he has a number of moles he has no previous history of melanoma and no known family history of melanoma. On examination the dermatologist noted that the lesion was 6 mm × 8 mm, raised and consisted of light brown, dark brown and black colours. It had irregular borders and was not ulcerated. On palpation of Mark’s groin he noted there was no lymphadenopathy.
The lesion was excised and a full-thickness biopsy was performed. Histopathology showed proliferation of atypical melanocytes that extended into the papillary layer of the dermis. It also showed lymphocytes present surrounding the malignant cells in the dermis. The Breslow thickness was 0.35 mm and the TNM stage was given as T1a. The diagnosis of malignant melanoma was confirmed.