15 ALTERATIONS OF IMMUNE FUNCTION ACROSS THE LIFE SPAN
INTRODUCTION
Immunity is a complex interrelated series of events that provides constant protection for the body. Even when an individual’s immune system is functioning normally, the person may still experience infection if they are exposed to a sufficient enough load of the pathogenic microorganism. It should be stressed that, although the individual will exhibit signs and symptoms, their immune system is responding appropriately. In the majority of cases, the immune system will be able to combat the pathogenic microorganism and restore the individual back to homeostasis. However, sometimes the immune system does not respond appropriately — there may be not enough immunity or the response may be excessive and inappropriate. Inappropriate immune responses may be:
All these responses can be serious or life-threatening.
The first part of this chapter explores diseases where immunity is either exaggerated or inappropriate; while the second part looks at the less prevalent, but devastating, diseases that cause immunodeficiency. We start by examining hypersensitivity reactions, which are likely to be the most commonly encountered altered immune responses across different healthcare settings.
HYPERSENSITIVITY REACTIONS
Hypersensitivity refers to an immune response that is exaggerated or activated inappropriately, resulting in disease or damage to the individual. Hypersensitivity reactions are the most common altered immune responses, but fortunately they are usually the least life-threatening. There are four different types of hypersensitivity reactions:
The four mechanisms are interrelated and in most hypersensitivity reactions several mechanisms may be at work simultaneously or sequentially. Autoimmunity arises when the immune system attacks the body’s own cells because there is a failure to recognise self. Autoimmune responses can be caused by hypersensitivity mechanisms, but hypersensitivity reactions can occur without autoimmunity. Therefore, for the purposes of clarity, autoimmunity is discussed later in the chapter in the discussion on autoimmune diseases.
As with all immune responses, hypersensitivity reactions require sensitisation against a particular antigen, which results in a primary immune response. Disease symptoms appear after an adequate secondary immune response occurs. Hypersensitivity reactions are immediate or delayed, depending on the time required to elicit clinical symptoms after re-exposure to the antigen. Reactions that occur within minutes to a few hours after exposure to an antigen are termed immediate hypersensitivity reactions. Delayed hypersensitivity reactions may take several hours to appear and are at maximum severity days after re-exposure to the antigen.
The incidence of allergies in Australia and New Zealand
Australia and New Zealand have some of the highest rates of allergy in the world. Allergies are a major cause of disability and are a major public health problem, costing an estimated $7 billion per year. It is thought that approximately 20% of the population have at least one allergy, with most sufferers aged between 15 and 64 years of age. In addition, many children have allergies: in children aged 6–7 years, 1 in 6 have eczema and 1 in 10 have allergic rhinitis. Food allergies are also high and have been estimated to affect approximately 11% of children, although allergy testing was not confirmed in these cases. Most alarming is the rapid increase in the number of children suffering from peanut allergy, with the number diagnosed doubling between 2003 and 2008. The exact reasons for the high prevalence of allergies in Australia and New Zealand remain unknown.
Source: Australian Institute of Health and Welfare. Australia’s health, 2008. Cat. no. AUS 99. Canberra: AIHW; 2008; Australasian Society of Clinical Immunology and Allergy. Is it allergy? Available at www.allergy.org.au; Crooks C et al. The changing epidemiology of food allergy — implications for New Zealand. NZMJ 2008; 121(1271):74–82; Mullins R. The economic impact of allergic disease in Australia: not to be sneezed at. Access Economics and Australasian Society of Clinical Immunology and Allergy; 2009.
Allergy refers to a hypersensitivity to environmental antigens called allergens. It is not known why some antigens are allergens and others are not. Typical allergens include pollens, moulds and fungi, foods, animals, cigarette smoke and components of house dust. Often the allergen is contained within a particle that is too large to be phagocytosed or is surrounded by a protective non-allergenic coat. The actual allergen is released after enzymatic breakdown (usually by lysozyme in secretions) of the larger particle. Allergies are the most common hypersensitivity diseases.1 The majority of allergies are type I reactions that lead to annoying symptoms such as a runny nose, sneezing and other relatively mild reactions.
Type I: IgE-mediated hypersensitivity reactions
Type I reactions are the most common reactions and are mediated by antigen-specific immunoglobulin E (IgE) and the products of tissue mast cells (see Figure 15-1).2 In addition, most type I reactions occur against environmental antigens and are therefore allergic. Because of this strong association, many healthcare professionals use the term allergy to indicate only IgE-mediated reactions. However, IgE can contribute to some autoimmune diseases, and many common allergies (e.g. cows’ milk causing enteropathy) are not mediated by IgE.
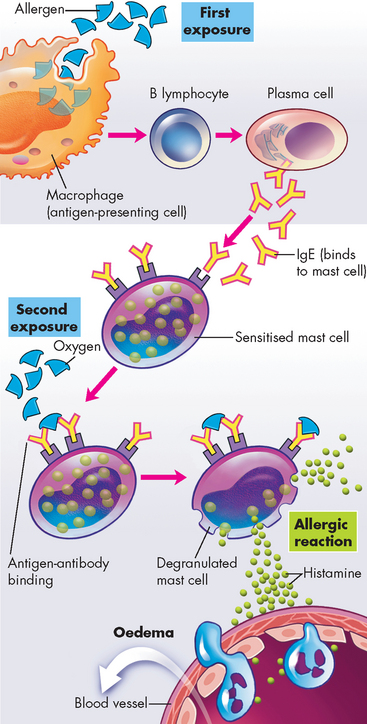
FIGURE 15-1 The mechanism of type I IgE-mediated hypersensitivity reactions.
First exposure to an allergen stimulates B lymphocytes to mature into plasma cells that produce IgE. The IgE attaches to the surface of the mast cell by binding with IgE-specific receptors. When an adequate amount of IgE is bound, the mast cell is ‘sensitised’. During a second exposure, the allergen cross-links the surface-bound IgE and causes degranulation of the mast cell. The initial phase is characterised by vasodilation, vascular leakage and smooth muscle spasm or glandular secretions, usually within 5–30 minutes after exposure to the antigen. The late phase occurs 2–8 hours later without additional exposure to the antigen and results from infiltration of tissues with inflammatory cells, including eosinophils, neutrophils and basophils.
Before an IgE-mediated hypersensitivity reaction occurs, the allergen is digested by an antigen-presenting cell (such as a macrophage), which presents the antigen fragment to a helper T cell. The helper T cell assists the B lymphocyte to convert into a plasma cell, which produces IgE. Immunoglobulin E has a relatively short life span in the blood because it rapidly binds to receptors on mast cells. In predisposed individuals, large amounts of IgE are produced by the plasma cells which bind to mast cells and basophils, with the individual considered to be sensitised. Upon further exposure of the sensitised individual to the allergen, degranulation of the mast cell occurs with the release of mast cell products (discussed in Chapter 13) (see Figure 15-1). A flood of substances are released from the mast cells, which cause the signs and symptoms of the type I hypersensitivity reaction.
Mechanisms of IgE-mediated hypersensitivity
The most potent mediator of IgE-mediated hypersensitivity is histamine, which affects several key target cells.3 Histamine contracts bronchial smooth muscles (bronchial constriction), increases vascular permeability (oedema) and causes vasodilation (increased blood flow) (see Chapter 16). In addition, the interaction of histamine with receptors in the stomach results in increased gastric acid secretion. Some type I allergic responses can be controlled by blocking histamine receptors with antihistamines.
CLINICAL MANIFESTATIONS
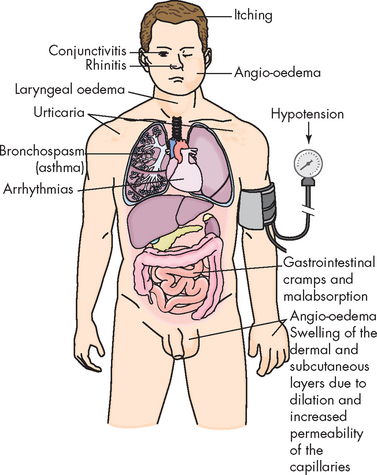
The clinical manifestations of type I reactions are attributable mostly to the biological effects of histamine. The tissues most commonly affected by type I responses contain large numbers of mast cells and are sensitive to the effects of histamine released from them. These tissues are found in the gastrointestinal tract, the skin and the respiratory tract (see Figure 15-2).
Gastrointestinal allergy is caused primarily by allergens that enter through the mouth — usually foods or oral medications. Symptoms include vomiting, diarrhoea or abdominal pain. Foods most often implicated in gastrointestinal allergies are peanuts, milk, chocolate, citrus fruits, eggs, wheat, nuts and fish. When food is the allergen, the active immunogen may be an unidentifiable product of food breakdown by digestive enzymes. Sometimes the allergen is a drug, an additive or a preservative in the food. For example, cows are sometimes given penicillin to treat infection and trace amounts of the antibiotic can be present in the milk. Thus, hypersensitivity apparently caused by milk proteins may instead be the result of an allergy to penicillin.
Urticaria, or hives, is a dermal (skin) manifestation of allergic reactions (see Figure 15-3). The underlying mechanism is the localised release of histamine and increased vascular permeability, resulting in limited areas of oedema. Urticaria is characterised by white fluid-filled blisters surrounded by areas of redness. This is usually accompanied by itching. Urticaria is not only caused by immunological reactions, but it can also result from exposure to cold temperatures, emotional stress, medications, systemic diseases or malignancies (e.g. lymphomas).
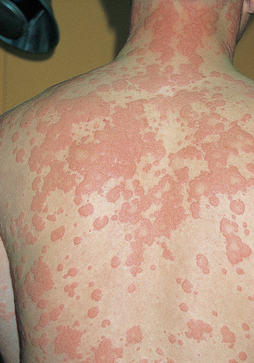
FIGURE 15-3 Urticaria due to type I hypersensitivity reaction.
The skin lesions have raised edges and develop within minutes or hours, with resolution occurring after about 12 hours.
Source: Male D et al. Immunology. 7th edn. St Louis: Mosby; 2006.
Allergens can also cause rhinitis (inflammation of the mucous membrane of the nose) and conjunctivitis (inflammation of the membrane lining the eyelids). The more serious effects of type I hypersensitivity reactions are vasodilation, hypersecretion of mucus, oedema and swelling of the respiratory mucosa (see Figure 15.2). Because the mucous membranes lining the respiratory tract are continuous, they are all adversely affected. This can cause obstruction of the large and small airways (bronchi) of the lower respiratory tract by bronchospasm (constriction of smooth muscle in airway walls), oedema and thick secretions. This leads to ventilatory insufficiency, wheezing and difficult or laboured breathing.
Certain individuals are genetically predisposed to develop allergies and are called atopic. In families in which one parent has an allergy, allergies develop in about 40% of the offspring. If both parents have allergies, the incidence may be as high as 80%. Atopic individuals tend to produce higher quantities of IgE and to have more receptors for IgE on their mast cells. The airways and skin of atopic individuals have increased responsiveness to a wide variety of both specific and nonspecific stimuli.
EVALUATION AND TREATMENT
It must be emphasised that the majority of allergic reactions are not life-threatening, but often the individual affected feels quite debilitated. For instance, allergic rhinitis affects the eyes and nasal passages and causes itching, swelling and increased mucus production. Hay fever, which affects a large percentage of the population, occurs when pollen enters the upper airway and causes a type I reaction. These conditions can be treated with antihistamines to primarily alleviate the symptoms, or individuals can undergo allergy testing to identify the specific allergen. Clinical desensitisation to allergens can be achieved in some individuals. Minute quantities of the allergen to which the person is sensitive are injected in increasing doses over a prolonged period. This procedure may reduce the severity of the allergic reaction in the treated individual.
In some cases, an allergic reaction can be life-threatening; therefore, it is essential that severely allergic individuals be made aware of the specific allergen against which they are sensitised and instructed to avoid contact with that material. If these individuals are exposed to that specific allergen, it may induce a severe allergic reaction that can be life-threatening, and immediate treatment is required.
Anaphylaxis
The most rapid and severe immediate hypersensitivity reaction is anaphylaxis. Anaphylaxis occurs within minutes of re-exposure to the antigen and produces a severe reaction. Anaphylactic shock is a life-threatening condition that causes contraction of bronchial smooth muscle, oedema of the throat, breathing difficulties, decreased blood pressure and ultimately death without immediate rescue therapy.
One of the main problems with anaphylaxis is that it results in widespread vasodilation (increased diameter of blood vessels). The circulatory system usually works by a combination of vasoconstriction and vasodilation in different organs at any one time, to redirect blood flow to areas of most need. However, with mass vasodilation all blood vessels have a much wider diameter than usual, which results in low blood pressure and an insufficient amount of blood going to the vital organs of the brain, heart and lungs. To try to compensate for this, the heart rate becomes rapid (tachycardia).
An example of systemic anaphylaxis is an allergic reaction to peanuts, which has a high prevalence in children (1:50).4 Accordingly, health- and child-care workers should be aware of children’s susceptibility to peanut allergy and many institutions try to prohibit peanuts and other nuts from their facilities.
Individuals with severe systemic anaphylaxis are required to avoid exposure to the particular environmental trigger. In addition, they may need to carry adrenaline with them at all times, in case of a life-threatening allergic episode. An auto-injector called an Epipen® (Epi indicates epinephrine — the American term for adrenaline) is a self-injecting pen that is given subcutaneously or intramuscularly in the case of anaphylaxis. Adrenaline is an adrenergic agonist that when administered provides bronchodilation to correct breathing difficulties, as well as vasoconstriction to increase cardiac output and provide adequate blood flow back to the vital organs, thereby temporarily alleviating the effects of the anaphylactic reaction. Prompt hospitalisation is also required. Individuals are taught how to self-inject with the pen, which reduces the intervention time and has been shown to decrease mortality.5
Type II: tissue-specific hypersensitivity reactions
Type II hypersensitivities are generally reactions against a specific cell or tissue. Cells express a variety of antigens on their surfaces, some of which are called tissue-specific antigens because they are expressed on the plasma membranes of only certain cells. Antibodies are produced that bind to the antigen on these tissues that cause the hypersensitivity reaction. The symptoms of many type II diseases are determined by which tissue or organ expresses the particular antigen. Environmental antigens (e.g. drugs) may bind to the plasma membranes of specific cells (especially erythrocytes and platelets) and function as targets of type II reactions.
There are four general mechanisms by which type II hypersensitivity reactions can affect cells (see also Figure 15-4):
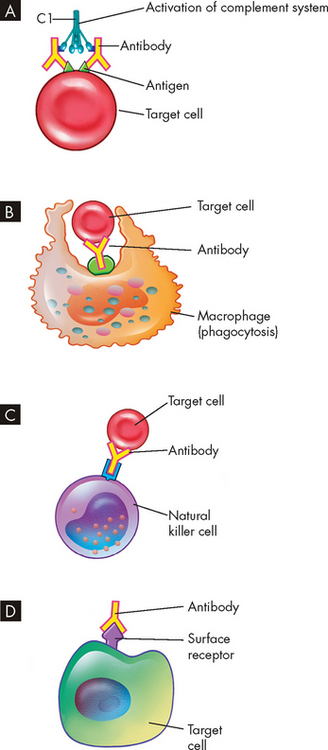
FIGURE 15-4 The mechanisms of type II tissue-specific hypersensitivity reactions.
A The antigen-antibody (immune) complex causes activation of the complement system. B Phagocytosis of the targeted cell occurs when bound with antibody. C The antibody attracts natural killer cells, which cause lysis of the cell when attached. D The antibody binds to a surface receptor and inhibits usual cell function, which destroys the receptor and target cell.
Type III: immune complex–mediated hypersensitivity reactions
Most type III hypersensitivity diseases are caused by antigen-antibody (immune) complexes that are formed in the circulation and deposited later in vessel walls or other tissues (see Figure 15-5).6 The primary difference between type II and type III mechanisms is that in type II hypersensitivity the antibody binds to the antigen on the cell surface, whereas in type III the antibody binds to soluble antigen that was released into the blood or body fluids and the complex is then deposited in the tissues.
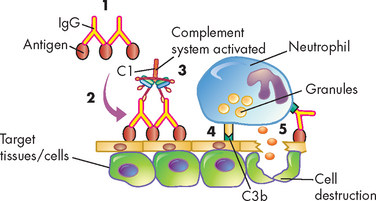
FIGURE 15-5 The mechanism of type III immune-complex-mediated hypersensitivity reactions.
(1) Immune complexes form in the blood from circulating antigen and antibody and (2) are deposited in certain target tissues. (3) The complexes activate the complement system via C1 and generate fragments that attract neutrophils to the site. (4) The neutrophils attach to the IgG and C3b in the immune complexes and (5) release a variety of degradative enzymes that destroy the healthy tissues.
Type III reactions are not organ-specific and symptoms have little to do with the particular antigenic target of the antibody. The harmful effects of immune complex deposition are caused by activation of the complement system, particularly through the generation of chemotactic factors (chemicals that attract immune cells) for neutrophils. The neutrophils bind to the antibody and C3b (a component of the complement system) contained in the complexes and attempt to ingest the immune complexes. They are often unsuccessful because the complexes are bound to large areas of tissue. During the attempted phagocytosis, large quantities of lysosomal enzymes are released into the inflammatory site. The attraction of neutrophils and the subsequent release of lysosomal enzymes cause most of the resulting tissue damage. Type III hypersensitivity reactions are the cause of many of the conditions seen in systemic lupus erythematosus, an autoimmune disease discussed later in the chapter.
Type IV: cell-mediated hypersensitivity reactions
Whereas types I, II and III hypersensitivity reactions are mediated by antibody, type IV reactions are mediated by T lymphocytes and do not involve antibody (see Figure 15-6). Type IV mechanisms occur through either cytotoxic T lymphocytes or cytokine-producing helper T (TH1) cells.7 Cytotoxic T cells attack and destroy cellular targets directly. TH1 cells produce cytokines that recruit and activate phagocytes, especially macrophages. Destruction of the tissue is usually caused by direct killing by cytotoxic T cells or the release of soluble factors, such as lysosomal enzymes from activated macrophages.
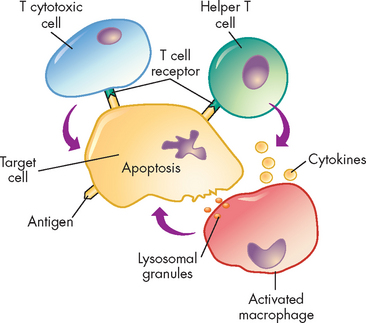
FIGURE 15-6 The mechanism of type IV cell-mediated hypersensitivity reactions.
Antigens from target cells stimulate T cells to differentiate into T cytotoxic cells, which have direct cytotoxic activity, and helper T cells, which produce cytokines (especially interferon-gamma) that activate macrophages. The macrophages can attach to targets and release enzymes that induce apoptosis of the target cell.
Latex allergy in healthcare workers
Latex is a natural rubber derived from the rubber tree, Hevea brasiliensis. The protein concentration in raw latex is very low, but is associated with a number of allergens. In the general population, the prevalence of latex allergy is low. However, in healthcare workers, especially nurses, the prevalence of latex allergy is considerably higher and is estimated to be between 5 and 15% — it is also expected to increase. The primary reason for the higher rate among healthcare workers is their increased exposure to latex compared to the general population. Healthcare workers routinely apply gloves during numerous clinical procedures and this greater usage of latex gloves increases the risk of developing a latex allergy. The most common reported symptoms include erythema, itch and swelling, typical of IgE-mediated allergy clinical manifestations. In addition, type IV cell-mediated hypersensitivity reactions are common and are related to contact dermatitis. It has been reported that a number of individuals with a positive latex skin-prick test are asymptomatic upon latex exposure with the skin. The reasons for this are unclear. The best treatment to date consists of avoiding latex gloves and it is recommended that healthcare workers use non-latex powdered gloves to decrease latex allergy.
Source: Douglas R et al. Prevalence of IgE-mediated allergy to latex in hospital nursing staff. Aus New Zeal J Med 1997; 27:165–169; Thien F. Latex allergy for health professionals. The Australasian Society of Clinical Immunology and Allergy; 2007. Available at www.allergy.org.au.
Clinical examples of type IV hypersensitivity reactions include graft rejection, the skin test for tuberculosis (Mantoux test) and allergic reactions resulting from contact with such substances as nickel, plants and latex. These are called allergic contact dermatitis and examples can be seen in Figure 15-7. A type IV component also may be present in many autoimmune diseases. For example, T cells against collagen (a protein present in joint tissues) contribute to the destruction of joints in rheumatoid arthritis, and T cells against an antigen on the surface of pancreatic beta cells (the cells that normally produce insulin) are responsible for beta cell destruction in insulin-dependent (type 1) diabetes mellitus. Therefore, type IV reactions are delayed hypersensitivity reactions as they take hours to days to appear, whereas type I reactions exhibit immediate allergic hypersensitivity reactions.
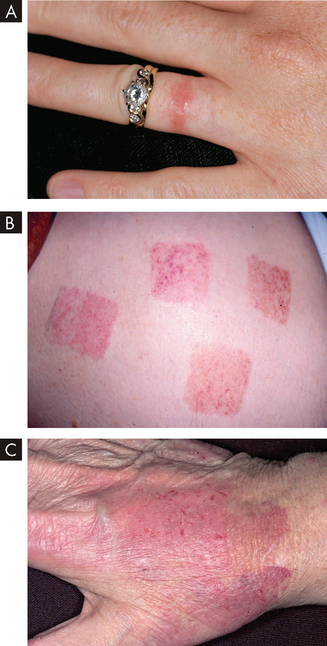
FIGURE 15-7 Allergic contact dermatitis from different allergens.
A Nickel is the most common cause of contact dermatitis in Australia. B Skin-contacting nicotine patches can cause allergic dermatitis. C Latex glove contact can cause allergic dermatitis, especially in healthcare workers (see the box ‘Health alert: latex allergy in healthcare workers’).
Source: A Weston WL. Color textbook of pediatric dermatology. 4th edn. St Louis: Mosby; 2007. B Rakel RE. Textbook of family medicine. 7th edn. Philadelphia: Saunders; 2007. C Habif TP. Clinical dermatology: a color guide to diagnosis and therapy. 5th edn. St Louis: Mosby; 2010.
TRANSPLANTATION
Despite Australia and New Zealand having low rates of organ donation,8 approximately 30,000 tissue and organ transplants have occurred in Australia since 1963.9 For these transplants to be successful, individuals have to be closely tissue-matched with the donor, meaning that the major histocompatibility complex (MHC), the molecules found on all nucleated cells that are self-antigens, must be closely matched between the recipient and donor. This reduces the capacity of the recipient’s immune system to attack the transplanted organ, because it is less ‘foreign’. In addition, the recipient receives immunosuppressive medication to reduce their immune system’s response to the transplanted organ. Blood also needs to be matched between the recipient and donor to avoid serious immune reactions. In most clinical situations, the transfusion of blood is not associated with any major immune reactions.
Organ transplantation is successful because technology enables close matching of tissue types between the donor and recipient and the level of immunosuppressive medication is carefully calculated to achieve optimal health for the recipient. However, the recipient’s immune system does mount a response to transplanted tissues and we now examine this in more detail.
Transplantation rejection
The MHC molecules, also referred to as human leucocyte antigens (HLA), are responsible for displaying proteins on the surface of every nucleated cell in the body, so that the immune system can recognise the cells as ‘self’ — that is, normal cells. Each individual has unique MHC molecules. Within the human population, the number of possible different MHC combinations is enormous and so finding two individuals with similar MHC antigens is difficult.
The diversity of MHC molecules becomes clinically relevant during organ transplantation. The recipient of a transplant can mount an immune response against the foreign MHC antigens on the donor tissue, resulting in rejection. To minimise the chance of tissue rejection, the donor and recipient are required to have the same blood group and are tissue-typed to identify differences in MHC antigens.10 Because of the large number of different MHC antigen combinations, it is highly unlikely that a perfect match will be found between an individual who needs a transplant and a potential donor from the general population. However, the more similar two individuals are in their MHC antigen tissue type, the more likely a transplant from one to the other will be successful. Because MHC antigens are inherited from parents, siblings are usually closely matched and identical twins are genetically similar and therefore the closest match.
Transplant rejection may be classified as hyperacute, acute or chronic, depending on the amount of time that elapses between transplantation and rejection.
 Hyperacute rejection is immediate and rare. When the circulation is re-established to the grafted area, the graft may immediately turn white (so-called white graft) instead of a normal pink colour. Hyperacute rejection usually occurs because of pre-existing antibody (type II reaction) to antigens on the vascular endothelial cells in the grafted tissue.
Hyperacute rejection is immediate and rare. When the circulation is re-established to the grafted area, the graft may immediately turn white (so-called white graft) instead of a normal pink colour. Hyperacute rejection usually occurs because of pre-existing antibody (type II reaction) to antigens on the vascular endothelial cells in the grafted tissue. Acute rejection is a cell-mediated immune response that occurs within days to months after transplantation. This type of rejection occurs when the recipient develops an immune response against unmatched HLAs after transplantation. A biopsy of the rejected organ usually shows an infiltration of lymphocytes and macrophages characteristic of a type IV reaction.
Acute rejection is a cell-mediated immune response that occurs within days to months after transplantation. This type of rejection occurs when the recipient develops an immune response against unmatched HLAs after transplantation. A biopsy of the rejected organ usually shows an infiltration of lymphocytes and macrophages characteristic of a type IV reaction.Blood transfusion reactions
Erythrocytes (red blood cells) express several important surface antigens, known collectively as the blood group antigens, which can be targets of immune reactions.11 More than 80 different red cell antigens are grouped into several dozen blood group systems. The most important of these, because they provoke the strongest immune response, are the ABO and Rhesus systems.
The ABO blood group system
The ABO blood group consists of two major carbohydrate antigens, labelled A and B (see Figure 15-8). These are codominant so that both A and B can be simultaneously expressed, resulting in an individual having any one of four different blood types. The erythrocytes of persons with blood type A have the type A antigen (i.e. carry the A antigen), those with blood type B carry the B antigen, those with blood type AB carry both A and B antigens, and those with blood type O carry neither the A nor the B antigen.
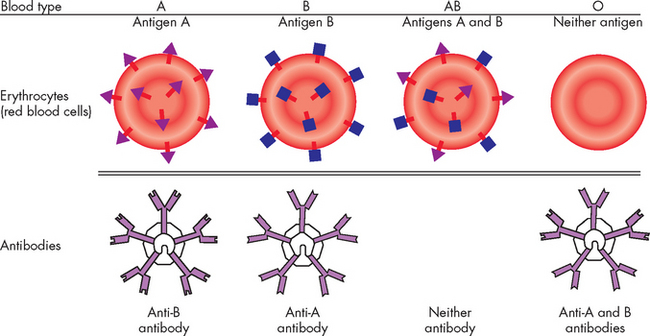
FIGURE 15-8 The ABO blood group.
The relationship of antigens and antibodies associated with the ABO blood groups. The surfaces of erythrocytes of individuals with blood group A have the A antigenic carbohydrate. The blood of these individuals has IgM antibodies against the B antigen. In individuals with blood group B, the red blood cells have the B antigenic carbohydrate, and the blood contains IgM antibodies against the A antigen. In individuals with blood group AB, the same cells have both the A and B antigens. These individuals do not have antibody to either A or B antigens. The erythrocytes of blood group O individuals have neither antigen, but their blood contains antibodies to both A and B.
A person with type A blood also has circulating antibodies to the B carbohydrate antigen, called anti-B antibody. If this person receives blood from a type AB or B individual, a severe transfusion reaction occurs and the transfused erythrocytes are destroyed by agglutination (literally clumping of the cells together, not to be confused with the blood clotting process) or complement-mediated lysis (where the cell membrane is destroyed and the cell contents leak out causing cell death). Similarly, a type B individual (whose blood contains anti-A antibodies) cannot receive blood from a type A or AB donor. Type O individuals, who have neither antigen but have both anti-A and anti-B antibodies, cannot accept blood from any of the other three types.
Because individuals with type O blood lack both types of antigens, they are considered universal donors, meaning that anyone can accept their red blood cells (for this reason, type O blood is usually kept in stock in hospitals to allow transfusion without losing time for blood typing). Similarly, type AB individuals are considered universal recipients because they lack both anti-A and anti-B antibodies and can be transfused with any ABO blood type. Agglutination and lysis cause harmful transfusion reactions that can be prevented only by complete and careful ABO matching between donor and recipient. In clinical practice, cross-matching of blood is routine and severe transfusion reactions occur only if an individual is transfused with an incompatible blood group.
The Rhesus system
The Rhesus (Rh) blood group is a group of antigens expressed only on red blood cells. This is the most diverse group of red cell antigens, consisting of at least 45 separate antigens, although only one is considered very important. The most important is the D antigen. Individuals who express the D antigen on their red cells are considered Rh-positive, whereas individuals who do not express the D antigen are Rh-negative. About 85% of the population are Rh-positive and these people can receive both positive and negative blood. Those who are Rh negative can receive only Rh-negative blood. The Rh blood group is checked before individuals receive a blood transfusion. Therefore, blood to be transfused into individuals is checked for both ABO and Rh factor.
A disease called haemolytic disease of the newborn is most commonly caused by IgG anti-D antibody produced by Rh-negative mothers against erythrocytes of their Rh-positive fetuses (see Chapter 17). This may occur if the maternal Rh-negative blood comes into contact with the fetus’ Rh-positive blood. The mother’s antibody will coat the fetal red blood cells and destroy them. The occurrence of this particular form of the disease has decreased dramatically because of the use of prophylactic anti-D immunoglobulin (i.e. Rhogam). By mechanisms that are still not completely understood, administration of anti-D antibody within a few days of exposure to RhD-positive erythrocytes completely prevents sensitisation against the D antigen.
AUTOIMMUNE DISEASES
The final aspect of exaggerated or inappropriate immune responses is that related to autoimmune diseases. Autoimmunity is a disturbance in the immunological tolerance of self-antigens. The immune system normally is able to distinguish the individual’s own antigens against foreign antigens. Healthy individuals of all ages, but particularly the elderly, may produce low quantities of antibodies against their own antigens (these are termed autoantibodies) without developing overt autoimmune disease. Therefore, the presence of low quantities of autoantibodies does not necessarily indicate a disease state. Autoimmune diseases occur when the immune system reacts against self-antigens to such a degree that autoantibodies and cytotoxic T cells damage the individual’s own tissues. The reasons for the autoimmunity occurring are not entirely clear, but it appears that genetic inheritance and an environmental trigger, such as infection causing activation of antigen-presenting cells, may promote activation of cytotoxic T cells and subsequent attack on the body’s tissues (see Figure 15-9).
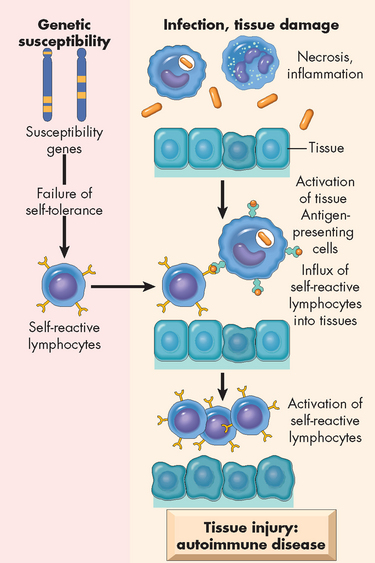
FIGURE 15-9 The proposed pathogenesis of autoimmunity.
Autoimmunity can result from multiple factors, including susceptibility genes that may interfere with self-tolerance and environmental triggers (inflammation, other inflammatory stimuli) that promote lymphocyte entry into tissues, activation of lymphocytes and tissue injury.
Source: Kumar V. Robbins & Cotran pathologic basis of disease. 8th edn. Philadelphia: Saunders; 2010.
Many clinical disorders are associated with autoimmunity and are generally referred to as autoimmune diseases (see Table 15-1). It is fairly well established that autoimmune diseases can be familial. Affected family members may not all develop the same disease, but several members may have different disorders characterised by a variety of hypersensitivity reactions, including autoimmune and allergic.
Table 15-1 AUTOIMMUNE DISORDERS
| SYSTEM DISEASE | ORGAN OR TISSUE | PROBABLE SELF-ANTIGEN |
|---|---|---|
| Endocrine system | ||
| Hyperthyroidism (Graves’ disease) | Thyroid gland | Receptors for thyroid-stimulating hormone on plasma membrane of thyroid cells |
| Hashimoto hypothyroidism | Thyroid gland | Thyroid cell surface antigens, thyroglobulin |
| Insulin-dependent diabetes | Pancreas | Islet cells, insulin, insulin receptors on pancreatic cells |
| Addison’s disease | Adrenal gland | Surface antigens on steroid-producing cells; microsomal antigens |
| Skin | ||
| Pemphigus vulgaris | Skin | Intercellular substances in stratified squamous epithelium |
| Vitiligo | Skin | Surface antigens on melanocytes (melanin-producing cells) |
| Neuromuscular tissue | ||
| Multiple sclerosis | Neural tissue | Surface antigens of nerve cells |
| Myasthenia gravis | Neuromuscular junction | Acetylcholine receptors; striations of skeletal and cardiac muscle |
| Cardiovascular system | ||
| Rheumatic fever | Heart | Cardiac tissue antigens that cross reaction with group A streptococcal antigen |
| Gastrointestinal system | ||
| Ulcerative colitis | Colon | Mucosal cells |
| Pernicious anaemia | Stomach | Surface antigens of parietal cells; intrinsic factor |
| Primary biliary cirrhosis | Liver | Cells of bile duct |
| Chronic active hepatitis | Liver | Surface antigens of hepatocytes, nuclei, smooth muscle |
| Connective tissue | ||
| Ankylosing spondylitis | Joints | Sacroiliac and spinal apophyseal joint |
| Rheumatoid arthritis | Joints | Collagen, IgG |
| Systemic lupus erythematosus | Multiple sites | Numerous antigens in nuclei, organelles and extracellular matrix |
| Renal system | ||
| Immune complex glomerulonephritis | Kidney | Numerous immune complexes |
| Goodpasture’s syndrome | Kidney | Glomerular basement membrane |
| Haematological system | ||
| Idiopathic neutropenia | Neutrophil | Surface antigens on polymorphonuclear neutrophils |
| Autoimmune haemolytic anaemia | Erythrocytes | Surface antigens on erythrocytes |
| Autoimmune thrombocytopenic purpura | Platelets | Surface antigens on platelets |
The breakdown of tolerance
Each individual is usually tolerant to their own antigens. This is termed self-tolerance. Self-tolerance is a state of immunological control so that the individual does not make a detrimental immune response against their own cells and tissues. Autoimmune disease results from a breakdown of this tolerance.
Although many theories exist concerning the initial cause of autoimmune diseases, only one example is known: acute rheumatic fever. In a small number of individuals with group A streptococcal sore throats, proteins in the bacterial capsule mimic normal heart antigens and induce antibodies that also react with proteins in the heart valve, damaging the mitral valve in particular.12 Thus, rheumatic fever is a type II autoimmune hypersensitivity. Additionally, some streptococcal skin or throat infections release bacterial antigens into the blood that form circulating immune complexes. The complexes may deposit in the kidneys and initiate an immune complex glomerulonephritis (inflammation of the kidney). Thus, streptococcal antigens (an environmental antigen) may also cause a type III allergic hypersensitivity.
Many of the autoimmune diseases are presented in the chapter associated with a particular body system (for instance, rheumatoid arthritis is discussed in Chapter 21 on the musculoskeletal system, and rheumatic fever is discussed in Chapter 23 on the cardiovascular system). However, one disease is examined in detail here as it is difficult to categorise into a single body system — systemic lupus erythematosus.
Systemic lupus erythematosus
Systemic lupus erythematosus (SLE) is a complex and serious autoimmune disorder that affects more than 17,000 individuals in Australia.13 It is characterised by the production of a large variety of antibodies (autoantibodies) against self-antigens, including nucleic acids, erythrocytes, coagulation proteins, phospholipids, lymphocytes, platelets and many other self-components.14 The most characteristic autoantibodies are against nucleic acids (e.g. single-stranded DNA, double-stranded DNA), ribonucleoproteins and other nuclear materials. The blood normally contains many of these products of cellular turnover and breakdown. Excessive levels of autoantibodies react with the circulating antigen and form circulating immune complexes. The deposition of circulating DNA/anti-DNA complexes in the kidneys can cause severe kidney inflammation. Similar reactions can occur in the brain, heart, spleen, lungs, gastrointestinal tract, peritoneum and skin. Thus, some of the symptoms of SLE result from a type III hypersensitivity reaction. Other symptoms, such as destruction of red blood cells (anaemia), lymphocytes and other cells, may be type II hypersensitivity reactions.
SLE, like most autoimmune diseases, occurs more often in women (approximately a 10:1 predominance of females), especially in the 20–40-year-old age group (see the box ‘Health alert: autoimmune diseases affect women more than men’). A genetic predisposition for the disease has been implicated on the basis of increased incidence in twins and the existence of autoimmune disease in the families of individuals with SLE.
Clinical manifestations are often many and include arthralgia (joint pain) or arthritis (90% of individuals), vasculitis and rash (70–80% of individuals), renal disease (40–50% of individuals), haematological abnormalities (50% of individuals, with anaemia being the most common complication) and cardiovascular diseases (30–50% of individuals) (see Figure 15-10). As with most autoimmune diseases, SLE is characterised by frequent remissions and exacerbations. Because the signs and symptoms affect almost every body system and tend to come and go, SLE is extremely difficult to diagnose. This has led to the development of a list of 11 common clinical findings. The serial or simultaneous presence of at least four of these indicates that the individual has SLE. They are as follows:15
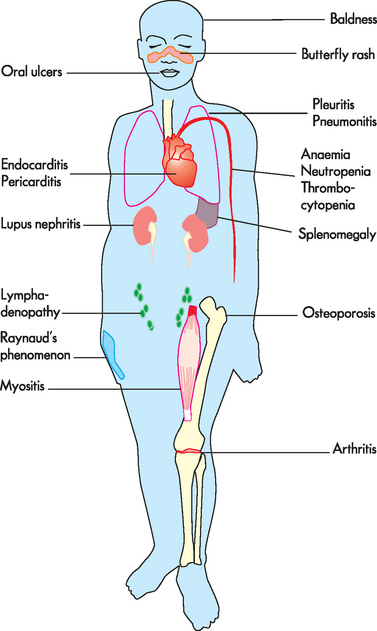
FIGURE 15-10 Systemic clinical manifestations of systemic lupus erythematosus.
Source: Based on Damjanov I. Pathology for the health professions. 3rd edn. Philadelphia: Saunders; 2006.
Autoimmune diseases affect women more than men
To explain why women are especially susceptible to autoimmune disorders, researchers are investigating whether the sex hormones oestrogen and testosterone affect the immune system. Although results are inconclusive, several findings support the idea that differences between gender are based on sex hormones. Disappointingly, however, treating affected individuals with sex hormones has had inconclusive results. Other hormones may affect the progress of autoimmune diseases. In mice, the cells of males and females differ in how they process newly made proteins. These differences may contribute to the increased susceptibility to autoimmune diseases in women.
Source: Christensen D. Vaccine verity: new studies weigh benefits and risks. Sci News 2001; 16:110–111; Cohen-Solal JF et al. Sex hormones and SLE: influencing the fate of autoreactive B cells. Curr Top Microbiol Immunol 2006; 305:67–88; Tomassini V, Pozzilli C. Sex hormones: a role in the control of multiple sclerosis? Expert Opin Pharmacother 2006; 7(7):857–868.
There is no cure for SLE or most other autoimmune diseases. The goals of treatment are to control symptoms and prevent further damage by suppressing the autoimmune response. Non-steroidal anti-inflammatory drugs, such as aspirin, ibuprofen and naproxen, reduce inflammation and relieve pain. Corticosteroids are often prescribed for more serious active disease. Immunosuppressive drugs (e.g. methotrexate, azathioprine or cyclophosphamide) are used to treat severe symptoms involving internal organs. Ultraviolet light can worsen symptoms (known as flares) and protection from sun exposure is helpful. Improved outcomes may be available in the future with continued advances in medical research and the use of stem cell treatments.16
IMMUNE DEFICIENCIES
Thus far we have explored the pathophysiology of enhanced immunity. In these conditions, the immune responses are present but they are either exaggerated or inappropriate. In contrast, immune deficiencies (or insufficient immunity) occur when the immune system or inflammatory responses fail to function normally, resulting in increased susceptibility to pathogenic microorganisms and cancers. Primary immune deficiencies are congenital — that is, they are caused by a genetic defect, whereas secondary immune deficiencies are acquired by another condition, such as cancer, infection or normal physiological changes like ageing. Acquired forms of immune deficiency are far more prevalent than the rare primary immune deficiencies.
Primary immune deficiencies
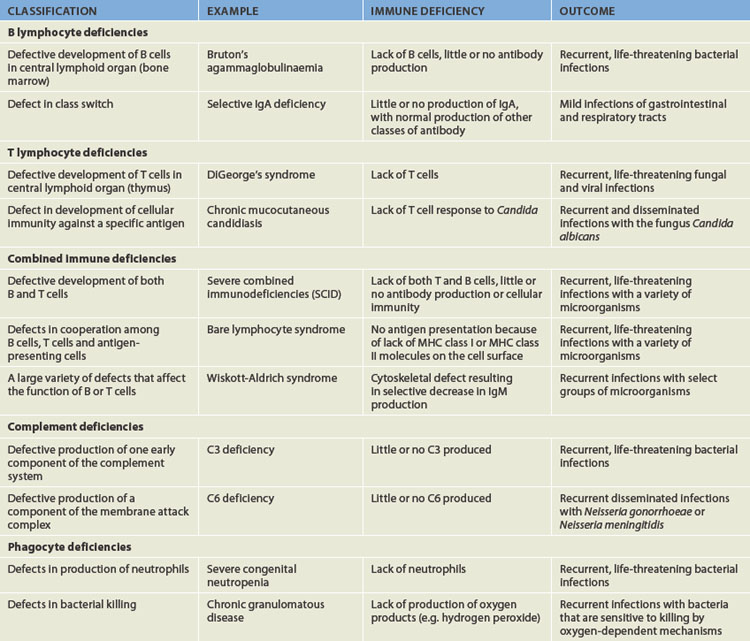
Most primary immune deficiencies are the result of a single gene defect (see Table 15-2).17 Generally, the mutations are sporadic and not inherited: a family history exists in only about 25% of individuals. The sporadic mutations occur before birth, but the onset of symptoms may be early or later, depending on the particular syndrome. In some instances, symptoms of immune deficiency appear within the first 2 years of life. Other immune deficiencies are progressive, with the onset of symptoms appearing in the second or third decade of life.
The clinical hallmark of immune deficiency is a tendency to develop unusual or recurrent severe infections.18,19 Preschool and school-age children may have 6–12 infections per year and adults may have 2–4 infections per year. Most of these are not severe and are limited to viral infections of the upper respiratory tract, recurrent streptococcal pharyngitis or mild otitis media (ear infections).
Individually, primary immune deficiencies are rare. There are approximately 120 different primary immunodeficiency disorders, and a central register has been developed in Australia and New Zealand to account for those individuals with primary immunodeficiency diseases.20 Through the registry, the Australasian Society for Clinical Immunology and Allergy has identified 1209 individuals diagnosed with a primary immunodeficiency disease across both countries — a combined prevalence (Australia and New Zealand) of 4.9 per 100,000 of the population.
Primary immune deficiencies are classified into five groups, based on which principal component of the immune or inflammatory systems is defective:
 B lymphocyte deficiencies result from defects in B cell immune responses.21 This results in lower levels of circulating immunoglobulins (hypogammaglobulinaemia) or occasionally totally or nearly absent immunoglobulins (agammaglobulinaemia).
B lymphocyte deficiencies result from defects in B cell immune responses.21 This results in lower levels of circulating immunoglobulins (hypogammaglobulinaemia) or occasionally totally or nearly absent immunoglobulins (agammaglobulinaemia). T lymphocyte deficiencies are defects in the development and function of T lymphocytes.21 Because helper T cells are obligatory in the development of many B lymphocyte responses, antibody production is often diminished, although the B cells are fully capable of producing an adequate antibody response. Immunodeficiency of T cell function contributes to failure to thrive, oral infections (e.g. candidiasis), chronic diarrhoea, pneumonia and skin rashes.
T lymphocyte deficiencies are defects in the development and function of T lymphocytes.21 Because helper T cells are obligatory in the development of many B lymphocyte responses, antibody production is often diminished, although the B cells are fully capable of producing an adequate antibody response. Immunodeficiency of T cell function contributes to failure to thrive, oral infections (e.g. candidiasis), chronic diarrhoea, pneumonia and skin rashes. Combined immune deficiencies result from defects that directly affect the development of both T and B lymphocytes. Some combined deficiencies result in major defects in both the T and B cell immune responses, whereas others are ‘partial’ and more adversely affect T cells than B cells. The most severe are called severe combined immunodeficiencies. Most individuals with severe combined immunodeficiencies have few detectable lymphocytes in the circulation and secondary lymphoid organs (spleen, lymph nodes). The thymus usually is underdeveloped because of the absence of T cells. Immunoglobulin levels, especially IgM and IgA, are absent or greatly reduced. Children with this deficiency develop serious, life-threatening infections and usually die before the age of 5 years.
Combined immune deficiencies result from defects that directly affect the development of both T and B lymphocytes. Some combined deficiencies result in major defects in both the T and B cell immune responses, whereas others are ‘partial’ and more adversely affect T cells than B cells. The most severe are called severe combined immunodeficiencies. Most individuals with severe combined immunodeficiencies have few detectable lymphocytes in the circulation and secondary lymphoid organs (spleen, lymph nodes). The thymus usually is underdeveloped because of the absence of T cells. Immunoglobulin levels, especially IgM and IgA, are absent or greatly reduced. Children with this deficiency develop serious, life-threatening infections and usually die before the age of 5 years. Complement deficiencies result from deficient or abnormal levels of complementary plasma proteins. Individuals with complement deficiencies have an increased risk of infection and autoimmune-like disorders. The complement system is integral to a normal immune response and so individuals with genetic abnormalities that affect the complement system are not able to mount immune responses to pathogenic microorganisms.
Complement deficiencies result from deficient or abnormal levels of complementary plasma proteins. Individuals with complement deficiencies have an increased risk of infection and autoimmune-like disorders. The complement system is integral to a normal immune response and so individuals with genetic abnormalities that affect the complement system are not able to mount immune responses to pathogenic microorganisms.Secondary immune deficiencies
Secondary, or acquired, immune deficiencies are far more common than primary deficiencies. These deficiencies are not related to genetic defects but are complications of other physiological or pathophysiological conditions. Conditions associated with secondary immunodeficiencies are listed in Table 15-3.
Table 15-3 CONDITIONS ASSOCIATED WITH SECONDARY IMMUNODEFICIENCIES
| GROUP | CONDITION |
|---|---|
| Normal physiological conditions | |
| Psychological stress | |
| Dietary insufficiencies | |
| Malignancies | |
| Physical trauma | |
| Medical treatments | |
| Other diseases or genetic syndromes |
Although secondary immunodeficiencies are common, many are not clinically significant. In many cases, the degree of the immune deficiency is relatively minor and without any apparent increased susceptibility to infection. Alternatively, the immune system may be substantially suppressed, but only for a short duration, thus minimising the incidence of clinically relevant infections.
However, some secondary immune deficiencies are extremely severe and may result in recurrent life-threatening infections, such as malignancy and burns. The most destructive secondary immunodeficiency is acquired immunodeficiency syndrome (AIDS). The following section explores the pathophysiology of AIDS and the virus that causes the disease, the human immunodeficiency virus (HIV).
Acquired immunodeficiency syndrome
The human immunodeficiency virus (HIV) infects and destroys helper T cells, which are necessary for the development of both plasma cells and cytotoxic T cells. Therefore, HIV suppresses the immune response against itself and secondarily creates a generalised immune deficiency by suppressing the development of immune responses against other pathogens and opportunistic microorganisms, leading to the development of acquired immunodeficiency syndrome (AIDS).
An estimated 33 million individuals worldwide are living with HIV (see Figure 15-11).22 Approximately 7400 people become infected every day, which equated to about 2.7 million new HIV infections and 2 million AIDS-related deaths in 2007.22 The majority of new cases are in sub-Saharan Africa, but the epidemic is worldwide and the number of new cases is increasing. Unfortunately, this trend is likely to continue as universal access to treatment is not equitable. For instance, for every two individuals who commence treatment for HIV, five more become infected.22
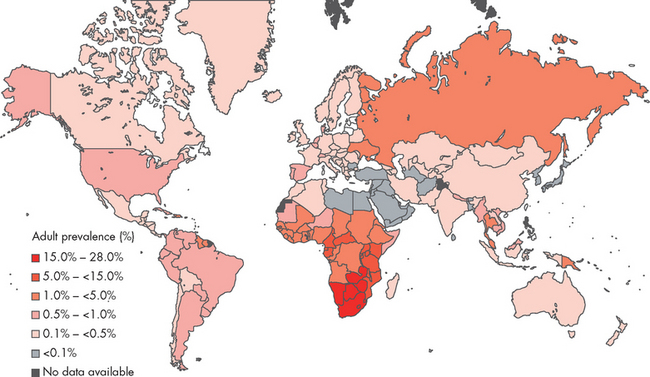
FIGURE 15-11 A global view of HIV infection.
The countries of the Oceanic region, including Australia and New Zealand, all have very low rates of HIV infection except for Papua New Guinea. In 2007, 33 million people were living with HIV worldwide.
Source: UNAIDS. 2008 report on the global AIDS epidemic. Available at www.unaids.org.
In Oceania, which contains both Australia and New Zealand, an estimated 74,000 individuals are living with HIV. Most of the region has a relatively small number of HIV-infected individuals compared with other regions; however, Papua New Guinea has an HIV epidemic, with approximately 54,000 of its 6 million residents living with HIV as at the end of 2007, up from 10,000 in 2001.22 By comparison, between the inception of HIV and the end of 2007, Australia recorded 27,331 cases of HIV infection, 10,303 cases of AIDS and 6767 deaths out of a total population of 22 million. In 2007, the number of individuals living with HIV in Australia was approximately 17,000, 12,000 of whom were in the 15–49 age group.23
Epidemiology
HIV is a blood-borne pathogen with the typical routes of transmission: blood or blood products, intravenous drug abuse, both heterosexual and homosexual activity and maternal–child transmission before or during birth (see Table 15-4). Worldwide, the most common route of transmission is through heterosexual contact, but in Australia and New Zealand homosexual contacts account for the largest proportion of new diagnoses (see Figure 15-12). In 2007, 1051 new diagnoses of HIV were made in Australia, while 184 new diagnoses were made in New Zealand in 2008.23,24 The rate of new diagnoses has remained stable for many years. Unfortunately, in both countries the rate of new diagnoses is proportionally higher in the Indigenous populations than in the non-Indigenous populations.23,24 However, overall the rates of HIV infection are low compared to other Western countries and reflect the effectiveness of public health campaigns instituted as early as 1987.
Table 15-4 POSSIBLE TRANSMISSION ROUTES OF HIV INFECTION
| ROUTES | SPECIFIC TRANSMISSION |
|---|---|
| Known routes of transmission | |
| Inoculation in blood | |
| Sexual transmission | |
| Perinatal transmission | |
| Routes not involved in transmission | |
| Close personal contact | |
Source: Murray PR. Medical microbiology. 5th edn. St Louis: Mosby; 2005.
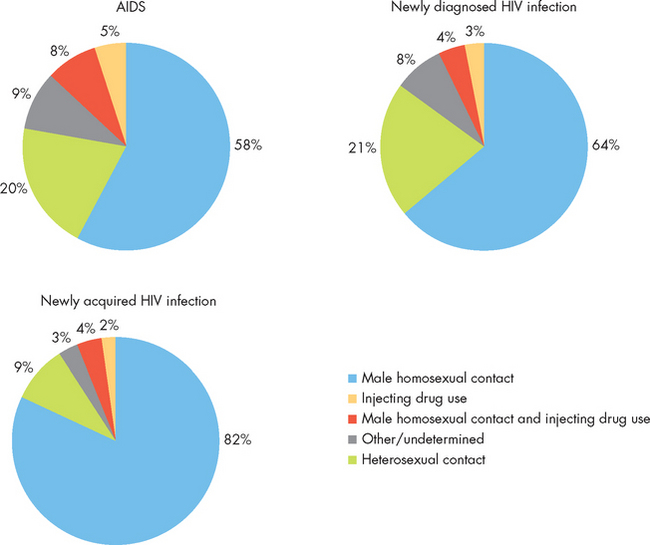
FIGURE 15-12 The proportion of newly diagnosed and newly acquired cases of HIV infection in Australia, 2003–2007.
Source: Based on National Centre in HIV Epidemiology and Clinical Research. HIV/AIDS, viral hepatitis and sexually transmissible infections in Australia: annual surveillance report 2008. Sydney: The University of New South Wales; 2008.
PATHOGENESIS
HIV is a member of a family of viruses called retroviruses, which carry genetic information in the form of RNA rather than DNA. Retroviruses use a viral enzyme, reverse transcriptase, to convert RNA into double-stranded DNA. Using a second viral enzyme, an integrase, the new DNA is inserted into the infected cell’s genetic material, where it may remain dormant. If the cell is activated, translation of the viral information may be initiated, resulting in the formation of new virions, lysis and death of the infected cell, and shedding of infectious HIV particles. If, however, the cell remains relatively dormant, the viral genetic material may remain latent for years and is probably present for the life of the individual (see Figure 15-13).
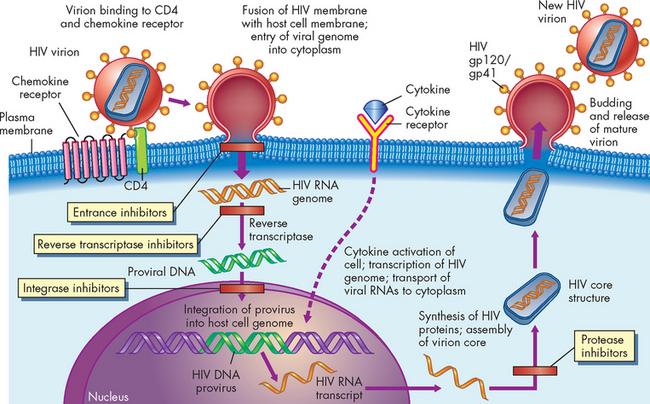
FIGURE 15-13 The life cycle and possible sites of therapeutic intervention of human immunodeficiency virus.
The HIV virion consists of a core of two identical strands of viral RNA encoated in a protein structure with viral proteins gp41 and gp120 on its surface (envelope). HIV infection begins when a virion binds to CD4 and chemokine co-receptors on a susceptible cell and follows the process described here. The provirus may remain latent in the cell’s DNA until it is activated (e.g. by cytokines). The HIV life cycle is susceptible to blockage at several sites (see the text for further information), including entrance inhibitors, reverse transcriptase inhibitors, integrase inhibitors and protease inhibitors.
Source: Kumar V et al. Robbins & Cotran pathologic basis of disease. 7th edn. Philadelphia: Saunders; 2005.
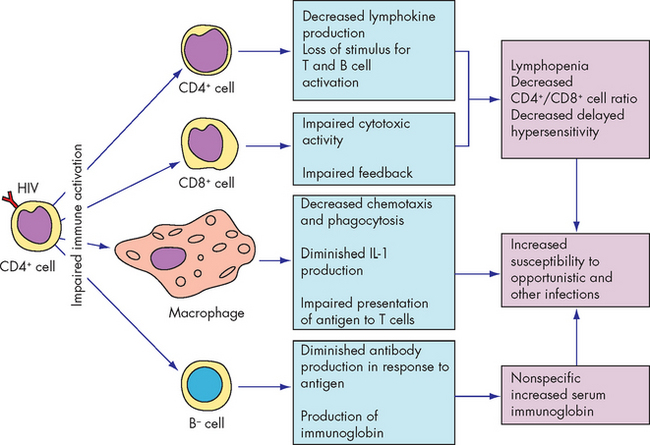
The primary surface receptor on HIV is the envelope protein gp120, which binds to the molecule CD4 on the surface of helper T cells. Several other necessary co-receptors have been identified on target cells. Thus, the major immunological finding in AIDS is the striking decrease in the number of CD4+ helper T cells (see Figure 15-14). Individuals who are not HIV-infected typically have a range of 600–1200 CD4+ cells per cubic millimetre of blood (count/mm3). In individuals with HIV the initial decline reduces the CD4+ count to about 500–600/mm3 and generally the CD4+ count declines as HIV disease progresses. The count is often used as a guide to initiating treatment.
CLINICAL MANIFESTATIONS
Depletion of CD4+ cells has a profound effect on the immune system, causing a severely diminished response to a wide array of infectious pathogens and malignant tumours (see Box 15-1). At the time of diagnosis, the individual may present with one of several different conditions: serologically negative (no detectable antibody), serologically positive (positive for antibody against HIV) but asymptomatic, early stages of HIV disease or AIDS (see Figure 15-15).
Box 15-1 AIDS-DEFINING OPPORTUNISTIC INFECTIONS AND NEOPLASMS FOUND IN HIV-INFECTED INDIVIDUALS
INFECTIONS
Protozoal and helminthic infections
Cryptosporidiosis or isosporiasis (enteritis)
Pneumocystosis (pneumonia or disseminated infection)
Toxoplasmosis (pneumonia or central nervous system infection)
Candidiasis (oesophageal, tracheal or pulmonary)
Cryptococcosis (central nervous system infection)
Coccidioidomycosis (disseminated)
Mycobacteriosis (atypical, e.g. M. avium-intracellulare, disseminated or extrapulmonary; M. tuberculosis, pulmonary or extrapulmonary)
Nocardiosis (pneumonia, meningitis, disseminated)
Salmonella infections (disseminated)
Cytomegalovirus (pulmonary, intestinal, retinitis or central nervous system infections)
Herpes simplex virus (localised or disseminated)
Varicella zoster virus (localised or disseminated)
Progressive multifocal (leucoencephalopathy)
B cell non-Hodgkin’s lymphomas
Invasive cancer of the uterine cervix
Source: Kumar V, Abbas A, Fausto N. Robbins & Cotran pathologic basis of disease. 7th edn. Philadelphia: Saunders; 2005.
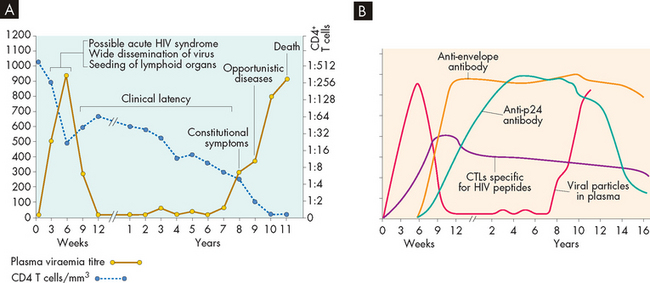
FIGURE 15-15 Typical progression from HIV infection to AIDS in untreated individuals.
A Clinical progression begins within weeks after infection; the person may experience symptoms of acute HIV syndrome. During this early period, the virus progressively infects T cells and other cells and spreads to the lymphoid organs, with a sharp decrease in circulating CD4+ T cells. During a period of clinical latency, the virus replicates and T cell destruction continues, although the person is generally asymptomatic. The individual may develop HIV-related disease (constitutional symptoms) — a variety of symptoms of acute viral infection that do not involve opportunistic infections or malignancies. When the number of CD4+ cells is critically suppressed, the individual becomes susceptible to a variety of opportunistic infections and cancers with a diagnosis of AIDS. The length of time for progression from HIV infection to AIDS may vary considerably from person to person. B Laboratory tests are changing throughout infection. Antibody and Tc cell (cytotoxic T lymphocytes [CTLs]) levels change during the progression to AIDS. During the initial phase, antibodies against HIV-1 are not yet detectable (window period), but viral products, including proteins and RNA, and infectious virus, may be detectable in the blood a few weeks after infection. Most antibodies against HIV are not detectable in the early phase. During the latent phase of infection, antibody levels against p24 and other viral proteins, as well as HIV-specific CTLs, increase, then remain constant until the development of AIDS.
Source: A Fauci AS, Lane HC. Human immunodeficiency virus disease: AIDS and related conditions. In: Fauci AS et al (eds). Harrison’s principles of internal medicine. 14th edn. New York: McGraw-Hill; 1997. B Kumar V et al. Robbins & Cotran pathologic basis of disease. 8th edn. Philadelphia: Saunders; 2010.
The presence of circulating antibody against the HIV indicates infection by the replicating virus, although many of these individuals are asymptomatic. Antibody appears rather rapidly after infection through blood products, usually within 4–7 weeks. After sexual transmission, however, the individual can be infected yet seronegative for 6–14 months or, in at least one case, for years. The period between infection and the appearance of antibody is referred to as the window period. Although an individual may not have antibody, they may have virus growing, have virus in the blood and body fluids, and be infectious to others.
Those with the early stages of HIV disease (early-stage disease) usually present with relatively mild symptoms resembling influenza, such as night sweats, swollen lymph glands, diarrhoea or fatigue. The early stage may last as long as 10 years. Although individuals appear to be in clinical latency, the virus is actively proliferating in the lymph nodes (see Figure 15-16).
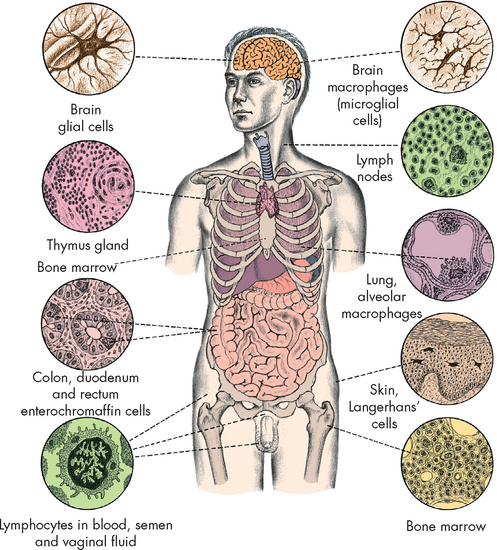
FIGURE 15-16 The distribution of tissues that can be infected with HIV.
Source: Weber JN, Weiss RA. HIV infection: the cellular picture, in the science of AIDS: readings from Scientific American. New York: Freeman; 1989.
The currently accepted definition of AIDS relies on both laboratory tests and clinical symptoms. The most common laboratory test is for antibodies against HIV. If the individual is seropositive, the diagnosis of AIDS is made in association with various clinical symptoms (see Box 15-1 and Figure 15-17). The symptoms include atypical or opportunistic infections and cancers, as well as indications of debilitating chronic disease (e.g. wasting syndrome, recurrent fevers). Most commonly, new cases of AIDS are diagnosed initially by decreased CD4+ T cell numbers. The average time from infection to development of AIDS has been estimated at just over 10 years. Some estimates are that approximately 99% of untreated HIV-infected individuals will eventually progress to AIDS. As the disease progresses, body systems become increasingly affected (see Figure 15-18).
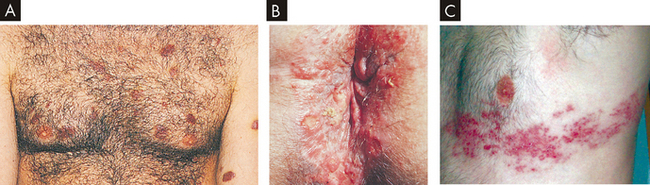
FIGURE 15-17 Clinical manifestations of acquired immunodeficiency syndrome.
A Kaposi’s sarcoma lesions over chest and arms. B Perianal lesions of herpes simplex infection. C Herpes zoster infection.
Source: A & B Morse SA, Ballard RC, Holmes KK et al (eds). Atlas of sexually transmitted diseases and AIDS. 3rd edn. Edinburgh: Mosby; 2003. C Walsh D. Palliative medicine. Philadelphia: Saunders; 2008.
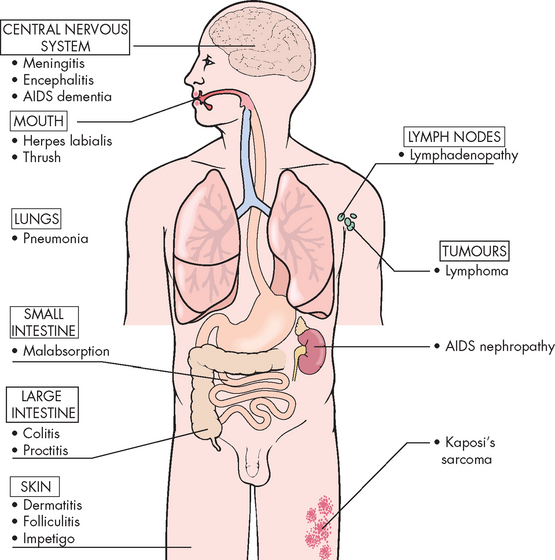
FIGURE 15-18 Systemic changes associated with acquired immunodeficiency syndrome.
Source: Damjanov I. Pathology for the health professions. 3rd edn. Philadelphia: Elsevier; 2006.
Neurological and cardiac complications are detailed in Box 15-2.
BOX 15-2 COMPLICATIONS OF AIDS
Neurological complications
Some 40–60% of all individuals with AIDS have neurological complications. The most common neurological disorder is HIV-associated cognitive dysfunction (HIV encephalopathy). It may affect adults or children and is characterised by progressive cognitive dysfunction with motor and behavioural alterations. The syndrome typically develops later in the disease, but may be an early or singular manifestation in some individuals.
It is believed that HIV-associated cognitive dysfunction results from direct brain tissue infection by the virus. HIV is found mostly in white matter sub-cortical areas causing an immune demyelinating-mediated process (literally, stripping the myelin), but some viral replication occurs in glial cells (central nervous system support cells) and, occasionally, neurons.
The cognitive dysfunction is insidious in onset and unpredictable in its course. Most individuals experience a steady progression with abrupt accelerations of signs over several months to more than one year, although some individuals experience an abrupt onset or an accelerated course. Early clinical manifestations may be vague. Impaired concentration and memory deficits are common, and apathy, lack of motivation, social withdrawal, irritability and emotional lability appear. Later, difficulties with language, spatial or temporal disorientation and visual construction are present.
Diagnosis is difficult, especially in early stages. A history with physical examination findings and supporting cerebrospinal fluid, CT scan and MRI data help establish the diagnosis. Treatment consists of antiviral agents and supportive measures.
Cardiac complications
Individuals infected with HIV and AIDS are at risk for cardiac complications, including dilated cardiomyopathy, myocarditis, pericardial effusion, endocarditis, pulmonary hypertension and non-antiretroviral drug-related cardiotoxicity. In addition to HIV infection, cardiac involvement may be induced by the accompanying inflammatory response or by various bacterial, viral, protozoan, mycobacterial and fungal pathogens. Malignancies, such as lymphoma and Kaposi’s sarcoma, are often seen in individuals with AIDS and can affect the heart. Furthermore, treatment with highly active antiretroviral therapy (HAART) can cause hyperlipidaemia and atherosclerotic disease. Left heart failure is the most common complication of HIV infection and is related to left ventricular dilation and dysfunction. Pericardial effusion, ventricular arrhythmias, electrocardiographic changes and right ventricular dilation and hypertrophy are other less common findings.
Opportunistic infections
Opportunistic infections may be bacterial, fungal or viral in origin and may produce disease. Typically, bacterial infections are caused by unusual microorganisms. Cryptococcal infection is the most common fungal disorder and the third leading cause of neurological disease in individuals with AIDS. The symptoms are vague, such as fever, headache and malaise. Cytomegalovirus encephalitis is common in persons with AIDS. Toxoplasmosis (a protozoal infection) is a common central nervous system disorder that occurs in one-third of those with AIDS.
Source: Zareba KM, Miller TL, Lipschultz SE. Cardiovascular disease and toxicities related to HIV infection and its therapies. Exp Opinion Drug Safety 2005; 4(6):1017–1025. Belman A, Marett-Savatic M. Human immunodeficiency virus and acquired immunodeficiency syndrome. In: Goetz C (ed.). Textbook of clinical neurology. Philadelphia: Saunders; 2003.
TREATMENT AND PREVENTION
The current regimen for treatment of HIV infection is a combination of drugs, termed highly active antiretroviral therapy (HAART). The combination includes inhibitors of reverse transcriptase (reverse transcriptase inhibitors) and of the viral protease (protease inhibitors) (see Figure 15-13). The clinical benefits of HAART are profound and durable. Death from AIDS-related diseases has been reduced significantly since the introduction of HAART. However, resistant variants to these drugs have been identified.
Drug therapy for AIDS is difficult because, like most retroviruses, the AIDS virus incorporates into the genetic material of the host and may never be removed by antimicrobial therapy. Therefore, drug administration to control the virus may have to continue for the lifetime of the individual. Additionally, HIV may persist in regions where the antiviral drugs are not as effective, such as the central nervous system. Inhibitors of the initial viral entrance into the target cell (entrance inhibitors) and inhibitors of the viral integrase (integrase inhibitors) have undergone clinical trials and are being added to the combination.
Vaccine development for preventing HIV infection is ongoing. Most of the common viral vaccines (e.g. rubella, mumps, influenza) induce protective antibodies that block the initial infection. Only one vaccine (rabies) is used after the infection has occurred. That approach is successful because the rabies virus proliferates and spreads very slowly. Whether an HIV vaccine would successfully prevent or treat HIV infection is questionable for several reasons. First, HIV is genetically and antigenically variable, like the influenza virus, so that a vaccine created against one variant may not provide protection against another variant. Second, although individuals with AIDS have high levels of circulating antibodies against the virus, these antibodies do not appear to be protective. Therefore, even if a circulating antibody response can be induced by vaccination, that response might not be effective.
Hypersensitivity reactions
 Hypersensitivity is an inappropriate immune response misdirected against the host’s own tissues or directed against beneficial foreign tissues, such as transfusions or transplants; or it can be an exaggerated response against environmental antigens (allergy).
Hypersensitivity is an inappropriate immune response misdirected against the host’s own tissues or directed against beneficial foreign tissues, such as transfusions or transplants; or it can be an exaggerated response against environmental antigens (allergy). Mechanisms of hypersensitivity are classified as type I IgE-mediated hypersensitivity reactions, type II tissue-specific hypersensitivity reactions, type III immune complex-mediated hypersensitivity reactions and type IV cell-mediated hypersensitivity reactions.
Mechanisms of hypersensitivity are classified as type I IgE-mediated hypersensitivity reactions, type II tissue-specific hypersensitivity reactions, type III immune complex-mediated hypersensitivity reactions and type IV cell-mediated hypersensitivity reactions. Hypersensitivity reactions can be immediate (developing within seconds or hours) or delayed (developing within hours or days).
Hypersensitivity reactions can be immediate (developing within seconds or hours) or delayed (developing within hours or days). Anaphylaxis, the most rapid immediate hypersensitivity reaction, is an explosive reaction that occurs within minutes of re-exposure to the antigen and can lead to cardiovascular shock.
Anaphylaxis, the most rapid immediate hypersensitivity reaction, is an explosive reaction that occurs within minutes of re-exposure to the antigen and can lead to cardiovascular shock. Type I hypersensitivity reactions occur after antigen reacts with IgE on mast cells, leading to mast cell degranulation and the release of histamine and other inflammatory substances.
Type I hypersensitivity reactions occur after antigen reacts with IgE on mast cells, leading to mast cell degranulation and the release of histamine and other inflammatory substances. Type II hypersensitivity reactions are caused by four possible mechanisms: complement-mediated lysis; opsonisation and phagocytosis; antibody-dependent cell-mediated cytotoxicity; and modulation of cellular function.
Type II hypersensitivity reactions are caused by four possible mechanisms: complement-mediated lysis; opsonisation and phagocytosis; antibody-dependent cell-mediated cytotoxicity; and modulation of cellular function. Type III hypersensitivity reactions are caused by the formation of immune complexes that are deposited in target tissues, where they activate the complement cascade, generating chemotactic fragments that attract neutrophils into the inflammatory site.
Type III hypersensitivity reactions are caused by the formation of immune complexes that are deposited in target tissues, where they activate the complement cascade, generating chemotactic fragments that attract neutrophils into the inflammatory site. Type IV hypersensitivity reactions are caused by specifically sensitised T cells, which either kill target cells directly or release lymphokines that activate other cells, such as macrophages.
Type IV hypersensitivity reactions are caused by specifically sensitised T cells, which either kill target cells directly or release lymphokines that activate other cells, such as macrophages. Clinical manifestations of allergic reactions are usually confined to the areas of initial intake or contact with the allergen. Ingested allergens induce gastrointestinal symptoms, airborne allergens induce respiratory or skin manifestations and contact allergens induce allergic responses at the site of contact.
Clinical manifestations of allergic reactions are usually confined to the areas of initial intake or contact with the allergen. Ingested allergens induce gastrointestinal symptoms, airborne allergens induce respiratory or skin manifestations and contact allergens induce allergic responses at the site of contact.Transplantation
Autoimmune diseases
Immune deficiencies
 Immunodeficiencies are either primary (congenital) or secondary (acquired). Primary immunodeficiencies are caused by genetic defects that disrupt lymphocyte development, whereas acquired immunodeficiencies are secondary to disease or other physiological alterations.
Immunodeficiencies are either primary (congenital) or secondary (acquired). Primary immunodeficiencies are caused by genetic defects that disrupt lymphocyte development, whereas acquired immunodeficiencies are secondary to disease or other physiological alterations. The clinical hallmark of immunodeficiency is a propensity to unusual or recurrent severe infections. The type of infection usually reflects the immune system defect.
The clinical hallmark of immunodeficiency is a propensity to unusual or recurrent severe infections. The type of infection usually reflects the immune system defect. The most common infections in individuals with defects of cell-mediated immune response are fungal and viral, whereas infections in individuals with defects of the humoral immune response or complement function are primarily bacterial.
The most common infections in individuals with defects of cell-mediated immune response are fungal and viral, whereas infections in individuals with defects of the humoral immune response or complement function are primarily bacterial. Severe combined immunodeficiency is a total lack of T cell function and a severe (either partial or total) lack of B cell function.
Severe combined immunodeficiency is a total lack of T cell function and a severe (either partial or total) lack of B cell function. Defects in B cell function are diverse, ranging from a complete lack of the human bursal equivalent, the lymphoid organs required for B cell maturation, to deficiencies in a single class of immunoglobulins.
Defects in B cell function are diverse, ranging from a complete lack of the human bursal equivalent, the lymphoid organs required for B cell maturation, to deficiencies in a single class of immunoglobulins. Acquired immunodeficiencies are caused by superimposed conditions, such as malnutrition, medical therapies, physical or psychological trauma or infections.
Acquired immunodeficiencies are caused by superimposed conditions, such as malnutrition, medical therapies, physical or psychological trauma or infections.Mohammed is 26 years old and is allergic to the antibiotic, penicillin. He was exposed to penicillin as a child (aged 6) when it was prescribed to treat an infected abscess on his leg. At the time he developed a whole body rash and had itchy skin — he did not have skin testing but was informed that an allergic reaction to penicillin was the most likely cause. Mohammed is currently in hospital after being involved in a motor vehicle accident. He was trapped in the vehicle and sustained a fractured right femur requiring orthopaedic surgery. His medical notes clearly document that he is allergic to penicillin. On returning to the ward after an open reduction and internal fixation to correct the fractured femur he is prescribed cefazolin (intravenously), a cephalosporin antibiotic with some similarities to penicillin. Shortly after the first dose of cefazolin has started he rings his buzzer, and the nurse immediately notices that he has a rash, an audible wheeze, angio-oedema and pale skin. His blood pressure is low, his heart rate elevated and his breathing rapid and shallow. An emergency is called and Mohammed is successfully resuscitated using adrenaline.