31 THE STRUCTURE AND FUNCTION OF THE REPRODUCTIVE SYSTEMS
INTRODUCTION
Human beings are sexually reproductive organisms; parts of the genetic material from the mother and father combine to produce offspring that each have a unique and distinguishing set of DNA. With the exception of fraternal twins (and other multiple births), which are derived from the same fertilised egg, each individual human being has a one-of-a-kind combination of DNA. The production of this exclusive DNA code is made possible by the process of sexual reproduction — only half of the mother’s DNA goes into each egg (ovum = singular, ova = plural) and only half of the father’s DNA goes into each sperm, and for each egg or sperm, a different mix of chromosomes make the DNA. This explains our incredible diversity of appearance, ability and genetic susceptibility to some diseases.
Sexual reproduction involves two fundamental processes:
The sex cells (sperm and ova), collectively known as gametes, are special cells in the body as they have only half the number of chromosomes that all other types of body cells (somatic cells) have. When two gametes fuse during fertilisation, the normal chromosome number is reached. The cell that is created by the fusion of the male and female gametes is initially known as a zygote and goes on to develop into an embryo, then a fetus and finally the newborn from the time of birth.
The primary reproductive organs that create the gametes are known as the gonads (i.e. gametogenic organs): the testes in the male and the ovaries in the female. These have two functions:
Although the gonads are the primary reproductive organs, the reproductive system requires other organs and tissues to deliver sperm to the female and to transport and support the growing fertilised embryo. In both males and females there are ducts (tubes) for the transport of gametes. In males there are glands for the secretion of fluids (collectively semen) essential for fertilisation; and in females there is the uterus to support, protect and nurture the embryo until birth, and breasts to feed the newborn infant. The genitalia of both males and females are comprised of internal and external structures; while most people are familiar with the names and shapes of the external genitalia, the internal genitalia are less well known to the layperson.
In this chapter we introduce the anatomical and functional aspects of the adult male and female reproductive systems and explore the role of hormones in the control of reproductive development in the early embryo, children and adults. We also consider how gametes are formed in both males and females and outline the stages of the sexual process that brings the gametes together. Finally, we focus on stages of conception, gestation and parturition.
We begin by looking at the anatomy and function of the male and female reproductive organs.
THE STRUCTURE AND FUNCTION OF THE MALE REPRODUCTIVE SYSTEM
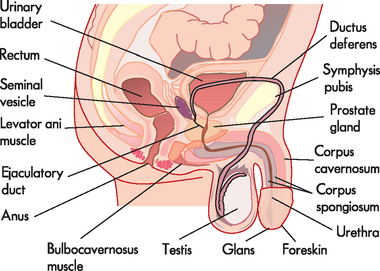
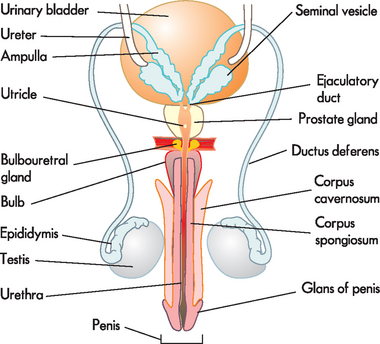
In males, the external organs that are visible are the scrotum (containing the testes) and the penis.1 The external genitalia perform the major functions of reproduction (see Figure 31-1). Sperm (spermatozoa) are produced in the testes (plural; singular = testis) and are delivered by the penis. The internal male genitalia consist of tubes to transport the sperm, and fluid-producing glands to aid in the transport of sperm from the testes to the urethral opening of the penis.
External structures
Testes
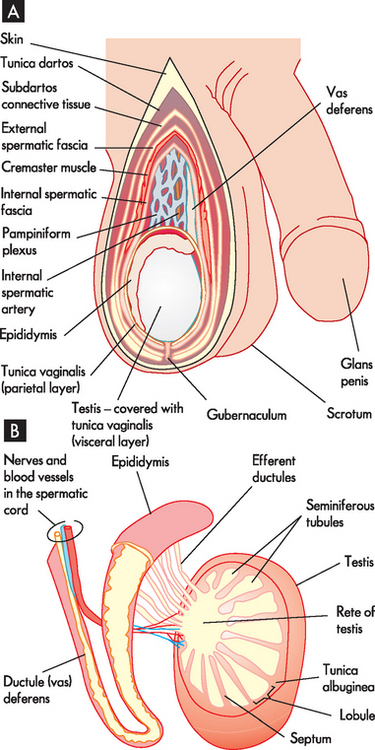
The testes (see Figure 31-2) are the source of both sperm and the male sex hormone, testosterone. There are usually two testes, oval in shape and approximately 4–5cm long, located outside the body cavity in the scrotum (a pouch of skin). The cremaster muscles in the scrotum can raise the testes closer to the body (in a cold environment) or lower them further from the body (in a hot environment). The temperature in the scrotum is approximately 3°C lower than normal core body temperature (see Figure 31-3) — this is essential for the survival of sperm. During fetal development, the testes develop in the upper body cavity and at 7 months’ gestation they move through the inguinal canal to the scrotum together with the spermatic cord.
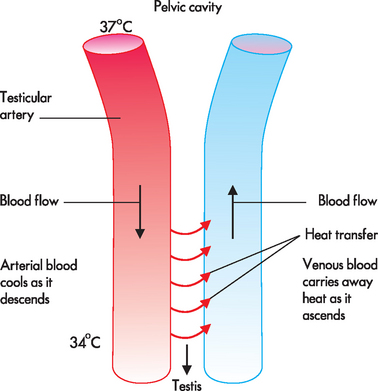
FIGURE 31-3 The heat exchanger of the testis.
As blood descends from the body through the testicular artery, heat is transferred into nearby venous blood, so the arterial blood cools as it descends. The blood that reaches the testes is approximately 3°C cooler than blood in the rest of the body.
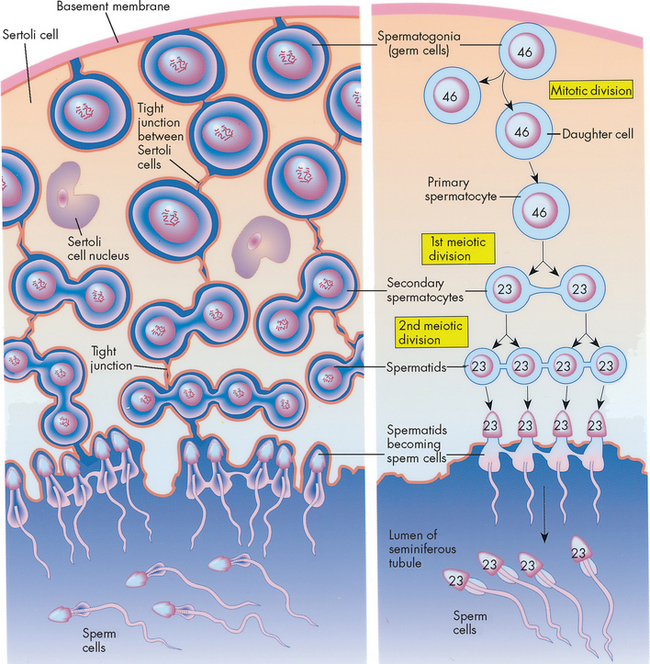
Each testis is divided into about 250 internal compartments called lobules, each of which contains 1 to 3 long, thin tightly coiled hollow tubes called seminiferous tubules. The seminiferous tubules are the sites of sperm production and contain two major types of cells: (1) spermatogonia, which are germ cells that line the walls and develop into spermatozoa; and (2) sustentacular (Sertoli) cells, which support this process. Adjacent sustentacular cells form tight junctions in which the walls of two adjacent cells are ‘stuck’ together very firmly. These tightly joined cells create a blood–testis barrier, which prevents immune blood cells from entering the seminiferous tubules. This barrier is required because the developing sperm have surface antigens that could be recognised as foreign by the immune system; in the absence of the barrier, an immune attack would be mounted against the sperm, causing inflammation and possible male sterility.
Located between the tightly packed seminiferous tubules are cells called interstitial (Leydig) cells. These cells secrete testosterone when stimulated by luteinising hormone (LH).
Penis
The human male penis (see Figure 31-4) performs two major functions: (1) the elimination of urine; and (2) the delivery of mature spermatozoa and associated fluids to the female reproductive tract. The structure of the penis is such that it is a small soft organ for the majority of the time, but becomes greatly enlarged and stiffened for the purpose of reproduction.
The penis contains 3 cylindrical columns of erectile tissue — that is, ‘spongy’ tissue that becomes rigid when congested with blood (supplied to the penis from the internal iliac artery via the internal pudendal or penile artery). On each side of the penis there is an erectile corpus cavernosum and medially there is the corpus spongiosum, the tip of which is enlarged to form the glans penis. The penile urethra runs through the corpus spongiosum and the glans is covered by a double fold of skin, the prepuce (foreskin).
The nerve supply to the penis is from the second, third and fourth sacral spinal nerves, through the pelvic plexuses and the internal pudendal nerve. These are the nerves that are involved in the different stages of the male sexual response. These nerves can be damaged through trauma (often car or motorcycle accidents) and can result in altered sexual function.
Internal structures
Spermatic ducts
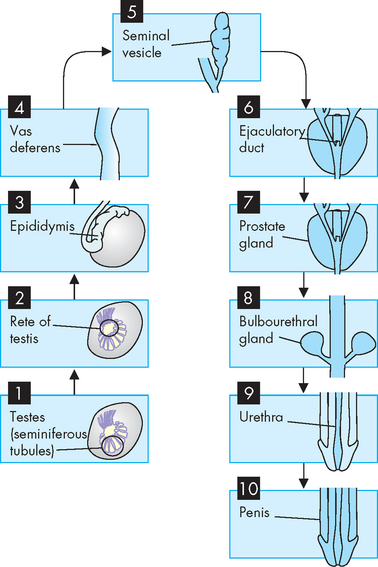
Sperm cells must travel from the testis to the tip of the penis in order to be delivered into the female reproductive system. This is achieved by a series of dedicated tubes (see Figure 31-2) through which the sperm move.
Sperm leave the seminiferous tubules, travel through the efferent ducts (coiled tubules) and reach the epididymis. The epididymis is attached to the posterior side of the testis. It consists of a head, body and tail region, the tail continuing into the ductus deferens. Within the epididymis there is a highly coiled tube called the ductus epididymis, which is the site of sperm maturation and storage.
Mature sperm move from the epididymis into the pelvic cavity through the ductus deferens (previously known as the vas deferens), which passes upwards behind the testis and epididymis, through the inguinal canal and into the pelvic cavity. Here it turns back over the ureter and tunnels through the urinary bladder (although the contents of the ductus deferens do not mix with the bladder; refer to Figure 31-1). The last section of the ductus deferens is dilated to form the ampulla. Mature sperm are stored here, awaiting ejaculation. The ductus deferens together with blood vessels, lymphatics, autonomic nerves and cremaster muscle make up the spermatic cord. The cremaster muscle elevates the testes during sexual arousal or during exposure to cold and lowers the testes when body temperature rises.
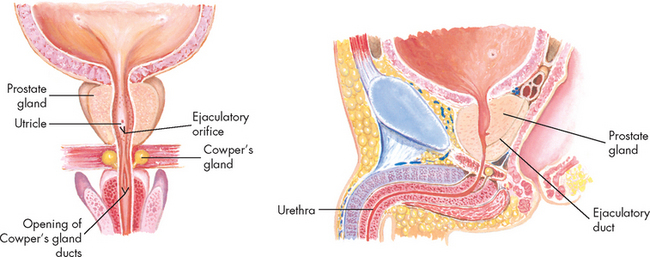
Located at the distal end of the ampulla is the ejaculatory duct. This short tube (2 cm) tunnels through the prostate gland (without mixing with prostate tissue) to join the urethra. Note that it is within the prostate gland that the tubules that contain reproductive contents (including sperm) become shared with the tubules that contain urine (urethra). The urethra transports these fluids to exit the penis at different times such that they do not mix. Note that the urethra in males comprises three segments with distinct names: prostatic (in the prostate), membranous (in the pelvic floor) and penile (in the penis).
Accessory glands
The male reproductive system includes three accessory glands (see Figure 31-5). These glands produce the fluids that that are added to the sperm to produce the final mixture known as semen.
 Seminal vesicles. These two pouches behind the bladder, alongside the ampullae, secrete and store the seminal fluid. The seminal fluid is viscous and alkaline, and it helps to neutralise the acid environment of the female vagina. The fluid also contains fructose (a sugar energy source for sperm) and fibrinogen (to clot the semen and attach it to the wall of the vagina). The fluid is emptied into the ejaculatory ducts to join the sperm from the ductus deferens and forms a major part of the ejaculate (60% of semen volume).
Seminal vesicles. These two pouches behind the bladder, alongside the ampullae, secrete and store the seminal fluid. The seminal fluid is viscous and alkaline, and it helps to neutralise the acid environment of the female vagina. The fluid also contains fructose (a sugar energy source for sperm) and fibrinogen (to clot the semen and attach it to the wall of the vagina). The fluid is emptied into the ejaculatory ducts to join the sperm from the ductus deferens and forms a major part of the ejaculate (60% of semen volume). Prostate gland. This gland is located at the base of the bladder, surrounding the first part of the urethra. It may be palpated through the rectum in a prostate examination. The prostate is often enlarged in old age (obstructing the flow of urine from bladder). The prostate gland secretes a milky, slightly acidic fluid that contains acid phosphatase, clotting enzymes and fibrinolysin (which breaks the clot of sperm to allow sperm to move into the uterus). This contributes to sperm motility and viability and makes up about 25% of semen volume. Many small ducts within the prostate deliver this fluid to the urethra.
Prostate gland. This gland is located at the base of the bladder, surrounding the first part of the urethra. It may be palpated through the rectum in a prostate examination. The prostate is often enlarged in old age (obstructing the flow of urine from bladder). The prostate gland secretes a milky, slightly acidic fluid that contains acid phosphatase, clotting enzymes and fibrinolysin (which breaks the clot of sperm to allow sperm to move into the uterus). This contributes to sperm motility and viability and makes up about 25% of semen volume. Many small ducts within the prostate deliver this fluid to the urethra.
It is important to note the spelling (and hence pronunciation) of the word prostate, as it is sometimes confused with the word prostrate, which means lying face-down.
 Bulbourethral glands (Cowper’s glands). These glands are the size of peas and lie immediately below the prostate. The ducts empty into the penile urethra below the prostate. The fluid produced by these glands is clear, mucous and alkaline and makes up 10% of the semen. This small volume of secretion is released shortly before ejaculation, serving to neutralise the acidic pH of the penile urethra.
Bulbourethral glands (Cowper’s glands). These glands are the size of peas and lie immediately below the prostate. The ducts empty into the penile urethra below the prostate. The fluid produced by these glands is clear, mucous and alkaline and makes up 10% of the semen. This small volume of secretion is released shortly before ejaculation, serving to neutralise the acidic pH of the penile urethra.THE STRUCTURE AND FUNCTION OF THE FEMALE REPRODUCTIVE SYSTEM
In females the major sexual functions of gametogenesis and gestation are carried out by the internal structures.2–4 4 6 A general overview of the female reproductive system is shown in Figure 31-6.
External structures
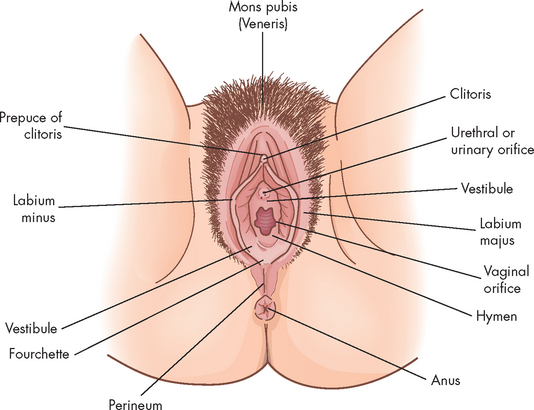
The vulva is the collective name for the external female genitalia comprising the mons pubis, clitoris, vestibule, introitus (entrance), Bartholin’s glands and the labia (lips) majora and minora (see Figure 31-7).
The mons pubis is a pad of fat that covers and protects the pubic symphysis. The clitoris is erectile tissue and it is the female equivalent of the male penis, in that it arises from the same embryonic tissue, has a similar (but much smaller) structure and becomes engorged with blood during arousal. It consists of a spongy tissue that becomes rigid when congested with blood (during arousal) and is highly innervated, being the major site of female sexual stimulation and orgasm. The vestibule contains the openings of the urethra, vagina and Bartholin’s glands (which produce lubricating secretion). The introitus is the vaginal orifice itself, and is covered by a thin membrane (hymen) in females prior to their first sexual encounter.
The labia majora (plural; singular = labium majorum) are two folds of skin that extend from the mons pubis to the fourchette. They are highly sensitive to temperature, touch, pressure and pain and are homologous to the male scrotum. Sebaceous glands on the medial (inner) surfaces secrete lubricants and the labia protect the structures of the vulva. The labia minora (plural; singular = labium minorum) are two smaller and thinner folds of skin within the labia majora. They form the prepuce (foreskin) of the clitoris, are hairless and secrete a bactericidal lubricant. When aroused, the labia become flushed with blood and enlarge slightly. The labia minora are also highly innervated and provide stimulation during coitus (sexual intercourse).
Posterior to the vestibule are the perineum and anus, the perineum being the area of skin lying between the vaginal orifice and the anus — it has very little subcutaneous fat, little hair and sebaceous glands.
Internal structures
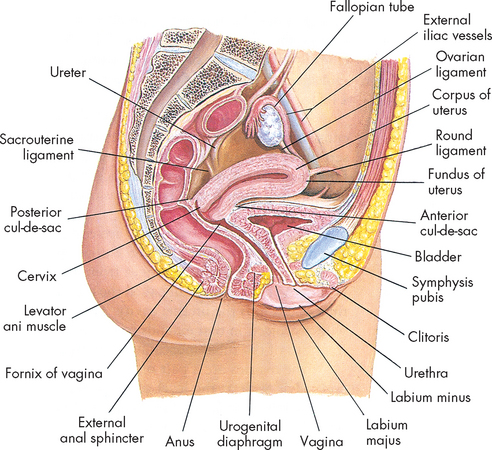
The internal structures of the female reproductive system (see Figure 31-6) are the vagina, cervix, uterus, fallopian tubes and ovaries.
Vagina
The vagina is an elastic, fibromuscular canal that is 9–10 cm in length. It extends up and back from the introitus to the lower portion of the uterus. As Figure 31-6 shows, the vagina lies between the urethra (and part of the bladder) and the rectum. Mucosal secretions from the upper genital organs, menstrual fluids and products of conception leave the body through the vagina, which also receives the penis during coitus. During sexual excitement, the vagina lengthens and widens and the anterior third becomes congested with blood.
The vaginal wall consists of a mucous membrane lining of squamous epithelial cells that allows the vagina to stretch during coitus and childbirth. There is also a thick layer of smooth muscle that assists with these functions.
The upper part of the vagina surrounds the cervix, the lower end of the uterus (see Figure 31-8). The recessed space around the cervix is called the fornix of the vagina. The posterior fornix is deeper than the anterior fornix because of the angle at which the cervix meets the vaginal canal, which is usually about 90°. A pouch called the cul-de-sac separates the posterior fornix and the rectum.
The vagina’s elasticity and relatively sparse nerve supply enhance its function as the birth canal. During sexual arousal, however, the vaginal wall becomes engorged with blood, like the labia minora and clitoris. Engorgement pushes some fluid to the surface of the mucosa, enhancing lubrication. The vaginal wall does not contain mucus-secreting glands; rather, secretions drain into the vagina from the endocervical glands or enter from the vestibule from the Bartholin’s glands.
Uterus, cervix and fallopian tubes
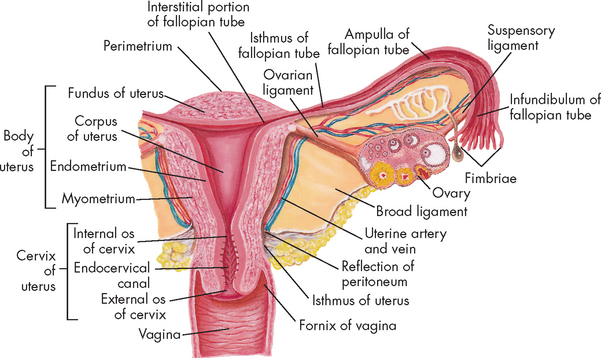
The uterus is a hollow, pear-shaped organ with thick muscular walls and a small central cavity (see Figure 31-8). Its major function is the anchorage and protection of a developing embryo, as well as the expulsion of the baby at birth. The uterus of a non-pregnant female is about 7–9 cm long and 6.5 cm at its widest part. It is held loosely in place by ligaments, folds of the peritoneum and the pressure of surrounding organs (bladder, sigmoid colon and rectum). In most women the uterus is tipped forwards, resting on the urinary bladder (anteverted), but it may be in various positions from anteverted to retroverted (tipped backwards).
There are two principal parts of the uterus: the corpus (or body) and the cervix. The cervix is the lowest part of the uterus and projects into the vagina. A continuous channel of the cervix connects the uterine cavity from the internal (uterine) cervical opening (internal os) to the external (vaginal) cervical opening (external os).
The uterus is a three-layered organ (see Figure 31-8):
Two fallopian tubes (also known as uterine tubes or oviducts) extend from the superior region of the uterus and end by curving around the ovaries. The lumen of each tube connects the uterine cavity to the peritoneal cavity. The lumen is straight and narrow as it leaves the uterus, but expands and becomes funnel-shaped towards the ovary. The ovarian end is fringed with fimbriae (small, finger-like projections), which waft the egg into the tube.
Each fallopian tube has three sections: (1) the uterine end is the isthmus; (2) the middle section is the ampulla; and (3) the ovarian end is the infundibulum. The fallopian tubes are lined with beating cilia, which beat rhythmically towards the uterine cavity. The combined action of smooth muscle peristalsis and the beating cilia serve to convey the ovulated ova to the uterine cavity for either implantation or expulsion (via the vagina). Fertilisation usually occurs closer to the ovarian end of the tube, whereby the fertilised ovum, now known as a zygote, is conveyed to the uterine cavity. If the tube is dysfunctional, a zygote may implant in the fallopian tube; this is a life-threatening outcome known as an ectopic or tubal pregnancy.
Ovaries
The ovaries are the female gonads responsible for the production of the gametes, as well as the production of the major female sex hormones, oestrogen and progesterone (see Figure 31-9). There are two small, oval-shaped ovaries, one on each side of the uterus. In women of reproductive age, each ovary is 3–5 cm long, 2.5 cm wide and 2 cm thick and weighs 4–8 g. Size and weight vary somewhat from phase to phase of the menstrual cycle. Each ovary is secured by ligaments, including the mesovarian, ovarian and suspensory ligaments, while the blood supply comes from the ovarian artery.
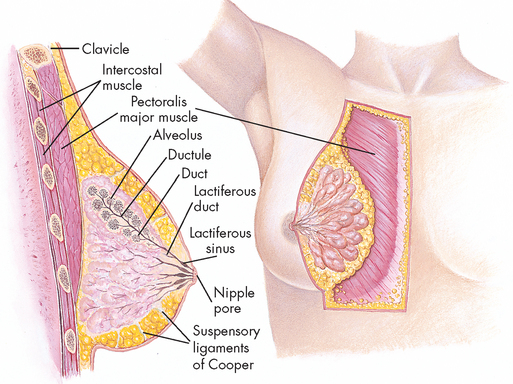
Breast structure
The female breast is composed of 15–20 pyramid-shaped lobes that are separated and supported by Cooper’s ligaments (see Figure 31-10). Each lobe contains 20–40 lobules, which subdivide into many functional units called acini (plural; singular = acinus). Each of these is lined with epithelial cells capable of secreting milk and subepithelial cells that contract to squeeze milk from the acinus. The acini empty into a network of ducts that reach the skin surface through openings (pores) in the nipple. The lobes and lobules are surrounded by muscle strands and fatty connective tissue. The amount of fatty connective tissue varies from individual to individual, depending on weight, genetic and endocrine factors.
An extensive capillary network surrounds the acini, with supply from the internal and lateral thoracic arteries and intercostal arteries, while venous return follows the arterial supply and empties into the superior vena cava. Lymphatic drainage of the breast occurs largely through axillary nodes, but approximately 25% occurs through transpectoral and internal mammary routes. The breasts receive sensory innervation from branches of the second to sixth intercostal nerves and the cervical plexus. This accounts for the fact that breast pain may be referred to the chest, back, scapula, medial arm and neck.
The nipple is a pigmented, cylindrical structure, which has multiple openings, one from each lobe. The areola is the pigmented, circular area around the nipple. A number of sebaceous glands, the glands of Montgomery, are located within the areola and aid in lubrication of the nipple during lactation (milk production). The nipple and areola contain smooth muscles, which receive motor innervation from the sympathetic nervous system. Sexual stimulation, breastfeeding and exposure to cold cause the nipple to become erect.
At the onset of puberty in the female, oestrogen secretion stimulates mammary growth. Breast development, or thelarche, is usually the first sign of puberty. During the reproductive years, the breast undergoes cyclic changes in response to changes in the levels of oestrogen and progesterone associated with the menstrual cycle. Oestrogen promotes development of the lobular ducts; progesterone stimulates development of cells lining the acini. Lactation occurs after childbirth in response to increased levels of prolactin. Prolactin secretion increases by continued breastfeeding. Oxytocin, another hormone released after delivery, controls milk ejection (let down) from acini cells.
During the proliferative phase of the menstrual cycle, high oestrogen levels increase the vascularity of breast tissue and stimulate proliferation of ductal and acinar tissue. This effect is sustained into the luteal phase of the cycle. During this phase, progesterone levels increase and contribute to the breast changes induced by oestrogen. Specific effects of progesterone include dilation of the ducts and conversion of the acinar cells into secretory cells. Most women experience some degree of premenstrual breast fullness, tenderness and increased breast nodularity. Breast volume may increase as much as 10–30 mL. Because the length of the menstrual cycle does not allow for complete regression of new cell growth, breast growth continues at a slow rate until approximately 35 years of age. Because of the cyclic changes that occur in breast tissue, breast examination should be conducted at the conclusion of or a few days after the menstrual cycle, when hormonal effects are minimal and breasts are at their smallest.
The function of the female breast is primarily to provide a source of nourishment for the newborn. Physiologically, breast milk is the most appropriate nourishment for newborns. Not only does its composition change over time to meet the changing digestive capabilities and nutritional requirements of the infant, but it also contains specific immunoglobulins, especially IgA, and nonspecific antimicrobial factors, such as lysosomes and lactoferrin, that protect the infant against infection. During lactation, high prolactin levels interfere with hypothalamic-pituitary hormones that stimulate ovulation. This mechanism suppresses the menstrual cycle and prevents ovulation.5 In underdeveloped countries, breastfeeding is the major means of contraception.
PUBERTY IN MALES AND FEMALES
In both males and females the sexually mature period begins with the onset of puberty.2 Puberty is the name given to the process of development and growth that leads to a reproductively capable adult. The end of the reproductive years (particularly for females) is called the climacteric and is commonly known as the menopause. Males do not reach such an obvious end to their reproductive years, although there is a general decline in function with ageing (see ‘Ageing and the reproductive systems’ below).
The process of sexual maturation usually begins earlier in females than in males, but in both it begins between the ages of 8 and 12 years and continues during the early ‘teens’. Sexual maturation begins with the secretion of gonadotropin-releasing hormone (GnRH) from the hypothalamus and the process of maturation is completed in males with the first complete ejaculation and in females with the first ovulation.
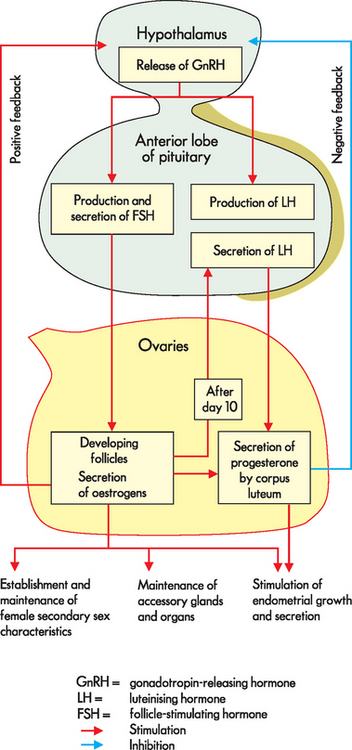
Puberty involves dramatic changes in the male and female body shape, as well as the beginning of the activity of the gonads to produce gametes. Puberty and continued reproductive function are coordinated by the hypothalamus, the endocrine system (the anterior pituitary) and the gonads (refer to Chapter 10). This is called the hypothalamic-pituitary-gonadal axis. Males and females commence puberty in response to the same set of hormones released from the hypothalamus and anterior pituitary gland. The hypothalamus releases gonadotropin-releasing hormone (GnRH).2,4,6,7 This name is descriptive, in that gonado- refers to the gonads, while tropin means growth-stimulating — hence this hormone causes the release of hormones that will ultimately result in growth of the gonads. In response to GnRH, the anterior pituitary releases follicle-stimulating hormone (FSH) and luteinising hormone (LH), as seen in Figure 31-11. These hormones target the gonads.
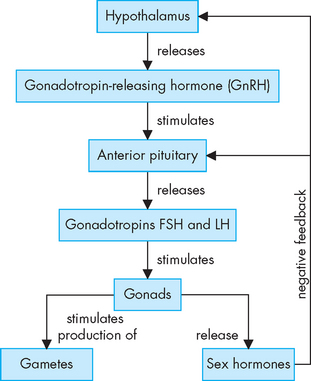
FIGURE 31-11 The hypothalamic-pituitary-gonadal axis.
The hypothalamus releases GnRH, which travels down the hypothalamo-pituitary portal system (two capillary beds connected in series by venules) to the anterior pituitary. The anterior pituitary releases the gonadotropic hormones follicle-stimulating hormone (FSH) and luteinising hormone (LH) into the systemic blood flow. When the gonadotropins are detected by receptors in the gonads, they respond by producing sex hormones and these influence the production of gametes. Since this is a pathway that relies on each step working, a dysfunction in any part (hypothalamus, anterior pituitary or the gonads) can result in failure of the gonads to produce sex hormones and/or gametes.
In both males and females, puberty follows the same pattern but with differences according to sex. In males, spermatogenesis starts and testosterone production increases dramatically. Puberty leads to the adolescent growth spurt, which requires testosterone and growth hormone from the anterior pituitary. Differences in the growth rate of different regions of the body lead to the male build (wide shoulders, narrow pelvis).
In females, oogenesis starts, oestrogen levels rise and the ovarian and menstrual cycles start — the first menstrual cycle is known as menarche. The ovarian and menstrual cycles work together to produce (at least) one mature oocyte and to prepare the uterus for the potential implantation of a fertilised embryo every month, until the reproductive years cease. In both sexes, there is a corresponding development of secondary sex characteristics.
Testosterone and oestrogen cause huge changes throughout the male and female bodies. These hormones also cause changes in the brain (such as moodiness), which may be particularly evident during puberty.
The exact cause of the rise in GnRH levels is not entirely understood but it has been linked to the presence of the hormone leptin. Leptin is secreted by adipose tissue (refer to Chapter 35), and precocious (early) puberty is associated with obesity. This implies that the body becomes reproductively active when it is ‘large’ enough, but this is not necessarily the only trigger.
The effects of testosterone in males
Testosterone is a very powerful hormone causing major changes in the male body. Testosterone is essential for spermatogenesis and the changes seen in the young male when puberty commences. After puberty, it is necessary to maintain the function and size of the reproductive organs. The effects of castration (removal of the testes, also known as orchidectomy) include both depression of sexual function and a reduction in the size of the accessory organs; this is evidence of the necessity for the continued production of testosterone throughout adult life.
Testosterone stimulates the development and maintenance of the male secondary sex characteristics of hair growth (facial, pubic and axillary or underarm), growth of the larynx resulting in a deepening of the voice, secretion of skin oil glands, the male pattern of fat distribution and the male libido (sex drive). In individuals with a genetic predisposition, higher levels of testosterone are thought to be associated with baldness. Higher levels of testosterone also cause negative feedback to the hypothalamus and anterior pituitary (to lower the secretion of GnRH, FSH and LH).
A major function of testosterone is to drive anabolism — namely, increased muscle and bone growth. These effects have been exploited by athletes who use anabolic steroids (similar to testosterone) to gain muscle mass and hence sports controlling bodies commonly test for anabolic steroids. An increased level of aggression is often associated with testosterone; however, this is a hotly debated topic and there is no clear evidence to show that high levels of testosterone lead to violent tendencies. Note that androgens are the male sex hormones, the main one being testosterone.
The effects of oestrogen and progesterone in females
Oestrogen and progesterone are powerful hormones that cause major changes in the female body. Unlike testosterone, they are not required to be produced in the fetus to develop a female reproductive anatomy, so the absence of testosterone in a fetus results in female development. (Remember that the sex of the fetus is determined at conception by the combination of chromosomes from the mother and father, and the hormones are produced to match this sex.
Oestrogen is analogous with testosterone in that it stimulates the growth of the ovaries and primordial follicles (germ cells that become oocytes, the immature eggs). In addition, it stimulates the growth of smooth muscle and the proliferation of the epithelial linings of the reproductive tract, growth of the external genitalia and breast growth (particularly the lactiferous ducts and fat deposition). Oestrogen promotes development of the female body configuration (narrow shoulders, broad hips), the female fat distribution pattern and the development of pubic, axillary and body hair. Another effect is on bone growth, and oestrogen is protective against osteoporosis. Oestrogen is the main hormone referred to although it also usually indicates oestradiol as well.
Progesterone is predominantly a sexual cycle hormone, as it stimulates the endometrial glands of the uterus during the menstrual cycle induces the production of thick sticky cervical mucus, decreases contractions of the uterine tubes and myometrium (essential for the prevention of miscarriage), stimulates breast growth (particularly glandular tissue) and has feedback effects on the hypothalamus and anterior pituitary to maintain control of the sexual cycles.
GAMETOGENESIS
General principles
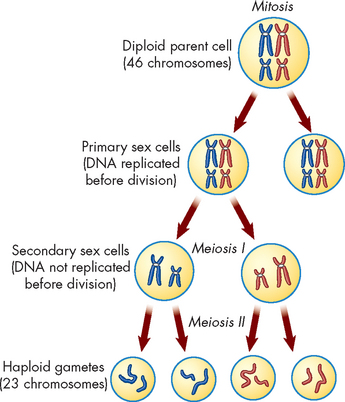
Adult males and females produce a set of specialised reproductive cells called gametes. Cells that give rise to gametes undergo a different type of cell division in which the amount of genetic material received by each daughter cell is halved. This type of division is called meiosis and it occurs solely in the gonads (see Figure 31-12). Thus gametes contain one half of the genetic material of somatic cells: one copy of each type of chromosome. The total number of chromosomes in all other body cells is 46, comprising 22 pairs of autosomes and 2 sex chromosomes: XX in females (homologous) and XY in males.
In the gametes, the egg (ovum) and sperm cells each contain half of the genetic material of all other body (somatic) cells, as they have only 23 chromosomes instead of 23 pairs of chromosomes. When an ovum (from the mother) and sperm (from the father) combine to undergo fertilisation, a new cell with the full human genetic complement is produced (that is, with 23 pairs of chromosomes). This cell is called a zygote and it then undergoes many cycles of cell division to give rise to a new individual. At each cycle, genetic material is precisely copied for transmission to the new daughter cells. Products of any one cell division are genetically identical to each other, to their parent cell and to the original fertilised egg (zygote). This type of cell division is known as mitosis and it continues throughout life in growth, turnover and repair (see Chapter 5).
Meiosis
Meiosis can essentially be thought of as two rounds of mitosis in which the synthesis phase (namely, replication of the chromosomes) is skipped in the second round, resulting in a halving of the genetic material. The phases of the cell cycle are shown in Figure 31-12 and you can see that the second round of division (meiosis II) begins immediately after telophase I without the duplication of chromosomes so that the daughter cells each receive only half the chromosome number. In males this meiotic event is called spermatogenesis and in females it is called oogenesis. Gamete formation in both males and females is controlled by the sex hormones. In both sexes the same set of hormones triggers the process and the end result is the production of sperm and testosterone in the male and the production of ova, oestrogen and progesterone in the female. In addition, in females the hormones that trigger oogenesis are also responsible for the changes of uterine thickness during the menstrual cycle.
Spermatogenesis
Spermatogenesis is the formation of spermatids and it takes place in the seminiferous tubules of the testes under the influence of testosterone (see Figures 31-13 and 31-14). Spermatogonia (plural; singular = spermatogonium) are primitive germ cells that line the walls of the seminiferous tubules. They progress through various stages of development to become more developed spermatocytes; each spermatocyte then undergoes meiosis to give rise to four spermatids that reach the lumen of the seminiferous tubules. These four spermatids are immature sperm cells and are not free-swimming. They need to mature in a process called spermiogenesis to ultimately give rise to four mature free-swimming spermatozoa (commonly abbreviated to sperm). It takes about 2 months for spermatogonia to become mature sperm and a healthy adult male will produce several million mature sperm each day; these mature sperm are stored in the ductus deferens (including the ampulla) awaiting ejaculation.
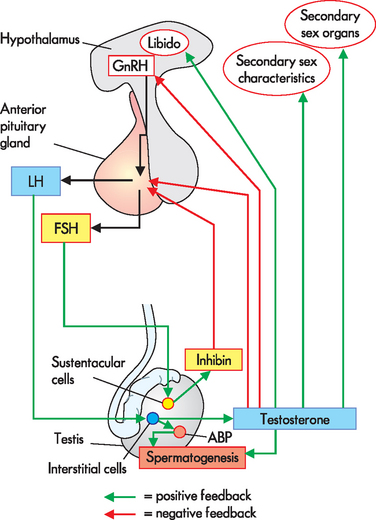
FIGURE 31-14 Hormonal control of spermatogenesis in a sexually mature male.
ABP = androgen-binding protein; LH= luteinising hormone; FSH = follicle-stimulating hormone; GnRH = gonadotropin-releasing hormone.
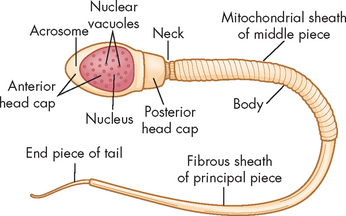
Mature sperm have named parts each with a specific function (see Figure 31-15): the head contains the genetic material, the midpiece contains mitochondria, which provide energy for sperm movement; and the tail provides locomotion.
The pathway of sperm from the testes to the tip of the penis is shown in Figure 31-16. The transport of sperm from the epididymis to the ductus deferens is due to peristalsis of smooth muscle in the epididymis and ductus deferens, rather than the sperm’s own motility. Mature sperm, together with other components of semen, are ejaculated by contraction of smooth muscle in the epididymis, ductus deferens, ejaculatory ducts, prostate and seminal vesicles (sympathetic stimulation). Each ejaculate is a volume of about 3 mL containing about 300 million sperm.
The complete process, from hormonal roles through to spermatogenesis, is shown in Figure 31-17.
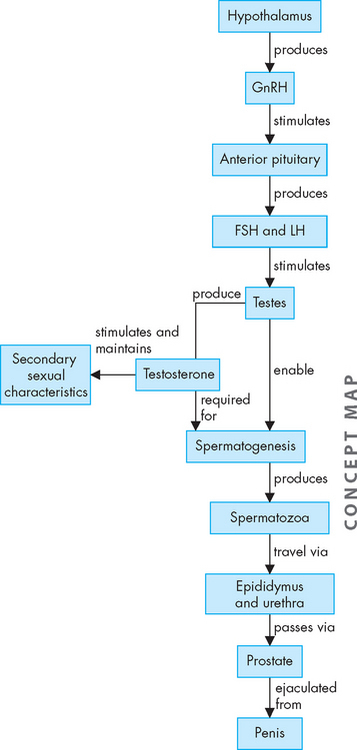
FIGURE 31-17 Hormonal changes and sperm production.
In response to signals from the hypothalamus, follicle-stimulating hormone (FSH) and luteinising hormone (LH) are released, which in turn stimulate the testes to produce testosterone necessary for sexual maturation, as well as spermatogenesis. Following spermatogenesis, the spermatozoa travel through the ducts to exit via the penis. GnRH = gonadotropin-releasing hormone.
Oogenesis
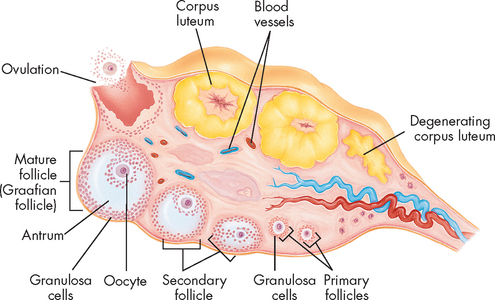
The same hormones that control male sexual development control female sexual development and menstrual and ovarian cycling (see Figure 31-18). At birth, a female has all of the oocytes she will ever have — approximately 2 million. No new oocytes are subsequently produced, so a female is born with all her immature gametes already present — a situation that is in contrast to male gamete production. Most of these will degenerate so that at puberty she has 200,000–400,000 left. Over a woman’s reproductive lifetime of about 30–40 years, several hundred oocytes will complete differentiation and be released from the ovary (ovulation).
The gametes are all present in each of the ovaries in the form of primordial follicles. The actual gamete is surrounded by a layer of cells called the granulosa cells in the cortex of the ovary. The oocyte and the granulosa cells together comprise the follicle. During a woman’s reproductive years, every month 1 follicle develops and is ovulated. In 1–2% of cycles more than 1 oocyte develops to this stage, potentially resulting in multiple births. The oocyte degenerates within a few days if it is not fertilised by sperm.
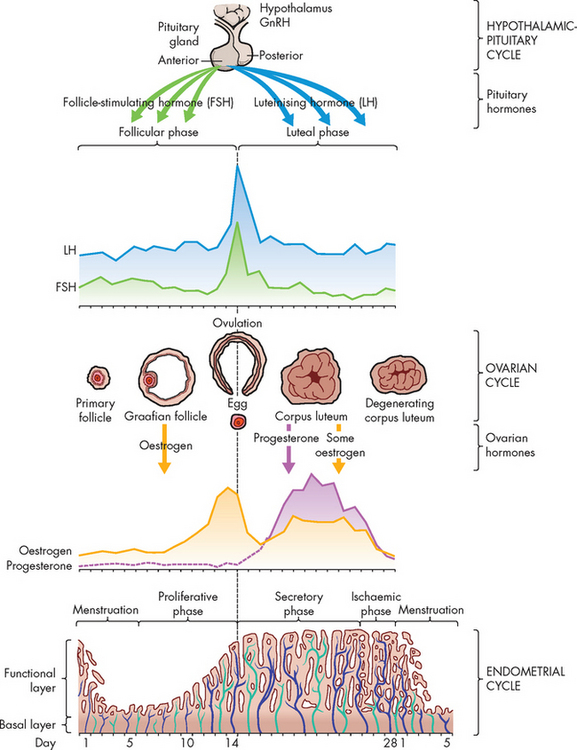
The ovarian cycle
The ovarian cycle is the series of changes in the ovarian follicles that include oocyte development and ovulation. The follicles respond to FSH by growing and maturating, such that 1 follicle bulges out from the ovary. The granulosa cells within this follicle proliferate and a zona pellucida (clear area or zone) forms between the oocyte in the middle and the surrounding granulosa cells, while a fluid-filled antrium appears. The granulosa cells increase in number and produce increasing amounts of oestrogen until the follicle bursts and the oocyte is released. Ovulation is stimulated by the surge in LH (see Figure 31-19). The oocyte is surrounded by a layer of granulosa cells (corona radiata) at ovulation and the remains of the follicle stay in the ovary and become the corpus luteum. Oestrogen and progesterone are secreted from the corpus luteum for about 10 days if a pregnancy does not occur.
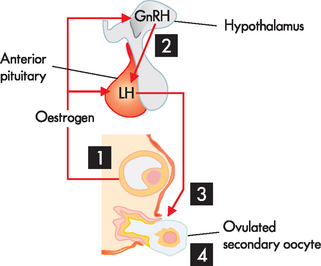
FIGURE 31-19 Hormonal control of ovulation.
As the follicle matures, more and more oestrogen is produced from the larger number of granulosa cells surrounding the ovum. When the concentration of oestrogen in the blood reaches a certain level it has a positive feedback action on the hypothalamus (1) triggering a ‘burst’ in production of GnRH (2) and a consequent rapid increase in LH concentration. This rise in LH concentration is essential for the wall of the follicle to burst (3) and release the oocyte (4).
The menstrual cycle
The menstrual (or uterine) cycle lasts for approximately 28 days and is controlled by oestrogen and progesterone secreted by the ovaries (see Figure 31-20).2,8,9 The ovarian and menstrual cycles occur in concert with each other as the developing follicle produces oestrogen, which stimulates the uterine endometrium to proliferate. This is necessary to prepare the uterus to receive a zygote (fertilised egg).
The menstrual cycle is divided into three stages:
During both the menstrual and the proliferative stages, the follicle is developing under the influence of FSH and oestrogen levels are rising as the number of granulosa cells in the follicle increases. The levels of oestrogen rise until a positive feedback loop between oestrogen and the anterior pituitary results in a large ‘spike’ in LH concentration. This spike causes the follicle to rupture and the oocyte to be released. Hence, these stages are started by FSH, which causes a rise in oestrogen (progesterone remains low), and this rise in oestrogen causes an LH spike and the mature egg is ovulated.
The luteal stage is controlled by the corpus luteum and is under the influence of LH. The corpus luteum produces more progesterone than oestrogen, so this phase is dominated by progesterone. Progesterone causes the endometrial glands to fill with glycogen, the endometrial blood vessels to grow and it inhibits smooth muscle activity in the myometrium.
Changes in hormone levels are quite dramatic throughout the cycle (Figure 31-20) and can be measured to monitor the cycle and potential pregnancy. The complete process, from hormonal roles through to ovulation, is shown in Figure 31-21.
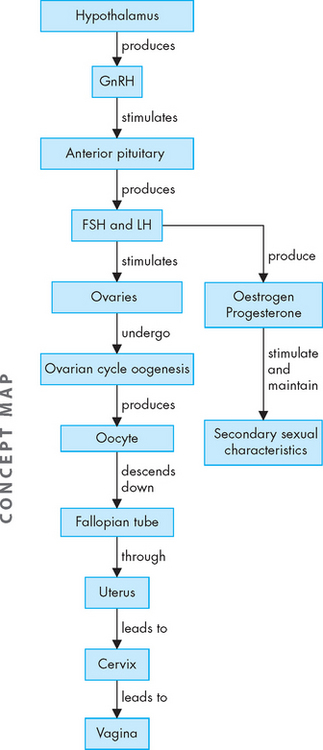
FIGURE 31-21 Hormonal changes and oocyte production.
In response to signals from the hypothalamus, FSH and LH are released, which in turn stimulate the ovaries to produce oestrogen and progesterone necessary for sexual maturation, as well as oogenesis. Following ovulation, the oocyte travels through the fallopian tube and exits the body via the vagina. The corpus luteum, which remains in the ovary, continues producing hormones for several days before degenerating.
Cervical mucus plays a role in disease prevention, contraception (to an extent) and the sexual act itself. In the menstrual and proliferative stages, which are under the influence of oestrogen, the mucus is abundant, clear and non-viscous. This is most pronounced at the time of ovulation and the freely flowing liquid allows sperm easy access from the vagina, through the uterus and to the fallopian tubes. During the luteal phase under the influence of progesterone the cervical mucus is thick and sticky. This can form a barrier to sperm movement and create a plug to seal off the uterus (and embryo if implantation occurs) from infection.
Ovarian and menstrual cycle timing
The ovarian and menstrual cycles occur in concert with each other, as the ovarian cycle drives the menstrual cycle. The developing primordial germ cells in the ovary begin releasing oestrogen in increasing amounts as their development proceeds. The granulosa cells, which produce oestrogen, increase in number throughout the development process and the endometrial layer of the uterus responds to the increasing levels of oestrogen in the blood. When a follicle is fully matured and ready to ovulate, it is producing a relatively high amount of oestrogen, which causes a positive feedback on the hypothalamus (see Figure 31-19). The hypothalamus responds by increasing the GnRH pulses and, in turn, the anterior pituitary produces large quantities of LH. This sharp rise in LH blood concentration is the trigger necessary for the oocyte to burst out of the ovary (ovulate). In assisted reproductive technology this increase in LH must be artificially created (by LH injections) to stimulate ovulation for egg collection (refer to Chapter 32).
After ovulation has occurred, oestrogen levels drop and the remains of the follicle in the ovary (the corpus luteum) produce a relatively higher concentration of progesterone. One important effect that progesterone has is to prevent muscular contraction of the myometrium (the middle layer of smooth muscle of the uterus). This is essential for the uterus to hold and carry a baby to full term; if the uterine myometrium contracts, then the zygote or more developed embryo could be expelled and the pregnancy fail.
The corpus luteum will persist in the ovary and secrete progesterone for about 10 days before it degenerates. If an egg is fertilised soon after ovulation (within hours) it will take the zygote about 5–6 days to reach the uterus and another day to become embedded in the endometrium (implantation) and produce sufficient hCG to establish the pregnancy. The hCG produced maintains the corpus luteum for a longer period until the placenta is established, at which time the placenta produces the progesterone necessary to maintain the quiet state of the myometrium. So the corpus luteum produces progesterone long enough for a possible pregnancy to establish and then degenerates into the corpus albicans (which does not produce progesterone). After 10 days, if no implantation is established, the levels of progesterone fall and the myometrium begins to contract to expel the excess endometrium, which is not needed. This is the menstrual flow. hCG will continue to be produced by an implanted embryo and this is the basis of a pregnancy test: hCG detection in the urine (or blood, for greater sensitivity) is positive 2 weeks after fertilisation.
The interplay of the ovarian and menstrual cycles may at first seem very complex, but when you focus on the reasons for the events you will see that this elegant system brings about maturation of an oocyte and prepares the uterus to receive the oocyte if it is fertilised. The hormonal interplay also seems complex, but essentially the pre-ovulation phase is dominated by oestrogen (preparing the uterus) while the post-ovulation phase is dominated by progesterone (holding the prepared uterus ready) in case fertilisation and implantation occur. If there is no fertilisation, both hormone levels are relatively low and the thickened uterus, which is not needed, is expelled.
MALE AND FEMALE SEXUAL RESPONSES
The most widely discussed reference on the sexual responses of men and women is the four-stage model: (1) excitement, (2) plateau, (3) orgasm and (4) resolution.10 There are no really clear distinctions between the stages or time points at which one phase ends and the next begins. Although biologically accurate, this model does not account for the psychological aspects of the sexual response.
In both males and females, initiation of the sexual response may include visual, mental and physical stimuli. The sexual responses of males and females have many similarities. Two basic physiological responses to sexual stimulation are vasocongestion and myotonia. Vasocongestion occurs when body tissues fill with blood and swell in size. This is responsible for the erection of the penis and also causes a woman’s breasts to increase in size and the vagina to lubricate. Other body parts that may be affected are the labia, testicles, clitoris and nipples. Myotonia is the increased muscle tension that happens during sexual arousal. This includes both flexing (which is voluntary) and contractions (which are involuntary). The most obvious examples of this are the muscle contractions that occur during both male and female orgasm.
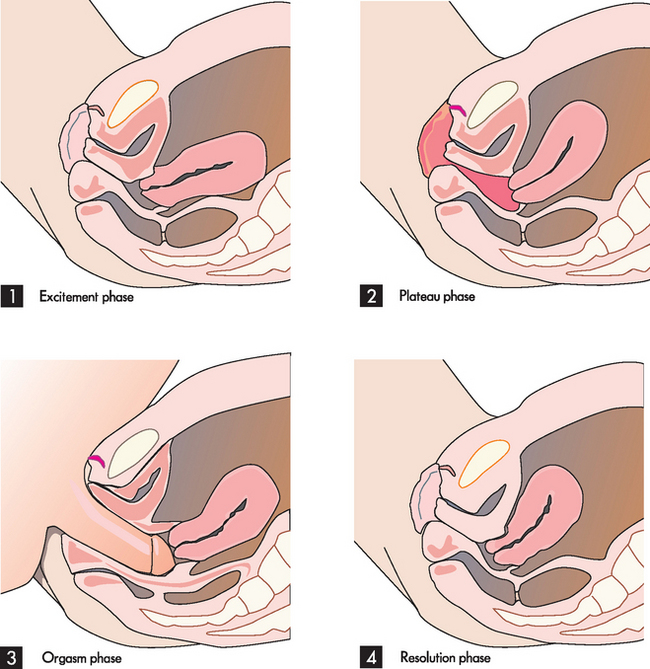
The female sexual response
When not sexually aroused, the uterus lies in its normal anteverted position over the bladder, the vagina is relatively narrow and the labia minora are retracted (see Figure 31-22). In the excitement phase, sexual stimulation causes the uterus to stand more vertically and the inner end of the vagina dilates. The labia minora become vasocongested and may extend beyond the labia majora. A vaginal transudate moistens the vagina and vestibule. The plateau phase is a continuation of the excitement phase up to the moment of orgasm and resolution. In this phase the uterus is vertical and the cervix is withdrawn from the vagina. The clitoris is engorged and the labia are bright red and flushed with blood, and the lower third of the vagina constricts the penis. This phase can continue for a long or short time depending on the couple and the circumstances of the sexual activity. During the orgasm phase, the vagina contracts rhythmically and the uterus has peristaltic contractions. Finally, at resolution the uterus returns to its original anteverted position and the cervix protrudes into the top of the vagina; it may contact a pool of sperm as it does so, thereby allowing the sperm to gain access to the uterus and uterine tubes.
The male sexual response
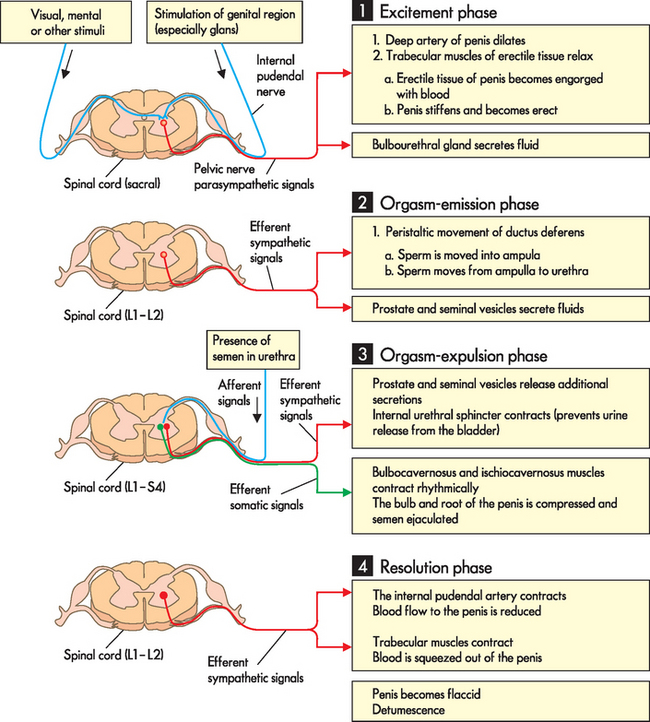
The physiological response of the male can also be thought of in terms of four stages. Figure 31-23 details both the nerve impulses responsible and the actions they bring about in males.
With tactile (touch) stimulation of the genital region (the excitement phase), signals are transmitted via the internal pudendal nerve to the sacral level of the spinal column, which in turn sends efferent parasympathetic signals to the internal pudendal artery of the penis and the trabecular muscles of the erectile tissue. The trabecular muscles relax, allowing blood to flow into the erectile tissue, with the result that the penis becomes erect. A second consequence of this is the release of a small amount of fluid from the bulbourethral glands. During the plateau phase this heightened state of arousal, accompanied by widespread muscle myotonia, is maintained and built up further.
The male orgasm phase is divided into two parts: the orgasm-emission phase and the orgasm-expulsion phase. During the orgasm-emission phase sympathetic nerve signals arising in the L1–L2 level of the spinal cord induce peristaltic movement of the ductus deferens, which moves sperm from the epididymis to the ampulla and from there into the prostatic urethra. In addition, these nerve signals induce release of seminal and prostatic fluids from the seminal vesicles and prostate gland, respectively. The orgasm-expulsion phase is triggered by the presence of semen in the urethra, which is detected by sensory receptors and signalled to the central nervous system; nervous signals are integrated in the L1–S4 level of the spinal cord. Efferent sympathetic and efferent somatic signals arise from this integration of sensory input. The sympathetic signals cause further release of seminal and prostatic fluid and close the internal urethral sphincter, preventing the release of urine. The somatic signals innervate the bulbocavernosus and ischiocavernosus muscles, which contract rhythmically around the bulb and root of the penis. This rhythmical compression of the penis forces the semen out through the penile urethra (ejaculation).
Finally, during the resolution phase, efferent sympathetic signals arising from the L1–L2 level of the spinal cord cause the internal pudendal artery to contract, reducing blood flow to the erectile tissue. At the same time the trabecular muscles contract and the remaining blood is squeezed out of the penis as it returns to its flaccid state.
CONCEPTION, GESTATION AND PARTURITION
During intercourse increased blood flow to the vagina causes transudation of fluid through the walls, providing lubrication. Mucous lubricant is secreted by the Bartholin’s glands (at the vaginal entrance). Sperm are deposited in the vagina near the cervix and from there must travel through the uterus to the fallopian tubes. The cervical mucus is non-viscous at the time of ovulation, as the production of progesterone is relatively low — this aids the free movement of sperm. Sperm move due to the motility of the sperm tail and ciliary and peristaltic activity in the uterus. There are millions of sperm in each ejaculate, but very few reach the fallopian tubes; however, those few do not take long to get there.
Fertilisation
The oocyte is ‘fertilisable’ for only a few hours after ovulation and entering the fallopian tube; however, sperm can live for 2–3 days after ejaculation into the vagina. The sperm meet the oocyte at fertilisation at the ovarian end of the fallopian tube, and the zygote implants in the uterus (see Figure 31-24). At ovulation, an oocyte completes its first meiotic division. As it is released from the ovary, it is surrounded by the zona pellucida and granulosa cells (corona radiata), both of which are a barrier to the sperm. The sperm undergo capacitation in the female tract, which ‘primes’ them for the meeting with the oocyte. It actually takes many sperm to bind to the zona pellucida to release the acrosome that gradually breaks down this barrier. When one sperm finally penetrates the zona pellucida and reaches the oocyte cell membrane, the membrane alters immediately to prevent any more sperm binding to it. This is termed the fast block and is essential to prevent the entry of more than one sperm ‘head’. The fertilised ovum now contains the oocyte nucleus (with 23 chromosomes) and the sperm nucleus (with 23 chromosomes) and these fuse together to form one cell nucleus containing the full complement of 46 chromosomes. At this stage the fertilised egg is called a zygote.
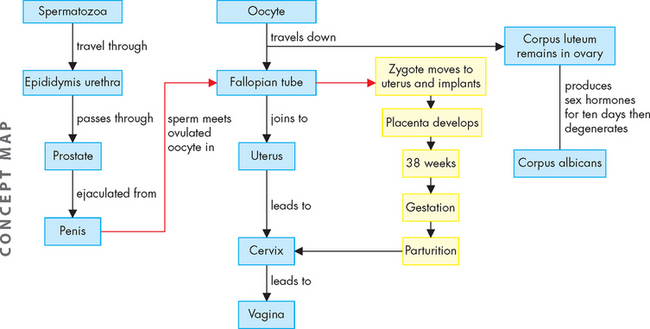
FIGURE 31-24 Fertilisation, gestation and parturition.
After sperm fertilise the oocyte within the fallopian tube, the zygote continues travelling until it reaches the uterus. Here, it implants and grows, while the placenta also develops, until parturition (labour). The fetus is expelled from the uterus through the cervix and vagina.
The zygote travels along the fallopian tube to the uterus (this takes 3–4 days) and as it does so, it undergoes a series of rapid mitotic divisions to become known as an embryo. The embryo reaches the uterine cavity as a tiny hollow ball of cells termed a blastocyst. After several more days, this implants in the uterine wall. At this stage the uterus is primed by progesterone and oestrogen (from the corpus luteum) such that the endometrium is thickened and rich in nutrients and blood flow. As discussed above, oestrogen and progesterone are vital for the maintenance of a pregnancy. When fertilisation occurs, the corpus luteum is stimulated by hCG secreted from the developing embryo to secrete oestrogen and progesterone for 2–3 months.
Implantation
Implantation is the process whereby the blastocyst adheres to and then sinks into the endometrium. Implantation is completed early in the second week (after fertilisation) and normally occurs in the upper part of the uterus; if the blastocyst implants lower, near to the cervix, there may be complications during birth (parturition). The outer cell layers of the blastocyst proliferate and ‘invade’ the endometrium. These cells produce enzymes that break down the endometrial cells, allowing the blastocyst to ‘sink’ into the endometrium until it becomes completely embedded. The blastocyst initially derives its nutrition from the endometrial cells that are broken down — the blastocyst literally ‘eats’ its way into the thickened endometrium and digests the released products to continue growing. As the blastocyst develops into an embryo, it eventually forms the placenta and obtains its nutrition from maternal blood, via the placenta.
The development and function of the placenta
The placenta is a complex organ that is derived partly from the developing embryo and partly from the maternal endometrium. The embryo-derived part is the chorion, and it is derived from the outer cells of the blastocyst. The mature placenta secretes hCG, oestrogen and progesterone. The oestrogen has negative feedback on the hypothalamus, which reduces the production of GnRH, thus reducing the production of FSH and LH. In this way, with very little FSH, there is no stimulation of the primordial germ follicles in the ovaries and the ovarian and menstrual cycles stop.
After implantation, the embryo is completely embedded in the endometrium. The continued invasion of the endometrium results in eroded spaces that become filled with maternal blood; these spaces are termed sinuses or lacunae (literally, empty spaces). Lacunae receive blood from the uterine arteries and drain into the uterine veins. Nutrients, gases and wastes thus diffuse to and from the embryo and maternal blood across a layer of blastocyst cells (now termed chorionic cells), but without direct connection between the two circulations — at this and all subsequent stages of development, maternal blood is separated from embryonic and fetal tissues, including embryonic blood, by a layer of chorion.
By the end of the second week after fertilisation, chorionic villi (projections of the chorion) have grown over the entire surface of the embedded embryo and the menstrual period is missed. During the third week, embryonic blood vessels begin to appear in the chorionic villi and soon establish connections with the blood vessels developing in the embryo itself via the umbilical blood vessels (in the umbilical cord). By the end of the third week, when the embryo’s heart begins to beat, blood begins to circulate from the embryo, through the umbilical arteries to capillaries in the chorionic villi.
Up to the eighth week of gestation, chorionic villi cover the entire surface of the embryonic sac. After this time, they begin to disappear, eventually remaining only where they form the fetal part of the mature placenta; the rest of the chorion becomes smooth as the villi disappear.
As the embryo and its sac grow, they begin to bulge from the uterine wall into the uterine cavity, and by the end of the tenth week the fetus completely obliterates the uterine cavity.
Formation of the placenta is completed by the end of the first trimester. The fetal component of the placenta is that part of the chorion that retained its villi — these branch as they continue to grow, providing a large surface area for diffusional exchange between fetal and maternal blood (see Figure 31-25). The maternal component of the placenta is the endometrium. Over the second and third trimesters, the placenta increases in size, in the degree of branching of the villi and in the volume of the intervillous space (i.e. the continuous space between the villi, filled with maternal blood). At term, the placenta weighs 500–600 g and is 18–20 cm in diameter.
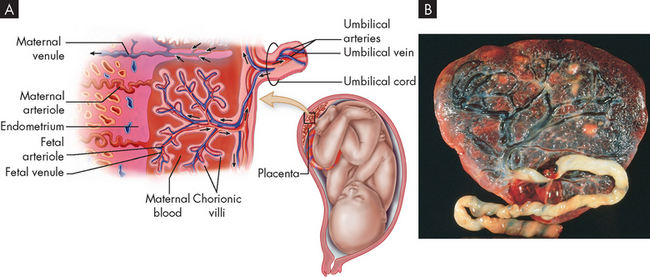
FIGURE 31-25 Structural features of the placenta.
The close placement of the fetal blood supply and maternal blood supply permits diffusion of nutrients and other substances. It also forms a thin barrier to prevent diffusion of most harmful substances. No mixing of fetal and maternal blood occurs. A Diagram showing a cross-section of the placental structure. B Photograph of a normal, full-term placenta (fetal side) showing the branching of the placental blood vessels.
Source: Patton KT, Thibodeau GA. Anatomy & physiology. 7th edn. St Louis: Mosby; 2010.
Fetal placental circulation
Blood from the fetus, low in oxygen and high in carbon dioxide and other wastes, passes through the two umbilical arteries to the placenta. Here the arteries branch to enter the villi. In the villi, blood flows in the capillaries. The villi are surrounded by a ‘lake’ of maternal blood and the capillaries in the villi bring fetal blood close to maternal blood; exchange of gases, wastes and nutrients can thus be effected across the placental (chorionic) membrane, which at all times separates the fetal and maternal circulations. Fetal blood, now rich in oxygen and nutrients and low in carbon dioxide, leaves the villi and returns to the fetus in the single umbilical vein, which feeds directly into the fetal right atrium via the inferior vena cava.
Maternal placental circulation
Maternal blood enters the intervillous space through spiral arteries in the decidua basalis, and it leaves through endometrial veins. Maternal blood pressure is low in the placenta and the flow of blood is correspondingly slow: this aids diffusional exchange between maternal and fetal blood.
Transfer (exchange) across the placenta
The welfare of the fetus depends above all else on adequate bathing of its chorionic villi by maternal blood. If maternal placental circulation is reduced, fetal hypoxia, growth retardation or fetal death may ensue.
Most blood-borne substances can cross the placental ‘barrier’ between the fetal and maternal bloodstreams. Many large molecules, such as immunoglobulin G (IgG), pass the barrier readily, but a few such as heparin and immunoglobulin M are excluded. Transfer of the following occurs readily by a variety of mechanisms:
 some antibodies such as IgG, which serve to confer passive immunity to a variety of pathogens on the newborn (but which can also harm the baby in the case of Rhesus incompatibility)
some antibodies such as IgG, which serve to confer passive immunity to a variety of pathogens on the newborn (but which can also harm the baby in the case of Rhesus incompatibility)From the perspective of the fetus, the placenta acts as a lung (gas exchange), gut, liver (nutrient absorption and metabolism) and kidney (excretion, water and electrolyte homeostasis) — the main limiting factor for the efficiency of all these functions being the maternal placental circulation.
The embryonic sac
From the time the embryonic (amniotic) sac begins to bulge into the uterine cavity until the time it completely occupies the cavity some 10 weeks after fertilisation, it has the following three layers:
 The innermost layer of the sac is the amnion. Extensions of the amnion cover the fetal surface of the placenta and the umbilical cord. The amnion is continuous with the skin of the fetus at the umbilicus.
The innermost layer of the sac is the amnion. Extensions of the amnion cover the fetal surface of the placenta and the umbilical cord. The amnion is continuous with the skin of the fetus at the umbilicus.These membranes constitute a barrier to infection of the fetus through the vagina and cervix. Within the sac, the fetus floats freely in amniotic fluid, anchored through the umbilical cord.
Note that all the cells derived from the fertilised egg, which includes the fetus itself, the fetal components of the placenta and the fetal membranes other than the decidua capsularis, are genetically identical to each other, but genetically different from the mother. Thus, if a biopsy sample of the chorionic villi is taken, we can look at the karyotype and genetic composition of the baby, enabling prenatal testing for genetic diseases.
The origin, composition and significance of amniotic fluid
Amniotic fluid is derived in part as a filtrate of maternal blood and in part from fetal urine. At term, about 1 L of amniotic fluid is present in the sac. The composition of amniotic fluid is similar to that of extracellular fluid, with the addition of:
 hormones, enzymes and other fetal metabolites — many of these metabolites can be measured in a sample of amniotic fluid derived by amniocentesis to give a prognosis of the health of the baby (e.g. alpha (α)-fetoprotein as an indicator of neural tube disorder, bilirubin as an indicator of haemolysis)
hormones, enzymes and other fetal metabolites — many of these metabolites can be measured in a sample of amniotic fluid derived by amniocentesis to give a prognosis of the health of the baby (e.g. alpha (α)-fetoprotein as an indicator of neural tube disorder, bilirubin as an indicator of haemolysis) cells shed from the fetus — some of these are viable and can be cultured for genetic and biochemical analysis.
cells shed from the fetus — some of these are viable and can be cultured for genetic and biochemical analysis.The functions of the amniotic fluid are of significance in terms of the normal development of the fetus. The fluid mechanically cushions the fetus from external shock and the mother’s physical activity (walking, exercise, etc.), and it also helps maintain a constant temperature of 37°C. But perhaps one its most important functions involves allowing the external growth of the fetal limbs and the free movement essential for musculoskeletal development.
As the fetal central nervous system develops, reflex actions of swallowing and urination begin and the amniotic fluid circulates through the fetus as well as surrounding it. Circulation of the amniotic fluid involves exchange between 3 fluid compartments: the amniotic cavity, the fetus (gastrointestinal tract, blood, urinary tract) and the mother (blood). This circulation is essential to the normal development of the fetus, and the level of amniotic fluid (checked by ultrasound) can be diagnostic for the development of the fetus.
The mother’s adaptations to pregnancy
Weight gain
During pregnancy a mother usually gains 10–12 kg, mainly during the second and third trimesters. The usual components of this weight gain are shown in Table 31-1, but of course this is different for each pregnancy, so the values given are merely examples.
Table 31-1 TYPICAL WEIGHT GAINS DURING PREGNANCY
| Maternal body | Uterus | 1 kg |
| Breasts | 0.5–1 kg | |
| Extra fat and protein | 3 kg | |
| Blood and intracellular fluids | 2 kg | |
| Uterine contents | Fetus | 3.5 kg |
| Placenta | 1 kg | |
| Amniotic fluid | 0.5 kg |
Endocrine changes
Many of the anatomical and physiological changes of pregnancy are driven by maternal hormonal changes. The principal changes are the high levels of oestrogen and progesterone produced by the placenta and corpus luteum; other hormonal changes are summarised in Table 31-2. In the first two months, the corpus luteum, under the influence of chorionic gonadotropin, is almost entirely responsible for the secretion of these hormones; thereafter, they are produced by the placenta.
Table 31-2 HORMONAL CHANGES OTHER THAN OESTROGEN AND PROGESTERONE DURING PREGNANCY
| SITE OF PRODUCTION | HORMONE | EFFECTS |
|---|---|---|
| Anterior pituitary | Prolactin | Breast development Lactation |
| Adrenocorticotropic hormone (ACTH) | Stimulates the adrenal cortex | |
| Melanocyte-stimulating hormone | Causes some skin darkening, especially around the nipple | |
| Posterior pituitary | Oxytocin | Uterine contractions during labour Letdown of milk post parturition |
| Antidiuretic hormone (ADH) | Fluid retention by kidneys | |
| Thyroid | Thyroid hormones (T3 and T4) | Increases basal metabolic rate Increases pulse rate Increases CO2 production (due also to increased metabolism) |
| Adrenal cortex | Aldosterone | Salt retention by kidney |
| Kidney | Erythropoietin (EPO) | Increased erythrocyte production |
Among the many effects of oestrogen on the mother in pregnancy are growth of the uterus and breasts, fluid retention and widespread vasodilation, which assists in preventing blood pressure from increasing as blood volume increases in pregnancy. The effects of progesterone include reduced smooth muscle activity in the uterus, gastrointestinal tract, airways and arterioles; growth of the breasts; an increase in the basal metabolic rate; and stimulation of the respiratory centre in the medulla of the brain.
Changes in reproductive and other organs during gestation
The uterus and breasts increase in size in the pregnant female, but the degree of increase depends on a variety of factors. For example, a small baby will not expand the uterus quite as much as a larger baby.
Uterus
The uterus expands to contain the ever-growing fetus. At 12 weeks the height of the fundus of the uterus is at the level of the pubic symphysis, by 20 weeks it is at the level of the umbilicus and between 36 and 40 weeks it is at the level of the xiphoid process of the sternum. The uterus enlarges mainly by hypertrophy (enlargement) of existing cells. In late pregnancy, stretching leads to thinning of the uterine wall. Irregular contractions of the uterine muscle occur throughout pregnancy; these are known as Braxton-Hicks contractions.
Cervix
The cervix enlarges and softens, and the cervical canal is filled by a mucous plug (operculum). The operculum not only helps to hold the fetus and placenta within the uterus, but it also acts as a physical barrier to infection. At the onset of labour the operculum is expelled as a ‘show’ and is a clear indication of impending parturition.
Breasts
There is hyperproliferation of breast tissue, increasing the size of the breasts. This is physiologically normal growth, not to be confused with the rapid growth of breast tissue experienced in breast cancer (see Chapter 32). This growth may be accompanied by a feeling of fullness and tenderness. There will also be dilation of the veins, a deepening in colour of the nipples and areola, and the appearance of Montgomery follicles (enlarged sebaceous glands) in the areola.
Respiratory changes
In pregnancy, there is an increase in the depth of breathing (at rest), without an increase in the rate of breathing. This change leads to better clearance of carbon dioxide and improved oxygenation of maternal blood; this is clearly favourable for fetal gas exchange in the placenta. Progesterone acts on the respiratory centre in the medulla of the brain to increase its sensitivity to carbon dioxide: the result is an increase in tidal volume, thereby increasing the depth of breathing. Thus ventilation increases, without an increase in respiratory rate. This is aided by the relaxing effect of progesterone on the smooth muscle of the airways (reducing airway resistance).
Late in pregnancy, the growing uterus lifts up the diaphragm, leading to more effective ‘emptying’ of the lungs (lower lung residual volume), improving clearance of carbon dioxide from the maternal blood. In addition to increased concentrations of oxygen (and reduced concentrations of carbon dioxide), oxygen carriage by maternal blood is increased because the blood volume and the total number of erythrocytes (oxygen-carrying red blood cells) are increased in pregnancy.
Cardiovascular changes
The cardiovascular changes of pregnancy ensure increased blood flow to the placenta and uterus. The fundamental change is a large increase in blood volume. Plasma volume increases (by 45%) to a greater extent than does the number of erythrocytes (by 25%); thus there are apparent falls in values for red blood cells and haemoglobin (haemodilution), with an actual increase in oxygen-carrying capacity. Haemodilution is beneficial in that it results in better circulation of the blood. One of the effects of this improved circulation is more efficient loss of heat, from both the fetus and the mother, through the mother’s skin. The increased blood volume automatically causes increased cardiac output, as both stroke volume (blood ejected from each ventricle at each contraction) and heart rate increase. While increased blood flow to the placenta and the uterus is effected in this way, it is achieved without corresponding increases in blood pressure, as the relaxing effect of progesterone on the smooth muscle of the arterioles leads to reduced peripheral resistance. Thus blood pressure does not in general change with pregnancy and may in fact fall slightly due to the vasodilative effect of progesterone.
Fetal blood has a higher concentration of haemoglobin than does maternal blood, and fetal haemoglobin has a greater affinity for oxygen than does adult haemoglobin: these differences favour the transfer of oxygen from maternal blood to fetal blood in the placenta.
Water and electrolytes
In pregnancy there are increases in both body water content and urine output. The increase in body water comprises increases in plasma volume and increased extracellular fluid (i.e. widespread oedema). With the increased blood volume, there is increased renal blood flow and thus the glomerular filtration rate increases. This leads to increased urine output, even though aldosterone causes reabsorption of sodium and antidiuretic hormone causes reabsorption of water at markedly high levels during pregnancy. Increased urine output, of course, leads to a greater frequency of micturition. Another contributor to this effect is the increasing pressure of the growing uterus on the bladder. Increased thirst and drinking in pregnancy maintain the supply of water for these processes.
Nutrition and metabolism
In pregnancy food intake increases, as does anabolism, and there is some increase in the basal metabolic rate. In early pregnancy the mother may experience nausea, but appetite and thirst subsequently increase to high levels and these are maintained throughout pregnancy. The increase in food intake supplies the required nutrients for:
 laying down of fat and glycogen stores in the fetus, late in pregnancy — at the beginning of independent life glycogen stores are used as metabolic fuels and the fat as insulation.
laying down of fat and glycogen stores in the fetus, late in pregnancy — at the beginning of independent life glycogen stores are used as metabolic fuels and the fat as insulation.There may also be some problems associated with nutrition in pregnancy:
 If the increased requirements for special nutrients (e.g. calcium, iron, folic acid) are not met, these may be reflected in abnormal development, such as congenital defects of the spinal chord.
If the increased requirements for special nutrients (e.g. calcium, iron, folic acid) are not met, these may be reflected in abnormal development, such as congenital defects of the spinal chord. A reduction in gut motility, caused by the relaxing effect of progesterone on smooth muscle, can lead to constipation.
A reduction in gut motility, caused by the relaxing effect of progesterone on smooth muscle, can lead to constipation. Stomach reflux and heartburn may be frequent as the lower oesophageal sphincter has reduced tone (an effect of progesterone) and the stomach is ‘crowded’ by the uterus.
Stomach reflux and heartburn may be frequent as the lower oesophageal sphincter has reduced tone (an effect of progesterone) and the stomach is ‘crowded’ by the uterus.Fetal development
The fetus’ organ systems are established by the end of the eighth week after fertilisation. In the following 30 weeks the fetus changes in proportion, as growth of the body ‘catches up’ to the relatively large head. In addition the fetus also grows in size — the increase in length is especially marked from 9 to 16 weeks and the increase in weight is especially marked in the last few weeks of pregnancy.
In the first half of pregnancy, all babies grow at the same rate, so measurement of size, by ultrasound, gives accurate dating of the pregnancy within this period. However, in the second half of pregnancy, individual differences in the sizes of different fetuses appear.
Some features of the fetal period:
 9–12 weeks
9–12 weeks
 17–20 weeks
17–20 weeks
 26–29 weeks
26–29 weeks
 30–38 weeks
30–38 weeks
The average pregnancy is 266 days (38 weeks) after conception or 40 weeks after the last menstrual period. Pregnancy may be prolonged up to 2 weeks; a baby born this late will be thin, with dry skin (‘postmature’).
Development of the reproductive systems in utero
The structure and function of both the male and the female reproductive systems depend on the sex hormones. Hormonal effects on the reproductive systems begin during embryonic development and continue in varying degrees throughout life.
Until the eighth week of gestation, the initial reproductive structures of male and female embryos appear the same.2 At about 7–8 weeks of gestation, the gonads of genetically male embryos begin to produce testosterone. Under its influence, the male gonads develop into the testes, which produce sperm after puberty. The presence of oestrogen or the absence of testosterone cause the female gonads to develop into ovaries. By 9 months of gestation, the internal and external genital structures are all present for both sexes and the male gonads (the testes) have descended into the scrotum.
The immune system of the fetus
The structural components of the immune system (the thymus, spleen and lymphatic system) appear early in the embryo and become functional in the fetus in the second trimester. However, production of antibodies does not reach effective levels until several months after birth. Thus in the first few months after birth, the baby relies for immune protection on passive immunity from IgG, transferred across the placenta in fetal life. In addition, IgA in colostrum and milk provides protection in the lumen of the baby’s digestive tract (but is not taken up into the baby’s bloodstream).
Note that during pregnancy, the mother may produce antibodies to some fetal cells (e.g. fetal erythrocytes that have escaped into the maternal circulation), but she does not produce antibodies to the fetal part of the placenta.
The neonate
Changes in the cardiovascular system at birth
Profound changes in the pattern of circulation must occur rapidly at birth. The fundamental causes of these changes are the cessation of circulation in the placenta and the inflation of the baby’s lungs with air.
Cessation of placental circulation occurs as the umbilical arteries constrict at birth, so there is no loss of the newborn’s blood to the placenta. The remnants of the umbilical arteries are recognisable throughout life as the umbilical ligaments. The umbilical vein continues to carry fetal blood from the placenta to the newborn for a minute or so after birth; it is eventually transformed into the ligamentum teres, running from the umbilicus to the liver. The ductus venosus (liver bypass) ceases to carry blood after birth and is transformed into the ligamentum venosus, running from the portal vein to the inferior vena cava, through the liver.
As the newborn’s lungs fill with air, resistance to blood flow through the lungs falls. Thus pulmonary blood flow increases and the volume of blood that returns from the lungs to the left atrium increases. Pressure in the left atrium increases and rises to exceed pressure in the right atrium, a reversal of the situation before birth. As left atrial pressure rises above right atrial pressure, the valve-like foramen ovale in the interatrial septum closes so that blood can no longer move directly from the right atrium to the left atrium. The closure is at first ‘functional’ — that is, a valve-like closure. It eventually becomes ‘anatomical’ by the growth of fibrous tissue, the site of the foramen ovale becoming part of the interatrial septum.
The ductus arteriosus in the fetus is a bypass from the pulmonary trunk (high right ventricular pressure) to the aorta (low pressure). At birth, with inflation of the lungs, the ductus arteriosus constricts (at the same time as the foramen ovale closes). Thus all of the blood pumped by the right ventricle travels to the lungs. There is subsequently no vascular connection between the pulmonary trunk and the aorta and the ductus arteriosus is transformed into the ligamentum arteriosus, running from the left pulmonary artery to the aorta.
In some individuals, the foramen ovale may remain partly open after birth, allowing movement of some deoxygenated blood from the right atrium to the left atrium. In premature babies, especially those experiencing respiratory difficulties (and in those whose mother had rubella), the ductus arteriosus may remain patent for some time after the birth; the flow of blood in this bypass will, however, now be reversed as blood flows from high pressure (aorta, after birth) to low (pulmonary trunk).
In the fetus, the right side of the heart is at higher pressure than the left, because relatively little blood flows from the lungs to the left atrium. The right ventricle of the fetus works harder than the left and the muscle of the right ventricular wall is correspondingly thicker. Relative pressures are reversed at birth and the left ventricular wall becomes thicker than the right ventricular wall by the end of the first month.
Changes in the respiratory system at birth
In the fetus, the lungs are not collapsed, but are filled and expanded by a fluid (mainly sodium chloride solution) secreted by the lungs themselves. Breathing movements occur before birth and are apparently required in order for the fetal lungs to be maintained in their expanded, fluid-filled state. At birth, the fluid in the lungs is cleared by pressure on the thorax during delivery, the fluid draining through the nose and mouth; absorption into the blood of the pulmonary capillaries; and absorption into the pulmonary lymphatics.
Breathing normally commences within 1 minute of birth as the amniotic fluid in the lungs is squeezed out or absorbed. Respiration is initiated by multiple stimuli as the baby suddenly moves from a warm quiet dark environment (inside the mother) to a colder brighter and noisier outside world. The trigger to breathe comes from: (1) chemoreceptors responding to low blood oxygen, high carbon dioxide and low pH as the blood flow from the placenta stops; (2) thermoreceptors responding to reduced ambient temperature; and (3) other receptors responding to movement, pain, handling, light, noise and gravity. It is not necessary to hold the baby upside down and smack its backside as is commonly depicted, although this will not actually harm the baby and can be used to assist lung fluid drainage and stimulation of breathing. These stimuli act through the respiratory centre in the brain to trigger breathing. Diaphragm activity usually becomes settled within a few hours, with a breathing rate of about 40/min — before settling, it may be irregular for some time, with higher rates such as 60/min.
Aeration and expansion of the lungs with air immediately lead to a reduced resistance to blood flow through the lungs (hence the increase in pulmonary blood flow). This gives rise to an increase in left atrial pressure (higher than the right atrial pressure) and closure of the foramen ovale and ductus arteriosus, as discussed above.
Fully mature alveoli do not appear until some time after birth and most of the alveoli that will eventually be present in the adult develop within the child’s first eight years. However, the alveoli present at birth, although fewer and different in structure from those of the adult, are adequate for gas exchange. Functioning of the alveoli, of whatever age, depends on surfactant, and this does not reach adequate levels for efficient lung function until shortly before the end of pregnancy.
Changes in liver function at birth
The liver of the newborn is relatively large. One of its major functions is the storage of iron: if the mother’s iron intake has been adequate during pregnancy, considerable iron stores are present in the baby’s liver at birth. These stores are drawn upon until solid feedings with a high iron content commence. The newborn’s liver also has a store of glycogen, which provides energy in the first few days after birth, until abundant carbohydrate becomes available from milk. The glycogen store is especially rapidly depleted if the newborn is hypoxic or hypothermic.
In addition, the liver of the newborn must metabolise bilirubin, a waste produce of red blood cell breakdown. This is extensive after birth, as fetal red blood cells are replaced with new adult red blood cells. This substance must be metabolised in the liver and then excreted in the bile. If, as is frequently the case, the liver is not yet proficient at metabolising bilirubin at birth, a mild jaundice may be evident. Persistently high levels of bilirubin in the blood of the newborn may pass into the brain and cause permanent damage (kernicterus). Potentially damaging high levels of bilirubin can be reduced by phototherapy in which the infant is exposed to bright light, which modifies bilirubin as it flows through the skin into a form that can be excreted by the kidneys.
Production of blood-clotting factors by the liver requires vitamin K. In later life, this vitamin is produced by intestinal bacteria, but these are not present at birth. As a result, vitamin K is administered prophylactically to all newborns to avoid the possibility of spontaneous bleeding.
CONTROL OF FERTILITY
A range of techniques are available to prevent conception. These techniques interfere with the ability of sperm and ova to meet and implant in the uterus, and may be structural or hormonal barriers. Some options are more reliable than others.
Surgical sterilisation
Sterilisation procedures involve surgical interruption of the reproductive ducts in either the male or the female. Overall, they should be considered as permanent, as they cannot be reliably reversed.
Vasectomy in males is the removal of part of the ductus deferens, which was previously known as the vas deferens — hence the term vasectomy refers to the severing of this duct. This procedure is performed under local anaesthetic as an outpatient. The ejaculate is free of sperm after 2–3 months; otherwise, sexual function remains unchanged. There may be adverse effects, such as leakage from the cut end of the ductus deferens, which may cause chronic inflammation and tenderness; and leakage of sperm into the blood, which may elicit an (auto-) immune response resulting in inflammation and a danger of destruction of the spermatocytes. Males are often worried about a vasectomy and fear that they will become impotent; this may stem from the mistaken belief that infertility results in impotence.
Tubal ligation in females is essentially interruption of the fallopian tubes by ligation (tying), electrocoagulation or surgical section (cutting). It is usually performed laparoscopically under general anaesthetic. This operation prevents sperm from reaching the oocyte and the oocyte from travelling down the fallopian tubes into the uterus. The adverse effects are associated with surgical trauma and anaesthetics.
The Essure method of tubal interruption is an alternative permanent sterilisation technique. A catheter-like instrument is guided through the cervix and uterus into the fallopian tubes. A microinsert (a soft, springlike coil made of polyester fibres and nickel titanium alloy) is placed in each fallopian tube. The microinsert has an inner coil and an outer coil and polyethylene fibres in a tightly wound configuration. Once in place, the outer coil expands to anchor the microinsert in the fallopian tube. Over a period of approximately 3 months, tissue grows into the coil to permanently block the fallopian tube and hence the oocyte can no longer meet the sperm. An X-ray is taken 3 months after the procedure to assess whether the blockage is complete. The first clinical trial of this technique in Australia showed the method to be safe and highly acceptable to women with 100% efficacy.11 Although there is at least one report of failure,12 this is a safer method than tubal ligation, as it does not involve laparoscopic surgery.
Barrier methods
Barrier methods of contraception are designed to prevent sperm from reaching the oocyte and are reliable only if properly used.
The condom needs to be put on the penis prior to physical contact between the penis and vagina; its reliability can be increased by concurrent use of a spermicide. The condom has the added advantage of providing protection for both partners against sexually transmitted infections.
The cervical cap fits around the cervix and is held there by suction. Similarly, the diaphragm has a sprung margin that allows it to block the top of the vagina as well as the cervix itself. Both the cap and diaphragm can be inserted a considerable time before intercourse and must be left in place for 6 hours after intercourse as the sperm will remain in the vagina for some time. The cap or diaphragm should be used only in combination with a spermicide, since they are unreliable on their own. Spermicides contain a chemical in either a cream or a foam that will kill sperm. The spermicide must be applied shortly before intercourse and does not provide reliable contraception on its own.
The disadvantages of the cap or diaphragm plus spermicide include: they need to be in place before sexual intercourse and so forethought is required; the cap and diaphragm must be carefully fitted to prevent sperm accessing the uterus; and spermicides can cause irritation of the vagina and cervix.
Hormonal methods
Oral contraceptive medications are referred to as the contraceptive pill (or ‘the pill’). Combined oral contraceptives contain both oestrogen and progesterone. These hormones suppress ovulation by feeding back on the hypothalamus and anterior pituitary to inhibit the secretion of FSH and LH. Thus follicles do not mature (lack of FSH) and oocytes are not released (lack of LH). The hormones in the combined pill thus mimic the actions of the corpus luteum in the luteal phase of a normal cycle or of the placenta in pregnancy. Typically, the pill is taken for 21 days, during which FSH and LH are suppressed and follicle development does not occur; however, the endometrium develops and is maintained by the steroids in the pill. For the next 7 days, no active pills are taken and the endometrium breaks down in a ‘simulation’ of normal menstruation.
Progesterone alone has a number of contraceptive effects: it inhibits ovulation in some (but not all) cycles; it inhibits the development of the endometrium under the influence of oestrogen (as seen in the proliferative phase of the menstrual cycle); and, most importantly, it causes thickening of the cervical mucus, making it impenetrable by sperm. The minipill contains progesterone only. The contraceptive action on cervical mucus lasts only 22–26 hours, so the pill must be taken at the same time each day (successive pills no more than 24 hours apart).
Other means of delivery of progesterone-only preparations are by implantation, such as Norplant capsules or Depot injection (e.g. Depo-Provera). Norplant implants may last for 5 years, while Depo-Provera lasts for 3 months.
The most significant side effects of oral contraceptives are an increased risk of blood clotting and hypertension in women who smoke or have a history of cardiovascular disease. The risk was higher in older forms of combined contraceptives, which incorporated much higher hormone concentrations than are currently used. Irregular bleeding is often associated with the use of progesterone-only preparations. The association of steroid contraceptives with cancers of the breast and reproductive system is controversial. Major life-threatening side effects are rare.
Contraceptives for men containing testosterone are in the experimental stage. Testosterone feeds back on the hypothalamus and anterior pituitary to inhibit the secretion of FSH and LH. Without FSH, sustentacular cells cannot carry out their crucial functions in supporting spermatogenesis; thus sperm concentrations in semen fall below those required for fertility. This method of contraception appears effective, with minor side effects of weight gain and acne; long-term effects on the prostate gland are under investigation.
Other methods of contraception
Coitus interruptus (withdrawal) requires the penis to be withdrawn prior to ejaculation. If practised diligently, there is some contraceptive effect. However, often the penis is either not withdrawn at all or only partially withdrawn, and this practice will almost inevitably lead to a high failure rate.
In the rhythm method, the couple monitors the female’s menstrual cycle over a few months to ensure a good record of the cycle. They then pick the point of the cycle where they are relatively certain that they will have little chance of fertilising an oocyte. This could be at least 6 days before ovulation (sperm can survive for up to 5 days inside the female body) or 2 days post-ovulation (when the oocyte is no longer capable of being fertilised). The problem with this method of contraception is that few couples really understand the female cycle well enough to successfully chart the progress. Furthermore, most women do not have an absolutely regular cycle and, since timing is crucial, the failure rate is high.
The intrauterine device (IUD) acts as foreign body in the uterus to produce a low-grade, chronic inflammatory response. In this state, the uterus inhibits sperm transport and prevents successful implantation of a fertilised egg. Modern IUDs are now made of plastic rather than copper. Adverse effects of IUDs include cramping and bleeding and increased risk of infection. If pregnancy occurs, there is an increased risk that it may be ectopic.
The ‘morning after’ pill interferes with transport of the fertilised egg and with implantation. A combined pill is given in two doses, 12 hours apart.
Mifepristone (also known as RU486) is a progesterone antagonist that blocks progesterone receptors in the uterus. This progesterone-blocking action in the endometrium could prevent implantation; however, the more significant use of this drug is to produce early abortion. Administration of the drug within 2 months of conception causes erosion of the endometrium and increased contraction of the myometrium. The administration of RU486 is followed after 48 hours by administration of prostaglandin, to complete the process of expulsion of the embryo.
The failure of contraception
Failure rates for contraception are shown in Table 31-3. Several of the techniques in Table 31-3 are quite reliable, with sterilisation procedures being the most successful. However, sterilisation procedures are often avoided due to the difficulty in reversing them at a later stage. Oral contraceptive pills and implants based on hormones are widely used, as they are effective and can be ceased relatively easily if conception is desired. The only completely reliable method of preventing conception is abstinence (avoiding sexual intercourse). If no method is used at all, the failure rate is 80–90% in women under the age 35 and 40–50% in women over the age of 35.
Table 31-3 FAILURE RATES FOR CONTRACEPTION (NUMBER OF PREGNANCIES PER 100 WOMEN PER YEAR)
| TECHNIQUE | PRINCIPLE | FAILURE RATE |
|---|---|---|
| Sterilisation | Male: vasectomyFemale: tubal ligation | Male: 0–0.2%Female: 0–0.5% |
| Condom | Physical barrier, worn on the penis | Up to 15% |
| Cap or diaphragm | Physical barrier, inserted into the vagina | Up to 15% |
| Spermicide | Cream or foam, kills sperm | Up to 25% |
| Oral contraceptive pill | Hormonal control, main effect is by preventing ovulation | Oestrogen and progesterone: up to 3%Progesterone only: up to 4% |
| Progesterone implant or injection | Hormonal control | Up to 1% |
| Intrauterine device | Inserted into the uterus (by a doctor) | Up to 5% |
| Withdrawal | Removal of the penis from the vagina prior to ejaculation | Up to 20% |
| Rhythm method | Avoiding sexual intercourse around the day of ovulation | Up to 25% |
AGEING AND THE REPRODUCTIVE SYSTEMS
In males, testosterone levels peak at 20 years of age and then slowly decline as the number and function of the interstitial and sustentacular cells decrease. A reduction in testosterone (and inhibin) results in a rise in FSH and LH levels as the negative feedback by testosterone is reduced, resulting in higher levels of FSH and LH being released from the anterior pituitary. The effect of the reduction in testosterone and increase in FSH and LH causes a male climacteric (mood changes and hot flushes), which is much less well-defined and physically apparent than menopause in women. Impotence (inability to maintain an erection sufficient for intercourse) becomes more common with increasing age.
In females, a regular monthly cycling of hormones continues until sometime in the fifth decade, when the menstrual cycle becomes less regular.8 Oestrogen is not secreted by follicle cells in sufficient amounts to cause an LH surge, so ovulation may fail to occur (climacteric). Finally, the two cycles cease at menopause due to the decreasing number of follicles and the reduced responsiveness of the follicles to FSH.13–18 14 15 16 17 18 After menopause, diminishing levels of oestrogen are secreted from the ovary. This results in atrophy of the sex organs — for example, the breasts lose much of the fat and milk-producing ducts. In addition to its effects on the reproductive organs, the lack of oestrogen leads to a decrease in bone density (because oestrogen is required for the maintenance of bone). Loss of bone density can lead to brittleness of the bones, which may break easily in older women.
The structure and function of the male reproductive system
 The scrotum is a pouch of skin that contains the testes and epididymis. It is maintained at a cooler temperature than that of the body core.
The scrotum is a pouch of skin that contains the testes and epididymis. It is maintained at a cooler temperature than that of the body core. The testes are the male gonads; they are filled with seminiferous tubules, which contain primordial germ cells for sperm production. The sustentacular (Sertoli) cells support sperm production, while the Leydig cells produce testosterone.
The testes are the male gonads; they are filled with seminiferous tubules, which contain primordial germ cells for sperm production. The sustentacular (Sertoli) cells support sperm production, while the Leydig cells produce testosterone. The epididymis is the site for sperm maturation and storage. Sperm then move through the ductus deferens (vas deferens), the ejaculatory duct, prostate gland and urethra.
The epididymis is the site for sperm maturation and storage. Sperm then move through the ductus deferens (vas deferens), the ejaculatory duct, prostate gland and urethra.The structure and function of the female reproductive system
 The vulva is the collective name for the external female genitalia. It includes the clitoris, which is an erectile tissue that is important in sexual stimulation.
The vulva is the collective name for the external female genitalia. It includes the clitoris, which is an erectile tissue that is important in sexual stimulation. The vagina is an elastic fibromuscular canal that receives the penis and ejaculate during sexual intercourse, as well as being the route for parturition of the fetus.
The vagina is an elastic fibromuscular canal that receives the penis and ejaculate during sexual intercourse, as well as being the route for parturition of the fetus. The uterus has thick muscular walls that consist of the endometrium, the myometrium and the serosa. Its function is for gestation of the fetus.
The uterus has thick muscular walls that consist of the endometrium, the myometrium and the serosa. Its function is for gestation of the fetus. The fallopian (or uterine) tubes each extend from the uterus and end by curving around the ovary. They transport the oocyte or zygote to the uterus.
The fallopian (or uterine) tubes each extend from the uterus and end by curving around the ovary. They transport the oocyte or zygote to the uterus. The ovaries are the female gonads. They are the site of oogenesis and the production of the sex hormones oestrogen and progesterone.
The ovaries are the female gonads. They are the site of oogenesis and the production of the sex hormones oestrogen and progesterone.Puberty in males and females
 Puberty commences when the hypothalamus produces gonadotropin-releasing hormone, which stimulates the anterior pituitary to produce the gonadotropins follicle-stimulating hormone (FSH) and luteinising hormone (LH).
Puberty commences when the hypothalamus produces gonadotropin-releasing hormone, which stimulates the anterior pituitary to produce the gonadotropins follicle-stimulating hormone (FSH) and luteinising hormone (LH). The gonadotropins are released into the general circulation; the ovaries or testes (gonads) respond by producing sex hormones (oestrogen, progesterone and testosterone) and gametes (oocytes and spermatozoa).
The gonadotropins are released into the general circulation; the ovaries or testes (gonads) respond by producing sex hormones (oestrogen, progesterone and testosterone) and gametes (oocytes and spermatozoa).Gametogenesis
 Most body cells contain 23 pairs of (or 46) chromosomes. The ova and sperm each contain 23 chromosomes.
Most body cells contain 23 pairs of (or 46) chromosomes. The ova and sperm each contain 23 chromosomes. Spermatogenesis is the process by which the germ cells within the seminiferous tubules undergo development into spermatids. Spermiogenesis allows them to mature into spermatozoa (sperm).
Spermatogenesis is the process by which the germ cells within the seminiferous tubules undergo development into spermatids. Spermiogenesis allows them to mature into spermatozoa (sperm). Sperm consist of a head (which contains chromosomes), a midpiece (which contains mitochondria to produce energy) and a tail (for propulsion).
Sperm consist of a head (which contains chromosomes), a midpiece (which contains mitochondria to produce energy) and a tail (for propulsion). Sperm travel through the epididymis, ductus deferens, ejaculatory ducts and urethra to exit the male body.
Sperm travel through the epididymis, ductus deferens, ejaculatory ducts and urethra to exit the male body. The ovarian cycle occurs in response to FSH and LH. FSH causes the development of the follicle, and a surge in LH causes ovulation.
The ovarian cycle occurs in response to FSH and LH. FSH causes the development of the follicle, and a surge in LH causes ovulation. The menstrual cycle occurs in response to oestrogen and progesterone, and consists of the menstrual stage (days 1–5), the proliferative stage (days 6–14) and the luteal stage (days 15–28). This cycle allows the uterus to be prepared for implantation (in the event of fertilisation), or for the endometrium to be shed (in the event of no fertilisation)
The menstrual cycle occurs in response to oestrogen and progesterone, and consists of the menstrual stage (days 1–5), the proliferative stage (days 6–14) and the luteal stage (days 15–28). This cycle allows the uterus to be prepared for implantation (in the event of fertilisation), or for the endometrium to be shed (in the event of no fertilisation)Male and female sexual responses
Conception, gestation and parturition
 Capacitation of sperm allows them to be physically ready to meet the oocyte. Sperm break down the zona pellucida until one sperm penetrates it and reaches the oocyte cell membrane.
Capacitation of sperm allows them to be physically ready to meet the oocyte. Sperm break down the zona pellucida until one sperm penetrates it and reaches the oocyte cell membrane. Fusion of the 23 chromosomes from the sperm with the 23 chromosomes from the oocyte results in the fertilised egg, referred to as the zygote.
Fusion of the 23 chromosomes from the sperm with the 23 chromosomes from the oocyte results in the fertilised egg, referred to as the zygote. The zygote undergoes cell divisions to enlarge and eventually implants in the endometrium as the embryo.
The zygote undergoes cell divisions to enlarge and eventually implants in the endometrium as the embryo. Umbilical arteries transport nutrient-rich oxygenated blood from the placenta to the fetus, while the umbilical vein transports deoxygenated blood from the fetus to the placenta.
Umbilical arteries transport nutrient-rich oxygenated blood from the placenta to the fetus, while the umbilical vein transports deoxygenated blood from the fetus to the placenta. The fetal membranes contain amniotic fluid, which allows the fetus to float and provides it with physical protection.
The fetal membranes contain amniotic fluid, which allows the fetus to float and provides it with physical protection. Major changes occur to the mother’s body during pregnancy. These include changes in her reproductive organs, as well as increases in both respiratory and cardiovascular system functions. The mother’s needs for adequate nutrition, fluids and electrolytes all increase with pregnancy.
Major changes occur to the mother’s body during pregnancy. These include changes in her reproductive organs, as well as increases in both respiratory and cardiovascular system functions. The mother’s needs for adequate nutrition, fluids and electrolytes all increase with pregnancy.Control of fertility
 Surgical sterilisation procedures are vasectomy in the male, and tubal ligation and Essure in the female.
Surgical sterilisation procedures are vasectomy in the male, and tubal ligation and Essure in the female.Anthea is 28 years old. When she missed her last menstrual cycle, she thought she might be pregnant for the first time, and a home pregnancy test confirmed her suspicions. After seeing her doctor she was given further tests, which confirmed her pregnancy, and scheduled for ultrasound examination. As the pregnancy developed she was given further tests (blood tests, glucose tolerance test, urine and vaginal swabs) to determine how well her body was coping and how the baby was progressing. A friend told her that she should begin to feel the baby moving by 14 weeks and she was worried when she felt nothing. However, at her regular ultrasound examination she was assured that all was progressing well. At term, Anthea’s waters broke and she began to experience regular contractions which become stronger and more frequent, so she immediately attended the maternity hospital. The staff examined her: her cervix had dilated to 10 cm, so she was taken to a delivery room and told she can have some medication (epidural pethidine) for the pain if she wanted. The frequency of contractions was timed and the fetal heart rate was monitored at 120 bpm. She was told that she should deliver soon. The baby was delivered without complication and started breathing almost immediately; after a minute, the umbilical cord was cut. Some 20 minutes later the placenta was delivered and examined. Anthea had a healthy baby girl and was left to bond with her.