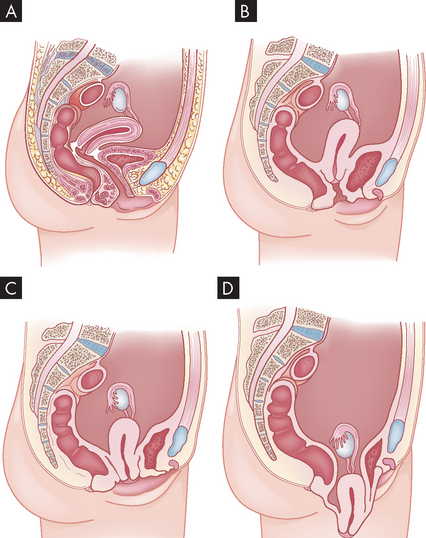
32 ALTERATIONS OF THE REPRODUCTIVE SYSTEMS ACROSS THE LIFE SPAN
INTRODUCTION
Alterations of the reproductive systems span a wide range of concerns, from delayed sexual development and suboptimal sexual performance to structural and functional abnormalities. Many common reproductive disorders carry potentially serious physiological and psychological consequences. For example, sexual dysfunction such as impotence can dramatically affect self-confidence, relationships and overall quality of life. Conversely, organic and psychosocial problems, such as alcoholism, depression, chronic illness and medications, can affect ovulation and menstruation, sexual performance and fertility. In addition, these alterations may be risk factors for the development of some types of reproductive tract cancers. Unfortunately, cancers of the reproductive system in both sexes often cause considerable alteration to homeostasis and are potentially life-threatening. In Australia and New Zealand, breast cancer is one of the leading causes of cancer deaths in women and affects many women at a young age, and prostate cancer is one of the most common cancers in men. Therefore, a thorough understanding of the pathogenesis of these cancers is fundamental when caring for the individuals affected by them.
In this chapter we consider problems in both male and female reproductive structure and function. We briefly explore the likely causes of these problems and outline some of the symptoms and treatments. The reproductive systems and sexual function are often taboo subjects outside of the healthcare profession and this in itself is a major issue when dealing with associated problems. Many people find it difficult to talk about reproductive problems with their health professional — let alone their partner. Diagnosis and treatment of reproductive system disorders are often complicated by the stigma associated with the reproductive organs and by emotions related to reproductive health. Treatment and/or diagnosis may be delayed because of embarrassment, guilt, fear or denial.
CLASSIFICATION OF REPRODUCTIVE SYSTEM ALTERATIONS
Alterations of the reproductive systems that are most commonly seen are broadly the result of one of three different initial causes that display similar symptoms. The most common causes of sexual dysfunction are: (1) growths, (2) problems associated with the endocrine system, and (3) structural and functional alterations of the reproductive system itself.
Growths
Growths within different parts of the reproductive system can be benign, pre-cancerous or cancerous. The growths themselves can be the problem (particularly if they are cancerous) or the effects of the growths can be the problem (if, for example, the growths impinge on another organ). Some growths lead to overproduction of hormones, resulting in a more varied set of symptoms in other parts of the reproductive system.
The endocrine system
Problems associated with the endocrine system can be present in utero and thus lead to abnormal structural development. For example, during development a genetically male fetus (XY) can fail to produce testosterone for a variety of reasons — some genetic, some environmental (see Chapter 37). This lack of testosterone during early fetal development can lead to partially functional female and, in some cases, male genitalia. Although this is an endocrine (or hormonal) dysfunction it leads to poor reproductive development in utero and to physical and psychological problems later in life.
In other cases, endocrine function may be normal during development in utero but then starts too early or too late during adolescence, leading to precocious (early) puberty or delayed puberty. At first, precocious puberty may seem to be less of a health issue and more of a psychosocial issue, but it can be an indication of hypothalamic dysfunction or tumour growth.1 It can also result in a reduced stature as the growth of bones ceases prematurely.2 In addition, precocious puberty has been associated with mental health problems in middle adolescence.3
During adulthood, endocrine dysfunction can result in the failure of normal menstrual and ovarian cycling in females or the failure to produce sperm in males. This can bring about infertility and/or result in pain during menstruation, intercourse and emptying of the bladder or bowel. In addition, an incorrect hormone balance throughout the body can have adverse effects on secondary sexual characteristics, such as the growth of facial hair in females or the growth of breasts in males.
The reproductive system
Structural and functional abnormalities of the reproductive system can be the result of the above-mentioned hormonal problems during development or they can arise as the result of trauma to the body or bacterial or viral infection of the reproductive tract. Trauma can give rise to ectopic implants of endometrial tissue in the myometrium, leading to dysfunctional bleeding and pain. This may also occur following a caesarean section. Infection causes inflammation in which immune cells are recruited to the site of infection and release inflammatory cytokines (see Chapter 13). Inflammation can lead to scarring or adhesions developing and these can interfere with normal ovarian or uterine function.
CANCER
Breast cancer is the most common cancer in females but is uncommon in males. Breast cancer has an incidence rate of almost 28% (expressed as a percentage of all cancers) — just over 12,000 new cases are detected in women in Australia each year.4 To put this in perspective, the number of females diagnosed with breast cancer each year is more than double the number diagnosed with colorectal cancer, the second most common cancer in females. In males, prostate cancer is by far the most commonly diagnosed cancer and accounts for 29.1% of all cancers.4 Like breast cancer in females, the rate of prostate cancer in males is more than double the rate of colorectal cancer, the second most common cancer in males.
Therefore, cancers of the reproductive system in both sexes are the most prevalent of all cancers. Breast cancer and prostate cancer are not the only cancers of the reproductive tract in either sex, but the incidence of other cancers is extremely low. For instance, of all female cancers, the incidence rates of other cancers of the reproductive tract are as follows: cervical cancer 1.7%, uterine cancer 3.8%, ovarian cancer 2.7%, vulvar cancer 0.6%, vaginal cancer 0.2% and cancer of all other gynaecological sites 0.2%.4 In males, the rates are testicular cancer 1.2%, breast cancer 0.2% and penile cancer 0.1%.4 The following discussion therefore focuses mainly on breast cancer and prostate cancer, which you are more likely to encounter in the healthcare setting. Additional discussion of general features of cancer can be found in Chapter 36.
Cancers of the female reproductive system
Breast cancer
Breast cancer is a disease in which abnormal cells in the breast tissues multiply and form an invasive or malignant tumour. Such tumours can invade and damage the tissue around them and then spread to other parts of the body through the lymphatic or vascular systems. If the spread is not controlled, they can result in death. Not all tumours are invasive; some are benign tumours that are not life-threatening.
The risk of being diagnosed with breast cancer before 85 years of age is 1 in 9, which equates to 35 females per day being diagnosed. Although some younger high-profile women have been diagnosed with breast cancer, the average age at diagnosis is 60 years. Unfortunately, of all cancers, breast cancer has a high mortality rate, second only to lung cancer, and in just one year in Australia, 2707 women die of breast cancer.5
Mammographic screening every 2 years is recommended for women aged 50–69 years, although it is also available to women from 40 years of age onwards. Younger women in high-risk groups may be screened by MRI. Symptoms can include: new lumps or thickening in the breast or under the arm, nipple sores, nipple discharge, breast skin dimpling, rash or red swollen breasts. Pain is rare, so regular checking of the breasts is recommended. If any symptoms are found, further diagnostic options such as imaging and biopsy are required.
Staging is rated according to the TMN (tumour, metastasis, node) classification system, which makes use of information on the size of the primary tumour (T), lymph node involvement (N) and the absence or presence of distant metastases (M). Invasive breast cancers are classified in a range from stage I (early disease) to stage IV (advanced disease). If the cancer is limited to the breast, 98% of patients will survive (survival is considered as being free of cancer 5 years after the cancer is detected). If the cancer has spread to the regional lymph nodes, the survival rate drops to 83%.6
Causes of breast cancer are similar to causes for many cancers in that there is a hereditary component, but there are also cases (known as sporadic cases) in which there is no family history (see below). Other factors are increasing age, inheritance of specific gene mutations (in the genes BRCA2, BRCA1 and CHEK2), exposure to female hormones (natural and administered), past exposure to radiation, obesity (diet and exercise) and excess alcohol consumption.
Risk factors
Risk factors and possible causes of breast cancer can be classified as reproductive, hormonal, environmental and familial (see Table 32-1).
Table 32-1 FACTORS ASSOCIATED WITH INCREASED RISK OF BREAST CANCER
| CATEGORY | RISK FACTOR | RELATIVE RISK* |
|---|---|---|
| Family history | Postmenopausal in first-degree relative | ≤ 2.0 |
| Breast cancer in first-degree relative before age 60 years | 2.0–3.0 | |
| BRCA1 or BRCA2 gene | ≥ 4.0 | |
| Premenopausal or bilateral breast cancer | > 4.0 | |
| p53 (tumour-suppressor gene) | > 4.0 | |
| Breast cancer in 2 first-degree relatives | 4.0–6.0 | |
| Previous medical history | Moderate or florid mammary hyperplasia | 1.5–2.0 |
| Mammary papilloma | 1.5–2.0 | |
| Atypical mammary hyperplasia | 4.0–5.0 | |
| Ductal carcinoma in situ or lobular carcinoma in situ | 8.0–10.0 | |
| Oestrogen exposure | Early menarche (before age 12 years) | 1.1–1.9 |
| Late menopause (after age 55 years) | 1.1–1.9 | |
| Postmenopausal oestrogen therapy | 1.4 | |
| Oral contraceptive use | 1.5 | |
| Pregnancy | Nulliparous or late first pregnancy (after age 35 years) | 1.1–1.9 |
| Radiation | Repeated fluoroscopy (X-ray with a fluorescent screen) | 1.5–2.0 |
| Obesity and stature | Postmenopausal | 1.2 |
| Tallness | ≤ 2.0 | |
| Dietary/alcohol | High alcohol consumption | 1.4–2.0 |
| High energy intake | ≤ 2.0 | |
| Advanced age | 2.0–4.0 | |
| Social | Higher socioeconomic status | ≤ 2.0 |
| Low physical activity | ≤ 2.0 | |
| Smoking | 2.0–4.0 | |
| Environmental | Chemical carcinogens | ≤ 2.0 |
| Infectious agents | ≤ 2.0 | |
| * Relative risk is different to absolute risk (overall risk of developing a disease over time). Relative risk is the incidence rate of the disease among individuals exposed to the risk factor compared to the incidence rate of the disease in individuals not exposed to the risk factor. For instance, smokers have a higher risk of developing breast cancer than non-smokers. | ||
Source: Kumar V. Robbins & Cotran pathologic basis of disease. 7th edn. Philadelphia: Saunders; 2005.
Reproductive factors
A woman’s age when her first child is born affects her risk for developing breast cancer: the younger she is, the lower the risk. The main mechanism for the protective effect of pregnancy is controversial. The most widely accepted explanation proposes that the development and differentiation of the breast are completed only by the end of the first term of pregnancy. The protection seems to be the interval of time between menarche and the first pregnancy, because a greater risk is noted with an interval of more than 14 years. The protection conveyed early persists into old age, possibly because of lasting genetic change through differentiation.7 However, this is not entirely clear and research has shown evidence against this as well.
The duration of a woman’s reproductive life also affects her risk of developing breast cancer. Late menarche and early menopause (i.e. a short reproductive life) reduce risk. Menarche marks the onset of the mature hormonal environment — that is, cyclic hormonal changes that result in ovulation, menstruation and cellular proliferation in the breast. Thus, the younger the age at menarche, the earlier a young woman experiences steroid hormone levels and ovulatory cycles. Although data are limited, women with earlier menarche may have higher levels of endogenous oestrogen.8,9 Age of menarche is a relatively weak risk factor overall.
Hormonal factors
Endogenous hormones have long been implicated in the development of breast cancer. Most significant are the findings of: (1) the protective effect of an early first pregnancy; (2) the protective effect of bilateral oophorectomy (surgical removal of the ovary) before age 45 years; (3) the increased risk associated with early menarche, late menopause and nulliparity (never having carried a pregnancy); and (4) the hormone-dependent development and differentiation of mammary gland structures. A vast majority of breast cancers are initially hormone-dependent with oestrogen playing a crucial role in their development.10
Studies have shown that postmenopausal women who have elevated plasma levels of androgen of adrenal or ovarian origin or oestrogens have an increased risk of breast cancer.11 Studies assessing plasma levels of hormones among premenopausal women have produced conflicting results. These studies are more difficult because circulating levels of hormones vary greatly during the menstrual cycle and the low numbers of women with breast cancer among premenopausal women.
Insulin-like growth factors (IGFs) regulate cellular functions involving cell proliferation, differentiation and apoptosis. Insulin-like growth factor-1 (IGF-1) is a protein hormone with a structure similar to insulin. The growth hormone-IGF-1 axis can stimulate proliferation of both breast cancer and normal breast epithelial cells.12 IGF-1 levels seem to be more of a risk factor for premenopausal women. In addition, premenopausal mammographic breast density (a risk factor for breast cancer) has been positively correlated with IGF-1 levels. This relationship has not been found in postmenopausal women.13
Controversy remains about the relationship between oral contraceptive use and breast cancer risk. In contrast, the efficacy of oral contraceptives in protecting against ovarian cancer and endometrial cancer is well established.
Environmental factors
The environmental causes of breast cancer probably affect the glandular epithelial cells of the breast during the early differential stages from undifferentiated cells to alveolar buds and lobules. During these early phases, mitotic activity and cell division are greater than later in life.14
 Radiation. High doses of ionising radiation are associated with an increased risk of all cancers including breast cancer, especially if exposure occurs during adolescence or pregnancy, when breast cells are proliferating rapidly. Ionising radiation causes damage to a cell’s DNA (that is, it alters the sequence of genes). The genes most often affected are those that control the rate of cell division or the rate of cell death (apoptosis). Radiological exposure of the upper spine, heart, ribs, lungs, shoulders and oesophagus also exposes breast tissue to radiation.
Radiation. High doses of ionising radiation are associated with an increased risk of all cancers including breast cancer, especially if exposure occurs during adolescence or pregnancy, when breast cells are proliferating rapidly. Ionising radiation causes damage to a cell’s DNA (that is, it alters the sequence of genes). The genes most often affected are those that control the rate of cell division or the rate of cell death (apoptosis). Radiological exposure of the upper spine, heart, ribs, lungs, shoulders and oesophagus also exposes breast tissue to radiation. Smoking. This is known as a risk factor for development of all forms of cancer, not specifically breast cancer. The association is probably due to the harmful chemicals in cigarette smoke, which cause DNA damage and thereby initiate the development of a tumour.
Smoking. This is known as a risk factor for development of all forms of cancer, not specifically breast cancer. The association is probably due to the harmful chemicals in cigarette smoke, which cause DNA damage and thereby initiate the development of a tumour. Diet. The association between individual foods and breast cancer is inconsistent and new data on dietary patterns are emerging. The prudent pattern includes a higher intake of fruits, vegetables, whole grains, low-fat dairy products, fish and poultry. The Western pattern includes a higher intake of red and processed meats, refined grains, sweets and desserts, and high-fat dairy products. In general, however, neither pattern has been associated with an overall risk of postmenopausal breast cancer. However, a lower risk of oestrogen receptor-negative cancer has been observed in those on a prudent diet.15
Diet. The association between individual foods and breast cancer is inconsistent and new data on dietary patterns are emerging. The prudent pattern includes a higher intake of fruits, vegetables, whole grains, low-fat dairy products, fish and poultry. The Western pattern includes a higher intake of red and processed meats, refined grains, sweets and desserts, and high-fat dairy products. In general, however, neither pattern has been associated with an overall risk of postmenopausal breast cancer. However, a lower risk of oestrogen receptor-negative cancer has been observed in those on a prudent diet.15Most studies have not supported a link between fibre intake and breast cancer.16,17 Carbohydrate quality, however, rather than absolute amount, may be important for breast cancer risk, especially for premenopausal women. Substantial evidence exists that alcohol consumption increases breast cancer risk.18 Differences in alcohol intake, however, explain only a small fraction of breast cancer rates.19,20 The mechanisms by which alcohol intake increases the risk of breast cancer are unknown. It is not known whether decreasing or stopping alcohol consumption in midlife decreases the risk of breast cancer.
 Obesity. Obesity has been associated with a reduced risk of premenopausal breast cancer. One mechanism suggested is the direct relationship between irregular menstrual cycling, especially anovulatory (lack of ovulation) cycling, and obesity. Anovulatory cycling would result in a decrease in both oestrogen and progesterone and thus a decreased risk of breast cancer. Some obese women have polycystic ovaries. With this condition they may have anovulatory cycling, abnormal menstrual periods, elevated androgens and hyperinsulinaemia (see Chapter 35). It is possible that higher insulin levels increase the enzymatic conversion of testosterone to dihydrotestosterone, rather than oestradiol (a type of oestrogen), lowering their oestrogen levels.21
Obesity. Obesity has been associated with a reduced risk of premenopausal breast cancer. One mechanism suggested is the direct relationship between irregular menstrual cycling, especially anovulatory (lack of ovulation) cycling, and obesity. Anovulatory cycling would result in a decrease in both oestrogen and progesterone and thus a decreased risk of breast cancer. Some obese women have polycystic ovaries. With this condition they may have anovulatory cycling, abnormal menstrual periods, elevated androgens and hyperinsulinaemia (see Chapter 35). It is possible that higher insulin levels increase the enzymatic conversion of testosterone to dihydrotestosterone, rather than oestradiol (a type of oestrogen), lowering their oestrogen levels.21
Obesity is associated with poor survival among women with breast cancer and the association of obesity with mortality from breast cancer appears to be stronger than its association with incidence.22,23 Obesity, however, is weakly related to increasing the risk of breast cancer in postmenopausal women:24 despite strong links with endogenous oestrogen levels, body fat has been consistently but weakly related to increased postmenopausal risk.25 This observation has been surprising because obese postmenopausal women have endogenous oestrogen levels nearly double those of lean women.26,27 This weak association is possibly due to the following: the premenopausal reduction in breast cancer risk related to being overweight may persist, opposing the adverse effect of elevated oestrogen after menopause. Thus, weight gain should be more strongly related to postmenopausal breast cancer risk than attained weight. The increase in breast cancer risk with increasing body mass index (see Chapter 35) among postmenopausal women is possibly the result of increases in oestrogen, especially with oestradiol.24,27 Studies do not support the concept that fat intake in middle life has a major relationship with breast cancer risk.28
 Physical activity. Regular physical activity may reduce the overall risk of breast cancer, especially in premenopausal or young postmenopausal women.29–31 4 6 Activity may also reduce invasive breast cancer.32 The mechanisms for this protective effect are not known but include alterations in endogenous free radical formation and oxidative damage (see Chapter 4), effects on DNA repair capacity, increased intestinal transit times (that is, reduced exposures to carcinogens), weight loss and changes in endogenous sex hormone levels.30,31
Physical activity. Regular physical activity may reduce the overall risk of breast cancer, especially in premenopausal or young postmenopausal women.29–31 4 6 Activity may also reduce invasive breast cancer.32 The mechanisms for this protective effect are not known but include alterations in endogenous free radical formation and oxidative damage (see Chapter 4), effects on DNA repair capacity, increased intestinal transit times (that is, reduced exposures to carcinogens), weight loss and changes in endogenous sex hormone levels.30,31Familial factors
Genetically, breast cancer can be divided into three main groups: (1) sporadic — the majority, or 40%, of women with breast cancer have no known family history; (2) inherited autosomal dominant cancer gene syndromes; and (3) polygenic, where there is a family history but it is not passed on to future generations as a dominant gene.
A history of breast cancer in first-degree relatives (mother or sister) increases a woman’s risk two to three times. Risk increases even more if two first-degree relatives are involved, especially if the disease occurred before menopause and was bilateral. A small proportion of breast cancers (5–10%) are the result of highly penetrant dominant genes (that is, hereditary breast cancers).
The most important of the dominant genes are the breast cancer susceptibility genes: BRCA1 and BRCA2. BRCA1 is located on chromosome 17 and BRCA2 is located on chromosome 13. A family history of both breast and ovarian cancer increases the risk that an individual with breast cancer carries a BRCA1 mutation. Up to age 40, a woman with a BRCA1 mutation is estimated to have a 20-times greater risk of breast cancer compared to the general population and a lifetime risk of 60–85%.31 Carriers of the BRCA1 gene are also at higher risk for ovarian cancer. BRCA1 is a tumour-suppressor gene; therefore, any mutation in the gene may inhibit or retard its suppressor function, leading to uncontrolled cell proliferation.33 Interestingly, men who develop breast cancer are more likely to have a BRCA2 mutation than a BRCA1 mutation.
Another tumour suppressor gene, p53, is mutated in approximately 20–40% of individuals with breast cancer.34 p53 is a regulatory gene (that is, it acts to turn mechanisms on or off) that increases DNA repair and, if damage is great, cell death (apoptosis) occurs in mutated cells. Thus, it helps to get rid of cancer proliferating cells. When p53 is mutated, its regulatory properties are radically altered, conferring a loss of tumour-suppressor activity and, possibly, allowing tumour growth.
PATHOGENESIS
Most breast cancers (70%) arise from the epithelial linings of the lactiferous ducts. The reason that these types of tumours account for a high mortality is most likely due to the regular cycles of hormone-induced proliferation (see Chapter 31). It is important to realise that these types of tumours do not grow to a large size, but they often metastasise early, which makes treatment more difficult and contributes to an increased mortality. The pathogenesis (like that of other cancers) involves several main steps:
Unlike most human organs that are differentiated at the end of fetal life, the mammary glands develop and differentiate after puberty. Factors that affect full differentiation of the breast may be essential for countering the development of breast cancer.
Mammary epithelial cells achieve rapid renewal by a small number of mitotic divisions of immortal stem cells. Because the number of mutations is proportional to the rate and number of stem cell divisions, factors that accelerate cell division can have a carcinogenic effect. Hormones may act as accelerators, as well as initiators, and influence the susceptibility of the breast epithelium to environmental carcinogens, because hormones control the differentiation of the mammary gland epithelium and thereby regulate the rate of stem cell division.
In each ovulatory cycle between puberty and either the first full-term pregnancy or menopause among nulliparous women, mammary epithelium stem cells show their greatest rate of division during the luteal phase. In this phase progesterone levels predominate and the breast cells display increased mitosis (the reasons for this are not yet clear).35,36 During the oestrogen follicular phase, terminal ductules are few and there is no mitotic activity. During the luteal phase, because of increased progesterone levels perhaps resulting from the oestrogen priming or as a result of cooperation between the two hormones, there is increased mitotic activity.37,38
Emerging is the understanding that the biological and structural features of carcinomas usually begin at the in situ stage.39 The in situ lesion closely resembles the developing invasive carcinoma.39 For example, low-grade ductal carcinoma in situ (cells that have changed in the lining of the ducts of the breast) with well-differentiated carcinomas, high-grade ductal carcinoma in situ with high-grade carcinomas and lobular carcinomas are associated with lobular carcinoma in situ. Ductal carcinoma in situ is a clonal proliferation (genetically identical cells that rapidly multiply) usually confined to a single ductal system. Lobular carcinoma in situ, unlike ductal carcinoma in situ, has a uniform appearance in which the cells occur in non-cohesive clusters in ducts and lobules. It is important to understand that these are not invasive breast cancers, but rather abnormal changes in the cells. However, both are associated with an increased risk of developing invasive breast cancer.
The majority of carcinomas of the breast occur in the upper outer quadrant, where most of the glandular tissue of the breast is located. The lymphatic spread of cancer to the opposite breast, to lymph nodes in the base of the neck and to the abdominal cavity is caused by obstruction of the normal lymphatic pathways or destruction of lymphatic vessels by surgery or radiotherapy. The less common inner quadrant tumours may spread to mediastinal nodes, which are located between the pectoral muscles.
Internal mammary chain nodes are also common sites of metastasis. Metastases from the vertebral veins can involve the vertebrae, pelvic bones, ribs and skull. The lungs, kidneys, liver, adrenal glands, ovaries and pituitary gland are also sites of metastasis.
CLINICAL MANIFESTATIONS
The first sign of breast cancer is usually a painless lump. Lumps caused by breast tumours do not have any classic characteristics. Other presenting signs include palpable nodes in the axilla (underarm), retraction of tissue (dimpling) or bone pain caused by metastasis to the vertebrae. Table 32-2 summarises the clinical manifestations of breast cancer. Manifestations vary according to the type of tumour and stage of disease.
Table 32-2 CLINICAL MANIFESTATIONS OF BREAST CANCER
| CLINICAL MANIFESTATION | PATHOPHYSIOLOGY |
|---|---|
| Local pain | Local obstruction caused by the tumour |
| Dimpling of the skin | Can occur with invasion of the dermal lymphatics because of retraction of Cooper’s ligament or involvement of the pectoralis fascia |
| Nipple retraction | Shortening of the mammary ducts |
| Skin retraction | Involvement of the suspensory ligament |
| Oedema | Local inflammation or lymphatic obstruction |
| Nipple/areolar eczema | Paget’s disease |
| Pitting of the skin (similar to the surface of an orange) | Obstruction of the subcutaneous lymphatics, resulting in the accumulation of fluid |
| Reddened skin, local tenderness and warmth | Inflammation |
| Dilated blood vessels | Obstruction of venous return by a fast-growing tumour; obstruction dilates superficial veins |
| Nipple discharge in a non-lactating woman | Spontaneous and intermittent discharge caused by tumour obstruction |
| Ulceration | Tumour necrosis |
| Haemorrhage | Erosion of blood vessels |
| Oedema of the arm | Obstruction of lymphatic drainage in the axilla |
| Chest pain | Metastasis to the lung |
Source: Based on Griffiths MJ, Murray KH, Russo PC. Oncology nursing: pathophysiology, assessment, and intervention. New York: Macmillan; 1984.
EVALUATION AND TREATMENT
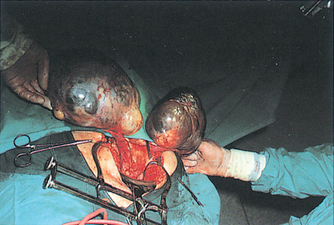
Mammography, ultrasound, percutaneous needle aspiration, biopsy or minimally invasive biopsy called Mammotome, palpation and hormone receptor assays are generally used in evaluating breast alterations and cancer (see Figure 32-1). Biopsy is the definitive diagnostic test (see Figure 32-2).
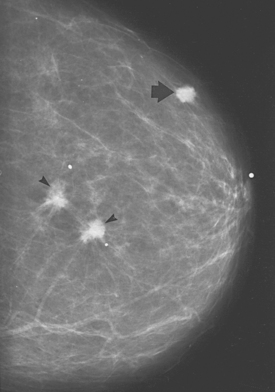
FIGURE 32-1 Invasive breast carcinoma.
Two irregular carcinomas (arrowheads) are present in one quadrant, representing ‘multifocal’ carcinoma. The presence of a third lesion (arrow) in another quadrant leads to the designation ‘multicentric’.
Source: Bassett LW. Diagnosis of diseases of the breast, 2nd edn. Philadelphia: Saunders; 2005.
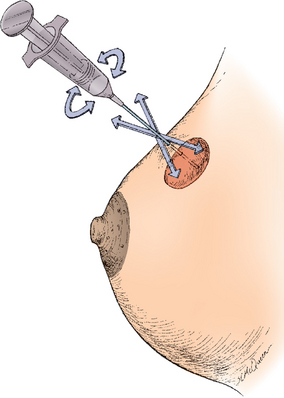
FIGURE 32-2 Needle biopsy and aspiration with negative pressure.
The needle is rotated, moved back and forth, and slightly in and out to aspirate representative specimen.
Source: Katz VL et al. Comprehensive gynecology. 5th edn. St Louis: Mosby; 2007.
Treatment is based on the extent or stage of the cancer. The extent of the tumour at the primary site, the presence and extent of lymph node metastasis and the presence of distant metastases are all evaluated to determine the stage of disease. For localised breast cancer, the most extensive surgical option would be removing the breast and lymph nodes under the arm. Removing the lump and just a section of the breast followed by radiotherapy results in the same rate of survival. If the first draining lymph node can be identified using dye or a nuclear medicine scan, it can be sampled and, if negative, further surgery avoided.
For tumours at greater risk of recurrence, such as bigger more aggressive-looking tumours that have spread to the lymph nodes, additional treatment (adjuvant therapy) can be given after surgery. This may include hormone therapy of aromatase inhibitors or tamoxifen for women whose tumours have hormone receptors on their surfaces, chemotherapy and targeted therapies such as trastuzumab for those 25% of tumours that are HER2-positive (that is, have the target for trastuzumab on their surfaces). Patients presenting with locally extensive cancer will have chemotherapy and radiotherapy initially to see whether they can shrink the cancer to become operable. If breast cancer returns after initial treatment, local disease may be treated with surgery, while more widespread disease will be treated with combinations of similar drugs to those listed for the adjuvant setting, as is the case for patients who present with widespread disease. Common chemotherapy drugs include anthracyclines and taxanes. Patients with bone disease can receive bisphosphonates such as zoledronate to slow the erosion of bone as well as local radiotherapy for pain.
Cervical cancer
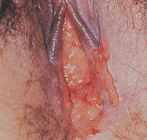
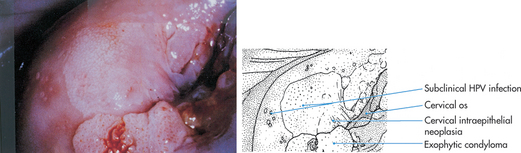
Cervical intraepithelial neoplasia (CIN), also known as cervical dysplasia, is the potentially premalignant transformation and abnormal growth (dysplasia) of squamous cells on the surface of the cervix.40 It is more common than invasive cancer and occurs more often in younger women, with 1 in 8 young women having cervical dysplasia by the age of 20 years. The premalignant features of CIN, such as genetic abnormalities, loss of cellular function and some phenotypic characteristics of cancer, predict the risk of developing an invasive cancer. Human papillomavirus (HPV) is a necessary precursor to the development of the vast majority of cases of cervical cancer (see the box ‘Health alert: vaccine offers promise of cervical cancer prevention’).41,42
Some risk factors have been found to be important in developing CIN — for example, having multiple sexual partners, being infected with higher risk types of HPV, smoking and being immunodeficient. In addition, engaging in sexual intercourse before the age of 16 and having intercourse with a male partner who has had multiple partners place a woman at risk.
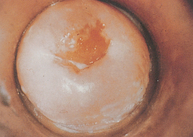
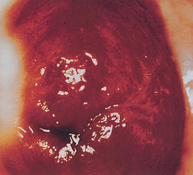
Cervical cancer is a progressive disease, moving from normal cervical epithelial cells to dysplasia to CIN to invasive cancer. Figure 32-3 summarises the progressive degrees of CIN. Premalignant lesions usually occur 10–12 years before the development of invasive carcinoma. Cervical neoplasms are often asymptomatic, so regular Pap smears are necessary to monitor development. About 90% of cervical cancers can be detected early through the use of Pap smears and HPV testing. The incidence of cervical cancer is in fact already declining in Australia and New Zealand, which is likely to be due to regular pap smear tests (see Chapter 36).
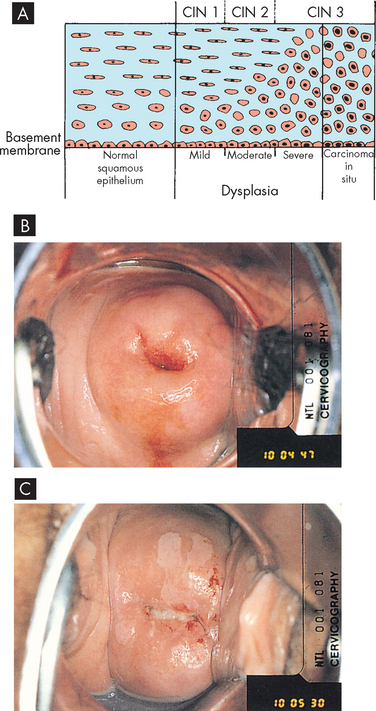
FIGURE 32-3 Cervical intraepithelial neoplasia.
A A diagram of cervical endothelium showing progressive degrees of CIN. B Normal multiparous cervix. C CIN stage 1. Note the white appearance of part of the anterior lip of the cervix associated with neoplastic changes.
Source: A Herbst AL et al. Comprehensive gynecology. 2nd edn. St Louis: Mosby; 1992. B & C Symonds EM, Macpherson MBA. Color atlas of obstetrics and gynecology. London: Mosby; 1994.
Vaccine offers promise of cervical cancer prevention
In 2006 the TGA approved a vaccine (Gardisil) developed in Australia and produced by Merck against human papillomavirus (HPV) types 6, 11, 16, and 18. HPV types 16 and 18 are responsible for 70% of all cervical cancers, and HPV types 6 and 11 are associated with benign genital warts. The vaccine is given in a series of 3 shots to girls and young women aged 9–26. In clinical trials, the vaccine was 90% effective in preventing these types of HPV. It does not, however, replace the need for screening with a Pap test.
Source: Joura EA et al. Efficacy of a quadrivalent prophylactic human papillomavirus (types 6, 11, 16 and 18) L1 virus-like-particle vaccine against high-grade vulval and vaginal lesions: a combined analysis of three randomised clinical trials. Lancet 2007; 369(9574):1693–1927; The FUTURE II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med 2007; 356(19):1915–1927; Villa LL et al. Prophylactic quadrivalent human papillomavirus (types 6, 11, 16 and 18) L1 virus-like particle vaccine in young women: a randomised double-blind placebo-controlled multicentre phase II efficacy trial. Lancet 2005; 6(5):271–278.
There are often no symptoms but, if present, they may include a change in vaginal discharge or bleeding either after intercourse or between menstrual periods. At times, women will complain of abnormal menses or postmenopausal bleeding. A less common symptom may be a yellowish vaginal discharge. A new or foul odour also may be present. Pelvic or epigastric pain is experienced only with large lesions. Severe bleeding may cause anaemia. Advanced disease may cause urinary or rectal symptoms and pelvic or back pain. When dysplasia is detected, cervical biopsy and endocervical curettage are required. Colposcopy (visualisation of the cervix using a camera and light source) is used to suggest sites for biopsy. If invasive carcinoma is found, lymphangiography (X-ray of lymph nodes and lymph vessels), CT scanning, ultrasonography or radio-immunodetection methods (use of radioactive antibodies to detect neoplasms) are used to assess lymphatic involvement.
The treatment depends on the degree of neoplastic change, the size and location of the lesion and the extent of metastatic spread. With early detection and treatment, prognosis for invasive cervical cancer is excellent. Overall, the 5-year survival rate is 70% and it increases to 92% with early diagnosis.
Ovarian cancer
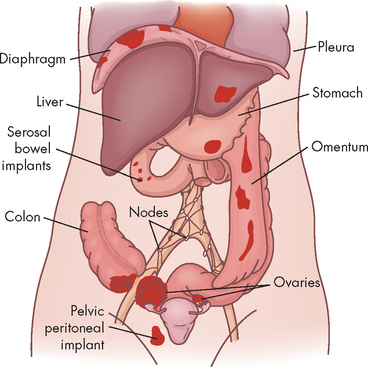
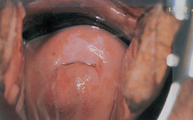
The cause of ovarian cancer is unknown, but multiple studies agree that an increased risk of epithelial ovarian cancer has been linked to advancing age, a family history of breast or ovarian cancer and frequency of ovulation.43 The dismal overall prognosis for women with ovarian cancer results from an inability to detect ovarian cancer early when treatment might result in cure and from our incomplete understanding of the early changes in the ovary before the development of cancer and the initiators of these changes.43 More than 90% of ovarian cancers arise from the ovarian surface epithelium,44 although cancers can also arise from germ cells of the ovarian stroma (connective tissue). Germ cell tumours occur in younger women, whereas those from epithelial tissue occur in women over 40 (see Figure 32-4).
Ovarian cancers exhibit a distinctive pattern of progression, spreading intra-abdominally over the surface of the peritoneum. It is often considered a silent disease, meaning that by the time the individual experiences symptoms and seeks treatment, the disease has spread beyond the primary site (see Figure 32-5). The most obvious symptoms are pain and abdominal swelling (ascites) that arise from the primary ovarian tumour mass. Gastrointestinal complaints resulting from mechanical obstruction by the tumour may include dyspepsia (abdominal discomfort, such as indigestion), vomiting and alterations in bowel habits. Abnormal vaginal bleeding may occur if the postmenopausal endometrium is stimulated by a hormone-secreting tumour. The tumour may also cause ulcerations through the vaginal wall that result in bleeding. There also can be a feeling of pressure in the pelvis and leg pain.45
Because ovarian cancer has no early symptoms and there are no effective screening techniques to detect it, the disease is usually advanced by the time treatment is sought. Transvaginal ultrasound and a tumour marker (CA-125) may assist diagnosis but are not recommended for routine screening. The initial approach to treatment is surgery, performed to determine the stage of disease and to remove as much of the tumour as possible. Radiation therapy may follow if the tumour is smaller than 2 cm in size and is confined to the abdominopelvic area without involvement of the kidneys or liver. The success of chemotherapy depends on the extent of disease, whether the tumour is a discrete mass and whether there has been prior exposure to chemotherapeutic agents.
Cancers of the male reproductive system
Prostate cancer
Prostate cancer is the most common cancer in men, with 85% of cases being diagnosed in those over the age of 65 years. Just over 16,000 cases of prostate cancer (nearly 30% of male cancers) are diagnosed each year in Australia.46 The risk of prostate cancer rises with age, increasing rapidly after age 50. Family history increases the chances of developing the disease. There has been some association with a diet high in fats and low in fresh fruit and vegetables. Men of African descent are at higher risk than men of European descent and there is an association with high testosterone levels. Other possible causes are those of genetic predisposition (familial and hereditary forms). As with breast cancer, there are a number of risk factors, with tobacco use having a significant impact on the occurrence of fatal prostate cancer.47
Risk factors
Diet
The worldwide distribution of prostate cancer suggests that diet may play a role in its development, especially if the diet affects hormone levels. Consistency across studies indicates that a high intake of fat (total and especially saturated fat) is a risk factor for prostate cancer.48–50 4 6 Several hypotheses exist concerning the enhancing effect of fat on prostate carcinogenesis, including hormonal and the generation of free radicals (see Chapter 4). Fat intake from dairy products increases calcium, itself a proposed risk factor. Calcium can suppress circulating levels of vitamin D, a possible protective factor for prostate cancer.51 In addition, a low intake of dietary fibre and complex carbohydrates and a high intake of protein are associated with an increased risk of prostate cancer.50 It remains controversial whether obesity or an increased body mass index is a risk factor for prostate cancer.
Nutritional and chemopreventive agents for risk reduction of prostate cancer
Several areas of investigation are identifying prospective therapies for prostate cancer prevention. Several studies found antioxidants to be potential chemopreventive agents. Individuals with high antioxidant levels (such as vitamin E and selenium) have a 42% reduction in prostate cancer risk. The effect was higher (59% reduction) in those with high-grade or high-stage cancer risk. Many chemopreventive agents that have been identified in laboratory studies — including silibinin (from milk thistle), inositol hexaphosphate (a form of carbohydrate in cereals, legumes, oil seeds and nuts), apigenin and acacetin (fruits and vegetables), grape seed extract, curcumin and green tea — could be useful against prostate cancer.
Source: Kantoff P. Prevention, complementary therapies, and new scientific developments in the field of prostate cancer. Rev Urol 2006; 8(suppl 1):S9–S14; Singh RP, Agarwal R. Mechanisms of action of novel agents for prostate cancer chemoprevention. Endocr Relat Cancer 2006; 13(3):751–778, review.
Individual nutrients or foods and their associations with prostate cancer risk are not strong, yet migration of individuals from low-risk geographic areas of the world, such as Japan, to high-risk countries, such as Australia and New Zealand, increases risk considerably. These changes in risk probably reflect differences in lifestyle and dietary habits. Geographically, individuals who reside in regions with less sunlight have a higher risk of prostate cancer. The highest rates of mortality from prostate cancer are in Scandinavian countries, where exposure to ultraviolet light is low; the possible link is less vitamin D induced by less sun exposure.
Hormones
Prostate cancer develops in an androgen-dependent epithelium and is usually androgen sensitive. In addition, a few case reports of prostate cancer in men who used androgenic steroids as anabolic agents or for medical purposes suggest a causal relationship.49,52–54 4 6 Population studies have not, however, provided clear and convincing patterns involving associations between circulating hormone concentrations and prostate cancer risk.48,55
Investigations directed at understanding the hormonal basis of prostate (as well as breast) carcinogenesis have numerous problems. The complexities of interacting hormones and separating out the effects of a single hormone are profound. In addition, only single blood samples are generally available, tissue hormone samples are not consistently measured and within-subject variations over time and differences in circadian rhythms cannot be adequately measured. Therefore, while the role of hormones in the implication of prostate cancer development may be relevant, the evidence is yet to find a direct link.
Chronic inflammation
The results of a 5-year, longitudinal study of the influence of chronic inflammation and prostate cancer have been reported.56 Biopsies revealed prostatic hyperplasia (see ‘Disorders of the male reproductive system’ below) and inflammatory atrophy in those with chronic inflammation. Upon repeat biopsy, new cancers were diagnosed.56 In contrast, of the men initially showing no inflammation, only a small number were found to have adenocarcinoma. Thus, chronic inflammation may be an important risk factor for prostatic adenocarcinoma. An inflammatory process could possibly account for the evidence that antioxidants and non-steroidal anti-inflammatory drugs (NSAIDs), such as aspirin, may be protective.51,56,57
Familial factors
Other possible causes are those of genetic predisposition (familial and hereditary forms). Genetic studies suggest that strong familial predisposition may be responsible for 5–10% of prostate cancers.58 Hereditary cancer differs from the familial form, which occurs in individuals with a positive family history but who do not exhibit early age of onset.59 The hereditary form constitutes about 9% of all prostate cancers and approximately 43% of cancers in men younger than 55 years of age.60 There is no clear evidence of a causal link between benign prostatic hyperplasia and prostate cancer, even though they may often occur together.
PATHOGENESIS
More than 95% of prostatic neoplasms are adenocarcinomas,61 and most occur in the periphery of the prostate. The biological aggressiveness of the neoplasm appears to be related to the degree of differentiation rather than the size of the tumour (see Box 32-1).
Box 32-1 PROSTATIC CANCER GRADES
Grade 1. The cancer cells closely resemble normal cells. They are small, uniform in shape, evenly spaced and well differentiated (that is, they remain separate from one another).
Grade 2. The cancer cells are still well differentiated, but they are arranged more loosely and are irregular in shape and size. Some of the cancer cells have invaded the neighbouring prostate tissue.
Grade 3. This is the most common grade. The cells are less well differentiated (some have fused into clumps) and are more variable in shape.
Grade 4. The cells are poorly differentiated and highly irregular in shape. Invasion of the neighbouring prostate tissue has progressed further.
Grade 5. The cells are undifferentiated. They have merged into large masses that no longer resemble normal prostate cells. Invasion of the surrounding tissue is extensive.
Although steroid hormonal factors are strongly implicated in prostate carcinogenesis, little is known about their involvement. Just as the testicles are the male equivalent of the female ovaries, the prostate is the male equivalent of the female uterus.
Testosterone is the major circulating androgen, whereas dihydrotestosterone (a biological active metabolite of testosterone, important in the development of male sexual characteristics) predominates in prostate tissue and binds to the androgen receptor with greater affinity than does testosterone.62 Testosterone is the major androgen that comes from the interstitial cells of the testis (Leydig cells). The adrenal cortex produces far less potent androgen than the testis.
There are significant age-dependent alterations in the hormones, although these vary in different prostate tissues. In epithelium, the level of dihydrotestosterone decreases with age; whereas in stroma (connective tissue), the dihydrotestosterone level is rather constant. Interestingly, the oestrogen content (males do produce small amounts of oestrogen) in the stroma increases with age. Therefore, the ratio of oestrogen to androgens alters substantially with age. In animal studies, chronic exposure to testosterone plus oestradiol is strongly carcinogenic.48 The mechanism is not clearly understood and may involve oestrogen-generated oxidative stress and DNA toxicity.48
From all of these observations, the following multifactorial general hypothesis of prostate carcinogenesis emerges. Androgens act as strong tumour promoters to enhance the weak but continuously present carcinogen to affect genes and possibly unknown environmental carcinogens. All of these factors are modulated by diet and genetic determinants, including multifactorial inheritance which is influenced by multiple genes (see Chapter 37). These genes encode receptors and enzymes involved in the metabolism and action of steroid hormones.48
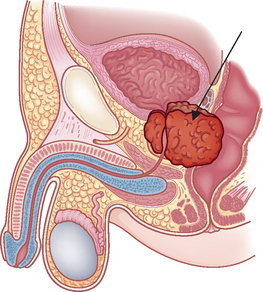
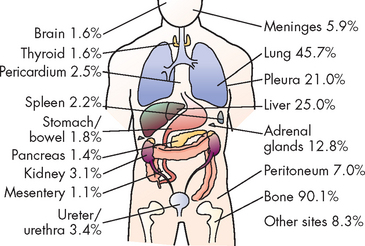
The microenvironment (stroma) surrounding the prostatic tumour actively fuels the progression of prostate cancer from localised growth, to invasion, to development of distant metastases.63 The most common sites of distant metastasis are the lymph nodes, bones, lungs, liver and adrenals. The pelvis, lumbar spine, femur, thoracic spine and ribs are the most common sites of bone metastasis. Local extension is usually posterior, although late in the disease the tumour may invade the rectum or encroach on the prostatic urethra and cause bladder outlet obstruction (see Figure 32-6). The spread of cancer through blood vessels is illustrated in Figure 32-7.
CLINICAL MANIFESTATIONS
Prostatic cancer often causes no symptoms until it is far advanced. Therefore, routine screening is recommended for asymptomatic men beginning at age 50 — or 45 if they are considered at high risk. The first manifestations of disease are those of bladder outlet obstruction. Since the urethra passes through the prostate (see Chapter 31) any enlargement will have an effect on the flow of urine from the bladder. Symptoms therefore include frequent low-volume urination (particularly at night), pain on urination, blood in the urine and a weak stream. Local extension of prostatic cancer can obstruct the upper urinary tract ureters as well. Rectal obstruction also may occur, causing the individual to experience large bowel obstruction or difficulty in defecation. Symptoms of late disease include bone pain at the sites of bone metastasis, oedema of the lower extremities, enlargement of the lymph nodes, liver enlargement, pathological bone fractures and mental confusion associated with brain metastases. Prostatic cancer and its treatment can affect sexual functioning.
EVALUATION AND TREATMENT
Diagnosis is made using a digital rectal examination to feel the prostate and a blood test for prostate-specific antigen (PSA) (see Box 32-2). More than 95% of prostatic neoplasms are adenocarcinomas31 and most occur in the periphery of the prostate. Bone scans and CT scans are used to determine spread.
In Australia, it is clear that the profile of prostate cancer has risen significantly due to media coverage and that many more men over the age of 40 are seeking PSA and digital rectal exam (DRE) testing. There is growing pressure on state health authorities to initiate widespread screening, but this is being resisted for several reasons. For example, multiple stages of testing (PSA/DRE and biopsy) are required before a definitive diagnosis of prostate cancer can be given. In addition, PSA levels can be elevated for up to 10 years before any prostate cancer has developed, and invasive procedures (biopsy) can lead to urological problems and infections giving otherwise healthy men immediate problems. There is no clear evidence from population-based studies that a positive (raised) PSA level indicates that prostate cancer will develop. Thus, there can be no real justification for widespread PSA screening of all men over 40 years. The issue will be resolved only with more research into prostate cancer and more definitive and less invasive subsequent testing.
Source: Holden CA et al. Men in Australia Telephone Survey (MATeS): predictors of men’s help-seeking behaviour for reproductive health disorders. MJA 2006; 185:418–422; Carrière P et al. Cancer screening in Queensland men. MJA 2007; 186(8): 404–407; Australian Institute of Health and Welfare. BreastScreen Australia monitoring report, 2004–2005. Cancer series no. 42. Catalogue no. CAN 37. Canberra: AIHW; 2008; Smith D et al. Prostate cancer and prostate-specific antigen testing in New South Wales. MJA 2008; 189(6):315–318; www.andrologyaustralia.org/docs/AndrologyAustralia_PSAposition_webversion_140509.pdf; www.betterhealth.vic.gov.au/bhcv2/bhcarticles.nsf/pages/Prostate_cancer_and_the_PSA_test.
Treatment of prostate cancer depends on the stage of the disease (see Box 32-1), the anticipated effects of treatment and the age, general health and life expectancy of the individual. Options range from hormonal or radiation therapy or chemotherapy to surgery (contemporary nerve sparing or robotic surgery), any combination of these or no treatment. Low-grade disease confined to the prostate can be watched (surveillance) if not causing symptoms. It is desirable to avoid the side effects of surgery; however, early surgery may be of benefit. Surgery with curative intent removes the whole prostate (radical prostatectomy). The main side effects are impotence and incontinence. Radical radiotherapy can also be given with curative intent, either with external radiation or by implanting radioactive seeds (brachytherapy). Side effects are similar to surgery, and bowel problems may also occur.
For widespread disease, hormone therapy reduces the stimulus of the male hormones. Removal of the testis or injecting luteinising hormone-releasing hormone (LHRH) or anti-androgen hormones can hold the disease for 3–4 years and may improve outcomes if undertaken early with radiation in high-risk patients. When hormone resistance occurs, chemotherapy with docetaxel can be used or mitoxantrone can control symptoms. Bisphosphonates (e.g. zoldedronate) can be use to help control bone metastases. Nearly all patients who present with localised disease will live beyond 5 years, with the 10- and 15-year survival rates being 93% and 77%, respectively.64
Treatment for prostate cancer may lead to loss of urinary control, which can return to normal after several weeks or months. Mild stress incontinence can occur after surgery and mild urge incontinence after radiation therapy. Prostate cancer and its treatment can affect sexual functioning. Most men will need assistance (medication) with obtaining an erection for 3–12 months after surgery. Sensation of orgasm is not usually affected, but smaller amounts of ejaculate will be produced or men may experience a ‘dry’ ejaculate because of retrograde ejaculation.
Palliative treatment is aimed at relieving urinary, bladder outlet or colon obstruction, spinal cord compression and pain.
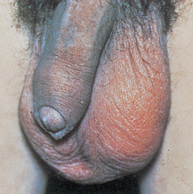
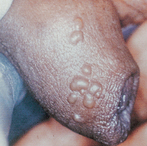
Testicular cancer
Testicular cancer is among the most curable of cancers, with cure rates greater than 95%. Overall, testicular cancer is uncommon, accounting for approximately 1.2% of all male cancers. However, it is the most common solid tumour of young adult men.65 Cancer of the testis occurs most commonly in men between the ages of 15 and 35 years.65 Testicular tumours are slightly more common on the right side than on the left, a pattern that parallels the occurrence of cryptorchidism (lack of one testis or both testes from the scrotum) and they are bilateral in 1–3% of cases. The profile of testicular cancer in the general public is high due mainly to one man: Lance Armstrong. An American professional road cyclist, Armstrong was diagnosed with testicular cancer at the age of 25. He underwent surgery and chemotherapy for a metastasised tumour, and has gone on to win the Tour de France, the most popular cycle race in the world, seven times, as well as to set up a cancer foundation, which has raised the profile of all cancers worldwide.
PATHOPHYSIOLOGY
Some 90% of testicular cancers are germ cell tumours, arising from the male gametes. Germ cell tumours include seminomas, embryonal carcinomas, teratomas and choriosarcomas. Testicular tumours can also arise from specialised cells of the gonadal stroma. These tumours, which are named for their cellular origins, are the Leydig cell, sustentacular (Sertoli) cell, granulosa cell and theca cell tumours.
The cause of testicular neoplasms is unknown. A genetic predisposition is suggested by the fact that the incidence is higher among brothers, identical twins and other close male relatives. Genetic predisposition is supported by statistics showing that the disease is relatively rare among Africans, Asians and indigenous New Zealanders. A history of trauma or infection is also associated with the development of testicular neoplasms, but it may be that coexisting testicular tumours are discovered by accident in men who undergo examination because of trauma or infections.
CLINICAL MANIFESTATIONS
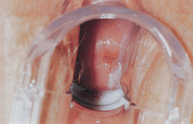
Painless testicular enlargement is commonly the first sign of testicular cancer (Figure 32-8). Occurring gradually, it may be accompanied by a sensation of testicular heaviness or a dull ache in the lower abdomen. Occasionally acute pain occurs because of rapid growth resulting in haemorrhage and necrosis. Of those affected, 10% have epididymitis (see ‘Disorders of the scrotom, testis and epididymis’ below), 10% have hydroceles (fluid collection in the scrotal sac) and 5% have breast enlargement (gynaecomastia). The testicular mass is usually discovered by the individual or by his sexual partner. At the time of initial diagnosis, approximately 10% of individuals already have symptoms related to metastases. Lumbar pain may also be present and is usually caused by retroperitoneal node metastasis. Signs of metastasis to the lungs include cough, dyspnoea (difficulty breathing) and haemoptysis (bloody sputum). Supraclavicular node involvement may cause difficulty swallowing (dysphagia) and neck swelling. With metastasis to the central nervous system, alterations in vision or mental status and seizures may be experienced.
EVALUATION AND TREATMENT
Evaluation begins with careful physical examination, including palpation of the scrotal contents with the individual in the erect (standing) and supine positions. Signs of testicular cancer include abnormal consistency, nodularity or irregularity of the testis. The abdomen and lymph nodes are palpated to seek evidence of metastasis and tumour type is identified after orchiectomy. Testicular biopsy is not recommended because it may cause dissemination of the tumour and increase the risk of local recurrence. Primary testicular cancer can be assessed rapidly and accurately by scrotal ultrasonography. Tumour markers, alpha (α)-fetoprotein and beta (β)-subunit gonadotropin, and lactate dehydrogenase are usually elevated. Chest X-ray films, lymphangiograms, intravenous pyelograms, abdominal ultrasound or CT scan and measurement of serum markers are used in clinical staging of the disease.
Besides surgery, treatment involves radiation and chemotherapy singly or in combination. Factors influencing the prognosis include histology of the tumour stage of the disease and selection of appropriate treatment. Most patients treated for cancer of the testis can expect a normal life span; some have persistent paraesthesia (tingling or numbness over the skin) or infertility. Almost 90% of disease-related deaths occur in the first 2 years after cessation of therapy. A person who is disease-free after 3 years is considered to be cured. Orchiectomy does not affect sexual function.
DISORDERS OF THE FEMALE REPRODUCTIVE SYSTEM
Benign growths and proliferative conditions
Benign ovarian cysts
Benign (non-cancerous) cysts of the ovary may occur at any time of life but are most common during the reproductive years and, in particular, at the extremes of those years. An increase in benign ovarian cysts occurs when hormonal imbalances are more common, around puberty and menopause. Two common causes of benign ovarian enlargement in ovulating women are follicular cysts and corpus luteum cysts. These are called functional cysts because they are caused by variations of normal physiological events. Follicular and corpus luteum cysts are usually unilateral and are produced when a follicle or a number of follicles are stimulated but no dominant follicle develops and completes the maturity process to ovulation. Benign cysts of the ovary are typically 5–6 cm in diameter but can grow as large as 8–10 cm.
Follicular cysts can be caused by a transient condition in which the dominant follicle fails to rupture or one or more of the non-dominant follicles fail to regress. This disturbance is not well understood. It may be that the hypothalamus does not receive or send a message strong enough to increase follicle-stimulating hormone (FSH) levels to the degree necessary to develop or mature a dominant follicle. Clinical symptoms of follicular cysts or even a single cyst are pelvic pain, a sensation of feeling bloated or irregular menses. After several subsequent cycles in which hormone levels once again follow a regular cycle and progesterone levels are restored, cysts are usually absorbed or will regress. Follicular cysts can be random or recurrent events.
Corpus luteum cysts can develop due to an intracystic haemorrhage that occurs in the vascularisation stage; the affected cyst then consists of blood. In normal cycles, the vascularisation is replaced by a clear fluid that accumulates in the cavity of the corpus luteum. Corpus luteum cysts are less common than follicular cysts, but luteal cysts typically cause more symptoms, particularly if they rupture. Manifestations include dull pelvic pain and amenorrhoea (absence of menstruation) or delayed menstruation, followed by irregular or heavier-than-normal bleeding. Rupture can cause massive bleeding with excruciating pain and can require immediate surgery. Corpus luteum cysts usually regress spontaneously in non-pregnant women. The combined contraceptive pill may be used to prevent cysts from forming as the contraceptive prevents the development of the follicles.
Endometrial polyps
An endometrial polyp is a mass of endometrial tissue containing a variable amount of glands, stroma and blood vessels. Endometrial polyps are usually found singly at the fundus but about 20% are multiple or originate from lower in the uterine body or upper endocervix and contain mixed epithelium. Most polyps are asymptomatic. However, some endometrial polyps cause intermenstrual bleeding or even excessive menstrual bleeding. Although polyps most often develop in women between the ages of 40 and 60 years, they can occur at all ages.66
Leiomyomas
Leiomyomas (or uterine fibroids) are benign tumours that develop from smooth muscle cells in the myometrium. They are the most common benign tumours of the uterus and usually occur in multiples in the fundus; the majority remain small and asymptomatic. Prevalence increases in women aged 30–50 years but decreases with menopause.
PATHOPHYSIOLOGY
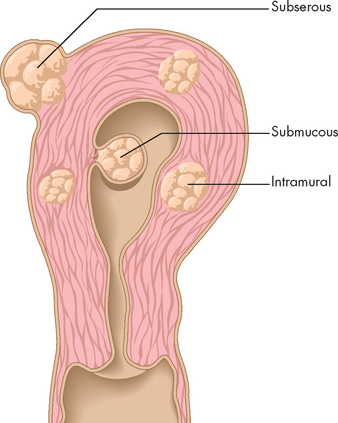
The cause of uterine leiomyomas is unknown, although their size appears to be related to hormonal fluctuations (particularly oestrogen). Uterine leiomyomas are not seen before menarche and those that develop during the reproductive years generally shrink after menopause. Leiomyomas are classified as subserous, submucous or intramural, according to their location within the various layers of the uterine wall (see Figure 32-9). Degenerative changes, such as ulceration and necrosis, may occur when the leiomyoma outgrows its blood supply and are therefore more common in larger tumours.
CLINICAL MANIFESTATIONS
Although leiomyomas rarely present problems, they occasionally cause cramping, excessive bleeding and symptoms related to pressure on nearby structures. Because the tumour is relatively slow growing, enabling adjacent structures to adapt to pressure, symptoms of abdominal pressure develop slowly. Pressure on the bladder may contribute to urinary frequency, urgency and dysuria. If the tumour is large enough, pressure on the lower colon may lead to constipation and abdominal heaviness.
EVALUATION AND TREATMENT
Uterine leiomyomas are suspected when manual examination finds irregular, non-tender nodes on the uterus. Scanning, using MRI, confirms the diagnosis. Treatment depends on symptoms, tumour size and the individual’s age, reproductive status and overall health. Most leiomyomas can be treated conservatively by shrinking the tumour using pharmacological agents, such as the gonadotropin-releasing hormone agonist, progesterone, or oral contraceptives. Mifepristone, an antiprogesterone, may also be useful as a conservative treatment.
Adenomyosis
In this condition endometrial tissue (the inner lining of the uterus) is present within the myometrium (the thick, muscular layer of the uterus). Some endometrial tissue is therefore ectopic (out of place), possibly as a result of uterine trauma. Adenomyosis commonly develops during the late reproductive years, with the highest incidence among women in their 40s and women taking tamoxifen (used to treat hormone-positive breast cancer). It may be asymptomatic or it may be associated with abnormal menstrual bleeding, dysmenorrhoea, uterine enlargement and uterine tenderness during menstruation. Secondary dysmenorrhoea becomes increasingly severe as the disease progresses. On examination, the uterus is enlarged, globular and most tender just before or after menstruation. Diagnosis is confirmed with ultrasonography or MRI. Treatment, when necessary, includes surgical resection of localised areas of adenomyosis or, if severe, hysterectomy. Adenomyosis is typically unresponsive to hormone treatment.
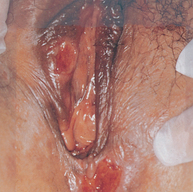
Endometriosis
Endometriosis is a condition in which endometrial-like cells appear and flourish in areas outside the uterine cavity (see Figure 32-10). Like normal endometrial tissue, the ectopic endometrial implants respond to the hormonal fluctuations of the menstrual cycle. Thus they behave like normal endometrium and proliferate, break down and bleed in conjunction with the normal menstrual cycle. The bleeding causes inflammation and pain in surrounding tissues. The inflammation may lead to fibrosis, scarring and adhesions. Endometriosis affects 10–15% of reproductive-age women and 50% of infertile women.67
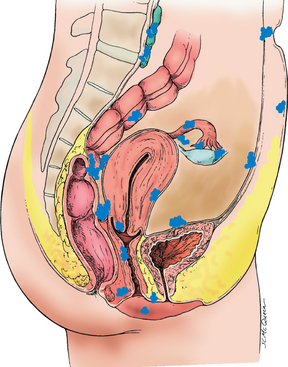
FIGURE 32-10 Common pelvic sites of endometriosis.
Source: Katz VL et al. Comprehensive gynecology, 5th edn. St Louis: Mosby; 2007.
The clinical manifestations of endometriosis vary in frequency and severity and include primarily infertility and pain, dysmenorrhoea, dyschezia (pain on defecation), dyspareunia (pain on intercourse) and, less commonly, constipation and abnormal vaginal bleeding. Dyschezia, a hallmark symptom of endometriosis, occurs with bleeding of ectopic endometrium in the rectosigmoid musculature and subsequent fibrosis. Medical therapies for endometriosis include suppression of ovulation with various medications.
Up to one-third of individuals with endometriosis are infertile, presumably because of a combination of the following:
Polycystic ovary syndrome
Polycystic ovary syndrome is a condition in which excessive androgen production is triggered by inappropriate secretion of gonadotropins (see Chapter 31 for normal cycling of gonadotropins). It is the most common endocrine disturbance affecting women. This hormonal imbalance prevents ovulation and causes enlargement and cyst formation in the ovaries (Figure 32-11), excessive endometrial proliferation and often hirsutism (excessive female hair growth in areas where hair growth is minimal or absent). It is defined as the presence of any two of the following:
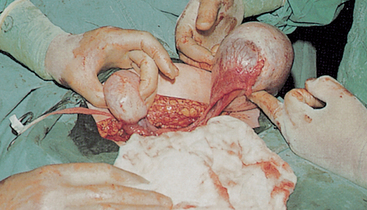
FIGURE 32-11 Polycystic ovary. Both ovaries shown are enlarged with multiple cysts.
Source: Symonds EM, Macpherson MBA. Color atlas of obstetrics and gynecology. London: Mosby; 1994.
Polycystic ovary syndrome affects an estimated 400,000 Australian women, approximately 5–10% of the reproductive age group.68 Eighty per cent of women with normal ovaries experience one or more symptoms of polycystic ovary syndrome and the signs and symptoms of women with polycystic ovary syndrome may change over time.
PATHOPHYSIOLOGY
Although common, polycystic ovary syndrome remains poorly understood with no clear aetiology. While it has been classified as an ovarian disease, it is associated with other long-term health issues, including hypertension, dyslipidaemia and hyperinsulinaemia.69 Hyperinsulinaemia plays a key role in androgen excess, anovulation and pathogenesis of polycystic ovary syndrome.70 Insulin stimulates androgen secretion by the ovarian stroma and reduces serum sex hormone-binding globulin directly and independently. The net effect is an increase in free testosterone levels. Excessive androgens affect follicular growth and insulin affects follicular decline by suppressing apoptosis and enabling follicles, which would normally disintegrate, to survive (see Figure 32-12). Furthermore, there seems to be a genetic ovarian defect in polycystic ovary syndrome that makes the ovary either more susceptible to or at least sensitive to insulin’s stimulation of androgen production in the ovary.71
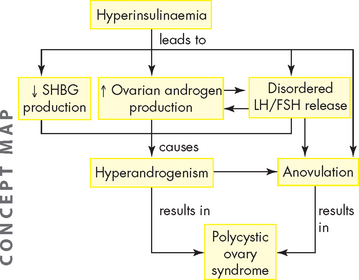
FIGURE 32-12 Insulin resistance and hyperinsulinaemia in polycystic ovary syndrome.
See text for explanation. SHBG = sex hormone-binding globulin; LH = luteinising hormone; FSH = follicle-stimulating hormone.
Inappropriate gonadotropin secretion triggers the beginning of a vicious cycle that perpetuates anovulation. Typically, levels of FSH are low or below normal and the LH level is elevated. Persistent LH elevation causes an increase in androgens. Androgens are converted to oestrogen in peripheral tissues and increased testosterone levels cause a significant reduction (approximately 50%) in sex hormone-binding globulin, which, in turn, causes increased levels of free oestradiol. Elevated oestrogen levels trigger a positive-feedback response in LH and a negative-feedback response in FSH. Because FSH levels are not totally depressed, new follicular growth is continuously stimulated, but not to full maturation and ovulation (see Figure 32-12).71
CLINICAL MANIFESTATIONS
Clinical manifestations of polycystic ovary syndrome are related to anovulation and elevated testosterone levels and include dysfunctional bleeding or amenorrhoea, hirsutism and infertility (see Box 32-3). Approximately 38% of women with polycystic ovary syndrome are obese and 20% are asymptomatic. In addition, 30% of women with polycystic ovary syndrome will develop diabetes by the age of 30 years. Other pathological conditions resulting from polycystic ovary syndrome include type 2 diabetes mellitus, cardiovascular disease and endometrial carcinoma. Pregnant women with polycystic ovary syndrome may be at increased risk for glucose intolerance.
Box 32-3 CLINICAL MANIFESTATIONS OF POLYCYSTIC OVARY SYNDROME
Presenting signs and symptoms (percentage of women affected)
Hormonal disturbances
Increased insulin (independent of obesity)
Increased androgens (testosterone androstenedione)
Increased leptin, especially in obesity (independent of insulin)
Possible decreased oestrogen receptors (intraovarian and along hypothalamic-pituitary axis)
EVALUATION AND TREATMENT
Diagnosis of polycystic ovary syndrome is based on evidence of androgen excess, chronic anovulation and inappropriate gonadotropin secretion. Treatment with insulin sensitisers seems to increase fertility while decreasing predisposition to type 2 diabetes. Adding an anti-androgen agent may enhance results. Hormonal contraception may be used to suppress androgen production and reduce endometrial hyperplasia.
Hormonal and menstrual alterations
Dysmenorrhoea
Primary dysmenorrhoea is painful menstruation associated with the release of prostaglandins in ovulatory cycles but not with pelvic disease. Between 50% and 75% of women aged 15–25 years are affected — some (up to 15%)72 are affected severely enough to cause missed work or school. Primary dysmenorrhoea begins with the onset of ovulatory cycles. The incidence steadily rises, peaks in women in their mid-20s and decreases slowly thereafter.
Primary dysmenorrhoea results from excessive prostaglandin F production by the secretory endometrium. Prostaglandins are lipid-based hormones that can increase contractions of the myometrium and constrict endometrial blood vessels. Reduced blood flow to the endometrium causes ischaemia resulting in the shedding of the endometrium. In addition, prostaglandins and prostaglandin metabolites can cause gastrointestinal complaints, headache and syncope (fainting).
The chief symptom of dysmenorrhoea is pelvic pain associated with the onset of menses. The severity is directly related to the length and amount of menstrual flow, and pain often radiates into the groin and may be accompanied by backache, anorexia, vomiting, diarrhoea, syncope and headache. Usually, the discomfort begins shortly before the onset of menstruation and rarely persists beyond the second day. Dysmenorrhoea may be relieved with hormonal contraceptives, which stop ovulation and prevent growth of the endometrium, thereby decreasing prostaglandin synthesis and myometrial contractility. Prostaglandin inhibitors work in a majority of women with primary dysmenorrhoea and should be taken before or at the onset of bleeding or cramping. Regular exercise seems to prevent or reduce symptoms. Other comfort measures include local application of heat, massage or relaxation techniques. Orgasm may relieve or worsen symptoms.
Secondary dysmenorrhoea is related to pelvic pathology, manifests later in the reproductive years and may occur any time in the menstrual cycle. Secondary dysmenorrhoea results from disorders such as endometriosis, pelvic adhesions, pelvic inflammatory disease, uterine fibroids or adenomyosis.
Primary amenorrhoea
Amenorrhoea means lack of menstruation from any cause. Primary amenorrhoea, however, is defined as either the failure of menarche and the absence of menstruation by age 14 years with no development of secondary sex characteristics, or the absence of menstruation by age 16 years regardless of the presence of secondary sex characteristics. Causes include congenital defects of gonadotropic hormone production, genetic disorders (such as Turner’s syndrome), congenital or acquired central nervous system defects (e.g. hydrocephalus, trauma, infection and tumours) and congenital malformations of the reproductive system (e.g. absence of vagina or uterus). A major cause of primary amenorrhoea is the failure of the hypothalamic-pituitary-gonadal axis (see Chapter 31), as a result of which the ovaries do not receive the hormonal signals (FSH and LH) that normally initiate menarche. Essentially, any defect in the development of the gonads or their ability to respond to the gonadotropins FSH and LH will result in primary amenorrhoea.
Diagnosis of primary amenorrhoea is based on the history and physical examination to investigate:
Treatment involves correction of any underlying disorders and hormone replacement therapy to induce the development of secondary sex characteristics. Although surgical alteration of the genitalia may be undertaken to correct abnormalities, it should be postponed until the individual can make a truly informed decision.
Secondary amenorrhoea
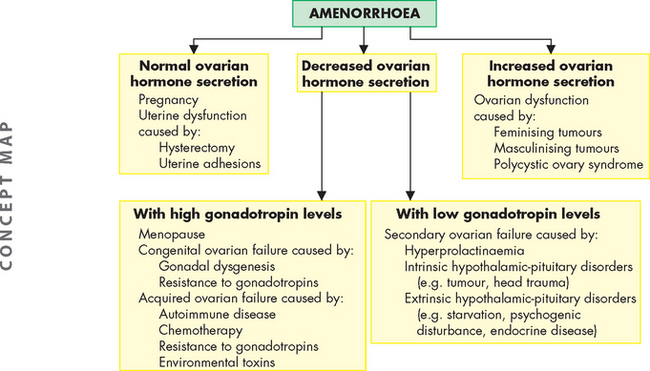
Secondary amenorrhoea is the absence of menstruation for a time equivalent to 3 or more cycles or 6 months in women who have previously menstruated. Many disorders and physiological conditions are associated with secondary amenorrhoea. Secondary amenorrhoea is normal during early adolescence, pregnancy, lactation and in older women approaching menopause. Pregnancy is the most common cause of secondary amenorrhoea and must be ruled out before any further evaluation. The pathophysiology of secondary amenorrhoea is summarised in Figure 32-13.
The major manifestation of secondary amenorrhoea is the absence of menses. Depending on the underlying cause of the amenorrhoea, infertility, vasomotor flushes (dilation of blood vessels leading to ‘hot flushes’), vaginal atrophy, acne and hirsutism may also be present. Diagnosis involves identifying underlying hormonal or anatomical alterations. Depending on the cause of the amenorrhoea, treatment may involve hormone replacement therapy or a corrective procedure, such as surgical removal of pituitary tumours.
Abnormal uterine bleeding
The most common cause of abnormal uterine bleeding is failure to ovulate related to age or pathology of the endocrine (hormone) function. Common causes of abnormal bleeding based on age group and frequency are presented in Table 32-3.
Table 32-3 COMMON CAUSES OF ABNORMAL VAGINAL/GENITAL BLEEDING
| AGE GROUP | CAUSE |
|---|---|
| Prepubescence | |
| Adolescence | |
| Reproductive years | |
| Premenopause | |
| Postmenopause | Malignancy |
Dysfunctional uterine bleeding is abnormal uterine bleeding resulting from a disturbance of the menstrual cycle, usually anovulation. Although dysfunctional uterine bleeding may occur at any time during the reproductive years, it is most likely to affect women at the extremes of the reproductive years.
Abnormal bleeding in ovulatory cycles is less common and mechanisms underlying this bleeding are unclear. Excessive fibrinolytic activity and changes in prostaglandin production may be implicated. Infection or structural abnormalities may also be present.
Dysfunctional uterine bleeding is characterised by unpredictable and variable bleeding in terms of amount and duration. When a woman is close to menopause, dysfunctional bleeding may involve flooding and the passing of large clots.73 Heavy bleeding may be preceded by episodes of amenorrhoea and be perceived by individuals as a miscarriage. Dysfunctional uterine bleeding is the diagnosis when other conditions that could cause abnormal bleeding are eliminated. Goals of therapy are to control bleeding, prevent hyperplasia, prevent or treat anaemia and treat concurrent endocrine problems if present. The usual therapy is treatment with hormones.
Premenstrual syndrome
Premenstrual syndrome (PMS) is much misunderstood in the general population. It is characterised by quite distressing pain and mood swings in the luteal phase of the menstrual cycle (post-ovulation, in which the corpus luteum produces progesterone and oestrogen; see Chapter 31). Premenstrual symptoms affect many adolescent females, with studies suggesting an incidence range of 31–61% in this age group.74,75 An estimated 5–10% of menstruating women have severe to disabling symptoms, and 3–8% of these have a cyclic exaggerated feeling of depression (dysphoria), known as premenstrual dysphoric disorder, which requires treatment. It seems that symptoms are experienced to some degree by all ovulating adolescent and adult women, and they may occur throughout the menstrual phases — the presence and severity of symptoms in any one woman may be inconsistent from month to month and the menstrual phase for peak symptom severity may differ depending on the population studied.76,77
PATHOPHYSIOLOGY
The exact aetiology (cause) of PMS is unknown but it is considered to be multifactorial. Fluctuating oestrogen and progesterone levels may trigger this biological response but are not sufficient alone to cause PMS. Research suggests that serotonin levels play a role in the type and severity of symptoms. Low-dose fluoxetine, a selective serotonin re-uptake inhibitor, significantly reduces premenstrual mood-related symptoms, and higher doses also decrease breast tenderness, bloating and joint/muscle pain.
A predisposition for PMS runs in families, perhaps because of genetics or shared environment. A woman’s menstrual experience tends to be similar to her mother’s or her sister’s experience. Further evidence supports a relationship between the severity and frequency of premenstrual symptoms and reports of major affective disorders, personality characteristics and family conflict. In turn, when premenstrual symptoms are perceived as distressing, the quality of interpersonal relationships and self-image are negatively affected.
CLINICAL MANIFESTATIONS
The pattern of symptom frequency and severity is more important than specific complaints. More than 200 physical, emotional and behavioural symptoms have been attributed to PMS. Emotional symptoms — particularly depression, anger, irritability and fatigue — have been reported as the most prominent and the most distressing, whereas physical symptoms seem to be the least prevalent and problematic. Approximately 6% of women have classic PMS (distressing luteal symptoms) and 7% report premenstrual magnification of symptoms that are present during the entire cycle. A typical premenstrual symptom pattern may appear after the treatment of a systemic disease. Likewise, underlying physical or psychological disease may be aggravated before menstruation.
EVALUATION AND TREATMENT
Diagnosis of PMS is based on the health history and symptoms. Research and diagnostic criteria for premenstrual dysphoric disorder are presented in Box 32-4. Current treatment for PMS is symptomatic because the cause is complex and cannot be reduced to a single biological explanation and because the occurrence and severity are mediated by lifestyle, social and psychological factors. Non-pharmacological therapies, with or without medication, tend to be more effective in controlling symptoms than medication alone.
Box 32-4 GENERAL CRITERIA FOR PREMENSTRUAL DYSPHORIC DISORDER
Premenstrual dysphoria is the predominant feature of premenstrual dysphoric disorder and is triggered (not caused) by the endocrine changes that occur in the late luteal phase of the menstrual cycle. Although premenstrual dysphoric disorder is not an accepted diagnostic entity in the DSM-IV-TR diagnostic and statistical manual of mental disorders, recognition is given to the severe and incapacitating dysphoria that characterises the disorder by listing premenstrual dysphoric disorder as an example under ‘Mood disorders, depression, not otherwise specified’. To encourage further research, premenstrual dysphoric disorder remains in the appendix of DSM-IV. Criteria for premenstrual dysphoric disorder include a rigorous prospective assessment confirming a regular premenstrual pattern of severe depressive symptoms.
Source: DSM-IV-TR diagnostic and statistical manual of mental disorders. 4th edn. Washington, DC: American Psychiatric Association; 2000.
Initial treatment focuses on education about PMS, self-help techniques and elimination of contributing factors or coexisting disorders. The following dietary changes are beneficial — eating 6 small meals each day; increasing the intake of complex carbohydrates, fibre and water; and decreasing caffeine, alcohol, sugar and animal fat consumption.
If the criteria for diagnosis of premenstrual dysphoric disorder are met, drugs such as selective serotonin re-uptake inhibitors, antiprostaglandins and alprazolam may be prescribed. Selective serotonin reuptake inhibitors relieve symptoms in about 60% of women and may be given continuously or only during the premenstrual period. Oedema associated with PMS is a result of local fluid shifts rather than fluid retention, so diuretics are not recommended. In severe cases, menses is abolished, which eliminates cyclic ovarian hormones and thus the biological trigger for PMS. This is accomplished by the use of oral contraceptives.
Infection and inflammation
Infections of the genital tract by bacteria or fungi lead to inflammation due to the increased blood flow and the presence of immune cells, which are attracted to the site of infection. The bacteria or fungi causing the infection may be an overgrowth of the flora normally resident in the vagina, bowel or vulva (endogenous) or they may be foreign organisms (exogenous) transmitted to the site of infection. Exogenous pathogens are most often sexually transmitted, but can be the result of poor hygiene. Endogenous causes of infection occur if resident microorganisms migrate to a new location or overproliferate. Infections can result in inflammation of almost any part of the reproductive tract of both men and women, and given the anatomy of the reproductive system (Chapter 31), can spread easily to other parts of the reproductive system and to sexual partners.
Skin disorders affect the female more frequently than the male, and this may affect the vulva. Most infections of the vulva and vagina are sexually transmitted (see the section on sexually transmitted infections later in the chapter). Infection and inflammation can occur in the vagina (vaginitis), the cervix (cervicitis), the vulva (vulvitis) and the Bartholin’s glands (bartholinitis). The labia can also become inflamed and oedematous if irritated or if infection is present in the vagina or vulva. The following is a summary of the most common presentations in healthcare settings in Australia and New Zealand.
Pelvic inflammatory disease
Pelvic inflammatory disease (PID) is an acute inflammatory process caused by infection (see Figure 32-14). It may involve any organ, or combination of organs, of the upper genital tract (the uterus, fallopian tubes or ovaries) and, in its most severe form, the entire peritoneal cavity. Inflammation of the fallopian tubes is termed salpingitis (see Figure 32-15); inflammation of the ovaries is called oophoritis.
FIGURE 32-14 Pelvic inflammatory disease.
A A drawing depicting involvement of both ovaries and fallopian tubes. B Total abdominal hysterectomy and bilateral salpingo-oophorectomy specimen showing unilateral pyosalpinx.
Source: A Seidel HM et al. Mosby’s guide to physical examination. 6th edn. St Louis: Mosby; 2006. B Morse SA, Ballard RC, Holmes KK, Moreland AA. Atlas of sexually transmitted diseases and AIDS. 3rd edn. Edinburgh: Mosby; 2003.
A Note the swollen fallopian tubes. B Bilateral, retort-shaped, swollen sealed tubes and adhesions of ovaries are typical of salpingitis.
Most cases of pelvic inflammatory disease are caused by sexually transmitted microorganisms that migrate from the vagina to the uterus, fallopian tubes and ovaries.78 Pelvic inflammatory disease is generally the result of a polymicrobial (many microorganisms) infection and although mostly initiated by gonorrhoea or Chlamydia (50–60% of cases), mixed bacteria (Gardnerella vaginalis, Haemophilus influenzae and streptococci) also contribute. Neisseria gonorrhoeae and Chlamydia trachomatis induce necrosis with repeated infections and predispose an individual to develop pelvic inflammatory disease. After one episode of pelvic infection, 15–25% of women develop long-term complications such as infertility, ectopic pregnancy, chronic pelvic pain, dyspareunia (painful intercourse), pelvic adhesions, perihepatitis (inflammation of the serous or peritoneal coating of the liver) and fallopian tube and ovary abscess.
The clinical manifestations of pelvic inflammatory disease vary from sudden, severe abdominal pain with fever to no symptoms at all. The pain of pelvic inflammatory disease may worsen with walking, jumping or intercourse, and dysuria (difficult or painful urination) and irregular bleeding are often present. An asymptomatic cervicitis (inflammation of the cervix) may be present for some time before pelvic inflammatory disease develops. The first sign of the ascending infection may be the onset of low bilateral abdominal pain, often characterised as dull and steady with a gradual onset. Symptoms are more likely to develop during or immediately after menstruation.
The diagnosis of pelvic inflammatory disease is based on a number of factors including the history, abdominal tenderness, the presence of uterine and cervical movement, tenderness on bimanual pelvic examination, mucopurulent (mucus and pus) discharge at the cervical os (opening), white blood cells in the cervical discharge, leucocytosis (an increase in the number of white blood cells) and an increased erythrocyte sedimentation rate. To support the diagnosis, tests for Chlamydia and gonorrhoea are done. Other conditions that cause pelvic pain must be excluded, including ectopic pregnancy, threatened abortion or appendicitis.
Because of the significance of the complications of pelvic inflammatory disease, aggressive treatment (bed rest, avoidance of intercourse and combined antibiotic therapy) is recommended. Between 25% and 40% of women with pelvic inflammatory disease require hospitalisation for intravenous antibiotics and treatment of peritonitis or a tubo-ovarian abscess. To prevent recurrence, sexual partners are also treated with antibiotic combinations.
Vaginitis
Vaginitis is usually caused by sexually transmitted pathogens, bacterial vaginosis or Candida albicans. The incidence of sexually transmitted vaginitis remains highest in women 10–24 years of age.78 Vaginitis can occur when either the skin of the vagina is damaged or the pH of the vaginal secretions changes. The pH of the vagina depends on cervical secretions and the presence of normal flora (Lactobacillus acidophilus) that help maintain an acidic environment. The acidic environment acts as a barrier to infectious organisms; however, if the pH of the secretions becomes higher (less acidic), then the vagina becomes predisposed to infection. The vaginal pH can be altered by douching; use of soaps, spermicides, feminine hygiene sprays or deodorant menstrual pads or tampons; conditions associated with increased glycogen content of vaginal secretions, such as pregnancy or diabetes; and conditions that compromise the immune system.
In addition, the use of antibiotics for other conditions may kill the normal vaginal flora, resulting in an increase in alkalinity and making the vagina more susceptible to trichomoniasis (bacterial vaginosis) and an overgrowth of Candida albicans.
Cervicitis
Cervicitis is a nonspecific term used to describe inflammation of the cervix before the identification of pathogens. Mucopurulent cervicitis is usually caused by one or more sexually transmitted pathogens, such as Trichomonas, Neisseria gonorrhoeae, Chlamydia, Mycoplasma or Ureaplasma. Infection causes the cervix to become red and oedematous. A mucopurulent (mucus- and pus-containing) exudate drains from the external cervical os and may be accompanied by vague pelvic pain, bleeding or dysuria. The infectious organisms are cultured or identified and then treated by oral antibiotic therapy. Sexual partners are usually treated to prevent reinfection.78
Vulvitis
Acute vulvitis is an inflammation of the skin (dermatitis) of the vulva and often of the perianal area. It can be caused by contact with perfumed and scented hygiene products (soaps, detergents, lotions, hygienic sprays, shaving creams, menstrual pads or toilet paper) and can be aggravated by non-absorbent or tight-fitting clothes. The inflammation can increase susceptibility to a vaginal infection or may be caused by a vaginal infection that spreads to the labia. The vulva also can be affected by other skin diseases, such as tinea cruris, lichen sclerosis, psoriasis and inflammation of the apocrine (sweat) glands (see Chapter 19).
Avoidance of irritants, wearing loose cotton clothing and appropriate antimicrobial/antifungal treatment for recurrent vaginitis are usually effective cures for acute vulvitis. Chronic vulvitis is usually treated with fluorinated hydrocortisone. Biopsy specimens of persistent lesions are examined for the presence of malignancy.
Bartholinitis
Bartholinitis (Bartholin’s cyst) is an inflammation of one or both of the ducts that lead from the introitus (vaginal opening) to the Bartholin’s glands (see Figure 32-16). The usual causes are microorganisms that infect the lower female reproductive tract, such as streptococci, staphylococci and sexually transmitted pathogens. Acute bartholinitis may be preceded by an infection, such as cervicitis, vaginitis or urethritis. Cultures for gonorrhoea and Chlamydia are recommended. Infection is treated with antibiotics and pain is relieved with analgesics and warm sitz baths (where the pelvic region is immersed in warm water; from the German word sitzen mean ‘to sit’). If an abscess forms, it may be surgically drained.
Pelvic relaxation disorders
The uterus, bladder, urethra and rectum are supported by the endopelvic fascia and perineal muscles. This muscular and fascial tissue loses tone and strength with ageing and after giving birth and may fail to maintain the pelvic organs in the proper position. Uterine displacement can be caused by progressive relaxation of the pelvic support structures or trauma such as childbirth or pelvic surgery that damages or weakens the supporting structures. The bladder, urethra or rectum (and hence the uterus) may become displaced many years after an initial injury to the supporting structure. A strong familial tendency and, possibly, a multifactorial genetic component place some women at risk for the development of prolapse.
Vaginal prolapse can result from one of the following:
Figure 32-17 shows vaginal prolapse caused by cystocele and rectocele.
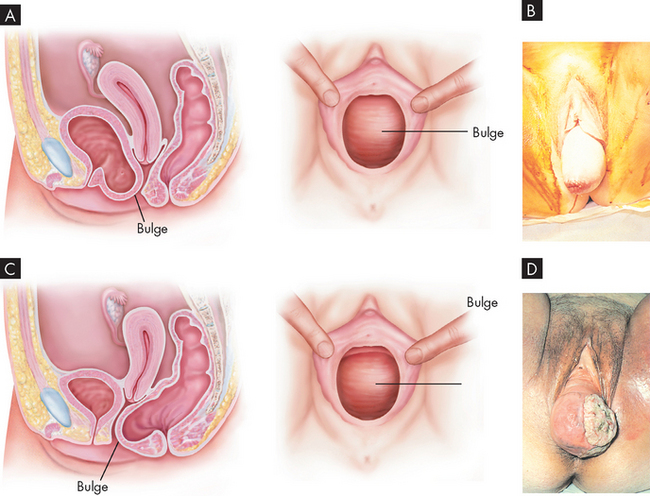
FIGURE 32-17 Vaginal prolapse.
A Anatomical positioning involving cystocele. B Large cystocele. C Anatomical positioning involving rectocele. D Rectocele associated with ulceration of vaginal wall.
Source: A & C Seidel HM et al. Mosby’s guide to physical examination. 6th edn. St Louis: Mosby; 2006. B & D Symonds EM, Macpherson MBA. Color atlas of obstetrics and gynecology. London: Mosby; 1994.
In severe cases of cystocele the bladder and anterior vaginal wall bulge outside the introitus (vaginal opening). Cystocele can cause stress incontinence in which the woman leaks urine when she laughs, sneezes, coughs or does anything that strains the abdominal muscles. Cystocele is usually accompanied by urethrocele, or sagging of the urethra. Urethrocele is usually caused by the shearing effect of the fetal head on the urethra during childbirth. If rectocele is severe, defecation can be difficult and may be accomplished only by applying manual pressure to the posterior vaginal wall. Enterocele is usually associated with other pelvic relaxation disorders, such as uterine prolapse, cystocele and rectocele. Table 32-4 summarises the causes, symptoms and treatment of cystocele, urethrocele and rectocele.
Uterine prolapse is descent of the cervix or entire uterus into the vaginal canal (see Figure 32-18). In severe cases, the uterus falls completely through the vagina and protrudes from the introitus. First-degree uterine prolapse is not treated unless it causes discomfort. Second- and third-degree prolapses cause feelings of fullness, heaviness and collapse through the vagina. Symptoms of other pelvic relaxation disorders may also be present. Treatment in these cases is the insertion of a pessary, which is a removable mechanical device that holds the uterus in position.79 The pelvic fascia may be strengthened through Kegel exercises (repetitive, isometric tightening and relaxing of the pubococcygeal muscles) or by a course of oestrogen therapy, particularly if the woman is past menopause. Surgical repair with or without hysterectomy may be necessary. Prevention of constipation, maintaining a healthy body mass index and early treatment of respiratory ailments that cause coughing may help.
Reproductive and sexual dysfunction
Increased awareness of female sexual dysfunction is relatively new. Most of what is known comes from clinical observations and anecdotal reports from women, as adequate research is lacking. Both organic and psychosocial disorders can be implicated in sexual dysfunction. Organic problems may be the underlying cause in 10–20% of cases and may contribute to another 15%. Chronic illness can affect sexual functioning and response. Table 32-5 outlines possible effects of specified chronic diseases on sexual functioning.
Table 32-5 POSSIBLE EFFECTS OF CHRONIC DISEASE ON SEXUAL FUNCTIONING IN WOMEN
| DISEASE | SEXUAL FUNCTION |
|---|---|
| Cerebral palsy | |
| Stroke | |
| Diabetes mellitus | |
| End-stage kidney disease | |
| Rheumatoid arthritis | |
| Systemic lupus erythematosus | |
| Acute myocardial infarction | |
| Multiple sclerosis | |
| Spinal cord injury |
Disorders of desire (inhibited sexual desire, decreased libido) may be a biological manifestation of depression, alcohol or other substance abuse, prolactin-secreting pituitary tumours or testosterone deficiency. Beta (β)-adrenergic blocker medication, such as propranolol and metoprolol, used in the treatment of heart disease may also inhibit sexual desire.
Vaginismus is an involuntary muscle spasm of the pubococcygeal muscle in response to attempted penetration. Common psychological causes include prior sexual trauma and fear of sex. Organic causes are similar to those that cause dyspareunia. Even after the underlying organic problem is detected and successfully treated, vaginismus may persist.
Anorgasmia (orgasmic dysfunction) is the inability of a woman to reach or achieve orgasm. Specific disorders that may block orgasm are diabetes, alcoholism, neurological disturbances, hormonal deficiencies and pelvic disorders, such as infections, trauma and surgical scarring. Other inhibitors include drugs such as narcotics, tranquillisers, antidepressants and antihypertensive medications.
Dyspareunia (painful intercourse) is common; women may experience pain at any time from the beginning of arousal to after intercourse. The pain may have a burning, sharp, searing or cramping quality and may be described as external, vaginal, deep abdominal or pelvic. A variety of psychosocial and organic causes have been identified. Inadequate lubrication may make penetration or intercourse difficult or painful. Drugs with a drying effect, such as antihistamines, tranquillisers and marijuana, and disorders such as diabetes mellitus, vaginal infections and oestrogen deficiency can decrease lubrication. Other causes include skin problems in various locations.
Sexual dysfunction may develop as a coping mechanism. Women with a history of sexual trauma such as rape or incest often have problems with desire, arousal or orgasm or experience pain with sexual activity. In extreme cases, total sexual aversion may develop. At other times, sexual dysfunction may be a symptom of marital or relationship problems; often, unresolved anger manifests as inhibited desire or diminished arousal.
DISORDERS OF THE MALE REPRODUCTIVE SYSTEM
Male reproductive disorders can arise from hormonal dysfunction, structural abnormalities or infections. For example, the testes are easily damaged by trauma, which can lead to infertility as well as further hormonal balance changes if testosterone-producing cells are damaged. Men are on the whole less likely to seek medical assistance for problems and so the incidence of male reproductive disorders is underreported.
Disorders of the urethra
Urethritis
Urethritis is a common disorder of the urethra and is an inflammatory process that is usually, but not always, caused by a sexually transmitted infection. Infectious urethritis caused by Neisseria gonorrhoeae is often called gonococcal urethritis. Urethritis caused by other microorganisms is called nongonococcal urethritis. Non-sexual origins of urethritis include inflammation or infection as a result of urological procedures, insertion of foreign bodies into the urethra, anatomical abnormalities or trauma.
Symptoms of urethritis include a tingling, itching or burning sensation of the urethra, and the frequency and urgency of urination increases. The individual may note a purulent or clear mucus-like discharge from the urethra. Early detection of Neisseria gonorrhoeae and Chlamydia trachomatis in urine tests is possible. Treatment consists of appropriate antibiotic therapy for infectious urethritis and avoidance of future exposure or mechanical irritation.
Urethral strictures
A urethral stricture is a narrowing of the urethra caused by scarring. The scars may be congenital but are more likely to result from trauma (e.g. injury by urological instrument) or untreated or severe urethral infections. Prostatitis and infection secondary to urinary stasis are common complications. Severe and prolonged obstruction can result in hydronephrosis and renal failure. (Hydronephrosis is distension and dilation of the renal pelvis and calyces, usually caused by obstruction of the free flow of urine from the kidneys, leading to progressive atrophy of the kidneys.80)
Clinical manifestations include a desire to urinate frequently, but often with a hesitant urine flow with lower force than normal, dribbling after voiding and nocturia. Urethral stricture is diagnosed on the basis of the history, physical examination and cystoscopy. Treatment is usually surgical and may involve urethral dilation, urethrotomy or a variety of open surgical techniques. The choice of surgical intervention depends on the age of the individual and the severity of the problem.
Disorders of the penis
Phimosis and paraphimosis
Phimosis and paraphimosis are both disorders in which the foreskin (prepuce) is ‘too tight’ to move easily over the glans penis (see Figure 32-19). Phimosis is a condition in which the foreskin cannot be retracted back over the glans. The inability to retract the foreskin is normal in infancy (caused by congenital adhesions between the foreskin and glans). During the first 3 years of life these congenital adhesions separate naturally with penile erections and are not an indication for circumcision. Phimosis can occur at any age and is most commonly caused by poor hygiene and chronic infection. It rarely occurs with normal foreskin. Reasons for seeking treatment include oedema, erythema, tenderness of the prepuce and purulent discharge; inability to retract the foreskin is a less common complaint. Circumcision, if needed, is performed after infection has been eradicated. Complications of phimosis include inflammation of the glans (balanitis) or prepuce (posthitis) and paraphimosis. There is a higher incidence of penile carcinoma in uncircumcised males, but chronic infection and poor hygiene are usually the underlying factors in such cases.
FIGURE 32-19 Phimosis and paraphimosis.
A Phimosis: the foreskin has a narrow opening that is not large enough to permit retraction over the glans. B Lesions on the prepuce secondary to infection cause swelling, and retraction of foreskin may be impossible. Circumcision is usually required. C Paraphimosis: the foreskin is retracted over the glans but cannot be reduced to its normal position. Here it has formed a constricting band around the penis.
D This oedematous foreskin is the result of paraphimosis.
Source: Roberts JR, Hedges JR. Clinical procedures in emergency medicine. 5th edn. Philadelphia: Saunders; 2009.
Box 32-5 outlines the use of circumcision in Australia and New Zealand.
Box 32-5 CIRCUMCISION IN AUSTRALIA AND NEW ZEALAND
Circumcision of males has been undertaken for religious and cultural reasons for many thousands of years and remains an important ritual in some religious and cultural groups. In Australia and New Zealand, the circumcision rate has fallen considerably in recent years and it is estimated that currently only 10–20% of male infants are routinely circumcised. Circumcision is generally performed with local or general anaesthesia, and when the procedure is carried out for a medical indication this is usually outside the neonatal period. The best-recognised medical indication for circumcision is phimosis.
The Paediatrics and Child Health Division of the Royal Australasian College of Physicians (RACP) has carried out a study into the risk and benefits of routine circumcision of the neonate. The review revealed that there was evidence that routine circumcision may provide some benefit in the prevention of urinary tract infections, HIV and later cancer of the penis, but there is ‘no evidence of benefit outweighing harm for circumcision as a routine procedure in the neonate’. For example, urinary tract infections affect 1–2% of boys and may be about five times less frequent in circumcised boys. However, the complication rate of neonatal circumcision is around 1–5%. Complications range from local infection, bleeding and damage to the penis to more serious complications such as bleeding, septicaemia and meningitis occasionally causing death. Circumcision may provide some protection from HIV in areas where rates of HIV are high, such as Sub-Saharan Africa (although data are conflicting), but Australia and New Zealand have low incidence rates of HIV due to increased public awareness and the relatively extensive use of condoms (a good preventative measure against sexually transmitted infections including HIV). Finally, since penile cancer is a rare disease with an incidence rate of about 1 per 100,000 in developed countries, the rarity of the condition does not justify the routine circumcision of males, even though evidence suggests that circumcision reduces the risk ten-fold.
As a result, in 2004 the RACP released a policy statement stating: ‘After extensive review of the literature the RACP reaffirms that there is no medical indication for routine neonatal circumcision.’ The policy statement also acknowledged that there may be legal implications of performing circumcision on a minor where there is no proven medical benefit in so far as the procedure may contravene the human rights of the child.
The RACP has since issued a set of guidelines for physicians in Australia stating: ‘If the operation is to be performed, the medical attendant should ensure this is done by a competent operator, using appropriate anaesthesia and in a safe child-friendly environment … In all cases where parents request a circumcision for their child the medical attendant is obliged to provide accurate information on the risks and benefits of the procedure. Up-to-date, unbiased written material summarising the evidence should be widely available to parents.’
Source: Paediatrics and Child Health Division, The Royal Australasian College of Physicians. Policy statement on circumcision 2004. Available at: www.racp.edu.au.
Paraphimosis is a condition in which the foreskin is retracted and cannot be moved forward (reduced) to cover the glans. This can constrict the penis, causing oedema of the glans. If the foreskin cannot be reduced manually, surgery must be performed to prevent necrosis of the glans caused by constricted blood vessels. Severe paraphimosis is a surgical emergency.
Peyronie’s disease
Peyronie’s disease (‘bent nail syndrome’) is a connective tissue disorder involving the growth of fibrous plaques in the soft tissue of the penis affecting as many as 1–4% of men.81 Specifically, the fibrosing process occurs in the tunica albuginea, a fibrous envelope surrounding the penile corpora cavernosa causing an abnormal curvature of the penis (see Figure 32-20). A dense, fibrous plaque is usually palpable on the dorsal side of the penile shaft whether erect or flaccid. The problem usually affects middle-aged men and is associated with painful erection, painful intercourse (for both partners) and poor erection distal to the involved area. In some cases, impotence or unsatisfactory penetration occurs. When the penis is flaccid, there is no pain.
FIGURE 32-20 Peyronie’s disease.
This individual complained of pain and deviation of the penis to one side during erection.
Source: Taylor PK. Diagnostic picture tests in sexually transmitted diseases. London: Mosby; 1995.
A local vasculitis-like inflammatory reaction occurs and decreased tissue oxygenation results in fibrosis and calcification. The exact cause is unknown. Peyronie’s disease is associated with Dupuytren contracture (a flexion deformity of the fingers or toes caused by shortening or fibrosis of the palmar or plantar fascia), diabetes, a predisposition to keloids and, in rare cases, use of β-blocker medications.82
Surgical treatments include intracavernosal plaque excision, including newer minimally invasive repair strategies.83
Priapism
Priapism is an uncommon condition of prolonged penile erection that is usually painful and is not associated with sexual arousal (Figure 32-21). Priapism is idiopathic (it has no known cause) in 60% of cases. The remaining 40% of cases can be associated with spinal cord trauma, sickle cell disease, leukaemia, pelvic tumours or intracavernous injection therapy for impotence.
This patient experienced 18 hours of priapism after penile self-injection of papaverine as therapy for impotence.
Source: Roberts JR, Hedges JR. Clinical procedures in emergency medicine. 5th edn. Philadelphia: Saunders; 2009.
Priapism must be considered as a urological emergency as the erect penis prevents the easy passage of urine. Treatment within hours is effective and prevents impotence. Conservative approaches include iced saline enemas, ketamine administration and spinal anaesthesia. Newer drug therapy for recurrent ischaemic priapism includes phosphodiesterase 5 inhibitors (these drugs block the enzyme phosphodiesterase, which breaks down the bonds between nucleic acids).84 Needle aspiration of blood from the corpus through the dorsal glans is often effective and is followed by catheterisation and pressure dressings to maintain decompression. More aggressive surgical treatments include the creation of vascular shunts to maintain blood flow. Erectile dysfunction results in up to 50% of cases.
Disorders of the scrotum, testis and epididymis
Disorders of the scrotum
The scrotum is a multilayered skin sac housing the testes, epididymis and blood vessels supplying the testes. Swelling of the testes or scrotum is fairly common and has a number of underlying causes, the three most common being varicocele, hydrocele and spermatocele:
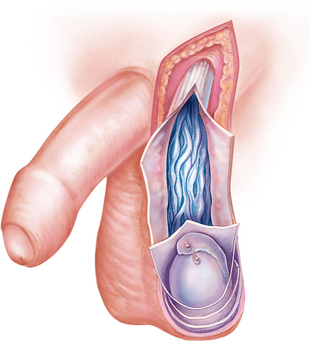
FIGURE 32-22 Schematic of a variocele.
Dilation of the veins within the spermatic cord.
Source: Seidel HM et al. Mosby’s guide to physical examination. 6th edn. St Louis: Mosby; 2006.
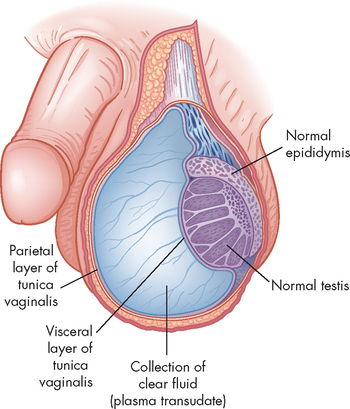
FIGURE 32-23 Schematic of an hydrocele.
Accumulation of clear fluid between the visceral (inner) and parietal (outer) layers of the tunica vaginalis (covering of the testis).
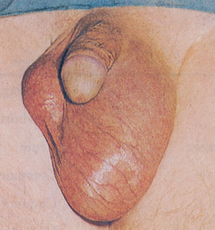
Retention cyst of the head of the epididymis or an aberrant tubule or tubules of the rete testis. The spermatocele lies outside the tunica vaginalis; therefore, on palpation it can be readily distinguished and separated from the testis.
Source: Lloyd-Davis RW et al. Color atlas of urology. 2nd edn. London: Wolfe medical; 1994.
Cryptorchidism
In cryptorchidism, one or both testes fail to descend into the scrotum. This is the most common congenital condition involving the testes, with about 3–6% of all full-term males and 20–30% of all premature males having undescended testes at birth. The testes may remain in the abdomen or descent may be arrested in the inguinal canal or the puboscrotal junction. In approximately 75–90% of infants with cryptorchidism, the testes descend into the scrotum by 1 year of age, leaving a true incidence of 0.8% of the male population.
Cryptorchidism may result from a developmental delay, a defect of the testis, deficient maternal gonadotropin stimulation or some mechanical factor that prevents descent through the inguinal canal. Mechanical possibilities include a short spermatic cord, fibrous bands or adhesions in the normal path of the testes, or a narrowed inguinal canal. Chromosomal studies do not support a genetic component.
Untreated cryptorchidism is associated with a lowered sperm count and, therefore, impaired fertility. Impaired spermatogenesis is caused by higher temperatures within the abdomen. Cryptorchidism does not prevent puberty or maintenance of secondary sex characteristics if the testis is otherwise normal. Undescended testes are susceptible to neoplastic processes. The risk of testicular cancer is 35–50 times greater for men with cryptorchidism or a history of cryptorchidism than for the general male population.
Physical examination discloses the absence of one or both testes in the scrotum. Ultrasonography or a CT scan can help clinicians locate a non-palpable testis that has migrated intra-abdominally. Treatment often begins with hormonal therapy. If hormonal therapy is not successful, to preserve fertility, surgical correction (orchiopexy) of cryptorchidism is attempted when the child is about 2 years of age. Orchiopexy is recommended no later than age 5 or 6 years. Placement of the cryptorchid testis into the scrotal sac does not decrease the potential for malignancy, but it does facilitate examination and tumour detection.
Torsion of the testis
In torsion of the testis, the testis rotates on its vascular pedicle, interrupting its blood supply (Figure 32-25). Torsion of the testis is one of several conditions that cause an acute scrotum (testicular pain and swelling). This event can occur at any age but is most common among neonates and adolescents, particularly at puberty.87 Onset may be spontaneous or follow physical exertion or trauma. Torsion twists the arteries and veins in the spermatic cord, reducing or stopping circulation to the testis. Vascular engorgement and ischaemia develop, causing scrotal swelling and pain not relieved by rest or scrotal support. Diagnostic testing includes urinalysis (for infection) and colour Doppler ultrasonography.88–90 4 6 Torsion of the testis is a surgical emergency. If it cannot be reduced manually (scrotal elevation), surgery must be performed within 6 hours after the onset of symptoms to preserve normal testicular function.
Orchitis
Orchitis is an acute inflammation of the testes (see Figure 32-26) and is uncommon except as a complication of systemic infection. Infectious organisms may reach the testes through the blood or the lymphatics or, most commonly, by ascent through the urethra, ductus deferens and epididymis. Mumps is the most common infectious cause of orchitis and usually affects postpubertal males. The onset is sudden, occurring 3–4 days after the onset of parotitis (inflammation of one or both parotid glands, the major salivary glands located on either side of the face). Signs and symptoms include high fever, reaching 40°C, marked prostration, bilateral or unilateral erythema, oedema and tenderness of the scrotum and leucocytosis. An acute hydrocele may develop. Atrophy with irreversible damage to spermatogenesis may result in 30% of affected testes.
Impairment of sperm production and quality
This is a major cause of male infertility (see Table 32-6 later in the chapter). Spermatogenesis requires:
Table 32-6 KNOWN CAUSES OF MALE INFERTILITY
| Sperm production problems | |
| Blockage of sperm transport | |
| Sperm antibodies | |
| Sexual problems (erection and ejaculation problems) | |
| Hormonal problems |
Inadequate secretion of gonadotropins has a variety of causes related to the function of the hypothalamic-pituitary-gonadal axis in males. In the absence of adequate gonadotropin levels, the Leydig cells are not stimulated to secrete testosterone and inhibin, and sperm maturation is not promoted in the sustentacular cells.
Spermatogenesis also depends on an appropriate response by the testes, so defects in the testicular response to the gonadotropins result in decreased secretion of testosterone and inhibin B. In the absence of adequate testosterone levels, spermatogenesis is impaired. Defects in the testes can be congenital, caused by trauma, infection, atrophy of the testes, systemic illness involving high fever, ingestion of various drugs, exposure to environmental toxins and cryptorchidism.
Fertility is adversely affected if spermatogenesis is normal but the sperm are chromosomally or morphologically abnormal or are produced in insufficient quantities. Chromosomal abnormalities are caused by genetic factors and by external variables, such as exposure to radiation or toxic substances.
Treatment for impaired spermatogenesis involves correcting any underlying disorders, avoiding radiation and toxins, and using hormones to enhance spermatogenesis. In addition, semen can be modified to improve sperm motility; modifications are followed by artificial insemination.
Epididymitis
Epididymitis, or inflammation of the epididymis, generally occurs in sexually active young males (younger than 35 years of age) and is rare before puberty. Approximately 54,200 patient encounters for epididymitis take place in Australian general practice each year.91
The pathogenic microorganism usually reaches the epididymis by ascending the ductus deferens from an already infected urethra or bladder. The main symptom is scrotal or inguinal pain caused by the resulting inflammatory response in the epididymis and surrounding tissues (see Figure 32-27). The pain is usually acute and severe. Flank pain may occur if, as the urethra passes over the spermatic cord, oedematous swelling of the cord obstructs the urethra. The individual may have pyuria, bacteriuria and a history of urinary symptoms, including urethral discharge.
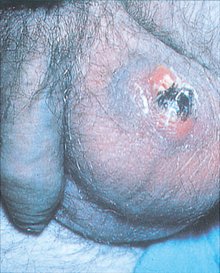
FIGURE 32-27 Epididymitis secondary to gonorrhoea or non-gonococcal urethritis.
This infection spread to the testes, and rupture through the scrotal wall is threatened.
Source: Taylor PK. Diagnostic picture tests in sexually transmitted disease. London: Mosby; 1995.
A history of recent urinary tract infections or urethral discharge suggests the diagnosis of epididymitis. Definitive diagnosis is based on culture or Gram stain of a urethral swab. Treatment includes antibiotic therapy for the infection itself and various measures to provide symptomatic relief. Complete resolution of swelling and pain may take several weeks to months. The individual’s sexual partner should be treated with antibiotics if the causative microorganism is a sexually transmitted pathogen.
Disorders of the prostate gland
Benign prostatic hyperplasia
Benign prostatic hyperplasia is the enlargement of the prostate gland (see Figure 32-28). In clinical practice it is often called benign prostatic hypertrophy, which is technically incorrect: because the major prostatic changes are caused by hyperplasia (abnormal expansion of cell numbers), not hypertrophy (normal expansion of cell size), benign prostatic hyperplasia is the preferred term. This condition becomes problematic as prostatic tissue compresses the prostatic urethra, resulting in an interrupted urine flow and increased frequency of lower urinary tract symptoms. About 80% of men will have prostatic enlargement before age 80 years and there is a 25–30% lifetime chance of needing prostatectomy for benign prostatic hyperplasia once a man reaches 50 years of age. At birth, the prostate is pea-sized and growth of the gland is gradual until puberty. At that time, there is a period of rapid development that continues until the third decade of life when the prostate reaches adult size. At about 40–45 years of age, benign hyperplasia begins and continues slowly until death.
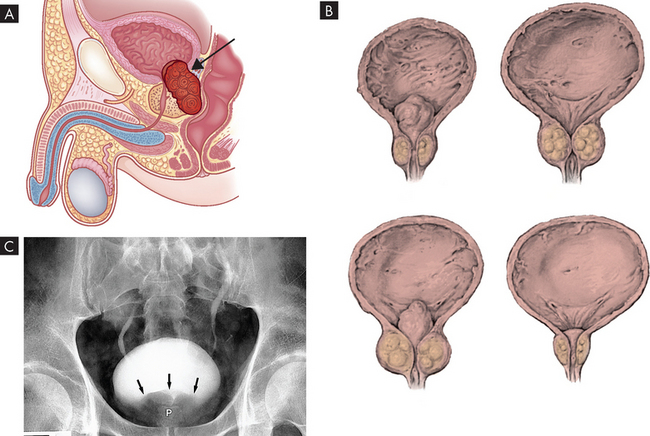
FIGURE 32-28 Benign prostatic hyperplasia.
A The condition becomes a problem as prostatic tissue compresses the urethra (arrow indicates prostate). B Gross appearance of hyperplastic prostatic tissue obstructing the prostatic urethra, forming ‘lobes’. C A view of the bladder obtained during an intravenous pyelogram shows a smooth defect impressing on the inferior aspect of the bladder (arrows) caused by a benign enlargement of the prostate (P).
Source: A & B Wein AJ. Campbell-Walsh urology. 9th edn. Philadelphia: Saunders; 2007. C Mettler FA. Essentials of radiology. 2nd edn. Philadelphia: Saunders; 2005.
Benign prostatic hyperplasia begins in the periurethral glands, which are the inner glands or layers of the prostate. The prostate enlarges as nodules form and grow (nodular hyperplasia) and glandular cells enlarge (hypertrophy). As nodular hyperplasia and cellular hypertrophy progress, tissues that surround the prostatic urethra compress it, usually but not always causing bladder outflow obstruction.
Symptoms include the urge to urinate often, some delay in starting urination and decreased force of the urinary stream. As the obstruction progresses, often over several years, the bladder cannot empty all the urine and the increasing volume leads to long-term urine retention. The volume of urine retained may be great enough to produce uncontrolled ‘overflow incontinence’ with any increase in intra-abdominal pressure. At this stage, the force of the urinary stream is significantly reduced and much more time is required to initiate and complete voiding.
Digital rectal examination and prostate-specific antigen (PSA) are conducted to determine hyperplasia. PSA density is helpful in differentiating benign prostatic hyperplasia from prostatic cancer. PSA density is calculated by dividing PSA serum levels by the volume of prostate tissue, which is determined by transrectal ultrasound. Treatment depends on the severity of symptoms, including post-void (after urination) residual urine, pressure-flow study, creatinine and blood nitrogen levels, and subjective symptoms score. Thirty per cent of men with mild to moderate symptoms improve with watchful waiting. Candidates for surgical intervention include those with post-void residual urine, severe symptoms or complications or those who fail to improve with medical therapy.
Prostatitis
Prostatitis is an inflammation of the prostate. Some degree of prostatic inflammation is present in 4–36% of the male population, increasing to 50% in older men. Inflammation is usually limited to a few of the gland’s excretory ducts. Prostatitis is characterised as one of the following:
Bacterial prostatitis
Acute bacterial prostatitis is an ascending infection of the urinary tract that tends to occur in men between the ages of 30 and 50 years but is also associated with benign prostatic hyperplasia in older men. Infection stimulates an inflammatory response in which the prostate becomes enlarged, tender, firm or boggy. The onset of prostatitis may be acute and unrelated to previous illnesses or it may follow catheterisation or cystoscopy.
Clinical manifestations of acute bacterial prostatitis are those of urinary tract infection or pyelonephritis (see Chapter 30). Sudden onset of malaise, low back and perineal pain, high fever (up to 40°C) and chills is common, as are dysuria, an inability to empty the bladder, nocturia and urinary retention. The individual may also have symptoms of lower urinary tract obstruction, such as a slow, small, ‘narrowed’ urinary stream, which may be a medical emergency. Acute inflammatory prostatic oedema can compress the urethra, causing urinary obstruction. Systemic signs of infection include sudden onset of high fever, fatigue, arthralgia and myalgia (pain in the joints and muscles, respectively). Prostatic pain may occur, especially when the individual is in an upright position, because the pelvic floor muscles tighten with standing and compression of the prostate gland occurs. Some individuals experience low back pain, painful ejaculation and rectal or perineal pain. Palpation discloses an enlarged, extremely tender and swollen prostate that is firm, indurated (hardened as a reaction to inflammation, hyperaemia or neoplastic infiltration) and warm to the touch.
Infections causing prostatitis are treated with long-term, broad-spectrum antibiotic therapy (up to 6 weeks). In severe cases, the individual is hospitalised and treated with intravenous antibiotics (aminoglycosides and ampicillin) for 7 days, followed by 4–6 weeks of oral antibiotics.
Chronic bacterial prostatitis is characterised by recurrent urinary tract symptoms and persistence of pathogenic bacteria (usually gram-negative) in urine or prostatic fluid. This form of prostatitis is the most common recurrent urinary tract infection in men. Symptoms may be similar to those of an acute bladder infection: frequency, urgency, dysuria, perineal discomfort, low back pain, myalgia, arthralgia and sexual dysfunction. The prostate may be only slightly enlarged or boggy, but it may be fibrotic because repeated infections can cause it to be firm and irregular in shape. A pelvic x-ray or transurethral ultrasound may show prostatic calculi (stones formed in the body; see Figure 32-29).
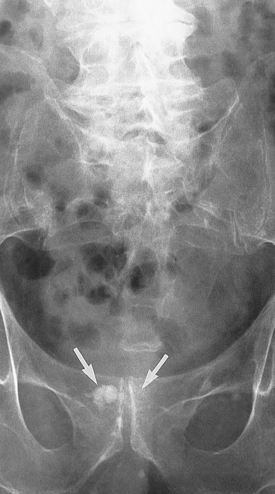
FIGURE 32-29 Prostatic calcification.
Calcification situated immediately behind the pubis (arrows) in a male patient usually represents the sequelae of previous prostatitis.
Source: Mettler FA. Essentials of radiology. 2nd edn. Philadelphia: Saunders; 2005.
The treatment of chronic bacterial prostatitis is difficult as it is often caused by prostatic calculi. Calculi harbour pathogens and, consequently, pathogens cannot be eradicated from the urinary tract. Permanent cure is achieved by surgical removal of the stones through transurethral prostatectomy, which may not be a viable option for young men. More common symptoms are tempered with chronic suppressive therapy. Comfort measures include NSAID therapy and the liberal use of sitz baths.
Non-bacterial prostatitis
Non-bacterial prostatitis is the most common prostatitis syndrome and is essentially prostatic inflammation without evidence of bacterial infection. Symptoms tend to be milder but are persistent and annoying. Presumably, non-bacterial prostatitis is caused by reflux of sterile urine into the ejaculatory ducts because of high-pressure voiding.92 Quinolones are the treatment of choice, because of their bioavailability and penetration into prostatic tissue. Drug therapy lasts for a minimum of 3–4 weeks. If symptoms do not subside, other infectious microorganisms are considered and treated accordingly.91
Men with non-bacterial prostatitis may complain of pain or a dull ache that is continuous or spasmodic in the suprapubic, infrapubic, scrotal, penile or inguinal area. Other symptoms are pain on ejaculation and urinary symptoms, such as frequency of urination. The prostate gland generally feels normal on palpation.
Non-bacterial prostatitis is a diagnosis by exclusion of bacterial presence. Digital examination of the prostate, bacterial cultures of the urogenital tract, microscopic examination of expressed prostatic fluid, urethroscopy and urodynamic studies are used to verify the diagnosis. There is no generally accepted treatment for non-bacterial prostatitis. Hot sitz baths, bed rest, alpha-blockers, anticholinergics and anti-inflammatory drugs can relieve symptoms.
Sexual dysfunction
In males, the normal sexual response involves erection, emission and ejaculation. Sexual dysfunction is the impairment of any or all of these processes and can be caused by various physiological, psychological and emotional factors. Until the late 1970s, most cases of male sexual dysfunction were considered psychogenic — that is, the origin was thought to be in the individual’s mind. Now there is evidence that some 90% of cases involve organic factors such as:
 iatrogenic factors (inadvertent adverse effects resulting from medical treatment or advice), such as surgery and pharmacological therapies.93
iatrogenic factors (inadvertent adverse effects resulting from medical treatment or advice), such as surgery and pharmacological therapies.93Sexual dysfunction can have a specific physiological cause, can be associated with many chronic diseases and their treatment, or may be related to low energy levels, stress or depression. For example, vascular disease may cause impotence and endocrine disorders or conditions that cause decreased testosterone levels or testicular atrophy can diminish sexual functioning or libido. In addition, neurological disorders and spinal cord injuries can interfere with sympathetic, parasympathetic and central nervous system mechanisms required for erection, emission and ejaculation.
Drug-induced sexual dysfunction manifests as decreased desire, decreased erectile ability or decreased ejaculatory ability. Alcohol and other central nervous system depressants, antihypertensives, antidepressants, antihistamines and hormonal preparations are commonly used drugs that affect sexual functioning. Other pharmacological agents may diminish the quality or quantity of sperm or cause priapism.
Evaluation of sexual dysfunction includes a thorough history and physical examination. Particular attention is given to drug history and examination of the genitalia, prostate and nervous system. Basic laboratory tests are used to identify the presence of endocrinopathies or other underlying disorders that can cause dysfunction. Psychological evaluation is indicated for younger men with a sudden onset of sexual dysfunction or men of any age who can achieve but not maintain an erection. If no physiological cause is found and the condition does not improve with psychotherapy, the man is referred for further investigation of organic causes.
Treatments for organic sexual dysfunction include both medical and surgical approaches. The drug Viagra (sildenafil) has created much enthusiasm for its ability to help a man maintain an erection. For a small percentage of men (1%), however, this improvement in sexual function is accompanied by myocardial infarction and sudden death. Whether these effects are the result of sexual performance or Viagra has been controversial. Non-surgical approaches include correction of underlying disorders, particularly drug-induced dysfunction and endocrinopathy-related dysfunction (such as reduced testosterone associated with chronic renal failure). Vasodilators and cessation of smoking can benefit individuals with erectile dysfunction arising from vascular origins. Surgical approaches include penile implants, penile revascularisation and correction of other anatomical defects contributing to sexual dysfunction.
DISORDERS OF THE BREAST
Disorders of the female breast
Galactorrhoea (inappropriate lactation) is the persistent and sometimes excessive secretion of a milky fluid from one or both breasts of non-pregnant, non-lactating women. It has been defined as ‘nipple discharge’.94 Galactorrhoea, which can also occur in men, may involve one or both breasts and is not associated with breast cancer.95
Galactorrhoea is not a breast disorder but, rather, a manifestation of pathophysiological processes elsewhere in the body. These processes are chiefly hormone imbalances caused by hypothalamic-pituitary disturbances, pituitary tumours or neurological damage. Exogenous causes include drugs, oestrogen (for example, in oral contraceptives) and manipulation of the nipples.
Most women with galactorrhoea experience menstrual abnormality. If a pituitary process is involved, the woman usually experiences hirsutism and infertility; if a hypothalamic lesion is present, she may report central nervous system symptoms, such as intractable headache, visual field disturbances, sleep disturbances and abnormal temperature, thirst or appetite.96
Disorders of the male breast
Gynaecomastia is the overdevelopment of breast tissue in a male and accounts for approximately 85% of all masses that develop in the male breast. If only one breast is involved, it is typically the left. Incidence is greatest among adolescents and men older than 50 years. Gynaecomastia results from hormonal alterations, which may be idiopathic or caused by systemic disorders, drugs or neoplasms. It is usually caused by an imbalance of the oestrogen/testosterone ratio. The normal oestrogen/testosterone ratio can be altered in one of two ways:
Gynaecomastia can also be caused by increased or decreased sensitivity of breast tissue to hormone levels. Breast tissue may have increased responsiveness to oestrogen or decreased responsiveness to androgen. Alterations of responsiveness may cause many cases of idiopathic gynaecomastia. Systemic disorders associated with gynaecomastia include cirrhosis of the liver, infectious hepatitis, chronic renal failure, chronic obstructive lung disease, hyperthyroidism, tuberculosis and chronic malnutrition. It may be that these disorders ultimately alter the oestrogen/testosterone ratio, initiating the gynaecomastia.
The diagnosis of gynaecomastia is based on physical examination. Identification and treatment of the cause are likely to be followed by resolution of the gynaecomastia. The man should be taught to perform breast self-examination and is re-examined at 6- and 12-month intervals if the gynaecomastia persists.
IMPAIRED FERTILITY
Infertility affects approximately 15% of all couples and is defined as the inability to conceive after 1 year of unprotected intercourse. Fertility can be impaired by factors in either the man or the woman, or both partners.
Male factors include diminished quality and production of sperm (see Table 32-6). Causes include infections or inflammation, endocrine or hormonal disorders, immunological problems in which men produce antibodies to their own sperm, and environmental or lifestyle factors. Female infertility factors are associated with malfunctions of the fallopian tubes, the ovaries or the reproductive hormones. Adhesions from pelvic infection may cause blockage of one or both fallopian tubes, preventing the sperm from accessing the ovum. Hormonal or local factors may disrupt ovulation or prevent a fertilised egg from implanting. Endometriosis may also contribute to infertility.
Having an understanding of the normal structure and function of the male and female reproductive systems will help you to grasp the reasons for impaired fertility. Male and female factors are equally responsible: data from the Infertility Treatment Authority indicate that about 40% of the time the cause is one of the male factors, 40% of the time it is one of the female factors, 10% of the time it involves both male and female factors and 10% of the time it is idiopathic.97
A number of diagnostic procedures are required in the routine investigation of the infertile couple. Initial procedures include semen analysis and determination of ovulation. Treatment is aimed towards correcting problems identified during diagnosis. Each couple is different and the specific cause of the infertility must be assessed by health professionals. However, altering lifestyle choices such as giving up smoking (which negatively impacts on spermatogenesis) or reducing kilojoule intake and exercising more to reduce weight (obesity negatively impacts on spermatogenesis and oogenesis) could have significant benefits for some couples. In addition, immediate treatment of a sexually transmitted infection will help prevent scarring and the formation of adhesions in the reproductive tract of either the man or the woman and so reduce the chances of impaired fertility developing as a consequence of the infection.
ASSISTED REPRODUCTIVE TECHNOLOGIES
Assisted reproductive technology is a general term referring to methods used to achieve pregnancy by artificial or partially artificial means. Assisted reproductive technologies are increasing in both number and success as we understand more about the processes of fertilisation and implantation. Knowledge of hormonal control of the ovarian and menstrual cycles and spermatogenesis, combined with understanding of the anatomical aspects of both men and women (see Chapter 31), is leading to the development of new technologies and the refinement of existing technologies.
In vitro fertilisation (IVF) is the original method of assisted reproductive technology pioneered in Australia in the late 1970s, although the technology has improved since then. In IVF, the oocyte is collected by manipulating the female cycle with hormonal (gonadotropin) injections designed to cause multiple oocyte proliferation, and sperm are collected from the male. One isolated sperm and oocyte are then brought together in an artificial vessel for fertilisation to occur; when this has been achieved, the resultant embryo is implanted into the female. Obviously, the uterus must be made receptive to implantation and this is where an understanding of the ovarian and menstrual cycles has proved invaluable.
A common treatment for infertility involves the use of fertility medications (based on the gonadotropins) to stimulate the female’s ovaries to develop multiple eggs. This is followed by an injection of hCG (human chorionic gonadotropin) to release the oocytes and then transvaginal aspiration to collect them (termed transvaginal ovum retrieval). The male is required to donate sperm, the quality of which is assessed by microscopic examination. The examination looks at both the quantity and the quality of sperm produced. There are a variety of abnormal morphologies (shapes) of sperm and this could be the cause of infertility in the male. If this is the case, healthy individual sperm can be isolated and injected into the oocyte (termed intracytoplasmic sperm injection). If the male is not producing enough sperm, which could be the cause of the infertility, intracytoplasmic sperm injection can be performed or the sperm can be concentrated and IVF carried out.
The zygote (the result of successful fertilisation) is incubated until the 4- or 8-cell stage blastocyst develops. Before transferring the blastocyst to the female, cells are taken for pre-implantation genetic diagnosis if there is a likelihood of a problem. Pre-implantation genetic diagnosis is currently used for two principal reasons:
 to screen for aneuploidy (an abnormal chromosome number) in the gametes of those couples who have experienced recurrent failure using assisted reproductive technologies or recurrent miscarriage, or who are of advanced age, are known carriers of chromosomal rearrangements or have a previous history of fetal aneuploidy
to screen for aneuploidy (an abnormal chromosome number) in the gametes of those couples who have experienced recurrent failure using assisted reproductive technologies or recurrent miscarriage, or who are of advanced age, are known carriers of chromosomal rearrangements or have a previous history of fetal aneuploidy to test for single gene disorders (X-linked, autosomal dominant or autosomal recessive; see Chapter 37) in those couples with a family history or who have previously had an affected child.
to test for single gene disorders (X-linked, autosomal dominant or autosomal recessive; see Chapter 37) in those couples with a family history or who have previously had an affected child.Other uses of intracytoplasmic sperm injection — such as sex selection and tissue-matching for bone marrow donation — must be approved by the relevant authority.
The choice of transfer site in the female is crucial for successful implantation. For example, in zygote intrafallopian transfer, the zygote is transferred to the fallopian tube and allowed to make its way naturally to the uterus for implantation. If the cause of infertility is idiopathic, then gametes collected can be co-introduced into the fallopian tube where fertilisation takes place more or less naturally and the zygote travels along the fallopian tube to the uterus where implantation takes place. This is termed gamete intrafallopian transfer.
A couple can use their own gametes, but third-party conception is possible with donor procedures (donated oocytes, sperm or embryos) or surrogate arrangements. In New Zealand and Victoria, children conceived as a result of such procedures have the right to be told the identity of the donor (biological) parents when they turn 18 years of age.
Gametes and embryos can be stored (frozen) but storage is regulated under state legislation. Time limits are imposed (5 years for embryos, 10 years for gametes) to ensure that those who produce the gametes and embryos retain the responsibility for decision making as to the fate of those gametes and embryos.
Although scientific progress has led to a huge improvement in the success of assisted reproductive technologies, the science is not the only issue that society has to deal with in this field. This success has opened up the possibilities of surrogacy, genetic screening and single (younger or older) individuals having offspring, and it challenges the current cultural and ethical values of many in society.
MAJOR SEXUALLY TRANSMITTED INFECTIONS
Infectious diseases of the reproductive system are often transmitted through sexual activity. About 70,000 cases of sexually transmitted infections are reported each year in Australia and approximately 10,000 cases are reported in New Zealand — including HIV, the virus that can cause AIDS. However, reporting of sexually transmitted infections is not mandatory in Australia or New Zealand, so the actual number of cases may be higher. In fact, the number may be even higher still as many people who have had unprotected sex have not been tested for infection.98
A wide range of organisms, such as bacteria, viruses, fungi and protozoa, may cause sexually transmitted infections, the effects of which range from asymptomatic to lethal. The pathogens of many sexually transmitted infections do not survive well outside their hosts, thus intimate contact is usually required for infection to occur. Fomites such as toilet seats are not likely sources of infection!
The current increase in the severity and incidence of sexually transmitted infections can be attributed to demographic, lifestyle and behavioural factors. First, indulgence in high-risk sexual behaviours and poor health habits, such as failure to use a condom, non-monogamous relationships, drug use and douching, increases an individual’s risk of exposure or the severity of infection if exposed. Second, many infected individuals do not seek treatment because symptoms are absent, minor or transient. Finally, a rise in the number of single or never-married individuals, involvement in premarital or extramarital sexual affairs and bisexuality contribute to an increase in the number of lifetime sexual partners and the increased exposure to sexually transmitted infections. Perhaps partly as a result of risk-taking behaviour, adolescents tend to be at highest risk for sexually transmitted infections.
The following points should be appreciated.
 Infections may be transmitted by means other than sexual activity, blood–blood contact being the most important (for example, HIV infection).
Infections may be transmitted by means other than sexual activity, blood–blood contact being the most important (for example, HIV infection). Sexual contact with sites outside the reproductive system — such as the mouth or anus — may cause infection and disease of those areas (for example, pharyngeal or rectal gonorrhoea).
Sexual contact with sites outside the reproductive system — such as the mouth or anus — may cause infection and disease of those areas (for example, pharyngeal or rectal gonorrhoea). Sexually transmitted infections can affect organs and systems in addition to the reproductive system — for example, syphilis may affect the nervous system and HIV affects the immune system.
Sexually transmitted infections can affect organs and systems in addition to the reproductive system — for example, syphilis may affect the nervous system and HIV affects the immune system. Long-term sequelae of untreated or undertreated sexually transmitted infections may be disastrous and can impact a person’s physical, emotional and financial wellbeing.
Long-term sequelae of untreated or undertreated sexually transmitted infections may be disastrous and can impact a person’s physical, emotional and financial wellbeing. Apart from considerations of sexual behaviour, control of sexually transmitted infections in the community is difficult because many infections may be asymptomatic for extended periods and so may be spread ‘silently’ by carriers; and different infections may occur together in the one host, with one infection facilitating transmission of another — for example, infections that cause open sores provide portals of entry for HIV.
Apart from considerations of sexual behaviour, control of sexually transmitted infections in the community is difficult because many infections may be asymptomatic for extended periods and so may be spread ‘silently’ by carriers; and different infections may occur together in the one host, with one infection facilitating transmission of another — for example, infections that cause open sores provide portals of entry for HIV. Fortunately, barrier methods of contraception, especially the careful use of condoms, are effective in preventing the spread of sexually transmitted infections.
Fortunately, barrier methods of contraception, especially the careful use of condoms, are effective in preventing the spread of sexually transmitted infections.We now look at some of the more common sexually transmitted infections — see also Table 32-7 and the images that follow the table.
Table 32-7 MAJOR SEXUALLY TRANSMITTED INFECTIONS
Gonorrhoea
Gonorrhoea is caused by the gram-negative coccus, Neisseria gonorrhoeae (gonococcus). The bacteria attach by pili to epithelial cells in the mucous membrane of the urethra (males) or cervix (females), multiply and ascend into deeper tissue layers. In this process, they resist, by means of their pili, the tendency of urine or vaginal secretions to wash them away and defend themselves against immunoglobulin A (IgA) of the mucosal surfaces by producing an enzyme (IgA protease) that breaks down this antibody. Tissue damage and inflammation ensue and symptoms usually develop within a few days of infection.
In females, infection is often mild or asymptomatic. In symptomatic infection, vaginal discharge occurs and pain on urination (dysuria) or intercourse (dyspareunia) is common. Even when infection appears to be asymptomatic, inflammation of the fallopian tubes may occur. Inflammation of the tubes (salpingitis) and other pelvic sites such as the ovaries is termed pelvic inflammatory disease. Damage to the fallopian tubes resulting from such infection can lead to infertility or ectopic pregnancy. Other pathogens, notably Chlamydia trachomatis, can also cause pelvic inflammatory disease. In males, infection may be mild or asymptomatic, but it usually causes urethral discharge with dysuria. The diagnosis of gonorrhoea is made from culture of appropriate specimens (for example, discharge) and microscopic identification of intracellular gram-negative cocci. People with gonorrhoea may be suffering concurrently from other sexually transmitted infections. Infection can be treated with antibiotics.
Gonorrhoea can be transmitted from a mother to her baby (congenital infection). This occurs during passage of the baby through the birth canal and usually results in a characteristic eye infection (ophthalmic gonorrhoea).
Syphilis
Syphilis is caused by the spirochaete (spiral-shaped bacterium) Treponema pallidum. It is transmitted from lesions in the skin or mucous membranes of infected people to mucous membranes or small skin lesions in the recipient. This pathogen grows more slowly than Neisseria gonorrhoeae and its incubation period is longer (average 3 weeks). If untreated, the disease typically follows a course of 3 stages (but this is not seen in all cases):
 Primary syphilis: 2–10 weeks after infection, a lesion develops at the site of infection (penis or cervix) and develops into a painless hard chancre. These lesions contain large numbers of bacteria and are highly infectious. They result from the response of neutrophils (and later lymphocytes and macrophages) to replicating bacterial cells at the site of entry. Chancres appear to heal spontaneously within a few weeks. In 50% of cases, the disease progresses to secondary syphilis.
Primary syphilis: 2–10 weeks after infection, a lesion develops at the site of infection (penis or cervix) and develops into a painless hard chancre. These lesions contain large numbers of bacteria and are highly infectious. They result from the response of neutrophils (and later lymphocytes and macrophages) to replicating bacterial cells at the site of entry. Chancres appear to heal spontaneously within a few weeks. In 50% of cases, the disease progresses to secondary syphilis. Secondary syphilis: some weeks later, the bacteria have spread from the site of entry through local lymph nodes to the blood. A skin rash, which is itself infectious, may appear anywhere on the body, accompanied by malaise and mild fever. In addition to the skin, many other organs may be involved, such as the liver, joints or muscles. These symptoms, like those of primary syphilis, disappear spontaneously.
Secondary syphilis: some weeks later, the bacteria have spread from the site of entry through local lymph nodes to the blood. A skin rash, which is itself infectious, may appear anywhere on the body, accompanied by malaise and mild fever. In addition to the skin, many other organs may be involved, such as the liver, joints or muscles. These symptoms, like those of primary syphilis, disappear spontaneously. Tertiary syphilis: after an asymptomatic period of years, renewed growth of bacteria and, more importantly, the development of cell-mediated hypersensitivity cause characteristic lesions in a number of organs. These lesions are soft granulomas termed gummas. Invasion and tissue damage of the aorta and nervous system are particularly significant. Damage to the thoracic section of the aorta may lead to aneurysm and rupture. Damage to the nervous system may cause dementia, paresis (muscle weakness or paralysis) or tabes dorsalis (poor muscle coordination and unstable gait).
Tertiary syphilis: after an asymptomatic period of years, renewed growth of bacteria and, more importantly, the development of cell-mediated hypersensitivity cause characteristic lesions in a number of organs. These lesions are soft granulomas termed gummas. Invasion and tissue damage of the aorta and nervous system are particularly significant. Damage to the thoracic section of the aorta may lead to aneurysm and rupture. Damage to the nervous system may cause dementia, paresis (muscle weakness or paralysis) or tabes dorsalis (poor muscle coordination and unstable gait).Many aspects of the pathogenesis of syphilis remain unclear. It is not known, for example, how lesions develop or resolve or how host defence responses give rise to the symptoms of tertiary syphilis. Syphilis is diagnosed by microscopic identification of Treponema pallidum from primary or secondary lesions. Special microscopic techniques are required, as the organism is too thin to be seen in a standard gram-stained preparation. If lesions are not present, serological testing of blood can be performed. Infection is treated with antibiotics.
Syphilis can be transmitted from a mother to her baby through the placenta, and it can cause malformation of the developing baby.
Chlamydia trachomatis
Chlamydia trachomatis is an unusual, small bacterium that replicates only within host cells. It has only recently been shown to cause urethritis. Some varieties cause trachoma, an eye infection that can lead to blindness, while others cause genital infections. Chlamydia trachomatis is extremely common — perhaps the most common of all sexually transmitted infections — and many of the resulting infections are asymptomatic. Infection in males may cause urethritis. In females, symptomatic infection may be evident as cervicitis (inflammation of the cervix) or as salpingitis and pelvic inflammatory disease. These symptoms are similar to those of other sexually transmitted infections such as those caused by Neisseria gonorrhoeae (which may indeed be concurrent with the chlamydial infection). Therefore, laboratory examination of specimens is required for diagnosis. Chlamydial infection can be treated with antibiotics.
Chlamydia trachomatis can infect the baby during birth, causing eye disease and sometimes pneumonia.
Herpes simplex virus
There are two major types of herpes simplex virus (HSV):
HSV2 is transmitted by contact with mucosal surfaces or through secretion of infected people. It is able to invade intact mucous surfaces and minimally damaged skin. Infection may in many cases be asymptomatic. Symptomatic infection is characterised by the development of small vesicles filled with clear fluid on the genital or anal region and surrounded by an area of inflammation. The vesicles break down, leaving painful ulcers; while these lesions heal, the virus itself travels up the local sensory nerve to the sensory (dorsal) root ganglion. There it may persist for life, ‘hidden’ from the body’s defences. At times it may reactivate, travel back down the nerve and produce new lesions. The frequency of such recurrent attacks varies greatly.
Carriers of the virus are infectious when lesions are present but may also be infectious when asymptomatic. HSV2 infection is diagnosed when the virus can be cultured from vesicle fluid or other samples. There is no cure for HSV infection although acute outbreaks can be managed with the use of antiviral drugs such as acyclovir. Safe sex practices are required to prevent transmission.
HSV2 can be transmitted from a mother to her baby before, during or after birth. This may have severe consequences for the baby such as mental retardation, blindness or deafness or it may be fatal to the baby. For this reason, delivery by caesarean section may be recommended if lesions are present.
Human papillomavirus
Papillomaviruses cause warts of the skin or mucous membranes. Many varieties are known, of which a restricted number are regularly associated with genital infections. Genital or anal warts caused by these viruses may appear after an incubation period of months and then disappear within 1–2 years. Asymptomatic infection is also very common. The major significance of human papillomavirus (HPV) is that certain varieties are strongly associated with cervical cancer. Damage to cervical cells may occur even where warts are not present; this damage can often be detected in a Pap smear. A vaccine, Gardasil™, is now available to young women to prevent HPV infection and reduce the risk of cervical cancer (see Chapter 36).
Human immunodeficiency virus
The human immunodeficiency virus (HIV) is the infective agent and cause of the fatal disease known as AIDS (acquired immunodeficiency syndrome). An estimated 17,444 people were living with HIV in Australia at the end of 2008. Up until the end of 2008, there had been 28,330 diagnoses of HIV and 10,348 diagnoses of AIDS in Australia, with 6765 AIDS-related deaths.99
HIV is transmitted through direct contact of a mucous membrane or the bloodstream with a body fluid containing HIV, such as blood, semen, vaginal fluid, preseminal fluid or breast milk.100 The three main transmission routes of HIV are (1) sexual contact, (2) exposure to infected body fluids or tissues and (3) from mother to fetus or child during the perinatal period. The virus infects the cells of the immune system that are normally responsible for fighting infections (CD4+ T lymphocytes). Over time, infection of these cells leads to their death and thus the individual’s immune system is compromised and cannot fight other infections. The individual is essentially immunocompromised and as a result acquires infections and tumours (opportunistic infections) that would not normally infect a person with a healthy immune system (see Chapter 15).
A person infected with HIV passes through stages of infection.
 The first stage lasts for a few weeks and is often accompanied by flu-like symptoms as the virus rapidly replicates and the body produces anti-HIV antibodies and cytotoxic lymphocytes. This process is known as seroconversion. If an HIV antibody test is done before seroconversion is complete, it may not be positive (that is, the virus may not be detected).
The first stage lasts for a few weeks and is often accompanied by flu-like symptoms as the virus rapidly replicates and the body produces anti-HIV antibodies and cytotoxic lymphocytes. This process is known as seroconversion. If an HIV antibody test is done before seroconversion is complete, it may not be positive (that is, the virus may not be detected). The second stage can last for 10 years or more and is generally asymptomatic. HIV is not dormant during this stage, but is very active in the lymph nodes. A test is available to measure the small amount of HIV that escapes the lymph nodes. This test, which measures HIV RNA (HIV genetic material), is referred to as the viral load test and it has an important role in the treatment of HIV infection.99
The second stage can last for 10 years or more and is generally asymptomatic. HIV is not dormant during this stage, but is very active in the lymph nodes. A test is available to measure the small amount of HIV that escapes the lymph nodes. This test, which measures HIV RNA (HIV genetic material), is referred to as the viral load test and it has an important role in the treatment of HIV infection.99 In the third stage the viral load is very high and the number of CD4+ T lymphocytes is so low that the individual begins to acquire opportunistic infections. As this progresses and the immune system becomes more compromised the diagnosis of AIDS is made and the individual suffers more debilitating illnesses from the opportunistic infections.
In the third stage the viral load is very high and the number of CD4+ T lymphocytes is so low that the individual begins to acquire opportunistic infections. As this progresses and the immune system becomes more compromised the diagnosis of AIDS is made and the individual suffers more debilitating illnesses from the opportunistic infections.Although treatments for AIDS and HIV can slow the course of the disease, there is currently no vaccine or cure and AIDS is ultimately fatal. Antiretroviral treatment reduces both the mortality and the morbidity of HIV infection, but these drugs are expensive and routine access to such medication is not available in all countries.101 Due to the difficulty in treating HIV infection, preventing infection is a key aim in controlling the AIDS pandemic, with health organisations promoting safe sex and needle-exchange programs in an attempt to slow the spread of the virus.
Other infections
Nonspecific urethritis in males is urethral inflammation other than that caused by Neisseria gonorrhoeae or Chlamydia trachomatis. The symptoms are urethral discharge and dysuria, similar to those of gonorrhoea.
Vaginosis is vaginal discharge, with or without inflammation of the vaginal mucosa (vaginitis). It may result from opportunistic infection by a variety of microbes that normally form part of the flora of this part of the body. The healthy vagina is acidic with a mixed flora of bacteria and fungi. The major component is Lactobacillus, a bacterium that ferments glycogen to produce acid. If the acidity of the vagina is reduced, minor components of the flora may overgrow to cause disease. The usual reason for such a change is disturbance of the normal flora, especially Lactobacillus, by antibiotic treatment for a problem elsewhere in the body or by immunosuppression, pregnancy or diabetes. Disease symptoms are discharge and often soreness or itchiness, and there may follow the overgrowth of bacteria such as Gardnerella vaginalis or fungi such as Candida albicans (commonly known as thrush). Diagnosis may be made by microscopic examination of the discharge. Treatment differs according to the causative agent. It may also be necessary to treat sexual partners of the affected woman.
Classification of reproductive system alterations
 The most common causes of sexual dysfunction are: (1) growths, (2) problems associated with the endocrine system, and (3) structural and functional alterations of the reproductive system.
The most common causes of sexual dysfunction are: (1) growths, (2) problems associated with the endocrine system, and (3) structural and functional alterations of the reproductive system. Growths within the reproductive system can be benign, pre-cancerous or cancerous. The growths themselves can be the problem or the effects of the growths can be the problem.
Growths within the reproductive system can be benign, pre-cancerous or cancerous. The growths themselves can be the problem or the effects of the growths can be the problem.Cancer
 Breast cancer is the most prevalent cancer in women. The major risk factors for breast cancer are reproductive factors, such as nulliparity; hormonal factors and growth factors, such as excessive oestradiol; familial factors, such as a family history of breast cancer; and environmental factors, such as ionising radiation. Physical activity and human chorionic gonadotropin hormone may be protective factors.
Breast cancer is the most prevalent cancer in women. The major risk factors for breast cancer are reproductive factors, such as nulliparity; hormonal factors and growth factors, such as excessive oestradiol; familial factors, such as a family history of breast cancer; and environmental factors, such as ionising radiation. Physical activity and human chorionic gonadotropin hormone may be protective factors. Most breast cancers arise from the ductal epithelium and may metastasise to the lymphatics, opposite breast, abdominal cavity, lungs, bones, kidneys, liver, adrenal glands, ovaries and pituitary glands.
Most breast cancers arise from the ductal epithelium and may metastasise to the lymphatics, opposite breast, abdominal cavity, lungs, bones, kidneys, liver, adrenal glands, ovaries and pituitary glands. Most cancers of the female genitalia involve the uterus (particularly the endometrium), the cervix and the ovaries. Cancer of the vagina is rare. Cervical cancer arises from the cervical epithelium and is triggered by human papillomavirus (HPV).
Most cancers of the female genitalia involve the uterus (particularly the endometrium), the cervix and the ovaries. Cancer of the vagina is rare. Cervical cancer arises from the cervical epithelium and is triggered by human papillomavirus (HPV). Prostate cancer is the most prevalent cancer in men. Even at an early stage, it results in impaired urine flow and sperm transport. Routine screening is recommended for early detection of disease.
Prostate cancer is the most prevalent cancer in men. Even at an early stage, it results in impaired urine flow and sperm transport. Routine screening is recommended for early detection of disease.Disorders of the female reproductive system
 The female reproductive system can be altered by benign or malignant proliferative conditions, hormonal imbalances, infectious microorganisms, inflammation and structural abnormalities.
The female reproductive system can be altered by benign or malignant proliferative conditions, hormonal imbalances, infectious microorganisms, inflammation and structural abnormalities. Benign ovarian cysts develop from mature ovarian follicles that do not release their ova (follicular cysts) or from a corpus luteum that persists abnormally instead of degenerating (corpus luteum cysts). Cysts usually regress spontaneously.
Benign ovarian cysts develop from mature ovarian follicles that do not release their ova (follicular cysts) or from a corpus luteum that persists abnormally instead of degenerating (corpus luteum cysts). Cysts usually regress spontaneously. Endometrial polyps consist of overgrowths of endometrial tissue; they often cause abnormal bleeding in the premenopausal woman.
Endometrial polyps consist of overgrowths of endometrial tissue; they often cause abnormal bleeding in the premenopausal woman. Leiomyomas, also called uterine fibroids, are benign tumours arising from the muscle layer of the uterus, the myometrium.
Leiomyomas, also called uterine fibroids, are benign tumours arising from the muscle layer of the uterus, the myometrium. Endometriosis is the presence of functional endometrial tissue (i.e. tissue that responds to hormonal stimulation) at sites outside the uterus. Endometriosis causes an inflammatory reaction at the site of implantation and is a cause of infertility.
Endometriosis is the presence of functional endometrial tissue (i.e. tissue that responds to hormonal stimulation) at sites outside the uterus. Endometriosis causes an inflammatory reaction at the site of implantation and is a cause of infertility. Polycystic ovary syndrome is a condition in which excessive androgen production is triggered by inappropriate secretion of gonadotropins. This hormonal imbalance prevents ovulation and causes enlargement and cyst formation in the ovaries, excessive endometrial proliferation and often hirsutism. Hyperinsulinaemia plays a key role in androgen excess.
Polycystic ovary syndrome is a condition in which excessive androgen production is triggered by inappropriate secretion of gonadotropins. This hormonal imbalance prevents ovulation and causes enlargement and cyst formation in the ovaries, excessive endometrial proliferation and often hirsutism. Hyperinsulinaemia plays a key role in androgen excess. Primary dysmenorrhoea is painful menstruation not associated with pelvic disease. It results from excessive synthesis of prostaglandin F. Secondary dysmenorrhoea results from endometriosis, pelvic adhesions, inflammatory disease, uterine fibroids or adenomyosis.
Primary dysmenorrhoea is painful menstruation not associated with pelvic disease. It results from excessive synthesis of prostaglandin F. Secondary dysmenorrhoea results from endometriosis, pelvic adhesions, inflammatory disease, uterine fibroids or adenomyosis. Primary amenorrhoea is the continued absence of menarche and menstrual function by 14 years of age without the development of secondary sex characteristics or by 16 years of age if these changes have occurred.
Primary amenorrhoea is the continued absence of menarche and menstrual function by 14 years of age without the development of secondary sex characteristics or by 16 years of age if these changes have occurred. Secondary amenorrhoea is the absence of menstruation for a time equivalent to more than 3 cycles or 6 months in women who have previously menstruated. Secondary amenorrhea is associated with anovulation.
Secondary amenorrhoea is the absence of menstruation for a time equivalent to more than 3 cycles or 6 months in women who have previously menstruated. Secondary amenorrhea is associated with anovulation. Dysfunctional uterine bleeding is heavy or irregular bleeding caused by a disturbance of the menstrual cycle.
Dysfunctional uterine bleeding is heavy or irregular bleeding caused by a disturbance of the menstrual cycle. Premenstrual syndrome is the cyclic recurrence of physical, psychological or behavioural changes distressing enough to disrupt normal activities or interpersonal relationships. Emotional symptoms — particularly depression, anger, irritability and fatigue — are reported as the most distressing symptoms; physical symptoms tend to be less problematic. Treatment is symptomatic and includes self-help techniques, lifestyle changes, counselling and medication.
Premenstrual syndrome is the cyclic recurrence of physical, psychological or behavioural changes distressing enough to disrupt normal activities or interpersonal relationships. Emotional symptoms — particularly depression, anger, irritability and fatigue — are reported as the most distressing symptoms; physical symptoms tend to be less problematic. Treatment is symptomatic and includes self-help techniques, lifestyle changes, counselling and medication. Infection and inflammation of the female genitalia can result from microorganisms from the environment or overproliferation of microorganisms that normally populate the genital tract.
Infection and inflammation of the female genitalia can result from microorganisms from the environment or overproliferation of microorganisms that normally populate the genital tract. Pelvic inflammatory disease is an acute ascending infection of the upper genital tract caused by a sexually transmitted pathogen. If untreated, it can lead to infertility.
Pelvic inflammatory disease is an acute ascending infection of the upper genital tract caused by a sexually transmitted pathogen. If untreated, it can lead to infertility. Vaginitis, a vaginal infection, is usually caused by sexually transmitted pathogens or Candida albicans, which causes candidiasis.
Vaginitis, a vaginal infection, is usually caused by sexually transmitted pathogens or Candida albicans, which causes candidiasis. Cervicitis, which is infection of the cervix, can be acute (mucopurulent cervicitis) or chronic. Its most common cause is a sexually transmitted pathogen.
Cervicitis, which is infection of the cervix, can be acute (mucopurulent cervicitis) or chronic. Its most common cause is a sexually transmitted pathogen. Vulvitis is an inflammation of the skin of the vulva. It can be caused by chemical irritants, allergens, skin disorders, irritation from tight-fitting clothing or spread of vaginal infections such as candidiasis.
Vulvitis is an inflammation of the skin of the vulva. It can be caused by chemical irritants, allergens, skin disorders, irritation from tight-fitting clothing or spread of vaginal infections such as candidiasis. Bartholinitis is an infection of the ducts that lead from the Bartholin’s glands to the surface of the vulva. Infection blocks the glands, preventing the outflow of glandular secretions.
Bartholinitis is an infection of the ducts that lead from the Bartholin’s glands to the surface of the vulva. Infection blocks the glands, preventing the outflow of glandular secretions. The pelvic relaxation disorders — uterine displacement, uterine prolapse, cystocele, rectocele and urethrocele — are caused by the relaxation of muscles and fascial supports, usually with age or after childbirth or other trauma, and are more likely to occur in women with a familial or genetic predisposition.
The pelvic relaxation disorders — uterine displacement, uterine prolapse, cystocele, rectocele and urethrocele — are caused by the relaxation of muscles and fascial supports, usually with age or after childbirth or other trauma, and are more likely to occur in women with a familial or genetic predisposition.Disorders of the male reproductive system
 Disorders of the urethra include urethritis (infection of the urethra) and urethral strictures (narrowing or obstruction of the urethral lumen caused by scarring).
Disorders of the urethra include urethritis (infection of the urethra) and urethral strictures (narrowing or obstruction of the urethral lumen caused by scarring). Most cases of urethritis result from sexually transmitted pathogens. Urethritis causes urinary symptoms, including a burning sensation during urination (dysuria), frequency, urgency, urethral tingling or itching, and clear or purulent discharge.
Most cases of urethritis result from sexually transmitted pathogens. Urethritis causes urinary symptoms, including a burning sensation during urination (dysuria), frequency, urgency, urethral tingling or itching, and clear or purulent discharge. The scarring that causes urethral stricture can be caused by trauma or by severe untreated urethritis. Manifestations of urethral stricture include those of bladder outlet obstruction: urinary frequency and hesitancy, diminished force and calibre of the urinary stream, dribbling after voiding and nocturia.
The scarring that causes urethral stricture can be caused by trauma or by severe untreated urethritis. Manifestations of urethral stricture include those of bladder outlet obstruction: urinary frequency and hesitancy, diminished force and calibre of the urinary stream, dribbling after voiding and nocturia. Phimosis and paraphimosis are penile disorders involving the foreskin (prepuce). In phimosis, the foreskin cannot be retracted over the glans. It is caused by poor hygiene and chronic infection and can lead to paraphimosis. In paraphimosis, the foreskin is retracted and cannot be reduced (returned to its normal anatomic position over the glans). It can constrict the penile blood vessels, preventing circulation to the glans.
Phimosis and paraphimosis are penile disorders involving the foreskin (prepuce). In phimosis, the foreskin cannot be retracted over the glans. It is caused by poor hygiene and chronic infection and can lead to paraphimosis. In paraphimosis, the foreskin is retracted and cannot be reduced (returned to its normal anatomic position over the glans). It can constrict the penile blood vessels, preventing circulation to the glans. Peyronie’s disease consists of fibrosis affecting the corpora cavernosa, which causes penile curvature during erection. Fibrosis prevents engorgement on the affected side, causing a lateral curvature that can prevent intercourse.
Peyronie’s disease consists of fibrosis affecting the corpora cavernosa, which causes penile curvature during erection. Fibrosis prevents engorgement on the affected side, causing a lateral curvature that can prevent intercourse. Priapism is a prolonged, painful erection that is not stimulated by sexual arousal. The corpora cavernosa (but not the corpus spongiosum) fill with blood that does not drain out, probably because of venous obstruction. Priapism is associated with spinal cord trauma, sickle cell disease, leukaemia and pelvic tumours. It can also be idiopathic.
Priapism is a prolonged, painful erection that is not stimulated by sexual arousal. The corpora cavernosa (but not the corpus spongiosum) fill with blood that does not drain out, probably because of venous obstruction. Priapism is associated with spinal cord trauma, sickle cell disease, leukaemia and pelvic tumours. It can also be idiopathic. A varicocele is an abnormal dilation of the veins within the spermatic cord caused by either congenital absence of valves in the internal spermatic vein or acquired valvular incompetence.
A varicocele is an abnormal dilation of the veins within the spermatic cord caused by either congenital absence of valves in the internal spermatic vein or acquired valvular incompetence. A hydrocele is a collection of fluid between the testicular and scrotal layers of the tunica vaginalis. Hydroceles can be idiopathic or caused by trauma or infection of the testes.
A hydrocele is a collection of fluid between the testicular and scrotal layers of the tunica vaginalis. Hydroceles can be idiopathic or caused by trauma or infection of the testes. A spermatocele is a cyst located between the testis and epididymis that is filled with fluid and sperm.
A spermatocele is a cyst located between the testis and epididymis that is filled with fluid and sperm. Cryptorchidism is a congenital condition in which one or both testes fail to descend into the scrotum. Uncorrected cryptorchidism is associated with infertility and significantly increased risk of testicular cancer.
Cryptorchidism is a congenital condition in which one or both testes fail to descend into the scrotum. Uncorrected cryptorchidism is associated with infertility and significantly increased risk of testicular cancer. Testicular torsion is the rotation of a testis, which twists blood vessels in the spermatic cord. This interrupts the blood supply to the testis, resulting in oedema and, if not corrected within 6 hours, necrosis and atrophy of testicular tissues.
Testicular torsion is the rotation of a testis, which twists blood vessels in the spermatic cord. This interrupts the blood supply to the testis, resulting in oedema and, if not corrected within 6 hours, necrosis and atrophy of testicular tissues. Orchitis is an acute infection of the testes. Complications of orchitis include hydrocele and abscess formation.
Orchitis is an acute infection of the testes. Complications of orchitis include hydrocele and abscess formation. Spermatogenesis (sperm production by the testes) can be impaired by disruptions of the hypothalamic-pituitary-testicular axis that reduce testosterone secretion and by testicular trauma, infection or atrophy from any cause.
Spermatogenesis (sperm production by the testes) can be impaired by disruptions of the hypothalamic-pituitary-testicular axis that reduce testosterone secretion and by testicular trauma, infection or atrophy from any cause. Epididymitis, an inflammation of the epididymis, is usually caused by a sexually transmitted pathogen that ascends through the vasa deferentia from an already infected urethra or bladder.
Epididymitis, an inflammation of the epididymis, is usually caused by a sexually transmitted pathogen that ascends through the vasa deferentia from an already infected urethra or bladder. Benign prostatic hyperplasia is the enlargement of the prostate gland. This condition becomes symptomatic as the enlarging prostate compresses the urethra, causing symptoms of bladder outlet obstruction and urine retention.
Benign prostatic hyperplasia is the enlargement of the prostate gland. This condition becomes symptomatic as the enlarging prostate compresses the urethra, causing symptoms of bladder outlet obstruction and urine retention. Bacterial prostatitis is an infection of the prostate. Acute bacterial prostatitis causes an inflammatory response in which the prostate becomes enlarged, tender and firm. Infection may spread to the bladder. Chronic bacterial prostatitis is recurrent prostatic infection that eventually causes fibrosis.
Bacterial prostatitis is an infection of the prostate. Acute bacterial prostatitis causes an inflammatory response in which the prostate becomes enlarged, tender and firm. Infection may spread to the bladder. Chronic bacterial prostatitis is recurrent prostatic infection that eventually causes fibrosis.Disorders of the breast
 Galactorrhoea, or inappropriate lactation, is the persistent secretion of a milky substance by the breasts of a woman who is not in the postpartum state or nursing an infant. It is a manifestation of pathophysiological processes elsewhere in the body, chiefly hormone imbalances caused by hypothalamic-pituitary disturbances, pituitary tumours or neurological damage.
Galactorrhoea, or inappropriate lactation, is the persistent secretion of a milky substance by the breasts of a woman who is not in the postpartum state or nursing an infant. It is a manifestation of pathophysiological processes elsewhere in the body, chiefly hormone imbalances caused by hypothalamic-pituitary disturbances, pituitary tumours or neurological damage.Impaired fertility
Major sexually transmitted infections
 The aetiology of a sexually transmitted infection may be bacterial, viral, protozoan, parasitic or fungal. Although the majority of sexually transmitted infections can be treated, viral-induced infections are considered incurable.
The aetiology of a sexually transmitted infection may be bacterial, viral, protozoan, parasitic or fungal. Although the majority of sexually transmitted infections can be treated, viral-induced infections are considered incurable.Shaun and Karin are in their mid-30s (Shaun is 34 and Karin 36). They have been trying to have a baby together for more than 2 years without success. They are attending an infertility clinic to seek advice and possibly intervention with assisted reproductive technology. Karin is a non-smoker and works as a secretary in a small accountancy business. She has had a previous ectopic pregnancy and had to have a fallopian tube removed. She has also had a number of reproductive tract infections. Shaun works in a chemicals manufacturing plant. He is a cigarette smoker and has a heavy cough. He has an undescended testicle and has also previously had a sexually transmitted infection. Upon examination, it is found that Shaun has a slightly enlarged prostate, but he says that his urine flow is normal.