10 THE STRUCTURE AND FUNCTION OF THE ENDOCRINE SYSTEM
INTRODUCTION
Most people have some understanding of the endocrine system because of the changes that their body undergoes during puberty — hormones gave rise to some very obvious physical changes. This is only a small part of the endocrine system, however, as almost every cell of the body is influenced by hormones. A substantial number of processes are controlled by the endocrine system — examples of such diverse functions include regulation of blood pressure, control of the sleep–wake cycle, maintenance of adequate calcium in the blood and bones, and even assisting with the long-term stress response.
The endocrine system is composed of various glands located throughout the body (see Figure 10-1). The main endocrine glands are the pancreas, adrenals, hypothalamus, pituitary, thyroid and parathyroid (other glands that secrete hormones are considered throughout this textbook). The endocrine glands produce and release hormones as chemical messengers. The endocrine system has four general functions:
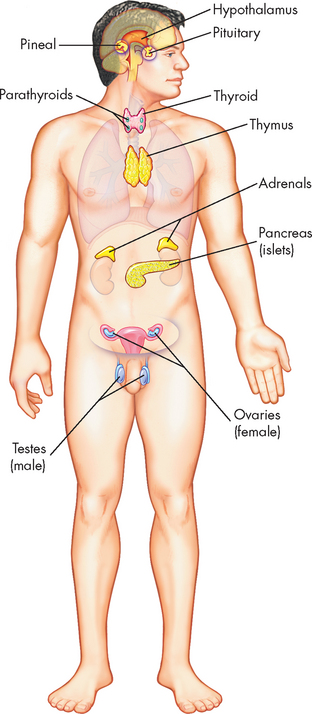
FIGURE 10-1 The principal endocrine glands.
Source: Thibodeau GA, Patton KT. Anatomy & physiology. 6th edn. St Louis: Mosby; 2007.
Hormones convey specific regulatory information among cells and organs and are integrated with the nervous system to maintain communication and control. The main mechanism of communication by hormones is the endocrine system, where hormones are released and travel through the bloodstream to reach their target cells. The endocrine system works with the nervous system in the control and regulation of body processes: the endocrine system is involved in long-term control (such as regulation of growth), while the nervous system is mainly responsible for control of activities that require a quick response (such as coordination of muscle activity so that you can walk smoothly and effortlessly; see Chapter 6). The endocrine and nervous systems work together to regulate responses to the internal and external environments.
The endocrine cells release hormones into the bloodstream and, since the hormones travel through the circulation, they are potentially able to reach every cell of the body. The hormones then cause an intracellular response at their target cells. Some hormones are tropic hormones, which means that their function is to increase or decrease the secretion of another hormone. In contrast, the remaining hormones (which are non-tropic) have direct effects on the body’s cells. In the following section we explore the factors involved in stimulating the release of hormones from the endocrine cells.
MECHANISMS OF HORMONAL REGULATION
There are specific rates and rhythms of hormonal secretion. The three basic secretion patterns are:
Diurnal, pulsatile and cyclic patterns of hormone release involve consistent patterns of secretion.
All hormones share certain general characteristics:
Regulation of hormone release
Hormones are released either in response to an altered cellular environment or in the maintenance of a regulated level of another hormone or substance. One or more of the following mechanisms regulates hormone release: (1) chemical factors (such as blood glucose or calcium levels); (2) endocrine factors (a hormone from one endocrine gland controls another endocrine gland); and (3) neural control. For example, insulin is secreted in response to increased glucose levels (a chemical stimulus), to direct stimulation of the insulin-secreting cells of the pancreas by the autonomic nervous system (a neural stimulus) and to the secretion of cortisol by the adrenal medulla, a form of endocrine regulation.
Feedback systems provide precise monitoring and control of the cellular environment. The most common feedback system, negative feedback, occurs because the rising hormone level negates the initiating change that triggered the release of the hormone (refer to Chapter 2 to revise negative feedback). Negative-feedback systems are important in maintaining hormones within physiological ranges. The lack of negative-feedback inhibition on hormonal release often results in pathological conditions. As discussed in Chapter 11, various hormonal imbalances and related conditions are caused by excessive hormone production, which is the result of failure to ‘turn off’ the system.
Mechanisms of hormone action
The sensitivity of the target cell to a particular hormone is related to the total number of receptors per cell: the more receptors, the more sensitive the cell. Low concentrations of hormone increase the number of receptors per cell; this is called up-regulation. High concentrations of hormone decrease the number of receptors; this is called down-regulation (see Figure 10-2). Thus, the cell can adjust its sensitivity to the concentration of the signalling hormone. The receptors on the cell membrane are continuously produced and degraded, so that changes in receptor concentration may occur within hours. Various conditions can affect both the receptor number and the affinity (attraction) for which the hormone binds to its receptor.
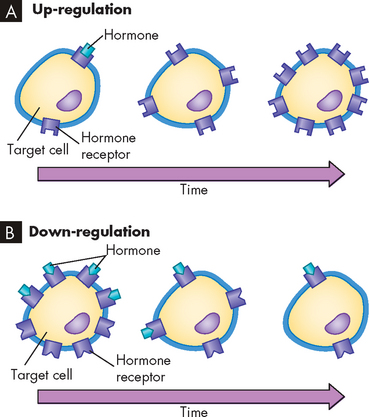
FIGURE 10-2 Regulation of target cell sensitivity.
A Low hormone level and up-regulation, or an increase in number of receptors. B High hormone level and down-regulation, or a decrease in number of receptors.
Source: Thibodeau GA, Patton KT. Anatomy & physiology. 6th edn. St Louis: Mosby; 2007.
Hormone receptors
Although a hormone is distributed throughout the body via the bloodstream, only those cells with appropriate receptors for that hormone are affected (see Figure 10-3). Hormone receptors of the target cell have two main functions: (1) to recognise and bind specifically and with high attraction (or affinity) to their particular hormones; and (2) to initiate a signal to appropriate intracellular effectors.
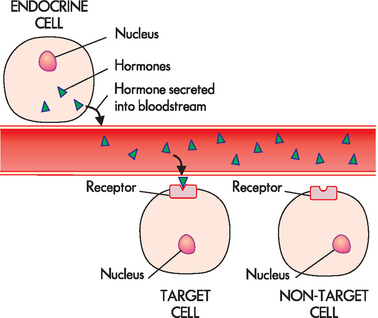
FIGURE 10-3 Hormone release and target cell specificity.
The hormone (represented by triangles) is released from the endocrine cell into the bloodstream. It travels through the blood and can bind to receptors on the target cells. The hormone cannot bind to cells without receptors for that particular hormone (non-target cells).
Some hormone receptors are located in the cell membrane of the target cell. Hormones that are protein-based (consist of proteins) are water soluble and therefore they cannot easily diffuse through the lipids of the cell membrane. The receptors for these types of hormones are on the cell surface, where the hormone can bind with the receptor (see Figure 10-4A). Most of the hormones in the human body are these protein-based hormones.
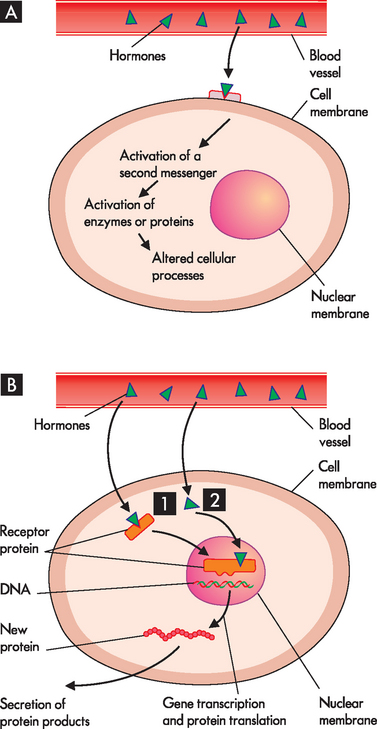
FIGURE 10-4 The signalling process for protein- and steroid-based hormones.
A Protein-based hormones bind to receptors located on the cell membrane, activate a second messenger and alter cellular processes. B Steroid-based hormones readily diffuse across the cell membrane and attach to a receptor in the cytosol (1) or in the nucleus (2).
Other hormone receptors are located inside the target cell. This means that the hormone actually diffuses through the cell membrane, where it binds to receptors located within the cell. This process applies to the lipid-based or steroid-based hormones, which are derived from cholesterol, such as the sex hormones as well as those from the adrenal cortex. These can diffuse through the lipids of the cell membrane and bind with receptors in the cytoplasm or on the nucleus (see Figure 10-4B). The hormone-receptor complex then binds to a specific region in the DNA and stimulates the expression of a specific gene.
Types of hormones are summarised in Table 10-1.
Table 10-1 CLASSIFICATION OF THE MAJOR HORMONES
| PROTEIN-BASED HORMONES | STEROID-BASED HORMONES |
|---|---|
Protein-based hormones
Receptors for the protein-based (water-soluble) hormones are located in the cell membranes. The hormone is the first messenger secreted into the bloodstream and carries the message from the endocrine gland to the target cell. At the target cell it interacts with the receptor on the cell membrane. As the protein-based hormones cannot get into the cell, they must pass the message on by using a second messenger (see Figure 10-4A). The hormone–receptor interaction initiates a signal that generates this second messenger inside the cell, which takes the signal from the receptor (at the cell membrane) to the cytoplasm and nucleus of the cell. The second messenger controls the effect of the hormone on the target cell — for example, membrane transport, contractile proteins, enzyme activation, production of proteins (protein synthesis) and cellular growth. Consequently, the second messenger directs the actions or products of specific cells.
Steroid-based hormones
The lipid-soluble hormones are steroid-based hormones and are produced from cholesterol. They include androgens, oestrogens, progestins, glucocorticoids, mineralocorticoids and thyroid hormones. Because they are relatively small, lipophilic (lipid-loving), hydrophobic molecules, they can cross the lipid cell membrane by simple diffusion (see Chapter 3). Receptors for steroid-based hormones are in the cytoplasm and nucleus (see Figure 10-4B). Their role is to trigger events that increase or decrease the expression of specific genes. This modulation of gene expression can take hours to days.
THE STRUCTURE AND FUNCTION OF THE ENDOCRINE GLANDS
In this section, we explore the names and functions of specific hormones. For each hormone, we also consider the specific stimulus that causes the hormone to be secreted, as well as the signal that limits further hormone secretion when adequate levels have been reached — in this way, homeostasis of hormone levels is maintained.
The hypothalamic-pituitary system
The hypothalamic-pituitary system (or hypothalamic-pituitary axis) produces a number of hormones that affect a diverse variety of body functions. The hypothalamic-pituitary axis forms the structural and functional basis for central integration of the neurological and endocrine systems. The hypothalamus controls the function of the pituitary gland.
The hypothalamus is located at the base of the brain; just inferior to it (below it) is the pituitary gland, connected to the hypothalamus by the infundibulum (pituitary stalk). The cells of the hypothalamus are like other neurons in that they have similar electrical properties, membranes and synapses (refer to Chapter 6). However, the hypothalamus also contains neurosecretory cells — these are specialised neurons that produce and secrete hormones.
The pituitary gland is located in a depression of the sphenoid bone at the base of the skull. It weighs approximately 0.5 g, except during pregnancy when its weight approaches 1 g. It is composed of two distinctly different lobes: (1) the anterior pituitary and (2) the posterior pituitary (see Figure 10-5). These two lobes differ in their cell types and functional relationship to the hypothalamus.
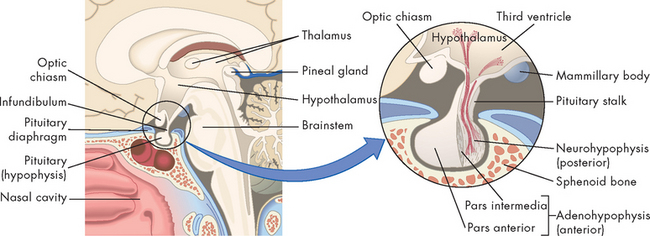
FIGURE 10-5 Location and structure of the pituitary gland (hypophysis).
The pituitary gland is located within the sella turcica of the skull’s sphenoid bone and is connected to the hypothalamus by a stalk-like infundibulum. The pituitary stalk passes through a gap in the portion of the dura mater that covers the pituitary (the pituitary diaphragm). The inset shows that the pituitary is divided into an anterior portion, the adenohypophysis, and a posterior portion, the neurohypophysis.
Source: Thibodeau GA, Patton KT. Anatomy & physiology. 6th edn. St Louis: Mosby; 2007.
The anterior pituitary
The anterior pituitary (adenohypophysis) accounts for 75% of the total weight of the pituitary gland. Blood vessels known as the hypophyseal portal system link the hypothalamus to the anterior pituitary, so the hypothalamus secretes hormones into the blood to reach target cells within the anterior pituitary, thereby controlling this part of the pituitary gland (see Figure 10-6). The hypophyseal portal system is the primary blood supply to the pituitary gland. It is known as a portal system because the capillary bed from the hypothalamus drains into the capillary bed of the anterior pituitary — an unusual arrangement, as capillaries generally drain into a venous system returning towards the heart (see Chapter 22).
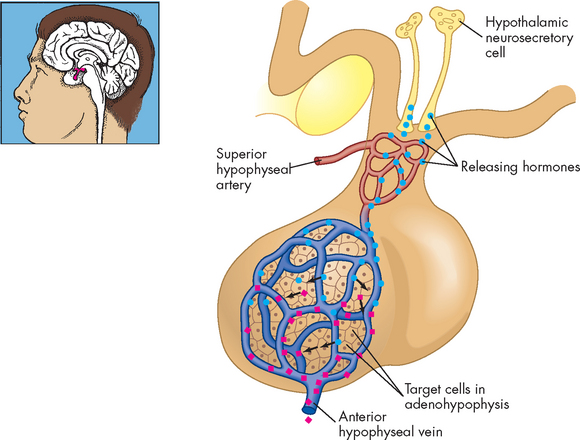
FIGURE 10-6 The relationship of the hypothalamus and anterior pituitary.
Neurons in the hypothalamus secrete releasing hormones into veins that carry them directly to the vessels of the adenohypophysis, thus bypassing the normal circulatory route.
Source: Thibodeau GA, Patton KT. Anatomy & physiology. 6th edn. St Louis: Mosby; 2007.
The major hypothalamic hormones that control the secretion from the anterior pituitary are listed in Table 10-2. In response to each of these hypothalamic hormones, the anterior pituitary secretes growth hormone (GH), prolactin (PRL), adrenocorticotropic hormone (ACTH), thyroid-stimulating hormone (TSH), follicle-stimulating hormone (FSH) and luteinising hormone (LH). Each hormone affects the physiological function of the specific target organ (see Figure 10-7).
Table 10-2 MAJOR HORMONES OF THE HYPOTHALAMUS AND ANTERIOR PITUITARY
| HYPOTHALAMUS RELEASES | ANTERIOR PITUITARY RELEASES | TARGET |
|---|---|---|
| Growth hormone–releasing hormone (GHRH) | Growth hormone (GH) | Bones, muscle, liver, other |
| Prolactin-releasing hormone (PRH) | Prolactin (PRL) | Breasts |
| Corticotropin-releasing hormone (CRH) | Adrenocorticotropic hormone (ACTH) | Adrenal gland (hormone release) |
| Thyrotropin-releasing hormone (TRH) | Thyroid-stimulating hormone (TSH) | Thyroid gland (hormone release) |
| Gonadotropin-releasing hormone (GnRH) | Follicle-stimulating hormone (FSH) Luteinising hormone (LH) | Reproductive organs |
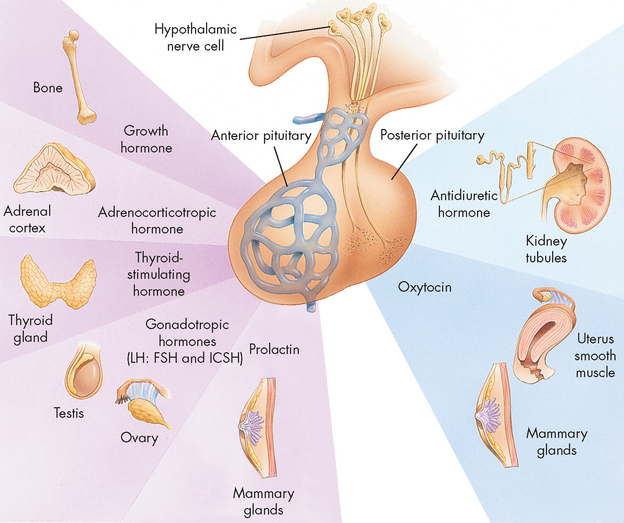
FIGURE 10-7 Anterior pituitary hormones and their target hormones.
LH = luteinising hormone; ICSH = interstitial cell-stimulating hormone (male); FSH = follicle-stimulating hormone (female).
Source: Thibodeau GA, Patton KT. Anatomy & physiology. 6th edn. St Louis: Mosby; 2007.
Growth hormone
Function of growth hormone
Growth hormone is secreted by the anterior pituitary and, as its name suggests, it is important for growth and development of muscles and bones. It causes the cells to increase in size and divide, resulting in increased mass of bones and muscles. The highest levels of growth hormone are found throughout adolescence, when growth is at its peak. In addition, growth hormone actually targets nearly every body cell, where it is necessary for normal metabolism. Growth hormone also targets the liver to increase blood glucose and fatty acid levels, making these fuels available for working cells.
Regulation of growth hormone secretion
The regulation of growth hormone secretion from the anterior pituitary is by growth hormone–releasing hormone from the hypothalamus. In addition, growth hormone secretion has a daily cycle, with high peaks seen during sleep (see Figure 10-8). High levels of secretion also occur during exercise.
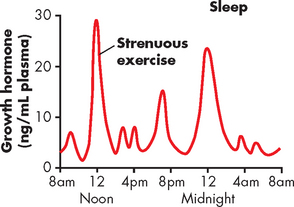
FIGURE 10-8 Typical variations in growth hormone secretion throughout the day.
The graph demonstrates the high rate of growth hormone secretion that occurs during the first few hours of deep sleep, as well as the especially powerful effect of strenuous exercise on growth hormone secretion.
Source: Guyton AC, Hall JE. Textbook of medical physiology. 11th edn. Philadelphia: Saunders; 2006.
Other anterior pituitary hormones
The anterior pituitary releases adrenocorticotropic hormone, which targets the cells of the adrenal cortex (adrenal gland) — the effects of this are discussed later in the section on the adrenal gland; similarly, thyroid-stimulating hormone is discussed in the section on the thyroid gland. The remaining anterior pituitary hormones — follicle-stimulating hormone, luteinising hormone and prolactin — have several functions specific to the reproductive system and are discussed in Chapter 31.
The posterior pituitary
The posterior pituitary (neurohypophysis) is a neural extension of the hypothalamus and the hypothalamus controls this part of the gland with action potentials (neural signals; see Figure 10-9). These neurons have their cell bodies within the hypothalamus and the axons of those neurons extend through the infundibulum to the axon terminals in the posterior pituitary. Hormones are stored in these axon terminals. When signals (action potentials) come from the hypothalamus, they travel through the infundibulum on the neurons and, on reaching the axon terminal in the posterior pituitary, cause the release of those hormones from the neurons.
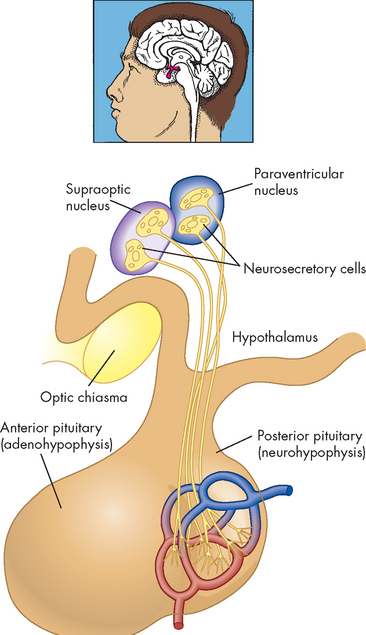
FIGURE 10-9 The relationship of the hypothalamus and the posterior pituitary.
Neurosecretory cells have their cell bodies in the hypothalamus and their axon terminals in the posterior pituitary. Thus, hormones synthesised in the hypothalamus are actually released from the posterior pituitary.
Source: Thibodeau GA, Patton KT. Anatomy & physiology. 6th edn. St Louis: Mosby; 2007.
The posterior pituitary secretes two protein-based hormones: antidiuretic hormone (discussed next) and oxytocin (discussed in Chapter 31 on the reproductive system). These hormones are structurally very similar. They are produced in the hypothalamus, packaged in secretory vesicles and moved down the axons to the posterior pituitary for storage. The posterior pituitary can be seen as a storage and releasing site for hormones produced in the hypothalamus.
Antidiuretic hormone
Function of antidiuretic hormone
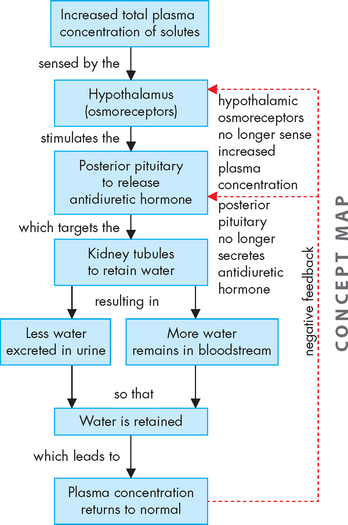
The major homeostatic function of the posterior pituitary is the control of plasma osmolality (concentration) as regulated by antidiuretic hormone (ADH). Antidiuretic hormone released into the bloodstream travels to the kidneys, where its effect is to increase the permeability of the distal renal tubules and collecting ducts (see Chapter 28). This increased permeability leads to increased water reabsorption and more concentrated urine. As a result, more water is retained in the bloodstream, rather than being excreted in the urine (see Figure 10-10). The name antidiuretic hormone describes its function: diuresis is the production of urine, hence antidiuretic hormone decreases the production of urine.
Antidiuretic hormone was originally named vasopressin because in extremely high doses it causes vasoconstriction and increased arterial blood pressure. These levels are not reached physiologically, but high doses of antidiuretic hormone (as the drug vasopressin) may be administered to promote vasoconstriction during haemorrhage. Interestingly, it is also used in clinical trials as an alternative to adrenaline for the management of cardiac arrest (refer to Chapter 23).
Regulation of antidiuretic hormone secretion
The secretion of antidiuretic hormone is regulated primarily by the osmoreceptors of the hypothalamus. These osmoreceptors are specialised neurons that can sense the concentration of body fluids and they become activated by increased osmolality. As plasma osmolality increases, the rate of antidiuretic hormone secretion increases. This hormone has no direct effect on electrolyte levels, but by increasing water reabsorption, the serum electrolyte concentrations may decrease because of a dilutional effect.
Antidiuretic hormone secretion is also increased by changes in intravascular volume, which are monitored by baroreceptors in the left atrium, the carotid and aortic arches. A volume loss of 7–25% stimulates antidiuretic hormone secretion. Stress, trauma, pain, exercise, nausea, nicotine, exposure to heat and drugs such as morphine also increase its secretion.
Antidiuretic hormone secretion decreases with decreased plasma osmolality, when the blood is already sufficiently diluted (see Figure 10-10). Its release is also inhibited by increased intravascular volume and hypertension. In addition, ingestion of alcohol inhibits antidiuretic hormone, which results in loss of fluid via the urine. This can lead to dehydration, worsening the effects of intoxication and hangover.
The thyroid and parathyroid glands
The thyroid gland, located in the anterior part of the neck just below the larynx, produces hormones that control the rates of metabolic processes throughout the body. The four parathyroid glands are near the posterior side of the thyroid and function to control serum calcium levels (see Figure 10-11).
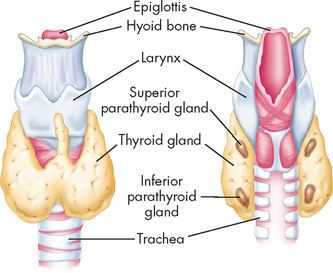
FIGURE 10-11 The thyroid and parathyroid glands.
Note their location in relation to each other and to the larynx and trachea.
Source: Thibodeau GA, Patton KT. Anatomy & physiology. 6th edn. St Louis: Mosby; 2007.
The thyroid gland
The two lobes of the thyroid gland lie on either side of the trachea, inferior to the thyroid cartilage and joined by the isthmus, giving it a ‘butterfly-shaped’ appearance (see Figure 10-11). The normal thyroid gland is not visible on inspection, but it may be palpated on swallowing, which causes it to be displaced upward.
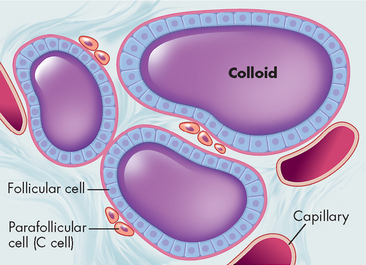
The internal structure of the thyroid gland is organised into follicles. These contain follicular cells that surround a viscous substance called colloid (see Figure 10-12). The follicular cells produce and secrete the thyroid hormones. Neurons terminate on blood vessels within the thyroid gland and on the follicular cells themselves, so neurotransmitters may directly affect the secretory activity of follicular cells.
Thyroid hormone
Function of thyroid hormone
Thyroid hormone is available in the body as either thyroxine (T4, 90% of thyroid hormone) or triiodothyronine (T3, 10% of thyroid hormone). The thyroid gland produces thyroid hormone using iodide (which is converted to iodine) and thyroglobulin. Either three or four molecules of iodine may be needed for production of thyroid hormones: triiodothyronine (T3) has three iodine molecules and thyroxine (T4) has four. Although most hormone produced by the gland is thyroxine, at the target tissues it becomes converted to triiodothyronine, which acts on the target cell.
Thyroid hormone affects most body tissues by increasing the rate of protein, fat and glucose metabolism; this increases the metabolic rate and as a result increases heat production and body temperature. Normal growth requires thyroid hormone, as do the central and autonomic nervous systems1 — normal neural tissue growth and function require thyroid hormone, so it is important across the life span. Thyroid hormone also increases the sympathetic nervous system response by increasing the number of adrenergic receptors present on the surface of cells. In this way, thyroid hormone enhances the sympathetic response.
Regulation of thyroid hormone secretion
The thyroid gland produces thyroid hormone when stimulated by pituitary thyroid-stimulating hormone (TSH), low serum iodide levels or drugs interfering with the thyroid gland’s uptake of iodide from the blood.
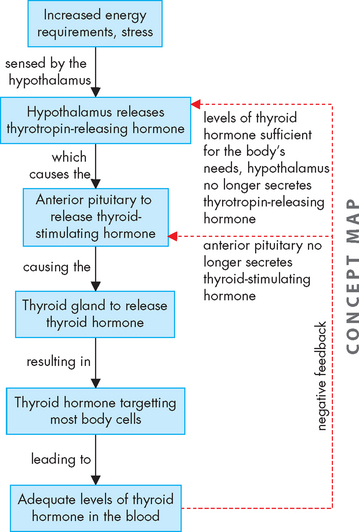
Thyrotropin-releasing hormone levels from the hypothalamus increase in response to increased energy requirements, such as with exposure to cold, stress and pregnancy, as well as when there are decreased levels of thyroxine. Thyrotropin-releasing hormone promotes the release of thyroid-stimulating hormone from the anterior pituitary. Thyroid-stimulating hormone’s effects include: (1) an immediate increase in the release of stored thyroid hormone; (2) an increase in iodide uptake and conversion to iodine; (3) an increase in thyroid hormone production; and (4) growth of the thyroid gland. Thyroid gland hormones and their regulation and function are summarised in Table 10-3.
Table 10-3 REGULATION AND FUNCTIONS OF THYROXINE (T4) AND TRIIODOTHYRONINE (T3)
| REGULATION | FUNCTIONS |
|---|---|
| Thyroxine and triiodothyronine levels are controlled by thyroid-stimulating hormone Influences on amount secreted: |
Source: Monohan FD et al. Phipps’ medical-surgical nursing: health and illness perspective. 8th edn. St Louis: Mosby; 2007.
Thyroid hormone is regulated through a negative-feedback loop involving the hypothalamus, anterior pituitary and thyroid gland. Thyrotropin-releasing hormone, which is produced and stored within the hypothalamus, initiates this loop. Thyrotropin-releasing hormone is released into the hypothalamic-pituitary portal system and circulates to the anterior pituitary, where it stimulates the release of thyroid-stimulating hormone (see Figure 10-13).
Calcitonin
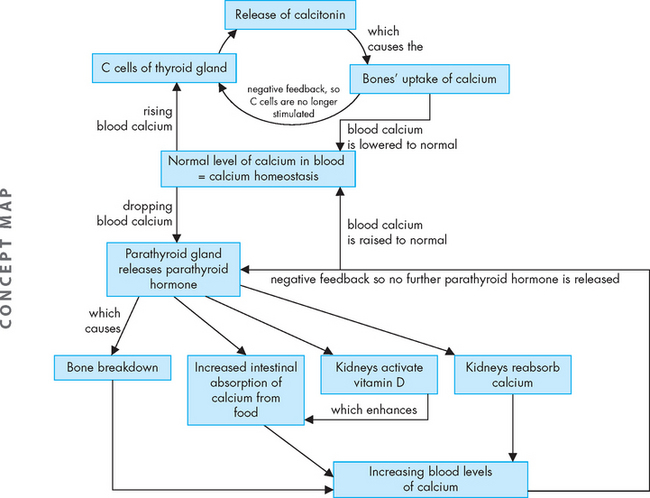
Also found in the thyroid gland are parafollicular or C cells (see Figure 10-12). C cells secrete various polypeptides, including calcitonin and somatostatin. Calcitonin (also called thyrocalcitonin) lowers serum calcium levels by stimulating calcium uptake from the blood into the bones, as well as by inhibiting bone-resorbing osteoclasts (bone resorption is explained in Chapter 14). Calcitonin and parathyroid hormone together regulate calcium balance (see Figure 10-14).
The parathyroid glands
Normally two pairs of small parathyroid glands are present behind the upper and lower poles of the thyroid gland (see Figure 10-11). However, their number may range from two to six.
Parathyroid hormone
Function of parathyroid hormone
The parathyroid glands produce parathyroid hormone, which is the single most important factor in the regulation of serum calcium. The overall effect of parathyroid hormone secretion is to increase serum calcium and decrease serum phosphate. Parathyroid hormone increases calcium levels in the blood by stimulating breakdown of the bone matrix, calcium reabsorption (and a corresponding decrease in phosphate reabsorption) at the kidney and increased intestinal absorption of calcium from food (see Figure 10-14). Furthermore, parathyroid hormone stimulates the kidneys to activate vitamin D, which further increases intestinal absorption of calcium.
Regulation of parathyroid hormone secretion
Parathyroid hormone is released in response to low serum calcium. The relationship between serum calcium and serum phosphate means that an increase in serum phosphate decreases serum calcium by causing calcium-phosphate deposition into soft tissue and bone. As this will lower the calcium in the blood, it will stimulate parathyroid hormone secretion.
When calcium levels in the blood are adequate, parathyroid hormone secretion is inhibited.
Homeostasis of blood calcium levels
Maintaining homeostasis of calcium in the blood is achieved by a balance between parathyroid hormone and calcitonin. Of these two hormones, parathyroid hormone has the main control (see Figure 10-14). While you are no doubt aware that calcium is necessary for bones, it is actually essential that neurons can obtain sufficient calcium from the blood in order to function correctly — neurons require calcium for synaptic transmission (see Chapter 6), so insufficient calcium in the blood can be detrimental.
The pancreas
The pancreas produces hormones that are involved in glycaemic control (control of blood glucose levels) — the main ones being insulin and glucagon. The importance of the pancreas cannot be emphasised strongly enough, as one of the major health crises of our time — diabetes mellitus — is linked to abnormal secretion or function of insulin.
The pancreas is located behind the stomach, between the spleen and duodenum. It houses the islets of Langerhans (see Figure 10-15). The islets of Langerhans have two main types of hormone-secreting cells: alpha cells, which secrete glucagon; and beta cells, which secrete insulin. Other hormones secreted by this organ include somatostatin and pancreatic polypeptide. These hormones regulate most carbohydrate, fat and protein metabolism. Nerves from both the sympathetic and the parasympathetic divisions of the autonomic nervous system innervate the pancreatic islets. (The pancreas also functions as an exocrine gland, producing and secreting pancreatic juice and digestive enzymes into ducts. This is not part of the endocrine system and is discussed in Chapter 26.)
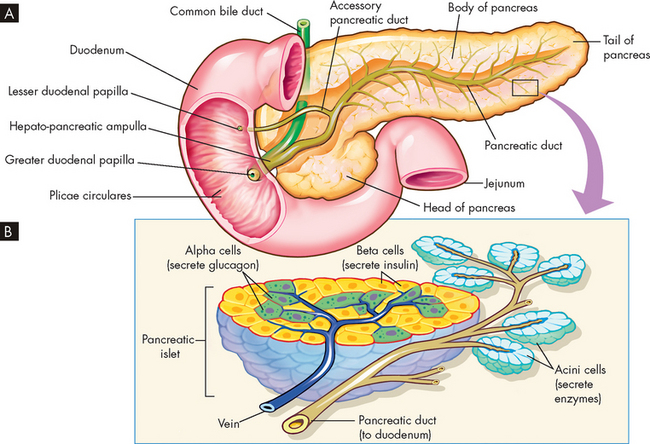
A Pancreas dissected to show main and accessory ducts. The main duct may join the common bile duct, as shown here, to enter the duodenum by a single opening at the major duodenal papilla, or the two ducts may have separate openings. The accessory pancreatic duct is usually present and has a separate opening into the duodenum. B Exocrine glandular cells (around small pancreatic ducts) and endocrine glandular cells of the pancreatic islets (adjacent to blood capillaries). Exocrine pancreatic cells secrete pancreatic juice, alpha endocrine cells secrete glucagon and beta cells secrete insulin.
Source: Thibodeau GA, Patton KT. Anatomy & physiology. 6th edn. St Louis: Mosby; 2007.
Insulin
Function of insulin
The beta cells of the pancreas produce and secrete insulin from peptides (small proteins made of a few amino acids). During the production of insulin, C peptide is released. C peptide can be measured in the blood as an indirect measure of serum insulin production.2 This can be used clinically to assist with determining the function of the pancreatic beta cells, as the presence of C peptide in the blood indicates the active production of insulin.
At the target cell, insulin binds with the insulin receptor on the cell surface. When insulin binds to the receptor, it causes signals within the cell that result in glucose transporters being available in the cell membrane. The glucose transport molecules are stored in the cell cytoplasm and are only inserted into the cell membrane in the presence of insulin. These glucose transport molecules allow glucose to be taken from the blood stream into body cells, thereby lowering blood glucose levels (see Figure 10-16).
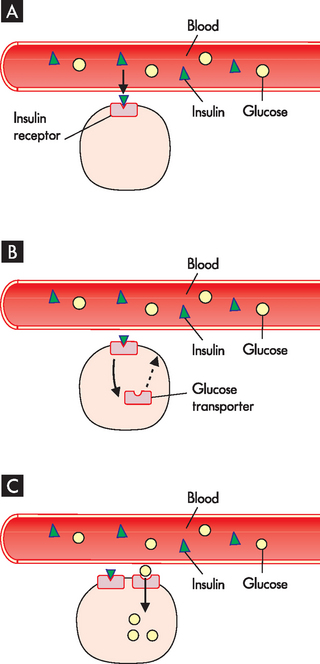
FIGURE 10-16 The effects of insulin on glucose uptake.
A Insulin binds to its receptors on the target cells, mainly skeletal muscle and liver cells. B In the presence of insulin, glucose transport molecules become available in the cell membrane. C Glucose is now able to enter the cell through the glucose transport molecules.
After a meal has been digested and the glucose molecules absorbed into the bloodstream, the blood glucose level will rise. The main function of insulin is promoting glucose uptake into body cells, particularly into liver and muscle tissue. In this way, glucose that is not required immediately is taken out of the bloodstream and stored in the form of glycogen (many molecules of glucose attached together). If the blood glucose levels are higher than normal, then hyperglycaemia may result. This can occur when blood glucose levels are in excess of 7 mmol/L)3 and may indicate diabetes (refer to Chapter 35).
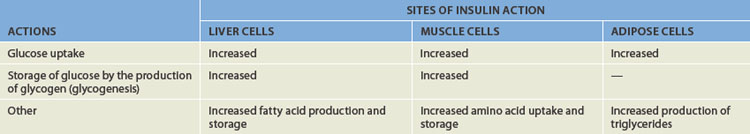
Insulin also causes these tissues to manufacture molecules for storing excess proteins, carbohydrates and lipids. Table 10-4 summarises the actions of insulin. The net effect of insulin in these tissues is to stimulate protein and fat storage and decrease blood glucose.
The brain, red blood cells, kidney and lens of the eye do not require insulin for glucose transport. Glucose is able to enter these cells on a continual basis from the bloodstream. Because the brain is dependent on glucose as an energy source, yet does not store any glucose, it must be able to continually obtain adequate glucose from the bloodstream. This is an important reason why glycaemic control is so critical.
Regulation of insulin secretion
Secretion of insulin is regulated by chemical, hormonal and neural control. The main stimulus for the pancreas to secrete insulin into the bloodstream is hyperglycaemia. Increased levels of amino acids in the bloodstream will also stimulate insulin secretion. Both glucose and amino acids are usually increased in the bloodstream following meals, when those nutrients have been absorbed from the food. Other hormones of the gastrointestinal system can also stimulate the release of insulin — these are gastrin, cholecystokinin and secretin (see Chapter 26). Finally, the parasympathetic nervous system responsible for control of ‘rest and digest’ functions stimulates secretion of insulin, which allows glucose to be taken into cells and stored as part of the body’s restoration process.
Insulin secretion diminishes in response to hypoglycaemia (blood glucose levels below 3 mmol/L), when there is no further requirement for insulin to move glucose out of the blood and into cells for storage. Similarly, high levels of insulin switch off further insulin secretion. Finally, the sympathetic nervous system, which mediates the stress response, inhibits secretion of insulin, as adequate or high blood levels of glucose are necessary to facilitate the stress response — this will also occur during exercise, when the sympathetic nervous system is activated, thereby allowing glucose and fatty acids to remain in the blood as fuels for working muscles.4
Glucagon
Function of glucagon
Glucagon is produced by the alpha cells of the pancreas (as well as by cells lining the gastrointestinal tract). Glucagon acts primarily in the liver and increases blood glucose by stimulating the breakdown of the glycogen stores (glycogenolysis), thereby releasing glucose into the bloodstream. It is easy to confuse the words glucagon and glycogen due to their similar spelling — you can remember that glucagon is the hormone, as glucagon ends in the letters ‘on’, also found in the word ‘hormone’. Glucagon can also signal the liver and muscle to use other non-carbohydrate sources (such as amino acids) to make glucose (gluconeogenesis). Finally, in addition to increasing blood glucose levels, glucagon promotes release of fatty molecules to the blood from stores in adipose cells (lipolysis).
Regulation of glucagon secretion
The main stimulus for glucagon release by the pancreas is hypoglycaemia, a decline in blood glucose levels. Some amino acids also stimulate glucagon secretion. The sympathetic nervous system stimulates glucagon release to cause an increase in blood glucose levels, which assists in dealing with stressful circumstances.
Glucagon release is inhibited by high glucose levels, when there is no requirement to further increase blood glucose levels.
Homeostasis of blood glucose levels
In euglycaemia, blood glucose levels are normal. In someone who has been fasting, euglycaemia occurs from 3.0 to 5.4 mmol/L, whereas after eating up to 7.7 mmol/L is still considered normal.5
Insulin and glucagon have opposing effects on blood glucose levels:
There is a constant balancing between levels of insulin and glucagon to maintain homeostasis of blood glucose levels. Insulin is the only hormone responsible for lowering blood glucose levels. While glucagon is usually considered the main hormone for increasing blood glucose levels, other hormones that contribute to this function include adrenaline, cortisol, growth hormone and thyroid hormone (see Table 10-5). Because the brain depends on a constant supply of glucose from the blood, it is necessary to have alternative hormones that can raise blood glucose if necessary.
Table 10-5 HORMONES DIRECTLY INVOLVED IN THE REGULATION OF BLOOD GLUCOSE LEVELS
The adrenal glands
The adrenal glands are paired, pyramid-shaped organs behind the peritoneum and close to the upper pole of each kidney. Each adrenal gland consists of two separate portions: an inner medulla and an outer cortex. These two portions have different structures and hormonal functions. In effect, each adrenal gland functions like two separate glands (see Figure 10-17).
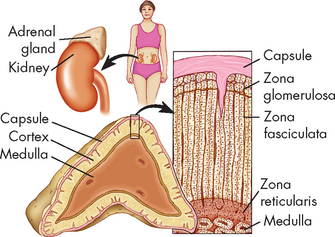
FIGURE 10-17 The structure of the adrenal gland showing cell layers (zonae) of the cortex.
Zona glomerulosa secretes aldosterone. Zona fasciculata secretes abundant amounts of glucocorticoids, chiefly cortisol. Zona reticularis secretes minute amounts of sex hormones and glucocorticoids. A portion of the medulla is visible at the bottom of the drawing.
Source: Thibodeau GA, Patton KT. Anatomy & physiology. 6th edn. St Louis: Mosby; 2007.
The adrenal cortex, or outer region of the gland, accounts for 80% of the weight of the adult gland. The cortex is subdivided into the following three zones:
The adrenal medulla, which accounts for the remaining 20% of the gland’s total weight, secretes the catecholamines adrenaline and noradrenaline. Both sympathetic and parasympathetic cholinergic fibres innervate the adrenal medulla (see Chapter 6 on the neurological system).
The adrenal cortex
The adrenal cortex secretes several steroid hormones, including cortisol (a glucocorticoid), aldosterone (a mineralocorticoid) and the adrenal androgens and oestrogens (gonadocorticoids). These hormones are all produced from cholesterol — although cholesterol can increase the risk of heart disease if too much is present in the blood, it is important that you realise that some cholesterol is essential for the normal structure and function of the body. The cells of the adrenal cortex are stimulated by adrenocorticotropic hormone from the pituitary gland.6
Cortisol
Functions of cortisol
The adrenal cortex produces the glucocorticoid cortisol. Cortisol is the main secretory product of the adrenal cortex and it is needed to maintain life and protect the body from stress (see Chapter 34 for a focus on the stress response). Cortisol is a steroid hormone that has metabolic, anti-inflammatory and growth-suppressing effects and influences levels of awareness and sleep patterns. These functions are summarised in Box 10-1. In the liver, cortisol acts primarily to stimulate glucose formation — this increase in blood glucose levels assists with the stress response. In extra-hepatic tissues (those outside of the liver), cortisol stimulates the breakdown of proteins to assist with glucose production.
Cortisol acts at several sites to influence immune and inflammatory reactions. One major immune suppressant effect is the decrease in the proliferation of T lymphocytes, primarily T helper lymphocytes. There is a greater effect on T helper 1 cytokine production (including antiviral interferons) than there is T helper 2 cytokine production and therefore greater depression of cell-mediated immunity than humoral immunity (see Chapter 12). Cortisol also has anti-inflammatory effects related to decreased natural killer cell function, suppression of inflammatory cytokines and stabilisation of lysosomal membranes, which decreases the release of proteolytic enzymes.7
Other effects of cortisol include inhibition of bone formation, inhibition of antidiuretic hormone secretion and stimulation of gastric acid secretion. Cortisol appears to potentiate the effects of adrenaline and noradrenaline (catecholamines), thyroid hormone and growth hormone on adipose tissue. A metabolite of cortisol may act like a barbiturate and depress nerve cell function in the brain, accounting for the noted effects on mood associated with steroid fluctuation in disease or stress.
Pathologically high levels of cortisol increase circulating erythrocytes (leading to polycythaemia), increase the appetite, promote fat deposition in the face and cervical (neck) areas, increase uric acid excretion, decrease serum calcium levels (possibly by inhibiting gastrointestinal absorption of calcium), suppress the production and secretion of adrenocorticotropic hormone, and interfere with the action of growth hormone so that growth is inhibited. They also have important ‘permissive’ effects, sensitising arterioles to the vasoconstrictive effects of noradrenaline.
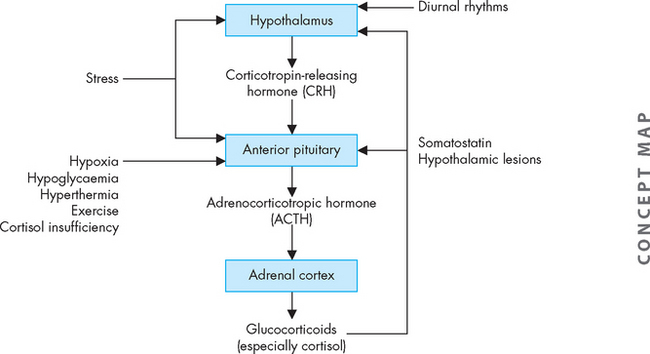
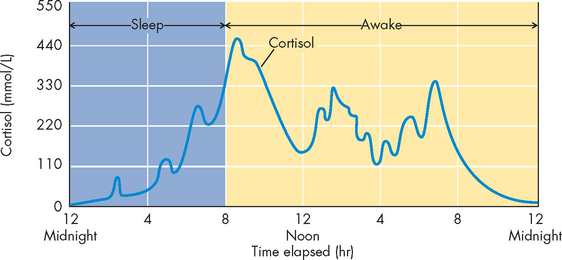
Regulation of cortisol secretion
Cortisol secretion is regulated primarily by the hypothalamus and anterior pituitary (see Figure 10-18). Corticotropin-releasing hormone is produced by the hypothalamus. Once released, it travels through the portal vessels to stimulate the production and secretion of adrenocorticotropic hormone by the anterior pituitary. Adrenocorticotropic hormone is the main regulator of cortisol secretion and adrenocortical growth.
Once adrenocorticotropic hormone stimulates the cells of the adrenal cortex, cortisol production and secretion occur immediately. In the healthy person, the secretory patterns of adrenocorticotropic hormone and cortisol are nearly identical. Adrenocorticotropic hormone is rapidly inactivated in the circulation, and the liver and kidneys remove the deactivated hormone.
Three factors appear to be primarily involved in regulating the secretion of adrenocorticotropic hormone:
Aldosterone
Functions of aldosterone
Aldosterone is the most potent naturally occurring mineralocorticoid. Its target is the kidneys — it causes the kidneys to retain sodium and water in the blood, rather than being lost in the urine. As a result, potassium is excreted from the body in the urine.
Aldosterone maintains extracellular volume by acting on the kidney cells to increase sodium reabsorption and potassium and hydrogen excretion. This renal effect takes 90 minutes to 6 hours. Other effects of aldosterone include enhancement of cardiac muscle contraction, possible stimulation of ectopic ventricular activity through secondary cardiac pacemakers in the ventricles, stiffening of blood vessels and increased vascular resistance, and decreased fibrinolysis.8–10 4 6
Regulation of aldosterone secretion
Aldosterone production and secretion are regulated primarily by the renin-angiotensin-aldosterone system (described in Chapter 28). The renin-angiotensin-aldosterone system is activated by sodium and water depletion, increased potassium and a diminished blood volume (see Figure 10-20). Angiotensin II is the primary stimulant of aldosterone production and secretion; however, sodium and potassium levels may also directly affect aldosterone secretion. Adrenocorticotropic hormone may transiently stimulate aldosterone production but does not appear to be a major regulator of secretion.
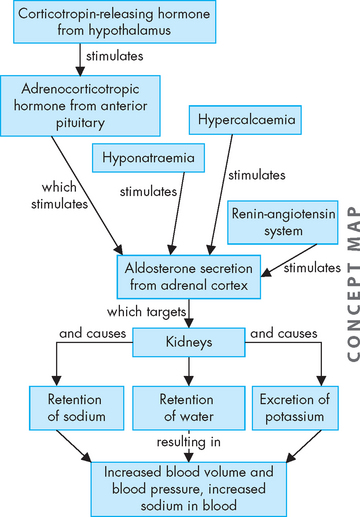
FIGURE 10-20 Secretion of aldosterone.
The main stimulus for aldosterone secretion is from the renin-angiotensin system (see Chapter 28).
Oestrogens and androgens
The healthy adrenal cortex secretes minimal amounts of oestrogens and androgens. Adrenocorticotropic hormone appears to be the major regulator. Some of the weakly androgenic substances secreted by the cortex (dehydroepiandrosterone, androstenedione) are converted by peripheral tissues to stronger androgens, such as testosterone, thus accounting for some androgenic effects initiated by the adrenal cortex. Peripheral conversion of adrenal androgens to oestrogens is enhanced in some cases, including ageing, obesity, liver disease and hyperthyroidism.11 The biological effects and metabolism of the adrenal sex steroids do not vary from those produced by the gonads (see Chapter 31).
The adrenal medulla
The adrenal medulla is located deep in the adrenal gland and receives inputs from the sympathetic nervous system (the fight-or-flight or stress response). It mainly produces adrenaline and noradrenaline, which are secreted to enhance the actions of the sympathetic nervous system.
Adrenaline and noradrenaline
Function of adrenaline and noradrenaline
The adrenal medulla works together with the sympathetic division of the autonomic nervous system. The major hormones secreted by the adrenal medulla are the catecholamines adrenaline and noradrenaline, which are produced from the amino acid phenylalanine. Only 30% of circulating adrenaline comes from the adrenal medulla; the other 70% is released from nerve terminals of neurons. The medulla is only a minor source of noradrenaline, as most of this substance is released from neurons too. The adrenal medulla functions to enhance the stress response mediated by the neurons of the sympathetic nervous system.
Catecholamines have diverse effects on the entire body. Their release and the body’s response have been characterised as the fight-or-flight response (see Chapter 6). Adrenaline is 10 times more potent than noradrenaline in exerting metabolic effects. The metabolic effects of catecholamines promote hyperglycaemia through a variety of mechanisms including interference with the usual glucose regulatory feedback mechanisms.
Regulation of adrenaline and noradrenaline secretion
Stimuli to adrenal medullary secretion include sympathetic nerve stimulation, hypoglycaemia (low blood glucose), hypoxia (low tissue oxygen), hypercapnia (high tissue carbon dioxide), acidosis, haemorrhage, glucagon, nicotine, histamine and angiotensin II. In addition, adrenocorticotropic hormone and cortisol increase adrenal catecholamine secretion.
Neuroendocrine response to stressors
The endocrine system acts together with the nervous and immune systems to respond to stressors. Perception that an event is stressful may be essential to emotional arousal and initiation of the stress response. Some events, such as bacterial invasion, activate the stress response without emotional arousal. The stress response characterised by the sympathetic nervous system is discussed in Chapter 6. As stress is so commonplace in modern-day Australia and New Zealand and it increases the likelihood of developing some chronic diseases, we focus on stress in Chapter 34.
The pineal gland
The pineal gland secretes melatonin, which is an important hormone in the regulation of circadian rhythm, whereby fluctuations in body function and hormone levels occur around a 24-hour cycle. As such, it is a regulator of the sleep–wake system (sleep is discussed in Chapter 6). Peak levels of melatonin occur during night-time, as they induce drowsiness.
The thymus gland
The thymus gland is located within the thoracic cavity near the chest. This gland has a role in the normal development of T lymphocytes as part of the immune system (see Chapter 12). The thymus gland is most active in childhood when the immune system is undergoing extensive development.
The testes and ovaries
The testes (in males) and ovaries (in females) secrete hormones that have specific roles in the reproductive system. These are discussed in Chapter 31.
AGEING AND THE ENDOCRINE SYSTEM
In general, with ageing there is atrophy (shrinkage) of endocrine organs, which can result in decreased secretion of hormones. In addition, there is often decreased clearance or removal of hormones by the liver.
Pancreatic alterations with ageing include the development of glucose intolerance or diabetes (refer to Chapter 35). The thyroid gland is prone to fibrosis and infiltration by inflammatory processes, which disrupts thyroid function. Possible changes in thyroid hormone are difficult to determine because of concurrent disease in the elderly: there may be decreased thyroxine secretion and turnover, a decline in triiodothyronine (especially in men), diminished thyroid-stimulating hormone secretion and reduced response of plasma thyroid-stimulating hormone concentration to thyroid-releasing hormone administration (especially in men).
The declining function of the adrenal glands leads to decreased levels of dehydroepiandrosterone (a steroid involved in many functions), which leads to decreased production of androgen-derived oestrogen and testosterone, decreased metabolic clearance of cortisol, decreased cortisol secretion and decreased levels of aldosterone.
After menopause, women have decreased levels of oestrogen and progesterone, increased levels of follicle-stimulating hormone and relative increases in androgen levels; these changes have numerous physiological and pathophysiological consequences (see Chapter 32); in men, there is a gradual decrease in serum testosterone levels leading to decreased sexual activity, decreased muscle strength and decreased bone mineralisation.
In the posterior pituitary, a decrease in size is seen, accompanied by reduced antidiuretic hormone secretion. In the anterior pituitary, there is increased fibrosis and a moderate increase in the size of the gland, as well as a decline in growth hormone release.
Mechanisms of hormonal regulation
 The endocrine system has diverse functions, including sexual differentiation, growth and development, and continuous maintenance of the body’s internal environment.
The endocrine system has diverse functions, including sexual differentiation, growth and development, and continuous maintenance of the body’s internal environment. Most hormone levels are regulated by negative feedback, in which hormone secretion raises the level of a specific hormone, ultimately causing secretion to subside.
Most hormone levels are regulated by negative feedback, in which hormone secretion raises the level of a specific hormone, ultimately causing secretion to subside. Hormones affect only target cells with appropriate receptors and then act on these cells to initiate specific cell functions or activities.
Hormones affect only target cells with appropriate receptors and then act on these cells to initiate specific cell functions or activities. Receptors for hormones may be located on the cell membrane or in the intracellular compartment of a target cell.
Receptors for hormones may be located on the cell membrane or in the intracellular compartment of a target cell.The structure and function of the endocrine glands
 The hypothalamus is an endocrine gland that exerts control over the hormone production of most of the other endocrine glands of the body. It controls their function by releasing hormones into the blood, which bind with receptors on the target gland cells.
The hypothalamus is an endocrine gland that exerts control over the hormone production of most of the other endocrine glands of the body. It controls their function by releasing hormones into the blood, which bind with receptors on the target gland cells. The pituitary gland, consisting of anterior and posterior portions, is connected to the central nervous system through the hypothalamus.
The pituitary gland, consisting of anterior and posterior portions, is connected to the central nervous system through the hypothalamus. The hypothalamus regulates anterior pituitary function by secreting hormones into the hypophyseal portal circulation, a network of blood vessels.
The hypothalamus regulates anterior pituitary function by secreting hormones into the hypophyseal portal circulation, a network of blood vessels. Hormones of the anterior pituitary are mainly regulated by secretion of hypothalamic-releasing hormones or by negative feedback from hormones secreted by target organs.
Hormones of the anterior pituitary are mainly regulated by secretion of hypothalamic-releasing hormones or by negative feedback from hormones secreted by target organs. The hormones of the anterior pituitary are adrenocorticotropic hormone, growth hormone, prolactin, follicle-stimulating hormone, luteinising hormone and thyroid-stimulating hormone.
The hormones of the anterior pituitary are adrenocorticotropic hormone, growth hormone, prolactin, follicle-stimulating hormone, luteinising hormone and thyroid-stimulating hormone. Growth hormone is secreted by the anterior pituitary for growth during childhood and adolescence and normally fluctuates throughout the day.
Growth hormone is secreted by the anterior pituitary for growth during childhood and adolescence and normally fluctuates throughout the day. The posterior pituitary is connected to the hypothalamus through neurons, whose cell bodies are in the hypothalamus and axons extend into the posterior pituitary. Hormones are manufactured in the hypothalamus, travel down the axon and are released from the posterior pituitary.
The posterior pituitary is connected to the hypothalamus through neurons, whose cell bodies are in the hypothalamus and axons extend into the posterior pituitary. Hormones are manufactured in the hypothalamus, travel down the axon and are released from the posterior pituitary. Antidiuretic hormone controls serum osmolality (total concentration of solutes in the plasma), increases permeability of the renal tubules to water and thereby causes water to be retained in the body by the kidneys rather than lost through urine.
Antidiuretic hormone controls serum osmolality (total concentration of solutes in the plasma), increases permeability of the renal tubules to water and thereby causes water to be retained in the body by the kidneys rather than lost through urine. The thyroid gland contains follicles that secrete the thyroid hormones thyroxine (T4) and triiodothyronine (T3). It also contains C cells, which secrete calcitonin.
The thyroid gland contains follicles that secrete the thyroid hormones thyroxine (T4) and triiodothyronine (T3). It also contains C cells, which secrete calcitonin. Thyroid hormone secretion is regulated by thyroid-releasing hormone through a negative-feedback loop that involves the anterior pituitary and hypothalamus.
Thyroid hormone secretion is regulated by thyroid-releasing hormone through a negative-feedback loop that involves the anterior pituitary and hypothalamus. Thyroid-stimulating hormone, which is produced and stored in the anterior pituitary, stimulates secretion of thyroid hormone by activating intracellular processes, including uptake of iodine necessary to make thyroid hormone.
Thyroid-stimulating hormone, which is produced and stored in the anterior pituitary, stimulates secretion of thyroid hormone by activating intracellular processes, including uptake of iodine necessary to make thyroid hormone. Thyroid hormones alter protein synthesis (or production) at target cells and have a wide range of metabolic effects on proteins, carbohydrates, lipids and vitamins. Thyroid hormone also affects heat production and cardiac function.
Thyroid hormones alter protein synthesis (or production) at target cells and have a wide range of metabolic effects on proteins, carbohydrates, lipids and vitamins. Thyroid hormone also affects heat production and cardiac function. Calcitonin stimulates calcium uptake from the blood into the bones, thereby lowering blood calcium levels.
Calcitonin stimulates calcium uptake from the blood into the bones, thereby lowering blood calcium levels. The paired parathyroid glands are normally located behind the upper and lower poles of the thyroid. These glands secrete parathyroid hormone, an important regulator of serum calcium levels.
The paired parathyroid glands are normally located behind the upper and lower poles of the thyroid. These glands secrete parathyroid hormone, an important regulator of serum calcium levels. In bone, parathyroid hormone causes bone breakdown and resorption. In the kidneys, parathyroid hormone increases reabsorption of calcium, so that less is lost from the body in urine.
In bone, parathyroid hormone causes bone breakdown and resorption. In the kidneys, parathyroid hormone increases reabsorption of calcium, so that less is lost from the body in urine. The pancreas contains islets of Langerhans, which secrete hormones responsible for controlling blood glucose levels.
The pancreas contains islets of Langerhans, which secrete hormones responsible for controlling blood glucose levels. Beta cells of the pancreas secrete insulin, which is critical for lowering blood glucose concentrations and also has a role in overall body metabolism of fat, protein and carbohydrates.
Beta cells of the pancreas secrete insulin, which is critical for lowering blood glucose concentrations and also has a role in overall body metabolism of fat, protein and carbohydrates. Pancreatic alpha cells produce glucagon, which is secreted when blood glucose concentrations are low.
Pancreatic alpha cells produce glucagon, which is secreted when blood glucose concentrations are low. Insulin and glucagon work together to maintain homeostasis of blood glucose levels, known as euglycaemia.
Insulin and glucagon work together to maintain homeostasis of blood glucose levels, known as euglycaemia. The paired adrenal glands are situated superior to (above) the kidneys. Each gland consists of an adrenal medulla, which secretes catecholamines, and an adrenal cortex, which secretes steroid hormones.
The paired adrenal glands are situated superior to (above) the kidneys. Each gland consists of an adrenal medulla, which secretes catecholamines, and an adrenal cortex, which secretes steroid hormones. The steroid hormones secreted by the adrenal cortex are produced from cholesterol. These hormones include cortisol, aldosterone and adrenal androgens and oestrogens.
The steroid hormones secreted by the adrenal cortex are produced from cholesterol. These hormones include cortisol, aldosterone and adrenal androgens and oestrogens. Cortisol directly affects carbohydrate metabolism by increasing blood glucose concentration through causing the liver to release glucose into the blood. Cortisol also inhibits immune and inflammatory responses.
Cortisol directly affects carbohydrate metabolism by increasing blood glucose concentration through causing the liver to release glucose into the blood. Cortisol also inhibits immune and inflammatory responses. Secretion of cortisol is regulated by corticotropin-releasing hormone from the hypothalamus and adrenocorticotropic hormone from the anterior pituitary. Cortisol levels fluctuate throughout the day.
Secretion of cortisol is regulated by corticotropin-releasing hormone from the hypothalamus and adrenocorticotropic hormone from the anterior pituitary. Cortisol levels fluctuate throughout the day. Aldosterone is a steroid hormone that directly affects the kidneys, where it causes sodium reabsorption into the blood and potassium and hydrogen excretion with urine.
Aldosterone is a steroid hormone that directly affects the kidneys, where it causes sodium reabsorption into the blood and potassium and hydrogen excretion with urine. Aldosterone secretion is regulated primarily by the renin-angiotensin system and serum sodium concentration.
Aldosterone secretion is regulated primarily by the renin-angiotensin system and serum sodium concentration. Androgens and oestrogens secreted by the adrenal cortex act in the same way as those secreted by the gonads. Most of these hormones are secreted by the gonads.
Androgens and oestrogens secreted by the adrenal cortex act in the same way as those secreted by the gonads. Most of these hormones are secreted by the gonads. The adrenal medulla secretes the catecholamines adrenaline and noradrenaline. Adrenaline is 10 times more potent than noradrenaline in exerting metabolic effects. Their release is stimulated by sympathetic nervous system stimulation and cortisol.
The adrenal medulla secretes the catecholamines adrenaline and noradrenaline. Adrenaline is 10 times more potent than noradrenaline in exerting metabolic effects. Their release is stimulated by sympathetic nervous system stimulation and cortisol.One of your friends has a 16-year-old boy, Sam, who is undergoing rapid growth and getting taller very quickly. Sam’s doctor says that the pains Sam is experiencing in his legs are just growing pains and are nothing to worry about, because they are probably just due to hormones. Sam knows that the pimples and blemishes on his face are also due to hormones and thinks that hormones are really annoying. Fortunately for Sam, you have just been reading the endocrine chapter in your textbook and can reassure him that hormones actually are of great benefit.