Chapter 18 Psychotropic Agents
Patients with a major psychosis, such as schizophrenia, depression or mania, are usually prescribed drug therapy. To optimise treatment, health-care professionals must be familiar with the functions of the central nervous system and its role in mood and emotions, the changes that occur in psychiatric disorders and the mechanisms of action, main effects and adverse effects of psychotropic drugs. This is one of the more complex and rapidlychanging areas of pharmacology.
In this chapter, the relationships between neurotransmitter levels in the brain and mood, emotions and behaviour are described, and the pathogenesis of the major psychoses is discussed. This background assists understanding the pharmacological properties of the main groups of psychotropic drugs: antipsychotic agents used in schizophrenia and antidepressants and lithium used in affective disorders. These are potent drugs with actions on many receptor types, and thus with the potential for inducing serious adverse reactions and drug interactions. Reference texts should be consulted for more detailed information on individual drugs.
Key abbreviations
ADHD attention defi cit hyperactivity disorder
AMH Australian Medicines Handbook
BAD bipolar affective disorder
CBT cognitive behaviour therapy
5-HT 5-hydroxytryptamine (serotonin)
MAOI monoamine oxidase inhibitor
RIMA reversible inhibitor of MAO-A
Key background: psychiatry and cns neurotransmitters
Psychiatry
PSYCHIATRY is the branch of medicine dealing with treatment of disorders of the mind. Traditionally in psychiatry disorders were classified into the major conditions: the psychoses, which affect a person’s whole mind and mental state; and the less pervasive conditions, the neuroses, in which a person’s mental state is only partly changed. There are two major types of psychosis: schizophrenia and the mood disorders (depression, mania and bipolar affec tive disorder). The neuroses include conditions such as anxiety, obsessive–compulsive disorder and phobias; in these disorders, responses to stress are considered to be at the extreme of the normal range rather than abnormal. Psychiatrists also see patients with organic mental dis orders (dementias, delirium and drug-related disorders), developmental disorders (autism, mental retardation and specific disorders of speech, attention etc) and personality disorders involving maladaptive responses to circumstances and unusual behaviour patterns.
These conditions are very common (see Clinical Interest Box 18-1). They are not static but are defined in terms of relationships and the person’s responses to the environment and usually have a remitting/relapsing course. There is often a high risk of suicide, hence the importance of early effective treatment. The general clinical features are of disordered thought, perception, emotion, behaviour, intellect and personality. Most people with a psychosis can live in the community but may require long-term treatment; long-term adverse effects make safe effective treatment and compliance difficult.
Clinical interest box 18-1 Prevalence of mental disorders
A major survey was carried out in Australia in 2007 to determine the prevalence of mental disorders in the community, both over a person’s lifetime and within the preceding 12-month period. The term ‘mental disorder’ excluded schizophrenia and related disorders; prevalence refers to the proportion of a population with a medical condition at a point in time. In summary, extrapolating to the general population:
Source: Data adapted from Australian Bureau of Statistics, article 4326.0: National Survey of Mental Health and Wellbeing, 2007, Summary of Results: http://www.health.gov.au/internet/mentalhealth/publishing.nsf/Content/national-surveys-1.
Models used in psychiatry
Various models are used in psychiatry to help describe conditions and rationalise therapy:
Overall, an eclectic approach that borrows from all models where relevant is most useful. In the context of pharmacology and attempts to explain the mechanism of a psychoactive drug’s actions, we inevitably tend to emphasise the biological model, particularly pathological imbalances in CNS neurotransmitter levels that can be corrected by drugs.
Drugs used in psychiatry
Psychotropic literally means ‘affecting the mind’. The term ‘psychotropic agents’ could thus include all drugs that primarily affect the mind, such as sedatives and hypnotics, antianxiety agents, central nervous system (CNS) stimulants, general anaesthetics and social drugs, including alcohol, marijuana and caffeine, as well as the drugs used to treat the major psychiatric disorders. In this text, however, ‘psychotropic’ is used in the narrower sense to refer to drugs used to treat the major psychoses. Although the term ‘antipsychotic’ could apply to drugs used in all psychoses, including affective (mood) disorders, it has come to refer only to drugs used in schizophrenia; the other term used similarly is ‘neuroleptic’, meaning a drug that can modify abnormal psychotic behaviour.
The main emphasis in this chapter will be on the antipsychotic drugs used in the treatment of schizophrenia and the antidepressant and antimanic drugs used in mood disorders. (See Clinical Interest Box 18-2 for the historical background to psychiatric treatments.)
Clinical interest box 18-2 Historical background to psychiatric drugs
Historically, people perceived as being mad or insane were driven out of towns and villages, hidden in cellars or attics in their homes (as in the classic 1847 English novel Jane Eyre, by Charlotte Bronte) or committed to custodial care in gaols or in lunatic asylums. Physical restraints (stone walls, straitjackets, tranquilliser chairs); shock therapy with water, ice packs, insulin or electricity; or major CNS surgery such as lobotomy were often the only ways to deal with severe mental illness. As sedating medicines became available, sufferers could be drugged into oblivion with narcotics (opioids) or early hypnotics (bromides, alcohols, paraldehyde, chloral hydrate and the barbiturates).
Psychiatry as a specialist branch of medicine started in the late 19th century, and scientific psychiatry in the 1920s, as studies were carried out to define and classify types of mental illness and relate them to inheritance or traumatic events. Electroconvulsive therapy (ECT) was discovered as an effective form of treatment of severe depression in 1938.
The first specific drug treatment was discovered in 1949: lithium was recognised by Dr John Cade, a Melbourne psychiatrist, as being effective in mania (Cade 1979; see also Clinical Interest Box 18-13). Reserpine (from the plant Rauwolfia serpentina), an Indian drug used to encourage meditation and introspection, was demonstrated to have tranquillising and antihypertensive properties (due to inhibition of storage of monoamines in vesicles, see Figure 12-1) but caused severe depression and parkinsonian effects. The first safe ‘major tranquilliser’, or neuroleptic drug (chlorpromazine), was developed in 1952 and an effective antidepressant drug (imipramine) soon afterwards.
These drugs, and better understanding of the roles of CNS neurotransmitters, revolutionised the treatment of mental illness and led to many other new psychotropic agents. People successfully treated could remain in their families, jobs and communities. Even more specific, safe and effective drugs are continually being developed, tested and used clinically, e.g. the selective serotonin reuptake inhibitors (SSRIs) such as fluoxetine in depression and the atypical antipsychotics in schizophrenia.
The central nervous system, the mind and emotions
It has become increasingly difficult in practice to separate the functions of the mind from those of the body. The CNS is responsible for consciousness, behaviour, memory, recognition, learning and the more highly developed integrative and creative processes such as imagination, abstract reasoning and creative thought. In addition, it serves to coordinate vital regulatory functions such as blood pressure, heart rate, respiration, salivary and gastric secretions, muscular activity and body temperature. The interrelationships among the various circuits in the brain produce patterns of behaviour that can be modified by conscious choice, by external situations, by internal adjustments or by drugs. This allows the individual to adapt to changes in both the external and internal environ ments. To understand the actions of drugs in treating the symptoms of mental illness, it is important to have knowledge of the functioning of the nervous system. Refer to Chapter 14 for a review of the anatomy, physiology and functions of the various components of the CNS (Figures 14-1 and 14-2), including the CNS functional systems (e.g. limbic system [Figure 16-3] and extrapyramidal system).
Relevant neurotransmitter mechanisms
Knowledge of the major CNS neurotransmitter systems (catecholamines, 5-hydroxytryptamine [5-HT, serotonin] and acetylcholine, see Figures 14-5 and 14-6 and Table 14-1) and their proposed relationships to psychiatric disorders is necessary for understanding psychotropic drug actions. Because the monoamine neurotransmitters are particularly involved in the aetiology, pathogenesis and pharmacological treatment of schizophrenia and depression, these will be described in more detail in this context. Virtually all psychotropic drugs interact with catecholamine-containing neurons by some mechanism. It should be recognised that, while there may be evidence that a transmitter is depleted in a condition (e.g. 5-HT in depression, dopamine in Parkinson’s disease) and that enhancement of that transmitter improves the patient (e.g. selective serotonin reuptake inhibitor antidepressants and levodopa plus a dopa decarboxylase inhibitor in antiparkinson therapy), many links in the cause–effect–cure chain remain to be completed.
Noradrenaline
High concentrations of noradrenaline (NA) are located in neurons in the hypothalamus, pons (locus coeruleus), medulla and cranial nerve nuclei; these noradrenergic neurons innervate virtually the entire CNS from the cerebral cortex to all spinal levels (Figure 14-5). Noradrenergic pathways are thought to have global activating functions in responses to various sensory stimuli, maintaining attention and vigilance. The tricyclic antidepressants (TCAs) are thought to enhance nor adrenergic transmission by inhibiting reuptake of NA into nerve terminals.
Dopamine
The relationship of dopamine to the major psychoses has received much attention. Dopamine is found in high concentrations in the striatum and caudate nucleus and especially in the basal ganglia and extrapyramidal tracts. It is both a neurotransmitter in its own right and a precursor for noradrenaline synthesis (see Figure 12-1). There are various types of dopamine receptors in the brain, especially in the basal ganglia and limbic areas, but D1 and D2 receptors are the primary receptor types influenced by the antipsychotic agents. Although both receptor types are involved with movement disorders in the basal ganglia, tardive dyskinesia, a severe adverse effect of chronic treatment with antipsychotic agents, is thought to be due to supersensitivity of D2 receptors. In schizophrenia, the density of D2 receptors in the caudate and putamen brain regions is consistently high. Newer antipsychotic agents with low affinity for D2 receptors but high affinity for D4 receptors, such as clozapine, are less apt to cause extrapyramidal effects. Further research in this area may result in the development of more specific agents with fewer adverse effects.
5-Hydroxytryptamine (serotonin)
Areas rich in neurons containing 5-HT include the hypothalamus, pineal gland and midbrain, with pathways projecting especially to the spinal cord, limbic system and thalamus. In most cells, 5-HT causes a decrease in discharge rate and hence is inhibitory. There are many types of 5-HT receptors (at least 15 distinct types have been cloned), with more than eight types found in brain regions. The 5-HT1-type receptors are involved particularly in thermoregulation, regulation of the cardiovascular system and hypotension, sexual behaviour and the serotonin syndrome; 5-HT2 receptors mediate excitation rather than inhibition. (In the peripheral nervous system, 5-HT is particularly involved in aggregation of blood platelets and contraction of smooth muscle in the gut.)
5-HT appears to coordinate complex cognitive, sensory and motor patterns. Alterations of 5-HT levels in the nervous system are associated with changes in behaviour and mood: 5-HT activity levels are highest during waking arousal and lowest during REM sleep. Clinical conditions that are influenced by 5-HT levels include affective disorders, ageing and neurodegenerative disorders, anxiety, developmental disorders, eating disorders, vomiting, migraine, obsessive–compulsive disorder, pain sensitivity, sexual disorders, sleep disorders and substance abuse. 5-HT is also involved in many interactions with dopaminergic and glutamatergic pathways, to modulate states of consciousness and contribute to psychotic disorders.
Many drugs mimic or block the action of 5-HT on peripheral tissues and produce changes in mood and behaviour, which suggests that they interfere with the action of 5-HT in the brain. For example the Indian drug reserpine, used to treat hypertension, causes severe depression; and many hallucinogenic agents, including lysergic acid diethylamide (LSD), are chemically related to 5-HT (see Figure 21-4). Ecstasy, or 3,4-methylenedioxymethamphetamine (MDMA), a neurotoxic ‘party drug’, decreases 5-HT turnover in the brain and causes a loss of 5-HT-containing axons. The efficacy of the selective serotonin reuptake inhibitors (SSRIs) in treating major depression is good evidence that 5-HT function is impaired in depressive illness.
Histamine
Histamine, although not a catecholamine, is included among the monoamine transmitters. The fact that systemically administered antihistamines cause CNS effects (sedation, hunger) is evidence for roles of histamine in the brain. Histamine-containing neurons in the posterior hypothalamus send long projecting fibres to many areas, including the cortex, hippocampus, striatum and thalamus. Histamine is thought to be involved in altering food and water intake and in thermoregulation, autonomic activity and hormone release; its effects are mediated by histamine H1, H2 and H3 receptors. As many of the psychotropic agents (antipsychotics and antidepressants) have antagonistic activity on histamine receptors, there are frequently other effects such as sedation, weight gain and antiemetic actions.
Acetylcholine
Acetylcholine is the neurotransmitter in many short interneurons in the CNS, especially in the spinal cord. There are also two major cholinergic tracts in the brain, starting in the basal forebrain and the pons–tegmental areas. Acetylcholine may participate in pain perception, and cholinergic dysfunction has been implicated in degenerative diseases, including Huntington’s chorea and Alzheimer’s disease (see Chapter 20).
Side effects in the peripheral nervous system
The monoamine neurotransmitters and acetylcholine are also neurotransmitters in the peripheral nervous system, particularly in the autonomic and enteric nervous systems. It is highly likely, therefore, that drugs given for their central effects in disorders affecting the mind will have potentially major adverse effects in the periphery. Adverse effects on blood pressure (orthostatic hypotension), gastrointestinal tract functions (dry mouth, constipation, weight gain), sexual function (impotence, decreased libido) and eye functions (blurred vision) are common with antipsychotic and antidepressant drugs.
Clinical aspects of drug therapy in psychiatry
Prescribing of drugs
Indications for drug therapy
Drugs play an important role in psychiatric care. Although many people with mild mental disorders can be treated successfully with psychotherapies, patients with moderate and severe disorders usually require drugs or electroconvulsive therapy (ECT). Clinically, drug therapy reduces or alleviates symptoms and allows the patient an opportunity to participate more easily in other forms of treatment that may produce a permanent change in mood and behaviour. The environment can potentiate the effectiveness of drug treatment or detract from it.
Guidelines for prescribing psychotropic drugs suggest:
Current research into genetic bases for varying aetiologies of psychoses and responsiveness to antipsychotic therapies may soon enhance prescribing for individuals; however, at present pharmacogenetic studies are rarely applied to individual patients.
Drug selection
Generally, prescribers select psychotherapeutic agents on the basis of the patient’s symptoms and diagnostic cat egory, e.g. schizophrenia, bipolar affective disorder or psychoneurosis, and the likely adverse effects. Since their introduction, the antipsychotic and antidepressant agents have been widely prescribed, and frequently over-prescribed, particularly in the elderly. Inappropriate prescribing exposes the older person to an increased risk of adverse reactions and drug interactions that are often detrimental to the person’s cognitive and functional health status.
Informed consent
Informed consent is usually taken to imply that the patient has agreed to participate in particular treatment after being given adequate information to assist in making the decision. In the context of mental illness, however, the concept of informed consent can be difficult. A person suffering severe anxiety may be in no state to weigh up potential benefits or adverse effects of treatment; a person in acute mania or delirium may need restraint before long-term clinical plans can be instituted; and the disordered thought patterns of schizophrenia may make it difficult for the person to make reasoned objective judgements about possible therapies. Patients need to be assisted to make wise and balanced decisions about their treatment—the patient’s involvement in and ‘owning’ of the treatment plan will improve compliance with it. Negotiations should be documented, which may involve signing of appropriate consent forms.
Improving compliance
Ongoing compliance with long-term antipsychotic therapy is often a problem, as many of these medications have unpleasant and disabling adverse effects and the treatment might seem to the patient to be worse than the disease. It is noteworthy in this context that animals in the laboratory situation will self-administer many drugs (for reward), including alcohol, cocaine, opioids, nicotine and amphetamines, but will not self-administer antipsychotic agents such as phenothiazines. In the clinical context, patients may not have sufficient insight into their own condition to recognise the need for medication. Compliance can be improved by:
Discontinuation of therapy and rebound effects
After discontinuation of therapy, there are often withdrawal effects that may be related more to rebound phenomena1 than to any dependence on the drug. For example, after discontinuation of use of antipsychotics, there may be nausea, vomiting, restlessness and excessive cholinergic stimulation effects; after cessation of antidepressant therapy, there may be agitation and insomnia; and after abrupt withdrawal of lithium, relapse of mania. These effects can often be avoided by slow tapering off of the drug. Many patients eventually relapse and require renewed drug treatment.
Adverse effects
Adverse drug reactions
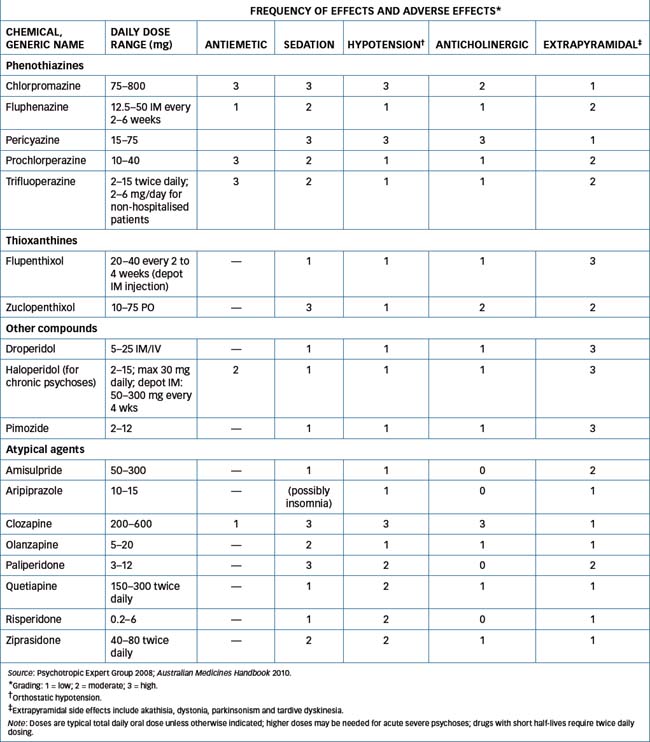
The adverse effect profile of a drug is a useful tool to help a prescriber select an appropriate antipsychotic agent. Drugs that antagonise neurotransmitter receptors in both central and peripheral nervous systems are liable to have wide-ranging effects. This problem is compounded in the cases of antipsychotic and antidepressant drugs by the fact that many of the drug groups act at receptors for several transmitters. For example the earliest antischizophrenic agents, the phenothiazines, are notoriously ‘dirty’ drugs as they antagonise receptors for dopamine (D2), acetylcholine (muscarinic), noradrenaline (α-), 5-HT and histamine (H1). Table 18-1 summarises some of these adverse effects.
Table 18-1 Antipsychotic medications: adverse reactions
| RELATIVELY FREQUENT ADVERSE REACTIONS |
| Sleepiness, dizziness, dry mouth, constipation and nasal congestion reported with phenothiazines and thioxanthines Weight gain commonly reported with most antipsychotics |
| GENERAL ADVERSE REACTIONS |
| Visual changes and hypotensive episodes (more common with phenothiazines and thioxanthines) |
| Dystonia and/or parkinsonian effects, including shuffle in walk, arm or leg stiffness, tremors, mask-like facial expression, dysphagia, imbalance and muscle spasms or unusual twisting effects of face, neck or back (more common with phenothiazines, thioxanthines, risperidone and haloperidol) |
| Akathisia (abnormal motor restlessness and agitation), increased pacing and insomnia (more often reported with haloperidol and thioxanthines). This may be misinterpreted as worsening of agitation associated with the psychosis |
| Tardive dyskinesia, a late-developing serious adverse reaction in about 20% of patients, especially older women, who show involuntary repetitive hyperkinetic movements, usually of the mouth and face; possibly due to supersensitivity to dopamine following upregulation of receptors. There is no effective treatment |
| Neuroleptic malignant syndrome, a rare but potentially fatal adverse effect; features include high temperature, muscle rigidity and altered consciousness |
| Hyperkinesia, agitation and aggressive behaviour |
Treatment of adverse drug reactions
In some cases, adverse effects are sufficiently severe to require treatment if administration of the antipsychotic or antidepressant drug is to continue. Specific drugs are used where possible, e.g. diazepam or propranolol to decrease severe agitation and benztropine to treat anticholinergic or parkinsonian effects.
Adverse drug interactions
There are long lists of potential interactions with psychiatric drugs (e.g. see Drug Interactions 18-1, 18-2, 18-3 and Tables in Therapeutic Guidelines: Psychotropics and Australian Medicines Handbook, Appendix). Elderly patients are particularly at risk, because of the likelihood of renal impairment leading to prolonged half-lives of drugs and because of polypharmacy (excessive use of many drugs) to treat multiple pathologies. In general, due to their mechanisms of action in altering the levels of CNS neurotransmitters, psychotropic agents are likely to interact with all other drugs affecting the central or autonomic nervous systems. These include opioids, anxiolytics, cardiovascular drugs, antihistamines, anaesthetics, sedatives, antiepileptics, endocrine drugs, stimulants, antiemetics, sympathomimetic amines, muscle relaxants, anticholinergics and social drugs such as alcohol and tobacco. Lithium, because of its effects on the kidney, has specific interactions with other drugs affecting the kidneys, such as diuretics, sodium salts and non-steroidal anti-inflammatory agents. In addition, there can be pharmacokinetic interactions based on altered metabolism or excretion of either drug.
It is impossible for anyone—health-care professional or student—to learn all these potential drug interactions. It is safer to understand the general principles and look up reference texts for specific interactions of drugs prescribed concurrently.
Psychiatric therapy in special groups
Psychiatric disorders in childhood
It is estimated that 15% of children show symptoms leading to psychosocial impairment and requiring treatment. Common conditions are anxiety disorders, depressive disorders, attention deficit hyperactivity disorder (ADHD) and conduct disorders. In general, girls are more likely to have emotion-type problems and boys to have behaviourtype problems. Contributing factors are thought to include genetic factors, socioeconomic problems, family disruption, child abuse and stressful life experiences. The prevalence of some disorders decreases with age (e.g. ADHD), whereas for others it increases with age (e.g. depression, schizophrenia, substance abuse).
The increasing use of psychotropic medications in children and adolescents is of concern (see Clinical Interest Box 18-3 and discussion of ADHD in Chapter 19).
Clinical interest box 18-3 Paediatric psychiatric therapy
Children are at a greater risk than adults of developing neuromuscular or extrapyramidal adverse effects from antipsychotic agents, especially dystonias; therapy should be monitored closely. Extrapyramidal effects may be confused with CNS signs of encephalopathy or Reye’s syndrome. Avoid use of phenothiazine antiemetic therapy in such patients.
Children and young adolescents may suffer depression; however, tricyclic antidepressants are not recommended for children, in whom an acute overdose can be potentially fatal. Adverse effects include changes in electrocardiogram patterns, increased nervousness, sleep disorders, complaints of tiredness, hypertension and mild stomach distress.
Adolescents often require a lower dose because of their sensitivity to this drug group. Compliance with long-term medication regimens can be a problem, particularly with teenagers, who usually do not like feeling different from their peer group.
Lithium may decrease the bone density or bone formation in children. If it must be used, serum levels and signs of toxicity must be closely monitored.
Psychiatric disorders in the elderly
Although most elderly people are healthy, fit and active, many old people have physical or mental limitations on their independence. Multiple pathologies and polypharmacy can confound diagnosis of mental illness, as can gradually increasing neurodegenerative or endocrine disorders, anxiety, dementia or depression. Psychotropic drugs need to be used with caution because of the likelihood of impaired renal function, prolonged half-lives and possible drug toxicity (see Clinical Interest Box 18-4).
Clinical interest box 18-4 Geriatric psychiatric therapy
Elderly people prescribed antipsychotic and antidepressant drugs tend to develop higher plasma drug levels because of changes in drug distribution resulting from a decrease in lean body mass, less total body water, less serum albumin for binding, a relative increase in body fat, hyponatraemia and impaired renal and hepatic clearance mechanisms. Therefore, these patients often require a lower drug dose and a more gradual drug dose titration than younger adult patients.
Geriatric patients are more prone to adverse effects such as orthostatic hypotension, anticholinergic effects, extrapyramidal effects and sedation. They should be carefully evaluated before starting therapy and, if an antipsychotic agent is necessary, the person generally should receive only half the recommended adult dose. When clinical improvement is noted, attempts at tapering the dose and discontinuing the drug should be instituted.
The tricyclic antidepressants may cause increased anxiety in geriatric patients. If the patient has cardiovascular disease, the use of tricyclic antidepressants increases the risk of inducing arrhythmias, tachycardia, stroke, congestive heart failure and myocardial infarction.
Lithium is more toxic in geriatric patients so lower lithium dosages, a lower lithium plasma level and very close monitoring are critical in this age group. Generally, excessive thirst and polyuria may be early adverse effects of lithium toxicity, and CNS toxicity, lithium-induced goitre and clinical hypothyroidism may develop.
Psychiatric disorders specific to women
It is now recognised that there are gender issues in mental health. Some are psychosocial: e.g. in certain societies girls and women may have inferior status and roles, less opportunity for education, paid employment or health care, and may be more at risk of violence and abuse. Biological differences in hormones and stresses due to menstruation, pregnancies, breastfeeding, child care and menopause can all increase risk of mental illness. Syndromes specific to women include:
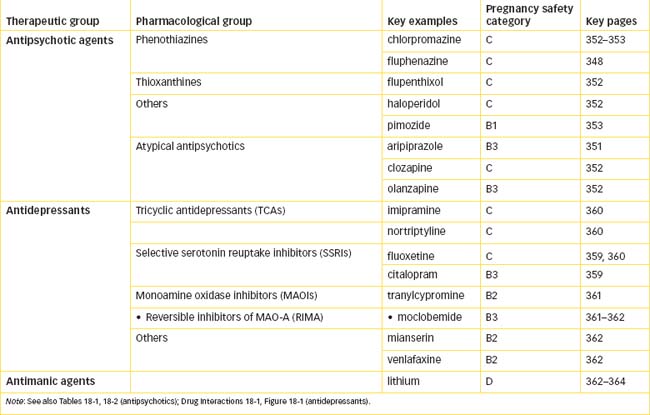
Drugs should be avoided during pregnancy if possible; however, the health of the mother and baby are paramount, so if the mother’s psychiatric condition is so serious as to warrant medication during pregnancy or breastfeeding, then the safest drugs should be used at the lowest effective doses (see Drugs at a Glance 18). Most antipsychotic drugs will be secreted in breast milk, and are sedating, so the infant should be monitored for lethargy and delayed development.
Psychiatric disorders in indigenous populations
Indigenous communities have suffered major disruption after colonisation of their lands, with subsequent discrimination, dispossession, poverty, poor health, suppression of traditional cultures and family supports and lack of educational and employment opportunities. These are all established risk factors for psychological distress and mental illness, particularly for depression, anxiety and substance abuse (see Clinical Interest Box 18-5).
Clinical interest box 18-5 Indigenous mental health
In Australia, Aboriginal people are the most disadvantaged group in socioeconomic terms and frequently have unequal access to health services, education and employment, due to isolation. There is a different understanding of health and illness: spiritual factors, family groups and relationship with the land are seen as important, hence Western psychiatric classifications and treatment methods may not be appropriate. Depres sion, substance abuse and post-traumatic stress disorder are much more prevalent in Aboriginal com munities than in the general population, as are deaths in custody (while rates of schizophrenia and bipolar disorder are similar). Mental health services need to be sensitive to cultural differences and provide appropriate training, policies, resources and ongoing programs to redress the imbalance in mental health.
In New Zealand, the Maori people also place high value on spiritual and family dimensions in life and health; in the traditional community, people with mental illness and epilepsy were not shunned. After dispossession of land and culture, near-extinction, then more supportive public health policies, Maori people still suffer disadvantage and poverty. Maori men have much higher rates of readmission to psychiatric hospitals than Maori women or non-Maori men, and youth suicide rates are almost double those in the non- Maori community. Drug and alcohol misuse is common, as are melancholy and anxiety. Maori participation in mental health services is being encouraged and increased, with culturally based assessments and treatments that acknowledge the importance of traditional culture, spirituality and the extended family.
Non-drug therapy
Detailed discussion of non-drug therapeutic modalities is beyond the scope of this pharmacology text. Because the psychotropic drugs are generally used only after other therapies have been unsuccessful, most patients taking psychotropics have already experienced non-drug therapies, possibly on an ongoing basis, so these therapies are described briefly here.
Psychotherapies
Psychotherapies include various types of treatment based on a relationship between a person needing help for psychological distress or disturbed behaviour or relationships and a trained health-care professional (e.g. psychologist, psychiatrist, social worker, occupational therapist) who systematically uses psychological principles in therapy (see Clinical Interest Box 18-6).
Clinical interest box 18-6 Principles of psychotherapy
Features of psychotherapies (clinical psychological interventions without drugs) include:
Objectives of therapies might be to deal with a crisis, improve specific symptoms, facilitate self-awareness or provide longterm supportive help.
Types of therapies include psycho analysis (frequent long intense sessions over a prolonged period), group psychotherapy, self-help groups, family therapy, couple therapy, sex therapy, crisis intervention, counselling, behav iour modification therapy, cognitive therapy and cognitive behaviour therapy (which challenges patterns of negative thought and behaviour to improve thinking, mood and relationships). Art therapy and music therapy are also included.
Electroconvulsive therapy
Electroconvulsive therapy is used mainly as a safe, highly effective treatment for severe depression unresponsive to drugs, and can also be used for mania and schizophrenia. It was originally introduced (in the 1930s) for schizophrenia, on the rationale that inducing a series of epileptic-type fits would help the disordered CNS functions. Early techniques were primitive and potentially dangerous; however, in current practice anaesthetics and muscle relaxants are used, dosage of the electrical current is more accurately determined and applied and EEG is routinely monitored. The convulsion induced by the current is essential; ECT is thought to act by causing readjustment in monoamine levels in the brain.
There is a higher positive response from ECT (80%) than from antidepressants (60%) in severe depression, especially when associated with psychotic features, sui cidal ideas, psychomotor slowing and weight loss. ECT is relatively contraindicated in people with severe cardiovascular and respiratory disorders, or in conditions with raised intracranial pressure. The main adverse effects are memory impairment and confusion, plus muscle pains.
Psychosurgery
In the early 20th century, the technique of prefrontal lobotomy was used to treat severe schizophrenia by severing the connections between the frontal lobes and the rest of the brain. The much more specific tech nique currently used, limbic system surgery, targets the connections between the frontal lobes and particular components of the limbic system (see Chapter 14 and Figure 16-3). It is sometimes used in severe cases of depression and obsessive–compulsive disorder that are unresponsive to other treatments.
Antipsychotic agents
Schizophrenia
Schizophrenia (sometimes erroneously referred to as split personality) is manifested by disordered emotion, speech, thought, perception and volition, leading to delusions, withdrawal and loss of insight (see Clinical Interest Box 18-7). It is thought to be due to abnormal brain circuitry or disturbed neurotransmission, especially overactivity of the mesolimbic dopaminergic pathways. It has an insidious onset in young adults (aged 15–35 years) and a prevalence of about 1% in virtually all societies. It causes considerable morbidity, lost work time and mortality (from suicide).
Clinical interest box 18-7 Signs and symptoms of schizophrenia
Patients with schizophrenia have a wide variety of symptoms and signs:
A deficit of willed action can explain many of the negative symptoms, while the bizarre positive symptoms may be due to a deficit in self-monitoring (inhibitory) systems. The target symptoms are used as monitoring parameters to evaluate the person’s response to a drug.
Most antipsychotic agents produce useful effects on the positive symptoms; however, the negative symptoms are usually less responsive to drug therapy, as are the cognitive aspects. Newer atypical antipsychotic drugs, such as clozapine and risperidone, appear to be more effective than other neuroleptic agents against the negative symptoms.
See publications by the Schizophrenia Fellowship of New Zealand (http://www.sfnat.org.nz/) and the Mental Illness Fellowship of Australia (http://www.mifa.org.au).
Aetiological factors have been studied at length: there is a strong biological basis and genetic vulnerability, and environmental associations with perinatal complications (i.e. first few days of the patient’s life) or stressful relationships or life events. Involvement of 5-HT is proposed, due to the similarity between psychoses and the hallucinations produced by serotonergic drugs like lysergic acid diethylamide (LSD, see Figure 21-4). Studies of molecular mechanisms implicate genes coding for proteins involved in dopamine, glutamate and GABA pathways and also immune and signalling networks. The current view is that many cases of schizophrenia are caused by a defect in early brain development. There is a well-established strong association between use of cannabis (marijuana) in adolescence and increased risk of developing schizophrenia as a young adult. A related condition, schizophreniform psychosis, has an acute onset related to drugs or trauma (physical or emotional).
Antipsychotic drug groups
Antipsychotic, or neuroleptic, agents are the mainstay of treatment of schizophrenia. They are also used in acutely disturbed patients in the manic phase of bipolar disorder or in acute agitation or delirium. The first antipsychotic agent, which is still the prototype phenothiazine drug, was chlorpromazine, well known by its trade name Largactil. This was the first effective tranquilliser (a drug prescribed to calm an agitated or anxious individual) without serious sedating actions. It was released in the early 1950s and revolutionised treatment. Hundreds of analogues were developed as ‘me-too drugs’, of which a few are still in use (see Table 18-2). More recent antipsychotics tend to be grouped as ‘second-generation’ or ‘atypical’ agents.
Classification of antipsychotics
Antipsychotics are sometimes classified into groups using various criteria; however, it is difficult to generalise as there are still many differences between agents within a group.
High–low potency
Antipsychotic medications (neuroleptics, major tranquillisers) have been classified (based on average dose required) as low-potency, intermediate-potency and highpotency drugs. For example, 100 mg chlorpromazine (low-potency agent) is considered to be about equivalent to 5 mg trifluoperazine or 2 mg haloperidol (highpotency agents). The low-potency agents tend to have predominant sedating, hypotensive and anticholinergic effects with fewer extrapyramidal side effects, whereas the high-potency drugs cause more problems with extrapyramidal side effects but are less sedating, hypotensive or anticholinergic.
Typical–atypical
On the basis of chronology, antipsychotics are also classified as being first-generation agents (i.e. the older agents), such as the phenothiazines, thioxanthines and haloperidol-type drugs, which thus came to be considered typical or conventional, and second-generation agents, such as clozapine, olanzapine and risperidone, which appear to have rather different profiles of actions (see Table 18-2) and are known as the atypical antipsychotics; they are less likely to induce extrapyramidal side effects, but more likely to cause metabolic effects such as weight gain and diabetes.
Actions and mechanisms of antipsychotics
Therapeutic actions
Antipsychotics are particularly effective against the positive symptoms of schizophrenia. They decrease hallucinations, delusions, initiative, emotion, aggression, responses to external stimuli and thought disorder, and can prevent relapses. The patient may become drowsy but is readily arousable without confusion. There is good evidence that the mechanism of action of antipsychotics is by antagonism of dopamine receptors, especially the D2 subclass, which mediate the main inhibitory effects of dopamine in the CNS, particularly in the nigrostriatal, mesolimbic and tuberoinfundibular systems. This action leads to useful therapeutic effects (slower thinking and movements and antiemetic actions) and also to common adverse reactions including extrapyramidal effects (see Clinical Interest Box 18-7) and hyperprolactinaemia, which infrequently results in swelling of the breast and milk secretion.
Clinically, it is known that many antipsychotic drugs (and antidepressants) take many weeks before their actions are most effective, even though their biochemical actions may be immediate. It is thought that the delay for antipsychotics may be due to a transient increase in dopaminergic activity, which changes after about three weeks to inhibition, when the antipsychotic effects ‘kick in’.
Adverse drug reactions
As discussed earlier, the phenothiazines also block receptors for acetylcholine, noradrenaline (α-receptors), histamine, and 5-HT, so there are wide-ranging adverse effects, including sedation, anticholinergic and gastrointestinal effects, hypotension and movement disorders. Partly because of the adverse effects, there is often poor compliance with antipsychotics and they have no abuse potential. Long-acting depot IM injections of oily solutions of some of the agents help overcome compliance problems. New drugs, and old drugs being used for new indications, require post-marketing monitoring for adverse effects (see Clinical Interest Box 18-8).
Clinical interest box 18-8 Post-marketing monitoring of thioridazine and moclobemide
Thioridazine and arrhythmia: prescribing changes
Thioridazine, a phenothiazine tranquilliser, increases the risk of arrhythmias from QT prolongation. After reviewing international and New Zealand data, the NZ Medicines Adverse Reactions Committee recommended that thioridazine should not be prescribed if there are interacting medicines or predisposing conditions:
Thioridazine has since dropped out of favour due to serious cardiac arrhythmias; and has been withdrawn from use in Australia (2007).
Moclobemide can put the pressure up
A reduction in blood pressure associated with postural hypotension, as well as spontaneous hypertension, are consid ered to be typical side effects of conventional, irreversible and non-selective MAO (A+B) inhibitor anti depressants. The new generation of reversible MAO-A inhibitors is, however, expected to have negligible cardio vascular effects and low propensity to induce either blood pressure increases or decreases. However, the true incidence of such changes is largely unknown since observation studies, assessing the frequency of blood pressure changes in selected populations of patients treated in practice, are lacking. The frequency of hyper tension in patients treated with moclobemide was reported to be 0.5/100, whereas there were no reports of hyper tension associated with the SSRI antidepressant fluoxetine.
Source: NZ Prescriber Update No. 21, pp 4–7; June 2001.; Source: Delini-Stula et al, 1999.
As can be seen from Tables 18-1 and 18-2, the adverse effect profiles of various antipsychotic agents can be compared and tabulated to aid in choice of drug. For example, if a drug with a strong sedative property is desired, chlorpromazine might be prescribed, whereas haloperidol or pimozide is less likely to cause daytime drowsiness. However, the drugs with sedative effects impair psychomotor performance and thus can impair driving skills, ability to operate machinery and reaction times to dangerous stimuli. Sedative effects of other CNS depressants taken concurrently, including alcohol, will be increased. If anticholinergic effects (such as dry mouth, blurred vision, constipation and urinary retention) are disturbing to the patient, the prescriber could select an agent with less potential for inducing such effects, such as fluphenazine, thiothixene or haloperidol.
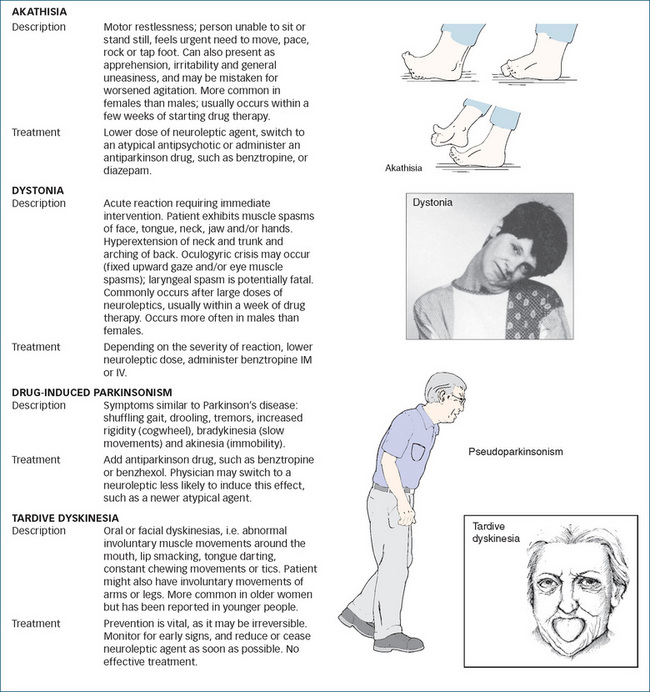
Extrapyramidal effects
An important group of common adverse effects from antipsychotics are the extrapyramidal effects, i.e. those involving marked motor stimulation mediated via pathways in the extrapyramidal system, the indirect descending motor pathways in the spinal cord (see Figure 18-1). Motor effects including dystonias, akathisia, parkinsonism and dyskinesia are described in Clinical Interest Box 18-7. If extrapyramidal effects are troublesome, one of the atypical agents might be chosen. An anticholinergic drug (e.g. benztropine) is sometimes required to reduce excessive motor stimulation.
Neuroleptic malignant syndrome
This is a rare but potentially fatal adverse effect occurring in 0.5%–1% of patients on typical antipsychotics. It involves high temperature, muscle rigidity, altered consciousness and impaired autonomic homeostasis. Treatment requires withdrawal of the drug, hydration and sometimes bromocriptine (a dopamine agonist) and dantrolene to control muscle spasms.
Other adverse effects
All groups of antipsychotics can cause metabolic disturbances such as weight gain, diabetes and dyslipidaemia; clozapine, olanzapine and quetiapine particularly are associated with type 2 diabetes. The atypicals are sometimes prescribed for behavioural disturbances in people with dementia; this use is associated with increased risk of morbidity and mortality from stroke. Other antipsychotics have individual adverse reactions profiles; e.g. chlorpromazine is associated with skin reactions and photosensitivity, and several agents with prolonged cardiac QT interval.
Treatment of adverse drug reactions
Extrapyramidal effects and/or parkinsonian effects, generally related to overactivity of cholinergic pathways, are treated with antimuscarinic agents such as benztropine or benzhexol (see Chapters 11 and 20). Akathisia (motor restlessness) may possibly be avoided by switching to a different antipsychotic drug or treated with propranolol or diazepam. People with schizophrenia are at greater than usual risk of diabetes, exacerbated by antipsychotic drugs, so should be managed with appropriate education, lifestyle changes and monitoring of blood glucose levels and other physiological parameters.
Other indications for antipsychotic agents
While antipsychotics are most commonly used in treatment of schizophrenia, there are several other conditions in which the drugs are indicated. For example, chlorpromazine and haloperidol are indicated for short-term management of severe anxiety and for intractable hiccups, droperidol as an adjunct in anaesthesia and olanzapine, zuclopenthixol or quetiapine in bipolar disorder as an adjunct to lithium. As indicated in Table 18-2, the conventional agents have useful antiemetic actions (presumably due to antidopamine actions in the CNS); those most commonly prescribed as antiemetics are droperidol and haloperidol; prochlorperazine is a phenothiazine that is now used only as an antiemetic. Haloperidol and pimozide are indicated for treatment of choreas (repetitive behaviours) such as tics and Tourette’s syndrome, a rare CNS disorder that presents as involuntary, rapid and repetitive motor movements, tics (facial grimaces and blinking) and vocal noises.
Behavioural emergencies
In behavioural emergencies, such as when a person is threatening assault or self-harm or is excessively agitated, hostile, aggressive or intimidating, medical intervention may be necessary (as may physical assistance from security staff or police). Depending on the aetiology and situation, pharmacological management may include use of sedatives, such as diazepam or midazolam, or antipsychotics, such as chlorpromazine, droperidol, olanzapine or risperidone. Drugs may have to be administered by IM or IV injection, and close monitoring of the patient’s vital signs is necessary.
Delirium
Delirium is a state characterised by impaired cognitive function and ability to maintain attention, often with agitation, delusions and disturbed sleep patterns. Aetiologies include CNS infections and metabolic disturbances, and drug toxicity (especially from alcohol, anticholinergic drugs or narcotic analgesics). Haloperidol is indicated in pharmacological management.
Childhood psychoses
Schizophrenia is rare in children; however, there are other disorders that may indicate the need for antipsychotic agents in children. These include disruptive behaviours and some developmental disorders. Risperidone in parti cular is used in severe behavioural disorders, associated with autism or intellectual disabilities, and in Tourette syndrome.
Atypical antipsychotic agents
The second-generation atypical antipsychotics have less potential than earlier neuroleptics to cause extrapyramidal effects or sedation and are becoming more commonly prescribed; however, they are more likely to cause metabolic adverse effects (increased blood glucose, dyslipidaemias) and weight gain. They are not a homogeneous class, but have widely differing adverse effect profiles (see Table 18-2). Amisulpride, aripiprazole, clozapine, olanzapine, paliperidone, quetiapine, risperidone and ziprasidone are the drugs in this class. They are better at treating the negative symptoms of schizophrenia than the typical agents, and are less likely to cause extrapyramidal effects, tardive dyskinesia or neuroleptic malignant syndrome. These drugs are still relatively new so efficacy and safety in children, pregnancy or lactation are not well established. They are generally more expensive than older drugs. Clozapine and olanzapine are most likely to cause metabolic effects and weight gain.
Aripiprazole
Aripiprazole will be described as an example of the atypical antipsychotics (see Drug Monograph 18-1); however, none is really representative as they have varying adverse effect profiles; aripiprazole is one of the safest.
Drug monograph 18-1 Aripiprazole
The antischizophrenia activity of aripiprazole is thought to be due to its partial agonist actions at dopamine D2 and 5-HT1A receptors, plus antagonist actions at 5-HT2A receptors. Clinical improvement may take several days to some weeks.
Pharmacokinetics
Aripiprazole is highly lipid-soluble, so is well absorbed orally (delayed by food) with peak plasma levels reached in 3–5 hours; oral bioavailability is about 87%. It is widely distributed in the body, with an apparent volume of distribution of 4.9 L/kg. It is highly bound to plasma proteins (88%–99%). It is extensively metabolised, mainly by enzymes CYP3A4 and 2D6. The main metabolite, dehydroaripiprazole, has similar affinity for D2 receptors as the parent drug, thus prolonging its duration of action. Some unchanged drug is excreted via the faeces, and metabolites via urine and faeces. The half-life of the main active metabolite is about 100 hours; steady state is not reached for about 14 days. No dosage adjustment is required in severe renal or hepatic impairment.
Adverse effects
See the earlier discussion on adverse effects of psychotropic agents and Tables 18-1 and 18-2. Common adverse effects include headache and lightheadedness, akathisia (motor restlessness) and constipation; less common is orthostatic hypotension. Rare cases of neuroleptic malignant syndrome, seizures or metabolic disturbances have occurred. A small weight gain (1–3 kg) is likely over 52 weeks, especially for patients with a low body mass index to start.
Drug interactions
See general statement in text. In particular, aripiprazole is likely to interact with inhibitors of enzymes CYP3A4 (especially ketoconazole) and 2D6 (including fluoxetine and paroxetine), which can decrease its metabolism and increase effects and toxicity thus requiring reduced dosage. Inducers of CYP3A4 (notably carbamazepine) can enhance metabolism of aripiprazole and thus require higher doses.
Warnings and contraindications
Elderly patients with dementia-associated psychosis are at increased risk of death (from cardiovascular disorders) from atypical antipsychotics; use with caution in patients with recent history of myocardial infarction or with unstable heart disease. Contraindicated in patients hypersensitive to the drug or any tablet ingredients. Use with caution in children (see Clinical Interest Box 18-3), in the elderly (see Clinical Interest Box 18-4), in pregnant women (category B3, see Drugs at a Glance 18) or during lactation.
Dosage and administration
Typical dosage (oral tablets) is 10–15 mg once daily; at least 2 weeks should be allowed for reaching steady state. Tablets are available in strengths of 5, 10, 15, 20 or 30 mg, allowing close titration of dose. Patients can be safely switched from another antipsychotic to aripiprazole without a wash-out period.
Clozapine
Clozapine differs from the other neuroleptics by antagonising D1, D2 and D4 dopamine receptors, with less affinity for D2 receptors, so it is less apt to induce extrapyramidal effects. It also antagonises 5-HT2, α1-adrenoceptors and histamine H1 receptors. It may be the best antipsychotic for management of negative symptoms; however, because it has the potential for causing neutropenia, agranulocytosis, seizures, cardiomyopathies and severe constipation, this drug is reserved for treatment-resistant schizophrenia or for when the adverse effects of other drugs preclude their continued use. Treatment with clozapine is closely monitored. The manufacturer recommends dispensing only weekly supplies and performing weekly white blood cell counts for the first 18 weeks. Other common adverse effects include drowsiness and seizures, orthostatic hypotension when treatment is started and type 2 diabetes. In Australia, there is a national distribution system requiring registration of doctors, pharmacists and patients involved with the clinical use of clozapine.
Olanzapine and risperidone
Olanzapine and risperidone also block both 5-HT2 and dopamine D2 receptors. Compared with clozapine, they are less sedating, cause fewer anticholinergic effects and do not have the same potential to cause serious agranulocytosis. There are clinically significant drug interactions with CNS depressants, antihypertensives, dopamine agonists, the new antidepressants and drugs that inhibit or enhance drug-metabolising enzymes. Olanzapine has also been approved for IM use in treatment of acute manic episodes associated with bipolar disorder, agitation and behavioural symptoms in dementia; it is available as tablets, injection and also a wafer form for acutely psychotic patients.
Others
Quetiapine is an antagonist at many CNS neurotransmitter receptors, such as 5-HT (5-HT1A and 5-HT2), dopamine (D1 and D2), histamine (H1) and adrenergic (α1 and α2) receptors. It has low potency and has a short half-life, so twice-daily doses are required. Other ‘atypicals’ include amisulpride, paliperidone (an active metabolite of risperidone) and ziprasidone; their places in the range of antipsychotic drugs are not fully established.
Drug interactions
As with the conventional antipsychotics (Drug Interactions 18-1), there are a multitude of potential drug interactions with atypical agents, especially with all drugs that depress the CNS, alter cardiac QT interval, cause hypotension, lower the seizure threshold, are dopamine agonists or antagonists, have anticholinergic effects, affect blood glucose concentration or either inhibit or enhance the metabolism of the antipsychotic. Reference texts (e.g. the Australian Medicines Handbook) should be consulted for specific interactions.
Conventional (typical) antipsychotics
Phenothiazine derivatives
Chlorpromazine will be considered as the prototype phenothiazine antipsychotic drug (see Drug Monograph 18-2) and brief information will be given on other phenothiazines.
Drug monograph 18-2 Chlorpromazine
Indications
Chlorpromazine is indicated in the treatment of schizophrenia and other acute and chronic psychoses, intractable hiccups and short-term management of anxiety, agitation or disturbed behaviour in non-psychotic disorders.
Pharmacokinetics
Phenothiazines generally are lipid-soluble so are well absorbed orally and concentrate in the CNS. Chlorpromazine is subject to first-pass meta bolism and oral bioavailability ranges from 10% to 80%. Peak plasma levels are reached in 1–4 hours after oral administration or in 15–30 minutes after IM injection. The onset of antipsychotic effect is achieved gradually over several weeks, and peak therapeutic effect occurs between 6 weeks and 6 months. Duration of action ranges from 6 to 24 hours or more depending on dosage and frequency of drug administration. Chlorpromazine is metabolised in the liver to various metabolites, which are generally inactive and are excreted primarily by the kidneys.
Adverse effects
See the earlier discussion on adverse effects of psychotropic agents and Tables 18-1 and 18-2. Common adverse effects include orthostatic hypotension, sedation, anticholinergic and extrapyramidal effects (see Clinical Interest Box 18-2)—note that tardive dyskinesia has no known effective treatment so early assessment and diagnosis are crucial to prevent progression. Chlorpromazine can cause cholestatic jaundice and phototoxic skin reactions. IM and SC injections are not recommended as they are painful and can cause muscle necrosis.
Drug interactions
See Drug Interactions 18-1. In addition, chlorpromazine is a substrate for CYP2D6, so interactions with inducers, inhibitors or substrates of this enzyme are likely. Lithium can decrease concentration of chlorpromazine, whereas propranolol and chlorpromazine can increase the concentration of each other.
Warnings and contraindications
Use with caution in patients with breast cancer, cardiovascular disease, severe liver impairment, hyperthyroidism, Parkinson’s disease, chronic respiratory disease or epilepsy, in glaucoma and other conditions involving problems of parasympathetic control, in children (see Clinical Interest Box 18-3) and in the elderly (see Clinical Interest Box 18-4).
Avoid use in patients with phenothiazine hypersensitivity, phaeochromocytoma, profound CNS depression, in alcohol abusers, in pregnant women (see Drugs at a Glance 18), during lactation and in people often exposed to sunlight.
Dosage and administration
The dosage of antipsychotic agents varies according to the individual, the indication for treatment and the patient’s response to the medication. It is best to titrate from a low dose, increasing when necessary to produce a therapeutic response. When stopping antipsychotic therapy, the dosage should be reduced gradually over 2 or 3 weeks, otherwise rebound nausea, vomiting, dizziness, tremors and dyskinesias may occur. Chlorpromazine is available in tablet, oral liquid and injection (not recommended) formulations; the maximum adult daily dose (all routes) is 600–800 mg.
Drug interactions 18-1 Conventional antipsychotic drugs
| Drug | Possible effects and management |
| Other CNS depressants, e.g. benzodiazepines, anaesthetics, lithium, opioids and alcohol | Additive CNS depression (may be useful in acutely disturbed patients), respiratory depression and hypotensive effects. The drug dosage should be reduced |
| Drugs that lower seizure threshold | May cause seizures—avoid combination |
| Levodopa (L-dopa), other dopamine agonists | Concurrent use with antipsychotic agents can render levodopa ineffective in controlling Parkinson’s disease and antipsychotics ineffective in schizophrenia |
| Antihypertensive agents | Concurrent drug use with the antipsychotics may result in an exacerbation of hypotensive effects |
| Drugs with anticholinergic effects | Concurrent drug use may result in an increase in anticholinergic adverse effects, including delirium |
| Drugs that prolong the QT interval, such as antiarrhythmic agents, many antimicrobials, tyrosine kinase inhibitors etc | Additive prolongation with antipsychotics droperidol, haloperidol, pimozide (also amisulpride, ziprazidone)—potentially fatal ventricular arrhythmia |
Other phenothiazines
Tables 18-1 and 18-2 show the properties of various phenothiazines. Fluphenazine and trifluoperazine cause extrapyramidal reactions relatively frequently. Flu phenazine is available as a depot IM injectable for chronic use. Pericyazine is a low-potency drug and is recommended in low doses for behavioural disturbances in children, the elderly and in dementias.
Prochlorperazine is a phenothiazine that is mainly used for its antiemetic actions: it is usually more effective than simple antihistamines in severe vomiting, especially in vertigo and migraine. It is available as tablets, injections and suppositories.
Thioxanthines
The thioxanthines flupenthixol and zuclopenthixol resemble the piperazine phenothiazines (such as fluphenazine) in their antipsychotic effects, including the high incidence of extrapyramidal effects (see Table 18-2). Their antipsychotic indications, adverse effects, precautions and drug interactions are similar to those for the phenothiazines. Zuclopenthixol is available in three different chemical and pharmaceutical forms: as tablets, as a short-acting depot injectable preparation (for initial use in highly disturbed patients, for 2–3 days only) and in a long-acting depot form.
Other conventional antipsychotics
Butyrophenone derivatives: haloperidol, droperidol
Haloperidol and droperidol, although structurally different from the other antipsychotic agents, have similar properties in terms of antipsychotic efficacy, adverse effects and drug interactions. Haloperidol appears to have a selective CNS effect. It competitively blocks D2 receptors in the meso limbic system and causes an increased turnover of brain dopamine to produce its antipsychotic effect. It is associated with a significant degree of extrapyramidal effects but has less effect on noradrenergic receptors. It is a useful antipsychotic and antiemetic and is used to treat severe behavioural problems in children, in acute mania and in Tourette’s syndrome.
Droperidol is used as an adjunct in anaesthesia and in short-term management of disturbed behaviour and severe anxiety. It is available only as an injection.
Pimozide
Pimozide antagonises dopamine receptors in the CNS and increases dopamine turnover. It has very weak α-blocking and hypotensive actions. It is indicated for treating psychoses and for treating severe motor and vocal tics in people with Tourette’s syndrome who have failed to respond to haloperidol. Extrapyramidal effects are common and there is a risk of arrhythmias when used in combination with drugs that affect heart rate or if the pimozide plasma level becomes high, so ECG should be monitored regularly.
Drug interactions with conventional antipsychotics
Many interactions are possible; reference texts should be consulted for specific interactions. Some of the more common effects that can occur when a conventional antipsychotic such as a phenothiazine is given with other drugs are listed in Drug Interactions 18-1. In addition to these typical group pharmacodynamic interactions, each individual phenothiazine may have specific interactions related to its pharmacokinetic properties, such as which CYP450 isoenzyme is involved in its metabolism and whether or not its elimination is enhanced or inhibited.
New drugs on the horizon
Despite the many drugs that have been developed for treatment of schizophrenia, none is ideal. There are still many concerns over safety and efficacy, and new agents and formulations are required. Some drugs in the pre-marketing stage (in 2010) are:
Treatment of affective disorders
Affective disorders, or mood disturbances, include depression (the most common) and mania; bipolar affective disorder (BAD) involves mood swings between these conditions.
Clinical aspects
Depression and mania
Over the years many classifications of depression have been used, such as the time during life at which depression occurred (childhood, adolescent, postnatal or senile depression), or the reason for the depression, such as exogenous (from outside; reactive or secondary) depression or endogenous depression.
Exogenous depression may be a person’s response to a loss, such as the loss of a loved one or loss of a job, or to the presence of a serious illness or disappointment. The response may manifest as lack of pleasure or interest in activities and everyday living. This is usually referred to as normal depression or ‘the blues’ and generally improves within a few weeks or months without the use of antidepressant medications. The mobilisation of support systems and, if necessary, psychotherapy are useful in exogenous depression, and benzodiazepines can help with associated anxiety.
Endogenous (from within) depression is characterised by the absence of obvious external causes for depression. This type of depression may be caused by genetic determination and biochemical alterations, and can be relieved by antidepressant medications.
The current classification of depressive disorders has eliminated the use of the terms ‘exogenous’ and ‘endogenous’. Instead, major affective disorders are defined as bipolar affective disorders (BAD; manic–depressive psychosis, including one or more manic or hypomanic episodes) or major unipolar depressive disorders (single major depressive episode or recurrent episodes). There are also atypical affective disorders, and depression can occur along with neurotic and personality disorders or schizophrenia.
Criteria for major depression include the presence of:
Mania, the opposite pole of bipolar depressive illness, is characterised by wild mood swings, excessive energy, high pressure of speech, extravagant gestures and gifts and seeming lack of need for sleep.
It is estimated that in Australia at any time one in five people has depressive symptoms, many of which are transient and normal. Clinical BAD has a prevalence of about 10% and is being recognised as a serious public health issue as there is a high risk of suicide (10%–19% in people with BAD).
Measures to treat depression include reduction of environmental stressors, ECT, psychotherapy and drug therapy. In some patients, antidepressant drug therapy in combination with one or more adjunct measures is more effective than drug therapy alone. Herbal remedies, such as St John’s wort, have become popular (see Clinical Interest Box 18-9). Referral to a specialist psychiatrist is advisable in cases of severe depression when the patient has not responded to antidepressants or other measures.
Clinical interest box 18-9 St john’s wort and other complementary and alternative therapies in psychiatry
Extracts of the plant St John’s wort (Hypericum perforatum) have been used for more than 2000 years for their medicinal properties and the plant is believed to be the most widely prescribed herbal medicine worldwide. About 450 products containing St John’s wort are listed in Australia. St John’s wort has been shown to block reuptake of monoamine neurotransmitters, bind to GABA receptors, upregulate 5-HT receptors, and inhibit MAO and COMT enzymes.
There is a significant amount of evidence for the effec tiveness of St John’s wort in depression. Many double-blind, randomised controlled trials subjected to meta-analysis have shown St John’s wort to be more effective than placebo but less effective than tricyclic antidepressants. Rates of adverse effects are low but ‘serotonin syndrome’ and drug interactions can occur, particularly with other antidepressants, as they also increase 5-HT levels. Hypericum extracts are potent inducers of hepatic drug-metabolising enzymes and can reduce the levels and efficacy of important drugs such as warfarin, digoxin, theophylline, anti-retroviral agents, cyclosporin and oral contraceptives.
Frequent problems with the use of natural products and complementary therapies in mental disorders are that:
Other complementary and alternative therapies used in mental illnesses include folate, tryptophan, tyrosine, 5-adeno sylmethionine, phenylalanine, acupuncture, aroma therapy, prayer, t’ai chi exercise and homeopathy as adjuncts to antidepressants; Ginkgo biloba for dementia; kava for anxiety; valerian for insomnia and stress; omega-3 polyunsaturated fatty acids as mood stabilisers; and hypnotherapy, movement therapies, acupuncture and dietary interventions in schizophrenia. Cognitive behaviour therapy is significantly effective in depression, insomnia, anxiety and panic disorders. Interestingly, there appear to be no proven effective herbal remedies for schizophrenia.
Patients and consumers should be asked about their use of all remedies—prescription, non-prescription and complementary.
Aetiologies of affective disorders
No single factor has been identified as the cause of affective disorders. Psychiatrists who emphasise psychosocial therapies will identify stressful events or mental conflicts (divorce, death of a parent or partner, inadequate parenting, physiological stressors, illness, infection or childbirth) that preceded the onset of depression. Others adhering to the biological theory tend to explain affective disorders by reference to the monoamine theory, i.e. reduced levels of catecholamine (noradrenaline, dopamine, adrenaline) and indoleamine (5-HT) transmitters in the CNS, or to changes in hormone or sodium levels. Functional polymorphisms in the promoter region of the serotonin transporter gene have been found to moderate the influences of stressful life events on a person’s liability to suffer depression or tendency to suicide. A combination of genetic, psychosocial and biological factors probably leads to a common pathway that results in an affective disorder.
Many drugs themselves can evoke depression, probably by altering amine neurotransmitter levels in the CNS. Drug groups implicated include sedatives (alcohol, benzodiazepines, barbiturates), antipsychotics, antihypertensives (reserpine, β-blockers), hormones (corticosteroids, oral contraceptives), opioids and hallucinogens.
The monoamine theory in affective disorders
Imbalances in the centrally acting monoamine neurotransmitters, especially noradrenaline and 5-HT, have been theorised to be the cause of depression and mania. A deficiency in central noradrenaline or 5-HT has been associated with depression, whereas an excess of dopamine or noradrenaline is believed to be related to mania (see Figure 14-5). This theory is borne out by the efficacy as antidepressants of drugs that increase levels of monoamine transmitters, as described later.
Antidepressant drugs
Indications for antidepressant drugs
Antidepressants are indicated for treatment of mood disorders plus many other conditions: post-traumatic stress disorder, neuroses (some anxiety disorders, panic disorder, obsessive–compulsive disorder and eating disorders), in the management of enuresis and incontinence, premenstrual syndrome and as adjunctive therapy in neuropathic pain, migraine and ADHD in children.
Antidepressant drug groups
The first antidepressant drugs were discovered by serendipity (sheer good luck): iproniazide, an antitubercular agent, and imipramine, a drug being tested as an antischizophrenic, were found to elevate the mood in subjects taking the drugs. This led to studies of the actions and mechanisms of similar drugs, which came to be called the tricyclic antidepressants (TCAs, imipramine-like drugs) and MAO inhibitors (iproniazide-like drugs), respectively.
Mechanisms of action of antidepressants
The storage, release, action on receptors and inactivation of amine neurotransmitters are shown diagrammatically in Figure 12-5 (in the peripheral nervous system), and proposed mechanisms of action of antidepressants are shown in Figure 18-2. Although there are inconsistencies in some of the theories (e.g. why is cocaine not an effective antidepressant, and why and how is mianserin effective?), it is generally accepted that:
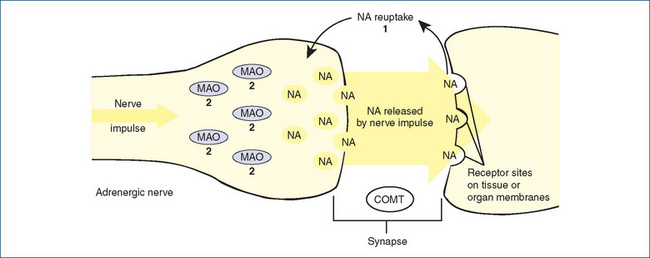
Figure 18-2 Proposed mechanisms of action of antidepressant drugs. Normally, noradrenaline (NA) is released from storage sites in vesicles within the noradrenergic nerve ending by the arrival of a nerve impulse. The released NA diffuses across the synaptic cleft and acts on adrenoceptors on the postsynaptic nerve or other cell. Released NA is inactivated mainly by reuptake back into the nerve ending (uptake 1 process) and then stored again in vesicles. Released NA may also be metabolised by catechol-O-methyltransferase (COMT) enzymes located in the synaptic cleft or by the enzyme monoamine oxidase (MAO) within the nerve terminal.
Antidepressant drug therapy: 1 tricyclic antidepressants block the reuptake of released NA and prevent it from re-entering the adrenergic nerve, thus there is more NA available to act on receptors; 2 MAO inhibitors block MAO located on the surface of the mitochondria within the cell, leaving more NA available for release. Selective serotonin reuptake inhibitors (SSRIs) selectively block the reuptake process for 5-hydroxytryptamine (5-HT, serotonin), analogous to mechanism 1 for NA, thus allowing more 5-HT to act on postsynaptic 5-HT receptors.
Other drugs acting on monoamine transmission in the CNS include reserpine, which blocks storage of catecholamines and hence inhibits aminergic transmission; cocaine, which is a powerful inhibitor of the NA reuptake process; amphetamine and tyramine, which are accumulated by uptake 1 and stored in vesicles, displacing NA, which is free to act; and entacapone, a COMT inhibitor used in Parkinson’s disease.
Adverse drug reactions
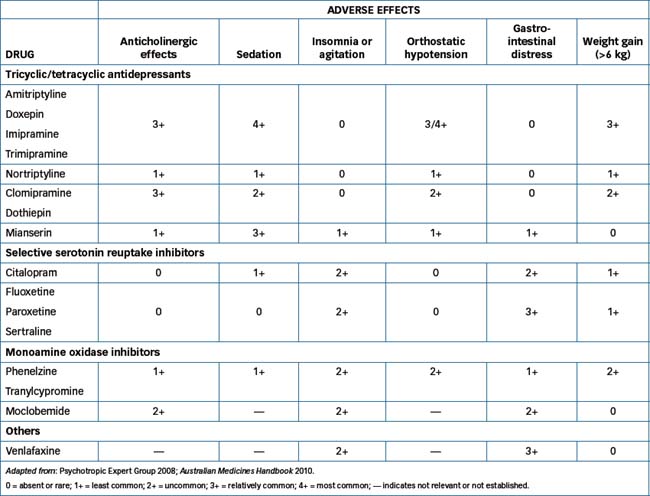
Due to their effects of enhancing neurotransmission by monoamines, transmitters present in many areas of the peripheral, enteric and central nervous systems, these drugs (like the antipsychotics) have many adverse effects (see Table 18-3). (The relative places of newer drugs in such comparative tables are not well established).
Serotonin syndrome
An adverse effect due to excessive stimulation of 5-HT2A receptors by serotonergic drugs is the serotonin syndrome or serotonin toxicity. This is characterised by mental state changes (confusion, delirium, hypomania), GI tract effects (diarrhoea), neuromuscular hyperactivity (hyperreflexia, incoordination, tremor), autonomic instability, sweating, fever and shivering. It occurs particularly when MAOIs are combined with SSRIs. Other drugs that enhance 5-HT transmission, including drugs used in migraine, opioid analgesics, CNS stimulants, many illicit drugs and St John’s wort, can also cause or exacerbate the syndrome. The implicated drugs must be stopped immediately as the syndrome is serious and deaths have occurred.
Drug interactions
As for the antipsychotics, with antidepressants there are potentially many drug interactions due mainly to their effects on many neurotransmitter systems; reference texts should be consulted for interactions with individual drugs, especially for effects on CYP drug-metabolising enzymes. Typical interactions are as shown in Drug Interactions 18-2.
Drug interactions 18-2 Antidepressants
| Drug | Possible effects and management |
| Other serotonergic drugs | With TCAs or SSRIs or MAOIs: risk of serotonin syndrome; implicated drugs must be discontinued |
| Other CNS depressants | Enhanced CNS depression and orthostatic hypotension |
| Drugs that lower seizure threshold | With TCAs or SSRIs: may cause seizures—avoid combination |
| Other drugs with sympathomimetic effects | With TCAs or MAOIs: additive effects, including hypertension |
| Drugs with anticholinergic effects | With TCAs: concurrent drug use may result in an increase in anticholinergic adverse effects, including delirium |
| Other drugs that prolong the QT interval | With TCAs: increased risk of cardiac arrhythmias; avoid combination |
| Other drugs affecting blood glucose | With SSRIs: can increase blood glucose concentration; monitor blood glucose level |
| Drugs affecting platelet aggregation | ‘With SSRIs: added risk of bleeding |
| Other drugs that lower blood pressure | With MAOIs: can cause hypotension; monitor closely when co-administered |
| Tyramine-containing foods and drinks (see Clinical Interest Box 18-11) | Potentially dangerous drug–food interactions with MAOIs; avoid those foods or drinks. |
Selection of an antidepressant
Overall, the antidepressants appear to have similar efficacies, although there is a wide variability in individ ual patient responses to particular drugs. Therapeutic Guidelines: Psychotropic (2008) recommends as first-line therapy the SSRIs, RIMA (moclobemide), mirtazepine, reboxetine and venlafaxine. Only after unsuccessful trials of at least two of these agents are the second-line agents indicated: the TCAs, mianserin, then the non-selective MAOIs, which have a large number of potentially serious drug and food interactions. When changing drugs, gradual withdrawal then a drug-free interval ranging from 1–2 days up to 2–5 weeks is recommended, depending on the drugs involved.
Selection of an antidepressant is empirical, taking into consideration the adverse effect potential of each antidepressant compared with the medical problems of the individual patient and the patient’s previous responses. For example, a prescriber might select a sedating antidepressant (amitriptyline, doxepin or mianserin) for an agitated depressed person or a drug less likely to cause sedation or hypotension (nortriptyline or an SSRI or MAOI) for an elderly patient. SSRIs and non-selective MAOIs are relatively activating, so best administered early in the day. Potential toxicity in overdose is also important, especially if the patient is at risk of attempting a deliberate overdose—SSRIs are safer than TCAs. Patients concerned about weight gain might avoid TCAs and mirtazepine.
Plasma levels and compliance
Plasma levels of the TCAs can vary widely between individuals and, with the possible exceptions of nortriptyline and imipramine, levels often do not correlate with dose or therapeutic response. Prescribers may order plasma concentration measurements in order to monitor therapy and to help identify non-compliant patients. A lower than expected plasma level should initially indicate the need to interview the person to verify adherence to the prescribed schedule. The possible reason for noncompliance or low level (such as intolerable adverse effects, misunderstanding of directions, potential drug interaction or inadequate finances to purchase prescriptions) can then be identified and perhaps resolved.
If compliance is verified but plasma concentration remains low, dosage adjustments may be necessary or the prescriber might consider switching to a different anti depressant. If the person is non-responsive to a predomin antly noradrenaline-potentiating medication, a 5-HT- potentiating agent might be indicated.
Antidepressant therapy in special groups
Elderly people often have reduced levels of liver drug metabolising enzymes, and thus normal doses can lead to higher plasma drug concentrations and a greater potential for adverse effects. Many prescribers start geriatric patients at one-third to one-half the usual adult dosage, adjusting as necessary according to therapeutic response or presence of undesirable effects (see Clinical Interest Box 18-4).
In children, antidepressants should be reserved for those with severe conditions not manageable with psychotherapy, and supervised by a specialist child psychiatrist—there have been few trials of antidepressants in children; however, SSRIs are considered the first-line drugs (Clinical Interest Box 18-3).
In the context of safety in pregnancy and breastfeeding (see Drugs at a Glance 18), the risks of depression to both mother and baby must be considered, as well as the risks from antidepressants. With respect to safety in pregnancy, most antidepressants are classified as C; however, some are considered safer, e.g. mianserin and moclobemide; venlafaxine is contraindicated. Postnatal or perinatal depression affects about 10%–20% of mothers, who may need drug treatment. The SSRIs (except fluoxetine) appear to be safest with respect to transfer into breast milk.
As antidepressants generally elevate the mood and raise levels of ‘stimulating’ CNS neurotransmitters, they can cause unwanted effects in patients with other conditions. For example, TCAs and mianserin lower the seizure threshold and can precipitate epileptic seizures, provoke manic episodes in patients with bipolar disorder and cause arrhythmias or angina in those with cardiac disease.
Delayed onset of action
These drugs tend to have a long-delayed onset of antidepressant action, due to their long half-lives and mechanism of action (alteration of neurotransmission in CNS pathways and subsequent elevation of mood), and possibly also due to negative-feedback effects on the presynaptic 5-HT(1A) receptors. A trend towards improvement in clinical symptoms may be apparent in 2–3 weeks and full effects may not appear for 6–8 weeks; this time corresponds with an inhibition rather than facilitation of monoaminergic transmission. During this period, patients are at great risk of deepening depression (‘nothing is ever going to help me’) and might need adjunct treatment with psychotherapy or ECT. The initial drug tried should be given in therapeutic doses for an adequate period, and compliance checked, before changing to another class of antidepressant. After symptoms improve, therapy should be continued for at least 6 months.
Selective serotonin reuptake inhibitors (SSRIs)
SSRIs are as effective as other antidepressants and considerably safer than TCAs because they increase levels of 5-HT but have much less effect on NA levels, hence fewer autonomic effects. The first, fluoxetine (see Drug Monograph 18-3), was so successful that soon after its release it took over the market for antidepressants (see Clinical Interest Box 18-10). Fluoxetine was soon followed by sertraline, fluvoxamine, paroxetine and citalopram then the latter’s active isomer escitalopram. Sertraline appears to show good balance between benefits, acceptability and cost.
Clinical interest box 18-10 Prozac beats the blues
Fluoxetine (Prozac) was released in the USA in 1987. By 1990 it had made the covers of both Newsweek and The New Yorker as the new wonder drug for depression, and by 1993 sales had reached US$1.2 billion worldwide. It was so widely prescribed in the USA that in some towns most of the population were said to be taking the ‘happy pill’.
In Australia, prescriptions for antidepressants surged by 35% in the 1990s, from 5.1 million in 1990 to 6.9 million in 1996. There was a rapid market uptake of SSRIs, with only a small decrease in prescribing of TCAs. General practitioners (GPs) are the major providers of treatment for depression in Australia; however, a significant proportion of prescriptions for SSRIs were actually outside the PBS restrictions for use, in which SSRIs were listed only for ‘major depressive disorders’. Later data by the Australian Institute of Health and Welfare revealed that the number of prescriptions for antidepressants increased 4.6% per year between 2000/01 and 2005/06, and that overall more people were seeking help for mental health problems.
Specialist physicians were unsure whether the prevalence of depression had risen or whether people were more aware of the condition, more prepared to consult their doctors about it or more aware that there are drugs to treat it. A depression institute (beyondblue: www.beyondblue.org.au/index.aspx) has been set up to study the disorder.
Source: McManus et al 2000; McManus et al 2003.
Drug monograph 18-3 Fluoxetine
Fluoxetine (well known by its original trade name, Prozac) is a selective serotonin reuptake inhibitor (SSRI) antidepressant; it is much more potent at inhibiting reuptake of serotonin than of noradrenaline. It is much less effective at antagonism of acetylcholine, histamine and α-adrenergic receptors than are the tricyclic antidepressants. It helps elevate mood, relieve other symptoms and reduce social impairment.
Indications
Fluoxetine is indicated for treatment of major depression, obsessive–compulsive disorder and premenstrual syndrome. It can also be used for bulimia nervosa, panic disorder and post-traumatic stress disorder.
Pharmacokinetics
Fluoxetine is readily absorbed after oral administration and reaches peak plasma levels after about 6–8 hours. It is highly protein-bound and has a very high volume of distribution. It has non-linear kinetics as it inhibits its own metabolism. It is extensively metabolised in the liver; one metabolite (norfluoxetine) is active as an antidepressant. Metabolites are excreted via the kidneys. With chronic administration, the half-lives of fluoxetine and norfluoxetine are respectively 4–6 and 9–16 days, hence it takes some weeks for the concentration at steady state to stabilise.
Drug interactions
Fluoxetine inhibits metabolism by the CYP2D6 isoenzymes, hence it raises plasma levels of drugs metabolised by that enzyme, including many antiepileptic drugs, antipsychotics, benzodiazepines, tricyclic antidepressants and St John’s wort. There are protein-binding interactions with warfarin (see also general drug interactions section).
Adverse reactions
Common reactions include rashes, anxiety, dizziness, weight loss, nausea and headaches; seizures are rare. There is some debate as to whether antidepressants may increase the risk of suicide in depression, or whether this and worsening symptoms occur in the interval between dosing and when the drug’s effects begin to occur.
Warnings and contraindictions
Patients need to be warned of possible adverse effects, of the delay before therapeutic effects and of caution required if driving or operating machinery. Withdrawal reactions can occur after cessation of the drug, due to its long half-life. The dose needs to be reduced in severe liver disease. Fluoxetine is in pregnancy category C as it crosses the placenta and can lead to withdrawal reactions in the neonate. It is not recommended during lactation due to its lipid solubility and long half-life. Antidepressants are not generally indicated for treatment of childhood depression.
Indications and actions
The SSRIs are generally indicated to treat depression and anxiety disorders such as obsessive–compulsive disorder and panic disorder; fluoxetine and sertraline are also indicated for treating bulimia nervosa and premenstrual syndrome. They have similar delayed onset of action to the TCAs. Unlike the TCAs, which often cause weight gain, the SSRIs (except paroxetine) can cause anorexia and weight loss. They have little affinity for receptors for DA, acetylcholine, histamine or NA and are the least toxic antidepressants in overdose.
Adverse effects are typically as shown for fluoxetine. Early suggestions that use of fluoxetine is associated with enhanced suicidal behaviour have not been supported by later studies.
As well as being a marketing phenomenon, fluoxetine is interesting from a pharmacokinetic viewpoint. While most SSRIs have half-lives of about 24 hours, fluoxetine has an active metabolite with a half-life of up to 16 days. Consequently, it can take weeks to achieve steady-state concentration or to eliminate the active metabolite after discontinuation of the drug, and there is an extended period for drug interactions.
Drug interactions
There are potential drug interactions, especially with other antidepressants and with drugs implicated in the serotonin syndrome, and they can inhibit the metabolism of many drugs (see Drug Interactions 18-2).
Tricyclic antidepressants (TCAs)
Drugs in this group tend to have names ending in -mipramine or -tryptyline; their chemical structures have three rings, hence their group name. All the TCAs act by the same mechanism and appear to have similar efficacies. Although they were the first major group of drugs successful in treating depression, they have many adverse effects (see Table 18-3) and are unsafe in overdose. TCA toxicity manifests as dilated pupils, extrapyramidal signs, CNS excitement or depression, life-threatening arrhythmias and seizures; the cause of death from overdose is usually cardiac failure secondary to arrhythmias and anoxaemia. Consequently TCAs have been largely overtaken by newer, safer drugs (e.g. the SSRIs such as fluoxetine); they are currently used as second-line agents if the newer agents do not bring benefit.
Typical characteristics of the TCAs such as imipramine are as follows:
Indications and precautions
The TCAs are indicated in treatment of major depression and also in other conditions: as adjunct therapy in pain management, in prophylaxis of migraine, for nocturnal enuresis and urge incontinence and as third-line therapy of ADHD.
Precautions need to be taken in people with many other conditions, including other psychoses; seizure disorders; prostatic hypertrophy and urinary retention; cardiac, liver, renal or thyroid disease; glaucoma; and during pregnancy or breastfeeding. Dosage is started low and gradually increased while monitoring therapeutic and adverse effects.
Other tricyclic antidepressants
Imipramine and trimipramine are fairly typical TCAs. Nortriptyline is less likely than others to cause sedation, hypotension or anticholinergic effects, so is safer in the elderly. Dothiepin is thought to be the most toxic in overdose and doxepin is the most sedating.
Clomipramine, an analogue of imipramine, is a more selective inhibitor of 5-HT reuptake (over noradrenaline) compared to the other TCAs, hence it may have fewer autonomic effects but is more likely to provoke the serotonin syndrome. It is indicated for the treatment of obsessive–compulsive disorders and premenstrual tension.
Monoamine oxidase inhibitor antidepressants
Monoamine oxidase (MAO)
MAO, an enzyme found in mitochondrial membranes in nerve terminals, the liver and the brain, is involved in the inactivation and degradation of various monoamines. Tyramine, catecholamines (noradrenaline [NA], adrenaline and dopamine [DA]), 5-HT and several amine drugs are all substrates for the enzyme. Compounds that inhibit the enzyme thus interfere with the inactivation of amine neurotransmitters and may potentiate their actions, particularly the vasopressor effects causing high blood pressure.
Two types of MAO enzymes have been identified: MAO-A and MAO-B. MAO-A preferentially metabolises 5-HT, NA and DA and is located throughout the body (high concentrations are present in the human placenta). MAO-B is contained mainly in human platelets; about equal amounts of both types are found in the liver and brain. DA and tyramine, a monoamine with sympathomimetic effects found in many foodstuffs, are inactivated by both MAO-A and MAO-B. Hence an inhibitor that selects for the MAO-A form is likely to be better clinically as an antidepressant, as it will raise the levels of neurotransmitter amines (see Figure 18-2) while allowing inactivation of tyramine and other potentially toxic monoamines.
MAO inhibitors (irreversible MAOIs)
The early MAOIs were irreversible and non-selective in their inhibitory effects, i.e. they inhibited both MAO-A and MAO-B, for 2–3 weeks. Current research indicates that the MAOIs also desensitise the α2- or β-adrenoceptors and 5-HT receptors (downregulation). Examples are the drugs phenelzine and tranylcypromine. They raise the level of monoamines and elevate the mood, but there is a long delay in mood improvement. There are many serious adverse reactions, including autonomic and sexual dysfunction, severe hypertension and insomnia.
MAOIs impair the metabolism of many other drugs, including adrenaline and sympathomimetic amines, DA (including that formed after administration of levodopa), methyldopa and pethidine, and enhance the activity of drugs that cause release of amine transmitters. Interactions can occur with prescription-only and over-the-counter medications, caffeine and tyramine-containing foods and beverages (the tyramine reaction: see Clinical Interest Box 18-11). The major adverse reaction with these agents is the occurrence of sudden and possibly very severe hypertension that if untreated can progress to vascular collapse and fatality. (Reference texts should be consulted for potential drug interactions whenever another drug is added to a regimen containing an MAOI.)
Clinical interest box 18-11 Tyramine-containing substances
Patients taking old-style non-selective MAOIs are at risk of the tyramine reaction if they take tyramine-containing preparations or foods, as the tyramine (usually inactivated by MAO-B) can build up to high levels and raise the blood pressure by a sympathomimetic action.
The tyramine content of foods varies because of different conditions or preparation of the foods, different food samples or different producers or manufacturers. The major goal should be to advise the patient to avoid foods and drinks with reported moderate to high tyramine contents as follows:
Some foods that contain tyramine or other pressor amines, if eaten in moderation when fresh, are less apt to cause a serious reaction. Such foods include yoghurt, sour cream, cream cheese, cottage cheese, chocolate and soy sauce.
(As a colleague who was taking tranylcypromine once complained: ‘I’m going to a cocktail party, and I won’t be able to eat or drink anything!’)
Because of the large number of adverse drug reactions and interactions, the old non-selective MAOIs are now indicated only as second- or third-line antidepressants for the treatment of depression that does not respond to other, safer drugs. Some patients, however, respond only to MAOIs.
Reversible inhibitors of MAO-A
Moclobemide is a reversible inhibitor of MAO-A (RIMA), a new antidepressant drug group. It is much less likely to cause tyramine reactions, as tyramine and other amines can still be inactivated by MAO-B. The fact that it is reversible means that MAO activity is restored within 1–2 days of stopping administration of the drug. Nausea, headache and insomnia are adverse effects and there may be adverse interactions with sympathomimetic amines and pethidine. A tyramine-free diet is not required, although large quantities of tyramine-rich foods should be avoided. Moclobemide is relatively safe in overdose.
Other miscellaneous antidepressants
There are other new antidepressants, which act by various mechanisms affecting amine neurotransmitter levels. Some (such as duloxetine) are known as ‘serotonin and noradrenaline reuptake inhibitors’ (SNRIs), a mechanism similar to that of the old TCAs. In bipolar depression, other drugs sometimes useful are the anticonvulsant lamotrigine and antipsychotic olanzapine.
Mianserin and mirtazepine
Mianserin has a tetracyclic chemical structure, rather than tricyclic. It does not inhibit the reuptake of monoamine transmitters but enhances postsynaptic 5-HT1A receptors, hence its antidepressant actions. It shares some of the other properties of the TCAs, as it antagonises α1-adrenoreceptors and histamine H1 receptors, but has less anticholinergic action so has fewer cardiovascular adverse effects. A rare adverse effect unrelated to the antidepressant actions is reversible neutropenia, which is monitored by blood counts before and during therapy.
Mirtazapine was released for general use in mid-2001. It is chemically related to mianserin and has different mechanisms of action from those of the TCAs and MAOIs. Mirtazapine, by selective blockade of histamine H1 receptors, α2-adrenoceptors and 5-HT2A, 5-HT2C and 5-HT3 receptors, appears to enhance noradrenergic activity and 5-HT activity at 5-HT1A receptors, which gives it better antidepressant efficacy and fewer peripheral and central adverse effects. In particular, it is safer in overdose, has fewer anticholinergic effects, and does not cause nausea or diarrhoea, insomnia or sexual dysfunction. However, markedly increased appetite and carbohydrate cravings can lead to serious weight gain.
Reboxetine and venlafaxine
Two other relatively new SNRI antidepressants are reboxetine and venlafaxine (and its metabolite desvenlafaxine, also used as an antidepressant), respectively more selective for noradrenaline and serotonin reuptakes. Their precise mechanisms of action differ but both affect various other neurotransmitters, and hence adverse effects include autonomic, CNS and sexual dysfunctions. In theory there might be fewer drug interactions with desvenlafaxine than with the parent drug; however, the precautions are similar for both drugs.
Antimanic therapy
Mania
Bipolar affective disorder involves mood swings between the poles of depression and mania; patients with unipolar mania are rare. Bipolar disorders are much less common than unipolar depression, with bipolar conditions accounting for only about 10% of affective disorders. The peak age of onset of bipolar illness is in the late 20s, about 15 years earlier than that of unipolar depression. In predisposed persons, mania can be provoked by drugs, including the antidepressants, corticosteroids, ACE inhibitors, dopaminergic agents and various illicit drugs.
Mania is characterised by speech and motor hyperactivity; reduced sleep requirements; flights of ideas; grandiose or paranoid thoughts; elated, irritable or angry mood; poor judgement; aggressiveness and hostility; overspending and possibly promiscuity. (The hypomanic state may cause social problems, with overactivity, uncompleted tasks, irritability, excessive untidy bright clothing, poor judgement and increased sexual interest.) Then the person with bipolar disorder may suffer a mood swing to depression, which can persist for several months (see Clinical Interest Box 18-12).
Clinical interest box 18-12 Van gogh’s affect and art
Vincent van Gogh, the great Dutch artist, suffered what would most likely now be classified as severe bipolar affective disorder, or manic–depressive psychosis.
Vincent was born in 1853 into an austere religious and artistic Dutch family. There was a history of mental illness (epilepsy, nervous complaints) on both sides of his family. His childhood was dominated by his mother’s grief over the earlier death of her first son. Vincent became an art dealer and preacher but his self-martyrdom and solitude were found to be unacceptable, so he turned to painting.
His four important relationships with women—two of whom were ‘mentally unbalanced’—all ended in shame and humiliation. His friendship and collaboration with the painter Gauguin in Arles (Provence, France) was short-lived; Vincent suffered a severe mental disturbance and cut off part of his own ear and sent it to a prostitute.
There are many references to his self-abasement, misery, moodiness, melancholy, neglect of his appearance and health, excessive drinking and feeling a prisoner. At other times he was remarkably energetic, with strong exalted emotions, swarms of ideas for his work and extravagant gestures. He painted prolifically but was financially dependent on his brother. He suffered many episodes of mental disorder, with confusions, hallucinations, delusions, paranoia and aggression, or anguish, immobility and guilt, identifying with the suffering and rejection of Jesus Christ. He committed himself to a mental asylum for a year and committed suicide a few months after leaving it, aged 37.
Many of the swings in Vincent’s moods can be traced in his paintings, which vary between brilliant yellow sunny scenes, such as sunflowers and harvests, and dark sombre visions of gloomy peasant life, ruined churches or flocks of black birds foreboding evil.
Genetic features played a part in Vincent’s illness, as did his unhappy childhood, rejection by women and peers, unhappy events, physical ill-health and heavy drinking of alcohol and absinthe (known to contain the neurotoxins thujone and santonin). There are other theories: that he had epilepsy or Ménière’s disease, was poisoned by drinking turpentine, had gonorrhoea or syphilis and glaucoma. He was treated for epilepsy with digitalis and it has been suggested that the bright yellow–green colours and swirling halos around lights in his later paintings are due to altered colour vision indicative of severe digitalis toxicity. Inevitably, Vincent’s affective disorder, of whatever aetiology, infl uenced his art. Perhaps if effective treatment for bipolar disorder had been available then, he would have lived much longer, but his art might have been less imaginative and creative?
After: Wolf 1994 and various sources.
Counselling, psychotherapy and drug therapy are useful for treating bipolar disorders. ECT may be required for severe mood disturbance or for suicidal depression. Antipsychotic drugs (e.g. phenothiazines) are useful for sedation and control of the mania symptoms, and antidepressants in the depressive phases for at least 6 months. Olanzapine and quetiapine, both atypical anti psychotic agents, have been approved for use in treatment of acute manic episodes associated with bipolar disorder, in which they help control disruptive behaviour and cause the patient to become more settled and sleep better.
Lithium is the specific treatment for prevention of recurrences of manic episodes (see Clinical Interest Box 18-13, and Drug Monograph 18-4). Other mood-stabilising drugs include the anti-epileptic agents sodium valproate, lamotrigine and carbamazepine (see Chapter 17), sometimes used in combination with lithium or with olanzapine.
Clinical interest box 18-13 Therapeutic value of lithium discovered in melbourne
Chemically, lithium (Li) is a simple metal, first isolated in 1817. It is the third lightest element, related in its properties to the other alkaline earth metals sodium and potassium. Lithium salts were once used for gout and as sedatives and a salt substitute. It was known that excess lithium caused cardiac depression, nausea and upset stomach and mental depression. How lithium came to be used in psychiatric medicine is an interesting story.
In 1948 Dr John Cade, a Melbourne psychiatrist, was doing research into the chemical basis of mania. He established that patients’ urine contained a ‘manic toxin’ that had excitant effects when injected into guinea pigs.
Working on the theory that urea was the neurotoxin and that urates would enhance the toxicity, he tested the lithium salt, lithium urate, but found it caused the animals to become lethargic and unresponsive to stimuli.
To his surprise, Cade found that other lithium salts had similar protective, calming effects and concluded it was lithium, not urate, that was the pharmacologically important ion.
He tested lithium clinically in 10 patients with mania and showed that lithium salts have specific effects in controlling mania but are ineffective in schizophrenia or acute depression. Cade recognised that the adverse effects (abdominal dis comfort, slurred speech, ataxia, depression) could be fatal if the drug administration was not immediately stopped.
Cade speculated ‘as to the possible aetiological significance of a deficiency in the body of lithium ions in the genesis of this disorder’, and reported that lithium treatment was ‘much preferred to prefrontal leucotomy’. He published his work in the Medical Journal of Australia (1949), but it met with little interest. Was the journal (then) too obscure? the remedy too simple? the drug not patentable? the cure potentially too toxic?
By the 1960s, lithium had been re-evaluated and its efficacy in reducing the prevalence, severity and duration of recurrent manic episodes proven. There is an 80%–85% response rate when lithium is used prophylactically in manic–depressive psychosis.
After: Cade, 1979.
Indications
Lithium is indicated for prevention of manic or depressive episodes in bipolar affective disorder and in treatment of acute mania. It is also used as adjunctive therapy in schizophrenia and treatment-resistant depression. It is a difficult drug clinically due to its low therapeutic index; it must be monitored closely.
Pharmacokinetics
With the exception of the slow-release dosage form, lithium is rapidly absorbed and reaches peak plasma concentrations in 1–3 hours. It has a long half-life: in adults 24 hours, in adolescents 18 hours, and in geriatric patients up to 36 hours, hence steady-state plasma concentrations are not reached for 5–7 days. Lithium is not metabolised, so it is excreted unchanged by the kidneys; it is partly reabsorbed from the proximal tubule along with sodium.
Therapeutic drug monitoring
Lithium has a very narrow therapeutic range, with concentrations only 1.5 times therapeutic concentration causing severe toxicity, so plasma levels must be monitored regularly. Samples are taken 12 hours post-dose to measure trough levels. Therapeutic plasma concentrations for the treatment of bipolar disorder are: acute, 0.8–1.2 mmol/L; and main tenance, 0.6–0.8 mmol/L. A clinical response is usually reported in 1–3 weeks. Levels are monitored weekly during dosage adjustment, then every 1–3 months once stabilised (see Clinical Interest Box 18-14 for other factors affecting lithium serum levels).
Adverse effects
These include tremors of hands, thirst, nausea, increased urination, diarrhoea, weight gain and irregular pulse rate. Long-term effects include acne, psoriasis, hypothyroidism and renal damage. A specific effect is nephrogenic diabetes insipidus, in which lithium inhibits the actions of antidiuretic hormone on the distal tubule cells, leading to polyuria. Early signs of toxicity include confusion, vomiting, tremors, slurred speech and drowsiness. Later signs are blurred vision, convulsions, severe trembling, ataxia, arrhythmias and increased production of urine. Prolonged toxic levels can lead to irreversible brain damage, and even relatively low plasma levels can be fatal. Treatment of toxicity is by gastric lavage, forced diuresis and dialysis.
Warnings and contraindications
Lithium should be used with caution in patients with diabetes mellitus, hypothyroidism, goitre or psoriasis, and in pregnant or severely debilitated patients or patients on a sodium-restricted diet. Avoid use in people with a history of lithium hypersensitivity or with severe dehydration or renal impairment, and during lactation.
Dosage and administration
Lithium, as the carbonate salt, is available as tablets and controlled-release tablets. The usual adult dose of lithium for acute mania is 250–500 mg, 3 times daily, adjusted according to the patient’s response and tolerance up to a maximum dose of 2 g/day. The maintenance dose is 1000–2000 mg daily in divided doses. Geriatric patients usually require a much lower dosage (one-third to one-half).
Drug interactions 18-3 Lithium
| Drug | Possible effects and management |
| Antithyroid drugs or iodides | Can enhance the hypothyroid goitrogenic effects of lithium or these medications; monitor closely for lethargy or intolerance to cold |
| Non-steroidal antiinflammatory agents | Can decrease excretion of lithium, leading to raised lithium levels and toxicity; monitor closely for blurred vision, confusion and dizziness |
| Phenothiazines, fluoxetine, haloperidol | Lithium levels may be altered, with risk of neurotoxicity; monitor physical symptoms and drug serum levels closely |
| Diuretics, especially thiazides | Decreased lithium excretion results in a raised lithium level and toxicity; a reduction in lithium dosage may be indicated; monitor closely |
Lithium: the antimanic drug
Lithium’s mechanism of action has still not been established. Sodium in the cells has been reported to increase by as much as 200% in manic patients. Lithium and sodium are both actively transported across cell membranes but lithium cannot be pumped out of the cell as effectively as sodium can. Lithium can impair sodium actions in many physiological processes. It inhibits or slows down G-protein coupling with receptors, adenylate cyclase activity, phosphoinositol cycling and various phosphokinase activities, and affects neuroprotective proteins. Overall, it inhibits transmitter release (especially dopamine) at synapses, increases the turnover of NA and 5-HT in the brain and decreases postsynaptic receptor sensitivity, with the result that the presumed overactive catecholamine systems in mania are corrected. It has little effect in people not suffering from mania.
Key points
 Health-care professionals involved in treating mental illness need some understanding of the pathogenesis and clinical manifestations of the major psychoses schizophrenia and bipolar affective disorder, and the relevance of imbalances in brain neurotransmitters, especially the monoamines noradrenaline, dopamine and serotonin.
Health-care professionals involved in treating mental illness need some understanding of the pathogenesis and clinical manifestations of the major psychoses schizophrenia and bipolar affective disorder, and the relevance of imbalances in brain neurotransmitters, especially the monoamines noradrenaline, dopamine and serotonin. Important clinical aspects include the importance of informed consent and compliance with therapy; the frequency of serious adverse drug reactions and interactions; concomitant non-drug therapy such as psychotherapy, electroconvulsive therapy and psychosurgery; and the selection of appropriate therapy for patients in special groups—children, elderly, female and indigenous people.
Important clinical aspects include the importance of informed consent and compliance with therapy; the frequency of serious adverse drug reactions and interactions; concomitant non-drug therapy such as psychotherapy, electroconvulsive therapy and psychosurgery; and the selection of appropriate therapy for patients in special groups—children, elderly, female and indigenous people. Since the discovery of antipsychotic tranquillisers in the 1950s, there has been a marked decrease in the length of institutionalisation for psychiatric disorders; many people are now treated as outpatients at community mental health centres.
Since the discovery of antipsychotic tranquillisers in the 1950s, there has been a marked decrease in the length of institutionalisation for psychiatric disorders; many people are now treated as outpatients at community mental health centres. The main antipsychotic (antischizophrenic) agents are the phenothiazine derivatives, thioxanthines and atypical antipsychotics. Although the exact mechanisms of their antipsychotic effect are unknown, a primary effect is dopamine blockade in specific areas of the CNS. Receptors for many other neurotransmitters are also likely to be blocked.
The main antipsychotic (antischizophrenic) agents are the phenothiazine derivatives, thioxanthines and atypical antipsychotics. Although the exact mechanisms of their antipsychotic effect are unknown, a primary effect is dopamine blockade in specific areas of the CNS. Receptors for many other neurotransmitters are also likely to be blocked. Major adverse effects of antipsychotics occur in the central, autonomic and motor nervous systems, including sedation, hypotension, behaviour changes, dystonias and parkinsonian effects and akathisia. Serious adverse effects are tardive dyskinesia and neuroleptic malignant syndrome.
Major adverse effects of antipsychotics occur in the central, autonomic and motor nervous systems, including sedation, hypotension, behaviour changes, dystonias and parkinsonian effects and akathisia. Serious adverse effects are tardive dyskinesia and neuroleptic malignant syndrome. The major affective disorders are depression and bipolar affective disorder. The monoamine theory of affective disorders suggests that during depressive episodes levels of monoamines, especially noradrenaline and serotonin, are low in parts of the brain related to mood.
The major affective disorders are depression and bipolar affective disorder. The monoamine theory of affective disorders suggests that during depressive episodes levels of monoamines, especially noradrenaline and serotonin, are low in parts of the brain related to mood. Antidepressant drug groups include the tricyclic antidepressants, the selective serotonin reuptake inhibitors and the monoamine oxidase inhibitors. All appear to act by increasing brain levels of serotonin and noradrenaline.
Antidepressant drug groups include the tricyclic antidepressants, the selective serotonin reuptake inhibitors and the monoamine oxidase inhibitors. All appear to act by increasing brain levels of serotonin and noradrenaline.Review exercises
References and further reading
Anonymous. NZ Prescriber Update; June 2001; no. 21: 4-7.
Australian Bureau of Statistics. Article 4326.0: National Survey of Mental Health and Wellbeing, 2007.
Australian Medicines Handbook 2008. Adelaide: AMH, 2008.
Bishara D., Taylor D. Upcoming agents for the treatment of schizophrenia: mechanism of action, efficacy and tolerability. Drugs. 2008;68(16):2269-2292.
Bloch S., Singh B.S. Foundations of Clinical Psychiatry, 3rd edn. Melbourne: Melbourne University Press; 2007.
Braun L., Cohen M. Herbs and Natural Supplements: An Evidence-Based Guide, 2nd edn. Sydney: Elsevier Mosby; 2007.
Cade J.F. Lithium salts in the treatment of psychotic excitement. Medical Journal of Australia. 1949;2(10):349-352.
Cade J.F.J. Mending the Mind: A Short History of Twentieth Century Psychiatry. Melbourne: Sun Books; 1979.
Caswell A., editor. MIMS Annual June 2005. Sydney: CMPMedica Australia, 2005.
Cipriani A., Furukawa T.A., Salanti G., et al. Comparative efficacy and acceptability of 12 new-generation antidepressants: a multiple-treatment meta-analysis. Lancet. 2009;373(9665):746-758.
Cooper J.R., Bloom F.E., Roth R.H. The Biochemical Basis of Neuropharmacology, 8th edn. New York: Oxford University Press; 2003.
Delini-Stula A., Baier D., Kohnen R., et al. Undesirable blood pressure changes under naturalistic treatment with moclobemide, a reversible MAO-A inhibitor: results of the drug utilization observation studies. Pharmacopsychiatry. 1999;32(2):61-67.
Geyer M.A., Vollenweider F.X. Serotonin research: contributions to understanding psychoses. Trends in Pharmacological Sciences. 2008;29(9):445-453.
Jureidini J.N. Suicide and antidepressants in children. Australian Prescriber. 2005;28(5):110-111.
Lang U.E., Puls I., Muller D.J., Strutz-Seebohm N., Gallinat J. Molecular mechanisms of schizophrenia. Cellular Physiology & Biochemistry. 2007;20(6):687-702.
Leucht S., Corves C., Arbter D., Engel R.R., Davis J.M. Secondgeneration versus first-generation antipsychotic drugs for schizophrenia: a meta-analysis. Lancet. 2009;373(9657):31-41.
McManus P., Mant A., Mitchell P.B., et al. Recent trends in the use of antidepressant drugs in Australia, 1990-1998. Medical Journal of Australia. 2000;173:458-461.
McManus P., Mant A., Mitchell P., et al. Use of antidepressants by general practitioners and psychiatrists in Australia. Australian & New Zealand Journal of Psychiatry. 2003;37:184-189.
Malhotra A.K., Murphy G.M., Kennedy J.L. Pharmacogenetics of psychotropic drug response. American Journal of Psychiatry. 2004;161:780-796.
Psychotropic Expert Group. Therapeutic Guidelines: Psychotropics, version 6. Melbourne: Therapeutic Guidelines Limited; 2008.
Singh A., Berk M. Acute management of bipolar disorders. Australian Prescriber. 2008;31(3):73-76.
Spencer J.W., Jacobs J.J. Complementary/Alternative Medicine: An Evidence-Based Approach. St Louis: Mosby; 1999.
Sun-Edelstein C., Tepper S.J., Shapiro R.E. Drug-induced serotonin syndrome: a review. Expert Opinions in Drug Safety. 2008;7(5):587-596.
Tiller J.W.G. Cognitive behaviour therapy in medical practice. Australian Prescriber. 2001;24:33-37.
Various authors. Depression and the community. Medical Journal of Australia. 2002;176(10 Suppl):S60-S101.
Wolf P.L. If clinical chemistry had existed then…. Clinical Chemistry. 1994;40:328-335.
Australian National Survey of Mental Health and Wellbeing, 2007: www.health.gov.au/internet/mentalhealth/publishing.nsf/Content/national-surveys-1
beyondblue, the national depression initiative: www.beyondblue.org.au/index.aspx
Mental Illness Fellowship of Australia: http://www.mifa.org.au
Mental Illness Fellowship of Victoria (formerly Schizophrenia Fellowship of Victoria): www.mifellowship.org/
New Zealand Medicines and Medical Devices Safety Authority: www.medsafe.govt.nz
Schizophrenia Fellowship of New Zealand: www.sfnat.org.nz/
More weblinks at: http://evolve.elsevier.com/AU/Bryant/pharmacology