Chapter 14 Central Nervous System Overview and Anaesthetics
The central nervous system (CNS), comprising the brain and spinal cord, regulates all body functions, allowing the person to adapt, both consciously and subconsciously, to the internal and external environments and to carry out complex functions such as integration, reasoning, memory, behaviour and expression of mood and personality. A broad knowledge of the anatomy, physiology and neurochemistry of the CNS is necessary for understanding the various drugs used to treat diseases that affect this system.
Anaesthesia is the loss of the sensations of pain, pressure, temperature or touch, in a part or the whole of the body. Anaesthetic drugs cause unconsciousness or insensitivity to pain by a reversible action, i.e. cells return to normal when the drug is eliminated from the cells. The discovery of anaesthesia and the development of anaesthetic drugs have proven invaluable in limiting pain and suffering during surgical procedures, and have resulted in many advances in modern surgical techniques. The two major categories of anaesthetic agents are the general anaesthetics, which depress consciousness and cause generalised loss of sensation, and the local anaesthetics, which block nerve conduction in a body region or localised area when applied locally or to nerve pathways and thus relieve pain locally. Many other drugs are also used during surgery to maintain the patient in a stable physiological state and relieve or prevent pain, anxiety and post-operative nausea.
Key abbreviations
EMLA eutectic mixture for local anaesthesia
GA general anaesthesia/anaesthetic
5-HT 5-hydroxytryptamine (serotonin)
IVRA intravenous regional anaesthesia
LA local anaesthesia/anaesthetic
MAC minimum alveolar concentration (for anaesthesia)
RAS reticular activating system
Key background
THE nervous system consists of the central nervous system (CNS) and the peripheral nervous system (PNS; see Figure 14-1). When a drug is described as having a central action, this means that it has an action on the brain or the spinal cord—these drugs are summarised in Clinical Interest Box 14-1. The particular response caused by a drug depends on many factors, including specific attributes of the drug, the personality and emotional and physiological state of the individual, any concurrent disease or drug therapy and even the environment in which the drug is administered.
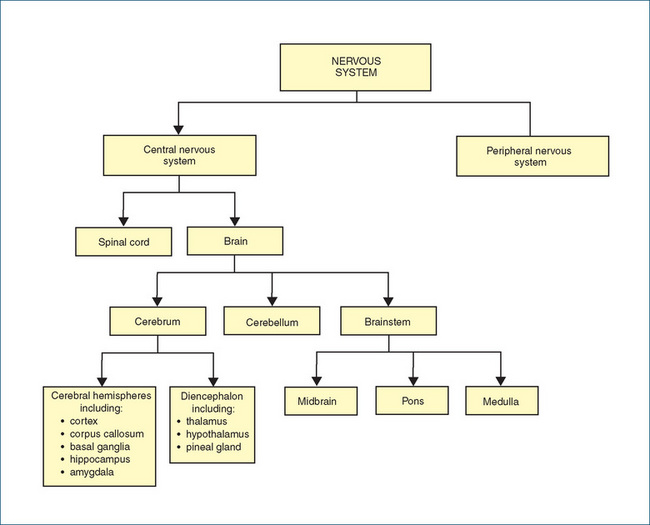
Figure 14-1 Organisation of the nervous system, showing the major anatomical subdivisions of the central nervous system. (Details of the peripheral nervous system subdivisions are shown in Figure 11-1.)
Clinical interest Box 14-1 Drugs affecting the CNS
The major drug groups with actions on the CNS include:
Other drugs may be administered to prevent or treat physical pathologies to brain tissue, e.g. cytotoxic agents for tumours, antibiotics for infections or anti-inflammatory agents in cerebral oedema. Many drugs given for peripheral effects may cross the blood–brain barrier and have side effects on the CNS, e.g. autonomic drugs, antihistamines and local anaesthetics. Drugs taken for social rather than medical reasons (tobacco, alcohol, marijuana, stimulants, psychedelics, cocaine) are used for their pleasurable central effects.
Drugs affecting the CNS are of particular importance in pharmacology. Not only are they often prescribed for treatment of common clinical conditions (pain, headache, anxiety, epilepsy, sleeplessness, depression, psychoses) but they are also the commonest self-administered drugs—as analgesics, tobacco, alcohol and caffeine. Unfortunately, it is not easy to study CNS-active drugs in the laboratory and extend the results to human medicine. This is partly because other animals may respond very differently from humans and cannot tell us how they are feeling or thinking. Also, actions at the cellular level may bear little obvious relationship to effects on the whole person in terms of complex functions such as emotions, memory, thought processes, personality and behaviour. Conse quently some of the most commonly used CNS-active drugs, such as general anaesthetics and drugs affecting mood and behaviour, are those about which we understand the least in terms of their mechanisms of action.
Composed of the brain and spinal cord, the CNS essentially controls all functions in the body. The PNS consists of all nervous tissue outside the brain and spinal cord—cranial and spinal nerves; somatic, sensory, autonomic and enteric neurons; and ganglia and receptors. (The PNS is discussed in greater detail in Unit 3.) The PNS is the network that transmits information to and from the CNS. Sensory information from the periphery is transmitted via afferent pathways,1 alerting the CNS to internal and external changes such as muscle tension, joint position, blood pressure, pain, fever, sound, smell, taste, touch and sight. This information is integrated in the CNS and messages are then relayed via peripheral efferent pathways to appropriate cells or tissues to produce the necessary actions and adjustments. Information concerning these actions and adjustments is again fed back into the CNS, permitting continuous adjustments to be made in various tissues to ensure effective balanced control of body functions (i.e. homeostasis).
CNS structure and function
Brain
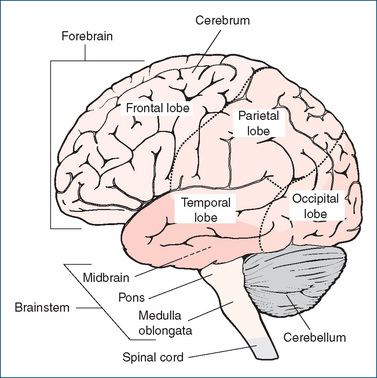
The human brain weighs about 1400 g and is estimated to contain around 100 billion neurons (see Figure 11-3), each of which connects with around 10,000 others (on average) in branching networks. The brain is suspended in cerebrospinal fluid (CSF), and surrounded and protected by membranes called the meninges. CSF helps keep the brain in a very stable environment, acts as a fluid shockabsorber and circulates compounds such as neurotransmitters and other mediators. The parts of the brain can be divided and discussed in various ways; a simplified approach is to consider the major component areas (see Figure 14-2):
In the following sections, the major areas of the brain are described briefly, especially those areas affected by drug therapies. The ‘special senses’ (sight, hearing, smell) and drugs affecting the eye or the ear are discussed in Chapters 31 and 32.
Brainstem
The brainstem is composed of the midbrain, pons and medulla oblongata and is the source of cranial nerves III–XII; the exceptions are the olfactory and optic nerves, which have origins in the nasal mucosa and retina, respectively. It is the most primitive part of the brain and is essential for life. The major long-fibre tracts running through it convey afferent sensory fibres from the PNS and efferent motor fibres from the cerebral cortex; fibres from the cerebellum are also channelled through the brainstem. The medulla, pons and midbrain contain important correlation centres (grey matter), as well as ascending and descending pathways (white matter). Tests for brain death, e.g. after severe CNS trauma or anoxia, all involve testing of brainstem functions, such as the gag and cough reflexes, ocular and vestibular reflexes and spontaneous breathing. Brain death is defined as irreversible cessation of brainstem functions.
The midbrain serves as a relay station between higher areas of the brain and the spinal cord. It is the source of the third (oculomotor) and fourth (trochlear) cranial nerves; centres for visual and auditory reflexes are also located here.
The pons helps bridge the left and right sides of the cerebellum. All information to and from the cerebellum passes through the pons, which also contains ascending sensory and descending motor tracts. The lower pons and medulla contain centres that control involuntary respiratory regulation; the upper pons and medulla contain the reticular activating system.
The medulla oblongata contains several vital centres: the respiratory, vasomotor, cardiac and vomiting centres. (Such centres are referred to as vital because they are necessary for survival—see Clinical Interest Box 14-2). Other essential functions also originate here, such as sneezing, coughing and swallowing reflexes. If the respiratory centre is depressed by drugs it will discharge fewer impulses down nerve pathways to the muscles of respiration, and respiration will be depressed. Other centres in the medulla that respond to drugs are the cough and vomiting centres. Within the pyramids of the medulla, the large motor tracts from the motor areas of the cerebral cortex to the spinal cord cross over. This is known as the decussation of the pyramids and explains why damage at a high level to motor areas (e.g. a stroke) causes motor impairment or paralysis on the contralateral side.
Clinical interest Box 14-2 Raised intracranial pressure (ICP)
The brain is enclosed within a rigid sphere of skull bones, with only slight room for expansion; thus any increase in the volume of the components inside the skull (brain tissue, interstitial fluid, blood within vessels or CSF within the ventricles) will raise the pressure inside the skull and put pressure on the other components.
Common causes of raised ICP are generalised oedema or a space-occupying lesion, which could be a tumour, infection, haemorrhage, haematoma, hydrocephalus or abscess.
Clinical manifestations include headache (which worsens with coughing or leaning forward), drowsiness, vomiting, confusion and papilloedema. Raised ICP can lead to blind ness (from compression of optic nerves) or death (from compression of vital centres in the brainstem).
Localised expansion of the brain may cause the brainstem to herniate through a foramen (hole) in the skull; com press nerves; compress blood vessels, causing ischaemia; or damage blood vessels, leading to rupture and bleeding, hence further raising ICP. This is a life-threatening condi tion and urgent treatment is required.
Treatment may be surgical, to reduce pressure, or pharmacological, with corticosteroids to relieve inflammation, osmotic dehydrating agents to reduce oedema or diuretics to reduce fluid load.
Cerebellum
Located in the posterior cranial fossa behind the brain stem, the cerebellum contains more neurons than all the rest of the brain, with centres for muscle coordination, maintenance of posture and muscle tone. It receives afferent impulses from the vestibular nuclei, as well as the cerebrum, and plays an important role in the maintenance of posture and skilled muscular activity. The cerebellum is an error-detector for all movements, and a lesion or damage to it leads to ataxia (postural instability). Drugs that disturb the cerebellum or vestibular branch of the eighth cranial nerve cause dizziness and loss of equilibrium.
Thalamus
The thalamus is composed of sensory nuclei and serves as the major relay centre for impulses to and from the cerebral cortex. With only a few important exceptions (including olfaction, the sense of smell), all information from the periphery is transmitted via the thalamus before being consciously perceived or processed; thus sensations such as pain, temperature, touch and other sensory impulses are relayed via the thalamus to the cerebral cortex.
The thalamus enables a person to have impressions of pleasantness or unpleasantness and plays a role in acquisition of knowledge and (with the reticular activating system) in arousal or alerting signals. Drugs that depress cells in the thalamus may interrupt the free flow of impulses to the cerebral cortex; this is one way in which pain may be relieved.
Hypothalamus
The hypothalamus lies below the thalamus and is a major controller of homeostasis of many body functions. It is a link between higher centres in the brain and both the auto nomic nervous system (ANS) and the endocrine system. Functions of the hypothalamus can be summarised as:
For example, tricyclic antidepressant agents often also reverse autonomic symptoms associated with depression, such as weight loss, anorexia, decreased libido and insomnia. Some hypnotic drugs depress hypothalamic centres. Other psychotherapeutic agents may cause a range of hypo thalamic side effects, including breast engorgement, lactation, amenorrhoea, appetite stimulation and alterations in temperature regulation.
Cerebrum
The cerebrum, the largest and uppermost section of the brain, is the highest functional area, where sensory, integrative, emotional, language, memory and motor functions are controlled (see Clinical Interest Box 14-3). The cerebrum consists mainly of right and left hemispheres connected by thick fibrous tracts. Each hemisphere is involved in functions and sensations of the opposite side of the body, i.e. contralateral control. The superficial, massively folded layer of the cerebrum is called the cerebral cortex (Latin: bark, rind), or grey matter of the brain, and covers the four lobes into which each hemisphere is divided. (These lobes are named for the bones of the skull under which they lie: frontal, parietal, occipital and temporal; see Figure 14-2.) The white matter is so-called as it is largely made up of myelinated axons, whereas the grey matter comprises dendrites, cell bodies and supportive tissues.
Clinical interest Box 14-3 Brain size does matter
Does brain size determine intelligence? If so, why is an elephant, with a larger brain, less intelligent than a human (assuming it is)? At what stage in evolution did primates develop consciousness and speech? How does brain size relate to body size? These and similar fascinating questions are discussed by the worldrenowned British biologist Richard Dawkins in his recent book, The Ancestor’s Tale: A Pilgrimage to the Dawn of Life (2004).
In a study of brain mass compared with body mass in a wide range of placental mammal species, from tiny shrews to massive whales, a clear straight-line correlation is found when the (logarithm of) brain mass is plotted against the (logarithm of) body mass. So body size does determine brain size. However, there are ‘outliers’—most notably primate species (humans, apes and monkeys) and dolphins—in which the brain is larger than would be expected. In fact, the human brain is about six times bigger than it ‘should’ be, from the graph; humans truly are brainier.
What drove the amazing evolution of the primate brain, such that humans can express personality, philosophise, play chess, paint masterpieces, design cities and computers and compose, memorise and perform whole musical concertos? Dawkins suggests various positive Darwinian selection pressures, including rising onto the hind limbs for walking, which allows manual dexterity, communal cooperative living, which allows for specialisation and the development of language.
And what is the relevance of this to pharmacology? Dawkins explains clearly why responses (such as growth in brain size or metabolic rate) are related to the logarithm of the body mass: because as the mass of a body is inflated, its surface area is squared, which is a doubling on a logarithmic scale. In the classic pharmacodynamic graph (Figure 5-5), response to a drug is plotted against the logarithm of the dose (or concentration) of the drug—not only does this turn a sigmoidal plot into a straight line, making it easier to compare effects, this also makes sense, as the number of sites for drug action (e.g. receptors) is more likely to be related to the surface area of the tissue or cells exposed to the drug, rather than to the amount or mass.
The cortex can be broadly classified into motor areas and sensory areas. The frontal lobe contains the motor and speech areas and areas for intellectual functions, affective behaviour (mood) and abstract thinking. The sensory areas are located in the parietal lobe, the visual cortex in the occipital lobe and the auditory cortex and memory areas in the temporal lobe. Association areas, which deal with complex integrative functions, lie near these lobes and act in conjunction with them. In addition, large parts of the cortex are concerned with higher mental activity—reasoning, creative thought, judgement and memory.3 The limbic lobe is the most primitive component of the cerebral cortex (see ‘Limbic system’, below) and is responsible for emotions, activities and drives required for the survival of the individual and the species.
Even simple tasks require simultaneous interactions among many parts of the brain, plus general functions of consciousness, attention and decision making. In righthanded people (about 90% of the population), the left hemisphere is dominant and controls speech and language, ability with sequential mathematical problems and aggressive or cheerful moods, while the right hemisphere is more concerned with emotional inflections of speech, consciousness, appreciation of music, three-dimensional relationships and introspective depressed moods.
Drugs that depress cerebral cortical activity (CNS depressants such as general anaesthetics and alcohol) may decrease acuity of sensation and perception, inhibit motor activity, decrease alertness and concentration, depress higher mental functions such as cognition and memory, depress autonomic functions such as cardiovascular control and respiration and promote drowsiness and sleep. Drugs that stimulate the cortical areas (such as caffeine or amphetamines) may cause more vivid impulses to be received, greater awareness of the surrounding environment, increased muscle activity and restlessness and autonomic stimulation.
CNS functional systems
Specific types of signals are processed in particular brain regions, described not so much by anatomical boundaries as by overall functional aspects. Generally, sensory areas receive and interpret information from receptors for touch, temperature, pain and proprioception; motor areas integrate all voluntary movements, including speech; and association areas have complex integrative functions in memory, emotions, willpower, intelligence and personality. Four major CNS functional systems affected by CNS-active drugs include the reticular activating system, the limbic system, the extrapyramidal system and the basal ganglia.
Reticular activating system
The reticular activating system (RAS) is a diffuse system of nuclei in the reticular formation of the brainstem that permits communication between the spinal cord, thalamus and cerebral cortex. The primary functions of the RAS are:
Inactivation of the RAS results in sleep, and injury or disease to the RAS may produce a lack of consciousness or a comatose state; in deep coma, even reflexes are lost. 5-Hydroxytryptamine (5-HT; serotonin) is a neurotransmitter in many pathways in the RAS.
Many drugs act on the RAS: anaesthetics dampen its activity and induce sleep, whereas amphetamines stimulate or activate the system. Lysergic acid diethylamide (LSD; see Figure 21-4) and other hallucinogenic agents may act on the RAS by interacting with serotonergic pathways, thus interfering with its ability to filter out stimuli; hence a person taking this substance is bombarded by stimuli. In contrast, the phenothiazine tranquillisers such as chlorpromazine enhance the filtering activity of the RAS, thus reducing hallucinations in psychotic patients or people taking LSD.
Limbic system
The limbic system is a border of subcortical structures that surround the corpus callosum around the top of the brainstem (Figure 16-3). Among the components of the limbic system are the olfactory bulbs, hippocampus, cingulate gyrus, hypothalamic nuclei and amygdala. The limbic system is extremely complex in its functioning, interacting with other parts of the brain to influence or normalise expressions of emotions, such as anger, fear, anxiety, pleasure and sorrow, or to affect the biological rhythms, sexual behaviour and motivation of a person. In addition, learning and memory have been associated with the hippocampus.
Drugs that affect the limbic system include the benzodiazepines and opioids. The benzodiazepines are believed to suppress the limbic system, preventing it from activating the reticular formation, and thus cause drowsiness and sleep, especially in patients with anxiety. Morphine is thought to alter subjective reactions to pain as well as abolishing pain stimuli received by special areas within the limbic system.
Extrapyramidal system
The extrapyramidal system is a series of indirect CNS motor pathways that are outside the main motor pathways that traverse the pyramids in the thalamus (hence the term ‘extrapyramidal’). The system consists of many pathways or tracts (with names such as the lateral reticulospinal tract and the rubrospinal tract) that coordinate posture and movements of muscles in the limbs, head and eyes. Antipsychotic agents that block dopamine receptors may produce adverse effects related to this system; these are referred to as extrapyramidal side effects and may mimic the signs of parkinsonism (see Chapters 18 and 20, and Clinical Interest Box 18-7).
Basal ganglia
The basal ganglia are a series of paired nuclei in each cerebral hemisphere that coordinate gross automatic muscle movements and regulate muscle tone; the main components are the corpus striatum (made up of the caudate nucleus and putamen) and the globus pallidus. The substantia nigra and the red nuclei of the mid-brain are sometimes included as components of the basal ganglia. They are connected with the cerebral cortex, thalamus and hypothalamus, and regulate the tone and characteristics of all voluntary movements; thus damage to the basal ganglia, such as occurs commonly in Parkinson’s disease, can lead to increased muscle tone, rigidity and tremors.
Spinal cord
The spinal cord is a thick band of nerve fibres surrounded by the three meningeal membranes (dura mater, arachnoid mater, pia mater) that surround the entire CNS; it lies within the spinal canal formed by the protective vertebrae. It functions in the transmission of impulses to and from all parts of the brain and is also a centre for reflex activity. Ascending tracts of afferent nerves conduct impulses up from peripheral receptors and nerves to the brain, and descending tracts conduct efferent impulses down from the brain to synapse with peripheral motor and autonomic nerves.
A cross-section of the spinal cord (Figure 14-3) reveals an internal mass of grey matter (cell bodies, dendrites, axon terminals, unmyelinated axons and neuroglia) enclosed by white matter (tracts or columns of myelinated nerve fibres). The butterfly-shaped grey matter is divided into horns; the afferent (sensory) nerve fibres are located in the dorsal (posterior) horns, while the efferent (motor and autonomic) nerve fibres exit from the ventral (anterior) horns. When a pain impulse reaches the dorsal horn, for example, the impulse will be transmitted towards the brain along specialised tracts (lateral spinothalamic tracts) to the thalamus, and thence to other areas of the brain. The brain responds by means of the descending efferent fibre pathways to inhibit or modify other incoming pain stimuli (see the discussion of the gate theory of pain in Chapter 15). Through this pathway, the perception of pain can be blunted by stress, stoic determination, the ‘heat of battle’ or analgesic drugs. Small doses of spinal stimulants may increase reflex excitability; larger doses may cause convulsions.

Figure 14-3 Transverse section of the spinal cord; neural components of a spinal reflex are shown in darker blue on the right-hand side.
Some sensory afferent neurons synapse with motor efferents in the grey matter of the spinal column, forming reflex arcs. An example is the flexor (withdrawal) reflex, which is important in preventing injury. If the hand touches a hot object, for example, then even before the ascending impulse reaches the brain and allows conscious thought about dangers, synapses between sensory neurons onto ipsilateral motor efferents within the spinal dorsal horn (via interneurons) relay signals back to the appropriate muscle groups, initiating movement to pull the hand away.
Blood–brain barrier
The blood–brain barrier is a selectively permeable filter between the blood circulation and the cells of the brain and spinal cord, which tends to exclude from the CNS large water-soluble molecules, microorganisms and other toxins. The existence of a barrier between the blood and the brain, preventing easy passage of molecules from the systemic circulation into the CNS, was first postulated to account for the fact that acidic dyes (after being injected IV into animals to stain tissues for histological studies) did not stain the brain cells. Other clinical evidence was that many antimicrobial drugs useful in peripheral infections were ineffective in treating infections of the CNS.
The blood–brain barrier is now attributed to tight junctions between endothelial cells in the cerebral capillaries, a covering formed from the foot-like processes of the glial cells (astrocytes) that encircle the brain’s capillary walls, and the almost complete absence of pinocytotic vesicles in the capillary endothelial cells (see Figure 14-4).
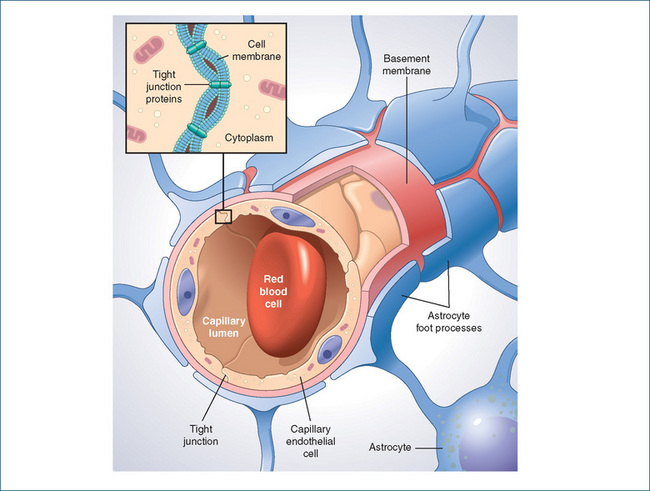
Figure 14-4 The blood–brain barrier, showing tight junctions between capillary endothelial cells and astrocyte foot processes.
Source: netterimages.com; used with permission.
The barrier presumably evolved for protective functions, as it prevents passage of many potentially toxic large molecules into the CNS and keeps the brain and spinal cord in a remarkably stable internal environment. This barrier function is not absolute but is selectively permeable, as it will allow small molecules (such as water, alcohol, oxygen and carbon dioxide), lipid-soluble substances and gases to penetrate but excludes water-soluble and large molecules. There is also active transport and secretion of compounds between the brain and blood: nutrients such as D-glucose and precursors to neurotransmitter substances (such as choline and the amino acids phenylalanine, tyrosine and L-dopa) pass across or are actively transported. Such selective processing allows the brain a degree of security against the toxic effects of some ingested poisons or drugs on the CNS, while substances essential to energy supply or metabolic pathways are permitted. The barrier also effectively prevents active compounds such as neurotransmitters released in one site in the CNS or in the periphery from being taken up into the bloodstream, transported to other CNS sites and acting inappropriately. Some of the important clinical implications of the existence of the blood–brain barrier are summarised in Clinical Interest Box 14-4.
Clinical interest Box 14-4 Brain size does matter
The barrier is ‘broken down’ in most focal injuries to the brain, e.g. inflammation, convulsions, trauma, tumours or infection; this has the useful effect that drugs that might not otherwise pass across into the brain, such as many antibiotics, are more likely to penetrate infected or inflamed tissue.
The barrier is underdeveloped at birth; hence infants are at risk of CNS side effects from any drugs administered, or indeed from drugs taken by the mother during pregnancy or while breastfeeding.
A consequence of the previous point is that infants are at risk of accumulating bilirubin, a breakdown product of cell metabolism, in the brain; the neonate’s liver is too immature to deal with large amounts of bilirubin, which can pass across into the brain and cause kernicterus and permanent brain damage. (Such infants are often placed under a UV lamp, as the energy from the UV source helps break down bilirubin and prevent its accumulation.)
As a general summary rule, the drugs that do pass the blood–brain barrier are uncharged compounds (not ionised), have high lipid solubility and are not highly protein-bound; an exception is alcohol (ethanol) which is a very water-soluble molecule, but so small that it crosses membranes as readily as does water.
A focus of current research is on methods to increase the permeability of the blood–brain barrier to specific therapeutic agents, such as antibiotics or antineoplastic agents needed to treat localised brain infections or brain tumours.
Nerve cells and synaptic transmission in the CNS
The two major cell types in the CNS are neurons, or nerve cells, and glial cells (neuroglia). The functions of the glial cells are not fully understood: they do not conduct action potentials, but may express a range of receptors and transporters, serve to nurture, support and assist neurons in the transfer and integration of information in the CNS and assist in maintaining nutrients, forming myelin, protecting against disease and helping form the blood–brain barrier. Recent studies indicate that glial cells play a role in neural plasticity, in protection from or recovery after injury (by taking up excitatory amino acids), and may also be crucial to memory formation.
Neurons in the CNS have the same basic structure as those in the PNS: dendrites, cell body, axon and nerve terminals (see Figure 11-3). However there are two major differences:
The CNS can be envisioned as an incredibly complex series of wired connections among neurons, with an estimated 100 billion neurons in a human brain. The connections, however, are incomplete: between each nerve terminal and the next cell is a gap, or synapse, and electrical impulses cannot jump directly across. Instead, chemical messengers are released from the terminal end of the first neuron (the presynaptic side) and cross the gap to receptors in the membrane of the cell on the post-synaptic side, which may be a neuron or (in the PNS) another cell that carries out some function stimulated by the nerve (an effector cell). This process is known as synaptic transmission and the chemical messenger is termed a neurotransmitter. Neurotransmission is described in more detail in Chapter 11 and Figures 11-6 and 11-7 in the context of transmission in the PNS. Neurotransmission in the CNS is a similar process but there are many more connections: each CNS neuron may synapse directly or indirectly with around 10,000 others.
Action potentials and ion channels
Most information transmitted in the CNS is due to alterations in electrical currents. The electrical properties of nerve cells are generated by various ions, pumps and channels located in the cell membrane (see Chapter 11 and Figure 11-4 for more detail). The pumps maintain an electrical difference or potential across the cell membrane by maintaining imbalances in the ionic concentrations of sodium, potassium and chloride across the membrane. They are capable of actively moving charged ions from one side of the membrane to the other side through ion channels (membrane pores) that allow the passage of specific ions. Channels are described as voltage-gated if they open in response to changes in membrane potential (voltage), e.g. during the generation and conductance of action potentials. By comparison, a ligand-gated channel opens and closes in response to a specific chemical stimulus (a ligand is something that binds, e.g. a neurotransmitter, hormone or drug that binds to a specific receptor or channel).
The movement and concentration of ions in and around the cell are the primary determinants affecting the membrane potential of the nerve cells. In the resting state, sodium and chloride are found in large amounts outside the cell, while potassium is in high concentration within the cell. The concentration gradients are stabilised by the sodium–potassium adenosine triphosphatase (ATPase) pump, which trades three sodium ions from the intracellular fluid for two potassium ions from the extracellular fluid. Overall, there is a small build-up of negative ions (phosphate and proteins) in the cytosol inside the cell membrane, and an equal build-up of positive ions (mainly sodium) just outside the membrane. This helps to maintain the resting membrane potential, i.e. the voltage difference across the membrane, normally at about −70 mV, as the inside of the cell is negative compared with the extra cellular fluid.
An important property of a nerve cell therefore is that it is polarised (electrically charged) in the resting state. However, a depolarising potential or other stimulus may reduce the membrane potential, i.e. depolarise the cell, to a critical level. This depolarisation will result in the rapid opening of voltage-gated sodium channels, allowing sodium to flow into the cell, which causes further depolarisation of the nerve cell along its length; this reduction in membrane potential generates an action potential (see Figure 11-5). Voltage-gated potassium channels open slightly later, allowing potassium ions to rush out of the cell, producing repolarisation. As the action potential moves very rapidly along the membrane of an axon, the nerve impulse is propagated along the nerve towards the terminals. The ATPase pump then restores the resting potential.
Many drugs act either directly on the ion channels or via receptors that affect ion channels; for example, local anaesthetics enter nerve cells and physically block sodium channels. This effectively reduces sodium influx, preventing generation of the action potentials and conduction of nerve impulses, especially in neurons that carry messages from pain receptors. Sedative drugs such as benzodiazepines and barbiturates modulate the binding of the inhibitory transmitter GABA to the GABAA receptor and thus enhance opening of the chloride channel, producing inhibitory postsynaptic potentials and synaptic inhibition.
Criteria for central neurotransmitter status
The criteria for a chemical to be classed as a central neurotransmitter are:
CNS neurotransmitters
There are about 40 different types of CNS neurons (classified by neurotransmitter) that use chemical transmitters for rapid communication across synapses. Some of the chemicals that have been identified as acting as CNS neurotransmitters are:
Pathways (tracts) of neurons containing a particular transmitter have been identified and tracked through brain areas (see Figure 14-5). For example, there are dopaminergic pathways (neurons that use DA as a transmitter) from the substantia nigra to the striatum, involved in motor control; from the ventral tegmental area to the limbic system and the frontal cortex, involved in cognition and emotion; and from the hypothalamus to the pituitary, controlling release of pituitary hormones. Drugs that affect DA transmission will therefore have major actions and/or side effects on motor control, thought processes and emotions, and endocrine functions.
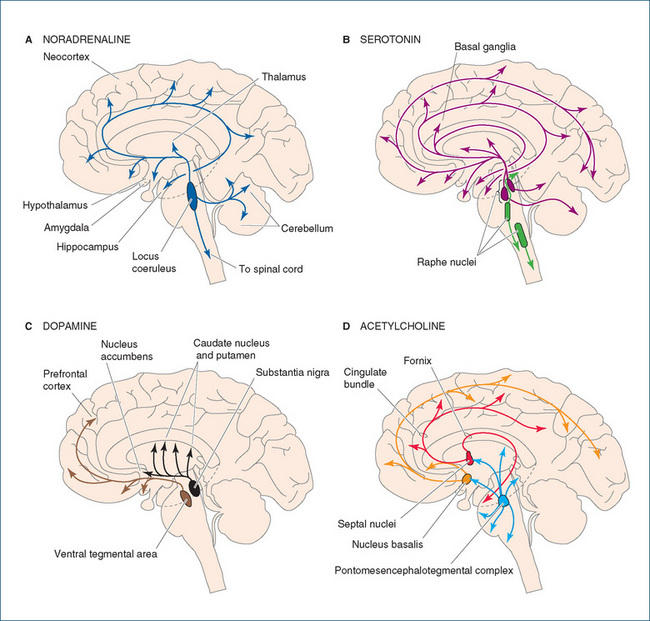
Figure 14-5 Sagittal sections of the brain, indicating major pathways of central neurons utilising important neurotransmitters: A noradrenaline; B 5-HT (serotonin); C dopamine; D acetylcholine.
Source: Boron & Boulpaep 2005; used with permission.
Acetylcholine
Acetylcholine (ACh) was the first identified and is the best known chemical transmitter of nerve impulses. In the PNS, ACh is the neurotransmitter at all autonomic ganglia, at parasympathetic (and sympathetic cholinergic) neuroeffector junctions and at the neuromuscular junction. However, not all parts of the CNS contain ACh; CNS areas with high concentrations of cholinergic neurons are the reticular formation, the basal forebrain, basal ganglia and anterior spinal roots (Figure 14-5D). In the CNS, ACh is mainly excitatory and is involved in cognition, memory, consciousness and motor control. Levels appear to be low in Huntington’s disease and in dementias such as Alzheimer’s disease.
Monoamines
The monoamine transmitters are NA, DA, adrenaline,6 5-HT and histamine. DA is particularly involved in motor control, behaviour, reward systems and endocrine control and is present in high concentrations in the ventral tegmental area, the substantia nigra and the caudate nucleus (Figure 14-5C). In the CNS, NA is mainly inhibitory; cell bodies for noradrenergic neurons are found in the pons and medulla. NA is present in central autonomic pathways, particularly in the hypothalamus and medullary centres, and is involved in central autonomic control, arousal, mood and reward systems (Figure 14-5A).
Important 5-HT pathways run between the midbrain and cortex, with extensive innervation of virtually all parts of the CNS. Cell bodies are especially prevalent in the raphe nuclei of the brainstem (Figure 14-5B). 5-HT actions are mediated through a wide range of receptor types and may be excitatory or inhibitory; 5-HT is involved in cognition, behaviour, sleep–wake cycles, mood, vomiting and pain (especially in the aetiology of migraine).
Although the effects of catecholamines (NA, DA and adrenaline) injected into the CNS are slight in comparison with their effects in the ANS, rises in levels of catecholamines and 5-HT do cause cerebral stimulation. Drugs such as reserpine and methyldopa that deplete the 5-HT and NA levels in the brain have a cerebral depressing effect. Centrally acting α2-adrenoceptor stimulants such as clonidine paradoxically reduce blood pressure by inhibiting peripheral sympathetic stimulation. The roles of monoamine transmitters in psychiatric disorders (schizophrenia and depression) and the effects of psychotropic drugs on monoaminergic transmission are discussed in greater detail in Chapter 18, and DA’s role in Parkinson’s disease in Chapter 20.
Amino acid transmitters
Amino acids are probably the most ancient (from an evolutionary viewpoint) type of neurotransmitter, being particularly prevalent in the spinal cord. For example, GABA is an important inhibitory transmitter in many interneurons in the spinal cord and in the cerebellum and hippocampus. GABA is involved particularly in motor control, in spasticity and in sleep/wakefulness. Inhibitory control is necessary to avoid such excessive excitation as occurs during seizures and epilepsy.
The excitatory amino acids (EAA)—glutamate, aspartate, cysteic acid and homocysteic acid—are present in virtually all regions and are thought to be implicated in the neuronal injury involved in many neurological disorders. Overactivation of receptors for L-glutamate, the major excitatory transmitter, mediates excitotoxicity leading to neuronal death in both acute brain injury such as stroke and chronic disorders such as motor neurone disease. Monosodium glutamate (MSG), a flavour enhancer present in many Asian foods and meals, causes in susceptible people the ‘Chinese restaurant syndrome’, with CNS stimulation, flushing and nausea. Other excitotoxins may be involved in chronic degenerative diseases such as Huntington’s chorea, in dysfunction after CNS viral infections and in neurological syndromes linked to plant neurotoxins.
Neuropeptides
Neuroactive peptides are derived from secretory proteins formed in the cell body; they may be considered as neuromodulators, neurohormones or neurotransmitters. Peptides may cause excitation or inhibition of target neurons. The parenteral or intracerebral injection of these chemicals causes potent behavioural effects. Some of these peptides also exist in tissues other than the CNS, primarily in the gastrointestinal tract cells or in the pituitary gland.
There are several families of neuropeptides: peptides in the same family contain long stretches of identical amino acid chains. Examples are vasopressin and oxytocin, the secretins, the tachykinins, the somatostatins and the opioid peptides. (The opioids, including enkephalins and endorphins, are considered in greater detail in Chapter 16, in the context of pain and analgesic drugs.) Many of the neuropeptides that have been demonstrated to be present in the brain have currently no specific pharmacological antagonists, so it is difficult to identify their functions. In the process of co-transmission, classic neurotransmitters and several neuroactive peptides may be released simultaneously from the same neuron, e.g. ACh and vasoactive intestinal peptide (VIP), enkephalin, substance P or galanin.
Other CNS neurotransmitters
Other chemicals that may act as neurotransmitters or neuromodulators include eicosanoids, such as prostaglandins, and purine nucleotides, such as adenosine and ATP. Nitric oxide undoubtedly has neurotransmitter-like functions in the CNS, but it does not classify as a typical transmitter by the ‘classical’ criteria listed above. There may indeed be many other chemicals with neurotransmitter functions in the CNS but these are as yet unidentified.
Receptors for neurotransmitters
The effect of a transmitter at any synapse is determined by the nature of the receptor to which it binds; thus ACh may have fast excitatory effects at nicotinic receptors and slower effects via G-proteins and second messengers at muscarinic receptors. Some transmitters may have inhibitory effects, e.g. by hyperpolarising postsynaptic membranes or by inhibiting further release of transmitter from the pre synaptic terminal by actions on autoreceptors (see below).
The field of CNS neurotransmitter receptor types is one of the most active and rapidly developing in all pharmacology: a bewildering number of types and subtypes of receptors for many CNS transmitters have been discovered, cloned and, for many, the amino acid sequence identified, and chemicals acting as specific agonists or antagonists synthesised. The pharmacological significance and clinical importance of these discoveries are still being elucidated. Table 14-1 summarises some of the better-known receptors and pharmacologically relevant data in this field.
Table 14-1 Summary of types of receptors for CNS neurotransmitters, emphasising those involved in pharmacological activity of clinically-important CNS-active drugs*
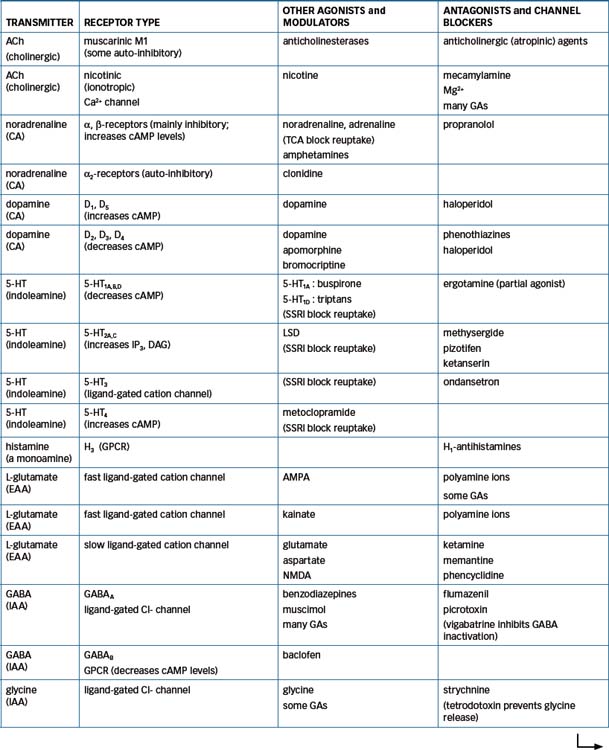
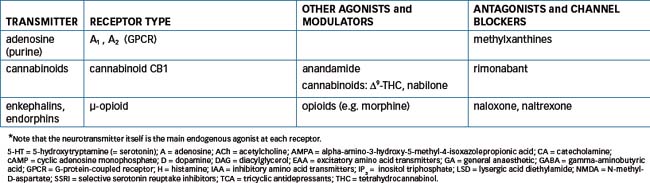
Several types of receptors for EAA such as glutamate have been identified, including receptors for NMDA (N-methyl-D-aspartate) and kainate (a constituent of seaweed); these may be involved in the aetiology of epilepsy (see Chapter 17). The GABAA receptor has sites for bind ing GABA and also sites that bind benzodiazepines, barbiturates, neurosteroids and picrotoxin, a GABA antagonist. Dopamine receptors have been classified into several subtypes (D1–5); development of agonists or antagonists specific for the different receptor types will be beneficial clinically and assist research into dopaminergic mechanisms. There are at least seven main types of 5-HT receptors, involved in functions as diverse as sleep and wakefulness, mood, feeding, behaviour and hallucinations.
Autoreceptors
Release of some transmitters can be modulated by the transmitter acting back on autoreceptors located on the presynaptic side of the nerve ending and on the dendrites and axons (analogous to α2-adrenoreceptors in the sympathetic nervous system; see Figure 12-2). The mechanisms by which a transmitter inhibits its own release have not been fully elucidated and may be different from postsynaptic transduction mechanisms. Presynaptic receptors may also be involved in modulating the release of other transmitters; for example, NA release can be inhibited by agonists acting on muscarinic, opioid and DA receptors and can be facilitated by agonists on β2-adrenergic, ACh-nicotinic and angiotensin II receptors. There are also presynaptic DA auto receptors that inhibit DA synthesis and release and thus slow the firing of dopaminergic neurons; these may be involved in the on–off effects in levodopa therapy for Parkinson’s disease. The presynaptic inhibitory and facilitatory receptors on the same nerve terminals are thought to allow for fine-tuning of transmitter release in various physiological (and pharmacological) situations.
Neurotransmitter imbalances in disease states
In many disorders of the CNS, it appears there are imbalances between levels of different neurotransmitters in particular parts of the brain. In some conditions, chemical analysis of the brains of patients who have died from a disease has shown that tracts or neurons had degenerated in particular areas. In a (simplistic) attempt to describe an overview of these conditions, the following scheme can be proposed (see Figure 14-6): the effects of monoamines (NA, DA, adrenaline, 5-HT) are envisaged to ‘balance’ (as on a see-saw; not in strict molecular equivalents) the effects of ACh, particularly on motor control, mood and thought processes. Thus in depression there is a relative deficiency of NA and 5-HT in areas of the brain related to mood (affect) and an excess of ACh. The depressed mood can be improved by antidepressant drugs such as the selective serotonin reuptake inhibitors (SSRIs), tricyclic antidepressants (TCAs) and monoamine oxidase inhibitors (MAOIs), all of which, by differing mechanisms, increase the levels of monoamines at synapses in the CNS. By contrast, in Parkinson’s disease there appears to be damage to DA-containing neurons and a relative deficiency of DA or excess of ACh. The main drugs used in treatment of Parkinson’s disease either raise the levels of DA or block the actions of ACh (atropinic drugs). This concept (and Figure 14-6) will be referred to again in later chapters on clinical use of drugs in neurological and psychiatric disorders.
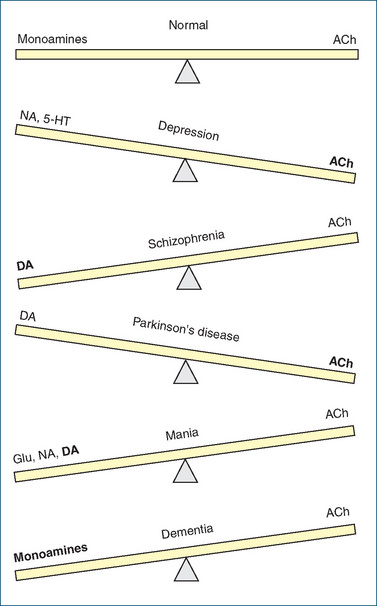
Figure 14-6 Neurotransmitter balances in CNS disorders. In the normal state, effects of monoamine transmitters are ‘balanced’ by those of acetylcholine. In various CNS disorders, imbalances occur and drugs are used in attempts to bring the levels back into balance. 5-HT = 5-hydroxytryptamine (serotonin); ACh = acetylcholine; DA = dopamine; Glu = gluta mate; NA = noradrenaline.
How drugs modify neurotransmission
The stages of neurochemical transmission, including the synthesis, storage, release and inactivation of transmitters, have been described in some detail, as have also the processes of generation and propagation of action potentials. In between are the complicated mechanisms whereby a transmitter activates a specific receptor and initiates a chain of events in the postsynaptic cell. These may include second messenger systems, G-proteins, ion channels, intracellular enzymes, transport systems (carriers and pumps), transcription factors that activate genes, the genes that code for synthesis of all the proteins involved, the enzymes involved in the biosynthetic pathways and the receptors themselves (see Chapter 5).
Virtually every step in these processes and every enzyme or receptor involved can potentially malfunction or be affected by other chemicals, i.e. by drug actions. It is therefore not surprising that there are many pathological conditions in which impaired neurotransmission may be implicated, many drugs that have actions (therapeutic and/or adverse effects) on the CNS and even more situations in which we do not as yet know how the physiological, pathological or pharmacological effects occur. However, wherever possible in the following chapters, the clinical use of drugs will be related back to the level of the synapse and to the drugs’ effects on neurochemical transmission and transmitter receptors.
General anaesthesia
 A general anaesthetic is a drug that produces a reversible state of unconsciousness, with absence of pain sensation over the entire body; such agents have been described as the drugs that remove the most precious of human attributes—consciousness. Before the era of modern medicine and the development of effective anaesthetics and analgesics (see Clinical Interest Box 14-5), successful major surgery was virtually impossible, owing to the devastating effects of pain, blood loss and infection; the patient was usually tied or held down, or rendered unconscious by hypoxia, concussion or high doses of natural CNS depressants such as alcohol or opium. Nowadays, anaesthetists are said to be the medical profession’s best clinical pharmacologists, as they administer a wide range of potent and specific drugs, often in emergency or intensive care situations, continually monitoring the patient for pharmacological effects, adverse reactions and drug interactions.
A general anaesthetic is a drug that produces a reversible state of unconsciousness, with absence of pain sensation over the entire body; such agents have been described as the drugs that remove the most precious of human attributes—consciousness. Before the era of modern medicine and the development of effective anaesthetics and analgesics (see Clinical Interest Box 14-5), successful major surgery was virtually impossible, owing to the devastating effects of pain, blood loss and infection; the patient was usually tied or held down, or rendered unconscious by hypoxia, concussion or high doses of natural CNS depressants such as alcohol or opium. Nowadays, anaesthetists are said to be the medical profession’s best clinical pharmacologists, as they administer a wide range of potent and specific drugs, often in emergency or intensive care situations, continually monitoring the patient for pharmacological effects, adverse reactions and drug interactions.
Clinical interest Box 14-5 A brief history of anaesthesiology
Early techniques for pain relief included:
In the 15th and 16th centuries:
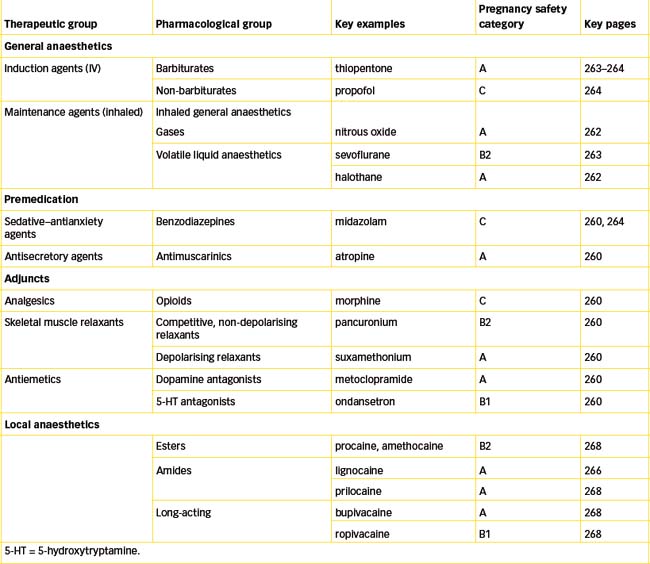
For a drug to be useful as a general anaesthetic, its actions must be of rapid onset, extendable for the duration of the surgical procedure, then rapidly reversible; this means that only CNS depressants that have short half-lives and can be continually administered are useful as general anaesthetics. (Hence depressants such as alcohol, longacting barbiturates and most benzodiazepines are not useful as anaesthetics.) General anaesthesia is usually induced by intravenous injection of a solution of anaesthetic agent such as thiopentone or propofol, and then maintained by inhalation of a gas (nitrous oxide) mixed with the vapour of a volatile liquid (halothane, sevoflurane).
Depressant effects of general anaesthetics
General anaesthetics depress all excitable tissues of the body at concentrations that produce anaesthesia. The pattern of depression is irregular and descending, with higher cortical functions (conscious thought, memory, motor control, perception of sensations) depressed first and medullary centres depressed last, which is fortunate as the medulla contains the vital centres that maintain cardiovascular and respiratory control. It should be noted that a drug may have useful anaesthetic actions without being a good analgesic (pain reliever), and vice versa.
Stages of general anaesthesia
The four stages of CNS depression during general anaesthesia were first described in detail by American anaesthetist Dr Arthur Guedel,7 who observed the effects on the eyes of slowly deepening unconsciousness induced with early anaesthetics such as ether and chloroform. However, the stages of anaesthesia vary with the choice of anaesthetic, speed of induction and skill of the anaesthetist. Stages 1 and 2 constitute induction of anaesthesia. It is now recognised that stage 2 (excitation) can be dangerous, so the current practice is to induce general anaesthesia rapidly with an intravenously administered anaesthetic, then maintain the stage of surgical anaesthesia (stage 3) by inhalation of an anaesthetic gas.
Stage 1: Analgesia
Stage 2: Excitement
Stage 3: Surgical anaesthesia
The third stage is divided into four planes of increasing depth of anaesthesia. The anaesthetist continually monitors the patient’s respirations, eye movements, pupil size and the degree to which reflexes (such as responses to painful stimuli) are present. Most operations are done with the patient in plane 2 or in the upper part of plane 3.
Stage 4: Medullary paralysis (toxic stage)
This is the stage of impending overdose, respiratory arrest and vasomotor collapse.8
Mechanisms of action of general anaesthetics
No GA receptor
General anaesthetics (GAs) vary widely in their chemical structures and in the concentration necessary for each to produce anaesthesia. GAs have been studied and used for more than 150 years and many theories of anaesthesia have been proposed. As there appears to be no simple chemical structure–activity relationship among different GAs, it is assumed that there is no one ‘anaesthetic receptor’. Any theory of GA action must take into account the clearest fact emerging from mechanism studies: the potency of anaesthetic effect is strongly correlated with the lipid solubility of the compound, with very lipid-soluble compounds being very potent. Indeed, the inverse correlation between lipid solubility and dose (expressed as minimal alveolar concentration [MAC] to achieve anaesthesia) is one of the most powerful correlations in biology, extending over a 100,000-fold dose range, across species ranging from goldfish to humans. For any given GA, there is a very narrow band of concentrations at which consciousness is lost. Early theories of GA mechanisms of action vaguely suggested that GAs either increase the fluidity of lipid membranes or form ‘water crystals’ in membranes, thus stabilising them.
Targets for GA actions
There is still much ongoing research into the mechanisms of action of GAs; research using ‘knock-out’ mice with mutations in particular genes has indicated particular protein targets important in GA actions. The current best theory of GA actions is that they modulate the activity of transmitter-gated ion channels. At the molecular and receptor level, there appear to be three main targets to which GAs bind (see Table 14-1):
Other potential molecular targets for GAs include glycine receptors (inhibitory in the lower brainstem and spinal cord), cyclic nucleotide-gated cation channels and presynaptic inhibition of some sodium channels. Different GAs appear to have varying selectivities for the above molecular targets. The fact that more than one mechanism is involved in general anaesthesia explains why there is no one single ‘GA receptor’ or specific GA antagonist drug.
Overall, GAs cause loss of consciousness by decreasing the functions of excitatory neurotransmitters, including ACh (nicotinic), 5-HT, glutamate and NMDA; increasing the functions of inhibitory transmitters, including GABA and glycine; and possibly interacting with peptidergic transmission, opioid receptors, the nitric oxide–cyclic GMP transduction pathway and reactive oxygen species. The most sensitive areas of the CNS are the sensory pathways from the thalamus to the cortex, leading to inhibition of arousal pathways and potentiation of sleep pathways (hence loss of consciousness), and the hippocampus (hence the amnesia caused by GAs). Immobility is mediated primarily via multiple molecular targets in the spinal cord.
Pharmacokinetic aspects
For anaesthesia to be rapidly controllable, it is important that the concentration of anaesthetic in lungs or blood should equilibrate rapidly with the concentration in the CNS tissues. This increases the speeds of both induction and recovery of anaesthesia, and allows the anaesthetist to adjust the depth of anaesthesia quickly. The depth of anaesthesia depends on the partial pressure of the anaesthetic gas, or the concentration of an injected drug, in the brain. Study of the ideal pharmacokinetics of anaesthetic gases is complicated, involving consideration of the solubility of the agent both in blood and tissues (the blood–gas partition coefficient) and in lipids (the oil–gas partition coefficient), as well as physiological factors that determine the efficiency of respiration and circulation.

Figure 14-7 Potency and solubility of inhaled general anaesthetics. The minimal alveolar concentration [% v/v] required to produce anaesthesia in 50% of patients [MAC] is plotted against the solubility of the anaesthetic drug in blood (expressed as blood–gas partition coefficient). Drugs with high blood solubility (such as ether) are relatively slow in onset of and recovery from anaesthesia, whereas drugs that have lower blood solubility (desflurane, nitrous oxide) are rapid in onset and recovery. The most potent anaesthetics (halothane, isoflurane) are those with low MAC values, whereas nitrous oxide requires >100% concentration for anaesthesia and is usually used at 50% concentration as an analgesic and carrier gas.
Note: Data are plotted with logarithmic scale on each axis.
Data from: Speight and Holford 1997; Oberoi and Phillips 2000.
Clinical aspects
Thanks to recent advances in drugs, monitoring devices and delivery systems, general anaesthesia is now very well tolerated in virtually all patients, allowing new surgical techniques to be made available to all ages of patients. However, most drugs used are both potent and potentially toxic, so patients must be monitored and managed well before, during and after the operation; drugs and techniques must be chosen carefully; and the implications of any concurrent diseases and drug regimens must be considered.
Administration
Most patients will be administered an anaesthetic by inhalation, so the first rule of anaesthesiology is to keep a clear airway. Airway obstruction will lead to anoxia and impaired gas intake, and hence to decreased absorption of anaesthetic drugs and the risk of the patient regaining consciousness too early. A typical set-up of equipment for administration of gas and volatile liquid anaesthetics via the mouth and larynx is shown diagrammatically in Figure 14-8.

Figure 14-8 Diagrammatic representation of the arrangement of a typical general anaesthetic machine. Gases from the cylinders are admitted by opening the cylinder keys (CK), and the pressures are measured with gauges (PG) and lowered by reduction valves (RV). The flows of gases are controlled by flow control valves (FCV) and monitored by flowmeters (FM). Gases other than those shown may be available (e.g. 5% CO2 in O2), and there may be additional volatilisers for generating anaesthetic vapour in the replenishment line or in the circuit.
Source: Bowman & Rand 1980; used with permission.
Methoxyflurane is available in an inhaler, to facilitate easy administration in an emergency situation. It is now used only as an analgesic, not for full anaesthesia, due to nephrotoxicity associated with higher doses.
Endotracheal intubation
Airways obstruction can be caused by the tongue falling back, laryngeal spasm, airways disease or mechanical faults. For these reasons, most patients will be intubated, i.e. have an endotracheal tube passed via the larynx into the upper part of the trachea (see Figure 28-6), to maintain a reliable airway. The tube is usually cuffed to help prevent inhalation of secretions or vomit. A dose of a skeletal muscle relaxant (see below, ‘Adjuncts to anaesthesia’) is normally administered to facilitate intubation.
Balanced anaesthesia
Maintaining the surgery patient insensible to pain, while supporting life functions and balancing mechanisms for fluid, electrolyte and metabolic homeostasis, requires the administration of many drugs concurrently by non-oral routes. No single anaesthetic drug can produce analgesia, relief of anxiety, muscle relaxation, amnesic effects, suppression of reflexes, physiological stability and rapid, maintained, reversible anaesthesia. The induction of anaesthesia by using a combination of drugs, each for its specific effect, rather than by using a single drug with multiple effects, is termed balanced anaesthesia (see Clinical Interest Box 14-6).
Clinical interest Box 14-6 In the anaesthetist’s drug trolley
A typical anaesthetic drug trolley may contain supplies of the following drugs, for use by the anaesthetist (only two or three examples of each type are given here):
It would be a useful pre-examination exercise for the student to ‘play anaesthetist’ and consider the actions, indications for use and common adverse effects of all these drugs, and attempt to predict any potentially major drug interactions and problems in elderly or renal-impaired patients.
The specific drugs and dosages used depend on the procedure to be carried out, the physical condition of the patient and the patient’s responses to the medications. The advantages of balanced anaesthesia include a safer induction, quicker recovery and lower reported incidence of postoperative nausea, vomiting and pain.
Neuroleptanalgesia and day surgery procedures
An anaesthetic technique formerly frequently used, but now dropping out of favour, is neuroleptanalgesia, or neuroleptanaesthesia. This is a state of deep sedation, analgesia and amnesia produced by a combination of a neuroleptic agent (i.e. an antipsychotic, see Chapter 18), such as droperidol, and a narcotic analgesic, most commonly fentanyl. Neuroleptanalgesia was used for minor surgical procedures requiring the patient’s cooperation, such as endoscopies. However, there was a high incidence of postoperative sedation and restlessness, so newer anaesthetic, anxiolytic and analgesic agents are considered to provide better pain relief and recovery. A typical current IV sedative/analgesic regimen for minor day procedures such as colonoscopy, and those in which the patient needs to remain conscious, is propofol and midazolam (for induction/sedation) and fentanyl (for analgesia). There is a potential for synergistic effects on respiratory depression, and patients must be warned not to drive, sign important documents or operate machinery for at least 24 hours.
Adverse effects and toxicity of general anaesthetics
Although each individual drug has its particular adverse effects, there are adverse effects that are common to all GAs as depressants of the cardiovascular and respiratory systems and reflexes. GAs may also cause postoperative convulsions, headache, nausea and vomiting, kidney or liver toxicity (hepatotoxicity especially with chloroform and halothane), malignant hyperthermia (see Clinical Interest Box 14-7) and hypersensitivity reactions. They are relatively contraindicated during pregnancy (see Drugs at a Glance 14); however, they may be carefully administered when required as maintenance of the mother’s health is important to the wellbeing of the fetus.
Clinical interest Box 14-7 Malignant hyperthermia
Malignant hyperthermia, or hyperpyrexia, is a rare but potentially fatal condition occurring in susceptible patients with an inherited abnormality in muscle sarcoplasmic reticulum. It appears to be precipitated by the combination of a depolarising neuromuscular blocking agent (suxamethonium) with a halogenated GA agent. Trigger drugs include all volatile GAs, xanthines (including caffeine), phenothiazines and possibly sympathomimetics; safe drugs include the benzodiazepines and barbiturates. It is a potentially fatal syndrome of acute accelerated metabolism in skeletal muscle, with rapid fever, acidosis, hyperkalaemia, muscle rigidity and dysfunction of many organ systems.
The predisposition to the condition can be diagnosed by muscle biopsy and in-vitro testing by challenge with caffeine and halothane. While it occurs in only 1 in 6000 to 200,000 persons due to mutations in the ryanodine receptor gene (which codes for the muscle cell Ca2+-release channel), mortality is 70% if specific treatment is not given. Emergency treatment consists of substituting the volatile GA agent with propofol infusion, actively cooling the patient and administering dantrolene (a direct inhibitor of muscle contractions), bicarbonate, an antiarrhythmic agent and appropriate electrolyte and fluid replacements. The patient needs to be cared for in an intensive care unit for at least 24 hours.
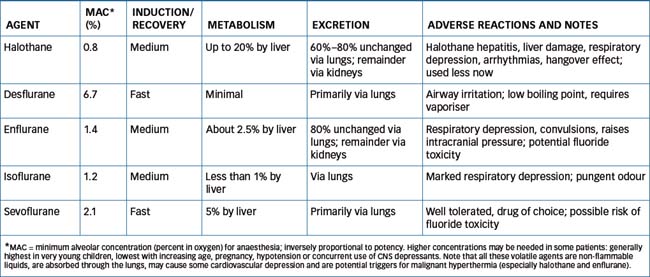
Studies on chemical series of halogenated hydrocarbon compounds have shown that, overall, fluorinated compounds are more potent and less toxic than others. Two recently introduced drugs, sevoflurane and desflurane, appear to have optimal properties as anaesthetics, compared with earlier agents such as ether, chloroform and halothane (Figure 14-7).
Other surgery-related problems
Many other problems unrelated to the anaesthetics used can occur during surgical procedures, such as:
In addition, the staff in the operating theatre are subject to potentially harmful levels of waste gases, even though exhaust gases are extracted from anaesthetic circuits (see Clinical Interest Box 14-8).
Clinical interest Box 14-8 Waste anaesthetic gases as an occupational health hazard
Chronic exposure of staff in hospital operating rooms, dental surgeries and veterinary clinics to waste anaesthetic gases (nitrous oxide and vapours from volatile halogenated agents) can present a significant occupational health hazard. Potential adverse effects include nausea, dizziness, headaches, cancers, liver and kidney disease, impaired mental performance, fatigue and irritability. Studies have demonstrated an increased incidence of spontaneous abortions among women exposed to nitrous oxide, as well as among wives of men who are exposed.
Operating theatre staff should be aware of the potential risks and protect themselves by avoiding the area within about 20 cm of the patient’s mouth and nose when the breath contains exhaled anaesthetic agents. Health-care institutions need to be active in ensuring that all exhaust gases are scavenged and vented to the outside air and in establishing exposure monitoring programs to detect unsafe levels caused by faulty equipment or unsafe practices.
Source: Safety and health topics: waste anesthetic gases (http://osha.gov/SLTC/wasteanestheticgases/index.html [28 November 2008]).
Significant drug interactions
Among the dangers facing a surgical patient are the adverse effects of, and interactions between, all the many drugs likely to be used during surgery, plus any drugs the patient has been taking for concurrent illness, whether prescribed, over-the-counter or complementary therapies, or for social rather than medical purposes. Anaesthetists need to be familiar with the significant interactions between anaesthetics and the maintenance drug therapies used in a wide range of illnesses.
As a general guideline, if a drug is needed for treatment preoperatively, it should be continued through surgery. Unnecessary drugs are discontinued for a period at least five times the half-life of the drug before surgery. Drugs having significant interactions with anaesthetic agents are replaced, where possible, with an alternative medication before surgery. An overview of potentially significant drug interactions during anaesthesia is given in Drug Interactions 14-1; reference texts (e.g. the Australian Medicines Handbook) should be consulted for likely effects of specific combinations. Not all drug interactions are adverse: the additive CNS-depressant effects of opioid analgesics and GAs can be useful in allowing lower doses of the GA, provided the interaction is anticipated and monitored. Multiple drug interactions are also possible with the many other drugs likely to be used during general anaesthesia, in particular between anticholinesterases or aminoglycoside antibiotics and neuromuscular blocking agents.
Drug interactions 14–1 General anaesthetics
| Drug or drug group | Likely effects and management |
| Anticoagulants such as heparin and warfarin | Usually discontinued 6 and 48 hours (respectively) before surgery to reduce the risk of haemorrhage |
| CNS depressants such as alcohol, antihistamines, antianxiety agents, opioids and sedatives/hypnotics | Intensify the cardiovascular-, respiratory- and CNS-depressant effects of GAs; monitor carefully and reduce GA dose if necessary |
| Antiarrhythmic agents | May exacerbate cardiovascular system depression and hypotension caused by GAs |
| Calcium channel blockers and β-blockers | Enhance cardiovascular suppression and arrhythmias; monitor blood pressure and reduce GA dose if necessary |
| Corticosteroids taken chronically | Produce adrenal gland suppression, may result in hypotension during surgery and lack of ability to respond to stress; corticosteroids are usually resumed in patients who have recently stopped exogenous corticosteroid therapy |
| Drugs that inhibit CYP3A4 enzymes, e.g. azole antifungals, protease inhibitors, macrolide antibiotics | May inhibit metabolism of midazolam and enhance its CNS depressant actions; dose of midazolam must be reduced |
| Drugs that affect blood pressure or heart rate | Interact with ketamine, which increases BP and HR; monitor carefully |
Special anaesthesia considerations
Many disease states and risk factors can alter a person’s response to anaesthesia. The preoperative assessment of the patient’s health status should consider acute and chronic medical conditions.
Young age
The physical characteristics of a neonate predispose to upper airway obstruction or laryngospasm during anaesthesia or resuscitation. A (relatively) large body water compartment, immature liver and kidneys, a rapid metabolic rate and undeveloped blood–brain barrier all contribute to the susceptibility to adverse reactions to CNS-active drugs and indicate the need for careful monitoring of the infant or paediatric patient. Drug dosages and administered fluids must be carefully calculated, using recommended paediatric dose regimens. Halothane and nitrous oxide are commonly used in paediatrics because the incidence of hepatitis in children is considered low. Neonates are usually more sensitive to the non-depolarising muscle-relaxing agents.
Advanced age
Ageing results in a generalised decline in organ function, decreased organ reserve capacities and often the existence of chronic disease processes and polypharmacy, with many drugs taken to treat concurrent diseases; the latter results in greater potential for drug interactions and adverse effects. Generally, an increased and prolonged drug effect is seen in the elderly; drug-induced confusion is more likely (especially after midazolam). Mortality rates for elderly patients undergoing major surgery may be many times higher than those for younger people.
Pregnancy and childbirth
Because CNS-active drugs are lipid-soluble, they are likely to cross the placenta and reach significant levels in the fetal bloodstream. Hence inhaled and IV GAs, LAs, analgesics and sedatives must be dosed and monitored carefully if used during pregnancy. For analgesia during childbirth, nitrous oxide (‘gas’) is commonly selfadministered by the mother. Opioid analgesics used during childbirth can lead to respiratory depression in the neonate, so doses are kept to a minimum and effects may need to be reversed by administration of naloxone to the infant. Caesarean section may require general anaesthesia, but can often be carried out under epidural anaesthesia with lignocaine and fentanyl, which allows the mother to remain conscious throughout the birth.
Before any drug is used, the expected drug benefits should be considered against the possible risk to the fetus (see the pregnancy safety categories in Drugs at a Glance 14).
Obesity
Overweight and obese patients may have cardiac insufficiency, respiratory problems, atherosclerosis, hypertension or an increased predisposition to diabetes, liver disease or thrombophlebitis. In such patients, obtaining the desired depth of anaesthesia and muscle relaxation may be a problem. Generally, highly fat-soluble anaesthetics, especially those with toxic metabolites such as methoxyflurane, should be avoided.
Smoking
People who smoke have increased risks of coronary heart disease, peripheral vascular disease and compromised lung function (e.g. bronchitis, emphysema or carcinoma). Postoperative complications are six times more common in smokers than in non-smokers. Smoking also increases a patient’s sensitivity to muscle relaxants.
Alcohol intake
People who are heavy or regular drinkers of alcohol may have a variety of associated disease states, including liver dysfunction, pancreatitis, gastritis and oesophageal varices. Anaesthetic requirements may be increased because of the increase in liver drug-metabolising enzymes and the development of cross-tolerance. Alcoholic patients need to be monitored closely during the post-anaesthetic period for alcohol withdrawal syndrome, as its onset may be delayed by the administration of analgesics. Diazepam or other sedatives may be required to prevent withdrawal symptoms.
Concurrent disease conditions
Whenever possible, concurrent diseases should be treated and pathologies corrected before surgery. Implications of common diseases for drug use in anaesthesia are summarised below.
Preoperative management
The preoperative visit to the patient by the anaesthetist and care of the patient by other health-care professionals should include taking a thorough medical history and ascertaining any relevant information such as drug allergies and concurrent disease. Questions (in words the patient can understand) are asked about:
Preoperative management also includes general aspects such as correct identification of the patient and obtaining written consent; providing information on the proposed procedures, risks and equipment; allaying of anxieties; and teaching of exercises for breathing, coughing and movement postoperatively.
Premedication
‘Premed’ (i.e. preoperative medication) was introduced in the early days of anaesthetic practice to prevent or treat some of the problems associated with the early inhaled GAs such as ether and chloroform. It is no longer considered essential and is often omitted or prescribed only when specifically indicated. Rationales for ‘premed’ include: to allay anxiety (allows lower doses of anaesthetics); to decrease secretions (salivary, gastric and bronchial); to reduce postoperative vomiting; to overcome CNS depression; and to provide prophylactic analgesia and sedation. Table 14-2 gives an overview of the common agents used.
Table 14-2 Premedication agents
| Drug classification | Agents frequently used | Desired effect |
| Opioid analgesics | morphine, fentanyl | Sedation to decrease anxiety; provide analgesia and decrease amount of anaesthetic used |
| Benzodiazepines | midazolam, flunitrazepam | Antianxiety, sedative, rapid induction, amnesia |
| Phenothiazines | prochlorperazine, promethazine | Sedative, antihistaminic, antiemetic, decreased motor activity |
| Anticholinergics | atropine, glycopyrrolate | Inhibition of secretions; reduced vomiting and laryngospasms |
Adjuncts to anaesthesia: muscle relaxants
Many surgical procedures, especially those on the abdomen, require inhibition of voluntary muscle tone and reflexes to stop muscles contracting when stimulated, to provide surgeons with easier access or to aid intubation. This can be achieved with deep general anaesthesia or with nerve block regional anaesthesia, but both these techniques carry risks. More simply, selective skeletal muscle relaxants can be administered once the patient is lightly anaesthetised and adequate analgesia provided. Artificial mechanical ventilation must be administered as the respiratory muscles are paralysed by neuromuscular blocking agents. The pharmacology of these drugs is considered in detail in Chapter 13; the two main groups of drugs used are summarised below.
Non-depolarising neuromuscular blockers
Depolarising neuromuscular blockers
Postoperative aspects
There are many potential complications following surgical operations. Nausea and vomiting can be induced by pain, drugs (especially opioids), suggestion, irritation, ketosis or dehydration. Refraining from food is sometimes helpful; if nausea and vomiting are severe, antiemetics (metoclopramide, ondansetron) can be given. Postoperative pain is common, particularly after procedures involving the thorax or abdomen, episiotomy after childbirth and haemorrhoidectomy. Adequate pain relief must be maintained to facilitate recovery and ease of coughing and defecation; remifentanil (an opioid analgesic) and nonsteroidal anti-inflammatory agents (such as parecoxib) are useful.
Respiratory depression often follows the use of narcotic analgesics (opioids); treatment may be with the opioid antagonist naloxone. Chest complications are exacerbated in smokers and patients with chronic airways diseases and by sputum retention, dehydration, ongoing use of opioids and pain that inhibits coughing. Physiotherapy and rehydration are helpful. The inactivity caused by long surgical procedures and prolonged bed-rest predisposes to thrombosis; early ambulation and antithrombotic drugs (aspirin) or anticoagulants help prevent thrombosis and embolism. Clonidine and dexmedetomidine are sometimes administered for their sedative and cardiovascular stabilising actions.
More general aspects of postoperative care include monitoring of cardiovascular and respiratory functions and fluid balance, supportive nursing and provision of adequate information. Doses of concurrent drugs may need to be lower than usual in the postoperative period, until the patient’s functioning returns to normal.
Reversal of neuromuscular blockade
A new drug used post-operatively is sugammadex, which reverses the neuromuscular (NM) blockade caused by the non-depolarising neuromuscular blockers rocuronium and vecuronium. It is a modified cyclodextrin that forms a complex with these drugs (but not other NM blockers) and thus reduces their binding to nicotinic receptors and speeds recovery from muscle relaxation. It is injected IV and has a rapid effect; recovery of muscle function occurs within 5 minutes, compared to 50 minutes after reversal with neostigmine (an anticholinesterase). Adverse effects include disturbances in taste and allergic reactions; interactions are likely to occur with flucloxacillin, fusidic acid, toremifene and progestogens; women using hormonal contraception should be warned to take extra contraceptive precautions for 7 days after sugammadex administration.
Types of general anaesthetics
General anaesthetics are usually divided into two groups: (1) the inhalation anaesthetics, which include gases and volatile liquids; and (2) intravenous general anaesthetics, such as thiopentone and propofol.
Inhalation anaesthetics
Inhalation anaesthetics are gases or volatile liquids that can be administered by inhalation when mixed with oxygen. These rapidly reach a concentration in the blood and brain sufficient to depress the CNS and cause anaesthesia. This is expressed as the minimum alveolar concentration (MAC) for anaesthesia and is inversely related to potency as an anaesthetic (Figure 14-7). Inhalation anaesthetics have the following characteristics:
Early inhaled anaesthetics included ether and chloroform as volatile liquids, and cyclopropane and nitrous oxide as gases; of these only nitrous oxide is still in clinical use today (in developed countries with advanced facilities) (see Drug Monograph 14-1).
Drug monograph 14-1 Nitrous oxide
Nitrous oxide, commonly referred to simply as ‘gas’, is a simple inorganic molecule with the chemical formula N2O. (It should not be confused with nitric oxide [NO], now recognised as a gas generated in many body cells, and involved in vasodilation, immune responses and as a chemical mediator in the CNS in neurotransmission and neurodegeneration.) Its analgesic action is similar to that of opioids and may be mediated via opioid receptors, while the anxiolytic action is mainly due to enhancement of GABA-mediated CNS depression.
INDICATIONS Nitrous oxide is the most commonly used agent for dental surgery, minor surgery and obstetric analgesia. It is a powerful analgesic, useful anxiolytic but a weak anaesthetic, so is often combined with other (volatile) anaesthetics to enhance its effects when it is also used extensively in major surgery. It is presented as a compres sed gas, for example in a 50:50 mixture with oxygen (Entonox), in blue cylinders (see Clinical Interest Box 28-3 and Figure 28-2).
PHARMACOKINETICS Nitrous oxide is inhaled and absorbed via the lungs; it has low solubility in blood and tissues, so has a rapid onset of action and recovery time. It is excreted 100% unchanged through the lungs.
ADVERSE EFFECTS AND DRUG INTERACTIONS It is non-irritant and virtually without odour. Its few adverse effects consist primarily of hypoxia, mild cardiac depression and postoperative nausea, vomiting or delirium; it has no known significant drug interactions. There is abuse potential, and escaped gas should be scavenged to avoid occupational exposure and contribution to the greenhouse effect. It is considered safe in pregnancy and is very widely used as an inhaled analgesic in child birth, as it can be self-administered during painful contractions then released, and does not accumulate or cause respiratory depression in the neonate.
WARNINGS AND CONTRAINDICATIONS There is a risk of hypoxia if inadequate oxygen is provided. At the termination of nitrous oxide anaesthesia, the rapid movement of large amounts of nitrous oxide from the circulation into the lungs may dilute the oxygen in the lungs (diffusion hypoxia). To prevent this, the anaesthetist usually administers 100% oxygen for 3–5 minutes to clear the nitrous oxide from the lungs.
DOSAGE AND ADMINISTRATION For general anaesthesia, the recommended dosage is 70% nitrous oxide with 30% oxygen for induction and 30%–70% nitrous oxide with oxygen for maintenance. In obstetrics, women self-administer a mixture of N2O/O2 that is 50/50, and in dental procedures a 25% concentration may be used.
Volatile liquid anaesthetics
When volatile liquid anaesthetics such as ether or chloroform were first used, they were administered by placing a pad soaked in the liquid over the patient’s mouth and nose, so that the fumes of the liquid were inhaled. This unpleasant procedure caused struggling, skin reactions, uncertain levels of dosage and absorption and a slow progression through the stages of anaesthesia. The more civilised technique used now involves controlled vaporisation of the volatile liquid into a flow of gas (oxygen with or without nitrous oxide), so that a known concentration of volatile agent in oxygen is administered via a mask or endotracheal tube.
Chloroform is hepatotoxic and ether and cyclopropane are highly flammable; these agents have now generally been replaced by safer anaesthetics. In 1956 halothane, a non-flammable chemical, largely replaced the older volatile liquids. However, halothane has been associated with hepatic dysfunction and failure, so safer analogues of the halogenated hydrocarbon series have been developed: methoxyflurane, desflurane, enflurane, isoflurane and sevoflurane (see Table 14-3). Sevoflurane has become the drug of choice for most procedures owing to its fast action and low toxicity (see Drug Monograph 14-2). Metho xyflurane is an effective analgesic and is used during acute trauma, patient transport and wound dressing; however, renal toxicity limits its regular use.
Drug monograph 14-2 Sevoflurane
Sevoflurane is non-irritant and has a pleasant smell and a rapid onset of action and recovery, so it is suitable for inhalation anaesthesia, particularly in children and in day surgery.
INDICATIONS Sevoflurane is indicated for induction and maintenance of general anaesthesia during surgery.
PHARMACOKINETICS Sevoflurane has a faster uptake, distribution and rate of elimination than isoflurane and halothane (but slightly slower than desflurane). About 5% is metabolised in the liver to an inactive derivative that is rapidly eliminated. Inorganic fluoride, released during metabolism of sevoflurane, has an elimination half-life in the range 15–23 hours.
ADVERSE EFFECTS Cardiac and respiratory depression and shivering and salivation can occur, as well as postoperative nausea and vomiting.
DRUG INTERACTIONS Few significant interactions have been reported. Concurrent administration of other CNS depressants (opioid analgesics, benzodiazepines, inhaled anaesthetics) allows reduction in sevoflurane dosage.
WARNINGS AND CONTRAINDICATIONS Sevoflurane is contraindicated in patients with susceptibility to malignant hyperthermia and used with caution in those with renal failure. A fluoro-ether derivative of sevoflurane (known as compound A), formed after passage of the exhaust gas over lime absorbers for carbon dioxide, is potentially toxic. Levels can be minimised by using high gas flow rates.
DOSAGE AND ADMINISTRATION Sevoflurane is administered from a vaporiser in a stream of oxygen, with or without nitrous oxide. While the induction dose is individualised, the usual inhalation dose for maintenance is up to 5% in adults and 7% in children; surgical anaesthesia is achieved in less than 2 minutes.
Intravenous anaesthetics
Intravenous anaesthetic agents are used for induction or maintenance of general anaesthesia, to induce amnesia and as adjuncts to inhalation-type anaesthetics. The major groups include ultrashort-acting barbiturates (thiopentone) and non-barbiturates (propofol and ketamine). The benzodiazepine midazolam is mainly a sedative–antianxiety agent, but is considered here as it is frequently used as an adjunct to IV anaesthesia. Intravenous anaesthetics are valuable in allaying emotional distress (because many patients fear having a tight mask placed over the face while they are fully conscious) and in reducing the amount of inhalation anaesthetic required. Clinical Interest Box 14-9 lists the advantages and disadvantages of intravenous anaesthetics.
Clinical interest Box 14-9 Advantages and disadvantages of intravenous anaesthetics
Pharmacokinetics
The intravenous anaesthetics are rapidly taken up by brain tissue because of their high lipid solubility. For example, equilibrium between brain and blood levels occurs within one arm–brain circulation time (patients are usually asked to count backwards from 10 as the agent is injected; they rarely reach 4 or 3). Shortness of action results from the drug being quickly redistributed into the fat depots of the body. The amount of body fat affects drug action, due to two-compartment distribution of the drug: the greater the amount of body fat, the briefer the effect of a single IV dose. With prolonged administration or large doses, however, saturation of fat depots leads to prolonged drug action, then slow rate of drug release back into the circulation to be eliminated (10%–15% per hour) results in delayed recovery.
Pharmaceutics
Intravenous anaesthetic agents present an interesting pharmaceutical formulation problem: an IV anaesthetic agent must be highly lipid-soluble (to cross the blood– brain barrier and act) yet sufficiently water-soluble to be formulated as a solution that can be safely injected IV. This problem has been solved for some drugs with very low water solubilities by formulating them as oil-in-water emulsions (similar to milk), e.g. diazepam or propofol in a soya oil/egg lecithin/glycerol emulsion.
Total intravenous anaesthesia (TIVA)
It is possible to carry out surgical procedures under total intravenous anaesthesia (i.e. using the IV agent throughout the operation with no inhaled agents). Both thiopentone and propofol are used this way. A constant plasma drug level is achieved with a bolus initial dose, then an infusion that can be altered depending on the patient’s responses. There are automated, computer-controlled infusion pumps that can be programmed to take into account the mathematically modelled pharmacokinetic parameters of the drug, patient parameters such as weight, age, liver and renal functions, the desired blood concentrations of drug, adjunct analgesics and type of surgical operation.
Ultrashort-acting barbiturates
The prototype ultrashort-acting barbiturate-type agent is thiopentone, which is a CNS depressant that produces hypnosis and anaesthesia without analgesia, and hence is often combined with a muscle relaxant and analgesic in balanced anaesthesia. Being very lipid-soluble, it has a rapid action then short duration due to redistribution, mainly to muscle tissues. Thiopentone also has anticonvulsant effects and reduces intracranial pressure; it is particularly useful in emergency anaesthesia. General anaesthesia with ultrashort-acting barbiturates is believed to result from suppression of the reticular activating system.
The most common adverse effects during the recovery period are shivering and trembling and additive effects with other CNS depressants. Less frequently reported are nausea, vomiting, prolonged somnolence and headache. Serious adverse reactions include emergence delirium (increased excitability, confusion, hallucinations), cardiac arrhythmias or depression, allergic responses and respiratory depression.
Non-barbiturates
Non-barbiturate intravenous anaesthetic agents include the short-acting hypnotics propofol and ketamine, and the benzodiazepines midazolam, diazepam and lorazepam. Propofol will be considered as the prototype drug of this group (see Drug Monograph 14-3).
Propofol is a rapidly acting, non-barbiturate hypnotic, formulated in an emulsion for IV injection or infusion. It has no analgesic effects. Propofol can be used for total intravenous anaesthesia. Its mechanism of action is not known for certain; the CNS depression is probably mediated through GABA receptors.
INDICATIONS It is used for the induction and maintenance of general anaesthesia and for conscious sedation, especially in day-surgery procedures.
PHARMACOKINETICS It has a rapid onset of action within 40 seconds and the duration of effect is only 3–5 minutes, owing to rapid redistribution from the brain to other body tissues; hence there is a short recovery period and few hangover effects. It is almost completely metabolised to the glucuronide, with a long terminal elimination half-life of 3–8 hours.
ADVERSE EFFECTS This agent is a respiratory and cardiac depressant and can produce apnoea, bradycardia and hypotension, depending on dose, rate of administration and drugs concurrently administered. Nausea, vomiting and involuntary muscle movement are commonly reported.
DRUG INTERACTIONS Sedative effects of other CNS depressants are increased; there are no other clinically significant interactions with drugs likely to be used in anaesthesia.
WARNINGS AND CONTRAINDICATIONS Pain on injection can occur; there is potential for abuse.
DOSAGE AND ADMINISTRATION IV dose for adults and children over 3 years is 2–2.5 mg/kg. Dosage regimens for total intravenous anaesthesia are calculated and controlled by the infusion system.
Ketamine is an effective analgesic as well as anaesthetic and is useful for brief procedures such as changing burns dressings; it can be administered IV, IM or orally. It has been called a dissociative anaesthetic as it causes analgesia and amnesia without loss of respiratory function or reflexes; it produces a cataleptic state in which the patient appears to be awake but is detached from the environ ment and unresponsive to pain. Because dreams and hallucinations can occur, it is sometimes subject to abuse (see Chapter 21).
The opioid analgesics fentanyl, sufentanil and alfentanil (see Chapter 15) are sometimes included in this classification as they can be used in high doses to induce anaesthesia.
Benzodiazepines (midazolam, diazepam, lorazepam)
Benzodiazepines are given intravenously as premedication (antianxiety and sedative effects), for induction of anaesthesia, for their amnesic actions and for seizure control. (This group of drugs is considered in detail in Chapter 16.) Diazepam and lorazepam are not watersoluble, so their non-aqueous solutions (emulsions) for injection can cause local irritation; they have very long elimination half-lives hence are long-acting, with prolonged recovery times.
Midazolam is water-soluble and thus is less irritating locally. Intravenously midazolam has a rapid onset of action (2–4 minutes) and a short elimination half-life of about 1–2 hours. It is metabolised in the liver and excreted by the kidneys. It is commonly used in conjunction with propofol and fentanyl in day-surgery procedures where only minimal or moderate sedation is required.
Concurrent use of benzodiazepines with alcohol or CNS-depressant drugs may result in hypotension and enhanced respiratory depression; a reduction in drug dosage and close monitoring are indicated.
Local anaesthesia
 Local anaesthesia refers to the direct administration of an agent to tissues to induce the absence of pain sensation in that part of the body. Unlike general anaesthesia, local anaesthesia does not depress consciousness. As most sensations are not lost, the term ‘anaesthesia’ (total lack of sensation) is strictly speaking inappropriate, and some pharmacologists prefer the term ‘local analgesia’. However, 150 years of usage sanction the terms ‘local anaesthesia’ and ‘local anaesthetic’. Local anaesthetic (LA) drugs reversibly prevent both the generation and conduction of impulses in excitable membranes, particularly in sensory nerves, and hence decrease the sensitivity to pain. They are used in many surgical procedures and for pain relief.
Local anaesthesia refers to the direct administration of an agent to tissues to induce the absence of pain sensation in that part of the body. Unlike general anaesthesia, local anaesthesia does not depress consciousness. As most sensations are not lost, the term ‘anaesthesia’ (total lack of sensation) is strictly speaking inappropriate, and some pharmacologists prefer the term ‘local analgesia’. However, 150 years of usage sanction the terms ‘local anaesthesia’ and ‘local anaesthetic’. Local anaesthetic (LA) drugs reversibly prevent both the generation and conduction of impulses in excitable membranes, particularly in sensory nerves, and hence decrease the sensitivity to pain. They are used in many surgical procedures and for pain relief.
An ideal LA would produce nerve blockade only in sensory nerves when administered topically or parenterally (by injection) and would be rapidly reversible, non-toxic to both local tissue and major organs, with rapid, painless onset of action for a reasonable operating time. While no LA is perfect, the two most commonly used are lignocaine (also known as lidocaine or Xylocaine) and the longeracting bupivacaine.
Local anaesthetic drugs were developed following the introduction of the natural compound cocaine into medicine and ophthalmic surgery in the 1870s and 1880s (as described in Clinical Interest Boxes 14-4 and 14-10). The main problems with cocaine were its acute toxicity and addictive properties, so other benzoic acid esters were studied for LA activity. The amide compound lignocaine, developed in 1943, rapidly became widely used and is still considered the prototype LA (see Drug Monograph 14-4). A drug from the bupivacaine family is used when longer duration of activity is required, and more recently a combination of lignocaine and prilocaine (EMLA: eutectic mixture for local anaesthesia) has been formulated as a cream and a patch for topical application (see under ‘Formulations of LAs’).
Clinical interest Box 14-10 Cocaine—the original local anaesthetic
Drug monograph 14-4 Lignocaine
Lignocaine (also known as lidocaine) is an amide-type local anaesthetic, preventing the initiation and propagation of nerve impulses. It also has antiarrhythmic properties because it stabilises all potentially excitable membranes, including the conduction system of the heart.
INDICATIONS Lignocaine is used commonly for production of local anaesthesia by topical, infiltration, nerve block, epidural or spinal routes. It is also used to treat or prevent ventricular arrhythmias.
PHARMACOKINETICS Onset of action is rapid (5–10 minutes) and duration of nerve blockade is 1–1.5 hours. After absorption into the general circulation or after IV injection, the drug is redistributed rapidly to tissues, especially the heart. Metabolism occurs in the liver and excretion via the kidneys; less than 10% is excreted unchanged. The elimination half-life is 90–120 minutes.
ADVERSE EFFECTS Excessive dosage, rapid absorption or delayed elimination can lead to toxic depressant effects in the central, autonomic and peripheral nervous systems and the cardiovascular and respiratory systems. Allergic reactions are rare.
DRUG INTERACTIONS Other anti-arrhythmics, phenytoin and alcohol may potentiate the cardiovascular effects of lignocaine. The clearance of lignocaine may be reduced by drugs including β-blockers, cimetidine, erythromycin and intraconazole.
WARNINGS AND CONTRAINDICATIONS Reduced doses should be given to children, elderly patients and those with cardiac, neurological, liver or kidney disease; cardiovascular function should be monitored during IV administration. Lignocaine is contraindicated in patients with hypersensitivity to amide LAs, inflammation or sepsis at the site of injection, severe shock or hypotension, diseases of the CNS or supraventricular arrhythmias.
DOSAGE AND ADMINISTRATION For local anaesthesia, the lowest effective dosage should be used, depending on the area to be anaesthetised, technique to be used, vascularity of the tissues and patient factors. The typical maximum safe dose is quoted as 3 mg/kg (solution without adrenaline), or 7 mg/kg with adrenaline. When used as an anti-arrhythmic, plasma levels should be monitored, with not more than 300 mg infused during 1 hour.
Topical local anaesthesia may also be achieved by freezing, as low temperatures in living tissues produce diminished sensation; hence the use of ice-packs to relieve pain, as in the first-aid acronym RICE: Rest, Ice, Compression, Elevation. This form of anaesthesia is sometimes used for minor operative procedures. However, tissues that are frozen too intensely for too long can be destroyed. Ethyl chloride is a volatile liquid formerly used to produce this effect by evaporative cooling.
Local anaesthetic drugs
Chemistry and dissociation of LA drugs
Chemically, LA drugs are closely related compounds: they generally have at one end an aromatic (phenyl) group, joined through an intermediate chain of carbons to an amine (nitrogen-containing) group. The aromatic group helps make that end of the molecule lipid-soluble (lipophilic) and the amine group makes the other end water-soluble (hydrophilic). This property appears to be essential, as it allows the LA molecules to align and act within nerve cell membranes (which can be considered as phospholipid bilayers).
The intermediate carbon chains contain either an ester link (CO–O) or an amide link (CO–N), which has some important clinical implications. The ester-type LAs (cocaine, procaine, amethocaine and benzocaine) are metabolised rapidly by plasma esterase enzymes to p-aminobenzoic acid (PABA) metabolites, which are mainly responsible for allergic reactions in some patients; ester LAs are not often used now. The amide anaesthetics (such as lignocaine, prilocaine, bupivacaine and ropivacaine) are not metabolised to PABA derivatives, and allergic reactions induced by these anaesthetics are rare. Articaine is interesting in that it has both an amide link and is presented as a methyl ester derivative, hence is metabolised both in the plasma by esterases and in the liver.
As the LAs are all amines (except benzocaine), they can exist in solution as the uncharged amine form (analogous to ammonia, NH3) or as the charged quaternary amine form (like the ammonium ion, N+H4). The forms are in equilibrium, as shown in the dissociation reaction below:
The proportion of each form depends on the chemistry of the individual LA molecule and the pH of the solution or tissue it is in. Clinically this is important for the following reasons:
Mechanism of action
Local anaesthetics reversibly prevent the generation and conduction of impulses in excitable membranes and thus decrease sensitivity to pain. The basic mechanism of action of these drugs has been studied in detail: they enter the cell by diffusion through membranes, bind to a modulatory site in the voltage-dependent sodium channel (see Figure 11-4), block the sodium channel and interfere with the transient opening of these channels, thus preventing the transient inrush of sodium. Hence threshold potential is not reached, the cell membrane is not depolarised, the development of the action potential and its propagation are prevented and the nerve is blocked.
It is important to remember that all potentially excitable membranes are affected, so LAs have actions not only on sensory nerve cells but also on autonomic and motor nerves, muscle cells (cardiac, smooth, skeletal), secretory cells and neurons in the CNS.
The susceptibility of a nerve to LA action depends on the fibre diameter, myelination, tissue pH and length of nerve fibre exposed to LA solution. Local anaesthetics are capable of abolishing all sensation, but autonomic and sensory fibres are blocked preferentially because they are thinner, unmyelinated and more easily penetrated by these drugs. Loss of pain is followed in sequence by loss of response to temperature, proprioception (position of body parts), touch and pressure. Most motor fibres may also be anaesthetised if an adequate concentration of the drug is present over sufficient time.9
The LA drugs are said to be ion channel modulators or membrane stabilisers. Other drug groups with similar actions are the anti-arrhythmic agents and anticonvulsants (lignocaine is in fact used for these effects). Natural toxins, such as those of the puffer fish (tetrodotoxin), blue-ringed octopus (maculotoxin) and marine organisms (saxitoxin), also block nerve transmission, particularly in skeletal muscle, often causing fatal paralysis (see Figure 13-3).
Pharmacokinetics
The purpose of giving an LA is to localise its analgesic action in the tissue or nerve pathway into which it is injected. It is only later that the drug is absorbed from the tissues into the bloodstream and distributed around the body, where it may have effects in other systems and be metabolised and excreted (see Figure 14-9). This is in contrast to the more usual situation of an orally administered drug, which is absorbed from the gastrointestinal tract and passes through the liver before being distributed via the bloodstream to the tissues where it acts.
An injected LA will first undergo local dispersion (i.e. moving around) in the tissue. The onset of action is determined by the speed with which it diffuses into nerve cells, which depends largely on its lipid solubility, which depends in turn on the pH of the tissue and the degree of ionisation of the LA molecules (a function of the pKa [negative logarithm of ionisation constant] of the drug). Binding of the LA to tissue proteins and the presence of a vasoconstrictor in the solution help retain the drug in the tissues for longer action. Other potential factors include the volume and concentration of solution injected, speed of injection and local blood flow.
Local action is terminated by diffusion away, dilution and uptake into the vasculature (i.e. systemic absorption from the tissue). Lipid solubility is again the major determining factor and a vasoconstrictor, by decreasing blood flow in the area, will decrease the rate of absorption into the general circulation. Studies have shown that the peak plasma concentration of lignocaine (plain, i.e. without adrenaline) is reached about 20 minutes after injection; at this time systemic adverse effects are most likely to occur. During distribution around the body, effects may occur in other tissues, the drug may bind in tissues and it may be transferred across the blood–brain barrier or the placenta. Ester LAs (procaine and benzocaine) are rapidly metabolised by esterase enzymes in the plasma, red cells and liver and have few systemic adverse effects. Rapid metabolism of amide LAs occurs on first pass through the liver; this explains why LAs are inactive if taken orally (besides which, oral administration does not allow for localised actions except in the mouth). Inactive metabolites are excreted via the kidneys.
Overall, the onset and duration of action of an injected LA depends on all the above factors and on the patient’s cardiovascular and liver functions. The half-lives of LAs are generally short (1–2 hours); however, the bupivacaine type LAs have high lipid solubility and are more highly proteinbound, so have longer durations of action. The choice of LA for a particular procedure depends largely on the duration of drug action desired; Table 14-4 summarises the properties of several commonly used short-, intermediate- and long-acting LAs. Another, proxymetacine, is available in eye-drop formulations for use as an ocular local anaesthetic (see Chapter 31).
Clinical aspects
Indications and contraindications for use of local anaesthetics
Local anaesthetics are indicated for surgical procedures when the patient’s cooperation and consciousness are required or desired, for minor superficial and body surface procedures when general anaesthesia would be unnecessary or hazardous and for sympathetic blockade or postoperative analgesia.
Contraindications to the use of local anaesthesia include extensive surgery that would require potentially toxic doses, known allergy or hyper sensitivity to the LA agent, lack of cooperation from the patient and local inflammation, infection or ischaemia at the injection site. As usual, precautions may be required in paediatric, elderly or pregnant patients and in patients with liver disease.
Dosage
The lowest effective dose should be used, noting that maximum safe doses are only guides (see Clinical Interest Box 14-11). Because a dose that is safe when injected SC may be toxic if injected IV, the dose should be injected slowly, with frequent aspirations (applying suction to syringe) to avoid intravascular injection.
Clinical interest Box 14-11 Calculating the safe dose of a local anaesthetic
The strength of a solution of a local anaesthetic formulated for injection is usually expressed in % terms, e.g. lignocaine 2% w/v. This means that there are 2 g solid drug dissolved in 100 mL solution. It follows that each 1 mL contains 20 mg of drug. Adrenaline concentration is usually expressed differently, e.g. as 1 in 200,000. This means that 1 g is present in 200,000 mL solution, or 1 mg in 200 mL.
Doses of LAs depend on many factors, including the drug, type of regional anaesthesia intended, weight and state of the patient and whether a vasoconstrictor is present; doses quoted are guidelines.
The minimum dose that results in effective anaesthesia should be used. The average dose of an LA is usually quoted in mL solution, rather than mg/kg body weight; thus for a brachial plexus block in an average 70 kg adult, 20–40 mL of a 1% lignocaine solution is the maximum recommended.
The maximum safe dose of lignocaine plain (i.e. without adrenaline) given as a single dose is about 3 mg/kg. Thus for an average 70 kg adult, 210 mg will be the maximum dose, which is contained in 21 mL of 1% solution, available in 5 mL ampoules, so no more than 4.2 × 5 mL ampoules should be needed. (If calculations show that 42 or 420 ampoules are required, warning bells should ring and the calculations should be checked!)
As a vasoconstrictor localises the LA in tissues and prevents a rapid bolus of drug being absorbed into the bloodstream, larger doses can be used than are safe in the absence of the vasoconstrictor. Thus the maximum dose of lignocaine in the presence of adrenaline is 7 mg/kg.
Use of a vasoconstrictor
Most LAs produce vasodilation by direct action on blood vessels and by anaesthetising sympathetic vasoconstrictor fibres. This can cause rapid absorption of the drug into the systemic circulation; when the rate of absorption exceeds the rate of elimination, toxic effects can occur. Hence vasoconstrictors, such as adrenaline or felypressin, are sometimes formulated in with the LA solution to decrease systemic absorption and hence prolong the contact of the drug with local tissue, prolong the anaesthetic’s duration of action and reduce risk of systemic toxicity. Another example is the combination of phenylephrine, an α-adrenoceptor agonist, with lignocaine in a nasal spray for use in nasal/pharyngeal anaesthesia.
Vasoconstrictors are not used for nerve blocks in areas where there are end-arteries (fingers, toes, ears, nose or penis) because ischaemia may develop, resulting in gangrene. The dose of vasoconstrictor must be carefully determined to prevent ischaemic necrosis at the injection site. Other potential risks include cardiovascular stimulation (from stimulation of cardiac β1-adrenoceptors by adrenaline), so caution is required in patients with cardiovascular or thyroid disease or those taking antidepressants.
Some LAs do not require the use of a vasoconstrictor: cocaine itself has vasoconstrictor actions (due to its sympathomimetic effects) and bupivacaine and related drugs inherently have long contact times in tissues, allowing tissue binding and prolonging their actions.
Formulations of local anaesthetics
Interestingly, there are no formulations for oral administration and systemic absorption (although there are topical formulations for mouth and gastric ulcers). This is because LAs are rapidly metabolised in the circulation or first-pass effect and because it would be impossible to localise their effects once absorbed into the systemic circulation.
Parenterals
For infiltration and nerve block techniques, the LA must be formulated in an injectable form (i.e. as a parenteral solution). Such solutions must be sterile, particle-free, stable and preferably isotonic and buffered to the pH of body solutions. Particular cases are those of LA solutions with added vasoconstrictor (e.g. adrenaline, 1 in 200,000); and heavy solutions (for spinal anaesthesia) such as Marcain Spinal 0.5% Heavy Injection, a hyperbaric bupivacaine solution containing glucose at 80 mg/mL. Parenteral solutions of LA may also be formulated with an opioid analgesic, such as fentanyl or pethidine.
Topicals
For topical administration, almost every dose form known to pharmaceutical science has been used, including creams, jellies, paints, lotions, ointments, adhesive ointments, sprays, dressings, lozenges, eye/ear-drops, viscous solu - tions, emulsions and suppositories. Some interesting topical combination formulations are lignocaine with chlorhexidine in a gel, for use as a lubricant in urological procedures, lignocaine with benzalkonium chloride in a skin spray, for sunburn relief, and benzocaine and clove oil in a solution for application to a tooth cavity to relieve throbbing persistent toothache (Oral-Eze).
Adverse drug reactions and drug interactions
Adverse drug reactions
Because LAs are potentially toxic drugs, a patient’s age, weight, physical condition and liver function must be taken into account in determining drug dosage. Adverse reactions can occur very quickly, so patients must never be left alone. Most reactions to LAs result from overdosage, inadvertent IV administration, rapid absorption into systemic circulation or individual hypersensitivity or allergic response. The lowest effective dose should be used and IV injections must be avoided by aspirating gently and injecting slowly.
Procaine is considered the least toxic LA and cocaine the most toxic. In order of increasing toxicity, the common LAs are procaine, prilocaine, mepivacaine, lignocaine, etidocaine, bupivacaine, amethocaine, cinchocaine, cocaine. Levobupivacaine has fewer cardiovascular and CNS adverse effects than does bupivacaine.
Adverse reactions can be classified as:
Adverse reactions may require treatment. For minor reactions, conservative resuscitation and first-aid efforts are effective. For major reactions, oxygen, assisted ventilation and IV infusion of fluids and drugs to counteract convulsions, cardiovascular and respiratory depression may be necessary.
Significant drug interactions
Significant drug interactions and unexpected responses can occur (Drug Interactions 14-2), so close observation is needed.
Drug interactions 14–2 Local anaesthetics
| Drug or drug group | Likely effects and management |
| Drugs that depress CVS, including phenytoin | Additive CV depressant effects if LA is significantly absorbed; use LA cautiously and monitor effects |
| Antihypertensive agents | Enhanced hypotension with epidural or spinal LA |
| Drugs that predispose to methaemoglobinaemia (sulfonamides, primaquine, sodium nitroprusside) | Exacerbate methaemoglobinaemia from prilocaine; use cautiously |
| Drugs that cause arrhythmias or prolong QT interval | Effects exacerbated by LA; avoid combination |
| Ciprofloxacin, fluvoxamine | Increase concentration, duration of action and toxicity of ropivacaine; avoid prolonged administration of the LA |
| Cimetidine, erythromycin, fluvoxamine, itraconazole, metoprolol, propranolol | May inhibit metabolism of lignocaine and/or increase its concentration (depends on route of LA administration); monitor for effects and toxicity and decrease LA dose if necessary |
| Suxamethonium | Lignocaine IV may decrease plasma cholinesterase activity and prolong muscle relaxation; monitor respiratory function |
| Anticholinesterase drugs | May inhibit the metabolism of ester-type LAs (procaine) |
Anaesthetics containing a vasoconstrictor are used with caution in patients receiving drugs that alter blood pressure, such as antihypertensives, monoamine oxidase inhibitors and tricyclic antidepressants, as the combination may produce hypertension. Cardiac arrhythmias may occur when catecholamine vasoconstrictors (e.g. adrenaline) are used in patients receiving general anaesthesia with cyclopropane or halothane.
Reversal of local anaesthesia
Recovery of sensation after local anaesthesia (administered plus a vasoconstrictor) can be accelerated by administration of phentolamine. This is particularly important in dental practice, in order to reduce the number of soft tissue injuries in children from biting their lips or tongue, and to reduce difficulties eating, drinking and speaking. Phentolamine, an α-adrenoceptor antagonist (α-blocker), injected by infiltration into the same site as the LA overcomes the vasoconstriction, enhances the clearance of lignocaine from oral tissues and markedly reduces the time to recovery of full lip sensation (see Moore et al [2008]).
Techniques for local anaesthesia
There are several techniques by which LAs are administered (see Figures 14-10 and 14-11 and Table 14-5). They can be applied to an area or injected into tissues where they produce their effect in the immediate area only, hence the term ‘local anaesthesia’. They can also be injected around a nerve or nerve trunk (e.g. nerve block, spinal or epidural techniques) to produce anaesthesia in a large region of the body (regional anaesthesia).
Figure 14-10 The routes of administration of local anaesthetic drugs. Half of a cross-section of the spinal column is shown with a mixed spinal nerve composed of examples of the main types of efferent and afferent fibres. IA = group IA afferent axon from annulospiral ending of muscle spindle; II = group II afferent axon from flower spray ending of muscle spindle; α = α axon (lower motoneuron) to extrafusal muscle fibres; γ = γ-efferent axon to intrafusal fibres of muscle spindle. Note that intrathecal injection into the subarachnoid space (spinal anaesthesia) is made in the lumbar region below the termination of the spinal cord (not as shown here for convenience).
Source: Bowman & Rand 1980; used with permission.
Figure 14-11 Technique for Bier’s block: IV regional anaesthesia of the upper limb. A Cuff has been positioned, IV cannula inserted and limb exsanguinated by winding an Esmarch bandage proximally up the arm. B Cuff has been inflated and the bandage removed, allowing injection of local anaesthetic.
Topical or surface anaesthesia
The use of surface, or topical, anaesthesia is restricted to mucous membranes, damaged skin surfaces, wounds and burns. Topical local anaesthesia is used to relieve pain and itching; to anaesthetise mucous membranes of the eye, nose, throat or urethra for minor surgical procedures; to facilitate instrumentation; and before venepuncture or split skin grafting. Many dose forms are available, including eye- and ear-drops, solutions, ointments, gels, creams, sprays or powders.
Topical anaesthetics do not effectively penetrate unbroken skin, except for the combination cream EMLA (eutectic mixture for local anaesthesia), which contains 25 mg/g each of lignocaine and prilocaine. Cocaine in a 4%–10% solution is still used topically for nasal anaesthesia.
A number of local anaesthetic agents cannot be injected because they are too insoluble (benzocaine) or too toxic (cocaine, amethocaine). However, because they are only slowly absorbed, they can usually be used safely on open wounds, ulcers and mucous membranes. They occasionally cause dermatitis and allergic sensitisation, which necessitates their discontinuance. Absorption is increased from mucous membranes and broken skin (e.g. abrasions, trauma and ulcers), leading to the possibility of systemic effects; deaths have occurred from absorption via the urethra. When they are used in the oral cavity (mouth and pharynx), interference with swallowing may occur and aspiration is a risk. The patient is assessed for a returning gag reflex by gentle touching of the back of the pharynx with a tongue blade. All food and fluids are withheld until the reflex returns.
Infiltration anaesthesia
Infiltration anaesthesia is the use of LAs in a small area that circles the operative field; it is produced by injecting dilute solutions (0.1%) of the agent into the skin and then subcutaneously into the region to be anaesthetised. Adrenaline is often added to the solution as a vasoconstrictor to intensify the anaesthesia in a limited region and to prevent excessive bleeding and systemic effects. Repeated injection extends the anaesthesia as long as needed. The sensory nerve endings are anaesthetised, but not motor nerves. This method of administration is used for minor surgery such as for skin lesions, skin incision and drainage or excision of a cyst, and sometimes for more major procedures including dental extractions.
Intravenous regional anaesthesia (Bier’s block)
Intravenous regional anaesthesia (IVRA) is a specialised technique for anaesthesia of the upper limb; the technique is as follows (see Figure 14-11):
The tourniquet is kept tight during the operation (and for a minimum of 20 minutes) to reduce blood flow to and from the area, which localises the LA and facilitates tissue binding. It is proposed that the injected LA diffuses from the vein into adjacent arteries and thence into the tissues, causing rapid analgesia and muscle relaxation within 10–15 minutes; the whole limb distal to the cuff is anaesthetised, allowing major surgery. This technique is considered safer than a major nerve block of the upper limb. It is not used in children, who do not tolerate well the discomfort of the tourniquet, but is very useful in emergency situations, e.g. treatment of Colles’ fracture, and in developing countries.
In theory, Bier’s block can also be used for the lower limb, but the large muscle masses in the leg make the method unsatisfactory in practice.
Nerve (conduction) block anaesthesia
For a nerve block, the LA is injected into the vicinity of a nerve trunk and inhibits the conduction of impulses to and from the area supplied by that nerve, the region of the operative site. The injection can be made at some distance from the surgical site. A single nerve may be blocked, or the anaesthetic may be injected where several nerve trunks emerge from the spinal cord (paravertebral block). During peripheral nerve block, motor nerves are usually blocked as well as sensory pathways. A concentrated solution is required because of the thickness of nerve trunk fibres; overall less LA is needed than for infiltration technique. This method of anaesthesia is often used for foot and hand surgery, eye surgery, pudendal block for obstetric procedures and postoperative pain relief.
Central nerve block: epidural and spinal anaesthesia
Two specialised types of central nerve blocks are the epidural and spinal blocks, in which spinal roots are blocked where they emerge from the spinal canal. These techniques are used for abdominal, pelvic and lower limb surgery. Autonomic nerves may also be blocked, so there is a risk of autonomic adverse effects. To increase analgesia, an opioid such as morphine, pethidine or fentanyl is often also administered by these techniques. Central nerve block is contraindicated if there is systemic anticoagulation or coagulation abnormality or if there is raised intracranial pressure.
Epidural (extradural) anaesthesia
An epidural is an injection of LA into the space between the dura mater and the ligamentum flavum, at spinal cord levels C7–T10 (see Figure 14-10). The ‘space’ is actually filled with loose adipose tissue, lymphatics and blood vessels; the solution tends to remain localised at the level at which it is injected. It is commonly used for obstetrics, urology and thoracic, abdominal and perineal surgery. The solution does not contact the spinal cord or CSF, so there is less risk of CNS infection than with spinal injection. Postoperative urinary retention is common, due to blockade of parasympathetic nerves.
Caudal anaesthesia is an epidural procedure in which the anaesthetic solution is injected into the caudal canal, the sacral part of the vertebral canal containing the cauda equina or the bundle of spinal nerves that innervate the pelvic viscera. It is used in obstetrics and for pelvic or genital surgery.
Spinal (subarachnoid) anaesthesia
In spinal anaesthesia (also called subarachnoid, intra dural or intrathecal block), the LA is injected into the CSF in the subarachnoid space, below the level of termination of the spinal cord (i.e. at L3–4 or L4–5) and affects the lower part of the spinal cord and nerve roots. As the needle and solution come into contact with the CSF, sterility and aseptic procedures are required to prevent infections such as meningitis. The onset of anaesthesia usually occurs within 1–2 minutes of injection. The duration is 1–3 hours, depending on the anaesthetic used. Spinal anaesthesia is used for surgical procedures on the lower abdomen, inguinal area or lower extremities and is often the method of choice for elderly patients or those with severe respiratory problems or liver, kidney or metabolic disease, in whom general anaesthesia is contraindicated. The success and safety of spinal anaesthesia depend primarily on the anaesthetist’s skill and knowledge.
The LA can spread through the spinal canal, so the specific gravity of the LA solution and the patient’s position determine the level of anaesthesia. For example, for low spinal anaesthesia, the patient is placed in a flat or Fowler’s position (head and knees elevated) and a solution with a specific gravity greater than that of CSF is used, as it tends to diffuse downward.
Marked hypotension, decreased cardiac output and respiratory depression tend to occur because of depression of medullary centres and sympathetic pathways; they are considered disadvantages of this method. Hypotension may be treated by injection with sympathomimetic agents such as ephedrine or metaraminol. Postoperatively, headache is the most common complaint and may be accompanied by difficulty in hearing or seeing. Headache may be postural and occur only in the head-up or sitting or standing position. This symptom is thought to be the result of the opening in the dura made by the large spinal needle. The opening may persist for days or weeks, permitting loss of CSF and risk of infection (meningitis). Paraesthesias such as numbness and tingling may occur after spinal anaesthesia; they are usually limited to the lumbar or sacral areas and disappear within a relatively short time.
Key points
 The CNS is a complex system that monitors and regulates all body functions, allowing adaptation to changes in both the internal and external environments.
The CNS is a complex system that monitors and regulates all body functions, allowing adaptation to changes in both the internal and external environments. Essentially, the CNS integrates information it receives from the PNS and then transmits messages via the PNS to organs and tissues in the body to maintain homeostasis and control higher functions.
Essentially, the CNS integrates information it receives from the PNS and then transmits messages via the PNS to organs and tissues in the body to maintain homeostasis and control higher functions. The CNS is composed of the brain (cerebrum, cerebellum and brainstem) and the spinal cord. Component anatomical areas and functional systems have specific and interrelated neurophysiological effects.
The CNS is composed of the brain (cerebrum, cerebellum and brainstem) and the spinal cord. Component anatomical areas and functional systems have specific and interrelated neurophysiological effects. Four of the major CNS functional systems are the reticular formation, limbic system, extrapyramidal system and basal ganglia. These are responsible for many functions: consciousness and attention; personality, emotions and behaviour; learning, memory and decision making; sensory perception; and motor control, muscle tone and coordination. All of these systems may be affected by drugs.
Four of the major CNS functional systems are the reticular formation, limbic system, extrapyramidal system and basal ganglia. These are responsible for many functions: consciousness and attention; personality, emotions and behaviour; learning, memory and decision making; sensory perception; and motor control, muscle tone and coordination. All of these systems may be affected by drugs. The blood–brain barrier maintains the CNS in a highly stable chemical environment, allowing passage of required nutrients, transmitters and lipid-soluble substances while excluding large water-soluble and potentially toxic compounds. The barrier is less efficient in young infants and in conditions of focal damage to CNS tissue.
The blood–brain barrier maintains the CNS in a highly stable chemical environment, allowing passage of required nutrients, transmitters and lipid-soluble substances while excluding large water-soluble and potentially toxic compounds. The barrier is less efficient in young infants and in conditions of focal damage to CNS tissue. The resting electrical potential maintained across neuronal membranes is maintained by ionic pumps and ion channels. Action potentials generated in nerve cells are propagated along axons to nerve terminals, where a synapse intervenes between adjacent neurons.
The resting electrical potential maintained across neuronal membranes is maintained by ionic pumps and ion channels. Action potentials generated in nerve cells are propagated along axons to nerve terminals, where a synapse intervenes between adjacent neurons. Messages are transmitted across synapses by neurotransmitters—chemicals that are synthesised, stored, then released at nerve terminals to cross the synapse and initiate an action at receptors in the postsynaptic neurons to increase or decrease their activity.
Messages are transmitted across synapses by neurotransmitters—chemicals that are synthesised, stored, then released at nerve terminals to cross the synapse and initiate an action at receptors in the postsynaptic neurons to increase or decrease their activity. Of the many chemicals proposed as neurotransmitters in the CNS, the most important in the context of drug actions are acetylcholine, the catecholamines (dopamine, noradrenaline and adrenaline), 5-HT, some amino acids and neuroactive peptides.
Of the many chemicals proposed as neurotransmitters in the CNS, the most important in the context of drug actions are acetylcholine, the catecholamines (dopamine, noradrenaline and adrenaline), 5-HT, some amino acids and neuroactive peptides. In many clinically important neurological and psychiatric disorders, imbalances in levels of neurotransmitters have been proposed as aetiological or pathological factors.
In many clinically important neurological and psychiatric disorders, imbalances in levels of neurotransmitters have been proposed as aetiological or pathological factors. General anaesthesia is the loss of all sensations and consciousness; it can be achieved by inhalation or injection of rapidly acting, reversible CNS depressants.
General anaesthesia is the loss of all sensations and consciousness; it can be achieved by inhalation or injection of rapidly acting, reversible CNS depressants. General anaesthetics are lipid-soluble agents that act by modulation of transmitter-gated ion channels; the main mechanisms are enhancement of GABA-mediated CNS inhibition and inhibition of NMDA-mediated excitation.
General anaesthetics are lipid-soluble agents that act by modulation of transmitter-gated ion channels; the main mechanisms are enhancement of GABA-mediated CNS inhibition and inhibition of NMDA-mediated excitation. Speed of onset of and recovery from anaesthetic action depends on the solubility of the agent in lipids and in blood and on the person’s alveolar ventilation function.
Speed of onset of and recovery from anaesthetic action depends on the solubility of the agent in lipids and in blood and on the person’s alveolar ventilation function. Inhalation agents include the gas nitrous oxide and volatile agents such as halothane and sevoflurane; IV anaesthetics include propofol and thiopentone.
Inhalation agents include the gas nitrous oxide and volatile agents such as halothane and sevoflurane; IV anaesthetics include propofol and thiopentone. Balanced anaesthesia is the use of a combination of agents to achieve unconsciousness, analgesia, muscle relaxation and amnesia. Premedication may include an antianxiety agent (midazolam) and atropine to suppress secretions. Antiemetics and opioids are used for postoperative nausea and pain.
Balanced anaesthesia is the use of a combination of agents to achieve unconsciousness, analgesia, muscle relaxation and amnesia. Premedication may include an antianxiety agent (midazolam) and atropine to suppress secretions. Antiemetics and opioids are used for postoperative nausea and pain. Common adverse effects and interactions are the potentiation of CNS depression and the risk of malignant hyperthermia from suxamethonium plus a general anaesthetic.
Common adverse effects and interactions are the potentiation of CNS depression and the risk of malignant hyperthermia from suxamethonium plus a general anaesthetic. Local anaesthetics such as lignocaine inhibit action potential transmission in all excitable tissues, especially in sensory nerves, by blocking voltage-dependent sodium channels.
Local anaesthetics such as lignocaine inhibit action potential transmission in all excitable tissues, especially in sensory nerves, by blocking voltage-dependent sodium channels. The local anaesthetic drug acts locally in the tissue to which it is administered before being absorbed into the general circulation; ester-type agents are metabolised in the bloodstream and amides in the liver.
The local anaesthetic drug acts locally in the tissue to which it is administered before being absorbed into the general circulation; ester-type agents are metabolised in the bloodstream and amides in the liver. A vasoconstrictor agent may be added to the solution to localise and prolong the action of the drug and minimise systemic adverse effects; adrenaline is commonly used in the proportion of 1:200,000.
A vasoconstrictor agent may be added to the solution to localise and prolong the action of the drug and minimise systemic adverse effects; adrenaline is commonly used in the proportion of 1:200,000. Local anaesthesia is achieved by various techniques, including topical application or by subcutaneous infiltration of the selected operative area. Regional anaesthesia is the injecting of a local anaesthetic drug near a peripheral nerve trunk (nerve block) or around the spinal column to anaesthetise spinal nerve roots (epidural or subarachnoid techniques).
Local anaesthesia is achieved by various techniques, including topical application or by subcutaneous infiltration of the selected operative area. Regional anaesthesia is the injecting of a local anaesthetic drug near a peripheral nerve trunk (nerve block) or around the spinal column to anaesthetise spinal nerve roots (epidural or subarachnoid techniques).Review exercises
References and further reading
Analgesic Expert Group. Therapeutic Guidelines: Analgesic, version 5. Melbourne: Therapeutic Guidelines Limited; 2007.
Australian Medicines Handbook 2010. Adelaide: AMH, 2010.
Boron W.F., Boulpaep E.L. Medical Physiology: A Cellular and Molecular Approach, Updated edn. Philadephia: Elsevier Saunders; 2005.
Bowman W.C., Rand M.J. Textbook of Pharmacology, 2nd edn. Oxford: Blackwell; 1980. [chs 7, 16]
Calmes S.H. Arthur Guedel, MD, and the eye signs of anesthesia. American Society of Anesthesiologists Newsletter. 2002;66(9):17-19.
Caswell A., editor. MIMS Annual June 2005. Sydney: CMPMedica Australia, 2005.
Cooper J.R., Bloom F.E., Roth R.H. The Biochemical Basis of Neuropharmacology, 8th edn. New York: Oxford University Press; 2002.
Cousins M.J., Bridenbaugh P.O. Neural Blockade in Clinical Anaesthesia and Management of Pain, 3rd edn. Philadelphia: Lippincroft-Raven; 1998.
Dawkins R. The Ancestor’s Tale: A Pilgrimage to the Dawn of Life. London: Weidenfeld & Nicolson; 2004.
Emmanouil D.E., Quock R.M. Advances in understanding the actions of nitrous oxide. Anesthesia Progress. 2007;54(1):9-18.
Fischer H.B.J., Pinnock C.A. Fundamentals of Regional Anaesthesia. Cambridge: Cambridge University Press; 2004.
Franks N.P. General anaesthesia: from molecular targets to neuronal pathways of sleep and arousal. Nature Reviews Neuroscience. 2008;9:370-386.
Ge S., Song L., Pachter J.S. Where is the blood–brain barrier—really? Journal of Neuroscience Research. 2005;79(4):421-427.
Moore P.A., Hersh E.V., Papas A.S., et al. Pharmacokinetics of lidocaine with epinephrine following local anesthesia reversal with phentolamine mesylate. Anesthesia Progress. 2008;55:40-48.
Oberoi G., Phillips G. Anaesthesia and Emergency Situations: A Management Guide. Sydney: McGraw-Hill; 2000.
Padley A.P. Westmead Pocket Anaesthetic Manual, 2nd edn. Sydney: McGraw-Hill; 2004.
Pardridge W.M. The blood–brain barrier: bottleneck in brain drug development. NeurRx. 2005;2(1):3-14.
Phillips G.D. Defining moments in medicine: anaesthesia. Medical Journal of Australia. 2001;174:17-18.
Rudolph U., Antkowiak B. Molecular and neuronal substrates for general anaesthetics. Nature Reviews Neuroscience. 2004;5:709-720.
Schug S., Dodd P. Perioperative analgesia. Australian Prescriber. 2004;27(6):152-154.
Speight T.M., Holford N.H.G., editors. Avery’s Drug Treatment, 4th edn., Auckland: Adis, 1997.
Stahl S.M. Essential Psychopharmacology: Neuroscientific Basis and Practical Applications, 2nd edn. Cambridge: Cambridge University Press; 2000. [chs 1-4]
Stowell K.M. Malignant hyperthermia: a pharmacogenetic disorder. Pharmacogenomics. 2008;9(11):1657-1672. Nov
New Zealand medicines and medical devices safety authority: www.medsafe.govt.nz (medicine data sheets accessed 21 January 2009).
Waste anaesthetic gases: www.osha.gov/SLTC/wasteanestheticgases/index.html [21 January 2009].
More weblinks at: http://evolve.elsevier.com/AU/Bryant/pharmacology