Chapter 27 Drugs affecting the haemopoietic system
The haemopoietic system comprises principally blood and bone marrow and the accessory organs: the liver, spleen and kidneys. Blood is the common element that serves every system in the body. It is a multipurpose medium that not only delivers oxygen and nutrients to tissues, removes waste products from cells, regulates pH, adjusts body temperature and affords protection against disease. It is crucial for the maintenance of homeostasis—perturbations of the haemopoietic system may manifest in illnesses such as anaemia, haemophilia, thromboembolic disease (Chapter 26) and leukaemia (Chapter 42). Numerous drugs are available that act on the blood, including those that treat anaemia. Equally the cancer chemotherapeutic agents depress bone marrow function as an adverse effect. This chapter reviews the haematinic agents and erythropoietin used in the treatment of anaemia and the haemopoietic colony-stimulating factors.
Key abbreviations
Key background
THE haemopoietic system comprises primarily the blood and bone marrow, complemented by the liver (storage of vitamin B12 for erythrocyte production), spleen (removal of expired blood cells and storage of platelets) and kidneys (erythropoietin production). Blood is the major transport system in the body carrying drugs and nutrients absorbed from the gastrointestinal (GI) tract, oxygen from the lungs and hormones, electrolytes etc to cells throughout the entire body. In addition, it transports metabolic products from cells to the liver, kidneys and lungs for excretion. Blood further helps to regulate body temperature in concert with changes in the vascular system that varies blood flow through the skin. Buffering is also an important function of blood; the pH of human blood ranges from 7.35 to 7.45. In addition to its transportation and regulatory roles, blood is integral to the process of coagulation (Chapter 26) and aids in immunity by producing antibodies (Chapter 47).
Blood composition
Blood is composed of two components, cells and plasma—the fluid portion in which the cells are suspended. Although blood volume can vary among individuals and between males and females, the blood volume of an average-sized adult is approximately 5 L, of which about 3 L is usually plasma and the remainder is primarily red blood cells (RBCs). Together, these two components are responsible overall for the viscosity of blood, which is greater than that of water and thus blood tends to flow more slowly than water. Changes in various physiological and pathological states may affect blood viscosity; for example, an increase in RBCs (polycythaemia) increases viscosity. A common laboratory test used is the haematocrit (the proportion of packed erythrocytes in blood after centrifugation). The normal range of haematocrit for adult men is 0.4–0.54 and for adult women 0.37–0.47. The higher the haematocrit, the greater the blood viscosity; for example, a person with polycythaemia may have a haematocrit of 0.6 or 0.7, while a person with anaemia may have a significant drop in haematocrit.
Blood is composed principally of three types of blood cells: red blood cells (RBCs), or erythrocytes, which transport oxygen and carbon dioxide; white blood cells (WBCs), or leucocytes, which defend the body against bacteria and infections; platelets, or thrombocytes, which are necessary for blood coagulation. Plasma, a strawcoloured fluid easily seen after centrifuging blood, is about 92% water and 8% plasma proteins (e.g. albumin, globulins and fibrinogen). Plasma proteins (e.g. albumin, α1 acid glycoprotein, fatty acid binding proteins) play an important role in maintaining the osmotic pressure of blood, which is important for fluid exchange through capillary walls. Albumin is important for the transport of some steroid hormones and in the binding of numerous drugs (refer to Chapter 6). Fatty acid binding proteins transport fatty acids and bind some drugs. The globulins include the immunoglobulins, also called antibodies, which are important in the body’s defence against viruses and bacteria. Fibrinogen is essential for blood clotting and is converted to fibrin by thrombin in the presence of calcium ions. In addition to the proteins, plasma may contain thousands of other substances, such as glucose, electrolytes, vitamins, hormones and products of metabolism.
Blood cell formation
Haemopoiesis, or blood cell production, occurs within certain parts of bone, principally the red bone marrow. During fetal development, many tissues (e.g. liver, spleen and thymus gland) participate in blood cell production but, after birth, haemopoiesis occurs only in the red bone marrow and, after 20 years of age, principally in the bone marrow of the vertebrae, sternum, ribs and ilia. Differentiation and proliferation of precursor cells into the various types of blood cells is regulated by haemopoietic growth factors such as erythropoietin (EPO), thrombopoietin and the cytokines, which include colony-stimulating factors (CSFs) and interleukins (see Chapter 47).
Secretion by the kidneys of EPO, a hormone that regulates production of RBCs by the bone marrow, is stimulated by hypoxia and/or blood loss. With maximal bone marrow stimulation, RBC production can be increased nearly seven times over the normal rate. Thrombopoietin, another hormone, is produced by the liver and substantially increases platelet production, while CSFs and interleukins stimulate formation of leucocytes. A simplified diagram of blood cell differentiation is shown in Figure 27-1.
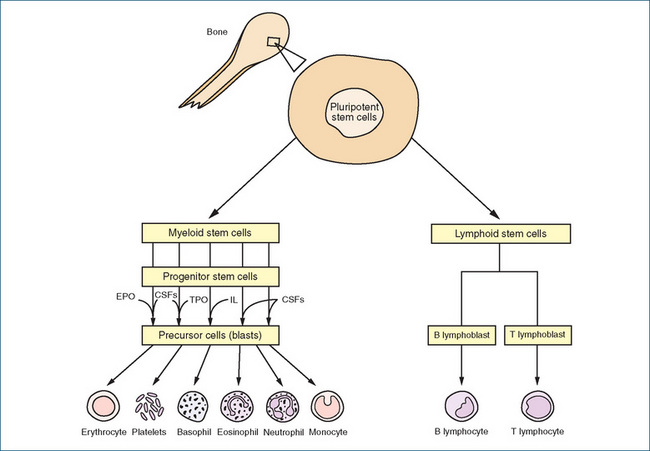
Figure 27-1 A simplified diagram of blood cell production (haemopoiesis) and the involvement of growth factors. Cells originating from myeloid stem cells are produced in the bone marrow. Lymphoid stem cells arise in bone marrow but development of lymphocytes is completed in lymphatic tissue. CSFs = colony-stimulating factors; EPO = erythropoietin; IL = interleukin; TPO = thrombopoietin.
Red blood cells (erythrocytes)
RBCs, or erythrocytes, are small, non-nucleated, biconcave disc-shaped cells present in large quantities in the bloodstream. Without a nucleus, RBCs have negligible synthetic capacity and hence their lifespan is short, about 120 days. Expired cells are removed from the circulation and destroyed by phagocytes resident in the liver and spleen. It has been estimated that more than 100 million RBCs are produced every minute during adulthood. The normal healthy adult has 4.0–6.0 × 1012 cells/L of blood. The body balances production (erythropoiesis) and destruction of these cells to maintain a relatively constant level of RBCs.
Within the cytosol of RBCs are haemoglobin molecules, the major function of which is the transport of oxygen. Each haemoglobin molecule consists of a protein called globin, composed of four polypeptide chains, four non-protein haem pigment molecules and four iron atoms. Each polypeptide chain is associated with one haem and one iron ion (Fe2+), thus one haemoglobin molecule combines in total with four oxygen molecules. Oxygen is transported from the lungs to the tissues where it is released from the haemoglobin and diffuses through the interstitial fluid into the cells. Haemoglobin can also combine with carbon dioxide that is carried from the cells to the lungs for excretion. More recently, haemoglobin has been reported to be involved in blood pressure regulation by transporting the vasodilator nitric oxide (NO), produced by endothelial cells that line blood vessels. Males tend to have more haemoglobin (range 130–180 g/L) in their blood than females (115–165 g/L).
White blood cells (leucocytes)
There are five types of nucleated leucocytes found in the blood. They are produced primarily in the bone marrow and are classified according to the presence or absence of granules in the cell cytoplasm (see Figure 27-1). There are three types of granular leucocytes: neutrophils, eosinophils and basophils; aged cells that have different-shaped nuclear lobes and an increased number of nuclei (>2, >3 or >5) are referred to as polymorphonuclear leucocytes, or polymorphs. The other two types of leucocytes are lymphocytes and monocytes, which are produced mainly in lymph tissues and organs such as the spleen, thymus, tonsils and various other lymphoid tissues in the bone marrow, GI tract and elsewhere. Blood of a healthy person usually contains 4.0–11.0 × 109 leucocytes/L. As each type of leucocyte plays a specific role, a differential WBC count may be used to detect infections or inflammatory conditions; for example, in acute appendicitis, the percentage of neutrophils increases, as does the total leucocyte count. Leucopenia refers to an abnormally low number of leucocytes; leucocytosis refers to an increase in WBCs (e.g. in response to infection).
Neutrophils, basophils, monocytes and lymphocytes are very mobile; they leave the capillaries and migrate to sites of infection. The neutrophils and monocytes ingest and destroy the pathogens, a process known as phagocytosis, while the lymphocytes defend the body against bacteria, fungi and viruses (see Chapter 47 for an overview of the immune system). In contrast, eosinophils play a dominant role during allergic reactions and parasitic infections.
The lifespan of granulocytes is estimated to be 4–8 hours in the bloodstream and 3–5 days in body tissues. If involved in phagocytosis of pathogens, this lifespan can be reduced to a few hours because they can also be destroyed. Monocytes also have a short lifespan in the blood, but in body tissues they can increase in size and differentiate to become tissue macrophages (the major phagocytic cells of the immune system that ingest foreign antigens and cell debris), providing a first line of defence against tissue infections. The agranular T and B lymphocytes may live for several years.
Platelets
Unlike RBCs and WBCs, platelets, or thrombocytes, are small, disc-shaped, non-nucleated colourless cell fragments that split off from the megakaryocytes produced by the bone marrow (see Figure 27-1). They have a short lifespan of about 5–8 days. Time-expired platelets are engulfed by resident macrophages in the spleen and liver. Platelets are essential for coagulation (see Chapter 26). The normal platelet concentration is 150–400 × 109/L. People with a low concentration of platelets have thrombocytopenia. Such people tend to bleed and their skin usually displays small purple spots (hence the name thrombocytopenia purpura). Thrombocytopenia is often induced by irradiation injury to the bone marrow, or results from aplasia of the bone marrow induced by specific drugs.
Haematinics
A condition in which there is a reduced oxygen-carrying capacity of the blood (due to reduced amount or functionality of haemoglobin) is referred to as anaemia and often manifests as fatigue. Different types of anaemia exist and are classified on the basis of size and number of functional RBCs and haemoglobin concentration. A combination of measuring serum ferritin, iron, vitamin B12 and folic acid, microscopic examination of a blood smear and a bone marrow smear allows a precise diagnosis of the type of anaemia.
A blood smear provides evidence of:
A bone marrow smear increases diagnostic precision by providing evidence of:
Agents used to treat anaemias include the haematinics: iron, folic acid and vitamin B12 and the erythropoietin agonists.
Iron deficiency anaemia
Iron deficiency anaemia is characterised by small RBCs with reduced haemoglobin. Before commencing therapy, other causes of iron deficiency should be excluded. These include, but are not limited to, blood loss (e.g. chronic NSAID use and GI ulceration), blood donation, pregnancy and lactation (increased iron requirement), malabsorption (e.g. after gastric surgery), inadequate diet (e.g. due to socioeconomic status, low consumption of meat or a vegetarian lifestyle) and previous history of iron deficiency. Iron deficiency not only causes anaemia: iron is also an essential component of myoglobin, enzymes with a haem moiety (e.g. cytochromes and peroxidases) and metalloflavoproteins such as xanthine oxidase, which is involved in purine metabolism (refer to section on hyperuricaemia and gout, Chapter 47).
Iron
Iron is a transition metal and the majority of iron (∼65%) in the human body circulates as haemoglobin, which contains four haem moieties, each having one iron atom to which one oxygen molecule binds reversibly. In general, iron is obtained through a meat-containing diet, creating a problem for cultures reliant on grain as a major food source. Iron is absorbed from the duodenum and upper jejunum and carried in plasma bound to transferrin. Iron that leaves the plasma is used for synthesis of haemoglobin by red cell precursors that bind the transferrin molecules, releasing them after the uptake of the iron. Iron as ferritin is stored in all cells and, on average, plasma contains approximately 4 mg iron; the daily turnover is ∼30 mg. The majority of the iron is stored in erythrocytes, with the next highest concentrations occurring in liver and bone marrow (stored as ferritin and haemosiderin) and in muscle, with small amounts in the spleen and bound in enzymes. Haemosiderin is a degraded form of ferritin. Iron concentration is tightly controlled by the absorptive process as the body has virtually no mechanism for excreting iron.
Once iron deficiency anaemia has been diagnosed, iron is administered orally but can also be given parenterally if required. Iron dosage is expressed in terms of elemental iron:
Iron is also available in combination with folic acid for the prevention and treatment of iron and folate deficiency during pregnancy. To prevent an excessive intake of iron, the oral and parenteral formulations of iron should not be used together. In addition, although a rare occurrence there is a risk of an anaphylactoid reaction with parenteral iron preparations. Doses of iron sucrose appear to be well tolerated and provide improved haemoglobin concentration in nondialysis-dependent patients with iron deficiency anaemia (Wall & Pauly 2008).
Iron causes most commonly GI disturbances (e.g. abdominal pain, nausea, vomiting, diarrhoea and blackcoloured faeces). As acute iron toxicity can be serious or even fatal in small children, iron formulations should be kept well out of reach and preferably locked away. In cases of iron overdose, the iron chelator desferrioxamine is administered. This forms water-soluble complexes with the iron, which are then excreted in urine.
Folic acid and vitamin B12
In general, folic acid deficiency occurs through poor diet, while vitamin B12 deficiency arises from absorptive problems in the terminal ileum (e.g. in Crohn’s disease). Folic acid and vitamin B12 are both obtained through the diet and both are interrelated in the synthesis of DNA. Folic acid is essential for DNA synthesis, and dietary folic acid is reduced to tetrahydrofolate (FH4). Vitamin B12 is required for conversion of methyl-FH4 to FH4, hence a deficiency of either results in defective DNA synthesis.
Folic acid is used to treat folate-deficiency anaemia, to prevent neural tube defects in the growing fetus and to treat or prevent toxicity from methotrexate (Chapter 42). Always exclude vitamin B12 deficiency before prescribing folic acid to treat megaloblastic anaemia. In addition, check medications, as some drugs (e.g. antiepileptics and dihydrofolate reductase inhibitors such as methotrexate [Chapter 42] and trimethoprim [Chapter 44]) cause folic acid deficiency. Except in special circumstances, folic acid is administered orally. Adverse reactions with folic acid are rare.
Vitamin B12 (available as hydroxocobalamin and cyanocobalamin) is used principally to treat pernicious anaemia and optic neuropathies (the vitamin is essential to nerve development). Confirm diagnosis before use, as vitamin B12 may mask the clinical signs of folic acid deficiency. Hydroxocobalamin is administered IM while cyanocobalamin is available both as an oral and an injectable (IM) formulation. In cases of malabsorption syndrome, oral formulations are inappropriate and vitamin B12 injections will be required. As with folic acid, adverse reactions are rare.
Haemopoietics
The continuous replacement of RBCs is called haemopoiesis and is regulated by growth factors such as erythropoietin (EPO), thrombopoietin and the cytokines, which include colony-stimulating factors (CSFs). EPO is not the sole haemopoietic growth factor but it is important, and in its absence severe anaemia is invariably observed. EPO stimulates the division and differentiation of erythroid progenitors in the bone marrow. When the haemoglobin level decreases or tissue oxygenation is low plasma EPO concentration rises and within 3–4 days circulating RBCs begin to rise. It is produced in the kidney, and production is impaired in chronic renal failure, giving rise to anaemia in that specific condition.
Erythropoietin agonists
Recombinant human erythropoietins (epoetin alpha and epoetin beta) are almost identical to the human hormone, while darbepoetin alpha, also a recombinant hormone, is a slightly larger version of EPO (due to glycosylation) but nevertheless has the same actions. Epoetin alpha and darbepoetin alpha act specifically through the erythropoietin receptor on the surface of erythroid progenitor cells, stimulating erythropoiesis, increasing reticulocyte count and increasing haematocrit and haemoglobin concentration. The EPO receptor is also expressed on mast cells and in gastric mucosa and brain neurons. It is thought to inhibit cell death in neurons following cerebral ischaemia and to exert an antioxidant effect (Henry et al 2004).
The duration of action of darbepoetin alpha is longer, allowing once-weekly dosing, while epoetin alpha is administered three times weekly. These drugs are used to treat anaemia associated with chronic renal failure; surgery with expected blood loss; in cancer chemotherapy; and to stimulate RBC production prior to autologous blood collection in patients with anaemia who are undergoing elective surgery.
Adverse reactions are common and include hypertension (due to a rapid rise in haemoglobin), flu-like symptoms (e.g. headache, bone pain, myalgia and fever) and rash, peripheral oedema, dyspnoea and GI disturbances (e.g. nausea, vomiting and diarrhoea). Administered SC/IV pain at the injection site is more common with darbepoetin alpha, while the development of epoetin antibodies (which may limit usefulness) has been reported.
Colony-stimulating factors
Colony-stimulating factors (CSFs) are cytokines that regulate cell proliferation, differentiation and growth through an action on progenitor cells. Currently available CSFs include ancestim, a recombinant stem cell factor that is produced by bone marrow fibroblasts, and the granulocyte colony-stimulating factors (G-CSFs) filgrastim and pegfilgrastim and lenograstim. G-CSF is primarily regarded as a haematopoietic growth factor of the granulocyte lineage controlling the development of neutrophils. The G-CSFs are indicated for the treatment of chemotherapy/drug-induced neutropenia, severe chronic neutropenia and autologous and allogenic bone marrow transplantation. The combination of ancestim and G-CSF is indicated for stem cell mobilisation in patients at risk of a poor stem cell mobilisation response. The G-CSFs shorten the period of severe neutropenia after high-dose chemotherapy and improve outcomes in terms of reduced hospitalisation rates for opportunistic bacterial and fungal infections and decreased frequency of interruptions to chemotherapy protocols through hospitalisation for febrile neutropenia. Rare adverse effects include cardiovascular toxicity, pulmonary oedema, pericardial effusion, splenic rupture and toxic epidermal necrolysis. Of serious concern is the suggestion of a link with secondary malignancies although a causal relationship has not been established.
Ancestim
Ancestim is a recombinant nonglycosylated human stem cell factor produced in culture using Escherichia coli. As monotherapy ancestim has little colony-stimulating activity but in combination it acts synergistically to increase G-CSF, granulocyte macrophage-CSF, EPO, megakaryocyte growth and development factor and interleukin-2. In general it stimulates the differentiation of primitive progenitor cells that can mature into blood cell types including neutrophils and mast cells. The latter is of concern because of the possibility of mast cell activation in patients with a history of severe allergic reactions or asthma. Premedication with histamine receptor antagonists (H1 and H2) and inhaled salbutamol is recommended to reduce the risk of allergic symptoms. Exercise extreme caution and monitor carefully. Monotherapy is not recommended and ancestim is administered SC only in combination with G-CSF.
G-CSF
Filgrastim is a recombinant nonglycosylated form of human G-CSF produced in culture using Escherichia coli while pegfilgrastim is filgrastim complexed with a polyethylene glycol moiety at the N-terminus of the recombinant protein. Pegylation of filgrastim reduces the clearance by glomerular filtration, increases the half-life thus prolonging the action and allowing less frequent dosing. Lenograstim is a recombinant glycosylated form of human G-CSF produced using Chinese hamster ovary (CHO) cells. Filgrastim and pegfilgrastim are administered SC/IV while lenograstim is administered SC. Specialist protocols should be consulted for information on dos age, route of administration and precautions prior to administration. Adverse effects are common and include headache, bone pain, fever, injection site reactions and splenomegaly.
Key points
 The haemopoietic system comprises primarily the blood and bone marrow, complemented by the liver, which stores vitamin B12 for erythrocyte production, the spleen, which removes expired blood cells and stores platelets, and the kidneys, which produce erythropoietin.
The haemopoietic system comprises primarily the blood and bone marrow, complemented by the liver, which stores vitamin B12 for erythrocyte production, the spleen, which removes expired blood cells and stores platelets, and the kidneys, which produce erythropoietin. Blood is the major transport system in the body carrying drugs and nutrients absorbed from the gastrointestinal tract, oxygen from the lungs and hormones and electrolytes etc to cells throughout the entire body.
Blood is the major transport system in the body carrying drugs and nutrients absorbed from the gastrointestinal tract, oxygen from the lungs and hormones and electrolytes etc to cells throughout the entire body. Blood is composed principally of plasma and three types of blood cells: red blood cells, or erythrocytes, which transport oxygen and carbon dioxide; white blood cells, or leucocytes, which defend the body against bacteria and infections; platelets, or thrombocytes, which are necessary for blood coagulation.
Blood is composed principally of plasma and three types of blood cells: red blood cells, or erythrocytes, which transport oxygen and carbon dioxide; white blood cells, or leucocytes, which defend the body against bacteria and infections; platelets, or thrombocytes, which are necessary for blood coagulation. Different types of anaemia exist and are classified on the basis of size and number of functional red blood cells and haemoglobin concentration.
Different types of anaemia exist and are classified on the basis of size and number of functional red blood cells and haemoglobin concentration. Iron concentration is tightly controlled by the absorptive process as the body has virtually no mechanism for excreting iron.
Iron concentration is tightly controlled by the absorptive process as the body has virtually no mechanism for excreting iron. Agents commonly used to treat anaemias include the haematinics (folic acid, iron and vitamin B12) and the erythropoetin agonists.
Agents commonly used to treat anaemias include the haematinics (folic acid, iron and vitamin B12) and the erythropoetin agonists. Haemopoiesis, or blood cell production, occurs within certain parts of bone, principally the bone marrow.
Haemopoiesis, or blood cell production, occurs within certain parts of bone, principally the bone marrow. Differentiation and proliferation of precursor cells into the various types of blood cells is regulated by haemopoietic growth factors such as erythropoietin (EPO), thrombopoietin and the cytokines, which include colonystimulating factors (CSFs) and interleukins.
Differentiation and proliferation of precursor cells into the various types of blood cells is regulated by haemopoietic growth factors such as erythropoietin (EPO), thrombopoietin and the cytokines, which include colonystimulating factors (CSFs) and interleukins. Erythropoetin agonists include recombinant human erythropoietin (epoetin alpha and epoetin beta), which are almost identical to the human hormone, while darbepoetin alpha, also a recombinant hormone, is a slightly larger version of EPO but nevertheless has the same actions.
Erythropoetin agonists include recombinant human erythropoietin (epoetin alpha and epoetin beta), which are almost identical to the human hormone, while darbepoetin alpha, also a recombinant hormone, is a slightly larger version of EPO but nevertheless has the same actions. Colony-stimulating factors (CSFs) are cytokines that regulate cell proliferation, differentiation and growth through an action on progenitor cells.
Colony-stimulating factors (CSFs) are cytokines that regulate cell proliferation, differentiation and growth through an action on progenitor cells.Review exercises
Australian Medicines Handbook 2010. Adelaide: AMH, 2010.
Hartung T., von Aulock S., Schneider C., et al. How to leverage an endogenous immune defense mechanism: the example of granulocyte colony-stimulating factor. Critical Care Medicine. 2003;31(Suppl):S65-S75.
Henry D.H., Bowers P., Romano M.T., et al. Epoetin alfa. Archives of Internal Medicine. 2004;164:262-276.
Kaushansky K., Kipps T.J. Hematopoietic agents growth factors and minerals and vitamins. In Brunton L.L., Lazo J.S., Parker K.L., editors: Goodman & Gilman’s The Pharmacological Basis of Therapeutics, 11th edn, New York: McGraw-Hill, 2006. [ch 53]
Rang H.P., Dale M.M., Ritter J.M., Flower R.J. Pharmacology, 6th edn. Edinburgh: Churchill Livingstone; 2007. [ch 22]
Wall G., Pauly R.A. Evaluation of total-dose iron sucrose infusions in patients with iron deficiency anemia. American Journal of Health-System Pharmacy. 2008;65:150-153.


