Chapter 25 NURSING ASSESSMENT: respiratory system
1. Describe and differentiate the structures and functions of the upper respiratory tract, the lower respiratory tract and the chest wall.
2. Describe the process that initiates and controls inspiration and expiration.
3. Describe the process of gas diffusion within the lungs.
4. Outline the respiratory defence mechanisms.
5. Outline the significance of arterial blood gas values and the oxygen–haemoglobin dissociation curve in relation to respiratory function.
6. Identify the signs and symptoms of inadequate oxygenation and the implications of these findings.
7. Describe age-related changes in the respiratory system and relate them to differences in assessment findings.
8. Discuss the significant subjective and objective data related to the respiratory system that should be obtained from a patient.
9. Select appropriate techniques to use in a physical assessment of the respiratory system.
10. Differentiate normal from common abnormal findings in a physical assessment of the respiratory system.
11. Describe the purpose, significance of results and nursing responsibilities related to diagnostic studies of the respiratory system.
Structures and functions of the respiratory system
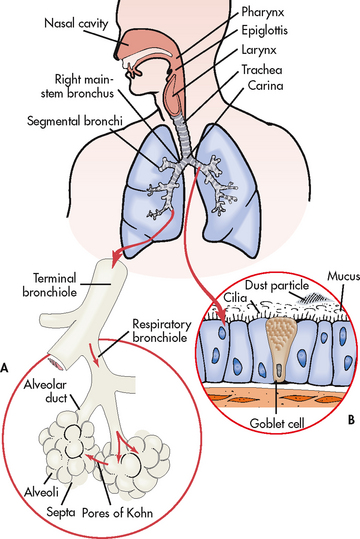
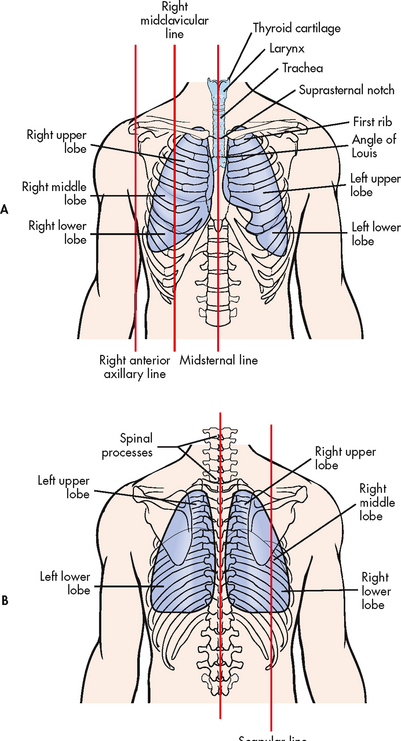
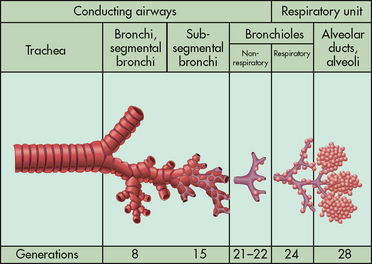
The primary purpose of the respiratory system is gas exchange, which involves the transfer of oxygen and carbon dioxide between the atmosphere and the blood. The respiratory system is divided into two parts: the upper respiratory tract and the lower respiratory tract (see Fig 25-1). The upper respiratory tract includes the nose, pharynx, adenoids, tonsils, epiglottis, larynx and trachea. The lower respiratory tract consists of the bronchi, bronchioles, alveolar ducts and alveoli. With the exception of the right and left main-stem bronchi, all lower airway structures are contained within the lungs. The right lung is divided into three lobes (upper, middle and lower) and the left lung into two lobes (upper and lower) (see Fig 25-2). The structures of the chest wall (ribs, pleura, muscles of respiration) are also important for respiration.
UPPER RESPIRATORY TRACT
Air enters the respiratory tract through the nose, which is made of bone and cartilage and is divided into two nares by the nasal septum. The inside of the nose is shaped into rolling projections called turbinates that increase the surface area of the nasal mucosa for warming and moistening air. The internal nose opens directly into the sinuses. The nasal cavity connects with the pharynx, a tubular passageway that is subdivided into three parts: the nasopharynx, the oropharynx and the laryngopharynx.
Breathing through the narrow nasal passages (rather than mouth breathing) provides protection for the lower airway. The nose is lined with mucous membranes and small hairs. Air entering the nose is warmed to near body temperature, humidified to nearly 100% water saturation and filtered of particles larger than 10 μm (e.g. dust, bacteria).
The olfactory nerve endings (receptors for the sense of smell) are located in the roof of the nose. The adenoids are found in the nasopharynx and the tonsils in the oropharynx; both of these are small masses of lymphatic tissue.
After passing through the oropharynx, air moves through the laryngopharynx, then the epiglottis and the larynx, where the vocal cords are located, and down into the trachea. The epiglottis is a small flap of tissue at the base of the tongue. During swallowing, the epiglottis covers the larynx, preventing solids and liquids from entering the lungs.
The trachea is a cylindrical tube about 10–12 cm long and 1.5–2.5 cm in diameter. The support of U-shaped cartilages keeps the trachea open but allows the adjacent oesophagus to expand for swallowing. The trachea bifurcates into the right and left main-stem bronchi at a point called the carina. The carina is located at the level of the manubriosternal junction, also called the angle of Louis. The carina is highly sensitive and touching it during suctioning causes vigorous coughing.1
LOWER RESPIRATORY TRACT
Once air passes the carina, it is in the lower respiratory tract. The main-stem bronchi, pulmonary vessels and nerves enter the lungs through a slit called the hilus. The right main-stem bronchus is shorter, wider and straighter than the left main-stem bronchus. For this reason, aspiration is more likely in the right lung than in the left lung.
The main-stem bronchi subdivide several times to form the lobar, segmental and subsegmental bronchi. Further divisions form the bronchioles. The most distant bronchioles are called the respiratory bronchioles. Beyond these lie the alveolar ducts and alveolar sacs (see Fig 25-3). The bronchioles are encircled by smooth muscles that constrict and dilate in response to various stimuli. The terms bronchoconstriction and bronchodilation are used to refer to a decrease or increase in the diameter of the airways caused by contraction or relaxation of these muscles.1
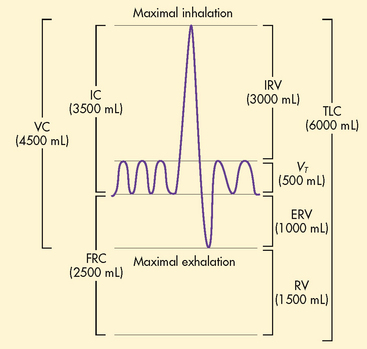
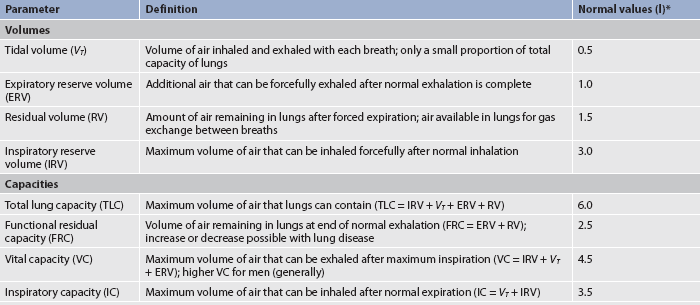
Oxygen and carbon dioxide exchange takes place in the respiratory bronchioles. The area of the respiratory tract from the nose to the respiratory bronchioles serves only as a conducting pathway and is termed the anatomical dead space (VD). The air filling this space with every breath is not available for gas exchange. In adults, a normal tidal volume (VT), or volume of air exchanged with each breath, is about 500 mL. Of each 500 mL inhaled, about 150 mL is VD.
After moving through the anatomical dead space, air reaches the respiratory bronchioles and alveoli (see Fig 25-4). Alveoli are small sacs that are the primary site of gas exchange in the lungs. The alveoli are interconnected by the pores of Kohn, which allow movement of air from alveolus to alveolus (see Fig 25-1). Bacteria can also move through these pores, resulting in an extension of respiratory tract infection to previously non-infected areas. The 300 million alveoli in the adult have a total volume of about 2500 mL and a surface area for gas exchange that is about the size of a tennis court. The alveolar–capillary membrane (see Fig 25-5) is very thin (<5 μm) and is the site of gas exchange. In conditions such as pulmonary oedema, excess fluid fills the interstitial space and alveoli, markedly impairing gas exchange.1,2 This may be due to disruption of the epithelial barrier formation, which can lead to increased permeability and subsequent fluid accumulation.
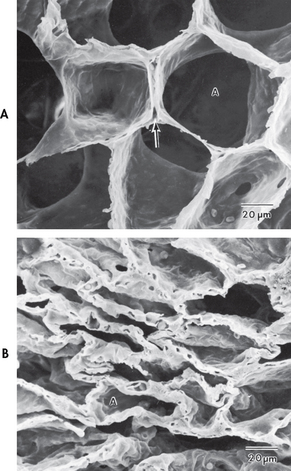
Figure 25-4 Scanning electron micrograph of lung parenchyma. A, Alveoli (A) and alveolar capillary (arrow). B, Effects of atelectasis. Alveoli (A) are partially or totally collapsed.
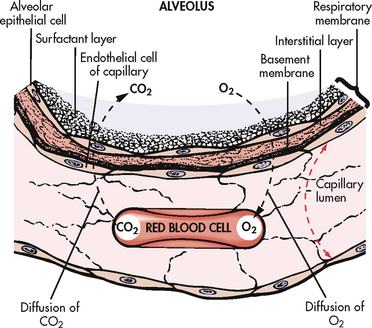
Figure 25-5 A small portion of the respiratory membrane greatly magnified. An extremely thin interstitial layer of tissue separates the endothelial cell and basement membrane on the capillary side of the respiratory membrane from the epithelial cell and surfactant layer on the alveolar side. The total thickness of the respiratory membrane is approximately 5 μm or 0.005 mm.
Surfactant
The lung can be conceptualised as a collection of 300 million bubbles (alveoli), each 0.3 mm in diameter. Such a structure is inherently unstable and, as a consequence, the alveoli have a natural tendency to collapse. The alveolar surface is composed of cells that provide structure and cells that secrete surfactant (see Fig 25-5). Surfactant, a lipoprotein that lowers the surface tension in the alveoli, reduces the amount of pressure needed to inflate the alveoli and decreases the tendency of the alveoli to collapse. Normally, a person takes a slightly larger breath, termed a sigh, after every five to six breaths. This sigh stretches the alveoli and promotes surfactant secretion.
When insufficient surfactant is present, the alveoli collapse. The term atelectasis refers to collapsed airless alveoli (see Fig 25-4). The postoperative patient is at risk of atelectasis because of the effects of anaesthesia and restricted breathing with pain (see Ch 19). In acute respiratory distress syndrome (ARDS), lack of surfactant contributes to widespread atelectasis (see Ch 67).1
Blood supply
The lungs have two different types of circulation: pulmonary and bronchial. The pulmonary circulation provides the lungs with blood for gas exchange. The pulmonary artery receives deoxygenated blood from the right ventricle of the heart and branches so that each pulmonary capillary is directly connected with many alveoli. Oxygen–carbon dioxide exchange occurs at this point. The pulmonary veins return oxygenated blood to the left atrium of the heart, which supplies the arteries of the systemic circulation.
The bronchial circulation starts with the bronchial arteries, which arise from the thoracic aorta. The bronchial circulation provides oxygen to the bronchi and other pulmonary tissues. Deoxygenated blood returns from the bronchial circulation through the azygos vein into the superior vena cava and left atrium.
CHEST WALL
The chest wall is shaped, supported and protected by 24 ribs (12 on each side). The ribs and the sternum protect the lungs and heart from injury and are sometimes called the thoracic cage. The structures of the chest wall include the thoracic cage, pleura and respiratory muscles.
The chest cavity is lined with a membrane called the parietal pleura and the lungs are lined with a membrane called the visceral pleura. The parietal and visceral pleurae are joined and form a closed, double-walled sac. The visceral pleura does not have any sensory (afferent) pain fibres or nerve endings. The parietal pleura, however, does have sensory pain fibres. Therefore, irritation of the parietal pleura causes severe pain with each breath.
The space between the pleural layers, termed the intrapleural space, is a potential space. Normally, this space is filled with 20–25 mL of fluid, which serves two purposes: (1) it provides lubrication, allowing the pleural layers to slide over each other during breathing; and (2) it increases cohesion between the pleural layers, thereby facilitating expansion of the pleura and lungs during inspiration.
Fluid drains from the pleural space by the lymphatic circulation. Several pathological conditions may cause the accumulation of greater amounts of fluid, termed a pleural effusion. Pleural fluid may accumulate because of blockage of lymphatic drainage (e.g. from malignant cells) or when there is an imbalance between intravascular and oncotic fluid pressures, such as occurs in congestive heart failure. Purulent pleural fluid with bacterial infection is called empyema.
The diaphragm is the major muscle of respiration. During inspiration, the diaphragm contracts, increasing thoracic volume and pushing the abdominal contents downwards. At the same time, the external intercostal muscles and scalene muscles contract, increasing the lateral and anteroposterior dimensions of the chest. This causes the size of the thoracic cavity to increase (see Fig 25-6) and intrathoracic pressure to decrease, so air enters the lungs.
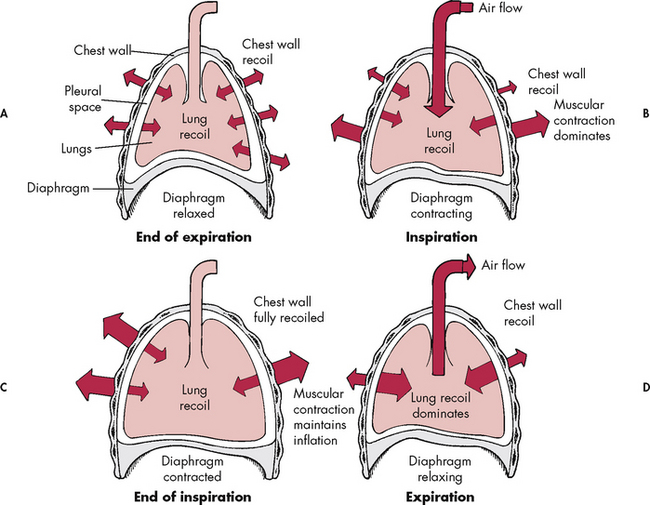
Figure 25-6 The interaction of forces during inspiration and expiration. A, Outward recoil of the chest wall equals inward recoil of the lungs at expiration. B, During inspiration, contraction of respiratory muscles, assisted by chest wall recoil, overcomes the tendency of lungs to recoil. C, At the end of inspiration, respiratory muscle contraction maintains lung expansion. D, During expiration, respiratory muscles relax, allowing elastic recoil of the chest wall to deflate the lungs.
The diaphragm is made up of two hemidiaphragms, each innervated by the right and left phrenic nerves. The phrenic nerves arise from the spinal cord between C3 and C5, the third and fifth cervical vertebrae. Injury to the phrenic nerve results in hemidiaphragm paralysis on the side of the injury. Complete spinal cord injuries above the level of C3 result in total diaphragm paralysis and mechanical ventilator dependence.3
PHYSIOLOGY OF RESPIRATION
Ventilation
Ventilation involves inspiration (movement of air into the lungs) and expiration (movement of air out of the lungs). Air moves in and out of the lungs because of intrathoracic pressure changes in relation to pressure at the airway opening. Contraction of the diaphragm and intercostal and scalene muscles increases chest dimensions, thereby decreasing intrathoracic pressure. Gas flows from an area of higher pressure (atmospheric) to one of lower pressure (intrathoracic) (see Fig 25-6). When inspiration is difficult (dyspnoea), neck and shoulder muscles can assist the effort. Some conditions (e.g. phrenic nerve paralysis, rib fractures, neuromuscular disease) may limit diaphragm or chest wall movement and cause the patient to breathe with smaller tidal volumes. As a result, the lungs do not fully inflate and gas exchange is impaired.
In contrast to inspiration, expiration is passive. Due to elastic fibres found in the alveolar walls and surrounding the bronchioles and capillaries, elastic recoil of the chest wall and lungs allows the chest to passively decrease in volume. Intrathoracic pressure rises, causing air to move out of the lungs. Exacerbations of asthma or chronic obstructive pulmonary disease (COPD) cause expiration to become an active, laboured process (see Ch 28). Abdominal and intercostal muscles assist in expelling air during laboured breathing.
Compliance
Compliance (distensibility) is a measure of the ease of expansion of the lungs. It is a product of the elasticity of the lungs and the elastic recoil of the chest wall. When compliance is decreased, the lungs are more difficult to inflate. Examples include conditions that increase fluid in the lungs (e.g. pulmonary oedema, ARDS, pneuomonia), conditions that make lung tissue less elastic (e.g. pulmonary fibrosis, sarcoidosis) and conditions that restrict lung movement (e.g. pleural effusion). Compliance is decreased as a result of ageing and when there is destruction of alveolar walls and loss of tissue elasticity, as in COPD.
Diffusion
Oxygen and carbon dioxide move back and forth across the alveolar capillary membrane by diffusion. The overall direction of movement is from the area of higher concentration to the area of lower concentration. Thus oxygen moves from alveolar gas (atmospheric air) into the arterial blood and carbon dioxide from the arterial blood into the alveolar gas. Diffusion continues until equilibrium is reached (see Fig 25-5).
The ability of the lungs to oxygenate arterial blood adequately is determined by examination of the arterial oxygen tension (PaO2) and arterial oxygen saturation (SaO2). Oxygen is carried in the blood in two forms: dissolved oxygen and haemoglobin-bound oxygen. The PaO2 represents the amount of oxygen dissolved in the plasma and is commonly expressed in conventional units as millimetres of mercury (mmHg). Increasingly, however, it is being expressed in SI units as kilopascals (kPa). The SaO2 is the amount of oxygen bound to haemoglobin in comparison with the amount of oxygen the haemoglobin can carry. The SaO2 is expressed as a percentage. For example, if the SaO2 is 90%, this means that 90% of the haemoglobin attachments for oxygen have oxygen bound to them.
Oxygen–haemoglobin dissociation curve
The affinity of haemoglobin for oxygen is described by the oxygen–haemoglobin dissociation curve (see Fig 25-7). Oxygen delivery to the tissues depends on the amount of oxygen transported to the tissues and the ease with which haemoglobin gives up oxygen once it reaches the tissues. The upper flat portion of the curve represents the conditions in the lungs. Fairly large changes in the PaO2 cause small changes in haemoglobin saturation. For this reason, if the PaO2 drops from 13 to 8 kPa (100 to 60 mmHg), the saturation of haemoglobin changes only 7% (from the normal 97% to 90%). Thus the haemoglobin remains 90% saturated despite a 5 kPa (40 mmHg) drop in the PaO2. This portion of the curve also explains the reason the patient is considered adequately oxygenated when the PaO2 is greater than 8 kPa (60 mmHg). Increasing the PaO2 above this level does little to improve haemoglobin saturation.
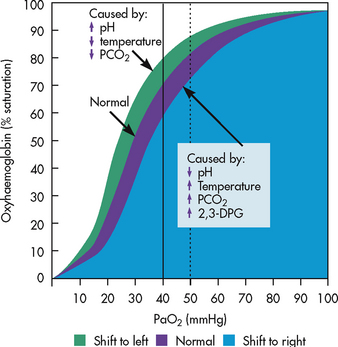
Figure 25-7 The oxygen–haemoglobin dissociation curve. The effects of acidity and temperature changes are shown. 2,3-DPG, 2,3-diphosphoglycerate; PaO2, partial pressure of oxygen in arterial blood; PCO2, pressure of carbon dioxide.
The lower portion of the curve represents oxygen binding by haemoglobin at the level of the peripheral tissues. As haemoglobin arrives at the tissues, it is desaturated and larger amounts of oxygen are released for tissue use. This is an important method of maintaining the pressure gradient between the blood and the tissues. It also ensures an adequate oxygen supply to the peripheral tissues, even if oxygen delivery is compromised.
Many factors alter the affinity of haemoglobin for oxygen. When the oxygen dissociation curve shifts to the left, blood picks up oxygen more readily in the lungs but delivers oxygen less readily to the tissues. Conditions such as alkalosis, hypothermia and a decrease in arterial carbon dioxide tension (PaCO2) can cause a shift to the left. The patient with a condition that causes a leftward shift of the curve, such as hypothermia following open heart surgery, may be given higher concentrations of oxygen until the body temperature normalises. This helps compensate for decreased oxygen unloading in the tissues. When the curve shifts to the right, the opposite occurs. Blood picks up oxygen less rapidly in the lungs but delivers oxygen more readily to the tissues. This is seen in acidosis, hyperthermia and when the PaCO2 is increased.4
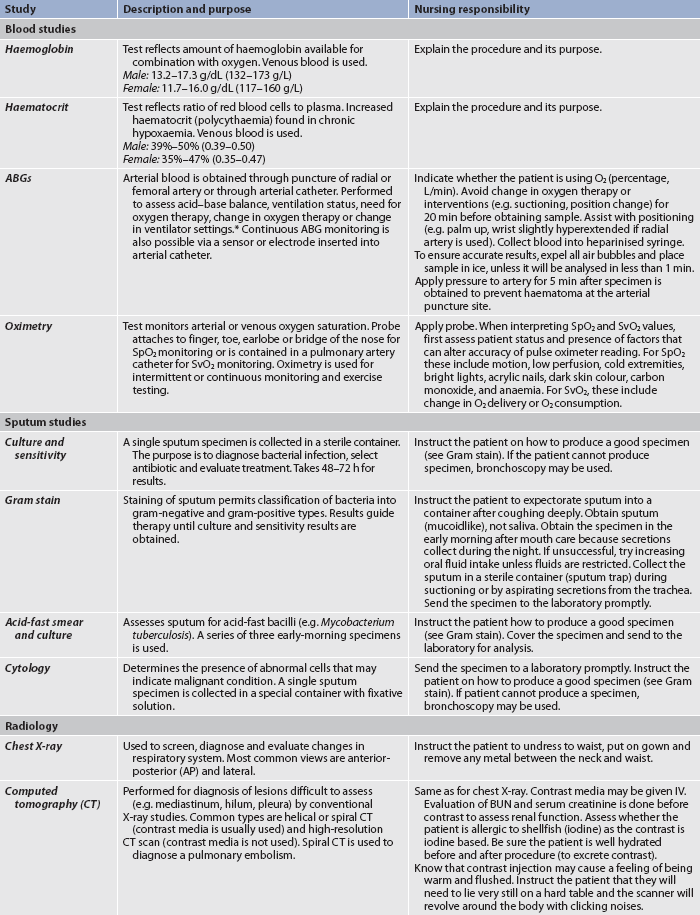
Three methods can be used to assess the efficiency of gas transfer in the lungs: analysis of arterial blood gases (ABGs), mixed venous blood gases and oximetry. These measures are usually adequate if the patient is stable and not critically ill.
Arterial blood gases
ABGs are measured to determine oxygenation status and acid–base balance. ABG analysis includes measurement of the PaO2, PaCO2, acidity (pH) and bicarbonate level (HCO3−) in arterial blood. The SaO2 is either calculated or measured during this analysis.
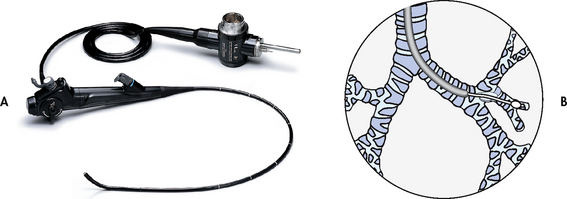
Blood for ABG analysis can be obtained by arterial puncture or from an arterial catheter in the radial or femoral artery. Both techniques are invasive and allow only intermittent analysis. Continuous intraarterial blood gas monitoring is also possible via a fibreoptic sensor or an oxygen electrode inserted into an arterial catheter. An arterial catheter permits ABG sampling without repeated arterial punctures.
Normal values for ABGs are given in Table 25-1. The normal PaO2 decreases with advancing age. It also varies in relation to the distance above sea level. At higher altitudes, the barometric pressure is lower, resulting in a lower inspired oxygen pressure and a lower PaO2 (see Table 25-1).
TABLE 25-1 Normal arterial and venous blood gas values*
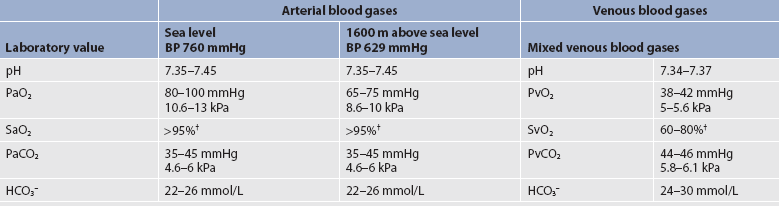
BP, barometric pressure; HCO3−, bicarbonate; PvCO2, partial pressure of CO2 in venous blood; PvO2, partial pressure of oxygen in venous blood; SvO2, venous oxygen saturation.
* assumes patient is ≤60 years of age and breathing room air.
† The same normal values apply when SpO2 and SvO2 are obtained by oximetry.
Mixed venous blood gases
For the patient with a normal or near-normal cardiac status, an assessment of PaO2 or SaO2 is usually sufficient to determine adequate oxygenation. The patient with impaired cardiac output or haemodynamic instability may have inadequate tissue oxygen delivery or abnormal oxygen consumption. The amount of oxygen delivered to the tissues or consumed can be calculated.
A catheter positioned in the pulmonary artery, termed a pulmonary artery (PA) catheter, is used for mixed venous sampling (see Ch 64). Blood drawn from a PA catheter is termed a mixed venous blood gas sample because it consists of venous blood that has returned to the heart from all tissue beds and ‘mixed’ in the right ventricle. Normal mixed venous values are given in Table 25-1. When tissue oxygen delivery is inadequate or when inadequate oxygen is transported to the tissues by the haemoglobin, the PvO2 and SvO2 fall.
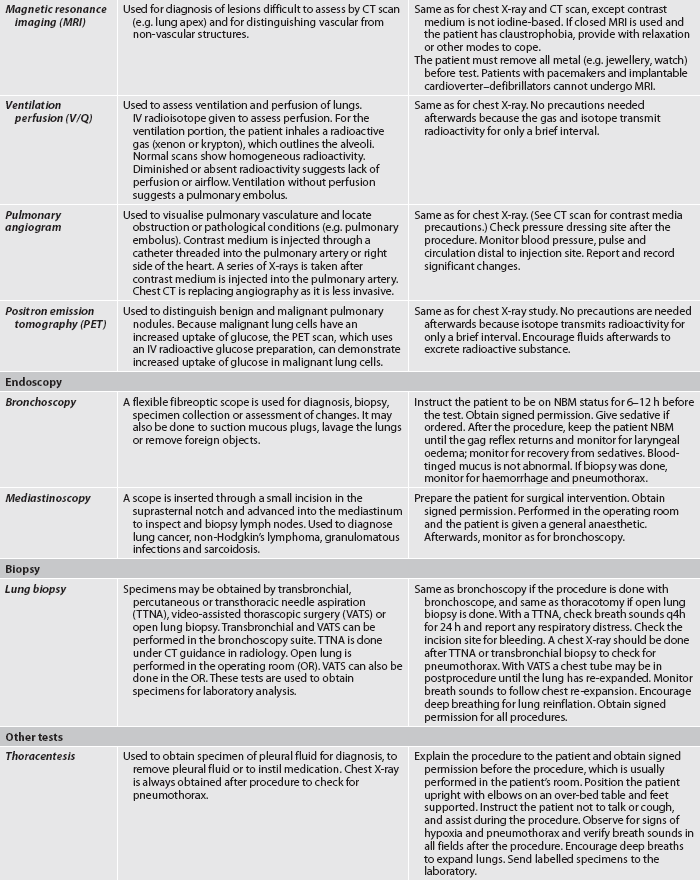
Oximetry
ABG values provide accurate information about oxygenation and acid–base balance.5 However, the technique is invasive, requires laboratory analysis and exposes the patient to the risk of bleeding from an arterial puncture. Arterial oxygen saturation can be monitored continuously using a pulse oximetry probe on the finger, toe, ear, forehead or bridge of the nose (see Fig 25-8).6
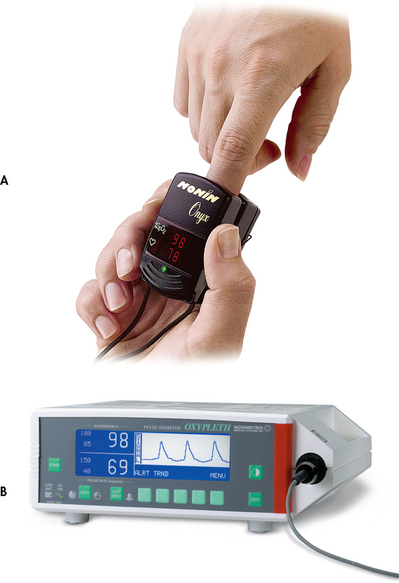
Figure 25-8 A, A portable pulse oximeter displays oxygen saturation and pulse rate (SpO2). B, A pulse oximeter displays the oxygen saturation and pulse rate as a digital reading.
The abbreviation SpO2 is used to indicate the oxygen saturation value obtained by pulse oximetry. SpO2 and heart rate are displayed on the monitor as digital readings (see Fig 25-8, B). The normal SpO2 is greater than 97%.
Pulse oximetry is particularly valuable in intensive care and perioperative areas where sedation or decreased consciousness might mask hypoxia (see Table 25-2). The SpO2 is assessed with each routine vital signs check in many inpatient areas. Changes in SpO2 can be detected quickly and treated (see Table 25-3). Oximetry is also used during exercise testing and when adjusting flow rates during long-term oxygen therapy. Pulse oximetry alone does not provide information about ventilation status and acid–base balance. Therefore, ABGs are also needed periodically.
TABLE 25-3 Critical values for PaO2 and SpO2*
| PaO2 (kpa) | SpO2 (%) | Considerations |
|---|---|---|
| ≥9.5 | ≥94 | Adequate unless the patient is haemodynamically unstable or haemoglobin (Hb) has difficulty releasing oxygen to the tissues (i.e. oxygen–haemoglobin dissociation curve shifts to the left). Higher O2 values may be desired with a low cardiac output, dysrhythmias, a leftward shift of the oxygen–haemoglobin dissociation curve or carbon monoxide inhalation. Benefits of a higher blood O2 value need to be balanced against the risk of O2 toxicity. |
| 8.1 | 90 | Adequate in almost all patients. Values are at steep part of oxygen–haemoglobin dissociation curve. Provides adequate oxygenation but with less margin of error than above. |
| 7.4 | 88 | Adequate for patients with chronic hypoxaemia if no cardiac problems occur. These values are also used as criteria for prescription of continuous O2 therapy. |
| 5.4 | 75 | Inadequate but may be acceptable on a short-term basis if the patient also has CO2 retention. In this situation, respirations may be stimulated by a low PaO2. Thus the PaO2 cannot be raised rapidly. O2 therapy at a low concentration (24–28%) will gradually increase the PaO2. Monitoring for dysrhythmias is necessary. |
| <5.4 | <75 | Inadequate. Tissue hypoxia and cardiac dysrhythmias can be expected. |
*The same critical values apply for SpO2 and SaO2. Values pertain to rest or exertion.
Values obtained by pulse oximetry are less accurate if the SpO2 is less than 70%. At this level, the oximeter may display a value that is ± 4% of the actual value. For example, if the SpO2 reading is 70%, the actual value can range from 66% to 74%. Pulse oximetry is also inaccurate if haemoglobin variants (e.g. carboxyhaemoglobin, methaemoglobin) are present. Other factors that can alter the accuracy of pulse oximetry include motion, low perfusion, anaemia, cold extremities, bright fluorescent lights, intravascular dyes, thick acrylic nails and dark skin colour. If there is doubt about the accuracy of the SpO2 reading, an ABG analysis should be obtained to verify accuracy.
Oximetry can also be used to monitor SvO2 via a PA catheter or an oesophageal Doppler. A decrease in SvO2 suggests that less oxygen is being delivered to the tissues or that more oxygen is being consumed. Changes in SvO2 provide an early warning of a change in cardiac output or tissue oxygen delivery. Normal SvO2 is 60–80%.
CONTROL OF RESPIRATION
The respiratory centre in the brainstem’s medulla oblongata responds to chemical and mechanical signals from the body. Impulses are sent from the medulla to the respiratory muscles through the spinal cord and phrenic nerves.
Chemoreceptors
A chemoreceptor is a receptor that responds to a change in the chemical composition (PaCO2 and pH) of the fluid around it. Central chemoreceptors are located in the medulla and respond to changes in the hydrogen ion (H+) concentration. An increase in the H+ concentration (acidosis) causes the medulla to increase the respiratory rate and tidal volume (VT). A decrease in H+ concentration (alkalosis) has the opposite effect. Changes in PaCO2 regulate ventilation primarily by their effect on the pH of the cerebrospinal fluid. When the PaCO2 level is increased, more CO2 is available to combine with H2O and form carbonic acid (H2CO3). This lowers the cerebrospinal fluid pH and stimulates an increase in respiratory rate. The opposite process occurs with a decrease in PaCO2 level.
Peripheral chemoreceptors are located in the carotid bodies at the bifurcation of the common carotid arteries and in the aortic bodies above and below the aortic arch. They respond to decreases in PaO2 and pH and to increases in PaCO2. These changes also cause stimulation of the respiratory centre.
In a healthy person an increase in PaCO2 or a decrease in pH causes an immediate increase in the respiratory rate. The process is extremely precise. The PaCO2 does not vary more than about 0.4 kPa (3 mmHg) if lung function is normal. Conditions such as COPD alter lung function and may result in chronically elevated PaCO2 levels. In these instances, the patient will be relatively insensitive to further increases in PaCO2 as a stimulus to breathe and may be maintaining ventilation largely because of a hypoxic drive from the peripheral chemoreceptors (see Ch 28).
Mechanical receptors
Mechanical receptors (juxtacapillary and irritant) are located in the lungs, upper airways, chest wall and diaphragm. They are stimulated by a variety of physiological factors, such as irritants, muscle stretching and alveolar wall distortion. Signals from the stretch receptors aid in the control of respiration. As the lungs inflate, pulmonary stretch receptors activate the inspiratory centre to inhibit further lung expansion. This is termed the Hering-Breuer reflex and it prevents overdistension of the lungs. Impulses from the mechanical sensors are sent through the vagus nerve to the brain. Juxtacapillary (J) receptors are believed to cause the rapid respiration (tachypnoea) seen in pulmonary oedema. These receptors are stimulated by fluid entering the pulmonary interstitial space.
RESPIRATORY DEFENCE MECHANISMS
Respiratory defence mechanisms are efficient in protecting the lungs from inhaled particles, microorganisms and toxic gases. The defence mechanisms include filtration of air, the mucociliary clearance system, the cough reflex, reflex bronchoconstriction and alveolar macrophages.
Filtration of air
Nasal hairs filter the inspired air. In addition, the abrupt changes in direction of airflow that occur as air moves through the nasopharynx and larynx increase air turbulence. This causes particles and bacteria to contact the mucosa lining these structures. Most large particles (>5 μm in diameter) are removed in this manner.
The velocity of airflow slows greatly after it passes the larynx, facilitating the deposition of smaller particles (1–5 μm in size). They settle out similarly to sand in a river, a process termed sedimentation. Particles less than 1 μm in size are too small to settle in this manner and are deposited in the alveoli. One example of small particles that can build up is coal dust, which can lead to pneumoconiosis (see Ch 27). Particle size is important. Particles greater than 5 μm in size are less dangerous because they are removed in the nasopharynx or bronchi and do not reach the alveoli.
Mucociliary clearance system
Below the larynx, movement of mucus is accomplished by the mucociliary clearance system, commonly referred to as the mucociliary escalator. This term is used to indicate the interrelationship between the secretion of mucus and the ciliary activity. Mucus is continually secreted at a rate of about 100 mL per day by goblet cells and submucosal glands. It forms a mucous blanket that contains the impacted particles and debris from distal lung areas (see Fig 25-1). The small amount of mucus normally secreted is swallowed without being noticed.1 Secretory immunoglobulin A (IgA) in the mucus contributes to protection against bacteria and viruses.1
Cilia cover the airways from the level of the trachea to the respiratory bronchioles (see Fig 25-1). Each ciliated cell contains approximately 200 cilia, which beat rhythmically about 1000 times per minute in the large airways, moving mucus towards the mouth. The ciliary beat is slower further down the tracheobronchial tree. As a consequence, particles that penetrate more deeply into the airways are removed less rapidly. Ciliary action is impaired by dehydration, smoking, inhalation of high oxygen concentrations, infection and ingestion of drugs such as atropine, anaesthetics, alcohol, cocaine or crack. Patients with COPD and cystic fibrosis have repeated upper respiratory tract infections. Cilia are often destroyed during these infections, resulting in impaired secretion clearance, a chronic productive cough and frequent respiratory tract infections.
Cough reflex
The cough is a protective reflex action that clears the airway by a high-pressure, high-velocity flow of air. It is a back-up for mucociliary clearance, especially when this clearance mechanism is overwhelmed or ineffective. Coughing is only effective in removing secretions above the subsegmental level (large or main airways). Secretions below this level must be moved upwards by the mucociliary mechanism or by interventions such as postural drainage and associated techniques of percussion and vibration before they can be removed by coughing. As noted in the COPD-X plan,7 there are several techniques available for section removal and the choice of technique will depend on the patient’s condition.8
Reflex bronchoconstriction
Another defence mechanism is reflex bronchoconstriction. In response to the inhalation of large amounts of irritating substances (e.g. dusts, aerosols), the bronchi constrict in an effort to prevent entry of the irritants. A person with hyperreactive airways, such as a person with asthma, experiences bronchoconstriction after inhalation of cold air, perfume or other strong odours.
Alveolar macrophages
Because ciliated cells are not found below the level of the respiratory bronchioles, the primary defence mechanism at the alveolar level is alveolar macrophages. Alveolar macrophages rapidly phagocytise inhaled foreign particles such as bacteria. The debris is moved to the level of the bronchioles for removal by the cilia or removed from the lungs by the lymphatic system. Particles (e.g. coal dust, silica) that cannot be adequately phagocytised tend to remain in the lungs for indefinite periods and can stimulate inflammatory responses (see Ch 27). Because alveolar macrophage activity is impaired by cigarette smoke, smokers who are employed in occupations with heavy dust exposure (e.g. mining, foundries) are at an especially high risk of lung disease.
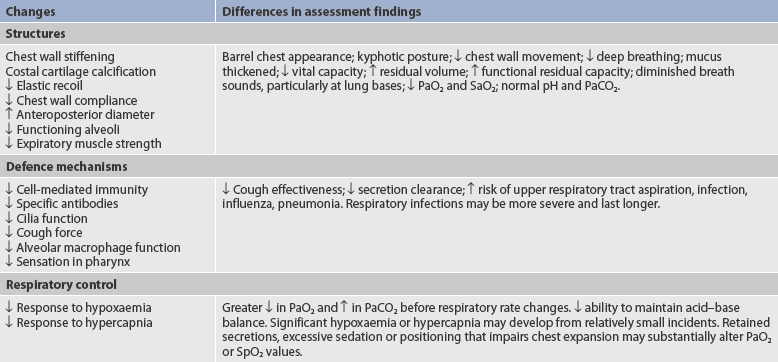
Gerontological considerations: effects of ageing on the respiratory system
Age-related changes in the respiratory system can be divided into alterations in structure, defence mechanisms and respiratory control (age-related changes in the respiratory system and differences in assessment findings are presented in Table 25-4). Structural alterations include a decrease in elastic recoil of the lungs and a decrease in chest wall compliance. The anteroposterior diameter of the thoracic cage increases. Within the lungs there is a decrease in the number of functional alveoli. Small airways in the lung bases close earlier in expiration. As a consequence, more inspired air is distributed to the lung apices and ventilation is less well matched to perfusion, causing a lowering of the PaO2. The PaO2 associated with a given age can be calculated by means of the following equation:
PaO2 (kPa) = 14 – (0.06 × age in years)
or
PaO2 (mmHg) = 103.5 – (0.42 × age in years)
For example, the normal PaO2 for a patient 80 years of age is 9.2 kPa (or 69.9 mmHg) (14 – [0.06 × 80] = 9.2 kPa) as compared with a PaO2 of 12.5 kPa (or 93 mmHg) for a 25-year-old (14 – [0.06 × 25] = 12.5 kPa).
Respiratory defence mechanisms are less effective because of a decline in cell-mediated immunity and formation of antibodies. The alveolar macrophages are less effective at phagocytosis. An older patient has a less forceful cough and fewer and less functional cilia. Mucous membranes tend to be drier. Retained mucus predisposes the elderly to respiratory infections. Formation of secretory IgA, an important mechanism in neutralising the effect of viruses, is diminished.
Respiratory control is altered, resulting in a more gradual response to changes in blood oxygen or carbon dioxide level. The PaO2 drops to a lower level and the PaCO2 rises to a higher level before the respiratory rate changes.
There is much variability in the extent of these changes in persons of the same age. The older patient who has a significant smoking history, is obese and is diagnosed with a chronic illness is at greatest risk of adverse outcomes.9
Assessment of the respiratory system
Correct diagnosis depends on an accurate health history and a thorough physical examination. A respiratory assessment can be done as part of a comprehensive physical examination or as an examination in itself. Judgement must be used in determining whether all or part of the history and physical examination will be completed, based on problems presented by the patient and the degree of respiratory distress. If respiratory distress is severe, only pertinent information should be obtained and a thorough assessment should be deferred until the patient’s condition stabilises.
SUBJECTIVE DATA
Important health information
Past health history
It is important to determine the frequency of upper respiratory tract problems (e.g. colds, sore throats, sinus problems, allergies) and whether seasonal changes have an effect on these problems. The patient with allergies should be questioned about possible precipitating factors, such as medications, pollen, mould, smoke or pet exposure. Characteristics of the allergic reaction, such as runny nose, wheezing, scratchy throat or tightness in the chest, and severity should be documented. The frequency of asthma exacerbations and causes or triggers, if known, should also be determined. Prior use of a peak expiratory flow rate (PEFR) meter and personal best values can be helpful information in determining the patient’s current asthma status, as can changes in the patient’s level of activity and ability to undertake everyday tasks. It is important to inquire about any history of lower respiratory tract problems, such as asthma, COPD, pneumonia and tuberculosis. Respiratory symptoms are often manifestations of problems that involve other body systems. Therefore, the nurse should ask the patient if there is a history of other health problems in addition to those involving the respiratory system. For example, the patient with cardiac dysfunction may experience dyspnoea (shortness of breath) due to heart failure. The patient with human immunodeficiency virus (HIV) infection may have frequent respiratory tract infections due to compromised immune function.
Medications
The nurse should take a thorough medication history. Prescription and over-the-counter medications should be assessed and documented. The nurse should obtain information about the reason for taking each medication, its name, the dose and frequency, the length of time taken, its effect and any side effects. The nurse should also assess for overuse of short-term bronchodilators as a key indicator of symptom control. It will help to guide the management. The nurse should inquire too about the use of angiotensin-converting enzyme (ACE) inhibitors, as cough is a relatively common side effect of this class of drugs. The use of natural or alternative therapies must also be ascertained, as some can enhance or decrease the effects of conventional medications.
If the patient is using oxygen to ease a breathing problem, the nurse should document the fraction of inspired oxygen concentration (FIO2), litre flow, method of administration, number of hours used each day and effectiveness of the therapy. Safety practices related to using oxygen should also be assessed, including the patient’s mechanical and cognitive ability related to using oxygen.10
Surgery or other treatment
The nurse should determine whether the patient has been hospitalised for a respiratory problem. If so, the dates, therapy (including surgery) and current status of the problem should be recorded. The nurse should determine also whether the patient has ever been intubated because of a respiratory problem, and ask about the use and results of respiratory treatments, such as a nebuliser or humidifier and airway clearance modalities, including a Flutter valve, high-frequency chest oscillation, postural drainage and percussion.
Functional health patterns
Health history questions to ask a patient with a respiratory problem are presented in Table 25-5.
Health perception–health management pattern
The patient should be asked if there has been a perceived change in health status within the last several days, months or years. In COPD, lung function declines slowly over many years. The patient may not notice this decline because activity is altered to accommodate reduced exercise tolerance. If an upper respiratory tract infection is superimposed on a chronic problem, dyspnoea and decreased exercise tolerance may occur very quickly. In asthma, symptoms may occur or worsen in the presence of exercise, animals or changes in temperature, causing the patient to avoid these activities or exposures.
Common cues that alert the nurse to the possibility of respiratory problems should be explored and documented (see Table 25-6). The course of the patient’s illness, including when it began, the type of symptoms and factors that alleviate or aggravate these symptoms should be described. Because of the chronic nature of respiratory problems, the patient may relate a change in symptoms rather than the onset of new symptoms when describing the present illness. Such changes should be carefully documented because they often suggest the cause of illness. For example, increased shortness of breath or a change in the volume, tenacity (thickness) or colour of sputum may suggest the onset of an acute exacerbation of COPD.
TABLE 25-6 Cues to respiratory problems
| Manifestation | Description |
|---|---|
| Shortness of breath (dyspnoea) | Distressful sensation of uncomfortable breathing. Most common complaint of people with respiratory problems. Person may become accustomed to sensation and not recognise its presence. Difficult to evaluate because it is a subjective experience. |
| Wheezing | May or may not be heard by the patient. May be described as chest tightness. |
| Pleuritic chest pain | Described on a continuum from discomfort during inspiration to intense, sharp pain at the end of inspiration. Pain is usually aggravated by deep breathing and coughing. Pain is very localised versus diffuse. |
| Cough | Characteristics of cough are important diagnostic cues. |
| Sputum production | Material coughed up from lungs. Contains mucus, cellular debris or microorganisms and may contain blood or pus. Amount, colour and constituents of sputum are important diagnostic information. |
| Haemoptysis | Coughing up of blood; either gross, frankly bloody sputum or blood-tinged sputum. Precipitating events should be investigated. |
| Voice change | Hoarseness, stridor (whistling sound during inspiration), muffling or a barking cough may indicate abnormalities of upper airway, vocal cord dysfunction or gastro-oesophageal reflux disease. |
| Fatigue | Sense of overwhelming tiredness not completely relieved by sleep or rest. |
If dyspnoea is present, the nurse should determine if it occurs at rest or with physical exertion. The nurse should also explore if the patient has difficulty breathing in a certain position or if relief of dyspnoea can be obtained by assuming a different position. To determine the intensity of dyspnoea, the use of a Borg scale or a visual analogue scale (VAS) may be helpful (see Fig 25-9).
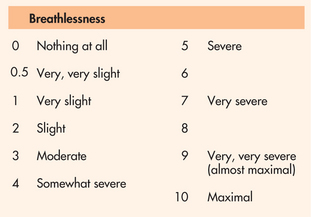
Figure 25-9 Borg category-ratio scale. Using this scale from 0 to 10, how much shortness of breath do you have right now?
If a cough is present, the nurse should evaluate the quality of the cough. For example, a loose-sounding cough indicates the presence of secretions; a dry, hacking cough indicates airway irritation or obstruction; a harsh, barky cough suggests upper airway obstruction from inhibited vocal cord movement related to subglottic oedema. The nurse should assess whether the cough is weak or strong and whether it is productive or unproductive of secretions. Determining whether the cough is acute or chronic is helpful in the differential diagnosis process. The pattern of the cough is determined by asking questions such as: What is the pattern of coughing? Has it been regular, paroxysmal, related to a time of day or weather, certain activities, talking, deep breaths? Has there been any change over time? What efforts have been tried to alleviate the coughing? Were any prescription or over-the-counter drugs tried?
If the patient has a productive cough, the following characteristics of sputum should be evaluated: amount, colour, consistency and odour. The amount should be quantified in teaspoons, tablespoons or cups per day. Any recent increases or decreases in the amount should be noted. The normal colour is clear or slightly whitish.
If a patient is a cigarette smoker, the sputum is usually clear to grey with occasional specks of brown. The patient with COPD may exhibit clear, whitish or slightly yellow sputum, especially in the morning on rising. If the patient reports any change from baseline to yellow, pink, red, brown or green sputum, pulmonary complications should be suspected. Changes in the consistency of sputum to thick, thin or frothy should be noted. These changes may indicate dehydration, postnasal drip or sinus drainage or possible pulmonary oedema. Normally sputum should be odourless. A foul odour suggests an infectious process. The nurse should ask the patient if the sputum was produced along with a position change (e.g. increased with lying down) or a change in activity.
The patient should be questioned about a history of coughing up blood (haemoptysis), which can range from slight streaking of blood in the mucus to massive coughing up of blood in which the patient loses 100–600 mL of blood in a 24-hour period—this situation is a medical emergency.
Frequently the patient cannot differentiate between haemoptysis and haematemesis (vomiting blood). Carefully questioning and testing for an acidic pH (present with haematemesis) can differentiate between the two. Haemoptysis can be found with a variety of conditions, such as pneumonia, tuberculosis, lung cancer and severe bronchiectasis.
The nurse should assess for a history of wheezing. Wheezes are musical sounds that are audible to the patient and the nurse. Wheezing indicates some degree of airway obstruction such as asthma, foreign body aspiration and emphysema. The nurse should also determine if there is a family history of respiratory problems that may be genetic or have familial tendencies, such as asthma, emphysema resulting from alpha-1-antitrypsin deficiency or cystic fibrosis. A history of family exposure to tuberculosis bacilli should be noted.
The nurse should ask where the patient has lived and travelled. Risk factors for tuberculosis include prior residence in Asia, Africa, the former Soviet Union, South America or any developing country. The nurse should also ask about current and past smoking habits and quantify exposure in pack-years. This is done by multiplying the number of packs smoked per day by the number of years smoked. For example, a person who smoked 1 pack per day for 15 years has a 15 pack-year history. The risk of lung cancer rises in direct proportion to the number of cigarettes smoked. Smoking increases the risk of COPD and lung cancer and exacerbates symptoms of asthma and chronic bronchitis. In addition to asking about cigarette use, it is important to find out about the use of any tobacco products, including cigars, pipes, chewing tobacco and smokeless tobacco products. It is also important to know about exposure to second-hand smoke. The nurse should determine if efforts have been made to stop smoking, including prescription, over-the-counter and herbal remedies.
It is important to assess if the patient has received immunisation for influenza (flu) and pneumococcal pneumonia. Influenza vaccine should be administered yearly in the autumn.11 Pneumovax is recommended for persons 65 years or older or those individuals with certain chronic diseases, such as cardiovascular disease, COPD, immunocompromised state and diabetes mellitus. Revaccination is currently advised only if the patient received the vaccine more than 5 years previously and was less than 65 years old at the time of vaccination. For immunocompromised persons (e.g. transplant recipients), who are at higher risk of serious pneumococcal infection, an initial vaccine is recommended followed by revaccination every 5 years.
The nurse should ask the patient about the use of equipment to manage respiratory symptoms (e.g. home oxygen therapy equipment, metered-dose inhaler [MDI] with spacer or nebuliser for medication administration, positive airway pressure device for relief of sleep apnoea). The patient should be questioned about the type of equipment used, the frequency of use, its effect and any side effects, and also asked to demonstrate use of the MDI or the dry powder inhaler. Many patients do not know how to use these devices correctly (see Ch 28).
Nutritional–metabolic pattern
Weight loss is a symptom of many respiratory diseases. The nurse should determine if weight loss was intentional and, if not, if food intake is altered by anorexia (from medications), fatigue (from hypoxaemia, increased work of breathing), feeling full too quickly (from lung hyperinflation) or social isolation. Anorexia, weight loss and chronic malnutrition are common symptoms in patients with COPD, acquired immunodeficiency syndrome (AIDS), lung cancer, tuberculosis and chronic severe infection (bronchiectasis). Fluid intake should also be noted. Dehydration can result in thickened mucus, which can cause airway obstruction.
Excessive weight interferes with normal ventilation and may contribute to the development of obstructive sleep apnoea (see Ch 26). Morbidly obese individuals may hypoventilate while awake or asleep, and weight loss can help improve blood gases. Rapid weight gain from fluid retention may decrease pulmonary gas exchange.
Elimination pattern
Healthy elimination habits depend on the ability to reach a toilet when necessary. Activity intolerance secondary to dyspnoea could result in incontinence. Dyspnoea can also be the cause of limited mobility, which can cause constipation. The patient with dyspnoea should be questioned about both of these possibilities. Those with a chronic cough, especially women, may be troubled by urinary incontinence during bouts of coughing.
Activity–exercise pattern
The nurse should determine whether the patient’s activity is limited by dyspnoea at rest or during exercise.12 The nurse should also note whether the patient’s housing (e.g. number of steps, levels) poses a problem that increases social isolation.
It is important to record and measure whether the patient is able to carry out activities of daily living without dyspnoea or other respiratory symptoms. If unable, the amount and type of care needed should be documented. Self-care strategies to minimise dyspnoea should be reinforced. Immobility and sedentary habits can be risk factors for hypoventilation leading to atelectasis or pneumonia, especially if the patient is overweight.13
Sleep–rest pattern
The nurse should determine whether the patient can sleep throughout the night. The patient with asthma or COPD may awaken at night with chest tightness, wheezing or coughing. This suggests a need for a longer-acting bronchodilator or other medication change. The patient with cardiovascular disease (e.g. heart failure) may sleep with the head elevated on several pillows to prevent respiratory problems brought on by lying flat (orthopnoea). The patient with sleep apnoea may have snoring, insomnia and daytime drowsiness. Night sweats may be a manifestation of tuberculosis.
Cognitive–perceptual pattern
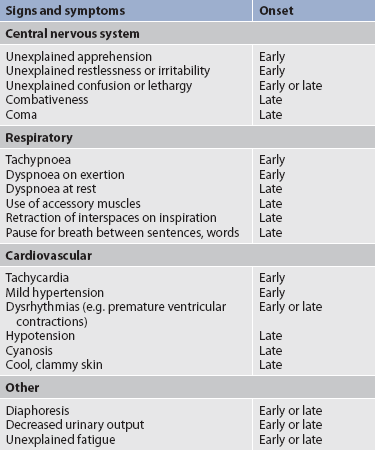
Because hypoxia can cause neurological symptoms, the patient should be asked about apprehension, restlessness, memory changes and irritability, which can indicate inadequate cerebral oxygenation (see Table 25-2). Hypoxaemia interferes with the ability to learn and retain information. For this reason, teaching may be more effective if another person is present during the teaching session to provide reinforcement at a later date.
The nurse should assess the patient’s cognitive ability and functional capacity to cooperate with treatment, including language of choice. Failure or inability to participate in needed therapy can result in exacerbation of respiratory problems; this may arise, for example, from an inability to fully understand instructions about medication, especially since in Australasia these are usually written in English and this might not be the patient’s first language.
The nurse should inquire about any discomfort or pain with breathing. A complaint of chest pain must be explored carefully to rule out cardiac involvement. Respiratory system problems such as pleurisy, fractured ribs and costochondritis cause chest pain. Pleuritic pain is described as a sharp, stabbing pain associated with movement or deep breathing. Fractured ribs cause localised sharp pain associated with breathing. The pain of costochondritis is along the borders of the sternum and is associated with breathing.
Self-perception–self-concept pattern
Dyspnoea limits activity, impairs ability to fulfil normal developmental role functions and often alters self-esteem. Concern about a highly visible nasal cannula and the difficulty of managing equipment may cause the patient to resist using oxygen in public. The nurse should discuss with the patient their personal views relating to their body image. Referral to a support group or pulmonary rehabilitation program may be beneficial in developing a support system and coping strategies.
Role–relationship pattern
Acute or chronic respiratory problems can seriously affect performance at work and in other activities. The nurse needs to ask about the impact of medications, oxygen and special routines (e.g. pulmonary hygiene for cystic fibrosis) on the patient’s family, job and social life.
The nature of the patient’s work and the frequency and intensity of exposure to fumes, toxins, asbestos, coal, fibres or silica should be documented. It is important to inquire whether symptoms are worse in specific situations (e.g. home versus work environments). Patient-specific allergens such as dust or fumes, which could be present in the work environment, should be investigated. Hobbies such as woodworking (sawdust) or pottery (silica) and exposure to animals (allergies) may also cause respiratory problems. Because of hyperreactive airways, exposure to fumes, smoke and other chemicals may trigger wheezing, especially in the asthmatic patient.
Sexuality–reproductive pattern
Most patients can continue to have good sexual relationships despite marked physical limitations. The nurse should tactfully determine whether breathing difficulties have caused alterations in sexual activity. If so, teaching can be provided about positions that decrease dyspnoea during sexual activity, including wearing oxygen therapy equipment if needed, and alternative strategies for sexual fulfilment.
Coping–stress tolerance pattern
Dyspnoea causes anxiety and anxiety exacerbates dyspnoea. The result is a vicious cycle—the patient avoids activities that cause dyspnoea, becoming more deconditioned and more dyspnoeic. The outcome is often physical and social isolation. Inquire about how often the patient leaves home and interacts with others. Referral to a support group or pulmonary rehabilitation program may be beneficial.
The chronic nature of respiratory problems such as COPD and asthma can cause prolonged stress. The nurse should inquire about the patient’s coping strategies to manage this stress.
Value–belief pattern
The nurse should determine the patient’s adherence to the management regimen and explore reasons for lack of adherence, including conflict with culturally specific beliefs, financial constraints (costs of prescriptions), failure to note benefit or other reasons.14 Including the family or carer in the planning of care can improve compliance.