Chapter 13 Genetics, altered immune responses and transplantation
1. Define common terms related to genetics and genetic disorders: autosome, carrier, heterozygous, homozygous, mutation, recessive and sex-linked.
2. Compare and contrast the most common classifications of genetic disorders.
3. Describe the functions and components of the immune system.
4. Compare and contrast humoral and cell-mediated immunity regarding the lymphocytes involved, the types of reactions and the effects on antigens (HLA).
5. Characterise the five types of immunoglobulins.
6. Differentiate among the four types of hypersensitivity reactions in terms of immunological mechanisms and resulting alterations.
7. Identify the clinical manifestations and emergency management of a systemic anaphylactic reaction.
8. Describe the assessment and multidisciplinary care of a patient with chronic allergies.
9. Explain the relationship between the human leucocyte antigen system and certain diseases.
10. Describe the aetiological factors, clinical manifestations and treatment modalities of autoimmune diseases.
11. Describe the aetiological factors and categories of immunodeficiency disorders.
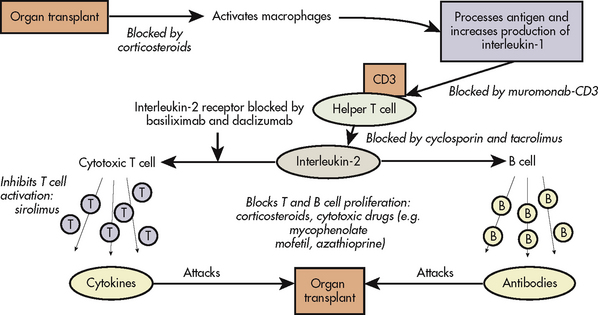
12. Differentiate among the types of rejections following transplantation.
13. Identify the types and side effects of immunosuppressive therapy.
Genetics
Genetics has a profound impact on health and disease. The study of genetics has become increasingly important for healthcare professionals. More than 4000 diseases are thought to be related to mutated genes. Common disorders such as heart disease and most cancers arise from a complex interplay between multiple genes and between genes and factors in the environment.
The identification of a genetic basis for many diseases has affected the study of genetics and its relevance to nurses. This has directly influenced the care of patients at risk of or diagnosed with a disease that has a genetic basis. Nurses need to know the basic principles of genetics, be familiar with the impact that genetics has on health and disease, and be prepared to assist the patient and family in dealing with genetic issues.
BASIC PRINCIPLES OF GENETICS
In the 1860s, a monk named Gregor Mendel discovered how traits are transmitted from parents to offspring while experimenting with pea plants. This discovery led to the study of genetics, which is also known as the study of inheritance. (Common terms used in the study of genetics are listed and defined in Table 13-1.)
TABLE 13-1 Glossary of genetic terms
| Term | Definition |
|---|---|
| Allele | One of two or more alternative forms of a gene that can occupy a particular chromosomal locus |
| Autosome | Any chromosome that is not a sex chromosome |
| Carrier | Individual who carries a copy of a mutated gene for a recessive disorder |
| Chromosome | Gene-carrying structure in the nucleus of all human cells consisting of Dna and protein |
| Codominance | Two dominant versions of a trait that are both expressed in the same individual |
| Congenital | Condition present at birth |
| Dominant allele | Gene that is expressed in the phenotype of a heterozygous individual |
| Gene | Unit of hereditary information located on a specific part of a chromosome |
| Genetics | Study of inheritance; study of individual genes and their impact on relatively rare single gene disorders |
| Genome | Complete genetic information of an organism |
| Hereditary | Transmission of a disease or condition from parent to offspring |
| Heterozygous | Having two different alleles for one given gene |
| Homozygous | Having two identical alleles for one given gene |
| Locus | Position of a gene on a chromosome |
| Mutation | Change in the DNA sequence of a gene affecting the original expression of the gene |
| Oncogene | Gene that is able to initiate and contribute to the conversion of normal cells to cancer cells |
| Pedigree | Family tree that contains the genetic characteristics and disorders of that particular family |
| Phenotype | Clinically expressed traits of an individual |
| Protooncogene | Normal cellular genes that are important regulators of normal cellular processes; mutations can activate them to become oncogenes |
| Recessive allele | Allele that has no noticeable effect on the phenotype in a heterozygous individual |
| Sex-linked gene | Gene located on a sex chromosome |
| Trait | Physical characteristic that one inherits, such as hair and eye colour |
Genes
Genes are the basic units of heredity. There are approximately 20,000–25,000 genes in each person’s genetic make-up, or genome. Any change in gene structure leads to a mutation that may alter the type and amount of protein produced. Genes are arranged in a specific linear formation along a chromosome. Each gene has a specific location on a chromosome, termed a locus. An allele is one of two or more alternative forms of a gene that occupy corresponding loci on homologous chromosomes (a pair of chromosomes having corresponding deoxyribonucleic acid [DNA] sequences with one coming from the mother and the other from the father). Each allele codes for a specific inherited characteristic. When two gene pairs are different alleles, the allele that is fully expressed is called the dominant allele. The other allele lacks the ability to express itself in the presence of a dominant allele and is called the recessive allele. Physical traits expressed by a person are termed the phenotype, and the actual genetic make-up of the person is termed the genotype.
Chromosomes
Chromosomes are contained in the nucleus of a cell and occur in pairs. There are 23 pairs of chromosomes; 22 of the 23 pairs of chromosomes are said to be homologous and are termed autosomes. Autosomes are the same in both males and females. The sex chromosomes make up the twenty-third pair of chromosomes. A female has two X chromosomes, and a male has one X and one Y chromosome. One chromosome of each pair is inherited from the mother and one from the father. Half of each child’s chromosomes (and therefore its genetic make-up) comes from the father and half from the mother.
DNA
Genes are made up of a nucleic acid called DNA. DNA stores genetic information and encodes the instructions for synthesising specific proteins that are needed to maintain life. DNA also dictates the rate at which proteins will be made. The DNA molecule is double-stranded and is identified as a double helix. Each DNA molecule is made up of many smaller molecules, including sugar, nitrogenous bases and phosphate units. The four nitrogenous bases making up DNA are adenine, thymine, guanine and cytosine.
RNA
Ribonucleic acid (RNA) is very similar to DNA. Like DNA, RNA contains the nitrogenous bases adenine, guanine and cytosine. However, although they are very similar, there are some significant differences: RNA lacks the nitrogenous base thymine and instead contains uracil; RNA is single-stranded and contains ribose instead of deoxyribose sugar; and RNA transfers the genetic information obtained from DNA to the proper location for protein synthesis and plays a critical role during the synthesis of proteins.
Protein synthesis
Protein synthesis, or the making of proteins, occurs in two steps: transcription and translation. (Transcription and translation are shown in Fig 13-1.) Transcription is the process where messenger RNA (mRNA) is synthesised from single-stranded DNA. The mRNA becomes attached to a ribosome, where translation occurs. At this point another specialised type of RNA, transfer RNA (tRNA), arranges the amino acids in the correct sequence to assemble the protein. Once the protein is made, it is released from the ribosome and is able to perform its specific function.
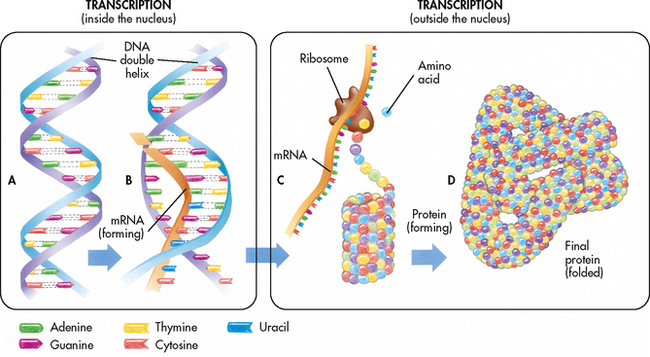
Figure 13-1 A, The DNA molecule contains a sequence of genes. B, During transcription, the DNA code is transcribed as mRNA. C, During translation, the mRNA code is translated at the ribosome and the proper sequence of amino acids is assembled. The amino acid strand coils or folds as it is formed. D, The coiled amino acid strand folds again to form a protein molecule with a specific complex shape.
Source: Thibodeau GA, Patton KT. The human body in health and disease. 3rd edn. St Louis: Mosby; 2009.
Mitosis
Mitosis is a type of cell division that results in the formation of genetically identical daughter cells. Before cell division the chromosomes duplicate, and each new cell (called a daughter cell) receives an exact replica of the chromosomes from the original cell (called the parent cell).
Meiosis
Meiosis occurs only in sexual reproductive cells. In meiosis, the number of chromosomes is reduced, resulting in half of the usual number of chromosomes. Therefore, oocytes and sperm contain only a single copy of each chromosome, whereas all other body cells contain duplicates of each chromosome.
In meiosis, a process known as crossing over may occur. Crossing over occurs when genetic material is exchanged between the two chromosomes in the cell. Because one chromosome is from the mother and the other from the father, the recombination from the process of crossing over creates a greater amount of diversity in the genetic make-up of the oocytes and sperm. During meiosis, a pair of chromosomes normally separates. However, sometimes this does not occur completely. Non-disjunction, the failure of the two chromosomes to separate during meiosis, causes an abnormal number of chromosomes. The result is an oocyte or sperm with two copies of the same chromosome, or sometimes a copy of a chromosome is missing. Examples of disorders caused by chromosome abnormalities include Down syndrome and Turner’s syndrome. These disorders are characterised by physical and/or mental defects.
INHERITANCE PATTERNS
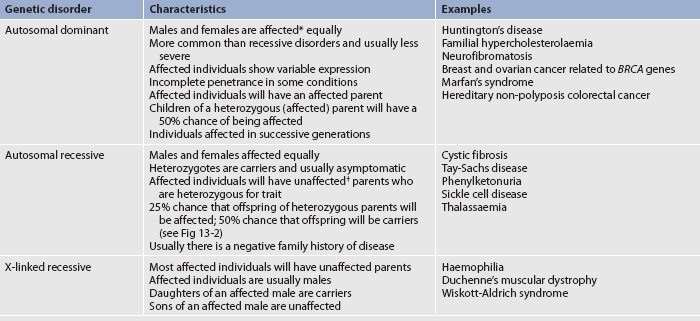
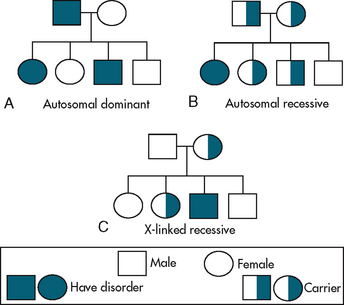
Genetic disorders can be categorised as autosomal dominant, autosomal recessive or sex-linked (X-linked) recessive disorders (see Table 13-2). If the mutant gene is located on an autosome, the genetic disorder is called autosomal. If the mutant gene is on the X chromosome, the genetic disorder is called X-linked. Family pedigrees for autosomal dominant, autosomal recessive and X-linked recessive disorders are shown in Figure 13-2.
Autosomal dominant disorders are caused by a mutation of a single gene pair (heterozygous) on a chromosome. A dominant allele prevails over a normal allele. Autosomal dominant disorders show variable expression. Variable expression means that the symptoms expressed by the individuals with the mutated gene vary from person to person even though they have the same mutated gene. Although autosomal dominant disorders have a high probability of occurring in families, sometimes these disorders cause a new mutation or skip a generation. This is termed incomplete penetrance.
Autosomal recessive disorders are caused by mutations of two gene pairs (homozygous) on a chromosome. A person who inherits one copy of the recessive allele does not develop the disease because the normal allele predominates. However, such a person is a carrier.
X-linked recessive disorders are caused by a mutation on the X chromosome. Usually only men are affected by these disorders because women who carry the mutated gene on one X chromosome have another X chromosome to compensate for the mutation. However, women who carry the mutated gene can transmit the mutated gene to their offspring. It is possible for women to have X-linked recessive disorders, and this can occur when an affected male mates with an unaffected female carrier. This points to the importance of testing the carrier status of the female mate of affected males. X-linked dominant disorders do exist but they are very rare.1
Multifactorial inherited conditions are caused by a combination of genetic and environmental factors. These disorders run in families but do not show the same inherited characteristics as the single-gene mutation conditions. Multifactorial conditions are poorly understood but include diabetes mellitus, obesity, hypertension, cancer and coronary artery disease.
HUMAN GENOME PROJECT
The Human Genome Project (HGP), which was completed in 2003, mapped the human genome (see the Resources on p 276). Analysis of the data will continue for many years. The knowledge gained through the HGP will: (1) help improve the diagnosis of diseases; (2) allow for earlier detection of genetic predisposition to diseases; and (3) play a critical role in determining risk assessment for genetic-related diseases. In addition, the results of the HGP will assist in matching organ donors with transplant recipients.
GENETIC TESTING
Genetic testing includes any procedure done to analyse chromosomes, genes or any gene product that can determine a mutation or a predisposition to a condition. Genetic tests include direct testing, linkage testing, biochemical testing and karyotyping. Direct testing examines the DNA for any mutations. Biochemical testing includes analysing gene products, such as enzymes and proteins. Karyotyping investigates the number, form, size and arrangement of the chromosomes. A blood sample or buccal smear (skin or hair can also be used) is frequently used to obtain samples for genetic testing. Tissues and cells can also be obtained prenatally.
Genetic testing has been very useful in healthcare (see Table 13-3). Some tests are used in diagnosing an illness or a risk for a disorder and provide the basis for appropriate treatments. Other tests allow families to avoid having children with devastating diseases or to identify people who are at high risk of conditions that may be prevented by monitoring or having surgery. For example, aggressive monitoring for and removal of colon growths in those inheriting a gene for familial adenomatous polyposis has saved many lives.
TABLE 13-3 Use of genetic tests
| Type of test | Example and description |
|---|---|
| Carrier screening | Sickle cell, haemophilia. Identifying unaffected individuals who carry one copy of a gene. |
| Preimplantation genetic diagnosis (PGD) | Fertilised embryos tested before implantation. Allows embryos free of particular disorders to be placed into the uterus. Embryos that test positive for genetic disorders can be destroyed. |
| Prenatal diagnostic testing | Fluid from amniocentesis or tissue from chorionic villus (placenta) used to obtain fetal cells. The tissue has the same genetic make-up as the fetus. |
| Newborn screening | Phenylketonuria. Allows for early diagnosis and treatment by diet. |
| Presymptomatic testing for predicting adult-onset disorders | Huntington’s disease, adult polycystic kidney disease. Codominant genetic disorders that have their onset in adulthood. |
| Presymptomatic testing for estimating the risk of developing disorder | Genetic testing for BRCA1 and BRCA2 mutations in women at risk for developing breast cancer. Testing allows for prophylactic measures (e.g. Performing a mastectomy or oophorectomy) to prevent the development of cancer. |
| Confirmational diagnosis of a symptomatic individual | Used to confirm findings when the patient’s signs and symptoms suggest a genetic disorder. |
| Identity testing | Paternity testing |
CLINICAL PRACTICE
Situation
A 30-year-old woman informs you that she is 3 months pregnant. She has two children with her current husband and her youngest child has cystic fibrosis (CF). This pregnancy was unplanned. She expresses concern regarding the possibility of having another child with CF. She mentions that she would like to have genetic testing on her fetus. Her husband asks you whether they will have another child with CF.
Important points for consideration
• With complete and accurate information, the woman and her husband can make a decision on their own, without coercion from others.
• Genetic counselling is a requirement before and after obtaining genetic testing because of the complexity of the information and the emotional issues involved.
• Knowing that CF is an autosomal recessive condition, the nurse can use Punnett squares (see Fig 13-3) or a family pedigree (see Fig 13-2) to show the woman and her husband the probability of having another child with CF.
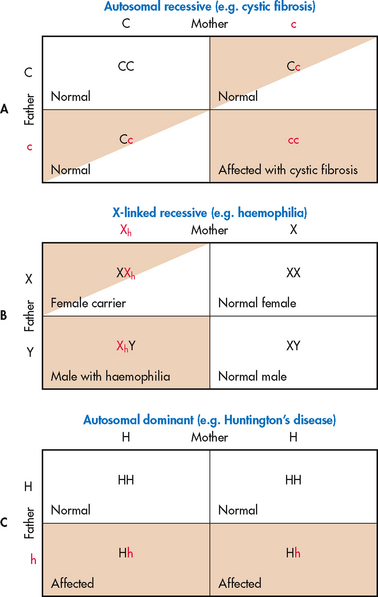
Figure 13-3 Punnett squares can be used to determine inheritance possibilities. A, If the mother and father are both carriers for cystic fibrosis, there is a 25% chance that their offspring will have cystic fibrosis. B, If the mother is a carrier for the haemophilia gene and the father has a normal genotype, there is a 50% chance that any male offspring will have haemophilia. There is a 50% chance that any female offspring will be a carrier. C, If the mother has a normal genotype and the father has Huntington’s disease, there is a 50% chance that their offspring will have the disease.
Critical thinking questions
1. What information would you give the patient regarding genetic testing in order for this couple to make a decision?
2. What options are available for this couple?
3. How would you assist this couple in making a decision about possibly terminating the pregnancy based on the results of the genetic testing?
At the same time, genetic testing of individuals opens the door for ethical and social issues. People making decisions about genetic testing should be aware that when test results are placed in their medical records, the results may not be kept private. If an individual is tested, it may uncover information about a family member who was not tested and these individuals are frequently not a part of the decision to undergo testing. Similarly, if a whole family is tested, the results may indicate that the biological relationship is not what the family believed it to be.
Widespread genetic testing has limitations. It is difficult to interpret a positive result because some people who have the genetic mutation never develop the disease. For example, many people who would test positive for the apolipoprotein E gene (ApoE-4) will never develop Alzheimer’s disease (see Ch 59).
Populations of people may be tested for multiple reasons. It may be a public health matter that propels the testing, such as the practice of testing newborn children. In Australia and New Zealand, all newborns are offered testing for phenylketonuria, congenital hypothyroidism, cystic fibrosis, galactosaemia and some rare metabolic disorders. Also, population testing may be performed as a matter of practice, such as the testing offered to all prenatal women. These group testings identify a treatable disease before the onset of symptoms, which can be especially helpful in diseases where early identification improves the outcome.2
The website of the Royal Australian College of Pathologists has more information about genetic tests available in Australia,3 and the National Institute of Health in the US maintains useful information about genetic testing on the GeneTests website (see the Resources on p 276).4
GENE THERAPY
Gene therapy is an experimental technique that is used to replace or repair defective or missing genes with normal genes. A normal gene can be inserted into a human chromosome to counteract the effects of a missing or abnormal gene. A carrier molecule called a vector must be used to deliver the therapeutic gene to the patient’s target cells. Currently, the most common vector is a virus that has been genetically altered to carry normal human DNA. The vector unloads its genetic material containing the therapeutic human gene into the target cell. The functional protein product from the therapeutic gene restores the target cell to a normal state. Although gene therapy is a promising treatment option for a number of diseases (including inherited disorders, some types of cancer and certain viral infections), the technique remains risky and is still under study to make sure that it is safe and effective. Gene therapy is currently being tested only for the treatment of diseases that have no other cure.5
Genetic discrimination
• Genetic discrimination is a term used to describe the differential treatment of individuals or their relatives on the basis of their actual or perceived genetic make-up. A person’s genetic make-up may be identified by DNA testing. It may also be inferred from the family’s health history.
• In Australia and New Zealand, discrimination on the grounds of genetic status is dealt with under the framework of existing Commonwealth, state and territory anti-discrimination laws.
• The issue of using genetic information for health insurance does not apply in Australia, as health insurance is community-rated—that is, people pay the same premium regardless of their personal or family health history or genetic test results, a situation similar to that in the UK and Canada.
• However, genetic information may be taken into account when applying for life insurance products such as cover for death or income protection, because these types of insurance are risk-rated. However, any risks calculated by the insurer that determine premium costs would have to be substantiated actuarially. In Australia, the insurance industry has agreed that it will not require people to have DNA tests before taking out life insurance, but individuals who have had a DNA test must report the results in their life insurance application.
Source: National Health and Medical Research Council. Genetic discrimination. Available at www.nhmrc.gov.au/node/329, accessed 25 March 2011.
 NURSING MANAGEMENT: GENETICS
NURSING MANAGEMENT: GENETICS
It is imperative that nurses are knowledgeable about the fundamentals of genetics. By understanding the profound influence that genetics has on health and disease, the nurse can assist the patient and family in making critical decisions related to genetic issues, such as genetic testing. In addition, the nurse needs to collaborate with the healthcare team or doctor to involve a genetic counsellor. The nurse should be able to give patients and families accurate information pertaining to genetics, genetic diseases and probabilities of genetic disorders.6,7
Nurses can assess inheritance patterns and explain them to the patient and family through the use of family pedigrees (see Fig 13-2) and Punnett squares (see Fig 13-3). It is important to maintain the patient’s confidentiality and respect the patient’s values and beliefs because genetic information may have major health and social implications.
Information related to various genetic disorders can be found in the following boxes throughout the book
HEALTH DISPARITIES
| Genetic disorder | Chapter |
|---|---|
| α-1 antitrypsin (AAT) deficiency | 28 |
| Alzheimer’s disease | 59 |
| Ankylosing spondylitis | 64 |
| Breast cancer | 51 |
| Cancer | 15 |
| Cystic fibrosis | 28 |
| Duchenne’s muscular dystrophy | 63 |
| Familial adenomatous polyposis (FAP) | 42 |
| Familial hypercholesterolaemia | 33 |
| Haemochromatosis | 30 |
| Haemophilia A and B | 30 |
| Hereditary non-polyposis colorectal cancer (HNPCC) | 42 |
| Huntington’s disease | 58 |
| Ovarian cancer | 53 |
| Polycystic kidney disease | 45 |
| Sickle cell disease | 30 |
| Types 1 and 2 diabetes mellitus | 48 |
Genetic testing may raise many psychological issues. Knowing that they are the carrier of a genetic disorder may influence people’s career plans and decisions for marriage and childbearing. It may also affect significant others in grappling with serious life and healthcare issues. For example, how should a husband deal with his wife who has tested positive for Huntington’s disease and shows early signs of cognitive impairment but does not yet show any other neurological manifestations of the disease?
Furthermore, there are ethical concerns. Who should know the results of a genetic test? Who should protect individuals’ privacy of test results and protect individuals from discrimination? Genetic information should not be misused to stigmatise individuals or particular groups. Attention must be paid to understanding the psychosocial needs of individuals and societal responses and healthcare policy related to genetic testing. People may be reluctant to share or disclose information about family history or genetic test results. They may fear that they are potentially vulnerable to discrimination based on their DNA. Nurses can provide information to patients about where to turn for help to discuss concerns about discrimination. Resources on genetics for nurses and nurse educators are available on p 276.
Stem cells
Stem cells are the subject of much discussion because they may offer treatment for many chronic illnesses. The use of stem cells may allow the regeneration of lost tissue and the restoration of function in various diseases. Stem cells are cells in the body that have the ability to divide and (1) remain a stem cell or (2) differentiate into specialised cells such as a brain cell or a muscle cell. In some body organs such as the gastrointestinal (GI) tract and bone marrow, stem cells divide to repair and replace damaged or old tissues. In other organs such as the pancreas and heart, stem cells divide only under special conditions.
Stem cells can be divided into two types: embryonic and adult (or somatic). Embryonic stem cells have the ability to become any one of the hundreds of types of cells in the human body. They are derived from human embryo cells that are 4–5 days old. These stem cells are pluripotent and can differentiate into any cell type that they are stimulated to become.
Adult stem cells are undifferentiated cells that are found in small numbers in many adult organs and tissues including the brain, bone marrow, peripheral blood, blood vessels, skeletal muscle, skin, teeth, heart, gut, liver, ovarian epithelium and testis. They are thought to reside in a specific area of each tissue called a stem cell niche. The primary roles of adult stem cells are to maintain and repair tissues in which they are found. They are usually thought of as multipotent cells, giving rise to a closely related family of cells within the tissue. For example, skin stem cells produce new skin cells. Haematopoietic stem cells found within the bone marrow are capable of forming all the various cells in blood. These cells are prolific by design and are already being used for bone marrow transplants (see Ch 15).
Perhaps the most important potential application of human stem cells is the generation of tissues that could be used for cell-based therapies. Currently, there are not enough available donated organs to meet the demand for transplants. In addition, stem cells could be directed to differentiate into specific cell types. They would then become a renewable source of replacement cells and tissues to treat diseases such as Alzheimer’s disease, spinal cord injury, stroke, burns, heart disease, diabetes, osteoarthritis and rheumatoid arthritis. For example, it may become possible to generate healthy heart muscle cells in the laboratory and then transplant those cells into patients with chronic heart disease. Whether these cells can generate heart muscle cells or stimulate the growth of new blood vessels is under investigation.8
Normal immune response
Immunity is a state of responsiveness to foreign substances, such as microorganisms and tumour proteins.9 Immune responses serve three functions:
1. Defence. The body protects against invasions by microorganisms and prevents the development of infection by attacking foreign antigens and pathogens.
2. Homeostasis. Damaged cellular substances are digested and removed. Through this mechanism the body’s different cell types remain uniform and unchanged.
3. Surveillance. Mutations continually arise in the body but are normally recognised as foreign cells and destroyed.
TYPES OF IMMUNITY
Immunity is classified as innate (natural) or acquired. Innate immunity exists in a person without prior contact with an antigen. It is present at birth and its primary role is first-line defence against pathogens. This type of immunity involves a non-specific response, and neutrophils and monocytes are the primary white blood cells (WBCs) involved. Innate immunity is not antigen-specific so it can respond within minutes to an invading microorganism without prior exposure to that organism. Acquired immunity is the development of immunity, either actively or passively (see Box 13-1).
Active acquired immunity
Active acquired immunity results from the invasion of the body by foreign substances, such as microorganisms, and the subsequent development of antibodies and sensitised lymphocytes. With each reinvasion of the microorganisms, the body responds more rapidly and vigorously to fight off the invader. Active acquired immunity may result naturally from a disease or artificially through inoculation with a less virulent antigen (e.g. immunisations). Because antibodies are synthesised, immunity takes time to develop but is long lasting.
HEALTH PROMOTION
• Can help control the spread of infections within communities.
• Can prevent disability and death from infectious disease for individuals.
• Reduces and can possibly eliminate polio, measles and other diseases with widespread use.
Note: The Australian government provides free immunisation against pneumococcal disease and a yearly immunisation against influenza for adults aged 65 years and older. These immunisations are also provided free for Indigenous people aged over 50 years and for those aged 15–49 years who are at high risk of these diseases and their complications.
Passive acquired immunity
Passive acquired immunity implies that the host receives antibodies to an antigen rather than synthesising them. This may take place naturally through the transfer of immunoglobulins across the placental membrane from mother to fetus. Artificial passive acquired immunity occurs through injection with gamma-globulin (serum antibodies). The benefit of this immunity is its immediate effect. Unfortunately, passive immunity is short lived because the host did not synthesise the antibodies and consequently does not retain memory cells for the antigen.
ANTIGENS
An antigen is a substance that elicits an immune response. Most antigens are composed of protein. However, other substances, such as large polysaccharides, lipoproteins and nucleic acids, can act as antigens. All of the body’s cells have antigens on their surface that are unique to that person and enable the body to recognise self. The immune system becomes ‘tolerant’ to the body’s own molecules and therefore is normally non-responsive to self-antigens.
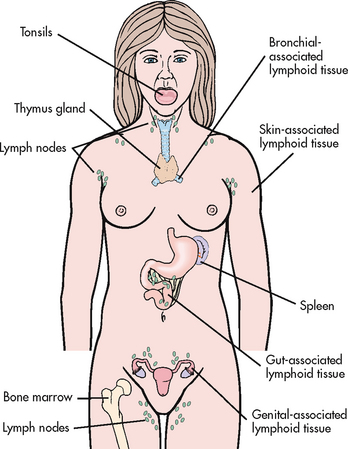
LYMPHOID ORGANS
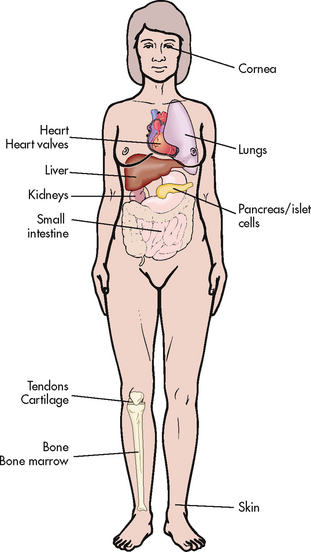
The lymphoid system is composed of central (or primary) and peripheral lymphoid organs. The central lymphoid organs are the thymus gland and bone marrow. The peripheral lymphoid organs are the tonsils; gut-, genital-, bronchial- and skin-associated lymphoid tissues; lymph nodes; and spleen (see Fig 13-4).
Lymphocytes are produced in the bone marrow and eventually migrate to the peripheral organs. The thymus is important in the differentiation and maturation of T lymphocytes and is therefore essential for a cell-mediated immune response. During childhood the gland is large, but it shrinks with age and is a collection of reticular fibres, lymphocytes and connective tissue in older people.
Lymphoid tissue is found in the submucosa of the respiratory (bronchial-associated), genitourinary (genital-associated) and gastrointestinal (gut-associated) tracts. This tissue protects the body from external microorganisms. The tonsils are a typical example of lymphoid tissue.
The skin-associated lymphoid tissue primarily consists of lymphocytes and Langerhans’ cells (a type of resident macrophage) found in the epidermis of skin. When Langerhans’ cells are depleted, the skin can neither initiate an immune response nor support a skin-localised delayed hypersensitivity response.
When antigens are introduced into the body, they may be carried by the bloodstream or lymph channels to regional lymph nodes. The antigens interact with B and T lymphocytes and macrophages in the lymph nodes. The two important functions of lymph nodes are: (1) filtration of foreign material brought to the site; and (2) circulation of lymphocytes.
The spleen is important as the primary site for filtering foreign substances from the blood. It consists of two kinds of tissue: white pulp containing B and T lymphocytes, and red pulp containing erythrocytes. Macrophages line the pulp and sinuses of the spleen.
CELLS INVOLVED IN THE IMMUNE RESPONSE
Mononuclear phagocytes
The mononuclear phagocyte system includes monocytes in the blood and macrophages found throughout the body. Mononuclear phagocytes have a critical role in the immune system. They are responsible for capturing, processing and presenting the antigen to the lymphocytes. This stimulates a humoral or cell-mediated immune response. Capturing is accomplished through phagocytosis. The macrophage-bound antigen, which is highly immunogenic, is presented to circulating T or B lymphocytes and thus triggers an immune response (see Fig 13-5).
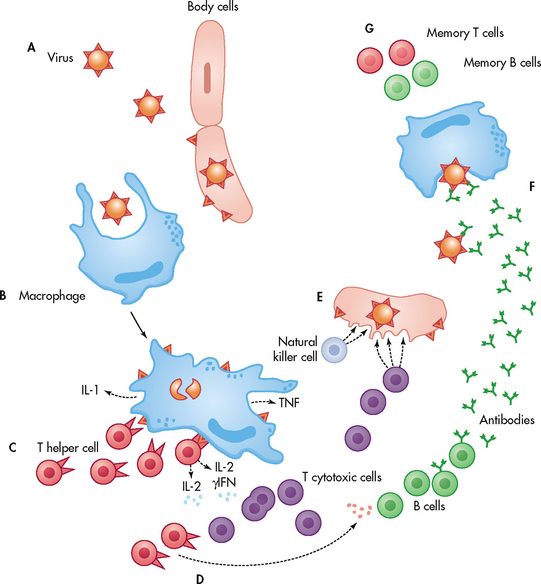
Figure 13-5 The immune response to a virus. A, A virus invades the body through a break in the skin or another portal of entry. The virus must make its way inside a cell in order to replicate itself. B, A macrophage recognises the antigens on the surface of the virus. The macrophage digests the virus and displays pieces of the virus (antigens) on its surface. C, A T helper cell recognises the antigen displayed and binds to the macrophage. This binding stimulates the production of cytokines (interleukin-1 [IL-1] and tumour necrosis factor [TNF]) by the macrophage and interleukin-2 (IL-2) and gamma-interferon (γIFN) by the T cell. These cytokines are intracellular messengers that provide communication between the cells. D, IL-2 instructs other T helper cells and T cytotoxic cells to proliferate (multiply). T helper cells release cytokines, causing B cells to multiply and produce antibodies. E, T cytotoxic cells and natural killer cells destroy infected body cells. F, The antibodies bind to the virus and mark it for macrophage destruction. G, Memory B and T cells remain behind to respond quickly if the same virus attacks again.
Lymphocytes
Lymphocytes are produced in the bone marrow and lymph nodes (see Fig 13-6). They then differentiate into B lymphocytes and T lymphocytes.
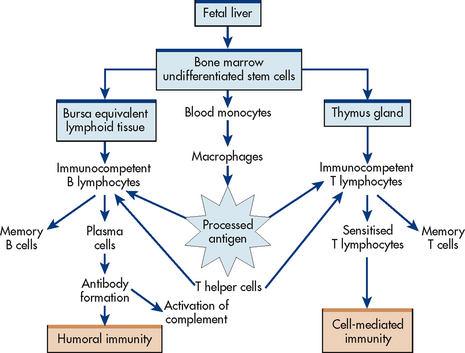
Figure 13-6 Relationships and functions of macrophages, B lymphocytes and T lymphocytes in an immune response.
B lymphocytes
In the early research on B lymphocytes (bursa-equivalent lymphocytes) in birds, it was discovered that they mature under the influence of the bursa of Fabricius; hence the name of B cells. However, this lymphoid organ does not exist in humans. The bursa-equivalent tissue in humans is the bone marrow. B cells differentiate into plasma cells when activated. Plasma cells produce antibodies (immunoglobulins) (see Table 13-4).
T lymphocytes
Cells that migrate from the bone marrow to the thymus differentiate into T lymphocytes (thymus-dependent cells). The thymus secretes hormones, including thymosin, which stimulate the maturation and differentiation of T lymphocytes. T cells comprise 70–80% of the circulating lymphocytes and are primarily responsible for immunity to intracellular viruses, tumour cells and fungi. T cells live from a few months to the life span of an individual and account for long-term immunity.
T lymphocytes can be categorised into T cytotoxic cells and T helper cells. Antigenic characteristics of WBCs have now been classified using monoclonal antibodies. These antigens are classified as clusters of differentiation, or CD antigens. Many types of WBCs, especially lymphocytes, are referred to by their CD designations. All mature T cells have the CD3 antigen.
T cytotoxic cells
T cytotoxic (or cytolytic) (CD8) cells are involved in attacking antigens on the cell membrane of a foreign pathogen and releasing cytolytic substances that destroy the pathogen. These cells have antigen specificity and are sensitised by exposure to the antigen. Similar to B lymphocytes, some sensitised T cells do not attack the antigen but remain as memory T cells. As in the humoral immune response, a second exposure to the antigen will result in a more intense and rapid cell-mediated immune response.
T helper cells
T helper (CD4) cells are involved in the regulation of cell-mediated immunity and the humoral antibody response. T helper cells differentiate into subsets of cells that produce distinct types of cytokines (discussed in the next section). These subsets are called TH1 cells and TH2 cells. TH1 cells stimulate phagocyte-mediated ingestion and killing of microbes, the key component of cell-mediated immunity. TH2 cells stimulate phagocyte-independent, eosinophil-mediated immunity, which is effective against parasites, and are involved in allergic responses.
Natural killer cells
Natural killer (NK) cells are also involved in cell-mediated immunity. These cells are not T or B cells but are large lymphocytes with numerous granules in the cytoplasm. NK cells do not require prior sensitisation for their generation. These cells are involved in recognition and killing of virus-infected cells, tumour cells and transplanted grafts. The mechanism of recognition is not fully understood. NK cells have a significant role in immune surveillance for malignant cell changes.
Dendritic cells
Dendritic cells make up a system of cells that are important to the immune system, especially the cell-mediated immune response. They have an atypical shape with extensive dendritic processes that form and retract. They are found in many parts of the body, including the skin (where they are called Langerhans’ cells) and the lining of the nose, lungs, stomach and intestine. Especially in the immature state, they are found in the blood. They primarily function to capture antigens at sites of contact with the external environment (e.g. skin, mucous membranes) and then transport the antigen until it encounters a T cell with specificity for the antigen. In this role, dendritic cells can have an important function in activating the immune response.
CYTOKINES
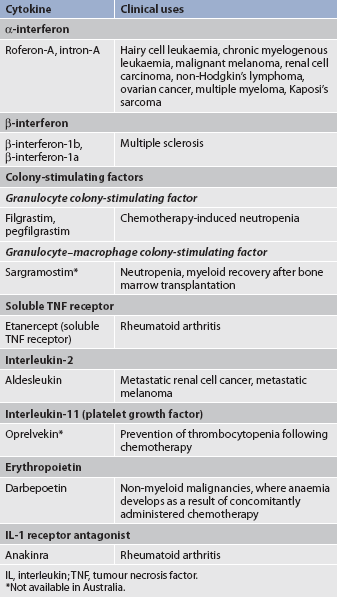
The immune response involves complex interactions of T cells, B cells, monocytes and neutrophils. These interactions depend on cytokines (soluble factors secreted by WBCs and a variety of other cells in the body), which act as messengers between the cell types. Cytokines instruct cells to alter their proliferation, differentiation, secretion or activity. There are currently more than 100 different known cytokines, and they can be classified into distinct categories. Some of these cytokines are listed in Table 13-5. In general, the interleukins act as immunomodulatory factors, colony-stimulating factors act as growth-regulating factors for haematopoietic cells, and interferons are antiviral and immunomodulatory.
TABLE 13-5 Types and functions of cytokines*
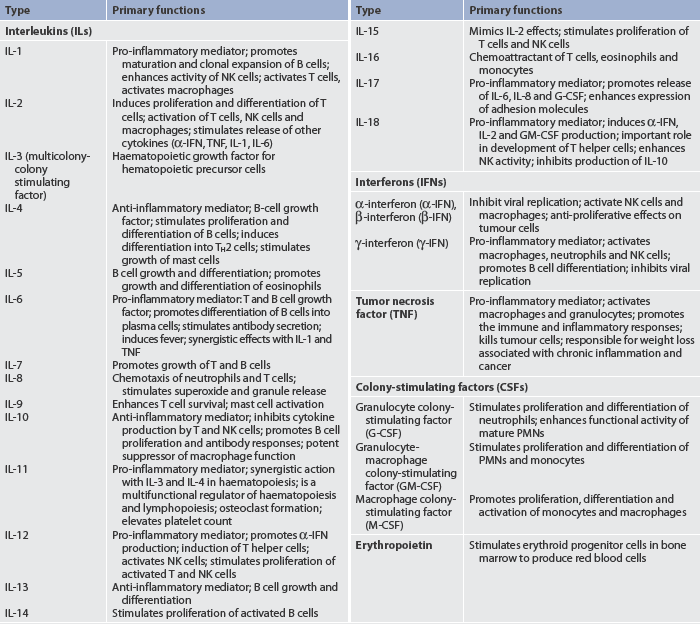
NK, natural killer; PMN, polymorphonuclear neutrophil.
* A more comprehensive presentation of cytokines is available at www.rndsystems.com/molecule_group.aspx?g=704&r=4.
The net effect of an inflammatory response is determined by a balance between pro-inflammatory and anti-inflammatory mediators. Sometimes cytokines are classified as pro-inflammatory or anti-inflammatory (see Table 13-5). However, it is not that clear-cut, as many other factors (e.g. target cells, environment) influence the inflammatory response to a given injury or insult.
Cytokines have a beneficial role in haematopoiesis and immune function. They can also have detrimental effects, such as those seen in chronic inflammation, autoimmune diseases and sepsis. Cytokines such as erythropoietin (see Ch 46), colony-stimulating factors (see Table 15-15), interferons (see Table 15-14) and interleukin-2 (see Table 15-14) are used clinically to: (1) stimulate haematopoiesis; (2) stimulate the bone marrow to make WBCs; and (3) treat various malignancies. In addition, inhibitors of cytokines, such as soluble tumour necrosis factor receptor antagonist and interleukin-1, are being used in clinical trials as anti-inflammatory agents. Clinical uses of cytokines are listed in Table 13-6.
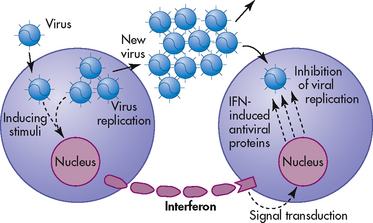
Interferon helps the body’s natural defences attack tumours and viruses. Three types of interferon have now been identified (see Table 13-5). In addition to their direct antiviral properties, interferons have immunoregulatory functions. These include enhancement of NK cell production and activation and inhibition of tumour cell growth. Interferon is not directly antiviral but produces an antiviral effect in cells by reacting with them and inducing the formation of a second protein termed antiviral protein (see Fig 13-7). This protein mediates the antiviral action of interferon by altering the cell’s protein synthesis and preventing new viruses from becoming assembled.
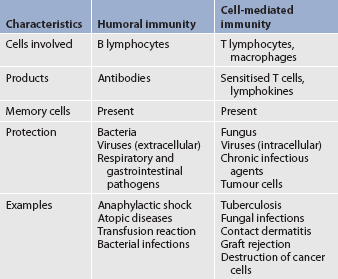
COMPARISON OF HUMORAL AND CELL-MEDIATED IMMUNITY
Humans need both humoral and cell-mediated immunity to remain healthy. Each type of immunity has unique properties and different methods of action, and reacts against particular antigens. Table 13-7 compares humoral and cell-mediated immunity.
Humoral immunity
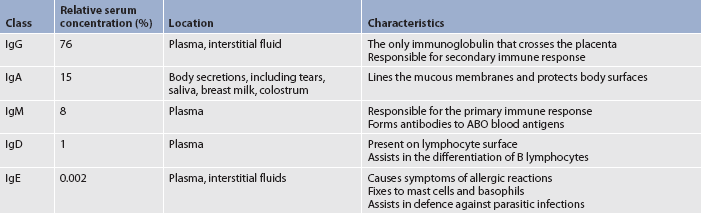
Humoral immunity is antibody-mediated immunity. The term humoral comes from the Greek word humor, which means body fluid. Antibodies are produced by plasma cells (differentiated B cells) and found in plasma; therefore, the term humoral immunity is used. Production of antibodies is an essential component in a humoral immune response. Each of the five classes of immunoglobulins—IgG, IgA, IgM, IgD and IgE—has specific characteristics (see Table 13-4).
When a pathogen (especially bacteria) enters the body, it may encounter a B lymphocyte that is specific for antigens located on that bacterial cell wall. In addition, a monocyte or macrophage may phagocytise bacteria and present its antigens to a B lymphocyte. The B lymphocyte recognises the antigen because it has receptors on its cell surface that are specific for that antigen. When the antigen comes in contact with the cell surface receptor, the B cell becomes activated, and most B cells will differentiate into plasma cells (see Fig 13-6). The mature plasma cell secretes immunoglobulins. Some stimulated B lymphocytes remain as memory cells.
The primary immune response develops in 4–8 days after the initial exposure to the antigen (see Fig 13-8). IgM is the first type of antibody formed. Because of the large size of the IgM molecule, this immunoglobulin is confined to the intravascular space. As the immune response progresses, IgG is produced and can move from intravascular to extravascular spaces.
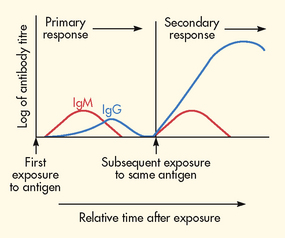
Figure 13-8 Primary and secondary immune responses. The introduction of antigen induces a response dominated by two classes of immunoglobulins, IgM and IgG. IgM predominates in the primary response, with some IgG appearing later. After the host’s immune system is primed, another challenge with the same antigen induces the secondary response, in which some IgM and large amounts of IgG are produced.
When an individual is exposed to an antigen a second time, a secondary antibody response occurs, which is faster (1–3 days), stronger and lasts for longer than the primary response. Memory cells account for the memory of the first exposure to the antigen and the more rapid production of antibodies. IgG is the primary antibody formed in the secondary immune response.
IgG crosses the placental membrane and provides the newborn with passive acquired immunity for at least 3 months. Infants may also have some passive immunity from IgA in breast milk and colostrum.
Gerontological considerations: effects of ageing on the immune system
With advancing age there is a decline in the immune system (see Box 13-2). The primary clinical evidence for this immunosenescence is the high incidence of tumours in older adults. In addition, a greater susceptibility occurs to infections (e.g. influenza, pneumonia) from pathogens that the older person had been relatively immunocompetent against earlier in life. Bacterial pneumonia is the leading cause of death from infections in older adults. The antibody response to immunisations (e.g. flu vaccine) in older adults is considerably lower than in younger adults.10
Immunoglobulin levels decrease with age and therefore lead to a suppressed humoral immune response in older adults. Thymic involution (shrinking) occurs with ageing along with decreased numbers of T cells. These changes in the thymus are probably a primary cause of immunosenescence. Both T and B cells show deficiencies in activation, transit time through the cell cycle and subsequent differentiation. However, the most significant alterations involve T cells. As thymic output of T cells diminishes, the differentiation of T cells increases. Consequently, there is an accumulation of memory cells rather than new precursor cells responsive to previously unencountered antigens.
The delayed hypersensitivity response, as determined by skin testing with injected antigens, is frequently decreased or absent in older adults. This altered response reflects anergy (an immunodeficient condition characterised by lack of or diminished reaction to an antigen or a group of antigens). The clinical consequences of a decline in cell-mediated immunity are evident.
Cell-mediated immunity
Immune responses that are initiated through specific antigen recognition by T cells are termed cell-mediated immunity. Although these reactions were initially considered to be solely mediated by T cells, several cell types and factors are involved in cell-mediated immunity. The cell types involved include T lymphocytes, macrophages and NK cells. Cell-mediated immunity is of primary importance in: (1) immunity against pathogens that survive inside cells, including viruses and some bacteria (e.g. Mycobacterium); (2) fungal infections; (3) rejection of transplanted tissues; (4) contact hypersensitivity reactions; and (5) tumour immunity.
Altered immune response
Immunocompetence exists when the body’s immune system can identify and inactivate or destroy foreign substances. When the immune system is incompetent or underresponsive, severe infections, immunodeficiency diseases and malignancies may occur. When the immune system overreacts, hypersensitivity disorders such as allergies and autoimmune diseases may occur.
HYPERSENSITIVITY REACTIONS
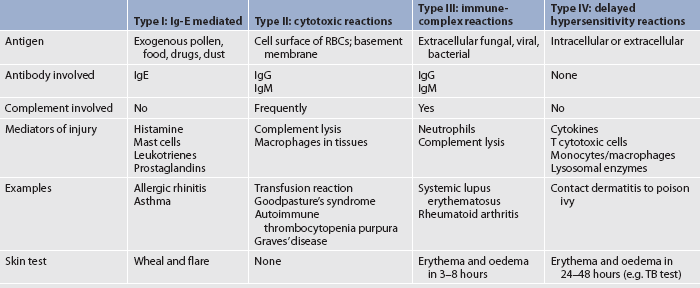
Sometimes the immune response is overreactive against foreign antigens or fails to maintain self-tolerance and this results in tissue damage. This is termed a hypersensitivity reaction. Autoimmune diseases, a type of hypersensitivity response, occur when the body fails to recognise self-proteins and reacts against self-antigens. Classification of hypersensitivity reactions may be done according to the source of the antigen, the time sequence (immediate or delayed) or the basic immunological mechanisms causing the injury. Basically, four types of hypersensitivity reactions occur. Types I, II and III are immediate and are examples of humoral immunity. Type IV is a delayed hypersensitivity reaction and is related to cell-mediated immunity. Table 13-8 summarises the four types of hypersensitivity reactions.
Type I: Ig-E mediated reactions
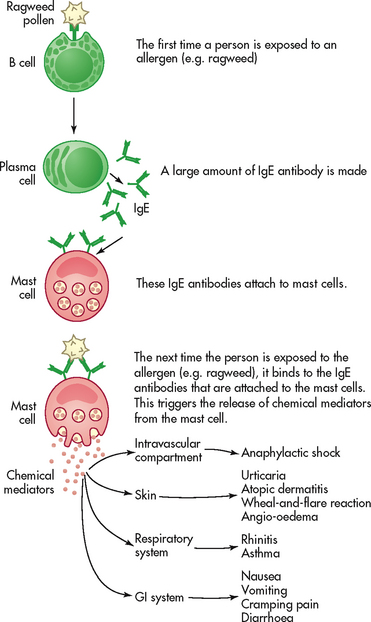
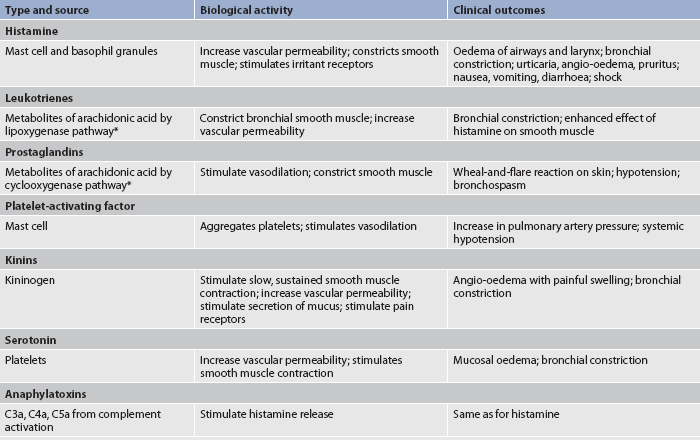
Anaphylactic reactions are type I reactions that occur only in susceptible persons who are highly sensitised to specific allergens. IgE antibodies, which are produced in response to the allergen, have a characteristic property of attaching to mast cells and basophils (see Fig 13-9). Within these cells are granules containing potent chemical mediators (histamine, serotonin, eosinophil chemotactic factor of anaphylaxis [ECF-A], kinins and bradykinin). (Chemical mediators of inflammation are discussed in Ch 12.) On the first exposure to the allergen, IgE antibodies are produced and bind to mast cells and basophils. On any subsequent exposures, the allergen links with the IgE bound to mast cells or basophils and triggers degranulation of the cells and the release of chemical mediators from the granules. In this process, the mediators that are released bind to target organs, causing clinical allergy symptoms. These effects include smooth muscle contraction, increased vascular permeability, vasodilation, hypotension, increased secretion of mucus and itching. Fortunately, the mediators are short acting and their effects are reversible. (The mediators and their effects are summarised in Table 13-9.)
A genetic predisposition to the development of allergic diseases exists.11 The capacity to become sensitised to an allergen appears to be the inherited trait rather than the specific allergic disorder. For example, a father with asthma may have a son who has allergic rhinitis.
The clinical manifestations of an anaphylactic reaction depend on whether the mediators remain local or become systemic or whether they affect particular organs. When the mediators remain localised, a cutaneous response termed the wheal-and-flare reaction occurs. This reaction is characterised by a pale wheal containing oedematous fluid surrounded by a red flare from the hyperaemia. The reaction occurs in minutes or hours and is usually not dangerous. A classic example of a wheal-and-flare reaction is the mosquito bite. The wheal-and-flare reaction serves a diagnostic purpose as a means of demonstrating allergic reactions to specific allergens during skin tests.
Common allergic reactions include anaphylaxis and atopic reactions.
Anaphylaxis
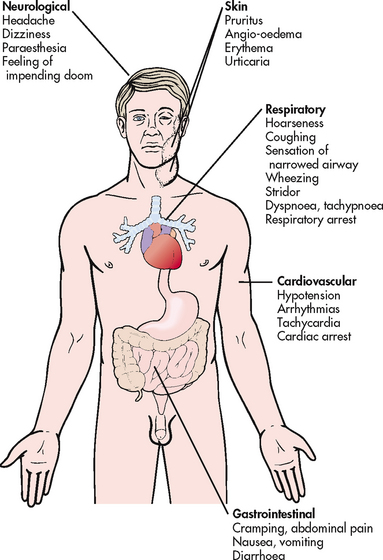
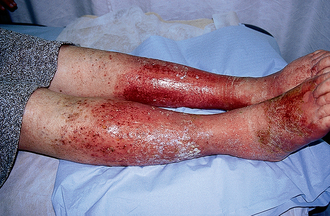
Anaphylaxis can occur when mediators are released systemically (e.g. after injection of a drug, after an insect sting). The reaction occurs within minutes and can be life-threatening because of bronchial constriction and subsequent airway obstruction and vascular collapse.12,13 The target organs affected are shown in Figure 13-10. Initial symptoms include oedema and itching at the site of the exposure to the allergen. Shock can occur rapidly and is manifested by rapid weak pulse, hypotension, dilated pupils, dyspnoea and possibly cyanosis. This is compounded by bronchial oedema and angio-oedema. Death will occur if emergency treatment is not initiated. Some of the important allergens leading to anaphylactic shock in hypersensitive persons are listed in Box 13-3.
Atopic reactions
An estimated 20% of the population is atopic, having an inherited tendency to become sensitive to environmental allergens. The atopic diseases that can result are allergic rhinitis, asthma, atopic dermatitis, urticaria and angio-oedema.
Allergic rhinitis, or hay fever, is the most common type I hypersensitivity reaction. It may occur year-round (perennial allergic rhinitis) or it may be seasonal (seasonal allergic rhinitis). Airborne substances such as pollens, dust or moulds are the primary cause of allergic rhinitis. Perennial allergic rhinitis may be caused by dust, moulds and animal dander. Seasonal allergic rhinitis is commonly caused by pollens from trees, weeds or grasses. The target areas affected are the conjunctiva of the eyes and the mucosa of the upper respiratory tract. Symptoms include nasal discharge, sneezing, lacrimation, mucosal swelling with airway obstruction, and pruritus around the eyes, nose, throat and mouth. (Treatment of allergic rhinitis is discussed in Ch 26.)
Many patients with asthma have an allergic component to their disease. These patients frequently have a history of atopic disorders (e.g. infantile eczema, allergic rhinitis, food intolerances). Inflammatory mediators produce bronchial smooth muscle constriction, excessive secretion of viscoid mucus, oedema of the mucous membranes of the bronchi and decreased lung compliance. Because of these physiological alterations, patients manifest dyspnoea, wheezing, coughing, tightness in the chest and thick sputum. (Pathophysiology and management of asthma are discussed in Ch 28.)
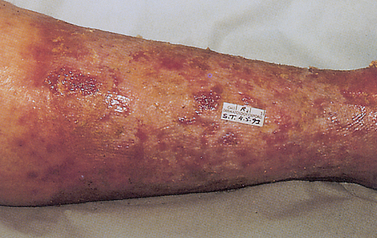
Atopic dermatitis is a chronic, inherited skin disorder characterised by exacerbations and remissions.14 It is caused by several environmental allergens that are difficult to identify. Although patients with atopic dermatitis have elevated IgE levels and positive skin tests, the histopathological features do not represent the typical, localised wheal-and-flare type I reactions. The skin lesions are more generalised and involve vasodilation of blood vessels, resulting in interstitial oedema with vesicle formation (see Fig 13-11). (Dermatitis is discussed in Ch 23.)
Urticaria (hives) is a cutaneous reaction against systemic allergens occurring in atopic persons. It is characterised by transient wheals (pink, raised, oedematous, pruritic areas) that vary in size and shape and may occur throughout the body. Urticaria develops rapidly after exposure to an allergen and may last minutes or hours. Histamine causes localised vasodilation (erythema), transudation of fluid (wheal) and flaring. Flaring is due to blood vessels on the edge of the wheal dilating in response to a reaction augmented by the sympathetic nervous system. Histamine is responsible for the pruritus associated with the lesions. (Urticaria is discussed in Ch 23.)
Angio-oedema is a localised cutaneous lesion similar to urticaria but involving deeper layers of the skin and the submucosa. The principal areas of involvement include the eyelids, lips, tongue, larynx, hands, feet, GI tract and genitalia. Swelling usually begins in the face and then progresses to the airways and other parts of the body. Dilation and engorgement of the capillaries secondary to release of histamine cause the diffuse swelling. Welts are not apparent as in urticaria; the outer skin appears normal or has a reddish hue. The lesions may burn, sting or itch and can cause acute abdominal pain if in the GI tract. The swelling may occur suddenly or over several hours and usually lasts for 24 hours.
Type II: cytotoxic and cytolytic reactions
Cytotoxic and cytolytic reactions are type II hypersensitivity reactions involving the direct binding of IgG or IgM antibodies to an antigen on the cell surface. Antigen–antibody complexes activate the complement system, which mediates the reaction. Cellular tissue is destroyed in one of two ways: (1) activation of the complement cascade resulting in cytolysis; or (2) enhanced phagocytosis.
Target cells frequently destroyed in type II reactions are erythrocytes, platelets and leucocytes. Some of the antigens involved are the ABO blood group, Rh factor and drugs. Pathophysiological disorders characteristic of type II reactions include ABO incompatibility transfusion reaction, Rh incompatibility transfusion reaction, autoimmune and drug-related haemolytic anaemias, leucopenias, thrombocytopenias, erythroblastosis fetalis (haemolytic disease of the newborn) and Goodpasture’s syndrome. The tissue damage usually occurs rapidly.
Haemolytic transfusion reactions
A classic type II reaction occurs when a recipient receives ABO-incompatible blood from a donor. Naturally acquired antibodies to antigens of the ABO blood group are in the recipient’s serum but are not present on the erythrocyte membranes (see Table 29-10). For example, a person with type A blood has anti-B antibodies, a person with type B blood has anti-A antibodies, a person with type AB blood has no antibodies, and a person with type O blood has both anti-A and anti-B antibodies.
If the recipient is transfused with incompatible blood, antibodies immediately coat the foreign erythrocytes, causing agglutination (clumping). The clumping of cells blocks small blood vessels in the body, uses existing clotting factors and depletes them, leading to bleeding. Within hours, neutrophils and macrophages phagocytose the agglutinated cells. As complement is fixed to the antigen, cytolysis occurs. Cellular lysis causes the release of haemoglobin into the urine and plasma. In addition, a cytotoxic reaction causes vascular spasms in the kidneys that further block the renal tubules. Acute renal failure can result from the haemoglobinuria. (Blood transfusions are discussed in Ch 30.)
Goodpasture’s syndrome
Goodpasture’s syndrome is a disorder involving the lungs and kidneys. An antibody-mediated autoimmune reaction occurs involving the glomerular and alveolar basement membranes.15 The circulating antibodies combine with tissue antigen to activate complement, which causes deposits of IgG to form along the basement membranes of the lungs or kidneys. This reaction may result in pulmonary haemorrhage and glomerulonephritis. (Goodpasture’s syndrome is discussed in Ch 45.)
Type III: immune-complex reactions
Tissue damage in immune-complex reactions, which are type III reactions, occurs secondary to antigen–antibody complexes. Antigens combine with immunoglobulins of the IgG and IgM classes to form complexes that are too small to be effectively removed by the mononuclear phagocyte system. Therefore, the complexes deposit in tissue or small blood vessels. They cause the fixation of complement and the release of chemotactic factors, which leads to inflammation and destruction of the involved tissue.
Type III reactions may be local or systemic and immediate or delayed. The clinical manifestations depend on the number of complexes and the location in the body. Common sites for deposit are the kidneys, skin, joints, blood vessels and lungs. Severe type III reactions are associated with autoimmune disorders, such as systemic lupus erythematosus (SLE), acute glomerulonephritis and rheumatoid arthritis (RA). (SLE and RA are discussed in Ch 64, and acute glomerulonephritis is discussed in Ch 45.)
Type IV: delayed hypersensitivity reactions
A delayed hypersensitivity reaction—a type IV reaction—is a type of cell-mediated immune response. Although cell-mediated responses are usually protective mechanisms, tissue damage occurs in delayed hypersensitivity reactions. The tissue damage does not occur in the presence of antibodies or complement. Rather, sensitised T lymphocytes attack antigens or release cytokines. Some of these cytokines attract macrophages into the area. The macrophages and enzymes released by them are responsible for most of the tissue destruction. The delayed hypersensitivity response takes 24–48 hours for a reaction to occur.
Clinical examples of a delayed hypersensitivity reaction include contact dermatitis (see Fig 13-12); hypersensitivity reactions to bacterial, fungal and viral infections; and transplant rejections. Some drug sensitivity reactions also fit this category.
Contact dermatitis
Allergic contact dermatitis is an example of a delayed hypersensitivity reaction involving the skin. The reaction occurs when the skin is exposed to substances that easily penetrate the skin to combine with epidermal proteins. The substance is then recognised as antigenic. Over a period of 7–14 days, memory cells form to the antigen. On subsequent exposure to the substance, a sensitised person develops eczematous skin lesions within 48 hours. The most common potentially antigenic substances encountered are metal compounds (e.g. nickel, mercury), rubber compounds, cosmetics and some dyes.
In acute contact dermatitis the skin lesions appear erythematous and oedematous and are covered with papules, vesicles and bullae. The involved area is very pruritic but may also burn or sting. When contact dermatitis becomes chronic, the lesions resemble atopic dermatitis because they are thickened, scaly and lichenified. The main difference between contact dermatitis and atopic dermatitis is that contact dermatitis is localised and restricted to the area exposed to the allergens, whereas atopic dermatitis is usually widespread.
Microbial hypersensitivity reactions
The classic example of a microbial cell-mediated immune reaction is the body’s defence against the tubercle bacillus. Tuberculosis results from invasion of lung tissue by the highly resistant tubercle bacillus. The organism itself does not directly damage the lung tissue. However, antigenic material released from the tubercle bacilli reacts with T lymphocytes, initiating a cell-mediated immune response. The resulting response causes extensive caseous necrosis of the lung. After the initial cell-mediated reaction, memory cells persist, so subsequent contact with the tubercle bacillus or an extract of purified protein from the organism causes a delayed hypersensitivity reaction. This is the basis for the purified protein derivative (PPD) tuberculosis skin test read 48–72 hours after the injection. (Tuberculosis is discussed in Ch 27.)
Allergic disorders
Although an alteration of the immune system may be manifested in many ways, allergies or type I hypersensitivity reactions are seen most frequently.
ASSESSMENT
For a thorough assessment of a patient with allergies, a complete database must be obtained. This consists of a comprehensive patient history, physical examination, diagnostic examination and skin testing for allergens.
A comprehensive history that covers family allergies, past and present allergies, and social and environmental factors is essential. The information can be obtained from the patient or the patient’s carer. Family history, including information about atopic reactions in relatives, is especially important in identifying at-risk patients. The nurse should assess the specific disorder, clinical manifestations and treatments prescribed. Past and present allergies should be noted. Identifying the allergens that may have triggered a reaction is essential to control allergic reactions. Determination of the time of year that an allergic reaction occurs can be a clue to a seasonal allergen. It is also important to obtain information about any over-the-counter or prescription medications used to treat the allergies.
In addition to identifying the allergen, the nurse should obtain information about the clinical manifestations and course of the allergic reaction. If the patient is female, assessment of symptoms during pregnancy, menstruation or menopause may be important. Social and environmental factors, especially the physical environment, are important. Questions about pets, trees and plants on property; pollutants in the air; and floor coverings, house plants, and cooling and heating systems in the home and workplace can provide valuable information about allergens. In addition, a daily or weekly food diary with a description of any untoward reactions is important. Of particular interest is a screening for any reaction to medication. Finally, questions about the patient’s lifestyle and stress level should be reviewed in connection with the appearance of allergic symptoms.
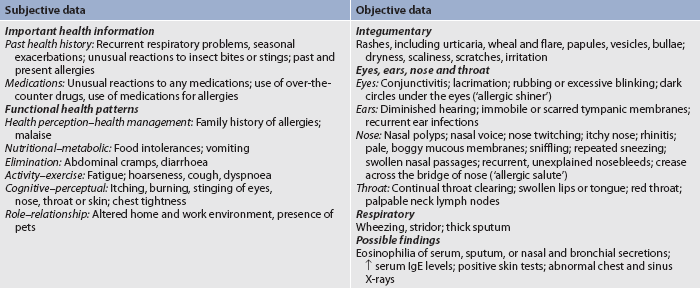
A comprehensive head-to-toe physical examination should be given to a patient with allergies, with particular attention focused on the site of the allergic manifestations. The nurse should obtain a comprehensive assessment that includes subjective and objective data (see Table 13-10).
DIAGNOSTIC STUDIES
Many specialised immunological techniques can be performed to detect abnormalities of lymphocytes, eosinophils and immunoglobulins. A full blood count (FBC) and serology tests are commonly done.
An FBC with a WBC differential is required, with an absolute lymphocyte count and eosinophil count. Cellular immunodeficiency is diagnosed if the lymphocyte count is below 1.2 × 109/L. T cell and B cell quantification is used to diagnose specific immunodeficiency syndromes. The eosinophil count is elevated with type I hypersensitivity reactions involving IgE immunoglobulins. Serum IgE level is also generally elevated in type I hypersensitivity reactions and serves as a diagnostic indicator of atopic diseases.
The radioallergosorbent test (RAST) is an in-vitro diagnostic test for IgE antibodies to specific allergens. It is safe but less sensitive and takes longer than skin tests for detecting allergens. RAST is helpful in confirming reactivity to various foods or drugs in individuals with a history of severe anaphylactic reactions.
Sputum, nasal and bronchial secretions may also be tested for the presence of eosinophils. If asthma is suspected, pulmonary function tests for vital capacity, forced expiratory volume and maximum mid-expiratory flow rates are helpful.
Skin tests
Skin testing is generally used to confirm specific sensitivity in patients with atopic disease after the history has suggested possible allergens for testing. With empirical allergy medications as the treatment of choice for most allergic rhinitis, it has become common practice to omit skin testing for specific allergens in these patients. However, diagnosing an allergy to a specific antigen enables the patient to avoid an allergen and makes them a candidate for immunotherapy. Unfortunately, skin testing cannot be performed on patients who cannot be removed from medications that suppress the histamine response or patients with food allergies.
Procedure
Skin testing may be done by one of three methods: (1) a scratch or prick; (2) an intradermal injection; or (3) a patch test. The areas of the body usually used in testing are the arms and back. Allergen extracts are applied to the skin in rows with a corresponding control site opposite the test site. Saline or another diluent is applied to the control site. In the scratch test the epidermal skin layer is then pricked with a pricking device so that the allergen can enter the skin. In the intradermal method the allergen extract is injected under the skin similar to a PPD test for TB. In the patch test, an allergen is applied to a patch on the skin (see Ch 22). In the scratch and intradermal tests, the reaction occurs in 5–10 minutes. In the patch test, the patches need to be worn for 48–72 hours.
Results
If the person is hypersensitive to the allergen, a positive reaction will occur within minutes after insertion in the skin and may last for 8–12 hours. A positive reaction is manifested by a local wheal-and-flare response. The size of the positive reaction does not always correlate with the severity of allergy symptoms. False-positive and false-negative results may occur. Negative results from skin testing do not necessarily mean the person does not have an allergic disorder, and positive results do not necessarily mean that the allergen was causing the clinical manifestations. Positive results imply that the person is sensitised to that allergen. Therefore, correlating skin test results with the patient’s history is important.
Precautions
A highly sensitive person is always at risk of developing an anaphylactic reaction to skin tests. Therefore, a patient should never be left alone during the testing period. Sometimes skin testing is completely contraindicated and the RAST test is used. If a severe reaction does occur with a cutaneous test, the extract is immediately removed and anti-inflammatory topical cream is applied to the site. For intradermal testing, the arm is used so that a tourniquet can be applied during a severe reaction. A subcutaneous injection of adrenaline may also be necessary.
MULTIDISCIPLINARY CARE
After an allergic disorder is diagnosed, the therapeutic treatment is aimed at reducing exposure to the offending allergen, treating the symptoms and, if necessary, desensitising the person through immunotherapy. All healthcare workers must be prepared for the rare but life-threatening anaphylactic reaction, which requires immediate medical and nursing interventions. It is extremely important that all of a patient’s allergies be listed on the chart, the nursing care plan and the medication record.
Anaphylaxis
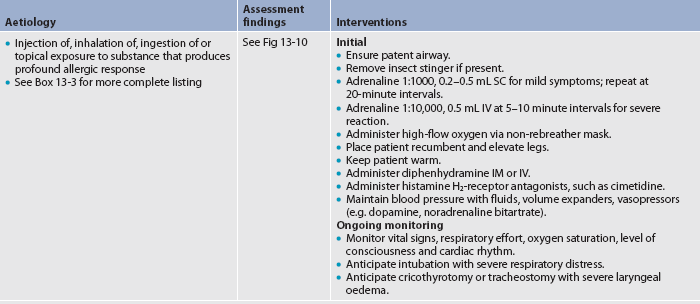
Anaphylactic reactions occur suddenly in hypersensitive patients after exposure to the offending allergen. They may occur following parenteral injection of drugs (especially antibiotics) or blood products and following insect stings. The cardinal principle in therapeutic management is speed in: (1) recognising the signs and symptoms of an anaphylactic reaction; (2) maintaining a patent airway; (3) preventing spread of the allergen by using a tourniquet; (4) administering drugs; and (5) treating for shock. Table 13-11 summarises the emergency treatment of anaphylactic shock.
In severe cases of anaphylaxis, hypovolaemic shock may occur because of the loss of intravascular fluid into interstitial spaces that occurs secondary to increased capillary permeability. Peripheral vasoconstriction and stimulation of the sympathetic nervous system occur to compensate for the fluid shift. However, unless shock is treated early, the body will no longer be able to compensate, and irreversible tissue damage will occur, leading to death. (Hypovolaemic shock is discussed in Ch 66.)
Chronic allergies
Most allergic reactions are chronic and are characterised by remissions and exacerbations of symptoms. Treatment focuses on the identification and control of allergens, the relief of symptoms through drug therapy and hyposensitisation of the patient to the offending allergen.
Allergen recognition and control
The nurse plays an important role in helping the patient to make lifestyle adjustments so that there is minimal exposure to offending allergens. The nurse must reinforce that, even with drug therapy and immunotherapy, the patient will never be desensitised or completely symptom-free. The nurse can initiate various preventative measures that will help control the allergic symptoms.
Of primary importance is the need to identify the offending allergen. Sometimes this is done through skin testing. In the case of food allergies, an elimination diet may be valuable. If an allergic reaction occurs, all foods eaten should be eliminated and gradually reintroduced one at a time until the offending food is detected.
Many allergic reactions, especially asthma and urticaria, may be aggravated by fatigue and emotional stress. The nurse can be instrumental in initiating a stress management program with the patient. Relaxation techniques can be practised when the patient comes for frequent immunotherapy treatments.
Sometimes control of allergic symptoms requires environmental control, including changing an occupation, moving to a different climate or giving up a favourite pet. In the case of airborne allergens, sleeping in an air-conditioned room, damp dusting daily, covering mattresses and pillows with hypoallergenic covers, and wearing a mask outdoors may be helpful.
If the allergen is a drug, the patient should be instructed to avoid the drug. The patient also has the responsibility to make the drug intolerance well known to all healthcare providers. The patient should wear a medical alert bracelet listing the particular drug allergy and have the offending drug listed on all medical and dental records.
For a patient allergic to insect stings, commercial bee-sting kits containing pre-injectable adrenaline and a tourniquet are available. The nurse has the responsibility to instruct the patient about the technique of applying the tourniquet and self-injecting the subcutaneous adrenaline. The patient should also wear a medical alert bracelet and carry a bee-sting kit whenever going outdoors.
Drug therapy
The major categories of drugs used for symptomatic relief of chronic allergic disorders include antihistamines, sympathomimetic drugs, corticosteroids, antipruritic drugs, mast cell-stabilising drugs and leukotriene receptor antagonists. Many of these drugs may be obtained over-the-counter and are often misused by patients.
Antihistamines
Antihistamines are the best drugs for the treatment of allergic rhinitis and urticaria (see Ch 26). They are less effective for severe allergic reactions. They act by competing with histamine for H1-receptor sites and thus block the effect of histamine. Best results are achieved if they are taken as soon as allergy signs and symptoms appear. Antihistamines can be used effectively to treat oedema and pruritus but are relatively ineffective in preventing bronchoconstriction. With seasonal rhinitis, antihistamines should be taken during peak pollen seasons. (Antihistamines are discussed in Ch 26.)
Sympathomimetic drugs
The major sympathomimetic drug is adrenaline, which is the drug of choice to treat an anaphylactic reaction. Adrenaline is a hormone produced by the adrenal medulla that stimulates α- and β-adrenergic receptors. Stimulation of the α-adrenergic receptors causes vasoconstriction of peripheral blood vessels. β-receptor stimulation relaxes bronchial smooth muscles. Adrenaline also acts directly on mast cells to stabilise them against further degranulation. The action of adrenaline lasts only a few minutes. For the treatment of anaphylaxis the drug must be given parenterally (usually subcutaneously).
Several specific, minor sympathomimetic drugs differ from adrenaline because they can be taken orally or nasally and last for several hours. Included in this category are phenylephrine and pseudoephedrine. The minor sympathomimetic drugs are used primarily to treat allergic rhinitis.
Corticosteroids
Nasal corticosteroid sprays are very effective in relieving the symptoms of allergic rhinitis (see Table 26-1). Occasionally, patients have such severe manifestations of allergies that they are truly incapacitated. In these situations, a brief course of oral corticosteroids can be used.
Antipruritic drugs
Topically applied antipruritic drugs are most effective when the skin is not broken. These drugs protect the skin and provide relief from itching. Common over-the-counter drugs include calamine lotion, coal tar solutions and camphor. Menthol and phenol may be added to other lotions to produce an antipruritic effect. Some more potent drugs that require a prescription include promethazine and trimeprazine: they should be used with great caution because of the associated risk of agranulocytosis.
Mast cell-stabilising drugs
Sodium cromoglycate and nedocromil are mast cell-stabilising agents that inhibit the release of histamines, leukotrienes and other agents from the mast cell after antigen–IgE interaction. They are available as an inhalant in a metered dose pump pack, a nasal spray or an oral pill. They are used in the management of asthma (see Ch 28) and in the treatment of allergic rhinitis (see Ch 26). An important feature of these drugs is a very low incidence of side effects.
Leukotriene receptor antagonists
Leukotriene receptor antagonists (LTRAs) block leukotriene, one of the major mediators of the allergic inflammatory process. These medications can be inhaled or taken orally. They may be used in the treatment of allergic rhinitis and asthma. For more information, refer to Chapter 26.
Immunotherapy
Immunotherapy is the recommended treatment for control of allergic symptoms when the allergen cannot be avoided and drug therapy is not effective. Relatively few patients with allergies have symptoms so intolerable that they require allergy immunotherapy. Immunotherapy is absolutely indicated only in individuals with anaphylactic reactions to insect venom. It involves administration of small titres of an allergen extract in increasing strengths until hyposensitivity to the specific allergen is achieved. For best results the patient should continue to avoid the offending allergen whenever possible because complete desensitisation is impossible. Unfortunately, not all allergy-related conditions respond to immunotherapy. Food allergies cannot be safely treated with this therapy and eczema may worsen with immunotherapy.
Mechanism of action
IgE immunoglobulin level is elevated in atopic individuals. When IgE combines with an allergen in a hypersensitive person, a reaction occurs, releasing histamine in various body tissues. Allergens more readily combine with IgG immunoglobulin than with other immunoglobulins. Therefore, immunotherapy involves injecting allergen extracts that will stimulate increased IgG levels. The binding of IgG to allergen-reactive sites interferes with allergen binding to mast cell-bound IgE, preventing mast cell degranulation, and thus reduces the number of reactions that cause tissue damage. The goal of long-term immunotherapy is to keep ‘blocking’ IgG levels high. In addition, allergen-specific T suppressor cells develop in individuals receiving immunotherapy.
Method of administration
The allergens included in immunotherapy are chosen on the basis of the results of skin testing with a panel of allergens found in the local geographic area. Immunotherapy involves the subcutaneous injection of titrated amounts of allergen extracts biweekly or weekly. The dose is small at first and is increased slowly until a maintenance dosage is reached. Generally it takes 1–2 years of immunotherapy to reach the maximal therapeutic effect. Therapy may be continued for about 5 years. After that, consideration is given to discontinuing therapy. In many patients a decrease in symptoms is sustained after the treatment is discontinued. For patients with severe allergies or sensitivity to insect stings, maintenance therapy is continued indefinitely. Best results are achieved when immunotherapy is administered throughout the year.
Sublingual immunotherapy involves allergen extracts taken under the tongue. This method of immunotherapy has a lower risk of severe adverse reaction than the traditional subcutaneous administration. Although available in commercial preparations in Europe, sublingual immunotherapy is not yet available in Australia or New Zealand.
 NURSING MANAGEMENT: IMMUNOTHERAPY
NURSING MANAGEMENT: IMMUNOTHERAPY
The nurse is often the person responsible for administering immunotherapy and should always anticipate adverse reactions, especially when using a new-strength dose, after a previous reaction or after a missed dose. Early signs and symptoms indicative of a systemic reaction include pruritus, urticaria, sneezing, laryngeal oedema and hypotension. Emergency measures for anaphylactic shock should be initiated immediately. A local reaction should be described according to the degree of redness and swelling at the injection site. If the area is greater than 1 cm in an adult, the reaction should be reported to the healthcare provider so that the allergen dosage may be decreased.
Immunotherapy always carries the risk of a severe anaphylactic reaction. Therefore, a healthcare provider, emergency equipment and essential drugs should be available whenever injections are given. Record-keeping must be accurate and can be invaluable in preventing an adverse reaction to the allergen extract. Before giving an injection, the nurse should check the patient’s name with the name on the vial and determine the vial strength, the amount of the last dose, the date of the last dose and any reaction information.
The nurse should always administer the allergen extract in an extremity away from a joint so that a tourniquet can be applied for a severe reaction. The site should be rotated for each injection. The nurse must aspirate for blood before giving an injection to ensure that the allergen extract is not injected into a blood vessel. An injection directly into the bloodstream can potentiate an anaphylactic reaction. After the injection is given, the patient should be carefully observed for 20 minutes because systemic reactions are most likely to occur immediately. However, the nurse should warn the patient that a delayed reaction can occur as much as 24 hours later.
LATEX ALLERGIES
Allergies to latex products have become a problem of increasing proportion, affecting both patients and healthcare professionals. The increase in allergic reactions has coincided with the sharp increase in glove use. It is estimated that 5–18% of healthcare workers who are regularly exposed to latex are sensitised. The more frequent and prolonged the exposure to latex, the greater the likelihood of developing a latex allergy.16 In addition to gloves, many latex-containing products are used in healthcare, such as blood pressure cuffs, stethoscopes, tourniquets, IV tubing, syringes, electrode pads, oxygen masks, tracheal tubes, colostomy and ileostomy pouches, urinary catheters, anaesthetic masks and adhesive tape. Latex proteins can become aerosolised through powder on gloves and can result in serious reactions when inhaled by sensitised individuals. It is recommended that all healthcare facilities use powder-free gloves to avoid respiratory exposure to latex proteins.
Types of latex allergies
Two types of latex allergies can occur: type IV allergic contact dermatitis and type I allergic reactions. Type IV contact dermatitis is caused by the chemicals used in the manufacturing of latex gloves. It is a delayed reaction that occurs within 6– 48 hours. Typically, the person first has dryness, pruritus, fissuring and cracking of the skin, followed by redness, swelling and crusting at 24–48 hours. Chronic exposure can lead to lichenification, scaling and hyperpigmentation. The dermatitis may extend beyond the area of physical contact with the allergen.
A type I allergic reaction is a response to the natural rubber latex proteins and occurs within minutes of contact with the proteins. These types of allergic reactions can manifest as various reactions ranging from skin redness, urticaria, rhinitis, conjunctivitis or asthma to full-blown anaphylactic shock. Systemic reactions to latex may result from exposure to latex protein via various routes, including the skin, mucous membranes, inhalation or blood.
Latex-food syndrome
Some proteins in rubber are similar to food proteins, so some foods may cause an allergic reaction in people who are allergic to latex. This is called latex-food syndrome. The most common of these foods are bananas, avocados, chestnuts, kiwi fruit, tomatoes, water chestnuts, guava, hazelnuts, potatoes, peaches, grapes and apricots. In people with latex allergy, at least 70% will have a positive allergy test to at least one related food.
 NURSING AND COLLABORATIVE MANAGEMENT: LATEX ALLERGIES
NURSING AND COLLABORATIVE MANAGEMENT: LATEX ALLERGIES
The identification of patients and healthcare workers sensitive to latex is crucial in the prevention of adverse reactions. A thorough health history and history of any allergies should be collected, especially on patients with any complaints of latex contact symptoms. Not all latex-sensitive individuals can be identified, even with a careful and thorough history. The greatest risk factor is long-term multiple exposures to latex products (e.g. healthcare personnel, individuals who have had multiple surgeries, rubber industry workers). Additional risk factors include a patient history of hay fever, asthma and allergies to certain foods (see above).
Latex precaution protocols should be used for those patients who are identified as having a positive latex allergy test or a history of signs and symptoms related to latex exposure. Many healthcare facilities have created latex-free product carts that can be used for patients with latex allergies. Occupational health and safety guidelines have been published by the various states and territories in Australia (see Box 13-4).
BOX 13-4 Guidelines for preventing allergic latex reactions
1. Use non-latex gloves for activities that are not likely to involve contact with infectious materials (food preparation and housekeeping).
2. Use powder-free gloves with reduced protein content.
3. Do not use oil-based hand creams or lotions when wearing gloves.
4. After removing latex gloves, wash hands and dry thoroughly.
5. Frequently clear work areas that are contaminated with latex-containing dust.
6. Know the symptoms of latex allergy, including skin rash; hives; flushing; itching; nasal, eye or sinus symptoms; asthma; and shock.
7. If symptoms of latex allergy develop, avoid direct contact with latex gloves and products. Wear a medical alert bracelet and carry an adrenaline pen.
Source: Adapted from National Institute for Occupational Safety and Health. Preventing allergic reactions to natural rubber latex in the workplace. Available at www.cdc.gov/niosh/docs/97-135/; and NSW Health. Latex allergy—policy framework and guidelines for prevention and management. Sydney: NSW Health; 2005. Available at www.health.nsw.gov.au/policies/pd/2005/PD2005_490.html, accessed 25 March 2011.
Because of the potential for severe symptoms of food allergy, patients should be taught to avoid those foods. Other recommendations for people with latex and food allergies include wearing a medical alert bracelet or necklace and carrying an injectable adrenaline pen.
MULTIPLE CHEMICAL SENSITIVITIES
Multiple chemical sensitivities (MCS) is an acquired disorder in which certain people exposed to various chemicals and foods in the environment have many symptoms related to multiple body systems.17 These symptoms are usually subjective and are not found during physical examination. The patient experiences wide-ranging symptoms, but evidence of pathology or physiological dysfunction is lacking.
Primarily, MCS is found in women. Symptoms include fatigue, headache, nausea, pain, irritable bowel symptoms, dizziness, mouth irritation, disorientation and cough. Almost any chemical can initiate the symptoms of MCS. Odour seems to be the principal trigger. Gas exhaust, perfumes, cigarette smoke, plastics, pesticides and industrial solvents are some of the most common odours associated with MCS. Also, food additives, drugs and naturally occurring foods, including drinking water, often cause sensitivity. The uniqueness of MCS is that symptoms occur at levels below the established guidelines of toxic levels and concentrations.
The causes of MCS are thought to be from immunological, psychological, toxicological and sociological factors. Diagnosis is usually made on the basis of the patient’s health history. There is no established test used to diagnose MCS. Diagnostic tests that are used include provocation–neutralisation and immunological testing (e.g. FBC, lymphocyte subsets, antibody titres), but immunological testing has not been widely accepted as a diagnostic test. The provocation–neutralisation test is done by exposing the patient to certain environmental substances to produce symptoms and then at higher and lower doses to initiate the disappearance of symptoms.
The most effective treatment for MCS is to avoid the chemicals that may trigger the symptoms and create a chemical-free home/workplace. However, this can be difficult in a chemical-dependent society. Other commonly recommended treatments include regular exercise, physiotherapy, massage, prayer and meditation.
Autoimmunity
Autoimmunity is an immune response against the self. The immune system no longer differentiates self from non-self. For some unknown reason, immune cells that are normally unresponsive (tolerant to self-antigens) are activated. Both T cells and B cells have the ability for tolerance to self-antigens. Therefore, an alteration in T cells alone or in both B cells and T cells can produce autoantibodies and autosensitised T cells to cause pathophysiological tissue damage. The particular autoimmune disease manifested depends on which self-antigen is involved.
The cause of autoimmune diseases is still unknown. Age is thought to play some role, because the number of circulating autoantibodies increases in people over the age of 50. However, the principal factors in the development of autoimmunity are: (1) the inheritance of susceptibility genes, which may contribute to the failure of self-tolerance; and (2) the initiation of autoreactivity by triggers, such as infections, which may activate self-reactive lymphocytes.
Autoimmune diseases tend to cluster, so that a given person may have more than one autoimmune disease (e.g. rheumatoid arthritis, Addison’s disease), or the same or related autoimmune diseases may be found in other members of the same family. This observation has led to the concept of genetic predisposition to autoimmune disease. Most of the genetic research in this area correlates certain human leucocyte antigen types with an autoimmune condition (human leucocyte antigens are discussed later in this chapter).
Even in a genetically predisposed person, some trigger is required for the initiation of autoreactivity. This may include infectious agents such as a virus. Viral infections can cause an alteration of cells or tissues that are not normally antigenic. The virally induced changes can make the cells or tissues antigenic. Viruses may be involved in the development of diseases such as multiple sclerosis and type 1 diabetes mellitus. Rheumatic fever and rheumatic heart disease are autoimmune responses triggered by streptococcal infection and mediated by antibodies against group A β-haemolytic streptococci that cross-react with heart muscles and valves and synovial membranes.
Drugs can be precipitating factors in autoimmune disease. Haemolytic anaemia can result from methyldopa administration. Procainamide can induce the formation of antinuclear antibodies and cause a lupus-like syndrome. Gender and hormones also have a role in autoimmune disease. More women than men have autoimmune diseases. During pregnancy, many autoimmune diseases get better. Following delivery, the woman with an autoimmune disease frequently has an exacerbation.
AUTOIMMUNE DISEASES
Generally, autoimmune diseases are grouped according to organ-specific and systemic diseases. (Box 13-5 presents examples of autoimmune diseases.) Systemic lupus erythematosus is a classic example of a systemic autoimmune disease characterised by damage to multiple organs. It occurs most frequently in women aged 20–40 years. The aetiology is unknown, but there appears to be a loss of self-tolerance for the body’s own DNA antigens. In SLE, tissue injury appears to be the result of the formation of antinuclear antibodies. For some reason (possibly a viral infection), the cell membrane is damaged and DNA is released into the systemic circulation, where it is viewed as non-self. This DNA is normally sequestered inside the nucleus of cells. On release into the circulation, the DNA antigen reacts with an antibody. Some antibodies are involved in immune complex formation and others may cause damage directly. Once the complexes are deposited, complement is activated and further damages the tissue, especially the renal glomerulus. (SLE is discussed in Ch 64.)
APHERESIS
Apheresis has been used effectively to treat autoimmune diseases and other diseases and disorders.18 Apheresis is the use of a procedure to separate components of the blood, followed by the removal of one or more of these components. Compound words are often used to describe any particular apheresis procedure, depending on the blood components being collected.19 Plateletapheresis is the removal of platelets, usually for collection from normal individuals to infuse into patients with low platelet counts (e.g. patients taking chemotherapy who develop thrombocytopenia). Leucocytapheresis is a general term indicating the removal of WBCs and is used in chronic myelogenous leukaemia to remove high numbers of leukaemic cells. Lymphocytapheresis is used to decrease high lymphocyte counts, such as in individuals with chronic lymphocytic leukaemia. Another type of apheresis is peripheral stem cell collection (also called haematopoietic progenitor cell collection), which is used to collect stem cells from peripheral blood. These stem cells can then be used to repopulate a person’s bone marrow after high-dose chemotherapy (see section on haematopoietic stem cell transplants in Ch 15).
Plasmapheresis
Plasmapheresis is the removal of plasma containing components causing or thought to cause disease. It can also be used to obtain plasma from healthy donors to administer to patients as replacement therapy. When plasma is removed, it is replaced by substitute fluids such as saline, fresh frozen plasma or albumin. Therefore, the term plasma exchange more accurately describes this procedure.
Plasmapheresis has been used to treat autoimmune diseases such as SLE, glomerulonephritis, Goodpasture’s syndrome, myasthenia gravis, thrombocytopenic purpura, rheumatoid arthritis and Guillain-Barré syndrome. Many disorders for which plasmapheresisis used are characterised by circulating autoantibodies (usually of the IgG class) and antigen–antibody complexes. The rationale for performing therapeutic plasmapheresis in autoimmune disorders is to remove pathological substances present in plasma. Immunosuppressive therapy has been used to prevent recovery of IgG production, and plasmapheresis has been used to prevent antibody rebound.
In addition to removing antibodies and antigen–antibody complexes, plasmapheresis may also remove inflammatory mediators (e.g. complement) that are responsible for tissue damage. In the treatment of SLE, plasmapheresis is usually reserved for the patient having an acute attack who is unresponsive to conventional therapy.
Plasmapheresis involves the removal of whole blood through an IV device and then the blood circulates through the apheresis machine. Inside the machine, the blood is divided into plasma and its cellular components by centrifugation or membrane filtration. The plasma is generally replaced with normal saline, Hartmann’s solution, fresh frozen plasma, plasma protein fractions or albumin. When blood is manually removed, only 500 mL may be taken at one time. However, with the use of apheresis procedures, more than 4 L of plasma can be pheresed in 2–3 hours.
As with administration of other blood products, it is important to be aware of the side effects associated with plasmapheresis. The most common complications are hypotension and citrate toxicity. Hypotension is usually the result of a vasovagal reaction or transient volume changes. Citrate is used as an anticoagulant and may cause hypocalcaemia, which may manifest as headache, paraesthesias and dizziness.
Immunodeficiency disorders
When the immune system does not adequately protect the body, immunodeficiency exists. Immunodeficiency disorders involve an impairment of one or more immune mechanisms, which include: (1) phagocytosis; (2) humoral response; (3) cell-mediated response; (4) complement; and (5) a combined humoral and cell-mediated deficiency. Immunodeficiency disorders are primary if the immune cells are improperly developed or absent and secondary if the deficiency is caused by illnesses or treatment. Primary immunodeficiency disorders are rare and often serious, whereas secondary disorders are more common and less severe.
PRIMARY IMMUNODEFICIENCY DISORDERS
The basic categories of primary immunodeficiency disorders are: (1) phagocytic defects; (2) B cell deficiency; (3) T cell deficiency; and (4) a combined B cell and T cell deficiency (see Table 13-12).
TABLE 13-12 Primary immunodeficiency disorders
| Disorder | Affected cells | Genetic basis |
|---|---|---|
| Chronic granulomatous disease | PMNs, monocytes | Sex-linked |
| Job syndrome | PMNs, monocytes | |
| Bruton’s X-linked hypogammaglobulinaemia | B | Sex-linked |
| Common variable hypogammaglobulinaemia | B | |
| Selective IgA, IgM or IgG deficiency | B | Some sex-linked |
| DiGeorge’s syndrome (thymic hypoplasia) | T | |
| Severe combined immunodeficiency disease | Stem, B, T | Sex-linked or autosomal recessive |
| Ataxia telangiectasia | B,T | Autosomal recessive |
| Wiskott-aldrich syndrome | B,T | Sex-linked |
| Graft-versus-host disease | B,T |
PMNs, polymorphonuclear neutrophils
SECONDARY IMMUNODEFICIENCY DISORDERS
Some of the important factors that may cause secondary immunodeficiency disorders are listed in Box 13-6. Drug-induced immunosuppression is the most common. Immunosuppressive therapy is prescribed for patients to treat autoimmune disorders and to prevent transplant rejection. In addition, immunosuppression is a serious side effect of drugs used in cancer chemotherapy. Generalised leucopenia often results, leading to a decreased humoral and cell-mediated response. Therefore, secondary infections are common in immunosuppressed patients.
Malnutrition alters cell-mediated immune responses. When protein is deficient over a prolonged period, atrophy of the thymus gland occurs and lymphoid tissue decreases. In addition, an increased susceptibility to infections always exists.
Hodgkin’s lymphoma greatly impairs the cell-mediated immune response, and patients may die from severe viral or fungal infections. (Hodgkin’s lymphoma is discussed in Ch 30.) Viruses, especially rubella, may cause immunodeficiency by direct cytotoxic damage to lymphoid cells. Systemic infections can place such a demand on the immune system that resistance to a secondary or subsequent infection is impaired.
Radiation can destroy lymphocytes either directly or through depletion of stem cells. As the radiation dose is increased, more bone marrow atrophies, leading to severe pancytopenia and suppression of immune function. Splenectomy in children is especially dangerous and may lead to septicaemia simply from respiratory infections.
Stress may alter the immune response. This response involves interrelationships among the nervous, endocrine and immune systems.
Human leucocyte antigen system
The antigens responsible for rejection of genetically unlike tissues are called the major histocompatibility antigens. These antigens are products of histocompatiblity genes. In humans they are called the human leucocyte antigen (HLA) system. The genes for the HLA antigens are linked and occur together on the sixth chromosome. HLAs are present on all nucleated cells and platelets. The HLA system is primarily used in matching organs and tissues for transplantation.
An important characteristic of HLA genes is that they are highly polymorphic (variable). Each HLA locus can have many different possible alleles. With many alleles possible at each HLA locus, many combinations exist. Each person has two alleles for each locus, one inherited from each parent. Both alleles of a locus are expressed independently (i.e. they are codominant). The proteins encoded by certain genes are known as antigens. The entire set of A, B, C, D and DR genes located on one chromosome is termed a haplotype. A complete set is inherited as a unit (haplotype). One haplotype is inherited from each parent (see Fig 13-13). This means that a person is 50% identical to each parent and has a 25% chance of being identical to a sibling.
Figure 13-13 Patterns of HLA inheritance. A, HLA genes are located on chromosome 6. B, The two haplotypes of the father are labelled P1 and P2 and the haplotypes of the mother are labelled M1 and M2. Each child inherits two haplotypes, one from each parent. C, Therefore only four combinations—P1M1, P1M2, P2M1, P2M2—are possible, and 25% of the offspring will have identical HLA haplotypes.
In organ transplantation A, B and DR are used primarily for compatibility matching. The specific allele at each locus is identified by a number. For example, a person could have an HLA of A2, A6, B7, B27, DR4 and DR7.
HUMAN LEUCOCYTE ANTIGEN AND DISEASE ASSOCIATIONS
The early interest in HLA was stimulated by its potential role in matching donors and recipients of organ transplants. During the last few years, interest in the association between HLA and disease has grown. Strong associations between HLA type and susceptibility to certain diseases have been demonstrated.20 HLA disease associations mean that the frequency of a defined HLA allele is significantly increased in patients with a certain disease when compared with ethnically matched controls. Most of the HLA-associated diseases are classified as autoimmune disorders. Examples of HLA types and disease associations include: (1) HLA-B27 and ankylosing spondylitis; (2) HLA-DR2 and HLA-DR3 and SLE; and (3) HLA-DR3 and HLA-DR4 and diabetes mellitus.
The discovery of HLA associations with certain diseases is a major breakthrough in understanding the genetic bases of these diseases. It is now known that at least part of the genetic basis of HLA-associated diseases lies in the HLA region, but the actual mechanisms involved in these associations are still unknown. However, most individuals who inherit an HLA type associated with a disease will never develop the disease.
The association between HLA and certain diseases is presently of little practical clinical importance. Nevertheless, there is promise for the development of clinical applications in the future. For example, with certain autoimmune diseases it may be possible to identify members of a family at greatest risk of developing the same or a related autoimmune disease. These persons would need close medical supervision, preventative measures implemented (if possible), and early diagnosis and treatment instituted to prevent chronic complications.
Organ transplantation
Transplantation success has improved with advances in surgical technique, advances in histocompatibility testing and more effective immunosuppressants (e.g. cyclosporine). Now, most organs and tissues are transplanted successfully with good survival rates.
Common tissue transplants include corneas, skin, bone marrow, heart valves, bone and connective tissues (see Fig 13-14). Cornea transplants can prevent or correct blindness. Skin grafts are used to assist in managing burn patients. Bone marrow is donated to help patients with leukaemia and other malignancies.
Transplanted organs currently come from many different body systems. These organs include the heart, lung, liver, kidneys, pancreas and intestine. Certain organs can be transplanted together, such as kidney and pancreas, kidney and liver, and kidney and heart.21 For example, many patients who receive a pancreas transplant also receive a kidney transplant because a patient with diabetes not only may have lost the ability to produce insulin but also may have renal failure as a result of the diabetes.
Some organs can be transplanted in parts or segments instead of transplanting an entire organ. Liver and lung lobes (rather than the whole organ) may be transplanted, or an intestine may be used in segments, thus allowing for one person’s organ donation to benefit many recipients. This technique also enables living donors to donate part of an organ while maintaining the functional organ themselves.
Organ donations are taken from two sources: deceased (cadaveric) and living donors. Most organs and tissues currently originate from deceased donors. However, because of the shortage of organs from deceased donors, the use of both related and living unrelated donor organs is increasing. In Australia, living donors accounted for one-third of transplants performed in 2008.
Individuals may make a decision to become a donor when they sign their driver’s licence expressing their wish to donate organs. However, ultimately the person’s legal next of kin must consent to the donation regardless of whether the donor card is signed. That is why it is extremely important for potential donors to notify their next of kin about their willingness to donate organs or tissues at the time of their death.22 The Australian Organ Donor Register became a register of legal consent in 2005, and individuals can register their wish to donate on this site.23 On 1 January 2010, there were 1650 people in Australia and 482 people in New Zealand on organ transplant waiting lists. In 2009, 1187 organs were transplanted from 247 donors in Australia, and 181 organs were transplanted from 43 donors in New Zealand. The organs with the highest demand are kidneys, hearts and livers; these are also the most commonly transplanted organs.24
Organ and tissue donations are regulated by relevant legislation in Australia and New Zealand in order to ensure fair and consistent transplantation laws for all. Patients are matched to available donors based on a number of factors: ABO blood and HLA typing, medical urgency, time on the waiting list and geographic location.23
TISSUE TYPING
The recipient must receive a transplant from an ABO blood group–compatible donor (see Table 29-10). The donor and recipient do not need to share the same Rh factor.
HLA typing
HLA typing is done on all potential donors and recipients. Currently only the A, B and DR antigens are thought to be clinically significant for transplantation. Because there are two alleles at each locus that encode for antigens, a total of six antigens are identified. In transplantation, an attempt is made to match as many antigens as possible between the HLA-A, HLA-B and HLA-DR loci. Antigen matches of five and six antigens and certain four-antigen matches have been found to have better clinical outcomes (i.e. the patient is less likely to reject the transplanted organ), especially in kidney and bone marrow transplants.
The degree of HLA matching required or deemed suitable for successful solid organ transplantation is dependent on the type of organ. Certain organ and tissue transplants require a closer histocompatibility match than other organs. For example, a cornea transplant can be accepted by nearly any individual because the corneas are avascular and therefore no antibodies reach the cornea to cause rejection. In kidney and bone marrow transplantation, HLA matching is very important, as these transplants are at high risk for graft rejection. On the other hand, for liver transplants, HLA mismatches have little impact on graft survival. For heart and lung transplants, they fall somewhere between, but minimising HLA mismatches significantly improves survival. In addition, for liver, lung and heart transplants, there are fewer donors available for these organs and it is difficult to get good HLA matches.
As immunosuppression has improved, to some extent HLA matching has become less important for successful transplantation overall. Another major consideration is the time and distance that an HLA-compatible organ has to be preserved. Increased storage time can result in damage to the donor organ. Therefore, the need to have the ‘best’ match has to be balanced against the time it takes to get the donated organ and transplant it.
Panel of reactive antibodies
A panel of reactive antibodies (PRA) indicates the recipient’s sensitivity to various HLAs before receiving a transplant. To detect preformed antibodies to HLA, the recipient’s serum is mixed with a randomly selected panel of donor lymphocytes to determine reactivity. The potential recipient may have been exposed to HLA antigens by means of previous blood transfusions, pregnancy or a previous organ transplant.
For the PRA, the results are calculated in percentages. A high PRA indicates that the person has a large number of cytotoxic antibodies and is highly sensitised, which means that there is a poor chance of finding a cross-match–negative donor. In patients awaiting transplantation, a PRA panel is usually done on a regular basis. Plasmapheresis and IV immune globulin have been used to lower the number of preformed HLA antibodies in highly sensitised patients.
Cross-match
A cross-match uses serum from the recipient mixed with donor lymphocytes to test for any preformed anti-HLA antibodies to the potential donor organ. The cross-match can be used as a screening test when multiple possible living donors are being considered or once a cadaver donor is selected. A final cross-match is done just before transplant.
A positive cross-match indicates that the recipient has cytotoxic antibodies to the donor and is an absolute contraindication to transplantation. If transplanted, the organ would undergo hyperacute rejection. A negative cross-match indicates that no preformed antibodies are present and it is safe to proceed with transplantation. Cross-matching is especially important for kidney transplants and may not be done for lung, liver and heart transplants.
TRANSPLANT REJECTION
Rejection is one of the major problems following organ transplantation. Rejection of organs will occur as a normal immune response to foreign tissue. The rejection can be controlled by immunosuppression therapy, ABO and HLA matching, and ensuring that the cross-match is negative. Unfortunately, many different HLAs exist and a perfect match is nearly impossible unless the tissue is from oneself or an identical twin. Rejection can be hyperacute, acute or chronic. Prevention, early diagnosis and treatment of rejection are essential for long-term graft function.
Hyperacute rejection
Hyperacute (antibody-mediated, humoral) rejection occurs minutes to hours after transplantation because the blood vessels are rapidly destroyed. It occurs because the person had pre-existing antibodies against the transplanted tissue or organ. There is no treatment for hyperacute rejection, and the transplanted organ is removed.
The kidney is most susceptible to hyperacute reaction. Fortunately, it is a rare event because the final cross-match just before transplant will usually identify whether the recipient is sensitised to any of the donor HLAs. On occasion, for unclear reasons, the final cross-match does not detect these preformed antibodies and hyperacute rejection occurs.
Acute rejection
Acute rejection most commonly manifests in the first 6 months after transplantation. This type of rejection is usually mediated by the recipient’s lymphocytes, which have been activated against the donated (foreign) tissue or organ (see Fig 13-15). Another type of acute rejection occurs when anti-donor antibodies develop after transplantation.
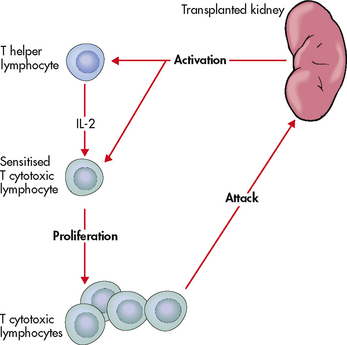
Figure 13-15 Mechanism of action of T cytotoxic lymphocyte activation and attack on transplanted tissue. The transplanted organ (e.g. kidney) is recognised as foreign and activates the immune system. T helper cells are activated to produce interleukin-2 (IL-2), and T cytotoxic lymphocytes are sensitised. After the T cytotoxic cells proliferate, they attack the transplanted organ.
It is not uncommon to have at least one rejection episode, especially with organs from deceased donors. These episodes are usually reversible with additional immunosuppressive therapy that may include increased corticosteroid doses or polyclonal or monoclonal antibodies. Unfortunately, immunosuppressants increase the risk for infection. To combat acute rejection, all patients with transplants require long-term use of immunosuppressants, putting them at a high risk for infection, especially in the first few months after transplant when the immunosuppression doses are highest.
Chronic rejection
Chronic rejection is a process that occurs over months or years and is irreversible. Chronic rejection can occur for unknown reasons or from repeated episodes of acute rejection. The transplanted organ is infiltrated with large numbers of T and B cells characteristic of an ongoing, low-grade, immune-mediated injury. Chronic rejection results in fibrosis and scarring. In heart transplants, it manifests as accelerated coronary artery disease. In lung transplants, it manifests as bronchiolitis obliterans. In liver transplants, it is characterised by loss of bile ducts. In kidney transplants, it manifests as fibrosis and glomerulopathy.
There is no definitive therapy for this type of rejection. Treatment is mainly supportive. This type of rejection is difficult to manage and is not associated with the optimistic prognosis of acute rejection. Patients with chronic rejection should be put on the transplant list in the hope that they can be retransplanted.
IMMUNOSUPPRESSIVE THERAPY
Immunosuppressive therapy requires a balance. On the one hand, the immune response needs to be suppressed to prevent rejection of the transplanted organ. On the other hand, an adequate immune response needs to be maintained to prevent overwhelming infection and the development of malignancies. Many of the medications used to achieve immunosuppression have significant side effects. Two are of particular concern: (1) increased risk of infection; and (2) increased risk of malignancies. Furthermore, because transplant recipients must take immunosuppressants for life, the risk of toxicity continues for the rest of their lives.
Immunosuppressant drugs are listed in Table 13-13. By using a combination of agents that work in different phases of the immune response (see Fig 13-16), lower doses of each drug produce effective immunosuppression while minimising side effects. The major immunosuppressive agents are: (1) calcineurin inhibitors, including cyclosporin and tacrolimus; (2) corticosteroids (prednisone, methylprednisolone); (3) mycophenolate mofetil; and (4) sirolimus. Azathioprine and cyclophosphamide have been used in the past, but are not commonly used now because they have been replaced with safer, more effective drugs. Antilymphocyte globulin (ALG) and muromonab-CD3 are IV medications used for short periods to prevent early rejection or reverse acute rejection.
TABLE 13-13 Immunosuppressive therapy
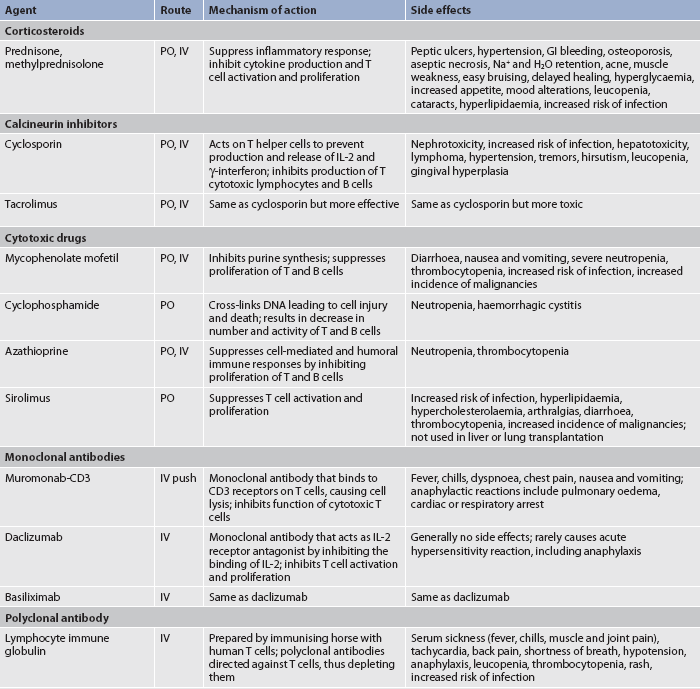
GI, gastrointestinal; IL, interleukin; IV, intravenous; PO, by mouth.
Immunosuppressive protocols are highly variable among transplant centres, with different combinations of medications being used. Most patients are initially on triple therapy. The standard triple therapy usually includes a calcineurin inhibitor, a corticosteroid and mycophenolate mofetil. The doses of immunosupressant drugs will be reduced over time. Patients taking corticosteroids may be weaned off after a few years. There is a trend in many transplant centres to use immunosuppression protocols that do not contain corticosteroids because of their many side effects.
Calcineurin inhibitors
This group of drugs includes cyclosporine and tacrolimus. They are the most effective immunosuppressants available.25 These drugs prevent a cell-mediated attack against the transplanted organ (see Fig 13-16) and they do not cause bone marrow suppression or alterations of the normal inflammatory response. They are generally used in combination with corticosteroids, mycophenolate mofetil and sirolimus. Many of the side effects of calcineurin inhibitors are dose related. These drugs are potentially nephrotoxic. Drug levels are followed closely to prevent toxicity. Microemulsions of cyclosporin are replacing Sandimmune because of better and more consistent absorption.26 Neoral and Sandimmune are not biocompatible and should never be interchanged for one another.
Mycophenolate mofetil
Mycophenolate mofetil is a lymphocyte-specific inhibitor of purine synthesis with suppressive effects on both T and B lymphocytes. This drug appears to be most effective when used in combination with tacrolimus or cyclosporin. Its effects are additive because it acts later in the lymphocyte activation pathway by a different mechanism. It has also been shown to decrease the incidence of late graft loss. The major limitation of this drug is its GI toxicities, including nausea, vomiting and diarrhoea. In many cases the side effects can be diminished by lowering the dose or giving smaller doses more frequently.
Sirolimus
Sirolimus is an immunosuppressive agent approved for use in renal transplant recipients. It is used in combination with corticosteroids and cyclosporin. It is also used in combination with tacrolimus.
Monoclonal antibodies
Monoclonal antibodies are used for preventing and treating acute rejection episodes. (Fig 13-17 shows how monoclonal antibodies are made.) Muromonab-CD3 was the first of these monoclonal antibodies to be used in clinical transplantation. It is a mouse monoclonal antibody that binds with the CD3 antigen found on the surface of human thymocytes and mature T cells. It is an anti–antigen receptor antibody that interferes with the function of the T lymphocyte, the pivotal cell in the response to graft rejection. It is administered via IV push daily for 7–14 days. All T cells are affected rather than just the subset active in graft rejection. Within minutes after the initial infusion of muromonab-CD3, the number of circulating T cells decreases significantly.
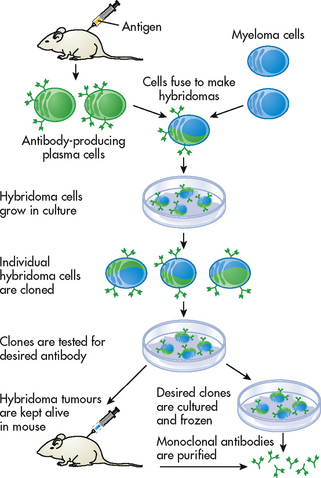
Figure 13-17 Monoclonal antibodies are identical antibodies made by clones of a single antibody-producing cell. The target antigen is injected into a mouse. Plasma cells are harvested from the spleen of the mouse and fused with myeloma cells. The fused cells, or hybridomas, are then cloned. A clone can secrete monoclonal antibodies over a long period of time.
A flu-like syndrome occurs during the first few days of treatment due to cytokine release. Side effects include fever, rigors, headache, myalgias and various GI disturbances. To reduce the expected side effects of muromonab-CD3, patients should receive acetaminophen, diphenhydramine and IV corticosteroids before administering the dose.
Newer generation monoclonal antibodies include daclizumab and basiliximab. These monoclonal antibodies are a hybrid of mouse and human antibodies and have fewer side effects than muromonab-CD3 because they have been humanised.
Polyclonal antibodies
Lymphocyte immune globulin is used as induction therapy or to treat acute rejection. The purpose of induction therapy is to severely immunosuppress an individual immediately after transplantation to prevent early rejection. It is made by immunising horses with human lymphocytes. The antibody made against the human lymphocytes is then purified and administered IV.
Allergic reactions to the foreign proteins from the host animal, manifested by fever, arthralgias and tachycardia, are common but usually not severe enough to preclude use. These side effects can be attenuated by administering the preparation slowly, over 4–6 hours, and premedicating patients with acetaminophen, diphenhydramine and methylprednisolone. The main toxicities of polyclonal antibodies are lymphopenia and thrombocytopenia caused by antibody contaminants that are not completely removed during preparation of the antibodies.
Graft-versus-host disease
Graft-versus-host disease (GVHD) occurs when an immunoincompetent (immunodeficient) patient is transfused or transplanted with immunocompetent cells.27 A GVHD response may result from the infusion of any blood product containing viable lymphocytes, such as in therapeutic blood transfusions, and from the transplantation of fetal thymus, fetal liver or bone marrow. In most transplantation situations, the biggest concern is the patient’s (host’s) rejection of the organ or transplant. However, in GVHD disease, the graft rejects the host or recipient tissue.
The GVHD response may have its onset 7–30 days after transplantation. Once the reaction is started, little can be done to modify its course. The exact mechanism involved in this reaction is not completely understood. However, it involves donor T cells attacking and destroying vulnerable host cells.
The target organs for the GVHD phenomenon are the skin, liver and GI tract. The skin disease may be a maculopapular rash, which may be pruritic or painful. It initially involves the palms and soles of the feet but can progress to a generalised erythema with bullous formation and desquamation (shedding of the outer layer of skin). The liver disease may range from mild jaundice with elevated liver enzymes to hepatic coma. The intestinal disease may be manifested by mild to severe diarrhoea, severe abdominal pain, GI bleeding and malabsorption. The biggest problem with GVHD is infection, with different types of infections seen in different periods. Bacterial and fungal infections predominate immediately after transplantation when granulocytopenia exists. The development of interstitial pneumonitis is the primary concern later in the course of the disease.
There is no adequate treatment of GVHD once it is established. Although corticosteroids are often used, they enhance the susceptibility to infection. The use of immunosuppressive agents (e.g. methotrexate, cyclosporin) has been most effective as a preventative rather than a treatment measure. Radiation of blood products before they are administered is another measure to prevent T-cell replication.
1. If a person is heterozygous for a given gene, it means that the person:
2. A father who has a sex-linked recessive disorder and a mother with a normal genotype will:
3. The function of monocytes in immunity is related to their ability to:
4. One function of cell-mediated immunity is:
5. The reason newborns are protected for the first 3 months of life from bacterial infections is because of the maternal transmission of:
6. In a type I hypersensitivity reaction, the primary immunological disorder appears to be:
7. The nurse is alerted to possible anaphylactic shock immediately after a patient has received intramuscular penicillin by the development of:
8. The nurse advises a friend who asks him to administer his allergy shots that:
9. Association between HLA antigens and diseases is most commonly found in what disease conditions?
10. A patient is undergoing plasmapheresis for treatment of systemic lupus erythematosus. The nurse explains that plasmapheresis is used in her treatment to:
11. The most common cause of secondary immunodeficiencies is:
12. Which of the following accurately describes rejection following transplantation?
13. In a person having an acute rejection of a transplanted kidney, which of the following would help the nurse understand the course of events?
1 Maradiegue A. A resource guide for learning about genetics. Online J Issues Nurs. 2008:13.
2 Bradley AN. Utility and limitations of genetic testing and information. Nurs Stand. 2005;20(2):52–55.
3 Royal Australian College of Pathologists. Catalogue of genetic tests and laboratories. Available at http://genetictesting.rcpa.edu.au/. accessed 25 March 2011.
4 National Center for Biotechnology Information, National Library of Medicine, National Institutes of Health. Gene tests. Available at www.ncbi.nlm.nih.gov/sites/GeneTests/?db=GeneTests. accessed 25 March 2011.
5 US National Library of Medicine, National Institutes of Health, Genetics Home Reference. Handbook of gene therapy. Available at http://ghr.nlm.nih.gov. accessed 21 September 2009.
6 Juglen LM, Pestka EL, Clawson ML, et al. Incorporating genetics and genomics into nursing practice: a demonstration. Online J Issues Nurs. 2008:13.
7 Vandenbusche LM. Integrating genetics into primary care: family history is key. Adv Prac Nurs eJournal. 2008.
8 US Department of Health and Human Services, National Institutes of Health. Stem cell basics. Available at http://stemcells.nih.gov/staticresources/info/basics/SCprimer2009.pdf. accessed 25 March 2011.
9 Coico R, Sunshine G. Immunology: a short course, 6th edn. Hoboken, NJ: John Wiley & Sons, 2009.
10 Goodwin K, Viboud C, Simonsen L. Antibody response to influenza vaccination in the elderly: a quantitative review. Vaccine. 2005;24(8):1159–1169.
11 Hummell D. Common pediatric allergy-related disorders. Contemp Pediatr. 2008;25:40.
12 Peavy RD, Metcalfe DD. Understanding the mechanisms of anaphylaxis. Curr Opin Allergy Clin Immunol. 2008;8:310–314.
13 Bryant H. Anaphylaxis: recognition, treatment, and education. Emerg Nurse. 2007;15:24–28.
14 Hunter S, Anderson JW, Langemo D, Hanson D, Thomson P. When your patient has atopic dermatitis. Nursing. 2008;38:55–56.
15 Goligher EC, Detsky AS. Migratory pulmonary infiltrates: Goodpasture syndrome. Can Med Assoc J. 2009;180:75–77.
16 Bundesen IM. Natural rubber latex: a matter of concern for nurses. AORN J. 2008;88:197–210.
17 Cooper C. Multiple chemical sensitivity in the clinical setting. Am J Nurs. 2007;107:40–47.
18 McLeod BC. Evidence based therapeutics apheresis in autoimmune and other hemolytic anemias. Curr Opin Hematol. 2007;14:647–654.
19 Leighton SC. The spin on apheresis. Nursing. 2008;38:29–31.
20 Turner D. The human leucocyte antigen (HLA) system. Vox Sang. 2004;87(suppl 1):87–90.
21 Tan J, Yang S, Cai J, et al. Simultaneous islet and kidney transplantation in seven patients with type 1 diabetes and end-stage renal disease using a glucocorticoid-free immunosuppressive regimen with alemtuzumab induction. Diabetes. 2008;57:2666–2671.
22 Linda EB. Speaking up for organ donors. Nursing. 2009;39:28.
23 Donate Life. Available at www.donatelife.gov.au accessed 25 March 2011.
24 Australia and New Zealand Dialysis and Transplant Registry. Annual report of the Australia and New Zealand Organ Donation Registry. Available at www.anzdata.org.au, 2010. accessed 25 March 2011.
25 Moore J, Middleton L, Cockwell P, Adu D, Ball S, et al. Calcineurin inhibitor sparing with mycophenolate in kidney transplantation: a systemic review and meta-analysis. Transplantation. 2009;87:591–605.
26 Zand MS. Immunosuppression and immune monitoring after renal transplantation. Semin Dial. 2005;18(6):511–519.
27 Hahn T, McCarthy PL, Zhang MJ, Wang D, Arora M, et al. Risk factors for acute graft-versus-host disease after human leukocyte antigen-identical sibling transplants for adults with leukemia. J Clin Oncol. 2008;28:5728–5734.
American Society of Human Genetics. www.ashg.org/genetics/ashg/ashgmenu.htm
Asthma and Allergy Foundation of America. www.aafa.org
Australasian Society of Clinical Immunology and Allergy. www.allergy.org.au
Australian Infection Control Association. www.aica.org.au
Australian Society for Microbiology. www.theasm.com.au
Australian Stem Cell Centre. www.stemcellcentre.edu.au
Centers for Disease Control and Prevention: resources and tools for genetics and genomics training. www.cdc.gov/genomics/training/resources.htm
Essential Nursing Competencies and Curricula Guidelines for Genetics and Genomics. www.genome.gov/17517146
GeneTests. www.geneclinics.org
Human Genome Project. www.ornl.gov/sci/techresources/Human_Genome/home.shtml
International Society of Nurses in Genetics. www.isong.org
National Coalition for Health Professional Education in Genetics. www.nchpeg.org
National Human Genome Research Institute. www.nhgri.nih.gov
National Institute of Allergy and Infectious Diseases. www.niaid.nih.gov
Nursing Competencies Relating to Genetics. www.genome.gov/27527634
Royal Australian College of Pathologists: catalogue of genetic tests and laboratories. http://genetictesting.rcpa.edu.au/
Understanding Gene Testing. www.accessexcellence.org/AE/AEPC/NIH