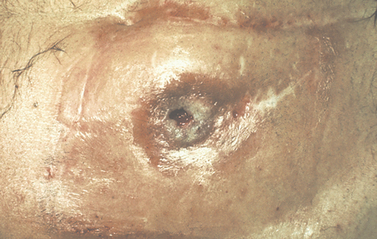
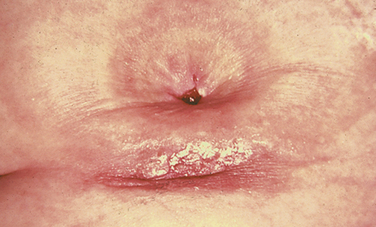
Strictures
A stricture is a narrowing of the lumen of the ureter or urethra.
URETERAL STRICTURES
Ureteral strictures can affect the entire length of the ureter, from the PUJ to the VUJ. These strictures are usually an unintended result of surgical intervention, often secondary to adhesions or scar formation. Depending on its severity, ureteral obstruction can threaten the function of the kidney. Clinical manifestations of a ureteral stricture include mild to moderate colic. This pain may be of moderate to severe intensity if the patient consumes a large volume of fluids (such as alcohol) over a brief period. Infection is unusual unless a calculus or foreign object such as a stent or nephrostomy tube is present.
The discomfort and obstruction of a ureteral stricture may be temporarily bypassed by placing a stent under endoscopic control or by diverting urinary flow via a nephrostomy tube inserted into the renal pelvis of the affected kidney. Definitive correction requires dilation with a balloon or catheter. If the stricture is severe or recurs after initial balloon or catheter dilation, it may be incised under endoscopic control (endoureterotomy). In selected cases, an open surgical approach may be required to excise the stenotic area and reanastomose the ureter to the contralateral ureter (ureteroureterostomy) or to the renal pelvis. Alternatively, distal ureteral strictures may be treated by a ureteroneocystostomy (reimplantation of the ureter into the bladder wall).
URETHRAL STRICTURE
A urethral stricture is the result of fibrosis or inflammation of the urethral lumen. Causes of urethral strictures include trauma, urethritis (particularly following gonococcal infection), iatrogenic (following surgical intervention or repeated catheterisations) or a congenital defect in the canalisation of the urethra. Once the process of inflammation and fibrosis begins, the lumen of the urethra narrows, and its compliance (ability to close or open in response to bladder filling or micturition) is compromised. Meatal stenosis, a narrowing of the urethral opening, is also common. A urethral stricture creates symptoms when it creates voiding dysfunction or bladder outlet obstruction.
Clinical manifestations associated with a urethral stricture include a diminished force of the urinary stream, straining to void, sprayed stream, postvoid dribbling or a split urine stream. The patient may report feelings of incomplete bladder emptying with urinary frequency and nocturia. Moderate to severe obstruction of the bladder outlet may lead to acute urinary retention. The patient may report a history of urethritis, difficulty with insertion of a urinary catheter, or trauma involving the penis or perineum. However, many patients are unable to recall any such events, thus leading to a diagnosis of an idiopathic stricture. A history of a UTI is not uncommon, particularly if the stricture involves the distal urethra. Retrograde urethrography (RUG) and voiding cystourethrography (VCUG) will identify stricture length, location and calibre.
Initial management of a stricture may be based on dilation. A metal instrument (urethral sound) may be placed or a series of progressively enlarging stents (filiforms and followers) can be placed into the urethra to expand its lumen in a stepwise fashion. Although initially successful, recurring stenosis is frequent. Recurrences may be managed by teaching the patient to repeatedly dilate the urethra by self-catheterisation using a soft (coudé-tip, red rubber) catheter every few days. Alternatively, an endoscopic or open surgical procedure (urethroplasty) may be performed to provide a more durable solution to an obstructive urethral stricture. Shorter strictures may be treated by resection of the fibrotic area with primary reanastomosis. Longer strictures may require autotransplantation of a substitute segment such as a skin flap.
RENAL TRAUMA
A continual increase in the incidence of traumatic renal injuries is related to the increase in violent crimes and injuries and in the mechanisation and speed of transportation. The majority of incidents occur in men younger than 30 years of age. Blunt trauma is the most common cause. Injury to the kidney should be considered in multiple or sports injuries, traffic accidents and falls. It is especially likely when the patient injures the abdomen, flank or back, as the kidney is injured in 5% of abdominal trauma cases, making it the most susceptible genitourinary organ.34 Penetrating injuries may result from violent encounters (e.g. gunshot or stabbing incidents) or from iatrogenic injuries.
Clinical findings include a history of trauma to the area of the kidneys. Gross or microscopic haematuria may be present. Diagnostic studies include urinalysis, IVP with cystography, and ultrasound, CT or MRI evaluation. Renal arteriography may also be used. Both the injured kidney and the non-involved kidney should be evaluated to provide information for further management. The severity of renal trauma depends on the extent of the injury. Treatments range from bed rest, fluids and analgesia to surgical exploration and repair or nephrectomy.
Nursing interventions vary with the type of renal trauma sustained and the extent of any associated injuries. Interventions related to renal trauma include assessing the cardiovascular status, monitoring for shock (e.g. penetrating injury), ensuring increased fluid intake, monitoring intake and output, providing comfort measures, observing for haematuria, determining the presence of myoglobinuria and monitoring potentially nephrotoxic antibiotics.
RENAL VASCULAR PROBLEMS
Vascular problems involving the kidney include: (1) nephrosclerosis; (2) renal artery stenosis; and (3) renal vein thrombosis.
Nephrosclerosis
Nephrosclerosis consists of sclerosis of the small arteries and arterioles of the kidney. There is decreased blood flow, which results in patchy necrosis of the renal parenchyma. Ischaemic necrosis and destruction of glomeruli with subsequent fibrosis also occur.
Benign nephrosclerosis usually occurs in adults 30–50 years of age. It is caused by vascular changes resulting from hypertension and from the atherosclerosis process. Atherosclerotic vascular changes account for most of the loss of renal function associated with ageing. There is a direct relationship between the degree of nephrosclerosis and the severity of hypertension. The patient with benign nephrosclerosis may have normal kidney function in the early stages. The only detectable abnormality may be hypertension.
Accelerated nephrosclerosis, or malignant nephrosclerosis, is associated with malignant hypertension, a complication of hypertension characterised by a sharp increase in blood pressure with a diastolic pressure greater than 130 mmHg. The patient is usually a young adult, with a male-to-female predominance of 2:1. Renal insufficiency progresses rapidly.
Treatment for benign nephrosclerosis is the same as that for essential hypertension (see Ch 32). Malignant nephrosclerosis is treated with aggressive antihypertensive therapy (see Ch 32). The availability and use of antihypertensive drugs have improved the prognosis for patients with benign and malignant nephrosclerosis. Renal dysfunction and renal failure are two major complications of hypertension. The prognosis for a patient with untreated or refractive malignant hypertension is poor, with the major cause of death related to kidney failure.
Renal artery stenosis
Renal artery stenosis is a partial occlusion of one or both renal arteries and their major branches. It can be due to atherosclerotic narrowing or fibromuscular hyperplasia. Renal artery stenosis accounts for 1–2% of all cases of hypertension.
When hypertension develops rather abruptly, renal artery stenosis should be considered as a possible cause, especially in the patient under 30 or over 50 years of age and in a patient with no familial history of hypertension. This contrasts with the age distribution for essential hypertension, which is 30–50 years of age. A renal arteriogram is the best diagnostic tool for identifying renal artery stenosis.
The goals of therapy are to control the blood pressure and restore perfusion to the kidney. Percutaneous transluminal renal angioplasty is the procedure of first choice, especially in older patients who are poor surgical risks. Surgical revascularisation of the kidney is indicated when blood flow is decreased enough to cause renal ischaemia or when evidence indicates that renovascular hypertension is present and surgical intervention may result in the patient becoming normotensive. The surgical procedure usually involves anastomoses between the kidney and another major artery, usually the splenic artery or aorta. In selected cases of unilateral renal involvement with high renin production, unilateral nephrectomy may be indicated.
Renal vein thrombosis
Renal vein thrombosis may occur unilaterally or bilaterally. Trauma, extrinsic compression (e.g. tumour, aortic aneurysm), renal cell carcinoma, pregnancy, contraceptive use and nephrotic syndrome are associated with renal vein thrombosis.
The patient has symptoms of flank pain, haematuria or fever, or has nephrotic syndrome. Anticoagulation (e.g. heparin, warfarin) is important in treatment because there is a high incidence of pulmonary emboli. Corticosteroids may be used for the patient with nephrotic syndrome. Surgical thrombectomy may be performed instead of or along with anticoagulation.
HEREDITARY RENAL DISEASES
Hereditary renal diseases involve developmental abnormalities of the renal parenchyma. These abnormalities are either isolated or part of more complex malformation syndromes. The majority of inherited structural abnormalities are cystic. However, cysts may also develop as a result of obstructive uropathies, metabolic derangements or neurological diseases. Cysts may be evaluated to rule out any tumour content.
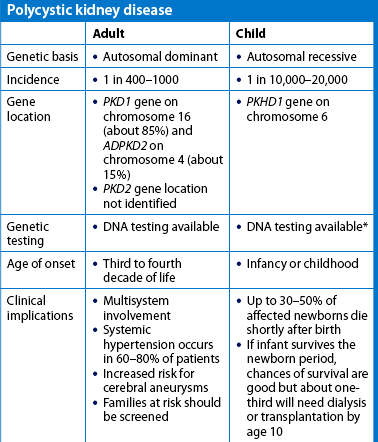
Polycystic kidney disease
Polycystic kidney disease (PKD) is the most common life-threatening genetic disease in the world, affecting 12.5 million people worldwide.35 It is the fourth highest cause of kidney failure in New Zealand and Australia.23 PKD accounts for 10–15% of chronic kidney disease. There are two forms of hereditary PKD: it may be manifested in childhood or adulthood. The childhood form of PKD is a rare autosomal recessive disorder that is often rapidly progressive (see the Health disparities box).
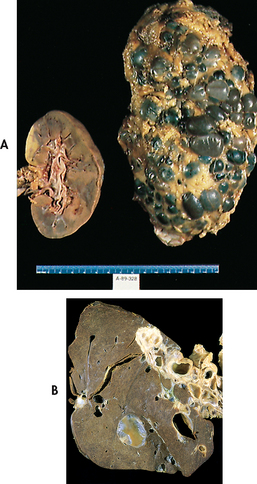
The adult form of PKD is an autosomal dominant disorder (see Table 13-2 and Fig 13-2). If one parent has the disease, there is a 50% chance that the disease will pass to the child. It is latent for many years and is usually manifested between 30 and 40 years of age. However, PKD has also been found in newborns. It involves both kidneys and occurs in both men and women. The cortex and the medulla are filled with large, thin-walled cysts that are several millimetres to several centimetres in diameter (see Fig 45-8, A). The cysts enlarge and destroy surrounding tissue by compression. The cysts are filled with fluid and may contain blood or pus. PKD kidneys examined during autopsy appear as if each is filled with golf balls.
Early in the disease there are generally no symptoms. Symptoms appear when the cysts begin to enlarge. Often the first manifestations are hypertension, haematuria (from rupture of cysts) or a feeling of heaviness in the back, side or abdomen. However, the first manifestations can also be a UTI or urinary calculi. Chronic pain is one of the most common problems experienced by individuals with PKD. The pain can be constant and severe. Bilateral, enlarged kidneys are often palpable on physical examination. In many people there are no symptoms and the disease is not diagnosed.
Diagnosis is based on clinical manifestations, family history, IVP, ultrasound (best screening measure) or CT scan. The disease usually progresses with loss of kidney function to the point of end-stage kidney disease occurring by age 60 in 50% of patients.35 PKD can also affect the liver (liver cysts; see Fig 45-8, B), heart (abnormal heart valves), blood vessels (aneurysms) and intestines (diverticulosis). The most serious complication is a cerebral aneurysm, which can rupture.
 NURSING AND COLLABORATIVE MANAGEMENT: POLYCYSTIC KIDNEY DISEASE
NURSING AND COLLABORATIVE MANAGEMENT: POLYCYSTIC KIDNEY DISEASE
There is no specific treatment for PKD. A major aim of treatment is to prevent infections of the urinary tract and/or to treat them with appropriate antibiotics if they occur. Nephrectomy may be necessary if pain, bleeding or infection becomes a chronic, serious problem. Dialysis and kidney transplant may be needed to treat end-stage kidney disease (see Ch 46).
When the patient begins to experience progressive kidney failure, the interventions are determined by the remaining kidney function. Nursing measures are those used for management of end-stage kidney disease. They include diet modification, fluid restriction, drugs (e.g. antihypertensives), assisting the patient to accept the chronic disease process and assisting the patient and family to deal with financial concerns and other issues related to the hereditary nature of the disease. The patient who has adult PKD often has children by the time the disease is diagnosed. The patient will need appropriate counselling regarding plans for having more children. In addition, genetic counselling information should be provided for the children.
Medullary cystic disease
Medullary cystic disease is a hereditary disorder that occurs in two forms. The autosomal recessive form is associated with kidney failure before age 20; the autosomal dominant form is associated with kidney failure after age 20. Most cysts are located in the medulla. The kidneys are asymmetrical in shape and are significantly scarred. There are defects in the concentration ability of the kidneys. Polyuria, progressive kidney failure, severe anaemia, metabolic acidosis and poor sodium conservation are common. Hypertension can be a terminal event. Genetic counselling may be helpful in family planning. Treatment measures are those related to end-stage kidney disease (see Ch 46).
Alport’s syndrome
Alport’s syndrome is also known as chronic hereditary nephritis. Two forms of the disease exist: (1) classic Alport’s syndrome, which is inherited as a sex-linked disorder with haematuria, sensorineural deafness and deformities of the anterior surface of the lens; and (2) non-classic Alport’s syndrome, which is inherited as an autosomal trait that causes haematuria but not deafness or lens deformities. Men are affected earlier and more severely than women. The disease is often diagnosed in the first decade of life. The basic defect is a mutation in a gene for collagen that results in altered synthesis of the GBM. The patient most commonly has haematuria and progressive uraemia. Treatment is supportive. Corticosteroids and cytotoxic drugs are not effective. The disease does not recur after kidney transplantation.
RENAL INVOLVEMENT IN METABOLIC AND CONNECTIVE TISSUE DISEASES
Various metabolic and connective tissue disease processes may have an effect on kidney function. The pathophysiological effects on the renal parenchyma are not always specific to each process. The clinical course of kidney involvement is that of chronic progressive nephropathy, which can result in uraemia and death. Management includes treatment of the primary disorder along with symptomatic relief of kidney involvement. If kidney involvement progresses to end-stage kidney disease, management includes dialysis or transplantation. Nursing interventions include teaching the patient about the primary disease process, the kidney involvement and the resulting need to comply with dietary and fluid restrictions and drug regimens.
Diabetic nephropathy is the primary cause of end-stage kidney failure in New Zealand and Australia.23 Diabetes mellitus may affect the kidneys in several ways. Microangiopathic changes in diabetes consist of diffuse glomerulosclerosis, involving thickening of the GBM, and nodular glomerulosclerosis (Kimmelstiel-Wilson syndrome), which is characterised by nodular lesions. Nodular glomerulosclerosis is reasonably specific for type 1 diabetes mellitus. The diabetic patient prone to glomerulonephropathy (e.g. the presence of trace proteinuria or retinopathy) requires careful monitoring of glucose levels and insulin requirements. Diabetes mellitus is discussed in Chapter 48.
Gout is a syndrome of acute attacks of arthritis caused by hyperuricaemia (see Ch 64). Monosodium urate crystals deposited in joints are responsible for the syndrome. Renal disease may develop as a result of damage caused by deposition of uric acid crystals in the renal interstitium and tubules.
Amyloidosis is a group of disorders manifested by impaired organ function from the infiltration of tissues with a hyaline substance (amyloid). The hyaline bodies consist largely of protein. Kidney involvement is common in amyloidosis. Proteinuria is often the first clinical manifestation.
Systemic lupus erythematosus is a connective tissue disorder characterised by the involvement of several tissues and organs, particularly the joints, skin and kidneys. Systemic lupus erythematosus is discussed in Chapter 64. Clinical manifestations of lupus nephritis are similar to those of other forms of glomerulonephritis. Kidney failure frequently occurs in systemic lupus erythematosus and has a poor prognosis.
Systemic sclerosis (scleroderma) is a disease of unknown aetiology characterised by widespread alterations of connective tissue and vascular lesions in many organs (see Ch 64). In the kidneys, vascular lesions are associated with fibrosis. An immune complex mechanism has been postulated as a possible aetiological factor. The severity of kidney involvement varies. The patient who develops severe renal lesions has a poor prognosis.
Kidney cancer
Approximately 2200 new cases of kidney cancer are diagnosed each year and it is the tenth most frequently seen cancer in Australia.36 In New Zealand, kidney cancer accounts for approximately 2% of all cases of cancer. Kidney cancers arise from the cortex or pelvis (and calyces). Tumours arising from both areas may be benign or malignant. However, malignant tumours are more frequent. Renal cell carcinoma (adenocarcinoma) is the most common type (see Fig 45-10). Adenocarcinoma occurs twice as often in men as in women, and is typically discovered when the person is 50–70 years of age. Cigarette smoking is the most significant risk factor for the development of renal cell carcinoma. An increased incidence has also been seen in first-degree relatives. Other risk factors include obesity, hypertension and exposure to asbestos, cadmium and petroleum.36 Risk for renal cancer is also increased for individuals who have acquired cystic disease of the kidney associated with end-stage kidney disease (see Ch 46).
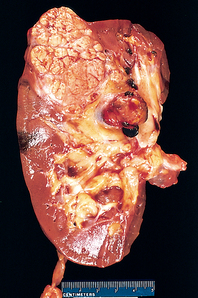
Figure 45-10 Cross-section of kidney with renal cell carcinoma. The carcinoma is on the pole of the kidney. Note that the renal vein is involved and thrombosed.
CLINICAL MANIFESTATIONS AND DIAGNOSTIC STUDIES
Since there are no characteristic early symptoms of kidney cancer, many patients go undiagnosed until the disease has significantly progressed. Kidney cancer can cause symptoms by compressing, stretching or invading structures near or within the kidney. The most common presenting manifestations are haematuria, flank pain and a palpable mass in the flank or abdomen. Other clinical manifestations include weight loss, fever, hypertension and anaemia.
About 30% of patients have metastasis at the time of diagnosis. Local extension of kidney cancer into the renal vein and vena cava is common (see Fig 45-10). The most common sites of metastases include the lungs, liver and long bones. Many kidney cancers are diagnosed as incidental findings on imaging studies used to evaluate symptoms for unrelated conditions.
CT scan is commonly used in the diagnosis of kidney cancer. Ultrasound examinations have improved the ability to differentiate between a solid mass tumour and a cyst. This is significant because the majority (90%) of masses detected on imaging are cysts. Angiography, percutaneous needle aspiration, MRI and IVP are also used in the diagnosis of renal tumours. Small renal tumours are being found earlier due to the increased use of CT and MRI scans. Radionuclide isotope scanning is used to detect metastases.
 NURSING AND COLLABORATIVE MANAGEMENT: KIDNEY CANCER
NURSING AND COLLABORATIVE MANAGEMENT: KIDNEY CANCER
Robson’s system is used to stage renal carcinoma and provides a basis for determining treatment options (see Table 45-5). The treatment of choice for renal cancer is a radical nephrectomy for patients with stage I or II tumours and selected stage III tumours. Nephrectomy is performed by the standard open approach or laparoscopically. Radical nephrectomy is the removal of the kidney, adrenal gland, surrounding fascia, part of the ureter and the draining lymph nodes.37 Radiation therapy is used palliatively in inoperable cases and when there is metastasis to bone or lungs.
TABLE 45-5 Robson’s system of staging renal carcinoma
| Stage | Description |
|---|---|
| I | Limited to kidney; small tumour <7 cm |
| II | Spreading to perirenal fat but confined within fascia |
| III | Tumour invades renal vein or vena cava, regional lymph node involvement, or both |
| IV | Presence of metastases |
Other treatment options include cryoablation (freezing technique) and radiofrequency ablation (destroying tumour by using radiofrequency heat). These procedures are frequently used when surgery is not an option (e.g. the patient has coexisting conditions).
Chemotherapy is used as a treatment in metastatic disease. These drugs include 5-fluorouracil, floxuridine and gemcitabine. However, renal cell carcinoma is refractory to most chemotherapy drugs. Biological therapy, including α-interferon and interleukin-2 (IL-2), is another treatment in metastatic disease.37 (The use of α-interferon and IL-2 is discussed in Ch 15.) Targeted therapy is also used to treat metastatic kidney cancer and includes sunitinib, sorafenib, temsirolimus, everolimus, ofatumumab, bevacizumab and pazopanib. Although the survival rate is significantly decreased, patients with metastatic disease often remain stable for a prolonged period of time.
Bladder cancer
Bladder cancer accounts for about 1 in every 25 cancers diagnosed in Australia and New Zealand.38,39 The most frequent malignant tumour of the urinary tract is transitional cell carcinoma (TCC) of the bladder. Most bladder tumours are papillomatous growths within the bladder (see Fig 45-11). Cancer of the bladder is most common between the ages of 60 and 70 and is at least three times as common in men as in women. Risk factors for bladder cancer include cigarette smoking, exposure to dyes used in the rubber and cable industries, and chronic abuse of phenacetin-containing analgesics. Phenacetin, once a popular analgesic, has been removed from the market. Women treated with radiation for cervical cancer and patients receiving cyclophosphamide also have increased risk, but the reason is unknown.38
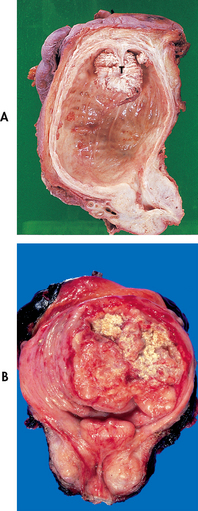
Figure 45-11 A, Papillary transitional cell carcinoma seen arising from the dome of the bladder as a cauliflower-like lesion. B, Opened bladder showing a bladder cancer at an advanced stage. Yellow areas represent ulcerations and necrosis.
Individuals with chronic, recurrent renal calculi (often bladder) and chronic lower UTIs have an increased risk of squamous cell cancer (SCC) of the bladder. Patients who have indwelling catheters for long periods can also develop bladder cancer.
CLINICAL MANIFESTATIONS AND DIAGNOSTIC STUDIES
Microscopic or gross, painless haematuria (chronic or intermittent) is the most common clinical finding. Bladder irritability with dysuria, frequency and urgency may also occur. When cancer is suspected, urine specimens can be obtained for cytology to determine the presence of neoplastic or atypical cells. Exfoliated cells from the epithelial surface of the bladder can be detected readily in voided specimens. Other urine tests assess for specific factors associated with bladder cancer, such as bladder tumour antigens. Bladder cancer can be detected using CT, ultrasound or MRI. However, the presence of cancer is confirmed by cystoscopy and biopsy. Cystoscopy is the most reliable test for detecting the presence of bladder tumours.39
The clinical staging of bladder cancer is determined by the depth of invasion of the bladder wall and surrounding tissue. The Jewett-Strong-Marshall classification system broadly classifies bladder cancer as superficial (carcinoma in situ [CIS], O, A), invasive (B1, B2, C) or metastatic (D1–D4) disease. Pathological grading systems are also used to classify the malignant potential of tumour cells, indicating a scale from well-differentiated to anaplastic categories. Eighty per cent of bladder tumours are ‘superficial’, meaning they do not invade the bladder wall. These low-stage, low-grade bladder cancers are the most responsive to treatment, which includes instillation of intravesical chemotherapy and transurethral resection of the bladder tumour (TURBT). Although patients with superficial tumours are more easily cured, periodic surveillance is important, as two-thirds of patients have tumour recurrence within 5 years and nearly 95% have recurrence within 15 years.40
 NURSING AND COLLABORATIVE MANAGEMENT: BLADDER CANCER
NURSING AND COLLABORATIVE MANAGEMENT: BLADDER CANCER
Multidisciplinary care of bladder cancer is outlined in Box 45-11.
 Surgical therapy
Surgical therapy
A variety of surgical procedures are used to treat or manage bladder cancer. Transurethral resection with fulguration (electrocautery) is used for the diagnosis and treatment of superficial lesions with a low recurrence rate. This procedure is also used to control bleeding in the patient who is a poor operative risk or who has advanced tumours. With this technique, the tumour mass is excised by means of a blade inserted through the cystoscope. The remaining portions of the tumour are cauterised.
A second technique, laser photocoagulation, is also used to treat superficial bladder cancers. This procedure can be repeated a number of times for recurrence. The advantages of laser include bloodless destruction of the lesion, minimal risk of perforation and lack of need for a urinary catheter. The primary disadvantage is destruction of the tumour so pathological evaluation for grading and staging cannot be completed.
A third technique is open loop resection (snaring of polyp types of lesion) with fulguration, which is used for the control of bleeding, for large superficial tumours and for multiple lesions. Treatment of large lesions entails a segmental resection of the bladder (segmental cystectomy).
Postoperative management of the patient who has had any of these surgical procedures includes instructions to drink a large volume of fluid each day for the first week following the procedure and to avoid intake of alcoholic beverages. The patient is taught to self-monitor the urine. It is anticipated that it will be pink during the first several days after the procedure, but it should not be bright red or contain blood clots. Approximately 7–10 days following tumour resection or ablation, the patient may observe dark-red or rust-coloured flecks in the urine. These are scabs from the healing tumour resection sites.
Opioid analgesics may be required for a brief period after the procedure, along with stool softeners. The patient should be encouraged to take a 15- to 20-minute sitz bath two or three times a day to promote muscle relaxation and to reduce the risk of urinary retention. The nurse should also help the patient and family cope with fears about cancer, surgery and sexuality, and should emphasise the importance of regular follow-up care. Follow-up cystoscopies are required every 3–6 months for 3 years and at least yearly thereafter.41
When the tumour is invasive or when it involves the trigone (the area where the ureters insert into the bladder) and the patient is free from metastasis beyond the pelvic area, a partial or radical cystectomy with urinary diversion is the treatment of choice (see the section on urinary diversion later in the chapter). A partial cystectomy includes resection of that portion of the bladder wall containing the tumour, along with a margin of normal tissue. A radical cystectomy involves removal of the bladder, prostate and seminal vesicles in men, and the bladder, uterus, cervix, urethra and ovaries in women.
 Radiation therapy and chemotherapy
Radiation therapy and chemotherapy
Radiation therapy is used with cystectomy or as the primary therapy when the cancer is inoperable or when surgery is refused.42 Increasingly, radiation therapy is being combined with systemic chemotherapy. Sometimes combination systemic chemotherapy is used for bladder cancer, usually preoperatively or before radiation therapy, or is used to treat distant metastases. Chemotherapy drugs used in treating invasive bladder cancer include cisplatin, vinblastine, doxorubicin and methotrexate.
 Intravesical therapy
Intravesical therapy
Chemotherapy with local instillation of chemotherapeutic or immune-stimulating agents can be delivered directly into the bladder by a urethral catheter.43 Protocols vary, but intravesical therapy is usually initiated at weekly intervals for 6–12 weeks. The chemotherapeutic agents are instilled directly into the patient’s bladder and retained for about 2 hours. The patient’s bladder must be empty before instillation. The patient’s position may be changed every 15 minutes for maximum contact in all areas of the bladder, especially if the tumour occurs on the bladder dome. The use of maintenance therapy after the initial induction regimen may be beneficial.
BCG, a weakened strain of Mycobacterium bovis, is the treatment of choice for carcinoma in situ. BCG stimulates the immune system rather than acting directly on cancer cells in the bladder. If BCG fails, α-interferon may be used in addition to BCG. Other treatments that can be used when BCG fails include thiotepa, an alkylating agent, and valrubicin, an antineoplastic antibiotic.44
Most patients have irritative voiding symptoms and haemorrhagic cystitis following intravesical therapy. Thiotepa can significantly reduce WBC and platelet counts in some individuals when absorbed into the circulation from the bladder wall. BCG may cause flu-like symptoms, increased urinary frequency, haematuria or systemic infection. Other side effects usually associated with chemotherapy, such as nausea, vomiting and hair loss, are not experienced with intravesical chemotherapy.
Nursing responsibilities include encouraging the patient to increase the daily fluid intake and to quit smoking, assessing the patient for secondary UTI and stressing the need for routine urological follow-up. The patient may have fears or concerns about sexual activity or bladder function that will need to be addressed.
Urinary retention
Urinary retention is the inability to empty the bladder despite micturition or the accumulation of urine in the bladder because of an inability to urinate.45 In certain cases, it is associated with urinary leakage or postvoid dribbling, called overflow UI. Acute urinary retention is the total inability to pass urine via micturition; it is a medical emergency. Chronic urinary retention is defined as incomplete bladder emptying despite urination. The postvoid residual (PVR) volumes in patients with chronic urinary retention vary widely. Normal PVR is between 50 and 75 mL, and findings over 100 mL indicate the need to repeat the measurement. An abnormal PVR in the older patient of ≥200 mL obtained on two separate occasions requires further evaluation.45 Even smaller volumes may justify evaluation when the patient has lower urinary tract symptoms suggestive of UTIs or these small volumes occur in a context of recurring UTIs.
Is pelvic floor muscle training helpful in treating urinary incontinence?
EVIDENCE-BASED PRACTICE
Clinical question
For women with urinary incontinence (P), is pelvic floor muscle training (I) effective in reducing incontinence episodes (O)?
Critical appraisal and synthesis of evidence
• Incontinence risk factors were examined in women (n = 1007 studies).
• Lifestyle risk factors including obesity, limited physical activity, poor diet and smoking were related to incontinence.
• Pelvic floor exercises were effective in reversing incontinence during the first year after childbirth with some short-term effectiveness in older women.
Implications for nursing practice
• Screen for urinary incontinence using simple questions such as, ‘How often do you leak urine?’
• Counsel patients about healthy lifestyle changes to help them prevent and treat incontinence.
• Inform patients that pelvic floor exercises, biofeedback and surgical treatments may help with incontinence.
P, patient population of interest; I, intervention or area of interest; O, outcome(s) of interest.
Landefeld CS, Bowers BJ, Feld AD, et al. National Institutes of Health state-of-the-science conference statement: prevention of fecal and urinary incontinence in adults. Ann Intern Med. 2008;148:449.
Shamliyan T, et al. Prevention of fecal and urinary incontinence in adults. Evid Rep Technol Assess. 2007;161:1.
Urinary retention is caused by two different dysfunctions of the urinary system: bladder outlet obstruction and deficient detrusor (bladder muscle) contraction strength. Obstruction leads to urinary retention when the blockage is sufficiently severe so that the bladder can no longer evacuate its contents despite a detrusor contraction. A common cause of obstruction in men is an enlarged prostate. Deficient detrusor contraction strength leads to urinary retention when the muscle is no longer able to contract with enough force or for a sufficient period of time to completely empty the bladder.
Common causes of deficient detrusor contraction strength are neurological diseases affecting sacral segments 2, 3 and 4; long-standing diabetes mellitus; overdistension; chronic alcoholism; and drugs (e.g. anticholinergic drugs).
Diagnostic studies
The basic evaluation for urinary retention includes a focused history, physical assessment and a bladder log or voiding record whenever possible.46
A urinalysis is used to identify possible factors contributing to urinary retention (or transient UI) (e.g. UTI, diabetes mellitus). PVR urine must be measured in the patient undergoing evaluation for urinary retention (and UI). The PVR volume is obtained by asking the patient to urinate, followed by catheterisation within a relatively brief period (preferably 10–20 minutes). Alternatively, and more commonly, a bladder ultrasound can be used to estimate the residual volume.
Urodynamic testing is indicated in selected cases of urinary retention (and UI). Imaging studies of the upper urinary tract (e.g. ultrasound) are obtained when retention (or UI) is associated with UTIs or when there is evidence of upper urinary tract involvement.
MULTIDISCIPLINARY CARE
Behavioural therapies may be used in the management of urinary retention.33 Scheduled toileting and double voiding may be effective in chronic urinary retention with moderate PVR volumes. Double voiding is an attempt to maximise bladder evacuation. The patient is asked to urinate, sit on the toilet for 3–4 minutes and urinate again before exiting the bathroom.
For acute or chronic urinary retention, catheterisation may be required. Ideally, intermittent catheterisation is used to manage urinary retention. It allows the patient to remain free of an indwelling catheter with its associated risk of UTI and urethral irritation. Despite these potential advantages, an indwelling catheter is preferred in certain cases (e.g. the patient who is unwilling or unable to perform intermittent catheterisation). An indwelling catheter is also used when urethral obstruction renders intermittent catheterisation uncomfortable or infeasible.
Drug therapy
Several drugs may be administered to promote bladder evacuation. For the patient with obstruction at the level of the bladder neck, an α-adrenergic blocker may be prescribed. These drugs relax the smooth muscle of the bladder neck, the prostatic urethra and possibly the dual-innervated rhabdosphincter, diminishing urethral resistance. Examples of α-adrenergic blocking agents are listed in Table 45-9. They are indicated in patients with benign prostatic hyperplasia, bladder neck dyssynergia or detrusor sphincter dyssynergia.
Surgical therapy
Surgical interventions are often useful when managing urinary retention caused by obstruction. Transurethral or open surgical techniques are used to treat benign or malignant prostatic enlargement, bladder neck contracture, urethral strictures or bladder neck dyssynergia in selected patients. Pelvic reconstruction using an abdominal or transvaginal approach can be used to correct bladder outlet obstruction in women with severe pelvic organ prolapse.
Unfortunately, surgery has a minimal role in the management of urinary retention caused by deficient detrusor contraction strength. Attempts to create a bladder stimulator (implanted device capable of stimulating micturition) have proved largely unsuccessful because of the difficulty in achieving a coordinated detrusor contraction associated with pelvic muscle and striated sphincter relaxation.
 NURSING MANAGEMENT: URINARY RETENTION
NURSING MANAGEMENT: URINARY RETENTION
Acute urinary retention is a medical emergency that requires prompt recognition and bladder drainage.47 The nurse should insert a catheter (as prescribed) unless otherwise directed. A catheter with a retention balloon is used in anticipation of the need for an indwelling catheter.
The patient with acute urinary retention (as well as the patient predisposed to these episodes) should be taught strategies to minimise risk, including avoiding intake of large volumes of fluid over a brief period. Instead, the patient should drink small volumes throughout the day. In addition, the patient should be instructed to warm up before attempting urination when chilled and to avoid large volumes of alcohol intake because it leads to polyuria and a diminished awareness of the need to urinate until the bladder is distended. The patient who is unable to urinate should be advised to drink a cup of coffee or brewed caffeinated tea to create or maximise urinary urgency and to sit in a tub of warm water or take a warm shower and attempt to urinate while in the bathtub or shower. The patient can be reassured that they can easily bathe immediately following bladder evacuation. If this does not lead to successful urination, the patient should be advised to seek immediate care.
Patients with chronic urinary retention may be managed by behavioural methods, indwelling or intermittent catheterisation, surgery or drugs.48 Scheduled toileting and double voiding are the primary behavioural interventions used for chronic retention. Scheduled toileting is used to reduce rather than expand bladder capacity. In this case, the patient voids every 3–4 hours regardless of the desire to urinate. This intervention is particularly useful in the patient with chronic overdistension, diabetes mellitus or chronic alcoholism characterised by a large bladder capacity and diminished or delayed sensations of bladder filling and urgency.
Urinary incontinence
Urinary incontinence (UI) is an uncontrolled leakage of urine.49 Among young adult to middle-aged women, prevalence is 30–40% and it increases to 30–50% in older women. In contrast, UI in men tends to be considerably lower, ranging from 1% to 5% in young adult men and increasing to 9–34% in older men.49 The prevalence of incontinence is higher among older women and older men, but it is not a natural consequence of ageing. Although UI has traditionally been viewed as a social or hygienic problem, it is now known to affect quality of life, as well as contributing to serious health problems in older adults.46
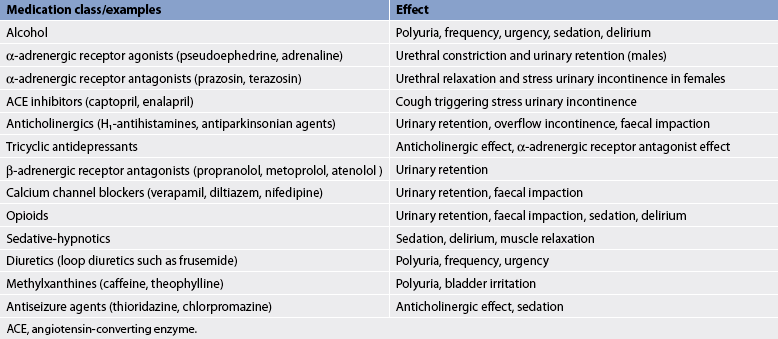
Anything that interferes with bladder or urethral sphincter control can result in UI. Causes can include confusion or depression, infection, atrophic vaginitis, urinary retention, restricted mobility, faecal impaction or drugs. (Table 45-6 presents a list of drugs that can affect the lower urinary tract.) Patients may have more than one type of incontinence (see Table 45-7).46
Drugs influencing lower urinary tract function
| Medication class/examples | Effect |
|---|---|
| Alcohol | Polyuria, frequency, urgency, sedation, delirium |
| α-adrenergic receptor agonists (pseudoephedrine, adrenaline) | Urethral constriction and urinary retention (males) |
| α-adrenergic receptor antagonists (prazosin, terazosin) | Urethral relaxation and stress urinary incontinence in females |
| ACE inhibitors (captopril, enalapril) | Cough triggering stress urinary incontinence |
| Anticholinergics (H1-antihistamines, antiparkinsonian agents) | Urinary retention, overflow incontinence, faecal impaction |
| Tricyclic antidepressants | Anticholinergic effect, α-adrenergic receptor antagonist effect |
| β-adrenergic receptor antagonists (propranolol, metoprolol, atenolol) | Urinary retention |
| Calcium channel blockers (verapamil, diltiazem, nifedipine) | Urinary retention, faecal impaction |
| Opioids | Urinary retention, faecal impaction, sedation, delirium |
| Sedative-hypnotics | Sedation, delirium, muscle relaxation |
| Diuretics (loop diuretics such as frusemide) | Polyuria, frequency, urgency |
| Methylxanthines (caffeine, theophylline) | Polyuria, bladder irritation |
| Antiseizure agents (thioridazine, chlorpromazine) | Anticholinergic effect, sedation |
ACE, angiotensin-converting enzyme.
TABLE 45-7 Types of urinary incontinence
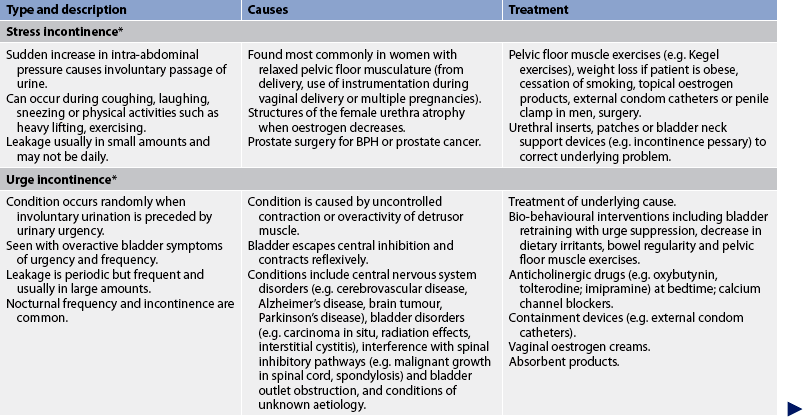
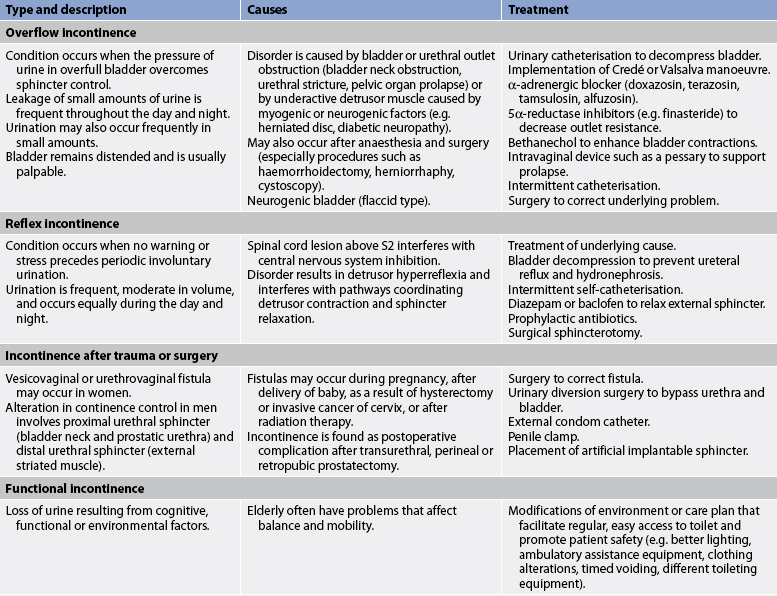
* Patients can have a combination of stress and urge incontinence referred to as mixed incontinence. BPH, benign prostatic hyperplasia.
DIAGNOSTIC STUDIES
As well as the studies outlined above under ‘Urinary retention’, there are some more specific diagnostic studies that are used to evaluate UI. Information should be obtained about the onset of UI, factors that provoke urinary leakage and associated conditions. Special attention should be paid to factors known to produce transient UI, particularly when a relatively sudden onset of urine loss is reported. The physical examination begins with an assessment of general health and functional issues associated with urinary function, including mobility, dexterity and cognitive function. A pelvic examination includes careful inspection of the perineal skin for signs of erosion or rashes related to UI and the existence of pelvic organ prolapse. Local innervation and pelvic floor muscle strength should also be evaluated, including a digital examination of the pelvic floor muscle to determine weakness or tension. Whenever possible, the patient is asked to keep a bladder log or voiding diary documenting the timing of urinations, episodes of urinary leakage and the frequency of nocturia for a period of 1–7 days.46 This record can be kept by nursing staff if the patient is in an inpatient or long-term care facility.
MULTIDISCIPLINARY CARE
An estimated 80% of incontinence can be cured or significantly improved. Transient, reversible factors are corrected initially, followed by management of the type of UI (see Table 45-7). In general, less invasive treatments are attempted before more invasive methods (e.g. surgery) are used. Nevertheless, the choice of initial treatment is highly individualised and based on patient preference, the type and severity of UI, and associated anatomical defects.
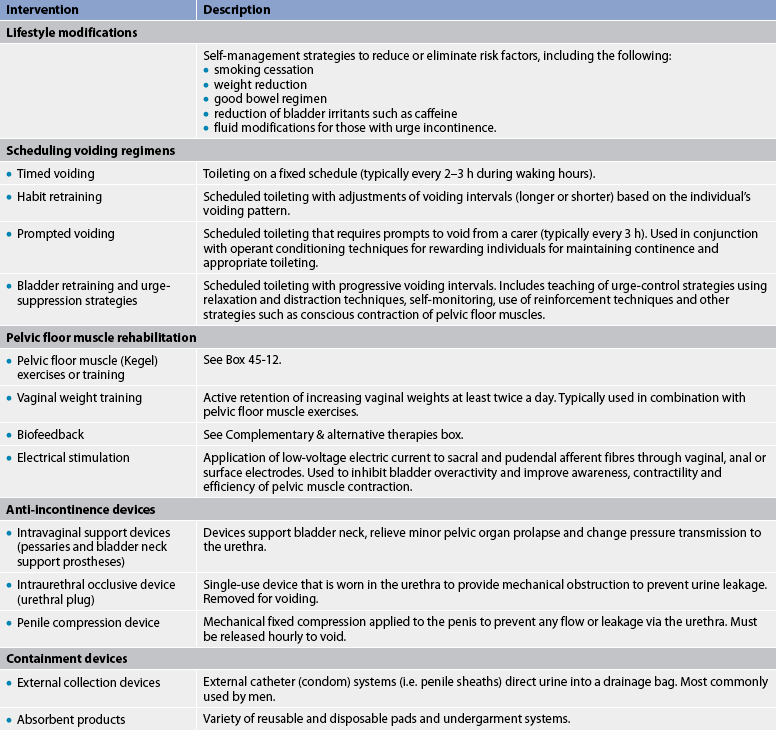
Several behavioural therapies may be employed to improve urinary incontinence.46 These interventions are outlined in Table 45-8. Pelvic floor muscle training (Kegel exercises) is used to manage stress, urge or mixed UI (see Box 45-12). Biofeedback is used to assist the patient to identify, isolate, contract and relax the pelvic muscles (see the Complementary & alternative therapies box).
BOX 45-12 Pelvic floor muscle or Kegel exercises
PATIENT TEACHING GUIDE
Include the following instructions when teaching the patient to perform Kegel exercises.
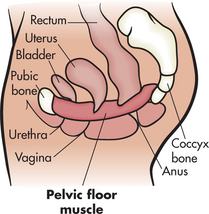
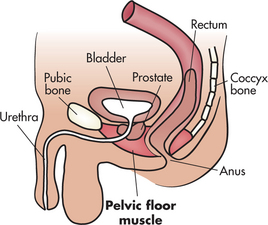
What is the pelvic floor muscle?
• Your pelvic floor muscle provides support for your bladder and rectum and, in women, the vagina and the uterus.
• If it weakens or is damaged, it cannot support these organs and their position can change.
• This causes problems with normal bladder and rectal function.
• If you have a weak pelvic floor muscle, you may want to do special exercises to make the muscle stronger, prevent unwanted urine leakage and lessen urinary urgency.
Finding the pelvic floor muscle
• Without tensing the muscles of your leg, buttocks or abdomen, imagine that you are trying to control the passing of gas or pinching off a stool.
• Or imagine you are in an elevator full of people and you feel the urge to pass gas. What do you do?
• You tighten or pull in the ring of muscle around your rectum—your pelvic floor muscle.
• You should feel a lifting sensation in the area around the vagina or a pulling in of your rectum.
How to do the exercises
There are two different kinds of exercises—short squeezes and long squeezes.
1. To do the short squeezes, tighten your pelvic floor muscle quickly, squeeze hard for 2 seconds and then relax the muscle. Also, when you have strong urinary urges, try to tighten your pelvic floor muscle quickly and hard several times in a row until the urge passes.
2. To do the long squeezes, tighten the muscle for 5–10 seconds before you relax.
Drug therapy
Drug therapy varies according to the UI type (see Table 45-9). Drugs have a very limited role in the management of stress UI. α-adrenergic agonists can be used to increase bladder sphincter tone and urethral resistance. Unfortunately, they exert a limited beneficial effect and they are associated with adverse effects including exacerbation of hypertension and tachycardia. Drugs play a more central role in the management of urge or reflex UI. Anticholinergic drugs and newer, more specific, muscarinic receptor antagonists relax the bladder muscle and inhibit overactive detrusor contractions.46 There are several preparations of these drugs, including: immediate and extended-release tolterodine; immediate, extended-release and transdermal oxybutynin; twice-daily trospium chloride; extended-release solifenacin; and darifenacin. Efficacy is similar for these medications, and side effects include dry mouth, dry eyes, constipation, blurred vision and somnolence. A recent drug for the treatment of overactive bladder is fesoterodine. This drug differs from the previously mentioned drugs in that it is a prodrug. (A prodrug is a drug that is administered in an inactive [or significantly less active] form. Once administered, the prodrug is metabolised in vivo [i.e. within the body] into an active metabolite. The rationale behind the use of a prodrug is generally to optimise absorption, distribution, metabolism and excretion.) Fesoterodine is rapidly and completely metabolised to the primary metabolite of tolterodine.45
Surgical therapy
Surgical techniques vary according to the type of UI.50 Surgical correction of stress UI may reposition the urethra and/or create a backboard of support or otherwise stabilise the urethra and bladder neck to be more receptive to changes in intraabdominal pressure. Another technique for stress UI augments the urethral resistance of the intrinsic sphincter unit with a sling or periurethral injectables. Consensus as to the best surgical procedure is lacking. Few data are available on complications, the treatment of complications and the frequency and effectiveness of additional treatment. Retropubic colposuspension and pubovaginal sling placement, with overall cure rates in the 80–90% range, appear to be most effective overall.51 Typically, both procedures are performed through low transverse incisions. Complications specific to retropubic suspensions include postoperative voiding dysfunction, urgency and vaginal prolapse.
Biofeedback for urinary incontinence
COMPLEMENTARY & ALTERNATIVE THERAPIES
Scientific evidence
Many studies have shown biofeedback to be an effective treatment for urinary incontinence.
Nursing implications
• Feedback from monitoring equipment can teach patients to control certain involuntary body responses, such as urinary incontinence.
• Although considered safe, patients should consult a qualified professional before using biofeedback.
Based on a systematic review of scientific literature. Available at www.naturalstandard.com.
Placement of a suburethral sling, using autologous fascia, cadaveric fascia or a synthetic material, is also used to correct stress UI in women. Complications include vascular and bowel injury, urinary retention, mesh/sling erosion, infection, urgency and bladder perforation. Suburethral slings are less invasive outpatient procedures that have success rates comparable to colposuspensions/slings and are associated with a more rapid return to normal activity. An artificial urethral sphincter can be used in men with intrinsic sphincter deficiency and severe stress UI.
Alternatively, one of several bulking agents can be injected underneath the mucosa of the urethra to correct stress UI in women or men. Bulking agents include glutaraldehyde cross-linked bovine collagen (GAX collagen), small silicone beads (Durasphere) or polytetrafluoroethylene (Teflon). Because of the risk of migration of Teflon particles, GAX collagen or Durasphere injections are most commonly used today. Although treatment with suburethral compounds avoids the risks associated with open surgery, reinjection after a period of several years is typically required.
A recent treatment involves the injection of autologous stem cells into the rhabdosphincter and urethral submucosa of women with stress UI. This technique increases urethral closure pressure and periurethral electromyography activity. However, the injection requires use of transurethral ultrasonography for correct placement of the stem cells.52
 NURSING MANAGEMENT: URINARY INCONTINENCE
NURSING MANAGEMENT: URINARY INCONTINENCE
The nurse needs to recognise both the physical problems and the emotional problems associated with UI.51 The patient’s dignity, privacy and feelings of self-worth must be maintained or enhanced. This often involves a two-step approach comprising containment devices to manage existing urinary leakage and a definitive plan of management designed to reduce or resolve the factors leading to UI.
Management options are reviewed in Table 45-7. They include lifestyle interventions such as teaching the patient about consumption of an adequate volume of fluids and reduction or elimination of bladder irritants (particularly caffeine and alcohol) from the diet. The patient should be advised to maintain a regular, flexible schedule of urination (usually every 2–3 hours while awake) and to quit smoking (if relevant), because this habit increases the risk of stress UI. In addition, patients should be counselled about the relationship between constipation, UI and urinary retention. Aggressive management of constipation is recommended, by ensuring adequate fluid intake, increasing dietary fibre, undertaking light exercise and judiciously using stool softeners. (The management of constipation is discussed in Ch 42.)
Behavioural treatments include scheduled voiding regimens (timed voiding, habit training and prompted voiding), bladder retraining and pelvic floor muscle training. The nurse should assess strategies that the patient uses to contain UI and offer advice concerning alternative devices when indicated. When attempting to manage UI, many women use feminine hygiene pads, and many men and women use household products such as rags, paper towels or folded toilet tissue. Unfortunately, none of these products is adequately designed to wick urine away from the skin, prevent soiling of clothing and reduce or eliminate odour. Instead, the nurse should provide information on products specifically designed to contain urine. For example, patients with mild to moderate UI often benefit from using incontinence pads containing super-absorbent material, which is specifically designed to absorb many times its weight in water. Patients with higher-volume urine loss or those with both urinary and faecal incontinence may benefit from using disposable or reusable incontinence protective underwear, briefs or pad/pant systems designed for more severe cases.
In inpatient or long-term care facilities, nursing management of UI includes maximising toilet access. This assistance may take the form of offering the urinal or bedpan or assisting the patient to the bathroom every 2–3 hours or at scheduled times. Patient toilets should be accessible to patients and adequate privacy should be allowed to ensure effective urine elimination.
Instrumentation
Reasons for short-term urinary catheterisation are listed in Box 45-13. Two reasons that are not indications for catheterisation are: (1) routine acquisition of a urine specimen for laboratory analysis; and (2) the convenience of nursing staff or the patient’s family. Catheterisation for sterile urine specimens may occasionally be indicated when patients have a history of complicated UTI. These specimens have to be as free of contaminants as possible. The risks of HAI are too high to allow catheterisation of a patient for the convenience of hospital personnel or family members. Increased prevalence of complications seen with long-term use (more than 30 days) of indwelling catheters include bladder spasms, periurethral abscess, pain, urosepsis, UTIs, urethral trauma/erosion, fistula/stricture formation and stones.53 A catheter should be the final means of providing the patient with a dry environment to prevent skin breakdown and protect dressings or skin lesions.
BOX 45-13 Indications for urinary catheterisation
Indwelling catheter
• Relief of urinary retention caused by lower urinary tract obstruction, paralysis or inability to void
• Bladder decompression preoperatively and operatively for lower abdominal or pelvic surgery
• Facilitation of surgical repair of urethra and surrounding structures
• Splinting of ureters or urethra to facilitate healing after surgery or other trauma in area
• Accurate measurement of urinary output in the critically ill patient
• Measurement of residual urine after urination (referred to as post-void residual) if portable ultrasound not available
• Contamination of stage III or IV pressure ulcers with urine that has impeded healing, despite appropriate personal care for the incontinence (indwelling)
• Terminal illness or severe impairment, which makes positioning or clothing changes uncomfortable, or which is associated with intractable pain (indwelling)
Urinary catheterisation is commonly used in the management of hospitalised patients, but it is not without serious risks. The urinary tract is the most common site of HAIs. Urinary catheterisation is a major cause of UTIs.53 Scrupulous aseptic technique is mandatory when a urinary catheter is inserted. After insertion, maintenance and protection of the closed drainage system are major nursing responsibilities. Irrigation of the catheter should not be performed routinely and should be done only with medical direction.54
While the patient has a catheter in place, nursing actions should include maintaining patency of the catheter, managing fluid intake, providing for the comfort and safety of the patient and preventing infection. Attention should be given to the psychological implications of urinary drainage. Patient concerns include embarrassment related to exposure of the body, altered body image and fear concerning the care of the catheter that results in increased dependency.
Catheters vary in construction materials, tip shape (see Fig 45-12) and size of the lumen. Catheter material includes Teflon-coated latex (polytetrafluoroethylene [PTFE]), 100% silicone elastomer and hydrogel-coated silicone. Catheters coated with silver or antimicrobial agents may lead to fewer UTIs in short-term catheter use.55 A coudé-tip catheter is commonly used in men. Catheters are sized according to the French scale. Each French unit (F) equals 0.33 mm of diameter. The diameter measured is the internal diameter of the catheter. The size used varies with the size of the individual and the purpose of catheterisation. In women, urethral catheter sizes 12F–16F are the most common; in men, sizes 14F–18F are used. Balloon sizes are either 5 mL (instilled with 5 mL of sterile water) or 30 mL (instilled with 30 mL of sterile water).54 The primary problem resulting from too large a catheter is tissue erosion secondary to excessive pressure on the meatus or urethra. Four routes are used for urinary tract catheterisation: urethral, ureteral, suprapubic and via a nephrostomy tube.
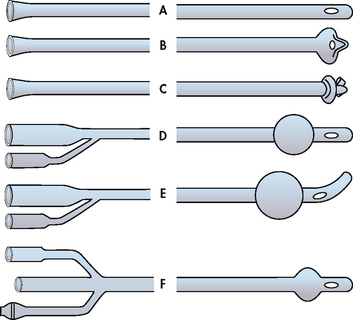
Figure 45-12 Different types of urinary catheters. A, Simple urethral catheter. B, Mushroom-tip de Pezzer catheter (can be for suprapubic catheterisation). C, Wing-tip Malecot catheter. D, Indwelling urethral catheter with inflated balloon. E, Indwelling Tiemann catheter with coudé tip–inflated balloon. F, Three-way indwelling catheter (third lumen can be used for irrigation of the bladder).
URETHRAL CATHETERS
The most common route of catheterisation is insertion of the catheter through the external meatus into the urethra, past the internal sphincter and into the bladder. Principles that should be considered in the management of the patient with an indwelling urethral catheter include the following:53,54
1. The catheterised patient, particularly the patient who is ambulatory, should receive appropriate instruction regarding catheter care.
2. A sterile, closed drainage system should always be used in short-term catheterisation. The distal urinary catheter and the proximal drainage tube should not be disconnected except for necessary catheter irrigation. Unobstructed downhill flow must be maintained (i.e. clamping of catheters should be avoided). The collecting bag should be emptied regularly (when urine drainage reaches 400 mL) and kept below the level of the bladder. A poorly functioning catheter should be replaced. The leg bag should not be used for short-term patients in the hospital setting because the risk of bacterial infection is great when the catheter is disconnected and the drainage bags are exchanged.
3. Perineal care (once or twice a day and when necessary) should include cleaning of the meatus-catheter junction with soap and water. Lotion or powder should not be used near the catheter.
4. All catheters should be anchored using some type of securement device. It is recommended that the catheter be anchored to the upper thigh in women and to the lower abdomen in men to prevent catheter movement and urethral tension.
5. Sterile technique must be used whenever the collecting system is opened. Catheter irrigation is performed only when blood clots are suspected. If frequent irrigations are necessary in short-term catheterisation for catheter patency, a triple-lumen catheter may be preferable, permitting continuous irrigations within a closed system.
6. Small volumes of urine can be aspirated for culture from the catheter sampling port by means of a sterile syringe and a 21-gauge needle. The puncture site must first be prepared with an alcohol solution.
7. When the patient is catheterised for less than 2 weeks, routine catheter change is not necessary. For long-term use of an indwelling catheter, catheter replacement should be based on patient assessment and not on a routine changing schedule.
8. With long-term use of a catheter, a leg bag may be used. If the collection bag is reused, it should be washed in soap and water and rinsed thoroughly. When not reused immediately, it should be filled with half a cup of vinegar and drained. The vinegar is effective against Pseudomonas and other organisms and eliminates odours.
9. The catheter should be removed at the earliest moment possible. Intermittent catheterisation and external catheters are alternatives that may be associated with less bacteriuria and/or UTIs than chronic indwelling urethral catheters.
URETERAL CATHETERS
The ureteral catheter is placed through the ureters into the renal pelvis. The catheter is inserted by either: (1) being threaded up the urethra and bladder to the ureters under cystoscopic observation; or (2) being surgically inserted through the abdominal wall into the ureters. The ureteral catheter is used after surgery to splint the ureters and to prevent them from being obstructed by oedema. The urine volume from the ureteral catheter should be recorded separately from other urinary catheters. The patient is usually kept on bed rest while a ureteral catheter is in place until specific orders indicate that ambulation is permissible. The self-retaining ureteral catheter is often inserted after a lithotripsy procedure has been done or when ureteral obstruction from adjacent tumours or fibrosis threatens renal function. The double-J ureteral catheter is frequently used and allows the patient to ambulate. One end coils up in the kidney pelvis, while the other coils in the bladder.
The nurse needs to check the placement of the ureteral catheter frequently, and avoid tension on the catheter. The catheter drains urine from the renal pelvis, which has a capacity of 3–5 mL. If the volume of urine in the renal pelvis increases, tissue damage to the pelvis will result from pressure. Therefore, the ureteral catheter should not be clamped. If the doctor orders irrigation of the ureteral catheter, strict aseptic technique is required. If output is decreased, the doctor should be notified immediately. Drainage should be checked often (at least every 1 or 2 hours). It is normal for some urine to drain around the ureteral catheter into the bladder. Accurate recording of urine output from both the ureters and the urethral catheter is essential. Sometimes a ureteral catheter may be used as a stent and is not expected to drain. It is important to check with the treating doctor as to the type of catheter and what to expect.
SUPRAPUBIC CATHETERS
Suprapubic catheterisation is the simplest and oldest method of urinary diversion. The two methods of insertion of a suprapubic catheter into the bladder are: (1) through a small incision in the abdominal wall; and (2) by the use of a trocar. A suprapubic catheter is placed while the patient is under general anaesthesia for another surgical procedure or at the bedside with a local anaesthetic. The catheter may be sutured into place. The nursing responsibility includes taping the catheter to prevent dislodgement. The care of the tube and catheter is similar to that of the urethral catheter. A pectin-based skin barrier (e.g. Stomahesive) is effective around the insertion site in protecting the skin from breakdown.
The suprapubic catheter is used in temporary situations such as bladder, prostate and urethral surgery. It is also used long term in selected patients (e.g. a male quadriplegic patient who tends to form penoscrotal fistulas).
A suprapubic catheter is prone to poor drainage because of mechanical obstruction of the catheter tip by the bladder wall, sediment and clots. Nursing interventions to ensure patency of the tube include: (1) preventing the tube from kinking by coiling the excess tubing and maintaining gravity drainage; (2) having the patient turn from side to side; and (3) milking the tube.54 If these measures are not effective, the catheter is irrigated with sterile technique after a doctor’s order has been obtained.
If the patient experiences bladder spasms that are difficult to control, urinary leakage may result. Oxybutynin or other oral antispasmodics or belladonna and opium (B&O) suppositories may be prescribed to decrease bladder spasms.
NEPHROSTOMY TUBES
The nephrostomy tube (catheter) is inserted on a temporary basis to preserve kidney function when complete obstruction of a ureter is present. It is inserted directly into the pelvis of the kidney and attached to connecting tubing for closed drainage. The principle is the same as with the ureteral catheter; that is, the catheter should never be kinked, compressed or clamped. If the patient complains of excessive pain in the area or if there is excessive drainage around the tube, the catheter should be checked for patency. If irrigation is ordered, strict aseptic technique is required. No more than 5 mL of sterile saline solution should be instilled gently at one time to prevent overdistension of the kidney pelvis and renal damage. Infection and secondary stone formation are complications associated with the insertion of a nephrostomy tube.
INTERMITTENT CATHETERISATION
An alternative approach to a long-term indwelling catheter is intermittent catheterisation, often referred to as ‘straight’ catheterisation or ‘in-and-out’ catheterisation.53,54 It is being used with increasing frequency in conditions characterised by neurogenic bladder (e.g. spinal cord injuries, chronic neurological diseases) or bladder outlet obstruction in men. This type of catheterisation is used in the oliguric and anuric phases of acute kidney injury to reduce the possibility of infection from an indwelling catheter. Intermittent catheterisation is also used postoperatively, after a surgical procedure for female incontinence or following radioactive seed implantation into the prostate for cancer. The main goal of intermittent catheterisation is to prevent urinary retention, stasis and compromised blood supply to the bladder caused by prolonged pressure.
The technique consists of inserting a urethral catheter into the bladder every 3–5 hours. Some patients do intermittent catheterisation only once or twice a day to measure residual urine and to ensure an empty bladder. Two main catheter designs are available: those that have a hydrophilic coating (which becomes slippery when immersed in water to aid insertion); and those with no coating (polyvinyl chloride [PVC]). The hydrophilic catheter was introduced in the past decade and is indicated for single use.56 Some catheters show a marked propensity to adhere to the wall of the urethra depending on differences in the slipperiness of catheter coatings. The design of single-use, self-lubricating, silicone-coated (closed-sterile) systems is useful for patients who have recurrent UTIs or need to catheterise while at work or during travel. Patients should be instructed to wash and rinse the catheter and their hands with soap and water before and after catheterisation. Lubricant is necessary for men and may make catheterisation more comfortable for women. The catheter may be inserted by the patient or the healthcare provider. Once the bladder is emptied, the catheter should be removed. The catheter can be dried and placed in a carrying pouch or folded in a paper towel until needed. In general, patients should change the catheter every 7 days.57
Sterile technique is used for catheterisation in the hospital or long-term care facility. For home care, a clean technique that includes good hand-washing with soap and water is used. There has been no significant increase in infection with the use of an appropriate clean technique as compared with sterile technique. The patient should be taught to observe for signs of UTI so that treatment can be instituted early. If indicated, some patients are placed on a regimen of prophylactic antibiotics. Urethral damage from intermittent catheterisation in men is similar to problems seen with indwelling catheterisation. Complications include urethritis, urethral sphincter damage (especially if there is forceful catheterisation against a closed sphincter), urethral stricture and creation of a false passage.58
Renal and ureteral surgery
The most common indications for nephrectomy are a renal tumour, polycystic kidneys that are bleeding or severely infected, massive traumatic injury to the kidney, and the elective removal of a kidney from a donor. Surgery involving the ureters and kidneys is most commonly performed to remove calculi that become obstructive, correct congenital anomalies and divert urine when necessary. Increasingly a laparoscopic surgical technique is used for a nephrectomy.
PREOPERATIVE MANAGEMENT
The basic needs of the patient undergoing renal and ureteral surgery are similar to those of any patient who experiences surgery (see Chs 17–19 Ch 18 Ch 19). In addition, it is especially important preoperatively to ensure adequate fluid intake and a normal electrolyte balance. The patient should be told that there will probably be a flank incision on the affected side and that surgery will require a hyperextended, side-lying position. This position frequently causes the patient to experience muscle aches after surgery. If a nephrectomy is planned, it is important that the patient has one working kidney to maintain normal kidney function.
POSTOPERATIVE MANAGEMENT
Specific postoperative needs of the patient are related to urine output, respiratory status and abdominal distension.
Urine output
In the immediate postoperative period, the urine output should be measured and recorded at least every 1 or 2 hours. Drainage from the various catheters should be measured and recorded separately. It is important not to clamp or irrigate the catheter or tube without a specific order. The total urine output should be at least 0.5 mL/kg per hour. It is important to assess for urine drainage on the dressing and to estimate this amount, as well as to observe and monitor the colour and consistency of the urine. Urine with increased amounts of mucus, blood or sediment may occlude the drainage tubing or catheter.
The patient should be weighed daily using the same scale and wearing similar clothing and dressings each time. A significant change in daily weight measurement can indicate retention of fluids, which places the patient at cardiovascular risk for developing heart failure. The kidneys regulate the volume and composition of extracellular fluid, excrete waste products, control blood pressure, produce erythropoietin, activate vitamin D and regulate acid–base balance. Retention of fluids can increase the work required of the remaining kidney to perform these functions.
Respiratory status
Renal surgery may require an open rather than a laparoscopic approach and involve a flank incision just below the diaphragm and removal of the twelfth rib. Postoperatively, it is important to ensure adequate ventilation. The patient is often reluctant to turn, cough and breathe deeply because of the incisional pain. The nurse should give adequate pain medication to ensure the patient’s comfort and ability to perform coughing and deep-breathing exercises. Frequently, additional respiratory devices such as an incentive spirometer are used every 2 hours while the patient is awake. In addition, early and frequent ambulation assists in maintaining adequate respiratory function.
Abdominal distension
Abdominal distension is present to some degree in most patients who have had surgery on their kidneys or ureters. It is most commonly due to paralytic ileus caused by manipulation and compression of the bowel during surgery. Oral intake is restricted until bowel sounds are present (usually 24–48 hours after surgery). IV fluids are given until the patient can take oral fluids. Progression to a regular diet follows.
LAPAROSCOPIC NEPHRECTOMY
Laparoscopic nephrectomy can be performed in selected situations to remove a diseased kidney. It can also be used to obtain a kidney from a living donor to be transplanted into a person with end-stage kidney disease. In contrast to the open incision of about 18 cm required in a conventional nephrectomy, a laparoscopic nephrectomy is performed using five puncture sites—one of these is used to view the kidney and another is used to dissect it. The laparoscope contains a miniature camera so that the surgeons can watch what they are doing on a video monitor. Once dissected, the kidney is manoeuvred into a nylon impermeable sack and its contents are safely removed from the patient. Compared with conventional nephrectomy, the laparoscopic approach is less painful, requires no sutures or staples, involves a shorter hospital stay and has a much faster recovery period.
Urinary diversion
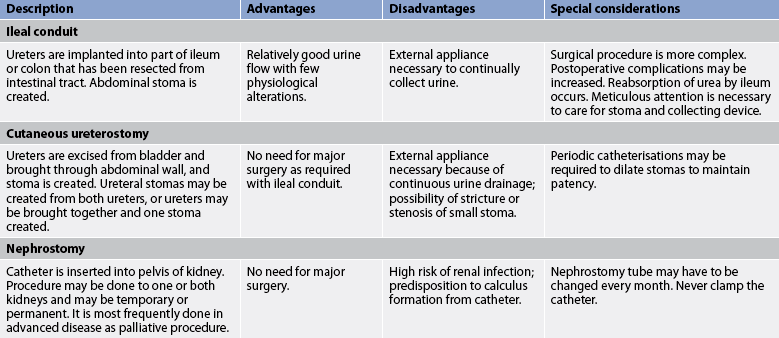
Urinary diversion may be performed with and without cystectomy. Urinary diversion procedures are performed to treat cancer of the bladder, neurogenic bladder, congenital anomalies, strictures, trauma to the bladder and chronic infections with deterioration of renal function. Numerous urinary diversion techniques and bladder substitutes are possible, including an incontinent urinary diversion, a continent urinary diversion catheterised by the patient and an orthotopic bladder reconstruction so that the patient voids urethrally.59 Types of surgical procedures for urinary diversion are presented in Table 45-10 and Figure 45-13.
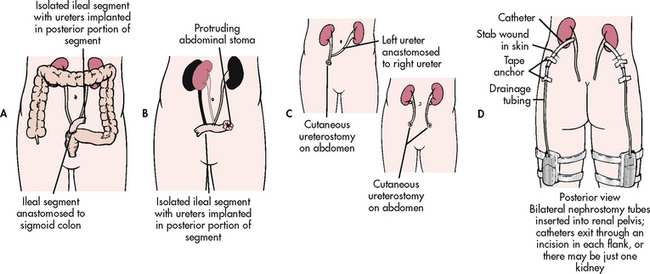
Figure 45-13 Methods of urinary diversion. A, Ureteroileosigmoidostomy. B, Ileal loop (ileal conduit). C, Ureterostomy (transcutaneous ureterostomy and bilateral cutaneous ureterostomies). D, Nephrostomy.
INCONTINENT URINARY DIVERSION
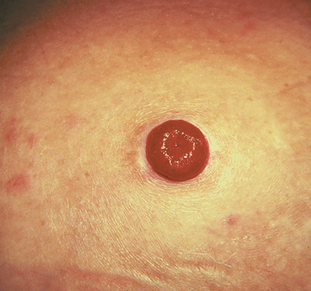
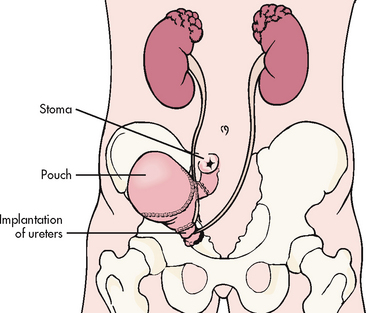
An incontinent urinary diversion is diversion to the skin, requiring an appliance. The simplest form is the cutaneous ureterostomy, but scarring and strictures of the ureter have led to the use of ileal or colonic conduits. The most commonly performed incontinent urinary diversion procedure is the ileal conduit (ileal loop). In this procedure a 15- to 20-cm segment of the ileum is converted into a conduit for urinary drainage. The colon (colon conduit) can be used instead of the ileum. The ureters are anastomosed into one end of the conduit, and the other end of the bowel is brought out through the abdominal wall to form a stoma (see Fig 45-14). Although the segment of bowel remains supported by the mesentery, it is completely isolated from the intestinal tract. The bowel is anastomosed and continues to function normally. Because there is no valve and no voluntary control over the stoma, drops of urine flow from the stoma every few seconds, requiring the use of a permanent external collecting device. The visible stoma and the need for external collection devices are obvious disadvantages of this procedure. The lifelong care and dealing with the stoma and collection devices may be psychologically difficult. These problems have stimulated the increasing use of continent urinary diversions and orthotopic bladder substitutes.
CONTINENT URINARY DIVERSIONS
A continent urinary diversion is an intraabdominal urinary reservoir that can be catheterised or that has an outlet controlled by the anal sphincter.59 Continent diversions are internal pouches created similarly to the ileal conduit. Reservoirs have been constructed from the ileum, ileocaecal segment or colon. Large segments of bowel are altered to prevent peristaltic action. A continence mechanism is formed between this large, low-pressure reservoir and the stoma by intussuscepting a portion of bowel. In this way, a patient does not leak involuntarily. The patient with a continent reservoir needs to self-catheterise every 4–6 hours but does not need to wear external attachments. Patients may wear a small bandage on the stoma to collect any mucous drainage or excess drainage. Examples of continent diversions are the Kock (see Fig 45-15), Mainz, Indiana and Florida pouches. The main difference among the various diversions is the segment of bowel used. For example, the Indiana pouch uses the right colon as a reservoir and has become a popular form of continent urinary diversion.
ORTHOTOPIC BLADDER RECONSTRUCTION
Orthotopic bladder reconstruction, or orthotopic neobladder, is the construction of a new bladder in the normal anatomical position of the bladder, with discharge of urine through the urethra. The reconstruction or neobladder can be derived from various segments of the intestines to create a low-pressure reservoir. An isolated segment of the distal ileum is often preferred. Various procedures include the hemi-Kock pouch, the Studer pouch and the W-shaped ileoneobladder. In these procedures the bowel is surgically reshaped to become a neobladder.59 The ureters and urethra are sutured into the neobladder. Orthotopic bladder reconstruction has become a more viable option for both men and women if cancer does not involve the bladder neck or urethra. Ideal candidates for this procedure have normal renal and liver function, life expectancy longer than 1–2 years, adequate motor skills and no history of inflammatory bowel disease or colon cancer. Obese patients and those with inflammatory bowel disease are not good candidates for this procedure. The advantage of orthotopic bladder substitution is that it allows for natural micturition. Incontinence is a possible problem with this technique, and intermittent catheterisation may be required.
 NURSING MANAGEMENT: URINARY DIVERSION
NURSING MANAGEMENT: URINARY DIVERSION
 Preoperative management
Preoperative management
The patient awaiting cystectomy and urinary diversion must be given a great deal of information. The nurse must assess the patient’s ability and readiness to learn before initiating a teaching program. If the patient is not ready to learn, the teaching plan should be adjusted. The patient’s anxiety and fear may be decreased by the information. However, the anxiety and fear may also interfere with learning. The patient’s family should be involved in the teaching process (see Table 45-11 for a patient and family teaching guide). A discussion of the social aspects of living with a stoma (including clothing, changes in body image and sexuality, exercise and odour) provides the patient with facts that may allay some fears. The patient with a continent diversion (e.g. Indiana pouch) should be taught to catheterise at least every 6 hours and to irrigate the pouch daily, and should be able to adhere to a strict catheterisation schedule. The patient with an orthotopic neobladder may have problems with incontinence. The patient’s concerns about sexual activities should also be discussed. The enterostomal therapy nurse should be involved in the preoperative phase of the patient’s care. Additional interventions are presented in NCP 45-3.
 Postoperative management
Postoperative management
Nursing interventions during the postoperative period should be planned to prevent surgical complications such as postoperative atelectasis and shock (see Ch 20). See NCP 45-3 for care after an ileal conduit. After pelvic surgery, there is an increased incidence of thrombophlebitis, small bowel obstruction and UTI. With removal of part of the bowel, the incidence of paralytic ileus and small bowel obstruction is increased, the patient is kept on nil-by-mouth status and a nasogastric tube is necessary for a few days.
The nurse should give specific attention to preventing injury to the stoma and maintaining urine output. The patient should be advised that mucus in the urine is a normal occurrence. The mucus is secreted by intestinal mucosa, used to create the ileal conduit, in response to the irritating effect of urine. A high fluid intake is encouraged to ‘flush’ the ileal conduit or continent diversion.
When an ileal conduit is created, the skin around the stoma requires meticulous care. Alkaline encrustations with dermatitis may occur when alkaline urine comes in contact with exposed skin (see Fig 45-16). Other common peristomal skin problems include yeast infections, product allergies and shearing-effect excoriations.60 A properly fitting appliance is essential to prevent skin problems. The appliance should be about 0.2 cm larger than the stoma. It is normal for the stoma to shrink within the first few weeks after surgery. The urine is kept acidic to prevent alkaline encrustations.
Patients with a neobladder may have postoperative urinary retention and require catheterisation.59 It may take up to 6 months for them to regain daytime bladder control, but nocturnal enuresis is seen in 25% of patients. Patients empty their neobladder by relaxing their outlet sphincter muscles and bearing down with their abdominal muscles. As there is no longer neurological feedback between reservoir and brain, patients should not expect a normal desire to void. To avoid bladder overdistension, patients should void at least every 2–4 hours, sit during voiding and practise pelvic floor muscle relaxation to aid voiding. Follow-up studies include a ‘pouchogram’ 3–4 weeks after surgery to assess for extravasations. The presence of extravasations indicates that the reservoir’s suture lines are not yet healed.
Acceptance of the surgery and of alterations in body image is needed to ensure the patient’s best adjustment. Meeting and sharing feelings with similar patients can enhance the patient’s adjustment to a urinary diversion. Patient concerns include fear that the stoma will be offensive to others and will interfere with sexual, personal, professional and recreational activities. The patient should be advised that few activities will be restricted as a result of the urinary diversion.
Discharge planning after an ileal conduit includes teaching the patient the symptoms of obstruction and infection and care of the ostomy. The patient with an ileal conduit is fitted for a permanent appliance 7–10 days after surgery and may need to be refitted at a later time, depending on the degree of stoma shrinkage. Appliances are made of a variety of products, including natural and synthetic rubbers, plastics and metals. Most appliances have a faceplate that adheres to the skin, a collecting pouch and an opening to drain the pouch. The faceplate may be secured to the skin with glues, adhesives or adhering synthetic wafers. Some appliances do not require adhesives, but their design relies on pressure to keep the pouch in place. If improperly fitted or applied, the faceplate may cause skin problems (see Fig 45-17).60 The patient needs information on where to purchase supplies, emergency telephone numbers, the location of ostomy clubs and follow-up visits with an enterostomal therapist. Medical follow-up is imperative to monitor and correct homeostatic abnormalities and to prevent complications and renal function deterioration.
The patient with a urinary tract infection
CASE STUDY
Patient profile
Sally Olds, a 28-year-old woman, is seen by a nurse practitioner for a history of painful, frequent urination.
Subjective data
• Has a history of painful, frequent urination with passage of small volumes of urine for 3 days
• Has had intermittent fever, chills and back pain over the last 3 days as well
• Was frightened when she saw blood in her urine
• Reports that this is her third attack of painful urination and back pain in 4 months
CRITICAL THINKING QUESTIONS
1. What are the most common organisms that cause UTIs?
2. What factors predispose a patient to a UTI?
3. What is the difference between upper and lower UTIs?
4. What are the priority nursing interventions for this patient?
5. Why might this patient be having recurrent bouts of UTIs? What other diagnostic tests may be indicated?
6. What can you do to help this patient to prevent another UTI?
7. Based on the data presented, write one or more appropriate nursing diagnoses. Are there any collaborative problems?
1. In teaching a patient with pyelonephritis about the disorder, the nurse informs the patient that the organisms that cause pyelonephritis most commonly reach the kidneys through:
2. The nurse teaches the female patient who has frequent UTIs that she should:
3. The immunological mechanisms involved in glomerulonephritis include:
4. One of the most important roles of the nurse in relation to acute post-streptococcal glomerulonephritis is to:
5. The oedema that occurs in nephrotic syndrome is due to:
6. A patient is admitted to the hospital with severe renal colic caused by renal lithiasis. The nurse’s first priority in management of the patient is to:
7. The nurse recommends genetic counselling for the children of a patient with:
8. The nurse encourages strict diabetic control in the patient prone to diabetic nephropathy knowing that the renal tissue changes that may occur in this condition include:
9. The nurse identifies a risk factor for kidney and bladder cancer in a patient who relates a history of:
10. In planning nursing interventions to increase bladder control in the patient with urinary incontinence, the nurse includes:
11. A patient with a ureterolithotomy returns from surgery with a nephrostomy tube in place. Postoperative nursing care of the patient includes:
12. A patient has had a cystectomy and ileal conduit diversion performed. Four days postoperatively, mucous shreds are seen in the drainage bag. The nurse should:
1 Abrahamian FM, Moran GJ, Talan DA. Urinary tract infections in the emergency department. Infect Dis Clin North Am. 2008;22:73–87.
2 Norrby SR. Approach to the patient with urinary tract infection. In Goldman L, Ausiello D, eds.: Cecil textbook of medicine, 23rd edn., Philadelphia: Saunders, 2007.
3 Lin K, Fajardo K. Screening for asymptomatic bacteriuria in adults: evidence for the US Preventive Services Task Force reaffirmation recommendation statement. AHRQ pub no. 08-05120-EF-3. Rockville, MD: Agency for Healthcare Research and Quality; 2008. Available at www.uspreventiveservicestaskforce.org/uspstf/uspsbact.htm accessed 4 October 2010.
4 Lin K, Fajardo K. US Preventive Services Task Force. Screening for asymptomatic bacteriuria in adults: evidence for the US Preventive Services Task Force reaffirmation recommendation statement. Ann Intern Med. 2008;149(1):W20–W24.
5 Rahn DD. Urinary tract infections: contemporary management. Urological Nursing. 2008;28(5):333–341.
6 Nicolle LE. Uncomplicated urinary tract infection in adults including uncomplicated pyelonephritis. Urol Clin North Am. 2008;35(1):1–12.
7 Shuman EK, Chenoweth CE. Recognition and prevention of healthcare-associated urinary tract infections in the intensive care unit. Crit Care Med. 2010;38(8 suppl):S373–S379.
8 Hooten TM, Bradley SF, Cardenes DD, Colgan R, Geerlings S, et al. Diagnosis, prevention, and treatment of catheter-associated urinary tract infection in adults: 2009 international clinical practice guidelines from the infectious diseases society of America. Clin Infect Dis. 2010;50:625–663.
9 Bradway C, Coyne KS, Irwin D, Kopp Z. Lower urinary tract symptoms in women—a common but neglected problem. J Am Acad Nurse Pract. 2008;20(6):311–318.
10 Juthani-Mehta M, Quagliarello V, Perrelli E, Towle V, Van Ness PH, Tinetti M. Clinical features to identify urinary tract infection in nursing home residents: a cohort study. J Am Geriatrics Soc. 2009;57(6):963–970.
11 Wu X. Urinalysis: a review of methods and procedures. Crit Care Nurs Clin North Am. 2010;22(1):121–128.
12 Jepson R, Craig JC. Cranberries for preventing urinary tract infections. Cochrane Database Syst Rev. 23(1), 2008. CD001321
13 Shields J, Maxwell AP. Acute pyelonephritis can have serious complications. Practitioner. 2010;254(1728):19. 21, 23–24.
14 Wagenlehner FME, Pilatz A, Naber KG, Weidner W. Therapeutic challenges of urosepsis. Eur J Clin Invest. 2008;38(suppl 2):45–49.
15 Peterson J, Kaul S, Khashab M, Kahn JB. A double-blind, randomized comparison of levofloxacin 750 mg once-daily for five days with ciprofloxacin 400/500 mg twice-daily for 10 days for the treatment of complicated urinary tract infections and acute pyelonephritis. Urol. 2008;71(1):17–22.
16 Tolkoff-Rubin NE, Rubin R. Urinary tract infection, pyelonephritis, and reflux nephropathy. In Brenner BM, Levine SA, eds.: Brenner and Rector’s the kidney, 7th edn., Philadelphia: Saunders, 2007.
17 Workowski KA, Berman SM. Sexually transmitted diseases treatment guidelines, 2006. Morb Mortal Wkly Rep. 2006;55(RR-11):1–94.
18 Giannitsas K, Athanasopoulos A. Female urethral diverticula: from pathogenesis to management. An update. Expert Review Obst Gyne. 2010;5(1):57–66.
19 Butrick CW, Howard FM, Sand PK. Diagnosis and treatment of interstitial cystitis/painful bladder syndrome: a review. J Women’s Hlth. 2010;19(6):1185–1193.
20 Homma Y, Ueda T, Tomoe H, Lin ATL, Kuo H-C, et al. Clinical guidelines for interstitial cystitis and hypersensitive bladder syndrome. Int J Urol. 2009;16(7):597–615.
21 McDermott P. Painful bladder syndrome/interstitial cystitis: history, epidemiology, symptoms, diagnosis and treatments. Urol Nurs. 2009;3(1):16–23.
22 Maurya DP, Sultana Y, Manju S. Extrapulmonary tuberculosis. Its management and control. Curr Drug Therapy. 2010;5(1):67–74.
23 McDonald S, Excell L, Livingston B. Australia and New Zealand Dialysis and Transplant Registry Report, 2009. Adelaide: Australia and New Zealand Dialysis and Transplant Registry, 2010.
24 Tomino Y. Immunoglobulin A nephropathy and chronic kidney disease. Nephrol. 2010;15:23–26.
25 Kanjanabuch T, Kittikowit W, Ejam-Ong S. An update on acute postinfectious glomerulonephritis worldwide. Nature Rev Nephrol. 2009;5:259–269.
26 Goligher EC, Detsky AS. Migratory pulmonary infiltrates. Goodpasture syndrome. Can Med Assoc J. 2009;180(1):75–77.
27 Kodner C. Nephrotic syndrome in adults: diagnosis and management. Am Fam Phys. 2009;80(10):1129–1134.
28 Johri N, Cooper B, Robertson W, Choong S, Rickards D, Unwin R. An update and practical guide to renal stone management. Nephron Clin Pract. 2010;116:c159–c171.
29 Johnston R, Lin A, Du J, Mark S. Comparison of kidney-ureter-bladder abdominal radiography and computed tomography scout films for identifying renal calculi. Brit J Urol Int. 2009;104:670–673.
30 Thomas M. Caring for Australians with renal impairment (CARI) guideline: clinical diagnosis of kidney stones. Nephrol. 2007;12:S1–S3.
31 Seitz C, Liatsikos E, Francesco Porpiglia F, Tiselius H-G, Zwergel U. Medical therapy to facilitate the passage of stones: what is the evidence? Europ Urol. 2009;56:455–471.
32 Traver MA, Passman CM, LeRoy T, Passmore L, Assimos DG. Is the Internet a reliable source for dietary recommendations for stone formers? J Endourol. 2009;23(4):715–717.
33 Steggal MJ, Omara M. Urinary stones: types, nursing care and treatment options. Brit J Nurs. 2008;17(9):S20–S23.
34 Elashry OM, Dessouky BA. Conservative management of major blunt renal trauma with extravasation: a viable option? Euro J Trauma Emerg Surg. 2009;35(2):115–123.
35 Chapman A. Polycystic kidney disease: the cadence of kidney growth in ADPKD. Nature Rev Nephrol. 2009;5:311–312.
36 Lipworth L, Tarone RE, Lund L, McLaughlin JK. Epidemiological characteristics and risk factors for renal cell cancer. Clin Epid. 2009;1:33–43.
37 Gerbershagen HJ, Dagtekin O, Rothe T, Heidenreich A, Gerbershagen K, et al. Risk factors for acute and chronic postoperative pain in patients with benign and malignant renal disease after nephrectomy. Eur J Pain. 2009;13(8):853–860.
38 Anderson B, Naish W. Bladder cancer and smoking. Part 1: addressing the associated risk factors. Br J Nurs. 2008;17(18):1182–1186.
39 Kivrak AS, Kiresi D, Emlik D, Odev K, Kilinc M. Comparison of CT virtual cystoscopy of the contrast material-filled bladder with conventional cystoscopy in the diagnosis of bladder tumours. Clin Radiol. 2009;64(1):30–37.
40 Colombel M, Soloway M, Akaza H, Buckley R, Lamm D, et al. Epidemiology, staging, grading, and risk stratification of bladder cancer. Eur Urol Suppl. 2008;7(10):618–626.
41 Anderson B, Naish W. Bladder cancer and smoking. Part 2: diagnosis and management. Br J Nurs. 2008;17(19):1240–1245.
42 Søndergaard J, Høyer M, Petersen JB, Wright P, Grau C, Muren LP. The normal tissue sparing obtained with simultaneous treatment of pelvic lymph nodes and bladder using intensity-modulated radiotherapy. Acta Oncol. 2009;48(2):238–244.
43 Walz J, Shariat SF, Suardi N, Perrotte P, Lotan Y, et al. Adjuvant chemotherapy for bladder cancer does not alter cancer-specific survival after cystectomy in a matched case-control study. BJU Int. 2008;101(11):1356–1361.
44 Takenaka A, Yamada Y, Miyake H, Hara I, Fujisawa M. Clinical outcomes of bacillus Calmette-Guérin instillation therapy for carcinoma in situ of urinary bladder. Int J Urol. 2008;15(4):309–313.
45 Wyndaele J, Goldfischer ER, Morrow JD, Gong J, Tseng L, Guan Z, Choo M. Effects of flexible-dose fesoterodine on overactive bladder symptoms and treatment satisfaction: an open-label study. Int J Clin Prac. 2009;63(4):560–567.
46 Peplar J, Wragg L. Development of a multidisciplinary continuing care continence assessment tool and continence care pathway. Aust NZ Continence J. 2010;16(2):14–16. 18, 20.
47 Thorne MB, Geraci SA. Acute urinary retention in elderly men. Am J Med. 2009;122:815–819.
48 Willson M, Wilde M, Webb M, Thompson D, Parker D, et al. Nursing interventions to reduce the risk of catheter-associated urinary tract infection. Part 2: staff education, monitoring, and care techniques. J Wound Ostomy Continence Nurs. 2009;36(2):137–154.
49 Rahn DD, Roshanravan SM. Pathophysiology of urinary incontinence, voiding dysfunction and overactive bladder. Obst Gynec Clin N Am. 2009;36(3):463–474.
50 Hinoul P, Roovers JP, Ombelet W, Vanspauwen R. Surgical management of urinary stress incontinence in women: a historical and clinical overview. Eur J Obstet Gynecol Reprod Biol. 2009;145(2):219–225.
51 Sørbye LW, Finne-Soveri H, Ljunggren G, Topinkova E, Garms-Homolova V, Jensdóttir AB, Bernabei R. Urinary incontinence and use of pads: clinical features and need for help in home care at 11 sites in Europe. Scandinavian J Caring Sci. 2009;23(1):33–44.
52 Frauscher F, Ulmer H, Hering S, Bartsch G, Strasser H. Adult stem cell therapy of female stress urinary incontinence. Eur Urolog. 2008;53(1):169–175.
53 Niel-Weise BS, van den Broek PJ. Urinary catheter policies for short-term bladder drainage in adults. Cochrane Database Syst Rev. (3):2006. CD004203.
54 Madeo M, Roodhouse AJ. Reducing the risks associated with urinary catheters. Nurs Stand. 2009;23(29):47–55.
55 Cardenas DD, Hoffman JM. Hydrophilic catheters versus noncoated catheters for reducing the incidence of urinary tract infections: a randomized controlled trial. Arch Physical Med Rehab. 2009;90(10):1668–1671.
56 Jonsson O, Kaps HP, Chapple CR. A multicenter, double-blind, randomized, parallel group study comparing polyvinyl chloride and polyvinyl chloride-free catheter materials. J Urol. 2009;182(6):2794–2798.
57 Newman DK. CAUTION: carefully manage indwelling catheters: review best practices for reducing catheter-associated urinary tract infections. Nurs Manag. 2009;40:20–22.
58 Herter R, Kazer MW. Best practices in urinary catheter care. Home Healthcare Nurs. 2010;28(6):342–349.
59 Xiong E, Song B. Better compliance contributes to better nocturnal continence with orthotopic ileal neobladder than ileocolonic neobladder after radical cystectomy for bladder cancer. Urol. 2009;73(4):838–843.
60 Rudoni C, Dennis H. Accessories or necessities? Exploring consensus on usage of stoma accessories. Brit J Nurs. 2009;18(18):1106. 1108, 1110–1112.
Australian Council of Stoma Associations. www.australianstoma.com.au
Continence Foundation of Australia. www.continence.org.au/
Department of Health and Ageing: National Continence Management Strategy. www.health.gov.au/internet/main/publishing.nsf/Content/ageing-continence-phasethree.htm
Interstitial Cystitis Support Group (Australia and New Zealand). www.painful-bladder.org/globalgroups_etc.html
Kidney Health Australia. www.kidney.org.au
Lions Australian Prostate Cancer. www.prostatehealth.org.au
New Zealand Continence Association. www.continence.org.nz
New Zealand Kidney Foundation. www.nzkidneyfoundation.co.nz
Urological Society of Australia and New Zealand. www.usanz.org.au
Women’s Hospital Melbourne: interstitial cystitis. www.thewomens.org.au/Interstitialcystitis
Also see resources for Chapter 46.