Topical Analgesics for Persistent (Chronic) Pain
IT is important to distinguish between the terms topical and transdermal (Stanos, 2007). Although both routes require a drug to cross the stratum corneum to produce analgesia (Figure 24-1), transdermal drug delivery (e.g., long-acting transdermal fentanyl patch) requires absorption to achieve systemic effects, whereas topical agents (e.g., lidocaine patch 5% and other local anesthetics, capsaicin) are intended to produce effects in the tissues immediately under the site of application (Stanos, 2007). Transdermal analgesic drugs provide systemic analgesia, and topical analgesics provide what is referred to as “targeted peripheral analgesia,” or pain relief achieved by “dampening” pain mechanisms within the peripheral nervous system (Argoff, 2003). Transdermal delivery systems typically have a slow onset and extended duration of analgesia. Topical analgesics are faster acting and dissipate relatively soon after removal of the drug.
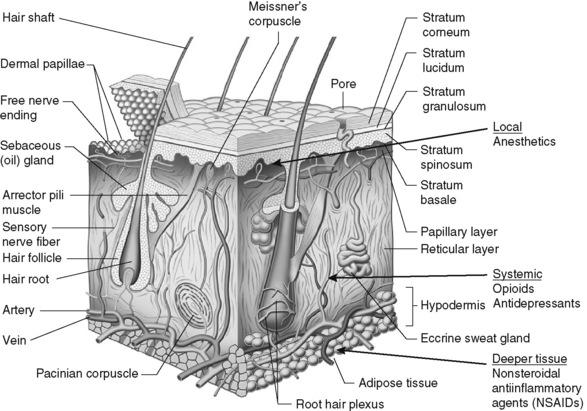
Figure 24-1 Anatomy and physiology of the skin: potential analgesic action sites. Adapted from Brown, M. B., Martin, G. P., Jones, S. A., et al. (2006). Dermal and transdermal drug delivery systems: Current and future prospects. Drug Deliv, 13(3), 175-187. In S. P. Stanos. (2007). Topical agents for the management of musculoskeletal pain. J Pain Symptom Manage, 33(3), 345.
There are significant benefits to using topical analgesics, including patient acceptance, ease of administration, reductions in systemic adverse effects, and fewer drug interactions (Argoff, 2003; McCleane, 2008; Stanos, 2007). Some preparations (e.g., capsaicin, L.M.X.4) can be obtained without a prescription; others (e.g., lidocaine patch 5%, clonidine, EMLA, diclofenac patch) require a prescription. Some topical analgesics (e.g., antidepressants, anticonvulsants, ketamine) are not commercially available but may be prepared by a compounding pharmacy. The topical therapies discussed here are those used primarily for persistent pain and include the lidocaine patch 5% and other local anesthetics, capsaicin, antidepressants, anticonvulsants, clonidine, and ketamine. A bupivacaine patch (Eladur) was under development at the time of publication. Local anesthetics used for procedural pain are discussed in Chapter 28. Topical formulations containing aspirin or NSAIDs are discussed in Chapter 7. A list of topical agents can be found in Table 24-1.
Lidocaine Patch 5%
The topical lidocaine patch 5% (Lidoderm) is 10 cm by 14 cm and contains 700 mg of lidocaine in an aqueous base. After removing a polyethylene terephthalate film release liner, the patch is placed directly over or adjacent to the painful area (Pasero, 2003b) (see Box 24-1 for guidelines on the use of the lidocaine patch 5%). The patch is not sterile so should not be placed in wounds, but they are often placed around painful wounds. They may be cut to fit smaller areas as well. For example, a patch can be cut into strips and wrapped around painful fingers or toes. A trial period of three weeks is recommended to determine effectiveness of lidocaine patch 5% (Dworkin, O’Connor, Backonja, et al., 2007).
Lidocaine and other local anesthetics relieve pain primarily by suppressing the activity of peripheral sodium channels thereby reducing ectopic, paroxysmal discharges and pathologic pain transmission (see Section I and Figure I-2 on pp. 4-5). A major advantage of analgesics such as the lidocaine patch 5% is that they work peripherally rather than systemically, and thus are associated with a low incidence of systemic adverse effects (Stanos, 2007). Although lidocaine is a local anesthetic, penetration of the drug following topical application is sufficient to produce an analgesic effect but not a sensory block. Patients should be told to expect pain relief, not anesthesia. At this time, the lidocaine patch 5% is available only by prescription. (See lidocaine patch 5% patient medication information, Form V-8 on pp. 773-774, at the end of Section V.)
Guidelines on the treatment of neuropathic pain recommend the lidocaine patch 5% as a first-line (Dubinsky, Kabbani, El-Chami, et al., 2004; Dworkin, O’Connor, Backonja, et al., 2007) or second-line (Moulin, Clark, Gilron, et al., 2007) drug for postherpetic neuralgia. However, a Cochrane Collaborative Review called for further research comparing it with other medications for the treatment of this condition (Khaliq, Alam, Puri, 2007). Nevertheless, it is approved for postherpetic neuralgia, and the safety, effectiveness, and convenience of this drug for treatment of this condition led to its use for a wide variety of other types of pain and pain syndromes. Below is a list of case reports, reviews, and research.
• Postherpetic neuralgia (Argoff, 2000; Argoff, Galer, Jensen et al., 2004; Dubinsky, Kabbani, El-Chami, et al., 2004; Dworkin, Schmader, 2003; Gammaitoni, Alvarez, Galer, 2003; Galer, Rowbotham, Perander, 1999; Katz, Gammaitoni, Davis, et al., 2002; Khaliq, Alam, Puri, 2007; Pasero, 2003b; Rowbotham, Davies, Verkempinck et al., 1996)
• Acute herpes zoster (within 4 weeks of infection onset) (Lin, Fan, Huang, et al., 2008)
• Variety of other focal peripheral neuropathic pain syndromes (see list in Meier, Wasner, Faust, et al., 2003)
• Painful diabetic neuropathy (Argoff, Backonja, Belgrade, et al., 2006; Argoff, Galer, Jensen, et al., 2004; Barbano, Herrmann, Hart-Gouleau, et al., 2004; Devers, Galer, 2000; Veves, Backonja, Malik, 2008)
• Idiopathic distal sensory polyneuropathy (Hermann, Barbano, Hart-Gouleau et al., 2005)
• Myofascial pain (Argoff, 2002; Dalpiaz, Dodds, 2002; Gammaitoni, Alvarez, Galer, 2003)
• Neuroma (Devers, Galer, 2000)
• Meralgia paresthetica (Devers, Galer, 2000)
• Intercostal neuralgia (Devers, Galer, 2000)
• Traumatic rib fracture (Ingalls, Horton, Bettendorf, et al., 2009)
• Erythromelalgia (Davis, Sandroni, 2002)
• Complex regional pain syndrome (CRPS types 1 and 2) (Devers, Galer, 2000)
• Cervical pain (Gammaitoni, Alvarez, Galer, 2003)
• Back pain (Gammaitoni, Alvarez, Galer, 2003; Gimbel, Linn, Hale, et al., 2005; Hines, Keaney, Moskowitz, et al., 2002)
• Radiculopathy (Devers, Galer, 2000)
• Variety of persistent pains (head, neck, extremity, back, knee) (Fishbain, Lewis, Cole, et al., 2006)
• Cancer-related neuropathic pain (Wilhelm, Griessinger, Koppert, et al., 2005)
• Osteoarthritis (OA) (Gammaitoni, Galer, Onawola, et al., 2004)
• Carpal tunnel syndrome (Nalamachu, Crockett, Gammaitoni, et al., 2006)
• Laparoscopic ventral hernia repair (postoperative pain) (Saber, Elgamal, Rao, et al., 2008)
• Radical retropubic prostatectomy (Habib, Polascik, Weizer, et al., 2009)
• Inguinal hernia repair (Lockhart, 2006)
• Persistent neuropathic postsurgical pain (thoracotomy, mastectomy) (Devers, Galer, 2000)
Many patients with neuropathic pain experience allodynia, or pain with a nonnoxious stimulus (see Section I). Allodynia can be so severe in some patients that even air currents and wearing clothing are excruciatingly painful. Understandably, individuals who suffer allodynia can become housebound in an effort to avoid wearing clothing or being exposed to other elements that increase pain (Rowbotham, Davies, Verkempinck, et al., 1996). In addition to relief of pain, after the initial patch application, the protective nature of the lidocaine patch 5% over allodynic skin is often cited as a reason for patient satisfaction with this method of analgesia.
Common misconceptions are that topical analgesics are capable of relieving only surface neuropathic pain, such as allodynia, and systemic analgesics are required to relieve other types of neuropathic pain (e.g., sharp, hot, or deep-quality pain). Research has refuted this thinking. A randomized controlled trial of patients with postherpetic neuralgia with and without allodynia (Galer, Jensen, Ma, et al., 2002) and another randomized controlled trial of patients with painful diabetic neuropathy with or without allodynia (Dworkin, Hart-Gouleau, Galer, et al., 2003) demonstrated that the lidocaine patch 5% relieved both types of neuropathic pain.
Older individuals are at highest risk for developing some of the conditions most responsive to treatment with the lidocaine patch 5%, such as postherpetic neuralgia, painful diabetic neuropathy, and OA. They are also among the highest risk for adverse effects related to systemic analgesics that are often used to treat these conditions (e.g., antidepressants, anticonvulsants, NSAIDs). A follow-up survey of older adults with postherpetic neuralgia who used the lidocaine patch 5% on a long-term basis after participating in its research revealed a mean duration of usage of 7.6 years and a high rate of satisfaction overall and in all subcategories, including convenience and ability to do normal activities (Galer, 2003). Erythema was the most common adverse effect, but most (75%) reported no adverse effects whatsoever. Pooled analysis of data from three open-label trials showed that the lidocaine patch 5% reduced pain intensity and pain interference and improved sleep and other quality of life indicators with a low risk of systemic adverse effects in geriatric patients (Gammaitoni, Onawola, Galer, 2005). Again, the most common adverse effect was dermal reactions.
The lidocaine patch 5% has been used safely in patients as early as 4 weeks after the onset of acute herpes zoster. Patients with moderate to severe acute pain were randomized to receive a vehicle patch (placebo) or lidocaine patch 5% applied on the torso or limbs with intact skin (without vesicles) by a 12-hours-on, 12-hours-off regimen (Lin, Fan, Huang, et al., 2008). Improvements in pain during rest and movement and patients’ global impression were greater in those who received the lidocaine patch 5%. Adverse effects were similarly low in both groups.
The lidocaine patch 5% can be used with a systemic drug regimen, potentially providing additive relief. For example, it can provide further relief when response to gabapentin and other systemic analgesics is only partial (Meier, Wasner, Faust, et al., 2003).
Although the risks associated with the lidocaine patch 5% are very small, it should be used with caution in patients taking antiarrhythmic medications (e.g., mexiletine, IV lidocaine) to prevent the potential for additive toxicity (Pasero, 2003b). Patients who are sensitive to amide local anesthetics (e.g., bupivacaine, ropivacaine) should not use the lidocaine patch 5%. The product has not been tested in pregnant or nursing women. Patients must be told to avoid putting heat (e.g., heating pads, hot packs) directly on the lidocaine patch because this will increase absorption of lidocaine and can lead to toxicity (Shemirani, Tang, Friedland, 2010) (see Chapter 23 for local anesthetic adverse effects).
Pharmacokinetics
Patients typically report feeling the onset of analgesia within 30 minutes of application of the lidocaine patch 5% and significant improvements in pain within days of regular use (Pasero, 2003b). The majority of patients in one study responded within the first week of continuous treatment, reporting better pain relief on day 7 than on day 0 (Katz, Gammaitoni, Davis, et al., 2002). Steady-state lidocaine plasma concentrations are reached by the second day of patch application. Pharmacokinetic studies show that maximal lidocaine plasma concentrations ranged from 0.13 mcg/mL (three patches 12-hours-on, 12-hours-off regimen) to 0.225 mcg/mL (four patches 12-hours-on, 12-hours-off regimen) (Gammaitoni, Alvarez, Galer, 2003). These levels are well below those associated with cardiac antiarrhythmic activity (1.5 mcg/mL) and toxicity (5 mcg/mL) (Lema, 1996), and lidocaine concentration does not increase with daily use. Every-12-hour administration of four patches was associated with slightly higher minimum and maximum plasma concentrations at steady state compared with every-24-hour administration of four patches. The plasma half-life of the lidocaine patch 5% is approximately 6 to 8 hours.
Dosing Regimen
The manufacturer of the lidocaine patch 5% (Endo) recommends a dosing regimen of up to 3 patches worn for 12 hours, then removed and not replaced until another 12 hours has elapsed (12 hours on, 12 hours off). The pharmacokinetic studies have established the safety of continuous daily use of three to four patches worn 12 hours/day (Devers, Galer, 2000; Gammaitoni, Alvarez, Galer, 2002), 18 hours/day (Gammaitoni, Davis, 2002), and 24 hours/day (Gammaitoni, Alvarez, Galer, 2002). In an open-label trial of 332 patients with postherpetic neuralgia, 28 days of treatment (12 hours on, 12 hours off) was shown to be safe (Katz, Gammaitoni, Davis, et al., 2002). The most common adverse effect was localized rash, which was a reason for discontinuation of treatment in three patients (1%). No systemic adverse effects were reported. A case report described the safe and effective use of up to four lidocaine patches 5% for 19 months followed by 10 patches for 4 months, worn continuously (24 hours/day), in a patient with cancer-related neuropathic pain (Wilhelm, Griessinger, Koppert, et al., 2005). Serum lidocaine plasma levels were low throughout treatment (less than 0.5 mcg/mL) (see discussion of serum lidocaine levels in Chapter 23).
EMLA
Eutectic mixture of local anesthetics (EMLA) is used most often for procedural pain management (see Chapter 28). It has limited use in persistent pain because of its relatively short duration of action, and there are very few studies on its use for this type of pain. Some studies reported that EMLA could provide pain relief for 5 to 6 hours for postherpetic neuralgia (Wallace, Galer, Gammaitoni, 2006). A case report described reduction in painful neuropathy of a forearm and improvement in hand function when EMLA was applied to the patient’s forearm (Rosen, Bjorkman, Lundborg, 2008). This led the authors to randomize 40 volunteers to receive a 2-hour application of EMLA or placebo cream to a lower leg (Rosen, Bjorkman, Weibull, et al., 2009). EMLA produced significant improvement in touch thresholds and sensation, which suggests therapeutic potential for neuropathies that cause disturbances in sensitivity.
Further research is needed to determine a potential role for EMLA in the prevention of persistent neuropathic postsurgical pain. A randomized controlled trial of women undergoing breast surgery demonstrated no differences between EMLA and placebo treatment on pain at rest and movement; however, analgesic consumption was reduced on the second to fifth day, and the incidence of persistent pain was less in those who received EMLA (Fassoulaki, Sarantopoulos, Melemeni, et al., 2000).
As explained in a more detailed discussion in Chapter 28, a relatively thick application of EMLA is needed for at least 1 hour to create an area of dense sensory loss. The effect is enhanced by application of an occlusive dressing over the cream. A 5 gm tube of EMLA can be used to spread 2.5 gm over a 20 to 25 cm2 [2 inch by 2 inch] area. Clinical experience suggests that an application time of 90 to 120 minutes produces better results, particularly for treatment of persistent pain states. These guidelines are difficult if the area of pain is larger or is adjacent to the face or a mobile region of the body.
No evidence exists that cutaneous anesthesia is necessary to gain benefit from a topical local anesthetic and, anecdotally, some patients seem to respond favorably to a thin application applied without a dressing. In the absence of any systematic evaluation of dosing techniques, the patient should be encouraged to try various modes of administration in an effort to identify a salutary approach. If possible, one of these trials should include EMLA for a duration of at least 1 hour under an occlusive dressing of some type (ordinary plastic wrap can be used for large areas).
Other Local Anesthetics
Novel approaches have been used to administer local anesthetics for persistent pain. A case report described the use of a 5% lidocaine plaster applied via a 12-hours-on, 12-hours-off regimen for 2 weeks in combination with an oral antidepressant and anticonvulsant to reduce persistent postorchiectomy pain by 70% (DeMello, Desai, 2009). A randomized, cross-over trial administered a metered dose of 8% lidocaine spray (not available in the United States) or saline placebo spray to completely cover the painful skin in 24 patients with postherpetic neuralgia (Kanai, Kumaki, Niki, et al., 2009). After 7 days, the patients crossed over to the alternative treatment. The lidocaine spray produced significantly greater decreases in pain compared with placebo, and the median analgesic duration was 4.5 hours after application. A second part of this study included an open-label trial of 100 patients who were given metered doses of 8% lidocaine spray as previously described for 2 weeks. These patients also experienced significant pain relief and no systemic adverse effects. Convenience and rapid analgesia were cited as benefits of the topical lidocaine spray. A topical bupivacaine patch (Eladur), intended to provide 3 days of pain relief from a single application, was in development at the time of publication.
A wide variety of other topical local anesthetics are available over the counter (OTC). Local anesthetics in various concentrations (e.g., lidocaine, benzocaine, tetracaine) are formulated alone and with other ingredients, such as aloe and hydrocortisone, in creams, ointments, gels, lozenges, and sprays. Lidocaine patches are commercially available without a prescription. For example, MediPad-L Plus is a 3 inch by 5 inch pad containing 4% lidocaine with 0.2% menthol. Anecdotal experience with commercially available, relatively low concentrations of local anesthetic topical ointments (e.g., Foille Plus Medicated First Aid with benzocaine 5%) and sprays (e.g., Medi-Quick First Aid with lidocaine 2%) has not been favorable, however, unless the painful area involves mucosal surfaces. The effectiveness and safety of these products vary widely (Zilbert, 2002), and research is needed to establish a role in relieving persistent pain. An excellent resource for available nonprescription products is the American Pharmacists Association’s Handbook of Nonprescription Drugs (Berardi, Kroon, McDermott, et al., 2006). See Chapter 28 for topical local anesthetics that are used for procedural pain treatment.
Capsaicin
Capsaicin (e.g., Qutenza 8%, Arthritis Formula Capiscum, Capzasin, Trixaicin, Zostrix, and others) is a naturally-occurring constituent of the chili pepper that produces its pungent taste. The mechanism of action of capsaicin is not entirely understood, but when applied topically, it affects function in primary afferent nociceptive neurons by inhibiting the release of substance P and other inflammatory neurochemicals (Argoff, Backonja, Belgrade, et al., 2006; Alvarez, Galer, Gammaitoni, 2006; Knotkova, Pappagallo, Szallasi, 2008; Lussier, Portenoy, 2004) (see Section I and Figure I-2, A and B on pp. 4-5). Regular application of capsaicin to the skin stimulates the release of substance P until it is eventually depleted from the C-fiber terminals. When depleted, desensitization occurs and pain transmission is diminished. Depending on concentration, capsaicin can selectively activate, desensitize, or produce a neurotoxic effect on the small diameter afferent C fibers while leaving larger diameter afferent fibers unaffected (Sawynok, 2003). Animal research suggests that capsaicin has a central mechanism, affecting prostaglandins at the spinal cord level as well (Minami, Bakoshi, Nakano, et al., 2001).
Cream preparations of capsaicin are available in varying concentrations to be applied to intact skin, usually three to five times daily. Pain relief is dependent on regular application, and the rubbing required for application may contribute to an analgesic effect (Simon, Lipman, Caudill-Slosberg, et al., 2002). A capsaicin skin patch (Qutenza patch 8%) containing 8% capsaicin (179 mg/patch) was approved in the United States in 2009 for the treatment of postherpetic neuralgia. The drug requires a prescription, and the manufacturer recommends application only by health care professionals under the supervision of a physician (NeurogesX, Inc, 2009). Local anesthetic cream (e.g., EMLA, L.M.X.4) should be applied to the painful intact skin prior to patch application. Nitrile (not latex) gloves are worn to apply up to 4 patches for 60 minutes every 3 months. (Application should be no more frequent than every 3 months; see the package insert). After the patch is removed, a cleansing gel (included with each patch) is applied for 1 minute then removed, and the skin is wiped dry (NeurogesX, Inc, 2009) (see research later in chapter).
Capsaicin has been used in a variety of persistent pain conditions including arthritis pain, postmastectomy pain, myofascial pain, simple back pain, postherpetic neuralgia, painful diabetic neuropathy, and HIV-related peripheral neuropathy (Argoff, 2002; Argoff, Backonja, Belgrade, et al., 2006; Paice, Ferrans, Lashley, et al., 2000; Robbins, 2000; Sawynok, 2003; Simon, Lipman, Caudill-Slosberg, et al., 2002; Simpson, Estanislao, Brown, et al., 2008; Stanos, 2007). A systematic review of the use of capsaicin concluded that although the drug is better than placebo, it has moderate to poor efficacy for treatment of persistent musculoskeletal and neuropathic pain but might be appropriate in some patients with pain refractory to other treatments (Mason, Moore, Deery, et al., 2004).
The United Kingdom’s National Collaborating Centre for Chronic Conditions discussed the research to date, including economic analysis, and suggested capsaicin as a second-line pharmacologic option for OA pain (National Collaborating Centre for Chronic Conditions, 2008). Others have similarly supported its use for OA (Hunter, Lo, 2008). One study showed efficacy for knee pain from both rheumatoid arthritis (RA) and OA (Deal, Schnitzer, Lipstein, et al., 1991), and another supported its use for hand pain of OA but not RA (McCarthy, McCarty, 1992). It is noted to be more effective than analgesic balms, such as menthol preparations, for arthritis pain (Simon, Lipman, Caudill-Slosberg, et al., 2002).
An early randomized controlled trial of patients with HIV distal symmetrical peripheral neuropathy assessed a variety of pain outcomes and found no significant differences between those who received capsaicin 0.025% and those who received placebo; those who received capsaicin had higher current pain scores and a higher withdrawal rate (Paice, Ferrans, Lashley, et al., 2000).
An open-label study was conducted to examine a single, 1-hour application of the high-concentration capsaicin patch (Qutenza) (640 mcg/cm2) in 12 HIV patients with peripheral neuropathy (Simpson, Estanislao, Brown, et al., 2008). There was a significant reduction (mean 40%) in pain throughout the 12-week study period, and one-third of the patients experienced 50% or greater reduction. Pretreatment with a local anesthetic cream was applied 1 hour prior to capsaicin patch application to address treatment-associated pain in this study, and all patients were able to complete at least 90% of the application time. One patient was hospitalized for severe pain after patch application that was thought to be related to the capsaicin, and 67% of the patients reported at least one pain score 30% higher than baseline, but application-related increased pain resolved within 1 week, and treatment was generally well-tolerated by the majority of patients. It is unlikely that this treatment with high concentration capsaicin and the usual topical treatment with 0.025% or 0.075% cream can be meaningfully compared.
Capsaicin is listed as a topical analgesic option in a consensus guideline on the treatment of painful diabetic neuropathy (Argoff, Backonja, Belgrade, et al., 2006) but is not listed in other neuropathic pain treatment guidelines (Dworkin, O’Connor, Backonja, et al., 2007; Moulin, Clark, Gilron, et al., 2007). Nonetheless, a large randomized controlled trial of patients (N = 200) with a variety of types of persistent neuropathic pain (McCleane, 2000) noted that topical capsaicin 0.025% yielded similar results as topical doxepin 3.3%, and the combination of the two drugs, when applied daily. Pain ratings were unchanged for placebo but decreased by 0.9, 1.12, and 1.07 in the doxepin, capsaicin, and combination groups, respectively. Burning pain decreased for all three treatment groups after 4 weeks of treatment, and sensitivity ratings and shooting pain decreased in those who received capsaicin or the combination cream.
Topical capsaicin also has been discussed as an option for postoperative pain and prevention of persistent neuropathic postsurgical pain. One randomized, placebo-controlled trial instilled ultrapurified capsaicin intraoperatively into the wound after hernia repair in 41 men and found significantly lower pain scores for the first 3 postoperative days but no difference at 1 and 4 weeks postoperatively (Aasvang, Hansen, Malmstrom, et al., 2008). There were no significant adverse effects except a mild, transient elevation in liver enzymes in those who received capsaicin. Further research is needed to define a role in this setting.
Adverse Effects
Topical capsaicin is associated with a minimal risk of systemic toxicity (Argoff, Backonja, Belgrade et al., 2006; Simon, Lipman, Caudill-Slosberg et al., 2002) but a larger risk of localized burning, pricking, or itching sensation. This may be caused by vasodilation at the site of application (Mason, Moore, Deery, et al., 2004), as well as the direct neurostimulatory effects of substance P release in the periphery. Although the effect can wane over time with repeated administration, it can be severe enough to force discontinuation of the therapy, and even if mild, can be quite bothersome to some patients (Alvarez, Galer, Gammaitoni, 2006; Argoff, 2002). As a result, there may be poor adherence with treatment. One in three patients experience local adverse effects, which contribute to a high withdrawal rate from capsaicin studies (Mason, Moore, Deery, et al., 2004).
Unfortunately, decreased doses in the capsaicin cream do not reduce the burning associated with capsaicin application (Argoff, Backonja, Belgrade, et al., 2006). Co-administration of topical local anesthetics has been reported to have no effect on the burning (Sawynok, 2003); however, as mentioned, the application of L.M.X.4 (local anesthetic) cream 1 hour prior to application is recommended to reduce burning associated with the high concentration formulation (Simpson, Estanislao, Brown, et al., 2008), and, anecdotally, some patients report benefit from this strategy. As mentioned, application of local anesthetic cream is advised prior to application of the capsaicin 8% patch.
Transient increases in blood pressure (BP) may occur during and shortly after application of the capsaicin 8% patch. The manufacturer recommends monitoring BP during and following the treatment procedure. Further research and clinical use are needed to more fully evaluate both the safety and effectiveness of this formulation. The full prescribing information should be read prior to the use of this product (NeurogesX, Inc, 2009).
Patient teaching is essential to successful treatment with capsaicin. Prior to application, patients must be told about the burning sensation and not to be alarmed by it. They can be reassured that it probably will subside and be replaced with analgesia as the substance P is depleted; however, it is important for them to understand that depletion of neurotransmitters is dependent on regular, uninterrupted application of the cream and that repeated application after a holiday from therapy may result in renewed burning.
Another key teaching point is that pain relief is not achieved rapidly; it is gradual and can take several days to weeks to be realized. A trial application three to four times daily for at least 4 weeks may be necessary to fully evaluate effect.
Capsaicin should never be applied to eyes, mouth, nostrils, genitals, or open or broken skin areas or close to areas where skin breakdown is present. This is especially important when it is used for herpes zoster pain; it should be used only after the skin lesions are fully healed. Patients should be told to wash their hands well after application. Cotton tip applicators or gloves may be used to apply the cream formulation but are not necessary if hands are washed well. If the hands are the site being treated, the cream should be applied and hands washed at least 30 seconds later.
Antidepressants
Although not listed in neuropathic guidelines as a pharmacologic option, the tricyclic antidepressants doxepin (Prudoxin, Zonalon) and amitriptyline (Elavil) have been claimed to be somewhat effective when applied topically for treatment of persistent pain (de Leon-Casasola, 2007; Lockart, 2004; Mays, 2006; McCleane, 2000). As with other topical analgesics, there is minimal systemic absorption and adverse effects (McCleane, 2008). The underlying mechanism of antidepressants was discussed earlier in Chapter 22 (also see Section I). Doxepin cream 5% is commercially available, but topical preparations of amitriptyline 1% to 5% are not and must be compounded for use.
The first use of amitriptyline applied to skin was reported in a patient with depression and pain from inflammatory bowel disease who could no longer take oral medications (Scott, Letrent, Hager, et al., 1999). The patient’s mood, but not pain, improved with the use of amitriptyline gel, suggesting systemic absorption. This was followed by several animal studies and research in healthy volunteers that demonstrated topical effects with little systemic absorption (Gerner, Kao, Srinivasa, et al., 2003).
A trial comparing topical amitriptyline 1%, topical ketamine 0.5%, or a combination of the two in patients with postherpetic neuralgia, painful diabetic neuropathy, or posttrauma neuropathic pain found no differences in pain relief following the 2-day application phase, but significant reductions after the 7-day open trial, which suggested a longer duration of treatment was indicated for effective analgesia (Lynch, Clark, Sawynok, 2003). A larger randomized, placebo-controlled trial using higher doses of these drugs applied three times daily for 3 weeks in patients (N = 92) with these same types of neuropathic pain observed no difference in pain relief between groups (Lynch, Clark, Sawynok, et al., 2005). The researchers speculated that even higher concentrations of the drugs are needed for effect, which may be consistent with observations suggesting that amitriptyline 4% and ketamine 2% are efficacious in patients with postherpetic neuralgia (Lockart, 2004).
Other studies also yielded mixed results. In a cross-over study of 35 patients with postherpetic neuralgia, painful diabetic neuropathy, or postsurgical neuropathic pain who received 7-day treatments of twice-daily topical amitriptyline 5%, lidocaine 5%, or placebo in randomized sequence with 7-day washout periods between treatments, topical amitriptyline was not effective in relieving pain (Ho, Huh, White, et al., 2008).
Doxepin 5% cream applied three times daily has been suggested to relieve chemotherapy-induced polyneuropathy (Mays, 2006). It has also been reported to reduce pain associated with complex regional pain syndrome (CRPS) (McCleane, 2002). Topical doxepin 3.3%, capsaicin 0.025%, a combination of the two, or placebo applied three times daily produced similar improvements in pain ratings in all treatment groups in a large (N = 200) randomized controlled trial of patients with a variety of types of persistent neuropathic pain (McCleane, 2000). Pain ratings were unchanged for placebo but decreased by 0.9, 1.12, and 1.07 in the doxepin, capsaicin, and combination groups, respectively. Burning pain decreased for all three treatment groups after 4 weeks of treatment, and sensitivity ratings and shooting pain decreased in those who received capsaicin or the combination cream.
Animal research and the presence of oral numbness following doxepin oral rinse suggests that the drug has a local anesthetic effect (de Leon-Casasola, 2007). A study of 51 patients with painful oral mucositis from cancer and cancer therapy found that 90% of the patients experienced pain reduction (oral numbness) following oral doxepin rinse (5 mg/mL doxepin in a solution containing 0.1% alcohol and sorbitol) (Epstein, Epstein, Epstein, et al., 2006). The maximum effect was 70% reduction in pain, and relief lasted over 4 hours in 37% of the patients.
Anticonvulsants
There are no commercially available preparations of topical anticonvulsants and little research regarding their use. A randomized controlled study instructed 41 patients with spontaneous burning oral mucosal pain associated with stomatodynia to suck a tablet of clonazepam (Klonopin) 1 mg or placebo and hold the saliva near the site of pain inside the mouth without swallowing for 3 minutes, then spit out the contents (Gremeau-Richard, Woda, Navez, et al., 2004). This was repeated 3 times daily for 14 days. Pain scores at 2 weeks were significantly reduced in those receiving clonazepam (2.4 points on 0 to 10 scale) compared with placebo (0.6 points).
Clonidine
The potential systemic mechanisms of clonidine analgesia were discussed in Chapter 22. Clonidine is available as a transdermal (Catapres-TTS) formulation, which is approved for treatment of hypertension and smoking cessation, and can be used to provide systemic clonidine as a treatment for pain.
The clonidine patch has been suggested to have topical analgesic effects when applied to areas of pain (Davis, Treede, Raja, et al., 1991; McCleane, 2008). Early research in patients with persistent noncancer pain and hyperalgesia utilized clonidine patches (30 mcg/cm2/day) for 7 days and found significant reductions or complete resolution of hyperalgesia (Davis, Treede, Raja, et al., 1991). The clonidine patch was studied in a randomized controlled trial that included 41 patients with painful diabetic neuropathy; there was little effect overall, but a secondary analysis suggested that patients with sharp and shooting pain may benefit (Byas-Smith, Max, Muir, et al., 1995). Others have found benefit with clonidine cream for orofacial neuropathic pain described as lancinating and sharp more than burning and aching (Epstein, Grushka, Le, 1997). Given the size of the patch and the occurrence of systemic absorption, the utility of topical clonidine is very limited.
Ketamine
Although research is generally lacking, case reports describe compounding the NMDA receptor antagonist ketamine into a gel for treatment of various types of pain. It is thought that topical ketamine acts on a variety of receptors and calcium, sodium, and potassium channels to produce analgesia (de Leon-Casasola, 2007; Sawynok, 2003). A randomized controlled trial of 20 patients with CRPS demonstrated inhibition of allodynia but no pain reduction after application of topical ketamine to the symptomatic limb (Finch, Knudsen, Drummond, 2009). Topical ketamine also has been administered as an oral rinse preoperatively to reduce posttonsillectomy pain in children (Canbay, Celebi, Uzun, et al., 2008). Research in healthy volunteers found that ketamine gel had no effect on immediate burning after injection of capsaicin but produced a significant reduction in mechanical hyperalgesia; this suggests a central mechanism of action, rather than a clear topical effect (Poyhia, Vainio, 2006).
Various compounded formulations of ketamine have been developed and used in cancer-related pain (Wood, 2000). Clinical observations have suggested that other types of persistent pain also may respond favorably. Five patients with CRPS, lumbar radiculopathy, or postherpetic neuralgia applied varying amounts of ketamine gel (0.093 mg/kg to 9.33 mg/kg) depending on involved surface area and reported dose-related changes (Gammaitoni, Gallagher, Welz-Bosna, 2000). At lower doses, patients reported alterations in temperature sensation, and as the dose was increased, feelings of relaxation and decreased tension in the painful area were reported. At higher doses, patients felt significant reductions in pain. Others have reported similar results for CRPS (Sawynok, 2003; Ushida, Tani, Kanbara, et al., 2002) and postherpetic neuralgia (Quan, Wellish, Gilden, 2003).
As mentioned earlier, topical ketamine has also been co-administered with topical amitriptyline for postherpetic neuralgia and CRPS (de Leon-Casasola, 2007; Lockart, 2004; Lynch, Clark, Sawynok, 2003; Lynch, Clark, Sawynok, et al., 2005). These studies yielded uncertain results.
A remarkable case report described immediate relief of refractory chemotherapy-induced neuropathic pain with three-times-daily applications of a gel combining ketamine 5%, clonidine 0.5%, and gabapentin 6% (Prommer, 2009). An increased dose of ketamine to 10% resulted in further improvement. Ultimately, all other analgesics could be discontinued.
Summary of Indications for Topical Analgesics for Persistent Pain
On the basis of these data, a trial of lidocaine patch 5% should be considered for well-localized pain, particularly peripherally-mediated neuropathic pain, such as postherpetic neuralgia and painful diabetic neuropathy. A number of other types of pain may be responsive to this drug as well. The other topical drugs discussed in this section may be considered for pains that are refractory to other treatments. With the exception of topical capsaicin in the treatment of pain caused by disease of small joints, the usefulness of the topical analgesics for nociceptive pains caused by injury of the skin, subcutaneous tissues, muscles, or joints is limited.
Summary of Adverse Effects of Topical Analgesics for Persistent Pain
The adverse effects associated with topical analgesic therapy are rare because they are associated with minimal systemic absorption. The most common are cutaneous reactions. As explained, capsaicin can cause local burning, which is sometimes intense. Although this symptom is not related to tissue damage and poses no risk to the patient, it can create significant discomfort and lead to discontinuation of the treatment.
Conclusion
Topical drug delivery produces effects in the tissues immediately under the site of application, whereas transdermal drug delivery requires absorption to achieve systemic effects. Topical analgesics are also faster acting and dissipate relatively soon after removal of the drug compared with transdermal analgesics. Benefits of topical analgesics include high patient acceptance and satisfaction, ease of administration, reductions in systemic adverse effects, and fewer drug interactions.