Individualizing the Selection of Nonopioid Analgesics
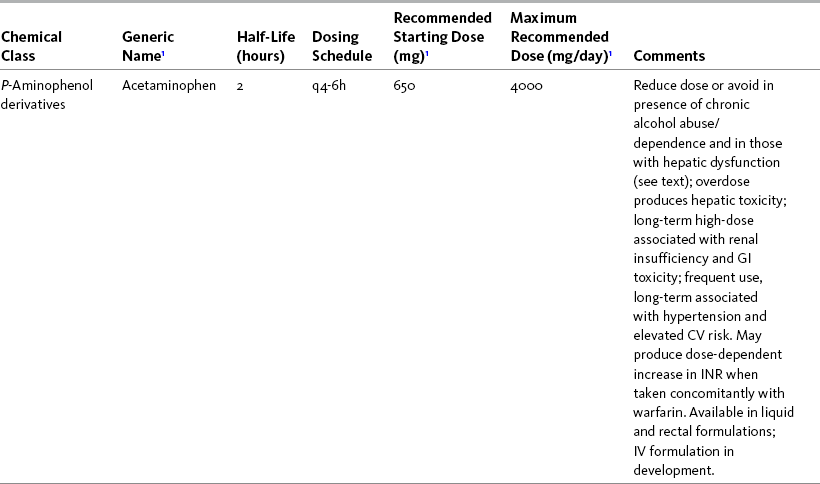
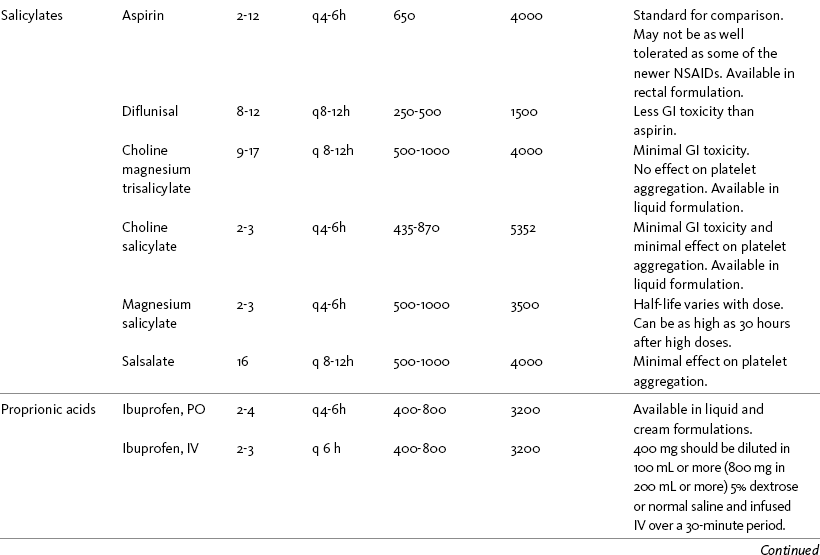
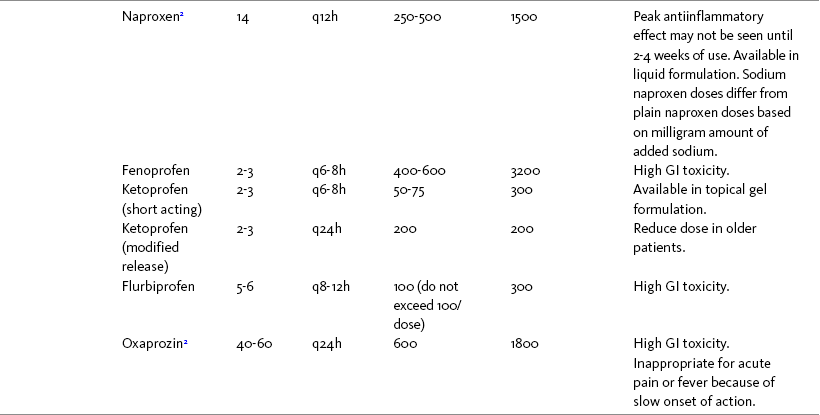
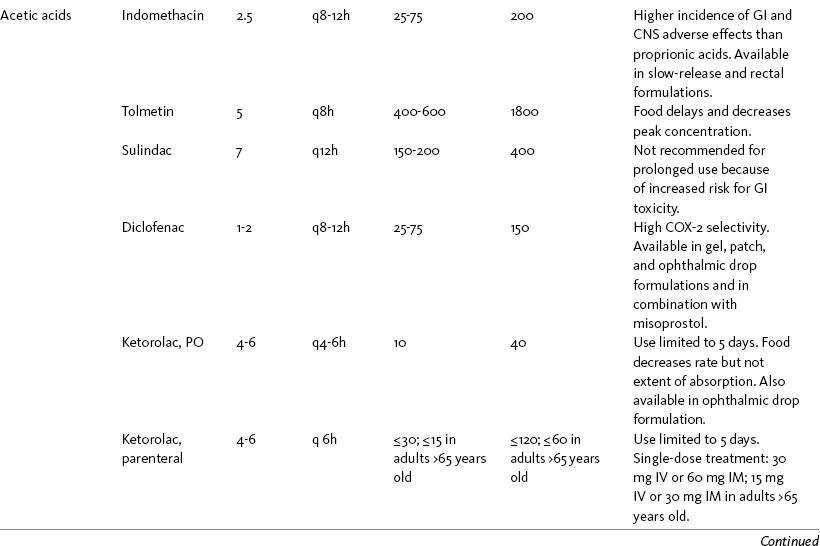
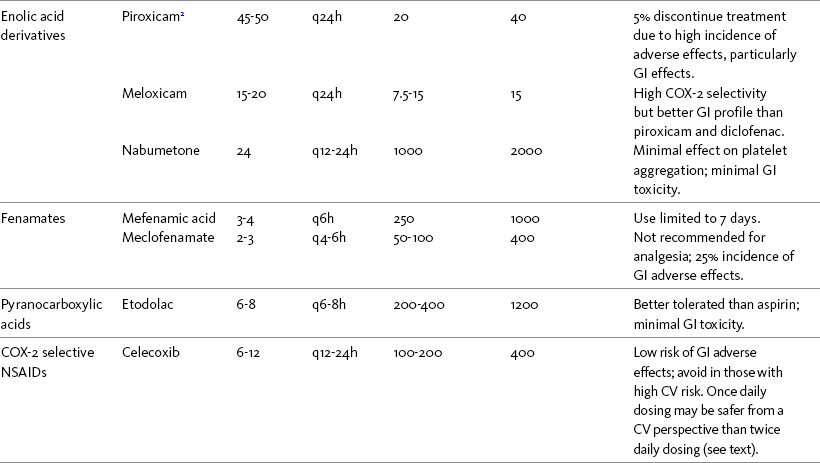
THE POTENTIAL risks and benefits of nonopioid analgesic therapy should be considered in developing a plan of care for all patients with pain. Most clinicians will consider trying a nonopioid unless there is clear evidence of nonefficacy or increased risk. Once the decision is made to try one of these drugs, the goal is to select an agent and a dose that offers satisfactory pain relief with a low risk of adverse effects. Decisions about drug and dose may be influenced by the patient’s past experience, pain syndrome, and the assessed risk factors for adverse effects. The patient’s ability to pay for nonopioid therapy also should be evaluated. Table 7-1 is a guide to generic and brand names of nonopioids and may be used in conjunction with Table 7-2, which details dosing information and other characteristics for acetaminophen and NSAIDs. See Chapter 6 regarding individualizing the selection of nonopioid analgesics based on GI risk and cardiovascular (CV) risk. See patient medication information in Forms III-1 through III-5 on pp. 250-259.
Table 7-1
Nonopioids Listed Alphabetically by Generic Name Followed by Brand Name
| Generic Name(s) | Brand Name(s) |
| Acetaminophen | Tylenol, many other brand names |
| Aspirin | Bayer, many other brand names |
| Celecoxib | Celebrex |
| Choline magnesium trisalicylate | Choline magnesium trisalicylate |
| Choline salicylate | Arthropan |
| Diclofenac | Cataflam (short acting for acute pain), Voltaren Delayed Release, Voltaren-XR (extended release for chronic pain therapy), Pennsaid (topical drops); Voltaren topical gel, Arthrotec (combined with misoprostol), Flector (topical patch) |
| Diflunisal | Dolobid |
| Etodolac | Lodine, Lodine XL |
| Etoricoxib | Arcoxia |
| Fenoprofen calcium | Nalfon, Nalfon 200 |
| Flurbiprofen | Ansaid |
| Ibuprofen | Advil, Motrin, Tab-Profen, Vicoprofen (combined with hydrocodone), Combunox (combined with oxycodone) |
| Indomethacin | Indocin, Indocin SR, Indo-Lemmon, Indomethagan |
| Ketoprofen | Orudis, Oruvail Extended-Release |
| Ketorolac | Toradol |
| Lumiracoxib | Prexige |
| Magnesium salicylate | Arthriten, Doan’s, Doan’s Extra Strength, Momentum |
| Meclofenamate sodium | Meclomen |
| Mefenamic acid | Ponstel |
| Meloxicam | Mobic |
| Nabumetone | Relafen |
| Naproxen | Aleve, Anaprox, Anaprox DS, Naprosyn, EC-Naproxyn, Naprelan, Naprapac (co-packaged with lansoprazole) |
| Oxaprozin | Daypro |
| Piroxicam | Feldene |
| Salsalate | Disalcid |
| Sulindac | Clinoril |
| Tolmetin | Tolectin, Tolectin DS, Tolectin 600 |
From Pasero, C., & McCaffery, M. Pain assessment and pharmacologic management, p. 210, St. Louis, Mosby. Pasero C, McCaffery M. May be duplicated for use in clinical practice.
General Considerations
Acute versus Persistent (Chronic) Pain
When a nonopioid is expected to be used with relatively infrequent dosing, or at low doses, or for only a short period of time (e.g., postoperatively), adverse effects are less problematic than with long-term use. Adverse effects increase dramatically with regular dosing for a period of days or more. Therefore, the importance of the risk:benefits analysis increases over time, and administration of the lowest effective dose for the shortest time needed is a key principle of nonopioid use (American Pain Society [APS], 2003).
Analgesic History
Talking with the patient about previous use of nonopioid analgesics for pain can provide valuable information as to which nonopioid to recommend. Whichever nonopioid has worked well for the patient and has caused minimal or no adverse effects is often the best place to begin with drug selection.
Pain Intensity
Several Cochrane Collaboration Reviews underscore the importance of evaluating the patient’s pain intensity when determining the appropriate nonopioid to administer. As mentioned earlier, acetaminophen has been shown to produce better pain relief than placebo, but NSAIDs were superior for osteoarthritis (OA) pain (Towheed, Maxwell, Judd, et al., 2006) (see pp. 220-221 for more on OA). Another Cochrane Collaboration review evaluating 51 studies and 5762 patients with moderate to severe postoperative pain concluded that acetaminophen provided effective analgesia for just half of the patients for 4 to 6 hours (Toms, McQuay, Derry, et al., 2008). These reviews support the appropriateness of acetaminophen as a first-line choice for mild pain and NSAIDs alone or in combination with other analgesics, including acetaminophen, for more severe pain.
Current Analgesic Response
Patients vary in response to nonopioids. If one nonopioid is ineffective after appropriate dose adjustment, it is worthwhile to try another. There is very little evidence to guide these so-called sequential trials, and assessment of risk and benefit should precede each decision about a new drug and dose.
A nonopioid should not be considered ineffective until the dose-response relationship has been explored and the clinician is certain that the dose has been increased either to (1) the highest dose conventionally accepted with the drug in question, (2) the dose associated with adverse effects, or (3) the dose that confirms that no additional analgesia is occurring with increases (i.e., a dose above the ceiling dose for analgesia). Many clinicians believe that it takes a few days to a week to evaluate the analgesia produced at a specific dose of an NSAID (Burke, Smyth, Fitzgerald, 2006), and a trial of at least 2 to 3 gm of acetaminophen per day for several weeks is recommended to evaluate the effectiveness of this drug (Simon, Lipman, Caudill-Slosberg, et al., 2002). Obviously, if severe adverse effects occur, the nonopioid should be stopped and another tried or the appropriateness of nonopioid therapy reconsidered.
Frequency of Dosing
Acetaminophen and NSAIDs may be given PRN for occasional pain or around the clock (ATC) for ongoing pain. Acetaminophen has a short half-life and usually must be given every 4 hours for ongoing pain. The half-lives of NSAIDs differ, and dosing intervals range from every 4 hours to once a day (see Table 7-2). For persistent pain, the use of once or twice a day dosing usually is more convenient and more likely to result in the patient taking all prescribed doses, which will lead to better pain control. This requires an NSAID with a long half-life or one that is formulated for modified release. However, it is recommended that full doses of naproxen, piroxicam, and oxaprozin be avoided in older adults because of their long half-life and an increased risk of GI toxicity (Fick, Cooper, Wade, et al., 2003; Hanlon, Backonja, Weiner, et al., 2009) (see Chapter 6 and later discussion about half-life in this chapter). When patients are taking other analgesics or medications, consideration should be given to NSAIDs that allow for scheduling as many doses as possible at the same time.
Research has shown that once-daily dosing of celecoxib may be safer from a CV perspective than twice-daily dosing, even of the same total daily dose (Vardeny, Solomon, 2008). The maximum plasma concentration of celecoxib is reached in 90 minutes after intake, and the drug has a short half-life of just 1.5 hours (Paulson, Hribar, Liu, et al., 2000). The COX-2 effect of celecoxib (and other NSAIDs) inhibits prostacyclin, a vasodilator that antagonizes platelet aggregation, and prostacyclin levels require approximately 12 hours to recover following a single oral dose of celecoxib (McAdam, Catella-Lawson, Mardini, et al., 1999). Once-daily dosing may allow enough prostacyclin recovery and normalization to attenuate any thrombotic effect (Grosser, Fries, FitzGerald, 2006; Vardeny, Solomon, 2008). Although further research is needed, the findings in other studies that utilized once-daily dosing support this theory (Sowers, White, Pitt, et al., 2005; Whelton, Fort, Puma, et al., 2001). See Chapter 6 for an extensive discussion of this and the other CV effects of NSAIDs.
Routes of Administration
NSAIDs are taken most often by the oral route of administration. All NSAIDs are available orally, and a few are available parenterally, rectally, and topically. Currently in the United States the only NSAIDs available for parenteral administration are ketorolac (Toradol), ibuprofen (Caldolor), and indomethacin (Indocin). Ketorolac is widely used parenterally as an analgesic for short-term pain (e.g., postoperative), and IV ibuprofen is approved for treatment of acute pain and fever; parental indomethacin is used primarily in infants for closure of patent ductus arteriosus (Burke, Smyth, Fitzgerald, 2006). Other countries have many nonopioids available parenterally, including acetaminophen, aspirin, ketoprofen, parecoxib, and diclofenac. At the time of publication, IV acetaminophen (Acetavance) (http://www.cadencepharm.com/products/apap.html) and injectable diclofenac (Dyloject) were in development for approval in the United States (Colucci, Wright, Mermelstein, et al., 2009). An intranasal formulation of ketorolac in a disposable, metered spray device for ambulatory patients with acute pain was also in development in the United States (Brown, Moodie, Bisley, et al., 2009; Moodie, Brown, Bisley, et al., 2008). See Chapter 8 for more on these nonopioid formulations.
Rectal Nonopioid Administration
Rectal NSAIDs are used far more often in countries other than the United States. Relatively few are available commercially for rectal administration in the United States, but most pharmacies can compound them as rectal suppositories. Oral formulations can also be administered rectally, either by using the intact tablet or by placing the intact or crushed tablet in a gelatin capsule and inserting the capsule into the rectum (Pasero, McCaffery, 1999). (Note that modified-release nonopioids should not be crushed.) Because rectal NSAIDs have an 80% to 90% oral bioavailability, higher rectal than oral doses may be required to achieve similar effects (Beck, Schenk, Hagemann, et al., 2000). (See Chapter 8 for discussion of rectal administration of perioperative nonopioids and Chapter 14 for rectal administration technique.)
Topical NSAIDs
Of all of the drugs administered topically, the largest amount of clinical evidence exists for NSAIDs (Stanos, 2007). They are used and researched more extensively in Europe than in the United States. The NSAIDs that are administered topically most often are diclofenac, ibuprofen, ketoprofen, piroxicam, and naproxen, but not all are available commercially in topical formulation in the United States (Moore, Derry, McQuay, 2008). Diclofenac is available in both gel and patch formulations. Other topical NSAIDs, such as ketoprofen and naproxen, are in development and are likely to become commercially available in the United States (McCleane, 2008). Compounding pharmacies sometimes prepare topical mixtures that contain NSAIDs when they are not available commercially (Coyne, Hansen, Watson, 2003).
Although cyclooxygenase (COX) inhibition is a primary mediator of NSAID analgesia by any route of administration, research is ongoing to elucidate all of the underlying mechanisms of action of topical NSAIDs. For example, in addition to COX inhibition, animal research has demonstrated that topical diclofenac inhibits peripheral NMDA (N-methyl-d-aspartate) receptors (Dong, Svensson, Cairns, 2009), and high tissue concentrations of diclofenac are capable of blocking sodium channels to mediate local anesthetic-like effects (Cairns, Mann, Mok, et al., 2008) (see Section I for more on the role of sodium channels and NMDA receptor antagonism in analgesia). The therapeutic effect of topical NSAIDs is the result of high concentrations of drug in the tissue rather than the systemic circulation (Galer, Rowbotham, Perander, et al., 2000; Mazieres, 2005; Sawynok, 2003; Stanos, 2007). The best use of topical NSAIDs, therefore, is for well-localized pain, such as arthritic joint or soft-tissue injury pain (Galer, Rowbotham, Perander, et al., 2000; McCleane, 2008). The effectiveness of a topical NSAID depends on its ability to reach the tissue generating nociception (Moore, Derry, McQuay, 2008). (See Chapter 24 for discussion of topical vs. transdermal drug delivery and Figure 24-1 on p. 685)
The bioavailability of an NSAID after topical application is 5% to 10% compared with equivalent oral administration (Heyneman, Lawless-Liday, Wall, 2000; Mazieres, 2005). For comparison, oral NSAIDs have a 50% to 70% oral bioavailability after first-pass effect (Wilbeck, Schorn, Daley, 2008). Thus, compared with other routes of administration, topical NSAIDs provide the benefits of a low incidence of systemic adverse effects and the potential for fewer drug-drug interactions (Bannwarth, 2006; Galer, Rowbotham, Perander, et al., 2000; Heyneman, Lawless-Liday, Wall, 2000; McCleane, 2008; Rainsford, Kean, Ehrlich, 2008).
Given their low systemic distribution, topical NSAIDs may be particularly advantageous in patients who have well-localized painful conditions that may respond to NSAID therapy but are at high risk for NSAID adverse effects, such as older adults with OA (McCleane, 2008) (see pp. 220-221 for more on OA). Patients should be advised that they may take acetaminophen concurrently but should avoid other NSAIDs while taking topical NSAIDs because this can increase the incidence of adverse effects (Galer, Rowbotham, Perander, et al., 2000; McCleane, 2008).
Although topical NSAIDs have a lower risk of adverse effects than systemically-administered NSAIDs, it is important to understand that some drug is absorbed and the risk of serious toxicity is not nil. GI adverse effects from a topical NSAID are more likely in individuals who have experienced a previous GI adverse effect, in a manner similar to orally-administered drugs (McCleane, 2008; Sawynok, 2003). Therefore, tolerability should be regularly assessed during topical NSAID use, particularly in patients with a history of GI adverse effects. Postmarketing surveillance identified reports of hepatotoxicity during the first months of treatment with diclofenac gel (Voltaren), prompting the revision of prescribing information for the drug to state that liver enzyme testing should be done within the first 4 to 8 weeks of treatment initiation (Dawson, 2010).
Topical NSAIDs are used for both acute and persistent (chronic) pain, but their effectiveness has been questioned over the years (McCleane, 2008; Moore, Derry, McQuay, 2008; Simon, 2008). A systematic review of the use of rubefacients (counterirritants) containing salicylates concluded that there is a lack of well-designed and controlled research regarding their use and adverse effects and reported poor efficacy for persistent pain and limited evidence of efficacy for acute pain treatment (Mason, Moore, Edwards, et al., 2004b). One major drawback is that many of the currently available or compounded topical NSAIDs are in short-acting gel or cream formulations only, which have a relatively brief duration of action (McCleane, 2008). This limits their usefulness, particularly for persistent pain. Gels and creams can also be messy and result in inexact dosing and disruption if the preparation is rubbed off. Gels are reported to be more effective than creams (Moore, Derry, McQuay, 2008).
Topical patch formulations seem to be a better option because they allow uniform application, which results in more controlled delivery than is possible with gels or creams (Rainsford, Kean, Ehrlich, 2008; Stanos, 2007). Steady state maximum plasma concentration of the diclofenac epolamine 1.3% patch (Flector) is reached in 5.4 hours following application of the patch, and it has a half-life of 26.4 hours. Significant accumulation of topical diclofenac occurs in the synovial fluid and musculature (Moore, Derry, McQuay, 2008). There are no clinically relevant metabolites, and mild GI symptoms and dermal reactions occur in 2% and 10% of patients, respectively (Rainsford, Kean, Ehrlich, 2008). Patches must be changed every 12 hours for continuous relief.
Patients often like the idea of applying medication directly to a painful area (Underwood, Ashby, Cross, et al., 2008); however, not all pain is responsive to topical NSAIDs; if pain relief is not apparent within a few hours, sustained use is not justified and alternative methods of pain control should be implemented (McCleane, 2008). As with all NSAIDs, the lowest effective dose for the shortest time necessary should be used.
Topical NSAIDs for Acute Pain: A systematic review of 26 double-blind, placebo-controlled trials analyzed data from 2853 patients to evaluate the efficacy and safety of a variety of topical NSAIDs for acute pain conditions, such as sprains and strains (Mason, Moore, Edwards, et al., 2004c). Compared with oral NSAIDs, topical NSAIDs provided better pain relief and similar adverse effects and treatment success. Withdrawals due to an adverse event were rare. Ketoprofen was more effective than topical ibuprofen, piroxicam, and indomethacin; there was no comparison data on diclofenac.
Zacher and colleagues (2008) performed a review of 19 double-blind, randomized, placebo- or active-controlled trials (> 3000 patients) of topical diclofenac. They concluded that this formulation is effective in reducing pain and inflammation associated with acute soft-tissue injury; benefits included functional improvement, a low incidence of mild dermal adverse effects, and fewer adverse effects than other topical and oral NSAIDs. Banning (2008) found that topical diclofenac in a variety of formulations was either superior or equivalent to oral diclofenac or placebo for orthopedic soft-tissue injury pain. Topical diclofenac produced significant reductions in pain and morning stiffness, and improved physical function. Again, minor dermatitis-type adverse effects were reported. Randomized, placebo-controlled trials that have focused specifically on the diclofenac 1.3% patch formulation have reported similar positive results for sports-related soft-tissue injuries (Galer, Rowbotham, Perander, et al., 2000; Predel, Koll, Pabst, et al., 2004).
At the time of publication, a topical patch containing 100 mg of ketoprofen was in development in the United States. Phase III trials in patients with traumatic soft-tissue injuries and other musculoskeletal pains have shown the patch to be more effective than placebo and similar to the diclofenac patch, to produce minor dermal adverse reactions, and to be associated with a low incidence of GI adverse effects (Mazieres, 2005). For example, a randomized, double-blind, 14-day study of 172 patients showed that the ketoprofen patch was safe and effective in relieving tendonitis (Mazieres, Rouanet, Guillon, et al., 2005). Other research has suggested that the ketoprofen patch may yield slightly better pain relief during activity and higher patient satisfaction than the diclofenac gel (Esparza, Cobian, Jimenez, et al., 2007).
Ibuprofen cream also has been used as a topical analgesic for acute pain. One study found no significant differences in pain relief at rest and with movement between oral ibuprofen 400 mg and ibuprofen 5% gel, both administered 3 times daily, for acute soft-tissue injuries (Whitefield, O’Kane, Anderson, 2002). A placebo-controlled study found that application of 5% ibuprofen cream 2 hours before elective external direct current cardioversion safely and effectively reduced pain and inflammation (Ambler, Zidema, Deakin, 2005); the researchers concluded that it should be used routinely prior to this procedure.
A foam dressing permeated with ibuprofen (Biatain Ibu) is used outside of the United States for the care of painful wounds. One randomized, controlled study showed that the use of ibuprofen foam dressings reduced pain and improved quality of life with minimal adverse effects in patients with painful exuding wounds (Palao, Domenech, Romanelli, et al., 2008). Another study found similar results with the combined use of the ibuprofen-releasing foam dressing and silver-releasing contact layer on locally infected, exuding venous leg ulcers (Jorgensen, Gottrup, Karlsmark, et al., 2008). Pain during dressing change was reduced, and persistent pain decreased from 6.2 to 3.0 (0 to 10 scale) with minimal adverse effects.
Topical NSAIDs for Persistent (Chronic) Pain: Numerous high-quality studies have shown the superiority of topical NSAIDs compared with placebo for persistent pain (McCleane, 2008; Moore, Derry, McQuay, 2008). An extensive, systematic literature review that included 14 randomized, double-blind trials with data on over 1500 patients comparing topical NSAIDs with either placebo or other active treatment concluded that topical NSAIDs were safe and effective for persistent musculoskeletal pain (Mason, Moore, Edwards, et al., 2004c). Local and systemic effects were minimal and similar to placebo.
Topical NSAIDs are presented as first-line analgesic options in a variety of clinical guidelines (Hunter, Lo, 2008). The United Kingdom’s National Collaborating Centre for Chronic Conditions recommends that topical NSAIDs be the core treatment for knee and hand OA and, with acetaminophen, should be tried before oral NSAIDs (NCC-CC, 2008). Others have supported this recommendation (Moore, Derry, McQuay, 2008). However, an extensive literature review on the use of topical NSAIDs for the treatment of OA pain concluded that topical NSAIDs produced better pain relief than placebo but inferior pain relief compared with oral NSAIDs (Lin, Zhang, Jones, et al., 2004). A controversial finding of this study was that the pain relief from topical NSAIDs did not extend beyond 2 weeks. This review has been criticized for many reasons, primarily design flaws, and the finding that topical NSAIDs work for only 2 weeks has not been supported by other research (Moore, Derry, McQuay, 2008).
Topical diclofenac has been extensively evaluated in placebo-controlled trials of longer duration. A 4-week randomized, placebo-controlled trial (N = 248) evaluating the effect of topical 1.5% diclofenac solution on OA knee pain found that patients taking diclofenac experienced significantly less pain on walking and improved physical function, and that patient global assessments were superior (Bookman, Williams, Shainhouse, 2004). Adverse effects, such as localized dry skin, were minor. Active drug and placebo groups had similar rates of GI and renal adverse events, suggesting that the topical drug had minimal systemic absorption. A randomized placebo-controlled 8-week trial (N = 385) of 1% diclofenac gel use for hand osteoarthritis (OA) demonstrated significant improvements in pain and global rating of disease; treatment was well tolerated with mild adverse effects, such as application-site paresthesia (Altman, Dreiser, Fisher, et al., 2009). Placebo-controlled studies of 6-week (Baer, Thomas, Shainhouse, 2005) and 12-week (Roth, Shainhouse, 2004) durations evaluated 1.5% diclofenac solution in 216 and 326 patients with knee OA, respectively, and found similarly positive results on pain, function, and patient satisfaction, with no systemic adverse effects. A randomized, placebo-controlled study (N = 153) established that the diclofenac (60 mg) patch produced effective pain control, improved function, and minimal adverse effects for myofascial pain syndrome (Hsieh, Hong, Chern, et al., 2010).
Similar findings were obtained when topical diclofenac was compared with other NSAIDs. The previously mentioned review by Zacher and colleagues (2008) of 19 double-blind, randomized trials (more than 3000 patients) concluded that topical diclofenac produced outcomes in OA pain that were better than other topical NSAIDs and oral diclofenac, ibuprofen, or naproxen. Topical diclofenac has also been shown to be as effective as oral diclofenac in reducing morning stiffness (Banning, 2008). In contrast, another systematic review found no significant differences in effectiveness or systemic effects between topical diclofenac and oral diclofenac, ibuprofen, and indomethacin (Moore, Derry, McQuay, 2008), and a study of a gel formulation of topical 1.16% diclofenac produced comparable findings (Niethard, Gold, Solomon, et al., 2005). More head-to-head research is needed to better compare the effectiveness of topical and oral NSAIDs.
Longer trials of other topical NSAIDs are very limited, and there are no clear conclusions. A randomized, controlled study found that 200 mg of ibuprofen cream (5%) applied to the knee in a 10-cm strip 3 times daily for 1 week provided better pain relief at rest, and overall, than placebo in patients with knee OA (Trnavsky, Fischer, Vogtle-Junkert, et al., 2004). This study confirmed earlier placebo-controlled research on ibuprofen cream (Rovensky, Micekova, Gubzova, et al., 2001). An interesting randomized 1-year study required researchers to advise patients with knee OA to use either oral or topical ibuprofen depending on the patient’s preferred route of administration (Underwood, Ashby, Cross, et al., 2008); although patients preferred topical ibuprofen, and both oral and topical ibuprofen were safe, neither were particularly effective, presumably because of relatively poor adherence. The latter study suggests that the outcomes of controlled trials with high adherence may overestimate the benefits actually obtained in clinical practice.
Piroxicam 0.5% gel was found to produce similar effectiveness and tolerability when compared with a gel containing a variety of homeopathic ingredients (van Haselen, Fisher, 2000). The authors recommended concomitant PRN administration of other “simple” analgesics but offered no specific suggestions. Piroxicam is noted to be theoretically inferior in terms of penetration compared with other more commonly used NSAIDs, such as ibuprofen and ketoprofen (Moore, Derry, McQuay, 2008).
Cost
The cost of treatment with a nonopioid analgesic may be an important consideration, particularly when nonopioids are used for persistent pain. Nonprescription generic brands of nonopioids are almost always less expensive than brand names and prescription nonopioids. Further, the APS points out that equianalgesic doses of nonprescription NSAIDs are as effective as prescription NSAIDs (Simon, Lipman, Caudill-Slosberg, et al., 2002).
Analgesics often have wide ranges in cost. The cost of prescription NSAIDs varies from one pharmacy to another, but the newer NSAIDs without generic equivalents tend to be more expensive. There are numerous web sites, such as PharmacyChecker.com (http://www.pharmacychecker.com/), that compare drug costs worldwide. Some pain guidelines include tables displaying the costs of analgesics at the time of publication. Although listed prices are likely to be different from current prices, they provide an idea of relative cost. Based on the 2002 APS Guideline for the Management of Pain in Osteoarthritis, Rheumatoid Arthritis, and Juvenile Chronic Arthritis data, aspirin, ibuprofen, and salsalate were among the least expensive nonopioids (Simon, Lipman, Caudill-Slosberg, et al., 2002).
Many pharmaceutical companies have financial assistance programs that allow reduced purchase prices for patients who qualify. Prescribers can find information about this in the Physician’s Desk Reference and at most pharmaceutical company websites. If drug costs are a significant issue, prices should be checked with the pharmacy.
Gastroprotective co-therapy will increase the cost of NSAID therapy and must be considered as well. The cost of a COX-2 selective NSAID that produces less GI toxicity may be less than an NSAID plus a PPI (Simon, Lipman, Caudill-Slosberg, et al., 2002). Unfortunately, research comparing the gastroprotective strategies in patients receiving long-term NSAID therapy is sparse (Brown, Hooper, Elliott, et al., 2006) (see Chapter 6). More studies also are needed that compare the cost of the various gastroprotective therapies with the cost of treating a serious adverse GI event that could have been prevented had gastroprotective therapy been initiated.
Choice of Starting Dose and Dose Titration
Regardless of which nonopioid analgesic is selected, a principle of pain management that has been reinforced throughout this section is to give the lowest effective nonopioid dose for the shortest time needed. Following is a discussion of starting doses and titration for acetaminophen, followed by NSAIDs (Box 7-1; also see Box 6-1). See patient medication information in Forms III-1 through III-5 on pp. 250-259.
Acetaminophen
The recommended starting dose of acetaminophen is 650 mg every 4 hours, not to exceed 4000 mg in a 24-hour period (APS, 2003). The American Geriatrics Society (AGS) (2009) recommends a daily dose range of 2000 mg to 4000 mg for persistent pain in older adults. The most common adult daily dose of acetaminophen is 1000 mg (Burke, Smyth, Fitzgerald, 2006; Toms, McQuay, Derry, et al., 2008). This is also suggested as the optimal dose with a gradual decline in analgesic activity over a 6-hour period (Bannwarth, Pehourcq, 2003).
Doses of 1000 mg of acetaminophen 4 times a day have been reported to be as effective as ibuprofen at 1200 or 2400 mg per day doses for OA pain (Simon, Lipman, Caudill-Slosberg, et al., 2002) (see pp. 220-221 for more on OA). A systematic review of randomized, controlled trials comparing higher and lower doses of nonopioids concluded 1000 mg of acetaminophen provided statistically superior analgesia compared with 500 mg (McQuay, Moore, 2006). There is a ceiling on the analgesia of acetaminophen, and increasing each dose greater than 1000 mg will result in very little added analgesia (Motov, Ast, 2008). If pain cannot be controlled with recommended doses, an NSAID or an opioid analgesic should be considered.
Caution is recommended with long-term acetaminophen use, even at therapeutic doses (Bolesta, Haber, 2002) (see Chapter 6 for hepatic effects). Most experts recommend a reduction in daily dose in individuals who are at high risk for hepatotoxicity (Burke, Smyth, FitzGerald, 2006). For example, the AGS (2009) recommends a 50% to 75% reduction in dose in older adults with hepatic dysfunction. Others recommend avoiding acetaminophen entirely in patients with liver disease (Bannwarth, Pehourcq, 2003; Laine, White, Rostom, et al., 2008). Still others suggest it as the optimal analgesic for patients with stable chronic liver disease (Graham, Scott, Day, 2005).
Though some research shows that 4000 mg/24 h can be safely taken in individuals who regularly drink alcohol (Graham, Scott, Day, 2005; Kuffner, Dart, 2001), the APS recommends no more than 2500 mg/24 h in individuals who consume more than 2 ounces of alcohol daily because of the elevated risk of hepatotoxicity (Simon, Lipman, Caudill-Slosberg, et al., 2002). The United States Food and Drug Administration (U.S. FDA) requires acetaminophen product labeling to warn consumers of the increased risk of liver damage when acetaminophen is taken by those who consume three or more alcoholic drinks per day (U.S. FDA, 2009). The AGS lists chronic alcohol abuse/dependence as a relative contraindication to using acetaminophen (AGS, 2009). Liver function tests should be performed every 6 to 12 months in any individual at high risk for hepatotoxicity who is taking long-term acetaminophen (Bannwarth, 2006; Miaskowski, Cleary, Burney, et al., 2005; Simon, Lipman, Caudill-Slosberg, et al., 2002).
Daily acetaminophen doses of greater than 500 mg have been shown to diminish gastric mucosal protection (Rahme, Pettitt, LeLorier, 2002), and epidemiologic studies show doses of greater than 2000 mg/24 h produce increased risk of upper GI adverse effects (Bannwarth, 2006). This risk of GI events is underappreciated. Acetaminophen also has been associated with dose-dependent renal adverse effects, and chronic use should incorporate appropriate precautions and monitoring for renal toxicity (Curhan, Knight, Rosner, et al., 2004; Forman, Stampfer, Curhan, 2005) (see Chapter 6 for GI effects and for renal effects).
NSAIDs
If the target of therapy is pain (and not inflammation), and in the absence of significant co-morbid hepatic disease, NSAID therapy should be considered when pain cannot be controlled by acetaminophen. For most patients, the initial dose is consistent with the lower effective dose suggested by the manufacturer. Pain may not respond until a higher dose is given, however, and the analgesic dose is usually less than the antiinflammatory dose. Dose escalation may be undertaken, therefore, either to explore the dose-response for pain or to gain better control over inflammation. For example, 400 mg of ibuprofen is usually recommended for analgesia, but doses as high as 800 mg 4 times a day may be necessary for an antiinflammatory effect.
There is a ceiling on the analgesia of each NSAID, but it varies from one person to another. Single ibuprofen doses greater than 400 mg and daily doses greater than 1200 mg have been shown to produce little analgesic advantage, leading some researchers to recommend a dosing regimen of 400 mg every 8 hours rather than the customary 600 mg to 800 mg every 6 to 8 hours (Motov, Ast, 2008). Individual patients, however, may demonstrate a clear-cut benefit at relatively higher doses. Higher doses are associated with a higher incidence of adverse effects (Antman, Bennett, Daugherty, 2007; Bertagnolli, Eagle, Zauber, et al., 2006; Wilcox, Allison, Benzuly, et al., 2006), and for this reason, most clinicians explore the dose-response of an NSAID only until the highest dose recommended by the manufacturer or modestly higher is reached.
There is no certainty about the minimal effective analgesic dose, the ceiling dose, or the toxic dose for the individual patient. To avoid giving a patient a higher dose than is needed, dose titration should be considered and may be especially important in those with increased risk of NSAID toxicity (e.g., older adults) and those who are treated with the intention of long-term therapy.
If the NSAID dose is titrated from a relatively low starting dose, it typically requires a period of 5 to 7 days to evaluate the response at each dose level. The occurrence of increased analgesia after a dose increase implies that the ceiling dose has not been reached. Titration can be continued until a further dose increase provides no additional pain relief, adverse effects occur, or a dose conventionally considered the ceiling dose for the specific drug in question is reached. The lowest dose that provides satisfactory pain relief should be maintained.
The potential value of different dosing regimens for pains of different types has not been empirically evaluated for most drugs. Celecoxib may illustrate the utility of this type of data. The recommended and most frequently prescribed starting dose of celecoxib for acute pain is an initial single dose of 400 mg (e.g., given preoperatively for surgical patients) followed by another 200 mg on the day of surgery and twice daily doses on subsequent days (Recart, Issioui, White, et al., 2003). Single doses of 200 mg daily or 100 mg twice daily are recommended and most often prescribed for OA; doses of 100 mg to 200 mg twice daily are recommended for RA, and the most commonly prescribed daily dose is 400 mg (Schnitzer, Kong, Mitchell, et al., 2003). Generally, a starting dose of 200 mg daily is recommended for most patients with persistent pain (Laine, White, Rostom, et al., 2008). Higher doses may improve analgesia but can also result in more adverse effects (Bertagnolli, Eagle, Zauber, et al., 2006).
Celecoxib efficacy does not appear to be influenced by whether the drug is dosed once or twice daily, but as discussed in Chapter 6, a twice-daily dosing regimen may increase the incidence of CV adverse effects. Once-daily dosing may be preferable, particularly in patients at high risk for CV adverse events (Grosser, Fries, FitzGerald, 2006; Sowers, White, Pitt, et al., 2005; Vardeny, Solomon, 2008; Whelton, Fort, Puma, et al., 2001).
Acute Pain
NSAIDs vary in time to onset and duration of analgesia. Generally, NSAIDs with a longer half-life have a slower onset of analgesia. NSAIDs with shorter half-lives, which have a more rapid onset, are used most often for acute pain management (Helstrom, Rostom, 2006). Usually, higher doses result in a faster onset of analgesia, higher peak effect, and longer duration of analgesia. These kinetics would support the conclusion that the treatment of acute pain is most effectively managed by initiating treatment with the highest approved dose of a short half-life drug and then adjusting the dose downward. As noted, however, the increased safety of dose titration suggests that the alternative strategy—low initial dose combined with gradual exploration of the dose-response—is appropriate if therapy is started with the intent of long-term treatment, or if patients have important risk factors for adverse effects (see Chapter 8 for perioperative use of nonopioids).
Persistent (Chronic) Pain
NSAIDs with long half-lives should be considered for persistent pain because they require less frequent dosing and may enhance adherence as a result. Although some clinicians recommend a priming dose of a long half-life drug to increase blood levels and shorten onset of analgesia, others start with low doses to minimize adverse effects, especially in those patients at risk for adverse effects (also see discussion of celecoxib dosing regimen earlier in the chapter). Caution is recommended in administering long-acting NSAIDs in older adults (Simon, Lipman, Caudill-Slosberg, et al., 2002). Many clinicians recommend avoiding full doses of naproxen, piroxicam, and oxaprozin in older adults because of their long half-life and increased risk of GI toxicity (Fick, Cooper, Wade, et al., 2003; Hanlon, Backonja, Weiner, et al., 2009) (see Chapter 6).
Several weeks are necessary to evaluate the effectiveness of an NSAID when it is used to treat grossly inflammatory conditions such as rheumatoid arthritis (RA) (Burke, Smythe, FitzGerald, 2006). However, analgesia can occur with the first dose, and typically a week or less is sufficient to evaluate the global analgesic benefit of an NSAID regimen at a selected dose. The foregoing discussion of selection and dosing of nonopioids is summarized in Box 7-1.
Special Circumstances and Conditions
OA is the most common form of arthritis in the United States (American College of Rheumatology (ACR). 2000). In 2000, the North American prevalence was 25 million; projections have this number doubling by 2020 (Hunter, Lo, 2008). OA is age-related, chronic, and often debilitating; pain is a major determinant of its impact on function and quality of life (AHRQ, 2006; Simon, Lipman, Caudill-Slosberg, et al., 2002).
The underlying disease process of OA is joint degeneration with loss of joint space, osteophyte formation, and subchondral sclerosis (Sun, Wu, Kalunian, 2007). OA can be asymmetrical and affect just one or multiple joints. The associated pain usually is classified as nociceptive (see Section I), and nonopioid analgesics have a major role in its relief. The challenge, as with all types of pain, is to select nonopioid analgesics that will provide the greatest degree of pain relief with the fewest adverse effects (AHRQ, 2006).
Several professional organizations, including the ACR (Altman, Hochberg, Moskowitz, et al., 2000), APS (Simon, Lipman, Caudill-Slosberg, et al., 2002), and European League Against Rheumatism (EULAR) (Jordan, Arden, Doherty, et al., 2003; Zhang, Doherty, Arden, et al., 2005; Zhang, Doherty, Leeb, et al., 2007), have released evidence-based treatment recommendations for OA pain. More recently, a panel composed of 16 experts from around the world appointed by the Osteoarthritis Research Society International (OARSI) released recommendations (Zhang, Moskowitz, Nuki, et al., 2008). The OARSI panel appraised already published guidelines, systematically reviewed the research for treatment of knee and hip OA through January 2006, and issued guidelines that are considered by some to contain the most up-to-date recommendations (Hunter, Lo, 2008). The reader is referred to the OARSI document.
All of the guidelines agree that acetaminophen at doses up to 4 g/day is first line for the pharmacologic treatment of mild to moderate OA pain (Altman, Hochberg, Moskowitz, et al., 2000; NCC-CC, 2008; Roddy, Doherty, 2003; Simon, Lipman, Caudill-Slosberg, et al., 2002; Zhang, Moskowitz, Nuki, et al., 2008). The AGS also recommends acetaminophen as a first-choice analgesic for mild to moderate persistent musculoskeletal pain (AGS, 2009). Further, the OARSI promotes acetaminophen as a safe long-term analgesic if pain relief is satisfactory. However, some researchers question the appropriateness of acetaminophen as a first-line choice for OA and call for more studies that compare the drug to specific NSAIDs for pain treatment (Case, Baliunas, Block, 2003). Sun and colleagues (2007) recommend an NSAID as a primary drug in the treatment of patients with an inflammatory phenotype of OA.
NSAIDs are recommended as the next choice of analgesic if pain is uncontrolled by acetaminophen (Altman, Hochberg, Moskowitz, et al., 2000; American Academy of Orthopaedic Surgeons, 2008; NCC-CC, 2008; Roddy, Doherty, 2003; Simon, Lipman, Caudill-Slosberg, et al., 2002; Zhang, Moskowitz, Nuki, et al., 2008). Randomized controlled studies comparing acetaminophen and NSAIDs support this recommendation with findings of superior analgesia with NSAIDs for OA pain (Battisti, Katz, Weaver, et al., 2004; Case, Baliunas, Block, 2003; Pincus, Koch, Lei, et al., 2004; Pincus, Koch, Sokka, et al., 2001). A Cochrane Collaboration Review of 15 randomized controlled trials involving nearly 6000 patients concluded that NSAIDs were superior to acetaminophen in reduction of pain, global assessments, and improvements in functional status for both knee and hip OA pain (Towheed, Maxwell, Judd, et al., 2006). Safety and tolerability were essentially the same between the two types of analgesics, except that those taking an NSAID were more likely to experience a GI adverse effect (see Chapter 6 for NSAID-induced GI adverse effects). The AHRQ (2006) executive summary on the best evidence comparing the various common OA pain treatments also notes the benefits of NSAIDs (AHRQ, 2006).
Some of the literature comparing NSAIDs and acetaminophen has focused on the COX-2 selective drugs. A 6-week study randomized 288 patients with OA of the knee to receive acetaminophen, rofecoxib, or celecoxib and found that all treatments were safe and well tolerated; however, more patients receiving acetaminophen than the COX-2 selective NSAIDs discontinued treatment due to lack of efficacy (Geba, Weaver, Polis, et al., 2002). Rofecoxib (no longer available), followed closely by celecoxib, was associated with the most improvement in outcome indicators (e.g., pain relief, morning stiffness, physical function). Although acetaminophen is generally reserved for mild OA pain, it has been shown to produce an additive effect (Sun, Wu, Kalunian, 2007), and some clinicians advocate the continuation of acetaminophen therapy during NSAID treatment as part of a multimodal pain treatment plan (Simon, Lipman, Caudill-Slosberg, et al., 2002).
As discussed in Chapter 6, the risks and benefits of initiating NSAID therapy must be considered on an individual basis. If initiated, all guidelines stress the importance of administering the lowest NSAID dose for the shortest time necessary. This principle is particularly applicable in patients with OA, as treatment is likely to be long-term and the risk for adverse effects increases over time. Older adults are the most likely to be afflicted with OA and are at high risk for NSAID-induced GI adverse effects, and it is reasonable to consider those NSAIDs with relatively more favorable GI risk profiles as particularly preferable. Topical NSAIDs produce fewer GI adverse effects than oral drugs, and guidelines also recommend that they be considered another means of reducing risk. The United Kingdom’s National Collaborating Centre for Chronic Conditions recommends that topical NSAIDs be the core treatment, along with acetaminophen, for knee and hand OA and should be tried before oral NSAIDs (NCC-CC, 2008).
For oral NSAID therapy, the OARSI recommends a COX-2 selective NSAID or a nonselective NSAID with a PPI or misoprostol, but cautions against long-term NSAID therapy because of the significant risk of GI adverse effects (Zhang, Moskowitz, Nuki, et al., 2008). Many older adults also have CV risk factors, and some have both GI and CV risk factors that must be considered. A placebo-controlled trial showed naproxen (440/660 mg/day), which is characterized as an NSAID with relatively low GI and CV adverse effect profiles, provided comparable satisfactory pain relief to ibuprofen (1200 mg/day) for mild to moderate OA knee pain (Schiff, Minic, 2004). Some researchers recommend avoiding full doses of NSAIDs with a long half-life, such as naproxen (Fick, Cooper, Wade, et al., 2003).
Several guidelines recommend the use of opioid analgesics for OA pain that is unrelieved by nonpharmacologic measures, acetaminophen, and NSAIDs (Altman, Hochberg, Moskowitz, et al., 2000; NCC-CC, 2008; Simon, Lipman, Caudill-Slosberg, et al., 2002; Zhang, Moskowitz, Nuki, et al., 2008). Tramadol is also suggested for more severe pain (Altman, Hochberg, Moskowitz, et al., 2000; Simon, Lipman, Caudill-Slosberg, et al., 2002). (See Section IV for more on the use of opioids and tramadol for OA pain.)
Rheumatoid Arthritis
RA is the second most common form of arthritis, with a prevalence of 1% to 2% of adults afflicted. It affects women more frequently than men and is diagnosed most often between the ages of 20 and 40 (Simon, Lipman, Caudill-Slosberg, et al., 2002). It is usually chronic, progressive, and can be extremely debilitating. Over 9 million physician visits and more than 250,000 hospitalizations annually are related to RA (Khanna, Arnold, Pencharz, et al., 2006).
RA is an autoimmune inflammatory disease with hallmark features of symmetrical erosive synovitis with an unknown etiology (Khanna, Arnold, Pencharz, et al., 2006). The disease begins in the synovial fluid but is systemic and can affect multiple organs and lead to premature death. Early and aggressive treatment is critical to prevent cartilage destruction (Simon, Lipman, Caudill-Slosberg, et al., 2002).
Pain, swelling, tenderness, and morning stiffness of various joints usually prompts individuals with RA to seek initial medical help. Urgent treatment with immunosuppressive agents, including a blocker of the cytokine tumor necrosis factor, typically is indicated. This treatment often is highly effective and may control pain to such a degree that further analgesic therapy is not needed. In some cases, other disease modifying therapies may be needed, among which is an NSAID at antiinflammatory doses. In other cases, synovitis appears to be stemmed by immunosuppressive therapy, but additional treatment for pain is necessary to allow optimal functioning.
A Cochrane Collaboration Review found only four studies comparing acetaminophen and NSAIDs for RA pain (Wienecke, Gotzsche, 2004). The poor quality of the studies made it impossible to draw conclusions about superiority; however, the patients in the trials preferred NSAIDs to acetaminophen far more often. Guidelines support the use of NSAIDs as first-line analgesics for RA pain; however, the choice of NSAID must be guided by thoughtful consideration of patient risk factors (Luqmani, Hennel, Estrach, et al., 2009; Simon, Lipman, Caudill-Slosberg, et al., 2002).
RA is associated with an increased risk for CV adverse events, such as myocardial infarction, and individuals with RA who have had a previous CV event or have other risk factors are of particular concern (Medscape Rheumatology, 2006) (see Table 6-4 and Chapter 6 for discussion of CV risk factors). Many individuals with RA take cardioprotective aspirin as a way of addressing the elevated CV risk. Aspirin therapy has the potential to produce an increase in GI adverse effects and also has implications for the choice of NSAID. Some clinicians favor the use of celecoxib given its pharmacologic profile (see discussion in Chapter 6), and a Cochrane Collaboration Review evaluating the use of celecoxib in patients with RA found that celecoxib produced pain relief similar to naproxen, diclofenac, and ibuprofen, and had fewer associated upper GI complications (Garner, Fidan, Frankish, et al., 2002). The latter benefit may or may not persist during long-term therapy, however, and presumably is attenuated or eliminated by aspirin co-therapy. Nonetheless, a recent study found a decline in NSAID-related GI adverse effects in patients with RA worldwide (Steen, Nurmohamed, Visman, et al., 2008), a change attributed to an increase in the use of COX-2 selective inhibitors, strict adherence to gastroprotective guidelines, and better general RA treatment regimens (see Chapter 6 for gastroprotective therapy and for selection of NSAID with consideration of CV and GI risk). Clearly, the selection of an NSAID for RA pain should favor those with relatively better risk profiles and the use of treatment strategies that minimize risk through careful dose selection and adverse effect management. Moreover, as with OA, the use of opioid analgesics should be considered when other analgesics do not produce adequate pain relief or are contraindicated by patient risk factors (Simon, Lipman, Caudill-Slosberg, et al., 2002). See Section IV for more on the use of opioid analgesics for RA.
Low Back Pain
At some point, most people will experience low back pain. It is reported that up to 90% of adults will have low back pain during their lifetime (Birbara, Puopolo, Munoz, et al., 2003). Next to upper respiratory infection, it is the most common reason for lost work. Although most acute low back pain is benign and resolves over time (Pepijn, Roelofs, Deyo, et al., 2008), it can persist and become disabling. The disability and costs associated with acute and persistent low back pain are in the range of 20 to 50 million dollars every year, with persistent pain accounting for up to 90% of this cost.
Nonopioid analgesics are commonly used to treat both acute and persistent low back pain, but the research supporting their efficacy for this type of pain is lacking. A Cochrane Collaboration Review of 65 trials (11,237 patients) found that acetaminophen and the various nonselective and COX-2 selective NSAIDs were more effective than placebo and equally effective to one another in reducing short-term acute low back pain and persistent low back pain without sciatica (Roelofs, Deyo, Koes, et al., 2008). In a joint clinical practice guideline, the American College of Physicians and the APS noted that acetaminophen is a less efficacious analgesic than NSAIDs but recommended it for initial low back pain treatment because of its more favorable safety profile (Chou, Qaseem, Snow, et al., 2007). The guideline recommends NSAID therapy in the lowest effective dose after a careful assessment of CV and GI risk and opioids or tramadol for severe, disabling acute or persistent low back pain. European evidence-based guidelines for the management of persistent low back pain recommend short-term use (e.g., 3 months) of NSAIDs and opioid analgesics (Airaksinen, Brox, Cedraschi, et al., 2006). The guideline panel stated that more research, specifically of functional outcomes, is needed before recommendations for long-term use of acetaminophen and NSAIDs could be made.
At least two studies have been conducted to evaluate the effect of the COX-2 selective NSAID etoricoxib (Arcoxia) on persistent low back pain. The first study randomized 319 patients with persistent low back pain to receive 60 mg or 90 mg of etoricoxib or placebo daily for 12 weeks (Birbara, Puopolo, Munoz, et al., 2003). The patients who took either dose of etoricoxib demonstrated significantly more pain relief and improvement in multiple functional and quality of life indicators compared with placebo. Pain relief and reductions in disability were noticeable within 1 week, maximal at 4 weeks, and maintained over the 3-month study period. A multicenter (46 sites) study of very similar design also reported improvements in pain and physical functioning that were maintained over 3 months with etoricoxib (Pallay, Seger, Adler, et al., 2004).
Combination opioid-nonopioid formulations are frequently administered for acute and persistent low back pain, but more research is needed to support their use. A multicenter, randomized, controlled study of 147 individuals with moderate-to-severe acute low back pain found similar efficacy and tolerability with the combination of hydrocodone (7.5 mg) and ibuprofen (200 mg) and the combination of oxycodone (5 mg) and acetaminophen (325 mg) (Palangio, Morris, Doyle, et al., 2002). The analgesics were administered over a period of 8 days.
Renal Colic
Ureteral obstruction such as from urolithiasis (renal calculi) is usually accompanied by the acute severe flank or abdominal pain of renal colic (Serinken, Karcioglu, Turkcuer, et al., 2008). Prostaglandins mediate ureteral contractility and stretching, which potentiates the pain associated with the obstruction (Jerde, Calamon-Dixon, Bjorling, et al., 2005). This suggests that NSAIDs may be useful in this condition beyond their nonspecific analgesic effects.
A Cochrane Collaboration Review of 20 randomized controlled trials (1613 patients) concluded that both NSAIDs and opioids are effective for pain associated with renal colic (Holdgate, Pollock, 2005). Research comparing pain relief in 130 patients who were given ketorolac or morphine or a combination of the two showed that the combination of ketorolac and morphine produced superior pain relief and a need for less rescue analgesia compared with either drug alone (Safdar, Degutis, Landry, et al., 2006). Experimental research has shown that parenteral ketorolac significantly reduces ureteral contractility, which may account for the effectiveness of this drug on this type of pain (Wen, Coyle, Jerde, et al., 2008). Diclofenac also has been shown to be effective (Yencilek, Aktas, Goktas, et al., 2008), but one study failed to show any morphine-sparing effects with the drug when used for treatment of renal colic pain (Engeler, Ackermann, Osterwalder, et al., 2005). A randomized placebo-controlled trial compared IV acetaminophen (1 g) with IV morphine (0.1 mg/kg) in 146 patients presenting in the emergency department (ED) with renal colic and found that IV acetaminophen and IV morphine produced comparable pain relief with no adverse events in any patients (Bektas, Eken, Karadeniz, et al., 2009).
Biliary Colic
Obstruction to gallbladder drainage is the underlying mechanism for acute cholecystitis (Yusoff, Barkun, Barkun, 2003). Enhanced prostaglandin production is reported to mediate the associated inflammation, and NSAID-induced inhibition of prostaglandins has been shown to reduce this process and the pain that accompanies the condition (Yusoff, Barkun, Barkun, 2003). In the past, meperidine has been the drug of choice for treatment of biliary colic in the ED; however, the drug does not appear to offer any advantages over NSAIDs and may have disadvantages (see Chapter 13). One study showed that ketorolac (30 mg IV) and meperidine (50 mg IV) produced equivalent pain relief for treatment of acute biliary colic in the ED, but ketorolac was better tolerated; those receiving meperidine experienced more nausea and dizziness (Henderson, Swadron, Newton, 2002). Another small study (N = 30) showed similar effective analgesia when meperidine and ketorolac were compared; however, patients in the ketorolac group required less rescue medication (Dula, Anderson, Wood, 2001). A randomized, controlled trial comparing the agonist-antagonist opioid butorphanol (Stadol) and ketorolac reported excellent pain relief with both drugs and more dizziness and sedation with butorphanol and nausea with ketorolac (Olsen, McGrath, Schwarz, et al., 2008). Effective pain relief and a lack of sedation with ketorolac may be important in helping to achieve the goal of short length of stay in the ED.
Pregnancy
The use of nonopioid analgesics during pregnancy has increased and appears to be commonplace today (Alano, Ngougmna, Ostrea, et al., 2001; Freyer, 2008; Werler, Mitchell, Hernandez-Diaz, et al., 2005). A case-control study of 101 newborn infants revealed a high presence of NSAIDs (49.5%) in meconium, particularly aspirin (43.6%), ibuprofen (22.8%), and naproxen (18.8%) (Alano, Ngougmna, Ostrea, et al., 2001). Analysis of data on over 10,500 women from two case-control studies on birth defects revealed that acetaminophen and ibuprofen were among the most commonly used over-the-counter (OTC) medications during pregnancy in the United States, with 65% and 18% of the women saying they used acetaminophen and ibuprofen, respectively (Werler, Mitchell, Hernandez-Diaz, et al., 2005). An increase in the use of naproxen during pregnancy also has been noted (Werler, Mitchell, Hernandez-Diaz, et al., 2005). A Canadian study (N = 36,387) showed that the most common nonopioids used by pregnant women were naproxen (35%), ibuprofen (26%), rofecoxib (15%), diclofenac (9%), and celecoxib (9%) (Ofori, Oraichi, Blais, et al., 2006).
More research is needed to determine the short- and long-term effects of NSAIDs in pregnancy (Andrade, Gurwitz, Davis, et al., 2004; Larsen, Pedersen, 2006; Li, Lui, Odouli, 2003; Werler, Mitchell, Hernandez-Diaz, et al., 2005). At present, the U.S. FDA (2008) lists the nonselective NSAIDs in risk category B (no controlled studies showing adverse effect, or controlled studies in women fail to demonstrate risk) and the COX-2 selective NSAIDs in risk category C (no controlled studies in women) (Temprano, Bandlamudi, Moore, 2005). It should also be noted that the FDA lists misoprostol, a gastroprotective therapy sometimes coadministered with NSAIDs, as a Category X drug, which means research and clinical experience has shown a definite fetal risk that clearly outweighs any possible benefit (Andrade, Gurwitz, Davis, et al., 2004).
Although the lack of data is noteworthy, enough evidence exists to recommend against NSAID use during pregnancy (Temprano, Bandlamudi, Moore, 2005). Not all of the effects of NSAIDs in the fetus are known, but NSAIDs do cross the placental barrier and can have a long half-life in the fetus. Research has shown an association between NSAID use during the first trimester and interference with implantation and increased rates of miscarriage (Li, Liu, Odouli, 2003). Cardiac malformations (Larsen, Pedersen, 2006; Ofori, Oraichi, Blais, et al., 2006) and reduced renal function in the fetus have also been reported (Freyer, 2008). Through meconium analysis, the previously mentioned case-control study of 101 newborn infants confirmed an association between the maternal use of NSAIDs and the development of persistent pulmonary hypertension of the newborn (PPHN) (Alano, Ngougmna, Ostrea, et al., 2001). A case report described renal tubular dysgenesis, a rare and lethal autosomal recessive disorder, in a neonate exposed in utero to naproxen (Koklu, Gurgoze, Akgun, et al., 2006). A study evaluating neonatal morbidity associated with prolonged use of indomethacin during pregnancy in 124 women demonstrated that 6.5% of the neonates developed ductal constriction and 7.3% had oligohydraminos (Savage, Anderson, Simhan, 2007). Composite neonatal morbidity was 29%. Ibuprofen also has been associated with premature ductal closure and oligohydraminos (Freyer, 2008). Aspirin has been associated with premature closure of the ductus arteriosis as well as fetal gastroschisis (James, Brancazio, Price, 2008).
Taken during the last trimester, NSAIDs, and particularly aspirin, even in low doses, can cause maternal and fetal bleeding and increase the risk of placental abruption (James, Brancazio, Price, 2008; Moore, 2008). Through prostaglandin inhibition, NSAIDs can relax uterine contractions and prolong gestation, which is the rationale for the use of indomethacin for premature labor (Temprano, Bandlamudi, Moore, 2005). If an NSAID must be taken for a disease process, such as severe RA, it should be stopped preferably by 32 weeks gestation and no later than 8 weeks prior to delivery (Temprano, Bandlamudi, Moore, 2005).
It has been noted that pregnant women may not realize the potential dangers of NSAIDs, and many do not even know they are taking a nonopioid because the drug is often hidden within a formulation of a medication they are taking for symptoms other than pain relief (e.g., common cold remedies) (Alano, Ngougmna, Ostrea, et al., 2001). This underscores the need for obstetrical care providers to take time during the initial prenatal visit, if not earlier, to discuss the common OTC medications and their ingredients and explain the risks associated with taking them during pregnancy.
Acetaminophen is widely recommended as a safe alternative to NSAIDs during pregnancy (Freyer, 2008; Li, Liu, Odouli, 2003; Rebordosa, Kogevinas, Horvath-Puho, et al., 2008). Analysis of a Danish study of over 88,000 delivered women revealed no association between acetaminophen intake during pregnancy and congenital abnormalities (Rebordosa, Kogevinas, Horvath-Puho, et al., 2008). However, research has shown that children who were exposed in utero to acetaminophen taken by their mothers during middle to late (but not early) pregnancy have an increased risk of wheezing (Persky, Piorkowski, Hernandez, et al., 2008; Shaheen, Newson, Henderson, et al., 2005).
Opioids are recommended as an alternative to nonopioids for moderate to severe pain in the pregnant woman (Freyer, 2008). A large retrospective study (N = 152,531) found that acetaminophen is frequently prescribed with an opioid for relief of moderate pain during pregnancy (Andrade, Gurwitz, Davis, et al., 2004).
Breast-Feeding
The pharmacodynamic characteristics of a drug determine whether and how much of it will be absorbed by an infant during breast-feeding (Wilbeck, Schorn, Dailey, 2008). In general, oral drugs have poor bioavailability because of GI metabolism (“first-pass effect”), which results in less drug reaching the mother’s systemic circulation to be passed on to the infant via breast milk. On the other hand, parenteral administration results in rapid and 100% bioavailability (see Chapter 11 for more on first-pass effect and drug pharmacodynamics). Unlike other NSAIDs, ketorolac has 100% bioavailability by both the oral and parenteral routes of administration (Wilbeck, Schorn, Dailey, 2008). Topical analgesics are poorly absorbed into the plasma, making the dose transferred to the infant almost negligible.
Other factors that determine how much drug is transferred into breast milk include drug dose and dosing regimen, molecular weight, lipid solubility, and protein binding (Wilbeck, Schorn, Daley, 2008). As the dose of the drug increases, the serum concentration and diffusion into the milk compartment increases. For this reason, any drug administered during breast-feeding should be given at the lowest dose possible. Drugs with a long half-life produce a greater risk to the nursing infant of cumulative exposure, and drugs with a short half-life (e.g., acetaminophen, ibuprofen) are preferred. Medications that are not very fat soluble but are highly protein bound and have a high molecular weight (e.g., ibuprofen, ketorolac) also are less likely to transfer into breast milk.
Most NSAIDs are safe for use during lactation, although they have been known to displace bilirubin and increase the risk of jaundice and kernicterus in the newborn (Temprano, Bandlamudi, Moore, 2005). In its policy statement on the transfer of drugs and other chemicals into human milk, the American Academy of Pediatrics (AAP) approved the use of acetaminophen, ibuprofen, indomethacin, ketorolac, naproxen, and piroxicam and recommended caution in the use of aspirin (AAP, 2001). There are no nonopioids listed in the AAP policy statement as drugs that are of concern or that would require cessation of breast-feeding. General consensus is that an appropriate choice of nonopioid is one with a short half-life and inactive metabolites that are rapidly excreted, such as acetaminophen or ibuprofen (Wilbeck, Schorn, Daley, 2008).
In summary, a few basic principles can be applied to nonopioid use during breast-feeding. First, the safest drug should be selected such as acetaminophen or ibuprofen rather than aspirin. A good rule of thumb is that a drug that is safe to give to an infant is likely to be safe to give to a breast-feeding mother (Wilbeck, Schorn, Daley, 2008). Second, mothers can be advised to plan feedings around times when the drug concentration in the breast milk is lowest. Taking the medication immediately before (Wilbeck, Schorn, Daley, 2008) or immediately after (AAP, 2001) breast-feeding is recommended. Avoiding drugs with a long half-life is advised, but if this is not possible, the drug should be taken once daily and just prior to an infant’s lengthy sleep period (Wilbeck, Schorn, Daley, 2008). Finally, as always, the lowest effective nonopioid dose should be taken for the shortest time needed.
NSAIDs and Prevention of Cancer
Epidemiologic research has suggested a possible connection between NSAID use and the prevention or a lower incidence of some types of cancer (National Cancer Institute, 2004; Harris, 2009; Harris, Beebe-Donk, Alshafie, 2007, 2008; Harris, Beebe-Donk, Doss, et al., 2005; Pereg, Lishner, 2005). COX-2 activates signaling pathways that promote cell production and inhibit cell death through its mediation of prostaglandin E2 (PGE2). Though further research is needed to more clearly identify the underlying mechanisms, the inhibition of COX-2, such as by NSAIDs, is thought to prevent activation of this pathway and thereby decrease cell proliferation, reduce formation of vasculature to cancer cells, and alter the immune response (National Cancer Institute, 2004; Pereg, Lishner, 2005). A review of the literature revealed that daily intake of nonselective NSAIDs reduced risk for cancers of the colon (63%), breast (39%), lung (36%), esophagus (73%), stomach (62%), ovary (47%), and prostate (39%) (Harris, Beebe-Donk, Doss, et al., 2005). A more recent comprehensive review of the epidemiologic literature reported that regular intake of OTC nonselective NSAIDs produced significant risk reductions of 43% for colon cancer, 25% for breast cancer, 28% for lung cancer, and 27% for prostate cancer (Harris, 2009). A case control study revealed an association between COX-2 selective NSAID use and risk reductions for cancers of the breast (71%), prostate (55%), colon (70%), and lung (79%) with an overall 68% risk reduction for all 4 cancers (Harris, Beebe-Donk, Alshafie, 2007).
COX-2 is reported to be overexpressed in breast cancer tissues, and the greater the expression, the poorer the prognosis. This led researchers to study the impact of celecoxib on moderately and highly invasive breast cancer cell lines (Basu, Pathangey, Tinder, et al., 2005). In both cell types, celecoxib arrested cell growth and vascular channel formation and reduced vascular endothelial growth factor. A case control study demonstrated a 71% risk reduction for breast cancer with celecoxib and rofecoxib, but no significant reductions with acetaminophen or low-dose aspirin (Harris, Beebe-Donk, Alshafie, 2006). COX-2 selective NSAIDs in combination with standard breast cancer chemotherapy has shown promising results as well, but further research is needed to more clearly identify the role of COX-2 selective NSAIDs in cancer treatment (Pereg, Lishner, 2005).
Retrospective research has shown an association between nonselective NSAID use and reductions in breast cancer, with aspirin (analgesic doses) being the most commonly used NSAID in the research (Pereg, Lishner, 2005). A meta-analysis of six cohort studies and eight case-control studies showed an association between regular use of NSAIDs and a consistently reduced relative risk of breast cancer in the majority of the studies in the analysis (Khuder, Mutgi, 2001). Another meta-analysis (10 observational studies) also concluded that aspirin may reduce breast cancer; more frequent use was associated with lower risk (Mangiapane, Blettner, Schlattmann, 2008).
Studies have shown similar results in patients with lung cancer. A case-control study showed that regular use of NSAIDs over a 2-year period was associated with a 68% reduction in relative risk of lung cancer in heavy smokers (Harris, Beebe-Donk, Schuller, 2002). A similar effect was noted in another case-control study of 1038 patients (Muscat, Chen, Richie, et al., 2003). One-year intake of NSAIDs 3 or more times weekly demonstrated an odds ratio of 0.68 for the development of lung cancer. In contrast, another case-control study (N = 1884) showed that regular use (4 days or more week) of aspirin or other nonselective NSAIDs had no effect on risk of lung cancer (Kelly, Coogan, Strom, et al., 2008).
Numerous observational studies suggested a reduction in the incidence of colorectal cancer and cancer-related deaths with the use of NSAIDs. These findings led to the initiation of case-control studies and randomized controlled trials investigating the association (Bertagnolli, Eagle, Zauber, et al., 2006). The previously discussed Adenoma Prevention with Celecoxib (APC) trial (see Chapter 6) randomized over 2000 patients to receive twice-daily doses of either 200 mg or 400 mg celecoxib or placebo (Bertagnolli, Eagle, Zauber, et al., 2006). The cumulative incidence of adenomas by year 3 was 60.7%, 43.2%, and 37.5% in those receiving placebo, celecoxib 200 mg twice daily, and celecoxib 400 mg twice daily, respectively. The researchers concluded that celecoxib is an effective agent for the prevention of colorectal adenomas but could not recommend its routine use in prevention due to dose-related serious CV events. The Prevention of Colorectal Sporadic Adenomatous Polyps (PreSAP) trial randomized patients (N = 1561) to receive either 400 mg of celecoxib once daily or placebo (Arber, Eagle, Spicak, et al., 2006). Colonoscopy analysis revealed the cumulative rate of adenomas detected through year 3 was 33.6% and 49.3% in those who received celecoxib and placebo, respectively, and the cumulative rate of advanced adenomas through year 3 was 5.3% and 10.4% in those who received celecoxib and placebo, respectively.
A Cochrane Collaboration Review of 9 trials (24,143 patients) concluded that aspirin significantly reduced the recurrence of colorectal adenoma after 1 to 3 years; short-term studies also supported the regression but not elimination or prevention of colorectal adenoma (Asano, McLeod, 2004). A more recent case-control study of 326 patients with colon cancer demonstrated that both nonselective and COX-2 selective NSAIDs produced significant reductions in the risk of colon cancer and a potential for colon cancer prevention; no reduction was noted with acetaminophen or low-dose aspirin (Harris, Beebe-Donk, Alshafie, 2008).
In summary, there is no consensus at this time about the use of NSAIDs to reduce the risk of neoplasm. Concerns about cumulative risk of GI and CV events have prevented the promotion of routine treatment of asymptomatic patients for the purpose of cancer risk reduction, even with the positive findings noted.
NSAIDs and Long-Term Cognitive Function
Ongoing research in older adults has focused on a possible relationship between NSAID consumption and long-term cognitive function (Fotuhi, Zandi, Hayden, et al., 2008; Grodstein, Sharupski, Bienias, et al., 2008; Hayden, Zandi, Khachaturian, et al., 2007; Soininen, West, Robbins, et al., 2007), including a possible reduced risk of Alzheimer’s dementia (ADAPT Research Group, 2008). This relationship may be due in part to the role of COX in the metabolism of arachidonic acid in the brain (Hoozemans, Rozemuller, van Haastert, et al., 2008; Tassoni, Kaur, Weisinger, et al., 2008). Inflammation has also been proposed to have a key role in neurodegeneration and cognitive disorders (Peila, Launer, 2006; Rogers, 2008).
Findings vary, however, and more well-designed research is needed to draw any firm conclusions. For example, although epidemiologic surveys have shown that NSAIDs may protect against the development of Alzheimer’s disease, treatment trials in individuals with existing Alzheimer’s disease have shown little or no effect on slowing or stopping the disease (Hoozemans, Rozemuller, van Haastert, et al., 2008; Rogers, 2008). A large study of veterans aged 55 years and older with Alzheimer’s disease (49,349 cases and 196,850 controls) found that long-term (more than 5 years) NSAID use had a protective effect against the development of Alzheimer’s disease (Vlad, Miller, Kowall, et al., 2008). In contrast, a 12-year follow-up study of 1019 Catholic clergy found no relationship between NSAID use and Alzheimer’s disease or change in cognition (Arvanitakis, Grodstein, Bienias, et al., 2008). Similarly, the ADAPT Research Group (2008) conducted a randomized, double-blind trial in 2117 older men and women with a family history of Alzheimer’s disease to evaluate the effect of celecoxib (200 mg twice daily), naproxen (220 mg twice daily), or placebo on cognitive function. The researchers concluded that neither of the drugs improved cognitive function and that naproxen produced a weak detrimental effect on cognitive function. This led to their suggestion that naproxen and celecoxib not be used for the prevention of Alzheimer’s disease. A Cochrane Collaboration Review concluded that ibuprofen could not be recommended for the treatment of Alzheimer’s disease either (Tabet, Feldman, 2003). Another Cochrane Collaboration Review found no evidence that aspirin improves cognitive function or prognosis in individuals with vascular dementia and suggested that the risk of hemorrhage associated with aspirin intake could worsen patient outcome should it occur (Rands, Orrell, Spector, 2000).
Need for Minimal Antipyretic Effect: Masking Infection
Occasionally, the antipyretic effect of NSAIDs is an undesirable adverse effect because it might mask an infection. If an NSAID is needed, a rapid onset, short half-life nonselective or COX-2 selective drug, or acetaminophen, can be used (Burke, Smythe, FitzGerald, 2006), and a temperature reading may be obtained at the end of the drug’s duration of action. Alternatively, diflunisal (Dolobid) may be a preferred NSAID because it has minimal antipyretic effects (Burke, Smythe, FitzGerald, 2006). NSAID treatment also may be permissible if an infection can be detected by means other than a fever (e.g., visual inspection, increased pain, and elevated white blood cell count). Aspirin should be avoided for treatment of viral-related fever in individuals less than 20 years old due to an association with Reye’s syndrome (Burke, Smythe, FitzGerald, 2006). Two consecutive randomized controlled, open label trials in healthy infants advised against prophylactic administration of acetaminophen prior to vaccinations, stating that although febrile reactions significantly decreased, antibody responses to several vaccine antigens also were reduced (Prymula, Siegrist, Chilbek, et al., 2009).
Conclusion
A key principle of establishing an optimal pain treatment plan is to individualize the selection of analgesics. General considerations in determining the best nonopioid to initiate therapy include the patient’s type of pain, pain intensity, analgesic history, response to current treatments, and adverse effect risk profile, as well as the costs associated with nonopioid analgesic treatment. Doses are titrated according to patient response, and if an initial nonopioid is ineffective, another may be tried. In addition to their usefulness as primary analgesics for a wide variety of acute and persistent pain states, research is ongoing to determine whether or not nonopioids play a role in the prevention of other conditions, such as cancer and cognitive function decline.