Chapter 26
Oral Preparations
Drugs may be administered by a variety of routes but oral administration is adopted wherever possible. It is the safest, easiest and most economical route of drug administration. There are, however, limitations to the use of the oral route. It is obviously unsatisfactory for substances such as insulin and adrenaline which are inactivated or destroyed by the secretions of the gastrointestinal tract. Other substances, although not destroyed, may not be adequately absorbed from the alimentary tract and if required to produce a systematic effect must be administered by other routes. Such drugs may nevertheless be given by mouth to obtain a localized action in the large intestine and bowel. For example, neomycin and the less soluble sulphonamides are administered orally in the treatment of gastrointestinal infections or to reduce the bacterial flora prior to surgery of the bowel.
Another limitation to oral administration is the fact that some drugs irritate the gastric or intestinal mucosa, but this may be minimized by giving the drug after food. Enteric coating may be applied to tablets or capsules to prevent gastric irritation and some drugs, e.g. phenylbutazone and indomethacin, may be administered rectally as an alternative to the oral route.
Solid Preparations
For oral administration there is a choice between solid and liquid medicines. Solid forms of drug presentation include powders, cachets, tablets and capsules.
The most commonly used oral preparations, now a days, are tablets and capsules. Generally speaking, they are more stable and less prone to microbiological contamination than extemporaneously dispensed liquid medicines. However, liquid preparations have advantages for administering drugs to children and patients who have difficulty in swallowing solid preparations.
Powders
Drugs administered in powdered form are immediately available for absorption. However, the absorption of substances which are only poorly soluble may be affected by the particle size of the drug. The clinical aspects of the fineness of particles in pharmaceutical practice were discussed by Lees (1963). He gave a number of examples of drugs, of which the gastrointestinal absorption is affected by particle size, including the corticosteroids, sulphonamides and griseofulvin. The absorbability of the latter drug is directly related to the logarithm of the specific surface. The efficacy of other drugs acting locally in the gut may be enhanced by reduction of particle size. In veterinary medicine, the anthelmintic activity of phenothiazine has been shown to be related to particle size.
Dispensing of Powders
Powders may be ordered to be individually wrapped in single doses or in bulk. Individually wrapped powders are used for potent drugs and wherever it is necessary to ensure accurate measurement of the dose. They may be either simple (containing only one active ingredient) or compound (containing more than one active ingredient). Individually wrapped powders provide a useful method of drug presentation if the required dose is not available as a standard tablet or capsule.
Powders are administered by placing the contents of one powder paper on the tongue and swallowing with a drink of water. For administration to children, powders are sometimes mixed with jam, treacle or honey. If the drug is likely to be adversely affected by acids, the responsible person should be warned against the use of jam.
Simple individually wrapped powders: Powders are wrapped in white glazed paper cut to a suitable size, depending upon the bulk of powder contained in a single dose. Machine or guillotine-cut paper is preferable.
Select a number of papers corresponding to the number of powders ordered and of a suitable size. Turn up one of the longer edges of each paper about half an inch and place the papers in a convenient position on the dispensing bench so that each slightly overlaps the next.
Weigh out the total quantity of powdered drug to be dispensed and replace the bottle. If the drug is caked or lumpy, reduce it to a powder by grinding in a mortar. When six or more powders are to be sent, it is advisable to weigh a total quantity corresponding to one more than is required, because during the weighing there may be slight losses due to powder adhering to the knife or scale pan. Place the total quantity of powder on a large sheet of paper and weigh from this. The latter procedure prevents too many or too few powders being sent and is a check on the weighing.
Wrap the powders so that they are of a size that will fit loosely in the box (a white glazed, slide or hinge-lid box is generally used). Bend the far border over until it is about half an inch from the near border (Fig. 26.1). Turn the margin upwards with the thumb and bend over to form a flap. Fold the flap loosely over on itself to an extent which makes the packet slightly narrower than the interior of the box. Hold the packet over the drawer section of the box and turn the ends down lightly over the ends of the drawer so as to form a slight crease that will act as a guide to the length of the finished powder. Bend each end in turn sharply over a powder knife, holding the knife about 3mm inwards from the creases made by the ends of the box. Finally, place the folded packet on a sheet of white paper, cover it with another sheet of paper and pass a knife over the upper sheet. This last procedure distributes the powder evenly within the packet and prevents the escape of powder through the folds.
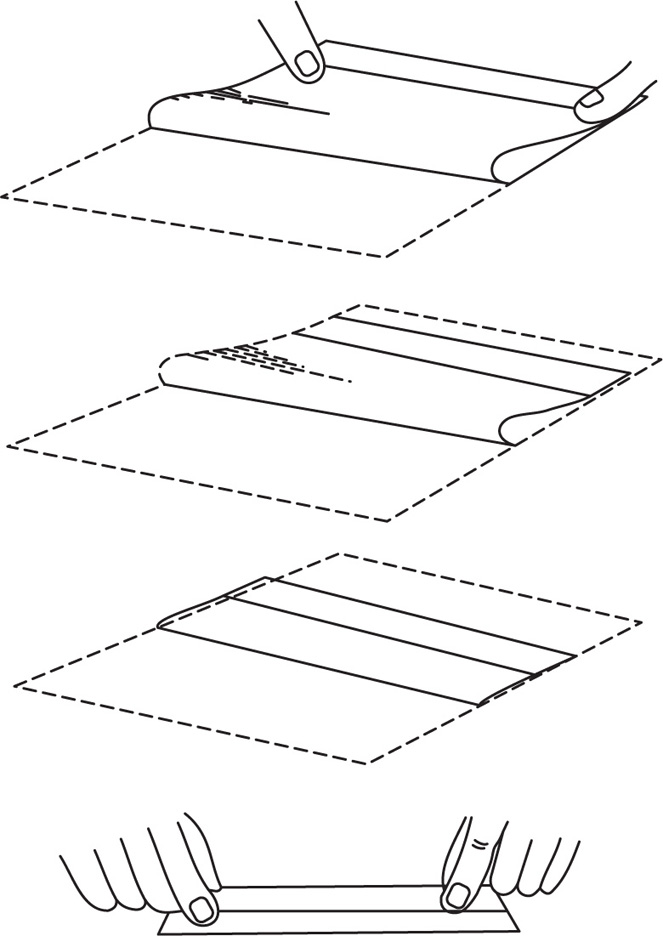
Fig. 26.1 Wrapping a powder.
When all the powders have been wrapped, arrange them in pairs, flaps to flaps and encircle the stack with an elastic band before placing them in the box.
Crystalline substances or substances in large or small lumps must be reduced to a fine powder to facilitate administration and hasten solution after administration.
Potent crystalline substances should be powdered in a glass mortar, preferably placed on a black surface. Wedgewood ware or composition mortars are liable to absorb potent drugs to some extent. Iodine is absorbed by composition mortars and stains them badly.
Hygroscopic substances must be powdered and weighed as rapidly as possible, avoiding undue exposure to moisture.
Powders of hygroscopic substances should be doubly wrapped, the inner wrap consisting of waxed paper to protect the powder from moisture.
Volatile substances must also be doubly wrapped in the same manner.
Triturations: A trituration is the term applied to a mixture or dilution of a potent substance with an inert one. Small quantities of finely powdered solids may be mixed on a sheet of white paper by means of a powder knife or spatula. A mortar should be used when the quantities are too large to be conveniently dealt with on paper. The invariable rule is to add a little of the substance present in greater amount to the whole of the substance present in lesser amount (usually the more potent substance), and mix intimately; the remainder of the substance present in greater amount is then introduced into the mixture in very small quantities at first, but gradually increasing the quantities, until the whole has been added. It is impossible to ensure intimate dispersion of one powder in another by mixing the two substances all at once.
Powders weighing less than 100mg must be made up to 100mg by adding an appropriate quantity of lactose. Consider the following prescription:
Small doses of potent substances must not be weighed on ordinary dispensing scales.
Substances having a maximal dose of less than 60mg should be regarded as potent substances. These should be weighed either on a chemical balance or upon a delicate pair of dispensing scales specially reserved for the purpose. In no case should a quantity less than 50mg be actually weighed. It may happen, of course, that a quantity of potent substance less than 50mg in weight is to be dispensed and the required amount should be obtained by the method described below.
The general procedure is as follows:
Weigh 100mg; gradually incorporate a convenient weighed quantity of lactose with this; then weigh a portion of the mixture that will contain the desired weight of the potent substance.
Examples:
l. 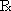 Prednisone 8mg
Prednisone 8mg
 Prednisone 8mg
Prednisone 8mgSend 4 powders
Method: Weigh 100mg of prednisone; mix on paper with 900mg of lactose = 1g total. 320mg of this mixture will contain 32mg of prednisone. Mix this quantity with 80mg of lactose and divide into 4 powders each weighing 100mg.
2. 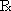 Pentobarbitone sodium 15mg
Pentobarbitone sodium 15mg
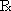 Pentobarbitone sodium 15mg
Pentobarbitone sodium 15mgSend 2 powders
Compound powders in doses: These are powders containing more than one active ingredient. Small quantities may be mixed on paper by means of a spatula or with a pestle and mortar. Any ingredients that are not finely subdivided should be reduced to powder form separately before being mixed.
In mixing compound powders it is essential to follow the general rule of gradually incorporating the ingredient present in greatest amount with the whole of the ingredient present in least amount, any other ingredients then being introduced in turn a little at a time. When using a pestle and mortar for mixing, trituration should be light; heavy trituration or grinding tends to make powders denser and less diffusible in fluids. Only hard, crystalline substances require heavy grinding. Caution should be exercised in handling mixtures of oxidizing and reducing agents in a mortar and pestle as an explosion may occur. If it is necessary to mix such substances, they should be powdered separately and then mixed very lightly on paper.
Sifting. Compound powders containing vegetable substances often require sifting to break up small masses of cohering particles. A no. 44 or no. 60 sieve should be chosen and the mixed powder brushed through or rubbed through with a spatula. Various mixers and sifters are obtainable for small quantities.
After sifting, all powders must again be lightly mixed, as there is sometimes a tendency for the ingredients to be separated by the sieve.
Fractional quantities: In some cases it may happen that after all the ingredients have been mixed an awkward fraction is obtained when the total weight is divided by the number of powders to be sent. This difficulty is easily overcome by adding a small quantity of lactose to bring the weight of each powder to a convenient figure. If the total weight of the ingredients is 3.7g and this is to be divided into 10 powders, it would be advisable to incorporate 0.3g of lactose (= 4g total) and divide into 10×0.4g powders.
Lactose intolerance: It should be noted that there is a rare condition in children which makes them intolerant to lactose. In such cases, an alternative inert diluent must be used (e.g. starch powder).
Powders in bulk: Sometimes, when the dose is not critical, powders may be ordered in bulk. Often powders of this kind are for use as antacids, e.g. Magnesium Trisilicate Powder, Compound BPC. The quantities are usually so large that it is necessary to use a mortar in mixing, even though the ingredients may be already in fine powder. They should be sent out in perfectly dry, wide mouthed, glass bottles with plastic screw caps or other suitable closure. If any of the ingredients are deliquescent or volatile, an airtight container should be used.
Cachets
Powders can be enclosed in small containers made of rice flour and water, known as cachets. They are available in various sizes holding from 0.2 to 1.5g of powder. Such preparations have advantages for the administration of nauseating drugs given in large doses. The cachet prevents the patient from tasting the powder and a larger dose can be enclosed in a cachet than in a tablet or a capsule. Sodium aminosalicylate, which is given in a dose of 12g daily in the treatment of tuberculosis, is commonly prescribed in cachets.
Before administration, a cachet should be immersed in water for a few seconds and then placed on the tongue and swallowed with a draught of water. In this way the outer shell of the cachet is softened, but it retains the enclosed powders long enough for the whole to be swallowed without permitting the powder to come into contact with the palate. After being swallowed, the cachet disintegrates and the powder is liberated.
There are two kinds of cachet; ‘wet seal’ cachets which are sealed by moistening the edges with water, and ‘dry seal’ cachets which, as the name implies, require no moisture for sealing.
Wet Seal Cachets
A wet seal cachet is composed of two similar concave halves, having fiat edges (see Fig. 26.2). The weighed powder is deposited into one half, the edges of the other half are moistened with water, inverted over the first half and the flat edges pressed together so that they adhere and enclose the powder.
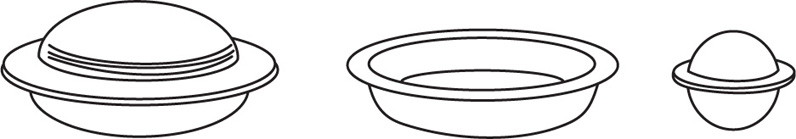
Fig. 26.2 Cachets.
Wet Seal Cachet Machine
This consists of three metal plates (Fig. 26.3), the two outermost plates being attached by hinges to the centre one. The halves of the cachets in which the powder is to be inserted are placed in holes in the centre plate, A, where they fit loosely. One of the other plates, B, is then swung over and placed on top of the cachets. This second plate also has holes, which are congruent with the holes in the first plate, and its object is to protect the edges of the cachets while they are being filled. A funnel, D, of the correct size, supplied with the machine, is next placed in each hole of the second plate in turn, and the powder introduced. The powder is pressed down with a small wooden plunger or metal thimble, E, also supplied with the machine.
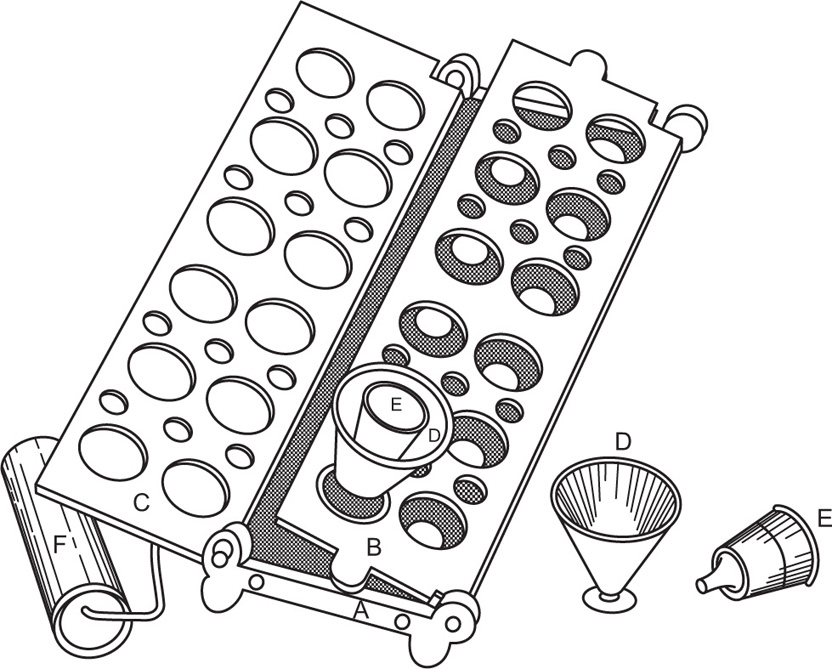
Fig. 26.3 Cachet machine.
When the lower halves have been filled, the second plate is folded back. The top halves of the cachets are then placed in the holes of the third late, C, on the opposite side of the centre plate. The holes in C are somewhat smaller than those of the centre plate, so that the tops of the cachets fit tightly. The edges of the top halves are then moistened with a roller, F, and the plate is swung over and pressed down firmly on the centre plate. The top halves of the cachets are thus brought exactly over the lower halves and the edges made to adhere. The upper plate is now lifted when it brings with it the completed cachets. The finished cachets are finally pushed out gently and boxed.
The amount of moisture used is very important and somewhat difficult to judge correctly too much causes the edges to crinkle and become discoloured; too little may produce an imperfect seal and allow the powder to escape at some point on the circumference, or cause the two halves to fall apart completely after the patient has received the cachets.
Dry Seal Cachets
Dry seal cachets consist of two halves, the upper half fitting over the lower half like the lid on a box (Fig. 26.4). The backs of both lower and upper halves have small projections which fit into the holes of the plates of the machine. Since the projections are the same size on all the sizes of cachets, only one machine is required for the whole range of sizes. The lower halves are fitted into the lower plate (Fig. 26.5) of the machine and filled. The upper halves are fitted into the upper plate, D, which is pressed down on the lower one, when the upper halves or ‘lids’ fit exactly over the lower halves. These cachets are much more substantial than the ordinary type, and there is no likelihood of the powder escaping. They can be prepared more quickly and are more hygienic.
Fig. 26.4 Dry-closing (Secca) cachets.
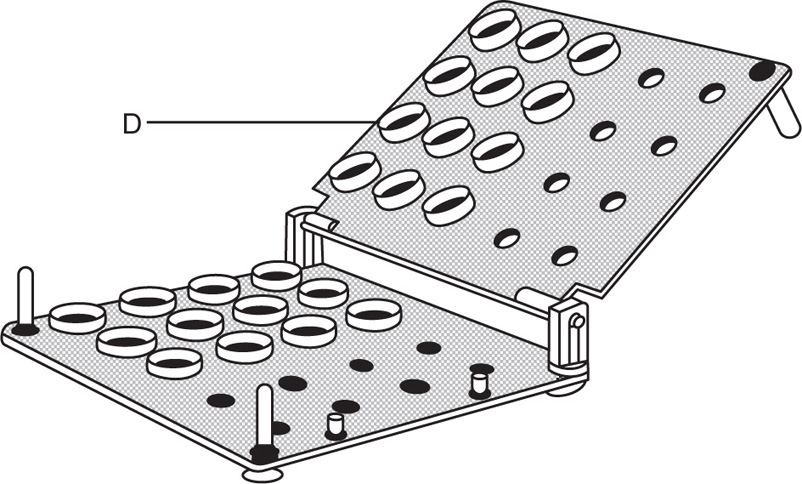
Fig. 26.5 Dry-closing machine (cacheteur Secca).
Dispensing Cachets
Cachets are dispensed in boxes or tins in which they are packed on their edges or lying flat. Some manufactured cachets are packed in plastic sleeves, each holding a day’s treatment. Cachets should be labelled with directions for administration, e.g. ‘Immerse in water for a few seconds and then swallow with water’.
Automatic and Semiautomatic Cachet Machines
The widespread use of cachets for the administration of sodium aminosalicylate has stimulated the industrial production of cachets in this country. Both dry and wet seal cachets may be filled and sealed by automatic (machine-fed) or semiautomatic (hand-fed) machines. Fig. 26.6 shows an automatic machine in which the adjustable four-punch dosing mechanism delivers a plug of compressed powder to the lower half of the cachet and to this the upper half (automatically moistened if wet sealed) is applied. To achieve consistent dosage from such machines it is essential that the bulk density, particle size and flow characteristics of the powder or blend of powders is accurately controlled.

Fig. 26.6 Automatic cachet machine (Smith and Nephew Ltd.).
Capsules
Capsules consist of a medicament enclosed in a shell of gelatin; methylcellulose has been used but is no longer recognized by the British Pharmacopoeia as a shell material. The shell is soluble in water at 37° and after the capsule has been swallowed, it dissolves and releases the medicament allowing it to be absorbed. However, the mucilaginous coating which forms around the drug as the capsule dissolves may delay absorption, so that its onset of action may be delayed. With very soluble drugs this may not be an important factor, but is more important when the drug is only very sparingly soluble.
The manufacture of hard gelatin and flexible gelatin capsules is described in Chapter 21. Small quantities of hard gelatin capsules may be extemporaneously dispensed by hand-filling.
Hard Capsules
Hard gelatin capsules are available in a range of sizes to hold varying doses of powdered drugs. They are formed of two cylindrical halves, one slightly larger but shorter in length than the other. The medicament is inserted in the longer narrower half, then the other half is fitted over the open end as a cap, held in place by moistening the edges.
Hand filling hard capsules: The smallest size capsule should be selected that will hold the quantity of medicament prescribed.
The lower halves of the capsules may be supported in the holes of a suppository mould or in a block of wood in which holes of a suitable size have been bored. The powders are prepared in the usual way and are poured into each capsule. This operation is facilitated by the use of a small glass or aluminium funnel and a thin wooden plunger that will pass down the stem of the funnel and prevent it from becoming clogged with powder. The outer edges of the lower halves of the capsules are then moistened with water, using a camel hair brush, and finally the caps are pressed on. This forms a seal which prevents the powder from leaking out of the capsules.
Enteric Coated Capsules
Enteric coating is applied to gelatin capsules when they are intended to pass through the stomach and release their contents in the small intestine. A 10-per cent solution of cellacephate (cellulose acetate phthalate) in acetone may be used for this purpose. The capsules are dipped in the solution, removed by means of tweezers and allowed to dry on a sieve. It is usually necessary to apply three coats.
Small scale manufacture of capsules: In hospital practice a hand-operated capsule filling machine may be found useful for the preparation of capsules, particularly special formulations required in the clinical trial of new drugs. The Tevopharm machine (see Fig. 26.7) is suitable for such purposes, and is essentially a device for locating the capsules into two plates which can be brought together to effect closure. Plates are available to accommodate the different sizes of empty capsules and each plate holds 60 capsules. A sorting device is used to drop the capsules, bottom downwards into the holes in the filling plates. The capsules are pushed firmly into the holes and the lower plate is then fixed by the screw on the front. The upper plate is removed, taking with it the tops of the capsules. The lower halves of the capsules are then pushed flush with the lower plate and are gravity filled with the powdered drug. If the dose required cannot be obtained by filling one of the standard capsule sizes a triturate in lactose must be prepared. After filling the capsules the top plate is replaced and the lever at the side of the machine operated to engage the rods under the base plate, pushing the bottoms of the capsules upwards at the same time as pressure is applied at the top to force the two halves of the capsules together. The bottom plate is again fixed and the top plate is removed bringing away the filled capsules.

Fig. 26.7 Tevopharm capsule filling machine (Anglo-Continental Machines Ltd.)
Liquid Preparations
Drugs administered in aqueous solution or suspension are presented to the gastrointestinal mucosa in a form that is immediately available for absorption. Liquid medicines are, however, generally less stable than solid forms of drug presentation, although, nonaqueous vehicles may be used for drugs which deteriorate in the presence of moisture. Nauseous drugs and those which are irritant to the gastric mucosa are usually unsuitable for presentation in liquid formulations unless well diluted.
Mixtures
Mixtures are liquid medicines which the patient divides into doses; he/she may or may not be directed to dilute each dose before taking. A draught is a liquid medicine consisting of one dose only. A linctus is a preparation intended for the treatment of a cough and is rendered viscous by the inclusion of glycerin or syrup; it should be administered undiluted in order to obtain the lubricant effect of the vehicle.
The British Pharmaceutical Codex 1973 gives the following metric dose volumes for liquid preparations: for linctuses and elixirs, 5ml: for adult mixtures, 10ml, or multiples thereof; and for draughts, 50ml.
Metric Medicine Bottles
Mixtures are dispensed in clear glass bottles which comply with British Standard No. 1679/6. The following range of sizes of metric bottles are available: 50, 100, 150, 200, 300 and 500ml.
If the mixture consists of a simple solution in water, it can be adjusted to volume in a measure before being transferred to the bottle. If the mixture contains an insoluble powder or is viscous, the bottle should be calibrated to the correct volume and the final adjustment to volume made in the bottle. This procedure enables the whole of the insoluble powder or a viscous liquid to he transferred to the final container.
Labelling
The dose should be stated in terms of the metric medicine spoon (B.S.3221/4). This has a capacity of 5ml and in the case of medicines having a 5ml dose-volume the label should read, one 5-ml spoonful to be taken...’. In the case of medicines having larger dose volumes, the dose should be stated in units of the 5ml medicine spoon, e.g. for a 10ml dose volume, the label should read, ‘Two 5-ml spoonfuls’.
For the medicines that are still prescribed in the apothecary system of weights and measures, the prescription must be transcribed into the metric system (see below) and the doses stated in terms of the metric medicine spoon. The use of domestic teaspoons and tablespoons should he abandoned for measuring medicines.
A plastic medicine spoon should be supplied with each bottle of mixture dispensed.
Mixtures containing an insoluble deposit should always be labelled with the direction, ‘Shake well before use’.
The British Pharmaceutical Codex 1973 gives the following directions for labelling mixtures issued on a medical prescription or for an individual patient:
The requirement to state a date after which the mixture should not be used applies to mixtures containing unstable ingredients. For example, elixir of phenoxymethylpenicillin prepared from granules which have been ready made by an industrial manufacturer. The granules are stable if stored in a cool place, but once the elixir has been prepared by the addition of water, it must be stored in a cool place and used within 7 days.
Tap water is bacteriologically controlled and is free from pathogenic organisms. Purified water, on storage, can become heavily contaminated with microorganisms. Purified water prepared in a deionizer is particularly liable to be contaminated if the water is allowed to stagnate in the resin bed. Purified water, prepared by distillation, should not be stored in large volume tanks or containers which permit it to become contaminated. Polythene tubes and containers may become contaminated and form a reservoir of infection which will proliferate and build up a heavy infection in stored water.
Medicinal Waters
Medicinal waters such as peppermint water and cinnamon water are used as flavouring agents and carminatives. Chloroform water is used for its sweet taste and also possesses excellent preservative properties (see Chapter 40).
Infusions
Fresh infusions are no longer used. They are now prepared by diluting concentrated infusions which are 10 times the strength of the original infusions (see Chapter 13).
Suspending Agents
Many insoluble substances, when shaken up, will remain suspended sufficiently long for a dose to be measured without the use of a suspending agent, but others will not and these require the use of a suspending agent. When no suspending agent is ordered by the prescriber, the pharmacist must use his judgement as to whether it is necessary to include one.
The following are the most commonly used suspending agents:
Compound Tragacanth Powder, BP: This is a mixture of powdered tragacanth, powdered acacia, starch and sucrose. It is used in quantities of 25 to 200mg for each 10ml of mixture. It is only in exceptional cases that more than 100mg for each 10ml is required.
The powder is mixed in a mortar with the substance to be suspended, and the vehicle added gradually with trituration.
Tragacanth Mucilage, BPC: This is employed in quantities up to 1ml for each 10ml of mixture, depending on the weight of insoluble matter to be suspended.
Sodium Carboxymethylcellulose BPC: This is available in various viscosity grades, depending upon the degree of polymerization. A medium viscosity grade is preferable for suspending powders in mixtures and is used in a concentration of 0.25–1 per cent. It is incorporated in the same manner as compound tragacanth powder.
Powdered tragacanth: This is less satisfactory as a suspending agent since it is liable to coagulate into unmanageable lumps unless it is mixed very intimately with an insoluble powder before addition of the vehicle. If it is prescribed in combination with an alcoholic liquid, tragacanth can be made into a mucilage by adding it to the alcoholic liquid in a dry bottle adding a large quantity of the aqueous vehicle all at once and shaking vigorously.
Liquids in Mixtures
Measurement of volume: The errors involved in measuring volumes of liquids are discussed by Capper and Dare in their report, Dispensing Tolerances in Liquid Medicines (1957). Government stamped metric measures complying with British Standard 1922: 1953 should be used for dispensing purposes.
Measures are graduated to contain and not to deliver. Consequently after the volume of a liquid has been measured, the measure should be rinsed out with some of the vehicle and the rinsings added to the mixture before adjusting to volume. To measure volume correctly the bottom of the meniscus should be in line with the graduation on the measure and this should be aligned with the sighting mark on the back of the measure.
If a viscous liquid is to be incorporated in a mixture, it should be measured, and then diluted in the measure with some of the vehicle, and mixed with a glass rod before transferring it from the measure. The measure should be finally rinsed with some more of the vehicle. However, glycerin or syrup if combined with an insoluble substance should not be too much diluted in the measure; if added to the powder in a mortar, they assist in producing a paste free from lumps and this can then be diluted with the vehicle to produce a uniform suspension.
Volatile liquids, such as compound spirit of orange, should always be added last to avoid loss of the volatile ingredients.
Soluble Solids in Mixtures
If there is sufficient water present to dissolve the substance completely, a solution can be prepared in a measure with the aid of a stirring rod. The solution should be filtered through sintered glass and the filter washed through with more of the water before making up to volume.
Large crystals such as those of ferrous sulphate should be powdered and dissolved in a mortar. Scale preparations, such as iron and ammonium citrate, dissolve more readily if they are added to water in a measure, small portions at a time with constant stirring.
Stock solutions: For convenience in dispensing, solutions of stable soluble salts may be kept ready prepared. However, solutions of some salts may become heavily contaminated with bacteria and fungi if stored for more than a few days.
The use of heat: Some substances dissolve only slowly and the rate of solution may be increased by the use of hot water. Care must be taken to ensure that there is sufficient water present in the mixture to keep the substance permanently in solution; and solution should be allowed to cool to room temperature before the final adjustment to volume. If the substance is present in excess of its solubility, the excess will crystallize out when the solution cools and probably form a mass of crystals at the bottom of the bottle. The method for dealing with substances present in excess of their solubility is described below.
Hot water should not be used for substances decomposed at only moderate temperatures, e.g. bicarbonates. It should also not be used if volatile ingredients are present.
Substances present in excess of their solubility: The substance is powdered as finely as possible in a mortar and triturated with some of the cold vehicle. The suspension is then transferred to a previously calibrated bottle and the mortar rinsed with further quantities of the cold vehicle, the rinsings being added to the contents of the bottle. The mixture is labelled ‘Shake well before use’.
Small doses of potent substances: The smallest quantity which can be weighed on a dispensing balance is 50mg and it may sometimes be necessary to make a liquid triturate in order to obtain the required quantity.
Hyoscine hydrobromide is freely soluble in water. The required quantity is 0.5×20 = 10mg. Weigh 50mg; dissolve in 100ml of chloroform water. Take 20ml of this solution and dilute with chloroform water to 100ml.
Insoluble Solids
Diffusible insoluble substances: The general procedure is to reduce the substance to a fine powder in a mortar. The powder is then triturated to a smooth cream with a small amount of the vehicle and this is then diluted by gradually adding about two-thirds of the vehicle required. The suspension is then transferred to a measure or calibrated bottle and the mixture is made up to volume with the remainder of the vehicle, washing out the mortar with successive small portions and adding to the bulk of the preparation. If syrup or glycerin is present in the mixture, it is diluted with a small portion of the vehicle and used to form the primary smooth cream.
This method can be applied to mixtures containing vegetable powders and diffusible solid drugs such as magnesium carbonate, magnesium trisilicate, light kaolin, etc.
Soluble substances with insoluble solids: The soluble substance can sometimes be mixed with the insoluble powder in a mortar before adding the vehicle. However it is usually preferable to dissolve the soluble ingredient separately and use the solution to triturate the insoluble powder in a mortar.
Indiffusible solids: Substances which do not remain in suspension sufficiently long for a dose to be measured accurately must be suspended by means of a suspending agent (see p. 666). Substances which require the use of a suspending agent include the sulphonamides (e.g. sulphadimidine and succinylsulphathiazole), aspirin and phenacetin. Corticosteroids such as hydrocortisone acetate may sometimes be prescribed as suspensions for administration to children but it is preferable for potent substances such as these to be supplied where possible in tablet form, with instructions that they be crushed and mixed with honey or syrup before administration.
The general procedure for indiffusible solids is to reduce the substance to a fine powder in a mortar, triturate with the suspending agent (usually compound tragacanth powder or sodium carboxymethylcellulose) and form a smooth cream with a small portion of the vehicle. The remainder of the vehicle is then added gradually.
Dispensing in the Metric System
As noted above, the liquid medicines in the British Pharmaceutical Codex and British National Formulary are formulated to a dose of 5ml or a multiple thereof. When a fractional dose is prescribed, the pharmacist is directed to dilute the medicine with the appropriate vehicle so that the patient is able to measure a full medicine spoonful. Consider the following prescription:
Send 100ml and label, ‘One 5-ml spoonful to be taken four times daily’.
The appropriate diluents for use in each case are stated in the monographs of the British Pharmaceutical Codex. Care should be taken to note those preparations which must not be diluted (e.g. Nystatin Mixture). Diluted preparations have only a limited stability and must not be used more than 14 days after the date of preparation. The above mixture therefore should also be labelled ‘Do not use after (date)’.
Interpretation of Prescriptions Written in Apothecary System
In order to effect the changeover to dispensing in the metric system, the Weights and Measures (Equivalents for Dealing with Drugs) Regulations, 1968 include six tables specifying how to convert a prescription from the apothecary system into the metric system. A useful series of articles dealing with the changeover to metric dispensing appeared in the Pharmaceutical Journal, February 1969, and a review of the problems involved was given by Thornton Jones (1969).
Emulsions
The theory of emulsions has been described in Chapter 20. In physical chemistry an emulsion is described as a system in which one immiscible liquid is dispersed in another, but in pharmacy the term is restricted to oil-in-water preparations for internal administration. An emulsion is a particularly convenient form in which to administer oily substances such as liquid paraffin and fixed oils. The oil is dispersed in a continuous surrounding aqueous medium in which flavouring agents may be incorporated to mask the nauseous properties of the oil and improve the palatability of the preparation. This chapter deals with the extemporaneous preparation of small volumes of emulsions. Equipment for the large scale production of emulsions is described in Chapter 20.
Small quantities of emulsions may be prepared either in a small emulsifying machine or by the mortar and pestle method. Many machines advertized for cream making can be used for the extemporaneous preparation of emulsions and the results are superior to those produced by the mortar and pestle method. The use of a machine enables a wider range of emulgents to be employed and smaller proportions of emulgents are required.
Hand Emulsifying Machines
The oil water and mucilage are shaken together to form a coarse emulsion which is poured into the bowl of the machine and pumped by a lever movement of the handle through a fine orifice at high pressure. This gives the shearing effect which produces a fine emulsion.
Mortar and Pestle Method
The most commonly used emulgent for preparing oil-in-water emulsions in powdered acacia, which can be used for fixed oils, volatile oils, oleo-resins and liquid paraffin. Emulsions may be prepared by hand in a mortar and pestle either by a dry gum method or a wet gum method.
The Dry Gum Method
A primary emulsion is prepared by mixing the oil, water and gum in the proportions indicated below. When the primary emulsion has been formed it may be diluted with more of the external aqueous phase.
To obtain a good primary emulsion, oil, water and gum must be mixed in the following proportions, depending on the nature of the oil:
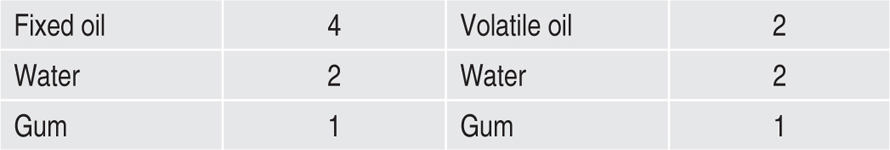
It will be observed that the proportion of gum is twice as great in an emulsion of a volatile oil as in one containing a fixed oil. The ratio of water to gum in the primary emulsion is always 2:1.
In preparing an emulsion by hand, an ample sized mortar should be selected with a corresponding pestle with a broad, flat head to provide the maximum amount of shear in use.
Method: Place in the mortar 12.5g of powdered acacia; measure 50ml of olive oil and pour it on to the gum in the mortar, allowing time for the measure to drain. Triturate the oil and gum together to obtain even dispersion of the gum (prolonged trituration is undesirable since it will result in the particles of gum becoming surrounded by oil and they will not readily be hydrated by water). Using a clean measure, add 25ml of chloroform water, all at once, and triturate vigorously, working in one direction only and using a whipping action.
As the primary emulsion forms, the preparation changes to a white homogeneous paste and the motion of the pestle produces a crackling sound. The trituration should be continued until a stable primary emulsion has formed. The remaining chloroform water should then be added, in small quantities at first but in increasing quantities as the preparation becomes dilute. Finally, transfer the emulsion to a measure, rinse the mortar with more chloroform water and adjust to volume.
A simple emulsion such as this should be white in appearance and when a few drops are diluted in a large volume of water there should be no visible oil globules on the surface. Emulsions should not cream for several hours and the oily layer should be easily diffused again by shaking.
Failure to obtain a satisfactory preparation may be due to one or more of the following causes: (1) the use of a mortar which is too small or which is too conical; (2) the use of a narrow-headed pestle which fails to shear the oil into globules; (3) measuring the oil in a wet measure; (4) measuring the water in an oily measure; (5) using an old sample of gum, which has acquired an acid reaction; (6) bad manipulation; (7) using incorrect proportions of oil, water and gum; and (8) diluting the primary emulsion before it is completely formed.
The Wet Gum Method
The proportions of oil water and gum used in the wet gum method are the same as those used for the dry gum method. Only the procedure is different.
Method: Weigh 12.5 of powdered acacia and transfer to a suitable sized mortar. Add 25ml of chloroform water and triturate until the gum has dissolved to form a mucilage. Add the cottonseed oil, drop by drop with constant trituration using a whipping motion. As the oil is added, the liquid in the mortar may acquire a ropy appearance and the oil may not be readily absorbed. When this occurs, add a few drops of chloroform water and triturate the mixture until it becomes homogeneous. Continue to add the oil as long as it is taken up homogeneously, adding a few drops of chloroform water if the preparation shows any tendency to become ropy.
When all the oil has been added, continue triturating until a stable primary emulsion has been obtained. Finally, dilute the primary emulsion with chloroform water, transfer to a measure, rinsing the mortar with further quantities of chloroform water, and adjust to volume.
The wet gum method is more time consuming than the dry gum method. The mucilage should be freshly prepared, since on storage it may become acid in reaction, lose viscosity and render emulsification impossible.
Emulsifying Agents
Powdered Tragacanth
This is inferior to acacia as an emulgent and its emulsifying properties depend mainly upon the viscosity it imparts to the continuous phase. Tragacanth emulsions are coarser than acacia emulsions and do not have their white colour. The main use of tragacanth in emulsions is as an ancillary emulgent in association with acacia. A mixture of tragacanth, 1 part, with acacia, 15 parts, may be used in place of powdered acacia in the extemporaneous preparation of emulsions.
Methylcellulose, BPC
This is a methyl ether of cellulose and is available in various viscosity grades which are denoted by a number following the name. The lower viscosity grades are used as emulsifying agents, methylcellulose 20 being the most commonly used member of the series for this purpose. The suffix ‘20’ indicates the approximate viscosity in centistokes of a 2.0 per cent w/v solution in water at 20°C.
A mucilage of methylcellulose may be prepared by adding the solid to about one-third the required amount of boiling water stirring constantly and adding the remainder of the water, preferably in the form of ice, when the powder is thoroughly hydrated. The mucilage can be used to make emulsions extemporaneously by the wet gum method but better results are obtained if a mechanical stirrer is used.
Methylcellulose 20 is used as the emulgent in Liquid Paraffin Emulsion, BPC.
Agar
This was formerly used extensively (in association with acacia and tragacanth) for the emulsification of liquid paraffin. It was included for its supposed therapeutic action but was present in too small a quantity to be effective. The British National Formulary allows Liquid Paraffin Emulsion to be supplied when Liquid Paraffin and Agar Emulsion is prescribed.
Casein
This is the protein which emulsifies fat in milk. Soluble casein or similar products obtained from milk protein may be used as emulgents in the proportion of 12g of casein for 100ml of fixed oil. The oil is added to the casein in a mortar and triturated to form a paste and the water is then added gradually with continuous trituration. Emulsions containing casein are particularly liable to microbiological contamination and a preservative should be included. Chloroform, 1:500, may be used for this purpose.
Substances Requiring Special Treatment
Male Fern Extract
Add it to its own weight of acacia and triturate thoroughly; then add twice the same amount of water with constant trituration. Take care that the extract does not come into contact with water until it is intimately mixed with gum. Otherwise, the emulsion will contain unsightly black particles.
Paraldehyde
This is now usually given by intramuscular injection but may occasionally be administered orally as a draught. One gram of paraldehyde dissolves in 9ml of water and it need only be emulsified if present in excess of its solubility. An emulsion can be prepared by adding the paraldehyde to compound powder of tragacanth (100mg for each 25ml of paraldehyde) in a dry bottle, shaking gently to disperse the powder and then adding twice the volume of water and shaking vigorously. The remainder of the water can then be added gradually, shaking after each addition.
References
Lees KA. Fine particles in pharmaceutical.. J. Pharm. Pharmacol. 1963;15:43T.
Thornton Jones AD. Changeover to the metric system.. Pharm. J. 1969;202:221.