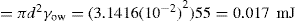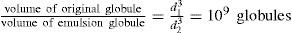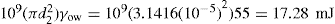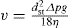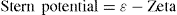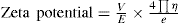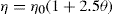Biphasic preparations involving a dispersed phase and a dispersion medium are necessitated because many drugs, solid and liquid, are either insoluble in or immiscible with water, which is almost always a vehicle. They probably present the biggest problem to dosage form formulation of the modern age because the present-day parameters required to assess their stability, acceptability, elegance and efficacy are much more rigid and involved than what they were in the past. The preparation and presentation of such drugs in forms in which they can be used or taken calls for newer understanding and manipulated skill on the part of industrial pharmacist because the application of physicochemical principles to facts relating to properties of biphasic systems have brought to the fore many new facts and ideas. Generally, two-phase liquid dosage forms include emulsion and suspension.
Emulsions
An emulsion has been defined by Becher (1965) as a heterogeneous system, consisting of at least one immiscible liquid dispersed in another in the form of droplets whose diameters, in general, exceed 0.1μm. Such systems possess a minimal stability, which may be accentuated by such additives as surface-active agents, finely-divided solids, etc. The liquid droplets, generally known as the emulsion globules, form the disperse phase (or internal phase) while the liquid in which they are dispersed is known as the continuous phase (or external phase). If oil globules are dispersed in water, the system is called an oil-in-water (o/w) emulsion; conversely, the dispersion of water in oil produces water-in-oil (w/o) emulsion. The ratio of internal phase volume to the total volume is known as the phase volume or the phase volume ratio.
It is not necessary to have an oil or indeed water to form an emulsion. When a coacervate (
Kruyt, 1949) first separates it does so in the form of colloid-rich globules. Conversely, both phases may be nonaqueous. Occasionally, a
multiple emulsion can be formed as when minute oil globules are dispersed in the water globules of a w/o emulsion: this might then be described as an o/w/o emulsion.
Globule sizes can vary enormously but are generally in the range 0.25–25μm diameter. Emulsions with predominantly large globules are sometimes referred to as coarse emulsions while those with globules of mean diameter below about 5μm are considered to be fine emulsions. Even finer emulsions can sometimes be formed with globule diameters as small as 10nm; these are known as microemulsions. Unlike ordinary emulsions which are generally ‘milky’, microemulsions may be transparent having globules only a little larger than micelles.
Except with some microemulsions, emulsification is generally not spontaneous and certainly not in the thermodynamic sense, and energy must be supplied in order to increase the interfacial area between the phases. Considering a 1cm diameter globule of liquid paraffin in water with an interfacial energy (γow) of 55mJ m–2, the interfacial energy is:
Interfacial area × energy per unit area
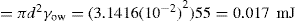
If this globule is broken into globules of 10μm diameter then:
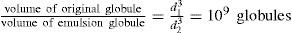
and the interfacial energy is then:
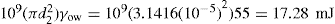
requiring an energy input of 17.28–0.017 = 17.26mJ. Reduction of globule size to 1μm diameter would have required an energy input of 172.8mJ or almost 330mJ for each ml of liquid paraffin emulsified. Much less expenditure of energy is required if the interfacial energy is decreased by the addition of surface-active agents to, say, 1mJ m–2 when only 330/55 = 6mJ would be needed for each ml of oil in order to make a fine emulsion (assuming, highly unrealistically, 100 per cent efficiency of the mechanical process!).
The mechanical disruption of the disperse phase may be achieved by shaking or stirring if
γow is very small, otherwise some kind of machine is required. Nonvigorous agitation generally leads to polydisperse globules with a wide range of sizes, so that the emulsion frequently needs further and more vigorous treatment in a homogenizer. Homogenization can be achieved by forcing the crude emulsion through a narrow orifice under high pressure, which produces extremely large shearing forces causing the globules to be strung out into high speed liquid jets which are ruptured into minute globules by the action of the interfacial tension on the perturbations. Colloid mills are also used. Alternatively, a fine emulsion can be achieved by means of an ultrasonic dispersator. Propellor mixers and shrouded turbines are favourite instruments on a small scale while the pestle and mortar may suffice for extemporaneous dispensing. Some emulsifying equipment is described at the end of this chapter.
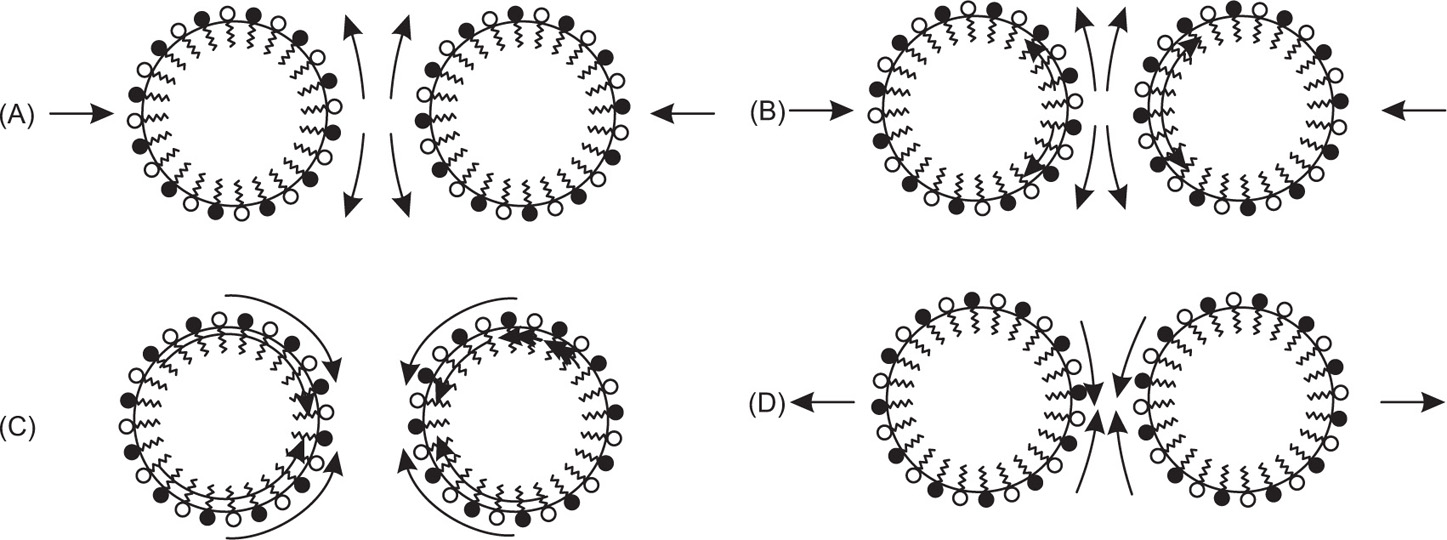
Since there is a positive interfacial tension between the internal and external phases, emulsions are thermodynamically unstable. Collision of unprotected globules will very probably result in their coalescence, thereby producing a rapid coarsening of the emulsion. This process is known as breaking or cracking. It follows that some barrier to coalescence is needed and this is achieved by the use of emulsifying agents otherwise known as emulgents.
Emulsifying Agents
There are basically three classes of these:
1. amphipaths such as soaps, long-chain amines and alcohols,
2. hydrophilic colloids such as acacia, sodium alginate, gelatin and methylcellulose and
3. finely-divided solids such as magnesium and aluminium hydroxide and bentonite.
All are surface-active in that they tend to congregate at an oil–water interface, but a marked lowering of
γow is found mainly with amphipaths whereas hydrophilic colloids generally produce only a moderate lowering. The mechanism of adsorption of the latter has been discussed in
Chapter 4 while that of fine particles is dealt with here.
The surface-activity and emulsifying power of single oil and water-soluble surfactants and blends of these have been classified semiempirically by
Griffin (1949,
1954) in terms of the
hydrophile–lipophile balance (HLB). This has been discussed by Becher (1965) and by
Sherman (1968). HLB numbers may be determined experimentally or calculated in a manner similar to the parachor (see
Chapter 5), and afford a means of blending surfactants so as to achieve a desired type of emulsion.
Oil soluble amphipaths have low HLB values whereas water-soluble ones have high values. Thus sorbitan mono-oleate has a value of 43 while polyoxyethylene sorbitan mono-oleate has a value between 10 and 15 depending upon the length of the oxyethylene chain. Blending of these amphipaths gives an HLB of intermediate value. For an o/w emulsion, an HLB value within the approximate range 8–18 is required, while within the range 3–6 a w/o emulsion would be produced. The HLB value of an individual surfactant is correlated with its dielectric constant and, for o/w emulsions, the more polar the oil, the more polar must be the emulgent. This means that the more polar oils need emulgent blends of higher HLB value. In connection with this, it is worth noting that the spreading coefficients of an oil on aqueous solutions of various surfactants, or of water on solutions in oil, have been correlated with the HLB value.
Preparation and Properties of Emulsions
In the labelling of medicines the term ‘emulsion’ is generally restricted to o/w emulsions intended for oral use, but it does in fact cover all aspects of the dispersion of one immiscible liquid in another and so includes creams and some lotions, liniments and applications.
o/w Emulsions Containing Amphipaths
Reference was made in
Chapter 5 to the work of Schulman and Cockbain who demonstrated that molecular association between an oil-soluble and a water-soluble surfactant was necessary for good emulsion stability and that stability of the film is a prerequisite for emulsion stability. These workers concluded that this stability was conferred by a charged liquid-condensed monolayer at the oil globule–water interface. Since strength and compactness of the interfacial film seemed important, ‘complex’ formation was considered necessary (
Chapter 5). This condition is achieved using mixtures of the anionic surfactant sodium lauryl sulphate with cetostearyl alcohol (as in aqueous cream) or of cationic cetrimide with cetostearyl alcohol (as in cetrimide cream). Since the Helmholtz double layer thickness is less than 10
nm in water, oil globules carrying an appreciable ξ-potential have an appreciable potential energy barrier to surmount before they can approach close enough to coalesce (see
Chapter 6). It is possible however for these globules to flocculate in the secondary minimum and this is commonly observed especially in concentrated emulsions such as many creams. Stabilization due to the electrical charge is supplemented by the Marangoni–Gibbs effect since close approach of two potentially colliding globules causes deformation of the opposing faces due to the resistance to ejection of the intervening medium. The resulting
localized increase in interfacial area causes depletion in monolayer packing with a concomitant increase in interfacial tension so that the distorted faces act like stretched membranes. The tension gradient which is produced then rapidly brings monolayer molecules from behind the globules to fill the weakened gaps and these molecules drag water with them thus opposing further thinning of the aqueous lamella between the globules. Delay in adsorption of more amphipath from the water allows time for elastic rebound and the globules will then have survived their encounter (
Fig. 20.1).
A liquid-condensed monolayer is not necessarily a prerequisite for good emulsion stability since dilute monolayers of sodium oleate with oleic acid have been shown to be effective. Nor is it necessary to use an ionic amphipath since equally good emulsions can be obtained using the nonionic surfactant cetomacrogol 1000 with cetostearyl alcohol (as in cetomacrogol cream). Globules of such an emulsion may still acquire an appreciable ξ-potential, presumably by adsorption of contaminant ions and friction with the aqueous medium. However, entropic stabilization appears to be more important with nonionics (Elworthy & Florence, 1969).
The three creams mentioned above may be prepared in two slightly different ways. Commonly the water-soluble and oil-soluble amphipaths are dissolved in their respective phases with the aid of gentle heat; the two phases are mixed at about 60–70° and then stirred gently till cool or passed, with cooling, through an emulsifier. Alternatively an
emulsifying wax is used which consists of the two amphipaths intimately mixed by melting and dissolution. This ‘wax’ is melted, dissolved in the warm oil phase and mixed with the warm aqueous phase. Emulsification
is then achieved as before since the water-soluble component rapidly partitions out of the oil.
The consistency of the product and its appearance may be influenced by the method of preparation. Since the concentration of water-soluble surfactant is in excess of the CMC, some of the cetostearyl alcohol partitions into the aqueous phase to form mixed micelles. Low concentrations of the alcohols give fluid emulsions but concentrations near or above the saturation concentration in the oil produce semisolid creams, i.e. they possess a yield stress (see
Chapter 10) and will not flow under their own weight. This
bodying action as it is called is due to gelation of the aqueous phase, probably as the result of the formation of liquid crystalline structures (
Lachampt, 1970) (see
Chapter 5). The viscoelastic gel network, which forms when the emulsion is first cooled, is partially disrupted when sheared, resulting in a reduced yield stress. However there may be some thixotropic recovery.
Evidence so far accumulated suggests that, for a given amount of oil-soluble component, ionic water-soluble surfactants produce stiffer creams than an equal molar concentration of a nonionic one. The consistency however depends largely on the rare of cooling of the cream during manufacture and on the mechanical dispersion process since this affects the degree of partitioning of the oil-soluble component before the structures are ‘frozen’ in position. It is possible that ionic surfactants behave rather differently from nonionic ones. Structure build-up is rapid with the former and occurs during the cooling process whereas structural changes occur over a period of several hours in cooled nonionic creams (
Barry & Saunders, 1971c). In order to obtain a cream with a smooth texture, the oil and water-soluble components should be readily capable of forming a ternary liquid crystal phase, otherwise granular textured creams may be produced containing waxy particles in the aqueous phase. These latter probably arise due to mechanical separation from the oil by the action of the homogenizer and may result when a low concentration of water-soluble component is used in conjunction with a fatty acid instead of an alcohol. Using cetrimide or cetomacrogol 1000, the optimal chain length of saturated fatty alcohols for a bodying action is C
I4 and C
16 or a mixture of C
I6 and C
18, but a marked viscoelasticity has been noted using shorter chain alcohols.
Unsaturated alcohols such as oleyl alcohol have no bodying action but do permit the production of stable creams. Increasing the volume of oil (producing a larger phase volume) has the effect of increasing the viscosity of the emulsion. This effect is supplemented by raising the proportion of oil-soluble component.
Stable emulsions may be produced in which the oil and water-soluble components are not chemically distinct. Soaps such as soft soap used in Turpentine Liniment partially hydrolyse so that coadsorption of ionized and unionized molecules at the o/w interface produces a complex film. The soap may also be formed in situ (nascent soap method) as when aqueous ammonia is added to oleic acid–paraffin oil in the preparation of Ammonia Liniment BPC 1949.
w/o Emulsions Containing Amphipaths
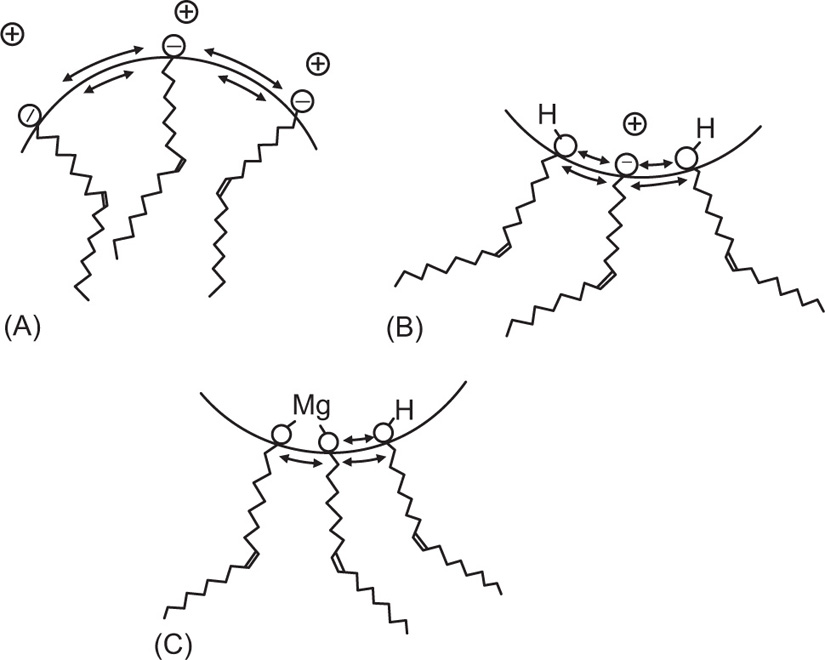
Schulman and Cockbain concluded that loss of charge together with rigidity in an interfacial monolayer leads to the formation of an emulsion where the globules are formed from the aqueous phase. Since an interfacial monolayer has two sides, one attracted strongly to the water and the other held by the oil, two different tensions, in effect, are operating and the film will
tend to curve towards the side of greatest tension. Thus a reduction in water
attracting power, for example by reducing ionization, may have the effect of causing an o/w emulsion to change or
invert to a w/o emulsion. A similar effect might be expected if the film becomes more bulky on the oil side as when the carboxyl groups of two fatty acids are joined by a divalent cation leaving the two hydrocarbon chains free to move on the oil side (
Fig. 20.2). Thus, shaking the o/w Ammonia Liniment with a little magnesium chloride causes complete inversion to a w/o emulsion. During shaking the least abundant phase would tend to be broken up but in the absence of the magnesium the aqueous phase globules readily coalesce so that the stable oil globules accumulate. Upon shaking the liniment with the magnesium salt, it is the turn of the oil globules to become unstable while the stabilized water globules accumulate.
A similar process occurs during the preparation of White Liniment. An o/w emulsion of turpentine oil is made using ammonium oleate (formed in situ) and this is then inverted upon shaking it with ammonium chloride dissolved in the rest of the water. Both w/o emulsions mentioned here contain emulgents whose water solubilities are low. In the first place, addition of the magnesium salt would cause precipitation of a magnesium oleate gel, while in the second ammonium chloride has a salting-out effect also potentially leading to a gel. Whether the film surrounding the water globules is a monolayer is obviously questionable since a gelatinous multilayer might well produce a suitable mechanical barrier. However, a surfactant mixture, such as lecithin–cholesterol in a ratio below 8:1, can form monolayer stabilized w/o emulsions. Close approach of two water globules leads to the formation of a lipid bilayer which, however, retains some oil and complete thinning of the oil lamella with consequent coalescence of the globules is resisted.
Water globules have been demonstrated to possess charges, the effect of which is long ranging due to the low permittivity of the intervening oil. In such instances, the Helmholtz double layer thickness can exceed 1μm producing a considerable energy barrier with respect to infinite separation of the globules. The globules are, however, not separated by vast distances so that geometric distribution of the globules necessarily means that part of the barrier is already surmounted and the net repulsion is considerably reduced.
Hydrophobic soaps produced by reaction between divalent and trivalent cations and fatty acids are nearly always formed in situ. Such is the case with zinc cream and oily calamine lotion, both of which contain calcium oleate formed by the interaction of calcium hydroxide solution and oleic acid. They will also contain traces of zinc oleate from the zinc oxide and calamine respectively. Emulsions prepared using these soaps alone tend to break readily, therefore in each case wool fat is added to enhance the stability. This contains long-chain alcohols and hydroxy sterols such as cholesterol, which on their own tend to produce w/o emulsions but may be used to stabilize both w/o and o/w emulsions. By contrast, it should be noted that calamine cream is an o/w emulsion utilizing sodium lauryl sulphate, the corresponding divalent salts of which are much more soluble in water than those of fatty acids.
Multiple Emulsions
These may form when a system does not strongly favour one particular emulsion type or when three phases are present. Certain aspects of their formation have been discussed by
Mulley and Marland (1970).
Emulsions Containing Hydrophilic Colloids
Macromolecular films at the oil–water interface have been described in
Chapter 5. Since the emulgents used are nontoxic and hydrophilic they are commonly employed in the preparation of o/w emulsions for oral use. A mechanically strong gel barrier forms around the outside of the oil globules while a considerable viscosity is imparted to the intervening aqueous phase thereby reducing the probability and energy of encounter between oil globules. The mechanical strength of the gel barrier largely determines the stability of the emulsion and this may be enhanced by ensuring physicochemical conditions for least solubility of the emulgent. Thus pH control may be important as demonstrated by the fact that gelatin is most effective at its isoelectric point. The rate of coalescence of oil globules protected by anionic hydrocolloids such as carboxy methyl cellulose is reduced at low pH where the interfacial film strengths are high even though the aqueous solution viscosities are reduced.
Since the interfacial energy of macromolecular film-stabilized emulsions is generally fairly high, perhaps in excess of 20
mJ m
–2, considerable energy needs to be expended in order to achieve a fine emulsion. Generally, the oil is dispersed in an aqueous solution of the hydrocolloid by means of a homogenizer or other suitable mechanical mixer which does not incorporate much air. For very small quantities of acacia stabilized emulsions, a pestle and mortar technique may be used where the oil–water–gum
proportions are initially carefully controlled so that the relative viscosities of the two phases allow adequate shearing of the interface. This is further described in
Chapter 26.
Since globule size reduction involves an increase in interfacial area, more hydrocolloid needs to be adsorbed. If there is an excess of hydrocolloid, coverage of the extra area need not be at the expense of the thickness of the interfacial film. In the presence of very thick films (of the order of 100
nm) such as occur in acacia stabilized liquid paraffin emulsions, the effective phase volume increases as the emulsion is made finer, resulting in an increased viscosity of the emulsion (
Shotton & White, 1963). Low viscosity grades of cellulose derivatives are desirable in order that these may be present in suitable concentration to produce a good coverage of the globules without increasing the viscosity of the external phase too much.
Emulsions Containing Solids
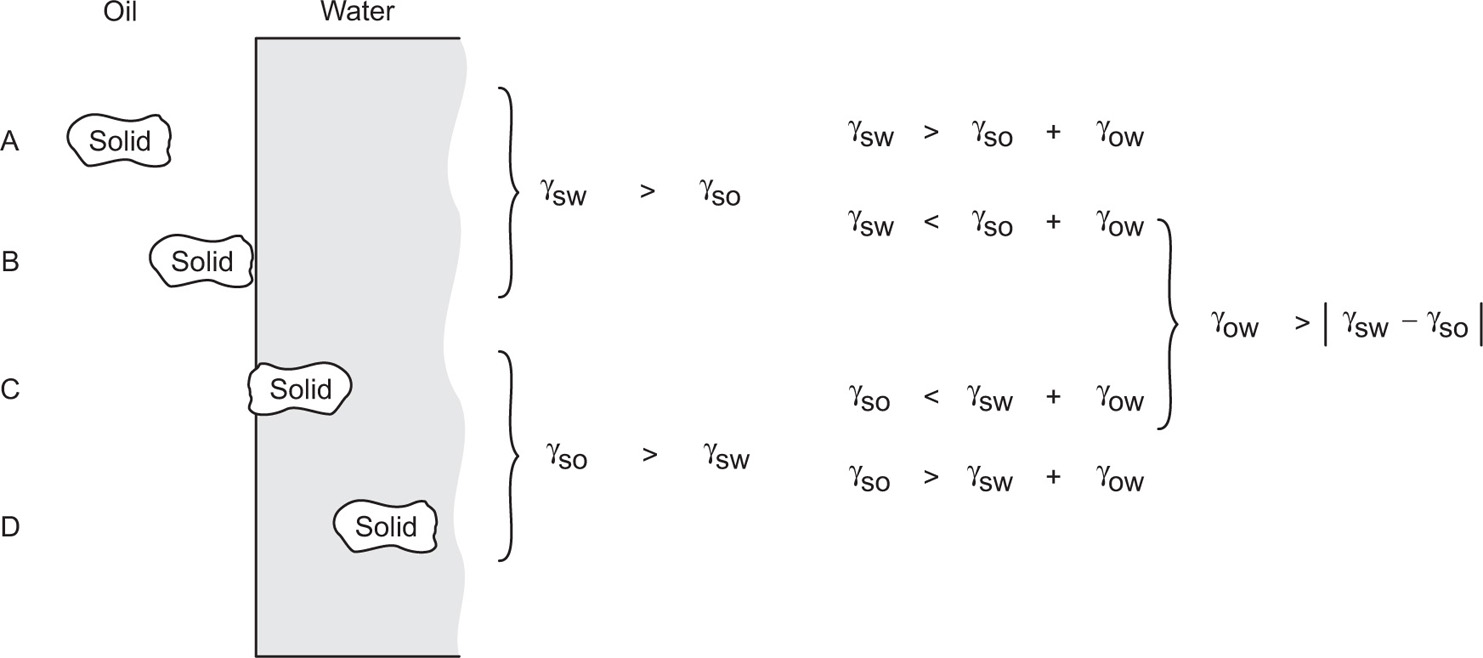
The extent of adsorption of solids, such as gelatinous precipitates, at an o/w interface depends very largely upon their physical state. Energy conditions for adsorption are illustrated in
Fig. 20.3, liquid paraffin and magnesium hydroxide emulsion BPC being an example of C. This is also illustrated in
Fig. 5.45, where a necessary condition is a nonzero angle of contact (
θ) such that |cos θ| < 1. In order to achieve this, the solids may be modified by the controlled addition of amphipaths or by reaction with a component of the oil in situ. The most stable emulsions are obtained when cos
θ ≈ 0.
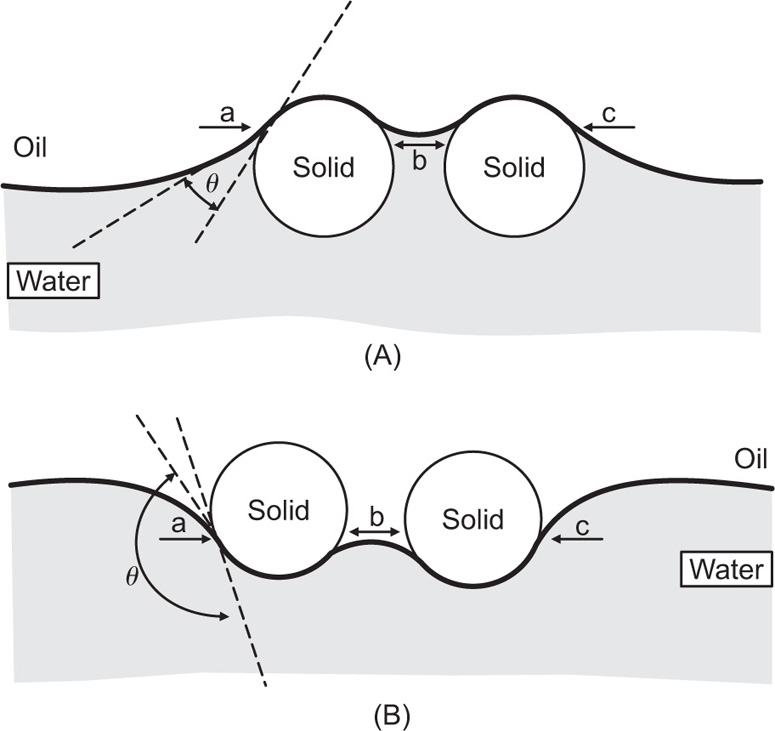
An extremely rigid interfacial film of closely packed particles can be produced by capillary forces as illustrated in
Fig. 20.4, the effect being enhanced with most particles by the irregularity of their surface. The phase having the greatest affinity for the solid forms the external phase. Thus magnesium and aluminium hydroxides may be employed, where appropriate, in o/w emulsions for oral use, whereas bentonite can be used in both o/w and o/w emulsions for external application.
These emulsions tend to be rather coarse but their stability can often be enhanced by the addition of other surface-active materials and these, together with the preparative technique, may influence the emulsion type. Thus a bentonite stabilized cream incorporating self-emulsifying Monostearin would be o/w whereas that made with calcium oleate might well be w/o.
It is probable that many w/o emulsions, whose stability is commonly attributed to monolayers, are actually stabilized by fine particles, probably with monolayers filling the gaps, and a similar mechanism might be postulated for some w/o emulsions where
gelatinous structures are known to exist in the aqueous phase (
Sanders, 1970;
Davis, 1971). A necessary criterion is that the solid particles shall be much smaller than the globules which they are to protect. Since their desorption involves, at least temporarily, an increase in interfacial energy, these emulsions can be very stable. This stability may be enhanced if the particles carry a charge, resulting in globules having a high ξ-potential.
Spontaneous Emulsification and Microemulsions
If some nonequilibrated oil and water phases are brought together, an emulsion may form near the interface without mechanical agitation. One of three mechanisms may be involved. Firstly, contact of an oily solution of a fatty acid with aqueous alkali may lead to interfacial turbulence owing to local variations in lowering of γow. Secondly, the diffusion of oil-soluble acid or alcohol into the aqueous surfactant may carry oil which then becomes stranded as the environment becomes predominantly aqueous. At the same time, water diffused into the alcohol laden oil becomes stranded as the alcohol passes out into the aqueous phase. The result is an o/w and a w/o emulsion on either side of the original interface. Emulsions formed spontaneously in this way are not thermodynamically stable.
The third mechanism leads to the formation of transparent or faintly turbid o/w or w/o
microemulsions with globule diameters in the range 8–80
nm; less than one order of magnitude larger than micelles. Low molecular weight hydrocarbons have been microemulsified probably due to the formation of an interfacial duplex film of low tension which is opposed by an imbalance of forces on either side of the film, tending to produce a negative interfacial tension. A nonnegative tension is then restored by the spontaneous curvature of the interface (
Prince, 1967). Such emulsions are therefore unique in being thermodynamically stable.
Stability of Emulsions
Since pharmaceutical emulsions have a positive interfacial energy they are thermodynamically unstable in the sense that breaking is the spontaneous process whereby globules coalesce to form larger globules and eventually separate completely into another layer with a minimum interfacial area. The rate of coarsening of an emulsion depends upon the effectiveness of the interfacial barrier to coalescence and upon the frequency and duration of globule encounter. The latter is increased if the globules collect into a smaller volume owing to a difference in density of the internal and external phases. This phenomenon is known as creaming by analogy with the concentration of oil globules at the top of a bottle of milk. By similar analogy, it will be appreciated that the process of creaming can be readily reversed by agitation whereas breaking cannot so easily be reversed.
Creaming should be minimized or eliminated since it tends to reduce the stability and elegance of an emulsion. Obviously, if an emulsion possesses a yield stress, the globules will be kept from moving by the structure of the external phase. In the absence of a yield stress, the Stokes’ equation points to the ways in which creaming may be controlled:
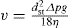
This shows that in a gravitational field of acceleration g, the velocity v of a globule can be reduced by decreasing its effective size (dst) and difference in density from the external phase (Δρ) and by increasing the viscosity (η) of the external phase. Reducing the effective globule size means not only that the individual globules should be as small as possible but that they should be kept from flocculating by means of a suitably high ξ-potential. It is not usual to reduce Δρ but this could be done by adjusting the oil density using a mixture of a light and a heavy oil such as a paraffin or vegetable oil and a brominated oil. The viscosity of the external phase may be increased by incorporating more bodying agent, such as a long-chain acid or alcohol in amphipath stabilized emulsions. The emulsifying waxes have a self-bodying action, i.e. the consistency of a cream may be controlled entirely by the proportion of ‘wax’ added. Thus emulsifying wax BP is used in both benzyl benzoate application and in aqueous cream but whereas the former contains only about 2 per cent ‘wax’ and is fluid and prone to creaming, the latter uses 9 per cent and is semisolid. Emulsions intended for oral use may include hydrocolloid thickening agent such as tragacanth or a small quantity of high molecular weight methylcellulose. Alternatively, sodium alginate may be thickened by the addition of traces of a calcium salt.
Phase volume may influence emulsion stability. Obviously if the internal phase volume is large, the mean distance between globules will be small. A large phase volume may involve considerable flocculation of globules and flattening of the regions of contact which will increase the chances of coalescence. The proportion of oil to water may occasionally decide whether an o/w or a w/o emulsion is obtained but with those emulgents where this is so the preparative technique may be the overriding factor. Sometimes it pays to prepare one type of emulsion, say a fine o/w emulsion, and then to invert this to w/o by the addition of more oil or by cooling. In this way, smaller water globules may be obtained than would otherwise be possible. White Liniment affords an example where the preparative technique involves inversion, in this instance due to an alteration in the nature of the emulgent.
Occasionally, however, only incomplete emulsification may be achieved. This might be due to a poor technique or to some unsuspected change in the quality of an ingredient. Instead of obtaining a white emulsion unsightly, immiscible, coarse mixture of o/w and w/o emulsions is obtained. It may be possible to break the emulsion by heating and then to make another attempt at emulsification, perhaps using another instrument, but usually it is best to start again. An example where difficulty might be encountered is in the preparation of Turpentine Liniment. If the soft soap is dispersed in the oil and this is then shaken vigorously with the water, an o/w emulsion may not be made successfully. However, o/w emulsification is readily produced with only a few shakes if about a one minute interval is allowed between each shake. This allows time for redistribution of the ionized soap and free fatty acid between the oil, the water and the interface
and results in a very thick stable o/w emulsion. Slight changes in pH may also lead to emulsion instability. With soft soap, this might result from the absorption of carbon dioxide.
Breaking of an emulsion during storage may be due to a number of interacting factors. Temperature fluctuations should be avoided. Elevated temperatures may lead to the emulsifier favouring a different emulsion type since changes in the interfacial film often become pronounced above 40°C. With o/w emulsions particularly, warm conditions enhance evaporation and chemical degradation processes such as oxidation of oils. The use of well-closed containers is therefore essential. The stability of most emulsions is also diminished by cooling to near or below 0°. Besides alteration of the emulgent properties, formation of ice crystals ruptures the interfacial film, thereby breaking o/w emulsions. In order to counteract loss of water from o/w dermatological creams, a humectant is frequently added. This is commonly glycerin or sorbitol. In sufficient amounts these compounds reduce the evaporation rate from the container and the skin, but the concentration is limited by their tendency to dry the skin.
Another source of instability is the growth of microorganisms. o/w Emulsions prepared with materials of natural origin such as gums, carbohydrates and proteins are particularly prone
to such spoilage, fungi, yeasts and bacteria all being implicated. Synthetic compounds such as cellulose and sorbitan derivatives are therefore to be preferred. Preservation requires the addition of one or more compatible, water-soluble, nontoxic compounds which have a long-lasting, wide spectrum of activity against microorganisms even in low concentration. Usually they should be undetectable by the senses. For oral use, benzoic acid is commonly employed, which may be supplemented with chloroform as in Liquid Paraffin Emulsion. Esters of
p-hydroxybenzoic acid are used for both oral and topical use. Sometimes, the emulgent itself has a preservative action as in cetrimide cream but in others a compound such as chlorocresol is a suitable additive. Since microbes grow in the aqueous phase, a sufficient preservative concentration must be found here and partitioning into the oil must be allowed for (
Bean, et al., 1969). Further aspects of preservation are discussed in
Chapter 40.
Instability of an emulsion is not associated merely with flocculation, creaming or breaking. Emulsions used as bases must not impair the activity of the medicament. Generally, an ionic emulgent will be incompatible with medicaments whose active ion is of opposite charge such as sodium lauryl sulphate with neomycin sulphate. Ionic incompatibility is not always obvious as for example the incompatibility of hexachlorophene with quaternary ammonium surfactants due to the anionic character of the phenolic group. Nonionic emulgents may be used when ionic reactions are at risk but even these are not immune from incompatibility due for example, to their micellar adsorption of phenolic compounds. Incompatibilities are not always visible although emulsion breaking may ensue. Sometimes a colour change may be seen as when proflavine hemisulphate is incorporated in an anionic base, but discoloration may develop only slowly as when hydrocortisone is incorporated in a cream stabilized with a triethanolamine soap. It is important to choose a base which is chemically unreactive and which gives the medicament the desired degree of skin penetration and release to the body tissues.
Another form of instability has been observed when the medicament is suspended in an emulsion. Liquid paraffin and phenolphthalein emulsion requires the phenolphthalein to be very finely dispersed otherwise it settles out and cannot easily be redispersed upon shaking.
Occasionally, emulsions are deliberately made with an inherent instability. An o/w barrier cream may be used which liberates a film of oil when applied to the skin. Sometimes, emulsion breaking is an essential part of a manufacturing process such as the separation of woolfat from wool scouring wastes. Yet another application is the formation of foams from emulsified aerosol propellants which vaporize on release from the container.
Globule Size
Microemulsions having globule diameters below about 50nm are transparent, while those with larger globules are decidedly turbid. If the globules are above about 100nm extremely white emulsions are produced but as the diameters increase much above 10μm a greyish or cream colour is developed. The whiteness of fine emulsions is due to the reflections and refractions at the many interfaces. If, however, the two phases have the same refractive index, a transparent emulsion is produced except when they differ in optical dispersive power and then a highly coloured chromatic emulsion results.
Globule size may be represented by any of the equivalent spherical diameters, the mean size generally being weighted by number. Although some size distributions may be approximately Gaussian, they are usually skewed but often capable of normalization by a logarithmic transformation in which case the distribution parameters are simply related by the equations of Hatch and Choate. Various other, largely empirical, distribution functions have also been proposed.
Size distribution is influenced by: (1) method of preparation of the emulsion, (2) temperature during mixing, (3) viscosity, (4) type of oil and its phase volume and (5) type and concentration of emulgent. Size reduction involves an increased interfacial area and interaction between globules resulting in appreciably higher viscosities only with the more concentrated emulsions (phase volume greater than 0.5). A more uniform size distribution also affords more resistance to flow. Thus concentrated emulsions having the same mean globule size may differ considerably in viscosity if they have different standard deviations. Reference has already been made to the increase in viscosity upon homogenizing emulsions stabilized by thick hydrocolloid films.
Size reduction may also lead to enhanced flocculation. This also increases emulsion viscosity. In the event of flocculation, care has to be taken in interpreting size parameters especially if nonmicroscopic methods of analysis are used.
Coarsening of an emulsion tends to follow first order kinetics and is observed as an increase in mean globule size. This may be accompanied by a broadening of the size distribution but sometimes a more nearly monodisperse system of large globules results.
Several methods are available for testing emulsion type. The dilution method involves placing a little of the emulsion in water. If the emulsion readily disperses it is o/w, otherwise a w/o type is indicated. Thick o/w creams may require slight agitation but usually emulsion type is readily observed especially as lumps of o/w cream appear dull in water whereas w/o creams are shiny and usually float to the top.
The dye solubilization method involves dusting a small amount of a water soluble dye, such as methylene blue, on the surface. Rapid dye diffusion indicates that water is the external phase. It is often useful to supplement this by dusting an oil-soluble dye such as scarlet red on another part of the emulsion surface. Dye diffusion then indicates contact with oil as in the case of w/o emulsions, but for this test to be valid the top surface of the emulsion should first be removed otherwise coloration may merely indicate a thin oil film on top of an o/w emulsion due to contamination from manufacturing equipment. Alternatively, it might indicate an unstable o/w emulsion.
Electrical conductivity is another method relying on the poor conductivity of oils compared with aqueous solutions. The conductivity may be measured by standard low frequency a.c. methods, and it will be low where oil is the continuous phase as in w /o emulsions. Ionic emulgents used for the production of the latter give higher conductivities than nonionic emulgents.
If a drop of emulsion is placed on filter paper, the external phase will tend to spread quickly if it is aqueous. The filter paper wetting method however, is useless for thick o/w creams or for emulsions of water in thin oils which themselves spread.
The tests mentioned above may be applied rapidly but the results must be interpreted with caution. They will, for instance, not indicate whether a multiple emulsion has been produced. This must be resolved by microscopy.
Emulsions in Pharmacy
Emulsions may be administered orally, rectally, parenterally and topically. Occasionally, a medicament forms all or most of one phase but generally, emulsions serve merely as carriers.
Oil-in-water emulsions are used for oral administration serving to mask the unpleasant taste of water-insoluble liquids such as liquid paraffin. Fine emulsification may enhance the absorption of lipid-soluble medicaments such as oil-soluble vitamins but these must use an absorbable oil as carrier: liquid paraffin would hinder their absorption. It should be noted that liquid paraffin emulsions should not be too fine since fine globules of paraffin can be absorbed. Hydrocolloids or nonionic amphipaths such as polyoxyethylene sorbitan esters are employed for oral use.
Emulsions used as enemas are also of the o/w type but may use soaps. The incorporation of a surfactant in suppositories has been reported to influence drug absorption either by increasing its rate or by prolonging it. The choice of surfactants for parenteral use is very limited and has already been discussed in
Chapter 4. Phytomenadione injection may be in the form of a fine o/w emulsion.
By far the greatest use of emulsions is in dermatology. Oilin-water creams and lotions form the washable variety whereas w/o creams may be used to act as barriers to aqueous solutions. Topical applications are common in the cosmetic field while in the medical treatment of diseased skin, emulsions are often used as carriers for a drug. Generally only a superficial drug action is required and undue penetration of the skin may need to be avoided as in the case of corticosteroid creams. Percutaneous absorption is mainly a, function of the drug itself and not of the vehicle containing it. Where penetration does occur, the hair follicles, sweat ducts and stratum corneum may all be implicated, absorption being greatest for those substances which have both a water and lipid solubility with the hydrophile–lipophile balance in favour of lipid. Since drug action requires partitioning out of the emulsion base, a base having a poor solvent action for the drug should be used. Percutaneous absorption is greatly affected by the condition of the skin such as its relative hydration and greasiness. It may be modified by vasodilation and by the accumulation of sweat during the use of occlusion dressings which involve covering the treatment area with a plastic film. Water-in-oil bases also may inhibit evaporation of sweat, reduce heat radiation and increase skin temperature.
The skin is a natural barrier with a pH between 5 and 6. Ideally, therefore, cream bases should have a neutral or slightly acid pH. Buffered cream BPC is one such o/w emulsion, this being similar to aqueous cream BP having the pH adjusted to about 6 with sodium phosphate and citric acid. The electrolyte content of the aqueous phase of a cream may be an important consideration when treating hypersensitive skins.
de Kay (1962) and Becher (1965) have surveyed much of the literature relating to the topical use of emulsions.
Emulsifying Equipment
A wide variety of machines is available for finely dispersing the internal phase of an emulsion. Among factors which influence the suitability of a particular machine are (1) the amount of emulsion to be processed, (2) the flow characteristics, (3) the need to blend ingredients such as powders, (4) the temperatures involved and (5) the desired rate of cooling if elevated temperatures are used.
Large and small batches may often be made conveniently by using propeller-mixers operating in suitable mixing vessels, the latter being steamjacketed if initial heating is required. If
a thick emulsion or cream is produced, the propeller-stirrer can be replaced by a gate-stirrer assisted by scraper arms. Fluid emulsions may also be prepared by means of a shrouded turbine such as a Silverson mixer-emusifier. Models are available for batch processing quantities from a few ml up to several thousand litres while continuous processing can be achieved by incorporation into a pipe-line.
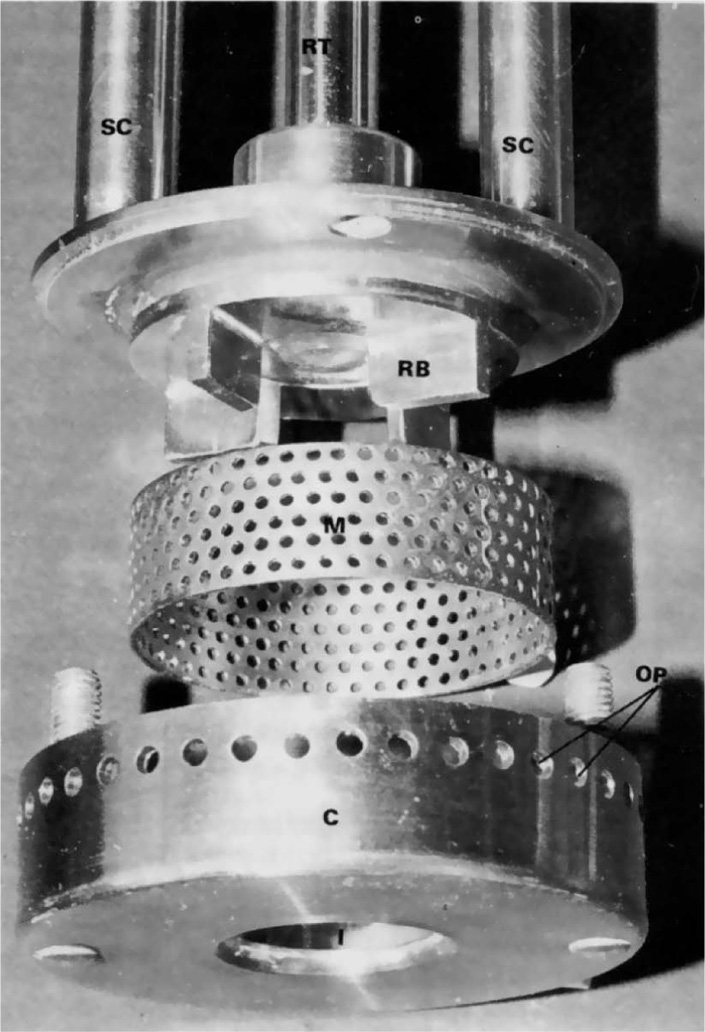
Fig. 20.5 shows the components of the working head of a large laboratory scale model. The columns SC support the head in which turbine blades RB are driven. These are powered by a motor above the supporting columns which act via the drive RT. Centrifugal forces expel the contents of the head through the mesh M and on to the cover C when large shearing and impaction forces produce a fine emulsion which emerges from the openings OP. Circulation of material through the head is maintained by the suction produced in the inlet I at the bottom of the head.
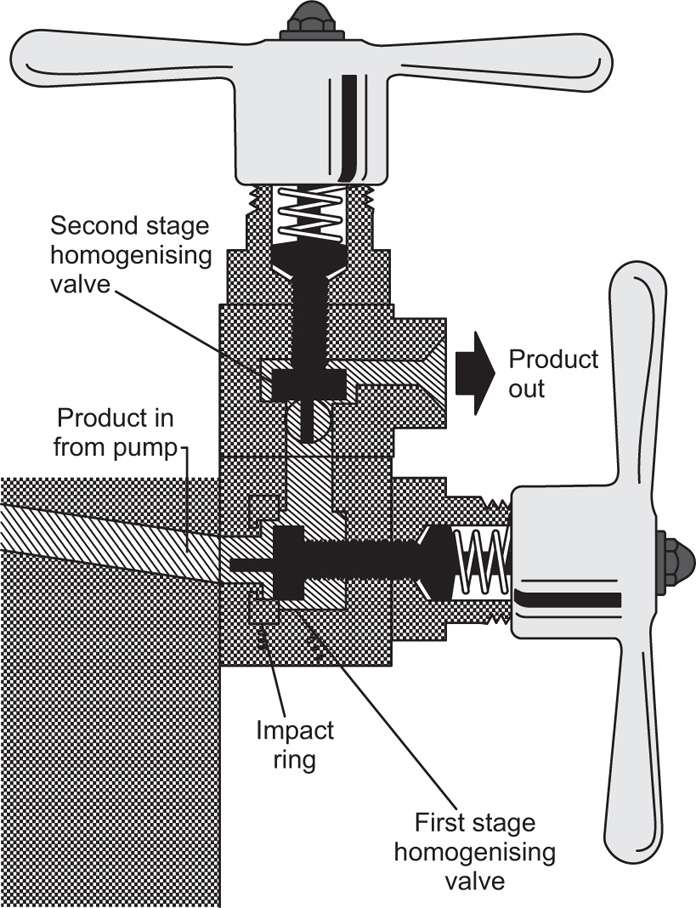
Homogenization can also be obtained by forcing the crude mix through a small annulus so that a high velocity jet is made to impinge on an impact ring set at right angles to the flow. One such instrument, the A.P.V. Gaulin homogenizer, uses a high pressure pump and the shearing action is divided between two independently adjustable stages (
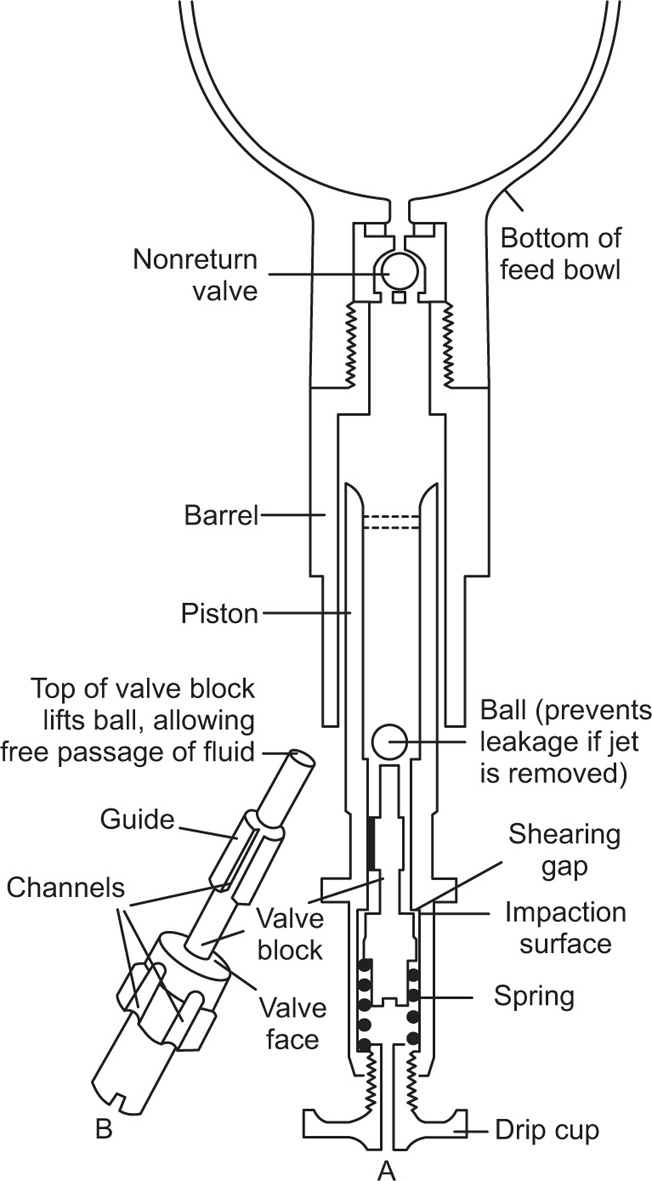
Fig. 20.6). Ormerod Q.P. homogenizers work on the same principle, the hand-operated model being commonly used for dispensing purposes. The plate jet, illustrated in
Fig. 20.7, is fitted into a reciprocating piston which is filled with the crude mix (held in the bowl) upon its downward stroke. The upward stroke causes expulsion via the shearing and impaction surfaces, the valve loading being controlled by rotation of the drip-cup.
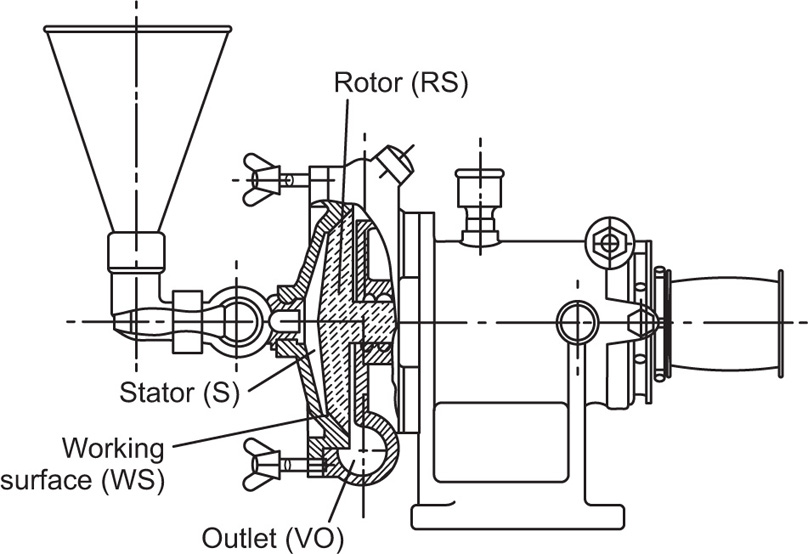
A premier colloid mill is shown in section in
Fig. 20.8, side and top loading models being obtainable.
The rotor RS runs at several thousand rev/min with its working surface WS in close proximity to the stator S, the gap being adjustable from about 50μm upward. Crude mix is fed via the funnel on to the centre of the rotor and is flung outwards via the shearing surfaces to be discharged at VO.
Cavitation effects the break up of liquid in ultrasonic equipment. In the Rapisonic homogenizer, a portable machine made by Ultrasonic Ltd., crude mix is sucked into one end of a long U-tube and ejected at the other end over a blade which vibrates at its natural frequency of about 30
kHz. The emulsifier head is illustrated in
Fig. 20.9.
Suspensions
Suspension is a dispersion of finely divided solids in a liquid. The lower limit of fineness of a solid particle in suspension has been arbitrarily fixed at 0.1
μm. Among the liquid suspension used in pharmaceutical practice may be mentioned mixtures or magmas for oral use, lotion or washes for external use and injections for parenteral use. The basic characteristics of such suspensions are:
• The rate of sedimentation of the dispersed particles should be so low that no distinct layer of the sediment or any appreciable separation of supernatant liquid on long storage and shelf life is observed.
• Any sedimentation taking place is easily dispersible by moderate shaking, i.e. the sediment does not cake or cement on setting.
• The flow property of the suspension is so balanced as to make it possible to pour easily from the container or to apply on the skin or inject it through hypodermic needles in suspension.
• The product must be chemically and physically stable, so as to maintain maximum therapeutic efficiency.
• The suspension should be acceptable to the consumers aesthetically, i.e. in regard to its taste, colors, smell and its cosmetic properties.
• In case the suspension is meant for parenteral use, it should not lose its efficacy when subjected to sterilization process.
To obtain a suspension with the above-mentioned characteristics, the following physical and physicochemical phenomena have to be taken into account:
• Surface properties of the suspended solid particles
• Electrokinetics of the system including particle–particle and particle–vehicle interaction
• Rate of sedimentation
• Caking or cementation
• Micromeritics
• Flow property or rheology of the product
Surface Properties of the Suspended Solid Particles
In accordance with the Stokes’ law, the rate of sedimentation of a suspended particle is directly proportional to its size. Hence, one way to reduce the rate of sedimentation or the settling of particles is to reduce the particle size. Another advantage of size reduction is that once the particles acquire a size below 5μm in diameter, they start manifesting Brownian movement, which helps to keep dispersed particles in suspension. On the other hand, on subdividing the dispersed solid into finer particles, their surface area increases immensely and so also the free energy associated with it. This increase in free surface energy energizes the particles greatly, and hence they tend to coalesce, thus reducing the surface area and the free energy. In this way the particle size reduction originally intended to give greater stability to the suspension by reducing the rate of sedimentation makes it unstable from the thermodynamic point of view. The increase in free surface energy, ΔF, is expressed mathematically as the product of the interfacial tension (η), expressed in dyne percentimetre, between the dispersed particles and the dispersion medium, and the increase in surface area (ΔA) due to reduction in particle size (ΔF = η × ΔA).
In the above equation, the electrical forces acting on the surface of the particle have been disregarded. The system can be made thermodynamically more stable by reducing either interfacial tension or the surface area. Reducing the surface area would mean increasing the size of suspended particles, which in turn would be detrimental to the stability of the suspension. The interfacial tension (
η) cannot be reduced to 0, but its magnitude can be appreciably decreased by means of a suitable surface-active agent (wetting agent). The indiscriminate use of such agents is, however, beset with another difficulty, i.e. increased deflocculation of the particles, which reduces their settling but ultimately leads to caking of the sediment. This
can be avoided by effecting partial occulation of the dispersed particles, whereby they loosely adhere to one another resulting into an open scaffold-like structure. The surface free energy thus reduced gives the system greater thermodynamic stability on the one hand and minimizes the risk of cake formation on the other.
Electrokinetics of the System
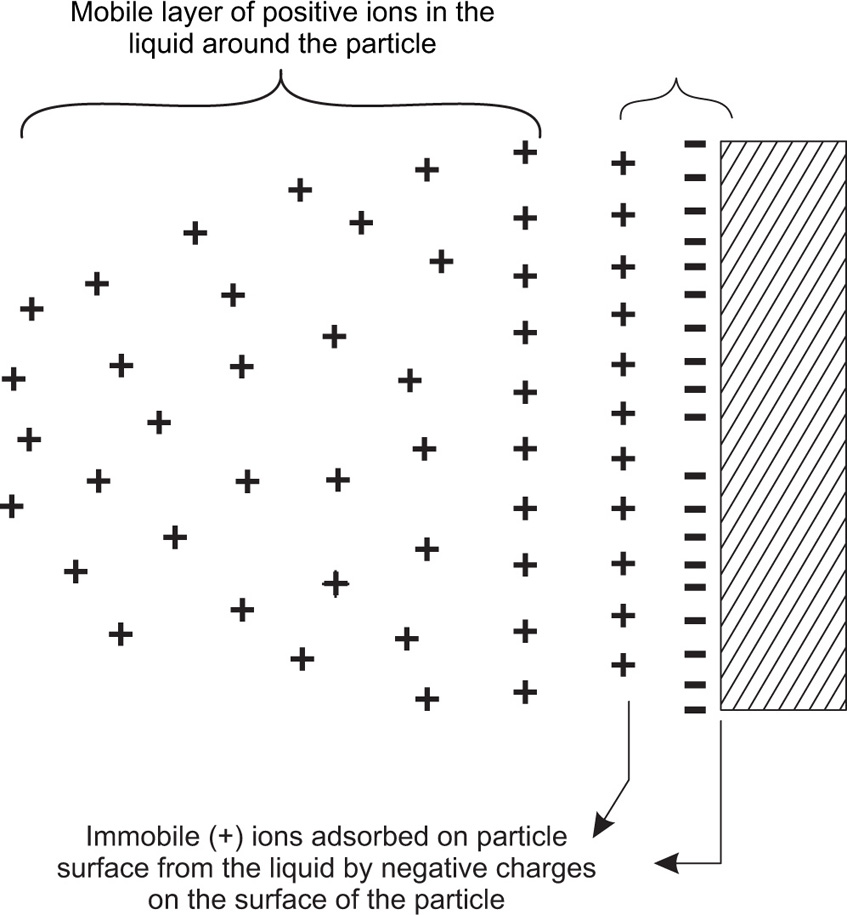
The finely subdivided particles having acquired electrical charge on their surface attract or repulse each other, which contributes to the stability or otherwise of the suspension system. The presence of electrical charge on the particle surface may be due to (1) dissociation of ionizable groups at the surface, (2) adsorption of some solute ions or solvent molecules from the solution, (3) friction between the particles and (4) the differential dielectric constant between the dispersed phase and the dispersion medium. The particles charged electrically due to any of the above reasons become surrounded by an ionic atmosphere of oppositely charged ions and formed an electrical double layer around the particles: (1) a stationary layer at the particle surface and (2) a mobile layer next to the stationary one (
Fig. 20.10).
Because of the surface charge on the suspended particles, a potential exists between the colloidal particles and the solution. The suspended particles in such a charged environment move when placed in an electrical field. The difference in the electrical potential between the moving positively charged particles in the surrounding medium and the immobile cations absorbed on the surface from the liquid is known as
zeta potential (
Fig. 20.11). It is a number and measured in units of potential.
When such a potential is high, the particles repel each other, remain deflocculated. i.e. remain in suspension for a long time and do not easily settle down. The addition to the system of any surfactant, yielding ions having similar charge (+ or −) as on the particle surface, helps to increase the zeta potential of the suspended particles and thus increase the force of repulsion between particles, causing still greater deflocculation. On the other hand, introducing such a body into the system possessing dissimilar charge will reduce the zeta potential causing flocculation of the particles to varying degrees.
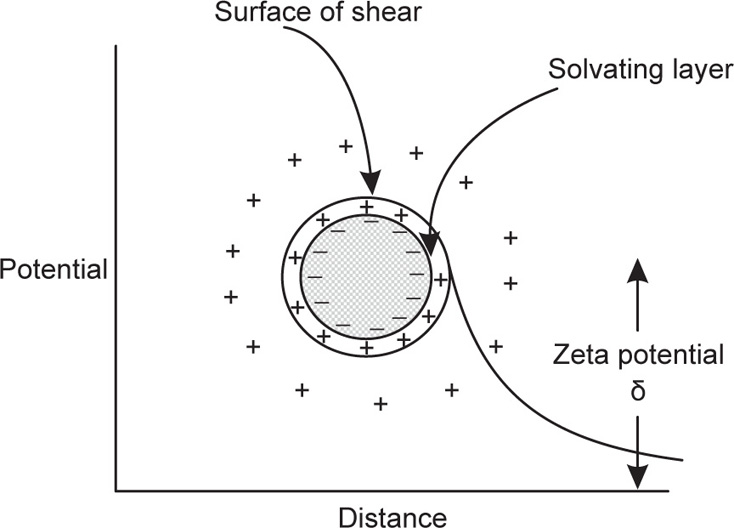
This term can be more precisely understood by referring to
Fig. 20.10, which illustrates Helmholtz–Gouy double layer. This theory explains the charge relationship at the surface of a dispersed particle. The potential difference across all the ion layers from the particle surface into the solution is known as the
Nernst electrothermodynamic or
electrochemical or
epsilon (
ε) potential, whereas the potential difference between the immobile layer of ions and the mobile layer is termed as the
electrokinetic or
zeta potential. The layer of adsorbed ions is often called the
Stern layer and the potential between the particle surface and the immobile layer is termed the
Stern potential. This is equal to the difference between the electrochemical or epsilon (
ε) potential and the electrokinetic or zeta potential, mathematically represented as:
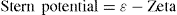
Therefore, electrochemical or epsilon potential is equal to the sum of Stern potential and zeta potential.
The measurement of zeta potential is done with the help of the electrophoresis cell, wherein the particles together with the rigidly bound double layer move under the influence of the applied direct current. The value of zeta potential, which is the difference in potential between the dispersion medium and the surface of the rigidly bound portion of the diffuse double layer surrounding the particle, is obtained from the electrophoresis experiments. And the directions in which the particles migrate indicate the sign of the charge borne by them. Zeta potential is mathematically expressed as under:
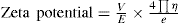
Wherein the zeta potential is expressed in volts,
V is the velocity of migration of the particle in cm/sec,
E is the potential gradient in volts/cm along the electrophoresis tube,
η is the viscosity of the dispersion medium in poises and
e is the dielectric constant of the medium.
Rate of Sedimentation
The velocity at which a suspended particle settles down has been expressed by the Stokes’ equation:
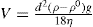
where
V represents the velocity of settling of an average particle,
d the mean diameter of the particle,
p the densities of the dispersed phase and medium,
g the gravitational force and η the viscosity of the suspension medium. This law is applicable only when the falling particles are perfectly and uniformly spherical and settle down in a streamline fashion, i.e. in an unhindered way. But the high concentration of the dispersed phase and other conditions such as the want of uniformity in shape and size of particles, the Brownian movement and the forces of attraction and repulsion of particles by particles, prevailing in the system, do not permit the application of the unmodified Stokes’ law. Nevertheless, the Law helps us to focus our attention on the factors that affect the stability of the dispersed system. The three factors most concerned are:
1. Gravitational force
2 Size of particles
2 Viscosity of the system including yield value and thixotropy
Increase in the first two factors accelerates sedimentation of the particleswhile increase in the third, i.e. viscosity, retards such tendency. Besides these above factors, the forces of attraction and repulsion of the particles themselves accelerate or prevent particle aggregation and consequent sedimentation.
From the above it can be seen that we can stabilize a suspension in the following three ways:
1. By decreasing the rate of sedimentation of dispersed particles
2. By increasing the consistency or viscosity of the dispersion medium
3. By preventing the particles from mutual aggregation
As we proceed further, it will become clear that the rate of sedimentation of suspended particles is not of that importance in suspension stability as are the phenomena of partial flocculation of particles to form an open scaffold-like structure and the bodying or structuring of the suspension medium.
Measurement of sedimentation rate is made in terms of the ratio of the ultimate height (Hu) of the sediment column to the original height (Ho) of the system containing the suspended particles dispersed all through, both readings taken from the same container (cylinder). This ratio Hu/Ho is called sedimentation ratio and its values against time have been used as an index of the settling rate of dispersed particles. It will be evident from the above that larger the Hu/Ho ratio value, slower is the sedimentation rate and greater is the degree of flocculation of the particles. This ratio assumes the maximum value when the optimum concentration of the flocculating agent has been reached.
In recent times, a device known as sedimentation kymograph has been employed to determine the rate of sedimentation. This is only a modification of the Oden’s balance and helps in maintaining a continuous record of the settling rate on a smoked drum rotating at a slow speed of about 0.1mm per minute. The pointer attached to the disc on which particles settle records the rate in terms of the height of the curve on the kymograph.
Caking of Suspensions
Caking or cementation of a suspension may be defined as the formation of indispensable solid cake of the sedimented particles. A caked suspension is a lost suspension, because it gets damaged beyond repair so far as no force or agitation can reconstitute it to the original system. Hence the phenomenon of caking has been said to be a scourge for suspension makers. For a clear understanding of this phenomenon, we have to recall the electrokinetics or zeta potential of the suspended particles, a mention of which has already been made. A highly deflocculated suspension, i.e. the one having dispersed particles with high value of zeta potential, may in one sense be desirable because in such a suspension the suspended particles remain discretely separate and thus dispersed in the system for a very long time. But once such a highly deflocculated suspension settles down, howsoever slowly this may happen, it invariably leads to cake formation.
According to the present theory, there are two types of opposite forces acting upon the dispersed particles in a suspension:
1. Force of attraction of the London van der Waals type
2. Force of repulsion due to the like electrical charges borne by the particles
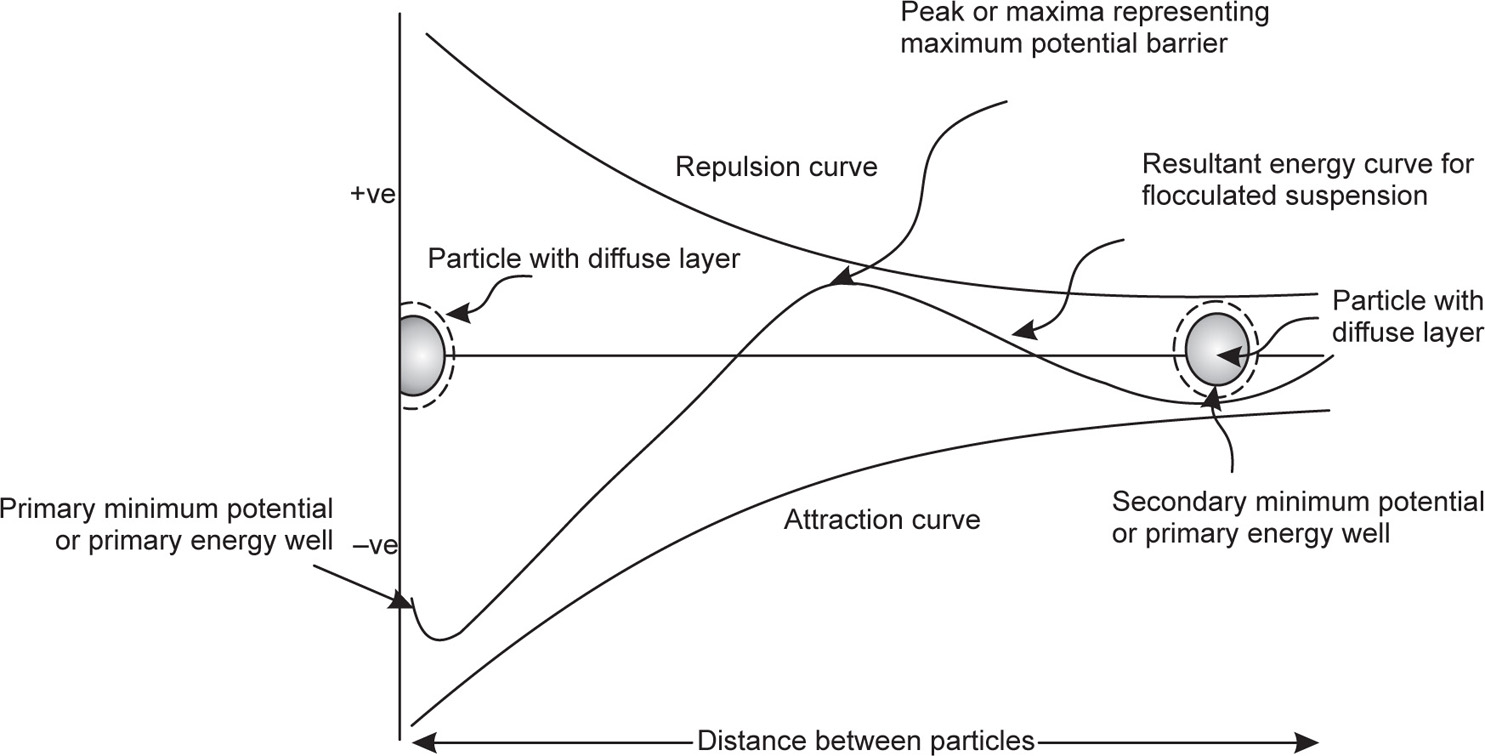
The energy diagram shown in
Fig. 20.12 depicts these forces; the attraction curve, lying in the lower area of the diagram or on the negative side of the potential energy, indicates that the force of attraction increases as the particles approach each other or as the distance between them decreases. On the other hand, the repulsion curve, lying as it does in the upper part of the diagram, i.e. on the positive side of the potential energy, also indicates that the repulsive force increases as the distance between the particles decreases. The third curve represents the resultant of the two opposing forces of attraction and repulsion acting on the suspended particles. The maxima or the peak of this curve lies in the upper part or the positive side of the diagram and represents the maximum repulsive force acting between the two particles, and indicates the maximum potential or energy barrier. The potential or energy barrier between two particles means the force of repulsion acting between them due to similar electrical charges borne by them and their diffuse layer of ions, the positive value of which indicates attraction. This third curve shows two potential minimas or energy wells.
The primary potential minimum or energy well shows the force of attraction of a much higher order, which exists when the distance between very fine and highly deflocculated particles is the minimum. At such a stage the potential or the energy barrier between them has cracked or vanished. This happens because the particles have come too close together, because of
• their settling down, howsoever it might happen,
• the smaller particles entering into the spaces caused between
• the larger ones where the particle-size distribution is wide and
• the lowermost particles of the sediment being gradually pressed together by the weight of those lying above.
Thus the potential or energy barrier, causing the particles to repel each other and remain dispersed, is altogether overcome causing the force of attraction between them become very great. This is the state that exists in the primary energy well and that leads the particles to adhere together so closely that their redispersion becomes almost impossible. This is the phenomenon that has been referred to as caking or cementation.
The secondary minimum potential or energy well also lies in the lower part, i.e. on the negative side of the diagram, representing a state of attraction between the particles, but here in this case the force of attraction is of much lower order and operates when the particles are comparatively far apart. Such a condition is brought about by the incorporation of a flocculating agent, i.e. an electrolyte that can furnish active ions carrying electrical charges opposite to that existing on the particle surface. When such ions get concentrated in the diffuse double layers of the dispersed particles, the force of repulsion between them is reduced to a certain extent causing an increase in the force of attraction even when they are at a greater distance, which has been estimated to be of the order of 1000–2000 Å. This condition prevailing in the secondary potential or energy well results in the building up of flocs or floccules, which are so structured with balanced forces of attraction and repulsion that though they settle down comparatively more quickly, their discrete particles are, at no stage, allowed to approach each other so close as to let the potential barrier existing between them to be overcome altogether, leading to the formation of a cake or a cemented sediment. The phenomenon of floc formation is known as flocculation, where the coarse and partially flocculated particles undoubtedly coalesce but remain redispersible, or reconstitute on gentle shaking because they have not actually caked or cemented.
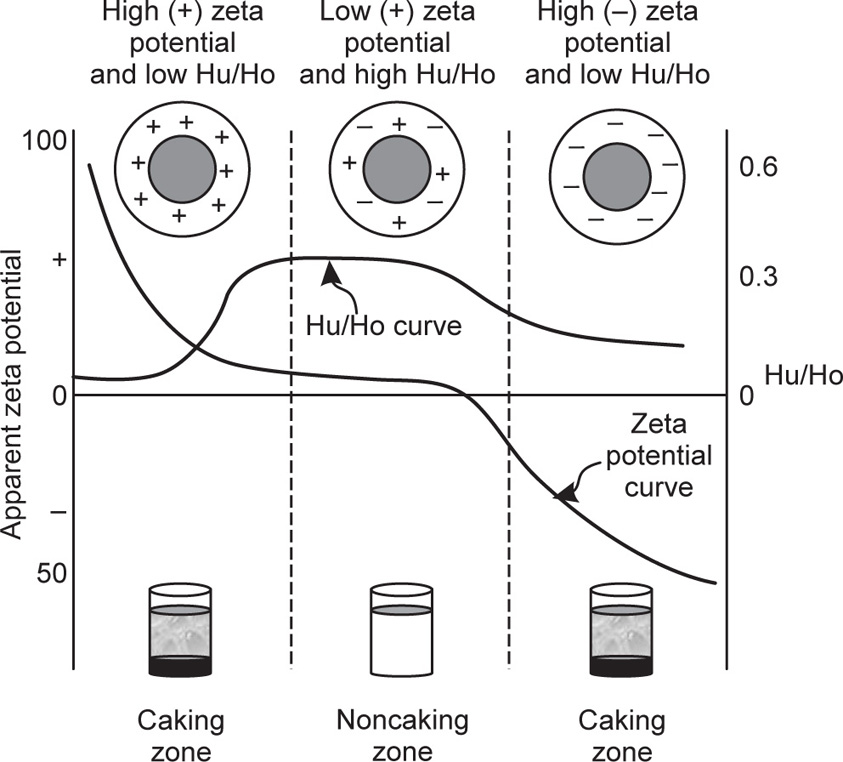
Fig. 20.13 shows a caking diagram of a bismuth subnitrate suspension being flocculated by adding to the system a dilute solution of dipotassium hydrogen phosphate as the flocculating agent. Initially, the dispersed particles of bismuth subnitrate have a
high positive zeta potential as indicated on the left-hand side of the diagram. On adding the flocculating agent (K
2HPO
4), the negative phosphate ions get adsorbed on positively charged particles of the dispersed phase and the charge on them is reduced; their zeta potential value is lowered to such an extent that there is maximum flocculation. By working out the sedimentation ratio, it is found that in the beginning
Hu/
Ho ratio is low, showing none or little flocculation. By introducing the flocculating agent into the system, one can reach a point at which the ratio assumes the highest value indicating the maximum flocculation. In other words, these values constitute the noncaking zone shown in the diagram as the middle region. Further addition of the flocculating agent to the suspension causes greater adsorption of the negative ions of the flocculants. Thus, the zeta potential is raised again though with a changed sign of the charge from positive to negative. The
Hu/
Ho ratio is also reduced because the particles are once again deflocculated. This event is accompanied by the shifting of the system again into the caking zone. Thus it can be concluded that both, the sedimentation ratio and the zeta potential value, can provide caking indices for suspensions. It is also concluded that controlled flocculation of the particles of the dispersed phase, caused by introducing a flocculating agent bearing electrical charge of the sign opposite to that on the particles, helps a good deal in preventing the cake formation or cementation.
There are some other factors that cause caking such as:
1. Crystal growth: When crystals grow or change their form or habit they do so in an interlocked fashion, which results in an intertwined structure leading to caking.
2. Temperature cycling: During the shelf life of the product when the environmental temperature changes take place, the dispersed phase gets dissolved to some extent by the rise in temperature and when the temperature falls in the next cycle, the dissolved substance reprecipitates or recrystallizes giving rise to cake formation, because it does so in an interlocked fashion.
3. Wide range of particle size distribution: In such an event the smaller particles having higher intrinsic solubility get dissolved and then get reprecipitated into bigger granules or crystals, which cause cementation of the suspended particles.
4. Chemical reactions: These have also been known to cause caking of suspension, for example, if a system contains magnesium oxide and sodium bicarbonate, there occurs conversion of magnesium oxide into magnesium carbonate resulting in caked sediment.
Micromeritics in Suspension
The size of particles of the dispersed phase, as stated earlier, is a very important factor in suspension making. The smallness of the particle size minimizes the velocity of sedimentation, firstly due to its lower magnitude and secondly by increasing the velocity of the system and thereby improving the quality of suspensions. This is explained by the Einstein’s equation, which relates viscosity to the effective volume fraction of the dispersed phase in the system. Einstein’s equation is:
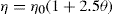
In which
η is the viscosity of the suspension,
η0 the viscosity of the dispersion medium and
θ the volume fraction
* of the dispersed phase.
Reduction of the particle size also contributes to the therapeutic usefulness of the product, because with reduction in the size of the particles, the surface area and hence the intrinsic dissolution rate increases leading to better and quicker absorption of the drug into system. There are numerous examples to prove this statement. For instance, it has been experimentally shown that quicker and higher peak of plasma level of spironolactone is reached when it is administered as micronized powder than when it is given as ordinary powder. There are a few exceptions also to this general rule. Besides the average size of the particles, the particle size distribution (p.s.d.) has its own effect on the physical stability of the suspension system. In the above narration, mention has been made of the wide range of particle size distribution leading to cake formation. In the next section, it will be shown how the particle size distribution affects the viscosity of suspensions.
Particle Size Considerations
The mean particle diameter and the particle size distributions of suspended insoluble drugs are important considerations in formulating stable pharmaceutical suspensions. Hiestand defines the lower limit of coarse suspensions as those containing particles larger than 0.1mm. Except for a number of clays, oxides, charcoal and pigments, the average particle size of most drugs and pharmaceuticals rarely falls below 1mm. Although most submicron inorganic excipients appear to behave like hydrophilic solids, most insoluble drugs and pharmaceutical excipients are usually soft, organic, essentially crystalline hydrophobic solids ranging in particle size from several microns to several hundred or more. Drug particle size is an important factor influencing product appearance, settling rates, drug solubility, in vivo absorption, resuspendability and overall stability of pharmaceutical suspensions. Insoluble drug particles are seldom uniform spheres or cubes, even after size reduction and classification. Wide distributions in particle size often lead to high-density suspensions. Systems with widely differing particle shapes (plates, needles, filaments and prisms) frequently produce low-density slurries. The growth over time of unprotected, slightly soluble drug solids and changes in their particle size distribution in suspension are a serious problem. Crystal growth of particles is usually attributed to one or more of the following mechanisms:
Oswalt ripening or the growth of large particles at the expense of smaller ones, because of a difference in solubility rates of different size particles. For example, the increase in the solubility rate of a 0.2-mm particle is 13%; for a 2-mm particle, it is 1%; and for particles above 20
mm, it is negligible. Crystal growth due to temperature fluctuations on storage is of minor
importance, unless suspensions are subjected to temperature variations of 20°C or more.
A polymorphic form may change to another more stable crystalline form; changes in crystal habit may be related to the degree of solvation or hydration. Crystal growth may also arise when the more energetic amorphous or glassy forms of a drug exhibit significantly higher initial solubility in water than the corresponding crystalline forms.
Size reduction by crushing and grinding can produce particles whose different surfaces exhibit high and low solubility rates. This effect can be related to differences in the free surface energy introduced during comminution (grinding). Crystal growth and changes in particle size distribution can generally be controlled by employing one or several of the following procedures and techniques:
1. Selection of particles with a narrow range of particle sizes, such as microcrystals between 1 and 10mm.
2. Selection of a stable crystalline drug form that usually exhibits lower solubility in water. The crystalline form that is physically most stable usually has the highest melting point.
3. High-energy milling should not be used during particle size reduction. Microcrystals are best formed by controlled precipitation techniques or shock cooling.
4. A water-dispersible surfactant wetting agent dissipates the free surface energy of particles by reducing the interfacial tension between the solid and the suspending vehicle.
5. A protective colloid, such as gelatin, gum or a cellulosic derivative, is used to form a film barrier around the particles, inhibiting dissolution and subsequent crystal growth.
6. The viscosity of the suspending vehicle is increased to retard particle dissolution and subsequent crystal growth.
7. Temperature extremes during product storage (freeze–thaw conditioning) must not occur.
8. Supersaturation favours the formation of needle-like crystals and should be avoided.
9. Rapid or shock cooling and high agitation favour the formation of thin, small crystals and should be avoided. Slow crystallization by evaporation yields compact crystals.
10. Experimentation with different crystallizing solvents is recommended to change crystal size and shape.
11. Impurities and foreign substances during crystallization affect the reproducibility and aggregation potential of many drug particle systems.
12. Constant crystallizing conditions are essential. Batch-to-batch variation in crystal size and shape is often associated with poor control of processing and crystallization procedures.
Variations in assay results can be avoided by the preparation of homogeneous, well-mixed or nonsettling fine particle suspensions (size 1–10mm). Particle size reduction results in slow, more uniform settling rates.
The bioavailability of drugs is improved by reducing the size of suspension particles. Furthermore, drug particles smaller than 20mm produce less pain and tissue irritation when injected parenterally. However, fine particles may have a deleterious effect on chemical stability because of their high dissolution rate.
Rheology of Suspensions
Dilute suspensions have higher viscosity values than the pure medium and follow the Newtonian pattern of flow slightly modified by the Einstein’s equation; on the other hand, concentrated suspensions even without any bodying agent or viscosity-increasing substance, show non-Newtonian character of flow, mostly of the plastic nature. Further, if suspended particles interact to form flocs, the suspension acquires thixotropic quality. Evidently, therefore, flow of such systems cannot be expressed in terms of one-point viscosity or Newtonian viscosity.
Furthermore, when the suspending agents are added, the system becomes complex and pseudoplastic with or without thixotropy.
The study of rheology of suspensions has been helpful in their formulation. Notable areas where rheology plays a significant role in pharmaceutical formulations, particularly with reference to suspensions, are (1) mixing, (2) particle size reduction of dispersed phase, (3) passage through orifices including filling in or pouring out of containers and passage through hypodermic needles and (4) physical and chemical stability of disperse systems.
Thixotropy and yield value are intimately related to suspension stability. These provide sufficient consistency to the product when at rest, and thus prevent its physical and chemical deterioration. While the product is at rest, the only shear that is acting is due to the inherent tendency of the suspended particles to fall and since the stress caused hardly ever exceeds the yield value of the system, its mobility is highly restricted. This explains the physical stability of the system. The restricted diffusion of the drug particles minimizes the chances of any chemical reaction and thereby contributes to the chemical stability of suspensions. But when the preparation is shaken and poured out of its container, the yield value is far exceeded, shear thinning takes place, and it becomes easily pourable or usable. Due to thixotropy the building up process is slower, thus providing enough time for use either orally or topically or parenterally. When the container is set at rest, the system again assumes its structure and becomes almost immobile to maintain its physical and chemical character intact. Hence, only one-point viscosity determination is not sufficient, but the study of total rheology of the suspension is essential for making a good choice of a suitable system.
With regard to caking, it has been observed that plastic and pseudoplastic materials with thixotropy possess the property of preventing or minimizing this phenomenon. However, in such a system, if caking once starts, it is more accelerated than retarded, and cakes formed in rheological systems are much more formidable than those formed otherwise. Based upon the above considerations, it has been possible to make permanent suspensions founded in rheological parameters like yield value and thixotropy. Attempts have been made to make stable suspensions by the use of a combination of both plastic
and pseudoplastic materials for giving body to the system. By doing so, the suspension system enjoys both the advantages: (1) of remaining pourable on the basis of its pseudoplasticity and (2) of producing a permanent suspension on the basis of yield value and thixotropy of the plastic material. For this purpose, pseudoplastic materials like tragacanth, sodium alginate and sodium carboxymethylcellulose could well be combined with advantage with plastic substances like veegum and bentonite.
Several workers have suggested that dispersed-phase particles could be kept in suspension more permanently if a vehicle, having molecular aggregates formed into a cell-like structure, were to be used. Such vehicles have been called structured vehicle. Carbopol 934, a carboxyvinyl polymer, has been shown to provide such a structured vehicle in aqueous solutions, having plastic flow with high yield value. The cell-like structure can keep the dispersed particles or their floccules entrapped and thus bring about their permanent suspension in the medium.
Designing, Preparation and Stabilization of Pharmaceutical Suspensions
From the above discussions, it must have become clear that the present-day designing of pharmaceutical suspensions is not merely mixing a drug powder with a gum powder or the so-called suspending agent and incorporating the mixture in a vehicle, but is a well-designed technique used to produce a stable and acceptable suspension. Such technique is now mainly based on three-pronged attack on the problem, viz. (1) wetting with or dispersion into vehicle of finely powdered drug in uniformly sized particles, (2) controlled flocculation and (3) bodying or giving a structure to the system.
Dispersion
The first step in suspension making is the introduction of the solid particles into the fluid, wherein comes the question of their wettability and dispersion, i.e. whether the particles are lyophilic or lyophobic. In the former case, there is not much of difficulty but in the latter case, particularly if the particles are light and lyophobic, their wetting, meaning the replacement of the solid–air interface by the solid–liquid one, becomes difficult because such particles clump together and float on the surface of the liquid. Therefore, one has to look for a suitable surface active agent that can reduce the angle of contact and make the particles wettable and dispersible. For this, the surface tension of the liquid has also to be reduced to the critical surface tension value or below, because above this value the liquid would fail to spread on the solid surface. For selecting out the best surfactant for the particular powder, the latter is placed at one end of a long trough, coated inside with paraffin, while at the other end of the trough a solution of the best surfactant is poured, and the rate of penetration of the solution is noted and compared with data obtained with other surfactants. The comparative data, so obtained, help in choosing the best surfactant to act as dispersant or wetting agent. Sometimes wettability of lyophobic powders may be increased by passing them through a colloid mill in the presence of a wetting agent. Alcohol, glycerin and other hygroscopic liquids may be used in the beginning for the displacement of air from the surface of the particles and thus makes them more dispersible.
In the act of dispersion, due to the presence of a surfactant, the zeta potential of the particles is enhanced, which becomes usually deflocculated leading to the formation of hard, indispersible sediment known as cake.
Flocculation
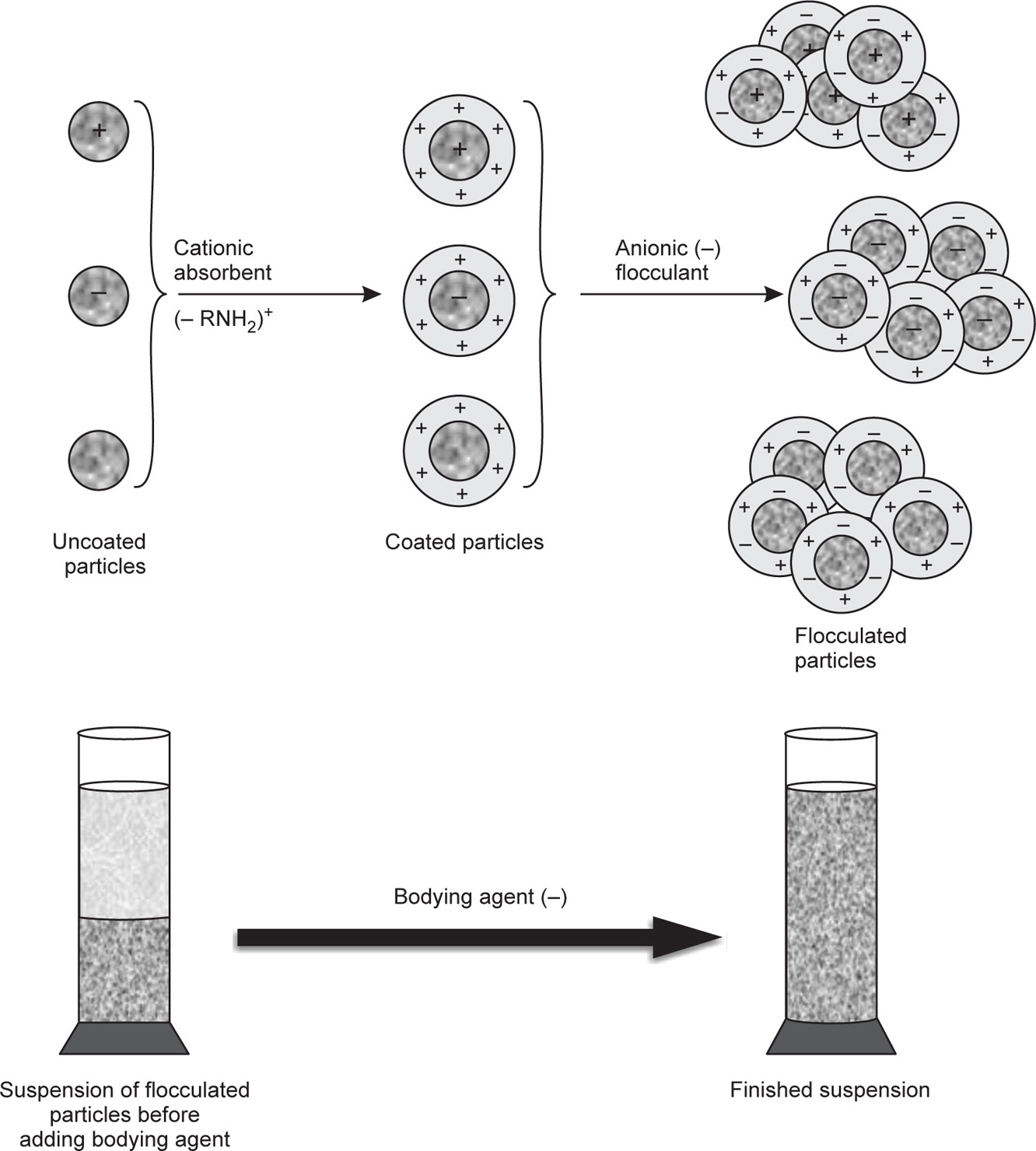
Partial or controlled flocculation has been possible in case of positively charged particles like those present in bismuth subnitrate suspensions by adding a very small quantity of an anionic flocculating agent like monobasic potassium phosphate. The phosphate ions deposit on the positively charged surface of bismuth subnitrate particles, bring about controlled reduction of their zeta potential and thus cause their partial flocculation. In the same way, when aluminium chloride is added to a sulphonamide suspension of negatively charged particles, the Al
3+ cations deposit partially on the surface, slightly reduce the zeta potential, and bring about partial flocculation. In the latter case when the flocculant is a cationic one, difficulty arises in the choice of the bodying agent or the protective colloid, because most of them are polyelectrolytes of anionic character. This causes incompatibility by causing the precipitation of the protective colloid. In the eventuality of the primary particles being negatively charged, they have first to be given a coating of positively charged ions and then flocculated by means of an anionic flocculant and thus avoid the incompatibility with the negatively charged protective colloid. Such positive coating has been possible by precipitating sodium sulphathiazole in acid solution in presence of gelatin. The product, known as
microform sulphathiazole, when suspended yields positively charged particles, which can be easily processed without any difficulty of incompatibility with the bodying agent. Another method of obtaining the primary dispersed particles with positive charge on their surface is to coat them with some fatty acid amines or quaternary amines and then flocculate them with an anionic flocculating agent. Finally, the system is given a body or structure by means of one or more protective colloids. This technique of first coating the primary suspension particles with positively charged substance followed by flocculation with anionic flocculant and finally stabilizing them with negatively charged polyelectrolytes is illustrated in
Fig. 20.14.
So far, suspensions have been made by using of electrolytes like potassium phosphate and aluminum chloride for controlled flocculation of suspension particles. These agents, besides lowering the zeta potential of the particles, have also been known to form chemical bonds between themselves and the suspension particles, which account for their flocculating effect by forming bridges between particles. Besides electrolytes, some anionic and nonionic detergents such as polyoxyethylated nonyphenols have also been used as flocculants.
Lyophilic polymers commonly used as suspending agents also manifest the property of causing flocculation of suspension particles. The polymers known as polyelectrolytes have active groups spaced along their entire chain. Part of the long-chain molecules get adsorbed on the particles leaving the extended portion projected from the particles surfaces. The protruding ends may get adsorbed on another particle, bridging the two into floccules, or it may do so on the same particles forming a loop. The latter may offer an avenue for another polymer chain adsorbed on other particle to get interwined resulting in the formation of particle flocs. The hydrocolloids that are polyelectrolytes possess some definite optimum for their flocculating and protective action. Therefore, the reaction of the suspension medium becomes an important factor in the formulation.
Bodying or Structuring of Suspensions
The final stage in the fabrication of suspension is of giving higher viscosity or body or structure to the system. This is necessary to support the dispersed phase and prevent it from quick sedimentation. As shown in the Stokes’ equation, the rate of sedimentation is inversely proportional to the viscosity of the system, and hence, by raising the viscosity, the sedimentation rate is lowered or minimized. During discussion of rheology of suspensions, it was shown that the function of natural or synthetic gums and polymers was not that of simply raising the viscosity of the system but also to give the desired rheological character to the product, for which the total product rheology had to be taken into account. In addition to these functions, such substances build up strong mechanical barrier or envelop
around the particles, which prevents them from agglomerating or forming cakes.
It has been stated earlier that a flocculated or partially flocculated suspension is better than a completely deflocculated one from the point of view of its noncaking or anticaking tendency. But this is also true that such suspension settles down comparatively more rapidly leaving a clear supernatant liquid at the top, which gives an unsightly or unacceptable appearance to the product. This defect is compensated by the use of bodying agent or hydrocolloids like carboxymethylcellulose, tragacanth, carrageen (chondrus, Irish moss), sodium alginate, carbopol, bentonite, veegum, etc. These substances, while supporting the flocs in the suspension, bestow desirable rheological properties to the product.
In making the suspension, it is necessary to aim at producing a uniform product of fine, well-dispersed floccules and having easy pourability, i.e. any possible sediment can be dispersed by simple inversion of the container or mild agitation. A suspension having a very high consistency cannot yield uniform doses, does not pour well from the container and leaves a film in the mouth causing an unpleasant after-feeling or taste. Therefore, it is best to have a partial flocculation of the system blended with the use of a small quantity of carefully chosen bodying agent.
In addition to the insoluble active ingredient or ingredients and the suspension stabilizers, viz. dispersants, surfactants, flocculants and protective colloids, the present-day suspension formulation also includes colouring and flavouring substances, preservatives and in case of oral ones, sweeteners also.
Various suspension stabilizers are effective only within a certain pH range and hence the adjustment of pH by use of buffers to obtain the optimum effect is necessary for all suspensions. Further, the viscosity and keeping quality are markedly influenced by pH variation.
The following outline of a scheme for the performance check of suspension will show the modern trends in suspension technology:
1. Shelf stability
Gross physical factors
Drug potency
2. Pharmacological evaluation
Toxicity
Therapeutic value (blood levels, etc.)
3. Patient acceptance
Oral suspension by taste panel
Parenteral (IM) suspensions (pain on injection and its persistence thereafter)
4. Clinical reports
Increasing use of physical data is being made in fabrication of suspension formulations, predicting the drug stability and correlation of the physical characterization of the formulation with its therapeutic usefulness. Such data may include (1) extent of purity of drug, (2) its solubility in water and other clinically usable solvents, (3) influence of pH on solubility and (4) its general therapeutic pattern in the temperature range of 5–75°C.