CHAPTER 46
Sensory Reeducation
BIRGITTA ROSÉN OT, PhD AND GÖRAN LUNDBORG, MD, PHD
CRITICAL POINTS
▪ After nerve injury the cortical representation of the hand becomes disorganized, diminishes, or may disappear, a fact that may seriously jeopardize hand function.
▪ The brain is much more plastic than was previously believed, possessing a large capacity for cortical functional reorganization even at the adult stage.
▪ The goal of sensory reeducation is to find ways to maintain or restore cortical hand representation after nerve injury and repair.
▪ Following repair of major nerve trunks, there is initially a period (phase 1) lasting for several months when no regenerating fibers have reached the senseless hand, followed by phase 2 representing reinnervation of the hand.
▪ It is our belief that sensory reeducation should start immediately after nerve repair (phase 1) to preserve the cortical hand representation.
Repair of injured nerve trunks in the upper extremity of adults usually results in incomplete recovery of sensory function in the hand. One explanation for this is that after nerve injury the cortical representation of the hand becomes disorganized, diminishes, or may disappear, a fact that may seriously jeopardize hand function.1-4 It is as if the hand “speaks a new language to the brain.” The purpose of sensory reeducation is to facilitate acquisition of the “new language” and to enhance recovery of sensory function in the hand.
It was long believed that the cortical body map was firmly established in the adult brain, but according to evolving concepts the brain is much more plastic than was previously believed, possessing a large capacity for cortical functional reorganization even at the adult stage. Rapid reorganizations occur as a result of changes in activity and sensory inflow. For example, a decrease of the cortical representation of a body part is seen as a result of anesthesia, amputation, and nerve injury. In addition, the brain possesses a cross-modal and multimodal capacity, implying that one sense can substitute for another.5-9
A major goal is thus to find ways to maintain or restore the cortical hand representation in such situations. Not only may sensory reeducation be of importance after a nerve reconstruction, it may be equally important in situations with only a slight change in the somatosensory cortex to maintain or restore the cortical somatosensory patterns so as to facilitate the sensorimotor neural networking.
Following repair of major nerve trunks, there is initially a period (phase 1) lasting for several months when no regenerating fibers have reached the senseless hand, followed by phase 2, representing reinnervation of the hand.10 Each of these phases requires a specific treatment strategy, and it is our belief that sensory reeducation should start immediately after nerve repair to preserve the cortical hand representation.
The Sensational Hand—Conceptual Framework
The richness in specific tactile information from the hand, combined with the flexible processing of the brain, has made the human hand a sophisticated instrument with an enormous capacity to perceive, to execute, and to express—simultaneously—in the explorative act of touch.11,12 A functioning sense of touch creates a base for the use of the hands, and such touch is a vital part of activity—activity that is a basic driving force in humans.
In Lettre sur les aveugles (“Letters on the Blind”), from 1749, the French philosopher Diderot discusses the role of learning in the development of normal perception, for example, that “touch gains in power owing to its use.”13 That is briefly what sensory reeducation and sensory relearning is about—“Use it or lose it”—and sensory reeducation is indeed a challenge in the efforts to improve a person’s function after a nerve injury.
Jean Ayres described in her sensory integration theory the dynamic relationship between behavior and neural processing of input from the senses.14 The brain must organize input from many sources into meaningful patterns that can be utilized for interaction with the environment and participation in daily-life activities.14 Such a continuous cross-modal and multimodal networking in the brain5,15 can be used in sensory relearning. Let other senses help when touch sensibility is weak. For example, touching a fruit can systematically be associated with its taste, smell, and color, creating “a tactile meal.”
Four major modalities of somatic sensibility can be defined: touch, proprioception, pain, and temperature sense.16 A hierarchy of touch functions can also be identified: If detection of touch is present, the next level is discrimination of touch, basic tactile gnosis.18,19 Localization of touch is also an aspect of discriminative touch, and identification of objects, shapes, and textures with active touching is the third level—a more refined tactile gnosis.18,20
Functionality of sensation has been analyzed. An adequate feedback system for control of grip force by the integration of sensory and motor mechanisms and the proprioceptive elements has been investigated in depth,21,22 and this knowledge is essential for sensory feedback. The superiority of sensibility in a well-coordinated hand is also emphasized, whereby sensibility and memory are discussed as key factors that control our acquired motor programs.23 Direct recording techniques from the cortical surface of the brain, and modern brain imaging techniques such as positron emission tomography, magnetic resonance imaging (MRI), magnetic encephalography, and transcranial magnetic stimulation have also made it possible to explore issues of tactile processing in the brain, and brain plasticity.16,17
Napier described the hand as “an organ of touch which feels round corners and sees in the dark.”24 Sterling Bunnell, the father of hand surgery, described sensation as the “eyes” of the fingers, illustrated by Moberg with the classic seeing fingertips, meaning that a hand without sensibility is blind.
The expression functional sensibility is frequently used in hand surgery literature, as a basic function for what the hand can do (i.e., purposeful use of the hand).25 It is the subtle sensibility that gives the grip “sight,” not just to perceive but to understand the touch. Brand and Yancey also pointed out the important functional protective aspect of hand sensibility: “Pain the gift nobody wants.”26 Wynn-Parry and later Dellon and Curtis express this holistic view on the hand and hand sensibility, and the importance of not only motor reeducation but also sensory reeducation programs to improve poor results after nerve injuries.27,28
Moberg established the term tactile gnosis in his classic articles from 1958 and 1962 as the specific aspect of functional sensibility representing the interplay between peripheral function of the nerve and the interpretation of sensory impressions in the brain. Tactile gnosis enables recognition of qualities and the character of objects without using vision.25,29,30 Tactile gnosis capacity is specifically addressed in sensory reeducation programs.
The hand is sometimes described as a sense organ strongly linked to the brain and to the personality.6 This approach has in recent years also gained importance in discussions of surgical techniques to improve poor results after nerve lesions.31 The emphasis in these discussions is not only on the importance of advanced surgical techniques but also on the rapidly expanding knowledge in neurobiology with several tools to influence injured neurons and to use the inherent plasticity of the neurons in further development of sensory reeducation.
Factors Influencing Outcome after a Nerve Injury and Sensory Reeducation
Nerve injuries may seriously interfere with an individual’s capacity to function adequately, and the acquired disability is often dramatic. A hand with limited sensibility is usually a hand with very limited function. There is a high probability of lost work capacity and impaired quality of life for the patient.32,33 There is often lifelong hand function impairment, pain, dysesthesia, allodynia, and cold intolerance.34 There is also a substantial economic impact of nerve injuries on the patient as well as on society.35
Age
Although the outcome from nerve repair is disappointing in adults, it is well-known that children usually achieve superior functional results without any formal sensory reeducation.36 The shorter regeneration distance in children and a better regeneration capacity in general contribute to these good outcomes. However, the superior ability of the cortex’s central adaptation to the new pattern of afferent impulses presented by misdirected axons also provides an explanation for superior recovery in children. There seems to be a critical age period for recovery of functional hand sensibility, with the best results being seen in those younger than the age of 10 years, followed by rapid decline leveling out after late adolescence. Interestingly, there is a striking analogy between this pattern and the pattern illustrating the scores of immigrants on a grammar test, plotted against the age at which they start to learn a new language.37 Thus, the critical period for regaining discriminative tactile capacity after nerve repair is analogous to a corresponding critical period for acquisition of a second language, indicating a strong learning component in acquisition of functional sensibility as well.
Timing of Repair, Type of Nerve, Level and Type of Injury
It is agreed that freshly transected nerves should be repaired acutely with no or minimal delay.38,39 Early repair will substantially reduce the postoperative nerve cell death.40
The type of nerve that is injured considerably influences the outcome. If a pure motor nerve is injured, the risk for mismatch between motor axons and sensory axons is eliminated, thus optimizing the accuracy in reinnervation. For pure sensory nerves such as a digital nerve, the situation is analogous.
After nerve transection there is an initial delay followed by sprouting and axonal outgrowth. A nerve outgrowth rate of 1 to 2 mm per day in humans has been suggested. In digital nerve injuries there is only a short distance separating the regenerating axons from their distal targets, while injuries at the upper arm level create different situations with longer time interval to regeneration of the hand. Nerve lesions at wrist level may require 3 to 4 months before the first signs of reinnervation in the hand occur.
A crush or compression lesion always results in better functional outcome than does total severance of a nerve trunk. The initial delay is shorter and the growth of axons proceeds at a faster rate after a crush injury than after a nerve transection. The Schwann cell basal lamina are still in continuity and can thus guide the axons back to their original peripheral targets. The correct peripheral reinnervation of crush injuries is reflected in a perfect restoration of the original cortical representational areas corresponding to the reinnervated body part.3,41
Central Nervous System Factors—Cognitive Capacity
The surgeon’s ambition is to use repair techniques that bring a maximal number of nerve fibers into correct peripheral cutaneous areas. However, there are at least three strong indications that central nervous system factors associated with cortical remodeling represent a major reason for the inferior functional outcome following nerve repair. First, children up to the age of 10 to 12 years usually present excellent recovery of functional sensibility in contrast to adult patients. This critical “age window” for perfect sensory recovery presented by children corresponds well with what is known from other types of learning processes, for instance, the ability to acquire a second language.37 Second, cognitive functions are important explanatory factors in adults for variations in recovery of tactile discrimination.42 Third, the peripheral repair technique in nerve lesions has not been found to influence the functional outcome in a clinical randomized study at a 5-year follow-up.43 Silicone tubular repair, leaving a short distance between the nerve cuts, was compared with the outcome from routine microsurgical repair in a clinical randomized prospective study. The study included 30 patients with median or ulnar nerve injuries in the distal forearm. Postoperatively, the patients were assessed regularly over a 5-year period with neurophysiologic and clinical assessments. After 5 years there was no significant difference in outcome between the two techniques except that cold intolerance was significantly less severe with the tubular technique. The most significant improvement of perception of touch occurred during the first postoperative year, while improvement of motor function could be observed much later.44 In the total group there was however an ongoing improvement of functional sensibility throughout the 5 years after repair, although there was no further impairment in nerve conduction velocity or amplitude after the first 2 years.41 This supports the thesis that central nervous system factors associated with the cortical remodeling after a nerve repair are important, and that efforts to improve the results following nerve repair in the future must address the brain as well as the peripheral nerve.
In addition to the large number of peripheral and central factors, active and conscious use of the hand in activities of daily life, combined with high motivation by the patient, has long been reported to be of great importance for useful return of functional sensibility.45 Bruyns et al. found that intensive education, high compliance to hand therapy, and an isolated injury predict quicker return to work in patients with median and/or ulnar nerve injuries.32 A recent meta-analysis showed that age, site, injured nerve, and delayed repair significantly influence the prognosis after nerve repair.46 Early psychological stress has also been found to influence the outcome in a negative direction.47
Cortical Remodeling—Response to Sensory Input
The human hand and the brain have developed into a sophisticated functional unit,48 and the hand is largely represented in the somatosensory cortex of the brain with arrangements of sharply divided territories receiving impulses from specific areas of the hand. This is reflected in the mapping of the body revealing great variation in tactile discrimination. The fingertips, lips, and the tongue, which occupy large areas, exhibit the highest resolution.49 However, the plasticity of the brain enables changes in the territories when the prerequisites and demands for sensory input are changed. This is a functional reorganization that is described by several authors.2-4,50-53
According to evolving concepts over the last decades, the brain is much more plastic than was formerly believed, possessing a substantial capacity for cortical functional reorganizations also at the adult stage.4,54 In primate experiments using techniques for direct recording from the brain cortex,55,56 strong evidence has been presented that there is a capacity for rapid cortical reorganization in the somatosensory cortex of adult primates that may occur for several reasons, such as changed sensory experience and performance of the hand, overuse, amputation, and local anesthesia. The brain can be sculpted by experience, and this dynamic is true for the entire lifetime. The brain’s capacity for remodeling is what makes sensory reeducation and sensory relearning possible. In various neuropathies, focal hand dystonia, and during immobilization, a disorganized somatosensory cortex may be one important reason for problems with hand function.
Experience-Dependent Cortical Remodeling
Effects of Increased Sensory Input
Direct recordings from the somatosensory cortex in monkeys demonstrated experience-induced cortical remodeling secondary to increased tactile stimulation of separate fingers.1,3 Simultaneous tactile stimulation of nearby separate receptive fields of the adult rat paw for a few hours also induces a selective enlargement of the cortical area representing the stimulated skin fields.57 This phenomenon has been demonstrated in experimental studies involving human subjects.58-60 Continuous co-activation of separate receptive fields in a fingertip for 2 to 3 hours results in an expansion of the fingertip cortical representation in the S1 area, a phenomenon that is linked to a significant improvement of two-point discrimination.58-60
This is interesting from a functional point of view. Tasks requiring increased discriminatory skill relate to an expansion of the cortical projection area corresponding to the fingers involved in the task. You can see the same phenomenon in fingertips subjected to long-term massive tactile stimulation. Patients with blindness using their index fingers for reading in Braille demonstrate an expansion of the finger representation.61 The string hand of violin players occupies enlarged projection areas in the somatosensory as well as motor cortex of the brain.51,62 These changes in representation have been demonstrated in other groups of professional musicians in both the somatosensory and auditory domains.63
When Increased Sensory Input Becomes a Problem
Stereotypic and repetitive fine motor movements can degrade the sensory representation of the hand in the somatosensory cortex and lead to a loss of normal motor control.64,65 This can be observed in musicians suffering from overuse syndromes such as functional dystonia (i.e., the inability to control and regulate individual finger movements). In such situations the cortical hand map is distorted and remodeled into a disorganized pattern.66-68 The physiologic basis is probably repetitive monotonous tactile stimulation and use of the hand over extended time periods. In monkeys, trained to perform monotonous repetitive hand movements involving simultaneous tactile stimulation of various parts of the hand, fusion of the normally well-separated cortical projection sites of individual fingers has been seen.69 The cortical “hand-glove” becomes a mitten. Reversal of the reorganization changes by use of specific training programs including very specific sensory discriminative tasks have shown good results in dystonia treatment.66,70,71 Some of this information is available to our readers in Chapter 135.
Effects of Decreased Sensory Input
Diminished or complete arrest of tactile input may result in degradation of cortical representations.9,72 Hands in persons with cerebral palsy that are contracted into severe flexion postures, devoid of sensory experiences, are associated with a diminished sensory capacity. Surgical procedures to open the hand allow for the possibility of new tactile experiences to wake up such sensibility in disguise.73 If the lower extremity is immobilized, its representation in the motor cortex decreases, a phenomenon that is reversible with regained mobility.74 Sensory reeducation may be helpful in this situation of prolonged immobilization.
Nerve Injury and Repair
After a major nerve injury there is a cortical response with an instant and long-standing reorganization of the somatosensory brain cortex. The silent area without sensory input triggers an expansion and invasion from adjacent cortical areas.3,9,75,76 This is the beginning of a dynamic interplay in the cortical neural networks, which is influenced by several biologic and psychological events during regeneration and reinnervation. These changes, which happen within minutes, are probably based on unmasking of normally occurring, but inhibited synaptic connections. There are reasons to believe that such connections are susceptible to further changes. During this postinjury period before regeneration occurs, the hand is without sensation and there is no sensory input from the areas normally innervated by the injured nerve. In the following sections this period will be named phase 1.
The microsurgical nerve repair techniques have been refined to an optimal level; however, there is still a significant disorientation of regenerating axons at the repair site. Therefore, the skin areas of the hand will, to a large extent, not be reinnervated by their original axons. The result is additional new changes in the cortical territory where the nerve is normally represented. The original well-organized hand representation is changed to a distorted pattern75 (Fig. 46-1). The nerve does not recapture all of its original territory. The former specific cortical representation of separate fingers disappears and changes into an overlapping pattern between the fingers.77 This knowledge is based primarily on primate experiments, but analogous findings have been made also in humans on the basis of functional MRI techniques.78 The specific cortical territories representing each finger in primate experiments have shown a completely changed pattern. The hand speaks a new distorted language to the brain, requiring a relearning process. This is referred to as phase 2, representing reinnervation of the hand. After a nerve injury at the wrist level, phase 2 usually begins 3 to 4 months after nerve repair. The timing for onset of sensory reeducation may be of critical importance. Each of the two phases following a nerve repair requires a specific treatment strategy, and we suggest that sensory reeducation should start immediately after nerve repair to preserve the cortical hand representation.
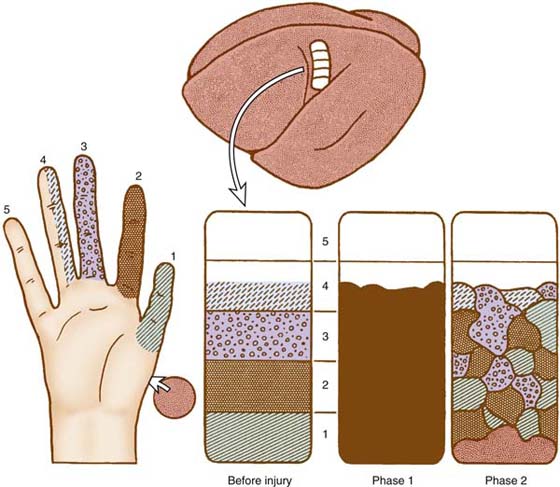
Figure 46-1 A schematic illustration of how the “hand map” in the brain changes after repair of the median nerve. Before injury each finger has its specific area; in phase 1 this area becomes “silent” and becomes occupied by adjacent areas. In phase 2, when the new axons have grown to the skin and to the muscles, the map is again changed, since the axons do not grow in exactly the same paths as before the injury. The map becomes unstructured and is difficult to interpret.
In summary, there are good reasons to look for factors in the central nervous system, in addition to the cellular and biochemical events, that are associated with degeneration and regeneration in the peripheral nervous system to explain the incomplete sensory recovery after nerve repair. A nerve injury in the upper extremity is followed by profound functional reorganization changes in the brain cortex, mainly due to misdirection of regenerating axons. These central events, which are an expression of the brain’s capacity for rapid plasticity, play a predominant role in the sensory reeducation programs of today. This is a challenging and important area for multidisciplinary clinical development and research in the field of rehabilitation following nerve injuries.
Sensory Reeducation—Principles and Planning
The practice of sensory reeducation has long existed in rehabilitation settings along with motor reeducation but was not recognized as such until Wynn-Parry and Dellon designed the first formal programs.27,28 The functional improvement seen after training may be based on normalization of the distorted hand map, or it may be due to adaptations in higher brain centers with a capacity to decipher the distorted hand map. Further study is needed to determine which explanation is correct.
Learning is a key word in the rehabilitation process after all injuries. New sensory and motor codes are presented to the brain with which the brain must cope for purposeful sensorimotor interaction and functional use. A relearning process is required to adapt to the new and distorted afferent sensory input when familiar objects are touched. The mind does not understand the new “sensory code” associated with specific textures and shapes. To facilitate and enhance this process, specific programs for sensory reeducation are routinely used for regaining tactile gnosis. According to these strategies, the brain is reprogrammed on the basis of a relearning process. Sensory reeducation is based on vision guiding touch and on higher cortical functions such as attention and memory during several daily short practice sessions occurring over several weeks or months.24,27 With active use of the hand, the patient learns to code the integrated passive and active afferent stimuli with slower and fewer conducting nerve fibers than normal.28,79
The sensory reeducation training is integrated in the overall rehabilitation program and individualized based on the patient’s level of sensory function. As mentioned previously, following repair of major nerve trunks there is initially a period (phase 1) lasting for several months when no regenerating fibers have reached the senseless hand, followed by phase 2 representing reinnervation of the hand.10 Each of these phases requires a specific treatment strategy.
In phase 1 there is no protective sensibility, and it is important to carefully watch the hand during use to prevent skin injuries. The lack of protective sensation is an important message to communicate to the patient in this early phase. In phase 2 the axons have reached the hand. Hypersensitivity to normal touch is common during this period. If so, desensitization exercises should precede the training sessions. Hyperesthesia and allodynia and its treatment are described elsewhere in Chapter 113, Chapter 114, Chapter 115 and Chapter 116.
Specific, simple, and repetitive sensory relearning exercises of increasing complexity and difficulty should be performed at home by the patient on a daily basis in frequent brief training sessions. Weekly training sessions with the therapist may be scheduled to provide guidance and feedback. Training in a quiet location for high attention is recommended, and active and conscious use of the hand in daily activities combined with high motivation by the patient are important factors. The rehabilitation after a nerve repair is a long process that can take several years, so it takes a lot of patience. The complexity after a nerve injury with interacting phenomena that depend on so many factors is not easy for the patient to understand. Therefore, the information and education of the patient about the injury and the sensory reeducation concept are crucial. If the patient does not understand the training concept, it is very difficult for him or her to be motivated to follow through with the retraining. A written home program should always be given to each patient. The patient should also be encouraged to use the affected hand very consciously in daily activities.
Timing—Onset of Sensory Reeducation
In the sensory reeducation program proposed in this chapter, the training technique is the classical one according to Wynn-Parry and Dellon,27,79 but focus is on the timing of initiation of the training program not only in phase 2 but also in phase 1. The strategy is to activate the cortical area representing the damaged nerve, thereby diminishing the cortical reorganization and maintaining the cortical hand map. Borsook et al.52 demonstrated that following amputation of the hand, touch of the face, being close to the hand in the cortical body map,49 gives rise to phantom sensations as soon as 24 hours after injury. Weiss et al. demonstrated plasticity after finger amputation within 10 days after amputation.80 An analogous phenomenon can also be seen after local anesthetic blocks that temporarily can induce shifts in neuronal receptive fields with cortical reorganization.81
The traditional procedure calls for the introduction of sensory reeducation once touch can be perceived in the involved area.27,79 However, at this point the reorganizing brain presents a random pattern, which may not be possible to reverse. Cheng et al. presented a prospective randomized study on early tactile stimulation after digital nerve repair (3 weeks after surgery) that showed excellent results and significantly better discriminative sensibility in the study group.82 If the “vacant” cortical area of a denervated hand could be provided relevant information from the hand using visual clues early after repair, it might help to minimize the synaptic reorganization. This may well make the brain better prepared for the relearning program once the nerve has regenerated and reinnervated the peripheral targets.
The design and protocols of the sensory reeducational programs have not changed over the last decades. This is surprising, considering the enormous advances in neuroscience and brain imaging techniques that have increased our understanding of mechanisms underpinning brain plasticity. We therefore have presented new strategies for sensory reeducation that utilize the capacity for cross-modal and multimodal capacity of the adult brain as well as the remodeling capacity of the brain.
Sensory Reeducation in Phase 1—Sensory Preparation
In phase 1 we focus on maintaining the cortical hand representation by using the brain’s capacity for sensory imagery as well as cortical visuo-tactile interaction. Phase 1 lasts until there is measurable sensibility present in the hand that can be assessed with Semmes–Weinstein monofilaments.83 To be able to start sensory reeducation at this critical early stage without any existing sensibility in the hand, we use the holistic organization of the brain with an extensive capacity for cross- and multimodality. The sensory relearning in this early phase, in combination with mobility training of the hand, is aimed at activating and maintaining the hand map in the brain to make the sensory relearning easier once the axons have regrown. This gives the brain an illusion of sensibility in the hand.
The use of vision to guide the retraining of sensation is the basis for classic sensory reeducation, but there is a continuous interplay among all senses. Multisensory neurons that receive more than one type of sensory input may be used to extract information from one sensory modality and use it in another by using polymodal association areas.5,15,84
The multimodal capacity of the brain and brain’s capacity for adaptation with deprivation of sense can be illustrated in individuals with blindness. When such a person reads Braille or carries out other tactile discrimination tasks, the primary visual cortex is activated together with the somatosensory cortex.85
Sensory Imagery
Similarities exist in the cortical functions between perception and imagery.86 Auditory cortical areas are recruited in the absence of sound during imagining music,86 the visual cortex is active during visual imagery,87 and imagination of odors is associated with increased activation in olfactory regions in the brain.88 Just imagining a movement activates the premotor cortex.89 The pattern of somatosensory activation during motor imagery is very similar to the pattern observed during a real movement execution.90 Few observations of sensory imagery with involvement of primary sensory cortical areas have been reported. Just thinking about stroking the dorsum of the hand activates the sensory cortex.91 It is recommended that the patient can try to imagine touch in the hand during phase 1.
Observation of Touch, Reading or Listening to “Sensory” Words, Observing “Sensory Pictures,” Mirror Training
Activation of motor neurons that may also serve as mirror neurons in the premotor cortex by the observation of hand activity is a well-known phenomenon, which is believed to play a fundamental role in both action and imitation.92 Mirror neuron areas are also involved in understanding the intention of actions.93 Reading or listening to action words related to hand movements activates hand representational areas in the motor cortex,94 and hypothetically reading or listening to “sensory” words or watching “sensory” pictures would relate to activity in the somatosensory cortex. Other ways to activate the somatosensory cortex include observing a body part being touched. Keysers showed this during observation of touching the legs,95 and we have demonstrated a visuo-tactile cortical interaction during mere observation of tactile stimulation of the hand.96 It is suggested that the somatosensory cortical areas, SI and SII cortex, are related to the mirror neuron system.91,97,98 The patient’s observation of his or her hand being touched is one component of early sensory training the first day after surgery, which might activate the cortical hand area due to visuo-tactile interaction (Fig. 46-2).
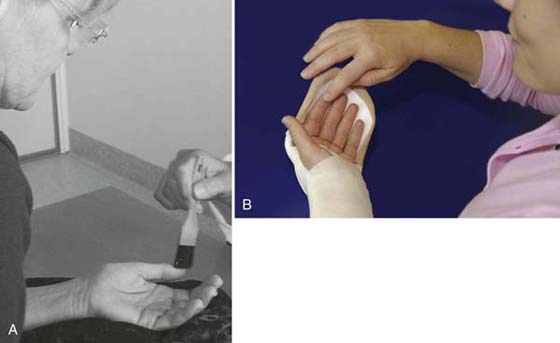
Figure 46-2 By touching the areas in the hand that have no sensibility in combination with concentrated watching, the hand map in the brain is activated. This is repeated several times per day. A, Someone else can touch the fingers without sensibility and corresponding fingers on the other hand simultaneously during careful watching. B, The patient can touch the fingers lacking sensibility using the corresponding finger area of the other hand, or someone else can do the stimulation.
The effect can hypothetically be further enhanced by using mirror training introduced by Ramachandran.99,100 A mirror is placed transversally in front of the patient with the nerve-injured hand hidden behind the mirror and the healthy hand being reflected in the position of the injured hand. Touching the healthy hand gives the illusion of touching the nerve-injured hand. In these training sessions, a clinical observation is that the patient often gets a perception of the tactile stimuli in the nerve-injured insensate hand by the combined mirror illusion and the true touch of the healthy hand. Exercises normally performed in phase 2, when there is sensibility in the hand, can be used (Fig. 46-3).

Figure 46-3 Mirror training positioned so the uninjured hand is reflected in the mirror looking like the injured hand. The injured hand is hidden behind the mirror. This creates an illusion that makes the brain think there is activity in the injured hand. A, Localization of touch; B, training with familiar objects.
Cortical Audio-Tactile Interaction
Another principle is to use another sense such as hearing to substitute for sensibility, thereby utilizing the brain’s multimodal capacity. This method allows early sensory relearning so as to feed the sensory cortex with “relevant” information.101 Cortical audio-tactile interaction has been recently demonstrated with functional MRI technique.102
In a prospective randomized multicenter study, significantly better tactile gnosis compared with controls was observed in persons with median or ulnar nerve repair.103 These observations were noted within a week after surgery with training in the Sensor Glove (Össur, Iceland) (www.ossur.com), which utilizes audio-tactile interaction.103 Microphones are mounted dorsally at fingertip levels or in a glove connected to earphones via a miniature stereo processor in the Sensor Glove system. With this system the patient can listen to what the hand feels. Auditory stimuli substitute for absent tactile stimuli. Specific and typical friction sounds are associated with touching of various textures. Exercises with identification of textures and localization were performed several times per day according to the training technique described above.103
Whatever method is chosen in phase 1 after the nerve repair, there are good reasons to start sensory relearning much earlier than we do today with the aim of minimizing or inhibiting the reorganization process in the somatosensory cortex induced by the nerve injury. The author’s experience is that the method of choice must be individualized. The patient must be comfortable with the exercises and must understand the purpose of such training in phase 1.
Sensory Reeducation in Phase 2
Techniques for Sensory Reeducation
Phase 2 starts when there is measurable sensibility in the palm (minimum Semmes–Weinstein monofilament no. 6.65, 300-g force). When there is some protective sensibility in the fingertips that can be localized correctly, discriminative exercises with identification of shapes, textures, and objects may be initiated. The classic training technique described by Wynn-Parry and Dellon is used. This technique is based on stimulation with varying and increasing difficulty with the eyes open and closed alternately. In this way, another sense (vision) assists the training and improves the deficient sense (sensation)27,79,104 (Fig. 46-4). Hyperesthesia/allodynia is common in the regeneration phase after a nerve injury, and in such cases desensitization exercises should precede sensory reeducation.
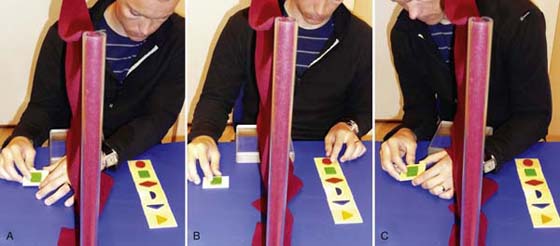
Figure 46-4 Combining visual and sensory information to teach the brain to understand the “new language” from the right hand. Examples of how the training can be performed with and without vision: A, Touch a hidden texture, shape, or object, and try to identify it. B, Touch a copy of the texture, shape, or object with the uninjured hand and the hidden object with the injured hand at the same time, and compare the feeling. C, Was it correct? If not, or if it was too difficult, touch and watch at the same time and then look in the other direction or close your eyes again.
Once the area with diminished sensibility has been established, the first modality to reeducate is the capacity to localize touch. Errors in localization of touch after a nerve reconstruction are part of the functional problem in identification of objects without vision. The points stimulated on the skin no longer match with their central projection, and the patient cannot interpret the modified sensation to a meaningful whole (Fig. 46-5). When localization has improved, touching and exploration of items—presenting various sizes, shapes, and textures—is introduced. A variety of tools and specifically designed products can be used for this (Fig. 46-6), but it is recommended also that real and familiar objects be used (Fig. 46-7). That makes it easier for the patient to incorporate the sensory relearning concept in daily activities, and that is of utmost importance. Exercises that incorporate both sensory and motor training are also highly recommended (Fig. 46-8).

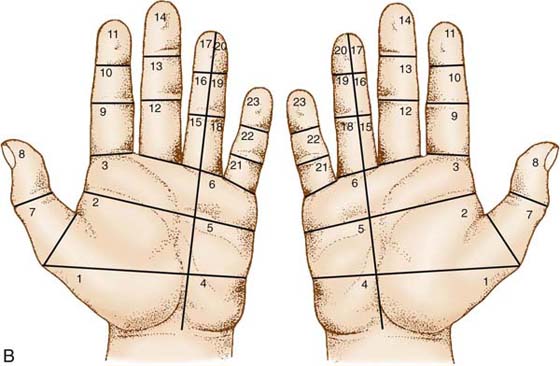
Figure 46-5 To localize touch, the area with diminished sensibility is identified. A, The patient is then instructed to touch the skin in one of the areas with a blunt object such as the end of a pen. The object can be pressed or moved hard enough to perceive the touch. The touch can also be compared to an area with normal sensibility. The patient should be instructed to concentrate on where, what, and how. Is it in the area where you touch or somewhere else? Is it static or moving touch? Does it feel different from the area with normal sensibility? The touch should be repeated several times, first with eyes open and then with eyes closed until the patient feels that he or she knows where the stimulus is. It is suggested to work on a few areas first until they can be localized correctly. Then, move to adjacent areas. It may also be useful to start to work with areas close to areas with normal sensibility. A good feedback is to get someone else to apply the stimulus to ensure that there is a sensory relearning. B, For this purpose the grid can be useful.
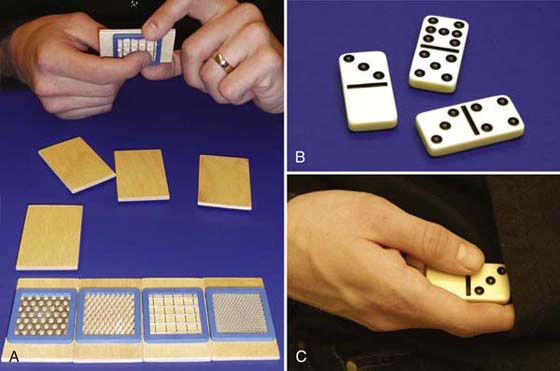
Figure 46-6 Combining identical textures (A) and domino brackets of various difficulties (B, C) for home training.

Figure 46-7 A, B, The patient should be encouraged to carry a few familiar objects in the pocket and try to identify them, and think about their shape, texture, weight, and which object is touched. The size of the objects is decided based on tactile gnosis assessment. If, for example, perception threshold in the fingertips is 4 g, and 15-mm objects cannot be identified correctly, the training objects must be simple and bigger.

Figure 46-8 Sensory and motor training with emphasis on coordination and identification. Here the patient with eyes closed holds identical keys in right and left (injured) hand, and tries to find a specific key with the left hand.
The first sensory reeducation program included timed identification of a series of familiar objects of increasing difficulty.79 In Dellon’s program the training is timed with the regeneration process and return of specific qualities of sensation. The selection of exercises is chosen based on neurophysiologic evidence of cutaneous sensibility. Training is performed depending on when the patient can perceive and identify moving and/or constant touch distally.27
Bilateral tactile stimulation, including the injured hand as well as the noninjured hand, might help to influence positively the central substrate for sensory relearning. Sensory input is normally processed to the greatest extent in the contralateral hemisphere, but there is also, to some extent, an ipsilateral activation.17,105 The whisker representational area in the somatosensory cortex of rats integrates information from both contralateral and ipsilateral whisker pads.106 In humans there is a transfer of improved tactile performance from a sensory-trained finger to the contralateral hand that has been demonstrated,107 and it has been shown that practice-related improvements in sensory discrimination can generate across skin location, hemisphere, and modality.108 So, there may be good reasons to use both hands in the training process. Use of additional ipsilateral pathways from the noninjured hand may provide correct tactile information to the hemisphere that is contralateral to the injured hand, thereby facilitating the learning process. A conscious sensory relearning in daily activities is very good to use in the bilateral training. Recovering patients should make it a rule to try to feel the structure and shape of everyday objects (Fig. 46-9).

Figure 46-9 A-E, It will help reeducate the sensibility if the patient makes it a rule to try to feel the structure and shape of everyday objects—preferably bilaterally.
Enhancing the Effects of Sensory Reeducation in Phase 2 Using the Rapid Plasticity of the Brain
It is reasonable to use the brain’s capacity for rapid redistribution of cortical resources as a component in the rehabilitation process. That has been well described in the literature in rehabilitation of patients following a stroke. For instance, it has been shown that selective anesthesia of ventral roots of C5–C8 (motor innervation of shoulder and elbow) results in enhanced motor function of the hand (innervation C8–C11).109 The mechanism is that deafferentation of a specific cortical area (shoulder and elbow representations) allows expansion of adjacent cortical representational areas (the hand). We have recently shown that selective cutaneous deafferentation of the forearm in healthy adults results in improved hand sensation and in expansion of the adjacent cortical hand representation.110 The rapid improvement in sensory functions that may occur within minutes after selective anesthesia is presumably due to unmasking of existing synapses that are normally inhibited. Long-lasting effects may be due to a long-term potentiation of synapses or even formation of new synaptic sites.111
So, in addition to traditional sensory reeducation in phase 2, we perform a selective temporary anesthesia of the forearm proximally to the nerve-injured hand during a limited period at a point when the patient has regained some protective sensibility at the fingertip level. This is established with regular follow-ups using a standardized test procedure.83 The method is used especially in cases with good reinnervation at fingertip level, but poor tactile gnosis.112 The purpose is to allow expansion of the cortical hand representation. Cutaneous anesthesia of the volar part of the forearm is induced by an anesthetic cream. The treatment is done under careful supervision at the clinic and after prior determination that the patient has no history of adverse reactions to local analgesics. The patient receives EMLA® (lidocaine plus prilocaine; AstraZeneca, Mississauga, ON, Canada), starting with frequent EMLA applications and then with a decreasing number of applications.112 The treatment is combined with an intense sensory reeducation program with assisted supervised training at the point when the “window” for training is wide open. A double-blind study showed that tactile gnosis (functional sensibility) improved significantly in patients with minimum Semmes–Weinstein monofilament no. 4.31 (1.4-g force) in the fingertips who had received this treatment in combination with intensive sensory reeducation compared with those who received intensive sensory reeducation with a placebo cream applied to their forearms. All patients improved their tactile gnosis, presumably as a result of the intensive sensory reeducation alone, but the EMLA® group was significantly better than the placebo group.112
The use of repeated selective temporary anesthesia in sensory training should hypothetically keep a larger cortical area available for the injured body part and, in turn, would increase the brain’s ability to interpret the signals from the territory of the injured nerve. This may open a window of opportunity during which it is possible for sensory reeducation to have a better and more long-lasting effect. The optimal frequency for application is yet to be determined in future studies. This is a new and original concept, which may enhance the effect of sensory reeducational programs.
How Is Brain Organization Influenced by Sensory Reeducation?
Little is known about the effects of sensory reeducation on brain organization. One possibility is that sensory training can reverse the disturbed cortical organization toward a normalized pattern. Another possibility is that the cortical hand map is permanently distorted and that the key is an improved processing and perceptual capacity in the sensory networks at a higher cortical level, facilitating interpretation of the distorted hand map. Although young individuals may have a greater capacity for normalization of the cortical hand map following nerve repair as compared with adults, such capacity for normalization of the cortical hand map by training probably does not exist in the mature brain. The topic was studied by Florence et al., investigating the effects of enriched environmental sensory experience on cortical organization on monkeys that had previously undergone nerve repair of the median nerve.113 One group was subjected to an enriched environmental sensory experience involving presentation of food treats on an artificial grass surface and toys while the control group was allowed only restricted mobility with no such enriched sensory experience. Using direct cortical recording techniques, it was demonstrated that the enriched sensory environment had a significant effect on receptor field size with smaller, normalized, and better located receptive fields likely to provide better resolution. In addition, the cortical hand maps were less distorted in the sensory-enriched animals compared with the sensory-restricted animals. Sensory relearning programs in humans, including an enriched environment, may thus hypothetically explain functional improvement after rehabilitation.
Environmental Influence on Sensory Reeducation
Several factors may influence the results from the sensory reeducational programs in a positive or negative way. For instance, active and conscious use of the hand in daily activities as well as attention during training combined with high motivation by the patient is extremely important. An enriched environment is fundamental to facilitate learning. It has been demonstrated that an enriched environment influences learning by stimulating formation of new synapses. Factors such as a stimulating environment, meaningful activities, and encouragement influence the molding of the brain in a positive direction.114-116 It has also been demonstrated that passive unattended and repetitive tasks lead to negative changes of the associated territories in the sensory cortex. These observations have been discussed especially as a genesis model for dystonia and repetitive-strain injury problems.2,65
Whether music stimulates learning is yet to be definitely demonstrated, but it is argued by several authors that music influences brain activity.63,117-119 It remains to be investigated if music can enhance sensory relearning and what kind of music would be best suited in such learning. The holistic organization of the brain and the continuous interplay between the brain regions speaks in favor of using all senses very consciously in the sensory relearning process.
Assessment—Results of Sensory Reeducation
Assessment of outcome provides important feedback for the patient and therapist during the rehabilitation period and should be performed in a standardized manner using evidence-based test instruments on a monthly or bimonthly basis. As discussed earlier, much of the recovery can be seen for a long time after the repair; so, after a nerve repair, for example at the wrist level, it can be recommended to follow the patient for at least one year after the injury. The sensory reeducation and relearning is usually a long process, and the patient needs constructive feedback along the way to keep up motivation for a training that has several abstract elements. Good clinical documentation can be a useful tool in this process, and the patient should take an active role in the evaluation of the assessments. This may help him or her to plan the training together with the therapist and to focus on the weak parts in the outcome. An instrument that is sensitive to subtle changes in details of specific functions as well as overall outcome over time is recommended. Several methods have been suggested over the years, and their pros and cons are discussed elsewhere in the literature.122,123 We suggest use of the “Model for Documentation of Outcome after Nerve Repair,” which includes separate domains for sensory, motor, pain–discomfort, and also a “total score.”83,120 Details in function and an overall score can be determined. This model instrument is standardized and is a tool suited for clinical as well as for scientific purposes.41,121-123 A long-term recovery curve in adults with estimated predicted values for an outcome score after repair of the median or ulnar nerve at the wrist level has been calculated, and can be good feedback during the sensory reeducation process.124
Much of the relearning process is probably gained by a conscious use of the hand in daily activities, using vision as a guide for the impaired sensibility. However, to facilitate and enhance this process, specific programs for sensory reeducation should be used as a clinical tool in adult patients for regaining functional sensibility. The therapist’s supporting and guiding role during the long and often very trying training period for the patient after a nerve injury must be emphasized.
Conclusion
Improvements of functional sensibility after sensory reeducation have been reported.79,82,112,125-129 However, the outcome after a nerve repair is often disappointing, especially tactile gnosis in adults. A recent systematic review also shows that very few solid scientific studies have been published investigating the effectiveness of such programs.129 Little is known about how such programs influence functional cortical organization. Hence, this is an important field for future research and for implementation of an evidence-based clinical routine for the treatment and documentation of recovery after nerve injuries to make clinical research in this field possible.
REFERENCES
1. Jenkins WM, Merzencih MM, Ochs MT, et al. Functional reorganization of primary somatosensory cortex in adult owl monkeys after behaviorally controlled tactile stimulation. J Neurophysiol. 1990;63:82–104.
2. Elbert T, Rockstroh B. Reorganization of human cerebral cortex: the range of changes following use and injury. Neuroscientist. 2004;10:129–141.
3. Merzenich MM, Jenkins WM. Reorganization of cortical representations of the hand following alterations of skin inputs induced by nerve injury, skin island transfers, and experience. J Hand Ther. 1993;6:89–104.
4. Bach-y-Rita P. The brain beyond the synapse: a review. Neuroreport. 1994;5:1553–1557.
5. Bavelier D, Neville H. Cross-modal plasticity: Where and how? Nat Neurosci. 2002;3:443–452.
6. Lundborg G, Richard P. Bunge Memorial Lecture. Nerve injury and repair—a challenge to the plastic brain. J Peripher Nerv Syst. 2003;8:209–226.
7. Calford MB, Tweedale R. Interhemispheric transfer of plasticity in the cerebral cortex. Science. 1990;249:805–807.
8. Chen R, Cohen LG, Hallett M. Nervous system reorganization following injury. Neuroscience. 2002;111:761–773.
9. Wall JT, Xu J, Wang X. Human brain plasticity: an emerging view of the multiple substrates and mechanisms that cause cortical changes and related sensory dysfunctions after injuries of sensory inputs from the body. Brain Res Brain Res Rev. 2002;39:181–215.
10. Lundborg G, Rosen B. Hand function after nerve repair. Acta Physiol (Oxford, England). 2007;189:207–217.
11. Katz D. The World of Touch. London: Lawrence Erlbaum Associates; 1989.
12. Gibson JJ. Observations on active touch. Psychol Rev. 1962;69:477–491.
13. Morgan M. Molyneux’s Question. New York: Cambridge University Press; 1977.
14. Fisher A, Murray E, Bundy A. Sensory Integration. Theory and Practice. Philadelphia: F.A. Davis; 1991.
15. Pascual-Leone A, Hamilton R. The metamodal organization of the brain. Prog Brain Res. 2001;134:427–445.
16. Kandel E, Schwartz J, Jessel T, Edshage S. Principles of Neural Science. 4th ed New York: McGraw-Hill; 2000.
17. Bodegård A, Geyer S, Naito E, Zilles K, Roland PE. Somatosensory areas in man activated by moving stimuli. Neuroreport. 2000;11:187–191.
18. Lundborg G, Rosen B. The two-point discrimination test—time for a re-appraisal? J Hand Surg [Br]. 2004;29:418–422.
19. Jerosch-Herold C. Assessment of sensibility after nerve injury and repair: a systematic review of evidence for validity, reliability and responsiveness of tests. J Hand Surg [Br]. 2005;30:252–264.
20. Jerosch-Herold C, Rosen B, Shepstone L. The reliability and validity of the locognosia test after injuries to peripheral nerves in the hand. J Bone Joint Surg Br. 2006;88:1048–1052.
21. Backlund Wasling H, Norrsell U, Gothner K, Olausson H. Tactile directional sensitivity and postural control. Exp Brain Res. 2005;166:147–156.
22. Edin B, Johansson N. Skin strain patterns privide kinaesthetic information to the human central nervous system. J Physiol (Lond). 1995;487:243–251.
23. Johansson RS, Flanagan R. Coding and use of the tactile signals from the fingers in object manipulation tasks. Nat Rev Neurosci. 2009;10:345–359.
24. Wynn-Parry CB. Rehabilitation of the Hand. London: Butterworths; 1981.
25. Moberg E. Objective methods for determining the functional value of sensibility in the hand. J Bone Joint Surg Am. 1958;40B:454–476.
26. Brand P, Yancey P. Pain: The Gift Nobody Wants. Grand Rapids, MI: Zondervan Publishing House; 1993.
27. Dellon A. Sensibility and Re-education of Sensation in the Hand. Baltimore: Williams & Wilkins; 1981.
28. Wynn-Parry CB. Rehabilitation of the Hand. London: Butterworths; 1966.
29. Moberg E. The unsolved problem—how to test the functional value of hand sensibility. J Hand Ther. 1991;4:105–110.
30. Moberg E. Criticism and study of methods for examining sensibility in the hand. Neurology. 1962;12:8–19.
31. Lundborg G, Rosén B, Dahlin LB, et al. Tubular repair of the median or ulnar nerve in the human forearm. A5-year follow-up. J Hand Surg. 2004;29:100–107.
32. Bruyns CN, Jaquet JB, Schreuders TA, et al. Predictors for return to work in patients with median and ulnar nerve injuries. J Hand Surg [Am]. 2003;28:28–34.
33. Jaquet JB, Luijsterburg AJ, Kalmijn S, et al. Median, ulnar, and combined median-ulnar nerve injuries: functional outcome and return to productivity. J Trauma. 2001;51:687–692.
34. Ruijs AC, Jaquet JB, van Riel WG, et al. Cold intolerance following median and ulnar nerve injuries: prognosis and predictors. J Hand Surg/Eurp Vol. 2007;32:434–439.
35. Rosberg HE, Carlsson KS, Höjgård S, et al. Injury to the human median and ulnar nerves in the forearm—analysis of costs for treatment and rehabilitation of 69 patients in southern Sweden. J Hand Surg. 2005;30B:35–39.
36. Birch R, Raji A. Repair of median and ulnar nerves—primary suture is best. J Bone Joint Surg. 1991;73B:154–157.
37. Lundborg G, Rosen B. Sensory relearning after nerve repair. Lancet. 2001;358:809–810.
38. Diao E, Vannuyen T. Techniques for primary nerve repair. Hand Clin. 2000;16:53–66. viii
39. Trumble TE, McCallister WV. Repair of peripheral nerve defects in the upper extremity. Hand Clin. 2000;16:37–52.
40. Ma J, Novikov LN, Kellerth JO, Wiberg M. Early nerve repair after injury to the postganglionic plexus: an experimental study of sensory and motor neuronal survival in adult rats. Scand. J Plast Reconstr Surg Hand Surg. 2003;37:1–9.
41. Lundborg G. Nerve Injury and Repair: Regeneration, Reconstruction, and Cortical Remodeling. 2nd ed Philadelphia: Elsevier; 2004.
42. Rosén B, Lundborg G, Dahlin LB, et al. Nerve repair: correlation of restitution of functional sensibility with specific cognitive capacities. J Hand Surg. 1994;19B:452–458.
43. Lundborg G, Rosen B, Dahlin L, et al. Tubular repair of the median or ulnar nerve in the human forearm: a 5-year follow-up. J Hand Surg [Br]. 2004;29:100–107.
44. Rosén B, Dahlin L, Lundborg G. Assessment of functional outcome after nerve repair in a longitudinal cohort. Scand J Plast Reconstr Surg Hand Surg. 2000;34:71–78.
45. Callahan AD. Methods of compensation and reeducation for sensory dysfunction. In: Callahan AD, ed. Rehabilitation of the Hand. St Louis: C.V. Mosby; 1995:701–714.
46. Ruijs AC, Jaquet JB, Kalmijn S, et al. Median and ulnar nerve injuries: a meta-analysis of predictors of motor and sensory recovery after modern microsurgical nerve repair. Plast Reconstr Surg. 2005;116:484–496.
47. Jaquet J, Kalmijn S, Kuypers P, et al. Early psychological stress after forearm nerve injuries: A predeictor for long-term functional outcome and return to work. Ann Plast Surg. 2002;49:82–90.
48. Wilson F. The Hand. How Its Use Shapes the Brain, Language, and Human Culture. New York: Pantheon Books; 1998.
49. Penfield W, Boldrey E. Somatic motor and sensory representations in the cerebral cortex of man as studied by electrical stimulation. Brain. 1937;60:389–443.
50. Rosenkranz K, Butler K, Williamon A, et al. Sensorimotor reorganization by proprioceptive training in musician’s dystonia and writer’s cramp. Neurology. 2008;70:304–315.
51. Schwenkreis P, El Tom S, Ragert P, et al. Assessment of sensorimotor cortical representation asymmetries and motor skills in violin players. Eur J Neurosci. 2007;26:3291–3302.
52. Borsook D, Becerra L, Fishman S, et al. Acute plasticity in the human somatosensory cortex following amputation. Neuroreport. 1998;9:1013–1017.
53. Werhahn KJ, Mortensen J, Van Boven RW, et al. Enhanced tactile spatial acuity and cortical processing during acute hand deafferentation. Nat Neurosci. 2002;5:936–938.
54. Buonomano D, Merzenich M. Cortical plasticity: from synapses to maps. Annu Rev Neurosci. 1998;21:149–186.
55. Merzenich MM, Nelson RJ, Kaas JH, et al. Variability in hand surface representations in areas 3b and 1 in adult owl and squirrel monkeys. J Comp Neurol. 1987;258:281–296.
56. Merzenich MM, Nelson RJ, Stryker MS, et al. Somatosensory cortical map changes following digit amputation in adult monkeys. J Comp Neurol. 1984;224:591–605.
57. Godde B, Spengler F, Dinse HR. Associative pairing of tactile stimulation induces somatosensory cortical reorganization in rats and humans. Neuroreport. 1996;8:281–285.
58. Godde B, Stauffenberg B, Spengler F, Dinse HR. Tactile coactivation-induced changes in spatial discrimination performance. J Neurosci. 2000;20:1597–1604.
59. Pleger B, Dinse HR, Ragert P, et al. Shifts in cortical representations predict human discrimination improvement. Proc Natl Acad Sci U S A. 2001;98:12255–12260.
60. Dinse HR, Ragert P, Pleger B, et al. Pharmacological modulation of perceptual learning and associated cortical reorganization. Science. 2003;301:91–94.
61. Pascual-Leone A, Amedi A, Fregni F, Merabet LB. The plastic human brain cortex. Annu Rev Neurosci. 2005;28:377–401.
62. Elbert T, Pantev C, Wienbruch C, et al. Increased cortical representation of the left hand in string players. Science. 1995;270:305–307.
63. Pantev C, Engelien A, Candia V, Elbert T. Representational cortex in musicians. Plastic alterations in response to musical practice. Ann N Y Acad Sci. 2001;930:300–314.
64. Byl N, Melnick M. The neural consequences of repetition: Clinical implications of a learning hypothesis. J Hand Ther. 1997;10:160–174.
65. Byl NN. Focal hand dystonia may result from aberrant neuroplasticity. Adv Neurol. 2004;94:19–28.
66. Candia V, Schafer T, Taub E, et al. Sensory motor retuning: a behavioral treatment for focal hand dystonia of pianists and guitarists. Arch Phys Med Rehabil. 2002;83:1342–1348.
67. Altenmuller E. Focal dystonia: advances in brain imaging and understanding of fine motor control in musicians. Hand Clin. 2003;19:523–538. xi
68. Bara-Jimenez W, Catalan MJ, Hallett M, Gerloff C. Abnormal somatosensory homunculus in dystonia of the hand. Ann Neurol. 1998;44:828–831.
69. Wang X, Merzenich MM, Sameshima K, Jenkins WM. Remodelling of hand representation in adult cortex determined by timing of tactile stimulation. Nature. 1995;378:71–75.
70. Byl NN, Nagajaran S, McKenzie AL. Effect of sensory discrimination training on structure and function in patients with focal hand dystonia: a case series. Arch Phys Med Rehabil. 2003;84:1505–1514.
71. Zeuner KE, Hallett M. Sensory training as treatment for focal hand dystonia: a 1-year follow-up. Mov Disord. 2003;18:1044–1047.
72. Xerri C, Coq JO, Merzenich MM, Jenkins WM. Experience-induced plasticity of cutaneous maps in the primary somatosensory cortex of adult monkeys and rats. J Physiol Paris. 1996;90:277–287.
73. Dahlin LB, Salgeback S, Komoto-Tufvesson Y, Lundborg G. Waking up a sleeping sensibility. Lancet. 1998;352:328.
74. Liepert J, Tegenthoff M, Malin JP. Changes of cortical motor area size during immobilization. Electroencephalogr Clin Neurophysiol. 1995;97:382–386.
75. Kaas JH, Merzenich MM, Killackey HP. The reorganization of somatosensory cortex following peripheral nerve damage in adult and developing mammals. Annu Rev Neurosci. 1983;6:325–356.
76. Silva AC, Rasey SK, Wu X, Wall JT. Initial cortical reactions to injury of the median and radial nerves to the hands of adult primates. J Comp Neurol. 1996;366:700–716.
77. Wall JT, Kaas JH, Sur M, et al. Functional reoganization in somatosensory cortical areas 3b and 1 of adult monkeys after median nerve repair: Possible relationships to sensory recovery in humans. J Neurosci. 1986;6:218–233.
78. Hansson T, Brismar T. Loss of sensory discrimination after median nerve injury and activation in the primary somatosensory cortex on functional magnetic resonance imaging. J Neurosurg. 2003;99:100–105.
79. Wynn-Parry CB, Salter M. Sensory re-education after median nerve lesions. Hand. 1976;8:250–257.
80. Weiss T, Miltner W, Huonker R, et al. Rapid functional plasticity of the somatosensory cortex after finger amputation. Exp Brain Res. 2000;134:199–203.
81. Rossini PM, Martino G, Narici L, et al. Short-term brain plasticity in humans: transient finger representation changes in sensory cortex somatotopy following ischemic anesthesia. Brain Res. 1994;642:169–177.
82. Cheng AS, Hung L, Wong JM, et al. A prospective study of early tactile stimulation after digital nerve repair. Clin Orthop. 2001.169–175.
83. Rosén B, Lundborg G. A model instrument for the documentation of outcome after nerve repair. J Hand Surg. 2000;25A:535–544.
84. Tanabe HC, Honda M, Sadato N. Functionally segregated neural substrates for arbitrary audiovisual paired-association learning. J Neurosci. 2005;25:6409–6418.
85. Gizewski ER, Gasser T, de Greiff A, et al. Cross-modal plasticity for sensory and motor activation patterns in blind subjects. Neuroimage. 2003;19:968–975.
86. Zatorre RJ, Halpern AR. Mental concerts: musical imagery and auditory cortex. Neuron. 2005;47:9–12.
87. Kosslyn SM, Ganis G, Thompson WL. Neural foundations of imagery. Nat Rev Neurosci. 2001;2:635–642.
88. Djordjevic J, Zatorre RJ, Petrides M, et al. Functional neuroimaging of odor imagery. Neuroimage. 2005;24:791–801.
89. Jeannerod M, Frak V. Mental imaging of motor activity in humans. Curr Opin Neurobiol. 1999;9:735–739.
90. Ehrsson HH, Geyer S, Naito E. Imagery of voluntary movement of fingers, toes, and tongue activates corresponding body-part-specific motor representations. J Neurophysiol. 2003;90:3304–3316.
91. Yoo SS, Freeman DK, McCarthy JJ 3rd, Jolesz FA. Neural substrates of tactile imagery: a functional MRI study. Neuroreport. 2003;14:581–585.
92. Rizzolatti G. The mirror neuron system and its function in humans. Anat Embryol (Berl). 2005;210:419–421.
93. Iacoboni M, Molnar-Szakacs I, Gallese V, et al. Grasping the intentions of others with one’s own mirror neuron system. PLoS Biol. 2005;3:e79.
94. Hauk O, Johnsrude I, Pulvermuller F. Somatotopic representation of action words in human motor and premotor cortex. Neuron. 2004;41:301–307.
95. Keysers C, Wicker B, Gazzola V, et al. A touching sight: SII/PV activation during the observation and experience of touch. Neuron. 2004;42:335–346.
96. Hansson T, Nyman J, Björkman A, et al. Sights of touching activates the somatosensory cortex in humans. Scand J Plast Reconstr Surg Hand Surg. 2009;43(5):267–269.
97. Mottonen R, Jarvelainen J, Sams M, Hari R. Viewing speech modulates activity in the left SI mouth cortex. Neuroimage. 2005;24:731–737.
98. Avikainen S, Forss N, Hari R. Modulated activation of the human SI and SII cortices during observation of hand actions. Neuroimage. 2002;15:640–646.
99. Ramachandran VS, Hirstein W. The perception of phantom limbs, The D.O. Hebb Lecture. Brain. 1998;121:1603–1630.
100. Rosén B, Lundborg G. Training with a mirror in rehabilitation of the hand. Scand. J Plast Reconstr Surg Hand Surg. 2005;39:104–108.
101. Lundborg G, Rosén B, Lindberg S. Hearing as substitution for sensation—a new principle for artificial sensibility. J Hand Surg. 1999;24A:219–224.
102. Lundborg G, Bjorkman A, Hansson T, et al. Artificial sensibility of the hand based on cortical audiotactile interaction: A study using functional magnetic resonance imaging. Scand J Plast Reconstr Surg Hand Surg. 2005;39:370–372.
103. Rosén B, Lundborg G. Enhanced sensory recovery after median nerve repair using cortical audio-tactile interaction. A randomised multicenter study. J Hand Surg/Europ Vol. 2007;32E:31–37.
104. Curtis RM, Dellon AL. Sensory re-education after peripheral nerve injury. In: Curtis RM, Dellon AL, eds. Management of Peripheral Nerve Problems. Philadelphia: W.B. Saunders; 1980:469–478.
105. Hansson T, Brismar T. Tactile stimulation of the hand causes bilateral cortical activation: a functional nagnetic resonance study in humans. Neurosci Lett. 1999;271:29–32.
106. Harris JA, Diamond ME. Ipsilateral and contralateral transfer of tactile learning. Neuroreport. 2000;11:263–266.
107. Harris J, Harris I, Diamond M. The topography of tactile learning in humans. J Neurosci. 2001;21:1056–1061.
108. Nagarajan SS, Blake DT, Wright BA, et al. Practice-related improvements in somatosensory interval discrimination are temporally specific but generalize across skin location, hemisphere, and modality. J Neurosci. 1998;18:1559–1570.
109. Muellbacher W, Richards C, Ziemann U, et al. Improving hand function in chronic stroke. Arch Neurol. 2002;59:1278–1282.
110. Bjorkman A, Weibull A, Rosén B, et al. Rapid cortical reorganisation and improved sensibility of the hand following cutaneous anaesthesia of the forearm. Eur J Neurosci. 2009;29:837–844.
111. Bjorkman A, Rosen B, Lundborg G. Acute improvement of hand sensibility after selective ipsilateral cutaneous forearm anaesthesia. Eur J Neurosci. 2004;20:2733–2736.
112. Rosen B, Bjorkman A, Lundborg G. Improved sensory relearning after nerve repair induced by selective temporary anaesthesia—a new concept in hand rehabilitation. J Hand Surg [Br]. 2006;31:126–132.
113. Florence SL, Boydston LA, Hackett TA, et al. Sensory enrichment after peripheral nerve injury restores cortical, not thalamic, receptive field organization. Eur J Neurosci. 2001;13:1755–1766.
114. Johansson BB, Belichenko PV. Neuronal plasticity and dendritic spines: effect of environmental enrichment on intact and postischemic rat brain. J Cereb Blood Flow Metab. 2002;22:89–96.
115. Nithianantharajah J, Hannan AJ. Enriched environments, experience-dependent plasticity and disorders of the nervous system. Nat Rev Neurosci. 2006;7:697–709.
116. Zhao LR, Mattsson B, Johansson BB. Environmental influence on brain-derived neurotrophic factor messenger RNA expression after middle cerbral artery occlusion in spontaneously hypertensive rats. Neuroscience. 2000;97:177–184.
117. Thompson BM, Andrews SR. An historical commentary on the physiological effects of music: Tomatis, Mozart and neuropsychology. Integr Physiol Behav Sci. 2000;35:174–188.
118. Schlaug G, Jancke L, Huang Y, et al. Increased corpus callosum size in musicians. Neuropsychologia. 1995;33:1047–1055.
119. Pascual-Leone A. The brain that plays music and is changed by it. Ann N Y Acad Sci. 2001;930:315–329.
120. Rosén B, Lundborg G. A new model instrument for outcome after nerve repair. Hand Clin. 2003;19:463–470.
121. Vordemvenne T, Langer M, Ochman S, et al. Long-term results after primary microsurgical repair of ulnar and median nerve injuries. A comparison of common score systems. Clin Neurol Neurosurg. 2007;109:263–271.
122. MacDermid J. Measurement of health outcomes following tendon and nerve repair. J Hand Ther. 2005;18:297–312.
123. Szabo RM. Outcome assessment in hand surgery: When are they meaningful. J Hand Surg. 2001;26A:993–1002.
124. Rosén B, Lundborg G. The long-term recovery curve in adults after median or ulnar nerve repair: A reference interval. J Hand Surg. 2001;26B:196–200.
125. Daniele HR, Aguado L. Early compensatory sensory re-education. J Reconstr Microsurg. 2003;19:107–110.
126. Dellon AL, Curtis RM, Edgerton MT. Reeducation of sensation in the hand after nerve injury and repair. Plastic Reconstr Surg. 1974;53:297–305.
127. Imai H, Tajima T, Natsumi Y. Succesful re-education of functional sensibility after median nerve repair at the wrist. J Hand Surg. 1991;16A:60–65.
128. Novak CB, Kelly L, Mackinnon SE. Sensory recovery after median nerve grafting. J Hand Surg [Am]. 1992;17:59–68.
129. Oud T, Beelen A, Eijffinger E, Nollet F. Sensory re-education after nerve injury of the upper limb: a systematic review. Clin Rehabil. 2007;21:483–494.









