Therapist’s Management of Complex Regional Pain Syndrome
▪ Early recognition of four sign and symptom factors determines success
▪ Pain modulation includes multiple techniques, patience, and perseverance
▪ Treatment avoids pain provocation
Complex regional pain syndrome (CRPS) types I and II is one of the greatest clinical challenges presented to the hand therapist. Treatments require the concerted effort of and close communication among the physician, therapist, and patient to obtain an optimal functional outcome. Historically, various categorizations of the syndrome have been formulated under the designation reflex sympathetic dystrophy (RSD). The term reflex sympathetic dystrophy implies that the sympathetic nervous system is causally involved and considers the clinical presentation the result of reflex activation of sympathetic neurons. This may not be the case, however, because these implied mechanisms have not been proven.1
Classically, RSD has been divided into five separate patient subtypes: minor causalgia, minor traumatic dystrophy, shoulder-hand syndrome, major causalgia, and major traumatic dystrophy.2 Betcher and Casten,3,4 in 1955, proposed a grading system progressing from grade 3 to 1, based on severity. More recently, Janig5 proposed three groups of RSD patients: 1, algodystrophy (a full-blown syndrome); 2, sympathetic dystrophy (RSD) without pain; and 3, sympathetic maintained pain (SMP), in which pain is the only overt manifestation. RSD had also been divided into three stages based on time frames and associated signs and symptoms with each stage.6 This staging has some merit; three distinct subgroups are recognized: (1) a limited syndrome predominated by neuropathic pain and sensory changes, (2) a limited syndrome predominated by vasomotor signs, and (3) “a florid CRPS syndrome similar to classical RSD descriptions.”7-9
As established by the International Association for the Study of Pain (IASP),10,11 the current recommended nomenclature is CRPS types I and II. CRPS I corresponds to RSD, and CRPS II designates causalgia that presents similarly but occurs with injury to a peripheral nerve or nerve branch. Within the CRPS categories, pain is regarded as sympathetically independent pain (SIP), occurring in the initial onset of the syndrome. SMP, by contrast, is defined as “a symptom of CRPS and not a clinical entity” occurring after a period of time of the onset of the syndrome.12 CRPS has strict inclusion criteria that do not encompass the presence or absence of SMP.13 The IASP diagnostic criteria for CRPS are listed in Box 116-1.11
Box 116-1 IASP Diagnostic Criteria
1. Presence of an inciting noxious event or a cause of immobilization.
2. Continuing pain, allodynia, or hyperalgesia with which pain is disproportionate to any inciting event.
3. Evidence at some time of edema, changes in skin blood flow or abnormal sudomotor activity in the region of pain.
4. This diagnosis is excluded by the existence of a condition that would otherwise account for the degree of pain and dysfunction.
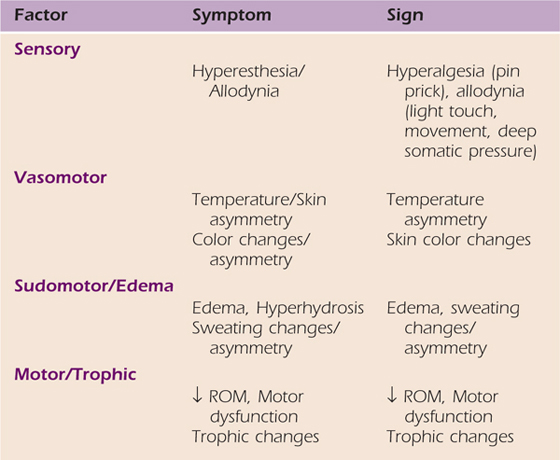
In an effort to further validate the clinical diagnosis of CRPS, a factor analysis was performed.7,14 As a result of this research new diagnostic criteria have been proposed.15 In addition to pain, four symptom and sign factors were identified: sensory, vasomotor, sudomotor–edema, and motor–trophic. The patient must report the presence of at least three of the symptom factors, and at least two of the sign factors must be evident at the time of examination. This would result in a sensitivity of .85 and specificity of 0.69.15,16 The symptom and sign factors are highlighted in Table 116-1.
Table 116-1 Four Sign and Symptom Factors Criteria
This chapter is devoted to the therapist’s recognition and management for CRPS. In the interest of clarity, discussion refers to CRPS I and II in place of RSD, unless referring to specific literature. The therapist must possess an in-depth understanding of the multiple components of this complex process on which to establish realistic goals, formulate appropriate treatment, and prognosticate the likelihood of successful functional outcome. This chapter emphasizes a problem-solving approach based on the patient’s presenting signs and symptoms as related to pain and the above four factors.
Lankford17 described the optimal treatment principle as follows: “Early diagnosis and concomitant early intervention of treatment is the most important factor in the eventual outcome of this disabling condition.” The hand therapist often is the first clinician to identify early onset, which may be precipitous and require immediate intervention. This point is supported by Birklein and associates, who noted that the early initiation of therapy after onset is important before structural changes occur in the affected limb that may compromise pain relief and function.18 Early recognition of CRPS will also help prevent exacerbation of the condition by the therapist and the patient. Finally the therapist must be mindful that recurrence has been reported to be 1.8%. This recurrence may occur spontaneously or 53% of the time may be related to a second traumatic incident.14 A delay in the diagnosis and treatment may also add to recurrence.18
CRPS may present after a seemingly minor injury, and the presentation will exceed the normally expected reaction and clinical characteristics.12 Examination should be ongoing and as thorough but as nonprovocative as possible; pain exacerbation must be avoided even if this necessitates delaying some aspects of the examination. It is necessary to rule out any underlying conditions or inciting lesions that would otherwise account for the degree of pain and dysfunction and to establish the presence of the diagnostic criteria listed in Table 116-1.11,15,19,20
CRPS signs and symptoms are dynamic (complex), extend beyond the area of injury (regional), always include disproportionate continuing (pain), and occur in variable combinations (syndrome).19 The diagnosis of CRPS is primarily clinical; however, predictive value of the symptoms has been reported. In a study of 155 CRPS assessments, Perez and colleagues evaluated the sensitivity and specificity of the diagnostic criteria. They determined that a Visual Analog Scale (VAS) > 3 cm, McGill Pain Questionnaire with more than 6 words, a checked temperature difference of more than 0.4°C, hand volume difference greater than 6.5%, and active range of motion (AROM) limitation greater than 15% could be used as diagnostic indicators for the presence of CRPS I.21 In addition, the VAS has diagnostically demonstrated a positive correlation with volumetrics, AROM, and joint pain in patients with CRPS.22
Pain has two basic components: sensory discriminative and affective-motivational.23,24 The physiologic component refers to strength and intensity; this may be neurogenically or non-neurogenically mediated by chemicals released in the body. Examples of neurogenic pain mediators are neuropeptides such as substance P and calcitonin gene-related peptides, which are also strong vasodilators. Non-neurogenic pain mediators such as bradykinins, certain antihistamines, prostaglandins, and leukotrienes are the result of connective tissue dysfunction. Dysesthetic pain results from damaged or regenerating nociceptive afferent fibers, which may also affect sympathetic function.25 The affective-motivational component is the emotional dimension, which allows pain to be perceived as pleasant or unpleasant. It is also the most likely source of variability of response to pain.
Pain assessment includes spatial representation, intensity, and qualitative and temporal components. Chapter 114 provides detailed information on the components of pain assessment. Clinical evaluation should include use of a body diagram indicating areas and types of pain (Fig. 116-1). This can be augmented by the McGill Pain Questionnaire for qualitative evaluation. Quantification of pain intensity can be accomplished with a verbal or visual analog scale. It is important to apply the scale or scales to each area and type of complaint the patient has delineated. The relationship of pain over time, progression since onset, and whether it is constant versus intermittent, also must be addressed. In a study of 145 CRPS patients, Birklein et al. reported that spontaneous pain was present in 75% of the patients. Pain was amplified in 93% of the patients by dependent position, striking, or AROM of the affected limb, greater at night, and with environmental temperature changes. Common descriptors were tearing, burning, stinging, and squeezing. The location was deep (63%), superficial (30%), and continuous (60%) and lancinating (27%).18
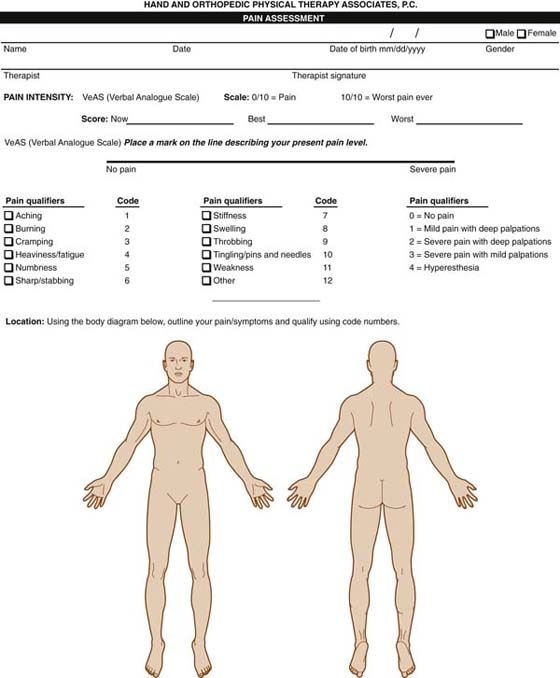
Figure 116-1 Pain profile. The patient colors or shades the spatial representation of his or her pain and completes the descriptor terms and visual analog scale 100-mm line. The verbal reporting scale and joint tenderness scale are used by the therapist to rate specific areas or types of pain.
Evaluation of pain provides an important baseline for measuring progress and helps determine whether the pain is sympathetic, dermatomal, peripheral nerve, or referral related, as from myofascial trigger points.26 Joint tenderness can be quantified with a four-point scale based on palpation22 (see Fig. 116-1). Gradl and Schurmann reported that sympathetic dysfunction occurs early in CRPS and normalizes with time.27 Therefore, early identification of vasomotor changes in conjunction with pain may lead to early diagnosis.
Pain examination in the future may be more objective, using specific tests such as: heat-induced hyperalgesias, low- and high-threshold mechanical allodynia, transcutaneous electrical nerve stimulation, or monofilament testing in slow temporal summation of mechanical allodynia. Patients in whom temporal summation occurred with these tests were found to have more intense spontaneous pain than those who did not.28 It may be possible to predict which patient’s neural pain threshold may be low, indicating hyperalgesia or allodynia. With evaluative measures, the therapist may be able to intervene earlier, possibly avoiding increased pain occurring with temporal summation or even being able to predict the potential for developing CRPS.
Examination of peripheral nerve function should include threshold (monofilament) and innervation density (two-point discrimination) tests to identify areas of altered response. Care must be exercised, because monofilament testing may increase allodynia.28 If the patient displays extreme hypersensitivity, testing should be delayed. Areas of tactile or mechanical allodynia and hyperalgesia should be carefully assessed and have been reported to occur in 88% of the patients.18 Sensory testing for these symptoms would include noting the distribution of the symptoms and the response to pinprick and light touch.18 Sensory examination may be used to serially chart changes in allodynia and may also help identify inciting lesions. Examination of peripheral nerve also includes neurodynamic assessment, patient resting posture, and any active or passive motion dysfunction. See Chapter 118 for more information on the assessment of adverse neural tension.
Systematic review should focus on the four sign factors. Changes such as fibrosis of the skin, connective, and skeletal tissue and vasomotor status should be determined, including comparison with the contralateral extremity. The observations of the skin, color, texture, temperature, hair growth, and other trophic changes should be noted. Sudomotor changes such as hypohidrosis or hyperhidrosis should be observed for any asymmetry. While taking the patient history, the therapist should inquire as to whether any diagnostic tests such as three-phase bone scan have been performed.
Vasomotor changes, whether vasodilated (hot, red, edematous), vasoconstricted (cold, pale), or mixed/fluctuating, should be assessed. Bilateral baseline circumferential and volumetric measurements should be obtained, and the type of edema (brawny or pitting) should be noted. The thoracic spine should be evaluated, considering the sympathetic chain from T2 to T6 and its relationship to the upper extremity. Note that sympathetic innervation does not correlate with dermatomes.
Decreased ROM, motor weakness, or movement dysfunction may be present. As patient tolerance permits, the entire skeletal system and articular structures from the cervical spine throughout the upper extremity should be examined. Manual muscle testing should be performed to ascertain muscle strength, integrity, innervation status, or the presence of any altered motor response. This, as with the pain evaluation, may uncover underlying precipitating lesions. Grip dynamometer and standard pinch meter testing will provide additional baseline data if the patient is able to tolerate testing. Connective tissue status should be assessed with palpation for fibrosis and joint tenderness.22 A careful screen for the presence of myofascial trigger points should also be performed, as this has been linked to CRPS.26
Goniometric measurement of AROM and passive range of motion (PROM) and assessment for arthrofibrosis, extrinsic, and intrinsic tightness provide additional information about level of involvement and effect on function. Any dystonia (abnormal posturing or movements), tremors, or akinesia/bradykinesia while attempting active motion should be noted.18 History taking should also determine the preexistence of any central movement disorders or predisposing factors. A “typical” RSD patient is depicted in Figure 116-2.
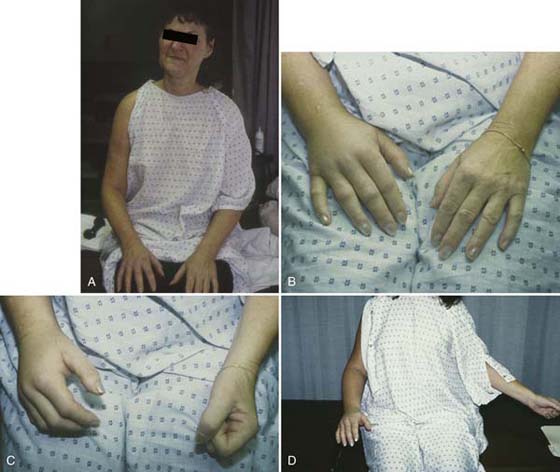
Figure 116-2 Complex regional pain syndrome patient. Note the entire extremity (A) and hand edema with metacarpophalangeal joints extended and proximal interphalangeal joints slightly flexed (B). Inability to make a complete fist (C) and significant loss of external rotation of the shoulder and extension of the elbow (D). The photos do not portray the coolness and pallor of the vasoconstricted extremity.
The history, pain and physical examination will establish the level of tissue irritability or sensitivity that creates the guidelines for formulating a treatment plan. The patient’s signs and symptoms may be dynamic, variable, and widespread. Clinical reasoning, ongoing observation, and assessment of tissue status and response to treatment determines the plan for care. Figure 116-3 is a diagrammatic representation of the clinical reasoning and the treatment options available to the therapist.
There is a paucity of any randomized controlled trials examining the effectiveness of therapy intervention. Regardless of this fact, almost every piece of literature on CRPS cites the use or need for therapy intervention.29-31 There have been multiple case reports that reported the successful treatment of CRPS with therapy intervention.32-34 However, these reports lacked the use of patient self-report outcome measures other than a pain analog scale and ROM measurements. The only prospective randomized clinical study of CRPS was reported in a series of four articles.34-37 In this series 145 patients with CRPS I with symptom duration less than 1 year were each randomly assigned to one of three treatments: physical therapy (PT), occupational therapy (OT), and control therapy (counseling by social worker; CT). Oerlemans and associates reported no difference in impairment percentages after 1 year35; however, PT and OT resulted in a significant and more rapid improvement in the impairment level sum score,36 PT resulted in a more rapid decrease in pain and was superior to OT and CT for increasing AROM34; and PT was more cost-effective.37 Specific interventions were not described but were defined based on the goals of each discipline. In a randomized clinical trial on the efficacy of spinal stimulation that included PT intervention, the effectiveness of PT was extrapolated, and it was reported that PT did not influence functional parameters in chronic (>1 year) CRPS I. The authors did, however, report that patients with lower pain scores and less motor and/or strength involvement responded better with the PT intervention.38
Through the examination, the therapist can determine the irritability of the patient’s involved tissues and formulate a treatment plan. If the therapist fails to make an accurate assessment of the patient’s signs and symptoms, in many instances he or she becomes the “pain terrorist.” Even with accurate assessment, there is no recipe for treating these patients. Treatment program development requires clinical reasoning, frequent if not daily observation of the patient, assessment of the patient’s response to previous treatments, and assessment of the patient’s level of irritability. The therapist must clinically decide at each visit the vasomotor state of the patient’s extremity. There is no support in the literature to suggest an appropriate dosage or duration for treatment. It is only through this ongoing evaluation process that these parameters can be determined and modified.
The therapist should choose a practical approach based on the major presenting signs and symptoms, within the context of the four factors previously discussed. Before the IASP classification of CRPS, the teachings and descriptions of RSD stages led the practitioner to believe that the patient is vasodilated at certain stages (stages 1 and 2) and vasoconstricted at other stages (stages 2 and 3). It is now recognized that CRPS is an ongoing vasomotor instability problem. The patient may be vasodilated, vasoconstricted, or a combination of both at any given time. In light of this unstable vasomotor tone, the therapist should clinically attempt to identify the current vasomotor state (at each treatment session) and avoid exacerbating the tone identified.39 A patient with a hot, red, swollen hand may be vasodilated. The therapist should choose treatment methods that will not accentuate the vasodilatation. In contrast, the patient with a cool and pale extremity may be vasoconstricted. The vasoconstriction may be assisting in perpetuating the syndrome, and treatment should use methods that create vasodilatation and avoid vasoconstriction.
The pursuit of a practical treatment approach should be individually geared toward treating the pain and accompanying four sign and symptom factors of sensory, vasomotor instability, sudomotor/edema, and motor/trophic changes. The therapist must recognize the emotional and psychological aspects of the RSD patient. Hardy and Merritt40 found that compared with non-RSD patients, RSD patients were significantly different in somatization, depression, interpersonal sensitivity, and anxiety but that pain scores showed no significant difference between the two groups. In contrast Ciccone et al. found no difference in psychological dysfunction between CRPS, chronic low back pain, and neuropathic pain patients.41
Pain-related fear of physical activity or re-injury has been identified in chronic pain42 and CRPS patients.43 This pain-related fear can often be more disabling than pain itself. Pain-related fear has led to the model of fear-avoidance, where “confrontation” and “avoidance” are the extremes of the response. Confrontation may lead to reductions in fear over time.44 As a result, a treatment model of graded exposure has been recommended.43,45 The application of graded exposure was studied by deJong et al. in a single-case experimental ABCD design of eight patients diagnosed with CRPS I. The program consisted of education to the fear avoidance model to increase willingness to move (exposure) and graded exposure to those movements or situations that provoked pain-related fear. They found this program decreased pain-related fear, pain intensity, disability, and physiologic signs or symptoms related to autonomic and vasomotor disturbances.43 The important point is that the treatment environment and the therapist also should address the psychological needs of the patients.
Every health-care professional treating patients with CRPS should consider the impact of this disease on their quality of life. This fact alone supports the need for therapeutic intervention. It has been demonstrated in acute and chronic (>3 years) CRPS that there is a marked decrease in the use of the involved extremity,46-48 especially in the case of involvement of the dominant hand.46 The greater the impairments in ROM and strength, the greater the limb activity was decreased especially in sitting. Patients with CRPS also tend to be less active overall with less intensity.47 This decrease in limb activity tended to correlate with disability and handicap. Geertzen and associates reported that 62% of their patients were limited in activities of daily living (ADLs) and these patients were significantly more handicapped as measured by the Groningen Activity Restriction Scale and RAND-36 (SF-36) questionnaire.49 Similar findings of reduced quality of life as measured with the Neuropathic Pain Scale, Modified Brief Pain Inventory, and a self-report questionnaire were reported by Galer et al.50 Both of these research groups confirmed that pain also played a major role in quality of life.
Recognition of the biopsychosocial aspects of CRPS has led to the concept of functional restoration, which advocates, as have I, for the treatment of the signs and symptoms of which therapy plays a major role.31 Stanton-Hicks and associates state that therapy must include five aspects: neuromodulation of pain, movement and muscle activation, isometric strength and stress load, and functional recovery.31 The dynamic nature of CRPS necessitates that each program is individually based and allows flexibility in the plan of care.
The management of pain is of primary importance, especially in the early onset of CRPS. Before any progress can be achieved, pain must be decreased. Pain modulation may include modalities as an adjunct and may be obtained by managing tissue changes, which may allow improvement in movement dysfunction. It is of the utmost importance that the therapist not exacerbate the patient’s pain during treatment17,31 To determine a meaningful reduction of pain in patients with CRPS I, Forouzanfar and associates studied 52 patients and measured their responses on the VAS and Perceived Global Effect Score.51 The authors determined their patients’ defined meaningful reduction in pain to be at least a 50% reduction and an absolute 3-cm change on the VAS. Furthermore, it is necessary to have this level of pain reduction for patients to report a successful treatment.
There is little evidence at this time that supports the use of transcutaneous electroanalgesia in the management of patients suffering with CRPS. The reader is referred to Chapter 114, “Pain Management: Principles of Therapist’s Interventions,” and Chapter 117, “The Use of Physical Agents in Hand Rehabilitation,” for a more in-depth discussion on the use of electroanalgesia and physical agents for pain modulation.
Active exercise may contribute to decreasing the patient’s discomfort through activation of large-diameter afferent fiber stimulation23,24,52 or through opioid stimulation. The use of thermotherapy to treat pain has been reported.53 Ultrasound is delivered over the affected area or the accompanying peripheral nerves.53 The reader is referred to Chapter 117 on physical agents for a more in-depth discussion on the use of physical agents as an adjunct to treatment intervention.
Mirror visual feedback (MVF) has been advocated for the treatment of CRPS to assist in somatosensory cortex reorganization.54,55 The technique utilizes a mirror to simulate movement of the affected limb by using the unaffected limb’s reflection while performing specific movements (Fig. 116-4). McCabe and associates in a descriptive study of eight patients treated with MVF reported a “striking reduction” in VAS during and after treatment in five patients suffering with CRPS for less than 2 years. Repeated use of MVF four to nine times per day resulted in progressive extension of the analgesic effect.54 In a pilot study, Tichelaar et al. treated three CRPS patients with MVF combined with a cognitive behavioral therapy and reported some success in decreasing the VAS and improving motion in two of the patients with symptoms of 8 and 30 months in duration. There was no change in the other patient, whose symptoms had been present for 9 years.55 A more in-depth discussion on MVF will occur later in this chapter and can also be found in Chapter 46, “Sensory Reeducation.”

Figure 116-4 Mirror visual feedback. The patient focuses on the image in the mirror of the noninvolved side performing active motion that the therapist chooses. The mirror is then removed with the patient continuing with the motion with both hands.
As discussed earlier many patients with CRPS demonstrate pain-related fear behavior. Use of graded exposure to the pain-related fear has been used successfully to treat patients with chronic low back pain.45 Recently deJong and colleagues treated patients with chronic CRPS I utilizing a graded exposure to overcome pain-related fear using a single-case experimental ABCD design. The treatments consisted of educating the patients on the fear avoidance model, then developing an individual graded exposure plan to the situations the patients described as dangerous. The patients agreed to expose themselves to these fearful activities until anxiety had decreased. The therapist gradually withdrew from the program. Although a relatively low study population (eight patients), self-reported pain-fear, pain intensity, disability, and physiologic signs and symptoms all improved.43
Treatment requires the patient’s diligent performance of a desensitization program incorporated into the clinic and home program. The desensitization program should be specifically outlined for the patient, including duration and frequency of each stimulus throughout the day. Desensitization should be initiated after determining the patient’s baseline hypersensitivity and the amount and type of tactile stimulation he or she is capable of tolerating without exacerbation of pain. Desensitization may include the use of textures, percussion, pressure, and vibration.
The therapist should initiate desensitization outside of the area of hypersensitivity and progressively work toward the area of greatest sensitivity. As previously discussed, Price et al.28 demonstrated the capability of some tactile stimuli to create temporal summation of mechanical allodynia. Intermittent tactile stimuli applied at intervals of 3 seconds have been found to increase the patient’s discomfort.28 In the clinic, the intermittent or cyclic application of tactile stimuli such as percussion or intermittent skin contact with desensitization modalities actually may increase the patient’s allodynia. Every effort should be made to maintain contact of the stimulus with the skin and avoid cyclic stimulation, as occurs when the therapist removes his or her hands with each stroke of retrograde massage.
Vibration is a component of desensitization. Bin et al.56 found that vibration of 200 Hz performed directly over the projected area of pain was most effective in relieving the pain produced by electrical stimulation of cutaneous fascicles of the median nerve. Pressure, cooling, and warming also were compared with vibration for the relief of this discomfort and were found to be less effective. Lundenberg57 treated 267 pain patients using 100-Hz vibration with a 60- and 200-cm probe. He found after 3 months of vibration that 50% of the patients reported more than 50% pain relief. After 6 months, 75% still using the device were reporting pain relief. In general, he found that the use of vibration decreased as the months progressed and relief was greatest in the musculoskeletal patients as compared with the neurogenic pain patients. Vibration used for a 45-minute period twice a day at 100 Hz also was found to be most effective for pain relief when compared with various vibratory rates from 50 to 600 Hz. In general, the greater the relief, the longer the relief lasted after vibration, and this relief was directly related to the amount of time vibration was applied. Finally, investigators found that stimulation of greater than 200 Hz caused radiation of discomfort.58 From the results of these experiments, vibration treatments at 100 Hz for periods of 20 to 45 minutes each for a total of 90 minutes per day with continuous modest pressure using a 6-cm square padded probe appears most effective in relieving pain and hypersensitivity. The benefits of vibration have been reported by Gay and associates. In a nonrandomized study with 11 subjects the authors utilized 14 points of stimulation on the hand that caused “kinesthetic illusions” at a frequency of 86 Hz for 1 minute in 4 15-second periods; this was repeated once and performed 5 days per week for 10 weeks.59 The authors reported a 50% reduction in pain as measured on the VAS and a 30% improvement in wrist and finger ROM following the 10-week treatment session.
A 10-step hierarchy program for desensitization has been advocated by Barber.60 For the CRPS patient, every attempt should be made to include desensitization as part of the AROM and functional activity program. Initially, desensitization may make up the major component of the treatment program to gain control of hypersensitivity. As hypersensitivity improves, the necessity for desensitization will diminish. The therapist should determine the tactile stimulus that just provokes the threshold response. Only after the patient accommodates to this stimulus is the patient progressed to the next stimulus in the hierarchy.
Retrograde massage performed for edema control is also part of a desensitization program. In most cases the patient should be encouraged to perform desensitization with the established modality for 20 to 45 minutes at a time, totaling 90 minutes per day. Desensitization often fails as a result of early abandonment of the program, inadequate time spent with each individual modality to produce sensory accommodation to the stimulus, or inappropriate stimulus choice. In those patients who cannot tolerate desensitization on the affected extremity, stimulating the identical area on the unaffected extremity may assist in decreasing sensitivity and assisting in cortical reorganization.61 This can be combined with MVF.
When desensitization fails, the therapist may need to provide protection to areas that are hypersensitive to enable the patient to perform ADLs and occupational requirements. Use of protective orthotic positioning, elastomer inserts, and padded gloves or garments may be the only alternative available to protect the hypersensitive area from environmental stimuli (Fig. 116-5).
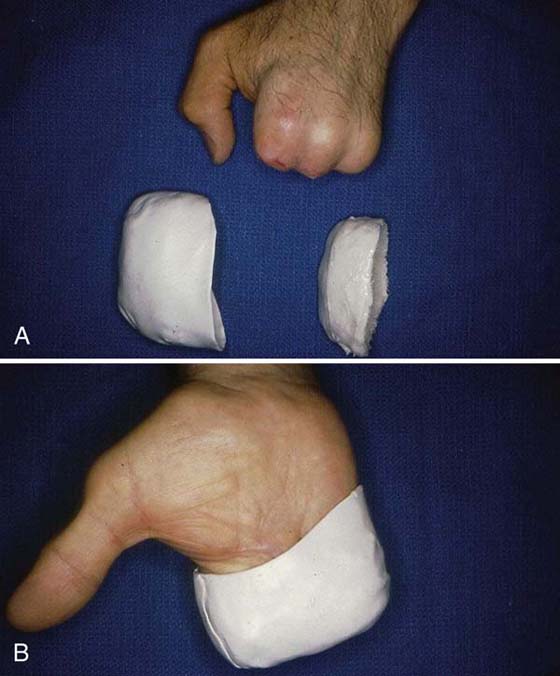
Figure 116-5 A, Hypersensitive amputation stumps. B, Application of Elastomer Putty (Paterson Medical/Sammon Preston, Bolingbrook, Illinois) insert for force absorption and hand thermoplastic cover for protection.
CRPS is recognized as presenting with an ongoing dynamic vasomotor instability problem. This vasomotor instability has been demonstrably affected by Valsalva maneuver, cold pressor test (water immersion at 0°C for 30 seconds), and the passive head-up tilt maneuver.62 Whether this vasomotor instability is the cause of patient discomfort or a manifestation of the syndrome remains in debate. However, because our approach is a practical one, it is best to address these vasomotor changes on a regional and holistic basis to provide sufficient stimulation to the sympathetic and circulatory systems to obtain stability. Many of these patients have been leading a sedentary life since injury, resulting in decreased activity and less tolerance for ADLs, occupational, and avocational activities. Low-impact aerobic activities to increase total body circulation and cardiac output may assist in providing stability to the vasomotor system while increasing circulation to the affected extremity indirectly.25
In keeping with the practical treatment approach, recognizing the patient’s vasomotor state at each session and educating the patient to recognize the vasomotor state is important. The physiologic effect on the peripheral vascular status must be considered when selecting modalities and treatment procedures. In the vasoconstricted state, the hand or extremity may be cool and pale; in this case, the therapist should consider using treatment techniques that increase circulation in the extremity by creating vasodilatation.
Massage and Superficial Thermal Agents. Retrograde massage,63 manual edema mobilization, and manual lymph drainage may assist in increasing circulation, while lending support to lymphatic and venous drainage by decreasing interstitial fluid volume. The topical application of hot packs and paraffin has been shown to increase blood flow in the affected extremity.64 Dry heat modalities such as a Fluidotherapy (Chattanooga Group, Encore Medical, West Chester, Pennsylvania) is an effective method to significantly increase tissue temperatures in the hand.65 In addition, the use of the dry particulate matter (Cellex medium; Chattanooga Group, Encore Medical, West Chester, Pennsylvania) may assist in decreasing hypersensitivity; however, one must be cautious because this level of stimulation also may increase hypersensitivity and pain. Although whirlpool use has been shown to increase tissue temperatures,65 the literature supports findings that whirlpool use increases edema in the upper extremity when administered at temperatures greater than 94°F and especially with the extremity in the dependent position.66 This significant increase in swelling occurs even with the use of active exercises,67 which may be compromised in the CRPS patient. In addition, any thermal effects are actually decreased by the end of a 20-minute treatment session because of the loss in temperature during the procedure.68 Therefore, the use of whirlpool as a superficial heating agent, especially in the treatment of CRPS, is usually not recommended because of the risk of increased edema.
Ultrasound. Ultrasound in the treatment of CRPS, especially for the vasoconstricted patient, has been advocated. Goodman69 reported that six of seven patients with RSD experienced marked to complete relief of pain, decreased edema, and concomitant return of function. Patients were treated with stationary-pulsed ultrasound at 1 to 1.5 W/cm2 with the beam directed over the stellate ganglion. This was combined with other treatments, including moist heat, whirlpool, AROM exercises, and functional activities.69 The use of ultrasound for the treatment of local tissues in RSD also has been advocated by Portwood et al.70 They reported three cases of lower extremity RSD treated successfully using ultrasound at 0.5 to 1.0 W/cm2 applied directly over the peripheral nerves in the affected foot.
Additional Measures. If the patient’s hypersensitivity does not allow the direct application of thermal modalities, other forms of remote heating may be beneficial, such as heating the contralateral limb or a warm bath for total body immersion at home. The use of contrast baths has been advocated. However, theoretically, a contrast bath creates an unstable vasomotor state. Furthermore, there is no support in the literature for its efficacy, and therefore its use must be questioned. All adjunctive modalities used to assist in increasing vasodilation should be immediately followed by an active exercise or stress-loading program (discussed later in this chapter) for the involved extremity. The therapist should counsel the patient to avoid the use of caffeine and nicotine, because these also have been demonstrated to increase vasoconstriction.
Biofeedback. Headley39 recommends the use of temperature biofeedback to treat the vasodilated or vasoconstricted condition. Temperature biofeedback alters sympathetic activity, thereby increasing or decreasing blood flow. This method of biofeedback could affect the sympathetic activity on a long-lasting or even permanent basis. Grunet et al.71 used a combination of thermal biofeedback, relaxation training, and supportive psychotherapy to reduce pain in RSD. They found a significant increase in post-treatment digital temperature, as well as a significant decrease in reported pain (VAS) after treatment sessions ranging from 15 to 60, averaging 23. This decrease was maintained at a 1-year follow-up, and 14 of 20 patients had returned to gainful employment by the 1-year follow-up.
In patients who present with a red, swollen, and warm hand, treatment modalities that increase this vasodilation may exacerbate pain. Modalities that help stabilize the vasomotor system and create vasoconstriction include superficial cryotherapy, such as cold packs or cold immersion baths at 18°C for 10 to 15 minutes.72 Cold modalities should be avoided if the response is exacerbation of pain. One must counsel these patients to avoid alcohol, which is known to have a vasodilatory effect.
Edema must be addressed assertively. It may be the result of vasomotor instability (vasodilation) and lack of active motion. There may also be an inflammatory component.73 Peacock and Van Winkle74 state that edema decreases motion and leads to remodeling of collagen in a shortened position, creating permanent motion loss. Fibrosis may be increased by compromised vascular flow, creating tissue hypoxia and increased cell permeability, and stagnating interstitial fluids containing quantities of protein.75 The control of edema should begin early and be continuous. The most important aspect of control may be in prevention, because interstitial fluid volume can increase 30% to 50% above normal before visible detection.76 In a systematic review Geurts and associates reported that no specific treatment has yet to be proven effective in treating hand edema in poststroke patients with shoulder–hand syndrome.73 Edema should be measured before and after treatment to assess treatment efficacy.
One of the simplest and most effective methods of edema control is through elevation of the hand above heart level on an incline to the elbow. Elevation decreases arterial hydrostatic pressure and assists in lymphatic and venous drainage, resulting in decreased interstitial volume.77 Caution should be exercised when using elevation with a CRPS patient to avoid positioning that exacerbates discomfort. Elevation also should not be emphasized to the point that the patient is no longer using the affected extremity. The use of a sling as a means of edema reduction should be avoided unless required for protection of other pathology.
Active exercise in conjunction with elevation is an effective means of edema control.78 AROM should be initiated early throughout the upper extremity, in full available nonsymptomatic range, to assist in overcoming edema as well as decreasing stiffness. Caution must be exercised, because movement that is too aggressive will result in joint reactivity, increased edema, progressive stiffness, and increasing pain-related fear behavior. Active exercises should be specifically directed at each joint through each plane, while providing support to adjacent joints. It is most effective to perform each of these exercises at low repetitions frequently throughout the day. In addition, active exercises should include differential tendon gliding.79
Retrograde massage is valuable in mobilizing both pitting and brawny edema.63 Manual edema mobilization may be effectively used in the management of a patient with CRPS. The reader is advised to review the extensive discussion on edema management in Chapter 63, “Edema Management,” Chapter 64, “Management of Lymphedema,” and Chapter 65, “Manual Edema Mobilization: Treatment for Edema in the Subacute Hand.” Caution must be exercised when massage is performed over an area of allodynia to avoid exacerbation of pain. Remember that some forms of gentle mechanical stimuli, such as retrograde massage, may increase temporal summation of the patient’s pain.28 This may be avoided by maintaining continuous contact with the patient’s skin. This avoids the intermittent tactile stimulation, which could potentially cause the temporal summation effect.
Joint stiffness is first approached through preventive means in the early phases of CRPS by providing a structured AROM program for the cervical and thoracic spine and entire upper extremity. In addition, active exercises that are activity-directed are extremely important to maintain the patient’s level of cooperation and motivation. Functional activities should center on ADLs and occupational activities that the patient is required to perform and finds most enjoyable. Standardized activities such as Minnesota Rate of Manipulation and Valpar Upper Extremity Work Sample or improvised activities such as using a weighted crate may be effective. It is also beneficial to include the uninvolved extremity in the AROM program as an active assist, such as in bilateral pushing/pulling activities or in the use of wand activities while supine or standing. Examples of these are shown in Figure 116-6. For specific small-joint stiffness, Lankford2 has recommended the use of isolated finger-blocking exercises for each joint of the hand.
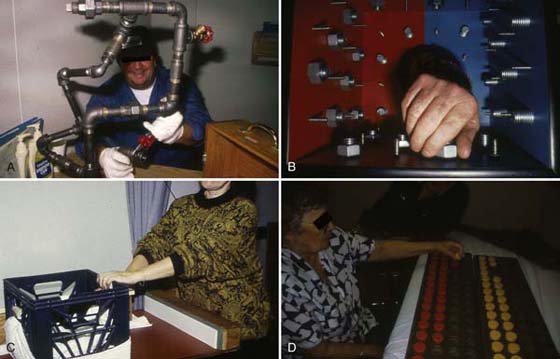
Figure 116-6 Functional activities. A, Pipe assembly and B, Valpar Upper Extremity ROM Work Sample require the use of all joints of the upper extremity. Both activities can be with or without hand tools. C, Weighted-crate push-pull activity is an example of closed-chain exercise or modified stress loading. D, Activity emphasizing shoulder motion and manipulation.
Orthotic positioning has been advocated as an appropriate method to assist in alleviating joint stiffness and contractures. The arthrofibrosis is often associated with the trophic changes seen in factor 4. Any positioning performed should be of a low enough force that it does not exacerbate pain or create increased tissue irritability and reactivity about the joints. Dynamic positioning (Fig. 116-7) at the appropriate force levels may assist in alleviating discomfort via stimulation of large-diameter afferent fibers.52 While in the dynamic positioning system, patients have a tendency to move against the traction, which may stimulate mechanoreceptors and other proprioceptors in the extremity. Dynamic positioning should be used only as an adjunctive technique and should not sacrifice the active functional use of the extremity. Therefore, dynamic positioning may be most appropriately used for short durations of approximately 30 minutes throughout the course of the day.80
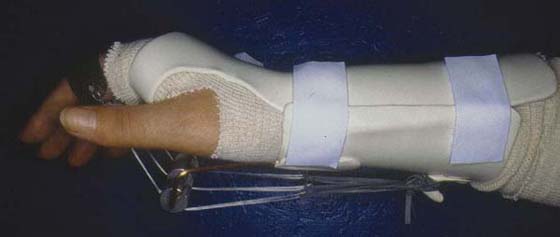
Figure 116-7 The pain-free application of a dynamic metacarpophalangeal joint flexion orthosis for treatment of extension contractures during the painful phase of CRPS.
To assist in edema control and pain management, a resting orthosis for the wrist or wrist and hand may be helpful. As a result of edema, the fingers may assume positions that lead to the development of permanent, nonfunctional contractures. Most common are thumb adduction (first metacarpal), finger metacarpophalangeal joint extension, and interphalangeal joint flexion contractures. Static positioning when appropriate should be used while sleeping to properly position the hand in the safe position (20-degree wrist extension, palmar abduction of the thumb, 70 degrees of metacarpophalangeal joint flexion, and the interphalangeal joints in 0 to 10 degrees). Static orthoses also are appropriate as an assistive measure to position joints for exercise or functional activities in the presence of motor impairment, dystonia, or joint pain. A resting wrist orthosis to maintain wrist position secondary to weakness of wrist extensors would allow for improved digital motion while performing functional activities. A static orthosis also can be used to block specific joint motion to assist the patient in providing more isolated motions to more distal joints or can be used for functional positioning of certain joints to improve hand function, such as an opponens orthosis when CRPS is accompanied by a median nerve injury.
As pain is controlled and the joint deformities become more isolated, static progressive orthotic positioning and serial positioning or casting may be appropriate for isolated contractures. Their ideal use is at night and for prolonged periods using the principles of low-load, prolonged stress. The therapist should avoid overwhelming the patient with many orthoses. This requires prioritizing joint motions that must be achieved to improve overall function of the patient’s hand and extremity. As a guideline, no more than two orthoses should be prescribed at any given time to improve function. Additional orthoses can be added to the patient’s program when previous orthoses are no longer necessary.
In rare instances, joint tightness as a result of CRPS also may be treated with continuous passive motion (CPM) devices. CPM has been used in stroke patients with a paretic hand to decrease edema and maintain joint motion.73 These devices should be used periodically throughout the day or at night to minimize their interference with the active use of the involved extremity that is most important. The CPM device should allow movement through the pain-free range for each of the joints being treated. CPM may contribute to pain relief via the gate-control theory and improve periarticular and cartilage nutrition. These devices may be used appropriately in patients who have accompanying dystonia or nerve paralysis.
PROM is most appropriately used only after the patient’s pain has been controlled. There are no data that support aggressive PROM following medical intervention such as regional or stellate ganglion blocks. Included in passive motion would be joint mobilization procedures to increase ROM and decrease pain.81 Use of these passive motion procedures is contraindicated if hand placement is required over areas of hypersensitivity or allodynia.
The combination of superficial heat and gentle, passive stretch has been shown to be most effective in increasing tissue extensibility when tissue temperatures are increased above 40°C.82 This heat and stretch can be effective in preconditioning the tissues for other activities and treating the stiff hand. The thermal effects of ultrasound also may assist in increasing tissue temperature and increasing its viscoelastic properties for stretching.53 The therapist must remember to apply the stretch, PROM, or AROM while the tissues are still warm and within asymptomatic range.
Controversy surrounding the existence of movement disorders associated with CRPS has dissipated, and they are now recognized as one of the four factors required for diagnosis. The literature now recognizes the presence of movement disorders in CRPS, with the incidence and type variable.83-87 van der Laan et al.88 reported an 88% incidence, Cardoso and Jankovic89 21.4%, Schwartzman and Kerrigan90 21.5%, and Jankovic and van der Laan91 36%. Reported disorders are in retrospective clinical designs with inherent weaknesses, except for those reported by Jankovic and van der Laan.91 Movement disorders have been described as: dystonia (focal dystonia (Fig. 116-8), myoclonus (or jerking movements), increased muscle tone, weakness and difficulty initiating movement (bradykinesia18,83-87,90), and various types of tremor. An example of these uncontrolled jerking motions can be seen in an online video. If a history of tremor, parkinsonism, or other central movement disorder exists,91 the therapist should anticipate the potential for movement disorder to develop.
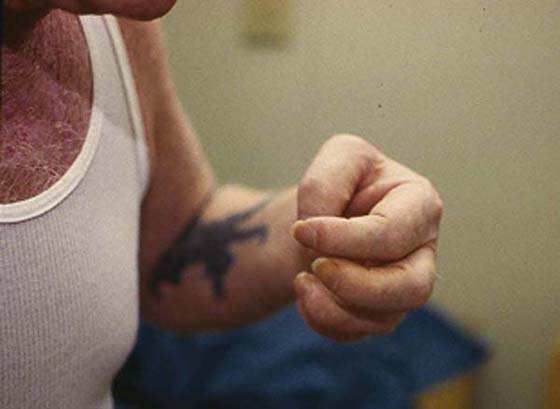
Figure 116-8 Example of a patient presenting with focal dystonia 4 years after onset of CRPS. Note the significantly flexed posture of the elbow, wrist, and digits.
The pathophysiology of movement disorders has been attributed to peripheral,88,92 sympathetic,90,93,94 or the central nervous system mechanisms.83-87 Peripheral factors appear to be involved when movement disorders occur early, while the sympathetic system has been suspected to play a role in both early and delayed onset. Recent literature appears to support the role of the central nervous system47,83-87 in the form of altered sensorimotor processing.83 In addition to motor impairment, many patients with CRPS demonstrate extremity neglect–like symptoms,95-99 which supports the altered sensorimotor theory. This has led to an effective treatment technique via graded motor imagery program (MIP).98,99 A three-step MIP follows a specific order, first requiring recognition of the hand laterality, then imaged hand movements, and finally mirror movements. Moseley reported on the use of MIP to treat 20 CRPS patients in a single blind randomized control trial. There was a 50% decrease in pain as measured by the neuropathic pain scale, and decreased disability as measured with a task-specific numerical rating system.98,99 It is beyond the scope of this chapter to discuss the specifics of this technique, and the reader is referred to the noted references. For the treating therapist the importance is recognizing that patients with CRPS may have difficulty in relating their injured extremity to the whole body.
Another potential theory for development of movement disorders in CRPS is the nociceptive sensitization of interneurons, the waking of “silent nociceptive neurons.”100 To combat this phenomenon, peripheral input from periarticular tissue proprioceptors other than type I and II mechanoreceptors may assist in modulating nociceptive input.24 Early institution of physiologic motion for the entire body may provide this input. For example, having the patient perform large, functional, multijoint movements such as reaching tasks, proprioceptive neuromuscular facilitation patterns, handling therapeutic balls, riding a stationary bike, or walking may provide beneficial input. These techniques also address the reduced activity seen in patients with CRPS as previously suggested. It is important to have the patient avoid exacerbation of pain or vasomotor instability by moving only in the pain-free range. Another potentially beneficial technique is the use of vibration.59 As previously mentioned, Gay et al. reported a 30% increase in ROM in 11 patients undergoing 10 weeks of vibration sessions. They theorized that this occurred as a result of “reestablishing consonance between sensory input and motor output at the cortical level.”
Central nervous system mechanisms might be influenced by clinical tactics such as in these patients presenting with bradykinesia a parkinsonian-type response.101 This motor inhibition or lack of internal drive requires the use of an external set to initiate motion. Techniques such as demonstrating performance of a task, verbal cueing for reaching, temporal cueing for cadence with any movement, or reaching for a moving object such as catching a ball can all be effective.101 Normalizing sensory input to the cortex may help allay the degradation of the somatic cortical representation, which appears to be present in chronic pain and movement disorders.102,103 This might be accomplished by attempting to peripherally desensitize the affected distribution of allodynia or hyperalgesia and also by avoiding stereotyping, repetitive motion tasks, or prolonged posturing. The use of other, unaffected limbs may help preserve the somatotrophic representation. As was discussed earlier, MVF via the use of mirror imagery has also been shown to increase motor function.54 Figure 116-4 demonstrates the use of MVF to assist in recovering motor function.
Conventional surface electromyographic biofeedback can be used in two ways: To inhibit muscle tone and decrease hypertonicity, which may be accentuating guarded posturing, immobilization, and pain. In those patients exhibiting motor inhibition, biofeedback could be used to facilitate specific muscle activation and assist in the performance of functional tasks (Fig. 116-9). When possible, biofeedback should be combined with functional activities to enhance muscle reeducation.
Watson and Carlson104 have reported successful treatment of RSD patients using a three-component stress-loading program. These components include compressive loading of the upper extremity, distraction, and use of other modalities for treatment, including orthotic positioning as necessary. They advise that other modalities and orthotic positioning be used cautiously, especially in the chronic patient. The initial home program they describe begins with 3 to 5 minutes of upper extremity compression via scrubbing with a brush, incrementally increased over a 2- to 3-week period until approximately 10 minutes of scrubbing three times a day is achieved. This is followed on an alternating basis by carrying activities starting with 1 to 5 pounds (.4 to 2.2 kg), increasing as tolerated up to a 10-minute period. The patient is encouraged to perform the carrying (distraction component) whenever ambulating or standing. Watson and Carlson104 reported an initial increase in pain and swelling; however, this decreased after a few days. In 41 patients with long-term follow-up, they reported an 88% improvement rate in pain, 95% improvement in motion, and 84% return to full work status at their same occupation. The object was to provide stress to the tissues while minimizing joint motion. Portwood et al.70 also supported this technique. They reported on three patients with lower extremity RSD who became symptom-free on a program of gradual weight-bearing in conjunction with ultrasound.
There are other forms of tissue stressing that are components of a CRPS program, such as functional activities and active and resisted ROM exercises. In addition, upper extremity closed-chain exercises also are a form of stress-loading. However, there is usually a motion component with these forms of tissue stressing that may tend to exacerbate pain in certain patients with CRPS. Therefore, it may be most appropriate initially to use the stress-loading program for those patients and introduce other forms of upper extremity tissue stressing as their symptoms come under control.
It has been postulated that some patients with CRPS may have associated myofascial pain syndrome.26 Examination of the patient may demonstrate the presence of active myofascial trigger points as a contributory source of the patients’ pain. Deactivation of these accompanying trigger points resulted in a reduction of VAS from 9.1 to 3.0 following treatment.26 Techniques that may be used for deactivation included: Spray and stretch, massage, and modalities such as thermal, electrical, and ultrasound.64,105 Following myofascial trigger point injections, the patient must be instructed in appropriate stretching techniques of the affected muscles to sustain the relief. The reader is referred to the work of Travell and Simonds for more specific treatment approaches.105
Investigators theorize that adverse tension in the sympathetic trunk may be a strong maintainer of sympathetic activity in the body.106 The nervous system is dynamic and must be capable of tolerating the tension placed on it by movements of the body and its tissues. Movement or mechanical tension of the sympathetic chain as it lies along the posterior aspect of the vertebral column may influence sympathetic outflow. The application of movement to the nervous system is not unfamiliar, as seen in the nerve mobilization and brachial plexus mobilization techniques.61 The need for the nervous system to elongate and glide can be applied to the sympathetic system. Treatment of the theorized hypomobile sympathetic system requires manual and stretching techniques that assist in increasing the sympathetic nervous system’s tolerance of adverse tension. This may also be accomplished by mobilization of the thoracic vertebrae or neural mobilization using slump techniques.107 This work is theoretically based at this time, and the reader is referred to Butler’s work106 for information regarding these techniques.
The therapist cannot treat this complex and devastating problem alone. A team approach of medical intervention through medication, regional and local blocks, surgical techniques, professional psychological support, and hand therapy is essential. Communication among all parties involved is of vital importance. The therapist plays a critical role in maintaining the lines of communication. Immediately after block anesthesia, the patient can be seen in therapy to begin an active therapy program.2
CRPS is a dynamic problem with multiple theories for its etiology and maintenance. It is probably the most challenging pain syndrome that the hand therapist will face. To assist the therapist in the clinical decision-making process, the sequence of examination and available treatment options are shown in Figure 116-3. Previously a lack of consensus on this syndrome’s diagnostic criteria presented a formidable challenge to the therapist in developing a treatment program; however, the recently proposed diagnostic criteria including pain plus the four sign and symptom factors have made this easier. These criteria have assisted my organization of a previous practical treatment approach of treating the signs and symptoms. This chapter has been organized with the new criteria in mind.
Management continues to require an understanding of the dimensions, components, and techniques available to recognize and modulate the patient’s pain. Early recognition is the key to alleviating most CRPS problems. Often it is the therapist who has the opportunity to recognize the symptomatology early. Treatment of patients should be holistic and not address a single component or affected tissue. The therapist must be cognizant of the biopsychosocial component to this syndrome, and treatment formulation must include this aspect to be successful. From a practical standpoint, treating the patients’ signs and symptoms seems to be the logical way of attacking this difficult problem.
The hand therapist is cautioned not to become the “pain terrorist” and to avoid exacerbating the patient’s symptoms. Lankford2 has stated, “The most important aspect of hand therapy is to make sure that the therapist does not use physical means that will produce pain.” This caution applies to examination as well as treatment. Any exacerbation that lasts beyond the treatment session is ill-advised. The program should be structured with very specific directions given for repetitions, duration, and timetables. These patients are usually overwhelmed by their symptoms, and the therapist must assist them in focusing on their treatment and avoid contributing to this feeling. The interventions available to the therapist have grown especially when it comes to pain and motor dysfunction. Finally, successful treatment of CRPS is directly related to active participation by the patient. The therapist must play a motivational role for the patients by assisting them with resolution of their symptoms.
1. Haddox JD. A call for clarity. In: Campbell JN, ed. Pain 1996—An Upated Review—Refresher Course Syllabus. Seattle: IASP Press; 1996:97–99.
2. Lankford L. Reflex sympathetic dystrophy. In: Hunter J, ed., et al. Rehabilitation of the Hand. St. Louis: CV Mosby; 1984:509–532.
3. Betcher A, Casten D. Reflex sympathetic dystrophy criteria for diagnosis and treatment. Anesthesiology. 1955;16:994–1003.
4. Casten D, Betcher A. Reflex sympathetic dystrophy. Surgery, Gynecology, Obstetrics. 1955;100:97–101.
5. Janig W. Pathobiology of reflex sympathetic dystrophy: some general considerations. In: Stanton-Hicks M, Janig W, Boas R, eds. Reflex Sympathetic Dystrophy. Kluwer: Boston; 1990:42–54.
6. Bonica J. Causalgia and other reflex sympathetic dystrophies. In: Bonica J, ed. The management of pain. Philadelphia: Lea & Febiger; 1990.
7. Bruehl S, Hardena RN, Galer BS, et al. External validation of IASP diagnostic criteria for complex regional pain syndrome and proposed research diagnostic criteria. Pain. 1999;81:147–154.
8. Bruehl S, Harden RN, Galer BS, et al. Complex regional pain syndrome: are there distinct subtypes and sequential stages of the syndrome? Pain. 2002;95:119–124.
9. Harden R, Bruehl S, Galer BS, et al. Complex regional pain syndrome: are the IASP diagnostic criteria valid and sufficiently comprehensive? Pain. 1999;83:211–219.
10. Bohm E. Transcutaneous electrical nerve stimulation in chronic pain after peripheral nerve injury. Acta Neurochir. 1978;40:277–287.
11. Mersky H, Bogduk N. Classification of Chronic Pain: Descriptions of Chronic Pain Syndromes and Definition of Pain Terms. 2nd ed Seattle: IASP Press; 1994. 2 line 3
12. Baron R, Blumberg H, Janig W. Reflex sympathetic dystrophy: a reappraisal. In: Janig W, Stanton-Hicks M, eds. Progress in Pain Research and Management. Seattle: IASP Press; 1996:25–48.
13. Campbell J. Complex regional pain syndrome and the sympathetic nervous system. In: Campbell J, ed. Pain 1996—An Updated Review—Refresher Course Syllabus. Seattle: IASP Press; 1996:89.
14. Veldman P, Goris R. Multiple reflex sympathetic dystrophy. which patients are at risk for developing a recurrence of reflex sympathetic dystrophy in the same or another limb. Pain. 1996;64:463–466.
15. Harden R, Bruehl S, Stanton-Hicks M, Wilson PR. Proposed new diagnostic criteria for complex regional pain syndrome. Pain Medicine. 2007;8(4):326–331.
16. Wilson P, Stanton-Hicks M, Harden R. CRPS: current diagnosis and therapy. In: Wilson PRM, Stanton-Hicks M, Michael MB, Norman HR, eds. Progress in Pain Research and Management. Seattle: IASP Press; 2005:45–58.
17. Lankford L. Reflex sympathetic dystrophy. In: Moer G, Spinner M, eds. Management of Peripheral Nerve Problems. Philadelphia: WB Saunders; 1980.
18. Birklein F., et al. Neurological findings in complex regional pain syndromes-analysis of 145 cases. Acta Neurologica Scandinavica. 2000;101:262–269.
19. Boas RA. Complex regional pain syndromes: symptoms, signs and differential diagnosis. In: Janig W, Stanton-Hicks M, eds. Reflex Sympathetic Dystrophy: A Reappraisal, Progress in Pain Research and Management. Seattle: IASP Press; 1994.
20. Wilson PR, Low PA, Bedder MD. Diagnostic algorithm for complex regional pain syndromes. In: Janig W, Stanton-Hicks M, eds. Reflex Sympathetic Dystrophy: A Reappraisal, Progress in Pain Research and Management. Seattle: IASP Press; 1996:93–105.
21. Perez R, Keijzer C, Bezemer PD, et al. Predictive value of symptom level measurements for complex regional pain syndrome type I. European Journal of Pain. 2005;9:45–56.
22. Danidoff G, Morey K, Amann M, Stamps J. Pain management in reflex sympathetic dystrophy syndrome. Pain. 1988;32:27–34.
23. Melzack R. The Puzzle of Pain. New York: Basic Books; 1973.
24. Melzack R, Wall P. Pain mechanisms: a new theory. Science. 1965;150:971.
25. Asbury A, Fields H. Pain due to peripheral nerve damage—a hypothesis. Neurology. 1984;34:1590–1597.
26. Imamura S, Lin, Teixeria, et al. The importance of myofascial pain syndrome in reflex sympathetic dystrophy (or complex regional pain syndrome). Physical Medicine and Rehabilitation Clinics of North America. 1997;8:207–210.
27. Gradl G, Schurmann M. Sympathetic dysfunction as a temporary phenomenon in acute posttraumatic CRPS I. Clin Auton Res. 2005;15:29–34.
28. Price D, Lang S, Huitt C. Sensory testing of pathophysiological mechanisms of pain in patients with reflex sympathetic dystrophy. Pain. 1992;36:163–173.
29. Berger P. The role of the physiotherapist in the treatment of complex peripheral pain syndromes. Pain Reviews. 1999;6:211–232.
30. Dommerholt J. Complex regional pain syndrome—2: physical therapy management. Journal of Bodywork Movement Therapies. 2004;8(4):241–248.
31. Stanton-Hicks M, Baron R, Boas R, et al. Complex regional pain syndromes: guidelines for therapy. The Clinical Journal of Pain. 1998;14(2):155–166. June 1998
32. Husslage P. Physiotherapy and its regimen in the treatment of reflex sympathetic dystrophy. Pain Clinic. 1995;8(1):77–79.
33. Mak P, Irwin M, Tsui S. Functional improvement after physiotherapy with a continuous infusion of local anaesthetics in patients with complex regional pain syndrome. ACTA Anaesthesiol Scand. 2003;47:94–97.
34. Oerlemans H, Oostendorp RA, de Boo T, Goris RJ. Pain and reduced mobility in complex regional pain syndrome I: outcome of a prospective randomised controlled clinical trial of adjuvant physical therapy versus occupational therapy. Pain. 1999;83:77–83.
35. Oerlemans H, Goris JA, de Boo T, Oostendorp RA. Do physical therapy and occupational therapy reduce the impairment percentage in reflex sympathetic dystrophy? American Journal of Physical Medicine & Rehabilitation. 1999;78(6):533–539. November/December 1999
36. Oerlemans H, Oostendorp RA, de Boo T, et al. Adjuvant physical therapy versus occupational therapy in patients with reflex sympathetic dystrophy/complex regional pain syndrome type I. Arch Phys Med Rehabil. 2000;81(January 2000):49–56.
37. Severens J, Oerlemans HM, Weegels AJ, et al. Cost-effectiveness analysis of adjuvant physical or occupational therapy for patients with reflex sympathetic dystrophy. Arch Phys Med Rehabil. 1999;80(September 1999):1038–1043.
38. Kemler MA, Rijks CPM, de Vet HCW. Which patients with chronic reflex sympathetic dystrophy are most likely to benefit from physical therapy? Journal of Manipulative and Physiological Therapeutics. 2001;24(4):272–277.
39. Headley B. Historical perspective of causalgia management of sympathetically maintained pain. Physical Therapy. 1987;67:1370–1374.
40. Hardy M, Merritt W. Physiological evaluation and pain assessment in patients with reflex sympathetic dystrophy. J Hand Ther. 1988;1:155–163.
41. Ciccone D, Bandilla E, Wu W. Psychological dysfunction in patients with relex sympathetic dystrophy. Pain. 1997;71:322–333.
42. Crombez G, Vlaeyen JW, Heuts PH, Lysens R. Pain related fear is more disabling than pain itself: evidence on the role of pain-related fear in chronic back pain disability. Pain. 1998;80:329–339.
43. deJong J, Vlaeyen JW, Onghena P, et al. Reduction of pain related fear in complex regional pain syndrome type I: the application of graded exposure in vivo. Pain. 2005;116:264–275.
44. Vlaeyen J, Linton S. Fear avoidance and its consequences in chronic musculoskeletal pain: a state of the art. Pain. 2000;85:317–332.
45. Boersma K, Linton S, Overmeer T, et al. Lowering fear-avoidance and enhancing function through exposure in vivo: a multiple baseline study across six patients with back pain. Pain. 2004;108:8–16.
46. Schasfoort F, Bussmann JB, Zandbergen AM, Stam HJ. Impact of upper limb complex regional pain syndrome type I on everyday life measured with a novel upper limb-activity monitor. Pain. 2003;101:79–88.
47. Schasfoort F, Bussmann JB, Stam HJ. Impairments and activity limitations in subjects with chronic upper-limb complex regional pain syndrome type 1. Arch Phys Med Rehabil. 2004;85(April):557–566.
48. Schasfoort F, Bussmann, Krijnen, et al. Upper limb activity over time in complex regional pain syndrome type I as objectively measured with an upper limb-activity monitor: an exploration multiple case study. European J Pain. 2006;10:31–39.
49. Geertzen J, Dijkstra PU, van Sonderen ELP, et al. Relationship between impairments, disability and handicap in reflex cympathetic dystrophy patients: a long-term follow up study. Clinical Rehabilitation. 1998;12:402–412.
50. Galer B, Henderson J, Perander J, Jensen MP. Course of symptoms and quality of life measurement in complex regional pain syndrome: a pilot study. Journal of Pain and Symptom Management. 2000;20:286–292.
51. Forouzanfar T, Weber WEJ, Kemler M, van Kleef M. What is a meaningful pain reduction in patients with complex regional pain syndrome type I? The Clinical Journal of Pain. 2003;19(5):281–285.
52. Manheimer J. Non-medical and non-invasive pain control techniques in the management of rheumatoid diseases and related musculoskeletal disorders. Journal Rheumatology. 1987;14:26–32.
53. Ziskin M, McDiaimid T, Michlovtiz S. Therapeutic ultrasound. In: Michlovitz S, ed. Thermal Agents in Rehabilitation. Philadelphia: FA Davis; 1990:134–166.
54. McCabe C, Haigh RC, Ring EF, et al. A controlled pilot study of the utility of mirror visual feedback in the treatment of complex regional pain syndrome (type I). Rheumatology. 2003;42:97–101.
55. Tichelaar V, Geertzen JHB, Keizer D, van Wilgen CP. Mirror box therapy added to cognitive behavioural therapy in three chronic complex regional pain syndrome type 1 patients: A pilot study. International Journal of Rehabilitation Research. 2007;30(2):181–188. June
56. Bin G, Cruccu G, Hagbath K. Analgesic effect of vibrations and cooling on pain induced by intraneural electrical stimulation. Pain. 1984;18:239–248.
57. Lundenberg T. Long term results of vibratory stimulation as a pain relief measure for chronic pain. Pain. 1984;20:13–23.
58. Lundenberg T, Nordemar R, Ottoson D. Pain alleviation by vibratory stimulation. Pain. 1984;20:25–44.
59. Gay A, Parratte S, Salazard B, et al. Proprioceptive feedback enhancement induced by vibratory stimulation in complex regional pain syndrome type I. An open comparative pilot study in 11 patients. Joint Bone and Spine. 2007;74:461–466.
60. Barber L. Desensitization of the traumatized hand. In: Hunter J, ed., et al. Rehabilitation of the Hand. St. Louis: CV Mosby; 1984:493–502.
61. Acerra N, Moseley L. Dysynchiria: watching the mirror image of the unaffected limb elicits pain on the affected side. Neurology. 2005;65:751–753.
62. Christensen K, Herrikson O. The reflex sympathetic dystrophy syndrome and experimental study of sympathetic reflex control of subcutaneous blood flow in the hand. Scand J Rheumatol. 1983;12:263–267.
63. Wood EC. Beard’s Massage Principles and Techniques. Philadelphia: WB Saunders; 1974.
64. Abramson D. Changes in blood flow, oxygen uptake, and tissue temperature produced by a topical application of wet heat. Archives of Physical Medicine and Rehabilitation. 1961;42:305.
65. Borrel R, Parker R, Henley EJ, et al. Comparison of in vivo temperatures produced by hydrotherapy paraffin wax treatment and fluidotherapy. Physical Therapy. 1980;60(10):1273–1276.
66. Walsh MT. Relationship of Hand Edema to Upper Extremity Position and Water Temperature during Whirlpool Treatments in Normals. Philadelphia: Temple University; 1983.
67. Schultz K. The Effect of Active Exercise during Whirlpool on the Hand. San Jose, California: San Jose State University; 1982.
68. Walsh M. Hydrotherapy: the use of water as a therapeutic agent. In: Michlovitz S, ed. Thermal Agents in Rehabilitation. Philadelphia: FA Davis Company; 1996:139–166.
69. Goodman C. Treatment of hand shoulder syndrome: combined ultrasonic application to stellate ganglion and physical medicine. NY State Journal of Medicine. 1971.559–562.
70. Portwood M, Lieberman J, Taylor R. Ultrasound treatment of reflex sympathetic dystrophy. Archives of Physical Medicine and Rehabilitation. 1987;68:116–118.
71. Grunet B, Devine CA, Sanger JR, et al. Thermal regulation for pain control in reflex sympathetic dystrophy syndrome. JHS. 1990;15A:615–618.
72. Michlovitz SL. Cryotherapy: The use of cold as a therapeutic agent. In: Michlovitz S L, ed. Thermal Agents in Rehabilitation. Philadelphia: FA Davis; 1986:73.
73. Geurts A, Visschers BA, van Limbeek J, Ribbers GM. Systematic review of aetiology and treatment of post-stroke hand oedema and shoulder-hand syndrome. Scand J Rehab Med. 2000;32:4–10.
74. Peacock ED, Van Winkle W. Surgery and Biology of Wound Repair. Philadelphia: WB Saunders; 1970.
75. Saferin E, Posch J. Secretans disease: post traumatic hand edema of the dorsum of the hand. Plastic Reconstructive Surgery. 1976;58:703.
76. Guyton A. Textbook of Medical Physiology. 6th ed Philadelphia: W. B. Saunders; 1975.
77. Vasudevan S, Melvin J. Upper extremity edema control rationale of treatment techniques. American Journal of Occupational Therapy. 1979;33:520.
78. Whitson T, Allen B. Management of the burned hand. Journal of Trauma. 1971;7:895.
79. Wehbe M, Hunter J. Flexor tendon gliding in the hand I: in vivo excursions II: differential gliding. Journal Hand Surgery. 1985;10A:570.
80. Mullins P. Reflex sympathetic dystrophy. In: Stanley B, Tribuzi S, eds. Concepts in Hand Rehabilitation. Philadelphia: FA Davis; 1992:446–470.
81. Maitland G. Peripheral Manipulation. 2nd ed London: Butterworths; 1977.
82. Lehman J. Effect of therapeutic temperatures on tendon extensibility. Archives of Physical Medicine and Rehabilitation. 1970;51:81.
83. Juottonen K., et al. Altered central sensorimotor processing in patients with complex regional pain syndrome. Pain. 2002;98:315–323.
84. Ribbers MT, Geurts A, den Otter R. Reflex sympathetic dystrophy of the left hand and motor impairments of the unaffected right hand: impaired central motor processing? Arch Phys Med Rehabil. 2002;83(January):81–85.
85. Rommel O., et al. Hemisensory impairment in patients with complex regional pain syndrome. Pain. 1999;80:95–101.
86. Schwenkreis P, Janssen F, Rommel O, et al. Bilateral motor cortex disinhibition in complex regional pain syndrome (CRPS) type I of the hand. Neurology. 2003;61(August):515–519.
87. Verdugo R, Ochoa J. Abnormal movements in complex regional pain syndrome: assessment of their nature. Muscle & Nerve. 2000;23:198–2005.
88. Van Der Laan L, Terlaak HJ, Gabreels-Festen A. Complex regional pain syndrome type I (RSD): pathology of skeletal muscle and peripheral nerve. Neurology. 1998;51:20–25.
89. Cardoso F, Jankovic J. Peripherally induced tremor and parkinsonism. Arch Neurol. 1991;52:263–270.
90. Schwartzman R, Kerrigan J. The movement disorders of reflex sympathetic dystrophy. Neurology. 1990;40:57–61.
91. Jankovic J, van der Laan C. Dystonia and tremor induced by peripheral trauma: predisposing factors. Journal Neurol Neurosurg Psychiat. 1988;51:1512–1519.
92. Eccles R, Kozak W, Wsterman RA. Enhancement of spinal monosynaptic reflex responses after denervation of synergic hind-limb muscles. Exper Neurology. 1962;6:451–464.
93. Deuschl G, Blumberg H, Lucking CH. Tremor in reflex sympathetic dystrophy. Arch Neurol. 1991;48:1247–1252.
94. Yokota T, Furukawa T, Tsukagoshi H. Motor paresis improved by sympathetic block: a motor form of reflex sympathetic dystrophy? Arch Neurol. 1989;46:683–687.
95. Forderreuther S, Sailer U, Straube A. Impaired self-perception of the hand in complex regional pain syndrome (CRPS). Pain. 2004;110:756–761.
96. Galer B, Jensen M. Neglect-like symptoms in complex regional pain syndrome: results of a self-administered survey. Journal of Pain and Symptom Management. 1999;18:213–217. No. 3(September)
97. Moseley G. Why do people with complex regional pain syndrome take longer to recognize their affected hand? Neurology. 2004;June(62):2182–2186.
98. Moseley G. Graded motor imagery is effective for long-standing complex regional pain syndrome: a randomised controlled trial. Pain. 2004;108(January):192–198.
99. Moseley G. Is successful rehabilitation of complex regional pain syndrome due to sustained attention to the affected limb? A randomised clinical trial. Pain. 2005;114:54–61.
100. Schott GD. Visceral afferents: their contribution to sympathetic dependent pain. Brain. 1994;117:397–413.
101. Majsak M, Kaminski T, Gentile AM, Flanagan JR. The reaching movements of patients with parkinson’s disease under self-determined maximal speed and visually cued conditions. Brain. 1998;121:755–766.
102. Byl NN, Melnick M. The neural consequences of repetition: clinical implications of a learning hypothesis. Journal of Hand Therapy. 1997;10:160–174.
103. Kaas JH, Merzenich MM, Killackey HP. The reorganization of somato sensory cortex following peripheral nerve damage in adult and developing mammals. Ann Rev Neurosci. 1983;6:325–356.
104. Watson H, Carlson L. Treatment of reflex sympathetic dystrophy of the hand with an active “stress loading” program. Journal Hand Surgery. 1987;12A:779–785.
105. Travell J, Simonds D. Myofascial pain and dysfunction. In: The Triggerpoint Manual. Baltimore: Williams and Wilkins; 1983:10–64.
106. Butler D. The Sensitive Nervous System. Adelaide, Australia: Noigroup Publications; 2000.
107. Lampen-Smith R. Complex regional pain syndrome i (rsd) and the physiotherapeutic intervention. New Zealand Journal of Physiotherapy. 1997.19–23. (April 1997)