The Sacrum, Sacroiliac Joint, and Coccyx
This chapter begins with a discussion of the bony sacrum. Then, because of its clinical significance, the sacroiliac joint is covered in detail. This is followed by a discussion of the anatomy of the coccyx.
The Sacrum
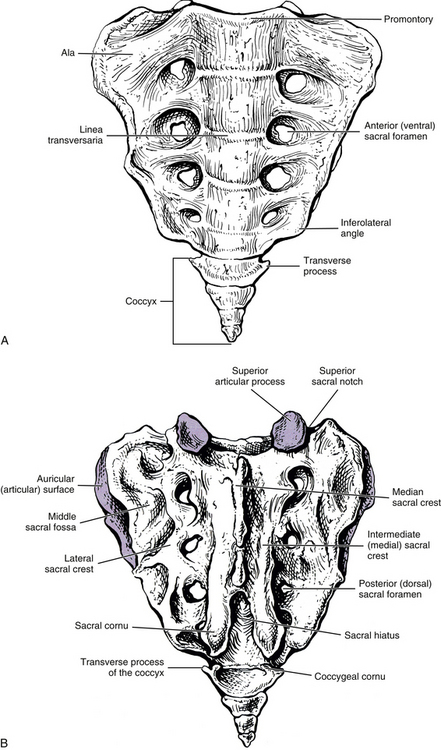
The sacrum is composed of five fused vertebral segments. The sacral segments decrease in size from the first to the fifth, making the sacrum triangular in shape. Consequently, the wider superior surface of the sacrum is known as the sacral base, and the smaller inferior surface is known as the apex (Figs. 8-1 and 8-2). The height of the sacrum is approximately 10.6 cm (4.2 inches) and its width at the base approximately 10.9 cm (4.3 inches). The height may be slightly larger in males and the width slightly larger in females (Hassanein, 2011).
The sacrum is concave anteriorly (kyphotic), and as with the other primary kyphosis (the thoracic region), its curvature helps to increase the size of a bony body cavity, in this case the pelvis. The sacrum is normally positioned so that its base is located anterior to its apex. Therefore the sacral curve faces anteriorly and inferiorly (Standring et al., 2008). The sacral curve is more pronounced in humans than in other mammals, including monkeys and apes. In addition, the human sacral curve is almost nonexistent in infants but becomes more pronounced with age (Abitbol, 1989). The combination of upright posture, supine sleeping posture, and a well-developed levator ani muscle is responsible for the increased sacral curve in humans. Abitbol (1989) also found that the frequency of sleeping in the supine posture and the younger the age when this posture was first assumed for sleeping were positively associated with the size of the sacral curve. An increased sacral kyphosis and increased slope of the superior S1 bony end plate have both been associated with L5-S1 spondylolisthesis in children and adolescents. The increased kyphosis and/or S1 slope enhances the distractive stress on the L5 pars interarticulares and also promotes slippage of the L5 vertebral body once the pars have failed. Consequently, these changes in sacral morphology enhance the progression of spondylolisthesis, and may even predispose individuals to developmental spondylolisthesis (Wang et al., 2008).
The sacrum ossifies much like any other vertebra, with one primary center located in the anterior with a centrally located primitive vertebral body and one primary center found in each posterior arch. Unique to the sacrum is that the costal elements develop separately and then fuse with the remainder of the posterior arch to form the solid mass of bone lateral to the pelvic sacral foramina. Secondary centers of ossification are complex, with centers developing on the superior and inferior aspects of each sacral vertebral body, the lateral and anterior aspects of each costal element, the spinous tubercles, and the lateral (auricular) surface of the sacrum. Most centers fuse by approximately the twenty-fifth year of life, but ossification and fusion of individual segments continue until later in life. Early in development, fibrocartilage forms between sacral bodies. These represent rudimentary intervertebral discs (IVDs) and usually become surrounded by bone as the sacral bodies fuse with one another. However, the central region of these “discs” usually remains unossified throughout life.
Sacral Base
The sacral base is composed of the first sacral segment. It has a large body that is the homologue of the vertebral bodies (see Figs. 8-1 and 8-2). This body is wider from left to right than from front to back. The anterior lip of the sacral body is known as the promontory. The vertebral foramen of the first sacral segment is triangular in shape and forms the beginning of the sacral canal. This canal extends the length of the sacrum. The pedicles of the first sacral segment are small and extend to the left and right laminae. The laminae of the first segment meet posteriorly to form the spinous tubercle. The transverse processes (TPs) extend laterally and fuse with the costal elements to form the large left and right sacral alae, which are also known as the lateral sacral masses. The bony trabeculae of the inner cancellous bone of the sacral ala are significantly less dense than those in the sacral bodies (Peretz, Hipp, & Heggeness, 1998). Each lateral sacral mass is concave on its anterior surface, allowing it to accommodate the psoas major muscle. The psoas major passes across the sacrum before inserting onto the lesser trochanter of the femur.
Extending superiorly from the posterior surface of the sacral base are the left and right articular processes. These processes generally face posteriorly and slightly medially. However, the plane in which these processes lie varies considerably (Dommisse & Louw, 1990). Their orientation ranges from nearly a coronal plane to almost a sagittal one. These processes also frequently are asymmetric in orientation, with one process more coronally oriented and the other more sagittally oriented. Such asymmetry is known as tropism and usually can be detected on standard anterior-posterior x-ray films. The superior articular processes possess articular facets on their posterior surfaces that articulate with the inferior articular facets of the L5 vertebra. The zygapophysial (Z) joints formed by these articulations are more planar than those between two adjacent lumbar vertebrae, and they usually are much more coronally oriented than the lumbar Z joints (see Chapter 7). However, because of the wide variation of the plane in which the superior articular processes lie, the orientation of the lumbosacral Z joints also varies in corresponding fashion.
Similar to lumbar superior articular processes and facets, a small percentage of sacral superior articular processes and facets can be hypoplastic, or rudimentary. Such hypoplastic facets have been linked to low back pain. In addition, rudimentary S1 facets are related to the development of ipsilateral accessory articulations between the transverse process of L5 and the superior aspect of the sacral ala. Bilateral rudimentary S1 superior articular facets are related to bilateral accessory L5-S1 articulations as well (Mahato, 2010a).
The left and right superior sacral notches are just lateral to the superior articular facets. These notches allow for passage of the left and right posterior primary divisions of the L5 spinal nerve.
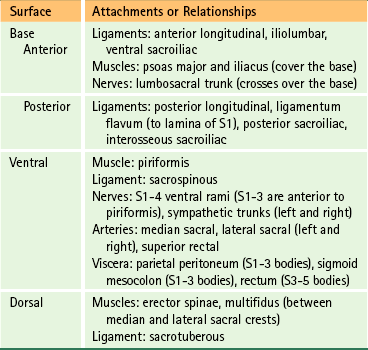
The muscular and ligamentous attachments to the sacral base and the anterior and posterior surfaces of the sacrum are listed in Table 8-1. This table also provides the key anatomic relationships between these regions of the sacrum, and the neural, muscular, and visceral structures that contact them.
Lateral Surface
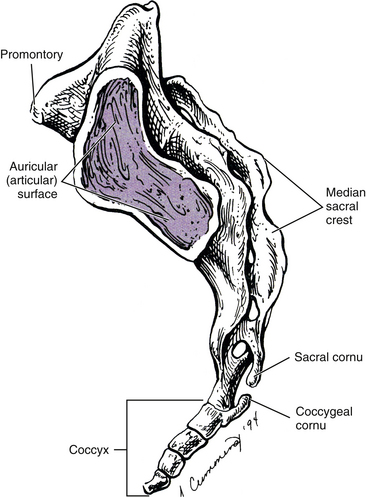
The lateral surface of the sacrum (see Fig. 8-2) is composed of the TPs of the five sacral segments, fused with the costal elements of the same segments. This surface contains the auricular surface. The auricular surface of the sacrum articulates with the auricular surface of the ilium. The sacral auricular surface is concave posteriorly and extends across the lateral aspects of three of the five sacral segments. Within the region surrounded by the concavity of the auricular surface are several elevations and depressions that serve as attachment sites for the ligaments that support the sacroiliac joint posteriorly. These ligaments and the sacroiliac joint are discussed in detail later in this chapter. Inferior to the auricular surface, the lateral surface of the sacrum curves medially and becomes thinner from anterior to posterior. The inferior and lateral angle of the sacrum is located at approximately the level of the junction of the fourth and fifth sacral segments. Below this angle the sacrum rapidly tapers to the sacral apex. The apex of the sacrum has an oval-shaped facet on its inferior surface for articulation with a small disc between the sacrum and coccyx.
Sacral Canal and Sacral Foramina
The sacral canal is composed of the vertebral foramina of the five fused sacral segments. The left and right lateral walls of the canal each contain four intervertebral foramina (IVFs). Each IVF is continuous laterally with a ventral (pelvic) and a dorsal sacral foramen. The sacral canal ends inferiorly as the sacral hiatus (see “Structures Exiting the Sacral Hiatus” and “Dorsal Surface”).
The cauda equina extends inferiorly through the beginning of the sacral canal and within the subarachnoid space. The arachnoid mater and dura mater end at about the level of the S2 spinous tubercle. The sacral roots exiting below this level must pierce the inferior aspect of the arachnoid and dura to continue inferiorly through the sacral canal. In the process, these roots receive a dural root sleeve. Dorsal and ventral roots unite within their dural sleeve to form a spinal nerve and then exit a sacral IVF.
Structures Exiting the Sacral Hiatus
Both the left and the right S5 nerves and the coccygeal nerve of each side exit the sacral hiatus just medial to the sacral cornua of the same side. They proceed inferiorly and laterally, wrapping around the inferior tip of the sacral cornua (see Dorsal Surface). The posterior primary divisions (PPDs) of these nerves pass posteriorly and inferiorly to supply sensory innervation to the skin over the coccyx. The S5 and coccygeal anterior primary divisions (APDs) pass anteriorly to pierce the coccygeus muscle and enter the inferior aspect of the pelvis. Here they are joined by the ventral ramus of the S4 nerve to form the coccygeal plexus. This small plexus gives off the anococcygeal nerves that help to supply the skin adjacent to the sacrotuberous ligament.
The end of the filum terminale also passes through the sacral hiatus. The filum terminale (see Chapter 3) originates from the most inferior aspect of the spinal cord, where it is known as the filum terminale internum. It passes through the lumbar cistern of cerebrospinal fluid (CSF), pierces the inferior aspect of the arachnoid and dura (at generally the level of the S2 segment), and then becomes known as the filum terminale externum (coccygeal ligament). After exiting the sacral hiatus, the filum terminale externum attaches to the posterior surface of the first coccygeal vertebral segment.
Ventral Surface
The junctions of the five fused sacral segments form lines that can be seen running across the central aspect of the anterior, or pelvic, surface of the sacrum. These junctions are known as the linea transversaria (also known as transverse lines, or transverse ridges). Remnants of the IVDs are located just deep to the transverse lines. These “discs” frequently remain throughout life and can be seen on standard x-ray films and magnetic resonance imaging (MRI) scans. The vertebral bodies of the five fused sacral segments lie between the transverse lines and medial to the pelvic sacral foramina (see Fig. 8-1).
The ventral surface of the sacrum displays four pairs of ventral (pelvic or anterior) sacral foramina. These foramina are continuous posteriorly and medially with the sacral IVFs. The sacral IVFs, in turn, are continuous with the more medially located sacral canal. The anterior primary divisions (APDs) of the S1 through S4 sacral nerves exit the pelvic sacral foramina. The APDs are accompanied within these openings by branches of the lateral and median sacral arteries and by segmental veins. Located between the pelvic sacral foramina of the same side are the costal elements. The cortical bone in these regions between the ventral sacral foramina is sturdy (dense), and the cortical bone between the S1 and S2 ventral sacral foramina is the most dense bone of the entire sacrum (Ebraheim et al., 2000). The costal elements fuse posteriorly with the TPs of the sacral segments.
“Sacral skewness” refers to a relatively common (incidence = 24%), but not frequently discussed, anomalous lateral curvature of the sacrum. The clinical significance of sacral skewness is currently unknown (Wu et al., 2009).
The muscular and ligamentous attachments to the anterior surface of the sacrum are listed in Table 8-1. Also, Figures 8-20 and 8-21 demonstrates the major arteries and nerves associated with the anterior surface of the sacrum.
Dorsal Surface
The dorsal surface of the sacrum is irregular in shape (see Fig. 8-1). The superior articular processes extend from the superior aspect of the sacral base (see Sacral Base).
Four pairs of dorsal (posterior) sacral foramina are located among the five fused sacral segments. These openings are continuous anteriorly and medially with the IVFs of the sacral segments. The PPDs of the S1 through S4 spinal nerves exit through these openings. The PPDs are accompanied by small segmental arteries and veins.
The posterior surface of the sacrum contains five longitudinal (vertical) ridges known as the median, intermediate (left and right), and lateral (left and right) sacral crests (see Fig. 8-1). These crests are homologous to the spinous processes, articular processes, and the TPs of the rest of the spine, respectively.
The median sacral crest is composed of four fused spinous tubercles that form the posterior boundary of the sacral canal. Each sacral tubercle is formed by the union of the left and right laminae of the sacral vertebral segments. Usually (82% of individuals) the S2 sacral tubercle is in the same horizontal plane as the left and right posterior superior iliac spines (PSISs) (McGaugh et al., 2007); consequently, the PSISs are used as landmarks for palpating this sacral tubercle. Once identified, the S2 sacral tubercle can be used as a landmark for palpating the S1 sacral tubercle and then the L5 spinous process. Of course, variations in lumbosacral anatomy can lead to inaccurate palpation of these landmarks.
The median sacral crest ends as the only normally occurring spina bifida of the vertebral column because the left and right laminae of the fifth (and usually the fouth) sacral segment(s) normally do not fuse, forming an opening at the inferior end of the sacral canal known as the sacral hiatus.
The extent of the sacral hiatus varies and may include only the S5 segment (approximately 34%, incidence varies among different populations) or the S4 and S5 segments (43%), and a sacral hiatus extending superiorly to the S3 segment (19%) is also considered to be within normal limits (Albrecht, Scutter, & Henneberg, 2007; Kumar & Tubbs, 2011). The hiatus can extend to the S2 segment (4%), and in rare instances include all sacral segments (Kumar & Tubbs, 2011); however, these conditions are considered to be outside the limits of normal variation (Kumar & Tubbs, 2011).
Spina bifida occulta of the sacrum above the level of a fused sacral hiatus occurs in 4% to 28% of the population (Fig. 8-3, C). The wide variation of incidence may be related to genetic factors of the populations studied, diets of the various populations (spina bifida has been related to a prenatal folic acid deficiency), and/or methods used in the various investigations (x-ray versus analysis of dried specimens) (Wu et al., 2009).
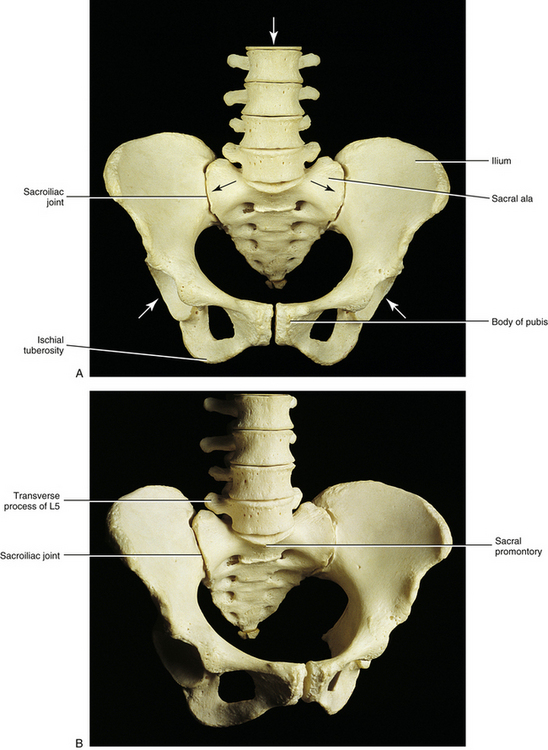
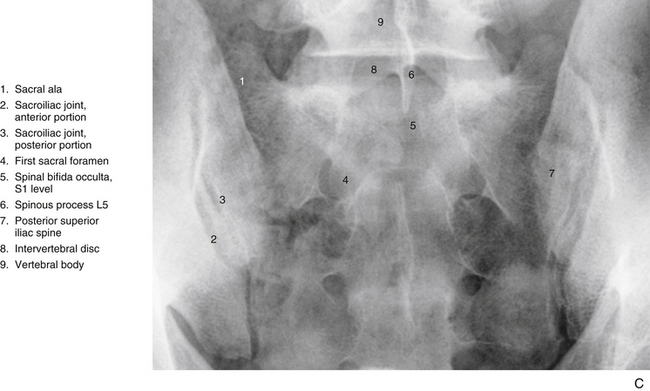
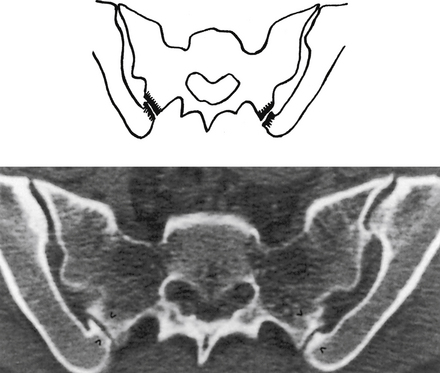
FIG. 8-3 A, Anterior and, B, anterior oblique views of the bony pelvis. C, Anterior-posterior “spot” x-ray view of the sacroiliac joints (SIJs). The anterior aspect of the left and right sacroiliac joints (SIJs) can be seen. The arrows in A indicate the forces transmitted to the SIJs. Notice that the SIJs receive forces from above and below. Those from above are generated primarily from carrying the weight of the trunk, from lifting objects with the upper extremities, and from forces generated during pushing or bending. Forces from below are generated primarily from the lower extremities during walking, running, and so on and are transmitted through the acetabula. Forces from below also can be transmitted through the ischial tuberosities during sitting.
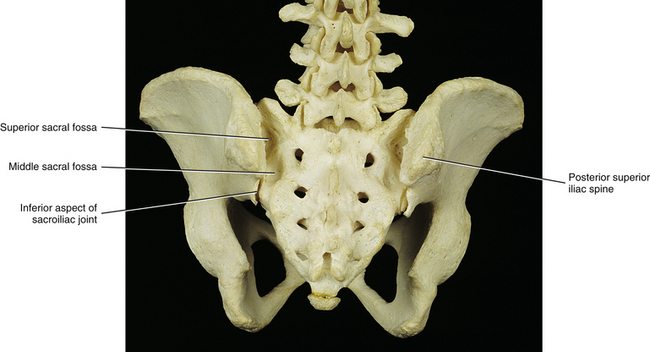
FIG. 8-4 Posterior view of the pelvis. Several sacral fossae associated with the sacroiliac joint are visible from this perspective.
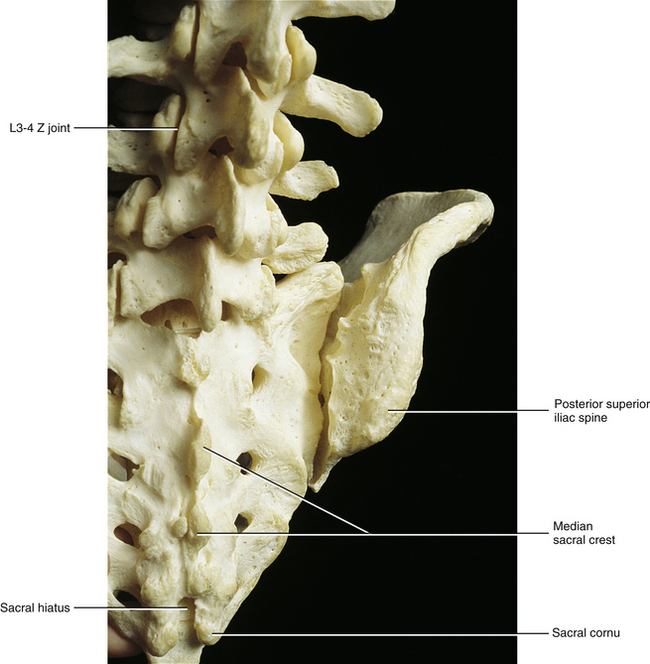
FIG. 8-5 Close-up of the posterior aspect of the sacroiliac joint, showing the normal anatomic relationships of the sacrum and the ilium.
The left and right intermediate, or medial, sacral crests are located just medial to the posterior (dorsal) sacral foramina. They are formed by four fused articular tubercles on each side of the sacrum (S2-5 tubercles; the S1 articular process is distinct). The left and right fifth articular tubercles extend inferiorly as the sacral cornua. The sacral cornua terminate as blunted tips inferiorly. They form the left and right inferior boundaries of the sacral hiatus (Fig. 8-1).
Finally, the left and right lateral sacral crests lie lateral to the dorsal sacral foramina. These crests are formed by the fused transverse tubercles of the five sacral segments.
The muscular and ligamentous attachments to the posterior surface of the sacrum are listed in Table 8-1.
Several differences exist between male and female sacra, although sometimes these changes may be subtle. Generally, the male sacrum is narrower from left to right and longer from superior to inferior than that of the female. The wider sacrum of the female results in a larger pelvic inlet (i.e., region bounded by the pecten of the pubis, arcuate line of the ilium, sacral ala, and sacral promontory). A larger pelvic inlet results in more room for the passage of the fetal head during childbirth. The sacrum in the female is also oriented slightly more horizontally than that of the male. This results in an increase of the lumbosacral angle. The ventral surface of the female sacrum is more concave than that of the male. This concavity provides more space in the pelvic cavity proper than would otherwise be the case, and it also results in a more prominent S2 spinous tubercle on the posterior surface of the female sacrum.
Fusion of the L5 vertebra with the sacrum (sacralization) is discussed in Chapter 7 and shown in Figure 12-14, G and H. As mentioned in Chapter 7, sacralization of the L5 vertebra is associated with decreased superior-inferior height of the “true sacrum” (Mahato, 2010b). The first sacral segment also may separate from the sacrum, known as lumbarization. The separation may be complete but usually is unilateral, and a joint may develop between the TP of the lumbarized segment and the remainder of the sacrum. Lumbarization (six lumbar vertebrae) has been associated with smaller dimensions, and more coronal orientation, of the superior articular facets of the (remaining) sacrum (Mahato, 2011). Lumbarization is also associated with altered function of the S1 nerve, which in lumbarization provides motor innervation to the muscles and supplies sensory innervation to the structures usually achieved by the L5 nerve (Chang & Nakagawa, 2004). A less common anomaly related to sacral segmental bony fusion is the first coccygeal segment fusing with the fifth sacral segment.
Clinical Implications
Sacral fractures are present in as many as 45% of pelvic fractures (Gibbons, Soloniuk, & Razack, 1990). These fractures may remain undetected if caudal, cephalic, and oblique x-ray examinations of the pelvis are not performed. Fractures of the sacrum can damage the APDs of the lumbosacral plexus, and fractures involving the sacral canal may affect the sacral roots before they are able to exit the sacrum.
Gibbons and colleagues (1990) found neurologic deficits in 34% of patients with sacral fractures. They also noted that the neurologic deficits usually improved with time. The presence and type of nerve injury correlated with the type of sacral fracture. Patients with injuries that involved only the sacral ala had the lowest incidence of neurologic deficit, although L5 or S1 radiculopathy was found in 24% of these patients. The mechanism of L5 radiculopathy was thought to be caused by entrapment of the APD of L5 between the fractured, superiorly displaced ala and the TP of L5.
Gibbons and colleagues (1990) also found that fractures involving the pelvic sacral foramina usually were vertical fractures that passed through all four foramina of one side. These injuries always were associated with other pelvic fractures and had a 29% incidence (two of seven fractures) of unilateral L5 or S1 nerve root involvement. However, bowel and bladder functions were maintained; a bilateral lesion is necessary to affect these functions.
Fractures involving the sacral canal can be either transverse (horizontal) or vertical and have the greatest chance of causing nerve damage (57% with horizontal and 60% with vertical). Vertical fractures usually are associated with other pelvic fractures and can result in bladder and bowel dysfunction (Gibbons, Soloniuk, & Razack, 1990; Ebraheim, Biyani, & Salpietro, 1996).
Horizontal fractures of the sacrum affecting the sacral canal are not necessarily associated with other pelvic fractures. They can be isolated injuries caused by a direct blow, as might occur from a long fall. The inferior fragment sometimes is considerably displaced, and severe neurologic deficits, involving bladder and bowel functions, can occur if the fracture is above the S4 segment (Gibbons, Soloniuk, & Razack, 1990).
Sacrococcygeal Joint
A fibrocartilaginous disc typically exists between the apex of the sacrum and the coccyx, making this joint a symphysis. However, occasionally a synovial joint develops here. At the other extreme, this region may completely fuse in older individuals (Standring et al., 2008).
Table 8-2 lists all the ligaments of the sacrococcygeal joint by their coccygeal attachments, as well as the muscles attaching to the coccyx.
Table 8-2
Attachments and Relationships to the Coccyx
| Surface | Attachments or Relationships |
| Anterior | Pubococcygeus, iliococcygeus, and ischiococcygeus (coccygeus) muscles; ventral sacrococcygeal ligament (similar to anterior longitudinal ligament) |
| Posterior | Gluteus maximus and sphincter ani externus (to tip of apex) muscles, intercornual ligaments, deep and superficial dorsal sacrococcygeal ligament,∗ filum terminale externum |
| Lateral | Lateral sacrococcygeal ligament (from transverse process of coccyx to inferolateral angle of sacrum) |
∗The superficial part runs between the sacral cornua (it closes the inferior aspect of the sacral canal at the sacral hiatus). The deep part is similar in location (and function) to the posterior longitudinal ligament. The filum terminale externum passes between the two parts of this ligament before attaching to the coccyx.
From Standring S et al. (2008). Gray’s anatomy: the anatomical basis of clinical practice (40th ed.). Edinburgh: Churchill Livingstone.
Sacroiliac Joint
The degree to which low back pain is caused by pathologic conditions or dysfunction of the sacroiliac joint (SIJ) has been discussed for many decades. The SIJ is now gaining added attention as a primary source of low back pain (Cassidy & Mierau, 1992; Daum, 1995; Schwarzer, Aprill, & Bogduk, 1995; Dreyfuss et al., 1996). One reason for this is that protrusion of the IVD is now considered to be a relatively infrequent cause of low back pain, accounting for less than 10% of the pain in this region (Cassidy & Mierau, 1992). On the other hand, pain arising from the SIJ is reported to account for 13% to 30% of low back pain (Kirkaldy-Willis, 1988; Eichenseer, Sybert, & Cotton, 2011) and may be implicated to some extent in more than 50% of patients with low back pain (Cassidy & Mierau, 1992). This makes the SIJ an area of significant clinical importance. An understanding of the unique and interesting anatomy of this joint is essential before a clinician can properly diagnose and treat pain arising from this articulation.
The pelvic ring possesses five distinctly different types of joints: the lumbosacral Z joints, the anterior lumbosacral (L5-S1 IVD), coxal (hip), SIJ, and symphysis pubis. The dynamic interactions between these joints are not well understood. Because the pelvic ring is complex and actually involves a total of eight joints (left and right Z joints, coxals, and SIJs; plus the single lumbosacral joint and the pubic symphysis), any change in the trunk or lower extremity is compensated in some way by the complicated dynamic mechanism of the pelvic ring (Lichtblau, 1962; Winterstein, 1972; LaBan et al., 1978; Sandoz, 1978; Grieve, 1981; Fidler & Plasmans, 1983; Wallheim, Olerud, & Ribbe, 1984; Drerup & Hierholzer, 1987). In fact, a survey of the pelvic rings of asymptomatic schoolchildren 7 to 8 years of age revealed distortion (asymmetry) in 40% of them. Surgical removal of bone graft material from the pelvic bone (iliac crest) also causes distortion of the pelvic ring (Grieve, 1975; Beal, 1982; Diakow, Cassidy, & DeKorompay, 1983).
The SIJ and symphysis pubis move a small amount. At first inspection, what little motion they have may seem enigmatic at best. Some authors have stated that the sole function of these two joints simply is to widen the pelvic ring during pregnancy and parturition. The joints are aided in this action by the hormone relaxin (Simkins, 1952; Bellamy, Park, & Rodney, 1983). However, mounting evidence over several decades indicates that the SIJs move in both males and females during many activities. An incomplete list of activities thought to enlist the movement of the SIJ includes the following: locomotion, spinal and thigh movement, and changes of position (from lying to standing, standing to sitting, for example).
The SIJ is thought to move a minimum of 2 mm and 2 degrees, but this small amount of movement is complex (Pitkin & Pheasant, 1936; Weisl, 1955; Colachis et al., 1963; Wood, 1985; Sturesson, Selvik, & Uden 1989; Brunner, Kissling, & Jacob, 1991; Barakatt et al., 1996; Smidt et al., 1997; Bussey, Milosavljevic, & Bell, 2009). Janse (1978) stated that the function of movement in the SIJ is to convert the pelvis into a resilient, accommodating receptacle essential to the ease of locomotion, weight bearing, and shock absorption. This is in agreement with other authors (DonTigny, 1990; King, 1991) who state that the function of SIJ is to buffer, absorb, direct, and compensate for forces generated from above (e.g., gravity, carrying the torso, and muscle action) and below (e.g., forces received during standing and locomotion). The section later in this chapter entitled Sacroiliac Joint Motion provides additional detail on the amount and types of movements that occur at the SIJ.
Unique Characteristics
Differences between humans and quadrupeds: The pelvis tilts anteriorly and inferiorly in the bipedal human, and the SIJ is aligned in parallel fashion with the vertebral column, whereas the pelvis of quadrupedal animals is tilted more posteriorly. The SIJ of the bipedal human has other differences associated with a two-legged stance. These include the articulating surfaces of the SIJ that are shaped like an inverted L, the interosseous ligaments that are more substantial and stronger posteriorly, and the many bony interlockings between the sacrum and the ilium that develop with age (Walker, 1986).
Differences among humans: The plane of articulation of SIJs can vary from one individual to another; and the left and right SIJs of one individual can even lie in different planes (Ebraheim et al., 1997b). The weight of the human trunk is transmitted by gravity through the lumbosacral joint and is then divided onto the right and left SIJs. In addition, the ground reaction, or bouncing force, is transmitted through the hip joints and also acts on the SIJs. Adapting to the bipedal requirements of mobility and stability may account for the tremendous amount of variation and asymmetry found in the human SIJ. The joint structure and the surface contours of the SIJ have changes associated with age, sex, and the mechanical loads that are placed on them (Walker, 1986; Bussey, Milosavljevic, & Bell, 2009). Therefore the functional requirements of the SIJ, to a great extent, may influence the structural changes found in them (Weisl, 1954a). For example, one may speculate that more mobility is necessary in the SIJ in younger persons, in females around the time of parturition, and in athletes. On the other hand, more stability is necessary in older persons, in males (generally larger body weight), and in those who frequently carry heavy weights. This stability may be provided by the development of stronger interosseous ligaments and a more prominent series of bony interlockings (Table 8-3). Also, the osteophytes (Enix & Smith, 2010) and ankylosis frequently seen in the SIJs of older individuals may develop to increase stability.
Table 8-3
“Tongue and Groove” Relationships (Bony Interlockings) between the Sacrum and Ilium∗
| Sacral E or D | Iliac E or D |
| Sacral groove (D) | Iliac ridge (E) |
| Middle sacral fossa (see text) (D) | Iliac tuberosity (E) |
| Sacral tuberosity (E) | Sulcus between iliac ridge and iliac tuberosity (iliac sulcus) (D) |
| Additional elevation on posterior and superior surface of sacral ala (alar tuberosity) (E) | Depression anterior and superior to iliac tuberosity (D) |
∗Compare with Figure 8-9.
Structure
The SIJ is an articulation between the auricular surface of the lateral aspect of the sacrum and the auricular surface of the medial aspect of the ilium (see Figs. 8-6 and 8-7). Previously this joint was classified as an amphiarthrosis. However, the SIJ is now classified as an atypical synovial joint with a well-defined joint space and two opposing articular surfaces (Cassidy & Mierau, 1992). Each auricular surface is shaped like an inverted L (Standring et al., 2008). Other authors have described it as being C-shaped (Cassidy & Mierau, 1992). The superior limb of this surface is oriented posteriorly and superiorly, and the inferior limb is oriented posteriorly and inferiorly (Figs. 8-2 to 8-9). The angle between the superior and inferior limbs is approximately 71 to 76 degrees (Enix & Smith, 2010) and is greater in females than in males (Bussey, Milosavljevic, & Bell, 2009). Consequently, the upper, middle, and lower regions of the SIJ have distinctly different appearances when viewed in the horizontal plane, as is done with MRI and computed tomography (CT) scans (Fig. 8-10). The location of the auricular surface is usually between the levels of the S1 and middle of the S3 sacral segments (bodies of the sacral segments). However, this location can vary from being positioned more superiorly, beginning above the body of the S1 segment to the superior aspect of the S3 segment; to more inferiorly, beginning at the lower aspect of the S1 segment and extending to the lower border of the S3 segment (Mahato, 2010c).
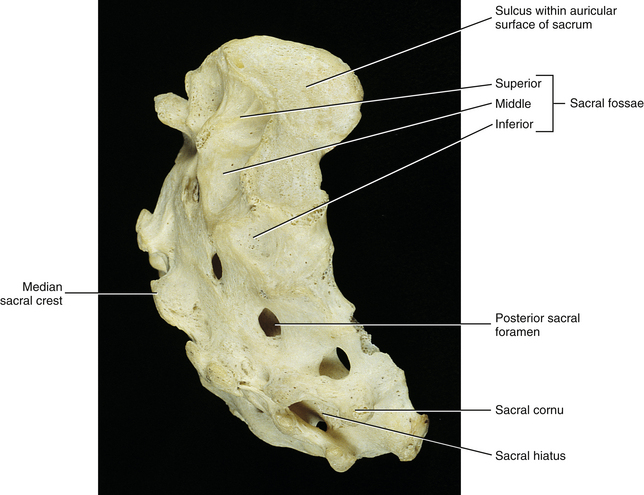
FIG. 8-6 Lateral view of the sacrum demonstrating several structures that help to form the sacroiliac joint.
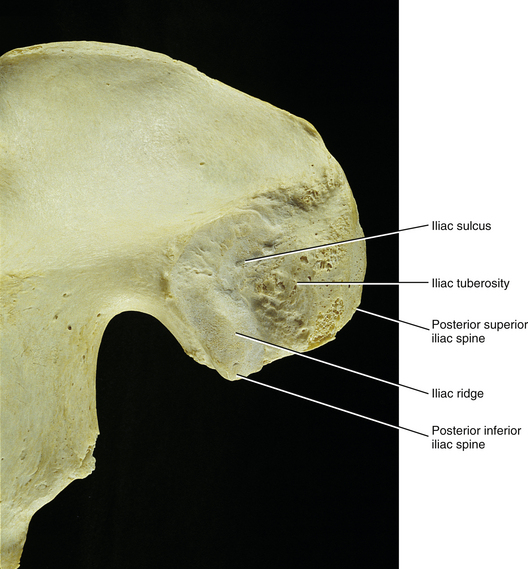
FIG. 8-7 Medial surface of the posterior ilium of a dried skeletal specimen. Several structures that participate in the formation of the sacroiliac joint are demonstrated.
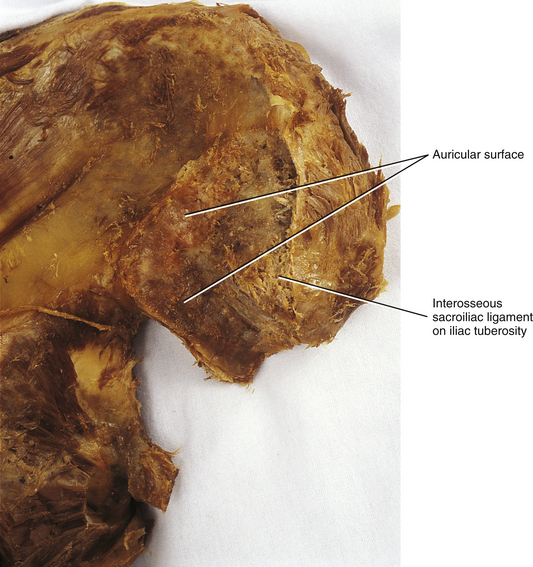
FIG. 8-8 View similar to that seen in Figure 8-7, only with more soft tissue visible on this cadaveric specimen. The auricular surface can be identified readily on this medial surface of the posterior ilium. Also notice the cut fibers of the interosseous sacroiliac ligament.

FIG. 8-9 Medial surfaces of the right side of the sacrum and right ilium showing the series of elevations and depressions associated with the sacroiliac joint (SIJ). These elevations and depressions are thought to help increase stability of the SIJ. Table 8-3 lists the important elevations and depressions of the SIJ.
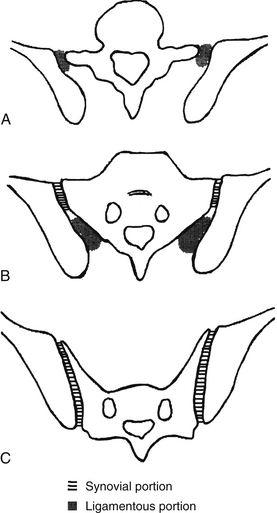
FIG. 8-10 Horizontal sections through, A, the upper, B, middle, and C, lower portions of the sacroiliac joint (SIJ). The diagram also demonstrates the synovial versus fibrous (ligamentous) portions of the SIJ. (From Prassopoulos PK et al. [1999]. Sacroiliac joints: anatomic variants on CT. J CAT, 23, 323-327.)
An articular capsule lines the SIJ’s anterior aspect, whereas the SIJ’s posterior aspect is covered by the interosseous sacroiliac (S-I) ligament. No articular capsule has been found along the posterior joint surface.
The sacral auricular surface has a longitudinal groove, known as the sacral groove, along its center that extends from the upper end to the lower end. The posterior rim of this groove is thick and is known as the sacral tuberosity. The iliac auricular surface has a longitudinal ridge, known as the iliac ridge, which corresponds to the sacral groove. The inferior end of this iliac ridge ends as the posterior inferior iliac spine (PIIS). The sacral groove and iliac ridge interlock for stability and help to guide movement of the SIJ (see Table 8-3 and Figs. 8-8 and 8-9).
The region of the sacrum within the posterior concavity of the SIJ is covered by the interosseous sacroiliac ligament and consists of three fossae (see Fig. 8-9). The middle fossa is the estimated location of the axis of SIJ rotation. It is in the region of this fossa that the iliac ridge moves circularly in the sacral groove. The iliac tuberosity is posterior to the auricular surface of the ilium. The anterior aspect of the iliac tuberosity inserts into the middle sacral fossa, creating a pivot around which the iliac ridge turns within the sacral groove (Bakland & Hanse, 1984) (see Table 8-3). There is a sulcus between the iliac tuberosity and iliac ridge. The sulcus promotes stability by interlocking with the sacral tuberosity (see the following discussion). Finally, anterior and superior to the iliac tuberosity is a depression that interlocks with an additional elevation on the posterior and superior surface of the sacral ala (alar tuberosity). Therefore stability of the SIJ is promoted by a series of “tongues and grooves.” Figures 8-8 and 8-9 show this series of interlocking elevations and depressions that aid the stability of the SIJ, and Table 8-3 summarizes the tongue and groove relationships between these “hills and valleys.” This series of interlocking prominences and depressions becomes more enhanced and irregular with age. The prominences and depressions are somewhat less dramatic in females than in males, which is thought to be one reason the SIJs of females allow more motion than the SIJs of males (Bussey, Milosavljevic, & Bell, 2009).
Ligaments
Articular capsule: The fibrous articular capsule of the SIJ is only located along the anterior surface of the joint (Fig. 8-11). Its outer portion is thick and tough, and its inner portion is lined internally with a synovial membrane (Jaovisidha et al., 1996). The articular capsule is innervated with nociceptive and proprioceptive (mechanoreceptive) nerve endings. No articular capsule is located along the posterior border of the SIJ.
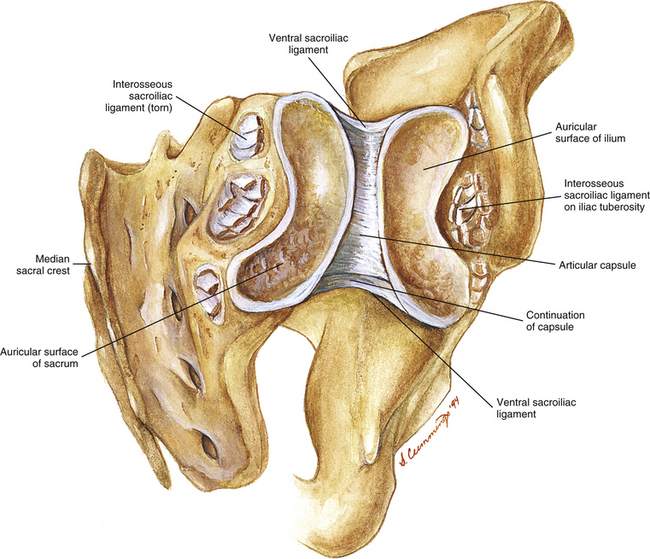
FIG. 8-11 Posterior view of an opened right sacroiliac joint demonstrating some of the important bony and soft tissue components. Notice that the articular capsule is found only anteriorly. Posteriorly the interosseous sacroiliac ligament supports the joint. This ligament is shown torn to illustrate the deeper structures of the opened joint.
Interosseous sacroiliac ligament: This ligament receives the greatest stresses of the ligaments associated with the SIJ (Eichenseer, Sybert, & Cotton, 2011). The interosseous ligament of each SIJ connects the three sacral fossae (see previous section) to the iliac tuberosity and the area around the tuberosity (Figs. 8-12 to 8-16; see also Figs. 8-8 to 8-11). The interosseous ligament consists of superficial and deep layers. Furthermore, the deep layer has a cranial band and a caudal band. The cranial band is oriented transversely, and the caudal band is oriented more vertically. The superficial layer is membranous and is covered by the posterior S-I ligaments (long and short). Nerves and blood vessels pass between the posterior sacroiliac ligament and the superficial layer of the interosseous ligament. Because there is no posterior joint capsule of the SIJ, the interosseous S-I ligament is what limits the SIJ posteriorly.
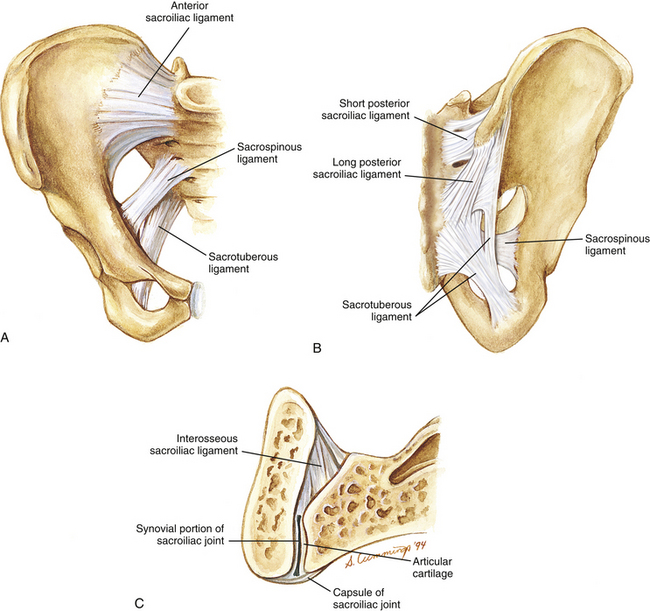
FIG. 8-12 Ligaments of the sacroiliac joint (SIJ). A, Anterior view. B, Posterior view. C, SIJ in horizontal section. Notice that the capsule of the SIJ joint is only present anteriorly.
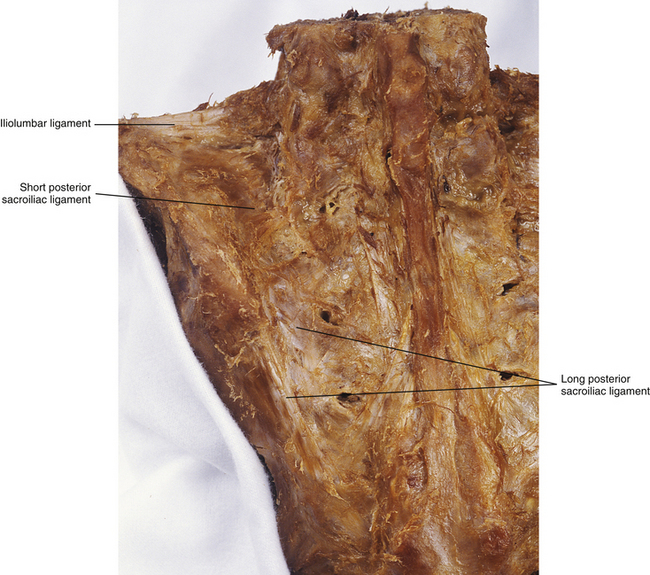
FIG. 8-13 Posterior view of the sacrum and the left posterior ilium, showing the iliolumbar ligament and the posterior sacroiliac ligament. The posterior sacroiliac ligament has long fibers, which are vertically oriented, and short fibers, which are horizontally oriented.
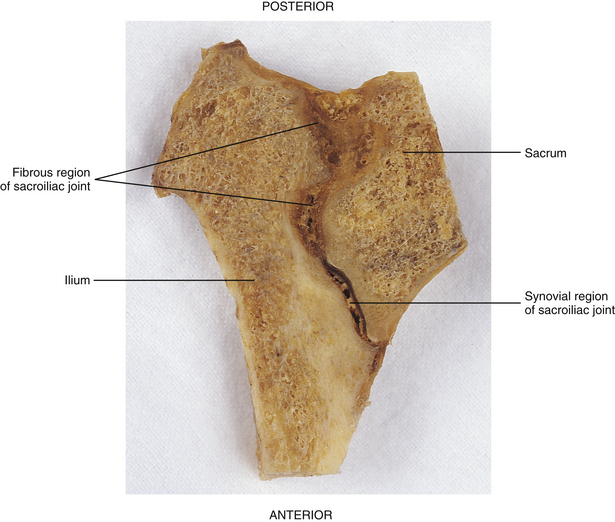
FIG. 8-14 Horizontal section through the right sacroiliac joint. Notice the synovial portion of the joint anteriorly and the fibrous portion posteriorly. The posterior fibrous portion of the joint is filled with the interosseous sacroiliac ligament.
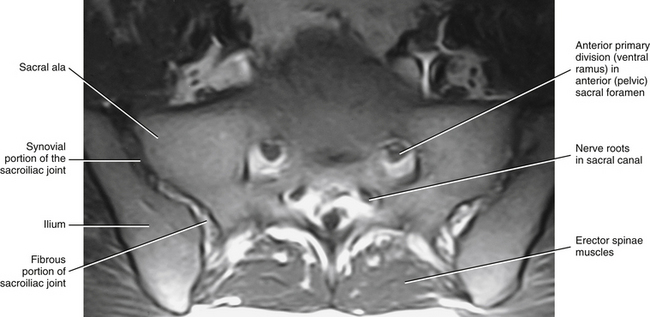
FIG. 8-15 Magnetic resonance imaging scan performed in a horizontal plane that shows the sacroiliac joint. (Image courtesy Dr. Dennis Skogsbergh.)
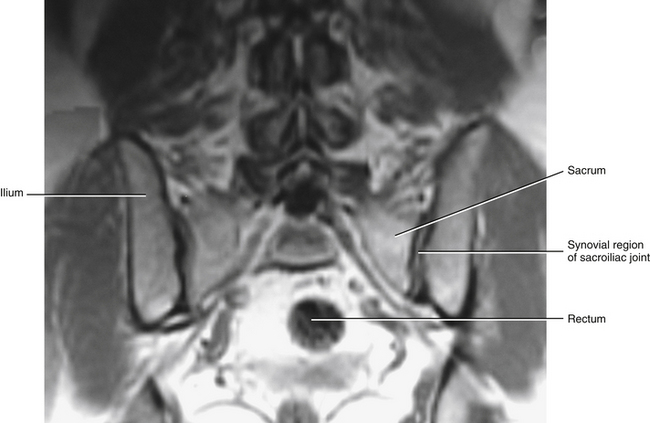
FIG. 8-16 Magnetic resonance imaging scan taken in a coronal plane that shows the sacroiliac joint. (Image courtesy Dr. Dennis Skogsbergh.)
A small band, known as the superior intracapsular ligament, or Illi’s ligament (Illi, 1951; Janse, 1976), has been found to extend between the superior aspects of the sacral and iliac auricular surfaces in 75% of 31 cadavers studied (Freeman, Fox, & Richards, 1990). This ligament may be an anterior and superior extension of the interosseous S-I ligament (Enix & Smith, 2010), but Freeman and colleagues (1990) found that it was quite distinct. The superior intracapsular ligament may have relatively little biomechanical value.
Anterior sacroiliac ligament: The pelvic surface and articular capsule of each SIJ is covered by the anterior, or ventral, S-I ligament (see Fig. 8-12). The fibers of the superior part of the ventral S-I ligament course in a slight superolateral direction from the sacrum to the ilium, the fibers in the middle of the ligament are horizontally oriented, and those of the inferior aspect of the ligament course inferolaterally (Jaovisidha et al., 1996). The ventral S-I ligament does not provide as much support to the SIJ as either the interosseous or the posterior S-I ligaments. The ventral S-I ligament blends with the anterior joint capsule of the SIJ. These two structures cannot be distinguished from each other during gross dissection, but can be distinguished from one another histologically by evaluating the different direction taken by the collagen fibers of each structure. The ventral S-I ligament and articular capsule combined are approximately 2 mm thick (Jaovisidha et al., 1996). The ligament–articular capsule complex is thicker inferiorly, near the region of the posterior inferior iliac spine, than superiorly (Weisl, 1954b; Freeman, Fox, & Richards, 1990; Jaovisidha et al., 1996). The APD of L5 supplies sensory innervation to the superior aspect of this ligament, and the APD of S2 (occasionally with additional fibers from the sacral plexus) supplies the lower part of the ventral S-I ligament (Jaovisidha et al., 1996).
Posterior sacroiliac ligament: The posterior (dorsal) sacroiliac (S-I) ligament is made up of two distinct parts (see Figs. 8-12 and 8-13):
• Long posterior sacroiliac ligament. Laterally, the long posterior S-I ligament (LPSL) attaches to the posterior superior iliac spine (PSIS) (where it blends with the superior attachment of the sacrotuberous ligament) and neighboring posterior aspect of the internal lip of the iliac crest. Medially, the LPSL attaches to the lateral sacral crests at the level of S3 and S4 (i.e., S3 and S4 lateral sacral tubercles). Some of the inferior fibers of the LPSL blend with the sacrotuberous ligament (Standring et al., 2008; Moore et al., 2010; personal communication, M. Kumka, MD, PhD, October 21, 2011). The LPSL can be palpated immediately inferior to the PSIS. The ligament is tough at this location and throughout its course from the PSIS to the lateral sacral crest. Many important structures attach to this ligament via fascial aponeuroses that may actually help to form the LPSL. These structures include the deep fascia of the gluteus maximus muscle (attaching to the superficial, lateral aspect of the LPSL), the deep fibers of the posterior layer of the thoracolumbar fascia (attaching to the superficial, medial aspect of the LPSL), the aponeurotic attachments of the common origin of the erector spinae muscle (also attaching to the superficial, medial aspect of the LPSL), and also a deep fascial layer from the sacral attachments of the multifidus muscle (attaches to the deep, medial aspect of the LPSL) (Vleeming et al., 1996; McGrath, Nicholson, & Hurst, 2009). The LPSL is under tension during the transmission of forces from the lower extremities to the trunk and vice versa. Vleeming and colleagues (1996) found that the ligament is tensed during counternutation of the sacrum (posterior rocking of the sacral base) and is slackened during sacral nutation (anterior rocking of the sacral base; see Sacroiliac Joint Motion). The LPSL is innervated by the posterior primary divisions (dorsal rami) of S2-S4 (occasionally S1) (McGrath & Zhang, 2005). Damage to the ligament and increased tensile stress to the ligament (e.g., during pregnancy) are considered to be potential sources of low back pain and pelvic pain associated with pregnancy (McGrath & Zhang, 2005; Moore et al., 2010). The S2-S4 PPDs pass from the dorsal sacral foramina through a layer of fibroadipose tissue before either entering the more superficial LPSL or continuing laterally to innervate other structures. Some authors have proposed that the lateral branches of the S2-S4 PPDs may be susceptible to compression as they pass through the space filled with fibroadipose tissue, leading to low back pain (entrapment neuropathy) in these individuals (McGrath, Nicholson, & Hurst, 2009).
• Short posterior sacroiliac ligament. This ligament originates from the intermediate and lateral sacral crests at the level of S1 and S2. It runs in the horizontal plane covering the SIJ posteriorly, and attaches to the medial aspect of the posterior surface of the iliac crest and the iliac tuberosity.
Accessory sacroiliac ligaments: The stability of the SIJ is enhanced by two accessory S-I ligaments (see Fig. 8-12). A third ligament, the iliolumbar ligament (see Chapter 7 and Fig. 8-13), also provides stability to the region.
• Sacrotuberous ligament. This ligament has a broad attachment to the PSIS (most lateral attachment): posterior aspect of the long posterior sacroiliac ligament, inferior aspect of the lateral sacral crest, and lateral edge of the inferior sacrum and lateral coccyx (most medial attachment). The strong ligament narrows as it passes inferiorly and laterally to attach to the medial aspect of the ischial tuberosity (Standring et al., 2008). The lesser sciatic foramen is formed between this ligament and the sacrospinous ligament.
• Sacrospinous ligament. The sacrospinous ligament courses from the anterior surface of the sacrum (fused second, third, and fourth segments) to the spine of the ischium (see Fig. 8-12). The greater sciatic foramen is located superior to this ligament.
The sacrotuberous and sacrospinous ligaments help to limit the small amount of anterior and inferior nodding (nutational) motion of the sacrum at the SIJ. This is accomplished by restricting the amount the sacral apex can move posteriorly and superiorly when the promontory of the sacrum moves anteriorly and inferiorly. These ligaments also help to stabilize the inferior aspect of the SIJ.
• Iliolumbar ligament. The iliolumbar ligament (see Chapter 7) connects the iliac crest with the adjacent TP of the L5 vertebra. Part of the iliolumbar ligament (lumbosacral ligament, see Figs. 7-23 and 8-20) attaches to the anterior and superior part of the sacrum. The iliolumbar ligament helps to limit lateral tilting of the pelvis and gapping of the superior aspect of the SIJ.
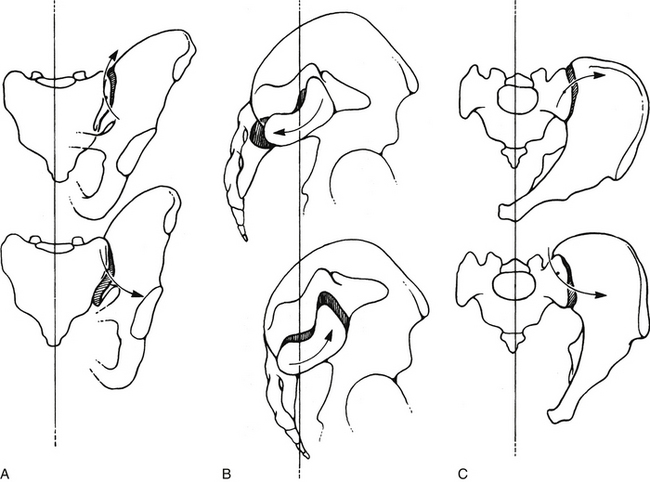
FIG. 8-17 Three types of sacroiliac joint (SIJ) motion. A, Superior and inferior aspects of the SIJ are shown gapping. B, Anterior and posterior rocking of the sacral base. This is sometimes known as nutation and counternutation, respectively. C, Movement of the ilium on the sacrum that takes place in the horizontal plane. A and C, Arrows show motion of the ilium. B, Arrows show sacral motion. These movements are accentuated for demonstrative purposes in this illustration.
Arterial Supply and Venous Drainage
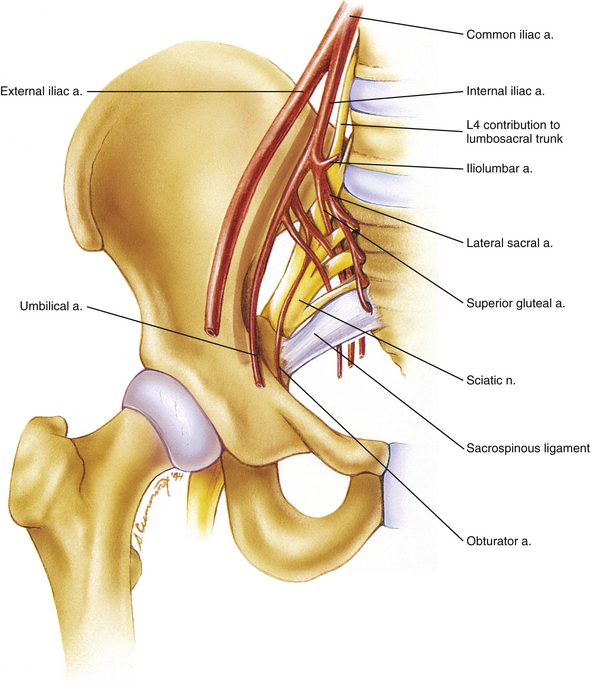
Both the anterior and the posterior aspects of the SIJ are served by the superior branch of the lateral sacral artery and vein, which are branches of the internal iliac artery and vein, respectively. These vessels anastomose with the superficial branch of the superior gluteal artery and vein (Standring et al., 2008). Figures 8-20 and 8-21 demonstrate the major arteries and nerves associated with the anterior surface of the SIJ.
Innervation
The SIJ receives sensory innervation, and the joint capsule (anteriorly located) and all the dorsally located sacroiliac ligaments (superficial, deep, and accessory ligaments) possess both nociceptors (pain receptors) and mechanoreceptors (for joint position sense, i.e., proprioception) (Grob, Neuhuber, & Kissling, 1995). This indicates that in addition to being pain sensitive, the sensory receptors of the SIJ relay information related to movement and joint position and in doing so may help to keep the body upright and balanced. Sakamoto and colleagues (2001) found many more nociceptive nerve endings in the SIJ than mechanoreceptive endings. In addition to ligaments, the sacral articular (auricular) cartilage is also most likely pain sensitive (Szadek et al., 2010), and the posterosuperior aspect of this cartilage is the region that appears to have the greatest nociceptive innervation (Murata et al., 2007). Consequently, this posterosuperior region of the sacral auricular cartilage is probably most often associated with intraarticular SIJ pain.
The specific innervation of the SIJ is variable, even between the left and right sides of the same individual. The anterior (pelvic) part of the SIJ is innervated by the APDs of L2 through S2 (Bernard & Cassidy, 1993), with L4 and L5 being the most frequent source of innervation (Cassidy & Mierau, 1992). The posterior part of the SIJ is innervated by PPDs of S1-S4. However, the innervation of this part of the joint is probably more extensive than just sacral segments and may include the lateral branches of the PPDs of L4 (Bernard & Cassidy, 1993) and L5 (Ro, 1990).
The variable innervation of the SIJ from person to person and even from the left to the right side of the same person may be one reason for the wide range of pain referral patterns described by patients experiencing discomfort of SIJ origin (Bernard & Cassidy, 1993). Furthermore, the wide variation of referral patterns may help to explain the difficulty researchers and clinicians have had in identifying the incidence with which SIJ dysfunction occurs.
A portion of the sacral plexus is formed along the anterior surface of the SIJ and most L4 to S3 nerve roots are proximate to the SIJ (Waikakul, Chandraphak, & Sangthongsil, 2010). Figures 8-20 and 8-21 demonstrate the relationship of the sacral plexus to the anterior aspect of the SIJ.
Microscopic Anatomy
The histologic composition of the cartilage lining the auricular surface of the sacrum differs from that lining the auricular surface of the ilium. The sacral surface is lined by hyaline cartilage, and the iliac surface is lined by what is best described as fibrocartilage. The hyaline cartilage of the adult sacral surface is three times thicker than the cartilage of the iliac surface. Large, round, paired chondrocytes are distributed throughout the hyaline matrix of the sacral auricular surface and are arranged in columns parallel to the articulating surface. The hyaline cartilage is homogeneous with a small amount of fibrous tissue. Its smooth surface aids the gliding motion of the joint.
The iliac cartilage is thin (≈1 mm) and contains smaller spindle-shaped chondrocytes clumped in a fibrous matrix. The cell columns are oriented at right angles to the surface. Postpartum the iliac cartilage degenerates early, and the amount of fibrous tissue increases. The sacral cartilage degenerates throughout life, and in later adult life it may appear fibrous as well (Sashin, 1929; Bowen & Cassidy, 1981; Paquin et al., 1983). These changes cause the synovial portion of the SIJ to narrow with age. Sometimes this narrowing can be seen on standard x-rays that include the SIJ (Sgambati et al., 1997).
Development
The SIJ begins development during the seventh week of fetal life with the ilia moving superiorly and also posteriorly to the sacrum. During the eighth week of development, the mesenchyme between the two bones becomes arranged into three layers. At the tenth week, multiple cavities develop in the mesenchyme. These cavities are separated by septa, which disappear by the time the fetus reaches term. The sacral hyaline cartilage develops first, followed by the development of the iliac surface. At birth the sacral hyaline cartilage is thick and almost fully developed, whereas the iliac cartilage is thin and irregular. The iliac surface has the appearance of fibrocartilage by the time of infancy (Cassidy & Mierau, 1992).
Before puberty, both sacral and iliac auricular surfaces are flat, straight, and vertically oriented (Beal, 1982). The joint can conceivably have a gliding movement in any direction, being restricted only by ligaments. After puberty the auricular surfaces change shape to form a horizontal and a vertical limb. The horizontal limb, which can be longer than the vertical limb, is possibly formed to aid stability (Otter, 1985). Also during this time the longitudinal groove is formed in the sacral auricular surface. This groove runs from top to bottom, down the center of this surface. The corresponding iliac ridge develops simultaneously on the iliac auricular surface. The interlocking groove and ridge limit the direction of motion, but increase stability.
The interosseous ligaments are strengthened during the third decade of life. The many bony tuberosities and corresponding grooves and fossae develop and probably increase the stability of the SIJ by their interlocking relationships (see Table 8-3 and Fig. 8-9). The differences in depth and height of the interlocking system of grooves and crests among different individuals cause a wide variation of normal appearances of the SIJ on CT scans (Prassopoulos et al., 1999). This led Prassopoulos and colleagues (1999) to conclude that all SIJs have a distinct appearance on CT (at least subtly).
Beginning in the fourth decade of life, marginal osteophytes frequently begin to develop, particularly on the anterior and superior portions of the SIJ along the articular capsule. These degenerative changes develop earlier in the male. They probably increase stability of the joint, at the expense of decreased joint mobility. In later life the cartilage undergoes degeneration and further marginal ankylosis develops. Total fibrous ankylosis eventually may occur. During the eighth decade of life, SIJ mobility usually is lost completely, making body movement stiffer than before fusion (Walker, 1986).
Sacroiliac Joint Motion
The SIJ provides substantial, yet resilient, stability to the region between the spine and the lower extremities while also allowing for slight mobility to occur between the sacrum and ilium. The SIJ has a small amount of movement (Solonen, 1957; Frigerio, Stowe, & Howe, 1974; Egund et al., 1978), and females have more SIJ movement than males (Bussey, Milosavljevic, & Bell, 2009). The movement is difficult to evaluate because of its location deep to the origin of the erector spinae muscle group, the posterior sacroiliac (S-I) ligament, and the interosseous S-I ligament. SIJ movement is three dimensional and contains several elements. The primary movements appear to be anteroinferior and posterosuperior nodding (called nutation and counternutation, respectively) of the sacral base in relation to the ilium (Fig. 8-17, B). This represents rotation along the sacral groove, with the center of rotation located in the middle sacral fossa of the SIJ. The full range of motion of the SIJ is not expressed until the extremes of hip motion are reached.
In a study evaluating SIJ motion in five unembalmed cadavers (age range 52 to 68), Smidt and colleagues (1997) found that each SIJ moved an average of 7.5 degrees (range, 3 to 17 degrees) in the sagittal plane during full flexion and extension of the hips. In addition, SIJ ranges of motion of 22 to 36 degrees have been reported in young gymnasts and nongymnasts (Barakatt et al., 1996).
Another type of movement is rotatory movement along an axis that passes longitudinally through the iliac ridge of the SIJ (Fig. 8-17, C). The movement of the posterior aspect of the ilium in this case is superomedial and inferolateral. Although the iliac ridge may move only 2 mm during this type of movement, the distance between the two anterosuperior iliac spines increases or decreases by as much as 10 mm (Hadley, 1952; Wilder, Pope, & Frymoyer, 1980; Ehara, El-Khoury, & Bergman, 1988).
Gapping of the superior and inferior aspects of the SIJ has also been described. This could be interpreted as a third type of SIJ motion (Fig. 8-17, A).
Initiation of SIJ movements is made by the vertebral column and lower extremities. The forces inducing SIJ motion are gravity (trunk weight), ground reaction (bouncing) force, and muscle contraction. Postural changes of the vertebral column (e.g., during lying, sitting, standing) and motion of the vertebral column (e.g., flexion, extension, rotation) cause the sacrum to move relative to the ilium. Change of thigh position (e.g., during sitting, standing, standing on one leg) and active motion of the thigh during flexion, extension, abduction, adduction, and rotation cause the iliac surface of the SIJ to move relative to the sacral surface of the SIJ. In addition, abduction and adduction of the thigh cause a certain amount of gapping motion. The mechanism of walking is extremely complex, thereby causing movements of the SIJ to be complicated.
Even though there appears to be no muscle specifically designed for movement of the SIJ, approximately 40 muscles can influence this joint. Some of the most important are the erector spinae, quadratus lumborum, multifidus lumborum, iliopsoas, rectus abdominis, gluteus maximus, and piriformis muscles (Fligg, 1986).
As mentioned, stability is increased and mobility is decreased with age. Until puberty, stability is maintained primarily by ligaments. After puberty, the bony interlockings that enhance stability begin to form (see Table 8-3 and Fig. 8-9). Recall that after the fourth decade of life, osteophytes are formed and ankylosis may begin to occur, increasing stability. Near the eighth decade of life, total fibrous degeneration develops for stability; consequently, SIJ movement usually completely stops at approximately this age.
Clinical Considerations
Disorders of the Sacroiliac Joint
Because of its role in weight bearing and perhaps also because of its unique anatomy, the SIJ can become a source of pain (Daum, 1995; Schwarzer, Aprill, & Bogduk, 1995; Dreyfuss et al., 1996). This section briefly highlights some of the most common causes of pain arising from this clinically important articulation. Brief mention also is made of the most important factors involved in the clinical evaluation of the SIJ. Also, some of the most common methods currently used to treat SIJ dysfunction are mentioned. However, a complete consideration of the pathologic conditions, diagnosis, and treatment of SIJ disorders is beyond the scope of this book.
Trauma and repeated minor forces can cause SIJ disorders. Examples of minor forces include those received while driving for long periods over rough terrain or while driving on poorly maintained roads in a vehicle with inadequate suspension (Abel, 1950). The SIJ receives its greatest stresses from below during sitting. This is because the ground reaction (bouncing) force reaches the SIJ directly without passing through any other joint (Johnson, 1964; Schuchman & Cannon, 1986; Bermis & Daniel, 1987).
An accessory SIJ may form within the posterior (fibrous) portion of the joint (Fig. 8-18). Such accessory joints are more common in obese individuals than in individuals of normal weight. Accessory SIJs also are more common in older than younger individuals, and are also associated with other signs of degeneration (e.g., sclerosis and roughening of the articular surfaces). Therefore accessory SIJs are most likely acquired rather than congenital in nature (Prassopoulos et al., 1999). Prassopoulos and colleagues (1999) found an accessory SIJ in 19.1% of the patients in their series of pelvic CT scans that excluded frank SIJ disease. Wu and colleagues (2009) found the incidence to be 12.3% from the examination of dried sacra of a Chinese population. However, the incidence may be lower (Fortin & Ballard, 2009). This anomaly was more frequently associated with periodic low back pain than other lumbosacral and pelvic anomalies.
Low back pain during pregnancy is common and may have a different etiology than low back pain unrelated to pregnancy (Kristiansson, Svärdsudd, & von Schoultz, 1996). Women appear to be more susceptible to SIJ syndrome (pain as a result of mechanical irritation) than men. This is probably caused by the actions of the hormone relaxin during menstruation and pregnancy, and for a short time after childbirth (Cassidy & Mierau, 1992). Relaxin decreases the tension of the S-I ligaments, allowing them to become more pliable (or lax). The best known SIJ disease is osteitis condensans ilii, which occurs secondary to pregnancy and parturition (Nykoliation, Cassidy, & Dupuis, 1984; Olivieri et al., 1990).
A partial list of disorders of the SIJ includes joint space widening or narrowing, cystic or erosive change, osteosclerosis, osteophytosis, and idiopathic hyperostosis. Some causes of SIJ dysfunction include trauma, disease of bone, infection, and arthropathy (Fryette, 1936; Romanus, 1955; Blumel, Evans, & Eggers, 1959; Resnik, Dwosh, & Niwayama, 1975; Dunn et al., 1976; DeCavalho & Graudal, 1980; Bose, 1982; Cone & Resnick, 1983; Blower & Griffin, 1984; Vogler et al., 1984; Resnik & Resnick, 1985; Jajic & Jajic, 1987; Burns, Mireau, & Howlett, 1995). Bacterial infection of the SIJ is a rare cause of low back pain. However, when the source of SIJ pathology is bacterial infection, immediate treatment with antibiotics is essential to prevent permanent destruction of adjacent bone (Burns, Mireau, & Howlett, 1995). Table 8-4 provides a more complete list of some of the causes of SIJ dysfunction.
Table 8-4
Causes of Sacroiliac Joint Dysfunction
| Type | Possible Causes |
| Trauma | Direct trauma, falls, locus minoris resistentiae |
| Disease of bone | Osteitis condensans ilii, infection |
| Arthropathies | Ankylosing spondylitis, enteropathic arthropathies, gouty arthritis |
| Other causes | Hyperparathyroidism, paraplegia, lower extremity disorders, activity-related (e.g., athletic activity), after hip surgery, neoplasm |
Evaluation and Treatment
Examination of the SIJ is challenging for many reasons. First, the SIJ is subject to a wide range of normal anatomic variation (Prassopoulos et al., 1999). Second, its unusual location and oblique position make direct palpation almost impossible. Also, evaluation is made more challenging because spinal radiography does not always correspond well with symptoms. In addition, pain patterns associated with SIJ disorders can be diverse (Dreyfuss et al., 1996). Outside of diagnostic anesthetic blocks of the SIJ, differentiating SIJ pain from pain arising from structures of the lumbar spine can be difficult. However, one potential clue is that pain arising from the SIJ usually does not radiate superiorly to the L5 level of the lumbar region (low back) (Dreyfuss et al., 1996).
As always, the patient’s history can be extremely revealing. The patient may complain of pain over the PSIS that radiates into the buttock and less frequently to the groin and lower extremity (Cassidy & Mierau, 1992). Neurologic signs are negative, and the pain is not of dermatomal distribution.
Useful methods of palpatory examination for the SIJ include the palpation of neighboring prominences (PSIS and the S2 spinous tubercle) during thigh flexion (motion palpation). In addition, several orthopedic tests for SIJ dysfunction exist (Lawrence, 1990). Potter and Rothstein (1985) investigated the reliability of many physical tests for SIJ dysfunction and found the most reliable test to be a measurable widening of the distance between the left and right anterior superior iliac spines from a standing to a supine position. Using this method, a 94% agreement was found between multiple observers. A measured narrowing of the distance between the left and right anterior superior iliac spines after compression in the side-lying posture was the second most reliable method, with a 76% agreement found among observers. Also, certain orthopedic tests may be helpful in evaluating disorders of the SIJ. Using bone scans, Cassidy and Mierau (1992) found SIJ dysfunction to be particularly correlated with orthopedic testing when at least two out of three of the following orthopedic tests for SIJ sprain were positive: Patrick-Faber (supine hip flexion, external rotation and abduction; useful for SIJ assessment after pathologic conditions of the hip were previously ruled out), Gaenslen’s test (forced thigh flexion), and Yeoman’s test (forced thigh extension). Szadek and colleagues (2009) found that combining the SI compression test, thigh thrust test, and “3 or more positive stressing tests showed discriminative power for diagnosing SI joint pain.”
Differentiation between enteric disorders, pelvic disorders, and inflammatory arthritides from SIJ dysfunction can be challenging, and two of these disorders may coexist. Such conditions include Crohn’s disease, psoriasis, Reiter’s syndrome, Behçet’s syndrome, and other inflammatory bowel disorders. Differential diagnosis may be aided by anesthetic injection into the SIJ, with relief of pain after anesthetic injection being an indication of SIJ dysfunction (McEwen et al., 1971; Russell et al., 1977; Davis, Thomson, & Lentle, 1978; Dekker-Saeys et al., 1978; Yazici, Tuzlaci, & Yurdakul, 1981; Olivieri et al., 1990; Ro, 1990).
Fortunately SIJ syndrome usually is self-limiting and responds well to rest. However, in some individuals the condition becomes chronic and disabling. Chiropractic manipulation has been used to treat SIJ disorders. More than 90% of patients disabled with chronic SIJ syndrome who presented to a university hospital responded favorably to a regimen of sacroiliac manipulation (Cassidy & Mierau, 1992). Some believe examination and treatment of the soft tissues surrounding the SIJ is the best way to manage spinal and SIJ problems (Lavignolle et al., 1983; Maltezopoulos & Armitage, 1984). Injection of local anesthetic not only may prove useful in the diagnosis of these disorders, but also may provide long-term relief in some patients who do not respond to other treatments (Cassidy & Mierau, 1992). The use of belts to stabilize the SIJ, injections of proliferant (to decrease mobility), adherence to prescribed exercises, and utilization of surgical fusion also have been used to treat SIJ disorders (Cassidy, 1993).
Increased stiffness of the SIJ can potentially protect against sprains of the SIJ ligaments. Richardson and colleagues (2002) assessed stiffness of the SIJ by measuring vibrations of the sacrum and ilium adjacent to the SIJ, after the application of vibrations with a frequency of 200 Hz to the anterior superior iliac spine. Through use of these methods, contraction of the left and right transversus abdominis muscles was found to increase stiffness of the sacroiliac joint. The work of Pel and colleagues (2008) supports the importance of the transversus abdominis muscles in stabilizing the SIJ.
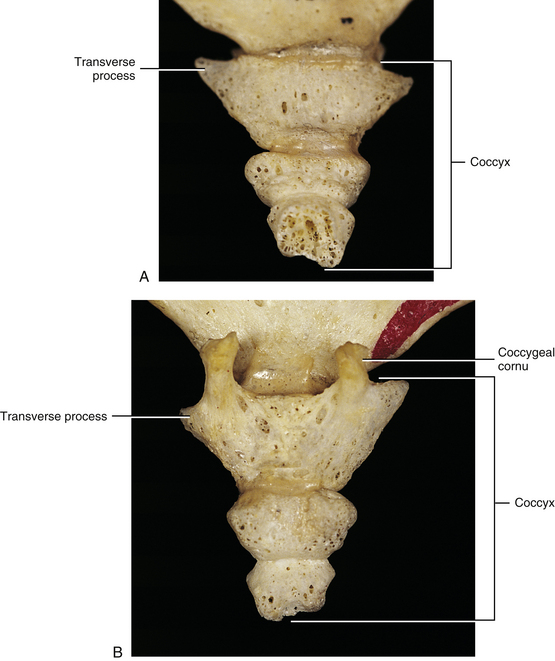
The Coccyx
The coccyx (Fig. 8-19) is formed by three to five fused segments (usually four), and each develops from one primary center of ossification. The coccygeal segments develop between the ages of 1 (first segment) and 20 (fourth segment) years, although the time when coccygeal ossification centers develop varies. Fusion of the coccygeal segments usually occurs in the late twenties but may be delayed considerably (Standring et al., 2008). Sometimes the first coccygeal segment does not fuse with the remainder of the coccyx at all. The first coccygeal segment has several prominences (see the following discussion), whereas the second through fifth coccygeal segments are rather simple and are homologous to vertebral bodies of typical vertebrae.
As with the sacrum, the coccyx is triangular in shape; with the superior surface the base and the inferior surface the apex (see Fig. 8-19). The base of the coccyx is formed by the first coccygeal segment. The top of the base has an articular facet for articulation with a small disc that intervenes between it and the apex of the sacrum. Also, a TP extends from the left and right lateral surfaces of the coccygeal base. Posteriorly the coccygeal cornua are in register with the sacral cornua. Intercornual ligaments connect the cornua of the sacrum with those of the coccyx. The S5 and coccygeal nerves of each side exit the sacral hiatus (see The Sacrum) and pass between the apex of the sacrum and intercornual ligament (i.e., the nerves pass deep to this ligament). They then give off the dorsal rami, which pass posterior to the TP of the coccyx.
A series of fibrocartilaginous discs develop, before and after birth, between the individual coccygeal segments. These discs eventually are replaced by bone as the segments fuse during the second or third decades of life.
Structures That Attach to the Coccyx and the Borders of the Pelvic Outlet
Table 8-2 lists the muscles and ligaments that attach to the coccyx. The apex of the coccyx helps to form the boundaries of the pelvic outlet. The boundaries of this outlet follow:
Nerves and Vessels Associated with the Sacrum and Coccyx
Location of Sacral Dorsal Root Ganglia
Ebraheim and Lu (1998) evaluated the locations of the first through the fourth sacral dorsal root ganglia (DRG) within the sacral canal and sacral foramina. They found that the S1 DRG was intraforaminal (defined as lateral to the medial border of the sacral pedicle) 55% to 60% of the time and “intracanalar” (defined as medial to the medial aspect of the sacral pedicles) 40% to 45% of the time. The S2 DRG was intraforaminal 15% to 50% of the time and intracanalar 50% to 85% of the time. The S3 and S4 DRG always were intracanalar. No S1 to S4 DRG were found to be extraforaminal. The S1 DRG were the largest, the DRG became smaller from S1 to S4, and the S1 and S2 DRG were significantly larger than the S3 and S4 DRG. Ebraheim and Lu (1998) were convinced that intracanalar S1 and S2 DRG were more susceptible than intraforaminal DRG to compression from L5-S1 IVD protrusions and other space-occupying lesions causing stenosis of the sacral canal. Kikuchi and colleagues (1994) found an even higher incidence (77.3%) of intraspinal “intracanalar” S1 DRG than did Ebraheim and Lu (1998).
Sacral Plexus
The sacral plexus is formed by the anterior primary divisions (ventral rami) of L4 and L5 (lumbosacral trunk), S1-3, and part of S4. Of note is that the lumbosacral trunk is approximately 30 mm in length. It crosses the SIJ approximately 2 cm inferior to the pelvic brim (i.e., 2 cm below the internal arcuate line of the ilium) and is fixed to the sacral ala by fibrous connective tissue. In addition, the APD of S1 is the widest APD (9.0 mm) contributing to the lumbosacral plexus (Ebraheim et al., 1997a). The anterior primary division of S4 also contributes to the coccygeal plexus.
The sacral plexus is located on the posterior pelvic wall, specifically, anterior to the piriformis muscle (Figs. 8-20 and 8-21). In fact, the S1 nerve root courses superior to the piriformis muscle as the root exits the pelvis, whereas the S2 and S3 nerve roots usually traverse the piriformis muscle (75% and 97% of the time, respectively), and the S4 nerve root exits inferior to the piriformis muscle (Russell et al., 2008). The branches of the sacral plexus follow (contributing spinal cord segments appear in parentheses):
• Posterior cutaneous nerve of the thigh (S1-3)
• Sciatic nerve (L4, L5, S1-3)
• Superior gluteal nerve (L4, L5, S1)
• Inferior gluteal nerve (L5, S1, S2)
• Nerve to the obturator internus (and superior gemellus) (L5, S1, S2)
• Nerve to the quadratus femoris (and inferior gemellus) (L4, L5, S1)
Chapters 9 and 10 provide further information on the nerves of the sacral plexus. Chapter 10 also discusses the relationship between the nerves of the sacral plexus and pelvic autonomic fibers.
Pelvic Autonomic Nerves
The S2 to S4 segments provide parasympathetic innervation to the pelvic viscera via nerves of the sacral plexus. In addition, each sympathetic trunk has five sacral ganglia that are located along the anterior surface of the sacrum. These ganglia supply sympathetic innervation to the pelvic viscera via gray rami. The two sympathetic chains join inferiorly on the anterior surface of the coccyx at a single ganglion, which is called the ganglion impar. The inferior hypogastric autonomic plexus, which receives contributions from the lumbar splanchnic nerves (sympathetic), sacral sympathetics, and pelvic splanchnic nerves (parasympathetic), helps to supply the pelvic viscera with autonomic fibers. Chapter 10 thoroughly discusses the pelvic autonomics.
Arteries Associated with the Sacrum and Coccyx
The aorta bifurcates at approximately the body of L4 into left and right common iliac arteries. Each common iliac artery, in turn, bifurcates into an internal and external iliac artery. The external iliac artery courses toward the inguinal ligament, giving off the inferior epigastric and deep circumflex iliac arteries before crossing under the inguinal ligament to become the femoral artery. The left and right internal iliac arteries supply the pelvic viscera, inferior aspect of the posterior abdominal wall, pelvic wall, gluteal region, ischioanal (ischiorectal) fossa, perineum, and adductor region of the thigh. Each internal iliac artery can be described as having an anterior, or visceral, division and a posterior, or somatic, division. The branches of each division of the internal iliac artery are listed in the following section.
Posterior Division of the Internal Iliac Artery
1. Iliolumbar artery. This is the first branch of the internal iliac artery and often (52%) originates from the internal iliac artery proper, rather than from the posterior division of the artery (Rusu et al., 2010). The iliolumbar artery further divides into two branches:
a. An iliac branch that passes along the superior border of the iliac crest to supply the iliacus and quadratus lumborum muscles.
b. A lumbar branch courses superiorly to help supply the psoas muscle.
2. Lateral sacral artery. This artery has been mentioned previously and is shown in Figure 8-21. It courses along the anterior and lateral surface of the sacrum, sending branches into the anterior sacral foramina. It serves as a major source of blood to the sacrum and sacral nerve roots.
3. Superior gluteal artery. This artery usually passes between the lumbosacral trunk and S1 ventral ramus to exit the pelvis. (In some cases it exits between the S1 and S2 ventral rami.) The superior gluteal artery helps to supply the gluteal region.
Anterior Division of the Internal Iliac Artery
1. Inferior gluteal artery. This artery usually exits the pelvis by passing between the S1 and S2, or the S2 and S3, ventral rami. As with the superior gluteal artery, this artery also helps to supply the buttock and thigh.
2. Internal pudendal artery. This artery usually exits the pelvis between the S2 and S3 ventral rami. It then passes around the posterior surface of the sacrospinous ligament to enter the ischioanal (ischiorectal) fossa, where it continues anteriorly within the pudendal (Alcock’s) canal. It terminates near the symphysis pubis by dividing into the deep and dorsal arteries of the penis.
3. Inferior vesical artery. This artery is only found in the male. In the female its place is taken by the vaginal artery. The inferior vesical (or a branch of the vaginal artery in the female) supplies the inferior aspect of the bladder.
4. Middle rectal artery. This artery usually is small and may arise from the inferior vesical artery or the internal pudendal artery. It helps to supply the rectum.
5. Obturator artery. The obturator artery has a rather long intrapelvic course before exiting the pelvis at the obturator foramen. It then supplies the adductor region of the thigh. Before exiting the pelvis, this artery gives a branch that anastomoses with the pubic branch of the inferior epigastric artery. This anastomosis frequently is called the accessory obturator artery. Occasionally the pubic branch of the inferior epigastric artery replaces the obturator artery.
6. Umbilical artery. This artery is the direct continuation of the internal iliac artery. It courses from the superior aspect of the bladder to the anterior abdominal wall, where its continuation forms the medial umbilical ligament. The superior vesical arteries are branches of the proximal portion of the umbilical artery. As the name implies, these arteries pass inferiorly from the umbilical artery to supply the superior aspect of the bladder.
Branches Found Only in the Female
1. Uterine artery. This artery courses beneath (inferior to) the ureter to reach the lateral aspect of the uterus, which it supplies.
2. Vaginal artery. This artery not only supplies the vagina, but also takes the place of the male inferior vesical artery. Therefore it also supplies the inferior aspect of the bladder.
Median sacral artery: The median sacral artery is another vessel associated with the anterior surface of the sacrum. The median (middle) sacral artery (see Chapter 7) is a tiny unpaired artery that arises from the posterior surface of the abdominal aorta just before the aorta bifurcates into right and left common iliac arteries. It then passes inferiorly along the midline of the anterior sacrum, sending branches into the anterior sacral foramina. These foraminal branches are accompanied by branches of the lateral sacral artery.
Veins Associated with the Sacrum and Coccyx
The venous drainage of the sacrum, coccyx, and pelvic viscera generally flows in the opposite direction as the arterial supply and drains into the internal iliac veins (left and right). The internal iliac veins usually course lateral to the internal iliac arteries (71% of the time on the left side and 93% on the right) (Bleich et al., 2007). Each internal iliac vein drains into the common iliac vein of the same side, and the common iliac vein of each side drains into the inferior vena cava. One exception to this is the superior rectal vein, which helps to form the inferior mesenteric vein. The inferior mesenteric vein, in turn, drains into either the splenic or superior mesenteric veins. The latter two veins combine to form the portal vein. Blood from the inferior rectal vein eventually drains into the inferior vena cava. The anastomosis between the superior rectal veins and the inferior and middle rectal veins forms an important portal-caval anastomosis.
The internal and external vertebral venous plexuses also help to drain the sacrum. These venous plexuses are discussed with the vertebral canal in Chapter 2.
References
Abel, M.S. Sacroiliac joint changes in traumatic paraplegics. Radiology. 1950;55:235–239.
Abitbol, M. Sacral curvature and supine posture. Am J Phys Anthropol. 1989;80:379–389.
Albrecht, T.L., Scutter, S.D., Henneberg, M. Radiographic method to assess the prevalence of sacral spina bifida occulta. Clin Anat. 2007;20:170–174.
Bakland, O., Hanse, J. The axial sacroiliac joint. Anat Clin. 1984;6:29–36.
Barakatt, E., et al. Interinnominate motion and symmetry: comparison between gymnasts and nongymnasts. J Orthop Sports Phys Ther. 1996;23:309–319.
Beal, M.C. The sacroiliac problem: review of anatomy, mechanics and diagnosis. J Am Osteopath Assoc. 1982:667–673. [June].
Bellamy, N., Park, W., Rodney, P.J. What do we know about the sacroiliac joint? Arthritis Rheum. 1983;12(3):282–309.
Bermis, T., Daniel, M. Validation of the long sitting test on subjects with sacroiliac dysfunction. J Orthop Sports Phys Ther. 1987;8:336–343.
Bernard, T., Cassidy, D. As quoted from: the sacroiliac joints revisited: report from the San Diego congress on the sacroiliac joint. Chiro Rep. 1993;7(2):1–6.
Bleich, A.T., et al. Posterior division of the internal iliac artery: anatomic variations and clinical applications. Am J Obstet Gynecol. 2007;197(658):e651–e655.
Blower, P.W., Griffin, A.J. Clinical sacroiliac joint test in ankylosing spondylitis and other causes of low back pain: 2 studies. Ann Rheum Dis. 1984;43:192–195.
Blumel, J., Evans, E.B., Eggers, G.W.N. Partial and complete agenesis or malformation of the sacrum with associated anomalies. J Bone Joint Surg. 1959;41-A:497–518.
Bose, R.N. Ankylosing spondylitis: treatment. Am Chiro. 1982:50. [June].
Bowen, V., Cassidy, J.D. Macroscopic and microscopic anatomy of sacroiliac joint from embryonic life until the eighth decade. Spine. 1981;6(6):620–628.
Brunner, C., Kissling, R., Jacob, H.A.C. The effects of morphology and histopathologic findings on the mobility of the sacroiliac joint. Spine. 1991;16(9):1111–1117.
Burns, S.H., Mireau, D.R., Howlett, E. Sacroiliac joint pain due to bacterial infection: a report of two cases. J Can Chiro Assoc. 1995;39:139–146.
Bussey, M.D., Milosavljevic, S., Bell, M.L. Sex differences in the pattern of innominate motion during passive hip abduction and external rotation. Man Ther. 2009;14:514–519.
Cassidy, D. As quoted from: the sacroiliac joints revisited: report from the San Diego congress on the sacroiliac joint. Chiro Rep. 1993;7(2):1–6.
Cassidy, J.D., Mierau, D.R. Pathophysiology of the sacroiliac joint. In Haldeman S., ed.: Principles and practice of chiropractic, 2nd ed., East Norwalk, Conn: Appleton & Lange, 1992.
Chang, H.S., Nakagawa, H. Altered function of lumbar nerve roots in patients with transitional lumbosacral vertebrae. Spine (Phila Pa 1976). 2004;29:1632–1635. [discussion 1635].
Colachis, S.C., et al. Movement of sacroiliac joint in adult male: a preliminary report. Arch Phys Med Rehabil. 1963;44:490–497.
Cone, R.O., Resnick, D. Roentgenographic evaluation of the sacroiliac joints. Orthop Rev. 1983;12(1):95–105.
Daum, W.J. The sacroiliac joint: an underappreciated pain generator. Am J Orthop. 1995:475–478. [June].
Davis, P., Thomson, A.B.R., Lentle, B.C. Quantitative sacroiliac scintigraphy in patients with Crohn's disease. Arthritis Rheum. 1978;21(2):234–237.
DeCavalho, A., Graudal, H. Sacroiliac joint involvement in classical or definite rheumatoid arthritis. Acta Radiol Diagn. 1980;21:417–423.
Dekker-Saeys, B.J., et al. Prevalence of peripheral arthritis, sacroiliitis and ankylosing spondylitis in patients suffering from inflammatory bowel disease. Ann Rheum Dis. 1978;37:33–35.
Diakow, P., Cassidy, J.D., DeKorompay, V.L. Post-surgical sacroiliac syndrome: a case study. J Can Chiro Assoc. 1983;27(1):19–21.
Dommisse, G.F., Louw, J.A. Anatomy of the lumbar spine. In: Floman Y., ed. Disorders of the lumbar spine. Rockville, Md, and Tel Aviv: Aspen and Freund, 1990.
DonTigny, R.L. Anterior dysfunction of sacroiliac joint as a major factor in the etiology of idiopathic low back pain syndrome. Phys Ther. 1990;70(4):250–265.
Drerup, B., Hierholzer, E. Movement of human pelvis and displacement of related anatomical landmarks on body surface. J Biomech (Br). 1987;20(19):971–977.
Dreyfuss, P., et al. The value of medical history and physical examination in diagnosing sacroiliac joint pain. Spine (Phila Pa 1976). 1996;21:2594–2602.
Dunn, E.J., et al. Pyogenic infections of the sacroiliac joint. Clin Orthop. 1976;118:113–117.
Ebraheim, N.A., Lu, J. Morphometric evaluation of the sacral dorsal root ganglia. Surg Radiol Anat. 1998;20:105–108.
Ebraheim, N.A., et al. Radiology of the sacroiliac joint. Spine. 1997;22:869–876.
Ebraheim, N.A., et al. The relationship of lumbosacral plexus to the sacrum and the sacroiliac joint. Am J Orthop. 1997:105–110. [Feb].
Ebraheim, N.A., et al. Internal architecture of the sacrum in the elderly. Spine. 2000;25:292–297.
Ebraheim, N.A., Biyani, A., Salpietro, B. Zone III fractures of the sacrum: a case report. Spine. 1996;21:2390–2396.
Egund, N., et al. Movement of sacroiliac joint, demonstrated with roentgen stereophotogrammetry. Acta Radiol Diagn. 1978;19:833–846.
Ehara, S., El-Khoury, G.Y., Bergman, R.A. The accessory sacroiliac joint, a common anatomic variant. AJR, Am J Roentgenol. 1988;150:857–859.
Eichenseer, P.H., Sybert, D.R., Cotton, J.R. A finite element analysis of sacroiliac joint ligaments in response to different loading conditions. Spine (Phila Pa 1976). 2011;36:E1446–E1452.
Enix, D., Smith, D. Variability in the morphology of the articular surface of the sacroiliac joint. J Chiro Educ. 2010;24:91.
Fidler, M.W., Plasmans, C.M.T. The effect of four types of support on segmental mobility of the lumbosacral spine. J Bone Joint Surg. 1983;65-A:943–947.
Fligg, D.B. Piriformis technique. J Can Chiro Assoc. 1986;30(2):87–88.
Fortin, J.D., Ballard, K.E. The frequency of accessory sacroiliac joints. Clin Anat. 2009;22:876–877.
Freeman, M.D., Fox, D., Richards, T. The superior intracapsular ligament of the sacroiliac joint: confirmation of Illi's ligament. J Manipulative Physiol Ther. 1990;13(7):384–390.
Frigerio, N.A., Stowe, R.R., Howe, J.W. Movement of sacroiliac joint. Acta J Chiro. 1974;8:161–166.
Fryette, H.H. Some reasons why sacroiliac lesions recur. J Am Osteopath Assoc. 1936;36(3):119–122.
Gibbons, K., Soloniuk, D., Razack, N. Neurological injury and patterns of sacral fractures. J Neurosurg. 1990;72:889–893.
Grieve, E. Lumbopelvic rhythm and mechanical dysfunction of the sacroiliac joint. Physiotherapy. 1981;67(6):171–173.
Grieve, G.P. The sacroiliac joint. Norfolk Norwich Hosp. 1975:384–401.
Grob, K.R., Neuhuber, W.L., Kissling, R.O. [Innervation of the sacroiliac joint of the human]. Z Rheumatol. 1995;54:117–122.
Hadley, L.A. Accessory sacroiliac articulations. J Bone Joint Surg. 1952;34-A:149–155.
Hassanein, G.H. Metric study of Egyptian sacrum for lumbo-sacral fixation procedures. Clin Anat. 2011;24:218–224.
Illi, F.W. The vertebral column: lifeline of the body. Chicago: National College of Chiropractic; 1951.
Jajic, I., Jajic, Z. The prevalence of osteoarthrosis. Clin Rheum. 1987;6:39–41.
Janse, J. Principles and practice of chiropractic: an anthology. Lombard, IL: National College of Chiropractic; 1976.
Janse, J. The clinical biomechanics of sacroiliac mechanism. ACA J Chiro. 1978;12:s1–s8.
Jaovisidha, S., et al. Ventral sacroiliac ligament: anatomic and pathologic considerations. Invest Radiol. 1996;31:532–541.
Johnson, J.W. Sacroiliac strain. J Am Osteopath Assoc. 1964;63:1132–1148.
Kikuchi, S., et al. Anatomic and radiographic study of dorsal root ganglia. Spine. 1994;19:6–11.
King, L. Incidence of sacroiliac joint dysfunction and low back pain in fit college students. J Manipulative Physiol Ther. 1991;14(5):333–334.
Kirkaldy-Willis, W. The pathology and pathogenesis of low back pain. In Kirkaldy-Willis W., ed.: Managing low back pain, 2nd ed., New York: Churchill Livingstone, 1988.
Kristiansson, P., Svärdsudd, K., von Schoultz, B. Back pain during pregnancy. Spine. 1996;21:702–709.
Kumar, A., Tubbs, R.S. Spina bifida: a diagnostic dilemma in paleopathology. Clin Anat. 2011;24:19–33.
LaBan, M.M., et al. Symphyseal and sacroiliac joint pain associated with pubic symphysis instability. Arch Phys Med Rehabil. 1978;59:470–472.
Lavignolle, B., et al. An approach to functional anatomy of sacroiliac joint in vivo. Anat Clin. 1983;5:169–176.
Lawrence, D.J., Low back pain: mechanism, diagnosis and treatment. Cox, J.M., eds. 5th ed. Baltimore: Williams & Wilkins; 1990.
Lichtblau, S. Dislocation of sacroiliac joint. J Bone Joint Surg. 1962;44-A:193–198.
Mahato, N.K. Association of rudimentary sacral zygapophyseal facets and accessory and ligamentous articulations: implications for load transmission at the L5-S1 junction. Clin Anat. 2010;23:707–711.
Mahato, N.K. Complete sacralization of L5 vertebrae: traits, dimensions, and load bearing in the involved sacra. Spine J. 2010;10:610–615.
Mahato, N.K. Variable positions of the sacral auricular surface: classification and importance. Neurosurg Focus. 2010;28:E12.
Mahato, N.K. Facet dimensions, orientation, and symmetry at L5-S1 junction in lumbosacral transitional states. Spine (Phila Pa 1976). 2011;36:E569–E573.
Maltezopoulos, V., Armitage, N. A comparison of four chiropractic systems in the diagnosis of sacroiliac malfunction. Eur J Chiro. 1984;32:4–42.
McEwen, C., et al. Ankylosing spondylitis and spondylitis accompanying ulcerative colitis, regional enteritis, psoriasis and Reiter's disease. Arthritis Rheum. 1971;14(3):291–318.
McGaugh, J.M., Brismee, J.M., Dedrick, G.S., et al. Comparing the anatomical consistency of the posterior superior iliac spine to the iliac crest as reference landmarks for the lumbopelvic spine: a retrospective radiological study. Clin Anat. 2007;20:819–825.
McGrath, M.C., Zhang, M. Lateral branches of dorsal sacral nerve plexus and the long posterior sacroiliac ligament. Surg Radiol Anat. 2005;27:327–330.
McGrath, C., Nicholson, H., Hurst, P. The long posterior sacroiliac ligament: a histological study of morphological relations in the posterior sacroiliac region. Joint Bone Spine. 2009;76:57–62.
Moore, A.E., et al. An anatomical ultrasound study of the long posterior sacro-iliac ligament. Clin Anat. 2010;23:971–977.
Murata, Y., et al. Innervation of the sacroiliac joint in rats by calcitonin gene-related peptide-immunoreactive nerve fibers and dorsal root ganglion neurons. Clin Anat. 2007;20:82–88.
Nykoliation, J.W., Cassidy, J.D., Dupuis, P. Osteitis condensans ilii, a stress phenomenon. J Can Chiro Assoc. 1984;28(1):21–24.
Olivieri, I., et al. Differential diagnosis between osteitis condensans ilii and sacroiliitis. J Rheum. 1990;17(1):1504–1512.
Otter, R. A review study of differing opinions expressed in the literature about the anatomy of the sacroiliac joint. Eur J Chiro. 1985;33:221–242.
Paquin, J.D., et al. Biomechanical and morphological study of cartilage from adult human sacroiliac joint. Arthritis Rheum. 1983;26(6):887–895.
Pel, J.J., et al. Biomechanical analysis of reducing sacroiliac joint shear load by optimization of pelvic muscle and ligament forces. Ann Biomed Eng. 2008;36:415–424.
Peretz, A.M., Hipp, J.A., Heggeness, M.H. The internal bony architecture of the sacrum. Spine. 1998;23:971–974.
Pitkin, H.C., Pheasant, H.C. A study of sacral mobility. J Bone Joint Surg. 1936;18-A:365–374.
Potter, N.A., Rothstein, J.M. Intertester reliability for selected clinical tests of the sacroiliac joint. Phys Ther. 1985;65(11):1671–1675.
Prassopoulos, P.K., et al. Sacroiliac joints: anatomical variants on CT. J CAT. 1999;23:323–327.
Resnik, C.S., Resnick, D. Radiology of disorders of sacroiliac joints. JAMA. 1985;253(19):2863–2866.
Resnik, D., Dwosh, I.L., Niwayama, G. Sacroiliac joint in renal osteodystrophy: roentgenographic-pathologic correlation. J Rheum. 1975;2(3):287–295.
Richardson, C.A., et al. The relation between the transversus abdominis muscles, sacroiliac joint mechanics, and low back pain. Spine. 2002;27:399–405.
Ro, C.S. Sacroiliac joint. In Cox J.M., ed.: Low back pain: mechanism, diagnosis and treatment, 5th ed., Baltimore: Williams & Wilkins, 1990.
Romanus, R. Pelvo-spondylitis ossificans. Chicago: Year Book; 1955.
Russell, A.S., et al. The sacroiliitis of acute Reiter's syndrome. J Rheum. 1977;4(3):293–296.
Russell, J.M., et al. Magnetic resonance imaging of the sacral plexus and piriformis muscles. Skeletal Radiol. 2008;37:709–713.
Rusu, M.C., et al. The iliolumbar artery-anatomic considerations and details on the common iliac artery trifurcation. Clin Anat. 2010;23:93–100.
Sakamoto, N., et al. An electrophysiologic study of mechanoreceptors in the sacroiliac joint and adjacent tissues. Spine. 2001;26:E468–E471.
Sandoz, R.W. Structural and functional pathologies of the pelvic ring. Ann Swiss Chiro Assoc. 1978:101–155.
Sashin, D. A critical analysis of the anatomy and the pathologic changes of the sacroiliac joints. Bull Hosp Joint Dis. 1929:891–910.
Schuchman, J.A., Cannon, C.L. Sacroiliac strain syndrome, diagnosis and treatment. Tex Med. 1986;82:33–36.
Schwarzer, A.C., Aprill, C.N., Bogduk, N. The sacroiliac joint in chronic low back pain. Spine. 1995;20:31–37.
Sgambati, E., et al. Morphometric analysis of the sacroiliac joint. It J Anat Embryol. 1997;102:33–38.
Simkins, C.S. Anatomy and significance of the sacroiliac joint. AAO Yearbook. 1952:64–69.
Smidt, G.L., et al. Sacroiliac motion for extreme hip positions. Spine. 1997;22:2073–2082.
Solonen, K.A. The sacroiliac joint in the light of anatomical, roentgenographical and clinical studies. Acta Orthop Scand. 1957;27:160–162.
Standring, S., et al. Gray's anatomy: the anatomical basis of clinical practice, 40th ed. Edinburgh: Churchill Livingstone; 2008.
Sturesson, B., Selvik, G., Uden, A. Movements of the sacroiliac joints. A roentgen stereophotogrammetric analysis. Spine (Phila Pa 1976). 1989;14:162–165.
Szadek, K.M., et al. Diagnostic validity of criteria for sacroiliac joint pain: a systematic review. J Pain. 2009;10:354–368.
Szadek, K.M., et al. Possible nociceptive structures in the sacroiliac joint cartilage: an immunohistochemical study. Clin Anat. 2010;23:192–198.
Vleeming, A., et al. The function of the long dorsal sacroiliac ligament. Spine. 1996;21:556–562.
Vogler, J.B., et al. Normal sacroiliac joint: a CT study of asymptomatic patients. Radiology. 1984;151(2):433–437.
Waikakul, S., Chandraphak, S., Sangthongsil, P. Anatomy of L4 to S3 nerve roots. J Orthop Surg (Hong Kong). 2010;18:352–355.
Walker, J.M. Age-related differences in the human sacroiliac joint: a histological study; implications for therapy. J Orthop Sports Phys Ther. 1986;7(6):325–334.
Wallheim, G.G., Olerud, S., Ribbe, T. Motion of symphysis in pelvic instability. Scand J Rehabil Med. 1984;16:163–169.
Wang, Z., et al. Influence of sacral morphology in developmental spondylolisthesis. Spine (Phila Pa 1976). 2008;33:2185–2191.
Weisl, H. The articular surface of sacroiliac joint and their relation to the movement of the sacrum. Acta Anat. 1954;22:1–14.
Weisl, H. The ligaments of sacroiliac joint examined with particular reference to their function. Acta Anat. 1954;20(30):201–213.
Weisl, H. The movement of the sacroiliac joint. Acta Anat. 1955;23:80–91.
Wilder, D.G., Pope, M.H., Frymoyer, J.W. The functional topography of sacroiliac joint. Spine. 1980;5(6):575–579.
Winterstein, J.F. Spinographic evaluation of pelvic and lumbar spine. Lombard, Ill: National College of Chiropractic; 1972.
Wood, J. Motion of the sacroiliac joint. Palmer Coll Res Forum, Spring. 1985:1–16.
Wu, L.P., et al. Variable morphology of the sacrum in a Chinese population. Clin Anat. 2009;22:619–626.
Yazici, H., Tuzlaci, M., Yurdakul, S. A controlled survey of sacroiliitis in Behçet's disease. Ann Rheum Dis. 1981;40:558–559.