Ligaments and Intervertebral Discs of the Lumbar Region
Most of the ligaments associated with the lumbar region have been discussed in previous chapters. The articular capsules of the Z joints, ligamenta flava, supraspinous ligament, interspinous ligaments, intertransverse ligaments, and anterior and posterior longitudinal ligaments are discussed in Chapters 5 and 6, and the IVDs are discussed in Chapter 2. The mamillo-accessory ligament was discussed previously in this chapter (see Articular Processes). This section is devoted to characteristics of the mentioned ligaments that are unique to the lumbar region. The iliolumbar ligaments (left and right), which are found only in the lower lumbar region, also are covered in this section.
Lumbar Anterior Longitudinal Ligament
The anterior longitudinal ligament (ALL) and posterior longitudinal ligament (PLL) have been collectively termed the intercentral ligaments because they connect the anterior and posterior surfaces of adjacent vertebral bodies (centra), respectively (Grenier et al., 1989b). They also help attach the vertebral bodies to the IVDs and are important in stabilizing the spine during flexion (PLL) and extension (ALL). They also function to limit flexion (PLL) and extension (ALL) of the spine.
The ALL is wider from side to side in the lumbar than in the thoracic region. It has also been found to be thicker than the PLL (Grenier et al., 1989b). The lumbar ALL extends across the anterior aspect of the vertebral bodies and IVDs to attach inferiorly to the sacrum (Fig. 7-18). Like the ALL throughout the vertebral column, the lumbar ALL is firmly attached to a significant portion of the superior and inferior bony end plates of the vertebral bodies, but is only lightly attached to the center of the vertebral bodies or IVDs (Cramer et al., 1996, 1998a). Table 7-9 demonstrates the percentage of the width of the spine taken up by the ALL at the superior-to-inferior center of the vertebral bodies and at the center of the IVD. Figure 7-18, A, shows the lumbar ALL drawn to scale. Notice that the ALL bows out slightly at the level of the lumbar IVDs. This flaring of the ALL at the IVDs is unique to the lumbar region of the vertebral column (Cramer et al., 1996, 1998a,b).
Table 7-9
Width Measurements∗ of the Anterior Longitudinal Ligament and Spine at Seven Locations†
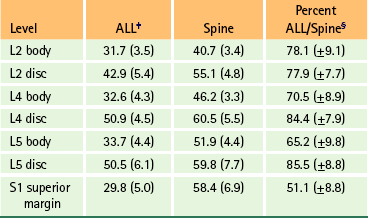
∗Measurements are in millimeters with standard deviations in parentheses.
†These data allowed for the development of the composite diagram of the lumbar spine and the ALL shown in Figure 7-18, A.
‡ALL, Anterior longitudinal ligament.
§These are the averages of the percentages obtained for each specimen (30 specimens), not results obtained from values of column two divided by those of column three. These values represent the percent of the width of the spine occupied by the ALL at the specified level.

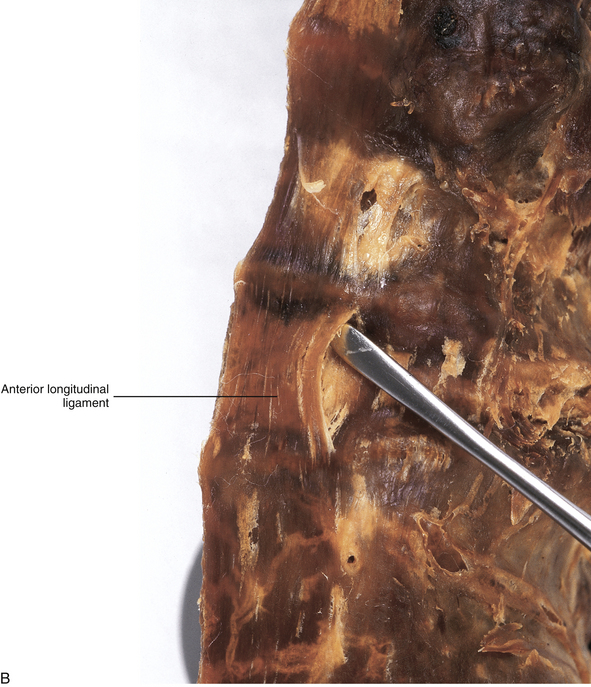
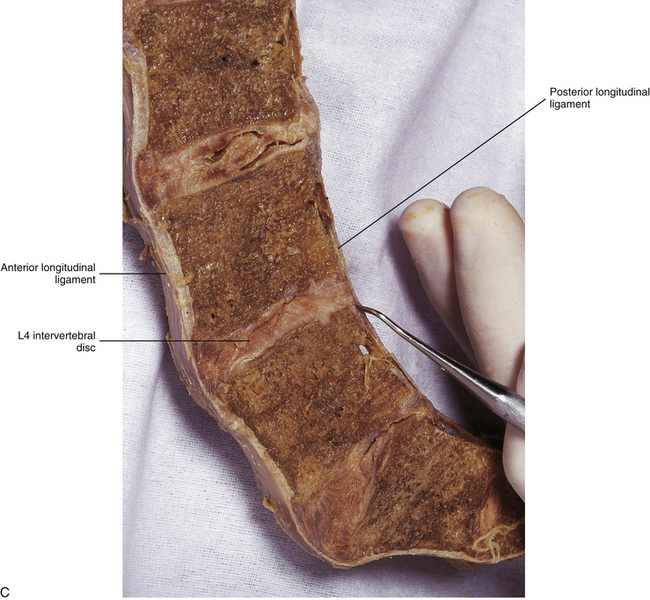
FIG. 7-18 A, Anterior longitudinal ligament (ALL) in the lumbar region. The ALL is illustrated to scale using the data of Table 7-9 and shows the lateral extensions of the ALL. B, Dissection of the lumbar ALL. Notice the probe passing posterior to the ligament, demonstrating its firm superior attachment to the bony end plate of the vertebral body. C, Midsagittal section of the lumbar region showing the anterior and posterior longitudinal ligaments. The probe is passing in front of (anterior to) the posterior longitudinal ligament.
The ALL functions to limit extension, and it may be torn during extension injuries of the spine. It receives sensory (nociceptive and proprioceptive) innervation from branches of the gray communicating rami of the lumbar sympathetic trunk; therefore damage to the ALL during extension injuries can be a direct source of pain.
Lumbar Posterior Longitudinal Ligament
The PLL in the lumbar region is denticulated in appearance (Figs. 7-19 and 7-20). That is, it is narrow over the posterior aspect of the vertebral bodies and flares laterally at each IVD, where it attaches to the posterior aspect of the anulus fibrosus. The lumbar PLL is composed of two strata of fibers, superficial and deep. The superficial fibers form a distinct midline band that spans several vertebral levels. The deep fibers are much shorter, and they converge on the IVD and extend laterally to the wide attachment sites of the PLL to the anulus fibrosus of the IVD (Parke & Schiff, 1993).
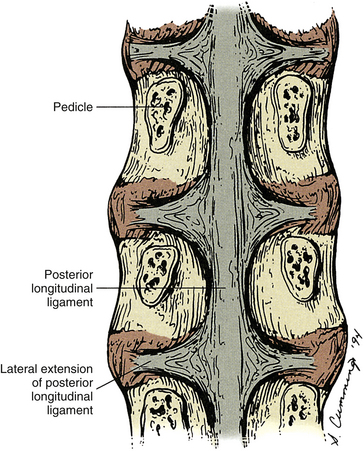
FIG. 7-19 Posterior longitudinal ligament (PLL) is shown coursing along the anterior aspect of the lumbar vertebral canal (along the posterior aspect of the vertebral bodies and the intervertebral discs). Contrast this with the shape of the PLL in the cervical region (Fig. 5-22).
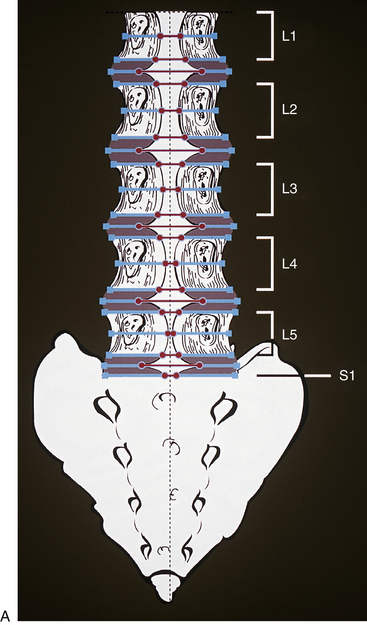
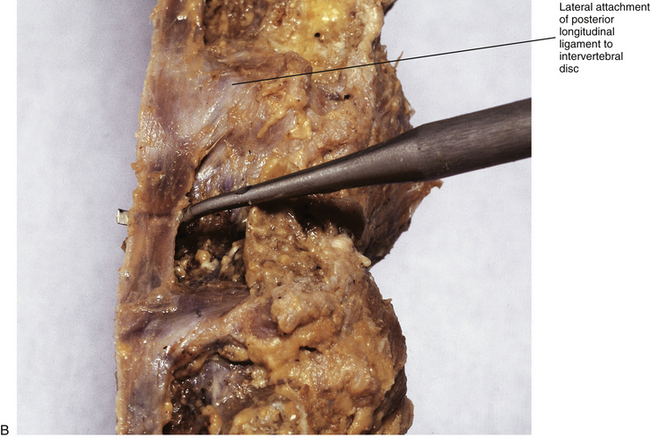
FIG. 7-20 A, Posterior longitudinal ligament (PLL) in the lumbar region. The PLL is illustrated to scale from the data presented in Table 7-10. B, Dissection of a midsagittally sectioned lumbar spine demonstrating the posterior longitudinal ligament. Notice how narrow the ligament is in the region of the vertebral body, where the probe is passing anterior to it, and how the ligament flares laterally as it firmly attaches to the intervertebral discs.
The width of the central attachment of the PLL to the lumbar vertebral bodies narrows as one moves down the spine, whereas the discal attachments remain relatively constant in width (Table 7-10 and Fig. 7-20). The PLL firmly attaches to the superior and inferior bony end plates of all lumbar vertebrae, and also firmly attaches to the superior aspect of the S1 segment. However, the PLL does not attach to the central regions of the L1-S1 vertebral bodies. The PLL is also firmly attached to the IVD along the peripheral margins of the PLL, but does not firmly attach to the IVD centrally to these peripheral attachments. This creates a rhomboid-shaped fascial cleft deep to the PLL in the center of the IVD. This fascial cleft may be important in helping to contain IVD extrusions (Oshima et al., 1993; Parke & Schiff, 1993; Yu et al., 1996; Cramer et al., 1998b).
Table 7-10
Width Measurements∗ of the Posterior Longitudinal Ligament and Spine at Six Locations†
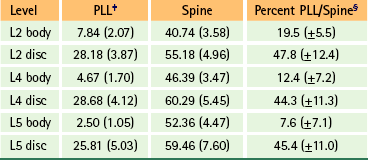
∗Measurements are in millimeters with standard deviations in parentheses.
†These data allowed for the development of the composite diagram of the lumbar spine and the PLL shown in Figure 7-20, A.
‡PLL, Posterior longitudinal ligament.
§These are the averages of the percentages obtained for each specimen (33 specimens), not results obtained from values of column two divided by those of column three. These values represent the percent of the width of the spine occupied by the PLL at the specified level.
The PLL receives sensory innervation from the recurrent meningeal nerve (sinuvertebral nerve). Substance P, a known sensory neurotransmitter that is usually associated with pain sensation, has been found in the terminal fibers of the sinuvertebral nerve innervating the lumbar PLL. Korkala and colleagues (1985) also found enkephalins, a known neuromodulator, in the PLL. Together these findings substantiate previous suppositions that the PLL is pain sensitive and may indicate that the PLL (at least in the lumbar region) is highly sensitive to pain. The pain sensitivity of the PLL has been demonstrated by mechanical irritation of the ligament in patients with only local anesthetics administered to the overlying skin. The pain was felt in the midline and radiated into the low back and superior aspect of the buttock (Edgar & Ghadially, 1976).
In some instances, posterior and posterolateral IVD extrusions may penetrate the PLL. This is a strong sign that the extrusion is not contained within the anulus fibrosis (see Herniated Disc in Chapter 11 and later discussion of Lumbar Intervertebral Discs in this chapter). This may be an indication for surgical removal of the disc. The penetrated PLL is able to be distinguished from a bulging anulus fibrosus on parasagittal MRI scans. The PLL appears as an area of low signal intensity on these images. Using MRI, Grenier and colleagues (1989b) were able to determine when the PLL was not disrupted (not penetrated by the anulus fibrosus or nucleus pulposus) 100% of the time and were able to determine when the PLL was disrupted 78% of the time. The authors concluded that MRI was useful in the detection of PLL disruption.
Lumbar Ligamenta Flava
The posterior ligaments of the thoracic and lumbar regions (Z joint capsules, ligamenta flava, interspinous and supraspinous ligaments, and also the thoracolumbar fascia) are highly important for maintaining the stability of the vertebral column (Vaccaro et al., 2009). The ligamenta flava of the lumbar region are the thickest (anterior to posterior thickness) of the entire spine. They extend between the laminae of adjacent vertebrae throughout the lumbar region, including the junction between the laminae of L5 and those of the S1 segment. In fact, thickness of the L5-S1 ligamenta flava is greater than that of L4-L5 (Safak et al., 2010). Each ligamentum flavum is thickest medially, and the overall thickness of the lumbar ligamenta flava increases during extension (Schmid et al., 1999). Laterally the ligament passes more anteriorly to form the anterior joint capsule of the Z joint, attaching to the superior and inferior articular processes that form this joint. The most lateral fibers attach to the pedicle of the vertebra below (Fig. 7-21).
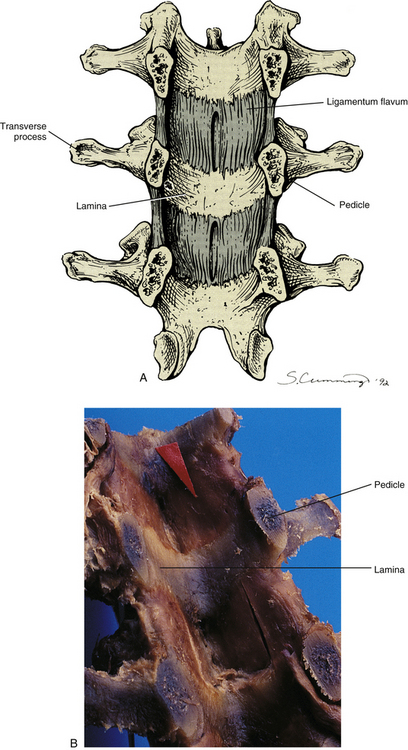
FIG. 7-21 Pedicles have been sectioned in a coronal plane to reveal the posterior aspect of the vertebral canal. A, Artist’s rendering. B, Cadaveric specimen. Notice the ligamenta flava (one example indicated by red arrow) passing between the adjacent laminae. (B, Courtesy National University of Health Sciences, Lombard, IL.)
The left and right sides of the lumbar ligamenta flava are actually continuous with one another in the midline (Olszewski, Yaszemski, & White, 1996), although the left and right sides of the lumbar ligamenta flava are usually asymmetric in thickness (Safak et al., 2010). A deep midline recess of several millimeters extends posteriorly in the midline. In the past this recess had been mistaken for a separation of the two sides of the ligamentum flavum. Each ligamentum flavum of the lumbar region has a superficial (posterior) and a deep (anterior) component. The two components adhere tightly to one another, except at their inferior attachment sites. Superiorly the adherent superficial and deep components attach to the anteroinferior aspect of the laminae above. The amount of the anterior surface of the laminae that is covered by the fibers extending superiorly increases from the upper to lower lumbar regions (a little less than 60% at L1 to approximately 70% at L5). Inferiorly, the attachments of the superficial and deep components of the ligamentum flavum differ. The superficial component attaches to the posterosuperior aspect of the lamina below; however, the deep component attaches to the anterosuperior aspect of the lamina below. Again, a much greater portion of the anterior surface of the lamina is covered by the deep component of the ligamentum flavum as one descends the lumbar region, with the combination of the superior attachment of the ligament below and the inferior attachment of the ligament above covering a little more than 60% of the lamina at L1 (the inferior aspect of the T12-L1 ligamentum flavum covers little of the anterior surface of L1), 75% to 80% at L3 and L4, and 100% of the lamina at L5 (Olszewski, Yaszemski, & White, 1996).
The exiting dorsal and ventral nerve roots of the lumbar region come in direct contact with the anterior aspect of the ligamentum flavum as the ligament forms the anterior capsule of the Z joint within the IVF (Hasue et al., 1983). A recess has been found in the lateral, articular portion of the ligament. Paris (1983) states that this recess may allow the synovium of the facet joint to pass through the ligamentum flavum. Under certain circumstances the synovium could then extend into the IVF, where it could conceivably compress the spinal nerve.
Sensory fibers, probably arising from the medial branch of the posterior primary division (Bogduk, 1983), have been found innervating the ligamenta flava (Edgar & Ghadially, 1976). Therefore damage to these ligaments may result in back pain. The sensory receptors within the ligamenta flava have been found to be both mechanoreceptors (i.e., Ruffini corpuscles, Ruffini end organs, Pacinian corpuscles), for proprioception, and free nerve endings, for nociception (Yahia, Newman, & Rivard, 1988; Yahia, Newman, & St-Georges, 1988). In fact, tissue samples of the ligamenta flava taken from low back pain patients at surgery have been found to contain a significant number of nociceptive fibers, similar in number to the Z joint capsule and significantly more than the IVD (Bucknill et al., 2002). Therefore tissue damage and inflammation of the ligamentum flavum may stimulate an additional ingrowth of sensory neurons, as has been found in other tissues (e.g., the IVD). However, as the ligamentum flavum undergoes aging and degeneration the number of nerve fibers decreases (Viejo-Fuertes et al., 1998). In addition, the role of the ligamenta flava as a secondary source of back pain in spinal stenosis (by compressing the nerve roots) is also well documented (see Lumbar Vertebral Foramen and Vertebral Canal; Lumbar Intervertebral Foramina and Nerve Root Canals).
Hypertrophy of the ligamenta flava can contribute to spinal canal stenosis. There are three primary patterns of ligamenta flava hypertrophy: (1) medially, which may be a cause of vertebral canal (spinal) stenosis; (2) laterally within the IVF, which could be a source of IVF foraminal stenosis; or (3) both medially and laterally (Safak et al., 2010). The ligamenta flava have been found to undergo hypertrophy in some individuals during simple aging of the spine. More frequently, hypertrophy of the ligamenta flava occurs as other spinal tissues, such as the IVDs and Z joints, undergo degenerative changes and decrease the stability of the motion segments. However, many instances of ligamenta flava hypertropy are probably the result of inflammation related to repeated microtears in the ligament. The microtears likely occur during years of physical stress to the ligment. The inflammation results in scar formation (fibrosis), and the scar tissue, which has a much greater volume than the tissue it replaces, causes the ligamentum flavum hypertrophy (Sairyo et al., 2007). Additionally, an increase in the growth factor transforming growth factor-beta 1 (TGF-β1) in ligamentum flavum fibroblasts is probably a part of the scar tissue and hypertrophy formation (Park, Chang, & Lee, 2001). Finally, a separate cause of ligamentum flavum hypertrophy is calcium pyrophosphate deposition disease (pseudogout) (Markiewitz et al., 1996).
Lumbar Interspinous Ligaments
The results of descriptive studies of these ligaments in the lumbar region have led to elaboration on their structure in this particular area of the spine. Therefore a brief discussion of the unique aspects of these ligaments is included here, even though the interspinous ligaments were described with the thoracic region (see Chapter 6).
Several authors have described the structure of a typical lumbar interspinous ligament as being composed of three parts: anterior, middle, and posterior (Behrsin & Briggs, 1988; Heylongs, 1978). These three parts run between adjacent spinous processes, filling the gap along the length of these processes (Fig. 7-22).

FIG. 7-22 Lumbar interspinous ligaments. Notice the anterior, middle, and posterior parts of each ligament.
The anterior portion of the interspinous ligament is paired (left and right) anteriorly, with each part attaching to the ligamentum flavum of the same side. A thin layer of adipose tissue separates the two halves. Posteriorly the two sides of this part of the interspinous ligament unite to form a single ligament. The fibers of this part of the interspinous ligament pass posteriorly and superiorly from their origin (ligamentum flavum) to attach to the anterior half of the inferior aspect of the spinous process of the vertebra above (Fig. 7-22).
The middle portion of the interspinous ligament is the most substantial region. It originates from the anterior half of the upper surface of a spinous process and passes posteriorly and superiorly to insert onto the posterior half of the lower surface of the spinous process of the vertebra above (Fig. 7-22). Although this orientation of fibers in the interspinous ligament would not seem to limit flexion of the lumbar region, the collagen fibers of this ligament have a complex interaction that increases stiffness of the ligament, helping it to limit flexion (Dickey, Bednar, & Dumas, 1996).
The posterior aspect of the interspinous ligament attaches to the posterior half of the upper surface of a spinous process and continues superiorly to pass behind (posterior to) the vertebra above, becoming continuous with the supraspinous ligament (see following discussion). Bogduk (2005) does not consider this posterior portion to be a true part of the interspinous ligament because it does not attach to two adjacent bones.
The interspinous ligament appears to be quite capable of being torn. One investigator found its fibers to be ruptured in 21% of cadavers examined. The middle fibers were found to be torn most frequently (Behrsin & Briggs, 1988). Because this ligament is supplied with sensory innervation containing both mechanoreceptive and nociceptive nerve endings (Yahia, Newman, & St-Georges, 1988), tearing of the ligament is likely a source of low back pain.
The interspinous ligaments and supraspinous ligament limit the end stage of lumbar flexion, and they are the first to sprain during hyperflexion of the lumbar region (Hutton, 1990).
Lumbar Supraspinous Ligament
The supraspinous ligament is strongest in the lumbar region. It is classically described as extending to the sacrum. However, Behrsin and Briggs (1988) believe that the supraspinous ligament ends at L5 and does not extend to the sacrum, and Paris (1983) states that it usually ends at L4 and rarely at L5, never extending to the sacrum. Paris (1983) has found that the strong fibers of origin of the lumbar erector spinae muscles and the thoracolumbar fascia take the place of this ligament inferior to the spinous process of L4. This fascia continues inferiorly to the median sacral crest. Bogduk (2005) states that the supraspinous ligament is not a true ligament in the lumbar region. He believes it is primarily made up of strong tendinous fibers of the longissimus thoracis and multifidus muscles, and crisscrossing fibers of the thoracolumbar fascia. In addition, a condensation of the membranous (deep) layer of the superficial fascia of the back forms the superficial layer of the supraspinous ligament (Bogduk, 2005). In spite of this, the term supraspinous ligament continues to be used quite frequently by clinicians and researchers alike. In such instances, they are probably referring to the tough combination of midline tendons of the longissimus thoracis muscle, intersecting fibers of the thoracolumbar fascia, and membranous layer of superficial fascia. The term lumbar supraspinous restraints would seem to reflect more accurately the true nature of the fibrous band of tissue that is found along the posterior aspect of the lumbar spinous processes and interspinous spaces. Regardless of the true origin of the tissue forming these supraspinous restraints, they do receive sensory innervation that consists of both mechanoreceptive and nociceptive nerve endings (Yahia, Newman, & St-Georges, 1988); therefore damage to these restraints is likely to cause low back pain.
Lumbar Intertransverse Ligaments
The general characteristics of the intertransverse ligaments are described in Chapter 5. Some authors describe the lumbar intertransverse ligaments as being thin membranous bands that connect two adjacent TPs (Behrsin & Briggs, 1988). Others consider the intertransverse ligaments to be rather discrete and well-defined bands; still other authors consider them to consist of two lamellae (Behrsin & Briggs, 1988). The latter view appears to be gaining acceptance (Bogduk, 2005).
The posterior lamella of the intertransverse ligament passes medially to the posterior aspect of the Z joint. It is pierced by the posterior primary division and continues medially to help reinforce the Z joint capsule from behind. Laterally the membranous intertransverse ligament also has a posterior layer. The posterior layer of the lateral aspect of the intertransverse ligament becomes continuous with the aponeurosis of the transversus abdominis muscle and then becomes continuous with the middle layer of the thoracolumbar fascia (Bogduk, 2005).
The anterior lamella of the intertransverse ligament passes medially to form a layer of fascia over the IVF, where it is pierced by the APD and the spinal branches of lumbar segmental arteries and veins. The anterior lamella then continues anteriorly and medially to become continuous with the ALL. The accessory (transforaminal) ligaments that span the IVF (see Chapter 2 and previous discussion on corporotransverse ligament under L5 NRC) are probably condensations of the anterior lamella of the intertransverse ligament (Bogduk, 2005). It should be recalled that laterally the membranous intertransverse ligament also has anterior and posterior layers. The anterior layer becomes continuous with the anterior layer of the thoracolumbar fascia and covers the anterior aspect of the quadratus lumborum muscle.
A V-shaped groove (with the apex of the V facing laterally) is formed by the medially located anterior and posterior lamellae of the intertransverse ligament. The region between the two lamellae is filled with a small amount of adipose tissue that is continuous with the adipose tissue within the Z joint. This V-shaped region is known as the superior articular recess (see Zygapophysial Joints). It aids the Z joint by allowing for its adipose contents to be displaced during extension of the spine (Bogduk, 2005).
Iliolumbar Ligaments
The left and right iliolumbar ligaments (ILLs) course from the left and right TPs of L5 (and rarely L4) to the sacrum and iliac crest of the same side. Each is composed of as many as five parts (Bogduk, 2005). The most prominent part consists of an inferior and superior band (Olsewski et al., 1991; Standring et al., 2008). The inferior band is present 97% of the time and is also known as the lumbosacral ligament (LSL). The LSL extends from the inferior aspect of the L5 TP and the body of L5 to the anterosuperior aspect of the sacral ala, where it blends with the ventral sacroiliac ligament (Fig. 7-23). The LSL extends from the TP and body of L5 to the sacral promontory at least 3% of the time (Olsewski et al., 1991).
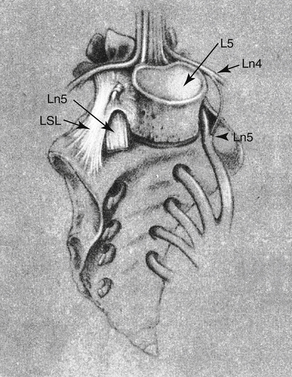
FIG. 7-23 The lumbosacral ligament (LSL). Notice the relationship between the LSL and the ventral ramus of L5 (Ln5). (From Olsewski JM et al. [1991]. Evidence from cadavers suggestive of entrapment of fifth lumbar spinal nerves by lumbosacral ligaments. Spine, 16, 336-347.)
The superior band of the ILL courses farther laterally than the LSL and attaches to the iliac tuberosity in front of the superior aspect of the sacroiliac joint. This band is continuous with the attachment of the quadratus lumborum muscle to the TP of L5, and usually consists of two distinct bands, anterior and posterior (Fig. 7-24), although one of the bands can be absent in some individuals. The anterior band is flat and fan-shaped, narrow medially and wide laterally. Its dimensions average 30 to 40 mm long, 8 to 10 mm wide, and 2 to 3 mm thick. The medial attachment of the anterior band is to the anterior, inferior, and lateral aspect of the TP of L5. From here the anterior band passes laterally, within the horizontal plane, to reach its lateral attachment to the medial aspect of the ilium, inferior to the iliac crest (specifically, to the upper and anterior-most aspect of the iliac tuberosity). The posterior band is more rod-shaped (Fig. 7-24). The medial attachment of this band is to the lateral tip of the L5 TP. From here the posterior band courses laterally, posteriorly (at a 45- to 55-degree angle to a horizontal line passing through the TP of L5), and superiorly, reaching its lateral attachment to the superior and anterior aspects of the iliac tuberosity, just above and behind the anterior band (Hanson & Sonesson, 1994; Basadonna, Gasparini, & Rucco, 1996; Rucco, Basadonna, & Gasparini, 1996). This is the band that is most commonly seen during typical dissections of the lower lumbar region using the standard posterior approach. When L5 is positioned more inferiorly in the pelvis, the anterior and posterior bands of the ILLs pass more obliquely superiorly, and when L5 is more superiorly positioned with respect to the pelvis, the anterior and posterior bands are shorter and have a more horizontal orientation (Rucco, Basadonna, & Gasparini, 1996). Other, less significant, portions of the ILL include inferior (not the LSL) and vertical parts. These are both said to originate from the TP of L5 and attach to the ilium (Bogduk, 2005); however, not all authors agree about their existence (Hanson & Sonesson, 1994).

FIG. 7-24 The iliolumbar ligament (ILL). A, Posterior view of a dissection showing the posterior band of the superior component of this ligament. S, Superior; I, inferior; L, left; R, right. B, Illustration (superior view) demonstrating the anterior and posterior bands of the superior component of the ILL. (B, Based on Rucco V, Basadonna PT, & Gasparini D. [1996] Anatomy of the iliolumbar ligament: a review of its anatomy and a magnetic resonance study. Am J Phys Med Rehabil, 75, 451-455.)
Previously the ILL was thought to develop as a result of metaplasia of the epimysium (outer covering) of the inferior fibers of the quadratus lumborum muscle. However, Uhthoff (1993) found this ligament to be well developed in 12 of 12 fetuses beyond 11.5 weeks of gestational age. Further, he found that the direction of the collagen fibers was 90 degrees to the muscle fibers of the quadratus lumborum muscle. He concluded that the ILL develops in a fashion similar to most of the other ligaments of the body and is not formed by metaplasia of the inferior fibers of the quadratus lumborum muscle. The findings of Hanson and Sonesson (1994), who fully dissected the ILLs in 100 spines, 50 of them in cadavers between birth and 10 years of age, confirm the results of Uhthoff (1993).
The ILLs probably function to stabilize the L5-S1 junction, helping to maintain the proper relationship of L5 on S1 (Olsewski et al., 1991), and also to prevent disc degeneration at this level (Aihara et al., 2002). Shorter posterior bands of the superior ILL, as measured in cadavers, are associated with less IVD degeneration at the L5-S1 level and greater disc degeneration at the L4-5 level. The greater degeneration at the L4-5 level is presumably the result of increased loads being transmitted there, secondary to decreased motion at the L5-S1 motion segment in specimens with a short posterior band of the superior ILL (Aihara et al., 2002). In addition to stabilizing the L5 vertebra on the first sacral segment, the ILL limits flexion (L5 motion increases 77.5% in flexion when ILL is experimentally cut), extension (20.4% increase when ILL is cut), lateral flexion (141.7% increase in contralateral lateral flexion when ILL is cut), and axial rotation of L5 on S1 (surprisingly, L5 motion increases only 5.3% in unilateral axial rotation when the ILL is experimentally cut) (Basadonna, Gasparini, & Rucco, 1996; Sims & Moorman, 1996).
The ILL is innervated by posterior primary divisions of the neighboring spinal nerves and consequently may be a primary source of back pain. Therefore sprain of the ILL can be painful, and enthesopathy of the posterior band of the superior ILL can develop. Enthesopathy refers to inflammation at the attachment site of a ligament to bone that frequently results in calcification of the ligament at the site of attachment (in this case, the ilium). The narrower attachment sites of the posterior aspect of this ligament may explain the development of such enthesopathy. Such a narrow attachment causes the loads to be more focused, placing more stress on the ligamentous attachment site (Basadonna, Gasparini, & Rucco, 1996; Sims & Moorman, 1996).
Lumbar Intervertebral Discs
Because of their tremendous clinical significance, much has been written in this chapter about the lumbar IVDs. In addition, Chapter 2 describes the composition of the IVDs and much of the clinical significance associated with their unique morphology, Chapter 11 discusses disc bulge and protrusion and their biologic consequences, and Chapter 14 discusses the microscopic anatomy of the IVDs and IVD aging and degeneration. This chapter focuses on the unique characteristics of the lumbar IVDs with an emphasis on their clinical importance.
In general, IVDs of the lumbar region are the thickest (tallest) of the spine. As discussed in Chapter 2, they consist of a central nucleus pulposus, an outer anulus fibrosus composed of 15 to 25 lamellae (Marchand & Ahmed, 1990; Twomey & Taylor, 1990), and the vertebral (cartilaginous) end plates that line the superior and inferior IVD borders. The thickness of the lamellae of the anulus fibrosus of lumbar IVDs varies considerably from one lamella to the next and also varies within the same lamella. In addition, the thickness of the lamellae also increases with age. Often lamellae are incomplete and do not completely encircle the region of the anulus fibrosus. Consequently, some lamellae are ending and some are beginning in any given 20-degree sector of the anulus fibrosus (Marchand & Ahmed, 1990).
The function of the lumbar IVDs is similar to the function of the IVDs throughout the spine. That is, they function with the vertebral bodies to absorb loads placed on the spine from above (axial loading) and also allow for some motion to occur (Hutton, 1990) while restricting too much motion. The lumbar discs become shorter during the day because they carry the load of the torso. They usually regain their shape within 5 hours of sleep. During active hours the discs require movement to maintain proper hydration. Decreased movement and decreased axial loading have been strongly associated with disc degeneration (Twomey & Taylor, 1990).
The IVDs do not always become narrower from superior to inferior with age (Twomey & Taylor, 1990). Their central region can become more convex with age and push into the central region of the adjacent vertebral bodies. The central aspects of the vertebral bodies lose transverse trabeculae with age and become somewhat shorter from superior to inferior. The peripheral margins of the bodies lose much less height, causing the bony end plates to become concave.
The lumbar IVDs are normally thicker (taller) anteriorly than posteriorly. This helps in the formation of the lumbar lordosis. Liyang and colleagues (1989) found that the shapes of the lumbar IVDs change significantly during flexion and extension of the lumbar region. Flexion was found to narrow the anterior aspect of the disc by approximately 1 to 5 mm and to increase the height of the posterior aspect of the disc by between approximately 1.5 and 3 mm. Consequently, lumbar flexion significantly increases tension on the posterior fibers of the anulus fibrosus (Hedman & Fernie, 1997). In addition, the nucleus pulposus tends to move posteriorly during flexion and anteriorly during extension (Fennell, Jones, & Hukins, 1996). Interestingly, IVDs with greater height and a smaller area (i.e., tall IVDs with small vertebral bodies) are the most susceptible to protrusion (Natarajan & Andersson, 1999).
Rather than considering IVD injury as the result of a single event, McGill (1997) and others (Yang et al., 1988; Gordon et al., 1991) describe lumbar IVD injury as usually occurring over a long period of time as a consequence of cumulative trauma (or “loading”) with the spine in a forward flexed position. Disc protrusion has been found to be rare when the spine is loaded in axial compression in the neutral position; under these circumstances the bony end plates of the vertebral bodies usually fracture before injury to the IVD can occur (McGill, 1997).
The IVDs usually are protected from anterior displacement, or shear stress, by the Z joints and the lumbar extensor muscles (Hutton, 1990). However, fracture of the pars interarticularis allows anterior displacement of the IVDs to occur.
Pain Originating from the Intervertebral Disc
Because each lumbar disc is in direct contact with two or three pairs of dorsal roots (Taylor, 1990), bulging, or protrusion, of the IVD is considered by most authors to be a major cause of radicular pain (Fig. 7-25). However, clinicians should keep in mind that each IVD is innervated by sensory nerve endings and, as a result, can be a primary source of back pain. The IVD receives both nociceptive and proprioceptive fibers (McCarthy, 1993) (see Chapter 2). The nociceptive fibers are particularly sensitive to inflammatory changes in the IVD (Ozawa et al., 2006). The posterior aspect of the disc receives innervation from the recurrent meningeal nerve (sinuvertebral nerve), and the lateral and anterior aspect of the disc is supplied by branches of the gray communicating rami of the lumbar sympathetic trunk (Edgar & Ghadially, 1976; Bogduk, Tynan, & Wilson, 1981).
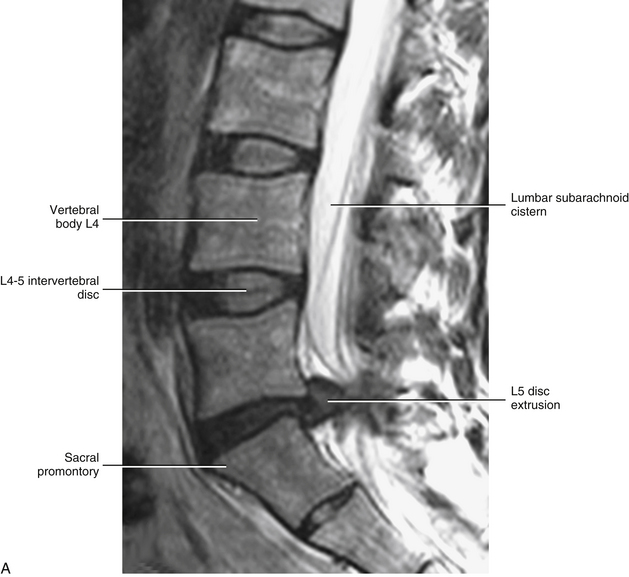
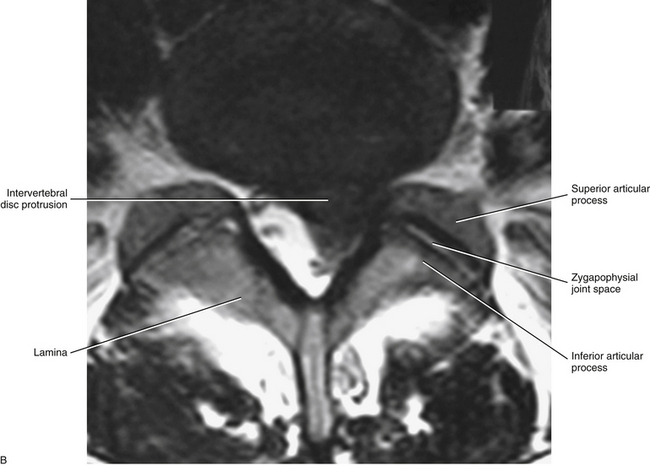
FIG. 7-25 Magnetic resonance imaging scans demonstrating a protrusion of the L5-S1 intervertebral disc. A, Sagittal view. B, Horizontal view. (Images courtesy Dr. Dennis Skogsbergh.)
Nociceptive fibers from that part of the IVD innervated by branches of the gray communicating rami (left and right at each level) probably course through the gray rami to the anterior primary divisions and then enter the dorsal horn of the spinal cord in a fashion similar to other nociceptive fibers of the somatic nervous system (Bogduk, 1983). However, some fibers innervating the anterior and lateral aspects of the discs, and to a lesser extent even the posterior aspect of the IVDs (Ohtori et al., 1999), have been found in animal models (rats) to ascend several segments in the sympathetic chain before passing through a gray communicating ramus, anterior primary division, and dorsal root and ganglion to enter the dorsal horn of the spinal cord several segments higher than the level of innervation. This is thought to explain the broad referral patterns (e.g., pain referred into the inguinal region from IVD pathology as low as L5-S1) seen in various presentations of low back pain of primary discal origin (Morinaga et al., 1996; Ohtori et al., 1999, 2001). For example, Yukawa and colleagues (1997) have reported that approximately 4.1% of patients with protrusion of the IVD at L4-5 or L5-S1 experience groin pain. (Chapters 9 and 11 discuss the central connections of fibers conducting nociception.) Bogduk (1990) has described a series of events that explain the mechanisms by which the IVD can be a primary source of pain without IVD herniation. He states that there are two mechanisms by which the disc causes pain without herniation: torsional injuries to the disc and compression of the IVD.
Torsional Injuries to the Lumbar Intervertebral Discs
Torsional injury to the IVD refers to a sprain of the outer layers of the anulus fibrosus after excessive axial rotation. Normally, tearing of the anulus does not occur because the collagen fibers of the 15 to 25 anular lamellae of a lumbar IVD are able to withstand more than 3 degrees of axial rotation without being stretched beyond their capacity. In fact, 22.6 degrees of unilateral axial rotation are needed to cause failure of the anular fibers. This amount of segmental rotation of a single lumbar motion segment only can occur under experimental conditions after complete removal of the Z joints (facetectomy) in unembalmed cadaveric spines (Farfan, 1973). Therefore rotation of the lumbar spine does not normally cause damage to the IVD (Hutton, 1990), because axial rotation between two adjacent segments is primarily limited by the Z joints and usually does not exceed 3 degrees (see Ranges of Motion in the Lumbar Spine). However, flexion significantly increases tension on the posterior aspect of the anulus fibrosus (Hedman & Fernie, 1997), and if flexion is added to axial rotation, the collagen fibers of the anulus fibrosus (AF) can be stretched beyond their limits, resulting in circumferential tears of the anulus (Fig. 7-26). Because of the nociceptive innervation of the outer third of the IVD, these tears can result in pain of discal origin. However, even though these injuries have been produced experimentally, have been identified in cadavers, and match the signs and symptoms expressed by many patients who have back pain after injuries involving rotation combined with flexion, no definitive studies irrevocably link isolated circumferential tears of the IVD with low back pain (Bogduk, 1990).
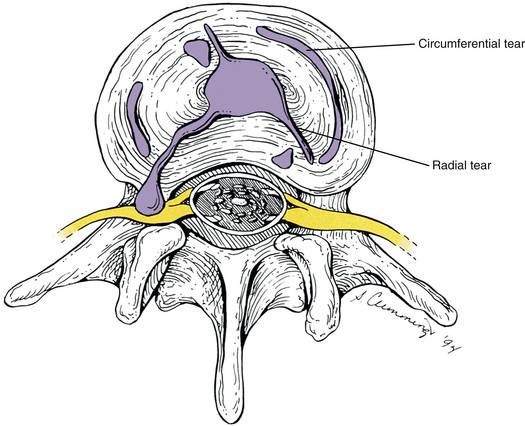
FIG. 7-26 Circumferential and radial tears of the intervertebral disc. Notice that one of the radial tears is allowing the nucleus pulposus to extrude posterolaterally on the left side.
Combined motions (e.g., combined lateral flexion and rotation or combined flexion and rotation) and asymmetric lifting (e.g., lifting an object from a laterally flexed position) increase the stresses on the AF (Natarajan et al., 2008), particularly the posterolateral aspect of the AF (Schmidt et al., 2007). If several episodes of excessive loading of the disc during flexion and axial rotation occur, the result may be circumferential tears of several lamellae of the anulus fibrosus. If enough anular lamellae tear in this way, the anulus fibrosus may be weakened to the point that the nucleus pulposus may be allowed to tear a path created by the circumferential tears of weakened adjacent anular lamellae. Such a path courses from the centrally located nucleus pulposus to the periphery through successive layers of the anulus fibrosus, and is known as a radial tear (see Fig. 7-26). A radial tear can result in protrusion and extrusion of nuclear contents into the vertebral canal. Once in the canal, entrapment or stretching of the neural elements can occur. As discussed, a protruding or extruding disc can affect the neural elements as they course within the vertebral canal, pass through the IVF, or both.
MRI is gaining wide acceptance in the evaluation of disc protrusion. However, a significant number of false-positive findings have been found with MRI. Therefore close correlation of MRI findings with other clinical findings is essential before a diagnosis of disc protrusion can be made with certainty (Boden et al., 1990).
Compression Injuries to the Intervertebral Discs and Internal Disc Disruption
The second type of injury that can result in pain originating from the IVD itself occurs from excessive compression of the IVD. Compression injuries can result in pain of discal origin by two mechanisms, chemical and mechanical (Bogduk, 1990). Bogduk (1990) has described a series of events that may occur after excessive compression of a lumbar IVD. These events are summarized in Box 7-3. Note that fracture of the cartilaginous and bony end plate is a key feature in this sequence of events. During compressive loading of the spine (forces being placed on the spine from directly above), the vertebral (cartilaginous) and bony end plates have been shown to fracture before tearing of the anulus fibrosus or protrusion of the nucleus pulposus occurs. Such fractures may heal completely and remain unnoticed. However, a dramatic repair response may occur if the fracture extends into the cancellous bone of the vertebral body and the IVD comes in contact with the vascular supply of the vertebral body. This response is characterized by the nucleus pulposus being treated as if it were foreign to the body. The result has been described as an autoimmune type of response (Bogduk, 1990). This response leads to destruction of the proteoglycan aggregates and proteoglycan monomers that compose the disc. The result of this destruction is a condition known as internal disc disruption (Kirkaldy-Willis et al., 1978).
Internal disc disruption can result in spinal stenosis (including IVF stenosis), protrusion of the nucleus pulposus, or both. The processes by which these two entities develop are summarized in Boxes 7-4 and 7-5.
Internal disc disruption also can result in resorption, or loss, of IVD material. The loss of discal material, over time, may make extrusion of the nucleus pulposus less likely. Kirkaldy-Willis and colleagues (1978) state that discography has shown a marked correlation between loss of disc height, the presence of traction spurs (osteophytes), and disruption of the disc. Therefore even if patients escape nuclear bulge or extrusion (see Box 7-5), they remain vulnerable to lateral recess stenosis (see Box 7-4).
In addition, as disruption continues toward the outer layers of the anulus fibrosus, pain of purely discal origin also can result. Bogduk (1990) has reviewed two mechanisms by which this can occur. These mechanisms are summarized in Boxes 7-6 and 7-7. The first mechanism that produces pain of discal origin is chemical in nature. Progression of the inflammatory process of internal disc disruption results in the direct stimulation of nociceptors in the outer third of the anulus fibrosus. The second process causes pain from the anulus as a result of its decreased ability to handle mechanical stress adequately. As the process of disruption progresses, the inner lamellae of the anulus deteriorate, causing the outer layers to absorb all the loads (including torsional loads) placed on the disc. The anulus is rendered more susceptible to circumferential tears, which are likely to produce pain. Therefore this type of pain, secondary to internal disc disruption, is the result of mechanical forces. The two mechanisms just described (pain of chemical and mechanical origin) can occur simultaneously (Bogduk, 1990).
Finally, Box 7-8 summarizes the clinical findings in a patient with early internal disc disruption. The neurologic findings are negative because the disc remains contained, especially in the early stages. Pain from internal disc disruption has characteristics similar to those of other somatic causes of low back pain (e.g., Z joint pathology), making the diagnosis more challenging. If the pain is prolonged or becomes severe, injection of radiopaque dye into the disc followed by CT (CT discography) usually shows disruption into the anulus fibrosus when internal disc disruption is present. In addition, CT discography reproduces the patient’s symptoms. Extrusion of dye into the anulus combined with provocation of the patient’s symptoms confirms the condition of internal disc disruption (Schwarzer et al., 1995). Additionally, a disc affected by internal disc disruption may be identified as an area of reduced signal intensity when viewed on sagittal MRI scans.
Intervertebral Disc Degeneration and Its Consequences
Decreased disc height and osteophyte formation are the best indicators of IVD degeneration on x-ray. A low signal in the IVD on MRI is also an indication of disc degeneration (Marchiori et al., 1994). It should be recalled that the IVD and the two Z joints between two adjacent vertebrae make up a three-joint complex. Pathologic conditions or dysfunction of one component can adversely affect the others (Kirkaldy-Willis et al., 1978; Haher et al., 1992) (see Fig. 7-6). As disc degeneration increases, segmental motion, particularly in axial rotation, also increases; however, in the final stages of degeneration, intersegmental motion decreases (Fujiwara et al., 2000; Krismer et al., 2000). The increased motion during the initial stages of IVD degeneration results in decreased IVD stiffness; however, during the later stages of IVD degeneration, stiffness increases as intersegmental motion decreases (Brown, Holmes, & Heiner, 2002). In addition, loss of disc height as a result of disc degeneration, protrusion, internal disc disruption, resorption of the disc, chemonucleolysis, and discectomy may lead to added loads being placed on the Z joint capsules and the articular processes, resulting in degenerative changes of the articular processes. Disc deterioration at one level also can lead to increased strain and possible degeneration of the discs immediately above or below the level of primary involvement (Kirkaldy-Willis et al., 1978).
Loss of disc height also can result in subluxation of the Z joints and upward and forward displacement of the superior articular processes (Kirkaldy-Willis et al., 1978). This in turn results in narrowing of the lateral recesses of the vertebral canal. Narrowing of the lateral recess may result in entrapment of the exiting nerve roots as they proceed to the medial aspect of the IVF proper. Therefore loss of disc height from any cause can result in abnormal joint position and abnormal motion. Such abnormal joint position and abnormal motion of both the interbody joint and the Z joints of two adjacent vertebrae has been termed instability (Dupuis et al., 1985). Instability, in turn, can lead to repeated entrapment of the spinal nerve exiting between the two adjacent segments.
Ranges of Motion in the Lumbar Spine
Several factors help to limit specific movements of the lumbar region. These include the unique configuration of the lumbar articular facets and the restraints of the Z joint capsules, ligaments of the lumbar region, deep back muscles, and lumbar IVDs. For example, flexion of the lumbar region is primarily limited by the Z joint capsules (Hutton, 1990) and the articular processes themselves (Taylor & Twomey, 1986). However, the ligamenta flava, IVDs, and interspinous and supraspinous ligaments also help to limit flexion. Hutton (1990) found that the interspinous and supraspinous ligaments (supraspinous restraints) were the first to tear during hyperflexion of the lumbar region.
A few items about lumbar flexion are worth noting. First, all of the lumbar vertebrae do not move simultaneously during flexion. In fact, lumbar flexion occurs in a stepwise fashion, beginning at L1-L2 and continuing through L5-S1 (Kanayama et al., 1996), with the upper lumbar segments contributing more than the lower segments during typical bending (different from total possible motion at each segmental level), excluding L5-S1 (Kozanek et al., 2009). Second, advancing age also can affect lumbar flexion. That is, lumbar flexion decreases with age, with the most marked change beginning in the sixth decade of life (McGregor, McCarthy, & Hughes, 1995).
Extension is limited by the ALL, the Z joint capsular ligaments (Dupuis et al., 1985), and the bony “stop” of the inferior articular processes coming against the pars interarticularis of the subjacent vertebra. Again, the upper lumbar segments contribute more than the lower lumbar segments during typical extension (different from total possible motion at each segmental level), excluding L5-S1 (Kozanek et al., 2009).
The tightly interlocking facets of this region dramatically limit axial rotation. However, the reciprocal concave and convex surfaces of the respective superior and inferior articular processes do allow for a very small amount of axial rotation to occur, enabling rotation of the lumbar segments to occur during normal locomotion (Rice et al., 2004). Gapping of the Z joint occurs on the same side of vertebral body rotation (i.e., gapping of right Z joint with right rotation) (Boszczyk et al., 2001; Cramer et al., 2002). Axial rotation is finally stopped by the impact of articular processes that comprise the Z joint of the side opposite that to which the vertebral body is rotating. This limitation usually occurs at 1 to 2 degrees of axial rotation between adjacent vertebrae from L1 to L4 (Hutton, 1990). The lower lumbar segments contribute more to rotation than the upper segments (Kozanek et al., 2009) and the L5-S1 segment attains more axial rotation than the other lumbar segments (see values at the end of this section).
The lower lumbar segments contribute more to lateral flexion than the upper segments (Kozanek et al., 2009). Lateral flexion in the lumbar region is limited primarily by the contralateral intertransverse ligaments. The contralateral capsular ligaments and ligamenta flava are also important in the limitation of lateral flexion. In addition, the configuration of the articular processes helps to limit this motion (Dupuis et al., 1985). Lateral flexion in the lumbar region usually is coupled with axial rotation such that left lateral flexion results in right rotation of the vertebral bodies (left rotation of the spinous processes), and vice versa (i.e., right lateral flexion is coupled with left rotation of the vertebral bodies). Probably this is caused by the sagittal orientation of the lumbar Z joints, combined with the effect of the relatively strong lumbar interspinous and supraspinous restraints. The latter restraints tend to hold the spinous processes together during lateral flexion. Abnormally loose posterior stabilizers (e.g., interspinous ligaments, supraspinous restraints, deep back muscles, Z joints) can result in abnormal coupling during lateral flexion so that left lateral flexion is coupled with left rotation of the vertebral body and right rotation of the spinous process (the opposite of the normal coupling pattern). This abnormal coupling pattern, which can be detected on standard x-ray films, is an indication of lumbar instability (Dupuis et al., 1985).
Lumbar instability (abnormally high intersegmental motion) may result in low back pain if abnormal stress is placed on the unstable segments. A patient with lumbar instability may have centralized low back pain without leg pain or central and lateral low back pain combined with radiation into the buttock and thigh. Some patients with lumbar instability have signs of nerve root entrapment. Differentiation between pain caused by lumbar instability and that caused by primary IVD or Z joint pathology may be challenging, because the latter structures may be receiving increased mechanical loading and thus may be generating pain themselves. Examination of dynamic x-ray films taken in flexion, extension, and lateral flexion that demonstrate increased motion have been found to aid in this differentiation (Dupuis et al., 1985).
Table 7-11 lists the total ranges of motion for the lumbar region as reported by several different authors.
Table 7-11
Total Ranges of Motion for the Lumbar Region
| Direction | Motions and Ranges (in Degrees) Reported in Literature |
| Flexion L4-5 segment |
60 39-55 (average: 45.95 ± 4.28,∗ 52 ± 18)† 14.5-19.0 (average: 15.95 ± 1.38)∗ |
| Extension | 20 16 ± 10† |
| Lateral flexion‡ | 25-30 |
| Axial rotation‡ | 10-15 5† |
∗Data from Liyang D et al. (1989). The effect of flexion-extension motion of the lumbar spine on the capacity of the spinal canal, an experimental study. Spine, 14, 523-525.
†Data from Bogduk N. (2005). Clinical anatomy of the lumbar spine (4th ed.). London: Churchill Livingstone.
The following is a list of ranges of motion (in degrees) for each lumbar segmental level (White & Panjabi, 1990).
Plamondon and colleagues (1988) used stereometry (method of measurement using sets of x-ray films taken at 90 degrees to one another) to determine the motion of individual lumbar vertebrae. The following list represents the amount of motion they found, per segment, for the L1 to L4 vertebrae:
It should be recalled that the L5-S1 articulation is the most movable segment in flexion, extension, and axial rotation in this region. The following is a list of the ranges of motion at this level (White & Panjabi, 1990):
However, some texts present data that show less motion at the L5-S1 region (Bogduk, 2005).
Soft Tissues of the Lumbar Region: Nerves and Vessels
The muscles associated with the lumbar region are discussed in Chapter 4. This includes a discussion of the diaphragm and the muscles of the anterior and posterior abdominal walls, including the abdominal obliques, transversus abdominis, rectus abdominis, quadratus lumborum, and psoas major and minor muscles. Consequently, this section on soft tissue structures of the lumbar region focuses on vessels and nerves related to the lumbar spine.
Nerves of the Lumbar Region
The innervation of the lumbar portion of the vertebral column and the soft-tissue structures of the lumbar region is a topic of supreme clinical importance. Having knowledge of the innervation of the spine gives the clinician a better understanding of the source of the patient’s pain. Perhaps Bogduk (1983) stated it best, “The distribution of the intrinsic nerves of the lumbar vertebral column systematically identifies those structures that are potential sources of primary low back pain.”
Because the basic neural elements associated with the spine are covered in Chapters 2, 3, 5, and 6, this chapter concentrates on those aspects of innervation unique to the lumbar region. However, many key features of the basic neural elements also are included here to maintain continuity and minimize the need to refer to previous chapters.
The cauda equina and exiting roots and spinal nerves have been discussed (see Lumbar Vertebral Foramen and Vertebral Canal and Lumbar Intervertebral Foramina and Nerve Root Canals, respectively). This section briefly covers the dorsal and ventral roots and the spinal nerve. It concentrates on the neural elements once they have left the confines of the IVF. Because the vast majority of spinal structures are innervated by either the recurrent meningeal nerves or the posterior primary divisions (PPDs), these nerves and the structures they innervate are covered in more detail. This is followed by a discussion of the anterior primary divisions (APDs) and the lumbar plexus. It should be recalled that the lateral and anterior aspects of the IVDs and the ALLs are innervated by direct branches of the lumbar sympathetic trunk and also by branches from the lumbar gray rami communicantes. The specific innervation of the IVD has been discussed in greater detail earlier (see Pain Originating from the Intervertebral Disc).
General Considerations
Three types of nerve endings have been found in almost all the innervated structures of the lumbar vertebral column: free nerve endings, other nonencapsulated endings, and encapsulated endings. This seems to indicate that most innervated structures of the spine are sensitive to pain, pressure, and proprioception (Jackson et al., 1966).
Of particular interest, and sometimes of particular frustration to clinicians and researchers alike, is that innervation overlaps throughout the spine. This has been particularly well documented in the lumbar region. Most spinal structures seem to be innervated by nerves from at least two adjacent vertebral levels. This led Edgar and Ghadially (1976) to state, “The poor localization of much low back pain and its tendency to radiate may be related to this neurological pattern.” This can at times make the task of identifying the cause of low back pain particularly challenging.
Dorsal and Ventral Roots and Spinal Nerves
The dorsal and ventral roots of the lumbar spine travel inferiorly as the cauda equina. They then course through the NRC before exiting the IVF (see previous material). The nerve roots can be irritated by many structures and pathologic processes (see previous discussions and Chapter 11). These include disc protrusion or other space-occupying lesions, structural lesions of the vertebral canal, chemical irritation, and intrinsic radiculitis (Bogduk, 1976). The dorsal and ventral roots unite to form a spinal nerve before exiting the IVF. Each lumber spinal nerve emerges from a lumbar IVF and immediately divides into an APD (ventral ramus) and PPD (dorsal ramus).
Dorsal Root Ganglia
Each dorsal root has a marked enlargement, known as the dorsal root (spinal) ganglion (DRG, both singular and plural), that is located immediately proximal to the union of the dorsal and ventral roots to form the spinal nerve. DRG increase in diameter from L1 to S1. Because the dural root sleeve of S1 is relatively short, the S1 DRG is frequently (77.3% to 79%) within the superior aspect of the sacral canal, rather than within the first sacral IVF. However, the locations of the lumbar and sacral DRG are variable, and can even be asymmetric from left to right. The positions of the DRG are clinically relevant. Kikuchi and colleagues (1994) studied the locations of the L4, L5, and S1 DRG, and Hasegawa and colleagues (1996) studied the location of the L1-5 DRG. They categorized the ganglia as being intraspinal (within the vertebral canal), intraforaminal (within the IVF), or extraforaminal (lateral to the IVF). The following is a breakdown of the percentage of DRG found in each category (the values of Hasegawa and colleagues [1996] are in parentheses):
Intraspinal: L4, 9.3%; L5, 19.2%; S1, 77.3%
Intraforaminal: (L1, 92%; L2, 98%; L3, 100%) L4, 86.1% (100%); L5, 72.8% (95%); S1, 22.7%
In addition, Hamanishi and Tanaka (1993) found values similar to those of Kikuchi and colleagues (1994) (i.e., more intraforaminal and extraforaminal DRG).
When a lumbar DRG is located within the IVF, it generally occupies 23% to 30% of the area of the IVF within a parasagittal plane passing through the DRG. The more superior DRG (L1 and L2) have a tendency to be more laterally placed within the IVF, or are located lateral to their IVF altogether (extraforaminal location), whereas the more inferior DRG (L5 and S1) generally are located in the medial aspect of the IVF, or can be within the vertebral (spinal) canal (intraspinal location), or in the case of S1 within the superior-most aspect of the sacral canal. Intraspinal L5 or S1 DRG have an increased incidence of DRG compression with associated radiculopathy. The DRG compressions in these instances usually are secondary to IVD bulging, Z joint articular facet arthropathy, or a combination of both conditions (Hasegawa et al., 1996). Compression of intraspinal DRG (indentation seen at postmortem examination) has been found to be more frequent with advancing age (Kikuchi et al., 1994).
Recurrent Meningeal (Sinuvertebral) Nerves
The recurrent meningeal nerves (RMNs) at each level innervate many structures located within the IVF and the vertebral canal. Because they have been found to carry fibers that conduct nociception (pain), structures innervated by RMNs are considered capable of producing back pain. However, in addition to nociceptive input, the RMNs also probably carry thermal sensation and proprioception (Edgar & Ghadially, 1976). Even though the RMNs are discussed in Chapters 5 and 6, they are included here because of their clinical importance.
The RMNs are found at each IVF of the vertebral column. They each originate from the most proximal portion of the APD just distal to the IVF that they eventually reenter. They receive a branch from the closest gray communicating ramus and then enter the anterior aspect of the IVF close to the pedicle that forms the roof of this opening. Usually, more than one RMN enters each IVF, and up to six have been found at one level. Consequently, compression of the RMNs within the confines of the IVF may be a cause of back pain (Edgar & Ghadially, 1976).
The RMNs ramify extensively on entering the IVF. Great variation is associated with their distribution within the vertebral canal (Groen, Baljet, & Drukker, 1990). Usually each gives off a large ascending branch and smaller descending and transverse branches, although the transverse branch is not always present. The ascending branch usually extends superiorly for at least one vertebral level above its level of entrance. The branches of the RMNs anastomose with those of adjacent vertebral segments, including those of the opposite side of the spine (Bogduk, 1976; Edgar & Ghadially, 1976; Groen, Baljet, & Drukker, 1990). They innervate the posterior aspect of the IVD, PLL, periosteum of the posterior aspect of the vertebral bodies, epidural venous plexus, and anterior aspect of the spinal dura mater (see Chapter 11). Therefore all these structures have been implicated as possible sources of back pain. In addition, compression of the RMNs in the vertebral canal may be a component of spinal stenosis (Edgar & Ghadially, 1976). However, because of the great variability in the distribution of the RMNs, the pattern of pain referral as a result of nociceptive input received from them also may be inconsistent.
Less frequently cited possible causes of back pain that receive innervation by the RMNs include venous congestion within the vertebral bodies (intravertebral venous congestion) and varicosities of the epidural veins (Edgar & Ghadially, 1976) and basivertebral veins (Bogduk, 1976). Edgar and Ghadially (1976) stated that, in addition to relieving pressure on nerve roots, decompression of the vertebral canal via laminectomy may reduce back pain by relieving venous congestion in the epidural and intravertebral veins.
Posterior Primary Divisions
Whereas the recurrent meningeal nerves innervate the structures located on the anterior aspect of the vertebral canal, the posterior primary rami innervate those structures of the posterior aspect of the vertebral canal (vertebral arch structures). This difference of innervation may be significant; the RMNs may be responsible for information related to potential or real harm to the neural elements of the vertebral canal, and the PPDs may be responsible for relaying information related to the structural integrity of the spine (Edgar & Ghadially, 1976).
Each PPD of the lumbar region leaves the spinal nerve at the lateral border of the IVF and passes over the TP of the lower vertebra participating in the formation of the IVF (e.g., the L3 nerve passes over the L4 TP). The PPD then passes through a small osteoligamentous canal. This canal, which is unique to the lumbar region, lies between the base of the anterior surface of the superior articular process medially and the posterior lamella of the intertransverse ligament laterally. The nerve then sends a twig to the intertransversarius medialis muscle and continues posteriorly, where it divides into a medial and a lateral branch. The medial branch passes deep to the mamillo-accessory ligament (see Articular Processes) and supplies sensory innervation to the Z joint and then motor innervation to the multifidi. This innervation to the lumbar multifidi has been found to be specific (Bogduk, Wilson, & Tynan, 1982). The medial branch of a PPD innervates those fibers that insert onto the spinous process “of the same segmental number as the nerve” (Bogduk, Wilson, & Tynan, 1982). For example, the medial branch of the L3 PPD innervates the multifidi muscles that insert onto the L3 spinous process. The medial branch then continues further medially to innervate the rotatores and interspinalis muscles and provide sensory innervation to the ligamentum flavum, interspinous ligament, supraspinous “ligament” (restraints), and periosteum of the posterior arch, including the spinous process (Box 7-9 and Fig. 7-27). Along its course, the medial branch anastomoses with medial branches of adjacent levels and sends an inferior branch to the Z joint of the level below and an ascending branch to the level above (Bogduk, 1976; Edgar & Ghadially, 1976). Therefore each Z joint typically is innervated by medial branches of three PPDs (Bogduk et al., 1982; Jeffries, 1988).
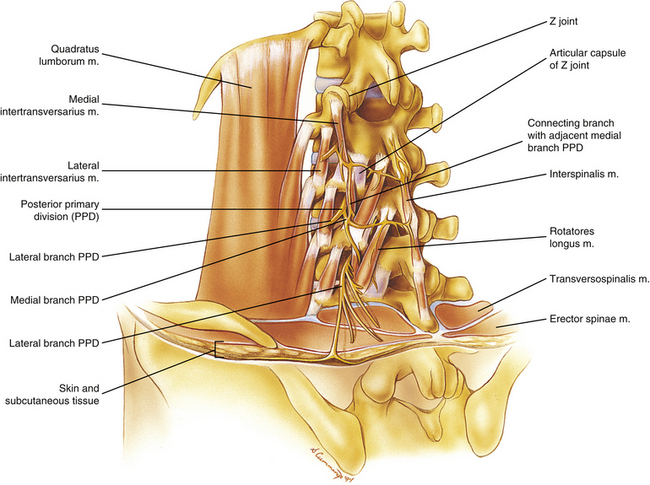
FIG. 7-27 Structures innervated by the posterior primary divisions of typical lumbar spinal nerves. The quadratus lumborum muscle, which is innervated by the anterior primary divisions, also is shown.
The lateral branch of the PPD supplies motor innervation to the erector spinae muscles. Bogduk (1983) found that the lateral branch supplies the iliocostalis lumborum muscle whereas an intermediate branch stems from the lumbar PPDs to supply the longissimus thoracis muscle. The longissimus thoracis muscle in the upper lumbar region is innervated by a dense network of nerve fibers, and touching these nerves with a needle can be a source of pain during a paramedian approach to epidural anesthesia (Saito et al., 2006). After innervating the longissimus thoracis muscle, the intermediate branches form an anastomosis with the intermediate branches of adjacent levels. The PPD of L5 has only two branches: a medial branch with a typical distribution and a more lateral branch that corresponds with the intermediate branches of higher levels because it innervates the longissimus thoracis muscle. Because the muscle fibers of the iliocostalis lumborum do not extend inferiorly to the level of the L5 nerve, the absence of a nerve corresponding to a typical lateral branch of higher levels is understandable (Bogduk, 1983). Neither the medial branches of the PPDs nor any branches of the L4 and L5 PPDs supply the skin of the back.
The (T11) T12, L1, L2, and L3 lateral branches are sometimes known as the superior clunial nerves. They supply sensory innervation to the skin over the upper buttocks. The medial superior cluneal nerve can become entrapped, resulting in pain localized to the iliac crest and radiating into the buttock (Maigne & Doursounian, 1997). The medial superior cluneal (MSC) nerve originates from L1 60% of the time and from L2 40% of the time, and occasionally receives a contributing branch from L3. The MSC courses over the iliac crest approximately 7 cm lateral of the midline. As it rests on the iliac crest, the MSC passes under a bridge of fibrous tissue formed by the thoracolumbar fascia. Occasionally the fibrous tissue wraps too tightly around the nerve at this location, causing constriction and the signs and symptoms of the entrapment neuropathy described previously (see sections, Neurogenic Claudication and Entrapment of the Neural Elements). Superior cluneal nerve injury also has been implicated as a cause of postoperative pain after iliac crest bone harvesting for spine fusion surgery (Maigne & Doursounian, 1997).
Anterior Primary Divisions and the Lumbar Plexus
The APDs, or ventral rami, branch from the spinal nerves at the lateral border of the IVF and immediately enter the psoas major muscle. The ventral rami of the first four lumbar nerves then branch within the substance of the psoas major muscle to form the lumbar plexus. As mentioned in this chapter, the psoas major muscle may provide some protection for the dorsal and ventral roots from traction forces placed on the peripheral nerves of the lumbar plexus (dePeretti et al., 1989).
The lumbar plexus is derived from the ventral rami of only the first four lumbar nerves. The ventral ramus of L5 unites with a branch of the ventral ramus of L4 to form the lumbosacral trunk. The lumbosacral trunk then enters the pelvis to unite with the APDs of the sacral spinal nerves and in doing so helps to form the sacral plexus (see Chapter 8). Frequently the twelfth thoracic (subcostal) nerve also participates in the lumbar plexus.
The branches of the lumbar plexus are listed next, along with the closely related subcostal nerve and lumbosacral trunk.
• Subcostal nerve (T12). The subcostal nerve provides sensory innervation to the region under the umbilicus and also provides motor innervation to the pyramidalis and quadratus lumborum muscles.
• Iliohypogastric nerve (L1, T12, or L2 may contribute; L3 occasionally contributes [Klaasen et al., 2011]). This nerve provides sensory innervation to the gluteal, inguinal, and suprapubic regions. It also provides some motor innervation to the muscles of the anterior abdominal wall.
• Ilioinguinal nerve (L1; T12 frequently contributes [Klaasen et al., 2011]). This nerve provides motor innervation to the muscles of the anterior abdominal wall.
• Genitofemoral nerve (L1 and L2). The femoral branch provides sensory innervation to the region of the femoral triangle of the thigh, and the genital branch provides motor innervation to the dartos and cremaster muscles of the male (no important innervation by this branch in the female).
• Lateral femoral cutaneous nerve (L2 and L3). This nerve provides sensory innervation to the lateral aspect of the thigh.
• Femoral nerve (L2, L3, and L4). The femoral nerve provides motor innervation to the psoas and iliacus muscles before leaving the abdominopelvic cavity posterior to the inguinal ligament. Distal to the inguinal ligament, this nerve innervates the quadratus femoris and pectineus muscles and supplies sensory innervation to the anterior thigh and medial leg. Spratt and colleagues (1996) found that aberrant slips of the psoas major or iliacus muscle pierced the femoral nerve unilaterally in 4 of 68 cadavers. In one case a slip of iliacus muscle originated from the iliolumbar ligament and pierced the femoral nerve before inserting onto the lesser trochanter. Spratt and colleagues (1996) felt that such anomalies could traction the femoral nerve, possibly leading to referred pain to the hip or knee or along the L2-4 dermatomes.
• Obturator nerve (L2, L3, and L4). The obturator nerve provides motor innervation to the adductor muscles of the thigh and supplies sensory innervation to the medial aspect of the thigh.
• Lumbosacral trunk (L4 and L5). The lumbosacral trunk is not officially a part of the lumbar plexus. This nerve passes inferiorly to participate in the sacral plexus. It therefore serves as a connection between the lumbar and sacral plexuses.
Autonomic Nerves of the Lumbar Region
The abdominal and pelvic viscera receive their motor innervation from autonomics derived from both the sympathetic and parasympathetic nervous systems. Sensory nerves originating from the same visceral structures also travel along the sympathetic and parasympathetic nerve fibers. The diffuse nature of the sympathetic and parasympathetic systems is responsible for the equally diffuse nature of the sensory innervation that travels along with them. This is one reason pain from an abdominal or pelvic viscus may “refer” to a region some distance from the affected organ.
Sympathetic innervation of the abdominal viscera is derived from two sources: the thoracic and lumbar splanchnic nerves. The parasympathetics are supplied either by the left and right vagus nerves or by the pelvic splanchnic nerves. The clinical relevance and specific nerves that comprise both the sympathetic and parasympathetic divisions of the autonomic nervous system are discussed in detail in Chapter 10.
Vessels of the Abdomen Related to the Spine
This synopsis of the arteries and veins of the abdomen is included because of the close relationship of the abdominal vessels to the anterior and anterolateral aspects of the lumbar vertebral bodies and lumbar IVDs. This section is by no means complete; it is meant to provide a ready reference for the student and clinician.
Abdominal Aorta and Its Branches
The abdominal aorta receives its name as the continuation of the thoracic aorta, after passing through the aortic hiatus, which is about in the midline at the level of the twelfth lumbar vertebral body. The abdominal aorta continues along the anterior aspect of the lumbar vertebral bodies and IVDs (which are covered anteriorly by the ALL) and subtly deviates to just left of center before bifurcating into the left and right common iliac arteries. Each of these latter arteries supplies the structures of the pelvis (via the internal iliac artery) and lower extremity (via the external iliac artery) of the same side. The bifurcation of the aorta occurs at the level of the vertebral body of L4 67% of the time, with variations extending from L3 to L5 in the remainder of cases (more often higher than lower). No variation in an individual’s age or sex has been found with respect to the location of the aortic bifurcation; however, the frequency of transitional lumbar segments makes for additional variation of the aortic bifurcation. When lumbarization of the first sacral segment is present, the aortic bifurcation occurs at L4 40% of the time, at the L4-5 IVD 33% of the time, and below this level in the remainder of the cases. When sacralization of the L5 vertebra is present, the aortic bifurcation occurs at L3 59% of the time, and below that level in the remainder of the cases (Chithriki, Jaibaji, & Steele, 2002).
Three large, unpaired branches of the aorta—the celiac trunk, the superior mesenteric artery, and the inferior mesenteric artery—exit the anterior aspect of the abdominal aorta and supply the gastrointestinal tract from the stomach to the superior aspect of the rectum. In addition, the celiac trunk supplies the spleen, liver, gallbladder, and a large part of the pancreas. Paired branches of the abdominal aorta also are present throughout its length and are closely related to the posterior abdominal wall. For this reason, they are more relevant to the current discussion (Fig. 7-28). The paired branches of the abdominal aorta are listed and briefly discussed next.
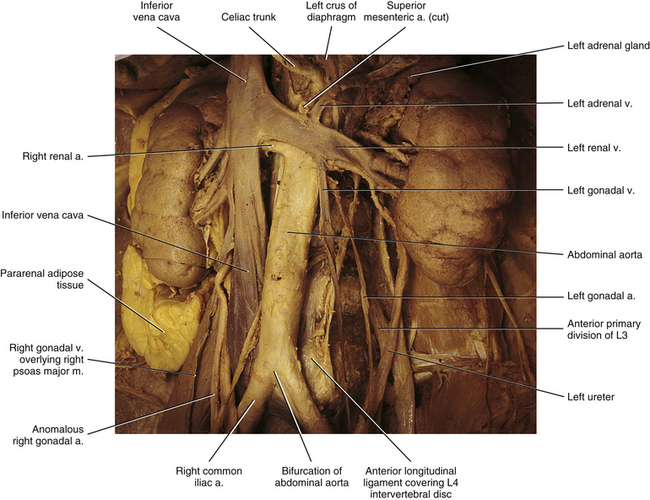
FIG. 7-28 Important vessels of the abdomen related to the lumbar vertebral column. The left ureter also is seen emerging posterior to a branch of the left renal artery coursing to the inferior pole of the left kidney.
• Inferior phrenic artery. The left and right inferior phrenic arteries branch from the aorta as it enters the abdominal cavity through the aortic hiatus. Each courses superiorly to supply the posteroinferior aspect of the diaphragm and, along its way, gives many superior suprarenal (adrenal) arteries.
• Middle suprarenal (adrenal) arteries. These are several paired branches that course directly to the adrenal gland.
• Renal artery. The large left and right renal arteries course behind the renal veins to enter the hilus of the left and right kidneys, respectively. At the hilus, each divides into five branches (four anterior and one posterior) that supply the five renal arterial segments.
• Gonadal artery (testicular artery in males and ovarian artery in females). The left and right gonadal arteries arise from the anterior aspect of the abdominal aorta in a staggered fashion. That is, one of the arteries (usually the left) originates up to several centimeters superior to the other. The gonadal vessels have a long inferolateral course within the abdomen and pelvis. The testicular artery of each side enters the deep inguinal ring on its way to the testes. The left and right ovarian vessels enter the pelvis by crossing the external iliac artery of the same side before supplying the ovary.
• Lumbar arteries and the blood supply to the lumbar region of the spine. These are four (occasionally five) paired segmental arteries that arise from the posterolateral aspect of the aorta. The left and right lumbar arteries (LAs) arise only a few millimeters apart from one another. Each runs laterally along the center of the anterior and lateral aspects of the vertebral bodies, sending branches to the psoas major muscle, the ALL, and the vertebral bodies themselves. They also supply many small branches to retroperitoneal tissues and the posterior peritoneum. In addition, each LA gives off short centrum branches that immediately enter the center of the vertebral body. Each LA also gives off ascending and descending branches that proceed toward the superior and inferior aspects of the vertebral body, respectively. These ascending and descending arteries form branches that enter the vertebral body along the way.
As an LA reaches the intervertebral foramen, it gives off three main sets of branches: (a) branches to the abdominal wall (sometimes called the anterior branches, these arteries pass behind the psoas major muscle, then course along the quadratus lumborum muscle, pierce the transversus abdominis muscle, and course between that muscle and the internal abdominal oblique muscle); (b) spinal canal branches (or “spinal branches” or “spinal rami”); and (c) branches to the posterior spinal elements (sometimes called posterior branches). Each spinal ramus of an LA passes through the IVF and divides into three branches: an anterior branch that courses to the vertebral body, a posterior branch that supplies the posterior arch structures, and a neural branch that courses along the spinal nerve and then divides into anterior and posterior radicular arteries, supplying these neural structures (see Chapters 2 and 3 and Fig. 2-4 for further details). The anterior branch to the vertebral body gives off large branches that enter the center of the posterolateral aspect of the body. Once within the vertebral body these branches send smaller branches that pass either superiorly or inferiorly within the body. Before sending these branches to the vertebral body, the anterior branch divides into an ascending and descending branch. These branches course along the external surface of the vertebral body. The ascending branch crosses the IVD above to unite with the descending branch from the level above. The descending branch passes near the pedicle, supplying it, before anastomosing with the ascending branch from the level below. Both ascending and descending branches send twigs into the posterior aspect of the vertebral body, and also supply the PLL (Crock & Yoshizawa, 1976) (Fig. 2-4, B).
The posterior branch of the spinal ramus courses to the inner surface of the lamina and ligamentum flavum, and then usually continues as a large branch that pierces the base of the spinous process and continues posteriorly within this process until it reaches the posterior tip of the spinous process. Along its course, the posterior branch sends some twigs to the extradural adipose tissue and others to the posterior aspect of the spinal dura mater. A large laminar branch enters the lamina very close to the union of the lamina with the pedicle. Once within the bone an ascending branch and descending branch (the longer of the two) course through the superior and inferior articular processes, respectively, sending smaller branches that reach the subchondral bone of the articular facets (Fig. 2-4). The posterior branches of the LA course posterior to the neural arch. These branches supply the posterior aspect of the arch structures and the transversospinalis and erector spinae muscles (Crock & Yoshizawa, 1976).
Therefore a series of relatively large arteries pierce the center of the vertebral bodies (VBs) along their entire circumference. On entering the VB, these arteries form a dense plexus of arteries within the central horizontal plane of the VB. From this central plexus many small branches ascend and descend to reach the superior and inferior margins of the VBs; these margins are adjacent to the cartilaginous end plates (Crock & Yoshizawa, 1976) (Fig. 2-4).
• Median (middle) sacral artery. This is a small, unpaired artery that arises from the posterior aspect of the abdominal aorta just before its bifurcation into right and left common iliac arteries. The median sacral artery passes inferiorly along the midline of the anterior sacrum. Although the median sacral artery is almost always present, it is rather variable with respect to its location, typically lying more than 5 mm to the left or right of the midline of the sacrum (Tribus & Belanger, 2001).
Interestingly, a much higher incidence of blocked lumbar segmental arteries and blockage of the middle sacral artery has been found in subjects with low back pain than in age-matched controls (Kauppila & Tallroth, 1993).
Veins of the Abdomen
The two large veins of the abdomen are the portal vein and the inferior vena cava (IVC). The portal vein receives the blood from the entire gastrointestinal tract and spleen and pancreas. The IVC receives blood from the remainder of the abdominal and pelvic viscera and the lower extremities. The IVC begins as the union of the left and right common iliac veins. These latter vessels course posteriorly and slightly to the right of the arteries of the same name. The common iliac arteries and veins form an inverted V over the vertebral body of L5. Tribus and Belanger (2001) found that the left common iliac vein and right common iliac artery were separated by an average of 33.5 mm at the level of the L5-S1 IVD, and that the left common iliac vein generally was found approximately 12 mm to the left of the midline at this level. In general, the venous return to the IVC follows a similar course to that of the arterial supply from the abdominal aorta. Some unique features of the abdominal venous system are listed next.
• Venous drainage of spinal structures. Large veins accompany each LA and drain into either the IVC or the left common iliac vein. These large veins are connected by vertical channels, known as the ascending lumbar veins. The relationship of the ascending lumbar veins to the azygos system of veins is described in Chapter 2. Intervertebral veins exit the IVFs and drain blood from the internal vertebral venous plexus to join this drainage system (i.e., entering lumbar veins and ascending lumbar veins). Veins also accompany the posterior branches of the LA. These veins also drain into the large veins that accompany the LAs themselves (Crock & Yoshizawa, 1976). The external vertebral venous plexus also drains into the ascending lumbar veins.
• Hepatic veins. These veins drain the liver and empty into the IVC as it passes through the fossa for the IVC on the posteroinferior surface of the liver.
• Renal veins. These tributaries of the IVC drain the kidneys. The right renal vein also communicates with the azygos vein, whereas the left renal vein communicates with the hemiazygos vein. The azygos and hemiazygos veins course along the right and left sides of the upper lumbar vertebral bodies, respectively.
• Ascending lumbar veins. As described, the right and left ascending lumbar veins course superiorly along the posterior and lateral aspects of the lumbar vertebral bodies. They receive tributaries from both the internal and external vertebral venous plexuses. Superiorly the right and left ascending lumbar veins communicate with the azygos and hemiazygos veins, respectively. The drainage of the azygos and hemiazygos veins and their specific relationship to the venous drainage of the vertebral column are described in Chapter 2, and a full discussion of the azygos system of veins is found in Chapter 6.
REFERENCES
Acharya, S., Dorje, T., Srivastava, A. Lower dorsal and lumbar pedicle morphometry in Indian population: a study of four hundred fifty vertebrae. Spine (Phila Pa 1976). 2010;35:E378–E384.
Aihara, T., et al. Does the morphology of the iliolumbar ligament affect lumbosacral disc degeneration? Spine. 2002;27:1499–1503.
Amundsen, T., et al. Lumbar spinal stenosis. Spine. 1995;20:1178–1186.
Aota, Y., et al. Presurgical identification of extradural nerve root anomalies by coronal fat-suppressed magnetic resonance imaging: a report of six cases and a review of the literature. J Spinal Disord. 1997;10:167–175.
Arnoldi, C.C., et al. Lumbar spinal stenosis and nerve root entrapment syndromes. Clin Orthop. 1976;115:4–5.
Ashton, I.K., et al. Morphological basis for back pain: the demonstration of nerve fibers and neuropeptides in the lumbar facet joint capsule but not in ligamentum flavum. J Orthop Res. 1992;10:72–78.
Bachop, W., Janse, J. The corporotransverse ligament at the L5 intervertebral foramen. Anat Rec. 205, 1983. [(abstract)].
Bachop, W.E., Ro, C.S. A ligament separating the nerve from the blood vessels at the L5 intervertebral foramen. Orthopaedic Transactions. 1984;8:437.
Bailey, P., Casamajor, L. Osteo-arthritis of the spine as a cause of compression of the spinal cord and its roots. J Nerv Ment Dis. 1911;38:588–609.
Bakkum, B., Mestan, M. The effects of transforaminal ligaments on the sizes of T11 to L5 human intervertebral foramina. J Manipulative Physiol Ther. 1994;17:517–522.
Basadonna, P.T., Gasparini, D., Rucco, V. Iliolumbar ligament insertions. Spine. 1996;21:2313–2316.
Bashline, S.D., Bilott, J.R., Ellis, J.P. Meningovertebral ligaments and their putative significance in low back pain. J Manipulative Physiol Ther. 1996;19:592–596.
Beaujeux, R., et al. Posterior lumbar epidural fat as a functional structure? Spine. 1997;22:1264–1269.
Behrsin, J.F., Briggs, C.A. Ligaments of the lumbar spine: a review. Surg Radiol Anat. 1988;10:211–219.
Bergmann, T.F., Peterson, D.H., Lawrence, D.J. Chiropractic technique: principles and procedures. New York: Churchill Livingstone; 1993.
Bian, Q., et al. Prolonged and repeated upright posture promotes bone formation in rat lumbar vertebrae. Spine (Phila Pa 1976). 2011;36:E380–E387.
Boden, S.C., et al. Orientation of the lumbar facet joints: association with degenerative disc disease. J Bone Joint Surg. 1996;78-A:403–411.
Boden, S.D., et al. Abnormal magnetic-resonance scans of the lumbar spine in asymptomatic subjects. J Bone Joint Surg. 1990;72:401–408.
Bogduk, N. The anatomy of the lumbar intervertebral disc syndrome. Med J Aust. 1976;1:878–881.
Bogduk, N. The lumbar mamillo-accessory ligament: its anatomical and neurosurgical significance. Spine. 1981;6:162–167.
Bogduk, N. The innervation of the lumbar spine. Spine. 1983;8:286–293.
Bogduk, N. Low back pain. Aust Fam Physician. 1985;14:1168–1171.
Bogduk, N. Pathology of lumbar disc pain. Manual Med. 1990;5:72–79.
Bogduk, N. The causes of low back pain. Med J Aust. 1992;156:151–153.
Bogduk, N. Clinical anatomy of the lumbar spine, 4th ed. London: Churchill Livingstone; 2005.
Bogduk, N., Tynan, W., Wilson, A. The nerve supply to the human lumbar intervertebral discs. J Anat. 1981;132:39–56.
Bogduk, N., Wilson, A., Tynan, W. The human lumbar dorsal rami. J Anat. 1982;134:383–397.
Bose, K., Balasubramaniam, P. Nerve root canals of the lumbar spine. Spine. 1984;9:16–18.
Boszczyk, B.M., et al. An immunohistochemical study of the dorsal capsule of the lumbar and thoracic facet joints. Spine. 2001;26(15):e338–e343.
Bougie, J.D., Franco, D., Segil, C.M. An unusual cause for lumbar radiculopathy: a synovial facet joint cyst of the right L5 joint. J Manipulative Physiol Ther. 1996;19:48–51.
Briggs, C.A., Chandraraj, S. Variations in the lumbosacral ligament and associated changes in the lumbosacral region resulting in compression of the fifth dorsal root ganglion and spinal nerve. Clin Anat. 1995;8:339–346.
Brown, M.D., Holmes, D.C., Heiner, A.D. Measurement of cadaver lumbar spine motion segment stiffness. Spine. 2002;27:918–922.
Bucknill, A.T., et al. Nerve fibers in lumbar spine structures and injured spinal roots express the sensory neuron-specific sodium channels SNS/PN3 and NaN/SNS2. Spine. 2002;27:135–140.
Cao, K.D., Grimm, M.J., Yang, K.H. Load sharing within a human lumbar vertebral body using the finite element method. Spine. 2001;26:E253–E260.
Caputo, L.A., Cusimano, M.D. Schwannoma of the cauda equina. J Manipulative Physiol Ther. 1997;20:124–129.
Cassidy, J., Kirkaldy-Willis, W. Manipulation. In Kirkaldy-Willis W., ed.: Managing low back pain, 2nd ed., New York: Churchill Livingstone, 1988.
Chen, D., et al. Increasing neuroforaminal volume by anterior interbody distraction in degenerative lumbar spine. Spine. 1995;20:74–79.
Chithriki, M., Jaibaji, M., Steele, R.D. The anatomical relationship of the aortic bifurcation to the lumbar vertebrae: a MRI study. Surg Radiol Anat. 2002;24:308–312.
Cinotti, G., et al. Stenosis of lumbar intervertebral foramen. Spine. 2002;27:223–229.
Clarke, G.A., et al. Can infant malnutrition cause adult vertebral stenosis? Spine. 1985;10:165–170.
Cox, J. Low back pain: mechanism, diagnosis, and treatment, 7th ed. Philadelphia: Wolters Kluwer/Lippincott Williams & Wilkins; 2011.
Cramer, G.D., et al. Generalizability of patient profiles from a feasibility study. J Can Chiropractic Assoc. 1992;36:84–90.
Cramer, G.D., et al. Comparison of computed tomography to magnetic resonance imaging in the evaluation of the lumbar intervertebral foramina. Clin Anat. 1994;7:173–180.
Cramer, G.D., et al. Identification of the anterior longitudinal ligament on cadaveric spines and comparison with appearance on MRI. Bournemouth, England: Proc 1996 Intern Conf Spinal Manipulation (ICSM); 1996. [October 17-19, pp 151–152].
Cramer, G.D., et al. Morphometric evaluation of the anterior longitudinal ligament on cadaveric spines by means of magnetic resonance imaging. J Chiropract Educ. 1998;11:141.
Cramer, G.D., et al. Morphometric evaluation of the posterior longitudinal ligament on cadaveric spines by means of magnetic resonance imaging. Vancouver, BC: Proc Intern Conf Spinal Manipulation; 1998. [July 16-19, pp 24–26].
Cramer, G.D., et al. Effects of side-posture positioning and side posture adjusting on the lumbar zygapophysial joints as evaluated by magnetic resonance imaging: a before and after study with randomization. J Manipulative Physiol Ther. 2000;23:380–394.
Cramer, G.D., et al. The effects of side posture positioning and spinal adjusting on the lumbar Z joints: a randomized controlled trial of 64 subjects. Spine. 2002;27:2459–2466.
Cramer, G.D., et al. Dimensions of the lumbar intervertebral foramina as determined from the sagittal plane magnetic resonance imaging scans of 95 normal subjects. J Manipulative Physiol Ther. 2003;26:160–170.
Cramer, G.D., et al. Zygapophyseal joint adhesions after induced hypomobility. J Manipulative Physiol Ther. 2010;33:508–518.
Crock, H.V. Normal and pathological anatomy of the lumbar spinal nerve root canals. J Bone Joint Surg. 1981;63:487–490.
Crock, H.V., Yoshizawa, H. The blood supply of the lumbar vertebral column. Clin Orthop. 1976;115:6–21.
Cvijanovic, O., et al. Age- and region-dependent changes in human lumbar vertebral bone: a histomorphometric study. Spine (Phila Pa 1976). 2004;29:2370–2375.
Dagenais, S., Caro, J., Haldeman, S. A systematic review of low back pain cost of illness studies in the United States and internationally. Spine. 2008;8:8–20.
Day, M.O. Spondylolytic spondylolisthesis in an elite athlete. Chiro Sports Med. 1991;5:91–97.
dePeretti, F., et al. Biomechanics of the lumbar spinal nerve roots and the first sacral root within the intervertebral foramina. Surg Radiol Anat. 1989;11:221–225.
De Carvalho, D.E., et al. Lumbar spine and pelvic posture between standing and sitting: a radiologic investigation including reliability and repeatability of the lumbar lordosis measure. J Manipulative Physiol Ther. 2010;33:48–55.
Dickey, J.P., Bednar, D.A., Dumas, G.A. New insight into the mechanics of the lumbar interspinous ligament. Spine. 1996;21:2720–2727.
Dommisse, G.F., Louw, J.A. Anatomy of the lumbar spine. In: Floman Y., ed. Disorders of the lumbar spine. Rockville, Md, and Tel Aviv: Aspen and Freund, 1990.
Dreyfuss, P.H., Dreyer, S.J. Lumbar zygapophysial (facet) joint injections. North American Spine Society. Contemp Concepts Spine Care. 2001:1–16.
Dunlop, R.B., Adams, M.A., Hutton, W.C. Disc space narrowing and the lumbar facet joints. J Bone Joint Surg. 1984;66:706–710.
Dupuis, P.R. The anatomy of the lumbosacral spine. In Kirkaldy-Willis W., ed.: Managing low back pain, 2nd ed., New York: Churchill Livingstone, 1988.
Dupuis, P.R., et al. Radiologic diagnosis of degenerative lumbar spinal instability. Spine. 1985;10:262–276.
Ebraheim, N.A., et al. Anatomic considerations of the lumbar isthmus. Spine. 1997;22:941–945.
Ebraheim, N.A., et al. Anatomic relations between the lumbar pedicle and the adjacent neural structures. Spine. 1997;22:2338–2341.
Edelson, J.G., Nathan, H. Stages in the natural history of the vertebral end plates. Spine. 1988;13:21–26.
Edgar, M., Ghadially, J. Innervation of the lumbar spine. Clin Orthop. 1976;115:35–41.
Einstein, S.M., et al. Innervation of the spondylolysis “ligament.”. Spine. 1994;19:912–916.
Engel, R., Bogduk, N. The menisci of the lumbar zygapophysial joints. J Anat. 1982;135:795–809.
Epstein, J.A., et al. Lumbar nerve root compression at the intervertebral foramina caused by arthritis of the posterior facets. J Neurosurg. 1973;39:362–369.
Ericksen, M.F. Some aspects of aging in the lumbar spine. Am J Phys Anthropol. 1976;45:575–580.
Ericksen, M.F. Aging in the lumbar spine. Am J Phys Anthropol. 1978;48:241–245.
Faingold, R., et al. Imaging of low back pain in children and adolescents. Semin Ultrasound CT MR. 2004;25:490–505.
Farfan, H.F. Mechanical disorders of the low back. Philadelphia: Lea & Febiger; 1973.
Farfan, H.F., Huberdeau, R.M., Dubow, H.I. Lumbar intervertebral disc degeneration. J Bone Joint Surg. 1972;54-A:492–510.
Fennell, A.J., Jones, A.P., Hukins, D.W.L. Migration of the nucleus pulposus within the intervertebral disc during flexion and extension of the spine. Spine. 1996;21:2753–2757.
Floman, Y., Mirovsky, Y. The physical examination of the lumbosacral spine. In: Floman Y., ed. Disorders of the lumbar spine. Rockville, Md, and Tel Aviv: Aspen and Freund, 1990.
Fujiwara, A., et al. The effect of disc degeneration and facet joint osteoarthritis on the segmental flexibility of the lumbar spine. Spine. 2000;25:3036–3044.
Fujiwara, A., et al. Morphologic changes in the lumbar intervertebral foramen due to flexion-extension, lateral bending, and axial rotation: an in vitro anatomic and biochemical study. Spine. 2001;26:876–882.
Giglio, G.C., et al. Anatomy of the lumbar spine in achondroplasia. Basic Life Sci. 1988;48:227–239.
Giles, L.G. Lumbo-sacral zygapophysial joint tropism and its effect on hyaline cartilage. Clin Biomech. 1987;2:2–6.
Giles, L.G. Human zygapophyseal joint inferior recess synovial folds: a light microscopic examination. Anat Rec. 1988;220:117–124.
Giles, L.G. A histological investigation of human lower lumbar intervertebral canal (foramen) dimensions. J Manipulative Physiol Ther. 1994;17:4–14.
Giles, L.G., Taylor, J.R. Human zygapophyseal joint capsule and synovial fold innervation. Br J Rheumatol. 1987;26:93–98.
Goel, V.K., et al. A combined finite element and optimization investigation of lumbar spine mechanics with and without muscles. Spine. 1993;18:1531–1541.
Gordon, S.J., et al. Mechanism of disc rupture: a preliminary report. Spine. 1991;16:450–456.
Grant, J.P., Oxland, T.R., Dvorak, M.F. Mapping the structural properties of lumbosacral vertebral end plates. Spine. 2001;26:889–896.
Grenier, N., et al. Isthmic spondylolisthesis of the lumbar spine: MR imaging at 1.5T. Radiology. 1989;170:489–493.
Grenier, N., et al. Normal and disrupted lumbar longitudinal ligaments: correlative MR and anatomic study. Radiology. 1989;171:197–205.
Grimes, P.F., Massie, J.B., Garfin, S.R. Anatomic and biomechanical analysis of the lower lumbar foraminal ligaments. Spine. 2000;25:2009–2014.
Groen, G., Baljet, B., Drukker, J. Nerves and nerve plexuses of the human vertebral column. Am J Anat. 1990;188:282–296.
Hadley, L.A. Apophysial subluxation. J Bone Joint Surg. 1948:428–433.
Haher, T.R., et al. Instantaneous axis of rotation as a function of the three columns of the spine. Spine. 1992;17:s149–s154.
Hall, L.T., et al. Morphology of the lumbar vertebral end plates. Spine. 1998;23:1517–1522.
Hamanishi, C., Tanaka, S. Dorsal root ganglia in the lumbosacral region observed from the axial views of MRI. Spine. 1993;18(13):1753–1756.
Hanson, P., Sonesson, B. The anatomy of the iliolumbar ligament. Arch Phys Med Rehabil. 1994;75:1245–1246.
Hasegawa, T., et al. Morphometric analysis of the lumbosacral nerve roots and dorsal root ganglia by magnetic resonance imaging. Spine. 1996;21:1005–1009.
Hasue, M., et al. Anatomic study of the interrelation between lumbosacral nerve roots and their surrounding tissues. Spine. 1983;8:50–58.
Hedman, T.P., Fernie, G.R. Mechanical response of the lumbar spine to seated postural loads. Spine. 1997;22:734–743.
Helms, C.A., Sims, R. Foraminal spurs: a normal variant in the lumbar spine. Radiology. 1986;160:153–154.
Herman, M.J., Pizzutillo, P.D., Cavalier, R. Spondylolysis and spondylolisthesis in the child and adolescent athlete. Orthop Clin North Am. 2003;34:461–467. [vii].
Heylongs, D.A. Supraspinous and interspinous ligaments of the human spine. J Anat. 1978;125:127–131.
Higashino, K., et al. Vertebral rounding deformity in pediatric spondylolisthesis occurs due to deficient of endochondral ossification of the growth plate: radiological, histological and immunohistochemical analysis of a rat spondylolisthesis model. Spine (Phila Pa 1976). 2007;32:2839–2845.
Hoyland, J.A., Freemont, A.J., Jayson, M.I.V. Intervertebral foramen venous obstruction: a cause of periradicular fibrosis. Spine. 1989;14:558–568.
Hsieh, C., et al. Lumbosacral transitional segments: classification, prevalence, and effect on disc height. J Manipulative Physiol Ther. 2000;23:483–489.
Hutton, W.C. The forces acting on a lumbar intervertebral joint. J Manual Med. 1990;5:66–67.
Ianuzzi, A., Pickar, J.G., Khalsa, P.S. Relationships between joint motion and facet joint capsule strain during cat and human lumbar spinal motions. J Manipulative Physiol Ther. 2011;34:420–431.
Inufusa, A., et al. Anatomic changes of the spinal canal and intervertebral foramen associated with flexion-extension movement. Spine. 1996;21:2412–2420.
Jackson, H.C., et al. Nerve endings in the human lumbar spinal column and related structures. J Bone Joint Surg. 1966;48:1272–1281.
Jeffries, B. Facet joint injections. Spine State Art Rev. 1988;2:409–417.
Jönsson, B., Stromqvist, B. Motor affliction of the L5 nerve root in lumbar nerve root compression syndromes. Spine. 1995;20:2012–2015.
Jonsson E. (2000). Back pain, neck pain. SBU Project Group 2000, Summary & Conclusions, Report, p 145.
Kalichman, L., et al. Facet orientation and tropism: associations with facet joint osteoarthritis and degeneratives. Spine (Phila Pa 1976). 2009;34:E579–E585.
Kanayama, M., et al. Phase lag of the intersegmental motion in flexion-extension of the lumbar and lumbosacral spine. Spine. 1996;21:1416–1422.
Kauppila, L.I., Tallroth, K. Postmortem angiographic findings for arteries supplying the lumbar spine: their relationship to low-back symptoms. J Spinal Disord. 1993;6:124–129.
Kikuchi, S., et al. Anatomic and clinical studies of radicular symptoms. Spine. 1984;9:23–30.
Kikuchi, S., et al. Anatomic and radiographic study of dorsal root ganglia. Spine. 1994;19:6–11.
Kirk, C.R., Lawrence, D.J. States manual of spinal, pelvic, and extravertebral techniques, 2nd ed. Baltimore: Waverly Press; 1985.
Kirkaldy-Willis, W.H., et al. Pathology and pathogenesis of lumbar spondylosis and stenosis. Spine. 1978;3:319–328.
Klaassen, Z., et al. Anatomy of the ilioinguinal and iliohypogastric nerves with observations of their spinal nerve contributions. Clin Anat. 2011;24:454–461.
Korkala, O., et al. Immunohistochemical demonstration of nociceptors in the ligamentous structures of the lumbar spine. Spine. 1985;10:156–157.
Kos, J., Hert, J., Sevcik, P. Meniscoids of the intervertebral joints [in Czech]. Acta Chir Orthop Traumatol Cech. 2002;69:149–157.
Kozanek, M., et al. Range of motion and orientation of the lumbar facet joints in vivo. Spine (Phila Pa 1976). 2009;34:E689–E696.
Krismer, M., et al. Motion in lumbar functional spine units during side bending and axial rotation moments depending on the degree of degeneration. Spine. 2000;25:2020–2027.
Lancourt, J.E., Glenn, W.V., Wiltse, L.L. Multiplanar computerized tomography in the normal spine and in the diagnosis of spinal stenosis. A gross anatomic-computerized tomographic correlation. Spine. 1979;4:379–390.
Lee, C.K., Rauschning, W., Glenn, W. Lateral lumbar spinal canal stenosis: classification, pathologic anatomy and surgical decompression. Spine. 1988;13:313–320.
Lee, H., et al. Morphometric study of the lumbar spinal canal in the Korean population. Spine. 1995;20:1679–1684.
Lew, P.C., Morrow, C.J., Lew, A.M. The effect of neck and leg flexion and their sequence on the lumbar spinal cord. Spine. 1994;19:2421–2425.
Limthongkul, W., et al. Volumetric analysis of thoracic and lumbar vertebral bodies. Spine J. 2010;10:153–158.
Lippitt, A.B. The facet joint and its role in spine pain: management with facet joint injections. Spine. 1984;9:746–750.
Liyang, D., et al. The effect of flexion-extension motion of the lumbar spine on the capacity of the spinal canal, an experimental study. Spine. 1989;14:523–525.
Macdonald, A., et al. Level of termination of the spinal cord and the dural sac: a magnetic resonance study. Clin Anat. 1999;12:149–152.
Madsen, R., et al. The effect of body position and axial load on spinal canal morphology: an MRI study of central spinal stenosis. Spine (Phila Pa 1976). 2008;33:61–67.
Mahato, N.K. Complete sacralization of L5 vertebrae: traits, dimensions, and load bearing in the involved sacra. Spine J. 2010;10:610–615.
Maigne, J.Y., Doursounian, L. Entrapment neuropathy of the medial superior cluneal nerve. Spine. 1997;22:1156–1159.
Maigne, J.Y., Maigne, R., Guerin-Surville, H. The lumbar mamillo-accessory foramen: a study of 203 lumbosacral spines. Surg Radiol Anat. 1991;13:29–32.
Malas, M.A., et al. The relationship between the lumbosacral enlargement and the conus medullaris during the period of fetal development and adulthood. Surg Radiol Anat. 2000;22:163–168.
Marchand, F., Ahmed, A. Investigation of the laminate structure of lumbar disc anulus fibrosus. Spine. 1990;15:402–410.
Marchiori, D.M., Firth, R. Tethered cord syndrome. J Manipulative Physiol Ther. 1996;19:265–267.
Marchiori, D.M., et al. A comparison of radiographic signs of degeneration to corresponding MRI signal intensities in the lumbar spine. J Manipulative Physiol Ther. 1994;17:238–245.
Markiewitz, A.D., et al. Calcium pyrophosphate dehydrate crystal deposition disease as a cause of lumbar canal stenosis. Spine. 1996;21:506–511.
Masharawi, Y., Salame, K. Shape variation of the neural arch in the thoracic and lumbar spine: characterization and relationship with the vertebral body shape. Clin Anat. 2011;24:858–867.
Masharawi, Y., et al. Facet orientation in the thoracolumbar spine: three-dimensional anatomic and biomechanical analysis. Spine (Phila Pa 1976). 2004;29:1755–1763.
Masharawi, Y., et al. Facet tropism and interfacet shape in the thoracolumbar vertebrae: characterization and biomechanical interpretation. Spine (Phila Pa 1976). 2005;30:E281–E292.
Masharawi, Y., et al. Vertebral body shape variation in the thoracic and lumbar spine: characterization of its asymmetry and wedging. Clin Anat. 2008;21:46–54.
Mayoux-Benhamou, M.A., et al. A morphometric study of the lumbar spine, influence of flexion-extension movements and of isolated disc collapse. Surg Radiol Anat. 1989;11:97–102.
Maxfield, B.A. Sports-related injury of the pediatric spine. Radiol Clin North Am. 2010;48:1237–1248.
McCarthy, P.W. The innervation of lumbar intervertebral discs: an update. Eur J Chiropract. 1993;41:21–29.
McGill, S.M. The biomechanics of low back injury: implications on current practice in industry and the clinic. J Biomechanics. 1997;30:465–475.
McGregor, A.H., McCarthy, I.D., Hughes, S.P. Motion characteristics of the lumbar spine in the normal population. Spine. 1995;20:2421–2428.
McNab, I. Negative disc exploration: an analysis of the causes of nerve-root involvement in sixty-eight patients. J Bone Joint Surg. 1971;53-A:891–903.
Mitra, S.R., Datir, S.P., Jadhav, S.O. Morphometric study of the lumbar pedicle in the Indian population as related to pedicular screw fixation. Spine. 2002;27:453–459.
Mooney, V., Robertson, J. The facet syndrome. Clin Orthop. 1976;115:149–156.
Morinaga, T., et al. Sensory innervation to the anterior portion of the lumbar intervertebral disc. Spine. 1996;21:1848–1851.
Morishita, Y., et al. Measurement of the local pressure of the intervertebral foramen and the electrophysiologic values of the spinal nerve roots in the vertebral foramen. Spine (Phila Pa 1976). 2006;31:3076–3080.
Mosner, E.A., et al. A comparison of actual and apparent lumbar lordosis in black and white adult females. Spine. 1989;14:310–314.
Nachemson, A.L. The lumbar spine, and orthopedic challenge. Spine. 1976;1:59–71.
Natarajan, R.N., Andersson, G.B.J. The influence of lumbar disc height and cross-sectional area on the mechanical response of the disc to physiological loading. Spine. 1999;24:1873.
Natarajan, R.N., et al. Biomechanical response of a lumbar intervertebral disc to manual lifting activities: a poroelastic finite element model study. Spine (Phila Pa 1976). 2008;33:1958–1965.
Nathan, H., Weizenbluth, M., Halperin, N. The lumbosacral ligament (LSL), with special emphasis on the “lumbosacral tunnel” and the entrapment of the 5th lumbar nerve. Int Orthop. 1982;6:197–202.
Neidre, A., MacNab, I. Anomalies of the lumbosacral nerve roots: review of 16 cases and classification. Spine. 1983;8:294–299.
Nicholson, A.A., Roberts, G.M., Williams, L.A. The measured height of the lumbosacral disc in patients with and without transitional vertebrae. Br J Radiol. 1988;61:454–455.
Nordström, D., et al. Symptomatic lumbar spondylolysis. Spine. 1994;19:2752–2758.
Nowicki, B.H., et al. Effect of axial loading on neural foramina and nerve roots in the lumbar spine. Radiology. 1990;176:433–437.
Nowicki, B.H., Haughton, V.M. Ligaments of the lumbar neural foramina: a sectional anatomic study. Clin Anat. 1992;5:126–135.
Ohtori, S., et al. Sensory innervation of the dorsal portion of the lumbar intervertebral disc in rats. Spine. 1999;24:2295.
Ohtori, S., et al. Neurones in the dorsal root ganglia of T13, L1 and L2 innervate the dorsal portion of lower lumbar discs in rats. A study using diI, an anterograde neurotracer. J Bone Joint Surg [Br]. 2001;83(8):1191–1194.
Olsewski, J.M., et al. Evidence from cadavers suggestive of entrapment of fifth lumbar spinal nerves by lumbosacral ligaments. Spine. 1991;16:336–347.
Olszewski, A.D., Yaszemski, M.J., White, A.A. The anatomy of the human lumbar ligamentum flavum: new observations and their surgical importance. Spine. 1996;21:2307–2312.
Oshima, H., et al. Morphologic variation of lumbar posterior longitudinal ligament and the modality of disc herniation. Spine. 1993;18:2408–2411.
Oxland, T.R., et al. Effects of end plate removal on the structural properties of the lower lumbar vertebral bodies. Spine. 2003;28:771–777.
Ozawa, T., et al. The degenerated lumbar intervertebral disc is innervated primarily by peptide-containing sensory nerve fibers in humans. Spine (Phila Pa 1976). 2006;31:2418–2422.
Pal, G.P., et al. Trajectory architecture of the trabecular bone between the body and the neural arch in human vertebrae. Anat Rec. 1988;222:418–425.
Papp, T., Porter, R.W., Aspden, R. The growth of the lumbar vertebral canal. Spine. 1994;19:2270–2273.
Paris, S. Anatomy as related to function and pain. Symposium on Evaluation and Care of Lumbar Spine Problems. 1983:475–489.
Parke, W. Role of epidural and radicular veins in chronic back pain and radiulopathy. In: Kambin P., ed. Arthroscopic and endoscopic spinal surgery text and atlas. 2nd ed. Totowa, New Jersey: Humana Press, Inc; 2005:151–165.
Park, J.B., Chang, H., Lee, J.K. Quantitative analysis of transforming growth factor-beta 1 in ligamentum flavum of lumbar spinal stenosis and disc herniation. Spine. 2001;26:E492–E495.
Parke, W.W., Schiff, D.C. The applied anatomy of the intervertebral disc. Orthop Clin North Am. 1993;2:309–324.
Paz-Fumagalli, R., Haughton, V.M. Lumbar cribriform fascia: appearance at freezing microtomy and MR imaging. Radiology. 1993;187:241–243.
Peterson, C.K., Boulton, J.E., Wood, A.R. A cross-sectional study correlating lumbar spine degeneration with disability and pain. Spine. 2000;25:218–223.
Plamondon, A., Gagnon, M., Maurais, G. Application of stereoradiographic method for the study of intervertebral motion. Spine. 1988;13:1027–1032.
Porter, R. Spinal stenosis and neurogenic claudication. Spine. 1996;21:2046–2052.
Postacchini, F., Perugia, D. Degenerative lumbar spondylolisthesis. It J Orthop Traumatol. 1991;17:165–173.
Putz, R.L., Müller-Gerbl, M. The vertebral column: a phylogenetic failure? A theory explaining the function and vulnerability of the human spine. Clin Anat. 1996;9:205–212.
Qian, Y., Qin, A., Zheng, M.H. Transforaminal ligament may play a role in lumbar nerve root compression of foraminal stenosis. Med Hypotheses. 2011;77:1148–1149.
Rauschning, W. Normal and pathologic anatomy of the lumbar root canals. Spine. 1987;12:1008–1019.
Revel, M., et al. Morphological variations of the lumbar intervertebral foramina during flexion-extension and disc collapse. Revue Rhumatisme. 1988;55:361–366.
Rice, J., et al. Movements at the low back during normal walking. Clin Anat. 2004;17:662–666.
Rizzo, J.A., Abbott, T.A., III., Berger, M.L. The labor productivity effects of chronic backache in the US. Medical Care. 1998;36(10):1471–1488.
Rucco, V., Basadonna, P.T., Gasparini, D. Anatomy of the iliolumbar ligament. Am J Phys Med Rehabil. 1996;75:451–455.
Safak, A.A., et al. The thickness of the ligamentum flavum in relation to age and gender. Clin Anat. 2010;23:79–83.
Sairyo, K., et al. Lumbar ligamentum flavum hypertrophy is due to accumulation of inflammation-related scar tissue. Spine (Phila Pa 1976). 2007;32:E340–E347.
Saito, T., et al. The medial branch of the lateral branch of the posterior ramus of the spinal nerve. Surg Radiol Anat. 2006;28:228–234.
Salbacak, A., et al. An investigation of the conus medullaris and filum terminale variations in human fetuses. Surg Radiol Anat. 2000;22:89–92.
Scapinelli, R. Morphological and functional changes of the lumbar spinous processes in the elderly. Surg Radiol Anat. 1989;11:129–133.
Scapinelli, R. Anatomical and radiologic studies on the lumbosacral meningovertebral ligaments of humans. J Spinal Disord. 1990;3:6–15.
Schlegel, J.D., et al. The role of distraction in improving the space available in the lumbar stenotic canal and foramen. Spine. 1994;19(18):2041–2047.
Schmid, M.R., et al. Changes in cross-sectional measurements of the spinal canal and intervertebral foramina as a function of body position: in vivo studies on an open-configuration MR system. AJR Am J Roentgenol. 1999;172:1095–1102.
Schmidt, H., et al. Intradiscal pressure, shear strain, and fiber strain in the intervertebral disc under combined loading. Spine (Phila Pa 1976). 2007;32:748–755.
Schwab, F.J., Farcy, J.C., Roye, D.P., Jr. The sagittal pelvic tilt index as a criterion in the evaluation of spondylolisthesis. Spine. 1997;22:1661–1667.
Schwarzer, A.C., et al. The prevalence and clinical features of internal disc disruption in patients with chronic low back pain. Spine. 1995;20:1878–1883.
Shirazi-Adl, A., Drouin, G. Load-bearing role of facets in a lumbar segment under sagittal plane loadings. J Biomech. 1987;20(6):601–613.
Silva, M.J., Keaveny, T.M., Hayes, W.C. Load sharing between the shell and centrum in the lumbar vertebral body. Spine. 1997;22:140–150.
Sims, J.A., Moorman, S.J. The role of the iliolumbar ligament in low back pain. Med Hypotheses. 1996;46:511–515.
Solaroglu, I., Okutan, O., Beskonakli, E. The ATA and its surgical importance: a newly described ligament lying between the dural sac and the ligamentum flavum at the L5 level. Spine (Phila Pa 1976). 2011;36:1268–1272.
Spencer, D.L., Irwin, G.S., Miller, J.A.A. Anatomy and significance of fixation of the lumbosacral nerve roots in sciatica. Spine. 1983;8:672–679.
Spratt, J.D., Logan, B.M., Abrahams, P.H. Variant slips of psoas and iliacus muscles, with splitting of the femoral nerve. Clin Anat. 1996;9:401–404.
Standring, S., et al. Gray's anatomy: the anatomical basis of clinical practice, 40th ed. Edinburgh: Churchill Livingstone; 2008.
Suseki, K., et al. Innervation of the lumbar facet joints. Spine. 1997;22:477–485.
Tague, R.G. Sacralization is not associated with elongated cervical costal process and cervical rib. Clin Anat. 2011;24:209–217.
Takahashi, K., et al. Epidural pressure measurements. Spine. 1995;20:650–653.
Takahashi, K., Shima, I., Porter, R.W. Nerve root pressure in lumbar disc herniation. Spine. 1999;24:2003.
Takemitsu, Y., et al. Lumbar degenerative kyphosis. Spine. 1988;13:1317–1326.
Tallroth, K., et al. Lumbar spine in Marfan syndrome. Skeletal Radiol. 1995;24:337–340.
Taylor, J.R. The development and adult structure of lumbar intervertebral discs. J Manual Med. 1990;5:43–47.
Taylor, J.R., Twomey, L.T. Age changes in lumbar zygapophyseal joints: observations on structure and function. Spine. 1986;11:739–745.
Theil, H.W., Clements, D.S., Cassidy, J.D. Lumbar apophyseal ring fractures in adolescents. J Manipulative Physiol Ther. 1992;15:250–254.
Torun, F., et al. The lumbar roots and pedicles: a morphometric analysis and anatomical features. J Clin Neurosci. 2008;15:895–899.
Toyone, T., et al. Facet joint orientation difference between cephalad and caudad portions: a possible cause of degenerative spondylolisthesis. Spine (Phila Pa 1976). 2009;34:2259–2262.
Tribus, C.B., Belanger, T. The vascular anatomy anterior to the L5-S1 disk space. Spine. 2001;26:1205–1208.
Tsuji, H., et al. Redundant nerve roots in patients with degenerative lumbar spinal stenosis. Spine. 1985;10:72–82.
Twomey, L., Taylor, J.R. Structural and mechanical disc changes with age. J Manual Med. 1990;5:58–61.
Uhthoff, H. Prenatal development of the iliolumbar ligament. J Bone Joint Surg. 1993;75:93–95.
Ulmer, J.L., et al. Lumbar spondylolysis without spondylolisthesis: recognition of isolated posterior element subluxation on sagittal MR. Am J Neuroradiol. 1995;16:1393–1398.
Vaccaro, A.R., et al. Injury of the posterior ligamentous complex of the thoracolumbar spine: a prospective evaluation of the diagnostic accuracy of magnetic resonance imaging. Spine (Phila Pa 1976). 2009;34:E841–E847.
Van Schaik, J., Verbiest, H., Van Schaik, F. The orientation of laminae and facet joints in the lower lumbar spine. Spine. 1985;10:59–63.
Vanharanta, H., et al. The relationship of facet tropism to degenerative disc disease. Spine. 1993;18:1000–1005.
Verbiest, H. A radicular syndrome from developmental narrowing of the lumbar vertebral canal. J Bone Joint Surg. 1954;36-B:230–237.
Viejo-Fuertes, D., et al. Morphologic and histologic study of the ligamentum flavum in the thoraco-lumbar region. Surg Radiol Anat. 1998;20(3):171–176.
Vital, J.M., et al. Anatomy of the lumbar radicular canal. Anat Clin. 1983;5:141–151.
Wang, Z., et al. Influence of sacral morphology in developmental spondylolisthesis. Spine (Phila Pa 1976). 2008;33:2185–2191.
Wang, J., Yang, X. Age-related changes in the orientation of lumbar facet joints. Spine (Phila Pa 1976). 2009;34:E596–E598.
Ward, C.V., et al. Radiographic assessment of lumbar facet distance spacing and spondylolysis. Spine (Phila Pa 1976). 2007;32:E85–E88.
White, A.W., Panjabi, M.M. Clinical biomechanics of the spine. Philadelphia: JB Lippincott; 1990.
Wilson, D.A., Prince, J.R. John Caffey award. MR imaging determination of the location of the normal conus medullaris throughout childhood. AJR Am J Roentgenol. 1989;152(5):1029–1032.
Wiltse, L.L. The etiology of spondylolisthesis. J Bone Joint Surg Am. 1962;44-A:539–560.
Wiltse, L.L., et al. Alar transverse process impingement of the L5 spinal nerve: the far-out syndrome. Spine. 1984;9:31–41.
Worril, N.A., Peterson, C.K. Effect of anterior wedging of L1on the measurement of lumbar lordosis: comparison of two roentgenological methods. J Manipulative Physiol Ther. 1997;20:459–467.
Xu, G.L., et al. Normal variations of the lumbar facet joint capsules. Clin Anat. 1991;4:117–122.
Xu, G.L., Haughton, V.M., Carrera, G.F. Lumbar facet joint capsule: appearance at MR imaging and CT. Radiology. 1990;177:415–420.
Yahia, H., Newman, N., St-Georges, M. Sensory nerve endings in the posterior ligaments of the lumbar spine. Electrophysiol Kinesiol. 1988:455–458.
Yahia, L.H., Newman, N., Rivard, C. Neurohistology of lumbar spine ligament. Acta Orthop Scand. 1988;59:508–512.
Yamashita, T., et al. Mechanosensitive afferent units in the lumbar facet joint. J Bone Joint Surg. 1990;72-A:865–870.
Yamashita, T., et al. A morphological study of the fibrous capsule of the human lumbar facet joint. Spine. 1996;21:538–543.
Yang, K.H., King, A.I. Mechanism of facet load transmission as a hypothesis for low-back pain. Spine. 1984;9:557–565.
Yang, K.H., et al. Annulus fibrosus tears. An experimental model. Orthop Trans. 1988;12:86–87.
You-Lu, Z., Wu, W. CT measurement of the normal cervical and lumbar spinal canal in Chinese. Chin Med J. 1988;101:898–900.
Yu, S.W., et al. Identification of the posterior longitudinal ligament on magnetic resonance imaging scans: comparison between MRI and a cadaveric specimen. Bournemouth, England: Proc 1996 Intern Conf Spinal Manipulation (ICSM); 1996. [October 17-19, pp 153–154].
Yukawa, Y., et al. Groin pain associated with lower lumbar disc herniation. Spine. 1997;22:1736–1740.