Unique Anatomic Features of the Pediatric Spine
Importance of the Pediatric Spine
Age-Related Anatomic Changes of the Pediatric Spine
Key Anatomic Features of the Adult Spine Leading to Foraminal Stenosis
Summary of Clinically Relevant Changes of the Zygapophysial Joints
Summary of Clinically Relevant Changes of the Intervertebral Foramina
Clinically Relevant Developmental Anatomy in Children
Developmental Anomalies of the Spine
Unique Development of the Atypical Vertebrae
Key Feature in the Development of Typical Vertebrae
Location of the Conus Medullaris on Magnetic Resonance Imaging Scans of Children
Common Pediatric Disorders Related to the Anatomy of the Spine
Importance of the Pediatric Spine
Understanding the spines of children is important for a number of reasons. Contrary to common belief, a fairly high number of children, particularly adolescents, suffer from back pain, and the pain is recurrent in many (Burton et al., 1996). A study of children in Finland, conducted by Taimela and colleagues (1997), showed the following prevalence of back pain among children:
Salminen and colleagues (1995) showed that recurrent or continuous low back pain was present in 8% to 18% of 14- to 16-year-old children. Other studies have reported the incidence of low back pain in children in Europe and North America to range from 20% to 51% (Faingold et al., 2004).
Taimela and colleagues (1997) found no difference in the incidence of low back pain between males and females. However, pain was identified as being chronic or recurrent in 26% of boys with low back pain and in 33% of girls with low back pain (Taimela et al., 1997).
Perhaps surprisingly, intervertebral disc (IVD) degeneration has been identified with magnetic resonance imaging (MRI) in 15-year-old children, and an unexpectedly high number of young people in their twenties have bulging IVDs that are thought to be the cause of their back pain (Kraemer, 1995; Salminen et al., 1995; Faingold et al., 2004). IVD protrusion is more prevalent in taller adolescents who have less ability to forward flex than in those of average height with better mobility in forward flexion (Salminen, Erkintalo-Tertti, & Paajanen, 1993). Discitis, attributable to inflammation and/or infection of the IVD, is most common in children 10 years of age or younger, probably because of the increased vascular supply to the IVD in young children (Faingold et al., 2004).
As might be expected, the incidence of low back pain among children who are less physically active is higher than in children who engage in the average level of physical activity (Salminen et al., 1995), although participation in organized sports increases the incidence of low back pain (Maxfield, 2010). Unfortunately, low back pain during childhood and adolescence may be a risk factor for back pain in adulthood (Jeffries, Milanese, & Grimmer-Somers, 2007).
The purpose of this chapter is to identify unique anatomic features related to the pediatric spine. A separate chapter on this topic is important because the spines of children are not just miniature versions of the spines of adults. For example, many of the differences related to the pediatric spine can be mistaken for fractures on the x-rays of children (Fesmire & Luten, 1989). Because normally children are not x-rayed unless the chief complaint is related to trauma, or suspected trauma or other serious pathology, the identification of fractures is a primary focus when viewing these x-ray films. Knowing the precise locations of the ossification centers and the locations of closure, or final union, of the primary and secondary centers of ossification is important because these sites are the regions that can be mistaken for fractures most easily.
Understanding pediatric spinal anatomy also is important for the proper planning of certain treatment procedures, such as spinal manipulation. For example, the zygapophysial (Z) joints are underdeveloped until approximately 10 years of age. Knowing the rate and stage of development of these structures allows a clinician to tailor the manipulation by significantly reducing the force in those individuals, and those age groups, when the joints of the spine are not yet fully developed.
This chapter begins with a discussion of the changes that occur with age in key anatomic structures of the spine. Next, clinically relevant developmental changes of the spine that are important to various diagnostic and treatment procedures are covered. The chapter ends with a brief discussion of common pediatric disorders related to the anatomy of the spine.
Age-Related Anatomic Changes of the Pediatric Spine
The age-related changes to key anatomic structures of the spine are covered in several ways in this chapter. First, Table 13-1 describes the changes of key anatomic structures for several pediatric age groups. Notice that the key anatomic structures included in Table 13-1 are the curves of the spine, vertebral bodies, IVDs, intervertebral foramina (IVFs), Z joints, and neurologic elements in the vertebral canal. The age groups of Table 13-1 are newborn, 3 months, 2 years, and 10 years. Next, the following sections briefly summarize the most important anatomic changes for the age groups just listed. Finally, age-related changes of the Z joints and the IVFs are described. These two anatomic structures are featured because they undergo unique and clinically important changes with age.
Table 13-1
Changes during Infancy and Childhood of Important Anatomic Structures
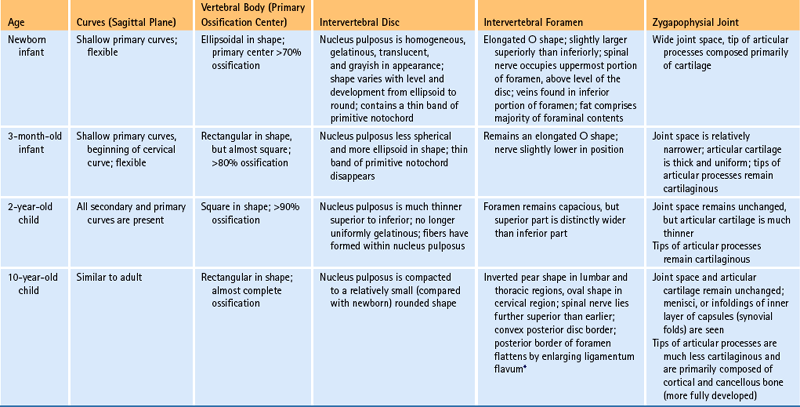
∗The ligamenta flava begin as thin structures that are convex anteriorly. They thicken dramatically with age and are relatively thick by age 2. They continue to thicken until the age of 10, at which time their anterior border has flattened into the adult appearance.
The Newborn Spine
The primary curves (thoracic and sacral kyphoses) of the newborn spine are developed; however, the secondary curves are rudimentary. The vertebral bodies of the newborn spine are more or less ellipsoidal in shape, although they are considerably ossified at birth (70%). The IVDs of the newborn are extremely large relative to their adult size, and the nucleus pulposus is more or less rounded in shape (Fig. 13-1).
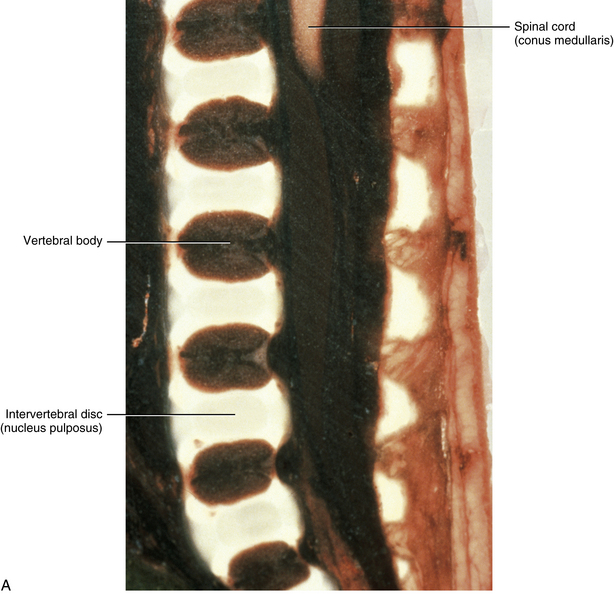
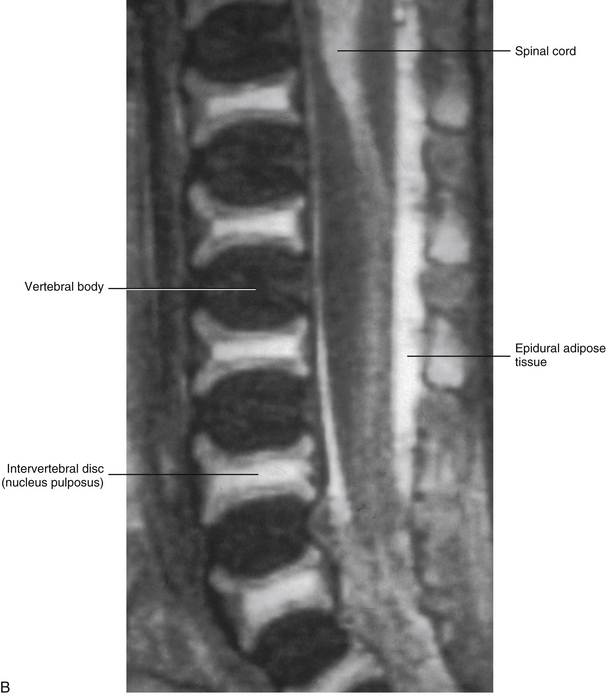
FIG. 13-1 A, Midsagittal section of a cadaveric newborn lumbar spine. Notice the large intervertebral discs and ellipsoidal vertebral bodies. (A, From Ho PS et al. [1988]. Progressive and regressive changes in the nucleus pulposus. Part I. The neonate. Radiology, 169[1], 87-91.)B, Midsagittal magnetic resonance imaging scan of same specimen.
At birth, the vertebral canal between L1 and L4 is 70% of its adult size; however, the L5 vertebral foramen and the L5-S1 vertebral canal are only 50% developed. The remaining 30% of the L1-4 vertebral canal development is completed by the end of the first year of infancy, whereas the L5 vertebral foramen and canal continues to develop until approximately 5 years of age. Of clinical importance is that the vertebral canal does not undergo “catch-up growth”; that is, if development is incomplete in infancy the vertebral canal will remain smaller than normal throughout life (Ursu, Porter, & Navaratnam, 1996).
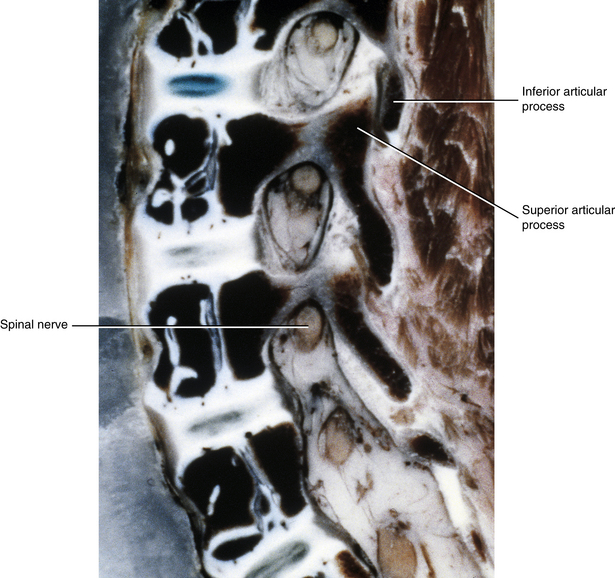
Newborn IVFs are relatively huge (Fig. 13-2). The term capacious is used sometimes to describe the size of the IVFs with respect to the relatively small foraminal contents of young children. Occasionally authors incorrectly apply the term capacious to the IVFs of adults (Crelin, 1973), whereas others have shown that this clearly is not the case (Hewitt, 1970; Giles, 1994). The main reason for the relatively large size of the IVFs in newborns is that the Z joints, which form the posterior border of the IVFs, are underdeveloped in young children (see Fig. 13-2). Both the superior and inferior articular processes are extremely small and the distal ends of both are primarily cartilaginous. The spines of newborns should be handled with extreme care for this reason.
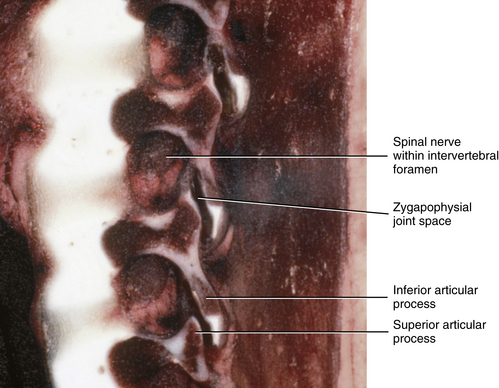
FIG. 13-2 Parasagittal section of a cadaveric newborn lumbar spine. Notice the large intervertebral foramina and underdeveloped articular processes forming the zygapophysial joints.
The plane of articulation of the newborn cervical Z joints is more horizontal in orientation than in the adult. This plane gradually becomes more vertically oriented until the age of 10 years, when the adult angle of approximately 45 degrees to the vertical plane is achieved (Kasai et al., 1996).
The 3-Month-Old Spine
The curves of the vertebral column at 3 months of age are already changing from those of the newborn. In addition to the primary curves of the thoracic and sacral regions (which have been present since birth), the cervical lordosis (which is a secondary curve) begins to develop at approximately 3 months of age. The formation of the cervical lordosis begins because the 3-month-old infant is beginning to lift up his or her head and look around.
The vertebral bodies are continuing to ossify at 3 months of age; they are now approximately 80% ossified, compared with the 70% ossification found in the newborn. The vertebral bodies also are becoming more rectangular in shape, rather than the ellipsoidal shape seen in newborns. The IVDs are narrowing from superior to inferior and the nucleus pulposus is assuming a more ellipsoidal shape, rather than the rounded shape of newborns (Fig. 13-3).
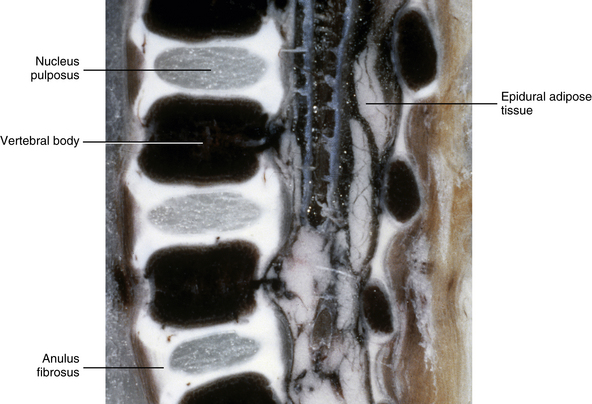
FIG. 13-3 Near midsagittal section (≈3 mm lateral to the midline; note medial aspects of laminae rather than spinous processes in section) of the lumbar region of a 3-month-old infant. The vertebral bodies are now more rectangular in shape than in the newborn, and the intervertebral discs have narrowed from superior to inferior.
The IVFs of 3-month-old spines remain large, and the articular processes of the Z joints remain relatively underdeveloped (Fig. 13-4). The distal ends of the superior and inferior articular processes are still primarily composed of cartilage (see Fig. 13-4).
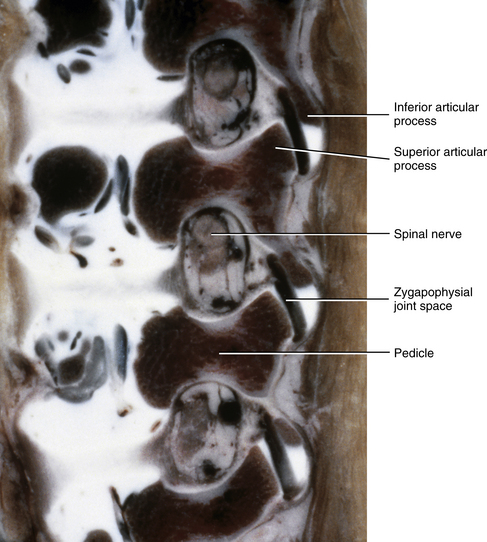
FIG. 13-4 Parasagittal section of the lumbar region of a 3-month-old spine. The intervertebral foramina remain large and the articular processes remain underdeveloped. Notice the great amount of cartilage on the tips of the inferior articular processes. (From Yu S, Haughton VM, & Rosenbaum AE. [1991]. Magnetic resonance imaging and anatomy of the spine. Radiol Clin North Am, 29[4], 691-710.)
The 2-Year-Old Spine
The primary thoracic and sacral curves are well-defined at 2 years of age. In addition, both secondary curves have developed at this age. That is, the cervical lordosis developed at approximately 3 months of age, and the lumbar lordosis began between 9 and 12 months of age. Like the cervical lordosis, the lumbar lordosis is created by postural changes. Specifically the lumbar lordosis begins when the infant starts to pull himself or herself up to stand, and then begins to walk. The cervical lordosis is greater in children younger than 5 years of age than in older children and adults. This is thought to be the result of holding up the comparatively heavy head with the relatively underdeveloped cervical vertebrae and cervical paraspinal musculature (Kasai et al., 1996).
The vertebral bodies of the 2-year-old child are almost 90% ossified, and are almost fully developed. They are more rectangular in shape than at 3 months, and the superior and inferior bony rims of the vertebral bodies are more “squared off” at this stage, rather than rounded, as in the 3-month-old infant. At 2 years the IVDs are narrowing from top to bottom, taking on more of an adult type of appearance, although significant further development remains to be completed (Fig. 13-5).
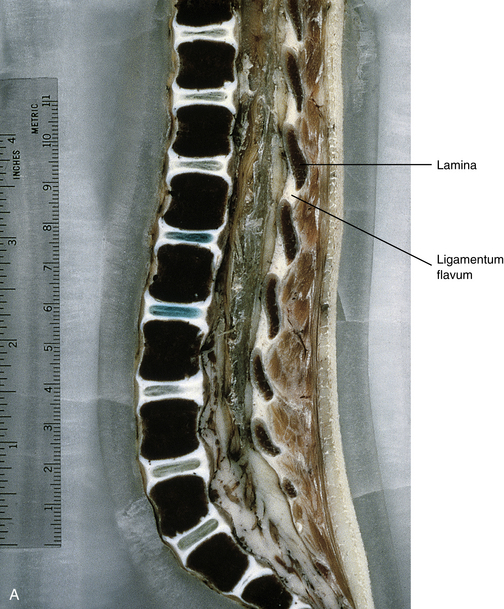
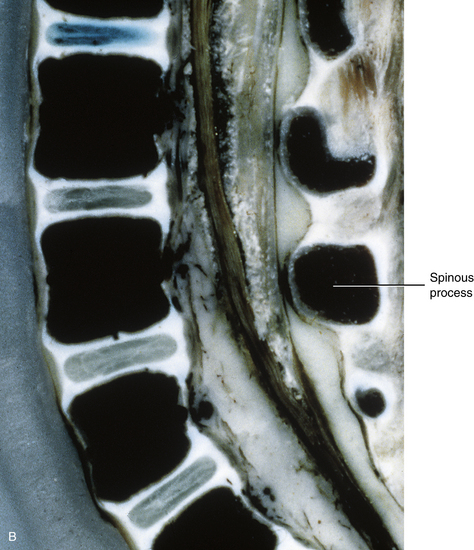
FIG. 13-5 A, Near midsagittal section (≈3 mm lateral to the midline; note laminae) of the lumbar region of a 2-year-old child. Note the relatively square shape of the vertebral bodies. B, Close-up of a midsagittal section (notice the spinous processes). The vertebral bodies are almost 90% ossified, and the intervertebral discs have narrowed considerably from 3 months of age.
The IVFs of the 2-year-old spine remain capacious; however, they begin to slightly narrow at their inferior aspect, particularly in the lumbar region (Fig. 13-6). The superior and inferior articular processes, which form the Z joints, begin to grow into the posterior and inferior aspect of the IVFs and then become more fully ossified during this stage of development. Even with this added growth and ossification, the Z joints are still relatively underdeveloped, leaving the IVFs comparatively large (see Fig. 13-6).
The 10-Year-Old Spine
The pedicles reach approximately 75% of their adult dimensions by 5 years of age (Kanna, Shetty, & Rajasekaran, 2011). The neurocentral synchondrosis persists as a plate of growth cartilage until it completes its fusion and is completely replaced with bone at 3 to 6 years of age. This cartilage plate, and later bony plate, which is located within the posterolateral aspect of the fully developed vertebral body, extends from the superior to inferior anular apophyses. The bony plate persists throughout life (Maat, Matricali, & van Persijn van Meerten, 1996). These plates are thought to be the reason that burst fractures of the vertebral body usually do not enter the posterolateral aspect of the anatomic vertebral body, and why during such fractures a central segment of the vertebral body frequently protrudes directly posteriorly, between the “wedge shape” formed by the left and right bony plates found at the neurocentral junction (Maat, Matricali, & van Persijn van Meerten, 1996).
The primary and secondary curves are fully developed at 10 years of age. Although the cervical lordosis may be prominent in early childhood, it diminishes after the age of 5 and is absent in 14% of the late childhood and adolescent population until approximately 16 years of age. This is partially because the cervical vertebral bodies can be wedge-shaped (shorter anteriorly) during this period of time. The anterior wedging of the vertebral bodies reduces the cervical lordosis and also can be mistaken for a compression fracture (Fesmire & Luten, 1989). However, the fact that the wedged vertebrae occur at multiple levels precludes fracture.
Ligamentous laxity typically exists in the cervical spines of children, especially those younger than 8 years of age. Such laxity can create a pseudosubluxation (i.e., the appearance of dislocation) during forward flexion (Kasai et al., 1996) as seen on lateral x-rays. This phenomenon is found in 46% of children 1 to 8 years old. The pseudosubluxation usually is most marked between the second and third cervical vertebrae (Fesmire & Luten, 1989).
The vertebral bodies (VBs) of the spines of 10-year-old children are almost 100% ossified and begin to look like “little adult” vertebral bodies. The growth in vertical height of the VBs is similar to growth of the long bones with primary and secondary growth spurts. Growth in boys terminates approximately 2 years later than that in girls (Kasai et al., 1996). The IVDs have more adultlike proportions as well, with further narrowing of the IVDs from superior to inferior and the nucleus pulposus more centrally located (Fig. 13-7). The continued growth of the vertebral bodies and IVDs in size (particularly growth in anterior-posterior depth and lateral width) during adolescence decreases spinal motion but increases the ability of the spine to withstand loads (i.e., increases the biomechanical “stiffness” of the spine) (Meijer et al., 2010).
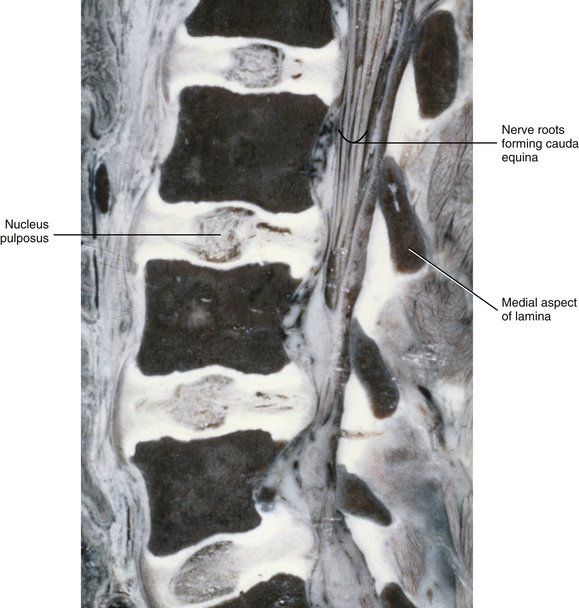
FIG. 13-7 Near midsagittal section (≈3 mm lateral to the midline; note laminae) of the lumbar region of a 10-year-old child. The vertebral bodies are almost fully developed, and the intervertebral discs are fully developed.
Severe spinal injuries in children are relatively rare. For example, fracture dislocation in the cervical region of children less than 15 years of age accounts for only approximately 2% of all such injuries. Seventy-five percent of these injuries in children result from motor vehicle and diving accidents. In children less than 8 years of age almost all fractures in the cervical region occur at C1 or C2. This is probably because of the relatively heavy head and relatively lax ligaments of infants and young children (Fesmire & Luten, 1989), making the upper cervical region relatively more mobile in children than in adults (Vialle & Vialle, 2005). Spinal cord injuries also are rare in children younger than 16 years of age, accounting for 1% to 3.3% of all spinal cord injuries, although some authors report a higher incidence in certain metropolitan areas. Approximately 20% (4% to 67%) of pediatric spinal cord injuries resulting from significant trauma show no radiographic abnormalities. This condition has been logically termed spinal cord injury without radiographic abnormalities (SCIWORA). Sometimes the neurologic deficits do not appear immediately; however, patients may report paresthesias after the trauma. For this reason all pediatric patients suffering from significant cervical spine trauma who describe feeling numbness and tingling after the trauma should be examined carefully and considered to have cervical spinal cord injury until it is definitively ruled out over time (Fesmire & Luten, 1989).
The IVFs of 10-year-old children begin to assume a more adult type of appearance. This IVF appearance occurs at approximately 6 to 10 years of age (notice the significant age range), and is an inverted pear shape in the lumbar and thoracic regions, being much more narrow inferiorly than superiorly (Fig. 13-8), and an oval shape in the cervical region. This dramatic change in IVF shape at this age results from the significant development and full ossification of the superior and inferior articular processes that form the Z joints. As the superior articular process continues to grow and develop, it presses into the posterior and inferior aspects of the IVF. The anulus fibrosus also protrudes slightly into the anterior and inferior aspects of the IVF. The result of these two structures moving into the inferior aspect of the IVF is a narrowing of that region. The ligamentum flavum continues to thicken since birth, and this ligament also helps narrow the posterior border of the IVF (see Fig. 13-8).
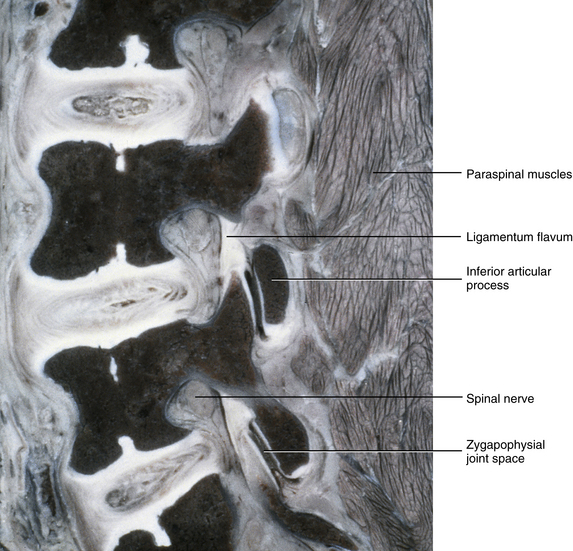
FIG. 13-8 Parasagittal section of the lumbar region of a 10-year-old child. The intervertebral foramina (IVFs) have attained the “inverted pear” shape of a fully developed lumbar IVF. The articular processes also have significantly advanced in development. Notice the ligamenta flava forming the posterior border of the IVFs. Also notice Z joint synovial folds protruding into the labeled Z joint space.
The IVF and articular process changes in this age range are important for a number of reasons. For example, once this age is reached, the Z joints are developed to the same proportions as adult Z joints. Therefore treatment procedures (e.g., spinal manipulation) can be employed in fashions more similar to those used with adults. Of course, the forces of manipulative procedures must be titrated to patients’ size, body type, and weight.
Radicular pain from pathology within the IVF before this stage of development is rare and should be examined carefully. Space-occupying lesions large enough to cause radicular pain in the capacious IVFs of young children need to be large. Such large space-occupying lesions could be the result of neoplasia, or a displaced bone fragment from a fracture if trauma was associated with the event. Fortunately, neoplasia (tumors) of the spine in children and adolescents younger than 16 years of age is uncommon. Such tumors usually are accompanied by back pain and neurologic deficits, and usually can be identified on x-rays (Beer & Menezes, 1997). After the developmental period when the child is 6 to 10 years old (usually closer to the 10-year age group), and after the IVFs have reached their adult shape, the possibility exists (although rare) for an IVD to protrude far laterally into the IVF, affecting the dorsal root ganglion or dorsal roots and causing radicular pain.
Key Anatomic Features of the Adult Spine Leading to Foraminal Stenosis
A better understanding of the process of spinal degeneration can be achieved by comparing and contrasting several of the key anatomic features of 10-year-old and adult spines. For example, the inverted pear shape of thoracic and lumbar IVFs and the oval shape of cervical IVFs are common to both healthy adult spines, as well as the spines of 10-year-old children. However, with advancing age the IVDs can narrow in some individuals, as a result of the breakdown of proteoglycans or secondary to trauma (see Chapter 14). As the IVD narrows, the anulus fibrosus can bulge into the region of the IVF. Narrowing of the IVD also causes the functional spinal unit (i.e., two vertebrae and the soft tissues between them) to narrow from top to bottom, causing the superior-to-inferior dimensions of the IVF to decrease. At the same time the bony end plates begin to widen as trabecula are laid down along the superior and inferior rims of the vertebral bodies. This bony change probably is secondary to the loss of fluid pressure in the nucleus pulposus; therefore more pressure is received by the anulus fibrosus and transferred along the rims of the vertebral bodies, causing the added trabeculae to be laid down in this region. This new bone frequently is dense and sclerotic and can appear more opaque on x-rays. The superior and inferior articular processes of the Z joint override one another as the two vertebrae surrounding the narrowing IVD come closer together and the IVF narrows. This overriding causes the superior articular process of the vertebra below to fill the inferior aspect of the IVF, causing further stenosis. In addition, osteophytic bone changes (bone spurs) can occur on the articular processes. These bone spurs can protrude into the IVF, again resulting in additional IVF narrowing. Finally, as the two adjacent vertebrae continue to approximate each other, the ligamentum flavum thickens or in some cases can even buckle into the IVF, again resulting in further constriction of this region. These changes can result in foraminal stenosis. The changes associated with foraminal stenosis are summarized in Figure 11-12.
Summary of Clinically Relevant Changes of the Zygapophysial Joints
Recall that the articular processes have little bone in the newborn and are cartilaginous. They remain quite cartilaginous until approximately 6 to 10 years of age (closer to 10 years), when the cartilages of the tips of the superior and inferior articular processes gradually are replaced by bone. They then begin to attain the same proportions as articular processes of adult spines. The precise age of this maturation of the articular processes varies considerably among individuals.
The Z joint space in the newborn is wide, but it narrows fairly quickly and by 3 months of age the Z joint space already has narrowed considerably. The articular cartilage becomes thick at 3 months of age and then gradually begins to become thinner over the next 2 years. This cartilage remains relatively unchanged in thickness from 2 years of age until late puberty; then the articular cartilage begins to gradually thicken until the early twenties, eventually reaching up to 2 mm in thickness at approximately 22 years of age.
Summary of Clinically Relevant Changes of the Intervertebral Foramina
As mentioned, the changes of the Z joints are related to the changes of the IVFs seen during infant and childhood development. Because the superior articular processes of infants and young children are relatively underdeveloped until approximately 6 to 10 years of age, they do not protrude into the IVFs as they do after this age period. Therefore the IVFs are rounded until approximately 6 to 10 years of age; after this age they gradually begin to take on their adult appearance (inverted pear-shaped in the thoracic and lumbar regions, oval in the cervical region). The thickening of the ligamenta flava also contributes to the development of the inverted pear-shaped adult lumbar and thoracic IVFs.
Clinically Relevant Developmental Anatomy in Children
Developmental anomalies of the spine often are first noted during childhood. Also, many of the unique features of spinal development that can be confused as fractures on children’s x-rays are related to spinal development or developmental anomalies of the vertebral column. Even though the topic of spinal development is fully covered in Chapter 12, the purpose of this section is to highlight the most clinically relevant aspects of spinal development that are most frequently encountered when evaluating the spines of children. Therefore this section begins with a brief review of some of the most common types of developmental anomalies. This is followed by a discussion of the normal development of the atypical vertebrae (C1 and C2), the relatively common developmental anomalies that occur in these vertebrae, and the potential consequences of these anomalies. The last topic is a brief description of the development of the ring apophyses in typical vertebrae. These structures can be misinterpreted as fractures on x-rays if the normal time sequence of their development is not understood (Fesmire & Luten, 1989).
Developmental Anomalies of the Spine
There are many different types of developmental anomalies of the spine. However, the majority of the spinal anomalies seen on children’s x-rays are within the following categories:
This section briefly discusses these anomalies.
Hemivertebra, or “half vertebrae,” is a relatively common vertebral anomaly (see Fig. 12-14, A and B) in which the left or right half of the vertebral body, and possibly the posterior arch of the same vertebrae, fails to develop (see Chapter 12). If the sclerotomes migrate properly, the next step of development is for a portion of the migrated sclerotomes to develop into the “prototypes” of vertebra, while the remaining portions develop into the ligaments (including the anulus fibrosus) between adjacent vertebrae. This process of part of the sclerotomes becoming bony vertebrae and the other portions becoming ligamentous is known as segmentation; this process allows the development of movable segments. If segmentation does not occur properly, then ossification continues along several bony segments without joints forming between the affected segments. The result is known as block vertebra (see Fig. 12-14, E and F). Lack of segmentaion in the cervical region is known as Klippel-Feil syndrome. See Chapter 12 for further details of Klippel-Feil syndrome.
In addition, variations of segmentation occur at the superior and inferior extremes of the vertebral column. These variations lead to anomalies of the occiput-C1 region and the L5-S1 region, and are discussed in Chapters 5 and 7, respectively, and also in Chapter 12.
Other developmental anomalies seen on pediatric spinal x-rays frequently are related to ossification centers: too many primary centers of ossification, too few primary centers of ossification, or asymmetry of the primary centers of ossification.
Unique Development of the Atypical Vertebrae
The first and second cervical vertebrae are atypical, even in the adult, and their development is atypical as well. The main, clinically relevant features of the development of these two important vertebrae are discussed next.
The Atlas
The first cervical vertebra (the atlas, or C1) develops from three primary centers of ossification: one in the anterior arch, and one in each lateral mass (Fig. 13-9). During development, these centers grow toward one another until the entire vertebra is ossified. The primary center of ossification in the anterior arch does not develop into a vertebral body, as is the case in other vertebrae (see Chapter 12). In addition, this ossification center does not appear until the first postnatal year. It fuses with the two lateral masses at 7 to 9 years of age (Fesmire & Luten, 1989). Sometimes there is no primary center of ossification within the anterior arch. When this occurs, bone grows anteriorly from the ossification center in each lateral mass. Fusion then may eventually occur at the center of the anterior arch. An anterior cleft remains in the anterior arch when the bone growth from the two lateral centers does not meet anteriorly (Fesmire & Luten, 1989). The incidence of cleft of the anterior arch is approximately 0.1%. Anterior clefts are usually associated with a cleft of the posterior arch. If initial imaging is conducted as a result of trauma, the combination of anterior and posterior cleft anomalies can resemble a compression fracture of the atlas caused by rapid loading on the vertex of the skull (Jefferson fracture) (de Zoete & Langeveld, 2007).
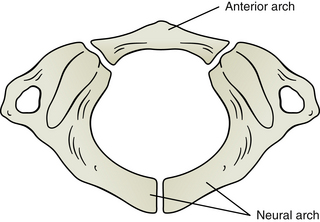
FIG. 13-9 Development of the atlas (first cervical vertebra, C1). The atlas develops from three primary centers of ossification: one that will become the anterior arch and one for each side of the neural arch. These latter centers will form the majority of the lateral masses and the posterior arch. The ossification center in the anterior arch appears during the first postnatal year and fuses with the centers in the lateral masses between 7 and 9 years of age. The left and right ossification centers of the neural arch will fuse posteriorly to complete the posterior arch of the atlas between the ages of 4 and 7 years. Failure of these latter two ossification centers to fuse posteriorly is termed agenesis of the atlas.
Normally the chondrification centers and then the primary centers of ossification for each lateral mass grow posteriorly and the ossification centers fuse in the center of the posterior arch at 4 to 7 years of age (Fesmire & Luten, 1989). A common anomaly of C1 (4% of the population) is a lack of fusion of the posterior arch of the atlas (de Zoete & Langeveld, 2007; Doukas & Petridis, 2010). Such lack of fusion is termed agenesis of the atlas. As with the the combination of anterior and posterior clefts discussed above, agenesis of the atlas alone can resemble a compression fracture of the atlas (Jefferson fracture), particularly if the initial imaging is conducted following trauma (Doukas & Petridis, 2010). Agenesis of the atlas has been statistically related to Arnold-Chiari malformation, or a descent of the cerebellar tonsils past the foramen magnum. For this reason a careful neurologic examination should be performed when an incidental finding of agenesis of the atlas is found on x-ray. Other anomalies related to C1 are too many primary centers of ossification or asymmetric primary centers of ossification.
A direct relationship exists between age and the concavity of the superior articular processes (SAPs) of C1, with SAP concavity being less in children and greater in adults. Approximately 90% of the concavity is achieved by the age of 8 years; however, the curvature continues to increase throughout life. The lack of SAP concavity in infants and young children is thought to decrease stability of the upper cervical region, making children more vulnerable to damage of the upper cervical ligaments, and potentially the spinal cord, during flexion-extension (whiplash) types of injuries, including shaken baby syndrome (Hallgren, Cattrysse, & Zrull, 2011).
Previously some investigators believed that because the nervous system is more fully developed than the skeletal system at birth, the spinal cord of newborns and young children might be more susceptible to compression at the C1-2 level than in adults. However, Jauregui et al. (1993), making measurements from MRI scans, found the midsagittal diameter of the vertebral canal at C1 in neonates to be 12.4 mm, leaving ample space for the spinal cord (13 mm is the accepted value in adults). They also found that the vertebral foramen of the atlas, the atlanto-odontoid interspace, and the space available for the spinal cord underwent accelerated growth until the age of 5 years, allowing for Steel’s rule of thirds for the vertebral foramen at C1 to be roughly maintained (i.e., one third of the foramen filled with spinal cord, one third with odontoid, and one third being “free space”). Recall from Chapter 5 that some ligamentous laxity in the upper cervical region is normal in children and the atlanto-odontoid interspace, which normally is less than 3 mm in adults, can be 3.5 to 5 mm in children 8 years old or younger (Fesmire & Luten, 1989).
The Axis
The second cervical vertebra (the axis, or C2) has an atypical development (Fig. 13-10). The axis has the same three primary centers of ossification that correspond with the primary centers of typical vertebra (i.e., one in the vertebral body and one on each side of the posterior [vertebral] arch). The two ossification centers in the posterior arch normally fuse posteriorly in the midline at 2 to 3 years of age. The same two centers of ossification fuse anteriorly with the body of C2 at 3 to 6 years of age (Fesmire & Luten, 1989). However, in addition to the primary centers of ossification found in typical vertebrae, the odontoid process (dens) of the axis (C2) has two primary centers of ossification of its own. These two ossification centers begin to fuse with one another by the eighth month of fetal development (Fesmire & Luten, 1989), but this newly united ossification center of the odontoid process does not begin to fuse with the body of C2 until 2 to 4 years of age. The region of fusion between the odontoid and the body of C2 is known as the dentocentral (subdental) synchondrosis (Fig. 13-11). Fusion usually occurs here along the outer rim of the odontoid process, leaving a cartilaginous remnant of an IVD within the confines of the rim of bone between the odontoid process and the body of C2. The rim of bony fusion around the subdental synchondrosis fuses by 4 years of age in 50% of individuals, and should be fused in all individuals by 6 years of age (Lefebvre et al., 1993); however, a remnant of nonunion may persist until late childhood, and can be seen on many anatomic specimens and x-rays until 11 to 12 years of age (Fesmire & Luten, 1989). This line of nonunion frequently is mistaken for a fracture, and it can be seen on x-ray throughout life in one third of the population. The centrally located subdental synchondrosis may never completely ossify and can be seen prominently on midsagittal cervical MRI scans of many individuals throughout their lifetimes (Fesmire & Luten, 1989).
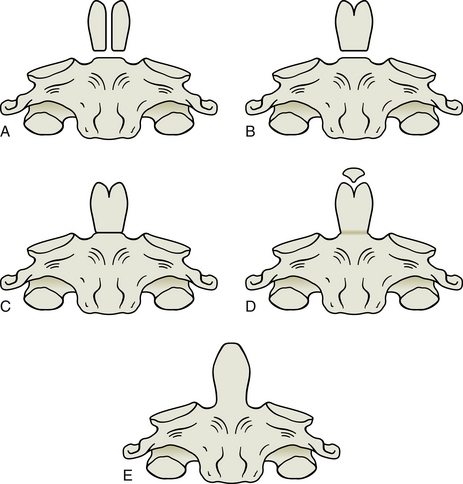
FIG. 13-10 Development of the odontoid process of the axis (second cervical vertebrae, C2). The odontoid process develops from two primary centers of ossification and one secondary center. The two primary centers are shown in A, before they have fused in the midline, which is depicted in B. The odontoid process has begun to fuse with the body of C2 in C. The secondary center of ossification, know as the ossiculum terminale, is beginning to develop in D. This usually occurs between 3 and 6 years of age. The ossiculum terminale normally fuses with the remainder of the odontoid, E, by approximately 12 years of age. Notice that E also shows that the odontoid has completed its fusion with the body of C2. Lack of fusion of the odontoid with the body of C2 (persistence of what is shown in B) is termed os odontoideum, and lack of fusion of the secondary center of ossification is logically termed persistent ossiculum terminale (persistence of what is shown in D).
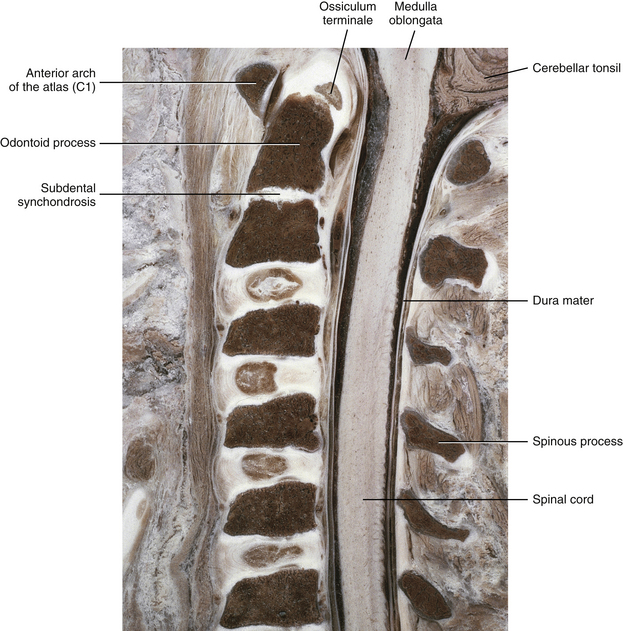
FIG. 13-11 Midsagittal section of the cervical region in a 10-year-old spine. Notice the unfused subdental synchondrosis. Also notice that the ossiculum terminale has not yet fused with the remainder of the odontoid process. This is normal; the ossiculum terminale usually fuses by 11 to 12 years of age. (From Yu S et al. [1989]. Comparison of MR and diskography in detecting radial tears of the annulus: a postmortem study. AJNR Am J Neuroradiol, 10[5], 1077-1081.)
There are times when the odontoid process never fuses with the body of C2, a condition known as os odontoideum (Figs. 13-10 through 13-12). However, some authors are convinced that most cases of os odontoideum are the result of a postnatal fracture (Lefebvre et al., 1993). This condition is discussed in detail in Chapters 5 (The Cervical Region) and 12 (Development of the Spine and Spinal Cord). Of particular clinical importance is that a cartilage bridge can exist between the unfused odontoid and the body of C2. Such a bridge adds stability to the unfused odontoid. However, this cartilage bridge does not always remain firmly connected between the odontoid and the body of C2, and in other cases the cartilage bridge can fracture. A surgical attachment is frequently indicated if no firm cartilaginous attachment exists.
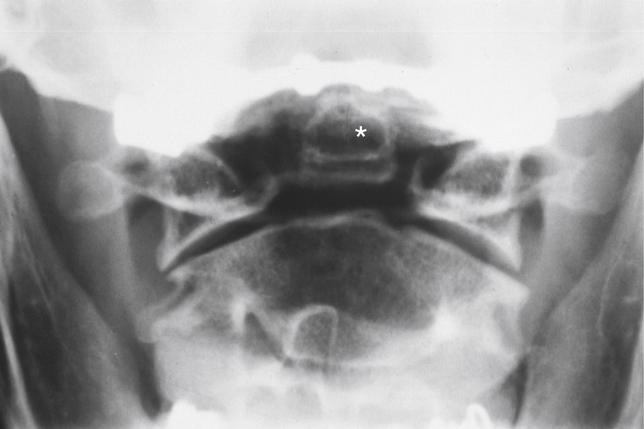
FIG. 13-12 Anteroposterior open-mouth cervical x-ray showing an os odontoideum (asterisk). Notice that the odontoid process lacks a bony attachment to the body of C2.
A secondary center of ossification called the ossiculum terminale develops at the tip of the odontoid process (Fig. 13-13; see also Figs. 13-10 and 13-11). The ossiculum terminale first appears at 3 to 6 years of age and completes its fusion with the rest of the odontoid by approximately 12 years of age (Fesmire & Luten, 1989). Sometimes the ossiculum terminale does not fuse with the odontoid; this is termed persistent ossiculum terminale. This condition can be confused with a type I odontoid fracture (see Chapter 5 and Fig. 5-13). Careful examination of the margins between the free tip, or what on x-ray appears to be the free tip of the odontoid, and the remainder of the odontoid process usually reveals either roughened edges on the adjacent surfaces of the odontoid and the ossiculum terminale, indicating a type I odontoid fracture, or smooth edges between these surfaces, indicating a true persistent ossiculum terminale.
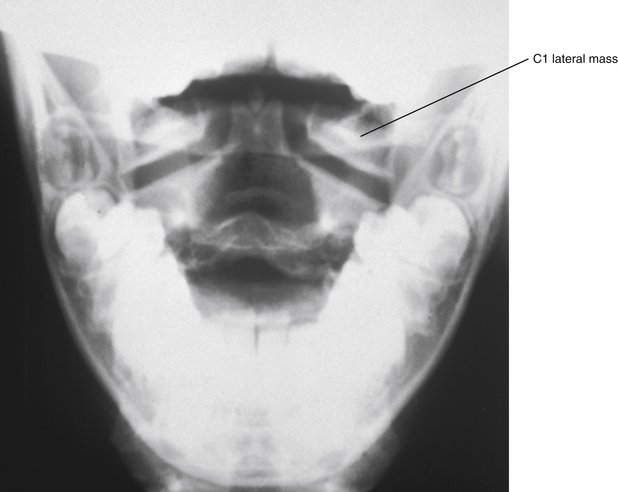
FIG. 13-13 Anteroposterior open-mouth cervical x-ray. Notice the odontoid process is apparent between the left and right lateral masses of the atlas (C1). The ossiculum terminale can be seen at the superior aspect of the odontoid.
Both the dentocentral synchondrosis and ossiculum terminale frequently can be seen on x-rays and MRI scans of the upper cervical region, and can be identified mistakenly as fractures or other pathology if the clinician does not remain aware of the patient’s age and the normal development of this vertebra.
Key Feature in the Development of Typical Vertebrae
The anular (ring) apophyses appear on typical vertebrae just before puberty. The ring apophyses are secondary centers of ossification, located along the superior and inferior rims of the vertebral bodies (see Fig. 12-11). They do not extend all the way to the edge of the vertebral bodies; this lack of extension to the edge of the vertebral bodies is apparent on x-rays. They first appear on x-rays as early as 7 years of age in some children, and their x-ray appearance can be mistaken for chip fractures (Fesmire & Luten, 1989). Although these structures do not fuse with the remainder of the vertebral body until approximately 25 years of age, they become difficult to see on x-rays after the late teenage years. Recall that further ossification and growth of the vertebral body do not occur once the ring apophyses fuse with the remainder of the vertebral body.
Location of the Conus Medullaris on Magnetic Resonance Imaging Scans of Children
MRI scans allow clear visualization of the spinal cord. Because of this, understanding the normal position of the spinal cord at different stages of childhood development becomes important. The conus medullaris (inferior-most part of the spinal cord) extends inferiorly to the second sacral segment in fetuses, but the rapid growth of the bony vertebral column during the fetal period causes the vertebral column to become longer than the spinal cord. Consequently, at full-term birth the conus medullaris is usually at the level of the L1-L3 lumbar vertebral bodies, and within the next several months to 2 years the spinal cord comes to lie at the level of the first to the second lumbar vertebral body, where it remains throughout adulthood (Macdonald et al., 1999; Malas et al., 2000; Schenk et al., 2006) (Fig. 13-14).
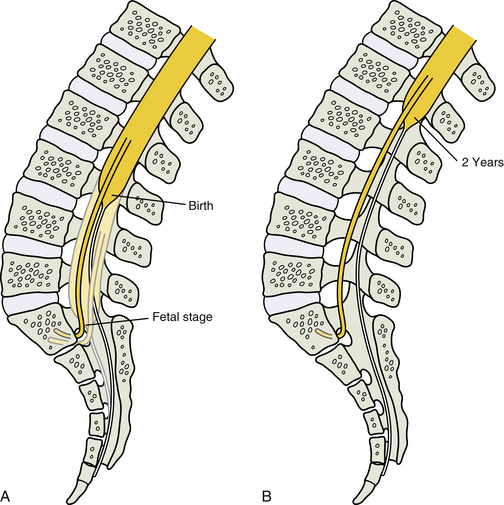
FIG. 13-14 Relative elevation of the conus medullaris during late fetal stage and early childhood development. A, The conus medullaris extends to the second sacral segment in fetuses, but the rapid growth of the bony vertebral column during the fetal period causes the vertebral column to become longer than the spinal cord. At birth the conus medullaris is usually at the level of the third lumbar vertebral body (although it can be higher in some instances). B, Within the next several months to 2 years the spinal cord comes to lie approximately at the level of the first-second lumbar vertebral body.
Common Pediatric Disorders Related to the Anatomy of the Spine
Spondylolysis and Spondylolisthesis
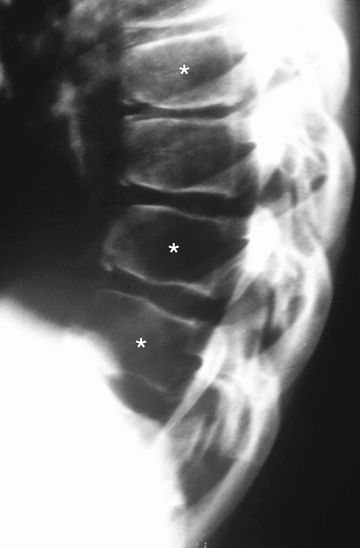
Although spondylolysis and spondylolisthesis can occur in the cervical region of children (Legaye & Horduna, 2009), these conditions are much more common in the lumbar spine; consequently, spondylolysis and spondylolisthesis are discussed in detail in Chapter 7. These clinical entities are mentioned here to highlight their occurrence in children. Spondylolysis, with or without spondylolisthesis, is the most common cause of low back pain in adolescents and in children more than 10 years of age (Faingold et al., 2004). These conditions probably occur with some frequency in young children as well (Fig. 13-15). The youngest reported case of spondylolisthesis was that of a 4-month-old infant, and there have been several reported cases of this condition in infants less than 2 years of age (Fig. 13-16). However, pain from this condition is uncommon before the age of 9 (Lucey & Gross, 1995). As discussed in Chapter 7, the lack of pain in early cases of spondylolysis is probably because the nerves innervating the posterior vertebral arch do not extend to the pars interarticularis until after the age of 9 years. Consequently, in young children spondylolisthesis usually first manifests as variations of gait (wide-based, shuffling, or spastic), and then usually is identified on standard x-rays (Lucey & Gross, 1995). Because changes of gait also can be caused by neurologic and orthopedic disorders of the pelvis, hip, and lower extremities, neurologic and orthopedic conditions, along with spondylolysis and spondylolisthesis, should be included in the differential diagnosis of infants with gait disorders with or without back pain.
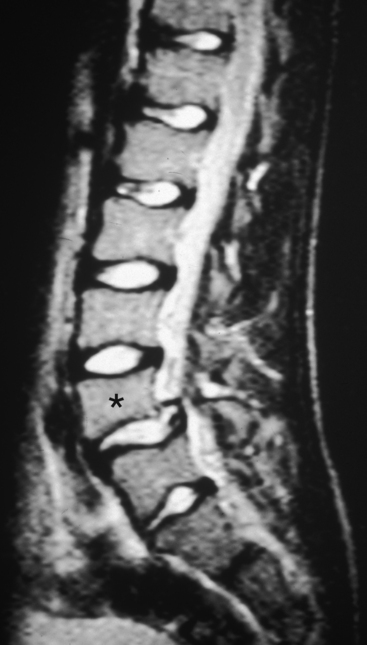
FIG. 13-15 Lumbar magnetic resonance imaging scan showing spondylolisthesis of L4 (asterisk on vertebral body of L4) in a 13-year-old boy.
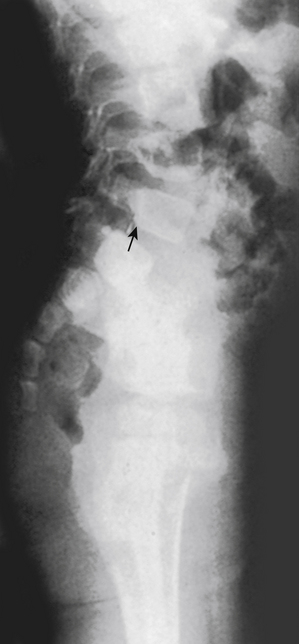
FIG. 13-16 X-ray showing spondylolisthesis of L5 (arrow) in a 2-year-old girl. (From Lucey SD & Gross R. [1995]. Painful spondylolisthesis in a two-year-old child. J Pediatr Orthop, 15, 199-201.)
Miyake and colleagues (1996) found that defects of the pars interarticularis in children resulted in retarded growth of the related articular processes and Z joint. When this occurred at L5, the result was more coronally facing L5-S1 articular facets that were more planar (i.e., the S1 superior articular facet was less concave and the L5 inferior articular facet was less convex) than normal.
The Normal Development of the Thoracic and Lumbar Curves and Scoliosis
A discussion of scoliosis can be found in Chapter 6. However, because idiopathic scoliosis is most commonly identified in the preteen or early teen years, the normal development of the thoracic and lumbar curves and scoliosis (Fig. 13-17) are briefly mentioned here.
Recall that the thoracic kyphosis normally increases during childhood, peaking at approximately 13 years of age. The lumbar lordosis decreases with age, becoming the smallest at approximately 14 years of age (Nissinen et al., 1995). Much individual variation is seen in the development of these curves. Idiopathic scoliosis can develop before and during this time period, and lateral curves developing during the preteen and teenage years should be monitored closely.
Scheuermann’s Disease
A more thorough discussion of Scheuermann’s disease is also found in Chapter 6. This, too, is a condition of adolescence and should be considered when prolonged pain in the thoracic region, with or without pain in the upper lumbar region, is experienced in this age group. Recall that the hallmark of this condition is the irregular appearance of the bony end plates as seen on lateral x-rays (Fig. 13-18).
References
Beer, S.J., Menezes, A.H. Primary tumors of the spine in children. Spine. 1997;22:649–659.
Burton, A.K., et al. The natural history of low back pain in adolescents. Spine. 1996;21:2323–2328.
Crelin, E.S. A scientific test of the chiropractic theory. Amer Sci. 1973;61:574–580.
de Zoete, A., Langeveld, U.A. A congenital anomaly of the atlas as a diagnostic dilemma: a case report. J Manipulative Physiol Ther. 2007;30:62–64.
Doukas, A., Petridis, A.K. A case of aplasia of the posterior arch of the atlas mimicking fracture: review of the literature. Clin Anat. 2010;23:881–882.
Faingold, R., et al. Imaging of low back pain in children and adolescents. Semin Ultrasound CT MR. 2004;25:490–505.
Fesmire, F.M., Luten, R.C. The pediatric cervical spine: developmental anatomy and clinical aspects. J Emerg Med. 1989;7:133–142.
Giles, L.G.F. A histological investigation of human lower lumbar intervertebral canal (foramen) dimensions. J Manipulative Physiol Ther. 1994;17:4–14.
Hallgren, R.C., Cattrysse, E., Zrull, J.M. In vitro characterization of the anterior to posterior curvature of the superior articular facets of the atlas as a function of age. Spine J. 2011;11:241–244.
Hewitt, W. The intervertebral foramen. Physiotherapy. 1970;56:332–336.
Jauregui, N., et al. Surgically related upper cervical spine canal anatomy in children. Spine. 1993;18:1939–1944.
Jeffries, L.J., Milanese, S.F., Grimmer-Somers, K.A. Epidemiology of adolescent spinal pain: a systematic overview of the research literature. Spine (Phila Pa 1976). 2007;32:2630–2637.
Kanna, P.R., Shetty, A.P., Rajasekaran, S. Anatomical feasibility of pediatric cervical pedicle screw insertion by computed tomographic morphometric evaluation of 376 pediatric cervical pedicles. Spine (Phila Pa 1976). 2011;36:1297–1304.
Kasai, T., et al. Growth of the cervical spine with special reference to its lordosis and mobility. Spine. 1996;21:2067–2073.
Kraemer, J. Presidential address: natural course and prognosis of intervertebral disc diseases. Spine. 1995;20:635–639.
Krishna, M., Upadhyay, S.S. Increased limb lengths in patients with shortened spine due to tuberculosis in early childhood. Spine. 1996;21:1045–1047.
Lefebvre, S., et al. Unstable os odontoideum in young children. J CCA. 1993;37:141–144.
Legaye, J., Horduna, M. Cervical spondylolysis in a child: a case with hypermobility. Spine J. 2009;9:e15–e19.
Lucey, S.D., Gross, R. Painful spondylolisthesis in a two-year-old child. J Pediatr Orthop. 1995;15:199–201.
Maat, G.J.R., Matricali, M., van Persijn van Meerten, E.L. Postnatal development and structure of the neurocentral junction. Spine. 1996;21:661–666.
Macdonald, A., et al. Level of termination of the spinal cord and the dural sac: a magnetic resonance study. Clin Anat. 1999;12:149–152.
Malas, M.A., et al. The relationship between the lumbosacral enlargement and the conus medullaris during the period of fetal development and adulthood. Surg Radiol Anat. 2000;22:163–168.
Maxfield, B.A. Sports-related injury of the pediatric spine. Radiol Clin North Am. 2010;48:1237–1248.
Meijer, G.J., Homminga, J., Hekman, E.E., et al. The effect of three-dimensional geometrical changes during adolescent growth on the biomechanics of a spinal motion segment. J Biomech. 2010;43:1590–1597.
Miyake, R., et al. Pathogenesis of sports-related spondylolisthesis in adolescents. Radiographic and magnetic resonance imaging study. Am J Sports Med. 1996;24(1):94–98.
Nissinen, M., et al. Left handedness and risk of thoracic hyperkyphosis in prepubertal schoolchildren. Int J Epidemiol. 1995;24(6):1178–1181.
Salminen, J.J., et al. Low back pain in the young. Spine. 1995;20:2101–2108.
Salminen, J.J., Erkintalo-Tertti, M.O., Paajanen, H.E.K. MRI findings of lumbar spine in the young: correlation with leisure time physical activity, spinal mobility and trunk muscle strength in 15-year-old schoolchildren with or with out low back pain. J Spinal Disord. 1993;6:386–391.
Schenk, J.P., Herweh, C., Gunther, P., et al. Imaging of congenital anomalies and variations of the caudal spine and back in neonates and small infants. Eur J Radiol. 2006;58:3–14.
Taimela, S., et al. The prevalence of low back pain among children and adolescents. Spine. 1997;22:1132–1136.
Ursu, T.R.S., Porter, R.W., Navaratnam, V. Development of the lumbar and sacral vertebral canal in utero. Spine. 1996;21:2705–2708.
Vialle, L.R., Vialle, E. Pediatric spine injuries. Injury. 2005;36(Suppl 2):B104–B112.