Microscopic Anatomy of the Zygapophysial Joints, Intervertebral Discs, and Other Major Tissues of the Back
Microscopic Anatomy of the Zygapophysial Joints
Zygapophysial Joint Articular Cartilage
Zygapophysial Joint Articular Capsule
Supporting Cells and Extracellular Matrix of Connective Tissue: Functional Components
Microscopic and Molecular Structure of the Intervertebral Discs
Much of the current anatomic research related to the spine is concerned with the zygapophysial (Z) joints and the intervertebral discs (IVDs). The gross anatomy of these structures is covered in detail in Chapter 2. The characteristics of these structures unique to the cervical, thoracic, and lumbar regions are covered in Chapters 5, 6, and 7, respectively. Because much of the current investigation related to the Z joints and IVDs has been carried out in the lumbar region, Chapter 7 describes the Z joints and IVDs in significant detail. However, a considerable amount of the research on these two tissues is associated with their microscopic anatomy and molecular structure. The results of these investigations provide a greater understanding of normal, as well as pathologic, structure and function at the microscopic, ultrastructural (electron microscopic), and molecular levels.
As more information becomes available on the precise composition and arrangement of the tissues of normal and diseased Z joints, IVDs, fascia, and other tissues of the back, a better understanding of the biologic basis for current treatment develops. Continued investigation should lead to an increase in the understanding of spinal dysfunction. With changing concepts on the mechanisms of spinal dysfunction, new therapeutic approaches will undoubtedly emerge, and it will be necessary to keep abreast of these changing concepts to be able to effectively apply the new therapeutic approaches. Therefore an understanding of the microscopic anatomy of the tissues of the spine is extremely important to the clinician and researcher alike.
The purpose of this chapter is to provide the reader with comprehensive information on the microscopic anatomy of the Z joints and IVDs. A discussion of the normal composition of connective tissue in general is also included. In addition, detailed descriptions of fascia and also of hyaline cartilage and fibrocartilage in association with the Z joints and IVDs are provided. Finishing out the chapter is a brief overview of the microscopic anatomy of the other major tissues in the region of the back.
Microscopic Anatomy of the Zygapophysial Joints
Bones in contact with one another are held together by connective tissue. This union forms a joint that, in some instances, is freely movable and lined by a synovial membrane. This type of joint is known as a synovial (diarthrodial) joint. The Z joints of the spine are of this type. The joints between contiguous vertebral bodies are formed by the IVDs and are classified as symphysis (symphyseal) joints. Symphysis joints are a type of cartilaginous joint or amphiarthrosis. The IVDs are tough, cushionlike pads consisting mainly of connective tissue, more specifically, specialized fibrocartilage. The IVDs are discussed later in this chapter.
As with all diarthrodial joints, the articular surfaces that form the Z joints are covered with shiny hyaline cartilage. This cartilage is lubricated by synovial fluid that allows the bones to glide smoothly over each other with minimal friction (Swann et al., 1974). A tough sleeve of dense connective tissue envelops the articular cartilages and joint cavity of the Z joints posteriorly. This connective tissue sleeve is known as the fibrous capsule. Anteriorly the ligamentum flavum takes the place of the articular capsule of the Z joint (Xu et al., 1991). A thin inner layer of highly vascularized connective tissue called the synovial membrane lines the joint capsule. Cells within the synovial membrane manufacture the synovial fluid.
This section discusses the microscopic anatomy of the articular cartilage, capsule, and synovial membrane of the Z joints. A working knowledge of connective tissue is important in treating pain of spinal origin because most tissues involved in the formation of the Z joints (and IVDs) are connective tissue, and pain arising from the Z joints is a significant cause of back pain (Mooney & Robertson, 1976; Kirkaldy-Willis, 1988). Therefore a section on connective tissue, including hyaline cartilage, immediately follows this section on Z joints.
Zygapophysial Joint Articular Cartilage
The articular cartilages lining the superior and inferior articular processes of each Z joint are similar in many respects to the articular cartilage associated with most synovial joints of the body. This means that the articular cartilage lining each of the articular processes of a Z joint is composed of a special variety of hyaline cartilage that is durable, lubricated by synovial fluid, compressible, and also able to withstand large compressive forces (Standring et al., 2008).
Recall that hyaline cartilage is not unique to the Z joints but is widely distributed in the body. In addition to lining the articular facets of Z joints, it also is found in a portion of the vertebral (cartilaginous) end plates of the IVDs, the nose, most of the laryngeal cartilages, C rings of the trachea, primary and secondary bronchi, costal cartilages of ribs, most articular cartilages of joints throughout the body, and the xiphoid process. It is also the type of cartilage present in the epiphyseal cartilage plates of growing long bones. Consequently, hyaline cartilage is essential for the growth and development of long bones before and after birth.
The purposes of Z joint articular cartilage are to protect the articular surfaces of the superior and inferior articular processes by acting as a shock absorber and to allow the articular surfaces to move across one another with little friction. Both functions are carried out efficiently. In fact, the coefficient of friction for typical articular surfaces is less than 0.002, which means that the two surfaces of a typical Z joint glide across each other with much greater ease than they would if they were both made of ice (the coefficient of friction for ice sliding on ice is <0.03) (Whiting, 1998).
The articular cartilage of a single Z joint surface is small; in fact, the lumbar articular surfaces measure approximately 8 × 10 mm (Giles, 1992a, b). The Z joint articular cartilage also is approximately 1 to 2 mm thick (Figs. 14-1, 14-2, and 14-3). The concavity of the cartilage on lumbar superior articular facets is thicker than the periphery of the same surfaces. This is the opposite from that typically found in other joints of the body where the concavity of a joint surface usually is lined by thinner cartilage than that surrounding the concavity.
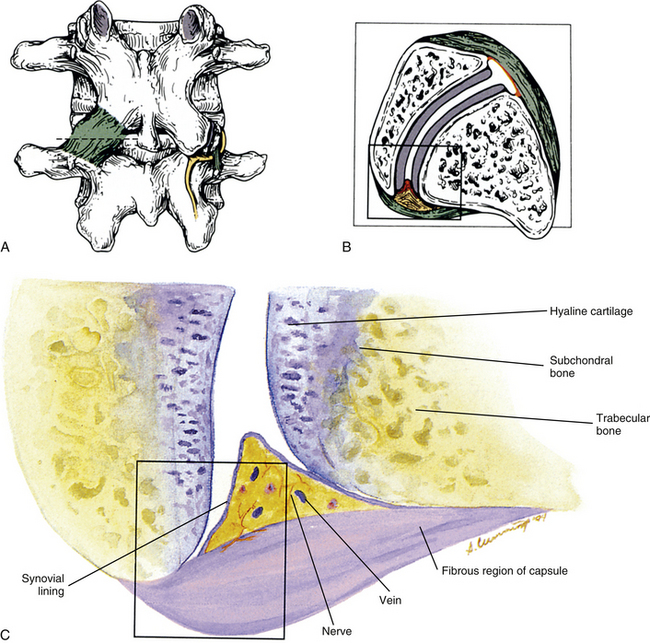
FIG. 14-1 Zygapophysial (Z) joint. A and B, Z joint from a posterior view and a horizontal section, respectively. C, Z joint after magnification by approximately a factor of 10. The articular cartilage, subchondral bone, and articular capsule are prominently displayed. In addition, a Z joint synovial fold is prominent. Notice that the articular capsule has an outer, tough fibrous region. The center of the synovial fold is more vascular and contains adipose tissue. A nerve can be seen passing through this latter region. A synovial lining can be seen on the deep surface of the articular capsule and the synovial fold. The box enclosing much of the synovial fold and a portion of the superior articular process is the region shown at higher magnification in Figure 14-7.
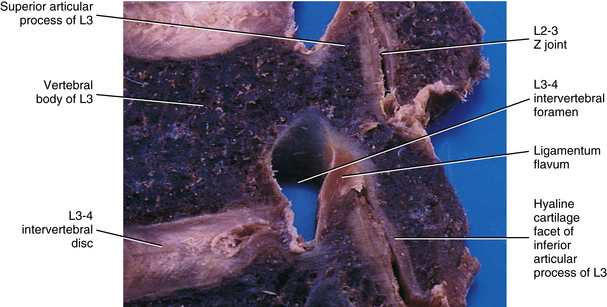
FIG. 14-2 Parasagittal section through the L3 and L4 region of a cadaveric spine. Notice the ligamentum flavum covering the anterior (inner) aspect of the zygapophysial (Z) joint.
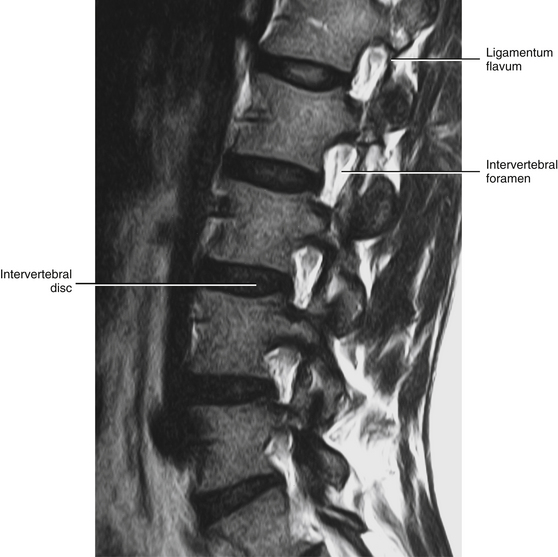
FIG. 14-3 Magnetic resonance imaging scan of the lumbar region performed in a parasagittal plane. The plane of section approximately corresponds to that of Figure 14-2. Notice the intervertebral discs, the intervertebral foramina, and their contents. (Magnetic resonance image courtesy Dr. Dennis Skogsbergh.)
Z joint articular cartilage is made up of 75% water and 25% solids (Giles, 1992a, b) and consists of cells embedded in an abundant and firm matrix (Fig. 14-4). The cells that produce the cartilage matrix are chondroblasts, and in mature cartilage they are known as chondrocytes (Table 14-1). The matrix consists of an intricate network of collagen fibers surrounded by proteoglycans and glycoproteins. The concentration of these constituents of articular cartilage differs from one part of the joint surface to another and also at different depths from the joint surface (Giles, 1992a, b).
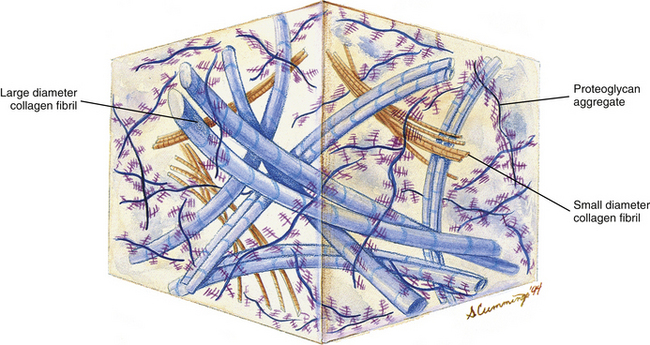
FIG. 14-4 Extracellular matrix of hyaline cartilage. Notice the abundance of collagen fibrils and proteoglycan aggregates.
Fresh hyaline cartilage is bluish white and translucent. In stained, fixed preparations, the matrix appears glassy, homogeneous, and smooth. Distributed throughout the matrix are spaces called lacunae, and within each lacuna is a chondrocyte. As with all articular cartilage, that of the Z joints has no nerve supply and no direct blood supply. Chondrocytes must receive nutrients by diffusion across the cartilage matrix from several sources. These sources include the blood vessels within the synovial membrane that is located along the peripheral margin of the nonarticular portion of the cartilage, the synovial fluid, and the blood vessels in the adjacent bone (Standing et al., 2008).
Chondrocytes are found singly in the lacunae. Commonly, especially in cartilage that is actively growing, the lacunae are grouped into clusters of two or more. These clusters are called cell nests or isogenous cell groups (Fig. 14-5). The cells within the nests have arisen from the mitotic activity of a single chondrocyte; therefore the presence of isogenous cell groups signifies interstitial cartilage growth. This is supported by electron microscopic findings revealing that the chondrocytes within a cell nest exhibit a well-developed rough endoplasmic reticulum, a Golgi complex, and a large amount of glycogen and lipid.
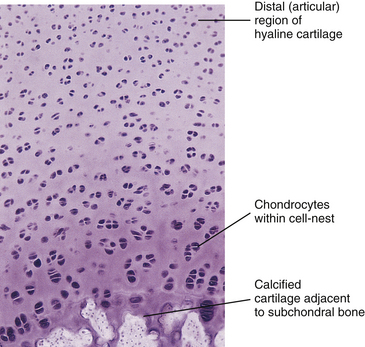
FIG. 14-5 Light micrograph of hyaline (articular) cartilage. The cartilage is from the distal tip of a fetal phalanx (magnification ×100). Developing articular cartilage of the zygapophysial joints is quite similar.
Articular cartilage differs from typical hyaline cartilage in that the articular surface does not possess a covering of perichondrium (Giles, 1992b; Standring et al., 2008). Instead the cells of the articular surface appear flat and are closer together than they are farther within the cartilage matrix. In addition, the matrix of the articular surface becomes dense and fibrous. The collagen fibers, which course perpendicular to the articulating surface from deep within the cartilage matrix, curve as they reach the joint surface and become oriented parallel to the free edge of the articular cartilage.
Cartilage Matrix
The cartilage matrix immediately surrounds the lacunae containing the chondrocytes. The matrix of hyaline cartilage consists of collagen (type II) fibers, a small number of elastic fibers, and an amorphous ground substance. The ground substance consists primarily of proteoglycans and glycoproteins. Each of these components of the cartilage matrix contributes to the strength, longevity, and resilience of this tissue.
Collagen: The collagen component of hyaline cartilage consists of type II collagen fibers. These fibers are relatively thin and course in all directions within the cartilage. Usually they are not visible with the light microscope because they are masked by another component of the cartilage matrix, the ground substance. The collagen fibers can be seen easily with an electron microscope.
Collagen functions to bind the cartilage together, protect the chondrocytes, allow for attachment of the articular cartilage to the subchondral bone, and help resist compressive loads (Giles, 1992a). Because collagen is an important constituent of all the connective tissue components of the Z joints and the IVDs, it is discussed in detail in Supporting Cells and Extracellular Matrix of Connective Tissue: Functional Components.
Ground substance: Chemical analysis of the ground substance of the extracellular matrix of hyaline cartilage reveals that it contains a small amount of glycoproteins and a high concentration of three types of glycosaminoglycans: hyaluronic acid, chondroitin sulfate, and keratan sulfate. The chondroitin and keratan sulfates are joined to a core protein to form a proteoglycan monomer. These macromolecules interact with the collagen and elastic fibers of the hyaline cartilage matrix (Fig. 14-6).
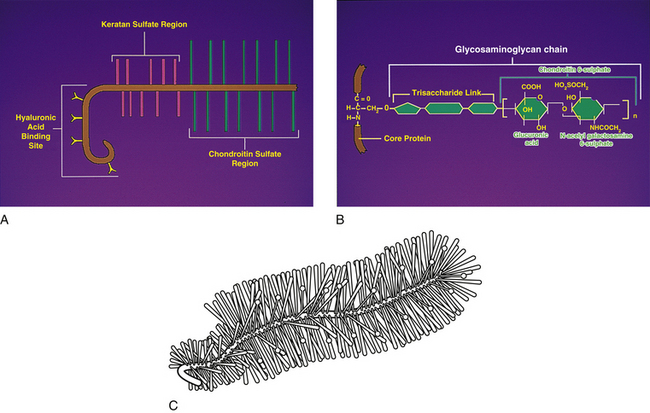
FIG. 14-6 A, Structure of a proteoglycan monomer. Notice several glycosaminoglycan chains (chondroitin sulfate and keratan sulfate) attached to a core protein. The protein molecule can attach to a long hyaluronic acid molecule to help form a proteoglycan aggregate. B, An example of an individual glycosaminoglycan chain, in this case chondroitin 6-sulfate, and its attachment to the core protein. C, The “bottle-brush” appearance of a proteoglycan monomer. (A and B, Courtesy Dino Juarez, National University of Health Sciences.)
The single core protein of the proteoglycan molecule has a molecular weight of 200,000 to 350,000 daltons (Da). The core proteins represent approximately 7% to 12% of the dry weight of cartilage. Bound to each core protein are 80 to 100 chondroitin 4-sulfate and chondroitin 6-sulfate chains, each with a molecular weight of 20,000 Da. These two glycosaminoglycans make up 80% to 85% of the dry weight of hyaline cartilage. In addition, approximately 50 chains of keratan sulfate, each with a molecular weight of 5000 Da, are also attached to the core protein. Keratan sulfate contributes approximately 7% of the total dry weight of hyaline cartilage.
At one end of each core protein is a hyaluronic acid–binding region (see Fig. 14-6). At this site the proteoglycan units are joined to hyaluronic acid molecules to form long proteoglycan–hyaluronic acid (PG-HA) aggregates. The interaction of the proteoglycan monomer with hyaluronic acid is strengthened by the presence of a link protein (see Fig. 14-6). Proteoglycans and glycosaminoglycans are discussed in further detail later in this chapter with regard to the IVD.
Chondronectin is a glycoprotein found in cartilage. Glycoproteins differ from proteoglycans by their low carbohydrate content, different repeating disaccharide units, and the absence of sulfate esters. Chondronectin participates in the adhesion of chondrocytes to type II collagen. Common glycoproteins found in other body tissues include laminin and fibronectin. Laminin is found in basal laminae and is partially responsible for the adhesion of epithelial cells. Fibronectin is found in blood, plasma, fibroblasts, and some epithelial cells and helps to mediate normal cell adhesion and migration (Table 14-2).
Clinical and biomechanical considerations: Normally fluid moves out of articular cartilage when it is compressed and back into the cartilage when the Z joint is distracted. Such movement may help nutrients diffuse through the matrix to the chondrocytes. Articular cartilage can deform considerably when heavy compressive loads are applied to a joint. However, it returns to its previous state when the load is removed. If injured, articular cartilage heals rather slowly (a 1-mm defect heals in approximately 4 weeks). Passive movement of the joint may stimulate cartilage regeneration, whereas immobility results in the development of adhesions. Intermittent light weight-bearing activity does not stimulate cartilage regeneration but does stop the development of adhesions (Giles, 2005).
Articular cartilage becomes yellow, thinner, and more brittle with age, and undulations that may develop into ragged projections appear as a result of “wear and tear” of the joint surface (Standring et al., 2008). Also with age, fissures or cracks may develop in the articular cartilage. The development of such fissures is known as fibrillation of articular cartilage. The fissures may extend from the joint surface to the subchondral bone.
Zygapophysial Joint Articular Capsule
The Z joint capsules attach to the margins of the opposed superior and inferior articular facets of adjacent vertebrae throughout the vertebral column. The capsules are longer and looser in the cervical region than in the lumbar and thoracic regions. The articular capsule of a typical Z joint covers the joint’s posterolateral surface. It consists of an outer layer of white and shining dense fibroelastic connective tissue with bundles of collagen fibers coursing parallel with one another. Deep to the outer fibrous layer is a vascular central layer that is softer and more extensible than the outer layer, and is made up of elastic fibers, similar to the ligamentum flavum, areolar tissue, and loose connective tissue. The third and deepest layer of the Z joint capsule is an inner smooth and shining layer consisting of a white synovial membrane (Giles & Taylor, 1987; Yamashita et al., 1996). The outer, connective tissue layer of the capsule is tough and is essentially composed of parallel bundles of collagen fibers that are primarily oriented in the horizontal plane. A few fibroblasts and fibrocytes and a small amount of ground substance also are found in this layer (see Supporting Cells and Extracellular Matrix of Connective Tissue: Functional Components). The collagen fibers of the capsule attach to the adjacent surfaces of the superior and inferior articular processes, just peripheral to the articular cartilage. In fact, a gradual transition occurs from the joint capsule to fibrocartilage and finally to the articular cartilage of the Z joint. The capsules have a rich sensory innervation, consisting of mechanoreceptors for proprioception and free nerve endings containing substance P for nociception (Giles & Taylor, 1987; Yamashita et al., 1996). However, they have a poor blood supply, which slows the healing of these structures once they are damaged (Giles, 1992b). The multifidus lumborum muscle attaches to the articular capsule, which lies just medial to the primary attachment of this muscle to the mamillary process. The multifidus lumborum muscle may put tension on the capsule and help keep it from being entrapped in the joint space (Taylor & Twomey, 1986).
The posterior and lateral aspect of each lumbar inferior articular process (IAP) has a “lip” that projects further posteriorly than the medial aspect of the IAP, which is more anteriorly located. Consequently, the articular capsule “wraps around” this posterior lip of the lateral aspect of the IAP before attaching to the more anteriorly positioned medial aspect of the IAP. The cervical and thoracic IAPs are oriented differently and do not have this posterior lip; consequently, their capsules do not have a wrap-around component. Boszczyk and colleagues (2001) found that this wrap-around region of the lumbar Z joint capsule was thicker and more fibrocartilaginous in nature (containing type II collagen, aggrecan, and link protein) than the thoracic Z joint capsules, which were found to be thinner and more purely fibrous (rather than fibrocartilaginous) in nature. The entheses (attachment sites) of the lumbar Z joint capsules to the lumbar inferior and superior articular processes were found to have the same fibrocartilaginous composition as the wrap-around portion, indicating that traction forces were placed on the entheses. The authors believed that the costovertebral (costocorporeal) and costotransverse articulations of the thoracic region, along with the spatial orientation of the thoracic articular processes, spared the thoracic capsules from the traction and compressive forces placed on the lumbar Z joint capsules (Boszczyk et al., 2001).
A detailed description of the fiber direction of the outer part of the lumbar Z joint capsules and the clinical significance of the fiber direction in the lumbar capsule is given in Chapter 7.
The articular capsules are thinner superiorly and inferiorly, where they form capsular recesses that cover fat-filled synovial pads. Defects exist within the superior and inferior aspects of the joint capsule and allow for the passage of small nerves and vessels. The synovial joint recesses and the development of synovial joint cysts are discussed in further detail with the lumbar region, where they have been studied the most extensively (see Chapter 7). Also, the specific innervation of the Z joint capsule by the medial branch of the posterior primary division (dorsal ramus) is discussed in Chapter 2.
Ligamentum Flavum
The ligamentum flavum takes the place of the joint capsule anteriorly and medially. As discussed, this ligament passes from the anterior and inferior aspect of the lamina of the vertebra above to the posterior and superior aspect of the lamina of the vertebra below. However, the lateral fibers of this ligament course anterior to the Z joint, attach to its margins, and form its anterior capsule. Synovial extensions, or cysts, protrude out of the Z joint and along the attachment sites of the ligamentum flavum to the adjacent superior and inferior articular processes.
The ligamentum flavum is 80% elastic fibers and 20% collagen fibers. The elastic fibers within the ligamentum flavum prevent it from buckling into the intervertebral foramen (IVF) and vertebral canal, thus sparing the contents of these regions. Degenerative change of the ligamentum flavum can result in elastic fibers being replaced with collagen. This replacement with collagen causes the ligamentum flavum to thicken (up to 10 times its normal width in some instances), which in turn can cause stenosis of the vertebral canal.
However, as described in Chapter 7, many instances of ligamenta flava hypertropy are probably the result of inflammation related to repeated microtears in the ligament. The inflammation then leads to hypertrophic scar formation (fibrosis) (Sairyo et al., 2007).
The ligamentum flavum can also ossify over a long period of time, which can lead to serious vertebral canal stenosis. Ossification of the ligamentum flavum has a higher prevalence in the Asian population than in other racial groups. The ossification process appears to be related to degeneration of the elastic fibers, which in the case of ossification of the ligamentum flavum appears to have a genetic component (Yayama et al., 2007).
Synovial Membrane
The synovial membrane, synovium, or joint lining is a condensation of connective tissue that covers the inner surface of the fibrous capsule, thus forming a sac that encloses the joint cavity (Fig. 14-7). Therefore the region of a diarthrodial joint surrounded by a synovium is known as the synovial, or joint, cavity. The synovium covers the nonarticular bone enclosed within the joint capsule and courses to the margin of the articular cartilage, where a transition zone exists between the synovium and articular cartilage. The synovium does not cover the load-bearing surface of the cartilage. The joint cavity normally contains a small amount of a highly viscous, hyaluronic acid–rich fluid that lubricates the joint surfaces. This fluid is known as synovial fluid and is produced by the cells within the synovial membrane (see Synoviocytes). The major function of the synovial membrane is to produce synovial fluid. Another function is to absorb waste products of metabolism and cellular debris before they can accumulate in the Z joint cavity.

FIG. 14-7 Portion of the zygapophysial (Z) joint at a magnification of approximately ×40. The region here is shown by the box in Figure 14-1. Portions of the articular cartilage, subchondral bone of the superior articular process and mamillary process, the articular capsule, and the Z joint synovial fold can be seen.
The innermost portion of the synovium is composed of one to three layers of specialized cells, known as synoviocytes or synovial lining cells. These cells form the intimal layer. Beneath this layer is a loose network of vascular areolar connective tissue that contains a rich blood supply. This layer is known as the synovial subintimal layer. It possesses many elastic fibers that probably serve to keep the synovium taut and prevent it from buckling into the joint cavity. The synovium is innervated by sensory nerve endings.
Typically projections of the synovial layer extend into the synovial cavity as Z joint synovial folds (Giles, 1992a). Their purpose is to fill in the small gaps along the periphery of the joint, where the articular cartilages of the opposing surfaces do not normally come in contact with one another. These folds also produce synovial fluid and provide an efficient mechanism for the distribution of this fluid directly into the joint cavity.
Z joint synovial folds contain a relatively large amount of adipose tissue at the region of their attachment to the fibrous layer of the articular capsule. They possess a nociceptive sensory nerve supply of free nerve endings containing substance P (Giles, 1987), and at times they may extend a considerable distance into the joint, in which case their central tips usually are fibrous. Entrapment of these folds between and extrapment of them peripheral to the articular surfaces of the Z joint have been implicated as possible causes of back pain (Mooney & Robertson, 1976; Giles & Taylor, 1987; Bogduk, 2005). Giles (1992a) also states that traumatic synovitis of these folds may cause the release of pain-mediating agents and subsequent back pain.
Synoviocytes
Transmission electron microscopy studies reveal that a discontinuous layer of cells, known as synoviocytes, lines the free surface of the synovial membrane. Although synoviocytes resemble other connective tissue cells, they differ from ordinary fibroblasts (see Table 14-1) in their ultrastructural features and metabolic activities.
Synoviocytes have been classified into two types based on their cellular morphologic structure: fibroblast-like cells, or type A synoviocytes, and type B synoviocytes. Type A synoviocytes are somewhat numerous and are characterized by the presence of abundant cytoplasmic organelles such as endoplasmic reticulum. These cells are involved in secretion and are believed to synthesize hyaluronic acid and glycoproteins (see following discussion). Type B synoviocytes are similar to macrophages and are involved in phagocytosis. Types A and B synoviocytes are not connected by junctional complexes and do not rest on a basement membrane; therefore they do not constitute an epithelial lining of the joint cavity, although they do create a smooth secreting surface for the synovium. Small folds of synovium, or synovial villi, can be found periodically along the surface of the synovial membrane.
The synovial fluid produced by the type A synoviocytes is rich in hyaluronic acid and also contains protein, although its protein content is less than that of blood plasma (Triano, 1992; Standring et al., 2008). The hyaluronic acid imparts synovial fluid with great viscosity. Coiling of the hyaluronic acid molecules and interlocking between different molecules allow the synovial fluid to act as a shock absorber during compressive loads. However, during shear forces the coiled hyaluronic acid molecules straighten and the interlocking between molecules decreases, resulting in smooth, low-friction movement between the adjacent Z joint surfaces.
Supporting Cells and Extracellular Matrix of Connective Tissue: Functional Components
Because the Z joints are composed of connective tissue, a brief discussion of the normal characteristics of this type of tissue is essential for a complete understanding of the structure and function of the Z joints. Therefore this section discusses the cellular and extracellular components of connective tissue.
Early Connective Tissue (Mesenchyme)
The connective tissue appearing in embryonic and early fetal development is called mesenchyme. When examined under the light microscope, this type of tissue is seen to be composed of large stellate or spindle-shaped cells that are separated by an abundant amount of intercellular substance. Early embryonic mesenchymal tissue does not contain fiber bundles. Instead, it is composed of fine reticular fibrils (type III collagen) embedded in a gelatinous, amorphous ground substance that is rich in glycosaminoglycans. Embryonic mesenchymal tissue is described as a multipotent or pluripotent tissue. This suggests that mesenchymal cells undergo extensive mitosis and are able to develop into many different types of connective tissue and related cells during fetal and adult life.
Mature Connective Tissue
Connective tissue is responsible for maintaining structural interrelationships between tissues and cells, including the tissues and cells of the spine. All connective tissue is composed of cells, extracellular fibers, an amorphous ground substance, and tissue fluid. The extracellular fibers and ground substance form the extracellular matrix. In contrast to other body tissues (e.g., epithelium, muscle), connective tissue contains fewer cells in proportion to the amount of extracellular matrix. Based on the composition of the extracellular matrix, adult connective tissue is classified into three main types: connective tissue proper, cartilage, and bone. The composition of the extracellular matrix varies among these three types. In connective tissue proper the extracellular matrix is soft; in cartilage it is much firmer, partially calcified, but flexible in nature; and in bone the matrix is rigid because of the presence of calcium salts, which are in the form of hydroxyapatite crystals.
Cartilage and bone are specialized types of connective tissue. Three histologic types of cartilage are encountered based on characteristics of the ground substance matrix:
Hyaline cartilage is discussed in the previous section along with the Z joints, and fibrocartilage is discussed with the IVD. There is no elastic cartilage in spinal tissues; therefore this type of cartilage is not discussed in this chapter.
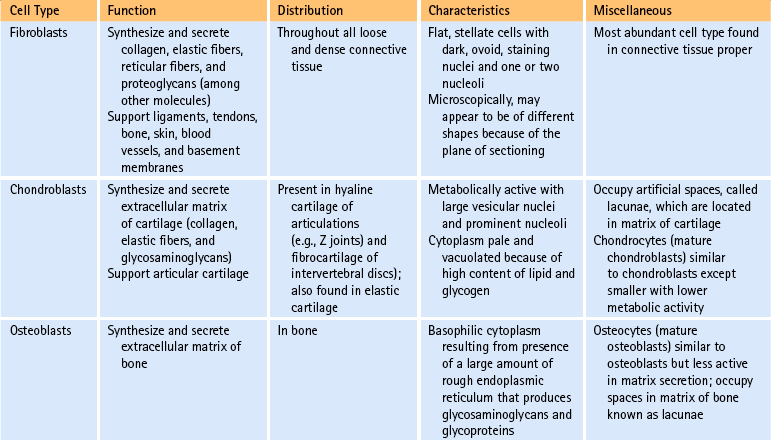
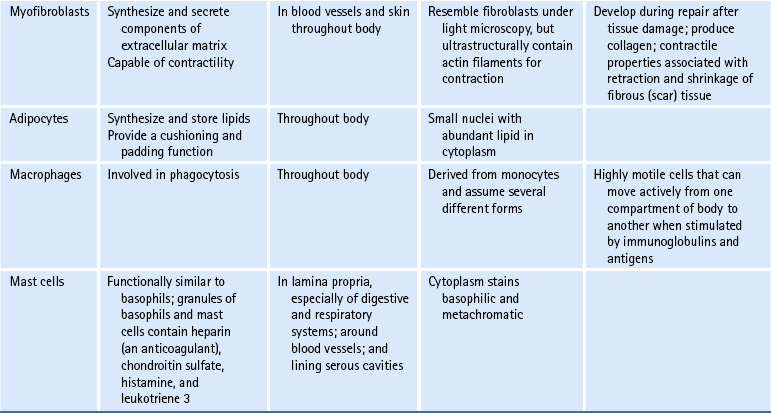
Cells of connective tissue: As mentioned, connective tissue consists of cells, fibers, and ground substance (including water). The type of supportive resident cells found in connective tissue varies considerably and may include fibroblasts; chondroblasts and chondrocytes; and osteoblasts, osteoclasts, and osteocytes. These cells are important when considering the connective tissue of spinal structures. Adipocytes, mast cells, macrophages, and myofibroblasts are also found in connective tissue in various parts of the body. The functions and primary characteristics of these cells are listed in Table 14-1.
In addition to the fixed or resident cells of connective tissue described in Table 14-1, connective tissue also contains transient or immigrant cells. These include all the formed cellular elements found in blood with the exception of erythrocytes. The immigrant cells include the neutrophils, eosinophils, basophils, monocytes, lymphocytes, and plasma cells. When inflammation occurs, these immigrant cells leave the circulation and join fibroblasts and other connective tissue resident cells, such as macrophages. Once in the connective tissue, they fight microorganisms that cause inflammation and clean up (phagocytize) the debris that results from this process.
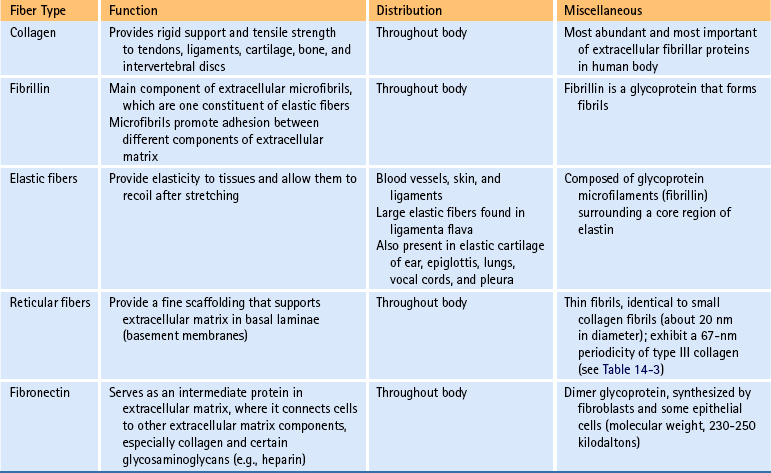
Fibers of connective tissue: The fiber component is another of the three elements of connective tissue. The types of fibers found in connective tissue are collagen, fibrillin, elastin, reticulum, and fibronectin. The functions of each of these are listed in Table 14-2.
Collagen Synthesis
Collagen is the most important fiber type of connective tissue. Collagen is a major component of connective tissue proper, cartilage, and bone. Collagen fibers are found in abundance throughout the articular capsule and hyaline cartilage of the Z joints and also throughout the IVDs. Collagen fibers are composed of collagen macromolecules, which are the most abundant protein in the human body. Collagen fibers are flexible and strong, and they are made up of a bundle of fine, threadlike subunits called collagen fibrils. Collagen is a stable protein under the physiologic conditions that exist in connective tissue; however, collagen is constantly being degraded and replenished by collagen-secreting cells.
It was believed for many years that collagen synthesis occurred primarily in fibroblasts, chondroblasts, osteoblasts, and odontoblasts; however, recent investigations in collagen biology indicate that many other cell types produce this unique protein. Collagen synthesis has been studied extensively in fibroblasts (Standring et al., 2008). Fibroblasts have the extensive rough endoplasmic reticulum and well-developed Golgi apparatus required of cells actively involved in protein synthesis. Labeled amino acids endocytosed by fibroblasts can be followed autoradiographically to the rough endoplasmic reticulum (rER), later to the Golgi complex, then to the outside of the fibroblast, and eventually to the newly formed collagen fibers. This evidence indicates that the collagen synthesis pathway is similar to that of other proteins. Fibroblasts synthesize collagen de novo and secrete it into the extracellular matrix. Fibroblasts also have the ability to break down collagen with specific degradative enzymes called collagenases.
Collagen is a ubiquitous substance that is extremely important in the integrity of both the Z joints and IVDs. Current research and possibly future treatments (nutritional and pharmacologic) related to these two regions of the spine may involve the individual steps of collagen synthesis. Because of the clinical importance of collagen synthesis, this pathway is discussed briefly here.
Collagen synthesis begins inside cells. However, the final processing and assembly into fibers takes place after collagen building blocks have been secreted outside the manufacturing cells. The intracellular events include synthesis of proalpha chains in the rER, hydroxylation and glycosylation of proalpha chains into triple helices in the Golgi apparatus, and formation of secretory granules (vesicles). The extracellular events include cleavage of extension peptides, fibrillogenesis and cross-linking, and assembly of fibrils into mature fibers (Fig. 14-8). Box 14-1 shows the events (steps) involved in collagen synthesis within the fibroblast (steps 1 through 9) and outside the fibroblast in the extracellular matrix (steps 10 through 12).
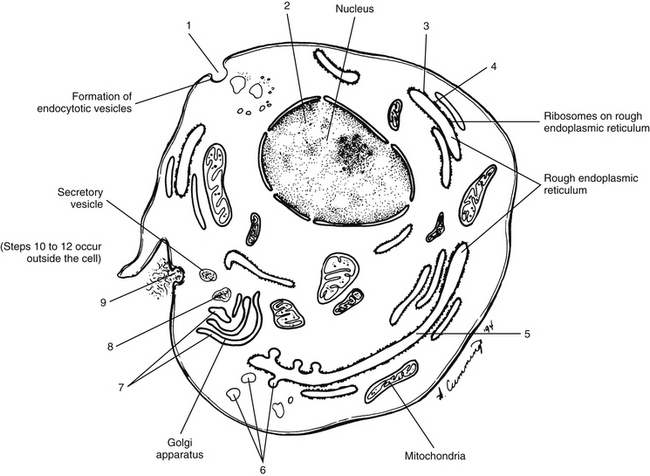
FIG. 14-8 Intracellular steps involved in collagen synthesis. The numbers refer to Box 14-1.
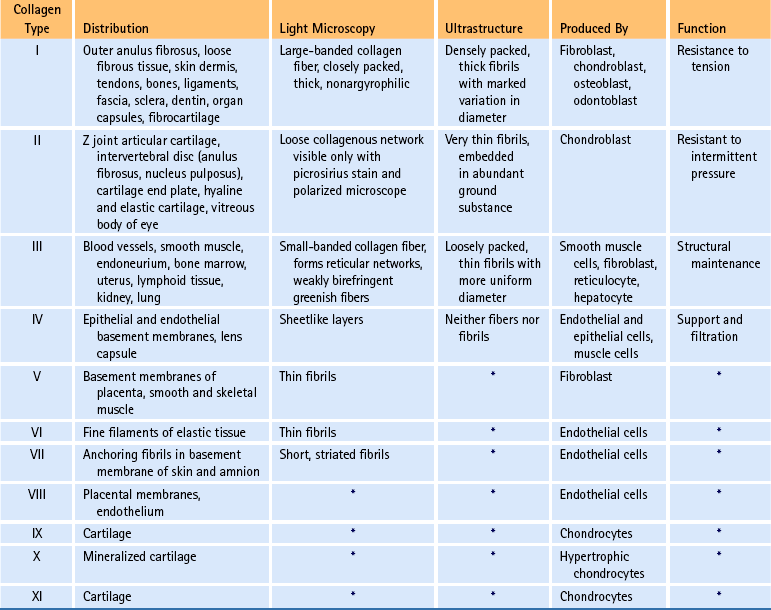
The amino acid composition of collagen is one of the features that make collagen such a unique protein. Four amino acids compose most of the polypeptides in the collagen macromolecules. The principal amino acids that make up collagen are glycine (35%), proline (12%), hydroxyproline (10%), and alanine (11%). In the cytoplasm of the fibroblast, approximately 250 to 300 amino acids are combined by polyribosomes associated with rER to form a polypeptide with a molecular weight of 30,000 Da. This step of translation is performed under the control of messenger ribosomal ribonucleic acid (mRNA). Three polypeptide chains are combined into polypeptide alpha triple helices with a molecular weight of approximately 100,000 Da. These triple helices are released into the cisternae of rER (see Box 14-1, steps 1 through 3). Glycine is the third amino acid in each alpha chain of the newly formed triple helix. The amino acid after glycine frequently is proline, and the amino acid preceding the glycine frequently is hydroxyproline. Differences in the chemical structure of the alpha chains are responsible for at least 19 different types of collagen identified to date (Ross et al., 2003). Specifics of the 11 most common types of collagen can be found in Table 14-3.
Several modifications of the polypeptide chains occur within the cisternae of rER and the Golgi apparatus (see Box 14-1, steps 4 to 6, and Fig. 14-8). Disulfide bonds are formed within each polypeptide chain and between adjacent chains. Vitamin C is necessary for the formation of the disulfide bonds, and its absence results in certain types of collagen-related diseases such as scurvy. This bonding gives shape and stability to the triple-helix collagen macromolecule. The structure formed now constitutes a procollagen molecule. The procollagen molecule moves to the exterior of the cell via secretory granules (see Box 14-1, steps 7 to 9, and Fig. 14-8). Further modifications are made outside the cell. For example, enzymes cleave most of the uncoiled amino acids, thereby converting procollagen to tropocollagen molecules. These eventually aggregate to produce collagen fibrils (see Box 14-1, steps 10 to 12). Cross-links between lysine and hydroxylysine are then formed, giving the molecule its tensile strength. Changes in collagen cross-links have been seen in IVD degeneration (Duance et al., 1998).
The tropocollagen molecules are 300 nm long and 1.5 nm in diameter. They consist of three polypeptide chains that are twisted around one another to form a right-handed superhelix with a head and a tail end. Numerous tropocollagen molecules lie end-to-end and also in parallel chains or rows. All the molecules face the same direction, and approximately one fourth of the length of the tropocollagen molecule overlaps between the parallel rows. Therefore a tropocollagen molecule of one row ends approximately one fourth of the distance along the length of another tropocollagen molecule of an adjacent row. This configuration results in a regular 64- to 67-nm periodicity that is clearly visible on an electron micrograph. Figure 14-9 shows collagen fibers within the IVD.
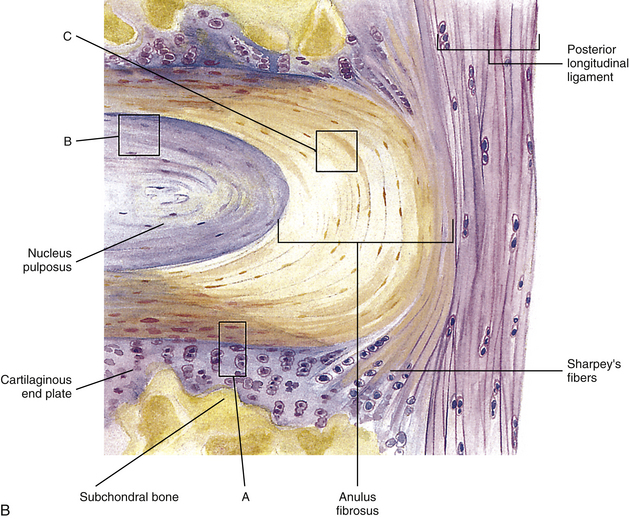
FIG. 14-9 A, Sagittal section of two adjacent vertebrae and the intervertebral disc between them. B, Illustration of the boxed region in A at higher magnification (magnification ≈×15). In addition to showing the anulus fibrosus (AF), nucleus pulposus, and cartilaginous end plate, the vertebral body and posterior longitudinal ligament are shown. Notice that the outer fibers of the AF attach to the cortical and subchondral bone of the vertebral body. These attachment sites are known as Sharpey’s fibers. The collagen fibers of the inner layers of the AF enter the end plate and curve to run parallel to the discal surface of the vertebral body. (The boxes labeled A, B, and C refer to the regions shown in Figure 14-10.)
The finest strand of collagen that can be seen with the light microscope is the fibril, which is approximately 0.2 to 0.3 µm in diameter. A fibril is made up of still smaller units that have a diameter of 45 to 100 nm. These are called microfibrils. Newly formed microfibrils are only approximately 20 nm in diameter, and evidence shows that they increase in size with age. Most microfibrils are visible only with the electron microscope and demonstrate the characteristic cross-banding with a periodicity of 64 to 67 nm. The parallel assembly of microfibrils forms fibrils. The fibrils in turn aggregate in bundles to form the thicker collagen fibers. These fibers have a diameter ranging from 1 to 12 µm or more.
Types of Collagen
At present, 19 different types of collagen have been positively identified. They are designated as types I through XIX. Types I to V are the most abundant types of collagen. Types VI to XIX are considered less important because they occur in small quantities. Several of the minor types of collagen (types VI, IX, X, XI, XII, and XIV) are present in small amounts in the IVD (Duance et al., 1998). Table 14-3 lists the characteristics of the 11 most important types of collagen.
Types I, II, and III are arranged as ropelike fibrils and are the main forms of fibrillar collagen. Type I collagen consists of two alpha-1 chains and one alpha-2 chain and represents 90% of all collagen fibers distributed in connective tissue. Because type I fibers resist tensile stresses, their orientation and cross-linking vary according to the local environment. Type I collagen is found in bone, tendon, and the anulus fibrosus (AF) of the IVD. It is also found in the skin and cornea (see Table 14-3).
Type II collagen fibers are small, banded fibrils averaging 20 nm in diameter. They help to form the extracellular matrix of hyaline cartilage, including that of the Z joints and cartilaginous end plates (CEPs) of the IVDs. Type II collagen is the main type of collagen found in the nucleus pulposus (NP) of the IVD. It is also found in elastic cartilage and the cornea and vitreous body of the eye. These fibers demonstrate a high electrostatic attraction for the chondroitin sulfate glycosaminoglycans. Type II collagen contains a higher degree of lysine hydroxylation than type I collagen.
Types III and IV collagen are well distributed throughout the body but are not found to any great extent in Z joints, IVDs, or other spinal tissues, although type III has been found in regions adjacent to spondylosis (Schollmeier, Lahr-Eigen, & Lewandrowski, 2000). The key features of these fibers and collagen types V through XI are listed in Table 14-3.
Ground Substance
The cells and fibers of connective tissue are surrounded by a translucent, fluidic, homogeneous, gel-like matrix called amorphous ground substance (Bloom & Fawcett, 1986). The ground substance exhibits no structural organization that is visible with light microscopy. Extracellular amorphous ground substance plays a vital role in the regulation of tissue nutrition, support, and maintenance of proper water content. Based on chemical analysis, the extracellular ground substance of connective tissue has the physical properties of a viscous solution or thin gel and consists of proteoglycans and glycosaminoglycans of various types. Proteoglycans and glycosaminoglycans are an important part of the hyaline cartilage of the Z joints and the cartilaginous (vertebral) end plates of the IVDs. They are also being studied with regard to the AF and NP of the IVD. Therefore glycosaminoglycans and proteoglycans are discussed in further detail with the articular cartilage of the Z joint (see previous discussion) and with the IVD (see following discussion).
Microscopic and Molecular Structure of the Intervertebral Discs
Symphyseal joints unite the vertebral bodies, and these joints are made up of the IVDs. The IVDs permit a limited amount of movement between the vertebral bodies while maintaining a union of great strength. The intrinsic stability of the motion segment (two adjacent vertebrae and the ligaments, including the disc, between them), and therefore of the whole spine, results mainly from the IVDs and the ligaments associated with them (Bogduk, 2005). The paraspinal and trunk muscles provide the spine’s extrinsic stability.
IVDs (see Fig. 14-9) are important parts of the spinal column and play an active and important role in the spine’s physiologic function. The physical properties, elasticity, and resiliency of the IVDs allow them to give support to the spine and allow motion to occur between adjacent vertebral segments, while also preventing too much motion from occurring between the same segments. The IVDs also allow the spine to return to its original shape after being compressed or stretched (Chai & Tang, 1987).
The IVD consists of three main parts: the outer AF, which consists of a series of fibrocartilaginous rings (except in the cervical region, where it is a solid, crescent-shaped, fibrocartilaginous structure); the inner gelatinous NP; and the CEPs of hyaline-like cartilage. The end plates are located between the bony vertebrae and other parts of the IVD (Ghosh, 1990).
Each IVD is reinforced peripherally by circumferential ligaments (see Fig. 14-9, A). A thick anterior longitudinal ligament extends down the anterior aspect of the spinal column and is attached to the vertebral end plates. It provides additional anterior support to the AF. A thinner posterior longitudinal ligament spans across the posterior aspect of each disc and is firmly attached to the IVD’s posterior aspect.
The IVD is specialized connective tissue designed to provide strength, mobility, and resistance to strain. All three parts of the IVD listed previously (NP, AF, and CEP, Fig. 14-10; see also Fig. 14-9, B) consist of water, cells, proteoglycans (PGs), and collagen. These components are found in varied concentrations in the three different regions of the disc. In fact, the varied concentrations of these basic components within the IVD make it a specialized type of connective tissue. For example, after an early age (≈2 years) the disc has no blood supply (except for the vessels within the vertebral bodies that are adjacent to the CEPs and remain until 11 to 12 years of age), and so receives its nutrition from the adjacent vertebral bodies. The PGs are essential in the process of attracting fluid and nutrition to the IVD from the adjacent vertebral bodies. The PGs are negatively charged and attract Na+, which then attracts water and other nutrients by osmotic flow. The PGs have been found to actually regulate the amount and type of molecules entering the IVD. Breakdown of PG molecules in the IVD has been associated with the decreased fluid and increased tissue breakdown found in disc degeneration. PGs are important to the health and treatment of the IVD. Because chondroitin sulfate is a major component of the PGs, this substance and a related molecule, glucosamine sulfate, are frequently given as part of the conservative treatment of disc degeneration.
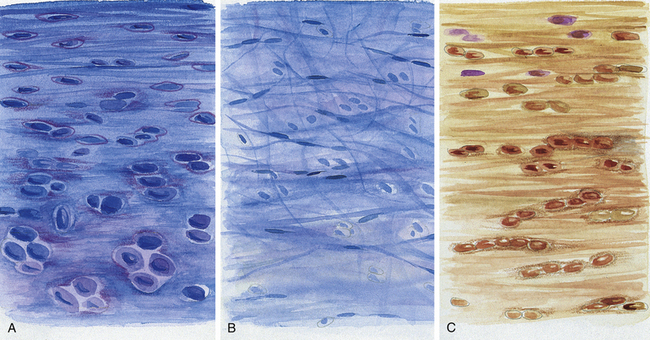
FIG. 14-10 Regions of the intervertebral disc. A, B, and C correspond to the respective lettered boxes of Figure 14-9, BA, Cartilaginous end plate. B, Nucleus pulposus. C, Anulus fibrosus. (A, B, and C represent a magnification ≈×100.)
Collagen is another of the important components of the IVDs. As mentioned, the main type of collagen in the AF is type I, and is a ropelike molecule that is tough and strong and gives the AF its ability to withstand the forces and loads placed on the IVD. The NP has a higher concentration of the less–well-organized type II collagen.
Cells (primarily fibroblasts and chondrocytes) make up the third important component of the IVD. Cells manufacture the collagen, as well as the PGs, of the disc. Because the CEP of the IVD has the greatest number of cells, this region may serve as the “manufacturing plant” for the important PGs and collagen of the IVD. The CEP also is permeable to fluids, thus allowing the fluids and metabolites from the adjacent vertebral bodies to diffuse into the NP and AF. The CEP has been found to be most permeable in its center, the part adjacent to the nucleus pulposus, and the NP has the greatest amount of fluid and hydrostatic pressure (fluid pressure) of the three parts of the IVD.
The hydrostatic pressure just mentioned is maintained by the fourth important constituent of the IVD: water. The water of the disc gives the disc the hydrostatic pressure needed to resist compression, while also allowing for adequate intersegmental movement. Remarkably the hydrostatic pressure of the IVD is also important in resisting too much movement between vertebrae. Too much movement, of course, would damage the articular processes and possibly the neural elements housed within the vertebral canal.
Therefore each of the building blocks of the IVD is important and clinically relevant. In addition, each of the four constituents is closely related to the others. For example, the collagen fibers within the IVD become taut during movements of the spine and tend to restrain the PGs. The PGs in turn allow the IVD to deform. Because of its ability to absorb fluid (swell) and then to maintain its hydration (water), the PG gel of the NP is able to resist compression under large external loads (Weiss, 1988). The cells in turn maintain the proper levels of PGs and collagen fibers. Therefore the IVD is able to act as a relatively thick lubricating pad that prevents adjacent vertebrae from being eroded by abrasive forces during movement of the spinal column. The hydrated gelatinous NP serves to a certain extent as a shock absorber to reduce the impact between adjoining vertebrae (Mescher, 2010), although the vertebral body is primarily responsible for this function.
The histologic changes that take place in the IVD with advancing age have been described in postmortem studies by several investigators (Brown, 1971; Pritzker, 1977; Roberts et al., 1989, 1996; Boos et al., 2002). These changes include loss of distinction between the NP and AF, desiccation and fibrosis of the NP with fibrillation of the matrix, brown discoloration of the nucleus, fissuring of the nucleus and AF, fractures of the vertebral end plate, and formation of osteophytes.
Figure 14-11 demonstrates a series of events associated with degeneration of the IVD. Based on plain x-ray films, the fundamental diagnostic features of disc degeneration are reported to be disc space narrowing and osteophytosis. Decreased hydration as demonstrated by a decreased signal intensity of the IVD on T1- and T2-weighted magnetic resonance imaging (MRI) scans is also an indication of IVD degeneration. The section entitled Normal Aging of the Intervertebral Discs and Intervertebral Disc Degeneration discusses the changes of IVD degeneration in further detail. In addition, Chapter 7 describes the consequences of these changes and the development of internal disc disruption. Chapter 2 describes the gross anatomic features of the IVD and the clinical relevance of these features, and Chapter 11 discusses IVD bulging, protrusion, and extrusion and their effects on the neural elements within the vertebral canal (i.e., cauda equina and dorsal root ganglia).
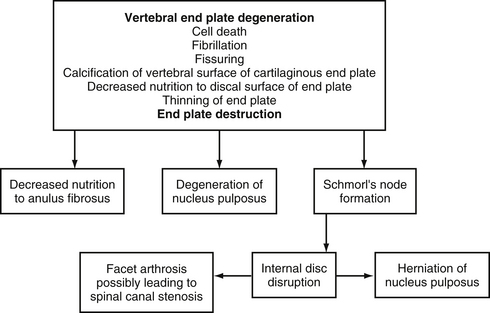
FIG. 14-11 Flowchart demonstrating a series of events leading to degeneration of the intervertebral disc.
The next section of this chapter focuses on the typical microscopic anatomy and the composition of the AF, NP, and cartilaginous vertebral end plate (CEP). This section concludes with subsections covering IVD aging and degeneration, PGs, and fibrocartilage. These last three subsections have been included for readers interested in acquiring a deeper understanding of the biology of the IVD.
Anulus Fibrosus
The AF is the rigid, outer series of rings (lamellae) that forms the peripheral portion of the IVD (Figs. 14-12 and 14-13). It functions to absorb pressure from the central well-hydrated (jellylike) NP. The tightly packed collagen fibers of the AF normally do not allow the large PG molecules of the NP to pass between them, even when the IVD is subjected to large compressive forces. The adult AF is not distinctly separated from the NP or cartilage of the vertebral end plates (Inerot & Axelsson, 1991).
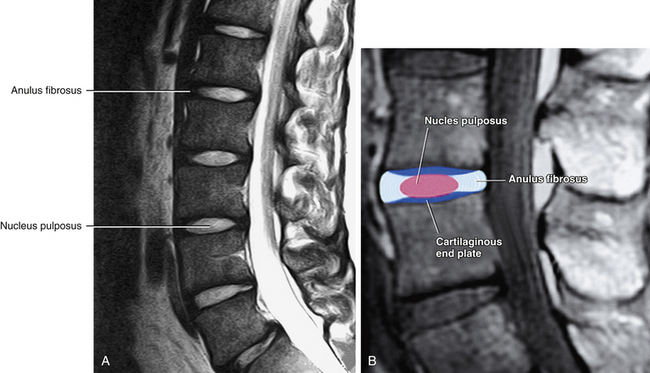
FIG. 14-12 A, Midsagittal magnetic resonance imaging scan of the lumbar region showing the intervertebral discs with adjacent vertebral bodies. B, Similar view with the parts of the intervertebral disc labeled. (Magnetic resonance images courtesy Dr. Dennis Skogsbergh.)
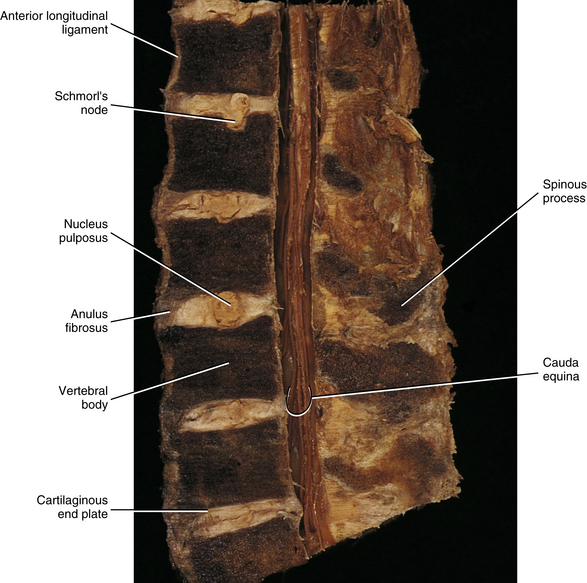
FIG. 14-13 Midsagittal section through a cadaveric lumbar spine. Notice that the cartilaginous end plates, the anuli fibrosi, and the nuclei pulposi can be seen at several levels. Also notice the Schmorl’s node (intravertebral herniation), which has been labeled. (Compare with Figs. 14-15 and 14-16.)
The outer ring of the AF consists of an external tough layer of dense collagenous connective tissue, whereas the remainder of the AF is primarily composed of overlapping concentric layers of fibrocartilage. The outer part of the AF attaches to the margins of adjacent CEPs in infancy and childhood and to the outer rims of adjacent vertebral bodies (region of the anular apophyses) in adolescence (see Fig. 14-9, B, Sharpey’s fibers). The attachments of the AF to the anular (ring) apophyses are considered to be a part of the intervertebral disc (Fardon, 2001).
Light and electron microscopy indicate that a typical lumbar AF is composed of fibrocartilage and has a lamellar structure. Anteriorly the AF consists of more than 20 moderately thick lamellae. The outer lamellae are entirely fibrous and contain thick, tightly packed bundles of type I collagen fibers (Ghosh, 1990; Schollmeier, Lahr-Eigen, & Lewandrowski, 2000). Although the outer AF is composed of type I collagen (see Table 14-3), the fibers of the inner AF are composed of type II collagen (Bishop, 1992; Schollmeier, Lahr-Eigen, & Lewandrowski, 2000). The lamellae of the inner part of the AF also have a richer PG ground substance associated with them (greater concentration of PGs in the posterior versus the anterior AF) (Iatridis et al., 2007), which increases the capacity to resist compression (McDevitt, 1988). The collagen fibers in each lamella are orientated parallel to one another and form an angle of inclination (≈25 to 30 degrees) with the horizontal axis of the bony vertebral rims. The fibers of each consecutive layer form approximately a 120- to 130-degree angle with the fibers of adjacent lamellae. The lamellar structure and the angle of inclination of the collagen fibers enable the AF to sustain the normal forces of compression, torsion, and flexion that occur during movements of the IVD (Chai & Tang, 1987). Elastic fibers have also been identified in the outer and inner aspects of the AF and may also play a role in the mechanical properties of the IVD (Yu et al., 2005).
As mentioned, the anterior and lateral parts of the AF are composed of more than 20 moderately thick lamellae. The outer lamellae are loosely attached to the strong anterior longitudinal ligament (Ghosh, 1990). The posterior and posterolateral parts of the AF are much thinner. They consist of 12 to 15 more closely arranged, thinner lamellae that follow the contour of the posterior parts of the adjacent vertebral bodies. The collagen fibers of the outer lamellae of the AF are fused with the lateral margin of the relatively thin posterior longitudinal ligament (Ghosh, 1990). As mentioned, the outer collagen fibers also attach to the posterior vertebral rims. The inner fibers of the AF are continuous with the CEPs (see following discussion and Fig. 14-9, B).
The cells of the AF are primarily chondrocytes. Although they produce cartilage, specific matrix proteins, the chondrocytes of the AF are of a different stage of differentiation than the chondrocytes of the growth plates of bones or of articular cartilage (Poiraudeau et al., 1999).
PG extraction from ground human lumbar AFs suggests that the PGs contain three regions: a chondroitin sulfate–rich region, a keratan sulfate–rich region, and a region that binds to hyaluronic acid (Table 14-4; see also Fig. 14-6). By binding to hyaluronic acid, the PGs are permitted to aggregate into PG macromolecules. Because of the immense clinical importance of PGs as they relate to the IVDs, a section devoted to this topic is found later in this chapter. However, characteristics of PGs specific to the AF are covered here.
Table 14-4
Glycosaminoglycans∗
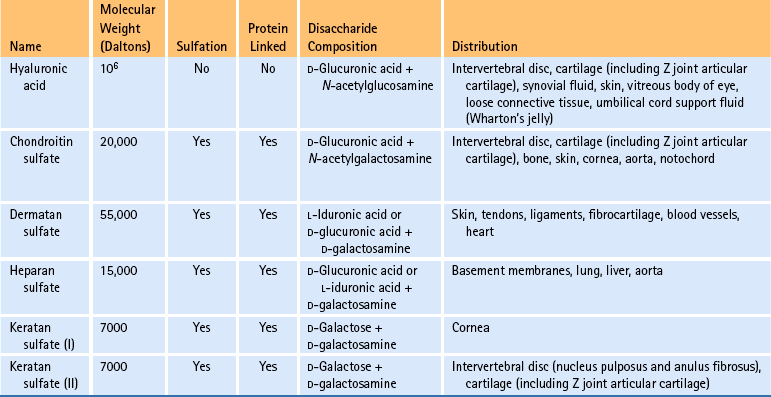
∗Five main groups of glycosaminoglycans with different tissue distributions exist. Chondroitin sulfate exists as chondroitin 4-sulfate and chondroitin 6-sulfate; both possess high levels of interaction with collagen type II. Dermatan sulfate demonstrates low levels of interaction, mainly with collagen type I. Heparan sulfate demonstrates intermediate levels of interaction with collagen types III and IV. Sulfation causes the molecules to be highly negatively charged and contributes to their ability to attract and bind Na+ and water.
Previous investigations of glycosaminoglycans and PGs of the IVDs have found that the PGs from the AF contain approximately 75% chondroitin sulfate and 25% keratan sulfate and hyaluronic acid (Antonopoulos et al., 1974; Stevens, Dondi, & Muir, 1979). These percentages are determined by analyzing the glucosamine/galactosamine ratios (see Table 14-4) (Inerot & Axelsson, 1991). Both the hyaluronic acid and the keratan sulfate concentrations are higher in the AF than in hyaline cartilage (Antonopoulos et al., 1964; Hardingham & Adams, 1976). Also, the keratan sulfate region appears to be larger in AF PGs than in hyaline cartilage PGs. Fibrocartilage of human knee joint menisci has been shown also to contain dermatan sulfate. This molecule has not been detected in the human AF. Biochemically the absence of dermatan sulfate and the presence of types I and II collagen fibers suggest that the AF may be classified as an intermediate between hyaline cartilage and fibrocartilage (Inerot & Axelsson, 1991).
A study of the aging of IVD PG composition of canines and humans has shown that the keratan sulfate–rich region of the PG core protein (Fig. 14-6) is more resistant to proteolysis than the chondroitin sulfate–rich region. In addition, the number of keratan sulfate–rich fragments in human disc tissue increases with aging (Cole, Ghosh, & Taylor, 1986).
Clinical Considerations
Fluid moves in and out of the NP during the day, providing nutrients to the disc. During sleeping hours, the NP fills with fluid and presses against the AF. Therefore when an individual arises in the morning, the AF is tense and less flexible. This increase in AF tension after approximately 5 hours of rest may render it more vulnerable to injury after the rest.
Sudden movements of the lumbar spine, especially torsion coupled with flexion, can produce small tears in the AF. These tears usually occur in the posterior part of the AF, where the distribution of collagen fibers is less concentrated. Sometimes, tears in the AF may allow some of the soft, jellylike NP to squeeze out into the vertebral canal. This latter condition is known as an extruded IVD (see Chapter 11). IVD extrusion is not as common a cause of back pain as once thought (see Chapter 7). However, the discs can be a source of pain without protrusion or extrusion (Bogduk, 1990). Contrary to previous reports (Malinsky, 1959; Wyke, 1987) that the IVD could not produce pain because it lacks nerve supply, several investigators (Yoshizawa, O’Brien, & Thomas-Smith, 1980; Bogduk et al., 1981) have confirmed that the lumbar discs do have a nerve supply and that nerve fibers and nerve endings have been demonstrated to exist in at least the outer third and possibly as far as the outer half of the AF. Most of these authors conclude that the lumbar disc is supplied with the necessary apparatus for the transmission of nociception, resulting in the subsequent perception of pain. Chapters 2, 7, and 11 discuss the gross anatomy, including the innervation, and the clinical relevance of the IVD (including the AF) in further detail.
Nucleus Pulposus
Both fetal and infant discs have large notochordal NPs with abundant fluid mucoid matrices. The nucleus of a young disc is encapsulated along the periphery by the AF and on the superior and inferior surfaces by the CEPs (see following discussion). Perinatally the AF and CEPs are vascular, but their blood supply declines dramatically with childhood growth (Taylor, 1990); by 11 to 12 years of age, even the blood vessels that earlier supplied the IVD by entering the deepest parts of the CEPs from the vertebral bodies cannot be found. In fact, the adult IVD (including the NP) is the largest avascular structure of the body. It receives nutrition primarily by means of diffusion from blood vessels within the subchondral bone of the adjacent vertebral bodies. This diffusion process by which the IVD receives its nutrients is known as imbibition.
The human NP is a highly hydrated tissue at birth, with a water content of 88%. This falls to 69% at 77 years of age. By comparison, the water content of the AF declines from 78% at birth to approximately 70% at 30 years, and thereafter it stays relatively constant (Gower & Pedrini, 1969). In adults, as the hydration declines with age, the tissues become firmer and lose their translucency, and the boundaries between the NP and AF become less distinguishable. Table 2-5 shows the relative concentrations of water, collagen, and PG (nonaggregated/aggregated ratio) of the NP and AF.
The higher water content of the NP, compared with that of the AF, is accompanied by a lower concentration of collagen in the NP. In addition, the collagen found in the NP is type II rather than type I, which is found in the AF. The individual fibrils of type II collagen are much smaller than those of type I (see Table 14-3). The fibers are also loosely arranged and are surrounded by a more abundant ground substance. In the NP, this ground substance contains a high percentage (65%) of hydrophilic, nonaggregated PGs (Iatridis et al., 2007).
Therefore the NP is a thick, jellylike region with a high concentration of fluid. It draws this fluid from the surrounding vertebral bodies. The fluid, a distillate of plasma, passes through the CEPs on its way to the NP. The NP also has relatively few cells. The cells are primarily notochordal cells in the young (see following discussion). These are then replaced by fibroblasts and chondrocytes. The adult NP comprises 35% to 50% of the IVD (Bishop, 1992). It normally lies slightly posterior to the IVD’s center. Normal nuclear material moves backward and forward with flexion and extension movements of the spine, respectively.
The region of the adult NP that is adjacent to the CEPs contains a relative abundance of chondrocytes. The matrix surrounding the chondrocytes stains deeply with safranin and Alcian blue because of the presence of abundant PG macromolecules. Also in this region, vertically oriented collagen fibers extend from the end plate to the NP (Oda, Tamaka, & Tsukuki, 1988). These collagen fibers seem to be independent of the anchoring fibers of the AF. The attachment of these fibers to the end plate and the NP of the IVD may give stability to the IVD at times when the CEP is calcified or replaced by bone.
The Controversial Role of the Notochord in the Formation of the Nucleus Pulposus
As mentioned, the NP is located in the center of the AF and occupies 35% to 50% of the IVD volume. In children the NP is large and is derived from the notochord (see Fig. 12-12). Gradually the transparent embryonic notochordal cells are replaced by a sparse population of chondrocytes and fibroblasts that originate in the CEP (Kim et al., 2003). In time these cells are partially replaced by fibrocartilage, which makes the NP more opaque and no longer transparent (Mescher, 2010).
Several investigators have proposed that embryonic notochordal cells undergo degeneration and disappear soon after birth and that these cells have no further participation in the formation of the NP. Virchow (1857) wrote that the NP was formed from connective tissue. Luschka (1856, 1857) maintained that both the notochordal cells and the liquefaction of the inner layers of the surrounding connective tissue contributed to the formation of the NP. Peacock (1951) concluded that the NP was produced by mucoid degeneration of notochordal cells, which caused the disappearance of these cells and increased the mucoid matrix. However, Woelf and colleagues (1975) studied enzymes present in the NP and described the presence of enzymes that were associated with PG synthesis and oxidative activity. This indication of PG synthesis led them to conclude that human notochordal cells do contribute to the matrix of the NP in fetal and postnatal life.
Oda and colleagues (1988) found that the NP was composed of tissue derived from the notochord in specimens collected from individuals ranging in age from 1 month to the midteens. They also found a fine fibrous tissue in the NP that was derived from the AF. No notochordal cells were demonstrated in the NP in the specimens that came from individuals 16 to 19 years of age. Also, the NP of the specimens had been replaced by fibrocartilage and dense collagenous fibrous tissue. These findings have been supported by those of Boos and colleagues (2002). Pritzker (1977) and Bishop (1992) suggested that the cells originating from the vertebral CEPs might be responsible for synthesizing the gelatinous matrix of the NP in mature IVDs. Kim and colleagues (2003) found in a rabbit model that the NP changes from having notochordal cells to chondrocyte-like cells. These cells were found to migrate from the adjacent CEP. The CEP chondrocytes began to migrate from the peripheral region of the CEP–NP interface; then the process progressed centrally, changing the notochordal NP into a fibrocartilaginous NP. The NP notochordal cells have also been found to stimulate migration of the CEP chondrocytes (Kim et al., 2009). Therefore notochordal cells probably contribute to the formation, development, and maintenance of the NP and to the migration of CEP chondrocytes into the maturing NP. However, this role dramatically declines as individuals reach ages 11-16 years. After this age, the vertebral end plate may continue to help the few cells left within the NP maintain the PG and collagen composition of the NP.
Cartilaginous End Plate
As mentioned, the IVD is composed of a tougher, peripheral fibrocartilaginous AF and a central gelatinous NP, both of which are located between the superjacent and subjacent CEPs (Chai & Tang, 1987) (Figs. 14-12 and 14-13). The adult CEP is a thin strip (≈0.6 to 3 mm thick) of hyaline-like cartilage (it transitions to fibrocartilage as it contacts the NP and AF) that contains many fine collagenous fibrils (similar to fibrocartilage) (Bishop, 1992; Roberts et al., 1996). The CEPs separate the NP and medial aspect of the AF from the subchondral bone of the adjacent vertebral bodies (see Figs. 14-9, 14-12, and 14-13). The subchondral bone of the vertebral bodies consists of a thin peripheral ring of compact bone that surrounds the CEP and a large central region that is cribriform in appearance, containing many small holes that pass to the cancellous bone of the vertebral body.
Developmentally each CEP is a part of the cartilage model of the vertebral body; however, the CEP does not have a firm attachment to the vertebral body. In fact, no fibrillar connections have been found between the CEP and adjacent vertebral body (Bishop, 1992), but the collagen fibers of the AF and NP enter the CEP and become enclosed in the CEP’s ground substance (see Fig. 14-9, B). In addition, the CEP plays a vital role in the nutritional support of the IVD and may be the source of PG synthesis for the NP and AF (Bishop, 1992). Because the CEP is more closely related to the AF and NP than to the subchondral bone of the adjacent vertebral body, usually it is considered to be an integral part of the IVD.
Each CEP is composed of parallel lamellae of cells (primarily chondrocytes) and collagen fibers, arranged horizontally (Ghosh, 1990). As mentioned, the collagen fibers from the AF appear to continue into the CEP at the AF–CEP junction (Roberts et al., 1989). The CEP’s ground substance consists of water within an amorphous matrix of PGs.
The CEPs have important mechanical functions. They contribute to the resilience of the motion segment. In addition, the CEPs participate in the hydrostatic distribution of the pressure absorbed by IVDs during loading (Broberg, 1983).
The CEPs are also thought to play an important role in the IVD’s nutrition. Nutrients must diffuse from the blood vessels within the vertebral bodies, which contact the periphery of each CEP, through the cartilage matrix, eventually to reach the cells deep within the CEP. Only 10% of the adult bony end plate of the vertebral body is perforated by small vascular buds that make contact with the CEP (Maroudas et al., 1975). The vascular contacts are more plentiful in the central part of the CEP than in the peripheral regions (Roberts et al., 1989). The CEP and the NP have both a close anatomic and a close physiologic relationship with each other. The physiologic relationship is demonstrated by the fact that degeneration of a CEP may initiate the “degeneration” of the NP (see Chapter 7).
The ability to transport nutrients through cartilaginous tissues is known to depend on the composition of the cartilage matrix, particularly the PG content of the matrix (Nachemson et al., 1970). The PG content (i.e., types of PGs present) and PG concentration control the diffusion rate and distribution of charged solutes and macromolecules within the cartilage matrix. The CEP near the NP has a higher PG and water concentration than does the CEP adjacent to the AF. The CEP close to the AF (but not overlying it) also has a higher concentration of PGs, as well as a lower concentration of collagen, than the neighboring AF (Roberts et al., 1989). Therefore permeability is enhanced close to the NP and probably tapers off near the periphery of the CEP. If PGs in the CEP degrade (as has been found to occur with age and degeneration) or are lost, solutes can enter the IVD that normally would be excluded (e.g., chemokines or enzymes that could injure the IVD); also solutes (e.g., nutrients) that should remain in the IVD could escape (Roberts et al., 1996). This process also may initiate internal disc disruption (Crock, 1986; Chai & Tang, 1987).
Formation of bone inside the CEP, which occurs in some CEPs of certain individuals, also initiates a reduction of the nutritional route to the IVD as a whole. Bone formation may first cause the destruction of the discal surface of the CEP, which eventually contributes to the degeneration of the NP (Oda, Tamaka, & Tsukuki, 1988).
Detailed histologic changes of the human cervical IVD from the neonate to the ninth decade, with special emphasis on the age changes of the NP and the CEP, were investigated by Oda and colleagues (1988). They found that the CEP can be divided into two regions: the growth cartilage layer, which corresponds to the growth plate of a growing long bone, and the articular cartilage layer, which faces the NP (Fig. 14-14). The articular cartilage layer has been called the “zone of resting cartilage” by other authors (Chandraraj, Briggs, & Opeskin, 1998). Oda and colleagues (1988) also noted a fine fibrous tissue between the material derived from the notochordal cells in the NP and the articular cartilage layer of the CEP of the cervical discs of 1-month-old infants. The CEPs of specimens collected from individuals 1 year old to the teenage years also contained the same two layers (growth cartilage layer and articular layer). The CEPs of specimens from individuals older than 20 years of age had lost the growth layer and were composed only of the articular layer. In people 20 to 30 years of age, the CEPs began to calcify, and the calcified areas were invaded by blood vessels from the adjacent vertebral bodies. Calcification of the vertebral CEP has been related to degenerative change within the IVD as a whole (Bishop, 1992) (see Fig. 14-11).
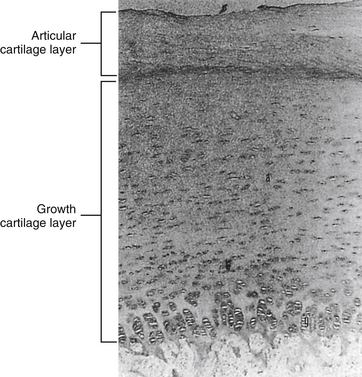
FIG. 14-14 Cartilaginous end plate of a newborn. Notice that it can be divided into two regions: the growth cartilage layer, which corresponds to the growth plate of a growing long bone, and the articular cartilage layer, which faces the nucleus pulposus. (From Oda J, Tamaka H, & Tsukuki N. [1988]. Intervertebral disk changes with aging of human cervical vertebra: from the neonate to the eighties. Spine, 13, 1205-1211.)
End Plate Fracture (Intravertebral Herniation, Schmorl’s Nodes)
Hydrostatic loading of the NP of the IVD causes bulges of the nucleus into the CEP. Fracture of the CEP can occur if the compressive force is great enough. CEP fractures, also known as traumatic intravertebral herniations (Fardon, 2001) or Schmorl’s nodes, have been noted in postmortem studies as features of disc degeneration (Vernon-Roberts & Pirie, 1977; Sachs et al., 1987) (see Fig. 14-13).
Schmorl’s nodes, or intravertebral disc herniations, are defined as herniations of the IVD through the CEP and bony end plate (Figs. 14-15 and 14-16; see also Fig. 14-13). They were first described in 1927 by a German pathologist, Christian G. Schmorl. These lesions are believed to be associated with trauma and occur most frequently in the lower thoracic and lumbar regions. Even though trauma is the most likely cause of Schmorl’s node formation, a possible congenital origin, such as notochordal cell “rests” (i.e., pockets of notochordal cells that remain after they are normally displaced by chondrocytes or osteocytes) within the subchondral bone adjacent to the CEP, also has been suggested (Taylor, 1990; Pate, 1991) (notochordal cell rests can be seen as the white-appearing structures in the region of the vertebral bodies adjacent to the IVDs in Fig. 13-8). Such congenital defects could predispose one to a later CEP fracture.

FIG. 14-15 X-ray demonstrating a Schmorl’s node. (Compare with Figs. 14-13 and 14-16.) (Image courtesy Dr. Dennis Skogsbergh.)
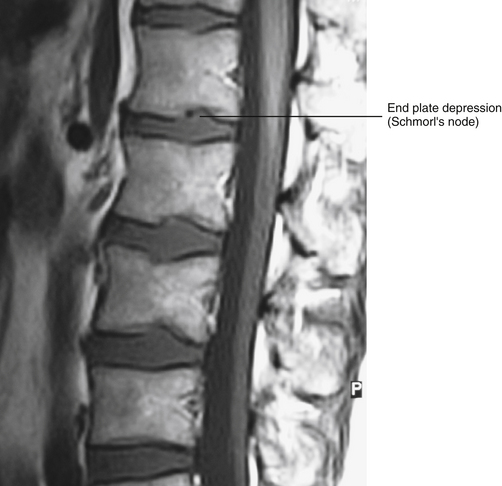
FIG. 14-16 Midsagittal magnetic resonance imaging scan demonstrating a Schmorl’s node. (Compare with Figs. 14-13 and 14-15.) (Image courtesy Dr. Dennis Skogsbergh.)
Reported incidence of Schmorl’s nodes ranges from 38% in radiologic studies to 76% in postmortem studies; however, the incidence varies rather dramatically in different populations. For example, the incidence in the Chinese population is 16.4% (Mok et al., 2010). Schmorl’s nodes are more common in the upper (L1-L2 and L2-L3) versus lower lumbar vertebrae (L3-L4 and L4-L5) and are least common at L5-S1 (Mok et al., 2010). Schmorl’s nodes are thought to occur most commonly between the ages of 20 and 40, when the IVD has a relatively high fluid pressure (Chandraraj, Briggs, & Opeskin, 1998). They are also more prevelent in taller and heavier individuals and in males (Mok et al., 2010).
Because the CEP, particularly the region adjacent to the NP, receives sensory innervation (Edgar, 2007), Schmorl’s nodes secondary to end plate fractures are likely a source of pain. In addition, Schmorl’s nodes probably predispose the IVD to early degenerative change, especially when observed in younger age groups. In fact, a dorsolumbar kyphosis, seen in adolescents, may be associated with Schmorl’s nodes. Therefore CEP fractures should be considered a possible etiologic cause when an active adolescent patient has back pain of the thoracolumbar region.
Compression injury frequently results in an end plate fracture. This may completely resolve in some patients, or in others, inflammatory repair processes may extend into the NP and result in disc degradation (see Chapter 7). Such inflammatory disc degradation initiates internal disc disruption, which may become symptomatic. If the AF remains intact, isolated IVD resorption may follow, but if fissures and tears develop in the AF, the degraded nuclear material may extrude (Bogduk, 1990).
Normal Aging of the Intervertebral Discs and Intervertebral Disc Degeneration
The preceding sections on the three regions of the IVD (AF, NP, and CEP) have hinted at the fact that IVD degeneration is difficult to define, and yet is distinct from the normal aging process of the IVD. Disc degeneration is not easy to define. Adams (2005) states that disc degeneration involves “gross structural changes” of the nucleus pulposus, anulus fibrosus, or vertebral end plate. The hallmarks of disc degeneration are disc space narrowing, loss of fluid pressure, disruption or breakdown of collagen and PGs, sclerosis of the CEP, osteophytosis, marrow changes in the adjacent subchondral bone, and changes in trabeculation of the adjacent subchondral bone (sclerotic changes seen on x-ray) (Adams, 2005; Kuisma et al., 2007). Most of these hallmark signs of IVD degeneration also can occur as part of the normal aging process of the IVD. For these reasons, disc degeneration and normal aging of the disc frequently are discussed interchangeably (Kraemer, 1995; Boos et al., 2002). Understanding these processes is helping researchers and clinicians understand the subtle differences between normal IVD aging and degeneration. Understanding these processes is also helping researchers and clinicians develop novel therapies for treating IVD degeneration (Zhang et al., 2011).
The IVD seems to age differently than other tissues of the body (probably as a result of its avascular nature), and many authors now conclude that the disc is unique in that it begins the degenerative process quite early in life (Videman & Nurminen, 2004), approximately in the second decade or even earlier (Kraemer, 1995). However, there is a wide variation in the aging and degenerative process of the disc; some septuagenarians have the IVDs typical of 30-year-old people, and vice versa.
The first part of this section focuses on the normal aging process of the IVD and the relationship of this aging process to IVD degeneration. The second part of this section concentrates on unique characteristics of IVD degeneration and certain conditions that can promote or initiate IVD degeneration. Figure 14-17 illustrates the changes that take place during aging and degeneration of the IVD.
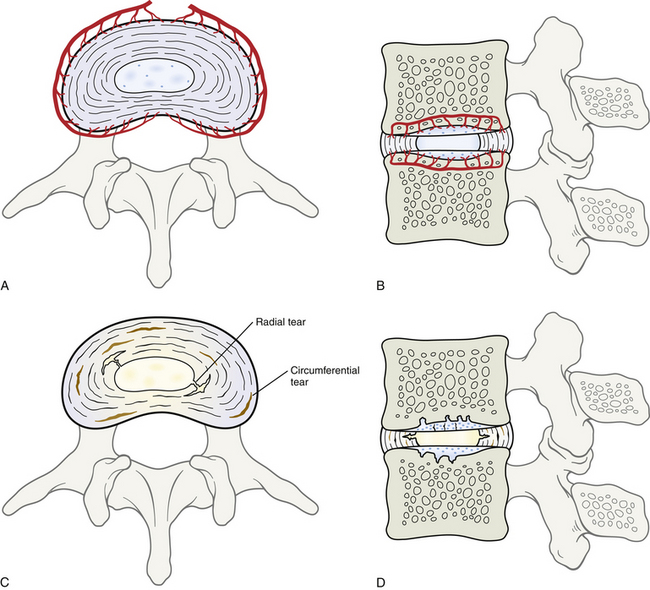
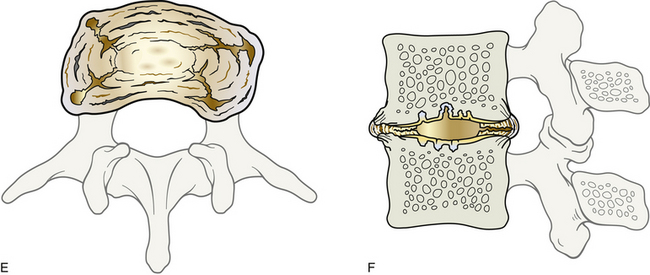
FIG. 14-17 Aging and degeneration of the intervertebral disc (IVD). Illustrations of superior views (left images: A, C, E) and midsagittal sections (right images: B, D, F) of the IVDs at the following three ages: A and B, newborn; C and D, second decade of life; E and F, sixth decade of life. Notice in the newborn that the IVD receives a blood supply. A shows the vessels that supply the periphery of the IVD and B shows those that supply the inner surfaces of the anulus fibrosus (AF), nucleus pulposus (NP), and cartilaginous end plates (CEPs). The large horizontal vascular channels within the adjacent vertebral bodies in B are supplied by ascending and descending branches of the central arterial plexus of the vertebral bodies. This plexus and the ascending and descending branches are shown in Figure 2-4, B. The arteries shown here will regress and become obliterated in the next few years of life. Notice in A that the lamellae of the AF are tightly packed, the NP has a few cells within it (the nuclei are seen as dark blue dots), and the CEPs (above and below the NP and AF in B) are smooth and contain cells (primarily fibroblasts and chondrocytes). The changes shown in C and D can begin as early as the first decade of life, but usually do not become apparent until the second or third decades. The IVD is less well hydrated in C and D as the vessels shown in A and B regress. Some of the lamellae of the AF seen in C are separating to form circumferential tears, and two small radial tears have also begun to protrude through the innermost layers of the AF to connect to circumferential tears. There are very few cells remaining in the NP, and the superior CEP shows early signs of cracking (fissuring). Extensions of the cartilage of the superior and inferior CEPs can be seen plugging the vascular channels left by the obliterated vessels of the vertebral bodies that were shown in B. The IVDs in E and F show dramatic signs of aging and degeneration. Notice that the IVD in E has “spread out” as it dehydrates and is seen bulging in several locations. The IVD in F is shorter from superior to inferior, and the vertebral bodies have developed osteophytes (bone spurs) that are beginning to cover the AF above and below. F also shows Sharpey’s fibers attaching the most peripheral layers of the AF to the adjacent bone of the osteophytes. The border between the AF and NP is indistinct in E and F, and many circumferential tears in the AF are connected by radial tears. This has allowed the NP to move through the radial tears (fissures) and fill the gaps between the lamellae of the AF created by the circumferential tears. Some of the radial tears extend all the way to the periphery of the AF, and some of the circumferential tears show signs of scarring (i.e., the dark, narrow irregular tears). The CEPs in F are much thinner now and have begun to show more significant signs of aging as they become dry and brittle. Notice several regions where the NP has pushed well into the CEPs and is beginning to enter the vertebral body. (Please note: The ages at which the changes depicted in these illustrations occur are quite variable and may not progress to E and F in some individuals or may not be seen at all vertebral levels in others.)
Summary of the Normal Aging of the Intervertebral Disc as a Whole
The normal aging (and degeneration) of the IVD is closely related to the number of vessels that reach the IVD, especially the CEP. As the numbers of vessels decrease, and the nutrition provided and waste removed by these vessels decrease, the changes associated with IVD aging and degeneration increase (Brown et al., 1997; Chandraraj, Briggs, & Opeskin, 1998; Horner & Urban, 2001; Boos et al., 2002) (see Fig. 14-17).
Small vessels surround the IVD during fetal development. These vessels begin to decrease in number during the first 2 years of life, and the remaining vessels that course within the subchondral bone to enter the deepest layers of the CEP are almost completely obliterated by 11 to 12 years of age, leaving the IVD as the largest avascular structure of the body. The first significant degenerative changes in the IVD appear shortly thereafter, as early as 11 to 16 years of age. This is when the last blood vessels leave the CEP. While the vessels decrease in number in the CEP, a rather dramatic decrease in the number of cells in the CEP, NP, and AF occurs (Liebscher et al., 2011). From this point on the NP and AF begin to show signs of degeneration (e.g., cell death, clefts, radial tears, degeneration of the extracellular matrix, and granular changes). Therefore even some teenagers may experience back pain as a result of IVD degeneration (Herzog, 1996). The increased cell death is replenished somewhat at this age by a marked increase in the proliferation of chondrocytes during this time (Boos et al., 2002).
The number of clefts and radial tears increases from 17 to 20 years of age. These changes are more prominent in the posterior than the anterior AF (Vernon-Roberts, Moore, & Fraser, 2007). In addition, the overall number of chondrocytes decreases as cell death, mucoid degeneration, and granular (scar tissue) changes begin to appear. These latter signs of degeneration increase throughout the second to the fourth decades of life. During the fifth and sixth decades of life the degenerative changes become the most severe; many clefts and tears filled with granular tissue become apparent throughout the IVD. Blood vessels (neovascularization) and nerve fibers able to conduct nociception have been found in many tears of elderly discs (Vernon-Roberts, Moore, & Fraser, 2007). Advanced tissue destruction and defects can be seen during this period of time. There is a high level of cell death (apoptosis) during these decades (Gruber & Hanley, 1998). Using a mouse model, Hutton and colleagues (1999) found that the rate of apoptosis in the CEP that occurs with aging and degeneration was also related to the rate of formation of osteophytes (spondylosis) along the superior and inferior margins of the vertebral bodies.
After 70 years of age the clefts and tears throughout the IVD have been filled with granular tissue, and the IVD assumes a homogeneous “burnt out” appearance, making the NP, AF, and CEP difficult to distinguish from one another (Boos et al., 2002).
There is no sex difference in the rate or severity of lumbar IVD degenerative changes, but the IVDs of whites show greater degenerative changes than the IVDs of individuals of African ethnicity. The L5-S1 IVDs show more degenerative changes than the other lumbar levels, particularly in individuals younger than 40 years of age (Siemionow et al., 2011).
Normal Aging of the Nucleus Pulposus and Anulus Fibrosus
The NP of fetal tissue contains many notochordal cells. The number of chondrocytes increases and the NP begins to assume slight mucoid characteristics during the first few months after birth. Subtle clefts develop in the NP during the next 2 years. The notochordal cells continue to die and the chondrocytes continue to increase in density between the ages of 3 and 10 years. There are virtually no notochordal cells left and there are significant clefts within the NP by 11 to 16 years of age. The number of clefts and radial fissures continues to increase until late in life (>70 years of age) when they have filled with granular (scar) tissue and are difficult to distinguish from the other tissue in the IVD. However, intranuclear clefts filled with fibrous (granular) tissue have been identified by means of T2-weighted MRI in patients just more than 30 years of age (Herzog, 1996). The outer lamellae of the AF are the last to show signs of degeneration, and these signs in the AF appear in the seventh decade of life (Boos et al., 2002).
Normal Aging of the Cartilaginous End Plate
The cartilaginous end plate (CEP) undergoes the earliest degenerative changes of the IVD. The CEP is well vascularized and slightly irregular in appearance during fetal development. Immediately after birth, the number of cells (primarily chondrocytes) increases dramatically and the cartilage takes on a disorganized appearance. During the next 16 years the number of blood vessels in the CEP steadily decreases, until there are no active vessels left by approximately 16 years of age (most are obliterated by 11 to 12 years of age) (Chandraraj, Briggs, & Opeskin, 1998; Boos et al., 2002). The decrease in active blood vessels is paralleled by the appearance of areas of obliterated vessels. These areas of obliterated vessels are already beginning to appear during the first 2 years of postnatal life. The number of regions of obliterated vessels dramatically increases until 16 years of age, when these regions are the most pronounced. The number of cells decreases significantly in the CEP until 16 years of age and then the numbers decline more gradually until the later decades of life (Liebscher et al., 2011). The number of PGs also decreases and the extracellular matrix becomes more disorganized as cell death increases (Antoniou et al., 1996; Roberts et al., 1996; Gruber & Hanley, 2002). Because the PGs dictate the solutes that are transported across the CEP and the rate of transport of these solutes, the decrease in the number of PGs may result in waste products accumulating in the IVD and nutrients being excluded from the IVD, both of which can lead to IVD degeneration.
Also between the ages of 11 and 16 years significant numbers of cracks appear in the CEP. The CEP cracks are accompanied by corresponding microfractures of the adjacent subchondral bone. These findings often are associated with new bone formation in the subchondral bone. The CEP cracks and the microfractures in the subchondral bone continue to increase during the second through the sixth decades, when the subchondral bone becomes sclerotic and regions of the CEP calcify. This leads to an increased vulnerability of the CEP to Schmorl’s node formation. In the seventh decade of life, the CEP stabilizes, and its appearance remains relatively the same throughout the remaining decades of life (Boos et al., 2002).
Normal Aging and Degeneration of the Bony End Plate
Even though the bony end plate is not a part of the IVD, the fates of the bony and cartilaginous end plates are so closely linked that a brief discussion of the normal aging and degeneration of the bony end plate is warranted. The relationship between degeneration of the bony end plate and formation of intravertebral herniations (Schmorl’s nodes) is also of clinical importance.
During the first few years of life, vessels within the vertebral body course into the cartilage growth cap (not the CEP, but subjacent to it) of the superior and inferior ends of the vertebral body. Vessels course within “vascular canals” within this cartilage. Each vascular canal contains several vessels that extend into the CEP. While the cartilage of the growth cap is replaced by bone, the vessels recede, leaving “cartilage nodes,” or areas of obliterated vessels, where the vascular canals once ended. These cartilage nodes fill with collagen from the CEP and NP and in some cases form what could be called “mini-Schmorl’s nodes.” Therefore the cartilage nodes represent weak spots in the vertebral bodies, and during the aging process they allow nuclear material to extrude through the thinning CEP and into and through the cartilage nodes; with further increase in IVD pressures, true Schmorl’s nodes can be formed (Chandraraj, Briggs, & Opeskin, 1998). In addition, multiple Schmorl’s nodes have been observed in individuals playing sports that create extensive axial loading (Hamanishi et al., 1994). Schmorl’s nodes are also sometimes seen in conjunction with typical disc protrusions, suggesting that axial loading is causing the NP to protrude in many directions (Chandraraj, Briggs, & Opeskin, 1998).
Unique Features of Intervertebral Disc Degeneration
Recall from previous chapters that degeneration of the IVDs is accompanied by nerve and vascular ingrowths into the disc. Schwann cells of the nerves innervating the outer layers of the AF appear to play a role in this ingrowth (Johnson et al., 2001). The increased innervation is attributable to free nerve endings containing increased amounts of substance P and calcitonin gene–related peptide (CGRP) (nociceptors). Therefore injured or degenerated discs are more pain sensitive than noninjured or nondegenerated IVDs (Coppes et al., 1997), and are particularly sensitive to inflammatory changes in the IVD (Ozawa et al., 2006). Also, remember from previous chapters that the IVDs thrive on reasonable motion within normal physiologic limits (Korecki, MacLean, & Iatridis, 2008). Consequently, frequent changes of posture improve and maintain the internal environment of the IVD, and an overly sedentary lifestyle is not beneficial for the IVD and the entire spine (Kraemer, 1995). Also, spending prolonged periods of time in postures that produce increases in IVD hydrostatic pressure, such as prolonged standing while carrying loads and prolonged sitting (again, an overly sedentary lifestyle), can affect PG and collagen synthesis, inhibit disc nutrition, and also lead to IVD degeneration (Kraemer, 1995; Hutton et al., 1998, 1999, 2002). In fact, hydrostatic pressure within the IVD has been found to have significant influences on IVD cell metabolism. Normal pressure in the IVD is approximately 3 atm (atmospheres). Pressures at this level were found in experiments (in vitro) conducted by Handa and colleagues (1997) to stimulate the synthesis of PGs and inhibit the production of matrix metalloproteinases (which degrade the PGs and collagen of the IVD) in both the NP and the inner AF. Pressures either greater than or less than 1 to 3 atm were found to increase the rate of disc degeneration (decreased production of PGs and increased production of matrix metalloproteinases). Lotz and colleagues (1998, 2000), using a mouse model, also found that prolonged graded compression resulted in graded IVD degeneration. They also noted that the IVDs showed signs of recuperation after having the increased pressures removed for 1 month. Other authors have found evidence that negative pressure, or distraction, may help the tissues of the IVD regenerate (e.g., increased hydration, increased numbers of cells that manufacture proteins, and up-regulation of gene expression for stimulating extracellular matrix production) (Guehring et al., 2006).
Conditions that may promote or initiate intervertebral disc degeneration: Several conditions have been identified that promote or even possibly initiate disc degeneration. For example, a high body mass index (BMI) and high levels of low-density lipoprotein cholesterol in the blood are associated with IVD degeneration (Hangai et al., 2008). In addition (and in some cases related to increased BMI), traumatic Schmorl’s node formation was previously mentioned as likely being able to initiate IVD degeneration. Also, extruded NP spontaneously produces increased amounts of matrix metalloproteinases, nitric oxide, interleukin-6, prostaglandin E2, and other chemokines (see Chapter 11). These products may be intimately involved in both the biochemistry of disc degeneration and the pathophysiology of radiculopathy (Herzog, 1996; Kang et al., 1996, 1997; Crean et al., 1997; Duance et al., 1998; Brisby et al., 2000) (see Chapter 11).
Another condition that may promote or initiate IVD degeneration is advanced aortic atherosclerosis. This condition presents as calcium deposits in the posterior wall of the aorta and has been found to increase a person’s risk of developing IVD degeneration, and is also associated with the occurrence of back pain (Kauppila et al., 1997).
Iwahashi and colleagues (2002) injected liquid nicotine into rabbits and then studied the IVDs. They found that nicotine reduced the vascular buds in the subchondral bone adjacent to the CEP and also decreased the lumen of the remaining vascular buds. In addition, nicotine administration resulted in necrosis in the NP and a “disturbance of the pattern of overlapping laminae” in the AF. These authors believe that a decrease in the vascular supply to the CEP led to a decrease in the oxygen tension in the CEP, which resulted in a decrease in the synthesis of PGs and collagen, both hallmarks of IVD degeneration (Iwahashi et al., 2002).
Potential treatments based on the basic science of the intervertebral disc: Adenovirus-mediated transfer of genes and the resultant production of therapeutic growth factors are being investigated as a means to further study the biology of the IVD and the potential for treatment for disc degeneration (Nishida et al., 1999); however, the low vascularity of the adult IVD may preclude the effective use of gene therapy in IVD disease (Boos et al., 2002). Other investigators are exploring the use of recombinant human osteogenic protein-1 as a means to treat IVD degeneration (Imai et al., 2007).
As mentioned, decreased PG synthesis is related to IVD degeneration. Maeda and Kokubun (2000) also found an increased sensitivity of IVD cells to interleukin-1α with age and felt that this may be associated with IVD degeneration. Inner AF cells were found to be more sensitive to interleukin-1α than outer AF cells after normal aging. They believe this may explain why NP and the inner AF are the first disc areas that undergo degeneration before the outer lamellae of the AF. Maeda and Kokubun (2000) hypothesized that an interleukin-1α receptor antagonist protein introduced into the IVD may be beneficial in decreasing IVD degeneration by blocking the degenerative effects of interleukin-1α.
Glycosaminoglycans and Proteoglycans
The topic of glycosaminoglycans (GAGs) and PGs was introduced with connective tissue earlier in this chapter. GAGs and PGs are covered in further detail here because of their extreme importance in the IVD’s proper functioning. Much of the relevant current research related to the IVD involves GAGs and PGs. In fact, “PGs are cellular indicators of disc functional capacity.” (Bishop, 1992).
The main function of GAGs and therefore PGs is structural; they interact with collagen fibers to provide support. In addition to providing support, GAGs, because of their ionic charge, are able to form electrostatic interactions with cationic molecules. This serves to transport electrolytes, water, and metabolites. The gel-like or viscous nature of the GAGs allows them to have a lubricating function in connective tissue and joints and also allows them to withstand compressive loads.
Proteoglycan Monomers and Proteoglycan Aggregates
PG monomers are complex macromolecules composed of many GAGs covalently bonded to a core protein of varying length. Three dimensionally, the side chains attached to the core protein form the shape of a “bottle brush” (see Fig. 14-6). The short branches of the bottle brush represent keratan sulfate, and the long ones represent chondroitin sulfate. PG monomers group together to form PG aggregates. The backbone of a PG aggregate is hyaluronic acid. Hyaluronic acid is a long coil-like chain composed of alternating molecules of glucuronic acid and glucosamine. The PG monomers are attached to hyaluronic acid by a link protein.
Glycosaminoglycans
GAGs refer to long-chain, unbranched carbohydrate polymers composed of repeating disaccharide units of glucosamine (or galactosamine) and glucuronic acid attached to a protein core. The hexosamine (glucosamine and galactosamine) usually is sulfated (see Table 14-4). However, an exception among the GAGs to this general pattern is hyaluronic acid, which is the longest GAG and has no sulfated hexosamines. Most of the carbohydrate molecules of GAG chains are negatively charged and repel each other. This negative charge attracts numerous Na+ ions, which are osmotically active. This causes a large amount of water to rush into the matrix and creates a swelling hydrostatic pressure. This hydrostatic pressure enables the matrix of cartilage and the IVD to withstand compressive forces.
GAGs are synthesized within the cells of the connective tissue in which they are found. For instance, the fibroblast is the primary cell type of connective tissue proper, and it produces and maintains the ground substance of this tissue, which primarily consists of GAGs. Chondroblasts are cells that produce the ground substance of cartilage, and osteoblasts produce the ground substance of bone. The GAGs for the entire IVD are probably primarily produced by the cells within the CEP. The time it takes to replace the PGs in the human IVD is considerable (approximately 3 years) and is longer than the replacement times found in other species (Table 14-5). Therefore much time is needed before complete repair of pathologic conditions or damage to the IVD can take place, and in some cases (especially if the CEP is damaged) repair may not be possible (Moore et al., 1996).
Table 14-5
Comparison of Turnover Time (Days) for Proteoglycan Molecules from Various Regions of the Intervertebral Discs of Dogs, Pigs, and Humans
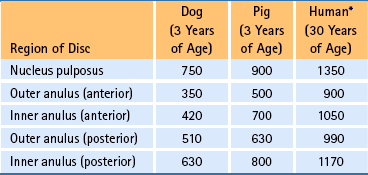
∗Note that for a 30-year-old human, it takes more than 3 years to replace proteoglycan molecules.
The principal GAGs of the extracellular matrix include the following:
These five types of GAGs differ in the following important ways: molecular weight, length of the chain, and type of disaccharide units. Table 14-4 summarizes the major characteristics of the principal GAGs. GAGs tend to exist in various configurations, which results in a variety of electrostatic charges among GAGs and allows them to participate in various degrees of interactions with adjoining chains. This variety of configurations and interactions of GAGs contributes to the formation of additional physical and chemical characteristics of the extracellular matrix.
Hyaluronic acid is by far the largest GAG, consisting of an estimated 2500 disaccharide units. Its molecular weight is approximately 106 Da. Hyaluronic acid is the major GAG of synovial fluid and many tissues (see Table 14-4). It is partially responsible for swelling within the extracellular matrix and also for attracting cells to the site of an injury. As mentioned previously, PG monomers also bind to hyaluronic acid to form PG aggregates.
Chondroitin 4-sulfate and chondroitin 6-sulfate consist of glucuronic acid and N-acetylgalactosamine. These two GAGs are similar in structure and function (see Table 14-4). Chondroitin 4-sulfate is the most abundant GAG found in the body and is present in immature cartilage. Chondroitin 6-sulfate is distributed in mature cartilage and other tissues. Decreased sulfation of the chondroitin sulfates has been related to IVD degeneration (Hutton et al., 1997).
Dermatan sulfate is the major GAG of skin, from which it derives its name. It is also distributed in tendons, ligaments, fibrocartilage, and other tissues. However, it is not found in the IVD or Z joints. Dermatan sulfate has a high affinity to associate closely with collagen type I fibers (see Tables 14-3 and 14-4).
Keratan sulfate is the shortest of the GAGs (see Table 14-4). It is found in skeletal tissue, as well as the cornea (keratan sulfate type I). Along with hyaluronic acid and chondroitin sulfate, keratan sulfate is a main contributor of cartilage PGs. It is also found in abundance in the NP of the IVD.
Heparan sulfate is associated with collagen type III reticular fibers, which are found in large amounts in basal laminae. Heparan sulfate is also distributed in the lungs, liver, and aorta. However, it is not found to any great extent in the Z joints or IVDs.
Fibrocartilage
Because the AF and NP of the IVDs are considered to be specialized fibrocartilage, a brief discussion of the general characteristics of this type of cartilage is included here.
Light microscopy reveals that fibrocartilage appears similar to dense connective tissue and hyaline cartilage (Fig. 14-18). Its matrix contains obvious thick bundles of type I collagen fibers (see Table 14-3). The collagen bundles are distributed in parallel beams among rows of chondrocytes. The chondrocytes are smaller than those of hyaline or elastic cartilage and are easily distinguished from the fibroblasts, also present in fibrocartilage, because chondrocytes lie within round or oval lacunae. Because of the presence of the collagen fibers and a lesser amount of GAGs, the matrix of fibrocartilage stains more eosinophilic than hyaline or elastic cartilage. Also, fibrocartilage, unlike nonarticular hyaline and elastic cartilage, is not enveloped by a perichondrium. Fibrocartilage is distributed in the IVD (see the previous discussions) and articular cartilages. It is also found in the pubic symphysis, ligamentum teres femoris capitis, glenoid ligament, and the intraarticular cartilages of some joints.
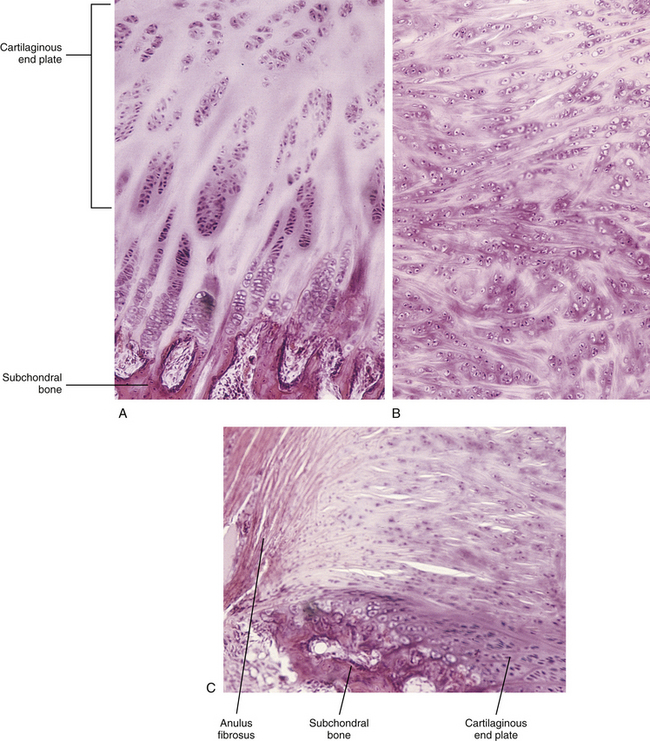
FIG. 14-18 Light micrographs demonstrating the fibrocartilaginous composition and distinguishing features of the three component parts of the intervertebral disc. This specimen is from a mammalian fetus. A, Cartilaginous end plate (magnification ×100). B, Developing nucleus pulposus (magnification ×100). Notice the haphazard arrangement of the cells and fibrous elements in this region. C, Portion of the anulus fibrosus.
Microscopic Anatomy of Other Major Tissues in the Back
The other tissues of the spine are similar in composition to these same tissues in other regions of the body. However, an understanding of these tissues is essential to a comprehension of the structure and function of the spine. Therefore the remainder of the chapter gives a cursory overview of the microscopic anatomy of the other major tissues in the region of the back. These include connective tissues other than those previously discussed, skeletal muscle, and neural tissue. The reader is encouraged to consult histology texts for more in-depth information concerning these tissues.
Connective Tissue (Including Fascia)
As stated, connective tissue is the tissue that binds together and provides support for the various structures of the body. All connective tissues have three components: cells, fibers, and ground substance (including water). The ground substance and fibers together are collectively known as the extracellular matrix. It is the relative amounts and structures of these components that determine the types of connective tissues found in the body. There are various classifications of connective tissues, but in general there are embryonic connective tissue, connective tissue proper, and specialized connective tissue (Ross et al., 2003). Each of these can be further subdivided:
Of the embryonic connective tissues, mesenchyme has been discussed. Mucous connective tissue is found only in the umbilical cord and is beyond the scope of this text. Also, blood, lymphatic, and hemopoietic tissues are not discussed here. Cartilage has been thoroughly discussed, but connective tissue proper, adipose tissue, and bone are briefly covered.
Connective Tissue Proper
Connective tissue proper is tissue that, rather intuitively, seems to fall into the category of tissue that holds things together. It can be subdivided into two types: loose and dense (Mescher, 2010). This is based mostly on the relative amounts of fibers that can be found in the extracellular matrix. Further, dense connective tissue can be thought of as having two varieties, based mostly on the arrangement of the fibers in the extracellular matrix: irregular and regular.
Loose or areolar connective tissue is characterized by a large amount of fluid ground substance (Fig. 14-19). There are moderate amounts of cells and fibers that are loosely, or not closely, arranged in this ground substance. The cells are predominantly fibroblasts and macrophages, but other cell types common to connective tissue also can be found, especially lymphatic cells and mast cells. All three connective tissue fiber types (elastic, collagen [mostly type I], and reticular) are represented. In general, connective tissues do not have as rich of a blood supply as other, more metabolically active tissues (e.g., neural tissue), but for a connective tissue, loose connective tissue is quite vascular. The functional characteristics of this type of tissue are that it is rather flexible but not very strong. It is commonly found in the body. In the region of the back, it mainly supports epithelia (e.g., papillary layer of the dermis), surrounds bundles of muscle cells (perimysium), and ensheaths lymphatic and blood vessels. Loose connective tissue, along with adipose tissue (see the following), also forms the superficial fascia.
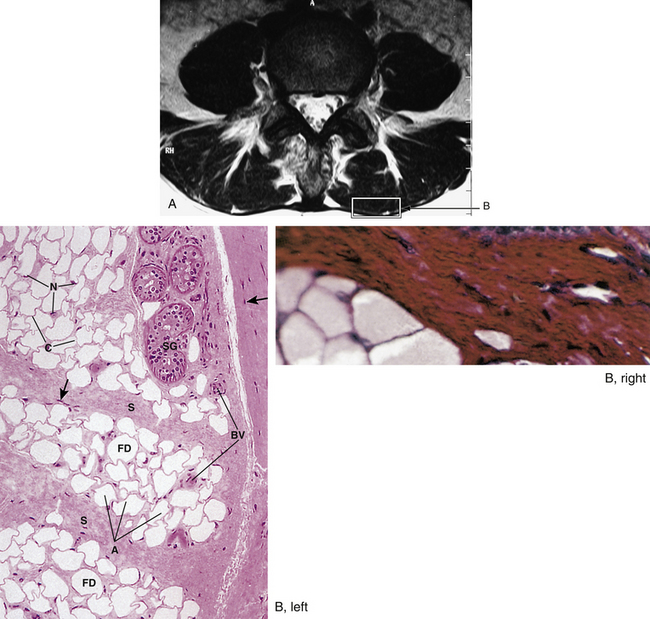
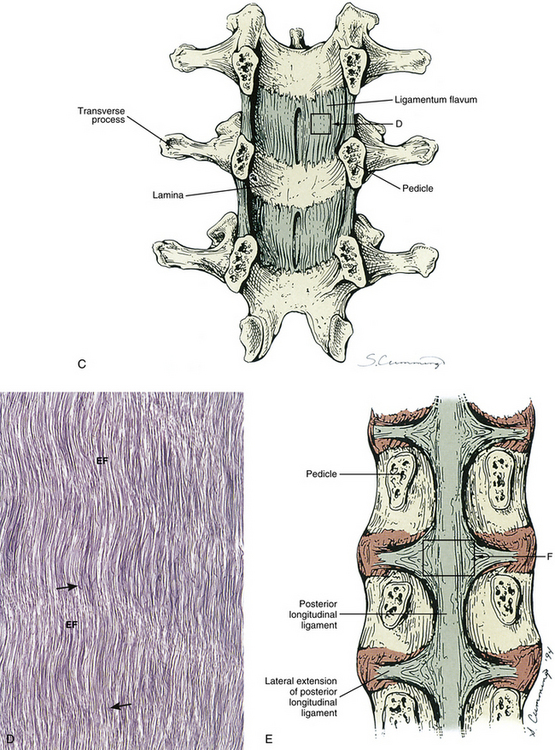
FIG. 14-19 Fascia and ligaments. A, Horizontal MRI scan through the lower lumbar region. Box labeled B indicates an area containing superficial and deep fascia, the focus of part B. B (left), Superficial fascia (×132 magnification). Notice the adipose cells and loose arrangement of collagen fibers. A, Adipocytes; BV, blood vessels; C, cytoplasm of adipocytes; FD, fat droplets; N, nuclei; S, connective tissue septa; SG, sweat gland; arrows, fibroblast nuclei. B (right), Deep fascia (×132 magnification). Notice the regularly dense arrangement of cells and collagen fibers (right side of the image). C, Illustration demonstrating the ligamentum flavum (an elastic ligament). Notice the box indicating the region depicted in part D. D, Ligamentum flavum (×132 magnification). Notice the regular, “wavy” arrangement of the elastic fibers (EF) in this ligament. Arrows, Fibroblasts. E, Illustration demonstrating the posterior longitudinal ligament (F), a typical spinal ligament from a histologic perspective. Notice the box indicating the region depicted in part F. F, A typical ligament (approximately ×100 magnification). Notice the elongated fibroblast nuclei between tightly packed bundles of regularly arranged collagen fibers. (B, left, From Gartner LP & Hiatt JL. [2000]. Color atlas of histology [3rd ed.]. Philadelphia: Lippincott Williams & Wilkins. B, right, From Junqueira LC & Carneiro L. [2002]. Basic histology text and atlas [10th ed.]. Chicago: McGraw-Hill. D, From Gartner LP & Hiatt JL. [2000]. Color atlas of histology (3rd ed.). Philadelphia: Lippincott Williams & Wilkins.)
Dense irregular connective tissue has large numbers of fibers that are arranged in bundles that are oriented in various directions (see Fig. 14-19). These fibers are mostly collagen fibers composed of type I collagen. There are relatively few cells, which are mostly fibroblasts, and little ground substance, when compared with loose connective tissue. Therefore it is less flexible but much stronger than loose connective tissue. The three-dimensional arrangement of the fibers gives this tissue good strength in all directions. In the region of the back, dense irregular connective tissue may be found mostly in the reticular or deep layer of the dermis. Most of the supporting tissue of peripheral nerves (epineurium and perineurium) and muscles (epimysium and thicker types of perimysium) also consist of dense irregular connective tissue. The fibrous layers of the periosteum and perichondrium are also considered to be this type of tissue.
The components of dense regular connective tissue are similar to those of dense irregular connective tissue, but their arrangement is different. In this case, the bundles of fibers are arranged linearly, with rows of cells, mostly fibroblasts, and a minimal amount of ground substance located between the bundles. This arrangement gives a tremendous amount of resistance to traction stresses in one direction (parallel to the fibers). In the back region, three main types of structures are composed of this tissue: tendons, ligaments, and deep fascia.
Tendons, the structures that connect muscles to bone, are composed of closely packed bundles of collagen fibers (see Fig. 14-23). These fibers are almost exclusively type I collagen. Between the bundles of fibers are rows of tendinocytes, which resemble fibroblasts. The bundles of collagen fibers are grouped together into fascicles, which are continuous with the corresponding fascicles of the muscle to which they attach (see the following). Each fascicle is invested or surrounded by a thin layer of loose connective tissue called the endotendineum (see Fig. 14-21). The endotendineum contains blood vessels and nerves. Surrounding the entire tendon is a thin layer of dense irregular connective tissue called the epitendineum.The endotendineum septa are continuous with the epitendineum. Recent advances are leading to a more integrated understanding of tendons and their relationship with fascia (see following section). This has led to a better appreciation of their function (Benjamin, Kaiser, & Milz, 2008).
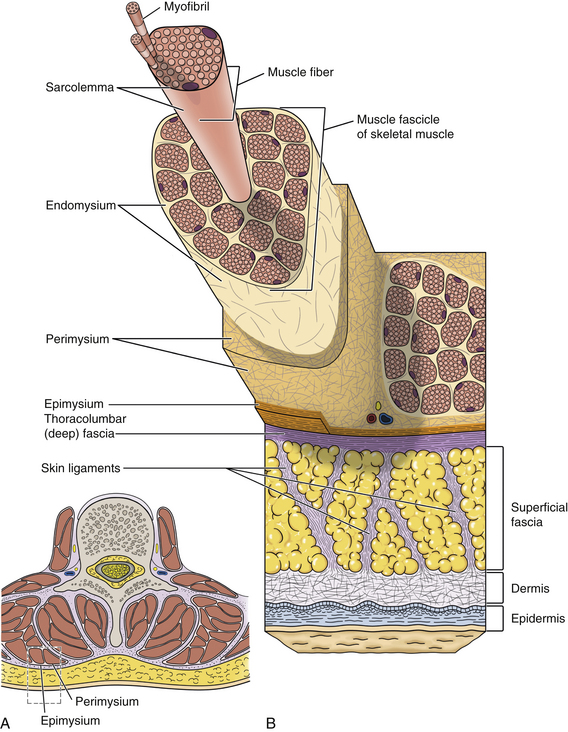
Fig. 14-20 Fascia. A, Transverse section through the lumbar region showing the fascicular arrangement of the deep back muscles. B, Closer view of the relationship between the skin, superficial fascia, deep fascia, and the connective tissues associated with skeletal muscle.
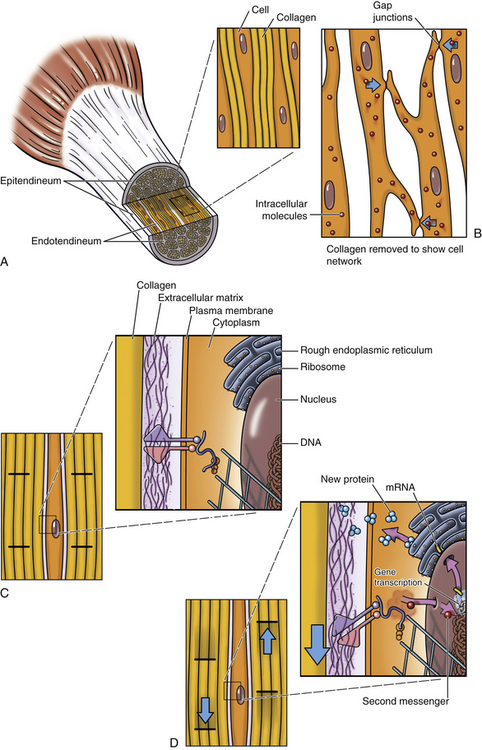
Fig. 14-21 Mechanotransduction. A, Longitudinal section of tendon showing arrangement of tendinocytes and collagen fibers. B, Similar section of tendon with collagen removed to show cell-cell interactions via gap junctions. C, Closer view of similar section showing possible interactions between extracellullar matrix, transmembrane proteins, and cytoskeletal structures. D, With shear motions, it is thought that deformation of transmembrane proteins can lead to second messenger signals, which can alter protein production.
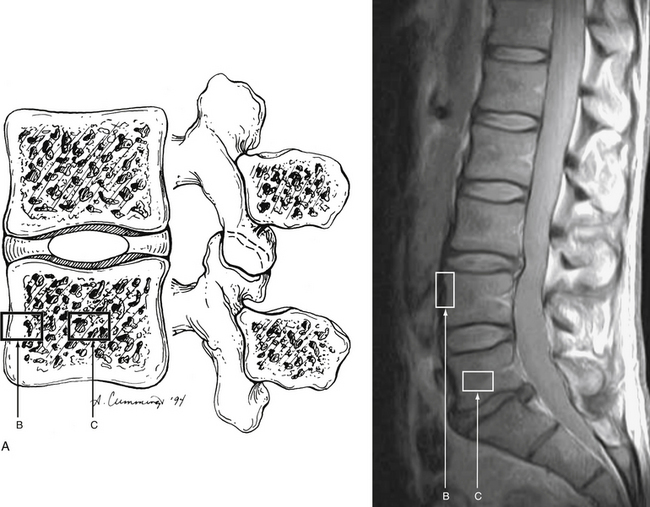
FIG. 14-22 Bone. A, Boxes indicate (B) cortical bone and (C) cancellous bone that are demonstrated in the corresponding parts of this figure. B, Illustration of cortical bone (compact bone). C, Light micrograph (approximately ×40 magnification) of cancellous bone (spongy bone, trabecular bone). See text for details. (B, From Gartner LP & Hiatt JL. [2000]. Color atlas of histology [3rd ed.]. Philadelphia: Lippincott Williams & Wilkins. C, From Eroschenko VP. [2005]. diFiore’s atlas of histology with functional correlations [10th ed.]. Philadelphia: Lippincott Williams & Wilkins.)

FIG. 14-23 Muscle and tendon. A, Boxes indicate the regions illustrated in parts B to D. B, Tendon (×270 magnification). Notice the rows of elongated fibroblast nuclei (N) between tightly packed bundles of very regularly arranged collagen fibers. C, Skeletal muscle (×540 magnification). D, Ultrastructure of a sarcomere from skeletal muscle (approximately ×130,000 magnification). The lines and bands have been labeled. See text for further details. (B, From Gartner LP & Hiatt JL. [2001]. Color textbook of histology [2nd ed.]. Philadelphia: Saunders. D, From Leeson TS & Leeson CR. [1981]. Histology [4th ed]. Philadelphia: WB Saunders.)
Ligaments connect bones or cartilages and serve to support and strengthen joints. Like tendons, the bundles of fibers are linearly arranged in ligaments, although not quite as uniformly (Ross et al., 2003). Also, unlike tendons, there is no fascicular arrangement of the bundles of fibers, making ligaments less vascular than tendons. In most ligaments of the body, the fibers are predominantly type I collagen fibers, giving a great amount of tensile strength, but no elasticity (see Fig. 14-19). Because of the high amount of collagen, these ligaments appear white to the unaided eye, and sometimes this dense regular collagenous tissue is called white connective tissue. One exception to this is the ligamentum flavum. It is composed of dense regular connective tissue, but most of the fibers in its extracellular matrix are elastic fibers, not collagen fibers. Under the microscope, longitudinal sections of these bundles of elastic fibers have a distinctive wavy appearance (see Fig. 14-19). This type of fiber gives the ligamentum flavum not only good tensile strength but also elasticity. Masses of elastic fibers have a distinct yellow-orange color when viewed by the unaided eye. Therefore dense regular elastic connective tissue sometimes is known as yellow connective tissue. This coloration is also responsible for the name flaval ligament (i.e., ligamentum flavum), because flavus means yellow in Latin.
The site where a tendon or ligament attaches to a bone is called an enthesis. Most authors recognize at least two types of enthesis: fibrous and fibrocartilaginous (Benjamin et al., 2006). In a fibrous enthesis, the dense regular connective tissue of the tendon or ligament attaches either directly to the bone or indirectly to it via the periosteum. The fibrocartilaginous enthesis is more common, especially for tendons (Benjamin, Evans, & Copp, 1986). Four zones are recognized as comprising a fibrocartilaginous enthesis. These include (from superficial to deep) (1) tendon/ligament, (2) fibrocartilage, (3) calcified fibrocartilage, and (4) bone. Recently the concept of an “enthesis organ” has been proposed (Benjamin et al., 2006). This term has been used to describe the collection of related tissues at or near an enthesis, which serve a common function of stress dissipation. The concept of the enthesis organ can have major implications for the clinician because this concept helps to explain patterns of injury and the diffuse nature of signs and symptoms associated with particular types of enthesopathies (i.e., enthesis injuries).
Fascia: Another type of connective tissue structure is fascia. Recent research has led to a significant change in the understanding of the importance of fascia in health and disease. Traditionally, fascia has been considered a fairly vague term derived from the Latin word for bandage and has been used to describe a spectrum of relatively undifferentiated connective tissues of mesenchymal origin that “wrap” around other more specialized structures or organs. For a long time, many health care professionals have considered it as rather inconsequential.
In recent years there has been a renaissance of sorts when it comes to fascial research. An important general consideration of fascia that has evolved from this research is that fascia is a connective tissue continuum that runs throughout the body and may form a body-wide cellular signaling system (Langevin, Cornbrooks, & Taaties, 2004). In addition, much of this evidence has shown that fasciae are actually active structures that play key roles in the function of many components of the body, especially in the musculoskeletal system (Benjamin, 2009). This research indicates that health care professionals, especially those that deal primarily with the neuromuscular and musculoskeletal systems, need to have a thorough understanding of the anatomy of fascia in order to be more effective in rendering care to their patients.
Given the renewed interest in fascia, there have been several attempts at better defining this tissue. Some people have expanded the definition of fascia to embrace all fibrous connective tissues of the body (including tendons and ligaments) (Findley & Schleip, 2007). However, in this text, a more traditional definition of a fascia will be used that includes the fasciae associated with the skin, those that invest muscles and groups of muscles, and the connective tissue portions of muscles themselves. There are also fasciae that are associated with the major organs of the body and the great body cavities, which are beyond the scope of this text. Likewise, there appear to be some special arrangements of fascia in the limbs that will not be discussed here (Benjamin, 2009).
For centuries, anatomists have distinguished between superficial and deep fasciae (see Figs. 14-19 and 14-20). The superficial fascia (tela subcutanea, hypoderm, subcutis) is the layer of loose connective tissue that lies deep to the dermis of the skin and binds it to the underlying structures. It, therefore, is a single continuous layer. There is a highly variable amount of adipose tissue (see below) associated with the superficial fascia. This adipose tissue is sometimes known as the panniculus adiposus. A prominent fatty layer of the skin is a characteristic of humans and may play a role in heat conservation as a compensation for the lack of a hairy coat, which is seen in many other mammals. The superficial fascia allows the skin to be mobile over the underlying structures, especially over highly mobile joints. This appears to be promoted by multiple sheets of collagen fibers that are linked to elastin (Kawamata et al., 2003). The relative independence of the collagen sheets allows for the mobility, whereas the elastin helps return the skin to its original position. The superficial fascia also conveys blood vessels and nerves to and from the more superfical portions of the skin. These neurovascular structures are very tortuous in the superficial fascia so that they can adapt to the deformations imposed upon them by the mobility of this layer.
When most clinicians think of fascia, though, they usually mean the deep fascia. Deep fascia is mainly dense regular connective tissue that is continuous with the deep surface of the superficial fascia, although there appears to be some loose connective tissue included in its composition. The histologic structure of deep fascia is similar to that of tendons, but instead of having bundles of collagenous fibers arranged in fasciculi, the fibers are arranged into multiple layers. The fibers of each layer are parallel to each other, but the fibers of the various layers usually have a different orientation to the fibers of adjacent layers. This arrangement gives deep fascia excellent tensile strength in many different directions in the same plane. Classically, different portions of the deep fascia are named regionally, for example, thoracolumbar fascia (see Chapter 4). Unfortunately, this may be misleading because the deep fascia forms a tough, continuous layer throughout the body. Deep fascia also forms intermuscular septa, which usually run fairly perpendicular to the body surface and separate muscle groups from each other.
There are connective tissue structures associated with skeletal muscles. These connective tissue layers are increasingly being defined as fascial structures and are collectively known as muscle fasciae (or intramuscular extracellular matrix/intramuscular connective tissue) (Purslow, 2010). Classically, three types of connective tissue are recognized associated with muscles: epimysium, perimysium, and endomysium (see Fig. 14-20).
Epimysium is the thick, dense collagenous connective tissue that surrounds an entire muscle. It is continuous with the epitendineum of the attached tendons and appears to act as a surface tendon—it helps transmit force alongs its length. In some places the epimysium is continuous with the deep fascia whereas in others (e.g., in the region of the thoracolumbar fascia) it is a separate layer.
Perimysium is a continuous layer of collagenous connective tissue that separates the skeletal muscle tissue into muscle fascicles (i.e., bundles of muscle cells). The perimysial network blends with the epimysium at the surface of the muscle. Muscle fascicles are of varying sizes in diameter, ranging from a few muscle cells to hundreds of muscle cells. This variation in fascicle diameter gives rise to a wide variation in the thickness and density of the perimysium associated with muscle fascicles. Perimysium surrounding smaller fascicles is rather thin with few collagen fibers, basically loose connective tissue, whereas perimysium surrounding large fascicles is quite thick (nearing the thickness of epimysium) and can be considered dense connective tissue. Each muscle fascicle generally runs the entire length of the muscle (i.e., from tendon to tendon) and, therefore, the perimysium is continuous with the endotendineum of the attached tendons. This means that the perimysium helps act like an “internal” tendon by helping transmit forces (Purslow, 2010).
Endomysium is the relatively thin reticular connective tissue layer that surrounds individual muscle cells. Consequently, endomysium is actually a single structure that fills the space between adjacent muscle cells, suggesting that it may also be involved in force transmission (Swatland, 1975).
The predominant cell found in fascia is the fibroblast. These cells appear to be very important in mechanotransduction. Mechanotransduction involves the processes whereby cells convert physiologic mechanical stimuli into biochemical responses (Khan & Scott, 2009) (Fig. 14-21). These processes can be categorized into three main categories: (1) Mechanocoupling—a physical load can cause physical perturbations to cells, which can trigger a wide array of responses, including changes to the cytoskeleton (Langevin et al., 2005; Langevin et al., 2006); (2) cell-cell communication—this can occur via gap junctions and allows a stimulus in one location to lead to changes in cells at a distance (Khan & Scott, 2009); (3) effector cell response—mechanical loading can lead to changes in protein synthesis (via alterations in gene expression; for example, Bouffard and colleagues [2008] demonstrated that brief stretching of fascia can decrease TGF-β1–mediated fibrillogenesis in its fibroblasts), which, in turn, can cause changes in the cell-extracellular matrix interface and matrix remodeling (Langevin et al., 2010; Langevin et al., 2011). Mechanotransduction appears to be an important mechanism in explaining some of the therapeutic effects of various mechanotherapies, including exercise, massage, acupuncture (Langevin et al., 2005; Langevin et al., 2006; Khan & Scott, 2009) and others.
Fascia appears to receive a rather rich nerve supply. Free and encapsulated nerve endings, including Ruffini endings and pacinian corpuscles, can be found in appreciable numbers in various regions of fascia, including the human thoracolumbar fascia (Stilwell, 1957; Yahia et al., 1992). However, the literature is not clear on whether the deep fascia itself is innervated or just the loose connective tissue and/or adipose tissue associated with the deep fascia. Either way, fasciae and/or their associated tissues appear to be sources of proprioceptive and nociceptive information. Recent evidence suggests that muscle fascia rather than muscle tissue is important in exercise-induced delayed-onset muscle soreness, which may offer an explanation for (and possibly more effective management of) this poorly understood, and commonly encountered, phenomenon (Gibson et al., 2009).
Unlike the traditional view of fascia being merely a “wrap” for more important structures, recent evidence has shown that fascia has more than a few other important functions. Because fascia is mostly a collagenous tissue, it does not stretch; consequently, fascia functions to restrict movement. This firm collagenous composition allows deep fascia to be able to contain and separate muscle groups into fairly well-defined compartments (see Fig. 14-20). Because this partitioning role is accomplished not only with specializations of deep fascia but also with bones and intermuscular septa (also formed by deep fascia), the regions defined by theses structures are sometimes known as osteofascial compartments.
Where the deep fascia and intermuscular septa attach to bones, they have an organization that is similar to that of an enthesis (see earlier discussion) (Benjamin, 2009). These attachments have been implicated in overuse injuries, especially in the limbs (Bouche & Johnson, 2007). In addition, the fasciae and intermuscular septae that help form the osteofascial compartments, along with the fascial structures incorporated into the muscles themselves (i.e., epimysium, perimysium, and endomysium), appear to contribute to the attachment of the muscles with which they are associated. The term “ectoskeleton” has been used to describe the arrangement of fascia between, around, and within muscles and their additional role in contributing to muscle attachment to bone (Wood Jones, 1944). This concept of fascia as an ectoskeleton leads to the idea of “myofascia,” where the muscle-ectoskeleton complex (myofascia) allows force transmission to occur within the skeletal muscles themselves (in addition to force transmission at the tendon-bone attachments) (Huijing, 2003). In other words, the contractile forces generated by the muscles are conveyed directly not only to their tendons but also to the connective structures associated with the muscles and groups of muscles (Huijing, 1999). This concept of myofascia leads to the notion that individual muscles, as defined anatomically, do not act alone and should not be considered as acting separately (Yucesoy & Huijing, 2007). Some authors are convinced that agonists and antagonists may even be considered to be mechanically coupled (Huijing, 2007).
The arrangement of various muscles attaching to a common fascia puts fascial structures in a position to help coordinate muscle activity. For example, Vleeming and colleagues (1995) have hypothesized that the thoracolumbar fascia is important in integrating the forces generated by muscles that have been traditionally considered as belonging only to the upper limb, lower limb, pelvis, and spine. Specifically, the thoracolumbar fascia may help coordinate the functions of the gluteus maximus and latissimus dorsi muscles to allow the contralateral pendulum-like motion of the upper and lower limbs that are characteristic of walking and swimming. Also, other authors have suggested that the thoracolumbar fascia may be an important link between the transversus abdominis muscle and the control of lumbar segmental motion (Barker et al., 2007). This relationship between the transversus abdominis muscle and the thoracolumbar fascia may provide a basis for recommending exercises that cause a submaximal contraction of the transversus abdominis muscle in the management of certain forms of low back pain (Barker et al., 2007). Strengthening of the transversus abdominis muscle may allow for better control of possibly altered lumbar intersegmental mechanics associated with low back pain.
Fascia has certainly gained a new prominence in the area of health care. It is the subject of intense research and has even led to two international research congresses that were devoted entirely to the subject of fascia (Findley & Schleip, 2007; Huijing et al., 2009). A better appreciation of this exciting field may lead to sweeping changes in the care and management of many clinical problems and especially the use and understanding of manual therapies.
Adipose tissue: Fat-storing cells, or adipocytes, can be found throughout most of the loose connective tissue of the body. In the regions where these adipocytes are the predominant cell type, the tissue is called adipose tissue. The triglycerides in adipose tissue represent the main store of excess caloric energy in the body. Lipids are an efficient form of energy storage, because more than 9 kcal of energy are in 1 gram of lipid, as opposed to approximately 4 kcal/g of either carbohydrate or protein. Also, this tissue acts as insulation for the body because fat is a poor conductor of heat and much of the adipose tissue is located subcutaneously. There are two types of adipose tissue found in the body: white and brown.
White adipose tissue, which actually looks yellow in humans, is characterized by having adipocytes with a single droplet of neutral fat. These cells are described as unilocular. White adipose tissue composes the vast majority of adipose tissue in the body. Most of the superficial fascia of the body is basically white adipose tissue. When closely packed together, the adipocytes are mostly round or polyhedral. The fat droplet takes up the majority of the volume of each cell, so much so that the cytoplasm forms only a thin rim on the periphery of the cell. Also, the nucleus is flattened and eccentrically located; therefore adipocytes are sometimes known as signet-ring cells. Each adipocyte is surrounded by a meshwork of reticular fibers that are composed of type III collagen. Adipose tissue is incompletely subdivided into lobules by loose connective tissue septa, which contain a rich vascular supply and network of nerves. Adipose tissue is highly vascularized, so much so that the ratio of blood volume to cytoplasm volume is greater in adipose tissue than in skeletal muscle (Mescher, 2010). The storage and mobilization of fat are regulated by a complex interplay of hormonal and neurologic mechanisms. Besides being an energy storage tissue, adipose tissue also has a secretory function. It produces a hormone known as leptin, which appears to be a circulating satiety factor involved in the regulation of food intake.
In brown adipose tissue, the adipocytes store fat in multiple droplets and are known as multilocular. The fat appears distinctly brown when seen by the unaided eye. Brown adipose tissue is important in thermoregulation. The oxidation of fatty acids in the mitochondria of brown fat cells is uncoupled from the production of adenosine triphosphate (ATP); therefore the energy produced by these mitochondria is dissipated as heat. Brown fat is found abundantly in animals that hibernate. In humans, it can be found in late-term fetuses and newborns and may be important in heat regulation for the first few months of life. By the end of the first decade of life, the amount of brown adipose tissue is greatly diminished, such that there are negligible amounts of it in the adult.
Bone
Bone is a specialized connective tissue in which the extracellular matrix is mineralized mostly by calcium phosphate in the form of hydroxyapatite crystals. This makes bone tissue hard, such that it forms the rigid support of the body as the skeleton. Bone also plays a vital role in the regulation of blood calcium levels by acting as a reservoir for both calcium and phosphate. Therefore unlike the dried skeletons that are studied in school, bone is a living, highly vascular, constantly changing tissue.
The organic portions of the extracellular matrix are composed of fibers and ground substance. The vast majority of the fibers are type I collagen fibers. These comprise the main structural component of the extracellular matrix of bone (Ross et al., 2003). The ground substance is composed of mostly GAGs that are similar to those of other connective tissues (see the preceding sections). Several special glycoproteins found only in the ground substance of bone tissue appear to be involved with the promotion of mineralization of the matrix. During the mineralization process, the mineral salts become associated not only with the ground substance but also with the collagen fibers.
Within the extracellular matrix are physical holes known as lacunae (singular, lacuna). Each contains a cell known as an osteocyte. Because there is little to no diffusion through the hardened extracellular matrix, there are tunnels in the matrix known as canaliculi, which contain cytoplasmic processes of osteocytes. At the point where the process of one osteocyte encounters the process of a different osteocyte in the canaliculus is a gap junction. This allows communication and diffusion of materials between the osteocytes.
Three other types of cells are associated with bone tissue:
Osteoprogenitor cells are found primarily on the outer and inner surfaces of bone tissue. They are pluripotential cells of mesenchymal origin that undergo a continual process of mitosis. They mostly give rise to osteoblasts, although during bone repair (e.g., after fracture) they may develop into other types of cells involved in that process.
Osteoblasts are the differentiated bone cells that give rise to bone matrix. They secrete both collagen and ground substance in an unmineralized form known as osteoid. This addition of new osteoid on the existing surface of bone tissue is known as appositional growth. Active osteoblasts exhibit the same cytoplasmic architecture as other cells that secrete large amounts of protein (e.g., abundant rough endoplasmic reticulum and a well-developed Golgi apparatus). In actively growing bones, osteoblasts are found alongside each other exclusively on the surfaces of bone tissue in a way reminiscent of simple cuboidal epithelium, but without any basal lamina. Although osteoblasts appear to be polar in nature and secrete osteoid on only one side, occasionally an osteoblast may become completely surrounded by osteoid. When this happens, a new lacuna is formed, and the cell becomes known as an osteocyte.
Osteoclasts are the bone cells responsible for resorption of bone matrix. They are large, multinucleated cells that are in physical contact with the surface of bone tissue. At this contact point the cytoplasm of these cells forms many folds that look like microvilli, giving it the appearance of a ruffled border. Lysosomal hydrolases are released from this ruffled border that digest the bone matrix outside the cell, creating shallow indentations in the bone matrix known as resorption bays or Howship’s lacunae. Osteoclasts appear to endocytose the digested bone matrix, because large numbers of coated vesicles can be found in the region of the ruffled border. Osteoclasts, unlike osteoprogenitor cells, appear to originate from the same mononuclear hemopoietic progenitor cells that differentiate into monocyte lineages. This makes osteoclasts closely related to macrophages, in both origin and function.
There are two main arrangements of bone tissue seen in mature bone (e.g., the vertebrae) (Fig. 14-22). A dense layer of compact bone forms the outer portion of bones. Spongy, or cancellous, bone, which consists of a network of bony trabeculae (from the Latin word for beam) and spicules, forms the interior of the vertebrae. The spaces between the trabeculae are known as the marrow cavities and are occupied by blood vessels and bone marrow, which is the main hemopoietic tissue of the body.
Compact bone consists of collections of cylindrical units of concentric lamellae (singular, lamella) or layers of mineralized extracellular matrix surrounding a centrally located canal. These units are known as osteons or haversian systems. The collagen fibers within a single lamella are arranged parallel with each other, but in different directions when compared with adjacent lamellae. This arrangement, reminiscent of plywood, gives compact bone tissue great strength. The centrally located canal is known as the osteonal or haversian canal and contains blood vessels, nerves, and some loose connective tissue. The haversian canals usually run parallel to the long axis of the bone. Lacunae, containing osteocytes, are located mostly between the concentric lamellae, although some may be located within the lamellae. Canaliculi, containing osteocyte processes, generally are arranged in a radial pattern with respect to the haversian canals. Perforating or Volkmann’s canals are channels carrying blood vessels and nerves that travel from the superficial surface of the bone to the marrow cavities. They usually run perpendicular or oblique to the haversian canals, such that the Volkmann’s canals also connect haversian canals with each other.
Spongy bone is similar in structure to compact bone, except that the matrix is arranged in trabeculae and spicules. If the trabeculae are relatively thick, they may contain true osteons; otherwise, the trabeculae are composed of irregularly arranged lamellae of matrix and lacunae. There are numerous interconnecting marrow cavities of various sizes between the portions of bony matrix. This honeycomb arrangement of the mineralized matrix gives cancellous bone great strength with relatively little weight.
The bones of the skeleton can be called organs because they are composed of several tissues. Not only are they composed of bone tissue, but also they have other connective tissues (e.g., hemopoietic and adipose tissues, blood vessels, nerves) and usually some sort of specialized articular regions (e.g., in the vertebrae; see the preceding discussions of Z joints and IVDs). A layer of dense, irregular connective tissue called periosteum covers the outer surface of bones, except where there are specializations for the formation of a joint. The periosteum has a more fibrous outer layer with fibroblasts and type I collagen fibers. The inner layer is more cellular and consists mostly of osteoprogenitor cells. There are special bundles of periosteal collagen fibers called Sharpey’s fibers that penetrate into the bone matrix and attach the periosteum to the bone. Periosteum contains a rich vascular supply and network of nerves. Endosteum is the connective tissue layer that lines the inner surface of bones and surrounds the marrow cavity. It is thinner than the periosteum, being mostly a single layer of osteoprogenitor cells with a minimal amount of extracellular matrix.
Skeletal Muscle
Muscle tissue is responsible for motion of the body as a whole and for the movements of various parts of the body in relation to each other. It is characterized by having elongated cells that are arranged parallel to one another. The primary physiologic property of muscle tissue is the ability to contract or forcefully shorten the length of its cells. There are three types of muscle tissue that can be recognized in the body. Skeletal muscle has cylindrical, multinucleated cells that exhibit cross striations. It is found in the named muscles of the body and is under voluntary control. Cardiac muscle also has cross striations, but the cells are branched and each has only one nucleus. It is found exclusively in the myocardium of the heart and is involuntary. Smooth muscle has spindle-shaped cells with no cross striations. It is mostly found in the walls of viscera and the vascular system, in the arrector pili muscles of the skin, and in the intrinsic muscles of the eye. It is under autonomic and hormonal control. Although there is smooth muscle in the walls of the blood vessels in the back, and the skin of the back has arrector pili muscles, neither smooth nor cardiac muscle is discussed here.
Skeletal muscle cells are known as muscle fibers (Fig. 14-23). Do not confuse this use of the term fiber with connective tissue fibers. In muscle, the term fiber refers to a cell, but in connective tissue, fibers are extracellular structures that are produced by fibroblasts. Each skeletal muscle fiber is enveloped by endomysium (see the previous section Fascia for a further description of the connective tissues and fascia associated with muscle). Skeletal muscle fibers are found grouped into fascicles or bundles by thicker connective tissue layers. Each of these connective tissue layers is called a perimysium (see Fascia section). An entire muscle consists of a variable number of muscle fascicles that are entirely surrounded by a dense connective tissue layer called epimysium (see Fascia). The rich blood, lymphatic, and nerve supply of a muscle penetrates and ramifies within these connective tissue layers. Also, these connective tissue layers, especially the endomysium, electrochemically isolate each skeletal muscle fiber from its neighbors. This means that, unlike in cardiac and smooth muscle, each skeletal muscle fiber must be specifically stimulated to contract by the nervous system. This allows a great amount of control of the force produced by a muscle.
Each skeletal muscle fiber is multinucleated because it developed from the fusion of multiple cells known as myoblasts. Myoblasts forming the muscles in the region of the back are of mesenchymal origin (see Chapter 12). The nuclei of muscle fibers are elongated and characteristically found in the periphery of the cytoplasm, just below the sarcolemma or plasma membrane of the muscle cell. The majority of the cytoplasm is filled with contractile elements known as myofibrils that extend the entire length of the muscle fiber (Ross et al., 2003). Myofibrils are composed of bundles of thick and thin myofilaments that are organized in a specific pattern. Each myofilament is composed of specific protein molecules (see the following). Surrounding each myofibril is a well-developed smooth endoplasmic reticulum known as the sarcoplasmic reticulum. Numerous mitochondria and glycogen deposits are in the regions between the myofibrils. The sarcolemma has numerous invaginations known as T (transverse) tubules that penetrate the cytoplasm to encircle each myofibril at regular intervals. Flanking the T tubules are expanded regions of the sarcoplasmic reticulum known as terminal cisternae. These function as Ca++ sinks. The sarcolemma can be depolarized by the neural stimulation at a special synapse known as the neuromuscular (myoneural) junction or motor end plate. A single axon may synapse only with one muscle fiber in muscles that need fine motor control (e.g., in some laryngeal muscles) or with up to a couple hundred fibers in muscles that have more mass actions (e.g., quadriceps femoris muscles) (Mescher, 2010).
When viewed in longitudinal section with some magnification, skeletal muscle fibers exhibit cross striations of alternating light and dark bands (see Fig. 14-23). The dark bands are known as A bands because they are anisotropic to polarized light. The light bands are known as I bands because they are isotropic to polarized light. Sometimes visible at the light microscopic level, but more commonly seen at the ultrastructural level, are Z bands (discs or lines) (German, zwischen, between), which bisect the I bands. A sarcomere is the functional unit of organization of the myofilaments within the myofibril. A sarcomere extends from Z band to Z band. The linear repetitions of these sarcomere units form the myofibrils. The cross striations seen at the light microscopic level are the result of the sarcomeres being arranged in register between adjacent myofibrils.
At the electron microscopic level, each sarcomere can be seen to consist of several bands that are created by the relationships between the thin and thick myofilaments (see Fig. 14-23). The I band is made up of thin myofilaments whose ends are anchored in the Z band. The A band is composed of thick myofilaments. The A band is bisected by a lighter area known as the H band (German, helle, light). In the middle of the H band is a narrow, dense region known as the M band (disc or line) (German, mitte, middle). The M band represents the anchored region of the middle of the thick myofilaments and is the center of the sarcomere. The thick and thin myofilaments partially overlap each other; therefore the dark portion of the A band actually is the region of overlap and consists of both thick and thin myofilaments. The H band is the region of the sarcomere that contains only thick myofilaments.
Thin myofilaments are composed of three proteins: F actin, tropomyosin, and troponin. G (globular) actin monomers polymerize into double-stranded, helically arranged F (filamentous) actin. In the groove between the two strands is a myosin-binding site on each G actin. Tropomyosin is a filamentous protein that lies in the groove of F actin and sterically inhibits the myosin-binding sites of the actin. Troponin is a globular protein that attaches to tropomyosin and also has a Ca++-binding site.
Thick myofilaments are composed of myosin that has a filamentous (“tail”) portion and a globular (“head”) portion. The tail portion is the part that is anchored at the M line. The head portion is located in the region of overlap of the thick and thin myofilaments and projects at nearly a right angle with respect to the tail portion of the molecule. The head has a binding site for actin and a separate binding site for ATP, which also acts as an ATPase.
Briefly the mechanism that explains how skeletal muscles contract that has received the most acceptance is the sliding filament hypothesis (Pollard & Earnshaw, 2002). The process is initiated when the sarcolemma is depolarized by neural activation of the neuromuscular junction. This depolarization is carried to the sarcoplasmic reticulum by the T tubules, which causes Ca++ release from the terminal cisternae. The Ca++ binds to troponin and causes a conformational change in that molecule. This conformational change in troponin causes the attached tropomyosin to shift position such that the myosin-binding site of actin is made available. Myosin and actin then bind to each other. Although there are many myosin heads projecting off a single thick myofilament, at any given time only a few are aligned in such a way that they can bind to actin. This binding of myosin and actin causes an ATP molecule (that is already bound to myosin) to be split into adenosine diphosphate (ADP) and inorganic phosphate (Pi). The resultant release of energy causes a conformational change in the myosin head in relation to the tail. This pulls the attached actin toward the center of the sarcomere, and creates contraction. The conformational change in the myosin head causes release of the ADP, and a new ATP molecule binds to the site. The binding of the new ATP molecule causes the release of actin and the myosin head to return to its original conformation. Therefore with contraction, the thin myofilaments are pulled and slide along the thick myofilaments toward the center of the sarcomere, hence the name of the hypothesis. This explains why the I and H bands get narrower and the Z bands get closer together during contraction. It must be remembered that a single muscle contraction (“twitch”) is the result of hundreds of these binding and unbinding cycles.
Not only do muscles receive motor innervation, which stimulates contraction, but also they have sensory innervation. The specialized proprioceptive element in muscle is called a muscle spindle, from its fusiform shape. A muscle spindle is a mechanoreceptor that consists of a connective tissue capsule that is filled with fluid. In the fluid-filled space are some specialized muscle fibers known as intrafusal fibers. The regular muscle fibers that comprise the majority of the muscle sometimes are known as extrafusal fibers. There are two types of intrafusal fibers, based on the arrangement of their nuclei. In the nuclear bag cells, the nuclei are clumped in the center of the cell. In the other type of cell, known as nuclear chain cells, the nuclei form a row. Each intrafusal fiber has a sensory neuron terminal associated with the center of the cell. In addition, the ends of the intrafusal fibers, which have contractile capability, receive motor innervation in the form of a gamma motor neuron. Muscle spindles are stretch receptors (see Chapter 9).
Tendons also have sensory innervation that plays a role in proprioception. This is in the form of encapsulated receptors known as Golgi tendon organs. These mechanoreceptors are typically found in the tendon near the tendon-muscle interface. Golgi tendon organs appear to signal tension on a tendon (see Chapter 9).
Neural Tissue
The nervous system is the chief organ system that correlates the adjustments and reactions of the body to internal and external environmental conditions. Anatomically it is divided into the central and peripheral nervous systems. The central nervous system (CNS) consists of the encephalon or brain and spinal medulla or cord. The peripheral nervous system (PNS) is the rest of the mass of neural tissue, which includes peripheral nerves, collections of nerve cell bodies known as ganglia, and specialized nerve endings. The nervous system also can be divided into functional units known as the somatic and autonomic nervous systems. The somatic nervous system supplies all of the parts of the body except viscera, smooth muscle, and glands. The autonomic nervous system supplies the targets that the somatic nervous system does not. It can be divided into a generally energy-mobilizing portion known as the sympathetic nervous system and a generally energy-sparing portion called the parasympathetic nervous system (see Chapter 10).
Nerve tissue consists of two main types of cells: neurons or nerve cells and neuroglia or supporting cells. Neurons are the functional units of the nervous system that can conduct electrochemical impulses known as action potentials or nerve impulses. Action potentials are transient depolarizations of the cell membrane of the neuron. Neurons are arranged into networks in which the impulses travel from one part of the system to another. There are specialized contacts between neurons that allow the transmission of signals from one neuron to the next that are called synapses.
Neuroglia cells are the nonconducting cells of the nervous system. They serve to support the neurons in various ways that include physical protection, trophic functions, participation in impulse conduction, and, in the CNS, defense.
Neurons
The human nervous system contains more than 10 billion neurons (Ross et al., 2003). Functionally, there are three general categories of neurons: afferent, efferent, and interneurons. Afferent neurons carry information, generally sensory in nature, from the periphery into the CNS. Efferent neurons carry information, much of which is motor, from the CNS to targets in the periphery. Interneurons, which make up the vast majority of the neurons in the nervous system, communicate between neurons within the CNS.
Each neuron consists of a cell body and processes, known as axons and dendrites (Fig. 14-24). The cell body (soma or perikaryon) contains the nucleus of the cell. Unfortunately, a couple of terms can be confusing. The term nucleus not only can refer to the organelle of the neuron that contains the genetic material, but also can be used to denote a collection of nerve cell bodies within the CNS. The term neuron not only is used for the conductive cells of the nervous system, but also is sometimes used to refer only to the nerve cell body. This latter definition of the term neuron is used more often when referring to the nerve cell bodies that are found in the nuclei of the CNS or in peripheral ganglia. Besides the nucleus of the cell, the cell body contains the usual cytoplasmic organelles present that maintain the cell. Prominent in the cytoplasm are stacks of rough endoplasmic reticulum or polyribosomes called Nissl substance or bodies. These are necessary because neurons synthesize a large amount of proteins. Mitochondria also are represented in large numbers because of the high metabolic activity associated with neurons.
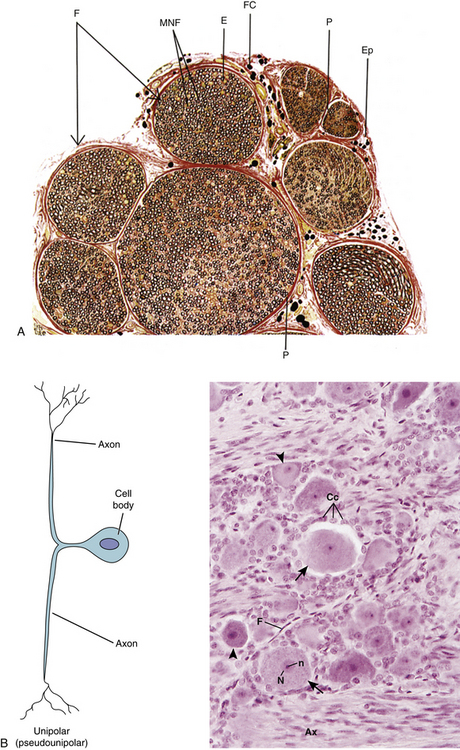
FIG. 14-24 Nervous tissue. A, Cross section of a peripheral nerve. E, Endoneurium; Ep, epineurium; F, fasciculi; FC, fat cells; MNF, myelinated nerve fibers; P, perineurium. B, Illustration (left) of a pseudounipolar neuron and a light micrograph (right, ×270 magnification) of a dorsal root ganglion showing large (arrows) and small (arrowheads) cell bodies and cell processes of many such neurons. Ax, Axons; Cc, cell bodies of satellite cells; F, fibroblasts; N, nucleus; n, nucleolus. C, Illustration (left) of a multipolar neuron and a light micrograph (right, ×612 magnification) showing the cell body (notice the axon hillock) and the proximal aspect of the cell processes (dendrites and axon) of a multipolar neuron. A, Axon; Axhi, axon hillock; D, dendrite; Pe, perikaryon. D, Cross section of the spinal cord (×21 magnification) with inset (for E) demonstrating the interface between the ventral gray horn and the adjacent white matter. A, Arachnoid mater; CC, central canal; DH, dorsal horn; DM, dura mater; DR, dorsal root; G, gray matter; Gc, anterior gray commissure; PM, pia mater; SS, subarachnoid space; VH, ventral horn; VR, ventral root; W, white matter. E, Region identified in the inset of D. This region includes both the gray matter of the ventral horn of the spinal cord and the adjacent white matter. BV, Blood vessel; CB, cell bodies of multipolar neurons; W, white matter; asterisks, interface between gray and white matter; arrowheads, nuclei of glial cells within the white matter; arrows, nuclei of glial cells within the gray matter (×132 magnification). (A, From Williams PL et al. [Eds.]. [1995]. Gray’s anatomy [38th ed.]. Edinburgh: Churchill Livingstone. B [left] and C [left], From Gartner LP & Hiatt JL. [2001]. Color textbook of histology [2nd ed.]. Philadelphia: Saunders. B [right], D, and E, From Gartner LP & Hiatt JL. [2000]. Color atlas of histology [3rd ed.]. Philadelphia: Lippincott Williams & Wilkins. C [right], From Bergmann RA & Afifi AK. [1974]. Atlas of microscopic anatomy: a companion to histology and neuroanatomy. Philadelphia: WB Saunders.)
Besides a cell body, each neuron has a variable number of processes. Each neuron has a single axon that carries impulses away from the soma and forms a synapse (or synapses) where it contacts another neuron or other effector target. Most axons ramify distally such that a typical neuron in the CNS forms more than 1000 synapses (Kandel & Siegelbaum, 2000). The specific portion of the axon that helps form a synapse is known as the axon terminal or terminal bouton. The region of the cell body from which the axon arises is known as the axon hillock. There is a characteristic lack of Nissl substance in this region. Just distal to the axon hillock is the initial segment of the axon. It is in this location that action potentials are generated. Most axons are relatively long, as compared with dendrites, and many are myelinated. Neurons almost always have dendrites that carry impulses toward the soma. Typically dendrites are short, thicker in diameter than axons, and unmyelinated. In most neurons the dendrites ramify extensively into what is known as the dendritic tree. Neurons may be classified by the number of processes they have. Multipolar neurons have one axon and two or more dendrites. Motor neurons and interneurons typically have this morphology. Bipolar neurons have one axon and one dendrite. They are only found associated with the neural circuitry of the special senses. Pseudounipolar or unipolar neurons have a single process that is axonal in structure. Actually, during development these cells had a separate dendrite and axon that fused together to make a single process attaching to the soma. This single process splits into a central process that enters the CNS and a peripheral process that terminates peripherally as a sensory receptor. Only primary somatosensory afferent neurons have this arrangement.
Because most synthetic activity is restricted to the soma of the neurons, it is necessary to have an axonal transport system to move materials along the processes of the neuron, including dendrites. This transport system uses mostly microtubules and intermediate filaments to move materials. Axonal transport is a bidirectional system. Anterograde transport carries material from the soma to the periphery of the cell, whereas retrograde transport moves molecules from the periphery to the cell body. It has been shown that various types of materials in the cell are transported at different speeds such that both slow and fast axonal transport systems are recognized in neurons.
A synapse is the site of functional apposition between neurons, at which an impulse is transmitted from one neuron (the presynaptic neuron) to another (the postsynaptic neuron). Synapses are also found between neurons and their nonneural effector (target) cells (e.g., muscle or gland cells). Synapses may be formed between various parts of neurons. Most commonly they are axodendritic, in which a presynaptic axon forms a synapse with a postsynaptic dendrite. Many times in the CNS, the postsynaptic dendrite has a special structure for the synapse known as a dendritic spine that may help modify synaptic activity. Synapses may also form between axons and cell bodies of neurons (axosomatic) and between axons of two neurons (axoaxonic). In vertebrates, almost all synapses are chemical synapses in which the conduction of impulses from one neuron to the next is achieved by the release of a chemical neurotransmitter (e.g., acetylcholine, norepinephrine, and glycine) by the presynaptic terminal. The neurotransmitter diffuses across the synaptic cleft, which is the 20- to 30-nm space that separates the two neurons involved in the synapse. The neurotransmitter then may either excite (depolarize) or inhibit (hyperpolarize) the cell membrane of the postsynaptic neuron. This is achieved by interaction of the neurotransmitter with ligand-gated ion channels that are located in the postsynaptic membrane. These ion channels appear to be organized by an electron-dense structure known as the postsynaptic density. There are a few electrical synapses known as nexuses that can be found in certain places of the vertebrate CNS. These allow action potentials to spread directly from one neuron to the next and are structurally and functionally identical to the gap junctions that occur between cardiac muscle cells and between smooth muscle cells.
A typical neuron in the CNS is postsynaptic to or receives thousands of synapses (Kandel & Siegelbaum, 2000). Ultimately, the ability of an action potential to be generated in the postsynaptic neuron depends on the summation of all of the synaptic activity on the neuron. If the synaptic activity sufficiently depolarizes the cell membrane to reach threshold, an action potential is generated in the initial segment of the axon and carried by the axon to the synapse(s) for which it is presynaptic. If threshold is not reached, the cell does not generate an action potential, and the impulse from the presynaptic neurons is not passed along the neural circuit.
Neuroglia
There are approximately 10 times more neuroglia or glial cells than neurons in the mammalian nervous system (Mescher, 2010). These supporting cells surround the nerve cell bodies and processes and furnish the microenvironment for the neurons. Sometimes only the supporting cells of the CNS are termed neuroglia, whereas the ones in the PNS are referred to only as supporting cells or peripheral neuroglia (Ross et al., 2003). The various types of neuroglia include the following:
Schwann cells are associated with axons in the PNS. These axons are either myelinated or unmyelinated. If the axon is unmyelinated, it is embedded within a simple cleft in the Schwann cell (Ross et al., 2003). This helps maintain the microenvironment of the unmyelinated axon. Unlike with myelinated axons, a single Schwann cell may be associated with multiple unmyelinated axons. A lipid-rich layer known as the myelin sheath surrounds myelinated axons and acts as an insulator (or more correctly, a capacitor) for the axon, allowing saltatory proliferation of the action potentials carried by the axon. The myelin sheath is composed of layers of the plasmalemma of the Schwann cells. Each Schwann cell wraps tightly around a single axon multiple times in a spiral fashion reminiscent of a cinnamon roll. The Schwann cell cytoplasm of each layer is removed such that the layers of the Schwann cell membrane fuse with each other to form the lipoprotein complex known as myelin. A thin cytoplasmic rim remains and surrounds the myelin. This rim, which contains the organelles of the Schwann cell, including its nucleus, is known as the neurilemma. Islands of cytoplasm found in the myelin sheath are known as clefts of Schmidt-Lanterman and appear to be necessary for metabolic maintenance of the myelin. The myelin sheath is interrupted at intervals by the nodes of Ranvier. These gaps between adjacent Schwann cells are necessary for saltatory conduction of action potentials. Saltatory conduction causes the axon cell membrane in the gap between two Schwann cells to depolarize; the depolarization then “skips” from one gap to the next along the axon. Saltatory conduction dramatically increases the conduction velocity of axons.
Oligodendrocytes or oligodendroglia are associated with axons in the CNS. They provide the myelination for these axons in a way similar to that of Schwann cells in the PNS. Unlike Schwann cells, which surround a single axon, oligodendrocytes surround more than one axon. Oligodendrocytes have several dendritelike processes, hence the name of the cell, each of which surrounds an axon. There are other biochemical differences in the myelination of CNS versus PNS axons that may be involved in the lack of regenerative ability of CNS axons.
Astrocytes are support cells of the CNS. These are by far the most numerous type of glial cell, and therefore the most numerous type of cell in the nervous system. Each has a cell body with numerous processes giving it a starlike shape, hence the name. Cells with fewer, longer processes are known as fibrous astrocytes and are located in the white matter. Cells with more numerous, shorter processes are called protoplasmic astrocytes and are found in the gray matter. Astrocytes completely surround the neurons of the CNS, except where they are myelinated by the oligodendrocytes. Astrocytes have a unique type of intermediate filament made of glial fibrillar acidic protein that helps these cells provide strong structural support to the neurons. Astrocytes also help relate neurons to capillaries. In fact, some astrocytes have processes with expanded end-feet that attach to capillaries. These appear to have a role in maintaining the blood-brain barrier. Astrocytes appear to help regulate the movement of metabolites and wastes into and out of neurons along with controlling the ionic concentration of the extracellular compartment. Therefore astrocytes appear to play a major role in maintaining the microenvironment of the CNS neurons and their synapses.
Satellite cells surround the soma of neurons in peripheral ganglia. Ganglia or peripheral ganglia are collections of nerve cell bodies in the PNS. Satellite cells appear to have a similar function to Schwann cells associated with unmyelinated axons in the PNS or astrocytes in the CNS. They help establish and maintain the microenvironment of the cell bodies of neurons in the ganglia.
Ependymal cells form the simple cuboidal to columnar epithelial lining of the ventricles of the brain and the central canal of the spinal cord, which are the remnants of the lumen of the neural tube. The basal surfaces of the ependymal cells interdigitate with astrocyte processes. There is no basal lamina associated with this epithelium. The apical surfaces of ependymal cells have cilia that may help move cerebrospinal fluid. Microvilli also can be found on the apical surface of these cells. These may be involved with absorption of cerebrospinal fluid.
Microglia are elongated cells that are relatively small and possess several irregular processes. They are actively phagocytic in regions of injury or disease. They represent the mononuclear phagocytic system in the CNS. As such they are derived from the bone marrow and migrate to the CNS by way of blood vessels.
Organization of the Central Nervous System
As stated, the CNS consists of the brain and spinal cord. All of the cells of the CNS, with the exception of microglia, are derived from the neural tube (see Chapter 12). When sectioned and viewed with the unaided eye, there are regions that look lighter in color (white matter) and darker in color (gray matter). White matter consists mostly of myelinated and unmyelinated nerve fibers along with their associated glial cells. In the CNS, a nerve fiber is an axon with its associated oligodendrocytes. The presence of large amounts of the fatty substance myelin is responsible for the lighter color. There are no neuron cell bodies in white matter. Gray matter is composed of nerve cell bodies, dendrites, axons (myelinated and unmyelinated), and associated glial cells. Sometimes the term neuropil is used to describe the mass of neuronal processes in the gray matter. Synapses are located in the regions of gray matter. The relative lack of myelin in these regions is responsible for their darker color. In much of the brain, the gray matter is located peripherally, whereas the white matter is more internal, although the regions of gray and white matter are not separated as simply in the brain stem. Discrete groups of functionally related nerve cell bodies in the CNS gray matter are called nuclei.
The relationship of the gray and white matter in the spinal cord is reversed when compared with that of most of the brain. The gray matter of the spinal cord is internal and forms the general shape of a capital H. The ventral cornua or horns contain neurons that are involved in motor function. Very large, multipolar neurons that innervate skeletal muscles are located here. These multipolar neurons are sometimes called lower motor neurons, and the axons of these neurons form the ventral roots of the spinal nerves. The dorsal horns have neurons, most of which are relatively smaller in size, that are concerned with sensory functions. Sensory input into the dorsal horns enters via the dorsal roots of the spinal nerves. Several discrete nuclei are recognized in the gray matter of the spinal cord. In the center of the spinal cord is the central canal, which is the remnant of the caudal portion of the neural tube lumen and is lined with ependymal cells.
The white matter surrounds the gray matter of the spinal cord. This tissue consists of various functionally related columns of nerve fibers called tracts or fasciculi that mostly run in a vertical fashion to carry information to and from the brain. Other groups of fibers called commissures also connect structures on opposite sides of the spinal cord. Many other intersegmental fibers can be found in the white matter of the spinal cord.
Few connective tissues are found within the brain and spinal cord, so they are soft and watery in nature; however, three layers of connective tissue, known as meninges (singular, meninx), surround the CNS. These layers are of mesenchymal origin. They are, from superficial to deep, dura mater, arachnoid, and pia mater. The dura mater or pachymeninx is the thickest and toughest of the meninges. It consists of dense, irregular collagenous connective tissue. Unlike around the brain, where it fuses with the inner periosteum of the skull, the dura mater surrounding the spinal cord is separated from the vertebrae by a fat-filled epidural space. The dura mater has a rich blood supply and is innervated.
The other two layers of the meninges are thin compared with the dura mater and are sometimes collectively known as the leptomeninges. The arachnoid is a thin avascular layer of connective tissue that is loosely adherent to the dura and separated from the dura mater by a potential space known as the subdural space. The arachnoid also has delicate trabeculae composed of loose connective tissue that connect it with the pia mater. These trabeculae give the arachnoid a spiderweb appearance, hence the name. The regions between the trabeculae are collectively called the subarachnoid space. The subarachnoid space is filled with cerebrospinal fluid. The pia mater is a very thin layer usually consisting of a single layer (or in some places several layers) of cells that is intimately attached to the entire surface of the CNS. It also surrounds the perivascular connective tissue of the arteries and veins, but not capillaries, of the brain and spinal cord.
The capillaries of the CNS are less permeable to certain substances than in other parts of the body. This decreased permeability of these capillaries is termed the blood-brain barrier. It is mostly the result of occluding junctions between the endothelial cells, making these continuous-type capillaries. These occluding junctions are morphologically similar to tight junctions of other types of epithelia. The end-feet of astrocytes that are associated with these capillaries also seem to be involved in this blood-brain barrier, especially in maintaining water homeostasis in the CNS (Ross et al., 2003).
Organization of the Peripheral Nervous System
The main components of the PNS are nerves, ganglia, and nerve endings. Other than the nerve endings described previously (i.e., muscle spindles and Golgi tendon organs), the structure of the other types of nerve endings, which are mostly cutaneous in nature, is beyond the scope of this discussion. See Peripheral Receptors in Chapter 9 for a discussion of this topic.
Nerves, or peripheral nerves, are bundles of nerve fibers, usually both myelinated and unmyelinated, held together by connective tissue. In the PNS, a nerve fiber is essentially an axon and the surrounding Schwann cells. In the spinal nerves, the cell bodies for the motor nerve fibers are located in the anterior horn of the spinal cord. The cell bodies for the sensory nerve fibers are located in the dorsal root ganglion (see the following).
Surrounding each Schwann cell–ensheathed axon is a thin layer of reticular fibers called the endoneurium. These fibers appear to be produced by the Schwann cells, because there is a lack of fibroblasts in the vicinity. The fibers run both parallel to and around the axons, functionally binding them into fascicles. Around each fascicle of axons is the perineurium. It is made up of one or more layers of flattened epithelial-like cells. When there is more than one layer of perineurial cells (e.g., in the larger-caliber nerve fascicles) these cells appear to produce collagen fibers. They may even be contractile, given the large amount of actin found in their cytoplasm (Ross et al., 2003). Tight junctions connect the perineurial cells. Therefore perineurium acts as a metabolic diffusion barrier for the nerve fibers and establishes a blood-nerve barrier similar to the blood-brain barrier of the CNS. This protective function of the perineurium seems important to the normal functioning of peripheral nerves. In fact, there is a dearth of classic connective tissue cells, including most immune system cells, within peripheral nerves. About the only cells other than Schwann cells that can be found within a peripheral nerve are the occasional fibroblast and some mast cells. Surrounding the entire nerve is a relatively thick, dense irregular collagenous connective tissue layer known as the epineurium. In larger nerves adipose tissue may be associated with the epineurium. Blood vessels (vasa nervorum) and nerves (nervi nervorum) supplying the nerve penetrate into the epineurium and travel in the perineurium. The tissue at the level of the endoneurium is poorly vascularized, and metabolic exchange must occur through diffusion from the perineurium. In the region of the intervertebral foramen, the epineurium of the spinal nerves becomes continuous with the dural root sleeve surrounding the spinal nerve roots (see Chapter 3).
Ganglia are collections of nerve cell bodies in the PNS that have a connective tissue framework and capsule for support. There are two varieties of ganglia. The first is sensory ganglia. The neuronal cells in these ganglia are pseudounipolar. Each cell body is surrounded by cuboidal-shaped satellite cells. The fibers associated with sensory ganglia are the peripheral and central processes of the pseudounipolar neurons. These fibers only travel through the ganglion and do not synapse. Dorsal root ganglia are the example of this type of ganglion in the region of the back. The second type of ganglion is the autonomic ganglion. The cell bodies of the multipolar postganglionic autonomic neurons reside in these ganglia. Usually there is a layer of relatively flattened satellite cells surrounding the cell bodies of these neurons. Unlike the sensory ganglia, in which no synapses occur, there is an abundance of synapses in autonomic ganglia. These are where the axons of preganglionic autonomic neurons synapse with the dendrites of the postganglionic autonomic neurons. The ganglia of the sympathetic gangliated chains are examples of this type of ganglion in the region of the back.
References
Adams, M., Pathophysiology of disc degeneration. Haldeman, S., eds. 3rd ed. New York: McGraw-Hill Companies, Inc; 2005:383–400.
Antoniou, J., et al. The human lumbar end plate. Spine. 1996;21:1153–1161.
Antonopoulos, C.A., et al. Determination of glycosaminoglycans from tissue on the microgram scale. Biochim Biophys Acta. 1964;83:1–19.
Antonopoulos, C.A., et al. Extraction and purification of proteoglycans from various types of connective tissue. Biochim Biophys Acta. 1974;338:108–119.
Barker, P.J., et al. The middle layer of the lumbar fascia and attachments to lumbar tranverse processes: implications for segmental control and fracture. Eur Spine J. 2007;16:2232–2237.
Benjamin, M., et al. Where tendons and ligaments meet bone: attachment sites ('entheses') in relation to exercise and/or mechanical load. J Anat. 2006;208:471–490.
Benjamin, M. The fascia of the limbs and back—a review. J Anat. 2009;214:1–18.
Benjamin, M., Evans, E.J., Copp, L. The histology of tendon attachments to bone in man. J Anat. 1986;149:89–100.
Benjamin, M., Kaiser, E., Milz, S. Structure-function relationships in tendons: a review. J Anat. 2008;212:211–228.
Bishop, P. Pathophysiology of the intervertebral disc. In Haldeman S., ed.: Principles and practice of chiropractic, 2nd ed., East Norwalk, Conn: Appleton & Lange, 1992.
Bloom, W., Fawcett, D. Textbook of histology, 3rd ed. Philadelphia: WB Saunders; 1986.
Bogduk, N. Pathology of lumbar disc pain. Manual Med. 1990;5:72–79.
Bogduk, N., et al. The nerve supply to the human lumbar intervertebral discs. J Anat. 1981;132:39–56.
Bogduk, N. Clinical anatomy of the lumbar spine, 4th ed. London: Churchill Livingstone; 2005.
Boos, N., et al. Classification of age-related changes in lumbar intervertebral discs: 2002 Volvo award in basic science. Spine. 2002;27:2631–2644.
Boszczyk, B.M., et al. An immunohistochemical study of the dorsal capsule of the lumbar and thoracic facet joints. Spine. 2001;26:E338–E343.
Bouche, R.T., Johnson, C.H. Medial tibial stress syndrome (tibial fasciitis): a proposed pathomechanical model involving fascial traction. J Am Podiatr Med Assoc. 2007;97:31–36.
Bouffard, N.A., et al. Tissue stretch decreases soluble TGF-beta1 and type-1 procollagen in mouse subcutaneous connective tissue: evidence from ex vivo and in vivo models. J Cell Physiol. 2008;214:389–395.
Brisby, H., et al. Nitric oxide as a mediator of nucleus pulposus-induced effects on spinal nerve roots. J Orthop Res. 2000;18:815–820.
Broberg, K.B. On the mechanical behavior of intervertebral disc. Spine. 1983;8:151–161.
Brown, M.D. The pathophysiology of disc disease. Orthop Clin North Am. 1971;2:359–370.
Brown, M.F. Sensory and sympathetic innervation of the vertebral end plate in patients with degenerative disc disease. J Bone Joint Surg Br. 1997;79(1):147–153.
Chai, B.-F., Tang X-, M. Electron microscopic observation of normal, protruding and ruptured lumbar intervertebral discs. Chin Med J. 1987;100(9):723–730.
Chandraraj, S., Briggs, C.A., Opeskin, K. Disc herniations in the young and end-plate vascularity. Clin Anat. 1998;11(3):171–176.
Cole, T.C., Ghosh, P., Taylor, T.K. Variations of the proteoglycans of the canine intervertebral disk with aging. Biochim Biophys Acta. 1986;19:209–219.
Coppes, M.H., et al. Innervation of 'painful' lumbar discs. Spine. 1997;22:2342–2349.
Crean, J.K.G., et al. Matrix metalloproteinases in the human intervertebral disc: role in disc degeneration and scoliosis. Spine. 1997;22:2877–2884.
Crock, H.V. Internal disc disruption: a challenge to disc prolapse fifty years on. Spine. 1986;1:650–653.
Duance, V.C., et al. Changes in collagen cross-linking in degenerative disc disease and scoliosis. Spine. 1998;23:2545–2551.
Edgar, M.A. The nerve supply of the lumbar intervertebral disc. J Bone Joint Surg Br. 2007;89:1135–1139.
Fardon, D.F. Nomenclature and classification of lumbar disc pathology. Spine (Phila Pa 1976). 2001;26:461–462.
Findley, T., Schleip, R. Introduction. In: Findley T., Schleip R., eds. Fascia research, basic science and implications for conventional and complementary health care. Munich: Elsevier, 2007.
Ghosh, P. Basic biochemistry of the intervertebral disc and its variation with ageing and degeneration. J Man Med. 1990;5:48–51.
Gibson, W., et al. Increased pain from muscle fascia following eccentric exercise: animal and human findings. Exp Brain Res. 2009;194:299–308.
Giles, L.G. The pathophysiology of the zygapophysial joints. In Haldeman S., ed.: Principles and practice of chiropractic, 2nd ed., East Norwalk, Conn: Appleton & Lange, 1992.
Giles, L.G. The surface lamina of the articular cartilage of human zygapophyseal joints. Anat Rec. 1992;233:350–356.
Giles, L.G.F. Pathophysiology of the posterior zygapophysial (facet) joints. In: Haldeman S., ed. Principles and practices of chiropractic. New York: McGraw Hill Companies Inc; 2005:401–417.
Giles, L.G., Taylor, J.R. Human zygapophyseal joint capsule and synovial fold innervation. Br J Rheumatol. 1987;26:93–98.
Gower, W.E., Pedrini, V. Age related variations in protein polysaccharides from human nucleus pulposus, anulus fibrosus, and costal cartilage. J Bone Joint Surg. 1969;51:1154–1162.
Gruber, H., Hanley, E.J. Analysis of aging and degeneration of the human intervertebral disc: comparison of surgical specimens with normal controls. Spine. 1998;23:751–757.
Gruber, H.E., Hanley, E.N., Jr. Ultrastructure of the human intervertebral disc during aging and degeneration: comparison of surgical and control specimens. Spine. 2002;27:798–805.
Guehring, T., Omlor, G.W., Lorenz, H., et al. Disc distraction shows evidence of regenerative potential in degenerated intervertebral discs as evaluated by protein expression, magnetic resonance imaging, and messenger ribonucleic acid expression analysis. Spine (Phila Pa 1976). 2006;31:1658–1665.
Hamanishi, C., et al. Schmorl's nodes on magnetic resonance imaging. Their incidence and clinical relevance. Spine. 1994;19(4):450–453.
Handa, T., et al. Effects of hydrostatic pressure on matrix synthesis and matrix metalloproteinase production in the human lumbar intervertebral disc. Spine. 1997;22:1085–1091.
Hangai, M., Kaneoka, K., Kuno, S., et al. Factors associated with lumbar intervertebral disc degeneration in the elderly. Spine J. 2008;8:732–740.
Hardingham, T.E., Adams, P.A. A method for the determination of hyaluronate in the presence of glycosaminoglycans and its application to human intervertebral disc. Biochem J. 1976;159:143–147.
Herzog, R.J. The radiologic assessment for a lumbar disc herniation. Spine. 1996;21:19S–38S.
Horner, H.A., Urban, J.P. 2001 Volvo Award Winner in Basic Science Studies: effect of nutrient supply on the viability of cells from the nucleus pulposus of the intervertebral disc. Spine. 2001;26(23):2543–2549.
Huijing, P.A. Muscular force transmission: a unified, dual, or multiple system? A review and some explorative experimental results. Arch Physiol Biochem. 1999;107:292–311.
Huijing, P.A. Muscular force transmission necessitates a multilevel integrative approach to the analysis of function of skeletal muscle. Exerc Sport Sci Rev. 2003;31:167–175.
Huijing, P.A. Epimuscular myofascial force transmission between antagonist and synergistic muscles can explain movement limitation in spastic paresis. J Electromyogr Kinesiol. 2007;17:297–311.
Huijing P.A., et al, eds. Fascia research II, basic science and implications for conventional and complementary health care. Munich: Elsevier, 2009.
Hutton, W.C., et al. Analysis of chondroitin sulfate in lumbar intervertebral discs at two different stages of degeneration as assessed by discogram. J Spinal Disord. 1997;10:47–54.
Hutton, W.C., et al. The effect of compressive force applied to the intervertebral disc in vivo: a study of proteoglycans and collagen. Spine. 1998;23:2524–2537.
Hutton, W.C., et al. The effect of hydrostatic pressure on intervertebral disc metabolism. Spine. 1999;24(15):1507–1515.
Hutton, W.C., et al. Effect of tail suspension (or simulated weightlessness) on the lumbar intervertebral disc: study of proteoglycans and collagen. Spine. 2002;27(12):1286–1290.
Iatridis, J.C., MacLean, J.J., O'Brien, M., et al. Measurements of proteoglycan and water content distribution in human lumbar intervertebral discs. Spine (Phila Pa 1976). 2007;32:1493–1497.
Imai, Y., Okuma, M., An, H.S., et al. Restoration of disc height loss by recombinant human osteogenic protein-1 injection into intervertebral discs undergoing degeneration induced by an intradiscal injection of chondroitinase ABC. Spine (Phila Pa 1976). 2007;32:1197–1205.
Inerot, S., Axelsson, I. Structure and composition of proteoglycans from human anulus fibrosus. Connect Tissue Res. 1991;26:47–63.
Iwahashi, M., et al. Mechanism of intervertebral disc degeneration caused by nicotine in rabbits to explicate intervertebral disc disorders caused by smoking. Spine. 2002;27(13):1396–1401.
Johnson, W.E.B., et al. Immunohistochemical detection of Schwann cells in innervated and vascularized human intervertebral discs. Spine. 2001;26:2550–2557.
Kandel, E.R., Siegelbaum, S.A. Overview of synaptic transmission. In Kandel E.R., et al, eds.: Principles of neural science, 4th ed., New York: McGraw-Hill, 2000.
Kang, J.D., et al. Herniated lumbar intervertebral discs spontaneously produce matrix metalloproteinases, nitric oxide, interleukin-6, and prostaglandin E2. Spine. 1996;21:271–277.
Kang, J.D., et al. Toward a biochemical understanding of human intervertebral disc degeneration and herniation: contributions of nitric oxide, interleukins, prostaglandin E2, and matrix metalloproteinases. Spine. 1997;22:1065–1073.
Kauppila, L.I., et al. Disc degeneration/back pain and calcification of the abdominal aorta: a 25-year follow-up study in Framingham. Spine. 1997;22:1642–1649.
Kawamata, S., et al. Structure of the rat subcutaneous connective tissue in relation to its sliding mechanism. Arch Histol Cytol. 2003;66:273–279.
Khan, S., Scott, A. Mechanotherapy: how physical therapists' prescription of exercise promotes tissue repair. Br J Sports Med. 2009;43:247–251.
Kim, K.W., et al. The origin of chondrocytes in the nucleus pulposus and histologic findings associated with the transition of a notochordal nucleus pulposus to a fibrocartilaginous nucleus pulposus in intact rabbit intervertebral discs. Spine. 2003;28(10):982–990.
Kim, K.W., Ha, K.Y., Lee, J.S., et al. Notochordal cells stimulate migration of cartilage end plate chondrocytes of the intervertebral disc in in vitro cell migration assays. Spine J. 2009;9:323–329.
Kirkaldy-Willis, W.H. The pathology and pathogenesis of low back pain. In Kirkaldy-Willis W., ed.: Managing low back pain, 2nd ed., New York: Churchill Livingstone, 1988.
Korecki, C.L., MacLean, J.J., Iatridis, J.C. Dynamic compression effects on intervertebral disc mechanics and biology. Spine (Phila Pa 1976). 2008;33:1403–1409.
Kraemer, J. Natural course and prognosis of intervertebral disc diseases. International Society for the Study of the Lumbar Spine Seattle, Washington, June 1994. Spine. 1995;20(6):635–639.
Kuisma, M., Karppinen, J., Niinimaki, J., et al. Modic changes in endplates of lumbar vertebral bodies: prevalence and association with low back and sciatic pain among middle-aged male workers. Spine (Phila Pa 1976). 2007;32:1116–1122.
Langevin, H.M., Cornbrooks, C.J., Taaties, D.J. Fibroblasts form a body-wide cellular network. Histochem Cell Biol. 2004;122:7–15.
Langevin, H.M., et al. Dynamic fibroblast cytoskeletal response to subcutaneous tissue stretch ex vivo and in vivo. Am J Physiol Cell Physiol. 2005;288:C747–C756.
Langevin, H.M., et al. Subcutaneous tissue fibroblast cytoskeletal remodeling induced by acupuncture: evidence for a mechanotransduction-based mechanism. J Cell Physiol. 2006;207:767–774.
Langevin, H.M., et al. Tissue stretch induces nuclear remodeling in connective tissue fibroblasts. Histochem Cell Biol. 2010;133:405–415.
Langevin, H.M., et al. Fibroblast cytoskeletal remodeling contributes to connective tissue tension. J Cell Physiol. 2011;226:1166–1175.
Liebscher, T., Haefeli, M., Wuertz, K., et al. Age-related variation in cell density of human lumbar intervertebral disc. Spine (Phila Pa 1976). 2011;36:153–159.
Lotz, J.C., Chin, B.A., Urban, J.P. Intervertebral disc cell death is dependent on the magnitude and duration of spinal loading. Spine. 2000;25:1477–1483.
Lotz, J.C., et al. Compression-induced degeneration of the intervertebral disc: an in vivo mouse model and finite-element study. Spine. 1998;23:2493–2506.
Luschka, H. Die altersveranderungen der zwischenwirbellnorpel. Virchows Arch. 1856;11:8.
Luschka, H. Uber gallertartige auswuchse am clivus blumenbachii. Virchows Arch. 1857;11:8.
Maeda, S., Kokubun, S. Changes with age in proteoglycan synthesis in cells cultured in vitro from the inner and outer rabbit annulus fibrosus. Responses to interleukin-1 and interleukin-1 receptor antagonist protein. Spine. 2000;25(2):166–169.
Malinsky, J. The ontogenetic development of nerve terminations in the intervertebral discs of man. Acta Anat. 1959;38:96–113.
Maroudas, A., et al. Factors involved in the nutrition of the human intervertebral disc. J Anat. 1975;120:13–130.
McDevitt, C.A. Proteoglycans of the intervertebral disc. In: Ghosh P., ed. Biology of the intervertebral disc. Boca Raton, Fla: CRC Press, 1988.
Mescher, A.L. Junqueira's basic histology, 12th ed. New York: McGraw-Hill; 2010.
Mok, F.P., Samartzis, D., Karppinen, J., et al. ISSLS prize winner: prevalence, determinants, and association of Schmorl nodes of the lumbar spine with disc degeneration: a population-based study of 2449 individuals. Spine (Phila Pa 1976). 2010;35:1944–1952.
Mooney, V., Robertson, J. The facet syndrome. Clin Orthop Res. 1976;115:149–156.
Moore, R.J., et al. The origin and fate of herniated lumbar intervertebral disc tissue. Spine. 1996;21:2149–2155.
Nachemson, A., et al. In vitro diffusion of dye through the end plates and the annulus fibrosus of human lumbar intervertebral disks. Acta Orthop Scand. 1970;41:589–607.
Nishida, K., et al. Modulation of the biologic activity of the rabbit intervertebral disc by gene therapy: an in vivo study of adenovirus-mediated transfer of the human transforming growth factor β1 encoding gene. Spine. 1999;24:2419.
Oda, J., Tamaka, H., Tsukuki, N. Intervertebral disk changes with aging of human cervical vertebra: from the neonate to the eighties. Spine. 1988;13:1205–1211.
Ozawa, T., Ohtori, S., Inoue, G., et al. The degenerated lumbar intervertebral disc is innervated primarily by peptide-containing sensory nerve fibers in humans. Spine (Phila Pa 1976). 2006;31:2418–2422.
Pate, D. Roentgen report: Schmorl's nodes. MPI's Dynam Chiro. 1991. [Sept 13].
Peacock, A. Observation on the prenatal development of the intervertebral disc in man. J Anat. 1951;185:260–274.
Pollard, T.D., Earnshaw, W.C. Cell biology. Philadelphia: WB Saunders; 2002.
Poiraudeau, S., et al. Phenotypic characteristics of rabbit intervertebral disc cells. Comparison with cartilage cells from the same animals. Spine. 1999;24:837–844.
Pritzker, P.H. Aging and degeneration in the lumbar intervertebral disc. Orthop Clin. 1977;8:65–77.
Purslow, P.P. Muscle fascia and force transmission. J Bdywrk Mvmnt Ther. 2010;14:411–417.
Roberts, S., et al. Biochemical and structural properties of the vertebral end plate and its relation to the intervertebral disc. Spine. 1989;14:166–174.
Roberts, S., et al. Transport properties of the human cartilage end plate in relation to its composition and calcification. Spine. 1996;21(4):415–420.
Ross, M.H., et al. Histology, 4th ed. Baltimore: Lippincott Williams & Wilkins; 2003.
Sachs, B.L., et al. Dallas discogram description: a new classification of CT/discography in low-back disorders. Spine. 1987;12:287–294.
Sairyo, K., Biyani, A., Goel, V.K., et al. Lumbar ligamentum flavum hypertrophy is due to accumulation of inflammation-related scar tissue. Spine (Phila Pa 1976). 2007;32:E340–E347.
Schollmeier, G., Lahr-Eigen, R., Lewandrowski, K.U. Observations on fiber-forming collagens in the anulus fibrosus. Spine. 2000;25(21):2736–2741.
Siemionow, K., An, H., Masuda, K., et al. The effects of age, sex, ethnicity, and spinal level on the rate of intervertebral disc degeneration: a review of 1712 intervertebral discs. Spine (Phila Pa 1976). 2011;36:1333–1339.
Standring, S., et al. Gray's anatomy: the anatomical basis of clinical practice, 40th ed. Edinburgh: Churchill Livingstone; 2008.
Stevens, R.L., Dondi, P.G., Muir, H. Proteoglycans of the intervertebral disk: absence of degradation during the isolation of proteoglycans from the intervertebral disk. Biochem J. 1979;179:573–578.
Stilwell, D.L. Regional variations in the innervation of deep fasciae and aponeuroses. Anat Rec. 1957;127:635–653.
Swann, D.A., et al. Role of hyaluronic acid in joint lubrication. Annu Rev Dis. 1974;33:318–326.
Swatland, H.J. Morphology and development of connective tissue in porcine and bovine muscle. J Animal Sci. 1975;41:78–86.
Taylor, J.R. The development and adult structure of lumbar intervertebral discs. J Man Med. 1990;5:43–47.
Taylor, J.R., Twomey, L.T. Age changes in lumbar zygapophyseal joints: observations on structure and function. Spine. 1986;11:739–745.
Triano, J. Interaction of spinal biomechanics and physiology. In Haldeman S., ed.: Principles and practice of chiropractic, 2nd ed., East Norwalk, Conn: Appleton & Lange, 1992.
Vernon-Roberts, B., Pirie, C.J. Degenerative changes in the intervertebral disks of the lumbar spine and their sequelae. Rheumatol Rehabil. 1977;16:13–21.
Vernon-Roberts, B., Moore, R.J., Fraser, R.D. The natural history of age-related disc degeneration: the pathology and sequelae of tears. Spine (Phila Pa 1976). 2007;32:2797–2804.
Videman, T., Nurminen, M. The occurrence of anular tears and their relation to lifetime back pain history: a cadaveric study using barium sulfate discography. Spine (Phila Pa 1976). 2004;29:2668–2676.
Virchow, R.L. Untersuchung uber die entwickelung des schadel grindes in gesunden und krankhaften zustande und uber den eintfuss derselben auf schadelform, gesichsbildun und gehirnbau. Berlin: G. Reimer; 1857.
Vleeming, A., et al. The posterior layer of the thoracolumbar fascia. Its function in load transfer from spine to legs. Spine. 1995;20:753–758.
Weiss, L. Histology, 6th ed. New York: McGraw-Hill; 1988.
Whiting, W., Zernicke, R. Biomechanics of musculoskeletal injury, 2nd ed. Champaign, IL: Human Kinetics Publishers; 2008.
Woelf, H.J., et al. Role of the notochord in human intervertebral disc. I. Fetus and infant. Clin Orthop Rel Res. 1975;39:205–212.
Wood Jones, F. Structure and function as seen in the foot. London: Baillière, Tindall & Cox; 1944.
Wyke, B. The neurology of back pain. In Jayson M.I.V., ed.: The lumbar spine and back pain, 3rd ed., New York: Churchill Livingstone, 1987.
Xu, G.L., et al. Normal variations of the lumbar facet joint capsules. Clin Anat. 1991;4:117–122.
Yahia, L., et al. Sensory innervation of human thoracolumbar fascia. An immunohistochemical study. Acta Orthop Scand. 1992;63:195–197.
Yamashita, T., et al. A morphological study of the fibrous capsule of the human lumbar facet joint. Spine. 1996;21:538–543.
Yayama, T., Uchida, K., Kobayashi, S., et al. Thoracic ossification of the human ligamentum flavum: histopathological and immunohistochemical findings around the ossified lesion. J Neurosurg Spine. 2007;7:184–193.
Yoshizawa, H., O'Brien, J., Thomas-Smith, W. The neuropathology of the intervertebral discs removed for low back pain. J Pathol. 1980;132:95.
Yu, J., Fairbank, J.C., Roberts, S., et al. The elastic fiber network of the anulus fibrosus of the normal and scoliotic human intervertebral disc. Spine (Phila Pa 1976). 2005;30:1815–1820.
Yucesoy, C.A., Huijing, P.A. Substantial effects of epimuscular myofascial force transmission on muscular mechanics have major implications on spastic muscle and remedial surgery. J Electromyogr Kinesiol. 2007;17:664–679.
Zhang, Y., Drapeau, S., An, H.S., et al. Histological features of the degenerating intervertebral disc in a goat disc-injury model. Spine (Phila Pa 1976). 2011;36:1519–1527.