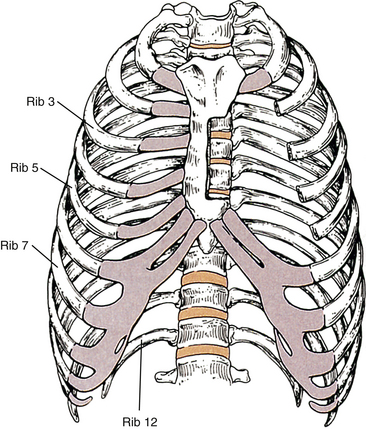
The Thoracic Region
The thoracic region contains the most vertebrae (12) of any of the movable regions of the spine. Consequently, it is the longest region of the spine. However, because of its relationship with the ribs, which attach anteriorly to the sternum, the thoracic region has relatively little movement. Many of the unique characteristics of the thoracic region result from its anatomic relationships with the ribs. The typical thoracic vertebrae are T2 through T8. T1, T9 (occasionally), T10, T11, and T12 perhaps can best be described as unique rather than “atypical.” The size of the thoracic vertebrae generally increases from the superior to the inferior vertebrae, just as the load they are required to carry increases from superior to inferior.
This chapter first discusses the typical thoracic curve, vertebrae, ribs, and sternum. This is followed by a discussion of the thoracic vertebrae that have unique features (T1, T9 to T12). Next, ligaments with distinctive features in the thoracic region are covered. Many ligaments are described with the cervical region in Chapter 5 and are not covered again here. This chapter also includes a brief discussion of lateral curves that may develop in the thoracic region (scoliosis). The last section is devoted to nerves, vessels, and visceral structures associated with the thoracic vertebrae and the thoracic cage.
Thoracic Curve (Kyphosis)
As stated in Chapter 2, the normal thoracic curve is a rather prominent kyphosis, which extends from T2 to T12 (Fig. 6-1, G, to 6-1, I). It is created by the larger superior-to-inferior dimensions of the posterior portion of the thoracic vertebrae (Masharawi et al., 2008).
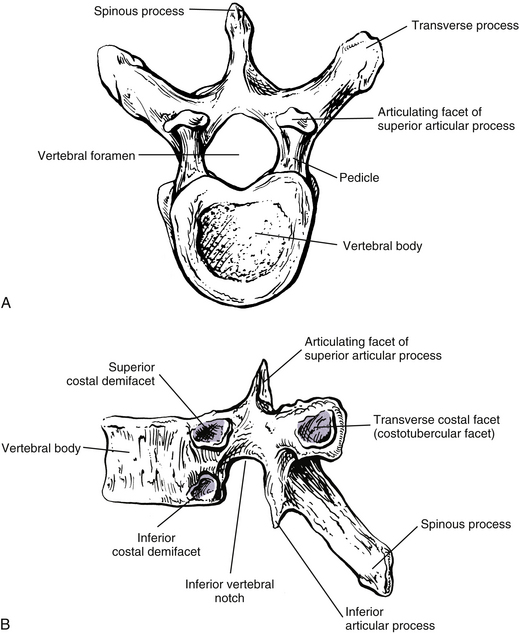
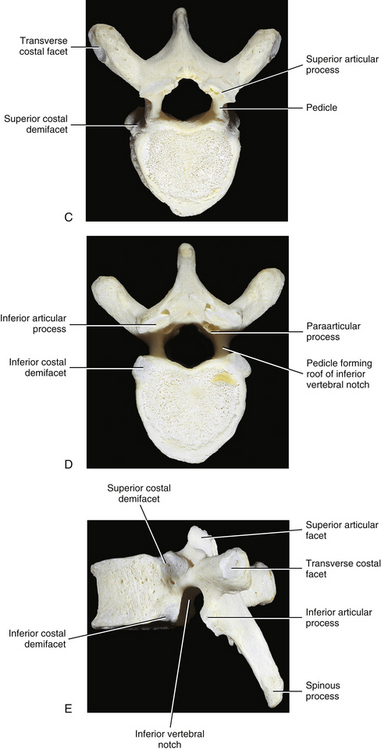
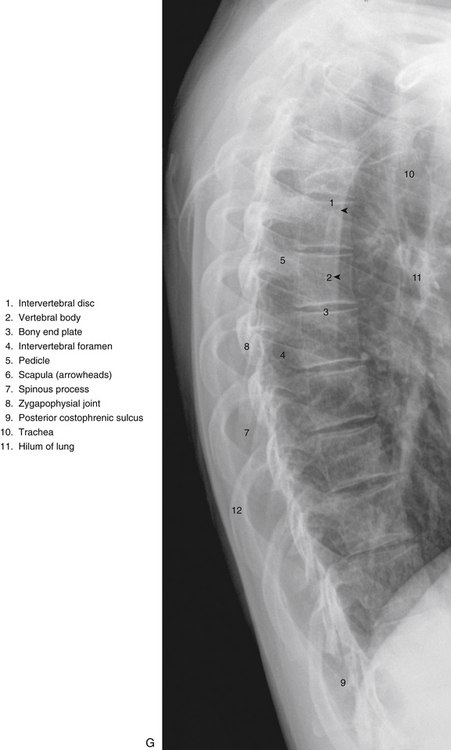
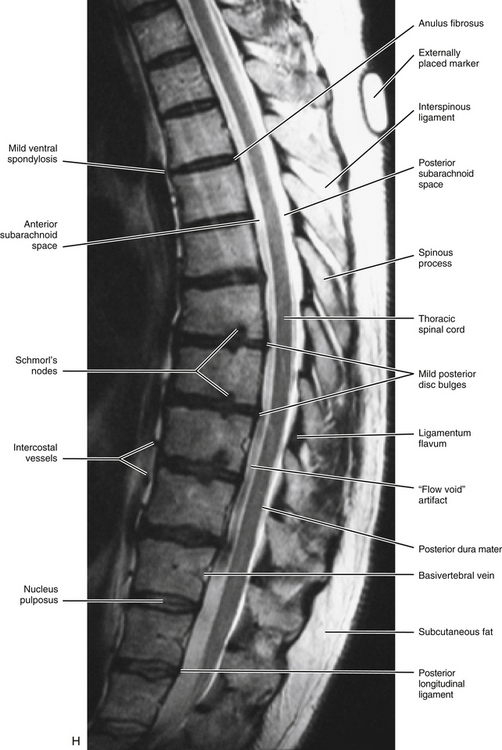
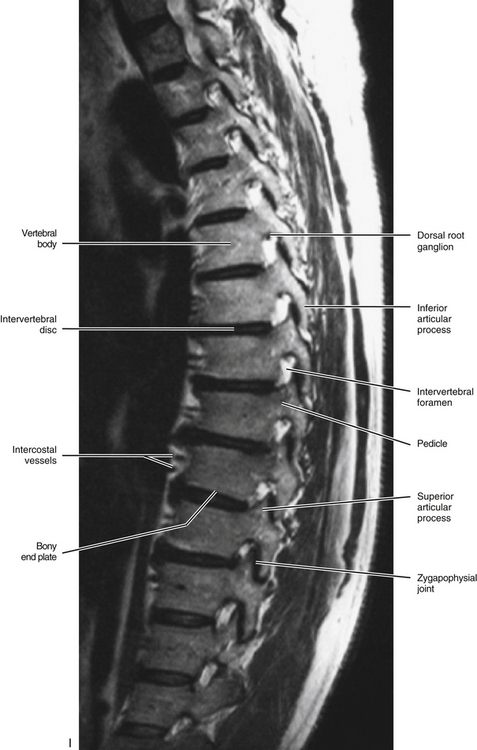
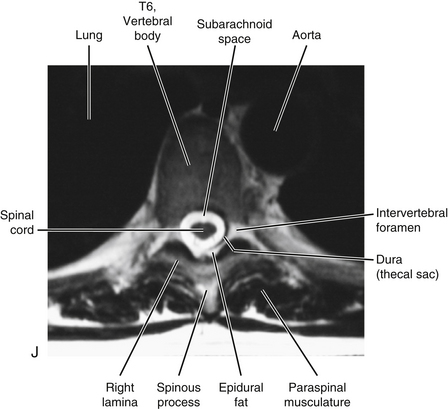
FIG. 6-1 Typical thoracic vertebra and x-ray and MRI images of the thoracic spine. A, Superior view. B, Lateral view.Photograph of a typical thoracic vertebra. C, Superior view. D, Inferior view. E, Lateral view. F, Anterior-posterior x-ray. G, Lateral x-ray. H, Midsagittal MRI of the upper to lower thoracic region (T2-weighted image). Mild spondylosis (bone spurs, osteophyte formation) is present along the anterior aspect of several vertebral bodies. I, Right parasagittal MRI of the thoracic region (approximately 1 cm right lateral toH). J, Horizontal (axial) MRI through the vertebral body of T6. (F-J, Courtesy Dr. William Bogar, National University of Health Sciences, Lombard, IL.)
Prolonged changes in the forces received by the thoracic region can result in postural changes and pain. For example, carrying book bags on one shoulder has been found to adversely affect posture and gait (Pascoe et al., 1997).
Occasionally the thoracic kyphosis is almost completely absent. This is logically called the straight back syndrome. This syndrome is associated with systolic heart murmurs and a distorted cardiac silhouette on x-ray film; as a result it can simulate organic heart disease. The straightening of the thoracic kyphosis results in a narrowing of the anterior-to-posterior dimension of the thoracic cage, which decreases the space available for the heart. The heart is forced to shift to the left, which leads to kinking of the great vessels. This results in a variety of heart murmurs. The straight back syndrome also has been associated with idiopathic mitral valve prolapse, a potentially life-threatening condition (Spapen et al., 1990).
Scheuermann’s Disease
Another clinical condition that can affect the thoracic kyphosis is Scheuermann’s disease. This disease is found in 0.4% to 8.3% of adolescents. Although its cause is unknown, there appears to be a genetic component to Scheuermann’s that is combined with increased physical stress and/or repeated trauma (Faingold et al., 2004). The condition has two forms based almost entirely on the location of the disease—classical Scheuermann’s disease, which affects the thoracic region and is seen in two thirds of cases; and Scheuermann’s disease of the lumbar region (one third of cases), which is generally considered to be the more painful form (Faingold et al., 2004). Both types usually occur during adolescence. Scheuermann’s disease is characterized by disruption of the cartilaginous end plate and fragmentation of the anular apophyses and the adjacent bone of the vertebral bodies (bony end plate) of many adjacent vertebral segments. The vertebral bodies become wedge-shaped (shorter anteriorly than posteriorly) if the disease is untreated. This results in an increase in the thoracic kyphosis, or in the case of the lumbar form of the disease, a continuation of the thoracic kyphosis into the upper lumbar region. As a result, the condition is the most common cause of a pathologic increase in the kyphosis of adolescents. Scheuermann’s disease affects males and females with equal frequency (Lemire et al., 1996).
Scheuermann’s disease usually begins at 10 to 12 years of age; however, radiologic changes are usually not apparent at this stage. The individual typically seeks treatment at 12 to 15 years of age because of aching back pain, an increase in thoracic kyphosis, or both. Irregularity and fragmentation of the bony end plates usually can be seen on x-rays at this time. The apex of the curve is at T8 in two thirds of the cases. The apex is usually in the upper lumbar region in the remainder of cases (lumbar type). Progression of the condition is slow in the beginning, increases as skeletal maturity of the spine occurs, and ends when vertebral growth is complete (up to 25 years of age). Multiple Schmorl’s nodes (end plate fractures) are another hallmark of the condition. Schmorl’s nodes typically are found in approximately 36% of all spines, but in up to 93% of spines with an increase in kyphosis resulting from Scheuermann’s disease. In addition, the intervertebral disc spaces usually narrow, especially in the segments affected by Schmorl’s nodes (Lemire et al., 1996).
In the classical form of Scheuermann’s disease, the affected individual may develop a stooped and round-shouldered appearance, with generally poor posture. Not all patients have pain; in those who do experience pain, it is frequently described as aching rather than constant and incapacitating. The pain almost always disappears as skeletal maturity is reached. The increased kyphosis that develops usually is rigid or fixed rather than flexible in nature (Lemire et al., 1996).
The severity of the condition typically is tracked by measuring the kyphosis and the vertebral wedging of affected vertebrae. The kyphosis is calculated by drawing lines anteriorly from the bony end plates at the superior and inferior boundaries of the kyphosis and then measuring the angle between these lines. The normal average is approximately 25 degrees (range, 10 to 40 degrees). A kyphosis greater than 45 degrees is indicative of Scheuermann’s disease. Vertebral wedging is measured by drawing a line anteriorly from the bony end plates of an individual vertebra. One or more vertebrae with wedging of greater than 5 degrees also is indicative of Scheuermann’s disease (Lemire et al., 1996).
Complications can occur in Scheuermann’s disease. The spinal cord may become compressed against the posterior surfaces of the vertebral bodies forming the peak of the kyphosis, leading to signs and symptoms of myelopathy (signs of a bilateral upper motor neuron lesion). In addition, in rare instances the condition may progress to form a significant deforming kyphosis (Lemire et al., 1996).
Treatment for Scheuermann’s disease includes bracing the spine in extension and providing palliative treatment for pain and discomfort. Exercise also has been found to be beneficial. Most patients respond well to such treatment (Lemire et al., 1996).
Typical Thoracic Vertebrae, Ribs, and Sternum
Vertebral Bodies
The size of the thoracic vertebral bodies is approximately the same in males and females of all races and of all adult ages (Masharawi et al., 2008; Limthongkul et al., 2010). The vertebral bodies of the typical thoracic vertebrae (T2 to T8) are larger than those of the cervical region (Fig. 6-1). The volume of the vertebral bodies increases from T1 to T12 (average 15.0 cm3; range 5.2 to 39.5 cm3) (Limthongkul et al., 2010). Thoracic vertebral bodies appear to be heart-shaped when viewed from above, primarily because of a marked concavity of the posterior aspect of the vertebral body in the region adjacent to the vertebral foramen. The posterior edge of the superior surface of upper thoracic vertebral bodies exhibits small remnants of the cervical uncinate processes (Dupuis et al., 1985).
The left-right (lateral) width of the thoracic vertebral bodies is greater than the anterior-posterior (length) and superior-inferior (height) dimensions. The lateral width decreases from T1 through T3 and then gradually increases throughout the thoracic region (continuing into the lumbar region until L4-L5). The decreasing width of the upper thoracic vertebral bodies may allow for increased lateral flexion (and possibly rotation) of the cervical region (Masharawi et al., 2008). In addition, the lateral width of a thoracic vertebra is smaller at the superior versus inferior vertebral margin of the vertebra and the lateral width of the inferior aspect of each vertebra is always larger than the superior width of the subjacent vertebra. Consequently, in a coronal (frontal) section the vertebral bodies have a trapezoid shape (i.e., narrower superiorly) and the intervertebral discs have an inverted trapezoid shape (i.e., narrower inferiorly) (Masharawi et al., 2008). Typical thoracic vertebrae also are more flattened on their left than right surfaces because of pressure from the thoracic aorta.
The thoracic vertebral bodies are wedged-shaped, the superior-inferior height being smaller anteriorly than posteriorly. The wedging increases from T1 to T7 and then begins to decrease incrementally until L2 (Masharawi et al., 2008). The posterior aspect of a typical thoracic vertebra is approximately five times the height of the posterior aspect of the intervertebral disc immediately above the vertebra (Harrison et al., 2003).
The superior-inferior height of most thoracic vertebral bodies is asymmetric with the right side usually greater than the left side. This “left lateral wedging” is typically found in one or several (usually 3) contiguous vertebrae, “interrupted” by a single vertebra of symmetric left and right heights, followed by additional vertebrae with left lateral wedging. This pattern is repeated throughout the thoracic region. Further investigation is needed to determine if the thoracic intervertebral discs normally compensate for the left lateral wedging of the vertebral bodies. The mild lateral wedging may help to provide stability to the thoracic region during the normal spinal loading (i.e., ground reaction force) that occurs throughout the day. The left lateral wedging is more pronounced in females (92% of females) than in males (86% of males). The female-predominant left lateral wedging may explain the clinical findings that the large thoracic curves of most adolescent idiopathic scolioses are most often found in girls and are usually convex to the right (Masharawi et al., 2008).
The superior-inferior height of the T1 and T2 vertebral bodies is greater than the anterior-posterior length. This ratio is reversed throughout the rest of the thoracic region. Consequently, the T1 and T2 vertebral bodies are more rounded when viewed from the left or right sides. This configuration may help to accommodate the large amount of flexion and extension that occurs in the cervical region (Masharawi et al., 2008). The T2 vertebral body is somewhat cervical in appearance, being slightly larger in transverse than anteroposterior diameter. The body of the T3 vertebra is the smallest of the thoracic region; the vertebral bodies gradually increase in size below this level. The vertebral bodies of T5 through T8 become more and more heart-shaped. This means that the concavity of the posterior aspect of the vertebral bodies becomes more prominent. The heart-shaped appearance also is accentuated because the anteroposterior dimension of the vertebral bodies increases more than the transverse (lateral) dimension at these levels.
The T9 through T12 vertebral bodies begin to acquire lumbar characteristics (see the following discussion) and enlarge more in their transverse than anteroposterior dimension. The T12 vertebral body is similar in shape to that of a lumbar vertebra.
The thoracic vertebral bodies become stronger from upper to lower thoracic vertebrae. This results from an increase in bone density that is probably a response to the increase in compressive forces placed on the successively lower vertebral bodies (Humzah & Soames, 1988).
The neurocentral synchondrosis (see Chapter 12), found between the neural arches and centra of developing vertebrae, fuses first in the lumbar and cervical regions and last in the thoracic region. This fusion may be incomplete in the thoracic region, even in adults (Edelson & Nathan, 1988).
Osteophytes are more prominent at T9-10 than at other thoracic levels. Generally, the aorta decreases the formation of osteophytes (bone spurs) on the thoracic vertebral bodies. Recall that the thoracic aorta courses along the left side of the thoracic vertebral bodies. It then begins to move to the anterior surface of the lower thoracic vertebrae, before passing through the aortic hiatus of the diaphragm. Consequently, osteophytes are found more on the right than left sides of the typical thoracic vertebral bodies (Nathan, 1962; Edelson & Nathan, 1988).
Typical thoracic vertebral bodies have four small facets, two on each side, for articulation with the heads of two adjacent ribs. These facets are known as costal demifacets (literally, “half-facets”) because the head of each rib articulates with both the superior demifacet of the vertebra with the same number and the inferior demifacet of the vertebra above (see Fig. 6-9). For example, the head of the sixth rib articulates with the superior demifacet of T6 and the inferior demifacet of T5. A ridge on the head of each rib, known as the crest of the head, is located between the two articular surfaces of the rib head. The crest of the head of each rib has a ligamentous attachment (intraarticular ligament) to the intervertebral disc (IVD) between adjacent thoracic vertebrae. A fibrous capsule surrounds each vertebral demifacet and continues to the rib surrounding the articular surface on the corresponding half of the rib head. The capsule is lined by synovium, making the costovertebral joint (costocorporeal joint) a synovial joint (diarthrosis). The radiate ligament extends from the head of each rib to the adjoining vertebral bodies and the surface of the intervening IVD (see Costocorporeal Articulations).
Several structures attach to the thoracic vertebral bodies. Table 6-1 summarizes these attachments.
Table 6-1
Attachments to Thoracic Vertebral Bodies
| Region | Structure(s) Attached |
| Anterior surface | Anterior longitudinal ligament, origin of longus colli muscle (T1, T2, T3, lateral to anterior longitudinal ligament) |
| Posterior surface | Posterior longitudinal ligament |
| Lateral surface | Origin of psoas major and minor muscles from T12 |
Pedicles
The pedicles of the thoracic spine are long and stout (Fig. 6-1). In fact, the pedicles in the lower thoracic region are larger than the pedicles of the upper lumbar vertebrae (Ofiram et al., 2007). The size of the thoracic pedicles varies considerably among both individuals and vertebrae, but the left and right pedicles of the same vertebra usually are similar (McLain, Ferrara, & Kabins, 2002). The T4 pedicles are the narrowest (left-to-right dimension) and then the pedicles of T5 to T12 become increasingly wider (Kretzer et al., 2011). The pedicles of males are wider than those of females (Pai et al., 2010; Kretzer et al., 2011), and there is ethnic variation in the size of the pedicles; for example, the dimensions of thoracic pedicles are smaller among East Indians and white populations (Acharya, Dorje, & Srivastava, 2010).
Unlike the pedicles of the cervical vertebrae, cancellous bone, rather than cortical bone, predominates in the thoracic pedicles. However, like the cervical pedicles, the cortical bone of the lateral wall of a typical thoracic pedicle is thinner than that of the medial wall (Kothe et al., 1996).
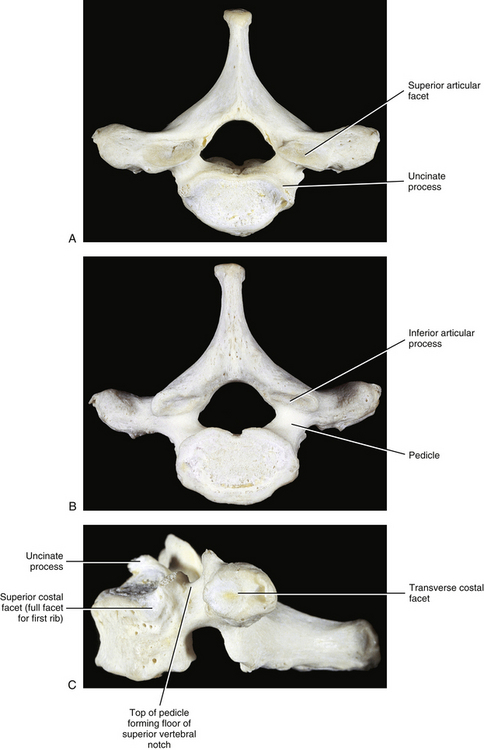
The thoracic pedicles become larger along their inferior surface from T1 to T12. Also, unlike the cervical pedicles, which attach at a significant lateral angle with the cervical vertebral bodies, the thoracic pedicles form only a slight lateral angle in the transverse plane with the thoracic vertebral bodies (and T12 forms no lateral angle with the vertebral body in this plane, i.e., a 90º angle to the vertebral body). The thoracic pedicles incline slightly superiorly in the sagittal plane (Marchesi et al., 1988). They also attach very high on their respective vertebral bodies; as a result, no superior vertebral notch is associated with typical thoracic vertebrae. T1, which is atypical, does have a superior vertebral notch (see First Thoracic Vertebra later in this chapter). On the other hand, the inferior vertebral notches of the typical thoracic vertebrae are very prominent.
Transverse Processes
The transverse processes (TPs) of typical thoracic vertebrae project obliquely posteriorly (see Chapter 2) (Fig. 6-1). They also lie in a more posterior plane than those of the cervical or lumbar regions, being located behind the pedicles, intervertebral foramina, and articular processes of the thoracic vertebrae (Standring et al., 2008). The TPs also become progressively shorter from T1 to T12; therefore the distance between the tips of the left and right TPs is the greatest at T1 and then decreases incrementally until T12, where the TPs are very small. This distance increases in the lumbar region (see Chapter 7). As with other components of thoracic vertebrae, the left and right transverse processes are asymmetric in length, with the left transverse process being longer than the right. In addition, the transverse process of males are larger than those of females (Masharawi & Salame, 2011).
Each thoracic TP possesses a facet for articulation with the articular tubercle of the corresponding rib (e.g., the TP of T6 articulates with the sixth rib). This facet is appropriately named the transverse costal facet, or costal facet of the transverse process, and is located on the anterior surface of the TP.
The first six transverse costal facets are concave and face not only anteriorly but also slightly laterally. The transverse costal facets inferior to T6 are more planar (flatter) in shape and face anteriorly, laterally, and superiorly. The forces applied to the ribs during movements, load carrying, or muscular contraction are transmitted through the TPs to the laminae of the thoracic vertebrae (Pal et al., 1988).
The TPs serve as attachment sites for many muscles and ligaments. Table 6-2 lists the most important attachments to the TPs of thoracic vertebrae.
Table 6-2
Attachments to Thoracic Transverse Processes
| Region | Structure(s) Attached |
| Anterior surface | Costotransverse ligament (medial to transverse costal facet) |
| Apex | Lateral costotransverse ligament |
| Posterior apex | Levator costarum muscle |
| Inferior surface | Superior costotransverse ligament |
| Superior border | Intertransversarius muscle (or remnant) Intertransverse ligament |
| Inferior border | Intertransversarius muscle (or remnant) Intertransverse ligament |
| Posterior surface | Deep back muscles (longissimus thoracis, semispinalis thoracis and cervicis, multifidus thoracis, rotatores thoracis longus and brevis) |
From Standring S et al. (2008). Gray’s anatomy: the anatomical basis of clinical practice (40th ed.). Edinburgh: Churchill Livingstone.
Articular Processes
The superior articular processes of the thoracic spine are small superior projections of bone oriented in a plane that lies approximately 60 to 75 degrees to the horizontal plane (25 to 40 degrees to the coronal [frontal] plane) (White & Panjabi, 1990; Masharawi et al., 2004). This makes them much more vertically oriented than the cervical superior articular processes. The thoracic superior articular processes and their facets face posteriorly, slightly superiorly, and laterally (see Fig. 6-1). The left and right superior articular facets are usually asymmetric with the right facets being slightly more vertically oriented and the left ones facing slightly more laterally and also being slightly longer from superior to inferior (Masharawi et al., 2004, 2005). The inferior articular processes and their facets match those of the superior ones facing in the opposite direction—that is, anteriorly, slightly inferiorly, and medially, with matching asymmetry as well. The facet orientation and asymmetry are the same for males and females of all ages and racial origins (Masharawi et al., 2004).
The orientation of the thoracic articular processes and their articulating facets allows a significant amount of rotation to occur in this region (see Ranges of Motion in the Thoracic Spine). Flexion and extension are limited primarily by the orientation of the thoracic facets (Oda et al., 1996), and lateral flexion is limited partly by the orientation of the facets. However, the firm attachments of the thoracic vertebrae to the relatively immobile thoracic cage, by means of the costocorporeal and costotransverse articulations, are the primary constraints to axial rotation and lateral flexion of the thoracic spine.
Spondylolysis is a fracture of the region between the superior and inferior articular processes (pars interarticularis). It is usually found in the lumbar region (see Chapter 7 for a discussion of this condition), has been rarely found in the cervical region, and is considered extremely rare in the thoracic region. The first case reported in the English literature was that of Shimada and colleagues (2006), who reported a case of bilateral spondylolysis at T11 with forward slippage of the T11 vertebral body (spondylolisthesis).
Zygapophysial (Z) Joints
The zygapophysial (Z) joints are important structures clinically. These joints are thought to be the source of pain in 48% of the cases of chronic thoracic pain (Schulte et al., 2010). The capsules of the thoracic Z joints are similar to those of the cervical and lumbar regions. However, there are fewer mechanoreceptors in the Z joint capsules of the thoracic region than in the cervical or lumbar regions (McLain & Pickar, 1998).
Z Joint Synovial Folds (Meniscoids)
Z joint synovial folds (meniscoids) have been found to protrude into approximately 62% of thoracic Z joints, which is a lower incidence than the cervical and lumbar regions. Some thoracic Z joints have more than one synovial fold (Ley, 1974; Schulte et al., 2010). The folds are usually located in the inferior (caudal) aspect of the joints, although they have been identified in all regions of the joint. The folds are smaller in the thoracic region than in the other regions of the spine, but as in the cervical and lumbar regions, the thoracic synovial folds are believed capable of becoming entrapped (i.e., trapped between the articular facets) or extrapped (i.e., trapped between the articular process and Z joint capsule), both situations being a theoretical source of back pain (Kos, Hert, & Sevcik, 2002). In addition, Z joint synovial folds have been identified as a source of intraarticular bleeding associated with destruction of facetal cartilage (Schulte et al., 2010).
Thoracic Z joint synovial folds usually originate from a base of well-vascularized connective tissue and extend as flat, solid structures between the articular facets. The synovial lining is found at the base of these folds and usually does not extend between the facetal surfaces, which is different from the lumbar region where the synovial lining has been found to extend along the entire surfaces of the folds. The thoracic folds are longest in the lower thoracic Z joints, are of intermediate length in the upper thoracic region, and are shortest in the mid-thoracic region. These synovial fold lengths correspond to the mobility of the thoracic Z joints (most mobile to least mobile = lower, upper, and middle thoracic Z joints, respectively) (Schulte et al., 2010).
Approximately 6% of thoracic Z joint synovial folds are fatty in nature, also well vascularized, but are found exclusively in the joint recesses and do not extend between the articular facets. Occasionally, the adipose tissue found in the base of the solid (preceding paragraph) and fatty types of synovial folds passes through the ligamentum flavum and becomes continuous with the epidural adipose tissue of the vertebral canal (Schulte et al., 2010).
Another 4% of thoracic Z joint synovial folds are simply well-defined thickenings of the joint capsule (Schulte et al., 2010).
Laminae
The laminae in the thoracic region are short from medial to lateral, broad from superior to inferior, and thick from anterior to posterior. As with other components of thoracic vertebrae, the left and right laminae are asymmetric in superior-to-inferior length, with the right laminae being longer than the left. In addition, the laminae of males are larger (superior-to-inferior) than those of females (Masharawi & Salame, 2011). They completely protect the vertebral canal from behind. Therefore no space exists between the laminae of adjacent vertebrae in a dried preparation. This is unique to thoracic vertebrae. The rotatores longus and brevis muscles partially insert on the laminae of the thoracic vertebrae.
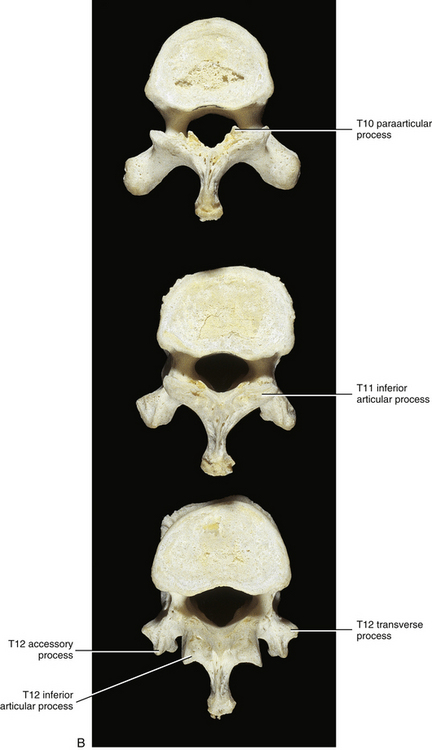
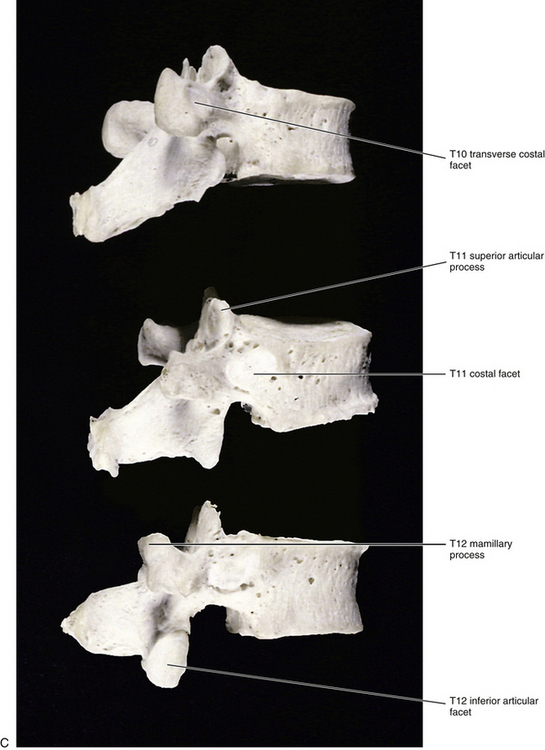
Paraarticular processes: Paraarticular processes are spurlike calcifications on the anterior and inferior aspects of the laminae of thoracic vertebrae (Figs. 6-1, D, and 6-6, B). They also have been called “laminar spurs” or “spicules,” and represent spur formations of the lateral-most attachment sites of the ligamentum flavum (LF) (Nathan, 1959). They are rarely found in the lumbar region, and they are almost never found in the cervical region. However, paraarticular processes are thought to be normal findings on thoracic vertebrae and are distinct from the condition known as ossification of the LF. Paraarticular processes have a relatively wide base and then taper to a dull point or blunt end inferiorly. They usually are paired and the left and right processes generally are of similar size, although they can be found unilaterally and also can be asymmetric in size. They are found throughout the thoracic region of the vertebral column, and their frequency increases as one descends the thoracic region from T1 to T10; the latter is the vertebra where they are most commonly found. The processes are also found at T11 and T12, but less frequently than at T10. Paraarticular processes occur with increasing frequency from 16 to 30 years of age, when they reach the maximum incidence; the incidence remains approximately the same throughout all succeeding age groups. The presence of paraarticular processes in younger age groups distinguishes them from osteophytes. The processes are thought to reinforce the attachment of the LF, adding strength to the attachment site. Nathan (1959) felt that their presence reflected increased strain placed on the LF as a result of the kyphotic architecture of the thoracic region.
More specifically, paraarticular processes attach to the lamina at the junction between the pedicle and inferior articular process and extend obliquely inferiorly and slightly anteriorly, forming a notch that opens inferiorly. The superior tip of the superior articular process of the vertebra below fits into this notch. Occasionally a bone spur extending from the superior articular process ascends and almost comes in contact with the paraarticular process. This latter process is a calcification of the inferior attachment of the LF. Other similar but smaller superior projections are found occasionally where the LF attaches along the superior margin of the laminae (Nathan, 1959).
The paraarticular processes vary in size from 1 to 15 mm in length, and the width of their attachment to the laminae ranges from 1 to 10 mm. They are found in 74.7% of spines, and are more common in whites than blacks or native Africans (whites, 82.1%; blacks, 63.8%; native Africans: Bantus, 62%; East Africans, 65%) (Nathan, 1959).
Occasionally paraarticular processes become quite large. These large processes could compress the exiting spinal nerve. However, a more likely scenario is that large paraarticular processes would predispose the exiting nerve to compression either from posterior osteophytes projecting from the vertebral body or possibly from IVD protrusion (Nathan, 1959).
Vertebral Canal
The vertebral canal in the thoracic region is more rounded in shape than in any other region (Fig. 6-1, A, C, and D). It is also smaller in the thoracic region than in either the cervical or the lumbar region (see Fig. 2-16). Compared to the other regions of the spinal cord, the thoracic spinal cord also is smaller.
Spinous Processes
The spinous processes of thoracic vertebrae generally are large (Fig. 6-1, B and E). The upper four thoracic spinous processes project almost directly posteriorly. The next four (T5 through T8) project dramatically inferiorly. The spinous process of T8 is the longest of this group. The last four thoracic spinous processes begin to acquire the characteristics of lumbar spinous processes by projecting more directly posteriorly and being larger in their superior-to-inferior dimension (see Unique Thoracic Vertebrae). The spinous processes of thoracic vertebrae serve as attachment sites for many muscles and ligaments. Because of the length of the thoracic region, attachments vary somewhat from the upper to lower thoracic vertebrae. Table 6-3 lists the attachments to the upper and lower thoracic spinous processes.
Table 6-3
Attachments to Thoracic Spinous Processes
| Region | Structure(s) Attached |
| Upper thoracic region | Ligaments: supraspinous, interspinous Muscles: trapezius, rhomboid major and minor, serratus posterior superior, deep back muscles (erector spinae and transversospinalis) |
| Lower thoracic region | Ligaments: supraspinous, interspinous Muscles: trapezius, latissimus dorsi, serratus posterior inferior, deep back muscles (erector spinae and transversospinalis) |
From Standring S et al. (2008). Gray’s anatomy: the anatomical basis of clinical practice (40th ed.). Edinburgh: Churchill Livingstone.
Intervertebral Foramina
The intervertebral foramina (IVFs) are covered in detail in Chapter 2. The IVFs in the thoracic region differ from those of the cervical region by facing directly laterally rather than obliquely anterolaterally. The lateral orientation of the thoracic IVFs is similar to that found in the lumbar region.
Unique to the thoracic region is that the T1 through T10 IVFs are associated with the ribs. The eleventh and twelfth ribs are not directly associated with IVFs. More precisely, the following structures are associated with the T1 through T10 IVFs: the head of the closest rib (e.g., T5-6 IVF associated with head of sixth rib), the articulation between the rib head and the demifacets of the vertebral bodies, including the associated ligamentous and capsular attachments with the vertebral bodies and the interposed IVD (see Costocorporeal Articulations). All these structures help to form the anterior and inferior boundaries of the first 10 thoracic IVFs. Pathologic conditions of these articulations may compromise the contents of the thoracic IVFs (Standring et al., 2008). For example, osteoarthritis (development of osteophytes) of the costocorporeal articulations may cause stenosis of the thoracic intervertebral foramina (Bailey & Casamajor, 1911).
Approximately one twelfth of the IVF contains a spinal nerve in the thoracic region, whereas up to 50% of the cross-sectional area of the IVF contains a spinal nerve (and dural root sleeve) in the cervical region (Sunderland, 1974), and approximately one third of the IVF is filled with a spinal nerve in the lumbar region. This may be one reason why radiculopathy as a result of IVD protrusion is much less common in the thoracic region than the lumbar or cervical areas. Thoracic disc protrusion is also less common than cervical or lumbar disc protrusion. One reason may be that the thoracic spine is rendered less movable than the cervical and lumbar regions. This is because the thoracic region is strongly supported by the ribs and sternum. The reduced motion may result in a reduction of stress on the thoracic IVDs.
Thoracic Cage
Discussing the bony elements of the thoracic cage here is appropriate because these elements are so intimately involved with the thoracic vertebrae. However, because the primary focus of this book is the spine, the ribs and sternum are not discussed in as much detail as the vertebrae. The intercostal muscles of the thoracic wall are covered in Chapter 4.
Components of the Thoracic Cage
The components of the thoracic cage (Fig. 6-2) include the following:
Superior Thoracic Aperture (Thoracic Inlet)
The thoracic cage is bounded superiorly by the superior thoracic aperture and inferiorly by the inferior thoracic aperture. The superior thoracic aperture is bounded by the following: T1, first ribs (left and right), and superior aspect of the sternum. The superior thoracic aperture allows connection of the anatomic structures of the thorax and the neck.
The term thoracic inlet has a slightly different meaning. It refers to the superior thoracic aperture, the region just above the first rib, and the opening between the clavicle and the first rib. Ironically the term thoracic outlet syndrome is frequently used to describe symptoms and signs arising from compromise of the neural or vascular structures as they pass through the region of the thoracic inlet. The symptoms associated with this syndrome typically are felt in the distal aspect of the upper extremity rather than the area of neurovascular compromise (Bland, 1987). The occurrence of thoracic outlet syndrome remains a matter of clinical debate, with some authorities stating that true compression of these structures is extremely rare. Others are convinced that such compression is relatively common. This section discusses the areas and structures typically associated with the thoracic outlet syndrome.
The right and left subclavian arteries and veins pass through the superior thoracic aperture. These vessels may be compromised by pathologic conditions of the lower cervical or upper thoracic viscera. Examples include lymphosarcoma affecting the lymphatics of the thoracic inlet (Moore, 1992) and tumors of the apex of the lung (Pancoast tumor), esophagus, and thyroid gland.
As the subclavian arteries and veins exit the superior thoracic aperture, they are met by the inferior structures of the brachial plexus. These neural structures include the anterior primary divisions of C8 and T1 and their union as the inferior trunk of the brachial plexus. All these vascular and neural structures pass over the first rib. The subclavian vein passes over the first rib in front of the anterior scalene muscle. The subclavian artery and lower (inferior) trunk of the brachial plexus course directly across the first rib between the insertions of the anterior and middle scalene muscles and are thought to be vulnerable in this region. Anomalous insertion of the scalenes or an anomalous inferior trunk of the brachial plexus that pierces either the anterior or the middle scalene muscles may provide the means by which these structures can become entrapped. Extension of the neck and rotation to the same side closes the interval between the anterior and middle scalene muscles, providing another possible mechanism of compromise. An elongated TP of C7 or a cervical rib (see Chapter 5) can dramatically crowd this region, and many believe that either one is a significant contributor to “thoracic outlet syndrome” (Bland, 1987; Foreman & Crofts, 1988). Cervical ribs range considerably in size, and even the smallest cervical rib can be associated with fibrous bands that course from the cervical rib to the first rib or sternum (van Es, Bollen, & van Heesewijk, 2010). Any or all of these structures could restrict the subclavian vessels and inferior trunk of the brachial plexus.
The subclavian artery becomes the axillary artery at the lateral border of the first rib. Surrounding the transition region of the subclavian artery to the axillary artery are the divisions of the brachial plexus, which soon combine into the cords of the plexus. The divisions and cords accompany the axillary artery beneath the clavicle and can be compressed between the clavicle and the first rib.
The axillary artery is surrounded by the cords of the brachial plexus as the artery passes beneath the coracoid process of the scapula. The axillary vein accompanies the artery in this region. The pectoralis minor muscle passes anterior to these structures as it inserts onto the coracoid process. The axillary artery, axillary vein, and the cords of the brachial plexus may be compressed against the coracoid process and the tendon of the pectoralis minor muscle during abduction and lateral rotation of the arm.
Terminology for Thoracic Outlet Syndrome
Thoracic outlet syndrome is characterized generally by numbness, paresthesias, pain, or a combination of these symptoms along the posterior aspect of the shoulder, axilla, and arm regions with or without vascular compression. However, there is much confusion related to the terminology surrounding thoracic outlet syndrome. Because of this confusion, Ranney (1996) has suggested a completely different nomenclature for the condition. Figure 6-3 illustrates the anatomic structures involved and the regions used in Ranney’s (1996) nomenclature.
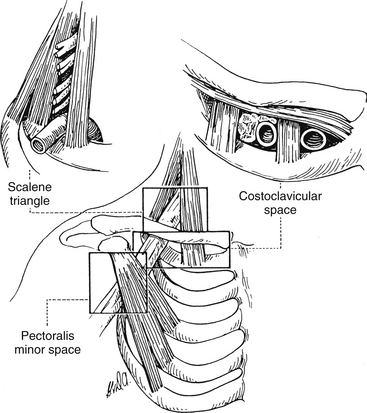
FIG. 6-3 Regions involved in the cervicoaxillary syndrome. (From Berger AC & Kleiner JM. [1991]. Work related vascular injuries and diseases. In ML Kasden [Ed.]. Occupational hand and upper extremity injuries and diseases. Philadelphia: Hunley & Belfus.)
Ranney (1996) first suggests that the term thoracic outlet syndrome be replaced with the term cervicoaxillary syndrome. Subdivisions of this syndrome would be named based on the specific anatomic region associated with the pathologic compression of neural or vascular tissues. Second, he suggests that the terms superior thoracic aperture and inferior thoracic aperture should be used instead of the clinically confusing terms thoracic inlet (for the superior thoracic aperture) and thoracic outlet (for the inferior thoracic aperture). Next, he proposes that the appropriate term scalene triangle be used for the space bordered by the anterior scalene muscle anteriorly, the middle scalene muscle posteriorly, and the first rib inferiorly. He then suggests that the superior aspect of this triangle, housing the C5, C6, and usually the C7 cervical nerve roots, be called the cervical outlet. (Compression of these structures in this region sometimes is called scalenus syndrome or scalenus anterior syndrome.) The term thoracic outlet would be reserved for the inferior aspect of the scalene triangle for the region that normally houses the C8 and T1 nerve roots and the subclavian artery. The subclavian artery or vein or the divisions or cords of the brachial plexus can be compressed in the more distal costoclavicular space. This space is bounded by the clavicle superiorly and first rib inferiorly. Finally, the pectoralis minor space is the region bounded superiorly by the pectoralis minor muscle and coracoid process to which it inserts, as well as the thoracic cage inferiorly. The term hyperabduction syndrome has been used to identify compression of the axillary artery and the cords of the brachial plexus against the pectoralis minor tendon during prolonged abduction of the upper extremity. With these terms and definitions in mind, Ranney (1996) suggests that the term cervicoaxillary syndrome (CAS) be used for compression of neural or vascular structures anywhere along the thoracic outlet, costoclavicular space, or pectoralis minor triangle, and that the more specific subtypes of CAS to include thoracic outlet, costoclavicular, and pectoralis minor syndromes be used once the precise location of compression is identified. The term cervical outlet syndrome would be used to describe compression of the C5, C6, or C7 nerve roots in the upper portion of the scalene triangle and the terms scalene syndrome and scalenus anticus syndrome would be discarded.
Subclavius Posticus Muscle
An anomalous muscle known as the subclavius posticus muscle (also known as the chondroscapular muscle or the scapulocostalis minor muscle of Gruber) can be associated with CAS. When present, the muscle usually originates along the superior border of the scapula and the transverse scapular ligament and passes anteriorly, inferiorly, and medially to insert onto the costal cartilage of the first rib. Because of its innervation from branches of the brachial plexus (e.g., the suprascapular nerve), the muscle is thought to be related to the subclavius muscle. Anatomic dissections have found the upper and middle trunks of the brachial plexus (see Fig. 5-33), as well as the subclavian artery (Forcada et al., 2001) and subclavian vein (Akita et al., 2000), to show signs of compression by this muscle, suggesting that the muscle could cause some cases of a variant of the costoclavicular syndrome of CAS (using Ranney’s terminology) and also Paget-von Schrötter syndrome (Akita et al., 2000; Forcada et al., 2001). The latter syndrome is thrombosis of the axillary vein, or its proximal continuation as the subclavian vein, that either can develop spontaneously or can be related to strenuous activity involving the upper extremity. The anomalous subclavius posticus muscle is common, being found unilaterally or bilaterally in 8.9% of cadavers (Akita et al., 2000). This anomaly should be visible on magnetic resonance imaging (MRI) (Collins et al., 1995; Akita et al., 2000).
Inferior Thoracic Aperture
The inferior thoracic aperture (thoracic outlet) is bounded by the following: T12, twelfth ribs, anterior costal margins, and xiphisternal joint. The inferior thoracic aperture contains the diaphragm, which serves as the boundary between the thorax and abdomen.
General Characteristics of the Thoracic Cage
The thoracic cage functions to protect underlying structures, support underlying structures (e.g., pericardium via sternopericardial ligaments), serve as attachment sites for overlying muscles, support the skin and breasts, and assist in respiration.
The adult thorax is wider from side to side than front to back. The anteroposterior diameter increases during inspiration. This is quite different than a child’s thorax, which is circularly-shaped and therefore does not allow change to occur during inspiration. In contrast to adults, children rely almost completely on the excursions of the diaphragm for respiration.
Ribs
Certain groups of cells throughout the spine, known as the costal elements, have the ability to develop into ribs (see Chapters 2 and 12 and Fig. 12-13) and do so in the thoracic region. These thoracic costal elements push through the ventral myotomal plates, which form the intercostal muscles. The costal elements further develop to become precartilaginous ribs, which, after undergoing chondrification and then ossification, become the ribs themselves. The TPs of the thoracic vertebrae grow behind the proximal ends of the developing ribs and are united to them by mesenchyme. This mesenchyme forms the articulations and ligaments of the costocorporeal and costotransverse joints. The fully developed ribs serve to protect the underlying thoracic viscera while providing attachment sites for a wide variety of muscles (Table 6-4).
Table 6-4
Relationships of the Thoracic Cage
| Region | Structure(s) Attached |
| Superiorly | Sternocleidomastoid, sternohyoid, sternothyroid, and anterior, middle, and posterior scalene muscles |
| Anteriorly | Pectoralis major and minor muscles, mammary glands |
| Posteriorly | Trapezius, rhomboid minor and major, scapula and all muscles related to it rest against thoracic cage; serratus posterior superior and inferior and deep back muscles |
| Laterally | Serratus anterior muscles |
| Inferiorly | Abdominal muscles attaching to thoracic cage (e.g., rectus abdominis, external and internal abdominal oblique, transversus abdominis) |
Typical Ribs
The typical ribs are ribs three through nine. Each consists of a head, neck, tubercle, and shaft (Fig. 6-4).
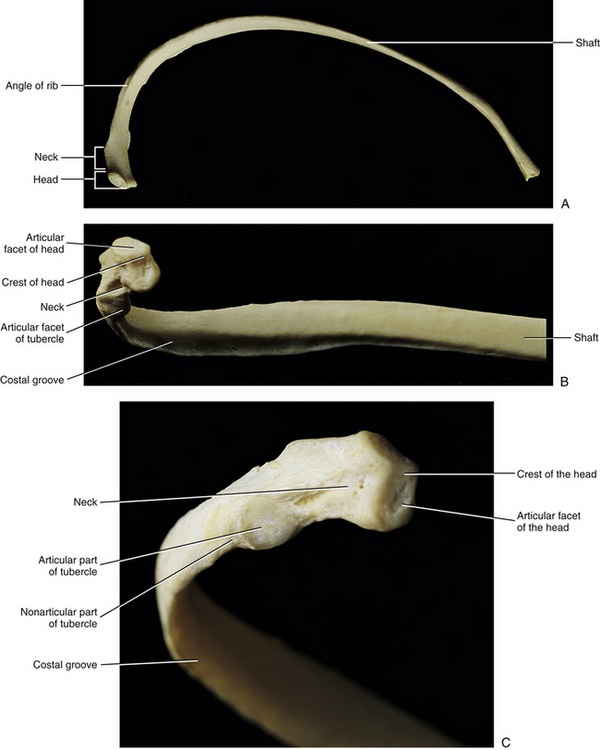
FIG. 6-4 Three views of a typical rib. A, Superior view. B, Head, angle, and shaft of a rib. C, Close-up of the head and neck of a rib.
The head of a typical rib articulates with two adjacent vertebral bodies (see Vertebral Bodies). Inferior and superior articular facets of the rib head articulate with the superior costal demifacet of the same-number vertebra as the rib and with the inferior costal demifacet of the vertebra above, respectively. The crest of the head is a ridge that runs between the two articular surfaces of the rib head. The crest is joined by the intraarticular ligament to the adjacent IVD. This creates the two separate components of the costocorporeal joints—one superior to the crest of the head of the rib and one inferior to the crest (see Costocorporeal Articulations and Fig. 6-9).
The neck of a typical rib is located between its head and tubercle. The neck serves as the attachment site for the costotransverse ligament and superior costotransverse ligament.
The tubercle of a rib is a process that forms the lateral boundary of the neck and the beginning of the shaft. It possesses an articular facet (articular portion) for articulation with the transverse costal facet on the TP of a typical thoracic vertebra. The tubercle of a rib articulates with the same-number vertebra as the rib (e.g., fourth rib articulates with TP of T4). The tubercle also contains a nonarticular part lateral to the articular portion. The nonarticular region serves as an attachment site for the lateral costotransverse ligament.
The shaft of a rib begins at the articular tubercle and extends distally to the end of the rib at its articulation with the costal cartilage. The typical ribs curve inferiorly and anteriorly. Much of this anterior curve is achieved at the angle of the rib. The angle of the rib is located a few centimeters distal to the articular tubercle and is where the shaft makes the sharpest anterior bend.
A costal depression or groove, located along the inferior aspect of each rib, shelters (from superior to inferior) the intercostal vein, artery, and nerve.
Anteriorly each typical rib attaches to a costal cartilage. The costal cartilage, in turn, joins each of the first through seventh ribs with the sternum. The eighth through tenth costal cartilages articulate with the costal cartilage immediately above. The xiphoid process, seventh costal cartilage, and union of the eighth through tenth costal cartilages together form the substernal angle.
Atypical Ribs
The first, second, tenth, eleventh, and twelfth ribs all have special features (Standring et al., 2008). The first rib is short, flat, and strong. It lies almost completely in the horizontal plane and does not angle inferiorly as do typical ribs. Its superior surface is marked by a scalene tubercle (for insertion of the anterior scalene muscle). The subclavian vein passes anterior to the scalene tubercle (and the anterior scalene muscle), and the subclavian artery and inferior trunk of the brachial plexus course posterior to this tubercle. The first rib usually articulates with only one vertebra (T1). Occasionally the head also articulates with the body of C7.
The second rib is much more typical than the first and is almost twice its size. The major distinguishing characteristic of the second rib is a tuberosity on its superior surface, which serves as the partial origin of the serratus anterior muscle.
The tenth rib has only a single facet, and no crest, on its head. The head articulates with the large, single costal facet on the lateral aspect of the body (close to the pedicle) of T10. Sometimes the head of the tenth rib also articulates with the IVD between T9 and T10.
The eleventh and twelfth ribs are short, and neither possesses a neck or tubercle. They are considered free, or floating, ribs because they do not attach to costal cartilage anteriorly. As with the first and tenth ribs, both the eleventh and twelfth ribs articulate with only one vertebra (T11 and T12, respectively).
Sternum
The sternum develops from left and right bars of mesenchyme that migrate to the midline and eventually fuse. The fully developed sternum is composed of a manubrium, body, and xiphoid process. The superior aspect of the manubrium is at the level of the T2-3 IVD. The manubrium possesses a superior concavity known as the jugular notch (see Fig. 6-2). Lateral to the jugular notch is the clavicular notch, which projects superolaterally, allowing its concavity to articulate with the clavicle. The apex of the lung extends above the sternoclavicular joint and the clavicle. The lung is vulnerable here and may be punctured from the anterior in this region. The articulation with the first costal cartilage is inferior to the clavicular notch, on the lateral aspect of the manubrium (see Fig. 6-2).
The inferior margin of the manubrium joins the body of the sternum. The manubriosternal joint is usually a symphysis, although occasionally it may develop a joint cavity, giving it characteristics of a synovial joint. The sternal angle (of Lewis) is formed by the angle between the manubrium and the body of the sternum at the manubriosternal symphysis (see Fig. 6-2). This angle makes the sternum slightly convex anteriorly. The second costal cartilage articulates with the sternum at this angle. The sternal angle is located on a horizontal plane that posteriorly passes approximately through the level of the T4-5 IVD. (This level varies from the vertebral bodies of T4 to T6; see Chapter 1.) Other anatomic structures are present at the general level of this plane. These include the bifurcation of the trachea into primary (main stem) bronchi, the hilus of the lung, and the superior extent of the aortic arch.
The body of the sternum is formed by the union of four segments known as sternebrae. The lateral margin is notched for articulation with costal cartilages of ribs. The inferior process of the sternum is the xiphoid process. It is joined with the body of the sternum by a symphysis that usually ossifies by 40 years of age. The xiphoid process also articulates with the costal cartilage of the seventh rib.
The thoracic cage serves as an attachment site for a variety of structures. See Table 6-4 for structures associated with various regions of the thoracic cage.
Unique Thoracic Vertebrae
Several thoracic vertebrae have distinct characteristics: T1, T9 (occasionally), T10, T11, and T12. They can best be considered as unique, not atypical, thoracic vertebrae.
First Thoracic Vertebra
T1 possesses two characteristics associated with cervical vertebrae but not normally found on typical thoracic vertebrae: uncinate processes and superior vertebral notches above the pedicles (Fig. 6-5). In addition, the vertebral body of T1 resembles that of a cervical vertebra, being rectangular in shape instead of heart-shaped, with the transverse diameter greater than the anteroposterior diameter.
Sixteen percent of T1 vertebrae have asymmetry of greater than 10 degrees of the plane of articulation of the left and right articular processes and their facets (Boyle, Singer, & Milne, 1996).
The superior facet on the vertebral body for articulation with the head of the first rib is usually a full facet (not a demifacet). Occasionally the superior facet is a demifacet, allowing the first rib to attach to both T1 and C7 vertebral bodies and the intervening IVD. Frequently a deep depression can be found on the vertebral body of T1 just inferior to the superior costal facet (Standring et al., 2008). The inferior demifacet of T1 is typical. The spinous process of T1 is large, projects directly posteriorly, and is often as long as, and sometimes longer than, the spinous process of C7.
Ninth Thoracic Vertebra
When the tenth rib does not articulate with the T9 vertebral body, the result is the absence of the inferior demifacet on T9. The other characteristics of T9 conform to those of typical thoracic vertebrae.
Tenth Thoracic Vertebra
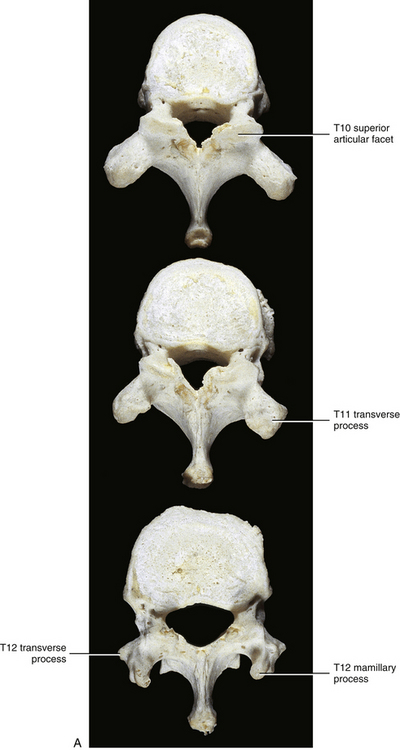
The vertebral body of T10 contains only a single facet on each side for articulation with the head of the left and right tenth ribs (Fig. 6-6). As stated, typical thoracic vertebrae possess two demifacets on each side for articulation with the rib of the same number and with the rib below. The single facet on T10 is usually oval or semilunar in shape. The precise shape depends on whether the tenth rib articulates with just the body of T10 or also with the body of T9 and the IVD between the two. The former results in an oval-shaped facet, and the latter results in a semilunar-shaped facet. The TP of T10 does not always have a facet for articulation with the articular tubercle of the tenth rib.
Eleventh Thoracic Vertebra
T11 also has only a single facet on each side for articulation with the head of the eleventh rib (see Fig. 6-6). However, this facet is located on the pedicle. There is also no articular facet on the TP for articulation with the articular tubercle of the rib. Therefore the eleventh rib does not articulate with the TP of T11. The vertebral body of T11 also resembles that of a lumbar vertebra. The spinous process of T11 is almost triangular in shape with a blunt apex (Standring et al., 2008).
The superior articular processes of T11 resemble those of other thoracic vertebrae. However, usually T11 represents the transition of thoracic-type articular processes to the lumbar type (Masharawi et al., 2004). Therefore the inferior articular processes usually are convex and face anteriorly and laterally. The articular processes of thoracic vertebrae allow for rotation to be the primary movement, whereas the lumbar articular processes limit rotation but encourage flexion and some extension. This transition of facet type also can occur at T12 or occasionally T10.
Degeneration of the T11 inferior articular process has been related to asymmetry of the plane of articulation of the T11-12 articular processes and their articular facets with degenerative changes predominating on the side of the articular process that is more closely oriented to the sagittal plane (Boyle, Singer, & Milne, 1996). The same has been found to be true at the L5-S1 articulation (Giles, 1987).
Twelfth Thoracic Vertebra
The vertebral body of T12 is large, but the TPs are small (see Fig. 6-6). In fact, each TP is actually replaced by three smaller processes (Standring et al., 2008). One process projects laterally and is the equivalent of a thoracic TP except that it is small. The largest of the three processes projects posteriorly and superiorly and is the homologue of the mamillary process of a lumbar vertebra. However, this mamillary process is not as closely related to the superior articular process as it is in the lumbar region. Finally, a small process that is homologous to the accessory process of lumbar vertebrae projects posteriorly and slightly inferiorly. T12 also has a single facet on each side for articulation with the head of the corresponding twelfth rib. The facet is circular and is located primarily on the pedicle but may extend onto the vertebral body. The small TP has no facet for articulation with the twelfth rib.
Thoracolumbar Junction
The left and right Z joints between the T12 and L1 vertebrae are unique. At this joint the L1 mamillary process (see Chapter 7) of each side overlaps the posterior aspect of the inferior articular process of T12. This usually occurs to a greater degree between these two vertebrae than at any other level. The result is that each inferior articular process of T12 fits closely into the superior articular process and overlying mamillary process of L1, much like a well-made carpenter’s joint (e.g., mortise and tenon joint). This configuration prevents almost any movement except flexion from occurring at this articulation (Singer & Giles, 1990; Singer, Giles, & Day, 1990). Singer and colleagues (1990) have shown large Z joint synovial folds (see Chapter 2) protruding into this joint (Fig. 6-7). They also emphasize that normally almost no rotation occurs at this articulation.
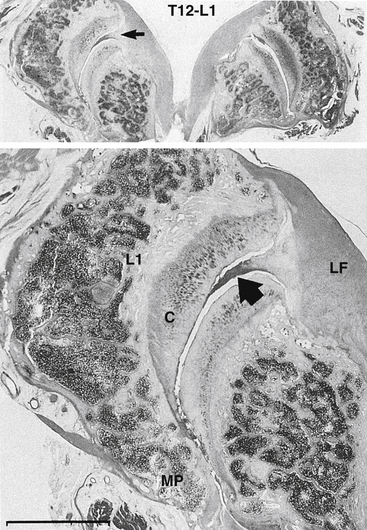
FIG. 6-7 Photomicrograph of the left (bottom image) Z joint at the thoracolumbar junction. L1 represents the superior articular process of L1; C, articular cartilage. Notice that MP, the mammillary process of L1, protrudes medially to overlap the inferior articular process of T12. Also notice the Z joint synovial fold (arrows) protruding into the joint space from LF (the ligamentum flavum). (From Singer KP, Giles LGF, & Day RE. [1990]. Intra-articular synovial folds of thoracolumbar junction zygapophyseal joints. Anat Rec, 226, 147-152.)
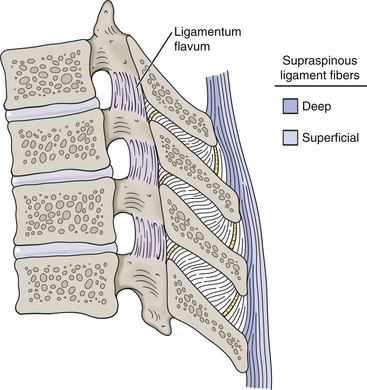
FIG. 6-8 The thoracic supraspinous ligament. Notice the ligament is composed of two layers: superficial (light blue) and deep (dark blue). The deep fibers span adjacent vertebrae and become continuous with the interspinous ligaments. The superficial fibers span many segments.
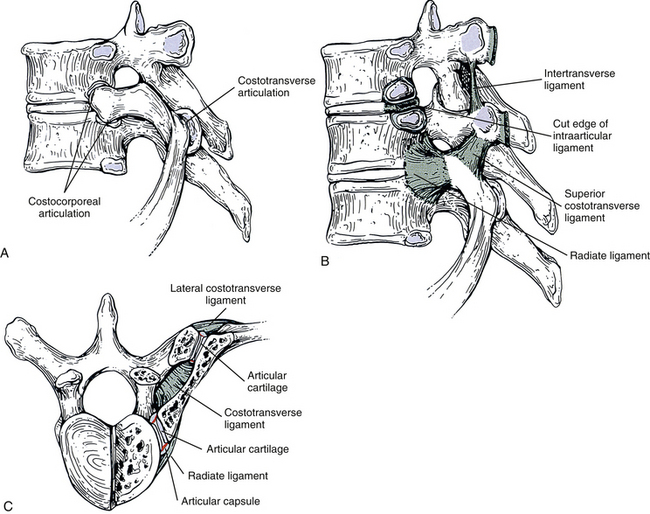
FIG. 6-9 Costovertebral articulations. A, Bony costocorporeal and costotransverse articulations. B, Ligamentous attachments of these joints. C, Superior view. The vertebra and rib have been horizontally sectioned on the right half of the illustration to demonstrate further the costocorporeal and costotransverse articulations.
FIG. 6-10 Bottom, Posterior view of a scoliotic spine. Top, Superior view of a single vertebra and the articulating ribs. Notice the asymmetry between the left and right costocorporeal and costovertebral articulations (compare with Fig. 6-5). (Modified from Netter F. (1990). Atlas of Human Anatomy. Summit, N.J.: CIBA-GEIGY Corp.)
Ligaments and Joints of the Thoracic Region
Several ligaments found in the thoracic spine are also present in the cervical spine and are discussed in Chapter 5. These include the ligamenta flava (LF), anterior longitudinal ligament (ALL), posterior longitudinal ligament (PLL), and interspinous ligaments. Because the interspinous ligaments in the thoracic region differ from those in the cervical region, they are discussed more fully in this section. In addition, further information is provided on the anterior longitudinal ligament, posterior longitudinal ligament, thoracic IVDs, and LF in this section. Also, the supraspinous ligament is discussed for the first time here. Finally, because the joints between the thoracic vertebrae and ribs are unique to the thoracic region, much of this section is devoted to these interesting and important articulations and the ligaments that support them. This section concludes with a discussion of the sternocostal and interchondral articulations.
Anterior and Posterior Longitudinal Ligaments
The anterior longitudinal ligament (ALL) in the thoracic region (see Figs. 6-11, 6-12, and 6-13, B) is thicker from anterior to posterior and thinner from side to side than in either the cervical or the lumbar regions. The ALL attaches firmly to the superior and inferior bony end plates of the thoracic vertebrae, but has only weak attachments to the remainder of the thoracic vertebral bodies. However, firm attachments of the ALL to the thoracic IVDs have been found in more than 50% of spines (Cramer et al., 1996). The firm attachments of the ALL to the IVDs may help prevent anterior protrusion of the IVDs in this region.
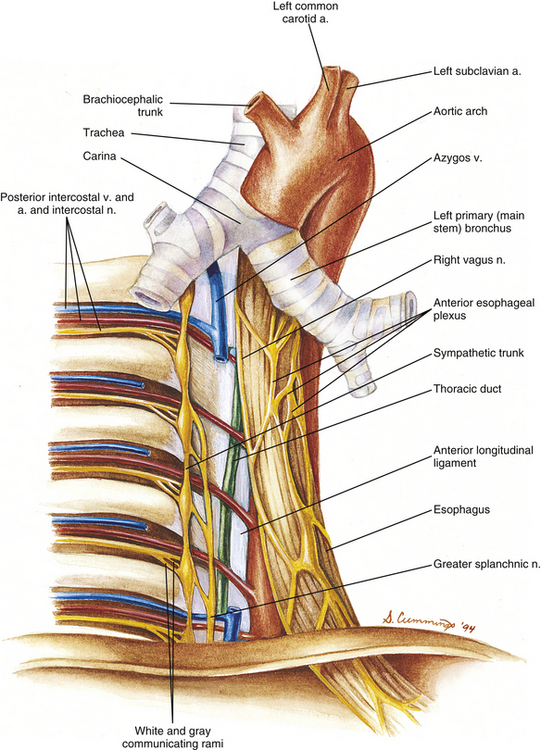
FIG. 6-11 Posterior thoracic wall showing the relationship of the vertebrae and ribs to the vessels and nerves of the thorax. The right vagus nerve is shown sending a few branches to the anterior esophageal plexus. The more abundant and important contributions of the right vagus nerve to the posterior esophageal plexus cannot be seen from this perspective.
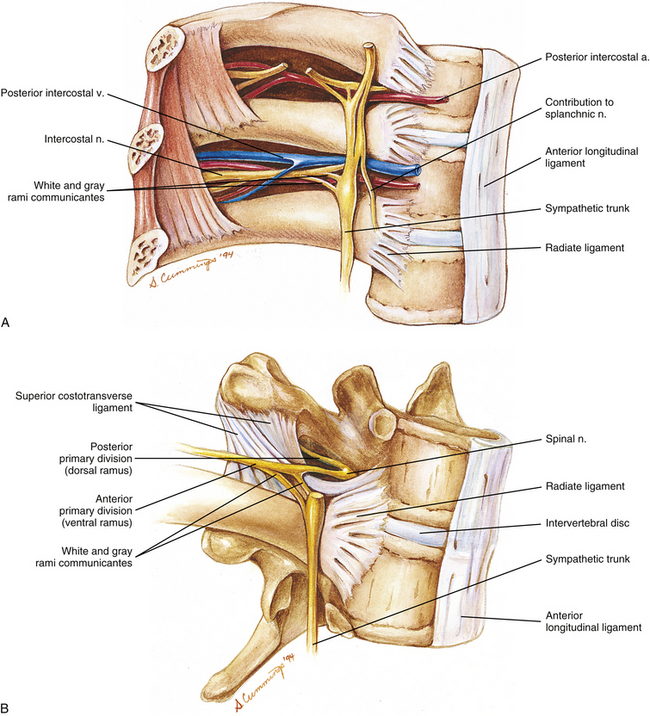
FIG. 6-12 A, Nerves and vessels related to three adjacent thoracic vertebrae and the ribs that articulate with them. B, Close-up of the nerves associated with a single thoracic motion segment (two adjacent thoracic vertebrae).
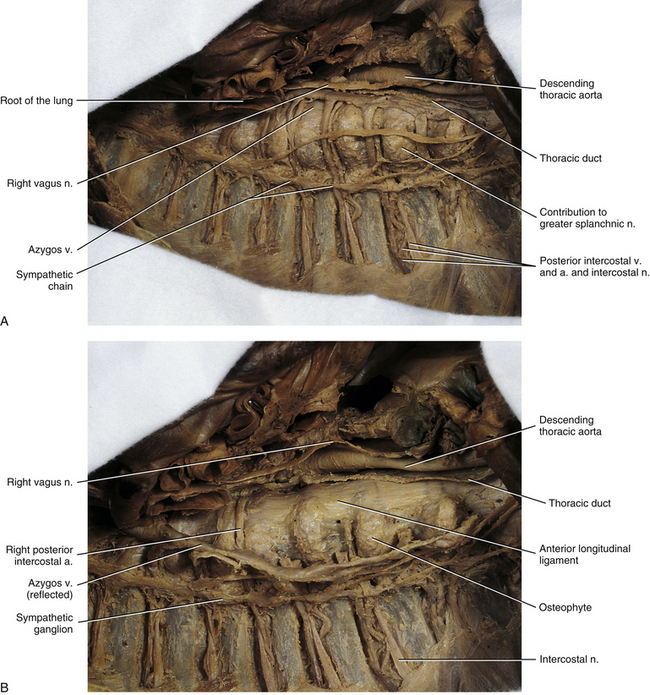
FIG. 6-13 A, Right side of the mediastinum and the thoracic vertebrae, ribs, and intercostal spaces associated with this region. B, Same specimen with the azygos vein and related intercostal veins reflected. This was done to show more clearly the anterior longitudinal ligament. Notice the large osteophytes extending from the right anterolateral aspects of the thoracic vertebrae. Typically such osteophytes are seen in dissections of this region and have been found more frequently on the right side versus the left side of the vertebral bodies (see text). Also notice the sympathetic ganglia connected to one another to form the sympathetic chain.
Ossification of the posterior longitudinal ligament (PLL) is much less common in the thoracic region than in the cervical region; however, when it does occur, it can be severe and lead to paraplegia (Fujimura et al., 1997; Vera et al., 1997).
Ligamentum Flavum
Viejo-Fuertes and colleagues (1998) studied the ligamenta flava (ligamentum flavum, sing.) in the region of the thoracolumbar junction in detail. They found that each LF had two layers, superficial (posterior) and deep (anterior). The fibers of the superficial layer were oriented obliquely, and those of the deep layer were more organized and oriented in a sagittal plane. The superficial and deep layers were separated by a potential space that Viejo-Fuertes and colleagues (1998) called a “gliding space.” Many nerve fibers were found to innervate the LF. These fibers were thought to originate from the posterior primary division of the spinal nerve, and the authors speculated that the deep layer of the LF also may receive fibers from the recurrent meningeal nerve. In addition, they found a distinct border between the superficial layers of the left and right LF and also a distinct border between the superficial layer of each LF and the more medially located interspinous ligament. They called the region between the left and right superficial layers of the LF the “zone of separation.” The deep layers of the left and right LF were continuous at the midline; however, the division between the left and right sides could be identified easily by a prominent posterior midline indentation (a “cleavage separation”). The lateral aspect of each LF was crossed by the longissimus thoracis muscle, and the medial aspect was crossed by the tendons of the rotatores longus and brevis muscles. Elastic fibers dominated the fiber type of typical LF, making up 80% of the fibers in them. The remaining 20% of the fibers were collagen fibers found in densely packed arrays. When the LF was in the neutral position, the significant amount of elastin resulted in a “pretension,” meaning that in the neutral position the length of the LF was increased by 15% from the length when no tension was placed on the LF. Therefore a typical ligament was stretched partially when in the neutral position. The percentage of elastin was found to be highest “at the end of fetal life and during the first years of development.” With age the pretension of the LF decreased and the elastic fibers became disorganized. Also, increased numbers of chondrocytes and small regions of calcification where the LF attached to bone could be seen under magnification in aging LF. With more advanced age and degeneration, 80% of the elastic fibers were replaced with collagen and chondrocytes. In specimens that showed injury to the ligaments, only minimal replacement of elastic fibers was found and collagen fibers predominated in these regions. No elastic fibers were seen in specimens showing advanced degeneration, and the remaining collagen fibers were disorganized and an increased number of chondrocytes were seen in these specimens. In addition, the number of nerve fibers innervating the LF decreased as the degree of degeneration increased.
Viejo-Fuertes and colleagues (1998) described two functions of the LF—biomechanical and neurologic functions. Biomechanically the LF decreases flexion and also helps with extension of the vertebral column. Degenerative changes can cause the LF to buckle into the vertebral canal. The authors felt that the neurologic function of the LF was both proprioceptive and nociceptive in nature; the proprioceptive information received from the LF would be important in providing the central nervous system with information used for segmental muscle reflexes, and the nociceptive information would be important in relaying information related to tissue damage to the central nervous system.
The LF can ossify. The cause of such ossification is unknown and the condition is poorly understood. Ossification of the ligamentum flavum (OLF) occurs most frequently in the thoracic region, including the thoracolumbar junction (Hasue et al., 1983). It may be related to repetitive stress (i.e., repetitive streatching) of the ligament over many years. The ossification is related to significant degeneration of elastic fibers (as described previously), including fragmentation of these fibers and disruption of elastic fiber bundles (Yayama et al., 2007). The condition appears to be more common in Asian populations (e.g., Japanese and Chinese populations). A full spine magnetic resonance imaging (MRI) study of 1736 Chinese volunteers (668 males and 1068 females) identified OLF in 3.8% of subjects. The T9-T10 and T10-T11 levels were the most often affected, but OLF was found at all thoracic levels, and was less often found at lower cervical or upper lumbar levels. Single-level OLF was found in 68.2% of those cases and multiple-level OLF in 31.8% (16.7% multiple-level OLF in contiguous vertebral levels and 15.2% in noncontiguous vertebral levels). The authors emphasized that noncontiguous multiple-level OLF could be missed if clinicians did not image the entire thoracic region, including the thoracolumbar junction (Guo et al., 2009). OLF can increase the size of the LF dramatically, to the point of causing paraplegia secondary to compression of the spinal cord from behind. Some degree of compression of the spinal cord is found in 62% of cases (Yamashita et al., 1990), most of the compressions are mild (56%), and only 6% are severe. Ossification of the LF is frequently associated with ossification of the PLL, and when the two occur together they usually are found at the same level (Hasue et al., 1983; Vera et al., 1997).
Interspinous Ligaments
The interspinous ligaments pass between adjacent spinous processes, filling the gap along the anterior-to-posterior length of these processes. Anteriorly each interspinous ligament is continuous with the left and right ligamenta flava, and posteriorly each is continuous with the supraspinous ligament. Even though the thoracic interspinous ligaments are thin and membranous in structure, they are more fully developed in the thoracic than cervical region. As discussed in Chapter 5, some authors dispute their existence in the cervical region altogether, stating that they are simply thin, fascial, anterior extensions of the ligamentum nuchae (Standring et al., 2008). Controversy also surrounds the precise orientation of these ligaments (Behrsin & Briggs, 1988; Standring et al., 2008). Some authors believe that the fibers of the interspinous ligament run from anterosuperior to posteroinferior, and others believe that the fibers making up this ligament run from posterosuperior to anteroinferior (Paris, 1983; Scapinelli, 1989). The latter scenario is more likely. The interspinous ligaments have been studied more fully in the lumbar region, where they are better developed (see Chapter 7).
Supraspinous Ligament
The supraspinous ligament (Fig. 6-8) limits flexion of the spine. It is classically described as forming a continuous band that passes from the spinous process of C7 to the sacrum (Standring et al., 2008). However, disagreement exists as to whether or not it extends all the way to the sacrum. Some investigators believe that it is almost nonexistent in the lumbar region (Behrsin & Briggs, 1988). The supraspinous ligament in the thoracic region is actually composed of two layers (Fig. 6-8), with the deeper fibers running between adjacent vertebrae and the more superficial fibers spanning several (up to four) vertebrae (Standring et al., 2008). The deepest fibers of the thoracic supraspinous ligament become continuous with the interspinous ligaments. The supraspinous ligament seems to warrant further investigation.
Thoracic Intervertebral Discs
The thoracic IVDs have the thinnest superior-to-inferior dimension of the spine. Also, the discs of the upper thoracic region are thinner than those of the lower thoracic region. The upper thoracic region is also the least movable area of the thoracic spine (although the small amount of motion appears to support end range motion of the cervical region). In contrast to the cervical and lumbar IVDs, which are thicker anteriorly than posteriorly, the thoracic IVDs are of more equal thickness. In addition, the left-right (lateral) width of each thoracic IVD is greater at the superior versus inferior aspect of the IVD. Consequently, in a coronal (frontal) section the thoracic IVDs have the shape of an inverted trapezoid (wider superiorly than inferiorly) (Masharawi et al., 2008).
Calcification of the IVD is found with greater frequency in the thoracolumbar region than in any other region of the spine. Radiographic surveys have found thoracolumbar IVD calcification in 5% to 6% of adults. Postmortem examinations have found such calcification in up to 70% of adults. Disc calcification usually is asymptomatic unless it is associated with protrusion into the vertebral canal, in which case neurologic compression symptoms result (Lipson & O’Connell, 1991).
Thoracic IVD protrusion is rather infrequent, accounting for only 0.15% to 1.8% of all disc protrusions (Alvarez, Roque, & Pampati, 1988; Bauduin et al., 1989). However, they may be more common than previously believed (Vernon, Dooley, & Acusta, 1993). When present, this condition usually affects the lower thoracic discs of individuals primarily between 30 and 60 years of age (Otani et al., 1988). Symptoms vary dramatically from none at all to motor and sensory deficits resulting from spinal cord compression (myelopathy). Pain, muscle weakness, and spinal cord dysfunction that can present with bowel and bladder dysfunction are the most common clinical symptoms. Computed tomography (CT), in conjunction with contrast enhancement of the subarachnoid space (CT myelography), and MRI are useful in the detection of these rare but significant lesions (Alvarez, Roque, & Pampati, 1988; Bauduin et al., 1989; Vernon, Dooley, & Acusta, 1993), and may allow for more frequent detection of thoracic IVD protrusion in the future.
Crean and colleagues (1997) found matrix metalloproteinases in degenerated IVDs and in IVDs at the peak of a scoliotic curve. The presence of these proteinases directly correlated with the degree of degeneration. These substances degrade all known extracellular matrix substances and may be associated with the “progressive nature” of IVD degeneration, including the IVD degeneration seen at the apex of scoliotic curves (Crean et al., 1997). A more thorough discussion of IVD degeneration can be found in Chapter 14.
Costovertebral Articulations
The ribs and vertebrae articulate in two locations. The first is the joint complex between the head of a rib and the adjacent vertebral bodies, known as the costocorporeal articulation. The second costovertebral articulation is between a rib and the TP, known as the costotransverse articulation. In addition to allowing the movements of the ribs so important to respiration, the costocorporeal and costotransverse articulations, along with the rib cage, provide stability to the thoracic region of the vertebral column. Whereas the articular processes of the thoracic region limit flexion and extension, the ribs and costovertebral (both costocorporeal and costotransverse) articulations limit lateral flexion and axial rotation (Oda et al., 1996).
Costocorporeal Articulations
The joint between the head of a rib and the adjoining typical thoracic vertebrae consists of articulations with the two adjacent vertebral bodies and interposed IVD (Fig. 6-9). The rib head articulates with the superior demifacet of the same-number vertebra and the inferior demifacet of the vertebra above (e.g., seventh rib articulates with superior demifacet of T7 and inferior demifacet of T6). The crest of the rib head is attached to the adjacent IVD by an intraarticular ligament. This short, flat ligament creates two distinct articular compartments (upper and lower) within the costocorporeal articulation. Both of these compartments are surrounded by a fibrous articular capsule lined with a synovial membrane. These synovial joints can best be classified as having ovoid articular surfaces, and the fibrous capsule extends around the ovoid articular surfaces of both the demifacet and adjacent articular half of the rib head (see Fig. 6-9). The capsule extends to the intraarticular ligament between the upper and lower compartments. The fibers of the fibrous capsule closest to the IVD blend with that structure, and the posterior fibers blend with the costotransverse ligament. In a fashion similar to that found in the Z joints, synovial folds, or menisci, protrude into the costocorporeal articulation, presumably to help lubricate the joint and help with the sliding and rotary motions of the joint (Meyer, 1972). The heads of the first, tenth (occasionally), eleventh, and twelfth ribs form single ovoid synovial articulations with their respective ribs.
The ligaments of this compound joint include the capsular, intraarticular (both described previously), and radiate. Each radiate ligament (see Fig. 6-9) associated with typical vertebrae attaches to the anterior aspect of the head of the articulating rib and the two vertebrae to which the head attaches. In addition, the radiate ligament attaches by horizontal fibers to the IVD between the two vertebrae. The superior fibers attach just above the superior demifacet and ascend to the vertebral body of the superior vertebra. Likewise, the inferior fibers attach just below the inferior demifacet and descend to the inferior vertebral body. The radiate ligament of the first rib has some superior fibers that attach to C7. The radiate ligaments of the tenth through twelfth ribs attach to only the vertebra with which the rib head articulates.
In addition to allowing motion of the ribs, the costocorporeal joints provide stability to the thoracic region during motions in the sagittal (flexion-extension), coronal (lateral flexion), and transverse planes (axial rotation) (Oda et al., 2002).
Confirming the work of Meyer (1972), Erwin and colleagues (2000) found large synovial folds protruding into the costocorporeal joints. In addition, they found that the synovial folds were innervated by free nerve endings and mechanoreceptors immunoreactive to substance P. Substance P is associated with pain transmission and functions in the perception of pain, related reflex muscle responses (flexion reflexes), and reflex responses to pain of the autonomic nervous system and endocrine system. Therefore the costocorporeal joints are similar to the Z joints with respect to being planar synovial joints with nociceptive (pain-sensitive) innervation of both the joint capsule and the synovial folds. The nerve endings are most likely sensitive to tissue strain or tissue damage of mechanical origin. The authors speculated that nociception transmitted from a lesion affecting these nerve endings would probably radiate to the midline thoracic region and anteriorly along the same rib. In addition, pain from the costocorporeal joints also may be associated with atypical chest and arm pain (Erwin, Jackson, & Homonko, 2000). Others (Groen et al., 1987) have identified fibers from the sympathetic chain extending to the costocorporeal joint. The fibers were found to form a dense network that surrounded the joint, and met the criteria of vasomotor fibers that would provide innervation to the vessels extending to the synovial lining of the joint capsule.
Costotransverse Articulation
This joint is composed of the costal (articular) tubercle of a rib articulating with the transverse costal facet of a TP (see Fig. 6-9). Recall that the eleventh and twelfth ribs do not articulate with the TPs of their respective vertebrae. The joint surfaces of the upper five or six costotransverse joints are curved, with the transverse costal facet being concave and the articular tubercle convex. The remaining joints are more planar in configuration (Meyer, 1972; Standring et al., 2008). A thin, fibrous capsule lined by a synovial membrane attaches to the two adjacent articular surfaces. A costotransverse foramen is found between the TP and the rib between the costotransverse and costocorporeal articulations. This foramen is filled by the costotransverse ligament. The costotransverse ligament passes from the posterior aspect of the rib neck to the anterior aspect of the adjacent TP (see Fig. 6-9). For example, the costotransverse ligament of the sixth rib attaches to the posterior aspect of that rib and to the anterior aspect of the TP of T6. Sensory nerve endings also have been found in the costotransverse ligament, indicating that it is pain sensitive (Erwin, Jackson, & Homonko, 2000). Sensory innervation to the lateral and superior costotransverse ligaments (discussed immediately below) has not been investigated adequately.
The ligaments of the costotransverse articulation include the articular capsule, costotransverse ligament (both described previously), superior costotransverse ligament, and lateral costotransverse ligament (see Fig. 6-9). The strong but short lateral costotransverse ligament courses directly laterally from the lateral margin of the TP to the nonarticular region of the costal tubercle of the adjacent rib (see Fig. 6-9). This ligament is found at every thoracic segment. The ligaments of the upper thoracic vertebrae course slightly superiorly, as well as laterally, whereas the lower ones run slightly inferiorly, as well as laterally.
A superior costotransverse ligament courses between the neck of each rib and the TP of the vertebra above. This ligament is divided into two parts (laminae), anterior and posterior. Both laminae course superiorly from a rib neck to the inferior border of the TP immediately above. The anterior lamina angles slightly laterally as it ascends and blends with the posterior intercostal membrane (see Fig. 6-12, B). The posterior lamina angles slightly medially. Because it is more posteriorly placed, this ligament blends laterally with the external intercostal muscle. The intercostal vein, artery, and nerve pass across the anterior surface of these ligaments. A lumbocostal ligament runs from the inferior border of the twelfth rib shaft to the superior surface of the TP of L1.
Three small muscles have been found between anterior and posterior laminae of the first three superior costotransverse ligaments (Darwish & Ibrahim, 2009). The triangle-shaped muscles attach to the lateral aspects of transverse processes superiorly (forming the broad bases of the triangles) and the necks of the subjacent ribs inferiorly (narrow apices of the triangles). More specifically, the first muscle attaches to the TP of C7 and the neck of the first rib (there is only a single lamina of the superior costotransverse ligament at this level that covers the muscle anteriorly), the second attaches to the TP of T1 and the neck of the second rib, and the third muscle attaches to the TP of T2 and and the neck of the third rib. Because of similar embryologic origin, these muscles may have a function similar to the intertransversarii muscles (Darwish & Ibrahim, 2009).
An accessory ligament is normally found medial to the superior costotransverse ligament. This accessory ligament is separated from the superior costotransverse ligament by a gap that is filled by the posterior primary division as it leaves the mixed spinal nerve to reach the more posterior structures of the spine. More specifically, the accessory ligament originates from the region of the neck of a rib medial to the attachment of the superior costotransverse ligament. It courses superiorly to reach the inferior articular process of the vertebra above, although some fibers reach the TP.
A “first thoracic rib ligament” is found on at least one side of 80% of individuals (Matullo et al., 2010). The 3-cm-long ligament attaches to the inner surface of the neck of the first rib and then distally along the inner surface of the shaft of the first rib. The T1 anterior primary division courses around the inferior surface of this ligament and then passes superiorly between the ligament and the shaft of the first rib to unite on top of the first rib with the anterior primary division of C8 to form the inferior (lower) trunk of the brachial plexus. The first thoracic rib ligament could conceivably be the cause of a type of thoracic outlet syndrome (see Superior Thoracic Aperture, previously) in which the anterior primary division of T1 becomes entrapped as it passes beneath or lateral to the ligament (Matullo et al., 2010).
Sternocostal and Interchondral Articulations
The costal cartilages of the first through seventh ribs articulate directly with the sternum at the sternocostal joints (see Fig. 6-2). The costal cartilages of the eighth through tenth ribs attach to the costal cartilage of the rib above at articulations known as the interchondral joints.
Sternocostal Joints
A complex type of synarthrosis exists between the first costal cartilage and manubrium. A thin piece of fibrocartilage is interposed between the two surfaces and is tightly adherent to both (Standring et al., 2008). A radiate ligament also unites the two surfaces. The joint between the second costal cartilage and sternum is synovial and is separated into two compartments by an intraarticular ligament. Small synovial joints are located between the costal cartilages of the third through seventh ribs and the sternum. The costal cartilages and sternum are united by the fibrous capsules of the joints and also by the radiate ligaments that pass from the anterior and posterior surfaces of each costal cartilage to the sternum. A small amount of rotation occurs at these joints. This rotation allows the thorax to expand and contract during inspiration and expiration, respectively.
Interchondral Joints
As mentioned, the eighth through tenth costal cartilages articulate with the costal cartilage immediately above. Small synovial joints are formed at the attachment sites for the eighth and ninth ribs. The costal cartilage of the tenth rib usually is continuous with the costal cartilage of the ninth rib, and no true joint unites the two. Occasionally no attachment exists between the tenth rib and the costal cartilage of the ninth rib. Although both the sixth and seventh costal cartilages attach directly to the sternum, their most distal portions also contact one another (see Fig. 6-2). Small synovial joints are also located where these cartilages are in contact with one another.
Ranges of Motion in the Thoracic Spine
As stated, the facets of the thoracic vertebrae are oriented approximately 60 to 75 degrees to the horizontal plane. Therefore they are more vertically oriented than the articular processes of the cervical region. This vertical orientation dramatically limits forward flexion. Extension is limited by the inferior articular processes contacting the laminae of the vertebrae below and also by contact between adjacent spinous processes. Rotation is the dominant movement in the thoracic region with the axis of rotation for each vertebra located in the midsagittal plane at the anterior aspect of the vertebral foramen (Molnar et al., 2006). However, the vertebrae are a part of the entire thoracic cage and even this motion is limited considerably. The relationship to the thoracic cage may help to explain why the lower thoracic region, with its relation to floating ribs and ribs with only an indirect attachment to the sternum, is the most mobile part of the thoracic spine. Ranges of motion of the thoracic spine include the following:
| Combined flexion and extension | 34 degrees |
| Unilateral lateral flexion | 15 degrees |
| Unilateral axial rotation | 35 degrees |
Motion of the Ribs
Motion of the ribs at the costocorporeal and costotransverse articulations is primarily one of rotation with a slight gliding motion. Motion is limited because of the strong ligamentous attachments. Upward and downward rotation is the primary movement of the upper six ribs, accompanied by slight superior and inferior gliding. Rotation of the seventh through tenth ribs is accompanied by more gliding than in the ribs above. This is because the transverse costal facets of T7 through T10 are more flat than those of the vertebrae above and also because the facets face superiorly, anteriorly, and laterally. Upward rotation of these lower ribs is accompanied by posterior and medial gliding, and downward rotation is accompanied by anterior and slightly lateral gliding. These movements tend to open and close the substernal angle, respectively (Standring et al., 2008).
Lateral Curvature of the Spine (Scoliosis)
Lateral curvature or lateral deviation of the spine is known as scoliosis (Fig. 6-10). A slight lateral curve with the convexity on the same side as handedness (i.e., convexity to the left in left-handed individuals) is normally found in the upper thoracic region (see Chapter 2). This is a result of the pull of the stronger musculature on the side of handedness. Lateral curves seen clinically, other than this mild upper thoracic curve, are considered to be a variation from normal spinal structure. These scolioses range from being barely perceptible and insignificant deviations to extremely dramatic and deforming curvatures.
Scoliosis can be found in any spinal region, but the thoracic region is usually the most prominently affected, partly because of its length and central location. However, the thoracic region is the most noticeably affected primarily because the attachment of the rest of the thoracic cage to the thoracic vertebrae can result in deformation of the entire thorax. In addition, curvatures of the thoracic spine are more or less “held in place” by the remainder of the thoracic cage (ribs and sternum), and proper evaluation of the ribs can be useful in the diagnosis of scoliosis. In fact, with full forward flexion of the spine, posterior elevation on one side of the thorax (“rib hump”) of 6 mm or greater has been used as one of the primary indicators of scoliosis. The incidence of such posterior thoracic elevation has been reported as 4.1% in fourth-grade schoolchildren with an average age of 10.8 years (Nissinen et al., 1989).
Osteophytes of vertebral bodies frequently develop on the concave side of the scoliotic curve and are rarely seen on the convex side (Nathan, 1962). Occasionally scoliosis is so severe that serious compromise of lung capacity and cardiac output may result.
Interestingly, individuals with spines shortened as a result of pathology during childhood or early adolescence (e.g., scoliosis or tuberculosis affecting the spine) have upper and lower extremities that are statistically significantly longer (≈2 cm for each extremity) than “healthy” individuals. This increase in upper and lower extremity lengths is thought to be the result of a possible “compensatory stimulatory growth mechanism” (Upadhyay et al., 1991; Krishna & Upadhyay, 1996).
Scoliosis has many causes, ranging from developmental and anatomic, such as hemivertebra (see Chapters 2 and 12), to unknown causes (idiopathic). Idiopathic scoliosis is typically characterized by the presence of a concomitant lordosis at the apex of the lateral curve (Deacon, Archer, & Dickson, 1987). Infantile idiopathic scoliosis (birth to 3 years of age) is uncommon, but is more frequent in boys and is characterized by the most prominent curve being a left-sided lower and mid-thoracic curve (convex to the left) with the vertebral bodies rotated to the left. Adolescent idiopathic scoliosis (10 to 16 years of age) is much more common overall, is more common in girls, and is characterized by the opposite curves and rotation (right-sided lower and mid-thoracic most prominent curve with vertebral bodies rotated toward the right) (Janssen et al., 2011). The reason for the right-sided curve is likely due to the normal left lateral wedging of most thoracic vertebral bodies (Masharawi et al., 2008) (see Vertebral Bodies, previously).
The cause of idiopathic scoliosis remains unknown, but it is thought to be the result of many factors. There is most likely a genetic component to this disorder with both autosomal and sex-linked elements, with variable penetrance (expression) and heterogeneity (two or more genes acting independently), contributing to the genetic predisposition for idiopathic scoliosis (Kesling & Reinker, 1997; Lowe et al., 2000). This genetic predisposition is probably linked to asymmetric processing of motor and sensory information in the cerebral cortex and possibly improper processing in the paramedian pontine reticular formation (Cook et al., 1986; Wyatt et al., 1986; Goldberg et al., 1995; Carpintero et al., 1997). Secondary factors are then probably necessary for the genetic predisposition and neurologic abnormalities to be fully expressed. Such secondary factors include a decrease in melatonin production (Kanemura et al., 1997; Wang et al., 1997; Cheung et al., 2003), which leads to an increase in circulating levels of the hormone calmodulin (Lowe et al., 2000). These hormonal changes are probably related to bone growth during the pubertal growth spurt. This growth spurt has been found to begin earlier in children with idiopathic scoliosis. For this reason, the more rapid and earlier growth spurt in females compared with males may explain the higher incidence of adolescent idiopathic scoliosis in girls, and the fact that adolescent scoliosis tends to develop somewhat later in boys than girls (Karol et al., 1993). Asymmetric new bone growth at the vertebral body growth plates (Dahia et al., 2011) and changes in fiber composition in intervertebral discs (Yu et al., 2005) are also probably related to the genetic predisposition to scoliosis in some children. However, the altered biomechanics associated with spinal curvatures (Lowe et al., 2000) are most likely related to changes in the following: skeletal muscle (Carpintero et al., 1997), connective tissue (Crean et al., 1997; Duance et al., 1998), lack of elastic fibers in the IVD anulus fibrosus (Yu et al., 2005), bone density, rib distortion (Lowe et al., 2000), decreased height of the posterior vertebral arch (Deane & Duthie, 1973), asymmetry of the neurocentral synchondrosis (Vital et al., 1989), and the relatively common finding (up to 26%) of syrinx formation and other neuroanatomic abnormalities in the spinal cord (Evans et al., 1996; Ghanem et al., 1997).
Additional support for the involvement of high centers in the central nervous system in the cause of idiopathic scoliosis is provided by evidence that a significantly lower incidence of idiopathic scoliosis exists among hearing-impaired children (1.2% in hearing-impaired children versus 4% to 10% in the general population) (Woods et al., 1995). Of note is that 80% of hearing-impaired children also have significant vestibular impairment. This vestibular impairment forces these children to shift to vision and proprioception as the primary means of detecting and establishing a vertical position in space. The vestibular system is thought to normally provide the baseline from which visual and proprioceptive information is compared when establishing the vertical posture. Woods and colleagues (1995) hypothesize that the central nervous system of individuals with idiopathic scoliosis may process normal vestibular information incorrectly; visual and proprioceptive information then would be compared with this consistently altered baseline information, causing the reflex efferent projections to postural muscles to be incorrect. The incorrect efferent stimulation of postural muscles then would result in the postural alterations of scoliosis. More work is still needed to verify this compelling hypothesis. Additional evidence for high central nervous system (cortical) involvement in the development of idiopathic scoliosis is that a significantly higher incidence of scoliosis is found in individuals with cerebral palsy (an incidence of 4% to 64%, dependent on the amount of muscle spasticity) (Dias et al., 1997). Possibly related to scoliosis in the cerebral palsy population is that congenital block vertebrae (lack of vertebral segmentation) in the cervical region, known as Klippel-Feil syndrome, is also more prevalent in this group of individuals (Theiss, Smith, & Winter, 1997). Much more work is still needed to unravel the cause of idiopathic scoliosis and to possibly find a way to cure this disorder.
Nerves, Vessels, and Viscera Related to the Thoracic Spine
The thoracic spine and the ribs are intimately related to many neural, vascular, and visceral structures (Figs. 6-11 and 6-12). The structures most closely related to the spine and ribs include posterior primary divisions; the intercostal arteries, veins, and nerves; azygos, hemiazygos, and accessory hemiazygos veins; thoracic duct and thoracic lymphatics; thoracic aorta; esophagus; trachea; vagus nerves; thoracic sympathetic chain; and splanchnic nerves. These structures are discussed briefly in this section. Chapter 1 and Table 1-1 relate many of the most important visceral structures to vertebral body levels to aid in the interpretation of scans presenting cross-sectional anatomy (e.g., CT, MRI). The intercostal muscles are discussed in Chapter 4.
Posterior Primary Divisions (Dorsal Rami)
The thoracic spinal nerves are formed within the IVF by the union of the thoracic dorsal and ventral roots. The roots in the upper thoracic region descend only slightly before entering the IVF, whereas the roots of the lower thoracic region may descend as much as two vertebral levels before entering the IVF. Once formed, the spinal nerves contain both sensory and motor fibers (see Fig. 3-3). Each mixed spinal nerve then divides into a posterior primary division (dorsal ramus) and an anterior primary division (ventral ramus) as it exits the IVF (see Fig. 6-12, B). The anterior primary division of a thoracic nerve becomes an intercostal nerve and the subcostal nerve at the level of T12 (see the following discussion). The posterior primary division (dorsal ramus) passes posteriorly across the lateral aspect of the Z joint, to which it sends fine branches. It then passes through a small but adequate opening bounded superiorly by the TP, inferiorly by the rib of the vertebra below (e.g., T5 nerve exits between T5 and T6 vertebrae and above the sixth rib), medially by the Z joint and the accessory superior costotransverse ligament, and laterally by the superior costotransverse ligament, which attaches to the rib below. This opening is known as the costotransverse foramen (of Cruveilhier). The dorsal ramus then divides into medial and lateral branches.
The lateral branch of the posterior primary division supplies the erector spinae muscles in the region and continues to provide sensory innervation to the skin of the back. Not all of the first six lateral branches of the posterior primary divisions reach the skin. However, the lower six lateral branches all have significant cutaneous distributions. They supply the skin superficial to the spinous processes via medial cutaneous branches (of the lateral branches of the posterior primary divisions) and supply sensory innervation to the skin several inches lateral to the midline via lateral cutaneous branches (of the lateral branches of the posterior primary divisions). The lateral cutaneous branches may descend as far as four ribs before reaching the skin. The lateral cutaneous branch of the posterior primary division of T12 reaches the upper border of the iliac crest.
A typical medial branch of a posterior primary division takes a rather tortuous course, passing between the multifidus muscle on its medial surface and the levator costarum muscle on its lateral side (Maigne, Maigne, & Guerin-Surville, 1991). It then continues posteromedially and slightly inferiorly, running medial to most of the longissimus thoracis muscle fibers. The medial branches of the dorsal rami of the second, third, and fourth thoracic nerves then pass through the tendon of the splenius cervicis muscle, continue through the rhomboid muscles, pierce the trapezius muscle, and then reach the skin adjacent to the spinous process of its vertebra of origin (e.g., T3 nerve innervating region of T3 spinous process). The medial branches of the fifth and sixth thoracic nerves also reach the skin of the back.
Maigne and colleagues (1991) found that the upper thoracic medial branches of the dorsal rami frequently appeared to be entrapped in tendons of the erector spinae or splenius cervicis muscle. They believed this helped to explain localized areas of thoracic discomfort with associated hypesthesia and paresthesia frequently seen in their clinical practice. Also, in 1 of the 16 cadavers studied, the authors found a bilateral anastomosis between the medial branch of the dorsal ramus of T2 and the accessory nerve (CN XI). They thought this might explain the occasional lack of paralysis of the trapezius muscle among certain individuals after transection of the accessory nerve during neck surgery.
Like the medial branches of the posterior primary divisions of the upper six thoracic nerves, those of the lower six pass posteriorly to innervate primarily the multifidi, rotatores, and longissimus thoracis muscles; however, they only occasionally reach the skin of the back (Standring et al., 2008).
Intercostal Nerves, Arteries, and Veins
The anterior primary divisions of the T1 to T11 nerves are the intercostal nerves (see Figs. 6-11 and 6-12). The ventral ramus of the T1 nerve splits, and the larger branch joins the ventral ramus of C8 to form the lower (inferior) trunk of the brachial plexus. The smaller branch of the ventral ramus of T1 forms the first intercostal nerve. The anterior primary division of the T12 nerve is the subcostal nerve.
Close to its origin, each intercostal nerve (and the subcostal and upper two lumbar nerves) sends a white ramus communicans to the sympathetic ganglion of the same level. These ganglia are located anterior to the intercostal nerves and lie along the lateral aspect of the vertebral bodies.
The intercostal nerves also receive gray rami communicantes from the neighboring sympathetic ganglia (see Fig. 6-12). This is similar to all other anterior primary divisions. The intercostal nerve then continues laterally along the subcostal groove, inferior to the intercostal vein and artery (Fig. 6-13; see also Fig. 6-11). The lateral course of the intercostal nerve, within the posterior intercostal space, is subject to a wide degree of variation. The intercostal nerve frequently runs within the middle of the intercostal space (73% of the time) and occasionally courses along the inferior aspect of the intercostal space just above the subjacent rib (Hardy, 1988).
Each intercostal nerve provides sensory, motor (somatic motor), and sympathetic (visceral motor to blood vessels and sweat glands) innervation to the thoracic or abdominal wall. This is accomplished by means of posterior, lateral, and anterior branches.
Posterior Intercostal Arteries
The third through eleventh intercostal arteries originate from the thoracic aorta and course laterally along the inferior aspect of the corresponding rib (see Figs. 6-11 and 6-13, A). The artery that courses below the twelfth rib is known as the subcostal artery because it lies inferior to the twelfth rib and not between two ribs. The first two intercostal arteries arise from the highest intercostal artery. The highest intercostal artery is a branch of the costocervical trunk, which arises from the subclavian artery.
The intercostal arteries course between the intercostal vein superiorly and the intercostal nerve inferiorly. Because the thoracic aorta lies to the left of the thoracic spine, the right intercostal arteries must cross over the thoracic vertebral bodies to reach the right intercostal spaces. This results in the right intercostal arteries being longer than the left ones.
Each posterior intercostal artery gives rise to dorsal and lateral branches that supply the dorsal (including deep back muscles) and lateral aspects of the intercostal spaces, respectively.
Anterior Intercostal Arteries
The upper six anterior intercostal arteries arise from the internal thoracic artery (internal mammary artery). The internal thoracic artery arises from the subclavian artery, and then courses inferiorly, behind and lateral to the sternocostal articulations, and divides into the superior epigastric artery and the musculophrenic artery. The musculophrenic artery, in turn, supplies the seventh, eighth, and ninth anterior intercostal arteries. The nine anterior intercostal arteries supply the intercostal muscles anteriorly, as well as the muscles of the anterior thoracic wall and breast.
Intercostal Veins and the Azygos System of Veins
Venous drainage of the thoracic cage is accomplished primarily by the intercostal veins. The intercostal venous blood generally courses in the opposite direction of the arterial supply and drains into the azygos system of veins (see Figs. 6-11 and 6-13, A).
The azygos vein originates from one or more of the following: inferior vena cava, right renal vein, and right ascending lumbar vein. The azygos vein passes through the diaphragm by means of the aortic hiatus. It then courses along the right anterior aspect of the thoracic vertebral bodies. Along its course this vein receives the right lower eight intercostal veins, right superior intercostal vein, and hemiazygos vein. In addition, the accessory hemiazygos vein frequently is a direct tributary of the azygos vein. The right superior intercostal vein drains the upper two to three intercostal spaces and can empty into either the azygos or the right brachiocephalic vein. Other tributaries of the azygos vein include esophageal, bronchial, mediastinal, and pericardial veins. The azygos vein courses superiorly and arches (from posterior to anterior) around the superior aspect of the right primary bronchus. It then terminates by entering the superior vena cava.
The hemiazygos vein originates from the left ascending lumbar vein, left renal vein, or both. The hemiazygos vein enters the thorax through the aortic hiatus. It then continues superiorly along the left anterior aspect of the vertebral bodies. Along its path the hemiazygos vein receives the lower four or five left intercostal veins. It also frequently receives the accessory hemiazygos vein. The hemiazygos vein helps to drain the left mediastinum and left lower esophagus. The hemiazygos vein crosses from left to right at approximately the level of T9 and terminates by emptying into the azygos vein.
The accessory hemiazygos vein connects the middle three or four intercostal veins. It occasionally receives the left superior intercostal vein, which drains the upper two to three intercostal spaces. However, the left superior intercostal vein normally is a tributary of the left brachiocephalic vein. The accessory hemiazygos vein ends either by draining into the hemiazygos vein or by crossing the vertebral column from left to right, just above the hemiazygos vein, to drain into the azygos vein.
Thoracic Duct
The thoracic duct is the largest and most important lymphatic channel of the body (see Figs. 6-11 and 6-13). The thoracic duct drains the lower extremities, pelvis, abdomen, left side of the thorax, left upper extremity, and left side of the head and neck. It originates at the cisterna chyli (when present) and terminates at the junction of the left subclavian and left internal jugular veins. The cisterna chyli is a large midline lymphatic collecting structure located just inferior to the aortic hiatus of the diaphragm. It collects lymphatics from the lower extremities via left and right lateral branches and from the intestinal tract via an intestinal branch. The cisterna chyli tapers at its superior aspect and becomes the thoracic duct. Most frequently the cisterna chyli is replaced by a confluence of lymph trunks in the abdominal region. The thoracic duct subsequently enters the thorax through the aortic hiatus just to the right of the aorta. On entering the thorax, the thoracic duct continues superiorly along the anterior aspect of the thoracic vertebral bodies. Between T7 and T5 it passes to the left side of the anterior aspect of the vertebral bodies. The thoracic duct continues superiorly to empty into the junction of the left subclavian and internal jugular veins.
The right lymphatic duct drains the right side of the thorax, right upper extremity, and right side of the neck and head. It usually empties into the right subclavian vein, the internal jugular vein, or the union of the two.
The lymphatics of the thoracic cage drain into mediastinal nodes, which in turn drain into either the right lymphatic duct or the thoracic duct. The lymphatics of the mediastinum are abundant and can be divided into four major nodes: superior mediastinal, diaphragmatic, posterior mediastinal, and tracheobronchial. These lymph nodes drain into nearby lymphatic channels. Those of the right side drain into the right lymphatic duct, and those of the left side drain into the thoracic duct.
Aortic Arch and Thoracic Aorta
The aortic arch begins at the heart as the outflow path of the left ventricle. It extends in front of the trachea, swings around the left primary bronchus, and comes to lie to the left of the midthoracic vertebrae (see Fig. 6-11). The arch then continues inferiorly as the descending thoracic aorta. There are three large branches from the aortic arch: the brachiocephalic (innominate) artery, left common carotid artery, and left subclavian artery.
Descending Thoracic Aorta
The descending thoracic aorta is the continuation of the aortic arch (see Figs. 6-11 and 6-13). It begins at approximately the T4-5 disc and continues inferiorly along the left side of the thoracic vertebrae. The thoracic aorta shifts to the midline in the lower thorax, lying on the anterior aspect of the lower thoracic vertebrae. The thoracic aorta gives off bronchial arteries (which supply the lungs) and all the posterior intercostal arteries except the first two on each side (supplied by the highest intercostal artery of the costocervical trunk). The descending thoracic aorta also gives off the left and right subcostal arteries.
Esophagus
The esophagus (see Fig. 6-11) originates posterior to the cricoid cartilage (approximately at the level of C6) and terminates at the cardia of the stomach (at the T11 vertebral body level). Therefore the esophagus has cervical, thoracic, and abdominal regions. It is approximately 25 cm (10 inches) in length. The esophagus lies nearly in the midline in the upper and middle thorax, where it is located posterior to the left atrium. It curves left at the esophageal hiatus (approximately T10 vertebral body level), where it lies anterior and slightly to the left of the thoracic aorta and its hiatus.
Trachea
The trachea (see Fig. 6-11) is a rigid tubular organ that lies anterior to the esophagus between the brachiocephalic artery (on the right) and the left common carotid artery (on the left). The brachiocephalic veins lie anterior to the trachea. The trachea begins at the cricoid cartilage (about the level of C6) and ends by bifurcating into the left and right primary bronchi at approximately the level of the T4-5 disc (see Fig. 6-11). The trachea is kept rigid and held open by 16 to 20 cartilaginous tracheal rings. These rings are C-shaped with the open end facing posteriorly. Fibroelastic tissue and smooth muscle (tracheal or trachealis muscle) span the posterior opening. The less rigid posterior surface allows the passage of food through the posteriorly located esophagus. Helping to form the tracheal bifurcation is the carina, the inverted V-shaped inferior border of the trachea formed by the last tracheal cartilage.
The trachea receives innervation from sympathetic and parasympathetic autonomic fibers. The parasympathetics arise from the vagus nerves and their recurrent laryngeal branches. Stimulation of these nerves results in constriction of the trachea and increased secretion of the mucous cells of the tracheal epithelium. Sympathetic innervation of the trachea is derived from branches of the thoracic sympathetic trunk. Stimulation of these nerves results in dilation of the trachea and decreased mucous secretions.
Vagus Nerves
The vagus nerves (right and left) run within the carotid sheath in the neck and enter the thorax medial to the phrenic nerves (right and left). Each vagus nerve is responsible for carrying preganglionic parasympathetic fibers to all the thoracic viscera. These fibers synapse in the walls of the organs they supply. General visceral afferent fibers from these same viscera also travel within the left and right vagus nerves. The parasympathetic nervous system is described in detail in Chapter 10.
Left Vagus Nerve
The left vagus nerve enters the thorax between the left common carotid and left subclavian arteries. It continues inferiorly by crossing the aortic arch, where it gives off the large left recurrent laryngeal nerve. This nerve loops around the aortic arch from anterior to posterior just lateral to the ligamentum arteriosum. It then runs superiorly in a groove between the esophagus and trachea (tracheoesophageal groove) to supply eight of the nine laryngeal muscles on the left side. The main trunk of the vagus nerve continues inferiorly from the arch of the aorta (giving branches to the cardiac plexus) and follows the aorta posteriorly, passing behind the root of the left lung, where it participates in the pulmonary plexus. The vagus nerve then courses medially and comes to lie on the anterior aspect of the esophagus, where it helps to form the anterior esophageal plexus. The anterior esophageal plexus coalesces inferiorly to form the anterior vagal trunk. This trunk exits the thorax by traveling along the anterior aspect of the esophagus through the esophageal hiatus of the diaphragm.
Right Vagus Nerve
The right vagus nerve enters the thorax by crossing the right subclavian artery. The right recurrent laryngeal nerve is given off at this point. This nerve loops around the right subclavian artery and continues superiorly in the right tracheoesophageal groove to supply eight of the nine muscles of the larynx on the right side. The right vagus nerve continues inferiorly in the thorax, contributing to the superficial and deep cardiac plexuses, and runs posterior to the root of the right lung. Here it sends several branches to the posterior pulmonary plexus. The right vagus nerve then travels medially to the posterior aspect of the esophagus, where it forms the posterior esophageal plexus (see Figs. 6-11 and 6-13). The nerves of the posterior esophageal plexus coalesce to form the posterior vagal trunk. The posterior vagal trunk exits the thorax by traveling along the posterior aspect of the esophagus through the esophageal hiatus of the diaphragm.
Thoracic Sympathetic Chain
The sympathetic nervous system is discussed in detail in Chapter 10. Because of the close anatomic relationship between the sympathetic trunk and the thoracic vertebrae, it also is discussed briefly here.
The thoracic sympathetic trunk is composed of a series of connected sympathetic ganglia that course along the left and right sides of the vertebral column in the thoracic region. The thoracic ganglia are generally associated with each vertebral level, but the number is variable and frequently there is even variation in the number of ganglia found on the left and right sides of the sympathetic trunk of the same individual (Zhang et al., 2009). The trunk extends from superior to inferior across the heads of the ribs and is covered by the costal pleura (see Figs. 6-11, 6-12, and 10-7, B). As it reaches the inferior aspect of the thorax, the trunk courses medially to be positioned along the lateral aspect of the lower thoracic vertebral bodies. The sympathetic trunk is composed of axons of neurons whose cell bodies are located in the intermediolateral cell column of the thoracic spinal cord. These axons exit the cord via a ventral root that unites with a dorsal root, forming a spinal nerve. This nerve exits the vertebral canal through the IVF. These preganglionic sympathetic fibers leave the ventral ramus close to its origin to form a white ramus communicans, which connects to the sympathetic ganglion. Once in the sympathetic ganglion, the preganglionic sympathetic fibers have several options to reach their destinations (effector organs) (see Chapter 10). One option is to form gray rami communicantes that course to the ventral rami of spinal nerves (Figs. 6-11 and 6-12). Another option is to help form the splanchnic nerves, which supply a large part of the abdominal viscera with sympathetic innervation.
Splanchnic Nerves
The thorax contains three splanchnic nerves: the greater, the lesser (see Figs. 6-11, 10-5, and 10-12), and the least. Each is formed from branches of the sympathetic chain. The splanchnic nerves course along the lateral aspects of the middle and lower thoracic vertebral bodies and exit the thorax by piercing the posterior aspect of the diaphragm. Considerable variation exists in the ganlgia of origin and course of the splanchnic nerves (Loukas et al., 2010). They then synapse in one of several prevertebral ganglia. The postganglionic fibers from these prevertebral ganglia provide sympathetic innervation to the vast majority of the abdominal viscera.
The three splanchnic nerves, their ganglia of origin, and the prevertebral ganglion in which they synapse are as follows:
• Greater splanchnic nerve. This nerve arises from thoracic ganglia 5 through 9 (see Figs. 6-11; 6-12, A; and 6-13, A) and synapses in the celiac ganglion. Some of its fibers do not synapse here but pass directly to the medulla of the adrenal gland, which they innervate.
• Lesser splanchnic nerve. The lesser splanchnic nerve arises from thoracic ganglia 10 and 11 (Standring et al., 2008). It synapses in the aorticorenal ganglion.
• Least splanchnic nerve. This nerve originates from thoracic ganglion 12 and synapses within ganglia of the renal plexus.
References
Acharya, S., Dorje, T., Srivastava, A. Lower dorsal and lumbar pedicle morphometry in Indian population: a study of four hundred fifty vertebrae. Spine (Phila Pa 1976). 2010;35:E378–E384.
Akita, K., et al. The subclavius posticus muscle: a factor in arterial, venous or brachial plexus compression? Surg Radiol Anat. 2000;22:111–115.
Alvarez, O., Roque, C.T., Pampati, M. Multilevel thoracic disk herniations: CT and MR studies. J Comput Assist Tomogr. 1988;12:649–652.
Bailey, P., Casamajor, L. Osteo-arthritis of the spine as a cause of compression of the spinal cord and its roots. J Nerv Ment Dis. 1911:588–609.
Bauduin, E., et al. Foraminal herniation of a thoracic calcified nucleus pulposus. Neuroradiology. 1989;31:287–288.
Behrsin, J.F., Briggs, C.A. Ligaments of the lumbar spine: a review. Surg Radiol Anat. 1988;10:211–219.
Bland, J. Disorders of the cervical spine. Philadelphia: WB Saunders; 1987.
Boyle, J.J.W., Singer, K.P., Milne, N. Morphological survey of the cervicothoracic junctional region. Spine. 1996;21:544–548.
Carpintero, P., et al. Scoliosis induced by asymmetric lordosis and rotation. Spine. 1997;22:2202–2206.
Cheung, M.C., et al. Effect of melatonin suppression on scoliosis development in chickens by either constant light or surgical pinealectomy. Spine. 2003;28:1941–1944.
Collins, J.D., et al. Compromising abnormalities of the brachial plexus as displayed by magnetic resonance imaging. Clin Anat. 1995;8:1–16.
Cook, S.D., et al. Upper extremity proprioception in idiopathic scoliosis. Clin Orthop. 1986;213:118–123.
Cramer, G., et al. Identification of the anterior longitudinal ligament on cadaveric spines and comparison with appearance on MRI. Bournemouth, England: Proc 1996 Internat Conf Spinal Manipulation (ICSM); 1996. [October 17-19, pp 151–152].
Crean, J.K.G., et al. Matrix metalloproteinases in the human intervertebral disc: role in disc degeneration and scoliosis. Spine. 1997;22:2877–2884.
Dahia, C.L., et al. Intercellular signaling pathways active during and after growth and differentiation of the lumbar vertebral growth plate. Spine (Phila Pa 1976). 2011;36:1071–1080.
Darwish, H.H., Ibrahim, A.F. Three muscles in the upper costovertebral region: description and clinical anatomy. Clin Anat. 2009;22:352–357.
Deacon, P., Archer, J., Dickson, R.A. The anatomy of spinal deformity: a biomechanical analysis. Orthopedics. 1987;10:897–903.
Deane, G., Duthie, R.B. A new projectional look at articulated scoliotic spines. Acta Orthop Scand. 1973;44:351–365.
Dias, R.C., et al. Revision spine surgery in children with cerebral palsy. J Spinal Disord. 1997;10:132–144.
Duance, V.C., et al. Changes in collagen cross linking in degenerative disc disease and scoliosis. Spine. 1998;23:2545–2551.
Dupuis, P.R., et al. Radiologic diagnosis of degenerative lumbar spinal instability. Spine. 1985;10:262–276.
Edelson, J.G., Nathan, H. Stages in the natural history of the vertebral end plates. Spine. 1988;13:21–26.
Erwin, W.M., Jackson, P.C., Homonko, D.A. Innervation of the human costovertebral joint: implications for clinical back pain syndromes. J Manipulative Physiol Ther. 2000;23(6):395–403.
Evans, S.C., et al. MRI of 'idiopathic' juvenile scoliosis: prospective study. J Bone Joint Surg [Br]. 1996;78-B:314–317.
Faingold, R., et al. Imaging of low back pain in children and adolescents. Semin Ultrasound CT MR. 2004;25:490–505.
Forcada, P., et al. Subclavius posticus muscle: supernumerary muscle as a potential cause for thoracic outlet syndrome. Clin Anat. 2001;14:55–57.
Foreman, S.M., Crofts, A.C. Whiplash injuries: the cervical acceleration/deceleration syndrome. Baltimore: Williams & Wilkins; 1988.
Fujimura, Y., et al. Long-term follow-up study of anterior decompression and fusion for thoracic myelopathy resulting from ossification of the posterior longitudinal ligament. Spine. 1997;22:305–311.
Ghanem, I.B., et al. Chiari I malformation associated with syringomyelia and scoliosis. Spine. 1997;22:1313–1318.
Giles, L.G.F. Lumbo-sacral zygapophysial joint tropism and its effect on hyaline cartilage. Clin Biomech. 1987;2:2–6.
Goldberg, C.J., et al. Adolescent idiopathic scoliosis and cerebral asymmetry. Spine. 1995;20:1685–1691.
Groen, G.J., et al. Branches of the thoracic sympathetic trunk in the human fetus. Anat Embryol. 1987;176:401–411.
Guo, J.J., et al. Classification and management of the tandem ossification of the posterior longitudinal ligament and flaval ligament. Chin Med J (Engl). 2009;122:219–224.
Hardy, P.A. Anatomical variation in the position of the proximal intercostal nerve. Br J Anaesth. 1988;61:338–339.
Harrison, D.D., et al. Do alterations in vertebral and disc dimensions affect an elliptical model of thoracic kyphosis? Spine. 2003;28:463–469.
Hasue, M., et al. Anatomic study of the interrelation between lumbosacral nerve roots and their surrounding tissues. Spine. 1983;8:50–58.
Humzah, M.D., Soames, R.W. Human intervertebral disc: structure and function. Anat Rec. 1988;220:337–356.
Janssen, M.M., et al. Analysis of preexistent vertebral rotation in the normal infantile, juvenile and adolescent spine. Spine (Phila Pa 1976). 2011;36:E486–E491.
Kanemura, T., et al. Natural cause of experimental scoliosis in pinealectomized chickens. Spine. 1997;22:1563–1567.
Karol, L.A., et al. Progression of the curve in boys who have idiopathic scoliosis. J Bone Joint Surg. 1993;75-A:1804–1810.
Kesling, K.L., Reinker, K.A. Scoliosis in twins: a meta-analysis of the literature and report of six cases. Spine. 1997;22:2009–2014.
Kos, J., Hert, J., Sevcik, P. Meniscoids of the intervertebral joints [in Czech]. Acta Chir Orthop Traumatol Cech. 2002;69:149–157.
Kothe, R., et al. Internal architecture of the thoracic pedicle. Spine. 1996;21:264–270.
Kretzer, R.M., et al. A computed tomography-based morphometric study of thoracic pedicle anatomy in a random United States trauma population. J Neurosurg Spine. 2011;14:235–243.
Krishna, M., Upadhyay, S.S. Increased limb lengths in patients with shortened spine due to tuberculosis in early childhood. Spine. 1996;21:1045–1047.
Lemire, J.J., et al. Scheuermann's juvenile kyphosis. J Manipulative Physiol Ther. 1996;19(3):195–201.
Ley, F. Contribution to the study of thoracic vertebral articular cavities between the articular processes. Arch Anat Hist Embr. 1974;57:61–114.
Limthongkul, W., et al. Volumetric analysis of thoracic and lumbar vertebral bodies. Spine J. 2010;10:153–158.
Lipson, S.J., O'Connell, J.X. A 47-year-old woman with back pain and a lesion in a vertebral body. N Engl J Med. 1991;325:794–799.
Loukas, M., et al. A review of the thoracic splanchnic nerves and celiac ganglia. Clin Anat. 2010;23:512–522.
Lowe, T.G., et al. Current concepts review etiology of idiopathic scoliosis: current trends in research. J Bone Joint Surg. 2000;82-A:1157–1166.
Maigne, J.Y., Maigne, R., Guerin-Surville, H. Upper thoracic dorsal rami: anatomic study of their medial cutaneous branches. Surg Radiol Anat. 1991;13:109–112.
Marchesi, D., et al. Morphometric analysis of the thoracolumbar and lumbar pedicles, anatomico-radiologic study. Surg Radiol Anat. 1988;10:317–322.
Masharawi, Y., Salame, K. Shape variation of the neural arch in the thoracic and lumbar spine: characterization and relationship with the vertebral body shape. Clin Anat. 2011;24:858–867.
Masharawi, Y., et al. Facet orientation in the thoracolumbar spine: three-dimensional anatomic and biomechanical analysis. Spine (Phila Pa 1976). 2004;29:1755–1763.
Masharawi, Y., et al. Facet tropism and interfacet shape in the thoracolumbar vertebrae: characterization and biomechanical interpretation. Spine (Phila Pa 1976). 2005;30:E281–E292.
Masharawi, Y., et al. Vertebral body shape variation in the thoracic and lumbar spine: characterization of its asymmetry and wedging. Clin Anat. 2008;21:46–54.
Matullo, K.S., et al. Characterization of a first thoracic rib ligament: anatomy and possible clinical relevance. Spine (Phila Pa 1976). 2010;35:2030–2034.
McLain, R.F., Ferrara, L., Kabins, M. Pedicle morphometry in the upper thoracic spine: limits to safe screw placement in older patients. Spine. 2002;27:2467–2471.
McLain, R.F., Pickar, J.G. Mechanoreceptor endings in human thoracic and lumbar facet joints. Spine. 1998;23:168–173.
Meyer, P.R. Contribution to the study of costo-vertebral articular cavities. Arch Anat Hist Embr. 1972;55:283–360.
Molnar, S., et al. Ex vivo and in vitro determination of the axial rotational axis of the human thoracic spine. Spine (Phila Pa 1976). 2006;31:E984–E991.
Moore, K.L. Clinically oriented anatomy, 3rd ed. Baltimore: Williams & Wilkins; 1992.
Nathan, H. The para-articular processes of the thoracic vertebrae. Anat Rec. 1959;133:605–618.
Nathan, H. Osteophytes of the vertebral column: an anatomical study of their development according to age, race, and sex with considerations as to their etiology and significance. J Bone Joint Surg. 1962;44-A:243–268.
Nissinen, M., et al. Trunk asymmetry and scoliosis. Acta Paediatr Scand. 1989;78:747–753.
Oda, I., et al. Biochemical role of the posterior elements, costovertebral joints, and rib cage in stability of the thoracic spine. Spine. 1996;21:1423–1429.
Oda, I., et al. An in vitro human cadaveric study investigating the biomechanical properties of the thoracic spine. Spine. 2002;27:E64–E70.
Ofiram, E., et al. Is it safer to place pedicle screws in the lower thoracic spine than in the upper lumbar spine? Spine (Phila Pa 1976). 2007;32:49–54.
Otani, K., et al. Thoracic disc herniation: surgical treatment in 23 patients. Spine. 1988;13:1262–1267.
Pai, B.S., et al. Morphometric analysis of the thoracic pedicle: an anatomico-radiological study. Neurol India. 2010;58:253–258.
Pal, G.P., et al. Trajectory architecture of the trabecular bone between the body and the neural arch in human vertebrae. Anat Rec. 1988;222:418–425.
Paris, S. Anatomy as related to function and pain. Orthop Clin North Am. 1983;14:476–489.
Pascoe, D.D., et al. Influence of carrying book bags on gait cycle and posture of youths. Ergonomics. 1997;40:631–641.
Ranney, D. Thoracic outlet: an anatomical redefinition that makes clinical sense. Clin Anat. 1996;9:50–52.
Scapinelli, R. Morphological and functional changes of the lumbar spinous processes in the elderly. Surg Radiol Anat. 1989;11:129–133.
Schulte, T.L., et al. Intra-articular meniscoid folds in thoracic zygapophysial joints. Spine (Phila Pa 1976). 2010. [[Epub ahead of print]].
Shimada, Y., et al. Spondylolisthesis of the thoracic spine. Case report. J Neurosurg Spine. 2006;4:415–418.
Singer, K.P., Giles, L.G.F. Manual therapy considerations at the thoracolumbar junction: an anatomical and functional perspective. J Manipulative Physiol Ther. 1990;13:83–88.
Singer, K.P., Giles, L.G.F., Day, R.E. Intra-articular synovial folds of thoracolumbar junction zygapophyseal joints. Anat Rec. 1990;226:147–152.
Spapen, H.D., et al. The straight back syndrome. Neth J Med. 1990;36:29–31.
Standring, S., et al. Gray's anatomy: the anatomical basis of clinical practice, 40th ed. Edinburgh: Churchill Livingstone; 2008.
Sunderland, S. Meningeal-neural relations in the intervertebral foramen. J Neurosurg. 1974;40:756–763.
Theiss, S.M., Smith, M.D., Winter, R.B. The long-term follow-up of patients with Klippel-Feil syndrome and congenital scoliosis. Spine. 1997;22:1219–1222.
Upadhyay, S.S., et al. Disproportionate growth in girls with adolescent idiopathic scoliosis: a longitudinal study. Spine. 1991;8(suppl):s343–s347.
van Es, H.W., Bollen, T.L., van Heesewijk, H.P. MRI of the brachial plexus: a pictorial review. Eur J Radiol. 2010;74:391–402.
Vera, C.L., et al. Paraplegia due to ossification of ligamenta flava in x-linked hypophosphatemia. Spine. 1997;22:710–715.
Vernon, L., Dooley, J., Acusta, A. Upper lumbar and thoracic disc pathology: a magnetic resonance imaging analysis. J Neuromusculoskeletal Syst. 1993;1:59–63.
Viejo-Fuertes, D., et al. Morphologic and histologic study of the ligamentum flavum in the thoraco-lumbar region. Surg Radiol Anat. 1998;20:171–176.
Vital, J., et al. The neurocentral vertebral cartilage: anatomy, physiology and physiopathology. Surg Radiol Anat. 1989;11:323–328.
Wang, X., et al. Characterization of the scoliosis that develops after pinealectomy in the chicken and comparison with adolescent idiopathic scoliosis in humans. Spine. 1997;22:2626–2635.
White, A.W., Panjabi, M.M. Clinical biomechanics of the spine. Philadelphia: JB Lippincott; 1990.
Woods, L.A., et al. Decreased incidence of scoliosis in hearing impaired children. Spine. 1995;20:776–781.
Wyatt, M.P., et al. Vibratory response in idiopathic scoliosis. J Bone Joint Surg. 1986;68:714–718.
Yamashita, Y., et al. Spinal cord compression due to ossification of ligaments: MRI imaging. Radiology. 1990;175:843–848.
Yayama, T., et al. Thoracic ossification of the human ligamentum flavum: histopathological and immunohistochemical findings around the ossified lesion. J Neurosurg Spine. 2007;7:184–193.
Yu, J., et al. The elastic fiber network of the anulus fibrosus of the normal and scoliotic human intervertebral disc. Spine (Phila Pa 1976). 2005;30:1815–1820.
Zhang, B., et al. Anatomical variations of the upper thoracic sympathetic chain. Clin Anat. 2009;22:595–600.