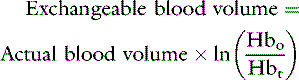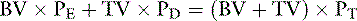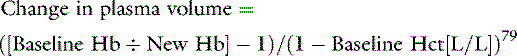Chapter 17 Perioperative Management of Fluid Therapy
The normal mechanisms of fluid homeostasis are disturbed when an animal undergoes anesthesia and surgery. Consequently, animals should receive fluids during the perioperative period to maintain proper fluid balance. Anesthetized animals should receive fluids:
1. To establish and maintain venous access. A minimal rate of fluid administration is necessary (e.g., 3 mL/hr) and will ensure rapid access to the circulation in the event of an emergency in the perioperative period.
2. To counter the physiologic changes associated with anesthetics. Most of the drugs and techniques used to anesthetize animals have some effect on the circulation.
3. To replace fluids lost during anesthesia and surgery. During the procedure, the animal cannot drink and its metabolic rate is reduced (decreased production of metabolic water). At the same time, the animal continues to produce urine, salivate, secrete fluid into the gastrointestinal tract, and lose water by evaporation from the respiratory tract. The aim should be at least to replace the expected insensible fluid losses.
4. To correct fluid losses caused by disease and replace ongoing losses attributed to the procedure. The volume of fluid lost or gained depends on the type of surgical procedure, the skill of the surgeon, the preoperative state of the animal, and the equipment used by the anesthetist. Trauma and surgery are associated with increased secretion of vasopressin, and additional secretion may occur as a result of hypotension or hypovolemia. Other stress hormones (e.g., cortisol, catecholamines, renin) released during the procedure also may play a role in upsetting normal fluid homeostasis and warrant perioperative fluid therapy.
Preoperative preparation of the patient
The animal’s fluid balance should be as close to normal as possible before anesthesia. Almost all anesthetics have some effects on circulatory and renal function, and it is important that the patient’s circulating volume be optimal so that these effects are not exacerbated. Disturbances that require attention may be classified by their urgency. Some can be corrected acutely (e.g., hypovolemia); some require more time to correct (e.g., hypernatremia); and a few require completion of the procedure before correction of the problem can occur (e.g., hypervolemia associated with acute renal failure in a patient being prepared for hemodialysis).
Changes in vascular volume
Hypovolemia
Hypovolemia may be caused by fluid loss directly from the vascular space (e.g., hemorrhage), a more general loss (e.g., dehydration), or changes in vascular tone. In all cases, fluid should be given to replace the loss. For a simple loss in which the composition of the vascular space is relatively normal, the loss can be replaced effectively using an isotonic crystalloid, a hypertonic crystalloid, an artificial colloid, or a blood product. The fluid used depends on the severity of the loss and the financial resources of the client. Acute blood loss of up to 30% of blood volume can be replaced adequately using a crystalloid solution (assuming normal hematocrit and total protein concentration before therapy), whereas a loss of 50% of blood volume or more will probably require blood component therapy and possibly additional crystalloid or colloid support. Occasionally, fluid therapy is not sufficient, and surgical management is required to stop bleeding (e.g., penetrating trauma with major blood vessel rupture). In these instances, it is crucial to have one or more large-gauge venous catheters in place in an attempt to keep pace with the loss. In cats and dogs weighing less than 5 kg, it usually is feasible to place an 18-gauge catheter in the jugular vein. In many dogs of 5 to 15 kg, it is feasible to place a 16-gauge catheter in a cephalic vein, whereas in dogs more than 15 kg, a 14-gauge catheter normally may be placed. After the catheter has been placed, the animal should be anesthetized using a technique that induces minimal disturbances in volume status and cardiovascular function. Some investigators advocate withholding fluids from trauma patients with major vessel rupture before surgical intervention. In one study of human patients, a marginal benefit was demonstrated using this approach.14 Others have advocated resuscitation to lower than normal blood pressures to minimize the chance of dislodging a fragile clot or increasing the rate of hemorrhage.55 It is likely to be a realistic approach only when blood loss is rapid and surgery can be performed immediately.157 In patients with major blood loss but no central vessel rupture, it is more appropriate to replace the volume deficit before anesthetizing the animal.
If the patient is expected to lose a large volume of blood during an anticipated elective surgery, the animal can donate blood in advance so as to have autologous blood available. The animal can donate 1 unit of blood and then return 3 weeks later, at which time the first unit of blood can be returned to the animal and 2 units of blood drawn. This procedure can be repeated to collect several units from the same animal. This approach usually is not possible because of the lead time needed to complete these multiple collections, but a single donation technique has been reported for cats undergoing partial craniectomy.64 Another alternative in an animal with relatively normal hematocrit and total protein concentration is to use acute normovolemic hemodilution, collect blood immediately before surgery, and replace it with three times the volume of crystalloid or the same volume of colloid. The expectation is that the animal will lose less protein and red cell volume during the surgery because of hemodilution, and the collected blood will be available for transfusion when it is needed after surgery. The formula for calculating the hemodilution was originally described by Bourke and Smith20:
where Hb0 is the original hemoglobin concentration, and Hbt is the target value. This formula tends to overestimate the exchangeable blood volume, and a more accurate iterative formula has been published that uses a more sophisticated calculation technique.121
Although these techniques may be beneficial under special circumstances, they have been evaluated in human medicine and have been found to be very expensive and to provide little benefit to the patient.21,37,73,100,163 Nevertheless, the American Society of Anesthesiologists (ASA) published guidelines for the use of packed red cells that include the following statements:143
1. When appropriate, preoperative autologous blood donation, intraoperative and postoperative blood recovery, acute normovolemic hemodilution, and measures to decrease blood loss (deliberate hypotension and pharmacologic agents) may be beneficial.
2. The indications for transfusion of autologous red blood cells (RBCs) may be more liberal than for allogeneic RBCs because of the lower (but still significant) risks associated with the former.
Hypervolemia
Hypervolemia is likely to be either iatrogenic or the result of oliguric renal failure or heart failure. In the former situation, it may simply be sufficient to monitor the patient carefully until its fluid volume status has normalized. In the case of oliguric renal failure, it is difficult to reduce the blood volume without dialysis. The primary risk of hypervolemia is related to hypertension and an increase in myocardial work, which could lead to failure in a heart with marginal reserve. Hypervolemia also may lead to pulmonary edema, in which case the circulating volume should be reduced by administration of a diuretic (if renal function is normal) or by phlebotomy if necessary.
Changes in content
Occasionally, changes in vascular volume do not affect the composition of blood, but in many cases changes in composition also occur and require attention.
Anemia
The major concern with anemic patients is the supply of oxygen to the tissues after the animal has been anesthetized with drugs that may impair cardiovascular function. In the chronically anemic animal, some compensation already has occurred to facilitate delivery of oxygen to the tissues. This compensation usually occurs as a result of an increase in cardiac output and a change in the affinity of hemoglobin for oxygen. When the animal is anesthetized, especially using drugs such as α2 agonists or inhalants, cardiac output is decreased, which reduces the delivery of oxygen to the tissues. Administration of 100% oxygen increases the amount of oxygen in solution (0.3 mL per 100 mL of blood per 100 mm Hg pressure), but this effect provides little compensation for the decline in cardiac output. Figure 17-1 illustrates the relationship between hemoglobin concentration and the cardiac index assuming a constant saturation of hemoglobin (99%) to deliver oxygen at a given rate (15 mL/kg/min). The second line shows the same relationship for a Pao2 of 500 mm Hg assuming a hemoglobin saturation of 100%. In acute anemia, the animal may have been able to increase cardiac output, but there has not been sufficient time for changes in hemoglobin affinity to occur, and the delivery of oxygen is likely to be decreased further. What is a “critical” hemoglobin concentration? In many experiments, carried out in dogs, the critical hemoglobin concentration is defined as the point at which oxygen delivery fails to keep up with tissue oxygen demand. In the healthy, lightly anesthetized dog, this concentration appears to be approximately 3 g/dL but varies with the anesthetic used and increases substantially at deeper planes of anesthesia.164,166 Many human patients are anesthetized and survive with hemoglobin concentrations as low as 3 to 4 g/dL, but anesthesia is not recommended in this situation unless great care is taken to ensure that the patient has adequate cardiovascular reserve and unless techniques can be used that minimize reduction in cardiac output.8,32 The ASA guidelines are based on the available literature in human medicine.143 The ASA recommendations for use of packed red cells include the following:
1. Transfusion is rarely indicated when the hemoglobin is greater than 10 g/dL and is almost always indicated when it is less than 6 g/dL, especially when the anemia is acute.
2. The determination of whether intermediate hemoglobin concentrations (6 to 10 g/dL) justify or require RBC transfusion should be based on the patient’s risk for complications of inadequate oxygenation.
3. The use of a single hemoglobin “trigger” for all patients and other approaches that fail to consider all important physiologic and surgical factors affecting oxygenation are not recommended.
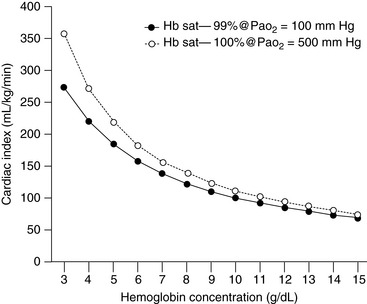
Figure 17-1 The graph indicates the alteration in cardiac index needed to provide an oxygen delivery of 15 mL/kg/min when the Pao2 is increased from 100 to 500 mm Hg, assuming that hemoglobin saturation increases from 99% to 100%. Note that the increased Pao2 begins to make a difference only when the hemoglobin decreases below about 5 g/dL.
A review in the Cochrane database examined the use of restrictive versus liberal transfusion practices and could identify no adverse effects of the use of transfusion triggers in the 7 to 9 g/dL range.84 Although hemoglobin concentration is reported on the complete blood count, it is more common for veterinarians to evaluate the hematocrit, which usually is approximately three times the hemoglobin concentration (expressed in g/dL). A scoring system for the rational use of packed RBCs in dogs was developed in an attempt to decrease unnecessary use.102 However, this scoring system did not account for blood transfusions under conditions of rapid blood loss and failure to maintain blood pressure. It is important to assess anemic dogs and cats carefully and to estimate the likelihood of blood loss during the procedure. A dog with a hematocrit of 18% and a healthy cardiovascular system about to undergo a noninvasive diagnostic procedure may be a candidate for anesthesia without previous transfusion. A patient with the same hematocrit but with clinically relevant mitral regurgitation and about to undergo an exploratory laparotomy for an undefined abdominal mass would be more likely to require a preoperative blood transfusion.
Polycythemia
Patients with polycythemia are at risk for complications because of the increased viscosity of their blood. High viscosity increases myocardial work and may lead to inadequate flow in some capillary beds, especially if the animal becomes hypotensive.9 The hematocrit should be reduced to at least 65% by removal of blood and replacement with an isotonic crystalloid before the polycythemic patient is anesthetized. Animals with polycythemia caused by chronic hypoxia (e.g., tetralogy of Fallot) must be monitored carefully for signs of inadequate oxygen delivery when such hemodilution is undertaken.
Hypoproteinemia
Many drugs given during anesthesia are highly protein bound, and hypoproteinemia may result in a greater fraction of the anesthetic being available. More profound depression thus may occur from a given dose in the hypoproteinemic patient. Most drugs bind to albumin, and it is this fraction of the proteins that is of greatest importance. However, if the drug is titrated to effect, the increased free fraction of drug is accounted for by close monitoring of anesthetic induction. Thus concerns about hypoproteinemia are greater when using intramuscular injection or bolus dose techniques.
Hypoproteinemia also may affect the balance between hydrostatic and colloid osmotic pressure, leading to increased loss of fluid from the capillaries. This effect is of particular concern to the anesthetist because it may increase the likelihood of pulmonary edema formation. Total plasma protein and colloid osmotic pressure typically decrease during anesthesia in dogs whether or not fluids are administered.52,198 Clinically, this effect is of limited importance unless there is a strong possibility that left atrial pressure is increased (e.g., low oncotic pressure in an animal with mitral regurgitation).
Hyperproteinemia
Increased plasma protein concentration is of concern only as a sign of hypovolemia. In normally hydrated dogs and cats with hyperproteinemia, it is the globulins that are increased, and this fraction has less impact on protein binding of drugs and oncotic pressure than does albumin. However, hyperproteinemia may be a cause of pseudohyponatremia if the total protein concentration exceeds 10 g/dL.
Hyponatremia
Rapid correction of hyponatremia may be necessary to treat cerebral edema (usually only when serum sodium is <130 mEq/L). With acute hyponatremia, rapid correction may not cause any complications in the brain, but with chronic hyponatremia, a rapid change in serum sodium concentration can lead to an osmotic demyelination syndrome or myelinolysis occurring one to several days after therapy.111,131 In both acute and chronic situations, the rate of change should be approximately 0.5 to 1 mEq/hr unless the patient is manifesting signs of cerebral edema, in which case initial therapy with 3% saline may be used to increase serum sodium concentration by 5 to 6 mEq/L over 2 to 3 hours. Ideally, hyponatremia should be corrected before surgery; however, given the required time frame, this is not always possible. Therefore the anesthetist must be prepared to monitor changes in serum sodium concentration carefully to prevent myelinolysis. It may be necessary to administer a diuretic to facilitate excretion of free water (see Chapter 3).
Hypernatremia
Rapid correction of hypernatremia can lead to acute cerebral edema. If the patient is severely hypovolemic, it is important to correct that deficit using a solution with a sodium concentration similar to that of the patient. If the animal is not severely dehydrated and the serum sodium exceeds 165 mEq/L, correction should proceed slowly to achieve a rate of change of 0.5 to 1 mEq/hr using 0.45% NaCl or 5% dextrose. In dogs, administration of 5% dextrose at 3.7 mL/kg/hr should decrease the serum sodium concentration by 1 mEq/hr. Hypernatremia may increase the minimum alveolar concentration of inhalants, and a higher dose may be required to maintain anesthesia.180
Hypokalemia
Hypokalemia can lead to muscle weakness, cardiac arrhythmias, hypotension, and renal insufficiency with associated metabolic acidosis in dogs and cats. In patients with mild hypokalemia but no clinical signs and no identifiable underlying cause, it probably is unnecessary to treat the animal. The patient with hypokalemia that is likely to have a whole-body deficit of potassium should be treated to correct this deficit if possible. The usual recommendation is to correct the deficit at a maximal rate of 0.5 mEq/kg/hr, although higher rates can be used if a severe deficit of total body potassium is suspected (up to 1.0 mEq/kg/hr). If the hypokalemic patient must be anesthetized, it is important to monitor for cardiac arrhythmias and to recognize that the heart will be refractory to class I antiarrhythmic drugs (e.g., quinidine, procainamide, lidocaine) and more sensitive to the toxic effects of digitalis glycosides. Hypotension may occur because there is a decrease in systemic vascular resistance possibly related to decreased sensitivity to angiotensin II.66 The pressor response to norepinephrine is normal. If muscle relaxants are to be used, it is prudent to start with a dose that is 30% to 50% lower than the normal dose and titrate the final dose to effect. Care should be taken administering glucose, sodium bicarbonate, or β2-agonists because they tend to decrease serum potassium concentration. If a potassium-supplemented solution is to be used during anesthesia to correct the deficit, it should be used in conjunction with a solution containing a normal concentration of potassium (4 to 5 mEq/L), and the two solutions should be clearly labeled. If the animal requires a bolus of fluid during anesthesia, the solution with normal potassium concentration should be used, thus reducing the risk of iatrogenic hyperkalemia. Solutions containing more than 60 mEq/L of potassium should be given via a central vein.
Hyperkalemia
Hyperkalemia also is associated with muscle weakness and cardiac arrhythmias. If these signs are present, it is crucial to reduce the effects of hyperkalemia even though it is not possible to reduce total body potassium content without treating the primary condition (e.g., oliguric renal failure, urethral obstruction). Animals with moderate hyperkalemia (6 to 7 mEq/L) are more likely to develop arrhythmias during anesthesia even if they have not demonstrated electrocardiographic abnormalities earlier. Therapy for hyperkalemia includes administration of calcium to alter the threshold potential of cells, sodium bicarbonate to alter the flux of potassium across the cell membrane, and glucose to facilitate movement of potassium into cells. Insulin may be used with glucose to prevent hyperglycemia, but the blood glucose concentration must be monitored for several hours to avoid hypoglycemia. β-Adrenergic agonists such as albuterol and salbutamol have been used to manage hyperkalemia, and their activity may be enhanced with the use of insulin.6,113 One study in dogs documented the effect of epinephrine and ritodrine in reducing hyperkalemia.61 After the animal is anesthetized, ventilation should be monitored and controlled if necessary because hypercapnia may decrease pH and facilitate potassium efflux from cells. Depolarizing muscle relaxants (e.g., succinylcholine) should be avoided because they may cause release of potassium from cells. Nondepolarizing relaxants should be used cautiously (50% to 70% of the normal dose) to prevent prolonged effects. The patient should be monitored carefully by electrocardiography and frequent measurements of serum glucose, potassium, and ionized calcium concentrations and acid-base status (see Chapter 5).
Hypocalcemia
Decreased calcium concentrations are associated with increased neuromuscular excitability. In the heart, this may manifest itself as a prolonged QT–interval and other arrhythmias (e.g., ventricular premature contractions, ventricular fibrillation). As with the other electrolytes, the rate of change is an important factor in the type of clinical signs seen. It is important to treat a patient with hypocalcemia and clinical signs before anesthesia. This can be achieved rapidly while the electrocardiogram is monitored for signs of overly rapid correction (bradycardia). Hyperthermia associated with hypocalcemic seizure activity also should be treated before anesthesia. Hypocalcemic patients are at increased risk from the toxic manifestations of digoxin therapy, and this risk should be taken into consideration when preparing cardiac patients for anesthesia.
Hypercalcemia
Signs of muscle weakness also may be seen with hypercalcemia, but arrhythmias are relatively uncommon. When they do occur, cardiovascular manifestations include bradycardia with prolonged PR–interval, wide QRS complex, and shortened QT–interval. Hypercalcemia is difficult to treat acutely and usually requires treatment for at least 24 hours before anesthesia (see Chapter 6).
Hyperosmolality
Hyperosmolality usually is associated with hypernatremia, hyperglycemia, ketoacidosis, uremia, or the presence of exogenous toxins (e.g., ethylene glycol). In some cases, it may be impossible to reverse the hyperosmolar state adequately before anesthesia because therapy (e.g., hemodialysis) may require an invasive procedure. Hyperosmolality may be associated with disruption of the blood-brain barrier leading to greater uptake of some drugs.200 This is unlikely to affect most anesthetics because they readily cross the blood-brain barrier normally. The hyperosmolar state associated with hypernatremia may increase the dose of inhalant required for anesthesia.180
Hypoosmolality
This invariably is associated with an excess of free water and hyponatremia and should be managed as described previously.
Hypoglycemia
Hypoglycemia in an awake patient usually is manifested by somnolence progressing to coma. In the anesthetized animal, there may be no outward signs, and unless blood glucose concentration is being monitored, it is unlikely that hypoglycemia would be detected. Hence, it is important to recognize and manage hypoglycemia preoperatively. Most animals regulate their blood glucose concentration closely, but this may not be the case in very young animals, those with insulinomas, and animals with portosystemic shunts. It usually is unnecessary to remove very young animals from their dam until the time of premedication if they are receiving a liquid diet only. If they have been orphaned or are ill and have not been taking in fluids, it is best to check blood glucose concentration before anesthesia and treat accordingly. If blood is difficult to obtain, the animal can be given some oral glucose in the form of Karo syrup (ACH Food Companies, Inc., Memphis, Tenn.) or some other clear dextrose-containing fluid.58 Intraoperatively, it may be best to use a 2.5% to 5% glucose solution intravenously. Postoperatively, these patients should be monitored carefully or given additional Karo syrup until they can return to their previous feeding regimen. Animals with insulinomas can have resting blood glucose concentrations of 30 to 40 mg/dL and may tolerate these low glucose concentrations quite well. If exogenous glucose is administered as a bolus to an animal with hyperinsulinism, massive release of insulin may trigger a hypoglycemic crisis. Therefore it is important to use relatively dilute solutions of glucose and administer them as an infusion rather than as a bolus. We typically administer 2.5% glucose to these patients the night before surgery at 1 to 1.5 times the normal maintenance rate. Intraoperatively, blood glucose concentration is monitored carefully, and glucose infusions are continued as necessary. After the tumor is removed, blood glucose concentration usually returns rapidly to the normal range. Animals with portosystemic shunts may become hypoglycemic, and glucose supplementation may be needed in the perioperative period. In one retrospective series, 2 of 13 dogs with portosystemic shunts were reported to have developed hypoglycemia intraoperatively.105 Postoperative administration of dexamethasone (0.1 to 0.2 mg/kg IV) may be helpful in managing hypoglycemia in these cases.87
Hyperglycemia
Hyperglycemia typically occurs in diabetic dogs and cats, and in stressed cats. Hyperglycemia itself may not be dangerous; however, if blood glucose concentration exceeds 400 mg/dL, it may contribute to a hyperosmolar diuresis with subsequent dehydration. With diabetic animals, it is ideal if anesthesia can be postponed until blood glucose concentration can be better regulated. If this is not feasible, the animal should be treated with insulin and glucose to stabilize blood glucose concentration between 200 and 300 mg/dL. In patients with brain trauma or those suffering from focal or global brain ischemia during surgery, hyperglycemia may be detrimental to the neurologic outcome.36,165,166,191 In animal models, blood glucose concentrations as low as 150 to 200 mg/dL have been shown to have negative effects on outcome, but the threshold for cerebral damage seems to be approximately 200 mg/dL.115,166 In a study of dogs, dextrose administration was associated with greater renal damage after an ischemic insult than lactated Ringer’s solution (LRS).128 It is thought that increased intracellular glucose contributes to lactic acidosis in the cell, decreasing the chance of cell survival.
Metabolic acidosis
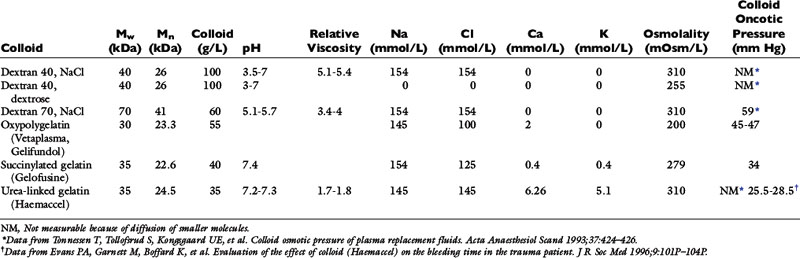
Dogs and cats generally tolerate moderate acidosis reasonably well. However, severe acidosis is likely to lead to reduced activity of enzyme systems in the body with subsequent alterations in energy production and metabolism of drugs. Acidosis also may alter the activity of some anesthetic drugs because more of the un-ionized active form of anionic drugs is available at lower pH values. In patients with acidosis arising from insufficient oxygen delivery to tissues because of inadequate circulating volume, correction of the volume deficit may reverse acidosis without the need for further therapy. Dogs and cats with diabetic ketoacidosis rarely require exogenous alkali if fluid therapy and insulin administration are managed appropriately. In cases in which the underlying condition is difficult to reverse (e.g., hypoxemia related to airway pathology, heart failure, pheochromocytoma), it is important to manage the acidosis before anesthesia. This is normally done using sodium bicarbonate, but Carbicarb and tromethamine may also be used (see Chapter 10). Sodium bicarbonate usually is available as an 8.4% solution with 1 mEq bicarbonate per milliliter and an osmolality of 2000 mOsm/L. In animals that are hyperosmolar or hypernatremic, it may be advisable to dilute bicarbonate to an isosmotic solution to prevent further exacerbation of the animal’s condition. An osmolality of 300 mOsm/L can be achieved by diluting 1.5 mL of the 8.4% solution in 8.5 mL of sterile water. Sodium bicarbonate also should not be administered through the same intravenous line as catecholamines because it inactivates them (Table 17-1). Care should be taken when administering sodium bicarbonate to patients with respiratory depression because it increases the production of CO2. If the animal is unable to increase its ventilation in response to increased production of CO2, there may be little overall change in pH.
Table 17-1 Compatibility of Intravenous Solutions with Other Drugs That Might Be Administered During Anesthesia
| Solution | Comments |
|---|---|
| 5% Dextrose | The pH of the solution ranges from 3.5 to 6.5, so alkaline solutions may precipitate. |
| Lactated Ringer’s | Slightly acidic and contains calcium. Do not administer with blood products. Sodium bicarbonate may also react with the calcium and form calcium carbonate. |
| Acetated polyionic | If it contains no calcium, can be used with blood products and sodium bicarbonate. |
| Sodium chloride 0.9% | Usually slightly acidic but is compatible with most intravenous solutions; may cause precipitation if added to mannitol. |
| Sodium bicarbonate | Alkaline solution—incompatible with dobutamine, dopamine, isoproterenol, norepinephrine, and epinephrine. May react with calcium in solution (e.g., lactated Ringer’s, acetated Ringer’s, some polygelatins). |
| Dextrans | Slightly acidic—may degrade acid-labile drugs and may form drug complexes but appear to be compatible with most intravenous solutions. |
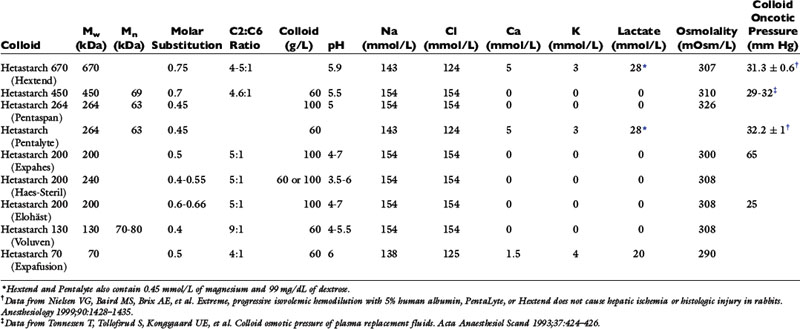
| Hetastarch | May be incompatible with some antibiotics—crystals formed with amikacin, cefamandole, cefoperazone, and tobramycin. |
| Polygelatins | Some preparations contain calcium, and these should not be used with blood products or sodium bicarbonate. |
| Blood and plasma | Do not administer through the same line as calcium salts. |
Metabolic alkalosis
Conditions that cause metabolic alkalosis may be associated with a high mortality rate, and 10 of 20 dogs with primary alkalemia died in one study.151 Induction of anesthesia in an alkalotic patient may be associated with an increased dose requirement because of a decreased amount of un-ionized drug. In most cases, management of metabolic alkalosis requires the administration of chloride-containing solutions. This normally is achieved using 0.9% NaCl supplemented with KCl. Mild alkalosis may be caused by hypoalbuminemia, and correction of serum albumin concentration may be sufficient to correct the alkalosis.
Changes in distribution
Dehydration
Dehydration reduces vascular volume and results in changes in the volume of the intracellular space. The type and extent of change in the various compartments depend on the type of fluid lost. With pure water loss, volume contraction occurs in the intracellular compartment, whereas with hypotonic dehydration, an increase in the volume of the intracellular compartment may occur. With pure water loss, it is relatively simple to replace the circulating volume, but it takes longer to replenish the volume lost from the rest of the body. These concepts are discussed further in Chapter 3.
Peripheral edema
Peripheral edema usually is a reflection of poor circulation, leaky capillaries, or low oncotic pressure. Peripheral edema may have little impact on the course of anesthesia and surgery, but edema in certain locations may make induction and maintenance of anesthesia difficult for the anesthetist. If the limbs are edematous, it may be difficult to achieve venous or arterial access. In such cases, it may be necessary to use the jugular vein to place an intravenous catheter because the neck usually is less affected than are the limbs. Occasionally, dogs suffer damage to or occlusion of the jugular veins that can be associated with edema of the head and neck, potentially including the airway. Great care should be taken when performing endotracheal intubation in edematous animals because the affected tissue often is very fragile. It may be necessary to create a tracheostomy if the upper airway becomes obstructed and there is no way to improve venous drainage. Therapy aimed at improving local (e.g., hot packs, massage) and general (e.g., positive inotropes) circulation or increasing colloid osmotic pressure may reduce peripheral edema.
Pulmonary edema
Pulmonary edema is of great concern to the anesthetist because it impairs gas exchange in the lungs and potentially reduces uptake of inhaled anesthetics. Formation of edema in the pulmonary circulation is a result of increased hydrostatic pressure, decreased colloid osmotic pressure, or damage to the endothelium allowing leakage of fluid. Increased hydrostatic pressure may be caused by absolute (e.g., volume overload) or relative (e.g., redistribution of blood to the pulmonary circulation) hypervolemia, increased pulmonary venous pressure (e.g., left ventricular failure, mitral regurgitation), or increased pulmonary flow (e.g., left-to-right shunt, anemia). Volume overload should be treated with diuretics or phlebotomy as described earlier (hypervolemia). In animals with left ventricular failure or mitral regurgitation, the aim of therapy is to promote forward flow by using vasodilators or positive inotropes. In the acute setting, dobutamine is a suitable positive inotrope because it increases myocardial contractility while tending to decrease systemic vascular resistance. Nitroprusside or nitroglycerin can be used to decrease peripheral vascular resistance and can be titrated to effect. Ideally, therapy should be monitored using a catheter that allows measurement of pulmonary capillary wedge pressure (PCWP).
A decrease in colloid osmotic pressure rarely causes pulmonary edema acutely in dogs and cats, but it is important to take low colloid osmotic pressure into account when designing an anesthetic regimen because pulmonary edema may occur with smaller increases in pulmonary hydrostatic pressure. Both ketamine and large doses of oxymorphone have been shown to increase pulmonary vascular pressures.42,82 If it is thought that low colloid osmotic pressure is contributing to pulmonary edema, therapy should be instituted to increase colloid osmotic pressure (e.g., plasma, dextrans, hetastarch [HES], polygelatins). In the case of pulmonary edema related to leaky membranes, therapy should be aimed at reducing pulmonary vascular pressure (e.g., nitroprusside, diuretics) and providing supportive care for the animal. Supportive care involves provision of oxygen, suction of froth from the airway, and institution of positive-pressure ventilation if necessary. Mechanical ventilation may improve gas exchange in patients with pulmonary edema. Positive-pressure ventilation with the addition of positive end-expiratory pressure (PEEP) or continuous positive airway pressure (CPAP) may not reduce lung water but may increase access to previously collapsed regions of the lung and may increase the capacity of the interstitium to hold fluid.
Pleural fluid
Pleural fluid acts as a space-occupying lesion and impairs ventilation. In most cases, pleural fluid should be drained before anesthetizing the animal. If there appears to be a continuous air leak from the lung, it is best to place a chest drain before anesthesia or place a large-gauge catheter (e.g., 14 gauge) that can be aspirated rapidly to remove any accumulated air. In cases of hemothorax, blood is defibrinated during its residence in the pleural space. Accumulated blood can be aspirated from the pleural space and given back to the animal intravenously without providing additional anticoagulants. Autotransfusion should only be performed if there is minimal risk of bacterial contamination of the blood and no risk of the blood containing cancer cells that could metastasize to other areas of the body. The blood should be passed through a filter to remove clots before it is autotransfused. Cats with pleuritis appear to be in great pain and often are very fractious. It may be beneficial to provide sedation and analgesia (e.g., oxymorphone) and oxygen before attempting to drain the chest.
Peritoneal fluid
A large volume of fluid in the abdomen can increase intraabdominal pressure (so-called abdominal compartment syndrome) and should be drained before anesthesia if feasible. Abdominal compartment syndrome is associated with a number of physiologic changes, including hypoventilation with reduced pulmonary compliance; tachycardia; low cardiac output; and increased central venous pressure (CVP), mean pulmonary artery pressure, and PCWP. In the abdomen, the increased pressure reduces urine output and decreases blood flow to the abdominal wall and the splanchnic vascular beds. Intraabdominal hypertension also may increase intracranial pressure (ICP) with a decrease in cerebral perfusion pressure.94
Drainage of the abdomen usually is achieved by placing a catheter in the abdominal cavity and drawing off the fluid with a syringe. It is helpful if the catheter has additional side holes cut in it before insertion so that there is less likelihood of the catheter being obstructed by the omentum. Most affected animals have greater respiratory distress lying on their backs, and the catheter usually is inserted with the animal on its side. I usually place the catheter about halfway between the last rib and the ischium, 1 to 4 inches off the ventral midline. Draining fluid in this manner can take a long time, but this is actually advantageous because rapid removal can result in mesenteric vasodilatation and cardiovascular collapse.94 In the case of hemoabdomen, the blood may have been defibrinated, but it is best to collect it in an anticoagulant (e.g., heparin, citrate). Collected blood should be used only if there is no gross contamination of the abdomen and no risk of neoplasia. The blood should be passed through a filter to remove clots before it is autotransfused. In cases of massive trauma, it may be better to leave the blood in the abdomen until the surgeon is ready to stop the bleeding. Although the accumulated blood may compromise ventilation during this time, the increased intraabdominal pressure may reduce the rate of hemorrhage.
Increased intracranial pressure
Increased ICP requires careful management in terms of fluid balance. The cranial vault is a relatively fixed cavity, and any accumulated fluid tends to increase the pressure. An increase in the fluid content of the brain or in the volume of blood or cerebrospinal fluid in the cranial vault promotes an increase in ICP. In situations in which the cause is medically reversible (e.g., hyponatremia), therapy should be carried out before anesthesia. In cases in which the diagnosis or treatment requires anesthesia, the preoperative assessment of the patient must include a detailed examination of fluid balance. Animals with an acute increase in ICP caused by trauma also may be hypovolemic because of other injuries. Judicious use of hypertonic resuscitation fluids is appropriate for these patients because such fluids promote a reduction in ICP while restoring circulating volume.103,199 Patients with chronically increased ICP often have had decreased food and water intake for some time and may have been treated with diuretics to reduce ICP. Consequently, such patients often are dehydrated and may have electrolyte disturbances. Whenever possible, preoperative assessment should include examination of the animal for signs of dehydration, an assessment of the cause of increased ICP, an evaluation of renal function, and measurement of serum electrolytes, hematocrit, total proteins, osmolality, and colloid osmotic pressure. If the animal clearly is dehydrated, it should be given fluids before anesthesia to increase its circulating volume. If plasma osmolality is less than 320 mOsm/kg, it may be beneficial to treat the animal with mannitol (0.25 to 1 g/kg).
Increased intraocular pressure
Patients with glaucoma often are treated similarly to patients with an increased ICP (i.e., diuretics), but they also are given carbonic anhydrase inhibitors (e.g., methazolamide, Teva, Sellersville, Pa.), which can cause metabolic acidosis over the course of 12 to 24 hours. Although correction of the acidosis may not be essential in many of these animals, treatment with sodium bicarbonate may decrease the risk associated with anesthesia. The combination of dehydration and acidosis may substantially reduce the dose of thiopental required for induction, and care should be taken to titrate this drug to effect in these patients.
Age
As animals get older, body water and cardiovascular reserve decrease. These changes make older animals more susceptible to fluid overload in the perioperative period. Geriatric patients admitted to the hospital several days before anesthesia and surgery may not have been drinking well (i.e., low tolerance for a new environment) and may be dehydrated.
Pregnancy
Pregnancy is associated with many changes in fluid balance. In women, the typical changes associated with pregnancy include hyponatremia; decreased blood urea nitrogen and creatinine concentrations; respiratory alkalosis; decreased serum calcium, magnesium, and protein concentrations; and decreased hematocrit. Similar changes have been documented in dogs. Serum protein concentrations tend to decrease during pregnancy, with the most marked change being a decrease in serum albumin concentration.28 Hematocrit decreases with a proportionately greater decrease with increasing numbers of fetuses.5,99 The pregnant dog has a decreased baroreceptor response to hypotension and is more susceptible to hypotension with blood loss.22,23 Thus the pregnant animal may be more susceptible to the negative circulatory effects of anesthetics and may require an increased volume of fluids during a surgical procedure. In bitches and queens that have been in labor for some time, dehydration and endotoxemia also may be present and add to circulatory instability. Affected patients may benefit from fluid therapy before anesthesia.
Changes in function
Cardiovascular disease
If the heart is failing, it may not tolerate an increased fluid load. Increased preload in this setting may not result in increased cardiac output because of changes in the Frank-Starling curve. Conversely, even a failing heart does not function optimally if preload is allowed to decrease too much. In a prospective study of human patients, it was found that the frequency of postoperative heart failure was highest in patients who had received less than 500 mL/hr of fluids intraoperatively.34 The most common cause of congestive heart failure in dogs is mitral insufficiency. This condition is characterized by excessive retrograde flow with an increasing volume load on the heart. Treatment often involves use of vasodilators (e.g., nitroglycerin, hydralazine, angiotensin-converting enzyme inhibitors) to decrease afterload, and diuretics and salt restriction to decrease circulating volume. Consequently, cardiac patients have the potential to be hypovolemic. The diagnosis of relative hypovolemia in these patients is based on clinical signs, such as skin turgor, mucous membrane color, capillary refill time (CRT), and jugular venous distention. Evaluation of renal function (including urine output) may assist in deciding whether the animal is adequately hydrated. Thoracic radiographs can be used to help assess pulmonary venous distention (i.e., lack of pulmonary venous distention implies lower left atrial pressure and hence a lack of excessive preload). The most useful measurement in these patients is PCWP. PCWP is obtained by inserting a balloon-tipped catheter into the pulmonary vein from either the jugular or femoral vein. Such invasive monitoring certainly is warranted in some cardiac patients and provides the best guide to fluid therapy. If the animal has right-sided heart failure, monitoring CVP provides similar information. In one study, use of CVP or PCWP was associated with more aggressive fluid therapy (>500 mL/hr), which in turn was associated with a lower risk of postoperative congestive heart failure.34 In the past, it has been recommended that fluids containing low concentrations of sodium be administered to cardiac patients (e.g., 0.45% saline in 2.5% dextrose). Most of these patients have an increase in total body sodium and an increase in total body water. The latter tends to exceed the former, and affected patients may be hyponatremic.7 Thus it seems illogical to give a solution that contains additional free water. If such a patient is hypovolemic, it is more appropriate to use a balanced electrolyte solution. If the patient is not hypovolemic or it already has excessive volume, fluids may not be needed.
In other myocardial diseases, it also is important to assess the patient preoperatively for signs of dehydration and heart failure (e.g., distended jugular veins, slow jugular emptying, jugular pulses, ascites, pulmonary edema, pleural effusion). Invasive monitoring as described earlier may be necessary to optimize fluid therapy during anesthesia and surgery. Blood may flow best at a hematocrit of 25% to 30%, but it may be necessary to maintain higher values to maintain optimal tissue oxygenation. If an animal with heart failure also is anemic, consideration should be given to preloading the animal with packed red cells to optimize oxygen delivery.
Coagulation defects
Any coagulation defect that is likely to increase intraoperative blood loss should be corrected before surgery if possible. If an animal has a known coagulation defect (e.g., hemophilia, hepatic failure, coumarin poisoning, von Willebrand’s disease), it should be given the appropriate therapy such as fresh frozen plasma, cryoprecipitate, fresh plasma, or fresh whole blood, and vitamin K in the case of coumarin poisoning. These treatments should be given within a few hours of surgery because the half-lives of most clotting factors are relatively short. Although fresh frozen plasma and fresh plasma may have sufficient clotting factors to reverse the coagulation defect, such therapy often fails in animals with severe defects. In dogs with von Willebrand’s disease, infusion of cryoprecipitate is a more effective treatment than fresh frozen plasma alone.35,175 Therapy with plasma from donors receiving desmopressin (DDAVP) may be more effective than plasma from untreated donors.96 When DDAVP is given to dogs with typ. 1 von Willebrand’s disease, there is a measurable increase in the binding of von Willebrand factor to collagen, suggesting an improvement in clotting ability during surgery.97 Cryoprecipitate often is prepared from a number of donors and therefore has the potential to provide greater antigenic stimulation or transmit disease. Cryoprecipitate contains 10 to 20 times the normal amount of clotting factors and can be given in a small volume. Thus it may be useful in animals in which volume overload may be a concern (e.g., Doberman pinschers with von Willebrand’s disease and cardiomyopathy). I have used cryoprecipitate but also have successfully managed dogs with von Willebrand’s disease using fresh frozen plasma in mildly affected dogs or by treating both the plasma donor and recipient with DDAVP (1 μg/kg subcutaneously) in more severely affected dogs. Recommendations for the dosage of fresh frozen plasma range from 6 to 30 mL/kg and for cryoprecipitate from 1 U/5 to 15 kg.175 DDAVP also may be useful in restoring platelet function in some cases of iatrogenic platelet dysfunction. It has been used to treat increased bleeding times associated with aspirin administration and also platelet defects associated with cardiopulmonary bypass.138,156,193
Animals with thrombocytopenia or dysfunctional platelets may require platelet infusion before surgery. Platelet life span in immune-mediated thrombocytopenia is considerably shortened, and platelet infusions may be effective for only a matter of hours. Although it is commonplace for platelet-rich plasma to be prepared for affected people, this is relatively rare in veterinary medicine. Platelet preparations have a short half-life (<5 days) and must be maintained on a rocker in a very narrow range of temperatures so it is hard to maintain adequate supplies in veterinary medicine. Consequently, most patients that are thrombocytopenic or have platelet dysfunction are treated with fresh whole blood. The amount of blood needed (TV) depends on the platelet count of the patient (PE), the platelet count of the donor blood (PD), the target platelet count (PT), and the blood volume (BV) of the patient:
Note: Volumes must be expressed in the same units (i.e., blood volume in microliters if platelet count is per microliter or platelet count per liter if blood volume is in liters). The dosage of a platelet-rich plasma could be determined using a similar approach but there is uncertainty about the actual dosage required.168
The ASA guidelines for infusion of platelets are as follows:143
1. Prophylactic platelet transfusion is rarely indicated when thrombocytopenia is caused by increased platelet destruction (e.g., idiopathic thrombocytopenic purpura).
2. Prophylactic platelet transfusion is rarely indicated when thrombocytopenia is caused by decreased platelet production when the platelet count is greater than 100 × 109/L and is usually indicated when the platelet count is less than 50 × 109/L. The determination of whether patients with intermediate platelet counts (50 to 100 × 109/L) require therapy should be based on the risk of bleeding.
3. Surgical and obstetric patients with microvascular bleeding usually require platelet transfusion if the platelet count is less than 50 × 109/L and rarely require therapy if it is greater than 100 × 109/L. With intermediate platelet counts (50 to 100 × 109/L), the determination should be based on the patient’s risk for more significant bleeding.
4. Operative procedures ordinarily associated with insignificant blood loss may be undertaken in patients with platelet counts less than 50 × 109/L.
5. Platelet transfusion may be indicated despite an apparently adequate platelet count if a known platelet dysfunction and microvascular bleeding are present.
More recent guidelines also suggest that platelet numbers should not be allowed to decrease to less than 50 × 109/L during massive transfusion and should be greater than 100×109/L in patients with multiple trauma or central nervous system injury.77 A platelet count as low as 5 x 109/L is an effective transfusion trigger in human patients with thrombocytopenia who are not undergoing invasive procedures.168
Patients with disseminated intravascular coagulation (DIC) may need surgical intervention to correct the initiating cause of the DIC. Restoration of circulating volume with fresh whole blood or fresh frozen plasma is the mainstay of preoperative therapy for patients with DIC. If heparin is used, it should be given at a dosage that does not cause significant prolongation of bleeding time (e.g., 75 U/kg every 8 hours subcutaneously). If heparin is added to the blood or plasma (same dosage), the activated partial thromboplastin time (APTT) should be determined before surgery to ensure that it is not excessively prolonged (i.e., not more than twice normal).
Renal disease
Patients with chronic renal insufficiency are at risk for having their disease exacerbated by the hemodynamic changes during anesthesia and surgery. Affected animals should be managed carefully during the perioperative period. They should be allowed access to water until the time of premedication. Any dehydration present should be corrected before anesthesia.
Patients with severe oliguric renal insufficiency are of concern because they have severely limited ability to excrete an extra fluid load and may already be hypervolemic and hypertensive. If possible, it is advantageous to monitor CVP as a guide to fluid therapy in these animals. Monitoring CVP provides information on how well the heart is able to pump the existing circulating volume and allows the anesthetist to watch the response to fluid therapy in the perioperative period.
Hepatic disease
Mild hepatic insufficiency rarely causes clinically relevant disturbances in fluid balance, but substantial alterations occur as the severity of hepatic injury progresses. The liver synthesizes many proteins, and hypoalbuminemia and deficiencies of clotting factors may occur as hepatic insufficiency progresses. These alterations are managed as described earlier. Blood ammonia concentrations are increased in patients with portosystemic shunts and in those with hepatic failure. Consequently, it is important not to administer additional ammonia by the use of stored blood products that may have increased ammonia content.
Endocrine disease
Diabetes insipidus
Animals with diabetes insipidus must be monitored carefully during the preoperative period to be sure they continue to drink water. The owner should be asked how much water the animal is consuming to ensure that a similar volume can be administered intraoperatively. Animals with complete central diabetes insipidus can become markedly dehydrated within a matter of hours (5% dehydration may occur after 4 hours of water deprivation). Consequently, affected animals should have access to water until the time of premedication, and intraoperative management should take into account the actual urine production of that animal so it is best to place a urinary catheter and use a closed collection system to monitor urine volumes.
Hyperadrenocorticism
Animals with hyperadrenocorticism are polyuric and polydypsic and should have access to water until the time of premedication. Some dogs with hyperadrenocorticism have mildly increased serum sodium and mildly decreased serum potassium concentrations, but these rarely are of sufficient magnitude to be of concern. Animals with hyperadrenocorticism tend to be hypertensive, which may exacerbate underlying cardiac disease (e.g., mitral regurgitation), and they may have increased sensitivity to vasoconstrictive drugs. They also bruise easily, and special care should be taken when placing intravenous catheters. If the affected animal is being anesthetized for major surgery, hypercoagulability and increased risk of pulmonary thromboembolism are concerns. Prophylactic therapies for hypercoagulability may include the use of regular or low molecular weight heparins, plasma, and HES.
Hypoadrenocorticism
Hyponatremia, hypochloremia, hyperkalemia, hypovolemia, hypoglycemia, metabolic acidosis, and azotemia commonly are associated with hypoadrenocorticism. These abnormalities are associated with hypotension and decreased sensitivity to positive inotropes and vasoconstrictive drugs. The fluid of choice for managing these animals is 0.9% NaCl, which tends to correct all of the preceding abnormalities except the hypoglycemia and metabolic acidosis, which should be monitored during therapy and corrected as necessary by administration of glucose and sodium bicarbonate. Hypotension can be especially difficult to manage in these patients intraoperatively, and steroid replacement should be started before induction of anesthesia.
Diabetes mellitus
In controlled diabetes, there rarely is any major concern about fluid balance preoperatively. The animal’s normal feeding regimen and insulin dose are used on the day before surgery. On the morning of surgery, the animal receives one third to one half of its daily dose of insulin, and blood glucose concentration is monitored throughout the procedure.106 The animal is treated with glucose, insulin, or some combination of these as determined by serial blood glucose measurements. Animals with uncontrolled diabetes may be dehydrated and may require fluid therapy before anesthesia.
Hypothyroidism
Patients with hypothyroidism rarely have any electrolyte disturbances but can be hypotensive and have a poor response to positive inotropes and vasoconstrictors. If possible, the animal should be adequately treated for hypothyroidism for at least 1 to 2 weeks before it is anesthetized.
Hyperthyroidism
Animals with hyperthyroidism tend to be in a hyperdynamic state and are at risk for fatal, catecholamine-mediated arrhythmias when anesthetized. It is best if the animal is treated with methimazole for at least 2 weeks before anesthesia.140
Access to the circulation
The technical aspects of fluid administration are covered in Chapter 15. In the perioperative period, access to the circulation via the intravenous or intraosseous route should be available so that fluids can be given rapidly should the need arise. As discussed earlier, the diameter of the catheter should be sufficient to allow fluids to be administered rapidly enough for the expected deficits. It also is important that the connections to the animal be set up carefully and that they are secure. If the fluid line becomes disconnected with the animal draped for surgery, it may not be detected quickly, and the animal may experience substantial blood loss from the catheter. When the patient is prepared for a surgical procedure, the anesthetist should make sure to set up the fluid lines so that an injection port is accessible without the need to reach under the drapes. The animal also should be positioned in such a way that the fluids can flow easily. Drugs added to the fluids and administered through the same line must be compatible (see Table 17-1). If an animal will be receiving several drugs, it may be necessary to create additional access sites to prevent incompatible drugs from being administered through the same line. Consideration also must be given to the site of access. In cats undergoing declawing of the front paws, it is advisable to place the catheter in the hind leg so that it does not interfere with the surgery. When the caudal vena cava is to be occluded during surgery, it is important to have the catheter in the forelimb or neck so that fluids reach the remaining circulation during the occlusion. In an emergency in an anesthetized animal with no venous access, the most visible vessel usually is the sublingual vein. This vein can be catheterized rapidly if necessary.
Thermodynamic considerations
Infusion of fluids with temperatures less than normal body temperature requires that the animal warms the fluid, and this effect cools the animal. If we assume that the specific heat of water (and most of the crystalloid solutions used in fluid therapy) is 1 kcal/kg/° C, it would cost the animal 18 kcal to increase the temperature of 1 L of fluid from 20° C to 38° C. If the specific heat of the body is 0.83 kcal/kg/° C, 1 L of fluid at 20° C would cool a 21.7-kg dog by 1° C.106 Stated in another way, a fluid infusion rate of 10 mL/kg/hr at 20° C would cost the patient 0.18 kcal/hr and would tend to cool the body by approximately 0.2° C/hr. These losses are relatively minor in comparison with the body heat lost via radiation but may become more important when massive fluid volumes are required or the infused fluid is much colder (e.g., stored blood products).
Effects of anesthesia
Some drugs may alter sympathetic activity and thus affect blood volume and the distribution and excretion of body fluids. Acepromazine is a potent α1-antagonist, and even low doses of the drug (0.001 mg/kg) induce this effect. In the healthy patient, this effect is associated with minor decreases in arterial blood pressure and hematocrit.45 In an animal with increased sympathetic tone, however, the administration of acepromazine may result in profound hypotension. Acepromazine also is a dopamine antagonist and may inhibit the effect of dopamine to increase renal blood flow. Such an effect has been demonstrated with chlorpromazine,24 but dopamine maintained its vasodilatory effect in the presence of acepromazine, suggesting that this did not hold true for acepromazine.123 The α2-agonists have profound effects on the circulation and on renal function. In dogs and cats, administration of these drugs, even at low doses, causes a substantial decrease in cardiac output (40% to 60%). They also have a direct effect on the kidney, the end result of which is marked diuresis (urine output increases three fold to tenfold). The mechanism for this effect appears to be related to antagonism of vasopressin, and this dehydrating effect may be even more relevant in a patient that is avidly conserving water. The opioids have a variety of actions. The μ-agonists (e.g., morphine, oxymorphone, meperidine) have an antidiuretic effect, whereas the κ-agonists (e.g., butorphanol, pentazocine, nalbuphine) tend to promote diuresis. The antidiuresis associated with the μ-agonists may be the result of stimulation of vasopressin release. Release of vasopressin may be stimulated in the awake patient, but there is a reduction in the release of vasopressin in anesthetized patients receiving large doses of potent opioids (causing a reduced stress response).49 The dissociative drugs (e.g., ketamine, tiletamine) tend to decrease urine output despite increases in cardiac output and blood pressure.60 These drugs also tend to decrease baroreceptor responses, and this may be important in the anesthetized patient with relative hypovolemia that undergoes changes in body position.
Drugs that are used for the induction and maintenance of anesthesia all tend to decrease urine output, mainly through their hemodynamic effects.93 Thiopental has been shown to alter renal sodium resorption, leading to increased sodium and water losses in dogs, but in human patients there is either no change or a decrease in urine output.65,93 Thiopental also decreases hematocrit (which may be important in an anemic patient), but it has little effect on plasma volume.185 Propofol causes hypotension if given rapidly, and it may cause some reduction in the glomerular filtration rate and urine flow.139 In normal sheep, there was minimal effect on renal function, but there was a significant detrimental effect during sepsis.18 Etomidate preserves circulation better than most other drugs administered intravenously for induction, but it may alter renal function by virtue of the base in which it is constituted. Etomidate usually is supplied in propylene glycol, which can induce renal failure if enough is given. This would be unlikely with an induction dose of the drug, but continuous infusion might be associated with nephrotoxicity from the propylene glycol or the hemolysis that is likely to occur. Severe renal insufficiency was reported in dogs after an infusion of etomidate.124 All of the inhalants are associated with a decrease in renal function, but this effect can be prevented to some extent by preloading the animals with fluids. There is some concern that sevoflurane can react with soda lime or baralyme, releasing a polyvinyl compound (compound A) that is nephrotoxic, but this has not yet been seen to be a clinically important issue.
Positive-pressure ventilation has been associated with changes in renal function. A reduction in urine output occurs with the institution of positive-pressure ventilation, with CPAP or PEEP.98 The techniques of PEEP and CPAP increase CVP, mean pulmonary artery pressure, and PCWP.120 The increase in CVP tends to increase renal vein pressure, which may alter interstitial pressure within the kidney.153 The use of intermittent positive-pressure ventilation (IPPV) and PEEP or CPAP is associated with an increase in vasopressin secretion, but it is likely that increased renal interstitial pressure has a more important effect because the decrease in urine output can be seen without changes in vasopressin.152
Regional anesthetic techniques also may result in volume-responsive hypotension. This is particularly true with epidural or intrathecal techniques. In people, the spinal cord ends at vertebral level L1/L2, and it is necessary to inject enough drug to extend high into the thoracic region to block enough spinal segments for abdominal surgery. As a result, there is a significant block of sympathetic outflow from the thoracic and lumbar spinal cord segments, which can result in hypotension. The cord ends at vertebral level L6/L7 in dogs and at S1/S2 in cats. Thus it is feasible to achieve an effective abdominal block in dogs and cats without substantial loss of sympathetic tone. However, hypotension can occur with this technique, and the animal should be monitored accordingly.
Intraoperative blood loss is affected by blood pressure and body temperature. In some situations in which it is difficult to control blood loss, it may be possible to reduce the loss by maintaining pressure at a lower than normal value for the period of concern. It would be advantageous to be able to monitor lactate concentrations to ensure that global perfusion was not being adversely affected by this approach. Hypothermia has been shown to alter coagulation. The mechanism for this effect appears to be mainly related to platelet function until body temperature decreases to less than 33° C when the effects on enzymes become manifest.197 In dogs, it has been noted that platelet counts decrease by up to 70% between 37° C and 32° C because of splenic sequestration, but the defective release of thromboxane A2, down-regulation of platelet glycoprotein Ib-IX, and up-regulation of platelet surface protein GMP-140 also alter platelet aggregation.83,122 Several studies in humans have shown increased blood loss during procedures normally associated with hemorrhage, even with small changes in body temperature (e.g., 30% greater loss with intraoperative temperature differences of <2° C).160,196 Other studies in humans have not been able to repeat these findings, and there are no similar studies in dogs and cats.76,141
Monitoring fluid therapy
Determining the best fluid regimen and judging the adequacy of therapy are dependent on monitoring the patient. In human medicine, the term “goal-directed therapy” has been used to indicate that the parameters of the ideal perfusion state can be defined and the type and volume of fluid necessary to achieve this state then can be administered.85,95 To some extent, this approach has been used in veterinary medicine for many years, but the primary goal has been to increase blood pressure because it often is the only parameter measured. Inadequate perfusion of tissues occurs at low pressures but the converse may not be true (i.e., adequate perfusion may not necessarily occur at normal pressures). Normal arterial pressures can be achieved with very low cardiac output if peripheral resistance is increased sufficiently because arterial blood pressure is the product of cardiac output and systemic vascular resistance.
Monitoring changes in volume
Methods for monitoring intravascular volume are not available in routine practice. Most of the techniques that have been used in the laboratory involve dye dilution and require sophisticated measuring techniques and calculations to determine intravascular volume. Even if such information was available, it is unlikely that absolute values for vascular volume would be of much use because it is unlikely that a normal volume measurement for the animal in question would be available before the procedure. However, trends over time may be helpful. A simple method for estimating a change in plasma volume is to use the change in hematocrit with time:
This calculation ideally would be based on hemoglobin and hematocrit values taken soon after the induction of anesthesia because substantial decreases in hematocrit and hemoglobin can occur during anesthesia. Devices that measure changes in blood volume are available on sophisticated hemodialysis machines and provide a guide to therapy in situations in which blood volumes can change rapidly. In general, however, changes in blood volume must be inferred from clinical signs. An interesting new approach to this problem is to use the response of the patient’s hemoglobin concentration to a fluid challenge to differentiate dehydration from hypovolemia.78 This method still is not very precise, but with refinement may prove useful in a clinical setting.
Loss of skin turgor is a helpful sign when present, but in many animals skin turgor changes little until volume depletion is severe80; skin turgor is not useful in monitoring hypervolemia. Radiographic signs of hypovolemia include microcardia and a decrease in the size of the caudal vena cava and pulmonary vessels. CRT is used to monitor the microcirculation and, if prolonged, implies poor tissue perfusion. Poor tissue perfusion may be the result of hypovolemia, heart failure, vasoconstriction, or endotoxemia. This clinical sign has been examined carefully in humans and was found to be a poor predictor of volume status.162 CRT is significantly affected by body temperature and ambient temperature.10,74 CRT also can appear normal immediately after cardiac arrest. In dogs and cats, it is usual to use the mucous membranes of the mouth for testing capillary refill, and this technique may avoid some of the changes occurring in people as a result of alterations in ambient temperature because the temperature of the mouth remains relatively constant. The ability to assess CRT accurately is affected by the presence of pigment in the mucous membranes of some animals, making it impossible to obtain a result in these individuals.
Heart rate increases in response to hypovolemia but is a nonspecific sign. In anesthetized animals that develop unexplained tachycardia, I often give a fluid bolus to determine whether the animal is hypovolemic. A decreased heart rate after fluid infusion without resumption of tachycardia is indicative of preexisting hypovolemia.
Low CVP, PCWP, and systemic blood pressure all can imply low circulating volume but also can change for other reasons. The CVP and PCWP probably are better measurements of volume status because they are affected by cardiac preload, which is largely dependent on blood volume. Static pressures such as these, however, still are not very good predictors of overall volume status (see Chapters 15 and 16 for more information on CVP measurement and interpretation). In dogs and cats receiving IPPV and direct arterial pressure monitoring, systolic pressure may vary because of the effect of intrathoracic pressure changes on venous return. Although not totally predictable, significant decreases in systolic pressures associated with ventilation are indicative of hypovolemia (assuming ventilation pressure is 10 to 20 cm H2O). In one study, the systolic pressure variation was approximately 6%, with a 5% loss of blood volume, and it increased linearly to approximately 11% with 30% loss of blood volume.136 Systolic pressure variation was much less in hypotension without hypovolemia.142 Plethysmographic techniques are being developed to monitor this parameter noninvasively, but the results have not been very promising to date.31 The PCWP was a better predictor of responders to a fluid bolus than was the systolic pressure variation in one study in human cardiac patients.12
Cardiac output tends to decrease with hypovolemia, but this is a relatively nonspecific change because cardiac output also decreases with increased systemic vascular resistance or myocardial failure. Evaluation of cardiac output in conjunction with pressure measurements allows the clinician to interpret volume status more readily. Determination of cardiac output and PCWP can be carried out by placement of a thermistor and pressure port in the pulmonary artery and taking the measurements with sophisticated and expensive equipment. Placement of these catheters in small patients (<5 kg) is particularly difficult and makes it virtually impossible to obtain such readings in a clinical setting. A newer, simpler technique uses access to a vein and an artery with injection of lithium into a vein and withdrawal of blood from the artery while measuring lithium concentration (LiDCO, Cambridge, UK). This method can provide a limited number of cardiac output measurements in medium- to large-sized dogs. A method for providing continuous cardiac output measurements based on pulse contour analysis (PulseCO, Cambridge, UK) also has been developed, but it does not respond well to rapid changes in cardiac output.41,53 In humans, transesophageal echocardiography has been used to estimate cardiac output and to set goals for stroke volume to improve fluid therapy intraoperatively.70,190 An ideal stroke volume for a particular patient is established by giving fluid boluses and assessing the stroke volume response. If stroke volume does not increase after a fluid bolus, additional fluid therapy would not likely be helpful. An echo/Doppler machine has been used in cats to measure cardiac output.148 Measurements correlated well with cardiac output determined by thermodilution but consistently underestimated cardiac output.148
Urine output decreases with hypovolemia but also decreases with hypotension or low cardiac output. If urine output remains relatively normal, it is unlikely that the animal is hypovolemic. Measurement of urine volume requires time, and it is difficult to obtain accurate measurements at shorter time intervals than every hour. Consequently, measurement of urine volume cannot be used to monitor acute changes in circulating volume. The only available method for the measurement of urine output involves the insertion of a urinary catheter, and this involves some risk of introducing a urinary tract infection (UTI).133,146,173,194 The risk of UTI with catheterization is greater in female than in male dogs.15 If monitoring urine output is necessary, a sterile urinary catheter should be inserted aseptically and immediately connected to a closed drainage system.112 The reservoir of the urinary collection system should be maintained below the level of the patient. If the animal is being moved, it is best to clamp the drainage system so that urine cannot reflux up the tubing into the bladder. The urinary catheter should be left in the patient for the shortest duration possible because the risk of a UTI increases with every day the catheter is left in place. Ideally, the animal should not receive antibiotics while the catheter is in place (unless the UTI already has been diagnosed) because use of antibiotics increases the likelihood of antibiotic-resistant UTI. Withholding antibiotics may not be feasible in a surgical setting, and it is important to monitor for development of UTI using urinalysis and urine culture.
Monitoring changes in composition
Blood samples must be obtained to monitor changes in the composition of the blood. The results of sodium, potassium, chloride, calcium, bicarbonate, pH, carbon dioxide tension (Pco2), Po2, osmolality, colloid osmotic pressure, hematocrit, protein, glucose, urea, and creatinine determinations may affect fluid therapy decisions. When a patient requires monitoring of the composition of blood, it is important to determine how blood samples are to be obtained intraoperatively. It often is difficult to obtain samples from peripheral venous catheters (particularly in small patients), and other sites must be used. Samples can be obtained from the jugular vein with relative ease, and a jugular catheter should be placed if several samples are likely to be required. If it is not necessary to measure CVP, a short intravenous catheter can be used (1.5 to 2 inches). Also useful in the anesthetized patient are the lingual veins. These vessels usually are readily accessible during anesthesia and can be sampled several times without the insertion of a catheter. All of these measurements can be obtained using such samples, but care must be taken with interpretation of Po2.134 Single arterial samples can be obtained from the lingual, femoral, ulnar, auricular, coccygeal, or dorsal pedal arteries. If several samples will be required and it is advisable to know the Pao2, an arterial catheter should be placed. In most dogs and cats, the most accessible vessel for this purpose is the dorsal pedal artery over the metatarsal area. If this vessel is inaccessible (e.g., bilateral tibial fractures) or cannot be catheterized, it is feasible to use the other vessels mentioned. If a femoral arterial catheter is placed, great care is needed because it is relatively easy for such catheters to pull out of the vessel while still attached to the skin. Unless a long stiff catheter has been placed in the femoral artery, it is not advisable to allow the animal to recover with the catheter still in place. The ulnar artery is difficult to catheterize because the shape of the limb makes it difficult to approach the site at a sufficiently narrow angle. The auricular arteries are useful in dogs and can be used into the postoperative period, although there is some risk of ischemia with prolonged catheterization. A catheter can be placed in the lingual artery after induction of anesthesia, but it must be removed before the end of surgery and the vessel held off for 15 minutes after the catheter has been removed to prevent the formation of a sublingual hematoma. Care must be taken when flushing auricular and lingual arterial catheters to prevent the injection of air because air could be introduced into the carotid arteries, resulting in air embolism of the cerebral arteries.
The electrocardiogram is used to presumptively identify changes in serum electrolyte concentrations. The electrocardiogram is useful in this regard because the magnitude of electrocardiographic changes is dependent both on the rate of change and on the actual serum concentration of the electrolyte.
Determination of venous saturation with oxygen allows assessment of a combination of volume and composition. The assumption with this method is that blood entering the tissue must have sufficient oxygen content (Hb × saturation %) and be flowing rapidly enough to provide adequate oxygen delivery to the tissue. The lower the supply, the greater the depletion in oxygen content and the lower the venous oxygen saturation. Determination of mixed venous oxygen saturation would be the ideal measurement from a whole-animal perspective, but would require placement of a pulmonary arterial catheter. The principle, however, can be applied to individual organs. For example, does the venous saturation of blood leaving the kidneys, heart, or the brain suggest adequate blood supply? Fluid therapy in a pig model designed to provide mixed venous saturation (So2) of 60% showed that HES provided better tissue oxygenation than did LRS.85 Samples are easier to obtain from a central venous site than the pulmonary artery, and the measurement of central venous saturation (ScvO2) may provide a good estimate of mixed venous saturation.147 Studies in humans suggest a target ScvO2 of 70%.
Monitoring changes in distribution
Dehydration is monitored using the clinical signs described earlier. The presence or absence of peripheral edema and ascites should be readily apparent. In some cases, it may be helpful to measure limb or abdominal circumference to determine whether the fluid accumulation is increasing or decreasing. Measuring the size of the abdomen is particularly difficult but still may be of use in individual patients. An indelible marker can be used to identify the site of measurement for future reference and thus improve accuracy. Pleural fluid accumulation can be monitored only by thoracic radiography or by draining the fluid on an intermittent or continuous basis.
ICP can be measured and can play a crucial role in the management of patients with increased ICP. The catheter is inserted into the cranial vault and attached to a measuring device. The simplest approach is to use a fluid-filled catheter, which can provide sensitive measurements of ICP and also allow measurement of intracranial compliance. The latter can be helpful because it can provide an estimate of the risk of brain herniation. A fiberoptic catheter that measures pressure indirectly can be inserted directly into the brain. The objective measurement of intraocular pressure with a Schiøtz or applanation tonometer may help guide fluid therapy in patients with high intraocular pressure.
Monitoring changes in function
The end result of failure in fluid management is that organs begin to fail. In human medicine, a relatively noninvasive test has been introduced to monitor the functional ability of the liver to clear foreign substances from the plasma. This system (LiMON, Pulsion Medical Systems, Munich, Germany) uses pulse densitometry to monitor the arterial concentration of an administered dye (indocyanine green [ICG]). The rate of clearance of ICG is a measure of hepatic function. In human patients with sepsis, mortality was 80% when clearance of ICG was less than 8%, whereas it was 11% when clearance was greater than 24%.91
Intraoperative fluid management
Intraoperative fluid management depends on:
1. How well the patient has been prepared beforehand
2. How much fluid loss occurs normally (insensible loss)
3. How much fluid loss occurs because of the equipment used (e.g., dry gas causes greater water loss than humidified gas)
4. Changes in vascular tone and cardiac output
5. The amount and nature of the tissue exposed during surgery
In most patients, crystalloid solutions are used first, and colloids and blood products are added as required.
Crystalloids
The anesthetized animal has ongoing fluid losses of approximately 132 × BW0.75 mL/day for the dog and 80 × BW0.75 mL/day for the cat, where BW is body weight in kilograms. It is likely that losses will be less than predicted by these formulas because the metabolic rate of most anesthetized animals is less than in the awake resting state. A maintenance solution would be appropriate merely to replace this loss. However, it is expected that fluid losses will increase during anesthesia because of increased loss from the respiratory tract and that there will be changes in hemodynamics that will require fluid therapy (see Effects of Anesthesia section). Consequently, it has been traditional to use isotonic replacement solutions during anesthesia and to expect that the kidneys will excrete any excess sodium in the postoperative period. Replacement solutions do not contain high concentrations of potassium and can be given rapidly if necessary without risk of potassium toxicity.
The rate of administration often is set arbitrarily at 10 mL/kg/hr. This rate of administration is based on research in humans in the 1960s suggesting that this rate was appropriate for losses occurring during major abdominal surgery. I have used this approach in many dogs and cats with few apparent adverse effects. In the original studies, blood volume was measured using radioactive tracers.164,187 These techniques are accurate in a steady state but may not be accurate when volumes are changing during fluid infusion. Later studies evaluated the dilution of hemoglobin or albumin, or the change in blood water content to assess acute changes in blood volume but these may not be accurate either because they do not account for the full circulating volume.79,172,177 Although these initial studies were performed in healthy human volunteers, they provide some useful information. In one study, infusions were carried out at different rates using two different volumes.79 The interstitial fluid space is roughly twice the volume of the intravascular space, and isotonic replacement solutions redistribute, leaving approximately 33% of the infused volume in the vascular space. In this study, the volume retained in the vascular space 15 minutes after the end of the infusion was approximately 20%, and it was approximately 15% after 30 minutes, indicating rapid redistribution of crystalloid solutions. The volume of distribution for the balanced electrolyte solution was similar to the expected plasma volume but only 50% to 70% of the expected volume for the interstitial space. Regions of the interstitial space with poor blood supply or rigid structure (e.g., bone) may be less likely to take up fluid, and this may account for the difference in calculated volumes.
The authors of these volume-kinetic studies proposed that their data could be used to calculate infusion rates that would expand the plasma compartment (bolus) and maintain it at this volume (infusion). To increase blood volume by 5%, the patient would receive 36 mL/kg/hr for 20 minutes and an ongoing infusion of 15 mL/ kg/hr.177 In another study, nomograms were presented for men and women showing the infusion rate and time required to achieve a specific blood volume expansion and the infusion rate required to maintain this expansion.79 Whether these data apply to anesthetized animals is uncertain, but the results suggest that a fluid rate of 10 mL/kg/hr is relatively conservative if an expansion of circulating volume is the aim.
In a study of healthy dogs undergoing elective ovariohysterectomy or castration, the rate of polyionic fluid administration was examined to determine how it affected hematocrit, total protein concentration, glucose concentration, and systolic blood pressure.67 The authors tested an acetated polyionic solution given at 0, 5, 10, and 15 mL/kg/hr for 1 to 2 hours. They saw no differences among groups, suggesting that there was no advantage to fluid therapy in these instances. Even at the highest rate of fluid administration neither packed cell volume (PCV) nor total protein concentration (TP) decreased significantly. Cardiac output and renal function were not evaluated, and so it is not possible to say whether fluids affected these functions. Crystalloid fluid administration at 11 mL/kg/hr for 60 minutes to halothane-anesthetized cats did not result in any changes in PCV or TP.16 These cats had undergone thoracotomy for placement of catheters and did not start the study with normal values (PCV = 25%, TP = 4.9 g/dL, colloid osmotic pressure = 10.2 mm Hg) and thus may be regarded as similar to compromised animals in a clinical situation. In a clinical setting, however, the PCV and TP often decrease over time due to a combination of fluid dilution and blood loss. In a study of dogs undergoing a tibial plateau leveling operation, the PCV decreased from approximately 48% to approximately 32% and the TP from approximately 6.8 to approximately 5 g/dL with administration of LRS at 10 mL/kg/hr over a 4-hour period.19
Studies in sheep have examined the redistribution of 0.9% NaCl during isoflurane anesthesia, and the results showed a similar rate of redistribution away from the vascular space, but there was much greater retention in the interstitial space when compared with the awake animal.40 This observation was accounted for by a dramatic reduction in urine output during isoflurane anesthesia, and a recent study in dogs corroborated this finding.19 In that study, dogs undergoing a routine orthopedic procedure received LRS at 10 mL/kg/hr but urine output remained less than 0.5 mL/kg/hr. Fluid was retained in the extracellular space and a significant increase in body weight occurred. These data suggest that fluid accumulates in the interstitium during anesthesia to the detriment of the patient.144 Further work by this latter group in elderly human trauma patients suggests that excretion of fluid also is decreased in the postoperative period.178 Careful measurement of respiratory function in awake 59- to 67-year-old people showed some impairment of respiratory function when they were given 40 mL/kg LRS over 3 hours.88 In studies of humans, this issue has been examined further by using less (restrictive) or more (liberal) fluid in the perioperative period, but the definitions of the terms “restrictive” and “liberal” has varied substantially from study to study and consequently the results are hard to interpret. Regardless, the fluid retention has been associated with harm to the patient.33 The excessive fluid administration has been implicated in longer hospital stays, decreased wound healing, delayed postoperative gastrointestinal activity and even increased postoperative pain.27
This information suggests that use of isotonic fluids at 10 mL/kg/hr is probably excessive under most situations encountered in routine practice, but as of yet no evidence-based criteria for a new approach has been presented. One recommendation is to provide for ongoing losses using crystalloids (1 to 2 mL/kg/hr) and manage relative hypovolemia using colloid solutions.33 If a crystalloid is used, a decision still must be made about which crystalloid to use. Commonly available crystalloids include normal saline (0.9% NaCl), a lactated polyionic fluid (e.g., LRS), an acetated polyionic fluid (e.g., acetated Ringer’s solution, Normosol-R, Plasma-Lyte 148, Isolyte S, Polyionic R), or 5% dextrose in water, saline, or polyionic solutions.
Normal saline
Normal saline is used widely as a replacement solution intraoperatively. It is the solution of choice for patients with hypercalcemia or hypochloremic alkalosis. This solution contains higher amounts of chloride than plasma and tends to decrease the strong ion difference, leading to acidosis. In classical terms, it dilutes the concentration of bicarbonate and provides large amounts of chloride for reabsorption from the glomerular filtrate, thus leading to hyperchloremic acidosis. The degree of acidosis is not likely to be a problem in the healthy patient but may exacerbate acidosis in a compromised patient. Evidence from some studies in humans indicates that urine output may be decreased when patients receive the same volume of normal saline as compared with LRS.159,195
Lactated Ringer’s solution (Hartmann’s solution)
LRS is a balanced electrolyte solution containing lactate that contributes to the correction of acidosis and is my fluid of choice for most anesthetized patients. Potential disadvantages of this solution are as follows:
1. It contains calcium and because blood products generally are stored using a compound that chelates calcium, it is not ideal to administer LRS through the same intravenous line as blood products. A 1:10 mixture of blood and LRS resulted in clot formation within 2 minutes at 37° C (see Table 17-1).155
2. The osmolality of LRS is 272 mOsm/L and the sodium content is 130 mEq/L, which means it is a hypotonic solution. This hypotonicity could lead to greater loss of fluid into the intracellular compartment, which in turn may be detrimental in patients with cerebral edema. In models of traumatic brain injury, infusion of LRS was associated with an increase in ICP.145,152 In a model of closed-head trauma in rats, use of LRS did not affect neurologic outcome or formation of brain edema.59 However, the low sodium content has been implicated in postoperative hyponatremia in human patients, particularly children, sometimes with disastrous outcomes.126
3. It contains lactate, which mostly is metabolized in the liver (approximately 56% of normal lactate metabolism occurs in the liver). In some LRS, the lactate is in the form of L-lactate (e.g., the lactate in Baxter’s product is derived from fermentation), whereas in others, a racemic mixture with equal amounts of the D- and L-lactate is used (e.g., the lactate in Hospira’s product is derived from chemical production of lactate). The L-form is more readily metabolized than is the D-form.81 It is stated on the bag of LRS that it should not be used in patients with a lactic acidosis, but infusion of LRS was not associated with an increase in blood lactate concentrations even when there was considerable impairment of hepatic function.50,71 However, hepatic removal of lactate is a saturable process, and infusion of lactate in patients with severe hyperlactatemia (>9 mmol/L) may result in an increase in blood lactate concentration.130 However, at concentrations of lactate greater than 9 mmol/L, the peripheral tissues remove more lactate than the liver, and peripheral metabolism of lactate is not saturable.130 In clinical patients with initial lactate concentrations greater than 10 mmol/L, infusion of LRS and other volume support was always associated with a decrease in blood lactate concentrations.30 Some patients with cancer may be hyperlactatemic and have increased ability to recycle lactate to glucose.192 In some patients with cancer cachexia, concern has been expressed that the metabolism of lactate consumes energy and thus lactated solutions should not be used. It has been shown that dogs with lymphoma have a transient inability to cope with the lactate load imposed by infusion of LRS.186 Although this finding may be valid in unusual cases, the amount of lactate provided with LRS at 10 mL/kg/hr is approximately 36% of the basal production or utilization rate, and it is likely that any negative effect is transient.3
The metabolism of lactate is either by gluconeogenesis or by oxidation, and hydrogen ions are consumed in both instances. It takes approximately 30 minutes for this alkalinizing effect to be accomplished.81 The alkalinizing effect is not as great as that seen with acetate (approximately 50%).
Acetated polyionic solutions
It is thought that acetate is metabolized rapidly throughout the body, and the alkalinizing effect of this solution is more readily available. As with lactate, the effect takes approximately 30 minutes to be evident.81 In some commercial solutions, gluconate also is used. There is little information on the effects of gluconate, but it does appear to cause a slight increase in pH.104 Acetated Ringer’s solution suffers from the same disadvantage as LRS in terms of its sodium content, but some of the commercial solutions have higher sodium content and osmolalities (e.g., Plasmalyte-148, Na = 140 mEq/L and osmolality = 294 mOsm/L), and these are much closer to the reference intervals in dogs and cats. Many of the commercial solutions are calcium-free and can be given through the same line as blood products. The main disadvantage of solutions containing acetate is the vasodilatation that can occur with rapid administration.75,92 In a normal healthy patient, a bolus of acetated polyionic solution usually results in an increase in heart rate but little change in blood pressure, but in a patient that is already hypovolemic, dramatic decreases in blood pressure can be seen (Figure 17-2).158 Acetate-containing solutions also are contraindicated in patients with diabetic ketoacidosis because they tend to increase blood ketone concentrations.4
Figure 17-2 Administration of an acetated solution (Plasma-Lyte 148) to a 16-kg dog being anesthetized for cataract surgery. The 50-mL bolus was given before the start of surgery. Hypotension occurred, and the dog was given 0.5 μg/kg of epinephrine intravenously when the mean pressure had leveled off at 33 mm Hg (approximately 10 minutes).
5% Dextrose
Five percent dextrose in water contains no electrolytes, and only water remains when the dextrose is metabolized. Five percent dextrose may be the solution of choice for patients that have suffered from pure water loss, but it is rarely indicated as the prime replacement solution during anesthesia and surgery. Apart from the fact that the volume of distribution of the 5% dextrose is likely to be larger than that of a balanced electrolyte solution (which would result in a diminished ability to maintain circulating volume), the glucose itself may be detrimental in certain circumstances.150 In both acute renal and acute cerebral injury, high concentrations of glucose may be detrimental.108,115,128 Concentrations of glucose more than 200 mg/dL may be of concern in animals with cerebral ischemia.108,115
2.5% Dextrose in half-strength ionic solution
Dextrose (5%) can be mixed with any of the preceding ionic solutions in a 1:1 ratio to halve the ionic strength. Such solutions may be of use in the management of patients with hypernatremia. These solutions are designed to increase the free-water content of the body, and it is important to monitor electrolyte concentrations to ensure that excessive dilution does not occur.
Hypertonic solutions
These solutions may provide rapid resuscitation in the preoperative period but are seldom used intraoperatively. They may be needed in special circumstances such as for an animal with rapid hemorrhage when blood products are unavailable, an animal with a high ICP, or a patient with hyponatremia. Most of these solutions have very high sodium concentrations, and it is important to monitor serum sodium concentrations before and after their administration. Maintenance with an isotonic crystalloid usually is required after administration of these solutions.
Colloids
Dextrans, HES, polygelatins, and plasma are the main colloid solutions available. They are used to correct hypovolemia, provide colloid osmotic pressure, and in the case of fresh frozen plasma they provide clotting factors. The synthetic colloids are polydisperse colloids that, by definition, contain particles of several different molecular weights. In the past, the average molecular weight (Mw) of such solutions was described, but this approach favors the high molecular weight particles. It now is common to describe the solution according to the number molecular weight (Mn), which is the total weight of all the molecules divided by the number of molecules. In the case of dextran 70, the Mw is 70,000, but the Mn is 41,000 (Table 17-2). Use of Mn allows recognition of the smaller molecular weight particles in the solution. The terms have clinical significance because the oncotic pressure exerted by the solution depends on the number of particles present, whereas the duration of effect depends on the size of the particles present. The duration of effect of a colloid is short if the particles rapidly leak through the endothelium.
Dextrans
The dextran molecule is a linear polysaccharide produced by certain strains of Leuconostoc bacteria growing in sucrose-containing media. Dextrans are supplied in low and high molecular weight forms (dextran 40 and 70, respectively) with plasma half-lives estimated at 1 to 3 and 2 to 6 hours.182 In dogs with normal renal function, 70% of a dose of dextran 40 and 40% of a dose of dextran 70 are excreted unchanged in urine within 24 hours. The remaining molecules are metabolized slowly to glucose by dextranase in the liver. Some of these molecules may be present in the body weeks after their administration. The plasma volume expansion achieved per gram of dextran is roughly the same, regardless of molecular weight (approximately 20 to 25 mL water/g dextran).86 Clinically, however, dextran 40 has a greater concentration per milliliter and provides greater plasma volume expansion initially.
Concerns about the use of dextrans include effects on hemostasis and allergic reactions. Dextrans tend to prolong bleeding times by interfering with fibrin clot formation, reducing factor VIII and von Willebrand’s factor, diluting clotting factors, and interfering with platelet function. In dogs, rapid infusion of dextran 70 caused a decrease in von Willebrand’s factor antigen and factor VIII activity and increases in APTT and buccal mucosal bleeding time.39,69 Dextrans and HES also alter the structure of the fibrin clot, giving it a weaker, more chaotic appearance.72 These effects suggest that dextrans may not be the best choice for fluid therapy when major surgery is planned. Clinically, it seems that infusions of dextrans have been associated with increased bleeding, but no studies have documented increased blood loss when dextrans have been used. Allergic reactions have been reported in human patients, but the frequency appears to be less than 0.1%, and such reactions have not been reported in dogs or cats.
In humans, dextran 40 has been used to reduce the occurrence of deep vein thrombosis. It is thought that this effect is caused by decreased viscosity of blood after dextran administration. There also is some evidence that low molecular weight dextrans alter red cell aggregation and decrease clumping of red cells in the microcirculation. Use of dextran 70 also reduced the frequency of fatal postoperative pulmonary embolism from 2.0% to 0.35%. In these studies, dextrans were given on the day of surgery. The only common conditions in dogs and cats complicated by pulmonary thromboembolism are hyperadrenocorticism and the nephrotic syndrome, and the use of dextrans has not been investigated in these settings in veterinary medicine.
A number of reports have linked dextrans to renal failure. This complication has been attributed to increased viscosity of the glomerular filtrate associated with early excretion of low molecular weight particles.107,201 Experimental studies in dogs identified changes in proximal tubular cells but no effect on renal function. Affected human patients have received large doses of dextrans and have had an associated increase in oncotic pressure. Treatment by exchange transfusion to lower oncotic pressure has been successful, suggesting that the renal changes are not structural but functional.189,201 There are no reports of renal failure after dextran administration in dogs or cats.
Hetastarch
HES is a synthetic polymer of glucose (amylopectin) that closely resembles glycogen and contains predominantly α-1,4 linkages. Starch normally is metabolized by amylases, and by adding hydroxyethyl groups to positions 2, 3, or 6 on the glucose molecules, the rate of metabolism can be reduced. Metabolic breakdown is slower with increased substitution and a higher ratio of C2:C6 substitution. This understanding has led to development of different molecules that are described by their molecular weight, the proportion of substitution, and their C2:C6 ratio (Table 17-3).51 In the literature, the molecular weight and substitution ratio usually are used to define the product. For example, HES 200/0.5 represents HES with an average molecular weight of 200 kDa and a molar substitution ratio of 5 hydroxyethyl groups per 10 molecules of glucose. It would be preferable to include the C2:C6 ratio of the molecule because HES 200/0.5 with a substitution ratio of 13.4:1 behaved very differently than HES 200/0.5 with a C2:C6 substitution ratio of 5.7:1.183 The original commercially available preparation of HES has an average molecular weight (Mw) of 450 kDa with a number molecular weight (Mn) of 69 kDa. This solution was made up in normal saline. A newer high molecular weight HES (Hextend, BioTime, Inc., Emeryville, Calif.) is made up in a balanced electrolyte solution so that infusion is less likely to be associated with hyperchloremic acidosis, and the presence of calcium may reduce the occurrence of clotting abnormalities. However, this solution, at a dosage of 20 mL/kg administered over 1 hour had an effect on platelet function for at least 5 hours and in some individual dogs up to 24 hours.169 The newer HESs have even lower molecular weights, and HES 130/0.4 (Voluven, Fresenius Kabi Norge AS, Holden, Norway, and Hospira Inc., Lake Forest, Ill.) is thought to have close to ideal properties because it does not remain in plasma as long as bulkier molecules, but it also does not interfere with coagulation as much. In any of these solutions, the smaller molecules (molecular weight, <59 kDa) are excreted by the kidneys or pass through the vascular endothelium into the interstitial space. Molecules that reach the interstitial space are taken up by macrophages and are slowly metabolized by cellular lysozymes. The larger molecules are slowly broken down by α-amylases. Dogs have approximately three times as much amylase as do humans, and HES 450/0.7 is broken down faster. In dogs, 31.5% of administered HES was excreted in urine, and 38% remained in plasma after 24 hours.182 The half-life of HES 450/0.7 in humans varies with time after administration and dose (e.g., the half-life is 1.5 to 3.6 days during the first 3 days after administration and 13 to 17 days between 7 and 42 days after administration). After three consecutive daily doses, the excretion of 41% to 46% of this HES took 168 hours compared with 48 hours after a single dose. This dependence on time and dose has not been demonstrated in dogs.171 In hypoalbuminemic dogs, the administration of HES 450/0.7 was associated with an increase in colloid osmotic pressure and a reduction in peripheral edema in most treated patients. There was no apparent correlation between the dose of HES 450/0.7 or the change in colloid osmotic pressure and resolution of edema. A few dogs showed prolongation of APTT and a decrease in platelet numbers, but it was unclear whether this effect was caused by the HES treatment. Some dogs with abnormal hemostasis before treatment actually became normal after treatment.170 In another trial using HES in 30 hypoalbuminemic dogs, there was an increase in colloid osmotic pressure with the administration of 7.7 to 43.9 mL/kg, but this effect lasted less than 12 hours. It was suggested that maintenance of colloid osmotic pressure would require additional HES or administration of other colloids.125
As with dextrans, there has been concern about the effect of HES on coagulation.176 In early experiments in dogs, infusion of 10 mL/kg was not associated with increased blood loss or any change in bleeding time. With infusions of 20 to 30 mL/kg, however, bleeding time and quantity of blood lost increased. These effects were more pronounced with dextrans than with HES 450/0.7.101 Factor VIII complex consistently is decreased after HES 450/0.7 administration, and it is advised that HES 450/0.7 not be given to dogs with known or suspected von Willebrand’s disease.171 In a study in which very large doses (110 to 120 mL/kg) of HES 450/0.7 were used, prolonged bleeding times were identified. Platelets appeared swollen and shiny and had decreased adhesion.114 Clots were friable and had weak tensile strength. These effects were presumably caused by more than just hemodilution, and these findings should be borne in mind when using HES 450/0.7 at the time of surgery. Studies in humans undergoing surgery have not documented any increase in blood loss associated with the administration of HES 450/0.7.13,188 The lower molecular weight HES is associated with fewer alterations in coagulation. HES 130/0.4 causes fewer effects on coagulation than HES 200/0.5 and may reduce the need for blood transfusions in human orthopedic patients.110 I have not seen increased bleeding tendency in dogs given HES 450/0.7, but the dosage used has not exceeded 20 mL/kg.
Other concerns with HESs are their effects on renal and hepatic function. Renal function appears to be minimally affected if it was normal initially, but septic patients may be at increased risk for renal injury after HES administration.26,161 Hepatic failure has been noted in some human patients who have had repeated infusions of high molecular weight HES, but such usage does not appear to be a major risk factor in the perioperative period in veterinary patients.36 Serum amylase concentrations are expected to increase after the use of HES. Pruritus is another consequence of HES infusion and appears to be related to dose rather than HES type. If pruritus occurs, it can be of major concern to the patient and is refractory to treatment.11
HES may be beneficial to the patient by reducing the inflammatory response to surgery. In human patients undergoing abdominal surgery, concentrations of interleukin-6 and -8 and intercellular adhesion molecule-1 were lower when HES 130/0.4 was used for intravascular volume replacement instead of LRS.109 The effect on adhesion molecules also may alter the capillary leak that can occur in trauma and sepsis. The idea that HES may protect against vascular leakage is supported by a study showing that HES 200/0.5 did not appear in cerebrospinal fluid in patients with an impaired blood-brain barrier.51
HES administration increases plasma volume by 71% to 172% of the administered volume and generally increases plasma volume by at least the volume administered.171 The degree of expansion depends largely on the concentration of HES. Greater blood volume expansion (e.g., 130%) is seen with 10% as compared with 6% solutions. In this regard, HES is about equivalent to dextran 70 but has a slightly longer duration of action. In one study in dogs, 25 mL/kg of dextran 70 or HES 450/0.7 gave an almost identical increase in plasma volume compared with the volume infused (approximately 140%), but at 12 hours the dextran effect had decreased to 18%, whereas the HES effect had decreased to 38%. By 24 hours, the dextran effect had further decreased to 1%, whereas HES still caused a 16% increase in plasma volume compared with the volume infused.182 Greater expansion of blood volume occurs when HES is used to maintain blood volume in the face of ongoing loss as compared with trying to expand blood volume in the normovolemic patient. In the former situation, the volume effect can be up to 80% of the dose administered as compared with 40% in the latter situation.33 The incidence of anaphylactoid reactions with HES use in people is similar to that recorded for dextrans. Whereas antibodies to dextrans have been found in humans, no antibodies to HES have been found in dogs, cats, or humans even after chronic use. The frequency of life-threatening reactions appears to be lower for HES than for other colloids.171 No anaphylactoid reactions to HES have been reported in dogs or cats.
Gelatin solutions
Gelatin solutions are prepared by degradation of bovine collagen and come in several forms. The process involves exposure of the raw material to hydrochloric acid for several days, to saturated calcium hydroxide for several weeks, and finally to a temperature of at least 138° C. The three currently used preparations are oxypolygelatin (Vetaplasma/Geloplasma, Institut Merieux Benelux, Brussels, Belgium), succinylated gelatin (Gelofusine, B Braun Medical, Bethlehem, Pa.), and urea-linked gelatin (Haemaccel, Intervet, Milton Keynes, UK). Oxypolygelatin was available in the United States, and the other two forms have been used extensively in Europe. The main advantages of these solutions are that they have lower molecular weights than the other colloids (and hence are excreted rapidly), they appear to be minimally antigenic, and they have minimal effects on coagulation.117 In one report, the use of more than 79,000 units of succinylated gelatin in humans was summarized.117 The infusion of a solution of succinylated gelatin was associated with an increase in plasma volume equal to or approximately 10% less than the volume infused; hence there is little risk of volume overload. Of the infused volume, approximately 50% was present in the circulation after 4 to 5 hours, although it has been stated that the plasma half-life is approximately 8 hours.117 The plasma half-life of oxypolygelatin is 2 to 4 hours. The majority of the gelatin is excreted by the kidneys, with 71% of the urea-linked gelatin and 62% of the succinylated gelatin being found in the urine in people within 24 hours. In chimpanzees, 66% of a dose of oxypolygelatin was found in the urine within 24 hours. Mechanisms for the metabolism of the remaining molecules are not well defined, but it is thought that they are metabolized by proteolytic enzymes in the liver with some of the end products being excreted in the feces (approximately 15% of the total dose).117
Anaphylactoid reactions to gelatin solutions are rare. It is uncertain whether these reactions represent an immunologic response or are caused by histamine release. An overall incidence of allergic reactions to gelatins was reported to be 0.115%, with the highest incidence reported for oxypolygelatin (0.617%).149 In this report, it was also noted that the severity of the reactions was greater with the gelatins than with other colloids (0.038% vs. 0.008% for dextrans and 0.006% for HES). In a study of the release of histamine associated with use of urea-linked gelatin in anesthetized patients, a 26% incidence of histamine release was reported with 4 of 57 patients exhibiting life-threatening signs.116 Patients with malignant disease were twice as likely to release histamine and were seven times more likely to have a life-threatening episode. Pretreatment of patients with histamine blockers (H1 and H2) reduced the incidence of clinical signs to zero.116 The gelatin solution (500 mL) in this study was given over 20 minutes (approximately 20 to 25 mL/kg/hr), and it has been recommended that these solutions be administered slowly.
In the early reports of gelatin infusion, minimal effects on coagulation were identified.117 However, subsequent studies showed that the effects are somewhat similar to those observed with other colloids but of lesser magnitude. An increase in bleeding time was recorded in healthy people and in trauma victims and was attributed to a decrease in von Willebrand’s factor activity.48,57 In studies using thromboelastography to measure the dynamics of clot formation, dilution with gelatins resulted in more rapid onset of clot formation, more rapid strengthening of the clot, and some decrease in the maximal strength obtained.56,127 In both of these studies, gelatin was compared with hydroxyethyl starch, and the latter induced greater changes than did the gelatin solution. In one study, 50% dilution with dextran 40 prolonged most coagulation parameters to such an extent as to be unmeasurable.127 In a clinical study examining the use of gelatin as a priming solution before cardiopulmonary bypass, ristocetin-induced platelet agglutination was significantly impaired, and this effect was not corrected by the use of aprotinin as compared with the control group (albumin prime).179 There also was a direct correlation between postoperative blood loss and the amount of gelatin used during the operation with the greatest blood loss occurring in patients receiving more than 3.5 L of gelatin (approximately 45 mL/kg).179 In another study evaluating human patients undergoing orthopedic surgery, no major differences were noted between patients receiving similar volumes of 6% HES or 3% gelatin (<33 mL/kg/day) for colloid replacement.13 Despite these findings, gelatin infusions often are given rapidly to veterinary patients before or during surgery with little evidence of adverse effects on coagulation or histamine release.
Plasma protein
Plasma protein is available either as a fresh or frozen preparation or as liquid or frozen plasma that has been harvested during the collection and storage of blood. Fresh plasma may be prepared so that it contains platelets (platelet-rich plasma) and clotting factors. It must be used within 4 hours of preparation because of the risk of bacterial contamination at the recommended room temperature storage. Fresh frozen plasma contains clotting factors, which are destroyed if the unit has been thawed for more than 8 hours, but contains no platelets. Fresh frozen plasma can be used in any situation in which blood volume must be expanded, hematocrit is within an acceptable range, and no allergic reaction to foreign protein is anticipated. If there is no major concern about dilution of existing clotting factors, the stored form of the plasma can be used. The infusion of plasma tends to increase colloid osmotic pressure and increase both serum albumin and globulin concentrations. The main concerns about the use of plasma intraoperatively are cost and the potential for allergic reactions. Commercially, plasma is more expensive than any of the other colloids, but its use is justified in animals with marginal coagulation (e.g., use of fresh frozen plasma in a patient with low plasma protein concentration related to hepatic dysfunction) or in surgical cases in which there is concern about dilutional coagulopathy. Life-threatening allergic reactions to plasma infusions are not common, but urticaria may be observed. The author has not seen any episodes of profound hypotension associated with plasma infusions but has seen considerable swelling of the head and limbs develop. If such a reaction occurs, the plasma infusion should be stopped immediately and the animal treated with antihistamines (H1 and H2 blockers). Corticosteroids also may be administered if warranted by the severity of the reaction. This type of therapy rarely reverses the clinical signs but may prevent exacerbation of the condition. A note should be made in the patient’s medical record to ensure that it does not receive infusions of plasma products in the future. In dogs and cats with portosystemic shunts, there is concern about the ammonia content of stored plasma because it tends to increase with time. Clinical signs of encephalopathy in these patients are related in part to blood ammonia concentration, and it is advisable not to burden them with an additional source of ammonia.
Albumin
Concentrated solutions of human albumin have been used in dogs to increase colloid osmotic pressure and maintain blood volume. Early results appeared to be quite promising,119,184 but it has become evident that severe immune reactions may occur in response to infusion of human albumin solutions.38,62,118,184 A lyophilized canine albumin solution has now become commercially available (Animal Blood Resources International, Dixon, Calif.), but there are no reports yet on its safety or efficacy.
Packed red blood cells
Packed red cells are used primarily in patients with low hematocrits before surgery or in patients that are likely to have low tolerance for a decreased hematocrit that develops during surgery (e.g., a patient with minimal cardiovascular reserve). It is advisable to crossmatch both dogs and cats before transfusion. Crossmatching requires some time, and it is important to plan for the use of packed red cells by having the crossmatch results available before the animal requires transfusion. The indications for packed red cells are given in the earlier section on Anemia. Administration of packed red cells can be difficult because of the viscosity of the solution and can be facilitated by diluting the cells with warm normal saline, by using adult rather than pediatric administration sets, and by using the largest venous access possible (ideally >20 gauge). Smaller needles (<20 gauge) tend to impede the flow of the blood and may lead to hemolysis if external pressure is applied for the administration.
Whole blood
Ideally, whole blood is used when the animal needs all of the components present in whole blood. Practically, whole blood often is used because it is more convenient than individual component therapy. Fresh whole blood contains all of the normal clotting factors and active platelets. Clotting factors and platelets deteriorate within the first 24 hours, and stored whole blood is ineffective at restoring normal coagulation. Whole blood typically is used in patients that are bleeding actively or have already lost a large volume of blood and are likely to become severely hemodiluted if other fluids are used. Some concern has been expressed about the effect of blood transfusion on immune function. A beneficial effect was first noticed in renal transplant patients. Patients who had received blood transfusions in association with renal transplantation were less likely to reject the grafted organ.132 Additional studies in human patients showed an increased frequency of infections in patients receiving allogeneic blood transfusions.54 These included wound infections, UTI, and respiratory tract infections, and the frequency of infection increased with the number of units of blood received.107,181 Patients receiving their own blood did not have such an increase in infection rate, and studies have focused on reducing the white cell count in transfused blood to determine whether this will alter the infection rate.17 This approach seems to have met with success, but further analysis is required before its efficacy is understood.89 Another effect of immunosuppression caused by blood transfusions is its effect on cancer development. In several animal models, allogeneic infusions have been associated with increased tumor growth, but the results of studies in humans are not clear.17,63,154 Leukocyte removal before transfusion may reduce the effect on cancer growth.17 Leukoreduction has been used in collecting blood from dogs, but this procedure has not been reported with regard to its effect on cancer recurrence.25
Another concern with the administration of blood products is that citrate present in stored blood will decrease the availability of calcium in the recipient. In normal humans, the amount of citrate found in 1 unit of blood (approximately 32 mg/kg) can be metabolized in 3 to 5 minutes without the person developing hypocalcemia. However, the rate of metabolism of citrate decreases with decreased hepatic perfusion (e.g., shock), decreased hepatic function, and hypothermia. In these settings, plasma citrate concentration may increase rapidly. This effect is of concern mainly when blood is given rapidly (>30 mL/kg/hr), and calcium salts may be given when rapid transfusion of blood or plasma is required.2 Calcium must be given through a separate intravenous line because it may cause the transfused blood to clot in the line if it is given concurrently. Calcium chloride should be given at a dosage of 5 to 10 mg/kg and calcium gluconate at 18 to 35 mg/kg for an equivalent effect.43 The patient is less likely to have a hypotensive response if calcium can be given before or during the rapid administration of citrate-containing blood products.44 If serum ionized calcium concentration can be measured, sufficient calcium should be given to return the ionized calcium concentration to normal, but the animal should be treated only if serum ionized calcium concentration is decreased. If blood is not being given rapidly or is not needed on a continuous basis, it rarely is necessary to administer calcium because the serum calcium concentration will be corrected rapidly by the animal as a result of changes in parathyroid hormone concentration and by mobilization of calcium stores in the body.2,167
Stored blood usually is kept at 4° C and is more likely to cause arrhythmias and decreased cardiac output if administered without being warmed first. A 250-mL unit of blood at 4° C requires 7.2 kcal of heat to warm it to 38° C. Stated differently, an infusion of 25 to 30 mL/ kg of blood at 4° C can decrease body temperature by as much as 1° C. Given these facts, it is best if blood can be warmed before it is given. This can be achieved by placing the blood in warm water (up to 42° C but no higher) before infusion or by running the blood through a warming device as it is being infused. Warming can be as simple as running the line through a container of warm water or as sophisticated as using a device specifically designed to heat blood safely as it is being infused. The effectiveness of these techniques depends on the length of line exposed to the heat and the rate of infusion. Most of the commercial devices that are designed for this purpose require the addition of an extra length of line that conforms to the heating device. Such devices further increase the cost of blood or blood component therapy.
Hemoglobin solutions
Various hemoglobin solutions have been tested over the years, but only one has been licensed for veterinary use.47 Oxyglobin (Biopure Corporation, Cambridge, Mass.) is an ultrapure glutaraldehyde polymerized hemoglobin of bovine origin made up in a modified LRS. This hemoglobin solution has a P50 (oxygen tension at 50% saturation) of 35 mm Hg, a molecular weight of 64 to 500 kDa, and a colloid osmotic pressure of approximately 20 mm Hg.135 It comes as a purple-colored solution and contains 13 mg/dL of hemoglobin. The solution may be stored at room temperature and has a shelf life of 24 months. This latter feature makes it an attractive product for veterinarians who use canine or feline blood infrequently and who do not have access to blood donors of known status. When given to a patient, it acts as a colloidal solution but has the added advantage of providing oxygen-carrying capacity. It can be given intraoperatively in any situation in which blood would normally be used except in circumstances requiring clotting factors or platelets. Administration leads to jaundice and hematuria in many patients, and interference with a number of biochemical tests (e.g., sodium, potassium, chloride, blood urea) may occur.29 Monitoring the patient by use of pulse oximetry reflects changes in arterial hemoglobin saturation, but measurement of hematocrit alone no longer provides an accurate indication of hemoglobin content.90 Measurement of total protein concentration using a refractometer also will be affected because of the presence of free hemoglobin. The recommended rate of administration for Oxyglobin is 10 mL/ kg/hr in dogs and 5 mL/kg/hr in cats, but boluses of 1 to 2 mL/kg may be used in animals suffering from acute hypovolemia. Special care needs to be taken when giving Oxyglobin to cats because pulmonary edema has been reported in a number of cats and is probably related to acute circulatory overload.68 Some degree of systemic vasoconstriction may occur with Oxyglobin administration because of the scavenging effect of free hemoglobin on nitric oxide. This effect may be of benefit in some severely hypotensive and hypovolemic patients in which an immediate increase in blood pressure would be desirable. Oxyglobin also would be very useful in an animal that fails to crossmatch to existing donors and yet needs increased oxygen-carrying capacity intraoperatively.46 Experimentally, it has been shown that Oxyglobin results in a more rapid increase in muscle tissue oxygenation than occurs with the infusion of a similar dose of stored packed cells.174 This observation suggests that animals with severe shock, anemia, or ischemia may benefit from an infusion of Oxyglobin as an initial treatment that could then be followed by more Oxyglobin or the use of blood products.
Crystalloids versus colloids
There has been a long debate over the nature of the fluid that should be used for volume expansion. In the human medical literature, this question has been addressed in a number of reviews. The most recent review in the Cochrane database for the use of crystalloids or colloids in critically ill patients states, “There is no evidence from random controlled trials (RCTs) that resuscitation with colloids reduces the risk of death, compared to resuscitation with crystalloids, in patients with trauma, burns or following surgery.” As colloids are not associated with an improvement in survival, and as they are more expensive than crystalloids, it is hard to see how their continued use in these patients can be justified outside the context of RCTs.137 In isoflurane-induced hypotension (80 mm Hg systolic arterial blood pressure [SAP]) in dogs, an HES (6% HES 450) infusion of 40 mL/kg restored blood pressure in 4 of 6 animals but 80 mL/kg LRS did not successfully resuscitate any animal.1 In dogs made hypotensive (80 mm Hg SAP) by blood loss, restoration of blood pressure to within 10% of control took 194 mL of HES as compared with 749 mL of LRS. In the former experiment, the authors concluded that HES should be used rather than LRS to treat isoflurane-induced hypotension. However, in neither of these experiments were the cardiac indices different between treatments, suggesting that both solutions restored systemic blood flow. In the isoflurane experiment, oxygen delivery was no different between treatments either, further supporting the notion that blood pressure is not a particularly good goal for therapy.129 Colloidal solutions should increase vascular volume faster and for a longer period of time than crystalloids, and therefore may be useful when colloid osmotic pressure is low or when crystalloids are not working well because their effect is transient. However, given the evidence cited above, use of crystalloids as a primary form of fluid therapy still seems justified with the addition of colloids when necessary to improve COP, oxygen delivery, or to prolong the achieved increase in circulating volume.
Postoperative fluid management
The patient will continue to lose fluids over time and may have decreased food and water intake after surgery. Consequently, it is essential to consider fluid therapy in the postoperative period. The choice of fluid is governed by factors similar to those used before and during surgery. A main factor to consider is when the animal is likely to be able to regulate its own fluid balance. With minor surgical procedures, this may be almost immediately after surgery, but with procedures in which recovery is slow or oral intake is contraindicated, it is necessary to continue fluid therapy. Continuing fluid therapy may be particularly important in geriatric patients because they often are unwilling to drink in the hospital environment and may be at greater risk because of marginal renal function.
1 Aarnes T.K., Bednarski R.M., Lerche P., et al. Effect of intravenous administration of lactated Ringer’s solution or hetastarch for the treatment of isoflurane-induced hypotension in dogs. Am J Vet Res. 2009;70:1345-1353.
2 Abbott T.R. Changes in serum calcium fractions and citrate concentrations during massive blood transfusions and cardiopulmonary bypass. Br J Anaesth. 1983;55:753-759.
3 Adrogue H.J., Tannen R.L. Ketoacidosis, hyperosmolar states, and lactic acidosis. In: Kokko J.P., Tannen R.L., editors. Fluids and Electrolytes. 3rd ed. Philadelphia: W.B. Saunders; 1996:643-674.
4 Akanji A.O., Bruce M.A., Frayn K.N. Effect of acetate infusion on energy expenditure and substrate oxidation rates in non-diabetic and diabetic subjects. Eur J Clin Nutr. 1989;43:107-115.
5 Allard R.L., Carlos A.D., Faltin E.C. Canine hematological changes during gestation and lactation. Companion Animal Practice. 1989;19:3-6.
6 Allon M., Copkney C. Albuterol and insulin for treatment of hyperkalemia in hemodialysis patients. Kidney Int. 1990;38:869-872.
7 Anand I.S., Ferrari R., Kalra G.S., et al. Edema of cardiac origin. Studies of body water and sodium, renal function, hemodynamic indexes, and plasma hormones in untreated congestive cardiac failure. Circulation. 1989;80:299-305.
8 Asao Y., Hirasaki A., Matsushita M., et al. A patient who recovered successfully from severe anemia which continued for one hour. Masui. 1997;46:700-703.
9 Baer R.W., Vlahakes G.J., Uhlig P.N., et al. Maximum myocardial oxygen transport during anemia and polycythemia in dogs. Am J Physiol. 1987;252:H1086-H1095.
10 Baraff L.J. Capillary refill: Is it a useful clinical sign? Pediatrics. 1993;92:723-724.
11 Barron M.E., Wilkes M.M., Navickis R.J. A systematic review of the comparative safety of colloids. Arch Surg. 2004;139:552-563.
12 Bennett-Guerrero E., Kahn R.A., Moskowitz D.M., et al. Comparison of arterial systolic pressure variation with other clinical parameters to predict the response to fluid challenges during cardiac surgery. Mt Sinai J Med. 2002;69:96-100.
13 Beyer R., Harmening U., Rittmeyer O., et al. Use of modified fluid gelatin and hydroxyethylstarch for colloidal volume replacement in major orthopaedic surgery. Br J Anaesth. 1997;78:44-50.
14 Bickell W.H., Wall M.J., Pepe P.E., et al. Immediate versus delayed fluid resuscitation for hypotensive patients with penetrating torso injuries. N Engl J Med. 1994;331:1105-1109.
15 Biertuempfel P., Ling G., Ling G.A. Urinary tract infection resulting from catheterization in healthy adult dogs. J Am Vet Med Assoc. 1981;178:989-991.
16 Bjorling D.E., Rawlings C.A. Relationship of intravenous administration of Ringer’s lactate solution to pulmonary edema in halothane-anesthetized cats. Am J Vet Res. 1983;44:1000-1006.
17 Blajchman M.A. Immunomodulation and blood transfusion. Am J Ther. 2002;9:389-395.
18 Booke M., Armstrong C., Hinder F., et al. The effects of propofol on hemodynamics and renal blood flow in healthy and in septic sheep, and combined with fentanyl in septic sheep. Anesth Analg. 1996;82:738-743.
19 Boscan P., Pypendop B.H., Siao K.T., et al. Fluid balance, glomerular filtration rate, and urine output in dogs anesthetized for an orthopedic surgical procedure. Am J Vet Res. 2010;71:497-596.
20 Bourke D.L., Smith T.C. Estimating allowable hemodilution. Anesthesiology. 1974;41:609-612.
21 Brecher M., Rosenfeld M. Mathematical and computer modeling of acute normovolemic hemodilution. Transfusion. 1994;34:176-179.
22 Brooks V.L., Keil L.C. Changes in the baroreflex during pregnancy in conscious dogs: Heart rate and hormonal responses. Endocrinology. 1994;135:1894-1901.
23 Brooks V.L., Keil L.C. Hemorrhage decreases arterial pressure sooner in pregnant compared with nonpregnant dogs: role of baroreflex. Am J Physiol. 1994;266:H1610-H1619.
24 Brotzu G. Inhibition by chlorpromazine of the effects of dopamine on the dog kidney. J Pharm Pharmacol. 1970;22:664-667.
25 Brownlee L., Wardrop K.J., Sellon R.K., et al. Use of a prestorage leukoreduction filter effectively removes leukocytes from canine whole blood while preserving red blood cell viability. J Vet Intern Med. 2000;14:412-417.
26 Brunkhorst F.M., Engel C., Bloos F., et al. Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med. 2008;358:125-139.
27 Bundgaard-Nielsen M., Secher N.H., Kehlet H. Liberal” vs. “restrictive” perioperative fluid therapy: a critical assessment of the evidence. Acta Anaesthesiol Scand. 2009;53:843-851.
28 Cairoli F., Colombo G., Arrighi S. Variazioni di alcune componenti ematiche nella cagna di razza Beagle durante la gravidanza ed il puerperio. Clin Vet (Milano). 1980;103:267-283.
29 Callas D.D., Clark T.L., Moreira P.L., et al. In vitro effects of a novel hemoglobin-based oxygen carrier on routine chemistry, therapeutic drug, coagulation, hematology, and blood bank assays. Clin Chem. 1997;43:1744-1748.
30 Canizaro P.C., Prager M.D., Shires G.T. The infusion of Ringer’s lactate solution during shock. Changes in lactate, excess lactate, and pH. Am J Surg. 1971;122:494-501.
31 Cannesson M. Arterial pressure variation and goal-directed fluid therapy. J Cardiothorac Vasc Anesth. 2009.
32 Carson J.L., Poses R.M., Spence R.K., et al. Severity of anaemia and operative mortality and morbidity. Lancet. 1988;1:727-729.
33 Chappell D., Jacob M., Hofmann-Kiefer K., et al. A rational approach to perioperative fluid management. Anesthesiology. 2008;109:723-740.
34 Charlson M., MacKenzie C., Gold J., et al. Risk for postoperative congestive heart failure. Surg Gynecol Obstet. 1991;172:95-104.
35 Ching Y.N.L.H., Meyers K.M., Brassard J.A., et al. Effect of cryoprecipitate and plasma on plasma von Willebrand factor multimers and bleeding time in Doberman pinschers with type-I von Willebrand’s disease. Am J Vet Res. 1994;55:102-110.
36 Christidis C., Mal F., Ramos J., et al. Worsening of hepatic dysfunction as a consequence of repeated hydroxyethylstarch infusions. J Hepatol. 2001;35:726-732.
37 Clugston P., Fitzpatrick D., Kester D., et al. Autologous blood use in reduction mammaplasty: is it justified? Plast Reconstr Surg. 1995;95:824-828.
38 Cohn L.A., Kerl M.E., Lenox C.E., et al. Response of healthy dogs to infusions of human serum albumin. Am J Vet Res. 2007;68:657-663.
39 Concannon K.T., Haskins S.C., Feldman B.F. Hemostatic defects associated with two infusion rates of Dextran 70 in dogs. Am J Vet Res. 1992;53:1369-1375.
40 Connolly C.M., Kramer G.C., Hahn R.G., et al. Isoflurane but not mechanical ventilation promotes extravascular fluid accumulation during crystalloid volume loading. Anesthesiology. 2003;98:670-681.
41 Cooper E.S., Muir W.W. Continuous cardiac output monitoring via arterial pressure waveform analysis following severe hemorrhagic shock in dogs. Crit Care Med. 2007;35:1724-1729.
42 Copland V., Haskins S., Patz J. Oxymorphone: cardiovascular, pulmonary, and behavioral effects in dogs. Am J Vet Res. 1987;48:1626-1630.
43 Cote C.J., Drop L.J., Daniels A.L., et al. Calcium chloride versus calcium gluconate: comparison of ionization and cardiovascular effects in children and dogs. Anesthesiology. 1987;66:465-470.
44 Cote C.J., Drop L.J., Hoaglin D.C., et al. Ionized hypocalcemia after fresh frozen plasma administration to thermally injured children: effects of infusion rate, duration, and treatment with calcium chloride. Anesth Analg. 1988;67:152-160.
45 Coulter D.B., Whelan S.C., Wilson R.C. Determination of blood pressure by indirect methods in dogs given acetylpromazine maleate. Cornell Vet. 1981;71:76-84.
46 Crystal M.A., Mott J., Van Der Veldt P. Blood loss and no matching donor. Veterinary Forum. 1999;16:55-57.
47 Day T.K. Current development and use of hemoglobin-based oxygen-carrying (HBOC) solutions. J Vet Emerg Crit Care. 2003;13:77-93.
48 de Jonge E., Levi M., Berends F., et al. Impaired haemostasis by intravenous administration of a gelatin-based plasma expander in human subjects. Thromb Haemost. 1998;79:286-290.
49 de Lange S., Boscoe M.J., Stanley T.H., et al. Antidiuretic and growth hormone responses during coronary artery surgery with sufentanil-oxygen and alfentanil-oxygen anesthesia in man. Anesth Analg. 1982;61:434-438.
50 Didwania A., Miller J., Kassel D., et al. Effect of intravenous lactated Ringer’s solution infusion on the circulating lactate concentration: Part 3. Results of a prospective, randomized, double-blind, placebo-controlled trial. Crit Care Med. 1997;25:1851-1854.
51 Dieterich H.J., Reutershan J., Felbinger T.W., et al. Penetration of intravenous hydroxyethylstarch into the cerebrospinal fluid in patients with impaired blood-brain barrier function. Anesth Analg. 2003;96:1150-1154. table of contents
52 Dismukes D.I., Thomovsky E.J., Mann F.A., et al. Effects of general anesthesia on plasma colloid oncotic pressure in dogs. J Am Vet Med Assoc. 2010;236:309-311.
53 Duffy A.L., Butler A.L., Radecki S.V., et al. Comparison of continuous arterial pressure waveform analysis with the lithium dilution technique to monitor cardiac output in conscious dogs with systemic inflammatory response syndrome. Am J Vet Res. 2009;70:1365-1373.
54 Duffy G., Neal K. Differences in post-operative infection rates between patients receiving autologous and allogeneic blood transfusion: a meta-analysis of published randomized and nonrandomized studies. Transfusion Medicine. 1996;6:325-328.
55 Dutton R.P., Mackenzie C.F., Scalea T.M. Hypotensive resuscitation during active hemorrhage: impact on in-hospital mortality. J Trauma. 2002;52:1141-1146.
56 Egli G.A., Zollinger A., Seifert B., et al. Effect of progressive haemodilution with hydroxyethylstarch, gelatin and albumin on blood coagulation. Br J Anaesth. 1997;78:684-689.
57 Evans P.A., Garnett M., Boffard K., et al. Evaluation of the effect of colloid (Haemaccel) on the bleeding time in the trauma patient. J R Soc Med. 1996;89:101P-104P.
58 Faggella A.M., Aronsohn M.G. Anesthetic techniques for neutering 6- to 14-week-old kittens. J Am Vet Med Ass. 1993;202:56-62.
59 Feldman Z., Zachari S., Reichenthal E., et al. Brain edema and neurological status with rapid infusion of lactated Ringer’s or 5% dextrose solution following head trauma. J Neurosurg. 1995;83:1060-1066.
60 Fischer D., Omlor D., Kreuscher D. Influence of ketamine anaesthesia on renal and cardiovascular functions in mongrel dogs. Int Urol Nephrol. 1979;11:271-277.
61 Follett D.V., Loeb R.G., Haskins S.C., et al. Effects of epinephrine and ritodrine in dogs with acute hyperkalemia. Anesth Analg. 1990;70:400-406.
62 Francis A.H., Martin L.G., Haldorson G.J., et al. Adverse reactions suggestive of type III hypersensitivity in six healthy dogs given human albumin. J Am Vet Med Assoc. 2007;230:873-879.
63 Francis D.M., Shenton B.K. Blood transfusion and tumour growth: Evidence from laboratory animals. Lancet. 1981;2(8251):871.
64 Fusco J.V., Hohenhaus A.E., Aiken S.W., et al. Autologous blood collection and transfusion in cats undergoing partial craniectomy. J Am Vet Med Assoc. 2000;216:1584-1588.
65 Gagnon J.A., Felipe I., Nelson L.D. Influence of thiopental anesthesia on renal sodium and water excretion in the dog. Am J Physiol. 1982;243:F265-F270.
66 Galvez O.G., Bay W.H., Roberts B.W., et al. The hemodynamic effects of potassium deficiency in the dog. Circ Res. 1977;40(Suppl. 1):I-11-I-16.
67 Gaynor J.S., Wertz E.M., Kesel L.M., et al. Effect of intravenous administration of fluids on packed cell volume, blood pressure, and total protein and blood glucose concentrations in healthy halothane-anesthetized dogs. J Am Vet Med Assoc. 1996;208:2013-2015.
68 Gibson G.R., Callan M.B., Hoffman V., et al. Use of a hemoglobin-based oxygen-carrying solution in cats: 72 cases (1998-2000). J Am Vet Med Assoc. 2002;221:96-102.
69 Glowaski M.M., Moon-Massat P., Erb H., et al. Effects of oxypolygelatin and Dextran 70 on hemostatic variables in dogs. Vet Anaesth Analg. 2003;30:230-238.
70 Goddard N.G., Menadue L.T., Wakeling H.G. A case for routine oesophageal Doppler fluid monitoring during major surgery becoming a standard of care. Br J Anaesth. 2007;99:599.
71 Goldstein S.M., MacLean L.D. Ringer’s lactate infusion with severe hepatic damage: effect on arterial lactate level. Can J Surg. 1972;15:318-321.
72 Gollub S., Schaefer C. Structural alteration in canine fibrin produced by colloid plasma expanders. Surg Gynecol Obstet. 1968;127:783-793.
73 Goodnough L., Grishaber J., Monk T., et al. Acute preoperative hemodilution in patients undergoing radical prostatectomy: a case study analysis of efficacy. Anesth Analg. 1994;78:932-937.
74 Gorelick M.H., Shaw K.N., Baker M.D. Effect of ambient temperature on capillary refill in healthy children. Pediatrics. 1993;92:699-702.
75 Graefe U., Milutinovich J., Follette W.C., et al. Less dialysis-induced morbidity and vascular instability with bicarbonate in dialysate. Ann Intern Med. 1978;88:332-336.
76 Guest J.D., Vanni S., Silbert L. Mild hypothermia, blood loss and complications in elective spinal surgery. Spine J. 2004;4:130-137.
77 Guidelines for the use of platelet transfusions. Br J Haematol. 2003;122:10-23.
78 Hahn R.G., Andrijauskas A., Drobin D., et al. A volume loading test for the detection of hypovolemia and dehydration. Medicina (Kaunas). 2008;44:953-959.
79 Hahn R.G., Svensen C. Plasma dilution and the rate of infusion of Ringer’s solution. Br J Anaesth. 1997;79:64-67.
80 Hardy R.M., Osborne C.A. Water deprivation test in the dog: Maximal normal values. JAMA. 1979;174:479-483.
81 Hartsfield S.M., Thurmon J.C., Corbin J.E., et al. Effects of sodium acetate, bicarbonate and lactate on acid-base status in anaesthetized dogs. J Vet Pharmacol Ther. 1981;4:51-61.
82 Haskins S.C., Farver T.B., Patz J.D. Ketamine in dogs. Am J Vet Res. 1985;46:1855-1860.
83 Hessel E.A.2nd, Schmer G., Dillard D.H. Platelet kinetics during deep hypothermia. J Surg Res. 1980;28:23-34.
84 Hill S.R., Carless P.A., Henry D.A., et al. Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion. Cochrane Database Syst Rev. 2002. CD002042
85 Hiltebrand L.B., Kimberger O., Arnberger M., et al. Crystalloids versus colloids for goal-directed fluid therapy in major surgery. Crit Care. 2009;13:R40.
86 Hint H. Relationships between the chemical and physicochemical properties of Dextrans and its pharmacological effects. In: Derrick J.R., Guest M.R., editors. Dextrans Current concepts of basic actions and clinical applications. 1st ed. Springfield, Ill: Charles C. Thomas; 1971:3-26.
87 Holford A.L., Tobias K.M., Bartges J.W., et al. Adrenal response to adrenocorticotropic hormone in dogs before and after surgical attenuation of a single congenital portosystemic shunt. J Vet Intern Med. 2008;22:832-838.
88 Holte K., Jensen P., Kehlet H. Physiologic effects of intravenous fluid administration in healthy volunteers. Anesth Analg. 2003;96:1504-1509.
89 Houbiers J.G., van de Velde C.J., vande Watering L.M., et al. Transfusion of red cells is associated with increased incidence of bacterial infection after colorectal surgery: A prospective study. Transfusion. 1997;37:126-134.
90 Hughes G.S., Francom S.F., Antal E.J., et al. Effects of a novel hemoglobin-based oxygen carrier on percent oxygen saturation as determined with arterial blood gas analysis and pulse oximetry. Ann Emerg Med. 1996;27:164-169.
91 Inal M.T., Memis D., Kargi M., et al. Prognostic value of indocyanine green elimination assessed with LiMON in septic patients. J Crit Care. 2009;24:329-334.
92 Iseki K., Onoyama K., Maeda T., et al. Comparison of hemodynamics induced by conventional acetate hemodialysis, bicarbonate hemodialysis and ultrafiltration. Clin Nephrol. 1980;14:294-298.
93 Ishihara H., Ishida K., Oyama T., et al. Effects of general anaesthesia and surgery on renal function and plasma ADH levels. Can Anaesth Soc J. 1978;25:312-318.
94 Ivatury R.R., Diebel L., Porter J.M., et al. Intra-abdominal hypertension and the abdominal compartment syndrome. Surg Clin North Am. 1997;77:783-800.
95 Jans O., Tollund C., Bundgaard-Nielsen M., et al. Goal-directed fluid therapy: stroke volume optimisation and cardiac dimensions in supine healthy humans. Acta Anaesthesiol Scand. 2008;52:536-540.
96 Johnstone I.B., Crane S. The effects of desmopressin on hemostatic parameters in the normal dog. Can J Vet Res. 1986;50:265-271.
97 Johnstone I.B. Desmopressin enhances the binding of plasma von Willebrand factor to collagen in plasmas from normal dogs and dogs with type I von Willebrand’s disease. Can Vet J. 1999;40:645-648.
98 Kaczmarczyk G. Pulmonary-renal axis during positive-pressure ventilation. New Horiz. 1994;2:512-517.
99 Kaneko M., Nakayama H., Igarashi N., et al. Relationship between the number of fetuses and the blood constituents of Beagles in late pregnancy. J Vet Med Sci. 1993;55:681-682.
100 Kanter M., van Maanen D., Anders K., et al. Preoperative autologous blood donations before elective hysterectomy. JAMA. 1996;276:798-801.
101 Karlson K.E., Garzon A.A., Shaftan G.W., et al. Increased blood loss associated with administration of certain plasma expanders: Dextran 75, Dextran 40, and hydroxyethylstarch. Surgery. 1967;62:670-678.
102 Kerl M.E., Hohenhaus A.E. Packed red blood cell transfusions in dogs: 131 cases 1989. J Am Vet Med Assoc. 1993;202:1495-1499.
103 Kerwin A.J., Schinco M.A., Tepas J.J.3rd, Renfro W.H., Vitarbo E.A., Muehlberger M. The use of 23.4% hypertonic saline for the management of elevated intracranial pressure in patients with severe traumatic brain injury: a pilot study. J Trauma. 2009;67:277-282.
104 Kirkendol P.L., Starrs J., Gonzalez F.M. The effects of acetate, lactate, succinate and gluconate on plasma pH and electrolytes in dogs. Trans Am Soc Artif Intern Organs. 1980;26:323-327.
105 Komtebedde J., Forsyth S.F., Breznock E.M., et al. Intrahepatic portosystemic venous anomaly in the dog: Perioperative management and complications. Vet Surg. 1991;20:37-42.
106 Kronen P.W.M., Moon-Massat P.F., Ludders J.W., et al. Comparison of two insulin protocols for diabetic dogs undergoing cataract surgery. Vet Anaesth Analg. 2001;28:146-155.
107 Kurnik B.R., Singer F., Groh W.C. Case report: Dextran-induced acute anuric renal failure. Am J Med Sci. 1991;302:28-30.
108 Lam A.M., Winn H.R., Cullen B.F., et al. Hyperglycemia and neurological outcome in patients with head injury. J Neurosurg. 1991;75:545-551.
109 Lang K., Suttner S., Boldt J., et al. Volume replacement with HES 130/0.4 may reduce the inflammatory response in patients undergoing major abdominal surgery. Can J Anaesth. 2003;50:1009-1016.
110 Langeron O., Doelberg M., Ang E.T., et al. Voluven, a lower substituted novel hydroxyethylstarch (HES 130/0.4), causes fewer effects on coagulation in major orthopedic surgery than HES 200/0.5. Anesth Analg. 2001;92:855-862.
111 Laureno R., Karp B. Myelinolysis after correction of hyponatremia. Ann Intern Med. 1997;126:57-62.
112 Lees G. Use and misuse of indwelling urethral catheters. Vet Clin North Am: Small Anim Pract. 1996;26:499-505.
113 Lens X.M., Montoliu J., Cases A., et al. Treatment of hyperkalaemia in renal failure: salbutamol v. insulin. Nephrol Dial Transplant. 1989;4:228-232.
114 Lewis J.H., Szeto I.L.F., Bayre W.L., et al. Severe hemodilution with hydroxyethylstarch and Dextrans. Arch Surg. 1966;93:941-950.
115 Li L.P.A., Shamloo M., Katsura K.I., et al. Critical values for plasma glucose in aggravating ischaemic brain damage: correlation to extracellular pH. Neurobiol Dis. 1995;2:97-108.
116 Lorenz W., Duda D., Dick W., et al. Incidence and clinical importance of perioperative histamine release: Randomized study of volume loading and antihistamines after induction of anaesthesia. Lancet. 1994;343:933-940.
117 Lundsgaard-Hansen P., Tshirren B. Modified fluid gelatin as a plasma substitute. Prog Clin Biol Res. 1978;19:227-257.
118 Martin L.G., Luther T.Y., Alperin D.C., et al. Serum antibodies against human albumin in critically ill and healthy dogs. J Am Vet Med Assoc. 2008;232:1004-1009.
119 Mathews K.A., Barry M. The use of 25% human serum albumin: outcome and efficacy in raising serum albumin and systemic blood pressure in critically ill dogs and cats. J Vet Emerg Crit Care. 2005;15:110-118.
120 Matsumura L.K., Ajzen H., Chacra A.R., et al. Effect of positive pressure breathing on plasma antidiuretic hormone and renal function in dogs. Braz J Med Biol Res. 1983;16:261-270.
121 Meier J., Kleen M., Habler O., et al. New mathematical model for the correct prediction of the exchangeable blood volume during acute normovolemic hemodilution. Acta Anaesthesiol Scand. 2003;47:37-45.
122 Michelson A.D., MacGregor H., Barnard M.R., et al. Reversible inhibition of human platelet activation by hypothermia in vivo and in vitro. Thromb Haemost. 1994;71:633-640.
123 Monteiro E.R., Teixeira Neto F.J., Castro V.B., et al. Effects of acepromazine on the cardiovascular actions of dopamine in anesthetized dogs. Vet Anaesth Analg. 2007;34:312-321.
124 Moon P.F. Acute toxicosis in two dogs associated with etomidate-propylene glycol infusion. Lab Anim Sci. 1994;44:590-594.
125 Moore L.E., Garvey M.S. The effect of hetastarch on serum colloid oncotic pressure in hypoalbuminemic dogs. J Vet Intern Med. 1996;10:300-303.
126 Moritz M.L., Ayus J.C. Water water everywhere: standardizing postoperative fluid therapy with 0.9% normal saline. Anesth Analg. 2010;110:293-295.
127 Mortier E., Ongenae M., De Baerdemaeker L., et al. In vitro evaluation of the effect of profound haemodilution with hydroxyethylstarch 6%, modified fluid gelatin 4% and Dextran 40 10% on coagulation profile measured by thromboelastography. Anaesthesia. 1997;52:1061-1064.
128 Moursi M., Rising C.L., Zelenock G.B., et al. Dextrose administration exacerbates acute renal ischemic damage in anesthetized dogs. Arch Surg. 1987;122:790-794.
129 Muir W.W.3rd, Wiese A.J. Comparison of lactated Ringer’s solution and a physiologically balanced 6% hetastarch plasma expander for the treatment of hypotension induced via blood withdrawal in isoflurane-anesthetized dogs. Am J Vet Res. 2004;65:1189-1194.
130 Naylor J.M., Kronfeld D.S., Freeman D.E., et al. Hepatic and extrahepatic lactate metabolism in sheep: effects of lactate loading and pH. Am J Physiol. 1984;247:E747-E755.
131 O’Brien D., Kroll R., Johnson G., et al. Myelinolysis after correction of hyponatremia in two dogs. J Vet Intern Med. 1994;8:40-48.
132 Opelz G., Sengar D.P., Mickey M.R., et al. Effect of blood transfusions on subsequent kidney transplants. Transplant Proc. 1973;5:253-259.
133 Palacios A., Martainez M., Costela J., et al. Postoperative infection and anesthesia: analysis of various risk factors. Rev Esp Anestesiol Reanim. 1995;42:87-90.
134 Pang D.S., Allaire J., Rondenay Y., et al. The use of lingual venous blood to determine the acid-base and blood-gas status of dogs under anesthesia. Vet Anaesth Analg. 2009;36:124-132.
135 Paradis N.A. Dose-response relationship between aortic infusions of polymerized bovine hemoglobin and return of circulation in a canine model of ventricular fibrillation and advanced cardiac life support. Crit Care Med. 1997;25:476-483.
136 Perel A., Pizov R., Cotev S. Systolic pressure variation is a sensitive indicator of hypovolemia in ventilated dogs subjected to graded hemorrhage. Anesthesiology. 1987;67:498-502.
137 Perel P., Roberts I. Colloids versus crystalloids for fluid resuscitation in critically ill patients. Cochrane Database Syst Rev. 2007. CD000567
138 Peter F.W., Benkovic C., Muehlberger T., et al. Effects of desmopressin on thrombogenesis in aspirin-induced platelet dysfunction. Br J Haematol. 2002;117:658-663.
139 Petersen J., Shalmi M., Christensen S., et al. Comparison of the renal effects of six sedating agents in rats. Physiol Behav. 1996;60:759-765.
140 Peterson M.E. Feline hyperthyroidism. Vet Clin North Am: Small Anim Pract. 1984;14:809-826.
141 Pit M.J., Tegelaar R.J., Venema P.L. Isothermic irrigation during transurethral resection of the prostate: effects on peri-operative hypothermia, blood loss, resection time and patient satisfaction. Br J Urol. 1996;78:99-103.
142 Pizov R., Ya’ari Y., Perel A. Systolic pressure variation is greater during hemorrhage than during sodium nitroprusside-induced hypotension in ventilated dogs. Anesth Analg. 1988;67:170-174.
143 Practice guidelines for blood component therapy: A report by the American Society of Anesthesiologists task force on blood component therapy. Anesthesiology. 1996:732-747.
144 Prien T., Backhaus N., Pelster F., et al. Effect of intraoperative fluid administration and colloid osmotic pressure on the formation of intestinal edema during gastrointestinal surgery. J Clin Anesth. 1990;2:317-323.
145 Ramming S., Shackford S.R., Zhuang J., et al. The relationship of fluid balance and sodium administration to cerebral edema formation and intracranial pressure in a porcine model of brain injury. J Trauma. 1994;37:705-713.
146 Rebollo M., Bernal J., Llorca J., et al. Nosocomial infections in patients having cardiovascular operations: a multivariate analysis of risk factors. J Thorac Cardiovasc Surg. 1996;112:908-913.
147 Reinhart K., Rudolph T., Bredle D.L., et al. Comparison of central-venous to mixed-venous oxygen saturation during changes in oxygen supply/demand. Chest. 1989;95:1216-1221.
148 Rezende M.L., Pypendop B.H., Ilkiw J.E. Evaluation of transesophageal echo-Doppler ultrasonography for the measurement of aortic blood flow in anesthetized cats. Am J Vet Res. 2008;69:1135-1140.
149 Ring J., Messmer K. Incidence and severity of anaphylactoid reactions to colloid volume substitutes. Lancet. 1977;i:466-469.
150 Roberts J.P., Roberts J.D., Skinner C., et al. Extracellular fluid deficit following operation and its correction with Ringer’s lactate: A reassessment. Ann Surg. 1985;202:1-8.
151 Robinson E.P., Hardy R.M. Clinical signs, diagnosis, and treatment of alkalemia in dogs: 20 cases (1982-1984). J Am Vet Med Assoc. 1988;192:943-949.
152 Rossaint R., Jorres D., Nienhaus M., et al. Positive end-expiratory pressure reduces renal excretion without hormonal activation after volume expansion in dogs. Anesthesiology. 1992;77:700-708.
153 Rossaint R., Krebs M., Forther J., et al. Inferior vena caval pressure increase contributes to sodium and water retention during PEEP in awake dogs. J Appl Physiol. 1993;75:2484-2492.
154 Rusthoven J.J. Blood transfusion and cancer: Clinical studies. In: Singal D.P., editor. Immunological effects of blood transfusion. 1st ed. Ann Arbor, Mich: CRC Press Boca Raton; 1994:85-110.
155 Ryden S.E., Oberman H.A. Compatibility of common intravenous solutions with CPD blood. Transfusion. 1975;15:250-255.
156 Sakai M., Watari T., Miura T., et al. Effects of DDAVP administrated subcutaneously in dogs with aspirin-induced platelet dysfunction and hemostatic impairment due to chronic liver diseases. J Vet Med Sci. 2003;65:83-86.
157 Sapsford W. Should the “C” in “ABCDE” be altered to reflect the trend towards hypotensive resuscitation? Scand J Surg. 2008;97:4-11. discussion 12–13
158 Saragoca M.A., Bessa A.M., Mulinari R.A., et al. Sodium acetate, an arterial vasodilator: haemodynamic characterisation in normal dogs. Proc Eur Dial Transplant Assoc. 1985;21:221-224.
159 Scheingraber S., Rehm M., Sehmisch C., et al. Rapid saline infusion produces hyperchloremic acidosis in patients undergoing gynecologic surgery. Anesthesiology. 1999;90:1265-1270.
160 Schmied H., Kurz A., Sessler D.I., et al. Mild hypothermia increases blood loss and transfusion requirements during total hip arthroplasty. Lancet. 1996;347:289-292.
161 Schortgen F., Lacherade J.C., Bruneel F., et al. Effects of hydroxyethylstarch and gelatin on renal function in severe sepsis: a multicentre randomised study. Lancet. 2001;357:911-916.
162 Schriger D.L., Baraff L.J. Capillary refill - Is it a useful predictor of hypovolemic states? Ann Emerg Med. 1991;20:601-605.
163 Segal J.B., Blasco-Colmenares E., Norris E.J., et al. Preoperative acute normovolemic hemodilution: a meta-analysis. Transfusion. 2004;44:632-644.
164 Shires T., Williams J., Brown F. Acute changes in extracellular fluids associated with major surgical procedures. Ann Surg. 1961;154:803-810.
165 Sieber F.E., Traystman R.J. Special issues: glucose and the brain. Crit Care Med. 1992;20:104-114.
166 Sieber F.E. The neurologic implications of diabetic hyperglycemia during surgical procedures at increased risk for brain ischemia. J Clin Anesth. 1997;9:334-340.
167 Silberstein L.E., Naryshkin S., Haddad J.J., et al. Calcium homeostasis during therapeutic plasma exchange. Transfusion. 1986;26:151-155.
168 Slichter S.J. Evidence-based platelet transfusion guidelines. Hematology Am Soc Hematol Educ Program. 2007:172-178.
169 Smart L., Hopper K., Aldrich J., et al. The effect of hetastarch (670/0.75) on urine specific gravity and osmolality in the dog. J Vet Intern Med. 2009;23:388-391.
170 Smiley L.E., Garvey M.S. The use of hetastarch as adjunct therapy in 26 dogs with hypoalbuminemia: a phase two clinical trial. J Vet Intern Med. 1994;8:195-202.
171 Smiley L.E. The use of hetastarch for plasma expansion. Probl Vet Med. 1992;4:652-667.
172 Ståhle L., Nilsson A., Hahn R.G. Modelling the volume of expandable body fluid spaces during i.v. fluid therapy. Br J Anaesth. 1997;78:138-143.
173 Stamm W. Infections related to medical devices. Ann Intern Med. 1978;89:764-769.
174 Standl T., Freitag M., Burmeister M.A., et al. Hemoglobin-based oxygen carrier HBOC-201 provides higher and faster increase in oxygen tension in skeletal muscle of anemic dogs than do stored red blood cells. J Vasc Surg. 2003;37:859-865.
175 Stokol T., Parry B. Efficacy of fresh-frozen plasma and cryoprecipitate in dogs with von Willebrand’s disease or hemophilia A. J Vet Intern Med. 1998;12:84-92.
176 Strauss R.G. Review of the effects of hydroxyethylstarch on the blood coagulation system. Transfusion. 1981;21:299-302.
177 Svensén C., Hahn R.G. Volume kinetics of Ringer solution, Dextran 70, and hypertonic saline in male volunteers. Anesthesiology. 1997;87:204-212.
178 Svensen C., Ponzer S., Hahn R.G. Volume kinetics of Ringer solution after surgery for hip fracture. Can J Anaesth. 1999;46:133-141.
179 Tabuchi N., de Haan J., Gallandat Huet R.C., et al. Gelatin use impairs platelet adhesion during cardiac surgery. Thromb Haemost. 1995;74:1447-1451.
180 Tanifuji Y., Eger E.I. Brain sodium, potassium, and osmolality: effects on anesthetic requirement. Anesth Analg. 1978;57:404-410.
181 Tartter P.I. Blood transfusion and bacterial infections: Clinical studies. In: Singal D.P., editor. Immunological effects of blood transfusion. 1st ed. Ann Arbor, Mich: CRC Press Boca Raton; 1994:111-126.
182 Thompson W.L., Fukushima T., Rutherford R.B., et al. Intravascular persistence, tissue storage, and excretion of hydroxyethylstarch. Surg Gynecol Obstet. 1970;131:965-972.
183 Treib J., Haass A., Pindur G., et al. HES 200/0.5 is not HES 200/0.5. Influence of the C2/C6 hydroxyethylation ratio of hydroxyethylstarch (HES) on hemorheology, coagulation and elimination kinetics. Thromb Haemost. 1995;74:1452-1456.
184 Trow A.V., Rozanski E.A., Delaforcade A.M., et al. Evaluation of use of human albumin in critically ill dogs: 73 cases (2003-2006). J Am Vet Med Assoc. 2008;233:607-612.
185 Usenik E.A., Cronkite E.P. Effects of barbiturate anesthesia on leukocytes in normal and splenectomized dogs. Anesth Analg. 1965;44:167-170.
186 Vail D.M., Ogilvie G.K., Fettman M.J., et al. Exacerbation of hyperlactatemia by infusion of lactated Ringer’s solution in dogs with lymphoma. J Vet Intern Med. 1990;4:228-232.
187 Virtue R.W., LeVine D.S., Aikawa J.K. Fluid shifts during the surgical period: RISA and S35 determinations following glucose, saline or lactate infusion. Ann Surg. 1965;163:523-528.
188 Vogt N.H., Bothner U., Lerch G., et al. Large-dose administration of 6% hydroxyethylstarch 200/0.5 total hip arthroplasty: plasma homeostasis, hemostasis, and renal function compared to use of 5% human albumin. Anesth Analg. 1996;83:262-268.
189 Vos S.C., Hage J.J., Woerdeman L.A., et al. Acute renal failure during Dextran-40 antithrombotic prophylaxis: report of two microsurgical cases. Ann Plast Surg. 2002;48:193-196.
190 Wakeling H.G., McFall M.R., Jenkins C.S., et al. Intraoperative oesophageal Doppler guided fluid management shortens postoperative hospital stay after major bowel surgery. Br J Anaesth. 2005;95:634-642.
191 Wass C.T., Lanier W.L. Glucose modulation of ischemic brain injury: review and clinical recommendations. Mayo Clin Proc. 1996;71:801-812.
192 Waterhouse C. Lactate metabolism in patients with cancer. Cancer. 1974;33:66-71.
193 Weber C.F., Dietrich W., Spannagl M., et al. A point-of-care assessment of the effects of desmopressin on impaired platelet function using multiple electrode whole-blood aggregometry in patients after cardiac surgery. Anesth Analg. 2010;110:702-707.
194 Wenzel R., Osterman C., Hunting K. Hospital-acquired infections. II. Infection rates by site, service and common procedures in a university hospital. Am J Epidemiol. 1976;104:645-651.
195 Williams E.L., Hildebrand K.L., McCormick S.A., et al. The effect of intravenous lactated Ringer’s solution versus 0.9% sodium chloride solution on serum osmolality in human volunteers. Anesth Analg. 1999;88:999-1003.
196 Winkler M., Akca O., Birkenberg B., et al. Aggressive warming reduces blood loss during hip arthroplasty. Anesth Analg. 2000;91:978-984.
197 Wolberg A.S., Meng Z.H., Monroe D.M.3rd, et al. A systematic evaluation of the effect of temperature on coagulation enzyme activity and platelet function. J Trauma. 2004;56:1221-1228.
198 Wright B.D., Hopkins A. Changes in colloid osmotic pressure as a function of anesthesia and surgery in the presence and absence of isotonic fluid administration in dogs. Vet Anaesth Analg. 2008;35:282-288.
199 Zornow M.H., Prough D.S. Fluid management in patients with traumatic brain injury. New Horiz. 1995;3:488-498.
200 Zunkeler B., Carson R.E., Olson J., et al. Hyperosmolar blood-brain barrier disruption in baboons: an in vivo study using positron emission tomography and rubidium-82. J Neurosurg. 1996;84:494-502.
201 Zwaveling J.H., Meulenbelt J., van Xanten N.H., et al. Renal failure associated with the use of Dextran-40. Neth J Med. 1989;35:321-326.