chapter 76 Geriatric Incontinence and Voiding Dysfunction
Urinary incontinence is a major problem for the elderly (Chang et al, 2008). It afflicts 15% to 30% of older people living at home, one third of those in acute-care settings, and half of those in nursing homes (Fantl et al, 1996; McGrother, 1998). It predisposes to perineal rashes, pressure ulcers, urinary tract infections, urosepsis, falls, and fractures (Fantl et al, 1996; Tromp et al, 1998; Brown et al, 2000; Parsons et al, 2009). It is associated with embarrassment, stigmatization, isolation, depression, anxiety, sexual dysfunction, and risk of institutionalization (Fantl et al, 1996; Farage et al, 2008; Angner et al, 2009). Also, it cost more than $26 billion to manage incontinence in America’s elderly in 1995, the last time primary data was collected (Wagner and Hu, 1998). This amount exceeded the amount devoted to dialysis and coronary bypass surgery combined. Despite these considerations, geriatric incontinence remains neglected by patients and physicians alike.
Providers and older patients alike often neglect incontinence or dismiss it as a normal part of growing old (Branch et al, 1994; Mann et al, 2000; Chang et al, 2008; Lawhorne et al, 2008), but it is abnormal at any age (Resnick, 1988; Herzog and Fultz, 1990; Fantl et al, 1996). Although its prevalence increases, at no age does incontinence affect the majority of individuals, even over age 85 (Wetle et al, 1995). Moreover, its increased prevalence relates more to age-associated diseases and functional impairments than to age itself (Resnick et al, 1988; Herzog and Fultz, 1990; Resnick et al, 1995; Wetle et al, 1995). Regardless, incontinence is usually treatable and often curable at all ages, even in frail elderly (Ouslander and Schnelle, 1995; Resnick, 1995; Wagg and Malone-Lee, 1998; Weinberger et al, 1999), but the approach must differ significantly from that used in younger patients.
The Impact of Age on Incontinence
At any age, continence depends on not only the integrity of lower urinary tract function but also the presence of adequate mentation, mobility, motivation, and manual dexterity. Although incontinence in younger patients is rarely associated with deficits outside the urinary tract, such deficits occur commonly in older patients. It is crucial to detect them, both because they exacerbate and, occasionally, even cause incontinence in the elderly, and because design of an efficacious intervention requires that they be addressed.
In addition, the lower urinary tract changes with age, even in the absence of disease. Data from continent elderly are sparse and longitudinal data virtually nonexistent. However, it appears that although bladder capacity does not change with age (Pfisterer et al, 2006b), bladder sensation, contractility, and the ability to postpone voiding decline in both sexes, while urethral length and maximum closure pressure, as well as striated muscle cells in the rhabdosphincter and urogenital diaphragm, probably decline with age in women (Diokno et al, 1988; Resnick, 1988; Resnick et al, 1995; Strasser et al, 1999; Perucchini et al, 2002; Pfisterer et al, 2006a, 2006b; Trowbridge et al, 2007; Betschart et al, 2008). The prostate enlarges in most men, and appears to cause urodynamic obstruction in half (Resnick et al, 1995). In both sexes, the prevalence of involuntary detrusor contractions increases, whereas the postvoid residual (PVR) volume probably increases but to no more than 50 to 100 mL (Diokno et al, 1988; Resnick, 1988; Resnick et al, 1995; Bonde et al, 1996). In addition, the elderly often excrete most of their fluid intake at night, even in the absence of venous insufficiency, renal disease, heart failure, or prostatism (Miller, 2000; Morgan et al, 2000). This fact, coupled with an age-associated increase in sleep disorders, leads to one to two episodes of nocturia in the majority of healthy elderly (Miller, 2000; Morgan et al, 2000). Finally, at the cellular level, detrusor smooth muscle develops a “dense band pattern” characterized by dense sarcolemmal bands with depleted caveolae (Elbadawi et al, 1993a, 1993b, 1997a). This depletion may mediate the age-related decline in bladder contractility. In addition, an “incomplete dysjunction pattern” develops, characterized by scattered protrusion junctions, albeit not in chains; these changes may underlie the high prevalence of involuntary detrusor contractions (vide infra) (Resnick et al, 1995; Hailemariam et al, 1997; Elbadawi et al, 1997a).
None of these age-related changes causes incontinence, but they do predispose to it. This predisposition, coupled with the increased likelihood that an older person will encounter an additional pathologic, physiologic, or pharmacologic insult, explains why the elderly are so likely to become incontinent. The implications are equally important. The onset or exacerbation of incontinence in an older person is probably due to precipitant(s) outside the lower urinary tract that are amenable to medical intervention. Furthermore, treatment of the precipitant(s) alone may be sufficient to restore continence, even if there is coexistent urinary tract dysfunction. For instance, flare of hip arthritis in a woman with age-related detrusor overactivity may be sufficient to convert her urinary urgency into incontinence. Treatment of the arthritis, rather than the involuntary detrusor contractions, will not only restore continence but also lessen pain and improve mobility. These principles, depicted in Figures 76-1 and 76-2 (Resnick, 1996; Resnick and Marcantonio, 1997), provide the rationale in the older patient for adding a set of transient causes to the established lower urinary tract causes of incontinence. Because of their frequency, ready reversibility, and association with morbidity beyond incontinence, the transient causes are discussed first.
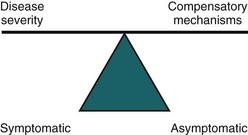
Figure 76–1 Incontinence results when the ability to compensate for bladder dysfunction is inadequate. For instance, in the intact older person, detrusor overactivity or sphincter weakness may not cause leakage if the patient is more attuned to bladder fullness, drinks less, voids more often, eliminates precipitants such as coughing, and stays close to a toilet. This explains how older patients may remain continent despite such abnormalities, and also suggests alternative therapeutic approaches independent of the urinary tract.
(From Resnick NM. An 89-year-old woman with urinary incontinence. JAMA 1996;276:1832–40.)
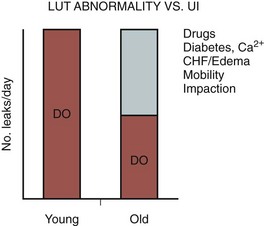
Figure 76–2 This graph shows why treatment of urinary incontinence (UI) in older adults has to differ and is often easier than in younger adults. Both of these patients with detrusor overactivity (DO) have the same frequency of leakage. However, in the younger patient with intact compensatory mechanisms, such leakage reflects solely the contribution of the DO. In the older adult, other comorbid conditions make it more difficult to appreciate bladder fullness, adjust fluid output, and reach the bathroom. Such conditions magnify the impact of DO and will be unaffected by bladder relaxant therapy. But addressing them will result in a marked improvement in the UI even without treatment of the DO and will make DO therapy, if still required, more effective. LUT, lower urinary tract.
(From Resnick NM, Marcantonio ER. How should clinical care of the aged differ? Lancet 1997;350:1157–58.)
Causes of Transient Incontinence
Incontinence is transient in up to one third of community-dwelling elderly and in up to half of acutely hospitalized patients (Resnick, 1988; Herzog and Fultz, 1990). Although most of the transient causes lie outside the lower urinary tract, three points warrant emphasis. First, the risk of transient incontinence is increased if, in addition to physiologic changes of the lower urinary tract, the older person also suffers from pathologic changes. Anticholinergic agents are more likely to cause overflow incontinence in persons with a weak or obstructed bladder, while excess urine output is more likely to cause urge incontinence in persons with detrusor overactivity and/or impaired mobility (Fantl et al, 1990; Diokno et al, 1991). Second, although termed “transient,” these causes of incontinence may persist if left untreated and cannot be dismissed merely because incontinence is longstanding. Third, similar to the situation for established causes (vide infra), identification of “the most common cause” is of little value. The likelihood of each cause depends on the individual, the clinical setting (community, acute hospital, nursing home), and referral pattern. Moreover, geriatric incontinence is rarely due to just one of these causes. Trying to disentangle which of the multiple abnormalities is “the” cause is more useful for metaphysics than for clinical practice.
The causes of transient incontinence can be recalled easily using the mnemonic “DIAPERS” (Table 76–1). In the setting of delirium (an acute and fluctuating confusional state due to virtually any drug or acute illness), incontinence is merely an associated symptom that abates once the underlying cause of confusion is identified and treated. The patient needs medical rather than bladder management (Resnick, 1988).
Table 76–1 Causes of Transient Incontinence
| CAUSE | NOTES |
|---|---|
| Delirium/confusional state | Results from almost any underlying illness or medication; incontinence is secondary and abates once the cause of confusion has been corrected |
| Infection—Urinary (only symptomatic) | Causes incontinence, but the more common asymptomatic bacteriuria does not |
| Atrophic urethritis/vaginitis | Characterized by vaginal erosions, telangiectasia, petechiae, and friability; may cause or contribute to incontinence. Now controversial but may be worth a 3- to 6-month trial of estrogen, especially topical application (if not contraindicated by breast or uterine cancer) |
| Pharmaceuticals | Includes many prescribed and nonprescribed agents, because incontinence can be caused by diverse mechanisms (see Table 76–2) |
| Excess urine output | Results from large fluid intake, diuretic agents (including theophylline, caffeinated beverages, and alcohol), and metabolic disorders (e.g., hyperglycemia or hypercalcemia); nocturnal incontinence also may result from mobilization of peripheral edema (e.g., congestive heart failure [CHF], venous insufficiency, drug side effect) |
| Restricted mobility | Often results from overlooked, correctable conditions such as arthritis, pain, foot problem, postprandial hypotension, or fear of falling |
| Stool impaction | May cause both fecal and urinary incontinence that remit with disimpaction |
Adapted from Resnick NM. Urinary incontinence in the elderly. Med Grand Rounds 1984;3:281–90.
Symptomatic urinary tract infection causes transient incontinence when dysuria and urgency are so prominent that the older person is unable to reach the toilet before voiding. Asymptomatic bacteriuria, which is much more common in the elderly, does not cause incontinence (Brocklehurst et al, 1968; Resnick, 1988; Baldassare and Kaye, 1991; Ouslander et al, 1995). Because illness can present atypically in older patients, however, incontinence is occasionally the only atypical symptom of a urinary tract infection (UTI). Thus, if otherwise asymptomatic bacteriuria is found on initial evaluation, it should be treated and the result recorded in the patient’s record to prevent future futile therapy.
Atrophic urethritis/vaginitis frequently causes lower urinary tract symptoms, including incontinence. Up to 80% of elderly women attending an incontinence clinic have atrophic vaginitis, characterized by vaginal mucosal atrophy, friability, erosions, and punctate hemorrhages (Robinson and Brocklehurst, 1984). Incontinence associated with this entity usually is associated with urgency and, occasionally, a sense of “scalding” dysuria that mimics a urinary tract infection, but both symptoms may be unimpressive. In demented individuals, atrophic vaginitis may present as agitation. Atrophic vaginitis also can exacerbate stress incontinence.
The importance of recognizing atrophic vaginitis is that it may respond to low-dose estrogen (Fantl et al, 1996; Cardozo et al, 2004). Moreover, as for other causes of transient incontinence, treatment has additional benefits; in the case of atrophic urethritis, treatment ameliorates dyspareunia and reduces the frequency of recurrent cystitis (Resnick, 1988; Raz and Stamm, 1993; Cardozo et al, 1998; Eriksen, 1999).
Reports from two large-scale randomized prospective trials, however, have recently challenged long-standing assumptions about the benefits and risks of hormone therapy. The Women’s Health Initiative (WHI) randomized more than 27,000 women, aged 50 to 79 years, to oral conjugated equine estrogen (CEE) 0.625 mg, with or without medroxyprogesterone (MPA) 2.5 mg (Hendrix et al, 2005). The Heart and Estrogen/Progestin Replacement Study (HERS) randomized nearly 2800 postmenopausal women of the same age to the same regimen (Grady et al, 2001). Both studies found that hormone therapy was associated with worsening of pre-existing incontinence. WHI investigators also found that hormone therapy, whether prescribed as CEE alone or with MPA, was associated with increased incidence of new incontinence. Because both studies also found that hormone therapy did not protect against heart disease, they advised against using hormone therapy for urinary incontinence.
In trying to apply the results of the WHI and HERS trials, several caveats should be noted. Postmenopausal hormone levels differ substantially, and in some individuals they are similar to levels seen in the follicular phase of premenopausal women (Kuchel et al, 2001). Tissue sensitivity also varies. Thus it is surprising that neither WHI nor HERS randomized or stratified results by the presence or absence of atrophic vaginitis, nor did investigators examine patients to determine whether the dose administered was sufficient to treat the condition if it was present. Dropout rates in WHI were also high: 42% in the combined hormone therapy group and 54% in the estrogen-alone group. In addition, incontinence data were based on self-report, which can be problematic in older adults, both for reproducibility and for determining the type of incontinence (Resnick et al, 1994; Kirschner-Hermanns et al, 1998). Urodynamic testing was not performed. Moreover, although drugs used to treat heart disease in such patients can cause or exacerbate incontinence (e.g., diuretics, α blockers, calcium channel blockers, and angiotensin-converting enzyme inhibitors (ACEI), their impact was not reported. Finally, other hormone doses, types, and routes of administration were not evaluated. For example, a recent randomized placebo-controlled study with ultralow-dose transdermal estrogen (0.014 mg) revealed no substantial effect on either the frequency of incontinence or the risk of developing at least weekly incontinence (Waetjen et al, 2005).
There is less evidence available from recent studies of nursing home patients, but limitations of sample size; selection bias; route, dose adequacy, and type of hormone used; and characterization and stratification by type of urinary infection (UI) all similarly preclude meaningful conclusions (DuBeau, 2001; Ouslander et al, 2001a).
The apparent lack of efficacy for conjugated equine estrogen demonstrated in these two studies, as well as the risks associated with long-term use of higher doses, must be balanced against the other benefits cited above—the lower doses and shorter duration of treatment advised for treatment of atrophic vaginitis, and the shorter life expectancy of many older women in whom the prevalence and severity of atrophic vaginitis are highest. Moreover, data from other randomized double-blind trials, which employed different estrogen preparations (estriol, estradiol, or quinestradol), demonstrated significant reduction in incontinence among nursing home residents (Judge, 1969) and postmenopausal women with sensory urge incontinence (Walter et al, 1978; Moehrer et al, 2003). Thus clinical judgment is warranted, and local administration of other types of estrogen may be best. Unfortunately, the use of vaginal estrogen has not yet been well-studied.
With adequate treatment, symptoms of atrophic vaginitis remit in a few days to several weeks, but the intracellular response takes longer (Semmens et al, 1985). The duration of therapy has not been well established (Pandit and Ouslander, 1997). One approach is to insert a ring containing estradiol. The advantage is that it is small and delivers the dose locally. Because it does not increase systemic estrogen concentrations, it is often considered for women with a past history of breast cancer. The disadvantage is that, despite its small size, it is not always feasible or well tolerated by frail older women, especially those with pronounced vaginal stenosis. Another option is to administer a low dose of estradiol orally or vaginally for 1 to 2 months and then taper it. Most patients probably can be weaned to a dose given as infrequently as 2 to 4 times per month. After 6 months, estrogen can be discontinued entirely in some patients, but recrudescence is common. Because the dose is low and given briefly, the carcinogenic effect is likely slight, if any. However, if long-term treatment is selected, a progestin probably should be added if the patient has a uterus. Hormone treatment (except for administration by a vaginal ring) is contraindicated for women with a history of breast cancer. For those without such a history, mammography should be performed prior to initiating hormone therapy. There appears to be a slightly increased risk of breast cancer among women using oral estrogen daily for more than 5 years, but fortunately, such high-dose, frequent, and long-term therapy is rarely required.
Pharmaceuticals are one of the most common causes of geriatric incontinence, precipitating leakage by a variety of mechanisms (Table 76–2 and Tsakiris et al, 2008). Experts often cite dosages and serum levels below which side effects are uncommon. Unfortunately, such rules are of limited use in the elderly, because they are generally derived from studies of healthy younger persons who have no other diseases and take no other medications. Of note, many of these agents also are used in the treatment of incontinence, underscoring the fact that most medications are “double-edged swords” for the elderly.
Table 76–2 Commonly Used Medications That May Affect Continence
| TYPE OF MEDICATION | EXAMPLES | POTENTIAL EFFECTS ON CONTINENCE |
|---|---|---|
| Sedatives/hypnotics | Long-acting benzodiazepines (e.g., diazepam, flurazepam) | Sedation, delirium, immobility |
| Alcohol | Polyuria, frequency, urgency, sedation, delirium, immobility | |
| Anticholinergics | Dicyclomine, disopyramide, antihistamines (sedating ones only, e.g., Benadryl) | Urinary retention, overflow incontinence, delirium, impaction |
| Antipsychotics | Thioridazine, haloperidol | Anticholinergic actions, sedation, rigidity, immobility |
| Antidepressants (tricyclics) | Amitriptyline, desipramine; not SSRIs | Anticholinergic actions, sedation |
| Anti-Parkinsonians | Trihexyphenidyl, benztropine mesylate (not L-dopa or selegiline) | Anticholinergic actions, sedation |
| Narcotic analgesics | Opiates | Urinary retention, fecal impaction, sedation, delirium |
| α-Adrenergic antagonists | Prazosin, terazosin, doxazosin | Urethral relaxation may precipitate stress incontinence in women |
| α-Adrenergic agonists | Nasal decongestants | Urinary retention in men |
| Calcium channel blockers | All dihydropyridines* | Urinary retention; nocturnal diuresis due to fluid retention |
| Potent diuretics | Furosemide, bumetanide (not thiazides) | Polyuria, frequency, urgency |
| NSAIDs | Indomethacin, COX-2 inhibitors | Nocturnal diuresis due to fluid retention |
| Thiazolidinediones | Rosiglitazone, pioglitazone | Nocturnal diuresis due to fluid retention |
| Anticonvulsants/analgesics | Gabapentin, pregabalin | Nocturnal diuresis due to fluid retention |
| Parkinson agents (some) | Pramipexole, ropinirole, amantadine | Nocturnal diuresis due to fluid retention |
| Angiotensin converting enzyme (ACE) inhibitors | Captopril, enalapril, lisinopril | Drug-induced cough can precipitate stress incontinence in women and in some men with prior prostatectomy |
| Vincristine | Urinary retention owing to neuropathy |
COX-2, cyclooxygenase-2; NSAIDs, nonsteroidal anti-inflammatory drugs; SSRIs, selective serotonin reuptake inhibitors.
* Examples include amlodipine (Norvasc), nifedipine, nicardipine, isradipine, felodipine, nimodipine.
Adapted from Resnick NM. Geriatric medicine. In: Isselbacher KJ, Braunwald E, Wilson JD, Martin JB, Fauci AS, Kasper DJ, editors. Harrison’s principles of internal medicine. New York: McGraw-Hill; 2004. p. 34.
Long-acting sedative/hypnotics, whose half-life can exceed 100 hours, are associated not only with incontinence (Landi et al, 2002) but also with falling, hip fractures, driving accidents, depression, and confusion. Alcohol causes similar problems, but, for a variety of reasons, physicians frequently fail to identify alcohol use by older people as a source of symptoms, including incontinence. Sequelae of alcohol abuse are often absent or attributed to other causes. In addition, because of age-related alterations in the pharmacokinetics and pharmacodynamics of alcohol disposal, as well as interactions with other commonly used drugs, as few as one or two drinks can pose a problem for older individuals.
Because anticholinergic agents are prescribed so often for the elderly, and are used even without prescription (e.g., sedating antihistamines used for allergies, coryza, and insomnia), it is important to ask about them. They cause or contribute to incontinence in several ways. In addition to provoking overt urinary retention, these agents often induce subclinical retention. The resultant decrease in available bladder capacity (i.e., total capacity minus residual) allows it to be reached more quickly, exacerbating incontinence due to DO, as well as that due to functional impairment. By increasing bladder capacity (and often residual volume), anticholinergic agents also may aggravate leakage due to stress incontinence by further increasing the challenge to the sphincter mechanism. Additionally, many of these drugs decrease mobility (e.g., antipsychotics that induce extrapyramidal stiffness) and precipitate confusion. Finally, several agents intensify the dry mouth that many elderly already suffer owing to an age-related decrease in salivary gland function; the resultant increased fluid intake contributes to incontinence. Attempts should be made to discontinue anticholinergic agents, or to substitute with ones having less anticholinergic effect (e.g., a selective serotonin uptake inhibitor [SSRI] for a tricyclic antidepressant; risperidone for chlorpromazine). Limited data suggest that bethanechol may be useful for nonobstructed patients whose urinary retention is associated with use of an anticholinergic that cannot be discontinued (Everett, 1975).
In men with asymptomatic prostatic obstruction, α-adrenergic agonists can provoke acute retention. Particularly problematic are nonprescribed decongestants. They often contain an (anticholinergic) antihistamine and are frequently taken with a nonprescribed hypnotic, all of which are also sedating antihistamines. Because older individuals often fail to mention non-prescribed agents to a physician, urinary retention due to use of a decongestant, nose drops, and a hypnotic may result in premature or even unnecessary prostatectomy.
By blocking receptors at the bladder neck, α-adrenergic antagonists (many antihypertensives) may induce stress incontinence in older women (Mathew et al, 1988; Marshall and Beevers, 1996) in whom urethral length and closure pressure decline with age. Because hypertension affects half of the elderly, use of these agents may increase. Before considering interventions for stress incontinence in such women, one should substitute an alternative agent and reevaluate the incontinence.
Calcium channel blockers also can cause incontinence. As smooth muscle relaxants, they may increase residual volume and may occasionally lead to overflow incontinence, particularly in obstructed men with coexisting detrusor weakness. The dihydropyridine class of these agents (e.g., nifedipine, amlodipine, nicardipine, isradipine, nimodipine) also can cause peripheral edema, which can exacerbate nocturnal polyuria and nocturnal incontinence.
Angiotensin-converting enzyme inhibitors (ACEI) are prescribed increasingly for age-associated conditions such as myocardial infarction, congestive heart failure, and hypertension. Because the risk of developing an ACEI-induced cough increases with age, these agents may exacerbate what otherwise would be minimal stress incontinence in older women.
A few reports impugn cholinesterase inhibitors, such as donepezil (Aricept) or rivastigmine, as a cause of urinary incontinence (Hashimoto et al, 2000; Starr, 2007). The association is plausible, because these agents block the breakdown of acetylcholine within the neuroeffector junctional cleft. However, the incontinence often ceases even if the drug is continued, the number of cases is small, and the descriptions and evaluations of these cases are sparse. In addition, prospective trials in which these agents were evaluated for their cognitive impact did not note an increase in incontinence. Nonetheless, the association has not been well-evaluated to date, and because incontinence may improve or resolve on decreasing the drug dose or discontinuation, such an association is worth keeping in mind.
Excess urine output commonly contributes to or even causes geriatric incontinence. Causes include excessive fluid intake; diuretics (including theophylline-containing fluids, lithium, and alcohol); metabolic abnormalities (e.g., hyperglycemia and hypercalcemia); and disorders associated with fluid overload, including congestive heart failure, peripheral venous insufficiency, hypoalbuminemia (especially in malnourished debilitated elderly), and drug-induced peripheral edema associated with an increasing array of medications, including nonsteroidal anti-inflammatory drugs (NSAIDs), dihydropyridine calcium channel blockers, thiazolidinediones (“glitazones”), pramipexole and ropinirol (for Parkinson disease), amantadine (for Parkinson disease and influenza), and β blockers. Fluid overload is a likely contributor when incontinence is associated with nocturia.
Restricted mobility commonly contributes to geriatric incontinence. It can result from numerous treatable conditions, including arthritis, hip deformity, deconditioning, postural or postprandial hypotension, claudication, spinal stenosis, heart failure, poor eyesight, fear of falling, stroke, foot problems, drug-induced disequilibrium or confusion, or being restrained in a bed or chair (Resnick, 2004). A careful search will often identify these or other correctable causes. If not, a urinal or bedside commode may still improve or resolve the incontinence.
Finally, stool impaction is implicated as a cause of urinary incontinence in up to 10% of older patients admitted to acute hospitals or referred to incontinence clinics (Resnick, 1988); the mechanism may involve stimulation of opioid receptors (Hellstrom and Sjoqvist, 1988). Patients present with urge or overflow incontinence and typically have associated fecal incontinence as well. Disimpaction restores continence.
These seven reversible causes of incontinence should be assiduously sought in every elderly patient. In one series of hospitalized elderly patients, when these causes were identified, continence was regained by most of those who became incontinent in the context of acute illness (Resnick, 1988). Regardless of their frequency, their identification is important in all settings because they are easily treatable and contribute to morbidity beyond incontinence.
Key Points: Transient Incontinence
Established Incontinence
Lower Urinary Tract Causes
If incontinence persists after transient causes have been addressed, the lower urinary tract causes should be considered. These are similar to the causes in younger individuals, but there are several significant differences.
Detrusor overactivity (DO) is the most common type of lower urinary tract dysfunction in incontinent elderly of either sex (Resnick, 1988; Resnick et al, 1989). DO has been associated with increased spontaneous activity of detrusor smooth muscle and with specific changes at the cellular level. Termed the “complete dysjunction pattern,” these changes include widening of the intercellular space, reduction of normal (intermediate) muscle cell junctions, and emergence of novel “protrusion” junctions and “ultraclose abutments” connecting cells together in chains. These junctions and abutments may mediate a change in cell coupling from a mechanical to an electrical mechanism, which could facilitate propagation of heightened smooth muscle activity and provide the “final common pathway” by which such spontaneous cellular contractions result in involuntary contraction of the entire bladder (Elbadawi et al, 1993c, 1997a, 1997b; Hailemariam et al, 1997; Tse et al, 2000). However, this remains speculative to date. Other potential mechanisms are also being investigated, including, among others, the role of ischemia (Azadzoi et al, 2007), suburothelial myofibroblasts (Fry et al, 2007), and changes in central nervous system (CNS) structural and functional control mechanisms (Griffiths et al, 2007, 2009; Poggesi et al, 2008; Tadic et al, 2008; Kuchel et al, 2009).
A distinction is generally made between detrusor overactivity that is associated with a CNS lesion and that which is not. In older patients, the distinction is often unclear, because involuntary detrusor contractions may be due to normal aging, a past stroke (even if clinically unapparent), or urethral incompetence or obstruction—even in a patient with Alzheimer disease. There is still no reliable way to determine the source of such contractions (Elbadawi et al, 2003). This obviously complicates treatment decisions. It also suggests that DO coexisting with urethral obstruction or stress incontinence is less likely to resolve postoperatively than in younger individuals without other reasons for detrusor overactivity (Resnick, 1988; Gormley et al, 1993).
Traditionally, detrusor overactivity has been thought to be the primary urinary tract cause of incontinence in demented patients. Although this is true, DO is also the most common cause in nondemented older patients; the three studies that examined it failed to find an association between cognitive status and DO (Castleden et al, 1981; Resnick et al, 1989; Dennis et al, 1991). This lack of association likely reflects the fact that, in the elderly, there are multiple causes of DO unrelated to dementia, including cervical disk disease or spondylosis, Parkinson disease, stroke, subclinical urethral obstruction or sphincter incompetence, and age itself. Moreover, demented patients also may be incontinent due to the transient causes discussed above. Thus it is no longer tenable to ascribe incontinence in demented individuals a priori to detrusor overactivity (Resnick, 1995).
Detrusor overactivity in the elderly exists as two physiologic subsets: one in which contractile function is preserved and one in which it is impaired (Resnick and Yalla, 1987; Yalla et al, 2007). The latter condition is termed detrusor hyperactivity with impaired contractility (DHIC) and is likely the most common form of DO in the elderly (Resnick et al, 1989; Elbadawi et al, 1993c). DHIC appears to represent the coexistence of DO and bladder weakness rather than a separate entity (Elbadawi et al, 1993c). Regardless of the cause, DHIC has several implications. First, because the bladder is weak, urinary retention develops commonly in these patients, and DHIC must be added to outlet obstruction and detrusor underactivity as a cause of retention. Second, even in the absence of retention, DHIC mimics virtually every other lower urinary tract cause of incontinence. For instance, if the involuntary detrusor contraction is triggered by or occurs coincident with a stress maneuver, and the weak contraction (often only 2 to 6 cm water) is not detected, DHIC will be misdiagnosed as stress incontinence or urethral instability (Resnick et al, 1996a); alternatively, because DHIC may be associated with urinary urgency, frequency, weak flow rate, elevated residual urine, and bladder trabeculation, in men it may mimic urethral obstruction (Resnick et al, 1996a). Third, bladder weakness often frustrates anticholinergic therapy of DHIC because urinary retention is induced so easily. Thus alternative therapeutic approaches are often required (see Therapy section).
Stress incontinence is the second most common cause of incontinence in older women. As in younger women, it is usually associated with urethral hypermobility. A less common cause is intrinsic sphincter deficiency (ISD) or type 3 stress incontinence (McGuire, 1981; Blaivas and Olsson, 1988). The prevalence of ISD may be lower than thought, however, because the diagnosis is often based solely on documenting a very low leak point or urethral closure pressure. Because urethral pressure decreases with age, even a closure pressure of less than 20 cm water does not establish the presence of ISD. Moreover, because urethral pressure normally decreases with detrusor contraction, leakage coinciding with low urethral pressure can be observed in patients with DHIC in whom the low pressure contraction is missed (Resnick et al, 1996a).
When it occurs, ISD is usually due to operative trauma. But a milder form also occurs in older women, resulting only from urethral atrophy superimposed on the age-related decline in urethral pressure. Instead of leaking, with any bladder volume, such women leak at higher amounts (e.g., >200 mL). Many become dry if bladder volume is kept below this level.
A rare cause of stress incontinence in older women is “urethral instability,” in which the sphincter paradoxically relaxes in the absence of apparent detrusor contraction (McGuire, 1978). However, most older women thought to have this condition actually have DHIC (Resnick et al, 1996a).
In men, stress incontinence is usually due to sphincter damage following radical prostatectomy. In both sexes, stress-associated leakage also can occur in association with urinary retention, but in this situation leakage is not due to outlet incompetence.
Incontinence in the setting of outlet obstruction is the second most common cause of incontinence in older men, although most obstructed men are not incontinent. When obstruction is associated with incontinence, it generally presents as urge incontinence owing to the associated DO; overflow incontinence owing to obstruction is uncommon. Especially in older adults, it is difficult to determine whether DO that occurs with obstruction is related to the obstruction or is simply coexistent owing to its increased prevalence with age, even in non-obstructed individuals. Nonetheless, DO is more common among obstructed patients, even older ones, (Resnick et al, 1995) and can cause incontinence. In most women, urethral elasticity decreases with age. In a small proportion of older women, this reduced elasticity may be compounded by fibrotic changes associated with atrophic vaginitis and can result in moderate urethral stenosis. Frank outlet obstruction, however, is as rare in older women as in younger women. When present, it is usually due to kinking associated with a large cystocele or to obstruction following bladder neck suspension. Rarely, bladder neck obstruction or a bladder calculus is the cause.
Detrusor underactivity is usually idiopathic, but more information on this condition is emerging (Taylor and Kuchel, 2006). In the absence of obstruction or overt neuropathy, detrusor underactivity is characterized at the cellular level by widespread degenerative changes of both muscle cells and axons, without accompanying regenerative changes (Elbadawi et al, 1993b; Hindley et al, 2002). When it causes incontinence, detrusor underactivity is associated with overflow incontinence (<10% of geriatric incontinence) (Diokno et al, 1988; Resnick, 1988; Resnick et al, 1989). Owing to the age-related decline in sphincter strength, however, the postvoid residual (PVR) volume in women with overflow incontinence is often lower than in younger women. A mild degree of bladder weakness occurs quite commonly in older individuals. Although insufficient to cause incontinence, it can complicate treatment of other causes (see Therapy section).
Causes Unrelated to the Lower Urinary Tract (“Functional” Incontinence)
“Functional” incontinence is often cited as a distinct type of geriatric incontinence and attributed to deficits of cognition and mobility. This concept is problematic for several reasons (Resnick and Marcantonio, 1997). First, “functional incontinence” implies that urinary tract function is normal, but studies of both institutionalized and ambulatory elderly reveal that normal urinary tract function is the exception even in continent subjects, and is rarely observed in the incontinent elderly (Ouslander et al, 1986; Resnick, 1988; Resnick et al, 1989; Resnick et al, 1995). Second, incontinence is not inevitable with either dementia or immobility. We found that 17% of the most severely demented institutionalized residents (mean age 89) were continent and, if they could merely transfer from a bed to a chair, nearly half were continent (Resnick et al, 1988). Third, because functionally-impaired individuals are the most likely to suffer from factors causing transient incontinence (Resnick et al, 1988; DuBeau and Resnick, 1995; Skelly and Flint, 1995; Brandeis et al, 1997), a diagnosis of functional incontinence may result in failure to detect reversible causes of incontinence. Finally, functionally-impaired individuals may still have obstruction or stress incontinence and benefit from targeted therapy (Resnick, 1988; Resnick et al, 1989; Gormley et al, 1993; DuBeau and Resnick, 1995).
Nonetheless, the importance of functional impairment as a factor contributing to incontinence should not be underestimated, because incontinence is also affected by environmental demands, mentation, mobility, manual dexterity, medical factors, and motivation (Jenkins and Fultz, 2005; Cigolle et al, 2007; Kikuchi et al, 2007) Although lower urinary tract function is rarely normal in such individuals, these factors are important to keep in mind because small improvements in each may markedly ameliorate both incontinence and functional status. In fact, once one has excluded causes of transient incontinence and serious underlying lesions, addressing causes of functional impairment may obviate the need for further investigation.
Key Points: Established Incontinence
Diagnostic Approach
Evaluation
The evaluation should identify transient and established causes of incontinence, assess the patient’s environment and available support, and detect uncommon but serious conditions that may underlie incontinence, including lesions of the brain and spinal cord, carcinoma of the bladder or prostate, hydronephrosis, bladder calculi, detrusor-sphincter dyssynergia, and decreased bladder compliance. Assessment must be tailored to the patient’s clinical status and goals, and tempered by the realization that not all detected conditions can be cured, that simple interventions may be effective even in the absence of a diagnosis, and that for many elderly patients, diagnostic tests are themselves often interventions. Because the evaluation generally requires a comprehensive approach, it should be conducted over more than one visit to ease the burden and obviate further evaluation in those who respond to simple measures.
History
In addition to the assessment outlined in Chapter 64, evaluation of the older patient should search for transient causes of incontinence (including nonprescribed medications) and functional impairment. It should be augmented by medical records, as well as input from caregivers. Functional assessment focuses on both basic activities of daily living (ADLs: i.e., transferring from a bed, walking, bathing, toileting, eating, and dressing) and more advanced “instrumental” activities of daily living (IADLs: i.e., shopping, cooking, driving, managing finances, using the telephone). The assessment is accomplished by using a questionnaire, which can be completed by the patient or caregiver prior to the evaluation, and by objective evaluation of the patient from the beginning of the encounter, noting affect, mobility, ability to sit and rise from a chair, ability to provide a coherent history, and amount of time and assistance required to dress and undress.
Of course, as for younger individuals, it also is important to characterize the voiding pattern and the type of incontinence. Although the clinical type of incontinence most often associated with detrusor overactivity (DO) is urge incontinence, “urge” is neither a sensitive nor specific symptom; it is absent in 20% of older patients with detrusor overactivity, and the figure is higher in demented patients (Resnick et al, 1989). “Urge” is also reported commonly by patients with stress incontinence, outlet obstruction, and overflow incontinence.
A better term for the symptom associated with DO is “precipitancy,” which can be defined in two ways. For patients with no warning of imminent urination (“reflex” or “unconscious” incontinence), the abrupt gush of urine in the absence of a stress maneuver can be termed precipitant leakage, and it is almost invariably due to DO. For those who do sense a warning, it is of less value to focus on the leakage, because the presence and volume of leakage in this situation depend on bladder volume, amount of warning, toilet accessibility, the patient’s mobility, and whether the patient can overcome the relative sphincter relaxation accompanying detrusor contraction (Dyro and Yalla, 1986). Instead, precipitancy should be defined as the abrupt sensation that urination is imminent, whatever the interval or amount of leakage that follows; defined in these two ways, precipitancy is both a sensitive and specific symptom (Resnick, 1990).
Similar to the situation for urgency, other symptoms ascribed to DO also can be misleading in the older person unless explored carefully. Urinary frequency (greater than seven diurnal voids) is common (Brocklehurst et al, 1968; Diokno et al, 1986; Resnick, 1988), and may be due to voiding habit, preemptive urination to avoid leakage, overflow incontinence, sensory urgency, a stable but poorly compliant bladder, excessive urine production, depression, anxiety, or social reasons (Resnick, 1990). Conversely, incontinent individuals may severely restrict their fluid intake so that even in the presence of DO they do not void frequently. Thus the significance of urinary frequency, or its absence, can be determined only in the context of more information.
Nocturia also can be misleading unless it is first defined (e.g., two episodes may be normal for the individual who sleeps 10 hours but not for one who sleeps 4 hours) and then approached systematically (Table 76–3). The three general reasons for nocturia—excessive urine output, sleep-related difficulties, and urinary tract dysfunction—can be differentiated by careful questioning and a voiding diary that includes voided volumes (Fig. 76–3). The record of voided volumes is inspected to determine the available bladder capacity (the largest single voided volume) and then compares this capacity to the volume of each nighttime void. For instance, if the available bladder capacity is 400 mL and each of three nightly voids is approximately 400 mL, the nocturia is due to excessive production of urine at night. If the volume of most nightly voids is much smaller than bladder capacity, nocturia reflects either: (1) a sleep-related problem (the patient voids because she is awake anyway), or (2) a problem with the lower urinary tract. Similar to excess urine output, sleep-related nocturia may also be due to treatable causes, including age-related sleep disorders, pain (e.g., bursitis, arthritis), dyspnea, depression, caffeine, or a short-acting hypnotic (e.g., triazolam). Bladder-related causes of nocturia are displayed in the table. Whatever the cause, the nocturnal component of incontinence can generally be ameliorated.
| Volume Related |
| Sleep Related |
| Lower Urinary Tract–Related |
COX-2, cyclooxygenase; NSAIDs, nonsteroidal anti-inflammatory drugs.
Adapted from Resnick NM. Noninvasive diagnosis of the patient with complex incontinence. Gerontology 1990;36(Suppl. 2):8–18.
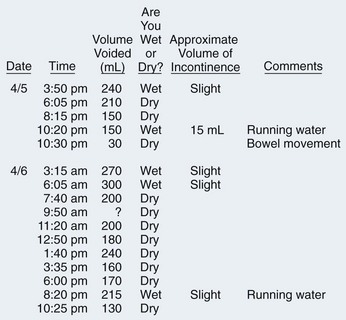
Figure 76–3 Voiding diary of an incontinent 75-year-old man. Urodynamic evaluation excluded urethral obstruction and confirmed a diagnosis of detrusor hyperactivity with impaired contractility (DHIC). Note the 24-hour urine output of nearly 3 L, due to the belief that drinking 10 glasses of fluid/day was “good for my health.” (He did not mention this until queried about the voiding record). Given the typical voided volume of 150 to 250 mL and a measured postvoid residual (PVR) of 150 mL, excess fluid intake was overwhelming his usual bladder capacity of 400 mL (150 + 250 mL). Although involuntary bladder contractions were present, the easily reversible volume component of the problem—combined with the risk of precipitating urinary retention with an anticholinergic agent—prompted treatment with volume restriction alone. After daily urinary output dropped to 1500 mL, frequency abated and incontinence resolved.
(Adapted from DuBeau CE, Resnick NM. Evaluation of the causes and severity of geriatric incontinence: a critical appraisal. Urol Clin North Am 1991;18:243–56.)
The symptoms of “prostatism” also warrant comment. Owing to the high prevalence of medication use, nocturnal polyuria (Reynard et al, 1998), constipation, and DHIC, as well as the impairment of bladder contractility that accompanies aging, “prostatic” symptoms are even less specific in older men than in younger men (DuBeau and Resnick, 1991).
When asking about leakage with stress maneuvers, it is important to ensure that the absence of such leakage is not due to simply the lack of coughing or sneezing. For those without such precipitants, it is useful to inquire about instantaneous leakage with lifting or bending over to put on a shoe or stockings.
Finally, patients or their caregivers should be asked which voiding symptom is most bothersome. For example, although a woman may have both stress and urge incontinence, the urge component may be her worst problem and should become the focus of evaluation and treatment. A man with “prostatism” may be most bothered by nocturia (DuBeau et al, 1995), which may be remedied without any consideration of his prostate (see Fig. 76–3). Failure to address symptom bother can lead to frustration for patient and provider alike.
Voiding Diary
One of the most helpful components of the history is the voiding diary. Kept by the patient or caregiver for 48 to 72 hours, the diary records the time of each void and incontinent episode. No attempt is made to alter voiding pattern or fluid intake. Many formats have been proposed; a sample is shown in Figure 76–3.
To record voided volumes at home, individuals use a measuring cup, coffee can, pickle jar, or other large-mouth container. Information regarding the volume voided provides an index of functional bladder capacity and, together with the pattern of voiding and leakage, can suggest the cause of leakage. For example, incontinence occurring only between 8 AM and noon may be caused by a morning diuretic. Incontinence that occurs at night in a demented man with congestive heart failure, but not during a 4-hour nap in his wheelchair, is likely due to neither dementia nor prostatic obstruction but to postural diuresis associated with his heart failure. A woman with volume-dependent stress incontinence may leak only on the way to void after a full night’s sleep, when her bladder contains greater than 400 mL—more than it ever does during her continent waking hours. A patient with impaired mobility may become incontinent if polyuria develops.
The voiding diary should also guide therapy. For instance, in a patient with DO or prostatic obstruction, excess nocturnal excretion may result in nocturnal incontinence, which is more severe and troublesome than daytime leakage; successful therapy must address the excess excretion. By contrast, another patient with the same urinary tract dysfunction and excretion—but with the ability to hold more urine when asleep—might be bothered more by daytime leakage. Shifting nocturnal excretion to the daytime will exacerbate his problem.
Targeted Physical Examination
Similar to the history, the physical examination is essential to detect transient causes, comorbid disease, and functional impairment. In addition to the standard neurourologic examination, one should check for signs of neurologic diseases that are more common in older people, such as delirium, dementia, stroke, Parkinson disease, cord compression, and neuropathy (autonomic or peripheral), as well as for atrophic vaginitis and general medical illnesses, such as heart failure and peripheral edema. The rectal exam checks for fecal impaction, masses, sacral reflexes, symmetry of the gluteal creases, and prostate consistency and nodularity; as noted in Chapter 92, the palpated size of the prostate is unhelpful. Many neurologically unimpaired elderly patients are unable to volitionally contract the anal sphincter, but, if they can, it is evidence against a spinal cord lesion. The absence of the anal wink is not necessarily pathologic in the elderly, nor does its presence exclude an underactive detrusor (due to diabetic neuropathy, for example).
Stress Testing and PVR Measurement
Several caveats apply to performing the stress test and measuring the PVR in older patients. Stress testing is performed optimally when the bladder is full, and the patient is relaxed (check the gluteal folds to corroborate) and in as close to the upright position as possible. The cough or strain should be vigorous and single, so one can determine whether leakage coincides with the increase in abdominal pressure or follows it. Stress-related leakage may be missed if any of these conditions is not met. Delayed leakage typical of stress-induced DO should be differentiated from leakage typical of stress incontinence, which is instantaneous and ceases as soon as abdominal pressure declines. To be useful diagnostically, leakage must replicate the symptom for which help is sought, because many older women have incidental but not bothersome leakage of a few drops. The test should not be performed if the patient has an abrupt urge to void because this is usually due to an involuntary detrusor contraction that will lead to a falsely positive stress test. Falsely negative tests occur when the patient fails to cough vigorously or to relax the perineal muscles, the bladder is not full, or the test is performed in the upright position in a woman with a large cystocele (that kinks the urethra). If performed correctly, the stress test is reasonably sensitive and quite specific (>90%) (Hilton and Stanton, 1981; Diokno, 1990; Kong et al, 1990).
Following the stress test, the patient is asked to void into a receptacle, and the PVR is measured. If the stress test was negative, the history suggests stress incontinence, and the combined volume of the void and PVR is less than 200 mL, the bladder should be filled with sterile fluid so that the stress test can be repeated at an adequate volume. There is no need to repeat a correctly performed positive stress test. Neither is there a need to repeat it in a woman whose history is negative for stress-related leakage; the sensitivity of the history for stress incontinence, unlike its specificity, exceeds 90% (Diokno et al, 1987; Jensen et al, 1994), making the likelihood of stress incontinence rare in this situation.
Optimally, the PVR is measured by portable ultrasonography, and within 5 minutes of voiding. Measuring it after an intentional void is better than after an incontinent episode, because many patients are able to partially suppress the involuntary contraction during the episode, and more than the true PVR remains. In cognitively impaired patients this may not be possible. Nonetheless, because the resulting artifact will lead to a falsely elevated PVR, a low value is still useful. The PVR will also be spuriously high if measurement is delayed (especially if the patient’s fluid intake was high or included caffeine), the patient was inhibited during voiding, or there is discomfort due to urethral inflammation or bladder infection. It will be spuriously low if the patient augmented voiding by straining (most important in women). If a catheter is used, the PVR will be spuriously low if the catheter is withdrawn too quickly or if the woman has a cystocele that allows urine to “puddle” beneath the reach of the catheter. Of note, relying on the ease of catheterization to establish the presence of obstruction can be misleading, because difficult catheter passage may be caused by urethral tortuosity, a “false passage,” or catheter-induced spasm of the distal sphincter, although catheter passage may be easy even in obstructed men (Klarskov et al, 1987).
Two other tests should be mentioned. The Q-tip (cotton swab) test for pelvic floor laxity is of little value in determining the cause of a patient’s leakage, and has a high false-negative rate in elderly women (DuBeau and Resnick, 1991). The Bonney (or Marshall) test is also of limited usefulness in the elderly, because vaginal stenosis is common and may lead to a false-positive result by precluding accurate finger placement. Furthermore, even if the test is performed correctly, a false positive result may occur if the first episode of leakage was due to a cough-induced detrusor contraction that, having emptied the bladder, does not recur during bladder base elevation.
Laboratory Investigation
(Resnick and Ouslander, 1990; DuBeau and Resnick, 1991; Fantl et al, 1996). BUN, creatinine, urinalysis, urine culture, and PVR should be checked in all older patients. Serum sodium, calcium, and glucose should be measured in patients with confusion. If the voiding record suggests polyuria, serum glucose and calcium (and albumin, to allow calculation of free calcium levels in sick or malnourished patients) should be determined. Sterile hematuria suggests partially/recently treated bacteriuria, malignancy, or calculus; tuberculosis should also be considered, because the elderly, particularly institutionalized residents, are an unappreciated reservoir of this infection (Stead, 1985). Finally, it is important to recognize when evaluating renal function that the age-related decline in glomerular flow rate (GFR)—30% by the 8th decade—is not associated with an increase in creatinine because of a concomitant decrease in muscle mass; thus normal creatinine levels do not imply a normal GFR.
Empiric Diagnostic Categorization
After transient and serious causes have been addressed, the optimal diagnostic strategy for persistent incontinence is unknown (Resnick and Ouslander, 1990; Fantl et al, 1996; Abrams et al, 2005; Fonda et al, 2005). “Bedside” cystometry has been proposed, but its utility is limited because it misses low-pressure contractions of DHIC; its feasibility and accuracy are low in frail elderly (Ouslander and Colling, 1992); and detected DO may be either incidental and unrelated to leakage, or due to urethral obstruction or incompetence and warrant different therapy. The following approach (Resnick, 1995), although still unproven, is relatively noninvasive, accurate, cost effective, and easily tolerated. A similar approach forms the basis for the Agency for Health Care Policy Research (AHCPR) Clinical Practice Guideline (Fantl et al, 1996), the International Consultation on Incontinence (Fonda et al, 2005), as well as the Minimum Data Set/Resident Assessment Instrument, which we designed and validated for use in all American nursing homes (Resnick et al, 1996b).
The first step is to identify individuals with overflow incontinence (e.g., PVR > 450 mL). Because obstruction and underactive detrusor cannot be differentiated clinically (Resnick et al, 1996b; DuBeau et al, 1998; Sonke et al, 2000), further assessment is warranted for those in whom it would affect therapy, while catheterization should be used for the rest. For the remaining 90% to 95% of patients, the next step depends on their sex. In women, the differential diagnosis is generally between stress incontinence and DO, because obstruction is rare in the absence of previous bladder neck suspension or prolapsing cystocele. If the contemplated intervention is nonoperative, the diagnosis usually can be established on clinical grounds alone, informed by the caveats mentioned earlier.
In men, stress incontinence is uncommon and, when present, results in a characteristic drip (similar to a “leaky faucet”) that is exacerbated by standing or straining. Thus the usual problem in men is differentiating unobstructed DO from obstruction. Uroflowmetry is helpful, but only if peak flow is normal (e.g., >12 mL/sec for voided volume of 200 mL); the age-related decrease in bladder contractility means that a normal unstrained flow rate, together with PVR <100 mL, effectively excludes clinically significant obstruction in an older man (DuBeau et al, 1998). The next step is to search for hydronephrosis in men whose PVR exceeds 200 mL and to decompress those in whom it is found (DuBeau and Resnick, 1992). Further evaluation is also reasonable for men without hydronephrosis who are appropriate candidates and would be amenable to surgery if obstructed. For the rest, it seems sensible to treat those with urge incontinence for presumed DO, provided they are compliant and can be taught signs of incipient urinary retention; bladder relaxants probably should be avoided in those with significantly elevated PVR (e.g., ≥ 150 mL). A similar approach is advocated for cognitively impaired men who can be closely observed (e.g., institutionalized residents) (Resnick et al, 1996b). Men without urge incontinence, those who fail empiric therapy, and those who are cognitively impaired and less supervised should be evaluated further if findings would affect therapy.
Urodynamic Testing
Although its precise role in the elderly is unclear, multichannel urodynamic evaluation is probably warranted when diagnostic uncertainty may affect therapy and when empiric therapy has failed and other approaches would be tried. Because conditions that closely mimic obstruction and stress incontinence are so common in the elderly, including altered fluid excretion (Reynard et al, 1998), medication use, DH, and DHIC (Resnick et al, 1996a), urodynamic corroboration of the diagnosis is strongly recommended if surgery will be performed (Resnick et al, 1991; Fantl et al, 1996; Resnick and Baumann, 1997). Whatever its role, however, urodynamic evaluation of even frail elderly patients is reproducible, safe, and feasible (Resnick et al, 1987; Resnick et al, 1989).
Key Points: Diagnostic Approach
Therapy
Similar to the diagnostic approach, treatment must be individualized, because factors outside the lower urinary tract so often affect therapeutic feasibility and efficacy. For instance, although both may have detrusor overactivity that can be managed successfully, a severely demented and bedfast woman must be treated differently from one who is ambulatory and cognitively intact. This section and Table 76–4 outline several treatments for each condition and provide guidance for their use. It is assumed that serious underlying conditions, transient causes of incontinence, and functional impairments have already been addressed.
Table 76–4 Stepwise Approach to Treatment of Urinary Incontinence*
| CONDITION | CLINICAL TYPE OF INCONTINENCE† | TREATMENT |
|---|---|---|
| Detrusor overactivity with normal contractility (DO) | Urge | 1. Bladder retraining or prompted voiding regimens |
| 2. ± Bladder relaxant medication, if needed and not contraindicated (see drug list below) | ||
| 3. Indwelling catheterization alone is often unhelpful because detrusor “spasms” often increase, leading to leakage around the catheter | ||
| 4. In selected cases, induce urinary retention pharmacologically and add intermittent or indwelling catheterization‡ | ||
| Detrusor hyperactivity with impaired contractility (DHIC) | Urge§ | 1. If bladder empties adequately, behavioral methods (as above) ± bladder relaxant medication (low doses; especially feasible if sphincter incompetence coexists) |
| 2. If residual urine ≥150 mL, augmented voiding techniques‖ or intermittent catheterization (± bladder relaxant medication). If neither feasible, undergarment or indwelling catheter‡ | ||
| 3. In selected cases, induce urinary retention pharmacologically and add intermittent or indwelling catheterization‡ | ||
| Stress incontinence | Stress | 1. Conservative methods (weight loss if obese; treatment of cough or atrophic vaginitis; physical maneuvers to prevent leakage [e.g., tighten pelvic muscles before cough, cross legs]; occasionally, use of tampon or pessary is useful) |
| 2. If leakage threshold ≥150 mL identified, adjust fluid excretion and voiding intervals appropriately | ||
| 3. Pelvic muscle exercises ± biofeedback/weighted intravaginal “cones”; must continue indefinitely | ||
| 4. Surgery (sling, artificial sphincter, periurethral bulking injections) | ||
| Urethral obstruction | Urge/overflow¶ | 1. Conservative methods (including adjustment of fluid excretion, bladder retraining/prompted voiding) if hydronephrosis, recurrent symptomatic UTI, and gross hematuria have been excluded |
| 2. α-Adrenergic antagonist | ||
| 3. Also consider adding a bladder relaxant if DO coexists, PVR is small, and surgery not desired/feasible; monitor PVR! | ||
| 4. Finasteride, if not contraindicated and the patient either prefers it or is not a surgical candidate | ||
| 5. Surgery (incision, prostatectomy) is an effective alternative before or after these steps | ||
| Underactive detrusor | Overflow | 1. Decompress for at least several days (the larger the PVR, the longer should be the decompression [up to a month]) and then perform a voiding trial |
| 2. Exclude urethral obstruction if this has not already been done | ||
| 3. If cannot void or PVR remains large, try augmented voiding techniques‖ | ||
| 4. ± α-Adrenergic antagonist, but only if some voiding possible; bethanechol rarely useful | ||
| 5. If fails, or voiding is not possible, intermittent or indwelling catheterization‡ |
Stress: Leakage that coincides instantaneously with stress maneuvers, in the absence of urinary retention or detrusor contraction.
Overflow: Frequent leakage of small amounts associated with urinary retention.
PVR, Postvoid residual; UTI, urinary tract infection.
* These treatments should be initiated only after adequate toilet access has been ensured, contributing conditions have been treated (e.g., atrophic vaginitis, UTI, fecal impaction, heart failure), fluid management has been optimized, and unnecessary or exacerbating medications have been addressed. For additional details, see text.
† Urge: Leakage in the absence of stress maneuvers and urinary retention, usually preceded by abrupt onset of desire to void.
‡ UTI prophylaxis can be used for recurrent symptomatic UTIs, but only if catheter is not indwelling.
§ May also mimic stress or overflow incontinence.
‖ Augmented voiding techniques include Credé (application of suprapubic pressure) and Valsalva (straining) maneuvers, and “double” voiding. They should be performed only after voiding has begun.
¶ Also can cause postvoid “dribbling” alone, which is treated conservatively (e.g., by sitting to void and allowing more time, “double voiding,” and in men by gently “milking” the urethra after voiding).
Adapted and updated in 2010 from Resnick NM. Voiding dysfunction and urinary incontinence. In: Beck JC, editor. Geriatric review syllabus. New York: American Geriatrics Society; 1991. p. 141–54.
It cannot be overemphasized that successful treatment of established incontinence, especially in the elderly, is usually multifactorial and requires addressing factors beyond the urinary tract. Figure 76–4, based on empiric data, illustrates the many factors that determine whether DO in an older adult will be associated with incontinence and, if so, the frequency of the related leakage (Miller et al, 2002; Rosenberg et al, 2005).
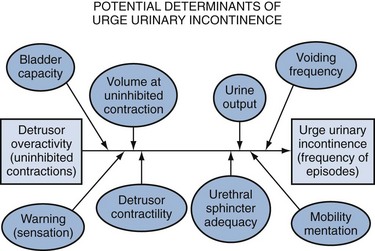
Figure 76–4 Factors that determine whether detrusor overactivity, found in nearly half of continent older adults, will be associated with urge incontinence and, if so, the frequency of the related leakage.
(Data from Miller KL, DuBeau CE, Bergmann M, et al. Quest for a detrusor overactivity index. J Urol 2002;167:578–85; Rosenberg LJ, Griffiths DJ, Resnick NM. Factors that distinguish continent from incontinent older adults with detrusor overactivity. J Urol 2005;174:1868–72.)
Detrusor Overactivity
The initial approach to detrusor overactivity is to identify and treat its reversible causes. Unfortunately, many of its causes are not amenable to specific therapy or a cause may not be found, so treatment usually must be symptomatic. Simple measures, such as adjusting the timing or amount of fluid excretion (see Fig. 76–3) or providing a bedside commode or urinal are often successful. If not, the cornerstone of treatment is behavioral therapy, which can be effective and avoids drug side effects. Some evidence suggests that it also may be more durable than drug therapy (Kafri et al, 2008). If the patient can cooperate, bladder training regimens will extend the voiding interval (Fantl et al, 1991; Burgio et al, 1998; Berghmans et al, 2000; Payne, 2000; Burgio et al, 2002). For instance, if the voiding record documents incontinence when the interval exceeds 3 hours, the patient is instructed to void every 2 hours and suppress urgency in between. Once dry, the patient can extend the interval by half an hour, and repeat the process until a satisfactory result or continence is achieved. Patients need not follow this regimen at night, because nighttime improvement parallels daytime success (Johnson et al, 2005). Biofeedback may be added (Berghmans et al, 2000), but its marginal benefit is unclear (Resnick, 1998; Burgio et al, 2002; Wilson et al, 2005; Shamliyan et al, 2008; Hartmann et al, 2009).
For cognitively impaired patients, “prompted voiding” is used (Fantl et al, 1996; Tannenbaum and DuBeau, 2004). Asked every 2 hours whether they need to void, patients are escorted to the toilet if the response is affirmative. Positive verbal reinforcement is employed, and negative comments are avoided. Prompted voiding reduces incontinence frequency in nursing homes by roughly 50%, and leakage can be virtually eliminated during daytime hours in one third of residents (Hu et al, 1989; Engel et al, 1990; Schnelle, 1990; Fink et al, 2008). The latter group can be identified within three days. When prompted hourly to void, they urinate into a toilet or commode more than two thirds of the time that they indicate the need to do so, or they become continent on more than 80% of checks. Response is maintained when the prompting interval is increased to two hours. Half of the remaining patients also improve with prompting, but they are still wet more than once during the daytime. For the quarter of patients who do not respond to prompting at baseline, little benefit is obtained by further prompting. Importantly, response does not correlate with the degree of dementia. In addition, these results were obtained without drugs, and urodynamic evaluations were not performed (Schnelle, 1990; Ouslander et al, 1995). Two additional studies found that adding oxybutynin or tolterodine improved results still further (Ouslander et al, 2001b; Fink et al, 2008).
The voiding record also can be helpful if it reveals that nocturnal incontinence correlates with nocturnal diuresis. If due to systolic congestive heart failure, it should improve with diuretic therapy. If due to obstructive sleep apnea, it may respond to continuous positive airway pressure (Guilleminault et al, 2004). If due to peripheral edema in the absence of heart failure and hypoalbuminemia (i.e., venous insufficiency), it should respond to pressure gradient stockings. If not associated with peripheral edema, it may respond to alteration of the pattern of fluid intake or to administration of a rapidly acting diuretic in the late afternoon or early evening (Pedersen and Johansen, 1988; Reynard et al, 1998). For patients with DHIC, whose voiding record and PVR suggest that involuntary detrusor contractions are provoked only at high bladder volume, augmented voiding techniques or catheterization at bedtime will remove the residual urine, thereby increasing functional bladder capacity and restoring both continence and sleep.
Drugs may augment behavioral intervention but do not supplant it, because they generally do not abolish involuntary detrusor contractions. Moreover, combining the two interventions generally improves outcome (Burgio et al, 2000; Mattiasson et al, 2003; Alhasso et al, 2006; Burgio et al, 2008). Timed toileting or bladder retraining in conjunction with a bladder relaxant is especially useful for older adults who have little warning before detrusor contraction (Wagg and Malone-Lee, 1998).
There are few data on comparative efficacy or toxicity of standard incontinence drugs in the elderly, but available studies show similar efficacy for most agents, except flavoxate, which fares poorly in controlled trials; (Fantl et al, 1996; Andersson et al, 2009a; Hartmann et al, 2009) no controlled data are available for hyoscyamine in any adult age group.
Data regarding use in the elderly are most extensive for oxybutynin and tolterodine. This includes dozens of trials that have included the elderly, several that have stratified results by age, and several that have focused exclusively on older adults, including patients who were frail and/or reside in nursing homes (Zinner et al, 2002; Ouslander, 2004; Sand et al, 2004; Sand et al, 2006; Lackner et al, 2008; Andersson et al, 2009a; DuBeau et al, 2009; Griebling et al, 2009; Hartmann et al, 2009). Such trials have established that these agents are likely as effective for improving incontinence and quality of life in older adults as they are in younger individuals and, especially in lower doses, as tolerable. Thus oxybutynin and tolterodine should be considered first-line pharmacotherapy in this population. As for any drug used in older adults, therapy should start with a low dose that is increased slowly, consistent with the agent’s pharmacokinetics and pharmacodynamics in older patients. Given the marked variability, however, dose escalation should continue until either a successful end point is reached or an intolerable side effect is encountered. Experience suggests that for oxybutynin IR, most older adults will respond best to 2.5 mg 3 to 4 times daily; for oxybutynin ER, 5 to 15 mg is optimal. At larger doses, side effects increase much more than therapeutic response. Immediate release oxybutynin, which has a short half-life, can be employed prophylactically if incontinence occurs at predictable times. Intravesical instillation of these agents is also effective in younger patients, but the need for self-catheterization makes this strategy less useful in the elderly.
The only currently used agents that have been evaluated in the nursing home setting are the immediate release formulations of tolterodine (1 to 2 mg twice daily) and oxybutynin (2.5 mg 3 to 4 times daily). Both have been evaluated during daytime hours when staff is available (7 AM to 7 PM). Overall, the reported reduction in leakage frequency is roughly 25%; roughly one third of such patients experience a decrease of at least 33%, and about 10% become continent during that time period (Ouslander et al, 2001b; DuBeau et al, 2009).
Antimuscarinic agents used to treat UI can induce confusion in older adults, but the incidence is rare, both in trials and in practice, and the very small risk should not preclude prescribing them for older adults, even those who are frail and/or cognitively impaired. There is justifiable concern about prescribing anticholinergic drugs in the elderly. CNS levels of acetylcholine decline with age and decline still further in the presence of many diseases. Many drugs prescribed for the elderly have anticholinergic properties, and anticholinergic agents are one of the most common causes of delirium in this age group, especially among individuals with preexisting cognitive, sensory, affective, or functional impairment. In addition, all currently-available agents used for incontinence either require metabolism by the hepatic cytochrome P450 system and/or renal excretion. Each of these functions declines with age and can be further impaired by other drugs and diseases.
Theoretical arguments have been marshaled for using one antimuscarinic over another. These are largely based on the drug’s characteristics, including muscarinic subtype affinity, lipophilicity, polarity, molecular size, protein binding, drug half-life, and active and passive transport across the blood-brain barrier. Also cited are experimental studies that observed a short-term impact on some components of detailed neuropsychological testing (Klausner and Steers, 2007; Kay and Ebinger, 2008), when continent volunteers were given doses of oxybutynin that are likely too high for older adults.
Yet understanding of the role and importance of CNS muscarinic subtypes in humans is still limited, and the blood brain barrier can be compromised by age and other drugs, as well as by a host of diseases that are common in the elderly, including diabetes, Alzheimer disease, stroke, Parkinson disease, and trauma (Klausner and Steers, 2007; Kay and Ebinger, 2008). The resulting permeability changes may dwarf the differences observed in experiments that involved younger and healthier elderly subjects. In addition, neuropsychologic impairments observed in older volunteers have generally been mild, and the subjects have been unaware of them, making it difficult to assess their relevance. Because the studies to date also have been short term, have been conducted in artificial conditions, and have used high doses of oxybutynin, it is impossible to know whether such effects would persist with chronic treatment. It is also worth noting that even darifenacin, which is widely marketed as being free of such effects, has been associated with significantly slowed reaction times in the Divided Attention Test (Kay et al, 2006), which could potentially predispose to problems in tasks that require such attention, such as driving and walking without falling.
Moreover, after decades of widespread use, evidence of clinical cognitive impairment still comprises only a handful of case reports, and clinical trials—which have involved relatively large numbers of older individuals—have observed only rare instances of drug-associated cognitive impairment (Tsao and Heilman, 2003; Andersson, 2004; Ouslander, 2004). For instance, analysis of the OPERA trial, which included approximately 120 patients over age 75 years, disclosed only 1 of 390 patients on oxybutynin 10 mg/day ER who reported confusion, and it was judged “mild” because it did not interfere with activities or require intervention; none of the 399 patients on tolterodine 4 mg LA reported confusion (Chu et al, 2005). Another study randomized 50 cognitively impaired nursing home residents, average age 89 years, to receive extended release oxybutynin 5 mg or placebo for 4 weeks. No changes were found in cognitive test performance, nor were there any cases of delirium. Another group of 48 nursing home residents responded well to tolterodine 1 to 2 mg administered twice daily in an 8-week trial. Side effects were minimal, and only 1 patient experienced worsening of baseline confusion (Ouslander et al, 2001b). In summary, the risk of drug-induced cognitive impairment appears to be real but small.
There is also a theoretical concern about prescribing an anticholinergic bladder relaxant to a patient taking a cholinesterase inhibitor. Fortunately, this has not proved to be a problem in clinical practice. Although a few case reports have appeared (Siegler and Reidenberg, 2004), neither a large observational nursing home study (Sink et al, 2008) nor a recent case series of 26 demented patients (Sakakibara et al, 2005) noted a deterioration in mental status when patients were also prescribed an antimuscarinic drug for incontinence. Furthermore, in some situations, the clinician and caregiver will decide that the positive impact on the incontinence warrants continuation of both agents regardless.
Finally, there is concern that an antimuscarinic-induced increase in heart rate may be dangerous. Apart from terodiline (which was withdrawn), however, there are no data to substantiate this concern at present, although the risk cannot yet be completely excluded. Among patients with established heart disease (who are generally not taking an anticholinergic agent), increased heart rates are associated with increased mortality. However, the risk in such patients likely reflects the extent of intrinsic heart disease and/or the drugs used to treat it. Heart rate increases induced by antimuscarinics are generally less than or equal to 5 beats/minute. An increase of this magnitude is of unknown significance and, to date, has not been associated with an increased mortality rate in antimuscarinic trials. Studies have also revealed little impact on the electrocardiogram (ECG) or Q-T interval (other than with terodiline). Moreover, because vagal influence on heart rate is substantially less in older adults than in younger adults, the likelihood of inducing an increased heart rate is less, especially because the appropriate dose of an antimuscarinic is also lower in the elderly.
Newer agents include darifenacin, solifenacin, trospium, and fesoterodine. Similar to the extended-release preparations of oxybutynin and tolterodine, and the newer preparations of oxybutynin (patch and gel), each is more convenient and better tolerated than the immediate release versions of oxybutynin and tolterodine. Not surprisingly, however, data for older people taking these drugs are still limited. To date, the only controlled trial of darifenacin in older adults focused on a relatively young (mean age 72 years) and fit group. Although the group randomized to placebo was younger than the darifenacin group (31% >75 years vs. 43%), urge incontinence improved similarly in both (Chapple et al, 2007). The only controlled data of solifenacin in older adults comprises a secondary analysis of pooled Phase 3 data, which revealed efficacy and safety similar to that seen among younger enrollees (Wagg et al, 2006); however, a higher rate of adverse effects was documented in a 12-week postmarketing surveillance study, especially among patients over age 80 years who were also taking multiple other medications (Michel et al, 2008). No controlled studies of fesoterodine or trospium have yet stratified the results by age, despite marketing for the latter agent that emphasizes its theoretical advantage in older adults as being less likely to cross the blood-brain barrier. Regardless, each agent has theoretical benefits and drawbacks for older adults; Table 76–5 notes some of the relevant caveats.
Table 76–5 Bladder Relaxant Medications Used to Treat Urge Incontinence*
| MEDICATION CLASS, NAME, AND DOSAGE | COMMENTS |
|---|---|
| Anticholinergic Agents | These agents are now the drugs of choice for older adults. Both oxybutynin and tolterodine have proved effective for older patients in controlled trials when used continuously; less controlled data are yet available for the other agents and still less for their efficacy, side effects, and drug interactions in frail older adults. Benefits of M3 selectivity remain theoretical. Concerns re: CNS side effects of oxybutynin and tolterodine emanate primarily from rare case reports and short-term, artificial experiments and likely are overstated. Immediate release oxybutynin has a rapid onset of action and can be tried prophylactically if incontinence occurs at predictable times. |
|
Darifenacin (7.5-15 mg qd)‡
|
|
| Smooth Muscle Relaxant | Obsolete; has not proved effective in placebo-controlled trials |
| Tricyclic Antidepressants¶ | Older controlled trial data support their use, but they are not first choice. May be helpful in women with coexistent stress incontinence. Orthostatic hypotension often precludes their use, but a tricyclic antidepressant may be preferred for a depressed incontinent patient without risk for orthostatic hypotension. Doxepin has more anticholinergic side effects than imipramine and can cause confusion. |
CNS, central nervous system; IR, immediate release; XL and LA, extended release.
* Each drug should be started at the lowest dose and increased slowly until encountering maximum benefit or intolerable side effects. The highest doses are generally not required and can cause problematic side effects for older patients.
† May also be applied intravesically in patients who can use intermittent catheterization.
‡ Toxicity can occur with concurrent use of potent cytochrome P450 3A4 inhibitors (e.g., amiodarone).
§ Dose reduction required for creatinine clearance ≤30 mL/min (e.g., in an 85-year-old woman with serum creatinine of 1.2 who weighs 50 kg).
‖ Levels may be affected by other commonly used drugs in the elderly such as digoxin or metformin, which compete for renal tubular secretion.
¶ May give as single daily dose of 25-100 mg after determining the optimal dose.
Adapted and updated in 2005 from Resnick NM. Urinary incontinence. Lancet 1995;346:94–9.
Other agents, formerly advocated for older incontinent adults, are no longer advised. Specifically, there are no appropriate trials for hyoscyamine (Roxburgh et al, 2007; Andersson et al, 2009a) and, as the active ingredient in atropine, hyoscyamine has a substantial risk of inducing confusion in older adults. Flavoxate has been not been found effective despite several controlled trials (Roxburgh et al, 2007). Calcium channel blockers, drugs long thought to be theoretically preferable for the elderly, were finally studied in a controlled trial and found to be ineffective (Naglie et al, 2002). For the incontinent and depressed older patient, nortriptyline or imipramine may be considered (amitriptyline and doxepin should not be given to older patients) (Fantl et al, 1996), but because evidence of their efficacy falls short of modern standards, they should be used only if depression has not already responded to an SSRI, there is no evidence of orthostatic hypotension, and the patient can be monitored for orthostasis and falls.
Regardless of which antimuscarinic is used, urinary retention may develop. The PVR and urine output should be monitored, especially with DHIC, in which the detrusor is already weak. Subclinical urinary retention also may develop, reducing available bladder capacity and attenuating, or even reversing, the drug’s benefit. Thus if incontinence worsens as the dose is increased, the PVR should be remeasured. Another reason for drug failure is excess fluid ingestion engendered by anticholinergic-induced xerostomia. For patients whose incontinence defies other remedies (such as those with DHIC), inducing urinary retention and using intermittent catheterization may be viable if catheterization is feasible.
Other remedies for urge incontinence, including electrical stimulation (see Chapter 70) and selective nerve blocks, are successful in selected situations. However, they have not been studied adequately in the elderly, and the limited preliminary data suggest that older patients may not fare as well (Amundsen and Webster, 2002; Amundsen et al, 2005; South et al, 2007; White et al, 2009). Trials of botulinum A toxin (BoNT) intradetrusor injection have included some older adults, but only a single study has focused on older patients (White et al, 2008). In that series, symptoms in 16 of the 21 patients over age 75 improved by at least 50%. However, incontinence was not specifically reported, and patients with PVR greater than 150 mL were excluded, as were those whose bladder failed to empty completely with a detrusor contraction on urodynamic testing. Moreover, a recent randomized clinical trial (RCT) of middle-aged women (Brubaker et al, 2008), mean age 65 years, found a much higher incidence of urinary retention and UTIs than previously reported. In 2009, the Food and Drug Administration (FDA) required a black-box warning for BoNT, owing to the risk of injected toxin migrating and causing paralysis, and even death. Thus BoNT appears promising, but given the very limited experience in older adults, the significant number who have impaired detrusor contractility, the non-feasibility of intermittent catheterization in many older patients, the risk, and the numerous remaining questions regarding optimal patient selection, dose, dilution, and injection site, BoNT is still best viewed as investigational (Brubaker et al, 2008; DuBeau et al, 2009; Hartmann et al, 2009).
Recent studies have suggested that desmopressin is relatively safe for older adults. However, these studies have generally been short term, have included very few subjects older than age 75, have included still fewer with comorbidity or diuretic use, and have sedulously screened and monitored all subjects. Moreover, they also have enrolled only patients who, during a prestudy run-in period, tolerated treatment with desmopressin, responded well to it, and did not lower their serum sodium (Weatherall, 2004). Despite these precautions, hyponatremia still developed in up to 22% of patients. Serum sodium levels decreased to less than 130 mMol/L in one fifth of these patients, almost all of whom were more than 65 years old (Abrams et al, 2002; Weatherall, 2004; Rembratt et al, 2006). More recently, a longer-term study documented the risk of hyponatremia at 6 months, even among patients who did not develop it earlier (Bae et al, 2007). Additional concerns include the fact that many older persons do not develop warning symptoms of hyponatremia, as well as recent data suggesting that even short-term desmopressin therapy can increase calcium excretion and decrease potassium excretion (Chang et al, 2007). If substantiated in longer-term studies, this effect would be problematic for older patients in whom the prevalence of both osteoporosis and renal insufficiency is considerable. Moreover, there is little evidence that vasopressin is effective for geriatric incontinence (Dequecker, 1965; Asplund and Aberg, 1993; Cannon et al, 1999). Thus the high prevalence of contraindications to its use (e.g., hyponatremia, renal insufficiency, heart failure [even subclinical]), the risk of inducing serious hyponatremia with fluid retention (Cannon et al, 1999), the potentially adverse impact on calcium and potassium excretion (Chang et al, 2007), and its lack of demonstrated efficacy all suggest that desmopressin should be used to treat urge incontinence in older adults with extreme caution, if at all.
Adjunctive measures, such as pads and special undergarments, are invaluable if incontinence proves refractory. Many types are now available, allowing the recommendation to be tailored to the individual’s problem (Fader et al, 2007; Fader et al, 2008; Cottenden et al, 2009). Most are included in an illustrated catalog (National Association for Continence (NAFC), PO Box 1019, Charleston, SC 29402; www.nafc.org). For bedridden individuals, a launderable bed pad may be preferable, while for those with a stroke, a diaper or pant that can be opened using the good hand may be preferred. For ambulatory patients with large gushes of incontinence, wood pulp–containing products can be superior to ones containing polymer gel, because the gel generally cannot absorb the large amount and rapid flow, while the wood-pulp product can be doubled up if necessary. Optimal products for men and women differ because of the location of the “target zone” of the urinary loss. Finally, the choice will be influenced by patient preference, cost, and the presence of fecal incontinence.
Condom catheters are helpful for men, but they are associated with skin breakdown, bacteriuria, and decreased motivation to become dry (Ouslander and Schnelle, 1995; Cottenden et al, 2009), and they are not feasible for the older man with a small or retracted penis. External collecting devices have been devised for institutionalized women, but whether they will adhere adequately in more active women remains to be determined. Indwelling urethral catheters are not recommended for detrusor overactivity because they usually exacerbate it. If they must be used (e.g., to allow healing of a pressure sore), a small catheter with a small balloon is preferable to avoid leakage around the catheter; such leakage almost invariably results from bladder contractions rather than from a catheter that is too small. Increasing catheter and balloon size only aggravates the problem and may result in urethral erosion and sphincter incompetence. If spasms persist, drugs such as oxybutynin can be tried. More potent anticholinergic agents, such as belladonna suppositories, should be avoided in the elderly.
Stress Incontinence
Urethral hypermobility, the most common cause of stress incontinence in older women, may be improved by weight loss if the patient is obese, (Brown et al, 2006; Hunskaar, 2008; Subak et al, 2009) by postural maneuvers (Norton and Baker, 1994), by therapy of precipitating conditions such as atrophic vaginitis or cough (e.g., due to an ACE inhibitor), and by insertion of a pessary (Suarez et al, 1991; Zeitlin and Lebherz, 1992; Donnelly et al, 2004; Cottenden et al, 2009). If the voiding diary reveals that leakage is volume dependent, it also may be improved by adjusting fluid excretion and voiding intervals to keep bladder volume below this threshold. However, if the threshold is less than 150 to 200 mL, this strategy, alone, is generally not sufficient.
Pelvic muscle exercises can decrease incontinence substantially in older women who are motivated, cognitively intact, and trained to perform them 30 to 200 times daily (Wells et al, 1991; Burns et al, 1993; Fantl et al, 1996; Goode et al, 2003; Choi et al, 2007; Hay-Smith et al, 2009). Unfortunately, such exercises must be pursued indefinitely, efficacy is limited for severe incontinence, only 10% to 25% of women become fully continent, and many older women are unable or unmotivated to follow such regimens. Adding vaginal cones, biofeedback, or electrical stimulation may enhance efficacy, but their marginal benefit remains unclear (Burns et al, 1993; Fantl et al, 1996; Burgio et al, 2002; Goode et al, 2003; Resnick and Griffiths, 2003; Neumann et al, 2006; Hay-Smith et al, 2009).
Effective pharmacotherapy for stress incontinence in older women remains elusive. Studies document, at best, modest efficacy for any of the available agents, and it is of unknown durability and often associated with significant side effects (Mariappan et al, 2007; Shamliyan et al, 2008; Andersson et al, 2009a). Phenylpropanolamine, the agent for which the most data are available, was withdrawn from the American market owing to reports of a small risk of hemorrhagic stroke (Haller and Benowitz, 2000). The FDA has added a “black-box” warning for duloxetine, and neither the FDA nor the United Kingdom’s National Institute for Clinical Excellence (NICE) agency have approved it to treat stress incontinence (Andersson et al, 2009a). However, for mild to moderate stress incontinence, pseudoephedrine is inexpensive and remains available without prescription. Because it is also contained in nonprescribed remedies for coryza and allergy, and some capsules also contain agents such as chlorpheniramine in doses that can be troublesome for elderly patients, the physician should prescribe the dose and guide the choice of preparation. The physician should also closely supervise its use in patients with hypertension, if it is used at all. Imipramine, which inhibits reuptake of both norepinephrine and serotonin, can also be useful for mild stress incontinence (Lin et al, 1999), but it should be considered only if symptoms and signs of postural hypotension have been excluded. Estrogen monotherapy has not proved effective for stress incontinence (Al-Badr et al, 2003), and although the addition of estrogen to α-adrenergic therapy may improve the latter’s efficacy, the magnitude of the effect is modest at best (Andersson et al, 2009a). Fewer and less promising data are available for pharmacotherapy of stress incontinence in older men.
If these methods fail or are unacceptable, surgical options should be considered. As recent data have challenged the long-term durability of bladder neck suspensions, suburethral slings and midurethral tape slings have become increasingly employed (Anger et al, 2009). Older women generally can tolerate a suburethral sling (Carey and Leach, 2004; Anger et al, 2007), but the ability to perform the midurethral procedures under regional, or even local, anesthesia on an outpatient basis makes them more feasible. In the few studies published to date, success rates for tension-free vaginal tape (TVT) procedures have been lower for older patients than for younger individuals, likely reflecting the higher prevalence of comorbidity, detrusor overactivity, intrinsic sphincter deficiency, decreased detrusor contractility, and prior operations in the elderly (Lo et al, 2002; Gordon et al, 2005; Sharp et al, 2006; Hellberg et al, 2007; Richter et al, 2009; Smith et al, 2009). Nonetheless, reported cure rates in older women have been 80% to 96% within the first year, declining to 60% to 70% at 2 to 3 years. Longer-term data are also available; to date, the lowest reported long-term cure rate is still 55% after a mean of 5 years in a group of 113 women at least age 75 (75 to 91 years) at the time of their TVT (Hellberg et al, 2007). In addition, investigators who have measured both cure and satisfaction have generally found that the level of satisfaction among older patients generally exceeds their cure rate (Sevestre et al, 2003; Tannenbaum et al, 2008; Richter et al, 2009). Complications, such as prolonged urinary retention and obstructive symptoms have generally ranged from 2% to 15%, while reported de novo urgency/DO has tended to be somewhat more common. Whether such results will be mirrored in routine practice remains to be seen, but long-term success rates for procedures available prior to the popularity of these procedures revealed “real-world” success rates of approximately 70% at 2 years and 40% at 10 years for women older than age 70 years (Diokno et al, 2003).
Other treatments for sphincter incompetence include periurethral bulking injections and insertion of an artificial sphincter (Shamliyan et al, 2008). Experience with these approaches has proved less auspicious than originally thought, but older women appear to benefit as much as younger women, although factors predicting success or failure in this group are still not entirely clear, and short-term and long-term data in women older than age 75 are still limited (Winters et al, 2000; Keegan et al, 2007; Smith et al, 2009). Yet, injections have several potential advantages over slings for older women. Injections are less invasive, can be performed in an office under local anesthesia, and are associated with fewer perioperative and postoperative complications. The ideal candidate appears to be an older woman whose mild to moderate stress incontinence is associated with intrinsic sphincter deficiency, absent or minimal detrusor overactivity/urgency, normal to moderately impaired detrusor contractility, and minimal urethral hypermobility; the last factor may be particularly important because it may substantially diminish the likelihood of success with TVT (Liapis et al, 2006). The woman should also understand that repeated injections will likely be required over time and that these may not be as effective as they were initially (Winters et al, 2000).
For men in whom these interventions fail, an artificial urinary sphincter (AUS) and “male sling” are potential options. Unfortunately, there are still little data regarding their use in older men (especially for the sling), for which techniques are still evolving and long-term data are lacking (Herschorn et al, 2009). For those who have mild postprostatectomy incontinence, have a strong detrusor, and have not had prior radiation, the sling is theoretically appealing because it is less invasive than the AUS, avoids a mechanical device, and is less expensive (Comiter, 2006). However, its theoretical advantages must be weighed against the known risks of sepsis, persistent perineal pain, and the need for explantation, as well as the risk of persistent urinary retention in older men whose detrusor contractility is more apt to be impaired. Although the same AUS model has been used successfully for many years, few studies have focused on its use in older patients or have examined the impact of age on outcome. In those few studies, carefully selected older patients seemed to do as well as younger ones, even up to 10 years (O’Connor et al, 2007, Kim et al, 2008). In addition, a sphincter can be used in older men with impaired detrusor contractility. Drawbacks include the higher complication and revision rates and the need for the patient to have adequate cognitive capacity and manual dexterity.
Prostheses such as condom catheters or penile clamps may be useful, but most of these require substantial cognitive capacity, and manual dexterity as well, and are often poorly tolerated. Penile sheaths (e.g., McGuire prosthesis or adhesive underwear liners) are an alternative. As discussed above, pads and undergarments are used as adjunctive measures. However, in these cases, polymer gel pads are frequently successful because the gel can more readily absorb the smaller amount of leakage. Some products can be flushed down the toilet, a convenient feature for ambulatory individuals.
Outlet Obstruction
For older men, conservative management of outlet obstruction often suffices. In the absence of urinary retention or upper tract damage, modification of fluid excretion and voiding habits may be effective. If not, α-adrenergic antagonists are useful and generally well-tolerated; they are actually beneficial for men with systolic (not diastolic) congestive heart failure. However, their use requires caution. With age, cardiac output depends less on cardioacceleration than on adequate ventricular filling. Unfortunately, the left ventricle also becomes stiffer, necessitating higher filling pressure. Thus, α blockers, which decrease preload as well as afterload, can result in symptomatic hypotension in older men, particularly those whose age-related left ventricular hypertrophy is exacerbated by hypertension or aortic stenosis. Medical consultation should be sought before prescribing an α blocker to such individuals. Although the newer agents, tamsulosin and alfuzosin, are more selective and appear to be less likely to cause orthostasis than terazosin and doxazosin in older patients (Mann et al, 2000; AUA Practice Guidelines Committee, 2003), further data are required. Because doxazosin was associated with an increased risk of heart failure when used to treat older men with hypertension in the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack (ALLHAT) Trial, it probably should be avoided. Recent studies also show that alpha 1α blockers, especially tamsulosin, increase the risk of serious ophthalmic events following iridectomy (Bell et al, 2009). Unfortunately, other α blockers are not free of such risk, and it is not yet clear how long these agents should be discontinued prior to surgery (Friedman, 2009). Fortunately, the risk is low, and new ophthalmologic strategies are under development.
Recent studies (Abrams, 2002; Lee et al, 2004; Kaplan et al, 2006; Blake-James et al, 2007; Rovner et al, 2008) have shown that anticholinergic therapy can be both effective and safe for obstructed men with concomitant DO who have not responded well to treatment with an α blocker, although the impact on incontinence has generally not been reported. Notably, all of these studies excluded men with severe obstruction, postvoid residual volume greater than 150 to 200 mL, and renal disease. Particular caution should be exercised for men who take other agents that have anticholinergic properties, for men with any degree of cognitive impairment, for men with PVR greater than 150 mL, and for men in whom close follow-up is questionable. Preliminary data on botulinum toxin A suggest that it too may have a role as further data become available (Maria et al, 2003; Kuo, 2005).
The 5α-reductase inhibitors (finasteride or dutasteride) have also proved effective as monotherapy, but fewer men appear to benefit, the effect is more modest, and the response occurs over months (Gormley et al, 1992). By contrast, and particularly for men with larger glands, combined therapy with an α blocker for several years is more efficacious than therapy with either drug alone. It also reduces the risk of urinary retention, although this appears to take at least a year. Devices and surgical approaches, as well as the approach to obstruction in women, are discussed elsewhere (see Chapters 69, 93, and 94). Of note, DO may resolve less often following removal of obstruction in older patients than in younger ones, but incontinence may still improve, even in cognitively impaired patients (Eastwood and Smart, 1985; Gormley et al, 1993; Mitterberger et al, 2007). In addition, less extensive resection or ablation often suffices for frail elderly men, in whom recurrence of symptoms with adenoma re-growth years later is often not an issue. This fact, coupled with surgical techniques that now permit resection under local anesthesia, has made surgery increasingly feasible for this population.
Underactive Detrusor
Management of detrusor underactivity is directed at reducing the residual volume, eliminating hydronephrosis (if present), and preventing urosepsis (Taylor and Kuchel, 2006; Yalla et al, 2007). The first step is to use indwelling or intermittent catheterization to decompress the bladder for up to a month (at least 7 to 14 days), while reversing potential contributors to impaired detrusor function (fecal impaction and medications). For patients presenting with acute retention, an α blocker should be used as well. Recent data suggest that such therapy increases the chances of success in men without another obvious cause of retention, even among men older than age 65 years and those with retained volume of 1000 to 1500 mL (Lucas et al, 2005; McNeill et al, 2005; Fitzpatrick and Kirby, 2006). Given the long-term success of combined therapy with an α blocker and a 5α-reductase inhibitor, addition of the latter should also be considered if the patient’s life expectancy is estimated to exceed one year.
If an indwelling catheter has been inserted, it should then be removed (Table 76–6). If decompression does not fully restore bladder function, augmented voiding techniques (such as double voiding and implementation of the Credé [application of suprapubic pressure during voiding] or Valsalva maneuver) may help if the patient is able to initiate a detrusor contraction or if there is coexistent stress incontinence, especially in women. Bethanechol (40 to 200 mg/day in divided doses) is occasionally useful in a patient whose bladder contracts poorly because of treatment with anticholinergic agents that cannot be discontinued (e.g., tricyclic antidepressant). In other patients, bethanechol may decrease the PVR if sphincter function and local innervation are normal, but evidence for its efficacy is equivocal at best (Barendrecht et al, 2007; Andersson et al, 2009a), and residual volume should be monitored to assess its effect.
Table 76–6 Removing an Indwelling Urethral Catheter*
|
Ensure that the bladder has been decompressed for at least several days, and 7 to 21 days if possible; the higher the residual volume, the longer the bladder should be decompressed.
Correct reversible causes of urinary retention: fecal impaction; pelvic/perineal pain; and use of anticholinergic, alpha adrenergic agonist, or calcium channel blocker medications. If an anticholinergic antidepressant/antipsychotic agent cannot be stopped, consider switching to one with fewer or no anticholinergic side effects.
An α blocker (e.g., alfuzosin or tamsulosin) increases the chances of success in men but is still unproven in women. It should be initiated several days before the catheter is removed. If life expectancy is estimated to exceed a year, initiation of finasteride should be considered as well.
Record output at 6- to 8-hour intervals for 2 days to establish a pattern of baseline urine excretion.
Remove the catheter at a time that permits accurate recording of urine output and allows for postvoiding recatheterization; clamping the catheter before removal is unnecessary and can be dangerous.
Reinsert the catheter only:
If the patient voids and the PVR volume is:
• 100-400 mL—watch for delayed retention and evaluate further, if appropriate*
|
PVR, postvoiding residual.
* Further evaluation is appropriate when the patient and physician feel that if a surgically correctable condition was found (e.g., urethral obstruction), an operation would be preferable to chronic catheterization or the other options described in the text.
Modified from Resnick NM. Incontinence. In: Beck JC, editor. Geriatric review syllabus. New York: American Geriatrics Society; 1991. p. 141–54.
On the other hand, if after decompression the detrusor is acontractile, these interventions are apt to be fruitless, and the patient should be started on intermittent catheterization or an indwelling urethral catheter. For individuals at home, intermittent self-catheterization is preferable and requires only clean, rather than sterile, catheter insertion. The patient can purchase two or three of these catheters inexpensively. One or two are used during the day and another is kept at home. The catheters are cleaned daily, allowed to air dry at night, sterilized periodically, and may be reused repeatedly. Antibiotic or methenamine prophylaxis against urinary tract infection is probably warranted if the individual gets more than an occasional symptomatic infection or has an abnormal heart valve (Chawla et al, 1988; Warren, 1990; Cottenden et al, 2005). Intermittent catheterization in this setting is generally painless, safe, inexpensive, and effective, and allows individuals to carry on with their usual daily activities. For debilitated patients, however, intermittent catheterization is usually less feasible, although sometimes possible (Hunt and Whitaker, 1990). If used in an institutional setting, sterile rather than clean technique should be employed until studies document the safety of the latter (Gammack, 2003).
Unfortunately, despite the benefits and proven feasibility of intermittent catheterization (Bennett and Diokno, 1984; Hunt and Whitaker, 1990; Bakke et al, 1992), most elderly patients choose indwelling catheterization instead. As in younger individuals, complications of chronic indwelling catheterization include renal inflammation and chronic pyelonephritis (Warren et al, 1994), bladder and urethral erosions, bladder stones and cancer, as well as urosepsis (Warren, 1990; Gammack, 2003; Cottenden et al, 2005). Principles of catheter care are summarized in Table 76–7.
Table 76–7 Principles of Indwelling Catheter Care
Adapted from Resnick NM. Voiding dysfunction and urinary incontinence. In: Beck JC, editor. Geriatric review syllabus. New York: American Geriatrics Society; 1991. p. 141–54.
When indicated, indwelling catheters can be extremely effective, but their use should be restricted, especially in nursing home settings (Johnson and Ouslander, 2006) (Center for Medicare and Medicaid Services: Surveyor Guidance for Urinary Incontinence and Catheters; available at: www.cms.hhs.gov/medicaid/survey-cert/sc0523.pdf). Catheters are indicated in acutely ill patients to monitor fluid balance, in the patient with a nonhealing pressure ulcer, for temporary bladder decompression in patients with acute urinary retention, and in the patient with overflow incontinence refractory to other measures. Even in long-term care facilities, they are probably indicated for only 1% to 2% of patients.
Key Points: Therapy
Summary
Regardless of age, mobility, mentation, or institutionalization, incontinence is never normal. By attenuating physiologic reserve, aging increases the likelihood of becoming incontinent in the setting of additional physiologic, pharmacologic, or pathologic insults. Because many of these problems lie outside the urinary tract, so too must the diagnostic and therapeutic focus. However, such a strategy, coupled with a multifactorial, creative, persistent, and optimistic approach, will increase the chances of a successful outcome and generally reward patient and physician alike.
Andersson KE, Chapple CR, Cardozo L, et al. Pharmacological treatment of overactive bladder: report from the International Consultation on Incontinence. Curr Opin Urol. 2009;19(4):380-394.
Chu FM, Dmochowski RR, Lama DJ, et al. Extended-release formulations of oxybutynin and tolterodine exhibit similar central nervous system tolerability profiles: a subanalysis of data from the OPERA trial. Am J Obstet Gynecol. 2005;192:1849-1855.
DuBeau CE, Kuchel GA, Johnson T, et al. Incontinence in the frail elderly. In: Abrams P, Cardozo L, Khoury S, Wein AJ, editors. Incontinence, 4th International Consultation on Incontinence. Plymouth (UK): Plymbridge Distributors; 2009:961-1024.
Fink HA, Taylor BC, Tacklind JW, et al. Treatment interventions in nursing home residents with urinary incontinence: a systematic review of randomized trials. Mayo Clin Proc. 2008;83(12):1332-1343.
Friedman AH. Tamsulosin and the intraoperative floppy iris syndrome. JAMA. 2009;301(19):2044-2045.
Griffiths D, Tadic SD, Schaefer W, Resnick NM. Cerebral control of the urinary bladder: how age-related changes may predispose to urge incontinence. Neuroimage. 2009;47:981-986.
Hartmann KE, McPheeters ML, Biller DH, et al. Treatment of overactive bladder in women. Evidence report/technology assessment no. 187, AHRQ Publication No. 09-E017. U.S. Department of Health and Human Services, Public Health Service, Agency for Healthcare Research and Quality, Rockville (MD), 2009.
Hay-Smith EJ, Berghmans B, Burgio KL, et al. Adult conservative management. In: Abrams P, Cardozo L, Khoury S, Wein AJ, editors. Incontinence, 4th International Consultation on Incontinence. Plymouth (UK): Plymbridge Distributors; 2009:1025-1120.
Pfisterer MH, Griffiths DJ, Schaefer W, Resnick NM. The effect of age on lower urinary tract function: a study in women. J Am Geriatr Soc. 2006;54:405-412.
Resnick NM. Clinical crossroads: urinary incontinence in an 89-year-old woman. JAMA. 1996;276:1832-1840.
Resnick NM, Marcantonio ER. How should clinical care of the aged differ? Lancet. 1997;350:1157-1158.
Smith ARB, Dmochowski R, Hilton P, et al. Surgery for urinary incontinence in women. In: Abrams P, Cardozo L, Khoury S, Wein AJ, editors. Incontinence, 4th International Consultation on Incontinence. Plymouth (UK): Plymbridge Distributors; 2009:1191-1272.
Taylor JA3rd, Kuchel GA. Detrusor underactivity: clinical features and pathogenesis of an underdiagnosed geriatric condition. J Am Geriatr Soc. 2006;54(12):1920-1932.
Abrams P. Tolterodine therapy in men with bladder outlet obstruction and symptomatic detrusor overactivity is not associated with urinary safety concerns. J Urol. 2002;167(Suppl.):1048.
Abrams P, Cardozo L, Khoury S, Wein A. Incontinence, 3rd ed. Plymouth (UK): Health Publications; 2005.
Abrams P, Mattiasson A, Lose G, Robertson GL. The role of desmopressin in the treatment of adult nocturia. BJU International. 2002;90(Suppl.):32-36.
Al-Badr A, Ross S, Soroka D, Drutz HP. What is the available evidence for hormone replacement therapy in women with stress urinary incontinence? J Obstet Gynaecol Can. 2003;25:567-574.
Alhasso AA, McKinlay J, Patrick K, Stewart L. Anticholinergic drugs versus non-drug active therapies for overactive bladder syndrome in adults. Cochrane Database Syst Rev 2006(4):CD003193.
Amundsen CL, Romero AA, Jamison MG, Webster GD. Sacral neuromodulation for intractable urge incontinence: are there factors associated with cure? Urology. 2005;66(4):746-750.
Amundsen CL, Webster GD. Sacral neuromodulation in an older, urge-incontinent population. Am J Obstet Gynecol. 2002;187(6):1462-1465. discussion 1465
Andersson KE. Antimuscarinics for treatment of overactive bladder. Lancet Neurology. 2004;3:46-53.
Andersson KE, Chapple C, Cardozo L, et al. Pharmacological treatment of urinary incontinence. In: Abrams P, Cardozo L, Khoury S, Wein AJ, editors. Incontinence, 4th International Consultation on Incontinence. Plymouth (UK): Plymbridge Distributors; 2009:631-699.
Andersson KE, Chapple CR, Cardozo L, et al. Pharmacological treatment of overactive bladder: report from the International Consultation on Incontinence. Curr Opin Urol. 2009;19(4):380-394.
Anger JT, Litwin MS, Wang Q, et al. The effect of age on outcomes of sling surgery for urinary incontinence. J Am Geriatr Soc. 2007;55(12):1927-1931.
Anger JT, Weinberg AE, Albo ME, et al. Trends in surgical management of stress urinary incontinence among female Medicare beneficiaries. Urology. 2009;74(2):283-287.
Angner E, Ray MN, Saag KG, Allison JJ. Health and happiness among older adults: a community-based study. J Health Psychol. 2009;14(4):503-512.
Asplund R, Aberg H. Desmopressin in elderly subjects with increased nocturnal diuresis. A two-month treatment study. Scand J Urol Nephrol. 1993;27(1):77-82.
AUA Practice Guidelines Committee. AUA Guideline on management of benign prostatic hyperplasia. Chapter 1: diagnosis and treatment recommendations. J Urol. 2003;170:530-547.
Azadzoi KM, Yalla SV, Siroky MB. Oxidative stress and neurodegeneration in the ischemic overactive bladder. J Urol. 2007;178(2):710-715.
Bae JH, Oh MM, Shim KS, et al. The effects of long-term administration of oral desmopressin on the baseline secretion of antidiuretic hormone and serum sodium concentration for the treatment of nocturia: a circadian study. J Urol. 2007;178(1):200-203.
Bakke A, Brun OH, Hoisaeter PA. Clinical background of patients treated with clean intermittent catheterization in Norway. Scand J Urol Nephrol. 1992;26:211-217.
Baldassare JS, Kaye D. Special problems in urinary tract infection in the elderly. Med Clin North Am. 1991;75:375-390.
Barendrecht MM, Oelke M, Laguna MP, Michel MC. Is the use of parasympathomimetics for treating an underactive urinary bladder evidence-based? BJU Int. 2007;99(4):749-752.
Bell CM, Hatch WV, Fischer HD, et al. Association between tamsulosin and serious ophthalmic adverse events in older men following cataract surgery. JAMA. 2009;301(19):1991-1996.
Bennett CJ, Diokno AC. Clean intermittent self-catheterization in the elderly. Urol. 1984;24:43-45.
Berghmans LCM, Hendriks HJM, DeBie RA, et al. Conservative treatment of urge urinary incontinence in women: a systematic review of randomized clinical trials. BJU International. 2000;85:254-263.
Betschart C, Scheiner D, Maake C, et al. Histomorphological analysis of the urogenital diaphragm in elderly women: a cadaver study. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19(11):1477-1481.
Blaivas JG, Olsson CA. Stress incontinence: classification and surgical approach. J Urol. 1988;139:727-731.
Blake-James BT, Rashidian A, Ikeda Y, Emberton M. The role of anticholinergics in men with lower urinary tract symptoms suggestive of benign prostatic hyperplasia: a systematic review and meta-analysis. BJU Int. 2007;99(1):85-96.
Bonde HV, Sejr T, Erdmann L, et al. Residual urine in 75-year-old men and women. A normative population study. Scand J Urol Nephrol. 1996;30:89-91.
Branch LG, Walker LA, Wetle TT, et al. Urinary incontinence knowledge among community-dwelling people 65 years of age and older. J Am Geriatr Soc. 1994;42:1257-1262.
Brandeis GH, Baumann MM, Hossain M, et al. The prevalence of potentially remediable urinary incontinence in frail older people: a study using the Minimum Data Set. J Am Geriatr Soc. 1997;45:179-184.
Brocklehurst JC, Dillane JB, Griffiths L, Fry J. The prevalence and symptomatology of urinary infection in an aged population. Gerontol Clin. 1968;10:242-253.
Brown JS, Vittinghoff E, Wyman JF, et al. Urinary incontience: does it increase risk for falls and fractures? J Am Geriatr Soc. 2000;48:721-725.
Brown JS, Wing R, Barrett-Connor E, et al. Lifestyle intervention is associated with lower prevalence of urinary incontinence: the Diabetes Prevention Program. Diabetes Care. 2006;29(2):385-390.
Brubaker L, Richter HE, Visco A, et al. Refractory idiopathic urge urinary incontinence and botulinum A injection. J Urol. 2008;180(1):217-222.
Burgio KL, Goode PS, Locher JL, et al. Behavioral training with and without biofeedback in the treatment of urge incontinence in older women. A randomized controlled trial. JAMA. 2002;288:2293-2299.
Burgio KL, Kraus SR, Menefee S, et al. Behavioral therapy to enable women with urge incontinence to discontinue drug treatment: a randomized trial. Ann Intern Med. 2008;149(3):161-169.
Burgio KL, Locher JL, Goode PS. Behavioral vs drug treatment for urge urinary incontinence in older women: a randomized controlled trial. JAMA. 1998;280:1995-2000.
Burgio KL, Locher JL, Goode PS. Combined behavioral and drug therapy for urge incontinence in older women. J Am Geriatr Soc. 2000;48(4):370-374.
Burns PA, Pranikoff K, Nochajski TH, et al. A comparison of effectiveness of biofeedback and pelvic muscle exercise treatment of stress incontinence in older community-dwelling women. J Gerontol. 1993;48:M167-M174.
Cannon A, Carter PG, McConnell AA, Abrams P. Desmopressin in the treatment of nocturnal polyuria in the male. BJU International. 1999;84:20-24.
Cardozo L, Bachmann G, McClish DK, et al. Meta-analysis of estrogen therapy in the management of urogenital atrophy in postmenopausal women: second report of the hormones and urogenital therapy committee. Obstet Gynecol. 1998;92:722-727.
Cardozo L, Lose G, McClish D, Versi E. A systematic review of the effects of estrogens for symptoms suggestive of overactive bladder. Acta Obstet Gynecol Scand. 2004;83:892-897.
Carey JM, Leach GE. Transvaginal surgery in the octogenarian using cadaveric fascia for pelvic prolapse and stress incontinence: minimal one-year results compared to younger patients. Urology. 2004;63:665-670.
Castleden CM, Duffin HM, Asher MJ. Clinical and urodynamic studies in 100 elderly incontinent patients. BMJ. 1981;282:1103-1105.
Chang CH, Gonzalez CM, Lau DT, Sier HC. Urinary incontinence and self-reported health among the U.S. Medicare managed care beneficiaries. J Aging Health. 2008;20(4):405-419.
Chang YL, Lin AT, Chen KK. Short-term effects of desmopressin on water and electrolyte excretion in adults with nocturnal polyuria. J Urol. 2007;177(6):2227-2279. discussion 2230
Chapple C, DuBeau C, Ebinger U, et al. Darifenacin treatment of patients > or = 65 years with overactive bladder: results of a randomized, controlled, 12-week trial. Curr Med Res Opin. 2007;23(10):2347-2358.
Chawla JC, Clayton CL, Stickler DJ. Antiseptics in the longterm urological management of patients by intermittent catheterization. British. J Urol. 1988;62:289-294.
Choi H, Palmer MH, Park J. Meta-analysis of pelvic floor muscle training: randomized controlled trials in incontinent women. Nurs Res. 2007;56(4):226-234.
Chu FM, Dmochowski RR, Lama DJ, et al. Extended-release formulations of oxybutynin and tolterodine exhibit similar central nervous system tolerability profiles: a subanalysis of data from the OPERA trial. Am J Obstet Gynecol. 2005;192:1849-1855.
Cigolle CT, Langa KM, Kabeto MU, et al. Geriatric conditions and disability: the Health and Retirement Study. Ann Intern Med. 2007;147(3):156-164.
Comiter CV. Surgery insight: management of failed sling surgery for female stress urinary incontinence. Nat Clin Pract Urol. 2006;3(12):666-674.
Cottenden A, Fader M, Getliffe K, et al. Management with continence products. In: Abrams P, Cardozo L, Khoury S, Wein A, editors. Incontinence. 3rd ed. Plymouth (UK): Health Publication; 2005:149-253.
Cottenden AM, Bliss DZ, Buckley B, et al. Management using continence products. In: Abrams P, Cardozo L, Khoury S, Wein AJ, editors. Incontinence, 4th International Consultation on Incontinence. Plymouth (UK): Plymbridge Distributors; 2009:1519-1642.
Dennis PJ, Rohner TJ, Hu TW, et al. Simple urodynamic evaluation of incontinent elderly female nursing home patients. A descriptive analysis. Urology. 1991;37:173-179.
Dequecker J. Drug treatment of urinary incontinence in the elderly. Gerontol Clin. 1965;7:311-317.
Diokno AC. Diagnostic categories of incontinence and the role of urodynamic testing. J Am Geriatr Soc. 1990;38:300-305.
Diokno AC, Brock BM, Brown M, Herzog AR. Prevalence of urinary incontinence and other urological symptoms in the non-institutionalized elderly. J Urol. 1986;136:1022.
Diokno AC, Brown MB, Brock BM, et al. Clinical and cystometric characteristics of continent and incontinent noninstitutionalized elderly. J Urol. 1988;140:567-571.
Diokno AC, Brown MB, Herzog AR. Relationship between use of diuretics and continence status in the elderly. Urol. 1991;38:39-42.
Diokno AC, Burgio K, Fultz NH, et al. Prevalence and outcomes of continence surgery in community dwelling women. J Urol. 2003;170:507-511.
Diokno AC, Wells TJ, Brink CA. Urinary incontinence in elderly women: urodynamic evaluation. J Am Geriatr Soc. 1987;35:940-946.
Donnelly MJ, Powell-Morgan S, Olsen AL, Nygaard IE. Vaginal pessaries for the management of stress and mixed urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2004;15(5):302-307.
DuBeau CE. Urinary incontinence management: new questions from old assumptions. J Am Geriatr Soc. 2001;49:829-830.
DuBeau CE, Kuchel GA, Johnson T, et al. Incontinence in the frail elderly. In: Abrams P, Cardozo L, Khoury S, Wein AJ, editors. Incontinence, 4th International Consultation on Incontinence. Plymouth (UK): Plymbridge Distributors; 2009:961-1024.
DuBeau CE, Resnick NM. Evaluation of the causes and severity of geriatric incontinence: a critical appraisal. Urol Clin North Am. 1991;18:243-256.
DuBeau CE, Resnick NM. Controversies in the diagnosis and management of benign prostatic hypertrophy. Adv Int Med. 1992;37:55-83.
DuBeau CE, Resnick NM. Urinary incontinence and dementia: the perils of guilt by association. J Am Geriatr Soc. 1995;43:310-311.
DuBeau CE, Yalla SV, Resnick NM. Most bothersome symptom in men presenting with prostatism: implications for outcomes research. J Am Geriatr Soc. 1995;43:985-993.
DuBeau CE, Yalla SV, Resnick NM. Primary care screening for outlet obstruction in men with voiding symptoms. J Am Geriatr Soc. 1998;46:1118-1124.
Dyro FM, Yalla SV. Refractoriness of urethral striated sphincter during voiding: studies with afferent pudendal reflex arc stimulation in male subjects. J Urol. 1986;135:732-736.
Eastwood HD, Smart CJ. Urinary incontinence in the disabled elderly male. Age Ageing. 1985;14:235-239.
Elbadawi A, Hailemariam S, Yalla SV, Resnick NM. Structural basis of geriatric voiding dysfunction. VI. Validation and update of diagnostic criteria in 71 detrusor biopsies. J Urol. 1997;157:1802-1813.
Elbadawi A, Hailemariam S, Yalla SV, Resnick NM. Structural basis of geriatric voiding dysfunction. VII. Prospective ultrastructural/urodynamic evaluation of its natural evolution. J Urol. 1997;157:1814-1822.
Elbadawi A, Resnick NM, Dorsam J, et al. Structural basis of neurogenic bladder dysfunction: I. Methods of a prospective ultrastructural study and overview of the findings. J Urol. 2003;169:540-546.
Elbadawi A, Yalla SV, Resnick NM. Structural basis of geriatric voiding dysfunction. I. Methods of a correlative study, and overview of the findings. J Urol. 1993;150:1650-1656.
Elbadawi A, Yalla SV, Resnick NM. Structural basis of geriatric voiding dysfunction. II. Aging detrusor: normal vs. impaired contractility. J Urol. 1993;150:1657-1667.
Elbadawi A, Yalla SV, Resnick NM. Structural basis of geriatric voiding dysfunction. III. Detrusor overactivity. J Urol. 1993;150:1668-1680.
Engel BT, Burgio LD, McCormick KA. Behavioral treatment of incontinence in the long-term care setting. J Am Geriatr Soc. 1990;38:361-363.
Eriksen B. A randomized, open, parallel-group study on the preventive effect of an estradiol-releasing vaginal ring (Estring) on recurrent urinary tract infections in postmenopausal women. Am J Obstet Gynecol. 1999;180(5):1072-1079.
Everett HC. The use of bethanechol chloride with tricyclic antidepressants. Am J Psychiat. 1975;132:1202-1204.
Fader M, Cottenden AM, Getliffe K. Absorbent products for light urinary incontinence in women. Cochrane Database Syst Rev 2007(2):CD001406.
Fader M, Cottenden AM, Getliffe K. Absorbent products for moderate-heavy urinary and/or faecal incontinence in women and men. Cochrane Database Syst Rev 2008(4):CD007408.
Fantl JA, Newman DK, Colling J, et al. Urinary incontinence in adults: acute and chronic management. Clinical practice guideline, no. 2, 1996 update, AHCPR Publicaion No. 96-0682. U.S. Department of Health and Human Services, Public Health Service, Agency for Health Care Policy and Research, Rockville (MD), 1996.
Fantl JA, Wyman JF, McClish DK. Efficacy of bladder training in older women with urinary incontinence. JAMA. 1991;265:609-613.
Fantl JA, Wyman JF, Wilson M, et al. Diuretics and urinary incontinence in community-dwelling women. Neurourol Urodyn. 1990;9:25-34.
Farage MA, Miller KW, Berardesca E, Maibach HI. Psychosocial and societal burden of incontinence in the aged population: a review. Arch Gynecol Obstet. 2008;277(4):285-290.
Fink HA, Taylor BC, Tacklind JW, et al. Treatment interventions in nursing home residents with urinary incontinence: a systematic review of randomized trials. Mayo Clin Proc. 2008;83(12):1332-1343.
Fitzpatrick JM, Kirby RS. Management of acute urinary retention. BJU Int. 2006;97(Suppl. 2):16-20. discussion 21–22
Fonda D, DuBeau CE, Harari D, et al. Incontinence in the frail elderly. In: Abrams P, Cardozo L, Khoury S, Wein A, editors. Incontinence. 3rd ed. Plymouth (UK): Health Publications; 2005:1163-1239.
Friedman AH. Tamsulosin and the intraoperative floppy iris syndrome. JAMA. 2009;301:2044-2045.
Fry CH, Sui GP, Kanai AJ, Wu C. The function of suburothelial myofibroblasts in the bladder. Neurourol Urodyn. 2007;26(Suppl. 6):914-919.
Gammack JK. Use and management of chronic urinary catheters in long-term care: much controversy, little consensus. J Am Med Dir Assoc. 2003;4(Suppl.):S53-S59.
Goode P, Burgio K, Locher JL, et al. The role of pelvic floor electrical stimulation in the behavioral treatment of stress incontinence in women: a randomized controlled trial. JAMA. 2003;290:345-352.
Gordon D, Gold R, Pauzner D, et al. Tension-free vaginal tape in the elderly: is it a safe procedure? Urology. 2005;65:479-482.
Gormley EA, Griffiths DJ, McCracken PN, et al. Effect of transurethral resection of the prostate on detrusor instability and urge incontinence in elderly males. Neurourol Urodyn. 1993;12:445-453.
Gormley GJ, Stoner E, Bruskewitz RC, et al. The effect of finasteride in men with benign prostatic hyperplasia. N Engl J Med. 1992;327:1185-1191.
Grady D, Brown JS, Vittinghoff E, et al. Postmenopausal hormones and incontinence: the Heart and Estrogen/Progestin Replacement Study. Obstet Gynecol. 2001;97:116-120.
Griebling TL, Kraus SR, Richter HE, et al. Tolterodine extended release is well tolerated in older subjects. Int J Clin Pract. 2009;63(8):1198-1204.
Griffiths D, Tadic SD, Schaefer W, Resnick NM. Cerebral control of the bladder in normal and urge-incontinent women. Neuroimage. 2007;37(1):1-7.
Griffiths D, Tadic SD, Schaefer W, Resnick NM. Cerebral control of the urinary bladder: how age-related changes may predispose to urge incontinence. Neuroimage. 2009;47:981-986.
Guilleminault C, Lin CM, Goncalves MA, Ramos E. A prospective study of nocturia and the quality of life of elderly patients with obstructive sleep apnea or sleep onset insomnia. J Psychosom Res. 2004;56(5):511-515.
Hailemariam S, Elbadawi A, Yalla SV, Resnick NM. Structural basis of geriatric voiding dysfunction. V. Standardized protocols for routine ultrastructural study and diagnosis of endoscopic detrusor biopsies. J Urol. 1997;157:1783-1801.
Haller CA, Benowitz NL. Adverse cardiovascular and central nervous system events associated with dietary supplements containing ephedra alkaloids. N Engl J Med. 2000;343:1833-1838.
Hartmann K, McPheeters M, Biller D, et al. Treatment of overactive bladder in women, AHRQ Publication No. 09-E017. Agency for Healthcare Research and Quality, Rockville (MD), 2009.
Hashimoto M, Imamura T, Tanimukai S, et al. Urinary incontinence: an unrecognised adverse effect wtih donepezil. Lancet. 2000;356:568.
Hay-Smith EJ, Berghmans B, Burgio KL, et al. Adult conservative management. In: Abrams P, Cardozo L, Khoury S, Wein AJ, editors. Incontinence, 4th International Consultation on Incontinence. Plymouth (UK): Plymbridge Distributors; 2009:1025-1120.
Hellberg D, Holmgren C, Lanner L, Nilsson S. The very obese woman and the very old woman: tension-free vaginal tape for the treatment of stress urinary incontinence. Int Urogynecol J. 2007;18:423-429.
Hellstrom PM, Sjoqvist A. Involvement of opioid and nicotinic receptors in rectal and anal reflex inhibition of urinary bladder motility in cats. Acta Physiol Scand. 1988;133:559-562.
Hendrix SL, Cochrane BB, Nygaard IE, et al. Effects of estrogen with and without progestin on urinary incontinence. JAMA. 2005;293:935-948.
Herschorn S, Bruschini H, Comiter C, et al. Surgical treatment of urinary incontinence in men. In: Abrams P, Cardozo L, Khoury S, Wein AJ, editors. Incontinence, 4th International Consultation on Incontinence. Plymouth (UK): Plymbridge Distributors; 2009:1121-1190.
Herzog AR, Fultz NH. Prevalence and incidence of urinary incontinence in community-dwelling populations. J Am Geriatr Soc. 1990;38:273-281.
Hilton P, Stanton SL. Algorithmic method for assessing urinary incontinence in elderly women. BMJ. 1981;282:940-942.
Hindley RG, Brierly RD, McLarty E, et al. A qualitative ultrastructural study of the hypocontractile detrusor. J Urol. 2002;168(1):126-131.
Hu TW, Igou JF, Kaltreider DL. A clinical trial of a behavioral therapy to reduce urinary incontinence in nursing homes. JAMA. 1989;261:2656-2662.
Hunskaar S. A systematic review of overweight and obesity as risk factors and targets for clinical intervention for urinary incontinence in women. Neurourol Urodyn. 2008;27(8):749-757.
Hunt GM, Whitaker RH. A new device for self-catheterization in wheelchair-bound women. British J Urol. 1990;66:162-163.
Jenkins KR, Fultz NH. Functional impairment as a risk factor for urinary incontinence among older Americans. Neurourol Urodyn. 2005;24(1):51-55.
Jensen JK, Nielsen FRJr, Ostergard DR. The role of patient history in the diagnosis of urinary incontinence. Obstet Gynecol. 1994;83(5 Pt. 2):904-910.
Johnson TM, Burgio KL, Redden TR, et al. Effects of behavioral and drug therapy on nocturia in older incontinent women. J Am Geriatr Soc. 2005;53:846-850.
Johnson TM2nd, Ouslander JG. The newly revised F-Tag 315 and surveyor guidance for urinary incontinence in long-term care. J Am Med Dir Assoc. 2006;7(9):594-600.
Judge TG. The use of quinestradol in elderly incontinent women. A preliminary report. Gerontol Clin. 1969;11(3):159-164.
Kafri R, Shames J, Raz M, Katz-Leurer M. Rehabilitation versus drug therapy for urge urinary incontinence: long-term outcomes. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19(1):47-52.
Kaplan SA, Roehrborn CG, Rovner ES, et al. Tolterodine and tamsulosin for treatment of men with lower urinary tract symptoms and overactive bladder: a randomized controlled trial. JAMA. 2006;296(19):2319-2328.
Kay G, Crook T, Rekeda L, et al. Differential effects of the antimuscarinic agents darifenacin and oxybutynin ER on memory in older subjects. Eur Urol. 2006;50(2):317-326.
Kay GG, Ebinger U. Preserving cognitive function for patients with overactive bladder: evidence for a differential effect with darifenacin. Int J Clin Pract. 2008;62(11):1792-1800.
Keegan PE, Atiemo K, Cody J, et al. Periurethral injection therapy for urinary incontinence in women. Cochrane Database Syst Rev 2007(3):CD003881.
Kikuchi A, Niu K, Ikeda Y, et al. Association between physical activity and urinary incontinence in a community-based elderly population aged 70 years and over. Eur Urol. 2007;52(3):868-874.
Kim SP, Sarmast Z, Daignault S, et al. Long-term durability and functional outcomes among patients with artificial urinary sphincters: a 10-year retrospective review from the University of Michigan. J Urol. 2008;179(5):1912-1916.
Kirschner-Hermanns R, Scherr PA, Branch LG, et al. Accuracy of survey questions for geriatric urinary incontinence. J Urol. 1998;159:1903-1908.
Klarskov P, Andersen JT, Asmussen CF, et al. Symptoms and signs predictive of the voiding pattern after acute urinary retention in men. Scand J Urol Nephrol. 1987;21:23-28.
Klausner AP, Steers WD. Antimuscarinics for the treatment of overactive bladder: a review of central nervous system effects. Curr Urol Rep. 2007;8(6):441-447.
Kong TK, Morris JA, Robinson JM, Brocklehurst JC. Predicting urodynamic dysfunction from clinical features in incontinent elderly women. Age Ageing. 1990;19:257-263.
Kuchel GA, Moscufo N, Guttmann CR, et al. Localization of brain white matter hyperintensities and urinary incontinence in community-dwelling older adults. J Gerontol A Biol Sci Med Sci. 2009;64(8):902-909.
Kuchel GA, Tannenbaum C, Greenspan SL, Resnick NM. Can variability in the hormonal status of elderly women assist in the decision to administer estrogens? J Wom Health Gend Based Med. 2001;10:109-116.
Kuo H-C. Prostate botulinum A toxin injection—an alternative treatment for benign prostatic obstruction in poor surgical candidates. Urology. 2005;65:670-674.
Lackner TE, Wyman JF, McCarthy TC, et al. Randomized, placebo-controlled trial of the cognitive effect, safety, and tolerability of oral extended-release oxybutynin in cognitively impaired nursing home residents with urge urinary incontinence. J Am Geriatr Soc. 2008;56(5):862-870.
Landi F, Cesari M, Russo A, et al. Benzodiazepines and the risk of urinary incontinence in frail older persons living in the community. Clin Pharmacol Ther. 2002;72(6):729-734.
Lawhorne LW, Ouslander JG, Parmelee PA, et al. Urinary incontinence: a neglected geriatric syndrome in nursing facilities. J Am Med Dir Assoc. 2008;9(1):29-35.
Lee JY, Kim HW, Lee SJ, et al. Comparison of doxazosin with or without tolterodine in men with symptomatic bladder outlet obstruction and an overactive bladder. BJU International. 2004;94(6):817-820.
Liapis A, Bakas P, Christopoulos P, et al. Tension-free vaginal tape for elderly women with stress urinary incontinence. Int J Gynaecol Obstet. 2006;92(1):48-51.
Lin HH, Sheu BC, Lo MC, Huang SC. Comparison of treatment outcomes for imipramine for female genuine stress incontinence. Br J Obstet Gynaecol. 1999;106(10):1089-1092.
Lo TS, Huang HJ, Chang CL, et al. Use of intravenous anesthesia for tension-free vaginal tape therapy in elderly women with genuine stress incontinence. Urology. 2002;59(3):349-353.
Lucas MG, Stephenson TP, Nargund V. Tamsulosin in the management of patients in acute urinary retention from benign prostatic hyperplasia. BJU International. 2005;95:354-357.
Mann RD, Biswas P, Freemantle S, et al. The pharmacovigilance of tamsulosin: event data on 12 484 patients. BJU International. 2000;85:446-450.
Maria G, Brisinda G, Civello IM, et al. Relief by botulinum toxin of voiding dysfunction due to benign prostatic hyperplasia: results of a randomized, placebo-controlled study. Urology. 2003;62:259-265.
Mariappan P, Alhasso A, Ballantyne Z, et al. Duloxetine, a serotonin and noradrenaline reuptake inhibitor (SNRI) for the treatment of stress urinary incontinence: a systematic review. Eur Urol. 2007;51(1):67-74.
Marshall HJ, Beevers DG. α-Adrenoceptor blocking drugs and female urinary incontinence: prevalence and reversibility. Brit J Clin Pharmacol. 1996;42:507-509.
Mathew TH, McEwen J, Rohan A. Urinary incontinence secondary to prazosin. Med J Aust. 1988;148:305-306.
Mattiasson A, Blaakaer J, Hoye K, Wein AJ. Simplified bladder training augments the effectiveness of tolterodine in patients with an overactive bladder. BJU Int. 2003;91(1):54-60.
McGrother C. Epidemiology and etiology or urinary incontinence in the elderly. World J Urol. 1998;16:S3-S9.
McGuire EJ. Reflex urethral instability. British J Urol. 1978;50:200-204.
McGuire EJ. Urinary incontinence. New York: Grune and Stratton; 1981.
McNeill SA, Hargreave TB, Roehrborn CG, Alfaur Study Group. Alfuzosin 10 mg once daily in the management of acute urinary retention: results of a double-blind placebo-controlled study. Urology. 2005;65:83-90.
Michel MC, Wetterauer U, Vogel M, de la Rosette JJ. Cardiovascular safety and overall tolerability of solifenacin in routine clinical use: a 12-week, open-label, post-marketing surveillance study. Drug Saf. 2008;31(6):505-514.
Miller KL, DuBeau CE, Bergmann M, et al. Quest for a detrusor overactivity index. J Urol. 2002;167:578-585.
Miller M. Nocturnal polyuria in older people: pathophysiology and clinical implications. J Am Geriatric Soc. 2000;48:1321-1329.
Mitterberger M, Pallwein L, Gradl J, et al. Persistent detrusor overactivity after transurethral resection of the prostate is associated with reduced perfusion of the urinary bladder. BJU Int. 2007;99(4):831-835.
Moehrer B, Hextall A, Jackson S. Oestrogens for urinary incontinence in women. Cochrane Database Syst Rev 2003(2):CD001405.
Morgan K, Bergmann M, DuBeau CE, Resnick NM. The voiding pattern of normal elders. J Am Geriatric Soc. 2000;48:S6.
Naglie G, Radomski SB, Brymer C, et al. A randomized, double-blind, placebo controlled crossover trial of nimodipine in older persons with detrusor instability and urge incontinence. J Urol. 2002;167:586-590.
Neumann PB, Grimmer KA, Deenadayalan Y. Pelvic floor muscle training and adjunctive therapies for the treatment of stress urinary incontinence in women: a systematic review. BMC Womens Health. 2006;6:11.
Norton PA, Baker JE. Postural changes can reduce leakage in women with stress urinary incontinence. Obstet Gynecol. 1994;84:770-774.
O’Connor RC, Nanigian DK, Patel BN, et al. Artificial urinary sphincter placement in elderly men. Urology. 2007;69(1):126-128.
Ouslander JG. Management of overactive bladder. N Engl J Med. 2004;350(8):786-799.
Ouslander JG, Colling J, PURT Study Group. Use of simple urodynamic tests in nursing homes. Neurourol Urodyn. 1992;11:159-160.
Ouslander JG, Greendale GA, Uman G, et al. Effects of oral estrogen and progrestin on the lower urinary tract among female nursing home residents. J Amer Geriatr Soc. 2001;49:803-807.
Ouslander JG, Hepps K, Raz S, Su HL. Genitourinary dysfunction in a geriatric outpatient population. J Am Geriatr Soc. 1986;34:507-514.
Ouslander JG, Maloney C, Grasela TH, et al. Implementation of a nursing home urinary incontinence management program with and without tolterodine. J Am Med Dir Assoc. 2001;2:207-214.
Ouslander JG, Schapira M, Schnelle JF, et al. Does eradicating bacteriuria affect the severity of chronic urinary incontinence in nursing home residents? Ann Intern Med. 1995;122:749-754.
Ouslander JG, Schnelle JF. Incontinence in the nursing home. Ann Intern Med. 1995;122:438-449.
Ouslander JG, Schnelle JF, Uman G, et al. Predictors of successful prompted voiding among incontinent nursing home residents. JAMA. 1995;273:1366-1370.
Pandit L, Ouslander JG. Postmenopausal vaginal atrophy and atrophic vaginitis. Am J Med Sci. 1997;314:228-231.
Parsons JK, Mougey J, Lambert L, et al. Lower urinary tract symptoms increase the risk of falls in older men. BJU Int. 2009;104(1):63-68.
Payne CK. Behavorial therapy for overactive bladder. Urology. 2000;55:3-6.
Pedersen PA, Johansen PB. Prophylactic treatment of adult nocturia with bumetanide. British J Urol. 1988;62:145-147.
Perucchini D, DeLancey JO, Ashton-Miller JA, et al. Age effects on urethral striated muscle. II. Anatomic location of muscle loss. Am J Obstet Gynecol. 2002;186(3):356-360.
Pfisterer MH, Griffiths DJ, Rosenberg L, et al. The impact of detrusor overactivity on bladder function in younger and older women. J Urol. 2006;175(5):1777-1783. discussion 1783
Pfisterer MH, Griffiths DJ, Schaefer W, Resnick NM. The effect of age on lower urinary tract function: a study in women. J Am Geriatr Soc. 2006;54(3):405-412.
Poggesi A, Pracucci G, Chabriat H, et al. Urinary complaints in nondisabled elderly people with age-related white matter changes: the Leukoaraiosis And DISability (LADIS) Study. J Am Geriatr Soc. 2008;56(9):1638-1643.
Raz R, Stamm WE. A controlled trial of intravaginal estriol in postmenopausal women with recurrent urinary tract infections. N Engl J Med. 1993;329:753-756.
Rembratt A, Riis A, Norgaard JP. Desmopressin treatment in nocturia; an analysis of risk factors for hyponatremia. Neurourol Urodyn. 2006;25(2):105-109.
Resnick NM. Voiding dysfunction in the elderly. In: Yalla SV, McGuire EJ, Elbadawi A, Blaivas JG, editors. Neurourology and urodynamics: principles and practice. New York: MacMillan Publishing; 1988:303-330.
Resnick NM. Noninvasive diagnosis of the patient with complex incontinence. Gerontology. 1990;36(Suppl. 2):8-18.
Resnick NM. Urinary incontinence. Lancet. 1995;346:94-99.
Resnick NM. An 89-year-old woman with urinary incontinence. JAMA. 1996;276:1832-1840.
Resnick NM. Improving treatment of urinary incontinence. JAMA. 1998;280:2034-2035.
Resnick NM. Geriatric medicine. In: Kasper DL, Braunwald E, Fauci AS, Hauser SL, Longo DL, Jameson JL, editors. Harrison’s principles of internal medicine. 16th ed. New York: McGraw-Hill; 2004:43-53.
Resnick NM, Baumann MM. Urinary incontinence. In: Morris JN, Lipsitz LA, Murphy K, Belleville-Taylor P, editors. Quality care in the nursing home. St Louis: CV Mosby; 1997:377-406.
Resnick NM, Baumann MM, Morris JN, Hawes C. Urinary incontinence and indwelling catheter. In: Minimum data set training manual and resource guide. Natick (MA): Eliot Press; 1991:F24-F31.
Resnick NM, Baumann MM, Scott M, et al. Risk factors for incontinence in the nursing home: a multivariate study. Neurourol Urodyn. 1988;7:274-276.
Resnick NM, Beckett LA, Branch LG, et al. Short-term variability of self report of incontinence in older persons. J Am Geriatr Soc. 1994;42(2):202-207.
Resnick NM, Brandeis GH, Baumann MM, et al. Misdiagnosis of urinary incontinence in nursing home women: prevalence and a proposed solution. Neurourol Urodyn. 1996;15:599-618.
Resnick NM, Brandeis GH, Baumann MM, Morris JN. A national assessment strategy for urinary incontinence in nursing home residents: reliability and validity of the Minimum Data Set and Resident Assessment Protocol. Neurourol Urodyn. 1996;15:583-598.
Resnick NM, Elbadawi A, Yalla SV. Age and the lower urinary tract: what is normal? Neurourol Urodyn. 1995;15:577-579.
Resnick NM, Griffiths DJ. Expanding treatment options for stress urinary incontinence in women. JAMA. 2003;290(3):395-397.
Resnick NM, Marcantonio ER. How should clinical care of the aged differ? Lancet. 1997;350:1157-1158.
Resnick NM, Ouslander JG. National Institutes of Health consensus development conference on urinary incontinence. J Am Geriatr Soc. 1990;38:263-386.
Resnick NM, Scherr PA, Wetle T, et al. Urinary incontinence and urgency among older persons in a geographically-defined community. JAMA. 1995. (submitted)
Resnick NM, Yalla SV. Detrusor hyperactivity with impaired contractile function. An unrecognized but common cause of incontinence in elderly patients. JAMA. 1987;257:3076-3081.
Resnick NM, Yalla SV, Laurino E. Feasibility, safety and reproducibility of urodynamics in the elderly. J Urol. 1987;137:189A.
Resnick NM, Yalla SV, Laurino E. The pathophysiology and clinical correlates of established urinary incontinence in frail elderly. N Engl J Med. 1989;320:1-7.
Reynard JM, Cannon A, Yang Q, Abrams P. A novel therapy for nocturnal polyuria: a double blind randomized trial of frusemide against placebo. BJU International. 1998;81:215-218.
Richter HE, Burgio KL, Chai TC, et al. Predictors of outcomes in the treatment of urge urinary incontinence in women. Int Urogynecol J Pelvic Floor Dysfunct. 2009. [Epub ahead of print]
Robinson JM, Brocklehurst JC. Evaluation of methods for assessment of bladder and urethral function. In: Brocklhurst JC, editor. Urology in the elderly. New York: Churchill Livingstone; 1984:19-54.
Rosenberg LJ, Griffiths DJ, Resnick NM. Factors that distinguish continent from incontinent older adults with detrusor overactivity. J Urol. 2005;174:1868-1872.
Rovner ES, Kreder K, Sussman DO, et al. Effect of tolterodine extended release with or without tamsulosin on measures of urgency and patient reported outcomes in men with lower urinary tract symptoms. J Urol. 2008;180(3):1034-1041.
Roxburgh C, Cook J, Dublin N. Anticholinergic drugs versus other medications for overactive bladder syndrome in adults. Cochrane Database Syst Rev 2007(3):CD003190.
Sakakibara R, Ito T, Uchiyama T, et al. Lower urinary tract function in dementia of Lewy body type. J Neurol Neurosurg Psychiatry. 2005;76(5):729-732.
Sand P, Zinner N, Newman D, et al. Oxybutynin transdermal system improves the quality of life in adults with overactive bladder: a multicentre, community-based, randomized study. BJU Int. 2006;99(4):836-844.
Sand PK, Miklos J, Ritter H, Appell R. A comparison of extended-release oxybutynin and tolterodine for treatment of overactive bladder in women. Int Urogynecol J. 2004;15(4):243-248.
Schnelle JF. Treatment of urinary incontinence in nursing home patients by prompted voiding. J Am Geriatr Soc. 1990;38:356-360.
Semmens JP, Tsai CC, Semmens EC, Loadholt CB. Effects of estrogen therapy on vaginal physiology during menopause. Obstet Gynecol. 1985;66:15-18.
Sevestre S, Ciofu C, Deval B, et al. Results of the tension-free vaginal tape technique in the elderly. Eur Urol. 2003;44(1):128-131.
Shamliyan TA, Kane RL, Wyman J, Wilt TJ. Systematic review: randomized, controlled trials of nonsurgical treatments for urinary incontinence in women. Ann Intern Med. 2008;148(6):459-473.
Sharp VJ, Bradley CS, Kreder KJ. Incontinence surgery in the older woman. Curr Opin Urol. 2006;16(4):224-228.
Siegler EL, Reidenberg M. Treatment of urinary incontinence with anticholinergics in patients taking cholinesterase inhibitors for dementia. Clin Pharmacol Ther. 2004;75:484-488.
Sink KM, Thomas J3rd, Xu H, et al. Dual use of bladder anticholinergics and cholinesterase inhibitors: long-term functional and cognitive outcomes. J Am Geriatr Soc. 2008;56(5):847-853.
Skelly J, Flint AJ. Urinary incontinence associated with dementia. J Am Geriatr Soc. 1995;43:286-294.
Smith ARB, Dmochowski R, Hilton P, et al. Surgery for urinary incontinence in women. In: Abrams P, Cardozo L, Khoury S, Wein AJ, editors. Incontinence, 4th International Consultation on Incontinence. Plymouth (UK): Plymbridge Distributors; 2009:1191-1272.
Sonke GS, Heskes T, Verbeek ALM, et al. Prediction of bladder outlet obstruction in men with lower urinary tract symptoms using artificial neural networks. J Urol. 2000;163:300-305.
South MM, Romero AA, Jamison MG, et al. Detrusor overactivity does not predict outcome of sacral neuromodulation test stimulation. Int Urogynecol J Pelvic Floor Dysfunct. 2007;18(12):1395-1398.
Starr JM. Cholinesterase inhibitor treatment and urinary incontinence in Alzheimer’s disease. J Am Geriatr Soc. 2007;55(5):800-801.
Stead WW. Tuberculosis as an endemic and nosocomial infection among the elderly in nursing homes. N Engl J Med. 1985;312:1483-1487.
Strasser H, Tiefenthaler M, Steinlechner M, et al. Urinary incontinence in the elderly and age-dependent apoptosis of rhabdosphincter cells. Lancet. 1999;354:918-919.
Suarez GM, Baum NH, Jacobs J. Use of standard contraceptive diaphragm in management of stress urinary incontinence. Urology. 1991;37:119-122.
Subak LL, Wing R, West DS, et al. Weight loss to treat urinary incontinence in overweight and obese women. N Engl J Med. 2009;360(5):481-490.
Tadic SD, Griffiths D, Schaefer W, Resnick NM. Abnormal connections in the supraspinal bladder control network in women with urge urinary incontinence. Neuroimage. 2008;39(4):1647-1653.
Tannenbaum C, Brouillette J, Corcos J. Rating improvements in urinary incontinence: do patients and their physicians agree? Age Ageing. 2008;37(4):379-383.
Tannenbaum C, DuBeau CE. Urinary incontinence in the nursing home: practical approach to evaluation and management. Clin Geriatr Med. 2004;20:437-452.
Taylor JA3rd, Kuchel GA. Detrusor underactivity: clinical features and pathogenesis of an underdiagnosed geriatric condition. J Am Geriatr Soc. 2006;54(12):1920-1932.
Tromp AM, Smit JH, Deeg DJH, et al. Predictors for falls and fractures in the Longitudinal Aging Study Amsterdam. J Bone Min Res. 1998;13:1932-1939.
Trowbridge ER, Wei JT, Fenner DE, et al. Effects of aging on lower urinary tract and pelvic floor function in nulliparous women. Obstet Gynecol. 2007;109(3):715-720.
Tsakiris P, Oelke M, Michel MC. Drug-induced urinary incontinence. Drugs Aging. 2008;25(7):541-549.
Tsao JW, Heilman KM. Transient memory impairment and hallucinations associated with tolterodine use. N Engl J Med. 2003;349:2274-2275.
Tse V, Wills E, Szonyi G, Khadra MH. The application of ultrastructural studies in the diagnosis of bladder dysfunction in a clinical setting. J Urol. 2000;163:535-539.
Waetjen LE, Brown JS, Vittinghoff E, et al. The effect of ultralow-dose transdermal estradiol on urinary incontinence in postmenopausal women. Obstet Gynecol. 2005;106(5 Pt. 1):946-952.
Wagg A, Malone-Lee J. The management of urinary incontinence in the elderly. British J Urol. 1998;82:11-17.
Wagg A, Wyndaele JJ, Sieber P. Efficacy and tolerability of solifenacin in elderly subjects with overactive bladder syndrome: a pooled analysis. Am J Geriatr Pharmacother. 2006;4(1):14-24.
Wagner TH, Hu TW. Economic casts of urinary incontinence. Urology. 1998;51:355-361.
Walter S, Wolf H, Barlebo H, Jensen HK. Urinary incontinence in postmenopausal women treated with estrogens. A double-blind clinical trial. Urol Int. 1978;33:135-143.
Warren JW. Urine collection devices for use in adults with urinary incontinence. J Am Geriatr Soc. 1990;38:364-367.
Warren JW, Muncie HL, Hebel JR, Hall-Craggs M. Long-term urethral catheterization increases risk of chronic pyelonephritis and renal inflammation. J Am Geriatr Soc. 1994;42:1286-1290.
Weatherall M. The risk of hyponatremia in older adults using desmopressin for nocturia: a systematic review and meta-analysis. Neurourol Urodyn. 2004;23:302-305.
Weinberger MW, Goodman BM, Carnes M. Long-term efficacy of nonsurgical urinary incontinence treatment in elderly women. J Gerontol. 1999;54A:M117-M121.
Wells TJ, Brink CA, Diokno AC, et al. Pelvic muscle exercise for stress urinary incontinence in elderly women. J Am Geriatr Soc. 1991;39:785-791.
Wetle TT, Scherr PA, Branch LG, et al. Difficulty with holding urine among older persons in a geographically-defined community: prevalence and correlates. J Am Geriatr Soc. 1995;43:349-355.
White WM, Mobley JD3rd, Doggweiler R, et al. Sacral nerve stimulation for refractory overactive bladder in the elderly population. J Urol. 2009;182(4):1449-1452.
White WM, Pickens RB, Doggweiler R, Klein FA. Short-term efficacy of botulinum toxin A for refractory overactive bladder in the elderly population. J Urol. 2008;180(6):2522-2526.
Wilson PD, Berghmans B, Hagen S, et al. Adult conservative management. In: Abrams P, Cardozo L, Khoury S, Wein A, editors. Incontinence. 3rd ed. Plymouth (UK): Health Publications; 2005:855-964.
Winters JC, Chiverton A, Scarpero HM, Prats LJJr. Collagen injection therapy in elderly women: long-term results and patient satisfaction. Urology. 2000;55(6):856-861.
Yalla SV, Sullivan MP, Resnick NM. Update on detrusor hyperactivity with impaired contractility. Curr Bladder Dysfunct Rep. 2007;2(4):191-196.
Zeitlin MP, Lebherz TB. Pessaries in the geriatric patient. J Am Geriatr Soc. 1992;40:635-639.
Zinner NR, Mattiasson A, Stanton SL. Efficacy, safety, and tolerability of extended-release once-daily tolterodine treatment for overactive bladder in older versus younger patients. J Am Geriatr Soc. 2002;50:799-807.