Chapter 12 Ocular Tumours
Benign epibulbar tumours
Conjunctival naevus
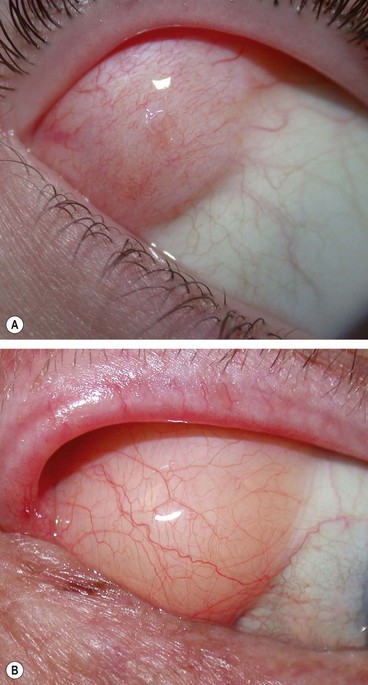
A conjunctival naevus is the most common melanocytic conjunctival tumour. The overall risk of malignant transformation is about 1%.
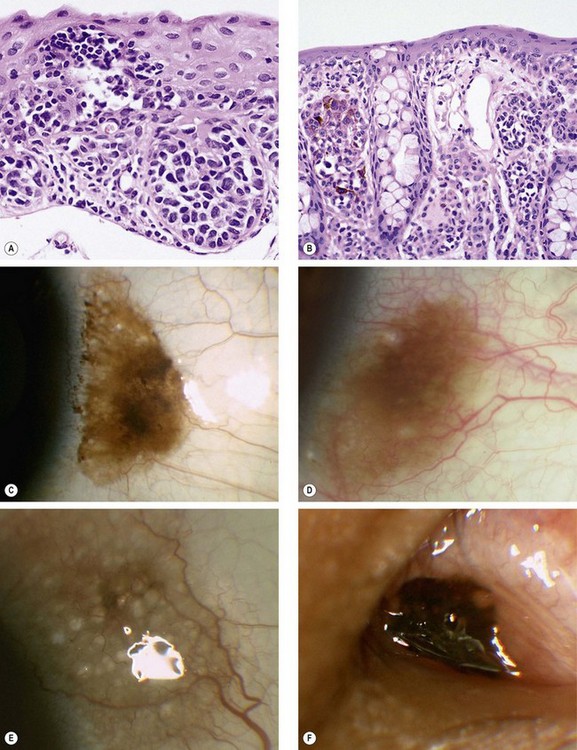
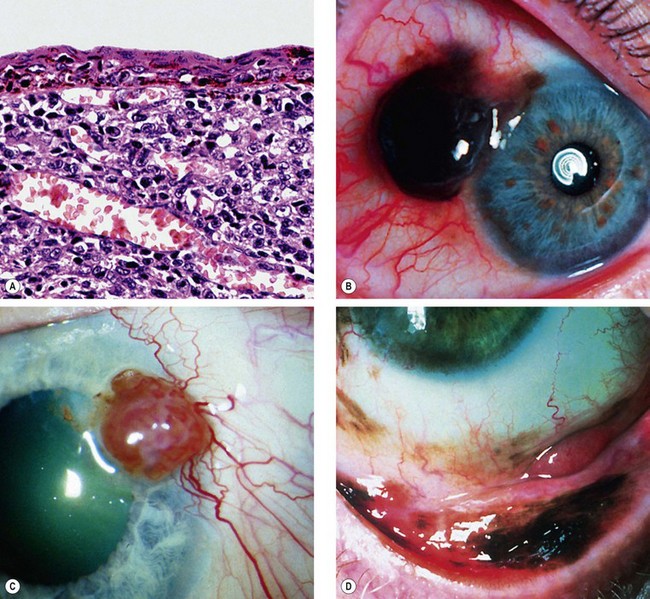
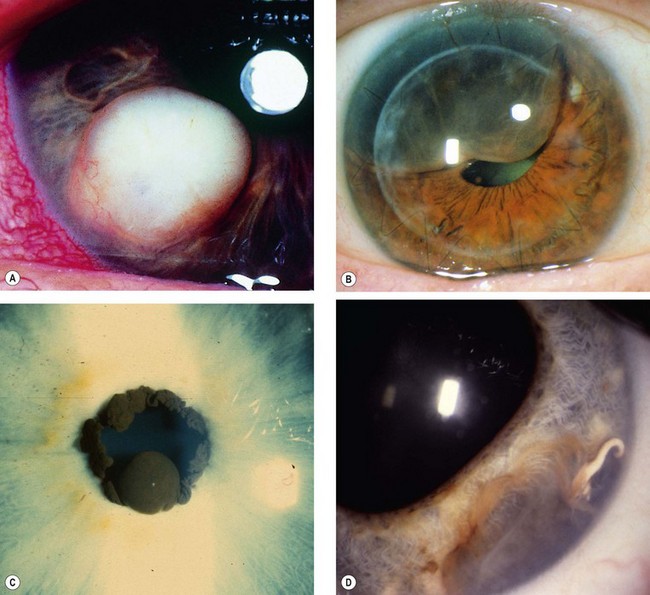
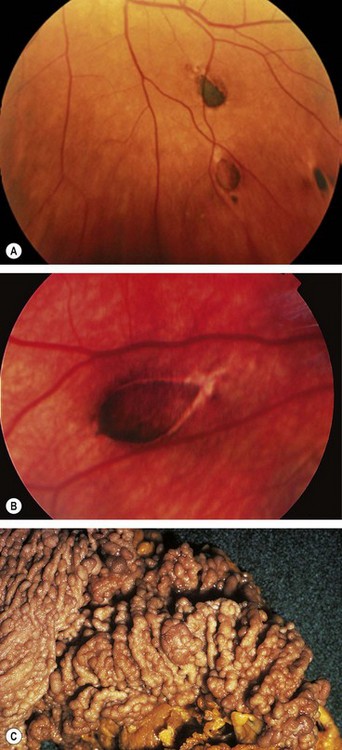
Fig. 12.1 Conjunctival naevus. (A) Histology of a junctional naevus shows nests of naevus cells at the epithelial/subepithelial junction; (B) histology of a compound naevus shows naevus cells at the epithelial/subepithelial junction and within the stroma, and downward proliferation of surface epithelium containing goblet cells (evident as clear spaces); (C) pigmented juxtalimbal naevus; (D) lightly pigmented juxtalimbal naevus; (E) naevus with cystic spaces; (F) naevus involving the caruncle
(Courtesy of J Harry and G Misson, from Clinical Ophthalmic Pathology, Butterworth-Heinemann 2001 – fig. A; J Harry – fig. B)
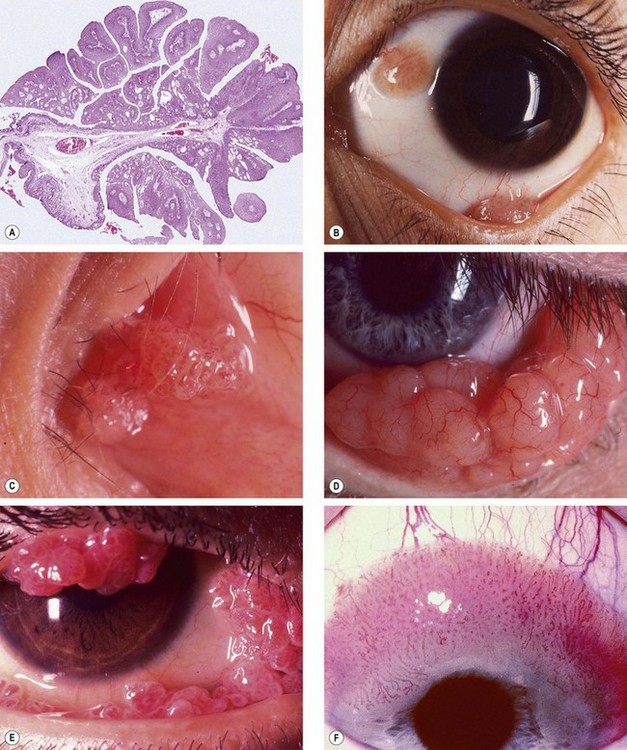
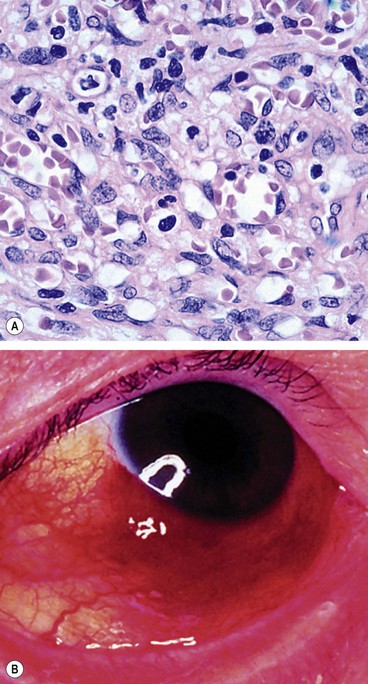
Fig. 12.2 Conjunctival papilloma. (A) Histology shows irregular proliferation of stratified squamous epithelium containing goblet cells, overlying a fibrovascular core; (B) small juxtalimbal and forniceal papillomas; (C) papillomas involving the plica and caruncle; (D) confluent papillomas; (E) large multiple papillomas interfering with eyelid closure; (F) large sessile papilloma encroaching onto the cornea
(Courtesy of J Harry – fig. A; U Raina – fig. B; R Bates – figs D and E)
Conjunctival papilloma
Conjunctival papillomas in childhood are caused by infection with human papillomavirus (types 6, 11 and 16) by mother-to-infant transmission at birth through an infected vagina. Adult papillomas are not infectious.
Dermoid
Diagnosis
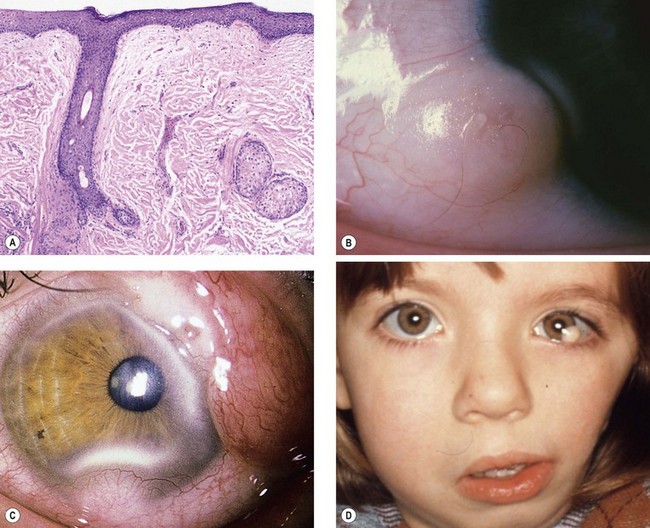
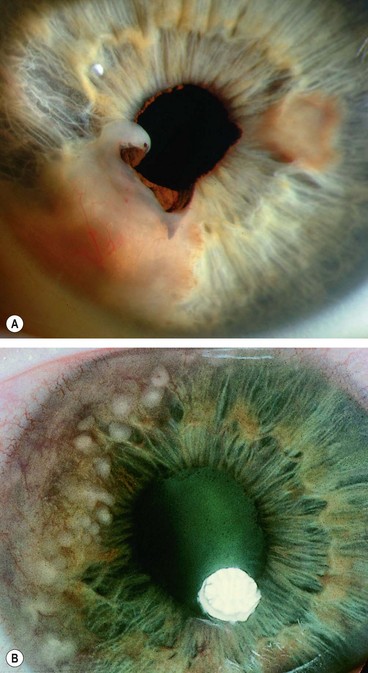
Fig. 12.3 Dermoid. (A) Histology shows a solid mass of collagenous tissue containing dermal elements and covered by stratified squamous epithelium; (B) typical dermoid with protruding hair; (C) complex choristoma; (D) dermoids in a patient with Goldenhar syndrome
(Courtesy of J Harry and G Misson, from Clinical Ophthalmic Pathology, Butterworth-Heinemann 2001 – fig. A)
Systemic associations
Systemic associations include Goldenhar syndrome (see below), and less commonly Treacher Collins syndrome (see Ch. 1) and naevus sebaceus of Jadassohn (see below).
Dermolipoma
Pyogenic granuloma
Conjunctival epithelial melanosis
Conjunctival (racial) epithelial melanosis is a benign condition due to increased melanin production. It is often seen in dark-skinned individuals. Both eyes are affected but the intensity may be asymmetrical.
Miscellaneous tumours
Malignant and premalignant epibulbar tumours
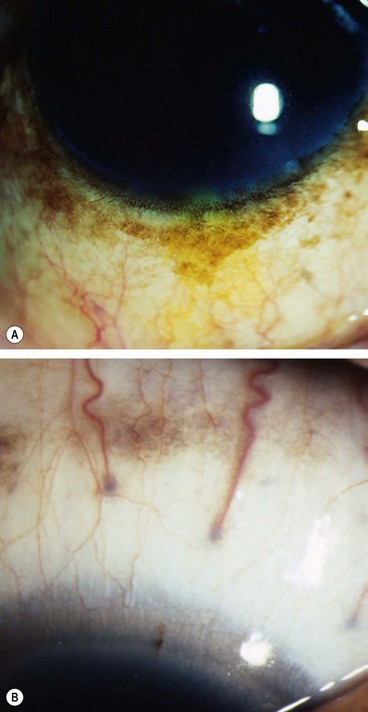
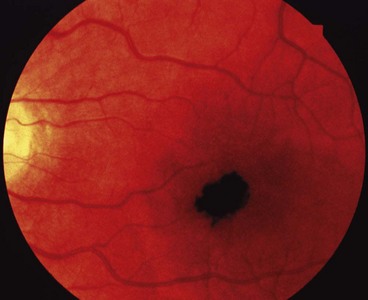
Primary acquired melanosis
Primary acquired melanosis (PAM) is a unilateral condition that typically affects white individuals with a fair complexion.
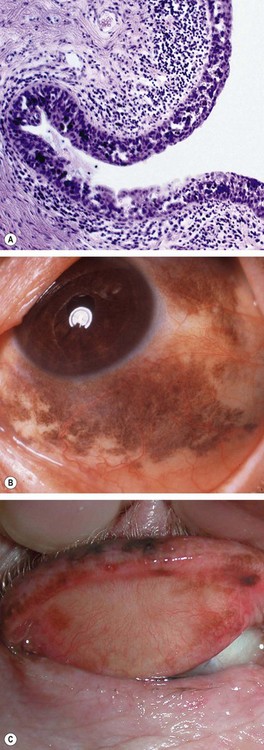
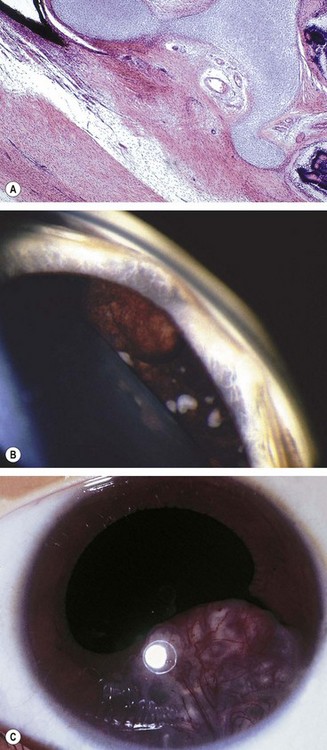
Fig. 12.8 Primary acquired melanosis (PAM). (A) Histology shows an intraepithelial proliferation of conjunctival epithelial melanocytes; (B) large area of PAM; (C) small area of PAM associated with lentigo maligna of the lid margin
(Courtesy of J Harry and G Misson, from Clinical Ophthalmic Pathology, Butterworth-Heinemann 2001 – fig. A; B Jay – fig. B; D Selva – fig. C)
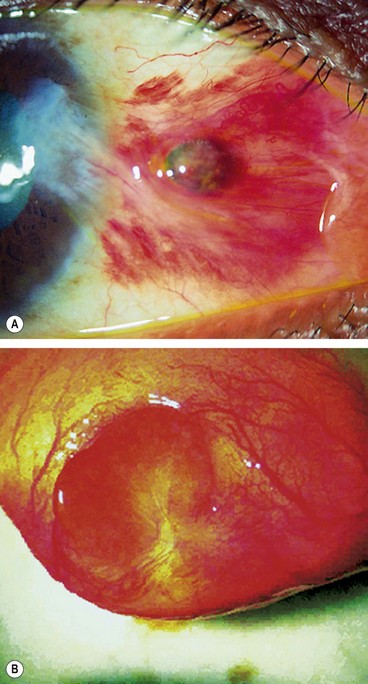
Melanoma
Conjunctival melanoma accounts for about 2% of all ocular malignancies.
Diagnosis
Treatment
Ocular surface squamous neoplasia
Definition
Ocular surface squamous neoplasia (OSSN) describes a spectrum of benign, pre-malignant and malignant unilateral slowly-progressive epithelial lesions of the conjunctiva and cornea. Risk factors include ultraviolet light exposure, human papilloma virus (type 16) infection, AIDS, xeroderma pigmentosum and stem cell therapy.
Diagnosis
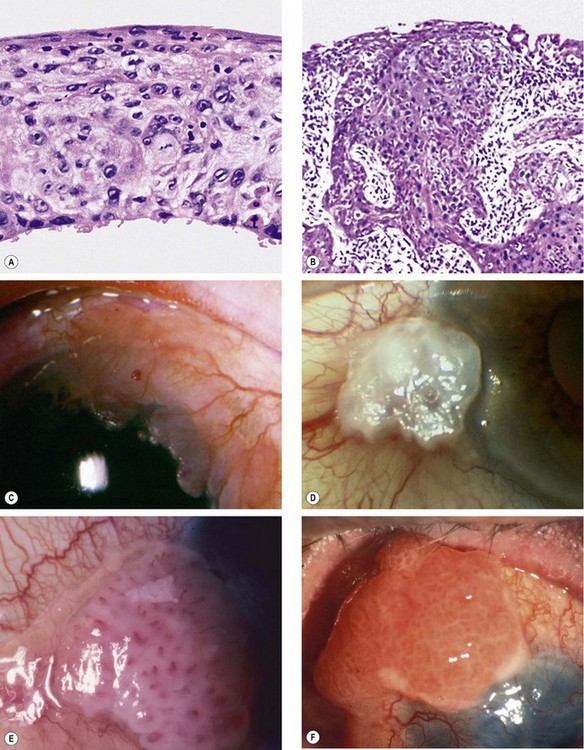
Fig. 12.10 Ocular surface squamous neoplasia. (A) Histology of carcinoma in situ shows dysplastic changes throughout the thickened epithelium; (B) histology of squamous cell carcinoma shows downward proliferation of irregular, dysplastic, squamous epithelium with infiltration of subepithelial tissue; (C) gelatinous lesion with surface vessels; (D) leukoplakic lesion; (E) papillomatous lesion; (F) very extensive carcinoma with corneal involvement
(Courtesy of J Harry – figs A and B; R Bates – fig. E; B Damato – fig. F)
Lymphoproliferative lesions
Most conjunctival lymphoproliferative lesions are reactive lymphoid hyperplasia, a proliferation of B and T cells with germinal follicle formation (Fig. 12.11A). Conjunctival lymphoma may arise in three clinical settings. (a) de novo, (b) extension from orbital lymphoma and (c) occasionally associated with systemic involvement. Sometimes reactive lymphoid hyperplasia undergoes transformation to lymphoma. Most conjunctival lymphomas are B cell lymphomas and arise from MALT (mucosa-associated lymphoid tissue).
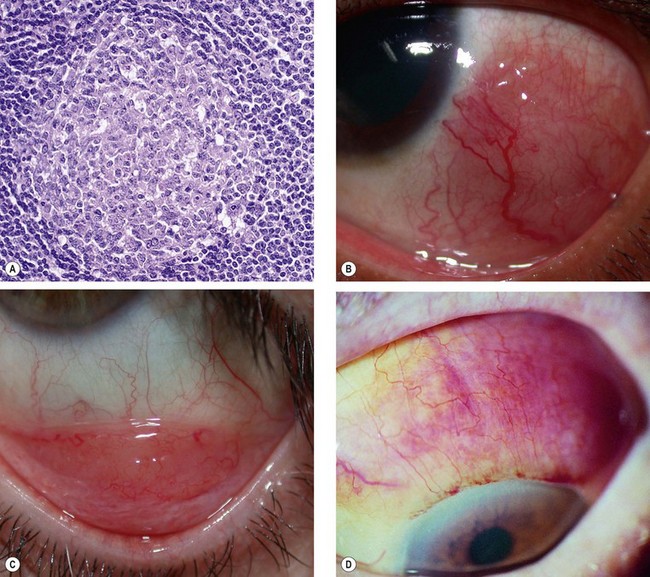
Fig. 12.11 Conjunctival lymphoproliferative lesions. (A) Histology of reactive lymphoid hyperplasia shows a germinal lymphoid follicle consisting of immature lymphoid cells at the centre and mature cells at the periphery; (B) epibulbar lymphoma; (C) forniceal lymphoma; (D) diffuse lymphoma
(Courtesy of J Harry and G Misson, from Clinical Ophthalmic Pathology, Butterworth-Heinemann 2001 – fig. A.)
Kaposi sarcoma
Kaposi sarcoma is a slow-growing tumour which occurs in patients with AIDS.
Iris tumours
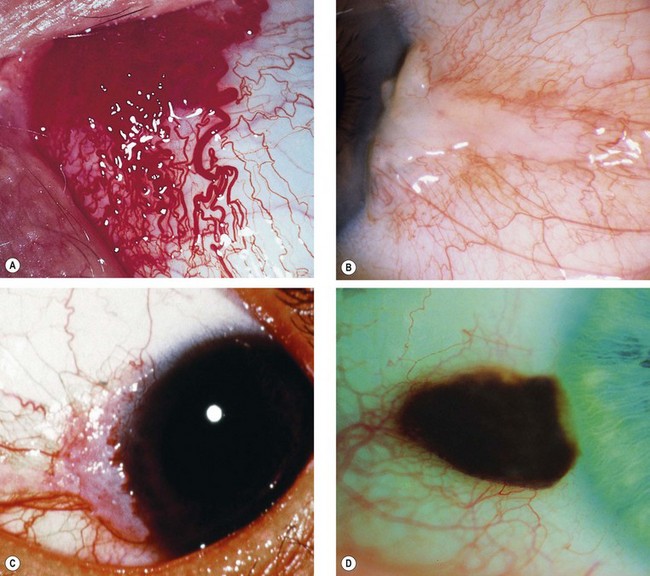
Iris naevus
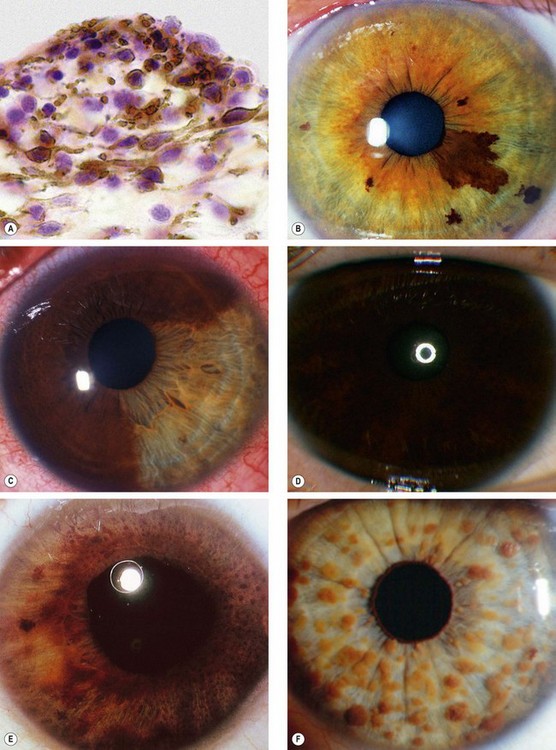
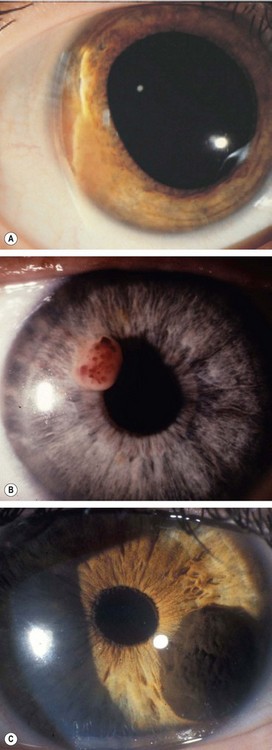
Fig. 12.13 Iris naevus. (A) Histology shows localized proliferation of melanocytes on the anterior iris stroma; (B) multiple iris freckles and a large naevus causing mild ectropion uveae; (C) sectoral diffuse naevus; (D) total diffuse iris naevus; (E) Cogan-Reese syndrome; (F) Lisch nodules
(Courtesy of J Harry – fig. A; B Damato – fig. C; P Gili – fig. F)
Iris melanoma
Overview
In general, uveal melanomas are three times more common in patients with blue/grey than brown irides. They are extremely rare in blacks and there is no sexual predominance. Conditions associated with or predisposing to uveal melanomas are: (a) fair skin, (b) light iris colour, (c) numerous cutaneous naevi, (d) congenital ocular melanocytosis, (e) oculodermal melanocytosis (naevus of Ota), (f) uveal melanocytoma, (g) dysplastic cutaneous naevi, (h) familial cutaneous melanoma and (i) NF1. About 8% of uveal melanomas arise in the iris. The prognosis is very good and only about 5% of patients develop metastases within 10 years of treatment.
Diagnosis
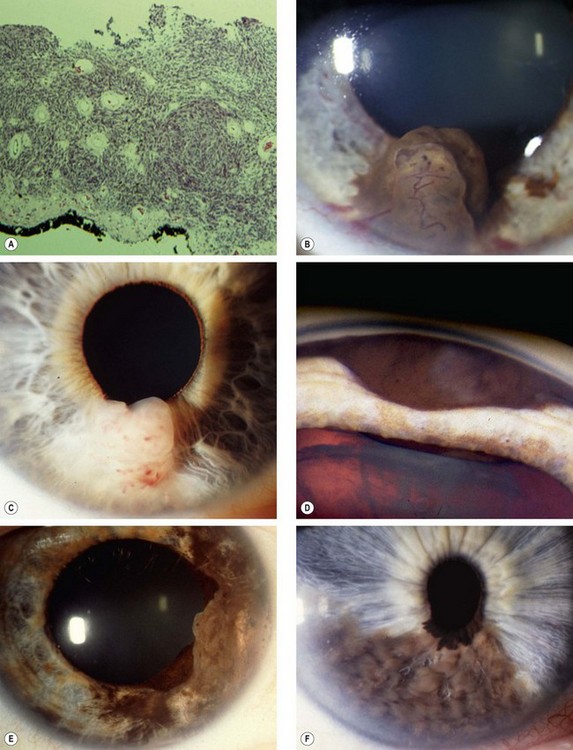
Fig. 12.14 Iris melanoma. (A) Histology shows infiltration of the entire thickness of the stroma; (B) highly pigmented tumour; (C) amelanotic tumour; (D) invasion of the angle; (E) extensive diffusely growing tumour; (F) ‘tapioca’ melanoma
(Courtesy of J Harry and G Misson, from Clinical Ophthalmic Pathology, Butterworth-Heinemann 2001 – fig. A; C Barry – fig. B; R Curtis fig. D; B Damato – fig. F)
Treatment
Metastatic tumours
Metastasis to the iris is rare and is characterized by a fast-growing white, pink or yellow mass (Fig. 12.15A) which may be associated with anterior uveitis and occasionally hyphaema. Small multiple deposits may also be seen (Fig. 12.15B).
Miscellaneous tumours
Iris cysts
Primary
Primary iris cysts are rare lesions arising from the iris epithelium or, rarely, the stroma. Epithelial cysts lie between the two layers of the pigment epithelium (Fig. 12.17A).
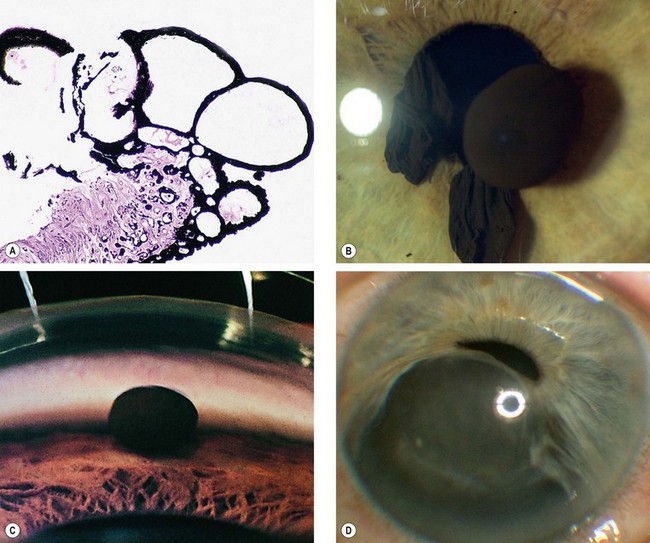
Fig. 12.17 Primary iris cysts. (A) Histology of epithelial cysts shows that they lie between the two layers of the pigment epithelium; (B) epithelial pupil margin cysts; (C) dislodged epithelial cyst in the angle; (D) enlarging stromal cyst
(Courtesy of J Harry and G Misson, from Clinical Ophthalmic Pathology, Butterworth-Heinemann 2001 – fig. A; J McAllister – fig. D)
Secondary
Secondary iris cysts develop as a result of the following:
Ciliary body tumours
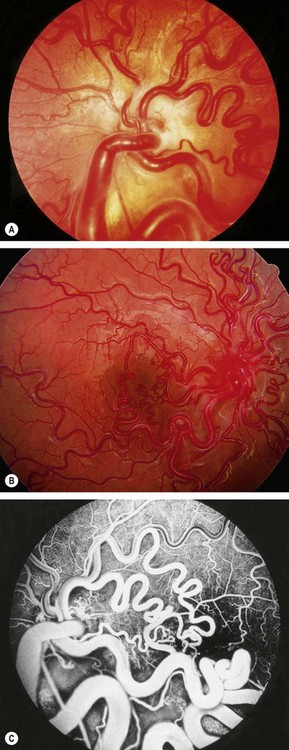
Ciliary body melanoma
Ciliary body melanomas comprise 12% of uveal melanomas.
Signs
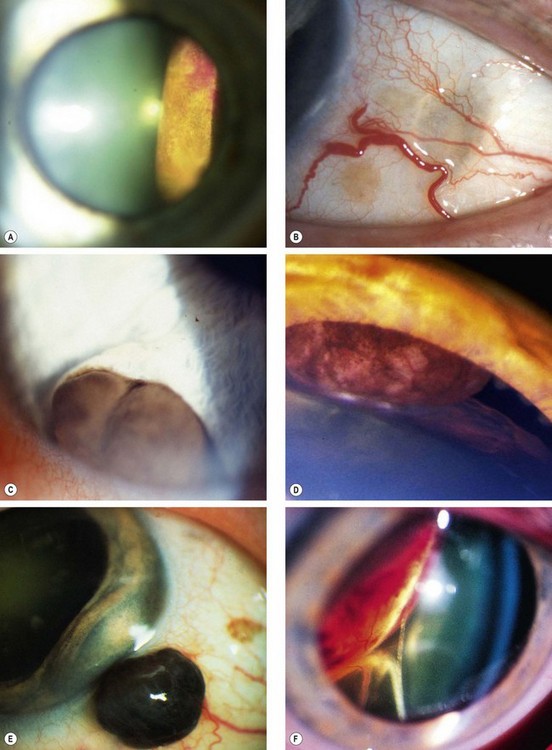
Fig. 12.19 Ciliary body melanoma. (A) Tumour seen on fundoscopy; (B) ‘sentinel’ vessels in the same quadrant as the tumour; (C) erosion of the tumour through the iris root; (D) pressure on the lens; (E) extraocular extension; (F) displacement of the lens and inferior retinal detachment
(Courtesy of B Damato – fig. B; R Curtis – fig. D)
Investigations
Treatment
Differential diagnosis
Medulloepithelioma
Medulloepithelioma (previously known as diktyoma) is a rare embryonal neoplasm that arises from the inner layer of the optic cup and can be benign or malignant. The latter may be fatal as a result of intracranial spread or metastatic disease.
Tumours of the choroid
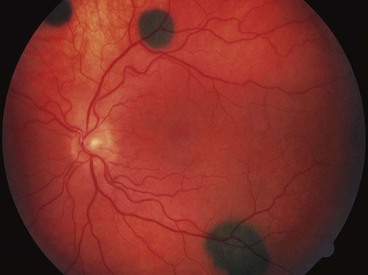
Choroidal naevus
Choroidal naevi are present in 5–10% of Caucasians but are very rare in dark-skinned races. They can be associated with NF1 and the dysplastic naevus syndrome. Although they are probably present at birth, growth occurs mainly during the pre-pubertal years and is extremely rare in adulthood. For this reason clinically detectable growth should arouse suspicion of malignancy.
Histology
The tumour is composed of a proliferation of spindle cell melanocytes (Fig. 12.21A).
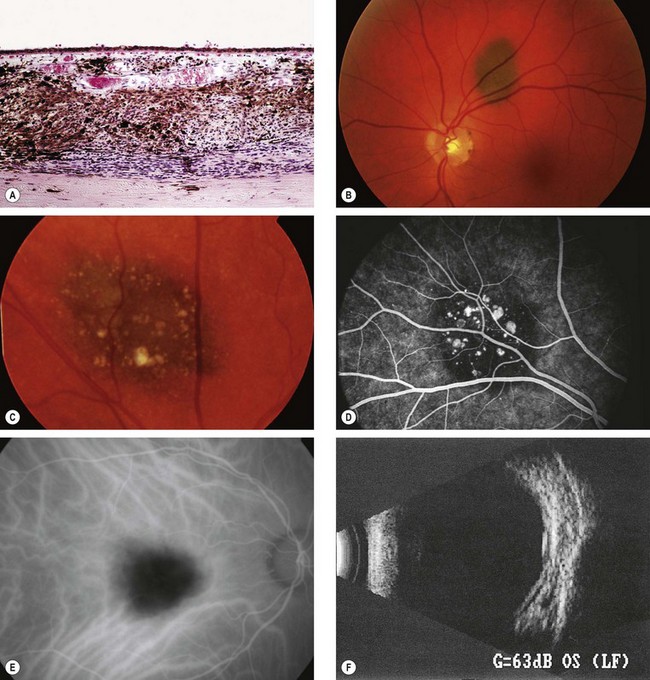
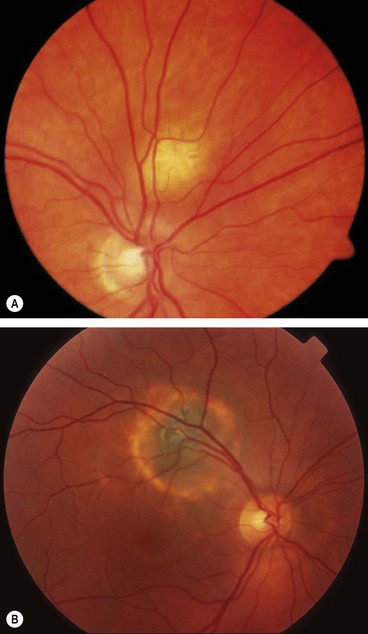
Fig. 12.21 Choroidal naevus. (A) Histology shows proliferation of melanocytes in the choroid but sparing the choriocapillaris; (B) typical naevus; (C) naevus with surface drusen; (D) FA shows hypofluorescence of the naevus and hyperfluorescence of drusen; (E) ICGA shows hypofluorescence relative to the surrounding choroid; (F) B-scan shows slight elevation with high internal acoustic reflectivity
(Courtesy of J Harry – fig. A; M Karolczak-Kulesza – fig. F)
Signs
Investigations
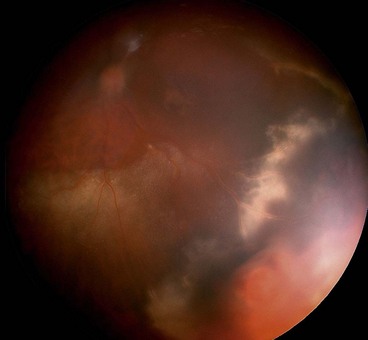
Choroidal melanoma
Choroidal melanoma has an overall incidence of 5–7.5 per million per year in western hemisphere countries with no significant gender difference. It is the most common primary intraocular malignancy in adults and accounts for 80% of all uveal melanomas.
Pathology
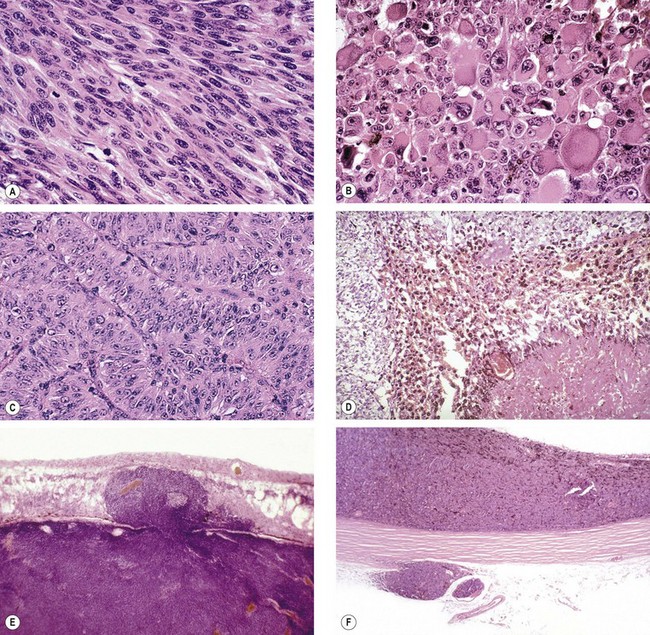
Fig. 12.23 Histology of choroidal melanoma. (A) Spindle cells – tightly arranged fusiform cells with indistinct cell membranes and slender or plump oval nuclei; (B) epithelioid cells – large pleomorphic cells with distinct cell membranes, large vesicular nuclei with prominent nucleoli, and abundant cytoplasm; (C) fascicular pattern – vasocentric; (D) necrotic tumour – cell type cannot be determined; (E) penetration of Bruch membrane in a ‘collar-stud’ fashion; (F) extraocular extension and an embolus of neoplastic cells within a blood vessel
(Courtesy of J Harry – figs A and B; J Harry and G Misson, from Clinical Ophthalmic Pathology, Butterworth-Heinemann 2001 – figs C, D, E and F)
Adverse prognostic factors
Signs
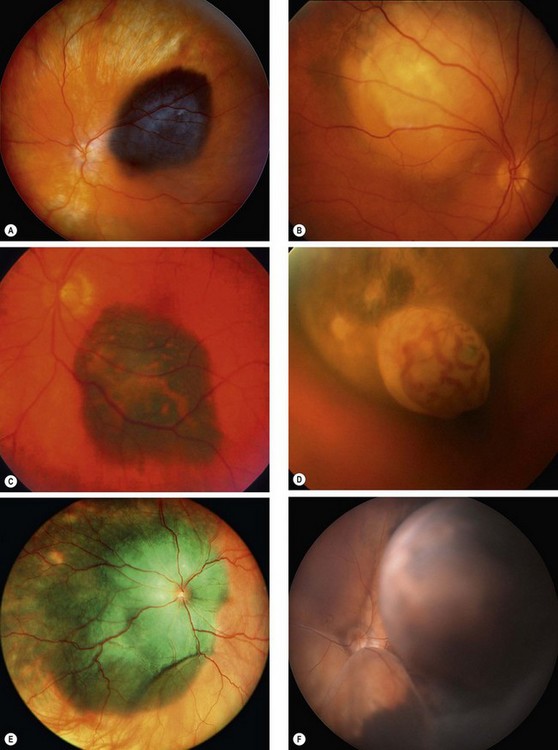
Fig. 12.24 Choroidal melanoma. (A) Highly pigmented melanoma; (B) amelanotic melanoma; (C) melanoma with surface orange pigment; (D) ‘collar-stud’ melanoma with intrinsic vessels; (E) diffuse melanoma; (F) large melanoma with subtotal retinal detachment
(Courtesy of B Damato – figs A, C and F); AD Singh, from Clinical Ophthalmic Pathology, Elsevier, 2007 – fig. E)
Special investigations
Although binocular indirect ophthalmoscopy combined with indirect slit-lamp biomicroscopy is sufficient for diagnosis in the vast majority of cases the following may be useful.
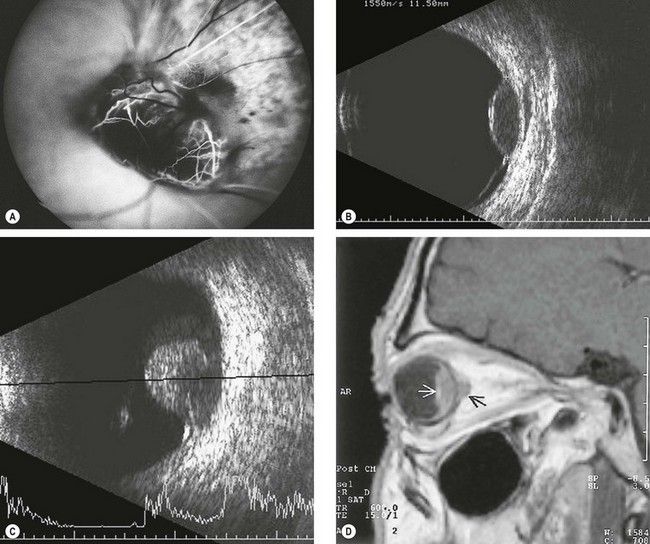
Fig. 12.25 Imaging in choroidal melanoma. (A) FA early phase of a ‘collar-stud’ tumour shows a ‘dual circulation’; (B) B-scan of a dome-shaped tumour shows choroidal excavation; (C) B-scan of a ‘collar-stud’ tumour; (D) T1-weighted MR shows a choroidal melanoma (white arrow) and extraocular extension (black arrow)
(Courtesy of B Damato – figs A and B; S Milewski – fig. C; M Karolczak-Kulesza – fig. D)
Systemic investigations
Systemic investigation is aimed at the following:
Principles of treatment
Treatment is performed to avoid the development of a painful and unsightly eye, preferably conserving as much useful vision as possible. Since it is not known when metastasis occurs it is uncertain as to whether or not ocular treatment influences survival. Theoretically the smaller the tumour the greater the opportunity for preventing metastasis and therefore the more urgent is the need for treatment. Management should be tailored to the individual patient taking the following factors into consideration:
Brachytherapy
Brachytherapy (episcleral plaque radiotherapy) with ruthenium-106 or an iodine-125 applicator (Fig. 12.26A) is usually the treatment of first choice because it is relatively straightforward and effective.
External beam radiotherapy
Irradiation with charged particles such as protons achieves a high dose in the tumour with a relatively small dose in the superficial tissues.
Stereotactic radiotherapy
Radiation is focused on the tumour by aiming multiple, highly collimated beams from different directions, either concurrently or sequentially, so that only the tumour receives a high dose of radiation. This is still a new technique, which is gaining in popularity in centres where proton beam radiotherapy is not available. The indications, contraindications and complications of these two methods are likely to be similar.
Transpupillary thermotherapy
Transpupillary thermotherapy (TTT) uses an infrared laser beam to induce tumour cell death by hyperthermia but not coagulation. It is a useful adjunct to radiotherapy.
Trans-scleral choroidectomy
Choroidectomy is a difficult procedure and is therefore not performed widely. It may be indicated for carefully selected tumours that that are too thick for radiotherapy but usually less than 16 mm in diameter. Complications include retinal detachment, ocular hypotony, wound dehiscence and local tumour recurrence.
Enucleation
Differential diagnosis
The following conditions should be considered in the differential diagnosis of atypical cases:
Circumscribed choroidal haemangioma
A circumscribed choroidal haemangioma is not associated with systemic disease. It may be dormant throughout life or may give rise to symptoms, usually as a result of exudative retinal detachment. Slight progressive enlargement can occur over many years.
Diagnosis
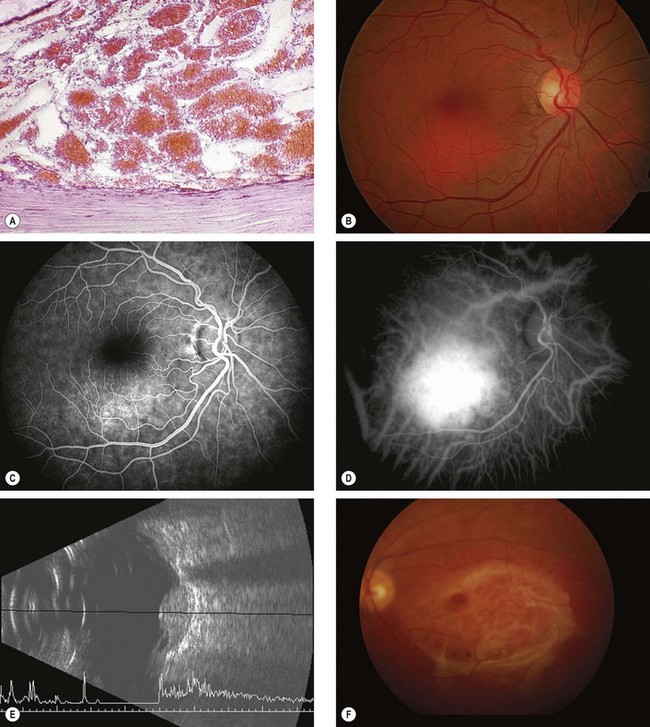
Fig. 12.27 Circumscribed choroidal haemangioma. (A) Histology shows varying-sized congested vascular channels forming a mass within the choroid; (B) clinical appearance; (C) FA early phase shows hyperfluorescence; (D) ICGA shows early hyperfluorescence; (E) B-scan shows an acoustically solid lesion with a sharp anterior surface and high internal reflectivity but without choroidal excavation and orbital shadowing; (F) surface fibrous metaplasia
(Courtesy of J Harry – fig. A; P Gili – figs B, C and D; B Damato – figs E and F)
Treatment
The following may be used to treat vision-threatening tumours.
Differential diagnosis
Diffuse choroidal haemangioma
Diffuse choroidal haemangioma usually affects over half of the choroid and enlarges very slowly. It occurs almost exclusively in patients with the Sturge–Weber syndrome ipsilateral to the naevus flammeus (see Ch. 1).
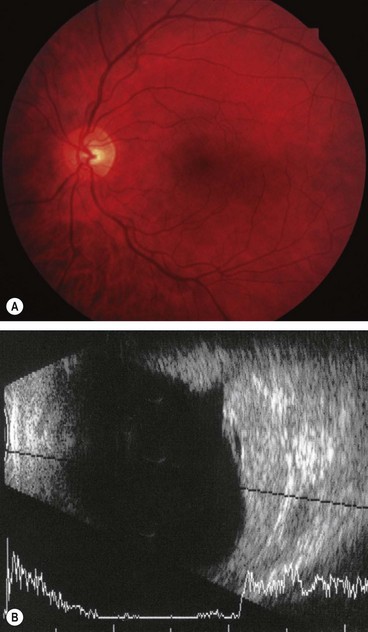
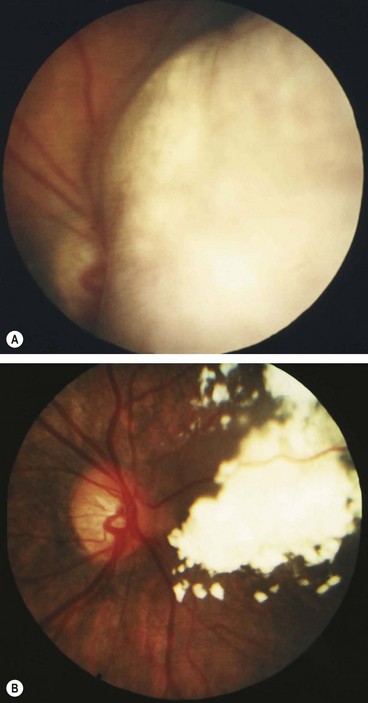
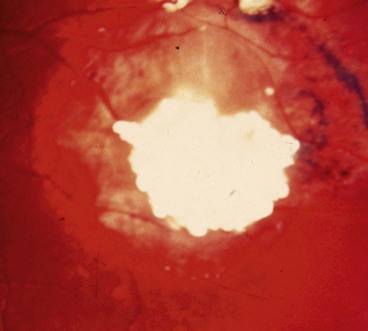
Optic disc melanocytoma
Melanocytoma (magnocellular naevus) is a rare, distinctive, unilateral, heavily pigmented congenital hamartoma which is seen most frequently in the optic nerve head but which can rarely arise anywhere in the uvea. In contrast to choroidal melanoma, melanocytomas are relatively more common in dark-skinned individuals and have a female predominance. In most cases the tumour is stationary with little tendency to change.
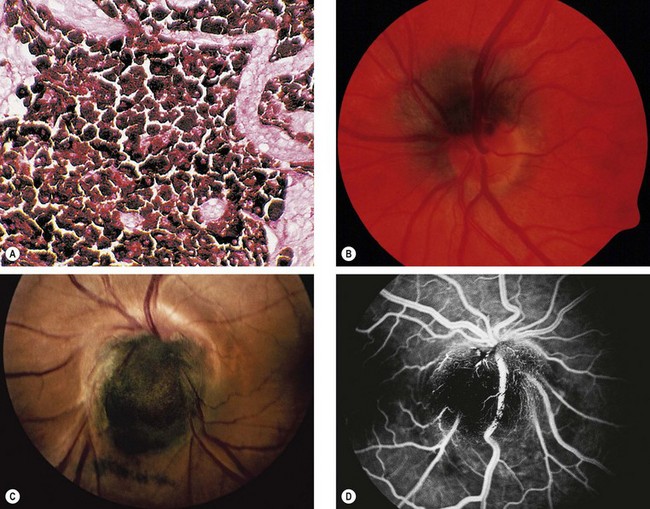
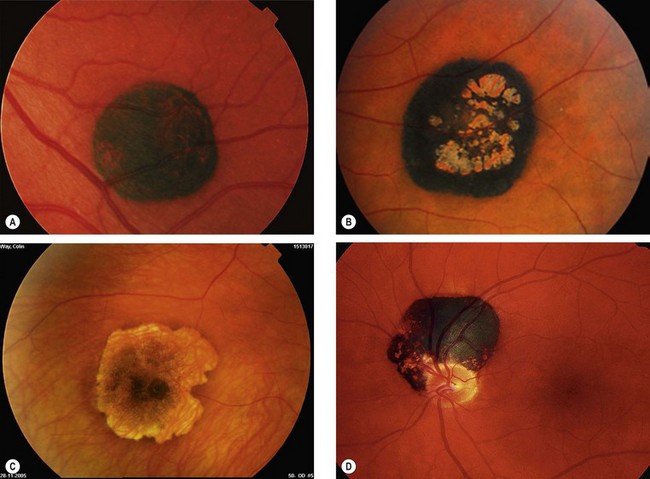
Choroidal osteoma
Choroidal osteoma is a very rare benign, slow-growing, ossifying tumour which has a very strong female preponderance. Both eyes are affected in about 25% of cases but not usually simultaneously.
Diagnosis
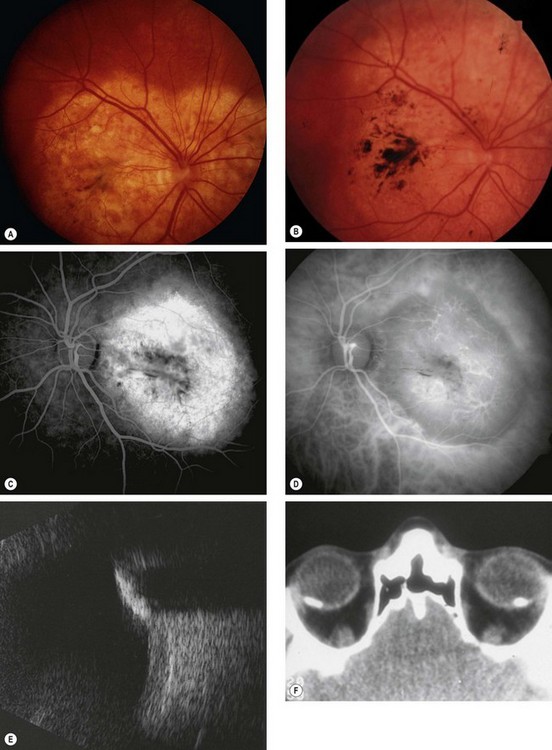
Fig. 12.30 Choroidal osteoma. (A) Early juxtapapillary lesion; (B) long-standing tumour with overlying RPE changes; (C) FA late phase shows mottled hyperfluorescence; (D) ICGA early phase shows hypofluorescence; (E) B-scan shows a highly reflective anterior surface and orbital shadowing; (F) axial CT demonstrates bilateral lesions that have the same consistency as bone
(Courtesy P Gili – figs C and D)
Differential diagnosis
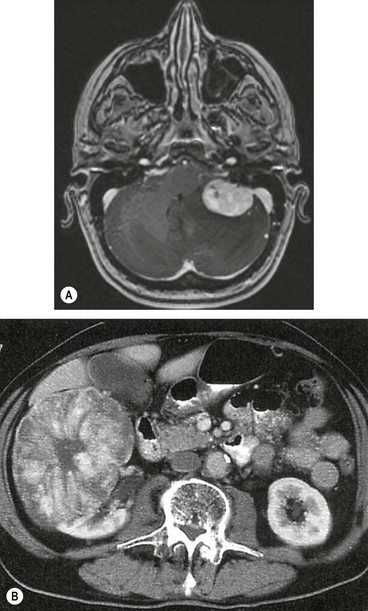
Metastatic tumours
The choroid is by far the most common site for uveal metastases accounting for about 90%, followed by the iris and ciliary body. The most frequent primary site is the breast and bronchus. A choroidal secondary may be the initial presentation of a bronchial carcinoma, whereas a past history of breast cancer is the rule in patients with breast secondaries. Other less common primary sites include the gastrointestinal tract, kidney and skin melanoma. The prostate is, however, an extremely rare primary site. Patient survival is generally poor, with a median of 8–12 months.
Diagnosis
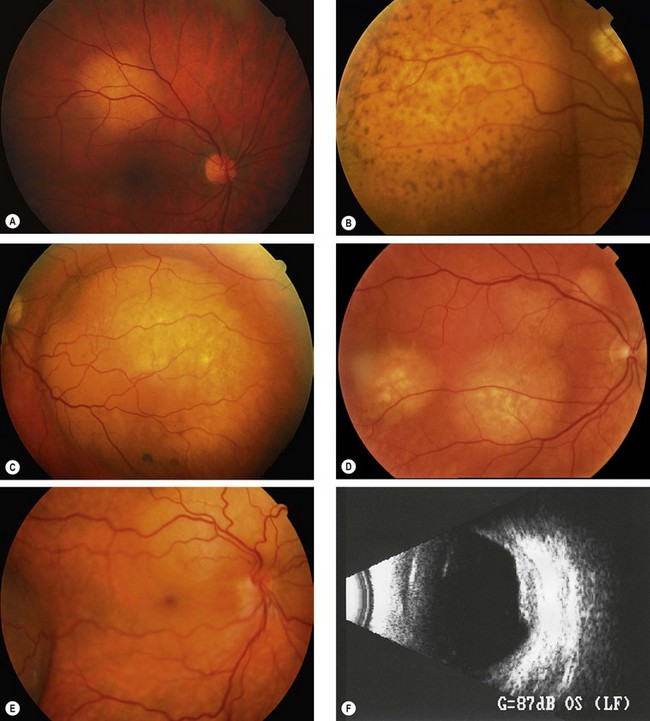
Fig. 12.31 Choroidal metastasis. (A) Small placoid deposit; (B) secondary pigment clumps on the surface of a large deposit; (C) large dome-shaped deposit; (D) multiple deposits; (E) deposits above the disc and in the temporal fundus with shallow inferior retinal detachment; (F) B-scan of a placoid lesion
(Courtesy of C Barry – figs A and E; B Damato – fig. B)
Systemic investigations
Systemic investigations are aimed at locating the primary tumour, if unknown, and other metastatic sites. This may include the following:
Neural retinal tumours
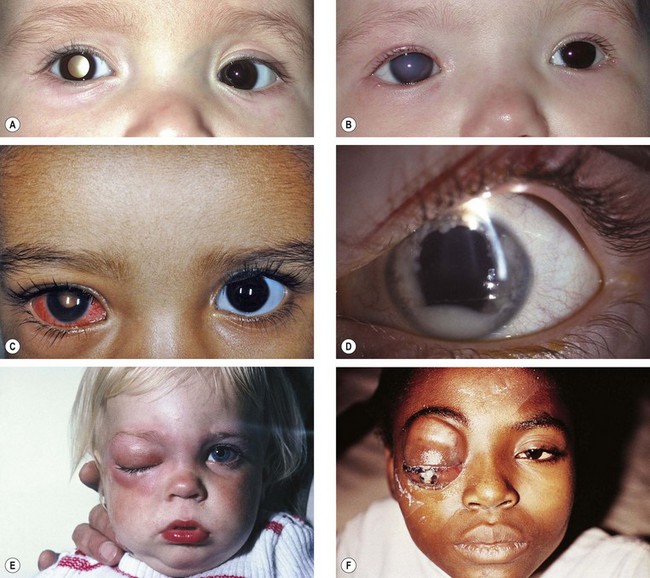
Retinoblastoma
Retinoblastoma is the most common primary intraocular malignancy of childhood and accounts for about 3% of all childhood cancers. Even so, it is rare, occurring in about 1 : 17 000 live births.
Pathology
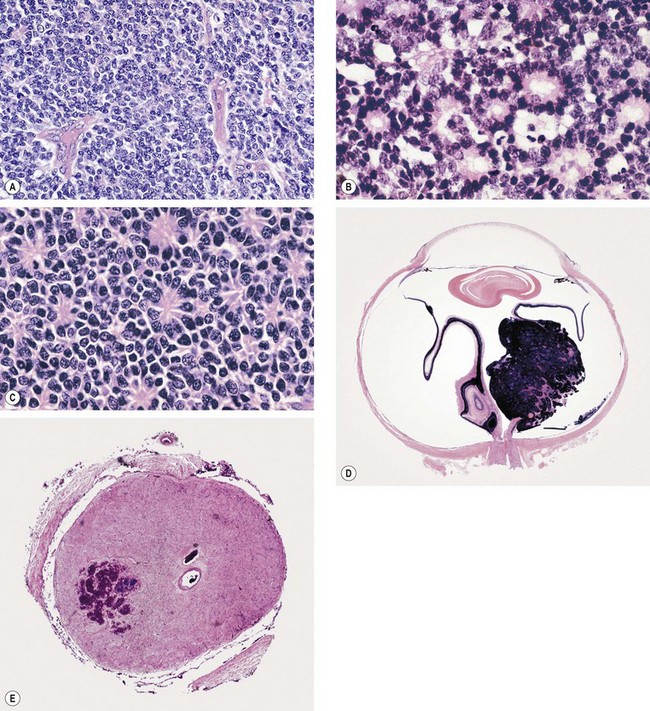
Fig. 12.32 Pathology of retinoblastoma: (A) Undifferentiated tumour; (B) well-differentiated tumour shows abundant Flexner-Wintersteiner rosettes; (C) fleurettes; (D) whole eye section shows a mixed endophytic (into the vitreous) and exophytic (into the subretinal space) growth pattern; (E) transverse section of the cut end of the optic nerve with an area of tumour infiltration
(Courtesy of J Harry and G Misson, from Clinical Ophthalmic Pathology, Butterworth-Heinemann 2001 – fig. A; courtesy of J Harry – figs B, C, D and E)
In both heritable and non-heritable retinoblastoma (see below), the risk of metastatic disease is greater if the tumour is advanced, and if there is retrolaminar optic nerve invasion, massive choroidal invasion, anterior chamber involvement and orbital spread. Repeated recurrences after conservative treatment also indicate an increased risk of metastasis.
Genetics
Retinoblastoma results from malignant transformation of primitive retinal cells before final differentiation. Because these cells disappear within the first few years of life, the tumour is seldom seen after 3 years of age. Retinoblastoma may be heritable or non-heritable. The gene predisposing to retinoblastoma (RB1) is at 13q14.
Siblings at risk of retinoblastoma should be screened by prenatal ultrasonography, and by ophthalmoscopy soon after birth and then regularly until the age of 4 or 5 years.
Presentation
Presentation is within the first year of life in bilateral cases and around 2 years of age if the tumour is unilateral.
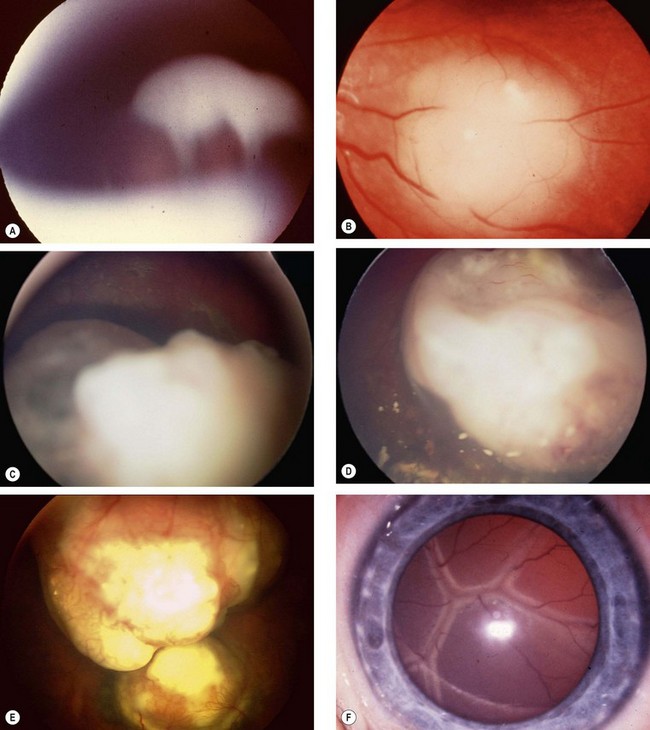
Signs
Indirect ophthalmoscopy with scleral indentation must be performed on both eyes after full mydriasis. This is because without indentation pre-equatorial tumours may be missed (Fig. 12.34A) and one eye may harbour multiple tumours. The clinical signs depend on tumour size and growth pattern.
Investigations
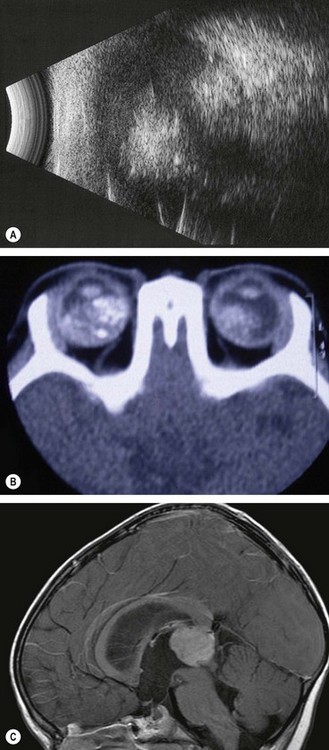
Fig. 12.35 Imaging of retinoblastoma. (A) B-scan with low gain shows echoes from calcification; (B) axial CT shows bilateral tumours and calcification; (C) sagittal MR shows a pinealoblastoma with secondary hydrocephalus
(Courtesy of K Nischal – fig. B; AD Singh, from Clinical Ophthalmic Pathology, Saunders Elsevier, 2007 – fig. C)
Treatment of small tumours
Tumours no more than 3 mm diameter and 2 mm thickness may be treated as follows:
Treatment of medium-size tumours
Tumours up to 12 mm wide and 6 mm thick may be treated as follows:
Treatment of large tumours
Treatment of extraocular extension
Follow-up
Differential diagnosis
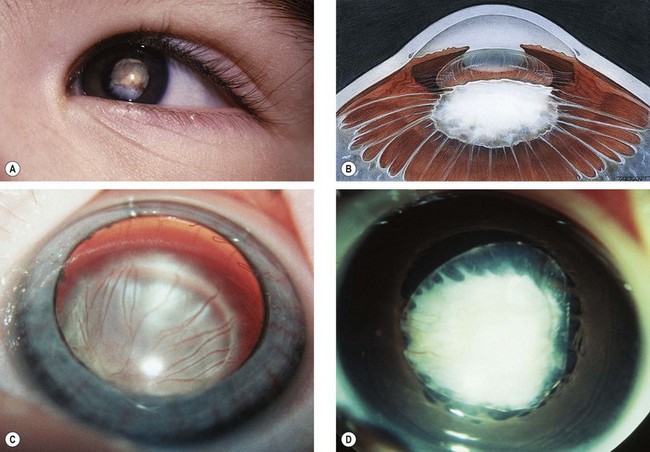
Fig. 12.37 Persistent anterior fetal vasculature. (A) Leukocoria; (B) retrolental mass with inserted ciliary processes; (C) early involvement; (D) advanced case with cataract
(Courtesy of K Nischal)
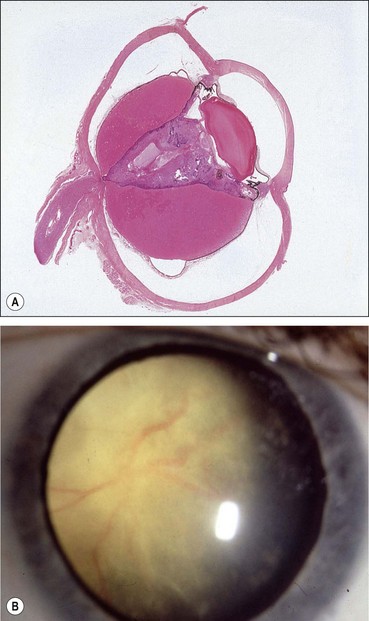
Fig. 12.39 Vitreoretinal dysplasia. (A) Pathological specimen; (B) clinical appearance
(Courtesy of J Harry and G Misson, from Clinical Ophthalmic Pathology, Butterworth-Heinemann 2001 – fig. A)
Astrocytoma
Astrocytoma of the retina and optic nerve head is a rare hamartoma, which does not usually threaten vision and does not require treatment. Most are endophytic, protruding into the vitreous, but exophytic subretinal tumours can occur. Astrocytomas may occasionally be encountered as incidental solitary lesions in normal individuals but are most frequently seen in tuberous sclerosis (see below) and occasionally in association with NF1 and retinitis pigmentosa. About 50% of patients with tuberous sclerosis have fundus astrocytomas which may be multiple and bilateral.
Diagnosis
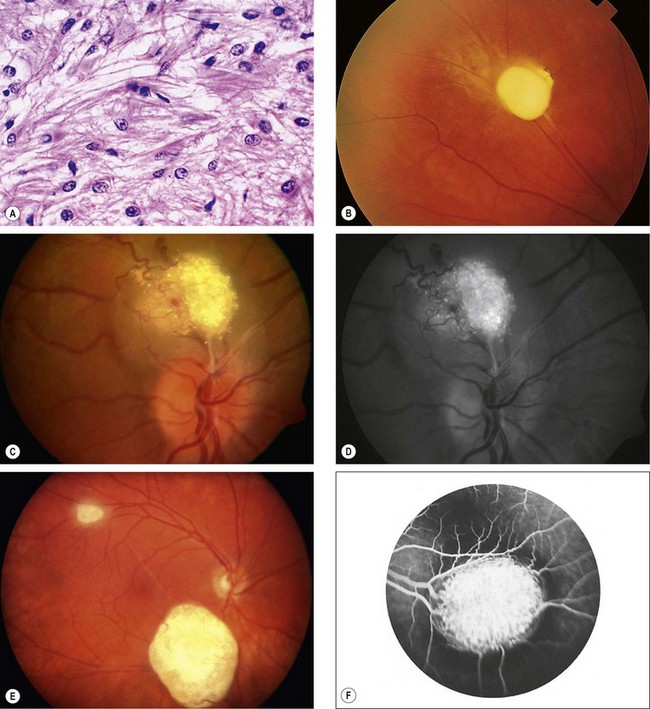
Fig. 12.42 Astrocytoma. (A) Histology shows proliferation of fibrous astrocytes with small oval nuclei and cytoplasmic processes; (B) small peripheral lesion; (C) juxtapapillary mulberry-like lesion; (D) red-free image shows autofluorescence; (E) two calcified lesions; (F) FA shows staining
(Courtesy of J Harry – fig. A; P Gili – figs C and D; J Donald M Gass, from Stereoscopic Atlas of Macular Diseases, Mosby 1997 – fig. F)
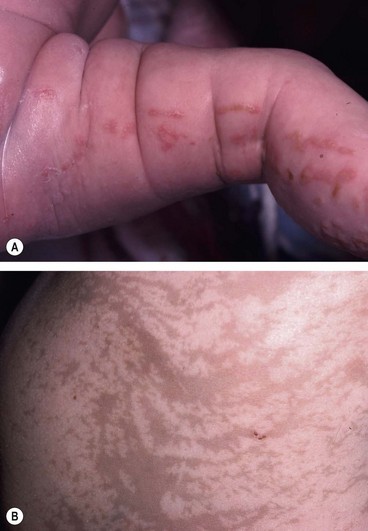
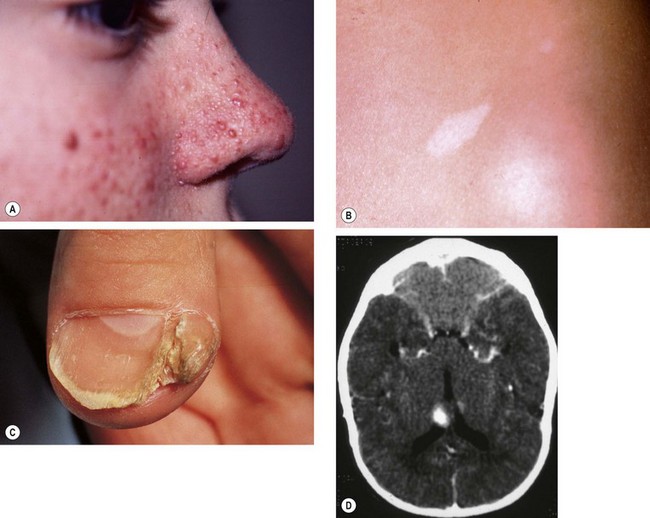
Tuberous sclerosis
Tuberous sclerosis (Bourneville disease) is an AD phacomatosis characterized by the development of hamartomas in multiple organ systems from all primary germ layers. The classic triad of (a) epilepsy, (b) mental retardation and (c) adenoma sebaceum is only present in a minority of patients, but is diagnostic. About 60% of cases are sporadic and 40% are AD.
Vascular retinal tumours
Capillary haemangioma
Overview
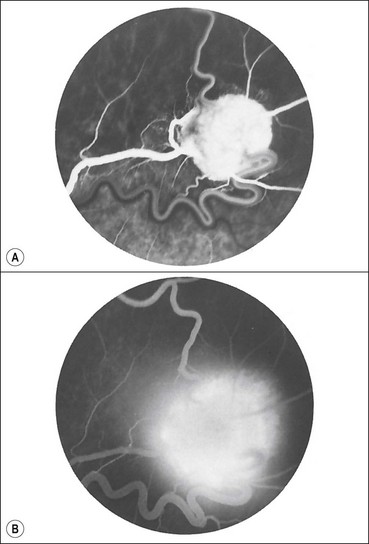
Retinal capillary haemangioma is a rare sight-threatening tumour that may occasionally occur in isolation, although about 50% of patients with solitary lesions and virtually all patients with multiple lesions have von Hippel–Lindau disease (VHL – see below). The prevalence of retinal tumours in VHL is approximately 60%. Vascular endothelial growth factor (VEGF) is important in the development of retinal lesions.
Diagnosis
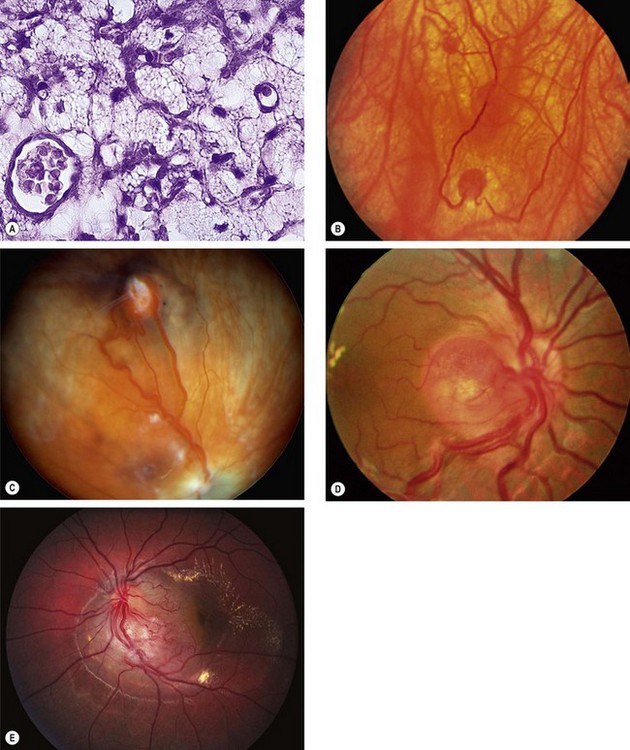
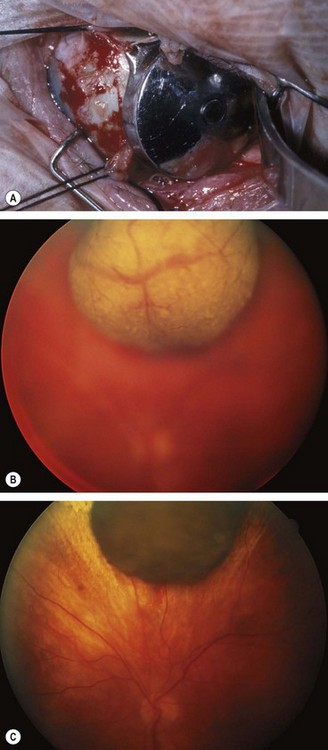
Fig. 12.44 Retinal capillary haemangioma. (A) Histology shows capillary-like vascular channels between large foamy cells; (B) early tumour; (C) more advanced tumour associated with vascular dilatation and tortuosity; (D) optic nerve head lesion; (E) sessile lesion
(Courtesy of J Harry and G Misson, from Clinical Ophthalmic Pathology, Butterworth-Heinemann 2001 – fig. A; B Damato – fig. C; J Donald M Gass, from Stereoscopic Atlas of Macular Diseases, Mosby 1997 – fig. D; P Saine – fig. E)
Treatment
Von Hippel–Lindau syndrome
Cavernous haemangioma
Cavernous haemangioma of the retina and optic nerve head is a rare, unilateral, congenital hamartoma. It is usually sporadic but occasionally can be inherited as AD with incomplete penetrance, in combination with lesions of the skin and CNS (‘neurooculocutaneous phacomatosis’ or ‘cavernoma multiplex’).
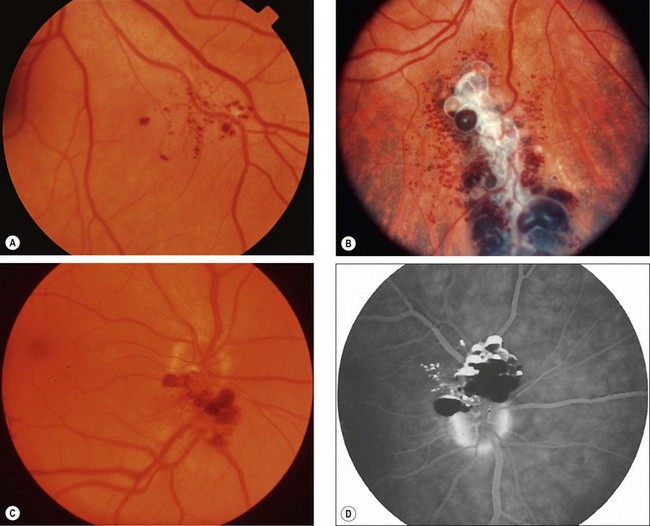
Fig. 12.48 Cavernous haemangioma. (A) Very small peripheral lesion; (B) larger peripheral lesion; (C) optic nerve involvement; (D) FA shows fluid levels due to separation of red cells (hypofluorescent) from plasma (hyperfluorescent)
(Courtesy of J Donald M Gass, from Stereoscopic Atlas of Macular Diseases, Mosby 1997 – fig. D)
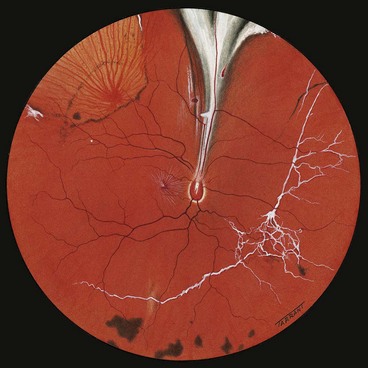
Racemose haemangioma
Racemose haemangioma (also known as arteriovenous malformation) of the retina and optic nerve head is a rare, sporadic, usually unilateral, congenital malformation involving direct communication between the arteries and veins without an intervening capillary bed. Some patients have similar ipsilateral lesions involving the midbrain, basofrontal region and posterior fossa (an association referred to as Wyburn–Mason syndrome). Brain involvement may lead to spontaneous haemorrhage or epilepsy. Occasionally, malformations may involve the maxilla and mandible, predisposing the patient to haemorrhage after dental treatment. Facial skin lesions have also been reported.
Vasoproliferative tumour
Retinal vasoproliferative tumour is a rare gliovascular lesion which can be primary or secondary to conditions such as intermediate uveitis, ocular trauma and retinitis pigmentosa. Secondary lesions may be multiple and occasionally bilateral depending on the underlying aetiology.
Primary intraocular lymphoma
Overview
Lymphoma is a group of conditions characterized by neoplastic proliferation of cells of the immune system typified by lymphadenopathy, constitutional symptoms, and occasionally CNS involvement. The main classification and ocular manifestations are as follows:
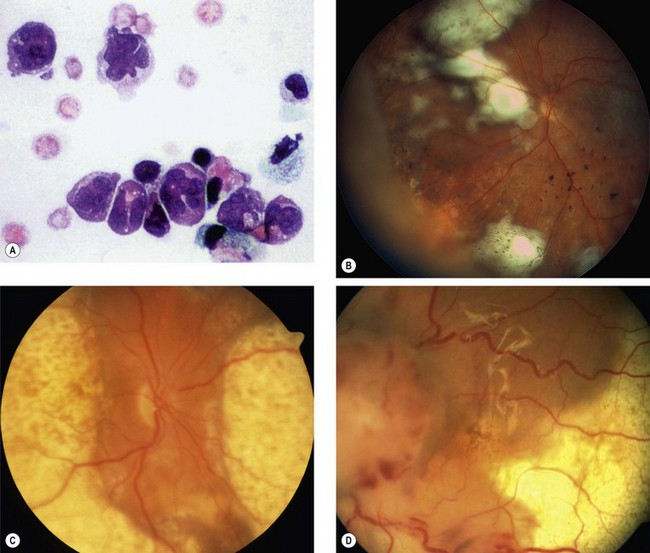
Fig. 12.51 Primary intraocular lymphoma. (A) Vitreous biopsy shows cells with irregular large nuclei and scanty cytoplasm; (B) multifocal subretinal infiltrates; (C) coalescent subretinal infiltrates; (D) shallow retinal detachment
(Courtesy of P Smith – fig. A; B Damato – figs B and C; A Turno-Krecicka – fig. D)
Ocular features
Neurological features
Investigations
Treatment
Tumours of the retinal pigment epithelium
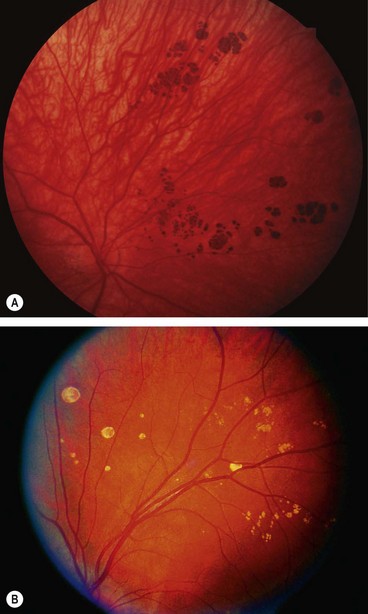
Typical congenital hypertrophy of the RPE
Congenital hypertrophy of the retinal pigment epithelium (CHRPE) is a common benign lesion which may be (a) typical, either solitary or grouped, or (b) atypical. It is important to differentiate between the two types because the latter may have important systemic implications.
Atypical congenital hypertrophy of the RPE
Signs
Systemic associations
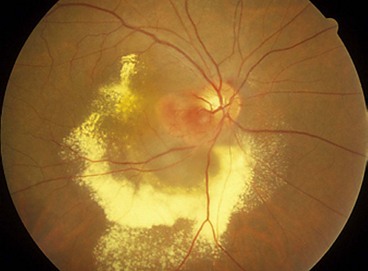
Combined hamartoma of the retina and RPE
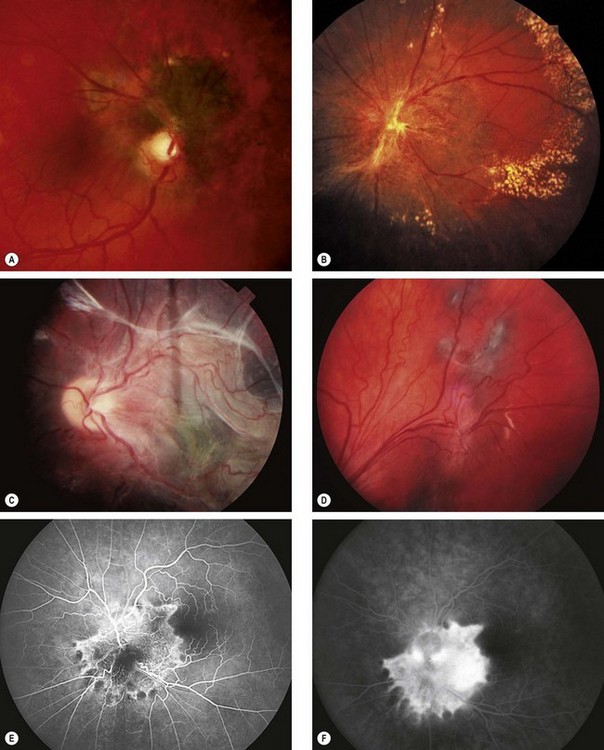
Fig. 12.55 Combined hamartoma of the retina and retinal pigment epithelium. (A) Small juxtapapillary lesion; (B) large peripapillary lesion with peripheral hard exudates; (C) large posterior pole lesion with ‘dragging’ of the disc; (D) peripheral lesion; (E) FA early venous phase shows hyperfluorescence of vascular lesions and blockage by pigment; (F) late phase shows intense hyperfluorescence due to leakage
(Courtesy of B Damato – fig. A; S Milewski – fig. C; C Barry – figs E and F)
Congenital hamartoma of the RPE
Congenital hamartoma of the RPE is a rare entity, usually incidentally diagnosed in asymptomatic children and young adults.
Paraneoplastic syndromes
Paraneoplastic retinopathies are rare diseases that might be missed or misdiagnosed by the unwary observer. Many of the patients present with visual symptoms before the primary malignancy is diagnosed. It is therefore important for clinicians to be familiar with these syndromes to detect the underlying malignancy as early as possible.
Bilateral diffuse uveal melanocytic proliferation
Bilateral diffuse uveal melanocytic proliferation (BDUMP) is a very rare paraneoplastic syndrome occurring usually in patients with systemic, often occult, malignancy. It is characterized by proliferation of benign melanocytes in the outer choroid.
Cancer-associated retinopathy
Cancer-associated retinopathy (CAR) is most frequently associated with small cell bronchial carcinoma, followed by gynaecological and breast cancer.
Melanoma-associated retinopathy
The presentation of melanoma-associated retinopathy (MAR) differs from CAR because the visual symptoms usually arise after, rather than prior to, the diagnosis of cutaneous melanoma. There may be concurrent vitiligo. The specific antigen responsible has not been identified, but autoantibodies from MAR sera react against bipolar cells in human retina. Clinical and electrophysiological data also implicate the bipolar cells as the disease target abnormality in MAR.