CHAPTER 4 Case Selection and Treatment Planning
The process of case selection and treatment planning begins after a clinician has diagnosed an endodontic problem. The clinician must determine whether the patient’s oral health needs are best met by providing endodontic treatment and maintaining the tooth or by advising extraction. The use of rotary instruments, ultrasonics, and microscopy as well as new materials has made it possible to predictably retain teeth that previously could not have been treated. Even teeth that have failed initial endodontic treatment can often be successfully retreated using nonsurgical or surgical procedures.
Increased knowledge concerning the importance of anxiety control, nonsteroidal antiinflammatory drug (NSAID) premedication, profound local anesthesia, appropriate occlusal adjustment, and biologically based clinical procedures enables clinicians to complete endodontic procedures without intra- or posttreatment pain.
Questions concerning tooth retention and possible referral can be answered only after a complete patient evaluation. The evaluation must include assessment of medical, psychosocial, and dental factors as well as consideration of the relative complexity of the endodontic procedure. Although most medical conditions do not contraindicate endodontic treatment, some can influence the course of treatment and require specific modifications. A number of valuable texts are available that review the subject of dental care for the medically compromised patient.32,40,78 The American Academy of Oral Medicine (Edmonds, WA) has an excellent website (http://www.aaom.com/) that can be used to elicit information about medically compromised patients.
Perhaps the most important advice for a clinician who plans to treat a medically compromised patient is to be prepared to communicate with the patient’s physician. The proposed treatment can be reviewed, and medical recommendations should be documented. Figure 4-1 depicts a sample medical consultation letter that can be modified as necessary.
The American Society of Anesthesiologists (ASA; Park Ridge, IL) Physical Status Classification system is commonly used to express medical risk (Box 4-1).
BOX 4-1 American Society of Anesthesiologists Physical Status Classification System
From: http://www.asahq.org/clinical/physicalstatus.htm
The ASA classification system remains the most widely used assessment method for preanesthetic patients despite some inherent limitations to its use as a peritreatment risk predictor. This classification system is a generally accepted and useful guide for pretreatment assessment of relative risk but does not advise appropriate treatment modifications. The clinician needs to go beyond the classification system and gather more information from the patient and physician, including the patient’s compliance with suggested medication, frequency of physician visits, and most recent visit. Typical questions include the following: Do you take medication as prescribed by your physician? Or, When was the last time you were examined by your physician? Other systems have been proposed that would better reflect the increasing number of medically complex patients treated by clinicians as Americans live longer.31 Regardless of the classification system used, these generalized guidelines need to be individualized for the patient under care.
An alternative means of considering risk assessment is to review the following issues:
A review of these areas provides the clinician with essential background data before initiating treatment.
Common Medical Findings That May Influence Endodontic Treatment Planning
Cardiovascular Disease
Patients with some forms of cardiovascular disease are vulnerable to physical or emotional stress that may be encountered during dental treatment, including endodontics. Patients may be confused or ill informed concerning the specifics of their particular cardiovascular problem. In these situations, consultation with the patient’s physician is mandatory before the initiation of endodontic treatment. Patients who have had a myocardial infarction (i.e., “heart attack”) within the past 6 months should not have elective dental care. Such patients have increased susceptibility to repeat infarctions and other cardiovascular complications and may be taking medications that could potentially interact with the vasoconstrictor in the local anesthetic. In addition, a vasoconstrictor should not be administered to patients with unstable angina pectoris or to patients with uncontrolled hypertension, refractory arrhythmias, recent myocardial infarctions (less than 6 mo), recent stroke (less than 6 mo), recent coronary bypass graft (less than 3 mo), uncontrolled congestive heart failure, and uncontrolled hyperthyroidism. Vasoconstrictors may interact with some antihypertensive medications and should be used only after consultation with the patient’s physician. For example, vasoconstrictors should be used with caution in patients taking digitalis glycosides (e.g., digoxin) because the combination of these drugs could precipitate arrhythmias. Local anesthetic agents with minimal or no vasoconstrictors are usually adequate for nonsurgical endodontic procedures (see also Chapter 20).40 A systematic review of the cardiovascular effects of epinephrine concluded that, although the quantity and quality of pertinent articles were problematic, the increased risk for adverse events among uncontrolled hypertensive patients was low, and the reported adverse events associated with epinephrine use in local anesthetics was minimal.6 Another review highlighted the advantages of including a vasoconstrictor in the local anesthesia, and stated that “pain control was significantly impaired in those patients receiving the local anesthetic without the vasoconstrictor as compared to those patients receiving the local anesthetic with vasoconstrictor.”12
A patient who has specific heart conditions may be susceptible to an infection of the heart valves, induced by a bacteremia. This infection is called infective or bacterial endocarditis and is potentially fatal. The American Heart Association (AHA; Dallas, TX) amended their recommendations in 2007 and recommends antibiotic prophylaxis in patients with prosthetic heart valves, a history of infective endocarditis, and a number of congenital heart abnormalities.77 The specific recommendations are summarized in a reference guide by the American Association of Endodontists (AAE; Chicago, IL) found online at http://www.aae.org/dentalpro/guidelines.htm. Because the AHA periodically revises its recommended antibiotic prophylactic regimen for dental procedures, it is essential that the clinician stay current concerning this important issue. A low compliance rate exists among at-risk patients regarding their use of the suggested antibiotic coverage before dental procedures. Therefore the clinician must question patients concerning their compliance with the prescribed prophylactic antibiotic coverage before endodontic therapy. If a patient has not taken the antibiotic as recommended, it may be administered up to 2 hours after the procedure.77
Patients with artificial heart valves are considered highly susceptible to bacterial endocarditis. Consulting the patient’s physician in such cases regarding antibiotic premedication is essential. Some physicians elect to administer parenteral antibiotics in addition to or in place of the oral regimen. The coronary artery bypass graft is a common form of cardiac surgery. Ideally, vasoconstrictors should be minimized during the first 3 months after surgery to avoid the possibility of precipitating arrhythmias. Ordinarily these patients do not require antibiotic prophylaxis after the first few months of recovery unless there are other complications.77
A clinician may be the first to detect elevated blood pressure if he/she routinely evaluates blood pressure before treatment. Furthermore, patients receiving treatment for hypertension may not be controlled adequately because of poor compliance or inappropriate drug therapy. Abnormal blood pressure readings may be the basis for physician referral.
Few conditions exist in which there is a possibility that dental treatment could seriously injure the patient or result in death. However, acute heart failure during a stressful dental procedure in a patient with significant valvular disease and heart failure or the development of infectious endocarditis represent two such life-threatening disorders.67 Careful evaluation of patients’ medical histories including the cardiac status of patients, the use of appropriate prophylactic antibiotics, and stress reduction strategies will minimize the risk of serious cardiac sequelae.
There is a widespread belief among dental clinicians and physicians that oral anticoagulation therapy in which patients receive drugs such as warfarin (Coumadin) must be discontinued before dental treatment to prevent serious hemorrhagic complications, especially during and after surgical procedures. Aspirin is a drug commonly used as an anticoagulant on a daily basis without the supervision of a physician. Clinical studies do not support the routine withdrawal of anticoagulant therapy before dental treatment for patients who are taking such medications.35,40 When patients report they are receiving an anticoagulant medication, they can benefit from the clinician using the following guidelines:
Another cardiac complication may occur in patients with Hodgkin’s disease or breast cancer, who often receive irradiation to the chest as an element of treatment. Although the therapy often cures the malignancy, it has been implicated in causing late-onset heart disease that may influence the development of a treatment plan and subsequent treatment. Therapeutic irradiation of the chest results in the inadvertent inclusion of the heart within the irradiation field. Some patients may experience pathologic changes of the heart valves that could predispose them to endocarditis, accelerated atherosclerosis of the coronary artery that increases their risk of experiencing a fatal myocardial infarction, or both. Clinicians must identify patients who have received irradiation to the chest and consult with patients’ physicians in order to determine whether that therapy has damaged the heart valves or coronary arteries. Patients with radiation-induced valvular disease may require prophylactic antibiotics when undergoing specific dental procedures that are known to cause a bacteremia and a heightened risk of developing endocarditis. Patients with radiation-induced coronary artery disease should be administered only limited amounts of local anesthetic agents containing a vasoconstrictor. They may require the administration of sedative agents and cardiac medications to preclude ischemic episodes. Consultation with the patient’s physician is an appropriate response when a patient presents with a history that includes prior radiation to the chest.27
Diabetes
The Centers for Disease Control and Prevention (CDC, Atlanta, GA) in 2006 reported a 6% increase in the incidence of diabetes mellitus in the United States in 1 year.30 This dramatic change has been linked to a remarkable increase in obesity among Americans during the last 10 years. Diabetes is the third leading cause of death in the United States, and it is estimated that nearly 20 million Americans, representing 6.5% of the population, have diabetes.45 Even more alarming is the fact that approximately 6 million of those cases have not been diagnosed. It is likely that diabetic patients requiring endodontic treatment will be increasingly common.
Diabetes mellitus appears to have multiple causes and several mechanisms of pathophysiology.40 It can be thought of as a combination of diseases that share the key clinical feature of glucose intolerance. Patients with diabetes, even those who are well controlled, require special consideration during endodontic treatment. The patient with diabetes who is well controlled medically and free of serious complications such as renal disease, hypertension, or coronary atherosclerotic disease, is a candidate for endodontic treatment. However, special considerations exist in the presence of acute infections. The non–insulin-controlled patient may require insulin, or the insulin dose of some insulin-dependent patients may have to be increased.57 When surgery is required, consultation with the patient’s physician is advisable in order to consider adjustment of the patient’s insulin dosage, antibiotic prophylaxis, and dietary needs during the posttreatment period.
The clinician should ask diabetic patients who self-monitor their glucose levels to bring a glucometer to each visit. If pretreatment glucose levels are below normal fasting range (80-120 mg/dl), it may be appropriate to take in a carbohydrate source.72 A source of glucose (e.g., glucose tablets, orange juice, or soda) should be available if signs of insulin shock (hypoglycemic reaction caused by overcontrol of glucose levels) occur.40 Signs and symptoms of hypoglycemia include confusion, tremors, agitation, diaphoresis, and tachycardia.72 The clinician can avoid a hypoglycemic emergency by taking a complete, accurate history of time and amount of the patient’s insulin and meals.
Appointments should be scheduled with consideration given to the patient’s normal meal and insulin schedule.57 Usually, a patient with diabetes who is well managed medically and is under good glycemic control without serious complications such as renal disease, hypertension, or coronary atherosclerotic heart disease can receive any indicated dental treatment.45 However, patients with diabetes who have serious medical complications may need a modified dental treatment plan.32 Moreover, studies suggest that diabetes is associated with a decrease in the success of endodontic treatment in cases with pretreatment periradicular lesions.11,26 These patients may require referral to an endodontist for alternative treatment considerations.
Pregnancy
Although pregnancy is not a contraindication to endodontics, it does modify treatment planning. Protection of the fetus is a primary concern when administration of ionizing radiation or drugs is considered. Of all the safety aids associated with dental radiography, such as high-speed film, digital imaging, filtration, and collimation, the most important is the protective lead apron with thyroid collar.7,76 Although drug administration during pregnancy is a controversial subject, Box 4-2 presents commonly used dental drugs usually compatible with both pregnancy and breast-feeding.40 A major concern is that a drug may cross the placenta and be toxic or teratogenic to the fetus. In addition, any drug that is a respiratory depressant can cause maternal hypoxia, resulting in fetal hypoxia, injury, or death. Ideally, no drug should be administered during pregnancy, especially during the first trimester. If a specific situation makes adherence to this rule difficult, then the clinician should review the appropriate current literature and discuss the case with the physician and patient.10,43,47
Further considerations exist during the postpartum period if the mother breast-feeds her infant. A clinician should consult the responsible physician before using any medications for the nursing mother. Alternative considerations include using minimal dosages of drugs, having the mother bank her milk before treatment, having her feed the child before treatment, or suggesting the use of a formula for the infant until the drug regimen is completed. There are limited data available on drug dosages and the effects on breast milk.40
In terms of treatment planning, elective dental care is best avoided during the first trimester because of the potential vulnerability of the fetus. The second trimester is the safest period in which to provide routine dental care. Complex surgical procedures are best postponed until after delivery.
Malignancy
Some malignancies may metastasize to the jaws and mimic endodontic pathosis, whereas others can be primary lesions (Fig. 4-2). A panoramic radiograph is useful in providing an overall view of all dental structures. When a clinician begins an endodontic procedure on a tooth with a well-defined apical radiolucency, it might be assumed to result from a nonvital pulp. Pulp testing is essential to confirm a lack of pulp vitality in such cases. A vital response in such cases is indicative of a nonodontogenic lesion.
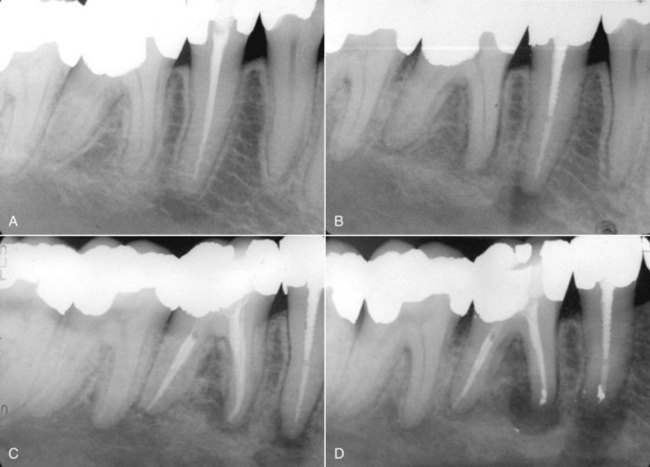
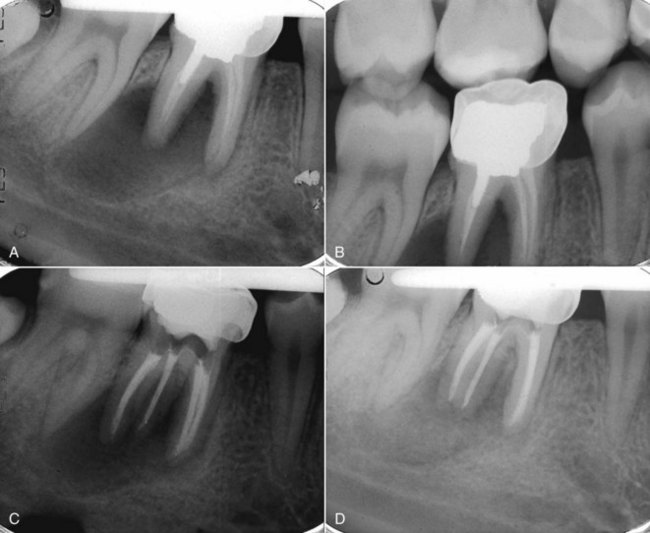
FIG. 4-2 A, Periapical view of tooth #29 after endodontic treatment by a general dental clinician. The diagnosis was irreversible pulpitis. B, Patient was referred to an endodontist 4 months later to evaluate radiolucencies of teeth #29 and #30. Symptoms indicated irreversible pulpitis of tooth #30, with concurrent lower right lip and chin paresthesia. Past medical history revealed breast cancer in remission. C, Nonsurgical endodontics was performed on tooth #30. Immediate referral was made to an oncologist/oral surgeon for biopsy to rule out nonodontogenic origin of symptoms. D, Surgical posttreatment radiograph of teeth #29 and #30. The biopsy report confirmed metastatic breast cancer.
(Courtesy Dr. R. Sadowsky, Dr. L. Adamo, and Dr. J. Burkes.)
Careful examination of pretreatment radiographs from different angulations is important because lesions of endodontic origin would not be expected to be shifted away from the radiographic apex in the various images.
A useful website for the differential diagnosis of radiographic lesions (Oral Radiographic Differential Diagnosis [ORAD] II) was created by Dr. Stuart White and is available online at http://www.orad.org/index.html. A definitive diagnosis of a periradicular osteitis can be made only after biopsy. When a discrepancy exists between the initial diagnosis and clinical findings, consultation with an endodontist is advisable.
Patients undergoing chemotherapy or radiation to the head and neck may have impaired healing responses. Treatment should be initiated only after the patient’s physician has been consulted. Resolving the question of endodontic treatment or extraction for preradiation patients often requires a dialogue between the dental clinician and the physician.
The effect of the external beam of radiation therapy on normal bone is to decrease the number of osteocytes, osteoblasts, and endothelial cells, thus decreasing blood flow. Pulps may become necrotic from this impaired condition.40 Toxic reactions during and after radiation and chemotherapy are directly proportional to the amount of radiation or dosage of cytotoxic drug to which the tissues are exposed. Delayed toxicities can occur several months to years after radiation therapy. Oral infections and any potential problems should be addressed before initiating radiation. It is advised that symptomatic nonvital teeth be endodontically treated at least 1 week before initiating radiation or chemotherapy, whereas treatment of asymptomatic nonvital teeth may be delayed.40 The outcome of endodontic treatment should be evaluated within the framework of the toxic results of radiation and drug therapy. The white blood cell (WBC) count and platelet status of a patient undergoing chemotherapy should also be reviewed before endodontic treatment. In general, routine dental procedures can be performed if the granulocyte count is greater than 2000/mm3 and the platelet count is greater than 50,000/mm3. If urgent care is needed and the platelet count is below 50,000/mm3, consultation with the patient’s physician is required.59
Bisphosphonate Therapy
Bisphosphonates offer great benefits to patients at risk of bone metastases and in the prevention and treatment of osteoporosis. A patient’s risk of developing osteonecrosis of the jaw while receiving oral bisphosphonates appears to be low, but there are factors known to increase the risk for bisphosphonate-associated osteonecrosis (BON) (Box 4-3).3,4
Because there are no studies that appropriately present the incidence of BON and treatment is unpredictable, prevention strategies are extremely important.3,4 For patients at higher risk of BON, surgical procedures such as extractions, endodontic surgery, or placement of dental implants should be avoided, if possible. Sound oral hygiene and regular dental care may be the best approach to lowering the risk of BON.3 Patients taking bisphosphonates and undergoing endodontic therapy should sign an informed consent form, inclusive of the risks, benefits, and alternative treatment plans.4 In case of any infection in a patient taking bisphosphonates, aggressive use of systemic antibiotics is indicated.40 Discontinuing bisphosphonate therapy may not eliminate any risk of developing BON.41,42,44 There is currently no valid diagnostic technique available to determine whether a patient is at risk of BON,3 although some clinicians have proposed use of the CTX (C-terminal telopeptide of type I collagen α1 chain) test (Quest Diagnostics, Madison, NJ) for assessing the risk of developing BON. For patients who have developed BON, close coordination with an oral maxillofacial surgeon or dental oncologist is highly recommended.
An astute awareness of the potential risk of BON in patients receiving bisphosphonate therapy is critical. Increased attentiveness to the prevention, recognition, and management of BON will allow the clinician to make the best treatment decisions. Our knowledge of BON is developing rapidly and it is essential that the clinician monitor published recommendation changes.3,4,41,42,44
Human Immunodeficiency Virus and Acquired Immunodeficiency Syndrome
It is important, when treating patients with acquired immunodeficiency syndrome (AIDS), that the clinician understand the patient’s level of immunosuppression, drug therapies, and potential for opportunistic infections. Although the effect of human immunodeficiency virus (HIV) infection on long-term prognosis of endodontic therapy is unknown, it has been demonstrated that clinicians may not have to alter their short-term expectations for periapical healing in patients infected with HIV.56 The clinical team must also minimize the possibility of transmission of HIV from an infected patient, and this is accomplished by adherence to universal precautions (for details see Universal Precautions for Prevention of Transmission of HIV and Other Bloodborne Infections, available at http://www.cdc.gov/ncidod/dhqp/bp_universal_precautions.html). Although saliva has not been demonstrated to transmit the virus in a dental situation, the potential for it to do so exists.14,40 Infected blood can transmit HIV, and during some procedures it may become mixed with saliva. Latex gloves and eye protection are essential for the clinician and staff. HIV can be transmitted by needlestick or via an instrument wound, but the frequency of such transmission is low, especially with small-gauge needles.40
A vital aspect of treatment planning for the patient with HIV/AIDS is to determine the current CD4+ lymphocyte count and level of immunosuppression. In general, patients having a CD4+ cell count exceeding 400 mm3 may receive all indicated dental treatment. Patients with a CD4+ cell count less than 200 mm3 will have increased susceptibility to opportunistic infections and may be effectively medicated with prophylactic drugs. Consultation with the patient’s physician is advisable before performing surgical procedures and before initiating complex treatment plans.40,65
End-Stage Renal Disease and Dialysis
Consultation with the patient’s physician is suggested before dental care is initiated for patients being treated for end-stage renal disease. Depending on the patient’s status and the presence of other diseases common to renal failure (e.g., diabetes mellitus, hypertension, and systemic lupus erythematosus), dental treatment may be best provided in a hospital setting. The goal of dental care for patients being treated for end-stage renal disease is to slow the progression of disease and preserve the patient’s quality of life.40,55
The most recent American Heart Association guidelines do not include a recommendation for prophylactic antibiotics before invasive dental procedures for patients receiving dialysis with intravascular access devices.5 Although controversy exists, antibiotic prophylaxis should be provided for patients receiving hemodialysis and who have known cardiac risk factors. When prophylaxis is used, the standard regimen of the American Heart Association is recommended.5
Some drugs frequently used during endodontic treatment are affected by dialysis. Drugs metabolized by the kidneys and nephrotoxic drugs should be avoided. Both aspirin and acetaminophen are removed by dialysis and require a dosage adjustment in patients with renal failure. Amoxicillin and penicillin also require dosage adjustment as well as a supplemental dosage subsequent to hemodialysis.55 It is advisable to consult the patient’s physician concerning specific drug requirements during endodontic treatment. Endodontic treatment is best scheduled on the day after dialysis, because on the day of dialysis patients are generally fatigued and could have a bleeding tendency.40
Prosthetic Implants
Patients with prosthetic implants are frequently treated in dental practices. The question concerning the need for antibiotic prophylaxis to prevent infection of the prosthesis has been debated for many years. A statement was issued jointly in 2003 by the American Dental Association (Chicago, IL) and the American Academy of Orthopaedic Surgeons (Rosemont, IL) in an attempt to clarify the issue.1 The statement concluded that scientific evidence does not support the need for antibiotic prophylaxis for dental procedures to prevent prosthetic joint infections. It went on to state that antibiotic prophylaxis is not indicated for dental patients with pins, plates, and screws, nor is it routinely indicated for most patients with total joint replacements. However, the statement indicated that some “high-risk patients” who are at increased risk for infection and undergoing dental procedures likely to cause significant bleeding should receive antibiotic prophylactic treatment. Such patients would include those who are immunocompromised or immunosuppressed, who have insulin-dependent (type I) diabetes, who are in the first 2 years following joint replacement, or who have previous joint infections, malnourishment, or hemophilia.1 The advisory statement concludes that the final decision on whether to provide antibiotic prophylaxis is the responsibility of the clinician, who must consider potential benefits and risks. It should be noted that although endodontics has been shown to be a possible cause of bacteremia,17,66 the risk is minimal in comparison with extractions, periodontal surgery, scaling, and prophylaxis.52 Consultation with the patient’s physician on a case-by-case basis is advisable to establish the need for prophylaxis.
Behavioral and Psychiatric Disorders
Stress reduction is an important factor in the treatment of patients with behavioral and psychiatric disorders. Sensitivity to the patient’s needs must be part of the dental team’s approach. Significant drug interactions and side effects are associated with tricyclic antidepressants, monoamine oxidase inhibitors, and antianxiety medications.40 Consultation with physicians in such cases is essential before using sedatives, hypnotics, antihistamines, or opioids.
Psychosocial Evaluation
The initial visit, during which medical and dental histories are gathered, provides an opportunity to consider the patient’s psychosocial status. Although some patients may want to maintain a tooth with a questionable prognosis, others may lack the necessary sophistication to comprehend the potential risks and benefits. It would be a mistake to lead patients beyond what they can appreciate, and patients should not be allowed to dictate treatment that has a poor prognosis.
The clinician should also assess the patient’s level of anxiety as an important part of preparation for the procedure to follow. It is reasonable to assume that most patients are anxious to some degree, especially when they are about to undergo endodontic treatment. A conversation describing the procedure and what the patient can expect is an important part of an anxiety reduction protocol. It is well documented that a high level of anxiety is a predictor of poor anesthesia and posttreatment pain.13,48 More than 200 studies indicate that behavioral intervention for the highly anxious patient before treatment decreases anxiety before and after surgery, reduces posttreatment pain, and accelerates recovery.13
Dental Evaluation and Development of the Endodontic Treatment Plan
The strategic value of a tooth in question should be considered before presenting alternative treatment plans to the patient. Although some decisions may be straightforward, considering alternative treatment options can be challenging as the clinician weighs multiple factors that will play a role in determining the ultimate success or failure of the case. Referral of the patient to a specialist should be considered when the complexity of a procedure is beyond the ability of a clinician. Factors that affect endodontic prognosis, including periodontal and restorative, must be considered. The alternative of a dental implant offers another choice when the endodontic prognosis is poor.
Periodontal Considerations
Extensive periodontal lesions frequently complicate the endodontic procedure being considered. Such lesions may necessitate consultation with an endodontist and/or periodontist in order to gather more information about the tooth’s prognosis. A tooth with a poor periodontal prognosis may have to be sacrificed, despite a favorable endodontic prognosis.
When establishing the prognosis of a tooth with an endodontic/periodontal lesion, there are essential factors to be considered. Determination of pulp vitality and the extent of the periodontal defect are central to establishing the prognosis and developing a treatment plan for a tooth with an endodontic/periodontal lesion (see also Chapter 18).
In primary endodontic disease the pulp is nonvital (Figs. 4-3 and 4-4), whereas in primary periodontal disease the pulp retains vitality. True combined endodontic–periodontal disease occurs less frequently. The combined lesion is found when the endodontic disease process advances coronally and joins with a periodontal pocket progressing apically. There is significant attachment loss with this type of lesion and the prognosis is guarded.61 The radiographic appearance of combined endodontic–periodontal lesions may be similar to that of a vertically fractured tooth. Therapy for true combined lesions requires both endodontic and periodontal therapy. Sequencing of treatment is based on addressing the initial chief complaint.
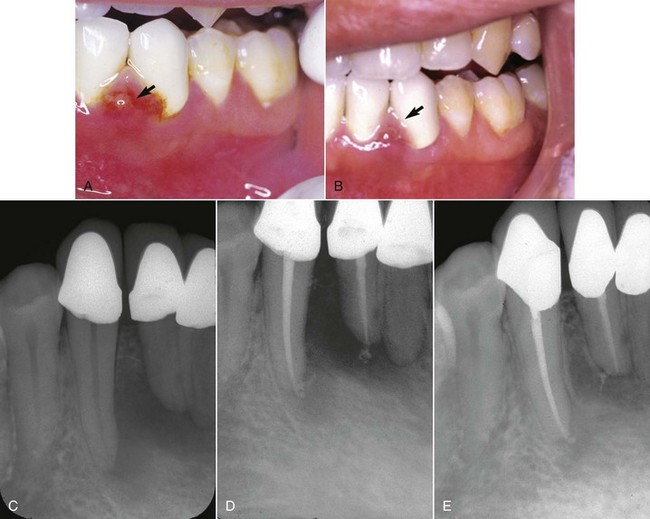
FIG. 4-3 A, Inflamed, edematous interproximal tissue (arrow) caused by acute endodontic pathosis. B, Soft tissue healing (arrow) 3 days after initiation of endodontic treatment. C, Periradicular pathosis. D, Completed endodontic therapy. E, Periradicular healing at 1-year recall.
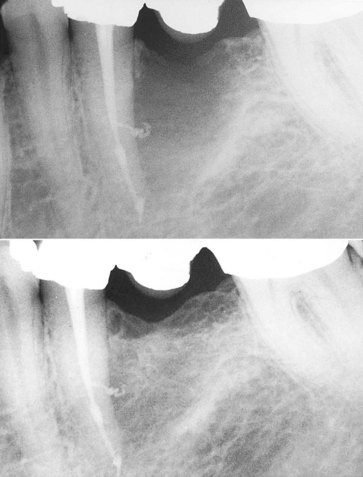
FIG. 4-4 A large, bony defect associated with tooth #20 healed after endodontic therapy. The tooth was nonvital, and no significant periodontal probing depth indicated pulpal disease.
The prognosis for and treatment of each type of endodontic–periodontal disease vary. Primary endodontic disease should be treated solely by endodontic therapy and the prognosis is usually good. Primary periodontal disease should be treated only by periodontal therapy and the prognosis varies depending on the seventy of the disease and patient’s response to treatment.61
Pathogenesis of the lesion can be better understood after vitality testing, periodontal probing, radiographic assessment, and evaluation of dental history. When extensive prostheses are planned, the potential risk of including a tooth with a questionable prognosis must be considered. It is not prudent to incorporate a chronic problem into a new complex prosthesis (Fig. 4-5).
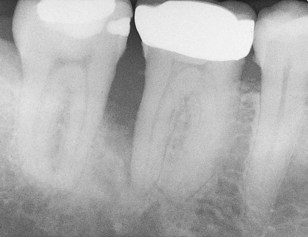
FIG. 4-5 Tooth #30 has a poor prognosis. Periodontal probing reached the apex of the distal root. Extraction is indicated and should be done as soon as possible to prevent further damage to the mesial bone associated with tooth #31. Implant site preservation is another consideration in treatment planning for this case.
Surgical Considerations
Surgical evaluation is particularly valuable in the diagnosis of lesions that may be nonodontogenic. Biopsy is the definitive means of diagnosing osseous pathosis, which may mimic a lesion of endodontic origin. When retreatment is being considered, the clinician must determine whether nonsurgical, surgical, or combined treatment is appropriate. This decision is influenced by the presence of complex restorations, posts, and the radiographic assessment of prior endodontic therapy.
Endodontic surgery is most often performed in an attempt to correct failure of nonsurgical therapy. Before surgery, it is essential that the clinician attempt to determine the cause of failure. For example, a deficient restoration or recurrent decay will result in microleakage into the root canal space. Unless that issue is addressed, apical surgery will not be successful (Fig. 4-6).
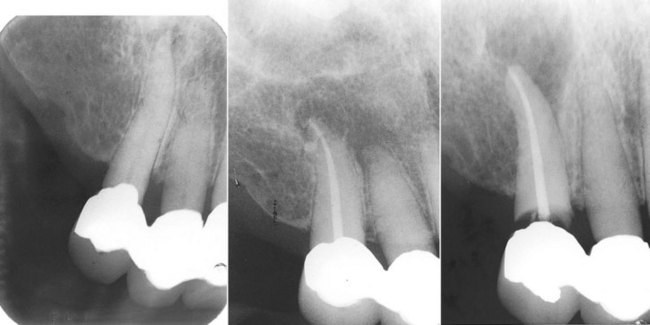
FIG. 4-6 Four years after endodontic therapy, the patient complained of pain and swelling associated with tooth #6. The initial impression was that apical surgery was indicated. However, further radiographs revealed the true cause of the endodontic failure. The initial endodontic access through the crown or caries damaged the coronal seal and recurrent decay followed.
Endodontic surgery (see Chapter 21) may also be performed as a primary procedure when there are complications such as calcific metamorphosis. In those cases, by using surgery as primary therapy, an apical seal can be established while preserving the crown of the tooth. The treatment plan for these cases is determined after reviewing multiple radiographs and considering the possibility of completing nonsurgical therapy without destroying an otherwise functional crown or natural tooth. Endodontic surgery without prior nonsurgical therapy should be a treatment of last resort and only when nonsurgical treatment is not possible.
The primary reason for apical surgery is to improve the quality of the apical seal. Dramatic changes in surgical technique and materials have occurred: The advent of microscopy, endoscopy, and ultrasonics, as well as of new retrograde materials such as mineral trioxide aggregate, represent important modifications of surgical technique.
Reviewing the best available evidence for alternative treatments is an important aspect of treatment planning for a tooth with failed endodontics. Evidence concerning healing potential after endodontic surgery is an important consideration in the management of posttreatment disease.28 Numerous studies have examined the outcome of apical surgery and the results vary considerably.73,74,80 This variability may reflect actual outcome differences or reflect variations in case selection techniques, recall periods, and methodology.
A study prospectively74 assessed the 4- to 8-year outcome of apical surgery on 155 teeth in 138 patients. The recall rate was 85% and the overall healed rate was 74%. The healed rate was significantly higher for teeth with small (≤5 mm) than larger pretreatment lesions (χ2, P = 0.02). Analysis indicated an increased odds ratio for disease persistence for teeth with larger pretreatment lesions and pretreatment root canal filling of adequate length. Pretreatment lesion size and root-filling length were significant predictors of the outcome of apical surgery.
One study assessed various predictors for healing 1 year after periapical surgery.73 The study evaluated pretreatment, surgical, and posttreatment variables as possible predictors for a 1-year healing outcome in periapical surgery. Only one parameter, pain at initial examination, reached a 0.05 level of significance in a logistic regression analysis, indicating that patients presenting with pain at the initial examination before surgery had a significantly lower rate of healing at the 1-year follow-up compared with patients having no pain at the initial examination.73
Restorative Considerations
A satisfactory restoration may be jeopardized by a number of factors. Subosseous root caries (perhaps requiring crown lengthening), poor crown-to-root ratio, and extensive periodontal defects or misalignment of teeth may have serious effects on the final restoration. These problems must be recognized before endodontic treatment is initiated. For complex cases, a restorative treatment plan should be in place before initiating endodontic treatment. Some teeth may be endodontically treatable but nonrestorable, or they may represent a potential restorative complication because of a large prosthesis. Reduced coronal tooth structure under a full-coverage restoration makes endodontic access more difficult because of reduced visibility and lack of radiographic information about the anatomy of the chamber. It is not unusual for restorations to be compromised during endodontic access (see Fig. 4-6). Whenever possible, restorations should be removed before endodontic treatment.
Endodontic Therapy or Dental Implant
The successful evolution of dental implants as a predictable replacement for missing teeth has had a positive impact on patient care. A clinician now has an additional possibility to consider when developing a treatment plan for a patient with a missing tooth or teeth. More challenging is the decision concerning whether or not to pursue endodontics for a tooth with a questionable prognosis or extract and use a single-tooth implant as a replacement. At this time, there are no randomized controlled outcome studies comparing endodontic therapy with single-tooth implants. Numerous studies have evaluated both nonsurgical endodontic therapy16,50,51,62,70,71 and endosseous dental implants.2,15,34,39
It is not possible to compare outcome studies because of variations in research methodologies, follow-up periods, and criteria associated with determining success or failure. A review of outcome studies points to the need for randomized controlled trials with standardized or similar methodologies that could provide a higher level of evidence to use in answering important clinical prognostic questions. A synthesis of available evidence indicates that both primary root canal treatment and single-tooth implants are highly predictable procedures when treatment is appropriately planned and implemented.
A systematic review of the literature examined the success and failure of nonsurgical endodontic therapy. The authors concluded that four conditions (pretreatment absence of a periapical radiolucency, root filling with no voids, root filling extending to 2 mm within the radiographic apex, and satisfactory coronal restoration) were found to improve the outcome of primary root canal treatment. They went on to note that the results of the review “should be interpreted with caution and cannot be considered to give definitive conclusions because of the retrospective and heterogeneous nature of the data. It does, however, provide strong clues about the factors likely to dominate outcomes and inform the design of future randomized trials.”50
Another study16 assessed clinical and radiographic success of initial endodontic therapy of 510 teeth over a 4- to 6-year period. It was found that 86% of teeth healed and 95% remained asymptomatic and functional. Absence of pretreatment apical periodontitis, single-rooted teeth, and the absence of intratreatment complications were significant predictors of better outcome in initial endodontic treatment.1 In 2004, a study considered the outcomes of endodontic treatment on 1,462,936 teeth. More than 97% of teeth were retained after 8 years. It should be noted that most of these cases were completed by general dental clinicians.62
The American Dental Association’s Council on Scientific Affairs has reported high survival rates for endosseous implants. An evaluation of 10 studies with more than 1400 implants demonstrated survival rates ranging from 94.4% to 99% with a mean survival rate of 96.7%.2 With such high survival rates reported for endodontics and single-tooth implants a clinician must consider a multiplicity of factors within the context of the best available evidence. Most current studies indicate no significant difference in the long-term prognosis between restored endodontically treated teeth and single-tooth implants.34
A retrospective cross-sectional comparison of initial nonsurgical endodontic treatment and single-tooth implants compared 196 implant restorations and 196 matched initial nonsurgical root canal–treated teeth. The results suggested that restored endodontically treated teeth and single-tooth implant restorations have similar failure rates, although the implant group showed a longer average and median time to function and a higher incidence of posttreatment complications requiring subsequent treatment intervention.19
A review summarized the best available evidence concerning factors influencing treatment planning involving preservation of a tooth with endodontic therapy or replacement by a single-tooth implant. Factors considered included prosthetic restorability of the natural tooth, quality of bone, esthetic concerns, cost-to-benefit ratio, systemic factors, potential for adverse effects, and patient preferences. The authors concluded that “endodontic treatment of teeth represents a feasible, practical and economical way to preserve function in a vast array of cases and that dental implants serve as a good alternative in selected indications in which prognosis is poor.”34
It seems clear that the patient is best served by retaining their natural dentition as long as the prognosis for long-term retention is positive. It is not reasonable to extract a tooth if endodontics with a good prognosis can be completed. It is also not reasonable for a patient to invest in root canal therapy, a post and crown if the prognosis is highly questionable and an implant with a good prognosis can be placed. An important advantage of providing endodontic therapy is to allow rapid return of the patient’s compromised dentition to full function and aesthetics. This rapid return is in marked contrast to the use of provisional restorations associated with dental implants while waiting for osseous integration.
Interestingly, some endodontic advanced education programs are now including implant training in their curriculum. This training will enable the endodontist to provide more value to the patient and referring dental clinician as treatment plans are determined. Such dually trained endodontists will be well positioned to provide endodontic therapy or place an implant as best serves the patient.
The scope of modern endodontics has been enhanced by the use of ultrasonics and microscopy as well as improved instruments and new materials. Teeth can be retained today that could not have been treated in the past. Biologically, it is clear that elimination of intraradicular infection is the key to endodontic success.
Vital Case
The acute vital case is best managed by a biologically based approach. Pain in such cases may be due to increased intrapulpal pressure and/or the release of inflammatory mediators and extension of the pathogenic process into the periodontal membrane. The challenge for the clinician is to treat inflamed and well-innervated tissue without causing additional pain. Performing a complete pulpotomy or, if time permits, establishing measurement control and completing a pulpectomy has a high degree of predictability in alleviating pain.8,33 If a canal has been entered, the clinician should be committed to removing all tissue. Partial instrumentation (i.e., leaving tissue remnants in the canal) may result in increased posttreatment pain.8,24,58 Teeth should be closed with a temporary filling at the conclusion of the visit in order to prevent bacterial contamination from saliva. After instrumentation of vital teeth with a history of pain and percussion sensitivity, occlusal reduction has been shown to be important in preventing posttreatment pain.60
Nonvital Case
The acute nonvital case represents a microbiologic challenge for the clinician (see also Chapters 9 and 15). A tooth that has had an asymptomatic nonvital pulp for some time may suddenly become acutely symptomatic. The cause of this dramatic change is due to an imbalance in the host–parasite relationship. This can be due to an increase in the virulence of bacteria, a change in the flora, or reduced host defense mechanisms.8 These changes can be initiated simply by opening the tooth and changing the environment of the bacterial flora. The therapeutic goals in such cases are to reduce the bacterial content in the root canal system and to promote decompression of the periradicular tissues by instrumentation and irrigation of the canal. Calcium hydroxide should be delivered into the root canal system as an intervisit dressing. Teeth should be closed with a cotton pledget in the chamber covered by a temporary seal. Endodontic treatment should be completed as soon as possible in order to prevent continued bacterial penetration into the canal. When a fluctuant swelling exists, incision and drainage may be performed in conjunction with canal instrumentation (Figs. 4-7 and 4-8).
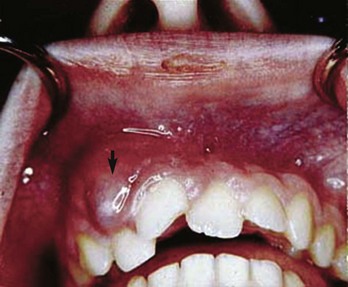
FIG. 4-7 Incision and drainage should be performed on this fluctuant swelling (arrow) in conjunction with canal instrumentation.
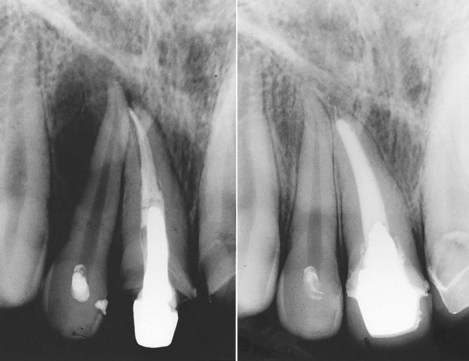
FIG. 4-8 Two years after endodontic therapy of tooth #8, the patient returned with pain and swelling. A clinician mistakenly began endodontic access on tooth #7, without confirming the apparent radiographic diagnosis by vitality testing. Tooth #7 was vital, and tooth #8 was successfully retreated after removal of the post.
(Courtesy Dr. Leon Schertzer.)
Single-Visit versus Multiple-Visit Treatment
Vital cases are often suitable for single-visit treatment. The number of roots, time available, and the clinician’s skills are factors to be considered. Severity of the patient’s symptoms is another important consideration. For example, a patient in severe pain, with or without swelling, should not experience a long visit including access, instrumentation, and obturation. Treatment in such cases should be directed at alleviating pain, with filling of the canal postponed until a later visit. The clinician’s judgment of what the patient can comfortably tolerate (regarding duration of the visit) is made on a case-by-case basis.
There are advantages in having a patient return for a second visit, after initially presenting with an endodontic emergency due to pain and/or swelling. The second visit allows the clinician to determine the effect of the therapy on the inflamed tissues. Deferring the filling of the canal(s) also leads to a shorter initial visit for the emergency patient. Although some researchers have reported less posttreatment pain in single-visit cases,24,58 a systematic review found that there was no detectable difference in the effectiveness of root canal treatment in terms of radiologic success between single and multiple visits.25 Neither single-visit nor multiple-visit root canal treatment can prevent 100% of short-term and long-term complications.25 The review found that the incidence of postobturation discomfort was similar in the single- and multiple-visit approaches although analgesic use was significantly less common in patients undergoing multiple-visit root canal treatment.
A systematic review examined the prevalence of posttreatment pain and flare-up among patients undergoing single- and multiple-visit endodontic treatment.63 The heterogeneity among the included studies was far too great to conduct a meta-analysis and yield meaningful results. The authors concluded that there was a lack of compelling evidence indicating a significantly different prevalence of posttreatment pain/flare-up with either single- or multiple-visit root canal treatment.
Teeth with nonvital pulps and apical periodontitis are often more complex than vital cases. Agreement is lacking concerning the appropriateness of single-visit endodontics for treating these patients. Some have postulated that the intervisit use of an antimicrobial dressing is essential to thoroughly disinfect the root canal system.68,69 In contrast, other researchers have found no statistically significant difference in success when using the single-visit or multivisit approach to the nonvital tooth with apical periodontitis.25,29,46,53,54,75 Two randomized controlled trials compared radiographic evidence of periapical healing after root canal treatment of necrotic teeth completed in one or two visits. The authors found no statistically significant difference in healing between the two groups.46,53
Treatment planning for an endodontic case should be based on biologic considerations. Patients who present with acute symptoms present a different set of biologic issues than those with an asymptomatic tooth. A swelling associated with an abscess, cellulitis, or presence of a stoma represent signs of biologic processes. The biologic significance of these conditions should be considered before determining specific goals for each visit. Although in most cases the clinical procedures required to complete endodontic treatment can be accomplished in a single visit, that does not mean it is the better course of treatment. What can be done and what should be done represent two very different approaches to endodontic treatment planning. The patient’s systemic health, level of anxiety, and symptoms, as well as the complexity of the root canal system, are factors that must be considered.
Agreement is lacking concerning long-term success rates associated with single-visit and multivisit procedures. Sjögren and associates68 investigated the influence of infection at the time of root filling on the outcome of endodontic treatment of teeth with apical periodontitis. Periapical healing was observed 5 years after completion of treatment. They concluded that “Complete periapical healing occurred in 94% of cases that yielded a negative culture. When the samples were positive before root filling, the success rate of treatment was just 68%—a statistically significant difference.” They concluded that the objective of eliminating bacteria from the root canal system “cannot be reliably achieved in a one-visit treatment because it is not possible to eradicate all infection from the root canal without the support of an interappointment antimicrobial dressing.” However, the findings of Friedman,29 Weiger and colleagues,75 and Peters and Wesselink54 contrast with those of Sjögren and colleagues.68 Those studies found no statistically significant differences in healing between teeth, with apical periodontitis, treated in one visit and two visits with the inclusion of calcium hydroxide as an intravisit medication.54,75 Sathorn and colleagues completed a systematic review of single- versus multiple-visit endodontic treatment of teeth with apical periodontitis.64 They found that, on the basis of current best available evidence, single-visit root canal treatment appeared to be slightly more effective than multiple-visit treatment (6.3% higher healing rate). However, the difference between these two treatment regimens was not statistically significant.64 This is a complicated issue because the inability to detect differences between groups might also be due to variations in research methodology, including sample size, duration of follow-up, and treatment methods.
It is possible that total elimination of bacteria may not be absolutely necessary for healing. Perhaps maximal reduction of bacteria, effective root canal filling, and a timely satisfactory coronal restoration can result in a high level of clinical success. However, regardless of the number of appointments, effective bacteriologic disinfection of the root canal system is critical.38
A study concerning the outcome of initial treatment noted the complexity of treating apical periodontitis.29 The author commented that “… treatment of this disease cannot be improved merely by changing treatment techniques. Because apical periodontitis results from interactions between microorganisms, their environment and the host immune system, only use of effective modifiers of any of these three factors might significantly improve the outcome of treatment.”
Retreatment Case
Retreatment cases offer a particular set of challenges to the clinician, and this topic is covered extensively in Chapter 26. Important questions to be considered before retreatment include the following:
A retreatment plan should be developed after the clinician has determined the cause of failure and weighed other factors that may affect the prognosis (e.g., root fracture, defective restoration) (Figs. 4-9 to 4-12). Retreatment cases may require surgical endodontics in combination with nonsurgical retreatment. Referral to a specialist is often helpful when planning treatment for complex cases.
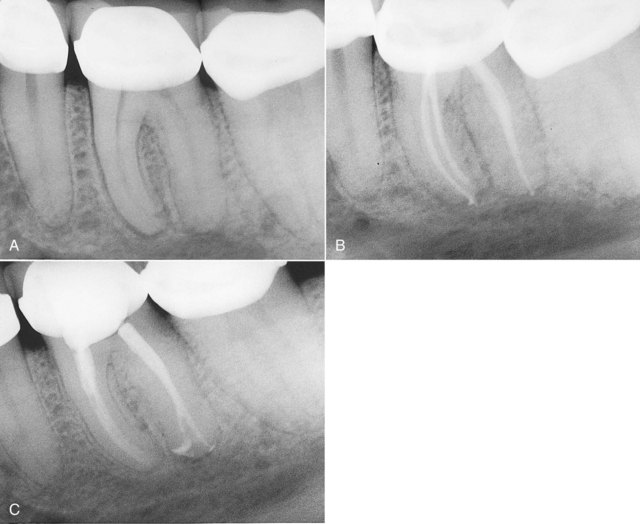
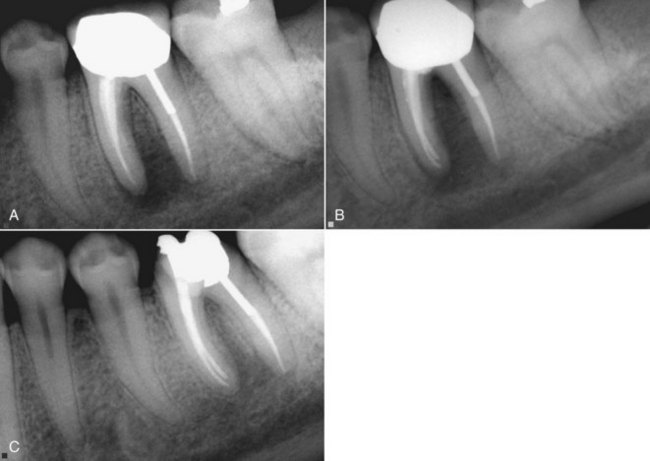
FIG. 4-9 Many years after endodontic treatment of tooth #19, the patient returned with a chief complaint of pain and an inability to chew with the tooth. Despite the radiographic appearance of excellent endodontic treatment, the tooth was retreated and the patient’s pain disappeared. Note the unusual distal root anatomy, which was not apparent during the initial procedure. A, Initial radiograph. B, Completion of initial endodontic therapy. C, Retreatment.
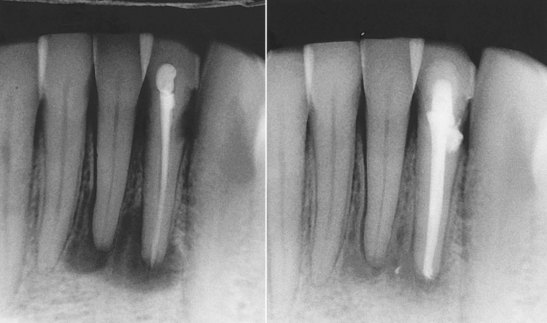
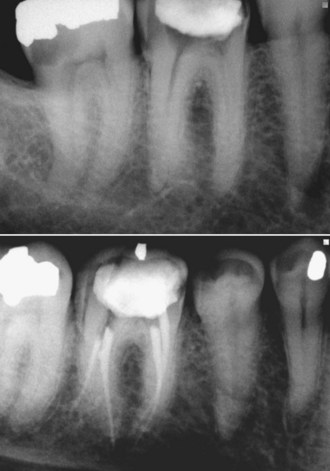
FIG. 4-10 Initial radiograph was misleading and implicated tooth #23 and tooth #24. Pulp testing indicated a vital pulp in tooth #24, and it was not treated. Retreatment of #23 resulted in healing of the periradicular lesion.
(Courtesy Dr. Leon Schertzer.)
Immature Teeth
Primary and immature permanent teeth may have pulpal pathosis caused by caries or trauma; preserving these young teeth is essential. Premature loss of an anterior tooth can lead to malocclusion, predispose the patient to tongue habits, impair aesthetics, and damage the self-esteem of the patient. See Chapters 16 and 24 for further information.
Other Factors That May Influence Endodontic Case Selection
A variety of factors may complicate proposed endodontic therapy. Calcifications, dilacerations, or resorptive defects may compromise endodontic treatment of a tooth with potentially strategic value (Fig. 4-13). The inability to isolate a tooth is also a problem and may result in bacterial contamination of the root canal system. Extra roots and canals pose a particular anatomic challenge that radiographs do not always reveal (Fig. 4-14). A bite wing radiograph is useful in providing an accurate image of the pulp chambers of posterior teeth. Retreatment cases offer particular mechanical challenges and are discussed in detail in Chapter 25. Ledges, perforations, or a post may be present, all of which complicate treatment and alter the prognosis. The clinician should recognize these potential problems and be able to manage and factor them into the decision concerning the tooth’s prognosis, including the possibility that the patient should be referred to a specialist.
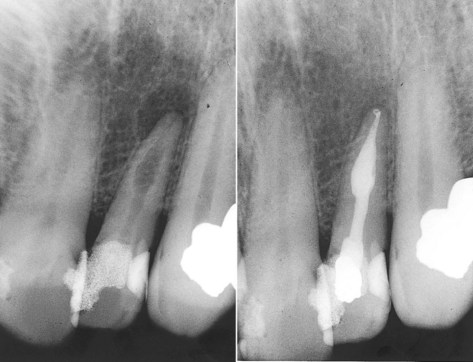
FIG. 4-13 Resorptive defects can be successfully treated. Early intervention, before there is perforation of the root, increases the chance of success.
(Courtesy Dr. Leon Schertzer.)
Some clinicians use a simple formula for determining which endodontic cases they treat and which they refer to a specialist. The number of roots may be the determining factor in a decision concerning referral, or the key factor may be the chronic or acute status of the case. Others consider the complexity of the ultimate prosthesis as a factor in considering an endodontic referral. The most important variables in determining whether to refer a patient to a specialist are the skills of the clinician and the complexity of the case.
The American Association of Endodontists (AAE) developed guidelines for assessing endodontic case difficulty (available at http://www.aae.org/dentalpro/CaseAssmtReferral.html) (see Fig. 2-1). The AAE Endodontic Case Difficulty Assessment Form enables a clinician to assign a level of difficulty to a particular case. The form describes cases of minimal, moderate, and high degrees of difficulty. This form lists criteria that can be used to identify cases that should be referred to a specialist. The use of surgical operating microscopes, endoscopes, and ultrasonics enables the specialist to predictably treat teeth that would not previously have been treatable.
Developing specific goals at each visit helps to organize the treatment. For example, for an uncomplicated molar or premolar, some clinicians will set a specific goal for the first visit that includes access and thorough instrumentation, while deferring the obturation to a second visit. Uncomplicated single-rooted, vital teeth may be planned for a single-visit approach. It is important that ample time be allowed so that the procedure can be adequately completed without undue stress. These recommendations have a biologic basis. Biologically, it is not reasonable to partially instrument root canal systems, thereby leaving residual inflamed pulpal remnants or necrotic debris in the canal, because such remnants may cause pain and be susceptible to infection. The clinician would be well advised to begin canal instrumentation only if time permits the extirpation of all pulp tissue.
Anxiety
Anxiety presents a problem at many levels of dental care (see also online Chapter 26). Avoidance of dental treatment due to anxiety appears to be associated with significant deterioration of oral and dental health.79 Even at the diagnostic stage, severe anxiety may confuse the process.21 Several studies support the hypothesis that pain or fear of pain is a primary source of anxiety as well as an obstacle to seeking dental care.37,79 Also, highly anxious patients appear to be more sensitive to pain.22,36 High levels of anxiety have been found to negatively affect clinical procedures including local anesthesia.48
It has been demonstrated that dental anxiety and expectation of pain had a profound effect on a patient’s ability to understand information provided.23 A person’s cognitive ability to process information is significantly affected by stress.23 A study found that 40% of patients who had minor oral surgery did not remember receiving both written and verbal instructions, contributing to 67% noncompliance with antibiotic prescriptions.9 Patients’ anxiety can compromise their understanding of complex treatment plans. Decisions made by a patient concerning options involving tooth retention or loss may be markedly affected by anxiety.
Unfortunately, the impact that a high level of anxiety can have on patient’s cognition, local anesthesia, and intra- and posttreatment experiences is not always recognized. Existing research has focused primarily on the effect of pretreatment information on reducing anxiety and stress during surgery.23 It was determined, using an anxiety virtual analog scale (AVAS), that higher AVAS scores were correlated with a greater number of injections needed to eliminate pain and that the higher the score, the less likely it was that pain would be eliminated during endodontic treatment.18
In a landmark medical study it was found that pretreatment discussion of surgical treatments and associated discomfort reduced by 50% the need for posttreatment morphine and reduced the time to discharge.20 More than 200 studies indicate that pre-emptive behavioral intervention decreases anxiety before and after surgery, reduces posttreatment pain intensity and intake of analgesics, and accelerates recovery.13 A calm setting, reassurance by the clinician and explanation of the treatment plan, as well as a discussion about pain prevention strategies are all important steps even before treatment starts.49 Written as well as a verbal description of the proposed treatment are helpful. It may also be of value to have a family member or friend accompany the patient for a discussion of the treatment plan.
Scheduling Considerations
If a vital case is to be treated by a multivisit approach, it is suggested that the clinician allow 5 to 7 days between canal instrumentation and obturation in order to allow periradicular tissues to recover. When a vital case is to be treated in a single visit, adequate time must be scheduled so that the clinician can comfortably complete the procedure. It is wise to schedule patients who require mandibular block anesthesia to arrive 15 to 20 minutes before their treatment visit. This avoids the frustration of “losing treatment time” while the anesthetic agent becomes fully effective (see also Chapter 20).
Appointments to fill nonvital cases should be scheduled approximately 1 week after instrumentation in order to maximize the antimicrobial effect of the intracanal dressing when calcium hydroxide is used.8,68,69 Acute (pain and/or swelling) nonvital cases should be seen every 24 to 48 hours in order to monitor the patient’s progress and bring the acute symptoms under control. Further cleaning and shaping are important components of the treatment as the clinician seeks to eliminate persistent microbes in the canal system. Long delays between visits contribute to the development of resistant microbial strains and should be avoided.
1. American Dental Association, American Academy of Orthopaedic Surgeons. Antibiotic prophylaxis for dental patients with total joint replacements. J Am Dent Assoc. 2003;134:895.
2. American Dental Association Council on Scientific Affairs. Dental endosseous implants: an update. J Am Dent Assoc. 2004;135:92.
3. Dental Management of Patients Receiving Oral Bisphosphonate Therapy—Expert Panel Recommendations. http://www.ada.org/prof/resources/topics/topics_osteonecrosis_bisphosphonate_report.pdf, July 2008. Available at
4. American Association of Endodontists http://www.aae.org/dentalpro/ClinicalTopics/
5. Baddour LM, Bettmann MA, Bolger AF, Epstein AE, et al. Nonvalvular cardiovascular device-related infections. Circulation. 2003;108:2015.
6. Bader JD, Bonito AJ, Shugars DA. A systematic review of cardiovascular effects of epinephrine on hypertensive dental patients. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;93:647.
7. Bean LRJr, Devore WD. The effect of protective aprons in dental roentgenography. Oral Surg Oral Med Oral Pathol. 1969;28:505.
8. Bergenholtz G H-BP, Reit C. Textbook of endodontology, Ed 1 Oxford. UK: Blackwell; 2003.
9. Blinder D, Rotenberg L, Peleg M, Taicher S. Patient compliance to instructions after oral surgical procedures. Int J Oral Maxillofac Surg. 2001;30:216.
10. Briggs GG, Freeman RK, Yaffe SJ. Drugs in Pregnancy and Lactation: A Reference Guide to Fetal and Neonatal Risk, ed 8. Philadelphia, PA: Lippincott Williams & Wilkins; 2008.
11. Britto LR, Katz J, Guelmann M, Heft M. Periradicular radiographic assessment in diabetic and control individuals. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;96:449.
12. Brown R, Rhodus N. Epinephrine and local anesthesia revisited. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;100:401.
13. Carr DB, Goudas LC. Acute pain. Lancet. 1999;353:2051.
14. Cottone JA, Molinari JA. State-of-the-art infection control in dentistry. J Am Dent Assoc. 1991;122:33.
15. Creugers NH, Kreulen CM, Snoek PA, de Kanter RJ. A systematic review of single-tooth restorations supported by implants. J Dent. 2000;28:209.
16. de Chevigny C, Dao TT, Basrani BR, et al. Treatment outcome in endodontics: the Toronto study. Phase 4: initial treatment. J Endod. 2008;34:258.
17. Debelian GJ, Olsen I, Tronstad L. Bacteremia in conjunction with endodontic therapy. Endod Dent Traumatol. 1995;11:142.
18. DiBernardo J, Bauer C, Monroe A, Rosenberg PA. Pre-operative levels of anxiety as a predictor of successful local anesthesia during endodontic treatment. J Endod. 2009. (in press)
19. Doyle SL, Hodges JS, Pesun IJ, Law AS, Bowles WR. Retrospective cross sectional comparison of initial nonsurgical endodontic treatment and single-tooth implants. J Endod. 2006;32:822.
20. Egbert LD, Battit GE, Welch CE, Bartlett MK. Reduction of postoperative pain by encouragement and instruction of patients. a study of doctor–patient rapport. N Engl J Med. 1964;270:825.
21. Eli I. Dental anxiety: a cause for possible misdiagnosis of tooth vitality. Int Endod J. 1993;26:251.
22. Eli I, Schwartz-Arad D, Baht R, Ben-Tuvim H. Effect of anxiety on the experience of pain in implant insertion. Clin Oral Implants Res. 2003;14:115.
23. Eli I, Schwartz-Arad D, Bartal Y. Anxiety and ability to recognize clinical information in dentistry. J Dent Res. 2008;87:65.
24. Fava LR. One-appointment root canal treatment: incidence of postoperative pain using a modified double-flared technique. Int Endod J. 1991;24:258.
25. Figini L, Lodi G, Gorni F, Gagliani M. Single versus multiple visits for endodontic treatment of permanent teeth: a Cochrane systematic review. J Endod. 2008;34:1041.
26. Fouad AF, Burleson J. The effect of diabetes mellitus on endodontic treatment outcome: data from an electronic patient record. J Am Dent Assoc. 2003;134:43.
27. Friedlander AH, Sung EC, Child JS. Radiation-induced heart disease after Hodgkin’s disease and breast cancer treatment: dental implications. J Am Dent Assoc. 2003;134:1615.
28. Friedman S. Considerations and concepts of case selection in the management of post-treatment endodontic disease (treatment failure). Endod Top. 2002;1:54.
29. Friedman S. Prognosis of initial endodontic therapy. Endod Top. 2002;2:59.
30. Geiss LS, Pan L, Cadwell B, Gregg EW, Benjamin SM, Engelgau MM. Changes in incidence of diabetes in U.S. adults, 1997–2003. Am J Prev Med. 2006;30:371.
31. Goodchild J, Glick M. A different approach to medical risk assessment. Endod Top. 2003;4:1.
32. Greenberg MS, Glick M, Ship JA. Burket’s oral medicine, ed 11. India: BC Decker; 2008.
33. Hasselgren G, Reit C. Emergency pulpotomy: pain relieving effect with and without the use of sedative dressings. J Endod. 1989;15:254.
34. Iqbal MK, Kim S. A review of factors influencing treatment planning decisions of single-tooth implants versus preserving natural teeth with nonsurgical endodontic therapy. J Endod. 2008;34:519.
35. Jeske AH, Suchko GD. Lack of a scientific basis for routine discontinuation of oral anticoagulation therapy before dental treatment. J Am Dent Assoc. 2003;134:1492.
36. Klages U, Kianifard S, Ulusoy O, Wehrbein H. Anxiety sensitivity as predictor of pain in patients undergoing restorative dental procedures. Community Dent Oral Epidemiol. 2006;34:139.
37. Lahmann C, Schoen R, Henningsen P, et al. Brief relaxation versus music distraction in the treatment of dental anxiety: a randomized controlled clinical trial. J Am Dent Assoc. 2008;139:317.
38. Lin LM, Lin J, Rosenberg PA. One-appointment endodontic therapy: biological considerations. J Am Dent Assoc. 2007;138:1456.
39. Lindh T, Gunne J, Tillberg A, Molin M. A meta-analysis of implants in partial edentulism. Clin Oral Implants Res. 1998;9:80.
40. Little JW, Falace DA, Miller C, Rhodus NL. Dental management of the medically compromised patient, ed 7. St. Louis, MO: Mosby; 2008.
41. Markiewicz MR, Margarone JE3rd, Campbell JH, Aguirre A. Bisphosphonate-associated osteonecrosis of the jaws: a review of current knowledge. J Am Dent Assoc. 2005;136:1669.
42. Melo MD, Obeid G. Osteonecrosis of the jaws in patients with a history of receiving bisphosphonate therapy: strategies for prevention and early recognition. J Am Dent Assoc. 2005;136:1675.
43. Michalowicz BS, DiAngelis AJ, Novak MJ, et al. Examining the safety of dental treatment in pregnant women. J Am Dent Assoc. 2008;139:685.
44. Migliorati CA, Casiglia J, Epstein J, Jacobsen PL, Siegel MA, Woo SB. Managing the care of patients with bisphosphonate-associated osteonecrosis: an American Academy of Oral Medicine position paper. J Am Dent Assoc. 2005;136:1658.
45. Miley D, Terezhalmy G. The patient with diabetes mellitus: etiology, epidemiology, principles of medical management, oral disease burden, and principles of dental management. Quintessence Int. 2005;36:779.
46. Molander A, Warfvinge J, Reit C, Kvist T. Clinical and radiographic evaluation of one- and two-visit endodontic treatment of asymptomatic necrotic teeth with apical periodontitis: a randomized clinical trial. J Endod. 2007;33:1145.
47. Moore PA. Selecting drugs for the pregnant dental patient. J Am Dent Assoc. 1998;129:1281.
48. Nakai Y, Milgrom P, Mancl L, Coldwell SE, Domoto PK, Ramsay DS. Effectiveness of local anesthesia in pediatric dental practice. J Am Dent Assoc. 2000;131:1699.
49. Ng SK, Chau AW, Leung WK. The effect of pre-operative information in relieving anxiety in oral surgery patients. Community Dent Oral Epidemiol. 2004;32:227.
50. Ng YL, Mann V, Rahbaran S, Lewsey J, Gulabivala K. Outcome of primary root canal treatment: systematic review of the literature. Part 2. Influence of clinical factors. Int Endod J. 2008;41:6.
51. Ng YL, Mann V, Rahbaran S, Lewsey J, Gulabivala K. Outcome of primary root canal treatment: systematic review of the literature. Part 1. Effects of study characteristics on probability of success. Int Endod J. 2007;40:921.
52. Pallasch TJ, Slots J. Antibiotic prophylaxis and the medically compromised patient. Periodontol 2000. 1996;10:107.
53. Penesis VA, Fitzgerald PI, Fayad MI, Wenckus CS, BeGole EA, Johnson BR. Outcome of one-visit and two-visit endodontic treatment of necrotic teeth with apical periodontitis: a randomized controlled trial with one-year evaluation. J Endod. 2008;34:251.
54. Peters LB, Wesselink PR. Periapical healing of endodontically treated teeth in one and two visits obturated in the presence or absence of detectable microorganisms. Int Endod J. 2002;35:660.
55. Proctor R, Kumar N, Stein A, Moles D, Porter S. Oral and dental aspects of chronic renal failure. J Dent Res. 2005;84:199.
56. Quesnell BT, Alves M, Hawkinson RWJr, Johnson BR, Wenckus CS, BeGole EA. The effect of human immunodeficiency virus on endodontic treatment outcome. J Endod. 2005;31:633.
57. Rhodus NL, Vibeto BM, Hamamoto DT. Glycemic control in patients with diabetes mellitus upon admission to a dental clinic: considerations for dental management. Quintessence Int. 2005;36:474.
58. Roane JB, Dryden JA, Grimes EW. Incidence of postoperative pain after single- and multiple-visit endodontic procedures. Oral Surg Oral Med Oral Pathol. 1983;55:68.
59. Robbins MA. Oral care of the patient receiving chemotherapy. In: Ord RA, Blanchaert RH, editors. Oral cancer: The dentist’s role in diagnosis, management, rehabilitation, and prevention. Chicago: Quintessence Publishing; 2000:120.
60. Rosenberg PA, Babick PJ, Schertzer L, Leung A. The effect of occlusal reduction on pain after endodontic instrumentation. J Endod. 1998;24:492.
61. Rotstein I, Simon J. The endo–perio lesion: a critical appraisal of the disease condition. Endod Top. 2006;13:34.
62. Salehrabi R, Rotstein I. Endodontic treatment outcomes in a large patient population in the USA: an epidemiological study. J Endod. 2004;30:846.
63. Sathorn C, Parashos P, Messer H. The prevalence of postoperative pain and flare-up in single- and multiple-visit endodontic treatment: a systematic review. Int Endod J. 2008;41:91.
64. Sathorn C, Parashos P, Messer HH. Effectiveness of single- versus multiple-visit endodontic treatment of teeth with apical periodontitis: a systematic review and meta-analysis. Int Endod J. 2005;38:347.
65. Scully C, Cawson RA. Medical problems in dentistry—immunodeficiencies and HIV disease, ed 5. Edinburg: Elsevier; 2005. p 683
66. Siqueira JFJr. Endodontic infections: concepts, paradigms, and perspectives. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;94:281.
67. Sirois DA, Fatahzadeh M. Valvular heart disease. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;91:15.
68. Sjögren U, Figdor D, Persson S, Sundqvist G. Influence of infection at the time of root filling on the outcome of endodontic treatment of teeth with apical periodontitis. Int Endod J. 1997;30:297.
69. Sjögren U, Hagglund B, Sundqvist G, Wing K. Factors affecting the long-term results of endodontic treatment. J Endod. 1990;16:498.
70. Torabinejad M, Goodacre CJ. Endodontic or dental implant therapy: the factors affecting treatment planning. J Am Dent Assoc. 2006;137:973.
71. Torabinejad M, Kutsenko D, Machnick TK, Ismail A, Newton CW. Levels of evidence for the outcome of nonsurgical endodontic treatment. J Endod. 2005;31:637.
72. Vernillo AT. Diabetes mellitus: relevance to dental treatment. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;91:263.
73. von Arx T, Jensen SS, Hanni S. Clinical and radiographic assessment of various predictors for healing outcome 1 year after periapical surgery. J Endod. 2007;33:123.
74. Wang N, Knight K, Dao T, Friedman S. Treatment outcome in endodontics—the Toronto Study. Phases I and II: apical surgery. J Endod. 2004;30:751.
75. Weiger R, Rosendahl R, Lost C. Influence of calcium hydroxide intracanal dressings on the prognosis of teeth with endodontically induced periapical lesions. Int Endod J. 2000;33:219.
76. White SC. 1992 assessment of radiation risk from dental radiography. Dentomaxillofac Radiol. 1992;21:118.
77. Wilson W, Taubert KA, Gewitz M, et al. Prevention of infective endocarditis: guidelines from the American Heart Association. Circulation. 2007;116:1736.
78. Wynn RL, Meiller TF, Crossley HL. Drug information handbook for dentistry, ed 14. Hudson, OH: Lexi-Comp; 2008.
79. Yu S, Bellamy H, Kogan M, Dunbar J, Schwalberg R, Schuster M. Factors that influence receipt of recommended preventive pediatric health and dental care. Am Acad Pediatr. 2002.
80. Zuolo ML, Ferreira MO, Gutmann JL. Prognosis in periradicular surgery: a clinical prospective study. Int Endod J. 2000;33:91.