Medical Nutrition Therapy for Low-Birth-Weight Infants
The management of low birthweight (LBW) infants requiring intensive care is continually improving. With new technologies, enhanced understanding of pathophysiologic conditions of the perinatal period (from 28 weeks of gestation to 4 weeks after birth), current nutrition management principles, and regionalization of perinatal care, the mortality rate during infancy—that period from birth to 1 year of age—remains stable in the United States. In particular, the development and use of surfactant—a mixture of lipoproteins secreted by alveolar cells into the alveoli and respiratory air passages that contributes to the elastic properties of pulmonary tissue—has increased the survival of preterm infants, as has the use of antepartum corticosteroids. Most premature infants have the potential for long and productive lives (Hack, 2009).
Nutrition can be provided to LBW infants in many ways, each of which has certain benefits and limitations. The infant’s size, age, and clinical condition dictate the nutrition requirements and the way they can be met. Because of the complexities involved in the neonatal intensive care setting, a team that includes a registered dietitian trained in neonatal nutrition should make the decisions necessary to facilitate optimal nutrition. In regionalized perinatal care systems, the neonatal nutritionist may also consult with health care providers in community hospitals and public health settings.
Infant Mortality and Statistics
In 2007 the infant mortality rate in the United States remains stable at 6.77 infant deaths per 1000 live births (Heron et al., 2010). More than 65% of these deaths occur in the neonatal period, with the leading causes being birth defects, prematurity, and LBW. The preterm birth rate was 12.7% and the incidence of LBW was 8.2%. Both rates are decreased from the 2006 rate and mark a change from the previous 20-year increase in premature and LBW infants.
The United States’ infant mortality rate remains higher than that for many industrialized countries (Heron et al., 2010). This discrepancy may be attributable to the inconsistent collection of mortality data among nations, which may falsely lower mortality rates in other countries. However the high incidence of premature infants born in the United States contributes to this high infant mortality rate (MacDorman and Mathews, 2009).
Physiologic Development
At birth an infant who weighs less than 2500 g (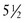 lb) is classified as having a low birthweight (LBW); an infant weighing less than 1500 g (
lb) is classified as having a low birthweight (LBW); an infant weighing less than 1500 g (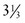 lb) has a very low birthweight (VLBW); and an infant weighing less than 1000 g (
lb) has a very low birthweight (VLBW); and an infant weighing less than 1000 g (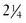 lb) has an extremely low birthweight (ELBW). LBW may be attributable to a shortened period of gestation, prematurity, or a restricted intrauterine growth rate, which makes the infant small for gestational age (SGA).
lb) has an extremely low birthweight (ELBW). LBW may be attributable to a shortened period of gestation, prematurity, or a restricted intrauterine growth rate, which makes the infant small for gestational age (SGA).
The term infant is born between the 37th and 42nd weeks of gestation. A premature (preterm) infant is born before 37 weeks of gestation, whereas a postterm infant is born after 42 weeks of gestation.
Antenatally, an estimate of the infant’s gestational age is based on the date of the mother’s last menstrual period, clinical parameters of uterine fundal height, the presence of quickening (the first movements of the fetus that can be felt by the mother), fetal heart tones, or ultrasound evaluations. After birth, gestational age is determined by clinical assessment. Clinical parameters fall into two groups: (1) a series of neurologic signs, which depend primarily on postures and tone; and (2) a series of external characteristics that reflect the physical maturity of the infant. The New Ballard Score (Ballard et al., 1991) examination is a frequently used clinical assessment tool. An accurate assessment of gestational age is important for establishing nutritional goals for individual infants and differentiating the premature infant from the term SGA infant.
An infant who is small for gestational age (SGA) has a birth weight that is lower than the 10th percentile of the standard weight for that gestational age. An SGA infant whose intrauterine weight gain is poor, but whose linear and head growth are between the 10th and 90th percentiles on the intrauterine growth grid, has experienced asymmetric intrauterine growth restriction (IUGR). An SGA infant whose length and occipital frontal circumference are also below the 10th percentile of the standards has symmetric IUGR. Symmetric IUGR, which usually reflects early and prolonged intrauterine deficit, is apparently more detrimental to later growth and development. Some infants can be SGA because they are genetically small, and these infants usually do well.
An infant whose size is appropriate for gestational age (AGA) has a birth weight between the 10th and 90th percentiles on the intrauterine growth chart. The obstetrician diagnoses IUGR when the fetal growth rate decreases. Serial ultrasound measurements document this reduction in fetal anthropometric measurements, which may be caused by maternal, placental, or fetal abnormalities. The future growth and development of infants who have had IUGR is diverse, depending on the specific cause of the IUGR and treatment. Some infants who suffered from IUGR are SGA, but many may plot as AGA infants at birth. Decreased fetal growth does not always result in an infant who is SGA.
An infant whose birth weight is above the 90th percentile on the intrauterine growth chart is large for gestational age (LGA). Box 43-1 summarizes the weight classifications. Figure 43-1 shows the classification of neonates based on maturity and intrauterine growth.
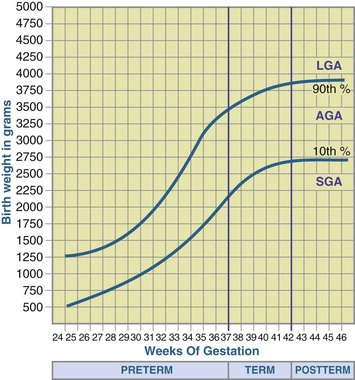
FIGURE 43-1 Classification of neonates based on maturity and intrauterine growth (small for gestational age [SGA], appropriate for gestational age [AGA], or large for gestational age [LGA]). (From Battaglia FC, Lubchenco LO: A practical classification of newborn infants by weight and gestational age, J Pediatr 71:159, 1967.)
Characteristics of Immaturity
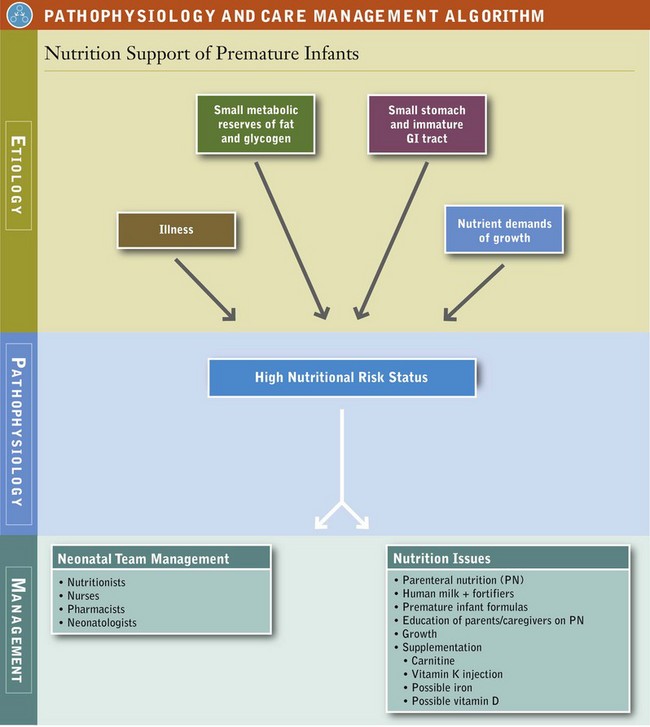
The premature or LBW infant has not had the chance to develop fully in utero and is physiologically different from the term infant (Figure 43-2). Because of this, LBW infants have various clinical problems in the early neonatal period, depending on their intrauterine environment, degree of prematurity, birth-related trauma, and function of immature or stressed organ systems. Certain problems occur with such frequency that they are considered typical of prematurity (Table 43-1). Premature infants are at high risk for poor nutrition status because of poor nutrient stores, physiologic immaturity, illness (which may interfere with nutritional management and needs), and the nutrient demands required for growth.
TABLE 43-1
Common Problems Among Premature Infants
| System | Problem |
| Respiratory | Respiratory distress syndrome, chronic lung disease (bronchopulmonary dysplasia) |
| Cardiovascular | Patent ductus arteriosus |
| Renal | Fluid and electrolyte imbalance |
| Neurologic | Intraventricular hemorrhage, periventricular leukomalacia (cerebral necrosis) |
| Metabolic | Hypoglycemia, hyperglycemia, hypocalcemia, metabolic acidosis |
| Gastrointestinal | Hyperbilirubinemia, feeding intolerance, necrotizing enterocolitis |
| Hematologic | Anemia |
| Immunologic | Sepsis, pneumonia, meningitis |
| Other | Apnea, bradycardia, cyanosis, osteopenia |
From Cloherty JP et al., editors: Manual of neonatal care, ed 6, Philadelphia, 2008, Wolters Kluwer/Lippincott Williams & Wilkins.
Most fetal nutrient stores are deposited during the last 3 months of gestation; therefore the premature infant begins life in a compromised nutritional state. Because metabolic (i.e., energy) stores are limited, nutrition support in the form of parenteral nutrition (PN), enteral nutrition (EN), or both should be initiated as soon as possible. In the preterm infant weighing 1000 g, fat constitutes only 1% of total body weight; by contrast the term infant (3500 g) has a fat percentage of approximately 16%. For example, a 1000-g AGA premature infant has a glycogen and fat reserve equivalent to approximately 110 kcal/kg of body weight. With basal metabolic needs of approximately 50 kcal/kg/day, it is obvious that this infant will rapidly run out of fat and carbohydrate fuel unless adequate nutrition support is established. The depletion time is even shorter for preterm infants weighing less than 1000 g at birth. Nutrient reserves are also depleted most quickly by tiny infants who have IUGR as a result of their decreased nutrient stores.
Theoretic estimates of survival time of starved and semistarved infants are shown in Table 43-2. These estimates assume depletion of all glycogen and fat and approximately one third of body protein tissue at a rate of 50 kcal/kg/day. The effects of fluids such as intravenously provided water (which has no exogenous calories) and 10% dextrose solution (D10W) are shown. Currently, PN fluids are started on the day of birth to provide energy and protein for the VLBW infant. Early protein intake promotes positive nitrogen balance, normal plasma amino acid levels, and glucose tolerance.
TABLE 43-2
Expected Survival Time of Starved (H2O Only) and Semistarved (D10W) Infants

D10W, Dextrose 10% in water; H2O, water.
Data from Heird WC et al: Intravenous alimentation in pediatric patients, J Pediatr 80:351, 1972.
The small premature infant is particularly vulnerable to undernutrition. Malnutrition in premature infants may increase the risk of infection, prolong chronic illness, and adversely affect brain growth and function. In fact, Lucas and colleagues (1998) reported that the type of milk used for the neonatal diet may be directly linked to neurodevelopment at 18 months of age. Human milk or premature infant formula fed the first month of life resulted in improved development.
Nutrition Requirements: Parenteral Feeding
Many critically ill preterm infants have difficulty progressing to full enteral feedings in the first several days or even weeks of life. The infant’s small stomach capacity, immature gastrointestinal tract, and illness make the progression to full enteral feedings difficult (see Pathophysiology and Care Management Algorithm: Nutrition Support of Premature Infants). PN becomes essential for nutrition support, either as a supplement to enteral feedings or as the total source of nutrition. Chapter 14 offers a complete discussion of PN; only aspects related to feeding of the preterm infant are presented here.
Fluid
Because fluid needs vary widely for preterm infants, fluid balance must be monitored. Inadequate intake can lead to dehydration, electrolyte imbalances, and hypotension; excessive intake can lead to edema, congestive heart failure, and possible opening of the ductus arteriosus. Additional neonatal clinical complications reported with high fluid intakes include necrotizing enterocolitis (NEC) and bronchopulmonary dysplasia (BPD) (see Chapter 35).
The premature infant has a greater percentage of body water (especially extracellular water) than the term infant (see Chapter 7). The amount of extracellular water should decrease in all infants during the first few days of life. This reduction is accompanied by a normal loss of 10% to 15% of body weight and improved renal function. Failure of this transition in fluid dynamics and lack of diuresis may complicate the course of preterm infants with respiratory disease.
Water requirements are estimated by the sum of the predicted losses from the lungs and skin, urine, and stool, and the water needed for growth. A major route of water loss in preterm infants is evaporation through the skin and respiratory tract. This insensible water loss is highest in the smallest and least mature infants because of their larger body surface area relative to body weight, increased permeability of the skin epidermis to water, and greater skin blood flow relative to metabolic rate. Insensible water loss is increased by radiant warmers and phototherapy lights and decreased by heat shields, thermal blankets, and humidified incubators. Insensible water loss can vary from 50 to 100 mL/kg/day on the first day of life and increase up to 120 to 200 mL/kg/day, depending on the infant’s size, gestational age, day of life, and environment. The use of humidified incubators can decrease insensible water losses and thereby reduce fluid requirements.
Excretion of urine, the other major route of water loss, varies from 24 to 72 mL/kg/day. This loss depends on the fluid volume and solute load presented to the kidneys. The infant’s ability to concentrate urine increases with maturity. Stool water loss is generally 5 to 10 mL/kg/day, and 10 to 15 mL/kg/day is suggested as optimal for growth (Dell and Davis, 2006).
Because of the many variables affecting neonatal fluid losses, fluid needs must be determined on an individual basis. Usually fluid is administered at a rate of 80 to 105 mL/kg/day the first day of life to meet insensible losses and urine output. Fluid needs are then evaluated by assessing fluid intake and comparing it with the clinical parameters of urine output volume; urine specific gravity; and serum electrolyte, creatinine, and urea nitrogen levels. Assessments of weight, blood pressure, peripheral perfusion, skin turgor, and mucous membrane moisture are performed daily. Daily fluid administration generally increases by 10 to 20 mL/kg/day. By the end of the second week of life, preterm infants may receive fluids at a rate of 140 to 160 mL/kg/day. Fluid restriction may be necessary in preterm infants with patent ductus arteriosus, congestive heart failure, renal failure, or cerebral edema. However, more fluids are needed by preterm infants who are placed under phototherapy lights or a radiant warmer or when the environmental or body temperature is elevated.
Energy
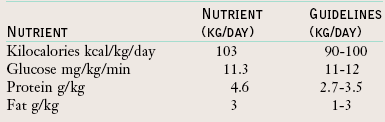
The energy needs of preterm infants fed parenterally are less than those of enterally fed infants because absorption loss does not occur when nutritional intake bypasses the intestinal tract. Enterally fed preterm infants usually require 105 to 130 kcal/kg/day to grow, whereas parenterally fed premature neonates can grow well if they receive 90 to 100 kcal/kg/day (American Academy of Pediatrics [AAP], 2009b). Minimum maintenance energy needs and adequate protein should be provided as soon as possible to prevent tissue catabolism. Providing VLBW infants with 1.5 to 2 g of protein and 30 to 50 kcal/kg/day promotes nitrogen balance during the first 3 days of life (AAP, 2004; AAP, 2009b). Up to 3 g/kg/day of protein is tolerated (Thureen et al., 2003).
Energy and protein intake should be increased as the infant’s condition stabilizes and growth becomes the goal (Table 43-3). Many VLBW infants are born AGA but at discharge from the hospital weigh less than the 10th percentile for their postmenstrual age. This new SGA status is called extrauterine growth restriction (EUGR). EUGR may occur as a result of poor energy and protein intakes, and the decreased growth associated with illness (Ehrenkranz, 2010).
TABLE 43-3
Comparison of Parenteral and Enteral Energy Needs of Premature Infants
| Parenteral | Enteral | |
| Maintenance | ||
| Gradually increase intake to meet energy needs by the end of the first week | 30-50 cal/kg/day | 50 kcal/kg/day |
| Growth | ||
| Meet energy needs as soon as the infant’s condition is stable | 90-100 cal/kg/day | 105-130 kcal/kg/day |
Glucose
Glucose or dextrose is the principal energy source (3.4 kcal/g). However, glucose tolerance is limited in premature infants, especially in VLBW infants, because of inadequate insulin production, insulin resistance, and continued hepatic glucose release while intravenous glucose is infusing. Hyperglycemia is less likely when glucose is administered with amino acids than when it is infused alone. Amino acids exert a stimulatory effect on insulin release. Prevention of hyperglycemia is important because it can lead to diuresis and dehydration.
To prevent hyperglycemia in VLBW infants, glucose should be administered in small amounts. The glucose load is a function of the concentration of the dextrose infusion and the rate at which it is administered (Table 43-4). The administration of exogenous insulin may be necessary for infants with persistent or very high glycemia, but changes in the infant’s blood glucose level are common problems associated with its use. In addition, protein synthesis may be inhibited by insulin administration in premature infants (Denne, 2007).
TABLE 43-4
Guidelines for Glucose Load in Premature Infants
| Initial Load (mg/kg/min)* | Daily Increments (mg/kg/min) | Maximum Load (mg/kg/min) |
| 4-6 | 1-2 | 11-12 |
*Use the following formula to calculate glucose load: (% Glucose × mL/kg/day) × (1000 mg/g glucose) ÷ (1440 min/day). For example, (0.10 × 150 mL/kg/day) × (1000 mg/g glucose) ÷ (1440 min/day) = 10.4 mg/kg/min.
In general, preterm infants should receive an initial glucose load of less than 6 mg/kg/min, with a gradual increase to 11 to 12 mg/kg/min. The glucose load can be advanced by 1 to 2 mg/kg/min/day. Hypoglycemia is not as common a problem as hyperglycemia, but it may occur if the glucose infusion is abruptly decreased or interrupted.
Amino Acids
Protein guidelines range from 2.7 to 3.5 g/kg/day (AAP, 2009b). Some ELBW infants need as much as 3.5 to 4 g/kg/day (AAP, 2009b; Tsang et al., 2005). Protein in excess of these parenteral requirements should not be administered because additional protein offers no apparent advantage, and increases the risk of metabolic problems. In practice preterm infants are usually given 1.5 to 3 g/kg/day of protein for the first few days of life, and then protein is provided as tolerated. Many nurseries stock starter PN, which is water, glucose, protein, and perhaps calcium and is available 24 hours a day. Infants can then be provided with protein immediately on admission to the nursery.
In the United States several pediatric PN solutions are available. The use of pediatric PN solutions results in plasma amino acid profiles similar to those of fetal and cord blood or to those of healthy infants fed breast milk (Schanler and Anderson, 2008). These solutions promote adequate weight gain and nitrogen retention. Standard amino acid solutions were not designed to meet the particular needs of immature infants and may provoke imbalances in plasma amino acid levels. For example, cysteine, tyrosine, and taurine levels in these solutions are low relative to the needs of the preterm infant; but the methionine and glycine levels are relatively high. Because premature infants do not effectively synthesize cysteine from methionine because of decreased concentrations of the hepatic enzyme cystathionase, a cysteine supplement has been suggested. Cysteine is insoluble and unstable in solution; thus it is added as cysteine hydrochloride when the PN solution is prepared.
In addition to plasma amino acid imbalances, other metabolic problems associated with amino acid infusions in preterm infants include metabolic acidosis, hyperammonemia, and azotemia. These problems can be minimized by using the crystalline amino acid products that are available and by keeping the protein load within the recommended guidelines (Table 43-5).
TABLE 43-5
Guidelines for Administration of Parenteral Amino Acids for Premature Infants
| Initial Rate (g/kg/day)* | Increments (g/kg/day) | Maximum Rate (g/kg/day) |
| 1.5-3 | Advance to meet needs | 3.5-4† |
For example: 2% amino acid parenteral solution provided at 150 mL/kg/day is × 0.02 × 150 mL/kg/day = 3 g/kg/day.
*Use the following formula to calculate protein load: % Protein × mL/kg/day = Protein g/kg/day.
†From Tsang RC et al: Summary of reasonable nutrient intakes. In Tsang RC: Nutrition of the preterm infant, ed 2, Cincinnati, OH, 2005, Digital Educational Publishing, Inc. 4 g/kg/day is recommended for infants weighing less than 1000 g.
Data from American Academy of Pediatrics, Committee on Nutrition: Nutritional needs of preterm infants. In Kleinman RE, editor: Pediatric nutrition handbook, ed 6, Elk Grove, IL, 2009, American Academy of Pediatrics.
Lipids
Intravenous fat emulsions are used for two reasons: (1) to meet essential fatty acid (EFA) requirements and (2 to provide a concentrated source of energy. EFA needs can be met by providing 0.5 to 1 g/kg/day of lipids. Biochemical evidence of EFA deficiency has been noted during the first week of life in VLBW infants fed parenterally without fat. The clinical consequences of EFA deficiency may include coagulation abnormalities, abnormal pulmonary surfactant, and adverse effects on lung metabolism.
Lipids can be initiated at 1 to 2 g/kg/day and should be provided over 24 hours (AAP, 2009b). Lipids can be advanced by 1 to 2 g/kg/day until a rate of 3 g/kg/day is reached (Table 43-6). Plasma triglycerides should be monitored because elevated triglyceride levels may develop in infants with a decreased ability to hydrolyze triglycerides. These infants usually have lower gestational age, SGA status, infection, surgical stress, or liver disease. Monitoring of serum triglyceride levels is indicated, and a rate of less than 3 g/kg/day of fat may be required to keep serum triglyceride levels under 200 mg/dL (AAP, 2009b). Once the infant is medically stable and additional energy is needed for growth, lipid loads can slowly be increased. Intralipids can be given to the infant with hyperbilirubinemia. At the present recommendation of 3 g/kg/day, given during 24 hours, the displacement of bilirubin from albumin-binding sites does not occur (AAP, 2009b).
TABLE 43-6
Guidelines for Administration of Parenteral Lipids for Premature Infants
| Initial Rate (g/kg/day)* | Increments (g/kg/day) | Maximum Rate (g/kg/day) |
| 1-2 | 1 | 3 |
*Use the following formula to calculate lipid load: % Lipid × mL/kg/day = Lipid g/kg/day. For example, 0.20 × 15 mL/kg = 3 g/kg/day.
Data from American Academy of Pediatrics, Committee on Nutrition: Nutritional needs of preterm infants. In Kleinman RE, editor: Pediatric nutrition handbook, ed 6, Elk Grove, IL, 2009, American Academy of Pediatrics.
The total lipid load is usually less than 30% to 40% of nonprotein calories, but it should not exceed 60% of nonprotein calories. (The lipid emulsions currently in use are described in Chapter 14). In preterm infants 20% solutions providing 2 kcal/mL are recommended because plasma triglyceride, cholesterol, and phospholipid levels are generally lower with these than with the 10% emulsions. The lower plasma fat levels may be attributable to a decreased phospholipid load per gram of fat in the 20% emulsion.
Intravenous fat emulsions are made from soybean oil and contain ω-6 fatty acids, linoleic acid and arachidonic acid (ARA). These EFAs increase the production of inflammatory mediators and increase the infant’s inflammatory state (Gura, 2010). There is a fish-oil base intravenous fat and it contains ω-3 fatty acids: eicosapentaenoic acid and docosahexaenoic acid (DHA). These ω-3 fatty acids are antiinflammatory and are helpful in the treatment of PN-associated liver disease (Gura, 2010). This fish-oil product is produced in Europe and requires approval for compassionate use by the Food and Drug Administration for patients in the United States. Investigations are ongoing to determine if this product can be used to prevent PN-associated liver disease, which presents with an elevated conjugated bilirubin.
Carnitine is frequently added to PN solutions provided to premature infants. Carnitine facilitates the mechanism by which fatty acids are transported across the mitochondrial membrane, allowing their oxidation to provide energy. Intravenous lipid does not contain carnitine and premature infants have limited ability to produce carnitine (Hay, 2008). Carnitine supplementation may be helpful for preterm infants who are receiving only PN for 2 to 4 weeks.
Electrolytes
After the first few days of life, sodium, potassium, and chloride are added to parenteral solutions to compensate for the loss of extracellular fluid. To prevent hyperkalemia and cardiac arrhythmia, potassium should be withheld until renal flow is demonstrated. In general, the preterm infant has the same electrolyte requirements as the term infant, but actual requirements vary, depending on factors such as renal function, state of hydration, and the use of diuretics (Table 43-7). Very immature infants may have a limited ability to conserve sodium and thus may require increased amounts of sodium to maintain a normal serum sodium concentration. Serum electrolyte levels should be monitored periodically.
Minerals
Calcium and phosphorus are important components of the PN solution. Premature infants who receive PN with low calcium and phosphorus concentrations are at risk for developing osteopenia of prematurity. This poor bone mineralization is most likely to develop in VLBW infants who receive PN for prolonged periods. Calcium and phosphorus status should be monitored using serum calcium, phosphorus, and alkaline phosphatase activity levels (see Appendix 30 for normal range). Alkaline phosphatase activity levels in premature infants are greater than the levels seen with adults. It is common to see levels up to 600 IU/L, which may reflect rapid bone growth (Mitchell et al., 2009). When alkaline phosphatase activity levels of 800 IU/L or more persist, knee or wrist radiographs should be examined for rickets (Mitchell et al., 2009). Elevation in alkaline phosphatase activity may also be seen with liver disease.
Preterm infants have higher calcium and phosphorus needs than term infants. However, it is difficult to add enough calcium and phosphorus to parenteral solutions to meet these higher requirements without causing precipitation of the minerals. Calcium and phosphorus should be provided simultaneously in separate PN solutions. Alternate-day infusions are not recommended because abnormal serum mineral levels and decreased mineral retention develop.
Current recommendations for parenteral administration of additional calcium, phosphorus, and magnesium are presented in Table 43-8. The intakes are expressed at a volume intake of 120 to 150 mL/kg/day, with 2.5 g/100 mL of amino acids or protein. Lower fluid volumes or lower protein concentrations may cause the minerals to precipitate out of solution. The addition of cysteine hydrochloride increases the acidity of the fluid, which inhibits precipitation of calcium and phosphorus.
TABLE 43-8
Guidelines for Administration of Parenteral Minerals for Premature Infants
| Minerals | Amount (mg/kg/day)* |
| Calcium | 60-80 |
| Phosphorus | 39-67 |
| Magnesium | 4.3-7.2 |
*These recommendations assume an average fluid intake of 120 to 150 mL/kg/day with 2.5 g of amino acids per 100 mL. The amino acid concentration prevents the precipitation of these minerals.
From American Academy of Pediatrics, Committee on Nutrition: Nutritional needs of preterm infants. In Kleinman RE, editor: Pediatric nutrition handbook, ed 6, Elk Grove, IL, 2009, American Academy of Pediatrics.
Trace Elements
Zinc should be given to all preterm infants receiving PN. If enteral feedings cannot be started by 2 weeks of age, additional trace elements should be added. However, the amounts of copper and manganese should be reduced for infants with obstructive jaundice; and the amounts of selenium, chromium, and molybdenum should be reduced in infants with renal dysfunction. Parenteral iron is not routinely provided because infants often receive blood transfusions soon after birth, and enteral feedings, which provide a source of iron, can often be initiated. If necessary, the dosage for parenteral iron is approximately 10% of the enteral dosage; guidelines range from 0.1 to 0.2 mg/kg/day (Rao and Georgieff, 2005). Recommended guidelines have not yet been established for parenteral administration of fluoride to preterm infants (Table 43-9).
TABLE 43-9
Guidelines for Administration of Parenteral Trace Elements for Premature Infants
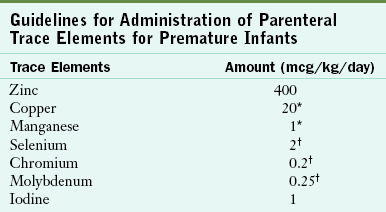
*Reduced or not provided for infants with obstructive jaundice.
†Reduced or not provided for infants with renal dysfunction.
From American Academy of Pediatrics, Committee on Nutrition: Parenteral nutrition. In Kleinman RE, editor: Pediatric nutrition handbook, ed 6, Elk Grove, IL, 2009, AAP.
Vitamins
Shortly after birth all newborn infants receive an intramuscular (IM) injection of 0.5 to 1 mg of vitamin K to prevent hemorrhagic disease of the newborn from vitamin K deficiency. Stores of vitamin K are low in newborn infants, and there is little intestinal bacterial production of vitamin K until bacterial colonization takes place. Because initial dietary intake of vitamin K is limited, neonates are at nutritional risk if they do not receive this IM supplement.
Only intravenous multivitamin preparations currently approved and designed for use in infants should be given to provide the appropriate vitamin intake and prevent toxicity from additives used in adult multivitamin injections. The American Academy of Pediatrics (AAP, 2009b) recommends 40% of the multivitamin for infusion (MVI)-pediatric 5-mL vial per kilogram of weight. The maximum dose of 5 mL is given to an infant with a weight of 2.5 kg (Table 43-10).
TABLE 43-10
Guidelines for Administration of Parenteral Vitamins for Premature Infants
| Preterm | |
| Percentage of one 5-mL vial of MVI-Pediatric/INFUVITE* | 40%/kg |
MVI, multivitamin for infusion.
Maximum volume intake is 5 mL/day, which is achieved at 2.5 kg body weight.
*MVI-Pediatric/INFUVITE (5 mL) contains the following vitamins: 80 mg of ascorbic acid, 2300 USP units of vitamin A, 400 USP units of vitamin D, 1.2 mg of thiamin, 1.4 mg of riboflavin, 1 mg of vitamin B6, 17 mg of niacin, 5 mg of pantothenic acid, 7 USP units of vitamin E, 20 mcg of biotin, 140 mcg of folic acid, 1 mcg of vitamin B12, and 200 mcg of vitamin K.
Data from American Academy of Pediatrics, Committee on Nutrition: Nutritional needs of preterm infants. In Kleinman RE, editor: Pediatric nutrition handbook, ed 6, Elk Grove, IL, 2009, American Academy of Pediatrics.
Respiratory distress syndrome (RDS) is a disease that occurs in premature infants shortly after birth because these infants are deficient in the lung substance surfactant. Surfactant is responsible for keeping the lung elastic while breathing; thus surfactant supplements are given to the infant to prevent RDS or to lessen the illness. Lipids and proteins are components of surfactant, and phospholipids are the major lipid. Choline is required for phospholipid synthesis, but choline supplementation does not increase the production of phospholipids (van Aerde and Narvey, 2006). Choline is a conditionally essential nutrient because the infant can synthesize choline (see Chapter 16 for a discussion of requirement for choline in pregnancy). Choline is added to premature infant formulas at the level contained in human milk. The upper level is extrapolated from the adult safe level of intake (Klein, 2002).
Bronchopulmonary dysplasia (BPD) is a chronic lung disease that commonly develops in the premature infant as a result of RDS and the mechanical ventilation and oxygen used to treat it. Because of the role of vitamin A in facilitating tissue repair, and because of reports of preterm infants having low vitamin A stores, large supplemental doses of vitamin A have been suggested for the prevention of BPD. One report suggests that providing ELBW premature infants with IM injections of vitamin A at 5000 units/day three times per week during the first month of life decreases the incidence of BPD (Tyson et al., 1999). Physicians may or may not use this supplementation. The decision will be based on the incidence of BPD in their nursery, the lack of proven additional benefits and the acceptability of using IM injections (Darlow and Graham, 2008). See Chapter 35 for discussion of BPD.
Transition from Parenteral to Enteral Feeding
It is beneficial to begin enteral feedings for preterm infants as early as possible because the feedings stimulate gastrointestinal enzymatic development and activity, promote bile flow, increase villous growth in the small intestine, and promote mature gastrointestinal motility. These initial enteral feedings can also decrease the incidence of cholestatic jaundice and the duration of physiologic jaundice and can improve subsequent feeding tolerance in preterm infants. At times small, initial feedings are used only to prime the gut and are not intended to optimize enteral nutrient intake until the infant demonstrates feeding tolerance or is clinically stable.
When making the transition from parenteral to enteral feeding, it is important to maintain parenteral feeding until enteral feeding is well established to maintain adequate net intake of fluid and nutrients. In VLBW infants it may take 7 to 14 days to provide a full enteral feeding, and it may take longer for infants with feeding intolerances or illness. The smallest, sickest infants usually receive increments of only 10 mL/kg/day. Larger, more stable preterm infants may tolerate increments of 20 to 30 mL/kg/day (see Chapter 14 for a more detailed discussion of transitional feeding).
Nutrition Requirements: Enteral Feeding
Enteral alimentation is preferred for preterm infants because it is more physiologic than parenteral alimentation and is nutritionally superior. Initiating a tiny amount of an appropriate breast milk feeding whenever possible is beneficial (Sisk et al., 2007). However, determining when and how to provide enteral feedings is often difficult and involves consideration of the degree of prematurity, history of perinatal insults, current medical condition, function of the gastrointestinal tract, respiratory status, and several other individual concerns (Table 43-11).
TABLE 43-11
Factors to Consider Before Initiating or Increasing the Volume of Enteral Feedings
| Category | Factors |
| Perinatal | Cardiorespiratory depression |
| Respiratory | Stability of ventilation, blood gases, apnea, bradycardia, cyanosis |
| Medical | Vital signs (heart rate, respiratory rate, blood pressure, temperature) |
| Gastrointestinal | Anomalies (gastroschisis, omphalocele), patency, gastrointestinal tract function (bowel sounds present, passage of stool), risk of necrotizing enterocolitis |
| Infection | Sepsis or suspect sepsis |
Data from Adamkin DH: Nutritional strategies for the very low birthweight infant, Cambridge, 2009,Cambridge University Press; Hay WW: Strategies for feeding the preterm infant, Neonatol 94:245, 2008.
Preterm infants should be fed enough to promote growth similar to that of a fetus at the same gestational age, but not so much that nutrient toxicity develops. Although the exact nutrient requirements are unknown for preterm infants, several useful guidelines exist. In general the requirements of premature infants are higher than those of term infants because the preterm infant has smaller nutrient stores, decreased digestion and absorption capabilities, and a rapid growth rate. Stress, illness, and certain therapies for illness may further influence nutrient requirements. It is also important to remember that, in general, enteral nutrient requirements are different from parenteral requirements.
Energy
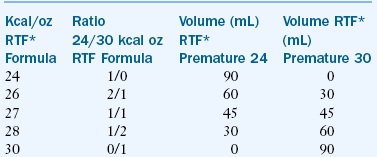
The energy requirements of premature infants vary with individual biologic and environmental factors. It is estimated that an intake of 50 kcal/kg/day is required to meet maintenance energy needs, compared with 105 to 130 kcal/kg/day for growth (Table 43-12). However, energy needs may be increased by stress, illness, and rapid growth. Likewise, energy needs may be decreased if the infant is placed in a neutral thermal environment (the environmental temperature at which an infant expends the least amount of energy to maintain body temperature). It is important to consider the infant’s rate of growth in relation to average energy intakes. Some premature infants may need greater than 130 kg/day to sustain an appropriate rate of growth. ELBW infants or those with BPD often require such increased amounts. To provide such a large number of calories to infants with a limited ability to tolerate large fluid volumes, it may be necessary to concentrate the feedings to a level of more than 24 kcal/oz (Box 43-2).
TABLE 43-12
Estimation of Energy Requirements of the Low-Birth-Weight Infant
| Activity | Average Estimation (kcal/kg/day) |
| Energy expended | 40-60 |
| Resting metabolic rate | 40-50* |
| Activity | 0-5* |
| Thermoregulation | 0-5* |
| Synthesis | 15† |
| Energy stored | 20-30† |
| Energy excreted | 15 |
| Energy intake | 90-120 |
Modified from American Academy of Pediatrics, Committee on Nutrition: Nutritional needs of preterm infants. In Kleinman RE, editor: Pediatric nutrition handbook, ed 6, Elk Grove, IL, 2009, AAP; Committee on Nutrition of the Preterm Infant, European Society of Paediatric Gastroenterology and Nutrition (ESPGAN): Nutrition and feeding of preterm infants, Oxford, 1987, Blackwell Scientific.
Protein
The amount and quality of protein must be considered when establishing protein requirements for the preterm infant. Amino acids should be provided at a level that meets demands without inducing amino acid or protein toxicity.
A reference fetus model has been used to determine the amount of protein that needs to be ingested to match the quantity of protein deposited into newly formed fetal tissue (Ziegler, 2007). To achieve these fetal accretion rates, additional protein must be supplied to compensate for intestinal losses and obligatory losses in the urine and skin. Based on this method for determining protein needs, the advisable protein intake is 3.5 to 4 g/kg/day. This amount of protein is well tolerated.
The quality or type of protein is an important consideration because premature infants have different amino acid needs than term infants because of immature hepatic enzyme pathways. The amino acid composition of whey protein, which differs from that of casein, is more appropriate for premature infants. The essential amino acid cysteine is more highly concentrated in whey protein, and premature infants do not synthesize cysteine well. In addition, the amino acids phenylalanine and tyrosine are lower, and the preterm infant has difficulty oxidizing them. Furthermore, metabolic acidosis decreases with consumption of whey-predominant formulas. Because of the advantages of whey protein for premature infants, breast milk or formulas containing predominately whey proteins should be chosen whenever possible.
Taurine is a sulfonic amino acid that may be important for preterm infants. Human milk is a rich source of taurine, and taurine is added to most infant formulas. Term and preterm infants develop low plasma and urine concentrations of taurine without a dietary supply. The premature infant may have difficulty with synthesizing taurine from cysteine. Although no overt disease has been reported in infants fed low taurine formulas, low taurine may affect the development of vision and hearing (Klein, 2002).
Energy must be provided at sufficient levels to allow protein to be used for growth and not merely for energy expenditure. A range of 10.2% to 12.4% of calories from protein has been suggested. Inadequate protein intake is growth limiting, whereas excessive intake causes elevated plasma amino acid levels, azotemia, and acidosis.
Lipids
The growing preterm infant needs an adequate intake of well-absorbed dietary fat to help meet the high energy needs of growth, provide EFAs, and facilitate absorption of other important nutrients such as the fat soluble vitamins and calcium. However, neonates in general, and premature and SGA infants in particular, digest and absorb lipids inefficiently.
Fat should constitute 40% to 50% of total calories. Furthermore, a diet that is high in fat and low in protein may yield more fat deposition than is desirable for the growing preterm infant. To meet EFA needs, linoleic acid should compose 3% of the total calories, and α-linolenic acid should be added in small amounts (AAP, 2009b). Additional longer-chain fatty acids—ARA and DHA—are present in human milk and are added to infant formulas for term and premature infants to meet federal guidelines.
The premature infant has a greater need than the term infant for ARA and DHA supplementation. These fatty acids accumulate in fatty tissue and the brain during the last 3 months of gestation; thus the premature infant has decreased stores. Premature infants fed formulas supplemented with ARA and DHA from birth to 92 weeks’ postmenstrual age (12 months after term) demonstrate greater gain in weight and length and higher psychomotor development scores than premature infants not receiving the fatty acid supplementation (Clandinin et al., 2005).
Preterm infants have low levels of pancreatic lipase and bile salts, and this decreases their ability to digest and absorb fat. Lipases are needed for triglyceride breakdown, and bile salts solubilize fat for ease of digestion and absorption. Because medium-chain triglycerides (MCTs) do not require pancreatic lipase and bile acids for digestion and absorption, they have been added to the fat mixture in premature infant formulas. Human milk and vegetable oils contain the EFA linoleic acid, but MCT oil does not. Premature infant formulas must contain vegetable oil and MCT oil to provide the essential long-chain fatty acids.
The composition of dietary fat also plays a role in the digestion and absorption of lipid. In general, infants absorb vegetable oils more efficiently than saturated animal fats, although one exception is the saturated fat in human milk. Infants digest and absorb human milk fat better than the saturated fat in cow’s milk or the vegetable oil in standard infant formulas. Human milk contains two lipases that facilitate fat digestion and has a special fatty acid composition that aids absorption.
Carbohydrates
Carbohydrates are an important source of energy, and the enzymes for endogenous production of glucose from carbohydrate and protein are present in preterm infants. Approximately 40% of the total calories in human milk and standard infant formulas are derived from carbohydrates. Too little carbohydrate may lead to hypoglycemia, whereas too much may provoke osmotic diuresis or loose stools. The recommended range for carbohydrate intake is 40% to 50% of total calories.
Lactose, a disaccharide composed of glucose and galactose, is the predominant carbohydrate in almost all mammalian milks and may be important to the neonate for glucose homeostasis, perhaps because galactose can be used for either glucose production or glycogen storage. Generally galactose is used for glycogen formation first, and then it becomes available for glucose production as blood glucose levels decrease. Because infants born before 28 to 34 weeks of gestation have low lactase activity, the premature infant’s ability to digest lactose may be marginal. In practice, malabsorption is not a clinical problem because lactose is hydrolyzed in the intestine or fermented in the colon and absorbed. Sucrose is another disaccharide that is commonly found in commercial infant formula products. Because sucrase activity early in the third trimester is at 70% of newborn levels, sucrose is well tolerated by most premature infants. Sucrase and lactase are sensitive to changes in the intestinal milieu. Infants who have diarrhea, are undergoing antibiotic therapy, or are undernourished, may develop temporary intolerances to lactose and sucrose.
Glucose polymers are common carbohydrates in the preterm infant’s diet. These polymers, consisting mainly of chains of five to nine glucose units linked together, are used to achieve the isoosmolality of certain specialized formulas. Glucosidase enzymes for digesting glucose polymers are active in small preterm infants.
Minerals and Vitamins
Premature infants require greater amounts of vitamins and minerals than term infants because they have poor body stores, are physiologically immature, are frequently ill, and will grow rapidly. Formulas and human milk fortifiers that are developed especially for preterm infants contain higher vitamin and mineral concentrations to meet the needs of the infant, obviating the need for additional supplementation in most cases (Table 43-13). One major exception is infants receiving human milk with a fortifier that does not contain iron. An iron supplement of 2 mg/kg/day should be sufficient to meet their needs (AAP, 2009b). The other exception is the use of donor human milk fortifier, which requires the addition of a multiple vitamin and an iron supplement.
TABLE 43-13
Recommendations for Enteral Administration of Vitamins for the Premature Infant
| Vitamin | Amount (kg/day) |
| Vitamin A | 700-1500 IU |
| Vitamin D | 150-400 IU* |
| Vitamin E | 6-12 IU |
| Vitamin K | 8-10 mcg |
| Ascorbic acid | 18-24 mg |
| Thiamin | 180-240 mcg |
| Riboflavin | 250-360 mcg |
| Pyridoxine | 150-210 mcg |
| Niacin | 3.6-4.8 mg |
| Pantothenate | 1.2-1.7 mg |
| Biotin | 3.6-6 mcg |
| Folate | 25-50 mcg |
| Vitamin B12 | 0.3 mcg |
Data from American Academy of Pediatrics, Committee on Nutrition: Nutritional needs of preterm infants. In Kleinman RE, editor: Pediatric nutrition handbook, ed 6, Elk Grove, IL, 2009, American Academy of Pediatrics.
Calcium and Phosphorus
Calcium and phosphorus are just two of many nutrients that growing premature infants require for optimal bone mineralization. Intake guidelines have been established at levels that promote the bone mineralization rate that occur in the fetus. An intake of 100-220 mg/kg/day of calcium and 60-140 mg/kg/day of phosphorus is recommended. Two thirds of the calcium and phosphorus body content of the term neonate is accumulated through active transport mechanisms during the last trimester of pregnancy. Infants who are born prematurely are deprived of this important intrauterine mineral deposition. With poor mineral stores and low dietary intake, preterm infants can develop osteopenia of prematurity, a disease characterized by demineralization of growing bones and documented by radiologic evidence of “washed-out” or thin bones. Very immature babies are particularly susceptible to osteopenia and may develop bone fractures or florid rickets with a prolonged dietary deficiency. Osteopenia of prematurity is most likely to develop in preterm infants who are (1) fed infant formula that is not specifically formulated for preterm infants, (2) fed human milk that is not supplemented with calcium and phosphorus, or (3) receiving long-term PN without enteral feedings.
Vitamin D
Human milk with human milk fortifier or infant formula for preterm infants provides adequate vitamin D when infants consume the entire calorie intake suggested. The current recommendations for intake range from 150 to 400 IU/day for preterm infants.
Vitamin E
Preterm infants require more vitamin E than term infants because of their limited tissue stores, decreased absorption of fat-soluble vitamins, and rapid growth. Vitamin E protects biologic membranes against oxidative lipid breakdown. Because iron is a biologic oxidant, a diet high in either iron or polyunsaturated fatty acids (PUFAs) increases the risk of vitamin E deficiency. The PUFAs are incorporated into the red blood cell membranes and are more susceptible to oxidative damage than when saturated fatty acids compose the membranes.
A premature infant with vitamin E deficiency may experience hemolytic anemia (oxidative destruction of red blood cells). However, this anemia is now uncommon today because of improvements in human milk fortifiers and infant formula composition. The human milk fortifiers and premature infant formulas now contain appropriate vitamin E/PUFA ratios for preventing hemolytic anemia.
Because the dietary requirement for vitamin E depends on the PUFA content of the diet, the recommended intake of vitamin E is commonly expressed as a ratio of vitamin E to PUFA. The recommendation for vitamin E is 0.7 IU (0.5 mg of d-α-tocopherol) per 100 kcal, and at least 1 IU of vitamin E per gram of linoleic acid.
Pharmacologic dosing of vitamin E (50 to 100 mg/kg/day) has not proven to be helpful in preventing BPD or retinopathy of prematurity by reducing the toxic effects of oxygen. Furthermore, high doses of vitamin E have been associated with intraventricular hemorrhage, sepsis, NEC, liver and renal failure, and death.
Iron
Preterm infants are at risk for iron deficiency anemia because of the reduced iron stores associated with early birth. At birth most of the available iron is in the circulating hemoglobin. Thus frequent blood sampling further depletes the amount of iron available for erythropoiesis. Transfusions of red blood cells are often needed to treat the early physiologic anemia of prematurity. Recombinant erythropoietin (EPO) therapy has been used to prevent anemia. Iron supplementation is indicated to facilitate red blood cell production, and a dosage of 6 mg/kg/day of enteral iron has been used (AAP, 2009b). This therapy has not consistently prevented anemia and the need for blood transfusions (AAP, 2009b).
In general the recommendation for iron intake is 2 to 4 mg/kg/day (AAP, 2009b). Infants fed human milk should be given ferrous sulfate drops beginning at 1 month of age (Baker and Greer, 2010). Formulas fortified with iron usually contain sufficient iron for preterm infants (AAP, 2009b).
Folic Acid
Premature infants seem to have higher folic acid needs than infants born at term. Although serum folate levels are high at birth, they decrease dramatically, probably as a result of high folic acid use by the premature infant for deoxyribonucleic acid and tissue synthesis needed for rapid growth.
A mild form of folic acid deficiency causing low serum folate concentrations and hypersegmentation of neutrophils is not unusual in premature infants. Megaloblastic anemia is much less common. A daily folic acid intake of 25 to 50 mcg/kg effectively maintains normal serum folate concentrations. Fortified human milk and formulas for premature infants meet these guidelines when full enteral feedings are established.
Sodium
Preterm infants, especially those with VLBW, are susceptible to hyponatremia during the neonatal period. These infants may have excessive urinary sodium losses because of renal immaturity and an inability to conserve adequate sodium. Furthermore, their sodium needs are high because of their rapid growth rate.
Daily sodium intakes of 4 to 8 mEq/kg or more may be required by some infants to prevent hyponatremia. Routine sodium supplementation of fortified human milk and infant formulas is not necessary. However, it is important to consider the possibility of hyponatremia and monitor infants by assessing serum sodium until the blood level is normal. Milk can be supplemented with sodium if repletion is necessary.
Feeding Methods
Decisions about breast-feeding, bottle-feeding, or tube-feeding depend on the gestational age and the clinical condition of the preterm infant. The goal is to feed the infant via the most physiologic method possible and supply nutrients for growth without creating clinical complications.
Gastric Gavage
Gastric gavage by the oral route is often chosen for infants who are unable to suck because of immaturity or problems with the central nervous system. Infants less than 32 to 34 weeks of gestational age, regardless of birth weight have poorly coordinated sucking, swallowing, and breathing abilities because of their developmental immaturity. Consequently they have difficulty with nipple-feeding.
With the oral gastric gavage method, a soft feeding tube is inserted through the infant’s mouth and into the stomach. The major risks of this technique include aspiration and gastric distention. Because of weak or absent cough reflexes and poorly developed respiratory muscles, the tiny infant may not be able to dislodge milk from the upper airway, which can cause reflex bradycardia or airway obstruction. However, electronic monitoring of vital functions and proper positioning of the infant during feeding minimize the risk of aspiration from regurgitation of stomach contents. Tiny, immature infants whose small gastric capacity and slow intestinal motility can impede the tolerance of large-volume bolus feeds may need bolus feedings provided with a pump for a 30 to 60 minute infusion to aid in feeding tolerance.
Occasionally elimination of the distention and vagal bradycardia requires the use of an indwelling tube for continuous gastric gavage feedings rather than intermittent administration of boluses. Continuous feedings may lead to loss of milk fat, calcium, and phosphorus, which deposit in the feeding tubing so that the infant does not receive the total amount of nutrition provided. Bolus feedings provided with the use of the pump infusion can decrease nutrient loss and promote better weight gain. (Hay, 2008; Rogers et al., 2010).
Nasal gastric gavage is sometimes better tolerated than oral tube-feeding. However, because neonates must breathe through the nose, this technique may compromise the nasal airway in preterm infants and cause an associated deterioration in respiratory function. This method is helpful for infants who are learning to nipple-feed. An infant with a nasal gastric tube can still form a tight seal on the bottle nipple, but it can be difficult if an oral feeding tube is in place during feedings (see Chapter 14).
Transpyloric Feeding
Transpyloric tube-feeding is indicated for infants who are at risk for aspirating milk into the lungs or who have slow gastric emptying. The goal of this method is to circumvent the often slow gastric emptying of the immature infant by passing the feeding tube through the stomach and pylorus and placing its tip within the duodenum or jejunum. Infants with severe gastrointestinal reflux do well with this method, which prevents aspiration of feedings into the lungs. This method is also used for infants whose respiratory function is compromised and who are at risk for milk aspiration. The possible disadvantages of transpyloric feedings include decreased fat absorption, diarrhea, dumping syndrome, alterations of the intestinal microflora, intestinal perforation, and bilious fluid in the stomach. In addition, the placement of transpyloric tubes also requires considerable expertise and radiographic confirmation of the catheter tip location. Although associated with many possible complications, transpyloric feedings are used when gastric feeding is not successful.
Nipple-Feeding
Nipple-feeding may be attempted with infants whose gestational age is greater than 32 weeks and whose ability to feed from a nipple is indicated by evidence of an established sucking reflex and sucking motion. Before this time they are unable to coordinate sucking, swallowing, and breathing. Because sucking requires effort by the infant, any stress from other causes such as hypothermia or hypoxemia diminishes the sucking ability. Therefore nipple-feeding should be initiated only when the infant is under minimum stress and is sufficiently mature and strong to sustain the sucking effort. Initial oral feedings may be limited to one to three times per day to prevent undue fatigue or too much energy expenditure, either of which can slow the infant’s rate of weight gain. Before oral feedings begin, a standardized oral stimulation program can help infants successfully nipple-feed more quickly (Fucile et al., 2005).
Breast-Feeding
When the mother of a premature infant chooses to breast-feed, nursing at the breast should begin as soon as the infant is ready. Before this time the mother must express her milk so that it can be tube-fed to her infant. These mothers need emotional and educational support for successful lactation. Studies report that premature breast-fed infants have better sucking, swallowing, and breathing coordination and fewer breathing disruptions than bottle-fed infants (Hurst, 2007). Kangaroo care—allowing the mother to maintain skin-to-skin contact while holding her infant—facilitates her lactation. In addition, this type of contact promotes continuation of breast-feeding and enhances the mother’s confidence in caring for her high-risk infant. The latter benefit may also apply to fathers who engage in kangaroo care with their infants (Stevens et al., 2010).
Feeding infants with cups instead of bottles to supplement breast-feeding has been suggested for preterm infants based on the rationale that it may prevent infant “nipple confusion” (i.e., confusion between nursing at the breast and from a bottle). Complications such as milk aspiration and low volume intakes need to be monitored. Cup feeding has been associated with successful breast-feeding at discharge, but increased length of stay in the hospital for the premature infant (Collins et al., 2008).
Tolerance of Feedings
All preterm babies receiving EN should be monitored for signs of feeding intolerance. Vomiting of feedings usually signals the infant’s inability to retain the provided amount of milk. When not associated with other signs of a systemic illness, vomiting may indicate that feeding volumes were increased too quickly or are excessive for the infant’s size and maturity. Simply reducing the feeding volume may resolve the problem. If not, or if the infant has signs of a systemic illness, feedings may need to be interrupted until the infant’s condition has stabilized.
Abdominal distention may be caused by excessive feeding, organic obstruction, excessive swallowing of air, resuscitation, sepsis (i.e., systemic infection), or NEC. Observing infants for abdominal distention should be a routine practice for nurses. Abdominal distention often indicates the need to interrupt feeding until its cause is determined and resolved.
Gastric residuals, measured by aspiration of the stomach contents, may be determined routinely before each bolus gavage feeding and intermittently in all continuous drip feedings. Whether a residual amount is significant depends partly on its volume in relation to the total volume of the feeding. For example, a residual volume of more than 50% of a bolus feeding or equal to the continuous infusion rate might be a sign of feeding intolerance. However, when interpreting the significance of a gastric residual measurement, it is important to consider other concurrent signs of feeding intolerance and the previous pattern of residual volumes established for a particular infant. Residuals are frequently present before feedings are initiated and as small volume feedings are started. As long as no signs of illness are present, feedings should not be held.
Bile-stained emesis or residuals frequently may be due to overdistention of the stomach with reflux of bile from the intestine or to a feeding tube that has slipped into the intestine, or may indicate that the infant has an intestinal blockage and needs additional evaluation (Schanler and Anderson, 2008). Bloody or bilious gastric residuals are more alarming than those that seem to be undigested milk.
The frequency and consistency of bowel movements should be constantly monitored when feeding preterm infants. Simple inspections can detect the presence of gross blood. All feeding methods for preterm infants have associated complications. Unless close attention is paid to symptoms that indicate poor feeding tolerance, serious complications may ensue. Certain diseases can be recognized by recognizing signs of feeding intolerance. For example, necrotizing enterocolitis (NEC) is a serious and potentially fatal disease associated with specific symptoms such as abdominal distention and tenderness, abnormal gastric residuals, and grossly bloody stools.
Selection of Enteral Feeding
During the initial feeding period premature infants often require additional time to adjust to EN and may experience concurrent stress, weight loss, and diuresis. The primary goal of enteral feeding during this initial period is to establish tolerance to the milk. Infants seem to need a period of adjustment to be able to assimilate a large volume and concentration of nutrients. Thus parenteral fluids may be necessary until infants can tolerate adequate amounts of feedings by mouth.
After the initial period of adjustment, the goal of enteral feeding changes from establishing milk tolerance to providing complete nutrition support for growth and rapid organ development. All essential nutrients should be provided in quantities that support sustained growth. The following feeding choices are appropriate: (1) human milk supplemented with human milk fortifier and iron and vitamins as indicated by fortifier used, (2) iron-fortified premature infant formula for infants who weigh less than 2 kg, or (3) iron-fortified standard infant formula for infants who weigh more than 2 kg.
Premature infants who are discharged from the hospital can be given a transitional formula. Additional vitamin D may be indicated to provide 400 IU per day (Wagner and Greer, 2008). Iron supplements may be needed for some infants with the use of this enriched formula (Baker and Greer, 2010). Breast-fed infants may be provided with two to three bottles of transitional formula daily to meet needs. The breast-fed premature infant should also receive 2 mg /kg/day of iron and a multiple vitamin for the first year of life (AAP, 2009b). Premature infants discharged home on standard formula should receive a multivitamin until the infant reaches 3 kg in weight (AAP, 2009b).
Human Milk
Human milk is the ideal food for healthy term infants and premature infants. Although human milk requires nutrient supplementation to meet the needs of premature infants, its benefits for the infant are numerous. During the first month of lactation, the composition of milk from mothers of premature infants differs from that of mothers who have given birth to term infants; the protein and sodium concentrations of breast milk are higher in mothers with preterm infants (Klein, 2002). When premature infants are fed their own mother’s milk, they grow more rapidly than infants fed banked, mature breast milk (Schanler et al., 2005).
In addition to its nutrient concentration, human milk offers nutritional benefits because of its unique mix of amino acids and long-chain fatty acids. The zinc and iron in human milk are more readily absorbed, and fat is more easily digested because of the presence of lipases. Moreover, human milk contains factors that are not present in formulas. These components include (1) macrophages and T and B lymphocytes; (2) antimicrobial factors - secretory immunoglobulin A, lactoferrin, and others; (3) hormones; (4) enzymes; and (5) growth factors. It has been reported that human milk as compared with premature infant formula fed to preterm infants reduces the incidence of NEC and sepsis, improves neurodevelopment, facilitates a more rapid advancement of enteral feedings, and leads to an earlier discharge (Sisk et al., 2007; Sisk et al., 2008). The use of mother’s own milk for her infant supplemented with liquid donor human milk fortifier and donor human milk is linked to decreased incidence of NEC (Sullivan et al., 2010).
However, one well-documented problem is associated with feeding human milk to preterm infants. Whether it is preterm, term, or mature, human milk does not meet the calcium and phosphorus needs for normal bone mineralization in premature infants. Therefore calcium and phosphorus supplements are recommended for rapidly growing preterm infants who are fed predominantly human milk. Currently three human milk fortifiers are available: powder bovine milk base, liquid bovine milk base, and liquid donor human milk base. The bovine products contain calcium and phosphorus, as well as protein, carbohydrates, fat, vitamins, and minerals, and are designed to be added to expressed breast milk fed to premature infants (Table 43-14). Vitamin supplements are not needed. One bovine fortifier is iron fortified and the other requires the addition of iron. The human-milk base product is made from donor human milk that has been pasteurized, concentrated, and supplemented with calcium, phosphorous, zinc, and electrolytes. A multivitamin and an iron supplement is needed with the use of the human-milk base fortifier.
TABLE 43-14
Comparison of the Nutritional Content of Human Milk and Formulas
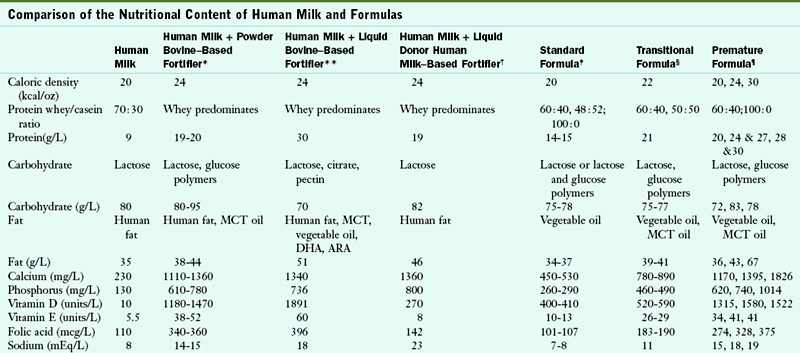
ARA, Arachidonic acid; DHA, docosahexaenoic acid; MCT, medium-chain triglyceride.
*Based on the composition of term human milk fortified with either powder Enfamil or Similac Human Milk Fortifiers at four packets per 100 mL.
**Based on the composition of term human milk fortified with liquid Enfamil Human Milk Fortifier at 1 vial + 25 mL milk.
†Based on the composition of term human milk fortified with Prolact +4.
‡Based on the composition of Enfamil Premium, Similac Advance, and Good Start Gentle Plus formulas.
§Based on the composition of Similac NeoSure, and Enfamil EnfaCare formulas.
¶Based on the composition of Enfamil Premature Lipil, Good Start Premature, and Similac Special Care formulas.
Data from American Academy of Pediatrics, Committee on Nutrition: Appendix C. Table C-1 Representative values for constituents of human milk. In Kleinman RE, editor: Pediatric nutrition handbook, ed 6, Elk Grove, Ill, 2009, American Academy of Pediatrics.
Providing human milk to a premature infant can be a very positive experience for the mother, one that promotes involvement and interaction. Because many preterm infants are neither strong enough nor mature enough to nurse at their mother’s breast in the early neonatal period, their mothers usually express their milk for several days (and occasionally for several weeks) before nursing can be established. The proper technique of expression, storage, and transport of milk should be reviewed with the mother (see Chapter 16). Many summaries of the special considerations for nursing a preterm infant have been published (AAP and the American College of Obstetricians and Gynecologists, 2006; Hurst, 2007).
Premature Infant Formulas
Formula preparations have been developed to meet the unique nutritional and physiologic needs of growing preterm infants. The quantity and quality of nutrients in these products promote growth at intrauterine rates. These formulas, which have caloric densities of 20, 24, and 30 kcal/oz, are available only in a ready-to-feed form. These premature formulas differ in many respects from standard cow’s milk–based formulas (see Table 43-14). The types of carbohydrate, protein, and fat differ to facilitate digestion and absorption of nutrients. These formulas also have higher concentrations of protein, minerals, and vitamins.
Transitional Infant Formulas
Formulas containing 22 kcal/oz have been designed as transition formulas for the premature infant. Their nutrient content is less than that of the nutrient-dense premature infant formulas and more than that of the standard infant formula (see Table 43-14). These formulas can be introduced when the infant reaches a weight of 2000 g or more, and they can be used throughout the first year of life. Not all premature infants need these formulas to grow appropriately. The AAP (2009a) suggests that the transitional formulas be continued until the infant’s weight for length is maintained at greater than the 25th percentile, or up to 9 or 12 months corrected age. It is not clear which premature infants need this specialized formula as studies have not always demonstrated improved growth with the use of transitional formula (AAP, 2009b). Transitional formulas are available in powder form and in ready-to-feed form.
Formula Adjustments
Occasionally it may be necessary to increase the energy content of the formulas fed to small infants. This may be appropriate when the infant is not growing quickly enough and is already consuming as much as possible during feedings.
Concentration
One approach to providing hypercaloric formula is to prepare the formula with less water, thus concentrating all its nutrients, including energy. Concentrated infant formulas with energy contents of 24 kcal/oz are available to hospitals as ready-to-feed nursettes. However, when using these concentrated formulas, it is important to consider the infant’s fluid intake and losses in relation to the renal solute load of the concentrated feeding to ensure that a positive water balance is maintained. This method of increasing formula density is often preferred because the nutrient balance remains the same; infants who need more energy also need additional nutrients. As mentioned, the transitional formulas are available in ready-to-feed and powder form and can be concentrated from 24 to 30 kcal/oz. However, this formula is still inadequate for infants who need additional calcium (e.g., infants with osteopenia).
A ready-to-feed 30 kcal/oz premature infant formula is available. It meets the nutritional needs for premature infants who must be fluid restricted because of illness. This 30 kcal/oz formula can be diluted with premature infant formula 24 kcal/oz to make 26, 27, or 28 kcal/oz milks (see Box 43-2). These milks are sterile and are the preferred source of providing concentrated milks to premature infants in the neonatal intensive care unit (NICU). Infant formula powder is not sterile and is not to be used with high-risk infants when a nutritionally adequate liquid, sterile product is available (Robbins and Beker, 2004).
Caloric Supplements
Another approach to increasing the energy content of a formula involves the use of caloric supplements such as vegetable oil, MCT oil, or glucose polymers. These supplements increase the caloric density of the formula without markedly altering solute load or osmolality. However, they do alter the relative distribution of total calories derived from protein, carbohydrate, and fat. Because even small amounts of oil or carbohydrate dilute the percentage of calories derived from protein, adding these supplements to human milk or standard (20 kcal/oz) formulas is not advised. Caloric supplements should be used only when a formula already meets all nutrient requirements other than energy or when the renal solute load is a concern.
When a high-energy formula is needed, glucose polymers can be added to a base that has a concentration of 24 kcal/oz or greater (either full-strength premature formula or a concentrated standard formula), with a maximum of 50% of total calories from fat and a minimum of 9% of total calories from protein. Vegetable oil may be added to a feeding or given as a oral medication. Vegetable oil added to a day’s supply of formula will separate out from the milk and cling to the milk storage container, and will not be in the feeding to the infant. See Table 45-6.
Nutrition Assessment and Growth
Dietary intake needs to be evaluated to ensure that the nutrition provided meets the infant’s needs. Parenteral fluids and milk feedings are advanced as tolerated, and the nutrient intakes must be reviewed to ensure that they are within the guidelines for premature infants and that the infant is thriving on the nutrition provided. Appropriate growth and growth charts are reviewed in the following paragraphs.
Laboratory Indices
Laboratory assessments usually involve measuring the following parameters: (1) fluid and electrolyte balance, (2) PN or EN tolerance, (3) bone mineralization status, and (4) hematologic status (Table 43-15). Hemoglobin and hematocrit will be monitored as medically indicated. The early decrease in hematocrit reflects the physiologic drop in hemoglobin after birth and blood drawings for laboratory assessments. Early low hemoglobins are treated with blood transfusions if needed. Dietary supplementation will not change this early physiologic drop in hemoglobin.
TABLE 43-15
Monitoring of the Feeding of the Premature Infant
| Monitor | Parenteral Nutrition | Enteral Nutrition |
| Fluid & electrolyte balance | Fluid intake | Fluid intake |
| Urine output | Urine output | |
| Daily weights | Daily weights | |
| Serum sodium, potassium and chloride | ||
| Serum creatinine | ||
| BUN | ||
| Glucose homeostasis | Serum glucose | Not routine |
| Fat tolerance | Serum triglycerides | Not indicated |
| Protein nutriture: BUN | Not helpful | Low levels with human milk–fed infants may indicate need for more protein |
| Osteopenia | Serum calcium | Serum calcium |
| Serum phosphorous | Serum phosphorous | |
| Serum alkaline phosphatase activity | Serum alkaline phosphatase activity | |
| Parenteral nutrition toxicity | Cholestasis: conjugated bilirubin | Not indicated |
| Liver function: ALT |
Growth Rates and Growth Charts
All neonates typically lose some weight after birth. Preterm infants are born with more extracellular water than term infants and thus tend to lose more weight than term infants. However, the postnatal weight loss should not be excessive. Preterm infants who lose more than 15% of their birth weight may become dehydrated from the inadequate fluid intake or experience tissue wasting from poor energy intake. An infant’s birth weight should be regained by the second or third week of life. The smallest and sickest infants take the longest time to regain their birth weights.
During the first 98 days of life the Ehrenkranz growth chart is commonly used to assess weight progress (Ehrenkranz et al., 1999) (Figure 43-3). This chart longitudinally depicts daily weight changes and actual growth curves for 1660 infants who were born with a weight of 501 to 1500 g ( to
to  lb). These infants received care in 12 different NICUs for various neonatal medical problems. Charts are also available for length, head circumference, and midarm circumference (see Useful Websites for a source to create a growth curve for an individual infant). These charts reflect how premature infants grow, and do not allow for the assessment of catch-up growth by the premature infant.
lb). These infants received care in 12 different NICUs for various neonatal medical problems. Charts are also available for length, head circumference, and midarm circumference (see Useful Websites for a source to create a growth curve for an individual infant). These charts reflect how premature infants grow, and do not allow for the assessment of catch-up growth by the premature infant.
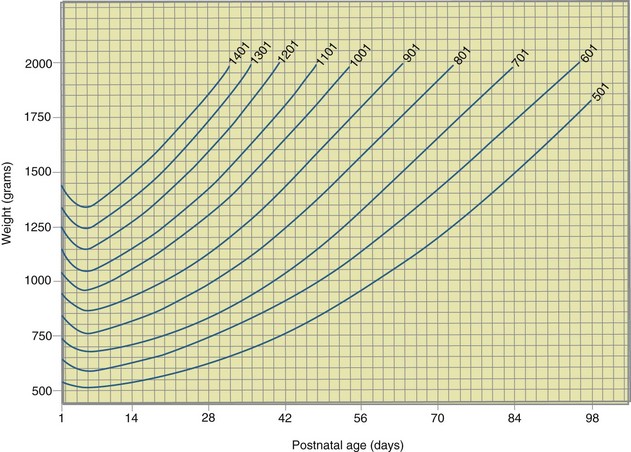
FIGURE 43-3 Weight chart for premature infants based on actual growth data. (From Ehrenkranz RA et al: Longitudinal growth of hospitalized very-low-birth-weight infants, Pediatrics 104:283, 1999.)
Intrauterine growth curves have been developed using birth weight, birth length, and birth head circumference data of infants born at several successive weeks of gestation. The intrauterine growth curves are the standard of growth recommended for premature infants. During the first week of life premature infants fall away from their birth weight percentile, which reflects the normal postnatal weight loss of newborn infants. After an infant’s condition stabilizes and the infant begins consuming all needed nutrients, the infant may be able to grow at a rate that parallels these curves. An intrauterine weight gain of 15 g/kg/day can be achieved.
Although weight is an important anthropometric parameter, measurements of length and head circumference can also be helpful. A growth curve can be used to evaluate the adequacy of growth in all three areas (Figure 43-4). This chart has a built-in correction factor for prematurity; the infant’s growth can be followed from 22 to 50 weeks of gestation and it represents cross-sectional data from Canada, Sweden, Australia, and the United States (Fenton, 2003).
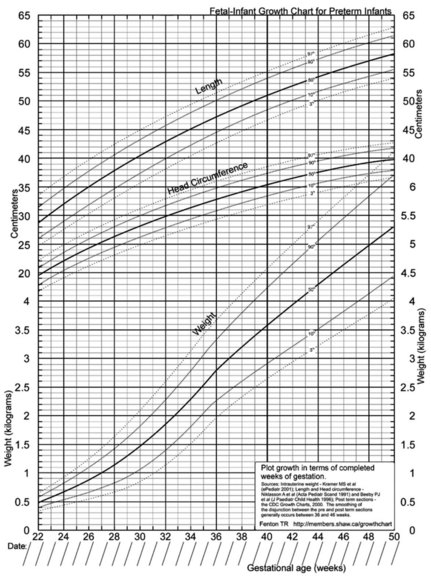
FIGURE 43-4 Example of a growth record of weight, length, and head circumference for infants from 22 to 50 weeks of gestation. This chart has a built-in correction factor for prematurity. http://members.shaw.ca/growthchart/ (From Fenton TR: A new growth chart for preterm babies: Babson and Benda’s chart updated with recent data and a new format, BMC Pediatr 3:13, 2003.)
Additional intrauterine growth charts based on the birthweight, birth length, and head circumferences of infants born in the United States have been developed (Olsen et al., 2010). Separate charts for male and female infants are available and infants can be plotted from 23 to 41 weeks’ gestation.
The 2006 World Health Organization Growth Charts designed for children from birth to 2 years of age should also be used for preterm infants once they reach 40 weeks’ gestation, as long as the age is adjusted (see Focus On: Long-Term Outcome for Premature Infants). For example, an infant born at 28 weeks of gestation is 12 weeks premature (40 weeks of term gestation minus 28 weeks of birth gestational age). Four months after birth, the growth parameters of a premature infant born at 28 weeks of gestation can be compared with those of a 1-month-old infant born at term (Box 43-3). When using growth grids, age should be adjusted for prematurity until at least  to 3 years of corrected age. In Figure 43-5 A.R.’s pattern of growth is shown through 18 years of age. These charts are based on term, healthy infants who were breast-fed the first year of life (Grummer-Strawn et al., 2010). By using this chart, the infant’s growth can be compared with the term infant to assess catch-up growth.
to 3 years of corrected age. In Figure 43-5 A.R.’s pattern of growth is shown through 18 years of age. These charts are based on term, healthy infants who were breast-fed the first year of life (Grummer-Strawn et al., 2010). By using this chart, the infant’s growth can be compared with the term infant to assess catch-up growth.
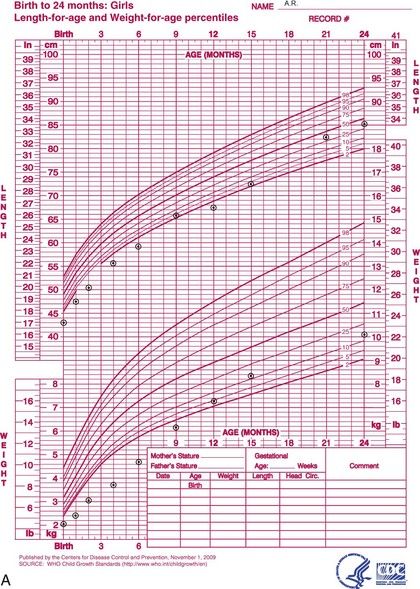
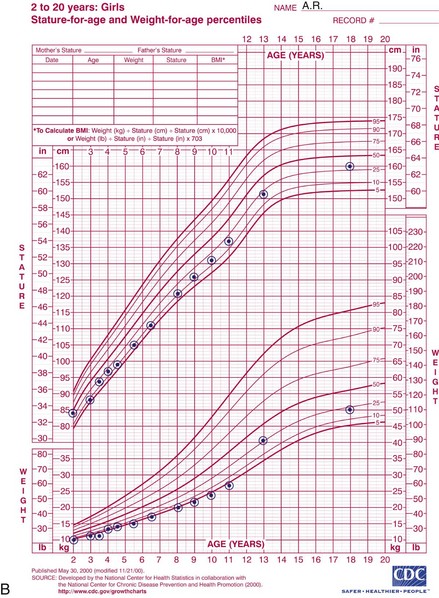
FIGURE 43-5 A, Graphs showing how A.R. (from Figure 43-2), who was born at 27 weeks of gestation, grew after leaving the neonatal unit 1 day before her due date at a weight of 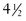 lb. Heights and weights until age of 24 months are plotted on the grid at “corrected age” points. A.R. experienced catch-up growth during the first 12 months. B, A.R.’s growth pattern from the age of 2 to 18 years. During the first 10 years she grew at the 5th percentile for weight and the 10th percentile for height. She followed her channel of growth but did not experience catch-up growth. However, between the ages of 10 and 13 she began to change growth channels and moved to the 25th percentile for weight and the 25th percentile for height (catch-up growth). At 18 years she crossed the 25th percentile for height and fell slightly below the 25th percentile for weight.
lb. Heights and weights until age of 24 months are plotted on the grid at “corrected age” points. A.R. experienced catch-up growth during the first 12 months. B, A.R.’s growth pattern from the age of 2 to 18 years. During the first 10 years she grew at the 5th percentile for weight and the 10th percentile for height. She followed her channel of growth but did not experience catch-up growth. However, between the ages of 10 and 13 she began to change growth channels and moved to the 25th percentile for weight and the 25th percentile for height (catch-up growth). At 18 years she crossed the 25th percentile for height and fell slightly below the 25th percentile for weight.
Discharge Care
Establishment of successful feeding is a pivotal factor determining whether a preterm infant can be discharged from the hospital nursery. Preterm infants must be able to (1) tolerate their feedings and usually obtain all of their feedings from the breast or bottle, (2) grow adequately on a modified-demand feeding schedule (usually every 3 to 4 hours during the day for bottle-fed infants or every 2 to 3 hours for breast-fed infants), and (3) maintain their body temperature without the help of an incubator. Medically stable premature infants who have delayed feeding development will go home on gavage feedings for a short period. In addition, it is important that any ongoing chronic illnesses, including nutrition problems, be manageable at home.
Most important, the parents must be ready to care for their infant. In hospitals that allow parents to visit their infants in the nursery 24 hours a day, staff can help parents develop their caregiving skills and learn to care for their infant at home. Often, parents are permitted to “room in” with their infant (i.e., stay with the infant all day and night) before discharge, which helps build confidence in their ability to care for a high-risk infant (Figure 43-6).
Many preterm infants who are discharged from the hospital weigh less than 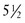 lb. Although these infants must meet certain discharge criteria before they can go home, the stress of a new environment may lead to setbacks. Small preterm infants should be followed very closely during the first month after discharge, and parents should be given as much information and support as possible. Within the first week of discharge, a home visit by a nurse, dietitian, or both and a visit to the pediatrician can be extremely helpful educationally; and they can provide opportunity for early intervention for developing problems.
lb. Although these infants must meet certain discharge criteria before they can go home, the stress of a new environment may lead to setbacks. Small preterm infants should be followed very closely during the first month after discharge, and parents should be given as much information and support as possible. Within the first week of discharge, a home visit by a nurse, dietitian, or both and a visit to the pediatrician can be extremely helpful educationally; and they can provide opportunity for early intervention for developing problems.
Factors that affect the feeding skills and behavior of preterm infants are particularly important after the infants have been discharged. Physical factors such as a variable heart rate, a rapid respiratory rate, and tremulousness are examples of physiologic events that interfere with feeding. In addition, infants weighing less than 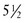 lb have poor muscle tone. Although muscle tone gradually improves as an infant becomes larger and more mature, it can deteriorate quickly in infants who are tired or weak. Feeding is often difficult for infants who have limited muscle flexion and strength and poor head and neck control, which are needed to maintain a good feeding posture. Positioning these infants in a manner that supports normal body flexion and ensures proper alignment of the head and neck during feedings is helpful. Premature infants may also need their chin and cheeks supported while bottle-feeding.
lb have poor muscle tone. Although muscle tone gradually improves as an infant becomes larger and more mature, it can deteriorate quickly in infants who are tired or weak. Feeding is often difficult for infants who have limited muscle flexion and strength and poor head and neck control, which are needed to maintain a good feeding posture. Positioning these infants in a manner that supports normal body flexion and ensures proper alignment of the head and neck during feedings is helpful. Premature infants may also need their chin and cheeks supported while bottle-feeding.
Small infants tend to sleep more than larger and term infants. It is much easier for preterm infants to feed effectively if they are fully awake. To awaken a preterm infant, the caregiver should provide one type of gentle stimulation for a few minutes and then change to a different type, repeating this pattern until the infant is fully awake. Lightly swaddling of the infant and then placing him or her in a semiupright position may also help.
The feeding environment should be as quiet as possible. Preterm infants are easily distracted and have difficulty focusing on feeding when noises or movements interrupt their attention. They also tire quickly and are easily overstimulated. When they are overstimulated, they may show only subtle signs of distress. It is important to teach parents of premature infants to recognize the subtle cues that indicate the need for rest or comfort and to respond to them appropriately.
After discharge, most preterm infants need approximately 180 mL/kg/day (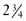 oz/lb/day) of breast milk or standard infant formula containing 20 kcal/oz. This amount of milk provides 120 kcal/kg/day (55 kcal/lb/day). Alternatively, transitional formula with a concentration of 22 kcal/oz can be provided at a rate of 160 mL/kg/day (or
oz/lb/day) of breast milk or standard infant formula containing 20 kcal/oz. This amount of milk provides 120 kcal/kg/day (55 kcal/lb/day). Alternatively, transitional formula with a concentration of 22 kcal/oz can be provided at a rate of 160 mL/kg/day (or 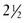 oz/lb/day). The best way to determine whether these amounts are adequate for individual infants is to compare their intake with their growth progress over time. Some infants may need a formula that provides 24 kcal/oz. As mentioned previously, powdered transitional formula can be readily altered to a concentration of 24 kcal/oz. The ready-to-feed premature formulas providing 24 kcal/oz are higher in most nutrients than are required postdischarge.
oz/lb/day). The best way to determine whether these amounts are adequate for individual infants is to compare their intake with their growth progress over time. Some infants may need a formula that provides 24 kcal/oz. As mentioned previously, powdered transitional formula can be readily altered to a concentration of 24 kcal/oz. The ready-to-feed premature formulas providing 24 kcal/oz are higher in most nutrients than are required postdischarge.
It is important to evaluate needs based on the three growth parameters: weight, length, and head circumference. Patterns of growth should be assessed to determine whether (1) individual curves at least parallel reference curves, (2) growth curves are shifting inappropriately across growth percentiles, (3) weight is appropriate for length, and (4) growth is proportionate in all three areas.
Neurodevelopmental Outcome
It is possible to meet the metabolic and nutritional needs of premature infants sufficiently to sustain life and promote growth and development. In fact, more tiny premature infants are surviving than ever before because of adequate nutrition support and the recent advances in neonatal intensive care technology. There is concern that the ELBW infant is often smaller at discharge than the infant of the same postmenstrual age who was not born prematurely. One report suggests that providing appropriate protein intake during week 1 of life to ELBW infants leads to improved growth of weight, length and head circumference at 36 weeks’ gestation, and improved head circumference in male infants at 18 months’ corrected age (Poindexter et al., 2006). Improved neurodevelopment and growth at 18 months has been reported with ELBW infants who gained more weight and had greater head circumference growth during their stay in the nursery (Ehrenkranz et al., 2006). The developmental outcome scores for ELBW infants have been higher as the intakes of human milk increase (Vohr et al., 2007).
The increased survival rate of ELBW infants has increased concerns about their short- and long-term neurodevelopmental outcomes. Many questions have been raised about the quality of life awaiting infants who receive neonatal intensive care. As a rule, VLBW infants should be referred to a follow-up clinic to evaluate their development and growth and begin early interventions (Wilson-Costello and Hack, 2006). The survival of ELBW infants has increased, with an increase in the number of children with neurodevelopmental disabilities, but also with an increase in the number of children who are developmentally normal (Wilson-Costello and Hack, 2006). Many of these premature infants reach adulthood with no evidence of any disability (Figure 43-7).
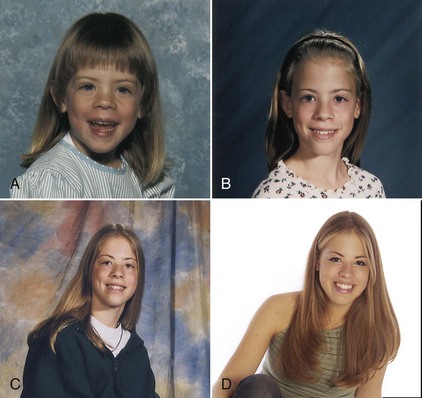
FIGURE 43-7 The premature infant A.R. (see Figures 43-2 and 43-6) as she grows up. A, 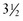 years. B, 10 years. C, 14 years. D, 18 years. (D Courtesy Yuen Lui Studio, Seattle, Wash.)
years. B, 10 years. C, 14 years. D, 18 years. (D Courtesy Yuen Lui Studio, Seattle, Wash.)
American Academy of Pediatrics
Ehrenkranz Growth Charts—National Institute of Child Health and Human Development, Neonatal Research Network
http://members.shaw.ca/growthchart/
Human Milk Banking Association of North American
http://www.nursing.upenn.edu/media/infantgrowthcurves/Documents/Olsen-NewIUGrowthCurves_2010permission.pdf
World Health Organization Growth Curves
http://www.cdc.gov/growthcharts/who_charts.htm
References
American Academy of Pediatrics (AAP) and the American College of Obstetricians and Gynecologists (ACOG). Breastfeeding infants with special needs. In: Schanler RJ, et al, eds. Breastfeeding handbook for physicians. Evanston, IL: American Academy of Pediatrics, 2006.
American Academy of Pediatrics (AAP), Committee on Nutrition. Nutritional needs of preterm infants. In Kleinman RE, ed.: Pediatric nutrition handbook, ed 5, Elk Grove, IL: American Academy of Pediatrics, 2004.
American Academy of Pediatrics (AAP), Committee on Nutrition. Failure to thrive. In Kleinman RE, ed.: Pediatric nutrition handbook, ed 6, Elk Grove, IL: American Academy of Pediatrics, 2009.
American Academy of Pediatrics (AAP), Committee on Nutrition. Nutritional needs of preterm infants. In Kleinman RE, ed.: Pediatric nutrition handbook, ed 6, Elk Grove, IL: American Academy of Pediatrics, 2009.
Baker, RD, Greer, FR, American Academy of Pediatrics (AAP)Committee on Nutrition, Clinical report-diagnosis and prevention of iron deficiency and iron-deficiency anemia in infants and young children (0-3 years of age). Pediatrics 2010:126. Accessed January 5, 2011 from. www.pediatrics.org/cgi/doi/10.1542/peds.2010-2576
Ballard, JL, et al. New Ballard score, expanded to include extremely premature infants. J Pediatr. 1991;119:417.
Clandinin, MT, et al. Growth and development of preterm infants fed infant formulas containing docosahexaenoic acid and arachidonic acid. J Pediatr. 2005;146:461.
Collins, CT, et al. Avoidance of bottles during the establishment of breast feeds in preterm infants (Review). Cochrane Database Syst Rev. 2008. [Issue 4. Art.No.:CD005252. DOI: 10.1002/14651858. CD005252.pub.2].
Darlow, BA, Graham, PJ. Vitamin A supplementation for preventing morbidity and mortality in very low birthweight infants. www.nichd.nih.gov/cochraneneonatal/, 2008. [Accessed 1 June 2010 from].
Dell, KM, Davis, ID. Fluid, electrolyte, and acid-base homeostasis. In Martin RJ, et al, eds.: Neonatal-perinatal medicine diseases of the fetus and infant, ed 8, Philadelphia: Mosby, 2006.
Denne, SC. Regulation of proteolysis and optimal protein accretion in extremely premature newborns. Am J Clin Nutr. 2007;85(Suppl):621S.
Doyle, LW, Anderson, PJ. Adult outcome of extremely preterm infants. Pediatrics. 2010;126:342.
Ehrenkranz, RA, et al. Longitudinal growth of hospitalized very low birth weight infants. Pediatrics. 1999;104:280.
Ehrenkranz, RA, et al. Growth in the neonatal intensive care unit influences neurodevelopment and growth outcomes of extremely low birth weight infants. Pediatrics. 2006;117:1253.
Ehrenkranz, RA. Early nutritional support and outcomes in ELBW infants. Ear Hum Dev. 2010;86:S21.
Fenton, TR. A new growth chart for preterm babies: Babson and Benda’s chart updated with recent data and a new format. BMC Pediatrics. 2003;3:13.
Fucile, S, et al. Effect of an oral stimulation program on sucking skill maturation of preterm infants. Dev Med Child Neurology. 2005;47:158.
Grummer-Strawn, LM, et al, Centers for Disease Control and Prevention, Use of World Health Organization and CDC growth charts for children aged 0-59 months in United States. MMWR. 2010;59(No. RR-9):1. Erratum in MMWR Recomm Rep. 59(36):1184, 2010
Gura, KM. Potential benefits of parenteral fish oil lipid emulsions in parenteral nutrition-associated liver disease. ICAN: Infant, Child, & Adolescent Nutrition. 2010;2:251.
Hack, M. Adult outcomes of preterm children. J Dev Behavioral Pediatr. 2009;30:460.
Hay, WW. Strategies for feeding the premature infant. Neonatology. 2008;94:245.
Heron, M, et al. Annual summary of vital statistics: 2007. Pediatrics. 2010;125:4.
Hovi, P, et al. Glucose regulation in young adults with very low birth weight. N Engl J Med. 2007;356:20.
Hurst, NM. The 3M’s of breast-feeding the preterm infant. J Perinat Neonat Nurs. 2007;21:234.
Klein, CJ. Nutrient requirements for preterm infant formula. editor. J Nutr. 2002;132:1395S.
Lucas, A, et al. Randomised trial of early diet in preterm babies and later intelligence quotients. Br Med J. 1998;317:1481.
MacDorman, MF, Mathews, TJ, Behind international rankings of infant mortality: how the United States Compares with Europe. NCHS Data Brief No. 23. 1-8. Hyattsville, MD: National Center for Health Statistics; 2009.
Mitchell, SM, et al. High frequencies of elevated alkaline phosphatase activity and rickets exist in extremely low birth weight infants despite current nutrition support. BMC Pediatrics. 2009;9:47.
Olsen, IE, et al. New intrauterine growth curves based on United States data. Pediatrics. 2010;125:e214.
Poindexter, BB, et al. Early provision of parenteral amino acids in extremely low birth weight infants: relation to growth and neurodevelopmental outcome. J Pediatr. 2006;148:300.
Rao, R, Georgieff, M. Microminerals. In Tsang RC, et al, eds.: Nutrition of the preterm infant, ed 2, Cincinnati, OH: Digital Educational Publishing, Inc., 2005.
Robbins ST, Beker LT, eds. Infant feedings: guidelines for preparation of formula and breastmilk in health care facilities. Chicago: American Dietetic Association, 2004.
Rogers, SP, et al. Continuous feedings of fortified human milk lead to nutrient losses of fat, calcium and phosphorous. Nutrients. 2010;2:230.
Saigal, S, et al. Comparison of current health, functional limitations, and health care use of young adults who were born with extremely low birth weight and normal weight. Pediatrics. 2006;119:e562.
Saigal, S, et al. Self-perceived health-related quality of life of former extremely low birth weight infants at young adulthood. Pediatrics. 2006;118:1140.
Schanler, RJ, et al. Randomized trial of donor human milk versus preterm formula as substitutes for mothers’ own milk in feeding of extremely premature infants. Pediatrics. 2005;116:400.
Schanler, RJ, Anderson, DM. The low-birth-weight infant: inpatient care. In Duggan C, et al, eds.: Nutrition in pediatrics, ed 4, Hamilton, Ontario: BC Decker Publishers, 2008.
Sisk, PM, et al. Early human milk feeding is associated with a lower risk of necrotizing enterocolitis in very low birth weight infants. J Peri. 2007;27:428.
Sisk, PM, et al. Human milk consumption and full enteral feeding among infants who weigh ≤ 1250 grams. Pediatrics. 2008;121:e1528.
Stevens, DC, et al. Achieving success in supporting parents and families in the neonatal intensive care unit. In McGrath J, Kenner C, eds.: Developmental care newborns and infants: a guide for health professionals, ed 2, Glenview, IL: National Association of Neonatal Nurses, 2010.
Sullivan, S, et al. An exclusively human milk-based diet is associated with a lower rate of necrotizing enterocolitis than a diet of human milk and bovine milk-based products. J Pediatr. 2010;156:562.
Thureen, PJ, et al. Effect of low versus high intravenous amino acid intake on very low birth weight infants in the early neonatal period. Pediatr Res. 2003;53:24.
Tsang, RC, et al. Summary of reasonable nutrient intakes. In Tsang RC, ed.: Nutrition of the preterm infant, ed 2, Cincinnati, OH: Digital Educational Publishing, Inc., 2005.
Tyson, JE, et al. Vitamin A supplementation for extremely-low-birth-weight infants. N Engl J Med. 1999;340:1962.
van Aerde, JE, Narvey, M. Acute respiratory failure. In Thureen PJ, Hay WW, eds.: Neonatal nutrition and metabolism, ed 2, Cambridge: Cambridge University Press, 2006.
Wagner, CL, Greer, FR, American Academy of Pediatrics, Section on BreastfeedingAmerican Academy of Pediatrics, Committee on Nutrition, Prevention of rickets and vitamin D deficiency in infants, children, and adolescents. Pediatrics. 2008;122(5):1142. published correction appears in Pediatrics 123(1):197, 2009
Wilson-Costello, DE, Hack, M, Follow-up for high-risk neonates. Philadelphia. ed 8. Fanaroff, AA, Martin, RJ, eds. Neonatal-perinatal medicine diseases of the fetus and infant. Mosby; 2006;vol 2.
Vohr, BR, et al. Persistent beneficial effects of breast milk ingested in the neonatal intensive care unit on outcomes of extremely low birth weight infants at 30 months of age. Pediatrics. 2007;120:e953.
Ziegler, EE. Protein requirements of very low birth weight infants. J Pediatr Gastroenterology and Nutrition. 2007;45:170.