Medical Nutrition Therapy for Pulmonary Disease
During fetal life, from birth to maturity and throughout adulthood, the pulmonary system is intertwined with nutrition. An optimal pulmonary system enables the body to obtain the oxygen needed to meet its cellular demands for energy from macronutrients, and to remove metabolic byproducts. Optimal nutrition permits the proper growth and development of the respiratory organs, supporting structures of the skeleton and muscles, and related nervous, circulatory, and immunologic systems. Overall, a person’s nutritional well being and proper metabolism of nutrients are essential for the formation, development, growth, maturity, and protection of healthy lungs and associated processes throughout life.
The Pulmonary System
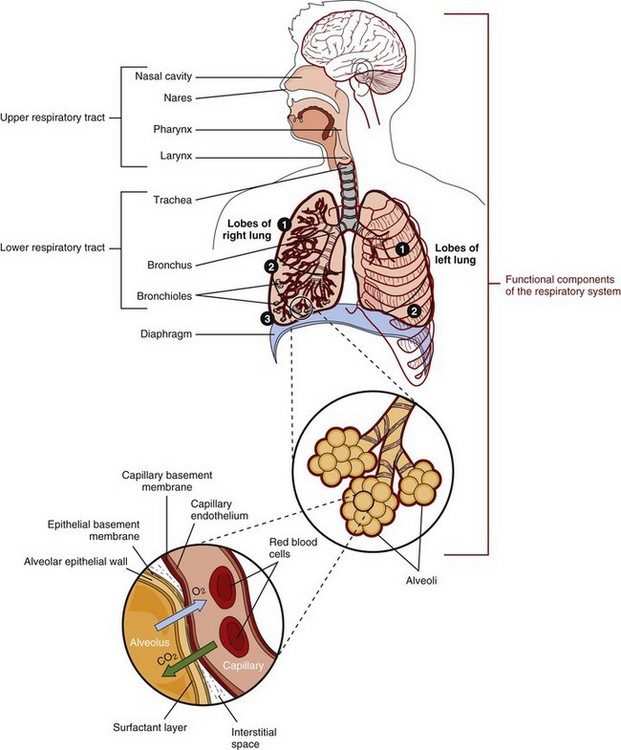
The respiratory structures include the nose, pharynx, larynx, trachea, bronchi, bronchioles, alveolar ducts, and alveoli (Figure 35-1). Supporting structures include the skeleton and the muscles (e.g., the intercostal, abdominal, and diaphragm muscles). Within a month after conception, pulmonary structures are recognizable. The pulmonary system grows and matures during gestation and childhood. No new alveoli are produced after approximately age 20. As aging occurs there is a loss of lung capillaries and the lungs lose elasticity.
Gas exchange is the major function of the pulmonary system (Figure 35-2). The lungs enable the body to obtain the oxygen needed to meet its cellular metabolic demands and to remove the carbon dioxide (CO2) produced by these processes. Healthy nerves, blood, and lymph are needed to supply oxygen and nutrients to all tissues. The lungs also filter, warm, and humidify inspired air.
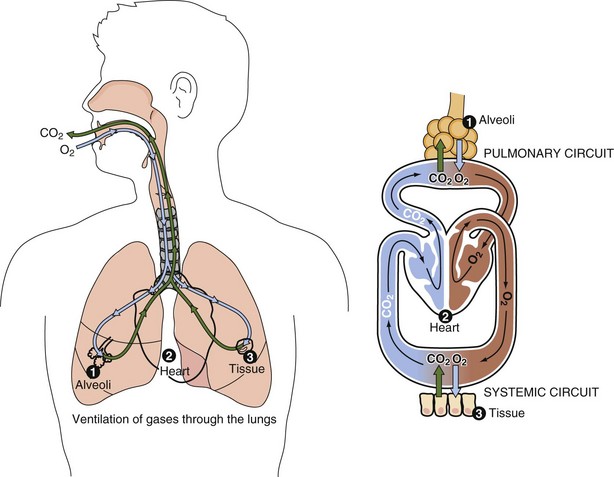
FIGURE 35-2 The major function of the respiratory tract is to provide the oxygen for cellular metabolism and to remove the carbon dioxide that is produced but not needed.
The lungs have several metabolic functions. For example, they help regulate the body’s acid-base balance. The body’s pH partially is maintained by the proper balance of CO2 and O2. The lungs synthesize arachidonic acid, that ultimately may be converted to prostaglandins or leukotrienes, a possible cause of bronchoconstriction in asthma. The lungs convert angiotension I to angiotensin II by the angiotension-converting enzyme (ACE) found mainly in the numerous capillary beds of the lungs. Angiotensin II increases blood pressure.
The alveolar cells secrete surfactant, a compound synthesized from proteins and phospholipids that serves to maintain the stability of pulmonary tissue by reducing the surface tension of fluids that coat the lung.
The lungs are an important part of the body’s immune defense system because inspired air is laden with particles and microorganisms. Mucus keeps the airways moist and traps the particles and microorganisms from inspired air. Most cells that line the trachea, bronchi, and bronchioles have cilia. These constantly beating cilia sweep the particles upward toward the pharynx so they can enter the gastrointestinal tract. Each time a person swallows, the particle- and microorganism-containing mucus passes into the digestive tract. The epithelial surface of the alveoli contains macrophages. By the process of phagocytosis, these alveolar macrophages engulf inhaled inert materials and microorganisms and digest them.
Medical Treatment
Pulmonary system disorders may be categorized as primary, such as tuberculosis (TB), bronchial asthma, and cancer of the lung; or secondary when associated with cardiovascular disease, obesity, human immunodeficiency virus (HIV) infection, sickle cell disease, and scoliosis. Conditions may also be acute or chronic. Examples of acute conditions include aspiration pneumonia, airway obstruction from foods like peanuts, and allergic anaphylaxis from consumption of shellfish. Examples of chronic conditions include cystic fibrosis (CF) and lung cancer.
The assessment of pulmonary status generally starts with physical examination using percussion and auscultation. These bedside techniques provide important information to the clinician on breathing. Numerous diagnostic and monitoring tests such as imaging procedures, arterial blood gas determinations, sputum cultures, and biopsies can also be employed. Signs and symptoms of pulmonary disease include cough, early satiety, anorexia, weight loss, dyspnea (shortness of breath), and fatigue.
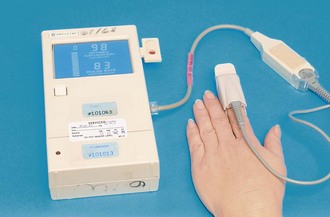
Pulmonary function tests are used to diagnose or monitor the status of lung disease; they are designed to measure the ability of the respiratory system to exchange oxygen andCO2. Pulse oximetry is one such test. A small device called a pulse oximeter, which uses light waves to measure the oxygen saturation of arterial blood, is placed on the end of the finger (Figure 35-3). Normal for a young, healthy person is 95% to 99%. Spirometry is another common pulmonary function test. This involves breathing into a spirometer that gives information on lung volume and the rate at which air can be inhaled and exhaled.
Medical Nutrition Therapy in Pulmonary Disease
Individualized nutrition assessment, diagnosis, and intervention, followed by routine monitoring and evaluation, are integral components of care for each patient with pulmonary disease. Concomitant assessment of the cardiovascular, renal, neurologic, and hematologic systems is important because diseases often produce complications affecting pulmonary anatomy, physiologic findings, and biochemistry. Nutrition assessment precedes any nutrition intervention or medical treatment, unless the treatment is emergent.
Effect of Malnutrition on the Pulmonary System
The relationship between malnutrition and respiratory disease has long been recognized. Malnutrition adversely affects lung structure, elasticity, and function; respiratory muscle mass, strength, and endurance; lung immune defense mechanisms; and control of breathing. For example, protein and iron deficiencies result in low hemoglobin levels which diminish the oxygen-carrying capacity of the blood. Low levels of calcium, magnesium, phosphorus, and potassium compromise respiratory muscle function at the cellular level. Hypoproteinemia contributes to the development of pulmonary edema by decreasing colloid osmotic pressure, allowing body fluids to move into the interstitial space. Decreased levels of surfactant contribute to the collapse of alveoli, thereby increasing the work of breathing. The supporting connective tissue of the lungs is composed of collagen, which requires ascorbic acid for its synthesis. Normal airway mucus is a substance consisting of water, glycoproteins, and electrolytes, and thus requires adequate nutritional intake.
Pulmonary disease substantially increases energy requirements. This factor explains the rationale for including body composition and weight parameters in medical, surgical, pharmacologic, and nutrition research studies. Weight loss from inadequate energy intake is significantly correlated with a poor prognosis in persons with pulmonary diseases. Malnutrition leading to impaired immunity places any patient at high risk for developing respiratory infections. Malnourished patients with pulmonary disease who are hospitalized are likely to have lengthy stays and are susceptible to increased morbidity and mortality.
The complications of pulmonary diseases or their treatments can make adequate intake and digestion difficult. Absorption and metabolism of most nutrients are affected. As pulmonary disease progresses, several conditions may interfere with food intake and overall nutrition status. For example, abnormal production of sputum, vomiting, tachypnea (rapid breathing), hemoptysis, thoracic pain, nasal polyps, anemia, depression, and altered taste secondary to medications are often present. Weight loss, low body mass index (BMI), and other adverse effects are listed in Box 35-1.
Aspiration
Pulmonary aspiration involves the movement of food or fluid into the lungs, which can result in pneumonia or even death. Besides liquids, foods that are most easily aspirated include those that have a round shape such as nuts, popcorn, hard candy, and hot dog pieces; or chunks of inadequately chewed foods such as meat or raw vegetables. Infants and toddlers are at increased risk for aspiration, as are older adults and persons with oral, upper gastrointestinal, neurologic, or muscular abnormalities. In addition, close attention must be given to people receiving enteral tube feedings (see Chapter 14). Because the primary reason for aspiration pneumonia is excessive lung secretions, pulmonary treatments and suctioning are critical to preventing aspiration.
Asthma
Asthma, a disease of bronchial hyperresponsiveness and airway inflammation, has increased in prevalence during the last three decades. The syndrome appears to result from complex interactions among genetic, immunologic, and environmental factors. It is characterized by (1) increased mucus secretion that can obstruct the airways, (2) inflammation and swelling, and (3) smooth muscle tightening that results in smaller airways. Asthma is characterized by airflow obstruction and is a leading cause of hospitalization and death worldwide (DHHS, 2010; Stevenson and Birrell, 2010).
Pathophysiology
Genetic attributes, environmental exposures, and gene-environmental interactions all play a role in asthma. Respiratory infections caused by viruses, Chlamydophila or Mycoplasma, have been hypothesized to have significant roles in the pathogenesis (Guilbert and Denlinger, 2010). Host immunity is also critical. A healthy diet during pregnancy and during the early years, and prolonged breastfeeding may decrease the risk of asthma in childhood. In children genetically at high risk of asthma, being overweight at age 1 year is often associated with a decreased risk of asthma and better lung function at ages 6 and 8 years; however, being overweight beyond infancy may confer a higher risk for asthma (Zhang et al., 2010). Thus obesity should be avoided after the toddler stage.
Medical Treatment
Asthma is categorized as allergic (extrinsic) and nonallergic (intrinsic). Allergic asthma is more common and is generally triggered by inhalation of pollen, pet dander, air pollution, cigarette smoke, or other inhalants. Nonallergic asthma may be triggered by factors such as ear infections, stress, viruses, and exercise. Asthmatic symptoms may be aggravated by exposure to allergens such as shrimp or sulfites (see Chapter 27); rhinoviruses; and botanicals such as citronella in insect repellents, rusty-leafed rhododendron in natural honeys, and strawberry leaf in herbal teas.
Eosinophilic airway inflammation is commonly observed; therefore removal of potential triggers and known sensitizers is an important measure. A life-threatening situation with flat airways, known as status asthmaticus, can result without proper intervention. Corticosteroid therapy is often prescribed, but chronic use may place the individual at risk for osteopenia, bone fractures, or steroid-induced hyperglycemia. New research proposes use of sublingual immunotherapy and other novel treatments (Peden and Bush, 2011.)
Medical Nutrition Therapy
Food and individual nutrients have possible roles in the treatment of asthma. Examples include soy, ω-3 fatty acids and ω-6 fatty acids (decreasing the production of bronchoconstrictive leukotrienes), antioxidant nutrients (protecting the airway tissues from oxidative stress), vitamin D (a molecular antiinfective nutrient), magnesium (a smooth-muscle relaxant and antiinflammatory agent), and methylxanthine bronchodilators such as caffeine (Baines et al., 2009; Barros, et al., 2008; Bede, et al., 2008; Kalhan, et al., 2008; Kazaks, et al., 2010; Lindemann, 2009; Schubert, et al., 2009). The dilemma for the nutrition care provider is the paucity of evidence-based research to support practice procedures (Allan and Devereux, 2011; Kealoha, 2009; Raviv and Smith, 2010; Sorkness, 2009). Scientific nutrition studies aimed at producing evidence-based results are desperately needed.
One illustration is the often-asked question: “Does milk cause an increase in mucus production in asthma?” A literature review found no cause-and-effect relationship, and avoiding dairy foods could lead to inadequate intake of nutrients (Wüthrich et al., 2005). However, until scientists clearly demonstrate the biologic foundations of people’s perceptions, clinicians will be asked the question and be expected to determine the proper course of treatment.
Nutrition assessment and therapy must take into account routinely prescribed medications. These include bronchodilators that relax the airway smooth muscle and antiinflammatory agents that suppress airway inflammation. Pulmonary patients experience side effects such as dry mouth and throat, nausea, early satiety, vomiting, diarrhea, increased serum glucose levels, sodium retention, hypokalemia, hand tremors, headache, and dizziness. Another possible side effect of medications or chronic coughing is gastroesophageal reflux. Chronic steroid use causes bone demineralization. Bone density tests should be part of the nutritional assessment when these drugs are chronically used (see Chapter 9).
Nutrition therapy should include individual evaluation for environmental or food triggers and strategies for their avoidance if necessary. In addition, there is need for a diet of wholesome foods to provide optimal energy and balance of nutrients; proper ratio of ω-3 and ω-6 fatty acids and phytonutrients; correction of diagnosed energy and nutrient deficiencies or excesses; careful attention to medication-food-nutrient interactions; frequent monitoring to maintain healthy pulmonary status; and education of the patient, family, and community (American Academy of Allergy, Asthma, and Immunology, 2005).
Chronic Lung Disease of Prematurity and Bronchopulmonary Dysplasia
Chronic lung disease of prematurity (CLD) and bronchopulmonary dysplasia (BPD) are closely related. Newborn lungs appear unable to respond to adverse situations. Immature lungs often cannot synthesize surfactant that permits inflation for gas exchange.
Pathophysiology
CLD and BPD are the result of incomplete recovery from lung injury during the neonatal period. CLD and BPD occur most frequently in infants who are extremely premature or of low birth weight (see Chapter 43). Other risk factors include perinatal infection, meconium aspiration, tracheoesophageal fistula, and generalized infections. Signs and symptoms of CLD and BPD include hypercapnia, tachypnea, wheezing, dyspnea, recurrent respiratory infections, cor pulmonale (right ventricular enlargement and heart failure), and a characteristic radiographic appearance of the lungs.
Medical Treatment
Because the pathophysiologic conditions of CLD and BPD are incompletely understood, medical treatment and nutrition intervention are empirically based and often have limited scientific rationale (Van Marter, 2009). Infants with severe disease often require prolonged intensive medical care, provided by an interdisciplinary team. Figure 35-4 shows student members of the interdisciplinary team practicing tube feeding a simulated infant with BPD. Therapies such as parenteral nutrition or enteral tube feedings, mechanical ventilation, supplemental oxygen, and medications may be required long after discharge from the hospital.
Medical Nutrition Therapy
Because of the fragile nature of affected infants, careful and consistent nutrition assessment is imperative. Infant growth is followed closely because it is a major outcome indicator of medical and nutrition therapy. Because lung size is stature-dependent, linear growth is important for the growth of healthy lung tissue and for the resolution of the condition. Observations of growth patterns of infants with CLD and BPD suggest that these infants have delayed growth, thereby requiring careful assessment of both respiratory and nutrition status (Box 35-2).
Possible reasons for growth failure among infants include increased energy needs combined with inadequate dietary intake, gastroesophageal reflux, emotional deprivation, and chronic hypoxia. Growth should be evaluated and compared with that of other infants of the same postconceptional age (see Chapter 43). Infants with CLD and BPD have special short- and long-term nutritional requirements related to both their prematurity and their pulmonary status. The general goals of nutrition care are to supply adequate nutrient intakes, promote linear growth, maintain fluid balance, and develop age-appropriate feeding skills. Meeting energy and nutrient needs is a major challenge in the care of infants and toddlers with BPD.
Energy
Increased energy needs are well recognized in infants with CLD and BPD. Resting energy expenditure for infants with CLD and BPD has been documented to be 25% to 50% greater than that in age-matched controls. Energy needs also vary during the course of the disease. In the acute phase, when infants are kept in controlled temperature environments, are fed parenterally, remain relatively inactive, and are not growing or are growing slowly, energy requirements may be 50 to 85 kcal/kg daily. In contrast, during the convalescent phase, when infants are growing rapidly, being fed orally, and using additional energy for temperature regulation, activity, and the work of breathing, they may require 120 to 130 kcal/kg or more daily.
Macronutrients
Protein intake should be within the advised range for infants of comparable postconceptional age. As the caloric density of the diet is increased by the addition of fat and carbohydrate, protein should provide 7% or more of total calories because lesser amounts may be inadequate for growth.
Additions of fat or carbohydrate are made to formula only after it has been concentrated to 24 kcal/oz to keep protein at an acceptable level. See Box 43-2 in Chapter 43. Fat provides essential fatty acids (EFAs) and helps meet energy demands when tolerance for fluid and CO2 load is limited. Excessive amounts of carbohydrate increase the respiratory quotient (RQ) (the ratio of CO2 expired to the volume of oxygen inspired) and the output of CO2. This would seem to make the work of breathing more difficult; however, the clinical application of manipulating nutrient mix to change RQ is controversial. Ongoing calculation of the proportions of the macronutrients related to respiratory status are major considerations in any nutritional evaluation.
Fluid
To maintain fluid balance, infants with CLD and BPD may require fluid restriction, sodium restriction, and diuretics, all of which have nutritional implications. When fluid intake is restricted, the use of parenteral lipids or calorically dense enteral feedings may help the infant meet energy needs. When calorically dense formulas are used (>24 kcal/oz), the adequacy of fluid intake and urinary output must be monitored closely.
Vitamins and Minerals
Adequate supplies of all vitamins and minerals are essential. Special attention is focused on those related to prematurity, infections, oxygen therapy, and drug-nutrient interactions. Adequate vitamin K is essential for bone development and should be monitored, especially when colon microflora are insufficient for the synthesis of this vitamin. Vitamin A is essential because of its role in the proper development and maintenance of the epithelial cells of the respiratory tract. In fact, some reports support the conclusion that vitamin A (either as an oral or intramuscular supplement) prevents or fully treats CLD and BPD, whereas others reject that conclusion (Darlow, 2007; Van Marter, 2009).
Mineral intake and retention should be monitored and supplemented as needed to maintain normal levels. Determination of mineral requirements is complicated by lack of adequate stores as a result of prematurity (e.g., iron, zinc, and calcium), growth delay, and the multiple medications prescribed for infants and toddlers with CLD and BPD. Medications may include diuretics, bronchodilators, antibiotics, cardiac antiarrhythmics, and corticosteroids. Collectively, these medications are associated with increased urinary loss of minerals, especially chloride, potassium, and calcium. Additional chloride losses may occur in infants with chronic CO2 retention and respiratory acidosis because of metabolic correction for the acidosis. Deficiencies of chloride or potassium are associated with muscle weakness and impaired growth (see Chapter 7). For infants sensitive to sodium loads, lower-sodium formulas can be used, and the sodium content of medications, water, and foods must be considered (see Chapter 34).
Infants with CLD and BPD are at risk for osteopenia (inadequate bone mineralization). Besides limited nutrient intake, other risk factors include inadequate stores of calcium and phosphorus related to prematurity, intermittent respiratory acidosis, chronic use of certain medications, and insufficient physical activity. See Chapter 25.
Feeding Strategies
Feeding difficulties frequently occur among infants with CLD and BPD. Risk factors include a history of unpleasant oral experiences (e.g., intubation, frequent suctioning, or recurrent vomiting), a history of parenteral and enteral nutrition, delayed introduction of solids, and discomfort or choking associated with eating solids. Infants may tire easily while breast-feeding or bottle-feeding. Useful approaches that facilitate feeding acceptance include providing a pleasant and calm mealtime environment, providing oral stimulation during tube feedings, using consistent and appropriate feeding techniques, and gradually introducing progressive texture and flavor changes.
Barriers to adequate intake include anorexia, fatigue, poor coordination of breathing and swallowing, and inability to suck. To meet energy needs, calorically dense formulas, small and frequent feedings, use of a soft nipple, and nasogastric or gastrostomy tube feedings may be needed. Gastroesophageal reflux is also common and may result in vomiting with expulsion of feedings, which leads to inadequate nutritional intake. Treatment includes upright positioning, medications such as antacids or histamine H2-receptor antagonists, and thickening of formula. In severe cases surgical fundoplication may be necessary.
Chronic Obstructive Pulmonary Disease
Chronic obstructive pulmonary disease (COPD) is characterized by slow, progressive obstruction of the airways. COPD may be subdivided into two categories: emphysema (type I), which is characterized by abnormal, permanent enlargement and destruction of the alveoli; and chronic bronchitis (type II), in which there is a productive cough with inflammation of the bronchi and other lung changes. Tobacco smoking or continual contact with second-hand smoke are the primary causative factors. Environmental air pollution (including cooking in a confined, unventilated space) and genetic susceptibility are other possible causal factors.
Pathophysiology
Patients with emphysema are thin and often cachectic. They are generally older and have mild hypoxemia but normal hematocrit values. Cor pulmonale (right ventricular enlargement and failure of the heart) develops late in the course of the disease. Conversely, patients with chronic bronchitis are of normal weight and, indeed, are often overweight. Hypoxemia is prominent in these patients, hematocrit values are increased, and cor pulmonale develops early.
Medical Treatment
Medical and surgical treatment approaches for persons with COPD are codified and periodically updated based on the latest research (Global Initiative for Chronic Obstructive Lung Disease, 2009). The four goals for effective management are to (1) assess and monitor disease, (2) reduce risk factors, (3) maintain stable COPD, and (4) manage any exacerbations. Early, accurate diagnosis is key to treatment. Once the disease progresses, in addition to pulmonary rehabilitation programs and oxygen therapy, numerous medications are prescribed, mainly bronchodilators, glucocorticosteroids, and mucolytic agents, along with antibiotics to treat infections. Surgical treatments for advanced COPD, including lung transplantation, may be options for some patients.
Medical Nutrition Therapy
Medical nutrition therapy (MNT) for people with COPD has been evaluated and recommendations have been made. (American Dietetic Association, 2010). The primary goals of nutrition care for patients with COPD are to facilitate nutritional well being, maintain an appropriate ratio of lean body mass to adipose tissue, correct fluid imbalance, manage drug-nutrient interactions, and prevent osteoporosis.
After fluid status, energy is a primary consideration. Because maintaining energy balance is crucial for combating this progressive disorder, accurate evaluation of both energy intake and energy expenditure is essential. On the energy intake side, decreased food intake is common. Morning headache and confusion from hypercapnia (excessive CO2 in the blood) may interfere with food preparation or intake. Other pertinent assessments focus on blood oxygen saturation, fatigue, anorexia, difficulty chewing and swallowing from dyspnea, constipation from low-fiber food selections, or diarrhea. Diarrhea occurs from impaired peristalsis, secondary to lack of oxygen to the gastrointestinal tract.
On the other hand, energy expenditure is generally elevated because of airflow obstruction, thus increasing the energy needs from the increased work of breathing. Gas diffusing capacity, CO2 retention, respiratory inflammation, and biochemical mediators such as hormones and cytokines also affect energy expenditure. Common outcomes are reduced respiratory and skeletal muscle strength and endurance along with increased muscle fatigability, altered pulmonary accessory muscle function, and increased susceptibility to infections. Malnourished patients with COPD, including those diagnosed with pulmonary cachexia, have a worse prognosis than those who are well nourished (King, 2008).
Nutritional depletion may be evidenced clinically by low body weight for height and reduced triceps fatfold measurements. Decreases in lean body mass may occur, even when actual weight appears stable. Calculation of BMI may be insufficient to detect alterations. Instead, determination of body composition helps to differentiate lean muscle mass from adipose tissue and overhydration from dehydration. In patients with cor pulmonale and the resultant fluid retention, weight maintenance or gain from fluid may camouflage actual wasting of lean body mass. Thus for patients retaining fluids, careful interpretation of both anthropometric measurements and biochemical indicators of nutrition status is necessary, especially because the latter are depressed by hemodilution (see Chapters 6 and 8).
The medication profile should be assessed for food and nutrient interactions. Examples of drugs with potential nutritional implications are bronchodilators, expectorants, and corticosteroids (see Chapter 9).
Energy
Meeting energy needs can be difficult. For patients participating in pulmonary inpatient or outpatient rehabilitation programs, adjusted energy requirements depend on the intensity and frequency of exercise therapy. Actual energy needs may be increased or decreased (Weekes, 2009). It is crucial to remember that energy balance and nitrogen balance are intertwined. Consequently, maintaining optimal energy balance is essential to preserving visceral and somatic proteins. Preferably, indirect calorimetry should be used to determine energy needs and to prescribe and monitor the provision of sufficient, but not excessive calories (American Dietetic Association, 2010). When energy equations are used for prediction of needs, increases for physiologic stress must be included. Caloric needs may vary significantly from one person to the next, and even in the same individual (see Chapter 2).
Macronutrients
In stable COPD, requirements for water, protein, fat, and carbohydrate are determined by the underlying lung disease, oxygen therapy, medications, weight status, and any acute fluid fluctuations. Attention to the metabolic side effects of malnutrition and the role of individual amino acids is necessary (Baldi, 2010). Determination of a specific patient’s macronutrient needs is made on an individual basis, with close monitoring of outcomes.
Sufficient protein of 1.2 to 1.7 g/kg of dry body weight is necessary to maintain or restore lung and muscle strength, as well as to promote immune function. A balanced ratio of protein (15% to 20% of calories) with fat (30% to 45% of calories) and carbohydrate (40% to 55% of calories) is important to preserve a satisfactory RQ from substrate metabolism use (see Chapter 2). Repletion but not overfeeding is particularly critical in patients with compromised ability to exchange gases as excess feeding of calories results in CO2 that must be expelled. Other concurrent disease processes such as cardiovascular or renal disease, cancer, or diabetes mellitus affect the total amounts, ratios, and kinds of protein, fat, and carbohydrate prescribed.
Vitamins and Minerals
As with macronutrients, vitamin and mineral requirements for individuals with stable COPD depend on the underlying pathologic conditions of the lung, other concurrent diseases, medical treatments, weight status, and bone mineral density. For people continuing to smoke tobacco, additional vitamin C is necessary. Research indicates that people who smoke approximately one pack of cigarettes per day appear to require approximately 16 mg more ascorbate per day, whereas those who smoke two packs need approximately 32 mg more than the recommended dietary allowance.
The role of minerals such as magnesium and calcium in muscle contraction and relaxation may be important for people with COPD. Intakes at least equivalent to the dietary reference intake (DRI) should be provided. Depending on bone mineral density test results, coupled with food intake history and glucocorticoid medications use, additional vitamins D and K also may be necessary (see Chapter 25).
Patients with cor pulmonale and subsequent fluid retention require sodium and fluid restriction. Depending on the diuretics prescribed, increased dietary intake of potassium may be required (see Chapter 9).
Feeding Strategies
Interdisciplinary team involvement is paramount. A modified oral diet is usually preferred. Adequate exercise, fluids, and easily-chewed dietary fiber enhance gastrointestinal motility. When abdominal bloating is a problem, limitation of foods associated with gas formation may help (see Chapter 29).
Patients and their families benefit from specific suggestions for enhancing appetite, promoting oral intake, and lessening fatigue when cooking or eating. Some suggestions are resting before meals, eating small portions of nutrient-dense foods, and planning medications and breathing treatments around mealtimes. For many patients using oxygen at mealtimes, eating slowly, chewing foods well, and engaging in social interaction all can enhance food intake, nutrient metabolism, and enjoyment of the experience. To prevent aspiration, special caution must be given to proper sequencing of breathing with swallowing as well as to proper sitting posture during eating. Patients with disease-related physical limitations may require assistance with food shopping, meal preparation, and linkage with community resources such as congregate meal programs or home-delivered meals (Fig. 35-5).

FIGURE 35-5 An individual with chronic obstructive pulmonary disease and oxygen being shown how to read a label.
Enteral tube feeding can be used to increase total caloric and nutrient intake for some patients with COPD. Decisions to implement this method of nutrition support should take into consideration the goal of the nutrition therapy, the ability of the caregivers, the attitude of the patient, and the cost (see Chapter 14).
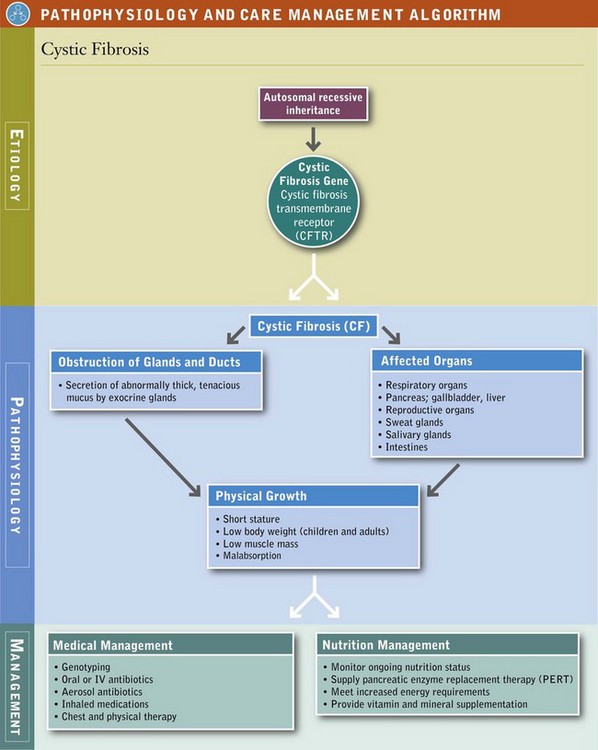
Cystic Fibrosis
Cystic fibrosis (CF) is a complex multisystem disorder that is inherited in an autosomal-recessive fashion. The underlying genetic basis of the disease has been identified, with more than 1400 mutations noted. CF remains one of the most common lethal genetic disorders in the white population, and it is expressed in other population groups as well. Approximately 2% to 5% of white populations are heterozygotes, with a CF incidence of 1 : 3500 live births.
CF was once thought to be only a pediatric disease, but the number of people surviving to or being diagnosed at 18 years or older is approximately 42%. Survival has dramatically improved because of scientific advancements and improvements in diagnostic and treatment procedures, including nutrition. The median age of patients is approximately 37 years. Many women with CF have delivered healthy infants, and some have chosen to breastfeed their unaffected infants.
Pathophysiology
Expression of the CF gene is largely restricted to epithelial cells. In CF, almost all exocrine glands are affected and secrete abnormally thick, tenacious mucus that obstructs glands and ducts in various organs. The clinical features are dominated by involvement of the respiratory tract, sweat and salivary glands, intestine, pancreas, liver, and reproductive tract. Pulmonary complications include acute and chronic bronchitis, bronchiectasis, pneumonia, atelectasis, and peribronchial and parenchymal scarring. Infection with Staphylococcus aureus and Pseudomonas aeruginosa is typical. Pneumothorax and hemoptysis are common. In advanced stages, cor pulmonale or infection with Burkholderia cepacia may be present, signifying a poor prognosis.
Medical Treatment
Several methods are available for diagnosing CF. For families with previously identified CF, prenatal analysis may be possible. Several countries and most states in the United States conduct routine neonatal screening for the disease. Genotyping is available and is a routine procedure. The most reliable clinical diagnostic test, known as the sweat test, is performed by pilocarpine iontophoresis.
CF can have a profound effect on the digestive system. Infants born with meconium ileus have the diagnosis of probable CF until differentiated from other causes. Approximately 85% to 90% of persons with CF have pancreatic insufficiency (PI), in which plugs of thick mucus reduce the quantity of digestive enzymes released from the pancreas into the small intestine. The resultant enzyme insufficiency causes maldigestion of food and malabsorption of nutrients. Decreased bicarbonate secretion can further reduce digestive enzyme activity. Decreased bile acid resorption contributes to fat malabsorption.
Distal intestinal obstruction syndrome (DIOS), recurrent intestinal impaction, sometimes occurs in children and adults. Prevention of DIOS involves intake of adequate enzymes, fluids, and dietary fiber and regular exercise; treatment includes adding stool softeners, laxatives, hyperosmolar enemas, or intestinal lavage.
The presence of excessive mucus lining the small intestinal tract may interfere with nutrient absorption by the microvilli. Gastrointestinal complications include bulky, foul-smelling stools; cramping and intestinal obstruction; rectal prolapse; and liver involvement. As the disease progresses, damage to the endocrine portion of the pancreas causes impaired glucose tolerance and development of CF-related diabetes mellitus (Moran, 2010). Pancreatic enzyme replacement therapy is essential (see Focus On: PERT Therapy).
Medical Nutrition Therapy
Because of the numerous intricate manifestations and complications, nutritional requirements and care must be individually determined for each patient. MNT must be coordinated with other treatments, including a variety of different kinds of medicines and chest physical therapy. The goals of nutrition care in CF are to control maldigestion and malabsorption, provide adequate nutrients, promote optimal growth or maintain weight for height, support pulmonary function, and prevent nutritional deficiencies. Individuals at especially high risk include infants, children, adolescents, and pregnant or lactating women, even when they are medically stable. They should have a nutrition assessment. Comprehensive MNT for people with CF has been evaluated scientifically, with recommendations published (Michel, 2009; Stallings, 2008). Periodic updated practice guidelines are available online from the various international CF organizations and should be consulted for the latest information.
Maldigestion and malabsorption, as well as the progressive complications of the disease, make it difficult to meet increased nutrient needs. Factors interfering with adequate intake and retention of nutrients include dyspnea, coughing and cough-induced vomiting, gastrointestinal discomfort, anorexia during episodes of infection, possible impaired sense of smell and taste, and glucosuria. Growth retardation and difficulty maintaining desired weight for height are common problems. Before diagnosis, infants with CF often demonstrate growth failure. With treatment, growth generally improves. When energy and nutrient intakes are adequate, growth nearly appropriate for age usually can be achieved (Leonard, 2010) (see Pathophysiology and Care Management Algorithm: Cystic Fibrosis).
As lung disease progresses, growth velocity in children and weight for height in adults may decline. The long-term relationship between nutrition support, growth, and survival is not known; however, improved nutrition status on a long-term basis continues to be suggested as a contributing factor to increased survival. Adults have similar medical, surgical, psychosocial, and nutrition assessment concerns, but they also have regular adult life issues. Thus they require nutrition information delivered by different educational approaches than those used with children (Morton, 2009; Watson, 2008).
Macronutrients
Energy needs vary widely from individual to individual, even in the same individual throughout the course of life based on gender, age, basal metabolic rate, physical activity, respiratory infection, severity of lung disease, and severity of malabsorption. When laboratory methods to determine energy requirements are unavailable, equations for calculating caloric recommendations are convenient to use (Magoffin, 2008). Patients with CF should not be encouraged to decrease their activity levels, but rather to increase their energy intake instead. Relatively healthy children with CF usually are able to maintain normal growth and energy stores when they eat a high-energy, moderate-fat diet complemented with sufficient pancreatic enzyme replacement therapy (PERT).
Dietary protein levels are increased in CF as a result of malabsorption; however, when energy needs are adequately met, individuals with CF generally can meet their protein needs. At least 15% to 20% of the total calories consumed as proteins or the appropriate DRI for protein for the individual’s gender, age, and height is suggested.
Fat intake should provide 35% to 40% or more of total kilocalories as tolerated. Dietary fat helps to provide the required energy, EFAs (i.e., linoleic acid and linolenic acid), and fat-soluble vitamins. Moreover, fat limits the volume of food required to meet energy demands and improves the palatability of the diet. Indications of fat intolerance include an increase in the number of stools, greasy stools, or abdominal cramping.
EFA deficiencies may be present, even among patients who are treated adequately with pancreatic enzymes to control malabsorption (Strandvik, 2010). Although clinical signs of EFA deficiency are rare, blood and tissue lipid levels may be abnormal (Aldamiz-Echievarria, 2009). Even if the visible signs of EFA deficiency (e.g., the typical skin lesions) are not noticeable, the clinician should consider routinely testing for abnormal blood lipid profiles. In addition, at-risk patients need to be encouraged to include sources of EFAs (e.g., canola, flaxseed, soybean, or corn oil, or fish) as part of their daily fat intake.
As the disease progresses, changes in carbohydrate intake may be necessary. Lactose intolerance may become evident (see Chapter 29), and pancreatic endocrine involvement may require carbohydrate adjustments.
Vitamins and Minerals
With pancreatic enzyme replacement, the water-soluble vitamins appear to be adequately absorbed in patients with CF. Requirements under normal conditions can usually be met by diet plus a standard age-appropriate multivitamin and mineral supplement; however, monitoring individual variations is important.
Even with pancreatic enzyme supplementation, fat-oluble vitamins usually remain inadequately absorbed. Low serum concentrations of vitamin A despite increased hepatic stores have been documented in CF, suggesting impaired mobilization and transport of the vitamin from the liver. Decreased levels of vitamin D metabolites have also been observed. This is one of several factors that may be related to the decreased bone mineral content seen in populations with CF. Low vitamin E levels have been associated with hemolytic anemia and abnormal neurologic findings. Individuals with CF may be at risk for vitamin K deficiency secondary to long-term use of antibiotics or liver disease, as well as malabsorption. Thus vitamin regimens should be adjusted based on routine serum monitoring of the individual.
Mineral intake should meet the gender and age recommendations according to the DRI. Special attention must be given to some of the minerals. Sodium requirements for infants, children, and adults are increased in CF because of increased losses in sweat. When sodium intake is inadequate, lethargy, vomiting, and dehydration may occur. Adequate salt is consumed by most children and adults who eat a typical North American diet with processed foods; however, supplemental salt is required under some conditions. Infants require extra salt because of the low-sodium content of breast milk, formula, and infant foods (Coates et al., 2009). Children and adults need additional salt during periods of fever, hot weather, or physical exertion. Table salt or proprietary electrolyte replacement solutions are used.
Other minerals are not routinely supplemented, although mineral status should be evaluated on an individual basis. Decreased bone mineralization starts during childhood and must be assessed and addressed (Haworth, 2010). Low iron stores and low magnesium levels have been described in CF. Plasma zinc levels may be low in cases of moderate to severe malnutrition.
Feeding Strategies
Diet modification focuses on meeting the increased nutritional requirements of CF. Along with adequate dietary modification, positive eating behaviors must be established (Stark et al., 2010). Parent educational materials are available online from the Cystic Fibrosis Foundation. For infants with CF and their families, the immunologic and psychosocial benefits of breast-feeding are well established, and breast-feeding should be encouraged. For the infant with pancreatic insufficiency, enzyme microspheres can be added to a small amount of baby food or placed directly in the infant’s mouth. Supplementation with high-calorie formula may be necessary to meet growth goals. For formula-fed infants, standard formulas (20-27 kcal/oz) given with supplemental enzymes are usually adequate.
For children and adults, intake can be enhanced by regular and enjoyable mealtimes, larger food portions at meals, extra snacks, and foods selected for high-nutrient density. Homemade or proprietary nutritional supplements such as fortified beverages and puddings also can help the individual with CF attain nutritional goals.
Supplementation by feeding tube is an alternative for those unable to meet nutritional needs by the oral route. Formulas are provided by continuous infusion through a nasogastric, gastrostomy, or jejunostomy tube, often while the person sleeps. Elemental (predigested) and nonelemental formulas with enzymes may be effective. See Chapter 14.
Intensive supplementation has been associated with improved weight gain, slowed decline in pulmonary function, decreased incidence of respiratory infection, and improved sense of well being. Although the short-term benefits of supplementation have been well documented, nutrition status is likely to regress when supplementation is discontinued. The long-term effect of intensive supplementation on the course of the disease has not been determined. Parenteral nutrition is best used for short-term support in patients with clearly evident needs such as those recuperating from gastrointestinal surgery.
Lung Cancer
The primary sites of lung cancer are usually the bronchi, with subsequent metastasis to other organs such as the bone, brain, liver, or skin. As new screening technologies become commonplace, early detection and diagnosis should improve.
Pathophysiology
Lung cancer is often associated with persistent tobacco smoking for many years, but other inhaled pollutants may initiate the malignant condition.
Medical Treatment
Currently the medical treatment of lung cancer involves radiation therapy, chemotherapy, and surgery, which are accompanied by various nutritional side effects. Patients with lung cancer experience the added stress of respiratory fatigue and diminished lung residual capacity. Smoking cessation sessions are part of most wellness programs and offer ideal settings for nutrition education.
Medical Nutrition Therapy
In cigarette smokers, food components and specific nutrients have been investigated as either preventive or therapeutic modalities for lung cancer. High-dose β-carotene supplements may have a negative effect, whereas increased consumption of fruits and vegetables may be beneficial (Hercberg, 2005). The possible role of whole foods or their various components, or botanicals, in lung cancer initiation, promotion, or treatment receives worldwide attention (Lambert et al., 2005).
Because of the pulmonary constraints in people with lung cancer, purchasing and preparing foods may be overwhelming tasks. Eating may become an unpleasant activity because of severe pain, dyspnea, and dyspepsia. Weight loss, along with associated declines in other anthropometric and laboratory indicators of cancer-related malnutrition, portends a worsening prognosis. Thus, providing foods, beverages, and nutritional supplements in the forms and at the times best tolerated by the patient is essential. Administering oral medications with calorically dense nutritional supplements is another means of supplying needed nutrients (Cranganu and Camporeale, 2009).
Pneumonia
Pneumonia usually occurs as an infection from bacteria, viruses, or fungi, or as a consequence of aspiration of food, fluid, or secretions such as saliva. Aspiration is common in infants, children, and adults who are frail, have frequent coughing spasms, are unable to effectively chew or swallow their foods and beverages, or have inadequate head and neck control during eating.
Pathophysiology
The infection or foreign material cause the alveoli to become inflamed. These air sacs fill with fluid or pus, which results in symptoms including cough (with phlegm), fever, chills, and labored breathing.
Medical Nutrition Therapy
The role of vitamin A in treating pneumonia in children yielded conflicting results based on study designs (Mathew, 2010). Because of their role in inflammation and immunity, EFAs in adults were investigated, and showed a possible protective effect against pneumonia by the ingestion of α-linolenic and linoleic acids (Merchant et al., 2005).
Optimal nutrition status and proper feeding techniques aid in preventing this pulmonary infection. Suggestions for preventing aspiration of secretions or food and fluids are in Chapter 41 and Appendix 35. Once pneumonia occurs, the goals of nutrition care are to provide adequate fluids and energy. Small, frequent meals of nourishing foods usually are better tolerated, coupled with proper positioning during eating.
Respiratory Failure
Respiratory failure (RF) occurs when the pulmonary system is unable to perform its functions. The causes may be traumatic, surgical, or medical. Multiple organ dysfunction syndrome (see Chapter 39) is the term used to denote abnormal interaction among the organ systems, culminating in relentless dysfunction of all organ systems.
Pathophysiology
Acute respiratory distress syndrome is a common complication of critical illness. Ultimately, in RF from any cause, the patient requires oxygen provided through a nasal cannula or by mechanical ventilator support for varying lengths of time and at various levels of oxygen.
Medical Treatment
Central factors in failure to wean from oxygen support or mechanical ventilation are respiratory muscle weakness and retention of CO2. The prognosis is precarious for patients with underlying chronic pulmonary disease such as CF or emphysema, or for those who are otherwise medically compromised, malnourished, or older. Lung transplantation (or cardiopulmonary transplantation) may be a viable option for some patients.
Medical Nutrition Therapy
Nutritional needs vary widely within this group of patients, depending on the underlying disease process, prior nutrition status, and the patient’s age. Hypercatabolism or hypermetabolism may be present.
As with most pulmonary diseases, body composition fluctuation is the hallmark nutrition assessment indicator for persons with RF. Most patients become severely underweight. Thus a series of accurate anthropometric measurements is crucial during the entire course of treatment, sometimes spanning the patient’s lifetime (see Chapter 6). When at all possible, more accurate estimations of energy requirements are recommended with the use of indirect calorimetry measurements (see Chapter 2). Accurate interpretation of laboratory results may be confounded by fluid imbalances, medications, and ventilator support. Other nutritionally relevant factors to assess include immunocompetence, chronic mouth breathing, aerophagia, dyspnea, exercise tolerance, and depression.
The goals of nutrition care in patients with RF are to meet basic nutritional requirements, preserve lean body mass, restore respiratory muscle mass and strength, maintain fluid balance, improve resistance to infection, and facilitate weaning from oxygen support and mechanical ventilation by providing energy substrates without exceeding the capacity of the respiratory system to clear CO2. Methods to provide nutrition support depend on the underlying disease, whether the patient is acutely or chronically ill, and whether ventilator support is necessary (see Chapter 14).
Energy
Because of hypercatabolism and hypermetabolism, energy needs are elevated in RF, and sufficient energy must be supplied to prevent the use of the body’s own reserves of protein and fat. Energy requirements fluctuate and thus are best determined by continuous individual assessment. To estimate initial caloric requirements using prediction equations, see Chapter 2. Indirect calorimetry is considered the “gold standard” because it is the most accurate estimation of energy needs. Overfeeding in this population is particularly deleterious. By general agreement the most important factor is to provide adequate but not excessive energy.
Macronutrients
Because the patient with RF may be in negative nitrogen balance, protein should be supplied to restore balance; however, enterally supplied protein does affect the RQ. The basic requirements for carbohydrate and fat as actual nutrients for nourishment are influenced by the underlying organ system decompensation, the patient’s respiratory status, and the ventilation methods used. Controversy persists concerning the optimal ratio of protein, fat, and carbohydrate supplied to patients with RF. Protein is calculated as 1.5 to 2 g/kg of dry body weight. Nonprotein calories are evenly divided between fat and carbohydrate. Daily monitoring of each patient’s intake is crucial.
Water requirements must be individualized based on the method of oxygen delivery and environmental factors, coupled with knowledge of underlying disease processes and prescribed medications.
Vitamins and Minerals
Exact requirements for specific vitamins and minerals in RF are unknown. It is assumed that vitamins and minerals need to be supplied at least at the levels of the DRI plus repletion, based on the gender and age of the patient. The intake of vitamins and minerals necessary for anabolism, wound healing, and immunity, and those with antioxidant functions may need to be increased. For example, during anabolism mineral balance must be monitored in an anticipatory manner to prevent the refeeding syndrome (see Chapter 14). Minerals that function as electrolytes need to be monitored closely, especially because of fluid imbalances and the occurrence of respiratory acidosis or alkalosis. As a side effect of medications, potassium, calcium, and magnesium may be lost in the urine.
Feeding Strategies
Diet composition and food selections should be planned to accommodate the nutritional requirements, individual preferences, and living arrangements of the patient. Some people participate in pulmonary rehabilitation programs. Most patients who are not intubated or who have tracheostomies will be able to meet all or most of their nutritional needs by mouth. Small portions and favorite foods enhance oral food intake. Consumption must be monitored to maintain appropriate calorie levels and a suitable ratio of protein, fat, and carbohydrate.
Provision of adequate oxygen is crucial for proper digestion and absorption of food. Patients receiving inadequate oxygen may complain of anorexia, early satiety, malaise, bloating, and constipation or diarrhea. Intubated patients usually require enteral tube feedings or parenteral feedings. In a hospital setting establishing a nutrition protocol increases the likelihood of appropriate enteral feeding, thus yielding better outcomes such as decreased duration of mechanical ventilation. The use of specially formulated pulmonary proprietary products should be reserved for patients fitting the specified criteria (see Chapter 14). Otherwise effort and expense may not provide the expected results.
Tuberculosis
Tuberculosis (TB) traditionally was diagnosed among economically disadvantaged population groups (e.g., immigrants, homeless persons, and children) or those living in close quarters (e.g., prisoners, refugees, and armed forces). At high risk are health care workers; residents in assisted living facilities, skilled nursing homes, or hospitals; and people who are immunocompromised such as those with cancer, chronic renal disease, or HIV (see Chapters 36 through 38).
Pathophysiology
Tuberculosis (TB) is a bacterial disease caused by mycobacteria, specifically Mycobacterium tuberculosis, M. bovis, or M. africanum. The disease is spread from inhalation of organisms dispersed as droplets from the sputum of infected persons (the bacteria-laden droplets can float in the air for several hours). TB yields prolonged cytokine production. Increased levels of interferon gamma, interleukin (IL)-10, and IL-6 are accompanied by a modest increase in the levels of cortisol, prolactin, and thyroid hormones and decreased testosterone and dehydroepiandrosterone levels (Bottasso et al., 2009).
TB produces profound abnormalities on the immune system. Although most people infected by the tubercle bacillus (90%) do not develop the disease during their lifetime, when there are coinfections with HIV, malnutrition, or diabetes, the risk of developing active disease increases considerably.
Medical Treatment
Pharmacologically, this pulmonary infection is treated with multiple medications, especially antibiotics. First-line drugs are isoniazid, rifampicin, ethambutol, and pyrazinamide. Each has drug-food-nutrient interactions (see Chapter 9). Because tubercle bacilli increasingly are becoming resistant to drug therapy; strains with increased virulence have emerged. New therapies are constantly under review.
Medical Nutrition Therapy
Signs and symptoms of TB with nutritional relevance include undernutrition, weight loss, night sweats, fatigue, dyspnea, and hemoptysis (Campbell and Bah-Sow, 2006; Villamor et al., 2006). People with chronic infections may need higher caloric intakes. Unless otherwise contraindicated, people with TB routinely require increased energy intake and fluids. Research has not found a role for vitamin A or zinc supplementation specifically, but vitamin C may have merit. Providing access to food and also to high-calorie, high-protein oral supplements is a less expensive, feasible medical option (Abba K, et al., 2008). Many patients require assistance with activities of daily living, such as shopping for food and meal preparation.
Because isoniazid absorption is reduced by food, it should be administered 1 hour before or 2 hours after meals. It depletes pyridoxine (vitamin B6) and interferes with vitamin D metabolism, which in turn can decrease absorption of calcium and phosphorus. Patients thus require increased vitamins B6 and D (Yamshchikov, et al., 2010), and minerals from meals or supplements.
American Association for Respiratory Care
Cystic Fibrosis Genetic Analysis Consortium (Cystic Fibrosis Mutation Database)
http://www.genet.sickkids.on.ca/cftr
National Asthma Education and Prevention Program
http://www.nhlbi.nih.gov/guidelines/asthma
National Cancer Institute (Lung Cancer)
http://www.cancer.gov/cancertopics/types/lung
National Institute of Diabetes and Digestive and Kidney Diseases—Cystic Fibrosis Research
References
Abba K, et al: Nutritional supplements for people being treated for active tuberculosis, Cochrane Database Syst Rev (4), 2008. Art. No.: CD006086. DOI: 10.1002/14651858.CD006086.pub2.
Aldamiz-Echievarria, L, et al. Persistence of essential fatty acid deficiency in cystic fibrosis despite nutritional therapy. Pediatric Res. 2009;66:585.
Allan, K, Devereux, G. Diet and asthma; nutrition implications from prevention to treatment. J Am Diet Assoc. 2011;111:258.
American Academy of Allergy, Asthma, and Immunology (AAAAI). Attaining optimal asthma control: A practice parameter. Accessed 22 October 2010 from www.aaaai.org, 2005.
American Dietetic Association, Evidence Analysis Library. COPD. Accessed 22 October 2010 from www.eatright.org, 2010.
Baines, KL, et al. The nutrigenomics of asthma: molecular mechanisms of airway neutrophilia following dietary antioxidant withdrawal. OMICS: Journal of Integrative Biology. 2009;13:355.
Baldi, S, et al. Fat-free mass change after nutritional rehabilitation in weight losing COPD: role of insulin, C-reactive protein and tissue hypoxia. Int J COPD. 2010;5:29.
Barros, R, et al. Adherence to the Mediterranean diet and fresh fruit intake are associated with improved asthma control. Allergy. 2008;63:917.
Bede, O, et al. Effects of magnesium supplementation on the glutathione redox system in atopic asthmatic children. Inflammation Res. 2008;57:279.
Bottasso, O, et al. Immunoendocrine alterations during human tuberculosis as an integrated view of disease pathology. Neuroimmunomodulation. 2009;16:68.
Campbell, IA, Bah-Sow, O. Pulmonary tuberculosis: diagnosis and treatment. Br Med J. 2006;332:1194.
Coates, AJ, et al. Evaluation of salt supplementation in CF infants. J Cys Fibr. 2009;8:382.
Cranganu, A, Camporeale, J. Nutrition aspects of lung cancer. Nutr Clin Pract. 2009;24:688.
Darlow BA, et al: Vitamin A supplementation to prevent mortality and short and long-term morbidity in very low birthweight infants, Cochrane Database Syst Rev 2007. CD000501.
DHHS (Department of Health and Human Services:) Action against asthma. a strategic plan for the Department of Health and Human Services. Accessed 22 October 2010 from http://www.aspe.hhs.gov/sp/asthma/.
Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease, Executive summary, updated. Accessed 22 October 2010 from http://www.goldcopd.org/, 2009.
Guilbert, TW, Denlinger, LC. Role of infection in the development and exacerbation of asthma. Expert Rev Respir Med. 2010;4:71.
Hercberg, S. The history of β-carotene and cancers: from observational to intervention studies. What lessons can be drawn for future research on polyphenols? Am J Clin Nutr. 2005;81:218S.
Haworth, CS. Impact of cystic fibrosis on bone health. Curr Opin Pulm Med. 2010;16:616.
Kalhan, R, et al. A mechanism of benefit of soy genistein in asthma: inhibition of eosinophil p38-dependent leukotriene synthesis. Clin Exper Allergy. 2008;38:103.
Kazaks, AG, et al. Effect of oral magnesium supplementation on measures of airway resistance and subjective assessment of asthma control and quality of life in men and women with mild to moderate asthma: a randomized placebo controlled trial. J Asthma. 2010;47:83.
Kealoha, MK. What’s new in alternative therapies for asthmatic children? J Comm Health Nurs. 2009;26:198.
King, DA, et al. Nutritional aspects of chronic obstructive pulmonary disease. Proc Am Thoracic Soc. 2008;5:519.
Lambert, JD, et al. Inhibition of carcinogenesis by polyphenols: evidence from laboratory investigations. Am J Clin Nutr. 2005;81:284S.
Leonard, A, et al. Description of a standardized nutrition classification plan and its relation to nutritional outcomes in children with cystic fibrosis. J Ped Psycol. 2010;35:6.
Lindemann, J, et al. Clinical study of the effects on asthma-related QOL and asthma management of a medical food in adult asthma patients. Curr Med Res Opin. 2009;25:2865.
Magoffin, A, et al. Longitudinal analysis of resting energy expenditure in patients with cystic fibrosis. J Pediatr. 2008;152:703.
Mathew, JL. Vitamin A supplementation for prophylaxis or therapy in childhood pneumonia: a systematic review of randomized controlled trials. Indian Pediatr. 2010;47:255.
Merchant, AT, et al. Intake of ω-6 and ω-3 fatty acids and fish and risk of community-acquired pneumonia in U.S. men. Am J Clin Nutr. 2005;82:668.
Michel, SH, et al. Nutrition management of pediatric patients who have cystic fibrosis. Pediatr Clin N Am. 2009;56:1123.
Moran, A, et al. Clinical care guidelines for cystic fibrosis-related diabetes. Diabetes Care. 2010;33:2697.
Morton, AM, et al. Symposium 6: Young people, artificial nutrition and transitional care. The nutritional challenges of the young adult with cystic fibrosis: transition. Proc Nutr Soc. 2009;68:430.
Peden, DB, Bush, RK. Advances in environmental and occupational respiratory disease in 2010. J Allergy Clin Immunol. 2011;127:696.
Raviv, S, Smith, LJ. Diet and asthma. Curr Opin Pulm Med. 2010;16:71.
Schubert, R, et al. Effect of ω-3 polyunsaturated fatty acids in asthma after low-dose allergen challenge. Int Arch Allergy Immunol. 2009;148:321.
Sorkness, RL. CAM and respiratory disease. Nutr Clin Pract. 2009;24:609.
Stallings, VA, et al. Evidence-based practice recommendations for nutrition-related management of children and adults with cystic fibrosis and pancreatic insufficiency: results of a systematic review. J Am Diet Assoc. 2008;108:832.
Stark, LJ, et al. The effects of an intensive behavior and nutrition intervention compared to standard of care on weight outcomes in CF. Pediatr Pulmonol. 2010;00:1.
Stevenson, CS, Birrell, MA. Moving towards a new generation of animal models for asthma and COPD with improved clinical relevance. Pharmacol Ther. 2011;130:93.
Strandvik, B. Fatty acid metabolism in cystic fibrosis: prostaglandins. Leukot Essent Fatty Acids. 2010;83:121.
Van Marter, LJ. Epidemiology of bronchopulmonary dysplasia. Semin Fetal Neonatal Med. 2009;14:358.
Villamor, E, et al. Wasting and body composition of adults with pulmonary tuberculosis in relation to HIV-1 coinfection, socioeconomic status, and severity of tuberculosis. Eur J Clin Nutr. 2006;60:163.
Weekes, CE, et al. Dietary counseling and food fortification in stable COPD: a randomized trial. Thorax. 2009;64:326.
Watson, H, et al. A randomized controlled trial of a new behavioral home-based nutrition education program, “Eat Well with CF,” in adults with cystic fibrosis. J Am Diet Assoc. 2008;108:847.
Wüthrich, B, et al. Milk consumption does not lead to mucus production or occurrence of asthma. J Am Coll Nutr. 2005;24:547S. [6].
Yamshchikov, AV, et al. Vitamin D status and antimicrobial peptide cathelicidin (LL-37) concentrations in patients with active pulmonary tuberculosis. Am J Clin Nutr. 2010;92:603.
Zhang, Z, et al. Early childhood weight status in relation to asthma development in high-risk children. J Allergy Clin Immunol. 2010;126:1157.