31 Obesity
Overview
Obesity is a growing health issue around the world and is reaching epidemic proportions in some nations. The problem is not restricted to the inhabitants of the affluent countries, to the adult population or to any one socioeconomic class. Body fat represents stored energy, and obesity occurs when the homeostatic mechanisms controlling energy balance become disordered or overwhelmed. In this chapter, we explore first the endogenous regulation of appetite and body mass, and then consider the main health implications of obesity and its pathophysiology. We conclude with a discussion of the two drugs currently licensed for the treatment of obesity, and glance at the future of pharmacological treatment of this condition.
Introduction
Survival requires a continuous provision of energy to maintain homeostasis even when the supply of food is intermittent. Evolution has furnished a mechanism for storing any excess energy latent in foodstuffs in adipose tissue as energy-dense triglycerides, such that these can be easily mobilised when food is scarce. This mechanism, controlled by the so-called thrifty genes, was an obvious asset to our hunter-gatherer ancestors. However, in many societies a combination of sedentary lifestyle, genetic susceptibility, cultural influences and unrestricted access to an ample supply of calorie-dense foods is leading to a global epidemic of obesity, or ‘globesity’ as it sometimes called.
Definition of Obesity
If the ‘ideal weight’ of an individual is that which maximises life expectancy, ‘obesity’ may be defined as an illness where the health (and hence life expectancy) is adversely affected by excess body fat.1 But at what point does an individual become ‘obese’? The generally accepted benchmark is the body mass index (BMI). The BMI is expressed as W/h2, where W = body weight (in kg), h = height (in metres). Although it is not a perfect index (e.g. it does not distinguish between fat and lean mass), the BMI is generally well correlated with other measurements of body fat, and it is widely employed in obesity studies. While there are problems in defining a ‘healthy’ weight for a particular population, the World Health Organization (WHO) classifies people with a BMI of < 18.5 kg/m2 as ‘underweight’, and those with a BMI of 18.5–24.9 kg/m2 as of ‘acceptable’ or ‘normal’ weight. A BMI in the range of 25.0–29.9 kg/m2 signifies ‘grade 1 overweight’. If it is between 30.0 and 39.9 kg/m2, the patient is deemed to be obese or ‘grade 2 overweight’, while those with a BMI of > 40 kg/m2 are said to be ‘grade 3 overweight’ or morbidly obese. Childhood obesity is more difficult to assess.
As the BMI obviously depends on the overall energy balance, another operational definition of obesity would be that it is a multifactorial disorder of energy balance in which calorie intake over the long term exceeds energy output.
The Homeostatic Mechanisms Controlling Energy Balance
A common view, and one that is implicitly encouraged by authors of numerous dieting books as well as the enormously lucrative dieting industry in general, is that obesity is simply the result of bad diet or wilful overeating (hyperphagia). In truth, however, the situation is more complex and, on its own, dieting does not usually provide a lasting solution. The failure rate of such diets is high (probably 90%), with most dieters eventually returning to their original starting weight. This suggests the operation of some intrinsic homeostatic system that strives to maintain a particular set weight. This mechanism is normally exceptionally precise, and it has been calculated that it is capable of regulating energy balance to 0.17% per decade (Weigle, 1994). An astonishing feat considering the day-to-day variations in food intake.
When exposed to the same dietary choices some individuals will become obese whereas others will not. Studies of obesity in monozygotic and dizygotic twins have established a strong genetic influence on the susceptibility to the disease, and studies of rare mutations in mice (and more recently in humans) have led to the discovery and elucidation of the neuroendocrine pathways that match food intake with energy expenditure, and to the concept that it is, in fact, disorders of this system that are largely responsible for the onset and maintenance of the disease of obesity.
The Role of Gut and Other Hormones in Body Weight Regulation
At the beginning of the 20th century, it was observed that patients with damage to the hypothalamus tended to gain weight. In the 1940s, it was also shown that discrete lesions in the hypothalamus of rodents caused them to become obese or exhibit unusual feeding behaviour. As early as 1953, Kennedy proposed, on the basis of experiments on rats, that a hormone released from adipose tissue acted on the hypothalamus to regulate body fat and food intake. These seminal findings set the stage for future discoveries in this area.
It also was observed that mice could become obese as a result of mutations in certain genes. At least five of these have now been characterised, including the ob (obesity), tub (tubby), fat and db (diabetes) genes. Mice that are homozygous for mutant forms of these genes—ob/ob mice and db/db mice—eat excessively, have low energy expenditure, become grossly fat and have numerous metabolic and other abnormalities. Weight gain in an ob/ob mouse is suppressed if its circulation is linked to that of a normal mouse, implying that the obesity is caused by lack of a blood-borne factor.
An important conceptual breakthrough came in 1994, when Friedman and his colleagues (see Zhang et al., 1994) cloned the ob gene and identified its protein product as leptin.2 When recombinant leptin was administered to ob/ob mice, it strikingly reduced food intake and body weight. It had a similar effect when injected directly into the lateral or the third ventricle, implying that it acted on the regions of the brain that control food intake and energy balance. Recombinant leptin has similar effects in humans (see Fig. 31.1).
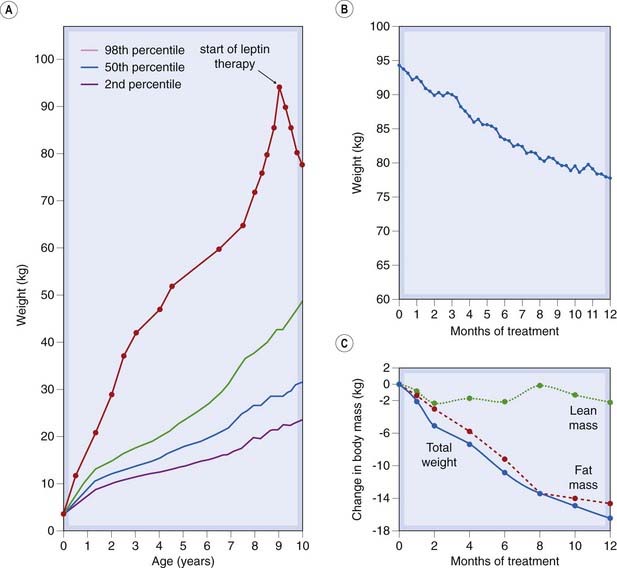
Fig. 31.1 The effect of recombinant leptin on body weight in a 9-year-old severely obese child deficient in endogenous leptin because of a frame shift mutation in the leptin gene.
Although of normal birth weight, the child began gaining weight at 4 months and was constantly demanding food. When treatment was initiated, the child weighed 94.4 kg. Weight loss began after 2 weeks’ treatment, and her eating pattern returned to normal. She had lost 15.6 kg of body fat after 1 year of treatment.
(Data and figure adapted from Farooqi et al. 1999).
Leptin mRNA is expressed in adipocytes; its synthesis is increased by glucocorticoids, insulin and the oestrogens, and it is reduced by β-adrenoceptor agonists. In humans, the release of leptin is pulsatile and varies according to the fat stores and BMI in normal subjects. Insulin (see Ch. 30) also functions in a similar manner although it is probably less important than leptin.
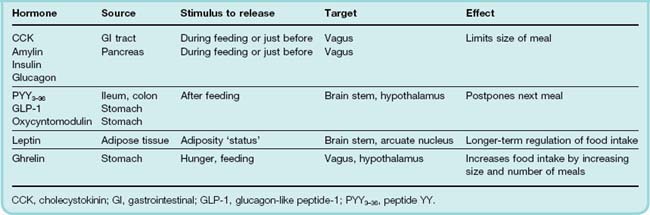
Today, it is recognised that in addition to leptin and insulin, several other peripheral hormones originating mainly from the gastrointestinal (GI) tract, play a crucial role in determining food intake, meal size and the feeling of satisfaction produced.3 Peptide hormones secreted by cells in the wall of the small intestine in response to the arrival of food (see Ch. 30) are important in this connection. Table 31.1 and Figure 31.2 summarise the main characteristics of these hormones.
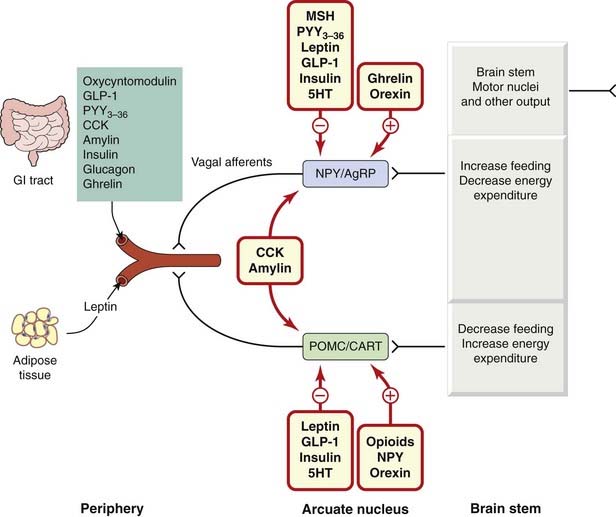
Fig. 31.2 A simplified representation of the role of peripheral hormones and other mediators in the regulation of energy balance and fat stores.
The primary level of hypothalamic control is vested in two groups of neurons, with opposing actions, in the arcuate nucleus (ARC). In one group, the peptides neuropeptide Y (NPY) and agouti-related protein (AgRP) are co-localised; the other contains the polypeptides prepro-opiomelanocortin (POMC) and cocaine- and amphetamine-related transcript (CART), which release α-melanocyte-stimulating hormone (MSH). Blood-borne hormones arising from the gastrointestinal (GI) tract or adipose tissue are sensed by receptors on vagal and other afferents and are relayed through the nucleus tractus solitarius to modify the activity of these neuronal circuits. The influence of hormones on each neuronal group is indicated. Hormones marked in blue (e.g. leptin) arise from the peripheral blood and influence the ARC neurons directly or indirectly through neuronal signals; mediators in green (e.g. 5HT, orexin) originate within the central nervous system itself. Activation of the NPY/AgRP group by, for example, a fall in leptin or an increase in ghrelin levels results in increased food intake and decreased energy expenditure. In the POMC/CART group of neurons, increased leptin or other hormone levels triggered by overfeeding produces a predominately inhibitory effect on feeding behaviour. A number of other hormones such as cholecystokinin (CCK) and amylin also alter the properties of the ARC neurons although the mechanism is not clear. GLP-1, glucagon-like peptide-1.
(Modified from Adan et al. 2008.)
The majority of these factors are released either during, or in anticipation of, eating and most are inhibitory in nature producing either satiety or satiation. Two exceptions are the gastric hormone, ghrelin, which promotes hunger, and leptin itself, which is controlled by the amount of adipose tissue and is thus more involved with the longer-term energy status of the individual. The main targets for these hormones are receptors on vagal afferent fibres or within the hypothalamus (or elsewhere in the central nervous system [CNS]). Here, they modulate the release of other neurotransmitters that exert a fine regulation over eating behaviour, energy expenditure and body weight. Other actions of these peptide hormones include the release of insulin by the incretins, namely glucagon-like peptide-1 (GLP-1) and gastric inhibitory peptide (GIP).
Neurological Circuits That Control Body Weight and Eating Behaviour
Control of Food Intake
The manner in which all these hormonal signals are processed and integrated with other viscerosensory, gustatory or olfactory information within the CNS is complex. Many sites within the CNS are involved in different aspects of the process and some 50 hormones and neurotransmitters are implicated. The account we present here is therefore necessarily an oversimplification: the Further Reading list should be consulted for a more complete picture.
As early lesioning studies predicted, the hypothalamus is the main brain centre that regulates appetite, feeding behaviour and energy status, although other sites in the brain such as the nucleus accumbens (NAc), the amygdala and especially the nucleus tractus solitarius (NTS) in the medulla, are also crucial. Within the hypothalamus, the arcuate nucleus (ARC), situated in the floor of the third ventricle, is a key site. It receives afferent inputs originating from the GI tract and contains receptors for leptin and other significant hormones. It also has extensive reciprocal connections with other parts of the hypothalamus involved in monitoring energy status, in particular the paraventricular nucleus and the ventromedial hypothalamus. Figure 31.2 summarises some of the complex interactions that occur in the ARC but it must be realised that this is only part of the overall control system and is presented in a simplified fashion.
Within the ARC are two groups of functionally distinct neurons that exert opposite effects on appetite. One group, termed anorexigenic (appetite suppressing), secrete pro-opiomelanocortin (POMC)-derived peptides (such as α-melanocyte-stimulating hormone; α-MSH) or cocaine- and amphetamine-regulated transcript (CART)4-derived peptides. The other group, termed orexigenic (appetite promoting) neurons, secrete neuropeptide Y (NPY) or agouti-related peptide (AgRP). As these groups of neurons have opposing actions, energy homeostasis depends, in the first instance, on the balance between these actions whose final effects are realised by the brain stem motor system as changes in feeding behaviour.
Monoamines such as noradrenaline, 5-hydroxy-tryptamine (5-HT) and dopamine also play a role in the modulation of satiety signals. Noradrenaline is co-localised with NPY in some neurons and greatly potentiates its hyperphagic action. Deficit of dopamine impairs feeding behaviour, as do agonists at the 5-HT2C receptor; antagonists at this receptor have the reverse effect.
Many neural signals arising from the GI tract are integrated, and relayed on to the hypothalamus, by the NTS in the medulla. Some of these signals, including those of gustatory, olfactory, mechanical and viscerosensory signals, arise from vagal and other spinal afferents originating in the GI tract or liver. Endocrine signals have more complex signalling pathways. For example, cholecystokinin (CCK) is secreted by the duodenum in response to the process of eating and digestion of (especially fatty) foodstuffs. CCK acts locally on CCKA receptors in the GI tract to stimulate vagal afferents and may also act on CCKB receptors in the brain in order to function as a satiety factor. Ghrelin also has complex actions. It stimulates growth hormone release (Ch. 32) and also has a direct action on neurons in the ARC to modify feeding behaviour. Blood ghrelin levels normally fall after eating but not in obese individuals (English et al., 2002). Interestingly, polymorphisms in the ghrelin gene may be important in the pathogenesis of the Prader–Willi syndrome, which predisposes to morbid obesity.
Leptin also targets these neurons in the ARC. Falling leptin levels activate the orexigenic neurons, resulting in increased food intake, and synthesis and storage of fat (anabolism), as well as decreased energy expenditure. Conversely, rising leptin levels activate the second group of neurons, producing the opposite anorexigenic and catabolic effect.
Inputs from other parts of the CNS also influence feeding behaviour. Of importance to us is the input from the NAc. This centre seems to regulate those aspects of eating that are driven by pleasure or reward—the so-called ‘hedonic’ aspects of eating (see also Ch. 48). The endocannabinoid system is important in this response. The hypothalamus contains large amounts of 2-arachidonyl glycerol and anandamide as well as the CB1 receptor (Ch. 18). Administration of endogenous or exogenous (e.g. Δ9-THC) cannabinoids provokes a powerful feeding response.5 This system in turn may be modulated by ‘stress’ and other factors in the environment.
Control of Energy Expenditure
Balancing food intake is the energy expenditure required to maintain metabolism, physical activity and thermogenesis (heat production). The metabolic aspects of energy expenditure include, among other things, cardiorespiratory work and the actions of a multitude of enzymes. Physical activity increases all these, as well as increasing energy expenditure by the skeletal muscles. Exposure to cold or feeding also stimulates thermogenesis, and the reverse is also true. The, often dramatic (20–40% increase), thermogenic effects of feeding may provide a partial protection against developing obesity.
The sympathetic nervous system (sometimes in concert with thyroid hormone) plays a significant part in the regulation of energy expenditure in cardiovascular and skeletal muscle function during physical activity, as well as in the thermogenic response of adipose tissue and the response to cold. Both ‘white’ and ‘brown’ (the colour is apparently caused by the high density of mitochondria) fat cells (but especially the latter) have a major role in thermogenesis. Brown fat, which is densely innervated by the sympathetic nervous system, is abundant in rodents and human infants, although in human adults these cells are generally to be found more interspersed with white fat cells. Because of their abundant mitochondria, they are remarkable heat generators, producing more heat and less ATP than white fat cells. The basis for this, as determined in mice, is the presence of mitochondrial uncoupling proteins (UCP). Three isoforms, UCP-1, -2 and -3, are known and have different distributions, although all are found in brown fat. These proteins ‘uncouple’ oxidative phosphorylation, so that mitochondria continue oxidative metabolism but produce much less ATP, thus promoting net energy loss as heat. As one might anticipate, exposure to cold or leptin administration increases both the activity and (after prolonged stimulation) the amount of UCP-1 in brown fat. Noradrenaline, acting on β-adrenoceptors (mainly β3) in brown fat, increases the activity of the peroxisome proliferator-activated receptor-γ (PPARγ) transcription factor which, in turn, activates the gene for UCP-1. The expression of β3-adrenoceptors is decreased in genetically obese mice.
Energy balance ![]()
Energy balance depends on food intake, energy storage in fat and energy expenditure. In most individuals, the process is tightly regulated by a homeostatic system that integrates inputs from a number of internal sensors and external factors. Important components of the system include the following:
Obesity as a Health Problem
Obesity is a growing and costly global health problem. According to the WHO (2005 figures), there are already more than 1.6 billion overweight adults, approximately one-quarter of whom are obese according to the criteria outlined above, and this is expected to rise to 2.3 billion overweight and 700 million obese people by 2015. National obesity levels vary enormously, being less than 5% in China, Japan and parts of Africa, to a staggering 75% in parts of Samoa. Obesity levels in the USA, Europe and the UK (among others) have increased three-fold since 1980, with figures of 31% being quoted for the USA and about 25% for many other industrialised nations (Padwal et al., 2003). The disease is not confined to adults: some 22 million children under 5 years old are estimated to be overweight. In the USA, the number of overweight children has doubled and the number of overweight adolescents has trebled since 1980. Ironically, obesity often co-exists with malnutrition in many developing countries. All socioeconomic classes are affected. In the poorest countries, it is the top socioeconomic classes in whom obesity is prevalent, but in the West it is usually the reverse.
 While obesity itself is rarely fatal, it brings with it the risk of increased susceptibility to a host of metabolic and other disorders, the most important of which are type 2 diabetes, metabolic syndrome, hypertension and cardiovascular conditions, cancers (particularly hormone dependent), respiratory (particularly sleep apnoea) and digestive problems, as well as osteoarthritis. One commentator (Kopelman, 2000) has remarked that obesity ‘… is beginning to replace under-nutrition and infectious diseases as the most significant contributor to ill health’. The total costs of obesity-related illness are hard to estimate. Figures in the range of 2–7% of the total healthcare budget are often given but are probably an underestimate. Increasingly, social stigma is suffered by obese individuals, leading to a sense of psychological isolation.
While obesity itself is rarely fatal, it brings with it the risk of increased susceptibility to a host of metabolic and other disorders, the most important of which are type 2 diabetes, metabolic syndrome, hypertension and cardiovascular conditions, cancers (particularly hormone dependent), respiratory (particularly sleep apnoea) and digestive problems, as well as osteoarthritis. One commentator (Kopelman, 2000) has remarked that obesity ‘… is beginning to replace under-nutrition and infectious diseases as the most significant contributor to ill health’. The total costs of obesity-related illness are hard to estimate. Figures in the range of 2–7% of the total healthcare budget are often given but are probably an underestimate. Increasingly, social stigma is suffered by obese individuals, leading to a sense of psychological isolation.
The risk of developing type 2 diabetes (which represents 85% of all cases of the disease) rises sharply with increasing BMI. The WHO reports that 90% of those diagnosed with the disease are obese. In a study of the disease in women, the risk of developing diabetes was closely correlated with BMI, increasing five-fold when the BMI was 25 kg/m2, to 93-fold when the BMI was 35 kg/m2 or above (Colditz et al., 1995). Cardiovascular disease is also increased in the obese individual, and the increased thoracic and abdominal adipose tissue reduces lung volume and makes respiration difficult. Obese subjects also have an increased risk of colon, breast, prostate, gall bladder, ovarian and uterine cancer. Numerous other disorders are associated with excess body weight, including osteoarthritis, hyperuricaemia and male hypogonadism. Gross obesity (BMI over 40 kg/m2) is associated with a 12-fold increase in mortality in the group aged 25–35 years compared with those in this age group with a BMI of 20–25 kg/m2.
The pathophysiology of human obesity
In most adult subjects, body fat and body weight remain more or less constant over many years, even decades, in the face of very large variations in food intake and energy expenditure—amounting to about a million calories per year. The steady-state body weight and BMI of an individual is, as has been stressed above, the result of the integration of multiple interacting regulatory pathways. How, then, does obesity occur? Why is it so difficult for the obese to lose weight and maintain the lower weight?
The main determinant is manifestly a disturbance of the homeostatic mechanisms that control energy balance, but genetic endowment underlies this disturbance. Other factors, such as food availability and lack of physical activity, contribute, and there are, of course, social, cultural and psychological aspects. We will deal below with the imbalance of homeostatic mechanisms and genetic endowment in obesity. The role of social, cultural and psychological aspects we will leave (with a profound sigh of relief) to the psychosociologists!
Food Intake and Obesity
As Spiegelman & Flier (1996) point out, ‘one need not be a rocket scientist to notice that increased food intake tends to be associated with obesity’. A typical obese subject will usually have gained 20 kg over a decade or so. This means that there has been a daily excess of energy input over energy requirement of 30–40 kcal initially, increasing gradually to maintain the increased body weight.
The type of food eaten, as well as the quantity, can disturb energy homeostasis. Fat is an energy-dense foodstuff, and it may be that the mechanisms regulating appetite react rapidly to carbohydrate and protein, but too slowly to fat to stop an individual consuming too much before the satiety systems come into play.
However, when obese individuals reduce their calorie intake as part of a diet regime, they shift into negative energy balance. When they lose weight, the resting metabolic rate decreases, and there is a concomitant reduction in energy expenditure. Thus an individual who was previously obese and is now of normal weight generally needs fewer calories to maintain that weight than an individual who has never been obese. The decrease in energy expenditure appears to be largely caused by an alteration in the conversion efficiency of chemical energy to mechanical work in the skeletal muscles. This adaptation to the caloric reduction contributes to the difficulty of maintaining weight loss by diet.
Physical Exercise and Obesity
It used to be said that the only exercise effective in combating obesity was pushing one’s chair back from the table. It is now recognised that physical activity—i.e. increased energy expenditure—has a much more positive role in reducing fat storage and adjusting energy balance in the obese, particularly if associated with modification of the diet. An inadvertent, natural population study provides an example. Many years ago, a tribe of Pima Indians split into two groups. One group settled in Mexico and continued to live simply at subsistence level, eating frugally and spending most of the week in hard physical labour. They are generally lean and have a low incidence of type 2 diabetes. The other group moved to the USA—an environment with easy access to calorie-rich food and less need for hard physical work. They are, on average, 57 lb (26 kg) heavier than the Mexican group and have a high incidence of early-onset type 2 diabetes.
Obesity as a disorder of the homeostatic control of energy balance
The long-term regulation of energy balance by adiposity signals such as leptin and insulin must obviously occur against a background of day-to-day variations in meal size, frequency and content.6 Because the homeostatic control of energy balance is extremely complex, it is not easy to determine exactly what goes wrong in obesity. When the leptin story unfolded, it was thought that alterations in leptin kinetics might provide a simple explanation. There is a considerable interindividual variation in sensitivity to leptin, and some individuals seem to produce insufficient amounts of this hormone. Paradoxically, however, plasma leptin is often higher in obese individuals compared with non-obese subjects, not lower as might be expected. The reason for this is that resistance to leptin rather than insufficient hormone is more prevalent in obesity. Such resistance could be caused by defects in leptin carriage in the circulation, transport into the CNS, in leptin receptors in the hypothalamus (as occurs in db/db mice) or in postreceptor signalling.
Mediators other than leptin are certainly implicated in obesity. For example, TNF-α, a cytokine that can relay information from fat tissue to brain, is increased in the adipose tissue of insulin-resistant obese individuals. Another pathophysiological alteration in obesity is a reduced insulin sensitivity of muscle and fat, and decreased β3-adrenoceptor function in brown adipose tissue (see above) may also occur; alternatively, UCP-2, one of the proteins that uncouple oxidative phosphorylation in adipocytes, may be dysfunctional in obese individuals.
A further suggestion is that alterations in the function of specific nuclear receptors, such as PPARα, β and γ, may play a role in obesity. These receptors regulate gene expression of enzymes associated with lipid and glucose homeostasis, and they also promote the genesis of adipose tissue. PPARγ is expressed preferentially in fat cells and synergises with another transcription factor, C/EBPα, to convert precursor cells to fat cells (see Spiegelman & Flier, 1996). The gene for UCP (see above) in white fat cells also has regulatory sites that respond to PPARα and C/EBPα. The thiazoladinediones bind to and activate PPARγ (see Ch. 30). One of these, pioglitazone, is licensed in the UK for treatment of type 2 diabetes and both cause weight gain. The pathophysiology of obesity could involve disturbance(s) in any of the multitude of other factors involved in energy balance.
Genetic Factors and Obesity
Analyses of large-scale (> 100 000) studies in human monozygotic and dizygotic twin pairs indicate that 50–90% of the variance of BMI can be attributed to genetic factors, and suggest a relatively minor role for environmental factors (Barsh et al., 2000). This conclusion may seem surprising, but feeding studies using laboratory rodents where food intake is held constant have demonstrated the importance of genetic background to body weight regulation, and this is especially true for high-fat diets. The prevailing viewpoint is that susceptibility to obesity is largely determined by genetic factors, while environmental factors determine the expression of the disease.
The discovery that spontaneous mutations arising in single genes (e.g. the ob/ob genotype) produced obese phenotypes in mice led to a search for equivalent genes in humans. A review (Pérusse et al., 2005) identified over 170 human obesity cases that could be traced to single gene mutations in 10 different genes. Leptin receptor or POMC mutations are sometimes observed, but melanocortin (MC)4 receptor mutations seem to be more prevalent (3–5%) in obese patients (e.g. see Barsh et al., 2000). In general, however, human obesity should be regarded as a polygenic disorder involving the interaction of many genes. At the time of writing, some 600 genes, markers and chromosomal regions are under investigation for linkage to human obesity (Pérusse et al., 2005).
Other genes that appear to be involved include the β3-adrenoceptor and the glucocorticoid receptor. Decreased function of the β3-adrenoceptor gene could be associated with impairment of lipolysis in white fat or with thermogenesis in brown fat. A mutation of this gene has been found to be associated with abdominal obesity, insulin resistance and early-onset type 2 diabetes in some subjects and a markedly increased propensity to gain weight in a separate group of morbidly obese subjects. Alterations in the function of the glucocorticoid receptor could be associated with obesity through the permissive effect of glucocorticoids on several aspects of fat metabolism and energy balance. The significance of polymorphisms in the ghrelin gene has already been mentioned.
Obesity ![]()
Pharmacological Approaches to the Problem of Obesity
The first weapons in the fight against obesity are diet and exercise. Unfortunately, these often fail or show only short-term efficacy, leaving only surgical techniques (such as gastric stapling or bypass)7 or drug therapy as a viable alternative. Surgery is much more effective than currently licensed drugs.
The attempt to control appetite with drugs has had a long and largely undistinguished history. Many types of ‘anorectic’ (e.g. appetite suppressant) agents have been tested in the past, including the uncoupling agent dinitrophenol (DNP), amphetamines and fenfluramine. However, these are no longer used and the only drug currently (2010) licensed in the UK for the treatment of obesity is orlistat (see below). It should not be used without concomitant dietary and other therapy (e.g. exercise). As might be imagined, the quest for further effective antiobesity agents is the subject of a prodigious effort by the pharmaceutical industry.
Sibutramine
Sibutramine inhibits the reuptake of 5-HT and noradrenaline at the hypothalamic sites that regulate food intake.8 Its main effects are to reduce food intake and cause dose-dependent weight loss (see Fig. 31.3), the weight loss being associated with a decrease in obesity-related risk factors. Sibutramine enhances satiety and is reported to produce a reduction in waist circumference (i.e. a reduction in visceral fat), a decrease in plasma triglycerides and very-low-density lipoproteins, but an increase in high-density lipoproteins. In addition, beneficial effects on hyperinsulinaemia and the rate of glucose metabolism are said to occur. There is some evidence that the weight loss is associated with higher energy expenditure, possibly through an increase in thermogenesis mediated by the sympathetic nervous system.
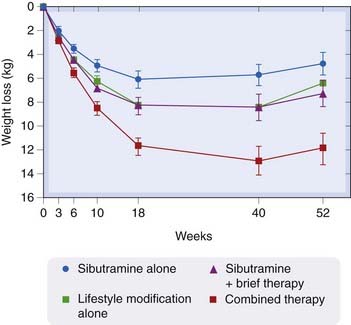
Fig. 31.3 The effect of treatment with sibutramine alone or in combination with lifestyle modification.
In this study, 224 obese patient were given sibutramine alone, lifestyle modification counselling alone or sibutramine together with a ‘brief’ or more extensive programme of lifestyle counselling. The Y-axis shows the weight loss in kg (± SE) over time (X-axis). It is evident that sibutramine is far more effective as a weight-loss therapy when combined with changes in patients’ lifestyle. This is a common experience when treating obesity
(From Wadden et al. 2005.)
A meta-analysis of three long-term treatment studies comparing sibutramine with placebo (Padwal et al., 2003) concluded that there was a 4.6% loss of weight after 1 year’s treatment with the drug, and a 15% increase in the number of patients who lost more than 10% of their body weight. Sibutramine was much more effective when combined with lifestyle modification (Wadden et al., 2005) and it was usually recommended only in conjunction with such measures.
The marketing authorisation for sibutramine was recently suspended by the European Medicines Agency because of concerns that its cardiovascular risks (see below) outweighed its benefits.
Pharmacokinetic Aspects and Unwanted Effects
Sibutramine is given orally; it is well absorbed and undergoes extensive first-pass metabolism. The metabolites are responsible for the pharmacological actions. Steady-state blood levels of the metabolites occur within 4 days. The active metabolites are inactivated in the liver, and 85% of the inactive residues are excreted in the urine and faeces.
Sibutramine increases heart rate and blood pressure and is contraindicated in hypertension, which often co-exists with obesity. Other unwanted effects include dry mouth, constipation, insomnia and drug interactions (e.g. antidepressants, see Ch. 46).
Orlistat
In the intestine, orlistat reacts with serine residues at the active sites of gastric and pancreatic lipases, irreversibly inhibiting these enzymes and thereby preventing the breakdown of dietary fat to fatty acids and glycerols. It therefore decreases fat absorption and correspondingly increases faecal fat excretion up to some 30% of dietary fat. Given in conjunction with a low-calorie diet in obese individuals, it produces a modest but consistent loss of weight compared with placebo-treated control subjects. In a meta-analysis of 11 long-term placebo-controlled trials encompassing over 6000 patients, orlistat was found to produce a 2.9% greater reduction in body weight than in the control group, and 12% more patients lost 10% or more of their body weight compared with the controls (Padwal et al., 2003).
Orlistat is also reported to be effective in patients suffering from type 2 diabetes and other complications of obesity, to reduce leptin levels and blood pressure, to protect against weight loss-induced changes in biliary secretion, to delay gastric emptying and gastric secretion, to improve several important metabolic parameters and not to interfere with the release or action of thyroid and other important hormones (Curran & Scott, 2004). It does not induce changes in energy expenditure.
Pharmacokinetic Aspects and Unwanted Effects
Virtually all (97%) of orlistat is excreted in the faeces (83% unchanged), with only negligible amounts of the drug or its metabolites being absorbed.
Abdominal cramps, flatus with discharge and faecal incontinence can occur, as can intestinal borborygmi (rumbling) and oily spotting. Surprisingly, in view of the possibility of these antisocial effects occurring, the drug is well tolerated. Supplementary therapy with fat-soluble vitamins may be needed. The absorption of contraceptive pills and ciclosporin (see Ch. 26) may be decreased. The former is probably not clinically significant but the latter is more serious. Given its good safety record, orlistat has recently been licensed for inclusion in some over-the-counter medicines for weight loss.
New Approaches to Obesity Therapy
Rare cases of leptin deficiency in patients have been successfully treated by long-term treatment with the hormone, but this is an unusual intervention and unlikely to be of more than limited use in the future. Many other approaches are being piloted; in fact, a comprehensive review of the area estimated that there were more than 150 novel agents under development (Kaplan, 2005). Some of these aim to exploit the action or production of neuroendocrine satiety signals such as CCK to produce appetite suppression. Many of these GI satiety hormones produce such effects when given systemically to humans or rodents although these are not always useful; for example, CCK reduces meal size but increases meal frequency (West et al., 1984).
Other strategies aim to alter the CNS levels of neurotransmitters such as NPY or melanocortins, which transduce changes in these hormonal signals (Halford, 2006). The tractability of the MC4 receptor itself as a drug target, coupled with the observation that defects in MC4 signalling are prevalent in obesity, has attracted much interest from the pharmaceutical industry.
Given the importance of the sympathetic nervous system in the control of energy regulation, one might predict that β3-adrenoceptor agonists might be useful therapeutics. This field has been extensively researched (see Arch, 2008, for a recent review) but disappointingly has so far failed to produce an acceptable drug. The search continues.
Another novel approach originated from research in the cannabinoid field (see Ch. 18 for further details). As noted above, the endocannabinoid system is involved in the regulation of feeding behaviour and from this observation arose the idea that this could be a useful site of pharmacological intervention. Such a drug was the CB1 receptor antagonist rimonabant that was originally developed for smoking cessation. This drug was introduced into therapy following some encouraging clinical trails (see Fig. 18.5) but was eventually withdrawn in 2008 because of adverse effects on mood seen in some patients.
References and Further Reading
Adan R.A., Vanderschuren L.J., la Fleur S.E. Anti-obesity drugs and neural circuits of feeding. Trends Pharmacol. Sci.. 2008;29:208-217. (Very accessible overview of the area. Recommended)
Ahima R.S., Flier J.S. Leptin. Annu. Rev. Physiol.. 2000;62:413-437. (Comprehensive review of leptin: its expression, actions in hypothalamus, role in energy homeostasis and other actions)
Ahima R.S., Osei S. Molecular regulation of eating behaviour: new insights and prospects for future strategies. Trends Mol. Med.. 2001;7:205-213. (Praiseworthy short review; excellent figures and useful tables of the mediators involved in stimulation and inhibition of feeding behaviour)
English P.J., Ghatei M.A., Malik I.A., et al. Food fails to suppress ghrelin levels in obese humans. J. Clin. Endocrinol. Metab.. 2002;87:2984-2987.
Farooqi I.S., Jebb S.A., Langmack G., et al. Effects of recombinant leptin therapy in a child with congenital leptin deficiency. N. Engl. J. Med.. 1999;341:879-884. (A classic clinical paper on the role of leptin in the control of feeding behaviour and weight control)
Frühbeck G., Gómez-Ambrosi J., Muruzábal F.J., Burrell M.A. The adipocyte: a model for integration of endocrine and metabolic signalling in energy metabolism regulation. Am. J. Physiol. Endocrinol. Metab.. 2001;280:E827-E847. (Detailed review covering receptors on and the factors secreted by the fat cell, and the role of these factors in energy homeostasis)
Kennedy G.C. The role of depot fat in the hypothalamic control of food intake in the rat. Proc. R. Soc. Lond. B. Biol. Sci.. 1953;140:578-592. (The paper that put forward the proposal, based on experiments on rats, that there was a hypothalamus-based homeostatic mechanism for controlling body fat)
Schwartz M.W., Woods S.C., Porte D.J., et al Central nervous control of food intake Nature 404:2000:661-671 (Outlines a model that delineates the roles of hormones and neuropeptides in the control of food intake. Outstanding diagrams. Note that there are several other excellent articles in this Nature Insight supplement on obesity)
Spiegelman B.M., Flier J.S. Adipogenesis and obesity: rounding out the big picture. Cell. 1996;87:377-389.
Spiegelman B.M., Flier J.S. Obesity regulation and energy balance. Cell. 2001;104:531-543. (Excellent review with up-to-date coverage of the CNS control of energy intake/body weight, monogenic obesities, leptin physiology, central neural circuits, the melanocortin pathway, the role of insulin and adaptive thermogenesis)
Weigle D.S. Appetite and the regulation of body composition. FASEB J.. 1994;8:302-310.
Colditz G.A., Willett W.C., Rotnitzky A., Manson J.E. Weight gain as a risk factor for clinical diabetes mellitus in women. Ann. Intern. Med.. 1995;122:481-486.
Kopelman P.G. Obesity as a medical problem. Nature. 2000;404:635-643.
Barsh G.S., Farooqi I.S., O’Rahilly S. Genetics of body-weight regulation. Nature. 2000;404:644-651.
Loos R.J., Rankinen T. Gene–diet interactions on body weight changes. J. Am. Diet. Assoc.. 2005;105(Suppl. 1):S29-S34. (Discusses gene–environment studies relating to obesity, drawing on data from monozygotic twins and candidate gene approaches)
Pérusse C., Rankinen T., Zuberi A., et al. The human obesity gene map: the 2004 update. Obes. Res.. 2005;13:381-490. (Detailed review of the genes, markers and chromosomal regions that have been shown to be associated with human obesity)
Zhang Y., Proenca R., Maffei M., et al. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425-432.
Appolinario J.C., Bueno J.R., Coutinho W. Psychotropic drugs in the treatment of obesity: what promise? CNS Drugs. 2004;18:629-651.
Chiesi M., Huppertz C., Hofbauer K.G. Pharmacotherapy of obesity: targets and perspectives. Trends Pharmacol. Sci.. 2001;22:247-254. (Commendable, succinct review; table of the potential targets, and useful, simple figures of the central and peripheral pathways of energy regulation and of the regulation of thermogenesis)
Clapham J.C., Arch J.R.S., Tadayyon M. Anti-obesity drugs: a critical review of current therapies and future opportunities Pharmacol. Ther. 89:2001:81-121 (Comprehensive review covering, under energy intake; biogenic amines, cannabinoids, neuropeptides, leptin, gastrointestinal tract peptides and inhibitors of fat absorption; and under energy expenditure, β3-adrenoceptor agonists and uncoupling proteins)
Collins P., Williams G. Drug treatment of obesity: from past failures to future successes? Br. J. Clin. Pharmacol.. 2001;51:13-25. (Overview—from a clinical perspective—of currently available antiobesity drugs and potential future drugs; well written)
Crowley V.E.F., Yeo G.S.H., O’Rahilly S. Obesity therapy: altering the energy intake-and-expenditure balance sheet. Nat. Rev. Drug Discov.. 2002;1:276-286. (Review stressing that pharmacological approaches to obesity therapy necessitate altering the balance between energy intake and expenditure and/or altering the partitioning of nutrients between lean tissue and fat)
Curran M.P., Scott L.J. Orlistat: a review of its use in the management of patients with obesity. Drugs. 2004;64:2845-2864.
James W.P.T., Finer N., Kopelman P., et al. Effect of sibutramine on weight maintenance after weight loss: a randomised trial. Lancet. 2000;256:2119-2125. (Report of the results of a multicentre, randomised, double-blind clinical trial)
Padwal R., Li S.K., Lau D.C. Long-term pharmacotherapy for overweight and obesity: a systematic review and meta-analysis of randomized controlled trials. Int. J. Obes. Relat. Metab. Disord.. 2003;27:1437-1446.
Wadden T.A., Berkowitz R.I., Womble G., et al. Randomized trial of lifestyle modification and pharmacotherapy for obesity. N. Engl. J. Med.. 2005;353:2111-2120.
Future drug treatments for obesity
Arch J.R. The discovery of drugs for obesity, the metabolic effects of leptin and variable receptor pharmacology: perspectives from beta3-adrenoceptor agonists Naunyn. Schmiedebergs Arch. Pharmacol. 378:2008:225-240 (A comprehensive review that focuses on the quest for antiobesity drugs that act through the β3-adrenoceptor. Useful comments and insights into the field as a whole)
Di Marzo V., Matias I. Endocannabinoid control of food intake and energy balance. Nat. Neurosci.. 2005;8:585-589. (A discussion of the putative role of endocannabinoids in this complex physiological mechanism; also considers therapeutic applications arising from this area)
Fong T.M. Development of anti-obesity agents: drugs that target neuropeptide and neurotransmitter systems. Expert Opin. Investig. Drugs. 2008;17:321-325. (Deals with drugs in late-stage development that target the regulatory neuropeptide pathways discussed in this chapter)
Halford J.C. Obesity drugs in clinical development. Curr. Opin. Investig. Drugs. 2006;7:312-318.
Kaplan L.M. Pharmacological therapies for obesity. Gastroenterol. Clin. North Am.. 2005;34:91-104.
Lefebvre P.J., Scheen A.J. Obesity: causes and new treatments. Exp. Clin. Endocrinol. Diabet.. 2001;109(Suppl. 2):S215-S224.
Mertens I.L., Van Gaal L.F. Promising new approaches to the management of obesity. Drugs. 2000;60:1-9.
West D.B., Fey D., Woods S.C. Cholecystokinin persistently suppresses meal size but not food intake in free-feeding rats. Am. J. Physiol.. 1984;246:R776-R787.
Wilding J.P.H. Pharmacotherapy of obesity. (Ed.). Parnham M.J., Bruinvels J., editors. Milestones in drug therapy. Basel: Birkhäuser, 2008. (This book covers a wide variety of topics connected with obesity and its therapy. The contributors are experts in the field)
http://www.who.int (This is the World Health Organization Web page that carries data about the prevalence of ‘globesity’ and its distribution around the world; click on the Health topics link and navigate to Obesity in the alphabetical list of topics for further information)
1‘Persons who are naturally very fat are apt to die earlier than those who are slender’ observed Hippocrates.
2The word is derived from the Greek leptos, meaning thin.
3The language can be confusing. ‘Hunger’ obviously refers to the desire to eat; ‘satiation’ is the feeling that you have eaten enough. ‘Satiety’ refers to the feeling that one will postpone the next meal.
4So called because the administration of cocaine or amphetamine stimulates the transcription of this gene. Its expression in the hypothalamus is related to nutritional status implicating it in the control of appetite. Its receptor is unknown but it probably modulates the action of NPY and leptin.
5This effect is responsible for the ‘munchies’, a common side effect of smoking cannabis.
6Even the type of gut flora has come under scrutiny as a potential determining factor in obesity. The notion that this could be supplemented with probiotics to modify the risk is obviously attracting attention. ‘Holy shit!’ was the title of one magazine article on the subject (The Economist, 12 November 2009).
7Such bariatric (weight loss) surgery owes at least part of its efficacy to the changes in blood levels of the hormones that regulate feeding behaviour.
8Many antidepressant drugs act by the same mechanism (see Ch. 46), and also cause weight loss by reducing appetite. However, sibutramine does not have antidepressant properties. Furthermore, depressed patients are often obese, and antidepressant drugs are used to treat both conditions (see Appolinario et al., 2004).